Abstract
Introduction
Low sanitary conditions characterize the rural and urban households in Sub-Saharan African region. Those environmental conditions propitiate the transmission of bacterial infections between animals and humans. Campylobacter spp. is a zoonotic bacterium and cause of human gastroenteritis worldwide, whose main symptom is diarrhea. It is normally found in the digestive tract of many farm animals as a commensal but some species cause diseases in animals. It is important to understand the occurrence of these bacteria in animals, as they may also play a role in transmission to humans. The main objective of this review was to describe the prevalence of Campylobacter in animals in Sub-Saharan Africa. We also report findings on antibiotic resistance.
Methods
We followed PRISMA guidelines to find studies about occurrence of Campylobacter spp. in animals in all countries from Sub-Saharan Africa. PubMed, Cochrane Library, CINAHL, African Index Medicus, African Journals Online, Google Scholar and Science Direct were searched for studies published between 2000 and 2019.
Results
We found 70 studies that described occurrence of Campylobacter spp. in animals in 18 out of 53 countries of Sub-Saharan Africa. Campylobacter jejuni and C. coli were the predominant species isolated. The majority of studies were found in Western Africa. Middle Africa had the lowest amount of data. Most records presented data from Nigeria (n = 25), South Africa (n = 14) and Tanzania (n = 11). Cattle and chickens appear to be important hosts and may be playing an important role in transmitting to humans. Most Campylobacter isolates were resistant to erythromycin (44%), ampicillin (39%), tetracycline (33%), nalidixic acid (31%) and ciprofloxacin (30%).
Conclusion
Several studies about Campylobacter spp. in animals have been published in the last 19 years but information on the epidemiology of campylobacteriosis is scarce in most Sub-Saharan African countries. Antibiotic resistance is an increasing concern in many countries. Measures should be taken to prevent infection by this pathogen in the region and to control antibiotic resistance.
Keywords: Microbiology, Epidemiology, Infectious disease, Public health, Campylobacter spp., Antibiotic resistance, Africa
Microbiology; Epidemiology; Infectious disease; Public health; Campylobacter spp.; Antibiotic resistance; Africa.
1. Introduction
The genus Campylobacter is a group of zoonotic bacteria that cause diseases in animals and humans, although some are commensal in the intestinal tract of birds and ruminants (Sahin et al., 2017).
The genus has currently 39 species and 16 subspecies (http://www.bacterio.net/campylobacter.html, accessed 21.10.2019).
Campylobacter was described first in 1886 from stools of children who suffered diarrhea. At that time, it was reported in other studies and was associated with causes of enteric diseases. From 1913, the pathogen was isolated from aborted bovine fetuses, cows and calves with intestinal disorders and from swine with dysentery. From the 1970s Campylobacter species were also recognized as human pathogens (García-Sánchez, Melero, and Rovira, 2018).
The most common Campylobacter species are C. jejuni and C. coli, which are associated with diarrheal disease in humans (García-Sánchez et al., 2018). This bacterium is also reported to cause enteritis, mainly characterized by diarrhea in many animals such as chicken (Humphrey et al., 2014), sheep (Pintar et al., 2015; Sahin et al., 2017), dogs, cats, goats, equids and pigs (Kaakoush et al., 2015; Pintar et al., 2015).
C. fetus subsp. fetus is a cause of reproductive failures in ruminants. In cattle, C. fetus subsp. venerealis causes bovine genital campylobacteriosis, a sexually transmitted infection that is characterized by early embryonic deaths and abortion (Sahin et al., 2017).
Most human infections are due to consumption of poultry meat and poultry meat derived products, but also direct contact with animals (Berthenet et al., 2019; Cheng and Fischer, 2018; Kaakoush et al., 2015; Nichols et al., 2012; Sheppard et al., 2009; Sheppard, et al., 2014b; Thépault et al., 2017, 2018). Most countries from Sub-Saharan Africa are characterized by poor hygiene and sanitation (Osbjer et al., 2016). Moreover, the contact with animals or derived products contributes to the spread of this pathogen among animals and to human exposure.
It is important to know in which other animals besides poultry and cattle there are reports of the occurrence of Campylobacter in Sub-Saharan Africa, as well as the outcomes. It is equally important to know if exposure to these carrier animals constitutes a risk to humans.
Understanding the distribution of Campylobacter in animals in Sub-Saharan Africa is important in order to reduce morbidity and mortality associated with diarrheal disease. This is of particular interest in low and middle income countries where the epidemiology of this bacteria is poorly understood.
Incidence and prevalence of Campylobacter are thought to be increasing globally (Connerton and Connerton, 2017; Kaakoush et al., 2015), but an extensive overview of its distribution and epidemiology in animals in Sub-Saharan Africa is not available. The available animal systematic reviews are restricted to one country (Komba et al., 2013), to a single Campylobacter species (Pike et al., 2013; Wilkinson et al., 2018), or to household pets (Pintar et al., 2015). Moreover, it is known that different C. jejuni strains are found in multiple animal species (Sheppard et al., 2010, 2011; Sheppard, et al., 2014a).
This review provides an extensive and systematic overview of the epidemiology of Campylobacter spp. in animals in Sub-Saharan Africa from 2000 to 2019. To our knowledge, this is the first study of this magnitude that addresses such topic in Sub-Saharan Africa in the last 19 years. We report on the study site, Campylobacter prevalence, identified species, infected animals, diagnostic method and samples analyzed. We also discuss about antibiotic resistance findings due to the increasing reports of such occurrence (Sproston et al., 2018).
2. Materials and methods
2.1. Identifying research evidence
We followed Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) guidelines to find data about animal Campylobacter infection available from all countries of Sub-Saharan Africa. We used the UN macro-geographical definition of Africa to define the geographical boundaries of this review (https://unstats.un.org/unsd/methodology/m49/). According to this UN definition, the Sub-Saharan Africa is divided into Eastern Africa, Middle Africa, Southern Africa and West Africa, as described below:
-
•
Eastern Africa: British Indian Ocean Territory, Burundi, Comoros, Djibouti, Eritrea, Ethiopia, French Southern Territories, Kenya, Madagascar, Malawi, Mauritius, Mayotte, Mozambique, Réunion, Rwanda, Seychelles, Somalia, South Sudan, Uganda, United Republic of Tanzania, Zambia and Zimbabwe.
-
•
Middle Africa: Angola, Cameroon, Central African Republic, Chad, Congo, Democratic Republic of the Congo, Equatorial Guinea, Gabon and Sao Tome and Principe.
-
•
Southern Africa: Botswana, Eswatini (former Swaziland), Lesotho, Namibia and South Africa.
-
•
Western Africa: Benin, Burkina Faso, Cabo Verde, Côte d’Ivoire, Gambia, Ghana, Guinea, Guinea-Bissau, Liberia, Mali, Mauritania, Niger, Nigeria, Saint Helena, Senegal, Sierra Leone and Togo.
PubMed, Cochrane Library, CINAHL, African Index Medicus, African Journals Online, Google Scholar and Science Direct were searched for studies published up to October 8, 2019 without language restrictions. The systematic review protocol is in Supplementary material 1 and the search strategy in Supplementary material 2. Supplementary material 3 presents the PRISMA checklist.
Selection criteria for inclusion of studies were:
-
-
The study population consisted of any group of animals in Sub-Saharan Africa, which had been tested for Campylobacter spp.,
-
-
Descriptive, cross-sectional studies, prospective, or retrospective studies and case reports and series in which the prevalence rate of Campylobacter in any country in Sub-Saharan Africa was reported;
-
-
Conference abstracts; and
-
-
Only studies published from 2000 onwards.
The primary outcome was estimation of the prevalence of Campylobacter in animals from countries in Sub-Saharan Africa. The secondary outcome was to identify the antibiotics to which the different Campylobacter isolates were resistant.
2.2. Data extraction (selection and coding)
Titles and abstracts were screened for location, study population and general correlation with the research objectives. Full versions of potentially relevant articles were obtained to assess eligibility. These were then independently evaluated for inclusion. Cross-references of the full text retrieved articles were also searched. Data were collected independently from each publication and captured using a standardised Word document form. We extracted data from text, tables and figures.
2.3. Data analysis
In reports where the numerator and denominator of the study sample were available, prevalence data were calculated, if not already provided. When not presented in the manuscript, the 95% exact confidence intervals (CI) were calculated, using the “binom.test” function (“stats” package) in R 3.5.1.
3. Results and discussion
3.1. Search results
A total of 980 records were obtained from the database search (see Figure 1). One article was obtained through a previous search on https://www.google.com/. After removal of duplicate records (n = 229), 752 records were screened based on title and, thereafter, abstract. Thus, 100 records fulfilled the eligibility criteria for evaluation and 30 were excluded. All records were published in English.
Figure 1.
PRISMA flow diagram of study selection.
From the 70 animal studies (Aboaba and Smith, 2005; Abubakar et al., 2019; Achá et al., 2004; Akwuobu et al., 2010; Bartkowiak-Higgo et al., 2006; Basardien, 2012; Bernadette et al., 2012; Bester and Essack, 2008, 2012; Cardinale et al., 2003, 2004; 2002; Chuma et al., 2016; Conan et al., 2017; Ewnetu and Muhret, 2010; Garin et al., 2012; Gwimi et al., 2015; Henry et al., 2011; Jonker and Picard, 2012; Julien et al., 2013; Kagambèga et al., 2018; Kalema-Zikusoka and Rothman, 2005; Kambuyi, 2018; Karama et al., 2019; Karikari et al., 2017b; Karshima et al., 2016; Karshima and Bobbo, 2016; Kashoma et al., 2015a, Kashoma et al., 2015b; Kassa et al., 2007; Kaur et al., 2011; Komba et al., 2014; Kusiluka et al., 2005; Madoroba et al., 2011; Mai et al., 2013b, 2013a; Mai et al., 2015; Mdegela et al., 2006, 2011; Mokantla et al., 2004; Mpalang et al., 2014; Mshelia et al., 2010, 2012; Ngotho et al., 2006; Ngulukun et al., 2010, 2011; Nizeyi et al., 2001; Njiro et al., 2012; Nonga and Muhairwa, 2009; Nonga et al., 2009; Nwankwo et al., 2016; Ofukwu et al., 2008; Ogbor et al., 2019; Okunlade et al., 2015; Olabode et al., 2017; Raji et al., 2000; Salihu et al., 2008, 2010; 2012; Salihu et al., 2009a, Salihu et al., 2009b, Salihu et al., 2009c; Swai et al., 2005; Uaboi-Egbenni et al., 2008, 2010; 2011a, 2011b; 2012; Villers et al., 2008; Woldemariam et al., 2009), 67 were articles, 1 conference abstract and 2 Master of Science theses. In 2 of the 70 included animal studies, prevalence of Campylobacter was reported in more than one country. One of these reported data from Senegal, Cameroon and Madagascar (Garin et al., 2012). The other reported data from South Africa, Namibia, Zambia and Eswatini (Madoroba et al., 2011). That is why in Table 1 data are reported from 75 studies.
Table 1.
Overview of the studies included in the review.
| Countries | Sub-Saharan Africa region | Number of studies | Percentage (%) |
|---|---|---|---|
| Tanzania (11), Ethiopia (3), Madagascar (2), Kenya (3), Uganda (2), Mozambique (1), Réunion (1) and Zambia (1) | Eastern Africa | 24 | 32.0 |
| Cameroon (1) and Democratic Republic of the Congo (1) | Middle Africa | 2 | 2.7 |
| South Africa (14), Namibia (1) and Eswatini (1) | Southern Africa | 16 | 21.3 |
| Nigeria (25), Senegal (4), Burkina Faso (1), Côte d’Ivoire (2) and Ghana (1) | Western Africa | 33 | 44.0 |
| Total | 75 | 100.0 |
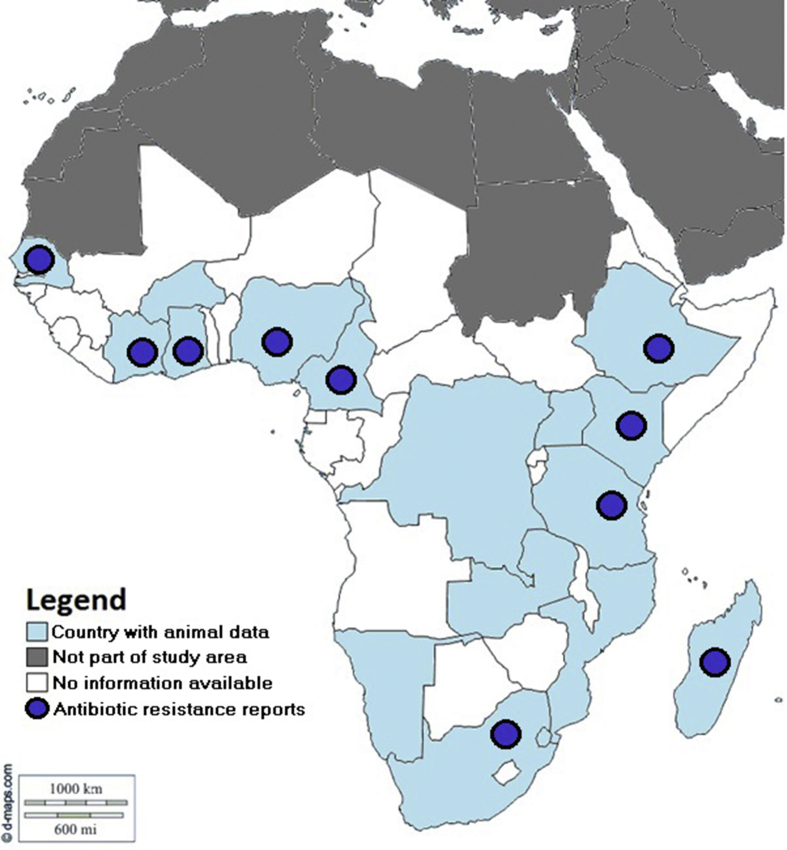
The studies included in this review were originated from 18 of 53 Sub Saharan African countries (Figure 2): Burkina Faso, Cameroon, Côte d’Ivoire, Democratic Republic of Congo, Eswatini, Ethiopia, Ghana, Kenya, Madagascar, Mozambique, Namibia, Nigeria, Réunion, Senegal, South Africa, Tanzania, Uganda and Zambia.
Figure 2.
Map with distribution of animal Campylobacter spp. studies published from Sub-Saharan Africa from 2000 to 2019.
The majority of records were from Nigeria (n = 25), South Africa (n = 14) and Tanzania (n = 11).
Table 2 summarizes the information about the Campylobacter species, prevalence in different regions, studied animals, samples, locations and resistance to antibiotics. Additionally, Supplementary material 4 provides detailed information about surveys of Campylobacter in animals from this region.
Table 2.
Summary of Campylobacter prevalence in different Sub-Saharan Africa regions; occurrence of the species in different animals, locations, samples and resistance to antibiotics. In brackets are the number of studies reporting such occurrence.
| C. jejuni | C. coli | C. lari | C. upsaliensis | C. fetus | C. hyointestinalis | C. sputorum | C.troglodytis | |
|---|---|---|---|---|---|---|---|---|
| Prevalence range per region | ||||||||
| EA | 0%–100% (19) | 0%–50% (17) | 0%–2.4% (2) | 4% (1) | 2.4%–5.1% (2) | - | - | 0%–87.5% (1) |
| MA | 13.6%–28.7% (2) | 24.8%–77.3% (2) | 0% (1) | 0% (1) | - | - | - | - |
| SA | 0.4%–73.3% (10) | 2%–48.2% (9) | - | 3%–13.1% (2) | 0%–2.1% (4) | - | - | - |
| WA | 0%–64.2% (23) | 0%–73% (25) | 0%–12.5% (11) | 0%–20.6% (6) | 0.1%–31.2% (6) | 0.4%–1.7% (3) | 0.7%–2.8% (2) | - |
| Animals | ||||||||
| Poultry (2) [chickens (25), ducks (3), guinea fowls (2), turkeys (1), pigeons (1)], cattle (11), camels (1), cats (2), crows (1), dogs (5), goats (5), guinea pigs (1), horses (1), quails (1), monkeys (1), pigs (8), rats (1), sheep (9) | Poultry (2) [chickens (26), ducks (2), guinea fowls (3)], cattle (11), camels (1), cats (1), crows (1), dogs (4), goats (7), grasscutters (1), guinea pigs (1), horses (1), pigs (9), quails (1), rats (1), sheep (9) | Camels (1), cattle (3), chickens (6), ducks (1), goats (1), guinea fowls (1), pigeons (1), pigs (1), sheep (3) | Camels (1), cats (1), cattle (1), chickens (2), dogs (2), pigs (1), sheep (1) | Cattle (12) | Cattle (2), pigs (1) | Camels (1), cattle (1) | Wild chimpanzees (1) | |
| Locations | ||||||||
| Abattoirs (16), farms (23), laboratory (1), rural or urban households (4), live bird markets (1), urban or peri-urban area (2), research institute (1), retail market/shop (1), rural area (2), state (4), town/metropolis (2), urban or rural veterinary clinics/premises (3), warehouses (1), wild (1) | Abattoirs (16), community (1), farms (24), households (3), laboratory (1), live bird markets (1), research institute (1), retail market/shop (2), rural area (2), state/town (7), veterinary clinics (2), warehouse (1) | Abattoirs (3), farms (3), live bird market (1), state (4) | Abattoirs (1), farms (2), households (2), state (3), veterinary clinics (2) | Farms (7), laboratory records (4), state (1) |
State (1), farms (2), households (1) | State (1), farms (1) | National parks (1) | |
| Samples tested for Campylobacter | ||||||||
| Caeca (5), carcass (11), cloaca (7), colon (1), feces (37), vaginal swab (1) | Caeca (4), carcass (14), cloaca (9), colon (1), feces (33) | Carcass (3), cloaca (3), feces (7), | Carcass (1), feces (7) | Cervico-vaginal mucus (1), rectal (1), preputial (10), vaginal mucus (1) | Feces (2), preputial washings and cervico-vaginal mucus (1) | Feces (1), preputial washings and cervico-vaginal mucus (1) | Feces (1) | |
| Resistance to antibiotics (above 10% of the isolates) | ||||||||
| Erythromycin (48.7%), Ampicillin (42.5%), Tetracycline (35.8%), Nalidixic Acid (32.0%), Ciprofloxacin (30.7%), Streptomycin (12.1%), Trimethoprim/Sulfametoxazole (10.6%), Gentamicin (10.5%) | Erythromycin (59.1%), Ampicillin (45.3%), Ciprofloxacin (40.2%), Nalidixic Acid (37.4%), Tetracycline (36.8%), Streptomycin (16.7%), Trimethoprim/Sulfametoxazole (14.7%), Chloramphenicol (12.9%), Gentamicin (12.9%) | Erythromycin (89.1%), Ampicillin (78.2%), Ciprofloxacin (51.2%), Nalidixic Acid (43.8%), Trimethoprim/Sulfametoxazole (19.4%), Tetracycline (17.9%), Cephalexin (16.7%), Chloramphenicol (16.5%), Cefotaxime (14.9%) | Erythromycin (90.4%), Ampicillin (73.9%), Ciprofloxacin (49.2%), Nalidixic Acid (46.7%) |
NR | NR | NR | NR | |
EA: Eastern Africa; MA: Middle Africa; SA: Southern Africa; WA: Western Africa; NR: Not reported.
3.2. General characteristics of studies (setting and methodology)
The included studies presented data that were collected from 1994 to 2017. Sixteen studies were recorded in the past 10 years. Twenty-one studies did not report about data collection time frame. Thirty studies were conducted in farms, nineteen in slaughterhouses or abattoirs, and the remaining ones in retail shops, markets, households, veterinary clinics, wild, national parks, reserves, urban or rural areas and some included records from laboratory. Seventy studies were cross-sectional, four retrospective and one longitudinal.
Cattle and chickens were the most studied animals. Other animals were camels, cats, goats, quails, horses, guinea fowls, guinea pigs, dogs, pigs, mountain gorillas, ring-tailed lemurs, sheep, rabbits, rats, mice, grasscutter, wild chimpanzees, monkeys, crows, donkeys, pigeons, turkeys and ducks.
Sixty-six studies used culture along with other methods to identify Campylobacter spp. in the samples. Other methods to identify the bacterium were biochemical tests, microscopy, haemagglutination tests, hemolysis, commercial identification tests [API CAMPY] and phenotypic tests. Twenty-three studies used PCR along with other methods to identify the bacteria, of which four used only PCR. One study used only ELISA test, whereas one other study did not report how Campylobacter was detected.
Collected samples were feces (droppings, cloacal swabs, rectal swabs and fecal sample), carcasses, sheath washings, preputial scrapings, intestinal contents, liver tissue, caeca, vaginal swab and cervico-vaginal mucus. The number of samples ranged from 2 to 1912.
The prevalence rates ranged from 0% to 100%. Identified species were: C. jejuni, C. coli, C. lari, C. upsaliensis, C. fetus, C. fetus subsp. fetus, C. f. venerealis biovar intermedius, C. fetus subsp. venerealis, C. hyointestinalis, C. troglodytis sp. nov, C. sputorum subsp. sputorum and other unidentified species. The most prevalent species in the studies were C. jejuni and C. coli; while the least prevalent were C. sputorum, C. hyointestinalis and C. troglodytis.
3.3. Epidemiology of Campylobacter spp. in animals in Sub-Saharan Africa
The information on the prevalence of Campylobacter in animals in Sub-Saharan Africa varies considerably because different methods were used to detect the bacterium, different animals were tested within each study and different isolates were found as well. However, we have found that Western Africa had majority of the data from Sub-Saharan Africa (44.0%, n = 33), mainly for Nigeria (n = 25), while Middle Africa had the least amount of data (2.7%, n = 2) from Cameroon and Democratic Republic of the Congo.
Comparing regions, Western Africa reported higher prevalence of different Campylobacter species such as C. jejuni, C. coli, C. lari, C. upsaliensis, C. fetus, C. hyointestinalis and C. sputorum. In this region a wide variety of animals were tested, mainly cattle and chickens. Other animals included: sheep, goats, pigs, camels, dogs, japanese quails, cats and grasscutters. This region was the most represented because of Nigeria that had 25 studies. This country had many published studies probably because the bacteria is a well-established cause of diarrhea, sometimes equal or exceeding Salmonella and Shigella rates (Aboaba and Smith, 2005; Aboderin et al., 2002). Still in this country, Campylobacter fetus subsp. venerealis was identified as a cause of bovine genital campylobacteriosis, that is characterized by reproductive failure and infertility in cattle (Mai et al., 2015; Mshelia et al., 2010). These reproductive problems are common occurrences amongst livestock in the country (Mshelia et al., 2010). Studies from Western Africa also reported about the transport of bacteria in different countries such as from Niger to Nigeria because of the large live bird market whereby poultry are transported unchecked (Nwankwo et al., 2016). This consequently leads to high prevalence rates in poultry and humans.
A high prevalence of different Campylobacter species was found in pigs (92.7%) (Gwimi et al., 2015). Authors stated that it might have been due to extensive system farming with indiscriminate defecation along with unhygienic disposal of human wastes in the environment and poor personal hygiene. One study from Senegal found an association between poor hygienic practices and Campylobacter occurrence (Cardinale et al., 2004).
The isolation of Campylobacter in poultry, camels, dogs, cats and quails was an indication that the presence of these animals was reservoir for human infection (Akwuobu et al., 2010; Karikari et al., 2017b; Karshima et al., 2016; Karshima and Bobbo, 2016; Ngulukun et al., 2010; Ogbor et al., 2019; Salihu et al., 2010; Salihu, et al., 2009b).
Probably in Nigeria there are many research projects focused in Campylobacter and other enteric bacteria. This contributes to the large number of publications. However, the other countries in the region with data on Campylobacter reported such occurrence mainly in poultry. This demonstrates that these food-animals constitutes an important reservoir of the bacterium.
Eastern Africa was the second region with the highest percentage of Campylobacter studies, where Tanzania stood out with 11 studies in total. In this region, as well as in Western Africa, a large diversity of animals were tested for the presence of Campylobacter with emphasis in cattle and poultry (including ducks and chicken), and other animals such as: pigs, guinea pigs, mice, rabbits, rats, goats, sheep, horses, camels, crows, mountain gorillas, ring-tailed lemurs, donkeys, dogs, cats and monkeys. Isolates were C. jejuni, C. coli, C. lari, C. upsaliensis, C. fetus and C. troglodytis that was a new species discovered in Tanzanian wild chimpanzees. This region was the only one that found 100% prevalence of C. jejuni in fecal samples from wild monkeys.
Studies from Eastern Africa reported possible cross-contamination between animals in farms, such as from pigs and chickens to horses and sheep, due to their proximity (Komba et al., 2014). There are probably many studies of the bacterium in Tanzania because it is one important cause of disease in children (Kashoma, et al., 2015a). Some studies addressing Campylobacter prevalence in pigs suggested a potential risk of infection to people through consumption of contaminated pork or contact with infected pigs (Mdegela et al., 2011). Some factors affecting prevalence are reported to be farming and slaughtering practices, geographical locations, and other risk factors, including the concentration of the farms in each location and their proximity to other livestock such as poultry (Kashoma, et al., 2015a).
Also in this region C. fetus subsp. venerealis was reported as the agent of enzootic infertility in smallholder herds (Swai et al., 2005).
After all, in one of the studies from Kenya the authors did not find any association between the presence of Campylobacter and other pathogens in household animals, and moderate to severe diarrhea in children (Conan et al., 2017). Nevertheless, it is known that these animals contribute to the transmission to humans.
Middle Africa region had the lowest amount of data from Sub-Saharan Africa, with only 2 studies in total (2.7%) from Cameroon and Democratic Republic of the Congo. Species found were C. jejuni and C. coli, although the presence of C. lari and C. upsaliensis was tested and had negative results. Slaughtered chicken and goats were the only tested animals and chicken had the higher prevalence of 92.7% (Garin et al., 2012). This emphasizes again the role of this food animal in Campylobacter epidemiology. In Lubumbashi city in Democratic Republic of the Congo, live goat and goat meat were the major sources of human and environmental contamination by Campylobacter spp., also because they are the major source of the meat supply in the city (Mpalang et al., 2014). In this region it was also found that slaughtering process is performed under poor hygienic conditions and this also contributes to bacterial proliferation (Mpalang et al., 2014). In general, Middle Africa is the second sub-region with the least countries, and probably for this reason it had fewer reports. On the other hand, the poor diagnostic capacity in Middle Africa may have limited the development of research on Campylobacter, since the published studies were supported by international collaborators.
Southern Africa had 21.3% of the total studies found in Sub-Saharan Africa, with more emphasis in South Africa that had 14 studies. South Africa was probably best represented because it has specialized research teams for zoonotic bacteria and appropriate diagnostic labs. Poultry and cattle were more studied in this region, although pigs, sheep, goats and dogs were also studied. Isolates of this region were C. jejuni, C. coli, C. upsaliensis and C. fetus. C. jejuni and C. coli had higher prevalence in diarrheic chicken and goats (Uaboi-Egbenni et al., 2010, 2012), indicating that they are potentially causers of diarrhea in these animals, although they are often known as commensals. The only study in non-food animals was in healthy dogs attending rural veterinary clinics, where almost half (41.6%) were positive for Campylobacter spp. In addition to C. jejuni and C. coli, C. upsaliensis was also found in these dogs. Living conditions influenced the occurrence of bacteria in these animals (Karama et al., 2019). Moreover, C. fetus was not identified as major problems of reproductive disorders in cattle from Southern Africa. However, this information should be carefully analyzed due to the low number of studies and representativeness (Madoroba et al., 2011; Njiro et al., 2012).
This systematic review confirmed that Campylobacter occurs in animals in Sub-Saharan Africa. Poor hygiene seems to be one of the major contributors to the spread of these bacteria in the region. The bacterium is commensal but also reported as potential cause of diarrhea in poultry and cattle and reproductive failure in cattle. Understudied animals such as quails, pigeons, camels, horses, grasscutters, guinea pigs, rats and especially crows deserve attention in future studies to know about the occurrence of different strains of this bacteria. The animals whose presence of Campylobacter was tested but was not found (0%) were donkeys (Conan et al., 2017), ring-tailed lemurs (Villers et al., 2008), mice and rabbits (Komba et al., 2014). All of these animals were tested only once and in Eastern Africa. Further research is needed to identify whether the bacteria occurs in these animals in different regions of Sub-Saharan Africa.
Countries such as Nigeria, Tanzania and South Africa are better represented in animal campylobacteriosis studies probably due to the economic development of the countries that have funds for research into Campylobacter. South Africa is best positioned in the human development index ranking, while Nigeria and Tanzania are ranked below (http://hdr.undp.org/en/content/2019-human-development-index-ranking). Many other Sub-Saharan African countries are in very low ranks. Less developed countries tend to invest less in research compared to most developed ones. In some studies, international partners were present as co-authors and probably supported the funding of the projects. In many Sub-Saharan African studies, local funding is insufficient to cover all areas of research. Most likely for this reason, many countries have few or no studies on Campylobacter.
3.4. Antibiotic resistance of Campylobacter spp. isolated from animals
From the 75 studies included in this review, a total of 26 studies from 10 countries have reports on antibiotic resistance, as described below:
-
•
Eastern Africa: Ethiopia (2 studies), Kenya (1), Madagascar (1) and Tanzania (4);
-
•
Middle Africa: Cameroon (1);
-
•
Southern Africa: South Africa (8)
-
•
Western Africa: Côte d’Ivoire (1), Ghana (1), Nigeria (5) and Senegal (2).
Supplementary material 5 summarizes the information of the antibiotic resistance studies. Campylobacter isolates for antibiotic resistance testing were found in ten different animals, namely: poultry (n = 2 studies; including chickens [16], ducks [1], guinea fowls [1]), cattle (8), pigs (5), sheep (4), goats (2), guinea pigs (1), horses (1) and rats (1).
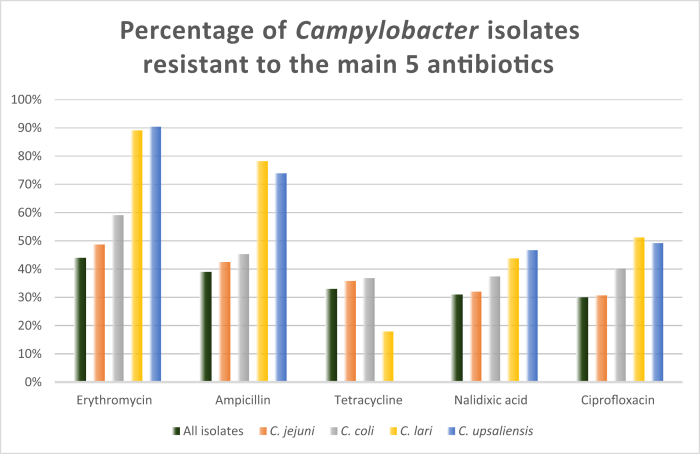
C. jejuni and C. coli were the most studied species, followed by C. lari and C. upsaliensis. A total of 49 different antibiotics were analyzed for 2481 isolates. Antibiotics with resistance in more than 10% of the isolates were: macrolides: erythromycin (44%); penincilins: ampicillin (39%); tetracycline (33%); fluoroquinolones: nalidixic acid (31%) and ciprofloxacin (30%); trimethoprim/sulfonamides: trimethoprim/sulfametoxazole (14%); aminoglycosides: streptomycin (13%) and gentamicin (12%) and cephalosporins: cephalothin (10%). Figure 3 shows the percentage of Campylobacter isolates resistant to erythromycin, ampicillin, tetracycline, nalidixic acid and ciprofloxacin.
Figure 3.
Percentage of Campylobacter isolates resistant to the main 5 antibiotics.
Fifteen antibiotics had no isolates with resistance, namely: aminoglycosides: neomycin; rifamycins: rifampicin; fluoroquinolones: levofloxacin, enrofloxacin and danofloxacin; lincosamides: lincomycin; tetracyclines: doxycycline; pleuromutilins: tiamulin; macrolides: telithromycin, tylosin, tulathromycin and tilmicosin; amphenicol: florfenicol and others: fosfomycin and spectinomycin.
The high percentage of isolates resistant to antibiotics, particularly for erythromycin (44%) is of particular concern as it is one of the drugs of choice for the clinical treatment of campylobacteriosis in complicated human cases (Igwaran and Ifeanyi, 2019; Zhou et al., 2016). The same is observed for ciprofloxacin that is used as therapeutic agent in countries from Sub-Saharan Africa (Abubakar et al., 2019; Bernadette et al., 2012; Ewnetu and Muhret, 2010; Kashoma et al., 2015b, Kashoma et al., 2015a; Komba et al., 2014; Uaboi-Egbenni et al., 2012). Resistance to ciprofloxacin is thought to have been caused by antibiotic use in humans, that is reported to be frequent in the region (Kashoma, et al., 2015a).
These resistances may be due to misuse in animal husbandry and also in human treatment (Kashoma, et al., 2015a). Poultry and cattle were the most tested animals for antibiotic resistance, followed by pigs and sheep. These are food animals commonly raised by both small breeders and large companies. The farmers commonly administer antibiotics to these animals as growth promoters and for prophylaxis purposes (Basardien, 2012; Karikari et al., 2017b; Uaboi-Egbenni et al., 2011a). In certain cases the farmers do not respect the withdrawal period and proper dosages (Ewnetu and Muhret, 2010; Olabode et al., 2017; Salihu et al., 2012) and this leads to antibiotic resistance. Moreover, many studies reported high levels of resistance to antibiotics commonly used in the poultry, cattle and swine industry (erythromycin, ciprofloxacin and tetracycline) (Akwuobu et al., 2010; Bester and Essack, 2012; Ewnetu and Muhret, 2010; Garin et al., 2012; Jonker and Picard, 2012; Kassa et al., 2007; Uaboi-Egbenni et al., 2011a, 2012).
Another fact that shows that large-scale food animals production contributes to antibiotic resistance is the significantly less resistance of the isolates from rurally raised chickens against ciprofloxacin (7.9%), erythromycin (0%) and tetracycline (21.6%) than those from commercially produced chickens in Kwazulu-Natal (Bester and Essack, 2012).
Prolonged exposure of animals to antibiotics may also contribute to increased resistance, such as observed in ducks in Tanzania (Nonga and Muhairwa, 2009).
Beta lactamase production by Campylobacter may be responsible for the high frequency of beta-lactam resistant strains (Jonker and Picard, 2012) such as to ampicillin (Kashoma, et al., 2015b; Nonga and Muhairwa, 2009). Additionally, treatment of Campylobacter using beta lactamases is not recommended as it is believed that C. jejuni wall is impenetrable for these antibiotics (Jonker and Picard, 2012).
High tetracycline resistance may be because it is the most widely used drug in veterinary medicine in some countries in the region, such as Tanzania and Ghana (Karikari et al., 2017a; Nonga and Muhairwa, 2009). It may also be due to overuse, which has led to the development of new resistance genes that have been transmitted between the bacteria (Nonga and Muhairwa, 2009). Resistance to many antibiotics may also be due to efflux pumps that remove antibiotics from the bacteria's cytosol (Jonker and Picard, 2012).
Antibiotic resistance was also found in isolates from animals not used for human consumption such as horses, guinea pigs and rats. Studies from other Sub-Saharan African countries, animals and other Campylobacter species (such as C. fetus, C. hyointestinalis, C. sputorum and C. troglodytis) did not test for antibiotic resistance. However, this does not indicate that there are no resistant circulating species in these cases. This should be the subject of future studies.
Multiple drug resistance is also commonly reported and a major concern (Abubakar et al., 2019; Akwuobu et al., 2010; Basardien, 2012; Cardinale et al., 2002; Jonker and Picard, 2012; Kambuyi, 2018; Karikari et al., 2017b; Kashoma et al., 2015a, Kashoma et al., 2015b; Kassa et al., 2007; Nonga and Muhairwa, 2009; Ogbor et al., 2019; Okunlade et al., 2015; Salihu et al., 2012; Uaboi-Egbenni et al., 2010, 2011a, 2012) as resistance genes can be shared between the bacteria (Uaboi-Egbenni et al., 2012) which leads to increasing resistant species. Some recent studies also report on antibiotic resistance genes (Abubakar et al., 2019; Kambuyi, 2018).
This antibiotic resistance can be transmitted to humans through the close contact between animals and humans and consumption of the contaminated food-animals meat (Nonga and Muhairwa, 2009; Uaboi-Egbenni et al., 2012). It is a major concern for both human and animal health.
The resistance scenario may be even more worrying than reported in this study, as the type of test influences the results. For example, in Tanzania, some isolates that were susceptible or intermediate to different antibiotic through disk diffusion were resistant to same antibiotics using broth microdilution methods (Kashoma et al., 2015a, Kashoma et al., 2015b).
As mentioned before, food-animals were more prominent in studies of antibiotic resistance. This shows that animal husbandry practices are having a major impact in increasing antibiotic resistance. Meanwhile, guinea pigs, horses and rats were also found to have resistant Campylobacter isolates. This also deserves particular attention in future studies as potential transmitters of resistant isolates to other animals and to humans.
This review reiterates that Campylobacter is resistant to fluoroquinolones, which was already a WHO concern (WHO, 2017), and there is also the concern of high resistance to macrolides as erythromycin (44%), penincilins as ampicillin (39%) and to tetracyclines (33%).
The use of antibiotics must be monitored and antibiotic surveillance must be done in order to reduce the spread of resistant strains into the environment. The antimicrobial resistance in both humans and animals leads to treatment failure and difficulty in case management (Ogbor et al., 2019). It will also limit the therapeutic choice, increasing hospitalization rates and cost of therapy, and decreasing the survival rate of patients (Jonker and Picard, 2012).
Bearing in mind the devastating consequences of antibiotic resistance within the African continent, countries like Nigeria, South Africa, Tanzania and Mozambique already have in place national plans to promote the rational use of antimicrobials in the context of “One Health” approach (DAFF, 2018; Federal Ministries of Agriculture Environment and Health, 2017; GARP-Moçambique, 2015; Ministry of Agriculture Livestock and Fisheries; and MoHCDEC, 2017).
For animals, the common objectives of these national plans include antimicrobial resistance surveillance at all levels of the agriculture food chain. Greater emphasis is given to food-producing animals such as meat, milk and eggs producing animals. The plans propose to set the minimum residue limits for antimicrobials in animals and animal products; promote appropriate use of antimicrobials through antimicrobial stewardship practices and controlled access to antimicrobials; establish a functioning vaccination program to prevent new infections and orient animal health workers on hygiene and safety standards and waste management.
However, the efficient implementation of those plans is still challenging due to limited access of financial and technical resources in most of Sub-Saharan African countries (IACG, 2018; ReAct, 2017).
4. Conclusions
The information on the prevalence of Campylobacter spp. in animals in Sub-Saharan Africa varies considerably because different methods were used to detect and identify the bacteria. Thus, the prevalences can be higher than those reported here, taking into account the different precision and accuracy of each method. For example, depending on the time and form of storage of the sample, the culture may not identify all bacteria that were previously present, since Campylobacter is anaerobic and extremely fragile. On the other hand, PCR can reveal very high prevalences, which may not correspond to a current infection, but may result from previous infections in which fragments of DNA from the bacteria are being eliminated after treatment. ELISA is a test used less frequently to detect Campylobacter and depending on the test, it may not discriminate between recent and old infections. Likewise, different sample matrices were used in the different studies. Some studies used fecal samples, sheath washings, preputial scrapings, intestinal contents, liver tissue, caeca, vaginal swabs and cervico-vaginal mucus, whereas others have used carcass samples at slaughterhouses. Therefore, the choice of matrix has significant impact on the outcome since each type of sample can hold different amounts of the bacteria and depending on the storage, the survival of the bacteria in the sample can be compromised, which influences its detection. Additionally different animals were tested using different sampling methods, in different geographic locations and conditions, which also influences the prevalence.
Overall, Campylobacter spp. infections are common in animals in Sub-Saharan Africa, mainly in cattle and chickens. Poor hygiene seems to be a major contributor to the spread of the bacteria in the region. However, food animals, household animals or animals that inhabit the forests harbor this bacteria in the region. These animals may be a source of contamination to humans. Frequently isolated species are C. jejuni and C. coli. New Campylobacter species were discovered and isolated from wild chimpanzees. The sub-region with the least studies was Middle Africa, where studies focused on food animals. It is necessary to know if household or other animals are also contaminated by Campylobacter in that region.
Understudied animals such as quails, pigeons, camels, horses, grasscutters, rats, guinea pigs and crows deserve attention in future studies to understand about the occurrence of different strains of these bacteria. Animals that had 0% of prevalence also deserve attention in future studies, namely: donkeys, ring-tailed lemurs, mice and rabbits.
Antibiotic resistance is a worrisome concern mainly in food-animals probably due to misuse by humans with disease and farmers. Erythromycin, ampicillin, tetracycline, nalidixic acid and ciprofloxacin are the drugs with most resistant isolates and this is a major public health concern.
Meanwhile, guinea pigs, horses and rats were also found to have resistant Campylobacter isolates. These also deserve particular attention in future studies as potential transmitters of resistant isolates to other animals and to humans.
Campylobacter species are not only a risk of disease to humans but are also associated with disease in animals such as C. fetus that causes reproductive failure in cattle; and C. jejuni and C. coli that are potentially causes of diarrhea in poultry and cattle.
Updated data on Campylobacter spp. epidemiology and antibiotic resistance are lacking in most of Sub Saharan African countries. Therefore, more research work is warranted to obtain an updated and detailed data on these subjects. These data would allow the development of control strategies to tackle campylobacteriosis in animals and humans in the region and to reinforce the implementation of antibiotic resistance control plans in each country.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Competing interest statement
The authors declare no conflict of interest.
Additional information
No additional information is available for this paper.
Acknowledgements
We thank Professor Virgílio do Rosário, retired Professor from Instituto de Higiene e Medicina Tropical, Universidade Nova de Lisboa for helping to draft and edit the manuscript.
Appendix A. Supplementary data
The following are the supplementary data related to this article:
References
- Aboaba O.O., Smith S.I. Occurrence of Campylobacter species in poultry forms in Lagos area of Nigeria. J. Environ. Biol. 2005;26(2):403–408. [PubMed] [Google Scholar]
- Aboderin A.O., Smith S.I., Oyelese A.O., Onipede A.O., Zailani S.B., Coker A.O. Role of Campylobacter jejunilcoli in Ile-Ife, Nigeria. East Afr. Med. J. 2002;79(8):423–426. [PubMed] [Google Scholar]
- Abubakar M.K., Muigai A.W.T., Ndung’u P., Kariuki S. Investigating carriage, contamination, antimicrobial resistance and assessment of colonization risk factors of Campylobacter spp. in broilers from selected farms in Thika, Kenya. Microbiol. Res. J. Int. 2019;27(6):1–16. [Google Scholar]
- Achá S.J., Kühn I., Jonsson P., Mbazima G., Katouli M., Möllby R. Studies on calf diarrhoea in Mozambique: prevalence of bacterial pathogens. Acta Vet. Scand. 2004;45(1):27–36. doi: 10.1186/1751-0147-45-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akwuobu C.A., Oboegbulem S.I., Ofukwu R.A. Characterization and antibiogram of local isolates of Campylobacter species from chicken in Nsukka area, southeast Nigeria. Am.-Eurasian J. Sustain. Agric. 2010;4(2):117–121. [Google Scholar]
- Bartkowiak-Higgo A.J., Veary C.M., Venter E.H., Bosman A.M. A pilot study on post-evisceration contamination of broiler carcasses and ready-to-sell livers and intestines (Mala) with Campylobacter jejuni and Campylobacter coli in a high-throughput South African poultry abattoir. J. S. Afr. Vet. Assoc. 2006;77(3):114–119. doi: 10.4102/jsava.v77i3.357. [DOI] [PubMed] [Google Scholar]
- Basardien L. University of the Western Cape; 2012. Molecular Characterization of Campylobacter Isolates from Free Range and Commercial Chicken in South Africa.http://etd.uwc.ac.za/xmlui/handle/11394/5068 [Google Scholar]
- Bernadette G., Essoh A.E., Solange K-N.E., Natalie G., Souleymane B., Sébastien N.L., Mireille D. Prevalence and antimicrobial resistance of thermophilic Campylobacter isolated from chicken in Côte d’Ivoire. Int. J. Microbiol. 2012;2012(150612):1–6. doi: 10.1155/2012/150612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthenet E., Thépault A., Chemaly M., Rivoal K., Ducournau A., Buissonnière A., Bénéjat L., Bessède E., Mégraud F., Sheppard S.K., Lehours P. Source attribution of Campylobacter jejuni shows variable importance of chicken and ruminants reservoirs in non-invasive and invasive French clinical isolates. Sci. Rep. 2019;9(1):1–8. doi: 10.1038/s41598-019-44454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bester L.A., Essack S.Y. Prevalence of antibiotic resistance in Campylobacter isolates from commercial poultry suppliers in KwaZulu-Natal, South Africa. J. Antimicrob. Chemother. 2008;62(6):1298–1300. doi: 10.1093/jac/dkn408. [DOI] [PubMed] [Google Scholar]
- Bester L.A., Essack S.Y. Observational study of the prevalence and antibiotic resistance of Campylobacter spp. from different poultry production systems in KwaZulu-Natal, South Africa. J. Food Protect. 2012;75(1):154–159. doi: 10.4315/0362-028X.JFP-11-237. [DOI] [PubMed] [Google Scholar]
- Cardinale E., Dromingny J.-A., Tall F., Ndieaye M., Konte M., Perrier Gros-Claude J.D. Antimicrobial susceptibility of Campylobacter strains isolated from chicken carcasses in Senegal. Rev. Elev. Méd. Vét. Pays Trop. 2002;55(4):259–264. [Google Scholar]
- Cardinale E., Tall F., Cisse M., Guèye E.H.F., Salvat G. Proceedings of the 10th International Symposium on Veterinary Epidemiology and Economics. 2003. Risk Factors for Salmonella Enterica Subsp. Enterica and Campylobacter Spp. Contamination of Chicken Carcasses in Senegal; pp. 1–3.http://www.sciquest.org.nz/elibrary/download/63233/Risk_Factors_for_Salmonella_enterica_subsp_enteric.pdf? [Google Scholar]
- Cardinale E., Tall F., Guèye E.F., Cisse M., Salvat G. Risk factors for Campylobacter spp. infection in Senegalese broiler-chicken flocks. Prev. Vet. Med. 2004;64(1):15–25. doi: 10.1016/j.prevetmed.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Cheng Y.W., Fischer M. Campylobacter. Ref. Modul. Biomed. Sci. 2018;1–5 [Google Scholar]
- Chuma I.S., Nonga H.E., Mdegela R.H., Kazwala R.R. Epidemiology and RAPD-PCR typing of thermophilic campylobacters from children under five years and chickens in Morogoro Municipality, Tanzania. BMC Infect. Dis. 2016;16:1–11. doi: 10.1186/s12879-016-2031-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conan A., O’Reilly C.E., Ogola E., Ochieng J.B., Blackstock A.J., Omore R., Ochieng L., Moke F., Parsons M.B., Xiao L., Roellig D., Farag T.H., Nataro J.P., Kotloff K.L., Levine M.M., Mintz E.D., Breiman R.F., Cleaveland S., Knobel D.L. Animal-related factors associated with moderate-to-severe diarrhea in children younger than five years in western Kenya: a matched case-control study. PLoS Neglected Trop. Dis. 2017;11(8) doi: 10.1371/journal.pntd.0005795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connerton I.F., Connerton P.L. Foodborne Diseases. Third Edition. Elsevier; 2017. Chapter 8 - Campylobacter Foodborne Disease. [Google Scholar]
- DAFF . South African Antimicrobial Resistance National Strategy Framework; A One Health Approach 2018 – 2024. Departments of Health and Agriculture, Forestry and Fisheries for the Republic of South Africa; 2018. pp. 1–22.http://www.health.gov.za/index.php/antimicrobial-resistance?download=3308:amr-national-action-plan-2018-2024 [Google Scholar]
- Ewnetu D., Mihret A. Prevalence and antimicrobial resistance of Campylobacter isolates from humans and chickens in Bahir Dar, Ethiopia. Foodborne Pathog. Dis. 2010;7(6):667–670. doi: 10.1089/fpd.2009.0433. [DOI] [PubMed] [Google Scholar]
- Federal Ministries of Agriculture Environment and Health . Nigeria; 2017. National Action Plan for Antimicrobial Resistance 2017–2022. [Google Scholar]
- García-Sánchez L., Melero B., Rovira J. Campylobacter in the food chain. Adv. Food Nutr. Res. 2018;86:215–252. doi: 10.1016/bs.afnr.2018.04.005. [DOI] [PubMed] [Google Scholar]
- Garin B., Gouali M., Wouafo M., Perchec A.M., Pham M.T., Ravaonindrina N., Urbès F., Gay M., Diawara A., Leclercq A., Rocourt J., Pouillot R. Prevalence, quantification and antimicrobial resistance of Campylobacter spp. on chicken neck-skins at points of slaughter in 5 major cities located on 4 continents. Int. J. Food Microbiol. 2012;157(1):102–107. doi: 10.1016/j.ijfoodmicro.2012.04.020. [DOI] [PubMed] [Google Scholar]
- GARP-Moçambique . Washington, DC e Nova Deli Centro para estudo de Dinâmica de Doenças, Economia e Política (CDDEP); 2015. Análise Situacional e Recomendações: Uso e Resistência Aos Antibióticos Em Moçambique; pp. 1–20.https://www.cddep.org/wp-content/uploads/2017/06/executive_summary_portugues_language_1.pdf [Google Scholar]
- Gwimi P.B., Faleke O.O., Salihu M.D., Magaji A.A., Abubakar M.B., Nwankwo I.O., Ibitoye E.B. Prevalence of Campylobacter species in fecal samples of pigs and humans from Zuru Kebbi state, Nigeria. Int. J. One Health. 2015;1:1–5. [Google Scholar]
- Henry I., Reichardt J., Denis M., Cardinale E. Prevalence and risk factors for Campylobacter spp. in chicken broiler flocks in Reunion Island (Indian Ocean) Prev. Vet. Med. 2011;100(1):64–70. doi: 10.1016/j.prevetmed.2011.03.007. [DOI] [PubMed] [Google Scholar]
- Humphrey S., Chaloner G., Kemmett K., Davidson N., Williams N., Kipar A., Humphrey T., Wigley P. Campylobacter jejuni is not merely a commensal in commercial broiler chickens and affects bird welfare. mBio. 2014;5(4) doi: 10.1128/mBio.01364-14. e01364-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IACG . Interagency Coordination Group on Antimicrobial Resistance; 2018. Antimicrobial resistance: national action plans; pp. 1–14.https://www.reactgroup.org/wp-content/uploads/2017/10/RAN_Conference-2017-Report.pdf [Google Scholar]
- Igwaran A., Ifeanyi A. Human campylobacteriosis: a public health concern of global importance. Heliyon. 2019;5 doi: 10.1016/j.heliyon.2019.e02814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonker A., Picard J.A. Antimicrobial susceptibility in thermophilic Campylobacter species isolated from pigs and chickens in South Africa. J. S. Afr. Vet. Assoc. 2012;81(4):228–236. doi: 10.4102/jsava.v81i4.153. [DOI] [PubMed] [Google Scholar]
- Julien C.K., Bernadette G., Stephane K.K., Stephane K.K., Forget K.G., Hortense F.K., Agathe F., Mireille D. Emergence of Campylobacter spp. in grasscutter (Thryonomys Swinderianus, Temminck, 1827) Asian Pac. J. Trop. Dis. 2013;3(4):320–322. [Google Scholar]
- Kaakoush N.O., Castaño-Rodríguez N., Mitchell H.M., Man S.M. Global epidemiology of Campylobacter infection. Clin. Microbiol. Rev. 2015;28(3):687–720. doi: 10.1128/CMR.00006-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kagambèga A., Thibodeau A., Trinetta V., Soro D.K., Sama F.N., Bako É., Bouda C.S., N’Diaye A.W., Fravalo P., Barro N. Salmonella spp. and Campylobacter spp. in poultry feces and carcasses in Ouagadougou, Burkina Faso. Food Sci. Nutr. 2018;6(6):1601–1606. doi: 10.1002/fsn3.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalema-Zikusoka G., Rothman J.M., Fox M.T. Intestinal parasites and bacteria of mountain Gorillas (Gorilla beringei beringei) in Bwindi Impenetrable National Park, Uganda. Primates. 2005;46(1):59–63. doi: 10.1007/s10329-004-0103-y. [DOI] [PubMed] [Google Scholar]
- Kambuyi K. University of Pretoria; 2018. Occurrence and antimicrobial resistance of Campylobacter spp. isolates from beef cattle in Gauteng and Northwest Provinces, South Africa.https://repository.up.ac.za/handle/2263/70601 [Google Scholar]
- Karama M., Cenci-Goga B.T., Prosperi A., Etter E., El-Ashram S., McCrindle C., Ombui J.N., Kalake A. Prevalence and risk factors associated with Campylobacter spp. occurrence in healthy dogs visiting four rural community veterinary clinics in South Africa. Onderstepoort J. Vet. Res. 2019;86(1):e1–e6. doi: 10.4102/ojvr.v86i1.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karikari A.B., Obiri-Danso K., Frimpong E.H., Krogfelt K.A. Antibiotic resistance in Campylobacter isolated from patients with gastroenteritis in a teaching hospital in Ghana. Open J. Med. Microbiol. 2017;7:1–11. [Google Scholar]
- Karikari A.B., Obiri-Danso K., Frimpong E.H., Krogfelt K.A. Antibiotic resistance of Campylobacter recovered from faeces and carcasses of healthy livestock. BioMed Res. Int. 2017;2017:1–9. doi: 10.1155/2017/4091856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karshima N.S., Benshak J.A., Tumba J.K. Isolation and molecular characterization of Campylobacter coli among trade pigs in Kafanchan, Kaduna state, Nigeria. Bull. Anim. Health Prod. Afr. 2016;64(3):319–326. [Google Scholar]
- Karshima S.N., Bobbo A.A. Isolation and PCR characterisation of thermophilic Campylobacter species in dogs presented to selected veterinary clinics in Jos, Nigeria. Alexandria J. Vet. Sci. 2016;50(1):70–77. [Google Scholar]
- Kashoma I.P., Kassem I.I., Kumar A., Kessy B.M., Gebreyes W., Kazwala R.R., Rajashekara G. Antimicrobial resistance and genotypic diversity of Campylobacter isolated from pigs, dairy, and beef cattle in Tanzania. Front. Microbiol. 2015;6(1240):1–11. doi: 10.3389/fmicb.2015.01240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashoma I.P., Mkomba F.D., Bunyaga A.S. Prevalence and antimicrobial resistance in Campylobacter from different stages of the chicken meat supply chain in Morogoro, Tanzania. Tanzan. Vet. J. 2015;30(2):41–60. [Google Scholar]
- Kassa T., Gebre-Selassie S., Asrat D. Antimicrobial susceptibility patterns of thermotolerant Campylobacter strains isolated from food animals in Ethiopia. Vet. Microbiol. 2007;119(1):82–87. doi: 10.1016/j.vetmic.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Kaur T., Singh J., Huffman M.A., Petrželková K.J., Taylor N.S., Xu S., Dewhirst F.E., Paster B.J., Debruyne L., Vandamme P., Fox J.G. Campylobacter troglodytis sp. nov., isolated from feces of human-habituated wild chimpanzees (Pan troglodytes schweinfurthii) in Tanzania. Appl. Environ. Microbiol. 2011;77(7):2366–2373. doi: 10.1128/AEM.01840-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komba E.V.G., Mdegela R.H., Msoffe P.L.M., Matowo D.E., Maro M.J. Occurrence, species distribution and antimicrobial resistance of thermophilic Campylobacter isolates from farm and laboratory animals in Morogoro, Tanzania. Vet. World. 2014;7:559–565. [Google Scholar]
- Komba E.V.G., Mdegela R.H., Msoffe P.L.M., Ingmer H. Human and animal Campylobacteriosis in Tanzania: A review. Tanzan. J. Health Res. 2013;15(1):1–13. doi: 10.4314/thrb.v15i1.6. https://www.ajol.info/index.php/thrb/article/viewFile/68676/74713 [DOI] [PubMed] [Google Scholar]
- Kusiluka L.J.M., Karimuribo E.D., Mdegela R.H., Luoga E.J., Munishi P.K.T., Mlozi M.R.S., Kambarage D.M. Prevalence and impact of water-borne zoonotic pathogens in water, cattle and humans in selected villages in dodoma rural and bagamoyo districts, Tanzania. Phys. Chem. Earth. 2005;30:818–825. [Google Scholar]
- Madoroba E., Awoke G., Tiny H., Mkhevu M. Prevalence of Campylobacter foetus and trichomonas foetus among cattle from southern Africa. Afr. Health Sci. 2011;10(50):10311–10314. [Google Scholar]
- Mai H.M., Irons P.C., Kabir J., Thompson P.N. Herd-level risk factors for Campylobacter fetus infection, Brucella seropositivity and within-herd seroprevalence of brucellosis in cattle in northern Nigeria. Prev. Vet. Med. 2013;111(3–4):256–267. doi: 10.1016/j.prevetmed.2013.05.016. [DOI] [PubMed] [Google Scholar]
- Mai H.M., Irons P.C., Kabir J., Thompson P.N. Prevalence of bovine genital campylobacteriosis and trichomonosis of bulls in northern Nigeria. Acta Vet. Scand. 2013;55:56. doi: 10.1186/1751-0147-55-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai H.M., Irons P.C., Thompson P.N. Brucellosis, genital campylobacteriosis and other factors affecting calving rate of cattle in three states of northern Nigeria. BMC Vet. Res. 2015;11(1):1–13. doi: 10.1186/s12917-015-0317-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mdegela R.H., Laurence K., Jacob P., Nonga H.E. Occurrences of thermophilic Campylobacter in pigs slaughtered at Morogoro slaughter slabs, Tanzania. Trop. Anim. Health Prod. 2011;43(1):83–87. doi: 10.1007/s11250-010-9657-4. [DOI] [PubMed] [Google Scholar]
- Mdegela R.H., Nonga H.E., Ngowi H.A., Kazwala R.R. Prevalence of thermophilic Campylobacter infections in humans, chickens and crows in Morogoro, Tanzania. J. Vet. Med. B. 2006;53:116–121. doi: 10.1111/j.1439-0450.2006.00926.x. [DOI] [PubMed] [Google Scholar]
- Ministry of Agriculture Livestock and Fisheries. MoHCDEC . Tanzania; 2017. The National Action Plan on Antimicrobial Resistance 2017-2022.https://www.flemingfund.org/wp-content/uploads/8b8fc897c422e11504c8c2ba126fac02.pdf [Google Scholar]
- Mokantla E., McCrindle C.M., Sebei J.P., Owen R. An investigation into the causes of low calving percentage in communally grazed cattle in Jericho, North West Province. J. S. Afr. Vet. Assoc. 2004;75(1):30–36. doi: 10.4102/jsava.v75i1.445. [DOI] [PubMed] [Google Scholar]
- Mpalang R.K., Boreux R., Melin P., Bitiang K.M.A., Daube G., De Mol P. Prevalence of Campylobacter among goats and retail goat meat in Congo. J. Infect. Dev. Ctries. 2014;8(2):168–175. doi: 10.3855/jidc.3199. [DOI] [PubMed] [Google Scholar]
- Mshelia G.D., Amin J.D., Egwu G.O., Woldehiwet Z., Murray R.D. The prevalence of bovine venereal campylobacteriosis in cattle herds in the lake Chad basin of Nigeria. Trop. Anim. Health Prod. 2012;44(7):1487–1489. doi: 10.1007/s11250-012-0092-6. [DOI] [PubMed] [Google Scholar]
- Mshelia G.D., Amin J.D., Egwu G.O., Yavari C.A., Murray R.D., Woldehiwet Z. Detection of antibodies specific to Campylobacter fetus subsp. venerealis in the vaginal mucus of Nigerian breeding cows. Vet. Ital. 2010;46(3):337–344. [PubMed] [Google Scholar]
- Ngotho M., Ngure R.M., Kamau D.M., Kagira J.M., Gichuki C., Farah I.O., Sayer P.D., Hau J. A fatal outbreak of Campylobacter jejuni enteritis in a colony of vervet monkeys in Kenya. Scand. J. Lab. Anim. Sci. 2006;33(4):205–210. [Google Scholar]
- Ngulukun S.S., Oboegbulem S.I., Fagbamila I.O., Bertu W., Odugbo M.O. Prevalence and molecular characterization of thermophilic Campylobacter species isolated from cattle in Plateau State, Nigeria. Niger. Vet. J. 2011;32(4):349–356. https://www.ajol.info/index.php/nvj/article/view/85623/75545 [Google Scholar]
- Ngulukun S.S., Oboegbulem S.I., Fagbamila I.O., Emennaa P.E., Ankeli P.I., Ardzard S.S., Okeke L.A., Ajayi O.T., Usman M., Muhammed M.J., Odugbo M.O., Okewole P.A. Isolation of thermophilic Campylobacter species from Japanese quails (Coturnix coturnix) in Vom, Nigeria. Vet. Rec. 2010;166(5):147–148. doi: 10.1136/vr.b4787. [DOI] [PubMed] [Google Scholar]
- Nichols G.L., Richardson J.F., Sheppard S.K., Lane C., Sarran C. Campylobacter epidemiology: a descriptive study reviewing 1 million cases in England and wales between 1989 and 2011. BMJ Open. 2012;2(4) doi: 10.1136/bmjopen-2012-001179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nizeyi J.B., Innocent R.B., Erume J., Kalema G.R., Cranfield M.R., Graczyk T.K. Campylobacteriosis, salmonellosis, and shigellosis in free-ranging human-habituated mountain gorillas of Uganda. J. Wildl. Dis. 2001;37(2):239–244. doi: 10.7589/0090-3558-37.2.239. [DOI] [PubMed] [Google Scholar]
- Njiro S.M., Kidanemariam A.G., Tsotetsi A.M., Katsande T.C., Mnisi M., Lubisi B.A., Potts A.D., Baloyi F., Moyo G., Mpofu J., Kalake A., Williams R. A study of some infectious causes of reproductive disorders in cattle owned by resource-poor farmers in gauteng province, South Africa. J. S. Afr. Vet. Assoc. 2012;82(4):213–218. doi: 10.4102/jsava.v82i4.76. [DOI] [PubMed] [Google Scholar]
- Nonga H.E., Muhairwa A.P. Prevalence and antibiotic susceptibility of thermophilic Campylobacter isolates from free range domestic duck (Cairina Moschata) in Morogoro Municipality, Tanzania. Trop. Anim. Health Prod. 2009;42(2):165–172. doi: 10.1007/s11250-009-9401-0. [DOI] [PubMed] [Google Scholar]
- Nonga H.E., Sells P., Karimuribo E.D. Occurrences of thermophilic Campylobacter in cattle slaughtered at Morogoro abattoir, Tanzania. Trop. Anim. Health Prod. 2009;42(1):73–78. doi: 10.1007/s11250-009-9387-7. [DOI] [PubMed] [Google Scholar]
- Nwankwo I.O., Faleke O.O., Salihu M.D., Magaji A.A., Musa U., Garba J. Epidemiology of Campylobacter species in poultry and humans in the four agricultural zones of Sokoto state , Nigeria. J. Publ. Health Epidemiol. 2016;8(9):184–190. [Google Scholar]
- Ofukwu R.A., Okoh A.E.J., Akwuobu C.A. Prevalence of Campylobacter jejuni in duck faeces around drinking water sources in Makurdi, north-Central Nigeria. Sokoto J. Vet. Sci. 2008;7(2):26–30. [Google Scholar]
- Ogbor O., Ajayi A., Zautner A.E., Smith S.I. Antibiotic susceptibility profiles of Campylobacter coli isolated from poultry farms in Lagos Nigeria – a pilot study. Eur. J. Microbiol. Immunol. 2019;9(2):32–34. doi: 10.1556/1886.2019.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunlade A.O., Ogunleye A.O., Jeminlehin F.O., Ajuwape A.T.P. Occurrence of Campylobacter species in beef cattle and local chickens and their antibiotic profiling in Ibadan, Oyo state, Nigeria. Afr. J. Microbiol. Res. 2015;9(22):1473–1479. [Google Scholar]
- Olabode H.O.K., Mailafia S., Ogbole M.E., Okoh G.R., Ifeanyi C.I.C., Onigbanjo H.O., Ugbaja I.B. Isolation and antibiotic susceptibility of Campylobacter species from cattle offals in Gwagwalada abattoir, Abuja-FCT Nigeria. Int. J. Curr. Microbiol. Appl. Sci. 2017;6(4):324–333. [Google Scholar]
- Osbjer K., Tano E.V.A., Chhayheng L., Mac-Kwashie A.O., Fernström L.L. Detection of Campylobacter in human and animal field samples in Cambodia. APMIS. 2016;124(5):508–515. doi: 10.1111/apm.12531. [DOI] [PubMed] [Google Scholar]
- Pike B.L., Guerry P., Poly F. Global Distribution of Campylobacter jejuni Penner Serotypes: A Systematic Review. PloS One. 2013;8(6) doi: 10.1371/journal.pone.0067375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintar K.D.M., Christidis T., Thomas M.K., Anderson M., Nesbitt A., Keithlin J., Marshall B., Pollari F. A systematic review and meta-analysis of the Campylobacter spp. prevalence and concentration in household pets and petting zoo animals for use in exposure assessments. PloS One. 2015;10(12) doi: 10.1371/journal.pone.0144976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raji M.A., Adekeye J.O., Kwaga J.K.P., Bale J.O.O. Bioserogroups of Campylobacter species isolated from sheep in Kaduna state, Nigeria. Small Rumin. Res. 2000;37(3):215–221. doi: 10.1016/s0921-4488(00)00125-5. [DOI] [PubMed] [Google Scholar]
- ReAct . Action on Antibiotic Resistance (ReAct) Africa Annual Conference 2017. ReAct; 2017. Moving Beyond Antimicrobial Resistance (AMR) National Action Plans Development to Implementation; p. 23.https://www.reactgroup.org/wp-content/uploads/2017/10/RAN_Conference-2017-Report.pdf [Google Scholar]
- Sahin O., Yaeger M., Wu Z., Zhang Q. Campylobacter-associated diseases in animals. Annu. Rev. Anim. Biosci. 2017;5(9):1–22. doi: 10.1146/annurev-animal-022516-022826. [DOI] [PubMed] [Google Scholar]
- Salihu M.D., Abdulkadir J.U., Oboegbulem S.I., Egwu G.O., Magaji A.A., Lawal M., Hassan Y. Isolation and prevalence of Campylobacter species in cattle from Sokoto state, Nigeria. Vet. Ital. 2009;45(4):501–505. [PubMed] [Google Scholar]
- Salihu M.D., Junaidu A.U., Abubakar M.B., Magaji A.A., Mohammed L.G. Isolation and characterization of Campylobacter species from camel (Camelus dramedarius) in Sokoto state, northwestern Nigeria. Int. J. Anim. Vet. Adv. 2009;1(1):25–27. [Google Scholar]
- Salihu M.D., Junaidu A.U., Oboegbulem S.I., Egwu G.O. Prevalence and biotypes of Campylobacter species isolated from sheep in Sokoto state, Nigeria. Int. J. Anim. Vet. Adv. 2009;1(1):6–9. [Google Scholar]
- Salihu M.D., Junaidu A.U., Magaji A.A., Yakibu Y. Prevalence and antimicrobial resistance of thermophilic Campylobacter isolates from commercial broiler flocks in Sokoto, Nigeria. Res. J. Vet. Sci. 2012;5(2):51–58. [Google Scholar]
- Salihu M.D., Magaji A.A., Abdulkadir J.U., Kolawale A. Survey of thermophilic Campylobacter species in cats and dogs in north-western Nigeria. Vet. Ital. 2010;46(4):425–430. [PubMed] [Google Scholar]
- Salihu M., Junaidu A., Oboegbulem S., Egwu G., Magaji A., Abubakar M., Ogbole A. Prevalence of Campylobacter spp. in Nigerian indigenous chicken in Sokoto state northwestern Nigeria. Internet J. Vet. Med. 2008;7(1):1–5. [Google Scholar]
- Sheppard S.K., Colles F.M., McCarthy N.D., Strachan N.J.C., Ogden I.D., Forbes K.J., Dallas J.F., Maiden M.C.J. Niche segregation and genetic structure of Campylobacter jejuni populations from wild and agricultural host species. Mol. Ecol. 2011;20(16):3484–3490. doi: 10.1111/j.1365-294X.2011.05179.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard S.K., Colles F., Richardson J., Cody A.J., Elson R., Lawson A., Brick G., Meldrum R., Little C.L., Owen R.J., Maiden M.C.J., Mccarthy N.D. Host association of Campylobacter genotypes transcends geographic variation. Appl. Environ. Microbiol. 2010;76(15):5269–5277. doi: 10.1128/AEM.00124-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard S.K., Cheng L., Méric G., De Haan C.P.A., Llarena A.K., Marttinen P., Vidal A., Ridley A., Clifton-Hadley F., Connor T.R., Strachan N.J.C., Forbes K., Colles F.M., Jolley K.A., Bentley S.D., Maiden M.C.J., Hänninen M.L., Parkhill J., Hanage W.P., Corander J. Cryptic ecology among host generalist Campylobacter jejuni in domestic animals. Mol. Ecol. 2014;23(10):2442–2451. doi: 10.1111/mec.12742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard S.K., Dallas J.F., MacRae M., McCarthy N.D., Sproston E.L., Gormley F.J., Strachan N.J.C., Ogden I.D., Maiden M.C.J., Forbes K.J. Campylobacter genotypes from food animals, environmental sources and clinical disease in Scotland 2005/6. Int. J. Food Microbiol. 2014;134:96–103. doi: 10.1016/j.ijfoodmicro.2009.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheppard S.K., Dallas J.F., Strachan N.J.C., MacRae M., McCarthy N.D., Wilson D.J., Gormley F.J., Falush D., Ogden I.D., Maiden M.C.J., Forbes K.J. Campylobacter genotyping to determine the source of human infection. Clin. Infect. Dis. 2009;48(8):1072–1078. doi: 10.1086/597402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sproston E.L., Wimalarathna H.M.L., Sheppard S.K. Trends in fluoroquinolone resistance in Campylobacter. Microb. Genom. 2018;4(8) doi: 10.1099/mgen.0.000198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swai E.S., Hulsebosch J., Van Der Heijden W. Prevalence of genital campylobacteriosis and trichomonosis in crossbred breeding bulls kept on zero-grazed smallholder dairy farms in the Tanga region of Tanzania. J. S. Afr. Vet. Assoc. 2005;76(4):224–227. doi: 10.4102/jsava.v76i4.431. [DOI] [PubMed] [Google Scholar]
- Thépault A., Guillaume M., Rivoal K., Pascoe B., Mageiros L., Touzain F., Rose V., Béven V., Chemaly M., Sheppard S.K. Segregating epidemiological markers for source attribution in Campylobacter. Appl. Environ. Microbiol. 2017;83(7) doi: 10.1128/AEM.03085-16. e03085-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thépault A., Rose V., Quesne S., Poezevara T., Béven V., Hirchaud E., Touzain F., Lucas P., Méric G., Mageiros L., Sheppard S.K., Chemaly M., Rivoal K. Ruminant and chicken: important sources of campylobacteriosis in France despite a variation of source attribution in 2009 and 2015. Sci. Rep. 2018;8(1):1–10. doi: 10.1038/s41598-018-27558-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uaboi-Egbenni P.O., Bessong P.O., Samie A., Obi C.L. Campylobacteriosis in sheep in farm settlements in the Vhembe district of South Africa. Afr. J. Microbiol. Res. 2010;4(20):2109–2117. [Google Scholar]
- Uaboi-Egbenni P.O., Bessong P.O., Samie A., Obi C.L. Prevalence, haemolysis and antibiograms of campylobacters isolated from pigs from three farm settlements in Venda region, Limpopo province, South Africa. Afr. Health Sci. 2011;10(4):703–711. [Google Scholar]
- Uaboi-Egbenni P.O., Bessong P.O., Samie A., Obi C.L. Prevalence and antimicrobial susceptibility profiles of Campylobacter jejuni and coli isolated from diarrheic and non-diarrheic goat faeces in Venda region, South Africa. Afr. J. Biotechnol. 2011;10(64):14116–14124. [Google Scholar]
- Uaboi-Egbenni P.O., Bessong P.O., Samie A., Obi C.L. Potentially pathogenic Campylobacter species among farm animals in rural areas of Limpopo province, South Africa: A case study of chickens and cattles. Afr. J. Microbiol. Res. 2012;6(12):2835–2843. [Google Scholar]
- Uaboi-Egbenni P.O., Okolie P.N., Adesanya O.D., Omonigbehin E., Sobande A.O. Epidemiological studies of the incidence of pathogenic Campylobacter spp. amongst animals in Lagos metropolis. Afr. J. Biotechnol. 2008;7(16):2852–2956. [Google Scholar]
- Villers L.M., Jang S.S., Lent C.L., Lewin-Koh S.C., Norosoarinaivo J.A. Survey and comparison of major intestinal flora in captive and wild ring-tailed Lemur (Lemur catta) populations. Am. J. Primatol. 2008;70(2):175–184. doi: 10.1002/ajp.20482. [DOI] [PubMed] [Google Scholar]
- WHO . 2017. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics.https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf 1–7. [Google Scholar]
- Wilkinson D.A., O’Donnell A.J., Akhter R.N., Fayaz A., Mack H.J., Rogers L.E., Biggs P.J., French N.P., Midwinter A.C. Updating the genomic taxonomy and epidemiology of Campylobacter hyointestinalis. Sci. Rep. 2018;8(2393):1–12. doi: 10.1038/s41598-018-20889-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woldemariam T., Asrat D., Zewde G. Prevalence of Thermophilic Campylobacter species in carcasses from sheep and goats in an abattoir in Debre Zeit area, Ethiopia. Ethiop. J. Health Dev. 2009;23(3):229–233. https://www.ajol.info/index.php/ejhd/article/view/53245/41826 [Google Scholar]
- Zhou J., Zhang M., Yang W., Fang Y., Wang G., Hou F. A seventeen-year observation of the antimicrobial susceptibility of clinical Campylobacter jejuni and the molecular mechanisms of erythromycin-resistant isolates in beijing , China. Int. J. Infect. Dis. 2016;42:28–33. doi: 10.1016/j.ijid.2015.11.005. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.