Abstract
Background
One in twenty adolescents experience excessive worry and evidence-based psychological therapies are not sufficiently widespread to reach most of those affected. In this multiple baseline evaluation, we assess the feasibility and preliminary efficacy of a scalable, online cognitive-behavioral intervention for adolescents with excessive worry (BIP worry).
Methods
Thirteen adolescents (age 13–17) with excessive worry underwent the 10-week online BIP worry intervention. The treatment also included an online intervention for parents. Completion rates, treatment satisfaction, and adverse events were measures of feasibility. Clinical outcomes included worry severity, symptoms of other anxiety and depression, and general functioning. To control for time and spontaneous fluctuations in symptoms, adolescents were randomized to a 2-, 6-, or 10-week baseline phase prior to treatment. A short measure of worry severity was administered weekly during the baseline and treatment phases. Outcomes were assessed before the baseline-phase, at pre-treatment, post-treatment, and at 1- and 3-month follow-ups.
Results
Twelve of 13 included adolescents, together with their parents, participated in BIP worry, with a mean completion rate of 9.8 of the 10 treatment modules. Adolescents reported an average of 4.4 exposures per week as homework during treatment. High levels of treatment adherence, credibility, and satisfaction, and no serious adverse events were reported. Therapists averaged 21 min per week communicating with each family. Linear mixed effects models indicated significant improvements in worry, anxiety, and general functioning from pre- to post-treatment, with these gains maintained at 1- and 3-months follow-up. Reductions in worry severity during treatment were significantly larger than during the baseline phase. The results from the multiple baseline evaluation suggested an association between the introduction of the BIP worry intervention and subsequent symptom change for some but not all adolescents.
Conclusions
BIP worry is a feasible and potentially effective treatment. As the treatment is scalable and involves limited therapist contact, it represents a low-cost method for treating adolescents with excessive worry and anxiety. Further investigation under randomized controlled trial (RCT) conditions is warranted.
Keywords: Adolescent, anxiety disorders, cognitive behavioral therapy (CBT), patient portals, treatment outcome
Introduction
Adolescence has been identified as a challenging developmental phase, with significantly increased stress arising from major changes both physiologically and socially (1). It is also the peak time for onset of psychiatric disorders that carry on into adulthood (2) and there is now widespread recognition that greater efforts are needed to address risk and protective factors in, and to develop effective treatments for, adolescents (3). However, the scale of the problem is such that there is insufficient capacity within healthcare and social systems to provide interventions to at-risk and affected individuals (3). This is particularly true of face-to-face psychological interventions, and alternative modes of delivery, including via the internet, are needed to address this capacity issue (4-6). This study evaluated an online intervention for excessive worry in adolescents.
Worries involve repetitive thoughts and images about future events whose outcomes are uncertain and/or negative; the worries involve negative affect and are often perceived as uncontrollable (7). Worrying about potential future failures and/or other negative outcomes is common among adolescents but epidemiological and longitudinal studies have found that excessive worries throughout childhood can interfere with daily functioning and increase the risk for psychiatric disorders (8-10). While excessive worry is the cardinal feature of Generalized Anxiety Disorder (GAD) (11) it is recognized as a primary underlying feature of all anxiety disorders in children and adolescents (12) and more broadly as a transdiagnostic construct in anxiety and affective disorders in adults (13). The mechanisms through which worry acts to impair functioning in children and adolescents remain unclear, but there is a growing body of evidence suggesting that frequent worrying can negatively alter cortisol responses and impair executive functioning in children and adolescents (14,15). Given the above, developing interventions that specifically target worry may have benefit for at-risk adolescents.
Our research group has developed an exposure-based psychological intervention for adolescents with excessive worry (“BIP worry”). As delivered in a face-to-face format, the content of BIP worry was found to be acceptable and efficacious in a pilot feasibility study with 12 adolescents with a variety of anxiety disorders (primarily GAD) and depressive symptoms (16). The next step, to ensure the scalability of the program, is to assess the feasibility and efficacy of this treatment in an online (only) format, thus making it more accessible to at-risk and/or affected adolescents. For the purposes of this feasibility study, only adolescents who had a diagnosis of GAD were included. This was done to test whether the treatment was acceptable and effective for adolescents for whom excessive worry was clearly impairing functioning. Based on the result of the face-to-face pilot study (16), we hypothesized that: (I) the majority of the adolescents (and their parents) would complete the BIP worry intervention and report high levels of treatment satisfaction; (II) the adolescents who completed the treatment would show statistically significant reductions in worry, other anxiety symptoms, and impaired functioning; and (III) a multiple baseline evaluation would show a decrease in adolescent-rated worry only when the intervention was introduced.
Methods
Study design
This study was a non-concurrent, multiple baseline design evaluation (17) with adolescents randomly allocated to a 2-, 6- or 10-week baseline assessment phase before receiving the BIP worry intervention. Using baselines of different lengths allowed us to investigate if reductions in worry could be specifically attributed to the intervention rather than the passage of time. Data collected during the baseline and treatment phases were compared, meaning adolescents acted as their own controls throughout the study. An independent researcher using a block randomization method conducted the randomization of participants to the different baseline allocations.
Participants
Thirteen adolescents (aged 13–17 years) with excessive worry were included. Excessive worry was defined as a score of ≥30 on the Penn State Worry Questionnaire for Children (PSWQ-C) (18). There is no agreed upon clinical cut off for the PSWQ-C; a score ≥30 was chosen based on previous studies comparing total scores on the PSWQ-C in normative and clinical samples (17,18) and treatment studies of youth with GAD (16,19-21). Additional inclusion criteria were: (I) the ability to read and write in Swedish; (II) having a parent or legal guardian able to co-participate in treatment; (III) fulfilling the DSM-5 criteria for GAD (11); (IV) if on psychotropic medication, having had a stable dose for six weeks prior to study inclusion; (V) and not currently receiving another psychological treatment for any disorder. Exclusion criteria were: (I) having symptoms other than excessive worry that were in need of more urgent treatment; (II) clinical indicators of autism, schizophrenia, bipolar disorder, anorexia nervosa, bulimia nervosa, or substance use disorder; (III) an elevated risk of suicide; (IV) ongoing domestic violence within the family; (V) and having completed >5 sessions of cognitive behavioral therapy (CBT), including in vivo exposure, for any anxiety disorder within the last 6 months. Participant characteristics are presented in Table 1.
Table 1. Demographic and clinical features of study participants (N=13).
| Characteristic | M/N | SD/% |
|---|---|---|
| Years of age, M (SD) | 14.6 | 1.4 |
| Gender, N (%) | ||
| Girls | 11 | 84.6 |
| Boys | 2 | 15.4 |
| Age of onset, M (SD) | 11.2 | 2.4 |
| Duration in years, M (SD) | 3.5 | 2.6 |
| Prior psychological treatment, N (%) | ||
| Non-CBT intervention | 6 | 46.2 |
| CBT | 5 | 38.5 |
| Ongoing psychotropic medication, N (%) | 1 | 7.7 |
| Comorbid disorders (CSR ≥4), N (%) | ||
| Specific phobia | 2 | 15.4 |
| Obsessive compulsive disorder | 2 | 15.4 |
| Panic disorder | 1 | 7.7 |
| Frequency of comorbid disorders, N (%) | ||
| 0 | 9 | 69.2 |
| 1 | 3 | 23.1 |
| 2 | 1 | 7.7 |
CBT, cognitive behavioral therapy; CSR, Clinician Severity Rating.
Measures
Feasibility, adherence, treatment credibility, and satisfaction
Feasibility was indicated by the number of online modules completed by the adolescents and their parents, and the number of adverse events. Occurrence of adverse events was assessed using a 21-item version of the Negative Effects Questionnaire, adapted for adolescents and parents (NEQ-C/P) (22). Adolescents and parents answer yes/no to having experienced a number of negative effects during treatment, rate their negative impact and report whether the negative effect can be attributed to the treatment (yes/no).
Adolescent’s adherence to BIP worry was assessed in two ways. First, the treating clinician completed the Internet Interventions Patient Adherence Scale (IIPAS) (23) after modules 5 (mid-treatment) and 10 (post-treatment). Each item is rated on a 4-point scale, higher scores indicate greater adherence. Second, adherence was indexed by the proportion of weeks that the adolescent reported having completed at least one exposure task (range from 0–100%). Treatment completion was defined as the adolescent and parent having accessed and worked with at least six of the ten BIP worry modules, since participants by then had been introduced to the most important treatment content.
A version of the 5-item Treatment Credibility and Expectancy Scale (24), adapted for children and parents (C-Scale-C/P) assessed treatment credibility. Each item is rated on a 10-point scale with higher scores indicating greater treatment credibility. Treatment satisfaction was measured with the 8-item Client Satisfaction Questionnaire (25), also adapted for children and parents (CSQ-C/P). Each item is rated on a 4-point scale; higher scores indicate higher treatment satisfaction. Each time a therapist logged in the online treatment platform to communicate with an adolescent or a parent, the platform automatically recorded therapist time, i.e., the time that the therapists spent communicating with each adolescent and parent. This automatic recording made it possible to calculate the exact time the therapists spent treating each participant during the treatment period.
Clinical outcome measures
Worry severity was assessed using the 14-item Penn State Worry Questionnaire for Children—Child and Parent Report Versions (PSWQ-C/P) (17). Each item is rated on a 4-point scale, yielding a total score of 0–42; higher scores indicate higher worry severity. Worry severity was also assessed weekly using the 5-item Brief Penn State Worry Questionnaire (brief PSWQ) (26). Each item is rated on the same 0–3 scale as the PSWQ-C, yielding a total score of 0–15. The 47-item Revised Children’s Anxiety and Depression Scale, Child and Parent Versions (RCADS-C/P) (27) is comprised of six anxiety subscales and one depression subscale. In this study, only the total scores for the anxiety subscales (omitting the GAD subscale as it mainly assesses level of worry) and the depression subscales were used. Each item on the RCADS is rated on a 4-point scale; yielding a total score of 0–90 on the 5 anxiety subscales used in this study, and a total score of 0–30 on the depression subscale; higher scores indicate greater anxiety and depression. The Work and Social Adjustment Scale—Child and Parent Versions (WSAS-Y-C/P), a 5-item scale adapted to youth and their parents, measured functional impairment (28,29). Each item is rated 0 to 8 with higher scores indicating more impaired functioning. The Children’s Global Assessment Scale (CGAS) is a single-item, clinician rated scale which yields a score of 0–100, higher scores indicate better functioning (30). The Clinical Global Impression—Improvement scale (CGI-I) is a clinician measure of improvement after treatment which yields a score of 1 (very much improved) to 7 (very much worse) (31).
Diagnostic assessment
Psychiatric disorders were assessed using the Anxiety Disorder Interview Schedule for DSM-IV, child and parent version (ADIS C/P) (32). Adolescents who meet the symptom criteria for GAD (or another disorder) then have the severity of the disorder rated by the interviewer using the Clinician Severity Rating (CSR). Using a 9-point scale [0–8], the interviewer rates the degree to which the symptoms impact the person’s overall levels of distress and functioning. A score ≥4 indicates that the individual has met both the symptom and impairment criteria according to DSM. In this trial, adolescents and parents were interviewed together using the ADIS-C.
Procedure
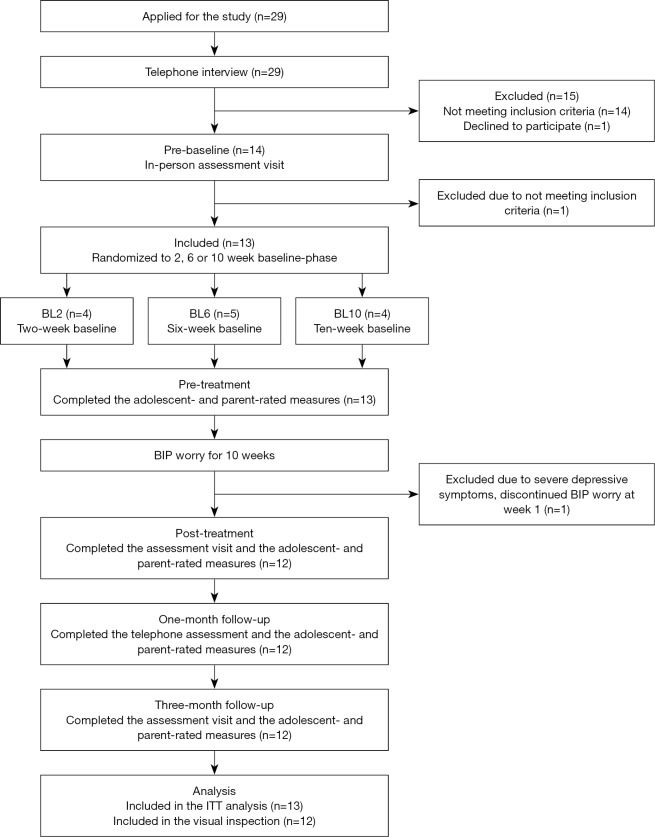
The study was conducted at the clinical research unit of the Child and Adolescent Mental Health Services in Stockholm, Sweden, approved by the regional ethics review board in Stockholm, and registered with www.clinicaltrials.gov (NCT03469453). Participants were recruited through advertisement on the clinic website and in a local newspaper and could self-refer via a secure internet platform where the adolescent completed the PSWQ-C (17). Adolescents who scored ≥30 on the PSWQ-C, and met all inclusion and no exclusion criteria, were scheduled for an interview at the clinic together with their legal guardians. During the interview, the adolescent and the parents were informed about the study aims and procedures, and written informed consent was obtained from both. Diagnostic status was assessed using the ADIS C/P (32). Two senior clinical psychologists conducted the diagnostic interviews. Of the 14 adolescents who completed the online screening and were offered an interview, one was excluded as her primary presenting complaint was social anxiety disorder, and she was referred for treatment of this condition. After inclusion in the study, we provided the adolescents and parents with separate personal login credentials to a secure internet platform (i.e., the BIP platform) where self- and parent-report assessments were conducted. Adolescents were then randomized to the 2-, 6-, or 10-week baseline phase and were informed about which baseline length they had been allocated to, and when they were going to commence the BIP worry treatment. After the 2-, 6- or 10-week baseline phase, the BIP worry intervention was provided via the online platform. See Figure 1 for participant flow.
Figure 1.
Participant flow.
Assessment points
Each week during the baseline and intervention phases, adolescents were invited by text message to complete self-report questionnaires assessing worry (brief PSWQ). The adolescents were also assessed by clinicians at four time-points; pre-baseline (before randomization to the baselines), post-treatment, 1-month, and 3-month follow-ups. Pre-baseline, we assessed diagnostic status in a face-to-face interview and administered adolescent-, parent- and clinician-rated symptom and functioning measures. Pre-treatment, adolescent- and parent-rated outcome measures were administered but the diagnostic interview was not repeated.
At week three of BIP worry, adolescents and parents rated treatment credibility and expectancy. At week five and post-treatment, adolescents’ adherence to treatment was assessed. At post-treatment, diagnostic status was re-assessed in a face-to-face interview and all adolescent-, parent- and clinician-report measures were re-administered, including the measure of adverse events.
At the 1-month follow-up assessment, self- and parent-report measures (PSWQ-C/P, RCADS-C/P) were administered and diagnostic status was assessed via telephone by the treating clinician. The ADIS can be reliably administered via telephone (33). The telephone call was also used as a “booster session” to increase compliance with the BIP worry interventions after treatment. At the 3-month follow-up, diagnostic status was assessed in a face-to-face interview, all clinical outcome measures were re-administered.
A clinician who had not participated in the patient’s treatment, but was aware of the patient’s medical history and participation in the study, conducted the post-treatment and 3-month follow-up assessments.
Intervention
BIP worry draws upon the intolerance of uncertainty (IU) model of pathological worry (34) which stipulates that excessive worriers have difficulty tolerating uncertainty and a tendency to interpret ambiguous situations involving uncertainty as threatening. In order to reduce the immediate experience of uncertainty, worriers engage in exaggerated control behaviors (e.g., excessively seeking reassurance from parents) and/or avoidance (e.g., trying to suppress worries, procrastination around tasks/homework), but over time, these behaviors increase hypervigilance to situations associated with uncertainty. To increase tolerance for uncertainty and reduce excessive worry, the main intervention is exposure to situations associated with uncertainty. The BIP worry intervention is influenced by the IU model, incorporating worry awareness training and exposure to uncertainty as the core interventions. The other interventions from the original IU model and its treatment (34,35) that target three putative mediators of worry (positive beliefs about worry, negative problem orientation and cognitive avoidance) are mostly omitted so that the focus of the BIP worry on worry awareness and exposure is very clear and accessible via the internet for adolescents and their parents.
BIP worry lasts for 10 weeks and consists of 10 different steps (modules) including texts, film clips, and illustrations delivered entirely online via the secure BIP platform. The adolescents and parents are encouraged to work with one module each week. Adolescents and their parents have separate login credentials to the site where they access different programs; one for adolescents and the other for their parents. Both adolescents and parents have email contact with the same designated therapist in the encrypted BIP platform. The parent program aims mainly at helping parents understand their child’s worrying, reducing unhelpful parent behaviors (e.g., giving excessive reassurances, helping the child avoid worry inducing situations) and increasing more supporting parenting styles such as showing warmth and active listening. The parent program includes information about the adolescents’ treatment as well as material specifically directed to the parents. The content of the program is the same, irrespective of the adolescent’s age. However, therapists can vary their recommendations to parents, for instance by underlining that younger adolescents may need more parental support through the treatment while older adolescents may benefit from working more independently.
The therapist’s primary responsibility is to answer questions, provide feedback and clarifications on assignments, and to encourage the participants to proceed through the treatment modules. An overview of the treatment modules for adolescents and parents is presented in Table 2. The treatment content, delivered in a face-to-face setting, has been described in detail in a previous study (16).
Table 2. Overview of the BIP worry content.
| Module | Adolescent program | Parent program |
|---|---|---|
| 1 | Psychoeducation about worry | Psychoeducation about worry |
| 2 | Worry behaviors | Worry behaviors |
| 3 | Introduction to exposure, setting goals for treatment | Exposure and common parental reactions to worry |
| 4 | Exposure to thoughts | Exposure to thoughts, alternative parental strategies |
| 5 | Being proactive when facing uncertainty | Being proactive, more alternative strategies for parents |
| 6 | Making decisions related to worry and uncertainty | Making decisions related to worry and uncertainty, and adolescent development related to worry |
| 7 | How to let go of control behaviors | Being supportive as a parent |
| 8 | Summary so far, evaluating goals | Summary so far |
| 9 | Relapse prevention | Relapse prevention |
| 10 | Planning for the future | Planning for the future |
Data analysis
Feasibility of BIP worry was assessed using summary statistics for completed modules and exposures conducted by the adolescents during treatment, the IIPAS, the C-scale-C/P, CSQ-C/P and the NEQ-C/P.
To calculate overall reductions on clinical outcome measures, we used piece-wise linear mixed models (36,37) to analyze pre- and post-treatment and follow-up data. All models included random intercept and random slope. No cases were excluded in accordance with the intent to treat principle (ITT). As post-hoc analyzes, we used linear regression to assess whether reduction in adolescent rated worry from pre- to post-treatment was associated with increased functioning and reduced anxiety and depression symptoms at 3-month follow-up. Cohen’s d was used to assess effect-sizes for all continuous outcome measures from pre-baseline to pre-treatment, and from pre-treatment to post-treatment, 1-month and 3-month follow-ups. Effect sizes were based on mean comparisons using observed data, and were considered small if d=0.20–0.49, medium if d=0.50–0.79 and large if d≥0.8 (38). Statistical significance was set to P<0.05. All statistical analyses were conducted in STATA 15.1 (39).
Individual trajectories of worry in the multiple baseline evaluation were analyzed visually according to guidelines (40). If the adolescents on longer baseline phases (BL6 and BL10) remained stable until after the adolescents on BL2 had a decrease in symptoms, an effect of the intervention on worry had been shown. The same needed to be true for adolescents on BL10 when those on BL6 showed symptom change.
Results
Feasibility
Summary statistics on all feasibility measures are presented in Table 3. Twelve out of 13 adolescents and their parents completed treatment (i.e., worked with at least six modules). One adolescent (P1, randomized to a 6-week baseline) experienced increased depressive symptoms during the baseline phase and dropped out after one week of BIP worry. During the active treatment phase, the proportion of weeks where adolescents reported completing exposures was high (96%) and clinician-rated adolescent adherence to BIP worry was high. Both adolescents and parents scored high on the treatment credibility scale and treatment satisfaction and all reported that they would probably or certainly recommend BIP worry to a friend with similar problems.
Table 3. Descriptive statistics with M and SD for the feasibility measures.
| Measure | Min-max | Adolescents | Parents |
|---|---|---|---|
| Completed modules (range) | 0–10 | 9.8 (8–10) | 9.8 (8–10) |
| Number of exposures (SD; range) | – | 31.0 (12.6; 13–60) | – |
| IIPAS mid-treatment | 0–20 | 17.3 (2.5) | – |
| IIPAS post-treatment | 0–20 | 16.8 (4.0) | – |
| C-Scale | 0–50 | 38.1 (7.0) | 35.0 (7.0) |
| CSQ | 8–32 | 27.8 (2.7) | 28.8 (3.2) |
| Therapist time, min/week | – | 11.6 (4.0) | 9.6 (1.8) |
IIPAS, Internet Interventions Patient Adherence Scale; C-Scale, Treatment Credibility and Expectancy Scale; CSQ, Client Satisfaction Questionnaire.
Adverse events analysis revealed five adolescents (P3, P7, P9, P11, P12) reporting increased stress related to the treatment. Three parents (those of P2, P3 and P9) also reported that their adolescents experienced increased stress related to treatment and three parents (to P2, P6 and P11) reported that their children had unpleasant memories resurfacing during the intervention. Furthermore, six adolescents (P2, P7, P8, P10, P12, P13) reported not always understanding the treatment content.
Efficacy
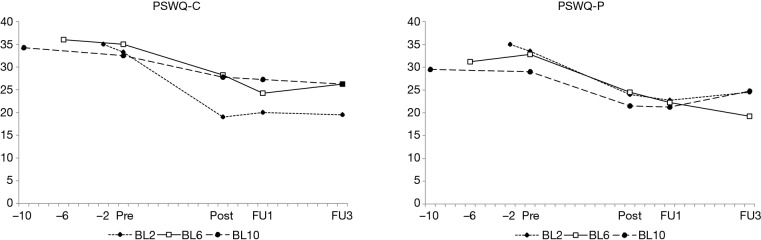
There were significant effects of time for the primary outcome variable (PSWQ-C/P). The overall reductions in adolescent-rated worry from pre-baseline to pre-treatment was d=0.41 (95% CI, –0.37–1.18). The corresponding drop from pre-treatment to post-treatment was d=1.38 (95% CI, 0.50–2.26). Follow-up assessments indicated sustained effects (pre-treatment to 1-month follow up d=1.80 (95% CI, 0.85–2.73), pre-treatment to 3-month follow up d=1.52 (95% CI, 0.61–2.41). Mean scores are shown in Figure 2 and further details are presented in Table 4. At post-treatment, seven adolescents (58.3%) were classified as much or very much improved on the CGI-I. The corresponding figures at the 1-month and 3-month follow-ups were 8 (66.7%) and 7 (58.3%) respectively.
Figure 2.
Mean reduction in observed values of adolescents’ worry as assessed with the PSWQ-C/P from pre-baseline (-10, -6 and -2 weeks respectively), Pre to Post, FU1 and FU3. PSWQ-C/P, Penn State Worry Questionnaire for Children—Child and Parent rated; Pre, pre-treatment; Post, post-treatment; FU1, 1-month follow-up; FU3, 3-month follow-up.
Table 4. Clinical outcome measures: Observed mean and standard deviation, estimated effect sizes (Cohen’s d based on observed values, estimated effects derived from the linear mixed model.
| Measure | Observed values | Estimated effects | |||||
|---|---|---|---|---|---|---|---|
| M | SD | Within group effect size d (95% CI) | B | z value | P value | ||
| PSWQ-C | |||||||
| Pre-baseline | 35.15 | 3.08 | |||||
| Pre-treatment | 33.69 | 3.95 | 0.41 (–0.37, –1.18) | ||||
| Post-treatment | 25.00 | 8.06 | 1.38 (0.50, 2.26) | –8.64 | –5.06 | <0.001 | |
| 1-month | 23.83 | 6.73 | 1.80 (0.85, 2.73) | –9.81 | –4.94 | <0.001 | |
| 3-month | 24.00 | 8.24 | 1.52 (0.61, 2.41) | –9.64 | –3.64 | <0.001 | |
| PSWQ-P | |||||||
| Pre-baseline | 31.85 | 3.85 | |||||
| Pre-treatment | 31.85 | 5.46 | 0.00 (–0.77, 0.77) | ||||
| Post-treatment | 23.33 | 4.05 | 1.76 (0.81, 2.68) | –8.37 | –5.01 | <0.001 | |
| 1-month | 22.08 | 4.23 | 1.99 (1.00, 2.94) | –9.62 | –4.88 | <0.001 | |
| 3-month | 22.83 | 9.13 | 1.21 (0.34, 2.06) | –8.87 | –3.31 | 0.001 | |
| RCADS-Anxiety-C | |||||||
| Pre-baseline | 36.70 | 10.19 | |||||
| Pre-treatment | 39.15 | 11.22 | –0.16 (–0.93, 0.61) | ||||
| Post-treatment | 26.08 | 14.72 | 0.74 (–0.08, 1.55) | –11.45 | –4.44 | <0.001 | |
| 1-month | 26.17 | 15.50 | 0.66 (–0.15, 1.46) | –11.37 | –4.00 | <0.001 | |
| 3-month | 23.92 | 13.57 | 0.91 (0.07, 1.72) | –13.62 | –3.87 | <0.001 | |
| RCADS-Anxiety-P | |||||||
| Pre-baseline | 29.31 | 7.17 | |||||
| Pre-treatment | 32.23 | 7.12 | –0.12 (–0.89, 0.65) | ||||
| Post-treatment | 22.27 | 8.34 | 1.02 (0.18, 1.85) | –9.49 | –5.02 | <0.001 | |
| 1-month | 19.00 | 10.85 | 0.86 (0.03, 1.67) | –12.66 | –6.10 | <0.001 | |
| 3-month | 17.10 | 9.74 | 1.07 (0.21, 1.90) | –14.57 | –5.72 | <0.001 | |
| RCADS-Dep-C | |||||||
| Pre-baseline | 13.38 | 5.73 | |||||
| Pre-treatment | 14.38 | 6.53 | –0.16 (–0.93, 0.61) | ||||
| Post-treatment | 9.75 | 5.88 | 0.74 (–0.80, 1.55) | –3.82 | –3.13 | 0.002 | |
| 1-month | 10.50 | 5.04 | 0.66 (–0.15, 1.46) | –3.07 | –2.35 | 0.019 | |
| 3-month | 9.00 | 5.24 | 0.91 (0.07, 1.72) | –4.57 | –2.98 | 0.003 | |
| RCADS-Dep-P | |||||||
| Pre-baseline | 10.62 | 2.82 | |||||
| Pre-treatment | 11.00 | 3.49 | –0.12 (–0.89, 0.65) | ||||
| Post-treatment | 7.83 | 2.59 | 1.02 (0.18, 1.85) | –2.89 | –3.45 | 0.001 | |
| 1-month | 7.67 | 4.25 | 0.86 (0.03, 1.68) | –3.06 | –3.32 | 0.001 | |
| 3-month | 7.00 | 4.02 | 1.07 (0.21, 1.90) | –3.72 | –3.28 | 0.001 | |
| WSAS-Y-C | |||||||
| Pre-baseline | 15.69 | 7.65 | |||||
| Pre-treatment | 16.85 | 8.86 | –0.40 (–0.91, 0.63) | ||||
| Post-treatment | 11.17 | 7.35 | 0.69 (–0.13, 1.49) | –4.81 | –3.22 | 0.001 | |
| 3-month | 10.75 | 9.69 | 0.66 (–0.16, 1.46) | –5.23 | –2.28 | 0.023 | |
| WSAS-Y-P | |||||||
| Pre-baseline | 13.00 | 4.55 | |||||
| Pre-treatment | 13.38 | 7.32 | –0.06 (–0.83, 0.71) | ||||
| Post-treatment | 10.25 | 4.14 | 0.49 (–0.31, 1.28) | –1.75 | –1.48 | 0.138 | |
| 3-month | 7.33 | 6.70 | 0.84 (0.01, 1.66) | –4.67 | –2.44 | 0.015 | |
| CGAS | |||||||
| Pre-baseline | 57.62 | 2.75 | |||||
| Post-treatment | 62.92 | 2.61 | 1.97 (0.99, 2.92) | 5.25 | 4.89 | <0.001 | |
| 1-month | 65.33 | 5.18 | 1.88 (0.92, 2.82) | 7.67 | 6.21 | <0.001 | |
| 3-month | 65.58 | 5.25 | 1.93 (0.95, 2.87) | 7.92 | 4.87 | <0.001 | |
Data from pre-baseline is used in the linear mixed models and d calculations for the CGAS. Higher scores on the CGAS indicate higher functioning. PSWQ-C, Penn State Worry Questionnaire for Children; C/P, Child and Parent rated; RCADS-Anxiety-C/P, Revised Children’s Anxiety Scale Child and Parent rated, anxiety subscales; RCADS-Dep-C/P, Revised Children’s Anxiety and Depression Scale Child and Parent rated, depression subscales; WSAS-Y-C/P, Work and Social Adjustment Scale—Youth version, Child and Parent rated; CGAS, Clinician Global Assessment Scale.
The piece-wise linear mixed effects model revealed significant effects of time on the secondary outcome measures; adolescent- and parent-rated anxiety, impaired functioning, and clinician-rated functioning (Table 4). Linear regression analyses revealed significant associations between reductions in adolescent-rated worry (PSWQ-C) from pre- to post-treatment and improved adolescent-rated functioning (F [2, 9]=4.22, P=0.026, R2=0.484), and reduced symptoms of other anxiety (F [2, 9]=7.05, P=0.006, R2= 0.610), and depression (F [2, 9]=6.88, P=0.007, R2=0.605) at the 3-month follow-up (when controlling for pre-treatment worry).
At post-treatment, 6 adolescents (50%) no longer fulfilled GAD diagnostic criteria, one no longer met obsessive compulsive disorder criteria, and one no longer had panic disorder. One adolescent met criteria for major depression at post-treatment. At 1-month follow-up, 8 adolescents (66.7%) did not meet GAD criteria, and only two had comorbid disorders (specific phobia and major depression). At the 3-month follow-up, 10 adolescents (83.3%) were free of GAD, while two adolescents had major depression and one had specific phobia and OCD.
Change between baseline and intervention phases in adolescents’ worry
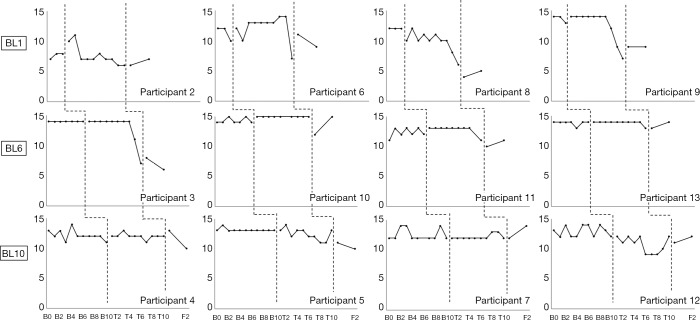
The visual analysis of weekly measures from the baseline phase compared to the intervention phase (Figure 3) yielded mixed results regarding the effect of BIP worry on worry severity. There were clear changes between the baseline phase and intervention phase on adolescent-reported worry (brief PSWQ) for four adolescents (P3, P6, P8, P9), with the differences between phases clearest for adolescents randomized to the 2-week baseline phase. As shown in Figure 3, two of the four adolescents randomized to BL2 experienced reductions in worry (brief PSWQ) and these occurred during the second half of the treatment. When the reductions in worry occurred for participants randomized to BL2, all participants in BL6 and BL10 remained stable. No intervention effects for worry were observed for one of four adolescents randomized to BL2 (P2) three of the four randomized to BL6 (P10, P11, P13,) and three of four randomized to BL10 (P4, P5, P7).
Figure 3.
Individual outcomes through baseline, during treatment, and at 1-month and 3-month follow-up. Each graph represents data for one participant. Each row represents one baseline length, i.e., 2, 6 and 10 weeks. B-PSWQ, Brief Penn State Worry Questionnaire; B-IUS, Brief Intolerance for Uncertainty Scale. B, baseline; T, treatment; F, follow-up.
Discussion
This study assessed the feasibility and preliminary clinical efficacy of an online intervention for adolescents with excessive worry and explored the processes of change during treatment. In accordance with our first hypothesis, the results indicated that BIP worry was feasible for adolescents with excessive worry. All participants (and their parents) who stayed in the study completed at least 80% of the treatment modules and a majority completed all modules. A single participant dropped out during the first week of treatment due reasons unrelated to the treatment, and while negative effects of going through treatment were reported by both adolescents and their parents, no serious adverse events associated with the BIP worry intervention were observed. Also, although half of the adolescents reported not always fully understanding the treatment at times, both adolescents and their parents rated BIP worry as credible and were highly satisfied with the intervention. Adherence to the treatment was high with adolescents reporting an average of four exposures per week during treatment, and 10 of 12 adolescents conducting at least one exposure every week. The total time needed for the therapist to treat patients averaged 20 min per family per week, which is about 30 min less when compared to the face-to-face version of the same treatment (16). These findings together indicate that online-delivered BIP worry is a feasible treatment for adolescents with excessive worry.
In accordance with the second hypothesis, we found significant reductions in the severity of adolescent- (primary outcome) and parent-reported worry. The effect sizes on worry severity (PSWQ-C) were large and on par with previous studies of face-to-face CBT for excessive worry in youth (16,19-21,41). The effects were maintained at both follow-ups. There were also significant effects on other symptoms of anxiety, depression, and overall functioning as rated by the adolescents, their parents, and the clinicians. The notion that worrying is a central aspect of psychopathology, and that targeting worry can help reduce other anxiety and depressive symptoms, and improve overall functioning has received support in previous studies (21,34, 42). Our study provides some additional preliminary support as reductions in worry from pre- to post-treatment were significantly associated with reductions in anxiety and depression and increased functioning at follow-up.
Regarding our third hypothesis, visual inspection suggested differences between baseline and intervention phases in level of worry for four (33.3%) of the twelve adolescents who stayed in treatment; three of the adolescents were randomized to the 2-week baseline, and one to the 6-week baseline. Thus, the overall findings for the multiple baseline evaluation did not to show a causal effect of the intervention, potentially because the average reduction in worry severity (measured with the PSWQ-C) during the baseline phase was equivalent to an effect size of d=0.41. It is possible that this reduction represents a regression towards the mean because adolescents in this trial were recruited based on their having a score on the PSWQ-C of >30; and their pre-baseline score (M =35.15; SD =3.08) was higher than what has previously been reported for youth with GAD, e.g., M =23.1, SD =6.3 (19); M =27.07, SD =5.43 (22). Another possibility is that this reduction is partly the result of the adolescents in this trial completing a measure of worry on a weekly basis during the baseline phase. However, wait-listed participants in the trial by Perrin and colleagues (21) also received weekly prompts to complete the PSWQ-C and no significant reductions in worry were observed.
The effects of the intervention on worry for P3, P6, P8 and P9 were also delayed, a finding consistent with our experience in the trial of the face-to-face version of the same treatment, wherein several adolescents only reported reductions in worry severity after several weeks of treatment (16). The changes in individual worry ratings were slightly clearer for adolescents randomized to the 2- and 6-week than the 10-week baseline assessment phases. Longer waiting times for an internet-delivered treatment may have dampened these participant’s enthusiasm for the treatment. There is evidence from the Improving Access to Psychological Therapies (IAPT) program in the England that longer waiting times are associated with poorer outcomes for adults receiving CBT for anxiety and depression (43).
Limitations
While our findings suggest that BIP worry is a feasible and potentially effective intervention for excessive worry in adolescents, certain limitations need to be noted. The sample size was small, there was no control for attention or placebo effects, the assessors were not blinded to treatment status, and the follow-up period was brief. A majority of participants (84.6%) were female, further limiting the generalizability of the findings. Also, while all of the participants had significant levels of worry, a diagnosis of GAD, and most had a prior history of mental health treatment, the participants were recruited online and, thus, may differ in important ways from those seeking treatment through routine psychiatric services in Sweden. There is no consensus in the literature on how to best measure worry on a repeated basis during treatment. It is possible that the brief, 5-item measure of worry used in this study is not sufficiently sensitive for use in treatment trials. Allocation to baseline was associated with a medium effect size (d=0.41) in respect of worry. This is the first trial to report such a finding and it may be sample specific.
Future research and clinical implications
Given that adolescence is a period of high stress and worry, and a peak onset period for mental health problems that continue into adulthood, interventions like BIP worry can play an important role in prevention and treatment, and potentially reducing the burden of disease. However, several important research topics need to be addressed further. Despite promising results regarding the feasibility and efficacy of the BIP worry, and the use of a multiple-baseline design to control for time and maturation, the results could not show a causal effect of the intervention on worry severity. Therefore, there is need for a randomized controlled trial (RCT) with a larger sample and an attention control group. An RCT could also help elucidate if the findings can be generalizable, e.g., to male adolescents and other high-worrier adolescent populations which were not included in this small feasibility trial.
Half of the adolescents in this study indicated that they had difficulties understanding the treatment content at some at point during the treatment phase. Clinically, this finding highlights the need for therapists who work with adolescents to continuously check in on the adolescents’ understanding of the treatment. Future studies should test the acquisition of knowledge and understanding and their relationship to outcome. Interestingly, one recent study on internet-delivered CBT for adolescents with depression could not find any clear association between an increase in knowledge about basic concepts concerning depression, anxiety and CBT and symptom reduction (44). We aim to further assess and improve the feasibility and efficacy of the BIP worry intervention by in-depth qualitative interviews with adolescents who have undergone BIP worry.
Finally, participants who waited the longest for treatment appeared to benefit the least. The relationship between wait-list length and subsequent outcomes in CBT for childhood anxiety has not been examined previously and further investigations are needed.
Conclusions
In conclusion, the psychological intervention BIP worry can be feasibly delivered online and is potentially effective in reducing worry and anxiety, and increasing general functioning for adolescents with excessive worry. Further large-scale investigations are warranted.
Acknowledgments
Funding: This work was supported by the Swedish Research Council for Health, Working Life and Welfare (Forte 2014-4052) and Stockholm County Council (HNSV 14099). Eva Serlachius was supported by the Stockholm County Council (clinical research appointment, grant number 20170605).
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted at the clinical research unit of the Child and Adolescent Mental Health Services in Stockholm, Sweden, approved by the regional ethics review board in Stockholm, and registered with www.clinicaltrials.gov (NCT03469453).
Footnotes
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- 1.Viner RM, Ozer EM, Denny S, et al. Adolescence and the social determinants of health. Lancet 2012;379:1641-52. 10.1016/S0140-6736(12)60149-4 [DOI] [PubMed] [Google Scholar]
- 2.Kessler RC, Berglund P, Demler O, et al. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005;62:593-602. 10.1001/archpsyc.62.6.593 [DOI] [PubMed] [Google Scholar]
- 3.World Health Organization. Health for the World’s Adolescents. 2014:1-20.
- 4.Holmes EA, Ghaderi A, Harmer CJ, et al. The Lancet Psychiatry Commission on psychological treatments research in tomorrow's science. The Lancet Psychiatry 2018;5:237-86. 10.1016/S2215-0366(17)30513-8 [DOI] [PubMed] [Google Scholar]
- 5.Kazdin AE. Addressing the treatment gap: A key challenge for extending evidence-based psychosocial interventions. Behav Res Ther 2017;88:7-18. 10.1016/j.brat.2016.06.004 [DOI] [PubMed] [Google Scholar]
- 6.Topper M, Emmelkamp PMG, Watkins E, et al. Prevention of anxiety disorders and depression by targeting excessive worry and rumination in adolescents and young adults: A randomized controlled trial. Behav Res Ther 2017;90:123-36. 10.1016/j.brat.2016.12.015 [DOI] [PubMed] [Google Scholar]
- 7.Borkovec TD, Robinson E, Pruzinsky T, et al. Preliminary exploration of worry: Some characteristics and processes. Behav Res Ther 1983;21:9-16. 10.1016/0005-7967(83)90121-3 [DOI] [PubMed] [Google Scholar]
- 8.Anniko MK, Boersma K, Tillfors M. Sources of stress and worry in the development of stress-related mental health problems: A longitudinal investigation from early- to mid-adolescence. Anxiety Stress Coping 2019;32:155-67. 10.1080/10615806.2018.1549657 [DOI] [PubMed] [Google Scholar]
- 9.Burstein M, Beesdo-Baum K, He JP, et al. Threshold and subthreshold generalized anxiety disorder among US adolescents: prevalence, sociodemographic, and clinical characteristics. Psychol Med 2014;44:2351-62. 10.1017/S0033291713002997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caes L, Fisher E, Clinch J, et al. The development of worry throughout childhood: Avon Longitudinal Study of Parents and Children data. Br J Health Psychol 2016;21:389-406. 10.1111/bjhp.12174 [DOI] [PubMed] [Google Scholar]
- 11.American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Arlington: American Psychiatric Association, 2013. [Google Scholar]
- 12.Weems CF, Silverman WK, La Greca AM. What Do Youth Referred for Anxiety Problems Worry About? Worry and Its Relation to Anxiety and Anxiety Disorders in Children and Adolescents. J Abnorm Child Psychol 2000;28:63-72. 10.1023/A:1005122101885 [DOI] [PubMed] [Google Scholar]
- 13.McEvoy PM, Watson H, Watkins ER, et al. The relationship between worry, rumination, and comorbidity. Evidence for repetitive negative thinking as a transdiagnostic construct. J Affect Disord 2013;151:313-20. 10.1016/j.jad.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 14.Arbel R, Spies Shapiro L, et al. Adolescents' Daily Worry, Morning Cortisol, and Health Symptoms. J Adolesc Health 2017;60:667-73. 10.1016/j.jadohealth.2017.01.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Geronimi EM, Patterson HL, Woodruff-Borden J. Relating Worry and Executive Functioning During Childhood: The Moderating Role of Age. Child Psychiatry Hum Dev 2016;47:430-9. 10.1007/s10578-015-0577-4 [DOI] [PubMed] [Google Scholar]
- 16.Wahlund T, Andersson E, Jolstedt M, et al. Intolerance of Uncertainty–Focused Treatment for Adolescents With Excessive Worry: A Pilot Feasibility Study. Cogn Behav Pract. 2019 doi: 10.1016/j.cbpra.2019.06.002. [Epub ahead of print] [DOI] [Google Scholar]
- 17.Pestle SL, Chorpita BF, Schiffman J. Psychometric Properties of the Penn State Worry Questionnaire for Children in a Large Clinical Sample. J Clin Child Adolesc Psychol 2008;37:465-71. 10.1080/15374410801955896 [DOI] [PubMed] [Google Scholar]
- 18.Chorpita BF, Tracey SA, Brown TA, et al. Assessment of worry in children and adolescents: An adaptation of the Penn State Worry Questionnaire. Behav Res Ther 1997;35:569-81. 10.1016/S0005-7967(96)00116-7 [DOI] [PubMed] [Google Scholar]
- 19.Holmes MC, Donovan CL, Farrell LJ, et al. The efficacy of a group-based, disorder-specific treatment program for childhood GAD--a randomized controlled trial. Behav Res Ther 2014;61:122-35. 10.1016/j.brat.2014.08.002 [DOI] [PubMed] [Google Scholar]
- 20.Payne S, Bolton D, Perrin S. A Pilot Investigation of Cognitive Therapy for Generalized Anxiety Disorder in Children Aged 7–17 Years. Cognit Ther Res 2011;35:171-8. 10.1007/s10608-010-9341-z [DOI] [Google Scholar]
- 21.Perrin S, Bevan D, Payne S, et al. GAD-Specific Cognitive Behavioral Treatment for Children and Adolescents: A Pilot Randomized Controlled Trial. Cognit Ther Res 2019. doi: . 10.1007/s10608-019-10020-3 [DOI] [Google Scholar]
- 22.Rozental A, Kottorp A, Boettcher J, et al. Negative Effects of Psychological Treatments: An Exploratory Factor Analysis of the Negative Effects Questionnaire for Monitoring and Reporting Adverse and Unwanted Events. PLoS One 2016;11:e0157503. 10.1371/journal.pone.0157503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lenhard F, Mitsell K, Jolsted M, et al. Development and psychometric evaluation of an adherence scale for guided internet-delivered behavioral interventions: The internet intervention Patient Adherence Scale (iiPAS). J Med Internet Res. 2019 doi: 10.2196/13602. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Borkovec TD, Nau SD. Credibility of analogue therapy rationales. J Behav Ther Exp Psychiatry 1972;3:257-60. 10.1016/0005-7916(72)90045-6 [DOI] [Google Scholar]
- 25.Attkisson CC, Zwick R. The client satisfaction questionnaire. Eval Program Plann 1982;5:233-7. 10.1016/0149-7189(82)90074-X [DOI] [PubMed] [Google Scholar]
- 26.Topper M, Emmelkamp PMG, Watkins E, et al. Development and assessment of brief versions of the Penn State Worry Questionnaire and the Ruminative Response Scale. Br J Clin Psychol 2014;53:402-21. 10.1111/bjc.12052 [DOI] [PubMed] [Google Scholar]
- 27.Chorpita BF, Yim L, Moffitt C, et al. Assessment of symptoms of DSM-IV anxiety and depression in children: A revised child anxiety and depression scale. Behav Res Ther 2000;38:835-55. 10.1016/S0005-7967(99)00130-8 [DOI] [PubMed] [Google Scholar]
- 28.Mundt JC, Marks IM, Shear MK, et al. The Work and Social Adjustment Scale: a simple measure of impairment in functioning. Br J Psychiatry 2002;180:461-4. 10.1192/bjp.180.5.461 [DOI] [PubMed] [Google Scholar]
- 29.Jassi A, Lenhard F, Krebs G, et al. The work and social adjustment scale, youth and parent versions: psychometric evaluation of a brief measure of functional impairment in young people. Available online: https://doi.org/ 10.31234/osf.io/f8zev [DOI] [PMC free article] [PubMed]
- 30.Shaffer D, Gould MS, Brasic J. A children's global assessment scale (CGAS). Arch Gen Psychiatry 1983;40:1228-31. 10.1001/archpsyc.1983.01790100074010 [DOI] [PubMed] [Google Scholar]
- 31.Guy W. ECDEU assessment manual for psychopharmacology. Rockville: National Institute of Mental Heath, 1976. [Google Scholar]
- 32.Silverman WK, Albano AM. Anxiety Disorders Interview Schedule for DSM-IV: Child interview schedule. Graywind Publications Incorporated, 1996. [Google Scholar]
- 33.Lyneham HJ, Rapee RM. Agreement Between Telephone and In-Person Delivery of a Structured Interview for Anxiety Disorders in Children. J Am Acad Child Adolesc Psychiatry 2005;44:274-82. 10.1097/00004583-200503000-00012 [DOI] [PubMed] [Google Scholar]
- 34.Dugas MJ, Gagnon F, Ladouceur R. Generalized anxiety disorder: A preliminary test of a conceptual model. Behav Res Ther 1998;36:215-26. 10.1016/S0005-7967(97)00070-3 [DOI] [PubMed] [Google Scholar]
- 35.Dugas MJ, Robichaud M. Cognitive-behavioral treatment for generalized anxiety disorder: from science to practice. New York: Routledge, 2007. [Google Scholar]
- 36.Gueorguieva R, Krystal JH. Move over ANOVA: progress in analyzing repeated-measures data and its reflection in papers published in the Archives of General Psychiatry. Arch Gen Psychiatry 2004;61:310-7. 10.1001/archpsyc.61.3.310 [DOI] [PubMed] [Google Scholar]
- 37.Hesser H. Modeling individual differences in randomized experiments using growth models: Recommendations for design, statistical analysis and reporting of results of internet interventions. Internet Interv 2015;2:110-20. 10.1016/j.invent.2015.02.003 [DOI] [Google Scholar]
- 38.Cohen J. Statistical power analysis for the behavioral sciences. 2nd ed. Hillsdale: Lawrence Earlbaum Associates, 1988. [Google Scholar]
- 39.Stata Statistical Software. College Station, TX: StataCorp LLC, 2017. [Google Scholar]
- 40.Kazdin AE. Single-case research designs: Methods for clinical and applied settings. 2nd edition. New York: Oxford University Press, 2011. [Google Scholar]
- 41.Léger E, Ladouceur R, Dugas MJ, et al. Cognitive-Behavioral Treatment of Generalized Anxiety Disorder Among Adolescents: A Case Series. J Am Acad Child Adolesc Psychiatry 2003;42:327-30. 10.1097/00004583-200303000-00013 [DOI] [PubMed] [Google Scholar]
- 42.Freeman D, Dunn G, Startup H, et al. Effects of cognitive behaviour therapy for worry on persecutory delusions in patients with psychosis (WIT): a parallel, single-blind, randomised controlled trial with a mediation analysis. Lancet Psychiatry 2015;2:305-13. 10.1016/S2215-0366(15)00039-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark DM, Canvin L, Green J, et al. Transparency about the outcomes of mental health services (IAPT approach): an analysis of public data. Lancet 2018;391:679-86. 10.1016/S0140-6736(17)32133-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Berg M, Rozental A, Johansson S, et al. The role of knowledge in internet-based cognitive behavioural therapy for adolescent depression: Results from a randomised controlled study. Internet Interv 2018;15:10-7. 10.1016/j.invent.2018.10.001 [DOI] [PMC free article] [PubMed] [Google Scholar]