Abstract
Background:
Few studies have examined combined or alternating treatment algorithms in eosinophilic esophagitis.
Aims:
We conducted a retrospective cohort study to ascertain the efficacy and adherence to a combined and alternating treatment approach with topical corticosteroids and two-food elimination diet for pediatric EoE.
Methods:
Patients were prescribed a two-food elimination diet (milk and soy) and topical corticosteroid (fluticasone or oral viscous budesonide) for 3 months, after which the steroid was discontinued and two-food elimination diet continued for 3 months. An EGD was performed at baseline, 3 months and 6 months. Clinical, endoscopic, and histologic data were extracted from electronic medical records. Non-parametric tests assessed adherence and outcomes.
Results:
Twenty-nine eosinophilic esophagitis cases were included (mean age 11.5 years, 61% male). Complete adherence to combined therapy and two-food elimination diet alone was 75% and 79%, respectively. Median eosinophil counts decreased from 51 to 2 eosinophils/hpf (p < 0.001) after combined treatment and rebounded to 31 (p =0.07) after 2FED alone. Dysphagia improved after both the combined and two-food elimination diet alone treatment approaches (52% vs. 11% and 10%; p = 0.001, 0.005). Non-significant improvements in endoscopic findings were documented across the length of follow-up.
Conclusions:
An initial combined treatment approach resulted in significant improvements in symptoms and histologic findings. While symptomatic improvements continued with two-food elimination diet alone, the histologic improvement was not maintained. While loss to follow-up may obscure the efficacy of two-food elimination diet alone, a combined/alternating treatment approach merits assessment in a larger prospective study.
Keywords: dysphagia, food bolus impaction, eosinophilic esophagitis, topical corticosteroids
Introduction
Eosinophilic esophagitis (EoE) is a chronic immune/antigen-mediated condition characterized histologically by eosinophilic-predominant esophageal inflammation and clinically by esophageal dysfunction [1]. The diagnosis requires at least 15 eosinophils per high-power field (eos/hpf) on esophageal biopsies and the exclusion of alternative etiologies of eosinophilia [1,2]. EoE represents a significant contributor to esophageal morbidity and the second leading cause of esophagitis [3–5]. Non-specific symptoms predominate in children including vomiting, regurgitation, heartburn and less frequently failure to thrive or growth failure [6]. Difficulty feeding, choking and refusal of food are more common in infants and toddlers [7,8].
EoE is a chronic disease, and without intervention the histologic and endoscopic disease activity persists [1,2,9]. Furthermore, the length of the symptomatic period prior to diagnosis correlates with stricture formation [10–12]. As such, early identification and treatment of children may decrease or eliminate the attendant consequences of untreated disease. The optimal treatment strategy for children, however, remains unclear. At present, the first-line treatment strategies for EoE in patients who do not respond to proton pump inhibition include topical corticosteroids (tCS) and food elimination diets (FED). Though both strategies improve the clinical and histologic features of EoE [13–17], patients frequently fail to respond to either strategy [15,18,19], may suffer deleterious side effects [20–24], experience disease recurrence with cessation of treatment [25,26], and incur significant quality of life burdens [27]. A combined treatment approach concurrently utilizing a tCS and FED may represent a preferential alternative to tCS and/or FED monotherapy in certain scenarios. For example, a tCS may be a potent means to achieve disease remission, while dietary elimination could be used to limit potential side effects in children. However, few data exist pertaining to a combined treatment approach with both corticosteroids and dietary food elimination [28], and to our knowledge no data report the efficacy of an alternating corticosteroid and dietary elimination approach for EoE.
Therefore, this study aimed to assess the overall efficacy of a combined and alternating treatment approach with topical corticosteroids and two-food elimination diet in a cohort of children with EoE. We also aimed to assess patient adherence to this treatment protocol.
Methods
We conducted a retrospective cohort study at Brenner Children’s Hospital from 2014 – 2017. Electronic medical records of each child with EoE treated at Brenner Children’s Hospital were searched to ascertain all patients meeting study inclusionary criteria. Included EoE cases met consensus guidelines for diagnosis [1,2] and were ≤18 years old at the time of protocol initiation. Additionally, for study inclusion, patients were required to have upper gastrointestinal tract symptoms attributable to pathologies of the upper gastrointestinal tract and at least 15 eos/hpf on esophageal biopsies after at least 6-week proton pump inhibitor (PPI) treatment. Patients were also required to have had a subsequent follow-up endoscopy with biopsy while on the combined/alternating treatment protocol at Brenner Children’s Hospital for inclusion. Included patients received instructions to initiate the treatment protocol assessed in this study, as described below.
Patients were treated with a two-food elimination diet (2FED) excluding milk and soy and a tCS (fluticasone or oral viscous budesonide, typically 440 mcg or 1 mg daily, respectively). Soy lecithin and soy oil were permitted in this protocol. Patients and/or their caregiver(s) created oral viscous budesonide suspension by mixing budesonide respules with Neocate Nutra in a ratio of 0.5 mg/2mL respules to 2.5 mL Neocate Nutra. This combined treatment was prescribed for 3 months, at which time the tCS was discontinued and patients were instructed to follow the 2FED alone for 3 months. An EGD was completed at baseline prior to combined therapy, and repeat EGDs were performed at 3 and 6 months following the combined and 2FED alone treatments.
Using a standardized data collection form, patient demographics, symptoms, previous treatments, endoscopic findings, and outcomes (symptomatic [yes/no], and endoscopic response [yes/no] were abstracted from the electronic medical record. As this was a retrospective study and validated patient reported outcome measures were not applied in clinical settings, symptoms were coded using dichotomous variables signifying their presence or absence [yes/no]. Additionally, as the EoE Endoscopic Reference Score (EREFS) [29] was not used in routine practice, endoscopic response was also coded using a dichotomous variable [yes/no] available from the medical records. Esophageal biopsy specimens were re-analyzed specifically for the purpose of this study by a single, non-blinded, gastrointestinal pathologist using a systematic approach [peak eosinophil count; % biopsies with < 15 eos/hpf; hpf size = 0.55 mm2]. Adherence to treatment was assessed utilizing an ordinal variable with three categories, complete, partial and inadequate, based on clinical report from the patient and as documented in the electronic medical record. Complete adherence signified complete FED and tCS adherence; partial adherence signified compliance to 50% or more meals, snacks, and tCS doses; inadequate compliance signified lack of tCS adherence or minimal adherence to the FED (0% - 50% of meals, snacks, and tCS doses). Sex and age standardized z-scores for height and weight were calculated using the Centers for Disease Control reference for child growth.
Descriptive statistics were used to characterize the cohort. Non-parametric, bivariate analysis was performed to describe the relationship between pre- and post-treatment outcome variables: McNemar’s X2 and Wilcoxon Signed-Rank test assessed pre- and post- treatment categorical variables and continuous variables, respectively. Paired t-tests were used to compare pre- and post-treatment height and weight z-scores. Non-paired assessment of categorical variables utilized Pearson’s X2 test and Student’s t-test for continuous variables. All data analyses were performed using Stata 14.1 (StataCorp, College Station, TX). The Wake Forest University Health Sciences Institutional Review Board approved this study for conduct.
Results
Baseline characteristics
A total of 29 patients met inclusion criteria for analysis in this study (Figure 1). At the start of the treatment protocol, the mean age of patients was 11.5 years, 61% were male, and 90% were white. Concurrent atopic conditions were reported in 59% of patients. Mean length of symptoms prior to diagnosis was 2.8 years. The mean height and weight z-scores at baseline were −0.10 and 0.38, respectively (Table 1).
Figure 1.
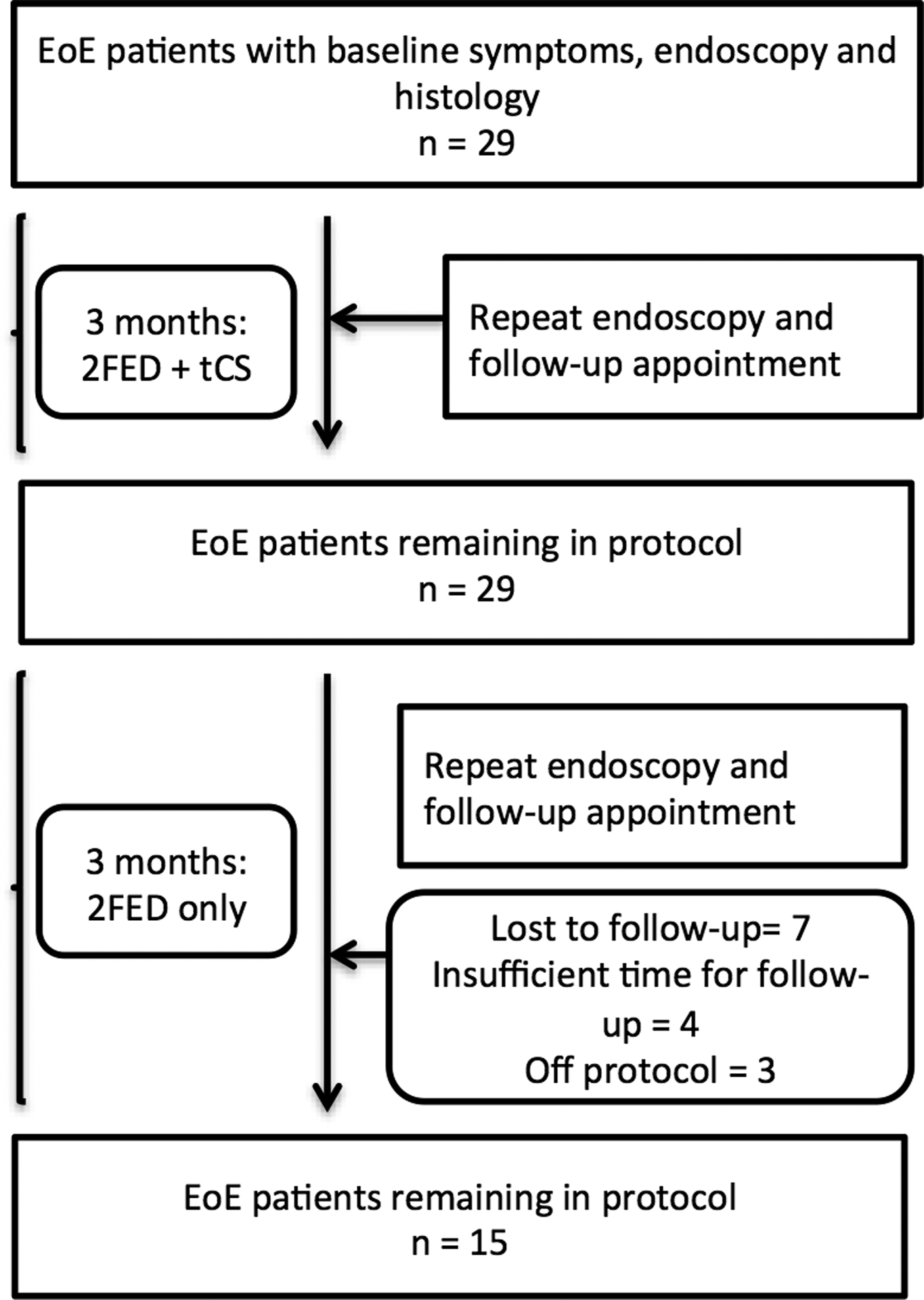
Patient flow throughout the treatment period for patients treated with topical corticosteroids (tCS) and 2-food elimination diet (2FED). At each point in the figure, the number of patients indicates those who remained on the treatment protocol and for which data were available for analysis.
Table 1.
Cohort demographic features, prior eosinophilic esophagitis (EoE)-specific therapies, and clinical history
| Demographic features | |
|---|---|
| Age at baseline (mean years ± SD1) | 11.5 ± 4.8 |
| Symptom duration prior to diagnosis (mean years ± SD) | 2.8 ± 3.1 |
| % Male | 61 |
| % White | 90 |
| % Atopic disease comorbidity | 59 |
| % MDI2 fluticasone/oral viscous budesonide at baseline | 82/18 |
| Insurance | |
| % Medicaid | 48 |
| % Private/commercial insurance | 52 |
| Baseline weight z-score (mean ± SD) | 0.38 ± 1.6 |
| Baseline height z-score (mean ± SD) | −0.10 ± 1.2 |
| Prior EoE-specific therapies in last 6 months (%) | |
| Any food elimination diet | 18 |
| Inhaled steroids | 11 |
| Systemic corticosteroids | 4 |
| Baseline symptoms (%) | |
| Dysphagia | 52 |
| Heartburn | 45 |
| Chest pain | 3 |
| Nausea | 24 |
| Vomiting | 41 |
| Abdominal pain | 45 |
| Baseline endoscopic findings (%) | |
| Rings | 0 |
| Furrows | 55 |
| Decreased vascularity | 0 |
| White plaques | 3 |
| Stricture | 0 |
| Erythema | 3 |
| Baseline histology findings (median peak eos/hpf3; IQR4) | 51; 32 – 71 |
| Adherence | |
| % Complete/partial adherence | |
| Following combined tCS5 + 2FED6 | 75/25 |
| Following 2FED | 79/21 |
standard deviation;
metered dose inhaler;
eosinphils per high-power field;
inter-quartile range;
topical corticosteroids;
2-food elimination diet;
Prior to treatment, the most common symptom was dysphagia (52%) followed by heartburn (45%) and abdominal pain (45%). Endoscopic findings of EoE, with the exclusion of esophageal furrowing, were relatively uncommon at baseline including 55% with furrows, 3% with white plaques, 3% with erythema, and no rings or strictures. The median peak eosinophil count at baseline was 51 eos/hpf (IQR: 32 – 71) (Table 1). Most patients were treatment naïve for EoE, while 5 patients (18%) were previously treated with a food elimination diet; no patients had received tCS (Table 1). At the initiation of the combined treatment protocol, 82% of patients were started on fluticasone, which was dosed at 440 mcg in the overwhelming majority of cases. The remaining 18% of patients were initiated on oral viscous budesonide typically at 1 mg per day (Table 1).
Response to combined topical corticosteroids and two-food elimination diet
The initiation of the treatment protocol, which consisted of combined tCS and 2FED, was followed by repeat endoscopic and clinical evaluation after approximately 3 months. At 3 months, all 29 patients had follow-up data. Significant decreases in symptoms of dysphagia (52% vs. 11%, p = 0.001), heartburn (45% vs. 7%, p = 0.01), and abdominal pain (45% vs. 15%, p = 0.03) were documented after starting the combined treatment protocol. Improvements in nausea (24% vs. 11%, p = 0.18) and vomiting (41% vs. 22%, p = 0.10) trended toward statistically significant improvements (Figure 2A). Height (−0.10 vs. −0.05, p = 0.13) and weight (0.38 vs. 0.18, p = 0.33) z-scores did not differ between the pre- and post-treatment periods. Complete adherence to the combined treatment strategy was documented in 75% of patients and partial adherence in 25% of patients (Table 1).
Figure 2.
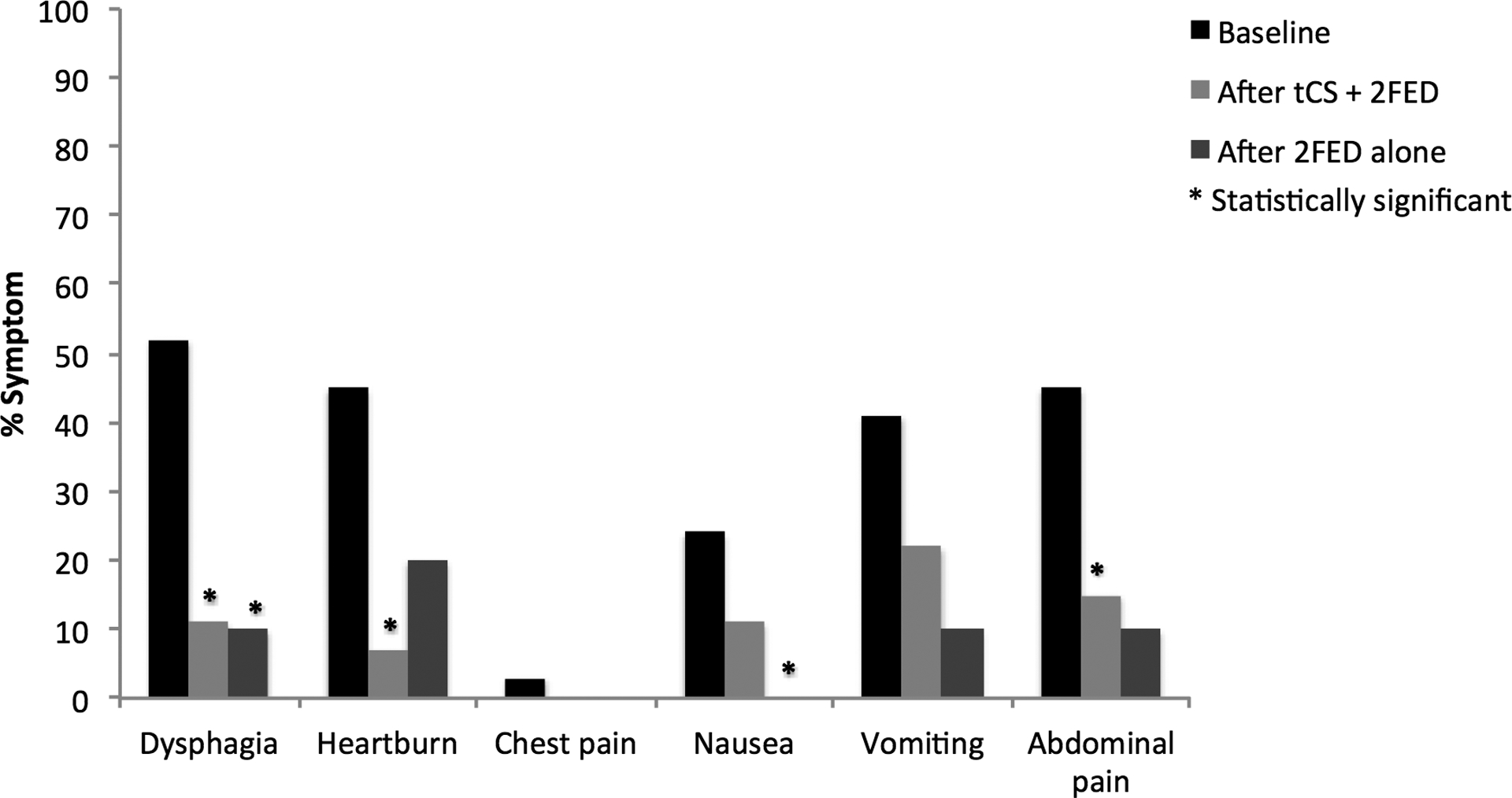
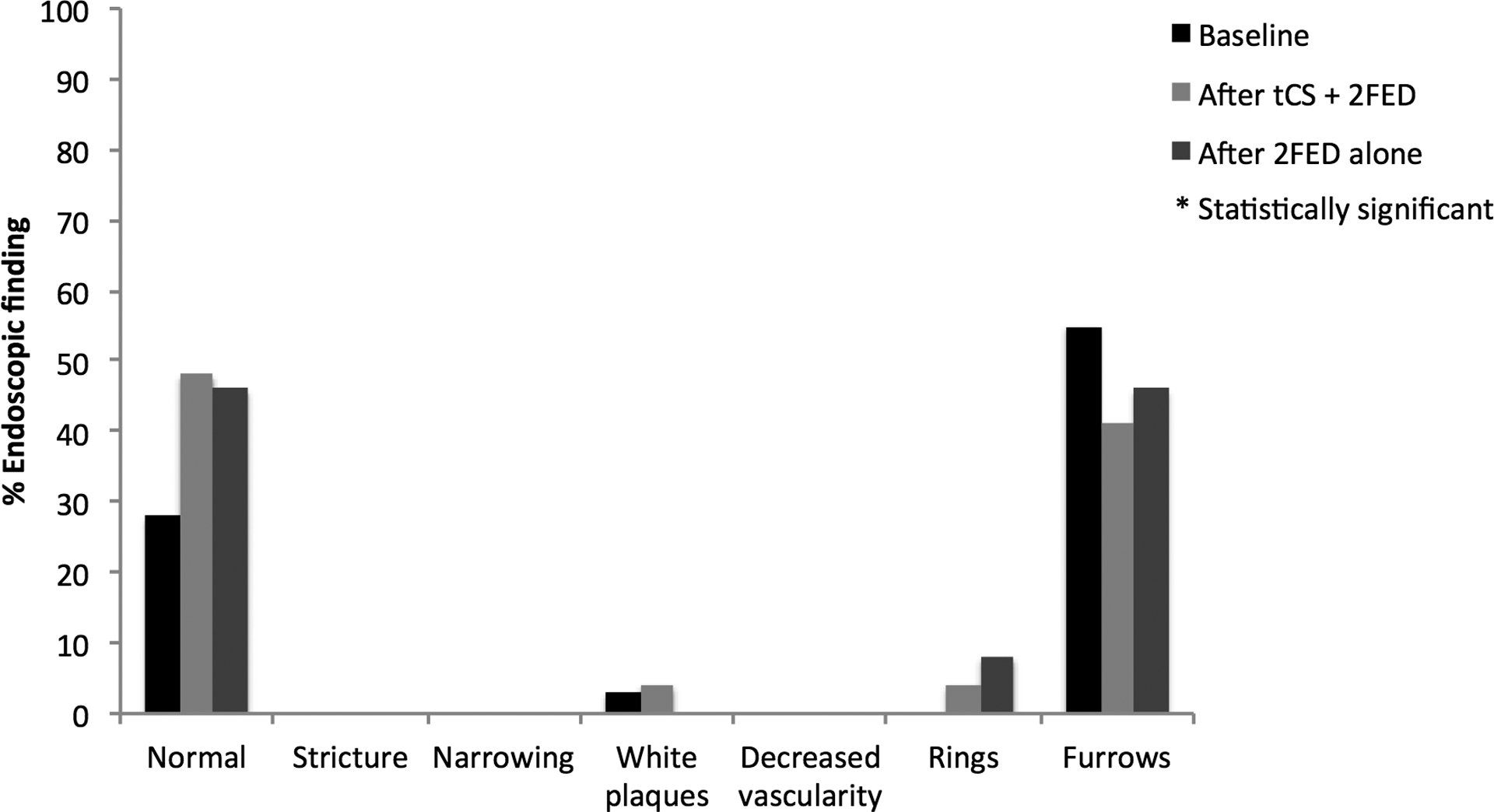
(A) Symptom reporting across the study period for patients treated with topical corticosteroids (tCS) and 2-food elimination diet (2FED). (B) Endoscopic findings across the study period for patients treated with topical corticosteroids (tCS) and 2-food elimination diet (2FED).
Following the initiation of the combined treatment strategy, the proportion of normal endoscopic examinations trended toward a significant difference (28% vs. 48%, p = 0.13), and there were fewer patients with appreciable esophageal furrowing (55% vs. 41%, p = 0.37). Observation of strictures, white exudate, and decreased vascularity, nearly absent at baseline, continued to be absent at combined treatment follow-up (strictures [0% vs. 0%], white plaques [3% vs. 4%, p = 1.0], and decreased vascularity [0% vs. 0%]) (Figure 2B). Also, of note, no patients required esophageal dilation at baseline or following treatment initiation nor did they exhibit evidence of esophageal candidiasis at either time point. The median peak eosinophil count decreased from 51 (IQR: 32–71) to 2 (IQR: 0–10) eos/hpf (p < 0.001). Of these 29 patients, 23 (79%) achieved a post-treatment eosinophil count of < 15 eos/hpf (Figure 3). After stratifying the cohort by partial to complete adherence versus inadequate adherence, no statistical differences were found for histologic, symptomatic, or endoscopic outcomes between the stratified groups.
Figure 3.
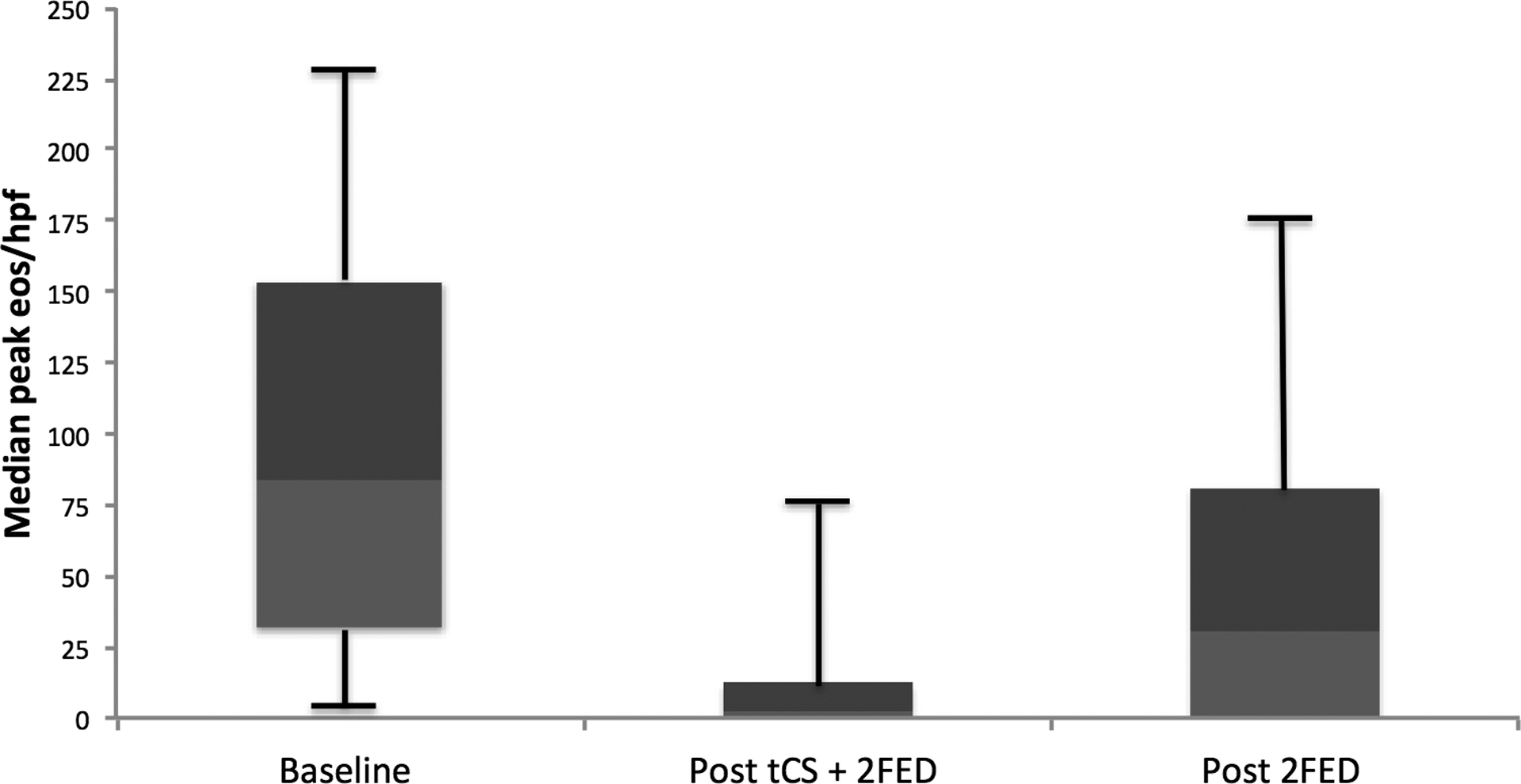
Histologic findings across the study period for patients treated with after topical corticosteroids (tCS) and 2-food elimination diet (2FED).
Response to a 2 food elimination diet alone
After approximately 3 months of combined tCS and 2FED, patients transitioned to a 2FED alone. The transition to 2FED mono-therapy was followed by an additional clinical and endoscopic evaluation at 3 months, which corresponded with a total elapsed time of approximately 6 months from protocol start. Of the 29 patients included in this study, 15 (52%) had a documented endoscopic and histologic evaluation for the final analysis of treatment outcomes. There were 7 patients (24%) who were lost to follow-up, 4 patients (14%) for whom the final endoscopy was not yet complete, and 3 patients (10%) for whom their provider did not continue the protocol. Symptom outcome data was available for 5 (36%) of the 14 patients who did not have a final endoscopy with biopsies. Patients not completing the protocol did not differ from patients who completed the protocol on post-combined therapy (tCS + 2 FED) mean peak eosinophil counts (10 non-completers vs. 9 completers eos/hpf, p = 0.92) or proportion with < 15 eos/hpf on initial follow-up endoscopy (85% non-completers vs. 80% completers, p = 0.26). These 2 groups also did not differ symptomatically by dysphagia (8% non-completers vs. 14% completers, p = 0.59), heartburn (14% non-completers vs. 0% completers, p = 0.13), or abdominal pain (15% non-completers vs. 14% completers, p = 0.94). Additionally, a similar proportion of patients in each group had normal endoscopic findings (absence of furrows, white exudate, and decreased vascularity) (46% non-completers vs. 50% completers, p = 0.84) after tCS with 2FED.
Among the 15 patients undergoing follow-up clinical and endoscopic evaluation following the 2FED alone, a significant and durable improvement was reported for dysphagia (53% vs. 13%, p = 0.01) and nausea (27% vs. 0%, p = 0.05) from baseline symptoms to after 2FED alone. Additionally, vomiting (33% vs. 7%, p = 0.10), abdominal pain (40% vs. 13%, p = 0.10), and heartburn (40% vs. 20%, p = 0.08) neared a statistical improvement at the end of the protocol (Figure 2A). The proportion of patients with normal endoscopic features at EGD (42%) was similar to that which was found after combined therapy (50%) and trended toward a significant improvement (42% vs. 27%, p = 0.18) from baseline findings. Again, no patients required an esophageal dilation there were no cases of and candidal esophagitis (Figure 2B). After withdrawal of tCS, eosinophil counts rebounded to a median peak count of 31 eos/hpf from a baseline of 53 eos/hpf (p = 0.07) (Figure 3). Similarly, a smaller proportion of patients (33%) maintained a histologic response of < 15 eos/hpf at the end of the protocol than following combined treatment (79%). Height (−0.57 vs. −0.54, p = 0.39) and weight (0.14 vs. −0.14, p = 0.88) z-scores were also unchanged from baseline. Adherence to a 2FED was recorded as complete in 11 (79%) and partial in 3 (21%) patients, respectively (Table 1). After stratifying by partial to complete adherence versus inadequate adherence, post-treatment eosinophil counts neared statistical significance (29 eos/hpf vs. 87 eos/hpf; p =0.07) and dysphagia differed between the groups (0% vs. 33.3%; p =0.05). No other differences were found by compliance for symptoms or endoscopic findings.
Discussion
Topical corticosteroids and food elimination diets improve the clinical and histologic findings of EoE and are regarded as the first-line therapies for management of this condition [13–16]. Not all patients, however, respond to tCS or food elimination strategies [18,19], and these treatments may produce side effects [20–24,30] and impose burdens on the quality of life of patients [27]. A combined treatment strategy may represent a viable alternative for EoE patients. Little data pertains to this form of treatment strategy [28], and no known studies report the efficacy of an alternating corticosteroid and dietary elimination approach for the disease. In this paper, we evaluated the efficacy of a combined treatment strategy with a 2FED consisting of soy and dairy elimination with tCS. As a part of the protocol for this treatment strategy, we minimized corticosteroid exposure by withdrawing tCS after 3 months of the protocol. We found that a combined and alternating treatment approach produced sustained and significant improvements in symptoms and endoscopic features of EoE. However, after withdrawal of tCS, histologic eosinophil counts rebounded substantially, but did not reach pre-treatment numbers. We also found, among the patients available for analysis, that adherence was acceptable in all phases of the treatment protocol. As such, patients with active disease requiring rapid relief of symptoms and/or patients without a clinical or histological response to monotherapy may represent those in which a combined approach should be utilized.
The significant and pronounced response to tCS plus the 2FED suggests it may be a viable management approach in children with EoE. Additionally, although the post-2FED mono-therapy eosinophil count increased toward pre-treatment levels, symptomatic and endoscopic improvements persisted through the final clinical and endoscopic evaluations. Such findings suggest that intermittent use of corticosteroids may mitigate the concern over long-term side effects, but further long-term data are needed to confirm this point. Furthermore, although it is plausible to presume that a proportion of patients did not have soy or dairy as sole trigger allergens, which partly explains the increase in eosinophil counts at the end of the protocol, the selection of a 2FED still benefited patients for whom these foods are triggers, and mitigated the follow-up burden imposed by a conventional 6FED. The loss of histologic response after 2FED alone, in a cohort largely consisting of adolescents, may also be subject to non-differential response bias, possibly attenuating estimates observed toward the null. As the patients without final follow-up data uniformly exhibited clinical, endoscopic, and histologic improvements after combined treatment, it is conceivable that their missing data reduced the power of our statistical testing, but not the validity of our conclusions. For this study, the 2FED consisted of dairy and soy. Cow’s milk and wheat are considered the most common food allergen triggers for EoE [1,2]. However, the elimination of milk and wheat from a child’s diet likely imposes a greater psychosocial burden than the elimination of milk alone [31]. In addition, elimination of dairy alone has been reported to produce clinical and histologic remission in approximately 65% of children [32]. Finally, further lending credence to our strategy, a previous study suggested that 25% - 60% of infants with non-IgE mediated reactions to dairy also react to soy [6,33–36].
Very minimal data currently exists similar to that which we present in this paper. In a recent study by Constantine et al. [28] 32 patients were treated with a combined treatment algorithm consisting of tCS with the targeted elimination of food triggers. Similar to the results observed in our study, they found a histological remission rate of 80%, which was defined as < 15 eos/hpf on repeat biopsies, in patients treated with the combined strategy. It is also notable that the combination treatment group in this study had previously failed single-agent treatment for EoE. However, the time between initiating combined treatment and repeat endoscopy with biopsies was longer at 0.7 years, increasing the potential for co-interventions and diminishing adherence to therapy. There also was only a single follow-up point reported.
Limitations exist for this study. Foremost, the study utilized a retrospective design, and data were obtained from the electronic medical record, so there is the potential for misclassification bias. There is also the potential for incomplete assessment of symptoms and clinical response. As most outcomes were assessed without validated patient reported outcome measures, measurement bias may exist for these variables. Using a single pathologist to re-read all of the biopsy samples minimized misclassification of histologic data, and is a strength of the study. The sample size for the study was relatively small, limiting the power to detect significant differences in treatment efficacy and adherence, particularly at the 6-month follow-up. Given the study was conducted at a single center site, there is potential that the results may not be generalizable to other pediatric populations. However, the clinical features are typical of other pediatric EoE populations, and 48% of patients in this cohort were covered by Medicaid, which is similar to the proportion of children in North Carolina covered by Medicaid overall. Strengths of this paper include the inclusion of patients meeting consensus disease definition, expert pathology interpretation, and the examination of a relatively homogenous cohort. Additionally, the study results are outside of a clinical trial, and give an approximation of how such a treatment strategy would work in ‘real-world’ clinical practice.
In summary, an initial treatment approach consisting of combined tCS and 2FED produced significant improvements in the histologic, symptomatic and endoscopic features of EoE. With a transition to 2FED alone, the histologic improvement was not maintained, though patients continued to report significant improvements in the other outcome domains. However, as a result of loss to follow-up, the histologic efficacy of such a treatment protocol may be underestimated. The efficacy of a combined and alternating treatment approach to EoE merits further assessment in a larger and prospective study.
Grant support:
This research was supported by NIH Awards T32 DK007634 (CCR) and R01 DK101856 (ESD).
Abbreviations:
- EoE
eosinophilic esophagitis
- eos/hpf
eosinophils per high-power field
- tCS
topical corticosteroids
- FED
food elimination diet
- PPI
proton pump inhibitor
- 2FED
two-food elimination diet
- EREFS
EoE Endoscopic Reference Score
Footnotes
Potential competing interests: None of the authors report and potential conflicts of interest with this study. Dr. Dellon is a consultant for Adare, Alivio, Allakos, Banner, Enumeral, GSK, Receptos/Celegene, Regeneron, Roche, and Shire, receives research funding from Adare, Meritage, Miraca, Nutricia, Receptos/Celgene, Regeneron, and Shire, and has received an educational grant from Banner.
References
- 1.Liacouras CA, Furuta GT, Hirano I, et al. Eosinophilic esophagitis: Updated consensus recommendations for children and adults. J Allergy Clin Immunol. 2011;128(1):3–20. doi: 10.1016/j.jaci.2011.02.040. [DOI] [PubMed] [Google Scholar]
- 2.Dellon ES, Gonsalves N, Hirano I, Furuta GT, Liacouras CA, Katzka DA. ACG clinical guideline: Evidenced based approach to the diagnosis and management of esophageal eosinophilia and eosinophilic esophagitis (EoE). Am J Gastroenterol. 2013;108(5):679–92; quiz 693. doi: 10.1038/ajg.2013.71. [DOI] [PubMed] [Google Scholar]
- 3.Desai TK, Stecevic V, Chang CH, Goldstein NS, Badizadegan K, Furuta GT. Association of eosinophilic inflammation with esophageal food impaction in adults. Gastrointest Endosc. 2005;61(7):795–801. doi: 10.1016/S0016-5107(05)00313-5. [DOI] [PubMed] [Google Scholar]
- 4.Dellon ES. Epidemiology of eosinophilic esophagitis. Gastroenterol Clin North Am. 2014;43(2):201–218. doi: 10.1016/j.gtc.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jensen ET, Kappelman MD, Martin CF, Dellon ES. Health-care utilization, costs, and the burden of disease related to eosinophilic esophagitis in the United States. Am J Gastroenterol. 2015;110(5):626–632. doi: 10.1038/ajg.2014.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liacouras CA, Spergel JM, Ruchelli E, et al. Eosinophilic esophagitis: A 10-year experience in 381 children. Clin Gastroenterol Hepatol. 2005;3(12):1198–1206. doi: 10.1016/S1542-3565(05)00885-2. [DOI] [PubMed] [Google Scholar]
- 7.Liacouras C a, Markowitz JE. Eosinophilic esophagitis: A subset of eosinophilic gastroenteritis. Curr Gastroenterol Rep. 1999;1:253–258. doi: 10.1007/s11894-999-0043-1. [DOI] [PubMed] [Google Scholar]
- 8.Noel RJ, Putnam PE, Rothenberg ME. Eosinophilic esophagitis. N Engl J Med. 2004;351(9):940–941. doi: 10.1056/NEJM200408263510924. [DOI] [PubMed] [Google Scholar]
- 9.Furuta GT, Liacouras C a, Collins MH, et al. Eosinophilic esophagitis in children and adults: a systematic review and consensus recommendations for diagnosis and treatment. Gastroenterology. 2007;133(4):1342–1363. doi: 10.1053/j.gastro.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 10.Dellon ES, Kim HP, Sperry SLW, Rybnicek DA, Woosley JT, Shaheen NJ. A phenotypic analysis shows that eosinophilic esophagitis is a progressive fibrostenotic disease. Gastrointest Endosc 2014;79(4). doi: 10.1016/j.gie.2013.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schoepfer AM, Safroneeva E, Bussmann C, et al. Delay in diagnosis of eosinophilic esophagitis increases risk for stricture formation in a time-dependent manner. Gastroenterology. 2013;145(6):1230–1236. doi: 10.1053/j.gastro.2013.08.015. [DOI] [PubMed] [Google Scholar]
- 12.Lipka S, Keshishian J, Kumar M. Time-dependent association of untreated eosinophilic esophagitis and the risk of esophageal stricture in a U.S. population. Gastroenterology. 2014;146((suppl 1)):S669–S670. [Google Scholar]
- 13.Aceves SS, Newbury RO, Chen D, et al. Resolution of remodeling in eosinophilic esophagitis correlates with epithelial response to topical corticosteroids. Allergy Eur J Allergy Clin Immunol. 2010;65(1):109–116. doi: 10.1111/j.1398-9995.2009.02142.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dellon ES, Liacouras CA. Advances in clinical management of eosinophilic esophagitis. Gastroenterology. 2014;147(6):1238–1254. doi: 10.1053/j.gastro.2014.07.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wolf WA, Jerath MR, Sperry SLW, Shaheen NJ, Dellon ES. Dietary elimination therapy is an effective option for adults with eosinophilic esophagitis. Clin Gastroenterol Hepatol. 2014;12(8):1272–1279. doi: 10.1016/j.cgh.2013.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arias Á, González-Cervera J, Tenias JM, Lucendo AJ. Efficacy of dietary interventions for inducing histologic remission in patients with eosinophilic esophagitis: A systematic review and meta-analysis. Gastroenterology. 2014;146(7):1639–1648. doi: 10.1053/j.gastro.2014.02.006. [DOI] [PubMed] [Google Scholar]
- 17.Cotton C, Eluri S, Wolf W, Dellon E. Six-Food Elimination Diet and Topical Steroid are Effectie for Eosinophilic Esophagitis: A Meta-Regression. Dig Dis Sci. 2017:In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Konikoff MR, Noel RJ, Blanchard C, et al. A randomized, double-blind, placebo-controlled trial of fluticasone propionate for pediatric eosinophilic esophagitis. Gastroenterology. 2006;131(5):1381–1391. doi:S0016-5085(06)01792-6 [pii]. [DOI] [PubMed] [Google Scholar]
- 19.Dellon E. Management of refractory eosinophilic oesophagitis. Nat Rev Gastroenterol Hepatol. 2017;14(8):479–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Konikoff MR, Noel RJ, Blanchard C, et al. A Randomized, Double-Blind, Placebo-Controlled Trial of Fluticasone Propionate for Pediatric Eosinophilic Esophagitis. Gastroenterology. 2006;131(5):1381–1391. doi: 10.1053/j.gastro.2006.08.033. [DOI] [PubMed] [Google Scholar]
- 21.Straumann A, Conus S, Degen L, et al. Budesonide is effective in adolescent and adult patients with active eosinophilic esophagitis. Gastroenterology. 2010;139(5):1526–1537. doi: 10.1053/j.gastro.2010.07.048. [DOI] [PubMed] [Google Scholar]
- 22.Lindberg GM, Van Eldik R, Saboorian MH. A case of herpes esophagitis after fluticasone propionate for eosinophilic esophagitis. Nat Clin Pract Gastroenterol Hepatol. 2008;5(9):527–530. doi: 10.1038/ncpgasthep1225. [DOI] [PubMed] [Google Scholar]
- 23.Alexander JA, Jung KW, Arora AS, et al. Swallowed Fluticasone Improves Histologic but Not Symptomatic Response of Adults With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2012;10(7):742–749. doi: 10.1016/j.cgh.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 24.Aceves SS, Bastian JF, Newbury RO, Dohil R. Oral viscous budesonide: A potential new therapy for eosinophilic esophagitis in children. Am J Gastroenterol. 2007;102(10):2271–2279. doi: 10.1111/j.1572-0241.2007.01379.x. [DOI] [PubMed] [Google Scholar]
- 25.Helou EF, Simonson J, Arora AS. 3-Yr-follow-up of topical corticosteroid treatment for eosinophilic esophagitis in adults. Am J Gastroenterol. 2008;103(9):2194–2199. doi: 10.1111/j.1572-0241.2008.01989.x. [DOI] [PubMed] [Google Scholar]
- 26.Straumann A, Conus S, Degen L, et al. Long-Term Budesonide Maintenance Treatment Is Partially Effective for Patients With Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2011;9(5). doi: 10.1016/j.cgh.2011.01.017. [DOI] [PubMed] [Google Scholar]
- 27.Franciosis J, Hommel K, Bendo C, et al. PedsQL™ Eosinophilic Esophagitis Module: Feasibility, Reliability and Validity. J Pediatr Gastroenterol Nutr. 2013;57(1):57–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Esophagitis E, Analysis AR, Constantine G, et al. Combination Steroid and Test-based Food Elimination for Eosinophilic Esophagitis: A retrospective analysis. J Pediatr Gastroenterol Nutr. 2017;64(6):933–938. doi: 10.1097/MPG.0000000000001584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hirano I, Moy N, Heckman MG, Thomas CS, Gonsalves N, Achem SR. Endoscopic assessment of the oesophageal features of eosinophilic oesophagitis: validation of a novel classification and grading system. Gut. 2013;62(4):489–495. doi: 10.1136/gutjnl-2011-301817. [DOI] [PubMed] [Google Scholar]
- 30.Muley P, Shah M, Muley A. Safety of inhaled fluticasone propionate therapy for pediatric asthma - a systematic review. Curr Drug Saf. 2013;8(3):186–194. doi: 10.2174/15748863113089990038. [DOI] [PubMed] [Google Scholar]
- 31.Teufel M, Biedermann T, Rapps N, et al. Psychological burden of food allergy. World J Gastroenterol. 2007;13(25):3456–3465. doi: 10.3748/wjg.v13.i25.3456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kagalwalla AF, Amsden K, Shah A, et al. Cow’s milk elimination: a novel dietary approach to treat eosinophilic esophagitis. J Pediatr Gastroenterol Nutr. 2012;55(6):711–716. doi: 10.1097/MPG.0b013e318268da40. [DOI] [PubMed] [Google Scholar]
- 33.Nutrition C on. American Academy of Pediatrics. Committee on Nutrition. Hypoallergenic infant formulas. Pediatrics. 2000;106(2 Pt 1):346–349. http://www.ncbi.nlm.nih.gov/pubmed/10920165. [PubMed] [Google Scholar]
- 34.Boyce JA, Jones SM, Rock L, et al. Guidelines for the Diagnosis and Management of Food Allergy in the United States: Report of the NIAID-Sponsored Expert Panel. Vol 126; 2010. doi: 10.1016/j.jaci.2010.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kagalwalla AF, Sentongo TA, Ritz S, et al. Effect of Six-Food Elimination Diet on Clinical and Histologic Outcomes in Eosinophilic Esophagitis. Clin Gastroenterol Hepatol. 2006;4(9):1097–1102. doi: 10.1016/j.cgh.2006.05.026. [DOI] [PubMed] [Google Scholar]
- 36.Spergel JM, Beausoleil JL, Mascarenhas M, Liacouras CA. The use of skin prick tests and patch tests to identify causative foods in eosinophilic esophagitis. J Allergy Clin Immunol. 2002;109(2):363–368. doi: 10.1067/mai.2002.121458. [DOI] [PubMed] [Google Scholar]


