Introduction
Discrepancies in healthcare resource delivery and outcomes with respect to ethnicity is a topic of concern for patients, providers and policy makers. The World Health Organization and the Institute of Medicine define quality of care as “the extent to which health care services provided to individuals and patient populations improve desired health outcomes ”1. This means that the care provided should “not vary in quality because of personal characteristics such as gender, ethnicity, geographic location, and socioeconomic status”2. However, discrepancies exist between non-Hispanic white patients and other races in the surgical options offered for treatment of pancreatic, prostate, colorectal and breast cancers3–6. Potential explanations for this disparity include the perception of increased likelihood of postoperative complications in a specific ethnicity7, increased patient-refusal of surgery4, 8, cultural beliefs9, and poor physician-patient communication10. Although the U.S. has a rapidly expanding Hispanic population, most racial disparity studies have focused on differences between African-Americans and non-Hispanic whites while less is known regarding disparities in care among the Hispanic population. Hispanics receive a proportionately different care in surgical treatment of breast cancer (breast-conserving versus mastectomy)3 but this has not been evaluated in the surgical treatment of other cancers.
Bladder cancer surgery offers an opportunity to assess ethnic disparities in the quality of surgical care given its multifaceted pre-surgical and surgical treatment approach. For instance, neoadjuvant chemotherapy provides a significant survival benefit for patients undergoing radical cystectomy and is the standard of care for cisplatin-eligible patients with muscle-invasive disease11. However, not all cisplatin-eligible patients are offered or accept neoadjuvant chemotherapy. Additional quality of care (QOC) metrics for radical cystectomy include the extent of nodal dissection, often estimated by the number of lymph nodes removed12, the use of continent urinary diversions13, and laparoscopic approach14.
While the incidence of bladder cancer is lower in Hispanics compared to non-Hispanics, Hispanics present with higher stage disease15–17, and the U.S. Hispanic population is growing rapidly, especially in urban cities near the Texas-Mexico border. According to the 2016 U.S. Census American Community Survey, over 60% of San Antonio identifies as Hispanic, which gives it the fourth highest Hispanic population of all U.S. cities18. Furthermore, the nearly 4.5 million residents of South Texas, two-thirds of whom are Hispanic, have unique cancer incidence and mortality rates that distinguish them from the rest of Texas.19 We sought to determine if disparities in QOC exist between Hispanics and non-Hispanics undergoing radical cystectomy in San Antonio, Texas.
Methods
This was a retrospective cohort study of patients’ records who underwent radical cystectomy for urothelial carcinoma of the bladder between January 2005 and July 2018 at two hospitals in San Antonio. Some patients participated in randomized controlled trials which influenced the type of surgical approach (i.e., open versus laparoscopic,)14 or the number of lymph nodes (i.e., extended versus standard node dissection, NCT01224665). Data were collected on clinical and pathologic features, including demographics, body mass index (BMI), Charlson comorbidity index20, receipt of neoadjuvant chemotherapy, and pathologic stage. We used patient self-reporting for determination of ethnicity and race. Distance to surgery was calculated using the patients’ self-reported zip code. Operative variables assessed included estimated blood loss, number of lymph nodes removed, operative time, radical cystectomy surgical approach (laparoscopic versus open), and type of urinary diversion (continent versus incontinent). Patients with missing data regarding ethnicity, pathologic stage, age, sex, type of urinary diversion or known receipt or non-receipt of neoadjuvant chemotherapy were excluded from analysis (n=19).
Summary statistics and Variable descriptions: The associations between ethnicity (Hispanic or non-Hispanic) and demographic or clinical characteristics were assessed with Fisher’s exact test and the two-sample Wilcoxon rank-sum test for categorical and continuous variables, respectively. Hierarchical clustering (complete linkage, Euclidian distance) was used to create a heatmap visual summary. Hierarchical clustering is an unsupervised learning technique that groups similar observations together by computing distances between individual observations and clusters, and groups them based on this distance. This analysis was used to determine whether we could identify clusters of patients based on a subset of the covariates. To construct surgical outcome variable, the continent orthotopic neobladder and non-orthotopic continent catheterizable pouches were categorized as continent urinary diversions and ileal or colon conduits were categorized as incontinent diversions.
Multivariable logistic regression was used to model ethnicity and quality metrics (i.e., receipt of neoadjuvant chemotherapy, continent diversion, and laparoscopic surgical approach)21. Linear regression analysis was used to evaluate the association between ethnicity and number of lymph nodes removed. Overlap weighting was used in order to ensure that covariates were balanced for the contrast between Hispanics and Non-Hispanics and to adjust for confounding22. Preoperative renal function was not available for some patients whose preoperative records did not indicate estimated glomerular filtration rate (eGFR) or serum creatinine. Because eGFR can influence receipt of neoadjuvant chemotherapy and the type of urinary diversion offered, subgroup analyses of the logistic regression with these outcome measures was performed using only patients with preoperative eGFR available. Subgroup analyses excluded such patients for evaluation of the laparoscopic versus open and number of lymph nodes removed outcome measures, respectively. Significance was set at 0.05 and all p values were two-sided. Statistics was performed using STATA 10.1 (StataCorp, College Station, Texas) or R version 3.5.3 (R Core Team, Vienna, Austria).
Results
Demographics
The cohort consists of 507 patients who underwent radical cystectomy for bladder cancer, including 136 (27%) Hispanics and 371 (73%) non-Hispanics. There were no significant differences observed between Hispanics and non-Hispanics across age, sex, or pathologic stage (Table 1). In addition, there were no significant differences between ethnicities observed in operative time or estimated blood loss. Charlson comorbidity scores were non-significantly higher in Hispanics compared to non-Hispanics (p=0.06). BMI was higher in Hispanics compared to non-Hispanics (28.16 kg/m2 versus 26.91 kg/m2; p=0.006), yet the BMI for both groups is still within the “overweight” BMI category range of 25–29.9 kg/m2. The distance from home to location of surgery was farther in Hispanics compared to non-Hispanics (96 miles versus 34 miles; p=0.02). Additionally, Hispanics had significantly lower median household income compared to non-Hispanics (40,837 versus 59,170; p=<0.001). Unsupervised hierarchical cluster analysis provides dendrograms that show clustering of patients based on variables BMI with Charlson comorbidity score and patient age with pathologic stage (Supplemental Figure S1). Distance from home to site of surgery clustered more closely with both BMI and comorbidity score than with age and pathologic stage (Supplemental Figure S1). A high proportion of Hispanic patients clustered towards the right-hand side of the heatmap which corresponds to higher BMI and higher comorbidity scores.
Table 1.
Baseline Characteristics of Hispanics and Non-Hispanics
| Variable | Hispanic | Non-Hispanic | P-Value |
|---|---|---|---|
| n | 136 | 371 | |
| Sex | 0.11 | ||
| Male | 102 (75.0%) | 304 (81.9%) | |
| Female | 34 (25.0%) | 67 (18.1%) | |
| Age | 69 61–75 |
69 61–75 |
0.67 |
| BMI (kg/m2) | 0.006 | ||
| Median | 28.16 | 26.91 | |
| Interquartile Range | 25.43–31.93 | 24.34–30.47 | |
| Charlson Comorbidity Index | 0.06 | ||
| Median | 1 | 0 | |
| Interquartile Range | 0–1 | 0–1 | |
| Estimated Blood Loss (mL) | 0.91 | ||
| Median | 700 | 700 | |
| Interquartile Range | 500–1000 | 450–100 | |
| Operative Time (minutes) | 0.43 | ||
| Median | 371 | 378 | |
| Interquartile Range | 313–436 | 310–463 | |
| Distance from home to surgery (miles) | 0.02 | ||
| Median | 95.9 | 34.4 | |
| Interquartile Range | 15.93–161.50 | 16.15–106.00 | |
| Diversion | 0.003 | ||
| Continent | 14 (10.3%) | 84 (22.6%) | |
| Incontinent | 122(89.7%) | 287 (77.4%) | |
| Median Income | <0.001 | ||
| Median | 40,837 | 59,170 | |
| Interquartile Range | 37,667–52,520 | 45,098–72,353 | |
| Education Percent | 0.60 | ||
| Median | 35.30 | 36.10 | |
| Interquartile Range | 30.30–41.30 | 28.17–40.10 | |
| Cystectomy Approach | 0.85 | ||
| Laparoscopic | 27 (19.9%) | 69 (18.6%) | |
| Open | 109 (80.1%) | 302 (81.4%) | |
| Neoadjuvant Chemotherapy | 0.34 | ||
| Yes | 49 (36.0%) | 153 (41.2%) | |
| No | 87 (64%) | 218 (58.8%) | |
| T Stage | 0.30 | ||
| <T2 | 54 (39.7%) | 156 (42.0%) | |
| T2 | 23 (16.9%) | 83 (22.4%) | |
| >T2 | 59 (43.4%) | 132 (35.6%) | |
| Number of Lymph Nodes Removed | 0.27 | ||
| Median | 21.50 | 19.00 | |
| Interquartile Range | 13.00–29.25 | 11.00–29.00 |
Neoadjuvant chemotherapy
Neoadjuvant chemotherapy was delivered in 202 (40%) of 507 patients. There was no significant difference in the receipt of neoadjuvant chemotherapy by Hispanics (36.0%) and non-Hispanics (41.2%) and the OR curves were nearly identical in appearance (Figure 1), suggesting that variability in the delivery of neoadjuvant chemotherapy was not influenced by Hispanic ethnicity. On multivariable analysis, Hispanic ethnicity was not associated with delivery of neoadjuvant chemotherapy (OR = 0.59, 95% CI 0.28–1.24, P = 0.17, Supplemental Table 1). However, decreased utilization of neoadjuvant chemotherapy was observed for older patients (P = 0.03, Supplemental Table 1). Stratification of the odds of receipt of neoadjuvant chemotherapy across both ethnicity (Figure 1) and sex (Supplemental Figure S2) revealed wide confidence intervals in the ORs for younger patients with more narrow confidence intervals in older patients. Surprisingly, serum creatinine prior to cystectomy was not associated with receipt of neoadjuvant chemotherapy in this cohort (Supplemental Table 1).
Figure 1.
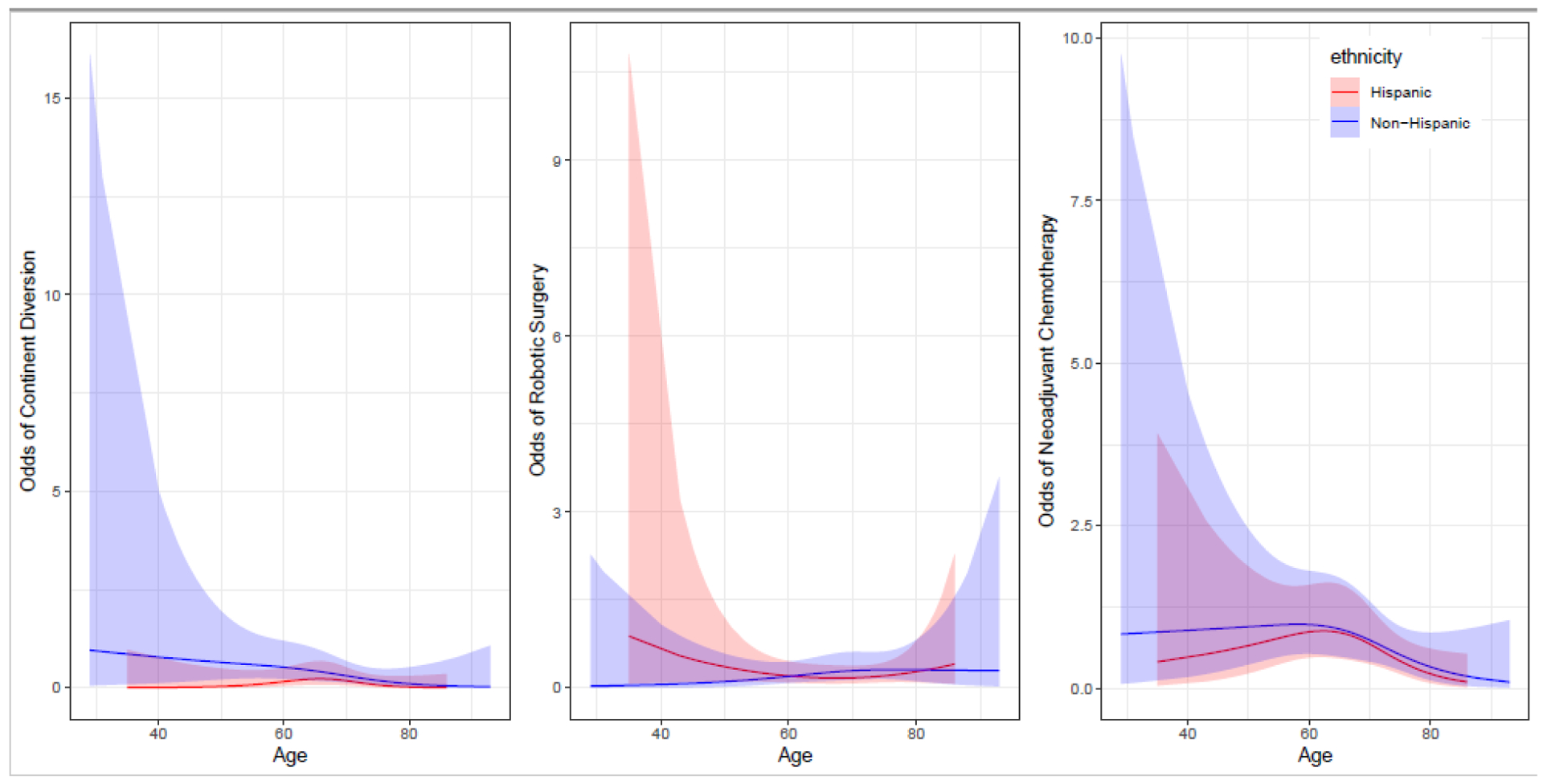
Adjusted odds ratio with 95% confidence interval looking at receipt of treatment modality across age range and ethnicity with all other variables in model held constant.
Laparoscopic approach
There was no significant association of surgical approach (i.e., laparoscopic versus open) across ethnicity on univariable (Table 1) or multivariable analysis which excluded patients enrolled in a randomized clinical trial comparing open versus laparoscopic surgery (Supplemental Table 2). The association between higher comorbidity scores and increased chance of undergoing an open surgical approach (OR 1.30) was not statistically significant (P = 0.11). When ORs were plotted by ethnicity and age, there appeared to be a wide confidence interval in receipt of laparoscopic surgery for younger Hispanic patients and older Hispanics (Figure 1), indicating increased variability in delivery of laparoscopic surgery for younger Hispanics compared to older Hispanics. ORs plotted by sex and age were nearly identical for males and females (Supplemental Figure S2).
Number of lymph nodes removed
The median number of lymph nodes removed during radical cystectomy was 21.5 (IQR 13.0 – 29.3) in Hispanics and 19 (IQR 11.0 – 29.0) in non-Hispanics (P = 0.27). On multivariable linear regression, ethnicity was not associated with the number of lymph nodes removed even after excluding patients enrolled in a randomized clinical trial studying extended versus standard lymph node dissection (Supplemental Table 3). Examination of the number of lymph nodes removed across age showed a biphasic trend of decreased number of nodes removed in youngest and oldest patients in Hispanics, but not in non-Hispanics (Figure 2).
Figure 2.
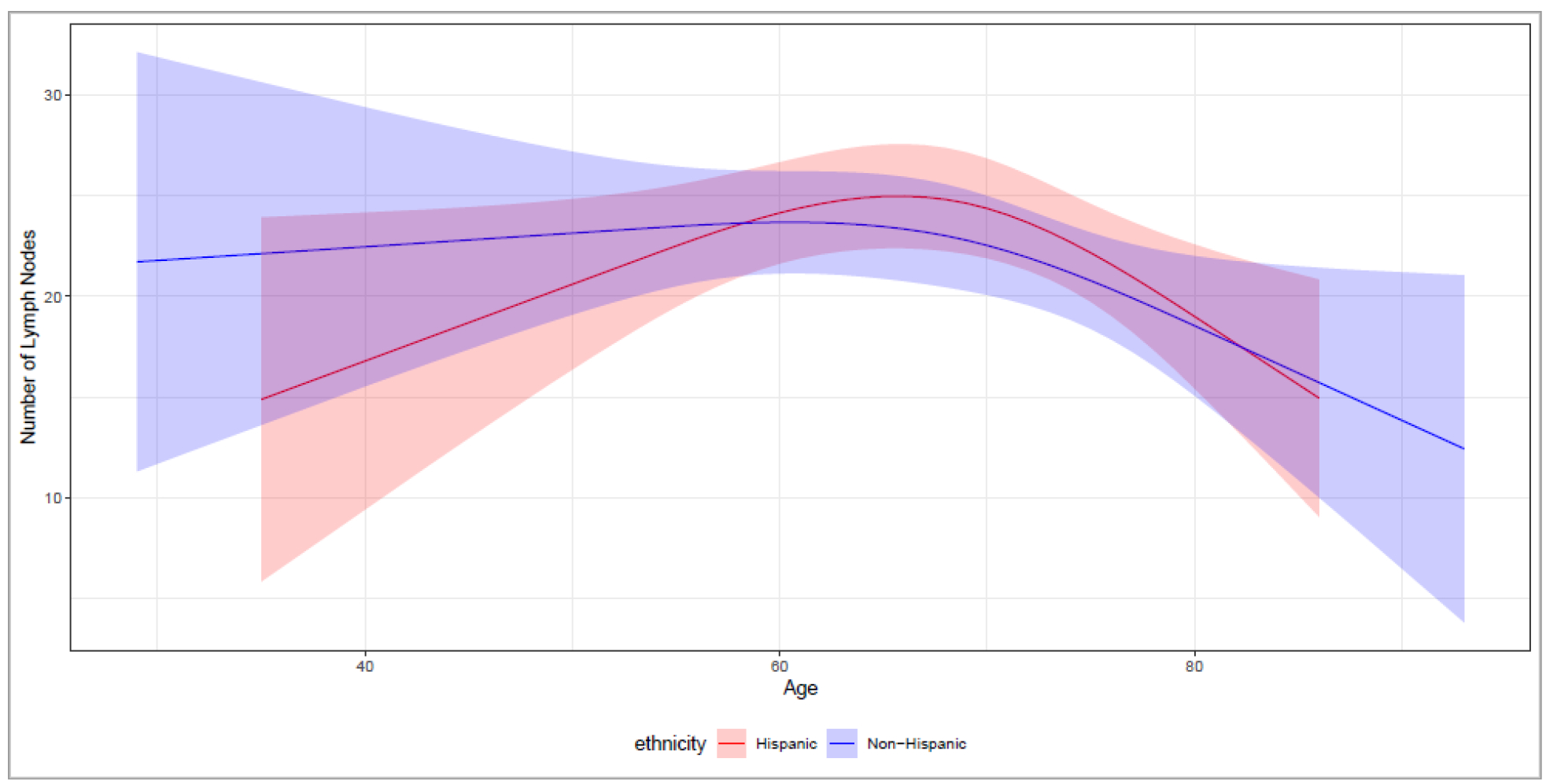
Predicted number of lymph nodes removed with 95% confidence interval across age range and ethnicity with all other variables in model held constant.
Urinary diversion
Continent urinary diversion was performed in 14 (10.3%) Hispanics and 84 (22.6%) non-Hispanics (P = 0.003) patients. On multivariable analysis that did not adjust for serum creatinine, Hispanics were less likely than non-Hispanics to receive a continent urinary diversion (OR 0.41, 95% CI 0.16 – 1.03, P = 0.05), (Supplemental Table 4). Graphical representation depicts the influence of age and ethnicity on the type of urinary diversion showing higher odds and wider confidence interval for younger non-Hispanics compared to older patients and compared to non-Hispanics (Figure 1). In a subset analysis of patients with serum creatinine available, Hispanics (OR 0.30, 95% CI 0.10–0.92, P = 0.03), and female sex (OR 0.12, 95% CI 0.02–0.90, P = 0.04) were significantly associated with decreased odds of receiving a continent urinary diversion (Table 2).
Table 2.
Multivariable logistic regression of association of clinical and pathologic variables with receipt of continent urinary diversion
| Variable | OR | 95% CI | P value |
|---|---|---|---|
| Hispanic Age | 0.30 | 0.10–0.92 | 0.03 |
| 0.95 | 0.89–1.01 | 0.09 | |
| Sex (female) | 0.12 | 0.02–0.90 | 0.04 |
| BMI (kg/m2) | 1.08 | 0.98–1.20 | 0.12 |
| Comorbidity Index | 0.46 | 0.21–0.98 | 0.04 |
| Distance (miles) | 1.03 | 0.69–1.54 | 0.87 |
| Pathologic T stage | 0.97 | 0.75–1.25 | 0.80 |
| Creatinine (mg/dL) | 0.23 | 0.04–1.26 | 0.09 |
| Median Income | 0.92 | 0.67–1.27 | 0.60 |
| Percent Education | 1.01 | 0.96–1.06 | 0.66 |
Discussion
We sought to identify potential disparities in the quality of surgical care delivered to Hispanic patients undergoing radical cystectomy for bladder cancer. No significant differences in delivery of neoadjuvant chemotherapy, laparoscopic surgery, or extent of nodal dissection were observed between Hispanics and non-Hispanics. However, Hispanics were less likely to receive a continent urinary diversion compared to non-Hispanics. Further, this disparity was mostly observed in younger Hispanic patients. We speculate that this disparity could be explained by (1) inadequate physician-patient communication when describing the urinary diversion, (2) surgeon’s selection potentially influenced by biases regarding comorbid illness in Hispanic patients, (3) other conscious or unconscious biases towards a specific urinary diversion, or (4) patient-centered preferences10, 23.
Poor communication contributes to patients’ incomplete understanding of potential benefits and risks of particular therapy, skewing their preference for one therapy over another. Interventions aimed to improve physician-patient communication, such as the use of culturally competent communicators and interpreting services, could lead to greater use of effective therapies and decrease disparities in treatment4, 24. Implementation of interpreter services for adults continuously enrolled in a staff model health maintenance organization showed an increase in delivery of health care to non English-speaking patients and significantly reduced disparities in rates of preventive services24. Language barriers to communication are unlikely in our study however, as the medical staff at the institutions in the study have a high number of Spanish-speaking nurses and physicians. Similarly, many Hispanic patients are bilingual, meaning assumptions that Hispanic patients are also Spanish-only speaking patients is invalid. Limited health literacy may hinder patients’ comfort with social interactions in medical settings and thus negatively affect decision-making behaviors. In a comprehensive review of the health status of the South Texas region, Ramirez et al. noted lower education and income levels of South Texans compared to the rest of the state, which has a known association lower health literacy levels25,19. In line with these findings, analysis of the education and income levels between non-Hispanic and Hispanic patients demonstrated no difference in educational level yet the income level of non-Hispanic patients was significantly higher (P < 0.001). Towards improving such interactions, pre-visit coaching of patients on how to ask questions and negotiate with their doctor showed that those who underwent coaching had better health outcomes 6 to 12 weeks after their visit26, 27.
Physician biases or preferences may have contributed to the type of urinary diversion offered. Continent urinary diversions are technically more challenging than incontinent urinary diversions and have higher risks of post-operative metabolic consequences. Thus, surgeon skill and biases influence the type of urinary diversion offered16. An evaluation of SEER-Medicare database reported that having a radical cystectomy at a National Cancer Institute-designated cancer center resulted in a 5-fold increased likelihood of receiving a continent diversion (OR 5.50, P = <0.001). This is presumably due to surgeons at these institutions being more likely to be fellowship trained and thus receiving more exposure to continent diversion procedures during training28. However, all physicians during the course of our study received fellowship training, making it less likely that surgical proficiency in performing continent diversions influenced the likelihood of offering this surgical option. Educating surgeons on ethnicity sensitivity during subspecialty training could help improve conscious and unconscious biases that lead to disparity in delivery of surgical care.
One potential confounder underlying the association of ethnicity with lower odds of continent diversion could be disparity in household income. Median household income could influence decisions on urinary diversion selection. Further, Hispanic patients had a significantly lower household income compared to non-Hispanic patients and including household income in one multivariable model impacted the significance of the ethnicity-diversion association.
Distance of travel to the site of surgery increases patients’ financial burden29. Interestingly, distance to surgery was not associated with quality of care delivered in this cohort. However, Hispanics travelled significantly farther distances than non-Hispanics to receive surgical care for bladder cancer. We speculate that this is because the demographics of surrounding cities in South Texas have higher proportions of Hispanic patients compared to the proportion of Hispanics in the city of San Antonio. In 2018, for example, Hispanics comprised 64% of the San Antonio population compared to other major South Texas cities such as McAllen (84.6%), Brownsville (93.2%), Corpus Christi (62.4%) and Laredo (95.6%). Although, distance was not significantly associated with receipt of incontinent urinary diversion, biases towards ethnicity based on perceived distance to travel for postoperative care could influence surgeons’ preference for urinary diversion type.
While our focus was on disparities across ethnicity, we note other important observations identified as opportunities for improvement in quality of care. First, women were significantly less likely to receive continent urinary diversions. We speculate that this may be because of fear of voiding dysfunction with orthotopic diversions28. Second, advanced pathologic stage was associated with decreased number of lymph nodes. This suggests that the value of nodal dissection may be perceived differently for advanced tumors.
Our retrospective review is subject to a few notable limitations and biases. Since data was collected from past patient records, only information that was properly charted by past physicians could be collected and patient preferences and concerns may not have been anecdotally noted. This narrows the breadth of complete data from our original sample size. Additionally, a loss to follow-up bias exists that limits the validity of data collection, as it especially skews patient data of those that may be naturally more non-adherent to medical advice. Future work should also include post-operative quality of life surveys to demonstrate whether or not observed differences in diversion impact quality of life measures. The lack of association between ethnicity and other surgical quality metrics could be influenced by the limited cohort size. Finally, data on primary language (English versus Spanish) was unavailable on a large percentage of patients in this cohort and therefore could not be included in our assessment. Collecting this information in future studies would be beneficial in order to assess possible causes of discrepancies.
Conclusions
Compared to non-Hispanic patients, Hispanic patients are less likely to undergo a continent urinary diversion during radical cystectomy for bladder cancer. Potential causes of this disparity in delivery of surgical care include physician biases, patient preferences, and physician-patient communication. Further investigation is necessary to understand the nature of this disparity and the impact of urinary diversion on quality of life across various ethnic groups.
Supplementary Material
Acknowledgements:
Max and Minnie Tomerlin Voelcker Fund
NIH 5K23CA178204-03
The Roger L. and Laura D. Zeller Charitable Foundation Chair in Urologic Cancer
Bladder Cancer Advocacy Network (BCAN)
CPRIT-funded institutional Research Training Award (RTA; RP170345)
Mays Cancer Center P30 Cancer Center Support Grant (National Cancer Institute) (CA054174)
Footnotes
Conflict of interest: The authors declare no conflicts of interest.
References
- 1.Geneva SWHO. World Health Organization. Quality of care: A process for making strategiec choies in health system. . 2006. [Google Scholar]
- 2.Braxton CC. Defining, measuring, and improving surgical quality: beyond teamwork and checklists to systems redesign and transformation. Surg Infect (Larchmt). 2012;13:312–316. [DOI] [PubMed] [Google Scholar]
- 3.Dehal A, Abbas A, Johna S. Racial disparities in clinical presentation, surgical treatment and in-hospital outcomes of women with breast cancer: analysis of nationwide inpatient sample database. Breast Cancer Res Treat. 2013;139:561–569. [DOI] [PubMed] [Google Scholar]
- 4.Landrum MB, Keating NL, Lamont EB, Bozeman SR, McNeil BJ. Reasons for underuse of recommended therapies for colorectal and lung cancer in the Veterans Health Administration. Cancer. 2012;118:3345–3355. [DOI] [PubMed] [Google Scholar]
- 5.Mort EA, Weissman JS, Epstein AM. Physician discretion and racial variation in the use of surgical procedures. Arch Intern Med. 1994;154:761–767. [PubMed] [Google Scholar]
- 6.Shavers VL, Brown ML. Racial and ethnic disparities in the receipt of cancer treatment. J Natl Cancer Inst. 2002;94:334–357. [DOI] [PubMed] [Google Scholar]
- 7.Lad SP, Bagley JH, Kenney KT, et al. Racial disparities in outcomes of spinal surgery for lumbar stenosis. Spine (Phila Pa 1976). 2013;38:927–935. [DOI] [PubMed] [Google Scholar]
- 8.McCann J, Artinian V, Duhaime L, Lewis JW Jr., Kvale PA, DiGiovine B. Evaluation of the causes for racial disparity in surgical treatment of early stage lung cancer. Chest. 2005;128:3440–3446. [DOI] [PubMed] [Google Scholar]
- 9.Margolis ML, Christie JD, Silvestri GA, Kaiser L, Santiago S, Hansen-Flaschen J. Racial differences pertaining to a belief about lung cancer surgery: results of a multicenter survey. Ann Intern Med. 2003;139:558–563. [DOI] [PubMed] [Google Scholar]
- 10.Gordon HS, Street RL Jr., Sharf BF, Kelly PA, Souchek J. Racial differences in trust and lung cancer patients’ perceptions of physician communication. J Clin Oncol. 2006;24:904–909. [DOI] [PubMed] [Google Scholar]
- 11.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. [DOI] [PubMed] [Google Scholar]
- 12.Rink M, Shariat SF, Xylinas E, et al. Does increasing the nodal yield improve outcomes in patients without nodal metastasis at radical cystectomy? World J Urol. 2012;30:807–814. [DOI] [PubMed] [Google Scholar]
- 13.Barocas DA, Alvarez J, Koyama T, et al. Racial variation in the quality of surgical care for bladder cancer. Cancer. 2014;120:1018–1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Parekh DJ, Reis IM, Castle EP, et al. Robot-assisted radical cystectomy versus open radical cystectomy in patients with bladder cancer (RAZOR): an open-label, randomised, phase 3, non-inferiority trial. Lancet. 2018;391:2525–2536. [DOI] [PubMed] [Google Scholar]
- 15.Manoharan M, Ayyathurai R, de Los Santos R, Nieder AM, Soloway MS. Presentation and outcome following radical cystectomy in Hispanics with bladder cancer. Int Braz J Urol. 2008;34:691–698; discussion 698. [DOI] [PubMed] [Google Scholar]
- 16.Yee DS, Ishill NM, Lowrance WT, Herr HW, Elkin EB. Ethnic differences in bladder cancer survival. Urology. 2011;78:544–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Weiner AB, Keeter MK, Manjunath A, Meeks JJ. Discrepancies in staging, treatment, and delays to treatment may explain disparities in bladder cancer outcomes: An update from the National Cancer Data Base (2004–2013). Urol Oncol. 2018;36:237 e239–237 e217. [DOI] [PubMed] [Google Scholar]
- 18.US Census. American Community Survey 2016.
- 19.Ramirez AG, Thompson IM, Vela L. The South Texas health status review: A health disparities roadmap: Springer; 2013. [PubMed] [Google Scholar]
- 20.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 21.Clavien PA, Barkun J, de Oliveira ML, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250:187–196. [DOI] [PubMed] [Google Scholar]
- 22.Li F, Morgan K, Zaslavsky A. Balancing Covariates via Propensity Score Weighting. Journal of the American Statistical Association. 2017;113:390–400. [Google Scholar]
- 23.Saha S, Arbelaez JJ, Cooper LA. Patient-physician relationships and racial disparities in the quality of health care. Am J Public Health. 2003;93:1713–1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mullins CD, Blatt L, Gbarayor CM, Yang HW, Baquet C. Health disparities: a barrier to high-quality care. Am J Health Syst Pharm. 2005;62:1873–1882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rikard R, Thompson MS, McKinney J, Beauchamp A. Examining health literacy disparities in the United States: a third look at the National Assessment of Adult Literacy (NAAL). BMC Public Health. 2016;16:975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Paasche-Orlow MK, Wolf MS. The causal pathways linking health literacy to health outcomes. Am J Health Behav. 2007;31 Suppl 1:S19–26. [DOI] [PubMed] [Google Scholar]
- 27.Ashton CM, Haidet P, Paterniti DA, et al. Racial and ethnic disparities in the use of health services: bias, preferences, or poor communication? J Gen Intern Med. 2003;18:146–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Farber NJ, Faiena I, Dombrovskiy V, et al. Disparities in the Use of Continent Urinary Diversions after Radical Cystectomy for Bladder Cancer. Bladder Cancer. 2018;4:113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Smith AB, Meyer AM, Meng K, et al. The relationship of travel distance with cystectomy access and outcomes. Urol Oncol. 2018;36:308 e301–308 e309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


