Abstract
Background
Chronic pain is frequently comorbid with depression in clinical practice. Recently, alterations in gut microbiota and metabolites derived therefrom have been found to potentially contribute to abnormal behaviors and cognitive dysfunction via the “microbiota–gut–brain” axis.
Methods
PubMed was searched and we selected relevant studies before October 1, 2019. The search keyword string included “pain OR chronic pain” AND “gut microbiota OR metabolites”; “depression OR depressive disorder” AND “gut microbiota OR metabolites”. We also searched the reference lists of key articles manually.
Results
This review systematically summarized the recent evidence of gut microbiota and metabolites in chronic pain and depression in animal and human studies. The results showed the pathogenesis and therapeutics of chronic pain and depression might be partially due to gut microbiota dysbiosis. Importantly, bacteria-derived metabolites, including short-chain fatty acids, tryptophan-derived metabolites, and secondary bile acids, offer new insights into the potential linkage between key triggers in gut microbiota and potential mechanisms of depression.
Conclusion
Studying gut microbiota and its metabolites has contributed to the understanding of comorbidity of chronic pain and depression. Consequently, modulating dietary structures or supplementation of specific bacteria may be an available strategy for treating chronic pain and depression.
Keywords: chronic pain, depression, gut microbiota, metabolites, short-chain fatty acids
Introduction
According to World Health Organization statistics, incidence rates of various pain symptoms range from 8% to 60% worldwide (Bair et al., 2003). Epidemiological data indicate that approximately 65% of patients with pain have experienced depression throughout their lifetime. Accordingly, patients with pain are 3 to 5 times more likely to suffer from depression than pain-free patients, while the prevalence of depression corresponds with the degree of pain. Similarly, reports have shown that the prevalence of chronic pain among patients with depression was 51.8% to 59.1% (Mohr et al., 2010; Hooten, 2016; Stubbs et al., 2017). In fact, chronic pain and comorbid depression have been frequently encountered clinically, while many studies have unraveled the close association between chronic pain and depression.
There are 1014 to 1015 microorganisms in the large intestine, which is approximately equal to the number of eukaryotic cells in the human body (de Vos and de Vos, 2012; Lozupone et al., 2012; Sender et al., 2016). Furthermore, some researchers have recognized gut microbiota as the second genome, which contains 100 times the number of genes in the human genome (Bäckhed et al., 2005). Host genes associated with the microbial genome are dependent on the overall metabolic status, which is vital for physiological and pathological conditions in humans.
Many studies have suggested that dysbiosis and metabolite alterations are associated with chronic pain and depression (Foster and McVey Neufeld, 2013; OʼMahony et al., 2017; Russo et al., 2018). Fecal microbiota transplantation of germ-free mice with “depression microbiota” derived from patients with major depressive disorder (MDD) resulted in similar depression-like behaviours and dysbiosis as well as host metabolites disturbance, especially for carbohydrate and amino acid metabolism (Zheng et al., 2016). This suggests that dysbiosis may play a causal role in the pathogenesis of depression via its influence on the host’s metabolism. Importantly, our previous study reported that anhedonia-susceptible rats had a significantly different gut microbiota composition compared with the sham or anhedonia-resilient rats who underwent spared nerve surgery (SNI) (Yang et al., 2019).
Taken together, the gut microbiota and its metabolites may be involved in the comorbidity of pain and depression. However, interactions between gut microbiota and host metabolism and their correlation with diseases remain ambiguous. The current review aimed to summarize alterations in the gut microbiota and its metabolites in chronic pain and depression and explore potential mechanisms of dysbiosis in the development of pain and depression from a perspective of bacteria-derived metabolites.
The Role of Gut Microbiota in Chronic Pain and Depression
It is acknowledged that gut microbiota imbalance plays a major role in the etiology of chronic pain and depression. With the development and progress of 16S rRNA gene sequencing and macrogenomics technologies, understanding the composition and function of gut microbiota has become convenient. To this end, 27 related studies, including 17 depression-related and 10 chronic pain-related preclinical and clinical studies, were enrolled. Subsequently, the major findings regarding gut microbiota during depression and pain will be discussed. The details of each study are shown in Tables 1 and 2.
Table 1.
Gut microbiota and metabolic processes associated with depression
| Model/disease | Subject | Sample size (M/F) | Measurements | Microbiota in depression | Metabolites involved |
|---|---|---|---|---|---|
| Depression (Naseribafrouei et al., 2014) | Human | HCs: 18 (7/11)Depressed: 37 (17/20) | ICD-10; MADRS | Phylum: Bacteroidetes↓Genus: Alistipes, Oscillibacter↑ | Not mentioned |
| MDD (Jiang et al., 2015) | Human | HCs: 30 (15/15)Active-MDD: 29 (18/11)Responsed-MDD: 17 (9/8) | DSM-IV; HAMDS; MADRS | Phylum: Bacteroidetes, Proteobacteria↑; Firmicutes↓ Genus: Alistipes, Parabacteroides, Clostridium↑;Bacteroides, Faecalibacterium↓ | Not mentioned |
| MDD (Kelly et al., 2016) | Human | HC: 33 (19/14)MDD: 34 (21/13) | HAMD-17; BDI | Genus: Eggerthella, Holdemania, Gelria, Turicibacter, Paraprevotella, Anaerofilum↑; Prevotella and Dialister↓ | SCFA;Kynurenine/tryptophan |
| MDD (Zheng et al., 2016) | Human | HCs: 63 (23/40) MDD: 39 (15/24) | DSM-IV; HAMDS | Phylum: Actinobacteria↑; Bacteroidetes↓ | Carbohydrate metabolismAmino acid metabolism |
| CSDS (Szyszkowicz et al., 2017) | C57BL/6 mice | Control: 6 (6/0)Susceptible: 10 (10/0)Resilient: 8 (8/0) | SIT | Phylum: Proteobacteria,Verrucomicrobia↑; Chloroflexi↓ Genus: Oscillospira and Turicibacter ↓ | Not mentioned |
| CSDS (Yang et al., 2017b) | C57BL/6 mice | Control: 6 (6/0)Model: 6 (6/0) | SIT; LMT; TST; FST; SPT | Phylum: Actinobacteria↑; Tenericute↓Genus: Butyricimonas↓ | Notmentioned |
| CVS (Yu et al., 2017) | Wistar rats | Control: 8 (8/0)Model: 8 (8/0) | None | Phylum: Bacteroidetes↑; Firmicutes↓Genus: Oscillibacter↑ | Amino acid metabolism; Fatty acid metabolism; Bile acid metabolism |
| MDD (Huang et al., 2018) | Human | HC: 27 (7/20)MDD: 27 (7/20) | ICD-10 | Phylum: Proteobacteria↑; Firmicutes↓Genus: Oxalobacter, Pseudomonas, Parvimonas, Bulleidia, Peptostreptococcus, Gemella↑;Lachnospiraceae, Ruminococcaceae, Coprococcus, Blautia, Clostridiaceae, Dorea↓ | Not mentioned |
| Flinders sensitive line rats (Tillmann et al., 2019) | Rats | FSL: 24 (24/0)FRL: 24 (24/0) | None | Phylum: Proteobacteria↑; Elusimicrobia and Saccharibacteria↓ Genus: Blautia, Subdoligranulum↑; Candidatus Saccharimonas, Alistipe, Roseburia↓ | Not mentioned |
| Depression (Skonieczna-Zydecka et al., 2018) | Women | Nondepressed: 69 (0/69)Depressed: 47 (0/47) | BDI-1 | Not mentioned | SCFA |
| MDD (Chen et al., 2018c) | Human | HCs: 10 (5/5)MDD: 10 (5/5) | DSM-IV; HAMDS | Phylum: Firmicutes, Actinobacteria, Lachnospiraceae↑; Bacteroidetes and Proteobacteria↓Genus: Faecalibacterium↓ | Glucose metabolism;Amino acid metabolism |
| CPSD (Ma et al., 2019) | Wistar rats | Control: 10 (10/0)Model: 10 (10/0) | OFT; TST; FST; SPT | Genus: Oscillospira, Parabacteroides, and Aggregatibacter↑;Phascolarctobacterium, Akkermansia, Ruminococcus↓ | Energy metabolismAmino acid metabolism |
| CUMS (Jianguo et al., 2019) | SD rats | Control: 6 (6/0)CUMS: 6 (6/0) | SPT | Genus: Blautia,Helicobacter↑; Lactobacillus, Porphyromonadaceae↓ | Amino acid metabolism |
| MDD (Chung et al., 2019) | Human | HCs: 37 (14/23)MDD: 36 (8/28) | DSM-V; BDI | Phylum: Firmicutes, Actinobacteria↑Genus: Bifidobacterium, Blautia ↑; Prevotella↓ | Not mentioned |
| MDD (Chen et al., 2018a) | Human | HCs: 44 (20/24)MDD: 44 (20/24) | HDRS-17 | Phylum: Firmicutes, Actinobacteria↑; Bacteroidetesa, Proteobacteria↓Genus: Faecalibacterium↓ | Not mentioned |
| MDD (Rong et al., 2019) | Human | HCs: 30 (14/16)MDD: 31 (9/22) | HAMD-17; DSM-V | Phylum: Firmicutes, Actinobacteria↑; Bacteroidete↓ Genus: Bacteroides, Clostridium, Bifidobacterium, Oscillibacter, Streptococcus↑ | Not mentioned |
| CSDS (McGaughey et al., 2019) | CD-1 mice | Control: 19 (19/0)Model: 20 (20/0) | SIT; OFT; FST; SPT | Genus: Ruminococcus, Dorea, Akkermansia↓ | Not mentioned |
Abbreviations: Active-MDD, group during major depressive episode; BDI, Beck Depression Inventory; CPSD, chronic paradoxical sleep deprivation; CSDS, chronic social defeat stress; CUMS, chronic unpredictated mild stress; CVS, chronic variable stress; DSM-IV/V, Diagnostic and Statistical Manual and Mental Disorders IV/V; FR/SL, flinders resilient/sensitive line; FST, forced swimming test; HAMD(S), Hamilton Depression Scale; HC(s), healthy controls; ICD-10, International Classification of Disease; HDRS-17, 17-item Hamilton Depression Rating Scale; LMT, locomotion test; MADRS, Montgomery Asberg Depression Rating Scale; MDD, major depressive disorder; OFT, open field test; Responsed-MDD, group in response to antidepressant treatment; SIT, social interaction test; SPT, sucrose preference test; TST, tail suspending test; ↑, increase; ↓, decrease.
Table 2.
Gut microbiota and metabolic processes associated with chronic pain.
| Model/Disease | Subject | Sample size (M/F) | Measurements | Microbiota in depression | Metabolites involved |
|---|---|---|---|---|---|
| CRPS (Reichenberger et al., 2013) | Women | HCs: 16 (0/16)CRPS: 16 (0/16) | International Association for the Study of Pain | Phylum: Proteobacteria↑; Firmicutes↓ | Not mentioned |
| CPPS (Shoskes et al., 2016) | Men | HC: 25 (25/0)CPPS: 25 (25/0) | NIH-Chronic Prostatitis Symptom Index; clinical phenotype with UPNIOT | Genus: Prevotella↓ | Not mentioned |
| IC (Braundmeier-Fleming et al., 2016) | Women | HCs: 16 (0/16)IC: 18 (0/18) | A female-specific MAPP genitourinary pain index (GUPI) questionnaire | Phylum: Actinobacteria, Verrucomicrobia↑; Firmicutes ↓Genus: Colinsella aerofaciens, Eggerthella sinensis, Faecalibacterium prasunitzii↓ | Glyceraldehyde;fatty acid metabolism;nicotinate/nicotinamide metabolism |
| ASD-FGID (Luna et al., 2017) | Human | NT: 6 (6/0)ASD-FGID: 14 (13/1)NT-FGID: 15 (13/2) | Autism Diagnostic Observation Schedule Questionnaire on Pediatric Gastrointestinal Symptoms-Rome III | Genus: Clostridiales↓ | Tryptophan metabolism |
| Abdominal pain (Hadizadeh et al., 2018) | Human | HC: 107 (42/65)Case: 52 (21/31) | Abdominal Symptom Questionnaire | Genus: Blautia, Streptococcus, Lactobacillus↑ Prevotella↓ | Not mentioned |
| SNI-induced anhedonia (Yang et al., 2019) | SD rats | Sham: 7 (7/0)Susceptible: 7 (7/0)Resilient: 7 (7/0) | Mechanical threshold;sucrose preference test | Phylum: Parcubacteria↑; Verrucomicrobia↓Genus: Butyricimonas, Parabacteroides, Prevotellaceae, Bilophila, Aggregatibacter↑ | Not mentioned |
| SNI (Guida et al., 2019) | C57 mice | Control: 5 (5/0)Model: 5 (5/0) | Mechanical threshold;tail flick test;thermal threshold | Phylum: Verrucomicrobia, Bacteroidetes↓ Firmicutes↑Genus: A. muciniphila↓ | Not mentioned |
| Fibromyalgia (Minerbi et al., 2019) | Women | HCs: 79 (0/79)Fibromyalgia: 77 (0/77) | 2016 Diagnostic Criteria for Fibromyalgia;interviewed by a specialized pain physician | Phylum: Firmicutes↑; Bacteroidetes↓Genus: Akkermansia muciniphila, Clostridium scindensB.desmolans, Parabacteroides merdae↑ F. prausnitzii, B. uniformis, Faecalibacteriumprausnitzii, Bacteroides uniformis, Prevotella copri, Blautia faecis↓ | SCFA |
| Fibromyalgia (Clos-Garcia et al., 2019) | Human | HCs: 54 (28/26) Fibromyalgia: 105 (32/73) | Widespread Pain Index; Severity Score | Phylum: Actinobacteria↓ Genus: Bifidobacterium, Eubacterium, Clostridum, Bacteroides↓ Dorea, Roseburia, and Alistipes↑ | Glutamate metabolism; Serine metabolism |
| Choronic abdominal pain in PCS (Kang et al., 2019) | Human | HCs: 8 (2/6)PCS with pain: 8 (3/5)PCS without pain: 8 (5/3) | Not mentioned | Phylum: Proteobacteria, Verrucomicrobia↑; Bacteroidetes, Firmicutes↓ | Not mentioned |
Abbreviations: ASD-FGID, functional gastrointestinal disorder in children with autism spectrum disorder; CPPS, chronic prostatitis/pelvic pain; CRPS, complex regional pain syndrome; GUPI, Genitourinary Pain Index; HC(s), healthy controls; IC, interstitial cystitis/bladder pain syndrome; MAPP, Multidisciplinary Approach to the Study of Chronic Pelvic Pain; NIH, National Institutes of Health; NT, neurotypical; NT-GFID, neurotypical children with functional gastrointestinal disorder; PCS, post-cholecystectomy syndrome; SCFA, short chain fatty acid; SNI, spared nerve injury; UPNIOT, urinary, psychosocial, organ-specific, infection, neurological/systemic and tenderness; ↑, increase; ↓, decrease.
Dysbiosis in Depression
Studies have reported that chronic social defeat stress (CSDS), chronic unpredictable mild stress (CUMS), and chronic variable stress (CVS) could effectively mimic animal models of depression, which are frequently utilized in preclinical studies. We previously compared gut microbiota composition in a CSDS model wherein increased phylum Actinobacteria and decreased phylum Tenericutes as well as a higher abundance of genus Bifidobacterium and Butyricimonas were associated with depression susceptibility (Yang et al., 2017b). Moreover, ketamine’s effect on alleviating depression may be attributed to the restoration of Bifidobacterium levels (Yang et al., 2017a). Another study (Szyszkowicz et al., 2017) also found that mice susceptible to chronic social defeat displayed prominent changes within particular sets of bacteria at the phylum and genus taxonomic ranks. At the phylum level, Verrucomicrobia and Proteobacteria increased, whereas Chloroflexi decreased. Interestingly, changes in the mRNA expression of interleukin (IL)-1β and IL-6 within the prefrontal cortex were associated with elevated Flavobacterium levels and reduced Turicibacter levels, which were also strongly correlated with social avoidance severity. Moreover, McGaughey et al. (McGaughey et al., 2019) demonstrated a reduction in Ruminococcus, Dorea, and Akkermansia and an increase in Prevotella and Parabacteroides among depression-susceptible animals. Meanwhile, further functional analyses predicted that an increase in Akkermansia was negatively related to G-protein-coupled receptors and behavior metrics in both anxiety and depression. Studies have also shown significant changes in Firmicutes and Bacteroidetes, indicators of gut microbiota “health,” among CUMS animals and patients with MDD. However, such studies have reported inconsistent changes in Firmicutes and Bacteroidetes, with 4 publications (Lin et al., 2017; Chen et al., 2018a; Rong et al., 2019; Taylor et al., 2019) showing increased Firmicutes and decreased Bacteroidetes in animals or patients susceptible to depression and others showing a higher proportion of Bacteroidetes and lower proportion of Firmicutes among patients with depression (Yu et al., 2017; Huang et al., 2018; Jianguo et al., 2019). These paradoxical results may be due to various factors, such as age, gender, severity of depression, complications, and drug use etc. Studies have also shown that other bacteria, including Alistipes, Oscillibacter, Blautia, and Faecalibacterium, were significantly associated with depression severity (Naseribafrouei et al., 2014; Jiang et al., 2015; Yu et al., 2017; Taylor et al., 2019).
In summary, most studies have shown dysbiosis among patients with depression or rodents with depression-like behaviors. However, inconsistent changes in gut microbiota have been described among the studies. Overall, higher Bacteroidetes, Actinobacteria, and Verrucomicrobia as well as lower Firmicutes and Proteobacteria were observed in depressive subjects. At the genus level, Alistipes, Oscillibacter, Blautia, Akkermansia, Ruminococcus, Prevotella, and Lactobacillus were closely associated with the severity of depression symptoms.
Dysbiosis in Chronic Pain
Currently, only 7 studies have investigated the association between chronic pain and gut microbiota. Although all such studies have indicated gut microbiota alterations among individuals with chronic pain, specific characteristics have remained inconsistent. Our previous study reported higher Parcubacteria and lower Verrucomicrobia in neuropathic pain combined with anhedonia rats. Importantly, antibiotic-treated pseudo germ-free mice received fecal microbiota from rats with chronic pain with anhedonia showed similar hypersensitivity and anhedonia as the donor rats (Yang et al., 2019). Therefore, gut microbiota could have likely played a major role in pain and depression-like phenotypes. In addition, alterations in gut microbiota were also observed in a chronic pain model of vitamin D deficiency with an increase in Firmicutes and decrease in Verrucomicrobia and Bacteroidetes. Furthermore, changes in gut bacterial composition were closely correlated with altered nociception and the endocannabinoid system in the spinal cord, suggesting that gut microbiota may be involved in the development of neuropathic pain induced by vitamin D deficiency (Guida et al., 2019). It has been reported that changes in the composition and physiologic functions of gut microbiota were closely associated with the endocannabinoid system in gut, neuroinflammation in hippocampus, and depression-like symptoms in antibiotic-induced dysbiotic mice (Guida et al., 2018). In particular, substances including N-acylethanolamines and N-acylserotonins are capable of enhancing functions of endocannabinoid systems to ameliorate abnormal behaviors of visceral pain and depression in rodents (Navarria et al., 2014; Bashashati et al., 2017). Thus, interactions between gut microbiota and the endocannabinoid system in chronic pain and depression need further investigation.
Complex Regional Pain Syndrome (CRPS) is a common neuropathic pain induced by a variety of conditions such as injury, illness, or surgery. Accordingly, Reichenberger et al. found more Proteobacteria and less Firmicutes in 16 patients with CRPS compared with 16 healthy controls (Reichenberger et al., 2013). Interestingly, studies have shown that the abundance of Prevotella was correlated with the severity of inflammatory bowel disease-induced functional abdominal pain as well as pain among men with chronic prostatitis/chronic pelvic pain syndrome (Shoskes et al., 2016; Cruz-Aguliar et al., 2019). In addition, a study on abdominal pain among the general population showed that gut microbiota composition, such as Akkermansia muciniphila, Blautia, Streptococcus, and Lactobacillus, was associated with the occurrence, frequency, duration, and intensity of abdominal pain (Hadizadeh et al., 2018). Functional gastrointestinal disorder (FGID) often occurred in children with Autism spectrum disorder (ASD). Another study showed that ASD-FGID had significantly higher levels of several mucosa-associated Clostridiales but markedly lower levels of Dorea, Blautia, and Sutterella compared with healthy children (Luna et al., 2017).
Emerging data on chronic pain suggest that altered host–microbe interaction may contribute to disease symptoms. Gut microbiota such as Prevotella in Bacteroidetes, Blautia in Firmicutes, Akkermansia muciniphila in Verrucomicrobia, and Lactobacillus tended to have a significant correlation with the severity and duration of chronic pain as well as depression.
The Role of Bacteria-Derived Metabolites in Chronic Pain and Depression
To evaluate the relationships between depression and fecal metabolome, 16s rRNA gene sequencing technology combined with ultra-high-performance liquid chromatography-mass spectrometry based on metabolomics was used to explore changes in gut microbiota metabolites in depression. A recent study found dysbiosis and fecal metabolite alterations in CUMS rats, while functional analysis demonstrated that fecal metabolome alterations occurred before changes in plasma metabolome and depressive-like symptoms. This seemingly suggests that fecal microbiota metabolites rather than blood metabolites possibly induce the pathogenesis of depression. Furthermore, several fecal and serum amino acids, such as alanine, serine, tyrosine, l-threonine, isoleucine, and oxidized proline, have demonstrated significant correlations with gut microbiota and behavioral indices of depression, suggesting that gut microbiota amino acid metabolites contributed toward changes in circulating amino acids and depressive behaviors (Jianguo et al., 2019). Rat models of CVS-induced depression also showed dysbiosis, with lower amino acid and fatty acid levels and higher bile acid, hypoxanthine, and stercobilin levels. In addition, altered fecal metabolites, especially the metabolic compounds of tryptophan and bile acids, showed substantial associations with perturbed microbiota genera and severity of depression (Yu et al., 2017). Studies have reported that short-chain fatty acids (SCFA), such as acetate, butyrate, and propionate, have multiple beneficial effects in humans and may play important roles in the pathology of depression. Although Skonieczna-Zydecka and Zheng found that common intestinal bacteria metabolites, such as SCFA, were negatively correlated with the severity of depressive symptoms (sample size of 10 and 58, respectively), Kelly et al. (Kelly et al., 2016) reported no difference in depressive patients (n = 34) (Zheng et al., 2016; Szyszkowicz et al., 2017). Apart from macrogenomics and metabolomics technologies, a comparative metaproteomics approach based on isobaric tags for relative and absolute quantification had been used to identify the host microbial signature in patients with MDD. The results showed that the relative abundance of Faecalibacterium was negatively correlated with the severity of depression and that carbohydrate and amino acid metabolism of fecal microbiota were important (Chen et al., 2018a). These findings were consistent with those presented in previous studies (Zheng et al., 2016; Ma et al., 2019).
Regarding bacteria-derived metabolites in pain-related studies, further microbiome-neuroimmune profile analysis was utilized in ASD-FGID patients to find that cytokines and tryptophan metabolites significantly increased in children with ASD and abdominal pain. Importantly, these proinflammatory cytokines and trytophan metabolites were significantly correlated with several Clostridiales (Luna et al., 2017). Microbiome, serum metabolome, and circulating cytokines were studied in fibromyalgia patients, and the results demonstrated that Bifidobacterium and Eubacterium genera were reduced and glutamine as well as serine metabolism were altered. It suggests that microbiota associated with neurotransmitter metabolism would contribute to the pathogenesis of fibromyalgia (Clos-Garcia et al., 2019). Moreover, another study in female fibromyalgia patients showed that SCFA, especially butyrate and propionate, in the serum and butyrate-producing bacteria in Clostridium genera were highly associated with chronic pain syndrome (Minerbi et al., 2019). Also, fatty acid metabolism and nicotinate/nicotinamide metabolism were reported to serve as new therapeutic strategies for treating chronic pelvic pain (Braundmeier-Fleming et al., 2016).
The relationship between fecal metabolome and depression as well as chronic pain has mainly focused on common metabolites, such as SCFA, amino acids, and bile acids, which need further investigation in future studies.
Potential Mechanism of Bacteria-Derived Metabolites in Chronic Pain and Depression
Accumulating evidence has indicated that nutritional substances obtained from diet could be metabolized by microbiota into a set of small molecular chemicals, such as SCFA, indole and its analogues, and bile acids, which interact with various physiological and pathological pathways in the gut and distant organs, such as the brain.
Influences of Diet on Microbiota and Its Metabolites
Diet, a major source of diverse nutritional component, could rapidly alter the microbial composition in the host (David et al., 2014; Kolodziejczyk et al., 2019). On the contrary, alterations in the microbiota and/or microbiome affect human health (Ursell et al., 2014; Sonnenburg and Bäckhed, 2016). The ultra-high-performance liquid chromatography-mass spectrometry method was performed to compare the metabolomics between mice transplanted with human fecal microbiota and conventional mice, and results showed that diet could remodel metabolites profile of their host (Marcobal et al., 2013). Moreover, omega-3 polyunsaturated fatty acid deficiency in early life could significantly alter the gut microbiota composition, HPA-axis activity, and inflammation, thereby inducing neurobehavioral dysfunction related to cognitive dysfunction and depression (Robertson et al., 2017). However, little is known about the role of bacteria-derived metabolites on behavioral performances and central nervous system function.
Bacteria-derived metabolites are important energy sources for colonocytes and regulate a range of processes, such as inflammatory response, immune modulation, and neurotransmitter synthesis, by acting on cell surface or nuclear receptors. Hence, we believe it is important to discuss the role of bacteria-derived metabolites, including SCFA, amino acid-derived metabolites, and secondary bile acids, in the comorbidity of chronic pain and depression as well as potential mechanisms for their relationship.
Role of SCFA
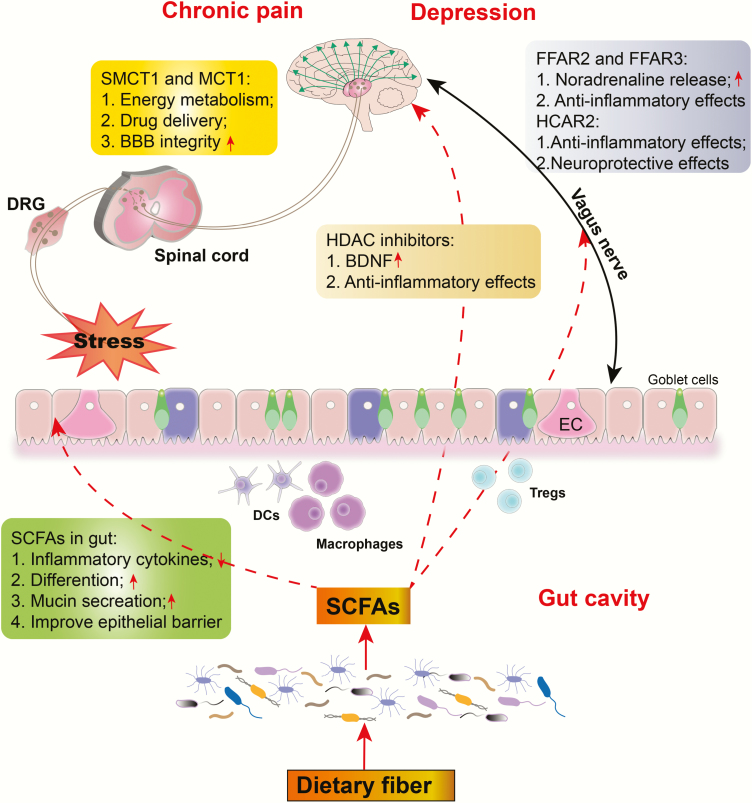
SCFA, including acetate, butyrate, and propionate, are important immunomodulatory and antiinflammatory molecules in the intestine that show promising effects against various diseases, including pain, depression, and neurodegenerative disease (Unger et al., 2016; Freidin et al., 2018; Deng et al., 2019). Regarding inflammation, studies have shown that high levels of proinflammatory cytokines may be associated with the pathogenesis of chronic pain and depression (Walker et al., 2014; Leonard, 2015). We previously reported that abnormities in inflammatory cytokines increased susceptibility to chronic neuropathic pain-induced anhedonia in a rat model of SNI (Yang et al., 2019). Thus, SCFA produced from microbiota may play an important role in the pathogenesis of chronic pain and depression, primarily due to their antiinflammatory effects (an outline for SCFA synthesis and its effects is presented in Figure 1).
Figure 1.
The effects of short-chain fatty acids (SCFAs) on chronic pain and depression. SCFAs indirectly affect the progression of chronic pain and depression by modulating intestinal inflammation and epithelial barrier function. They also directly impact the CNS by modulating energy metabolism, neuroinflammation, and blood–brain barrier (BBB) permeability via their receptors, transporters, and histone deacetylases (HDACs). DCs, dendritic cells; DRG, dorsal root ganglion; EC, enterochromaffin cell; FFAR 2/3, free fatty acid receptor 2 or 3; MCT1, monocarboxylate transporter 1; SMCT1, sodium-coupled monocarboxylate transporter 1; Tregs, regulatory T cells; ↑, increase; ↓, decrease.
Role of SCFA in the Gut
Irritable bowel syndrome (IBS), which is characterized by periodic abdominal pain, is a common gastrointestinal tract disorder. IBS and depression comorbidity is a common phenomenon in clinical practice, with co-occurrence rates of approximately 30% (Liu et al., 2016; Sibelli et al., 2016). The major risk factor for IBS was determined to be low-grade intestinal inflammation, which alters gut microbes and gut barrier permeability (Yamamoto et al., 2019). Microbial dysbiosis and gut barrier impairment enhance bacterial translocation and activation of the HPA axis, which is intrinsic to the pathology of depression (Zhuang et al., 2017).
SCFAs can promote antiinflammatory and immunoregulatory effects in different cells within the gastrointestinal tract. Accordingly, studies have shown that NLRP3 (NOD-, LRR-, and pyrin domain-containing protein 3) inflammasome in intestinal epithelial cells can be activated by SCFAs to enhance IL-8 secretion and subsequently improves the epithelia barrier integrity (Kalina et al., 2002; Macia et al., 2015). Moreover, germ-free mice transplanted with the Bacteroides thetaiotaomicron (acetate producer) could promote mucin production by goblet cells and maintain intestinal barrier integrity. Apart from intestinal epithelia cells, SCFAs can directly target immune cells to regulate the inflammatory response. Accordingly, butyrate and propionate block the genesis and differentiation of dendritic cells from bone marrow stem cells, which are responsible for immune dysfunction (Singh et al., 2010). However, butyrate and propionate but not acetate can potentiate the generation and differentiation of antiinflammatory regulatory T cells via inhibiting HDAC, affecting the balance of pro- and antiinflammatory mechanisms (Arpaia et al., 2013). Moreover, SCFAs can reduce the production of proinflammatory cytokines from lipopolysaccharide (LPS)-activated neutrophils and macrophages via HDAC inhibition (Chang et al., 2014).
Overall, SCFAs can regulate inflammatory and immunomodulatory response via affecting immune cells in the intestine as well as potentiate epithelial barrier integrity by promoting mucin secretion.
Role of SCFA in the Central Nervous System
More studies have reported that SCFA can impact the CNS through their effects on energy metabolism, neuro-inflammation, and the blood–brain barrier (BBB). To the best of our knowledge, only a few studies have investigated physiological concentrations of SCFAs in the brain or cerebrospinal fluid. Considering the relatively low levels of SCFAs in peripheral blood, we can speculate that SCFA concentration in the brain is extremely low. Evidence for the presence of SCFAs in the brain has been derived from the fact that numerous transmembrane neuronal proteins, receptors, and transporters typically bind to SCFAs to influence brain processes. Importantly, SCFAs can affect brain function by stimulating the peripheral nervous system or immune system without affecting the brain. We thus discuss relevant data regarding receptors, transporters, and histone deacetylases (HDACs) in the CNS.
SCFA Receptors
SCFAs have been found to activate 4 G protein-coupled receptors, namely GPR43 (also called free fatty acid receptor 2, FFAR2), GPR41 (FFAR3), GPR109a (also called hydroxycarboxylic acid receptor 2, HCAR2), and GPR164 (also called olfactory receptor family 51; Olfr558 in mice) (Bolognini et al., 2016). SCFA receptors can be found in several cell types, including neurons. Studies have shown that both FFAR2 and FFAR3 are expressed in norepinephrinergic sympathetic neurons and that binding of these receptors to propionate enhanced norepinephrine release (Kimura et al., 2011). Notably, recent findings have suggested that noradrenaline is extremely important for the inhibition of neuropathic pain and depression (Obata, 2017). Moreover, the main mechanism whereby antidepressants inhibit neuropathic pain is the increase in noradrenaline in the spinal cord and activation of the impaired descending noradrenergic inhibitory system (Obata, 2017). Furthermore, Lal et al. found that butyrate could directly activate afferent vagus nerve fibers and that FFAR3 was present in mouse brainstem vagal ganglion, the effects of which may be mediated by butyrate receptors (Lal et al., 2001). Importantly, dysfunctional vagus nerve-induced inflammatory imbalance has been closely associated with the etiology of chronic pain and depression (Chakravarthy et al., 2015; Kong et al., 2018). FFAR2 and FFAR3 had also been found to be expressed in dorsal root ganglia and trigeminal ganglia, which are necessary for pain transduction. The hypothalamus, a vital integration site during ascending pain transduction, has been identified to express HCAR2. Furthermore, HCAR2 upregulation in the substantia nigra of patients with Parkinson’s disease was responsible for the antiinflammatory and neuroprotective effects of the recently approved anti-multiple sclerosis drug dimethylfumarate (Chen et al., 2014; Fu et al., 2015; Offermanns and Schwaninger, 2015). Taken together, these studies suggest the potential mechanistic benefits of SCFA receptor activation in antiinflammation and pain transduction. However, considering the absence of studies directly investigating the role of SCFA receptors in pain and depression, further large-scale preclinical and clinical studies are urgently needed.
SCFA Transporters
SCFAs are transported across cell membranes with the help of H+-coupled monocarboxylate transporters (MCTs) and sodium-coupled monocarboxylate transporters (SMCTs). Interestingly, the distribution of these transporters in the brain is cell-specific such that SMCT1 is found mainly in neurons and MCT1 is predominantly expressed in glia cells, including astrocytes, microglia, and oligodendrocytes (Moreira et al., 2009; Lee et al., 2012). Under physiological conditions, MCTs and SMCTs are important for shuttling lactate and acetone bodies from astrocytes to the neurons for energy metabolism. Importantly, butyrate can be transferred from the circulation into glial cells and neurons to mediate direct effects in the brain (Vijay and Morris, 2014). In addition to monocarboxylates, these transporters play a critical role in brain drug delivery and can be blocked by nonsteroidal antiinflammatory drugs (Martin et al., 2006; Vijay and Morris, 2014). Hence, the relationship between these transporters and the pharmacological effects of antidepressants and analgesics remains to be clarified in the future. Interestingly, butyrate itself possesses the potential to maintain BBB integrity considering that colonization with butyrate-producing bacterium (Clostridium tyrobutyricum) and oral sodium butyrate administration (1000 mg/kg for 3 days) could repair BBB leakage by increasing the expression of tight junction proteins (Braniste et al., 2014). Given that only a few studies have investigated SCFA transporters in chronic pain and depression, further studies are warranted to elucidate underlying mechanisms and to determine contexts wherein SCFA transporters are beneficial for pain and depression.
Histone Deacetylase Inhibitors
Several lines of evidence have suggested that epigenetic factors, such as chromatin remodeling via histone methylation and acetylation, play an important role in chronic pain and depression. In this regard, epigenetic alterations in genes related to neuroprotection (e.g., brain-derived neurotrophic factor [BDNF]), inflammatory response (e.g., tumor necrosis factor-α). and oxidative stress (e.g., reactive oxygen species) have been identified in patients with chronic pain and depression (Kauer-Sant’Anna et al., 2009). Several studies have inferred that SCFA-induced inhibition of HDACs increases histone acetylation, thereby exerting analgesic and antidepressant effects (Negrete et al., 2017; Qiao et al., 2019). Yamawaki and colleagues found that repeated sodium butyrate treatment (1.2 g/kg for 7 days) alleviated LPS-induced extension of immobility time during the forced swimming test, which was associated with decreased expression ionized calcium-binding adapter molecule 1 in the hippocampus through acetylation of histones H3 and H4. Moreover, 1 study showed that butyrate alleviated depressive symptoms by increasing BDNF levels in the prefrontal cortex via its potential to inhibit histone deacetylation (Yamawaki et al., 2018). Although no direct evidence supports SCFA treatment for pain, HDAC inhibitors, such as trichostatin A, valproic acid, and suberoylanilide hydroxamic acid, were found to reverse C-fiber-related hypoesthesia and morphine resistance in neuropathic pain after peripheral nerve injury (Matsushita et al., 2013). Thus, the association between nociceptive transduction and SCFAs and HDACs should be investigated in future studies.
Overall, SCFAs may contribute to pain and depression relief through their antiinflammatory and immunomodulatory properties. SCFAs can also improve epithelial barrier function by stimulating mucin production and promoting reassembling of tight junctions (Braniste et al., 2014). However, except for inflammation, the underlying mechanism whereby SCFAs affect the comorbidity of chronic pain and depression remains unknown, thereby warranting further large-scale studies.
The Role of Amino Acid-Derived Metabolites
The gut microbiota is critical for the fermentation and absorption of amino acids derived from food or the host. Amino acid-derived metabolites have been considered important modulators in the pathogenesis of chronic pain and depression. Importantly, oral supplementation of specific amino acids, such as tryptophan, had protective and therapeutic effects on pain and depression. Hence, we will mostly discuss the role of tryptophan-derived metabolites in chronic pain and depression.
Tryptophan
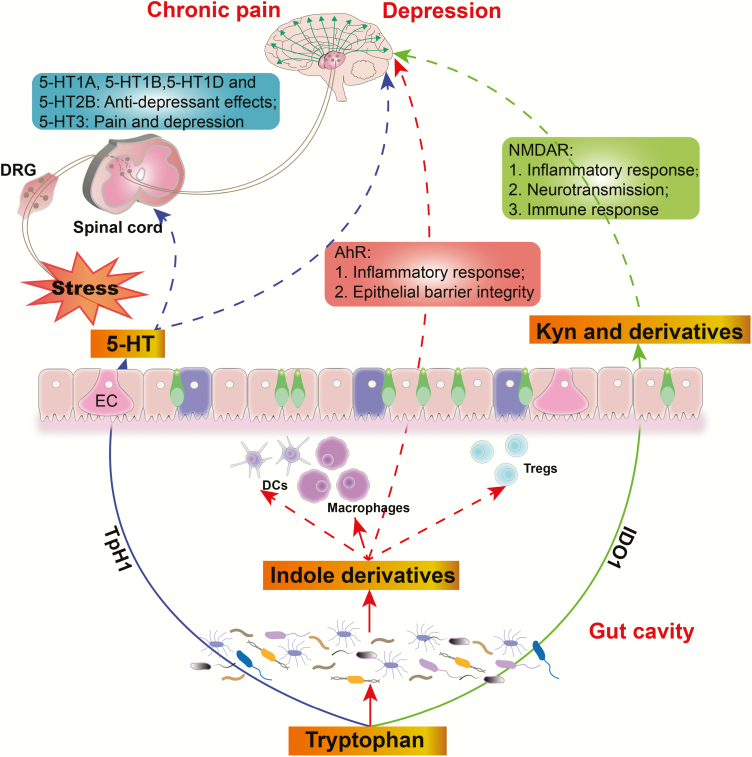
Tryptophan and tyrosine are 2 important elements for mood and emotion regulation given that they are precursors for several monoamine neurotransmitters, including serotonin (tryptophan) and dopamine, epinephrine, and norepinephrine (tyrosine) (Parker and Brotchie, 2011). Monoamine neurotransmitter deficiency has been considered the major mechanism underlying the comorbidity of chronic pain and depression (Thor et al., 2007; Benson et al., 2015). Tryptophan is essential and should be supplied externally, mostly through dietary supplementation. Accordingly, the World Health Organization recommends a daily tryptophan intake of 4 mg/kg. Three major pathways for tryptophan metabolism in the gastrointestinal tract exist: (1) several molecules metabolized from microbiota, including ligands for the aryl hydrocarbon receptor (AhR); (2) the kynurenine (Kyn) pathway via indoleamine 2,3-dioxygenase (IDO); and (3) 5-hydroxytryptamine (5-HT) synthesis via IDO (TpH1) (Agus et al., 2018) (an outline for tryptophan-derived metabolite synthesis and its effects is presented in Figure 2).
Figure 2.
The effects of tryptophan-derived metabolites on chronic pain and depression. Aryl hydrocarbon receptor (AhR) ligands metabolized from microbiota can improve epithelial function by activating AhR and modulate overall immune homeostasis. Kynurenine (Kyn) and its derivatives promote the progression of pain and depression by modulating inflammation, neurotransmission, and immune response. Another important product of tryptophan is 5-hydroxytryptamine (5-HT), synthesized in the EMC by tryptophan hydroxylase 1 (TpH1), which can promote or protect against chronic pain and depression by activating different receptors. DCs, dendritic cells; DRG, dorsal root ganglion; EC, enterochromaffin cell; IDO1, indoleamine 2,3-dioxygenase; NMDAR, N-methyl-D-aspartic acid receptor; Tregs, regulatory T cells.
AhR Ligand Pathway
Intestinal microorganisms can metabolize tryptophan into several molecules, such as indole and its derivatives. Many indole derivatives, including indole-3-acid-acietic, indole-3-aldehyde, indo-3-propionic acid, and indoleacrylic acid, are ligands for AhR. AhR signaling plays an important role in maintaining intestinal homeostasis by acting on many immune cells to modulate barrier integrity and inflammatory response (Lamas et al., 2018). AhR is a ligand-dependent transcription factor that mediates the expression of target genes, such as cytochrome P450, and a set of pro-/antiinflammatory cytokines. AhR deficiency or microbial dysbiosis has been shown to increase the severity of dextran sulfate sodium-induced colitis. In these models, AhR signaling dysfunction promotes colitis by decreasing the production of IL-22, a cytokine with well-known effects on intestinal homeostasis (Qiu et al., 2012; Zelante et al., 2013; Lamas et al., 2018). Moreover, reports have shown that oral supplementation of tryptophan or AhR ligand-producing Lactobacillus spp. can alleviate colitis symptoms, suggesting that tryptophan metabolites have an important role in mucosal immune homeostasis via AhR dependent IL-22 production. Notably, germ-free mice deficient in AhR agonists are susceptible to chronic stress and show anxiety and depression-like behaviors (Lukić et al., 2019). Our previous study also demonstrated that pseudo germ-free mice established using a broad-spectrum antibiotic cocktail presented lower mechanical withdraw threshold and sucrose preference loss (Yang et al., 2019). In this regard, we can speculate that tryptophan deficiency- or intestinal dysbiosis-induced alterations in the AhR signaling pathway, which lead to a reduction in antiinflammatory cytokines, might be involved in the pathogenesis of chronic pain and depression comorbidity. However, this remains to be validated in future studies.
Kyn Pathway
Tryptophan can be converted into Kyn and downstream products, such as quinolinic acid, niacin, nicotinamide adenine dinucleotide, and kynurenic acid, by the rate-limiting enzyme IDO (Cervenka et al., 2017; Kennedy et al., 2017). The gut microbiota plays a key role in modulating IDO activity, especially in germ-free and antibiotic-treated mice. Kyn and downstream products are implicated in numerous biological processes involving inflammation, neurotransmitter transmission, and immune response. Accordingly, studies have shown that patients with MDD had higher plasma Kyn concentrations compared with healthy controls and that variations in Kyn concentrations were closely associated with the severity of MDD (Savitz, 2017; Kuwano et al., 2018). Furthermore, a study using an LPS-induced depression model found that Kyn augmented systemic inflammation-induced monocyte trafficking in an AhR-dependent manner, which mediated neuroimmune dysregulation and depression-like behaviors (Zang et al., 2018). On the contrary, pharmacological AhR blockade and circulatory monocyte clearance reversed the LPS and Kyn effects on depressive symptoms, suggesting that the Kyn–AhR axis is important for immunoregulation and depression. Quinolinic acid, the end product of Kyn, is a neurotoxic N-methyl-d-aspartic acid receptor agonist that can directly contribute to depressive symptoms. Additionally, 1 study investigated IDO1 and Kyn levels in an SNI-induced pain and depression comorbidity model, with the results showing that neuropathic pain was closely associated with an increase in IDO1 and Kyn/tryptophan ratio in the liver but not the brain. Importantly, intrathecal IL-1 inhibitor IL-1RA reversed IDO1 levels in the liver, SNI-induced mechanical hyperalgesia, and depressive symptoms (Zhou et al., 2015). These findings support the possibility that Kyn derived from tryptophan via IDO plays a vital role in the comorbidity of chronic pain and depression.
5-Hydroxytryptamine Pathway
Tryptophan produces 5-hydroxytryptophan through catalysis of tryptophan hydroxylase, which can be inhibited by several factors, such as stress, inflammation, or insulin resilience (Turner et al., 2006). 5-hydroxytryptophan is then metalized into 5-HT (or serotonin), which affects numerous physiological functions, notably mood regulation, once released into the synaptic cleft. Interestingly, approximately 90% of 5-HT is produced in the gut, particularly by enterochromaffin cells (ECs), through the enzyme tryptophan hydroxylase 1 (TpH1). This observation suggests that ECs have potential effects on mood disorders, such as depression and anxiety, due to their regulation of 5-HT availability (Yang et al., 2018). Considering that the relationship between ECs and depression and chronic pain has rarely been studied, further studies are warranted to determine the role of ECs in the pathology of chronic pain and depression comorbidity. It is noteworthy that 5-HT cannot transverse the BBB under normal conditions, although ECs produce 90% of 5-HT in the gut (Martin et al., 2017; Lund et al., 2018). Furthermore, 5-HT3 receptor activation has been associated with pain and depression via the modulation of GABA and DA release into the CNS (Davies, 2011). Moreover, tricyclic antidepressants and SNRIs have been regularly used in treating chronic pain, such as neuropathic pain, partly due to the increase in 5-HT.
Overall, disturbed tryptophan metabolism in subjects with chronic pain or depression may be linked to abnormal inflammatory and metabolic processes, immune dysregulation, and neurotransmitter synthesis dysfunction. Hence, considering the complex and multifactorial relationship between tryptophan metabolites and comorbidity of chronic pain and depression, further studies are greatly needed.
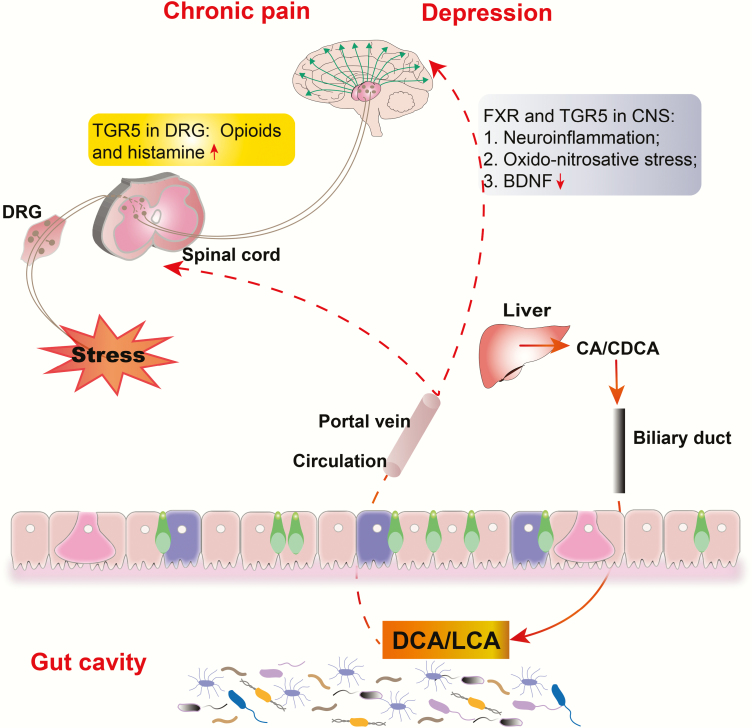
The Role of Secondary Bile Acids
Bile acids, approximately 85% of bile, are important components necessary for the emulsification and absorption of dietary fats. Bile acids are synthesized from cholesterol in the liver and thereafter metabolized into second bile acids by colonic bacteria through multiple and well-characterized enzymatic pathways (Lefebvre et al., 2009). Primary bile acids are the direct products of cholesterol metabolites in hepatocytes, such as cholic acid (CA) and chenodeoxycholic acid (CDCA). In response to cholecystokinin after feeding, primary bile acids are secreted by the liver into the small intestine to ensure assimilation of dietary lipids. Accordingly, 95% of the bile acids are actively absorbed in the terminal ileum and redirected into the portal circulation to reenter the liver, whereas a small proportion pass into the colon where they are transformed by bacteria into secondary bile acids—lithocholic acid, deoxycholic acid, and ursodeoxycholic acid—via deconjugation and 7α-dehydroxylation (Hofmann and Hagey, 2008; Bajor et al., 2010) (an outline for bile acid synthesis and its actions is presented in Figure 3). Studies have demonstrated that bile acids exert widespread physiologic effects via the activation of specific receptors in the nucleus and plasma membrane (Lieu et al., 2014). These receptors can mediate diverse pathophysiological processes, including glucose homeostasis, inflammation, and sensory transduction. Receptor-recognizing bile acids include nuclear receptors, for example, farnesoid X receptor (FXR), preganane X receptor, and vitamin D receptor; and surface receptors, for example, G protein-coupled bile acid receptor (GPBAR1 or TGR5), sphingosine 1 phosphate receptor 2, and muscarinic receptors 2 and 3. Nuclear receptors mediate the genomic effects of bile acids on glucose and lipid metabolism, while surface receptors mainly mediate rapid and nongenomic actions of bile acids, such as sensory transduction and inflammation (Lieu et al., 2014). The remainder of this section focuses mainly on FXR- and TGR5-mediated signaling in pain and depression.
Figure 3.
The effects of bile acids on chronic pain and depression. Deoxycholic acid (DCA)/lithocholic acid (LCA) metabolized from primary bile acids (cholic acid [CA]/chenodeoxycholic acid [CDCA]) by microbiota are absorbed in the terminal ileum and redirected into the portal vein. Bile acids can activate Takeda G-protein-coupled receptor 5 (TGR5) on the spinal neurons, inducing the release of opioids and histamine that transmit itch and analgesia. They also affect the CNS by activating farnesoid X receptor (FXR) in the neurons and TGR5 in glial cells, which modulate neuroinflammation, oxido-nitrosative stress, and brain-derived neurotrophic factor (BDNF) levels. DRG, dorsal root ganglion; ↑, increase; ↓, decrease.
FXR ligand activation is essential for the pathogenesis of depression. A recent study found that overexpression of hippocampal FXR through lentiviral gene modulation induced depression-like symptoms and decreased hippocampal BDNF expression in naïve rats. Moreover, knockout of hippocampal FXR completely prevented the effects of CUMS on depressive behaviors and BDNF expression (Chen et al., 2018b). This suggests that FXR plays a crucial role in the pathogenesis of depression via the modulation of BDNF levels. Similarly, tauroursodeoxycholic acid treatment could prevent LPS-induced depressive behaviors probably through the attenuation of neuroinflammation and oxido-nitrosative stress. Accordingly, the inhibition of glial nuclear factor-κB and activation of TGR5 in microglia have been revealed to mediate the effect of tauroursodeoxycholic acid on the production of proinflammatory cytokines (Yanguas-Casás et al., 2014).
Bile acids have been well recognized for their critical role in the process and treatment mechanisms of pain. Although morphine can be used to treat pain, chronic use thereof can induce several side effects, such as dependence, tolerance, immunosuppression, and gastrointestinal disorders, which limit their long-term use (Dominguez and Habib, 2013). However, the mechanisms underlying these side effects still remain unknown. A recent study suggested that microbial dysbiosis and bile acid imbalance contributed to the aforementioned side effects in mice receiving chronic morphine treatment, while further analysis demonstrated a liner correlation between morphine-induced microbial dysbiosis, bile acid dysregulation, gut barrier disruption, and systemic inflammation. This study also showed that microbiota transplantation and blockade of toll-like receptor 2/μ-opioid receptor signaling could restore gut homeostasis altered by morphine (Banerjee et al., 2016). Moreover, a study using a morphine dependence model revealed a significant shift in gut microbiome and metabolome within 1 day after morphine treatment, particularly expansion of Enterococcus faecalis and reduction of deoxycholic acid, contributing to deleterious effects during short-term opioid use (Wang et al., 2018). This suggests that gut microbiota and bile acids play a vital role in the pharmacological effects of opioid analgesics. Moreover, bile acids are often used as adjuvants to pharmaceutical drugs to increase their solubility. Studies have found that the administration of methyl ester of monoketocholic acid potentiates the analgesic effect of morphine by increasing morphine transport into the CNS (Shiffka et al., 2017). Lidocaine administration with cholic acids and its keto derivatives in rats has also been reported to increase the duration of local anesthesia (Posa et al., 2007). This analgesic effect of bile acids is assumed to be associated with their membrane-stabilizing action (Horváth et al., 2016).
Interestingly, bile acids themselves can modulate pain perception and sensory transduction. Patients with cholestatic liver disease exhibited severe pruritus and analgesia, which were probably mediated by TGR5 activation on sensory nerves, resulting in the release of neuropeptides, including opioids and histamine, in the spinal cord that transmit itch and analgesia. In this study, intrathecal and intraplantar bile acids and selective TGR5 agonists induced hypersensitivity of DRG neurons and scratching behaviors, while these symptoms were absent from Tgr5-KO mice (Dawson and Karpen, 2014). Admittedly, bile acids are crucial chemical molecules involved in the pain process. Visceral hypersensitivity in IBS has also been reported to be associated with colonic bile acid aggregation, which involves the FXR-nerve growth factor–transient receptor potential vanilloid 1 axis (Li et al., 2019).
In summary, bile acids are key signaling molecules involved in the pathogenesis of pain and depression. Although the mechanisms through which bile acids affect comorbidity of pain and depression remain unclear, further studies on the role of bile acids in the pathogenesis and treatment mechanisms of pain and depression are greatly needed.
Conclusions
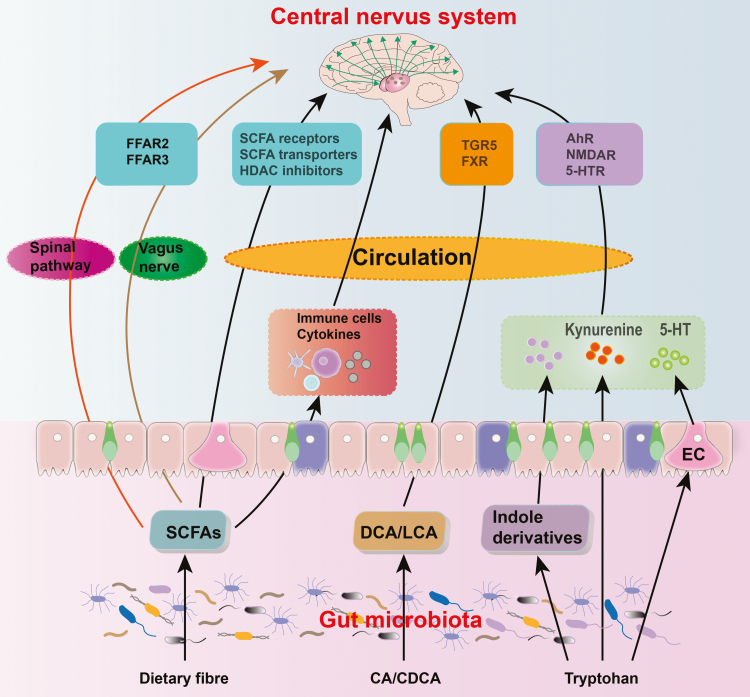
Both animal and human studies have revealed that alterations in gut microbiota and their metabolites can directly and indirectly affect neuroinflammation and neuro-immunity in the onset and transduction of pain and depression (the overview of gut microbiota and its metabolites in chronic pain and depression is shown in Figure 4). This review has focused on 3 classes of metabolites, namely SCFAs, amino acid-derived metabolites, and bile acids, which can act on epithelial and immune cells to modulate gut barrier permeability and inflammation, thereby indirectly affecting the progression of pain and depression. In addition, these substances are capable of crossing the epithelial barrier into the circulation, thus modulating distant organs, such as the brain and spinal cord, by activating or inhibiting specific receptors therein. In view of this, we are certain that more complete knowledge on the relationship between the microbiota and its metabolites will be crucial for the development of new treatment modalities for relevant diseases. Consequently, modulating dietary structures or supplementation of specific bacteria may be a new strategy to address chronic pain and depression. In addition, probiotics have been reported to alleviate stress and its related disorders such as anxiety and depression (Bercik et al., 2010; Slyepchenko et al., 2014; Wan and Jena, 2019). Future studies are required to investigate whether supplementation of specific deficient bacteria and its derived metabolites could provide a new therapeutic strategy to prevent and treat chronic pain and depression.
Figure 4.
Overview of “microbiota-gut-brain” axis in chronic pain and depression. AhR, aryl hydrocarbon receptor; CA, cholic acid; CDCA, chenodeoxycholic acid; DCA, deoxycholic acid; EC, enterochromaffin cell; FFAR 2/3, free fatty acid receptor 2 or 3; FXR, farnesoid X receptor; HDAC, histone deacetylase; 5-HT, 5-hydroxytryptamine; LCA, lithocholic acid; NMDAR, N-Methyl-D-aspartate receptor; SCFA, short-chain fatty acids; TGR 5, Takeda G-protein-coupled receptor 5.
Acknowledgments
This review was supported by grants from the National Natural Science Foundation of China (81771159 and 81571047 to A.L.; 81703482 and 81974171 to C.Y.).
Statement of Interest
None.
References
- Agus A, Planchais J, Sokol H (2018) Gut microbiota regulation of tryptophan metabolism in health and disease. Cell Host Microbe 23:716–724. [DOI] [PubMed] [Google Scholar]
- Arpaia N, Campbell C, Fan X, Dikiy S, van der Veeken J, deRoos P, Liu H, Cross JR, Pfeffer K, Coffer PJ, Rudensky AY (2013) Metabolites produced by commensal bacteria promote peripheral regulatory T-cell generation. Nature 504:451–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bäckhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI (2005) Host-bacterial mutualism in the human intestine. Science 307:1915–1920. [DOI] [PubMed] [Google Scholar]
- Bair MJ, Robinson RL, Katon W, Kroenke K (2003) Depression and pain comorbidity: a literature review. Arch Intern Med 163:2433–2445. [DOI] [PubMed] [Google Scholar]
- Bajor A, Gillberg PG, Abrahamsson H (2010) Bile acids: short and long term effects in the intestine. Scand J Gastroenterol 45:645–664. [DOI] [PubMed] [Google Scholar]
- Banerjee S, Sindberg G, Wang F, Meng J, Sharma U, Zhang L, Dauer P, Chen C, Dalluge J, Johnson T, Roy S (2016) Opioid-induced gut microbial disruption and bile dysregulation leads to gut barrier compromise and sustained systemic inflammation. Mucosal Immunol 9:1418–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bashashati M, Fichna J, Piscitelli F, Capasso R, Izzo AA, Sibaev A, Timmermans JP, Cenac N, Vergnolle N, Di Marzo V, Storr M(2017) Targeting fatty acid amide hydrolase and transient receptor potential vanilloid-1 simultaneously to modulate colonic motility and visceral sensation in the mouse: a pharmacological intervention with N-arachidonoyl-serotonin (AA-5-HT). Neurogastroenterol Motil 29. doi: 10.1111/nmo.13148. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Benson C, Mifflin K, Kerr B, Jesudasan SJ, Dursun S, Baker G (2015) Biogenic amines and the amino acids GABA and glutamate: relationships with pain and depression. Mod Trends Pharmacopsychiatry 30:67–79. [DOI] [PubMed] [Google Scholar]
- Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan WI, Corthesy-Theulaz I, Cherbut C, Bergonzelli GE, Collins SM (2010) Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology 139:2102–2112.e1. [DOI] [PubMed] [Google Scholar]
- Bolognini D, Tobin AB, Milligan G, Moss CE (2016) The Pharmacology and function of receptors for short-chain fatty acids. Mol Pharmacol 89:388–398. [DOI] [PubMed] [Google Scholar]
- Braniste V, Al-Asmakh M, Kowal C, Anuar F, Abbaspour A, Tóth M, Korecka A, Bakocevic N, Ng LG, Guan NL, Kundu P, Gulyás B, Halldin C, Hultenby K, Nilsson H, Hebert H, Volpe BT, Diamond B, Pettersson S (2014) The gut microbiota influences blood-brain barrier permeability in mice. Sci Transl Med 6:263ra158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braundmeier-Fleming A, Russell NT, Yang W, Nas MY, Yaggie RE, Berry M, Bachrach L, Flury SC, Marko DS, Bushell CB, Welge ME, White BA, Schaeffer AJ, Klumpp DJ (2016) Stool-based biomarkers of interstitial cystitis/bladder pain syndrome. Sci Rep 6:26083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervenka I, Agudelo LZ, Ruas JL(2017) Kynurenines: tryptophan’s metabolites in exercise, inflammation, and mental health. Science 357:1–8. [DOI] [PubMed] [Google Scholar]
- Chakravarthy K, Chaudhry H, Williams K, Christo PJ (2015) Review of the uses of vagal nerve stimulation in chronic pain management. Curr Pain Headache Rep 19:54. [DOI] [PubMed] [Google Scholar]
- Chang PV, Hao L, Offermanns S, Medzhitov R (2014) The microbial metabolite butyrate regulates intestinal macrophage function via histone deacetylase inhibition. Proc Natl Acad Sci U S A 111:2247–2252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Assmann JC, Krenz A, Rahman M, Grimm M, Karsten CM, Köhl J, Offermanns S, Wettschureck N, Schwaninger M (2014) Hydroxycarboxylic acid receptor 2 mediates dimethyl fumarate’s protective effect in EAE. J Clin Invest 124:2188–2192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JJ, Zheng P, Liu YY, Zhong XG, Wang HY, Guo YJ, Xie P (2018a) Sex differences in gut microbiota in patients with major depressive disorder. Neuropsychiatr Dis Treat 14:647–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen WG, Zheng JX, Xu X, Hu YM, Ma YM (2018b) Hippocampal FXR plays a role in the pathogenesis of depression: a preliminary study based on lentiviral gene modulation. Psychiatry Res 264:374–379. [DOI] [PubMed] [Google Scholar]
- Chen Z, Li J, Gui S, Zhou C, Chen J, Yang C, Hu Z, Wang H, Zhong X, Zeng L, Chen K, Li P, Xie P (2018c) Comparative metaproteomics analysis shows altered fecal microbiota signatures in patients with major depressive disorder. Neuroreport 29:417–425. [DOI] [PubMed] [Google Scholar]
- Chung YE, Chen HC, Chou HL, Chen IM, Lee MS, Chuang LC, Liu YW, Lu ML, Chen CH, Wu CS, Huang MC, Liao SC, Ni YH, Lai MS, Shih WL, Kuo PH (2019) Exploration of microbiota targets for major depressive disorder and mood related traits. J Psychiatr Res 111:74–82. [DOI] [PubMed] [Google Scholar]
- Clos-Garcia M, et al. (2019) Gut microbiome and serum metabolome analyses identify molecular biomarkers and altered glutamate metabolism in fibromyalgia. Ebiomedicine 46:499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cruz-Aguliar RM, Wantia N, Clavel T, Vehreschild MJGT, Buch T, Bajbouj M, Haller D, Busch D, Schmid RM, Stein-Thoeringer CK (2019) An open-labeled study on fecal microbiota transfer in irritable bowel syndrome patients reveals improvement in abdominal pain associated with the relative abundance of Akkermansia Muciniphila. Digestion 100:127–138. [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, Ling AV, Devlin AS, Varma Y, Fischbach MA, Biddinger SB, Dutton RJ, Turnbaugh PJ (2014) Diet rapidly and reproducibly alters the human gut microbiome. Nature 505:559–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies PA. (2011) Allosteric modulation of the 5-HT(3) receptor. Curr Opin Pharmacol 11:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson PA, Karpen SJ (2014) Bile acids reach out to the spinal cord: new insights to the pathogenesis of itch and analgesia in cholestatic liver disease. Hepatology 59:1638–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng FL, Pan JX, Zheng P, Xia JJ, Yin BM, Liang WW, Li YF, Wu J, Xu F, Wu QY, Qu CH, Li W, Wang HY, Xie P (2019) Metabonomics reveals peripheral and central short-chain fatty acid and amino acid dysfunction in a naturally occurring depressive model of macaques. Neuropsychiatr Dis Treat 15:1077–1088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominguez JE, Habib AS (2013) Prophylaxis and treatment of the side-effects of neuraxial morphine analgesia following cesarean delivery. Curr Opin Anaesthesiol 26:288–295. [DOI] [PubMed] [Google Scholar]
- Foster JA, McVey Neufeld KA(2013) Gut-brain axis: how the microbiome influences anxiety and depression. Trends Neurosci 36:305–312. [DOI] [PubMed] [Google Scholar]
- Freidin MB, Wells HRR, Potter T, Livshits G, Menni C, Williams FMK (2018) Metabolomic markers of fatigue: association between circulating metabolome and fatigue in women with chronic widespread pain. Biochim Biophys Acta Mol Basis Dis 1864:601–606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu SP, Liu BR, Wang JF, Xue WJ, Liu HM, Zeng YL, Huang BX, Li SN, Lv QK, Wang W, Liu JX (2015) β-Hydroxybutyric acid inhibits growth hormone-releasing hormone synthesis and secretion through the GPR109A/extracellular signal-regulated ½ signaling pathway in the hypothalamus. J Neuroendocrinol 27:212–222. [DOI] [PubMed] [Google Scholar]
- Guida F, Turco F, Iannotta M, De Gregorio D, Palumbo I, Sarnelli G, Furiano A, Napolitano F, Boccella S, Luongo L, Mazzitelli M, Usiello A, De Filippis F, Iannotti FA, Piscitelli F, Ercolini D, de Novellis V, Di Marzo V, Cuomo R, Maione S (2018) Antibiotic-induced microbiota perturbation causes gut endocannabinoidome changes, hippocampal neuroglial reorganization and depression in mice. Brain Behav Immun 67:230–245. [DOI] [PubMed] [Google Scholar]
- Guida F, Boccella S, Belardo C, Iannotta M, Piscitelli F, De Filippis F, Paino S, Ricciardi F, Siniscalco D, Marabese I, Luongo L, Ercolini D, Di Marzo V, Maione S(2019) Altered gut microbiota and endocannabinoid system tone in vitamin D deficiency-mediated chronic pain. Brain Behav Immun. doi: 10.1016/j.bbi.2019.04.006. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Hadizadeh F, Bonfiglio F, Belheouane M, Vallier M, Sauer S, Bang C, Bujanda L, Andreasson A, Agreus L, Engstrand L, Talley NJ, Rafter J, Baines JF, Walter S, Franke A, D’Amato M (2018) Faecal microbiota composition associates with abdominal pain in the general population. Gut 67:778–779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hofmann AF, Hagey LR (2008) Bile acids: chemistry, pathochemistry, biology, pathobiology, and therapeutics. Cell Mol Life Sci 65:2461–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooten WM. (2016) Chronic pain and mental health disorders: shared neural mechanisms, epidemiology, and treatment. Mayo Clin Proc 91:955–970. [DOI] [PubMed] [Google Scholar]
- Horváth G, Bencsura Á, Simon Á, Tochtrop GP, DeKoster GT, Covey DF, Cistola DP, Toke O (2016) Structural determinants of ligand binding in the ternary complex of human ileal bile acid binding protein with glycocholate and glycochenodeoxycholate obtained from solution NMR. Febs J 283:541–555. [DOI] [PubMed] [Google Scholar]
- Huang Y, Shi X, Li Z, Shen Y, Shi X, Wang L, Li G, Yuan Y, Wang J, Zhang Y, Zhao L, Zhang M, Kang Y, Liang Y (2018) Possible association of Firmicutes in the gut microbiota of patients with major depressive disorder. Neuropsychiatr Dis Treat 14:3329–3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang H, Ling Z, Zhang Y, Mao H, Ma Z, Yin Y, Wang W, Tang W, Tan Z, Shi J, Li L, Ruan B (2015) Altered fecal microbiota composition in patients with major depressive disorder. Brain Behav Immun 48:186–194. [DOI] [PubMed] [Google Scholar]
- Jianguo L, Xueyang J, Cui W, Changxin W, Xuemei Q (2019) Altered gut metabolome contributes to depression-like behaviors in rats exposed to chronic unpredictable mild stress. Transl Psychiatry 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalina U, Koyama N, Hosoda T, Nuernberger H, Sato K, Hoelzer D, Herweck F, Manigold T, Singer MV, Rossol S, Böcker U (2002) Enhanced production of IL-18 in butyrate-treated intestinal epithelium by stimulation of the proximal promoter region. Eur J Immunol 32:2635–2643. [DOI] [PubMed] [Google Scholar]
- Kang Z, Lu M, Jiang M, Zhou D, Huang H (2019) Proteobacteria acts as a pathogenic risk-factor for chronic abdominal pain and diarrhea in post-cholecystectomy syndrome patients: a gut microbiome metabolomics study. Med Sci Monit 25:7312–7320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauer-Sant’Anna M, Kapczinski F, Andreazza AC, Bond DJ, Lam RW, Young LT, Yatham LN (2009) Brain-derived neurotrophic factor and inflammatory markers in patients with early- vs. late-stage bipolar disorder. Int J Neuropsychopharmacol 12:447–458. [DOI] [PubMed] [Google Scholar]
- Kelly JR, Borre Y, O’ Brien C, Patterson E, El Aidy S, Deane J, Kennedy PJ, Beers S, Scott K, Moloney G, Hoban AE, Scott L, Fitzgerald P, Ross P, Stanton C, Clarke G, Cryan JF, Dinan TG (2016) Transferring the blues: depression-associated gut microbiota induces neurobehavioural changes in the rat. J Psychiatr Res 82:109–118. [DOI] [PubMed] [Google Scholar]
- Kennedy PJ, Cryan JF, Dinan TG, Clarke G (2017) Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 112:399–412. [DOI] [PubMed] [Google Scholar]
- Kimura I, Inoue D, Maeda T, Hara T, Ichimura A, Miyauchi S, Kobayashi M, Hirasawa A, Tsujimoto G (2011) Short-chain fatty acids and ketones directly regulate sympathetic nervous system via G protein-coupled receptor 41 (GPR41). Proc Natl Acad Sci U S A 108:8030–8035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolodziejczyk AA, Zheng D, Elinav E(2019) Diet-microbiota interactions and personalized nutrition. Nat Rev Microbiol 17:742–753. doi: 10.1038/s41579-019-0256-8. [DOI] [PubMed] [Google Scholar]
- Kong J, Fang J, Park J, Li S, Rong P(2018) Treating depression with transcutaneous auricular vagus nerve stimulation: state of the art and future perspectives. Front Psychiatry 9:20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuwano N, Kato TA, Setoyama D, Sato-Kasai M, Shimokawa N, Hayakawa K, Ohgidani M, Sagata N, Kubo H, Kishimoto J, Kang D, Kanba S (2018) Tryptophan-kynurenine and lipid related metabolites as blood biomarkers for first-episode drug-naïve patients with major depressive disorder: an exploratory pilot case-control study. J Affect Disord 231:74–82. [DOI] [PubMed] [Google Scholar]
- Lal S, Kirkup AJ, Brunsden AM, Thompson DG, Grundy D(2001) Vagal afferent responses to fatty acids of different chain length in the rat. Am J Physiol Gastrointest Liver Physiol 281:G907–G915. [DOI] [PubMed] [Google Scholar]
- Lamas B, Natividad JM, Sokol H(2018) Aryl hydrocarbon receptor and intestinal immunity. Mucosal Immunol 11:1024–1038. [DOI] [PubMed] [Google Scholar]
- Lee Y, Morrison BM, Li Y, Lengacher S, Farah MH, Hoffman PN, Liu Y, Tsingalia A, Jin L, Zhang PW, Pellerin L, Magistretti PJ, Rothstein JD (2012) Oligodendroglia metabolically support axons and contribute to neurodegeneration. Nature 487:443–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre P, Cariou B, Lien F, Kuipers F, Staels B (2009) Role of bile acids and bile acid receptors in metabolic regulation. Physiol Rev 89:147–191. [DOI] [PubMed] [Google Scholar]
- Leonard BE. (2015) Pain, depression and inflammation: are interconnected causative factors involved? Mod Trends Pharmacopsychiatry 30:22–35. [DOI] [PubMed] [Google Scholar]
- Li WT, Luo QQ, Wang B, Chen X, Yan XJ, Qiu HY, Chen SL (2019) Bile acids induce visceral hypersensitivity via mucosal mast cell-to-nociceptor signaling that involves the farnesoid X receptor/nerve growth factor/transient receptor potential vanilloid 1 axis. Faseb J 33:2435–2450. [DOI] [PubMed] [Google Scholar]
- Lieu T, Jayaweera G, Bunnett NW (2014) GPBA: a GPCR for bile acids and an emerging therapeutic target for disorders of digestion and sensation. Br J Pharmacol 171:1156–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin P, Ding B, Feng C, Yin S, Zhang T, Qi X, Lv H, Guo X, Dong K, Zhu Y, Li Q(2017) Prevotella and Klebsiella proportions in fecal microbial communities are potential characteristic parameters for patients with major depressive disorder. J Affect Disord 207:300–304. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhang L, Wang X, Wang Z, Zhang J, Jiang R, Wang X, Wang K, Liu Z, Xia Z, Xu Z, Nie Y, Lv X, Wu X, Zhu H, Duan L(2016) Similar fecal microbiota signatures in patients with diarrhea-predominant irritable bowel syndrome and patients with depression. Clin Gastroenterol Hepatol 14:1602–1611, e1605. [DOI] [PubMed] [Google Scholar]
- Lozupone CA, Stombaugh JI, Gordon JI, Jansson JK, Knight R(2012) Diversity, stability and resilience of the human gut microbiota. Nature 489:220–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lukić I, Getselter D, Koren O, Elliott E (2019) Role of tryptophan in microbiota-induced depressive-like behavior: evidence from tryptophan depletion study. Front Behav Neurosci 13:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luna RA, Oezguen N, Balderas M, Venkatachalam A, Runge JK, Versalovic J, Veenstra-VanderWeele J, Anderson GM, Savidge T, Williams KC (2017) Distinct microbiome-neuroimmune signatures correlate with functional abdominal pain in children with autism spectrum disorder. Cell Mol Gastroenterol Hepatol 3:218–230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund ML, Egerod KL, Engelstoft MS, Dmytriyeva O, Theodorsson E, Patel BA, Schwartz TW (2018) Enterochromaffin 5-HT cells - A major target for GLP-1 and gut microbial metabolites. Mol Metab 11:70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Song J, Wang H, Shi F, Zhou N, Jiang J, Xu Y, Zhang L, Yang L, Zhou M(2019) Chronic paradoxical sleep deprivation-induced depression-like behavior, energy metabolism and microbial changes in rats. Life Sci 225:88–97. doi: 10.1016/j.lfs.2019.04.006. [DOI] [PubMed] [Google Scholar]
- Macia L, et al. (2015) Metabolite-sensing receptors GPR43 and GPR109A facilitate dietary fibre-induced gut homeostasis through regulation of the inflammasome. Nat Commun 6:6734. [DOI] [PubMed] [Google Scholar]
- Marcobal A, Kashyap PC, Nelson TA, Aronov PA, Donia MS, Spormann A, Fischbach MA, Sonnenburg JL (2013) A metabolomic view of how the human gut microbiota impacts the host metabolome using humanized and gnotobiotic mice. Isme J 7:1933–1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin AM, Young RL, Leong L, Rogers GB, Spencer NJ, Jessup CF, Keating DJ (2017) The diverse metabolic roles of peripheral serotonin. Endocrinology 158:1049–1063. [DOI] [PubMed] [Google Scholar]
- Martin PM, Gopal E, Ananth S, Zhuang L, Itagaki S, Prasad BM, Smith SB, Prasad PD, Ganapathy V (2006) Identity of SMCT1 (SLC5A8) as a neuron-specific Na+-coupled transporter for active uptake of L-lactate and ketone bodies in the brain. J Neurochem 98:279–288. [DOI] [PubMed] [Google Scholar]
- Matsushita Y, Araki K, Omotuyi Oi, Mukae T, Ueda H (2013) HDAC inhibitors restore C-fibre sensitivity in experimental neuropathic pain model. Br J Pharmacol 170:991–998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGaughey KD, Yilmaz-Swenson T, Elsayed NM, Cruz DA, Rodriguiz RM, Kritzer MD, Peterchev AV, Roach J, Wetsel WC, Williamson DE (2019) Relative abundance of Akkermansia spp. and other bacterial phylotypes correlates with anxiety- and depressive-like behavior following social defeat in mice. Sci Rep 9:3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minerbi A, Gonzalez E, Brereton NJB, Anjarkouchian A, Dewar K, Fitzcharles MA, Chevalier S, Shir Y (2019) Altered microbiome composition in individuals with fibromyalgia. Pain 160:2589–2602. [DOI] [PubMed] [Google Scholar]
- Mohr P, Bitter I, Svestka J, Seifritz E, Karamustafalioglu O, Koponen H, Sartorius N (2010) Management of depression in the presence of pain symptoms. Psychiatr Danub 22:4–13. [PubMed] [Google Scholar]
- Moreira TJ, Pierre K, Maekawa F, Repond C, Cebere A, Liljequist S, Pellerin L (2009) Enhanced cerebral expression of MCT1 and MCT2 in a rat ischemia model occurs in activated microglial cells. J Cereb Blood Flow Metab 29:1273–1283. [DOI] [PubMed] [Google Scholar]
- Naseribafrouei A, Hestad K, Avershina E, Sekelja M, Linløkken A, Wilson R, Rudi K (2014) Correlation between the human fecal microbiota and depression. Neurogastroenterol Motil 26:1155–1162. [DOI] [PubMed] [Google Scholar]
- Navarria A, Tamburella A, Iannotti FA, Micale V, Camillieri G, Gozzo L, Verde R, Imperatore R, Leggio GM, Drago F, Di Marzo V (2014) The dual blocker of FAAH/TRPV1 N-arachidonoylserotonin reverses the behavioral despair induced by stress in rats and modulates the HPA-axis. Pharmacol Res 87:151–159. [DOI] [PubMed] [Google Scholar]
- Negrete R, García Gutiérrez MS, Manzanares J, Maldonado R (2017) Involvement of the dynorphin/KOR system on the nociceptive, emotional and cognitive manifestations of joint pain in mice. Neuropharmacology 116:315–327. [DOI] [PubMed] [Google Scholar]
- Obata H.(2017) Analgesic mechanisms of antidepressants for neuropathic pain. Int J Mol Sci 18:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Offermanns S, Schwaninger M (2015) Nutritional or pharmacological activation of HCA(2) ameliorates neuroinflammation. Trends Mol Med 21:245–255. [DOI] [PubMed] [Google Scholar]
- OʼMahony SM, Dinan TG, Cryan JF (2017) The gut microbiota as a key regulator of visceral pain. Pain 158 (Suppl 1):S19–S28. [DOI] [PubMed] [Google Scholar]
- Parker G, Brotchie H (2011) Mood effects of the amino acids tryptophan and tyrosine: ‘Food for Thought’ III. Acta Psychiatr Scand 124:417–426. [DOI] [PubMed] [Google Scholar]
- Posa M, Kevresan S, Mikov M, Cirin-Novta V, Kuhajda K (2007) Effect of cholic acid and its keto derivatives on the analgesic action of lidocaine and associated biochemical parameters in rats. Eur J Drug Metab Pharmacokinet 32:109–117. [DOI] [PubMed] [Google Scholar]
- Qiao M, Jiang QS, Liu YJ, Hu XY, Wang LJ, Zhou QX, Qiu HM (2019) Antidepressant mechanisms of venlafaxine involving increasing histone acetylation and modulating tyrosine hydroxylase and tryptophan hydroxylase expression in hippocampus of depressive rats. Neuroreport 30:255–261. [DOI] [PubMed] [Google Scholar]
- Qiu J, Heller JJ, Guo X, Chen ZM, Fish K, Fu YX, Zhou L (2012) The aryl hydrocarbon receptor regulates gut immunity through modulation of innate lymphoid cells. Immunity 36:92–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichenberger ER, Alexander GM, Perreault MJ, Russell JA, Schwartzman RJ, Hershberg U, Rosen G (2013) Establishing a relationship between bacteria in the human gut and complex regional pain syndrome. Brain Behav Immun 29:62–69. [DOI] [PubMed] [Google Scholar]
- Robertson RC, Seira Oriach C, Murphy K, Moloney GM, Cryan JF, Dinan TG, Paul Ross R, Stanton C (2017) Omega-3 polyunsaturated fatty acids critically regulate behaviour and gut microbiota development in adolescence and adulthood. Brain Behav Immun 59:21–37. [DOI] [PubMed] [Google Scholar]
- Rong H, Xie XH, Zhao J, Lai WT, Wang MB, Xu D, Liu YH, Guo YY, Xu SX, Deng WF, Yang QF, Xiao L, Zhang YL, He FS, Wang S, Liu TB (2019) Similarly in depression, nuances of gut microbiota: evidences from a shotgun metagenomics sequencing study on major depressive disorder versus bipolar disorder with current major depressive episode patients. J Psychiatr Res 113:90–99. [DOI] [PubMed] [Google Scholar]
- Russo R, Cristiano C, Avagliano C, De Caro C, La Rana G, Raso GM, Canani RB, Meli R, Calignano A (2018) Gut-brain axis: role of lipids in the regulation of inflammation, pain and CNS diseases. Curr Med Chem 25:3930–3952. [DOI] [PubMed] [Google Scholar]
- Savitz J. (2017) Role of kynurenine metabolism pathway activation in major depressive disorders. Curr Top Behav Neurosci 31:249–267. [DOI] [PubMed] [Google Scholar]
- Sender R, Fuchs S, Milo R (2016) Revised estimates for the number of human and bacteria cells in the body. Plos Biol 14:e1002533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiffka SJ, Kane MA, Swaan PW (2017) Planar bile acids in health and disease. Biochim Biophys Acta Biomembr 1859:2269–2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoskes DA, Wang H, Polackwich AS, Tucky B, Altemus J, Eng C (2016) Analysis of gut microbiome reveals significant differences between men with chronic prostatitis/chronic pelvic pain syndrome and controls. J Urol 196:435–441. [DOI] [PubMed] [Google Scholar]
- Sibelli A, Chalder T, Everitt H, Workman P, Windgassen S, Moss-Morris R (2016) A systematic review with meta-analysis of the role of anxiety and depression in irritable bowel syndrome onset. Psychol Med 46:3065–3080. [DOI] [PubMed] [Google Scholar]
- Singh N, Thangaraju M, Prasad PD, Martin PM, Lambert NA, Boettger T, Offermanns S, Ganapathy V (2010) Blockade of dendritic cell development by bacterial fermentation products butyrate and propionate through a transporter (Slc5a8)-dependent inhibition of histone deacetylases. J Biol Chem 285:27601–27608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skonieczna-Zydecka K, Grochans E, Maciejewska D, Szkup M, Schneider-Matyka D, Jurczak A, Loniewski I, Kaczmarczyk M, Marlicz W, Czerwinska-Rogowska M, Pelka-Wysiecka J, Dec K, Stachowska E(2018) Faecal short chain fatty acids profile is changed in polish depressive women. Nutrients 10:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slyepchenko A, Carvalho AF, Cha DS, Kasper S, McIntyre RS (2014) Gut emotions - mechanisms of action of probiotics as novel therapeutic targets for depression and anxiety disorders. CNS Neurol Disord Drug Targets 13:1770–1786. [DOI] [PubMed] [Google Scholar]
- Sonnenburg JL, Bäckhed F (2016) Diet-microbiota interactions as moderators of human metabolism. Nature 535:56–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stubbs B, Vancampfort D, Veronese N, Thompson T, Fornaro M, Schofield P, Solmi M, Mugisha J, Carvalho AF, Koyanagi A (2017) Depression and pain: primary data and meta-analysis among 237 952 people across 47 low- and middle-income countries. Psychol Med 47:2906–2917. [DOI] [PubMed] [Google Scholar]
- Szyszkowicz JK, Wong A, Anisman H, Merali Z, Audet MC (2017) Implications of the gut microbiota in vulnerability to the social avoidance effects of chronic social defeat in male mice. Brain Behav Immun 66:45–55. [DOI] [PubMed] [Google Scholar]
- Taylor AM, Thompson SV, Edwards CG, Musaad SMA, Khan NA, Holscher HD(2019) Associations among diet, the gastrointestinal microbiota, and negative emotional states in adults. Nutr Neurosci 1–10. doi: 10.1080/1028415X.2019.1582578. E-pub ahead of print. [DOI] [PubMed] [Google Scholar]
- Thor KB, Kirby M, Viktrup L (2007) Serotonin and noradrenaline involvement in urinary incontinence, depression and pain: scientific basis for overlapping clinical efficacy from a single drug, duloxetine. Int J Clin Pract 61:1349–1355. [DOI] [PubMed] [Google Scholar]
- Tillmann S, Abildgaard A, Winther G, Wegener G (2019) Altered fecal microbiota composition in the Flinders sensitive line rat model of depression. Psychopharmacology (Berl) 236:1445–1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner EH, Loftis JM, Blackwell AD (2006) Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan. Pharmacol Ther 109:325–338. [DOI] [PubMed] [Google Scholar]
- Unger MM, Spiegel J, Dillmann KU, Grundmann D, Philippeit H, Bürmann J, Faßbender K, Schwiertz A, Schäfer KH (2016) Short chain fatty acids and gut microbiota differ between patients with Parkinson’s disease and age-matched controls. Parkinsonism Relat Disord 32:66–72. [DOI] [PubMed] [Google Scholar]
- Ursell LK, Haiser HJ, Van Treuren W, Garg N, Reddivari L, Vanamala J, Dorrestein PC, Turnbaugh PJ, Knight R (2014) The intestinal metabolome: an intersection between microbiota and host. Gastroenterology 146:1470–1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijay N, Morris ME (2014) Role of monocarboxylate transporters in drug delivery to the brain. Curr Pharm Des 20:1487–1498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vos WM, de Vos EA (2012) Role of the intestinal microbiome in health and disease: from correlation to causation. Nutr Rev 70(Suppl 1):S45–S56. [DOI] [PubMed] [Google Scholar]
- Walker AK, Kavelaars A, Heijnen CJ, Dantzer R (2014) Neuroinflammation and comorbidity of pain and depression. Pharmacol Rev 66:80–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wan YY, Jena PK (2019) Precision dietary supplementation based on personal gut microbiota. Nat Rev Gastroenterol Hepatol 16:204–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F, Meng J, Zhang L, Johnson T, Chen C, Roy S (2018) Morphine induces changes in the gut microbiome and metabolome in a morphine dependence model. Sci Rep 8:3596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto M, Pinto-Sanchez MI, Bercik P, Britz-McKibbin P (2019) Metabolomics reveals elevated urinary excretion of collagen degradation and epithelial cell turnover products in irritable bowel syndrome patients. Metabolomics 15:82. [DOI] [PubMed] [Google Scholar]
- Yamawaki Y, Yoshioka N, Nozaki K, Ito H, Oda K, Harada K, Shirawachi S, Asano S, Aizawa H, Yamawaki S, Kanematsu T, Akagi H (2018) Sodium butyrate abolishes lipopolysaccharide-induced depression-like behaviors and hippocampal microglial activation in mice. Brain Res 1680:13–38. [DOI] [PubMed] [Google Scholar]
- Yang C, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017a) Bifidobacterium in the gut microbiota confer resilience to chronic social defeat stress in mice. Sci Rep 7:45942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Qu Y, Fujita Y, Ren Q, Ma M, Dong C, Hashimoto K (2017b) Possible role of the gut microbiota-brain axis in the antidepressant effects of (R)-ketamine in a social defeat stress model. Transl Psychiatry 7:1294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Gao J, Zhang J, Luo AL (2018) Enterochromaffin cells in the gut: a distant regulator of brain function? Gut 67:1557–1558. [DOI] [PubMed] [Google Scholar]
- Yang C, Fang X, Zhan G, Huang N, Li S, Bi J, Jiang R, Yang L, Miao L, Zhu B, Luo A, Hashimoto K (2019) Key role of gut microbiota in anhedonia-like phenotype in rodents with neuropathic pain. Transl Psychiatry 9:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanguas-Casás N, Barreda-Manso MA, Nieto-Sampedro M, Romero-Ramírez L (2014) Tauroursodeoxycholic acid reduces glial cell activation in an animal model of acute neuroinflammation. J Neuroinflammation 11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu M, Jia H, Zhou C, Yang Y, Zhao Y, Yang M, Zou Z (2017) Variations in gut microbiota and fecal metabolic phenotype associated with depression by 16S rRNA gene sequencing and LC/MS-based metabolomics. J Pharm Biomed Anal 138:231–239. [DOI] [PubMed] [Google Scholar]
- Zang X, Zheng X, Hou Y, Hu M, Wang H, Bao X, Zhou F, Wang G, Hao H (2018) Regulation of proinflammatory monocyte activation by the kynurenine-AhR axis underlies immunometabolic control of depressive behavior in mice. Faseb J 32:1944–1956. [DOI] [PubMed] [Google Scholar]
- Zelante T, Iannitti RG, Cunha C, De Luca A, Giovannini G, Pieraccini G, Zecchi R, D’Angelo C, Massi-Benedetti C, Fallarino F, Carvalho A, Puccetti P, Romani L (2013) Tryptophan catabolites from microbiota engage aryl hydrocarbon receptor and balance mucosal reactivity via interleukin-22. Immunity 39:372–385. [DOI] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, Zeng L, Chen J, Fan S, Du X, Zhang X, Yang D, Yang Y, Meng H, Li W, Melgiri ND, Licinio J, Wei H, Xie P (2016) Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Mol Psychiatry 21:786–796. [DOI] [PubMed] [Google Scholar]
- Zhou W, Dantzer R, Budac DP, Walker AK, Mao-Ying QL, Lee AW, Heijnen CJ, Kavelaars A (2015) Peripheral indoleamine 2,3-dioxygenase 1 is required for comorbid depression-like behavior but does not contribute to neuropathic pain in mice. Brain Behav Immun 46:147–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang X, Xiong L, Li L, Li M, Chen M (2017) Alterations of gut microbiota in patients with irritable bowel syndrome: a systematic review and meta-analysis. J Gastroenterol Hepatol 32:28–38. [DOI] [PubMed] [Google Scholar]