Abstract
The formation and characterization of positively surface charged TiN surfaces were investigated for improving dental implant survival. Surface nitrogen atoms of a traditional TiN implant were converted to a positive charge by a quaternization reaction which greatly increased the antibacterial efficiency. Ti, TiN, and quaternized TiN samples were incubated with human patient subgingival bacteria for 4 hours at 37°C in an anaerobic environment with an approximate 40% reduction in counts on the quaternized surface over traditional Ti and TiN. The samples were challenged with Streptococcus Mutans and fluorescent imaging confirmed significant reduction in the quaternized TiN over the traditional Ti and TiN. Contact angle measurement and X-Ray Photoelectron Spectroscopy (XPS) were utilized to confirm the surface chemistry changes. The XPS results found the charged quaternized nitrogen peak at 399.75 eV that is unique to the quaternized sample.
Keywords: antibiotics, quaternary, Titanium, peri-implantitis, Menschutkin
Graphical Abstract
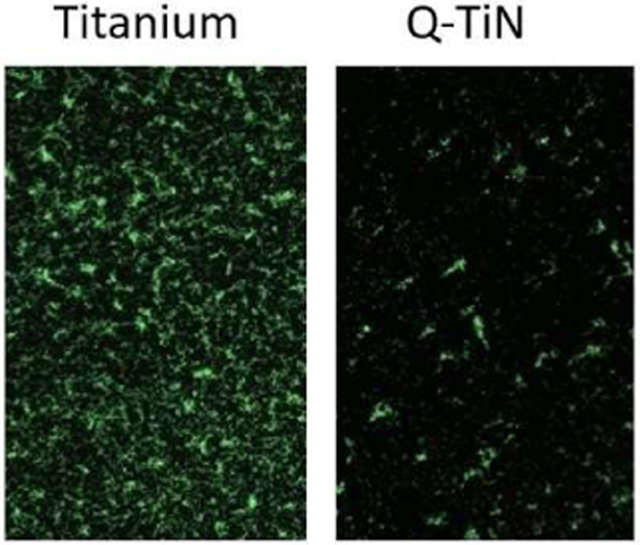
In this study, off the shelf Titanium Nitride implants were subjected to a single-step rapid reaction to improve their antibacterial efficiency by the conversion of surface nitrogen atoms to positively charged quaternary specie. This conversion greatly improved antibacterial efficiencies against general sub gingival bacteria along with early and late colonizers associated with peri-implantitis.
Introduction
Approximately 5% of all dental implants anchored by osseointegration will fail within 10 years.[1] A primary culprit for implant failure is peri-implantitis, which is site specific bacterial infection leading to bone loss around the implant and soft tissue inflammation. Controlling the inflammation through improved hygiene by reducing microbial activity is the only current method to combat potential implant failure, as there is no conclusive treatment to stop infection and progression of the disease.[2] Anywhere from 10% to 45% of all dental implants will have detectable peri-implantitis inflammatory reactions. Characterizing peri-implantitis has proven to be difficult due to many factors such as patients’ dental history, medical conditions, hygiene and eating habits.[3] For example, patients with previous history of periodontal disease have lower implant survival rates, but diseases such as osteoporosis or diabetes have not demonstrated consistent effects.[2a,b]
In addition, the bacterial flora has been explored to determine a prevalence of certain bacteria which may lend to formation of peri-implantitis. The sub-gingival flora of sites with peri-implantitis have similar flora to that of periodontitis with mostly gram-negative bacteria.[4] Persson et al. identified 19 bacterial species with higher counts at the site of peri-implantitis infection than at implant sites without infection, with seven bacteria strains accounting for 30% of bacterial flora at the infection site as compared to 14% at non-infected implants.[4a] These results indiciate that peri-implantitis may not directly result from a singular bacteria strain infecting the implant site, rather an imbalance of a healthy flora.
Use of antibacterial coatings may provide a method for improving implant lifetime. Even use of different metals in an implant has significant effect on bacterial growth. Titanium nitride (TiN) is the most promising metal as this promotes osseointegration while also having lower bacterial growth rate than traditional titanium (Ti) implants.[5] Current implants utilize several microns thick TiN by plasma spraying coatings on implant surfaces. Despite this, peri-implantitis still exists with these types of implants. Many groups have explored addition of metallic particles to the implant such as copper, silver, and metal oxides;[6] however, a galvanic effect was reported where patients who had gold or amalgam in their teeth concurrently with Ti implants displayed yellow nails and were found to have Ti in their nail clippings.[7] Thus, the additional metallic elements in Ti may enhance Ti and other metals to dissolve into the bodily environment. Primarily, the impact of metal dissolution into the surrounding tissue may facilitate peri-implant inflammatory reactions.[8]
Molecules with quaternary nitrogen atoms have shown the ability to disrupt the cell wall, leading to leakage of the cell contents and eventual apoptosis of the bacteria.[9] To minimize concerns over dissolute metallic species undergoing unfavorable interactions with the surrounding tissues, modifying the nitrogen atoms on TiN surface into quaternary nitrogen atoms may be a better alternative to inhibit peri-implant inflammatory reactions.
In this study, we aim to test the effectiveness of quaternized TiN on reducing bio-film growth by employing allyl bromide through the Menschutkin reaction to convert nitrogen atoms on the TiN surface into quaternary nitrogen. Sessile drop contact angle and x-ray photoelectron spectroscopy (XPS) were employed to confirm the nitrogen atoms were converted into quaternary nitrogen. The objectives of this study are to test the hypotheses that: (1) quaternized TiN will have greater antibacterial properties compared with Ti and TiN as a function of colony forming units and (2) bacterial film thickness will affect the anti-microbial properties of these coatings.
Results
ANOVA (R2=.288, adjusted R2=.250, F statistic=7.6 on 5 and 94 degrees of freedom) revealed that coating material is significantly associated with bacteria level (p<.0001). Pairwise comparisons among material groups show that across all thicknesses: (1) Quaternized TiN (Q) is significantly lower than Titanium (T) (p<.0001) and for any random thickness or experimental run, Q is estimated to be 45.6 units lower than T (95% CI=[−65.0, −26.2]); (2) Q is significantly lower than Titanium nitride (N) (p=.0003) and for any random thickness or experimental run, Q is estimated to be 37.1 units lower than N (95% CI=[−56.8, −17.4]); (3) T and N are not significantly different (p=.392) (Figure 1).
Figure 1.
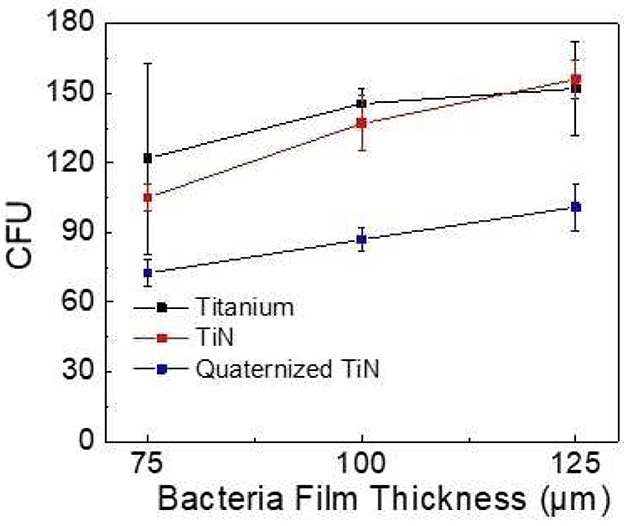
Colony forming unit (CFU) of bacteria growth plotted for Ti, TiN or quaternized TiN substrate and different film thicknesses.
Bacteria thickness is significantly associated with bacteria level (p=.002). Pairwise comparisons among thickness groups show that across all materials: (1) 100 μm yields marginally higher values than 75 μm (p=.060), and for any random material and experimental run, 100μm is estimated to be 18.9 units higher than 75 μm (95% CI=[−0.778, 38.6]); (2) 125 μm yields significantly higher values than 75 μm (p=.0004) and for any random material and experimental run, 125 μm is estimated to be 38.2 units higher than 75 (95% CI=[16.6, 55.7]); (3) 125 μm yields marginally higher values than 100 (p=.082) and for any random material and experimental run, 125 μm is estimated to be 17.3 units higher than 100 (95% CI=[−2.24, 38.8]) (Figure 2). The interaction between coating material and bacterial thickness is p=0.977 indicating that the anti-bacterial effectiveness of the coatings is not expected to be affected by the thickness of bacteria. Experimental run was not associated with bacteria level (p=.450).
Figure 2.
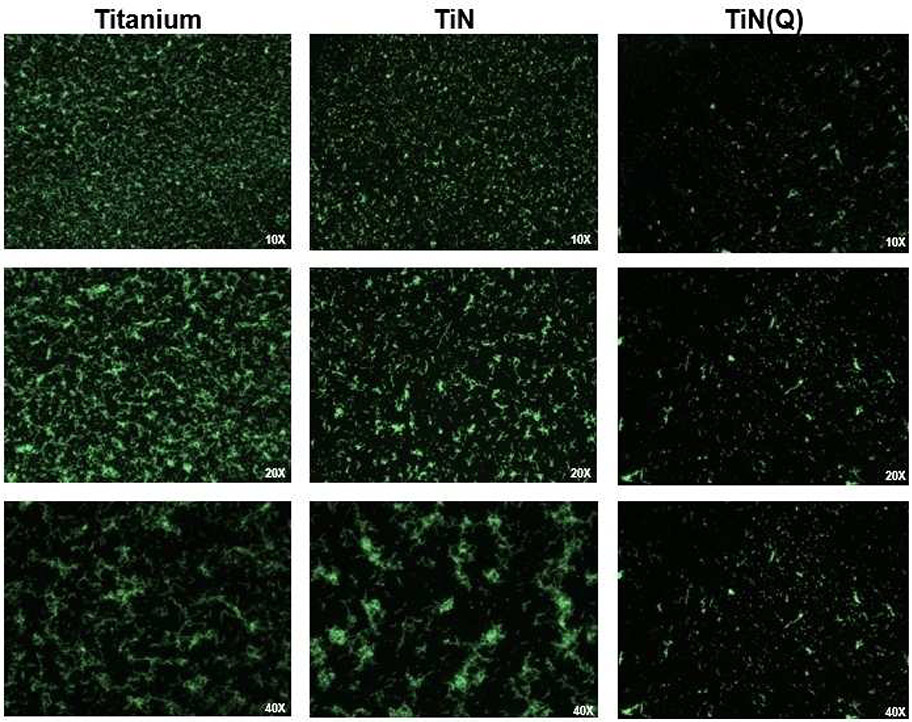
Fluorescence microscopy images of S. mutans cultured for 4 h on Ti, TiN or quaternized TiN substrate.
The result from the staining assay shows a significant reduction in the number of live bacteria, on the quaternized TiN samples, as shown by the fluorescence images on Figure 2. The calculation of live bacteria coverage indicate the bactericidal effect of the quaternized TiN samples against S. mutans after 4 h of culture, significantly decreased the number of live bacteria (Figure 4).
Figure 4.
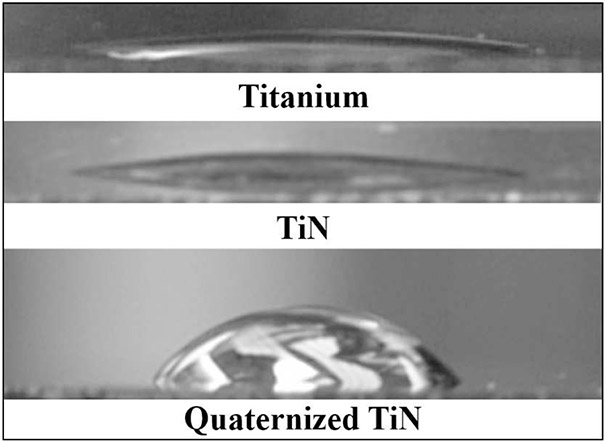
Contact angle images of Ti, TiN and quaternized TiN surface.
Pairwise comparisons among material groups show regard to live bacteria coverage: (1) Quaternized TiN (Q) (10.85%) is significantly lower than Titanium (T) (89.1%) (p=<.0001); (2) Q is significantly lower than Titanium nitride (N) (82.2%) (p<.0001); (3) T and N are not significantly different (p=.312) (Figure 3).
Figure 3.
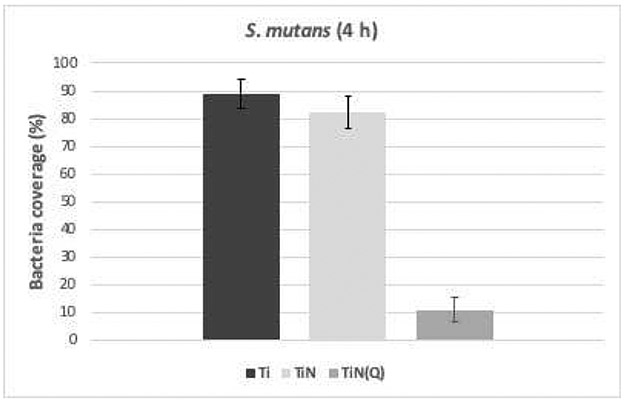
Live bacteria coverage of S. mutans cultured for 4 h on Ti, TiN or quaternized TiN substrate.
Sessile contact angle measurement images of part of a single water droplet placed on Ti, TiN or quaternized TiN surface are shown in Figure 4. For both Ti and TiN substrates, a small contact angle was observed indicating that Ti and TiN surface were hydrophilic and had good wettability (Figure 3). On the contrary, a larger contact angle was obtained on the quaternized TiN surface. Table 1 lists the specific average contact angles and standard deviations of Ti, TiN and quaternized TiN substrates. The average contact angle measurements were 12° for Ti; 16° for TiN and 67° for quaternized TiN substrates. zeta potential measurements were used to characterize the change in surface charge density of the samples. Surface charge density measurements were taken in a slightly acidic solution of pH 5.5. As deposited TiN and quaternized TiN exhibited surface charge densities of 1.19 × 10−7 C/cm2 and 1.81 × 10−7 C/cm2, respectively.
Table 1.
Sessile contact angle measurements performed on Ti, TiN and quaternized TiN substrate
| Sample | Contact Angle ( ° ) |
|---|---|
| Titanium after H2O2 clean | 12 ± 2 |
| TiN after clean | <6 |
| TiN in solvent 120 mins (no reagent) | 16 ± 2 |
| TiN Quaternization 30 mins | 67 ± 1 |
| TiN Quaternization 60 mins | 72 ± 3 |
| TiN Quaternization 120 mins | 71 ± 2 |
The stacked XPS survey scans of the TiN surface and the quaternized TiN surface are shown in Figure 5a. The spectra were aligned through the presence of adventitious carbon peak at 284.8 eV from the atmospheric exposure during handling and transport between deposition tools. The general surface chemistry between the two samples is similar as only Ti, O, N, and C were identified in both samples.
Figure 5.
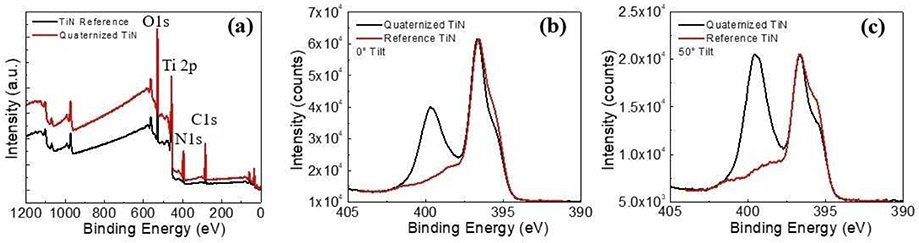
(a) Stacked XPS survey scans of reference TiN and quaternized TiN surface, (b) 0°, and (c) 50° tilted high resolution nitrogen 1 s XPS spectra of TiN and quaternized TiN surface.
The Nitrogen 1 s spectra acquired at different tilting angles for TiN and quaternized TiN sample are shown for 0° (Figure 5b) and 50° (Figure 5c). The main N peak corresponding to Ti─N bonds is located at 396.75 eV and there is an N satellite peak at 398.48 eV. The quaternized nitrogen, N+, peak is 399.75 eV. As compared to the 0° spectra, surface effects are more pronounced for the tilted spectra, and the penetration depth of the x-ray source is greatly reduced.
Discussion
Confirmation of Surface Reaction
The integration and longevity of a dental implant requires the interface between bone and implant remain bacteria free. TiN has been shown to greatly improve development of bone around an implant and decrease the bacteria count over Ti implants.[5] Prior to evaluating the effectiveness of quaternized TiN on biofilm reduction, the success of converting surface bound nitrogen from uncharged to positively charged nitrogen needs to be verified. As shown in Figure 4 and Table I, the contact angles of Ti and TiN substrates were very low. The titanium sample after treatment with hydrogen peroxide was expected to be very hydrophilic because of the presence of Ti-O─H and Ti=O on Ti surface. The TiN sample was also very hydrophilic owing to the high polarity of TiN and the ability for hydrogen bonding of the water droplet to the nitrogen rich surface of TiN. For the quaternized TiN surface, surface nitrogen was converted into a positively charged nitrogen through the Menschutkin reaction and the contact angle should be low. However, as shown in Figure 4 and Table I, the contact angle of the quaternized TiN surface dramatically increased from 16 to 67°. This increase in hydrophobicity is due to the carbon chains extending from the surface bound nitrogen, as illustrated in equation (1) and Figure 3.
| (1) |
Menschutkin reactions are known to progress very rapidly due to the lower enthalpy of formation of higher substituted amines, which is evident by the reaction saturating within an hour. Hence the Menschutkin reaction is well suited for formation of quaternary ammonium salts and is difficult to stop at secondary or tertiary products.[10]
XPS surface analysis was employed to further confirm and identify changes in surface chemistry of quaternized TiN surface. As shown in Figure 6, the wide range analyses were acquired from the surface of the TiN and quaternized TiN substrates and Ti, O, N and C were the only elements identified on these two samples. This indicates that the general surface chemistry of TiN and quaternized TiN samples are identical. Therefore, a detailed peak analysis was necessary for confirmation of the surface reaction. High resolution XPS spectra of the N 1s regions were acquired at 0 (Figure 6b) and 50° (Figure 6c) angle with respect to the normal for the TiN and quaternized TiN surface. The main N peak corresponding to Ti─N bonds was located at 396.75 eV and there was an N satellite peak at 398.48 eV. The shoulder peak of N for Ti─N at 394.5 eV was resultant of N on N─Ti-O. The XPS peak[11] for quaternized nitrogen bound to the allyl group (−CH3CH5), N+, was 399.75 eV. Surface effects were more pronounced for the spectra taken at a 50° tilt and the penetration depth of the x-ray source was greatly reduced, compared with the 0° spectra, . For the N spectra of TiN substrate, the peak corresponding to the N of N─Ti-O was more pronounced because of the presence of oxide on the TiN surface. For the N 1s spectra of the quaternized nitrogen, this effect was evident in the increased relative intensity of the quaternized nitrogen peak at 399.75 eV compared with the TiN peak at 396.5 eV at 50°; indicating that N+ atoms are situated on the surface of quaternized TiN. In addition, after a gentle sputtering with 500 eV Ar ions for 30 seconds to remove the top atomic layers of the quaternized TiN substrate, the quaternized nitrogen peak had completely disappeared. This also confirmed that quaternized nitrogen only exists on the top surface of the quaternized TiN substrate.
Impact of Quaternized TiN Surface on Biofilm Growth
As previously stated, the oral microbiome is incredibly diverse, and no singular bacteria has been identified as the primary causating agent of peri-implantitis. Thus, preventing all types of microbial growth near the implant site may provide the best avenue for implant survival. The variance experienced with efficacy is expected as each patient’s flora will vary day-to-day and may respond differently depending on environmental factors such as diet, hygiene, periodontal condition, etc.
This study shows the potential anti-microbial effect of quaternized TiN compared with Ti and TiN at reducing CFUs (Figure 2) and bacteria coverage (Figure 3). The antibacterial mechanism of quaternized nitrogen has been proposed to interact with the cell wall, destroy the cytoplasmic membrane leading to the leakage of intracellular components and consequent cell death.[12] The quaternized surface outperformed both the TiN and Ti surfaces significantly within the short 4-hour testing period for bacteria film thickness of 75 μm. Even with thick bacteria films that are 125μm thick, the quaternized surfaces still significantly reduced bacterial counts compared with the other two substrates, indicating that the surface has the ability to neutralize bacteria that are not directly in contact with the surface. The lack of statistical significance in interaction between coating type and thickness indicates that the superior anti-bacterial properties of quaternized TiN versus TiN and Ti is evident across different thicknesses of bacteria. Also, the fluorescent images showed the proliferation of live bacteria was lower in quaternized TiN compared to Ti and TiN. Implant surfaces should have the ability to affect bacteria that is not only in direct contact with the surface but also in the immediate vicinity. This is because during the process of osseointegration, the implant will initially be surrounded by saliva and soft tissue. Additionally, failure of an implant will be induced by an infection beginning near the soft tissue which will propagate downward towards the base of the implant. However, with the quaternized surface that propagation can be hindered or completely prevented.
Conclusion
A novel application of the well-known Menschutkin reaction has been applied to convert the surface nitrogen of a TiN coated implant from neutral to positively charged. The surface change was monitored and confirmed by Sessile contact angle and XPS measurements. Biofilm growth noted a 40–50% reduction in bacteria over traditional implant surfaces within 4 hours and noticeable effects many microns away. Considering current technology and other works pursuing high biocidal activity for implant structures, this methodology provides a simple method that would require little manufacturing line changes to accommodate and bring to market.
Supplementary Material
Acknowledgements
NIH-NIDCR Grant R01 DE025001 supported this study. Ceramic materials were supplied by Ivoclar Vivadent. Dektak was performed at the Nanoparticle Research Facility at the University of Florida. The XPS study was supported by a grant financed in the framework of Competitiveness Operational Program 2014–2020, ANCSI/MFE, project number MIFID ID P_39_366 Cod MySMIS 104809 and INFLPR Program NUCLEU-LAPLAS V.
Footnotes
Supporting Information Summary
Supporting information PDF contains experimental processes for fabrication of all sample types and details the characterization methodology used for each different section of the manuscript.
Conflict of Interest
US provisional patent (US2019/044556) in place for quaternized TiN
References
- [1] a).Mombelli A, Müller N, Cionca N, Clin Oral Implants Res 2012, 23, 67–76; [DOI] [PubMed] [Google Scholar]; b) Lindhe J, M. J J. Clin, Periodontol 2008, 35, 282–285; [DOI] [PubMed] [Google Scholar]; c) Mombelli A, Cionca N, Clin Oral Implants Res 2006, 17, 97–103. [DOI] [PubMed] [Google Scholar]
- [2] a).Safii SH, Palmer RM, Wilson RF, Clin Implant Dent Relat Res 2010, 12, 165–174; [DOI] [PubMed] [Google Scholar]; b) Prathapachandran J, Suresh N, Dent Res. J 2012, 9, 516–521; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Teles RP, Evidence Based Dentistry 2003, 4, 88; [Google Scholar]; d) Renvert S, Roos-Jansåker A-M, Claffey N, J. Clin. Periodontol 2008, 35, 305–315; [DOI] [PubMed] [Google Scholar]; e) Claffey N, Clarke E, Polyzois Iand Renvert S, J. Clin. Periodontol 2008, 35, 316–332; [DOI] [PubMed] [Google Scholar]; f) Quirynen M, Abarca M, Van Assche N, Nevins M, Van Steenberghe D, J. Clin. Periodontol 2007, 34, 805–815; [DOI] [PubMed] [Google Scholar]; g) Mombelli A, Lang NP, Clin Oral Implants Res 1992, 3, 162–168; [DOI] [PubMed] [Google Scholar]; h) Claffey N and Egelberg J, J. Clin. Periodontol 1995, 22, 690–696. [DOI] [PubMed] [Google Scholar]
- [3].Godoy-Gallardo M, Manzanares-Céspedes MC, Sevilla P, Nart J, Manzanares N, Manero JM, Gil FJ, Boyd SK, Rodríguez D, Mater. Sci. Eng.: C 2016, 69, 538–545. [DOI] [PubMed] [Google Scholar]
- [4] a).Mombelli A, Décaillet F, J. Clin. Periodontol. 2011, 38, 203–213; [DOI] [PubMed] [Google Scholar]; b) Persson GR, Renvert S, Clin Implant Dent Relat Res 2014, 16, 783–793; [DOI] [PubMed] [Google Scholar]; c) Mombelli A, A. C. Oosten M, Schürch E, Lang NP, Oral Microbiol. Immunol 1987, 2, 145–151. [DOI] [PubMed] [Google Scholar]
- [5] a).Berry CW, Flenry MTJSJACA, Wagner MJ, Implant Dent 1992, 1; [DOI] [PubMed] [Google Scholar]; b) van Hove RP, Sierevelt IN, van Royen BJ, Nolte PA, BioMed Res. Int 2015, 2015, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6] a).Pokrowiecki R, Zaręba T, Szaraniec B, Pałka K, Mielczarek A, Menaszek E, Tyski S, Int. J. Nanomed 2017, 12, 4285–4297; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu R, Memarzadeh K, Chang B, Zhang Y, Ma Z, Allaker RP, Ren L, Yang K, Sci. Rep 2016, 6, 29985; [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Vargas-Reus MA, Memarzadeh K, Huang J, Ren GG, Allaker RP, Int. J. Antimicrob. Agents 2012, 40, 135–139; [DOI] [PubMed] [Google Scholar]; d) Allaker RP, J. Dent. Res 2010, 89, 1175–1186. [DOI] [PubMed] [Google Scholar]
- [7].Berglund F, Carlmark B, Biol. Trace Elem. Res. 2011, 143, 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].a) Li X, Gao P, Wan P, Pei Y, Shi L, Fan B, Shen C, Xiao X, Yang Z. Guo K, Sci. Rep 2017, 7, 40755; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Khosravi F, Mansouri-Torshizi H, J. Biomol. Struct. Dyn 2018, 36, 512–531; [DOI] [PubMed] [Google Scholar]; c) Li G, Zhao Q.-m., Yang H.-l., Cheng L, Mater Res 2016, 19, 735–740; [Google Scholar]; d) Mei S, Wang H, Wang W, Tong L, Pan H, Ruan C, Ma Q, Liu M, Yang H, Zhang L, Cheng Y, Zhang Y, Zhao L, Chu PK, Biomaterials 2014, 35, 4255–4265; [DOI] [PubMed] [Google Scholar]; e) Liu R, Tang Y, Zeng L, Zhao Y, Ma Z, Sun Z, Xiang L, Ren L, Yang K, Dent. Mater 2018, 34, 1112–1126. [DOI] [PubMed] [Google Scholar]
- [9] a).Xue Y, Xiao H, Zhang Y, Int. J. Mol. Sci 2015, 16, 3626; [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Timofeeva L, Kleshcheva N, Appl. Microbiol. Biotechnol 2011, 89, 475–192; [DOI] [PubMed] [Google Scholar]; c) Hassan MS, Ibrahim HMM, Polym. Adv. Technol 2016, 27, 532–541; [Google Scholar]; d) Oosterhof JJH, Buijssen KJDA, Busscher HJ, van der Laan BFAM, van der Mei HC, Appl. Environ. Microbiol 2006, 72, 3673–3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10] a).Smith M, March J., March’s Advanced Organic Chemistry: Reactions, Mechanisms, and Structure 6th ed, John Wiley & Sons, Inc, Hoboken, NJ, 2007, p; [Google Scholar]; b) Harfenist M, J. Am. Chem. Soc 1957, 79, 4356–4358; [Google Scholar]; c) Stanger KJ, Lee J-J, Smith BD, J Org Chem 2007, 72, 9663–9668; [DOI] [PubMed] [Google Scholar]; d) Menschutkin N, Zeitschrift fur Physikalische Chemie 1890, 6, 1. [Google Scholar]
- [11] a).Craciun D, Socol G, Stefan N, Dorcioman G, Hanna M, Taylor CR, Lambers E, Craciun V, Appl. Surf. Sci 2014, 302, 124–128; [Google Scholar]; b) Jaeger D and Patscheider J, J. Electron Spectrosc. Relat. Phenom 2012, 185, 523–534. [Google Scholar]
- [12] a).Haidar J, Kondaiah P, Bhattacharya S, J. Med. Chem 2005, 48, 3823–3831; [DOI] [PubMed] [Google Scholar]; b) Rawlinson L-AB, Ryan SM, Mantovani G, Syrett JA, Haddleton DM, Brayden DJ, Biomacromolecules 2010, 11, 443–453. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.