Significance Statement
Analyses of entire glomeruli using a proteomic, transcriptomic, or other “omic” approach may obscure the molecular footprints of early and decisive processes in podocytes responding to injury. To pinpoint mechanisms underlying glomerulosclerosis, the authors performed ultrasensitive proteomics of purified podocyte fractions at early injury stages in mouse models of glomerular disease induced by doxorubicin or LPS. These analyses revealed an early stress response that involves upregulation of metabolic, proteostatic, and mechanoresponsive mechanisms. They also identified conserved upregulated proteins involved in the podocyte stress response, including the mechanosensor Filamin-B, and found a high correlation between proteinuria and Filamin-B levels. The work demonstrates that proteome integration at the single glomerulus and the individual organism levels can link “omics” datasets to physiological function at high resolution.
Keywords: podocyte, glomerular disease, renal injury
Abstract
Background
Understanding podocyte-specific responses to injury at a systems level is difficult because injury leads to podocyte loss or an increase of extracellular matrix, altering glomerular cellular composition. Finding a window into early podocyte injury might help identify molecular pathways involved in the podocyte stress response.
Methods
We developed an approach to apply proteome analysis to very small samples of purified podocyte fractions. To examine podocytes in early disease states in FSGS mouse models, we used podocyte fractions isolated from individual mice after chemical induction of glomerular disease (with Doxorubicin or LPS). We also applied single-glomerular proteome analysis to tissue from patients with FSGS.
Results
Transcriptome and proteome analysis of glomeruli from patients with FSGS revealed an underrepresentation of podocyte-specific genes and proteins in late-stage disease. Proteome analysis of purified podocyte fractions from FSGS mouse models showed an early stress response that includes perturbations of metabolic, mechanical, and proteostasis proteins. Additional analysis revealed a high correlation between the amount of proteinuria and expression levels of the mechanosensor protein Filamin-B. Increased expression of Filamin-B in podocytes in biopsy samples from patients with FSGS, in single glomeruli from proteinuric rats, and in podocytes undergoing mechanical stress suggests that this protein has a role in detrimental stress responses. In Drosophila, nephrocytes with reduced filamin homolog Cher displayed altered filtration capacity, but exhibited no change in slit diaphragm structure.
Conclusions
We identified conserved mechanisms of the podocyte stress response through ultrasensitive proteome analysis of human glomerular FSGS tissue and purified native mouse podocytes during early disease stages. This approach enables systematic comparisons of large-scale proteomics data and phenotype-to-protein correlation.
Podocytes are specialized epithelial cells at the kidney filtration barrier that enwrap the glomerular capillaries.1 Upon injury, podocytes dedifferentiate, lose their unique three-dimensional morphology, and detach into the urine. This response to injury of any kind is morphologically followed and accompanied by glomerular scarring and FSGS. Various molecular, chemical, and genetic stressors can induce such a response. Diverse animal models are used to study the disease. Commonly used models for identifying cellular pathways during podocyte injury include genetic models, and chemically induced podocyte damage such as the Doxorubicin nephrosis and LPS models. Although these models are widely used, it is currently not clear which parts of human podocyte disease are reflected in the animal models. Even with an increased understanding of the genomic landscape of FSGS,2,3 the immediate molecular response of podocytes in response to injury is still incompletely understood at a systems level.
Although proteomics technology is increasingly used to study glomerular disease,4–7 the three-cell architecture of the glomerulus limits data interpretation of glomerular omics data: podocyte injury leads to podocyte loss, and thereby alters the cellular composition of the glomerulus.8 In addition, the technical nature of proteomics requires analysis of pooled material from several animals with variable phenotypes, limiting feasibility of these studies. To improve this, we adapted an ultrasensitive proteome analysis9,10 of pure podocyte fractions from individual mice comprising as few as 10,000 cells to identify proteins that are regulated in the podocytes’ damage response.
The use of mouse models allows studying of early disease stages, when transcriptomic and proteomic changes are already measurable, but podocyte cell number is not yet diminished. The technique described herein can therefore be used to compare different disease stages in mouse models in order to identify genes and proteins involved in the early and late disease responses of podocytes.
Methods
Transgenic Mouse Models
The Doxorubicin study was performed with R26mTmG mice, which were mated with hNphs2.PodCre mice to achieve GFP expression exclusively in podocytes.11,12 For the LPS study Podocin.2A.iCre.2A.mTomato mice were used, which express tomato only in podocytes.13 In the Doxorubicin study we used only male mice, whereas in the LPS study we included mice from both sexes. Animals used in the Doxorubicin study were on a pure CD-1 background, whereas mice used in the LPS study were backcrossed for nine generations from C57BL/6 to CD-1 (95% CD-1). The mouse holding was done in the University of Cologne animal facility according to standardized specific pathogen–free conditions. The experimental protocol was examined and approved by the LANUV NRW (Landesamt für Natur, Umwelt und Verbraucherschutz Nordrhein-Westfalen, State Agency for Nature, Environment and Consumer Protection North Rhine-Westphalia, AZ 84–02.04.2013.A375).
Isolation of Primary Podocytes
Isolation of primary podocytes was performed after euthanizing mice and glomerular preparation was as previously described.14 A detailed protocol was described before. Mice were killed by cervical dislocation and isolated kidneys were manually perfused with Dynabeads suspension (200 μl/10 ml HBSS) and 500 μl Dynabeads in digestion buffer (containing collagenase 300 U/ml [Collagenase Type II; Worthington], 1 mg/ml pronase E [P6911; Sigma, Germany], and DNase I 50 U/ml [A3778; Applichem, Germany]). Kidneys were minced into 1-mm3 pieces and incubated in digestion buffer at 37°C for 15 minutes. The suspension was mildly pressed through a 100-μm straining sieve for 15 minutes with enough HBSS buffer (approximately 20 ml). The suspension was then pelleted by mild centrifugation (3000 rpm, 5 minutes), and the solution was resuspended. For primary podocyte isolation, glomeruli were resuspended in digestion buffer. For RNA isolation, glomeruli were transferred into Trizol until further processing. For proteomic analysis, glomeruli were digested until a single-cell suspension was obtained which was further used for FACs sorting. For this purpose, glomeruli were incubated at 37°C for 40 minutes, and the suspension was mixed by pipetting up and down every 10 minutes. Magnetic particles were discarded. Purity of cells was checked by fluorescence analysis. Cell suspension (2 ml) was sieved through a 40-μm mesh and washed with 10 ml HBSS. Cells were collected by centrifugation at 1500 rpm for 5 minutes at 4°C, resuspended in 0.5 ml HBSS, and supplemented with 0.1% BSA plus DAPI (1 μg/ml). To separate GFP-expressing (GFP+) and GFP-negative (GFP−) cells, glomerular cells were sorted by FACS for the respective dyes. The minimum number of sorted podocytes was approximately 12,500 podocytes/animal.
RNA Isolation and Quantitative PCR
Glomeruli used for quantitative PCR were isolated from 12-week-old pure Balb/C mice either treated with Doxorubicin (12 mg/kg body wt) or without treatment. RNA was isolated using the Direct-Zoll RNA MiniPrep Kit according to manufacturer’s instructions (Zymo, Irvine). A primer pair specific for murine Filamin-B was used to assess Filamin-B mRNA levels in glomeruli: sense primer: 5′-CAAAGCTGGGTCCAACATGC-3′, anti-sense primer: 5′-CGAGTCAAGTCTAGGGCACC-3′. For normalization, a primer pair specific for the house-keeping gene HPRT was used: sense: 5′-GCTGACCTGCTGGATTACAT-3′, anti-sense: 5′-TTGGGGCTGTACTGCTTAAC-3′. cDNA synthesis was performed using the High Capacity cDNA RT-Kit (Applied Biosystems, Foster City). Quantitative PCR was performed using SYBR Green (Thermo Fisher, Waltham) according to the manufacturer’s instructions.
Urinary Analysis
To measure proteinuria, urinary albumin levels were measured with a mouse albumin ELISA kit (ICL/Dunn Labortechnik GmbH, Asbach, Germany) according to the manufacturer. In parallel, urinary creatinine levels were measured with a urinary creatinine kit (Biomol, Hamburg, Germany).
Doxorubicin Study
Doxorubicin was injected once into the tail vein at a concentration of 6 mg/kg body wt (for CD-1 background). Doxorubicin was dissolved in 0.9% physiologic NaCl, which was also used as control treatment. Doxorubicin was obtained from the pharmacy of the University Hospital Cologne. The day of injection is counted as day 0. At days 7 and 14 urine was collected and used for urinary albumin measurement. Animals were euthanized either on day 6 (quantitative PCR) or on day 14 (proteomic analysis) and tissue was either collected for further podocyte isolation with FACS or collected for further immunohistochemistry. Animals used in this study were 12-week-old male mice.
LPS Study
LPS was injected into the peritoneal cavity once, at a final concentration of 20 µg/g body wt. LPS was dissolved in 0.9% NaCl, and appropriate vehicle treatment was performed as control. Urine was collected before the LPS injection and 24 hours after. Animals were euthanized 24 hours after the injection and tissue was either collected for podocyte isolation by FACS or harvested for further immunohistochemistry. Animals used were from both sexes and were 12 weeks old.
Histologic Analysis on Mouse Tissue
Mice were euthanized via perfusion with PBS via the heart. Kidneys were fixed in 4% paraformaldehyde overnight followed by embedding in paraffin. Periodic acid staining was performed according to standard methods on 2-µm-thick sections. All images were taken with a Leica SCN400 slidescanner and further processed using Aperio ImageScope v12.0.1.5030. Immunohistochemistry was performed using four different antibodies, listed in Table 1. After dehydration with increasing alcohol concentrations, sections were placed in a pressure cooker for antigen retrieval using Tris-EDTA pH 9.0. After blocking with 3% H2O2 and 1% BSA, the primary antibodies (Table 1) were incubated overnight at 4°C. On the next day, the biotin-conjugated secondary antibody was incubated for 1 hour at room temperature, followed by 3,3′-diaminobenzidine labeling and hematoxylin counterstain.
Table 1.
Antibodies
| Name | Company | Catalog No. | Host Species | Dilution IF |
|---|---|---|---|---|
| Anti-Duf | Provided by B. Denholm, University of Edinburgh, UK | N/A | Rabbit | 1:100 |
| Anti-Pyd | Developmental Studies Hybridoma Bank | PYD2 | Mouse | 1:25 |
| Anti-Filamin-B | Invitrogen | PA5–52098 | Rabbit | 1:200 |
| Anti-PSMB7 | Sigma | HPA052408 | Rabbit | 1:200 |
| Anti-STAT-1 | Sigma | SAB4300326 | Rabbit | 1:200 |
| Anti-TRIM32 | Proteintech | 10326–1-AP | Rabbit | 1:200 |
| Anti-rabbit-Cy3 | Jackson ImmunoResearch | 711–165–152 | Donkey | 1:250 |
| Anti-mouse-Cy5 | Jackson ImmunoResearch | 715–175–150 | Donkey | 1:250 |
| Biotin-conjugated anti-rabbit | Jackson ImmunoResearch | 711–065–152 | Donkey | 1:400 |
| Abberior STAR 635P | Abberior | ST635P | Rabbit | 1:250 |
| Abberior STAR 580 | Abberior | ST580 | Mouse | 1:250 |
IF, immunofluorescence; N/A, not applicable.
Histologic Analysis on Human Tissues
Embedded biopsy tissue from patients with FSGS was received from the Pathology Department of the University Hospital Cologne. As control, we used a cancer biopsy specimen without glomerular injury. Immunohistochemistry was performed as mentioned above using mouse tissue.
Drosophila melanogaster Strains and Holding
Fly strains were obtained from the Vienna Drosophila Resource Center. Here, we used mec2 (dPodocin) RNAi flies (VDRC ID: 104601), Cher (dFilamin-B) RNAi flies (VDRC ID: 107451), and Kirre (dNeph) RNAi flies (VDRC ID: 27227). To obtain nephrocyte-specific knockdown strains, RNAi strains were mated with an Sns (sticks and stones)-Gal4 driver strain. Flies were kept at 25°C.
Nephrocyte Dissection and Immunofluorescence
Dissection of Drosophila garland nephrocytes was performed with 3rd instar larvae. Isolated nephrocytes were fixed in 4% formaldehyde for 20 minutes followed by a 1-hour incubation in MeOH at room temperature. Afterward, cells were washed with staining PBS (PBS+5% BSA+0.01% Triton-X) three times for 20 minutes. Antibody staining was performed overnight at 4°C, followed by three washing steps. Antibodies were diluted with staining PBS. For antibody dilutions refer to Table 1. Afterward, cells were used for blocking with 5% NDS in staining PBS for 30 minutes at room temperature. The secondary antibody was diluted 1:250 in staining PBS and incubated for 1 hour at room temperature. Subsequently, cells were washed again three times and mounted with Vectashield. Super-resolution images were acquired using a STED microscope (TCS SP8 gSTED 3×, Leica Microsystems) equipped with a white-light laser for excitation and sensitive hybrid detectors (HyDs) for time-gated detection. A 100× oil immersion objective with a numeric aperture of 1.4 (PL Apo 100×/1.4 Oil STED, Leica Microsystems) was used. All images were deconvolved with Huygens Professional version 17.10 (Scientific Volume Imaging, The Netherlands, http://svi.nl).
Filtration Assay in Drosophila Nephrocytes
Nephrocytes of 3rd instar larvae were dissected and incubated for 1 minute in 0.2 mg/ml FITC-albumin as previously described,15 followed by 1-minute washing and 20-minute fixation in 4% formaldehyde. Afterward, the tissue was mounted with Vectashield and imaged with an Axiovert 200M microscope and further processed with ImageJ/Fiji 1.50f8 and Adobe Photoshop Version 11.0. Exposure times were identical for comparative analyses. To evaluate filtration function, the mean intensity of FITC was obtained by using ImageJ/Fiji 1.50f8. FITC intensity of SNS/Gal4 control cells was used for normalization and for statistical analysis.
Sample Preparation for Ultrasensitive Proteomics
Podocyte pellets were solubilized with 40 µl 8% SDS and heated at 95°C for 5 minutes to solubilize and denature all proteins. Next, 25 µU Benzonase was added to the solution. Microdissected garland nephrocytes from single 3rd instar larvae (five larvae per genotype) were solubilized with 40 µl 8% SDS followed by heating at 95°C for 5 minutes. Human and rat glomeruli were solubilized in 8% SDS and treated as previously described.9 Podocyte and nephrocyte samples were reduced with 5 mM DTT at 37°C for 30 minutes. Then, proteins were alkylated at room temperature with 10 mM IAA in the dark. Proteins were prepared and digested using Trypsin and LysC using the SP3 ultrasensitive proteomics technique as previously described.9
Mass Spectrometry
Proteomics data acquisition was performed on a quadrupole Orbitrap hybrid mass spectrometer (QExactive Plus, Thermo) coupled to an easynLC exactly as previously described using 2.5-hour (for podocytes) or 1-hour gradients (for nephrocytes).16
Study Approval and Human Patients
All investigations involving human subjects were conducted in accordance with the principles of the Declaration of Helsinki. All investigations were conducted after obtaining informed consent from the patients or their parents. All procedures were approved by local ethics committees in Cologne. The described pediatric patient with a steroid-resistant nephrotic syndrome and biopsy-proven FSGS (7 years old, male) was nephrectomized during living donor transplantation due to massive proteinuria, and the material was stored on ice, transported to the laboratory, and the kidney was microdissected. Exome sequencing using the Trusight one gene panel was performed and revealed a described TRPC6 variant (heterozygous) and an NPHS2 polymorphism resulting in an R229Q amino acid variant, and no further likely disease-causing variants could be identified using a comprehensive exome resequencing approach. The other two patients, whose material was used for proteomic analysis, were previously described.9 The patient material used for immunohistochemistry was obtained from renal biopsy specimens after routine diagnostics were performed. Informed consent was obtained from all patients.
Single-Glomerular Proteomics
Rats were treated with PAN (these were the same rats as in a previous study17) and a fraction was used for harvest of single glomeruli. Single glomeruli were harvested as previously described.17 In brief, glomeruli were isolated by sequential sieving and washed using ice-cold PBS. Single glomeruli were manually picked using 1-µl residual volume. Vehicle-treated controls were also collected and recorded. Glomeruli were then lysed in SDS and processed using the single-pot, solid-phase sample preparation (SP)3 protocol developed by Hughes et al.,10 including tryptic and LysC digestion. Then, the peptides were run using a parallel reaction monitoring approach in targeted proteomics mode as previously described,9 targeting the peptides listed in Supplemental Table 3. Quantification of abundance of peptides as well as merging of peptide expression into protein abundance was performed using Skyline software18 as previously described.9
Bioinformatic Analysis of Proteomics Data
Raw files generated by the mass spectrometry were processed using MaxQuant v. 1.5.3.8.19 Briefly, raw data generated by the mass spectrometer were searched against a database consisting of a mouse uniprot reference database without isoforms (downloaded in January of 2017), including common contaminants, or a drosophila uniprot reference database without isoforms, downloaded in January of 2018. Default search parameters were used, including mass accuracy of 2.5 ppm, methionine oxidation as a variable modification, cysteine alkylation as a fixed modification, and 0.01 FDR for peptide spectrum, site, and protein identification using the target decoy approach. Match between run options was enabled. The search option also included removal of all peptides that were identified by one peptide and by post-translational modification only. The label-free quantitative (LFQ) algorithm option was enabled to obtain label-free quantification intensities.20 Protein expression was further analyzed using Perseus 1.5.5.3 as well as homemade R-scripts. In brief, LFQ expression values were log2 transformed, and common contaminants, reverse peptides, and peptides only found by methionine oxidation were removed. Intensity-based absolute quantification (iBAQ), a parameter for absolute protein expression, was calculated as previously described.21 Data were filtered to contain at least two-thirds valid data. Samples including <50% of total identified proteins were excluded. Imputation of missing values was performed (downshift, 1.8 SD; width, 0.3). A two-tailed t test was used, and correction for multiple hypothesis testing was performed using the significance analysis of microarray approach using a Fudge factor (s0) and a permutation-based FDR.22 Parameters were s0=0.1 and FDR=0.05 (200 randomizations) if not otherwise indicated. Hierarchic clustering was performed by Euclidean distance (Figure 1) or maximum distance (Figure 2) using k-means preprocessing. Gene ontology (GO) term enrichments were performed using a Fisher exact test of increased and decreased population versus the nonchanged protein population after annotation with the proteins. For comparison of datasets, log2 ratios of the LPS model were mapped on the log2 ratios of the Doxorubicin model by Uniprot identifier. Two-dimensional GO and KEGG enrichment analysis was performed on the log2 ratios of LPS/control versus Doxorubicin/control (FDR threshold 0.05 with 200 randomizations).23 Visualization of LFQ intensity ratio on the canonical actin–associated cytoskeleton KEGG pathway was performed using Pathview with the default settings for normalization and visualization (pathview.r-forge.r-project.org).24 Homologene groups (NCBI) were used for annotation of ortholog protein groups between mouse, human, and rat. Z-scoring was performed using standard statistical procedures.
Figure 1.
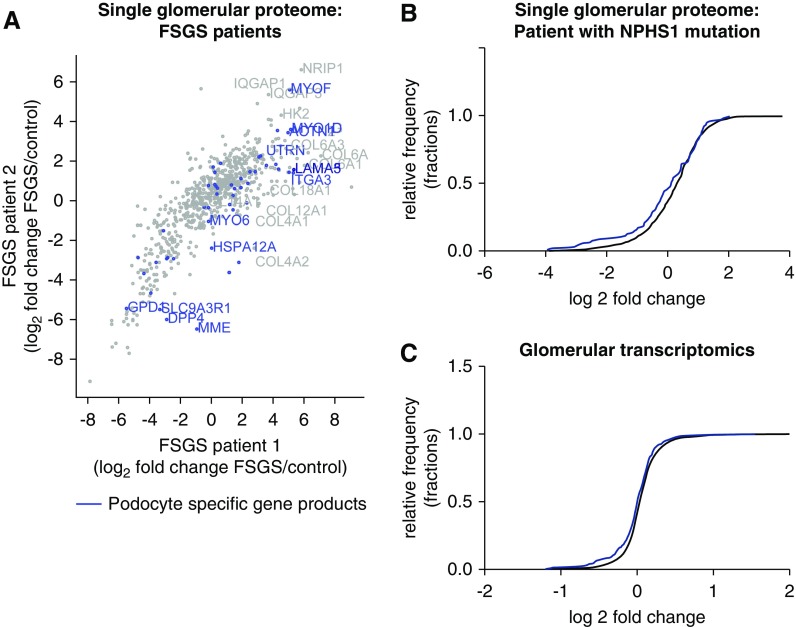
Glomerular omics data from patients with late-stage FSGS are biased by the effect of podocyte loss and dedifferentiation. (A) Comparison of single-glomerular proteomes from two patients with late-stage FSGS. The fold change of the regulation versus control is plotted. Podocyte-specific proteins are in blue and are either strongly up- or downregulated. (B) Protein distribution of single-glomerular proteomics from one patient with NPHS1 mutation presented as a cumulative histogram. This revealed an underrepresentation of podocyte-specific proteins in comparison with the whole glomerular proteome. Podocyte-specific proteins, n=85; other proteins, n=1079; Kolmogorov–Smirnov test: P<0.001. (C) Transcript distribution of glomerular transcriptomics revealed an underrepresentation of podocyte-specific proteins in comparison with the whole glomerular transcriptome. Podocyte-specific gene transcripts (corresponding to podocyte-specific proteins), n=308; other proteins, n=14,434; Kolmogorov–Smirnov test: P<0.001.
Figure 2.
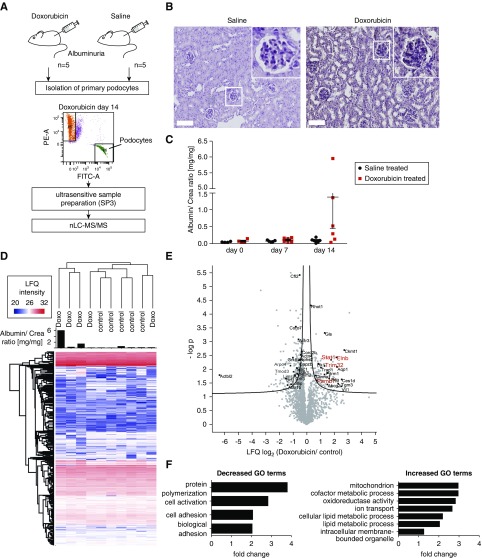
Proteomics analysis of isolated podocytes damaged by Doxorubicin revealed an upregulation of metabolic process, while adhesion processes were downregulated. (A) Workflow of Doxorubicin administration and sample preparation. NPHS2.Cre x mT/mG mice were treated with Doxorubicin (6 mg/kg body wt) or saline solution. FACS sorting of GFP-positive podocytes was performed. FACS-sorted podocytes were subjected to proteomic analysis by mass spectrometry. PE-A: tomato; FITC-A; GFP; red dots, Tomato-positive cells; green dots, GFP-positive cells; purple dots, cells out of gating. (B) Periodic acid-Schiff staining reveals morphologic changes upon Doxorubicin treatment in mice. Scale bar, 100 µm. (C) Albumin-to-creatinine ratios of animal urine after 7 and 14 days of Doxorubicin treatment. (D) Proteomic analysis of isolated podocyte fractions. Heatmap depicting LFQ protein expression between control and Doxorubicin-treated animals. Proteinuria per animal is depicted as a bar graph. (E) Volcano plot shows differentially regulated proteins when comparing proteinuric Doxorubicin versus control animals. Prioritized candidates such as Flnb (Filamin-B), STAT-1, PSMB7, and TRIM32 are depicted in red. (F) GO term enrichment analysis revealed an upregulation of metabolic processes, whereas actin-cytoskeleton–associated proteins were downregulated.
FSGS Signature
The human FSGS signature was created from datasets deposited into Nephroseq (www.nephroseq.org). Differential expression profiles were precomputed; FSGS versus normal kidney analysis was performed on the Hodgin FSGS Glom dataset and FSGS versus healthy living donor analysis was performed on the Ju CKD Glom dataset.25,26 A meta-analysis was performed on the two datasets to generate the top 200 over-expressing and top 200 underexpressing genes (by median P value across analyses).
Analysis of Mechanical Signature
Protein expression data from mechanically stressed human podocytes (grown on plastic) and podocytes grown on 12-kPa stiff dishes (not stressed) were reanalyzed.4 To find commonly regulated proteins, significantly increased proteins in Doxorubicin-treated, FACS-sorted isolated podocytes were matched with significantly regulated proteins on the basis of NCBI homologene (https://www.ncbi.nlm.nih.gov/homologene) groups.
Raw Data
Raw files of the study are deposited under PRIDE/ProteomExchange27 under the following identifiers: Project accession: PXD012064; PXD012063; PXD012025.
Statistical Analyses
Proteomics analysis, including correction for multiple testing, was performed as described under “bioinformatics analysis of proteomics data.” For the remaining data, all results are expressed as means±SEM. Statistical significance was evaluated using GraphPad Prism version 6 for Windows (GraphPad Software, San Diego, CA). For comparison of two groups, t test was used. A P value of <0.05 was considered significant. For one independent variable, one-way ANOVA combined with Tukey’s multiple comparison test was performed. A P value of <0.05 was considered significant. For two independent variables, two-way ANOVA combined with Sidak’s multiple comparison test was applied and a P value <0.05 was considered significant.
Results
Glomerular Omics Data from Patients with Late-Stage FSGS Are Biased by the Effect of Podocyte Loss and Dedifferentiation
In order to identify differentially regulated pathways and signatures in FSGS, we performed proteomics analysis of single glomeruli from two patients with FSGS (Figure 1A). Both patients received bilateral nephrectomy at the time of cadaveric kidney transplantation due to massive, therapy-resistant proteinuria. Nonsclerosed glomeruli were microdissected and subjected to single-glomerular proteomics analysis as previously described.9 Eighty-five putative podocyte markers, which were discovered by deep proteomic profiling of mouse podocytes and validated via human protein atlas staining,28 showed a dysregulation of several podocyte-specific proteins in both patients, including a downregulation of podocyte-specific proteases such as DPP4, and an increase in podocyte-specific adhesion proteins and cytoskeletal proteins such as integrins and myoferlin. Extracellular matrix proteins such as collagen A6 were increased (Figure 1A). Subsequent analysis of these putative podocyte markers in injured glomeruli of a patient with nephrin mutation revealed a normally distributed, but overall downshifted, regulation of these proteins, resulting in an underrepresentation of podocyte-specific proteins (Figure 1B). This can also be observed when analyzing transcriptomic levels in human FSGS tissue (glomeruli) from the studies by Ju and Hodgin combined in Nephroseq (Figure 1C).25,26 These data suggest that analysis of late-stage FSGS tissue might be biased by podocyte dedifferentiation and loss, and that an isolated window into early podocyte injury may be advantageous to identify molecular pathways involved in the podocyte stress response.
Proteomics Analysis of Isolated Podocytes after Doxorubicin Injury
To overcome these limitations, we aimed to generate podocyte-specific proteome datasets and utilized two commonly used FSGS disease models in mice, the Doxorubicin and LPS models. Our intent was to investigate proteomic changes in podocytes as soon as proteinuria was detectably increased. We induced podocyte injury by intravenous application of Doxorubicin. After 2 weeks, podocytes were isolated by FACS and subjected to proteomic analysis per individual podocyte mouse fraction using an ultrasensitive proteomics workflow9,10 (Figure 2, A–C). The experimental mice showed considerable variability in the degree of disease phenotype, most likely due to the early timepoint of termination. Ultrasensitive proteomic analysis identified 2200 proteins, of which 225 were significantly up- or downregulated (Figure 2, D and E). Among the increased proteins were several known glomerular disease–associated proteins, such as the transcription factors Stat-1 and Stat-329,30 (Figure 2E, Supplemental Table 1). Protein copy number estimations using the iBAQ parameter21 showed that the dynamic range of the podocyte proteome comprised five orders of magnitude (Supplemental Figure 1). GO enrichment revealed an upregulation of metabolic processes, whereas adhesion processes were downregulated (Figure 2F).
Proteomics Analysis of Pure Podocyte Injury in LPS Damage
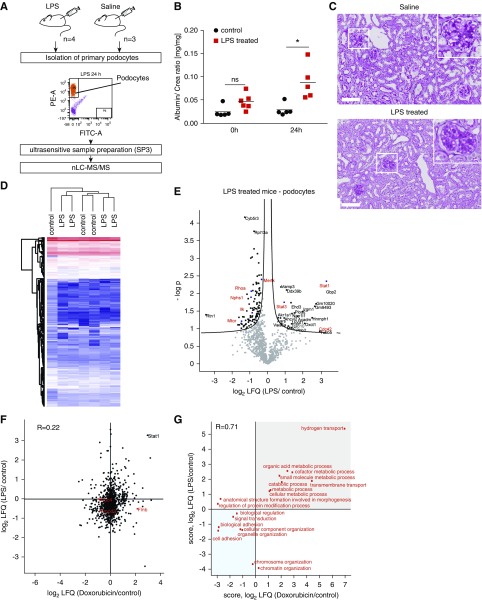
Next, we asked whether similar proteins would be changed in the LPS model of podocyte damage (Figure 3A, Supplemental Table 4). We injected LPS intraperitoneally into mice and analyzed protein expression in FACS-sorted podocytes. LPS administration resulted in slight proteinuria (Figure 3B) and only minor morphologic changes (Figure 3C). Differential proteomic data analysis revealed that 120 proteins were significantly changed upon podocyte injury. Although a similar increase of Stat-1 was observed in both models of podocyte injury (Figure 3, D and E), overall, there was only a weak correlation of damage-regulated protein expression between the two models (R=0.22; Figure 3F). In addition, we performed two-dimensional enrichment analysis using GO biologic processes (Figure 3G) and KEGG pathways (Supplemental Figure 2A). Both databases revealed damage-induced upregulation of proteins in essential metabolic pathways such as fatty acid metabolism, glycolysis, oxidative phosphorylation, and acetyl-CoA metabolic and catabolic processes. In addition, both models showed a significant downregulation of processes and pathways associated with the actin-cytoskeleton, such as focal adhesions (Supplemental Figure 2A) and actin-sensitive pathways (Supplemental Figure 2B).
Figure 3.
Proteomics analysis of isolated podocytes injured by LPS revealed a downregulation of pathways associated with the actin-cytoskeleton. (A) Workflow of LPS administration and sample preparation. Podocin.2A.iCre.2A.mTomato mice were treated with LPS (20 µg/g body wt) or saline solution. FACS sorting of Tomato-positive podocytes was performed. FACS-sorted podocytes were subjected to proteomic analysis by mass spectrometry. (B) Albumin-to-creatinine ratios show a significant increase in urinary albumin 24 hours after LPS administration. One-way ANOVA: *P<0.05. (C) Periodic acid staining reveals no severe morphologic changes in LPS-treated animals. Scale bar, 100 µm. (D) Heatmap representing the clustering of control and LPS-treated mice. (E) Volcano plot depicting differentially regulated proteins in FACS-sorted native podocytes after administration of LPS (24 hours). Candidates with direct physiologic links to podocyte disease are marked, including: Stat1, Stat3, and Nphs1. (F) Scatter plot analysis of the LFQ alterations of Doxorubicin- and LPS-treated animals reveals a common upregulation of Stat1; however, a common global regulation trend between the two models could not be observed. (G) GO term two-dimensional enrichment comparing Doxorubicin- and LPS-treated animals using the GO biologic processes dataset revealed an upregulation of metabolic signaling and a decrease in actin-cytoskeleton–associated pathways upon podocyte injury in both models.
Conserved and Individual Features of Podocyte Damage
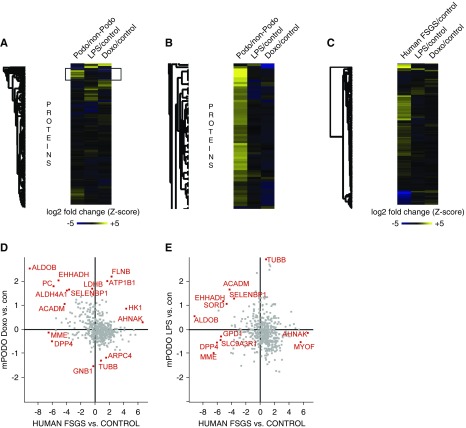
Next, we investigated whether podocyte-specific proteins were primarily affected by podocyte damage across both models. To this end, we aligned the fold change expression in the two models with quantitative proteomics data that profiled the podocyte proteome at a depth of >9000 proteins and compared it with nonpodocyte glomerular cells.28 Both podocyte-specific and podocyte-depleted proteins were almost equally affected by each injury stimulus, and both models differed strongly regarding individual protein regulation (Figure 4A). In our dataset, the majority of the detected proteins were in fact podocyte enriched (Figure 4B).
Figure 4.
Comparison of murine and human proteomic data sets identified conserved and individual features of podocyte damage. (A and B) Comparison of a wild-type mouse podocyte proteome with the Doxorubicin and the LPS datasets reveals that a majority of the detected proteins are podocyte specific and some of them are decreased upon injury. (C) Comparison of homologous protein expression in Doxorubicin- and LPS-treated FACS-sorted native podocytes with single human glomerular FSGS samples revealed only minor similarities. (D) Individual plotting and statistical outlier testing (FDR<0.01) revealed two interesting candidates, Filamin-B and ATP1B1. Both are significantly upregulated in the Doxorubicin model and the human FSGS sample. (E) Plotting of the LPS samples versus the human FSGS samples did not reveal increased statistical outliers in either condition.
We compared the changed proteins with the initial datasets from patients with glomerular disease (Figure 4C). Only a handful of proteins were significantly upregulated in the human tissue and in the injured podocytes by Doxorubicin (Figure 4D) and by LPS (Figure 4E). Among these were Filamin-B, Stat-1, and ATP1B1. Filamin-B was also increased in transcriptomic datasets of FSGS glomeruli as compared with controls (fold change=1.27, FDR Q value <0.001, obtained from Nephroseq datasets25,26). ATP1B1, a subunit of the sodium potassium ATPase, was not regulated in transcriptomic datasets, despite proven expression in podocytes.31
Next, we analyzed whether stress response proteins identified in these isolated podocyte proteomics experiments could have a broader relevance for podocyte injury. As a proof of concept, we decided to analyze the role of Filamin-B, a mechanical stress response protein, in different conditions of glomerular disease. We performed immunostaining of human biopsy samples from five patients with FSGS as well as controls from kidney biopsy samples, revealing strong expression of Filamin-B in certain, but not all, patients with FSGS. We did observe that Filamin-B staining intensity appeared to be concurrent with the degree of proteinuria at the time of biopsy (proteinuria parameters for biopsies of the depicted patients were: patient 1: 7676 mg/g albumin-to-creatinine; patient 2: 1715 mg/g albumin-to-creatinine; patient 3: 6333 mg/g albumin-to-creatinine). Representative results of the patients are presented in Supplemental Figure 3, A–C. For further corroboration, we also selected three additional candidates on the basis of their significant upregulation in podocytes isolated from Doxorubicin-treated mice. These candidates were the transcription factor Stat-1, the E3 ubiquitin ligase TRIM32, and the proteasome component PSMB7. All of these candidates were visualized on human kidney tissue from patients with FSGS and revealed an upregulation of the respective protein in podocytes in some, but not all, of the patients (Supplemental Figure 3, A–C). We also used immunohistochemistry to analyze Filamin-B expression in glomeruli of 3–4-week-old podocyte-specific Podocin knockout mice (Supplemental Figure 3, B–D), a genetic model of FSGS. We identified injured glomeruli with an increased Filamin-B signal in podocytes. In parallel, we assessed the expression levels of the three other candidates, STAT-1, TRIM32, and PSMB7 (Supplemental Figure 3D). In line with data obtained from the human FSGS samples, we observed an increase of all three proteins in injured glomeruli, suggesting that these proteins could be part of a stress responses in the podocytes.
Filamin-B Abundance Increases with Proteinuria in Animals and Single Glomeruli and Is Altered by Mechanical Stress
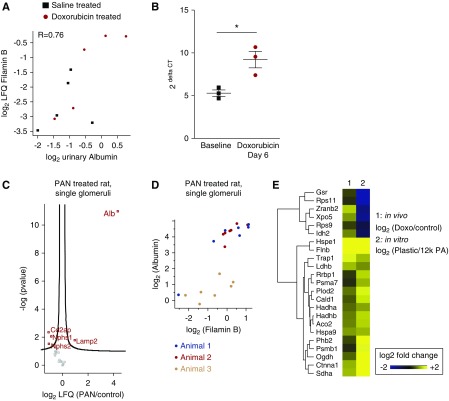
Intrigued by the upregulation of the mechanosensor Filamin-B in both human glomeruli and podocytes from Doxorubicin-treated mice, we looked at the correlation of expression levels with the amount of proteinuria in individual mice treated with Doxorubicin. This resulted in a high positive correlation (R=0.76, top 5% ranked percentile of correlation among all proteins in the dataset) and emphasized the potential role of Filamin-B during podocyte injury (Figure 5A). In addition, we investigated whether Filamin-B mRNA levels were altered by Doxorubicin-induced podocyte injury by performing quantitative PCR on glomeruli isolated from mice either without Doxorubicin treatment (baseline) or with Doxorubicin treatment (Figure 5B). This analysis revealed a significant upregulation of Filamin-B mRNA levels at day 6 after Doxorubicin administration.
Figure 5.
Filamin-B abundance increases with proteinuria in animals and single glomeruli and is altered by mechanical stress. (A) Correlation analysis revealed a strong positive correlation of podocyte Filamin-B protein with urinary albumin concentration (R, Pearson’s correlation coefficient; P<0.05). (B) Quantitative PCR of glomeruli isolated from Doxorubicin-treated mice revealed a significant increase of Filamin-B mRNA levels (2ΔCT) upon injury. Filamin-B levels are normalized to HPRT. t test: *P<0.05. (C) Volcano plot of PAN-treated single rat glomeruli reveals a significant increase of albumin (Alb), whereas podocyte-specific proteins such as Nphs2 and Nphs1 are downregulated. (D) Correlation analysis of Filamin-B levels with albumin reveals a positive correlation, R=0.76. (E) Comparison of protein abundance in our Doxorubicin-treated podocytes with cell culture podocytes exposed to increased mechanical stress (when grown on plastic as opposed to 12-kPa soft matrices) revealed similar signatures with regard to mechanosensation.
To further substantiate the functional role of Filamin-B in podocyte injury, we analyzed its amount in single glomeruli from the PAN proteinuria model in rats using targeted proteomics (Figure 5C). Glomerular-accumulated albumin is consistently found and quantified in glomerular proteomics studies.32 A single-glomerular proteomics approach allows to detect markers of glomerular injury such as albumin and to correlate them with the expression of individual proteins.9 When individual glomerular proteomes were resolved, Filamin-B correlated significantly with the amount of albumin detected in every glomerulus (Figure 5D). In line with our in vivo data, reanalysis of published in vitro data, podocytes mechanically stressed by cultivation on plastic dishes as opposed to physiological stiffness,4 also revealed an increase of Filamin-B protein upon elevated mechanical stress in podocytes, as part of a group of proteins that was also increased in the Doxorubicin-treated podocytes (Figure 5E).4
Taken together, our data obtained from human, rat, and mouse tissues as well as published in vitro data suggest a role of Filamin-B in the podocyte stress response.
Filamin-B/Cher Modulates Nephrocyte Function without Affecting Nephrocyte Slit Diaphragm Structure
Filamin-B could therefore contribute to the podocyte response to injury. To investigate this hypothesis further and to identify mechanisms influenced by altered Filamin-B levels, we employed the model organism Drosophila and its nephrocytes. We generated flies with a nephrocyte-specific depletion of Cher, the Filamin homolog in the fly, and analyzed nephrocyte morphology and function.
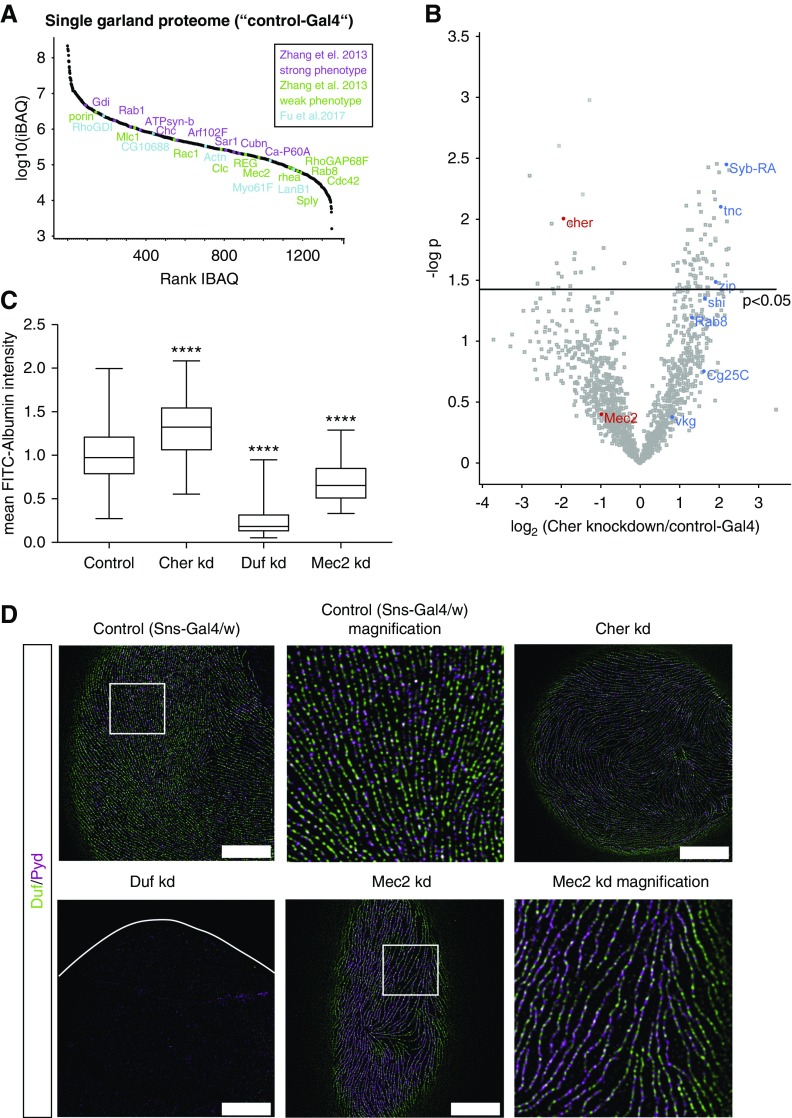
To identify downstream targets and signaling pathways regulated by Cher, we performed ultrasensitive proteome analysis of Cher-depleted garland nephrocytes from single larvae (3rd instar), comprising approximately 20 nephrocytes (Figure 6, A and B, Supplemental Table 2). The proteome was profiled at a depth of 1400 proteins over five orders of magnitude and contained several proteins associated with podocyte disease phenotypes, among these dMec2/Podocin and dActinin (Figure 6A, Supplemental Table 2).33,34 The proteome data also revealed a significant reduction of Cher expression in knockdown nephrocytes, proving the efficiency of the RNAi strain used (Figure 6B). In addition, we identified actin-cytoskeleton–associated and matrix-associated proteins to be upregulated upon loss of Cher. Among those are Syb-RA (Synaptobrevin), the Vamp2 ortholog; Zip, the Myh9/10 homolog; Shi, the Dynamin homolog; and Tnc, a matrix-associated protein (Figure 6B).
Figure 6.
Filamin-B/Cher modulates nephrocyte function and is not required for nephrocyte slit diaphragm structure. (A) Dynamic range of the nephrocyte proteome from a single fly covers 4.5 orders of magnitude. iBAQ is plotted. Proteins with a known phenotype from Zhang et al. and Fu et al. are marked.33,34 (B) Volcano plot depicting differentially regulated nephrocyte proteins upon Cher loss. Downregulated proteins such as Cher are depicted in red, whereas upregulated proteins such Syb-RA and tnc are shown in blue. n=4 flies per group. (C) Loss of Cher (dFilamin) causes a significantly increased FITC-albumin uptake when compared with control flies. As positive control, we used a Duf (dNeph) and a Mec2 (dPodocin) knockdown strain. n=3 (three independent matings and 5–7 animals per mating; total number of observations per group: Control: 244, Cher kd: 73, Duf kd: 58 and Mec2 kd: 108); ****P<0.001. (D) Nephrocyte-specific depletion of Cher (dFilamin) does not cause morphologic changes as depicted for Duf (dNeph) and Pyd (dZO-1) localization, when compared with control flies (Sns-Gal4/w). As positive control, Duf and Mec2 knockdown nephrocytes were imaged. Loss of Duf presented with a severe morphologic phenotype and an almost complete loss of the nephrocyte diaphragm. Scale bar, 5 µm; identical for all panels.
To assess filtration function, we performed FITC-albumin uptake assays (Figure 6C), which test albumin uptake in nephrocytes. Loss of two nephrocyte-specific proteins, Duf (dNEPH) and Mec2 (dPodocin), revealed a significant decrease in the FITC-albumin uptake capacity as previously described.15 Knockdown of Cher, in contrast, resulted in a significant increase of FITC-albumin uptake.
In order to observe whether these alterations were associated with a change in the structure of the nephrocyte diaphragm, a structure similar to the mammalian slit diaphragm,35,36 we also analyzed nephrocyte ultrastructure by super-resolution microscopy (Figure 6D). These data showed that the nephrocyte diaphragm structure was disturbed upon loss of Mec2, and more dramatically changed when Duf was depleted in nephrocytes. However, loss of Cher, the Filamin homolog, did not result in a changed ultrastructure of the nephrocyte diaphragm.
Discussion
A still unresolved challenge of any transcriptomic analysis is the insufficient prediction of protein expression abundance that can be directly linked to phenotypes of complex tissues.37 The advantage of the proteome as a rather close correlate to the phenotype is, however, largely limited by its practicability because it requires large preparations of renal cells from model organisms. Here, we expanded proteomic acquisition to a multitude of experimental systems to investigate model organisms of podocyte injury to improve the accessibility of proteomics (an overview of the studies is provided in Supplemental Figure 4). This allowed us for the first time in a proof-of-principle experiment to detect patterns of several proteins in multiple models in a scalable approach and to correlate the phenotype and the extremely variable degree of proteinuria with omics perturbations.
Podocyte injury causes a stress response followed by podocyte detachment and depletion, resulting in an underrepresentation of podocyte-specific proteins—a problem that involves several aspects contributing to a “bulk” glomerular omics datapoint: alterations of cell fractions in a glomerulus by podocyte loss, increase of mesangial and extracellular matrix, reactions of parietal epithelial cells (PECs), alterations in serum composition, alterations and depositions of complement and serum proteins, and many more. Although podocyte responses have been suggested as the key driver in FSGS, it appears to be difficult to extrapolate a podocyte response from late-stage FSGS samples, especially because podocyte-specific proteins are underrepresented (Figure 1). Here, we therefore aimed to observe the early fate determining factors of podocyte injury by performing podocyte proteomics from isolated podocyte fractions for the first time.
To gain an understanding in early models of podocyte injury, we profiled isolated podocytes from two distinct models of podocyte injury in mice. These are two widely used models of chemical podocyte injury, and the experiment was timed to catch immediate reactions at the first significant presence of proteinuria. From a mechanistic standpoint, the two models do not appear to have too much in common. Only computational pathway analysis identified perturbed pathways, which were not evident on the individual protein levels itself (Figure 3G). One of the few proteins found to be increased in both disease models was Stat-1, which is active in early stages of diabetic nephropathy in glomerular compartments in patients,29 and is also increased in kidney tissue of patients with FSGS,38 as confirmed also in this study (Supplemental Figure 3, A–C). The JAK/STAT pathway plays a crucial role in different processes such as cell division, cell death, and immunity. Moreover, JAK2 was described to be important in facilitating autophagy in podocytes.39 Notably, STAT activity was increased in the entire kidney, consistent with the cited previous reports.38 Of note, other models such as APOL1 and suPAR transgenic mice might be modeling human pathophysiology more closely.40–42
Two other interesting proteins, which were both upregulated upon Doxorubicin-induced injury and in human FSGS samples, are TRIM32 and PSMB7 (Figure 1, Supplemental Figure 3). TRIM32 functions as E3 ubiquitin protein ligase. One of its substrates is Dysbindin, which plays a role in actin-cytoskeleton reorganization and neurite outgrowth. Although we know from previous studies that E3 ubiquitin ligases play an important role in podocyte biology,16,43 nothing is known about the functional role of TRIM32 in podocytes. TRIM32 is a nuclear-localized E3 ligase that has been shown to regulate the antiviral response in neurons, in particular HIV response.44,45 PSMB7 is part of the 20S proteasome complex. Both of these are part of proteostatic responses in the kidney.46
The high-resolution approach provided here potentially allows the mathematic correlation of phenotypic parameters (proteinuria) and protein abundance. This approach can be used to find physiologic regulatory circuits and associations. As a proof of concept, we investigated the functional role of Filamin-B in greater detail. We discovered that the protein abundance of the mechanosensor protein Filamin-B correlated positively with elevated proteinuria in individual mice (Figure 5). In line with this, tissue from human patients with FSGS also showed increased Filamin-B on protein and transcript levels. Filamin-B is a cytoskeleton-associated protein that has been described as part of a mechanosensory complex at the slit diaphragm,47 and that is compensatorily upregulated via a selective autophagy–dependent mechanotransductive feedback loop involving YAP.48 It should be noted that high expression of Filamins, a ubiquitously expressed protein family, is strongly associated with mechanical stress conditions in other tissues and cells, including podocytes.49–52
Examination of individual glomeruli from the PAN model in rats revealed coregulation of Filamin-B with deposited albumin in individual glomeruli proteomes from proteinuric rats. Albumin is frequently found in glomerular tissue from proteinuric rat models, and the amount reflects the proteinuric degree of the individual animal.4,37 In addition, we utilized the model organism Drosophila and its nephrocytes and assessed effects after Cher (dFilamin) depletion. This revealed an increased filtration upon loss of Cher, whereas the nephrocyte diaphragm morphology was maintained. Although this model can be viewed as rather distant from human biology, the Drosophila nephrocyte is still one of the fastest models to analyze filtration function and nephrocyte diaphragm integrity and is used to model podocyte function.15,35,53
Taken together, the combined data suggest that protective mechanisms against mechanical forces play an important role in mechanotransduction and modulation of podocyte function and morphology. In addition, we advanced proteomics from the analysis of pooled glomerular samples from several mice to podocyte fractions from an individual mouse. Combined with single-glomerular data from human nephrectomies and animal models, this allows identification of functional and phenotype-associated protein expression patterns that govern the podocyte stress response.
Disclosures
Dr. Eddy reports other from Johnson and Johnson, other from Thermo Fisher Scientific, other from Gilead, and other from Abbvie, outside the submitted work. Dr. Kretzler reports grants from the National Institutes of Health and nonfinancial support from the University of Michigan, during the conduct of the study; grants from JDRF, grants from Astra-Zeneca, grants from NovoNordisk, grants from Eli Lilly, grants from Gilead, grants from Goldfinch Bio, grants from Merck, grants from Janssen, grants from Boehringer-Ingelheim, grants from Elpidera, grants from European Union Innovative Medicine Innitiative, grants from Certa, and grants from Chan Zuckerberg Initiative, outside the submitted work; in addition, Dr. Kretzler has a patent Biomarkers for CKD progression (encompassing urinary EGF as biomarker of CKD progression) issued. All of the remaining authors have nothing to disclose.
Funding
This study was supported by the German Research Foundation: clinical research unit (KFO 329, BR 2955/8-1 to Dr. Brinkkoetter, SCHE 1562/7-1 to Dr. Schermer, and BE 2212/23-1+2212/24-1 to Dr. Benzing). Dr. Rinschen was supported by a German Research Foundation fellowship (RI2811/1-1). This project was part of the work performed in FOR2743 to Dr. Höhfeld (HO1518/13-1), Dr. Rinschen (RI2811/2-1), and Dr. Benzing (BE2212/25-1). The Anti-Pyd mAb developed by Fanning54 was obtained from the Developmental Studies Hybridoma Bank, created by the National Institute of Child Health and Human Development of the National Institutes of Health, and maintained at The University of Iowa, Department of Biology, Iowa City, IA. Dr. Koehler received funding from the German Research Foundation (KO 6045/1-1), the Else-Kröner-Fresenius Stiftung (2017_A135), and the Köln Fortune Program of the University of Cologne, Germany.
Supplementary Material
Acknowledgments
We acknowledge the patients and their parents who donated biomaterial for this study. We thank V. Ludwig, R. Herzog, G. Rappl, the Cologne Excellence Cluster on Cellular Stress Responses in Aging-Associated Diseases (CECAD) imaging facility, and the CECAD proteomics facility for excellent technical support.
Dr. Koehler, Dr. Rinschen, and Dr. Brinkkoetter conceived the study; Dr. Koehler, Dr. Kuczkowski, Dr. Kuehne, Dr. Jüngst, and Dr. Rinschen performed experiments; Dr. Koehler and Dr. Rinschen analyzed the data; Dr. Hoehne, Dr. Grahammer, Dr. Eddy, Dr. Kretzler, and Dr. Beck contributed new tools, reagents, animal models, or biomaterial; Dr. Koehler and Dr. Rinschen made the figures and drafted the paper; Dr. Koehler, Dr. Rinschen, Dr. Brinkkoetter, Dr. Schermer, Dr. Benzing, and Dr. Höhfeld revised the paper; all authors approved the final version of the manuscript.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019030312/-/DCSupplemental.
Supplemental Figure 1. Dynamic range. Protein copy numbers of podocytes isolated from a single mouse.
Supplemental Figure 2. Different modes of injury cause an upregulation of metabolic signaling and a downregulation of actin-cytoskeleton–associated pathways.
Supplemental Figure 3. Immunohistochemistry on human FSGS and Podocin knockout tissue revealed an increased Filamin-B expression.
Supplemental Figure 4. Overview of this study, demonstrating the applicability of sensitive proteomics for phenotype-proteome correlations.
Supplemental Table 1. Proteome data from the doxorubicin study.
Supplemental Table 2. Nephrocyte proteome data.
Supplemental Table 3. List of peptides used in targeted proteomics.
Supplemental Table 4. Proteome data from the LPS study.
References
- 1.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003. [DOI] [PubMed] [Google Scholar]
- 2.Rosenberg AZ, Kopp JB: Focal segmental glomerulosclerosis. Clin J Am Soc Nephrol 12: 502–517, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bierzynska A, Saleem M: Recent advances in understanding and treating nephrotic syndrome. F1000 Res 6: 121, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rinschen MM, Grahammer F, Hoppe AK, Kohli P, Hagmann H, Kretz O, et al.: YAP-mediated mechanotransduction determines the podocyte’s response to damage. Sci Signal 10: eaaf8165, 2017. [DOI] [PubMed] [Google Scholar]
- 5.Azeloglu EU, Hardy SV, Eungdamrong NJ, Chen Y, Jayaraman G, Chuang PY, et al.: Interconnected network motifs control podocyte morphology and kidney function. Sci Signal 7: ra12, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rinschen MM, Wu X, König T, Pisitkun T, Hagmann H, Pahmeyer C, et al.: Phosphoproteomic analysis reveals regulatory mechanisms at the kidney filtration barrier. J Am Soc Nephrol 25: 1509–1522, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schordan S, Schordan E, Endlich N, Lindenmeyer MT, Meyer-Schwesinger C, Meyer TN, et al.: Alterations of the podocyte proteome in response to high glucose concentrations. Proteomics 9: 4519–4528, 2009. [DOI] [PubMed] [Google Scholar]
- 8.Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002. [DOI] [PubMed] [Google Scholar]
- 9.Höhne M, Frese CK, Grahammer F, Dafinger C, Ciarimboli G, Butt L, et al.: Single-nephron proteomes connect morphology and function in proteinuric kidney disease. Kidney Int 93: 1308–1319, 2018. [DOI] [PubMed] [Google Scholar]
- 10.Hughes CS, Foehr S, Garfield DA, Furlong EE, Steinmetz LM, Krijgsveld J: Ultrasensitive proteome analysis using paramagnetic bead technology. Mol Syst Biol 10: 757, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moeller MJ, Sanden SK, Soofi A, Wiggins RC, Holzman LB: Podocyte-specific expression of cre recombinase in transgenic mice. Genesis 35: 39–42, 2003. [DOI] [PubMed] [Google Scholar]
- 12.Muzumdar MD, Tasic B, Miyamichi K, Li L, Luo L: A global double-fluorescent Cre reporter mouse. Genesis 45: 593–605, 2007. [DOI] [PubMed] [Google Scholar]
- 13.Koehler S, Brähler S, Braun F, Hagmann H, Rinschen MM, Späth MR, et al.: Construction of a viral T2A-peptide based knock-in mouse model for enhanced Cre recombinase activity and fluorescent labeling of podocytes. Kidney Int 91: 1510–1517, 2017. [DOI] [PubMed] [Google Scholar]
- 14.Boerries M, Grahammer F, Eiselein S, Buck M, Meyer C, Goedel M, et al.: Molecular fingerprinting of the podocyte reveals novel gene and protein regulatory networks. Kidney Int 83: 1052–1064, 2013. [DOI] [PubMed] [Google Scholar]
- 15.Hermle T, Braun DA, Helmstädter M, Huber TB, Hildebrandt F: Modeling monogenic human nephrotic syndrome in the Drosophila garland cell nephrocyte. J Am Soc Nephrol 28: 1521–1533, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rinschen MM, Bharill P, Wu X, Kohli P, Reinert MJ, Kretz O, et al.: The ubiquitin ligase Ubr4 controls stability of podocin/MEC-2 supercomplexes. Hum Mol Genet 25: 1328–1344, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rinschen MM, Hoppe AK, Grahammer F, Kann M, Völker LA, Schurek EM, et al.: N-degradomic analysis reveals a proteolytic network processing the podocyte cytoskeleton. J Am Soc Nephrol 28: 2867–2878, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.MacLean B, Tomazela DM, Shulman N, Chambers M, Finney GL, Frewen B, et al.: Skyline: An open source document editor for creating and analyzing targeted proteomics experiments. Bioinformatics 26: 966–968, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cox J, Mann M: MaxQuant enables high peptide identification rates, individualized p.p.b.-range mass accuracies and proteome-wide protein quantification. Nat Biotechnol 26: 1367–1372, 2008. [DOI] [PubMed] [Google Scholar]
- 20.Cox J, Hein MY, Luber CA, Paron I, Nagaraj N, Mann M: Accurate proteome-wide label-free quantification by delayed normalization and maximal peptide ratio extraction, termed MaxLFQ. Mol Cell Proteomics 13: 2513–2526, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schwanhäusser B, Busse D, Li N, Dittmar G, Schuchhardt J, Wolf J, et al.: Global quantification of mammalian gene expression control. Nature 473: 337–342, 2011. [DOI] [PubMed] [Google Scholar]
- 22.Tusher VG, Tibshirani R, Chu G: Significance analysis of microarrays applied to the ionizing radiation response. Proc Natl Acad Sci U S A 98: 5116–5121, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cox J, Mann M: 1D and 2D annotation enrichment: A statistical method integrating quantitative proteomics with complementary high-throughput data. BMC Bioinformatics 13[Suppl 16]: S12, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Luo W, Brouwer C: Pathview: An R/Bioconductor package for pathway-based data integration and visualization. Bioinformatics 29: 1830–1831, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodgin JB, Borczuk AC, Nasr SH, Markowitz GS, Nair V, Martini S, et al.: A molecular profile of focal segmental glomerulosclerosis from formalin-fixed, paraffin-embedded tissue. Am J Pathol 177: 1674–1686, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ju W, Greene CS, Eichinger F, Nair V, Hodgin JB, Bitzer M, et al.: Defining cell-type specificity at the transcriptional level in human disease. Genome Res 23: 1862–1873, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Riverol Y, Csordas A, Bai J, Bernal-Llinares M, Hewapathirana S, Kundu DJ, et al.: The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res 47: D442–D450, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rinschen MM, Gödel M, Grahammer F, Zschiedrich S, Helmstädter M, Kretz O, et al.: A multi-layered quantitative In Vivo expression atlas of the podocyte unravels kidney disease candidate genes. Cell Reports 23: 2495–2508, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Berthier CC, Zhang H, Schin M, Henger A, Nelson RG, Yee B, et al.: Enhanced expression of Janus kinase-signal transducer and activator of transcription pathway members in human diabetic nephropathy. Diabetes 58: 469–477, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang H, Nair V, Saha J, Atkins KB, Hodgin JB, Saunders TL, et al.: Podocyte-specific JAK2 overexpression worsens diabetic kidney disease in mice. Kidney Int 92: 909–921, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kann M, Ettou S, Jung YL, Lenz MO, Taglienti ME, Park PJ, et al.: Genome-wide analysis of Wilms’ Tumor 1-controlled gene expression in podocytes reveals key regulatory mechanisms. J Am Soc Nephrol 26: 2097–2104, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rinschen MM, Benzing T, Limbutara K, Pisitkun T: Proteomic analysis of the kidney filtration barrier--Problems and perspectives. Proteomics Clin Appl 9: 1053–1068, 2015. [DOI] [PubMed] [Google Scholar]
- 33.Zhang F, Zhao Y, Han Z: An in vivo functional analysis system for renal gene discovery in Drosophila pericardial nephrocytes. J Am Soc Nephrol 24: 191–197, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fu Y, Zhu JY, Richman A, Zhao Z, Zhang F, Ray PE, et al.: A Drosophila model system to assess the function of human monogenic podocyte mutations that cause nephrotic syndrome. Hum Mol Genet 26: 768–780, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Weavers H, Prieto-Sánchez S, Grawe F, Garcia-López A, Artero R, Wilsch-Bräuninger M, et al.: The insect nephrocyte is a podocyte-like cell with a filtration slit diaphragm. Nature 457: 322–326, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Helmstädter M, Huber TB, Hermle T: Using the Drosophila nephrocyte to model podocyte function and disease. Front Pediatr 5: 262, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rinschen MM, Limbutara K, Knepper MA, Payne DM, Pisitkun T: From molecules to mechanisms: Functional proteomics and its application to renal tubule physiology. Physiol Rev 98: 2571–2606, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tao J, Mariani L, Eddy S, Maecker H, Kambham N, Mehta K, et al.: JAK-STAT signaling is activated in the kidney and peripheral blood cells of patients with focal segmental glomerulosclerosis. Kidney Int 94: 795–808, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Alghamdi TA, Majumder S, Thieme K, Batchu SN, White KE, Liu Y, et al.: Janus kinase 2 regulates transcription factor EB expression and autophagy completion in glomerular podocytes. J Am Soc Nephrol 28: 2641–2653, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beckerman P, Bi-Karchin J, Park AS, Qiu C, Dummer PD, Soomro I, et al.: Transgenic expression of human APOL1 risk variants in podocytes induces kidney disease in mice. Nat Med 23: 429–438, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bruggeman LA, Wu Z, Luo L, Madhavan SM, Konieczkowski M, Drawz PE, et al.: APOL1-G0 or APOL1-G2 transgenic models develop preeclampsia but not kidney disease. J Am Soc Nephrol 27: 3600–3610, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hahm E, Wei C, Fernandez I, Li J, Tardi NJ, Tracy M, et al.: Bone marrow-derived immature myeloid cells are a main source of circulating suPAR contributing to proteinuric kidney disease. Nat Med 23: 100–106, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Radón V, Czesla M, Reichelt J, Fehlert J, Hammel A, Rosendahl A, et al.: Ubiquitin C-Terminal Hydrolase L1 is required for regulated protein degradation through the ubiquitin proteasome system in kidney. Kidney Int 93: 110–127, 2018. [DOI] [PubMed] [Google Scholar]
- 44.Schwamborn JC, Berezikov E, Knoblich JA: The TRIM-NHL protein TRIM32 activates microRNAs and prevents self-renewal in mouse neural progenitors. Cell 136: 913–925, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fatima M, Kumari R, Schwamborn JC, Mahadevan A, Shankar SK, Raja R, et al.: Tripartite containing motif 32 modulates proliferation of human neural precursor cells in HIV-1 neurodegeneration. Cell Death Differ 23: 776–786, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer-Schwesinger C: The ubiquitin-proteasome system in kidney physiology and disease. Nat Rev Nephrol 15: 393–411, 2019. [DOI] [PubMed] [Google Scholar]
- 47.Venkatareddy M, Cook L, Abuarquob K, Verma R, Garg P: Nephrin regulates lamellipodia formation by assembling a protein complex that includes Ship2, filamin and lamellipodin. PLoS One 6: e28710, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ulbricht A, Eppler FJ, Tapia VE, van der Ven PF, Hampe N, Hersch N, et al.: Cellular mechanotransduction relies on tension-induced and chaperone-assisted autophagy. Curr Biol 23: 430–435, 2013. [DOI] [PubMed] [Google Scholar]
- 49.Klimek C, Kathage B, Wördehoff J, Höhfeld J: BAG3-mediated proteostasis at a glance. J Cell Sci 130: 2781–2788, 2017. [DOI] [PubMed] [Google Scholar]
- 50.Baudier J, Jenkins ZA, Robertson SP: The filamin-B-refilin axis - spatiotemporal regulators of the actin-cytoskeleton in development and disease. J Cell Sci 131: jcs213959, 2018. [DOI] [PubMed] [Google Scholar]
- 51.Kumar A, Shutova MS, Tanaka K, Iwamoto DV, Calderwood DA, Svitkina TM, et al.: Filamin A mediates isotropic distribution of applied force across the actin network. J Cell Biol 218: 2481–2491, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Nakamura F, Song M, Hartwig JH, Stossel TP: Documentation and localization of force-mediated filamin A domain perturbations in moving cells. Nat Commun 5: 4656, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Teumer A, Li Y, Ghasemi S, Prins BP, Wuttke M, Hermle T, et al.: Genome-wide association meta-analyses and fine-mapping elucidate pathways influencing albuminuria. Nat Commun 10: 4130, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Choi W, Jung KC, Nelson KS, Bhat MA, Beitel GJ, Peifer M, et al.: The single Drosophila ZO-1 protein Polychaetoid regulates embryonic morphogenesis in coordination with Canoe/afadin and Enabled. Mol Biol Cell 22: 2010–2030, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.