Abstract
The implicit paradigm that has governed the study and clinical management of preterm labor is that term and preterm parturition are the same processes, except for the gestational age at which they occur. Indeed, both share a common pathway composed of uterine contractility, cervical dilatation and activation of the membranes/decidua. This review explores the concept that while term labor results from physiological activation of the components of the common pathway, preterm labor arises from pathological signaling and activation of one or more components of the common pathway of parturition. The term ‘great obstetrical syndromes’ has been coined to reframe the concept of obstetrical disease. Such syndromes are characterized by: (1) multiple etiology; (2) long preclinical stage; (3) frequent fetal involvement; (4) clinical manifestations that are often adaptive in nature; and (5) gene–environment interactions that may predispose to the syndromes. This article reviews the evidence indicating that the pathological processes implicated in the preterm parturition syndrome include: (1) intrauterine infection/inflammation; (2) uterine ischemia; (3) uterine over-distension; (4) abnormal allograft reaction; (5) allergy; (6) cervical insufficiency; and (7) hormonal disorders (progesterone related and corticotrophin-releasing factor related). The implications of this conceptual framework for the prevention, diagnosis, and treatment of preterm labor are discussed.
Keywords: allergy, cervical insufficiency, inflammation, intrauterine infection, multiple etiology, prematurity, preterm birth, preterm labor, uterine ischemia, uterine over-distension
Introduction
The implicit paradigm that has governed much of the study of preterm parturition is that term and preterm labour are fundamentally the same process except for the gestational age at which they occur1,2 and share a ‘common pathway.’ The uterine components of this pathway include increased uterine contractility, cervical ripening (dilatation and effacement), and decidua/membrane activation.2,3
Nearly two decades ago, our group proposed that the fundamental difference between term and preterm parturition is that the former results from physiological activation of the common pathway, while preterm labor arises from pathological processes that extemporaneously activate one or more of the components of the common pathway of parturition. This article will review the evidence that preterm labor is a pathological condition with multiple etiologies. This has implications for the fundamental understanding of the biology of preterm parturition and the clinical strategies to diagnose, prevent, and treat spontaneous preterm labour.1,4 Some of these concepts were presented at the Premature Labour Study Group convened by the Royal College of Obstetricians and Gynaecologists and published in a contribution to the proceedings, as well as in a previously published book chapter.5,6
The common pathway of parturition: definition and components
We propose that the common pathway of human parturition be defined as the anatomical, physiological, biochemical, endocrinological, immunological, and clinical events that occur in the mother and/or fetus in both term and preterm labor. The common pathway of parturition is particularly evident when examining the uterine components. Parturition is accompanied by profound changes in other organ systems, and these are the extra-uterine components to the common pathway. Similarly, fetal physiopathologic adaptations associated with impending spontaneous birth are likely to occur, such as modifications in lung water distribution.7 These changes are difficult to study in humans, and most of the literature is confined to animal studies.
The uterine components include: (1) increased myometrial contractility; (2) cervical ripening (dilation and effacement); and (3) decidual/membrane activation. Examples of non-uterine features of the common pathway include changes in the concentrations of hormones such as corticotrophin-releasing factor (CRF) and cortisol, and in the caloric metabolic expenditures.8–17
The common pathway can be defined at different levels of complexity. The definition used above is based on a clinical perspective. A molecular and physiological approach to this definition could use high-dimensional biological techniques to describe the changes in messenger RNA (mRNA), proteins, metabolites, physiological parameters, etc., which occur during labor. We anticipate that transcriptomics (functional genomics), proteomics, metabolomics, physiomics, etc., will be used for a comprehensive description of the common pathway in the future.18,19 This approach has merit since the dissimilarities between term and preterm birth will provide insights into the mechanisms of disease responsible for preterm parturition. We have begun this process by examining the transcriptome of the chorioamniotic membranes in spontaneous labor at term20 and in preterm labor with and without inflammation (R Romero, unpublished observations).
For a comprehensive description of the common pathway of parturition, the reader is referred to other reviews in this area, in particular, to the proceedings of the Preterm Birth Study Group of the Royal College of Obstetricians and Gynaecologists.5 The proceedings contain learned discussions of each of the components of the pathway by experts in each particular field (Professors Bell, Norman, Calder, Bennett and Thornton).
Premature parturition: a syndrome
The current taxonomy of disease in obstetrics is based on the clinical presentation of the mother and not on the mechanism of disease responsible for the clinical manifestations. The term ‘preterm labor’ does not indicate whether the condition is caused by infection, a vascular insult, uterine over-distension, an abnormal allogenic recognition, stress, or some other pathological process. The same applies to pre-eclampsia, small for gestational age, fetal death, nausea and vomiting during pregnancy, and failure to progress in labor, in which the diagnoses simply describe the clinical manifestations without consideration of the specific etiology.
The lack of recognition that these conditions simply represent a collection of signs and symptoms with little reference to the underlying mechanisms of disease may be responsible for the expectation that one diagnostic test and treatment will detect and cure each of these conditions.
We have proposed that the term ‘syndrome’ is more apt to refer to the previously mentioned obstetrical disorders. The Oxford Medical Dictionary defines a syndrome as ‘a combination of symptoms and/or signs that form a distinct clinical picture indicative of a particular disorder.’ Implicit in this definition is that a syndrome can be caused by more than one mechanism of disease or etiology.
We have argued that obstetric disorders responsible for maternal death and perinatal morbidity and mortality are syndromes, hence, the designation of ‘the great obstetrical syndromes.’ Key features of these syndromes21 are (1) multiple etiology; (2) long preclinical stage; (3) frequent fetal involvement; (4) clinical manifestations which are often adaptive in nature; and (5) predisposition to a particular syndrome is influenced by gene-environment interaction and/or complex gene-gene interactions involving maternal and/or fetal genotypes.
This article will review the available evidence to support the concept that premature parturition has ‘multiple etiologies.’ However, preterm parturition meets all the criteria for a great obstetrical syndrome. For example, a sonographically short cervical length in the mid-trimester of pregnancy or high concentrations of fetal fibronectin in vaginal/cervical fluid are risk factors for subsequent spontaneous preterm labor and preterm birth.22–27 Since a short cervix or a positive fetal fibronectin generally occur weeks before the clinical recognition of spontaneous preterm labor and/or preterm prelabor rupture of membranes (PPROM), this can be taken as evidence that there is a subclinical stage in which pregnant women have abnormalities that may not be detected by standard clinical examination: a long preclinical stage. This also applies to intrauterine infection which can be clinically silent weeks or months before the onset of preterm parturition. Such infections have been detected at the time of routine mid-trimester amniocentesis for genetic indications in 0.4% of women and become clinically evident weeks later as either PPROM or preterm labour.28–30 ‘Fetal involvement’ in the context of infection has been shown in women with microbial invasion of the amniotic cavity (MIAC). Fetal bacteremia has been detected in 30% of women with PPROM and a positive amniotic fluid (AF) culture for microorganisms.31 Similarly, neonates born after spontaneous preterm labor or PPROM are more likely to be small for gestational age, indicating a pre-existing problem with the supply line, which results in fetal involvement.32–37 The ‘adaptive nature’ of the clinical manifestation has been proposed in the context of preterm labor with intrauterine infection. The onset of preterm labor can be considered a mechanism of host defense against intrauterine infection whereby the mother eliminates infected tissues (membranes, decidua, and/or fetus) to maintain reproductive fitness. When the fetus is mature, the onset of premature labor may also have survival value for it allows the fetus to escape a hostile intrauterine environment. The complexity of nature’s calculation to balance maternal and fetal interests in this context cannot be overemphasized.38,39 It is possible that other mechanisms of disease in preterm labor may also threaten the maternal and fetal pair (i.e. ischemia/hemostatic disorders) and a key question would be why some hosts resort to fetal growth restriction, others to pre-eclampsia, and yet others to the onset of preterm labor to deal with the underlying insult. If the clinical manifestations are adaptive, then treatment of the components of the terminal pathway (tocolysis, cerclage, etc.) could be considered as symptomatic and not aimed at the specific pathological process that causes preterm labor. Finally, the predisposition to use a specific mechanism of host defense (e.g. PPROM or preterm labor with intact membranes) may be determined by a ‘gene-environment interaction’ or ‘gene-gene interactions’ as in other complex disorders. Complexity is added during pregnancy by the presence, and even perhaps the conflicting interest, of two genomes (maternal and fetal).
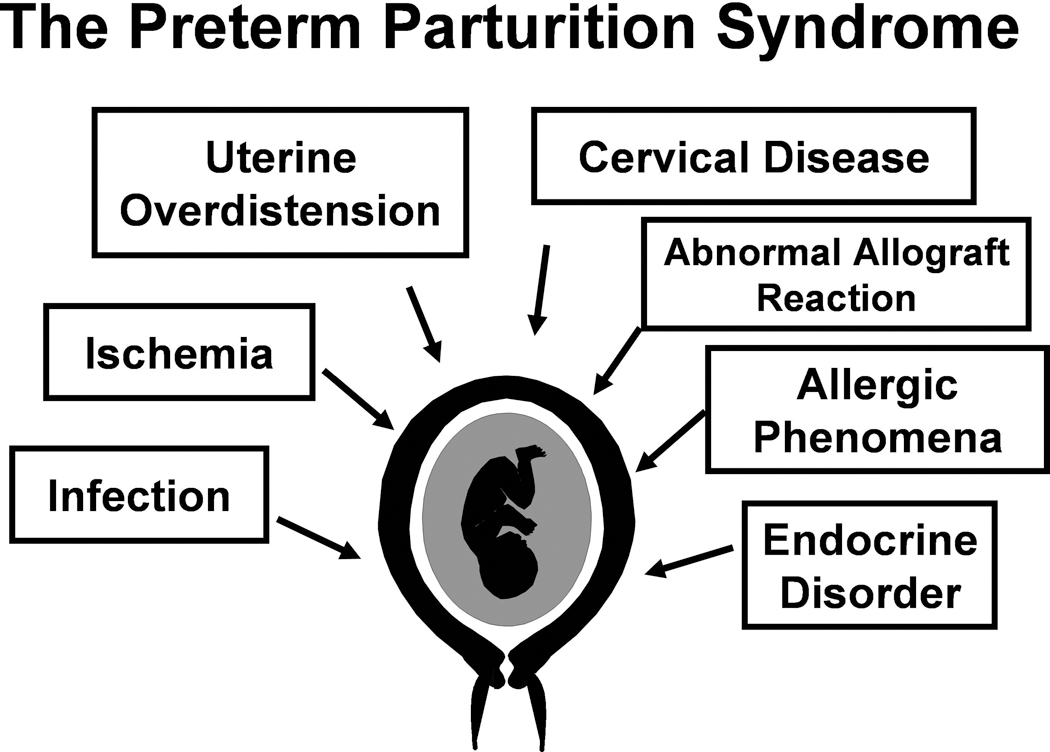
The pathological processes implicated in the preterm birth syndrome include intrauterine infection, uterine ischemia, uterine over-distension, abnormal allogenic recognition, allergic-like reaction, cervical disease, and endocrine disorders (Figure 1). The possibility that mechanisms of disease not yet described may be operative must be considered. Most of the understanding of the mechanisms of disease in obstetrics has been derived from observations in adults and children. The biology of pregnancy is unique since it requires the pacific co-existence of two hosts. The challenges presented by this intimate relationship could create conditions in which novel mechanisms of disease may emerge. Normal pregnancy is characterized by bi-directional traffic of cells (maternal and fetal). Increased fetal DNA has been reported in the maternal blood of women in preterm labor, leading to preterm birth.40,41 It is possible that abnormal fetal-maternal cell traffic poses challenges that can only be resolved, in some women, with preterm labor. Why excess fetal traffic is associated with pre-eclampsia42–45 in some women, and to preterm labor40,41 in others, is unclear. The following sections will review the evidence supporting different mechanisms of disease in preterm labor.
Figure 1.
Pathological processes implicated in the preterm parturition syndrome. (Reproduced with permission from reference 5.)
Infection as a cause of preterm labor
Intrauterine infection has emerged as a frequent and important mechanism of disease in preterm birth.46–49 It is the only pathological process for which a firm causal link with preterm birth has been established and for which a defined molecular pathophysiology is known.3
Evidence of causality
The evidence in support of a causal relationship between infection/inflammation and spontaneous preterm labor includes: (1) intrauterine infection or systemic administration of microbial products to pregnant animals can result in spontaneous preterm labor and preterm birth;47,50–62 (2) extra-uterine maternal infections, such as malaria,63–66 pyelonephritis,67–71 pneumonia,72–74 and periodontal disease,75–80 have been associated with preterm birth; (3) subclinical intrauterine infections are associated with preterm labor and preterm birth;81 (4) pregnant women with intra-amniotic infection28–30 or intrauterine inflammation (defined as an elevation of AF concentrations of cytokines82,83 and matrix degrading enzymes84) in the mid-trimester are at risk for subsequent preterm birth; (5) antibiotic treatment of ascending intrauterine infections can prevent preterm birth in experimental models of chorioamnionitis;58,85 and (6) treatment of asymptomatic bacteriuria prevents preterm birth.86,87
Infection versus inflammation
Microbiological studies suggest that infection may account for 25%–40% of preterm birth.49,88 Infection is difficult to detect due to the limitations of standard microbiological techniques (cultivation of microorganisms in the laboratory) and the difficulties in obtaining an informative sample (AF requires amniocentesis). Since infection is a major cause of inflammation, we often refer to women with proven infection and those with histological evidence of acute chorioamnionitis or elevated pro-inflammatory cytokines in the AF as belonging to an ‘inflammatory cluster.’
The frequency and clinical significance of intrauterine infection
Intrauterine infections caused by bacteria are considered to be the leading cause of infection-associated preterm birth. The amniotic cavity is considered sterile, as less than 1% of women not in labor at term will have bacteria in the AF. The isolation of bacteria in the AF is a pathological finding, which we have defined as microbial invasion of the amniotic cavity (MIAC). Most of these infections are subclinical in nature and cannot be detected without AF analysis. The frequency of MIAC depends on the clinical presentation and gestational age. In women with preterm labor and intact membranes, the rate of positive AF cultures is 12.8%.49 However, among those women in spontaneous preterm labor with intact membranes who deliver preterm, the frequency is 22%. Among women with PPROM, the rate of positive AF cultures at admission is 32.4%;49 however, at the time of the onset of labor, as many as 75% of women will have MIAC,89 suggesting that microbial invasion occurs during the latency period.
The frequency of MIAC among women presenting with the clinical picture of cervical insufficiency is up to 51%.90,91 If the cervix is short (as determined by a sonographic cervical length of less than 25 mm), MIAC occurs in 9% of women.92 Finally, the frequency of MIAC in twin gestations with preterm delivery is 11.9%.93 Of interest, in twin gestations in which MIAC is detected, the presenting sac is frequently involved, while the other amniotic cavity may not have MIAC.94
Women with MIAC are more likely to deliver preterm, have spontaneous rupture of the membranes, develop clinical chorioamnionitis, and have adverse perinatal outcome than those with preterm labor or PPROM with sterile AF.95 An interesting and consistent observation is that the lower the gestational age at presentation (preterm labor with intact membranes or PPROM), the higher the frequency of positive AF cultures.96,97
Microbiology of intrauterine infection
The most common microorganisms found in the amniotic cavity are genital Mycoplasma species and, in particular, Ureaplasma urealyticum.48,98 Other microorganisms found in the amniotic cavity include Streptococcus agalactiae, Escherichia coli, Fusobacterium species, and Gardnerella vaginalis.48 With the use of molecular microbiological techniques, organisms normally found in the oral cavity have been detected in the AF of women in preterm labor.99 This observation raises questions as to the pathway used by these organisms to reach the amniotic cavity (see below).
Significance of MIAC detected only by molecular microbiology techniques
The prevalence of MIAC described in the preceeding sections is based on the results of standard microbiological methods (i.e. cultivation techniques). A positive culture can only be obtained if the culture conditions in the laboratory are able to support the growth of a particular microorganism. Since the growth requirements of all microorganisms are unknown, a negative culture cannot be taken to definitively exclude the presence of microorganisms. In other words, while a positive culture is indicative of MIAC, a negative culture indicates that the laboratory was not able to grow bacteria from the specimen, either because bacteria were absent (a true-negative result) or because the laboratory conditions did not support the growth of a specific microorganism (a false-negative result). It is noteworthy that only 1% of the whole microbial world can be detected by cultivation techniques (‘the great plate count anomaly’).100–102
Consequently, the frequency of MIAC reported previously in the literature, using cultivation techniques, represents minimum estimates. These figures are likely to change with the introduction of more sensitive methods for microbial recovery and identification. Several investigators have shown that the prevalence of MIAC is higher when molecular microbiological techniques are used to detect conserved sequences in prokaryotes (e.g. bacterial 16S ribosomal DNA with polymerase chain reaction [PCR]) or specific probes.103–106
The clinical significance of MIAC detected purely by molecular microbiology techniques, but not by cultivation techniques, has been recently addressed. Women with a positive PCR for U. urealyticum, but a negative culture, have similar adverse outcomes to women with a positive AF culture and have worse outcomes than those with sterile AF and a negative PCR.107,108 Women with a positive PCR, but a negative culture, have the same degree of inflammation (AF interleukin [IL]-6, histological chorioamnionitis or funisitis) as those with a positive AF culture.108 Collectively, this evidence suggests that the presence of microbial footprints detected by PCR is associated with adverse outcomes.
Intrauterine infection can also be present without a positive AF culture for microorganisms or a positive PCR. If the infection is localized to the decidua or to the space between the amnion and the chorion, microorganisms may not be detected in the amniotic cavity.97 There is evidence that the rate of microbial colonization in the chorioamniotic space is higher than that observed in the amniotic cavity.97 Women with positive cultures in the membranes, but negative cultures in the AF, often have elevated AF concentrations of inflammatory markers such as IL-6.97 Some women with intra-amniotic inflammation, but negative cultures in the AF, may have intrauterine infection in the extra-amniotic space.
Microorganisms in the chorioamniotic membranes—is it always pathological?
The amniotic cavity is normally considered sterile for bacteria, even with the use of molecular microbiological techniques. In contrast, fluorescence in situ hybridization with a DNA probe specific for conserved regions of bacterial DNA (the 16S ribosomal RNA) has detected bacteria in the fetal membranes of up to 70% of women undergoing elective caesarean section at term.109 Bacteria are often present in the membranes of women in preterm labor and intact membranes and in women with PPROM. These findings suggest that the presence of bacteria alone is not sufficient to cause preterm labor and preterm birth and that microbial colonization of the chorioamniotic membranes may not always elicit a fetal or maternal inflammatory response.109
MIAC as a chronic process
Although chorioamnionitis is traditionally considered an acute process, evidence that MIAC exists for an extended period of time is mounting. Cassell et al.28 were the first to report the recovery of genital Mycoplasma species from 6.6% (4/61) of AF samples collected by amniocentesis between 16 and 21 weeks of gestation. Two women had positive cultures for M. hominis and two had positive cultures for U. urealyticum. Women with M. hominis delivered at 34 and 40 weeks without neonatal complications, while those with U. urealyticum had a preterm birth, neonatal sepsis and neonatal death at 24 and 29 weeks of gestation. Subsequently, Gray et al.29 reported a 0.37% prevalence (9/2461) of positive cultures for U. urealyticum in AF samples obtained during second-trimester genetic amniocentesis. After exclusion of a therapeutic abortion case, all women (8/8) with positive AF cultures had either a fetal loss within 4 weeks of amniocentesis (n = 6) or preterm birth (n = 2). All had histological evidence of chorioamnionitis. These observations suggest that microbial invasion could be clinically silent in the mid-trimester of pregnancy and that pregnancy loss/preterm birth could take weeks to occur. A similar finding was reported by Horowitz et al.30 who detected U. urealyticum in 2.8% (6/214) of AF samples obtained between 16 and 20 weeks of gestation. The rate of adverse pregnancy outcome (fetal loss, preterm birth and low birthweight) was significantly higher in women with a positive AF culture than in those with a negative culture (3/6 [50%] versus 15/123 [12%]; P = 0.035).
Intra-amniotic inflammation as a chronic process
High IL-6 concentrations in AF are considered a marker of intra-amniotic inflammation and are frequently associated with microbiological infection in the AF.110–113 Romero et al.82 reported the results of a case-control study in which IL-6 determinations were conducted in stored fluid of women who had a pregnancy loss after a mid-trimester amniocentesis and a control group who delivered at term. Women who had a pregnancy loss had a significantly higher median AF IL-6 concentration than those with a normal outcome. Similar findings were reported by Wenstrom et al.114 Of note is that maternal serum concentrations of IL-6 were not associated with adverse pregnancy outcome.114
The same approach was subsequently used to test the association between markers of inflammation in mid-trimester AF of asymptomatic women and preterm birth. The concentrations of matrix metalloproteinase (MMP)-8,84 IL-6,83 tumor necrosis factor alpha (TNF-α),115 and angiogenin116 in AF obtained at the time of mid-trimester amniocentesis were significantly higher in women who subsequently delivered preterm than in those who delivered at term.
Collectively, the evidence cited above suggests that a chronic intra-amniotic inflammatory process is associated with both miscarriage and spontaneous preterm labor and preterm birth. Whether intra-amniotic inflammation can be detected non-invasively remains to be determined. Goldenberg et al.117 showed that the maternal plasma concentration of granulocyte-colony-stimulating factor (G-CSF) at 24 and 28 weeks of gestation is associated with early preterm birth. To the extent that G-CSF may reflect an inflammatory process, this finding suggests that a chronic inflammatory process identifiable in the maternal compartment is associated with early preterm birth.
Pathways of intra-amniotic infection
Microorganisms may gain access to the amniotic cavity and fetus using any of the following pathways: (1) ascending from the vagina and the cervix; (2) hematogenous dissemination through the placenta (transplacental infection); (3) retrograde seeding from the peritoneal cavity through the fallopian tube; and (4) accidental introduction at the time of invasive procedures, such as amniocentesis, percutaneous fetal blood sampling, chorionic villus sampling, or shunting.118 The most common pathway of intrauterine infection is the ascending route (Figure 2).
Figure 2.
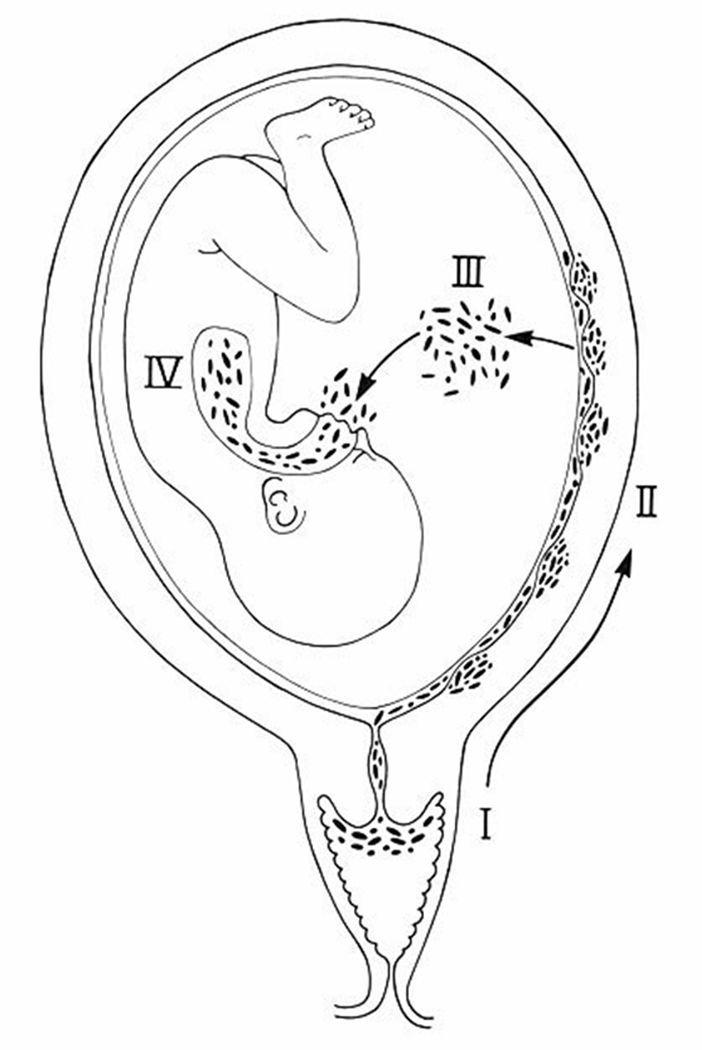
The most common pathway of intrauterine infection is the ascending route. (Reproduced with permission from reference 1.)
Accumulating evidence supports a relationship between periodontal disease and preterm labor and preterm birth.75–80,99,119–123 The mechanism underlying this association has not been established definitively; however, there is experimental evidence that microorganisms found in the gingival crevice can be isolated from the AF, suggesting that maternal bacteremia and transplacental passage could account for some of these infections. Indeed, a humoral fetal response has been demonstrated by Boggess et al.124
Microbial products in the amniotic cavity
The adverse events associated with microbial invasion can be due to the proliferation of intact microorganisms or bacterial products. The cell wall of Gram-negative bacteria contains lipopolysaccharide (LPS) or endotoxin. This potent agent is capable of inducing endotoxic shock and death.125 Gram-positive bacteria lack LPS but contain peptidoglycans and lipoteichoic acid, components of the bacterial cell wall.126 Mycoplasmas have products such as lipoglycans.127 Many of the effects of microorganisms are mediated by these products, which can be released during bacterial death. Thus, even nonviable bacteria may exert deleterious effects. LPS, peptidoglycans, and lipoglycans are recognized by Toll-like receptors (TLRs) and other pattern recognition molecules, and can elicit an inflammatory response.
Bacterial endotoxin in AF was first identified in 1987.128 Subsequently, it was found that the concentrations of these microbial products were significantly higher in women in preterm labor with ruptured membranes than in those with ruptured membranes but not in labor129 (Figure 3). There is a paucity of data about the AF concentration of other microbial products. A number of experimental studies have determined that endotoxin administration into the amniotic cavity,130 uterus,131,132 or intraperitoneally60,133 can result in an inflammatory response with potent biological effects in the fetal lung.134–136 Moreover, intrauterine bacterial inoculation, in an ascending model of intra-amniotic infection, was associated with histological evidence of brain white matter damage.137
Figure 3.
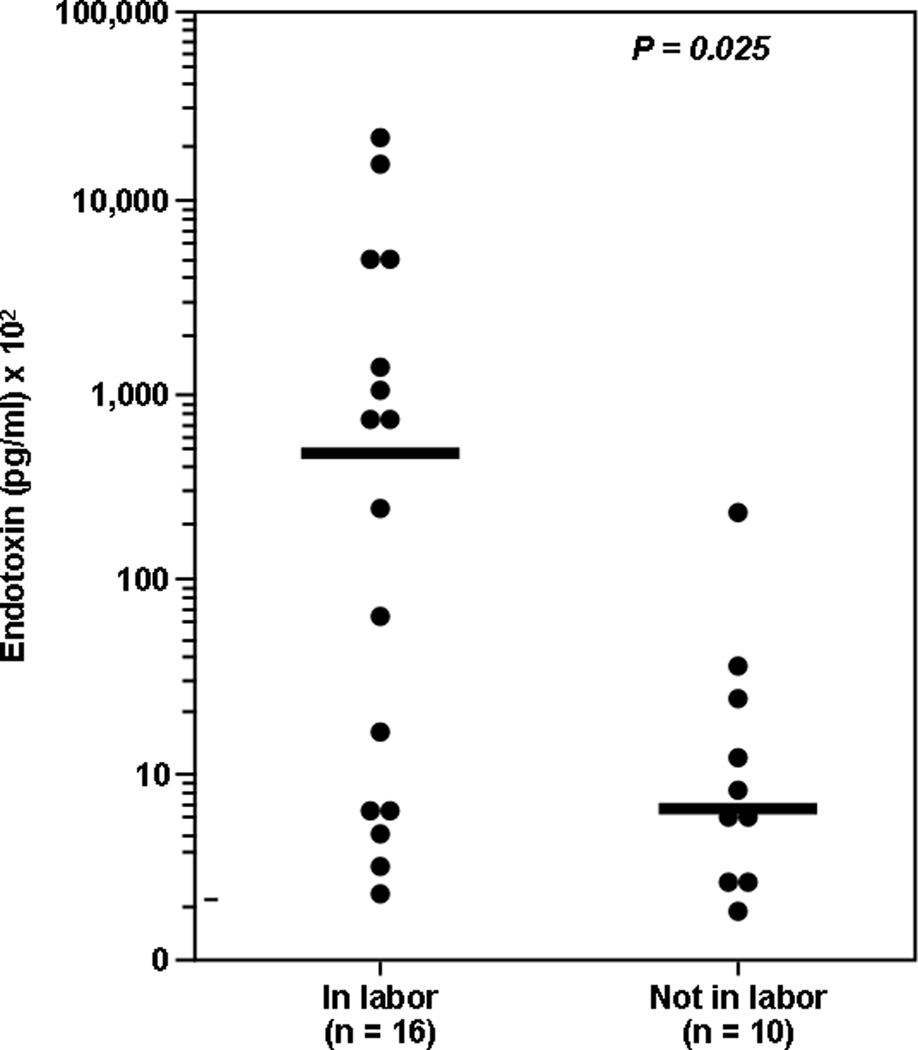
Concentrations of bacterial endotoxins are significantly higher in women with PPROM in labor than in those with PPROM not in labor. (Reproduced with permission from reference 129.)
Inflammation as a mechanism for preterm birth
An overview of the inflammatory response
The first line of defense against infection is provided by the innate immune system. Epithelial surfaces (mucous membranes) represent the first physical barrier between the body and the microorganisms. Injuries to the epithelial surface provide a point of entry for microorganisms. These injuries can result from accidents or physiological processes (e.g. menstruation). A sexually transmitted microorganism may cause infection if it gains access to the endometrium during menstruation. Bacteria can cross intact epithelial barriers. There is experimental138 and clinical evidence48,128 suggesting that bacteria can cross intact fetal membranes but epithelium represents more than a physical barrier against microorganisms. Most epithelia produce natural antimicrobial peptides (e.g. alpha-defensins and beta-defensins),139 which can kill bacteria by damaging their cell membrane.140–143 The fetal lung produces surfactant proteins (SP-A144,145 and SP-D144), which belong to the collectin family and can bind microorganisms and facilitate phagocytosis (opsonization). Moreover, SP-A and SP-D have been shown to be involved in clearance of bacteria, fungi, and apoptotic and necrotic cells, downregulation of allergic reaction, and resolution of inflammation.146
Another mechanism of host defense against infection derives from the metabolic products of bacteria. Lactobacilli, which colonise the vagina shortly after birth, produce lactic acid, which lowers the pH of the vagina. This unique partnership between vaginal tissues and species-specific strains of lactobacilli has been considered responsible for enabling internal fertilization in the evolution of mammals from amphibians.147 In addition to the low pH, some strains of lactobacilli also produce antimicrobial products (bacteriocin-like compounds), which prevent the growth of pathogenic bacteria.148,149
The innate component of the immune system also provides immediate protection from microbial challenge by recognizing the presence of microorganisms, preventing tissue invasion and/or eliciting a host response to limit microbial proliferation (inflammation).150 One of the mechanisms by which the innate immune system recognizes microorganisms is by using pattern recognition receptors (PRRs), which bind to patterns of molecular structures present on the surfaces of microorganisms.150 PRRs, which are classified according to their function and subcellular localization, include (1) soluble PRRs, such as ‘the acute-phase proteins,’ mannan-binding lectin and C-reactive protein, which act as opsonins to neutralize and clear pathogens through the complement and phagocytic systems; (2) transmembrane PRRs, which include scavenger receptors, C-type lectins, and TLRs; and (3) intracellular PRRs, including Nod1 and Nod2, retinoic-induced gene type 1 and melanoma differentiation associated protein 5, which mediate recognition of intracellular pathogens (e.g. viruses).151
Ten different TLRs have been recognized in humans.150 TLR-4 recognizes the presence of LPS (Gram-negative bacteria); TLR-2 recognizes peptidoglycans, lipoproteins, and zymosan (Gram-positive bacteria, Mycoplasmas, and fungi); and TLR-3 recognizes double-stranded RNA (viruses). The ligand for TLR-5 is flagellin.150,152,153
Ligation of TLRs results in activation of nuclear factor (NF)-kB, which, in turn, leads to the production of cytokines, chemokines, and antimicrobial peptides.150 Moreover, activation of the Toll pathway also induces surface expression of co-stimulatory molecules required for the induction of adaptive immune responses, such as CD-80 and CD-86. In combination with antigenic microbial peptides, these molecules presented by major histocompatability complex class II proteins in dendritic cells and macrophages can activate naïve CD4 T cells that initiate most adaptive immune responses.150
Innate immune receptors of the genital tract
TLR-1, −2, −3, −5, and −6 have been identified in epithelia from the vagina, ecto- and endocervix, endometrium, and uterine tubes.154 Of note, TLR-4 has been shown in the endocervix, endometrium, fallopian tubes and ectocervix.154,155 This has been interpreted as evidence that TLR-4 may participate in the modulation of the immune response in the genital tract of women and in host defense against infection.154 Similarly, trophoblast cells can recognize and respond to pathogens through TLRs. We have shown that trophoblast cells are able to recognize pathogens through the expression of TLR-2 and TLR-4. Activation of different TLRs generates distinct trophoblast cell responses. In vitro studies have shown that TLR-4 ligation promotes cytokine production, while TLR-2 ligation induces apoptosis in first trimester trophoblast cells.156 These findings suggest that a pathogen, through TLR-2, may directly promote trophoblast cell death,156 which is observed in a number of pregnancy complications including miscarriage,157 intrauterine growth restriction,158,159 and preeclampsia.159
The importance of TLRs in preterm parturition
Since TLRs are crucial for the recognition of microorganisms, it could be anticipated that defective signaling through this PRR will impair bacteria-induced preterm labor. A strain of mice that has a spontaneous mutation for TLR-4 is less likely to deliver preterm after intrauterine inoculation of heat-killed bacteria or LPS administration than wild-type mice.131,160 In pregnant women, TLR-2 and TLR-4 are expressed in the amniotic epithelium.161 Moreover, spontaneous labor at term or preterm with histological chorioamnionitis, regardless of the membrane status (intact or ruptured), is associated with an increased mRNA and protein expression of TLR-2 and TLR-4 in the chorioamniotic membranes.161 These observations suggest that the innate immune system plays a role in parturition.
The role of pro-inflammatory cytokines (IL-1 and TNF-a)
Strong evidence supports a role for inflammatory mediators in the mechanisms of preterm parturition. Major attention has been focused on the role of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL–8. Other pro-inflammatory and anti-inflammatory cytokines may also play a role, as can chemokines, platelet-activating factor, prostaglandins, and other inflammatory mediators. The current view is that during the course of ascending intrauterine infection, microorganisms may reach the decidua, where they can stimulate a local inflammatory reaction and the production of pro-inflammatory cytokines and inflammatory mediators (platelet-activating factor, prostaglandins, leukotrienes, reactive oxygen species, NO, etc.). If this inflammatory process is not sufficient to signal the onset of labor, microorganisms can cross intact membranes into the amniotic cavity, where they can also stimulate the production of inflammatory mediators by resident macrophages and other host cells. Finally, microorganisms that gain access to the fetus may elicit a systemic inflammatory response syndrome, characterized by increased concentrations of IL-638,39 and other cytokines,162,163 as well as cellular evidence of neutrophil and monocyte activation.164
A solid body of evidence indicates that cytokines play a central role in the mechanisms of inflammation/infection-induced preterm parturition.81,165–177 IL-1 was the first cytokine to be implicated in the onset of spontaneous preterm labor associated with infection.165 Evidence in support of the participation of IL-1 includes: (1) IL-1 is produced by human decidua in response to bacterial products;178 (2) IL-1 stimulated prostaglandin production by human amnion and decidua;179 (3) IL-1 concentration and bioactivity were increased in the AF of women with preterm labor and infection;180 (4) IL-1 could stimulate myometrial contractions181 (C. Bulletti, personal communication); and (5) administration of IL-1 to pregnant animals induced preterm labor and preterm birth,182 a phenomenon that could be blocked by the administration of its natural antagonist: the IL-1 receptor antagonist (IL-1ra).183
The evidence supporting the role of TNF-α in the mechanisms of preterm parturition includes: (1) TNF-α stimulates prostaglandin production by the amnion, decidua, and myometrium;47 (2) human decidua can produce TNF-α in response to bacterial products;184,185 (3) AF TNF-α bioactivity and immunoreactive concentrations are elevated in women in preterm labor and with intra-amniotic infection;186 (4) in women with PPROM and intra-amniotic infection, TNF-α concentrations are higher in the presence of labor;186 (5) TNF- α can stimulate the production of MMPs,187,188 which may play a role in membrane rupture189–191 and cervical ripening;187,192,193 (6) TNF-α application on the cervix induces changes that resemble cervical ripening;194 and (7) TNF- α is involved in the mechanisms of bacterial-induced preterm parturition in animal models.195,196
Redundancy in the cytokine network
Other cytokines (IL-6,97,110,197–200 IL-10,181,201,202 IL-16,203 IL-18,204 colony-stimulating factors,117,205,206 and macrophage migration inhibitory factor207) and chemokines (IL-8,206,208–210 monocyte chemotactic protein-1,211 epithelial-cell-derived neutrophil-activating peptide-78,212 and regulated on activation normal T-cell expressed and secreted213) have also been implicated in the mechanisms of disease in preterm labor and preterm birth. The redundancy of the cytokine network implicated in parturition is such that the blockade of a single cytokine is insufficient to prevent preterm birth in the context of infection. Preterm labor can occur in knockout (KO) mice for the IL-1 type I receptor after exposure to bacteria, suggesting that IL-1 administration is sufficient, but not necessary, for the onset of birth in the context of infection.214 However, blockade of both IL-1 and TNF-α signaling in a double KO mice model has been associated with a decreased rate of preterm birth after bacterial inoculation.215 This is compelling evidence of the importance of IL-1 and TNF-α in the mechanisms of preterm parturition associated with infection.
Anti-inflammatory cytokines and preterm labor
IL-10 is believed to be a key cytokine for the maintenance of pregnancy. IL-10 production is significantly reduced in the placenta at term without labor compared with that in first- and second-trimester tissues, suggesting that downregulation of IL-10 is a physiological event that favors an inflammatory state around the time of the onset of labour.201 IL-10 has also been implicated in the control of preterm parturition associated with inflammation.202 Indeed, IL-10 expression was reduced in the placental tissues of pregnancies complicated by preterm labor and chorioamnionitis when compared with that in placental tissues from normal controls.202 IL-10 inhibits cyclooxygenase type 2 (COX-2) mRNA expression in cultured placental explants from women following preterm labor and preterm birth but not in those from women in labor at term, indicating that the mechanisms involved in the regulation of the inflammatory response during term and preterm parturition may be different.202 Further evidence that IL-10 plays a role in down-regulation of the inflammatory response in preterm labor derives from a study in which pregnant rhesus monkeys (n = 13) were allocated to one of three groups: (1) intra-amniotic IL-1β infusion with maternal dexamethasone intravenously (n = 4); (2) intra-amniotic IL-1β + IL-10 (n = 5); or (3) intra-amniotic IL-1β administered alone (n = 5). Dexamethasone and IL-10 treatment significantly reduced IL-1β-induced uterine contractility (P < 0.05). The concentrations of TNF-α and leukocyte counts in AF were also attenuated by IL-10 treatment (P < 0.05).181 The administration of IL-10 in animal models of infection has been associated with improved pregnancy outcome.216,217
Fetal involvement
The most advanced and serious stage of ascending intrauterine infection is fetal infection. The overall mortality rate of neonates with congenital neonatal sepsis ranges between 25% and 90%.218–222 The wide range of results may reflect the effect of gestational age on the likelihood of survival. One study, which focused on infants born before 33 weeks of gestation, found that the mortality rate was 33% for those infected and 17% for non-infected fetuses.222 Carroll et al.29 have reported that fetal bacteremia is present in 33% of fetuses with positive AF culture and 4% of those with negative AF culture, indicating that subclinical fetal infection is far more common than traditionally recognized.
Inflammation and fetal injury: the fetal inflammatory response syndrome
While the traditional definition of inflammation describes ‘localized inflammation’ to a particular tissue, it is now recognized that inflammation may be present in the systemic circulation. Such a state is referred to as the ‘systemic inflammatory response syndrome’. This condition was originally described in adults and is often referred to by the acronym ‘SIRS.’ SIRS was introduced in 1992 by the American College of Chest Physicians and the Society of Critical Care Medicine to describe a complex set of findings, which often involved cardiovascular abnormalities believed to be the result of systemic activation of the innate immune system.223 The changes, which are characterized by fever, tachycardia, hyperventilation, and an elevated white blood cell count,223 have been attributed to the effects of cytokines and other pro-inflammatory mediators.224 In 2001, the same organization noted that the elevation of certain mediators, such as IL-6, may be associated with SIRS and this observation may bring about a new definition of the syndrome in adults, as the clinical and laboratory findings originally proposed to characterize SIRS were nonspecific.225 We defined the fetal counterpart of SIRS, the ‘fetal inflammatory response syndrome’ (FIRS), for the first time in 1997, using precisely the same parameter that was proposed in adults: an elevated IL-6 concentration (in fetal blood).38,226
FIRS was originally described in pregnancies complicated by preterm labor and PPROM and was operationally defined as a fetal plasma IL-6 concentration of >11 pg/ml. Fetuses with FIRS had a higher rate of severe neonatal morbidity (e.g. respiratory distress syndrome, suspected or proved neonatal sepsis, pneumonia, bronchopulmonary dysplasia, intraventricular hemorrhage, periventricular leukomalacia, or necrotizing enterocolitis)38 and a shorter cordocentesis-to-delivery interval.38,39 The original work describing FIRS was based on fetal blood samples obtained by cordocentesis.38,39 Many of the findings have since been confirmed by studying umbilical cord blood at the time of birth, including the elevation of pro-inflammatory cytokines and the relationship between these cytokines and the likelihood of clinical and suspected sepsis.227–229 Pathological examination of the umbilical cord is an alternative approach to determine whether fetal inflammation was present before birth. Funisitis and chorionic vasculitis are the histopathological hallmark of FIRS.230 Funisitis is associated with endothelial activation, a key mechanism in the development of organ damage,231 and neonates with funisitis are at increased risk for neonatal sepsis232 and long-term handicaps, such as bronchopulmonary dysplasia227 and cerebral palsy.233 Another approach to detect FIRS is to measure C-reactive protein concentration in umbilical cord blood, which has been shown to be elevated in women with AF infection, funisitis, and congenital neonatal sepsis.234 Since neutrophils in the AF are predominantly of fetal origin,235 the AF white blood cell count can also be used as an indirect index of fetal inflammation.235 Intra-amniotic inflammation is a risk factor for impending preterm birth and adverse perinatal outcome in women with PPROM, even in the absence of documented intra-amniotic infection.236
Among women with PPROM, an elevated fetal plasma IL-6 level is associated with the impending onset of preterm labor, regardless of the inflammatory state of the AF (Figure 4).39 This suggests that the human fetus plays a role in initiating the onset of labor. Maternal-fetal cooperation must occur for birth to be completed. Fetal inflammation has been linked to the onset of labor in association with ascending intrauterine infection. However, systemic fetal inflammation may occur in the absence of labor when the inflammatory process does not involve the chorioamniotic membranes and decidua. Such instances may take place in the context of hematogenous viral infections or other disease processes (e.g. rhesus alloimmunization).
Figure 4.
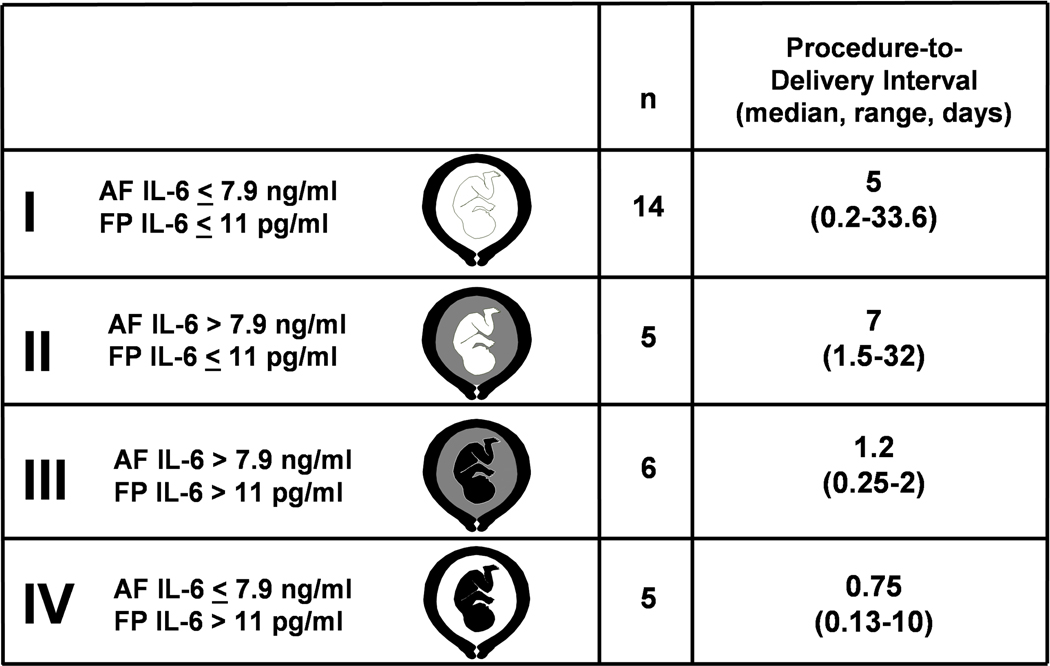
Classification and procedure-to-delivery interval of women according to AF and fetal plasma (FP) IL-6 concentrations. Analysis restricted to 30 women with available AF. White in the fetal or AF compartment represents a low FP or AF IL-6 concentration, respectively. Black in the fetal or AF compartment denotes elevated fetal plasma or AF IL-6 concentration, respectively. (Reproduced with permission from reference 39).
Gene-environment interaction
A gene-environment interaction is said to be present when the risk of a disease (occurrence or severity) among individuals exposed (to both genotype and an environmental factor) is greater or lower than that which is predicted from the presence of either the genotype or the environmental exposure.237,238 The most powerful evolutionary force shaping the development of the immune system is the microbial-host interaction. Bacterial vaginosis (BV) is a risk factor for spontaneous preterm delivery.239–241 However, meta-analysis and randomized clinical trials of antibiotic administration to prevent preterm birth have yielded contradictory results.239–251 Macones et al.252 recently reported the results of a case–control study in which participants were women who experienced spontaneous preterm labor and preterm birth and controls were women who delivered after 37 weeks. The environmental exposure was clinically diagnosed BV (symptomatic vaginal discharge, a positive whiff test, and clue cells on a wet preparation). The genotype of interest was TNF-α allele 2, given that carriage of this genotype had been shown by the authors to be associated with spontaneous preterm labor and preterm birth in previous studies.253 The key observations were that (1) clinically diagnosed BV was not associated with an increased risk for preterm birth (OR 1.6, 95% CI 0.8–3.5); and (2) women who carried the TNF-α allele 2 were also not at an increased risk for preterm birth (OR 1.8, 95% CI 1–3.1). In contrast, women with both BV and the TNF-α allele 2 had an odds ratio of 10 (95% CI 4.4–24) for spontaneous preterm labor and preterm birth, suggesting that a gene-environment interaction predisposes to preterm birth.254 Similar interactions may determine the susceptibility to intrauterine infection, microbial invasion of the fetus, and the likelihood of fetal injury. Gene-to-gene interactions may also play a role in modulating the inflammatory response, and therefore, may also play a role in preterm labor and delivery.
Uteroplacental ischemia
Women in spontaneous preterm labor can be classified into two groups: those with inflammatory lesions of the placenta and membranes and those without evidence of inflammation.255 A major challenge has been to identify the mechanisms of disease responsible for preterm parturition in the non-inflammatory group.
The most common pathological features in the placenta of women who belong to the non-inflammatory group are maternal and fetal vascular lesions.255 Maternal lesions observed in the placenta of patients with a spontaneous preterm delivery include failure of physiological transformation of the myometrial segment of the spiral arteries, atherosis, thrombosis of the spiral arteries (a form of decidual vasculopathy), and a combination of these lesions. Fetal lesions may include a decrease in the number of arterioles in the villi and fetal arterial thrombosis.
Maternal vascular lesions could lead to preterm labor by causing uteroplacental ischemia. Several lines of evidence support a role for uteroplacental ischemia as a mechanism of disease leading to preterm labor: (1) experimental studies designed to generate a primate model for pre-eclampsia by causing uterine ischemia showed that a proportion of animals had spontaneous preterm labor and preterm birth;256 (2) vascular lesions in decidual vessels attached to the placenta have been reported by Arias et al.257 in 34% of women in spontaneous preterm labor and intact membranes, in 35% of those with PPROM, and in only 12% of control women (term gestation without complications). Placental vascular lesions in the decidual vessels of the placenta are associated with a mean odds ratio of 3.8 and 4 for preterm labor with intact membranes and PPROM, respectively; (3) abruptio placenta, a lesion of vascular origin, is more frequent in women who deliver preterm with intact membranes257,258 or with rupture of membranes than in those who deliver at term;259–261 (4) women in preterm labor with intact membranes and those with PPROM who delivered preterm have a higher percentage of failure of physiological transformation in the myometrial segment of the spiral arteries than women who deliver at term;262,263 (5) women presenting with preterm labor and intact membranes, who have an abnormal uterine artery Doppler velocimetry, are more likely to deliver preterm than those with normal Doppler velocimetry.264,265 These results are similar to those reported by other investigators studying women before the onset of labour;266 and (6) the frequency of small-for-gestational-age infants is increased in women delivered after preterm labor with intact membranes and preterm PROM.32–37 Vascular lesions leading to compromise of the uterine supply line could account for both intrauterine growth restriction and preterm labor.
The precise mechanisms responsible for the onset of preterm parturition in women with uteroplacental ischemia have not been determined. A role for the renin–angiotensin system has been postulated as the fetal membranes are endowed with a functional renin-angiotensin system,267 and uterine ischemia increases the production of uterine renin.268,269 Angiotensin II can induce myometrial contractility directly270 or through the release of prostaglandins.271 When uteroplacental ischemia is severe enough to lead to decidual necrosis and hemorrhage, thrombin may activate the common pathway of parturition. Evidence in support of this includes: (1) decidua is a rich source of tissue factor, the primary initiator of coagulation;272 (2) intrauterine administration of whole blood to pregnant rats stimulates myometrial contractility,273 while heparinized blood does not (heparin blocks the generation of thrombin);273 (3) fresh whole blood stimulates myometrial contractility in vitro, and this effect is partially blunted by incubation with hirudin, a thrombin inhibitor;273 (4) thrombin stimulates myometrial contractility in a dose-dependent manner;273 (5) thrombin stimulates the production of MMP-1,274 urokinase-type plasminogen activator (uPA) and tissue-type plasminogen activator (tPA) by endometrial stromal cells in culture;275 MMP-1 can digest collagen directly, while uPA and tPA catalyse the transformation of plasminogen into plasmin, which in turn can degrade type III collagen and fibronectin,276 important components of the extracellular matrix in the chorioamniotic membranes;277 (6) thrombin/antithrombin (TAT) complexes, markers of in vivo generation of thrombin, are increased in the plasma278 and AF279 of women in preterm labor and with PPROM; (7) an elevation of plasma TAT complex concentration in the second trimester is associated with subsequent PPROM;280 (8) the presence of retroplacental hematoma detected by ultrasound examination in the first trimester is associated with adverse pregnancy outcomes, including preterm birth and fetal growth restriction;281 and (9) the presence of vaginal bleeding in the first or second trimester is associated with preterm birth and other adverse perinatal outcomes.282–284
Fetal vascular lesions (i.e. abnormal development due to defective angiogenesis or fetal thrombosis) have not been studied as thoroughly as maternal vascular lesions, but they could lead to fetal compromise and preterm labor. One study has reported that fetuses in preterm labor with an elevated umbilical systolic/diastolic ratio are more likely to deliver preterm.265 These results have not been confirmed.266,285
Although some investigators have proposed that fetal hypoxemia may be a cause of preterm labor, studies with cordocentesis have indicated that fetal hypoxemia and metabolic acidemia are not more frequent in women in preterm labor and with intact membranes who deliver preterm than in those who deliver at term.286 Similarly, Carroll et al.287 have shown that fetal hypoxemia is rare in women with PPROM. Uterine ischemia should not be equated with fetal hypoxemia, and no evidence currently shows that fetal hypoxemia is a cause of preterm parturition.
Uterine over-distension
Women with mullerian duct abnormalities,288 polyhydramnios,289,290 and multiple pregnancy291 are at increased risk for spontaneous preterm labor and preterm birth. Intra-amniotic pressure remains relatively constant throughout gestation despite the growth of the fetus and placenta.292,293 This has been attributed to progressive myometrial relaxation due to the effects of progesterone294 and endogenous myometrial relaxants such as nitric oxide.295 Stretching can, however, induce increased myometrial contractility,296 prostaglandin release,297 expression of gap junction protein or connexin-43,298 and increased oxytocin receptor in pregnant and non-pregnant myometrium.299 The stretch-induced contraction-associated protein gene expression during pregnancy is inhibited by progesterone.298 The effect of stretch increases in late gestation and is maximal during labor as a consequence of the relative reduction in uterine growth compared with fetal growth and of the declining circulating and/or local concentrations of progesterone.298,300,301 The effect of mechanical forces on muscle has been studied extensively in myocardium,302 vascular smooth muscle,303 bladder,304 and gastrointestinal smooth muscle305 but not in myometrium.
Mechanical stress induces activation of integrin receptors,306 stretch-activated calcium channels,305,307 phosphorylation of platelet-derived growth factor receptor,308 and activation of G proteins.308,309 Once mechanical force is sensed, it leads to activation of protein kinase C and mitogen-activated protein kinases, increased gene expression of c-fos and c-jun, and enhanced binding activity of transcription factor activator protein-1.310–315 Other effects of physical forces relevant to myometrium include increased expression of prostaglandin H synthase 2,316 superoxide dismutase, and nitric oxide synthase. The nature of force/pressure-sensing mechanisms of the myometrium has yet to be determined. A role for integrins and their ligands has been proposed for other organs.317,318 Stretch may not only induce increased myometrial contractility but may also modify the contractile response through ‘mechanoelectrical feedback’ similar to the one reported in the heart.319
The chorioamniotic membranes are distended by 40% at 25–29 weeks of gestation, 60% at 30–34 weeks of gestation, and 70% at term.320 Stretching of the membranes in vitro induces histological changes characterized by elongation of the amnion cells and increased production of collagenase activity and IL-8,321,322 while stretching of amnion cells in culture results in increased production of prostaglandin E2.323 Recent studies using an in vitro cell culture model for fetal membrane distension revealed upregulation of IL-8 and pre-B-cell colony-enhancing factor.324 When fetal membrane explants were distended in an in vitro distension device to mimic the situation in vivo, and the gene expressions of distended explants were compared with that of non-distended explants, three genes, namely IL enhancer binding factor 2, huntingtin-interacting protein 2, and interferon-stimulated gene encoding a 54 kDa protein, were found to be up-regulated.325 Collectively, these observations suggest that mechanical forces associated with uterine over-distention may result in activation of mechanisms leading to membrane rupture. Premature cervical ripening is also a feature of women with multiple gestations and those with certain mullerian duct anomalies (e.g. incompetent cervix in diethylstilbestrol [DES]-exposed daughters). IL-8,194,326–328 MMP-1,329 prostaglandins,330–332 and nitric oxide333 have been implicated in the control of cervical ripening. Inasmuch as these mediators are produced in response to membrane stretch, they may exert part of their biological effects in parturition by stimulating extracellular matrix degradation of the cervix.
Several lines of evidence indicate that women with twins and higher order multiple pregnancies also represent a heterogeneous group. Some women suffer preterm labor associated with MIAC.93,94,334 Others may have abnormalities of trophoblast invasion leading to vascular pathology with and without fetal growth disorders. These separate mechanisms of disease may operate in conjunction with uterine over-distension to activate the components of the common terminal pathway.
Abnormal allograft reaction
The fetoplacental unit has been considered nature’s most successful ‘graft.’ Reproductive immunologists have suggested that abnormalities in the recognition and adaptation to a set of foreign antigens (fetal) may be a mechanism of disease responsible for recurrent pregnancy loss, intrauterine growth restriction, and pre-eclampsia.335–338 Chronic villitis of unknown etiology has been proposed to be a lesion akin to ‘placental rejection.’ The presence of these lesions in a subset of women who deliver after spontaneous preterm labor provides indirect support for the concept that immune abnormalities may be responsible for preterm labor. We have observed that some women in preterm labor, in the absence of demonstrable infection, have elevated concentrations of the IL-2-soluble receptor.255 Elevated plasma concentrations of the IL-2 receptor are considered an early sign of rejection in women with renal transplants.339 Further studies are required to define the frequency and clinical significance of this pathological process in preterm labor. It is noteworthy that the traditional view of the fetus as an allograft has recently been challenged.340 The normal relationship between the mother and the conceptus has been likened to that of invertebrate allorecognition, in which cytotoxicity and rejection reactions are not inevitable consequences of exposure to foreign antigens. Pathological processes resulting from activation of the effector limb of the immune response (i.e. natural killer cells, macrophages, etc.) could still play an important role in the pathophysiology of preterm labor.
Over 10 years ago, Holmes proposed that a complement-mediated mechanism was required for fetal survival in humans.341–343 The complement system is a group of proteins that are activated during an inflammatory response triggered by foreign invaders. Recent studies in mice support the hypothesis that some components of the innate limb of the immune response may be suppressed during normal pregnancy.344,345 In mice, a cell surface protein called Crry suppresses the complement system, and its expression is essential for the survival of the embryo during pregnancy.345 When the Crry gene was inactivated (KO experiments), none of the Crry-deficient embryos lived to term (they died in utero). The dead embryos had a massive invasion of inflammatory cells and showed evidence of activated complement system in trophoblasts. Pregnant mice that were a complement-deficient strain gave birth to normal pups. These experiments suggest that the lack of Crry gene expression in trophoblasts led to activation of the complement system and inflammatory cells, which in turn destroyed the trophoblasts and the embryo. Although there is no gene structurally homologous to the Crry gene, humans have two other complement regulators, namely decay-accelerating factor and membrane cofactor protein, which are likely to serve the same functions as Crry in mice.341–350 A role for complement C5a receptors and neutrophils in fetal injury has been recently proposed in the antiphospholipid antibody syndrome.351 Whether or not this mechanism is operative in some women with preterm labor remains to be determined.
Allergic phenomena
Another potential mechanism for preterm labor and preterm birth is an immunologically mediated phenomenon induced by an allergic mechanism. We have previously proposed that an allergic-like immune response (type I hypersensitivity) may be associated with preterm labor.352 The term ‘allergy’ refers to disorders caused by the response of the immune system to an otherwise innocuous antigen.353 The antigen (allergen) cross-links immunoglobulin E (IgE) bound to high-affinity receptors on mast cells, causing degranulation of these cells and the initiation of inflammation.354 The required components of an allergic reaction are: (1) allergen; (2) production of IgE by B cells (Th2); and (3) the effector system composed of mast cells and the target organ mediators released by these cells, such as bronchial smooth muscle for asthma or smooth muscle in the gastrointestinal tract for food allergies.352
The evidence suggesting that an allergic-like phenomenon may operate in preterm labor is the following: (1) the human fetus is exposed to common allergens (i.e. house dust mite) as this compound has been detected in both AF in the mid-trimester of pregnancy and fetal blood. Moreover, the concentrations of the allergen are higher in fetal blood than in maternal blood;355 (2) allergen-specific reactivity has been shown in umbilical cord blood at birth and as early as 23 weeks of gestation;356 (3) pregnancy is considered a state in which there is preponderance of a Th2 cytokine response that favors the differentiation of naive CD4+ T cells to the Th2 phenotype with increased capacity for cytokine secretion in the IL-4 gene cluster and this predisposes to a switch to IgE production by B cells; (4) the uterus is a rich source of mast cells—the effector cells of allergic-like immunological reactions;357 (5) several products of mast cell degranulation can induce myometrial contractility (i.e. histamine and prostaglandins);358,359 (6) pharmacological degranulation of mast cells with a compound called ‘48/80’ induces myometrial and cervical contractility;360,361 (7) incubation of myometrial strips from sensitized and non-sensitized animals with an anti-IgE antibody increases myometrial contractility;360 (8) human myometrial strips obtained from women known to be allergic to ragweed show increased myometrial contractility when challenged in vitro by the allergen (RE Garfield, personal communication). Moreover, sensitivity of the myometrial strips of non-allergic women can be transferred passively by pre-incubation of the strips with human serum (RE Garfield, personal communication); (9) non-pregnant guinea pigs sensitized with ovo-albumin and then challenged with this antigen show increased uterine tone;360 (10) traditional descriptions of animals dying of anaphylactic shock show enhanced uterine contractility when an autopsy was performed immediately after death; (11) severe latex allergy in a pregnant woman after vaginal examination with a latex glove was followed by regular uterine contractions;362 (12) human decidua contains immune cells capable of identifying local foreign antigens, including macrophages, B cells, T cells,363,364 and dendritic cells;365 and (13) we have identified a subgroup of women in preterm labor who have eosinophils in the AF as the predominant white blood cell.352 Under normal circumstances, white blood cells are not present in AF. The presence of eosinophils, therefore, suggests an abnormal immune response and perhaps they are the markers of an allergic-like response in preterm labor. The antigen eliciting an abnormal immunological response remains to be identified. Recent evidence suggests that administration of ovo-albumin to sensitized pregnant guinea pigs can induce preterm labor and preterm birth and that this phenomenon can be prevented with treatment with antihistaminics.366
Cervical disorders
Although cervical insufficiency is traditionally considered a cause of mid-trimester abortion, accumulating evidence suggests that a wide spectrum of disease exists.367 This spectrum includes the well-recognized recurrent pregnancy loss in the mid-trimester, some forms of preterm labor (presenting with bulging membranes in the absence of significant uterine contractility or rupture of membranes), and probably precipitous labor at term. Cervical disease may be the result of a congenital disorder (i.e. hypoplastic cervix or DES exposure in utero), surgical trauma (i.e. conization resulting in substantial loss of connective tissue), or traumatic damage to the structural integrity of the cervix (i.e. repeated cervical dilatation associated with termination of pregnancy).368 Cervical insufficiency is a syndrome in which the predominant feature is cervical ripening. Some cases of cervical insufficiency in the mid-trimester may be caused not by a primary cervical disease leading to premature ripening but by another pathological process, such as infection. Intrauterine infection has been shown in nearly 50% of women with a clinical presentation consistent with acute cervical insufficiency.90 The reader is referred to a detailed review of cervical insufficiency and the role of cervical cerclage in the prevention of preterm birth recently published by the authors.369
Hormonal disorders
Progesterone is central to pregnancy maintenance.370 This biological effect is carried out in all the components of the common pathway of parturition. Specifically, progesterone promotes myometrial quiescence, down-regulates gap junction formation, inhibits cervical ripening, and decreases the production of chemokines (i.e. IL-8) by the chorioamniotic membranes, which is thought to be key to decidual/membrane activation.371–374 Progesterone is considered important for pregnancy maintenance in humans because inhibition of progesterone action could result in parturition. Administration of progesterone receptor antagonists [i.e. RU486 (Mifepristone) or ZK 98299 (onapristone)] to pregnant women,375 non-human primates,376 and guinea pigs372 can induce labour.370 Thus, a suspension of progesterone action is believed to be important for the onset of labor in humans. In contrast to the effects of progesterone, estrogens increase myometrial contractility and have been implicated in the induction of cervical ripening.371–373
In many species, a fall in maternal serum progesterone concentration (progesterone withdrawal) occurs prior to spontaneous parturition377 (see Table 1). However, in humans, non-human primates, and guinea pigs, a progesterone withdrawal is not apparent. The reader is referred to an excellent review by Young378 for a comparative physiology of parturition in mammals.
Table 1:
Source of steroids and mechanism for progesterone withdrawal before parturition in several species (Reproduced with permission from reference 377). Modified from Liggins, GC. Endocrinology of parturition. In: Novy, MJ & Resko, JA, editors. Fetal Endocrinology. New York: Academic Press. 1981, p. 211–37.
| Species | Sources of steroids in late pregnancy | Fall in maternal progesterone concentration | Mechanisms |
|---|---|---|---|
| Mouse | Corpus luteum | Yes | Luteolysis |
| Rat | Corpus luteum | Yes | Luteolysis |
| Rabbit | Corpus luteum | Yes | Luteolysis |
| Goat | Corpus luteum | Yes | P450 c17/ Luteolysis |
| Cow | Placenta | Yes | P450 c 17 |
| Sheep | Placenta | Yes | P450 c 17 |
| Guinea pig | Placenta | No | ? |
| Primates | Feto-placental unit | No | ? |
P450 C17 enzymes (17alpha-hydroxylase and C17–20 lyases activities) are induced in the placenta by an increase in fetal cortisol concentration. Luteolysis is provoked by prostaglandin F2α acting on fetal plasma receptor.
The mechanism by which progesterone action is suspended has eluded definition. Five potential mechanisms have been invoked to explain this paradox: 1) reduced bioavailability of progesterone by binding to a high affinity protein;379–380 2) increased cortisol concentration in late pregnancy that may compete with progesterone for binding to the glucocorticoid receptor;381 3) conversion of progesterone to an inactive form within the target cell before interacting with its receptor;382–383 4) quantitative and qualitative changes in progesterone receptor isoforms (PR-A, PR-B, PR-C);384–387 5) changes in progesterone receptor co-regulators;388 and 6) a functional progesterone withdrawal through NF-kB.389–391 The interested reader is referred to articles on this complex subject for details.379–399
Progesterone actions are mediated by multi-protein complexes including progesterone receptors, modifying components (co-regulators and adaptors) and effector proteins (RNA-polymerase, chromatin-remodeling and RNA-processing factors).388 In addition, non-genomic mechanisms have recently been proposed.388 The specific role of progesterone in the mechanism responsible for preterm labor remains to be elucidated.
The spectrum of reproductive abnormalities associated with progesterone deficiency is broad. Luteal phase deficiency (LPD) is widely believed to be a cause of infertility400,401 and is commonly implicated in the etiology of recurrent miscarriage.402,403 This disorder has been defined as either a defect of progesterone secretion by the corpus luteum or a defect in endometrial response to progesterone.404,405 In LPD, the ovary could function well enough to ovulate, but the function of the corpus luteum and/or endometrium would be below the physiological threshold sufficient for conception or pregnancy maintenance. Since there is no general agreement on the criteria for the diagnosis of LPD,400,401,406,407 the clinical significance and contribution of LPD to adverse pregnancy outcome remain uncertain. One retrospective study including 540 infertile women with a diagnosis of LPD (endometrial biopsy was out of phase by 2 days in two consecutive cycles) showed a high rate of preterm birth (31.2%) in women who had not received progesterone treatment.408 In contrast, women who were treated with progesterone (vaginal suppositories for at least 12 weeks) showed a lower rate of preterm
delivery at less than 32 weeks and less than 37 weeks of gestation (for 32 weeks, no progesterone: 12.5% [4/32] versus progesterone: 1.3% [6/469]; P < 0.01, and for 37 weeks, no progesterone: 31.2% [10/32] versus progesterone: 13.7% [64/469]; P < 0.01).408
Other than primary progesterone functional deficiency (e.g. LPD), reduction in progesterone function could result from other pathological mechanisms such as intrauterine infection. In animal models of ascending intrauterine infection, bacteria-induced and LPS-induced preterm birth are preceded by a significant fall in serum progesterone concentration,409 which was attributed to: (1) LPS- and pro-inflammatory cytokines-induced prostaglandin synthesis and subsequent leuteolysis; (2) direct anti-gonadotropic effect of pro-inflammatory cytokines and hence suppression of progesterone production; and (3) up-regulation of inducible form of nitric oxide synthase by pro-inflammatory cytokines and, thus, inhibition of steroidogenesis including progesterone production. Hirsch and Muhle410 have argued that the fall in serum progesterone concentration is unlikely to be a primary mechanism by which intrauterine inoculation with bacteria causes preterm parturition in mice since the mean interval to delivery is shorter in animals with intrauterine infection than in ovariectomized animals, despite a higher serum progesterone concentration was observed in the former.410
Intrauterine infection is associated with an increase in pro-inflammatory cytokines169,411 (i.e. IL-1, TNF-a, etc.) in AF,180,186,412,413 fetal membranes,414,415 decidua,165,184,416 and myometrium.417–420 In the context of infection-related preterm birth, the increased concentration of IL-1β in gestational tissue could stimulate NF-kB.389,421,422 The activation of NF-kB can increase COX-2 (mRNA and protein expression) and prostaglandin production,389 and also could repress progesterone activity, as proposed by Allport et al.391 (see above), resulting in a functional progesterone withdrawal and, thus, preterm parturition. Randomized clinical trials indicate that progesterone administration to women with a history of a previous preterm birth reduces the rate of spontaneous preterm birth.423,424 This data is discussed in detail in another article in this supplement. The mechanisms by which progesterone administration prevent preterm birth are unknown at this time.
Pregnancy and stress
Maternal stress, of exogenous or endogenous origin, is a risk factor for preterm delivery.425–429 The nature and timing of the stressful stimuli can range from a heavy workload to anxiety.430,431 The stressful insult could occur during pregnancy or in the pre-conceptual period.431–433 For example, starvation before pregnancy can lead to a spontaneous preterm delivery in sheep.434 Though the precise mechanism whereby stress induces preterm parturition is not known, a role for CRF has been proposed.10 Indeed, the trajectory in CRF serum concentrates identifies women at risk for preterm, term, and post-term delivery.12 Since this hormone is produced not only by the hypothalamus, but also by the placenta, the mechanisms regulating its production have been attributed to a ‘placental clock.’12 Maternal plasma concentrations of CRF are elevated in both term and preterm parturition. Moreover, patients with increased plasma concentrations of CRF in the midtrimester are at an increased risk for preterm delivery.12,433 The precise mechanisms by which CRF induces parturition have been subject to intensive investigation and involve the production of cortisol and prostaglandins.435,436
Summary
The emerging picture is that preterm labor, PPROM, and cervical insufficiency are syndromes. Multiple pathological processes may lead to myometrial contractions, membrane/decidual activation and cervical ripening. The clinical presentation (i.e. preterm labor, preterm cervical ripening without significant contractility, or PPROM) will depend on the nature and timing of the insults on the various components of the common terminal pathway. This view of preterm parturition has considerable implications for the understanding of the cellular and biochemical mechanisms responsible for the initiation of parturition, as well as the diagnosis, treatment, and prevention of preterm birth. Since preterm labor is a heterogeneous condition, it is unlikely that one treatment will prevent all cases of preterm birth in patients at risk. We consider interventions such as tocolysis, cerclage, and bedrest to represent attempted treatments for only one of the manifestations of the communal pathway of parturition (i.e. uterine contractility, membrane/decidual activation, and cervical disease) but not necessarily for the underlying pathological process responsible for this activation. For example, tocolysis can prolong pregnancy for up to 7 days,437 which may allow for the administration of steroids, accomplish maternal transfer to a tertiary care center, and institute other measures that may help improve pregnancy outcome (i.e. antibiotic administration to women with asymptomatic bacteriuria or other infection-related conditions). It is possible that tocolysis may reduce perinatal morbidity in a particular group of women, and research to identify such a group is urgently needed.
Acknowledgments
Funding: This work was funded entirely by the Intramural Program of the National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services.
References
- 1.Romero R, Mazor M. Infection and preterm labor. Clin Obstet Gynecol 1988;31:553–84. [DOI] [PubMed] [Google Scholar]
- 2.Romero R, Gomez R, Mazor M, Ghezzi F, Yoon BH. The preterm labor syndrome In: Elder MG, Romero R, Lamont RF, editors. Preterm labor. New York, NY: Churchill Livingstone; 1997. p. 29–49. [Google Scholar]
- 3.Romero R, Mazor M, Munoz H, Gomez R, Galasso M, Sherer DM. The preterm labor syndrome. Ann N Y Acad Sci 1994;734:414–29. [DOI] [PubMed] [Google Scholar]
- 4.Romero R, Avila C, Brekus CA, Mazor M. The role of systemic and intrauterine infection in preterm parturition In: Garfield RE, editor. Uterine contractility. Norwell, MA: Serono Symposia, USA; 1990. p. 319–53. [Google Scholar]
- 5.Romero R, Espinoza J, Mazor M, Chaiworapongsa T. The preterm parturition syndrome In: Critchely H, Bennett P, Thornton S, editors. Preterm Birth. London: RCOG Press; 2004. p. 28–60. [Google Scholar]
- 6.Romero R, Espinoza J, Santolaya J, Chaiworapongsa T, Mazor M. Term and preterm parturition In: Mor G, editor. Immunology of Pregnancy. New York: Springer, Landes Bioscience; 2006. p. 253–93. [Google Scholar]
- 7.Bland RD, Bressack MA, McMillan DD. Labor decreases the lung water content of newborn rabbits. Am J Obstet Gynecol 1979;135:364–67. [DOI] [PubMed] [Google Scholar]
- 8.Ohrlander S, Gennser G, Eneroth P. Plasma cortisol levels in human fetus during parturition. Obstet Gynecol 1976;48:381–87. [PubMed] [Google Scholar]
- 9.Genazzani AR, Petraglia F, Facchinetti F, Galli PA, Volpe A. Lack of beta-endorphin plasma level rise in oxytocin-induced labor. Gynecol Obstet Invest 1985;19:130–34. [DOI] [PubMed] [Google Scholar]
- 10.Petraglia F, Giardino L, Coukos G, Calza L, Vale W, Genazzani AR. Corticotropin-releasing factor and parturition: plasma and amniotic fluid levels and placental binding sites. Obstet Gynecol 1990;75:784–89. [PubMed] [Google Scholar]
- 11.Randall NJ, Bond K, Macaulay J, Steer PJ. Measuring fetal and maternal temperature differentials: a probe for clinical use during labour. J Biomed Eng 1991;13:481–85. [DOI] [PubMed] [Google Scholar]
- 12.McLean M, Bisits A, Davies J, Woods R, Lowry P, Smith R. A placental clock controlling the length of human pregnancy. Nat Med 1995;1:460–63. [DOI] [PubMed] [Google Scholar]
- 13.Challis JR. CRH, a placental clock and preterm labour. Nat Med 1995;1:416. [DOI] [PubMed] [Google Scholar]
- 14.Smith R Alterations in the hypothalamic pituitary adrenal axis during pregnancy and the placental clock that determines the length of parturition. J Reprod Immunol 1998;39:215–20. [DOI] [PubMed] [Google Scholar]
- 15.Korebrits C, Ramirez MM, Watson L, Brinkman E, Bocking AD, Challis JR. Maternal corticotropin-releasing hormone is increased with impending preterm birth. J Clin Endocrinol Metab 1998;83:1585–91. [DOI] [PubMed] [Google Scholar]
- 16.Leung TN, Chung TK, Madsen G, Lam PK, Sahota D, Smith R. Rate of rise in maternal plasma corticotrophin-releasing hormone and its relation to gestational length. Br J Obstet Gynaecol 2001;108:527–32. [DOI] [PubMed] [Google Scholar]
- 17.Florio P, Cobellis L, Woodman J, Severi FM, Linton EA, Petraglia F. Levels of maternal plasma corticotropin-releasing factor and urocortin during labor. J Soc Gynecol Investig 2002;9:233–37. [PubMed] [Google Scholar]
- 18.Romero R, Kuivaniemi H, Tromp G. Functional genomics and proteomics in term and preterm parturition. J Clin Endocrinol Metab 2002;87:2431–34. [DOI] [PubMed] [Google Scholar]
- 19.Romero R, Tromp G. High-dimensional biology arrives to obstetrics and gynecology: functional genomics with microarray studies. Am J Obstet Gynecol 2006;195:360–3. [DOI] [PubMed] [Google Scholar]
- 20.Haddad R, Tromp G, Kuivaniemi H, Chaiworapongsa T, Kim YM, Mazor M et al. Human spontaneous labor without histologic chorioamnionitis is characterized by an acute inflammation gene expression signature. Am J Obstet Gynecol 2006;195:394 e1–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Romero R The child is the father of the man. Prenat Neonat Med 1996;1:8–11. [Google Scholar]
- 22.Iams JD, Goldenberg RL,Meis PJ, Mercer BM, Moawad A, Das A, et al. The length of the cervix and the risk of spontaneous premature delivery. National Institute of Child Health and Human Development Maternal Fetal Medicine Unit Network. N Engl J Med 1996;334:567–72. [DOI] [PubMed] [Google Scholar]
- 23.Heath VC, Southall TR, Souka AP, Elisseou A, Nicolaides KU. Cervical length at 23 weeks of gestation: prediction of spontaneous preterm delivery. Ultrasound Obstet Gynecol 1998;12:312–17. [DOI] [PubMed] [Google Scholar]
- 24.Hassan SS, Romero R, Berry SM, Dang K, Blackwell SC, Treadwell MC, et al. Patients with an ultrasonographic cervical length < or =15 mm have nearly a 50% risk of early spontaneous preterm delivery. Am J Obstet Gynecol 2000;182:1458–67. [DOI] [PubMed] [Google Scholar]
- 25.Lockwood CJ, Senyei AE, Dische MR, Casal B, Shah KB, Thung SN, et al. Fetal fibronectin in cervical and vaginal secretions as a predictor of preterm delivery. N Engl J Med 1991;325:669–74. [DOI] [PubMed] [Google Scholar]
- 26.Goldenberg RL, lams JD, Das A, Mercer BM, Meis PJ, Moawad AH, et al. The Preterm Prediction Study: sequential cervical length and fetal fibronectin testing for the prediction of spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;182:636–43. [DOI] [PubMed] [Google Scholar]
- 27.Gomez R, Romero R, Medina L, Nien JK, Chaiworapongsa T, Carstens M, et al. Cervicovaginal fibronectin improves the prediction of preterm delivery based on sonographic cervical length in patients with preterm uterine contractions and intact membranes. Am J Obstet Gynecol 2005;192:350–59. [DOI] [PubMed] [Google Scholar]
- 28.Cassell GH, Davis RO, Waites KB, Brown MB, Marriott PA, Stagno S, et al. Isolation of Mycoplasma hominis and Ureaplasma urealyticum from amniotic fluid at 16–20 weeks of gestation: potential effect on outcome of pregnancy. Sex Transm Dis 1983;10:294–302. [PubMed] [Google Scholar]
- 29.Gray DJ, Robinson HB, Malone J, Thomson RB Jr. Adverse outcome in pregnancy following amniotic fluid isolation of Ureaplasma urealyticum. Prenat Diagn 1992;12:111–17. [DOI] [PubMed] [Google Scholar]
- 30.Horowitz S, Mazor M, Romero R, Horowitz J, Glezerman M. Infection of the amniotic cavity with Ureaplasma urealyticum in the midtrimester of pregnancy. J Reprod Med 1995;40:375–79. [PubMed] [Google Scholar]
- 31.Carroll SG, Papaioannou S, Ntumazah IL, Philpott-Howard J, Nicolaides KH. Lower genital tract swabs in the prediction of intrauterine infection in preterm prelabour rupture of the membranes. Br J Obstet Gynaecol 1996;103:54–59. [DOI] [PubMed] [Google Scholar]
- 32.Weiner CP, Sabbagha RE, Vaisrub N, Depp R. A hypothetical model suggesting suboptimal intrauterine growth in infants delivered preterm. Obstet Gynecol 1985;65:323–26. [PubMed] [Google Scholar]
- 33.MacGregor SN, Sabbagha RE, Tamura RK, Pielet BW, Feigenbaum SL. Differing fetal growth patterns in pregnancies complicated by preterm labor. Obstet Gynecol 1988;72:834–37. [DOI] [PubMed] [Google Scholar]
- 34.Ott WJ. Intrauterine growth retardation and preterm delivery. Am J Obstet Gynecol 1993;168:1710–15. [DOI] [PubMed] [Google Scholar]
- 35.Zeitlin J, Ancel PY, Saurel-Cubizolles MJ, Papiernik E. The relationship between intrauterine growth restriction and preterm delivery: an empirical approach using data from a European case-control study. BJOG 2000;107:750–58. [DOI] [PubMed] [Google Scholar]
- 36.Bukowski R, Gahn D, Denning J, Saade G. Impairment of growth in fetuses destined to deliver preterm. Am J Obstet Gynecol 2001;185:463–67. [DOI] [PubMed] [Google Scholar]
- 37.Morken NH, Kallen K, Jacobsson B. Fetal growth and onset of delivery: a nationwide population-based study of preterm infants. Am J Obstet Gynecol 2006;195:154–61. [DOI] [PubMed] [Google Scholar]
- 38.Gomez R, Romero R, Ghezzi F, Yoon BH, Mazor M, Berry SM. The fetal inflammatory response syndrome. Am J Obstet Gynecol 1998;179:194–202. [DOI] [PubMed] [Google Scholar]
- 39.Romero R, Gomez R, Ghezzi F, Yoon BH, Mazor M, Edwin SS et al. A fetal systemic inflammatory response is followed by the spontaneous onset of preterm parturition. Am J Obstet Gynecol 1998;179:186–93. [DOI] [PubMed] [Google Scholar]
- 40.Leung TN, Zhang J, Lau TK, Hjelm NM, Lo YM. Maternal plasma fetal DNA as a marker for preterm labour. Lancet 1998;352:1904–05. [DOI] [PubMed] [Google Scholar]
- 41.Farina A, LeShane ES, Romero R, Gomez R, Chaiworapongsa T, Rizzo N et al. High levels of fetal cell-free DNA in maternal serum: a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2005;193:421–25. [DOI] [PubMed] [Google Scholar]
- 42.Holzgreve W, Ghezzi F, Di Naro E, Ganshirt D, Maymon E, Hahn S. Disturbed feto-maternal cell traffic in preeclampsia. Obstet Gynecol 1998;91:669–72. [DOI] [PubMed] [Google Scholar]
- 43.Lo YM, Leung TN, Tein MS, Sargent IL, Zhang J, Lau TK et al. Quantitative abnormalities of fetal DNA in maternal serum in preeclampsia. Clin Chem 1999;45:184–88. [PubMed] [Google Scholar]
- 44.Zhong XY, Holzgreve W, Hahn S. Circulatory fetal and maternal DNA in pregnancies at risk and those affected by preeclampsia. Ann N Y Acad Sci 2001;945:138–40. [DOI] [PubMed] [Google Scholar]
- 45.Levine RJ, Qian C, LeShane ES, Yu KF, England LJ, Schisterman EF et al. Two-stage elevation of cell-free fetal DNA in maternal sera before onset of preeclampsia. Am J Obstet Gynecol 2004;190:707–13. [DOI] [PubMed] [Google Scholar]
- 46.Minkoff H Prematurity: infection as an etiologic factor. Obstet Gynecol 1983;62:137–44. [PubMed] [Google Scholar]
- 47.Romero R, Mazor M, Wu YK, Sirtori M, Oyarzun E, Mitchell MD et al. Infection in the pathogenesis of preterm labor. Semin Perinatol 1988;12:262–79. [PubMed] [Google Scholar]
- 48.Romero R, Sirtori M, Oyarzun E, Avila C, Mazor M, Callahan R et al. Infection and labor. V. Prevalence, microbiology, and clinical significance of intraamniotic infection in women with preterm labor and intact membranes. Am J Obstet Gynecol 1989;161:817–24. [DOI] [PubMed] [Google Scholar]
- 49.Goncalves LF, Chaiworapongsa T, Romero R. Intrauterine infection and prematurity. Ment Retard Dev Disabil Res Rev 2002;8:3–13. [DOI] [PubMed] [Google Scholar]
- 50.Zahl PA, Bjerknes C. Induction of decidua-placental hemorrhage in mice by the endotoxins of certain gram-negative bacteria. Proc Soc Exper Biol Med 1943;54:329–32. [Google Scholar]
- 51.Takeda Y, Tsuchiya I. Studies on the pathological changes caused by the injection of the Shwartzman filtrate and the endotoxin into pregnant rabbits. Jap J Exper Med 1953;21:9–16. [Google Scholar]
- 52.Fidel PL Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H, et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–75. [DOI] [PubMed] [Google Scholar]
- 53.McKay DG, Wong TC. The effect of bacterial endotoxin on the placenta of the rat. Am J Pathol 1963;42:357–77. [PMC free article] [PubMed] [Google Scholar]
- 54.Hirsch E, Saotome I, Hirsh B. A model of intrauterine infection and preterm delivery in mice. Am J Obstet Gynecol 1995;172:1598–603. [DOI] [PubMed] [Google Scholar]
- 55.Kullander S Fever and parturition. An experimental study in rabbits. Acta Obstet Gynecol Scand Suppl 1977;77–85. [DOI] [PubMed] [Google Scholar]
- 56.Gibbs RS, McDuffie RS Jr., Kunze M, Barr JM, Wolf DM, Sze CI, et al. Experimental intrauterine infection with Prevotella bivia in New Zealand White rabbits. Am S Obstet Gynecol 2004;190:1082–86. [DOI] [PubMed] [Google Scholar]
- 57.McDuffie RS Jr., Sherman MP, Gibbs RS. Amniotic fluid tumor necrosis factor-alpha and interleukin-1 in a rabbit model of bacterially induced preterm pregnancy loss. Am J Obstet Gynecol 1992;167:1583–88. [DOI] [PubMed] [Google Scholar]
- 58.Romero R, Munoz H, Gomez R, Ramirez M, Araneda H, Cutright J et al. Antibiotic therapy reduces the rate of infection-induced preterm delivery and perinatal mortality. Am J Obstet Gynecol 1994; 390. [Google Scholar]
- 59.Gravett MG, Witkin SS, Haluska GJ, Edwards JL, Cook MJ, Novy MJ. An experimental model for intraamniotic infection and preterm labor in rhesus monkeys. Am J Obstet Gynecol 1994;171:1660–67. [DOI] [PubMed] [Google Scholar]
- 60.Fidel PL Jr., Romero R, Wolf N, Cutright J, Ramirez M, Araneda H et al. Systemic and local cytokine profiles in endotoxin-induced preterm parturition in mice. Am J Obstet Gynecol 1994;170:1467–75. [DOI] [PubMed] [Google Scholar]
- 61.Elovitz MA, Mrinalini C. Animal models of preterm birth. Trends Endocrinol Metab 2004;15:479–87. [DOI] [PubMed] [Google Scholar]
- 62.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod 2003;69:1957–63. [DOI] [PubMed] [Google Scholar]
- 63.Gilles HM, Lawson JB, Sibelas M, Voller A, Allan N. Malaria, anaemia and pregnancy. Ann Trop Med Parasitol 1969;63:245–63. [DOI] [PubMed] [Google Scholar]
- 64.Herd N, Jordan T. An investigation of malaria during pregnancy in Zimbabwe. Cent Afr J Med 1981;27:62–8. [PubMed] [Google Scholar]
- 65.Osman NB, Folgosa E, Gonzales C, Bergstrom S. Genital infections in the aetiology of late fetal death: an incident case-referent study. J Trop Pediatr 1995;41:258–66. [DOI] [PubMed] [Google Scholar]
- 66.Kalanda BF, Verhoeff FH, Chimsuku L, Harper G, Brabin BJ. Adverse birth outcomes in a malarious area. Epidemiol Infect 2006;134:659–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hibbard L, Thrupp L, Summeril S, Smale M, Adams R. Treatment of pyelonephritis in pregnancy. Am J Obstet Gynecol 1967;98:609–15. [DOI] [PubMed] [Google Scholar]
- 68.Patrick MJ. Influence of maternal renal infection on the foetus and infant. Arch Dis Child 1967;42:208–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wren BG. Subclinical renal infection and prematurity. Med J Aust 1969;2:596–600. [DOI] [PubMed] [Google Scholar]
- 70.Cunningham FG, Morris GB, Mickal A. Acute pyelonephritis of pregnancy: A clinical review. Obstet Gynecol 1973;42:112–17. [PubMed] [Google Scholar]
- 71.Kaul AK, Khan S, Martens MG, Crosson JT, Lupo VR, Kaul R. Experimental gestational pyelonephritis induces preterm births and low birth weights in C3H/HeJ mice. Infect Immun 1999;67:5958–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Munn MB, Groome LJ, Atterbury JL, Baker SL, Hoff C. Pneumonia as a complication of pregnancy. J Matern Fetal Med 1999;8:151–54. [DOI] [PubMed] [Google Scholar]
- 73.Madinger NE, Greenspoon JS, Ellrodt AG. Pneumonia during pregnancy: has modern technology improved maternal and fetal outcome? Am J Obstet Gynecol 1989; 161:657–62. [DOI] [PubMed] [Google Scholar]
- 74.Benedetti TJ, Valle R, Ledger WJ. Antepartum pneumonia in pregnancy. Am J Obstet Gynecol 1982;144:413–17. [DOI] [PubMed] [Google Scholar]
- 75.Jeffcoat MK, Geurs NC, Reddy MS, Goldenberg RL, Hauth JC. Current evidence regarding periodontal disease as a risk factor in preterm birth. Ann Periodontol 2001;6:183–88. [DOI] [PubMed] [Google Scholar]
- 76.Offenbacher S Maternal periodontal infections, prematurity, and growth restriction. Clin Obstet Gynecol 2004;47:808–21. [DOI] [PubMed] [Google Scholar]
- 77.Goepfert AR, Jeffcoat MK, Andrews WW, Faye-Petersen O, Cliver SP, Goldenberg RL et al. Periodontal disease and upper genital tract inflammation in early spontaneous preterm birth. Obstet Gynecol 2004;104:777–83. [DOI] [PubMed] [Google Scholar]
- 78.Jarjoura K, Devine PC, Perez-Delboy A, Herrera-Abreu M, D’Alton M, Papapanou PN. Markers of periodontal infection and preterm birth. Am J Obstet Gynecol 2005;192:513–19. [DOI] [PubMed] [Google Scholar]
- 79.Xiong X, Buekens P, Fraser WD, Beck J, Offenbacher S. Periodontal disease and adverse pregnancy outcomes: a systematic review. BJOG 2006;113:135–43. [DOI] [PubMed] [Google Scholar]
- 80.Offenbacher S, Boggess KA, Murtha AP, Jared HL, Lieff S, McKaig RG et al. Progressive periodontal disease and risk of very preterm delivery. Obstet Gynecol 2006;107:29–36. [DOI] [PubMed] [Google Scholar]
- 81.Gomez R, Ghezzi F, Romero R, Munoz H, Tolosa JE, Rojas I. Premature labor and intra-amniotic infection. Clinical aspects and role of the cytokines in diagnosis and pathophysiology. Clin Perinatol 1995;22:281–342. [PubMed] [Google Scholar]
- 82.Romero R, Munoz H, Gomez R, Sherer DM, Ghezzi F, Gibbs RS et al. Two thirds of spontaneous abortion/fetal deaths after genetic amniocentesis are the result of a pre-existing sub-clinical inflammatory process of the amniotic cavity. Am J Obstet Gynecol 1995;172:S261. [Google Scholar]
- 83.Wenstrom KD, Andrews WW, Hauth JC, Goldenberg RL, DuBard MB, Cliver SP. Elevated second-trimester amniotic fluid interleukin-6 levels predict preterm delivery. Am J Obstet Gynecol 1998;178:546–50. [DOI] [PubMed] [Google Scholar]
- 84.Yoon BH, Oh SY, Romero R, Shim SS, Han SY, Park JS et al. An elevated amniotic fluid matrix metalloproteinase-8 level at the time of mid-trimester genetic amniocentesis is a risk factor for spontaneous preterm delivery. Am J Obstet Gynecol 2001;185:1162–67. [DOI] [PubMed] [Google Scholar]
- 85.Fidel P, Ghezzi F, Romero R, Chaiworapongsa T, Espinoza J, Cutright J et al. The effect of antibiotic therapy on intrauterine infection-induced preterm parturition in rabbits. J Matern Fetal Neonatal Med 2003;14:57–64. [DOI] [PubMed] [Google Scholar]
- 86.Romero R, Oyarzun E, Mazor M, Sirtori M, Hobbins JC, Bracken M. Meta-analysis of the relationship between asymptomatic bacteriuria and preterm delivery/low birth weight. Obstet Gynecol 1989;73:576–82. [PubMed] [Google Scholar]
- 87.Smaill F Antibiotics for asymptomatic bacteriuria in pregnancy. Cochrane Database Syst Rev 2001;CD000490. [DOI] [PubMed] [Google Scholar]
- 88.Romero R, Salafia CM, Athanassiadis AP, Hanaoka S, Mazor M, Sepulveda W et al. The relationship between acute inflammatory lesions of the preterm placenta and amniotic fluid microbiology. Am J Obstet Gynecol 1992;166:1382–88. [DOI] [PubMed] [Google Scholar]
- 89.Romero R, Quintero R, Oyarzun E, Wu YK, Sabo V, Mazor M et al. Intraamniotic infection and the onset of labor in preterm premature rupture of the membranes. Am J Obstet Gynecol 1988;159:661–66. [DOI] [PubMed] [Google Scholar]
- 90.Romero R, Gonzalez R, Sepulveda W, Brandt F, Ramirez M, Sorokin Y et al. Infection and labor. VIII. Microbial invasion of the amniotic cavity in patients with suspected cervical incompetence: prevalence and clinical significance. Am J Obstet Gynecol 1992;167:1086–91. [DOI] [PubMed] [Google Scholar]
- 91.Mays JK, Figueroa R, Shah J, Khakoo H, Kaminsky S, Tejani N. Amniocentesis for selection before rescue cerclage. Obstet Gynecol 2000;95:652–55. [DOI] [PubMed] [Google Scholar]
- 92.Hassan S, Romero R, Hendler I, Gomez R, Khalek N, Espinoza J et al. A sonographic short cervix as the only clinical manifestation of intra-amniotic infection. J Perinat Med 2006;34:13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Romero R, Shamma F, Avila C, Jimenez C, Callahan R, Nores J et al. Infection and labor. VI. Prevalence, microbiology, and clinical significance of intraamniotic infection in twin gestations with preterm labor. Am J Obstet Gynecol 1990;163:757–61. [DOI] [PubMed] [Google Scholar]
- 94.Mazor M, Hershkovitz R, Ghezzi F, Maymon E, Horowitz S, Leiberman JR. Intraamniotic infection in patients with preterm labor and twin pregnancies. Acta Obstet Gynecol Scand 1996;75:624–27. [DOI] [PubMed] [Google Scholar]
- 95.Romero R, Espinoza J, Chaiworapongsa T, Kalache K. Infection and prematurity and the role of preventive strategies. Semin Neonatol 2002;7:259–74. [DOI] [PubMed] [Google Scholar]
- 96.Watts DH, Krohn MA, Hillier SL, Eschenbach DA. The association of occult amniotic fluid infection with gestational age and neonatal outcome among women in preterm labor. Obstet Gynecol 1992;79:351–57. [DOI] [PubMed] [Google Scholar]
- 97.Andrews WW, Hauth JC, Goldenberg RL, Gomez R, Romero R, Cassell GH. Amniotic fluid interleukin-6: correlation with upper genital tract microbial colonization and gestational age in women delivered after spontaneous labor versus indicated delivery. Am J Obstet Gynecol 1995;173:606–12. [DOI] [PubMed] [Google Scholar]
- 98.Romero R, Mazor M, Morrotti R, Avila C, Oyarzun E, Insunza A et al. Infection and labor. VII. Microbial invasion of the amniotic cavity in spontaneous rupture of membranes at term. Am J Obstet Gynecol 1992;166:129–33. [DOI] [PubMed] [Google Scholar]
- 99.Bearfield C, Davenport ES, Sivapathasundaram V, Allaker RP. Possible association between amniotic fluid micro-organism infection and microflora in the mouth. Br J Obstet Gynaecol 2002;109:527–33. [DOI] [PubMed] [Google Scholar]
- 100.Amann RI, Ludwig W, Schleifer KH. Phylogenetic identification and in situ detection of individual microbial cells without cultivation. Microbiol Rev 1995;59:143–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Relman DA. The search for unrecognized pathogens. Science 1999;284:1308–10. [DOI] [PubMed] [Google Scholar]
- 102.Ranjard L, Poly F, Nazaret S. Monitoring complex bacterial communities using culture-independent molecular techniques: application to soil environment. Research in Microbiology 2000;151:167–77. [DOI] [PubMed] [Google Scholar]
- 103.Relman DA, Loutit JS, Schmidt TM, Falkow S, Tompkins LS. The agent of bacillary angiomatosis. An approach to the identification of uncultured pathogens. N Engl J Med 1990;323:1573–80. [DOI] [PubMed] [Google Scholar]
- 104.Jalava J, Mantymaa ML, Ekblad U, Toivanen P, Skurnik M, Lassila O et al. Bacterial 16S rDNA polymerase chain reaction in the detection of intra-amniotic infection. Br J Obstet Gynaecol 1996;103:664–69. [DOI] [PubMed] [Google Scholar]
- 105.Hitti J, Riley DE, Krohn MA, Hillier SL, Agnew KJ, Krieger JN et al. Broad-spectrum bacterial rDNA polymerase chain reaction assay for detecting amniotic fluid infection among women in premature labor. Clin Infect Dis 1997;24:1228–32. [DOI] [PubMed] [Google Scholar]
- 106.Gardella C, Riley DE, Hitti J, Agnew K, Krieger JN, Eschenbach D. Identification and sequencing of bacterial rDNAs in culture-negative amniotic fluid from women in premature labor. Am J Perinatol 2004;21:319–23. [DOI] [PubMed] [Google Scholar]
- 107.Yoon BH, Romero R, Kim M, Kim EC, Kim T, Park JS et al. Clinical implications of detection of Ureaplasma urealyticum in the amniotic cavity with the polymerase chain reaction. Am J Obstet Gynecol 2000;183:1130–37. [DOI] [PubMed] [Google Scholar]
- 108.Yoon BH, Romero R, Lim JH, Shim SS, Hong JS, Shim JY et al. The clinical significance of detecting Ureaplasma urealyticum by the polymerase chain reaction in the amniotic fluid of patients with preterm labor. Am J Obstet Gynecol 2003;189:919–24. [DOI] [PubMed] [Google Scholar]
- 109.Steel JH, Malatos S, Kennea N, Edwards AD, Miles L, Duggan P et al. Bacteria and inflammatory cells in fetal membranes do not always cause preterm labor. Pediatr Res 2005;57:404–11. [DOI] [PubMed] [Google Scholar]
- 110.Romero R, Avila C, Santhanam U, Sehgal PB. Amniotic fluid interleukin 6 in preterm labor. Association with infection. J Clin Invest 1990;85:1392–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Romero R, Sepulveda W, Kenney JS, Archer LE, Allison AC, Sehgal PB. Interleukin 6 determination in the detection of microbial invasion of the amniotic cavity. Ciba Found Symp 1992;167:205–20. [DOI] [PubMed] [Google Scholar]
- 112.Romero R, Yoon BH, Kenney JS, Gomez R, Allison AC, Sehgal PB. Amniotic fluid interleukin-6 determinations are of diagnostic and prognostic value in preterm labor. Am J Reprod Immunol 1993;30:167–83. [DOI] [PubMed] [Google Scholar]
- 113.Yoon BH, Romero R, Kim CJ, Jun JK, Gomez R, Choi JH et al. Amniotic fluid interleukin-6: a sensitive test for antenatal diagnosis of acute inflammatory lesions of preterm placenta and prediction of perinatal morbidity. Am J Obstet Gynecol 1995;172:960–70. [DOI] [PubMed] [Google Scholar]
- 114.Wenstrom KD, Andrews WW, Tamura T, DuBard MB, Johnston KE, Hemstreet GP. Elevated amniotic fluid interleukin-6 levels at genetic amniocentesis predict subsequent pregnancy loss. Am J Obstet Gynecol 1996;175:830–33. [DOI] [PubMed] [Google Scholar]
- 115.Ghidini A, Eglinton GS, Spong CY, et al. Elevated mid-trimester amniotic fluid tumor necrosis alpha levels: a predictor of preterm delivery. Am J Obstet Gynecol 1996;174:307. [Google Scholar]
- 116.Spong CY, Ghidini A, Sherer DM, Pezzullo JC, Ossandon M, Eglinton GS. Angiogenin: a marker for preterm delivery in midtrimester amniotic fluid. Am J Obstet Gynecol 1997;176:415–18. [DOI] [PubMed] [Google Scholar]
- 117.Goldenberg RL, Andrews WW, Mercer BM, Moawad AH, Meis PJ, Iams JD et al. The preterm prediction study: granulocyte colony-stimulating factor and spontaneous preterm birth. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 2000;182:625–30. [DOI] [PubMed] [Google Scholar]
- 118.Gomez R, Romero R, Mazor M, Ghezzi F, David C, Yoon BH. The role of infection in preterm labour and delivery In: Elder MG, Romero R, Lamont RF, editors. Preterm Labor. New York: Churchill Livingstone; 1997. p. 85–125. [Google Scholar]
- 119.Offenbacher S, Beck JD, Lieff S, Slade G. Role of periodontitis in systemic health: spontaneous preterm birth. Journal Of Dental Education 1998;62:852–58. [PubMed] [Google Scholar]
- 120.Jeffcoat MK, Geurs NC, Reddy MS, Cliver SP, Goldenberg RL, Hauth JC. Periodontal infection and preterm birth: results of a prospective study. J Am Dental Association 2001;132:875–80. [DOI] [PubMed] [Google Scholar]
- 121.Madianos PN, Lieff S, Murtha AP, Boggess KA, Auten RL Jr., Beck JD et al. Maternal periodontitis and prematurity. Part II: Maternal infection and fetal exposure. Ann Periodontol 2001;6:175–82. [DOI] [PubMed] [Google Scholar]
- 122.Offenbacher S, Lieff S, Boggess KA, Murtha AP, Madianos PN, Champagne CM et al. Maternal periodontitis and prematurity. Part I: Obstetric outcome of prematurity and growth restriction. Ann Periodontol 2001;6:164–74. [DOI] [PubMed] [Google Scholar]
- 123.Khader YS, Ta’ani Q. Periodontal diseases and the risk of preterm birth and low birth weight: A meta-analysis. J Periodontology 2005;76:161–65. [DOI] [PubMed] [Google Scholar]
- 124.Boggess KA, Madianos PN, Preisser JS, Moise J, Offenbacher S. Chronic maternal and fetal Porphyromonas gingivalis exposure during pregnancy in rabbits. Am J Obstet Gynecol 2005;192:554–57. [DOI] [PubMed] [Google Scholar]
- 125.Downey JS, Han J. Cellular activation mechanisms in septic shock. Front Biosci 1998;3:d468–d476. [DOI] [PubMed] [Google Scholar]
- 126.Wang JE, Dahle MK, McDonald M, Foster SJ, Aasen AO, Thiemermann C. Peptidoglycan and lipoteichoic acid in gram-positive bacterial sepsis: receptors, signal transduction, biological effects, and synergism. Shock 2003;20:402–14. [DOI] [PubMed] [Google Scholar]
- 127.Smith PF. Lipoglycans from mycoplasmas. Crit Rev Microbiol 1984;11:157–86. [DOI] [PubMed] [Google Scholar]
- 128.Romero R, Kadar N, Hobbins JC, Duff GW. Infection and labor: the detection of endotoxin in amniotic fluid. Am J Obstet Gynecol 1987;157:815–19. [DOI] [PubMed] [Google Scholar]
- 129.Romero R, Roslansky P, Oyarzun E, Wan M, Emamian M, Novitsky TJ et al. Labor and infection. II. Bacterial endotoxin in amniotic fluid and its relationship to the onset of preterm labor. Am J Obstet Gynecol 1988;158:1044–49. [DOI] [PubMed] [Google Scholar]
- 130.Grigsby PL, Hirst JJ, Scheerlinck JP, Phillips DJ, Jenkin G. Fetal responses to maternal and intra-amniotic lipopolysaccharide administration in sheep. Biol Reprod 2003;68:1695–702. [DOI] [PubMed] [Google Scholar]
- 131.Elovitz MA, Wang Z, Chien EK, Rychlik DF, Phillippe M. A new model for inflammation-induced preterm birth: the role of platelet-activating factor and Toll-like receptor-4. Am J Pathol 2003;163:2103–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Evaldson G, Malmborg AS, Nord CE, Ostensson K. Bacteroides fragilis, Streptococcus intermedius and group B streptococci in ascending infection of pregnancy. An animal experimental study. Gynecol Obstet Invest 1983;15:230–41. [DOI] [PubMed] [Google Scholar]
- 133.Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods 1998;39:147–54. [DOI] [PubMed] [Google Scholar]
- 134.Jobe AH, Newnham JP, Willet KE, Moss TJ, Gore EM, Padbury JF et al. Endotoxin-induced lung maturation in preterm lambs is not mediated by cortisol. Am J Respir Crit Care Med 2000;162:1656–61. [DOI] [PubMed] [Google Scholar]
- 135.Jobe AH, Newnham JP, Willet KE, Sly P, Ervin MG, Bachurski C et al. Effects of antenatal endotoxin and glucocorticoids on the lungs of preterm lambs. Am J Obstet Gynecol 2000;182:401–08. [DOI] [PubMed] [Google Scholar]
- 136.Jobe AH. Antenatal associations with lung maturation and infection. J Perinatol 2005;25 Suppl 2:S31–S35. [DOI] [PubMed] [Google Scholar]
- 137.Yoon BH, Kim CJ, Romero R, Jun JK, Park KH, Choi ST et al. Experimentally induced intrauterine infection causes fetal brain white matter lesions in rabbits. Am J Obstet Gynecol 1997;177:797–802. [DOI] [PubMed] [Google Scholar]
- 138.Galask RP, Varner MW, Petzold CR, Wilbur SL. Bacterial attachment to the chorioamniotic membranes. Am J Obstet Gynecol 1984;148:915–28. [DOI] [PubMed] [Google Scholar]
- 139.King AE, Critchley HO, Kelly RW. Innate immune defences in the human endometrium. Reprod Biol Endocrinol 2003;1:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Why Matsuzaki K. and how are peptide-lipid interactions utilized for self-defense? Magainins and tachyplesins as archetypes. Biochim Biophys Acta 1999;1462:1–10. [DOI] [PubMed] [Google Scholar]
- 141.Shai Y Mechanism of the binding, insertion and destabilization of phospholipid bilayer membranes by alpha-helical antimicrobial and cell non-selective membrane-lytic peptides. Biochim Biophys Acta 1999;1462:55–70. [DOI] [PubMed] [Google Scholar]
- 142.Yang L, Weiss TM, Lehrer RI, Huang HW. Crystallization of antimicrobial pores in membranes: magainin and protegrin. Biophys J 2000;79:2002–09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zasloff M Antimicrobial peptides of multicellular organisms. Nature 2002;415:389–95. [DOI] [PubMed] [Google Scholar]
- 144.Mori K, Kurihara N, Hayashida S, Tanaka M, Ikeda K. The intrauterine expression of surfactant protein D in the terminal airways of human fetuses compared with surfactant protein A. Eur J Pediatr 2002;161:431–34. [DOI] [PubMed] [Google Scholar]
- 145.Condon JC, Jeyasuria P, Faust JM, Mendelson CR. Surfactant protein secreted by the maturing mouse fetal lung acts as a hormone that signals the initiation of parturition. Proc Natl Acad Sci U S A 2004;101:4978–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kishore U, Bernal AL, Kamran MF, Saxena S, Singh M, Sarma PU et al. Surfactant proteins SP-A and SP-D in human health and disease. Arch Immunol Ther Exp (Warsz) 2005;53:399–417. [PubMed] [Google Scholar]
- 147.Costerton W, Veeh R, Shirtliff M, Pasmore M, Post C, Ehrlich G. The application of biofilm science to the study and control of chronic bacterial infections. J Clin Invest 2003;112:1466–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.McGroarty JA, Reid G. Detection of a Lactobacillus substance that inhibits Escherichia coli. Can J Microbiol 1988;34:974–78. [DOI] [PubMed] [Google Scholar]
- 149.Reid G, Burton J. Use of Lactobacillus to prevent infection by pathogenic bacteria. Microbes Infect 2002;4:319–24. [DOI] [PubMed] [Google Scholar]
- 150.Janeway C, Travers P, Walport M, Schlomchik M. Innate immunity In: Janeway C, Travers P, Walport M, Schlomchik M, editors. Immunobiology. New York: Garland Science Publishing; 2005. p. 37–102. [Google Scholar]
- 151.Hargreaves DC, Medzhitov R. Innate sensors of microbial infection. J Clin Immunol 2005;25:503–10. [DOI] [PubMed] [Google Scholar]
- 152.Pasare C, Medzhitov R. Toll-like receptors: linking innate and adaptive immunity. Microbes Infect 2004;6:1382–87. [DOI] [PubMed] [Google Scholar]
- 153.Way SS, Thompson LJ, Lopes JE, Hajjar AM, Kollmann TR, Freitag NE et al. Characterization of flagellin expression and its role in Listeria monocytogenes infection and immunity. Cell Microbiol 2004;6:235–42. [DOI] [PubMed] [Google Scholar]
- 154.Fazeli A, Bruce C, Anumba DO. Characterization of Toll-like receptors in the female reproductive tract in humans. Hum Reprod 2005;20:1372–78. [DOI] [PubMed] [Google Scholar]
- 155.Pioli PA, Amiel E, Schaefer TM, Connolly JE, Wira CR, Guyre PM. Differencial expression of Toll-like receptors 2 and 4 in tissues of the human female reproductive tract. Infect Immun 2004;5799–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Abrahams VM, Bole-Aldo P, Kim YM, Straszewski-Chavez SL, Chaiworapongsa T, Romero R et al. Divergent trophoblast responses to bacterial products mediated by TLRs. J Immunol 2004;173:4286–96. [DOI] [PubMed] [Google Scholar]
- 157.Vadillo OF, Avila Vergara MA, Hernandez GC, Arechavaleta VF, Beltran MJ. [Apoptosis in trophoblast of patients with recurrent spontaneous abortion of unidentified cause]. Ginecol Obstet Mex 2000;68:122–31. [PubMed] [Google Scholar]
- 158.Murthi P, Kee MW, Gude NM, Brennecke SP, Kalionis B. Fetal growth restriction is associated with increased apoptosis in the chorionic trophoblast cells of human fetal membranes. Placenta 2005;26:329–38. [DOI] [PubMed] [Google Scholar]
- 159.Huppertz B, Hemmings D, Renaud SJ, Bulmer JN, Dash P, Chamley LW. Extravillous trophoblast apoptosis--a workshop report. Placenta 2005;26 Suppl A:S46–S48. [DOI] [PubMed] [Google Scholar]
- 160.Wang H, Hirsch E. Bacterially-induced preterm labor and regulation of prostaglandin-metabolizing enzyme expression in mice: the role of toll-like receptor 4. Biol Reprod 2003;69:1957–63. [DOI] [PubMed] [Google Scholar]
- 161.Kim YM, Romero R, Chaiworapongsa T, Kim GJ, Kim MR, Kuivaniemi H et al. Toll-like receptor-2 and −4 in the chorioamniotic membranes in spontaneous labor at term and in preterm parturition that are associated with chorioamnionitis. Am J Obstet Gynecol 2004;191:1346–55. [DOI] [PubMed] [Google Scholar]
- 162.Berry SM, Gomez R, Athayde N, Ghezzi F, Mazor M, Yoon BH et al. The role of granulocyte colony stimulating factor in the neutrophilia observed in the fetal inflammatory response syndrome. Am J Obstet Gynecol 1998; S202. [Google Scholar]
- 163.Romero R, Maymon E, Pacora P, Gomez R, Mazor M, Yoon BH et al. Further observations on the fetal inflammatory response syndrome: a potential homeostatic role for the soluble receptors of tumor necrosis factor alpha. Am J Obstet Gynecol 2000;183:1070–77. [DOI] [PubMed] [Google Scholar]
- 164.Berry SM, Romero R, Gomez R, Puder KS, Ghezzi F, Cotton DB et al. Premature parturition is characterized by in utero activation of the fetal immune system. Am J Obstet Gynecol 1995;173:1315–20. [DOI] [PubMed] [Google Scholar]
- 165.Romero R, Durum SK, Dinarello CA, and et al. Interleukin-1: A signal for the initiation of labor in chorioamnionitis. Presented at the 33rd Annual Meeting for the Society for Gynecologic Investigation, 19–22 March 1986, Toronto, Ontario Canada. [Google Scholar]
- 166.Gibbs RS, Romero R, Hillier SL, Eschenbach DA, Sweet RL. A review of premature birth and subclinical infection. Am J Obstet Gynecol 1992;166:1515–28. [DOI] [PubMed] [Google Scholar]
- 167.Challis JR, Lye SJ, Gibb W, Whittle W, Patel F, Alfaidy N. Understanding preterm labor. Ann N Y Acad Sci 2001;943:225–34. [DOI] [PubMed] [Google Scholar]
- 168.Goldenberg RL, Andrews WW, Hauth JC. Choriodecidual infection and preterm birth. Nutr Rev 2002;60:S19–S25. [DOI] [PubMed] [Google Scholar]
- 169.Keelan JA, Blumenstein M, Helliwell RJ, Sato TA, Marvin KW, Mitchell MD. Cytokines, prostaglandins and parturition--a review. Placenta 2003;24 Suppl A:S33–S46. [DOI] [PubMed] [Google Scholar]
- 170.Menon R, Fortunato SJ. Fetal membrane inflammatory cytokines: a switching mechanism between the preterm premature rupture of the membranes and preterm labor pathways. J Perinat Med 2004;32:391–99. [DOI] [PubMed] [Google Scholar]
- 171.Mohan AR, Loudon JA, Bennett PR. Molecular and biochemical mechanisms of preterm labour. Semin Fetal Neonatal Med 2004;9:437–44. [DOI] [PubMed] [Google Scholar]
- 172.Yoshimura K, Hirsch E. Effect of stimulation and antagonism of interleukin- 1 signaling on preterm delivery in mice. J Soc Gynecol Investig 2005;12:533–8. [DOI] [PubMed] [Google Scholar]
- 173.Romero R, Espinoza J, Mazor M. Can endometrial infection/inflammation explain implantation failure, spontaneous abortion, and preterm birth after in vitro fertilization? Fertil Steril 2004;82:799–804. [DOI] [PubMed] [Google Scholar]
- 174.Hagberg H, Mallard C, Jacobsson B. Role of cytokines in preterm labour and brain injury. BJOG 2005;112 Suppl I:16–18. [DOI] [PubMed] [Google Scholar]
- 175.Hirsch E, Wang H. The molecular pathophysiology of bacterially induced preterm labor: insights from the murine model. J Soc Gynecol Investig 2005;12:145–55. [DOI] [PubMed] [Google Scholar]
- 176.Lindstrom TM, Bennett PR. The role of nuclear factor kappa B in human labour. Reproduction 2005;130:569–81. [DOI] [PubMed] [Google Scholar]
- 177.Vogel I, Thorsen P, Curry A, Sandager P, Uldbjerg N. Biomarkers for the prediction of preterm delivery. Acta Obstet Gynecol Scand 2005;84:516–25. [DOI] [PubMed] [Google Scholar]
- 178.Romero R, Wu YK, Brody DT, Oyarzun E, Duff GW, Durum SK. Human decidua: a source of interleukin-1. Obstet Gynecol 1989;73:31–34. [PubMed] [Google Scholar]
- 179.Romero R, Durum S, Dinarello CA, Oyarzun E, Hobbins JC, Mitchell MD. Interleukin-1 stimulates prostaglandin biosynthesis by human amnion. Prostaglandins 1989;37:13–22. [DOI] [PubMed] [Google Scholar]
- 180.Romero R, Brody DT, Oyarzun E, Mazor M, Wu YK, Hobbins JC et al. Infection and labor. III. Interleukin-1: a signal for the onset of parturition. Am J Obstet Gynecol 1989;160:1117–23. [DOI] [PubMed] [Google Scholar]
- 181.Sadowsky DW, Novy MJ, Witkin SS, Gravett MG. Dexamethasone or interleukin-10 blocks interleukin-1beta-induced uterine contractions in pregnant rhesus monkeys. Am J Obstet Gynecol 2003;188:252–63. [DOI] [PubMed] [Google Scholar]
- 182.Romero R, Mazor M, Tartakovsky B. Systemic administration of interleukin-1 induces preterm parturition in mice. Am J Obstet Gynecol 1991;165:969–71. [DOI] [PubMed] [Google Scholar]
- 183.Romero R, Tartakovsky B. The natural interleukin-1 receptor antagonist prevents interleukin-1-induced preterm delivery in mice. Am J Obstet Gynecol 1992;167:1041–45. [DOI] [PubMed] [Google Scholar]
- 184.Casey ML, Cox SM, Beutler B, Milewich L, MacDonald PC. Cachectin/tumor necrosis factor-alpha formation in human decidua. Potential role of cytokines in infection-induced preterm labor. J Clin Invest 1989;83:430–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Romero R, Mazor M, Manogue K, Oyarzun E, Cerami A. Human decidua: a source of cachectin-tumor necrosis factor. Eur J Obstet Gynecol Reprod Biol 1991;41:123–27. [DOI] [PubMed] [Google Scholar]
- 186.Romero R, Manogue KR, Mitchell MD, Wu YK, Oyarzun E, Hobbins JC et al. Infection and labor. IV. Cachectin-tumor necrosis factor in the amniotic fluid of women with intraamniotic infection and preterm labor. Am J Obstet Gynecol 1989;161:336–41. [DOI] [PubMed] [Google Scholar]
- 187.Watari M, Watari H, DiSanto ME, Chacko S, Shi GP, Strauss JF, III. Pro-inflammatory cytokines induce expression of matrix-metabolizing enzymes in human cervical smooth muscle cells. Am J Pathol 1999;154:1755–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Fortunato SJ, Menon R, Lombardi SJ. Role of tumor necrosis factor-[alpha] in the premature rupture of membranes and preterm labor pathways. Am J Obstet Gynecol 2002;187:1159–62. [DOI] [PubMed] [Google Scholar]
- 189.Athayde N, Edwin SS, Romero R, Gomez R, Maymon E, Pacora P et al. A role for matrix metalloproteinase-9 in spontaneous rupture of the fetal membranes. Am J Obstet Gynecol 1998;179:1248–53. [DOI] [PubMed] [Google Scholar]
- 190.Maymon E, Romero R, Pacora P, Gervasi MT, Gomez R, Edwin SS et al. Evidence of in vivo differential bioavailability of the active forms of matrix metalloproteinases 9 and 2 in parturition, spontaneous rupture of membranes, and intra-amniotic infection. Am J Obstet Gynecol 2000;183:887–94. [DOI] [PubMed] [Google Scholar]
- 191.Romero R, Chaiworapongsa T, Espinoza J, Gomez R, Yoon BH, Edwin S et al. Fetal plasma MMP-9 concentrations are elevated in preterm premature rupture of the membranes. Am J Obstet Gynecol 2002;187:1125–30. [DOI] [PubMed] [Google Scholar]
- 192.Osmers RGW, Adelmann-Grill BC, Rath W, Stuhlsatz HW, Tschesche H, Kuhn W. Biochemical events in cervical ripening dilatation during pregnancy and parturition. J Obstet Gynaecol 1995;21:185–94. [DOI] [PubMed] [Google Scholar]
- 193.Rath W, Winkler M, Kemp B. The importance of extracellular matrix in the induction of preterm delivery. J Perinat Med 1998;26:437–41. [DOI] [PubMed] [Google Scholar]
- 194.Chwalisz K, Benson M, Scholz P, Daum J, Beier HM, Hegele-Hartung C. Cervical ripening with the cytokines interleukin 8, interleukin 1 beta and tumour necrosis factor alpha in guinea-pigs. Hum Reprod 1994;9:2173–81. [DOI] [PubMed] [Google Scholar]
- 195.Hirsch E, Filipovich Y, Mahendroo M. Signaling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol 2006;194:1334–40. [DOI] [PubMed] [Google Scholar]
- 196.Kajikawa S, Kaga N, Futamura Y, Kakinuma C, Shibutani Y. Lipoteichoic acid induces preterm delivery in mice. J Pharmacol Toxicol Methods 1998;39:147–54. [DOI] [PubMed] [Google Scholar]
- 197.Cox SM, King MR, Casey ML, MacDonald PC. Interleukin-1 beta, −1 alpha, and −6 and prostaglandins in vaginal/cervical fluids of pregnant women before and during labor. J Clin Endocrinol Metab 1993;77:805–15. [DOI] [PubMed] [Google Scholar]
- 198.Hillier SL, Witkin SS, Krohn MA, Watts DH, Kiviat NB, Eschenbach DA. The relationship of amniotic fluid cytokines and preterm delivery, amniotic fluid infection, histologic chorioamnionitis, and chorioamnion infection. Obstet Gynecol 1993;81:941–48. [PubMed] [Google Scholar]
- 199.Gomez R, Romero R, Galasso M, Behnke E, Insunza A, Cotton DB. The value of amniotic fluid interleukin-6, white blood cell count, and gram stain in the diagnosis of microbial invasion of the amniotic cavity in patients at term. Am J Reprod Immunol 1994;32:200–10. [DOI] [PubMed] [Google Scholar]
- 200.Messer J, Eyer D, Donato L, Gallati H, Matis J, Simeoni U. Evaluation of interleukin-6 and soluble receptors of tumor necrosis factor for early diagnosis of neonatal infection. J Pediatr 1996;129:574–80. [DOI] [PubMed] [Google Scholar]
- 201.Hanna N, Hanna I, Hleb M, Wagner E, Dougherty J, Balkundi D et al. Gestational age-dependent expression of IL-10 and its receptor in human placental tissues and isolated cytotrophoblasts. J Immunol 2000;164:5721–28. [DOI] [PubMed] [Google Scholar]
- 202.Hanna N, Bonifacio L, Weinberger B, Reddy P, Murphy S, Romero R et al. Evidence for interleukin-10-mediated inhibition of cyclo- oxygenase-2 expression and prostaglandin production in preterm human placenta. Am J Reprod Immunol 2006;55:19–27. [DOI] [PubMed] [Google Scholar]
- 203.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Yoon BH et al. Interleukin 16 in pregnancy, parturition, rupture of fetal membranes, and microbial invasion of the amniotic cavity. Am J Obstet Gynecol 2000;182:135–41. [DOI] [PubMed] [Google Scholar]
- 204.Pacora P, Romero R, Maymon E, Gervasi MT, Gomez R, Edwin SS et al. Participation of the novel cytokine interleukin 18 in the host response to intra-amniotic infection. Am J Obstet Gynecol 2000;183:1138–43. [DOI] [PubMed] [Google Scholar]
- 205.Saito S, Kato Y, Ishihara Y, Ichijo M. Amniotic fluid granulocyte colony-stimulating factor in preterm and term labor. Clin Chim Acta 1992;208:105–09. [DOI] [PubMed] [Google Scholar]
- 206.Saito S, Kasahara T, Kato Y, Ishihara Y, Ichijo M. Elevation of amniotic fluid interleukin 6 (IL-6), IL-8 and granulocyte colony stimulating factor (G-CSF) in term and preterm parturition. Cytokine 1993;5:81–88. [DOI] [PubMed] [Google Scholar]
- 207.Chaiworapongsa T, Romero R, Espinoza J, Kim YM, Edwin S, Bujold E et al. Macrophage migration inhibitory factor in patients with preterm parturition and microbial invasion of the amniotic cavity. J Matern Fetal Neonatal Med 2005;18:405–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Romero R, Ceska M, Avila C, Mazor M, Behnke E, Lindley I. Neutrophil attractant/activating peptide-1/interleukin-8 in term and preterm parturition. Am J Obstet Gynecol 1991;165:813–20. [DOI] [PubMed] [Google Scholar]
- 209.Yoon BH, Romero R, Jun JK, Park KH, Park JD, Ghezzi F et al. Amniotic fluid cytokines (interleukin-6, tumor necrosis factor-alpha, interleukin-1 beta, and interleukin-8) and the risk for the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1997;177:825–30. [DOI] [PubMed] [Google Scholar]
- 210.Ghezzi F, Gomez R, Romero R, Yoon BH, Edwin SS, David C et al. Elevated interleukin-8 concentrations in amniotic fluid of mothers whose neonates subsequently develop bronchopulmonary dysplasia. Eur J Obstet Gynecol Reprod Biol 1998;78:5–10. [DOI] [PubMed] [Google Scholar]
- 211.Esplin MS, Romero R, Chaiworapongsa T, Kim YM, Edwin S, Gomez R et al. Monocyte chemotactic protein-1 is increased in the amniotic fluid of women who deliver preterm in the presence or absence of intra-amniotic infection. J Matern Fetal Neonatal Med 2005;17:365–73. [DOI] [PubMed] [Google Scholar]
- 212.Keelan JA, Yang J, Romero RJ, Chaiworapongsa T, Marvin KW, Sato TA et al. Epithelial cell-derived neutrophil-activating peptide-78 is present in fetal membranes and amniotic fluid at increased concentrations with intra-amniotic infection and preterm delivery. Biol Reprod 2004;70:253–59. [DOI] [PubMed] [Google Scholar]
- 213.Athayde N, Romero R, Maymon E, Gomez R, Pacora P, Araneda H et al. A role for the novel cytokine RANTES in pregnancy and parturition. Am J Obstet Gynecol 1999;181:989–94. [DOI] [PubMed] [Google Scholar]
- 214.Hirsch E, Muhle RA, Mussalli GM, Blanchard R. Bacterially induced preterm labor in the mouse does not require maternal interleukin-1 signaling. Am J Obstet Gynecol 2002;186:523–30. [DOI] [PubMed] [Google Scholar]
- 215.Hirsch E, Filipovich Y, Mahendroo M. Signalling via the type I IL-1 and TNF receptors is necessary for bacterially induced preterm labor in a murine model. Am J Obstet Gynecol 2006;194:1334–40. [DOI] [PubMed] [Google Scholar]
- 216.Terrone DA, Rinehart BK, Granger JP, Barrilleaux PS, Martin JN Jr., Bennett WA. Interleukin-10 administration and bacterial endotoxin-induced preterm birth in a rat model. Obstet Gynecol 2001;98:476–80. [DOI] [PubMed] [Google Scholar]
- 217.Rodts-Palenik S, Wyatt-Ashmead J, Pang Y, Thigpen B, Cai Z, Rhodes P et al. Maternal infection-induced white matter injury is reduced by treatment with interleukin-10. Am J Obstet Gynecol 2004;191:1387–92. [DOI] [PubMed] [Google Scholar]
- 218.Boyer KM, Gadzala CA, Kelly PD, Gotoff SP. Selective intrapartum chemoprophylaxis of neonatal group B streptococcal early-onset disease. III. Interruption of mother-to-infant transmission. J Infect Dis 1983;148:810–16. [DOI] [PubMed] [Google Scholar]
- 219.Placzek MM, Whitelaw A. Early and late neonatal septicaemia. Arch Dis Child 1983;58:728–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Ohlsson A, Vearncombe M. Congenital and nosocomial sepsis in infants born in a regional perinatal unit: cause, outcome, and white blood cell response. Am J Obstet Gynecol 1987;156:407–13. [DOI] [PubMed] [Google Scholar]
- 221.Gerdes JS. Clinicopathologic approach to the diagnosis of neonatal sepsis. Clin Perinatol 1991;18:361–81. [PubMed] [Google Scholar]
- 222.Thompson PJ, Greenough A, Gamsu HR, Nicolaides KH, Philpott-Howard J. Congenital bacterial sepsis in very preterm infants. J Med Microbiol 1992;36:117–20. [DOI] [PubMed] [Google Scholar]
- 223.American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med 1992;20:864–74. [PubMed] [Google Scholar]
- 224.Weiss M, Moldawer LL, Schneider EM. Granulocyte colony-stimulating factor to prevent the progression of systemic nonresponsiveness in systemic inflammatory response syndrome and sepsis. Blood 1999;93:425–39. [PubMed] [Google Scholar]
- 225.Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003;29:530–38. [DOI] [PubMed] [Google Scholar]
- 226.Gomez R, Ghezzi F, Romero R, et al. Two thirds of human fetuses with microbial invasion of the amniotic cavity have a detectable systemic cytokine response before birth. Am J Obstet Gynecol 1997;176:S14. [Google Scholar]
- 227.Yoon BH, Romero R, Kim KS, Park JS, Ki SH, Kim BI et al. A systemic fetal inflammatory response and the development of bronchopulmonary dysplasia. Am J Obstet Gynecol 1999;181:773–79. [DOI] [PubMed] [Google Scholar]
- 228.Chaiworapongsa T, Romero R, Kim JC, Kim YM, Blackwell SC, Yoon BH et al. Evidence for fetal involvement in the pathologic process of clinical chorioamnionitis. Am J Obstet Gynecol 2002;186:1178–82. [DOI] [PubMed] [Google Scholar]
- 229.Witt A, Berger A, Gruber CJ, Petricevic L, Apfalter P, Husslein P. IL-8 concentrations in maternal serum, amniotic fluid and cord blood in relation to different pathogens within the amniotic cavity. J Perinat Med 2005;33:22–26. [DOI] [PubMed] [Google Scholar]
- 230.Pacora P, Chaiworapongsa T, Maymon E, Kim YM, Gomez R, Yoon BH et al. Funisitis and chorionic vasculitis: the histological counterpart of the fetal inflammatory response syndrome. J Matern Fetal Neonatal Med 2002;11:18–25. [DOI] [PubMed] [Google Scholar]
- 231.D’Alquen D, Kramer BW, Seidenspinner S, Marx A, Berg D, Groneck P et al. Activation of umbilical cord endothelial cells and fetal inflammatory response in preterm infants with chorioamnionitis and funisitis. Pediatr Res 2005;57:263–69. [DOI] [PubMed] [Google Scholar]
- 232.Yoon BH, Romero R, Park JS, Kim M, Oh SY, Kim CJ et al. The relationship among inflammatory lesions of the umbilical cord (funisitis), umbilical cord plasma interleukin 6 concentration, amniotic fluid infection, and neonatal sepsis. Am J Obstet Gynecol 2000;183:1124–29. [DOI] [PubMed] [Google Scholar]
- 233.Yoon BH, Romero R, Park JS, Kim CJ, Kim SH, Choi JH et al. Fetal exposure to an intra-amniotic inflammation and the development of cerebral palsy at the age of three years. Am J Obstet Gynecol 2000;182:675–81. [DOI] [PubMed] [Google Scholar]
- 234.Yoon BH, Romero R, Shim JY, Shim SS, Kim CJ, Jun JK. C-reactive protein in umbilical cord blood: a simple and widely available clinical method to assess the risk of amniotic fluid infection and funisitis. J Matern Fetal Neonatal Med 2003;14:85–90. [DOI] [PubMed] [Google Scholar]
- 235.Sampson JE, Theve RP, Blatman RN, Shipp TD, Bianchi DW, Ward BE et al. Fetal origin of amniotic fluid polymorphonuclear leukocytes. Am J Obstet Gynecol 1997;176:77–81. [DOI] [PubMed] [Google Scholar]
- 236.Shim SS, Yoon BH, Romero R, Hong JS, Kim G, Sohn YK et al. The frequency and clinical significance on intra-amniotic inflammation in patients with preterm premature rupture of the membranes. Am J Obstet Gynecol 2003;189:S83. [Google Scholar]
- 237.Clayton D, McKeigue PM. Epidemiological methods for studying genes and environmental factors in complex diseases. Lancet 2001;358:1356–60. [DOI] [PubMed] [Google Scholar]
- 238.Tiret L Gene-environment interaction: a central concept in multifactorial diseases. Proc Nutr Soc 2002;61:457–63. [DOI] [PubMed] [Google Scholar]
- 239.Eschenbach DA, Gravett MG, Chen KC, Hoyme UB, Holmes KK. Bacterial vaginosis during pregnancy. An association with prematurity and postpartum complications. Scand J Urol Nephrol Suppl 1984;86:213–22. [PubMed] [Google Scholar]
- 240.Meis PJ, Goldenberg RL, Mercer B, Moawad A, Das A, McNellis D, et al. The preterm prediction study: significance of vaginal infections. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol. 1995;173:1231–35. [DOI] [PubMed] [Google Scholar]
- 241.Hillier SL, Nugent RP, Eschenbach DA, Krohn MA, Gibbs RS, Martin DH, et al. Association between bacterial vaginosis and preterm delivery of a low-birth-weight infant. The Vaginal Infections and Prematurity Study Group. N Engl J Med 1995;333:1737–42. [DOI] [PubMed] [Google Scholar]
- 242.Morales WJ, Schorr S, Albritton J. Effect of metronidazole in patients with preterm birth in preceding pregnancy and bacterial vaginosis: a placebo-controlled, double-blind study. Am J Obstet Gynecol 1994;171:345–7. [DOI] [PubMed] [Google Scholar]
- 243.McGregor JA, French JI, Parker R, Draper D, Patterson E, Jones W, et al. Prevention of premature birth by screening and treatment for common genital tract infections: results of a prospective controlled evaluation. Am J Obstet Gynecol 1995;173:157–67. [DOI] [PubMed] [Google Scholar]
- 244.Hauth JC, Goldenberg RL, Andrews WW, DuBard MB, Copper RL. Reduced incidence of preterm delivery with metronidazole and erythromycin in women with bacterial vaginosis. N Engl J Med 1995;333:1732–6. [DOI] [PubMed] [Google Scholar]
- 245.McDonald HM, O’Loughlin JA, Vigneswaran R, Jolley PT, Harvey JA, Bof A, et al. Impact of metronidazole therapy on preterm birth in women with bacterial vaginosis flora (Gardnerella vaginalis): a randomised, placebo controlled trial. Br J Obstet Gynaecol 1997;104:1391–7. [DOI] [PubMed] [Google Scholar]
- 246.Carey JC, Klebanoff MA, Hauth JC, Hillier SL, Thom EA, Ernest JM et al. Metronidazole to prevent preterm delivery in pregnant women with asymptomatic bacterial vaginosis. National Institute of Child Health and Human Development Network of Maternal-Fetal Medicine Units. N Engl J Med 2000;342:534–40. [DOI] [PubMed] [Google Scholar]
- 247.Koumans EH, Markowitz LE, Hogan V. Indications for therapy and treatment recommendations for bacterial vaginosis in nonpregnant and pregnant women: a synthesis of data. Clin Infect Dis 2002;35:S152–S172. [DOI] [PubMed] [Google Scholar]
- 248.Klebanoff MA, Guise JM, Carey JC. Treatment recommendations for bacterial vaginosis in pregnant women. Clin Infect Dis 2003;36:1630–31. [DOI] [PubMed] [Google Scholar]
- 249.Leitich H, Brunbauer M, Bodner-Adler B, Kaider A, Egarter C, Husslein P. Antibiotic treatment of bacterial vaginosis in pregnancy: a meta-analysis. Am J Obstet Gynecol 2003;188:752–58. [DOI] [PubMed] [Google Scholar]
- 250.McDonald H, Brocklehurst P, Parsons J, Vigneswaran R. Antibiotics for treating bacterial vaginosis in pregnancy. Cochrane Database Syst Rev 2003;CD000262. [DOI] [PubMed] [Google Scholar]
- 251.Lamont RF. Infection in the prediction and antibiotics in the prevention of spontaneous preterm labour and preterm birth. Br J Obstet Gynaecol 2003;110 Suppl 20:71–75. [DOI] [PubMed] [Google Scholar]
- 252.Macones G, Parry S, Elkousy M, Clothier B, Ural SH, Strauss JF III. A polymorphism in the promoter region of TNF and bacterial vaginosis: preliminary evidence of gene-environment interaction in the etiology of spontaneous preterm birth. Am J Obstet Gynecol 2004;190:1504–8. [DOI] [PubMed] [Google Scholar]
- 253.Roberts AK, Monzon-Bordonaba F, Van Deerlin PG, Holder J, Macones GA, Morgan MA et al. Association of polymorphism within the promoter of the tumor necrosis factor alpha gene with increased risk of preterm premature rupture of the fetal membranes. Am J Obstet Gynecol 1999;180:1297–302. [DOI] [PubMed] [Google Scholar]
- 254.Romero R, Chaiworapongsa T, Kuivaniemi H, Tromp G. Bacterial vaginosis, the inflammatory response and the risk of preterm birth: a role for genetic epidemiology in the prevention of preterm birth. Am J Obstet Gynecol 2004;190:1509–19. [DOI] [PubMed] [Google Scholar]
- 255.Romero R, Sepulveda W, Baumann P, Yoon BH, Brandt F, Gomez R, et al. The preterm labor syndrome: biochemical, cytologic, immunologic, pathologic, microbiologic, and clinical evidence that preterm labor is a heterogeneous disease. Am J Obstet Gynecol 1993;168:288. [Google Scholar]
- 256.Combs CA, Katz MA, Kitzmiller JL, Brescia RJ. Experimental preeclampsia produced by chronic constriction of the lower aorta: validation with longitudinal blood pressure measurements in conscious rhesus monkeys. Am J Obstet Gynecol 1993;169:215–23. [DOI] [PubMed] [Google Scholar]
- 257.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol 1993;168:585–91. [DOI] [PubMed] [Google Scholar]
- 258.Arias F Placental insufficiency: an important cause of preterm labor and preterm premature ruptured membranes. Presented at the 10th Annual Meeting of the Society of Perinatal Obstetricians, 23–27 January 1990, Houston, TX, USA. [Google Scholar]
- 259.Vintzileos AM, Campbell WA, Nochimson DJ, Weinbaum PJ. Preterm premature rupture of the membranes: a risk factor for the development of abruptio placentae. Am J Obstet Gynecol 1987;156:1235–38. [DOI] [PubMed] [Google Scholar]
- 260.Moretti M, Sibai BM. Maternal and perinatal outcome of expectant management of premature rupture of membranes in the midtrimester. Am J Obstet Gynecol 1988;159:390–96. [DOI] [PubMed] [Google Scholar]
- 261.Major C, Nageotte M., Lewis D. Preterm premature rupture of membranes and placental abruption: is there an association between these pregnancy complications? Am J Obstet Gynecol 1991;164:381. [DOI] [PubMed] [Google Scholar]
- 262.Kim YM, Chaiworapongsa T, Gomez R, Bujold E, Yoon BH, Rotmensch S et al. Failure of physiologic transformation of the spiral arteries in the placental bed in preterm premature rupture of membranes. Am J Obstet Gynecol 2002;187:1137–42. [DOI] [PubMed] [Google Scholar]
- 263.Kim YM, Bujold E, Chaiworapongsa T, Gomez R, Yoon BH, Thaler HT et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol 2003;189:1063–69. [DOI] [PubMed] [Google Scholar]
- 264.Brar HS, Medearis AL, DeVore GR, Platt LD. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: prediction of successful tocolysis. Am J Obstet Gynecol 1988;159:947–50. [DOI] [PubMed] [Google Scholar]
- 265.Brar HS, Medearis AL, De Vore GR, Platt LD. Maternal and fetal blood flow velocity waveforms in patients with preterm labor: relationship to outcome. Am J Obstet Gynecol 1989;161:1519–22. [DOI] [PubMed] [Google Scholar]
- 266.Strigini FA, Lencioni G, De Luca G, Lombardo M, Bianchi F, Genazzani AR. Uterine artery velocimetry and spontaneous preterm delivery. Obstet Gynecol 1995;85:374–77. [DOI] [PubMed] [Google Scholar]
- 267.Poisner AM. The human placental renin-angiotensin system. Front Neuroendocrinol 1998;19:232–52. [DOI] [PubMed] [Google Scholar]
- 268.Katz M, Shapiro WB, Porush JG, Chou SY, Israel V. Uterine and renal renin release after ligation of the uterine arteries in the pregnant rabbit. Am J Obstet Gynecol 1980;136:676–78. [DOI] [PubMed] [Google Scholar]
- 269.Woods LL, Brooks VL. Role of the renin-angiotensin system in hypertension during reduced uteroplacental perfusion pressure. Am J Physiol 1989;257:R204–R209. [DOI] [PubMed] [Google Scholar]
- 270.Lalanne C, Mironneau C, Mironneau J, Savineau JP. Contractions of rat uterine smooth muscle induced by acetylcholine and angiotensin II in Ca2+-free medium. Br J Pharmacol 1984;81:317–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Campos GA, Guerra FA, Israel EJ. Angiotensin II induced release of prostaglandins from rat uterus. Arch Biol Med Exp (Santiago ) 1983;16:43–49. [PubMed] [Google Scholar]
- 272.Lockwood CJ, Krikun G, Papp C, Toth-Pal E, Markiewicz L, Wang EY et al. The role of progestationally regulated stromal cell tissue factor and type-1 plasminogen activator inhibitor (PAI-1) in endometrial hemostasis and menstruation. Ann N Y Acad Sci 1994;734:57–79. [DOI] [PubMed] [Google Scholar]
- 273.Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol 2000;183:799–804. [DOI] [PubMed] [Google Scholar]
- 274.Rosen T, Schatz F, Kuczynski E, Lam H, Koo AB, Lockwood CJ. Thrombin-enhanced matrix metalloproteinase-1 expression: a mechanism linking placental abruption with premature rupture of the membranes. J Matern Fetal Neonatal Med 2002;11:11–17. [DOI] [PubMed] [Google Scholar]
- 275.Lockwood CJ, Krikun G, Aigner S, Schatz F. Effects of thrombin on steroid-modulated cultured endometrial stromal cell fibrinolytic potential. J Clin Endocrinol Metab 1996;81:107–12. [DOI] [PubMed] [Google Scholar]
- 276.Lijnen HR. Matrix metalloproteinases and cellular fibrinolytic activity. Biochemistry (Mosc ) 2002;67:92–98. [DOI] [PubMed] [Google Scholar]
- 277.Aplin JD, Campbell S, Allen TD. The extracellular matrix of human amniotic epithelium: ultrastructure, composition and deposition. J Cell Sci 1985;79:119–36. [DOI] [PubMed] [Google Scholar]
- 278.Chaiworapongsa T, Espinoza J, Yoshimatsu J, Kim YM, Bujold E, Edwin S et al. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med 2002;11:368–73. [DOI] [PubMed] [Google Scholar]
- 279.Gomez R, Athayde N, Pacora P, Mazor M, Yoon BH, Romero R. Increased Thrombin in Intrauterine Inflammation. Am J Obstet Gynecol 1998;178:S62. [Google Scholar]
- 280.Rosen T, Kuczynski E, O’Neill LM, Funai EF, Lockwood CJ. Plasma levels of thrombin-antithrombin complexes predict preterm premature rupture of the fetal membranes. J Matern Fetal Med 2001;10:297–300. [DOI] [PubMed] [Google Scholar]
- 281.Nagy S, Bush M, Stone J, Lapinski RH, Gardo S. Clinical significance of subchorionic and retroplacental hematomas detected in the first trimester of pregnancy. Obstet Gynecol 2003;102:94–100. [DOI] [PubMed] [Google Scholar]
- 282.Signore CC, Sood AK, Richards DS. Second-trimester vaginal bleeding: correlation of ultrasonographic findings with perinatal outcome. Am J Obstet Gynecol 1998;178:336–40. [DOI] [PubMed] [Google Scholar]
- 283.Williams MA, Mittendorf R, Lieberman E, Monson RR. Adverse infant outcomes associated with first-trimester vaginal bleeding. Obstet Gynecol 1991;78:14–18. [PubMed] [Google Scholar]
- 284.Funderburk SJ, Guthrie D, Meldrum D. Outcome of pregnancies complicated by early vaginal bleeding. Br J Obstet Gynaecol 1980;87:100–05. [DOI] [PubMed] [Google Scholar]
- 285.Ghezzi F, Ghidini A, Romero R, Gomez R, Galasso M, Cohen J et al. Doppler velocimetry of the fetal middle cerebral artery in patients with preterm labor and intact membranes. J Ultrasound Med 1995;14:361–66. [DOI] [PubMed] [Google Scholar]
- 286.Gomez R, Romero R, Ghezzi F, et al. Are fetal hypoxia and acidemia causes of preterm labor and delivery? Am J Obstet Gynecol 1997; S115. [Google Scholar]
- 287.Carroll SG, Papaioannou S, Nicolaides KH. Assessment of fetal activity and amniotic fluid volume in the prediction of intrauterine infection in preterm prelabor amniorrhexis. Am J Obstet Gynecol 1995;172:1427–35. [DOI] [PubMed] [Google Scholar]
- 288.Ludmir J, Samuels P, Brooks S, Mennuti MT. Pregnancy outcome of patients with uncorrected uterine anomalies managed in a high-risk obstetric setting. Obstet Gynecol 1990;75:906–10. [PubMed] [Google Scholar]
- 289.Hill LM, Breckle R, Thomas ML, Fries JK. Polyhydramnios: ultrasonically detected prevalence and neonatal outcome. Obstet Gynecol 1987;69:21–25. [PubMed] [Google Scholar]
- 290.Phelan JP, Park YW, Ahn MO, Rutherford SE. Polyhydramnios and perinatal outcome. J Perinatol 1990;10:347–50. [PubMed] [Google Scholar]
- 291.Besinger R, Carlson N. The physiology of preterm labor In: Keith L, Papiernik E, Keith D, Luke B, editors. Multiple Pregnancy: Epidemiology, Gestation and Perinatal Outcome. London: Parthenon Publishing; 1995. p. 415. [Google Scholar]
- 292.Sideris IG, Nicolaides KH. Amniotic fluid pressure during pregnancy. Fetal Diagn Ther 1990;5:104–08. [DOI] [PubMed] [Google Scholar]
- 293.Fisk NM, Ronderos-Dumit D, Tannirandorn Y, Nicolini U, Talbert D, Rodeck CH. Normal amniotic pressure throughout gestation. Br J Obstet Gynaecol 1992;99:18–22. [DOI] [PubMed] [Google Scholar]
- 294.Speroff L, Glass RH, Kase NG. The endocrinology of pregnancy In: Mitchell C, editor. Clinical Gynecologic Endocrinology and Infertility. Baltimore: Williams & Wilkins; 1994. p. 251–90. [Google Scholar]
- 295.Sladek SM, Westerhausen-Larson A, Roberts JM. Endogenous nitric oxide suppresses rat myometrial connexin 43 gap junction protein expression during pregnancy. Biol Reprod 1999;61:8–13. [DOI] [PubMed] [Google Scholar]
- 296.Laudanski T, Rocki W. The effects on stretching and prostaglandin F2alpha on the contractile and bioelectric activity of the uterus in rat. Acta Physiol Pol 1975;26:385–93. [PubMed] [Google Scholar]
- 297.Kloeck FK, Jung H. In vitro release of prostaglandins from the human myometrium under the influence of stretching. Am J Obstet Gynecol 1973;115:1066–69. [DOI] [PubMed] [Google Scholar]
- 298.Ou CW, Orsino A, Lye SJ. Expression of connexin-43 and connexin-26 in the rat myometrium during pregnancy and labor is differentially regulated by mechanical and hormonal signals. Endocrinology 1997;138:5398–407. [DOI] [PubMed] [Google Scholar]
- 299.Ou CW, Chen ZQ, Qi S, Lye SJ. Increased expression of the rat myometrial oxytocin receptor messenger ribonucleic acid during labor requires both mechanical and hormonal signals. Biol Reprod 1998;59:1055–61. [DOI] [PubMed] [Google Scholar]
- 300.Chow L, Lye SJ. Expression of the gap junction protein connexin-43 is increased in the human myometrium toward term and with the onset of labor. Am J Obstet Gynecol 1994;170:788–95. [DOI] [PubMed] [Google Scholar]
- 301.Ticconi C, Lye SJ. Placenta and fetal membranes in human parturition and preterm delivery--a workshop report. Placenta 2002;23 Suppl A:S149–S152. [DOI] [PubMed] [Google Scholar]
- 302.Watson PA, Hannan R, Carl LL, Giger KE. Contractile activity and passive stretch regulate tubulin mRNA and protein content in cardiac myocytes. Am J Physiol 1996;271:C684–C689. [DOI] [PubMed] [Google Scholar]
- 303.Barany K, Rokolya A, Barany M. Stretch activates myosin light chain kinase in arterial smooth muscle. Biochem Biophys Res Commun 1990;173:164–71. [DOI] [PubMed] [Google Scholar]
- 304.Steers WD, Broder SR, Persson K, Bruns DE, Ferguson JE, Bruns ME et al. Mechanical stretch increases secretion of parathyroid hormone-related protein by cultured bladder smooth muscle cells. J Urol 1998;160:908–12. [DOI] [PubMed] [Google Scholar]
- 305.Farrugia G, Holm AN, Rich A, Sarr MG, Szurszewski JH, Rae JL. A mechanosensitive calcium channel in human intestinal smooth muscle cells. Gastroenterology 1999;117:900–05. [DOI] [PubMed] [Google Scholar]
- 306.Tzima E, del Pozo MA, Shattil SJ, Chien S, Schwartz MA. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. EMBO J 2001;20:4639–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Holm AN, Rich A, Sarr MG, Farrugia G. Whole cell current and membrane potential regulation by a human smooth muscle mechanosensitive calcium channel. Am J Physiol Gastrointest Liver Physiol 2000;279:G1155–G1161. [DOI] [PubMed] [Google Scholar]
- 308.Hu Y, Bock G, Wick G, Xu Q. Activation of PDGF receptor alpha in vascular smooth muscle cells by mechanical stress. FASEB J 1998;12:1135–42. [DOI] [PubMed] [Google Scholar]
- 309.Li C, Xu Q. Mechanical stress-initiated signal transductions in vascular smooth muscle cells. Cell Signal 2000;12:435–45. [DOI] [PubMed] [Google Scholar]
- 310.Lefebvre DL, Piersanti M, Bai XH, Chen ZQ, Lye SJ. Myometrial transcriptional regulation of the gap junction gene, connexin-43. Reprod Fertil Dev 1995;7:603–11. [DOI] [PubMed] [Google Scholar]
- 311.Piersanti M, Lye SJ. Increase in messenger ribonucleic acid encoding the myometrial gap junction protein, connexin-43, requires protein synthesis and is associated with increased expression of the activator protein-1, c-fos. Endocrinology 1995;136:3571–78. [DOI] [PubMed] [Google Scholar]
- 312.Mitchell JA, Lye SJ. Regulation of connexin43 expression by c-fos and c-jun in myometrial cells. Cell Commun Adhes 2001;8:299–302. [DOI] [PubMed] [Google Scholar]
- 313.Mitchell JA, Lye SJ. Differential expression of activator protein-1 transcription factors in pregnant rat myometrium. Biol Reprod 2002;67:240–46. [DOI] [PubMed] [Google Scholar]
- 314.Oldenhof AD, Shynlova OP, Liu M, Langille BL, Lye SJ. Mitogen-activated protein kinases mediate stretch-induced c-fos mRNA expression in myometrial smooth muscle cells. Am J Physiol Cell Physiol 2002;283:C1530–C1539. [DOI] [PubMed] [Google Scholar]
- 315.Shynlova OP, Oldenhof AD, Liu M, Langille L, Lye SJ. Regulation of c-fos expression by static stretch in rat myometrial smooth muscle cells. Am J Obstet Gynecol 2002;186:1358–65. [DOI] [PubMed] [Google Scholar]
- 316.Wu WX, Ma XH, Yoshizato T, Shinozuka N, Nathanielsz PW. Differential expression of myometrial oxytocin receptor and prostaglandin H synthase 2, but not estrogen receptor alpha and heat shock protein 90 messenger ribonucleic acid in the gravid horn and nongravid horn in sheep during betamethasone-induced labor. Endocrinology 1999;140:5712–18. [DOI] [PubMed] [Google Scholar]
- 317.Lee HS, Millward-Sadler SJ, Wright MO, Nuki G, Al Jamal R, Salter DM. Activation of Integrin-RACK1/PKCalpha signalling in human articular chondrocyte mechanotransduction. Osteoarthritis Cartilage 2002;10:890–97. [DOI] [PubMed] [Google Scholar]
- 318.Shyy JY, Chien S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 2002;91:769–75. [DOI] [PubMed] [Google Scholar]
- 319.Ravens U Mechano-electric feedback and arrhythmias. Prog Biophys Mol Biol 2003;82:255–66. [DOI] [PubMed] [Google Scholar]
- 320.Millar LK, Stollberg J, DeBuque L, Bryant-Greenwood G. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol 2000;182:128–34. [DOI] [PubMed] [Google Scholar]
- 321.Maehara K, Kanayama N, Maradny EE, Uezato T, Fujita M, Terao T. Mechanical stretching induces interleukin-8 gene expression in fetal membranes: a possible role for the initiation of human parturition. Eur J Obstet Gynecol Reprod Biol 1996;70:191–96. [DOI] [PubMed] [Google Scholar]
- 322.Maradny EE, Kanayama N, Halim A, Maehara K, Terao T. Stretching of fetal membranes increases the concentration of interleukin-8 and collagenase activity. Am J Obstet Gynecol 1996;174:843–49. [DOI] [PubMed] [Google Scholar]
- 323.Kanayama N, Fukamizu H. Mechanical stretching increases prostaglandin E2 in cultured human amnion cells. Gynecol Obstet Invest 1989;28:123–26. [DOI] [PubMed] [Google Scholar]
- 324.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I. Differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am J Obstet Gynecol 2000;182:50–59. [DOI] [PubMed] [Google Scholar]
- 325.Nemeth E, Millar LK, Bryant-Greenwood G. Fetal membrane distention: II. Differentially expressed genes regulated by acute distention in vitro. Am J Obstet Gynecol 2000;182:60–67. [DOI] [PubMed] [Google Scholar]
- 326.Barclay CG, Brennand JE, Kelly RW, Calder AA. Interleukin-8 production by the human cervix. Am J Obstet Gynecol 1993;169:625–32. [DOI] [PubMed] [Google Scholar]
- 327.el Maradny E, Kanayama N, Halim A, Maehara K, Sumimoto K, Terao T. Interleukin-8 induces cervical ripening in rabbits. Am J Obstet Gynecol 1994;171:77–83. [DOI] [PubMed] [Google Scholar]
- 328.Sennstrom MK, Brauner A, Lu Y, Granstrom LM, Malmstrom AL, Ekman GE. Interleukin-8 is a mediator of the final cervical ripening in humans. Eur J Obstet Gynecol Reprod Biol 1997;74:89–92. [DOI] [PubMed] [Google Scholar]
- 329.Rajabi M, Solomon S, Poole AR. Hormonal regulation of interstitial collagenase in the uterine cervix of the pregnant guinea pig. Endocrinology 1991;128:863–71. [DOI] [PubMed] [Google Scholar]
- 330.Calder AA. Prostaglandins and biological control of cervical function. Aust N Z J Obstet Gynaecol 1994;34:347–51. [DOI] [PubMed] [Google Scholar]
- 331.Stjernholm YM, Sahlin L, Eriksson HA, Bystrom BE, Stenlund PM, Ekman GE. Cervical ripening after treatment with prostaglandin E2 or antiprogestin (RU486). Possible mechanisms in relation to gonadal steroids. Eur J Obstet Gynecol Reprod Biol 1999;84:83–88. [DOI] [PubMed] [Google Scholar]
- 332.Denison FC, Calder AA, Kelly RW. The action of prostaglandin E2 on the human cervix: stimulation of interleukin 8 and inhibition of secretory leukocyte protease inhibitor. Am J Obstet Gynecol 1999;180:614–20. [DOI] [PubMed] [Google Scholar]
- 333.Ekerhovd E, Weijdegard B, Brannstrom M, Mattsby-Baltzer I, Norstrom A. Nitric oxide induced cervical ripening in the human: Involvement of cyclic guanosine monophosphate, prostaglandin F(2 alpha), and prostaglandin E(2). Am J Obstet Gynecol 2002;186:745–50. [DOI] [PubMed] [Google Scholar]
- 334.Yoon BH, Park KH, Koo JN, Kwon JH, Jun JK, Syn HC et al. Intra-amniotic infection of twin pregnancies with preterm labor. Presented at the 17th Annual Meeting of the Society of Perinatal Obstetricians, 20–27 January 1997, Anaheim, CA, USA. Am J Obstet Gynecol 1997; 535. [Google Scholar]
- 335.McLean JM. Early embryo loss. Lancet 1987;1:1033–34. [DOI] [PubMed] [Google Scholar]
- 336.Kilpatrick DC. Immune mechanisms and pre-eclampsia. Lancet 1987;2:1460–61. [DOI] [PubMed] [Google Scholar]
- 337.Aksel S Immunologic aspects of reproductive diseases. JAMA 1992;268:2930–34. [PubMed] [Google Scholar]
- 338.Benirschke K, Kaufmann P. Villitis of unknown etiology In: Benirschke K, Kaufmann P, editors. Pathology of the Human Placenta. New York: Springer-Verlag; 1995. p. 596. [Google Scholar]
- 339.Soulillou JP, Peyronnet P, Le Mauff B, Hourmant M, Olive D, Mawas C et al. Prevention of rejection of kidney transplants by monoclonal antibody directed against interleukin 2. Lancet 1987;1:1339–42. [DOI] [PubMed] [Google Scholar]
- 340.Loke YW, King A. Immunology of human implantation: an evolutionary perspective. Hum Reprod 1996;11:283–86. [DOI] [PubMed] [Google Scholar]
- 341.Holmes CH, Simpson KL. Complement and pregnancy: new insights into the immunobiology of the fetomaternal relationship. Baillieres Clin Obstet Gynaecol 1992;6:439–60. [DOI] [PubMed] [Google Scholar]
- 342.Holmes CH, Simpson KL, Okada H, Okada N, Wainwright SD, Purcell DF et al. Complement regulatory proteins at the feto-maternal interface during human placental development: distribution of CD59 by comparison with membrane cofactor protein (CD46) and decay accelerating factor (CD55). Eur J Immunol 1992;22:1579–85. [DOI] [PubMed] [Google Scholar]
- 343.Simpson KL, Jones A, Norman S, Holmes CH. Expression of the complement regulatory proteins decay accelerating factor (DAF, CD55), membrane cofactor protein (MCP, CD46) and CD59 in the normal human uterine cervix and in premalignant and malignant cervical disease. Am J Pathol 1997;151:1455–67. [PMC free article] [PubMed] [Google Scholar]
- 344.Hagmann M Embryos attacked by mom’s natural defenses. Science 2000;287:408. [DOI] [PubMed] [Google Scholar]
- 345.Xu C, Mao D, Holers VM, Palanca B, Cheng AM, Molina H. A critical role for murine complement regulator crry in fetomaternal tolerance. Science 2000;287:498–501. [DOI] [PubMed] [Google Scholar]
- 346.Vanderpuye OA, Labarrere CA, McIntyre JA. The complement system in human reproduction. Am J Reprod Immunol 1992;27:145–55. [DOI] [PubMed] [Google Scholar]
- 347.Nishikori K, Noma J, Hirakawa S, Amano T, Kudo T. The change of membrane complement regulatory protein in chorion of early pregnancy. Clin Immunol Immunopathol 1993;69:167–74. [DOI] [PubMed] [Google Scholar]
- 348.Cunningham DS, Tichenor JR Jr . Decay-accelerating factor protects human trophoblast from complement-mediated attack. Clin Immunol Immunopathol 1995;74:156–61. [DOI] [PubMed] [Google Scholar]
- 349.Gonzalez NC, Chairez JA, Cueto SM. [Immunology of the fetal-maternal relationship]. Rev Alerg Mex 1996;43:18–22. [PubMed] [Google Scholar]
- 350.Pham TQ, Goluszko P, Popov V, Nowicki S, Nowicki BJ. Molecular cloning and characterization of Dr-II, a nonfimbrial adhesin-I-like adhesin isolated from gestational pyelonephritis-associated Escherichia coli that binds to decay-accelerating factor. Infect Immun 1997;65:4309–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 2003;112:1644–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Romero R, Mazor M, Avila C, et al. Uterine “allergy”: A novel mechanism for preterm labor. Am J Obstet Gynecol 1991; 375.1872343 [Google Scholar]
- 353.Holgate ST. The epidemic of allergy and asthma. Nature 1999;402:B2–B4. [DOI] [PubMed] [Google Scholar]
- 354.Corry DB, Kheradmand F. Induction and regulation of the IgE response. Nature 1999;402:B18–B23. [DOI] [PubMed] [Google Scholar]
- 355.Holloway JA, Warner JO, Vance GH, Diaper ND, Warner JA, Jones CA. Detection of house-dust-mite allergen in amniotic fluid and umbilical-cord blood. Lancet 2000;356:1900–2. [DOI] [PubMed] [Google Scholar]
- 356.Jones AC, Miles EA, Warner JO, Colwell BM, Bryant TN, Warner JA. Fetal peripheral blood mononuclear cell proliferative responses to mitogenic and allergenic stimuli during gestation. Pediatr Allergy Immunol 1996;7:109–16. [DOI] [PubMed] [Google Scholar]
- 357.Rudolph MI, Reinicke K, Cruz MA, Gallardo V, Gonzalez C, Bardisa L. Distribution of mast cells and the effect of their mediators on contractility in human myometrium. Br J Obstet Gynaecol 1993;100:1125–30. [DOI] [PubMed] [Google Scholar]
- 358.Padilla L, Reinicke K, Montesino H, Villena F, Asencio H, Cruz M et al. Histamine content and mast cells distribution in mouse uterus: the effect of sexual hormones, gestation and labor. Cell Mol Biol 1990;36:93–100. [PubMed] [Google Scholar]
- 359.Rudolph MI, Bardisa L, Cruz MA, Reinicke K. Mast cells mediators evoke contractility and potentiate each other in mouse uterine horns. Gen Pharmacol 1992;23:833–36. [DOI] [PubMed] [Google Scholar]
- 360.Garfield RE, Bytautiene E, Vedernikov YP, Marshall JS, Romero R. Modulation of rat uterine contractility by mast cells and their mediators. Am J Obstet Gynecol 2000;183:118–25. [DOI] [PubMed] [Google Scholar]
- 361.Bytautiene E, Vedernikov YP, Saade GR, Romero R, Garfield RE. Endogenous mast cell degranulation modulates cervical contractility in the guinea pig. Am J Obstet Gynecol 2002;186:438–45. [DOI] [PubMed] [Google Scholar]
- 362.Shingai Y, Nakagawa K, Kato T, Fujioka T, Matsumoto T, Kihana T et al. Severe allergy in a pregnant woman after vaginal examination with a latex glove. Gynecol Obstet Invest 2002;54:183–4. [DOI] [PubMed] [Google Scholar]
- 363.Bulmer JN, Pace D, Ritson A. Immunoregulatory cells in human decidua: morphology, immunohistochemistry and function. Reprod Nutr Dev 1988;28:1599–613. [DOI] [PubMed] [Google Scholar]
- 364.Lachapelle MH, Miron P, Hemmings R, Roy DC. Endometrial T, B, and NK cells in patients with recurrent spontaneous abortion. Altered profile and pregnancy outcome. J Immunol 1996;156:4027–34. [PubMed] [Google Scholar]
- 365.Kammerer U, Schoppet M, McLellan AD, Kapp M, Huppertz HI, Kampgen E et al. Human decidua contains potent immunostimulatory CD83(+) dendritic cells. Am J Pathol 2000;157:159–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 366.Bytautiene E, Romero R, Vedernikov Y, Saade G, Garfield R. An alergic reaction can induce premature labor and delivery, which can be prevented by treatment with antihistaminics and chromolyn sodium. Am J Obstet Gynecol 2004;191:1356–61. [DOI] [PubMed] [Google Scholar]
- 367.Iams JD, Johnson FF, Sonek J, Sachs L, Gebauer C, Samuels P. Cervical competence as a continuum: a study of ultrasonographic cervical length and obstetric performance. Am J Obstet Gynecol 1995;172:1097–103. [DOI] [PubMed] [Google Scholar]
- 368.Romero R, Mazor M, Gomez R. Cervix, incompetence and premature labor. Fetus 1993;3:1. [Google Scholar]
- 369.Romero R, Espinoza J, Erez O, Hassan S. The role of cervical cerclage in obstetric practice: can the patient who could benefit from this procedure be identified? Am J Obstet Gynecol 2006;194:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 370.Mesiano S Roles of estrogen and progesterone in human parturition. Front Horm Res 2001;27:86–104. [DOI] [PubMed] [Google Scholar]
- 371.Gorodeski IG, Geier A, Lunenfeld B, Beery R, Bahary CM. Progesterone (P) receptor dynamics in estrogen primed normal human cervix following P injection. Fertil Steril 1987;47:108–113. [DOI] [PubMed] [Google Scholar]
- 372.Chwalisz K The use of progesterone antagonists for cervical ripening and as an adjunct to labour and delivery. Hum Reprod 1994;9 Suppl 1:131–161. [DOI] [PubMed] [Google Scholar]
- 373.Stjernholm Y, Sahlin L, Akerberg S, Elinder A, Eriksson HA, Malmstrom A et al. Cervical ripening in humans: potential roles of estrogen, progesterone, and insulin-like growth factor-I. Am J Obstet Gynecol 1996;174:1065–71. [DOI] [PubMed] [Google Scholar]
- 374.Kelly RW, Leask R, Calder AA. Choriodecidual production of interleukin-8 and mechanism of parturition. Lancet 1992;339:776–7. [DOI] [PubMed] [Google Scholar]
- 375.Bygdeman M, Swahn ML, Gemzell-Danielsson K, Gottlieb C. The use of progesterone antagonists in combination with prostaglandin for termination of pregnancy. Hum Reprod 1994;9 Suppl 1:121–25. [DOI] [PubMed] [Google Scholar]
- 376.Puri CP, Patil RK, Elger WA, Pongubala JM. Effects of progesterone antagonist ZK 98.299 on early pregnancy and foetal outcome in bonnet monkeys. Contraception 1990;41:197–205. [DOI] [PubMed] [Google Scholar]
- 377.Bernal AL. Overview of current research in parturition. Exp Physiol 2001;86:213–22. [DOI] [PubMed] [Google Scholar]
- 378.Young IR. The comparative physiology of parturition in mammals In: Smith R, editor. The Endocrinology of Parturition. Basel, Switzerland: Reinhardt Druck; 2001. p. 10–30. [DOI] [PubMed] [Google Scholar]
- 379.Westphal U, Stroupe SD, Cheng SL. Progesterone binding to serum proteins. Ann N Y Acad Sci 1977;286:10–28. [DOI] [PubMed] [Google Scholar]
- 380.Schwarz BE, Milewich L, Johnston JM, Porter JC, MacDonald PC. Initiation of human parturition. V. Progesterone binding substance in fetal membranes. Obstet Gynecol 1976;48:685–9. [PubMed] [Google Scholar]
- 381.Karalis K, Goodwin G, Majzoub JA. Cortisol blockade of progesterone: a possible molecular mechanism involved in the initiation of human labor. Nat Med 1996;2:556–60. [DOI] [PubMed] [Google Scholar]
- 382.Milewich L, Gant NF, Schwarz BE, Chen GT, MacDonald PC. Initiation of human parturition. VIII. Metabolism of progesterone by fetal membranes of early and late human gestation. Obstet Gynecol 1977;50:45–48. [PubMed] [Google Scholar]
- 383.Mitchell BF, Wong S. Changes in 17 beta,20 alpha-hydroxysteroid dehydrogenase activity supporting an increase in the estrogen/progesterone ratio of human fetal membranes at parturition. Am J Obstet Gynecol 1993;168:1377–85. [DOI] [PubMed] [Google Scholar]
- 384.Pieber D, Allport VC, Hills F, Johnson M, Bennett PR. Interactions between progesterone receptor isoforms in myometrial cells in human labour. Mol Hum Reprod 2001;7:875–9. [DOI] [PubMed] [Google Scholar]
- 385.How H, Huang ZH, Zuo J, Lei ZM, Spinnato JA, Rao CV. Myometrial estradiol and progesterone receptor changes in preterm and term pregnancies. Obstet Gynecol 1995;86:936–40. [DOI] [PubMed] [Google Scholar]
- 386.Mesiano S, Chan EC, Fitter JT, Kwek K, Yeo G, Smith R. Progesterone withdrawal and estrogen activation in human parturition are coordinated by progesterone receptor A expression in the myometrium. J Clin Endocrinol Metab 2002;87:2924–30. [DOI] [PubMed] [Google Scholar]
- 387.Condon JC, Hardy DB, Kovaric K, Mendelson CR. Up-regulation of the progesterone receptor (PR)-C isoform in laboring myometrium by activation of nuclear factor-kappaB may contribute to the onset of labor through inhibition of PR function. Mol Endocrinol 2006;20:764–75. [DOI] [PubMed] [Google Scholar]
- 388.Zakar T, Hertelendy F. Progesterone withdrawal: key to parturition. Am J Obstet Gynecol 2007. Apr;196(4):289–96. [DOI] [PubMed] [Google Scholar]
- 389.Belt AR, Baldassare JJ, Molnar M, Romero R, Hertelendy F. The nuclear transcription factor NF-kappaB mediates interleukin-1beta-induced expression of cyclooxygenase-2 in human myometrial cells. Am J Obstet Gynecol 1999;181:359–66. [DOI] [PubMed] [Google Scholar]
- 390.Kalkhoven E, Wissink S, Van der Saag PT, van der BB. Negative interaction between the RelA(p65) subunit of NF-kappaB and the progesterone receptor. J Biol Chem 1996;271:6217–24. [DOI] [PubMed] [Google Scholar]
- 391.Allport VC, Pieber D, Slater DM, Newton R, White JO, Bennett PR. Human labour is associated with nuclear factor-kappaB activity which mediates cyclo-oxygenase-2 expression and is involved with the ‘functional progesterone withdrawal’. Mol Hum Reprod 2001;7:581–86. [DOI] [PubMed] [Google Scholar]
- 392.Rezapour M, Backstrom T, Lindblom B, Ulmsten U. Sex steroid receptors and human parturition. Obstet Gynecol 1997;89:918–24. [DOI] [PubMed] [Google Scholar]
- 393.Henderson D,Wilson T. Reduced binding of progesterone receptor to its nuclear response element after human labor onset. Am J Obstet Gynecol 2001;185:579–85. [DOI] [PubMed] [Google Scholar]
- 394.Gustafsson JA. An update on estrogen receptors. Semin Perinatol 2000;24:66–9. [DOI] [PubMed] [Google Scholar]
- 395.Warner M, Nilsson S, Gustafsson JA. The estrogen receptor family. Curr Opin Obstet Gynecol 1999;11:249–54. [DOI] [PubMed] [Google Scholar]
- 396.Pieber D, Allport VC, Bennett PR. Progesterone receptor isoform A inhibits isoform B-mediated transactivation in human amnion. Eur J Pharmacol 2001;427:7–11. [DOI] [PubMed] [Google Scholar]
- 397.Taylor AH, McParland PC, Taylor DJ, Bell SC. The progesterone receptor in human term amniochorion and placenta is isoform C. Endocrinology 2006;147:687–93. [DOI] [PubMed] [Google Scholar]
- 398.Wei LL, Norris BM, Baker CJ. An N-terminally truncated third progesterone receptor protein, PR(C), forms heterodimers with PR(B) but interferes in PR(B)-DNA binding. J Steroid Biochem Mol Biol 1997;62:287–97. [DOI] [PubMed] [Google Scholar]
- 399.Blanks AM, Vatish M, Allen MJ, Ladds G, de Wit NC, Slater DM, et al. Paracrine oxytocin and estradiol demonstrate a spatial increase in human intrauterine tissues with labor. J Clin Endocrinol Metab 2003;88:3392–400. [DOI] [PubMed] [Google Scholar]
- 400.Annos T, Thompson IE, Taymor ML. Luteal phase deficiency and infertility: difficulties encountered in diagnosis and treatment. Obstet Gynecol 1980;55:705–10. [PubMed] [Google Scholar]
- 401.Balasch J, Vanrell JA, Marquez M, Rivera F, Gonzalez-Merlo J. Luteal phase in infertility: problems of evaluation. Int J Fertil 1982;27:60–2. [PubMed] [Google Scholar]
- 402.Daya S Issues in the etiology of recurrent spontaneous abortion. Curr Opin Obstet Gynecol 1994;6:153–9. [PubMed] [Google Scholar]
- 403.Stephenson MD. Frequency of factors associated with habitual abortion in 197 couples. Fertil Steril 1996;66:24–9. [PubMed] [Google Scholar]
- 404.Jones GS. The luteal phase defect. Fertil Steril 1976;27:351–6. [DOI] [PubMed] [Google Scholar]
- 405.Jones GS. Luteal phase defect: a review of pathophysiology. Curr Opin Obstet Gynecol 1991;3:641–8. [PubMed] [Google Scholar]
- 406.Balasch J, Vanrell JA. Corpus luteum insufficiency and fertility: a matter of controversy. Hum Reprod 1987;2:557–67. [DOI] [PubMed] [Google Scholar]
- 407.Balasch J, Fabregues F, Creus M, Vanrell JA. The usefulness of endometrial biopsy for luteal phase evaluation in infertility. Hum Reprod 1992;7:973–7. [DOI] [PubMed] [Google Scholar]
- 408.Check JH, Lee G, Epstein R, Vetter B. Increased rate of preterm deliveries in untreated women with luteal phase deficiencies. Preliminary report. Gynecol Obstet Invest 1992;33:183–4. [DOI] [PubMed] [Google Scholar]
- 409.Fidel PI Jr, Romero R, Maymon E, Hertelendy F. Bacteria-induced or bacterial product-induced preterm parturition in mice and rabbits is preceded by a significant fall in serum progesterone concentrations. J Matern Fetal Med 1998;7:222–6. [DOI] [PubMed] [Google Scholar]
- 410.Hirsch E, Muhle R. Intrauterine bacterial inoculation induces labor in the mouse by mechanisms other than progesterone withdrawal. Biol Reprod 2002;67:1337–41. [DOI] [PubMed] [Google Scholar]
- 411.Dudley DJ, Collmer D, Mitchell MD, Trautman MS. Inflammatory cytokine mRNA in human gestational tissues: implications for term and preterm labor. J Soc Gynecol Investig 1996;3:328–35. [PubMed] [Google Scholar]
- 412.Romero R, Mazor M, Sepulveda W, Avila C, Copeland D, Williams J. Tumor necrosis factor in preterm and term labor. Am J Obstet Gynecol 1992;166:1576–87. [DOI] [PubMed] [Google Scholar]
- 413.Romero R, Mazor M, Brandt F, Sepulveda W, Avila C, Cotton DB, et al. Interleukin-1 alpha and interleukin-1 beta in preterm and term human parturition. Am J Reprod Immunol 1992;27:117–23. [DOI] [PubMed] [Google Scholar]
- 414.Fortunato SJ, Menon R, Swan KF. Expression of TNF-alpha and TNFR p55 in cultured amniochorion. AmJ Reprod Immunol 1994;32:188–93. [DOI] [PubMed] [Google Scholar]
- 415.Menon R, Swan KF, Lyden TW, Rote NS, Fortunato SJ. Expression of inflammatory cytokines (interleukin-1 beta and interleukin-6) in amniochorionic membranes. Am J Obstet Gynecol 1995;172:493–500. [DOI] [PubMed] [Google Scholar]
- 416.Romero R, LaFreniere D, Duff G, et al. Human decidua: a potent source of interleukin-1 like activity. Presented at the 32nd Annual Meeting for the Society for Gynecologic Investigation, 20–23 March 1985, Phoenix, AZ, USA. [Google Scholar]
- 417.Todd HM, Dundoo VL, Gerber WR, Cwiak CA, Baldassare JJ, Hertelendy F. Effect of cytokines on prostaglandin E2 and prostacyclin production in primary cultures of human myometrial cells. J Matern Fetal Med 1996;5:161–67. [DOI] [PubMed] [Google Scholar]
- 418.Sehringer B, Schafer WR, Wetzka B, Deppert WR, Brunner-Spahr R, Benedek E et al. Formation of proinflammatory cytokines in human term myometrium is stimulated by lipopolysaccharide but not by corticotropin-releasing hormone. J Clin Endocrinol Metab 2000;85:4859–65. [DOI] [PubMed] [Google Scholar]
- 419.Elliott CL, Slater DM, Dennes W, Poston L, Bennett PR. Interleukin 8 expression in human myometrium: changes in relation to labor onset and with gestational age. Am J Reprod Immunol 2000;43:272–77. [DOI] [PubMed] [Google Scholar]
- 420.Rauk PN, Chiao JP. Interleukin-1 stimulates human uterine prostaglandin production through induction of cyclooxygenase-2 expression. Am J Reprod Immunol 2000;43:152–9. [DOI] [PubMed] [Google Scholar]
- 421.Lappas M, Permezel M, Georgiou HM, Rice GE. Nuclear factor kappa B regulation of proinflammatory cytokines in human gestational tissues in vitro. Biol Reprod 2002;67:668–73. [DOI] [PubMed] [Google Scholar]
- 422.Yan X, Sun M, Gibb W. Localization of nuclear factor-kappa B (NF kappa B) and inhibitory factor-kappa B (I kappa B) in human fetal membranes and decidua at term and preterm delivery. Placenta 2002;23:288–93. [DOI] [PubMed] [Google Scholar]
- 423.Meis PJ, Klebanoff M, Thorn E, Dombrowski MP, Sibai B, Moawad AH, et al. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Prevention of recurrent preterm delivery by 17 alpha- hydroxyprogesterone caproate. N Engl J Med 2003;348:2379–85. [DOI] [PubMed] [Google Scholar]
- 424.da Fonseca EB, Bittar RE, Carvalho MH, Zugaib M. Prophylactic administration of progesterone by vaginal suppository to reduce the incidence of spontaneous preterm birth in women at increased risk: a randomized placebo-controlled double-blind study. Am J Obstet Gynecol 2003;188:419–24. [DOI] [PubMed] [Google Scholar]
- 425.Lockwood CJ. Stress-associated preterm delivery: the role of corticotropin- releasing hormone. Am J Obstet Gynecol 1999;180:S264–6. [DOI] [PubMed] [Google Scholar]
- 426.Wadhwa PD, Culhane JF, Rauh V, Barve SS. Stress and preterm birth: neuroendocrine, immune/inflammatory, and vascul mechanisms. Matern Child Health J 2001;5:119–25. [DOI] [PubMed] [Google Scholar]
- 427.Wadhwa PD, Culhane JF, Rauh V, Barve SS, Hogan V, Sandman CA, Hobel CJ, Chicz-DeMet A, Dunkel-Schetter C, Garite TJ, Glynn L Stress, infection and preterm birth: a biobehavioural perspective. Paediatr Perinat Epidemiol 2001;15(Suppl 2):17–29. [DOI] [PubMed] [Google Scholar]
- 428.Challis JR, Smith SK. Fetal endocrine signals and preterm labor. Biol Neonate 2001;79:163–7. [DOI] [PubMed] [Google Scholar]
- 429.Hobel CJ. Stress and preterm birth. Clin Obstet Gynecol 2004;47:856–80. [DOI] [PubMed] [Google Scholar]
- 430.Mozurkewich EL, Luke B, Avni M, Wolf FM. Working conditions and adverse pregnancy outcome: a meta-analysis. Obstet Gynecol 2000;95:623–35. [DOI] [PubMed] [Google Scholar]
- 431.Copper RL, Goldenberg RL, Das A, Elder N, Swain M, Norman G, et al. The preterm prediction study: maternal stress is associated with spontaneous preterm birth at less than thirty-five weeks’ gestation. National Institute of Child Health and Human Development Maternal-Fetal Medicine Units Network. Am J Obstet Gynecol 1996;175:1286–92. [DOI] [PubMed] [Google Scholar]
- 432.Wadhwa PD, Sandman CA, Porto M, Dunkel-Schetter C, Garite TJ. The association between prenatal stress and infant birth weight and gestational age at birth: a prospective investigation. Am J Obstet Gynecol 1993;169:858–65. [DOI] [PubMed] [Google Scholar]
- 433.Hobel CJ, Dunkel-Schetter C, Roesch SC, Castro LC, Arora CP. Maternal plasma corticotropin-releasing hormone associated with stress at 20 weeks’ gestation in pregnancies ending in preterm delivery. Am J Obstet Gynecol 1999;180(1 Pt3):S257–63. [DOI] [PubMed] [Google Scholar]
- 434.Bloomfield FH, Oliver MH, Hawkins P, Campbell M, Phillips DJ, Gluckman PD, et al. A periconceptional nutritional origin for noninfectious preterm birth. Science 2003;300:606. [DOI] [PubMed] [Google Scholar]
- 435.Jones SA, Challis JR. Local stimulation of prostaglandin production by corticotropin-releasing hormone in human fetal membranes and placenta. Biochem Biophys Res Commun 1989;159:192–9. [DOI] [PubMed] [Google Scholar]
- 436.Sandman CA, Glynn L, Schetter CD, Wadhwa P, Garite T, Chicz-DeMet A, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin releasing hormone (CRH): priming the placental clock. Peptides 2006;27:1457–63. [DOI] [PubMed] [Google Scholar]
- 437.Romero R, Sibai BM, Sanchez-Ramos L, Valenzuela GJ, Veille JC, Tabor B et al. An oxytocin receptor antagonist (atosiban) in the treatment of preterm labor: a randomized, double-blind, placebo-controlled trial with tocolytic rescue. Am J Obstet Gynecol 2000;182:1173–83. [DOI] [PubMed] [Google Scholar]