Abstract
Gokushoviruses are single-stranded, circular DNA bacteriophages found in metagenomic datasets from diverse ecosystems worldwide, including human gut microbiomes. Despite their ubiquity and abundance, little is known about their biology or host range: Isolates are exceedingly rare, known only from three obligate intracellular bacterial genera. By synthesizing circularized phage genomes from prophages embedded in diverse enteric bacteria, we produced gokushoviruses in an experimentally tractable model system, allowing us to investigate their features and biology. We demonstrate that virions can reliably infect and lysogenize hosts by hijacking a conserved chromosome-dimer resolution system. Sequence motifs required for lysogeny are detectable in other metagenomically defined gokushoviruses; however, we show that even partial motifs enable phages to persist cytoplasmically without leading to collapse of their host culture. This ability to employ multiple, disparate survival strategies is likely key to the long-term persistence and global distribution of Gokushovirinae.
Research organism: E. coli, Virus
Introduction
Single-stranded circular DNA (ssDNA) phages of the family Microviridae are among the most common and rapidly evolving viruses present in the human gut (Lim et al., 2015; Minot et al., 2013; Manrique et al., 2017; Shkoporov et al., 2019). Within the Microviridae, members of the subfamily Gokushovirinae are detected in metagenomic datasets from diverse environments, ranging from methane seeps to stromatolites, termite hindguts, freshwater bogs and the open ocean (Bryson et al., 2015; Desnues et al., 2008; Quaiser et al., 2015; Tikhe and Husseneder, 2018; Tucker et al., 2011).
Due to their small, circular genomes, full assembly of these phages from metagenomic data is easy (Creasy et al., 2018; Labonté and Suttle, 2013; Roux et al., 2012), and more than a thousand complete metagenome-assembled microvirus genomes have been deposited to NCBI as of beginning of 2020. In contrast, Microviridae have been isolated from very few hosts, hardly representative of their diversity as a whole, and the only readily cultivable member of this family is phiX174, which is classified to the distantly related subfamily Bullavirinae (Doore and Fane, 2016; Krupovic et al., 2016). While phiX and phiX-like phages are among the most well-studied groups of viruses, they are rare in nature and occupy a small specialist niche as lytic predators of select strains of Escherichia coli (Michel et al., 2010). Conversely, the Gokushovirinae and several other (though not formally described) subfamilies of Microviridae are seemingly abundant in the environment but almost exclusively known from metagenomic datasets (Creasy et al., 2018; Székely and Breitbart, 2016), although estimates of their actual numbers could be biased due to the methods used to prepare metagenomic samples (Kim and Bae, 2011; Roux et al., 2016). Despite their apparent prevalence in the environment, the only isolated gokushoviruses are lytic parasites recovered from the host-restricted intracellular bacteria Spiroplasma, Chlamydia and Bdellovibrio (Brentlinger et al., 2002; Garner et al., 2004; Ricard et al., 1980). Given their regular occurrence in metagenomes from diverse habitats, it seems unlikely that Gokushovirinae only infect intracellular bacteria, and their lack of recovery from other hosts is puzzling.
Typical gokushoviruses pack their 4000-6000 nt genomes, composed of 3–11 genes, into tailless icosahedral phage capsids (Roux et al., 2012). No gokushoviruses encode an integrase, which has led to the assumption that they are lytic phages. However, the presence of prophages belonging to several undescribed groups of microviruses within the genomes of some Bacteroidetes and Alphaproteobacteria (Krupovic and Forterre, 2011; Zhan and Chen, 2019; Zheng et al., 2018) raises the possibility that some can be integrated through the use of host-proteins, in a similar manner to the XerC/XerD dependent integration of ssDNA Inoviridae (Krupovic and Forterre, 2015).
All gokushovirus genomes assembled from metagenomic data lack multiple genes that are present in phiX-like phages (Roux et al., 2012). Markedly absent are: (i) a peptidoglycan synthesis inhibitor that leads to host cell lysis (although some phages appear to have horizontally acquired bacterial peptidases) and (ii) a major spike protein involved in host cell attachment (Doore and Fane, 2016; Roux et al., 2012). These proteins represent crucial elements in the infectious cycle of phiX, and their absence from other gokushoviruses indicates that these phages might operate quite differently on a molecular level.
Here we detect a large number of gokushovirus prophages integrated into the genomes of enterobacteria, contrasting with their predicted exclusively lytic lifestyle. Through transformation of a synthesized prophage genome into a laboratory strain of Escherichia coli, we cultivate a novel gokushovirus capable of lysogenizing enterobacteria. Using this experimental model system, we demonstrate that this phage is capable of passive integration via XerC/XerD mediated recombination of phage encoded dif-motifs with their bacterial counterparts. We also show that this capability has evolved independently in multiple lineages of gokushoviruses and demonstrate the existence of an intermediate, pseudolysogenic step between lytic and lysogenic lifestyle, indicating that gokushoviruses can lead a decidedly different existence from that of previously characterized members of the Gokushovirinae.
Results
A new, diverse group of gokushovirus prophages
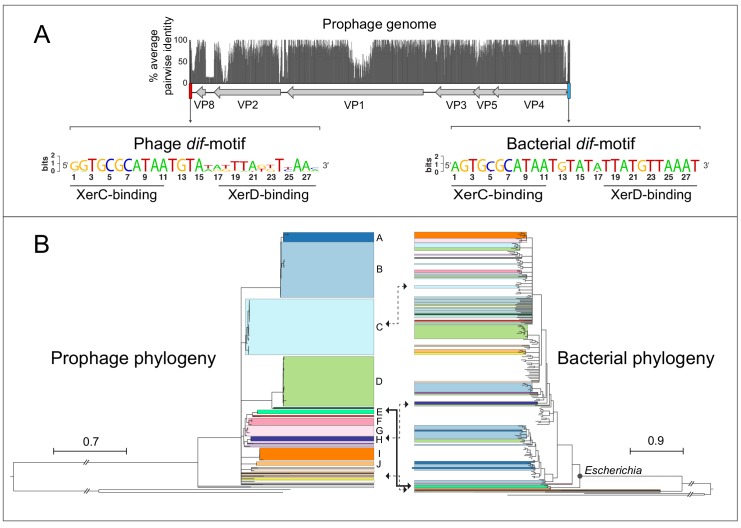
Querying fully assembled bacterial genomes with the major capsid protein VP1 of gokushovirus Chlamydia-phage 4 (NCBI Gene ID 3703676) returned 95 high-confidence hits (E-value <0.0001) within the Enterobacteriaceae (91 from Escherichia, and one each from Enterobacter, Salmonella, Citrobacter and Kosakonia; Supplementary file 1). Although Gokushovirinae were previously known only as lytic phages, inspection of each of the associated genomic regions revealed the presence of integrated prophages 4300–4700 bp in length and having a conserved six-gene arrangement: VP4 (replication initiation protein), VP5 (switch from dsDNA to ssDNA replication protein), VP3 (scaffold protein), VP1 (major capsid protein), VP2 (minor capsid protein) and VP8 (putative DNA-binding protein). Most of the variation in both the genome size and sequence of these prophages is confined to three regions: (i) near the C-terminus of VP2, (ii) in the non-coding region between VP8 and VP2, and (iii) within VP1, whose hypervariability is characteristic of the Gokushovirinae (Chipman et al., 1998; Diemer and Stedman, 2016; Figure 1A).
Figure 1. Gokushovirus prophages of Enterobacteria.
(A) Genome organization and average pairwise nucleotide identities of the gokushovirus prophages detected in Escherichia. Integrated prophage genomes range from 4316 to 4692 bp in length, with genes indicated by grey arrows, and flanking phage and bacterial dif-motifs indicated by red and blue bars. Nucleotide sequence logos of phage and bacterial dif-motifs, with corresponding XerC- and XerD-binding sites are shown. (B) Phylogeny of gokushovirus prophages and their enterobacterial hosts. Single lineages or clades comprising strains sharing >95% average nucleotide identity are individually colored, with colors in the bacterial phylogeny corresponding to those of their associated prophages. Clades not colored in the bacterial phylogeny correspond to Escherichia coli collection reference (ECOR) strains. Clades with bootstrap support values below 70% are collapsed. Arrows denote prophage-host pairs in which the corresponding phage was tested againstE. coliK-12, with the solid arrow indicating the prophage-host pair whose phage formed plaques in E. coli K-12 and subsequently used in experimental analyses. Tree scale bars correspond to nucleotide or amino acid substitutions/site for prophage and host trees, respectively, and ancestral branches with hatch-marks are truncated by the length of two scale bars. Accession numbers and details of prophages and their corresponding hosts are listed in Supplementary files 1 and 3.
Figure 1—figure supplement 1. Sequence similarities among gokushoviral prophages.
All detected prophages are flanked by dif-motifs, which are 28 bp palindromic sites that are known to be the targets of passive integration by phages and other mobile elements (Blakely et al., 1993; Das et al., 2013). The dif-motifs upstream of the insertions are highly conserved and individually differ by, at most, one nucleotide from the canonical dif-motif of Escherichia coli. These upstream dif-motifs consist of a central 6 bp spacer flanked by two 11 bp arms, previously been shown to bind tyrosine recombinases XerC/XerD during chromosome segregation and integration of mobile elements (Castillo et al., 2017). In contrast, the dif-motifs downstream of detected prophages are more variable, particularly in the spacer region and XerD-binding arms, representing the phage dif-motifs integrated along with the phage (Figure 1A).
A whole genome phylogeny of gokushovirus prophages shows a number of well-differentiated (bootstrap support 70% or higher) lineages, forming clades A-J that each contains members with >95% average nucleotide identity, and 14 singleton lineages with no close relatives (Figure 1B, Figure 1—figure supplement 1). Comparing the topology of the phage phylogeny with that of their E. coli host strains shows examples of prophage clades found only in specific branches of the E. coli phylogeny and clades with wider distributions. For example, whereas prophages belonging to clade A-D are found dispersed across the host phylogeny, prophage clade I is confined to a single E. coli clade (possibly as a result of the limited number of detected prophages). In all but one case, each E. coli host harbors only a single gokushovirus prophage. Although phage attachment and infection sometimes depend on O-antigenicity, there is no obvious association between the presence of gokushovirus prophages and particular E. coli O-serotypes among those strains for which information on O-antigens is available (Zhou et al., 2020). For example, four identical prophages belonging to clade C could be detected in E. coli strains MOD1-EC5200 (O76:H19), MOD1-EC5181 (O132:H8), NCTC9043 (O43:H2) and MOD1-EC6266 (O124:H21) (Supplementary file 1). Most gokushovirus prophages were detected in diverse E. coli strains isolated from various animals, mainly cattle and marmots, but five prophages were detected in isolates from humans, including one from a urinary tract infection (Supplementary file 1).
Prophages of enterobacteria form a distinct gokushovirus clade
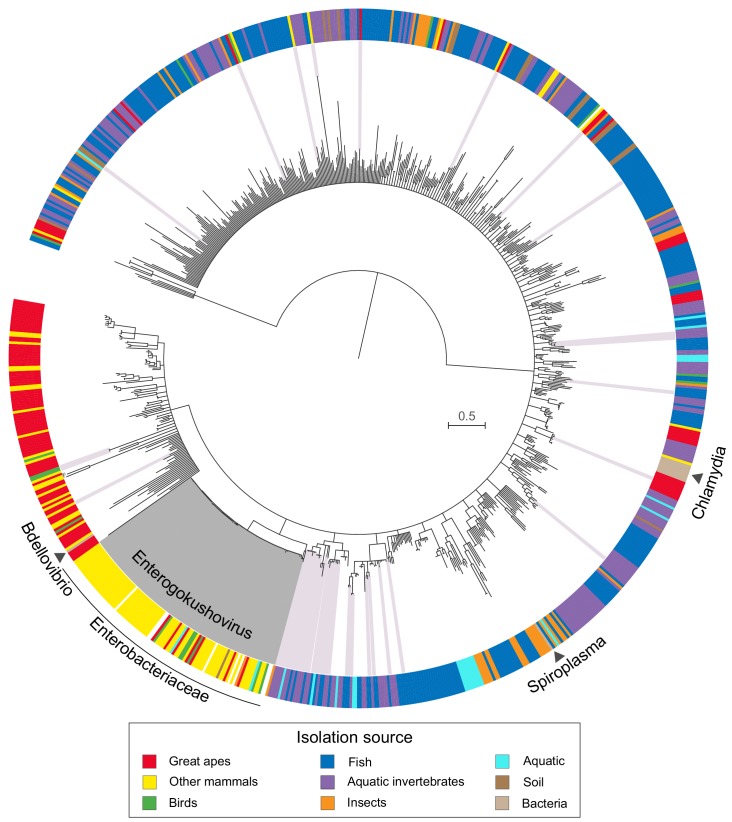
Phylogenetic analysis of available gokushovirus genomes based on an alignment of the conserved VP1 and VP4 proteins (n = 855; including the enterobacterial prophages discovered in this study, the previously sequenced lytic gokushoviruses from Chlamydia, Spiroplasma and Bdellovibrio, and the metagenomically assembled gokushovirus genomes available from NCBI) returned enterobacterial prophages as a well-supported (97% bootstrap support), monophyletic clade within the Gokushovirinae (Figure 2). The distinctiveness of this clade, whose members share a conserved (but not unique) gene order and display an average nucleotide identity of >50% (Figure 1—figure supplement 1 and Supplementary file 2), advocates the formation of a new proposed genus within the family Microviridae (subfamily Gokushovirinae), for which we suggest the name Enterogokushovirus on account of a distribution limited to members of the Enterobactericeae.
Figure 2. Phylogeny and sources of Gokushovirinae.
Maximum likelihood tree built from concatenated alignments of VP1 and VP4 protein sequences of 855 gokushovirus genomes. Tree is midpoint rooted, and branch support estimated with 100 bootstrap replicates. Branches with bootstrap support values below 70% are collapsed. Clades highlighted in grey indicate Enterogokushovirus prophages with recognizable dif-motifs; those highlighted in pink possess dif-motifs identified through an iterative HMM search. Outer ring indicates isolation source, with black triangles denoting the phylogenetic positions of officially described gokushoviruses. Scale bar corresponds to amino acid substitutions per site. Sample accession numbers are listed in Supplementary file 4.
Since a unique feature of enterogokushoviruses among the Gokushovirinae appears to be the ability to exist as lysogens, we searched all other gokushovirus MAGs in our dataset for dif-like sequence motifs that might be indicative of lysogenic ability. We detected similar motifs in 48 genomes distributed sporadically throughout the gokushovirus phylogeny. Aside from the enterogokushoviruses, there are two larger clades in which multiple genomes contain a dif-motif, with the rest of dif-bearing MAGs found largely as singletons (Figure 2; Supplementary file 4). The majority of these putative dif-motifs occurs in non-coding regions, and those detected within coding regions were typically at the 5’- or 3’-end of a predicted gene, with a nearby alternative start or stop codon that could preserve the genetic integrity of the phage through integration and excision. The overall dearth of dif-like sequences in gokushoviruses sampled from diverse geographic and ecological settings highlights the distinctiveness of Enterogokushovirus genomes and lifestyle.
We further attempted to determine the prevalence of enterobacterial gokushoviruses by interrogating 1839 samples from eight metagenomic studies of human and cattle gut microbiomes for the presence of closely related prophages. From these data, we were able to fully assemble only two integrated prophages corresponding to E. coli gokushoviruses, one from the fecal metagenome of an Austrian adult (0,05% of all reads in ERR688616) and the other from a Danish infant (0,02% of all reads in ERR525761) (see also Supplementary file 4).
Reconstituting viable phage from integrated prophages
The integrity of prophage structure in all enterobacteria and the lack of premature stop codons suggested that these sequences represent intact, functional insertions into bacterial hosts. To confirm the functionality of Escherichia gokushovirus prophages, characterize their biology and provide a type strain, we attempted to construct phages from genomic DNA of Escherichia strains MOD1-EC2703, MOD1-EC5150, MOD1-EC6098 and MOD1-EC6163, selected to represent the diversity of gokushovirus prophages and hosts (Figure 1B).
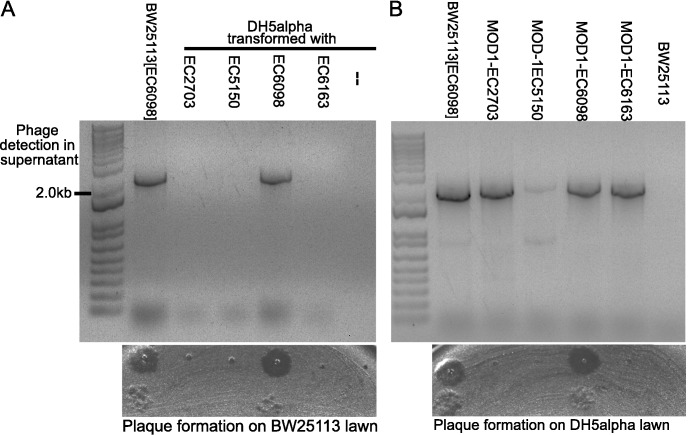
Sequences corresponding to prophages from these four Escherichia strains were amplified, circularized and transformed into E. coli DH5α (Figure 3A). Supernatants from the transformed DH5α culture were used in agar-overlay assays with E. coli K12 BW25113 hosts, resulting in plaques for only one of four reconstructed phage genomes (EC6098, derived from E. marmotae strain MOD1-EC6098 and belonging to prophage clade E (Figure 3B, Figure 3—figure supplement 1A). While both DH5α and BW25113 possess other prophages which could produce false positives when screening for gokushovirus production (Chen et al., 2018; Wang et al., 2010), DH5α alone did not produce plaques on BW25113 and vice versa (Figure 3—figure supplement 1). To corroborate the synthesis of EC6098 phages (and the inability to produce phages from the other three sequences), we additionally grew live cultures of MOD1-EC2703, MOD1-EC5150, MOD1-EC6098 and MOD1-EC6163 and via PCR detected the presence of circularized gokushovirus genomes in all four, but only MOD1-EC6098 produced plaques resembling those derived from synthetic EC6098 (Figure 3—figure supplement 1B). As only EC6098 formed plaques on K12 strains, we used this phage for further experimental characterization.
Figure 3. In vitro assembly and revival of enterobacterial gokushoviruses.
(A) Scheme used to produce viable phage from prophage inserts. The prophage region, with genes colored in grey, is amplified from the bacterial genome (black) using primers that incorporate the phage dif-motif but exclude the bacterial dif-motif (indicated by bent arrows). Circularization of the amplification product results in a molecule corresponding to the replicative dsDNA form of the phage. Transformation of this circular molecule into electrocompetent E. coli DH5α cells leads to expression of phage proteins, replication and packaging of ssDNA into infective virions. (B) Plaques formed by constructed bacteriophage EC6098 after infecting E. coli BW25113. (C) TEM images of bacteriophage EC6098 viewed at 175,000x magnification. (D) TEM images of bacteriophage EC6098 viewed at 300,000x magnification.
Figure 3—figure supplement 1. Synthesis of gokushoviruses in Escherichia coli DH5α and natural host strains.
Electron microscopic observation of pure EC6098 lysates recovered at a buoyant density of around 1.3 g/cm−3 in CsCl gradients (the range expected for members of the Microviridae, Thurber et al., 2009), revealed tailless icosahedral virions, 25–30 µm in size and displaying protrusions previously observed in other Gokushovirinae (Chipman et al., 1998; Diemer and Stedman, 2016; Figure 3D,E). Because Gokushovirinae have only been recovered as lytic particles from intracellular bacteria, this represents the first isolation of a gokushovirus able to infect free-living bacteria as well as being able to integrate as a lysogen into bacterial genomes.
Mechanisms of enterobacterial gokushovirus integration into host genomes
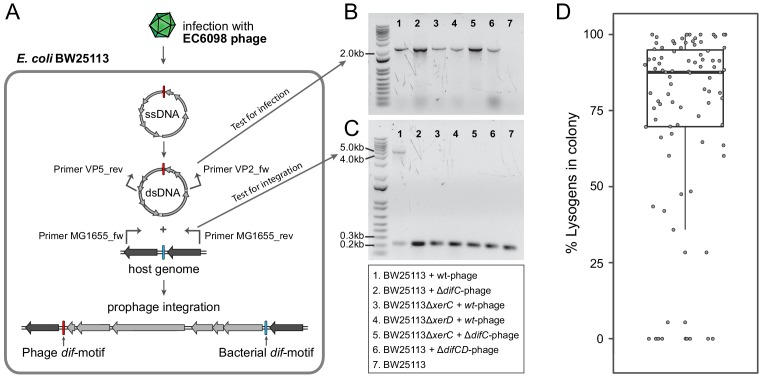
We next investigated the process by which gokushoviruses integrate into the bacterial host chromosome (Figure 4A). The presence of circularized phage genomes could readily be detected from surviving colonies in confluent plaques derived from agar-overlay assays (Figure 4B). Using primers that flank both sides of the dif-motif in host strain BW25113, we recovered products that were enlarged by the length of the phage relative to colonies lacking the prophage (Figure 4A,C). Sequencing confirmed that, in accordance with the integration site of detected prophages, phage EC6098 integrates downstream of the BW25113 dif-motif, which remains unchanged (corresponding to position 3,046,436–3,046,463 in the closed genome with accession number CP009273).
Figure 4. Integration of gokushoviruses into E. coli host genome.
(A) Schematic representation of phage integration process, and detection of circularized EC6098 phage genome and integrated prophages in E. coli BW25113. Upon infection, phage EC6098 releases ssDNA, with dif-motif denoted in red, leading to formation of dsDNA replicative genomes that integrate downstream of the bacterial dif-motif (blue). Primers VP2_rev and VP5_fw (indicated by bent arrows on EC6098 genome) anneal to genes flanking the phage dif-motif and amplify a ~ 2.1 kb product from closed circular phage genomes (corresponding to bands in panel B). Primers MG1655dif_fw and MG1655dif_rev (indicated by bent arrows on host genome) anneal to sites flanking the bacterial dif-motif and amplify either a 210 bp region of bacterial DNA when there is no phage integration or a ~ 5 kb region denoting the presence of an integrated prophage (corresponding to bands in panel C). (B) Detection of fragments indicating the presence of circularized phage. Numbered lanes correspond to samples listed in the box below the gel photographs. (C) Detection of fragments indicating the presence or absence of integrated phage from lysogenic colonies after infection of BW25113 strains with wild type or mutant phage. Numbered lanes are the same as in panel B, and correspond to samples listed in the box below the gel photograph. (D) Proportion of cells with integrated prophages in clonal lysogenic colonies. Box-and-whiskers plot shows median, 25th and 75th percentiles, and 1.5 inter-quartile range as well as individual datapoints for 87 independently sampled clonal colonies.
Because none of the gokushovirus prophages encodes an integrase, we predicted that host factors XerC and XerD might be responsible for prophage integration, similar to what has been hypothesized for microvirus-prophages in Bacteroidetes (Krupovic and Forterre, 2011). Neither ΔxerC nor ΔxerD mutants of E. coli host strain BW25113 resulted in integration, but the ΔxerC mutant was restored by complementation with a plasmid expressing the intact version of xerC. Similarly, phages with incomplete (i.e., lacking either their XerC or XerD binding site within the dif-motif, termed here ΔdifC and ΔdifD) or no dif-motifs (ΔdifCD) successfully infected hosts (as evidenced by the presence of circularized phage genomes in host colonies that had survived infection), but failed to integrate into host genomes, demonstrating the need for cooperative XerC/XerD binding for successful lysogeny (Table 1, Figure 4B,C). However, the retention of dif-motifs after integration indicates that this process is reversible: As evident in Figure 4C, there is a smaller fragment, in addition to that indicating prophage insertion, that corresponds to those cells in the same colony that do not harbor the integrated prophage. These cells persisted even after multiple rounds of re-streaking, and the median ratio of lysogens to non-lysogenic cells derived from clonal colonies approaches 4:1 (Figure 4D). Even in pure cultures, phages are thus continuously being excised and reintegrated, presumably as a result of XerC/XerD activity.
Table 1. Percentage of lysogenic colonies after phage infection*.
| Strain | Phage | Plasmid | % Lysogens |
|---|---|---|---|
| BW25113 | EC6098 | - | 17.71 |
| BW25113ΔxerC | EC6098 | - | 0 |
| BW25113ΔxerC | EC6098 | pJN105::xerC (induced†) | 14.58 |
| BW25113ΔxerC | EC6098 | pJN105::xerC (uninduced) | 0 |
| BW25113ΔxerD | EC6098 | - | 0 |
| BW25113ΔxerD | EC6098 | pJN105::xerD (induced†) | 0 |
| BW25113ΔxerD | EC6098 | pJN105::xerD (uninduced) | 0 |
| BW25113 | EC6098ΔdifC | - | 0 |
| BW25113 | EC6098ΔdifD | - | 0 |
| BW25113 | EC6098ΔdifCD | - | 0 |
*Assessed from screening 96 colonies for each strain.
†Expression induced by addition of 0.1% arabinose.
Integration into host genomes facilitates but is not required for long-term persistence
The removal of host factors xerC or xerD, the presence an incomplete phage dif-motif, or the lack of a dif-motif (as observed in the majority of Gokushovirinae) all prevent integration of phages into host genomes (Table 1, Figure 4C). However, almost all bacterial colonies that survived infections, regardless of whether they were lysogenized or not, were found to contain circular phage DNA (Figure 4B), and the presence of circular phage DNA alone, regardless of integration into the host genome, conferred superinfection immunity (Figure 5A). In addition, cultures in which phage DNA could only be detected in circularized form were capable of producing infectious particles at levels significantly higher than those of cultures containing integrated prophages (Figure 5B, Wilcoxon rank-sum test p<0.01).
Figure 5. Quantifying the effects of gokushovirus carriage.
(A) Superinfection immunity offered by integrated or non-integrated phages. Unimpeded growth in the presence of infecting EC6098 phage is shown for host cultures carrying lysogenic or lysogeny-deficient EC6098. Lysis of host by EC6098 in the absence of superinfection immunity is shown in grey for comparison. Averages and standard deviations are based on three replicates. (B) Phage production by lysogenic and non-lysogenic hosts. Plaque-forming units in culture supernatant were determined from six independent cultures of BW25113 or BW25113ΔxerC, each grown overnight at 37°C from an initial concentration of OD600 = 0.7. Box-and-whiskers plots show median, 25th and 75th percentiles (upper and lower hinges) and 1.5 inter-quartile range (whiskers). Outliers are shown as individual dots. P-values of <0.01 (Wilcoxon Rank-Sum test) in comparison to BW25113[EC6098] are indicated with asterisks. (C) Continuous phage production over the course of a month. Formation of plaques from supernatants of six replicate cultures of hosts carrying integrated or non-integrated phages is checked daily before 1:1000 dilution and growth for 18–24 hr at 37°C. In all cases, BW25113ΔfhuA served as the host strain for agar overlay assays.
The absence of alternative integration sites, as verified by inverse PCR, suggested that gokushoviruses might be able to persist in the host cytoplasm without integration into the host genome. To determine whether this persistence is a transient phenomenon that eventually leads to either the loss of phages or the collapse of bacterial cultures, we performed serial transfers (1:1000 dilution) with a variety of host-phage combinations over the course of a month. We enumerated plaque formation from culture supernatant on host strain BW25113ΔfhuA to avoid false positives from widespread contaminating dsDNA phages such as phi80 and SW-1, which unlike EC6098 require outer membrane receptor FhuA for successful infection (Rotman et al., 2012; Song et al., 2019). As expected, all lysogenic cultures (i.e., wild type cultures with integrated gokushovirus prophages) produced phage particles throughout the course of the experiments. However, a third of all cultures where gokushoviruses were initially present only in the cytoplasm (due to deletions in the host integration machinery or phage integration site) retained phage production over the course of a month despite the lack of integration (Figure 5C). Cumulatively, these results indicate that gokushoviruses do not exist solely as lytic particles, as previously believed, but can also exist as (pseudo)lysogens, in a carrier state that continuously produces phage particles without integrating into the genome.
Discussion
Through the analysis of whole genome sequences and metagenomic databases, we defined a unique genus of Gokushovirinae prophages and subsequently synthesized a viable gokushovirus capable of infecting and integrating into the genomes of enteric bacteria. Since Gokushovirinae were previously known as exclusively lytic predators of a few intracellular bacteria (King et al., 2011), the new proposed genus, Enterogokushovirus, offers new insights into our understanding of this ecologically widespread group of phages. First, by confirming the existence of Gokushovirinae prophages in free-living bacteria, we resolved a seeming paradox in which a diverse and widespread lineage within the Microviridae appeared to be confined to rare pathogenic and parasitic bacteria, such as Chlamydia (Wang et al., 2019). Second, by demonstrating the integration of a gokushovirus into the E. coli genome, we show that these phages employ survival strategies beyond the lytic infection of hosts.
Experimental characterization of the enterogokushovirus EC6098 isolated from an environmental strain of E. marmotae showed that these phages possess a dif-like recognition motif that, together with the host-encoded recombinases XerC/XerD, is required for lysogeny. In this manner, enterogokushoviruses appropriate a highly conserved bacterial chromosome concatemer-resolution system that enables their integration into host genomes via homologous recombination (Castillo et al., 2017). The exact mechanism of this integration process still remains to be elucidated: some phages and mobile elements are known to have different requirements for the bacterial XerC and/or XerD proteins (Midonet and Barre, 2015), which is perhaps the reason why we could only successfully reconstitute xerC but not xerD knockouts. We also show that phages with only partial DNA-binding motifs exist in a condition in which circularized phage genomes are present in the cytoplasm and virions are continuously released from the host. While it should be noted that this could be a laboratory-induced phenomenon, this strategy is similar to the pseudolysogenic state observed in crAssphage (Shkoporov et al., 2018) and other bacteriophages (Siringan et al., 2014), and helps explain the persistence of microviruses in the human gut (Minot et al., 2013; Shkoporov et al., 2019).
Despite an exhaustive sampling of gokushoviral diversity from metagenomic datasets, the occurrence of dif-positive gokushoviruses is rare outside of the enterogokushoviruses. However, the sporadic distribution of dif-motifs among other gokushoviruses indicates that the ability to lysogenize bacterial hosts has been independently gained and lost multiple times during the evolution of this taxon. For example, the exclusively lytic gokushoviruses of Chlamydia (Śliwa-Dominiak et al., 2013) might once have been able to integrate into their hosts’ genomes since extant Chlamydia genomes contain coding sequences similar to the gokushoviral replication initiation and minor capsid proteins (Read et al., 2000; Rosenwald et al., 2014).
We observed that lysogenic cultures produce a considerably lower number of phage particles than non-lysogenic cultures but are less prone to the loss of phage, indicating that the lytic and lysogenic lifecycles confer different tradeoffs. It has long been suggested that lysogeny is a ‘safe’ strategy for phages in situations where hosts are scarce, with vertical inheritance preventing the dilution of phage particles in the absence of hosts (e.g., Berngruber et al., 2010). Meanwhile, lysis (and its more abundant production of phage particles) is advantageous when host availability is high.
Lytic microviruses (i.e., those lacking dif-motifs) in the human gut have been predicted to infect Bacteroidetes and other dominant members of that community (Shkoporov et al., 2019). However, the enterobacterial hosts of the gokushovirus prophages described in this study generally do not reach very high abundances in the gut microbiome, usually constituting less than 0.1% of the bacterial population (Human Microbiome Project Consortium, 2012), prompting the evolution of lysogeny. Perhaps due to the high mutation rate of ssDNA phages, typically two orders of magnitude higher than in dsDNA phages (Sanjuán et al., 2010), and an estimated substitution rate of 10−5/ nucleotides/day in the human gut (Minot et al., 2013), the de novo evolution of the XerCD-binding motifs appears to be common, with similar systems existing in other families of phage and mobile-elements (Das et al., 2013).
Given the current depths of microbiome sampling and sequencing, it is surprising that enterogokushoviruses have not previously been identified in human metagenomes. Even among the more than 100,000 sequenced enterobacterial genomes that are currently available, we detected fewer than 100 gokushovirus prophages, and no gokushovirus prophages were detected in the sequenced genomes of other bacteria. This rarity of gokushoviruses existing as prophages might result from the process by which they integrate into genomes: although possession of dif-motifs provides a simple, passive integration system, they also facilitate prophage excision (and as a result, continuous production of phage particles) in the absence of external stressors. In fact, XerCD-mediated removal of genetic elements has attained application in molecular biology (Bloor and Cranenburgh, 2006). To prevent premature XerCD-mediated excision, the Vibrio cholerae phage CTX secures itself as a prophage by destroying the dif site upon insertion (Val et al., 2005), whereas Vibrio prophages that retain their dif-motifs are only rarely detected (Das et al., 2011). The abundance of gokushoviruses in the environment, as apparent from their regularity in metagenomic datasets, suggests that they are only transient residents of bacterial chromosomes and usually occur in pseudolysogenic or lytic states in the wild.
The discovery of this group of phages offers several new directions for the study of the Microviridae. First, the presence of numerous and diverse gokushovirus prophages in a wide variety of E. coli strains now makes it possible to elucidate aspects of gokushovirus biology in a comparative evolutionary framework. Furthermore, demonstrating that the host ranges of the Gokushovirinae extends beyond intracellular bacteria increases the likelihood that additional members of this prevalent group of phages will be isolated from appropriate hosts. Additionally, the amplification of entire ssDNA phage genomes and subsequent transformation into appropriate hosts, as conducted in this study and previously with de novo synthesized phiX (Smith et al., 2003) can aid in the description of other Microviridae subfamilies. A promising target for this would be the Alpavirinae, which have been detected as prophages of Bacteroidetes but so far remain without isolates and denied official recognition (Roux et al., 2012). In conclusion, the value of isolating enterogokushoviruses goes beyond their abundance in nature and provides a genetically manipulable model system that will further the understanding of this group as a whole (Shkoporov and Hill, 2019).
Materials and methods
Detection and phylogeny of gokushovirus prophages and their hosts
Using blastp, we queried the NCBI nr database (April 2019) with the Chlamydia Phage four major capsid protein VP1 (NCBI Gene ID 3703676) and downloaded the complete genomes of 95 strains within the Enterobacteriaceae containing sequences returning E-values less than 0.0001 (Supplementary file 1). Chromosome contigs containing the VP1 gene were visually inspected in Geneious R9 (www.geneious.com) for the presence of prophage insertion boundaries by searching for identical 17 bp sequences within 5 kb regions upstream and downstream of the VP1 gene. Prophage genes were annotated with GLIMMER3 (Delcher et al., 2007) using default settings, specifying a minimum gene length of 110 bp and a maximum overlap of 50 bp. Initial alignments of prophage regions (ranging in size from 4047 to 4692 bp) were made with ClustalO 1.2.4 using standard settings (Sievers et al., 2011), and were refined manually to accommodate hypervariable regions and the phage insertion sites at the 3’ and 5’ ends of the alignment. Average pairwise nucleotide identity at each position in the alignment was calculated and visualized using Geneious R9. Maximum likelihood phylogenetic trees of enterobacterial prophages were generated with RAxML 8.0.26 (Stamatakis, 2014) using the GTR+GAMMA substitution model and 100 fast-bootstrap replicates and visualized with FigTree 1.4.3 (http://tree.bio.ed.ac.uk/software/figtree/).
To evaluate the distribution of prophage hosts within the broad diversity of E. coli at large, we produced core genome alignments of prophage hosts and representative genomes from the Escherichia coli reference (ECOR) collection (Ochman and Selander, 1984; Supplementary files 1 and 3) based on protein families satisfying a 30% amino-acid identity cutoff (USEARCH 11, Edgar, 2010), which were aligned in MUSCLE 3.8.31 (Edgar, 2004) as implemented in the BPGA 1.3 pipeline (Chaudhari et al., 2016). The maximum likelihood phylogenetic tree of core genome alignments was built with IQTree 1.6.2 (Nguyen et al., 2015), using the JTT substitution model (Jones et al., 1992) and 100 bootstrap replicates.
Recovering prophages from metagenomes
To assemble prophages from metagenomic datasets, we downloaded SRA files from BioProjects PRJEB29491 (viral human, Moreno-Gallego et al., 2019), PRJNA362629 (cellular bovine, unpublished), PRJNA290380 (cellular human, Kostic et al., 2015), PRJNA352475 (cellular human, Ferretti et al., 2018), PRJEB6456 (cellular human, Bäckhed et al., 2015), PRJNA385126 (viral human, Stockdale et al., 2018),PRJEB7774 (cellular human, Feng et al., 2015), and PRJNA545408 (human viral, Shkoporov et al., 2019). We performed initial trimming and quality filtering with BBDuk (Bushnell, 2014a) with options ktrim = r k = 23 mink = 11 hdist = 1 tbe tbo. Reads having a minimum nucleotide sequence identity of 50% to sequences of enterobacterial prophages, as determined by BBMap (Bushnell, 2014b), were assembled into contigs using MEGAHIT 1.1.3 (Li et al., 2015) implemented with default settings, and those contigs > 1000 bp were retained.
Phylogenetic analysis of gokushovirinae
We downloaded a total of 1284 metagenome-assembled genomes (MAGs) of microviruses (Supplementary file 4), which were then reannotated in GLIMMER3 (Delcher et al., 2007) using default settings, with a minimum gene length of 110 bp and a maximum overlap of 50 bp. We recovered homologues to the conserved major capsid protein VP1 and replication initiation protein VP4 in the set of metagenome-assembled microviruses using PSI-BLAST searches and querying with VP1 and VP4 proteins from detected enterobacterial gokushoviruses, gokushovirus genomes of Chlamydia, Spiroplasma and Bdellovibrio, and Bullavirinae phage phiX174. After individual protein alignments using Clustal Omega 1.2.4 (standard settings), we concatenated the VP1 and VP4 alignments, and removed all sites with >10% gaps to decrease the amount of spuriously aligned sites using Geneious R9, for an alignment of 485 aa in length. The initial phylogenetic tree of all microviruses was built with IQTree 1.6.2 using the LG+F+R10 substitution model as determined by ModelFinder (Kalyaanamoorthy et al., 2017), and branch support was tested using 1000 ultra-fast bootstrap replicates (Hoang et al., 2018) and 1000 SH-aLRT tests. Collapsing all branches with <95% bootstrap support and <80% SH-aLRT support yielded a single, well-supported clade containing all known Gokushovirinae, and all subsequent alignments and phylogenetic trees were refined by including only those genomes represented in this clade, with branch support assessed with 100 bootstrap replicates. To complement already existing annotations of gokushovirus genomes for the purpose of comparing gene order in various branches of the phylogeny, iterative tblastx searches of individual enterogokushovirus EC6098 genes agains members of select branches were conducted. Hits with e-value <0.001 (lower than initial searches used to identify prophages to account for the larger phylogenetic distance or analyzed genomes) were considered homologs to enterogokushoviral genes.
Identification of dif-motifs in gokushovirus MAGs
To search for dif-motifs in enterobacterial prophages, we first performed an alignment of all enterobacterial prophage dif-motif sequences in the curated set of bacterial dif-motifs from Kono et al. (2011) using Clustal Omega 1.2.4. We used the resulting alignment to build a Hidden-Markov-Model using hmmer 3.2.1 (Wheeler and Eddy, 2013) and performed an iterative search for dif-like motifs in all gokushovirus-like MAGs. Due to the variation in phage and bacterial dif-motifs, the variation in these motifs among bacteria, and the short length of the target sites, only confirmed dif-motifs of enterobacterial prophages reached an E-value cutoff >0.005. A large number of hits fell below of this threshold and were treated as potential dif-motifs if they possessed at least 15 bp identical to confirmed dif-motifs and occurred in the short non-coding regions of MAGs. Hits within coding regions were removed as likely representing false positives (as integration would interrupt coding sequences), with the exception of those within the N-terminus of VP4 (as occasionally observed in Escherichia gokushovirus prophages).
Resurrection and modification of prophages
DNA fragments representing gokushovirus prophages were amplified from E. coli and E. marmotae strains MOD1-EC2703, MOD1-EC5150, MOD1-EC6098 and MOD1-EC6163 (Supplementary file 1) with Phusion polymerase (NEB) from 10 ng of genomic DNA using primer pairs listed in Supplementary file 5 and under the following PCR conditions: 98°C for 3 min; 30 cycles of 98°C for 15 s, 50°C for 15 s, 72°C 2:30 min; followed by 72°C 10 min. Amplified fragments of ~4.5 kb corresponding to gokushovirus prophages were purified from agarose gels using the Monarch DNA Gel Extraction Kit (NEB) and eluted in 20 µl ddH2O. Blunt ends of the purified linear fragment were phosphorylated with T4 Polynucleotide Kinase (ThermoFisher) followed by overnight treatment with T4 DNA Ligase (NEB) to form circular genomes. Ligation mixtures were heat-inactivated, desalted, transformed into E. coli DH5α and incubated for 1 hr in 1 ml SOC medium at 37°C. After this recovery period, cultures were grown overnight in 5 ml of LB medium at 37°C with mild shaking (200 rpm). Viable bacteriophages were harvested by centrifuging the culture for 5 min at 5000 g to pellet bacterial cells and then by filtering the supernatant through 0.45 µm syringe filters. The presence and identity of phages were confirmed through standard spot assays (see below) and Sanger sequencing (see Supplementary file 5).
Plasmid construction and complementation of knockout mutants
To construct complementation plasmids, we first amplified the xerC and xerD genes from Escherichia coli BW25113 with primers XerC_fw_EcoRI and XerC_rev _SacI or XerD_fw_EcoRI and XerD_rev_SacI (Supplementary file 5) under the conditions listed above. PCR products were purified using the Monarch DNA Gel Extraction Kit (NEB) and eluted in 20 µl ddH2O. PCR products and expression plasmid pJN105 (Newman and Fuqua, 1999) were digested with EcoRI and SacI (NEB) for 37°C for 1 hr, followed by heat inactivation for 10 min at 80°C and overnight ligation at a 1:3 vector-to-insert ratio using T4 DNA Ligase (NEB) at 4°C. One microliter of ligation mixtures were transformed into electrocompetent BW25113ΔxerC or ΔxerD mutants, and transformants were selected for growth on LB agar plates supplemented with 10 µg/ml gentamycin.
Phage and bacterial culture
Environmental Escherichia strains MOD1-EC2703, MOD1-EC5150, MOD1-EC6098 and MOD1-EC6163, Escherichia coli K12 derivates DH5α and BW25113, and KEIO collection strains BW25113ΔxerC (KEIO Strain JW3784-1), BW25133ΔxerD (KEIO Strain JW2862-1) BW25113ΔfhuA (KEIO strain JW0146-2) were grown at 37°C in LB liquid media (supplemented with of 50 µg/ml kanamycin for the KEIO knockout strains). Expression of xerC or xerD genes in BW25113ΔxerC and BW25133ΔxerD containing pJN::xerC or pJN::xerD was induced by addition of 0.1% arabinose.
To prepare agar-overlays, cells from 100 µl of overnight culture were pelleted, resuspended in PBS, combined with 100 µl of phage and incubated at room temperature for 5 min prior to addition 3 ml of 0.6% LB-agarose and plating onto LB agar. To increase the phage concentrations, we harvested phage lysates from plates exhibiting confluent lysis after overnight growth at 37°C. Phage titers were determined by spotting dilutions of lysates onto agar-overlay plates with 100 µl of overnight cultures of host strains and incubating plates overnight at 37°C. Liquid-infection assays were performed in 96-well plates by adding 2 µl of phage lysate (~108 pfu/ml) to 200 µl of overnight culture diluted with LB to OD600 = 0.4 and measuring growth and lysis at 37°C with 200 rpm shaking at 15 min intervals on a Tecan Spark 10M plate reader.
Serial transfers experiments were performed by inoculating lysogenic colonies (as identified by PCR, below) in LB, diluting overnight cultures to OD600 = 0.7, and then transferring 2 µl of the diluted culture into 2 ml of LB. After 18–24 hr incubation at 37°C with shaking, 2 µl of culture was transferred to 2 ml of fresh LB. This process was repeated for 28 days, and each day, phages were titered as described above.
Detection of circularized phages and prophages
The presence of lysogens and circularized phage was determined by PCR assays of liquid overnight cultures from surviving colonies in agar-overlay assays showing confluent lysis, using primers MG1655_fw and MG1655_rev, which flank the bacterial dif-motif, and VP2_fw and VP5_rev, which anneal up- and downstream the phage dif-motif in circularized phage genomes (Supplementary file 5). Ratios of lysogenic to non-lysogenic cells in individual cultures were measured by re-streaking single lysogenic colonies three times, selecting and resuspending a single resulting colony in LB, and re-plating it onto LB-agar plates. Colonies were grown in liquid culture in a 96-well plate overnight and assayed by PCR with primers MG1655_fw and MG1655_rev to detect prophage integration, as described above. PCR products were resolved on 1% agarose gels, and the intensity of the PCR products representing integrated prophage and non-integrated sites was measured with ImageJ 1.52a (http://imagej.nih.gov/ij).
Prophage integration sites were confirmed by inverse PCR (Ochman et al., 1988) as follows: 10 ng of DNA derived from colonies of BW25113 and BW25113ΔxerC infected with either EC6098 wild type or EC6098ΔdifC were cut with restriction enzyme HindIII (NEB) for 1 hr at 37°C. Reactions were heat inactivated at 80°C for 10 min, and then circularized with T4 DNA Ligase (NEB) overnight at 4°C. Primer VP2_rev, binding the 3’-end of VP2 and facing upstream, and primers Circle_1 and Circle_2, which bind to conserved regions downstream of VP2 and face downstream, were used to amplify circularized ligation products using Phusion polymerase (NEB) under the following PCR conditions described above. PCR products were resolved on 1% agarose gels, and all detected bands were extracted and Sanger-sequenced. Phage integration was confirmed by the presence phage and bacterial sequence in a single read.
Electron microscopy
Two milliliters of high-titer phage lysate were resuspended in PBS, layered on top of a CsCl step gradient (2 ml each of p1.6 to p1.2 in PBS) and centrifuged in a Beckman Coulter Optima L-100k Ultracentrifuge at 24,000 rpm for four hours. After centrifugation, fractions were collected in 0.5 ml steps, and to determine which fractions contained phage, PCR was performed using primers nocode_fw and VP2_rev using 1 µl of each fraction as template. Fractions containing phage were desalted with an Amicon Ultra-2ml Ultracel-30k filter unit and resuspended in water. For electron microscopy, viral suspensions were pipetted onto carbon-coated grids, negatively stained with 2% uranyl acetate, and imaged with a Tecnai BioTwin TEM operated at 80kV.
Acknowledgements
The authors thank the Federal Department of Agriculture for providing Escherichia spp. strains and DNA, Bentley A Fane for valuable discussion, Steven Kyle and Zachary A Martinez for assistance in experiments, Dwight Romanovicz for assistance with electron microscopy, and Kim Hammond for figure design. This study was funded by NIH award R35GM118038 to HO. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.’
Funding Statement
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Contributor Information
Paul C Kirchberger, Email: pkirchberger@utexas.edu.
María Mercedes Zambrano, CorpoGen, Colombia.
Karla Kirkegaard, Stanford University School of Medicine, United States.
Funding Information
This paper was supported by the following grant:
National Institutes of Health 26-1612-1250 to Howard Ochman.
Additional information
Competing interests
No competing interests declared.
Author contributions
Conceptualization, Data curation, Formal analysis, Investigation, Visualization, Methodology.
Resources, Supervision, Funding acquisition, Validation, Project administration.
Additional files
Data availability
The data used in this publication (accession numbers, sequences and alignments) are available in the manuscript, its supplementary files and on datadryad.org: https://doi.org/10.5061/dryad.z8w9ghx7s.
The following dataset was generated:
Kirchberger PC. 2020. Resurrection of a Global, Metagenomically Defined Gokushovirus. Dryad Digital Repository.
The following previously published datasets were used:
Moreno-Gallego JL, Chou SP, Di Rienzi SC, Goodrich JK, Spector TD, Bell JT, Youngblut ND, Hewson I, Reyes A, Ley RE. 2019. The virome in adult monozygotic twins with concordant or discordant gut microbiomes. NCBI BioProject. PRJEB29491
University of Alberta. 2018. metagenomic sequencing of calf gut content-associated microbiota. NCBI BioProject. PRJNA362629
Kostic AD, Gevers D, Siljander H, Vatanen T, Hyötyläinen T, Hämäläinen AM, Peet A, Tillmann V, Pöhö P, Mattila I, Lähdesmäki H. 2015. A longitudinal analysis of the developing gut microbiome in infants from Finland, Estonia, and Russian Karelia. NCBI BioProject. PRJNA290380
Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Armanini F, Truong DT, Manara S, Zolfo M, Beghini F. 2016. Mother-infant microbiome vertical transmission. NCBI BioProject. PRJNA352475
Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT. 2015. Dynamics and Stabilization of the Human Gut Microbiome during the First Year of Life. NCBI BioProject. PRJEB6456
Stockdale SR, Ryan FJ, McCann A, Dalmasso M, Ross PR, Hill C. 2018. Viral dark matter in the gut virome of elderly humans. NCBI BioProject. PRJNA385126
Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, Zhang D, Xia H, Xu X, Jie Z, Su L. 2015. Gut microbiome development along the colorectal adenoma-carcinoma sequence. NCBI BioProject. PRJEB7774
Shkoporov A, Clooney AG, Sutton TD, Ryan FJ, Daly KM, Nolan JA, McDonnell SA, Khokhlova EV, Draper LA, Forde A, Guerin E. 2019. Longitudinal study of the human gut virome. NCBI BioProject. PRJNA545408
References
- Bäckhed F, Roswall J, Peng Y, Feng Q, Jia H, Kovatcheva-Datchary P, Li Y, Xia Y, Xie H, Zhong H, Khan MT, Zhang J, Li J, Xiao L, Al-Aama J, Zhang D, Lee YS, Kotowska D, Colding C, Tremaroli V, Yin Y, Bergman S, Xu X, Madsen L, Kristiansen K, Dahlgren J, Wang J, Jun W. Dynamics and stabilization of the human gut microbiome during the first year of life. Cell Host & Microbe. 2015;17:690–703. doi: 10.1016/j.chom.2015.04.004. [DOI] [PubMed] [Google Scholar]
- Berngruber TW, Weissing FJ, Gandon S. Inhibition of superinfection and the evolution of viral latency. Journal of Virology. 2010;84:10200–10208. doi: 10.1128/JVI.00865-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakely G, May G, McCulloch R, Arciszewska LK, Burke M, Lovett ST, Sherratt DJ. Two related recombinases are required for site-specific recombination at dif and cer in E. coli K12. Cell. 1993;75:351–361. doi: 10.1016/0092-8674(93)80076-Q. [DOI] [PubMed] [Google Scholar]
- Bloor AE, Cranenburgh RM. An efficient method of selectable marker gene excision by xer recombination for gene replacement in bacterial chromosomes. Applied and Environmental Microbiology. 2006;72:2520–2525. doi: 10.1128/AEM.72.4.2520-2525.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brentlinger KL, Hafenstein S, Novak CR, Fane BA, Borgon R, McKenna R, Agbandje-McKenna M. Microviridae, a family divided: isolation, characterization, and genome sequence of phiMH2K, a bacteriophage of the obligate intracellular parasitic bacterium Bdellovibrio bacteriovorus. Journal of Bacteriology. 2002;184:1089–1094. doi: 10.1128/jb.184.4.1089-1094.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryson SJ, Thurber AR, Correa AM, Orphan VJ, Vega Thurber R. A novel sister clade to the enterobacteria microviruses (family Microviridae) identified in methane seep sediments. Environmental Microbiology. 2015;17:3708–3721. doi: 10.1111/1462-2920.12758. [DOI] [PubMed] [Google Scholar]
- Bushnell B. BBTools software package. 2014a http://sourceforge.net/projects/bbmap
- Bushnell B. BBMap: a fast, accurate, splice-aware aligner (No LBNL-7065E). Lawrence Berkeley National Laboratories (LBNL).2014b. [Google Scholar]
- Castillo F, Benmohamed A, Szatmari G. Xer site specific recombination: double and single recombinase systems. Frontiers in Microbiology. 2017;8:453. doi: 10.3389/fmicb.2017.00453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhari NM, Gupta VK, Dutta C. BPGA- an ultra-fast pan-genome analysis pipeline. Scientific Reports. 2016;6:24373. doi: 10.1038/srep24373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Li Y, Zhang K, Wang H. Whole-Genome sequence of Phage-Resistant strain Escherichia coli DH5α. Genome Announcements. 2018;6:e00097. doi: 10.1128/genomeA.00097-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chipman PR, Agbandje-McKenna M, Renaudin J, Baker TS, McKenna R. Structural analysis of the Spiroplasma virus, SpV4: implications for evolutionary variation to obtain host diversity among the Microviridae. Structure. 1998;6:135–145. doi: 10.1016/S0969-2126(98)00016-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creasy A, Rosario K, Leigh B, Dishaw L, Breitbart M. Unprecedented diversity of ssDNA phages from the family Microviridae Detected within the Gut of a Protochordate Model Organism (Ciona robusta) Viruses. 2018;10:404. doi: 10.3390/v10080404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Bischerour J, Barre FX. VGJphi integration and excision mechanisms contribute to the genetic diversity of Vibrio cholerae epidemic strains. PNAS. 2011;108:2516–2521. doi: 10.1073/pnas.1017061108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das B, Martínez E, Midonet C, Barre FX. Integrative mobile elements exploiting xer recombination. Trends in Microbiology. 2013;21:23–30. doi: 10.1016/j.tim.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Delcher AL, Bratke KA, Powers EC, Salzberg SL. Identifying bacterial genes and endosymbiont DNA with glimmer. Bioinformatics. 2007;23:673–679. doi: 10.1093/bioinformatics/btm009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnues C, Rodriguez-Brito B, Rayhawk S, Kelley S, Tran T, Haynes M, Liu H, Furlan M, Wegley L, Chau B, Ruan Y, Hall D, Angly FE, Edwards RA, Li L, Thurber RV, Reid RP, Siefert J, Souza V, Valentine DL, Swan BK, Breitbart M, Rohwer F. Biodiversity and biogeography of phages in modern stromatolites and thrombolites. Nature. 2008;452:340–343. doi: 10.1038/nature06735. [DOI] [PubMed] [Google Scholar]
- Diemer GS, Stedman KM. Modeling Microvirus capsid protein evolution utilizing metagenomic sequence data. Journal of Molecular Evolution. 2016;83:38–49. doi: 10.1007/s00239-016-9751-y. [DOI] [PubMed] [Google Scholar]
- Doore SM, Fane BA. The Microviridae: diversity, assembly, and experimental evolution. Virology. 2016;491:45–55. doi: 10.1016/j.virol.2016.01.020. [DOI] [PubMed] [Google Scholar]
- Edgar RC. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Research. 2004;32:1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar RC. Search and clustering orders of magnitude faster than BLAST. Bioinformatics. 2010;26:2460–2461. doi: 10.1093/bioinformatics/btq461. [DOI] [PubMed] [Google Scholar]
- Feng Q, Liang S, Jia H, Stadlmayr A, Tang L, Lan Z, Zhang D, Xia H, Xu X, Jie Z, Su L, Li X, Li X, Li J, Xiao L, Huber-Schönauer U, Niederseer D, Xu X, Al-Aama JY, Yang H, Wang J, Kristiansen K, Arumugam M, Tilg H, Datz C, Wang J. Gut microbiome development along the colorectal adenoma-carcinoma sequence. Nature Communications. 2015;6:6528. doi: 10.1038/ncomms7528. [DOI] [PubMed] [Google Scholar]
- Ferretti P, Pasolli E, Tett A, Asnicar F, Gorfer V, Fedi S, Armanini F, Truong DT, Manara S, Zolfo M, Beghini F, Bertorelli R, De Sanctis V, Bariletti I, Canto R, Clementi R, Cologna M, Crifò T, Cusumano G, Gottardi S, Innamorati C, Masè C, Postai D, Savoi D, Duranti S, Lugli GA, Mancabelli L, Turroni F, Ferrario C, Milani C, Mangifesta M, Anzalone R, Viappiani A, Yassour M, Vlamakis H, Xavier R, Collado CM, Koren O, Tateo S, Soffiati M, Pedrotti A, Ventura M, Huttenhower C, Bork P, Segata N. Mother-to-Infant microbial transmission from different body sites shapes the developing infant gut microbiome. Cell Host & Microbe. 2018;24:133–145. doi: 10.1016/j.chom.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garner SA, Everson JS, Lambden PR, Fane BA, Clarke IN. Isolation, molecular characterisation and genome sequence of a bacteriophage (Chp3) from Chlamydophila pecorum. Virus Genes. 2004;28:207–214. doi: 10.1023/B:VIRU.0000016860.53035.f3. [DOI] [PubMed] [Google Scholar]
- Hoang DT, Chernomor O, von Haeseler A, Minh BQ, Vinh LS. UFBoot2: improving the ultrafast bootstrap approximation. Molecular Biology and Evolution. 2018;35:518–522. doi: 10.1093/molbev/msx281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Human Microbiome Project Consortium Structure, function and diversity of the healthy human microbiome. Nature. 2012;486:207–214. doi: 10.1038/nature11234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DT, Taylor WR, Thornton JM. The rapid generation of mutation data matrices from protein sequences. Bioinformatics. 1992;8:275–282. doi: 10.1093/bioinformatics/8.3.275. [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS. ModelFinder: fast model selection for accurate phylogenetic estimates. Nature Methods. 2017;14:587–589. doi: 10.1038/nmeth.4285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Bae JW. Amplification methods Bias metagenomic libraries of uncultured single-stranded and double-stranded DNA viruses. Applied and Environmental Microbiology. 2011;77:7663–7668. doi: 10.1128/AEM.00289-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King AM, Lefkowitz E, Adams MJ, Carstens EB. Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses. San Diego: Elsevier; 2011. [Google Scholar]
- Kono N, Arakawa K, Tomita M. Comprehensive prediction of chromosome dimer resolution sites in bacterial genomes. BMC Genomics. 2011;12:19. doi: 10.1186/1471-2164-12-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostic AD, Gevers D, Siljander H, Vatanen T, Hyötyläinen T, Hämäläinen AM, Peet A, Tillmann V, Pöhö P, Mattila I, Lähdesmäki H, Franzosa EA, Vaarala O, de Goffau M, Harmsen H, Ilonen J, Virtanen SM, Clish CB, Orešič M, Huttenhower C, Knip M, Xavier RJ, DIABIMMUNE Study Group The dynamics of the human infant gut microbiome in development and in progression toward type 1 diabetes. Cell Host & Microbe. 2015;17:260–273. doi: 10.1016/j.chom.2015.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupovic M, Dutilh BE, Adriaenssens EM, Wittmann J, Vogensen FK, Sullivan MB, Rumnieks J, Prangishvili D, Lavigne R, Kropinski AM, Klumpp J, Gillis A, Enault F, Edwards RA, Duffy S, Clokie MR, Barylski J, Ackermann HW, Kuhn JH. Taxonomy of prokaryotic viruses: update from the ICTV bacterial and archaeal viruses subcommittee. Archives of Virology. 2016;161:1095–1099. doi: 10.1007/s00705-015-2728-0. [DOI] [PubMed] [Google Scholar]
- Krupovic M, Forterre P. Microviridae goes temperate: microvirus-related proviruses reside in the genomes of bacteroidetes. PLOS ONE. 2011;6:e19893. doi: 10.1371/journal.pone.0019893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krupovic M, Forterre P. Single-stranded DNA viruses employ a variety of mechanisms for integration into host genomes. Annals of the New York Academy of Sciences. 2015;1341:41–53. doi: 10.1111/nyas.12675. [DOI] [PubMed] [Google Scholar]
- Labonté JM, Suttle CA. Metagenomic and whole-genome analysis reveals new lineages of gokushoviruses and biogeographic separation in the sea. Frontiers in Microbiology. 2013;4:404. doi: 10.3389/fmicb.2013.00404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Liu CM, Luo R, Sadakane K, Lam TW. MEGAHIT: an ultra-fast single-node solution for large and complex metagenomics assembly via succinct de bruijn graph. Bioinformatics. 2015;31:1674–1676. doi: 10.1093/bioinformatics/btv033. [DOI] [PubMed] [Google Scholar]
- Lim ES, Zhou Y, Zhao G, Bauer IK, Droit L, Ndao IM, Warner BB, Tarr PI, Wang D, Holtz LR. Early life dynamics of the human gut virome and bacterial microbiome in infants. Nature Medicine. 2015;21:1228–1234. doi: 10.1038/nm.3950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manrique P, Dills M, Young M. The human gut phage community and its implications for health and disease. Viruses. 2017;9:141. doi: 10.3390/v9060141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel A, Clermont O, Denamur E, Tenaillon O. Bacteriophage PhiX174's ecological niche and the flexibility of its Escherichia coli lipopolysaccharide receptor. Applied and Environmental Microbiology. 2010;76:7310–7313. doi: 10.1128/AEM.02721-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Midonet C, Barre FX. Xer site-specific recombination: promoting verticaland horizontal transmission of genetic information. In: Craig NL, editor. Mobile DNA III. Washington: American Society of Microbiology Press; 2015. pp. 163–182. [Google Scholar]
- Minot S, Bryson A, Chehoud C, Wu GD, Lewis JD, Bushman FD. Rapid evolution of the human gut virome. PNAS. 2013;110:12450–12455. doi: 10.1073/pnas.1300833110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno-Gallego JL, Chou SP, Di Rienzi SC, Goodrich JK, Spector TD, Bell JT, Youngblut ND, Hewson I, Reyes A, Ley RE. Virome diversity correlates with intestinal microbiome diversity in adult monozygotic twins. Cell Host & Microbe. 2019;25:261–272. doi: 10.1016/j.chom.2019.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman JR, Fuqua C. Broad-host-range expression vectors that carry the l-arabinose-inducible Escherichia coli araBAD promoter and the araC regulator. Gene. 1999;227:197–203. doi: 10.1016/S0378-1119(98)00601-5. [DOI] [PubMed] [Google Scholar]
- Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ. IQ-TREE: a fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution. 2015;32:268–274. doi: 10.1093/molbev/msu300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Gerber AS, Hartl DL. Genetic applications of an inverse polymerase chain reaction. Genetics. 1988;120:621–623. doi: 10.1093/genetics/120.3.621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochman H, Selander RK. Standard reference strains of Escherichia coli from natural populations. Journal of Bacteriology. 1984;157:690–693. doi: 10.1128/JB.157.2.690-693.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quaiser A, Dufresne A, Ballaud F, Roux S, Zivanovic Y, Colombet J, Sime-Ngando T, Francez AJ. Diversity and comparative genomics of Microviridae in Sphagnum- dominated peatlands. Frontiers in Microbiology. 2015;6:375. doi: 10.3389/fmicb.2015.00375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Read TD, Brunham RC, Shen C, Gill SR, Heidelberg JF, White O, Hickey EK, Peterson J, Utterback T, Berry K, Bass S, Linher K, Weidman J, Khouri H, Craven B, Bowman C, Dodson R, Gwinn M, Nelson W, DeBoy R, Kolonay J, McClarty G, Salzberg SL, Eisen J, Fraser CM. Genome sequences of Chlamydia trachomatis MoPn and Chlamydia pneumoniae AR39. Nucleic Acids Research. 2000;28:1397–1406. doi: 10.1093/nar/28.6.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard B, Garnier M, Bové JM. Characterization of SpV3 from spiroplasmas and discovery of a new Spiroplasma virus (SpV4). International Organization for Mycoplasmology Meeting, Custer, États-Unis, 3/9 September.1980. [Google Scholar]
- Rosenwald AG, Murray B, Toth T, Madupu R, Kyrillos A, Arora G. Evidence for horizontal gene transfer between Chlamydophila pneumoniae and Chlamydia phage. Bacteriophage. 2014;4:e965076. doi: 10.4161/21597073.2014.965076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rotman E, Kouzminova E, Plunkett G, Kuzminov A. Genome of enterobacteriophage lula/phi80 and insights into its ability to spread in the laboratory environment. Journal of Bacteriology. 2012;194:6802–6817. doi: 10.1128/JB.01353-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S, Krupovic M, Poulet A, Debroas D, Enault F. Evolution and diversity of the Microviridae viral family through a collection of 81 new complete genomes assembled from virome reads. PLOS ONE. 2012;7:e40418. doi: 10.1371/journal.pone.0040418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux S, Solonenko NE, Dang VT, Poulos BT, Schwenck SM, Goldsmith DB, Coleman ML, Breitbart M, Sullivan MB. Towards quantitative viromics for both double-stranded and single-stranded DNA viruses. PeerJ. 2016;4:e2777. doi: 10.7717/peerj.2777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanjuán R, Nebot MR, Chirico N, Mansky LM, Belshaw R. Viral mutation rates. Journal of Virology. 2010;84:9733–9748. doi: 10.1128/JVI.00694-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkoporov AN, Khokhlova EV, Fitzgerald CB, Stockdale SR, Draper LA, Ross RP, Hill C. ΦCrAss001 represents the most abundant bacteriophage family in the human gut and infects Bacteroides intestinalis. Nature Communications. 2018;9:4781. doi: 10.1038/s41467-018-07225-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shkoporov AN, Clooney AG, Sutton TDS, Ryan FJ, Daly KM, Nolan JA, McDonnell SA, Khokhlova EV, Draper LA, Forde A, Guerin E, Velayudhan V, Ross RP, Hill C. The human gut virome is highly diverse, stable, and individual specific. Cell Host & Microbe. 2019;26:527–541. doi: 10.1016/j.chom.2019.09.009. [DOI] [PubMed] [Google Scholar]
- Shkoporov AN, Hill C. Bacteriophages of the human gut: the “Known Unknown” of the Microbiome. Cell Host & Microbe. 2019;25:195–209. doi: 10.1016/j.chom.2019.01.017. [DOI] [PubMed] [Google Scholar]
- Sievers F, Wilm A, Dineen D, Gibson TJ, Karplus K, Li W, Lopez R, McWilliam H, Remmert M, Söding J, Thompson JD, Higgins DG. Fast, scalable generation of high-quality protein multiple sequence alignments using clustal omega. Molecular Systems Biology. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siringan P, Connerton PL, Cummings NJ, Connerton IF. Alternative bacteriophage life cycles: the carrier state of Campylobacter jejuni. Open Biology. 2014;4:130200. doi: 10.1098/rsob.130200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Śliwa-Dominiak J, Suszyńska E, Pawlikowska M, Deptuła W. Chlamydia bacteriophages. Archives of Microbiology. 2013;195:765–771. doi: 10.1007/s00203-013-0912-8. [DOI] [PubMed] [Google Scholar]
- Smith HO, Hutchison CA, Pfannkoch C, Venter JC. Generating a synthetic genome by whole genome assembly: X174 bacteriophage from synthetic oligonucleotides. PNAS. 2003;100:15440–15445. doi: 10.1073/pnas.2237126100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song S, Guo Y, Kim J-S, Wang X, Wood TK. Phages mediate bacterial Self-Recognition. Cell Reports. 2019;27:737–749. doi: 10.1016/j.celrep.2019.03.070. [DOI] [PubMed] [Google Scholar]
- Stamatakis A. RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics. 2014;30:1312–1313. doi: 10.1093/bioinformatics/btu033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockdale SR, Ryan FJ, McCann A, Dalmasso M, Ross PR, Hill C. Viral dark matter in the gut virome of elderly humans. Preprints. 2018 doi: 10.20944/preprints201807.0128.v1. [DOI]
- Székely AJ, Breitbart M. Single-stranded DNA phages: from early molecular biology tools to recent revolutions in environmental microbiology. FEMS Microbiology Letters. 2016;363:fnw027. doi: 10.1093/femsle/fnw027. [DOI] [PubMed] [Google Scholar]
- Thurber RV, Haynes M, Breitbart M, Wegley L, Rohwer F. Laboratory procedures to generate viral metagenomes. Nature Protocols. 2009;4:470–483. doi: 10.1038/nprot.2009.10. [DOI] [PubMed] [Google Scholar]
- Tikhe CV, Husseneder C. Metavirome sequencing of the termite gut reveals the presence of an unexplored bacteriophage community. Frontiers in Microbiology. 2018;8:2548. doi: 10.3389/fmicb.2017.02548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker KP, Parsons R, Symonds EM, Breitbart M. Diversity and distribution of single-stranded DNA phages in the North Atlantic Ocean. The ISME Journal. 2011;5:822–830. doi: 10.1038/ismej.2010.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Val ME, Bouvier M, Campos J, Sherratt D, Cornet F, Mazel D, Barre FX. The single-stranded genome of phage CTX is the form used for integration into the genome of Vibrio cholerae. Molecular Cell. 2005;19:559–566. doi: 10.1016/j.molcel.2005.07.002. [DOI] [PubMed] [Google Scholar]
- Wang X, Kim Y, Ma Q, Hong SH, Pokusaeva K, Sturino JM, Wood TK. Cryptic prophages help Bacteria cope with adverse environments. Nature Communications. 2010;1:1–9. doi: 10.1038/ncomms1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Ling Y, Shan T, Yang S, Xu H, Deng X, Delwart E, Zhang W. Gut virome of mammals and birds reveals high genetic diversity of the family Microviridae. Virus Evolution. 2019;5:vez013. doi: 10.1093/ve/vez013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheeler TJ, Eddy SR. nhmmer: DNA homology search with profile HMMs. Bioinformatics. 2013;29:2487–2489. doi: 10.1093/bioinformatics/btt403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Chen F. The smallest ssDNA phage infecting a marine bacterium. Environmental Microbiology. 2019;21:1916–1928. doi: 10.1111/1462-2920.14394. [DOI] [PubMed] [Google Scholar]
- Zheng Q, Chen Q, Xu Y, Suttle CA, Jiao N. A virus infecting marine photoheterotrophic alphaproteobacteria (Citromicrobium spp.) Defines a New Lineage of ssDNA Viruses. Frontiers in Microbiology. 2018;9:1418. doi: 10.3389/fmicb.2018.01418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Z, Alikhan NF, Mohamed K, Fan Y, Achtman M, Agama Study Group The EnteroBase user's guide, with case studies on Salmonella transmissions, Yersinia pestis phylogeny, and Escherichia core genomic diversity. Genome Research. 2020;30:138–152. doi: 10.1101/gr.251678.119. [DOI] [PMC free article] [PubMed] [Google Scholar]