Abstract
Although overall survival in colorectal cancer (CRC) is increasing steadily due to progress in screening, therapeutic options and precise diagnostic tools remain scarce. As the understanding of CRC as a complex and multifactorial condition moves forward, the tumor microenvironment has come into focus as a source of diagnostic markers and potential therapeutic targets. The role of TGFβ in shifting the epithelial cancer compartment towards invasiveness and a pro-migratory phenotype via stromal signaling has been widely investigated. Accordingly, recent studies have proposed that CRC patients could be stratified into distinct subtypes and have identified one poor prognosis subset of CRC that is characterized by high stromal activity and elevated levels of TGFβ. The TGFβ superfamily member activin A is crucial for the pro-metastatic properties of the TGFβ pathway, yet it has been under-researched in CRC carcinogenesis. In this review, we will elucidate the signaling network and interdependency of both ligands in the context of the tumor microenvironment in CRC.
Keywords: TGFβ, activin A, tumor microenvironment, colorectal cancer
Introduction
Colorectal cancer (CRC) is among the deadliest cancers worldwide, approximately 50,000 annual cancer-associated deaths in the United States alone are attributed to CRC (1). Mortality is mainly due to metastatic disease, with 5-year survival rates as low as 14% in patients diagnosed at advanced stages (2). This circumstance has driven researchers to search for predictive biomarkers that indicate a tumor’s propensity to metastasize and ideally to develop therapeutic strategies that specifically combat metastasis-prone tumors. Recent advances have been made in the understanding of colorectal cancer in its entirety, where the paradigm of carcinogenesis being only attributed to faulty epithelial cells is being questioned. Investigation of the tumor microenvironment (TME) has given us important insights into the complex metastasis-driven forces that do not originate from but are potentiated by the epithelial axis.
The TME is seen as the non-malignant entity which is comprised of cellular components (fibroblasts, mesenchymal stem cells, osteoblasts, fat cells, blood and immune cells) as well as the extracellular matrix (ECM) scaffold surrounding the cancerous cells (3, 4). The TME has gained a lot of attention as a provider of growth factors, cytokines and other pro-metastatic and anti-apoptotic molecules that orchestrate the malignant cell’s ability to migrate. A body of evidence has emerged indicating that the interplay between the tumor and surrounding stroma is pivotal for a cancer to spread to distant sites, as first described by Paget’s ‘seed and soil theory’ (5). Rather than regarding the TME as a stationary compartment, it should be considered as equally prone to changes like its surrounding cancer cells, and there is likely a parallel co-evolution of cancer cells and the TME (6). To co-opt and promote the pro-tumorigenic effects of the TME is necessary for cancer cells to progress, and the more stroma-rich a tumor is, the more aggressive the tumor becomes. Therefore, gene signatures and overexpressed proteins in the cancer tissue that are associated with stromal activity provide potential biomarkers that are indicative of patient prognosis and disease progression (7–11).
Transforming Growth Factor β (TGFβ) is major player in tumorigenic stroma-cancer interactions, and the tumor stroma is well known to be a rich source of TGFβ (12, 13). Given the abundance of the molecule and its pro-metastatic effects, TGFβ was intensively studied and targeted in many clinical trials, although with mixed results (14). The underlying problem of its underperformance as a drug target may be due to its highly context-dependent behavior as a tumor suppressor and promoter, and many aspects of its signaling network are still unclear. TGFβ superfamily member activin A is involved in and necessary for some TGFβ effects. As such, it has been shown that the invasive, pro-metastatic CRC phenotype induced by TGFβ is activin A-dependent. Furthermore, the fact that TGFβ and activin A expression correlates on the mRNA level in colorectal tumors and co-occurring mutations in their receptors are frequent, suggests a relationship between these signaling pathways (15). TGFβ and activin A are indispensable players in CRC metastasis, and assessment of the two molecules could therefore yield important prognostic information. Since they are not only structurally related, but their signaling pathways are entwined, TGFβ and activin A pathways should be viewed as a network, and the ligands should be assessed together (15, 16). Especially in settings of uncertainty of disease relapse (high-risk patients in stage II), TGFβ and activin A could serve as biomarkers to stratify patients into tailored chemotherapy regimens, and given their pro-metastatic effects, TGFβ and activin A could be exploited as dual drug targets in advanced CRC.
1. The bigger picture: The colorectal cancer microenvironment
The initiative to define a molecular signature for colorectal cancer was undertaken to provide prognostic and predictive information that goes beyond the classical TNM (primary tumor, lymph node, metastasis) staging. However, aside from KRAS testing in metastatic CRC (mCRC) and assessment for microsatellite instability (MSI), a diagnostic tool that encompasses mutational characterization of the epithelium has not been implemented in the clinic. The TME has become a focus of interest in CRC, as it is becoming evident that much of the prognostic information in fact lies in the composition of the environmental factors supporting cancer cell growth and metastases. For example, a high stromal fraction in tumor tissues is associated with poor prognosis and higher tumor staging (10, 17), as evidenced by a correlation between tumor-infiltrating immune cells and patient outcome (18).
The tumor microenvironment initially acts in a tumor-suppressive manner by default; however, at some point in carcinogenesis the TME fails this task and starts promoting pro-tumorigenic pathways. Furthermore, metastatic spread would not be possible without a favorable TME. Thus, researchers in the field have endeavored to identify factors that prompt the TME to switch to a tumor-promoting milieu with pro-metastatic functions. Recent studies indicate that TGFβ and activin A are intimately involved in this process (14, 19, 20).
2. TGFβ
TGFβ, a member of the TGFβ superfamily, plays a role in a spectrum of physiologic processes including growth, differentiation, and migration, but is also associated with fibrosis, immune suppression, and carcinogenesis (21–24). Other members of the TGFβ superfamily include activins, bone morphogenetic proteins (BMPs), nodals and growth and differentiation factors (GDFs) (25). In the healthy colon epithelium, TGFβ is crucial for homeostasis, as increasing gradients from crypt to surface control enterocyte growth. Furthermore, it is a mediator of intestinal immunity (26). There are three isoforms of TGFβ: TGFβ 1, TGFβ 2, TGFβ 3, with TGFβ 1 being the most prominent. TGFβ signals through receptors that are serine-threonine kinases that comprise a heterotetramer of two type I and two type II receptor subunits (TGFBRI and TGFBRII). A cellular response is elicited through binding of TGFβ ligand that initiates the type II receptor phosphorylation of the type I receptor, which in turn allows association of the receptor SMADs (R-SMADs) SMAD2 and SMAD3 (27). After dimerization with SMAD4, the complex translocates to the nucleus to initiate a transcriptional response. Other stimulated pathways that are not SMAD-dependent are considered non-canonical. This heterogeneous group includes PI3K/Akt, MAPK/Erk, WNT/β-catenin, Rho-like GTPases and JNK/p38 pathways (28, 29). TGFβ signaling is highly context-dependent, where the cellular response may be influenced by tissue type, concentration of ligands and mutations in pathway components (30).
Germline mutations in components of TGFβ superfamily signaling pathways have been linked to increased risk of developing CRC. For example, increased susceptibility to CRC is observed in individuals harboring germline mutations in the BMPR1 and SMAD4 genes, leading to a condition known as juvenile polyposis syndrome (JPS) (31). Patients with JPS develop juvenile hamartomatous lesions in the stomach, small intestine, and colon and have a 50% lifetime risk of developing GI cancer (32). Germline variations in the TGFβ receptors are also associated with higher risk of developing CRC, although the extent of the effect is likely modest (32).
TGFβ signaling has been described as tumor-suppressive in early stages of carcinogenesis, based on observations of their SMAD-dependent growth inhibition through p21 activation and induction of apoptosis (33–35). However, various reports of TGFβ in metastatic CRC add to the complexity of its framework. Inactivating mutations in pathway components such as SMAD4 are seen in 30% of cancers and are typically considered a late stage event associated with metastatic CRC (36). Loss of SMAD4 seems to be an almost exclusive event in microsatellite stable (MSS) cancers. In a study of protein expression of SMAD4 in sporadic colorectal neoplasia, only 4% of MSI carcinomas showed depletion of SMAD4 expression (37). Loss of canonical SMAD expression may be the main factor to co-opt the TGFβ signaling network and circumvent the tumor-suppressing effects, leaving only the non-canonical pro-tumorigenic and pro-metastatic functions, such as enhanced cell migration, cell growth and resistance to apoptosis (24, 34, 38, 39). Interestingly, in a study aiming at identifying high risk stage I and II patients, patients with high tumor stroma and loss of SMAD4 had the most unfavorable prognosis compared to stroma low and SMAD4 intact patients (40).
The non-canonical mitogenic MAPK/Erk and the survival-promoting PI3K/Akt pathway are two prominent targets of TGFβ, and are known to drive cancer cell malignancy (28). One of the main factors by which TGFβ facilitates metastatic spread is by the induction of EMT, a process by which cancer cells lose their epithelial polarity and tight junctions and express mesenchymal proteins such as vimentin. EMT is a hallmark of metastasis and is necessary for cancer cells to acquire mesenchymal characteristics that endow them with the ability to migrate and invade distant tissues (41). In a study of a 5-FU-resistant colorectal cancer cell line, drug resistance was associated with upregulation of EMT markers and a change in cellular morphology. As TGFβ is a prominent inducer of EMT, this suggests another mechanism as to how TGFβ can render CRC more aggressive (42).
2.1. TGFβ in the TME as master regulator of CRC malignancy
TGFβ has been extensively studied and is now recognized a main driver of metastasis in CRC. Although many of the mechanisms that alter TGFβ’s behavior from tumor suppressor to promoter remain elusive, the field has reached consensus that TGFβ is predominantly over-represented in a late stage setting, and in combination with the TME, promotes disease progression and increases the likelihood of metastatic spread (43). TGFβ can be used as a prognostic biomarker in CRC, as it is indicative of survival and disease relapse (44). A recent meta-analysis concluded that CRC patients with either elevated TGFβ serum levels or high TGFβ protein or mRNA expression in the primary tumor have worse overall survival (with a hazard ratio (HR) of 1.68) compared to low TGFβ-expressing patients (44).This analysis strongly suggests that TGFβ is pivotal for cancer progression. However, mutations of TGFβ pathway components are frequent in CRC, such as inactivating mutations in TGFBRII (45). What may seem paradoxical at first can be explained by the notion that TGFβ exerts its pro-metastatic functions through effects on the TME rather than the epithelium. Calon et al. showed that epithelial cancer cells are able to initiate metastasis through a stromal TGFβ-guided response (12). According to their study, TGFβ response signatures (TBRS) in TME cell types (T-cells, macrophages, and fibroblasts) were predictors of disease relapse in stage I-III patients. This group also developed an in vivo model to investigate the metastatic action of TGFβ in the stroma. TGFβ signaling was inhibited in a CRC epithelial cell line by mutating TGFβ receptor 2. TGFβ ligand was then overexpressed in these cells and inoculated in the caecum of nude mice. Compared to mice with non-overexpressing tumors, mice with TGFβ-overexpressing tumors developed significantly more metastases. Thus, this study points to the importance of TGFβ signaling in the stroma specifically to promote metastasis and underscores the need for further study of the TME in CRC progression (12).
2.2. The immune landscape and its modulation by TGFβ
Suppressing antitumor immunity is a hallmark for cancer progression and is controlled by TGFβ on many levels. The cytokine represses cytolytic activity of CD8+ cytotoxic T-lymphocytes (CTL) by inhibiting granzyme B, perforin and FAS-L (46), inhibits T-cell proliferation, reduces antigen spreading (47), reduces T-cell activation, and mediates Treg induction and activity (48–50). The interplay between TGFβ and the adaptive immunity in CRC has been demonstrated in a study of an in vivo model of the metastatic consensus molecular subtype 4 (CMS4) which is defined by a TGFβ-rich stroma and first described by Guinney et al. (51). In this study, upon depletion of CD8+ CTL and CD4+ T-helper cells, metastatic tumors were no longer responsive to TGFBRI inhibitor Galunisertib, suggesting that the anti-tumor effect of TGFβ pathway inhibition is dependent on a functional adaptive immune system. Furthermore, mice receiving Galunisertib in combination with anti-PD-L1 antibodies had more remissions and longer disease-free survival than mice treated with Galunisertib alone. This finding might be crucial for combatting metastasis, as dual targeting of TGFβ and PD-1/PD-L1 might have synergistic effects (51, 52). The authors further demonstrated that in an MSS patient cohort, TGFβ1, 2 and 3 expression negatively correlated with the ratio of TH1/TH-naïve cells, implying that TGFβ might suppress T-cell maturation (51, 53). Another report showed that dysregulation of the ECM through TGFβ overexpression in ‘immunogenically hot’ tumors predicted failure of treatment with PD-1 inhibitors, again implying TGFβ to be a pivotal modulator of the adaptive immunity, and substantiating the rationale of dual targeting of TGFβ and PD-1/PD-L1 (54).
The effects by which TGFβ regulates the innate immunity are just as meaningful to promote cancer progression (55). As such, TGFβ acts as a chemoattractant for neutrophils and polarizes them to the pro-tumorigenic N2-phenotype (56). Subsequently, N2 TANs are able to undermine the antitumor immunity (57). Furthermore, cancer-associated fibroblast (CAFs), which are primarily induced by TGFβ, have been shown to shift macrophage populations in CRC towards M2 (58). Taken together, a main mode of action for TGFβ is fine-tuning the tumor-promoting effects of the immune landscape in colorectal cancer.
2.3. Desmoplastic tumor stroma and TGFβ
In wound healing, epithelial cells are physiologically able to migrate through the tissue by undergoing EMT. That, along with activated contractile myofibroblasts, is necessary for wound closure (59). Desmoplasia, a phenomenon that creates a rigid microenvironment that ‘forces’ tumor cells to undergo EMT and metastasize, is sustained by the pro-fibrotic effects of TGFβ (60). As such, TGFβ activates EMT in tumor cells, induces α-SMA expression in fibroblasts to transform them to the CAF phenotype, and promotes deposition of ECM components, ultimately leading to increased tissue rigidity through positive feedback loops (61, 62). As tumors progress, tissue density increases, and cancer cells are confronted with remarkable mechanical forces exerted by the contractile abilities of TGFβ-activated CAFs. A stiffening microenvironment further promotes CAF differentiation (63, 64) and increases TGFβ secretion (65), creating a vicious cycle and a self-sustained imbalance that ultimately increases the cancer cell’s propensity to metastasize.
3. The role of activin A in the TGFβ signaling network
Activin A, a member of the TGFβ superfamily, was originally described as a multifunctional protein in embryonic development as well as gonadal and pituitary physiology (66). The cytokine has been studied in the context of esophageal (67), skin (68), ovarian (69), lung (70), breast (71, 72), pancreatic (73) and colorectal cancer (74, 75) and may be critical in cancer cachexia (76). Similar to the TGFβ pathway, activin A binds to serine/threonine kinase receptors. Three type I receptors (ACVRIA, ACVRAIB, ACVRIC) and two type II receptors (ACVRIIA, ACVRIIB) exist (77). Dimerization of a type I and type II receptor after ligand binding allows phosphorylation of the type I receptor and leads to activation of the canonical SMAD2/3 cascade to elicit a transcriptional response (78).
In the context of CRC, inactivating mutations of ACVRIIA alongside TGFBRII mutations are very common in patients with MSI CRC. Microsatellite instability causes frequent frameshift mutations within the polyadenine tracts of exon 10 in ACVRIIA and exon 3 in TGFBRII (79, 80). It has been reported that stage III and high-risk stage II patients with MSI receiving adjuvant chemotherapy harboring defective TGFβ receptors have a better 5-year disease-free survival compared to non-TGFBRII mutated MSI patients (81). However, this survival advantage has not been shown for ACVRII (82, 83). One study observed mutations in ACVRII to be associated with metastasis and decreased survival (84). Conflicting with these reports, it has consequently been shown that overexpression of activin A in CRC tissues is associated with stage IV tumors and indicates lower overall survival (74, 85), and serum activin A levels positively correlate with disease stage (86). These discrepancies can be explained by the fact activin A signaling, similar to TGFβ signaling, is context dependent. As with TGFβ, activin A plays a much larger role in the TME than in the epithelial cells. Therefore, assessing the mutational status of activin pathway components in the epithelial compartment might not yield qualitative prognostic information.
The activation of canonical SMAD2/3 and subsequent dimerization with SMAD4 is the core similarity between activin A and TGFβ signaling pathways (87). Despite distinct receptors, activin A and TGFβ pathways in CRC are often seen as redundant due to shared canonical SMAD signaling. However, it has been reported that canonical as well as non-canonical signaling patterns are divergent. p21, a primary transcriptional target of activated SMADs, carries out different cellular effects depending on whether the activin A or TGFβ signaling pathway is activated. For example, activin A-associated p21 signaling induces apoptosis, whereas TGFβ promotes growth suppression (34).
Non-canonical pathways associated with activin A and TGFβ are also distinct. It has been shown that activin A induces a downstream PI3K/Akt response, and TGFβ engages the MAP/Erk pathway to enable EMT (88). Interestingly, activated Erk is able to phosphorylate SMAD2 and 3 on alternative sites, which impedes canonical, tumor-suppressive transcriptional activity (89). As concerning non-canonical activin A signaling, the beforementioned loss of SMAD4 causes upregulation of Akt, and in the clinical context, patients with SMAD4 mutations are more likely to show increased protein expression of phosphorylated Akt, which in turn predicts poor prognosis (90).
Activin A is increasingly recognized as a player in the metastatic process in CRC, as it carries out many of the malignant effects of TGFβ. As such, in a study of a CRC cell line, the pro-metastatic phenotype of TGFβ-treated cells was found to be activin A-dependent (15). The authors of the same study also report that activin A and TGFβ should be assessed together, as combined activin A and TGFβ protein expression scores in stage II patients yield a better prognostic information than either ligand alone (15). Acknowledging the differences in activin A and TGFβ while recognizing them as functionally intertwined is an important milestone and justifies continued investigation.
3.1. Activin A in the TME
Not only is activin A an important component in TGFβ signaling, the cytokine itself has many effects on cells of the TME to promote metastasis. Activin A is one of the first responders in wound healing, elevated mRNA expression of INHBA, the gene encoding for the β A subunit of activin A, is observed within 24 hours of wound infliction (91). However, perpetual activin A activity in tumors might add to the desmoplastic process, as overexpression of activin A is associated with excessive scarring and fibrosis (92). Strengthening the concept of activin A as a mediator in desmoplasia, CAFs secrete activin A in contrast to non-activated fibroblasts (93, 94), and activin A release can be potentiated by increased tissue stiffness (95). Strikingly, fibroblasts are a richer source of activin A than epithelial cells, and treatment with TGFβ leads to increased activin A release in epithelial and stromal cells, again underscoring a close reciprocal relationship of both ligands in the desmoplastic process (15).
The upregulation of MMPs by activin A provides another mechanistic insight into its function in facilitating metastasis. Induction of MMP-7 by activin A is necessary for dissolving the basement membrane and other components of the ECM to enable metastatic spread (96). Early increase of activin A in wounds and inflammatory processes establishes a logical link to its capacity to regulate inflammation and the innate immunity (97). This notion is corroborated by a study of LPS-stimulated mice, where the cytokines TNF-α and IL-1β were decreased after treatment with activin A antagonist follistatin (98).
It is still under debate whether activin A is considered a pro- or anti-inflammatory cytokine. The answer, as in many cases, may lie in the conditions where the molecule is active. Activin A was shown to have suppressive effects on pro-inflammatory IL-6 in a study of rheumatoid arthritis (99). Interestingly IL-6 regulation in amnion cells seems to be activin A dose-dependent; low quantities of activin A lead to a decrease in IL-6, whereas high amounts lead to a IL-6 increase, highlighting its complex role in coordinating inflammatory processes (100). INHBA is overexpressed in patients with inflammatory bowel diseases (101). The role of activin A in CRC-associated inflammation remains elusive. It has been reported that activin A is able to induce both the inflammatory M1 and the pro-tumorigenic M2 phenotype in macrophages (102, 103). Activin A modulates neutrophil function (104), but further studies are needed to investigate whether activin A influences their polarization.
Activin A has been demonstrated to influence the function of T-cells, as such it is able to induce Treg cells, which suppress antitumor immunity (105–107). Intriguingly, activin A is able to convert CD4+CD25- T-cells into iTreg that express FOXP3+ in a TGFβ-dependent manner (108), again hinting at a close relationship between activin A and TGFβ signaling. In line with these findings, a recent report showed that Treg induced by TGFβ increases mRNA expression of activin-receptor 1 (ACVRI) and activin A ligand (50). This suggests that TGFβ and activin A synergize to promote Treg activity and act in tandem to suppress anti-cancer immune responses.
3.2. TGFβ and activin A as drug targets
The context-dependent behavior and the various physiologic functions exerted by activin A and TGFβ yield challenges to targeting the pathways for cancer treatment. Nevertheless, the prominence of both molecules in advanced CRC makes them attractive targets in a late stage setting, or in situations with high likelihood of metastatic spread. However, patients need to be carefully selected for treatment to specifically combat pro-metastatic effects and ensure tumor-suppressive functions are not abolished. One approach could be to assess for activin A and TGFβ in tumor samples with emphasis on the stromal levels of the ligands. Another tool could be to use established fibroblast and T-cell response signatures (F-TBRS, T-TBRS) of TGFβ (12, 109) and activin A to help identify patients who would benefit the most from anti-activin A or anti-TGFβ treatment. Since the TGFβ and activin A signaling networks are intertwined and respective receptors are structurally related, small molecule inhibitors that target both activin A and TGFβ receptors could be the most effective option (110). Thus far, targeting the TGFβ pathway has been conducted by four different approaches: small molecule inhibitors, neutralizing antibodies, fusion proteins, and vaccines, whereas small molecule inhibitors and one ligand trap are the most promising tools for targeting activin A (Table 1). Current clinical trials are exploring the synergistic effects of drugs targeting the PD-L1 and TGFβ pathway, as the combination has been shown to be efficacious in various preclinical modalities of advanced cancer (51, 111). Two ongoing clinical trials are targeting TGFβ and PD-L1/PD-1 with separate drugs, and one utilizes a combined PD-L1/TGFβ ligand trap (https://clinicaltrials.gov : NCT02734160; NCT02423343; NCT03620201). As activin A has a similar potency in suppressing antitumor immunity as TGFβ, future studies will show whether combined inhibition of immune checkpoints and activin A can be effective in selected patient populations (68).
Table 1:
Therapeutic approaches to inhibit components of the activin A and TGFβ pathways in solid tumors. (ALK-1 activin receptor-like kinase, TGFBRI TGFβ receptor 1, ACVRI activin receptor 1)
| Name of drug | Target | Clinical phase | Type of cancer | Reference | Trial registration number | Status/Main outcome |
|---|---|---|---|---|---|---|
| TGFβ targeting agents | ||||||
| Small molecule inhibitors | ||||||
| PF-03446962 | ALK-1 | Phase I | Hepatocellular carcinoma (HCC) | (112) | NCT00557856 | Completed/No complete or partial responses. 50% had stable disease |
| TEW-7197 (Vactosertib) | TGFBRI | Phase I | Advanced stage solid tumors | (113) | NCT02160106 | Completed/Well tolerated, Vactosertib showed increased efficacy in patients with high fibroblast TGFβ response signature (F-TBRS) |
| LY3200882 | TGFBRI | Phase I | Solid tumors | NCT02937272 | recruiting | |
| LY2157299 (Galunisertib) | TGFBRI | Phase I/II | Metastatic colorectal cancer (mCRC) | NCT03470350 | recruiting | |
| Phase II | Metastatic Prostate cancer | NCT02452008 | recruiting | |||
| Ligand traps | ||||||
| AVID200 | TGFβ 1, TGFβ 3 | Phase I | Metastatic solid tumors | NCT03834662 | recruiting | |
| Vaccines | ||||||
| bi-shRNAifurin/GMCSF DNA/Autologous Tumor Cell Vaccine (FANG) | short hairpin RNAi (bi-shRNAi) targeting furin convert se to block TGFβ 1 and TGFβ 2 activation | Phase II | Metastatic colorectal cancer (mCRC) | NCT01505166 | terminated | |
| Antibodies | ||||||
| GC1008 (Fresolimumab) | TGFBRI | Phase II | Metastatic breast cancer | (114) | NCT01401062 | Completed/Fresolimumab + radiation therapy, patients receiving 10mg/kg had significantly higher median overall survival than patients with 1mg/kg |
| Activin A targeting Agents | ||||||
| Small molecule inhibitors | ||||||
| ACE-011 (Sotatercept) | ACVRII | Phase II | Non-small cell lung cancer (NSCLC) | (115) | NCT01284348 | terminated |
| Phase II | Metastatic breast cancer | NCT00931606 | terminated | |||
| Ligand traps | ||||||
| STM 434 | receptor-Fc fusion protein against activin A | Phase I | Advanced solid tumors | (116) | NCT02262455 | Completed/53.5% had stable disease |
Conclusion
Since the discovery of the multistep adenoma-to-carcinoma sequence, it took many years to uncover that cancer cells cooperate with and are controlled by various cues of the TME. This paradigm shift helped us understand the metastasis-driving forces outside the epithelium and has led to the discovery of predictive and prognostic biomarkers, as well as potential therapeutic targets. For successfully combatting CRC, combination therapies that entail specific targets for cancer cells as well as the TME will be the most efficient solution. Investigating activin A and TGFβ, key players in the TME, has provided insights into how these molecules are able to orchestrate the pro-metastatic actions in the TME. Their tumor-promoting behavior in late stage cancers is an excellent example how progressing cancer cells co-opt a signaling network that is tumor-suppressive by nature. Activin A and TGFβ could serve as biomarkers for risk stratification in early and advanced tumor stages (high -risk stage II and stage III) and may be promising drug targets in patients where cancers have already metastasized. Future research will elucidate the precise mechanisms by which activin A and TGFβ influence functions of all cell types in the TME and will help us understand the crosstalk and synergism of both molecules in CRC. Furthermore, high throughput screening of colorectal tumors will aid to distinguish between a specific subtype of CRC with an activin A/TGFβ enriched TME by identifying response signatures of both molecules. Taken together, activin A and TGFβ are promising markers and targets in CRC, and their further investigation will help us develop necessary individual tailored therapies.
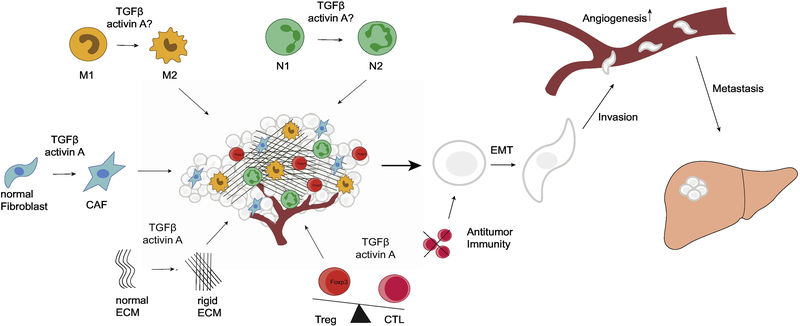
Figure 1:
The metastasis-promoting effects on the tumor microenvironment by activin A and TGFβ. Whereas TGFβ has been shown to shift neutrophil and macrophage populations towards N2/M2, the effects of activin A on those leukocytes are not as clear. Both molecules can induce the CAF-phenotype in fibroblasts, and reciprocally participate in the desmoplastic process in tumors. Desmoplasia is potentiated by a rigid ECM and the abundance of CAFs. Activin A and TGFβ are efficient suppressors of antitumor immunity and are able to induce immunosuppressive regulatory T-cells. The effects of activin A and TGFβ on the tumor microenvironment lead to increased cancer cells migration through induction of EMT, as well as angiogenesis and cancer cell invasiveness to ultimately accelerate the metastatic process.
(N1 classically activated neutrophils, N2 pro-tumor neutrophils, M1 classically activated macrophages, M2 pro-tumor macrophages, CAF cancer-associated fibroblasts CTL cytotoxic T-cells, Treg regulatory T-cells, EMT epithelial-to-mesenchymal transition)
Highlights.
The tumor microenvironment is a pivotal driver of colorectal cancer metastasis
TGFβ and activin A are the main determinants of a stroma-rich colorectal cancer subtype with poor prognosis
Assessing activin A and TGFβ in tumors can identify a metastasis-prone subset of colorectal cancer and guide individual-tailored therapies
Acknowledgements
JZS is supported by scholarships granted by the Medical University of Vienna and by the Foundation of Research and Education of Lower Austria (NÖ Forschungs- und Bildungsges.m.b.H.)
Funding
This work was supported by NIH RO1CA141057 awarded to BJ.
Abbreviations
- CAF
cancer-associated fibroblast
- CMS
consensus molecular subtype
- CRC
colorectal cancer
- CTL
cytotoxic T-lymphocyte
- ECM
extracellular matrix
- EMT
epithelial to mesenchymal transition
- IL
interleukin
- mCRC
metastatic colorectal cancer
- MMP
matrix metalloprotease
- MSI
microsatellite instability
- MSS
microsatellite stable
- PD1
programmed cell death protein 1
- PD-L1
programmed death-ligand 1
- TAM
tumor-associated macrophage
- TAN
tumor-associated neutrophil
- TBRS
TGFβ response signature
- TME
tumor microenvironment
- TNF-α
tumor necrosis factor α
- VEGF
Treg, regulatory T cell, vascular endothelial growth factor
- 5-FU
fluorouracil
Footnotes
Competing interests
The authors declare that they have no competing interests.
Declarations of interest: none
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA: A Cancer Journal for Clinicians. 2018;68(1):7–30.doi: 10.3322/caac.21442 [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Fedewa SA, Ahnen DJ, Meester RGS, Barzi A, et al. Colorectal cancer statistics, 2017. CA: A Cancer Journal for Clinicians. 2017;67(3):177–93.doi: 10.3322/caac.21395 [DOI] [PubMed] [Google Scholar]
- 3.Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenvironment. 2010;3:149–66.doi: 10.1007/s12307-010-0038-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Valkenburg KC, De Groot AE, Pienta KJ. Targeting the tumour stroma to improve cancer therapy. Nature Reviews Clinical Oncology. 2018;15:366–81.doi: 10.1038/s41571-018-0007-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stephen P The distribution of secondary growths in cancer of the breast. The Lancet. 1889;133:571–3.doi: 10.1016/S0140-6736(00)49915-0 [DOI] [Google Scholar]
- 6.Sleeman JP, Christofori G, Fodde R, Collard JG, Berx G, Decraene C, et al. Seminars in Cancer Biology Concepts of metastasis in flux : The stromal progression model. Seminars in Cancer Biology. 2012;22:174–86.doi: 10.1016/j.semcancer.2012.02.007 [DOI] [PubMed] [Google Scholar]
- 7.Sund M, Kalluri R. Tumor stroma derived biomarkers in cancer. Cancer and Metastasis Reviews. 2009;28(1):177–83.doi: 10.1007/s10555-008-9175-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sipos F, Germann TM, Wichmann B, Galamb O, Spisák S, Krenács T, et al. MMP3 and CXCL1 are potent stromal protein markers of dysplasia-carcinoma transition in sporadic colorectal cancer. European Journal of Cancer Prevention. 2014;23:336–43.doi: 10.1097/CEJ.0000000000000058 [DOI] [PubMed] [Google Scholar]
- 9.Bhome R, Goh R, Pillar N, Shomron N, Sayan E, Mirnezami A. ExomiRs can distinguish tumor-associated from normal stroma: Potential biomarkers in colorectal cancer. Cancer Research. 2018;78:5397 LP - doi: 10.1158/1538-7445.AM2018-5397 [DOI] [Google Scholar]
- 10.Danielsen HE, Hveem TS, Domingo E, Pradhan M, Kleppe A, Syvertsen RA, et al. Prognostic markers for colorectal cancer: estimating ploidy and stroma. Annals of Oncology 2018;29:616–23.doi: 10.1093/annonc/mdx794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cammarota R, Bertolini V, Pennesi G, Bucci EO, Gottardi O, Garlanda C, et al. The tumor microenvironment of colorectal cancer: stromal TLR-4 expression as a potential prognostic marker. Journal of Translational Medicine. 2010;8(1):112.doi: 10.1186/1479-5876-8-112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Calon A, Espinet E, Palomo-Ponce S, Tauriello DVF, Iglesias M, Céspedes MV, et al. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell. 2012.doi: 10.1016/j.ccr.2012.08.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Achyut BR, Yang L. Transforming Growth Factor-β in the Gastrointestinal and Hepatic Tumor Microenvironment. Gastroenterology. 2011;141(4):1167–78.doi: 10.1053/j.gastro.2011.07.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, et al. Targeting the TGFβ pathway for cancer therapy. Pharmacology and Therapeutics. 2015;147:22–31.doi: 10.1016/j.pharmthera.2014.11.001 [DOI] [PubMed] [Google Scholar]
- 15.Staudacher JJ, Bauer J, Jana A, Tian J, Carroll T, Mancinelli G, et al. Activin signaling is an essential component of the TGF-β induced pro-metastatic phenotype in colorectal cancer. Scientific Reports. 2017;7:1–9.doi: 10.1038/s41598-017-05907-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Loomans HA, Andl CD, Andl CD, Andl CD, Andl CD, Andl CD. Intertwining of activin a and TGFβ signaling: Dual roles in cancer progression and cancer cell invasion. Cancers. 2014;7:70–91.doi: 10.3390/cancers7010070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.van Pelt GW, Sandberg TP, Morreau H, Gelderblom H, van Krieken JHJM, Tollenaar RAEM, et al. The tumour–stroma ratio in colon cancer: the biological role and its prognostic impact. Histopathology. 2018;73(2):197–206.doi: 10.1111/his.13489 [DOI] [PubMed] [Google Scholar]
- 18.Type Galon J., Density, and Location of Immune Cells Within Human Colorectal Tumors Predict Clinical Outcome. Science. 2006;313(5795):1960–4.doi: 10.1126/science.1129139 [DOI] [PubMed] [Google Scholar]
- 19.Desmouliere A Transforming growth factor-beta 1 induces alpha-smooth muscle actin expression in granulation tissue myofibroblasts and in quiescent and growing cultured fibroblasts. 1993;122(1):103–11.doi: 10.1083/jcb.122.1.103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Calon A, Tauriello DVF, Batlle E. TGF-beta in CAF-mediated tumor growth and metastasis. Seminars in Cancer Biology. 2014;25:15–22.doi: 10.1016/j.semcancer.2013.12.008 [DOI] [PubMed] [Google Scholar]
- 21.Weiss A, Attisano L. The TGFbeta superfamily signaling pathway. Wiley Interdisciplinary Reviews: Developmental Biology. 2013;2:47–63.doi: 10.1002/wdev.86 [DOI] [PubMed] [Google Scholar]
- 22.Meng X-M, Nikolic-Paterson DJ, Lan HY. TGF-β: the master regulator of fibrosis. Nature Reviews Nephrology. 2016;12(6):325–38.doi: 10.1038/nrneph.2016.48 [DOI] [PubMed] [Google Scholar]
- 23.Wrzesinski SH, Wan YY, Flavell RA. Transforming Growth Factor- and the Immune Response: Implications for Anticancer Therapy. 2007;13(18):5262–70.doi: 10.1158/1078-0432.ccr-07-1157 [DOI] [PubMed] [Google Scholar]
- 24.Morikawa M, Derynck R, Miyazono K. TGF-β and the TGF-β Family: Context-Dependent Roles in Cell and Tissue Physiology. Cold Spring Harbor perspectives in biology;8(5):a021873.doi: 10.1101/cshperspect.a021873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wrana JL. Signaling by the TGF Superfamily. 2013;5(10):a011197–a.doi: 10.1101/cshperspect.a011197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.John A.Barnard, Ginger J.Warwick, Gold I, L. Localization of Transforming Growth Factor β lsoforms in the Normal Murine Small Intestine and Colon Gastroenterology. 1993;105(Issue 1):67–73 [DOI] [PubMed] [Google Scholar]
- 27.Massague J TGF-beta signal transduction. Annu Rev Biochem. 1998;67:753–91.doi: 10.1146/annurev.biochem.67.1.753 [DOI] [PubMed] [Google Scholar]
- 28.Zhang YE. Non-Smad pathways in TGF-β signaling. Cell Research. 2009;19:128–39.doi: 10.1038/cr.2008.328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang L, Zhou F, Ten Dijke P. Signaling interplay between transforming growth factor-β receptor and PI3K/AKT pathways in cancer. Trends in Biochemical Sciences. 2013;38(12):612–20.doi: 10.1016/j.tibs.2013.10.001 [DOI] [PubMed] [Google Scholar]
- 30.Massagué J TGFβ signalling in context. Nature Reviews Molecular Cell Biology. 2012;13:616.doi: 10.1038/nrm3434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elena M, Stoffel M and Kastrinos Fay. Familial CRC—Beyond the Lynch Syndrome. J Am Coll Surg. 2011;212:1049–60.doi: 10.1016/j.jamcollsurg.2011.02.017.Cost-Effective21444220 [DOI] [Google Scholar]
- 32.Jung B, Staudacher JJ, Beauchamp D. Transforming Growth Factor β Superfamily Signaling in Development of Colorectal Cancer. Gastroenterology. 2017;152:36–52.doi: 10.1053/j.gastro.2016.10.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schuster N, Krieglstein K. Mechanisms of TGF-β-mediated apoptosis. Cell and Tissue Research. 2002;307:1–14.doi: 10.1007/s00441-001-0479-6 [DOI] [PubMed] [Google Scholar]
- 34.Bauer J, Sporn JC, Cabral J, Gomez J, Jung B. Effects of Activin and TGFβ on p21 in Colon cancer. PLoS ONE. 2012;7:1–11.doi: 10.1371/journal.pone.0039381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jakowlew SB. Transforming growth factor-β in cancer and metastasis. Cancer and Metastasis Reviews. 2006;25:435–57.doi: 10.1007/s10555-006-9006-2 [DOI] [PubMed] [Google Scholar]
- 36.Woodford-Richens KL, Rowan AJ, Gorman P, Halford S, Bicknell DC, Wasan HS, et al. SMAD4 mutations in colorectal cancer probably occur before chromosomal instability, but after divergence of the microsatellite instability pathway. 2001;98(17):9719–23.doi: 10.1073/pnas.171321498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Salovaara R, Roth S, Loukola A, Launonen V, Sistonen P, Avizienyte E, et al. Frequent loss of SMAD4/DPC4 protein in colorectal cancers. 2002;51(1):56–9.doi: 10.1136/gut.51.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhao M, Mishra L, Deng C-X. The role of TGF-β/SMAD4 signaling in cancer. International journal of biological sciences. 2018;14(2):111–23.doi: 10.7150/ijbs.23230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Muñoz NM, Baek JY, Grady WM. TGF-β has paradoxical and context dependent effects on proliferation and anoikis in human colorectal cancer cell lines. Growth Factors. 2008;26:254–62.doi: 10.1080/08977190802291667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mesker WE, Liefers G-J, Junggeburt JMC, van Pelt GW, Alberici P, Kuppen PJK, et al. Presence of a high amount of stroma and downregulation of SMAD4 predict for worse survival for stage I-II colon cancer patients. Cellular oncology : the official journal of the International Society for Cellular Oncology. 2009;31(3):169–78.doi: 10.3233/CLO-2009-0478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ye X, Weinberg RA. Epithelial – Mesenchymal Plasticity : A Central Regulator of Cancer Progression. Trends in Cell Biology. 2015;25:675–86.doi: 10.1016/j.tcb.2015.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim AY, Kwak JH, Je NK, Lee Yh, Jung YS. Epithelial-mesenchymal transition is associated with acquired resistance to 5-fluorocuracil in HT-29 colon cancer cells. Toxicological Research. 2015;31:151–6.doi: 10.5487/TR.2015.31.2.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Calon A, Lonardo E, Berenguer-Llergo A, Espinet E, Hernando-Momblona X, Iglesias M, et al. Stromal gene expression defines poor-prognosis subtypes in colorectal cancer. Nature genetics. 2015;47:320–9.doi: 10.1038/ng.3225 [DOI] [PubMed] [Google Scholar]
- 44.Chen X-L, Chen Z-Q, Zhu S-L, Liu T-W, Wen Y, Su Y-S, et al. Prognostic value of transforming growth factor-beta in patients with colorectal cancer who undergo surgery: a meta-analysis. BMC Cancer. 2017;17(1).doi: 10.1186/s12885-017-3215-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Markowitz SD, Bertagnolli MM. Molecular Basis of Colorectal Cancer. New England Journal of Medicine. 2009;361(25):2449–60.doi: 10.1056/nejmra0804588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thomas DA, Massagué J. TGF-β directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80.doi: 10.1016/j.ccr.2005.10.012 [DOI] [PubMed] [Google Scholar]
- 47.Holmgaard RB, Schaer DA, Li Y, Castaneda SP, Murphy MY, Xu X, et al. Targeting the TGFβ pathway with galunisertib, a TGFβRI small molecule inhibitor, promotes anti-tumor immunity leading to durable, complete responses, as monotherapy and in combination with checkpoint blockade. Journal for ImmunoTherapy of Cancer. 2018;6(1).doi: 10.1186/s40425-018-0356-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kim R, Emi M, Tanabe K. Cancer immunoediting from immune surveillance to immune escape. Immunology. 2007;121(1):1–14.doi: 10.1111/j.1365-2567.2007.02587.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mougiakakos D Regulatory T Cells in Colorectal Cancer: From Biology to Prognostic Relevance. 2011;3(4):1708–31.doi: 10.3390/cancers3021708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ni X, Tao J, Barbi J, Chen Q, Park BV, Li Z, et al. YAP Is Essential for Treg-Mediated Suppression of Antitumor Immunity. Cancer Discovery. 2018;8(8):1026–43.doi: 10.1158/2159-8290.cd-17-1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tauriello DVF, Palomo-Ponce S, Stork D, Berenguer-Llergo A, Badia-Ramentol J, Iglesias M, et al. TGFβ drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554:538–43.doi: 10.1038/nature25492 [DOI] [PubMed] [Google Scholar]
- 52.Knudson KM, Hicks KC, Luo X, Chen J-Q, Schlom J, Gameiro SR. M7824, a novel bifunctional anti-PD-L1/TGFβ Trap fusion protein, promotes anti-tumor efficacy as monotherapy and in combination with vaccine. OncoImmunology. 2018;7(5):e1426519.doi: 10.1080/2162402x.2018.1426519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tauriello DVF, Batlle E. Targeting the Microenvironment in Advanced Colorectal Cancer. Trends in Cancer. 2016;2:495–504.doi: 10.1016/J.TRECAN.2016.08.001 [DOI] [PubMed] [Google Scholar]
- 54.Chakravarthy A, Khan L, Bensler NP, Bose P, De Carvalho DD. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nature Communications. 2018;9(1).doi: 10.1038/s41467-018-06654-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Monteleone G A Failure of Transforming Growth Factor- 1 Negative Regulation Maintains Sustained NF- B Activation in Gut Inflammation. 2003;279(6):3925–32.doi: 10.1074/jbc.m303654200 [DOI] [PubMed] [Google Scholar]
- 56.Mizuno R, Kawada K, Itatani Y, Ogawa R, Kiyasu Y, Sakai Y. The Role of Tumor-Associated Neutrophils in Colorectal Cancer. International Journal of Molecular Sciences. 2019;20(3):529.doi: 10.3390/ijms20030529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of Tumor-Associated Neutrophil Phenotype by TGF-ОІ: “N1” versus “N2” TAN. Cancer Cell. 2009;16(3):183–94.doi: 10.1016/j.ccr.2009.06.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang R, Qi F, Zhao F, Li G, Shao S, Zhang X, et al. Cancer-associated fibroblasts enhance tumor-associated macrophages enrichment and suppress NK cells function in colorectal cancer. Cell Death & Disease. 2019;10(4).doi: 10.1038/s41419-019-1435-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sorg H, Tilkorn DJ, Hager S, Hauser J, Mirastschijski U. Skin Wound Healing: An Update on the Current Knowledge and Concepts. European Surgical Research. 2017;58(1–2):81–94.doi: 10.1159/000454919 [DOI] [PubMed] [Google Scholar]
- 60.Wei SC, Yang J. Forcing through Tumor Metastasis : The Interplay between Tissue Rigidity and Epithelial – Mesenchymal Transition. 2016;26:111–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Leask A Targeting the TGFβ, endothelin-1 and CCN2 axis to combat fibrosis in scleroderma. 2008;20(8):1409–14.doi: 10.1016/j.cellsig.2008.01.006 [DOI] [PubMed] [Google Scholar]
- 62.Ling E, Robinson DS. Transforming growth factor-bbeta1: its anti-inflammatory and pro-fibrotic effects. Clinical Experimental Allergy. 2002;32(2):175–8.doi: 10.1046/j.1365-2222.2002.01287.x [DOI] [PubMed] [Google Scholar]
- 63.Hinz B Tissue stiffness, latent TGF-β1 Activation, and mechanical signal transduction: Implications for the pathogenesis and treatment of fibrosis. 2009;11(2):120–6.doi: 10.1007/s11926-009-0017-1 [DOI] [PubMed] [Google Scholar]
- 64.Johnson LA, Rodansky ES, Haak AJ, Larsen SD, Neubig RR, Higgins PDR. Novel Rho/MRTF/SRF Inhibitors Block Matrix-stiffness and TGF-β–Induced Fibrogenesis in Human Colonic Myofibroblasts. 2014;20(1):154–65.doi: 10.1097/01.mib.0000437615.98881.31 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pang M, Teng Y, Huang J, Yuan Y, Lin F, Xiong C. Substrate stiffness promotes latent TGF-β1 activation in hepatocellular carcinoma. 2016.doi: 10.1016/j.bbrc.2016.12.107 [DOI] [PubMed] [Google Scholar]
- 66.Xia Y, Schneyer AL. The biology of activin: Recent advances in structure, regulation and function. Journal of Endocrinology. 2009;202:1–12.doi: 10.1677/JOE-08-0549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Taylor C, Loomans HA, Bras GFL, Koumangoye RB, Romero-morales AI, Quast LL, et al. Activin a signaling regulates cell invasion and proliferation in esophageal adenocarcinoma. Oncotarget. 2015;6.doi: 10.18632/oncotarget.5349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Donovan P, Dubey OA, Kallioinen S, Rogers KW, Muehlethaler K, Müller P, et al. Paracrine Activin-A Signaling Promotes Melanoma Growth and Metastasis through Immune Evasion. Journal of Investigative Dermatology. 2017;137(12):2578–87.doi: 10.1016/j.jid.2017.07.845 [DOI] [PubMed] [Google Scholar]
- 69.Dean M, Davis DA, Burdette JE. Activin A stimulates migration of the fallopian tube epithelium, an origin of high-grade serous ovarian cancer, through non-canonical signaling. Cancer letters. 2017;391:114–24.doi: 10.1016/j.canlet.2017.01.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hoda MA, Rozsas A, Lang E, Klikovits T, Lohinai Z, Torok S, et al. High circulating activin A level is associated with tumor progression and predicts poor prognosis in lung adenocarcinoma. Oncotarget. 2016;7.doi: 10.18632/oncotarget.7796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Bashir M, Damineni S, Mukherjee G, Kondaiah P. Activin-A signaling promotes epithelial-mesenchymal transition, invasion, and metastatic growth of breast cancer. NPJ breast cancer. 2015;1:15007-.doi: 10.1038/npjbcancer.2015.7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Xie D, Liu Z, Wu J, Feng W, Yang K, Deng J, et al. The effects of activin A on the migration of human breast cancer cells and neutrophils and their migratory interaction. Experimental Cell Research. 2017;357:107–15.doi: 10.1016/J.YEXCR.2017.05.003 [DOI] [PubMed] [Google Scholar]
- 73.Togashi Y, Kogita A, Sakamoto H, Hayashi H, Terashima M, de Velasco MA, et al. Activin signal promotes cancer progression and is involved in cachexia in a subset of pancreatic cancer. Cancer Letters. 2015;356:819–27.doi: 10.1016/J.CANLET.2014.10.037 [DOI] [PubMed] [Google Scholar]
- 74.Wildi S, Kleeff J, Maruyama H, Maurer CA, Büchler MW, Korc M. Overexpression of activin A in stage IV colorectal cancer. Gut. 2001;49:409–17.doi: 10.1136/gut.49.3.409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Refaat B, El-Shemi AG, Mohamed AM, Kensara OA, Ahmad J, Idris S. Activins and their related proteins in colon carcinogenesis: Insights from early and advanced azoxymethane rat models of colon cancer. BMC Cancer. 2016;16:1–16.doi: 10.1186/s12885-016-2914-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Loumaye A, de Barsy M, Nachit M, Lause P, van Maanen A, Trefois P, et al. Circulating Activin A predicts survival in cancer patients. Journal of Cachexia, Sarcopenia and Muscle. 2017;8:768–77.doi: 10.1002/jcsm.12209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tsuchida K, Nakatani M, Uezumi A, Murakami T, Cui X. Signal Transduction Pathway through Activin Receptors as a Therapeutic Target of Musculoskeletal Diseases and Cancer. 2008;55(1):11–21.doi: 10.1507/endocrj.kr-110 [DOI] [PubMed] [Google Scholar]
- 78.Attisano L, Wrana JL, Montalvo E, Massagué J. Activation of signalling by the activin receptor complex. Molecular and cellular biology. 1996;16:1066–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jung B, Doctolero RT, Tajima A, Nguyen AK, Keku T, Sandler RS, et al. Loss of Activin Receptor Type 2 Protein Expression in Microsatellite Unstable Colon Cancers. Gastroenterology. 2004;126:654–9.doi: 10.1053/j.gastro.2004.01.008 [DOI] [PubMed] [Google Scholar]
- 80.Biswas S, Trobridge P, Romero-Gallo J, Billheimer D, Myeroff LL, Willson JKV, et al. Mutational inactivation ofTGFBR2 in microsatellite unstable colon cancer arises from the cooperation of genomic instability and the clonal outgrowth of transforming growth factor β resistant cells. 2008;47(2):95–106.doi: 10.1002/gcc.20511 [DOI] [PubMed] [Google Scholar]
- 81.Watanabe T, Wu T-T, Catalano PJ, Ueki T, Satriano R, Haller DG, et al. Molecular Predictors of Survival after Adjuvant Chemotherapy for Colon Cancer. New England Journal of Medicine. 2001;344(16):1196–206.doi: 10.1056/nejm200104193441603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yuza K, Nagahashi M, Ichikawa H, Nakajima M, Hanyu T, Ishikawa T, et al. Association of activin type II receptor mutation with microsatellite instability in gastric cancer. Journal of Clinical Oncology. 2017;35(15_suppl):e23191–e.doi: 10.1200/JCO.2017.35.15_suppl.e23191 [DOI] [Google Scholar]
- 83.Wodziński D, Wosiak A, Pietrzak J, Świechowski R, Jeleń A, Balcerczak E. Does the expression of the ACVR2A gene affect the development of colorectal cancer? Genetics and molecular biology. 2019;42(1):32–9.doi: 10.1590/1678-4685-GMB-2017-0332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhuo C, Hu D, Li J, Yu H, Lin X, Chen Y, et al. Downregulation of Activin A Receptor Type 2A Is Associated with Metastatic Potential and Poor Prognosis of Colon Cancer. Journal of Cancer. 2018;9(19):3626–33.doi: 10.7150/jca.26790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Okano M, Yamamoto H, Ohkuma H, Kano Y, Kim H, Nishikawa S, et al. Significance of INHBA expression in human colorectal cancer. Oncology Reports. 2013;30:2903–8.doi: 10.3892/or.2013.2761 [DOI] [PubMed] [Google Scholar]
- 86.Wu S, Qi Y, Niu LM, Xie DX, Cui XL, Liu ZH. Activin A as a novel biomarker for colorectal adenocarcinoma in humans. European Review for Medical and Pharmacological Sciences. 2015;19:4371–8 [PubMed] [Google Scholar]
- 87.Zhou S, Buckhaults P, Zawel L, Bunz F, Riggins G, Le Dai J, et al. Targeted deletion of Smad4 shows it is required for transforming growth factor and activin signaling in colorectal cancer cells. Proceedings of the National Academy of Sciences. 1998;95:2412–6.doi: 10.1073/pnas.95.5.2412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Bauer J, Ozden O, Akagi N, Carroll T, Principe DR, Staudacher JJ, et al. Activin and TGFβ use diverging mitogenic signaling in advanced colon cancer. Molecular Cancer. 2015;14:1–14.doi: 10.1186/s12943-015-0456-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kretzschmar M, Doody J, Timokhina I, Massagué J. A mechanism of repression of TGFbeta/ Smad signaling by oncogenic Ras. Genes & development. 1999;13(7):804–16.doi: 10.1101/gad.13.7.804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang B, Zhang B, Chen X, Bae S, Singh K, Washington MK, et al. Loss of Smad4 in colorectal cancer induces resistance to 5-fluorouracil through activating Akt pathway. 2014;110(4):946–57.doi: 10.1038/bjc.2013.789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hübner G, Hu Q, Smola H, Werner S. Strong Induction of Activin Expression after Injury Suggests an Important Role of Activin in Wound Repair. 1996;173(2):490–8.doi: 10.1006/dbio.1996.0042 [DOI] [PubMed] [Google Scholar]
- 92.Werner S, Alzheimer C. Roles of activin in tissue repair, fibrosis, and inflammatory disease. 2006;17(3):157–71.doi: 10.1016/j.cytogfr.2006.01.001 [DOI] [PubMed] [Google Scholar]
- 93.Gascard P, Tlsty TD. Carcinoma-associated fibroblasts: orchestrating the composition of malignancy. Genes & Development. 2016;30(9):1002–19.doi: 10.1101/gad.279737.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sobral LM, Bufalino A, Lopes MA, Graner E, Salo T, Coletta RD. Myofibroblasts in the stroma of oral cancer promote tumorigenesis via secretion of activin A. 2011;47(9):840–6.doi: 10.1016/j.oraloncology.2011.06.011 [DOI] [PubMed] [Google Scholar]
- 95.Emon B, Bauer J, Jain Y, Jung B, Saif T. Biophysics of Tumor Microenvironment and Cancer Metastasis - A Mini Review. Computational and Structural Biotechnology Journal. 2018;16:279–87.doi: 10.1016/j.csbj.2018.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yoshinaga K, Mimori K, Inoue H, Kamohara Y, Yamashita K, Tanaka F, et al. Activin A enhances MMP-7 activity via the transcription factor AP-1 in an esophageal squamous cell carcinoma cell line. International Journal of Oncology. 1992;33:453–9.doi: 10.3892/ijo_00000027 [DOI] [PubMed] [Google Scholar]
- 97.Sideras P, Apostolou E, Stavropoulos A, Sountoulidis A, Gavriil A, Apostolidou A, et al. Activin, neutrophils, and inflammation: just coincidence? 2013;35(4):481–99.doi: 10.1007/s00281-013-0365-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Jones KL, Mansell A, Patella S, Scott BJ, Hedger MP, De Kretser DM, et al. Activin A is a critical component of the inflammatory response, and its binding protein, follistatin, reduces mortality in endotoxemia. 2007;104(41):16239–44.doi: 10.1073/pnas.0705971104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yu Dolter, Shao Yu. Suppression of IL-6 biological activities by activin A and implications for inflammatory arthropathies. 1998;112(1):126–32.doi: 10.1046/j.1365-2249.1998.00522.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Keelan JA, Zhou RL, Mitchell MD. Activin A Exerts both Pro- and Anti-inflammatory Effects on Human Term Gestational Tissues. 2000;21(1):38–43.doi: 10.1053/plac.1999.0451 [DOI] [PubMed] [Google Scholar]
- 101.Hübner G, Brauchle M, Gregor M, Werner S. Activin A: a novel player and inflammatory marker in inflammatory bowel disease? Laboratory investigation; a journal of technical methods and pathology. 1997;77(4):311–8 [PubMed] [Google Scholar]
- 102.Ogawa K, Funaba M, Chen Y, Tsujimoto M. Activin A Functions as a Th2 Cytokine in the Promotion of the Alternative Activation of Macrophages. 2006;177(10):6787–94.doi: 10.4049/jimmunol.177.10.6787 [DOI] [PubMed] [Google Scholar]
- 103.Sierra-Filardi E, Puig-Kroger A, Blanco FJ, Nieto C, Bragado R, Palomero MI, et al. Activin A skews macrophage polarization by promoting a proinflammatory phenotype and inhibiting the acquisition of anti-inflammatory macrophage markers. Blood. 2011;117(19):5092–101.doi: 10.1182/blood-2010-09-306993 [DOI] [PubMed] [Google Scholar]
- 104.Qi Y, Ge J, Ma C, Wu N, Cui X, Liu Z. Activin A regulates activation of mouse neutrophils by Smad3 signalling. Open Biology;7(5):160342.doi: 10.1098/rsob.160342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Antsiferova M, Werner S. The bright and the dark sides of activin in wound healing and cancer. J Cell Sci. 2012;125(Pt 17):3929–37.doi: 10.1242/jcs.094789 [DOI] [PubMed] [Google Scholar]
- 106.Semitekolou M, Alissafi T, Aggelakopoulou M, Kourepini E, Kariyawasam HH, Kay AB, et al. Activin-A induces regulatory T cells that suppress T helper cell immune responses and protect from allergic airway disease. The Journal of experimental medicine. 2009;206(8):1769–85.doi: 10.1084/jem.20082603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tousa S, Semitekolou M, Morianos I, Banos A, Trochoutsou AI, Brodie TM, et al. Activin-A co-opts IRF4 and AhR signaling to induce human regulatory T cells that restrain asthmatic responses. Proceedings of the National Academy of Sciences. 2017;114(14):E2891–E900.doi: 10.1073/pnas.1616942114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Huber S, Stahl FR, Schrader J, Lüth S, Presser K, Carambia A, et al. Activin A Promotes the TGF-β-Induced Conversion of CD4+CD25− T Cells into Foxp3+ Induced Regulatory T Cells. The Journal of Immunology. 2009;182(8):4633.doi: 10.4049/jimmunol.0803143 [DOI] [PubMed] [Google Scholar]
- 109.Mariathasan S, Turley SJ, Nickles D, Castiglioni A, Yuen K, Wang Y, et al. TGFβ attenuates tumour response to PD-L1 blockade by contributing to exclusion of T cells. Nature. 2018;554(7693):544–8.doi: 10.1038/nature25501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fu K, Corbley MJ, Sun L, Friedman JE, Shan F, Papadatos JL, et al. SM16, an Orally Active TGF-β Type I Receptor Inhibitor Prevents Myofibroblast Induction and Vascular Fibrosis in the Rat Carotid Injury Model. Arteriosclerosis, Thrombosis, and Vascular Biology. 2008;28(4):665–71.doi:doi: 10.1161/ATVBAHA.107.158030 [DOI] [PubMed] [Google Scholar]
- 111.Lan Y, Zhang D, Xu C, Hance KW, Marelli B, Qi J, et al. Enhanced preclinical antitumor activity of M7824, a bifunctional fusion protein simultaneously targeting PD-L1 and TGF-β. [DOI] [PubMed]
- 112.Simonelli M, Zucali P, Santoro A, Thomas MB, De Braud FG, Borghaei H, et al. Phase I study of PF-03446962, a fully human monoclonal antibody against activin receptor-like kinase-1, in patients with hepatocellular carcinoma. 2016;27(9):1782–7.doi: 10.1093/annonc/mdw240 [DOI] [PubMed] [Google Scholar]
- 113.Keedy VL, Bauer TM, Clarke JM, Hurwitz H, Baek I, Ha I, et al. Association of TGF-β responsive signature with anti-tumor effect of vactosertib, a potent, oral TGF-β receptor type I (TGFBRI) inhibitor in patients with advanced solid tumors. Journal of Clinical Oncology. 2018;36(15_suppl):3031-.doi: 10.1200/JCO.2018.36.15_suppl.303130199311 [DOI] [Google Scholar]
- 114.Formenti SC, Lee P, Adams S, Goldberg JD, Li X, Xie MW, et al. Focal irradiation and systemic TGFb blockade in metastatic breast cancer. Clinical Cancer Research. 2018;24:2493–504.doi: 10.1158/1078-0432.CCR-17-3322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Raftopoulos H, Laadem A, Puccio M, Knight RD. A phase II/III study of sotatercept (ACE-011), an activin antagonist, for chemotherapy-induced anemia in patients with metastatic non-small cell lung cancer treated with first-line platinum-based chemotherapy. Journal of Clinical Oncology. 2011;29(15_suppl):TPS235–TPS.doi: 10.1200/jco.2011.29.15_suppl.tps235 [DOI] [Google Scholar]
- 116.Tao JJ, Cangemi NA, Makker V, Cadoo KA, Liu JF, Rasco DW, et al. First-in-Human Phase I Study of the Activin A Inhibitor, STM 434, in Patients with Granulosa Cell Ovarian Cancer and Other Advanced Solid Tumors. Clinical Cancer Research. 2019.doi: 10.1158/1078-0432.CCR-19-1065 [DOI] [PMC free article] [PubMed] [Google Scholar]