Abstract
Backgrounds/Aims
Approximately 60–80% of patients with intrahepatic cholangiocarcinoma (iCCA) are not suitable for surgical resection due to advanced disease at presentation. This review assesses the role of surgical resection followed by down staging treatment in the management of patients with locally advanced iCCA.
Methods
A systematic review and pooled analysis were performed of the relevant published studies published between January 2000-December 2018. The primary outcome measure was overall survival. Secondary outcome measures were rates of clinical benefit, margin-negative (R0) resections, overall and surgery-specific complications, and post-operative mortality.
Results
Eighteen cohort studies with 1880 patients were included in the review. The median overall survival in all patients was 14 months (range, 7–18 months). Patients undergoing resection following down staging had significantly longer survival than those who did not (median: 29 vs. 12 months, p<0.001). The Clinical Benefit Rate with this strategy (complete response+partial response+stable disease) was 64% (244/383), ranging from 33-90%. Thirty-eight percent of the patients underwent resections with a 60% R0 resection rate and 6% postoperative mortality.
Conclusions
Although the evidence to support the benefits of NAT for iCCA is limited, the review supports the use of down staging treatment and also surgical resection in the cohort with response to NAT in order to improve long-term survival in patients with locally advanced iCCA.
Keywords: Intrahepatic, Cholangiocarcinoma, Locally advanced, Down staging, Surgery
INTRODUCTION
The incidence of intrahepatic cholangiocarcinoma (iCCA) is about 0.7 cases per 100,000 adults in the USA.1 Despite advances in multimodality treatment, long-term survival is only seen in 10–20% of patients due to the advanced stage at presentation.2 Surgical resection remains the only potentially curative therapy for patients with iCCA; however, only 30–60% of patients are candidates for surgical resection due to locally advanced (large tumors with either hepatic inflow or outflow involvement) or metastatic disease, underlying chronic liver disease, or frailty.3 In patients undergoing surgical resection, the 5-year survival is 20-40%, and median survival is 25 months.3
In patients with unresectable disease, median survival is 12–15 months with a 5-year survival of 5–10%,4,5 with chemotherapy based on a combination of gemcitabine and platinum salts.6 There is no established standard treatment for patients with locally advanced biliary cancers. The role of chemotherapy or radiotherapy is considered mainly a palliative in unresectable iCCA. Neoadjuvant therapy (NAT) with a view to downstage has gained popularity in the last decade in the management of hepatobiliary and pancreatic cancers, particularly the latter in patients with borderline resectable and locally advanced (LA) cancers. Pooled analysis by Suker et al.7 in a systematic review demonstrated NAT with FOLFIRINOX for locally advanced pancreatic cancers had a median overall survival (OS) ranging between 10 and 33 months and resection rates of up to 43%. In resectable oesophageal cancers, NAT has been demonstrated standard treatment through high-quality randomized controlled trials.8 A recent network meta-analysis further demonstrated neoadjuvant CRT (chemoradiotherapy) followed by surgery to be the most effective strategy in improving long-term survival of resectable oesophageal cancers.9
To date, there is limited consensus for advocating NAT for patients with iCCA to further improve survival and surgical outcomes. For the purpose of the study, this was considered downstaging treatment given to patients with non-metastatic iCCA as NAT, although the intention might have been palliation given the clinical practice during the study period. Hence, the aim of this review is to determine the oncological and surgical outcomes of patients receiving NAT for borderline iCCA.
METHODS
Search strategy
A systematic search of PubMed, EMBASE and the Cochrane Library databases were conducted. The search terms used were ‘intrahepatic cholangiocarcinoma’ or ‘cholangiocarcinoma’, and ‘neoadjuvant therapy or ‘neoadjuvant radiotherapy’ or ‘neoadjuvant chemotherapy’ or ‘chemotherapy’ individually or in combination. Search terms used for this review are presented as shown in Supplementary Table 1. The ‘related articles’ function was used to broaden the search, and all citations were considered for relevance. A manual search of reference lists in recent reviews and eligible studies was also undertaken.
Inclusion and exclusion criteria
Inclusion criteria were: (1) studies reporting the use of NAT (by any modality) in human subjects with non-metastatic locally advanced iCCA; (2) published in the English language. Exclusion criteria were: (1) conference abstracts, review articles, and case reports (<5 patients); (2) publications with mixed populations where the outcomes of patients with cancers at another site could not be separated from those of patients with intrahepatic cholangiocarcinoma. After excluding duplicates, two authors (SK, BD) independently reviewed the titles and abstracts of studies identified by the literature search. Where a study was considered to be potentially relevant to the research question a full copy of the publication was obtained for further review. The references of all included studies were hand-searched in order to identify other potentially relevant studies. Any areas of disagreement between the two primary researchers were resolved through discussion. The intention of the NAT in this review might have been palliative treatment when offered to patients but patients have then progressed to surgical pathways where response has been noted. It is therefore important to note the differences in the terminology used in this review to the terms used in standard clinical practice. Patients who progressed to surgery following down staging treatment are considered to have had surgery following NAT, patients who received treatment but not surgery had palliative chemotherapy, and those who did not receive any treatment (no NAT group) were managed by best supportive care.
Data extraction
Two researchers (SK, BD) independently extracted data on study characteristics, patient demographics, definitions of borderline resection, modality and regimes of NAT, response to and the clinical benefit with NAT, progression to surgery and postoperative outcomes such as overall mortality and morbidity rates such as bile leak, liver failure, where reported.
Study outcomes
The primary outcome measure was OS in patients receiving NAT with or without subsequent surgical resection. Response to chemotherapy, where reported, was graded as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Secondary outcome measures were rates of overall resection rates and margin-negative (R0) resections, overall complications (Grade I-V) and major complications (>Grade III) reported according to Clavien-Dindo classification, surgery-specific complications (bile leak, intra-abdominal collections), and response to chemotherapy. A pooled analysis of the data was performed to assess the study outcomes and a comparative survival analysis was performed.
RESULTS
Study characteristics
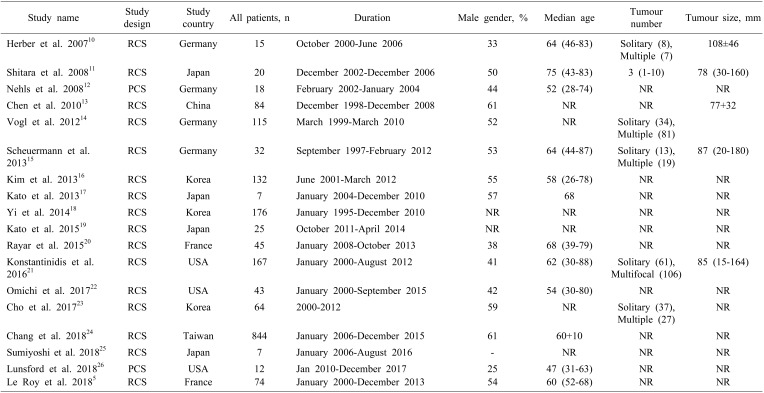
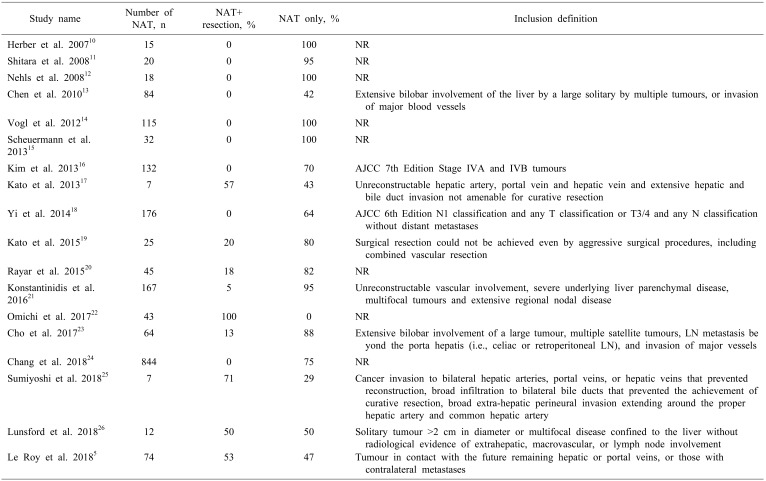
The literature search identified 18 cohort studies, including 1880 patients with locally advanced iCCA, of which two were prospective cohort studies. Baseline demographics of the included studies are presented in Table 1. Study quality was assessed using NewCastle Ottawa system. Ten studies provided the definitions for locally advanced or inoperable iCCA, and these are detailed in Table 2.
Table 1. Baseline demographics of included studies.
RCS, retrospective study; PCS, prospective study; NR, not reported
Table 2. Definitions of locally advanced iCCA used in the studies.
Chemotherapy and radiotherapy regimes
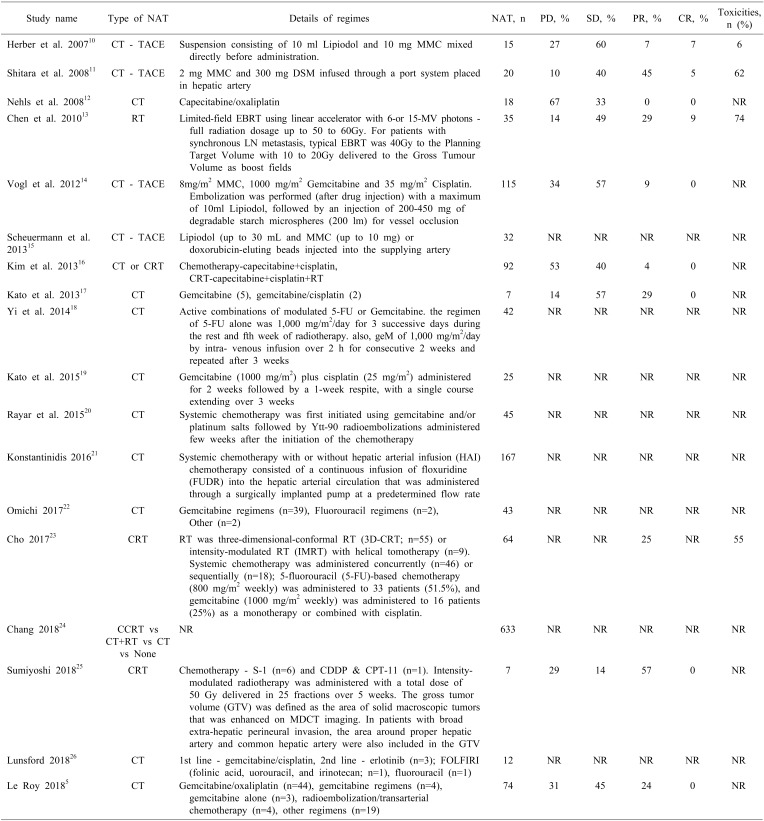
NAT regimes and tumor responses are presented in Table 3. Eleven studies reported the use of neoadjuvant chemotherapy, of which four10,11,14,15 reported the use of transarterial chemoembolization (TACE) with mitomycin C (MMC). One study14 used gemcitabine and cisplatin in addition to MMC for TACE. Eight studies5,12,17,18,19,20,22,26 evaluated combinations of chemotherapy (gemcitabine/capecitabine and platinum-based regimes). Of the remaining studies, one reported use of RT,13 three reported use of chemoradiotherapy (CRT),16,23,25 and one compared concurrent CRT, chemotherapy and RT, chemotherapy, and no treatment.24
Table 3. Treatment regimen used in the included studies and response to treatment.
PD, progressive disease; SD, stable disease; CR, complete response; PR, partial response; CT, chemotherapy; RT, radiotherapy; CRT, chemoradiotherapy; TACE, transarterial chemoembolisation
Response rates and the definitions of response
Eleven studies reported the criteria used to assess tumour response [RECIST (n=9),10,11,14,16,17,19,21,23,25 mRECIST (n=1),5 and WHO (n=1)].12 Nine studies5,10,11,12,13,14,16,17,25 (n=383 patients) provided response rates as the presence of progressive disease (PD), stable disease (SD), partial response (PR), and complete response (CR). The rates of PD were 36%, ranging from 10 to 67%. The Clinical Benefit Rate (CBR=CR+PR+SD) was noted in 64% (244/383) of the patients going for NAT, ranging from 33 to 90%.
Resectability rates
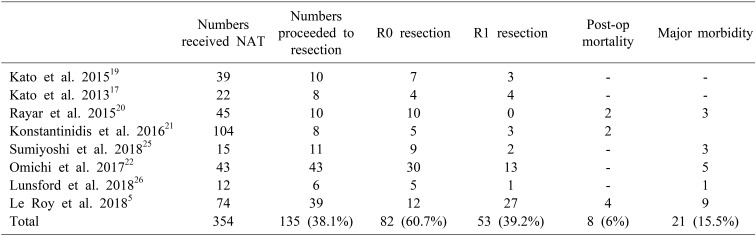
Of the nine studies5,10,11,12,13,14,16,17,25 (383 patients) where response rates were provided, 64% (244/383) showed a clinical benefit for NAT. None of the studies provided the number of patients who were explored for but failed to proceed to resection following NAT. Of the studies5,17,19,20,21,22,25,26 where post-NAT resection rates were reported, 135/354 patients (38%) underwent resections. Of these, 60% of the patients had R0 resections (82/135 patients), 40% were reported to have R1 resection, and R2 resection was performed in 1/135 patients.
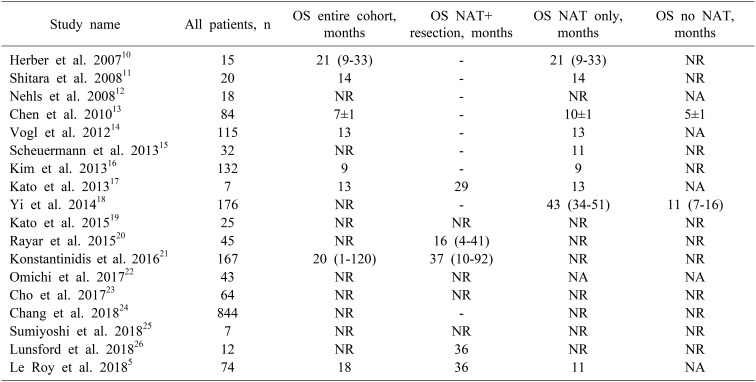
Overall survival
The OS of the entire cohort was 14 months (range, 7–18 months). OS was significantly longer in patients receiving NAT with resection (median: 29 months; range: 18–37 months) compared to NAT alone (median: 12 months; range: 5–43 months) or no NAT (median: 8 months; range: 5–11 months) (p<0.001) for locally advanced iCCA. In patients receiving chemotherapy only, the OS of the entire cohort was 18 months (range, 5–20 months). OS was significantly longer in patients receiving NAT with resection (median, 36 months; range, 18–37 months) compared to NAT alone (median, 12 months; range, 5–43 months, p=0.02). In patients receiving CRT only, the OS of the entire cohort was 12 months (range, 9–15 months). OS was longer in patients receiving NAT with resection (median, 21 months; range, 18–24 months) compared to NAT alone (median: 11 months; range: 9–43 months, p=0.8) (Table 4).
Table 4. Overall survival among the patients in the all the treatment pathways.
Postoperative outcomes
Overall post-surgical complications were reported in eight studies.5,17,19,20,21,22,25,26 The overall rate of postoperative mortality was 6% (8/135 patients) on pooled analysis. The rate of major complications was 15.5% (21/135 patients). Bile leaks (four patients), post-hepatectomy liver failure (four patients), intraabdominal collections (four patients), ascites (three patients), post-operative bleeding (one patient), pleural effusion (one patient), and acute kidney injury (one patient) were the reported complications (Table 5).
Table 5. Post-operative outcomes following resection surgery, where reported.
DISCUSSION
Over the last decade, the long-term survival of patients diagnosed with iCCA has been relatively poor, with a small proportion of patients undergoing curative surgical resection and a median survival of 20–30 months in the resected group.3 NAT for iCCA is an appealing option for suitable candidates to select the patient with less aggressive tumor biology, downstage the disease, increase the resectability rates, and improve the OS in the locally advanced iCCA. NAT therapy in iCCA lags behind other gastrointestinal cancers, such as oesophageal and pancreatic cancers, where this approach has shown significant improvement in long term-survival and increased the number of ongoing clinical trials.7,9 The present review highlights that current evidence for NAT in iCCA is limited to cohort studies, specifically retrospective case series. This review further highlights that NAT followed by resection has a superior survival rate than patients receiving NAT alone or no surgery in the group of patients deemed unresectable because of locally advanced disease. Le Roy et al.5 reported no significant difference in the patients who had primarily resectable and downstaged unresectable lesions in terms of postoperative complications, but despite the higher R1 resection rates (p=0.004), the OS was similar (HR 1.23, 0.77–1.97; p=0.391) between the two groups. The current review does not differentiate the outcomes of R1 and R0 resections but together the OS was significantly better in the cohort that proceeded for surgery following downstaging NAT.
iCCAs are usually peripherally located, away from the hilum and are usually dealt with by anatomical, non-anatomical resections based on the position of the lesion. When the lesions are larger or located centrally, the inflow or (more often) the outflow of the liver could be infiltrated. Such vascular or biliary involvement on the contralateral side of resection are usually considered the limiting factor for upfront resection.25 The group of patients with iCCA that is not suitable for surgical resections are usually put through a palliative pathway or best supportive care. Factors such as micro or macro vascular invasion, presence of lymph node metastases, and presence of satellite nodules represent poor pathological prognostic factors. The influence of pathological factors on their outcomes is not clear from this review. However, this study has shown a variable median OS in the group of patients receiving NAT (with or without curative resection) with an encouraging survival benefit. There is a lack of a definition for locally advanced iCCA, which might reflect the lack of consensus about the patients suitable for NAT and type of treatment in the context of multimodal treatment options (Table 2). We propose that the HPB surgical community should aim to obtain consensus for the definition of borderline resectability and selection of iCCA patients for NAT. In this group of patients, NAT might be able to increase resectability rates, with acceptable morbidity, mortality, and prolonged survival.
The response rates of iCCA to chemotherapy are variable and limited. Currently, there is no standard treatment for locally advanced cholangiocarcinoma, either in a NAT or palliative set up. The ABC-02 trial compared doublet therapy with gemcitabine and cisplatin to gemcitabine as a single agent in 410 patients with locally advanced or metastatic biliary tract cancer.6 After a median follow-up of 8.2 months, the combination group had a significantly improved OS (11.7 vs. 8.1 months). Similar results with combination chemotherapy were also reported by studies with a 70% response rate or stable disease and OS of up to 15 months.27 The results of ABC06 trial for 2nd line chemotherapy with FOLFOX regime are awaited but the unpublished results are promising.28 Other treatment options, such as chemoradiotherapy, TACE, and external beam RT, have also been associated with longer progression-free survival and OS than chemotherapy alone in a palliative setting for patients with unresectable advanced iCCA.29 Shitara et al.11 reported a 50% response rate and a median survival of 14.1 months with hepatic arterial infusion chemotherapy for unresectable iCCA. The review also reflects the variations in the NAT regimes used by the included studies, although the majority of studies used a gemcitabine-based chemotherapy regime and only one study used a standardized regime (gemcitabine/oxaliplatin) in all patients. Other limitations to this study include significant heterogeneity of the included studies with no clear definitions of locally advanced iCCA. Not all of the reported studies reported the survival data, and well-defined post-operative complications, limiting the quality of the meta-analysis to reliably analyze the impact of NAT in patients with and without surgical resection. However, the current study provides the base to plan future studies that would be of useful in the management of this group of patients.
In conclusion, although the evidence to support the benefits of NAT for iCCA is limited, the data from this review is very promising in improving the outcomes of patients with iCCA. International efforts are required to standardize the definitions and treatment regimens targeting locally advanced iCCA through randomized controlled trials.
Footnotes
- Conceptualization: BD.
- Data curation: SK, FG.
- Formal analysis: BD, SK, FG, KJR, RPS, PP, RM, NC, JI, DFM, PM.
- Methodology: BD, SK.
- Project administration: BD, KJR, PP, PM.
- Visualization: BD, DFM, RPS, RM.
- Writing — original draft: BD, SK.
- Writing — review & editing: BD, FG, KJR, RPS, PP, RM, NC, JI, DFM, PM.
SUPPLEMENTARY MATERIAL
Summary of search terms for literature review
References
- 1.Yao KJ, Jabbour S, Parekh N, Lin Y, Moss RA. Increasing mortality in the United States from cholangiocarcinoma: an analysis of the National Center for Health Statistics Database. BMC Gastroenterol. 2016;16:117. doi: 10.1186/s12876-016-0527-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kamarajah SK. Evaluation of the AJCC 8th edition staging system for pathologically versus clinically staged intrahepatic cholangiocarcinoma (iCCA): a time to revisit a dogma? A Surveillance, Epidemiology, and End Results (SEER) analysis. J Gastrointest Cancer. 2019;50:392–399. doi: 10.1007/s12029-018-0084-5. [DOI] [PubMed] [Google Scholar]
- 3.Endo I, Gonen M, Yopp AC, Dalal KM, Zhou Q, Klimstra D, et al. Intrahepatic cholangiocarcinoma: rising frequency, improved survival, and determinants of outcome after resection. Ann Surg. 2008;248:84–96. doi: 10.1097/SLA.0b013e318176c4d3. [DOI] [PubMed] [Google Scholar]
- 4.Nathan H, Pawlik TM. Staging of intrahepatic cholangiocarcinoma. Curr Opin Gastroenterol. 2010;26:269–273. doi: 10.1097/MOG.0b013e328337c899. [DOI] [PubMed] [Google Scholar]
- 5.Le Roy B, Gelli M, Pittau G, Allard MA, Pereira B, Serji B, et al. Neoadjuvant chemotherapy for initially unresectable intrahepatic cholangiocarcinoma. Br J Surg. 2018;105:839–847. doi: 10.1002/bjs.10641. [DOI] [PubMed] [Google Scholar]
- 6.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. ABC-02 Trial Investigators. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 7.Suker M, Beumer BR, Sadot E, Marthey L, Faris JE, Mellon EA, et al. FOLFIRINOX for locally advanced pancreatic cancer: a systematic review and patient-level meta analysis. Lancet Oncol. 2016;17:801–810. doi: 10.1016/S1470-2045(16)00172-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Burmeister BH, Smithers BM, Gebski V, Fitzgerald L, Simes RJ, Devitt P, et al. Trans-Tasman Radiation Oncology Group; Australasian Gastro-Intestinal Trials Group. Surgery alone versus chemoradiotherapy followed by surgery for resectable cancer of the oesophagus: a randomised controlled phase III trial. Lancet Oncol. 2005;6:659–668. doi: 10.1016/S1470-2045(05)70288-6. [DOI] [PubMed] [Google Scholar]
- 9.Pasquali S, Yim G, Vohra RS, Mocellin S, Nyanhongo D, Marriott P, et al. Survival after neoadjuvant and adjuvant treatments compared to surgery alone for resectable esophageal carcinoma: a network meta-analysis. Ann Surg. 2017;265:481–491. doi: 10.1097/SLA.0000000000001905. [DOI] [PubMed] [Google Scholar]
- 10.Herber S, Otto G, Schneider J, Manzl N, Kummer I, Kanzler S, et al. Transarterial chemoembolization (TACE) for inoperable intrahepatic cholangiocarcinoma. Cardiovasc Intervent Radiol. 2007;30:1156–1165. doi: 10.1007/s00270-007-9032-7. [DOI] [PubMed] [Google Scholar]
- 11.Shitara K, Ikami I, Munakata M, Muto O, Sakata Y. Hepatic arterial infusion of mitomycin C with degradable starch microspheres for unresectable intrahepatic cholangiocarcinoma. Clin Oncol (R Coll Radiol) 2008;20:241–246. doi: 10.1016/j.clon.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 12.Nehls O, Oettle H, Hartmann JT, Hofheinz RD, Hass HG, Horger MS, et al. Capecitabine plus oxaliplatin as first-line treatment in patients with advanced biliary system adenocarcinoma: a prospective multicentre phase II trial. Br J Cancer. 2008;98:309–315. doi: 10.1038/sj.bjc.6604178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen YX, Zeng ZC, Tang ZY, Fan J, Zhou J, Jiang W, et al. Determining the role of external beam radiotherapy in unresectable intrahepatic cholangiocarcinoma: a retrospective analysis of 84 patients. BMC Cancer. 2010;10:492. doi: 10.1186/1471-2407-10-492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vogl TJ, Naguib NN, Nour-Eldin NE, Bechstein WO, Zeuzem S, Trojan J, et al. Transarterial chemoembolization in the treatment of patients with unresectable cholangiocarcinoma: results and prognostic factors governing treatment success. Int J Cancer. 2012;131:733–740. doi: 10.1002/ijc.26407. [DOI] [PubMed] [Google Scholar]
- 15.Scheuermann U, Kaths JM, Heise M, Pitton MB, Weinmann A, Hoppe-Lotichius M, et al. Comparison of resection and transarterial chemoembolisation in the treatment of advanced intrahepatic cholangiocarcinoma--a single-center experience. Eur J Surg Oncol. 2013;39:593–600. doi: 10.1016/j.ejso.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 16.Kim YI, Park JW, Kim BH, Woo SM, Kim TH, Koh YH, et al. Outcomes of concurrent chemoradiotherapy versus chemotherapy alone for advanced-stage unresectable intrahepatic cholangiocarcinoma. Radiat Oncol. 2013;8:292. doi: 10.1186/1748-717X-8-292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kato A, Shimizu H, Ohtsuka M, Yoshidome H, Yoshitomi H, Furukawa K, et al. Surgical resection after downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer: a retrospective single-center study. Ann Surg Oncol. 2013;20:318–324. doi: 10.1245/s10434-012-2312-8. [DOI] [PubMed] [Google Scholar]
- 18.Yi SW, Kang DR, Kim KS, Park MS, Seong J, Park JY, et al. Efficacy of concurrent chemoradiotherapy with 5-fluorouracil or gemcitabine in locally advanced biliary tract cancer. Cancer Chemother Pharmacol. 2014;73:191–198. doi: 10.1007/s00280-013-2340-5. [DOI] [PubMed] [Google Scholar]
- 19.Kato A, Shimizu H, Ohtsuka M, Yoshitomi H, Furukawa K, Takayashiki T, et al. Downsizing chemotherapy for initially unresectable locally advanced biliary tract cancer patients treated with gemcitabine plus cisplatin combination therapy followed by radical surgery. Ann Surg Oncol. 2015;22 Suppl 3:S1093–S1099. doi: 10.1245/s10434-015-4768-9. [DOI] [PubMed] [Google Scholar]
- 20.Rayar M, Sulpice L, Edeline J, Garin E, Levi Sandri GB, Meunier B, et al. Intra-arterial yttrium-90 radioembolization combined with systemic chemotherapy is a promising method for downstaging unresectable huge intrahepatic cholangiocarcinoma to surgical treatment. Ann Surg Oncol. 2015;22:3102–3108. doi: 10.1245/s10434-014-4365-3. [DOI] [PubMed] [Google Scholar]
- 21.Konstantinidis IT, Groot Koerkamp B, Do RK, Gönen M, Fong Y, Allen PJ, et al. Unresectable intrahepatic cholangiocarcinoma: systemic plus hepatic arterial infusion chemotherapy is associated with longer survival in comparison with systemic chemotherapy alone. Cancer. 2016;122:758–765. doi: 10.1002/cncr.29824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omichi K, Cloyd JM, Yamashita S, Tzeng CD, Conrad C, Chun YS, et al. Neutrophil-to-lymphocyte ratio predicts prognosis after neoadjuvant chemotherapy and resection of intrahepatic cholangiocarcinoma. Surgery. 2017;162:752–765. doi: 10.1016/j.surg.2017.05.015. [DOI] [PubMed] [Google Scholar]
- 23.Cho Y, Kim TH, Seong J. Improved oncologic outcome with chemoradiotherapy followed by surgery in unresectable intrahepatic cholangiocarcinoma. Strahlenther Onkol. 2017;193:620–629. doi: 10.1007/s00066-017-1128-7. [DOI] [PubMed] [Google Scholar]
- 24.Chang WW, Hsiao PK, Qin L, Chang CL, Chow JM, Wu SY. Treatment outcomes for unresectable intrahepatic cholangiocarcinoma: nationwide, population-based, cohort study based on propensity score matching with the Mahalanobis metric. Radiother Oncol. 2018;129:284–292. doi: 10.1016/j.radonc.2018.09.010. [DOI] [PubMed] [Google Scholar]
- 25.Sumiyoshi T, Shima Y, Okabayashi T, Negoro Y, Shimada Y, Iwata J, et al. Chemoradiotherapy for initially unresectable locally advanced cholangiocarcinoma. World J Surg. 2018;42:2910–2918. doi: 10.1007/s00268-018-4558-1. [DOI] [PubMed] [Google Scholar]
- 26.Lunsford KE, Javle M, Heyne K, Shroff RT, Abdel-Wahab R, Gupta N, et al. Methodist-MD Anderson Joint Cholangiocarcinoma Collaborative Committee (MMAJCCC) Liver transplantation for locally advanced intrahepatic cholangiocarcinoma treated with neoadjuvant therapy: a prospective case-series. Lancet Gastroenterol Hepatol. 2018;3:337–348. doi: 10.1016/S2468-1253(18)30045-1. [DOI] [PubMed] [Google Scholar]
- 27.Eckmann KR, Patel DK, Landgraf A, Slade JH, Lin E, Kaur H, et al. Chemotherapy outcomes for the treatment of unresectable intrahepatic and hilar cholangiocarcinoma: a retrospective analysis. Gastrointest Cancer Res. 2011;4:155–160. [PMC free article] [PubMed] [Google Scholar]
- 28.Lamarca A, Palmer DH, Wasan HS, Ross PJ, Ma YT, Arora A, et al. ABC-06 | a randomised phase III, multi-centre, open-label study of active symptom control (ASC) alone or ASC with ox aliplatin / 5-FU chemotherapy (ASC+mFOLFOX) for patients (pts) with locally advanced / metastatic biliary tract cancers (ABC) previously-treated with cisplatin/gemcitabine (CisGem) chemotherapy. J Clin Oncol. 2019;37(Suppl 15):4003. [Google Scholar]
- 29.Currie BM, Soulen MC. Decision making: intra-arterial therapies for cholangiocarcinoma-TACE and TARE. Semin Intervent Radiol. 2017;34:92–100. doi: 10.1055/s-0037-1602591. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Summary of search terms for literature review