Abstract
The environmental hypoxia of high altitude (HA) increases the incidence of intrauterine growth restriction (IUGR) approximately threefold. The peroxisome proliferator-activated receptor γ (PPAR-γ), a ligand-activated nuclear receptor that promotes vasorelaxation by increasing nitric oxide and downregulating endothelin-1 (ET-1) production, has been implicated in IUGR. Based on our prior work indicating that pharmacologic activation of the PPARγ pathway protects against hypoxia-associated IUGR, we used an experimental murine model to determine whether such effects may be attributed to vasodilatory effects in the uteroplacental circulation. Using wire myography, ex vivo vasoreactivity studies were conducted in uterine arteries (UtA) isolated from pregnant mice exposed to hypoxia or normoxia from gestational day 14.5 to 18.5. Exposure to troglitazone, a high-affinity PPARγ agonist, induced vasorelaxation in UtA pre-constricted with phenylephrine, with HA-UtA showing increased sensitivity. Troglitazone blunted ET-1-induced contraction of UtA in hypoxic and normoxic dams equivalently. Immunohistological analysis revealed enhanced staining for ET-1 receptors in the placental labyrinthine zone in hypoxic compared to normoxic dams. Our results suggest that pharmacologic PPAR-γ activation, via its vasoactive properties, may protect fetal growth under hypoxic conditions by improving uteroplacental perfusion and thereby justify further investigation into PPARγ as a therapeutic target for IUGR in pregnancies complicated by hypoxia.
Keywords: hypoxia, thiazolidinedione, intrauterine growth restriction, vascular function
3. INTRODUCTION
Intrauterine growth restriction (IUGR) raises the risk of neonatal death up to 20-fold and is associated with increased morbidity and mortality rates across the lifespan.1–3 Despite its public health importance, the pathophysiology of IUGR remains unclear and, as a result, no effective therapies or preventative strategies exist. Impaired maternal oxygenation, such as that resulting from the chronic hypoxia of high altitude (> 2500 m) residence, reduces birth weight and increases the incidence of IUGR nearly threefold.4–7 For this reason, high-altitude research models provide a unique opportunity to identify the physiologic and molecular mechanisms by which hypoxia contributes to reduced fetal growth without the confounding effects of other pathologic changes present in IUGR.
In healthy pregnancy, widespread maternal vascular adaptations, including the extensive remodeling of the uterine spiral arteries and altered uterine artery vasoreactivity, collectively serve to reduce uteroplacental vascular resistance, enhance uterine artery blood flow and thereby maintain adequate uteroplacental perfusion for fetal development.8,9 Such effects are due, in part, to changes in the production of or sensitivity to vasodilators (e.g., nitric oxide [NO]) and vasoconstrictors (e.g. endothelin 1 [ET-1]).8 In contrast, high-altitude pregnancy and IUGR are marked by a reduction in the normal pregnancy-associated rise in uteroplacental blood flow, an effect that not only precedes slowed fetal growth but also directly corresponds to birth weight.10–13
Peroxisome proliferator-activated receptor gamma (PPARγ), a hypoxia-sensitive, ligand-activated transcription factor of the nuclear hormone receptor superfamily, is predominantly recognized for its role in the regulation of metabolic homeostasis and inflammation.14 However, PPARγ is also vital for establishing the uteroplacental villous circulation15, 16 and maintaining uterine vascular function.17, 18 PPARγ also exhibits vasoprotective properties, lowering blood pressure in hypertensive disease and improving endothelium-dependent vasorelaxation19 by suppressing ET-1 synthesis and prepro-ET −1 expression in human vascular endothelial cells,20 and increasing NO production.21, 22 In line with these functions, PPARγ is abundantly expressed in the rodent placental labyrinthine zone,23 human trophoblast,15, 24 and human vascular smooth muscle cells.25 Experimental animal studies also strongly demonstrate vascular sites of action for PPARγ. In rats, for instance, antagonizing PPARγ decreases fetal growth, reduces vasodilation of the radial uterine arteries and induces endothelial dysfunction.17, 18 In mice, PPARγ activation reduces vascular sensitivity to angiotensin II, lowers blood pressure and improves fetal growth in heterozygote Rgs5+/− mice, known to otherwise be susceptible to IUGR.26
An abundant literature implicates PPARγ for the development of vascular disorders of pregnancy. 17, 27–29 In humans, placental PPARγ mRNA expression is 2-fold lower in IUGR compared to controls, and directly related to fetal and placental weight.29 Our prior work demonstrates that high-altitude pregnancy also suppresses PPARγ pathway gene expression in maternal peripheral blood cells in parallel with reduced birth weight.30 Experimental animal studies further support the involvement of the PPARγ pathway for IUGR. In recent work, for example, we show that dietary supplementation with pioglitazone, a pharmacologic PPARγ agonist, partially prevented hypoxia-associated fetal growth restriction in mice.31 Here, we propose that one mechanism by which thiazolidinediones, a class of PPARγ agonists used clinically to treat diabetes and polycystic ovarian syndrome,32, 33 may protect fetal growth under hypoxic conditions is by promoting uterine artery vasorelaxation and, in turn, enhancing uteroplacental perfusion. Using a murine model of hypoxia-associated IUGR, we hypothesized that (1) PPARγ activation ex vivo induces vasorelaxation in uterine arteries pre-constricted with phenylephrine (PE) and that these effects are augmented in uterine arteries isolated from dams exposed to hypoxia during late gestation and, (2) hypoxia enhances vasocontractility to ET-1 and that this response is blunted by PPARγ activation.
4. MATERIALS AND METHODS
All animal procedures and protocols were completed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals34 and approved by the Institutional Animal Care and Use Committee at the University of Colorado (IACUC, Protocol Number: 113016(08)1E).
Mice and Late-Gestation Hypoxia Exposure
Female C57Bl/6 mice (Charles River, Wilmington, MA) were mated at 8–14 weeks of age. A copulatory plug was considered evidence of successful breeding and considered to be gestational day (GD) 0.5. From GD14.5 to GD18.5, pregnant mice were placed in hyperbaric (PB ~ 760 mmHg, sea level) or hypobaric (PB ~ 385 mmHg, 5300 m) chambers to simulate normoxia or hypoxia, respectively. Given the moderate altitude of Denver, Colorado (1609 m), a hyperbaric chamber had to be used for a true normoxic (i.e., sea level) exposure. On GD18.5, mice were euthanized by carbon dioxide asphyxiation and cervical dislocation. Directly after euthanasia, the main uterine arteries were excised, cleaned of surrounding connective tissue placed in an ice-cold PBS bath and immediately prepared for wire myography studies. Fetuses and placentas were excised, cleaned of fetal membranes, and weighed. Placentas were fixed in 4% paraformaldehyde overnight then embedded in paraffin or flash frozen in liquid nitrogen for homogenization and protein extraction. Our previous study utilizing the same model of hypoxia-associated fetal growth restriction reported that there was no effect of hypoxia on litter size, maternal food intake, or resorption number, indicating that dams likely tolerated the normoxic and hypoxic chambers equally well and fetal growth restriction was not due to maternal stress.31
Small-Vessel Wire Myography
For each dam, segments of each main UtA were mounted in a four-chamber, small-vessel wire myograph (DMT 610, Copenhagen, Denmark) with two wires (40 μm) threaded through the vessel lumen and connected to either a tension transducer or micrometer. Each vessel was normalized to a resting tension equivalent to 13.3 kPa for a minimum of 40 minutes in warm (37°C) Krebs solution (118 mmol/L NaCl, 4.7 mmol/L KCl, 1.2 mmol/L MgSO4, 1.2 mmol/L KH2PO4, 25 mmol/L NaHCO3, 11 mmol/L glucose, 2.5 mmol/L CaCl2) continuously bubbled with 95% O2, 5% CO2. To establish viability, UtAs were 1) constricted with 60 and 120 mmol/L KCl (Sigma Aldrich, St. Louis, MO), 2) a sub-maximal concentration of phenylephrine (PE, 10 μmol/L) and 3) exposed to the endothelial-dependent vasodilator, acetylcholine (1 μmol/L) to test for an intact endothelium. For our experimental protocol, vessels were preconstricted with 10 μmol/L PE (Sigma-Aldrich; % PEmax) and the vasodilator response to the high-affinity PPARγ agonist troglitazone (1–100 μmol/L) was established. Our rationale for using troglitazone is based on recent calls for revisiting the therapeutic value of thiazolidinediones for vascular disorders of pregnancy,35 and on-going human clinical trials for a wide array of vascular diseases.36 To assess the contribution of NO production for the vasodilatory effects of troglitazone, UtAs were incubated for 20 minutes with and without the NO synthase inhibitor L-NG-nitroarginine methyl ester (L-NAME; 10 μmol/L) prior to PE constriction and the application of troglitazone. Given that ET-1 is a potent vasoconstrictor implicated in hypoxic and hypertensive disorders of pregnancy37, 38 and is downregulated by PPARγ,39 we also determined the contractile response of the UtA to ET-1 (0.1–10 nmol/L) prior to and after a 20-minute incubation with troglitazone (1 μmol/L).
Placental Immunohistochemistry
Placental paraffin sections were deparaffinized in xylene and rehydrated in decreasing concentrations of ethanol (100 – 80%) and de-ionized water. For antigen retrieval, sections were submerged in 10mmol/L, pH 6.0 citrate buffer and boiled in a pressure cooker for 30 minutes at 45°C. Sections were blocked in 1% bovine serum albumin (BSA) in 1x Tris Buffered Saline (TBS) for 30 minutes. Primary antibodies against ET-1 receptor A and B (Thermo Fisher Scientific, Rockford, IL) were diluted 1:1000 in 1% BSA in 1x TBS and applied to sections overnight at 4°C. Secondary anti-HRP antibody (Dako, Santa Clara, CA) was applied for 1 hour at room temperature. DAB substrate (Dako) was then added and allowed to develop for 6 minutes. Sections were then counterstained with hematoxylin for 1 minute, dehydrated with increasing concentrations of ethanol (70–100%) and xylene, and then covered with cytoseal and cover slips.
Placental Protein Expression
Placenta total protein quantification was carried out using the Wes System (ProteinSimple, San Jose, CA). Placentas from dams at E18.5 were cleaned of fetal membranes and flash frozen. Placentas corresponding to the fetus closest to the litter mean fetal weight were homogenized using a sonicator in RIPA buffer (50 mmol/L Tris (pH 7.4), 2 mmol/L EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 150mmol/L NaCl, 50 mM NaF, 5 mmol/L sodium vanadate) containing a protease and phosphatase inhibitor cocktail (Thermo Fisher Scientific). Protein levels were determined by capillary electrophoresis size-based separation via the WES System (ProteinSimple) according to manufacturer’s instructions. Data were analyzed with Compass software (ProteinSimple). Primary antibodies against ET-1 Receptor A and ET-1 Receptor B (Thermo Fisher Scientific, PA3–065 and PA3–066, 1:100 dilution for both) were used and normalized to vinculin (Sigma-Aldrich).
Analytical Strategy
Fetoplacental weights were averaged per dam and are expressed as the group mean ± SEM with N representing the number of dams per group. Fetoplacental weights were compared between normoxic and hypoxic dams using Student’s unpaired t-tests (Prism v.8; GraphPad Software, San Diego, CA). Concentration-response curves were generated, and sigmoidal curves fitted to the data using the least squares method (Prism v.8; GraphPad Software). Uterine artery relaxation evoked by troglitazone is expressed as the % PEmax. The contractile force elicited by ET1 is normalized to that evoked by 60mM KCl (Kmax). Half-maximal effective concentration (EC50), maximal force and area under the curve (AUC) were compared using two-way analysis of variance (ANOVA) with Sidak’s multiple comparisons to determine the main effects of exposure during pregnancy (normoxia vs. hypoxia) and ex vivo treatment (e.g., control vs. troglitazone), or Student’s unpaired t-tests, as appropriate. Two-way ANOVA with repeated measures was performed for vascular dose–response curves. If significant interactions were observed, then individual group means were compared, with the level of significance adjusted by Bonferroni’s method to account for multiple comparisons, or Student’s unpaired t test were performed for post hoc comparisons. Significance was defined as a two-tailed p < 0.05.
5. RESULTS
Effectiveness of Hypoxic Protocol to Reduce Fetal Weight
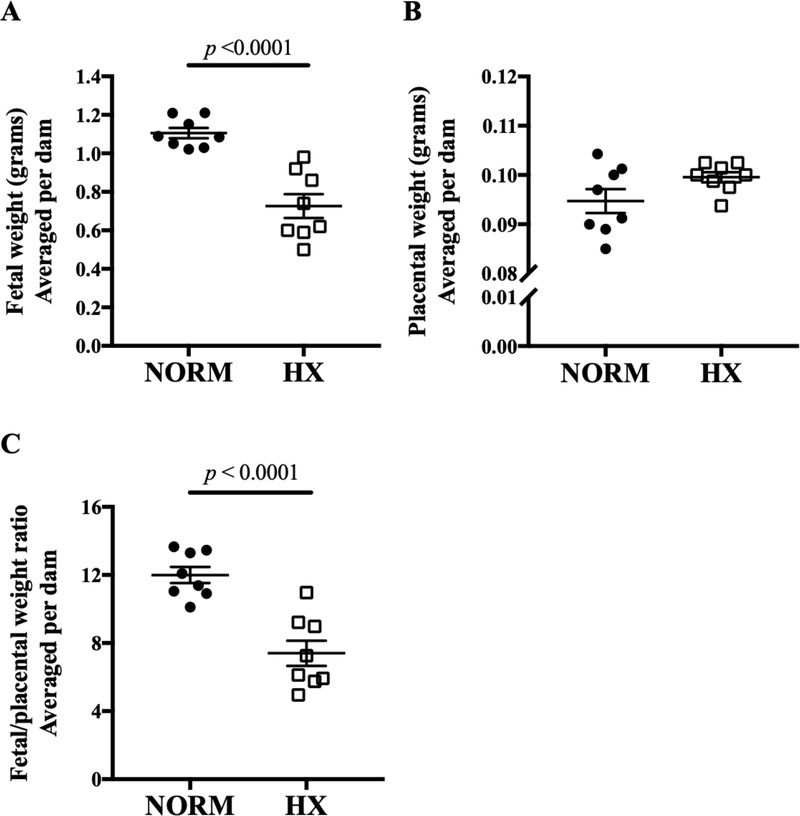
Maternal exposure to hypoxia from GD 14.5 to 18.5 reduced fetal weight by 38% (Fig. 1A; p < 0.0001). Compared to normoxic controls, mean placental weight tended to be higher (Fig. 1B; p = 0.08) and placental efficiency, expressed as the ratio of fetal to placental weight, lower in the hypoxic group (Fig. 1C; p < 0.0001).
Figure 1. Effects of late-gestation hypoxia on E18.5 feto-placental weights.
Hypoxia (HX) resulted in A) smaller fetal weights, B) similar placental weights, and C) reduced placental efficiency (ratio of fetal to placental weights) compared to normoxic (NORM) dams. Symbols are individual dams, bars are means ± SEM and compared by Student’s t-test; n = 8 NORM dams, 8 HX dams.
Vasodilatory Effects of Pharmacologic PPARγ Activation in Normoxic and Hypoxic Uterine Artery
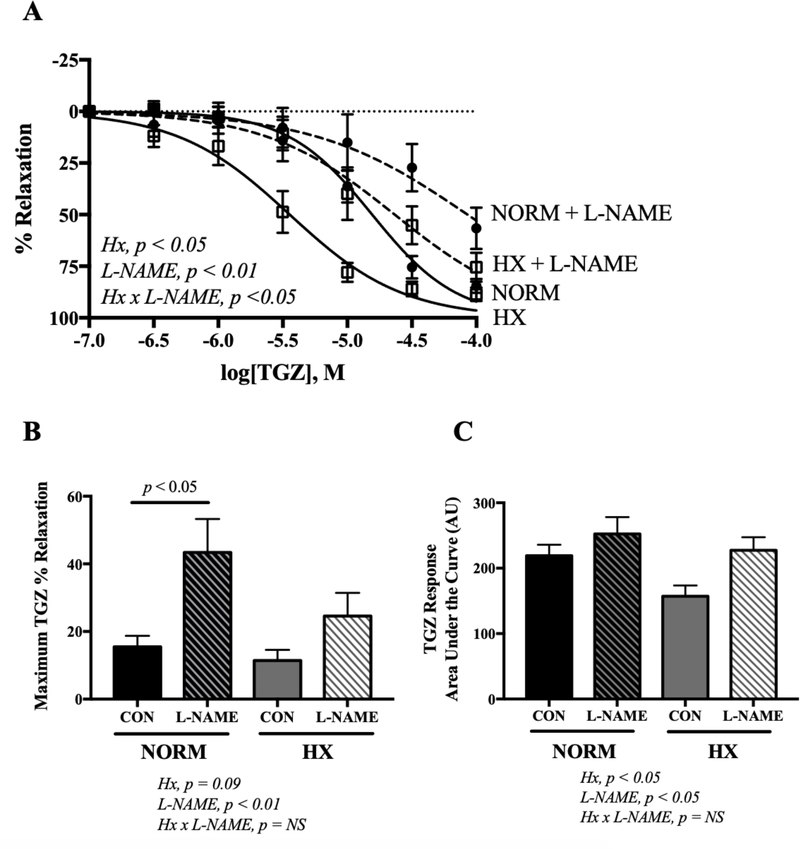
In normoxic and hypoxic dams, ex vivo exposure to the PPARγ agonist TGZ vasodilated uterine arteries pre-constricted with PE (Fig. 2A). Although maximal uterine artery vasorelaxation in response to TGZ was equivalent between normoxic and hypoxic mice (Fig. 2B), the overall sensitivity to TGZ (expressed by the AUC) was greater in the hypoxic group (Fig. 2C; 2-way ANOVA for hypoxic effect, p < 0.05) with an accompanying significant effect on the EC50 (12.1 ± 2.0 vs 12.9 ± 0.8 μmol/L for normoxic and hypoxic vessels, respectively; p < 0.001).
Figure 2. Effects of in vivo hypoxia and ex vivo L-NAME incubation on uterine artery troglitazone (TGZ)-induced vasorelaxation.
Uterine arteries were preconstricted with PE prior to TGZ or L-NAME exposure. A) Concentration response curves to troglitazone (TGZ; 0.1–100 μmol) for uterine arteries with pre-incubation with L-NAME (30 min; 10 μmol) or control (CON) in uterine arteries preconstricted with PE from normoxic (NORM) or hypoxic (HX) mice. HX and NORM dams are represented by open squares and solid circles, respectively. Curves generated without or with L-NAME are represented by solid or dashed lines, respectively. B) The maximum vasodilator response to TGZ (expressed as the % PE-induced constriction achieved by TGZ or TGZ + L-NAME) was blunted by L-NAME in normoxic dams. C) Overall sensitivity to TGZ as represented by area under the curve. Data are presented as means ± SEM and compared by 2-way ANOVA with differences between groups identified by Sidak’s multiple comparisons; n = 10 NORM CON, 10 NORM + L-NAME, 9 HX CON, 9 HX + L-NAME.
Inhibition of NO synthase by L-NAME raised the EC50 in normoxic and hypoxic groups (34.3 ± 13.1 vs 37.8 ± 18.9 μmol/L, respectively; p < 0.05 compared to controls), indicating that NO production contributed to the vasodilatory effects of TGZ in both groups. In normoxic mice, L-NAME reduced the maximum vasodilator response to TGZ by 33% (Fig. 2B; p < 0.05). In hypoxic mice, however, L-NAME had no effect on maximum vasodilator response to TGZ (Fig. 2B). These findings indicate a lesser contribution of NO to the effects of TGZ in hypoxic mice and suggest that NO production may be reduced by hypoxia.
Effects of Pharmacologic PPARγ Activation on ET1-Induced Contraction of the Uterine Artery
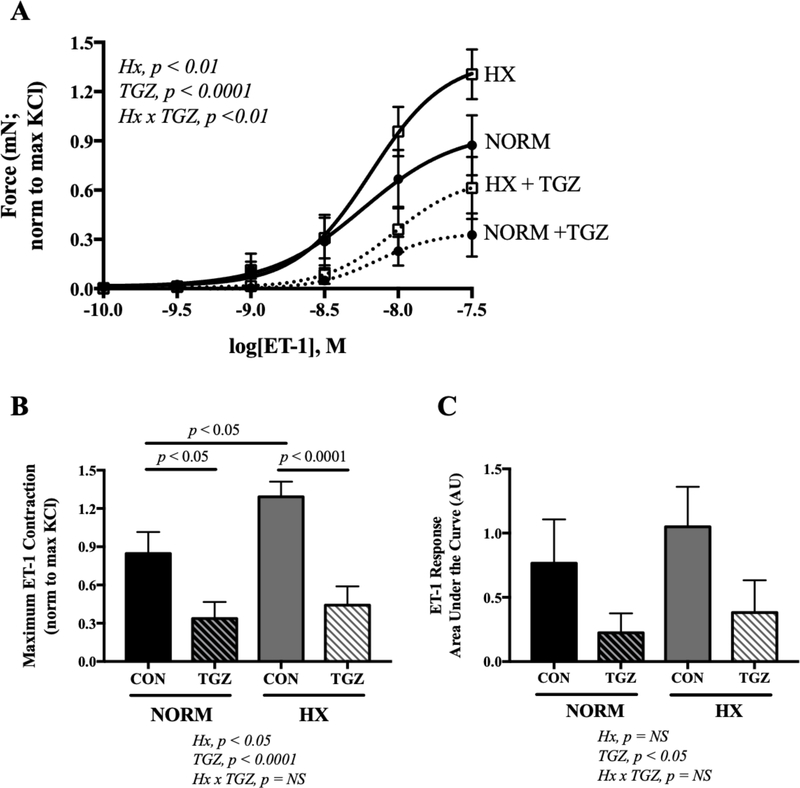
The uterine arteries from normoxic and hypoxic mice contracted in response to ET-1 (Fig. 3A). The maximum contractile response to ET-1 was augmented by hypoxia (Fig. 3B; p < 0.05), but the overall sensitivity remained the same as that in normoxic vessels (Fig. 3C). Preincubating vessels with TGZ to activate PPARγ reduced the maximal response to ET-1 in normoxic and hypoxic groups (Fig. 3B; p < 0.05 or p < 0.0001 respectively). It is notable that vessels obtained from hypoxic dams had a comparatively blunted, albeit not statistically significant, maximal ET-1 response after TGZ incubation compared to controls. Overall sensitivity to ET-1 was reduced by TGZ in both the normoxic and hypoxic groups (Fig. 3C; 2-way ANOVA for TGZ effect, p < 0.05). The contractile response to KCl was similar in normoxic and hypoxic mice (6.36 ± 0.48 vs 6.13 ± 0.38 mN, respectively, NS), indicating that the mechanical integrity of the uterine arteries was not affected by hypoxia.
Figure 3. Effects of in vivo hypoxia and ex vivo troglitazone on the uterine artery contractile response to endothlelin-1 (ET-1).
A) Concentration response curves to ET-1 (0.1–30 nmol/L) with 20 min pre-incubation with troglitazone (TGZ; 1 μmol/L) or control (CON) in uterine arteries from normoxic (NORM) or hypoxic (HX) mice. B) Maximum contractile response to ET-1 was higher in HX dams, and TGZ blunted the maximal response in NORM and HX dams. C) Overall sensitivity to ET-1 as represented by area under the curve. Data are presented as means ± SEM and compared by 2-way ANOVA with differences between groups identified by Sidak’s multiple comparisons; n = 11 NORM CON, 11 NORM + TGZ, 15 HX CON, 14 HX + TGZ.
Effect of Hypoxia on the Expression of ET1 Receptors in the Placenta and Uterine Artery
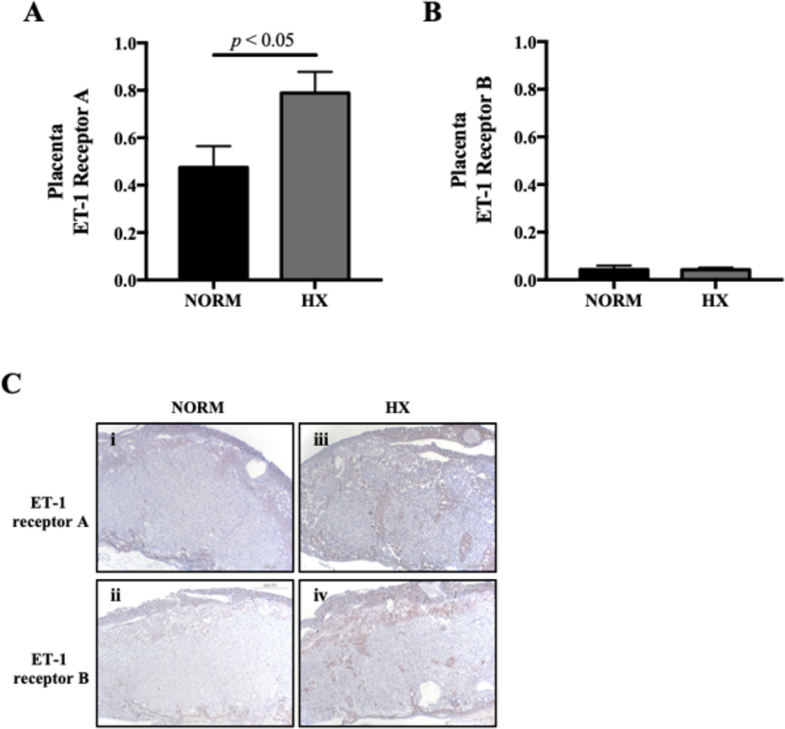
Given evidence suggesting that ET1 may be important for regulating uteroplacental blood flow,37, 40 we evaluated the effect of late-gestation hypoxia on the expression of ET1 receptors A and B in the placenta and uterine artery. In normoxic and hypoxic animals, placental ET-1 receptors A and B were detected (Fig. 4A, B). Compared to normoxic controls, placental ET-1 receptor A expression was greater in hypoxic dams (Fig. 4A). Using immunohistochemistry to localize ET-1 receptor expression in the placenta, we found that, in normoxic mice, ET1 expression was predominantly localized to the junctional zone (Fig. 4C panels i, ii). Qualitatively, ET-1 receptor staining was enhanced in placentas from hypoxic mice, and apparent in both the junctional zone and the labyrinthine zone (Fig. 4C panels iii, iv).
Figure 4. Effects of in vivo hypoxia on the expression of ET-1 receptors in the murine placenta.
A) ET-1 receptor A protein expression is higher in hypoxic (HX) compared to normoxic (NORM) mouse placental homogenates. B) ET-1 receptor B protein expression is unaffected by HX. C) Representative immunohistochemical staining of placentas from NORM (i and ii) and HX (iii and iv) mice reveal qualitatively higher expression of both ET-1 receptors A and B with HX. N = 6 NORM, 6 HX dams for each receptor in placenta.
6. DISCUSSION
Abundant evidence supports involvement of the PPARγ pathway for vascular disorders of pregnancy, including IUGR and preeclampsia. Our prior human and experimental animal studies suggest that impaired PPARγ signaling may also be important for hypoxia-associated fetal growth restriction. Dietary supplementation with a selective PPARγ agonist, for instance, appears to protect against hypoxia-associated fetal growth restriction in mice.31 In the present study, we have shown that ex vivo PPARγ activation evokes profound relaxation of the uterine arteries, and that vessels obtained from hypoxia-exposed dams are more sensitive to vasorelaxation in response to PPARγ activation compared to normoxic controls. In support of our observation, in an interventional experimental animal study, treatment with the PPARγ agonist troglitazone abolished hypertensive responses to angiotensin II infusion during pregnancy and normalized fetal growth in a genetic mouse model (Rgs5+/−) that is otherwise known to be susceptible to hypertension and IUGR.26
Our observation that uterine arteries from hypoxia-exposed dams are more sensitive to the vasodilatory effects of PPARγ activation may reflect increased sensitization to pharmacologic PPARγ agonist exposure due, in part, to reduced PPARγ expression under hypoxic conditions.27, 29–31 Our data indicate that inhibition of NO by L-NAME blunted vasorelaxation in response to TGZ incubation to a lesser extent in hypoxic compared to normoxic mice at higher TGZ concentrations (100uM), whereas as at lower TGZ concentrations inhibition of NO had a greater effect on inhibiting TGZ-induced vasorelaxation. PPARγ activation increases NO production via PPARγ-dependent mechanisms21 and increases eNOSser1177 phosphorylation via non-genomic mechanisms.22 Therefore, one possible explanation for our observations is that hypoxia increased sensitization of PPARγ activation-induced NO signaling, synthesis or bioavailability.41
An alternative explanation may be related to augmented vasocontractility to ET-1 in the uterine arteries of hypoxic dams. Circulating ET-1 is elevated in human HA pregnancy compared to low altitude pregnancy,40 and has been associated with lower birth weight in HA pregnancy.37 ET-1 is also elevated in pregnancies complicated by preeclampsia or small-for-gestational-age births,42, 43 and when its vasoconstrictor actions are inhibited with the use of ET-1 antagonists, hypoxia-associated reductions in uteroplacental blood flow and fetal growth in rat models are abolished.44 In the context of hypoxic pregnancy, therefore, it is plausible that PPARγ activation has an enhanced vasodilatory effect by reducing ET-1 signaling. In support of this hypothesis, PPARγ pathway activation enhances endothelium-dependent vasorelaxation (as previously reviewed),19 an effect that may be due to the suppression of ET-1 secretion,20, 45 ET-1 synthesis and prepro-ET-1 expression.20 While the brief troglitazone incubation implemented in our study likely excludes the possibility that changes in ET-1 synthesis or prepro-ET-1 expression affected vasoreactivity, significant changes in ET-1 secretion, as determined by circulating levels in the plasma, have been observed after only 5–6 minutes of low-grade exercise (recumbent cycling), indicating that ET-1 secretion shifts may occur rapidly.46 Additional studies are needed, however, to determine whether a 20-minute troglitazone exposure is sufficient to suppress ET-1 secretion. Chronic PPARγ activation, which we did not address in this experiment, may also have non-genomic effects in reducing ET-1 signaling through microRNA regulation, as it does in models of pulmonary hypertension.47
Our findings further indicate that pharmacologic activation of the PPARγ pathway blunts ET-1 induced contraction of the uterine artery in normoxic controls and hypoxia-exposed dams. From a therapeutic perspective, this effect may be considered advantageous. Most prominently, since ET-1 signaling may also be elevated in vascular disorders of pregnancy that are unrelated to maternal hypoxic exposure, our findings suggest that targeting PPARγ to improve uteroplacental blood flow is likely not limited to hypoxia-related disease. However, indicating that the effect of PPARγ activation to reduce ET-1-induced contraction of the UtA may be of particular importance for the vascular dysfunction characteristic of hypoxia-associated complications of pregnancy, the maximum contractile response to ET-1 was enhanced in uterine arteries of hypoxia-exposed dams.
Inhibition of NO production with L-NAME blunted the vasodilatory effects of troglitazone in uterine arteries from hypoxic and normoxic mice, indicating that elevated NO signaling contributed to the vasodilatory effects of troglitazone in both groups. However, the maximal vasorelaxation was blunted by L-NAME only in normoxic mice, indicating less NO production in hypoxic mice. This is consistent with previous findings of reduced NO signaling in uterine arteries from hypoxic animal models48–50 and in myometrial arteries from our recent HA human studies.51 In addition to enhanced NO signaling and blunted contractility as described above, troglitazone may be acting through endothelial-derived hyperpolarizing factor, cyclooxygenase-dependent mechanisms in the vascular endothelium to cause relaxation, or other non-genomic mechanisms including the regulation of calcium influx in vascular smooth muscle cells by, for instance, suppressing increased basal intracellular free calcium concentration or store-operated calcium entry as has been observed in pulmonary artery vascular smooth muscle cells;52 these mechanisms require further investigation.
Placental blood flow is also a major determinant of nutrient and oxygen delivery to the fetus.53 Placental vasomotor activity is largely dependent on the production of vasoactive compounds including ET-1 and expression of receptors including ET-1 receptors, allowing for autocrine and paracrine control of vascular function within the placenta.54–56 Upregulation of ET-1 and its receptors in the placenta is associated with preeclampsia,57, 58 a hypertensive disorder of pregnancy that often results in fetal growth restriction. For this reason, we assessed placental expression of ET-1 receptors A and B in normoxic and hypoxic mice with the hypothesis that increased placental ET-1 receptor expression would enhance ET-1 signaling, resulting in reduced placental perfusion and restricted nutrient delivery to the fetus. Indeed, compared to normoxic controls, we observed increased placental expression of both ET-1 receptors A and B in the junctional and labyrinthine zones of hypoxic mice. This observation supports the possibility that hypoxia may increase contractility in the portion of the placenta important for nutrient exchange between the dam and fetus. Our previous data in a similar murine model reported no change in placental vascular density or total vascular area with hypoxia exposure;31 these new data suggest that although the vessel number is similar, the in vivo contractile response might be augmented, resulting in increased placental vascular resistance. It is possible that ET-1 receptors are localized to placental cell types other than vascular cells, and so further investigation is needed to support these findings.
Given that the PPARγ pathway is a hypoxia-sensitive regulator of metabolic homeostasis and mediates the transcription of numerous inflammatory factors, our observations should be considered within a larger context. In particular, since hypoxia and inflammation are central to the pathophysiology of vascular disorders of pregnancy, PPARγ activation likely affects fetal growth in numerous ways, in addition to the potentially beneficial effects of blunting ET1-induced contraction of the UtA that we observed in this study. For instance, PPARγ activation mediates inflammatory responses by negatively regulating proinflammatory transcription factors [e.g., NF-κB, adaptor-related complex 1] and thereby attenuating cytokine production.59, 60 Additionally, PPARγ is a direct target of hypoxia-inducible factor 1α (HIF1α) and contains a highly conserved hypoxic response element upstream of the PPARγ transcriptional start site,61 although its regulation by HIF1α is complex and tissue-specific. In adipocyte differentiation, HIF1α inhibits PPARγ gene transcription,62 whereas in cardiomyocytes, HIF1α increases PPARγ expression.61 HIF1α is not always important in regulating effects of hypoxia on PPARγ; i.e., in murine trophoblast stem cells, hypoxia represses PPARγ expression in a HIF1α-independent fashion.63 Further studies are needed to determine whether impaired maternal oxygenation affects PPARγ expression or activation via HIF1α-independent mechanisms.
Together, our data suggest that one mechanism by which PPARγ activation in late pregnancy acts to protect against hypoxia-induced fetal growth restriction may be related to the vasodilatory properties of PPARγ within the uteroplacental circulation. Of potential clinical importance, our findings further implicate the PPARγ pathway as a potential therapeutic target for improving uteroplacental perfusion in hypoxic pregnancy. PPARγ agonists are in use clinically to treat type II diabetes and polycystic ovarian syndrome,64, 65 so their safety and efficacy have been established in non-pregnant women. Further studies are needed to determine PPARγ agonists’ specific mechanism of action in pregnant animal models. Additional studies are needed to determine the ability of PPARγ activation to regulate vasoreactivity in human resistance vessels in the uteroplacental circulation. Other future research avenues include establishing the role of PPARγ for the regulation of maternal, placental, and fetal metabolism, including glucose homeostasis or nutrient transporters,66–69 and the relationship of such effects for fetal growth in hypoxic pregnancy. Also, while their main target is PPARγ, thiazolidinedione drugs including troglitazone also directly activate AMP-activated protein kinase (AMPK) via a cell-autonomous effect and indirectly by increasing release of adiponectin.70, 71 Thus, the role of AMPK activation in the effects of PPARγ we observed here warrant further investigation. Together these studies address calls for investigating PPARγ agonists in treatment of vascular disorders of pregnancy72 in order to contribute to development of IUGR interventions using existing pharmacologic compounds.
7. ACKNOWLEDGMENTS
The authors would like to thank the veterinary technicians in the University of Colorado Vivarium and the University of Colorado Cardiovascular and Pulmonary Research Laboratory for their support.
SOURCES OF FUNDING
This work was supported by the American Heart Association Pre-Doctoral Award 7PRE33410652 (SLL), NIH R01 HL138181 (CGJ), and NIH R01 HD088590 (LGM, CGJ).
Nonstandard Abbreviations
- PPARγ
Peroxisome proliferator-activated receptor gamma
- ET-1
Endothelin-1
- NO
Nitric oxide
- IUGR
Intrauterine growth restriction
- TGZ
Troglitazone
Footnotes
DISCLOSURES
The authors have nothing to disclose.
REFERENCES
- 1.Gilbert W and Danielsen B. Pregnancy outcomes associated with intrauterine growth restriction. American journal of obstetrics and gynecology. 2003;188:1596–9; discussion 1599–601. [DOI] [PubMed] [Google Scholar]
- 2.Bjarnegard N, Morsing E, Cinthio M, Lanne T and Brodszki J. Cardiovascular function in adulthood following intrauterine growth restriction with abnormal fetal blood flow. Ultrasound Obstet Gynecol. 2013;41:177–84. [DOI] [PubMed] [Google Scholar]
- 3.Geva R, Eshel R, Leitner Y, Valevski AF and Harel S. Neuropsychological outcome of children with intrauterine growth restriction: a 9-year prospective study. Pediatrics. 2006;118:91–100. [DOI] [PubMed] [Google Scholar]
- 4.Jensen GM and Moore LG. The effect of high altitude and other risk factors on birthweight: independent or interactive effects? Am J Public Health. 1997;87:1003–1007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lichty JL, Ting R, Bruns PD and Dyar E. Studies of babies born at high altitude. AMA J Dis Child. 1957;93:666–669. [DOI] [PubMed] [Google Scholar]
- 6.Unger C, Weiser JK, McCullough RE, Keefer S and Moore LG. Altitude, low birth weight, and infant mortality in Colorado. Journal of the American Medical Association. 1988;259:3427–3432. [PubMed] [Google Scholar]
- 7.Julian CG, Vargas E, Armaza JF, Wilson MJ, Niermeyer S and Moore LG. High-altitude ancestry protects against hypoxia-associated reductions in fetal growth. Arch Dis Child Fetal Neonatal Ed. 2007;92:F372–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Osol G and Moore LG. Maternal uterine vascular remodeling during pregnancy. Microcirculation. 2014;21:38–47. [DOI] [PubMed] [Google Scholar]
- 9.Browne VA, Julian CG, Toledo-Jalden L, Cioffi-Ragan D, Vargas E and Moore LG. Uterine artery blood flow, fetal hypoxia and fetal growth. Philos Trans R Soc Lond B Biol Sci. 2015;370:20140068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zamudio S, Palmer SK, Droma T, Stamm E, Coffin C and Moore LG. Effect of altitude on uterine artery blood flow during normal pregnancy. J Appl Physiol. 1995;79:7–14. [DOI] [PubMed] [Google Scholar]
- 11.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J and Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol. 2008;295:R906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Browne VA, Toledo-Jaldin L, Davila RD, Lopez LP, Yamashiro H, Cioffi-Ragan D, Julian CG, Wilson MJ, Bigham AW, Shriver MD, Honigman B, Vargas E, Roach R and Moore LG. High-end arteriolar resistance limits uterine artery blood flow and restricts fetal growth in preeclampsia and gestational hypertension at high altitude. Am J Physiol Regul Integr Comp Physiol. 2011;300:R1221–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Konje JC, Howarth ES, Kaufmann P and Taylor DJ. Longitudinal quantification of uterine artery blood volume flow changes during gestation in pregnancies complicated by intrauterine growth restriction. BJOG: an international journal of obstetrics and gynaecology. 2003;110:301–5. [PubMed] [Google Scholar]
- 14.Ahmadian M, Suh JM, Hah N, Liddle C, Atkins AR, Downes M and Evans RM. PPARgamma signaling and metabolism: the good, the bad and the future. Nat Med. 2013;19:557–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parast MM, Yu H, Ciric A, Salata MW, Davis V and Milstone DS. PPARgamma regulates trophoblast proliferation and promotes labyrinthine trilineage differentiation. PloS one. 2009;4:e8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaiff WT, Carlson MG, Smith SD, Levy R, Nelson DM and Sadovsky Y. Peroxisome proliferator-activated receptor-gamma modulates differentiation of human trophoblast in a ligand-specific manner. The Journal of clinical endocrinology and metabolism. 2000;85:3874–81. [DOI] [PubMed] [Google Scholar]
- 17.McCarthy FP, Drewlo S, English FA, Kingdom J, Johns EJ, Kenny LC and Walsh SK. Evidence implicating peroxisome proliferator-activated receptor-gamma in the pathogenesis of preeclampsia. Hypertension. 2011;58:882–7. [DOI] [PubMed] [Google Scholar]
- 18.Gokina NI, Chan SL, Chapman AC, Oppenheimer K, Jetton TL and Cipolla MJ. Inhibition of PPARgamma during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation. Frontiers in physiology. 2013;4:184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ketsawatsomkron P, Pelham CJ, Groh S, Keen HL, Faraci FM and Sigmund CD. Does peroxisome proliferator-activated receptor-gamma (PPAR gamma) protect from hypertension directly through effects in the vasculature? J Biol Chem. 2010;285:9311–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P and Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. [DOI] [PubMed] [Google Scholar]
- 21.Polikandriotis JA, Mazzella LJ, Rupnow HL and Hart CM. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arterioscler Thromb Vasc Biol. 2005;25:1810–6. [DOI] [PubMed] [Google Scholar]
- 22.Kleinhenz JM, Kleinhenz DJ, You S, Ritzenthaler JD, Hansen JM, Archer DR, Sutliff RL and Hart CM. Disruption of endothelial peroxisome proliferator-activated receptor-gamma reduces vascular nitric oxide production. Am J Physiol Heart Circ Physiol. 2009;297:H1647–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barak Y, Sadovsky Y and Shalom-Barak T. PPAR Signaling in Placental Development and Function. PPAR Res. 2008;2008:142082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fournier T, Tsatsaris V, Handschuh K and Evain-Brion D. PPARs and the placenta. Placenta. 2007;28:65–76. [DOI] [PubMed] [Google Scholar]
- 25.Gong K, Xing D, Li P, Aksut B, Ambalavanan N, Yang Q, Nozell SE, Oparil S and Chen YF. Hypoxia induces downregulation of PPAR-gamma in isolated pulmonary arterial smooth muscle cells and in rat lung via transforming growth factor-beta signaling. American journal of physiology Lung cellular and molecular physiology. 2011;301:L899–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holobotovskyy V, Chong YS, Burchell J, He B, Phillips M, Leader L, Murphy TV, Sandow SL, McKitrick DJ, Charles AK, Tare M, Arnolda LF and Ganss R. Regulator of G protein signaling 5 is a determinant of gestational hypertension and preeclampsia. Sci Transl Med. 2015;7:290ra88. [DOI] [PubMed] [Google Scholar]
- 27.Waite LL, Louie RE and Taylor RN. Circulating activators of peroxisome proliferator-activated receptors are reduced in preeclamptic pregnancy. The Journal of clinical endocrinology and metabolism. 2005;90:620–6. [DOI] [PubMed] [Google Scholar]
- 28.Waite LL, Person EC, Zhou Y, Lim KH, Scanlan TS and Taylor RN. Placental peroxisome proliferator-activated receptor-gamma is up-regulated by pregnancy serum. The Journal of clinical endocrinology and metabolism. 2000;85:3808–14. [DOI] [PubMed] [Google Scholar]
- 29.Diaz M, Bassols J, Lopez-Bermejo A, Gomez-Roig MD, de Zegher F and Ibanez L. Placental expression of peroxisome proliferator-activated receptor gamma (PPARgamma): relation to placental and fetal growth. The Journal of clinical endocrinology and metabolism. 2012;97:E1468–72. [DOI] [PubMed] [Google Scholar]
- 30.Julian CG, Yang IV, Browne VA, Vargas E, Rodriguez C, Pedersen BS, Moore LG and Schwartz DA. Inhibition of peroxisome proliferator-activated receptor gamma: a potential link between chronic maternal hypoxia and impaired fetal growth. FASEB J. 2014;28:1268–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lane SL, Dodson RB, Doyle AS, Park H, Rathi H, Matarrazo CJ, Moore LG, Lorca RA, Wolfson GH and Julian CG. Pharmacologic Activation of Peroxisome Proliferator-Activated Receptor Gamma (PPARg) Protects Against Hypoxia-Associated Fetal Growth Restriction. FASEB J. 2019;33:8999–9007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Derosa G, Cicero AF, Dangelo A, Gaddi A, Ragonesi PD, Piccinni MN, Salvadeo S, Ciccarelli L, Pricolo F, Ghelfi M, Ferrari I, Montagna L and Fogari R. Thiazolidinedoine effects on blood pressure in diabetic patients with metabolic syndrome treated with glimepiride. Hypertens Res. 2005;28:917–24. [DOI] [PubMed] [Google Scholar]
- 33.Froment P and Touraine P. Thiazolidinediones and Fertility in Polycystic Ovary Syndrome (PCOS). PPAR Res. 2006;73986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Council N Guide for the Care and Use of Laboratory Animals. 2011. [Google Scholar]
- 35.Geach T PPAR agonists in pre-eclampsia and hypertension? Nat Rev Endocrinol. 2015;11:446. [DOI] [PubMed] [Google Scholar]
- 36.Kernan WN, Viscoli CM, Furie KL, Young LH, Inzucchi SE, Gorman M, Guarino PD, Lovejoy AM, Peduzzi PN, Conwit R, Brass LM, Schwartz GG, Adams HP Jr., Berger L, Carolei A, Clark W, Coull B, Ford GA, Kleindorfer D, O’Leary JR, Parsons MW, Ringleb P, Sen S, Spence JD, Tanne D, Wang D, Winder TR and Investigators IT. Pioglitazone after Ischemic Stroke or Transient Ischemic Attack. N Engl J Med. 2016;374:1321–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Julian CG, Galan HL, Wilson MJ, Desilva W, Cioffi-Ragan D, Schwartz J and Moore LG. Lower uterine artery blood flow and higher endothelin relative to nitric oxide metabolite levels are associated with reductions in birth weight at high altitude. Am J Physiol Regul Integr Comp Physiol. 2008;295:R906–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhou J, Xiao D and Hu Y. Gestational hypoxia induces preeclampsia-like symptoms via heightened endothelin-1 signaling in pregnant rats. Hypertension. 2013;62:599–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Delerive P, Martin-Nizard F, Chinetti G, Trottein F, Fruchart JC, Najib J, Duriez P and Staels B. Peroxisome proliferator-activated receptor activators inhibit thrombin-induced endothelin-1 production in human vascular endothelial cells by inhibiting the activator protein-1 signaling pathway. Circ Res. 1999;85:394–402. [DOI] [PubMed] [Google Scholar]
- 40.Bashir SO, Suekit H, Elkarib AO, Dafaalla MA, Abd Elrouf MB, Morsy MD and Eskandar M. The effect of high altitude on endothelial and vascular dysfunction markers in preeclamptic patients. Acta Physiol Hung. 2015;102:391–9. [DOI] [PubMed] [Google Scholar]
- 41.Ketsawatsomkron P and Sigmund CD. Molecular mechanisms regulating vascular tone by peroxisome proliferator activated receptor gamma. Curr Opin Nephrol Hypertens. 2015;24:123–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Erdem A, Erdem M, Himmetoglu O, Yildirim G and Arslan M. Maternal and fetal plasma endothelin levels in intrauterine growth restriction: relation to umbilical artery Doppler flow velocimetry. J Perinat Med. 2003;31:52–59. [DOI] [PubMed] [Google Scholar]
- 43.Furuhashi N, Kimura H, Nagae H and Yajima A. Maternal plasma endothelin levels and fetal status in normal and preeclamptic pregnancies. Gynecol Obstet Invest. 1995;39:88–92. [DOI] [PubMed] [Google Scholar]
- 44.Thaete LG, Dewey ER and Neerhof MG. Endothelin and the regulation of uterine and placental perfusion in hypoxia-induced fetal growth restriction. J Soc Gynecol Investig. 2004;11:16–21. [DOI] [PubMed] [Google Scholar]
- 45.Martin-Nizard F, Furman C, Delerive P, Kandoussi A, Fruchart JC, Staels B and Duriez P. Peroxisome proliferator-activated receptor activators inhibit oxidized low-density lipoprotein-induced endothelin-1 secretion in endothelial cells. J Cardiovasc Pharmacol. 2002;40:822–31. [DOI] [PubMed] [Google Scholar]
- 46.Barrett-O’Keefe Z, Ives SJ, Trinity JD, Morgan G, Rossman MJ, Donato AJ, Runnels S, Morgan DE, Gmelch BS, Bledsoe AD, Richardson RS and Wray DW. Taming the “sleeping giant”: the role of endothelin-1 in the regulation of skeletal muscle blood flow and arterial blood pressure during exercise. Am J Physiol Heart Circ Physiol. 2013;304:H162–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kang BY, Park KK, Kleinhenz JM, Murphy TC, Green DE, Bijli KM, Yeligar SM, Carthan KA, Searles CD, Sutliff RL and Hart CM. Peroxisome Proliferator-Activated Receptor gamma and microRNA 98 in Hypoxia-Induced Endothelin-1 Signaling. Am J Respir Cell Mol Biol. 2016;54:136–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White MM, McCullough RE, Dyckes R, Robertson AD and Moore LG. Chronic hypoxia, pregnancy, and endothelium-mediated relaxation in guinea pig uterine and thoracic arteries. Am J Physiol Heart Circ Physiol. 2000;278:H2069–H2075. [DOI] [PubMed] [Google Scholar]
- 49.Aljunaidy MM, Morton JS, Cooke CM and Davidge ST. Maternal vascular responses to hypoxia in a rat model of intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol. 2016;311:R1068–R1075. [DOI] [PubMed] [Google Scholar]
- 50.Mateev S, Sillau AH, Mouser R, McCullough RE, White MM, Young DA and Moore LG. Chronic hypoxia opposes pregnancy-induced increase in uterine artery vasodilator response to flow. Am J Physiol Heart Circ Physiol. 2003;284:H820–9. [DOI] [PubMed] [Google Scholar]
- 51.Lorca RA, Lane SL, Bales ES, Nsier H, Yi H, Donnelly MA, Euser AG, Julian CG and Moore LG. High Altitude Reduces NO-dependent Myometrial Artery Vasodilator Response During Pregnancy. Hypertension. 2019;73:1319–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang Y, Lu W, Yang K, Wang Y, Zhang J, Jia J, Yun X, Tian L, Chen Y, Jiang Q, Zhang B, Chen X and Wang J. Peroxisome proliferator-activated receptor gamma inhibits pulmonary hypertension targeting store-operated calcium entry. J Mol Med (Berl). 2015;93:327–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Krishna U and Bhalerao S. Placental Insufficiency and Fetal Growth Restriction. J Obstet Gynaecol India. 2011;61:505–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nielsen AH, Schauser KH and Poulsen K. Current topic: the uteroplacental renin-angiotensin system. Placenta. 2000;21:468–477. [DOI] [PubMed] [Google Scholar]
- 55.Nelson DM and Walsh SW. Thromboxane and prostacyclin production by different compartments of the placental villus. J Clin Endocrin Metab. 1989;68:676–83. [DOI] [PubMed] [Google Scholar]
- 56.Le SQ, Wasserstrum N, Mombouli JV and Vanhoutte PM. Contractile effect of endothelin in human placental veins: role of endothelium prostaglandins and thromboxane. American journal of obstetrics and gynecology. 1993;169:919–24. [DOI] [PubMed] [Google Scholar]
- 57.Walsh SW. Preeclampsia: an imbalance in placental prostacyclin and thromboxane production. American journal of obstetrics and gynecology. 1985;152:335–40. [DOI] [PubMed] [Google Scholar]
- 58.Walsh SW and Wang Y. Trophoblast and placental villous core production of lipid peroxides, thromboxane, and prostacyclin in preeclampsia. J Clin Endocrin Metab. 1995;80:1888–93. [DOI] [PubMed] [Google Scholar]
- 59.Ricote M, Li AC, Willson TM, Kelly CJ and Glass CK. The peroxisome proliferator-activated receptor-gamma is a negative regulator of macrophage activation. Nature. 1998;391:79–82. [DOI] [PubMed] [Google Scholar]
- 60.Jiang C, Ting AT and Seed B. PPAR-gamma agonists inhibit production of monocyte inflammatory cytokines. Nature. 1998;391:82–6. [DOI] [PubMed] [Google Scholar]
- 61.Krishnan J, Suter M, Windak R, Krebs T, Felley A, Montessuit C, Tokarska-Schlattner M, Aasum E, Bogdanova A, Perriard E, Perriard JC, Larsen T, Pedrazzini T and Krek W. Activation of a HIF1alpha-PPARgamma axis underlies the integration of glycolytic and lipid anabolic pathways in pathologic cardiac hypertrophy. Cell metabolism. 2009;9:512–24. [DOI] [PubMed] [Google Scholar]
- 62.Yun Z, Maecker HL, Johnson RS and Giaccia AJ. Inhibition of PPAR gamma 2 gene expression by the HIF-1-regulated gene DEC1/Stra13: a mechanism for regulation of adipogenesis by hypoxia. Dev Cell. 2002;2:331–41. [DOI] [PubMed] [Google Scholar]
- 63.Tache V, Ciric A, Moretto-Zita M, Li Y, Peng J, Maltepe E, Milstone DS and Parast MM. Hypoxia and trophoblast differentiation: a key role for PPARgamma. Stem Cells Dev. 2013;22:2815–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Froment P and Touraine P. Thiazolidinediones and Fertility in Polycystic Ovary Syndrome (PCOS). PPAR Res. 2006;2006:73986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kung J and Henry RR. Thiazolidinedione safety. Expert Opin Drug Saf. 2012;11:565–79. [DOI] [PubMed] [Google Scholar]
- 66.Towler MC and Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–41. [DOI] [PubMed] [Google Scholar]
- 67.Bugianesi E, McCullough AJ and Marchesini G. Insulin resistance: a metabolic pathway to chronic liver disease. Hepatology. 2005;42:987–1000. [DOI] [PubMed] [Google Scholar]
- 68.Zhang BB, Zhou G and Li C. AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell metabolism. 2009;9:407–16. [DOI] [PubMed] [Google Scholar]
- 69.Chen Z, He P, Ding X, Huang Y, Gu H and Ni X. PPARgamma stimulates expression of L-type amino acid and taurine transporters in human placentas: the evidence of PPARgamma regulating fetal growth. Sci Rep. 2015;5:12650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fryer LG, Parbu-Patel A and Carling D. The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct pathways. J Biol Chem. 2002;277:25226–32. [DOI] [PubMed] [Google Scholar]
- 71.Kubota N, Terauchi Y, Kubota T, Kumagai H, Itoh S, Satoh H, Yano W, Ogata H, Tokuyama K, Takamoto I, Mineyama T, Ishikawa M, Moroi M, Sugi K, Yamauchi T, Ueki K, Tobe K, Noda T, Nagai R and Kadowaki T. Pioglitazone ameliorates insulin resistance and diabetes by both adiponectin-dependent and -independent pathways. J Biol Chem. 2006;281:8748–55. [DOI] [PubMed] [Google Scholar]
- 72.Geach T PPAR agonists in pre-eclampsia and hypertension? Nat Rev Endocrinol. 2015;11:446. [DOI] [PubMed] [Google Scholar]