Significance
Water is the key limiting resource in the Earth’s arid and semiarid ecosystems, where its availability shapes plant diversity and primary productivity. Water-limited ecosystems are likely to become more arid under the combined impacts of higher temperatures and altered precipitation in the 21st century. Analyses of plant diversity in space and time in California, a biodiversity hotspot, support the expectation that diversity decreases as water availability declines. Not only numbers of species, but diversities of functional traits and evolutionary lineages represented in plant communities, are governed by water availability. Previous studies of climate and diversity have often focused on the effects of warming in cold, temperature-limited climates, and this study helps remedy the need for better predictions about water-limited ecosystems.
Keywords: aridification, climate change, drought, functional diversity, phylogenetic diversity
Abstract
Climate strongly shapes plant diversity over large spatial scales, with relatively warm and wet (benign, productive) regions supporting greater numbers of species. Unresolved aspects of this relationship include what causes it, whether it permeates to community diversity at smaller spatial scales, whether it is accompanied by patterns in functional and phylogenetic diversity as some hypotheses predict, and whether it is paralleled by climate-driven changes in diversity over time. Here, studies of Californian plants are reviewed and new analyses are conducted to synthesize climate–diversity relationships in space and time. Across spatial scales and organizational levels, plant diversity is maximized in more productive (wetter) climates, and these consistent spatial relationships are mirrored in losses of taxonomic, functional, and phylogenetic diversity over time during a recent climatic drying trend. These results support the tolerance and climatic niche conservatism hypotheses for climate–diversity relationships, and suggest there is some predictability to future changes in diversity in water-limited climates.
Plant diversity is shaped more strongly in space and time by climate than by any other factor. This control of diversity by climate is clearest when considering taxonomic diversity (species richness) over broad spatial scales. Globally, regions with climates that are both warm and wet support more species than regions where the climate is either cold or arid, and this broadscale climatic influence outweighs that of any other predictor of diversity (1–3). Understanding the basis of the climate–diversity relationship takes on particular urgency in light of contemporary climate change. Climates are now changing rapidly toward warmer temperatures, and in nearly half the Earth’s terrestrial surface, this is expected to lead to less water availability in the growing season (4). Simple extrapolation of present-day patterns into expected future climates leads to the prediction of diversity decline in much of the world, essentially wherever water is already strongly limiting (3). However, too many crucial aspects of the geographic climate–diversity relationship remain incompletely understood for accurate predictions.
Mechanisms underlying the climate–diversity relationship have been long debated and consensus is still elusive. One proposed explanation highlights the greater range of plant functional strategies that are capable of existing in warm and wet than cold or dry conditions (tolerance hypothesis) (5, 6). Another, nonexclusive explanation considers the greater age and historical extent of tropical-like climates, combined with intrinsic barriers limiting the rate of adaptive shifts into harsher climates (climatic niche conservatism hypothesis) (7, 8). Because the same climatic conditions that give rise to high species diversity also support high plant productivity, the climate–diversity relationship is sometimes attributed to the influence of an energetic carrying capacity on rates of speciation and extinction (productivity per se, climate-energy, or species-energy hypothesis) (5, 9). Finally, past climatic stability during the Pleistocene has left an important imprint on contemporary biotas, making some regions more diverse than expected based on their current climates (e.g., Cape Floristic Region) (2, 10).
Functional and phylogenetic diversity patterns are increasingly studied aspects of biodiversity that may influence how communities affect and are affected by the environment (11, 12) and that also offer new ways to examine hypotheses for the climate–diversity relationship. Functional diversity, or the variation in multiple resource-acquisition traits within assemblages of coexisting species, is expected to be highest in warm and wet climates under the tolerance hypothesis (6, 13–15). Phylogenetic diversity, or some measure of the amount of evolutionary history contained within assemblages using the branch lengths separating coexisting species, should generally be highest in warm and wet climates under the climatic niche conservatism hypothesis (7). Some progress is being made on testing these hypotheses, but large-scale patterns in functional and phylogenetic diversity are still in the process of being described and understood.
In addition to the unresolved questions about its underlying causes, and the organizational levels at which it is manifested, another aspect of the climate–diversity relationship that remains problematic is its spatial scale-dependence (16). In contrast to its strong influence at large regional scales, climate is a weak correlate of spatial variation in species diversity at the local community scale where species interact and share resources (17). Some evidence suggests that this scale-dependence is an artifact, arising partly because few studies of local community variation span large climate gradients, and partly because the signal of climate only emerges when more local sources of variation, such as disturbance and soil fertility, are accounted for (18). Alternatively, however, climate might operate on regional diversity mainly by promoting the persistence of geographically rare species (e.g., ref. 19), in which case it might have little direct influence on the diversity of local communities. It is also possible that climatic productivity tends to make local communities more dissimilar (higher β-diversity) (20, 21), which would also cause regional diversity to increase more strongly than local diversity along gradients of climate.
Recent studies in the Californian Floristic Province suggest some consistency in how contemporary climatic gradients affect plant diversity both in space, at large and small scales, and in recent time, at levels of organization that include taxonomic, functional, and phylogenetic diversity. This evidence is synthesized here using a combination of review and new analyses, with the goal of advancing predictions about how and why plant diversity varies with climate that may be relevant to other water-limited regions. Of particular interest are the predictions of the tolerance and niche conservatism hypotheses about how functional and phylogenetic diversity, respectively, should vary along gradients of water availability in parallel with taxonomic diversity.
Study System and Datasets
The Californian Floristic Province shares with the world’s four other Mediterranean climate ecosystems the defining features of cool wet winters and hot dry summers, and the floristic characteristics of high regional diversity and functional distinctiveness, striking species turnover along climatic and edaphic gradients, and moderate levels of local-scale plant diversity (10, 22). The historical assembly of its flora from older mesic-adapted elements and more recent xeric-adapted elements has been well characterized, and this history shares some of its basic elements with those of the other Mediterranean-type ecosystems (22). In contrast to the Cape Floristic Region, however, California’s regional plant diversity is not an outlier with respect to global climate–richness relationships (2). Within the Floristic Province there is substantial variation from cooler and wetter in the north-northwest to warmer and drier in the south-southeast. Current and anticipated climatic changes across this gradient are generally toward higher water deficit, with contributing factors including higher evapotranspiration, lower mean precipitation particularly in the south, and greater variability of precipitation, including more multiyear droughts (23–27).
Spatial Variation in Diversity.
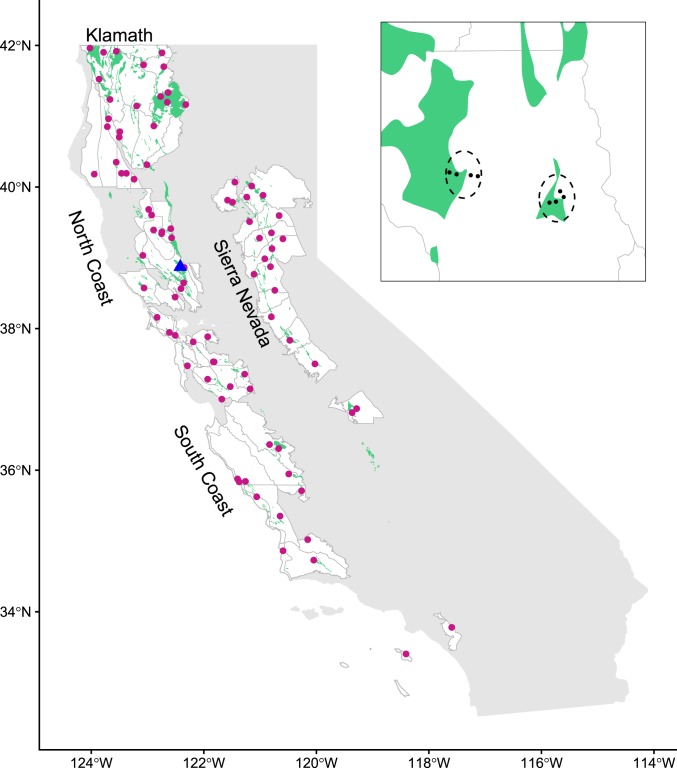
The dataset for geographic diversity patterns within California began as an examination of the spatial distribution of diversity on outcrops of serpentine (or ultramafic) rocks and soils (28). Serpentine is a chemically harsh and nutrient-poor substrate that constitutes 1.5% of the land area of the state of California, and contributes roughly 180 edaphic endemics to the 5,000 vascular plant species native to the Californian Floristic Province (29, 30). Although levels of α-diversity are not generally higher on serpentine than more fertile soils, high species turnover both among serpentine outcrops and between serpentine and neighboring nonserpentine soils adds notable β-diversity to Californian landscapes (28, 31, 32). Because of this original focus, the dataset spans only those parts of the state where serpentine soil is found, including most of the Californian Floristic Province (Klamath Mountains, North and South Coast Ranges, Sierra Nevada, Transverse Ranges) but largely or entirely excluding the Central Valley, Modoc Plateau, Peninsular Ranges, and deserts (Fig. 1). Vegetation across the study area varies from evergreen and deciduous forest, through woodland and chaparral, to open grasslands, and in any given location it is generally smaller-statured on serpentine than nonserpentine soil.
Fig. 1.
Statewide study regions (white areas) and sites (red dots). (Inset) One region with two sites (dashed ellipses), each of which consists of four plots (black dots), two of which are on serpentine soil (green area) and two of which are on nearby nonserpentine soil. Blue triangle represents grassland study location (McLaughlin University of California Reserve).
Using spatial floristic databases, the study area was subdivided into 78 regions (102- to 103-km2 area) for which regional floristic and environmental data could be derived. These regions span a roughly 200- to 1,800-mm gradient of mean annual precipitation that is positively correlated with a remotely sensed index of productivity (normalized difference vegetation index, NDVI) and negatively correlated with mean annual temperature. Local floristic and environmental data were subsequently sampled from hundreds of field plots (50 × 10 m), which were selected to be well-distributed across the regions, characteristic of the dominant regional vegetation type, and relatively undisturbed. Field plots were grouped into sites, or sets of four adjacent plots of which one plot was on each combination of two soils (serpentine, nonserpentine) and two topographic aspects (northerly, southerly). Functional traits (height, specific leaf area [SLA], wood density, and others not used here because we lack regional-scale coverage) were sampled from 10 individuals of each woody species. Regional database construction, climatic data, site selection and sampling, trait measurements, phylogeny construction, and earlier results on diversity are fully described elsewhere (28, 31–33).
In addition to reviewing relevant earlier results, the data for woody species are reanalyzed here to obtain parallel results for how taxonomic, functional, and phylogenetic diversity vary with climate at the regional, site, and plot scales. Trait data (height, SLA, wood density) for species found in the regions but not in the local sampling were supplemented using the TRY (34) and BIEN (Botanical Information and Ecology Network) (35) databases. Functional diversity was calculated as functional dispersion, which is the mean distance of species from the centroid of trait values (36). Regions were included if trait data were available for >70% of species, and sites and plots were included if trait data were available for >70% of the woody cover. The resulting sample sizes were 54 regions, 78 sites, and 224 plots. In this subset of regions, the spatiotemporal means of long-term (30 y) annual rainfall and temperature were negatively correlated (−0.60), and both were correlated with the spatial mean of the remotely sensed productivity index NDVI (rainfall, 0.54; temperature, −0.26) measured in August. Regional area was weakly correlated with climatic productivity (rainfall, 0.004; NDVI, 0.015) and its inclusion or exclusion did not qualitatively affect the outcomes of regional climate–diversity analyses; we excluded area and other variables from the models to maintain our focus on comparative climate patterns. Relationships between diversity and rainfall, and diversity and NDVI, were analyzed using separate generalized linear models (JMP v13.0) for each diversity metric at each scale. For the plot–scale analyses, we included site as a random factor and a main effect of soil type and its interaction with rainfall (or NDVI). For the site-scale analyses, we included region as a random factor and did not include soil as each site included both serpentine and nonserpentine plots.
Temporal Variation in Diversity.
The dataset for temporal trends in grassland community change was collected at the Donald and Sylvia McLaughlin University of California Reserve in Lake and Napa Counties, California Reserve (Fig. 1) (38.87°N, 122.43°W). The climate is Mediterranean, with mean temperatures of 7 °C in January and 25 °C in July and mean annual rainfall of 70 cm, falling mainly in November to April. Yearly weather data were recorded at a station within the study site (Western Regional Climate Center, Knoxville Creek Weather Station). Grassland communities consist of native and exotic forbs, most of which are annuals, in a matrix of grasses, most of which are exotic annuals. Native diversity is considerably higher and exotic grass dominance is lower on the serpentine soils.
Eighty sites were selected to include serpentine (n = 38) and nonserpentine (n = 42) soils, well interspersed across the 2,800-acre landscape. Each site consisted of five, 1-m2 plots separated by 10 m. Beginning in 2000, these sites were sampled twice annually in mid-April and late May/early June for species composition and (beginning in 2006) relative cover. Trait data including height, SLA, leaf dry matter content, and foliar C and N were collected from 10 adult individuals per species, separately for each soil. Site selection and sampling, functional trait measurements, phylogeny estimation, and earlier results on the relationships of diversity to soils, fire, grazing, and climate are described fully elsewhere (37–43).
In 2014, a long-term downward trend was detected in rainfall, cloud cover, and humidity during the winter (December to Feburary) period when annual species are present as small seedlings (40). Subsequent observational and experimental work linked this drying trend to changes in taxonomic (40, 41), functional (42), and phylogenetic (43) diversity. These results are reviewed here for comparison to the geographic diversity trends. In addition, the data are reanalyzed here to examine whether the declining trends are weaker on harsh serpentine soils than more fertile nonserpentine soils, as was the case in the geographic data (32). We used linear mixed models that accounted for temporal autocorrelations to test for effects of time, soil type, and their interaction on the three diversity metrics.
Taxonomic Diversity and Climate
Spatial Variation.
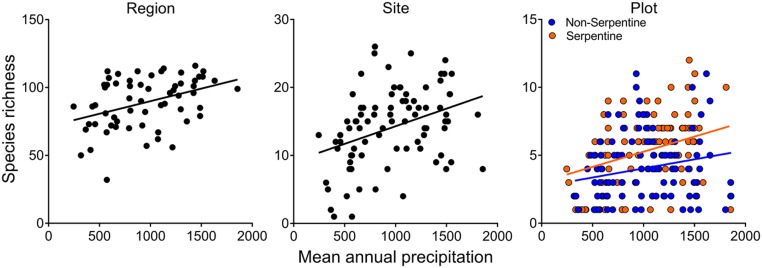
In the woody flora across California, taxonomic diversity increased with increasing mean annual rainfall at the regional, site (four plots), and plot scales, with the steepest slope at the largest scale (Fig. 2). These results were qualitatively unchanged when productivity (NDVI) was used instead of rainfall, and when log-transformed regional area was used as a covariate. This regional-scale relationship is in qualitative agreement with global climate–diversity patterns (1–3), and with the weaker but still significant climate–diversity relationships at local scales found in some although not all other studies (16, 17). Earlier analyses of our dataset, which focused only on the flora of serpentine soils and included both herbs and woody species, similarly found strong regional and weaker local climate–diversity relationships (28). Floristic dissimilarity—that is, β-diversity between communities in plots on serpentine and nonserpentine soils within the same site—also increased with climatic productivity (32), contributing to the stronger climatic gradient in site-scale than plot–scale diversity.
Fig. 2.
Taxonomic diversity (species richness) of woody plants versus mean annual rainfall at the regional scale (P = 0.002), site (P < 0.0001), and plot scale (rainfall P = 0.0001, soil type P = 0.001, interaction P = 0.12). Similar relationships (not shown) were found for taxonomic diversity versus NDVI at the regional (P < 0.0001), site (P = 0.0001), and plot scale (NDVI P < 0.0001, soil type P < 0.0001, interaction P = 0.08).
Woody species may tend to manifest simpler spatial patterns in local-scale diversity than herbs, since herbs respond both to climate itself and to the moderating influence of shading from woody species (44). Moreover, plant diversity in all of California (including the deserts), which is dominated by herbs, shows more complex large-scale geographic patterns than we found here, reflecting the strong influence of recent speciation in response to novel arid climates and rugged topography (45).
Temporal Change.
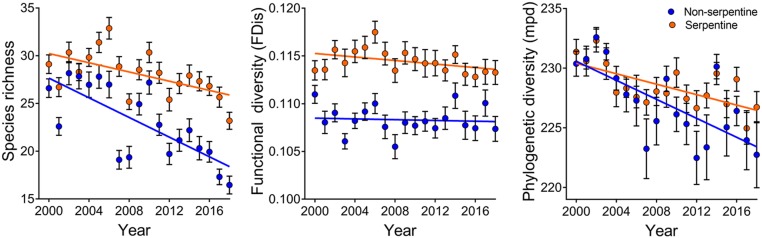
In the herbaceous flora of our northern Californian grassland study site, taxonomic diversity at the 5-m2 scale declined during a 15-y period of generally decreasing winter precipitation, humidity, and cloud cover. The decline mainly affected native annual forbs with a functional trait indicating drought intolerance (high SLA), although other groups of species were similarly affected to lesser extents. Using autoregressive time-series models, the diversity decline was statistically attributable to diminished winter rainfall (40, 41). Experimental evidence also supported the interpretation that drier winters caused higher seedling mortality and smaller size at maturity, thus depleting the seedbank and diminishing the responsiveness of the community to future wet winters (41, 46). Reanalysis of the data showed an interaction between soil and time, such that the decline in diversity was slower on infertile serpentine soils (Fig. 3).
Fig. 3.
Taxonomic, functional, and phylogenetic diversity of herbaceous plants versus time at 80 grassland study sites on contrasting soils (38 serpentine, 42 nonserpentine). Time and time–soil interaction are significant for taxonomic diversity (P < 0.0001 for year and year × soil) and phylogenetic diversity (P < 0.0001 for year, P = 0.035 for year × soil); only time is significant for functional diversity (P = 0.037).
Grassland diversity and composition often fluctuate in response to climatic water availability (e.g., refs. 47 and 48), but these changes are usually attributable to transitions between the dormant and nondormant states rather than to long-term gains or losses of species. Some directional trends in species richness in response to modern climate trends have been observed, including declines in arid climates (49–51) and increases in cold climates (52). In an especially revealing case, recent warming caused alpine plant diversity to increase on mountaintops in cold northern Europe but to decrease on mountaintops in arid southern Europe (53), just as would be predicted based on the global relationships of diversity to temperature and water availability (3).
In experimental studies, decreases in water availability tend to reduce diversity, and increases in water frequently increase diversity (54–57). Experimental warming tends to reduce diversity in climates that are relatively water-limited (58, 59), and watering sometimes offsets this effect of warming, consistent with the key role of water availability (55). Experimental watering effects may be highly dependent on within-season timing (59, 60), and may be complicated by indirect effects, such as the facilitation of N-fixers that raise soil fertility (61). Biomass and diversity often respond in broadly similar ways to water availability, although different functional groups within a community may be primarily responsible for biomass and diversity responses (56, 59).
Functional Diversity and Climate
Spatial Variation.
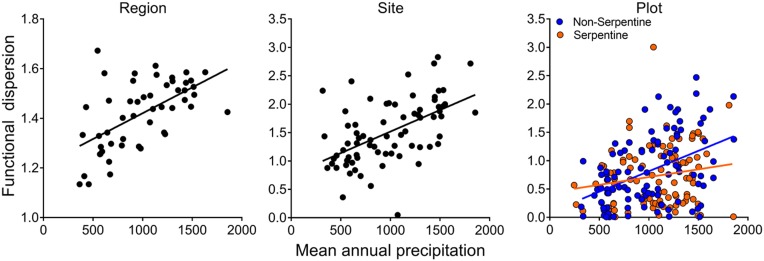
Multivariate functional diversity (measured as functional dispersion) in woody plants across California increased with increasing mean annual rainfall or NDVI at the regional, site, and local scales, with the increase again being strongest at larger scales (Fig. 4). At the plot scale, this pattern was seen in the flora of nonserpentine but not serpentine soils (Fig. 4), consistent with earlier findings for functional trait means (32), and consistent with the low functional diversity found on infertile soils in other Mediterranean-type climates (10, 22). Of the individual functional traits, plant height showed the most consistent pattern of increase in functional diversity with rainfall or NDVI across spatial scales, while SLA and wood density showed this pattern at some but not all scales (SI Appendix, Fig. S1). Mean plant height also increased consistently along the climate gradient (SI Appendix, Fig. S2).
Fig. 4.
Functional diversity (dispersion) of woody plants versus mean annual rainfall at the regional scale (P = 0.0001), site (P < 0.0001), and plot scale (rainfall P = 0.0001, soil type P = 0.001, interaction P = 0.02). Similar relationships (not shown) were found for functional diversity versus NDVI at the regional (P < 0.0001), site (P = 0.0001), and plot scale (NDVI P < 0.0001, soil type P = 0.36, interaction P = 0.20). Sites or plots with only one woody species (4 of 78 sites, 32 of 224 plots) were excluded from these analyses.
The results support the tolerance hypothesis (5, 6, 13–15), under which taxonomic diversity is highest in benign climates because a larger range of functional traits and strategies are compatible with persistence in these climates, while more extreme climates act as abiotic filters that exclude species lacking specialized traits conferring stress tolerance. Plant height is perhaps the trait that most clearly illustrates a mechanism for this hypothesis. Globally, mild climates support trees of a wide range of heights, including the tallest trees, while harsher climates support successively shorter trees; this may be because taller trees have larger maximum vessel diameters that convey higher vulnerability to embolism under water stress (62, 63). Multivariate combinations of traits, as well as individual traits, may become increasingly constrained in more demanding environments (64).
Trait-based tests have provided some evidence for the tolerance hypothesis both at large regional scales (6, 15), and at the scale of local field plots either across very large extents (13) or along more localized climatic gradients (63). Plant height, seed mass, foliar nutrient concentrations, and SLA, which are strongly correlated among species (65), all show some evidence for higher functional diversity in benign climates. Support for the hypothesis is not universal, however (13, 14, 66). Seasonality (66), long-term climatic stability (67), competition (14), and understudied traits or trait combinations may complicate relationships of climate to functional diversity.
Temporal Change.
In our grassland data, the long-term trend of decreased growing-season rainfall drove a decline in multivariate functional diversity (42) paralleling the loss of species richness. Native annual forbs with high SLA, a trait associated with drought sensitivity, were particularly vulnerable. Over time, native annual forb species with SLA values above the median for their local site disappeared from year to year more often than they reappeared, while species with below-median SLA disappeared and reappeared equally often (40). In turn, the loss of high-SLA native forbs drove a decline in multivariate functional diversity that was especially strong among native forbs, and weaker but still significant for the whole community (42). Reanalysis of this data found no interaction between soil and time when considering the whole community (Fig. 3). However, the decline was stronger on nonserpentine soils when considering only native annual forbs, which drove the overall decline but which are relatively sparse on these soils (42).
This grassland study may be unique in known observation of a climate-associated decline in functional diversity over time in a natural community, although many other studies have documented the relevance of functional traits in determining species and community responses to contemporary climatic trends (e.g., refs. 52 and 68). Several converse examples exist in which long-term increases in water availability led to increases in functional diversity (57, 69).
Phylogenetic Diversity and Climate
Spatial Variation.
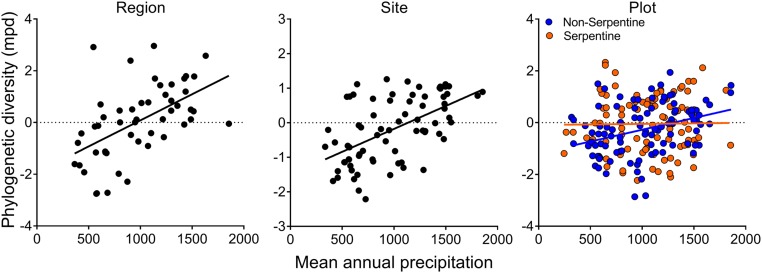
In our geographic dataset, phylogenetic diversity (measured as the mean branch length separating pairs of coexisting species) of the woody flora increased with increasing mean annual rainfall or NDVI at the regional, site, and local scales, with the increase once again being strongest at larger scales (Fig. 5). At the plot scale, this pattern was seen only in the flora of nonserpentine but not serpentine soils, similarly to functional diversity. These results are consistent with earlier published findings from this dataset (31), as well as broadly consistent with other analyses (70). The patterns are also consistent with the biogeographic history of the California Floristic Province, where the flora is interpreted as being a mixture of ancient warm-temperate lineages that have retreated toward wetter macro- and microclimates since the end of the Eocene, and more modern drought-adapted lineages that spread over that time and in some cases diversified rapidly (71, 72). The warm-temperate lineages are dominant in wetter regions and have greater collective phylogenetic diversity, more drought-intolerant functional traits, and considerably stronger responses of species diversity to the climatic gradient than the drought-adapted lineages (31, 33, 72).
Fig. 5.
Phylogenetic diversity (mean phylogenetic distance) of woody plants versus mean annual rainfall at the regional scale (P = 0.0001), site (P < 0.0001), and plot scale (rainfall P = 0.031, soil type P = 0.21, interaction P = 0.29). Similar relationships (not shown) were found for phylogenetic diversity versus NDVI at the regional (P < 0.0002), site (P = 0.0001), and plot scale (NDVI P = 0.81, soil type P = 0.15, interaction P = 0.045); the plot–scale relationship with NDVI was significant for nonserpentine soils but not for serpentine soils.
Global patterns of phylogenetic diversity in higher plants parallel the latitudinal gradient in species richness, supporting the interpretation that most modern lineages arose in tropical-like climates and have tended to conserve their climatic niches (8, 73). This so-called tropical conservatism (or climatic niche conservatism) hypothesis, implying limited rates of transition into cold or arid climates, also appears consistent with the climatic gradients in phylogenetic diversity within many regions [e.g., the Mediterranean Basin (74) and China (75)]. The same hypothesis is also capable of predicting alternative patterns under certain circumstances; for example, where cold or arid conditions have persisted for a long time, where particular lineages have proliferated unusually rapidly in harsh environments, or where geographic barriers have limited the opportunities for preadapted lineages to colonize novel climates, taxonomic, and phylogenetic diversity may be expected to be elevated in cold or arid climates, in contrast to the general global patterns (8, 76, 77).
Climate–richness relationships have sometimes been attributed to the effect of climate on speciation rates, perhaps through climatic productivity, setting a higher carrying capacity for diversity (i.e., the productivity per se or species-energy hypothesis) (9). Patterns of phylogenetic diversity in California make it clear, however, that rates of speciation have been highest in novel arid climates (69, 78) and within arid-adapted lineages (79), as is also true in the Mediterranean Basin (74). Recent speciation in the more arid parts of the Mediterranean climate biomes has apparently weakened the positive relationship of climatic productivity to taxonomic diversity while strengthening its relationship to phylogenetic diversity, which is consistent with climatic niche conservatism but not with the carrying capacity model.
Importantly, while the present analyses focus on explaining climatic diversity gradients within the Californian region, it is clear from phylogenetic and other evidence that contemporary climate cannot explain the elevated plant diversity of Mediterranean climate regions (particularly the Cape Floristic Region and Southwest Australia) compared to other regions (10). Soil infertility, the Miocene onset of frequent fire, and climatic stability during the Pleistocene have all played critical roles (22).
Temporal Change.
In our grassland data, the long-term drying trend was associated with declining phylogenetic diversity that closely paralleled the losses of taxonomic and functional diversity (43). The selective loss of native forb species with high SLA relative to other species in that group, as well as the loss of native forbs relative to other functional groups, contributed to leaving communities with lower phylogenetic diversity both among native forbs and for the community as a whole (43). This result indicates that species disappearing from each local community tended to be more distantly related to others in that community than the species that persisted. Reanalysis of the data showed an interaction between soil and time, such that the decline in phylogenetic diversity was slower on serpentine soils (Fig. 3).
Declining phylogenetic diversity in warm and dry climates that are becoming effectively more arid has been predicted in modeling studies, as the result of the disproportionate vulnerability of old, mesic-adapted elements within regional assemblages (80, 81).
Discussion
Geographic patterns in the diversity of Californian woody species showed striking consistency in their relationships with climate across spatial scales and organizational levels. Taxonomic diversity increased with increasing rainfall, or equivalently with increasing productivity despite decreased temperature, consistent with global climate–richness patterns. Functional diversity also increased with rainfall and productivity, as expected under the tolerance hypothesis, which proposes that more combinations of functional traits can inhabit benign than harsh climates. Phylogenetic diversity likewise increased with productivity as predicted by the climatic niche conservatism hypothesis, under which older mesic-adapted lineages with drought-intolerant functional traits are concentrated in benign climates. Each of these patterns manifested more strongly at the scale of large regions and less strongly within local communities. On an individual basis, the geographic patterns identified here have all been documented numerous times elsewhere.
Collectively these results are consistent with a view of large-scale diversity that emphasizes the greater age and historical stability of mesic climates, the relative rarity of evolutionary transitions in climatic tolerances, and the sharing of climate tolerances by related species (73), as well the stronger filtering effect of harsh than benign climates (6). In contrast, the results lend little support to the “productivity per se” or “species-energy” view of large-scale diversity (5) because this view is agnostic to the functional and phylogenetic structure of diversity gradients. The stronger regional than local effects found here are consistent with the view that regional species pools shape the diversity of local communities (82), but also consistent with the potential roles of environmental heterogeneity and β-diversity (32) and localized disturbances and competition (18) in strengthening regional climate–richness relationships and weakening local ones, respectively. The simplicity of the patterns detected here may have benefitted from the fairly one-dimensional climate gradient, along which rainfall, temperature, and productivity were correlated and the basic pattern of seasonality remained the same. In addition, woody species may show simpler climate–diversity patterns than herbs because of the complicating effects of woody canopies on the species richness, functional traits, and biogeographic affinities of herbs (43, 83).
Temporal patterns of decline in the taxonomic, functional, and phylogenetic diversity of the northern Californian grassland community during a prolonged drying period were consistent with one another and also with the geographic diversity patterns. Given that communities at any given point along the Californian climate gradient consist of mixes of species of different biogeographic affinities and climatic tolerances, it is straightforward to predict that under drying conditions, the most vulnerable species at any location will be those with the most mesic climatic affinities. If these mesic-adapted species are locally extirpated, their loss may drive down both functional diversity by removing particular sets of traits and phylogenetic diversity by removing species from older mesic lineages (80, 81). These expectations are at least partly met in several other studies in semiarid climates (49–51, 56), but the study reviewed here may be unique in supporting all of them. A water-limited rather than temperature-limited climate (53), a prolonged drying trend (25), and the lack of a woody overstory (44, 84, 85) may have contributed to the parallel patterns of climate-driven diversity decline at multiple organizational levels found in this study.
Climate-driven diversity changes are likely to be more complex in cold temperature-limited climates than in water-limited climates, such as the one studied here. While potential plant productivity and the “climatic capacity for species richness” (3) are predicted to increase with rising temperatures, actual changes in diversity will be strongly influenced by dispersal limitation, by potentially intensified competition from species with fast-growing functional traits, and by higher-order interactions, such as herbivory that may moderate plant community changes (86). Thus far, the predicted increases in diversity caused by warming in cold climates appear to have been most clearly realized on alpine mountaintops (52, 53), where a nearby pool of preadapted species is available at lower elevations.
The studies reviewed here originally began as comparative analyses of the diversity and ecological properties of plant communities on serpentine soils, which are chemically harsh and infertile and support generally low-statured plants with slow-growing functional traits, some of which are unique (endemic) to these soils and nearly all of which are natives rather than exotics. Thus the geographically distributed plots and the long-term grassland plots included roughly equal numbers of otherwise comparable locations on serpentine soil and on the nearest nonserpentine soil supporting the typical regional vegetation. As the focus of these studies shifted toward climate, an unexpected finding was that serpentine communities appeared less sensitive to climatic variation in both space and time, likely as the result of their more stress-tolerant functional traits. This conclusion is supported by other studies of floras on harsh soils (84, 87), as well as by water and nutrient manipulations in our grassland study system (88) and by the reanalyses presented here.
Climate-driven changes to biodiversity are immense and complex, and many aspects remain highly unpredictable (89). The studies here nevertheless hint at one possible aspect that is somewhat predictable: The directional loss in water-limited climates of plant community diversity at multiple levels of organization. This generalization may help guide the search for a more realistic understanding of the consequences of diversity loss for ecosystem function (90, 91).
Data Availability Statement.
Data for the temporal analyses are available on Figshare at https://figshare.com/articles/Climate_drives_loss_of_phylogenetic_diversity/9747455. Data for the spatial analyses are available on Figshare at https://figshare.com/articles/Data_from_Climate_and_Plant_Community_Diversity_in_Space_and_Time/11678076/2.
Supplementary Material
Acknowledgments
S.H. thanks her many collaborators on the work reviewed here. Helpful comments on earlier drafts were provided by H. V. Cornell, M. J. Donoghue, and J. Franklin. This work was supported by a National Science Foundation Opportunities for Promoting Understanding through Synthesis award (to S.H.).
Footnotes
Competing interest statement: M.J.S. and F.S.C. are coauthors on a 2018 research article.
Data deposition: Data for for the temporal analyses are available on Figshare at https://figshare.com/articles/Climate_drives_loss_of_phylogenetic_diversity/9747455. Data for the spatial analyses are available on Figshare at https://figshare.com/articles/Data_from_Climate_and_Plant_Community_Diversity_in_Space_and_Time/11678076/2.
See Profile on page 4439.
See online for related content such as Commentaries.
This article contains supporting information online at https://www.pnas.org/lookup/suppl/doi:10.1073/pnas.1921724117/-/DCSupplemental.
References
- 1.Hawkins B. A., et al. , Energy, water, and broad-scale geographic patterns of species richness. Ecology 84, 3105–3117 (2003). [Google Scholar]
- 2.Kreft H., Jetz W., Global patterns and determinants of vascular plant diversity. Proc. Natl. Acad. Sci. U.S.A. 104, 5925–5930 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sommer J. H., et al. , Projected impacts of climate change on regional capacities for global plant species richness. Proc. Biol. Sci. 277, 2271–2280 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin B. C., et al. , Hydrologic refugia, plants, and climate change. Glob. Change Biol. 23, 2941–2961 (2017). [DOI] [PubMed] [Google Scholar]
- 5.Currie D. J., et al. , Predictions and tests of climate-based hypotheses of broad-scale variation in taxonomic richness. Ecol. Lett. 7, 1121–1134 (2004). [Google Scholar]
- 6.Swenson N. G., et al. , The biogeography and filtering of woody plant functional diversity in North and South America. Glob. Ecol. Biogeogr. 21, 798–808 (2012). [Google Scholar]
- 7.Wiens J. J., Donoghue M. J., Historical biogeography, ecology and species richness. Trends Ecol. Evol. (Amst.) 19, 639–644 (2004). [DOI] [PubMed] [Google Scholar]
- 8.Donoghue M. J., Colloquium paper: A phylogenetic perspective on the distribution of plant diversity. Proc. Natl. Acad. Sci. U.S.A. 105 (suppl. 1), 11549–11555 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suding K. N., et al. , Scaling environmental change through the community-level: A trait-based response-and-effect framework for plants. Glob. Change Biol. 14, 1125–1140 (2008). [Google Scholar]
- 10.Rundel P. W., et al. , Mediterranean biomes: Evolution of their vegetation, floras and climate. Annu. Rev. Ecol. Evol. Syst. 47, 383–407 (2016). [Google Scholar]
- 11.Srivastava D. S., Cadotte M. W., MacDonald A. A. M., Marushia R. G., Mirotchnick N., Phylogenetic diversity and the functioning of ecosystems. Ecol. Lett. 15, 637–648 (2012). [DOI] [PubMed] [Google Scholar]
- 12.Rabosky D. L., Hurlbert A. H., Species richness at continental scales is dominated by ecological limits. Am. Nat. 185, 572–583 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Lamanna C. A., et al. , Functional space and the latitudinal species richness gradient. Proc. Natl. Acad. Sci. U.S.A. 111, 3745–13750 (2014).24567399 [Google Scholar]
- 14.Simova I., et al. , Shifts in trait means and variances in North American tree assemblages: Species richness patterns are loosely related to the functional space. Ecography 38, 649–658 (2015). [Google Scholar]
- 15.Echeverria-Londono S., et al. , Plant functional diversity and the biogeography of biomes in North and South America. Front. Ecol. Evol. 6, 219 (2018). [Google Scholar]
- 16.Keil P., Chase J. M., Global patterns and drivers of tree diversity integrated across a continuum of spatial grains. Nat. Ecol. Evol. 3, 390–399 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Field R., et al. , Spatial species-richness gradients across scales: A meta-analysis. J. Biogeogr. 36, 132–147 (2009). [Google Scholar]
- 18.Grace J. B., et al. , Integrative modelling reveals mechanisms linking productivity and plant species richness. Nature 529, 390–393 (2016). [DOI] [PubMed] [Google Scholar]
- 19.Sandel B., et al. , The influence of Late Quaternary climate-change velocity on species endemism. Science 334, 660–664 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Chase J. M., Leibold M. A., Spatial scale dictates the productivity-biodiversity relationship. Nature 416, 427–430 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Kraft N. J. B., et al. , Disentangling the drivers of β diversity along latitudinal and elevational gradients. Science 333, 1755–1758 (2011). [DOI] [PubMed] [Google Scholar]
- 22.Cowling R. M., Rundel P. W., Lamont B. B., Kalin Arroyo M., Arianoutsou M., Plant diversity in Mediterranean-climate regions. Trends Ecol. Evol. (Amst.) 11, 362–366 (1996). [DOI] [PubMed] [Google Scholar]
- 23.McIntyre P. J., et al. , Twentieth-century shifts in forest structure in California: Denser forests, smaller trees, and increased dominance of oaks. Proc. Natl. Acad. Sci. U.S.A. 112, 1458–1463 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cook B. I., Ault T. R., Smerdon J. E., Unprecedented 21st century drought risk in the American Southwest and Central Plains. Sci. Adv. 1, e1400082 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.MacDonald G. M., et al. , Prolonged California aridity linked to climate warming and Pacific sea surface temperature. Sci. Rep. 6, 33325 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang S. Y. S., Yoon J. H., Becker E., Gilles R., California from drought to deluge. Nat. Clim. Chang. 7, 465–468 (2017). [Google Scholar]
- 27.Swain D. L., Langenbrunner B., Neelin J. D., Hall A., Increasing precipitation volatility in twenty-first-century California. Nat. Clim. Chang. 8, 427–433 (2018). [Google Scholar]
- 28.Harrison S., Safford H. D., Grace J. B., Viers J. H., Davies K. F., Regional and local species richness in an insular environment: Serpentine plants in California. Ecol. Monogr. 76, 41–56 (2006). [Google Scholar]
- 29.Safford H. D., Viers J. H., Harrison S., Serpentine endemism in the Calfornia flora: A database of serpentine affinity. Madrono 52, 222–257 (2005). [Google Scholar]
- 30.Burge D. O., et al. , Plant diversity and endemism in the California Floristic Province. Madrono 63, 3–206 (2016). [Google Scholar]
- 31.Anacker B. L., Harrison S. P., Historical and ecological controls on phylogenetic diversity in Californian plant communities. Am. Nat. 180, 257–269 (2012). [DOI] [PubMed] [Google Scholar]
- 32.Fernandez-Going B. M., Harrison S. P., Anacker B. L., Safford H. D., Climate interacts with soil to produce beta diversity in Californian plant communities. Ecology 94, 2007–2018 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Harrison S., Grace J. B., Biogeographic affinity helps explain productivity-richness relationships at regional and local scales. Am. Nat. 170 (suppl. 2), S5–S15 (2007). [DOI] [PubMed] [Google Scholar]
- 34.Kattge J., et al. , TRY—A global database of plant traits. Glob. Change Biol. 17, 2905–2935 (2011). [Google Scholar]
- 35.Maitner B. S., et al. , The bien r package: A tool to access the Botanical Information and Ecology Network (BIEN) database. Methods Ecol. Evol. 9, 373–379 (2018). [Google Scholar]
- 36.Laliberté E., Legendre P., A distance-based framework for measuring functional diversity from multiple traits. Ecology 91, 299–305 (2010). [DOI] [PubMed] [Google Scholar]
- 37.Harrison S., Inouye B. D., Safford H. D., Ecological heterogeneity in the effects of grazing and fire on grassland diversity. Conserv. Biol. 17, 837–845 (2003). [Google Scholar]
- 38.Elmendorf S. C., Harrison S. P., Temporal variability and nestedness in California grassland species composition. Ecology 90, 1492–1497 (2009). [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Going B. M., Anacker B. L., Harrison S. P., Temporal variability in California grasslands: Soil type and species functional traits mediate response to precipitation. Ecology 93, 2104–2114 (2012). [DOI] [PubMed] [Google Scholar]
- 40.Harrison S. P., Gornish E. S., Copeland S., Climate-driven diversity loss in a grassland community. Proc. Natl. Acad. Sci. U.S.A. 112, 8672–8677 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Harrison S. P., LaForgia M. L., Latimer A. M., Climate-driven diversity change in annual grasslands: Drought plus deluge does not equal normal. Glob. Change Biol. 24, 1782–1792 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Miller J. E. D., Li D., LaForgia M. L., Harrison S., Functional diversity is a passenger but not driver of drought-related plant diversity losses in annual grasslands. J. Ecol. 107, 2033–2039 (2019). [Google Scholar]
- 43.Li D., Miller J. E. D., Harrison S., Climate drives loss of phylogenetic diversity in a grassland community. Proc. Natl. Acad. Sci. U.S.A. 116, 19989–19994 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Oberle B., Grace J. B., Chase J. M., Beneath the veil: Plant growth form influences the strength of species richness-productivity relationships in forests. Glob. Ecol. Biogeogr. 18, 416–425 (2009). [Google Scholar]
- 45.Baldwin B. G., et al. , Species richness and endemism in the native flora of California. Am. J. Bot. 104, 487–501 (2017). [DOI] [PubMed] [Google Scholar]
- 46.Harrison S., LaForgia M., Seedling traits predict drought-induced mortality linked to diversity loss. Proc. Natl. Acad. Sci. U.S.A. 116, 5576–5581 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cherwin K., Knapp A., Unexpected patterns of sensitivity to drought in three semi-arid grasslands. Oecologia 169, 845–852 (2012). [DOI] [PubMed] [Google Scholar]
- 48.Cleland E. E., et al. , Sensitivity of grassland plant community composition to spatial vs. temporal variation in precipitation. Ecology 94, 1687–1696 (2013). [DOI] [PubMed] [Google Scholar]
- 49.Damschen E. I., Harrison S., Grace J. B., Climate change effects on an endemic-rich edaphic flora: Resurveying Robert H. Whittaker’s Siskiyou sites (Oregon, USA). Ecology 91, 3609–3619 (2010). [DOI] [PubMed] [Google Scholar]
- 50.Harrison S., Damschen E. I., Grace J. B., Ecological contingency in the effects of climate change on forest herbs. Proc. Natl. Acad. Sci. U.S.A. 107, 19362–19367 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Slingsby J. A., et al. , Intensifying postfire weather and biological invasion drive species loss in a Mediterranean-type biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A. 114, 4697–4702 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Steinbauer M. J., et al. , Accelerated increase in plant species richness on mountain summits is linked to warming. Nature 556, 231–234 (2018). [DOI] [PubMed] [Google Scholar]
- 53.Pauli H., et al. , Recent plant diversity changes on Europe’s mountain summits. Science 336, 353–355 (2012). [DOI] [PubMed] [Google Scholar]
- 54.Gornish E. S., Tylianakis J. M., Community shifts under climate change: Mechanisms at multiple scales. Am. J. Bot. 100, 1422–1434 (2013). [DOI] [PubMed] [Google Scholar]
- 55.Cowles J., Boldgiv B., Liancourt P., Petraitis P. S., Casper B. B., Effects of increased temperature on plant communities depend on landscape location and precipitation. Ecol. Evol. 8, 5267–5278 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.DeMalach N., Zaady E., Kadmon R., Contrasting effects of water and nutrient additions on grassland communities: A global meta-analysis. Glob. Ecol. Biogeogr. 26, 983–992 (2017). [Google Scholar]
- 57.Raymundo D., et al. , Shifting species and functional diversity due to abrupt changes in water availability in tropical dry forests. J. Ecol. 107, 253–264 (2018). [Google Scholar]
- 58.Hoeppner S. S., Dukes J. S., Interactive responses of old-field plant growth and composition to warming and precipitation. Glob. Change Biol. 18, 1754–1768 (2012). [Google Scholar]
- 59.Pfeifer-Meister L., et al. , Climate change alters plant biogeography in Mediterranean prairies along the West Coast, USA. Glob. Change Biol. 22, 845–855 (2016). [DOI] [PubMed] [Google Scholar]
- 60.Jones S. K., Collins S. L., Blair J. M., Smith M. D., Knapp A. K., Altered rainfall patterns increase forb abundance and richness in native tallgrass prairie. Sci. Rep. 6, 20120 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Suttle K. B., Thomsen M. A., Power M. E., Species interactions reverse grassland responses to changing climate. Science 315, 640–642 (2007). [DOI] [PubMed] [Google Scholar]
- 62.Olson M. E., et al. , Plant height and hydraulic vulnerability to drought and cold. Proc. Natl. Acad. Sci. U.S.A. 115, 7551–7556 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fajardo A., McIntire E. J. B., Olson M. E., When short stature is an asset in trees. Trends Ecol. Evol. (Amst.) 34, 193–199 (2019). [DOI] [PubMed] [Google Scholar]
- 64.Dwyer J. M., Laughlin D. C., Constraints on trait combinations explain climatic drivers of biodiversity: The importance of trait covariance in community assembly. Ecol. Lett. 20, 872–882 (2017). [DOI] [PubMed] [Google Scholar]
- 65.Díaz S., et al. , The global spectrum of plant form and function. Nature 529, 167–171 (2016). [DOI] [PubMed] [Google Scholar]
- 66.Sakschewski B., et al. , Leaf and stem economics spectra drive diversity of functional plant traits in a dynamic global vegetation model. Glob. Change Biol. 21, 2711–2725 (2015). [DOI] [PubMed] [Google Scholar]
- 67.Ordoñez A., Svenning J. C., Consistent role of Quaternary climate change in shaping current plant functional diversity patterns across European plant orders. Sci. Rep. 7, 42988 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Soudzilovskaia N. A., et al. , Functional traits predict relationship between plant abundance dynamic and long-term climate warming. Proc. Natl. Acad. Sci. U.S.A. 110, 18180–18184 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Li D., Waller D., Fire exclusion and climate change interact to affect long-term changes in the functional composition of plant communities. Divers. Distrib. 23, 496–506 (2017). [Google Scholar]
- 70.Thornhill A. H., et al. , Spatial phylogenetics of the native California flora. BMC Biol. 15, 96 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raven P., Axelrod D., Origin and Relationships of the California Flora (University of California Press, Berkeley, 1978). [Google Scholar]
- 72.Ackerly D. D., Evolution, origin and age of lineages in the Californian and Mediterranean floras. J. Biogeogr. 36, 1221–1233 (2009). [Google Scholar]
- 73.Kerkhoff A. J., Moriarty P. E., Weiser M. D., The latitudinal species richness gradient in New World woody angiosperms is consistent with the tropical conservatism hypothesis. Proc. Natl. Acad. Sci. U.S.A. 111, 8125–8130 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Molina-Venegas R., Aparicio A., Lavergne S., Arroyo J., The building of a biodiversity hotspot across a land-bridge in the Mediterranean. Proc. Royal Soc. B Biol. Sci. 282, 20151116 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lu L. M., et al. , Evolutionary history of the angiosperm flora of China. Nature 554, 234–238 (2018). [DOI] [PubMed] [Google Scholar]
- 76.Wiens J. J., Graham C. H., Niche conservatism: Integrating evolution, ecology and conservation biology. Annu. Rev. Ecol. Evol. Syst. 36, 519–539 (2005). [Google Scholar]
- 77.Kozak K. H., Wiens J. J., Phylogeny, ecology, and the origins of climate–richness relationships. Ecology 93, S167–S181 (2012). [Google Scholar]
- 78.Kraft N. J. B., Baldwin B. G., Ackerly D. D., Range size, taxon age and hotspots of neoendemism in the California flora. Divers. Distrib. 16, 403–413 (2010). [Google Scholar]
- 79.Lancaster L. T., Kay K. M., Origin and diversification of the California flora: Re-examining classic hypotheses with molecular phylogenies. Evolution 67, 1041–1054 (2013). [DOI] [PubMed] [Google Scholar]
- 80.Thuiller W., et al. , Consequences of climate change on the tree of life in Europe. Nature 470, 531–534 (2011). [DOI] [PubMed] [Google Scholar]
- 81.González-Orozco C. E., et al. , Phylogenetic approaches reveal biodiversity threats under climate change. Nat. Clim. Chang. 6, 1110 (2016). [Google Scholar]
- 82.Cornell H. V., Harrison S., What are species pools and when are they important? Annu. Rev. Ecol. Evol. Syst. 45, 45–67 (2014). [Google Scholar]
- 83.Valiente-Banuet A., Rumebe A. V., Verdú M., Callaway R. M., Modern Quaternary plant lineages promote diversity through facilitation of ancient tertiary lineages. Proc. Natl. Acad. Sci. U.S.A. 103, 16812–16817 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Frenne P., et al. , Microclimate moderates plant responses to macroclimate warming. Proc. Natl. Acad. Sci. U.S.A. 110, 18561–18565 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Harrison S., Damschen E., Fernandez-Going B., Eskelinen A., Copeland S., Plant communities on infertile soils are less sensitive to climate change. Ann. Bot. 116, 1017–1022 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaarlejärvi E., Eskelinen A., Olofsson J., Herbivores rescue diversity in warming tundra by modulating trait-dependent species losses and gains. Nat. Commun. 8, 419 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Damschen E. I., Harrison S., Ackerly D. D., Fernandez-Going B. M., Anacker B. L., Endemic plant communities on special soils: Early victims or hardy survivors of climate change? J. Ecol. 100, 1122–1130 (2012). [Google Scholar]
- 88.Eskelinen A., Harrison S. P., Resource colimitation governs plant community responses to altered precipitation. Proc. Natl. Acad. Sci. U.S.A. 112, 13009–13014 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Bellard C., Bertelsmeier C., Leadley P., Thuiller W., Courchamp F., Impacts of climate change on the future of biodiversity. Ecol. Lett. 15, 365–377 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wardle D. A., Bardgett R. D., Callaway R. M., Van der Putten W. H., Terrestrial ecosystem responses to species gains and losses. Science 332, 1273–1277 (2011). [DOI] [PubMed] [Google Scholar]
- 91.Cavender-Bares J., Ackerly D. D., Hobbie S. E., Townsend P. A., Evolutionary legacy effects on ecosystems: Biogeographic origins, plant traits, and implications for management in the era of global change. Annu. Rev. Ecol. Evol. Syst. 47, 433–462 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data for the temporal analyses are available on Figshare at https://figshare.com/articles/Climate_drives_loss_of_phylogenetic_diversity/9747455. Data for the spatial analyses are available on Figshare at https://figshare.com/articles/Data_from_Climate_and_Plant_Community_Diversity_in_Space_and_Time/11678076/2.