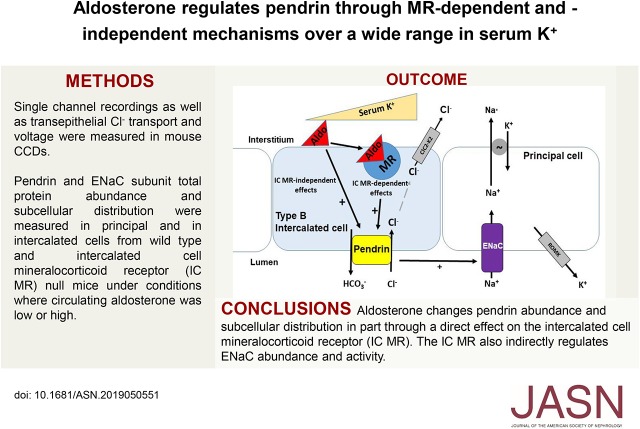
Significance Statement
The mineralocorticoid receptor within intercalated cells increases chloride transport in the mouse cortical collecting duct (CCD) through a mechanism involving the apical chloride/bicarbonate exchanger pendrin. In mouse studies, the authors demonstrated that ablating this receptor in intercalated cells markedly changes pendrin’s total abundance and subcellular distribution through a direct effect of the receptor, which occurs over a wide range in serum potassium concentration. The mineralocorticoid receptor within intercalated cells also indirectly modulates sodium channel activity in principal cells. Aldosterone increases pendrin through mechanisms both dependent and independent of the receptor. These data suggest that mineralocorticoid receptor antagonists increase NaCl excretion by the kidney, in part, by inhibiting intercalated cell pendrin-mediated chloride absorption directly and by inhibiting epithelial sodium channel–mediated sodium absorption indirectly through an effect of intercalated cell receptor blockade.
Keywords: Cell & Transport Physiology, ENaC, ion transport, hypertension
Visual Abstract
Abstract
Background
Aldosterone activates the intercalated cell mineralocorticoid receptor, which is enhanced with hypokalemia. Whether this receptor directly regulates the intercalated cell chloride/bicarbonate exchanger pendrin is unclear, as are potassium’s role in this response and the receptor’s effect on intercalated and principal cell function in the cortical collecting duct (CCD).
Methods
We measured CCD chloride absorption, transepithelial voltage, epithelial sodium channel activity, and pendrin abundance and subcellular distribution in wild-type and intercalated cell–specific mineralocorticoid receptor knockout mice. To determine if the receptor directly regulates pendrin, as well as the effect of serum aldosterone and potassium on this response, we measured pendrin label intensity and subcellular distribution in wild-type mice, knockout mice, and receptor-positive and receptor-negative intercalated cells from the same knockout mice.
Results
Ablation of the intercalated cell mineralocorticoid receptor in CCDs from aldosterone-treated mice reduced chloride absorption and epithelial sodium channel activity, despite principal cell mineralocorticoid receptor expression in the knockout mice. With high circulating aldosterone, intercalated cell mineralocorticoid receptor gene ablation directly reduced pendrin’s relative abundance in the apical membrane region and pendrin abundance per cell whether serum potassium was high or low. Intercalated cell mineralocorticoid receptor ablation blunted, but did not eliminate, aldosterone’s effect on pendrin total and apical abundance and subcellular distribution.
Conclusions
With high circulating aldosterone, intercalated cell mineralocorticoid receptor ablation reduces chloride absorption in the CCD and indirectly reduces principal cell epithelial sodium channel abundance and function. This receptor directly regulates pendrin’s total abundance and its relative abundance in the apical membrane region over a wide range in serum potassium concentration. Aldosterone regulates pendrin through mechanisms both dependent and independent of the IC MR receptor.
In the cortical collecting duct (CCD), Cl− absorption and HCO3− secretion occur through electroneutral apical Cl−/HCO3− exchange1–3 mediated by the Na+-independent anion exchanger, pendrin, encoded by Slc26a4.4,5 Aldosterone increases renal NaCl absorption by the CCD, in large part, by stimulating pendrin-mediated Cl−/HCO3− exchange in type B intercalated cells (ICs) in tandem with epithelial sodium channel (ENaC)-mediated Na+ absorption by principal cells.6–10 However, in CCDs from aldosterone-treated mice, pendrin gene ablation not only reduces Cl− absorption,5 but also reduces ENaC activity, ENaC subunit abundance, and ENaC’s relative abundance in the region of the apical membrane.11–13 The lower total ENaC subunit abundance, lower abundance in the region of the apical memberane, and lower ENaC activity observed in pendrin knockout (KO) mice occurs from a fall in luminal pH or HCO3− concentration,12 and/or increased ATP secretion by type B ICs,14 the latter of which acts on principal cell purinergic receptors to downregulate this channel.14 Although the signaling cascade by which aldosterone stimulates ENaC has been well studied, aldosterone’s signaling cascade in ICs is not fully understood.
When aldosterone binds to the principal cell mineralocorticoid receptor (MR), the aldosterone-MR complex is translocated to the nucleus,15 increasing transcription of ENaC subunits.16 The MR also regulates transporters such as ENaC through nongenomic mechanisms.17 Thus, either MR inhibitors or MR gene ablation reduces channel subunit abundance and function, particularly under treatment conditions associated with increased circulating aldosterone concentration, as occurs during dietary NaCl restriction.18–21 ENaC subunit labeling in the most apical region of principal cells is also reduced in MR null principal cells taken from NaCl-restricted mice.18,20
The distal convoluted tubule NaCl cotransporter, NCC, is upregulated by aldosterone and downregulated by MR inhibitors or MR gene ablation.18–20,22 However, aldosterone and the MR modulate NCC in part through changes in serum K+.18,20
The MR is highly expressed in ICs.23–25 However, because of a phosphorylation site (S843) seen only in this cell type, the IC MR has unique properties.25 In heterologous expression systems, either angiotensin II application or a fall in extracellular K+ concentration dephosphorylates the IC MR at S843, which promotes aldosterone (ligand) binding to the receptor. This complex is then translocated to the nucleus, which should increase gene transcription. In vivo, the MR antagonist spironolactone reduces pendrin total protein abundance in mouse kidney.25 What remains unclear, however, is whether the IC MR modulates CCD function, whether MR gene ablation changes pendrin protein abundance or subcellular distribution in vivo during high-aldosterone states through a direct effect of the MR within ICs, and whether this MR response is modulated by serum K+.
The purpose of this study was to determine (1) if the IC MR directly regulates pendrin, (2) if the IC MR modulates pendrin over the physiologic range in serum K+, (3) if the IC MR affects native CCD function, and (4) if IC MR gene ablation blunts the aldosterone-induced increment in pendrin total protein abundance and/or pendrin abundance in the region of the apical plasma membrane.
Methods
Animals
We generated mice in which the MR gene ablation occurred specifically within ICs. To do so, we bred floxed MR mice26 with mice expressing Cre-recombinase driven by the H+-ATPase B1 subunit promoter,27 which was carried through the female line. All experiments compared Cre(+) IC MR KO (MRloxloxcre) with Cre(−), sex-matched, wild-type littermates (MRloxlox).
Animal Conditioning
Treatment 1, NaCl-deficient diet: For 7 days, mice were ration-fed a balanced, NaCl-deficient diet (TD 90,228; Harlan Teklad) prepared as a gel (0.6% agar, 74.6% water, and 24.8% mouse chow), which provided mice with 0.015% Na+, 0.07% Cl−, 0.8% K+ per g diet or 0.03 meq/d Na+, 0.1 meq/d Cl−, and 0.9 meq/d K+.
Treatment 2, NaCl-replete diet: For 7 days, mice were ration-fed the gelled diet described in treatment 1, but supplemented with NaCl to give each mouse approximately 0.9 meq/d NaCl (0.5% Na+/g diet and 0.8% Cl−/g diet).
Treatment 3: Na+-deficient, high-K+ diet: For 7 days mice were ration-fed the gelled diet described in treatment 1, but supplemented with 1.0 meq/d K+ as KCl and 1.5 meq/d K+ as K3 citrate, giving mice a total of 3.4 meq/d K+ (2.9% K+ per gram Harlan Teklad diet) with 0.03 meq/d Na+ and 1 meq/d Cl−.
Treatment 4: Aldosterone infusion and a NaCl-replete diet: For 7–10 days before euthanasia, mice were ration-fed a NaCl-restricted diet (#53881300; Zeigler Brothers) prepared as a gel supplemented with NaCl to give approximately 0.8 meq NaCl per day (0.5% Na+ and 0.8% Cl−/g diet), and received either aldosterone (250 μg/kg body wt per day) or vehicle by minipump. In experiments involving CCDs perfused in vitro and in experiments using single-channel recordings, mice received the same diet but with aldosterone given by minipump at a dose of 200 μg/kg body wt per day for 5–7 days before euthanasia.
Treatment 5: Aldosterone infusion and a NaCl-replete diet with amiloride: For 7–10 days before euthanasia, mice were ration-fed a NaCl-restricted diet (#53881300; Zeigler Brothers) prepared as a gel supplemented with amiloride and NaCl, to give each mouse approximately 40 μg/d amiloride and 0.8 meq/d NaCl (0.5% Na+ and 0.8% Cl−/g diet). In addition, mice received aldosterone (250 μg/kg body wt per day) by minipump.
All treatment protocols were conducted in accordance with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and the Institutional Animal Care and Use Committee at Emory University approved all treatment protocols.
Measurement of Electrolytes, Arterial Blood Gases, and Aldosterone
Serum K+ was measured either by IDEXX or by using an iSTAT Alinity V (Abbot Point of Care, Princeton, NJ). Arterial blood gases were measured using an iSTAT Alinity V. Aldosterone was measured by ELISA using a kit (#IB79134; IBL America) by Sounddiagnostic Laboratories (Seattle, WA).
Measurement of Net Transepithelial Cl− Flux and Transepithelial Voltage in Mouse CCDs Perfused In Vitro
Mice were euthanized and CCDs were dissected from medullary rays and then perfused and bathed at flow rates of 2–3 nl/min in the presence of a symmetric, HCO3−-buffered physiologic solution containing (in mM): 125 NaCl, 24 NaHCO3, 2.5 K2HPO4, 2 CaCl2, 1.2 MgSO4, and 5.5 glucose. Tubules were equilibrated at 37°C for 30 minutes before starting the collections. All chemicals were purchased from Sigma-Aldrich (St. Louis, MO).
Cl− concentration was measured in perfusate and collected samples using a continuous-flow fluorimeter and the Cl−-sensitive fluorophore, 6 methoxy-N-(3-sulfopropyl) quinolinium (SPQ; Molecular Probes, Eugene, OR), as described previously.28,29 On the basis of the Cl− concentration measured in the collected samples and the flow rate, transepithelial Cl− flux was calculated as described previously.29 Transepithelial voltage (VT) was measured in the perfusion pipette connected to a high-impedance electrometer through an agar bridge saturated with 0.16 M NaCl and a calomel cell as described previously.30 The reference was an agar bridge from the bath to a calomel cell.
Patch-Clamp Electrophysiology in an Isolated, Split-Open Collecting Duct Preparation
Mice were euthanized and the kidneys immediately removed. CCDs were dissected, as described above, and put in ice-cold HBSS, pH 7.4, as described previously.31 Isolated collecting ducts were immobilized on 5×5-mm cover glass coated with Cell-Tak Adhesive. Cover glass–containing tubules were placed in a perfusion chamber on an inverted Nikon Eclipse TE200 microscope and superfused with room temperature solution containing (in millimolar) 150 NaCl, 5 KCl, 1 CaCl2, 2 MgCl2, 5 glucose, and 10 HEPES, pH 7.4. To access to the principal cell apical membrane, tubules were split open with sharpened micropipettes controlled with a micromanipulator. Isolated, split-open tubules were used for patch-clamp analysis within 2 hours after isolation.
A microelectrode was filled with physiologic buffer solution in which lithium was substituted for sodium, containing (in mM): 140 LiCl, 2 MgCl2, and 10 HEPES, pH 7.4.31 Gap-free single-channel current data from gigaohm seals (recording pipette resistance 7–8 MΩ) were acquired (and subsequently analyzed) with an Axopatch 1D (Axon Instruments) patch-clamp amplifier interfaced via a Digidata 14,440A (Axon Instruments) to a computer running the pClamp 10.3 (Axon Instruments). Currents were low-pass filtered at 100 Hz with an eight-pole Bessel filter (Frequency Devices). Unitary current (i) was determined from all-point amplitude histograms fitted with single- or multi-Gaussian curves by using the standard 50% threshold criterion to differentiate between events. The product of the number of channels in a patch and the channel open probability (NPo) were calculated from the single-channel record without any assumptions about the total number of channels in a patch or the Po of a single channel using the following relationship:
 |
where T is the total recording time and i is the number of channels open. Po was calculated by dividing NPo by the number of active channels within a patch as defined by all-point amplitude histograms. The recording times used in the Po measurements varied between 5 and 28 minutes, with the mean being >10 minutes for each group.
Antibodies
For all single- and double-labeling experiments in addition to immunoblot studies, we used antibodies to the MR,32 pendrin,33 AE1 (catalog no. AE11-A; Alpha Diagnostic International, San Antonio, TX).34 The MR antibody was a generous gift of Dr. Elise Gomez-Sanchez.32 The β- (catalog no. SPC-404) and γ- (catalog no. SPC-405) ENaC antibodies used have been described previously,7 and were obtained from StressMarq Biosciences. To quantify the abundance of the 30 kD fragment of α−ENaC, we used an antibody that recognizes amino acids 2–21 of the mouse α-ENaC sequence (MLDHTRAPELNLDLDLDVSNC), which was a generous gift of Dr. Johannes Loffing.35 To quantify the abundance of the 80 kD fragment of α-ENaC we used this antibody as well as an α-ENaC antibody described previously,7 which recognizes amino acids 46–68 of the rat α-ENaC sequence (NH2-LGKGDKREEQGLGPEPSAPRQPTC-COOH; catalog no. SPC-403; StressMarq Biosciences). A sheep anti-rat 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) antibody was obtained from EMD Millipore (catalog no. AB1296) and was used with a horse anti-goat IgG secondary antibody (Vector ImmPRESS; Vector Laboratories, Burlingame, CA).
Immunohistochemistry and Quantitative Analysis of Immunohistochemistry
For standard immunohistochemistry, kidneys were fixed in situ and embedded in paraffin or polyester wax (polyethylene glycol 400 distearate [Polysciences, Warrington, PA] and 10% 1-hexadecanol] and 2-μm-thick sections were cut and mounted on gelatin-coated glass slides, as described previously.34
Immunolocalization was accomplished using standard immunoperoxidase procedures. Briefly, the sections were dewaxed, rehydrated, and rinsed in distilled water. Endogenous peroxidase activity was blocked by incubating the sections in 3% H2O2 in distilled water for 45 minutes. For MR labeling, sections were blocked for 1 hour in MOM blocker (MKB-2213; Vector Laboratories) with an unconjugated Fab fragment goat anti-mouse IgG (115–007–003; Jackson ImmunoResearch, West Grove, PA), and for 15 minutes with Serum-Free Protein Block (X0909; Agilent Dako, Santa Clara, CA). Sections were then incubated at 4°C overnight with primary antibody diluted in Dako antibody diluent, washed in PBS, and incubated for 45 minutes in polymer-linked peroxidase-conjugated anti-mouse IgG diluted to 1:10 or 30 minutes in anti-rabbit IgG (Vector ImmPRESS), washed again with PBS, and exposed to diaminobenzidine (Vector DAB substrate kit) for 5 minutes. Sections were then washed in distilled water, dehydrated in graded ethanols and xylene, mounted, and observed by light microscopy.
Labeling was compared only between sections of the same thickness, which were from the same experiment done with identical reagents. Sections were examined using a Leica DM2000 microscope equipped with DIC optics and a Leica DFC425 digital camera and Leica DFC Twain Software and LAS application suite (Leica Microsystems, Buffalo Grove, IL).
Double immunolabeling was done using sequential immunoperoxidase procedures as described previously.34 Tissue sections were labeled with the anti-MR antibody. After the DAB reaction, sections were washed in PBS and blocked again using 3% H2O2 in methanol. A second immunolabeling procedure was done on the same sections using either anti-pendrin or anti-AE1 as the primary antibody, anti-rabbit Vector ImmPRESS as the secondary antibody, and Vector SG (Vector Laboratories) for the peroxidase substrate, which produces a blue reaction product easily distinguished from the DAB brown reaction product. Sections were then washed with glass-distilled water, dehydrated with graded ethanols and xylene, mounted, and observed by light microscopy.
Immunohistochemistry was done on both groups in the same experiment. Differences in wild-type and KO label were evaluated by two blinded observers who agreed on the overall differences in label distribution and intensity between the two groups.
Transporter subcellular distribution was quantified as described previously in brightfield light micrographs.34 High-resolution digital micrographs were taken of defined tubule segments using a Leica DM2000 microscope and a Leica DFC425 digital camera (14.4-megapixel images, ×63 objective) and Leica DFC Twain Software and LAS application suite (Leica Microsystems). Pixel intensity across a line drawn adjacent to the nucleus from the tubule lumen through the cytoplasm and across the basement membrane of an individual cell was quantified with ImageJ, version 1.52d software (National Institutes of Health). For pendrin labeling, background pixel intensity was calculated as the mean pixel intensity in the basal 20% of the cell and was subtracted from the pixel intensity at each point. Total cellular expression was determined by integrating net pixel intensity along the test line across the entire cell. Cell height was determined as the distance in pixels between the apical and the basal edges of the cell. Immunoreactivity expressed at zones throughout the cell was determined by integrating pixel intensity at this region. The individual performing the microscopy and quantifying the results was blinded as to the treatment group of each animal. Data from pendrin-positive cells in the connecting tubule (CNT) were averaged for each animal and used in the statistical analysis.
For comparisons of total pendrin immunolabel in MR-positive [MR(+)] versus MR-negative [MR(−)]cells in IC MR KO mice, cytoplasmic pendrin immunolabel was determined as the difference between whole cell and nuclear area and immunolabel in sections labeled for pendrin and MR. Individual cells and the respective nuclei were circumscribed using ImageJ software (version 1.52d; National Institutes of Health). Net intensity at each pixel was determined as the difference between absolute pixel intensity and mean background intensity. Immunolabel intensity was determined using custom-written software executed in Microsoft Excel 2010 to quantify integrated net pixel intensity. Cell and nuclear area were determined as the number of pixels within the outlined region. Background intensity was determined in each photomicrograph by circumscribing cytoplasm in an unlabeled cell in the same tubule. For each cell measured, nuclear area and pixel intensity were subtracted from whole cell area and pixel intensity to determine cytoplasmic area and pixel intensity. The redistribution ratio was the relative abundance of signal in the most apical 10% of the cell relative to the total signaling across the entire cell. For each IC MR null mouse, we studied 4 to 17 MR(+) and 5 to 20 MR(−) ICs. For each of these mice we thus quantified results in a mean of 9.2 MR(+) and 10.4 MR(−) ICs. Parameters were pooled for MR(+) versus MR(−) cells in each animal and differences were analyzed using paired t test.
Immunoblots
Immunoblots were performed using methods reported previously.11,36 Whole kidney lysates were isolated by harvesting mouse kidneys and placing them in an ice-cooled buffer (0.3 M sucrose, 25 mM imidazole, pH 7.2, containing 1× Roche Complete Protease Inhibitor Cocktail). Tissue was immediately homogenized using an Omni THQ Tissue Homogenizer (Omni International) and then centrifuged at 1000×g for 15 minutes at 4°C. To prepare whole cell lysates, ICs were homogenized in Gentle Lysis Buffer (10 mM Tris-HCl, 10 mM NaCl, 2 mM EDTA, 0.5% NP-40, 1% glycerol, and Na3VO4, with freshly added 0.18 μg/ml Na3VO4, 10 μg/ml PMSF, 5 μg/ml aprotinin, and 1 μg/ml leupeptin). To enable equal protein loading in each lane, protein content in the soluble fraction of homogenates was measured using a RC-PC protein assay kit (DC Protein Assay Kit; Bio-Rad, Hercules, CA) and then dissolved in Laemmli buffer.
Aliquots containing equal amounts of protein from these lysates were separated by SDS-PAGE on 8.5% acrylamide gels and then electroblotted to PVDF membranes (Immobilon; Millipore, Bedford, MA). Blots were blocked with Odyssey Blocking Buffer (LI-COR Biosciences) following the manufacturer’s instructions, and then incubated with primary antibody overnight at 4°C, followed by incubation for 2 hours at room temperature with Alexa Fluor 680–linked anti-rabbit IgG (Invitrogen). Pendrin protein was detected by immunoblot using a rabbit anti-rat pendrin antibody.33 To correct for possible differences between lanes in lysate protein loading, membranes were Coomassie Brilliant Blue-stained as reported previously (catalog no. 20279; ThermoFisher Scientific).37 Signals were visualized with an Odyssey Infrared Imaging System (LI-COR Biosciences). Immunoblot and Coomassie band densities were quantified using software program ImageJ (available at http://rsb.info.nih.gov/). Pendrin band density was normalized to the density of the Coomassie gel band with the same mobility. To confirm protein loading, actin immunoreactivity was quantified in the same blots, using a rabbit, anti-actin antibody (catalog no. A2066; Sigma Aldrich).
Statistical Analyses
Data are presented as the mean±SEM. Unless otherwise indicated, each n used in the statistical analysis represents data from separate animals. To test for statistical significance between two groups, a paired or unpaired t test was used, as appropriate. Multiple groups were compared by ANOVA with a Tukey or Hochberg post-test. The criterion for statistical significance was P<0.05.
Results
Creation and Characterization of IC MR Null Mice
To determine the role of the IC MR in aldosterone’s signaling cascade, we developed mice in which the MR is absent in ICs. To do so, we bred floxed MR mice26 with mice expressing Cre-recombinase driven by the H+-ATPase B1 subunit promoter.27 All experiments compared Cre(+) IC MR KO (MRloxloxcre) with age- and sex-matched Cre(−), wild-type littermates (MRloxlox), which we refer to as IC MR null and their wild-type littermates.
In wild-type mice, the MR is expressed in the thick ascending limb (TAL),20 the CNT,20 the collecting duct,20 and possibly in podocytes.38,39 To determine the extent of IC knockdown and to determine if this MR knockdown is limited to ICs, we compared MR labeling in cells from each of these segments in wild-type and IC MR KO mice given a NaCl-deficient diet for 7 days (treatment 1). In wild-type mice, we observed very weak MR labeling, not different from background, in podocytes and proximal tubule (Supplemental Figure 1). Clear nuclear MR labeling was, however, observed in virtually all cells of the TAL, DCT, CNT, and collecting duct of wild-type mice (Figure 1, Supplemental Figure 1, Table 1).
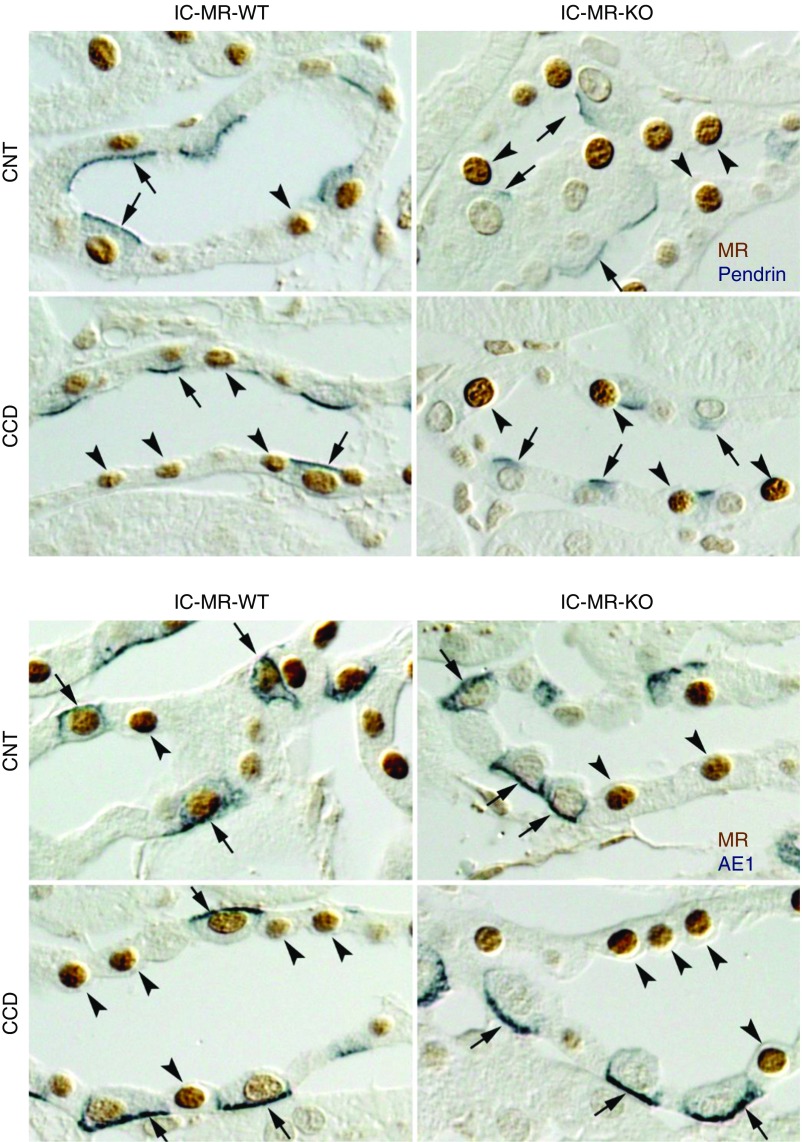
Figure 1.
MR label is absent in ICs from the IC MR null mice. This figure shows MR expression in principal, CNT, and IC cells from NaCl-restricted IC MR KO and wild-type (WT) littermates (treatment 1). The top panel shows pendrin (blue) as well as MR label (brown). As shown, in wild-type mice, MR label is seen in pendrin-positive cells (arrows), indicating MR expression in type B and non-A, non-B ICs. However, MR label is not seen in pendrin-positive cells from IC MR KO mice. The bottom panel shows AE1 label (blue), a marker of type A ICs, and MR label (brown). AE-positive cells are indicated by arrows. Arrowheads show MR(+), AE1-negative cells, which are predominantly principal or CNT cells. MR label is seen in AE1-positive cells from wild-type, but not in IC MR KO mice. Arrowheads show MR(+) cells that are either pendrin-negative (top panel) or AE1-negative (bottom panel). The absence of AE1 or pendrin label, in addition to cell morphology, indicates principal or CNT cells. The images shown are representative of the results obtained from three mice studied in each group.
Table 1.
Percentage of cells with MR label in TAL, CNT, and CCD from NaCl-restricted IC MR KO and wild-type mice
| Strain | TAL | CNT | CCD | |
|---|---|---|---|---|
| WT, n=3 | % ICs within this segment with MR label | NA | 89±1 | 82±5 |
| % of other cells within this segment with MR label | 81±1 | 78±2 | 79±4 | |
| IC MR KO, n=3 | % ICs within this segment with MR label | NA | 5±2 | 3±2 |
| % of other cells within this segment with MR label | 81±2 | 63±7 | 76±5 |
“Other cells” refer to cells in that segment that are not ICs. ICs were identified on the basis of AE1 and pendrin immunolabel. ICs had either AE1 or pendrin immunolabel, whereas non-ICs had neither label. WT, wild-type; NA, not applicable.
In IC MR KO mice, MR label was also observed in TAL cells, principal cells, and CNT cells (Figure 1, Table 1). However, while nuclear MR label was present in nearly all ICs from the wild-type mice, MR label was absent in nearly all ICs from the IC MR KO mice (Figure 1). Table 1 compares the percentage of cells with MR label for each cell type found within the TAL, CNT, and CCD, i.e., TAL cells, CNT cells, principal cells, and ICs, in NaCl-restricted wild-type and in IC MR KO mice (treatment 1). As shown, no large difference was detected between wild type and IC MR KO mice in the percent of TAL cells, CNT cells or CCD principal cells that expressed the MR. However, the percentage of ICs that express the MR was much lower in the IC MR KO than in their wild-type littermates. We conclude that within the aldosterone-sensitive region of the nephron, significant knockdown of the MR occurs in ICs from the CNT and CCDs of IC MR KO mice, without very significant knockdown in the other cell types.
IC MR Gene Ablation Reduces Cl−Absorption, and Concomitantly Reduces Principal Cell ENaC Activity and Subunit Abundance
In the CCD, transepithelial transport of Na+ occurs primarily across principal cells,13,40,41 whereas transepithelial Cl− transport occurs mainly across ICs.5,42 To determine the physiologic significance of IC MR gene ablation, we therefore measured Cl− absorption in CCDs from aldosterone-treated IC MR KO and their wild-type littermates. Figure 2A shows that Cl− absorption was approximately 40% lower in CCDs from aldosterone-treated IC MR KO than from wild-type littermates. Thus, the IC MR modulates Cl− absorption in mouse CCD.
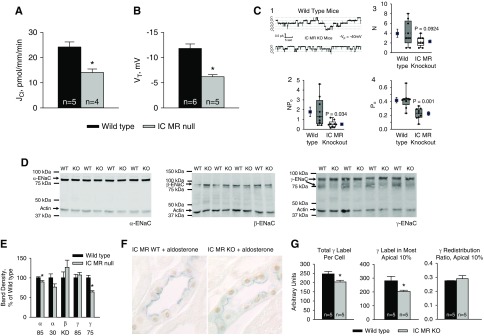
Figure 2.
IC MR gene ablation reduces Cl− absorption, VT, Na+ channel activity, as well as ENaC subunit abundance and distribution in aldosterone-treated mice. (A) Cl− absorption and (B) VT were measured in CCDs perfused in vitro that were taken from aldosterone-treated IC MR null and wild-type littermates (treatment 4). (C1) Single-channel records from principal cell patches of split-open collecting ducts from aldosterone-treated wild-type and IC MR null mice. In (C1), “c” marks the current level when all channels are closed; “o” marks levels at which one or more channels are open. These records show less activity in the MR KO than in wild-type patches [(C2) P=0.034]. (C3 and C4) Values for the number of channels at the cell surface (N) and the open probability (Po). N is not significantly different in wild-type versus MR KO patches, whereas Po is significantly different (P=0.001). ENaC activity was measured as the number of channels times the open probability (NPo) in nine patches from MR KO and nine patches from wild-type mice. Data are shown as box plots, where the boxes are at the 25th and 75 percentiles and whiskers are at the 10th and 90th. Solid lines in the boxes are the median values, whereas dotted lines represent the mean. Individual data points are superimposed on the box plots. To the left and right of the boxes are mean value±SEM (solid squares). (D and E) ENaC subunit immunoblots of whole kidney lysates from aldosterone-treated IC MR null and wild-type mice and their relative band density. The α-ENaC blot displayed used the antibody that recognizes amino acids 46–68 of the rat sequence detects the 85, but not the 30 kD α-ENaC fragment. To detect both the 85 and the 30 kD α-ENaC fragment we used an antibody that recognizes amino acids 2–21 of the mouse sequence (Supplemental Figure 3). The number of mice used to generate the data displayed in (E) are as follows: 30 kD α-ENaC, 6; 85 kD α-ENaC, 10; β-ENaC, 4; 85 and 75 kD γ-ENaC, 4. (F) MR (brown) and γ-ENaC (blue) double-labeling in CCDs from mice from mice in both of these groups. (G) γ-ENaC total label per cell, label in the most apical 10% of the cell and γ-ENaC redistribution ratio in CCDs taken from aldosterone-treated IC MR null and wild-type littermates. *P<0.05.
Because Cl− absorption in CCDs from aldosterone-treated mice is largely pendrin-dependent,12 and because pendrin is an electroneutral exchanger,43 we asked if the fall in Cl− absorption seen with IC MR gene ablation occurs without a change in VT. Figure 2B shows, however, that the lumen-negative VT was approximately 50% lower in CCDs from aldosterone-treated IC MR KO relative to their wild-type littermates, which indicates that IC MR gene ablation affects electrogenic pathways.
Because the lumen-negative VT observed in the CCD is generated primarily through ENaC-mediated Na+ absorption,13 we hypothesized that Na+ channel activity is reduced in the IC MR null mice. To test this hypothesis, we measured ENaC channel activity (NPo), channel open probability (Po) and the number of active channels on the cell surface (N) in principal cell apical membrane patches from CCDs of aldosterone-treated IC MR KO and wild-type mice. Single-channel records from principal cell patches of split-open collecting ducts from wild-type and IC MR null mice are shown in Figure 2C, panel 1. Currents are inward with long mean open and mean closed times, which is characteristic of ENaC (Supplemental Figure 2). Figure 2C, panels 2–4 show that IC MR gene ablation reduced ENaC channel activity (NPo) and open probability (Po), although the difference in channel surface density, N, was not statistically significant.
Because apical plasma membrane ENaC subunit abundance is regulated both by changes in ENaC subunit protein abundance as well as on changes in subunit subcellular distribution, we examined each in kidneys from aldosterone-treated IC MR null and in wild-type mice. As shown (Figure 2, D and E, Supplemental Figure 3), the abundance of the 85 kD fragment of α-ENaC was very slightly lower in the IC MR null than in the wild-type mice, although no difference in the abundance of the 30 kD fragment was detected. The abundance of β-ENaC and the 85 kD fragment of γ-ENaC were also similar in kidney lysates from aldosterone-treated IC MR KO and wild-type littermates. However, band density of the cleaved, 75 kD γ-ENaC fragment was lower in kidney lysates from the aldosterone-treated IC MR null than from wild-type littermates.
Further experiments explored γ-ENaC label intensity/cell and subcellular distribution in sections from aldosterone-treated IC MR null and wild-type mice (Figure 2, F and G). To do so, sections were labeled for both γ-ENaC and the MR to insure that γ-ENaC was quantified only in principal cells with documented MR immunolabel. Although the MR was expressed in all principal cells studied in both groups, γ-ENaC label intensity per cell was lower in principal cells from the aldosterone-treated IC MR KO relative to the wild-type mice. However, we detected no difference in γ-ENaC subcellular distribution in principal cells from IC MR null and wild-type mice. We conclude that IC MR gene ablation in aldosterone-treated mice, reduces ENaC channel activity primarily by reducing channel open probability. IC MR gene ablation also reduces total γ-ENaC label intensity per principal cell and the abundance of the cleaved, 75 kD fragment of γ-ENaC. However, IC MR gene ablation has no significant effect on β subunit and only slightly reduced α subunit abundance.
Pendrin Total Protein Abundance and Pendrin Abundance in the Region of the Apical Plasma Membrane Are Lower in IC MR KO Relative to Wild-Type Mice in Mice after an Aldosterone Infusion
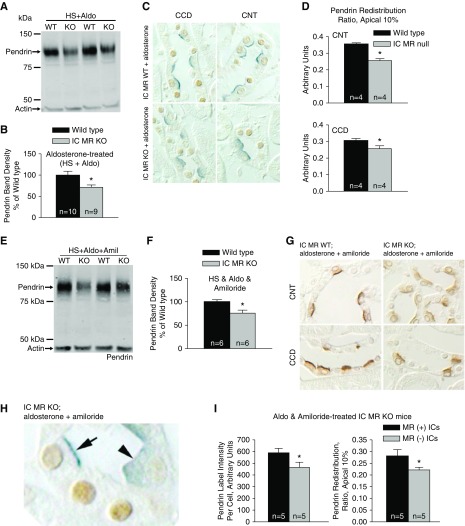
Because Cl− absorption in CCDs from aldosterone-treated mice is largely pendrin-dependent,5 we asked if pendrin abundance or subcellular distribution differs in kidneys from aldosterone-treated IC MR null and wild-type mice (treatment 4). As shown (Figure 3, A and B), pendrin protein abundance was 28% lower in kidney lysates from aldosterone-treated IC MR null than wild-type littermates. Similarly, pendrin label appeared more diffuse and less discrete in the apical region of cortical sections from aldosterone-treated IC MR KO relative to wild-type mice (Figure 3C). Quantitative immunohistochemistry showed that in both the CNT and CCD, pendrin label in the most apical 10% relative to label across the entire cell (redistribution ratio) was lower in ICs from aldosterone-treated IC MR KO relative to wild-type mice (Figure 3D). We conclude that in aldosterone-treated mice, IC MR gene ablation reduces pendrin total protein abundance and its relative abundance in the region of the apical plasma membrane.
Figure 3.
IC MR gene ablation reduces pendrin protein abundance and pendrin's relative abundance in the most apical 10% of ICs. in mice given either an aldosterone infusion alone or aldosterone plus amiloride. (A and B) Shown is a representative pendrin immunoblot (A) of kidney lysates from IC MR null and wild-type mice following a NaCl-replete diet with an aldosterone infusion (treatment 4), with its respective band density (B). (C) Pendrin and MR double-labeling in a CNT and a CCD from a representative cortical section taken from an aldosterone-treated IC MR null and a wild-type littermate. (D) Pendrin label in the most apical 10% relative to label across the entire cell (redistribution ratio) in both CCD and CNT. (E) A representative pendrin immunoblot of kidney lysates from IC MR null and wild-type mice after an aldosterone infusion with a NaCl-replete diet containing amiloride (treatment 5). Pendrin band density in lysates from each group is shown in (F). (G) Pendrin labeling in a representative cortical section from an amiloride and aldosterone-treated IC MR null and a wild-type littermate. (H) Pendrin label in MR(+) (arrows) and MR(−) (arrowheads) ICs from a cortical section of an IC MR null mouse. (I) Pendrin label per cell (left) and pendrin label in the most apical 10% relative to label across the entire cell (redistribution ratio, right) were quantified in both the MR(+) and MR(−) ICs. *P<0.05.
Eliminating Aldosterone-Induced Hypokalemia Does Not Abolish the Change in Pendrin Protein Abundance or Subcellular Distribution Observed with IC MR Gene Ablation
The Shibata model predicts that the low serum K+ observed after aldosterone administration (Table 2) magnifies the effect of IC MR gene ablation on pendrin expression.25 To exclude a possible role of hypokalemia in MR-dependent changes in pendrin expression, we examined pendrin abundance and subcellular distribution in aldosterone-treated mice when serum K+ was raised by administration of the ENaC inhibitor, amiloride (treatment 5). As shown, despite the higher serum K+ concentration seen in this treatment model (Table 2), pendrin total protein abundance was lower (Figure 3, E and F), and pendrin label was more diffuse and less discrete (Figure 3G) in ICs of the IC MR KO relative to the wild-type littermates.
Table 2.
Effect of IC MR gene ablation on serum K+, aldosterone, and arterial pH
| Strain | Serum K+, mEq | Serum Aldosterone, nM | Arterial Blood Gases | ||
|---|---|---|---|---|---|
| pH | pCO2, mm Hg | cHCO3−, mEq | |||
| NaCl-deficient diet supplemented with NaCl (treatment 2) | |||||
| Wild-type | 4.5±0.2 (n=16) | 0.56±0.02 (n=3) | 7.34±0.01 (n=5) | 42.5±1.1 | 23.0±0.9 |
| IC MR KO | 4.8±0.2 (n=13) | 0.57±0.02 (n=3) | 7.32±0.02 (n=4) | 47.0±1.9 | 24.1±1.2 |
| NaCl-deficient diet (treatment 1) | |||||
| Wild-type | 4.1±0.1 (n=12) | 1.98±0.34 (n=4) | 7.35±0.01 (n=4) | 41.6±1.5 | 22.7±0.7 |
| IC MR KO | 4.3±0.2 (n=9) | 2.09±0.22 (n=4) | 7.33±0.01 (n=4) | 44.4±1.5 | 23.3±0.6 |
| NaCl-deficient diet supplemented with KCl and K3 citrate (treatment 3) | |||||
| Wild-type | 5.2±0.4 (n=15) | 2.44±0.22 (n=9) | 7.40±0.04 (n=4) | 37.3±1.4 | 23.3±1.6 |
| IC MR KO | 5.5±0.3 (n=15) | 2.22±0.26 (n=9) | 7.35±0.01 (n=4) | 41.4±1.3 | 22.7±0.5 |
| NaCl-replete diet and aldosterone (treatment 4) | |||||
| Wild-type | 2.7±0.1 (n=10) | 1.95±0.33 (n=4) | 7.41±0.02 (n=5) | 43.2±2.3 | 27.1±0.8 |
| IC MR KO | 3.1±0.1 (n=12)a | 2.38±0.36 (n=4) | 7.42±0.02 (n=6) | 44.8±1.0 | 29.4±1.1 |
| NaCl-replete diet and aldosterone and amiloride (treatment 5) | |||||
| Wild-type | 4.1±0.1 (n=11) | 2.29±0.27 (n=5) | 7.35±0.01 (n=5) | 44.1±2.1 | 24.5±0.8 |
| IC MR KO | 5.1±0.3 (n=11)a | 2.27±0.40 (n=5) | 7.36±0.01 (n=5) | 44.6±1.2 | 25.3±0.9 |
cHCO3−, calculated serum HCO3−.
P<0.05.
Although we observed no significant effect of IC MR gene ablation on acid-base balance or serum aldosterone concentration under any of the treatment conditions examined, serum K+ was approximately 1.0 meq/L higher in the aldosterone and amiloride-treated IC MR KO relative to wild-type mice (Table 2; P<0.05). Therefore, to exclude a possible contribution of an indirect, systemic effect of IC MR gene ablation, such as changes in serum K+ concentration, we examined pendrin label intensity and subcellular distribution in MR(+) and MR(−) ICs taken from the same IC MR KO mouse. We studied ICs from the CNT, instead of the CCD, of these mutant mice because the abundance of MR(+) ICs in IC MR KO mice is likely somewhat greater in the former than the latter segment (Table 1). As shown, both pendrin label intensity per cell and pendrin’s relative abundance in the most apical 10% of the cell (Figure 3, H and I) were lower in the MR(−) than the MR(+) ICs taken from the same amiloride- and aldosterone-treated IC MR KO mice. We conclude that raising serum K+ does not eliminate the effect of the IC MR on pendrin abundance or subcellular distribution in aldosterone-treated mice.
The IC MR Regulates Pendrin Abundance and Subcellular Distribution Directly over a Wide Range in Serum K+
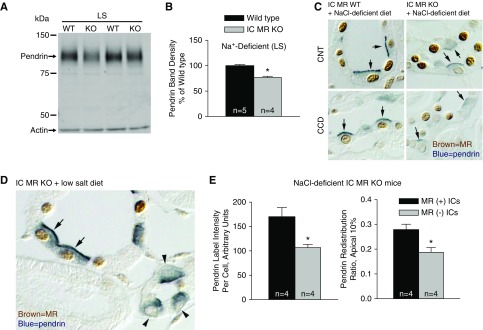
To determine if serum K+ or aldosterone concentration impact the effect of IC MR gene ablation on pendrin, further experiments examined pendrin total protein abundance and subcellular distribution in IC MR KO mice when serum concentrations of aldosterone and K+ were varied by manipulating dietary intake of Na+ and K+. In the first experiments, we examined pendrin expression in mice given a NaCl-deficient diet (treatment 1), which increases circulating aldosterone concentration (Table 2).11 In this model, pendrin total protein abundance was 23% lower in kidney lysates from the IC null relative to wild-type mice (Figure 4, A and B). Similarly, pendrin label in the region of the apical plasma membrane was less intense and more diffuse in kidneys from IC MR null relative to wild-type mice (Figure 4C).
Figure 4.
MR gene ablation reduces pendrin protein abundance and pendrin's relative abundance in the most apical 10% of the cell in mice given a NaCl-deficient diet. (A and B) Shown is a representative pendrin immunoblot (A) of kidney lysates from IC MR null and wild-type mice given a NaCl-deficient diet (LS, treatment 1) with pendrin band density (B). (C) Pendrin (blue, arrows) and MR labeling (brown) in the CNT or the initial collecting tubule (iCT) within the cortical labyrinth from a representative cortical section from an IC MR null and a wild-type littermate. (D) Pendrin (blue) and MR (brown) label in ICs that are either MR(+) (arrows) or MR(−) (arrowheads) in a cortical section from an IC MR null mouse given the Na+-deficient diet. (E) Pendrin label per cell (left) and pendrin label in the most apical 10% relative label across the entire cell (redistribution ratio, right) were quantified in both MR(+) and MR(−) ICs taken from the same NaCl-deficient IC MR null mouse. *P<0.05.
We did not detect a difference in serum aldosterone concentration between the wild-type and IC MR null mice under any of the treatment conditions studied (Table 2). Serum K+, however, was on average approximately 0.3–0.4 meq/L higher in the IC MR KO than the wild-type mice in this and the subsequent treatment groups tested (i.e., treatments 1–3), although none of these differences reached statistical significance. However, to exclude a possible influence of changes in serum K+, we examined pendrin label intensity per cell and pendrin’s relative abundance in the most apical region of MR(+) and MR(−) ICs from the same mouse. Figure 4, D and E show that pendrin label intensity per cell was 34% lower, whereas pendrin’s relative abundance in the most apical 10% of the cell was 33.0% lower in the MR(−) relative to the MR(+) ICs taken from the same Na+-deficient IC MR null mice. We conclude that after dietary Na+ restriction, MR gene ablation reduces pendrin protein abundance and reduces pendrin’s relative abundance in the region of the apical plasma membrane through a direct effect of the MR within ICs.
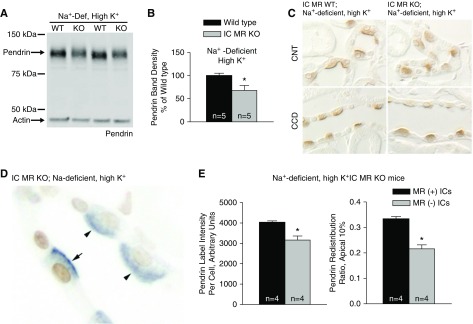
We asked if this MR response is eliminated when serum K+ is increased above the upper limit of normal. To do so, IC MR KO mice received the Na+-deficient gelled diet supplemented with K+ as KCl and K3 citrate (treatment 3), which raised serum K+ in the IC MR null mice from 4.3 to 5.5 meq/L. Despite the hyperkalemia observed in this treatment group, pendrin protein abundance was 32% lower in kidney lysates from the IC MR null relative to the wild-type mice (Figure 5, A and B). Moreover, pendrin label intensity per cell was 21% lower in the MR(−) relative to MR(+) ICs taken from the same IC MR null mice (Figure 5, D and E). IC MR gene ablation also modulated pendrin subcellular distribution in this treatment model. Figure 5C illustrates that pendrin label was more diffuse and less discrete in the region of the apical plasma membrane in the wild-type relative to the mutant mice. When quantified, we observed that pendrin’s relative abundance in the most apical 10% of the cell was 35.3% lower in MR(−) relative to MR(+) ICs (Figure 5, D and E), when taken from the IC MR null mice. Thus, despite a high serum K+ concentration, IC MR gene ablation reduced pendrin’s relative abundance in the region of the apical plasma membrane and reduced pendrin protein abundance.
Figure 5.
MR gene ablation reduces pendrin protein abundance and pendrin's relative abundance in the most apical 10% of the cell in mice given a Na+-deficient, high-K+ diet. (A and B) Shown is a representative pendrin immunoblot (A) of kidney lysates from IC MR null and wild-type mice following the Na+-deficient, high-K+ diet (treatment 3) with pendrin band density (B). (C) Single pendrin labeling of a representative cortical section from an IC MR null and a wild-type littermate is shown. (D) Pendrin (blue) labeling in ICs that were MR(+) (brown label, arrows) and MR(−) (arrowheads) in a representative cortical section from an IC MR null mouse given the Na+-deficient, high-K+ diet. (E) Pendrin label per cell (left) and pendrin label in the most apical 10% relative to label across the entire cell (redistribution ratio, right) were quantified in both the MR(+) and MR(−) ICs taken from the same Na+-deficient, high-K+ IC MR null mice. *P<0.05.
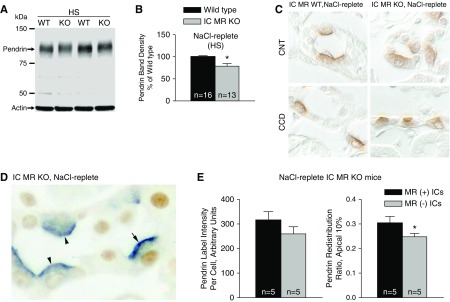
In the next experiments we explored the effect of IC MR gene ablation on pendrin abundance and subcellular distribution under conditions in which serum aldosterone concentration is low. To do so, we supplemented the Na+-deficient diet with NaCl (Table 2, treatment 2). As shown, pendrin total protein abundance was approximately 21% lower in kidneys from the IC MR null relative to the wild-type mice (Figure 6, A and B), although we detected no statistically significant difference in pendrin label intensity per cell in the MR(−) and MR(+) ICs from the IC MR KO mice (Figure 6, D and E, left panel). Pendrin label in cortical sections from these NaCl-replete IC MR KO and wild-type littermates is shown in Figure 6C. In sections from the IC MR KO mice, pendrin label in the most apical 10% relative to label across the entire cell (redistribution ratio) was 18% lower in the MR(−) than the MR(+) ICs (Figure 6, D and E). Thus in NaCl- and K+-replete mice, MR gene ablation reduces pendrin total protein abundance and pendrin’s relative abundance in the apical plasma membrane region. However, these differences are relatively small.
Figure 6.
MR gene ablation reduces pendrin protein abundance and pendrin's relative abundance in the most apical 10% of the cell in mice given a NaCl-replete diet. (A and B) Shown is a representative pendrin immunoblot (A) of kidney lysates from IC MR null and wild-type mice following the NaCl-replete diet (treatment 2) with pendrin band density (B). (C) Pendrin single-labeling in a representative cortical section from a NaCl-replete IC MR null and a wild-type littermate. (D) Pendrin (blue) and MR (brown) labeling of ICs in a representative cortical section from a NaCl-replete IC MR null mouse. (E) Pendrin label per cell (left) and pendrin label in the most apical 10% relative to label across the entire cell (right) were quantified in both the MR(+) (arrows) and MR(−) (arrowheads) ICs taken from the same NaCl-replete IC MR null mouse. *P<0.05.
IC MR Gene Ablation Does Not Eliminate the Increment in Pendrin Total Protein Abundance or Pendrin’s Relative Abundance in the Apical Regions of ICs
Although both aldosterone and glucocorticoids are MR ligands,44,45 MR binding sites are primarily occupied by the latter because circulating concentrations are higher for glucocorticoids than for mineralocorticoids. Aldosterone binding the MR is thus dependent on the oxidation of glucocorticoids, which occurs through the action of 11 β-hydroxysteroid dehydrogenase type 2 (11β-HSD2).45 As such, we examined 11β-HSD2 labeling in CNT cells, principal cells, and ICs. As shown (Figure 7A), although robust 11β-HSD2 labeling was observed in AE1/pendrin negative principal cells of the CNT and CCD, no 11β-HSD2 labeling was detected in either AE1 or pendrin positive ICs. We conclude that in mouse ICs, 11β-HSD2 is either not expressed or is expressed at very low levels, which is consistent with previous reports.23,46
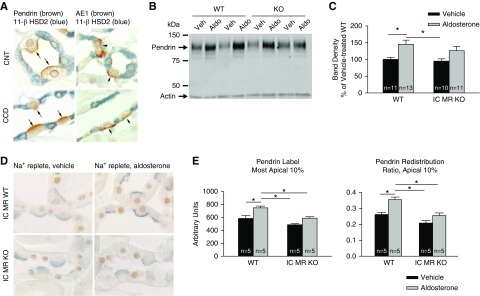
Figure 7.
IC MR gene ablation blunts, but does not eliminate, aldosterone’s effect on apical pendrin label and subcellular distribution. (A) 11β-HSD2 immunolabel (blue) in AE1- and pendrin-positive ICs (brown) and in AE1-/pendrin-negative principal cells or CNT cells from the CNT and CCD. As shown, no 11β-HSD2 label was observed in ICs from either segment. (B) Pendrin total protein abundance in a representative immunoblot of kidney lysates from aldosterone- and vehicle-treated wild-type and IC MR null mice. (C) Pendrin band density in kidney lysates from each group shown in (B). (D) Pendrin (blue) and MR (brown) immunolabel in sections of cortical labyrinth of aldosterone- and vehicle-treated wild-type and IC MR null mice. (E) The effect of aldosterone on pendrin’s abundance in the most apical 10% of ICs as well as pendrin label in the most apical 10% of the cell relative to label across the entire cell (pendrin redistribution ratio) in the CNT of wild-type and IC MR null mice. In immunoblots, groups were compared by ANOVA with a Tukey post-test. In immunohistochemistry studies, groups were compared by ANOVA with a Hochberg post-test. *P<0.05.
Although aldosterone increases pendrin total protein abundance and markedly increases pendrin’s abundance on the apical plasma membrane of ICs, because 11β-HSD2 expression in ICs is either very low or absent, we asked if aldosterone changes pendrin protein abundance and/or subcellular distribution in the absence of the IC MR. To answer this question, we examined the effect of an aldosterone infusion on pendrin total protein abundance and subcellular distribution in wild-type (floxed MR) and in IC MR null mice (treatment 4). Aldosterone increased pendrin total protein abundance in wild-type mice, although it did not produce a statistically significant increase in pendrin abundance in the IC MR null mice (Figure 7, B and C).
Further experiments explored the effect of aldosterone on apical pendrin label and pendrin subcellular distribution in these mutant and wild-type mice. As shown (Figure 7, D and E) aldosterone increased both pendrin label intensity in the most apical 10% of the cell as well as pendrin label in the most apical 10% of the cell relative to label across the entire cell (redistribution ratio) in wild-type (MR floxed) mice. However, no significant difference was observed in the IC MR null mice. We conclude that IC MR gene ablation blunts, but does not eliminate, aldosterone-induced changes in pendrin total protein abundance, apical abundance, and subcellular distribution.
Discussion
Although aldosterone’s signaling cascade has been studied extensively in principal cells and DCT cells, it is much less understood in ICs. Recent studies indicate, however, that these two cell types have very different MR signaling pathways.25 To explore the physiologic role of the IC MR in vivo, we developed mice lacking the IC MR. We observed lower Cl− absorption in CCDs from aldosterone-treated IC MR null relative to wild-type littermates, which was associated with reduced pendrin total protein abundance and reduced pendrin label in the most apical 10% relative to label across the entire cell. Our experiments in MR(+) and (−) ICs taken from the same IC MR KO mice demonstrate that the MR regulates pendrin abundance and function through a direct effect of the receptor within ICs. However, because pendrin mRNA was not measured in this study, the effect of IC MR gene ablation on pendrin transcription remains unresolved. Moreover, the effect of IC MR gene ablation on type A IC transporters as well as its effect on other type B IC transporters, remains the topic of future studies.
In heterologous expression systems, Shibata et al.25 observed that aldosterone binding to the IC MR is modulated by extracellular K+. Although we did not study the effect of K+ on aldosterone (ligand) binding to the IC MR directly, we explored the effect of IC MR gene ablation on pendrin abundance and subcellular distribution in a variety of treatment models associated with a wide range in serum K+ concentration. In treatment models associated with a high serum aldosterone concentration, we observed a significant reduction in pendrin label per cell and a fall in pendrin abundance in the most apical region of the cell, whether serum K+ was low or high. Therefore in treatment models associated with high serum aldosterone concentration, the IC MR regulates pendrin subcellular distribution over a wide range in serum K+ concentration.
Because angiotensin II should be higher in mice given a Na+-deficient diet than in mice given a NaCl-replete diet and aldosterone, it could be argued that when following a Na+-deficient diet supplemented with K+, the inhibitory effect of hyperkalemia on ligand (aldosterone) binding is offset by increased angiotensin II, which enhances aldosterone binding. However, previous rat and human studies make this unlikely.47,48 Sealey et al.47 observed that when rats were given a Na+-deficient diet, raising K+ intake reduced, whereas lowering K+ intake increased, plasma renin activity. These data therefore predict that in mice given a Na+-deficient diet, supplementing the diet with K+ should reduce plasma angiotensin II. Thus, our observation that IC MR-dependent changes in pendrin subcellular distribution were same or greater following a Na+-deficient diet supplemented with K+ relative to changes following the Na+-deficient diet alone, cannot be readily attributed to increased angiotensin II in the former relative to the latter treatment group.
The interaction of aldosterone and the IC MR requires further study. Because 11β-HSD2 has not been detected in ICs,23,46 whether aldosterone acts as an MR ligand in ICs and the role of 11β-HSD2 in this process have been debated. Although glucocorticoids may be the primary IC MR ligand in vivo, we observed that IC MR gene ablation blunts aldosterone-induced changes in pendrin total protein abundance and subcellular distribution, despite the apparent absence of 11β-HSD2 within ICs. These data, in addition to that of Shibata et al.,25 make it likely that aldosterone upregulates pendrin, at least in part, through a direct effect on the IC MR. Changes in IC MR phosphorylation may render the receptor’s apparent affinity for aldosterone greater than for cortisol/corticosterone, at least under some physiologic conditions.44 However, aldosterone may instead activate the IC MR through an intermediary, such as oxidative stress.49
We observed that a component of aldosterone’s effect on pendrin protein abundance and subcellular distribution occurs independently of the IC MR. Because aldosterone is a glucocorticoid receptor ligand,50 and because the glucocorticoid receptor is expressed within ICs,23 aldosterone may stimulate pendrin, in part, through its interaction with the glucocorticoid receptor. However, under each of the treatment conditions we studied, serum aldosterone concentration was significantly below the dissociation constant, Kd, reported for this receptor.50 As such, little aldosterone binding to this receptor is expected under these conditions. Other mechanisms for aldosterone’s IC MR-independent signaling cascade are also possible. For example, we cannot exclude the possibility that aldosterone regulates pendrin, in part, through indirect, systemic effects of this hormone.
Aldosterone-induced ENaC activation occurs through direct activation of the principal cell MR.18,20,21 As such, MR antagonists commonly used in clinical practice, such as spironolactone and eplerenone, induce a natriuresis, reduce BP, and increase serum K+, in part, by directly antagonizing aldosterone binding to the principal cell MR, which reduces ENaC function. The present study observed, however, that ablation of the MR within ICs also reduces ENaC channel activity and γ subunit label intensity per cell in mouse CCD. We cannot exclude the possibility that this fall in channel activity occurs in part from occasional MR gene ablation in principal cells, rather than from ablation of the MR within ICs alone. However, it is unlikely that the fall in ENaC channel activity we observed occurs only from MR ablation in principal cells because we observed relatively few principal cells lacking MR immunolabel in CCDs from these mutant mice. Moreover, we observed reduced γ-ENaC immunolabel in principal cells from the aldosterone-treated IC MR KO relative to the wild-type mice, despite documented MR expression in the principal cells studied from both groups. Our study thus provides evidence that MR blockade reduces ENaC-mediated Na+ absorption, in part, through its effect on IC function, which then secondarily inhibits ENaC channel activity.
Our observation that IC MR gene ablation reduces principal cell γ-ENaC label intensity per cell, 75 kD γ-ENaC abundance by immunoblot, and ENaC channel activity are all consistent with our previous reports that pendrin gene ablation reduces ENaC channel activity and 75 kD γ-ENaC abundance.11,13 These results are in contrast, however, with previous studies showing that random MR gene ablation in principal and CNT cells increases γ-ENaC subunit immunolabel.18 Consistent with these previous results, we also observed that γ-ENaC immunolabel intensity is higher in MR(−) than in MR(+) principal or CNT cells taken from aldosterone-treated IC MR null mice (not shown). Why γ-ENaC subunit immunolabel falls with IC MR gene ablation but increases with ablation of the MR in principal cells or CNT cells is unclear.
Serum K+ concentration was the same or higher in IC MR null relative to wild-type mice after each treatment condition tested. The higher serum K+ observed in the IC MR null mice might occur because the reduced ENaC channel activity seen in these mutant mice reduces the driving force for K+ secretion, such as through ROMK. However, other causes are also possible. Because the α and β4 subunits of large-conductance, Ca+-activated K+ channels (BK) are highly expressed in ICs, and because BKα subunit abundance is reduced in rat kidney after MR antagonist administration (spironolactone),51 IC MR gene ablation might increase serum K+ by reducing Maxi K+ channel-mediated K+ secretion.
Serum aldosterone concentration was similar in the wild-type and IC MR null mice under each treatment condition studied. Therefore, in the absence of the IC MR, intravascular volume is likely maintained through upregulation of other renal transporters. The effect of IC MR gene ablation on other transporters, particularly the thiazide-sensitive NaCl cotransporter, remains to be determined.
In conclusion, the IC MR modulates Cl− transport in the mouse CCD through a mechanism that involves the IC Cl−/HCO3− exchanger, pendrin. The IC MR directly regulates pendrin abundance and subcellular distribution and indirectly modulates Na+ channel activity and Na+ channel open probability in principal cells. Under treatment conditions associated with a high circulating aldosterone concentration, IC MR gene ablation directly reduces pendrin total protein abundance and pendrin’s relative abundance in the region of the apical plasma membrane over a wide range in serum K+. Aldosterone also stimulates pendrin through both IC MR-dependent and -independent mechanisms.
Disclosures
None.
Funding
This work was supported by National Institute of Diabetes and Digestive and Kidney Diseases grants DK119793 (to Dr. Wall) and DK110409 (to Dr. Eaton).
Supplementary Material
Acknowledgments
We thank Drs. David Ellison and Stefan Berger for providing the floxed MR mice, and Drs. Raoul Nelson and R. Lance Miller for providing the B1-H+-ATPase Cre mice. The MR antibodies were a generous gift of Dr. Elise Gomez-Sanchez.32 We thank Dr. Johannes Loffing for the α-ENaC antibody.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
See related editorial, “How Does Aldosterone Work in the β-Intercalated Cell?” on pages 451–452.
Supplemental Material
This article contains the following supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2019050551/-/DCSupplemental.
Supplemental Figure 1. MR labeling in the cortex of NaCl-deficient wild-type (floxed MR) mice.
Supplemental Figure 2. Current voltage relationship of single-channel recordings from CCD principal cells taken from aldosterone-treated IC MR null and wild-type littermates.
Supplemental Figure 3. Abundance of the 80 and 30 kD fragments of α-ENaC taken from aldosterone-treated IC MR null and wild-type littermates.
References
- 1.Star RA, Burg MB, Knepper MA: Bicarbonate secretion and chloride absorption by rabbit cortical collecting ducts. Role of chloride/bicarbonate exchange. J Clin Invest 76: 1123–1130, 1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knepper MA, Good DW, Burg MB: Mechanism of ammonia secretion by cortical collecting ducts of rabbits. Am J Physiol 247: F729–F738, 1984. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Austt J, Good DW, Burg MB, Knepper MA: Deoxycorticosterone-stimulated bicarbonate secretion in rabbit cortical collecting ducts: Effects of luminal chloride removal and in vivo acid loading. Am J Physiol 249: F205–F212, 1985. [DOI] [PubMed] [Google Scholar]
- 4.Royaux IE, Wall SM, Karniski LP, Everett LA, Suzuki K, Knepper MA, et al.: Pendrin, encoded by the Pendred syndrome gene, resides in the apical region of renal intercalated cells and mediates bicarbonate secretion. Proc Natl Acad Sci U S A 98: 4221–4226, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wall SM, Kim YH, Stanley L, Glapion DM, Everett LA, Green ED, et al.: NaCl restriction upregulates renal Slc26a4 through subcellular redistribution: Role in Cl- conservation. Hypertension 44: 982–987, 2004. [DOI] [PubMed] [Google Scholar]
- 6.Verlander JW, Hassell KA, Royaux IE, Glapion DM, Wang ME, Everett LA, et al.: Deoxycorticosterone upregulates PDS (Slc26a4) in mouse kidney: Role of pendrin in mineralocorticoid-induced hypertension. Hypertension 42: 356–362, 2003. [DOI] [PubMed] [Google Scholar]
- 7.Masilamani S, Kim GH, Mitchell C, Wade JB, Knepper MA: Aldosterone-mediated regulation of ENaC alpha, beta, and gamma subunit proteins in rat kidney. J Clin Invest 104: R19–R23, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Stoner LC, Burg MB, Orloff J: Ion transport in cortical collecting tubule; effect of amiloride. Am J Physiol 227: 453–459, 1974. [DOI] [PubMed] [Google Scholar]
- 9.Palmer LG, Frindt G: Amiloride-sensitive Na channels from the apical membrane of the rat cortical collecting tubule. Proc Natl Acad Sci U S A 83: 2767–2770, 1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pácha J, Frindt G, Antonian L, Silver RB, Palmer LG: Regulation of Na channels of the rat cortical collecting tubule by aldosterone. J Gen Physiol 102: 25–42, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim YH, Pech V, Spencer KB, Beierwaltes WH, Everett LA, Green ED, et al.: Reduced ENaC protein abundance contributes to the lower blood pressure observed in pendrin-null mice. Am J Physiol Renal Physiol 293: F1314–F1324, 2007. [DOI] [PubMed] [Google Scholar]
- 12.Pech V, Pham TD, Hong S, Weinstein AM, Spencer KB, Duke BJ, et al.: Pendrin modulates ENaC function by changing luminal HCO3-. J Am Soc Nephrol 21: 1928–1941, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pech V, Wall SM, Nanami M, Bao HF, Kim YH, Lazo-Fernandez Y, et al.: Pendrin gene ablation alters ENaC subcellular distribution and open probability. Am J Physiol Renal Physiol 309: F154–F163, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gueutin V, Vallet M, Jayat M, Peti-Peterdi J, Cornière N, Leviel F, et al.: Renal β-intercalated cells maintain body fluid and electrolyte balance. J Clin Invest 123: 4219–4231, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Faresse N, Ruffieux-Daidie D, Salamin M, Gomez-Sanchez CE, Staub O: Mineralocorticoid receptor degradation is promoted by Hsp90 inhibition and the ubiquitin-protein ligase CHIP. Am J Physiol Renal Physiol 299: F1462–F1472, 2010. [DOI] [PubMed] [Google Scholar]
- 16.Soundararajan R, Lu M, Pearce D: Organization of the ENaC-regulatory machinery. Crit Rev Biochem Mol Biol 47: 349–359, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thomas W, Harvey BJ: Mechanisms underlying rapid aldosterone effects in the kidney. Annu Rev Physiol 73: 335–357, 2011. [DOI] [PubMed] [Google Scholar]
- 18.Czogalla J, Vohra T, Penton D, Kirschmann M, Craigie E, Loffing J: The mineralocorticoid receptor (MR) regulates ENaC but not NCC in mice with random MR deletion. Pflugers Arch 468: 849–858, 2016. [DOI] [PubMed] [Google Scholar]
- 19.Nielsen J, Kwon TH, Masilamani S, Beutler K, Hager H, Nielsen S, et al.: Sodium transporter abundance profiling in kidney: Effect of spironolactone. Am J Physiol Renal Physiol 283: F923–F933, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Terker AS, Yarbrough B, Ferdaus MZ, Lazelle RA, Erspamer KJ, Meermeier NP, et al.: Direct and indirect mineralocorticoid effects determine distal salt transport. J Am Soc Nephrol 27: 2436–2445, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronzaud C, Loffing J, Bleich M, Gretz N, Gröne HJ, Schütz G, et al.: Impairment of sodium balance in mice deficient in renal principal cell mineralocorticoid receptor. J Am Soc Nephrol 18: 1679–1687, 2007. [DOI] [PubMed] [Google Scholar]
- 22.Kim GH, Masilamani S, Turner R, Mitchell C, Wade JB, Knepper MA: The thiazide-sensitive Na-Cl cotransporter is an aldosterone-induced protein. Proc Natl Acad Sci U S A 95: 14552–14557, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ackermann D, Gresko N, Carrel M, Loffing-Cueni D, Habermehl D, Gomez-Sanchez C, et al.: In vivo nuclear translocation of mineralocorticoid and glucocorticoid receptors in rat kidney: Differential effect of corticosteroids along the distal tubule. Am J Physiol Renal Physiol 299: F1473–F1485, 2010. [DOI] [PubMed] [Google Scholar]
- 24.Náray-Fejes-Tóth A, Rusvai E, Fejes-Tóth G: Minealocorticoid receptors and 11 beta-steroid dehydrogenase activity in renal principal and intercalated cells. Am J Physiol 266: F76–F80, 1994. [DOI] [PubMed] [Google Scholar]
- 25.Shibata S, Rinehart J, Zhang J, Moeckel G, Castañeda-Bueno M, Stiegler AL, et al.: Mineralocorticoid receptor phosphorylation regulates ligand binding and renal response to volume depletion and hyperkalemia. Cell Metab 18: 660–671, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berger S, Wolfer DP, Selbach O, Alter H, Erdmann G, Reichardt HM, et al.: Loss of the limbic mineralocorticoid receptor impairs behavioral plasticity. Proc Natl Acad Sci U S A 103: 195–200, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller RL, Lucero OM, Riemondy KA, Baumgartner BK, Brown D, Breton S, et al.: The V-ATPase B1-subunit promoter drives expression of Cre recombinase in intercalated cells of the kidney. Kidney Int 75: 435–439, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.García NH, Plato CF, Garvin JL: Fluorescent determination of chloride in nanoliter samples. Kidney Int 55: 321–325, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Wall SM, Fischer MP, Mehta P, Hassell KA, Park SJ: Contribution of the Na+-K+-2Cl- cotransporter NKCC1 to Cl- secretion in rat OMCD. Am J Physiol 280: F913–F921, 2001. [DOI] [PubMed] [Google Scholar]
- 30.Wall SM: NH+4 augments net acid secretion by a ouabain-sensitive mechanism in isolated perfused inner medullary collecting ducts. Am J Physiol 270: F432–F439, 1996. [DOI] [PubMed] [Google Scholar]
- 31.Bao HF, Thai TL, Yue Q, Ma HP, Eaton AF, Cai H, et al.: ENaC activity is increased in isolated, split-open cortical collecting ducts from protein kinase Cα knockout mice. Am J Physiol Renal Physiol 306: F309–F320, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gomez-Sanchez CE, de Rodriguez AF, Romero DG, Estess J, Warden MP, Gomez-Sanchez MT, et al.: Development of a panel of monoclonal antibodies against the mineralocorticoid receptor. Endocrinology 147: 1343–1348, 2006. [DOI] [PubMed] [Google Scholar]
- 33.Knauf F, Yang CL, Thomson RB, Mentone SA, Giebisch G, Aronson PS: Identification of a chloride-formate exchanger expressed on the brush border membrane of renal proximal tubule cells. Proc Natl Acad Sci U S A 98: 9425–9430, 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nanami M, Pham TD, Kim YH, Yang B, Sutliff RL, Staub O, et al.: The role of intercalated cell Nedd4-2 in BP regulation, ion transport, and transporter expression. J Am Soc Nephrol 29: 1706–1719, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sorensen MV, Grossmann S, Roesinger M, Gresko N, Todkar AP, Barmettler G, et al.: Rapid dephosphorylation of the renal sodium chloride cotransporter in response to oral potassium intake in mice. Kidney Int 83: 811–824, 2013. [DOI] [PubMed] [Google Scholar]
- 36.Klein JD, Martin CF, Kent KJ, Sands JM: Protein kinase C-α mediates hypertonicity-stimulated increase in urea transporter phosphorylation in the inner medullary collecting duct. Am J Physiol Renal Physiol 302: F1098–F1103, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Welinder C, Ekblad L: Coomassie staining as loading control in Western blot analysis. J Proteome Res 10: 1416–1419, 2011. [DOI] [PubMed] [Google Scholar]
- 38.Shibata S, Nagase M, Yoshida S, Kawachi H, Fujita T: Podocyte as the target for aldosterone: Roles of oxidative stress and Sgk1. Hypertension 49: 355–364, 2007. [DOI] [PubMed] [Google Scholar]
- 39.Nagase M, Fujita T: Role of Rac1-mineralocorticoid-receptor signalling in renal and cardiac disease. Nat Rev Nephrol 9: 86–98, 2013. [DOI] [PubMed] [Google Scholar]
- 40.Sauer M, Flemmer A, Thurau K, Beck FX: Sodium entry routes in principal and intercalated cells of the isolated perfused cortical collecting duct. Pflugers Arch 416: 88–93, 1990. [DOI] [PubMed] [Google Scholar]
- 41.Stoos BA, Garcia NH, Garvin JL: Nitric oxide inhibits sodium reabsorption in the isolated perfused cortical collecting duct. J Am Soc Nephrol 6: 89–94, 1995. [DOI] [PubMed] [Google Scholar]
- 42.Schlatter E, Greger R, Schafer JA: Principal cells of cortical collecting ducts of the rat are not a route of transepithelial Cl- transport. Pflugers Arch 417: 317–323, 1990. [DOI] [PubMed] [Google Scholar]
- 43.Shcheynikov N, Yang D, Wang Y, Zeng W, Karniski LP, So I, et al.: The Slc26a4 transporter functions as an electroneutral Cl-/I-/HCO3- exchanger: Role of Slc26a4 and Slc26a6 in I- and HCO3- secretion and in regulation of CFTR in the parotid duct. J Physiol 586: 3814–3824, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Funder JW, Feldman D, Edelman IS: The roles of plasma binding and receptor specificity in the mineralocorticoid action of aldosterone. Endocrinology 92: 994–1004, 1973. [DOI] [PubMed] [Google Scholar]
- 45.Kyossev Z, Walker PD, Reeves WB: Immunolocalization of NAD-dependent 11 beta-hydroxysteroid dehydrogenase in human kidney and colon. Kidney Int 49: 271–281, 1996. [DOI] [PubMed] [Google Scholar]
- 46.Chen L, Lee JW, Chou CL, Nair AV, Battistone MA, Păunescu TG, et al.: Transcriptomes of major renal collecting duct cell types in mouse identified by single-cell RNA-seq. Proc Natl Acad Sci U S A 114: E9989–E9998, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sealey JE, Clark I, Bull MB, Laragh JH: Potassium balance and the control of renin secretion. J Clin Invest 49: 2119–2127, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brunner HR, Baer L, Sealey JE, Ledingham JGG, Laragh JH: The influence of potassium administration and of potassium deprivation on plasma renin in normal and hypertensive subjects. J Clin Invest 49: 2128–2138, 1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ayuzawa N, Fujita T: Activation of mineralocorticoid receptor in salt-sensitive hypertension. Curr Hypertens Rep 17: 552, 2015. [DOI] [PubMed] [Google Scholar]
- 50.Arriza JL, Weinberger C, Cerelli G, Glaser TM, Handelin BL, Housman DE, et al.: Cloning of human mineralocorticoid receptor complementary DNA: Structural and functional kinship with the glucocorticoid receptor. Science 237: 268–275, 1987. [DOI] [PubMed] [Google Scholar]
- 51.Wen D, Cornelius RJ, Yuan Y, Sansom SC: Regulation of BK-α expression in the distal nephron by aldosterone and urine pH. Am J Physiol Renal Physiol 305: F463–F476, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.