Abstract
The objective of this study was to establish a large, densely-sampled, US population-based cohort of people with autism spectrum disorder (ASD). The Rhode Island Consortium for Autism Research and Treatment (RI-CART) represents a unique public-private-academic collaboration involving all major points of service for families in Rhode Island affected by ASD. Diagnosis was based on direct behavioral observation via the Autism Diagnostic Observation Schedule (ADOS-2). For the first 1,000 participants, ages ranged from 21 months to 64 years. Using Geographic Information System and published prevalence rates, the overall cohort is estimated to represent between 20% and 49% of pediatric-age persons in Rhode Island with ASD, with demographics representative of US Census. We observed a high rate of co-occurring medical and psychiatric conditions in affected individuals. Among the most prominent findings of immediate clinical importance, we found that females received a first diagnosis of ASD at a later age than males, potentially due to more advanced language abilities in females with ASD. In summary, this is the first analysis of a large, population-based US cohort with ASD. Given the depth of sampling, the RI-CART study reflects an important new resource for studying ASD in a representative US population. Psychiatric and medical comorbidities in ASD constitute a substantial burden and warrant adequate attention as part of overall treatment. Our study also suggests that new strategies for earlier diagnosis of ASD in females may be warranted.
Keywords: autism spectrum disorder, comorbidity, female autism, population study, registry
LAY SUMMARY
The Rhode Island Consortium for Autism Research and Treatment (RI-CART) represents a unique public-private-academic collaboration involving all major points of service for families in Rhode Island affected by autism spectrum disorder (ASD). Here we provide results from the first 1,000 participants, estimated to represent > 20% of affected families in the state. Importantly, we find a later age at first diagnosis of ASD in females, which potentially calls attention to the need for improved early diagnosis in girls. Also, we report a high rate of co-occurring medical and psychiatric conditions in affected individuals.
INTRODUCTION
Autism spectrum disorder (ASD) is a neurodevelopmental condition characterized by social impairment and restricted, repetitive behavior (RRB) (American Psychiatric Association, 2013; Lord, Cook, Lventhal, & Amaral, 2000; Lord, Elsabbagh, Baird, & Veenstra-Vanderweele, 2018). Despite the clear delineation of these core diagnostic features, ASD often manifests in a manner that can affect nearly all medical and functional domains, including emotional, behavioral, developmental, neurological, medical, and physical domains (Bishop-Fitzpatrick et al., 2018; Joshi et al., 2014; Lord et al., 2000; Lord et al., 2018; Maski, Jeste, & Spence, 2011; McElhanon, McCracken, Karpen, & Sharp, 2014). While diagnostic classifications can be made with a high level of reliability, the significant heterogeneity of ASD manifestations across multiple levels of analysis (e.g., genetic, neural, behavioral, cognitive) has created a notable degree of variability in well-designed research studies (Fein & Helt, 2017; Gillberg & Fernell, 2014; Rutter, 2014).
One of the possible contributors to ASD heterogeneity in research may relate to the availability of optimal methodologies for sample ascertainment (Joshi et al., 2014). ASD research is often conducted using modestly sized sample numbers derived from the clinic or, alternatively, using large sample numbers derived from epidemiological studies. While epidemiological studies are well-powered with reduced sampling bias, participants are often not directly evaluated, and characterization of the presentation is subsequently dependent on medical or educational records. Diagnoses and symptoms are therefore often based on insurance billing codes. The insurance billing code-based method limits opportunities for detailed characterization of participants and, additionally, is associated with bias, billing errors, and inter-provider diagnostic variability (Farmer, 2007; Tyrer & Heyman, 2016). On the other hand, clinic-based studies obtain a wealth of detailed information and well-characterized presentations, yet they may have limited sample size, be underpowered, and lack applicability to the whole ASD population (Georgiades, Szatmari, & Boyle, 2013; Lenroot & Yeung, 2013).
It is recognized that heterogeneity in clinical presentation is influenced by genetic variability, comorbidity, and sex (Beggiato et al., 2017; Bishop et al., 2017; Havdahl et al., 2016; Masi, DeMayo, Glozier, & Guastella, 2017), and small studies will sample unevenly across these dimensions, limiting both the reproducibility and generalizability of findings. In addition, the importance of demographic factors such as socioeconomic status (SES) is particularly important to address, given the strong link between demographic factors (e.g., SES, minority status) and presentation and outcomes in ASD (Dickerson et al., 2017; Zamora, Williams, Higareda, Wheeler, & Levitt, 2016). Namely, disadvantaged individuals are underrepresented in ASD research and, more generally, in all clinical research. A combination methodology that can appropriately characterize the participant presentation within a large, well-represented, population-based sample may yield a more optimal ASD research sample, although practical issues such as feasibility and funding are ongoing barriers (Fein & Helt, 2017).
Given these challenges associated with ASD research, the Rhode Island Consortium for Autism Research and Treatment (RI-CART) was developed. RI-CART represents a relatively unique public-private-academic collaboration in the state of Rhode Island and includes every major point of care and service to families affected by ASD in the state (Gerber, Morrow, Sheinkopf, & Anders, 2013). The goal of RI-CART was to create a major statewide population-based research registry for individuals with ASD and their families that also involves standardized, research-level, in-person autism assessments. Here we describe the results from the first 1,000 participants enrolled in the RI-CART registry, with a particular focus on: 1) the ability to obtain a large, well-characterized, population-based ASD sample that can generalize to the US population; 2) the overall presentation of these participants, particularly in females with autism; and 3) the manifestation of neuropsychiatric and medical comorbidities affecting participants.
METHODS
Participants and Enrollment Procedure
Study procedures were approved by the Institutional Review Boards (IRBs) at all relevant institutions, with Lifespan Health System representing the lead IRB. Written informed consent was obtained from all participants. Participants were recruited through a broad effort across the state of Rhode Island that included outreach to providers and advocacy organizations, including encounters in treatment settings and community events, and deliberate inclusion of community members in the research team. Recruitment strategies included distribution of information by way of fliers and word-of-mouth to all relevant points of care and service for families in Rhode Island. Participating sites are listed in the Acknowledgments section. Monthly meetings were held with representatives from participating service programs across the state. RI-CART staff also held annual outreach events for families and providers, as well as attended events of participating service programs.
Participants were eligible to participate in the registry study if: 1) the participant had previously been diagnosed as having ASD and/or 2) there was concern on the part of the participant, a family member, or a community clinician about a possible diagnosis of ASD or a related neurodevelopmental disorder. Enrollment for the analyses presented here was from March 2013 through January 2016. Most recruitment efforts focused on Rhode Island (all cities and towns), with additional participants enrolled from areas of Connecticut and Massachusetts that border Rhode Island, i.e., regions in the catchment area for Rhode Island-based hospitals and clinics. Assessments, typically conducted in a single visit, included: a demographics questionnaire; an individual and family medical, neuropsychiatric, and developmental history; the Vineland Adaptive Behavior Scales, Second Edition (VABS-II) (Sparrow, Balla, & Cicchetti, 2005); the Social Responsiveness Scale, Second Edition (SRS-2) (Constantino & Gruber, 2012); and the Autism Diagnostic Observation Schedule, Second Edition (ADOS-2) (Lord et al., 2012), which was administered by a research-reliable ADOS-2 assessor.
Measures
Demographics and Medical History Forms
Parents, caregivers, and/or participants completed a custom-designed individual and family medical history. Medications were divided into classes according to those assumed by the Physicians’ Desk Reference and are not necessarily representative of the prescribed indication (PDR Staff, 2017).
Adaptive Behavior and Verbal Status
The VABS-II (Sparrow et al., 2005) was completed by a parent or caregiver. This measure provides an overall adaptive behavior composite (ABC) score and standard scores in the domains of communication, daily living skills, and social skills. Categorization of a participant as verbal was based on parent response at the time of evaluation by RI-CART staff to the question: “Is your child verbal?”
Autism Classification and Symptoms
The ADOS-2 (Lord et al., 2012) was administered as a semi-structured interview and observation of ASD symptoms. Assessor reliability was established using standard methods; all ADOS-2 administrations were conducted by assessors who were trained to research reliability. The SRS-2 (Constantino & Gruber, 2012) was administered as an additional quantitative measure of individual differences in behaviors associated with ASD. The SRS-2 was completed by a parent or caregiver using one of three versions (i.e., preschool, school-age, or adult forms). Where appropriate, some adults completed the self-report version of this measure, either instead of or in addition to the caregiver report. Information concerning whether a participant had received a previous community-based diagnosis of ASD was also collected and, if so, the age at that diagnosis was recorded. The absence of a community ASD diagnosis could reflect that the community-based healthcare provider did not consider an ASD diagnosis, did not believe an ASD diagnosis was warranted, or was unsure about an ASD diagnosis; or that a formal ASD assessment was not conducted prior to study enrollment. Information concerning reasons for lack of evidence of a community ASD diagnoses was not recorded.
Geographic Information System (GIS) and Estimation of Depth of Population Sampling
In order to describe the geographic representativeness of the sample, place-of-residence for participants was mapped using ArcGIS software, Version Release 10.3.1 (ESRI ArcGIS Desktop, 2014). The resulting descriptive maps focus on all US Census tracts in Rhode Island and on selected regions from neighboring towns in Southeast Massachusetts. In order to estimate the percentage of total people with ASD ascertained, this analysis was restricted to participants between the ages of 3 years old and 21 years old and to Rhode Island Census tracts – the region of focus for recruitment efforts. For the current study, analyses were restricted to participants falling in the 3- to 21-year-old age range, because diagnoses are not generally made before an age of 3 years and because of challenges in enrollment of adults (Haas et al., 2016). We calculated four estimates of the predicted number of people in Rhode Island in this age range with ASD based on the range of prevalence statistics from recent studies (i.e., 1.0%, 1.5%, 2.0%, and 2.5%). A base rate of 1.0% of the population is consistent with ascertainment in clinical settings where ASD would be an identified condition (Christensen et al., 2016) and is relatively similar to the median prevalence estimates in Western developed nations (Elsabbagh et al., 2012). A base rate of 1.5% is similar to surveillance data with broader ascertainment from the Centers for Disease Control and Prevention Autism and Developmental Disabilities Monitoring Network (Baio, 2012; Baio et al., 2018). Base rates of 2.0% to 2.5% are consistent with recent research that has utilized total population ascertainment from health and medical records (Myers et al., 2019; Schendel & Thorsteinsson, 2018). This resulted in estimated numbers of possible individuals with ASD in the 3-year-old to 21-year-old age range for each Rhode Island Census tract. The actual number of participants in the RI-CART study with an ASD diagnosis in the 3- to 21-year-old age range for each tract was then divided by the estimated number of individuals with ASD, based on prevalence measures, resulting in an approximation of the percentages of people with ASD ascertained per Rhode Island Census block. Information about the population within each geographic unit, including age, sex, number of households, representation of different races and ethnicities, and poverty indicators was obtained (Missouri Census Data Center, 2016).
Statistical Analysis
Study data were managed using Research Electronic Data Capture (REDCap) tools (Harris et al., 2009). For each section, descriptive statistics are reported for all obtained variables within the total sample and, where appropriate, subgroups based on degree of concordance between community diagnosis and results of the ADOS-2. In general, chi-square tests and analyses of variance (ANOVA) were conducted to examine differences across diagnostic subgroups. For instances in which follow-up correlation or regression analyses were conducted, more detailed methods regarding statistical analyses are described in table legends.
RESULTS
ASD Diagnostic Subgroupings and ASD Features of Participants Assessed With ADOS-2
Overall RI-CART Study Participant Demographics
The data presented here reflect the first 1,000 participants enrolled in the RI-CART study (Table 1). The majority (67%, n = 671) had a formal diagnosis by a clinical professional of ASD at time of enrollment. The remainder (33%) did not report a formal diagnosis at time of enrollment. Age at enrollment ranged from 21 months to 64 years (Figure 1 and Table 1). Approximately seventy-eight percent (78.3%) of the participants were male. In terms of ethnicity, 72.8% identified as Not Hispanic/Latino, as compared to 73.3% as measured by US Census for the state of Rhode Island (US Census Bureau, 2017). Similarly, 12.4% of RI-CART study participants identified as Hispanic/Latino, as compared to 14.9% by US Census measures. Identification of race was as follows for RI-CART study participants vs US Census data for the state of Rhode Island: White/Caucasian, 71.8% vs 84.4%; Black/African American, 3.0% vs 8.1%; Asian alone, 1.0% vs 3.6%; and Multiracial, 8.2% vs 2.7%.
TABLE 1.
Demographic Characteristics of all RI-CART Study Participants
| Characteristic | Overall Group | Community Dx and Positive ADOS: ASD | Community Dx or Positive ADOS: ASD-Inclusive | Neither Community Dx nor Positive ADOS: Non-ASD |
|---|---|---|---|---|
| Participants, n | 1000a | 533 | 318 | 101 |
| Age, mean (SD), y | 13.62 (9.63) | 13.15 (9.02) | 13.97 (9.84) | 15.40 (11.80) |
| Sex, n (%) | ||||
| Male | 783 (78.3) | 429 (80.5) | 243 (76.4) | 68 (67.3) |
| Female | 217 (21.7) | 104 (19.5) | 75 (23.6) | 33 (32.7) |
| Ethnicity, n (%) | ||||
| Not Hispanic/Latino | 728 (72.8) | 429 (80.5) | 223 (70.1) | 71 (70.3) |
| Hispanic/Latino | 124 (12.4) | 70 (13.1) | 39 (12.3) | 14 (13.9) |
| Information not provided | 148 (14.8) | 34 (6.4) | 56 (17.6) | 16 (15.8) |
| Race, n (%) | ||||
| American Indian/Alaskan Native | 5 (0.5) | 2 (0.4) | 3 (0.9) | 0 (0.0) |
| Asian | 10 (1.0) | 6 (1.1) | 3 (0.9) | 1 (1.0) |
| Black or African American | 30 (3.0) | 22 (4.1) | 5 (1.6) | 3 (3.0) |
| White | 718 (71.8) | 424 (79.5) | 221 (69.5) | 67 (66.3) |
| Multiracial | 82 (8.2) | 46 (8.6) | 25 (7.9) | 11 (10.9) |
| Other | 20 (2.0) | 10 (1.9) | 6 (1.9) | 4 (4.0) |
| Information not provided | 135 (13.5) | 23 (4.3) | 55 (17.3) | 15 (14.9) |
| Community autism-related diagnosis, n (%) | ||||
| Autism | 189 (18.9) | 160 (30.0) | 29 (9.1) | 0 (0.0) |
| Asperger syndrome | 122 (12.2) | 91 (17.1) | 31 (9.7) | 0 (0.0) |
| PDD-NOS | 131 (13.1) | 98 (18.4) | 33 (10.4) | 0 (0.0) |
| ASD | 229 (22.9) | 184 (34.5) | 45 (14.2) | 0 (0.0) |
| No diagnosis | 189 (18.9) | 0 (0.0) | 109 (34.3) | 80 (79.2) |
| Unknown | 140 (14.0) | 0 (0.0) | 71 (22.4) | 21 (20.8) |
Abbreviations: ADOS, Autism Diagnostic Observation Schedule; ASD, autism spectrum disorder; Dx, diagnosis; PDD-NOS, pervasive developmental disorder-not otherwise specified; SD, standard deviation; y, years.
Neither an ADOS-2 nor a community diagnosis was available for 48 individuals in the registry. Therefore, these individuals were not included in any of the subgroups.
Figure 1. Ages of Study Participants at Time of Enrollment.
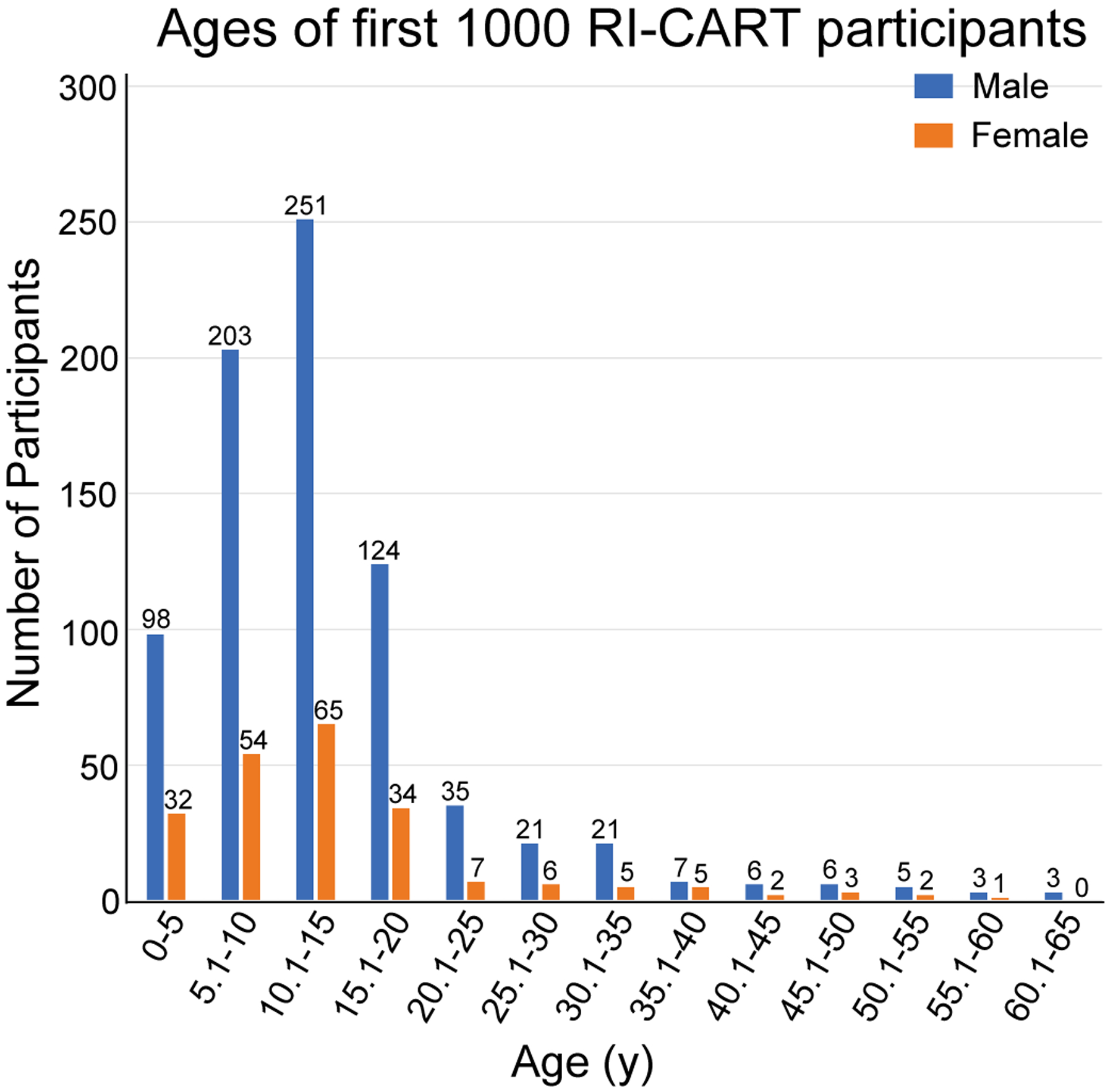
For the first 1,000 participants in the RI-CART study, ages at enrollment ranged from 21 months to 64 years, with a mean age of 13.62±9.63 years for the overall sample. Participants were largely male, reflecting 78.3% of the sample population, and the male:female ratio was approximately 4:1.
RI-CART Study Diagnostic Subgroups: ASD, ASD-Inclusive, and Non-ASD
Of the 671 participants who had a prior autism-related diagnosis given by their community-based clinician, specific diagnoses were reported as: autism (28.2%), Asperger syndrome (18.2%), pervasive developmental disorder-not otherwise specified (PDD-NOS; 19.5%), or ASD (34.1%) (Table 1). Most large-scale, population-based studies do not incorporate in-person autism assessments. As part of the RI-CART study, we were able to administer the ADOS-2 (Lord et al., 2012) by a research-reliable ADOS-2 assessor. Participants were, thereby, stratified according to their community diagnosis at the time of enrollment and their ADOS-2 score at the study visit. In addition to the Overall Group (n = 1,000), this resulted in 3 subgroups of participants, as can be seen in Table 1: 1) ASD (n = 533), participants with a community diagnosis of ASD that was confirmed with a positive ADOS-2 score; 2) ASD-Inclusive (ASD-I; n = 318), participants with either a) a community diagnosis that could not be confirmed with a positive ADOS-2 score (either because the ADOS-2 score was negative or could not be obtained) or b) a positive ADOS-2 score without a community diagnosis at the time of enrollment; and 3) Non-ASD (n = 101), participants who did not have or did not report a community diagnosis, and who did not have a positive ADOS-2 score. Notably, there was generally high concordance between community and research ASD diagnoses: 90.8% of participants with a community ASD diagnosis met diagnostic criteria for ASD based on the ADOS-2 (Tables S1 and S2; see Supporting Information for detailed analyses).
Regarding the ASD-I subgroup, this subgrouping reflects complex inclusion for patients with different types of evidence of ASD. This subgroup constitutes: participants with a community diagnosis of ASD but with a negative ADOS-2 score (n = 54 participants); participants with a community diagnosis of ASD but for whom RI-CART staff were not able to obtain an ADOS-2 score (n = 84 participants); and participants with a positive ADOS-2 score but lacking a community diagnosis, either because a diagnosis was not received, was unclear, or was not reported (n = 180 participants).
Diagnostic subgroups were significantly different in many scores on clinical measures (Table 2). There was an overall relationship between diagnostic subgroup and functional abilities, as measured by the VABS-II. Post-hoc tests revealed that the ASD subgroup was the most impaired in functional skills, followed by the ASD-I subgroup and then the Non-ASD subgroup. Differences in language abilities were found between subgroups. The Non-ASD subgroup had greater expressive language abilities as compared to the ASD or ASD-I subgroups, based on both the parent report and the observer ratings from the ADOS-2 administrations. Phenotypic variation across the three categories of participants within the ASD-I subgroup is presented in Tables S3 and S4 of the Supporting Information.
TABLE 2.
Phenotypic Presentation of Study Participants
| Subgroup Comparisons | |||||||
|---|---|---|---|---|---|---|---|
| Variable | Overall Group | ASD | ASD-Inclusive | Non-ASD | P Valuea | ||
| Participants, n | 1000b | 533 | 318 | 101 | |||
| Age at diagnosis, mean (SD), y | 5.92 (6.0) | 5.71 (5.8) | < | 6.80 (6.6) | N/A | .083 | |
| Age at enrollment, mean (SD), y | 13.7 (9.6) | 13.2 (9.0) | ≈ | 14.0 (9.8) | ≈ | 15.4 (11.8) | .079 |
| VABS-II, mean (SD) | |||||||
| Communication | 71.5 (22.0) | 68.05 (20.8)f | < | 75.23 (21.7)e | ≈ | 81.66 (24.5)e | <.001 |
| Daily living skills | 73.35 (21.2) | 70.37 (20.3)f | < | 77.69 (21.7)e | ≈ | 79.28 (20.9)e | <.001 |
| Social skills | 65.95 (19.7) | 63.19 (18.2)f | < | 69.50 (20.8)e | ≈ | 72.49 (21.2)e | <.001 |
| Motor skillsc | 79.85 (19.3) | 79.35 (18.9) | ≈ | 79.80 (20.9) | ≈ | 85.08 (18.0) | .619 |
| ABC | 68.64 (19.1) | 65.59 (17.7)f | < | 72.53 (20.0)e | ≈ | 75.96 (19.7)e | <.001 |
| ADOS-2, mean (SD) | |||||||
| Severity | 6.25 (2.5) | 7.31 (1.7)g | > | 5.47 (2.4)f | > | 2.10 (0.8)e | <.001 |
| ADOS-2 classification, n (%) | |||||||
| Autism | 600 (60.0) | 469 (88.0) | 131 (56.0) | 0 (0.0) | <.001 (χ2) | ||
| ASD | 113 (11.3) | 64 (12.0) | 49 (20.9) | 0 (0.0) | |||
| Non-spectrum | 141 (14.1) | 0 (0.0) | 54 (23.1) | 87 (86.1) | |||
| Data not available | 146 (14.6) | 84 (26.4) | 14 (13.9) | ||||
| SRS-2, mean (SD) | |||||||
| RRB | 76.01 (11.7) | 77.04 (10.5)f | ≈ | 74.97 (12.2) | ≈ | 73.16 (15.3)e | .012 |
| SCI | 74.35 (11.2) | 75.13 (10.1)f | ≈ | 74.09 (11.6)f | > | 70.56 (15.1)e | .005 |
| Total | 75.35 (11.1) | 76.24 (9.9)f | ≈ | 74.82 (11.4) | ≈ | 71.70 (15.2)e | .004 |
| Expressive language by parent report, n (%) | |||||||
| Verbal | 746 (74.8) | 422 (83.9) | 236 (88.7) | 88 (95.7) | .025 (χ2) | ||
| Nonverbal | 109 (11.0) | 76 (15.1) | 29 (10.9) | 4 (4.3) | |||
| Unsure | 6 (0.6) | 5 (1.0) | 1 (0.4) | 0 (0.0) | |||
| Data not available | 136 (13.6) | 30 (5.6) | 52 (16.4) | 9 (8.9) | |||
| Expressive language by ADOS-2 item A1, n (%)d | |||||||
| Minimally verbal | 115 (11.5) | 94 (17.6) | 19 (6.0) | 2 (2.0) | <.001 (χ2) | ||
| Single word speech | 92 (9.2) | 66 (12.4) | 21 (6.6) | 5 (5.0) | |||
| Phrase speech | 125 (12.5) | 92 (17.3) | 29 (9.1) | 4 (4.0) | |||
| Complex speech | 523 (52.3) | 281 (52.7) | 165 (51.9) | 77 (76.2) | |||
| Data not available | 145 (14.5) | 84 (26.4) | 13 (12.9) | ||||
Abbreviations: ABC, adaptive behavior composite; ADOS, Autism Diagnostic Observation Schedule; ADOS-2, Autism Diagnostic Observation Schedule, Second Edition; ASD, autism spectrum disorder; Dx, diagnosis; N/A, not applicable; RRB, restricted, repetitive behavior; SCI, social communication and interaction; SD, standard deviation; SRS-2, Social Responsiveness Scale, Second Edition; VABS-II, Vineland Adaptive Behavior Scales, Second Edition; y, years.
P values for overall tests are reported. Tests of mean differences utilized ANOVA. Chi-square tests are denoted in the table.
Neither an ADOS-2 nor a community diagnosis was available for 48 individuals in the registry. Therefore, these individuals were not included in any of the subgroups.
Motor skills scores were only collected for a subset of participants, namely, those who were either < 6 years old or > 50 years old.
Language level from ADOS-2 was defined as follows from item A1 on each module. Minimally verbal: Module 1 scores 4 or 3; Single word speech: Module 1 scores 2 or 1, Module 2 score 3; Phrase speech: Module 2 scores 2, 1, or 0, Modules 3 and 4 scores 2 or 3; Complex speech: Modules 3 and 4, scores 1 or 0.
Significant between subgroup post-hoc tests are indicated by letter notation with significant pairwise comparisons (Tukey) indicated by differing letter notations and by appropriate notation between columns (>, <, or ≈) to indicate direction of differences between subgroups.
Depth of ASD Sampling Across Rhode Island
Age-Restricted RI-CART Study Diagnostic Subgroups: ASD and ASD-I
In order to estimate the proportion of ASD cases across Rhode Island that were enrolled in the RI-CART study and the extent to which enrollment sampled neighborhood diversity in the state, participants were geocoded to point locations based on home street address at time of enrollment. The current sample is restricted to participants ranging in age from 3 years old to 21 years old who had a community diagnosis of ASD and/or an above threshold score on the ADOS-2 and who resided in Rhode Island. Thus, the ASD and ASD-I subgroups were classified as cases and counted in the numerator for these analyses. Using 2010 decennial US Census data, estimates of the number of people in this age range predicted to be diagnosed with ASD in each Rhode Island Census tract were generated (denominator). Four estimates were calculated, representing different ASD prevalence statistics (i.e., 1.0%, 1.5%, 2.0%, and 2.5%) (see Methods section). As presented in Figure 2A, the number of participants recruited per Rhode Island Census tract varied, with the preponderance of participants in the Providence urban core, but with higher pockets of enrollment in some more geographically distant areas.
Figure 2. Geographic Distribution Across Rhode Island and Neighboring Towns in Massachusetts of Study Participants and Enrollment Depth of Sample.
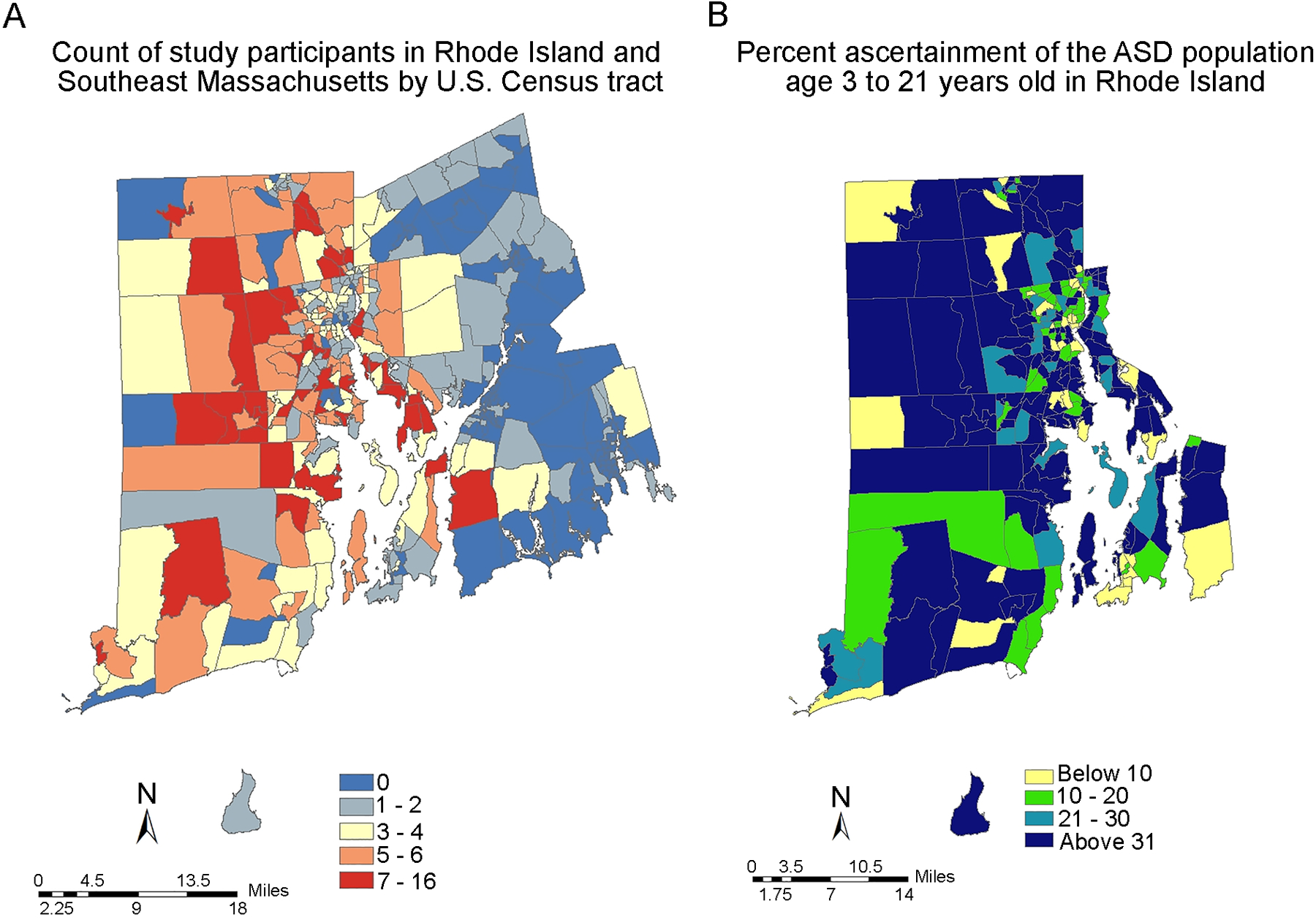
A, Numbers of study participants, as reflected by registry enrollees, of all ages by US Census tract for Rhode Island and neighboring geographic regions of Massachusetts. B, Variation in rate of ascertainment (i.e., depth of enrollment), as reflected by the number of RI-CART study enrollees in the ASD or ASD-I subgroups relative to the estimated number of cases, age 3 years old to 21 years old and assuming a base-rate prevalence of 1.5%, for each given Census block (see Methods section).
Ascertainment rates were somewhat positively skewed, indicating that, while ascertainment was high in some areas, a number of Rhode Island Census tracts existed with proportionally fewer children with ASD represented in the RI-CART study sample. Variation in ascertainment rate by Census tract is represented in Figure 2B. Here, the ascertainment rates are based on an assumed base-rate prevalence of 1.5%. These analyses demonstrated some reduced ascertainment in populations with low SES (see Supporting Information). Overall, the median state ascertainment estimates (as percentages of families sampled) were as follows for the four assumed base-rate prevalence levels of ASD: 49% ascertainment at 1.0% prevalence, 35% ascertainment at 1.5% prevalence, 24% ascertainment at 2.0% prevalence, and 20% ascertainment at 2.5% prevalence.
High Rate of Co-Occurring Medical and Psychiatric Conditions in ASD Participants
Notably, of the 879 total RI-CART study participants with available data, 47.0% of the participants reported another neurodevelopmental disorder (i.e., attention-deficit/hyperactivity disorder (ADHD) or intellectual disability), 44.1% reported a psychiatric disorder (i.e., bipolar disorder, depression, anxiety, obsessive-compulsive disorder (OCD), oppositional defiant disorder (ODD), conduct disorder (CD), or eating disorder), 42.7% reported a neurological condition (i.e., seizures/epilepsy, migraines, tics), 92.5% reported at least one general medical condition (i.e., non-neurological and non-psychiatric), and 28.4% reported other behavioral problems (i.e., self-injurious behavior or sensory processing problems) (Table 3). The rate of intellectual disability across the sample was low (19.3%) relative to other cohorts (Baio, 2012; Baio et al., 2018), which may reflect under-reporting as discussed below. Intellectual disability was higher (24%) in families wherein there was only one child affected (i.e., simplex families). (Comparison of simplex and multiplex families is described in greater detail in Supporting Information, and related data are presented in Table S5).
TABLE 3.
Psychiatric and Medical Co-Occurring Conditions in ASD Participants
| Variable | Total Sample | Community Dx and Positive ADOS: ASD | Community Dx or Positive ADOS: ASD-Inclusive | Neither Community Dx nor Positive ADOS: Non-ASD | P Valuea |
|---|---|---|---|---|---|
| Participants, n | 879 | 518 | 268 | 88 | |
| Neurodevelopmental, n (%) | |||||
| ADHD | 294 (33.4) | 146 (28.1) | 106 (40.0) | 42 (47.7) | <.001 |
| Intellectual disability | 170 (19.3) | 109 (21.0) | 49 (18.3) | 13 (14.7) | .431 |
| Any neurodevelopmental | 413 (47.0) | 228 (44.0) | 136 (50.7) | 49 (55.7) | .090 |
| Psychiatric, n (%) | |||||
| Eating disorder | 32 (3.6) | 21 (4.0) | 9 (3.4) | 2 (2.3) | .685 |
| Bipolar disorder | 44 (5.0) | 16 (2.9) | 20 (7.5) | 8 (9.1) | .005 |
| Depression | 133 (15.1) | 50 (10.0) | 61 (22.8) | 22 (25.0) | <.001 |
| Anxiety disorder | 309 (35.2) | 157 (30.0) | 116 (43.3) | 39 (44.3) | <.001 |
| OCD | 106 (12.1) | 56 (10.8) | 38 (14.2) | 12 (13.6) | .348 |
| ODD | 82 (9.3) | 33 (6.4) | 36 (13.4) | 14 (15.9) | <.001 |
| CD | 33 (3.8) | 14 (2.7) | 12 (4.5) | 7 (8.0) | .064 |
| Any psychiatric | 388 (44.1) | 197 (38.0) | 143 (53.4) | 48 (54.5) | <.001 |
| Neurological, n (%) | |||||
| Epilepsy | 40 (4.6) | 27 (5.2) | 10 (3.7) | 3 (3.4) | .551 |
| Seizures | 105 (11.9) | 69 (13.3) | 23 (8.6) | 12 (13.6) | .131 |
| Tics | 225 (25.6) | 132 (25.5) | 70 (26.1) | 25 (28.4) | .912 |
| Migraines | 112 (12.7) | 59 (11.4) | 33 (12.3) | 21 (23.9) | .004 |
| General medical, n (%) | |||||
| Vision or hearing problems | 426 (48.5) | 230 (44.4) | 144 (53.7) | 54 (61.4) | .001 |
| Skin problems | 285 (32.4) | 175 (33.8) | 81 (30.2) | 29 (32.9) | .631 |
| Sleep problems | 455 (51.8) | 264 (51.0) | 143 (53.4) | 49 (55.7) | .579 |
| GI problems | 459 (52.2) | 200 (38.6) | 110 (41.0) | 41 (46.6) | .302 |
| Feeding problems | 234 (26.6) | 152 (29.3) | 63 (23.5) | 19 (21.6) | .110 |
| Allergies | 445 (50.6) | 259 (50.0) | 138 (51.5) | 49 (55.7) | .554 |
| Any general medical | 794 (90.3) | 473 (91.3) | 242 (90.3) | 82 (93.1) | .705 |
| Other behavioral, n (%) | |||||
| Self-injury | 80 (9.1) | 35 (6.8) | 37 (13.8) | 8 (9.0) | .001 |
| Sensory processing problems | 205 (23.3) | 131 (25.3) | 61 (22.8) | 13 (14.8) | .056 |
| Any other behavioral | 250 (28.4) | 146 (28.2) | 87 (32.5) | 17 (19.3) | .016 |
| Medicationsb n (%) | |||||
| Antidepressants | 223 (25.5) | 129 (24.9) | 74 (31.1) | 23 (26.1) | .599 |
| AEDs/Mood stabilizers | 69 (7.9) | 43 (8.3) | 23 (8.6) | 5 (5.7) | .681 |
| Antipsychotics | 135 (15.4) | 78 (15.1) | 53 (19.8) | 9 (10.2) | .041 |
| Stimulants | 168 (19.2) | 86 (16.6) | 61 (22.8) | 22 (25) | .035 |
| Alpha-2 agonists | 75 (8.6) | 42 (8.1) | 31 (11.6) | 3 (3.4) | .042 |
| Anxiolytics | 31 (3.5) | 22 (4.2) | 9 (3.4) | 1 (1.1) | .743 |
| Any psychiatric medication | 446 (51.0) | 254 (49.0) | 154 (57.8) | 44 (50) | .026 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ADOS, Autism Diagnostic Observation Schedule; AEDs, antiepileptic drugs; ASD, autism spectrum disorder; CD, conduct disorder; Dx, diagnosis; GI, gastrointestinal; OCD, obsessive-compulsive disorder; ODD, oppositional defiant disorder.
P values for overall tests are reported.
For this variable, n = 874.
Analyses of co-occurring conditions across ASD subgroups revealed significant subgroup differences in psychiatric diagnoses (Table 3), with significant differences being found for bipolar disorder, depression, anxiety disorder, ODD, ADHD, self-injurious behavior, migraines, and vision or hearing problems. Across these conditions, the ASD subgroup predominantly had the lowest rate of the given condition and the Non-ASD subgroup had the highest rate of the given condition. The exception was for anxiety disorder and self-injury, where the ASD-I subgroup had the highest rate. Additionally, mood and anxiety disorders generally increased with age (Figure S1).
Approximately one half of the participants were prescribed one psychiatric medication at the time of enrollment (51.0%). The majority of these medications were prescribed to adolescents and adults, with 74.3% of adolescents and 64.4% of adults taking at least one prescription psychiatric medication or antiepileptic drug (AED). However, a substantial number of preadolescent children in the RI-CART study cohort were also being prescribed these medications (i.e., 38.2% of children in the sample were taking at least one prescription psychiatric medication or AED) (Table S6).
Statistically significant subgroup differences in medication were found for antipsychotics, stimulants, alpha-2 agonists, and any psychiatric medication (Table 3). Specifically, the ASD-I subgroup was more likely to have been prescribed these medications than the other two subgroups, perhaps reflecting the diagnostic and behavioral complexity of this subgroup. To understand this in greater depth, we analyzed the different subgroups, including specific categories of the ASD-I subgroup, for co-occurring conditions and medications (Table S7). Notably, the subgroup with the highest antipsychotic use was the subgroup with a community ASD diagnosis but for whom an ADOS-2 score was not available or ADOS-2 assessment was not tolerable, likely reflecting their behavioral acuity. This group also had the highest rate of intellectual disability (30.0%), bipolar disorder (9.5%), and OCD (24.3%).
Older Age at Diagnosis in Females With Autism, Potentially Due to More Advanced Language Abilities
Approximately seventy-eight percent (78.3%) of total study participants were male (representing a 3.5:1 male:female ratio) (Table 1). This ratio was 4.13:1 for the ASD subgroup; 3.24:1 for the ASD-I subgroup; and 2.06:1 for the Non-ASD subgroup. Notably, across the ASD and ASD-I groups combined, we observed no significant differences in ASD symptoms based on sex, although there was a trend toward an increased rate of nonverbal males with ASD (Table 4). Similarly, there were few notable differences in psychiatric or medical comorbidities in comparing males and females with ASD (Table 5).
TABLE 4.
ASD Symptoms and Adaptive Function by Sex in the Combined ASD and ASD-Inclusive Subgroups
| Characteristic | n | Males (n = 672) | Females (n = 179) | t/χ2 | P Value |
|---|---|---|---|---|---|
| Age at enrollment, mean (SD), y | 851 | 13.36 (9.17) | 13.84 (9.97) | −.612 | .541 |
| Age at diagnosis, mean (SD), y | 574 | 5.67 (5.57) | 6.95 (7.29) | −2.031 | .043 |
| Verbal “Yes” (parent report), n (%) | 658 | 512 (85.2) | 146 (90.1) | 2.616 | .106 |
| ADOS-2, mean (SD) | |||||
| Severity | 683 | 6.70 (2.21) | 6.85 (1.96) | −.699 | .485 |
| Social affect/Communication + Reciprocal social interaction | 767 | 10.84 (4.6) | 10.97 (4.5) | .−.320 | .749 |
| RRB/Stereotyped behaviors and restricted interests | 767 | 3.52 (2.2) | 3.26 (2.2) | 1.353 | .176 |
| ADOS-2 classification, n (%) | 767 | 2.624 | .269 | ||
| Autism | 600 | 470 (77.2) | 130 (82.3) | ||
| Autism spectrum | 113 | 92 (15.0) | 21 (13.3) | ||
| Non-spectrum | 54 | 47 (7.8) | 7 (4.4) | ||
| Expressive language by ADOS-2 item A1, n (%) | 740 | .909 | .823 | ||
| Minimally verbal | 105 | 86 (14.6) | 19 (12.8) | ||
| Single word speech | 86 | 71 (12.0) | 15 (10.1) | ||
| Phrase speech | 103 | 82 (13.9) | 21 (14.1) | ||
| Complex speech | 446 | 352 (59.6) | 94 (63.1) | ||
| SRS-2, mean (SD) | |||||
| Total | 701 | 75.59 (11.01) | 76.89 (10.24) | −1.311 | .190 |
| SCI | 701 | 74.65 (11.14) | 75.77 (10.38) | −1.117 | .264 |
| RRB | 701 | 76.07 (11.39) | 77.79 (11.49) | −1.644 | .101 |
| VABS-II, mean (SD) | |||||
| ABC | 632 | 67.85 (18.84) | 68.09 (18.66) | −.128 | .898 |
| Communication | 656 | 70.41 (21.11) | 70.73 (22.32) | −.159 | .874 |
| Daily living skills | 653 | 73.04 (21.46) | 72.08 (19.70) | .483 | .629 |
| Socialization | 640 | 64.91 (19.46) | 66.70 (18.81) | −.977 | .329 |
| Motor skills | 165 | 80.68 (19.29) | 74.44 (19.15) | 1.647 | .101 |
Abbreviations: ABC, adaptive behavior composite; ADOS-2, Autism Diagnostic Observation Schedule, Second Edition; ASD, autism spectrum disorder; RRB, restricted, repetitive behavior; SCI, social communication and interaction; SD, standard deviation; SRS-2, Social Responsiveness Scale, Second Edition; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
TABLE 5.
Psychiatric and Medical Co-Occurring Conditions by Sex in the Combined ASD and ASD-Inclusive Subgroups
| Variable | Males | Females | P Valuea |
|---|---|---|---|
| Participants, n | 672 | 179 | |
| Neurodevelopmental, n (%) | |||
| ADHD | 200 (29.8) | 52 (29.1) | .815 |
| Intellectual disability | 129 (19.2) | 29 (16.2) | .398 |
| Any neurodevelopmental | 295 (43.9) | 69 (38.5) | .229 |
| Psychiatric, n (%) | |||
| Eating disorder | 22 (3.3) | 8 (4.5) | .424 |
| Bipolar disorder | 31 (4.6) | 5 (2.8) | .292 |
| Depression | 83 (12.4) | 28 (15.6) | .196 |
| Anxiety disorder | 214 (31.8) | 59 (33.0) | .918 |
| OCD | 74 (11.0) | 20 (11.2) | .962 |
| ODD | 54 (8.0) | 15 (8.4) | .845 |
| CD | 24 (3.6) | 2 (1.1) | .093 |
| Any psychiatric | 269 (40.0) | 71 (39.6) | .888 |
| Neurological, n (%) | |||
| Epilepsy | 26 (3.9) | 11 (6.1) | .185 |
| Seizures | 75 (11.2) | 17 (9.5) | .524 |
| Tics | 169 (25.2) | 33 (18.4) | .061 |
| Migraines | 70 (10.4) | 22 (12.3) | .473 |
| General medical, n (%) | |||
| Vision or hearing problems | 286 (42.6) | 88 (49.2) | .114 |
| Skin problems | 211 (31.4) | 45 (25.1) | .105 |
| Sleep problems | 319 (47.5) | 88 (49.2) | .687 |
| GI problems | 256 (38.1) | 54 (30.2) | .050 |
| Feeding problems | 177 (26.4) | 38 (21.3) | .162 |
| Allergies | 315 (46.9) | 83 (46.4) | .904 |
| Any general medical | 572 (85.1) | 148 (82.7) | .422 |
| Other behavioral, n (%) | |||
| Self-injury | 52 (7.7) | 20 (11.2) | .137 |
| Sensory processing problems | 154 (22.9) | 38 (21.2) | .702 |
| Any other behavioral | 184 (27.4) | 49 (27.4) | .999 |
Abbreviations: ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CD, conduct disorder; GI, gastrointestinal; OCD, obsessive-compulsive disorder; ODD, oppositional defiant disorder.
P values for overall tests are reported.
Importantly, however, there was a statistically significant sex-based difference in age at first ASD diagnosis for the combined ASD and ASD-I groups (Table 4). Namely, on average, males received their diagnosis nearly 1.5 years earlier than females. Males (n = 672) were diagnosed by age 5.67±5.57 years on average, whereas females (n = 179) were not diagnosed until an approximate age of seven years old (age 6.95±7.29 years) (t[572] = −2.031; P = .043). Notably, this earlier age at time of diagnosis seemed to be driven largely by the ASD subgroup, as the ASD subgroup alone showed the strongest effect of female sex on age at diagnosis, and the ASD-I subgroup did not reveal a statistically significant result when analyzed alone (Tables S8 and S9).
We conducted a linear regression analysis of clinical predictors of age at ASD diagnosis across the entire sample, including those variables with suggestive evidence of differences between sexes such as verbal status and RRB, based on ADOS-2 assessment. This analysis revealed verbal status (but not RRB) to be an important predictor of age at ASD diagnosis (β = 0.177, P = .005) (Table 6). Notably, verbal status was found to be a strong predictor in males, but not in females (β = 0.198, P = .004 in males vs β = 0.135, P = .394 in females). These data suggest that more prominent language phenotypes in males with ASD may lead to earlier diagnosis. In order to account for language development that may be highly variable prior to age 5 years, we also conducted this regression analysis in participants 5 years old or greater and found similar results (Table S10). Other predictors did not differ between males and females, but an increased number of nonverbal males with ASD existed as compared to females (14.8% nonverbal males vs 9.9% nonverbal females; t/χ2 = 2.616, P = .106) (Table 4). To further examine the hypothesis that females are diagnosed at a later age due to more advanced language abilities than males, we conducted a logistic regression to test whether participant sex predicted verbal status in the whole RI-CART sample (n = 1,000). The overall model was statistically significant (χ2[2] = 25.544, P < .001; correctly classified 87.2% of cases), with both participant age (Wald = 15.043, P < .001) and participant sex (Wald = 4.449, P = .035) predicting verbal status. In addition, we conducted an analysis of covariance (ANCOVA) to examine sex differences in age at ASD diagnosis after controlling for age at enrollment and verbal status, due to concern that these variables may be confounding age at ASD diagnosis findings. After controlling for age at enrollment (F = 297.497, P < .001) and verbal status (F = 8.45, P = .004), there was a significant sex difference in age at ASD diagnosis (F[1,563] = 5.116 years, P = .024; estimated marginal means: males = 5.7 [.22] years and females = 6.9 [.45] years). Overall, our data support an interpretation of a delay in recognition of ASD in females in the RI-CART study sample, potentially due to more advanced verbal abilities in girls.
TABLE 6.
Linear Regression Analyses of Clinical Predictors of Age at Diagnosis
| Variable | Total Sample (n = 296) | Males (n = 240) | Females (n = 56) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model R2 | β | P Value | Model R2 | β | P Value | Model R2 | β | P Value | |
| Overall model | .108 | <.001 | .118 | <.001 | .119 | .161 | |||
| ADOS-2 RRB | −.114 | .062 | −.082 | .221 | −.248 | .115 | |||
| VABS-II ABC | −.119 | .051 | −.108 | .109 | −.180 | .232 | |||
| Verbal “Yes” | .177 | .005 | .198 | .004 | .135 | .394 | |||
| Number of psychiatric Dx | .168 | .005 | .195 | .003 | .065 | .676 | |||
Abbreviations: ABC, adaptive behavior composite; ADOS-2, Autism Diagnostic Observation Schedule, Second Edition; Dx, diagnoses; RRB, restricted, repetitive behavior; VABS-II, Vineland Adaptive Behavior Scales, Second Edition.
DISCUSSION
This study presents results from the first phase and the first 1,000 participants enrolled in the RI-CART (the Rhode Island Consortium for Autism Research and Treatment) study. The objective of the RI-CART study is to establish a densely-sampled, well-phenotyped, population-based cohort with ASD representative of the US population. This study is relatively unique, as it involves a sizable geographic population and reports a strong depth of sampling, estimated at 20% to as high as 49%, depending on the ASD base-rate prevalence used, of pediatric-age persons in Rhode Island. ASD research methodologies have faced many challenges with regard to recruitment. The RI-CART study utilizes a broad recruitment strategy that captures a heterogeneous range of clinical presentations, thereby providing an alternative method of investigation as compared to registries and cohorts with specific and more narrow case definitions. Strengths of this study include a large sample size (an initial sample of 1,000 individuals with enrollment continuing) that extends across the lifespan (21 months to 64 years of age), the inclusion of both community diagnosis and research criteria for ASD, and a geographically constrained area that primarily utilizes two collaborative health care systems.
RI-CART represents a recruitment method and scope that is complementary to other US cohorts. For example, the Study to Explore Early Development (SEED; Schendel et al., 2012) recruited a sizable and representative epidemiologic sample of children with ASD within a specific birth cohort (births between 2003 – 2006) and is optimized to examine potential early risk factors for ASD diagnosis. Similarly, influential studies from the Kaiser Health system in Northern California have been able to utilize large samples of individuals with ASD in a broad catchment area using medical records data (e.g., Croen et al., 2015; Kuzniewicz et al., 2014; Zerbo et al., 2015). The RI-CART study complements these other approaches by enrolling individuals with ASD across a broad age range and with demographic characteristics that are representative. The age range will support explorations of age-related trends in ASD presentation and will lay the foundation for longitudinal studies during a variety of developmental transition periods (e.g., from childhood to adolescence; from adolescence to early adulthood).
Development of a patient registry that includes in-person assessments of participants spanning a broad population and from within a specific geographic area requires grassroots enrollment and maintenance of relationships with community organizations. This creates challenges for developing demographic representativeness but has the benefit of developing relationships to support recontact and subsequent engagement of participants in ongoing research. We involved community stakeholders by making them partners in the entire research process. Important aspects of this were partnering with The Autism Project, a family support organization in Rhode Island, and also providing research outreach events designed to address interests in the community.
One focus of this study was to assess the ability to recruit a representative sample of individuals with ASD from across the state of Rhode Island. The sample is demographically comparable to the Rhode Island population in categories such as Not Hispanic/Latino (72.8% vs 73.3% for study sample vs Rhode Island Census) and Hispanic/Latino (12.4% vs 14.9% for study sample vs Rhode Island Census), while demonstrating relatively lower rates of participants expected to self-identify as Black/African American or Asian (US Census Bureau, 2017). However, it is noted that Rhode Island has a smaller proportion of residents who identify as Black than the overall US population (US Census Bureau, 2017). In addition, it is noted that a substantial number of participants identified as Multiracial (8.2%) or did not report race or ethnicity (13.5% overall). Maternal educational attainment was higher in the RI-CART study sample vs the general Rhode Island population (e.g., high school diploma or higher: 91.1% vs 85.4%; bachelor’s degree or higher: 46.4% vs 31.4%) (see Supporting Information).
Utilizing location mapping with GIS and cross-referencing this information with demographic data at the level of US Census tracts, case ascertainment was determined to be lower from neighborhoods with greater levels of poverty, which are also neighborhoods with higher proportions of minority residents (US Census Bureau, 2017). These findings are consistent with prior research demonstrating lower ASD ascertainment rates in minority racial and ethnic groups and lower SES (Dickerson et al., 2017). The underrepresentation of racial/ethnic minorities and impoverished individuals is unfortunately not uncommon in clinical research or medical care, yet this has critical implications for ASD research and treatment (Mandell et al., 2009). Initial efforts to systematically enhance ascertainment to specific minority groups (e.g., Latinos) have shown promise (Zamora et al., 2016). In the RI-CART study, we have subsequently implemented more aggressive ascertainment of Spanish-speaking populations. Implementation of such efforts into future population-based studies remains imperative, and the location data and mapping methods described in this study can be used to develop specific strategies to enhance future recruitment in underrepresented communities.
Importantly, our cohort and research approach have allowed for the study of agreement, or lack thereof, between community and research ASD diagnoses. We observed a high concordance between community diagnosis and research criteria for ASD, as 90.8% of those participants with a community diagnosis of ASD exceeded the ASD threshold on the ADOS-2. This is consistent with prior validation on the utility of the ADOS-2 in the ASD diagnostic evaluation process (Lord et al., 2012). Our results are also not consistent with gross over diagnosis of ASD by community clinicians. The 9.2% of participants with community diagnosis of ASD that did not have a positive ADOS-2 score were characterized by higher adaptive functioning, later age at time of ASD diagnosis, and less likely to identify as White. This may suggest that this cohort manifests as a milder phenotype, although the role of White/non-White status may also suggest some involvement of cultural or demographic factors. Of note, in this study, we defined the ASD-I subgrouping. While a subset of the ASD-I subgroup may have a milder ASD phenotype, it is notable that there was a high rate of caregiver-reported psychiatric diagnoses in this subgroup. Thus, in addition to the presence of individuals with a milder phenotype, participants within the ASD-I subgroup could also represent diagnostic uncertainties related to complex presentations.
We observed a male to female ratio of 4.13:1 for the ASD subgroup. However, in the overall sample, a 3.5:1 ratio was observed, which is more similar to recent meta-analytic results indicating the strongest estimate of biologic sex ratio in ASD is 3:25–3:32:1 (Lai, Lombardo, Auyeung, Chakrabarti, & Baron-Cohen, 2015; Loomes, Hull, & Mandy, 2017). Importantly, with regard to ASD features, we found no significant differences between males and females. That is, we did not find distinct cross-sectional ASD symptomology in males as compared to females, with the exception of a higher rate of nonverbal autism in males. Notably, a significant difference existed in age at first ASD diagnosis, as males on average had an earlier age at diagnosis (nearly 1.5 years younger). This effect seemed largely driven by the definitive ASD subgroup (Tables S2 and S3). The effects of biologic sex on clinical presentation, as reported in the literature, appear highly dependent on recruitment approaches (Howe, Yatchmink, Viscidi, & Morrow, 2014). The lack of sex differences in clinical symptoms has also been noted in some well-powered, larger studies more recently (Mussey, Ginn, & Klinger, 2017). In large, population-based studies (1,000+ participants), detection of differences between males and females in age at diagnosis has been somewhat inconsistent, with some studies identifying statistically significant differences between sexes (Begeer et al., 2013; May & Williams, 2018; Rosenberg, Landa, Law, Stuart, & Law, 2011; Shattuck et al., 2009), while others have not identified such differences (Giarelli et al., 2010; Hiller, Young, & Weber, 2016; D. Mandell et al., 2010; Mazurek, 2014). Our large, broad, population-based cohort is most consistent with no sex-based differences in autism phenotype at the age of enrollment; however, our data support sex-based differences in language development. Studies of sex differences in autism warrant a more developmental approach, as opposed to cross-sectional study designs, as suggested by our observations of distinct ages at first diagnosis. The first diagnosis of ASD in females at a later age is clearly of urgent clinical importance. Our initial analyses of predictors of age at diagnosis suggest that a reduced rate of nonverbal females may mean that fewer females are flagged early by lack of language. More aggressive and deliberate methods to diagnose ASD in females at earlier ages may be warranted.
Medical and psychiatric co-occurring conditions represent a sizable burden on participants and their families (Croen et al., 2015; Jokiranta-Olkoniemi et al., 2016; Soke, Maenner, Christensen, Kurzius-Spencer, & Schieve, 2018). Information reported was as follows: 74% with at least one co-occurring neurodevelopmental, psychiatric, or neurological condition and 93% with at least one general medical condition. This overall rate of co-occurring neuropsychiatric conditions among individuals with ASD is in line with rates reported by prior smaller population-based studies (Abdallah et al., 2011; Simonoff et al., 2008), as is the high percentage of the sample reporting medical co-occurring symptoms, with gastrointestinal problems, sleep problems, and allergies being among the most common (Bauman, 2010). The rates of individual neuropsychiatric conditions were generally consistent with prior population-based rates among individuals with ASD (Hudson, Hall, & Harkness, 2019; Simonoff et al., 2008), with the exception of intellectual disability (19%). This reported rate is approximately half of the previously reported prevalence rate (38%) (Baio, 2012). Limitations of community, clinical assessments of intellectual disability, reduced reporting to parents of such diagnoses, and/or reduced parental report of intellectual disability are likely, perhaps due in part to stigma. Whereas only 19% of participants were reported to have intellectual disability, nearly 50% were reported to have achieved only phrase speech or less. Also, a substantial percentage of the RI-CART study sample had ABC scores in the low or impaired range. Together, these data suggest that the reported rate of intellectual disability underestimates the significant degree of developmental impairments represented in the study sample.
Also, importantly, over half of the sample reported being prescribed at least one psychotropic medication, consistent with prevalence estimates in the United States for psychotropic medications that range from 34% to 81%, depending on age (Coury et al., 2012; Esbensen, Greenberg, Seltzer, & Aman, 2009; Frazier et al., 2011). These findings also support current recommendations by the medical community for a careful medical or psychiatric work-up in individuals with ASD, as clinically indicated (Isaksen et al., 2013).
When analyzing psychiatric co-occurring conditions of subgroups, the Non-ASD subgroup also reported high rates of neurodevelopmental and psychiatric conditions, especially ADHD, bipolar disorder, depression, and ODD. The ASD-I subgroup reported higher amounts of self-injurious behavior and anxiety and high rates of psychiatric medication use.
In summary, we have presented the first observations on autism phenotypes in a clinically-broad and deeply-sampled, population-based cohort with ASD. The information gathered from these initial 1,000 participants in the RI-CART study extends our knowledge of the vast heterogeneity of autism. Our findings of high rates of co-occurring neuropsychiatric disorders in individuals with ASD reflect provider challenges in diagnosing ASD in a clinically-complex presentation, as well as the heterogeneity of ASD presentation. The nature of this well-sampled, population-based cohort, therefore, mirrors contemporary clinical challenges regarding autism, which in turn affect diagnosis, treatment, and long-term outcomes. Future research on more aggressive and earlier diagnosis in females seems warranted, given the apparent risk of delayed diagnosis for this population.
Supplementary Material
ACKNOWLEDGMENTS
The authors foremost thank all of the families who took part in this study. In addition, the authors are indebted to the following agencies, centers, institutions, and individuals who comprise the Rhode Island Consortium for Autism Research and Treatment (RI-CART): The Autism Project, Johnston, RI (Alicia Ead, BS, Joanne G. Quinn, BA, and Susan B. Jewel, BA); Bailey’s Team for Autism, Attleboro, MA; Matthew Best, BFA, Hartford, CT; the Brown Center for the Study of Children at Risk, Providence, RI (Barry Lester, PhD, Todd P. Levine, MD, Cynthia Loncar, PhD, Christopher Phothisane, BA, Stephen J. Sheinkopf, PhD, and Kristen Sutton, BA); Butler Hospital, Providence, RI; the Center for Autism and Related Disorders, Providence, RI; Children’s Neurodevelopment Center, Hasbro Children’s Hospital, Providence, RI (Pei-Chi Wu, MD, Pamela High, MD, and Yvette Yatchmink, PhD, MD); Center for Autism and Developmental Disorders, Emma Pendleton Bradley Hospital (Brian C. Kavanaugh, PsyD, Giulia Righi, PhD, Daniel Moreno-De-Luca, MD, MSc, Carrie R. Best, MPH, Thomas F. Anders, MD, and Eric M. Morrow, MD, PhD); The Groden Network, Providence, RI (Robin Ringer, MEd and Cooper Woodard, PhD); Hassenfeld Child Health Innovation Institute, School of Public Health, Brown University, Providence, RI (Raul Smego Barranco, BA and Patrick Vivier, MD); The Museum of Work and Culture, Woonsocket, RI; the Neurodevelopmental Center, Providence, RI; The New England Pediatric Institute for Neurodevelopment, Pawtucket, RI (Rebecca L. McLean, PhD and Viren D’Sa, MD); Matthew Goodwin, PhD; Ocean State Libraries, Warwick, RI; Pathways Strategic Teaching Center/J. Arthur Trudeau Memorial Center, Warwick, RI; Department of Pathology and Laboratory Medicine, Rhode Island Hospital, Providence, RI (Ece D. Gamsiz Uzun, PhD); Paul V. Sherlock Center on Disabilities, Rhode Island College, Providence, RI (Amy Grattan, PhD and Paul LaCava, PhD); Perspectives Corporation, North Kingstown, RI; Rhode Island Parent Information Network, Cranston, RI; Rhode Island Department of Education, Providence, RI (Susan Constable, MA); Rhode Island Department of Health, Providence, RI; Special Olympics RI, Smithfield, RI; University of Rhode Island, South Kingstown, RI (Michelle Flippin, PhD, CCC-SLP, Dana Kovarsky, PhD, and Amy Laurent, PhD, OTR/L); and Women & Infants Hospital (James F. Padbury, MD). Also, the authors acknowledge the leadership of the Robert J. and Nancy D. Carney Institute for Brain Science at Brown University for institutional funding and support; Finally, the authors thank Greg Fritz, MD, former Academic Director of the Emma Pendleton Bradley Hospital.
FUNDING INFORMATION
This study was supported in part by a grant from the Simons Foundation Autism Research Initiative to E.M.M. (286756) and a gift through the Hassenfeld Child Health Innovation Institute at Brown University to E.M.M. and S.J.S. C.E.B.M. was supported by grants from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical and Translational Sciences Award (KL2 TR002530 and UL1 TR002529, PI: A. Shekhar). D.S. was supported by grants from the National Institutes of Health, National Institute of Mental Health (R25 MH101076, PI: A. Tyrka; T32 MH019927, PI: A. Spirito). The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
Footnotes
CONFLICT OF INTEREST
The authors report no biomedical financial interests or potential conflicts of interest.
REFERENCES
- Abdallah MW, Greaves-Lord K, Grove J, Norgaard-Pedersen B, Hougaard DM, & Mortensen EL (2011). Psychiatric comorbidities in autism spectrum disorders: findings from a Danish Historic Birth Cohort. Eur Child Adolesc Psychiatry, 20(11–12), 599–601. doi: 10.1007/s00787-011-0220-2 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association (2013). Diagnostic and Statistical Manual of Mental Disorders (5th ed.). Washington DC: American Psychiatric Association Publishing. [Google Scholar]
- Baio J (2012). Prevalence of autism spectrum disorders--Autism and developmental disabilities monitoring network, 14 sites, United States, 2008. MMWR Surveill Summ, 61, 1–19. [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, … Dowling NF (2018). Prevalence of Autism Spectrum Disorder Among Children Aged 8 Years - Autism and Developmental Disabilities Monitoring Network, 11 Sites, United States, 2014. MMWR Surveill Summ, 67(6), 1–23. doi: 10.15585/mmwr.ss6706a1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauman ML (2010). Medical comorbidities in autism: challenges to diagnosis and treatment. Neurotherapeutics, 7(3), 320–327. doi: 10.1016/j.nurt.2010.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begeer S, Mandell D, Wijnker-Holmes B, Venderbosch S, Rem D, Stekelenburg F, & Koot HM (2013). Sex differences in the timing of identification among children and adults with autism spectrum disorders. J Autism Dev Disord, 43(5), 1151–1156. doi: 10.1007/s10803-012-1656-z [DOI] [PubMed] [Google Scholar]
- Beggiato A, Peyre H, Maruani A, Scheid I, Rastam M, Amsellem F, … Delorme R (2017). Gender differences in autism spectrum disorders: Divergence among specific core symptoms. Autism Res, 10(4), 680–689. doi: 10.1002/aur.1715 [DOI] [PubMed] [Google Scholar]
- Bishop-Fitzpatrick L, Movaghar A, Greenberg JS, Page D, DaWalt LS, Brilliant MH, & Mailick MR (2018). Using machine learning to identify patterns of lifetime health problems in decedents with autism spectrum disorder. Autism Res, 11(8), 1120–1128. doi: 10.1002/aur.1960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop SL, Farmer C, Bal V, Robinson EB, Willsey AJ, Werling DM, … Thurm A (2017). Identification of Developmental and Behavioral Markers Associated With Genetic Abnormalities in Autism Spectrum Disorder. Am J Psychiatry, 174(6), 576–585. doi: 10.1176/appi.ajp.2017.16101115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen DL, Baio J, Braun KV, Bilder D, Charles J, Constantino JN, … Yeargin-Allsopp N (2016). Prevalence and characteristics of autism spectrum disroder among children aged 8 years- Autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ, 65, 1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constantino JN, & Gruber CP (2012). The Social Responsiveness Scale, Second Edition. Torrance, CA: Western Psychological Services. [Google Scholar]
- Coury DL, Anagnostou E, Manning-Courtney P, Reynolds A, Cole L, McCoy R, … Perrin JM (2012). Use of psychotropic medication in children and adolescents with autism spectrum disorders. Pediatrics, 130 Suppl 2, S69–76. doi: 10.1542/peds.2012-0900D [DOI] [PubMed] [Google Scholar]
- Croen LA, Zerbo O, Qian Y, Massolo ML, Rich S, Sidney S, & Kripke C (2015). The health status of adults on the autism spectrum. Autism, 19(7), 814–823. doi: 10.1177/1362361315577517 [DOI] [PubMed] [Google Scholar]
- Dickerson AS, Rahbar MH, Pearson DA, Kirby RS, Bakian AV, Bilder DA, … Slay Wingat M (2017). Autism spectrum disorder reporting in lower socioeconomic neighborhoods. Autism, 21(4), 470–480. doi: 10.1177/1362361316650091 [DOI] [PubMed] [Google Scholar]
- Elsabbagh M, Divan G, Koh YJ, Kim YS, Kauchali S, Marcin C, … Fombonne E (2012). Global prevalence of autism and other pervasive developmental disorders. Autism Res, 5(3), 160–179. doi: 10.1002/aur.239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esbensen AJ, Greenberg JS, Seltzer MM, & Aman MG (2009). A longitudinal investigation of psychotropic and non-psychotropic medication use among adolescents and adults with autism spectrum disorders. J Autism Dev Disord, 39(9), 1339–1349. doi: 10.1007/s10803-009-0750-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- ESRI ArcGIS Desktop (2014). Version Release 10.3.1 Redlands: Environmental Systems Research Institute. [Google Scholar]
- Farmer R (2007). The problems with some epidemiological studies. Maturitas, 57(1), 11–15. doi: 10.1016/j.maturitas.2007.02.003 [DOI] [PubMed] [Google Scholar]
- Fein D, & Helt M (2017). Facilitating Autism Research. J Int Neuropsychol Soc, 23(9–10), 903–915. doi: 10.1017/S1355617717001096 [DOI] [PubMed] [Google Scholar]
- Frazier TW, Shattuck PT, Narendorf SC, Cooper BP, Wagner M, & Spitznagel EL (2011). Prevalence and correlates of psychotropic medication use in adolescents with an autism spectrum disorder with and without caregiver-reported attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol, 21(6), 571–579. doi: 10.1089/cap.2011.0057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgiades S, Szatmari P, & Boyle M (2013). Importance of studying heterogeneity in autism. Neuropsychiatry, 3, 123–125. [Google Scholar]
- Gerber A, Morrow EM, Sheinkopf SJ, & Anders T (2013). The Rhode Island Consortium for Autism Research and Treatment (RI-CART): a new statewide autism collaborative. R I Med J, 97, 31–34. [PMC free article] [PubMed] [Google Scholar]
- Giarelli E, Wiggins LD, Rice CE, Levy SE, Kirby RS, Pinto-Martin J, & Mandell D (2010). Sex differences in the evaluation and diagnosis of autism spectrum disorders among children. Disabil Health J, 3(2), 107–116. doi: 10.1016/j.dhjo.2009.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillberg C, & Fernell E (2014). Autism plus versus autism pure. J Autism Dev Disord, 44(12), 3274–3276. doi: 10.1007/s10803-014-2163-1 [DOI] [PubMed] [Google Scholar]
- Haas K, Costley D, Falkmer M, Richdale A, Sofronoff K, & Falkmer T (2016). Factors Influencing the Research Participation of Adults with Autism Spectrum Disorders. J Autism Dev Disord, 46(5), 1793–1805. doi: 10.1007/s10803-016-2708-6 [DOI] [PubMed] [Google Scholar]
- Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, & Conde JG (2009). Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform, 42(2), 377–381. doi: 10.1016/j.jbi.2008.08.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havdahl KA, Hus Bal V, Huerta M, Pickles A, Oyen AS, Stoltenberg C, … Bishop SL (2016). Multidimensional Influences on Autism Symptom Measures: Implications for Use in Etiological Research. J Am Acad Child Adolesc Psychiatry, 55(12), 1054–1063 e1053. doi: 10.1016/j.jaac.2016.09.490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller RM, Young RL, & Weber N (2016). Sex differences in pre-diagnosis concerns for children later diagnosed with autism spectrum disorder. Autism, 20(1), 75–84. doi: 10.1177/1362361314568899 [DOI] [PubMed] [Google Scholar]
- Howe YJ, Yatchmink Y, Viscidi EW, & Morrow EM (2014). Ascertainment and gender in autism spectrum disorders. J Am Acad Child Adolesc Psychiatry, 53(6), 698–700. doi: 10.1016/j.jaac.2014.04.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson CC, Hall L, & Harkness KL (2019). Prevalence of Depressive Disorders in Individuals with Autism Spectrum Disorder: a Meta-Analysis. J Abnorm Child Psychol, 47(1), 165–175. doi: 10.1007/s10802-018-0402-1 [DOI] [PubMed] [Google Scholar]
- Isaksen J, Bryn V, Diseth TH, Heiberg A, Schjolberg S, & Skjeldal OH (2013). Children with autism spectrum disorders - the importance of medical investigations. Eur J Paediatr Neurol, 17(1), 68–76. doi: 10.1016/j.ejpn.2012.08.004 [DOI] [PubMed] [Google Scholar]
- Jokiranta-Olkoniemi E, Cheslack-Postava K, Sucksdorff D, Suominen A, Gyllenberg D, Chudal R, … Sourander A (2016). Risk of Psychiatric and Neurodevelopmental Disorders Among Siblings of Probands With Autism Spectrum Disorders. JAMA Psychiatry, 73(6), 622–629. doi: 10.1001/jamapsychiatry.2016.0495 [DOI] [PubMed] [Google Scholar]
- Joshi G, Faraone SV, Wozniak J, Petty C, Fried R, Galdo M, … Biederman J (2014). Examining the clinical correlates of autism spectrum disorder in youth by ascertainment source. J Autism Dev Disord, 44(9), 2117–2126. doi: 10.1007/s10803-014-2063-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuzniewicz MW, Wi S, Qian Y, Walsh EM, Armstrong MA, & Croen LA (2014). Prevalence and neonatal factors associated with autism spectrum disorders in preterm infants. J Pediatr, 164(1), 20–25. doi: 10.1016/j.jpeds.2013.09.021 [DOI] [PubMed] [Google Scholar]
- Lai MC, Lombardo MV, Auyeung B, Chakrabarti B, & Baron-Cohen S (2015). Sex/gender differences and autism: setting the scene for future research. J Am Acad Child Adolesc Psychiatry, 54(1), 11–24. doi: 10.1016/j.jaac.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenroot RK, & Yeung PK (2013). Heterogeneity within Autism Spectrum Disorders: What have We Learned from Neuroimaging Studies? Front Hum Neurosci, 7, 733. doi: 10.3389/fnhum.2013.00733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomes R, Hull L, & Mandy WPL (2017). What Is the Male-to-Female Ratio in Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. J Am Acad Child Adolesc Psychiatry, 56(6), 466–474. doi: 10.1016/j.jaac.2017.03.013 [DOI] [PubMed] [Google Scholar]
- Lord C, Cook EH, Lventhal BL, & Amaral DG (2000). Autism Spectrum Disorders. Neuron, 28, 355–363. [DOI] [PubMed] [Google Scholar]
- Lord C, Elsabbagh M, Baird G, & Veenstra-Vanderweele J (2018). Autism spectrum disorder. Lancet, 392(10146), 508–520. doi: 10.1016/s0140-6736(18)31129-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lord C, Rutter M, DiLavore PC, Risi S, Gotham K, & Bishop S (2012). Autism Diagnostic Observation Schedule, Second Edition Torrance, CA: Western Psychological Services. [Google Scholar]
- Mandell D, Morales K, Xie M, Lawer L, Stahmer A, & Marcus S (2010). Age of Diagnosis Among Medicaid-Enrolled Children With Autism, 2001–2004. Psychiatric Services, 61(8). doi: 10.1176/ps.2010.61.8.822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandell DS, Wiggins LD, Carpenter LA, Daniels J, DiGuiseppi C, Durkin MS, … Kirby RS (2009). Racial/ethnic disparities in the identification of children with autism spectrum disorders. Am J Public Health, 99(3), 493–498. doi: 10.2105/AJPH.2007.131243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masi A, DeMayo MM, Glozier N, & Guastella AJ (2017). An Overview of Autism Spectrum Disorder, Heterogeneity and Treatment Options. Neurosci Bull, 33(2), 183–193. doi: 10.1007/s12264-017-0100-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maski KP, Jeste SS, & Spence SJ (2011). Common neurological co-morbidities in autism spectrum disorders. Curr Opin Pediatr, 23(6), 609–615. doi: 10.1097/MOP.0b013e32834c9282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T, & Williams K (2018). Brief Report: Gender and Age of Diagnosis Time Trends in Children with Autism Using Australian Medicare Data. J Autism Dev Disord, 48(12), 4056–4062. doi: 10.1007/s10803-018-3609-7 [DOI] [PubMed] [Google Scholar]
- Mazurek MO, Handen BL, Wodka EL, Nowinski L, Butter E, Engelhardt CR (2014). Age at first autism spectrum disorder diagnosis: The role of birth cohort, demographic factors, and clinical features. Journal of Developmental and Behavioral Pediatrics, 35, 561–569. [DOI] [PubMed] [Google Scholar]
- McElhanon BO, McCracken C, Karpen S, & Sharp WG (2014). Gastrointestinal symptoms in autism spectrum disorder: a meta-analysis. Pediatrics, 133(5), 872–883. doi: 10.1542/peds.2013-3995 [DOI] [PubMed] [Google Scholar]
- Missouri Census Data Center (2016). Census 2010 SF1 [Dataset application]. Retrieved from http://mcdc.missouri.edu/. Accessed March 9, 2016.
- Mussey JL, Ginn NC, & Klinger LG (2017). Are males and females with autism spectrum disorder more similar than we thought? Autism, 21(6), 733–737. doi: 10.1177/1362361316682621 [DOI] [PubMed] [Google Scholar]
- Myers SM, Voigt RG, Colligan RC, Weaver AL, Storlie CB, Stoeckel RE, … Katusic SK. (2019). Autism Spectrum Disorder: Incidence and Time Trends Over Two Decades in a Population-Based Birth Cohort. J Autism Dev Disord, 49(4), 1455–1474. doi: 10.1007/s10803-018-3834-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- PDR Staff (2017). Physicians’ Desk Reference: PDR Network. [Google Scholar]
- Pizzo L, Jensen M, Polyak A, Rosenfeld JA, Mannik K, Krishnan A, … Girirajan S (2019). Rare variants in the genetic background modulate cognitive and developmental phenotypes in individuals carrying disease-associated variants. Genet Med, 21(4), 816–825. doi: 10.1038/s41436-018-0266-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson EB, Samocha KE, Kosmicki JA, McGrath L, Neale BM, Perlis RH, & Daly MJ (2014). Autism spectrum disorder severity reflects the average contribution of de novo and familial influences. Proc Natl Acad Sci U S A, 111(42), 15161–15165. doi: 10.1073/pnas.1409204111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg RE, Landa R, Law JK, Stuart EA, & Law PA (2011). Factors affecting age at initial autism spectrum disorder diagnosis in a national survey. Autism Res Treat, 2011, 874619. doi: 10.1155/2011/874619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M (2014). Addressing the issue of fractionation in autism spectrum disorder: a commentary on Brunsdon and Happe, Frazier et al., Hobson and Mandy et al. Autism, 18(1), 55–57. doi: 10.1177/1362361313513522 [DOI] [PubMed] [Google Scholar]
- Schendel DE, Diguiseppi C, Croen LA, Fallin MD, Reed PL, Schieve LA, … Yeargin-Allsopp M (2012). The Study to Explore Early Development (SEED): a multisite epidemiologic study of autism by the Centers for Autism and Developmental Disabilities Research and Epidemiology (CADDRE) network. J Autism Dev Disord, 42(10), 2121–2140. doi: 10.1007/s10803-012-1461-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schendel DE, & Thorsteinsson E (2018). Cumulative Incidence of Autism Into Adulthood for Birth Cohorts in Denmark, 1980–2012. JAMA, 320(17), 1811–1813. doi: 10.1001/jama.2018.11328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shattuck PT, Durkin M, Maenner M, Newschaffer C, Mandell DS, Wiggins L, … Cuniff C (2009). Timing of identification among children with an autism spectrum disorder: findings from a population-based surveillance study. J Am Acad Child Adolesc Psychiatry, 48(5), 474–483. doi: 10.1097/CHI.0b013e31819b3848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simonoff E, Pickles A, Charman T, Chandler S, Loucas T, & Baird G (2008). Psychiatric disorders in children with autism spectrum disorders: prevalence, comorbidity, and associated factors in a population-derived sample. J Am Acad Child Adolesc Psychiatry, 47(8), 921–929. doi: 10.1097/CHI.0b013e318179964f [DOI] [PubMed] [Google Scholar]
- Soke GN, Maenner MJ, Christensen D, Kurzius-Spencer M, & Schieve LA (2018). Prevalence of Co-occurring Medical and Behavioral Conditions/Symptoms Among 4- and 8-Year-Old Children with Autism Spectrum Disorder in Selected Areas of the United States in 2010. J Autism Dev Disord, 48(8), 2663–2676. doi: 10.1007/s10803-018-3521-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow S, Balla D, & Cicchetti D (2005). Vineland Adaptive Behavior Scales. Shoreview, MN: American Guidance Service. [Google Scholar]
- Tyrer S, & Heyman B (2016). Sampling in epidemiological research: issues, hazards and pitfalls. BJPsych Bull, 40(2), 57–60. doi: 10.1192/pb.bp.114.050203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- US Census Bureau (2017). Population Estimates, July 1, 2017. Retrieved from https://www.census.gov/quickfacts/fact/table/ri/PST045217. Accessed July 2018.
- Zamora I, Williams ME, Higareda M, Wheeler BY, & Levitt P (2016). Brief Report: Recruitment and Retention of Minority Children for Autism Research. J Autism Dev Disord, 46(2), 698–703. doi: 10.1007/s10803-015-2603-6 [DOI] [PubMed] [Google Scholar]
- Zerbo O, Qian Y, Yoshida C, Grether JK, Van de Water J, & Croen LA (2015). Maternal Infection During Pregnancy and Autism Spectrum Disorders. J Autism Dev Disord, 45(12), 4015–4025. doi: 10.1007/s10803-013-2016-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


