Abstract
The packaging of DNA in nucleosomes presents a barrier for biological transactions including replication, transcription and repair. However, despite years of research, how the DNA is freed from the histone proteins and thereby allows the molecular machines to access the DNA remains poorly understood. We are interested in global genomic nucleotide excision repair (GG-NER). It is established that the histones are obstacles to this process, and DNA lesions are repaired less efficiently in nucleosomes than in free DNA. In the present study, we utilized molecular dynamics simulations to elucidate the nature of the distortions and dynamics imposed in the nucleosome by a set of three topologically different lesions that vary in GG-NER efficiencies in free DNA and in nucleosomes [Shafirovich, Geacintov, et. al, 2019]. Two of these are bulky lesions derived from metabolic activation of the environmental carcinogen benzo[a]pyrene, the 10R (+)-cis-anti-B[a]P-N2-dG and the stereoisomeric 10S (+)-trans-anti-B[a]P-N2-dG, which respectively adopt base-displaced/intercalated and minor groove-aligned conformations in DNA. The third is a non-bulky GG-NER-susceptible lesion, the 5’R-8-cyclo-2’-deoxyguanosine cross-link, produced by reactive oxygen and nitrogen species; cyclopurine lesions are highly mutagenic. These adducts are placed near the dyad axis, and rotationally with the lesion-containing strand facing towards or away from the histones. While each lesion has distinct conformational characteristics that are retained in the nucleosome, a spectrum of structural and dynamic disturbances, from slight to substantial, are displayed that depend on the lesion’s topology and position in the nucleosome. We hypothesize that these intrinsic structural and dynamic distinctions provide different signals to initiate the cascade of chromatin-opening processes, including acetylation and other post translational modifications, remodeling by ATP-dependent complexes and spontaneous unwrapping that regulate the rate of access to the lesion; this may translate ultimately into varying GG-NER efficiencies, including repair resistance when signals for access are too weak.
Keywords: nucleosome, rotational and translational setting, nucleotide excision repair, DNA damage, 5’R-8-cyclo-2’-deoxyguanosine cross-link, benzo[a]pyrene diol epoxide-derived DNA adducts, rotational setting, molecular dynamics
Graphic Abstract
Introduction
Nucleosomal histones hinder the access of proteins to DNA, which slows or suppresses biological transactions such as replication, transcription and repair. This “access” problem has been of profound interest for many years and remains a challenging and significant topic of research that is still poorly understood. The focus of our interest is global genomic nucleotide excision repair (GG-NER), a mechanism that excises bulky DNA lesions as well as some small lesions. It has long been known that lesions are repaired much less efficiently by GG-NER in nucleosomes than in free DNA [1–11]. It is also well established that histone post translational modifications (PTMs), including acetylation [12, 13], as well as ATP-dependent remodeling complexes, and spontaneous unwrapping [14–17], play important roles in facilitating the access of DNA lesions to GG-NER factors, and other proteins [4, 12, 13, 18–29].
Previous molecular dynamics (MD) simulations [30–33] and experimental studies [34, 35] have shown that the acetylation of the histone tails releases them from the DNA surface and results in the opening of chromatin. However, how DNA damage in nucleosomes is first sensed to initiate its removal still remains unknown. A very limited number of crystal structures of lesion-containing nucleosomes have shed some light on this issue. Of particular interest are two crystal structures of nucleosomes containing CPD and 6–4 TT lesions [36, 37]; these highlighted the distinctions between these two structurally different UV-photo products, whose excision efficiencies in GG-NER differ significantly, with 6–4 TT excised much more efficiently than CPD [38–44]. Most recently, it has been shown that the 6–4 TT lesion can change the translational register of the nucleosome to enable its access for binding with the UV-DDB protein that recognizes photolesions to stimulate the subsequent GG-NER steps [45]. Overall, however, relatively little is known about how lesions with different chemical structures and different GG-NER efficiencies in free DNA are accommodated in the nucleosome.
The nature of the distortions imposed upon the nucleosome by a set of three topologically different lesions that vary in GG-NER efficiencies in free DNA and in nucleosomes [46] is the focus of the present study. Of particular interest is how the different lesions impact histone-DNA interactions as the lesion-containing strand faces towards (IN rotational setting) or away from the center of the nucleosome. We utilized molecular dynamics (MD) simulations to gain insights into the structural and dynamic features of the three different lesions that are positioned at different, but adjacent translational settings facing either toward the center of the histone octamer core (IN setting), or away in a more solvent-exposed site (OUT setting), and hence are in markedly different histone environments. In the OUT case, the translational position is half a helical turn further away from the dyad axis than in the IN setting (Figures 1A and S1).
Figure 1. Structure of unmodified nucleosome, and the chemical and solution structures of the lesions.
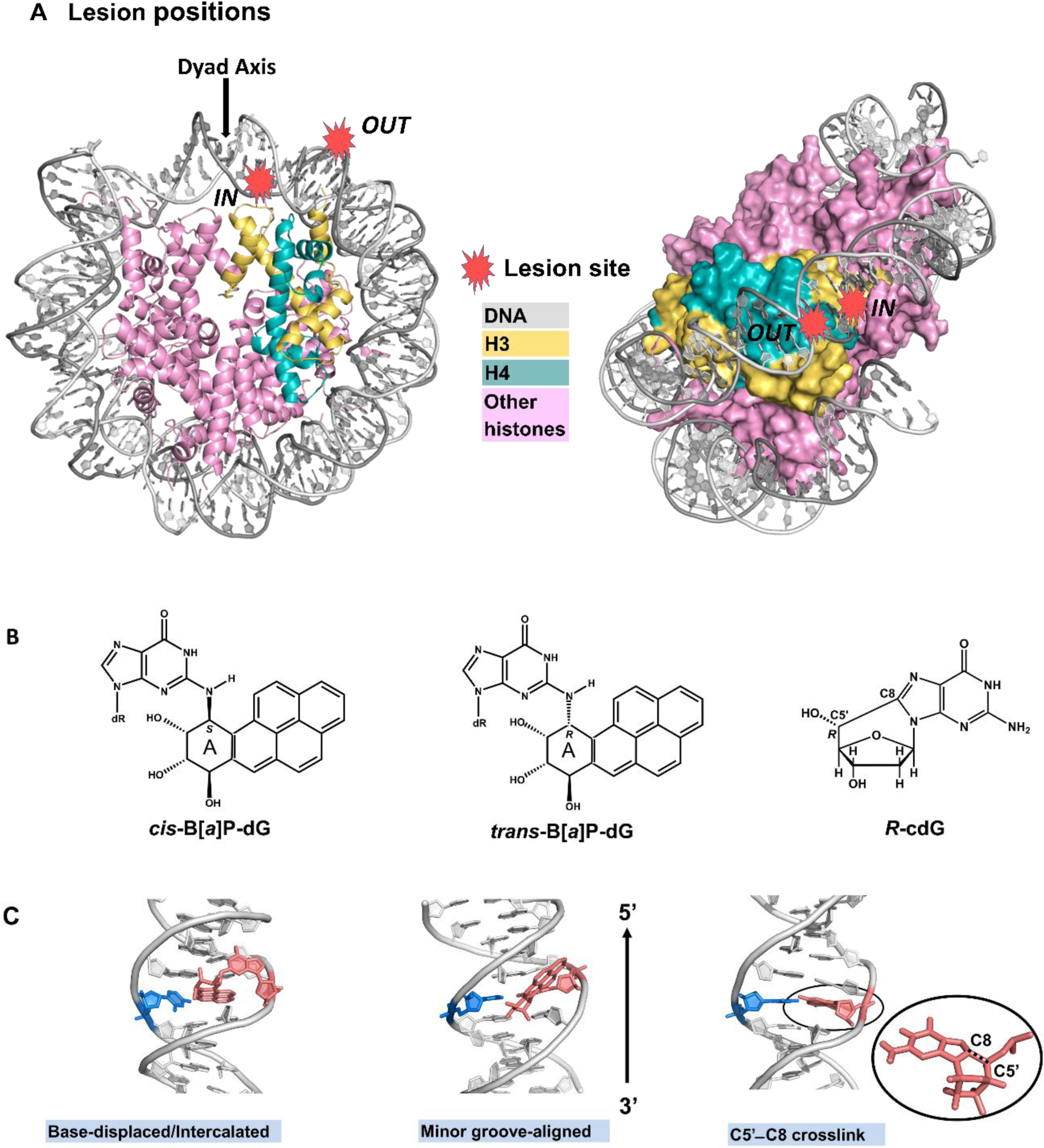
(A) Unmodified nucleosome modeled based on PDB [58] ID 1KX5 [59], see Methods. Lesion positions are designated as red stars. The IN position has the lesion two base pair steps from the dyad axis (at SHL = −0.25); the lesion-containing DNA strand facing histones and its partner faces the solvent. In the OUT position, the lesion is seven base pair steps from the dyad axis (at SHL = − 0.75); the lesion-containing DNA strand faces the solvent while its partner faces histones. Note that the lesion-containing DNA base pair confronts a different histone landscape in the IN and OUT positions. The left panel shows the view down the superhelix axis while the right panel shows a rotated view that reveals the two gyres with histones rendered in surface. Histones H3 and H4 are from Chains A and B respectively in PDB ID: 1KX5. A brief review of nucleosome structure is given by Cutter and Hayes [70]. (B) Chemical structures of lesions investigated. The benzylic ring of the B[a]P ring system is designated as “A”, and “dR” is deoxyribose. (C) Conformations of the lesion-containing DNAs [49, 53, 57]. Views are looking into the DNA minor groove. Color codes: DNA, grey, except damaged dG or its corresponding unmodified nucleoside, salmon, and its partner dC, marine; histone H3, yellow and H4, teal. Hydrogen atoms are omitted for clarity.
Two of the lesions studied are derived from the metabolic activation of the environmental carcinogen benzo[a]pyrene [47, 48]; these two lesions, the 10R (+)-cis-anti-B[a]P-N2-dG (cis-B[a]P-dG) and the stereoisomeric 10S (+)-trans-anti-B[a]P-N2-dG (trans-B[a]P-dG) (Figure 1B), have very different conformations in DNA (Figure 1C). The cis-B[a]P-dG stereoisomer assumes a base-displaced/intercalated conformation with ruptured Watson-Crick base pairs where the B[a]P ring system is attached to dG [49, 50]. The adducted dG is displaced into the minor groove, while its partner dC is displaced into the major groove; the polycyclic aromatic ring system of the benzo[a]pyrenyl moiety is intercalated into the DNA helix from the minor groove; this causes minor groove enlargement and DNA duplex untwisting on the 5’-side of the damaged nucleotide, with compensatory overtwisting on the 3’-side [51, 52]. By contrast, the trans-B[a]P-dG stereoisomer has the benzo[a]pyrenyl ring system positioned in an enlarged minor groove, and all Watson-Crick base pairs remain intact [50, 53]. Notably, the GG-NER excision efficiencies of cis-B[a]P is ~ two-fold greater than that of the stereoisomeric trans-adduct in free and in nucleosomal DNA, when placed at the same position in the nucleosome; however, the excision efficiencies are uniformly reduced in the nucleosome relative to the free solution environment [46]. The third DNA lesion, 5’R-8-cyclo-2’-deoxyguanosine (R-cdG), is a representative example of a non-bulky DNA lesion that is nonetheless a substrate of GG-NER. R-cdG is one of four cyclopurines (R/S-cdG, and R/S-cdA) that are produced by reactive oxygen and nitrogen species, and are known to cause mutations [54]; the cdG and cdA lesions contain a covalent cross-link between the C8 position of the purine and the 5’-deoxyribose group of the same nucleotide [55, 56] (Figure 1, B and C). As a result of this cross-link, the sugar pucker is constrained to an abnormal O4’-exo conformation and the C4’-C5’ backbone torsion angle γ also adopts an abnormal conformation. Furthermore, the base pair step between the cross-link-containing base pair and its 5’-neighboring base pair is overtwisted by ~ 17°; however, Watson-Crick pairing is maintained, although the base pairs are rotated by the cross-link, so that the R-cdG is exposed on the minor groove side, causing the groove to widen; concomitantly, the dC is more exposed on the major groove side. As a result, stacking with flanking base pairs is diminished due to the local overtwisting [57].
In the present work, we determined how the nucleosomal environments impact the conformations of these three different lesions, and how, in turn, these lesions affect the structure of the nucleosome, including interactions of the histones with the damaged base pair and the nearby DNA duplex. We found that each lesion has a different structural and dynamic impact on the nucleosome, which is strongly affected by its rotational setting, i.e. whether the lesion-containing strand faces the histone core (IN) or away (OUT) from the core (Figure 1A).
Methods
The rationale for design of the systems for the MD simulations is given in Supporting Information. The systems were designed to determine the intrinsic structural distortions/dynamics that our lesions impose on the nucleosome core structure when they are in different positions.
We have carried out ~ 2.0 μs MD simulations for the three lesion-containing systems: cis-B[a]P-dG, trans-B[a]P-dG and R-cdG, placed at the IN (superhelical location (SHL) = − 0.25) and the OUT (SHL = − 0.75) positions in an unmodified nucleosome core particle (PDB [58] ID: 1KX5 [59]), which is to our knowledge the highest resolution nucleosome core particle crystal structure available and hence most suitable for our modeling efforts (Figure 1A). The local DNA sequence contexts and numbering schemes are shown in Figure S1. We investigated a nucleosome model with N-terminal tails truncated according to trypsin digestion experiments [60], since MD simulations [30, 31] show that these tails in their unacetylated states condense on the DNA, including the lesions, and obscure the intrinsic impact of the lesions on nucleosome core structures. Furthermore, this condensation occurs in the simulated isolated nucleosome core particles because there are no other proteins or nucleosomes present in the system that would in vivo engage the tails. Amino acid sequences that remain in the truncated tails are shown in Fu et al. [61]. Unmodified nucleosomes were studied as a comparison to the lesion-containing ones.
The AMBER16 simulation package [62] with force field ff14SB [63] and previously published additional parameters for the lesions [49, 50, 53, 57] was utilized for carrying out the MD simulations. Table S1 shows the box sizes, number of water molecules, and the duration of the MD simulations. The CPPTRAJ module of the AMBER16 package [62] was used for structural analyses. The best representative structures were obtained using the cluster analysis in the CPPTRAJ module, with the average linkage hierarchical agglomerative method [64]. VMD [65] was used for molecular modeling and trajectory viewing. PyMOL (The PyMOL Molecular Graphic System, version 1.3x, Schrödinger, LLC) was used for visualizing structures, rendering images, and creating movies. Full details of the force field, parameters, modeling, MD protocols and further analyses are provided in the Supporting Information.
Results
cis-B[a]P-dG: partner dC is more dynamic when the lesion-modified strand faces IN.
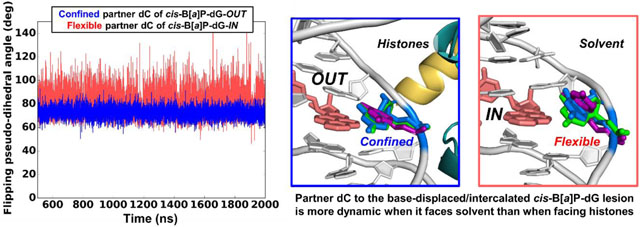
While the overall solution conformational features of the cis-B[a]P-dG adduct are maintained with the flipped-out partner dC residing in the major groove, the rotational setting of the lesion in the nucleosome environment has a very significant impact on the lesion dynamics and its interactions with histones. At the IN position, the damaged and displaced dG faces the histones, while the displaced partner dC faces the solvent (Figure 2A); the dC is therefore unrestrained and conformationally flexible (Figures 2A and 3A). By contrast, when the cis-B[a]P-dG-containing strand is at the OUT position, the dC faces towards the histone octamer, where it is constrained (Figures 2B and 3B). Furthermore, one of the backbone phosphate oxygen atoms is rotated toward the minor groove and forms a new hydrogen bond with Arg 45 of histone H4 (Figure 2A and Table S2). Hence, the dC nucleoside in this case is anchored, less dynamic, and less extruded into the major groove than when it is positioned at the OUT rotational setting, where it faces the solvent (Figures 3B, and S2). On the other hand, the damaged dG is inherently restrained due to its linkage to the intercalated benzo[a]pyrenyl ring system, whether it faces the histone or the solvent environment (Figure 2, A and B).
Figure 2. Best representative structures of lesion-containing nucleosomes and dynamics of partner dC.
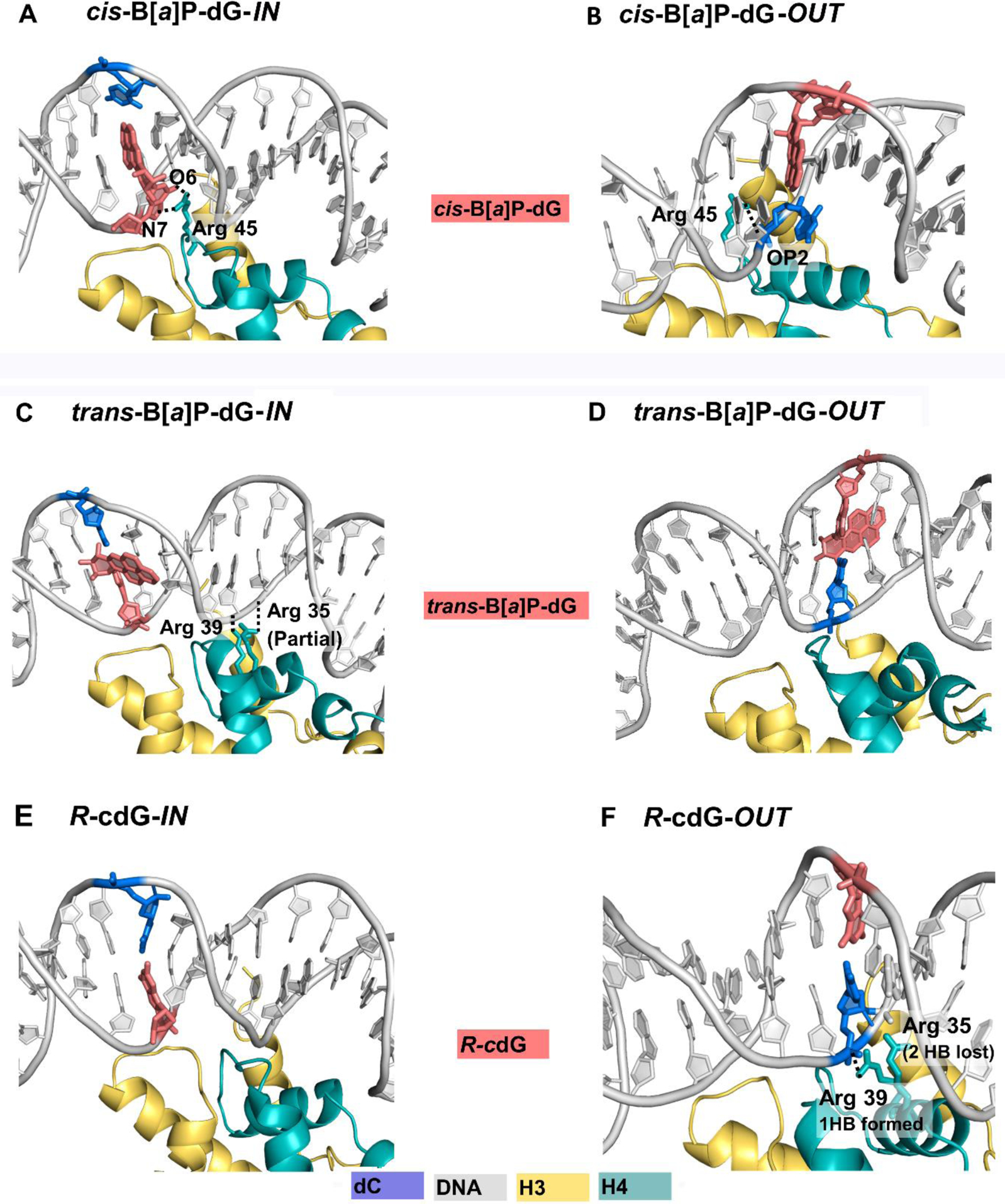
Base-displaced/Intercalated cis-B[a]P-dG with Watson-Crick pairing ruptured (A and B); (A): IN: the partner dC faces solvent, while the damaged dG in the minor groove faces the histones, forming two new hydrogen bonds with nearby Arg 45 of histone H4. (B) OUT: the confined partner dC faces histones with its backbone phosphate oxygens rotated toward the minor groove, and OP2, that is closer to H4 Arg 45, forms a new hydrogen bond with it. The adducted dG, now faces the solvent, and is inherently restrained due to its linkage to the benzo[a]pyrenyl ring system that is intercalated between adjacent base pairs. Minor groove-aligned trans-B[a]P-dG with Watson-Crick pairing maintained and minor groove enlarged (C and D); (C): IN: the histone-facing B[a]P rings that enlarge the minor groove cause shifting of the partner strand, which forms one partial and one full new hydrogen bond with Arg 35 and Arg 39 of the H4 histone. (D): OUT: the B[a]P rings face the solvent and there is no change in hydrogen bond contacts with histones. In both IN and OUT cases, the B[a]P rings are shielded on one face by the minor groove wall. Small and constrained cross-link R-cdG with Watson-Crick pairing maintained (E and F) (E): IN: hydrogen bond interactions are unchanged (F):OUT: two hydrogen bonds are lost between the DNA backbone of the partner strand and the nearby Arg 35 of histone H4, while one is gained between Arg 39 and this partner backbone, yielding a net loss of one hydrogen bond; this is caused by the shifting of the partner dC as it strives to maintain Watson-Crick pairing with R-cdG. Color codes are the same as in Figure 1. Movies S1 and S2 reveal the best representative structures of the cis-B[a]P-dG-OUT and -IN, respectively.
Figure 3. Partner dC flipped into the major groove is more dynamic for cis-B[a]P-dG-IN.
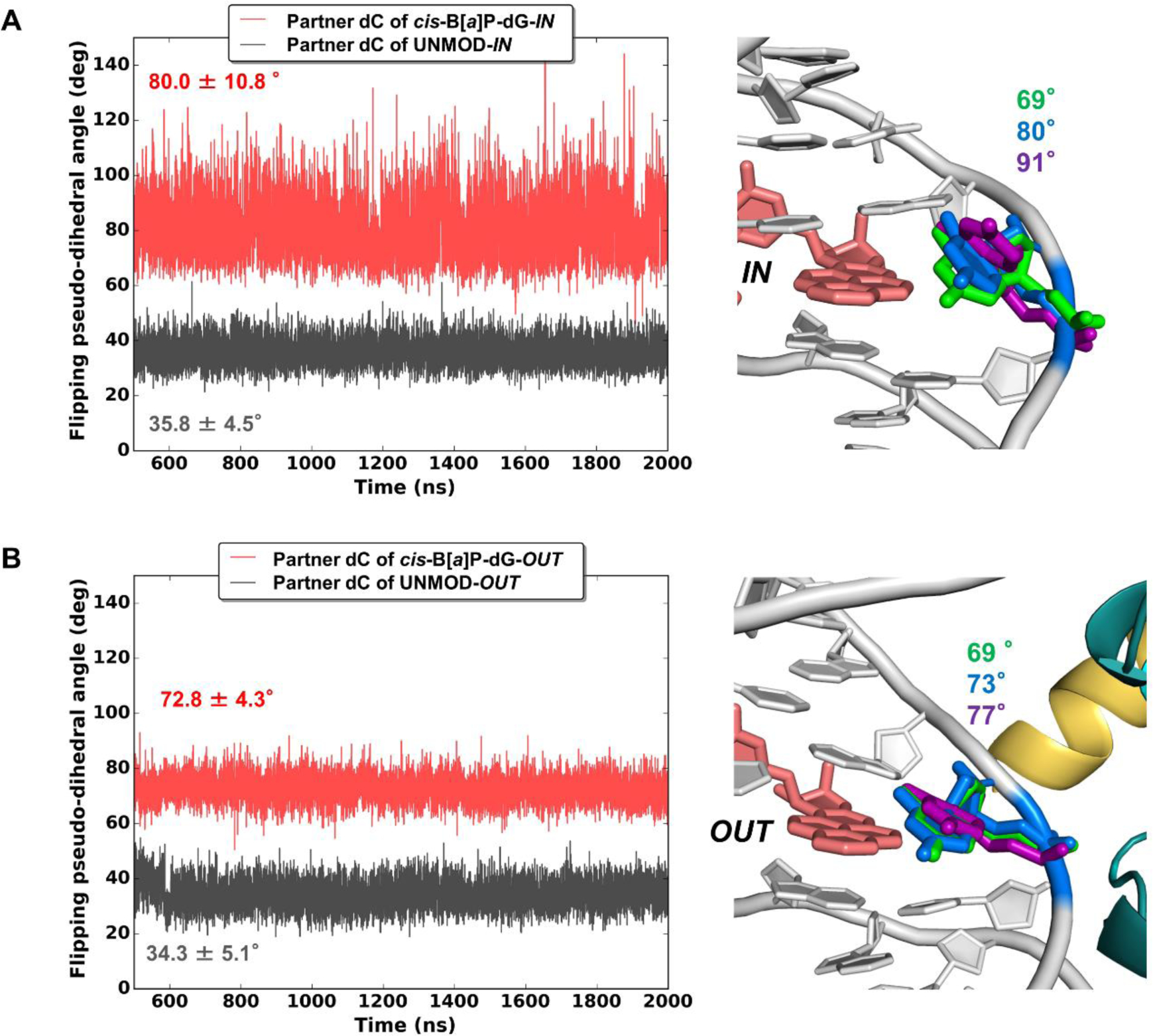
(Left Panels) Time dependence of flipping pseudo-dihedral angles of partner dC for (A) IN-facing lesion, and (B) OUT-facing lesion. Mean values and standard deviations are given. The much greater dynamics is revealed in the larger range and standard deviation for the IN-facing lesion where partner dC faces solvent. With this greater range, the dC samples a larger span in the major groove. This is shown in the right panels with superimposed positions of the dCs at pseudo-dihedral angles of the mean values (blue), and plus/minus their standard deviations (purple and green, respectively). These structures were superimposed by aligning all DNA atoms in the duplex trimer centered at the lesion-containing base pair. In the unmodified cases (left panels, black; structures not shown), the partner dC is stacked in; the flipping pseudo-dihedral angles, ~ 35 °, differ slightly for the IN vs. OUT cases, because they have different SHL positions and local sequence contexts. The definition of the flipping pseudo-dihedral angle is shown in Figure S2, in Supporting Information. The larger the flipping pseudo-dihedral angle, the greater the extrusion into the major groove. Color codes are the same as in Figure 2.
When facing the histones, the damaged dG forms two new hydrogen bonds with Arg 45 positioned in the nearby minor groove (Figure 2A and Table S2). The interactions of the Arg 45 with the damaged dG or partner dC nucleosides cause an enlargement of the minor groove (Figure S3). However, the minor groove is more enlarged and more flexible when the dC faces the solvent (IN) than when the dC is constrained by facing histones (OUT) (Figure S3 and Table 1). Similarly, the overtwisting or undertwisting imposed by the intercalated bulky B[a]P ring system is greater when the conformationally flexible dC faces the solvent (IN) than when the conformationally more restrained modified dG faces solvent (OUT) (Figure S4).
Table 1.
Summary of structural and dynamic features of lesion-containing nucleosomes
|
cis-B[a]P-dG Bulky multiple ring system |
trans-B[a]P-dG Bulky multiple ring system |
R-cdG Small cyclopurine cross-link |
||
|---|---|---|---|---|
| Conformational features maintained in solution and nucleosome |
|
|
|
|
| Properties in nucleosome | Lesion-containing strand IN, facing histones (SHL= −0.25) |
|
|
|
| Lesion-containing strand OUT, facing solvent (SHL=−0.75) |
|
|
|
|
Trans-B[a]P-dG: the minor groove is more enlarged when the lesion-modified strand faces OUT
In the case of the trans-B[a]P-dG adduct positioned in the DNA minor groove, Watson-Crick base pairing at the site of the lesion is also maintained in the nucleosome (Figure 2, C and D). However, here the rotational position plays a more modest role in its impact than in the case of the cis-B[a]P-dG adduct.
The key difference between the IN and OUT positions is in the extent of minor groove enlargement caused by the aromatic benzo[a]pyrenyl ring system. The enlargement of the minor groove is less prominent in the IN than the OUT case and ranges over a smaller number of base pairs because groove enlargement is impeded by the histones (Figures S3 and S5, and Table 1).
For the lesions facing IN, there are changes relative to the unmodified nucleosome simulation in histone-DNA interactions. Specifically, Arg 35 and Arg 39 of histone H4 (Figure 2C) form one partial and one full new hydrogen bond, respectively with nearby backbone phosphate oxygen atoms (Figure 2C, Table S2). These new hydrogen bonds involve residues in the unmodified partner strand on the 3’-side of the adduction site (Table 1). In addition, the trans-B[a]P-dG lesion induces the DNA duplex to untwist locally by ~ 8° at the IN and OUT sites (Figures 2, C and D, and S4, and Table S3).
R-cdG cyclopurine is structurally similar in nucleosomes and in solution and causes loss of a hydrogen bonding interaction with a nearby histone only at the OUT site.
For the R-cdG lesion embedded in the nucleosome (Figure 2, E and F), the intrinsic solution structural properties remain regardless of rotational setting (Table 1). Importantly, the Watson-Crick pairing remains intact, the overtwisting imposed by the dG C8–C5’ cross-link remains ~ 17° (Table S3) as in solution, and there is a modest minor groove widening (Figure S3), in the same range as in the solution structure (Table S5). However, the shifted translational settings of the IN and OUT positions, with differing histone landscapes (Figure 2, E and F), changes hydrogen bonding interactions involving Arg 35 and Arg 39 of histone H4. Specifically, hydrogen bonds with the backbone of the partner dC, and with its 5’-neighbor dA are altered, leading to a net loss of one hydrogen bond for the two arginines (Figure 2F, and Table S2). The loss is caused by the cross-link that rotates the R-cdG towards the minor groove and its partner dC towards the major groove, in order to maintain Watson-Crick pairing. This produces unstacking [57] and shifting of the partner backbone away from the Arg 35, with the loss of two hydrogen bonds. As a result, the backbone shifts towards Arg 39, to form one new hydrogen bond; this yields the net loss of one hydrogen bond (Table S2).
Table 1 summarizes our structural findings.
Discussion
Lesion-induced structural and dynamic disturbances in nucleosomes vary according to lesion topology and nucleosome positioning which may lead to differential lesion access.
Our goal is to provide structural and dynamic understanding of GG-NER studies in nucleosomes for our selected set of lesions, a pair of stereoisomeric bulky B[a]P-dG adducts with different conformations and the non-bulky R-cdG lesion. Their relative GG-NER efficiencies in nucleosomes containing HeLa histones were experimentally characterized [46] at the same translational positions and rotational settings as investigated in the present study. We delineate the intrinsically different structural and dynamic disturbances that our set of topologically distinct lesions impose upon the nucleosome, to signal for recruitment of the machinery that will provide access to the lesions for GG-NER. We hypothesize that the distinct structural and dynamic disturbances contribute to differences in access, by regulating the rates of acetylation [12, 13], other PTMs, remodeling by ATP-dependent complexes, and spontaneous unwrapping [14–17], which ultimately may lead to varying GG-NER efficiencies.
High GG-NER efficiency in the cis-B[a]P-dG adduct facing IN is attributed to flipped out and dynamic partner dC that faces the solvent.
Considering our observed structural impacts of the lesions in relation to their relative GG-NER efficiencies (Table S6), we first consider the finding that the cis-B[a]P-dG facing the histone core (IN) is surprisingly the most efficiently repaired one, as inward-facing lesions are expected to be obstructed by the histones. However, our simulations showed that the IN-facing cis-B[a]P-dG (i.e., the cis-B[a]P-dG-containing strand is facing OUT) has the partner dC in the solvent, where it is dynamic (Figure 3A). It is likely that the dynamic and flipped out partner dC is a powerful signal for invoking access to the lesion. Moreover, the damaged dG nucleoside that faces the histones is likely less responsible for stimulating such access to the lesion. This dG nucleoside is less mobile because it is linked to the intercalated B[a]P ring system, and because it forms two new hydrogen bonds with nearby H4 Arg 45 (Figure 2A and Table S2).
Lower GG-NER in the cis-B[a]P-dG lesion facing OUT is attributed to the histone-facing and obstructed partner dC.
By contrast, when the cis-B[a]P-dG-containing strand faces OUT, the GG-NER efficiency is lower by a factor of ~ 2 than in the IN case; in this position, the lesion-containing strand and the partner dC nucleoside faces the histone core (Table S6), which diminishes the dynamics of this base (Figures 2B and 3B). Moreover, an additional hydrogen bond between the flipped dC and Arg 45 in the minor groove further anchor this residue (Figure 2B and Table S2). These factors are consistent with diminished access by GG-NER proteins to lesions positioned at the OUT-setting. However, the GG-NER efficiency remains moderate (Table S6): the intrinsic conformational properties of the cis-B[a]P-dG lesion are still manifested in the nucleosome environment when facing OUT: the ruptured Watson-Crick pairing and flipped-out, albeit less mobile, partner dC with an accompanying twist distortion (Figure S4) and minor groove opening (Figure S3), are consistent with a moderate access rate. The importance of the flipped-out partner dC described here has been elucidated in our earlier work which showed that this dC residue can entrap a nearby H3 tail [30] that is considered to be a binding site for the XPC lesion-recognition protein [66] and is recognized by Rad4/yeast XPC via a conformational capture mechanism [67].
The trans-B[a]P-dG adducts feature intact Watson-Crick pairing with minor groove enlargement due to the minor groove positioning of the B[a]P rings.
Our results showed that this minor groove enlargement is somewhat smaller when the lesion-containing strand faces IN, since the groove enlargement is constrained by the adjacent histone amino acids (Figure 2C). Furthermore, in the IN case, there is one full and one partial new access-obstructing hydrogen bond between the DNA backbone of the damaged strand and the nearby histone H4 Arg 39 and Arg 35, respectively. By contrast, trans-B[a]P-dG-OUT is more accessible, has a more distorted minor groove, and is not confined like its IN counterpart (Figure 2D), consistent with its somewhat greater GG-NER efficiency (Table S6).
The nucleosome environment can significantly modulate relative GG-NER efficiencies for a given lesion depending on rotational and translational positions.
For the trans-B[a]P-dG adducts, the relative GG-NER efficiencies are in the same range as for the cis-B[a]P-dG-OUT (Table S6). We speculate that the nucleosome-imposed structural features may be similar in their impact on achieving access to the trans-B[a]P-dG as that of the cis-B[a]P-dG-OUT where the critical flipped out partner dC is confined and anchored by the nearby histones, providing a muted access signal in this rotational setting. We note that our lesions were placed in the vicinity of the dyad axis where mobility is limited, and future work is needed to investigate a wider range of positionings. In prior work with a ribonucleotide placed at SHL positions 0, 2.5 and 5.5 and facing toward or away from solvent, we found very different impacts of this small lesion depending on its position [58].
Limited impact of R-cdG on nucleosome structure is independent of rotational and translational setting and hinders its detection for GG-NER.
For the small and constrained cross-link R-cdG, GG-NER in the nucleosome experiment was not observed for this or any of the other cyclopurines [46]. However, they are all repaired by GG-NER in free DNA to various extents [57]. Furthermore, they are repaired to differing degrees in vivo in ERCC1-deficient mice over a long time frame [68]. The intrinsic structural phenomena--overtwisting, intact Watson-Crick pairing and other restraints on sugar pucker and DNA backbone structure imposed by the rigid cross-link--are retained in the nucleosome irrespective of the rotational and translational setting. The rigidity of this lesion with limited impact on the nucleosome structure and dynamics appears to inhibit the mechanisms that are necessary for inducing access to the lesion that is needed for successful GG-NER activity in human cell extracts [46]. However, the cyclopurine lesions do cause the rupture of two hydrogen bonds between the DNA backbone of the partner strand and a nearby arginine when the R-cdG-containing strand is facing OUT, away from the histones; this might help initiate slow chromatin opening processes to allow for repair once the DNA is free.
Conclusion
Each of our lesions has distinct structural and dynamic characteristics that are retained in the nucleosome, but that adapt to their rotational and translational positions which control their interactions with local histone amino acids. We hypothesize that the initial signal for triggering access to the lesion to initiate repair is provided by the disturbances that the lesion causes in the nucleosome structure. Differences in access rates resulting from weakened histone binding or eviction may translate ultimately to varying relative repair efficiencies and even repair resistance in cases where the signal for access is too weak [69].
Supplementary Material
Supplementary Methods: Rationale for design of systems for MD simulations; molecular modeling of lesion-containing nucleosomes; force field, protonation, MD protocols; structural analyses including minor groove widths, root mean square deviation, flipping pseudo-dihedral angles, N1–N3 distance to characterize Watson-Crick hydrogen bonding integrity, hydrogen bond occupancies and Twist angles computation
Supplementary Tables: Table S1. Box sizes, number of waters, and length of MD simulations; Table S2. Number of HBs between 13-mer DNA duplex centered at the lesion with nearby histones; Table S3. Ensemble average helicoidal Twist angles; Table S4. Means and standard deviations of the flipping pseudo-dihedral angle of the partner dC; Table S5. Ensemble average minor groove widths for the11-mer DNA duplex with the R-cdG lesion in the center; Table S6. Relative GG-NER efficiencies in HeLa cell experiments
Supplementary Figures: Figure S1. DNA sequence and numbering scheme; Figure S2. Definition of the flipping pseudo-dihedral angle; Figure S3. Ensemble average values of minor groove widths and their standard deviations; Figure S4. Ensemble average values for the Twist angles; Figure S5. Best representative structures of the trans-B[a]P-dG-OUT adduct superimposed against its unmodified control; Figure S6. Hydrogen bonds gained or lost due to lesion modifications; Figure S7. N1–N3 distances indicating Watson-Crick hydrogen bonding integrity
Supplementary Movies: Movies S1 and S2 reveal the best representative structures of the cis-B[a]P-dG-OUT and -IN, respectively.
Acknowledgments
We gratefully acknowledge resources provided by the Extreme Science and Engineering Discovery Environment (XSEDE), which is supported by National Science Foundation (NSF) Grant MCB060037 to S.B., and the NYU IT High Performance Computing Resources and Services.
Funding Information
This work was supported by National Institute of Environmental Health Sciences Grants R01-ES025987 (to S.B.) and 5R01ES024050 (to N.E.G.), and in party by National Cancer Institute Grant R01-CA75449 (to S.B.) and R01-CA168469 (to N.E.G.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
ABBREVIATIONS:
- B[a]P
benzo[a]pyrene
- GG-NER
global genomic nucleotide excision repair
- NCP
nucleosome core particle
- PTM
post translational modification
- PDB
protein data bank
- MD
molecular dynamics
- dG
deoxyguanosine
- cis-B[a]P-dG
10R (+)-cis-anti-B[a]P-N2-dG adduct
- trans-B[a]P-dG
10R (+)-trans-anti-B[a]P-N2-dG adduct
- R-cdG
5’R-8-cyclo-2’-deoxyguanosine
- SHL
superhelical locations
- HB
hydrogen bond
- UNMOD
unmodified
Footnotes
Conflict of interest
The authors declare no competing financial interest.
Supplementary Data
The Supplementary Data is available free of charge alongside the electronic version of the article in Elsevier, including ScienceDirect: http://www.sciencedirect.com.
References
- [1].Smerdon MJ, Lieberman MW, Nucleosome rearrangement in human chromatin during UV-induced DNA- repair synthesis, Proc. Natl. Acad. Sci. U. S. A, 75 (1978) 4238–4241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Wang ZG, Wu XH, Friedberg EC, Nucleotide excision repair of DNA by human cell extracts is suppressed in reconstituted nucleosomes, J. Biol. Chem, 266 (1991) 22472–22478. [PubMed] [Google Scholar]
- [3].Wellinger RE, Thoma F, Nucleosome structure and positioning modulate nucleotide excision repair in the non-transcribed strand of an active gene, EMBO J, 16 (1997) 5046–5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Smerdon MJ, Conconi A, Modulation of DNA damage and DNA repair in chromatin, Prog. Nucleic Acid Res. Mol. Biol, 62 (1999) 227–255. [DOI] [PubMed] [Google Scholar]
- [5].Hara R, Mo J, Sancar A, DNA damage in the nucleosome core is refractory to repair by human excision nuclease, Mol. Cell Biol, 20 (2000) 9173–9181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Liu X, Smerdon MJ, Nucleotide excision repair of the 5 S ribosomal RNA gene assembled into a nucleosome, J. Biol. Chem, 275 (2000) 23729–23735. [DOI] [PubMed] [Google Scholar]
- [7].Kosmoski JV, Ackerman EJ, Smerdon MJ, DNA repair of a single UV photoproduct in a designed nucleosome, Proc. Natl. Acad. Sci. U. S. A, 98 (2001) 10113–10118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Ura K, Araki M, Saeki H, Masutani C, Ito T, Iwai S, Mizukoshi T, Kaneda Y, Hanaoka F, ATP-dependent chromatin remodeling facilitates nucleotide excision repair of UV-induced DNA lesions in synthetic dinucleosomes, EMBO J, 20 (2001) 2004–2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wang D, Hara R, Singh G, Sancar A, Lippard SJ, Nucleotide excision repair from site-specifically platinum-modified nucleosomes, Biochemistry, 42 (2003) 6747–6753. [DOI] [PubMed] [Google Scholar]
- [10].Svedruzic ZM, Wang C, Kosmoski JV, Smerdon MJ, Accommodation and repair of a UV photoproduct in DNA at different rotational settings on the nucleosome surface, J. Biol. Chem, 280 (2005) 40051–40057. [DOI] [PubMed] [Google Scholar]
- [11].Polo SE, Almouzni G, Chromatin dynamics after DNA damage: The legacy of the access-repair-restore model, DNA Repair (Amst), 36 (2015) 114–121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Ray A, Khan P, Nag Chaudhuri R, Regulated acetylation and deacetylation of H4 K16 is essential for efficient NER in Saccharomyces cerevisiae, DNA Repair (Amst), 72 (2018) 39–55. [DOI] [PubMed] [Google Scholar]
- [13].Shogren-Knaak M, Ishii H, Sun JM, Pazin MJ, Davie JR, Peterson CL, Histone H4–K16 acetylation controls chromatin structure and protein interactions, Science, 311 (2006) 844–847. [DOI] [PubMed] [Google Scholar]
- [14].Clapier CR, Iwasa J, Cairns BR, Peterson CL, Mechanisms of action and regulation of ATP-dependent chromatin-remodelling complexes, Nat. Rev. Mol. Cell Biol, 18 (2017) 407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Fenley AT, Anandakrishnan R, Kidane YH, Onufriev AV, Modulation of nucleosomal DNA accessibility via charge-altering post-translational modifications in histone core, Epigenetics Chromatin, 11 (2018) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Fierz B, Poirier MG, Biophysics of Chromatin Dynamics, Annu. Rev. Biophys, 48 (2019) 321–345. [DOI] [PubMed] [Google Scholar]
- [17].Zhou K, Gaullier G, Luger K, Nucleosome structure and dynamics are coming of age, Nat. Struct. Mol. Biol, 26 (2019) 3–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Waters R, van Eijk P, Reed S, Histone modification and chromatin remodeling during NER, DNA Repair (Amst), 36 (2015) 105–113. [DOI] [PubMed] [Google Scholar]
- [19].Mao P, Wyrick JJ, Emerging roles for histone modifications in DNA excision repair, FEMS Yeast Res, 16 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Grunstein M, Histone acetylation in chromatin structure and transcription, Nature, 389 (1997) 349–352. [DOI] [PubMed] [Google Scholar]
- [21].Bannister AJ, Kouzarides T, Regulation of chromatin by histone modifications, Cell Res, 21 (2011) 381–395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Hodges AJ, Plummer DA, Wyrick JJ, NuA4 acetyltransferase is required for efficient nucleotide excision repair in yeast, DNA Repair (Amst), 73 (2019) 91–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Ramanathan B, Smerdon MJ, Changes in nuclear protein acetylation in u.v.-damaged human cells, Carcinogenesis, 7 (1986) 1087–1094. [DOI] [PubMed] [Google Scholar]
- [24].Allfrey VG, Faulkner R, Mirsky AE, Aceylation and methylation of histones and their possible role in the regulation of RNA synthesis, Proc. Natl. Acad. Sci. U. S. A, 51 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Koprinarova M, Schnekenburger M, Diederich M, Role of histone acetylation in cell cycle regulation, Curr. Top. Med. Chem, 16 (2016) 732–744. [DOI] [PubMed] [Google Scholar]
- [26].Bird AW, Yu DY, Pray-Grant MG, Qiu Q, Harmon KE, Megee PC, Grant PA, Smith MM, Christman MF, Acetylation of histone H4 by Esa1 is required for DNA double-strand break repair, Nature, 419 (2002) 411–415. [DOI] [PubMed] [Google Scholar]
- [27].Barnes CE, English DM, Cowley SM, Acetylation & Co: an expanding repertoire of histone acylations regulates chromatin and transcription, Essays Biochem, 63 (2019) 97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Mao P, Wyrick JJ, Organization of DNA damage, excision repair, and mutagenesis in chromatin: A genomic perspective, DNA Repair (Amst), (2019) 102645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Hodges AJ, Roberts SA, Wyrick JJ, Using yeast as a model organism to study the functional roles of histone acetylation in DNA excision repair, Methods Mol. Biol, 1983 (2019) 175–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cai Y, Fu I, Geacintov NE, Zhang Y, Broyde S, Synergistic effects of H3 and H4 nucleosome tails on structure and dynamics of a lesion-containing DNA: Binding of a displaced lesion partner base to the H3 tail for GG-NER recognition, DNA Repair (Amst), 65 (2018) 73–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Fu I, Cai Y, Geacintov NE, Zhang Y, Broyde S, Nucleosome histone tail conformation and dynamics: Impacts of lysine acetylation and a nearby minor groove benzo[a]pyrene-derived lesion, Biochemistry, 56 (2017) 1963–1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Kenzaki H, Takada S, Partial unwrapping and histone tail dynamics in nucleosome revealed by coarse-grained molecular simulations, PLoS Comput. Biol, 11 (2015) e1004443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Erler J, Zhang R, Petridis L, Cheng X, Smith JC, Langowski J, The role of histone tails in the nucleosome: a computational study, Biophys. J, 107 (2014) 2911–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Mishra LN, Pepenella S, Rogge R, Hansen JC, Hayes JJ, Acetylation mimics within a single nucleosome alter local DNA accessibility in compacted nucleosome arrays, Sci. Rep, 6 (2016) 34808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Morrison EA, Bowerman S, Sylvers KL, Wereszczynski J, Musselman CA, The conformation of the histone H3 tail inhibits association of the BPTF PHD finger with the nucleosome, Elife, 7 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Osakabe A, Tachiwana H, Kagawa W, Horikoshi N, Matsumoto S, Hasegawa M, Matsumoto N, Toga T, Yamamoto J, Hanaoka F, Thoma NH, Sugasawa K, Iwai S, Kurumizaka H, Structural basis of pyrimidine-pyrimidone (6–4) photoproduct recognition by UV-DDB in the nucleosome, Sci. Rep, 5 (2015) 16330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Horikoshi N, Tachiwana H, Kagawa W, Osakabe A, Matsumoto S, Iwai S, Sugasawa K, Kurumizaka H, Crystal structure of the nucleosome containing ultraviolet light-induced cyclobutane pyrimidine dimer, Biochem. Biophys. Res. Commun, 471 (2016) 117–122. [DOI] [PubMed] [Google Scholar]
- [38].Kropachev K, Kolbanovskii M, Cai Y, Rodriguez F, Kolbanovskii A, Liu Y, Zhang L, Amin S, Patel D, Broyde S, Geacintov NE, The sequence dependence of human nucleotide excision repair efficiencies of benzo[a]pyrene-derived DNA lesions: insights into the structural factors that favor dual incisions, J. Mol. Biol, 386 (2009) 1193–1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Reardon JT, Sancar A, Recognition and repair of the cyclobutane thymine dimer, a major cause of skin cancers, by the human excision nuclease, Genes Dev, 17 (2003) 2539–2551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Kobayashi T, Takeuchi S, Saijo M, Nakatsu Y, Morioka H, Otsuka E, Wakasugi M, Nikaido O, Tanaka K, Mutational analysis of a function of xeroderma pigmentosum group A (XPA) protein in strand-specific DNA repair, Nucleic Acids Res, 26 (1998) 4662–4668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Sancar A, DNA excision repair, Annu. Rev. Biochem, 65 (1996) 43–81. [DOI] [PubMed] [Google Scholar]
- [42].Koehler DR, Courcelle J, Hanawalt PC, Kinetics of pyrimidine(6–4)pyrimidone photoproduct repair in Escherichia coli, J. Bacteriol, 178 (1996) 1347–1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Szymkowski DE, Lawrence CW, Wood RD, Repair by human cell extracts of single (6–4) and cyclobutane thymine-thymine photoproducts in DNA, Proc. Natl. Acad. Sci. U. S. A, 90 (1993) 9823–9827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Young AR, Chadwick CA, Harrison GI, Hawk JL, Nikaido O, Potten CS, The in situ repair kinetics of epidermal thymine dimers and 6–4 photoproducts in human skin types I and II, J. Invest. Dermatol, 106 (1996) 1307–1313. [DOI] [PubMed] [Google Scholar]
- [45].Matsumoto S, Cavadini S, Bunker RD, Grand RS, Potenza A, Rabl J, Yamamoto J, Schenk AD, Schubeler D, Iwai S, Sugasawa K, Kurumizaka H, Thoma NH, DNA damage detection in nucleosomes involves DNA register shifting, Nature, 571 (2019) 79–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Shafirovich V, Kolbanovskiy M, Kropachev K, Liu Z, Cai Y, Terzidis MA, Masi A, Chatgilialoglu C, Amin S, Dadali A, Broyde S, Geacintov NE, Nucleotide excision repair and impact of site-specific 5’,8-cyclopurine and bulky DNA lesions on the physical properties of nucleosomes, Biochemistry, 58 (2019) 561–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Conney AH, Induction of microsomal enzymes by foreign chemicals and carcinogenesis by polycyclic aromatic hydrocarbons: G. H. A. Clowes Memorial Lecture, Cancer Res, 42 (1982) 4875–4917. [PubMed] [Google Scholar]
- [48].Phillips DH, Fifty years of benzo(a)pyrene, Nature, 303 (1983) 468–472. [DOI] [PubMed] [Google Scholar]
- [49].Cosman M, de los Santos C, Fiala R, Hingerty BE, Ibanez V, Luna E, Harvey R, Geacintov NE, Broyde S, Patel DJ, Solution conformation of the (+)-cis-anti-[BP]dG adduct in a DNA duplex: intercalation of the covalently attached benzo[a]pyrenyl ring into the helix and displacement of the modified deoxyguanosine, Biochemistry, 32 (1993) 4145–4155. [DOI] [PubMed] [Google Scholar]
- [50].Mocquet V, Kropachev K, Kolbanovskiy M, Kolbanovskiy A, Tapias A, Cai Y, Broyde S, Geacintov NE, Egly JM, The human DNA repair factor XPC-HR23B distinguishes stereoisomeric benzo[a]pyrenyl-DNA lesions, EMBO J, 26 (2007) 2923–2932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Mukherjee A, Lavery R, Bagchi B, Hynes JT, On the molecular mechanism of drug intercalation into DNA: A simulation study of the intercalation pathway, free energy, and DNA structural changes, J. Am. Chem. Soc, 130 (2008) 9747–9755. [DOI] [PubMed] [Google Scholar]
- [52].Li S, Cooper VR, Thonhauser T, Lundqvist BI, Langreth DC, Stacking interactions and DNA intercalation, J. Phys. Chem. B, 113 (2009) 11166–11172. [DOI] [PubMed] [Google Scholar]
- [53].Cosman M, de los Santos C, Fiala R, Hingerty BE, Singh SB, Ibanez V, Margulis LA, Live D, Geacintov NE, Broyde S, et al. , Solution conformation of the major adduct between the carcinogen (+)-anti-benzo[a]pyrene diol epoxide and DNA, Proc. Natl. Acad. Sci. U. S. A, 89 (1992) 1914–1918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Yuan B, Wang J, Cao H, Sun R, Wang Y, High-throughput analysis of the mutagenic and cytotoxic properties of DNA lesions by next-generation sequencing, Nucleic Acids Res, 39 (2011) 5945–5954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Zaliznyak T, Lukin M, de los Santos C, Structure and stability of duplex DNA containing (5’S)-5’,8-cyclo-2’-deoxyadenosine: an oxidatively generated lesion repaired by NER, Chem. Res. Toxicol, 25 (2012) 2103–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Huang H, Das RS, Basu AK, Stone MP, Structure of (5’S)-8,5’-cyclo-2’-deoxyguanosine in DNA, J. Am. Chem. Soc, 133 (2011) 20357–20368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Kropachev K, Ding S, Terzidis MA, Masi A, Liu Z, Cai Y, Kolbanovskiy M, Chatgilialoglu C, Broyde S, Geacintov NE, Shafirovich V, Structural basis for the recognition of diastereomeric 5’,8-cyclo-2’-deoxypurine lesions by the human nucleotide excision repair system, Nucleic Acids Res, 42 (2014) 5020–5032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Berman HM, Westbrook J, Feng Z, Gilliland G, Bhat TN, Weissig H, Shindyalov IN, Bourne PE, The Protein Data Bank, Nucleic Acids Res, 28 (2000) 235–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Davey CA, Sargent DF, Luger K, Maeder AW, Richmond TJ, Solvent mediated interactions in the structure of the nucleosome core particle at 1.9 a resolution, J. Mol. Biol, 319 (2002) 1097–1113. [DOI] [PubMed] [Google Scholar]
- [60].Brower-Toland B, Wacker DA, Fulbright RM, Lis JT, Kraus WL, Wang MD, Specific contributions of histone tails and their acetylation to the mechanical stability of nucleosomes, J. Mol. Biol, 346 (2005) 135–146. [DOI] [PubMed] [Google Scholar]
- [61].Fu I, Smith DJ, Broyde S, Rotational and translational positions determine the structural and dynamic impact of a single ribonucleotide incorporated in the nucleosome, DNA Repair (Amst), 73 (2019) 155–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Case DA, Darden TA, Cheatham TE 3rd, Simmerling CL, Wang J, Duke RE, Luo R, Walker RC, Zhang W, Merz KM, Roberts B, Wang B, Hayik S, Roitberg A, Seabra G, Kolossváry I, Wong KF, Paesani F, Vanicek J, Liu J, Wu X, Brozell SR, Steinbrecher T, Gohlke H, Cai Q, Ye X, Wang J, Hsieh MJ, Cui G, Roe DR, Mathews DH, Seetin MG, Sagui C, Babin V, Gusarov S, Kovalenko A, Kollman PA, AMBER 16, in, University of California, San Francisco., 2016. [Google Scholar]
- [63].Maier JA, Martinez C, Kasavajhala K, Wickstrom L, Hauser KE, Simmerling C, ff14SB: Improving the accuracy of protein side chain and backbone parameters from ff99SB, J. Chem. Theory Comput, 11 (2015) 3696–3713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Shao J, Tanner SW, Thompson N, Cheatham TE, Clustering molecular dynamics trajectories: 1. Characterizing the performance of different clustering algorithms, J. Chem. Theory Comput, 3 (2007) 2312–2334. [DOI] [PubMed] [Google Scholar]
- [65].Humphrey W, Dalke A, Schulten K, VMD: Visual molecular dynamics, J. Mol. Graph, 14 (1996) 33–38, 27–38. [DOI] [PubMed] [Google Scholar]
- [66].Kakumu E, Nakanishi S, Shiratori HM, Kato A, Kobayashi W, Machida S, Yasuda T, Adachi N, Saito N, Ikura T, Kurumizaka H, Kimura H, Yokoi M, Sakai W, Sugasawa K, Xeroderma pigmentosum group C protein interacts with histones: regulation by acetylated states of histone H3, Genes Cells, 22 (2017) 310–327. [DOI] [PubMed] [Google Scholar]
- [67].Mu H, Geacintov NE, Min JH, Zhang Y, Broyde S, Nucleotide excision repair lesion-recognition protein Rad4 captures a pre-flipped partner base in a benzo[a]pyrene-derived DNA lesion: How structure impacts the binding pathway, Chem. Res. Toxicol, 30 (2017) 1344–1354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Wang J, Clauson CL, Robbins PD, Niedernhofer LJ, Wang Y, The oxidative DNA lesions 8,5’-cyclopurines accumulate with aging in a tissue-specific manner, Aging Cell, 11 (2012) 714–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Geacintov NE, Broyde S, Repair-resistant DNA lesions, Chem. Res. Toxicol, 30 (2017) 1517–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Cutter AR, Hayes JJ, A brief review of nucleosome structure, FEBS Lett, 589 (2015) 2914–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods: Rationale for design of systems for MD simulations; molecular modeling of lesion-containing nucleosomes; force field, protonation, MD protocols; structural analyses including minor groove widths, root mean square deviation, flipping pseudo-dihedral angles, N1–N3 distance to characterize Watson-Crick hydrogen bonding integrity, hydrogen bond occupancies and Twist angles computation
Supplementary Tables: Table S1. Box sizes, number of waters, and length of MD simulations; Table S2. Number of HBs between 13-mer DNA duplex centered at the lesion with nearby histones; Table S3. Ensemble average helicoidal Twist angles; Table S4. Means and standard deviations of the flipping pseudo-dihedral angle of the partner dC; Table S5. Ensemble average minor groove widths for the11-mer DNA duplex with the R-cdG lesion in the center; Table S6. Relative GG-NER efficiencies in HeLa cell experiments
Supplementary Figures: Figure S1. DNA sequence and numbering scheme; Figure S2. Definition of the flipping pseudo-dihedral angle; Figure S3. Ensemble average values of minor groove widths and their standard deviations; Figure S4. Ensemble average values for the Twist angles; Figure S5. Best representative structures of the trans-B[a]P-dG-OUT adduct superimposed against its unmodified control; Figure S6. Hydrogen bonds gained or lost due to lesion modifications; Figure S7. N1–N3 distances indicating Watson-Crick hydrogen bonding integrity
Supplementary Movies: Movies S1 and S2 reveal the best representative structures of the cis-B[a]P-dG-OUT and -IN, respectively.


