Abstract
Non-coding RNA dysregulation is associated with many human diseases, including cancer. This study explored the effects of lncRNA SNHG5 on clear cell renal cell carcinoma (ccRCC). We found that lncRNA SNHG5 is upregulated in human ccRCC tissues and that lncRNA SNHG5 inhibition reduced ccRCC cell invasion and promoted apoptosis in vitro. Bioinformatics database searching revealed that lncRNA SNHG5 is predicted to regulate the interaction between miR-363-3p and Twist1. We further verified a ccRCC biomarker panel, which consists of lncRNA SNHG5, miR-363-3p, and Twist1 in ccRCC tissue samples. The direct SNHG5-miR-363-3p and Twist1-miR-363-3p interactions were confirmed via dual-luciferase reporter assays. Additionally, functional assays demonstrated that SNHG5 promotes cell invasion and inhibits apoptosis, while miR-363-3p inhibits cell invasion and promotes apoptosis via an interaction with Twist1. Furthermore, we found that Twist1 promotes tumor metastasis by regulating matrix metalloproteinase (MMP)2 and MMP9 levels. Together, these results suggest that lncRNA SNHG5 may predict ccRCC patient clinical outcome and serve as a novel anti-ccRCC therapeutic target.
Keywords: ccRCC, lncRNA SNHG5, miR-363-3p, Twist1, metastasis
Introduction
Renal cell carcinoma (RCC) is a common excretory system solid cancer, with high incidence and mortality rate [1,2]. Clear cell renal cell carcinoma (ccRCC) is the most frequent histological subtype of RCC, accounting for approximately 75% of cases [3]. Currently, surgical resection is the standard therapeutic option. As nearly 30% of patients experience local and/or distant recurrence and metastasis after tumor resection, the overall survival of RCC patients remains poor [4]. Thus, in-depth exploration of the molecular mechanisms underlying RCC tumor progression and discovery of reliable biomarkers that predict tumor metastasis and invasion are urgently needed.
Non-coding RNAs are RNA molecules that not code for proteins but play active roles in gene regulation. Non-coding RNAs are classified based on their length: microRNAs (miRNAs) have a short mature form of 21-24 nucleotides, and long non-coding RNAs (lncRNAs) refer to those that are longer than 200 nucleotides [5]. Non-coding RNA has been reported to be involved in the regulation of diverse cellular processes, including cell growth and differentiation, programmed cell death (apoptosis), and migration [6,7]. More specifically, some non-coding RNAs have been found to regulate the development and progression of human cancers [8-13]. For examples, the lncRNA SChLAP1 is a potential tissue-based biomarker for lethal prostate cancer [8], while the lncRNA HIF1A-AS2 is known to promote tumor invasion and lymph node metastasis in gastric cancer [9]. The lncRNA small nuclear RNA host gene 5 (SNHG5), which is 524 nucleotides in length, is comprised of six exons and encodes for the small nucleolar RNAs (snoRNAs) U50 and U50’ [14]. Recently, aberrant expression of SNHG5 has been reported in various human cancers, including colorectal cancer, malignant melanoma, and gastric cancer [15-17]; However, the underlying molecular mechanism of lncRNA SNHG5 in RCC remain unknown. Analysis with online software programs revealed that lncRNA SNHG5 may be a target of miR-363-3p. Thus, we sought to determine the function of lncRNA SNHG5 in promoting tumorigenicity in ccRCC.
Materials and methods
Human ccRCC specimens
Forty primary tissue samples, including tumor tissues and adjacent normal tissues, were obtained from ccRCC patients from Shanghai Ninth People’s Hospital. None of the patients had received chemotherapy or radiotherapy before surgery. The tumor specimens were collected immediately after surgical resection and stored in liquid nitrogen until usage. Clinical sample cohorts used for this study were approved by the Ethics Committee of Shanghai Ninth People’s Hospital.
Cell culture
The normal human epithelial kidney cell line HK-2 was obtained from the American Type Culture Collection (ATCC). The human RCC lines 786-O, ACHN, SW13, and Caki-1 were obtained from the Cell Bank of Type Culture Collection of the Chinese Academy of Sciences (CCCAS). HK-2, SW13, and 786-O cells were cultured in RPMI-1640 medium (Gibco) with 10% fetal bovine serum (FBS, Gibco), 50 U/ml penicillin, and 50 μg/ml streptomycin. ACHN cells and Caki-1 cells were cultured in MEM medium (Gibco) and McCOY’s 5A medium (Gibco), respectively, with 10% FBS, 50 U/ml penicillin and 50 μg/ml streptomycin. All cells were cultured and maintained in a sterile incubator at 37°C with 5% CO2.
Lentivirus infection
The 786-O cells were cultured in 10-cm dishes (5×105 cells/dish) overnight. The lentivirus was added into the dishes at a multiplicity of infection (MOI) of 50 when the cells reached approximately 60% confluence. The infection efficiency was detected after 48 h by analysis of EGFP. Cells used in experiments had a lentivirus infection efficiency of >90%.
Transwell invasion assays
The invasion assays were performed using Transwell chambers (Corning). The 786-O cells were seeded into upper chambers pre-coated with Matrigel (BD) in serum-free medium in triplicate. Medium containing 10% FBS was added to the lower chamber. After incubation for 24 h, cells from the upper chamber surface were removed, and the invaded cells on the lower chamber surface were fixed and stained with crystal violet.
Cell apoptosis
To determine apoptosis, cells were stained with Annexin V and propidium iodide according to the instructions for the Annexin V-APC detection kit (Shanghai R&S Biotechnology Co. Ltd.). Briefly, 300 µl binding buffer was added into each sample tube and mixed by shaking gently. Then, 5 µl Annexin V-APC/PI was added and incubated without light for 30 min. Finally, cell apoptosis was detected by flow cytometry.
Non-coding RNA target prediction and luciferase reporter assays
The miRNAs that may target Twist1 were predicted using TargetScan (http://genes.mit.edu/targetscan/), PicTar (http://pictar.mdc-berlin.de/), and miRanda (http://cbio.mskcc.org/cgi-bin/mirnaviewer/mirnaviewer.pl). The 786-O cells were seeded in 24-well plates and incubated overnight, and then the cells were co-transfected with miR-363 mimic (miR-363 sequence) and a 3’UTR reporter plasmid psiCHECK2-WT/MU. Cell transfections were performed using Lipofectamine® RNAiMAX (Invitrogen) with final concentrations of 50 nM miR-363-3p mimic and 200 ng psiCHECK2-WT/MU. The medium was changed 6 h after transfection, and 48 h later, these cells were collected for further luciferase reporter assay analysis. The luciferase activity was assessed using the Dual-Glo Luciferase Assay system (Promega) according to the manufacture’s protocol.
Quantitative RT-PCR
Total RNA was extracted using Trizol reagent (Invitrogen) according to the manufacturer’s protocol. For mRNA quantitative detection, total RNAs were reversely transcribed using the reverse transcription kit (Promega) according to the manufacturer’s protocol. Then, qRT-PCR was performed using the SYBR Green Master Mix (Takara), and hACTB was used as the internal control. For miRNA quantitative detection, All-in-One™ miRNA qRT-PCR Detection Kit (GeneCopoeia Inc.) was used according to the manufacturer’s instructions. The U6 snRNA was used as an endogenous control for miRNA. The ΔΔCt method was used to determine relative miRNA and mRNA expression, and fold change was determined as 2-ΔΔCt.
Western blot
Total proteins were extracted from cells according to protein extraction kit protocol (KEYGEN). The protein concentration of each sample was determined using a Bio-Rad protein assay system. Equivalent quantities of proteins were separated by 12% SDS-polyacrylamide gels, transferred to nitrocellulose membranes that were blocked with 10% non-fat milk, and incubated overnight with the appropriate primary antibodies. After incubation, membranes were washed and incubated with the corresponding horseradish peroxidase (HRP)-conjugated secondary antibody for 1 h at room temperature. ECL detection reagent (Amersham LifeSciences) was used to visualize the results. The following primary antibodies were used: anti-GAPDH, anti-Twist1, anti-matrix metalloproteinase (MMP) 2, and anti-MMP9 (CST).
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM) from three separate experiments. When two groups were compared, the differences between groups were analyzed using Student’s t-test. All statistical analyses were performed with SPSS 16.0 (SPSS Inc.). The differences were considered statistically significant at P<0.05.
Results
lncRNA SNHG5 is upregulated in ccRCC tissues
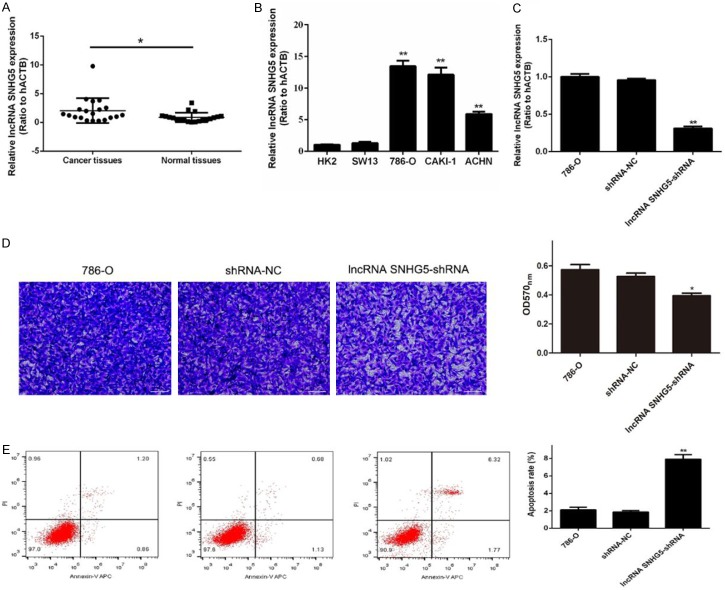
In order to explore the potential role of lncRNA SNHG5 in ccRCC, we analyzed 20 pairs of human ccRCC tissues and non-tumor tissues. The qRT-PCR analysis revealed that SNHG5 expression levels were significantly increased in ccRCC tissues compared to those in non-tumor tissues (Figure 1A). Next, we examined lncRNA SNHG5 expression levels in both non-malignant (HK-2) and malignant (786-O, Caki1, and ACHN) cell lines (Figure 1B). We found that lncRNA SNHG5 is overexpressed in the human RCC cell lines compared to the healthy epithelial kidney cell lines. These data suggest that SNHG5 is involved in tumor development.
Figure 1.
Effect of lncRNA SNHG5 on metastasis and apoptosis of RCC cells. (A) qRT-PCR results of lncRNA SNHG5 in human ccRCC tissues and non-tumor tissues. (B) The relative expression of lncRNA SNHG5 was determined in the non-malignant (HK-2 and SW-13) and malignant (786-O, Caki1, and ACHN) cell lines. (C) The expression of lncRNA SNHG5 in 786-O cells, cells treated with shRNA-NC, and cells treated with lncRNA SNHG5-shRNA was detected by qRT-PCR. (D) Transwell assay was used to determine the metastasis/invasion ability of the cells described for (C). (E) Apoptosis by the three cell groups was detected by flow cytometry. *P<0.05, **P<0.01.
Knockdown of lncRNA SNHG5 inhibited RCC cell invasion and promoted cell apoptosis by targeting miR-363-3p
In our study, 786-O RCC cells infected with shRNA-SNHG5 exhibited reduction of SNHG5 expression (Figure 1C) and significantly reduced metastasis ability (Figure 1D). Meanwhile, the apoptosis rate of shRNA-SNHG5-treated cells was significantly enhanced (Figure 1E). Our results showed that knockdown of the lncRNA SNHG5 inhibited RCC cell invasion and promoted cell apoptosis. To further explore the events following lncRNA SNHG5 knockdown, we performed bioinformatics analysis, revealing a correlation between the lncRNA SNHG5 and miR-363-3p. As expected, we found that miR-363-3p was downregulated in the carcinoma tissues compared to non-malignant tissue (Figure 2A). We examined the influence of lncRNA SNHG5 silencing on miR-363-3p in cultured cells and found that miR-363-3p was significantly increased by knockdown of lncRNA SNHG5 (Figure 2B). Furthermore, lncRNA SNHG5 targeted miR-363-3p in 786-O cells as assessed by a luciferase reporter assay. We found that miR-363-3p and lncRNA SNHG5 have binding targets. The luciferase assay data indicated that miR-363-3p mimics repress the expression of a reporter gene containing WT 3’-UTR of Twist1 (Figure 2C).
Figure 2.
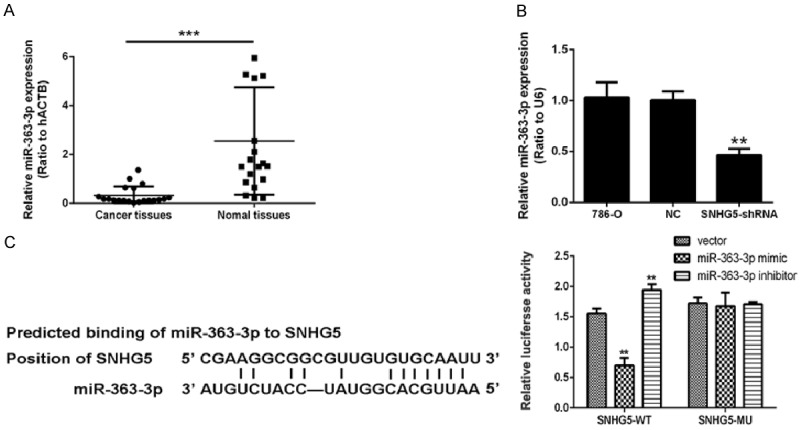
The miR-363-3p targeted the expression of lncRNA SNHG5 in 786-O cells. A. qRT-PCR results of miR-363-3p in human ccRCC tissues and non-tumor tissues. B. The expression of miR-363-3p in 780-O cells, cells treated with shRNA-NC, and cells treated with lncRNA SNHG5-shRNA was detected by qRT-PCR. C. The binding sites of miR-363-3p and lncRNA SNHG5-WT were detected using a luciferase reporter assay. *P<0.05, **P<0.01.
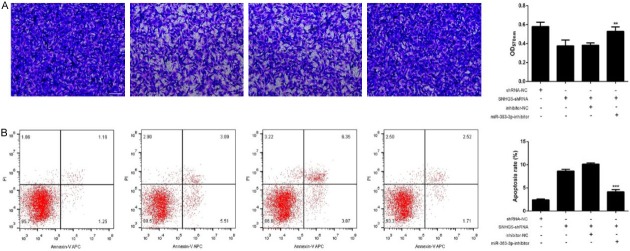
To confirm the role of the lncRNA SNHG5-miR-363-3p interaction in ccRCC cell progression, shRNA-SNHG5 was stably introduced into the 786-O cell line. We then assessed the effects of miR-363-3p and lncRNA SNHG5 on invasion and apoptosis of 786-O cells. We found that inhibition of SNHG5 expression induced a significant reduction in invasion ability and increased the apoptosis rate, while downregulation of miR-363-3p reversed these effects (Figure 3A and 3B). Thus, our results suggest that lncRNA SNHG6 promotes RCC cell invasion and inhibits ccRCC cell apoptosis by targeting miR-363-3p.
Figure 3.
Knockdown of lncRNA snhg5 inhibited RCC cell invasion and promoted cell apoptosis by targeting miR-363-3p. A. The effects of lncRNA SNHG5 on cell invasion via targeting of miR-363-3p. B. The apoptosis rates of cells treated with shRNA-NC, SNHG5-shRNA, mimic-NC+SNHG5-shRNA, and miR-363-3p-mimics+SNHG5-shRNA were detected by flow cytometry. **P<0.01, ***P<0.001.
Twist1 is a direct target of miR-363-3p
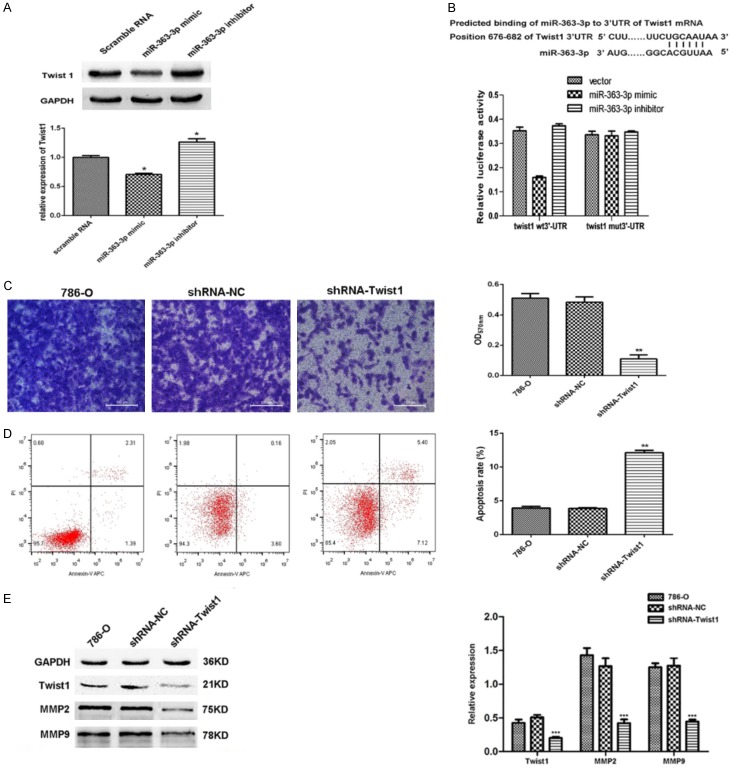
We next sought to examine the role of miR-363-3p in tumor progression. Bioinformatics analyses indicated that the transcription factor Twist1 was a good candidate for further investigation based on the results of correlation analysis. Firstly, to verify the correlation between miR-363-3p and Twist1, Twist1 expression was determined in the three sets of cells using western blotting: miR-363-3p mimic group, miR-363-3p inhibitor group, and control group. Twist1 was downregulated in the miR-363-3p-overexpression cells, whereas this factor was upregulated in the presence of miR-363-3p inhibitor compared to the control cells (Figure 4A). These data demonstrate that Twist1 expression is regulated by miR-363-3p. In addition, a luciferase reporter assay was performed to determine whether Twist1 is a direct target for miR-363-3p, as the 3’UTR region of the Twist1 mRNA contains a potential binding site for miR-363-3p (Figure 2B). The luciferase assay data indicated that miR-363-3p mimics repress the expression of a reporter gene containing WT 3’-UTR of Twist1 but not a reporter containing a mutated 3’-UTR (Figure 2B). These results demonstrate that Twist1 is a direct target for miR-363-3p.
Figure 4.
miR-363-3p affects the invasion and apoptosis of 786-O cells via its interaction with Twist1. A. Twist1 protein expression levels were increased in cells treated with the miR-363-3p inhibitor compared to those treated with NC and were decreased in cells treated with the miR-363-3p mimic. B. A potential binding site for miR-363-3p in Twist1 is shown, and binding was detected by a luciferase reporter assay. C, D. The effects of inhibition of Twist1 in 786-O cells on invasion and apoptosis were determined. E. The expression of MMP2 and MMP9 protein were measured and quantitated (right panel) following control shRNA treatment of shRNA downregulation of miR-363-3p in 786-O cells (The original western blot images reference to Supplementary Figure 1). *P<0.05, **P<0.01, ***P<0.001.
Effect of Twist1 on metastasis and apoptosis of RCC cells in vitro
In order to determine whether Twist1 is involved in ccRCC development, we established shRNA to interfere with the expression of Twist1 in the 786-O cell line and then assessed the effects of Twist1 on invasion of 786-O cells in a Transwell assay with Matrigel and on cell apoptosis using flow cytometry. Inhibition of Twist1 in 786-O cells induced a significant reduction in cell invasion (Figure 4C) and increased the apoptosis rate (Figure 4D). It is well known that invasion ability can promote tumor metastasis, which is the main cause of death in patients with ccRCC. Subsequently, immunohistochemistry analysis revealed that the Twist1 expression ratio (60%, n=29) in metastatic RCC specimens was significantly higher than that (30%, n=36) in non-metastatic RCC specimens (χ2=15, P=0.01) (Supplementary Figure 2). Furthermore, silencing miR-363-3p significantly decreased MMP2 and MMP9 protein levels in 786-O cells (Figure 4E). Together, these results demonstrate that Twist1 regulates RCC migration and invasion via the MMP2 and MMP9 pathway.
Discussion
Accumulating data indicate that lncRNAs are a critical regulator of cancer-related processes; however, the molecular mechanisms by which lncRNA modulates the behavior of cancer cells remain unknown. The lncRNA SNHG5 is one of the most well-studied lncRNAs, and previous studies showed that overexpression of lncRNA SNHG5 promotes imatinib resistance in chronic myeloid leukemia [18]. Depletion of SNHG5, alternatively, induces cell cycle arrest and apoptosis and limits tumor outgrowth in vitro [16]. In our study, qRT-PCR showed that SNHG5 expression levels in ccRCC tissues were significantly increased compared to normal tissues. Knockdown of SNHG5 inhibited RCC cell invasion and promoted cell apoptosis in vitro, in agreement with previous studies in other cell types. These findings indicate that SNHG5 may function as an oncogene, and overexpression of SNHG5 may contribute to RCC development.
Several studies suggested that lncRNAs are involved in tumor progression via competitive inhibition of miRNAs by functioning as a molecular sponge. For instance, lncRNA HOTAIR is involved in the regulation of glycolysis via miR-125/miR-143-HK2 in esophageal squamous cell carcinoma progression [19], and lncRNA-TTN-AS1 promoted Snail and FSCN1 expression by competitively binding to miR-133b [20]. In our study, miR-363-3p was downregulated in the ccRCC tissues compared to the para-cancerous ccRCC tissues. Furthermore, downregulation of miR-363-3p using shRNA promoted RCC cell invasion and inhibited cell apoptosis in vitro. Thus, we concluded that lncRNA SNHG5 regulated RCC cell invasion and cell apoptosis by targeting miR-363-3p.
Twist1, which is a basic helix-loop-helix (bHLH) domain-containing transcription factor [21,22], is indispensable for mesoderm specification and differentiation during embryonic development [22,23]. Twist1 plays a role in resistance to apoptosis of thyroid cancer cells [24], and more importantly, Twist1 is expressed in multiple types of invasive cancer cells, inducing the epithelial-mesenchymal transition (EMT) and enhancing cell invasion and metastasis [25,26]. Furthermore, Twist1 represses E-cadherin expression and upregulates Bmi1, AKT2, YB-1, and WNT5A to drive EMT, cell metastasis, and invasion [27-30]. Here, we confirmed a direct Twist1-miR-363-3p interaction in dual-luciferase reporter assays, and functional assays showed that Twist1 promotes cell invasion and inhibits cell apoptosis in RCC cells in vitro. Meanwhile, silencing Twist1 decreased the expression of MMP2 and MMP9. Subse-quently, immunohistochemistry analysis revealed that the Twist1 expression in metastatic RCC specimens was significantly higher than that in non-metastatic RCC specimens (Supplementary Figure 2), indicating that lncRNA SNHG5 is involved in metastasis of ccRCC in vitro and in vivo via regulation of the miR-363-3p-Twist1 interaction.
In conclusion, we report that lncRNA SNHG5 affects the invasion and apoptosis of RCC by regulating the expression of miR-363-3p, leading to aberrant expression of the transcription factor Twist1. Elucidation of the effect of lncRNA SNHG5 on ccRCC progression provides insight into the mechanisms underlying ccRCC carcinogenesis.
Disclosure of conflict of interest
None.
Supporting Information
References
- 1.Ljungberg B, Campbell SC, Choi HY, Cho HY, Jacqmin D, Lee JE, Weikert S, Kiemeney LA. The epidemiology of renal cell carcinoma. Eur Urol. 2011;60:615–621. doi: 10.1016/j.eururo.2011.06.049. [DOI] [PubMed] [Google Scholar]
- 2.Bex A, Jonasch E, Kirkali Z, Mejean A, Mulders P, Oudard S, Patard JJ, Powles T, Van Poppel H, Wood CG. Integrating surgery with targeted therapies for renal cell carcinoma: Current evidence and ongoing trials. Eur Urol. 2010;58:819–828. doi: 10.1016/j.eururo.2010.08.029. [DOI] [PubMed] [Google Scholar]
- 3.Banumathy G, Cairns P. Signaling pathways in renal cell carcinoma. Cancer Biol Ther. 2010;10:658–664. doi: 10.4161/cbt.10.7.13247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li M, Wang Y, Song Y, Bu R, Yin B, Fei X, Guo Q, Wu B. MicroRNAs in renal cell carcinoma: A systematic review of clinical implications. Oncol Rep. 2015;33:1571–1578. doi: 10.3892/or.2015.3799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Liz J, Portela A, Soler M, Gómez A, Ling H, Michlewski G, Calin GA, Guil S, Esteller M. Regulation of pri-miRNA processing by a long noncoding RNA transcribed from an ultraconserved region. Mol Cell. 2014;55:138–147. doi: 10.1016/j.molcel.2014.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Kim BK, Yoo HI, Choi K, Yoon SK. MiR-330-5p inhibits proliferation and migration of keratinocytes by targeting Pdia3 expression. FEBS J. 2015;282:4692–4702. doi: 10.1111/febs.13523. [DOI] [PubMed] [Google Scholar]
- 7.Shen F, Cai WS, Feng Z, Chen J, Feng J, Liu Q, Fang Y, Li K, Xiao H, Cao J, Xu B. Long non-coding RNA SPRY4-IT1 pormotes colorectal cancer metastasis by regulate epithelial-mesenchymal transition. Oncotarget. 2017;28:14479–14486. doi: 10.18632/oncotarget.10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mehra R, Udager AM, Ahearn TU, Cao X, Feng FY, Loda M, Petimar JS, Kantoff P, Mucci LA, Chinnaiyan AM. Overexpression of the long non-coding RNA SChLAP1 independently predicts lethal prostate cancer. Eur Urol. 2016;70:549–552. doi: 10.1016/j.eururo.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen W, Huang M, Kong R, Xu T, Zhang E, Xia R, Sun M, De W, Shu Y. Antisense long noncoding RNA HIF1A-AS2 is upregulated in gastric cancer and associated with poor prognosis. Dig Dis Sci. 2015;60:1655–1662. doi: 10.1007/s10620-015-3524-0. [DOI] [PubMed] [Google Scholar]
- 10.Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc Natl Acad Sci U S A. 2007;104:17719–17724. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cai X. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liang T, Hu XY, Li YH, Tian BQ, Li ZW, Fu Q. MicroRNA-21 regulates the proliferation, differentiation, and apoptosis of human renal cell carcinoma cells by the mTOR-STAT3 signaling pathway. Oncol Res. 2016;24:371–380. doi: 10.3727/096504016X14685034103356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tanaka R, Satoh H, Moriyama M, Satoh K, Morishita Y, Yoshida S, Watanabe T, Nakamura Y, Mori S. Intronic U50 small-nucleolar-RNA (snoRNA) host gene of no protein-coding potential is mapped at the chromosome breakpoint t(3;6)(q27;q15) of human B-cell lymphoma. Genes to Cells. 2000;5:277–287. doi: 10.1046/j.1365-2443.2000.00325.x. [DOI] [PubMed] [Google Scholar]
- 15.Ichigozaki Y, Fukushima S, Jinnin M, Miyashita A, Nakahara S, Tokuzumi A, Yamashita J, Kajihara I, Aoi J, Masuguchi S, Zhongzhi W, Ihn H. Serum long non-coding RNA, snoRNA host gene 5 level as a new tumor marker of malignant melanoma. Exp Dermatol. 2016;25:67–69. doi: 10.1111/exd.12868. [DOI] [PubMed] [Google Scholar]
- 16.Damas ND, Marcatti M, Côme C, Christensen LL, Nielsen MM, Baumgartner R, Gylling HM, Maglieri G, Rundsten CF, Seemann SE, Rapin N, Thézenas S, Vang S, Qrntoft T, Andersen CL, Pedersen JS, Lund AH. SNHG5 promotes colorectal cancer cell survival by counteracting STAU1-mediated mRNA destabilization. Nat Commun. 2016;7:13875. doi: 10.1038/ncomms13875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao L, Han T, Li Y, Sun J, Zhang S, Liu Y, Shan B, Zheng D, Shi J. The lncRNA SNHG5/miR32 axis regulates gastric cancer cell proliferation and migration by targeting KLF4. FASEB J. 2017;31:893–903. doi: 10.1096/fj.201600994R. [DOI] [PubMed] [Google Scholar]
- 18.He B, Bai Y, Kang W, Zhang X, Jiang X. LncRNA SNHG5 regulates imatinib resistance in chronic myeloid leukemia via acting as a ceRNA against miR-205-5p. Am J Cancer Res. 2017;7:1704–1713. [PMC free article] [PubMed] [Google Scholar]
- 19.Ma J, Fan Y, Feng T, Chen F, Xu Z, Li S, Lin Q, He X, Shi W, Liu Y, Liu Z, Zhu B, Cao X. HOTAIR regulates HK2 expression by binding endogenous miR-125 and miR-143 in oesophageal squamous cell carcinoma progression. Oncotarget. 2018;9:23843. doi: 10.18632/oncotarget.25377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lin C, Zhang N, Wang Y, Nice E, Guo C, Zhang E, Yu L, Li M, Liu C, Hu L, Hao J, Qi W, Xu H. Functional role of a novel long noncoding RNA TTN-AS1 in esophageal squamous cell carcinoma progression and metastasis. Clin Cancer Res. 2017;24:486–498. doi: 10.1158/1078-0432.CCR-17-1851. [DOI] [PubMed] [Google Scholar]
- 21.Thisse B, El Messal M, Perrin-Schmitt F. The twist gene: isolation of a Drosophila zygotle gene necessary for the establishment of dorsoventral pattern. Nucleic Acids Res. 1987;15:3439–3453. doi: 10.1093/nar/15.8.3439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vincentz JW, Firulli BA, Lin A, Spicer DB, Howard MJ, Firulli AB. Twist1 controls a cell-specification switch governing cell fate decisions within the cardiac neural crest. PLoS Genet. 2013;9:e1003405. doi: 10.1371/journal.pgen.1003405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen ZF, Behringer RR. Twist is required in head mesenchyme for cranial neural tube morphogenesis. Genes Dev. 1995;9:686–699. doi: 10.1101/gad.9.6.686. [DOI] [PubMed] [Google Scholar]
- 24.Orlandella FM, Di Maro G, Ugolini C, Basolo F, Salvatore G. TWIST1/miR-584/TUSC2 pathway induces resistance to apoptosis in thyroid cancer cells. Oncotarget. 2016;7:70575–70588. doi: 10.18632/oncotarget.12129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Qin Q, Xu Y, He T, Qin C, Xu J. Normal and disease-related biological functions of Twist1 and underlying molecular mechanisms. Cell Res. 2012;22:90–106. doi: 10.1038/cr.2011.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yang J, Mani SA, Donaher JL, Ramaswamy S, Itzykson RA, Come C, Savagner P, Gitelman I, Richardson A, Weinberg RA. Twist, a master regulator of morphogenesis, plays an essential role in tumor metastasis. Cell. 2004;117:927–939. doi: 10.1016/j.cell.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 27.Yang MH, Hsu DSS, Wang HW, Wang HJ, Lan HY, Yang WH, Huang CH, Kao SY, Tzeng CH, Tai SK, Chang SY, Lee OK, Wu KJ. Bmi1 is essential in Twist1-induced epithelial-mesenchymal transition. Nat Cell Biol. 2010;12:982–992. doi: 10.1038/ncb2099. [DOI] [PubMed] [Google Scholar]
- 28.Cheng GZ, Zhang W, Wang LH. Regulation of cancer cell survival, migration, and invasion by Twist: AKT2 comes to interplay. Cancer Res. 2008;68:957–960. doi: 10.1158/0008-5472.CAN-07-5067. [DOI] [PubMed] [Google Scholar]
- 29.Kalra J, Sutherland BW, Stratford AL, Dragowska W, Gelmon KA, Dedhar S, Dunn SE, Bally MB. Suppression of Her2/neu expression through ILK inhibition is regulated by a pathway involving TWIST and YB-1. Oncogene. 2010;29:6343–6356. doi: 10.1038/onc.2010.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi J, Wang Y, Zeng L, Wu Y, Deng J, Zhang Q, Lin Y, Li J, Kang T, Tao M, Rusinova E, Zhang G, Wang C, Zhu H, Yao J, Zeng YX, Evers BM, Zhou MM, Zhou BP. Disrupting the interaction of BRD4 with diacetylated twist suppresses tumorigenesis in basal-like breast cancer. Cancer Cell. 2014;25:210–225. doi: 10.1016/j.ccr.2014.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.