Abstract
Senescent cells (SCs) arise from normal cells in multiple organs due to inflammatory, metabolic, DNA damage, or tissue damage signals. SCs are non-proliferating but metabolically active cells that can secrete a range of pro-inflammatory and proteolytic factors as part of the senescence-associated secretory phenotype (SASP). Senescent cell anti-apoptotic pathways (SCAPs) protect SCs from their own pro-apoptotic SASP. SCs can chemo-attract immune cells and are usually cleared by these immune cells. During aging and in multiple chronic diseases, SCs can accumulate in dysfunctional tissues. SCs can impede innate and adaptive immune responses. Whether immune system loss of capacity to clear SCs promotes immune system dysfunction, or conversely whether immune dysfunction permits SC accumulation, are important issues that are not yet fully resolved. SCs may be able to assume distinct states that interact differentially with immune cells, thereby promoting or inhibiting SC clearance, establishing a chronically pro-senescent and pro-inflammatory environment, leading to modulation of the SASP by the immune cells recruited and activated by the SASP. Therapies that enhance immune cell-mediated clearance of SCs could provide a lever for reducing SC burden. Such therapies could include vaccines, small molecule immunomodulators, or other approaches. Senolytics, drugs that selectively eliminate SCs by transiently disabling their SCAPs, may prove to alleviate immune dysfunction in older individuals and thereby accelerate immune-mediated clearance of SCs. The more that can be understood about the interplay between SCs and the immune system, the faster new interventions may be developed to delay, prevent, or treat age-related dysfunction and the multiple senescence-associated chronic diseases and disorders.
Keywords: Senescent cells, Immune system, Cytokines, Chemokines, Senolytics
1. Introduction
Cellular senescence entails essentially irreversible loss of replicative capacity of proliferating cells in humans and other vertebrates [1–5]. Differentiated, non-dividing cells can also enter a senescent-like state [6–8]. Senescent cells (SCs) accumulate with aging in multiple tissues, particularly in association with age-related dysfunction. For example, senescent cell burden is higher in tissues of elderly women with frailty than in healthier elderly women [9]. SCs can also accumulate at sites of chronic diseases, even in younger individuals, and transplanting senescent cells into younger mice can induce development of age-related diseases [10–12]. Importantly, senolytic drugs, which promote removal of SCs by targeting SCAPs, alleviate a range of chronic diseases at least in mice, reduce frailty, improve healthspan (the period during life free of disability, pain, and independence-limiting dysfunction), delay onset of age-related diseases as a group, and extend remaining lifespan in old animals [10,13–24]. Therefore, understanding the mechanisms that control senescent cell burden is critical and could lead to development of diagnostic tests and clinical interventions with far-reaching health implications.
SCs are metabolically active, remain viable, and are resistant to apoptosis [1,25–27]. Rather than being removed directly through apoptosis, it appears that SCs are usually cleared by the immune system [28–34]. SCs generally exhibit downregulation of genes related to their original function when in the non-senescent state. Furthermore, SCs have altered heterochromatin patterns and cytoskeletal structure, up-regulation of lysosomal enzymes such as senescence-associated β-galactosidase (SA β-gal), and a metabolic shift from fatty acid utilization towards glycolysis [35–40].
Cells can become senescent in response to a variety of stressors. These stressors are of four main types: (1) DNA damage (e.g., oncogenic or other mutations, radiation, DNA-damaging chemotherapy, telomere shortening or dysfunction, oncogene activation, insertions [transposons, DNA viruses, or retro-transcribed RNA viruses], reactive oxygen species- [ROS] induced DNA damage or chromosomal instability), (2) metabolic stresses (e.g., high glucose, osmotic stress, peroxidized or saturated fatty acids or ceramides, ROS, etc.), (3) inflammation (e.g., inflammatory cytokines/chemokines), and (4) damage signals (e.g., damage-associated molecular pattern – DAMP – factors [nucleotides, urate, chromatin fragments], misfolded or aggregated proteins, etc.) [6,41]. SCs can develop a senescence-associated secretory phenotype (SASP) [42]. The array of SASP factors appears to vary among SC types and with the stimuli that originally drove the cells into senescence [43]. In general, SASP factors are inflammatory and can attract immune cells, promote matrix rearrangement, interfere with stem and progenitor cell function, cause thrombosis, and induce changes in the cellular composition of tissues due to growth factors [23,42,44–46].
There may be several beneficial roles of SCs, including for tissue regeneration, in guiding development, and in protection against cancer [29,47,48]. Oncogenic mutations, activated oncogenes, cancer-related metabolic byproducts, and DAMPs originating from cell debris within clusters of cancer cells may induce senescence in the tumor cells or nearby healthy cells. By becoming senescent, cancer cells are removed from the cell cycle, potentially slowing further cancer growth. Through their SASP, these SCs could kill neighboring cancer cells and attract immune cells that contribute to further removal of cancer cells. Additionally, senescence can spread from cell to cell [7,10], potentially slowing cancer growth. Consistent with these roles of SCs in cancer protection, interventions that interfere with the generation of SCs, such as knocking down p16Ink4a or p53 expression in mice, promote development of cancer [49]. Thus, it would appear to be more desirable to develop pharmacological interventions that eliminate SCs, rather than developing interventions designed to perturb the cellular machinery through which damaged cells become senescent or to restore the replicative potential of SCs already harboring potentially oncogenic mutations [50,51]. Immune clearance of SCs or senolytic drugs may prevent not only continuing damage to tissues by SCs because of their SASP, but such drugs and/or immune clearance could also remove SCs harboring oncogenic mutations that, with further mutations, might in theory otherwise re-enter the cell cycle as cancer cells. Consistent with the latter speculation, oncogenic SCs can escape from cell cycle arrest following in vitro manipulation of gene expression [51].
During aging and in multiple age-related diseases, SCs accumulate in numerous tissues [10,18,23,50,52–55]. This accumulation suggests that immune-mediated SC clearance might be impaired or overwhelmed, perhaps related to age-related changes in the immune system [56]. With aging, organs and compartments in which immune cells differentiate, mature, or circulate (bone marrow, thymus, spleen, lymph nodes, and blood) undergo morphological and functional changes that perturb immune cell quantity and quality [57,58]. There is also a general increase in circulating pro-inflammatory factors related to sterile, chronic, basal inflammation, inflammaging, with increases in circulating inflammatory cytokines such as IL-6, IL-1β, and TNFα [59]. The SASP may be a significant contributor to inflammaging [60–62].
2. The SASP
Through their SASP, SCs can impact cells and tissues around them and at a distance by inducing inflammation, attracting immune cells, spreading senescence to other cells, altering stem and progenitor cell function, and impacting on endocrine and metabolic function. The SASP varies among SCs, depending on the cell type the SCs originated from, how the SCs were induced, over time since induction, and in response to hormones, cytokines, drugs, and other factors. Among the more frequently observed SASP peptides and proteins are the cytokines IL-6, IL-8, IL-1β, and GM-CSF, the chemokines MCP-1 to 4, MIP-1α (MIP-3α), GROα (and γ), RANTES, RARRES2, matrix proteases and their inhibitors (MMP 1, 3, 9, and 12 and TIMPs), and factors that modulate stem cell function, tissue development, and angiogenesis, including activin A and TGF-β [42,46,63–65]. Bioactive SASP components also include microvesicles and exosomes, microRNAs and other non-coding RNAs, mitochondrial DNA fragments and other nucleotides, ROS, prostenoids, ceramides, bradykines, protein aggregates, and other factors that might also spread senescence and exacerbate inflammation [50,66–69]. SCs can attract, activate, and anchor immune system cells through SASP cytokines, chemokines, and other factors that control immune cell proliferation and development, proteases that remodel the extracellular matrix to facilitate immune cell migration or damage immune cell receptors or ligands, and production of neoantigens through protein modifications by these proteases, protein misfolding, protein aggregation, or release of intracellular contents [45,46,70].
Certain SASP components are recognized by receptors present on natural killer cells (NKs), monocytes/macrophages (MOs), and T-cells and have effects on other immune cell types, including neutrophils, basophils, mast cells (MCs), eosinophils, B-cells, and dendritic cells (DCs; Table 1). Immune cells have complex signaling and regulatory roles during innate and adaptive immune responses, but their primary function is to detect danger signals such as defective cells, intracellular components resulting from cell lysis, tumor-specific neoantigens, or pathogens. Immune cells can induce death of defective cells or pathogenic organisms by targeting membrane receptors and by releasing cytotoxic cytokines, ROS, cytolytic enzymes, or phagocytosis, leaving healthy neighboring cells alive. SASP factors can induce stem cell development and angiogenesis, facilitating regeneration of damaged tissue [71]. Furthermore, at least in an oncogene-induced senescence model, the SASP can progress through separate regenerative and pro-inflammatory phases (Hoare et al., 2016).
Table 1.
SASP Factors and Effects on Immune Cells.
| SASP factor | General function | Effects | References |
|---|---|---|---|
| IL-6 | Pleiotropic pro-inflammatory | Attract: | [78–85] |
| T-cells, B-cells, MOs, DCs, NKs, neutrophils, basophils (?), MCs, eosinophils | |||
| Other: | |||
| T-CD4+ regulatory cell (Treg) inhibition | |||
| Macrophage polarization to the M2 state | |||
| NK suppression | |||
| Neutrophil clearance | |||
| IL-8 | Pro-inflammatory | Attract: | [86–93] |
| T-cells, B-cells, MOs, DCs, NKs, neutrophils, basophils, MCs | |||
| Others: | |||
| Neutrophil trafficking and activation | |||
| CD8+ cytotoxic T lymphocyte (T-CD8+ CTL) activation | |||
| Basophil activation | |||
| IL-1β | Pro-inflammatory | Affect: | [94–97] |
| T-cells, NKs, neutrophils, MOs, MCs, eosinophils | |||
| Other: | |||
| MO migration and retention | |||
| T-CD4+ expansion and differentiation | |||
| NK cytotoxicity | |||
| Eosinophil and MC degranulation | |||
| TGF-β | Pleiotropic pro-inflammatory | Attract/Stimulate: | [98–100] |
| MOs, DCs, neutrophils, eosinophils, MCs (and Tregs) | |||
| Inhibit: Macrophages, DCs, T-CD8+ CTLs, B-cells, NKs, MCs | |||
| GM-CSF | Pleiotropic pro-inflammatory | Attract: | [92,101–103] |
| T-cells, MOs, DCs, neutrophils, basophils, eosinophils | |||
| Other: | |||
| Maturation of macrophages and granulocytes | |||
| Macrophage M1 polarization | |||
| DC maturation/Treg differentiation/Breg expansion | |||
| MCP-1 (CCL-2) | Pro-inflammatory | Attract: | [91,101,104–107] |
| T-cells, B-cells, MOs, DCs, NKs, neutrophils, basophils, MCs | |||
| Other: | |||
| Monocyte trafficking | |||
| T-CD8+ CTL activation | |||
| Macrophage polarization to the M2 state | |||
| DC maturation | |||
| Neutrophil activation | |||
| Basophil and MC degranulation | |||
| MIP-1α (CCL3) | Pro-inflammatory | Attract: | [92,105,108–114] |
| T-cells (T-CD8+ CTLs mainly), B-cells (few), MOs, DCs, NKs, neutrophils, basophils, MCs, eosinophils | |||
| Other: | |||
| Monocyte and NK trafficking | |||
| T-CD4+ polarization to the Th1 state | |||
| T cell-DC interaction | |||
| GROα (CXCL1) | Pro-inflammatory | Main: Neutrophil trafficking | [115,116] |
| Attract: T-cell (T-CD8+, CTLs mainly), B-cell (few), MOs, DCs, NKs, neutrophils, basophils, MCs, eosinophils | |||
| Other: | |||
| Monocyte trafficking | |||
| B-cell differentiation |
The major SASP factors, IL-6, IL-8, IL-1β, TGF-β, GM-CSF, MCP-1, MIP-1α, and GROα, have distinct effects on attracting immune cells and affecting their function. Some of their key effects are indicated. IL = Interleukin, TGF = Transforming Growth Factor, MCP = Macrophage/Monocyte Chemoattractant Protein, MIP = Macrophage Inflammatory Protein, GRO = Growth-Related Oncogene, MOs = Monocyte/Macrophage, DC = Dendritic Cell, NK = Natural Killer cell, MC = Mast Cell, T-CD8+ CTL = T-CD8+ Cytotoxic T Lymphocyte.
The main SASP factors, their functions, and how immune system cells are affected by them are shown in Table 1. Generally, secretion of type I (IFN-α, IFN-β) and II (IFN-γ) IFNs, as well as pro-inflammatory cytokines (TNF-α, IL-1β, IL-6, IL-8, IL-12, and IL-18) and chemokines (GM-CSF, CCL2, 3, 4, and 5, CXCL1, 9, 10, and 11) initiates innate responses, mediates inflammation and chemotaxis, augments NK activity, enhances HLA expression, increases phagocytosis and oxygen/nitrogen radical production, promotes eosinophil and neutrophil infiltration and action, drives maturation and activation of antigen-presenting cells (APCs), and augments cellular immunity. Immune cell receptors for SASP cytokines/chemokines have been systematically reviewed elsewhere [63].
Other SASP components such as exosomes (originating from endosomes) and ectosomes (direct budding of the membrane) can carry cytosolic and membrane constituents of SCs to distant sites [64,67,72–74]. Among these constituents are enzymes, bioactive lipids, ROS, miRNAs, and DNA fragments that can also affect immune cell function [7,67,75–77].
Cytokines have a key role in the regulation of immunity and coordinate diverse cellular responses. Cytokines can have dichotomous effects, depending on their environment, the length of time they have been acting on a cell, and the presence of other soluble factors at the sites of cytokine action. Accumulation of SCs and associated increases in SASP factor release can chronically damage the tissue in which the SCs reside and can suppress immune cell function over the long term. An example of this is IL-6. In the setting of acute tissue damage, IL-6 is pro-inflammatory, activates the anti-tumor phenotype of T-CD4+ helper cells and T-CD8+ cytotoxic lymphocytes (T-CD8+ CTLs), and suppresses anti-inflammatory T-CD4+ regulatory cells (Tregs) [117]. IL-6 also plays a role in resolution of inflammation, thereby protecting tissues against excessive injury and allowing restoration of homeostasis [118,119]. After the acute inflammatory response, IL-6 promotes a shift of T-CD4+ cells from the Th1 to Th2 state. This leads to attenuation of T-CD8+ CTL activity and generation of T-CD4+ Treg cells due to the action of anti-inflammatory DCs. IL-6 also shifts macrophages into the immune-suppressing M2 phenotype [120,121]. However, during chronic inflammation with persistent damage due to strong stimuli or impaired repair mechanisms, the anti-inflammatory reaction blocks efficient resolution of subsequent acute signals. This creates a feedback loop that spreads damage, delaying or impeding tissue regeneration [118]. Consistent with biphasic effects of cytokines in general, acute exposure to the SASP can promote regeneration, while chronic SASP exposure can inhibit regeneration and induce chronic tissue damage [71]. Although senescence has a critical role in suppressing replication of tumor cells, chronic expression of the SASP in tumors may lead to an immunosuppressive environment, stimulate tumor development, trap immune cells within the tumor, and stimulate tumor metastasis due to ROS, matrix degradation, and stimulation of angiogenesis [42,121–124].
Chronic inflammation stresses cells and these stresses can, in turn, induce senescence. Thus, a chronic pro-inflammatory SASP may promote propagation of senescence. Since SCs are resistant to apoptosis, being protected by senescent cell anti-apoptotic pathways (SCAPs) [24,26], they can endure and spread the senescent phenotype. Consistent with this, transgenic IL-10 knock-out mice (IL-10 is a cytokine with anti-inflammatory properties) have a premature aging-like phenotype and have increased SCs and SASP factors in their tissues [10]. Another example is the Nfκb1−/− mouse, which lacks suppressors of the pro-inflammatory activity of NF-kB (a major regulator of inflammation) and develops a progeroid phenotype together with SC accumulation [125,126]. Several other lines of evidence support the possibility that the SASP can promote senescence [127,128]. For example, the cytokine TNFα (implicated in systemic inflammation) is a SASP component in some cell types, such as fat cell progenitors [54]. TNFα has recently been shown to promote and sustain senescence [129]. TNFα induces ROS and activates senescence through a damage response JAK/STAT signaling pathway, which is self-reinforcing following withdrawal of TNFα [129]. The SASP is exacerbated in TNFα-mediated senescence. Thus, the SASP induced through TNFα-mediated senescence may spread senescence to adjacent cells.
3. Immune-mediated clearance of SCs
3.1. NKs
NKs remove compromised cells by inducing apoptosis through membrane receptors or by enzymatically disrupting target cell membranes. NKs recognize signs of cell dysfunction caused by damage or external pathogens through broad-affinity cell receptors, such as Toll-like receptors (TLRs) [130]. For example, in a model of liver fibrosis, hepatic stellate cells (HSCs – liver pericytes) become activated. These cells start to express alpha-smooth muscle actin (αSMA) and secrete extracellular matrix molecules that contribute to liver fibrosis [131]. These activated HSCs can become senescent-like, with up-regulation of the cell cycle inhibitors p16Ink4a and p21Cip1 and with impaired replicative capacity and accumulation of SA β-gal in lysosomes. These senescent HSCs attract NKs that clear them and promote resolution of fibrosis within 20 days [132]. The NK receptors, NKG2D and DNAM1, detect these senescent-like HSCs and induce their death through perforin exocytosis, rather than through the apoptosis-inducing death receptor, Fas ligand [33,133]. In another example, as part of the uterine changes required to support embryonic implantation, endometrial stromal cells become senescent-like. In response to the inflammation induced by these senescent-like endometrial cells, neighboring cells release IL-15 and attract NKs. These NKs then clear the SCs through NKG2D and DNAM1 receptors and induce perforin exocytosis in the same way as in the liver, thereby preparing endometrial tissue for the next phase of pregnancy [28].
3.2. Macrophages
Tissue-resident macrophages or circulating monocytes (which can differentiate into macrophages within tissues) are professional phagocytosing and antigen-presenting cells (APCs) that are attracted and stimulated by SASP factors/proteins such as MCP-1, MIP-1α, and GMCSF. MOs migrate in vitro in response to conditioned medium (CM) derived from senescent human fat cell progenitors, but not in response to CM from non-senescent fat cell progenitors [54]. MO-mediated SC clearance was first demonstrated during limb regeneration in salamanders [32]. MOs have been observed in direct contact with SCs, suggesting interaction through membrane surface receptors. Eliminating MOs prevented clearance of SCs in this model, indicating that MOs are essential for clearing SCs. The precise mechanism of SC killing by MOs is not fully understood. MOs can kill target cells by producing soluble cytotoxic factors such as ROS, TNFα, and nitric oxide in response to TLR signaling [134] or by phagocytosing Ig antibody (Ab)-coated cells (i.e., opsonization) recognized by Fc receptors [135,136]. CD47 in the membrane of target cells functions as a “don’t eat me signal” when recognized by signal regulatory protein α (SIRPα) of MOs. Loss of CD47 or disruption of CD47-clustered pattern in lipid rafts targets cells for phagocytosis, as happens to apoptotic cells and in removal of older red blood cells [137,138]. There are changes in the composition of lipid rafts in SCs [139] that may interfere with the clustering of the macromolecules acting as a “don’t eat me” signal [137]. This could stimulate or inhibit SC clearance, potentially affording SCs protection from clearance by MOs and contributing to the age-related increase in SC burden. Depending on whether MOs are in the classically-activated M1 or alternatively-activated M2 states, MOs can promote cell death through either cytotoxicity or phagocytosis, respectively. MOs in the M1 state secrete TNFα, IL-1β, and IL-6, potentially amplifying effects of the SASP [140,141]. Interactions of MOs with SCs and effects of these interactions on MO polarization warrant further investigation. The role of MOs in clearing SCs also needs to be examined more extensively.
3.3. Humoral immune system detection of SCs
The humoral immune system comprises soluble circulating components capable of recognizing a range of biomolecules and supports adaptive immune responses. Among these circulating components are proteins in the complement system, contact cascade elements, pentraxins, and naturally-occurring antibodies (NAbs) that recognize signs of cell death and signal the presence of microbial pathogens by attaching to damaged biomolecules, cell debris, and components of pathogens [142]. A NAb was found to be increased in plasma of mice after immunization with senescent lung fibroblasts. This NAb is a poly-reactive immunoglobulin that recognizes vimentin. This led to the finding that vimentin, a cytoskeletal molecule, can be exposed on the membranes of SCs, as can a form of vimentin modified by an oxidationspecific adduct, malondialdehyde (MDA) [143]. Vimentin can also be expressed on cell membranes of neutrophils, T-lymphocytes, and macrophages during apoptosis and activation [144,145] as well as when cleavage by metalloproteinases (normally present at sites of inflammation) generates “eat-me signals” that attract macrophages and enhance phagocytosis by them [146,147]. The presence of vimentin and its MDA-modified form on membranes of SCs together with opsonization of vimentin by NAbs may activate immune cells through Fc receptors (FcR), promoting phagocytosis. It is not yet clear if MDA-modified vimentin can lead to evasion of immune recognition. The NAb against vimentin is pre-produced by a subset of B-cells (B-1 cells), irrespectively of the presence of infection or other stimuli [148]. NAbs have broad reactivity to lipids, DNA, and other biomolecules, guaranteeing a reservoir of circulating Abs that can attach to dying, pathogen-infected, or malfunctioning cells, potentially including SCs. [148]. Although NAbs are poly-reactive and part of the innate immune system, future research may pinpoint specific anti-SC Abs made by adaptive B-cells. Furthermore, other as yet unknown antigenic characteristics of SCs may be discovered that could be used for identifying and targeting SCs and developing drugs and/or vaccines that eliminate SCs. This could inform the development of so-called “vaccine chips” that would be useful to rapidly evaluate the strength, type, duration, and quality of immune responses induced by such vaccines [149].
3.4. T-cells
T-cells are lymphocytes that have a unique, high-affinity, specific T-cell receptor (TCR) to a single antigenic peptide (epitope). To be activated, T lymphocytes must recognize naturally processed antigens, usually presented by APCs, and be able to retain immunological memory of prior encounters with antigens as part of the adaptive immune system. T lymphocytes include CD4+ helper cells, which orchestrate immune responses (polarization segregates T-helper cells into cells with distinct cytokine secretion profiles), CD8+ cytotoxic T lymphocytes (T-CD8+ CTLs) that promote killing similarly to NKs, and CD4+ Foxp3+ Treg cells that can suppress immune responses [150].
T-CD8+ CTLs can act through granular exocytosis as NKs, and express the same receptor, NKGD2, as that used by NKs to recognize SCs [33,151]. Therefore, T-CD8+ CTLs may contribute to immune surveillance of SCs as well. T-CD4+ cells polarized into the Th1-like profile produce cytotoxic inflammatory cytokines that could enhance the efficacy of senescence surveillance by immune cells. The Th2-like profile, on the other hand, involves secretion of IL-4 and TGF-β, which downregulate NKGD2 in NKs and T-CD8+ CTLs and, therefore, may impair SC clearance [151].
Hepatocytes in oncogene-induced tumors have a pre-malignant senescent-like phenotype, with a SASP capable of attracting T-CD4+ helper and T-CD8+ CTLs as well as neutrophils, NKs, MOs, and DCs [30,48]. In hepatic oncogene-induced tumors, the T-CD4+ cells and APCs are involved in clearing pre-malignant SCs, while the T-CD8+ CTLs and NKs, which are classically involved in tumor clearance, are not. It has been demonstrated that in subjects with cirrhosis due to hepatitis C infection, there is more SC accumulation when the subjects are also infected with HIV, which results in depletion of T-CD4+ cells. This observation indicates T helper cells are involved in SC clearance [30].
T-CD4+ Treg cells have the capacity to promote generation of senescent-like T lymphocytes in the tumor environment, with induction of DNA damage response (DDR) signaling through competing for glucose uptake [152]. These senescent T-cells cannot clear SCs, and themselves express SASP-like factors. Whether Treg cells promote senescence in other cell types has not, to our knowledge, been reported. Mechanisms of immune cell-mediated SC clearance are summarized in Table 2 and have recently been reviewed comprehensively [34,63].
Table 2.
SC Clearance by immune cells and unresolved questions.
| Sc | In vivo context | Clearance | Attracted by | Method | Reference |
|---|---|---|---|---|---|
| Hepatic stellate cells | Liver fibrosis | NKs | SASP(?) | Recognition of NKG2D. Granular exocytosis | [33,132] [63] |
| Hepatic tumor cells | Hepatic tumor | NKs | [33,48] [63] |
||
| Endometrial fibroblast decidual cells | Uterus phases | NKs | IL-15 (from neighboring cells) | Recognition of NKG2D. Granular exocytosis | [28] |
| Uterus SCs | Uterus postpartum | MOs | SASP(?) | Migrate towards SCs (TLR-mediated cytotoxicity? Ab FcR-mediated phagocytosis? Lack of CD47-induced phagocytosis?) | [31] |
| SCs cells in irradiated limbs | Limb regeneration (salamander) | MOs | SASP(?) | Direct contact (TLR-mediated cytotoxicity? Ab FcR-mediated phagocytosis? Lack of CD47-induced phagocytosis?) | [32] |
| Hepatic pre-malignant senescent-like cells | Liver oncogenic induction | T-CD4+ and APC (MOs, DCs, and HSCs) | SASP(?) | MHC/HLA class II - T-CD4+ stimulated clearance by macrophages? | [30,48]. |
| Lung senescent fibroblasts | Immunization model | Opsonization by natural antibodies (NKs, MOs?) | Vimentin and MDA- vimentin | Opsonization-mediated cytotoxicity (NKs, macrophages?), Ab FcR-mediated phagocytosis | [143] |
SCs appear to be cleared by different types of immune cells in different scenarios. We summarize some of the instances reported. Ab=Antibody, SC=Senescent Cell, MOs=Monocyte/Macrophage, DC=Dendritic Cell, NK=Natural Killer Cell, APC=Antigen Presenting Cell, IL=Interleukin, TLR=Toll-like Receptor.
4. Clearance of SCs by other immune system components
4.1. Pattern recognition receptors (PRRs)
The innate immune system conducts surveillance for signs of danger, such as cell membrane signals indicating the presence of cell damage, impending cell death, viruses, extracellular components of bacterial cell walls, or antigens expressed by fungi or helminths. Pattern recognition receptors (PRRs) trigger signaling pathways that respond to pathogen-associated molecular patterns (PAMPs – molecular signs of pathogen presence) and DAMPs (molecular signs of cell dysfunction or death) [153]. DAMPs can induce cellular senescence and can also be produced by SCs. Therefore, DAMPs are potential targets for immune cell- or humoral immune-mediated SC clearance [50]. For example, senescent fibroblasts secrete the DAMP oxidized high mobility group box 1 protein (HMGB1), which is recognized by TLR-4 and trans-membrane receptor for advanced glycation endproduct (RAGE) receptors by several immune cell types. A consequence of HMGB1 signaling, at least in macrophages, is inhibition of phagocytosis and increased secretion of IL-6 and other pro-inflammatory cytokines [154]. Furthermore, HMGB1 promotes senescence in non-senescent fibroblasts [41]. Thus, HMGB1 could protect SCs from clearance while spreading senescence, possibilities that warrant further investigation.
PRR signaling may contribute to the development of the SASP in SCs. Activation of cyclic GMP-AMP synthase (cGAS), an intracytoplasmic sensor of double-stranded DNA (dsDNA), detects genetic material of parasitic pathogens and due to reverse-transcribed transposable elements. Damage to the nuclear integrity of cells can release chromosomal DNA into the cytoplasm and trigger cGAS activity, promoting a SASP expression profile. SCs themselves have decreased nuclear lamin B1 and an unstable nuclear structure. SCs frequently contain cytoplasmic chromosomal fragments (CCF). This cytoplasmic DNA can lead to generation of a SASP [155,156]. Since bacterial or viral dsDNA can promote the SASP and the SASP itself can, in turn, reinforce and spread senescence, pathogen-infected cells might become senescent [157]. Indeed, virally-infected SCs host much less viral-induced proliferation than non-senescent cells, partly due to the anti-viral effects of SASP-generated inflammation. Supporting the suggestion that immune senescence surveillance mechanisms can limit viral infection, bleomycin-induced SCs in the lung in vivo did not contain detectible vesicular stomatitis virus (VSV), while infected non-senescent control cells had a high viral load [157].
4.2. Neutrophils
Neutrophils, which can respond to bacterial infections and DAMPs, are usually the first immune response cells to arrive at sites of inflammation [158,159]. IL-8, a major SASP cytokine, attracts neutrophils, which release microbicidal granules, liberating their cargo of nitric oxide and ROS [160]. Neutrophil recognition of humoral factors through FcR promotes phagocytosis and is related to NETosis, the cytotoxic process of releasing chromosomal DNA into the extracellular environment to attack pathogens. IL-8, TNFα, and IFNγ signaling are connected to NETosis [161]. Consistent with NETosis being related to senescence, in tumor cells made senescent by overexpressing p53, neutrophils were attracted to the tumor sites [48]. Although neutrophils may increase SC abundance by escalating inflammation due to local tissue damage, this could be counteracted by phagocytosis of SCs by neutrophils acting through FcR recognition. Neutrophil depletion with antibodies reduced SC clearance from liver, indicating that neutrophils contribute to SC surveillance [30].
4.3. Mast cells
Mast cells (MCs), which are usually tissue-resident, are rich in granules that contain histamine and other pro-inflammatory factors. MC degranulation induces permeability of blood vessels and lymphatics, stimulating migration of immune cells into the inflamed site. MCs can also boost inflammation in the absence of degranulation. Through TLRs, MCs activate the humoral immune system, attract neutrophils and eosinophils, and secrete MCP-1 and TNFα. MCs can also secrete IL-6 and IFNγ, stimulating matrix digestion and causing cytotoxicity in vascular cells [162]. MC abundance increases in several tissues during natural aging and in age-related chronic diseases in skin, blood vessels, endocrine organs, the thymus, and the liver [163–166]. Although MCs can be attracted by the SASP and facilitate migration of other immune cells involved in SC clearance, it is not clear if MCs have a direct role in clearing SCs. In the thymus during aging, MCs can interact with lipid-laden, lipofuscin-rich cells without undergoing degranulation [164]. These lipid-laden cells accumulate in the thymus with aging and may be senescent-like. During oncogene-induced accumulation of SCs in the skin, appearance of MCs has been observed, but only in old, not young mice [167]. The potential role of MCs in exacerbating the SASP warrants further investigation.
4.4. Basophils
Basophils are relatively rare cells that are associated with helminthic infections and allergies. Basophils can be activated and attracted by the DAMPs, PAMPs, cytokines, and complement factors [168] that are increased in the SC environment. Basophils can shift T-CD4+ helper cells from the pro-inflammatory Th1-like to the allergy-related Th2-like state [169], potentially downregulating the SASP. The SASP seems in general to stimulate the T-CD4+ Th1 state. There is an overall increase in Th1- vs. Th2-shifted cells during normal aging [170]. Therefore, increases in basophils could contribute to resistance against inflammaging and might be a possible target for therapeutic interventions [171].
4.5. Eosinophils
Eosinophils are, like basophils, related to allergic and anti-helminthic immune responses. SASP factors such as GM-CSF, eotaxin, IL-1β, and TNFα promote increased survival of eosinophils [159] Eosinophils also respond to the DAMPs that can be released by SCs.
Eosinophils have effector functions that are thought to contribute to the pathophysiology of asthma, including degranulation and superoxide production [172]. In chronic asthma, there is remodeling of airways associated with fibrosis. Eosinophils provide a pro-fibrotic stimulus, causing activated smooth muscle cells to produce extracellular molecules through TGF-β [173,174]. SC presence is required for lung airway remodeling (fibrosis) after injury, and blocking senescence also attenuates fibrosis. Based on these observations, it seems possible that SCs generated due to injury promote fibrosis in the lung in the same way as HSCs do in the liver. If injury responses or the SASP attract eosinophils, the activated fibrotic phenotype of senescent αSMA+ cells would be boosted. Instead of SC clearance, such eosinophils may promote an “anti-clearance” environment due to IL-4 secretion, which down-regulates expression of the NKGD2 receptor of NKs, shifts T cells into the Th2 state, and shifts MOs into the M2 profile. If this speculation is correct, eosinophils could impair immune clearance of SCs because of IL-4 and TGF-β in the setting of fibrosis [151,175]. Alternatively, since eosinophils induce switching into the Th2 state, like basophils, eosinophils may even contribute to healthy aging. However, this speculation remains to be tested.
4.6. Dendritic cells
Dendritic cells (DCs) are professional APCs that can assemble antigens from peripheral tissues and then migrate into secondary lymphoid organs and tissues, such as lymph nodes, where these DCs present the antigenic peptides to T- and B-cells, thus exerting an important role in immune surveillance. Macrophages have a similar function, but usually mostly in the tissues in which they reside [176]. DCs are potent inducers of NKs and TCTL activity and may enhance SC clearance promoted by these cell types [176]. However, in fibrotic lung airways, DCs appear to amplify the chronic environment that suppresses clearance of SCs [177]. Also, DCs can cause naïve T-CD4+ cells to become induced Treg cells (iTreg cells) in several tissues [178]. Given the already known immunosuppressive roles of Treg cells, DCs might indirectly suppress SC clearance and indirectly induce T-cell senescence, as seen in the tumor environment [179]. DCs are plastic and it is possible that DCs exacerbate the already pro-inflammatory environment in which they reside. However, the capacity of DCs to surveil the organism and migrate to lymphoid organs could make them initiators of T- and B-cell reactions against SC antigens. Indeed, it may prove to be possible to artificially activate DCs to develop cellular vaccine therapies [180].
4.7. B lymphocytes
B-cells have high-specificity and high-affinity receptors similar to those of T-cells. B-cells can also retain memory for previous infections and exposure to antigens. B-cells can produce a soluble version of their receptors, the immunoglobulins (Abs), which are secreted into the circulation. Antibodies recognize antigenic peptides on target cells and flag them for immune cell recognition and clearance. The first link connecting B-cells with possible SC clearance came from attempts to raise high-affinity Abs against SCs. However, SC clearance by B lymphocytes appears to be more closely linked to the primitive B1 cell lineage, which secretes the broad-affinity NAb that is known to react with SCs [143]. Mechanistic studies of the ability of such NAbs to mediate immune SC clearance in vivo could help in developing more highly-specific Abs with therapeutic potential.
4.8. SCs as immune cells
SCs develop in response to danger signals, the SASP of SCs impacts on multiple processes related to inflammation and cell survival in adjacent cells, and through the SASP, non-immune SC types (e.g., those originating from fibroblasts, endothelial cells, or stromal cells) modulate immune cell migration and state of activation and play a role in tissue repair, regeneration, and pathogen responses. Thus, SCs originating from cell types outside the formal immune system could essentially be considered as a component of the immune system. Consistent with this view, activated, senescent-like, αSMA+ HSCs have antigen-presenting activity in liver, can activate T cells, potently stimulate NK proliferation in vivo [181], and appear to regulate clearance of liver SCs [182]. Although the in vitro activation of HSCs into αSMA+ HSCs decreases antigen-presenting activity, this activity is rescued by type II interferon-gamma (INFγ) [183], which is highly secreted in liver fibrosis [184]. Since: (1) pre-malignant senescent HSCs in an oncogene-induced model express MHC/HLA class-II and (2) MHC/HLA class-II deficiency impairs clearance of senescent-like HSCs, perhaps SCs can present antigens/peptides in the context of liver fibrosis, a speculation meriting further study. While these senescent-like HSCs were not able to induce proliferation of T-cells as efficiently as professional immune system APCs, MHC/HLA class-II-related mechanisms appear to be relevant in regulating SC clearance [30]. Also consistent with the speculation that SCs can behave as part of the immune system, decidual stromal cells in the uterus potently inhibit DCs, preventing professional APCs from activating other immune cell types that could target implanted embryos. However, perhaps when stromal cells become senescent, they assume an APC-like activity, making clearance of SCs from the pregnant endometrium more precise, preserving the viability of the developing embryo. If these speculations are true, SCs of non-immune cell origin could induce selective activation of adaptive immune cells, such as T and B lymphocytes, through antigen processing and presentation by MHC/HLA molecules, over and above regulation of immune responses through SASP factors. These speculations about potential immune cell-like roles of SCs originating from outside the formal immune system are summarized in Fig. 1.
Fig. 1.
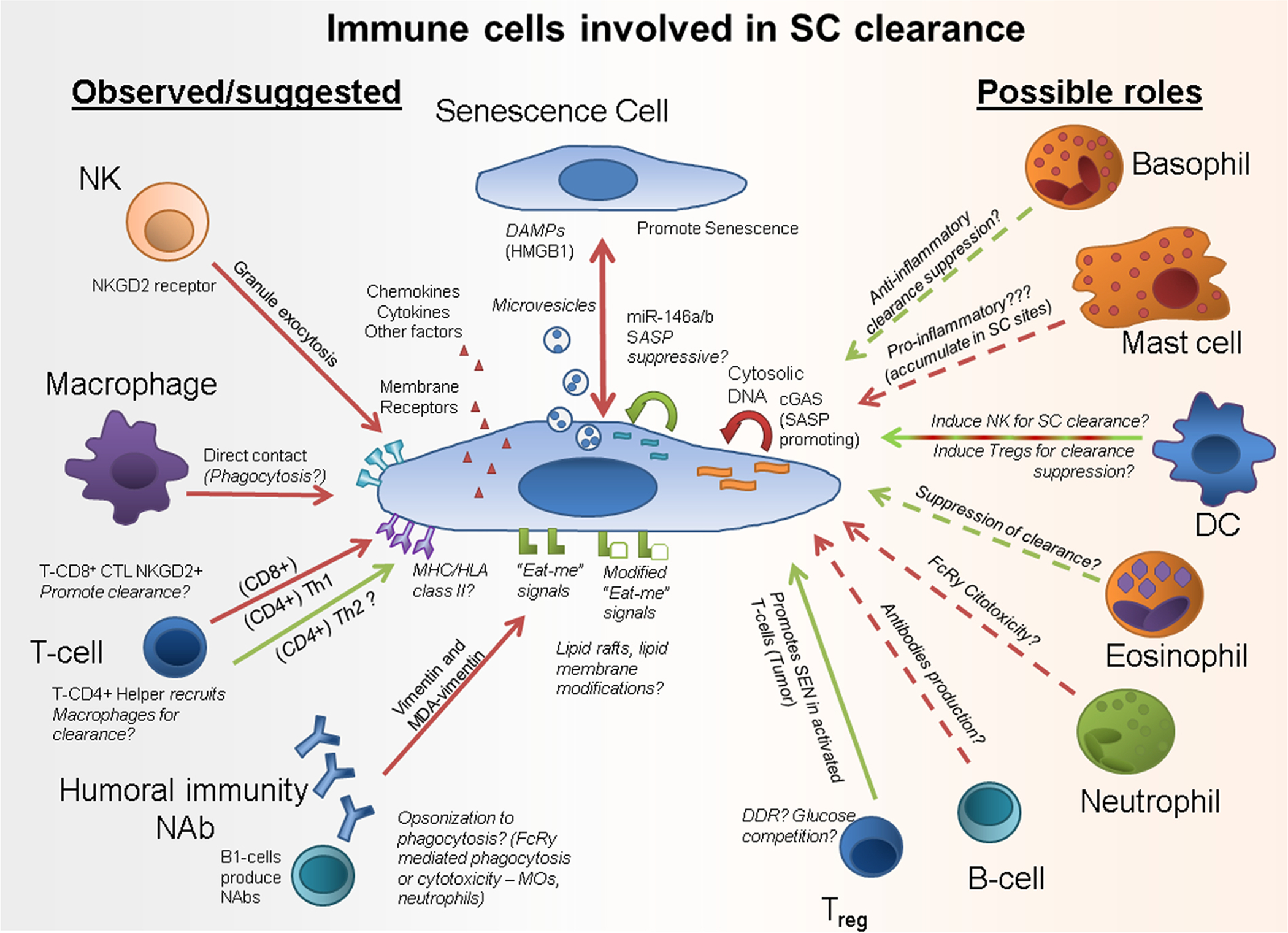
Senescent cell clearance mediated by the immune system cells: what is known and what has yet to be confirmed. Recent studies suggest several ways through which SCs may be cleared by immune cells. In a healthy immune surveillance scenario, SCs produce factors and provide signals that promote SC clearance. Interactions that could suppress SC clearance are shown in green and interactions that promote SC clearance are in red. Possible mechanisms are in Italics. NK = Natural Killer Cell; SC = Senescent Cell; DAMPs = Damage-Associated Molecular Patterns; T-CD8+ CTL = CD8+ Cytotoxic T Lymphocyte; MDA = Oxidative Adduct Malondialdehyde; Treg = T-CD4+ Regulatory Cell; SASP = Senescence-Associated Secretory Phenotype.
5. Which comes first, immune system dysfunction or SC accumulation?
Much of the work about SC surveillance has been conducted in the liver, which is immune-protected and has high regenerative potential, unlike most other organs [185]. Even though it is an immune-privileged organ, the liver is not spared from SC accumulation, such as that seen in non-alcoholic fatty liver disease, which can occur with obesity or aging [18]. SCs accumulate during chronological aging in adipose tissue, cartilage, arteries, lung, kidney, heart, skin, bone, and other tissues [12,20,24,52,54,186–190]. As SCs are generated by cellular damage and can spread senescence through their SASP (bystander senescence), SC abundance would need to exceed an immune clearance threshold in order to accumulate further. Alternatively, immune clearance could become impaired, allowing SCs to accumulate and spread.
5.1. The immune senescence hypothesis
A potential reason for the increasing SC burden that occurs with aging is that immune surveillance declines due to senescence of the immune system or its stem cell compartments [191]. Morphological or physiological changes in the differentiation niches from which new immune cells originate (bone marrow and thymus) or in tissues where immune cells reside or circulate (lymph nodes, spleen, blood, other organs) [192] could contribute to immune dysfunction [193,194] as well as intrinsic age-related changes in immune cells [192–197].
Hematopoietic niche morphology changes dramatically during aging, with the bone marrow becoming filled with bone marrow adipose tissue (MAT), bone loss, and inflammation [198]. Hematopoietic stem cells become biased toward myeloid as opposed to lymphoid lineages, potentially affecting the adaptive immune system and weakening responses to new infections and vaccines, while increasing the abundance of myeloid cells [193,194]. Altered cell localization in the spleen and lymph nodes with aging affects the cells’ access to antigens. This could interfere with migration of APCs and activation and secretion of antibodies by B-lymphocytes [58,192].
Although myeloid cell abundance is increased, differentiated myeloid cells become less effective with aging [195–197]. NK and MO chemotaxis and phagocytosis activity decreases with aging, as well as that of DCs [197,199]. In lymphocytes, co-receptors that promote sensitivity to antigen recognition become downregulated [195,200]. There is mounting evidence suggesting that with aging, memory T-CD8+ CTLs shift into a more NK-like state, becoming more frequently activated by receptors shared with NKs. This could serve to rescue surveillance in the face of decreased antigen recognition activity [200]. Understanding about senescence in immune cells remains incomplete [34,197,201]. Characteristics of immunosenescence are reviewed extensively elsewhere [34,56,202].
Immune clearance of SCs could also promote immune cell dysfunction, since debris from the SCs, including aggregated proteins, lipofuscin, nucleotides such as transposable elements or non-coding RNAs, and other persistent damage signals, may contribute to loss of immune cell function or to sustained inflammation and generation of additional SCs. Consistent with these possibilities, genetic or pharmacological clearance of SCs or targeting the SASP appears to alleviate inflammaging [15,20,23,24,54]. There is still much to learn about effects of reducing SC abundance on immune system function. Such information may be useful in devising novel therapeutic and vaccination strategies [200].
5.2. Research strategies for addressing links between SCs and immune aging
A number of questions remain about links between cellular senescence and age-related immune dysfunction. Several new experimental approaches could help provide answers. For example, transplantation of labeled non-immune SCs into younger or older recipient mice may help to identify mechanisms behind the age-related declines in the capacity of the immune system to clear SCs. Using such models, it may be feasible to determine if there is a threshold load of SCs that the immune system can clear and if such a threshold changes with age. Transplantation of labeled SCs into mice with different types of genetically-induced immune cell deficiencies could help to distinguish roles of the different immune cell types in clearing SCs. As an example, INK-ATTAC mice transplanted with wild-type bone marrow cells to create an immune system lacking the INK-ATTAC transgene could be treated with AP20187, a drug that clears p16Ink4a+ cells by dimerizing and activating the caspase-8 moieties of the ATTAC protein [203]. Such an experimental model could be used to test effects of clearing SCs from organs and tissues without affecting immune p16Ink4a+ cells, and allow investigation of consequences of this clearance on immune system function.
To address effects of senescence on immune system function, immune cell lineage-specific transgenic models may be informative. T lymphocyte immune responses in old animals were restored almost to those of young animals by suppressing the expression of p16Ink4a in T lymphocytes [204]. The opposite approach, promoting senescence in lineage-specific cells, could also be useful and could be achieved by isolating cells, irradiating them, and transplanting these autologous cells into young mice.
Organ-specific transgenic constructs that promote SC accumulation or that allow clearance of SCs from immune-related organs could be used to explore the relevance of these organs to immune system regulation of SC burden and overall aging. For example, thymic epithelial cells are responsible for maturation and selection of self-tolerant T-cells. The major sign of senescence in thymic epithelial cells is FoxN1 downregulation. Downregulation of FoxN1 gene expression in young mice promoted age-related morphological changes in the thymus and generation of dysfunctional T-cells, which are pro-inflammatory, infiltrate healthy organs, and cause increased IL-6 in the circulation, as occurs in inflammaging [205]. In this model, in which T-cells and their micro-environment were effectively “young” and the lymphoid organ where T-cells mature was “old”, T cell function was impaired [205]. Promoting senescence in immune cells isolated from young animals by sorting them, irradiating the cells to induce senescence, labeling them to allow them to be tracked, and then autologously re-implanting them, could be used to analyze effects of senescence of different immune cell types. These or other approaches need to be developed to answer fundamental questions about senescence in immune cells, the interaction of non-immune SCs with immune cells, and how these interactions affect aging and immune senescence (Fig. 2).
Fig. 2.
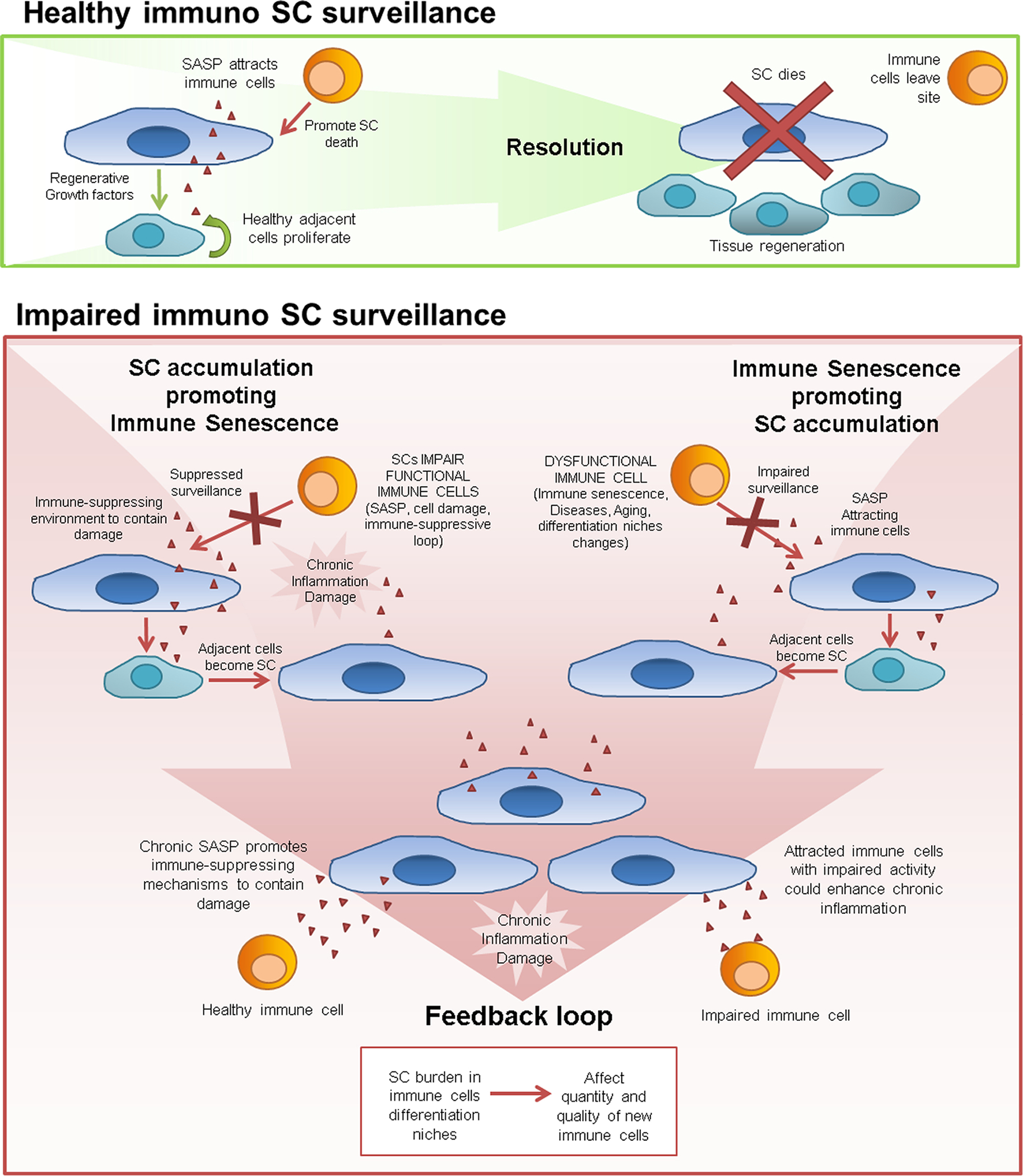
Effects of immune clearance of SCs in health and aging. Whether SC accumulation causes or is a consequence of age-related immune system dysfunction is an open question. Possible chains of events are shown. In younger individuals, there is continuing surveillance for SCs by the immune system, with clearance of SCs leading to attenuation of damage signals and the SASP. In older individuals, clearance of SCs by the immune system is impaired. SCs could accumulate and cause dysfunction of immune cells or, conversely, age-related changes in the immune system could allow SCs to accumulate. Once sufficient SCs accumulate and immune cell function becomes impaired by this SC accumulation, a feedforward cycle of further SC accumulation and immune system dysfunction might ensue.
5.3. Heterogeneous SC hypothesis
Cells of different types appear to vary in their likelihood to become senescent and in the nature of their SASP following exposure to different inducers of cellular senescence. There may also be changes over time in the composition of the SASP [206]. There could be a strong pro-inflammatory SASP developing shortly after senescence is stabilized that could progress to a low grade, chronically pro-inflammatory SASP over time, as suggested previously [43,207]. Thus, there could be differences in effects of different types and locations of SCs on immune cell function.
The existence of two states has been hypothesized to exist in non-immune SCs [43]: (1) immunogenic SCs that release immune cell-attracting SASP factors and express surface markers that promote clearance of the SCs and (2) SCs in an “anergic state”, in which the SCs do not stimulate immune responses [34]. These states may be interchangeable or fixed and may be induced by external stimuli. Anergic SCs could accumulate without causing symptoms but, during the course of aging or with disease, could become activated by triggers such as inflammation or infection, leading to their switching from an anergic state into one that is immunogenic. If so, this would amplify inflammation and potentially contribute to the more severe immune responses to infections or injuries seen in older than younger individuals. In senescence induced by the oncogene RAS, NOTCH1 signaling orchestrated a pre-SASP secretome. Initially, NOTCH1 suppressed C/EBPβ-IL1α. The SASP was mainly pro-regenerative (TGF-β, mitogens, etc.) and did not lead to attraction of immune cells. In a time-dependent manner, NOTCH1 activity was suppressed, giving rise to C/EBPβ-IL1α and classical SASP inflammatory cytokines able to attract and promote adhesion of lymphocytes to SCs [206,208]. NOTCH1 signaling seems to be part of the pre-senescence program. Its downregulation is associated with a fully senescent phenotype [206]. However, restoration of capacity to inhibit immune attraction when NOTCH1 is artificially re-induced in fully SCs suggests that there are mechanisms SCs could use to evade clearance. Though the NOTCH1 avoidance effect is mainly through downregulation of pro-inflammatory SASP factors, other mechanisms could contribute. SCs secrete microvesicles that can contain miRNAs, some of which can inhibit function of NKs and MOs, such as miRNA164a, and also inhibit expression of inflammatory cytokines (IL-6, IL-8, IL-1β, TNFα, and others) [64,72–74]. These paradoxical pro- and anti-inflammatory effects of miR146a, miR-30a, and other miRNAs secreted by SCs may have a role in SCs persistence and accumulation [67,69,74,209].
SCs can persist for years without being cleared by the immune system. For example, children can develop SCs in benign melanocytic nevi that may persist throughout their lives [4]. Whether these long-lived SCs have an attenuated SASP or develop other mechanisms to evade immune clearance is unclear [206,208]. Also, whether heterogeneity among SCs is due to differences in the signals that induced senescence, the originating cell type, time elapsed since the cell became senescent, the site where the SCs are located, exogenous signals in their microenvironment, or other mechanisms, remains to be investigated.
6. Open questions and therapeutic opportunities
Although several cytokines/chemokines that are part of the SASP can stimulate immune responses, relationships between SCs and the immune system are only beginning to become understood. Chemokine attraction of immune cells and clearance of SCs by the immune system are two of several potential interactions between SCs and immune function. SCs may be a cause of age-related immune system dysfunction, a possibility that has not been comprehensively explored. Potential interventions targeting SCs directly or through the immune system are summarized in Table 3 and described below.
Table 3.
Potential Therapies Targeting SCs, Senescence Surveillance, and the Immune-Senescence Axis.
| Treatment | Possible mechanism | Possible outcome |
|---|---|---|
| SASP inhibitors | Attenuate chronic inflammation | Recovery of immune surveillance mechanisms that were suppressed by chronic inflammation |
| Chemotherapeutic drugs | Promote senescence | Upregulate SASP factors that chemoattract immune cells and increase expression of surface markers that promote SC clearance |
| Vaccines | Induce immune clearance of SCs | Opsonization of SCs by antibodies, promoting immune recognition and clearance |
| Cellular vaccines | Induce immune clearance of SCs | Selectively sensitize autologous APC in vitro to generate high-affinity responses against SCs in vivo. |
| CAR-T | Generate T cells in vitro to attack SCs | Autologous T cells with engineered receptors against SCs |
| Senolytics (directly) | Facilitate apoptosis of SCs | Apoptosis of immune and non-immune SCs |
| Senolytics (indirectly: transplantation) | Eliminate SCs from organs or cells to be transplanted | Reduce induction of immune senescence and transplant rejection |
| Senolytics (indirectly – tumor immunotherapy) | Eliminate SCs during tumor expansion or before administering anti-tumor T cells | Improve replication kinetics and effectiveness of immunotherapy, less stimulation of immune senescence |
Based on recent findings of immune clearance of SCs and possible mechanisms linking SCs to immune senescence, potential interventions are indicated. SASP = Senescence-Associated Secretory Phenotype; SC = Senescent Cell.
6.1. SASP inhibitors
Drugs that inhibit pathways that lead to expression of constituents of the SASP by SCs, SASP inhibitors (also known as “senomorphic” drugs [17]) have been shown to alleviate aging phenotypes and cellular senescence-associated conditions. Among these agents are rapamycin, metformin, ruxolitinib, and p38MAPK inhibitors [10,17,23,50,54,210–214]. For example, alleviation of age-related osteoporosis by the JAK1/2 inhibitor, ruxolitinib, which attenuates the SASP [23,54] was found to closely parallel that by the senolytic combination, dasatinib plus quercetin, as well as that by induced genetic clearance of highly p16Ink4a-expressing cells from INK-ATTAC mice [15]. These SASP inhibitors may act in two ways. One mechanism could be by affecting the senescence-inducing secretome of SCs and subsequently the spread and accumulation of new SCs [212,215]. For example, p38 MAPK inhibitors, which have been shown to suppress the SASP in culture [216], are also able to protect cells from senescence and inhibit bystander spread of senescence in vitro [7,217]. The second mechanism could involve improvement of immune cell surveillance efficiency. If chronic inflammation can promote suppression of immune cells, then blocking inflammatory factors might rescue immune surveillance [218,219]. Indeed, rapamycin appears to rescue and enhance humoral immune responses to seasonal influenza vaccine in elderly humans [220]. However, at higher doses or after prolonged treatment, rapamycin can suppress immune responsiveness [50]. Furthermore, SASP inhibitors such as rapamycin segmentally attenuate the SASP and may not reduce production of all SASP factors. More studies of effects of SASP inhibitors on immunosenescence are needed.
6.2. Chemotherapeutic drugs
Chemotherapeutic drugs can promote a senescent-like state. For example, in myeloma cell lines, these agents can upregulate ligands for NKG2D and DNAM-1, sensitizing these senescent-like tumor cells to clearance by NKs [133]. However, senescence can also drive tumor progression and metastasis [221]. It remains to be determined if chemotherapy-induced induction of a senescent-like state in tumor cells sensitizes them to immune clearance and if this strategy can be exploited to decrease tumor burden.
6.3. Immunotherapies
It may become feasible to develop immunotherapies involving autologous transplantation of immune cells following activation of these immune cells in vitro against SC antigens. For example, chimeric antigen receptor T-cells (CAR-T) adoptive cell transfer (ACT), DC vaccines, development of Abs against MDA-vimentin, use of other Abs targeting SCs for removal by NKs, viral therapy using oncolytic viruses to enhance surveillance, and other potential immunological approaches for decreasing SC number have been reviewed recently [34,222].
6.4. Senolytics
Senolytic drugs are agents that can induce apoptosis in senescent cells while sparing non-senescent cells These include dasatinib, quercetin, navitoclax, A1331852, A1155463, fisetin, luteolin, HSP 90 inhibitors, and others being currently developed and tested [10,14,17,21,22,24,50]. Senoltyic agents act by transiently interfering with the pro-survival SCAPs that defend SCs against their own pro-apoptotic SASP as well as the increased intracellular drivers of apoptosis within senescent cells (DNA damage, ROS, cytoplasmic nucleotides, cGAS activation, etc.). Senolytic drugs are effective in delaying, preventing, or treating multiple age- and senescence-related conditions in mice [10,14–16,18,20,24,214,223,224]. Effects of senolytics on the immune system remain to be investigated. Potentially, these agents could enhance immune responsiveness by reducing SC burden. Senolytics may reduce abundance of immune cell types that produce pro-apoptotic factors by targeting them directly, although this appears not to occur in the case of activated macrophages [10]. More likely, senolytics may reduce abundance of activated immune cells indirectly through decreasing the burden of SCs that attract, anchor, and active immune cells. Whether there are direct effects of senolytics on immune organs remains to be determined.
Senolytics may have a role in reducing the impact of transplanting organs or cells from older individuals into younger recipients. SCs in organs transplanted from old donors could impede function of the recipient immune system or increase risk of transplant rejection or graft vs. host disease [225]. Before non-autologous bone marrow transplantation, recipients are treated with high doses of chemotherapy that can induce senescence and an accelerated aging-like phenotype [226]. The transplanted bone marrow cells are exposed to a microenvironment containing SCs that could compromise results of the transplantation. Potentially, intermittent treatment with senolytics may alleviate these effects, a possibility that warrants further research. Pretreatment with senolytics could turn out to increase effectiveness of vaccination, much as is the case for rapamycin (sirolimus) [220]. Senolytics might also be used to enhance effectiveness of adoptive cell transfer therapies involving use of tumor-infiltrating lymphocytes (TILs) or engineered CAR-T cells. The presence of senescent immune cells in the cell preparations used to make TILs or CAR-T cells could compromise their ability to proliferate in vitro and anti-tumor efficiency after implantation in vivo, since a fraction of these T cells could undergo replicative senescence during amplification before injection. Furthermore, some findings suggest that greater telomere length is correlated with TIL persistence and efficacy following transplantation [227,228]. Although there is controversy about effects of senescent T cells on ACT therapy [229,230], this possibility needs to be investigated further.
7. Conclusions
SCs and the SASP appear to be “root cause” contributors to multiple age-related diseases, geriatric syndromes (such as frailty), and loss of physiological resilience or ability to recover after illness, trauma, or physical stresses. Pharmacological clearance of SCs can delay, prevent, or alleviate many of these conditions and is associated with reduced tissue inflammation and fibrosis as well as increased healthspan and lifespan in mice [10,14,231]. SCs are cleared by the immune system in younger individuals, but accumulate with increasing age or disease severity. As SCs appear to differ depending on the cell type they originated from, how senescence was induced, and how long senescence persisted, there could be distinct effects on the immune system of these different types of SCs, perhaps contributing to variations in rate of SC clearance by the immune system, thereby resulting in different rates of accumulation of SCs. More research is needed regarding differences among SCs and how these differences are affected by or cause immune system dysfunction. Further work is needed about if and how SCs promote bystander senescence in immune cells and by what mechanisms. It may even be the case that SCs are an integral part of the immune system, orchestrating inflammation through attracting and anchoring formal immune cells and regulating their activation state. An understanding of the interplay between SCs and the rest of the immune system could lead to insights relevant to designing interventions to treat age-related dysfunction and chronic diseases. If senescence of the immune system indeed affects the capacity of the immune system to clear SCs, therapeutic approaches of at least two types can be envisaged: eliminating SCs directly or modulating immune responses to favor SC clearance. An understanding of the relationship between SCs and the immune system could be critical in elucidating the fundamental basis of aging changes and mechanisms accounting for development of age-related dysfunction and chronic diseases.
Acknowledgments
This work was supported by NIH grant AG13925, the Connor Group, and Robert J. and Theresa W. Ryan, and the Ted Nash Long Life and Noaber Foundations (JLK).
Abbreviations:
- Ab
antibody
- ACT
adoptive cell transfer
- APC
antigen-presenting cells
- CAR-T
chimeric antigen receptor T-cells
- CCF
cytoplasmic chromosomal fragments
- cGAS
cyclic GMP-AMP synthase
- CM
conditioned medium
- DAMP
damage-associated molecular pattern
- DC
dendritic cell
- DDR
DNA damage response
- dsDNA
double-stranded DNA
- HMGB1
high mobility group box 1 protein
- HSCs
hepatic stellate cells
- MAT
bone marrow adipose tissue
- MC
mast cells
- MDA
malondialdehyde
- MO
monocytes/macrophages
- NAb
naturally-occurring antibody
- NK
natural killer cell
- PAMPs
pathogen-associated molecular patterns
- PRR
pattern recognition receptors
- RAGE
receptor for advanced glycation endproducts
- ROS
reactive oxygen species
- SA β-gal
senescence-associated β-galactosidase
- SASP
senescence-associated secretory phenotype
- SC
senescent cells
- SCAP
senescent cell anti-apoptotic pathways
- SIRPα
signal regulatory protein α
- TCR
T-cell receptor
- T-CD8+ CTL
CD8+ cytotoxic T lymphocyte
- TIL
tumor-infiltrating lymphocytes
- TLR
toll-like receptor
- Treg
T-CD4+ regulatory cell
- VSV
vesicular stomatitis virus
- αSMA
alpha-smooth muscle actin
Footnotes
Competing financial interests
J.L.K. and T.T. have a financial interest related to this research. Patents on senolytic drugs are held by Mayo Clinic. I.G.O. holds three patents related to vaccinia and measles peptide research. This research has been reviewed by the Mayo Clinic Conflict of Interest Review Board and was conducted in compliance with Mayo Clinic Conflict of Interest policies. L.L.P. does not have relevant financial conflicts of interest.
References
- 1.Hayflick L, The limited in vitro lifetime of human diploid cell strains, Exp. Cell Res 37 (1965) 614–636. [DOI] [PubMed] [Google Scholar]
- 2.Stein GH, Dulic V, Origins of G1 arrest in senescent human fibroblasts, Bioessays 17 (6) (1995) 537–543. [DOI] [PubMed] [Google Scholar]
- 3.Gire V, Dulić V, Senescence from G2 arrest, revisited, Cell Cycle 14 (3) (2015) 297–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Michaloglou C, Vredeveld LC, Soengas MS, Denoyelle C, Kuilman T, van der Horst CM, Majoor DM, Shay JW, Mooi WJ, Peeper DS, BRAFE600-associated senescence-like cell cycle arrest of human naevi, Nature 436 (7051) (2005) 720–724. [DOI] [PubMed] [Google Scholar]
- 5.Wang C, Jurk D, Maddick M, Nelson G, Martin-Ruiz C, von Zglinicki T, DNA damage response and cellular senescence in tissues of aging mice, Aging Cell 8 (3) (2009) 311–323. [DOI] [PubMed] [Google Scholar]
- 6.Sapieha P, Mallette FA, Cellular senescence in postmitotic cells: beyond growth arrest, Trends Cell Biol (2018). [DOI] [PubMed] [Google Scholar]
- 7.Nelson G, Wordsworth J, Wang C, Jurk D, Lawless C, Martin-Ruiz C, von Zglinicki T, A senescent cell bystander effect: senescence-induced senescence, Aging Cell 11 (2) (2012) 345–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acosta JC, Banito A, Wuestefeld T, Georgilis A, Janich P, Morton JP, Athineos D, Kang TW, Lasitschka F, Andrulis M, Pascual G, Morris KJ, Khan S, Jin H, Dharmalingam G, Snijders AP, Carroll T, Capper D, Pritchard C, Inman GJ, Longerich T, Sansom OJ, Benitah SA, Zender L, Gil J, A complex secretory program orchestrated by the inflammasome controls paracrine senescence, Nat. Cell Biol 15 (8) (2013) 978–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Justice JN, Gregory H, Tchkonia T, LeBrasseur NK, Kirkland JL, Kritchevsky SB, Nicklas BJ, Cellular senescence biomarker p16INK4a+ cell burden in thigh adipose is associated with poor physical function in older women, J. Gerontol. A: Biol. Sci. Med. Sci 73 (7) (2018) 939–945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xu M, Pirtskhalava T, Farr JN, Weigand BM, Palmer AK, Weivoda MM, Inman CL, Ogrodnik MB, Hachfeld CM, Fraser DG, Onken JL, Johnson KO, Verzosa GC, Langhi LGP, Weigl M, Giorgadze N, LeBrasseur NK, Miller JD, Jurk D, Singh RJ, Allison DB, Ejima K, Hubbard GB, Ikeno Y, Cubro H, Garovic VD, Hou XN, Weroha SJ, Robbins PD, Niedernhofer LJ, Khosla S, Tchkonia T, Kirkland JL, Senolytics improve physical function and increase lifespan in old age, Nat. Med 24 (8) (2018) 1246–+. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xu M, Bradley EW, Weivoda MM, Hwang SM, Pirtskhalava T, Decklever T, Curran GL, Ogrodnik M, Jurk D, Johnson KO, Lowe V, Tchkonia T, Westendorf JJ, Kirkland JL, Transplanted senescent cells induce an osteoarthritis-like condition in mice, J. Gerontol. A: Biol. Sci. Med. Sci 72 (6) (2017) 780–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S, Identification of senescent cells in the bone microenvironment, J. Bone Miner. Res 31 (11) (2016) 1920–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tchkonia T, Kirkland JL, Aging, cell senescence, and chronic disease: emerging therapeutic strategies, JAMA 320 (13) (2018) 1319–1320. [DOI] [PubMed] [Google Scholar]
- 14.Yousefzadeh MJ, Zhu Y, McGowan SJ, Angelini L, Fuhrmann-Stroissnigg H, Xu M, Ling YY, Melos KI, Pirtskhalava T, Inman CL, McGuckian C, Wade EA, Kato JI, Grassi D, Wentworth M, Burd CE, Arriaga EA, Ladiges WL, Tchkonia T, Kirkland JL, Robbins PD, Niedernhofer LJ, Fisetin is a senotherapeutic that extends health and lifespan, EBioMedicine 36 (2018) 18–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S, Targeting cellular senescence prevents age-related bone loss in mice, Nat. Med 23 (9) (2017) 1072–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Moncsek A, Al-Suraih MS, Trussoni CE, O’Hara SP, Splinter PL, Zuber C, Patsenker E, Valli PV, Fingas CD, Weber A, Zhu Y, Tchkonia T, Kirkland JL, Gores GJ, Mullhaupt B, LaRusso NF, Mertens JC, Targeting senescent cholangiocytes and activated fibroblasts with B-cell lymphoma-extra large inhibitors ameliorates fibrosis in multidrug resistance 2 gene knockout (Mdr2(−/−)) mice, Hepatology 67 (1) (2018) 247–259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fuhrmann-Stroissnigg H, Ling YY, Zhao J, McGowan SJ, Zhu Y, Brooks RW, Grassi D, Gregg SQ, Stripay JL, Dorronsoro A, Corbo L, Tang P, Bukata C, Ring N, Giacca M, Li X, Tchkonia T, Kirkland JL, Niedernhofer LJ, Robbins PD, Identification of HSP90 inhibitors as a novel class of senolytics, Nat. Commun 8 (1) (2017) 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ogrodnik M, Miwa S, Tchkonia T, Tiniakos D, Wilson CL, Lahat A, Day CP, Burt A, Palmer A, Anstee QM, Grellscheid SN, Hoeijmakers JHJ, Barnhoorn S, Mann DA, Bird TG, Vermeij WP, Kirkland JL, Passos JF, von Zglinicki T, Jurk D, Cellular senescence drives age-dependent hepatic steatosis, Nat. Commun 8 (2017) 15691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schafer MJ, White TA, Iijima K, Haak AJ, Ligresti G, Atkinson EJ, Oberg AL, Birch J, Salmonowicz H, Zhu Y, Mazula DL, Brooks RW, Fuhrmann-Stroissnigg H, Pirtskhalava T, Prakash YS, Tchkonia T, Robbins PD, Aubry MC, Passos JF, Kirkland JL, Tschumperlin DJ, Kita H, LeBrasseur NK, Cellular senescence mediates fibrotic pulmonary disease, Nat. Commun 8 (2017) 14532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roos CM, Zhang B, Palmer AK, Ogrodnik MB, Pirtskhalava T, Thalji NM, Hagler M, Jurk D, Smith LA, Casaclang-Verzosa G, Zhu Y, Schafer MJ, Tchkonia T, Kirkland JL, Miller JD, Chronic senolytic treatment alleviates established vasomotor dysfunction in aged or atherosclerotic mice, Aging Cell 15 (5) (2016) 973–977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhu Y, Doornebal EJ, Pirtskhalava T, Giorgadze N, Wentworth M, Fuhrmann-Stroissnigg H, Niedernhofer LJ, Robbins PD, Tchkonia T, Kirkland JL, New agents that target senescent cells: the flavone, fisetin, and the BCL-XL inhibitors, A1331852 and A1155463, Aging (Albany NY) 9 (3) (2017) 955–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu Y, Tchkonia T, Fuhrmann-Stroissnigg H, Dai HM, Ling YY, Stout MB, Pirtskhalava T, Giorgadze N, Johnson KO, Giles CB, Wren JD, Niedernhofer LJ, Robbins PD, Kirkland JL, Identification of a novel senolytic agent, navitoclax, targeting the Bcl-2 family of anti-apoptotic factors, Aging Cell 15 (3) (2016) 428–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xu M, Palmer AK, Ding H, Weivoda MM, Pirtskhalava T, White TA, Sepe A, Johnson KO, Stout MB, Giorgadze N, Jensen MD, LeBrasseur NK, Tchkonia T, Kirkland JL, Targeting senescent cells enhances adipogenesis and metabolic function in old age, Elife 4 (2015) e12997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhu Y, Tchkonia T, Pirtskhalava T, Gower AC, Ding H, Giorgadze N, Palmer AK, Ikeno Y, Hubbard GB, Lenburg M, O’Hara SP, LaRusso NF, Miller JD, Roos CM, Verzosa GC, LeBrasseur NK, Wren JD, Farr JN, Khosla S, Stout MB, McGowan SJ, Fuhrmann-Stroissnigg H, Gurkar AU, Zhao J, Colangelo D, Dorronsoro A, Ling YY, Barghouthy AS, Navarro DC, Sano T, Robbins PD, Niedernhofer LJ, Kirkland JL, The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs, Aging Cell 14 (4) (2015) 644–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Macieira-Coelho A, Ponten J, Philipson L, Inhibition of the division cycle in confluent cultures of human fibroblasts in vitro, Exp. Cell Res 43 (1) (1966) 20–29. [DOI] [PubMed] [Google Scholar]
- 26.Wang E, Liu D, Characterization of senescence- and apoptosis-dependent forms of terminin as derived from a precursor found in replicating and nonreplicating cells, J. Cell. Biochem 60 (1) (1996) 107–120. [DOI] [PubMed] [Google Scholar]
- 27.Marcotte R, Lacelle C, Wang E, Senescent fibroblasts resist apoptosis by downregulating caspase-3, Mech. Ageing Dev 125 (10–11) (2004) 777–783. [DOI] [PubMed] [Google Scholar]
- 28.Brighton PJ, Maruyama Y, Fishwick K, Vrljicak P, Tewary S, Fujihara R, Muter J, Lucas ES, Yamada T, Woods L, Lucciola R, Hou Lee Y, Takeda S, Ott S, Hemberger M, Quenby S, Brosens JJ, Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium, Elife 6 (2017) e31274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Munoz-Espin D, Canamero M, Maraver A, Gomez-Lopez G, Contreras J, Murillo-Cuesta S, Rodriguez-Baeza A, Varela-Nieto I, Ruberte J, Collado M, Serrano M, Programmed cell senescence during mammalian embryonic development, Cell 155 (5) (2013) 1104–1118. [DOI] [PubMed] [Google Scholar]
- 30.Kang TW, Yevsa T, Woller N, Hoenicke L, Wuestefeld T, Dauch D, Hohmeyer A, Gereke M, Rudalska R, Potapova A, Iken M, Vucur M, Weiss S, Heikenwalder M, Khan S, Gil J, Bruder D, Manns M, Schirmacher P, Tacke F, Ott M, Luedde T, Longerich T, Kubicka S, Zender L, Senescence surveillance of pre-malignant hepatocytes limits liver cancer development, Nature 479 (7374) (2011) 547–551. [DOI] [PubMed] [Google Scholar]
- 31.Egashira M, Hirota Y, Shimizu-Hirota R, Saito-Fujita T, Haraguchi H, Matsumoto L, Matsuo M, Hiraoka T, Tanaka T, Akaeda S, Takehisa C, Saito-Kanatani M, Maeda KI, Fujii T, Osuga Y, F4/80+ macrophages contribute to clearance of senescent cells in the mouse postpartum uterus, Endocrinology 158 (7) (2017) 2344–2353. [DOI] [PubMed] [Google Scholar]
- 32.Yun MH, Davaapil H, Brockes JP, Recurrent turnover of senescent cells during regeneration of a complex structure, Elife 4 (2015) e05505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sagiv A, Biran A, Yon M, Simon J, Lowe SW, Krizhanovsky V, Granule exocytosis mediates immune surveillance of senescent cells, Oncogene 32 (15) (2013) 1971–1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burton DGA, Stolzing A, Cellular senescence: immunosurveillance and future immunotherapy, Ageing Res. Rev 43 (2018) 17–25. [DOI] [PubMed] [Google Scholar]
- 35.Yang OO, Lin H, Dagarag M, Ng HL, Effros RB, Uittenbogaart CH, Decreased perforin and granzyme B expression in senescent HIV-1-specific cytotoxic T lymphocytes, Virology 332 (1) (2005) 16–19. [DOI] [PubMed] [Google Scholar]
- 36.James EL, Michalek RD, Pitiyage GN, de Castro AM, Vignola KS, Jones J, Mohney RP, Karoly ED, Prime SS, Parkinson EK, Senescent human fibroblasts show increased glycolysis and redox homeostasis with extracellular metabolomes that overlap with those of irreparable DNA damage, aging, and disease, J. Proteome Res 14 (4) (2015) 1854–1871. [DOI] [PubMed] [Google Scholar]
- 37.Chen QM, Tu VC, Catania J, Burton M, Toussaint O, Dilley T, Involvement of Rb family proteins, focal adhesion proteins and protein synthesis in senescent morphogenesis induced by hydrogen peroxide, J. Cell Sci 113 (Pt 22) (2000) 4087–4097. [DOI] [PubMed] [Google Scholar]
- 38.Angello JC, Pendergrass WR, Norwood TH, Prothero J, Cell enlargement: one possible mechanism underlying cellular senescence, J. Cell Physiol 140 (2) (1989) 288–294. [DOI] [PubMed] [Google Scholar]
- 39.Narita M, Nuñez S, Heard E, Narita M, Lin AW, Hearn SA, Spector DL, Hannon GJ, Lowe SW, Rb-mediated heterochromatin formation and silencing of e2f target genes during cellular senescence, Cell 113 (6) (2003) 703–716. [DOI] [PubMed] [Google Scholar]
- 40.Lujambio A, To clear, or not to clear (senescent cells)? That is the question, Bioessays 38 (Suppl. 1) (2016) S56–S64. [DOI] [PubMed] [Google Scholar]
- 41.Davalos AR, Kawahara M, Malhotra GK, Schaum N, Huang J, Ved U, Beausejour CM, Coppe JP, Rodier F, Campisi J, p53-dependent release of Alarmin HMGB1 is a central mediator of senescent phenotypes, J. Cell Biol 201 (4) (2013) 613–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Coppe JP, Patil CK, Rodier F, Sun Y, Munoz DP, Goldstein J, Nelson PS, Desprez PY, Campisi J, Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor, PLoS Biol 6 (12) (2008) 2853–2868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hernandez-Segura A, de Jong TV, Melov S, Guryev V, Campisi J, Demaria M, Unmasking transcriptional heterogeneity in senescent cells, Curr. Biol 27 (17) (2017) 2652–2660 e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kuilman T, Michaloglou C, Vredeveld LC, Douma S, van Doorn R, Desmet CJ, Aarden LA, Mooi WJ, Peeper DS, Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network, Cell 133 (6) (2008) 1019–1031. [DOI] [PubMed] [Google Scholar]
- 45.Demaria M, Ohtani N, Youssef SA, Rodier F, Toussaint W, Mitchell JR, Laberge RM, Vijg J, Van Steeg H, Dolle ME, Hoeijmakers JH, de Bruin A, Hara E, Campisi J, An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA, Dev. Cell 31 (6) (2014) 722–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Borodkina AV, Deryabin PI, Giukova AA, Nikolsky NN, “Social life” of senescent cells: what is sasp and why study it? Acta Nat 10 (1) (2018) 4–14. [PMC free article] [PubMed] [Google Scholar]
- 47.Jun JI, Lau LF, The matricellular protein CCN1 induces fibroblast senescence and restricts fibrosis in cutaneous wound healing, Nat. Cell Biol 12 (7) (2010) 676–685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Xue W, Zender L, Miething C, Dickins RA, Hernando E, Krizhanovsky V, Cordon-Cardo C, Lowe SW, Senescence and tumour clearance is triggered by p53 restoration in murine liver carcinomas, Nature 445 (7128) (2007) 656–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sharpless NE, Bardeesy N, Lee KH, Carrasco D, Castrillon DH, Aguirre AJ, Wu EA, Horner JW, DePinho RA, Loss of p16Ink4a with retention of p19Arf predisposes mice to tumorigenesis, Nature 413 (6851) (2001) 86–91. [DOI] [PubMed] [Google Scholar]
- 50.Kirkland JL, Tchkonia T, Cellular senescence: a translational perspective, EBioMedicine 21 (2017) 21–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Milanovic M, Fan DNY, Belenki D, Dabritz JHM, Zhao Z, Yu Y, Dorr JR, Dimitrova L, Lenze D, Monteiro Barbosa IA, Mendoza-Parra MA, Kanashova T, Metzner M, Pardon K, Reimann M, Trumpp A, Dorken B, Zuber J, Gronemeyer H, Hummel M, Dittmar G, Lee S, Schmitt CA, Senescence-associated reprogramming promotes cancer stemness, Nature 553 (7686) (2018) 96–100. [DOI] [PubMed] [Google Scholar]
- 52.Ressler S, Bartkova J, Niederegger H, Bartek J, Scharffetter-Kochanek K, Jansen-Durr P, Wlaschek M, p16INK4A is a robust in vivo biomarker of cellular aging in human skin, Aging Cell 5 (5) (2006) 379–389. [DOI] [PubMed] [Google Scholar]
- 53.Jeyapalan JC, Ferreira M, Sedivy JM, Herbig U, Accumulation of senescent cells in mitotic tissue of aging primates, Mech. Ageing Dev 128 (1) (2007) 36–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xu M, Tchkonia T, Ding H, Ogrodnik M, Lubbers ER, Pirtskhalava T, White TA, Johnson KO, Stout MB, Mezera V, Giorgadze N, Jensen MD, LeBrasseur NK, Kirkland JL, JAK inhibition alleviates the cellular senescence-associated secretory phenotype and frailty in old age, Proc. Natl. Acad. Sci. U. S. A 112 (46) (2015) E6301–E6310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Minamino T, Orimo M, Shimizu I, Kunieda T, Yokoyama M, Ito T, Nojima A, Nabetani A, Oike Y, Matsubara H, Ishikawa F, Komuro I, A crucial role for adipose tissue p53 in the regulation of insulin resistance, Nat. Med 15 (9) (2009) 1082–1087. [DOI] [PubMed] [Google Scholar]
- 56.Ray D, Yung R, Immune senescence, epigenetics and autoimmunity, Clin. Immunol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chinn IK, Blackburn CC, Manley NR, Sempowski GD, Changes in primary lymphoid organs with aging, Sem. Immunol 24 (5) (2012) 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Thompson HL, Smithey MJ, Surh CD, Nikolich-Žugich J, Functional and homeostatic impact of age-related changes in lymph node stroma, Front. Immunol 8 (2017) 706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Franceschi C, Bonafe M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G, Inflamm-aging. An evolutionary perspective on immunosenescence, Ann. N. Y. Acad. Sci 908 (2000) 244–254. [DOI] [PubMed] [Google Scholar]
- 60.Tchkonia T, Zhu Y, van Deursen J, Campisi J, Kirkland JL, Cellular senescence and the senescent secretory phenotype: therapeutic opportunities, J. Clin. Invest 123 (3) (2013) 966–972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Prattichizzo F, Giuliani A, Recchioni R, Bonafe M, Marcheselli F, De Carolis S, Campanati A, Giuliodori K, Rippo MR, Bruge F, Tiano L, Micucci C, Ceriello A, Offidani A, Procopio AD, Olivieri F, Anti-TNF-alpha treatment modulates SASP and SASP-related microRNAs in endothelial cells and in circulating angiogenic cells, Oncotarget 7 (11) (2016) 11945–11958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Coppe JP, Desprez PY, Krtolica A, Campisi J, The senescence-associated secretory phenotype: the dark side of tumor suppression, Annu. Rev. Pathol 5 (2010) 99–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sagiv A, Krizhanovsky V, Immunosurveillance of senescent cells: the bright side of the senescence program, Biogerontology 14 (6) (2013) 617–628. [DOI] [PubMed] [Google Scholar]
- 64.Bhaumik D, Scott GK, Schokrpur S, Patil CK, Orjalo AV, Rodier F, Lithgow GJ, Campisi J, MicroRNAs miR-146a/b negatively modulate the senescence-associated inflammatory mediators IL-6 and IL-8, Aging (Albany NY) 1 (4) (2009) 402–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Freund A, Orjalo AV, Desprez P-Y, Campisi J, Inflammatory networks during cellular senescence: causes and consequences, Trends Mol. Med 16 (5) (2010) 238–246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawless C, Wang C, Jurk D, Merz A, Zglinicki T, Passos JF, Quantitative assessment of markers for cell senescence, Exp. Gerontol 45 (10) (2010) 772–778. [DOI] [PubMed] [Google Scholar]
- 67.Lei Q, Liu T, Gao F, Xie H, Sun L, Zhao A, Ren W, Guo H, Zhang L, Wang H, Chen Z, Guo AY, Li Q, Microvesicles as potential biomarkers for the identification of senescence in human mesenchymal stem cells, Theranostics 7 (10) (2017) 2673–2689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Venable ME, Lee JY, Smyth MJ, Bielawska A, Obeid LM, Role of ceramide in cellular senescence, J. Biol. Chem 270 (51) (1995) 30701–30708. [DOI] [PubMed] [Google Scholar]
- 69.Terlecki-Zaniewicz L, Lammermann I, Latreille J, Bobbili MR, Pils V, Schosserer M, Weinmullner R, Dellago H, Skalicky S, Pum D, Almaraz JCH, Scheideler M, Morizot F, Hackl M, Gruber F, Grillari J, Small extracellular vesicles and their miRNA cargo are anti-apoptotic members of the senescence-associated secretory phenotype, Aging (Albany NY) 10 (5) (2018) 1103–1132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Munoz-Espin D, Serrano M, Cellular senescence: from physiology to pathology, Nat. Rev. Mol. Cell. Biol 15 (7) (2014) 482–496. [DOI] [PubMed] [Google Scholar]
- 71.Ritschka B, Storer M, Mas A, Heinzmann F, Ortells MC, Morton JP, Sansom OJ, Zender L, Keyes WM, The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration, Genes Dev 31 (2) (2017) 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Huang C, Liu XJ, QunZhou, Xie J, Ma TT, Meng XM, J LiF, MiR-146a modulates macrophage polarization by inhibiting Notch1 pathway in RAW264.7 macrophages, Int. Immunopharmacol 32 (2016) 46–54. [DOI] [PubMed] [Google Scholar]
- 73.Olivieri F, Lazzarini R, Recchioni R, Marcheselli F, Rippo MR, Di Nuzzo S, Albertini MC, Graciotti L, Babini L, Mariotti S, Spada G, Abbatecola AM, Antonicelli R, Franceschi C, Procopio AD, MiR-146a as marker of senescence-associated pro-inflammatory status in cells involved in vascular remodelling, Age (Dordr) 35 (4) (2013) 1157–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Xu D, Han Q, Hou Z, Zhang C, Zhang J, miR-146a negatively regulates NK cell functions via STAT1 signaling, Cell. Mol. Immunol 14 (8) (2017) 712–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chiurchiù V, Leuti A, Maccarrone M, Bioactive lipids and chronic inflammation: managing the fire within, Front. Immunol 9 (2018) 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Olivieri F, Rippo MR, Monsurro V, Salvioli S, Capri M, Procopio AD, Franceschi C, MicroRNAs linking inflamm-aging, cellular senescence and cancer, Ageing Res. Rev 12 (4) (2013) 1056–1068. [DOI] [PubMed] [Google Scholar]
- 77.Barber GN, STING: infection, inflammation and cancer, nature reviews, Immunology 15 (12) (2015) 760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tanaka T, Narazaki M, Kishimoto T, IL-6 in inflammation, immunity, and disease, Cold Spring Harb. Perspect. Biol 6 (10) (2014) a016295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Mauer J, Chaurasia B, Goldau J, Vogt MC, Ruud J, Nguyen KD, Theurich S, Hausen AC, Schmitz J, Brönneke HS, Estevez E, Allen TL, Mesaros A, Partridge L, Febbraio MA, Chawla A, Wunderlich FT, Brüning JC, Signaling by IL-6 promotes alternative activation of macrophages to limit endotoxemia and obesity-associated resistance to insulin, Nat. Immunol 15 (2014) 423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fuster JJ, Walsh K, The good, the bad, and the ugly of interleukin-6 signaling, EMBO J 33 (13) (2014) 1425–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Fielding CA, McLoughlin RM, McLeod L, Colmont CS, Najdovska M, Grail D, Ernst M, Jones SA, Topley N, Jenkins BJ, IL-6 regulates neutrophil trafficking during acute inflammation via STAT3, J. Immunol 181 (3) (2008) 2189–2195. [DOI] [PubMed] [Google Scholar]
- 82.Vredevoe DL, Widawski M, Fonarow GC, Hamilton M, Martinez-Maza O, Gage JR, Interleukin-6 (IL-6) expression and natural killer (NK) cell dysfunction and anergy in heart failure, Am. J. Cardiol 93 (8) (2004) 1007–1011. [DOI] [PubMed] [Google Scholar]
- 83.Doganci A, Eigenbrod T, Krug N, De Sanctis GT, Hausding M, Erpenbeck VJ, Haddad el B, Lehr HA, Schmitt E, Bopp T, Kallen KJ, Herz U, Schmitt S, Luft C, Hecht O, Hohlfeld JM, Ito H, Nishimoto N, Yoshizaki K, Kishimoto T, Rose-John S, Renz H, Neurath MF, Galle PR, Finotto S, The IL-6R alpha chain controls lung CD4+CD25+ Treg development and function during allergic airway inflammation in vivo, J. Clin. Invest 115 (2) (2005) 313–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Okayama Y, Kawakami T, Development, migration, and survival of mast cells, Immunol. Res 34 (2) (2006) 97–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Heink S, Yogev N, Garbers C, Herwerth M, Aly L, Gasperi C, Husterer V, Croxford AL, Möller-Hackbarth K, Bartsch HS, Sotlar K, Krebs S, Regen T, Blum H, Hemmer B, Misgeld T, Wunderlich TF, Hidalgo J, Oukka M, Rose-John S, Schmidt-Supprian M, Waisman A, Korn T, Trans-presentation of inter-leukin-6 by dendritic cells is required for priming pathogenic T(H)17 cells, Nat. Immunol 18 (1) (2017) 74–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.David JM, Dominguez C, Hamilton DH, Palena C, The IL-8/IL-8R axis: a double agent in tumor immune resistance, Vaccines (Basel) 4 (3) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gesser B, Lund M, Lohse N, Vestergaad C, Matsushima K, Sindet-Pedersen S, Jensen SL, Thestrup-Pedersen K, Larsen CG, IL-8 induces T cell chemotaxis, suppresses IL-4, and up-regulates IL-8 production by CD4+ T cells, J. Leukoc. Biol 59 (3) (1996) 407–411. [DOI] [PubMed] [Google Scholar]
- 88.Krieger M, Brunner T, Bischoff SC, von Tscharner V, Walz A, Moser B, Baggiolini M, Dahinden CA, Activation of human basophils through the IL-8 receptor, J. Immunol 149 (8) (1992) 2662–2667. [PubMed] [Google Scholar]
- 89.Petering H, Götze O, Kimmig D, Smolarski R, Kapp A, Elsner J, The biologic role of interleukin-8: functional analysis and expression of CXCR1 and CXCR2 on human eosinophils, Blood 93 (2) (1999) 694–702. [PubMed] [Google Scholar]
- 90.Lima M, Leander M, Santos M, Santos AH, Lau C, Queirós ML, Gonçalves M, Fonseca S, Moura J, Teixeira MA, Orfao A, Chemokine receptor expression on normal blood CD56(+) NK-cells elucidates cell partners that comigrate during the innate and adaptive immune responses and identifies a transitional NK-cell population, J. Immunol. Res 2015 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Alter A, Duddy M, Hebert S, Biernacki K, Prat A, Antel JP, Yong VW, Nuttall RK, Pennington CJ, Edwards DR, Bar-Or A, Determinants of human B cell migration across brain endothelial cells, J. Immunol 170 (9) (2003) 4497–4505. [DOI] [PubMed] [Google Scholar]
- 92.Schneider E, Thieblemont N, De Moraes ML, Dy M, Basophils: new players in the cytokine network, Eur. Cytokine Netw 21 (3) (2010) 142–153. [DOI] [PubMed] [Google Scholar]
- 93.Hess C, Means TK, Autissier P, Woodberry T, Altfeld M, Addo MM, Frahm N, Brander C, Walker BD, Luster AD, IL-8 responsiveness defines a subset of CD8 T cells poised to kill, Blood 104 (12) (2004) 3463–3471. [DOI] [PubMed] [Google Scholar]
- 94.Arango Duque G, Descoteaux A, Macrophage cytokines: involvement in immunity and infectious diseases, Front. Immunol 5 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Whitcomb E, Dinarello C, Pincus S, Differential effects of interleukin-1 alpha and interleukin-1 beta on human peripheral blood eosinophils, Blood 73 (7) (1989) 1904–1908. [PubMed] [Google Scholar]
- 96.Rider P, Carmi Y, Guttman O, Braiman A, Cohen I, Voronov E, White MR, Dinarello CA, Apte RN, IL-1alpha and IL-1beta recruit different myeloid cells and promote different stages of sterile inflammation, J. Immunol 187 (9) (2011) 4835–4843. [DOI] [PubMed] [Google Scholar]
- 97.Cooper MA, Fehniger TA, Ponnappan A, Mehta V, Wewers MD, Caligiuri MA, Interleukin-1beta costimulates interferon-gamma production by human natural killer cells, Eur. J. Immunol 31 (3) (2001) 792–801. [DOI] [PubMed] [Google Scholar]
- 98.Li MO, Wan YY, Sanjabi S, Robertson AK, Flavell RA, Transforming growth factor-beta regulation of immune responses, Annu. Rev. Immunol 24 (1) (2006) 99–146. [DOI] [PubMed] [Google Scholar]
- 99.Fernando J, Ryan J, TGF beta suppresses mast cell function in vivo and in vitro, J. Immunol 182 (1 Suppl) (2009) 80.12. [Google Scholar]
- 100.Worthington JJ, Fenton TM, Czajkowska BI, Klementowicz JE, Travis MA, Regulation of TGFβ in the immune system: an emerging role for integrins and dendritic cells, Immunobiology 217 (12) (2012) 1259–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sierra-Filardi E, Nieto C, Dominguez-Soto A, Barroso R, Sanchez-Mateos P, Puig-Kroger A, Lopez-Bravo M, Joven J, Ardavin C, Rodriguez-Fernandez JL, Sanchez-Torres C, Mellado M, Corbi AL, CCL2 shapes macrophage polarization by GM-CSF and M-CSF: identification of CCL2/CCR2-dependent gene expression profile, J. Immunol 192 (8) (2014) 3858–3867. [DOI] [PubMed] [Google Scholar]
- 102.Hamilton JA, GM-CSF in inflammation and autoimmunity, Trends Immunol 23 (8) (2002) 403–408. [DOI] [PubMed] [Google Scholar]
- 103.Sheng JR, Quan S, Soliven B, CD1dhi CD5+ B cells expanded by GM-CSF in vivo suppress experimental autoimmune myasthenia gravis, J. Immunol 193 (6) (2014) 2669–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Balamayooran G, Batra S, Balamayooran T, Cai S, Jeyaseelan S, Monocyte chemoattractant protein 1 regulates pulmonary host defense via neutrophil recruitment during Escherichia coli infection, Infect. Immun 79 (7) (2011) 2567–2577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Deshmane SL, Kremlev S, Amini S, Sawaya BE, Monocyte chemoattractant protein-1 (MCP-1): an overview, J. Interferon Cytokine Res 29 (6) (2009) 313–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Flaishon L, Becker-Herman S, Hart G, Levo Y, Kuziel WA, Shachar I, Expression of the chemokine receptor CCR2 on immature B cells negatively regulates their cytoskeletal rearrangement and migration, Blood 104 (4) (2004) 933–941. [DOI] [PubMed] [Google Scholar]
- 107.Santos JC, de Brito CA, Futata EA, Azor MH, Orii NM, Maruta CW, Rivitti EA, Duarte AJS, Sato MN, Up-regulation of chemokine C–C ligand 2 (CCL2) and C-X-C chemokine 8 (CXCL8) expression by monocytes in chronic idiopathic urticaria, Clin. Exp. Immunol 167 (1) (2012) 129–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Schall TJ, Bacon K, Camp RD, Kaspari JW, Goeddel DV, Human macrophage inflammatory protein alpha (MIP-1 alpha) and MIP-1 beta chemokines attract distinct populations of lymphocytes, J. Exp. Med 177 (6) (1993) 1821–1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tumpey TM, Fenton R, Molesworth-Kenyon S, Oakes JE, Lausch RN, Role for macrophage inflammatory protein 2 (MIP-2), MIP-1alpha, and interleukin-1alpha in the delayed-type hypersensitivity response to viral antigen, J. Virol 76 (16) (2002) 8050–8057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pan Q, Feng Y, Peng Y, Zhou H, Deng Z, Li L, Han H, Lin J, Shi L, Wang S, An N, Yang C, Liu HF, Basophil recruitment to skin lesions of patients with systemic lupus erythematosus mediated by CCR1 and CCR2, Cell Physiol. Biochem 43 (2) (2017) 832–839. [DOI] [PubMed] [Google Scholar]
- 111.Foti M, Granucci F, Aggujaro D, Liboi E, Luini W, Minardi S, Mantovani A, Sozzani S, Ricciardi-Castagnoli P, Upon dendritic cell (DC) activation chemokines and chemokine receptor expression are rapidly regulated for recruitment and maintenance of DC at the inflammatory site, Inter. Immunol 11 (6) (1999) 979–986. [DOI] [PubMed] [Google Scholar]
- 112.Halova I, Draberova L, Draber P, Mast cell chemotaxis – chemoattractants and signaling pathways, Front. Immunol 3 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Berahovich RD, Lai NL, Wei Z, Lanier LL, Schall TJ, Evidence for NK cell subsets based on chemokine receptor expression, J. Immunol 177 (11) (2006) 7833–7840. [DOI] [PubMed] [Google Scholar]
- 114.Phillips RM, Stubbs VE, Henson MR, Williams TJ, Pease JE, Sabroe I, Variations in eosinophil chemokine responses: an investigation of CCR1 and CCR3 function, expression in atopy, and identification of a functional CCR1 promoter, J. Immunol 170 (12) (2003) 6190–6201. [DOI] [PubMed] [Google Scholar]
- 115.Sokol CL, Luster AD, The chemokine system in innate immunity, Cold Spring Harb. Perspect. Biol 7 (5) (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vries MH, Wagenaar A, Verbruggen SE, Molin DG, Dijkgraaf I, Hackeng TH, Post MJ, CXCL1 promotes arteriogenesis through enhanced monocyte recruitment into the peri-collateral space, Angiogenesis 18 (2) (2015) 163–171. [DOI] [PubMed] [Google Scholar]
- 117.Bettelli E, Carrier Y, Gao W, Korn T, Strom TB, Oukka M, Weiner HL, Kuchroo VK, Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells, Nature 441 (7090) (2006) 235–238. [DOI] [PubMed] [Google Scholar]
- 118.Nathan C, Ding A, Nonresolving inflammation, Cell 140 (6) (2010) 871–882. [DOI] [PubMed] [Google Scholar]
- 119.Lawrence T, Gilroy DW, Chronic inflammation: a failure of resolution? Int. J. Exp. Pathol 88 (2) (2007) 85–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Tang M, Diao J, Gu H, Khatri I, Zhao J, Cattral MS, Toll-like receptor 2 activation promotes tumor dendritic cell dysfunction by regulating IL-6 and IL-10 receptor signaling, Cell Rep 13 (12) (2015) 2851–2864. [DOI] [PubMed] [Google Scholar]
- 121.Tsukamoto H, Fujieda K, Senju S, Ikeda T, Oshiumi H, Nishimura Y, Immune-suppressive effects of interleukin-6 on T-cell-mediated anti-tumor immunity, Cancer Sci 109 (3) (2018) 523–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Ortiz-Montero P, Londono-Vallejo A, Vernot JP, Senescence-associated IL-6 and IL-8 cytokines induce a self- and cross-reinforced senescence/inflammatory milieu strengthening tumorigenic capabilities in the MCF-7 breast cancer cell line, Cell Commun. Signal 15 (1) (2017) 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Farsam V, Basu A, Gatzka M, Treiber N, Schneider LA, Mulaw MA, Lucas T, Kochanek S, Dummer R, Levesque MP, Wlaschek M, Scharffetter-Kochanek K, Senescent fibroblast-derived chemerin promotes squamous cell carcinoma migration, Oncotarget 7 (50) (2016) 83554–83569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aifuwa I, Giri A, Longe N, Lee SH, An SS, Wirtz D, Senescent stromal cells induce cancer cell migration via inhibition of RhoA/ROCK/myosin-based cell contractility, Oncotarget 6 (31) (2015) 30516–30531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, Greaves L, Saretzki G, Fox C, Lawless C, Anderson R, Hewitt G, Pender SLF, Fullard N, Nelson G, Mann J, van de Sluis B, Mann DA, von Zglinicki T, Chronic inflammation induces telomere dysfunction and accelerates ageing in mice, Nat. Commun 5 (2014) 4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Bernal GM, Wahlstrom JS, Crawley CD, Cahill KE, Pytel P, Liang H, Kang S, Weichselbaum RR, Yamini B, Loss of Nfkb1 leads to early onset aging, Aging (Albany NY) 6 (11) (2014) 931–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Severino V, Alessio N, Farina A, Sandomenico A, Cipollaro M, Peluso G, Galderisi U, Chambery A, Insulin-like growth factor binding proteins 4 and 7 released by senescent cells promote premature senescence in mesenchymal stem cells, Cell Death Dis 4 (2013) e911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Mosteiro L, Pantoja C, Alcazar N, Marion RM, Chondronasiou D, Rovira M, Fernandez-Marcos PJ, Munoz-Martin M, Blanco-Aparicio C, Pastor J, Gomez-Lopez G, De Martino A, Blasco MA, Abad M, Serrano M, Tissue damage and senescence provide critical signals for cellular reprogramming in vivo, Science 354 (6315) (2016). [DOI] [PubMed] [Google Scholar]
- 129.Kandhaya-Pillai R, Miro-Mur F, Alijotas-Reig J, Tchkonia T, Kirkland JL, Schwartz S, TNFα-senescence initiates a STAT-dependent positive feedback loop, leading to a sustained interferon signature, DNA damage, and cytokine secretion, Aging 9 (11) (2017) 2411–2435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Sivori S, Carlomagno S, Pesce S, Moretta A, Vitale M, Marcenaro E, TLR/NCR/KIR: which one to use and when? Front. Immunol 5 (105) (2014) 105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Suematsu M, Aiso S, Professor Toshio Ito: a clairvoyant in pericyte biology, Keio J. Med 50 (2) (2001) 66–71. [DOI] [PubMed] [Google Scholar]
- 132.Krizhanovsky V, Yon M, Dickins RA, Hearn S, Simon J, Miething C, Yee H, Zender L, Lowe SW, Senescence of activated stellate cells limits liver fibrosis, Cell 134 (4) (2008) 657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Soriani A, Zingoni A, Cerboni C, Iannitto ML, Ricciardi MR, Di Gialleonardo V, Cippitelli M, Fionda C, Petrucci MT, Guarini A, Foa R, Santoni A, ATM-ATR-dependent up-regulation of DNAM-1 and NKG2D ligands on multiple myeloma cells by therapeutic agents results in enhanced NK-cell susceptibility and is associated with a senescent phenotype, Blood 113 (15) (2009) 3503–3511. [DOI] [PubMed] [Google Scholar]
- 134.Aras S, Zaidi MR, TAMeless traitors: macrophages in cancer progression and metastasis, Br. J. Cancer 117 (11) (2017) 1583–1591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mosser DM, Zhang X, Measuring opsonic phagocytosis via Fcgamma receptors and complement receptors on macrophages, Curr. Protoc. Immunol Chapter 14 (2011) Unit 14 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Vicetti Miguel RD, Cherpes TL, Watson LJ, McKenna KC, CTL induction of tumoricidal nitric oxide production by intratumoral macrophages is critical for tumor elimination, J. Immunol 185 (11) (2010) 6706–6718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lv Z, Bian Z, Shi L, Niu S, Ha B, Tremblay A, Li L, Zhang X, Paluszynski J, Liu M, Zen K, Liu Y, Loss of cell surface CD47 clustering formation and binding avidity to sirpalpha facilitate apoptotic cell clearance by macrophages, J. Immunol 195 (2) (2015) 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Khandelwal S, van Rooijen N, Saxena RK, Reduced expression of CD47 during murine red blood cell (RBC) senescence and its role in RBC clearance from the circulation, Transfusion 47 (9) (2007) 1725–1732. [DOI] [PubMed] [Google Scholar]
- 139.Fulop T, Le Page A, Garneau H, Azimi N, Baehl S, Dupuis G, Pawelec G, Larbi A, Aging, immunosenescence and membrane rafts: the lipid connection, Longev. Healthspan 1 (2012) 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Lujambio A, Akkari L, Simon J, Grace D, Tschaharganeh DF, Bolden JE, Zhao Z, Thapar V, Joyce JA, Krizhanovsky V, Lowe SW, Non-cell-autonomous tumor suppression by p53, Cell 153 (2) (2013) 449–460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Biswas SK, Mantovani A, Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm, Nat. Immunol 11 (2010) 889. [DOI] [PubMed] [Google Scholar]
- 142.Shishido SN, Humoral innate immune response and disease, Clin Immunol 144 (2) (2012) 142–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Frescas D, Roux CM, Aygun-Sunar S, Gleiberman AS, Krasnov P, Kurnasov OV, Strom E, Virtuoso LP, Wrobel M, Osterman AL, Antoch MP, Mett V, Chernova OB, Gudkov AV, Senescent cells expose and secrete an oxidized form of membrane-bound vimentin as revealed by a natural polyreactive antibody, Proc. Natl. Acad. Sci. U. S. A 114 (9) (2017) E1668–E1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Bilalic S, Michlmayr A, Gruber V, Buchberger E, Burghuber C, Bohmig GA, Oehler R, Lymphocyte activation induces cell surface expression of an immunogenic vimentin isoform, Transpl. Immunol 27 (2–3) (2012) 101–106. [DOI] [PubMed] [Google Scholar]
- 145.Moisan E, Girard D, Cell surface expression of intermediate filament proteins vimentin and lamin B1 in human neutrophil spontaneous apoptosis, J. Leukocyte Biol 79 (3) (2006) 489–498. [DOI] [PubMed] [Google Scholar]
- 146.Nishida Y, Shibata K, Yamasaki M, Sato Y, Abe M, A possible role of vimentin on the cell surface for the activation of latent transforming growth factor-β, FEBS Lett 583 (2) (2009) 308–312. [DOI] [PubMed] [Google Scholar]
- 147.Starr AE, Bellac CL, Dufour A, Goebeler V, Overall CM, Biochemical characterization and N-terminomics analysis of leukolysin, the membrane-type 6 matrix metalloprotease (MMP25): chemokine and vimentin cleavages enhance cell migration and macrophage phagocytic activities, J. Biol. Chem 287 (16) (2012) 13382–13395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Holodick NE, Rodriguez-Zhurbenko N, Hernandez AM, Defining natural antibodies, Front. Immunol 8 (2017) 872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Pulendran B, Li S, Nakaya HI, Systems vaccinology, Immunity 33 (4) (2010) 516–529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Fulop T, Larbi A, Dupuis G, Le Page A, Frost EH, Cohen AA, Witkowski JM, Franceschi C, Immunosenescence and inflamm-aging as two sides of the same coin: friends or foes? Front. Immunol 8 (2017) 1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hu J, Batth IS, Xia X, Li S, Regulation of NKG2D+CD8+ T-cell-mediated antitumor immune surveillance: Identification of a novel CD28 activation-mediated, STAT3 phosphorylation-dependent mechanism, OncoImmunology 5 (12) (2016) e1252012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Liu X, Mo W, Ye J, Li L, Zhang Y, Hsueh EC, Hoft DF, Peng G, Regulatory T cells trigger effector T cell DNA damage and senescence caused by metabolic competition, Nat. Commun 9 (1) (2018) 249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Takeuchi O, Akira S, Pattern recognition receptors and inflammation, Cell 140 (6) (2010) 805–820. [DOI] [PubMed] [Google Scholar]
- 154.Schaper F, de Leeuw K, Horst G, Bootsma H, Limburg PC, Heeringa P, Bijl M, Westra J, High mobility group box 1 skews macrophage polarization and negatively influences phagocytosis of apoptotic cells, Rheumatology 55 (12) (2016) 2260–2270. [DOI] [PubMed] [Google Scholar]
- 155.Gluck S, Guey B, Gulen MF, Wolter K, Kang TW, Schmacke NA, Bridgeman A, Rehwinkel J, Zender L, Ablasser A, Innate immune sensing of cytosolic chromatin fragments through cGAS promotes senescence, Nat. Cell Biol 19 (9) (2017) 1061–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang H, Wang H, Ren J, Chen Q, Chen ZJ, cGAS is essential for cellular senescence, Proc. Natl. Acad. Sci. U. S. A 114 (23) (2017) E4612–E4620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Baz-Martinez M, Da Silva-Alvarez S, Rodriguez E, Guerra J, El Motiam A, Vidal A, Garcia-Caballero T, Gonzalez-Barcia M, Sanchez L, Munoz-Fontela C, Collado M, Rivas C, Cell senescence is an antiviral defense mechanism, Sci. Rep 6 (2016) 37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Drew W, Wilson DV, Sapey E, Inflammation and neutrophil immunosenescence in health and disease: Targeted treatments to improve clinical outcomes in the elderly, Exp. Gerontol 105 (2018) 70–77. [DOI] [PubMed] [Google Scholar]
- 159.Geering B, Stoeckle C, Conus S, Simon HU, Living and dying for inflammation: neutrophils, eosinophils, basophils, Trends Immunol 34 (8) (2013) 398–409. [DOI] [PubMed] [Google Scholar]
- 160.Singer M, Sansonetti PJ, IL-8 is a key chemokine regulating neutrophil recruitment in a new mouse model of shigella-induced colitis, J. Immunol 173 (6) (2004) 4197–4206. [DOI] [PubMed] [Google Scholar]
- 161.Kaplan MJ, Radic M, Neutrophil extracellular traps: double-edged swords of innate immunity, J. Immunol 189 (6) (2012) 2689–2695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kalesnikoff J, Galli SJ, New developments in mast cell biology, Nat. Immunol 9 11 (2008) 1215–1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gunin AG, Kornilova NK, Vasilieva OV, Petrov VV, Age-related changes in proliferation, the numbers of mast cells, eosinophils, and cd45-positive cells in human dermis, J. Gerontol. A: Biol. Sci. Med. Sci 66 (4) (2011) 385–392. [DOI] [PubMed] [Google Scholar]
- 164.Langhi LGP, Andrade LR, Shimabukuro MK, van Ewijk W, Taub DD, Borojevic R, de Mello Coelho V, Lipid-laden multilocular cells in the aging thymus are phenotypically heterogeneous, PLOS ONE 10 (10) (2015) e0141516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Grizzi F, Di Caro G, Laghi L, Hermonat P, Mazzola P, Nguyen DD, Radhi S, Figueroa JA, Cobos E, Annoni G, Chiriva-Internati M, Mast cells and the liver aging process, Immun. Ageing 10 (1) (2013) 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Chatterjee V, Gashev AA, Aging-associated shifts in functional status of mast cells located by adult and aged mesenteric lymphatic vessels, Am. J. Physiol. Heart Circ. Physiol 303 (6) (2012) H693–H702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Golomb L, Sagiv A, Pateras IS, Maly A, Krizhanovsky V, Gorgoulis VG, Oren M, Ben-Yehuda A, Age-associated inflammation connects RAS-induced senescence to stem cell dysfunction and epidermal malignancy, Cell Death Diff 22 (2015) 1764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Sektioglu IM, Carretero R, Bulbuc N, Bald T, Tuting T, Rudensky AY, Hammerling GJ, Basophils promote tumor rejection via chemotaxis and infiltration of CD8+ T cells, Cancer Res 77 (2) (2017) 291–302. [DOI] [PubMed] [Google Scholar]
- 169.Sakata-Kaneko S, Wakatsuki Y, Matsunaga Y, Usui T, Kita T, Altered Th1/Th2 commitment in human CD4(+) T cells with ageing, Clin. Exp. Immunol 120 (2) (2000) 267–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Uciechowski P, Kahmann L, Plümäkers B, Malavolta M, Mocchegiani E, Dedoussis G, Herbein G, Jajte J, Fulop T, Rink L, TH1 and TH2 cell polarization increases with aging and is modulated by zinc supplementation, Exp. Gerontol 43 (5) (2008) 493–498. [DOI] [PubMed] [Google Scholar]
- 171.van der Geest KS, Abdulahad WH, Tete SM, Lorencetti PG, Horst G, Bos NA, Kroesen BJ, Brouwer E, Boots AM, Aging disturbs the balance between effector and regulatory CD4+ T cells, Exp. Gerontol 60 (2014) 190–196. [DOI] [PubMed] [Google Scholar]
- 172.Mathur SK, Schwantes EA, Jarjour NN, Busse WW, Age-related changes in eosinophil function in human subjects, Chest 133 (2) (2008) 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Kolb M, Bonniaud P, Galt T, Sime PJ, Kelly MM, Margetts PJ, Gauldie J, Differences in the fibrogenic response after transfer of active transforming growth factor-beta1 gene to lungs of “fibrosis-prone” and “fibrosis-resistant” mouse strains, Am. J. Respir. Cell. Mol. Biol 27 (2) (2002) 141–150. [DOI] [PubMed] [Google Scholar]
- 174.Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C, A critical role for eosinophils in allergic airways remodeling, Science 305 (5691) (2004) 1776–1779. [DOI] [PubMed] [Google Scholar]
- 175.Wu J, Dong F, Wang R-A, Wang J, Zhao J, Yang M, Gong W, Cui R, Dong L, Central role of cellular senescence in TSLP-induced airway remodeling in asthma, PLOS ONE 8 (10) (2013) e77795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Rahman AH, Aloman C, Dendritic cells and liver fibrosis, Biochim. Biophys. Acta 1832 (7) (2013) 998–1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Bantsimba-Malanda C, Marchal-Somme J, Goven D, Freynet O, Michel L, Crestani B, Soler P, A role for dendritic cells in bleomycin-induced pulmonary fibrosis in mice? Am. J. Respir. Crit. Care Med 182 (3) (2010) 385–395. [DOI] [PubMed] [Google Scholar]
- 178.Kushwah R, Hu J, Role of dendritic cells in the induction of regulatory T cells, Cell Biosci 1 (2011) 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Ye J, Huang X, Hsueh EC, Zhang Q, Ma C, Zhang Y, Varvares MA, Hoft DF, Peng G, Human regulatory T cells induce T-lymphocyte senescence, Blood 120 (10) (2012) 2021–2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Saxena M, Bhardwaj N, Re-emergence of dendritic cell vaccines for cancer treatment, Trends Cancer 4 (2) (2018) 119–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Winau F, Hegasy G, Weiskirchen R, Weber S, Cassan C, Sieling PA, Modlin RL, Liblau RS, Gressner AM, Kaufmann SH, Ito cells are liver-resident antigen-presenting cells for activating T cell responses, Immunity 26 (1) (2007) 117–129. [DOI] [PubMed] [Google Scholar]
- 182.Schrader J, Fallowfield J, Iredale JP, Senescence of activated stellate cells: not just early retirement, Hepatology 49 (3) (2009) 1045–1047. [DOI] [PubMed] [Google Scholar]
- 183.Bomble M, Tacke F, Rink L, Kovalenko E, Weiskirchen R, Analysis of antigen-presenting functionality of cultured rat hepatic stellate cells and transdifferentiated myofibroblasts, Biochem. Biophys. Res. Commun 396 (2) (2010) 342–347. [DOI] [PubMed] [Google Scholar]
- 184.Attallah AM, El-Far M, Zahran F, Shiha GE, Farid K, Omran MM, Abdelrazek MA, Attallah AA, El-Beh AA, El-Hosiny RM, El-Waseef AM, Interferon-gamma is associated with hepatic dysfunction in fibrosis, cirrhosis, and hepatocellular carcinoma, J. Immunoassay Immunochem 37 (6) (2016) 597–610. [DOI] [PubMed] [Google Scholar]
- 185.Schildberg FA, Sharpe AH, Turley SJ, Hepatic immune regulation by stromal cells, Curr. Opin. Immunol 32 (2015) 1–6. [DOI] [PubMed] [Google Scholar]
- 186.Shimabukuro MK, Langhi LG, Cordeiro I, Brito JM, Batista CM, Mattson MP, Mello Coelho V, Lipid-laden cells differentially distributed in the aging brain are functionally active and correspond to distinct phenotypes, Sci. Rep 6 (2016) 23795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Martin JA, Buckwalter JA, Telomere erosion and senescence in human articular cartilage chondrocytes, J. Gerontol. A: Biol. Sci. Med. Sci 56 (4) (2001) B172–B179. [DOI] [PubMed] [Google Scholar]
- 188.Hashimoto M, Asai A, Kawagishi H, Mikawa R, Iwashita Y, Kanayama K, Sugimoto K, Sato T, Maruyama M, Sugimoto M, Elimination of p19(ARF)-expressing cells enhances pulmonary function in mice, JCI Insight 1 (12) (2016) e87732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Verzola D, Gandolfo MT, Gaetani G, Ferraris A, Mangerini R, Ferrario F, Villaggio B, Gianiorio F, Tosetti F, Weiss U, Traverso P, Mji M, Deferrari G, Garibotto G, Accelerated senescence in the kidneys of patients with type 2 diabetic nephropathy, Am. J. Physiol. Renal Physiol 295 (5) (2008) F1563–F1573. [DOI] [PubMed] [Google Scholar]
- 190.Waaijer ME, Parish WE, Strongitharm BH, van Heemst D, Slagboom PE, de Craen AJ, Sedivy JM, Westendorp RG, Gunn DA, Maier AB, The number of p16INK4a positive cells in human skin reflects biological age, Aging Cell 11 (4) (2012) 722–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Fulop T, Montgomery RR, Editorial overview: Immune senescence: known knowns and unknown unknowns, Curr. Opin. Immunol 29 (2014) vii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Turner VM, Mabbott NA, Influence of ageing on the microarchitecture of the spleen and lymph nodes, Biogerontology 18 (5) (2017) 723–738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Pang WW, Price EA, Sahoo D, Beerman I, Maloney WJ, Rossi DJ, Schrier SL, Weissman IL, Human bone marrow hematopoietic stem cells are increased in frequency and myeloid-biased with age, Proc. Natl. Acad. Sci. U. S. A 108 (50) (2011) 20012–20017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Kovtonyuk LV, Fritsch K, Feng X, Manz MG, Takizawa H, Inflamm-aging of hematopoiesis, hematopoietic stem cells, and the bone marrow microenvironment, Front. Immunol 7 (2016) 502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Akbar AN, Henson SM, Lanna A, Senescence of T lymphocytes: implications for enhancing human immunity, Trends Immunol 37 (12) (2016) 866–876. [DOI] [PubMed] [Google Scholar]
- 196.Lynch HE, Goldberg GL, Chidgey A, Van den Brink MR, Boyd R, Sempowski GD, Thymic involution and immune reconstitution, Trends Immunol 30 (7) (2009) 366–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Solana R, Tarazona R, Gayoso I, Lesur O, Dupuis G, Fulop T, Innate immunosenescence: effect of aging on cells and receptors of the innate immune system in humans, Sem. Immunol 24 (5) (2012) 331–341. [DOI] [PubMed] [Google Scholar]
- 198.Veldhuis-Vlug AG, Rosen CJ, Clinical implications of bone marrow adiposity, J. Intern. Med 283 (2) (2018) 121–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Plowden J, Renshaw-Hoelscher M, Engleman C, Katz J, Sambhara S, Innate immunity in aging: impact on macrophage function, Aging Cell 3 (4) (2004) 161–167. [DOI] [PubMed] [Google Scholar]
- 200.Pereira BI, Akbar AN, Convergence of innate and adaptive immunity during human aging, Front. Immunol 7 (2016) 445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Akbar AN, Henson SM, Are senescence and exhaustion intertwined or unrelated processes that compromise immunity? Nat. Rev. Immunol 11 (4) (2011) 289–295. [DOI] [PubMed] [Google Scholar]
- 202.Fulop T, Montgomery RR, Immune senescence: known knowns and unknown unknowns, Curr. Opin. Immunol 29 (2014) vii–ix. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Pajvani UB, Trujillo ME, Combs TP, Iyengar P, Jelicks L, Roth KA, Kitsis RN, Scherer PE, Fat apoptosis through targeted activation of caspase 8: a new mouse model of inducible and reversible lipoatrophy, Nat. Med 11 (7) (2005) 797–803. [DOI] [PubMed] [Google Scholar]
- 204.Liu Y, Johnson SM, Fedoriw Y, Rogers AB, Yuan H, Krishnamurthy J, Sharpless NE, Expression of p16(INK4a) prevents cancer and promotes aging in lymphocytes, Blood 117 (12) (2011) 3257–3267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Coder BD, Wang H, Ruan L, Su DM, Thymic involution perturbs negative selection leading to autoreactive T cells that induce chronic inflammation, J. Immunol 194 (12) (2015) 5825–5837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Hoare M, Narita M, NOTCH and the 2 SASPs of senescence, Cell Cycle 16 (3) (2017) 239–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Rodier F, Campisi J, Four faces of cellular senescence, J. Cell Biol 192 (4) (2011) 547–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hoare M, Ito Y, Kang T-W, Weekes MP, Matheson NJ, Patten DA, Shetty S, Parry AJ, Menon S, Salama R, Antrobus R, Tomimatsu K, Howat W, Lehner PJ, Zender L, Narita M, NOTCH1 mediates a switch between two distinct secretomes during senescence, Nat. Cell Biol 18 (2016) 979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Victoria B, Nunez Lopez YO, Masternak MM, MicroRNAs and the metabolic hallmarks of aging, Mol. Cell. Endocrinol 455 (2017) 131–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Laberge RM, Zhou L, Sarantos MR, Rodier F, Freund A, de Keizer PL, Liu S, Demaria M, Cong YS, Kapahi P, Desprez PY, Hughes RE, Campisi J, Glucocorticoids suppress selected components of the senescence-associated secretory phenotype, Aging Cell 11 (4) (2012) 569–578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Moiseeva O, Deschenes-Simard X, St-Germain E, Igelmann S, Huot G, Cadar AE, Bourdeau V, Pollak MN, Ferbeyre G, Metformin inhibits the senescence-associated secretory phenotype by interfering with IKK/NF-kappaB activation, Aging Cell 12 (3) (2013) 489–498. [DOI] [PubMed] [Google Scholar]
- 212.Wang R, Yu Z, Sunchu B, Shoaf J, Dang I, Zhao S, Caples K, Bradley L, Beaver LM, Ho E, Lohr CV, Perez VI, Rapamycin inhibits the secretory phenotype of senescent cells by a Nrf2-independent mechanism, Aging Cell 16 (3) (2017) 564–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Zhou F, Onizawa S, Nagai A, Aoshiba K, Epithelial cell senescence impairs repair process and exacerbates inflammation after airway injury, Respir. Res 12 (2011) 78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kirkland JL, Tchkonia T, Zhu Y, Niedernhofer LJ, Robbins PD, The clinical potential of senolytic drugs, J. Am. Geriatr. Soc 65 (10) (2017) 2297–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Correia-Melo C, Marques FD, Anderson R, Hewitt G, Hewitt R, Cole J, Carroll BM, Miwa S, Birch J, Merz A, Rushton MD, Charles M, Jurk D, Tait SW, Czapiewski R, Greaves L, Nelson G, Bohlooly-Y M, Rodriguez-Cuenca S, Vidal-Puig A, Mann D, Saretzki G, Quarato G, Green DR, Adams PD, von Zglinicki T, Korolchuk VI, Passos JF, Mitochondria are required for proageing features of the senescent phenotype, EMBO J (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Alimbetov D, Davis T, Brook AJ, Cox LS, Faragher RG, Nurgozhin T, Zhumadilov Z, Kipling D, Suppression of the senescence-associated secretory phenotype (SASP) in human fibroblasts using small molecule inhibitors of p38 MAP kinase and MK2, Biogerontology 17 (2) (2016) 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Hongo A, Okumura N, Nakahara M, Kay EP, Koizumi N, The effect of a p38 mitogen-activated protein kinase inhibitor on cellular senescence of cultivated human corneal endothelial cells, Invest. Ophthalmol. Vis. Sci 58 (9) (2017) 3325–3334. [DOI] [PubMed] [Google Scholar]
- 218.Wang D, DuBois RN, Immunosuppression associated with chronic inflammation in the tumor microenvironment, Carcinogenesis 36 (10) (2015) 1085–1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Shalapour S, Lin X-J, Bastian IN, Brain J, Burt AD, Aksenov AA, Vrbanac AF, Li W, Perkins A, Matsutani T, Zhong Z, Dhar D, Navas-Molina JA, Xu J, Loomba R, Downes M, Yu RT, Evans RM, Dorrestein PC, Knight R, Benner C, Anstee QM, Karin M, Inflammation-induced IgA+ cells dismantle anti-liver cancer immunity, Nature 551 (2017) 340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB, mTOR inhibition improves immune function in the elderly, Sci. Transl. Med 6 (268) (2014) 268ra179. [DOI] [PubMed] [Google Scholar]
- 221.Davalos AR, Coppe JP, Campisi J, Desprez PY, Senescent cells as a source of inflammatory factors for tumor progression, Cancer Metastasis Rev 29 (2) (2010) 273–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Weiland T, Lampe J, Essmann F, Venturelli S, Berger A, Bossow S, Berchtold S, Schulze-Osthoff K, Lauer UM, Bitzer M, Enhanced killing of therapy-induced senescent tumor cells by oncolytic measles vaccine viruses, Int. J. Cancer 134 (1) (2014) 235–243. [DOI] [PubMed] [Google Scholar]
- 223.Schafer MJ, White TA, Evans G, Tonne JM, Verzosa GC, Stout MB, Mazula DL, Palmer AK, Baker DJ, Jensen MD, Torbenson MS, Miller JD, Ikeda Y, Tchkonia T, van Deursen JM, Kirkland JL, LeBrasseur NK, Exercise prevents diet-induced cellular senescence in adipose tissue, Diabetes 65 (6) (2016) 1606–1615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Musi N, Valentine JM, Sickora KR, Baeuerle E, Thompson CS, Shen Q, Orr ME, Tau protein aggregation is associated with cellular senescence in the brain, Aging Cell (2018) e12840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Lau A, Kennedy BK, Kirkland JL, Tullius SG, Mixing old and young: enhancing rejuvenation and accelerating aging, J. Clin. Invest 129 (1) (2019) 4–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Cupit-Link MC, Kirkland JL, Ness KK, Armstrong GT, Tchkonia T, LeBrasseur NK, Armenian SH, Ruddy KJ, Hashmi SK, Biology of premature ageing in survivors of cancer, ESMO Open 2 (5) (2017) e000250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Shen X, Zhou J, Hathcock KS, Robbins P, Powell DJ, Rosenberg SA, Hodes RJ, Persistence of tumor infiltrating lymphocytes in adoptive immunotherapy correlates with telomere length, J. Immunother 30 (1) (2007) 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Li Y, Liu S, Hernandez J, Vence L, Hwu P, Radvanyi L, MART-1-specific melanoma tumor-infiltrating lymphocytes maintaining CD28 expression have improved survival and expansion capability following antigenic restimulation in vitro, J. Immunol 184 (1) (2010) 452–465. [DOI] [PubMed] [Google Scholar]
- 229.Radvanyi LG, Bernatchez C, Zhang M, Fox PS, Miller P, Chacon J, Wu R, Lizee G, Mahoney S, Alvarado G, Glass M, Johnson VE, McMannis JD, Shpall E, Prieto V, Papadopoulos N, Kim K, Homsi J, Bedikian A, Hwu WJ, Patel S, Ross MI, Lee JE, Gershenwald JE, Lucci A, Royal R, Cormier JN, Davies MA, Mansaray R, Fulbright OJ, Toth C, Ramachandran R, Wardell S, Gonzalez A, Hwu P, Specific lymphocyte subsets predict response to adoptive cell therapy using expanded autologous tumor-infiltrating lymphocytes in metastatic melanoma patients, Clin. Cancer Res 18 (24) (2012) 6758–6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Sim GC, Chacon J, Haymaker C, Ritthipichai K, Singh M, Hwu P, Radvanyi L, Tumor-infiltrating lymphocyte therapy for melanoma: rationale and issues for further clinical development, BioDrugs 28 (5) (2014) 421–437. [DOI] [PubMed] [Google Scholar]
- 231.Zhu Y, Armstrong JL, Tchkonia T, Kirkland JL, Cellular senescence and the senescent secretory phenotype in age-related chronic diseases, Curr. Opin. Clin. Nutr. Metab. Care 17 (4) (2014) 324–328. [DOI] [PubMed] [Google Scholar]


