Abstract
Multilevel factors impact HPV vaccine series initiation and completion among adolescents in the U.S. Synthesis of these factors is needed to inform intervention development and to direct future research. Current frameworks synthesizing factors focus on females only and do not include both series initiation and completion outcomes. We conducted a systematic review of reviews to identify modifiable individual-, provider-, and clinic-level factors associated with HPV vaccination outcomes among U.S. adolescents and developed a multilevel framework illustrating relations between factors to inform intervention development. We searched Medline, PsychInfo, Pubmed, CINAHL, and ERIC databases and included reviews published 2006 to July 2, 2018 describing individual-, provider-, or clinic-level factors quantitatively associated with HPV vaccination among U.S. adolescents. Two coders independently screened reviews, extracted data, and determined quality ratings. Sixteen reviews containing 481 unique primary studies met criteria. Factors synthesized into the multilevel framework included parent psychosocial factors (knowledge, beliefs, outcome expectations, intentions) and behaviors, provider recommendation, and patient-targeted and provider-targeted clinic systems. The scope of our framework and review advances research in two key ways. First, the framework illustrates salient modifiable factors at multiple levels on which to intervene to increase HPV vaccination. Second, the review identified critical gaps in the literature at each level. Future research should link the body of literature on parental intentions to vaccination outcomes, identify provider psychosocial factors associated with recommendation behaviors and subsequent vaccine uptake in their patient population, and understand clinic factors associated with successful implementation of patient- and provider-targeted system-level interventions.
Keywords: Human papillomavirus vaccine, adolescent vaccination, systematic review, multilevel intervention
Introduction
The human papillomavirus (HPV) vaccine protects against oncogenic types of HPV that can lead to cancer and genital warts.1,2 HPV-related cancers include cervical, anal, vaginal, vulvar, penile, and oropharyngeal cancers, with the most common being cervical cancer among women and oropharyngeal cancers among men.3 The CDC Advisory Committee for Immunization Practices (ACIP) recommends the HPV vaccine be administered to adolescents aged 11-12 years, although it is available for adolescents starting at age nine and adults up to age 45.4,5 Current HPV vaccination coverage lags behind the Health People 2020 national goal that 80% of 13-15 year olds complete the multi-dose series.6,7 National data show HPV vaccine initiation rates of 70% of females and 66% of males aged 13-17 years, and 54% of females and 49% of males aged 13-17 years are considered up to date on all doses.7
To increase vaccination, it is important to understand the diverse multilevel factors associated with HPV vaccine series initiation and completion. Synthesizing these factors in a multilevel framework that illustrates their associations can inform future interventions aiming to increase HPV vaccination coverage. The National Cancer Institute (NCI) specifically calls for multilevel interventions across the cancer control continuum.8 However, current frameworks and evidence syntheses of factors associated with HPV vaccination are limited in in their scope, outcomes, and populations.9-13 Fernandez and colleagues were the first to develop a multilevel logic model illustrating the relations between parental-, provider-, clinic-, community-, and policy-level factors associated with HPV vaccination acceptance and willingness to vaccinate.10 That model, however, has limited applicability to inform interventions focused on vaccine series initiation and completion. Although more recent frameworks illustrate relations between multilevel factors associated with behavioral outcomes, they were produced before the vaccine was recommended for males.9,11,14
An updated framework integrating multilevel factors associated with HPV vaccine series initiation and completion for male and female adolescents in the U.S. is needed. Therefore, our systematic review or reviews consolidates the large body of literature describing multilevel factors associated with HPV vaccination outcomes among U.S. adolescents aged 11-17 years, and we illustrate the relations among these factors in an empirically-based and theoretically-informed multilevel framework. We limit the scope of our review and framework to the individual-, provider-, and clinic-levels and do not include population levels such as the community or policy levels. While policies such as mandatory HPV vaccination for school entry have been successful at increasing coverage, particularly outside of the U.S., states are slow to adopt such policies which often have limited support.15-18 Instead, we focus on the individual-, provider-, and clinic-levels to inform the development of interventions targeting vaccination decision makers (parents and adolescents), vaccine administerers (providers), and clinic systems.
Methods
Methods are reported according to the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) checklist.19
Eligibility criteria
We included English-language peer-reviewed reviews (defined as comprehensive, integrated, or systematic literature reviews or meta-analyses) published between 2006, when the U.S. Food and Drug Administration approved the first available HPV vaccine,20 through the date of the final search (July 2, 2018). Those reporting modifiable correlates or predictors (factors) of HPV vaccine initiation and/or completion for U.S. adolescents ages 9-17 years via parental reports or electronic health records were eligible. We defined modifiable factors as those that have the potential to change through intervention including psychosocial, attitudinal, or behavioral variables at the individual- and provider-levels and procedural or systems variables at the clinic-level. We excluded the following: reviews with only HPV vaccine awareness, acceptability, and intention outcomes; commentaries, editorials, dissertations, abstracts, and conference proceedings; and reviews with only qualitative primary studies. However, reviews were included if they contained findings based on mixed methods research or if qualitative data were presented with quantitative data corroborating findings.
Information sources and search
We searched Pubmed (NLM), Medline and PsychInfo (Ovid), and CINAHL and ERIC (Ebsco). A health sciences librarian experienced in developing and documenting search strategies for systematic reviews assisted in developing the search strategy in Medline (see Supplemental Materials). Concepts in the search included: human papillomavirus, immunizations or vaccination, parents or guardians, and adolescents. The search was adapted for use in PsychInfo, Pubmed, CINAHL, and ERIC databases.
Study selection and data collection
Two reviewers used standardized forms to conduct title and abstract screening, full text reviews, and data extraction. First, the reviewers (blind to journal titles, authors, and author affiliations) independently screened titles and abstracts against eligibility criteria. We calculated interrater reliability based on a random sample of n=65 articles (κ=0.85). Next, reviewers assessed the full text of reviews considered for inclusion after title and abstract screening to determine final eligibility. After full text reviews, one reviewer searched reference lists of the final included reviews for additional records. Reviewers discussed all discrepancies during study selection and data collection to develop consensus.
The reviewers independently extracted review characteristics (aims, search period, eligibility criteria) and modifiable individual-, provider-, and clinic-level correlates and predictors (factors) of HPV vaccination initiation and completion. For reviews including multiple countries, reviewers extracted data associated with adolescents in the U.S. only. Reviewers extracted factors presented by authors in tables and/or text as associated with vaccination outcomes and supported by ≥2 quantitative primary studies. When a review labeled an outcome as “uptake,” we interpreted this as initiation only unless the authors explicitly stated they also assessed completion. For each review, modifiable factors were organized in a grid by positive or negative association with vaccination initiation or completion, adolescent sex when specified, and level (individual-, provider-, or clinic-level). Reviewers also coded factors with mixed or null results. For consistency, reviewers relabeled psychosocial factors that were similar (e.g., perceived risk and perceived susceptibility) where appropriate.
For systematic reviews, reviewers used the AMSTAR checklist to describe quality. AMSTAR is an 11-item validated tool used to assess methodological rigor of systematic reviews.21,22 Example items include: 1) was an “a priori” design provided, 2) was there duplicate study selection and data extraction, and 3) was the scientific quality of the included studies assessed and documented? Item response options included “yes,” “no,” “can’t answer,” and “not applicable.” We removed two items post hoc: 1) “were the methods used to combine the findings of the studies appropriate,” and 2) “was the likelihood of publication bias assessed?” Both required meta-analytic techniques, and no reviews included meta-analyses. Guidance on AMSTAR scoring for reviews without meta-analyses is to not calculate an overall score.23 Therefore, we present counts for each response option in the results.
During data collection and synthesis, we chose not to weigh findings from some reviews over others for two reasons. First, our eligibility criteria included multiple types of reviews (integrated reviews, systematic reviews, comprehensive literature reviews) with varying methodologies to capture a robust sample of studies. There is no objective measure to assess the quality of multiple types of reviews against one another, and AMSTAR response option counts for systematic reviews are for descriptive purposes only. Second, factors associated with vaccination outcomes were consistent across review types and primary studies included in the reviews. We therefore describe factors associated with vaccination outcomes from all of the reviews.
RESULTS
Study selection
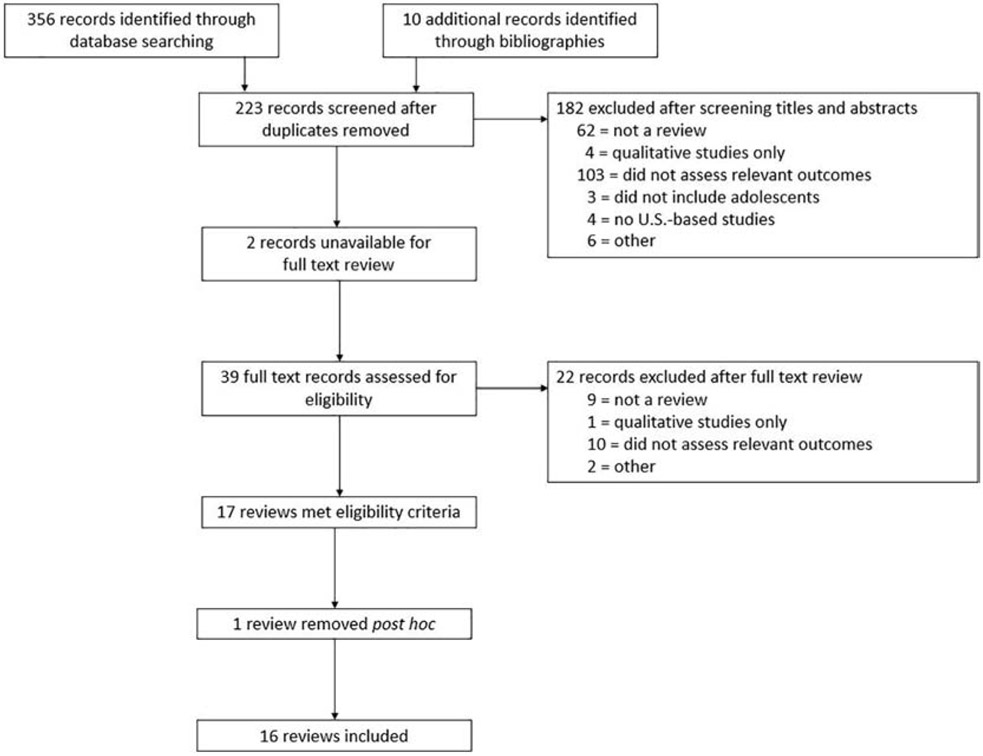
We identified 356 unique records from the database search and 10 additional records from reference lists. Seventeen met inclusion criteria after the two-step screening process (Figure 1). Principal reasons for exclusion were not being a review and not assessing HPV vaccination outcomes. One meta-analysis was excluded post hoc because it included only demographic correlates of vaccination outcomes.24 (Excluded citations are available from corresponding author.)
Figure 1.
PRISMA flowchart
Characteristics of reviews
Most reviews (n=11) included were systematic literature reviews (n=2 integrative reviews; n=3 literature reviews).25-35 None included meta-analyses of modifiable factors associated with adolescent HPV vaccination (Table 1). Among the systematic reviews, all but one28 conducted comprehensive literature searches (defined as listing at least two search sources and one supplemental search strategy) and provided characteristics of all studies included. No systematic reviews listed potential conflicts of interest for included studies, and none included a list of excluded studies. Responses for all other AMSTAR items varied. (AMSTAR checklist for all reviews available from corresponding author.)
Table 1.
Review characteristics and factors assessed by HPV vaccination outcomes
| Author, year; Search time period; review type |
# of studies/pubs (k); Study design |
Population;1 Country2 |
Modifiable factors assessed | AMSTAR3 |
|---|---|---|---|---|
| Outcome: HPV vaccine series initiation | ||||
|
Loke, 2017 Jan 2016 – Mar 4, 2017 Systematic review |
k=42 studies Cross-sectional; data-report; retrospective cohort; prospective cohort |
Parents of males and females 9-21 yrs U.S., United Kingdom, Norway, Hong Kong, Taiwan, Malaysia, Japan, Canada, Australia, Netherlands, Germany, France, Denmark, Latvia |
|
Yes: 5 No: 4 CA: 2 NA: 0 |
|
Radisic, 2017 Oct 2009 – Jul 10, 2015 Systematic review |
k=18 studies Quantitative, qualitative, mixed methods |
Parents of males ages 9-18 yrs U.S., Canada, Italy, Denmark |
|
Yes: 6 No: 3 CA: 2 NA: 0 |
|
Vollrath, 2017 Jan 1, 1999 – Mar 1, 2017 Integrative review |
k=37 publications Cluster randomized trial; cross-sectional; nonrandomized control trial; qualitative; pre/posttest; randomized intervention trial; retrospective chart review; systematic review |
Parents of adolescent males and females U.S., Australia, Canada, Georgia, Honduras, India, Iran, Italy, Pakistan |
|
NA |
|
Walling, 2016 Jan 1, 2006 – Apr 30, 2015 Systematic review |
k=51 publications Intervention studies |
Males and females 11-26 yrs U.S. |
|
Yes: 5 No: 4 CA: 2 NA: 0 |
|
Valentino, 2016 N/A Literature review |
N/A | Adolescent females; young adult females; parents of adolescent females; providers U.S. |
|
NA |
|
Small, 2014 Jan 1, 2009 – Jun 1, 2011 Literature review |
k=19 publications Longitudinal, cross-sectional |
Females; parents of adolescent females; providers U.S. |
|
NA |
|
Trim, 2012 2001-2011 Systematic review |
k=53 studies Cross-sectional |
Parents of adolescents U.S., EU, Canada, Asia, New Zealand, Australia |
|
Yes: 2 No: 6 CA: 2 NA: 1 |
|
Bartlett, 2011 2006 – Dec 30, 2010 Literature review |
k=14 studies Retrospective cohort, prospective cohort, randomized retrospective cohort, mixed methods |
Females ages 9-18 yrs; providers U.S. |
|
NA |
| Outcome: Vaccine series completion | ||||
|
Gallagher, 2016 Database inception – Feb 2014 Systematic review |
k=48 studies in 61 publications No study designs mentioned; included only those reporting multivariable analyses or qualitative findings |
Males and females 9-19 yrs U.S., Canada, Europe |
|
Yes: 8 No: 3 CA: 0 NA: 0 |
| Outcome: Vaccine series initiation and completion | ||||
|
Rosen, 2018 N/A – Aug 2016 Systematic review |
K=60 publications Cross-sectional; qualitative; retrospective study |
Healthcare clinicians defined as an individual qualified to deliver health care services to patients within recommended age group for vaccination U.S. |
|
Yes: 3 No: 6 CA: 2 NA: 0 |
|
Ryan, 2018 1996 – Nov 2016 Systematic review |
k=15 publications Intervention; randomized controlled trial; qualitative focus groups; survey |
Parents of adolescent males and females; women 18+ yrs; providers U.S. |
|
Yes: 3 No: 6 CA: 2 NA: 0 |
|
Kim, 2017 Jun 2006 – Feb 2015 Systematic review |
k=22 publications Cross-sectional, intervention, mixed methods, qualitative |
Immigrant parents of males and females age-eligible for HPV vaccine |
|
Yes: 4 No: 6 CA: 1 NA: 0 |
|
Galbraith, 2016 Database inception – Jan 2015 Integrative review |
k=67 studies Cross-sectional; qualitative; mixed methods; longitudinal cohort; intervention tests |
Parents of African-American and Latino adolescent females U.S. |
|
NA |
|
Niccolai, 2015 Database inception – Jul 2014 Systematic review |
k=14 studies Randomized controlled trials; quasi-experimental |
Males and females ≤ 18 yrs U.S. |
|
Yes: 5 No: 5 CA: 1 NA: 0 |
|
Holman, 2014 Jan 1, 2009 – Dec 31, 2012 Systematic review |
k=55 publications Quantitative, qualitative |
Parents of adolescents 11-17 yrs; health care professionals; under-served & disadvantaged populations; adolescent males U.S. |
|
Yes: 0 No: 8 CA: 2 NA: 1 |
|
Kessels, 2012 2006 – Mar 7, 2011 Systematic review |
k=25 studies in 33 publications Cross-sectional, longitudinal |
Females 9-18 yrs U.S. |
|
Yes: 6 No: 3 CA: 2 NA: 0 |
Extracted data related to vaccination among adolescents only.
Extracted data related to HPV vaccination in the U.S. only.
Assessed for systematic reviews only. CA = Can’t answer; NA = Not Applicable.
Five reviews examined outcomes among females only,25,36-39 one among males,34 and ten among males and females.26-33,35,40 Eight reviews assessed HPV vaccine uptake or initiation.26,31,33,34,37-40 One assessed series completion,30 and seven reviews assessed initiation and completion.25,27-29,32,35,36 Publication dates ranged from 201139 to 2018.27,35,40 Search end dates ranged from 201039 to March 1, 2017.40 Reviews included 481 unique primary studies, with 115 (28%) primary studies included in more than one review. We were unable to retrieve complete bibliographies for two reviews.30,37 (List of primary studies and citing reviews available from corresponding author.)
Factors positively associated with HPV vaccination outcomes
Parent and adolescent psychosocial factors, adolescent, parent, and provider behaviors, and clinic systems targeting providers and parents were positively associated with HPV vaccination outcomes in reviews (Table 2).
Table 2.
Factors positively associated with HPV vaccination outcomes among U.S. female and male adolescents ages 9-17 years
| Target population |
Outcomes | |||
|---|---|---|---|---|
| Initiation | Completion | Initiation and Completion | ||
| Female adolescents | Parent psychosocial factors | Parent behaviors | Parent psychosocial factors | |
| Knowledge | Seeking HPV vaccination information32 | Knowledge | ||
| HPV*25,36,38 | Familial decision making about vaccine32 | HPV vaccine30 | ||
| HPV vaccine*38 | Maternal experience with Pap tests39 | Provider behavior | ||
| Beliefs | Adolescent behaviors | Recommendation25,30 | ||
| Perceived benefits37 | Interaction with healthcare system | Adolescent behaviors | ||
| Perceived susceptibility28 | Regular visit with provider39 | Interaction with healthcare system*30 | ||
| Perceived vaccine effectiveness36,37 | Recent visit with provider25,38 | Parental behaviors | ||
| Perceived vaccine safety36 | Receipt of other adolescent vaccines25 | Maternal experience with Pap tests30 | ||
| Satisfaction with information25 | Provider behavior | |||
| Adolescent psychosocial factors | Recommendation28,36-39 | |||
| Knowledge | ||||
| HPV25 | ||||
| HPV vaccine25 | ||||
| Male adolescents | Parent psychosocial factor | |||
| Intentions to vaccinate34 | ||||
| Parent behavior | ||||
| Discussions about vaccine with son34 | ||||
| Adolescent behavior | ||||
| Interaction with healthcare system | ||||
| Visit with provider in past year34 | ||||
| Female and male adolescents | Parent psychosocial factors | Clinic systems | Clinic systems | |
| Acceptance33 | Provider-targeted | Provider-targeted | ||
| Knowledge | Assessment and feedback31 | Assessment and feedback31 | ||
| HPV vaccine32 | Education only*40 | Reminder/recall only35 | ||
| Beliefs | Education paired + other40 | Patient-targeted | ||
| Perceived effectiveness26 | Reminder/recall only40 | Reminder/recall29,31,40 | ||
| Perceived susceptability26 | Reminder/recall + other27,40 | |||
| Adolescent behaviors | Patient-targeted | |||
| Interaction with healthcare system | Reminder/recall31 | |||
| Receipt of other adolescent vaccines40 | ||||
| Provider behavior | ||||
| Recommendation27,32,33,40 | ||||
Mixed results found for association between factor and vaccination outcomes
Parent psychosocial factors
One review identified parental acceptance of the HPV vaccine as positively associated with initiation among male and female adolescents;33 however, the review did not define how acceptance was operationalized. Parental knowledge of the HPV vaccine was positively associated with series initiation for males and females32 and series completion for females.30 Among parents needing more information about the vaccine, satisfaction with the quality of information received was positively associated with initiation among females.25
Parental perceived benefits of the vaccine,37 perceived susceptibility of child to HPV or HPV-related diseases,26,28 perceived vaccine effectiveness,26,36,37 and perceived vaccine safety36 were associated with initiation among female adolescents. Perceived benefits included the prevention of cervical cancer and genital warts.37 One review found perceived effectiveness and perceived susceptibility to be positively associated with initiation among both males and females.26 Finally, parental intentions to vaccinate were positively associated with initiation among adolescent males.34
Adolescent psychosocial factors
Only one review identified adolescent psychosocial factors positively associated with vaccination outcomes with adolescent knowledge of HPV and the HPV vaccine associated with initiation among females.25
Parent behaviors
Reviews reported multiple parent behaviors positively associated with vaccination. For females, parental seeking of HPV vaccine information and familial decision making about the vaccine was associated with initiation among those with immigrant parents.32 Maternal experience obtaining Pap tests was associated with initiation and completion.39 For males, parents discussing the vaccine with sons was associated with initiation.34
Adolescent behaviors
Adolescent interaction with the healthcare system was positively associated with vaccination initiation among both males and females. Specifically, regular visits with a provider was associated with initiation among females,39 and recent visit with a provider and receipt of other adolescent vaccines were associated with initiation among males34,40 and females.25,38,40
Provider behavior
Reviews consistently identified provider recommendation of the HPV vaccine as positively associated with vaccination outcomes. Seven reviews found a positive association between a provider recommendation and series initiation28,36-39 and completion25,30 among females. Four reviews described the positive association between a provider recommendation and series initiation among both males and females.27,32,33,40
Clinic systems
Provider-targeted systems and patient-targeted reminder/recall systems were positively associated with vaccination outcomes among males and females. Specifically, provider assessment and feedback and reminder/recall systems were associated with initiation and completion.31,35,40 Provider education paired with additional provider-targeted systems, and reminder/recall paired with additional provider-targeted systems were associated with initiation only.27,40 Patient reminder/recall systems were associated with both initiation and completion among males and females.29,31,40 One review noted that provider-targeted interventions were more successful in increasing series initiation, and parent-targeted reminders were more successful for series completion.31
Factors negatively associated with vaccination outcomes
Reviews described parent psychosocial factors, adolescent behaviors, and provider behaviors negatively associated with vaccine initiation (Table 3). No reviews described factors negatively associated with completion.
Table 3.
Factors negatively associated with HPV vaccination outcomes among U.S. female and male adolescents ages 9-17 years
| Target population | Outcome |
|---|---|
| Initiation | |
| Female adolescents | Parent psychosocial factors |
| Lack of knowledge, require information37 | |
| HPV vaccine36 | |
| Beliefs | |
| Low perceived need36,37 | |
| Low perceived susceptibility36,37 | |
| Concerns about vaccine safety25,37,39 | |
| Concerns about vaccine effectiveness32,36,37 | |
| Concerns about cost39 | |
| Concerns about sexual activity*26,32 | |
| Dissatisfaction with information on vaccine25 | |
| Adolescent behavior | |
| Interaction with healthcare system | |
| Lack of recent preventive care visit25 | |
| Provider behavior | |
| Lack of recommendation36,37 | |
| Male adolescents | Parent psychosocial factors |
| Lack of knowledge28 | |
| Beliefs | |
| Low perceived benefit28 | |
| Low perceived need28 | |
| Provider behavior | |
| Lack of recommendation28 | |
| Female and male adolescents | Parent psychosocial factors |
| Lack of knowledge, require information28,33,40 | |
| Beliefs | |
| Low perceived need26,28,39,40 | |
| Low perceived susceptibility27,33,38 | |
| Concerns about vaccine safety32,33,38,40 | |
| Concerns about cost28,37,40 | |
| Parent behavior | |
| Previous refusal to vaccinate child26 | |
| Provider behavior | |
| Lack of recommendation28 |
Mixed results found for association between factor and vaccination outcomes
Parent psychosocial factors
Lack of knowledge about the HPV vaccine and requiring additional information were negatively associated with initiation for both males and females.28,33,36,37,40 Specific examples of parental knowledge gaps included not knowing the vaccine is available for males and28 the vaccine is a multi-dose series.28,36 Parental dissatisfaction with information received about the vaccine was negatively associated with initiation for females.25
Reviews consistently reported negative associations between parental beliefs and concerns about the HPV vaccine and vaccine series initiation. Low perceived need,26,28,36,37,39,40 low perceived susceptibility of child to HPV or HPV-related diseases,27,33,36-38 and concerns about vaccine safety25,32,33,37-40 were negatively associated with initiation in males and females. Reasons for not needing the vaccine were young age,26,28,39 being male,28 and no sexual activity.26,39 Examples of parental safety concerns included concerns about side effects25,39,40 and vaccine novelty.37,39 Additionally, parental concerns about vaccine effectiveness were negatively associated with initiation among females,32,36,37 and low parental perceived benefits of the vaccine was negatively associated with initiation among males.28 Finally, four reviews identified parental concerns about cost of the HPV vaccine as negatively associated with initiation for males and females.28,37,39,40
Parent behavior
One review identified previous parental refusal to vaccinate his/her child as negatively associated with HPV vaccine series initiation among males and females.26
Adolescent behavior
One review identified lack of adolescent interaction with the healthcare system, specifically a recent preventive care visit, as negatively associated with initiation among females.25
Provider behavior
Lack of provider recommendation for the HPV vaccination was negatively associated with HPV vaccine series initiation among males28 and females28,36,37 in three reviews.
Factors with mixed associations with vaccination outcomes
At the parent psychosocial level, reviews differed on whether parental knowledge of the HPV vaccine was associated with initiation38 and whether parental knowledge of the virus was associated with initiation and completion.25,30,36,38 Additionally, mixed associations were found between parental concerns about sexual activity and vaccination outcomes.26,32 While adolescent interaction with the healthcare system was associated with initiation among females, mixed associations were found between adolescent interaction with the healthcare system and vaccine series completion in one review.30 At the clinic level, although provider education paired with additional provider-targeted interventions was associated with initiation, there were mixed associations between provider education alone and vaccination outcomes.40
DISCUSSION
We conducted a systematic review of 16 reviews to synthesize multilevel factors associated with HPV vaccination among U.S. adolescents. The scope of the review advances research in two key ways. First, we can use the empirical evidence of modifiable factors associated with HPV vaccination and draw upon health behavior theory to develop a multilevel framework that can inform intervention development and increase HPV vaccine initiation and completion. Second, the review provides an up-to-date synthesis of salient factors associated with HPV vaccination and allows us to identify critical gaps in the literature.
Multilevel framework of HPV vaccination
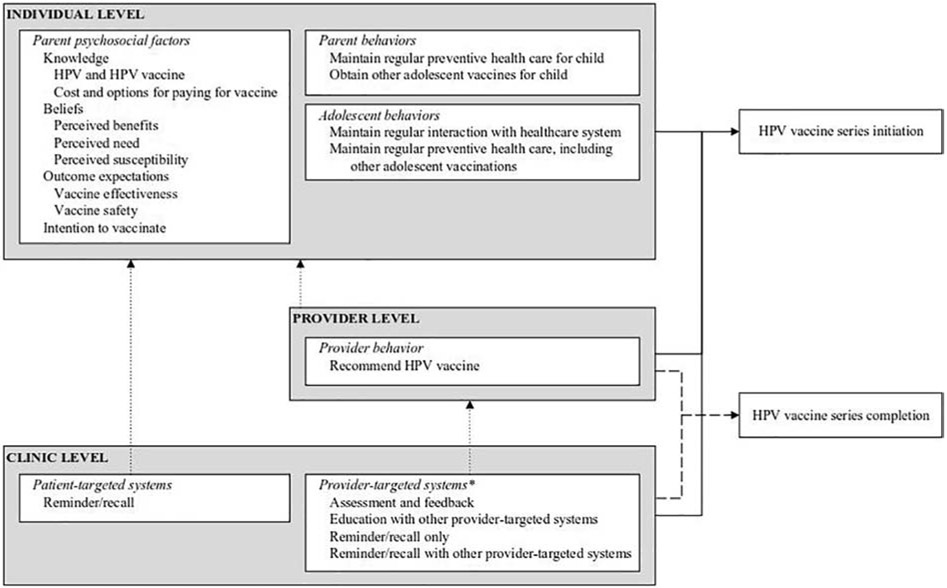
Drawing upon empirical evidence from the reviews and health promotion theories, we developed a multilevel framework illustrating hypothesized causal pathways between modifiable factors associated with HPV vaccination outcomes (Figure 2). Specifically, we drew upon Social Cognitive Theory (SCT),41 the Integrated Behavior Model (IBM),42 and the Health Belief Model (HBM)43 to build the multilevel framework. In short, according to the SCT, behavior change is influenced by personal cognitive influences (e.g., self-efficacy, knowledge, outcome expectations), environmental influences (e.g., normative beliefs, barriers and opportunities), and supporting behavioral factors (e.g., skills, intentions, reinforcement). Intention is the most important factor related to behavior change in the IBM, and intention is informed by attitudes, perceived norms, and personal agency.42 Finally, in the HBM, individual beliefs such as perceived susceptibility, severity, benefits, and barriers, and self-efficacy predict individual behaviors, and the model is often applied to preventive behaviors such as vaccination or screening.43,44
Figure 2.
Multilevel model of factors associated with HPV vaccination among adolescents ages 9-17 years in the U.S.
*Education with other provider-targeted systems and reminder/recall with other provider-targeted system were associated with series initiation only. All other clinic factors were associated with both initiation and completion.
The structure of our framework is based on one developed by Green and Kreuter used for organizing needs assessment information45 and adapted by Bartholomew Eldredge and colleagues to inform multilevel intervention development.46 We chose not to develop multiple frameworks by adolescent sex since factors associated with outcomes were similar across the sexes and reviews. Additionally, we did not include or exclude factors in the framework based on the number of reviews identifying them as associated with outcomes. Instead, we relied on health promotion theories to guide decision-making. This framework is the first to use empirical evidence and theory to organize multilevel correlates and predictors of HPV vaccine series initiation and completion for both male and female adolescents in the U.S.
Both SCT41 and the IBM42 highlight knowledge as an important precondition for behavior change. We synthesized review findings and theory to show that parents should have knowledge of HPV, the HPV vaccine, and the availability of options to pay for the vaccine.25,28,30,32,33,36,37,39,40 Parents also need to know available options to pay for the vaccine since cost was a barrier to initiation.28,37,39,40 According to the IBM, intention to perform a behavior is a significant predictor of the behavior,42 and our framework reflects this by including intention to vaccinate as a factor associated with series initiation.34
Based on review findings and guided by the Health Belief Model,43 we also included parental perceived benefits of the HPV vaccine and parental beliefs about their child’s susceptibility to HPV and HPV-related diseases in the framework.26-28,33,36,37 We included perceived need since beliefs that a child is too young for the vaccine26,28,39 or the vaccine is not needed if the child is not sexually active26,39 were barriers to initiation in reviews. Finally, guided by SCT, we synthesized parental beliefs about effectiveness and safety25,26,32,33,36-40 as outcomes expectations, or judgements about the likely outcome of vaccination.42 Our framework shows parents must expect that vaccination will be safe and effective in protecting against HPV and HPV-related diseases when initiating the series.
Adolescent interaction with the healthcare system and adolescent receipt of other adolescent vaccines were significantly associated with vaccine initiation.25,26,34,38-40 These factors are included as adolescent behaviors in the framework and as parent behaviors since adolescents often require caretakers to take them to appointments and consent to vaccinations. We chose not include additional parent behaviors that were associated with vaccination outcomes (familial decision-making, discussing the vaccine with son). Instead, we listed only those health-enhancing behaviors47 that involve the healthcare system.
Our framework includes only provider recommendation of the HPV vaccine at the provider level. In reviews, a provider’s recommendation was associated with both initiation and completion.25,27,28,30,32,33,36-40 In the framework, we link recommendations to the individual level as they can act as a cue to action43 influencing parental decisions to vaccinate. Finally, we include both patient-targeted systems and provider-targeted systems in the framework noting their associations with initiation and completion,27,29,31,35,40 and we show the influences patient reminder/recall systems can have on parents and the influences provider-targeted systems can have on provider behaviors.
Intervention development
Identifying factors associated with HPV vaccine initiation and completion and understanding the relations between levels is key to developing multilevel interventions that can increase HPV vaccination coverage and reduce HPV-related diseases. Our framework illustrates how factors relate to the outcomes and how factors can interact to create change. For example, parent reminders may act as motivational cues to action by including short messages addressing important psychosocial factors as opposed to stating only the date and time for an upcoming appointment. Strategically worded text messages have been shown to increase recipients’ perceived susceptibility to viruses and to increase parental intention to vaccinate against HPV.48,49
Because the framework combines salient factors associated with HPV vaccination from reviews focused on diverse populations, it provides a starting point for researchers and program planners interested in developing programs to increase vaccination in their communities. The framework can help researchers and planners avoid the “black box” in intervention development where mechanisms of change are unclear. Instead, planners can identify salient psychosocial factors, for example, and select appropriate behavior change techniques specific to the factor they aim to address.46,50 However, some factors specific to subpopulations may not be included in this framework. Researchers and planners must still engage with their communities and stakeholders to ensure their programs are appropriate.46 Specifically, engaging in community-based participatory research (CBPR) can ensure community members and stakeholders are involved in all stages of intervention development, implementation, evaluation, and dissemination.51 Researchers and program planners have successfully employed CBPR and partnered with communities to assess needs, develop interventions, and implement cancer prevention programs, including HPV vaccination interventions, in diverse settings.52-55
Future research
Our systematic review revealed gaps in the literature at the individual-, provider-, and clinic-levels. We describe future research needed to 1) link the significant body of literature on parental intentions to vaccination behavioral outcomes, 2) identify provider psychosocial factors associated with recommendation behaviors and subsequent uptake, 3) understand factors that influence implementation and adoption of system-level evidence-based interventions within clinics, 4) study factors associated with vaccine series completion, and 5) identify adolescent psychosocial and behavioral factors associated with vaccination outcomes.
First, primary studies in diverse populations have described correlates and predictors of parental intentions to vaccinate their adolescent children.56-61 However, only one review went beyond describing correlates of parental intentions and described the association between parental intentions and HPV vaccination outcomes.34 This lack of attention to the relation between parental intentions and HPV vaccination outcomes is surprising. This gap represents a lost opportunity to link previous findings about intentions to behavioral outcomes. Based on theory, this is an important gap since intentions may act as a mediator between psychosocial variables, such as attitudes and beliefs, and HPV vaccination.42 A clear understanding of the associations between intentions, other psychosocial factors, and vaccination outcomes can help refine the current framework of vaccination and inform future interventions.
Provider recommendation is a well-documented predictor of HPV vaccination.25,27,28,30,32,33,36-40 However, a shift in focus is needed moving away from focusing strictly on provider recommendation behaviors to examining why providers differentially recommend the vaccine for patients based on age, sex, or other factors and why they communicate differentially with parents.62 Studies examining the psychosocial factors associated with provider recommendations would provide a deeper understanding of the context of the patient-provider interaction.63-65 Identifying psychosocial correlates of provider behaviors was beyond the scope of this review. However, the few reviews that described findings from studies of predictors of provider recommendation found that provider knowledge, beliefs, personal discomfort discussing sexual health with parents, and concern about cost were associated with recommendation behaviors.28,37 Exploring these factors in more depth can inform provider-level interventions that can enhance vaccine recommendation quality and provider communication with parents.
Our review highlights important clinic-level systems associated with increased HPV vaccination coverage. These include provider assessment and feedback systems and patient reminder systems,31 both recommended by The Guide to Community Preventive Services (The Community Guide) to increase vaccination behaviors.66 Successful implementation of The Community Guide recommended strategies and of cancer control and prevention strategies, such as HPV vaccination interventions, often depend on clinic-level factors such as leadership engagement and organizational culture.67,68 Alternatively, clinic-level characteristics that can hinder implementation of these strategies include competing demands within the clinic, limited resources, limited organizational support, or other practices that work against or fail to incorporate the new systems.69,70 Thus, future research is needed to understand factors that influence the adoption and implementation of these clinic-level systems to increase HPV vaccination. Implementation science and implementation frameworks can help interventions developers or clinic stakeholders identify clinic-level factors relevant to adoption and implementation outcomes and develop implementation strategies to increase adoption and implementation of these systems.71
The lack of parent-level psychosocial factors and provider-level factors, other than recommendation, associated with completion in the reviews highlights the need for studies focused on these factors to inform multilevel intervention development. While clinic-level systems such as patient reminders are promising interventions to increase completion,31,72 a more robust understanding of the multilevel factors associated with completion is critical. This is important given ACIP recommendations moving from a three dose series to a two dose series for those under 15 years old.73
Finally, future research focusing on adolescent-level psychosocial factors and behaviors associated with HPV vaccination outcomes will be particularly important as policy-makers debate whether to eliminate parental consent for adolescent vaccinations.74,75 The Society for Adolescent Health and Medicine supports adolescent consent for vaccination,76 yet research is lacking on the effectiveness of adolescent consent in increasing vaccination rates.77 Identifying adolescent psychosocial and behavioral factors associated with HPV vaccine initiation and completion can significantly impact this research landscape.
Limitations
This review did not capture research not yet incorporated into reviews or reviews published since the search date. Additional themes or factors may emerge from continued research. While factors associated with vaccination may become more or less important over time, we chose not to weigh factors found in more recent reviews over factors identified in older reviews. Similarly, our findings do not include conclusions on the strength of the associations within reviews or across reviews. For example, Tables 2 and 3 depict factors associated with vaccination outcomes, but while consistency of assessment may signal the robustness of the association, frequency of assessment does not imply degree of the associations (effect size). Further, some clinic-level best practices for increasing vaccination, such as others described by The Community Guide,66 were not included in this systematic review. Because our focus was not specifically on interventions, this review did not capture some Community Guide best practices. Limiting the search to HPV vaccination alone may have affected our ability to identify best practices related to improving adolescent vaccination outcomes in general.
In our review, we listed all factors associated with vaccination outcomes supported by ≥2 primary studies from integrative, systematic, and literature reviews that included different populations, study designs, and research questions. This approach has some limitations. First, nuanced differences between subpopulations may be lost in data synthesis. Second, differing research questions, populations, settings, and methodologies may mean that reviews did not cover the entire literature body that assessed HPV vaccination outcomes among adolescents in the U.S. leaving out some factors from our synthesis. Finally, we chose not to weigh findings from some reviews over others based on review type or AMSTAR response options for systematic reviews. This approach has limitations if the methodological rigor of reviews significantly differed. However, as previously noted, no tool exists to compare rigor across review types since each type has different methods and objectives. Among systematic reviews, AMSTAR assessments revealed that only five included gray literature searches. This points to the potential for publication bias in the other reviews. AMSTAR responses were based solely on author reporting and may not reflect the methods actually used for a review if authors’ statements were less explicit than required to confirm AMSTAR elements.
Conclusion
In summary, an updated synthesis of the multilevel factors associated with HPV vaccine initiation and completion among U.S. adolescents is needed. Our systematic review and multilevel framework depict salient factors including parent knowledge, beliefs, outcome expectations, and intentions; parent behaviors; provider recommendation; and patient- and provider-targeted systems that can be modified and targeted through interventions to increase HPV vaccination coverage and reach Healthy People 2020 goals. Future research is needed to empirically link parental intentions to vaccinate and vaccination outcomes, to assess correlates of provider recommendation behaviors and subsequent vaccine uptake among patients, and to identify clinic factors associated with successful implementation of clinic-level systems shown to improve HPV vaccination.
Supplementary Material
Highlights.
Provider recommendation consistently associated with adolescent HPV vaccine uptake
Provider- and clinic-level factors most important for series completion
Research needed to identify provider-level factors associated with recommendation
Research needed on implementation strategies for effective clinic-level systems
Acknowledgements
The authors thank Helena VonVille, MPH for her assistance in creating the systematic review search criteria and managing the search and screening processes. SAR thanks Drs. Paula Cuccaro and Alan Nyitray for their feedback on iterations of this manuscript.
Funding sources
SAR was supported by a predoctoral fellowship from The University of Texas Health Science Center at Houston (UTHealth) School of Public Health Cancer Education and Career Development Program – National Cancer Institute (NCI)/National Institutes of Health (NIH) Grant R25 CA57712. She received partial support from the UTHealth School of Public Health Department of Health Promotion and Behavioral Sciences. SAR and DLM received partial support from the Cancer Prevention & Research Institute of Texas (CPRIT) Research Grants RR170493 and RR130459. The content is solely the responsibility of the authors and does not necessarily represent the official views of NCI, NIH, or CPRIT.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Paavonen J, Jenkins D, Bosch FX, et al. Efficacy of a prophylactic adjuvanted bivalent L1 virus-like-particle vaccine against infection with human papillomavirus types 16 and 18 in young women: an interim analysis of a phase III double-blind, randomised controlled trial. Lancet. 2007;369(9580):2161–2170. doi: 10.1016/S0140-6736(07)60946-5 [DOI] [PubMed] [Google Scholar]
- 2.Garland SM, Hernandez-Avila M, Wheeler CM, Perez G, Harper DM, Leodolter S, … & Koutsky LA. Quadrivalent Vaccine against Human Papillomavirus to Prevent Anogenital Diseases — NEJM. N Engl J Med. 2007;356(19):1928–1943. http://www.nejm.org.ezproxy.is.ed.ac.uk/doi/full/10.1056/NEJMoa061760. [DOI] [PubMed] [Google Scholar]
- 3.Viens LJ, Henley SJ, Watson M, Markowitz LE, Thomas CC. Human Papillomavirus – Associated Cancers — United States , 2008 – 2012. Morb Mortal Wkly Rep. 2016;65(26):2008–2012. [DOI] [PubMed] [Google Scholar]
- 4.Meites E, Kempe A, Markowitz LE. Use of a 2-Dose Schedule for Human Papillomavirus Vaccination — Updated Recommendations of the Advisory Committee on Immunization Practices. MMWR Morb Mortal Wkly Rep. 2016;65(49):1405–1408. doi: 10.15585/mmwr.mm6549a5 [DOI] [PubMed] [Google Scholar]
- 5.Meites E, Szilagyi PG, Chesson HW, Unger ER, Romero JR, Markowitz LE. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices Elissa. Morb Mortal Wkly Rep. 2019;68(32):698–702. doi: 10.15585/mmwr.mm6549a5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.U.S. Department of Health and Human Services Office of Disease Prevention and Health Promotion. Healthy People 2020: Immunization and Infectious Diseases. https://www.healthypeople.gov/2020/topics-objectives/topic/immunization-and-infectious-diseases. Published 2016. Accessed September 29, 2016.
- 7.Walker TY, Elam-Evans LD, Yankey D, et al. National, regional, state, and selected local area vaccination coverage among adolescents aged 13–17 years — United States, 2018. Morb Mortal Wkly Rep. 2019;68(33):718–723. doi: 10.15585/mmwr.mm6833a2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Cancer Institute. Understanding and Influencing Multilevel Factors Across the Cancer Care Continuum [Monograph]. J Natl Cancer Inst Monogr. 2012;44(May 2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Batista Ferrer H, Trotter C, Hickman M, Audrey S. Barriers and facilitators to HPV vaccination of young women in high-income countries: a qualitative systematic review and evidence synthesis. BMC Public Health. 2014; 14(1):700. doi: 10.1186/1471-2458-14-700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fernández ME, Allen JD, Mistry R, Kahn JA. Integrating clinical, community, and policy perspectives on human papillomavirus vaccination. Annu Rev Public Health. 2010;31(1):235–252. doi: 10.1146/annurev.publhealth.012809.103609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Katz IT, Ware NC, Gray G, Haberer JE, Mellins CA, Bangsberg DR. Scaling up human papillomavirus vaccination: a conceptual framework of vaccine adherence. Sex Health. 2010;7:279–286. doi: 10.1071/SH09130.Scaling [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batista Ferrer H, Audrey S, Trotter C, Hickman M. An appraisal of theoretical approaches to examining behaviours in relation to Human Papillomavirus (HPV) vaccination of young women. Prev Med (Baltim). 2015;81:122–131. doi: 10.1016/j.ypmed.2015.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brewer NT, Fazekas KI. Predictors of HPV vaccine acceptability: A theory-informed, systematic review. Prev Med (Baltim). 2007;45(2-3):107–114. doi: 10.1016/j.ypmed.2007.05.013 [DOI] [PubMed] [Google Scholar]
- 14.Tiro JA, Pruitt SL, Bruce CM, et al. Multilevel correlates for human papillomavirus vaccination of adolescent girls attending safety net clinics. Vaccine. 2012;30(13):2368–2375. doi: 10.1016/j.vaccine.2011.11.031 [DOI] [PubMed] [Google Scholar]
- 15.Calo WA, Gilkey MB, Shah PD, Moss JL, Brewer NT. Parents’ Support for School-Entry Requirements for Human Papillomavirus Vaccination: A National Study. Cancer Epidemiol Biomarkers Prev. 2016;25(9):1317–1325. doi: 10.1158/1055-9965.EPI-15-1159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vercruysse J, Chigurupati NL, Fung L, Apte G, Pierre-Joseph N, Perkins RB. Parents’ and providers’ attitudes toward school-located provision and school-entry requirements for HPV vaccines. Hum Vaccin Immunother. 2016; 12(6):1606–1614. http://ovidsp.ovid.com/ovidweb.cgi?T=JS&CSC=Y&NEWS=N&PAGE=fulltext&D=prem&AN=26934421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barraza L, Weidenaar K, Campos-Outcalt D, Yang YT. Human papillomavirus and mandatory immunization laws: What can we learn from early mandates? Public Health Rep. 2016; 131(5):728–731. doi: 10.1177/0033354916663184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brandt HM, Pierce JY, Crary A. Increasing HPV vaccination through policy for public health benefit. Hum Vaccines Immunother. 2016; 12(6):1623–1625. doi: 10.1080/21645515.2015.1122145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred Reporting Items for Systematic Reviews and Meta-Analyses: The PRISMA Statement. Annu Intern Med. 2009;151(4):264–269. doi: 10.1371/journal.pmed1000097 [DOI] [PubMed] [Google Scholar]
- 20.U.S. Food and Drug Administration. June 8, 2006 Approval Letter - Human Papillomavirus Quadrivalent (Types 6, 11, 16, 18) Vaccine, Recombinant. http://www.fda.gov/BiologicsBloodVaccines/Vaccines/ApprovedProducts/ucm111283.htm. Published 2009. Accessed August 10, 2016. [Google Scholar]
- 21.Shea BJ, Hamel C, Wells GA, et al. AMSTAR is a reliable and valid measurement tool to assess the methodological quality of systematic reviews. J Clin Epidemiol. 2009;62(10):1013–1020. doi: 10.1016/j.jclinepi.2008.10.009 [DOI] [PubMed] [Google Scholar]
- 22.Shea BJ, Bouter LM, Peterson J, et al. External validation of a measurement tool to assess systematic reviews (AMSTAR). PLoS One. 2007;2(12). doi: 10.1371/journal.pone.0001350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pieper D, Koensgen N, Breuing J, Ge L, Wegewitz U. How is AMSTAR applied by authors - A call for better reporting. BMC Med Res Methodol. 2018;18(1):1–7. doi: 10.1186/s12874-018-0520-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fisher H, Trotter CL, Audrey S, MacDonald-Wallis K, Hickman M. Inequalities in the uptake of human papillomavirus vaccination: A systematic review and meta-analysis. Int J Epidemiol. 2013;42(3):896–908. doi: 10.1093/ije/dyt049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kessels SJM, Marshall HS, Watson M, Braunack-Mayer AJ, Reuzel R, Tooher RL. Factors associated with HPV vaccine uptake in teenage girls: A systematic review. Vaccine. 2012;30(24):3546–3556. doi: 10.1016/j.vaccine.2012.03.063 [DOI] [PubMed] [Google Scholar]
- 26.Trim K, Nagji N, Elit L, Roy K. Parental Knowledge, Attitudes, and Behaviours towards Human Papillomavirus Vaccination for Their Children: A Systematic Review from 2001 to 2011. Obstet Gynecol Int. 2012;2012:1–12. doi: 10.1155/2012/921236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ryan C, Duvall KL, Weyant EC, Johnson KR, Wood D. Human Papillomavirus Vaccine Uptake, Knowledge, and Acceptance for Youth: A Systematic Review of Appalachia. J Community Health. 2018;43(3):616–624. doi: 10.1007/s10900-018-0500-6 [DOI] [PubMed] [Google Scholar]
- 28.Holman DM, Benard V, Roland KB, Watson M, Liddon N, Stokley S. Barriers to Human Papillomavirus Vaccination Among US Adolescents A systematic review. JAMA Pediatr. 2014;168(1):76–82. doi: 10.1001/jamapediatrics.2013.2752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niccolai LM, Hansen CE. Practice- and Community-Based Interventions to Increase Human Papillomavirus Vaccine Coverage: A Systematic Review. JAMA Pediatr. 2015;169(7):686–692. doi: 10.1001/jamapediatrics.2015.0310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gallagher KE, Kadokura E, Eckert LO, et al. Factors influencing completion of multi-dose vaccine schedules in adolescents: a systematic review. BMC Public Health. 2016; 16(1):172. doi: 10.1186/s12889-016-2845-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Walling EB, Benzoni N, Dornfeld J, et al. Interventions to Improve HPV Vaccine Uptake: A Systematic Review. Pediatrics. 2016;138(1):e20153863–e20153863. doi: 10.1542/peds.2015-3863 [DOI] [PubMed] [Google Scholar]
- 32.Kim K, LeClaire A-R. A systematic review of factors influencing human papillomavirus vaccination among immigrant parents in the United States. Health Care Women Int. 2017;0(0):00–00. doi: 10.1080/07399332.2017.1404064 [DOI] [PubMed] [Google Scholar]
- 33.Loke AY, Kwan ML, Wong Y-T, Wong AKY. The Uptake of Human Papillomavirus Vaccination and Its Associated Factors Among Adolescents: A Systematic Review. J Prim Care Community Health. 2017:215013191774229. doi: 10.1177/2150131917742299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Radisic G, Chapman J, Flight I, Wilson C. Factors associated with parents’ attitudes to the HPV vaccination of their adolescent sons: A systematic review. Prev Med (Baltim). 2016;95:26–37. doi: 10.1016/j.ypmed.2016.11.019 [DOI] [PubMed] [Google Scholar]
- 35.Rosen BL, Shepard A, Kahn JA. US Health Care Clinicians’ Knowledge, Attitudes, and Practices Regarding Human Papillomavirus Vaccination: A Qualitative Systematic Review. Acad Pediatr. 2018;18(2):S21–S22. doi: 10.1016/j.acap.2017.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Galbraith KV, Lechuga J, Jenerette CM, Moore LTCAD, Palmer MH, Hamilton JB. Parental acceptance and uptake of the HPV vaccine among African-Americans and Latinos in the United States: A literature review. Soc Sci Med. 2016;159:116–126. doi: 10.1016/j.socscimed.2016.04.028 [DOI] [PubMed] [Google Scholar]
- 37.Valentino K, Poronsky CB. Human Papillomavirus Infection and Vaccination. J Pediatr Nurs. 2016;31(2):e155–66. doi: 10.1016/j.pedn.2015.10.005 [DOI] [PubMed] [Google Scholar]
- 38.Small SL, Sampselle CM, Martyn KK, Dempsey AF. Modifiable influences on female HPV vaccine uptake at the clinic encounter level: a literature review. J Am Assoc Nurse Pract. 2014;26(9):519–525. doi: 10.1002/2327-6924.12057 [DOI] [PubMed] [Google Scholar]
- 39.Bartlett JA, Peterson JA. The uptake of human papillomavirus (HPV) vaccine among adolescent females in the United States: a review of the literature. J Sch Nurs. 2011;27(6):434–446. doi: 10.1177/1059840511415861 [DOI] [PubMed] [Google Scholar]
- 40.Vollrath K, Thul S, Holcombe J. Meaningful Methods for Increasing Human Papillomavirus Vaccination Rates: An Integrative Literature Review. J Pediatr Heal Care. 2018;32(2):119–132. doi: 10.1016/j.pedhc.2017.07.005 [DOI] [PubMed] [Google Scholar]
- 41.Bandura A Health promotion by social cognitive means. Heal Educ Behav. 2004;31(2):143–164. doi: 10.1177/1090198104263660 [DOI] [PubMed] [Google Scholar]
- 42.Montano DE, Kasprzyk D. Theory of Reasoned Action, Theory of Planned Behavior, an the Integrated Behavioral Model In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior: Theory, Research, and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:95–124. [Google Scholar]
- 43.Skinner C, Tiro J, Champion VL. The Health Belief Model In: Glanz K, Rimer B, Viswanath K, eds. Health Behavior: Theory, Research, and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:75–94. [Google Scholar]
- 44.Tanner-Smith EE, Brown TN. Evaluating the health belief model: A critical review of studies predicting mammographic and pap screening. Soc Theory Heal. 2010. doi: 10.1057/sth.2009.23 [DOI] [Google Scholar]
- 45.Green LW, Kreuter MW. Health Program Planning: An Educational and Ecological Approach. 4th ed. New York, NY: McGraw-Hill; 2005. [Google Scholar]
- 46.Bartholomew Eldredge LK, Markham CM, Ruiter RAC, Fernandez ME, Kok G, Parcel GS. Planning Health Promotion Programs: An Intervention Mapping Approach. 4th ed. San Francisco, CA: Jossey-Bass; 2016. [Google Scholar]
- 47.Kelder S, Hoelscher D, Perry CL. How individuals, environments, and health behavior inteact: Social Cognitive Theory. In: Glanz K, Rimer BK, Viswanath K, eds. Health Behavior: Theory, Research, and Practice. 5th ed. San Francisco, CA: Jossey-Bass; 2015:285–325. [Google Scholar]
- 48.McGlone MS, Stephens KK, Rodriguez SA, Fernandez ME. Persuasive texts for prompting action: Agency assignment in HPV vaccination reminders. Vaccine. 2017;35(34):4295–4297. doi: 10.1016/j.vaccine.2017.06.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McGlone MS, Bell R a, Zaitchik ST, McGlynn J. Don’t let the flu catch you: agency assignment in printed educational materials about the H1N1 influenza virus. J Health Commun. 2013;18(6):740–756. doi: 10.1080/10810730.2012.727950 [DOI] [PubMed] [Google Scholar]
- 50.Kok G, Gottlieb NH, Peters G-JY, et al. A taxonomy of behaviour change methods: an Intervention Mapping approach. Health Psychol Rev. 2016;10(3):297–312. doi: 10.1080/17437199.2015.1077155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Viswanathan M, Ammerman A, Eng E, et al. Community-based participatory research: assessing the evidence. Evid Rep Technol Assess (Summ). 2004. doi: 10.1037/e439622005-001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hou SI, Cao X. A Systematic Review of Promising Strategies of Faith-Based Cancer Education and Lifestyle Interventions Among Racial/Ethnic Minority Groups. J Cancer Educ. 2018. doi: 10.1007/s13187-017-1277-5 [DOI] [PubMed] [Google Scholar]
- 53.Baezconde-Garbanati L, Lienemann BA, Robles M, et al. Implementation of HPV vaccination guidelines in a diverse population in Los Angeles: Results from an environmental scan of local HPV resources and needs. Vaccine. 2017. doi: 10.1016/j.vaccine.2017.07.080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lai D, Bodson J, Davis FA, et al. Diverse Families’ Experiences with HPV Vaccine Information Sources: A Community-Based Participatory Approach. J Community Health. 2017;42(2):400–412. doi: 10.1007/s10900-016-0269-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braun KL, Stewart S, Baquet C, et al. The National Cancer Institute’s Community Networks Program Initiative to reduce cancer health disparities: Outcomes and lessons learned. Prog Community Heal Partnerships Res Educ Action. 2015. doi: 10.1353/cpr.2015.0017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lechuga J, Vera-Cala L, Martinez-Donate A. HPV Vaccine Awareness, Barriers, Intentions, and Uptake in Latina Women. J Immigr Minor Health. 2014:173–178. doi: 10.1007/s10903-014-0139-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lechuga J, Swain GR, Weinhardt LS. The Cross-Cultural Variation of Predictors of Human Papillomavirus Vaccination Intentions. J Women’s Heal. 2011;20(2):225–230. doi: 10.1089/jwh.2010.1993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kang HS, Moneyham L. Attitudes, intentions, and perceived barriers to human papillomavirus vaccination among Korean high school girls and their mothers. Cancer Nurs. 2011;34(3):202–208. doi: 10.1097/NCC.0b013e3181fa482b [DOI] [PubMed] [Google Scholar]
- 59.Dempsey AF, Butchart A, Singer D, Clark S, Davis M. Factors Associated With Parental Intentions for Male Human Papillomavirus Vaccination: Results of a National Survey. Sex Transm Dis. 2011:1. doi: 10.1097/OLQ.0b013e318211c248 [DOI] [PubMed] [Google Scholar]
- 60.Askelson NM, Campo S, Lowe JB, Smith S, Dennis LK, Andsager J. Using the Theory of Planned Behavior to Predict Mothers’ Intentions to Vaccinate Their Daughters Against HPV. J Sch Nurs. 2010;26(3):194–202. doi: 10.1177/1059840510366022 [DOI] [PubMed] [Google Scholar]
- 61.Baldwin AS, Bruce CM, Tiro JA. Understanding how mothers of adolescent girls obtain information about the human papillomavirus vaccine: Associations between mothers’ health beliefs, information seeking, and vaccination intentions in an ethnically diverse sample. J Health Psychol. 2013;18(7):926–938. doi: 10.1177/1359105312445078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shay LA, Baldwin AS, Betts AC, et al. Parent-Provider Communication of HPV Vaccine Hesitancy. Pediatrics. 2018. doi: 10.1542/peds.2017-2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Gilkey MB, Calo WA, Moss JL, Shah PD, Marciniak MW, Brewer NT. Provider communication and HPV vaccination: The impact of recommendation quality. Vaccine. 2016;34(9):1187–1192. doi: 10.1016/j.vaccine.2016.01.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gilkey MB, McRee A-LL. Provider communication about HPV vaccination: A systematic review. Hum Vaccines Immunother. 2016;5515(June):1–15. doi: 10.1080/21645515.2015.1129090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shay LA, Street RL, Baldwin AS, et al. Characterizing safety-net providers HPV vaccine recommendations to undecided parents: A pilot study. Patient Educ Couns. 2016;99(9):1452–1460. doi: 10.1016/j.pec.2016.06.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Community Preventine Services Task Force. The Community Guide. https://www.thecommunityguide.org/about/about-community-guide. Published 2017. Accessed July 16, 2017. [Google Scholar]
- 67.Liang S, Kegler MC, Cotter M, et al. Integrating evidence-based practices for increasing cancer screenings in safety net health systems: a multiple case study using the Consolidated Framework for Implementation Research. Implement Sci. 2015; 11(1):109. doi: 10.1186/s13012-016-0477-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Price RA, Zapka J, Edwards H, Taplin SH. Organizational factors and the cancer screening process. J Natl Cancer Inst - Monogr. 2010;(40):38–57. doi: 10.1093/jncimonographs/lgq008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Glasgow RE, Marcus AC, Bull SS, Wilson KM. Disseminating effective cancer screening interventions. Cancer. 2004;101(5 Suppl):1239–1250. doi: 10.1002/cncr.20509 [DOI] [PubMed] [Google Scholar]
- 70.Winterbauer NL, Bridger CM, Tucker A, Rafferty AP, Luo H. Adoption of Evidence-Based Interventions in Local Health Departments. Am J Prev Med. 2015;49(2):309–316. doi: 10.1016/j.amepre.2015.02.024 [DOI] [PubMed] [Google Scholar]
- 71.National Institutes of Health. Dissemination and Implementation Research in Health (R01). http://grants.nih.gov/grants/guide/pa-files/PAR-16-238.html. Published 2016.
- 72.Chao C, Preciado M, Slezak J, Xu L. A randomized intervention of reminder letter for human papillomavirus vaccine series completion. J Adolesc Heal. 2015;56(1):85–90. doi: 10.1016/j.jadohealth.2014.08.014 [DOI] [PubMed] [Google Scholar]
- 73.Centers for Disease Control and Prevention. CDC recommends only two HPV shots for younger adolescents. http://www.cdc.gov/media/releases/2016/p1020-hpv-shots.html. Published 2016. Accessed December 1, 2016.
- 74.Silverman RD, Opel DJ, Omer SB. Vaccination over parental objection - Should adolescents be allowed to consent to receiving vaccines? N Engl J Med. 2019;381(2):104–106. doi: 10.1056/NEJMp1905814 [DOI] [PubMed] [Google Scholar]
- 75.Haddad N, Allen RH, Szkwarko D, Forcier M, Paquette C. Eliminating parental consent for adolescents receiving human papillomavirus vaccination. R I Med J (2013). 2018; 101(7):12–14. http://www.ncbi.nlm.nih.gov/pubmed/30189697. [PubMed] [Google Scholar]
- 76.English A, Ford CA, Kahn JA, Kharbanda EO, Middleman AB. Adolescent consent for vaccination: A position paper of the society for adolescent health and medicine. J Adolesc Heal. 2013;53(4):550–553. doi: 10.1016/j.jadohealth.2013.07.039 [DOI] [PubMed] [Google Scholar]
- 77.Fisher H, Harding S, Hickman M, Macleod J, Audrey S. Barriers and enablers to adolescent self-consent for vaccination: A mixed-methods evidence synthesis. Vaccine. 2019;37(3):417–429. doi: 10.1016/j.vaccine.2018.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.