Abstract
Background:
In spring 2013, groundwater of a vast area of the Veneto Region (northeastern Italy) was found to be contaminated by perfluoroalkyl substances (PFAS) from a PFAS manufacturing plant active since the late 1960s. Residents were exposed to high concentrations of PFAS, particularly perfluorooctanoic acid (PFOA), through drinking water until autumn 2013. A publicly funded health surveillance program is under way to aid in the prevention, early diagnosis, and treatment of chronic disorders possibly associated with PFAS exposure.
Objectives:
The objectives of this paper are: a) to describe the organization of the health surveillance program, b) to report serum PFAS concentrations in adolescents and young adults, and c) to identify predictors of serum PFAS concentrations in the studied population.
Methods:
The health surveillance program offered to residents of municipalities supplied by contaminated waterworks includes a structured interview, routine blood and urine tests, and measurement of 12 PFAS in serum by high-performance liquid chromatography–tandem mass spectrometry. We studied 18,345 participants born between 1978 and 2002, 14–39 years of age at recruitment. Multivariable linear regression was used to identify sociodemographic, lifestyle, dietary, and reproductive predictors of serum PFAS concentrations.
Results:
The PFAS with the highest serum concentrations were PFOA [median , interquartile range (IQR) 19.3–84.9], perfluorohexanesulfonic acid (PFHxS) (median , IQR 1.9–7.4), and perfluorooctanesulfonic acid (PFOS) (median , IQR 2.6–5.8). The major predictors of serum levels were gender, municipality, duration of residence in the affected area, and number of deliveries. Overall, the regression models explained 37%, 23%, and 43% of the variance of PFOA, PFOS, and PFHxS, respectively.
Conclusions:
Serum PFOA concentrations were high relative to concentrations in populations with background residential exposures only. Interindividual variation of serum PFAS levels was partially explained by the considered predictors. https://doi.org/10.1289/EHP5337
Introduction
Perfluoroalkyl substances (PFAS) are manufactured chemicals widely used for a variety of commercial and industrial applications due to their grease-, stain-, and water-repelling properties. PFAS are extremely persistent and are widely distributed because of limited environmental degradation. Bioaccumulation varies among individual PFAS according to their chemical structure and differs among target organisms (Butenhoff et al. 2004; Olsen et al. 2007; Russell et al. 2013; Li et al. 2018).
In animals, PFAS are not metabolized and are eliminated mostly by renal excretion. In general, long-chain PFAS are excreted more slowly than are short-chain PFAS; however, their half-lives are also influenced by other chemical features and interspecies differences (Butenhoff et al. 2004; Olsen et al. 2007; Chang et al. 2008; Olsen et al. 2009; Russell et al. 2013; Li et al. 2018). In comparison with rodents and nonhuman primates, humans have a very poor capacity to excrete perfluorohexanesulfonic acid (PFHxS), perfluorooctanoic acid (PFOA), and perfluorooctanesulfonic acid (PFOS): Mean half-lives for these compounds in exposed workers have been estimated to be 8.5 y [95% confidence interval (CI): 6.4, 10.6], 3.8 y (95% CI: 3.1, 4.4), and 5.4 y (95% CI: 3.9, 6.9), respectively (Olsen et al. 2007). Recently, a study in a population with residential exposure estimated a mean half-life of 2.7 y (95% CI: 2.5, 2.9) for PFOA, 3.4 y (95% CI: 3.1, 3.7) for PFOS, and 5.3 y (95% CI: 4.6, 6.0) for PFHxS (Li et al. 2018).
Many studies conducted worldwide have reported measurable serum levels of PFHxS, PFOA, and PFOS in most of the general population (Calafat et al. 2007; Ingelido et al. 2010; Zhang et al. 2013; Cariou et al. 2015; CDC 2017). Food and dust are considered the major sources of background exposure (Lorber and Egeghy 2011; Fraser et al. 2013), but residential exposure due to drinking water contamination has also been described. The largest known incident involved more than 70,000 people in the mid–Ohio Valley (United States), where PFAS released from a fluoropolymer-producing plant contaminated surface water and groundwater (Emmett et al. 2006; Frisbee et al. 2009). In North Rhine–Westphalia, Germany, 40,000 inhabitants were exposed due to the use of PFAS-contaminated soil conditioners used on fields (Hölzer et al. 2008), and one-third of households in Ronneby, Sweden, (28,000 inhabitants), were supplied with drinking water contaminated with PFAS from firefighting foams used in a nearby airfield (Li et al. 2018).
PFOA and PFOS have endocrine-disruption properties and have been associated with several health conditions (EFSA CONTAM Panel 2018), including increased levels of serum cholesterol (Frisbee et al. 2010; Winquist and Steenland 2014a), impaired thyroid function (Lopez-Espinosa et al. 2012; Winquist and Steenland 2014b; Ballesteros et al. 2017), insulin resistance (Cardenas et al. 2017), gestational diabetes (Zhang C et al. 2015), and pregnancy-induced hypertension (Darrow et al. 2013).
Context: PFAS Contamination in the Veneto Region, Italy
In 2011, the Italian Ministry for the Environment, Land, and Sea commissioned the Institute of Water Research of the National Research Centre (IRSA–CNR) to study PFAS contamination in major Italian river basins (Valsecchi et al. 2015). Results of this study revealed surface water and groundwater in a large area of the Veneto Region in northeastern Italy was contaminated with PFAS that were also found in drinking water samples. The results of the study were communicated to the Regional Government in late spring 2013 (Polesello and Valsecchi 2013, unpublished data). In July 2013, the Agency for Environmental Prevention and Protection of the Veneto Region (ARPAV) set up an environmental monitoring plan that is still ongoing to determine the extent and level of groundwater and drinking water contamination and to identify its source. Twelve types of PFAS are measured in water matrices: perfluorobutanoic acid (PFBA), perfluorobutanesulfonic acid (PFBS), perfluoropentanoic acid (PFPeA), perfluorohexanoic acid (PFHxA), PFHxS, perfluoroheptanoic acid (PFHpA), PFOA, PFOS, perfluorononanoic acid (PFNA), perfluorodecanoic acid (PFDeA), perfluoroundecanoic acid (PFUnA), and perfluorododecanoic acid (PFDoA). A manufacturing plant located in the town of Trissino that produced PFAS since the late 1960s was identified as the only likely source of water contamination.
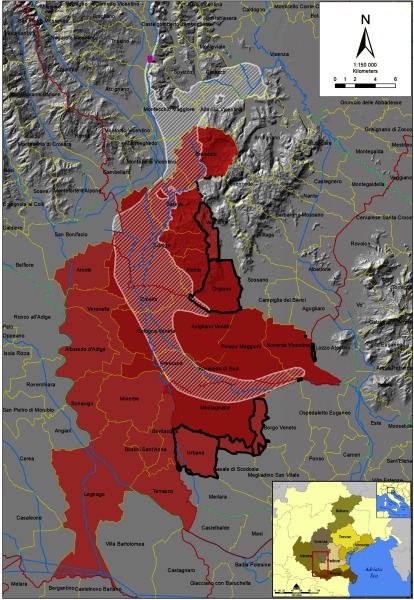
The groundwater contamination plume extends over an area of and affects both public waterworks and private wells. ARPAV estimated that the groundwater contamination plume reached public waterworks serving municipalities across the provinces of Vicenza, Verona, and Padua in 1980. The municipalities in the area of maximum exposure (referred to as the “Red Area”) are further divided into “Red Area A,” which includes municipalities served by the contaminated waterworks that are also located on the groundwater contamination plume; and “Red Area B,” which includes municipalities served by the contaminated waterworks but not located on the groundwater contamination plume. Initially, the Red Area was composed of 21 municipalities, with 126,000 inhabitants. In 2018, nine additional municipalities were added, some of which are only partially supplied by the contaminated waterworks (Figure 1). At present, the Red Area is wide and has a total population of approximately 140,000 people.
Figure 1.
Map of the PFAS-contaminated area of the Veneto Region. Note: Red Area: municipalities supplied by PFAS-contaminated waterworks. Red Area A (dark red): municipalities of the Red Area located on the groundwater contamination plume. Red Area B (light red): municipalities of the Red Area located outside the groundwater contamination plume. Dashed white area: groundwater contamination plume. Pink dot: location of the chemical plant that gave origin to groundwater contamination. Yellow lines: borders of municipalities. Red lines: borders of provinces. Blue lines: rivers. Black thick line: borders of the new areas included in the Red Area in 2018 (9 additional municipalities, some of them only partially included). The box on the lower right corner indicates the position of the represented area in the Veneto Region, northeastern Italy.
Measurements of 152 drinking water samples collected in July and August 2013 indicated that the main contaminants were PFOA (median , maximum ), PFBA (median , maximum ), and PFBS (median , maximum ), followed by PFPeA, PFHxA, PFOS, PFHpA, and PFHxS. The longer-chain PFAS congeners (PFNA, PFDA, PFUnA, PFDoA) were detected only in a minority of samples and at lower concentrations (Table 1). Between July and August 2013, water treatment plants were equipped with granular activated carbon (GAC) filters, which led to an abrupt reduction in PFAS concentrations in drinking water distributed by public waterworks, and the effectiveness of water treatment continued to improve so that by 2018 PFAS congeners were undetectable in the majority of samples (see Figures S1–S5). A comprehensive description of the management of this environmental disaster can be found elsewhere (WHO 2017).
Table 1.
Distribution of concentrations (ng/L) of the 12 measured PFAS congeners in 152 samples of drinking water taken during July and August 2013, before the full implementation of granular activated carbon filters.
| Congener | Min | 25th percentile | Median | 75th percentile | 95th percentile | Max | % samples |
|---|---|---|---|---|---|---|---|
| PFBA | 86.0 | 123.5 | 173.3 | 359.4 | 625.0 | 88.8% | |
| PFPeA | 45.0 | 70.0 | 100.0 | 210.6 | 370.0 | 90.1% | |
| PFHxA | 39.0 | 52.0 | 79.5 | 185.8 | 330.0 | 89.5% | |
| PFHpA | 14.0 | 23.0 | 56.0 | 151.0 | 67.1% | ||
| PFOA | 176.0 | 319.5 | 546.2 | 1,123.2 | 1,475.0 | 89.5% | |
| PFNA | 76.0 | 0.7% | |||||
| PFDeA | 81.0 | 0.7% | |||||
| PFUnA | 95.0 | 1.3% | |||||
| PFDoA | 10.0 | 0.0% | |||||
| PFBS | 66.5 | 91.5 | 183.2 | 382.1 | 765.0 | 89.5% | |
| PFHxS | 13.3 | 26.0 | 66.0 | 40.1% | |||
| PFOS | 11.0 | 18.0 | 29.3 | 66.5 | 117.0 | 77.6% |
Note: The LOQ is set at . LOQ, limit of quantification; Max, maximum; Min, minimum; PFBA, perfluorobutanoic acid; PFBS, perfluorobutanesulfonic acid; PFDeA, perfluorodecanoic acid; PFDoA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFPeA, perfluoropentanoic acid; PFUnA, perfluoroundecanoic acid.
In 2016, a biomonitoring study (Ingelido et al. 2018) was conducted on two randomly selected groups of people 20–51 years of age: 257 subjects living in the contaminated area and 250 living in a background area not affected by the contamination incident. The results showed that those living in the contaminated area had significantly higher serum PFAS concentrations than the control group had and that participants residing in municipalities served by contaminated waterworks had the highest serum PFAS concentrations.
To address public concerns about exposure to PFAS, and in accord with a precautionary principle, the regional health authorities established a health surveillance program for residents of the Red Area to aid in the prevention, early diagnosis, and treatment of some of the chronic disorders with epidemiological evidence of associations with PFAS exposures, i.e., dyslipidemia, hypertension, diabetes mellitus, liver dysfunction, metabolic syndrome, kidney dysfunction, and thyroid disorders.
The objectives of this paper are: a) to describe the organization of the health surveillance program, b) to report serum PFAS concentrations in adolescents and young adults age 14–39 y, and c) to identify predictors of serum PFAS concentrations in the study population.
Methods
Organization of the Health Surveillance Program
The health surveillance program is a free-of-charge population-based screening program. The initial target population was 84,795 people born between 1951 and 2002 who were residents of the 21 municipalities that comprised the Red Area as defined on 31 December 2016. The first invitations were sent by mail on first January 2017 to residents born in 2002, followed by invitations to progressively older birth cohorts. In December 2018, the regional government extended recruitment for the health surveillance program to the pediatric population, and in February 2019 recruitment began in nine additional municipalities that were added to the Red Area. At least one follow-up examination is planned for all participants in the first round of the health surveillance program. The regional government also offers current and former workers at the PFAS-producing plant a health surveillance program that includes collection of professional history and additional health examinations. Data on workers are not included in this paper.
Eligible subjects are identified through the regional health registry, which contains personal and residency data for the entire population of the Veneto region. Invitation letters indicating an appointment date and time are sent by mail, followed by a second invitation if there is no response to the first. Residents who decide to participate in the program complete a structured interview administered by a trained public health nurse, followed by blood pressure measurement, and blood and urine sampling. Program visits are performed at public health facilities that are located throughout the contaminated area to ensure easy accessibility.
Personal data collection and information system.
The structured interview investigates residential history, education, occupation, dietary habits (including consumption of self-produced food), drinking water intake and sources (public distribution system, private well, bottled water), smoking habits, alcohol consumption, physical activity, family and personal history of disease, medications, reproductive history, and self-reported height and weight [with automatic calculation of body mass index (BMI)]. During the interview, the public health nurse takes the opportunity to inform the patient about healthy lifestyle and recommend behavioral changes using motivational counselling techniques.
Blood pressure is measured according to the European Society of Hypertension recommendations (Williams et al. 2018). If the first measure is above for systolic blood pressure or for diastolic blood pressure, a second measurement is taken. If the second measure is normal, the participant is classified as having normal blood pressure, otherwise he or she is classified as having high blood pressure. Both pairs of measurements are recorded.
Nonfasting blood and urine samples collected from participants are sent to the Local Health Unit laboratory for analyzing serum creatinine, uric acid, aspartate aminotransferase (AST), alanine aminotransferase (ALT), glycated hemoglobin, total cholesterol, HDL cholesterol, triglycerides, thyroid-stimulating hormone (TSH), and urine albumin to creatinine ratio. Estimated glomerular filtration rate (eGFR) is calculated according to the CKD-EPI equation (Levey et al. 2009). Additional blood samples from each individual are centrifuged, and frozen serum samples are sent weekly to the ARPAV laboratory for measurement of 12 PFAS: PFBA, PFBS, PFPeA, PFHxA, PFHxS, PFHpA, PFOA, PFOS, PFNA, PFDeA, PFUnA, and PFDoA.
All data are collected using centralized web-based software connected with the regional health registry. The software allows extraction of lists of eligible residents, online compiling of interview and blood pressure data, and retrieval of laboratory test results.
Participants with normal blood pressure and clinical biochemical parameters and with serum PFOA and PFOS concentrations below the 95th percentiles of the general Italian population ( for PFOA and for PFOS) (Ingelido et al. 2010) receive only information and counseling on healthy lifestyle. Participants with abnormal blood pressure or clinical biochemical parameters but with serum PFOA and PFOS concentrations below the 95th percentile threshold are offered information and counseling on healthy lifestyle and then referred to their family physicians for subsequent evaluation or treatment. Participants with normal blood pressure and clinical biochemical parameters and serum PFOA or PFOS concentrations above the 95th percentile threshold are offered information and counseling on possible PFAS-related health conditions and on healthy lifestyle. Participants with abnormal blood pressure or clinical biochemical parameters and serum PFOA or PFOS concentrations above the 95th percentile threshold are referred to a dedicated specialist team of internists and cardiologists who, after an initial clinical evaluation, may prescribe additional medical investigations to diagnose existing disease and determine a tailored pathway of care for the patient. All additional examinations are provided free of charge.
Analytical method for serum PFAS measurement.
Blood samples collected in vacutainers containing a clot activator and separation gel undergo centrifugation prior to dispatch. Upon arrival at a lab facility, a robotic liquid handler (Microlab® STARlet; Hamilton Co.), following an in-house validated method, carries on sample preparation. Purification follows a protein-precipitation protocol by Acetonitrile (ACN) addition using Isotopic Labeled Internal Standard (ILIS) for quantitation. Uncapped thawed samples are distributed into a mapped 96-well deep-well plate for analysis by high-performance liquid chromatography–tandem mass spectrometry (HPLC-MS/MS) [Prominence UFLC XR 20 (Shimadzu) coupled to API 4000™ LC-MS/MS System (Sciex)]. Chromatographic separation uses ammonium formate buffer and methanol as eluents and Supelco™ Ascentis® Express RP-Amide , as analytical column. Method performances allow analytes to be detected as low as level of detection (LOD) and to be quantified above level of quantification (LOQ). Recoveries range from 70% to 120% for all analytes. Linearity of method is analyte-specific; for example, PFOA linear range extend up to and can be further extended by increasing the dilution factor.
Baseline PFAS Concentrations and Predictors
Study population.
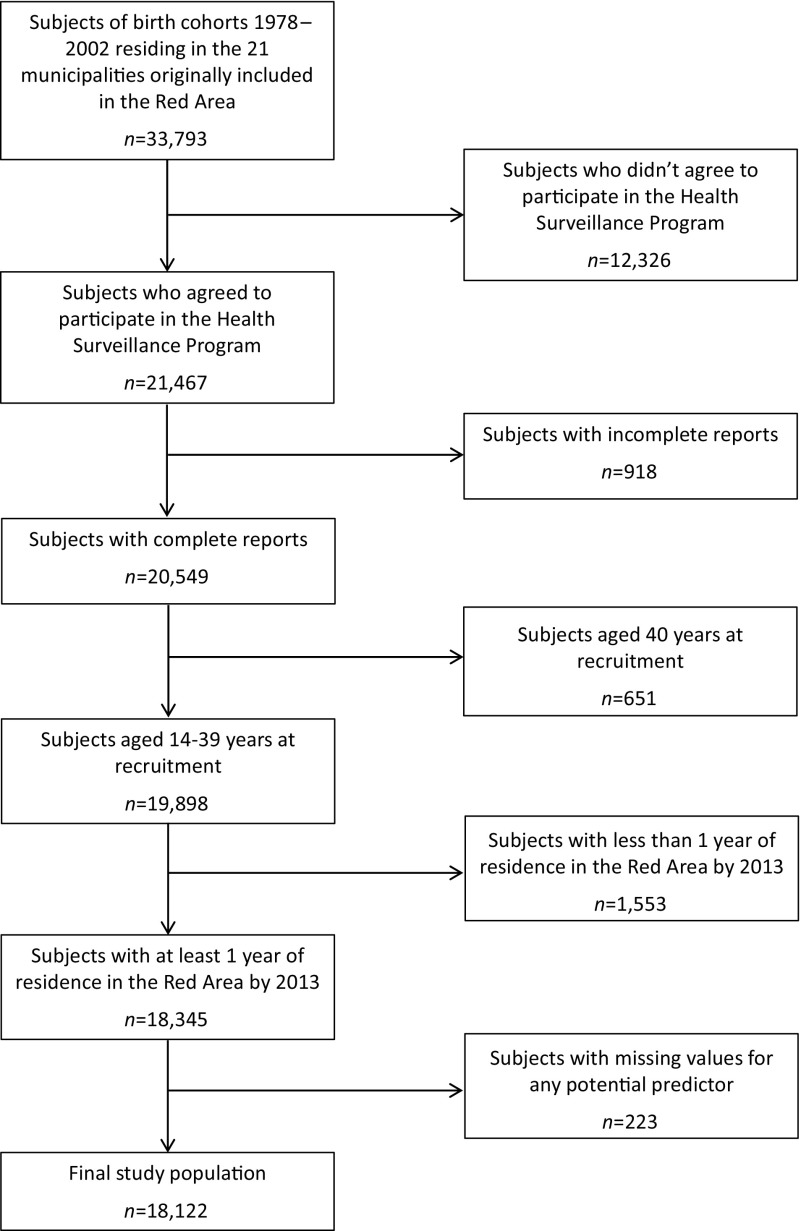
This analysis focuses on the subgroup of the surveillance program target population for whom enrollment has been completed, namely residents of the 21 municipalities in the original Red Area who were born between 1978 and 2002. Of the 33,793 residents eligible for this subgroup, 63.5% () agreed to participate in the surveillance program. Participants with incomplete reports (, 4.3%), who were 40 years of age at recruitment (), or who lived in the Red Area for less than 1 y prior to 2013 (), were excluded, leaving a total of 18,345 in the present analysis (Figure 2).
Figure 2.
Flow chart of the inclusion–exclusion process.
Potential predictors.
The following variables were considered as potential predictors of serum PFAS concentrations: gender, age at recruitment (14–19, 20–24, 25–29, 30–34, and 35–39 years of age), country of birth [Italy/other highly developed countries (HDC), high migratory pressure countries (HMPC)], educational attainment (primary/middle school, high school, university), occupation (farmer, nonfarmer), current municipality of residence (municipality where the subject resided at recruitment), predominant residential area (Red Area A vs. B), duration of residency in the Red Area (1–4, 5–9, 10–14, 15–19, 20–24, 25–29, ), time from the beginning of the study (1 January 2017) to the date of blood sampling (0–5, 6–10, 11–15, 16–20, ), BMI (, 18–24.9, 25–29.9, ), eGFR (, ), current pregnancy (yes, no), number of deliveries (0, 1, 2, ), smoking habits (current smoker, previous smoker, nonsmoker), physical activity (light, moderate, heavy), alcohol consumption [0, 1–2, 3–6, alcohol units (AU) per wk], water intake (, 1 , 1.5 , ), intake of meat, fish/seafood, milk/yogurt, cheese, eggs, bread/pasta/cereals, sweets/snacks/sweet beverages, fruits/vegetables, growing one’s own vegetables (yes, no), and raising animals for personal consumption (yes, no).
HDC were countries in North America, Israel, Oceania, Japan, and European countries outside of central-eastern Europe. HMPC were countries in Central-Eastern Europe (i.e., Albania, Bulgaria, Serbia and Montenegro, Poland, Romania, Ukraine, Hungary, Russian Federation, Estonia, Latvia, Lithuania, Croatia, Slovenia, Bosnia and Herzegovina, Macedonia, Moldova, Slovakia, Belarus, Czech Republic, Cyprus, Turkey), North Africa, sub-Saharan Africa, Central America, South America, Asian countries other than Japan, and did not include Israel. Duration of residence in the Red Area was calculated from residential history as the number of years spent in the Red Area between 1980 and 2013 (when GAC filters were installed). Predominant residential area was defined as the area (Red Area A vs. B) where the subject resided for the longest amount of time between 1980 and 2013. Degree of physical activity (light, moderate, or heavy) was defined based on an algorithm that combined information reported by the subject on intensity, duration, and frequency of all types of physical activity practiced during the week. Intake of food items was expressed as number of portions per week (with reference to usual intake) and categorized based on quartiles or tertiles.
Questionnaire contains a question related to the type of drinking water consumed with multiple choices (bottled water, water from a public water supply, well water, other). From this question we have created two variables as following: a) type of water categorized as only bottled water, only water from a public water supply, or a mix of any combination of the above-mentioned choices; and b) well water consumption categorized as yes or no. The latter source of water was contaminated only in the Red Area A, but not B. However, before August 2018, participants were asked about drinking water consumed at the time they completed the questionnaire, rather than their consumption before they became aware of the water contamination (when most residents switched to bottled water in 2013). Therefore, analyses of associations with the type of drinking water and consumption of well water was limited to participants enrolled after August 2018 (), when the questionnaire was revised to query participants about consumption before they were aware of contamination.
Statistical methods.
We calculated the percentage of samples with a quantifiable concentration of each PFAS () and focused our analysis on PFAS that were quantifiable in at least 80% of samples (PFOA, PFOS, and PFHxS). Samples below the LOQ were assigned a value equal to . Because PFAS concentrations were skewed to the right, concentrations were natural log-transformed to normalize the data distribution. Normality was checked using Shapiro–Wilk test. We used Spearman’s correlation to describe pairwise relationships between the three PFAS.
Associations between potential predictors and serum PFAS were evaluated in 18,122 subjects, excluding 223 subjects with missing values for any of potential predictors. A first assessment of relationship between each individual characteristic and serum concentrations of PFOA, PFOS, and PFHxS was conducted using Kruskal–Wallis test.
We fitted multivariable linear regression models to examine the relationship between potential determinants and each PFAS concentrations. We used a stepwise approach based on the Akaike information criterion (AIC) to define the optimal set of variables to retain in each model minimizing the AIC value. We specified a model including all possible predictors and then we used a stepwise approach based on a combination of forward and backward selections. By doing so, at each step, the algorithm chose the direction that led to the best AIC improvement and a single variable was added or dropped to minimize the AIC score. Because, in the Red Area, private wells are often used to water vegetables and animals, we also stratified analyses by predominant residential area to test whether the different status of groundwater contamination in Red Area A and B modified the association of growing vegetables and raising animals for personal consumption and serum PFAS levels. In addition, we conducted further analyses considering the type of water consumed on a restricted data set of participants recruited after August 2018 (, 19.9%), for whom questions about type of drinking water were specific to the time before they became aware of the contamination. The analyses on this restricted population were also stratified by predominant residential area.
Model goodness of fit was estimated using . We used analysis of variance (ANOVA) to estimate the contribution of each covariate to the overall variability of each PFAS concentration. Because outcome variables were natural log-transformed, we back-transformed coefficient estimates and confidence intervals using , and report effect estimates as percent differences in geometric mean PFAS concentrations relative to the reference group.
All analyses were performed using Stata (version 13.0; StataCorp) or R (version 3.6.1; R Development Core Team).
Results
Only three of the 12 PFAS were quantifiable in at least 80% of serum samples: PFOA (99.9%), PFOS (99.8%), and PFHxS (98.1%) (Table 2). The main contaminant was PFOA (median ), followed by PFHxS (median ) and PFOS (median ). Distributions were markedly right-skewed: For PFOA, the 95th percentile was , with a maximum of ; for PFHxS and PFOS, the 95th percentiles were and , respectively. Serum concentrations of the three PFAS congeners were strongly correlated, with Spearman’s correlation coefficients of 0.89 for PFOA and PFHxS, 0.66 for PFOS and PFHxS, and 0.62 for PFOS and PFOA.
Table 2.
Distribution of serum concentrations (ng/mL) of the twelve measured PFAS congeners and percentage of samples above the LOQ in the study population (18,345 subjects 14–39 years of age).
| Congener | Min | 5th percentile | 25th percentile | Median | 75th percentile | 95th percentile | Max | % samples |
|---|---|---|---|---|---|---|---|---|
| PFBA | 23.9 | 2.0% | ||||||
| PFPeA | 1.3 | 0.1% | ||||||
| PFHxA | 7.1 | 0.3% | ||||||
| PFHpA | 15.1 | 1.4% | ||||||
| PFOA | 5.1 | 19.3 | 44.4 | 84.9 | 189.7 | 1,400.0 | 99.9% | |
| PFNA | 0.5 | 0.6 | 1.0 | 39.7 | 51.3% | |||
| PFDeA | 0.8 | 8.3 | 22.0% | |||||
| PFUnA | 0.5 | 6.0 | 6.3% | |||||
| PFDoA | 4.5 | 0.8% | ||||||
| PFBS | 8.9 | 3.1% | ||||||
| PFHxS | 0.7 | 1.9 | 3.9 | 7.4 | 18.1 | 127.0 | 98.1% | |
| PFOS | 1.4 | 2.6 | 3.9 | 5.8 | 10.7 | 142.0 | 99.8% |
Note: LOQ, limit of quantification; Max, maximum; Min, minimum; PFBA, perfluorobutanoic acid; PFBS, perfluorobutanesulfonic acid; PFDeA, perfluorodecanoic acid; PFDoA, perfluorododecanoic acid; PFHpA, perfluoroheptanoic acid; PFHxA, perfluorohexanoic acid; PFHxS, perfluorohexanesulfonic acid; PFNA, perfluorononanoic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid; PFPeA, perfluoropentanoic acid; PFUnA, perfluoroundecanoic acid.
At bivariate analysis, all potential predictors were significantly associated with serum PFAS concentrations, except for cheese intake and raising animals for personal consumption for PFOA; eGFR, water intake, and milk/yogurt intake for PFOS; and fruit/vegetable intake and fish intake for PFHxS (Table S1).
Table 3 shows the results of the multivariable regression models for the entire population (). The total variance explained by the models for PFOA, PFOS, and PFHxS, was 37.4%, 22.6%, and 43.1%, respectively. Overall, gender and current municipality of residency were the most important predictors for all PFAS. Gender explained 11.5%, 9.9%, and 18.2% of the PFOA, PFOS, and PFHxS variability, respectively. In comparison with females, on average, males had 66% higher PFOA (95% CI: 61, 71), 42% higher PFOS (95% CI: 39, 45), and 93% higher PFHxS (95% CI: 88, 98). Current municipality of residency explained 12.6%, 6.7%, and 14.8% of the variance for PFOA, PFOS, and PFHxS, respectively. Overall, the municipalities in the Red Area A were associated with higher serum levels in comparison with those in the Red Area B. Predominant residency in the Red Area A was associated with 38% (95% CI: 27, 50) higher levels of PFOA and 42% (95% CI: 33, 51) higher levels of PFHxS, but there was no statistically significant difference in PFOS relative to Red Area B. Duration of residency in the Red Area was correlated with levels of all PFAS examined in this study, with a general trend toward higher levels with longer duration, especially for PFOA and PFHxS.
Table 3.
Multivariable linear regression models for the association between potential predictors and serum concentrations of PFOA, PFOS, and PFHxS in the entire study population ( subjects with complete records for all variables).
| Predictor | (%) | % difference (95% CI) | ||
|---|---|---|---|---|
| PFOA | PFOS | PFHxS | ||
| Gender | ||||
| Female | 8,892 (49.1) | Referent | Referent | Referent |
| Male | 9,230 (50.9) | 66 (61; 71) | 42 (39; 45) | 93 (88; 98) |
| Explained variance (%) | 11.52 | 9.94 | 18.17 | |
| Age (y) | ||||
| 14–19 | 4,478 (24.7) | Referent | Referent | Referent |
| 20–24 | 3,194 (17.6) | (; ) | (; 3) | (; 4) |
| 25–29 | 3,044 (16.8) | (; ) | 2 (; 7) | 10 (3; 16) |
| 30–34 | 3,304 (18.2) | (; ) | 8 (3; 14) | 14 (6; 21) |
| 35–39 | 4,102 (22.6) | (; 3) | 20 (14; 26) | 30 (21; 39) |
| Explained variance (%) | 2.96 | 0.13 | 0.74 | |
| Country of birth | ||||
| Italian, HDC | 16,696 (92.1) | Referent | Referent | Referent |
| HMPC | 1,426 (7.9) | (; ) | — | — |
| Explained variance (%) | 1.25 | — | — | |
| Educational level | ||||
| Primary/Middle school | 5,853 (32.3) | Referent | Referent | Referent |
| High school | 9,012 (49.7) | (; 0) | 1 (; 3) | (; 0) |
| University | 3,257 (18.0) | 2 (; 8) | 6 (3; 9) | 0 (; 4) |
| Explained variance (%) | 0.33 | 0.33 | 0.26 | |
| Occupational sector | ||||
| No farmer | 17,903 (98.8) | Referent | Referent | Referent |
| Farmer | 219 (1.2) | — | 8 (0; 16) | — |
| Explained variance (%) | — | 0.02 | — | |
| Predominant residential area | ||||
| Red Area A | 7,020 (38.7) | 38 (27; 50) | 5 (0; 10) | 42 (33; 51) |
| Red Area B | 11,102 (61.3) | Referent | Referent | Referent |
| Explained variance (%) | 0.20 | 0.01 | 0.30 | |
| Current municipality of residency (Red Area A or B) | ||||
| Terrazzo (B) | 288 (1.6) | Referent | Referent | Referent |
| Albaredo D’Adige (B) | 767 (4.2) | 160 (131; 194) | 14 (6; 23) | 126 (104; 150) |
| Arcole (B) | 899 (5.0) | 189 (156; 226) | 25 (16; 34) | 135 (113; 159) |
| Bevilacqua (B) | 216 (1.2) | 342 (278; 416) | 41 (28; 55) | 268 (224; 319) |
| Bonavigo (B) | 279 (1.5) | 170 (133; 213) | 16 (6; 27) | 137 (110; 168) |
| Boschi Sant’Anna (B) | 206 (1.1) | 202 (158; 254) | 22 (11; 35) | 155 (124; 191) |
| Legnago (B) | 2,945 (16.3) | 87 (68; 109) | 7 (0; 14) | 59 (45; 74) |
| Minerbe (B) | 628 (3.5) | 325 (276; 381) | 32 (23; 43) | 241 (208; 278) |
| Veronella (B) | 778 (4.3) | 340 (289; 397) | 35 (25; 46) | 248 (215; 285) |
| Alonte (A) | 346 (1.9) | 236 (186; 296) | 38 (25; 52) | 168 (134; 207) |
| Asigliano Veneto (A) | 161 (0.9) | 275 (210; 353) | 34 (19; 50) | 198 (155; 249) |
| Brendola (A) | 1,007 (5.6) | 125 (95; 160) | 21 (11; 32) | 31 (16; 47) |
| Cologna Veneta (A) | 1,208 (6.7) | 243 (199; 294) | 35 (24; 47) | 183 (153; 217) |
| Lonigo (A) | 2,569 (14.2) | 252 (206; 303) | 42 (31; 55) | 173 (144; 206) |
| Montagnana (A) | 1,146 (6.3) | 264 (216; 318) | 40 (29; 53) | 184 (153; 219) |
| Noventa Vicentina (A) | 1,410 (7.8) | 166 (132; 206) | 22 (12; 33) | 101 (79; 126) |
| Pojana Maggiore (A) | 767 (4.2) | 263 (214; 320) | 33 (22; 46) | 180 (148; 216) |
| Pressana (A) | 365 (2.0) | 272 (218; 336) | 29 (17; 42) | 192 (156; 232) |
| Roveredo Di Gua’ (A) | 263 (1.4) | 243 (190; 306) | 25 (13; 39) | 178 (142; 219) |
| Sarego (A) | 1,124 (6.2) | 197 (157; 242) | 46 (34; 59) | 118 (94; 146) |
| Zimella (A) | 750 (4.1) | 236 (192; 288) | 40 (28; 52) | 170 (140; 204) |
| Explained variance (%) | 12.57 | 6.70 | 14.84 | |
| Duration of residency in the Red Area (y) | ||||
| 1,082 (6.0) | Referent | Referent | Referent | |
| 5–9 | 1,375 (7.6) | 45 (35; 56) | 8 (3; 13) | 27 (20; 35) |
| 10–14 | 3,962 (21.9) | 97 (83; 111) | 17 (12; 22) | 68 (59; 79) |
| 15–19 | 3,380 (18.6) | 120 (104; 137) | 21 (16; 27) | 98 (86; 111) |
| 20–24 | 2,887 (15.9) | 120 (104; 137) | 26 (20; 31) | 111 (98; 124) |
| 25–29 | 2,663 (14.7) | 131 (115; 148) | 24 (19; 29) | 125 (113; 138) |
| more than 30 years | 2,773 (15.3) | 117 (102; 132) | 17 (12; 22) | 117 (105; 130) |
| Explained variance (%) | 3.40 | 0.91 | 5.34 | |
| Time-lag between the beginning of the study and blood sampling (months) | ||||
| Less than 6 months | 2,221 (12.3) | Referent | Referent | Referent |
| 6–10 | 5,152 (28.4) | 6 (0; 12) | (; ) | 13 (8; 18) |
| 11–15 | 5,283 (29.2) | 0 (; 7) | (; ) | 5 (; 11) |
| 16–20 | 4,156 (22.9) | (; ) | (; ) | (; 3) |
| 1,310 (7.2) | (; ) | (; ) | (; ) | |
| Explained variance (%) | 0.43 | 0.62 | 0.42 | |
| BMI (kg/m2) | ||||
| Underweight | 1,437 (7.9) | — | 0 (; 3) | (; 0) |
| Normal weight | 11,388 (62.9) | Referent | Referent | Referent |
| Pre-obesity | 3,895 (21.5) | — | (; ) | 3 (0; 6) |
| Obesity | 1,402 (7.7) | — | (; ) | 5 (1; 9) |
| Explained variance (%) | — | 0.53 | 0.06 | |
| eGFR (mL/min/1.73 m2) | ||||
| 15,691 (86.6) | Referent | Referent | Referent | |
| 2,431 (13.4) | 5 (1; 10) | 6 (3; 8) | 5 (1; 8) | |
| Explained variance (%) | 0.09 | 0.13 | 0.09 | |
| Number of deliveries | ||||
| 0 | 15,490 (85.5) | Referent | Referent | Referent |
| 1 | 1,100 (6.1) | (; ) | (; ) | (; ) |
| 2 | 1,213 (6.7) | (; ) | (; ) | (; ) |
| 319 (1.7) | (; ) | (; ) | (; ) | |
| Explained variance (%) | 3.42 | 1.15 | 2.19 | |
| Smoking habit | ||||
| Never | 11,364 (62.7) | Referent | Referent | Referent |
| Current | 4,775 (26.4) | 1 (; 5) | (; ) | 2 (; 4) |
| Former | 1,983 (10.9) | (; ) | (; ) | (; 1) |
| Explained variance (%) | 0.09 | 0.11 | 0.06 | |
| Alcohol intake (AU per week) | ||||
| Never | 5,525 (30.5) | Referent | Referent | Referent |
| 7,438 (41.1) | 2 (; 5) | 5 (3; 7) | (; 2) | |
| 3–6 | 3,232 (17.8) | 8 (3; 13) | 7 (4; 9) | 3 (; 7) |
| 1,927 (10.6) | 13 (7; 19) | 11 (7; 14) | 8 (3; 12) | |
| Explained variance (%) | 0.47 | 0.44 | 0.29 | |
| Water intake (L/d) | ||||
| Less than 1 | 1,739 (9.6) | Referent | Referent | Referent |
| 1–1.5 | 5,037 (27.8) | — | (; 0) | — |
| 1.5–2 | 9,463 (52.2) | — | (; ) | — |
| 1,883 (10.4) | — | (; ) | — | |
| Explained variance (%) | — | 0.41 | — | |
| Milk/Yogurt intake | ||||
| I quartile | 5,292 (29.2) | Referent | Referent | Referent |
| II quartile | 3,985 (22.0) | (; 2) | 0 (; 3) | (; 0) |
| III quartile | 7,176 (39.6) | (; 0) | (; 1) | (; 1) |
| IV quartile | 1,669 (9.2) | (; ) | (; ) | (; ) |
| Explained variance (%) | 0.14 | 0.19 | 0.09 | |
| Cheese intake | ||||
| I quartile | 5,433 (30.0) | Referent | Referent | Referent |
| II quartile | 4,574 (25.2) | — | 0 (; 2) | — |
| III quartile | 4,610 (25.4) | — | 0 (; 2) | — |
| IV quartile | 3,505 (19.4) | — | (; ) | — |
| Explained variance (%) | — | 0.05 | — | |
| Meat intake | ||||
| I quartile | 6,867 (37.9) | Referent | Referent | Referent |
| II quartile | 3,051 (16.8) | (; ) | 2 (; 4) | (; ) |
| III quartile | 6,813 (37.6) | (; ) | 2 (0; 4) | (; 0) |
| IV quartile | 1,391 (7.7) | (; ) | (; 0) | (; ) |
| Explained variance (%) | 0.12 | 0.06 | 0.09 | |
| Fish intake | ||||
| I tertile | 10,998 (60.7) | Referent | Referent | Referent |
| II tertile | 4,725 (26.1) | (; ) | 4 (3; 6) | — |
| III tertile | 2,399 (13.2) | (; 2) | 9 (6; 11) | — |
| Explained variance (%) | 0.02 | 0.22 | — | |
| Eggs intake | ||||
| I quartile | 5,211 (28.8) | Referent | Referent | Referent |
| II quartile | 7,701 (42.5) | — | 9 (7; 11) | — |
| III quartile | 3,545 (19.5) | — | 9 (6; 12) | — |
| IV quartile | 1,665 (9.2) | — | 13 (10; 17) | — |
| Explained variance (%) | — | 0.35 | — | |
| Sweets/snacks/sweet beverages intake | ||||
| I quartile | 6,340 (35.0) | Referent | Referent | Referent |
| II quartile | 4,936 (27.2) | (; 3) | (; 0) | (; 0) |
| III quartile | 4,555 (25.2) | (; 1) | (; 1) | (; ) |
| IV quartile | 2,291 (12.6) | (; ) | (; ) | (; ) |
| Explained variance (%) | 0.03 | 0.08 | 0.06 | |
| Bread/pasta/cereals intake | ||||
| I tertile | 7,497 (41.4) | Referent | Referent | Referent |
| II tertile | 7,880 (43.5) | 2 (; 6) | — | 1 (; 5) |
| III tertile | 2,745 (15.1) | 8 (3; 14) | — | 7 (3; 12) |
| Explained variance (%) | 0.02 | — | 0.02 | |
| Fruit/vegetables intake | ||||
| I tertile | 5,435 (30.0) | Referent | Referent | Referent |
| II tertile | 5,738 (31.7) | 0 (; 4) | 6 (4; 9) | 3 (0; 6) |
| III tertile | 3,776 (20.8) | 4 (0; 8) | 7 (4; 9) | 6 (3; 10) |
| IV tertile | 3,173 (17.5) | 4 (0; 9) | 8 (5; 11) | 7 (4; 11) |
| Explained variance (%) | 0.02 | 0.23 | 0.08 | |
| Growing vegetables for personal consumption | ||||
| No | 8,891 (49,1) | Referent | Referent | Referent |
| Yes | 9,231 (50.9) | 3 (0; 6) | 10 (8; 12) | 3 (1; 6) |
| Explained variance (%) | 0.33 | 2.52 | 0.73 | |
| Raising animals for personal consumption | ||||
| No | 14,334 (79.1) | Referent | Referent | Referent |
| Yes | 3,788 (20.9) | (; ) | 24 (21; 26) | — |
| Explained variance (%) | 0.05 | 1.19 | — | |
| Total variance explained (%) | 37.44 | 22.61 | 43.10 | |
Note: Red Area: the area of the Veneto Region including municipalities supplied by PFAS contaminated waterworks. Red Area A: part of Red Area whose municipalities are located on the groundwater contamination plume. Red Area B: part of Red Area whose municipalities are located outside the groundwater contamination plume. HDC were defined as not Central–Eastern Europe, North America, Oceania, Israel and Japan. HMPC were defined as Central-Eastern Europe, North Africa, sub-Saharan Africa, Asia except Israel and Japan, and Central and South America countries. Duration of residency in the Red Area was calculated as the number of years spent in the Red Area between 1980 and 2013 (when granular activated carbon filters were installed). Intake of food items was categorized based on quartiles or tertiles. Predictors shown for each PFAS were selected using a stepwise approach based on the Akaike information criterion (AIC). Values of % difference were derived from beta coefficients. —, no data. AU, alcohol units; BMI, body mass index; eGFR, estimated glomerular filtration rate; HDC, highly developed countries; HMPC, high migratory pressure countries; PFHxS, perfluorohexanesulfonic acid; PFOA, perfluorooctanoic acid; PFOS, perfluorooctanesulfonic acid.
Associations with age differed among the three PFAS (Table 3). PFOA levels were 9% (95% CI: , ), 12% (95% CI: , ) and 18% (, ) lower in the 20–24, 25–29, and 30–34 years of age categories, respectively, in comparison with the 14–19 years of age category, but there was no significant difference for the 35–39 years of age category. Serum levels of PFOS and PFHxS increased with age, with significant associations for the three oldest age groups for PFHxS and the two oldest groups for PFOS, relative to the 14–19 years of age group.
Regarding reproductive variables, the number of deliveries was inversely associated with serum PFAS levels and explained 3.4%, 1.2%, and 2.2% of the PFOA, PFOS, and PFHxS variability, whereas current pregnancy was not selected in any of the stepwise models (Table 3).
Serum PFOS progressively decreased with increasing time-lag between the beginning of the study and blood sampling, whereas a significant decrease was seen only with a time-lag of for PFOA and for PFHxS (Table 3).
All three PFASs were significantly higher when eGFR was (Table 3).
Regarding lifestyle factors, alcohol intake showed a significant positive association with all PFAS, especially PFOS and PFOA (Table 3). In comparison with concentrations in nonsmokers, PFOA concentrations were significantly lower in former smokers, and PFOS concentrations were significantly lower in both current and former smokers.
Associations between dietary predictors and the three PFAS varied and made a relatively small contribution to the explained variance overall (Table 3). PFOS concentrations were significantly higher with increasing intake of fish, eggs, and fruit/vegetables, and fruit/vegetables intake was also associated with increasing PFHxS concentrations. The highest quartiles of milk/yogurt and sweets/snacks/sweet beverages intakes were associated with lower concentrations of all PFAS. Meat consumption was associated with lower PFOA and PFHxS concentrations. In comparison with drinking , those who drank and had significantly lower PFOS concentrations.
Growing vegetables and raising animals for personal consumption were both associated with significantly higher serum PFOS, whereas only growing vegetables was associated with higher serum PFHxS, and raising animals was associated with lower serum PFOA (Table 3). With respect to the analyses stratified by predominant residential area, growing vegetables for personal consumption showed positive significant associations with serum PFOA and PFHxS in the Red Area A, but a negative significant association in the Red Area B, whereas for PFOS, the association was positive in both Area A and B. Raising animals for personal consumption was positively associated with PFOS in both Area A and B and negatively with PFOA in Red Area B (Table S2).
In the subset of population recruited after August 2018 (, 19.9%), the models explained 40.8%, 30.6%, and 50.8% of the total variance of serum PFOA, PFOS, and PFHxS, respectively (Table S3). The type of drinking water source (only public, only bottled, or mixed) and consumption of private well water (yes or no) explained about 3% of the variance regarding PFOA and PFHxS but accounted for only 0.4% of the variance in PFOS. When compared with consumption of water from mixed sources (any combination of public, bottled, and well water), consumption of only public water was associated with higher PFOA, PFHxS, and PFOS levels, whereas drinking only bottled water was associated with significantly lower PFOA and PFHxS levels. In the analysis stratified by predominant residential Area, consumption of private well water was associated with significantly lower serum PFOA and PFHxS levels in both Red Area A and B (Table S4). Growing vegetables and raising animals for personal consumption were associated with higher serum PFOS in both Red Area A and B; growing vegetables was associated with higher serum PFHxS only in Red Area A.
Discussion
For decades, residents of a vast territory of the Veneto Region have been inadvertently exposed to drinking water containing high concentrations of PFAS. Through public waterworks, contaminated groundwater was provided to roughly 140,000 people. The contamination herein described is one the largest cases of high residential exposure to PFAS ever reported, and it resembles in origin, extent, and characteristics the one that occurred in the Mid-Ohio Valley, in the United States (Frisbee et al. 2009). In both cases, PFAS released by a chemical plant spread in the environment primarily through water, which acted as the major exposure source for the local population.
Detailed information on the amount of different PFAS congeners produced by the Trissino plant has been provided by the owner company only for the period 2001–2016 and shows that PFOA was the most abundantly produced PFAS, with an average amount of 250 tons per year in the period 2001–2014 when the production was interrupted (Girardi and Merler 2019). PFOS was the second most produced congener, with an average amount of 37 tons per year in the period 2001–2011 and a peak of 88 tons in 2004; after that year, the production declined and then ceased in 2011 (Girardi and Merler 2019). Based on general information on productive processes, it is believed that the plant produced long-chain PFAS only, particularly PFOA and PFOS, from 1968 (when the plant became active) until 2001. In the period 2013–2016, the plant produced mostly short-chain PFAS congeners with four to six carbon atoms, with an overall production of 100 tons per year. The plant was eventually closed in 2018.
PFOA reached the highest concentrations both in drinking water and serum, consistent with previous reports from the Mid-Ohio Valley (Frisbee et al. 2009). PFBA and PFBS were found in high concentrations in drinking water but were detected only in a minority of serum samples at relatively low concentration, whereas PFOS and PFHxS, which were scarcely represented in drinking water, were detected in almost 100% of serum samples. This discrepancy may be explained by the exposure to PFOS and PFHxS from other sources, as demonstrated for the general population (Calafat et al. 2007; Cariou et al. 2015; CDC 2017; Ingelido et al. 2018; Sunderland et al. 2019), and by the longer human half-lives of PFOS and PFHxS (Li et al. 2018) in comparison with PFBA and PFBS (Chang et al. 2008; Olsen et al. 2009). Moreover, the exposure period to PFBA and PFBS was shorter in comparison with that of PFOA and PFOS.
The median serum PFOA concentration in this study () was substantially higher in comparison with that found in the C8 Health Project () (Frisbee et al. 2009), and 27 times higher than the median serum level () of nonexposed residents of the Veneto Region included in a previous biomonitoring study (Ingelido et al. 2018). However, in the C8 Health Project, there was a great heterogeneity in serum levels between the six water districts: In particular, the Little Hocking district that was closer to the point source showed a median serum PFOA concentration of , much higher than in our study, whereas the districts of Pomeroy and Mason County, farther from the point source, had median serum PFOA concentrations of 12.1 and , respectively (Steenland et al. 2009). This heterogeneity may depend on different levels and durations of groundwater contamination in different water districts, whereas the present study population was almost entirely served by a single water supply network that drained water from a single point of the aquifer, and therefore all members of the population were exposed to the same level of drinking water contamination since ∼1980, when the groundwater contamination plume reached public water supply. As a consequence, at variance with the C8 Health Project (Steenland et al. 2009), we did not observe a gradient of serum PFOA concentrations with increasing distance from the point source of contamination. Of note, a recent study based on the analysis of historical serum samples of different cohorts of residents in the Ohio River Valley has shown median serum PFOA concentrations () greater than those reported by the National Health And Nutrition Examination Survey (NHANES) for the general U.S. population (), indicating that PFOA released into the river has spread for hundreds of kilometers downstream from the point sources (Herrick et al. 2017).
In our multivariable analyses, the variability of PFOA, PFOS, and PFHxS serum concentrations was mainly explained by gender, duration of residency in the Red Area, current municipality of residency, and number of deliveries. Associations with these characteristics were stronger than age, education attainment, or behavioral factors (such as smoking, alcohol, and dietary habits). Females had significantly lower serum concentrations of PFOA, PFOS, and PFHxS than did males. This gender difference has been consistently reported across studies (Calafat et al. 2007; Hölzer et al. 2008; Frisbee et al. 2009; Ingelido et al. 2010; Ingelido et al. 2018) but its mechanisms have not been fully elucidated. Pregnancy, delivery, and lactation are known excretion routes for women (Kärrman et al. 2007; Zhang T et al. 2013; Mondal et al. 2014; Cariou et al. 2015; Manzano-Salgado et al. 2015), and previous childbirth and breastfeeding have been inversely associated with serum PFAS in several studies (Brantsæter et al. 2013; Lauritzen et al. 2016; Manzano-Salgado et al. 2016; Bartolomé et al. 2017). This finding is confirmed in the present study where serum concentrations of PFOA, PFOS, and PFHxS progressively decreased with increasing number of previous deliveries. However, reproductive history had a much smaller effect on variance than did female gender itself, indicating that some other mechanism contributes to the lower PFAS levels seen in females. Because PFAS accumulate in the plasma through binding to plasma proteins, blood loss through menstruation can be an important excretion route for this class of compounds (Harada et al. 2005). Organic anion transporters of the proximal renal tubule may mediate resorption of PFOA, PFOS, and PFHxS from the glomerular filtrate and may play a key role in prolonging the half-life of such substances in humans (Andersen et al. 2006). In rats, the expression and activity of organic anion transporters are under hormonal control (Kudo et al. 2002), and the half-life of PFOA, PFOS, and PFHxS is much shorter in females in comparison with males. However, no evidence exists that a similar hormonal mechanism is also operating in humans (Harada et al. 2005).
Other major predictors of serum PFAS concentrations were current municipality of residency and duration of residency in the Red Area. In general, municipalities comprised in Red Area A were associated with higher serum levels of PFOA, PFOS, and PFHxS, but this relation was not constant among all the municipalities. Predominant residency in Red Area A was associated with higher average serum PFOA and PFHxS concentrations (38% and 42%, respectively), whereas there was no significant association with serum PFOS. Because both Area A and B are served by the same waterworks, the reasons for such differences are not completely understood. We hypothesized that they may partially depend on the fact that groundwater is contaminated only in Red Area A, and therefore, the use of private wells to water vegetables or animals may have been an additional source of PFAS contamination for Red Area A residents. The analyses stratified by predominant residential Area partially fit with this hypothesis, showing an effect modification of the association between growing vegetables for personal consumption and serum PFOA and PFHxS levels (positive association in Red Area A and negative association in Red Area B).
Relative to the 14–19 years of age group, estimated mean concentrations of PFOA decreased with age until the 35–39 years of age group, in whom the concentration was not significantly different from that of the youngest group. This pattern is consistent with the J-shaped curve that was observed in the Mid-Ohio Valley population (Steenland et al. 2009; Frisbee et al. 2009) and also in the general population (Bartolomé et al. 2017). The explanation of this age-related pattern is unclear; however, we hypothesize that children may have a higher daily proportional intake of PFOA due to their higher proportional intake of water in comparison with that of adults. Therefore, they may be exposed to higher cumulative doses of PFOA during their early years of life and thereafter reach a lower steady state as they become young adults. Thereafter, continuing exposure may lead to a progressive increase in internal dose from mid-adulthood onward. For PFOS and PFHxS, on the contrary, serum levels tended to increase with age in our young population, suggesting progressive bioaccumulation across the lifespan, as shown in other studies (Bartolomé et al. 2017).
As expected, a longer time-lag between the beginning of the study and the date of blood sampling was associated with lower serum PFAS concentrations, indicating a spontaneous clearance of the PFAS body burden after the abrupt reduction of exposure through drinking water occurred in 2013. The decrease with time was more evident for PFOA and PFOS than for PFHxS, probably reflecting the longer half-life of this latter congener (Li et al. 2018). Glomerular filtration is one of the main excretion pathways for these substances; therefore, it is not surprising that impaired eGFR was associated with increased serum levels of the three PFAS.
Associations with overweight and obesity were in opposite directions for PFOS (negative association) and PFHxS (positive association). Current and former smoking was associated with significantly lower PFOS concentrations, whereas only former smoking was associated with significantly lower PFOA. The concentrations of all the three PFAS increased with alcohol intake. We think these associations should be taken cautiously because previous studies have shown varying results (Steenland et al. 2009; Nelson et al. 2010; Brantsæter et al. 2013; Lauritzen et al. 2016; Manzano-Salgado et al. 2016; Bartolomé et al. 2017; Lee et al. 2017), and the mechanisms are unclear.
Dietary predictors had a small effect on the explained variance of serum PFAS levels. Their contribution was relatively larger for PFOS, especially regarding consumption of fish and eggs. This finding is consistent with findings in previous literature because fish and eggs were found to be important contributors to the dietary intake of PFOS (EFSA CONTAM Panel 2018). Other food categories showed less consistent associations. The positive association between intake of fruits and vegetables and PFOS serum levels is in contrast with recent estimates that showed fruits and vegetables contribute little to the overall dietary intake of PFOS in the European population (EFSA CONTAM Panel 2018). We found it interesting that meat, milk, and dairy product intake were inversely associated with serum PFAS concentrations, a finding which is in the opposite direction of associations reported previously (EFSA CONTAM Panel 2018).
Because drinking water was the main source of exposure to PFOA for the studied population, it is somewhat surprising that water intake did not enter the model for PFOA, and that in the subset of the study population with data on type of water consumed before participants were aware of contamination, adding this information to the models increased explained variance by only 3%. Indeed, similar results were obtained also in the C8 Health Project (Steenland et al. 2009).
Strengths of the present study are the large size of the population and the comprehensive analysis of potential predictors of serum PFAS levels. However, this study has several notable limitations. The main limitation is the cross-sectional design, which precludes assessment of an important criterion to infer causality, i.e., the temporal relation between presumed exposures and outcomes. Second, information on almost all variables was gathered through a questionnaire, and this self-reporting might have resulted in an overestimation or underestimation of exposure, especially concerning food and water intake. Data on potentially important reproductive variables, such as breastfeeding and characteristics of menstrual bleeding, were not gathered in the current study. Another limitation is that the questionnaire did not explore the role of food packaging, and the questionnaire did not explore the consumption of unprocessed foods produced in the contaminated area: The only questions on the latter aspect regarded growing vegetables or animals for personal consumption.
Assessment of exposure to contaminated drinking water missed potentially important details. First, the questionnaire did not investigate whether people moved to noncontaminated areas during the day (for example to go to school or to work), thus limiting their exposure. The source of drinking water (public water supply, bottled water, private well) was assessed qualitatively, precluding the possibility of quantifying the intake of each source. Moreover, participants were not asked to report sources of drinking water before the discovery of contamination (instead of reporting on current consumption) until August 2018. This lack of reporting compromised the validity of data on source of drinking water gathered before August 2018, because most subjects shifted to bottled water as soon as tap water contamination was discovered.
In summary, the studied population, composed of more than 18,000 subjects 14–39 years of age who had been exposed to drinking water contaminated by PFAS, showed higher serum PFOA concentrations in comparison with other populations with residential exposure. Gender, residence area, and duration of residency were the main predictors of serum levels of PFOA, PFOS, and PFHxS, but the majority of variance remained unexplained by our large set of possible predictors, particularly for PFOS. This finding may be explained at least in part by the large interindividual variation of half-lives of PFOA, PFOS, and PFHxS (Li et al. 2018). Further studies are needed to better understand determinants of internal dose. The health surveillance program described in this report is generating a rich informative basis that will be exploited to study the association between the internal dose of PFAS and various health outcomes.
Supplementary Material
Acknowledgments
All phases of this study were funded by the Veneto Region (Regione del Veneto), Italy. The authors thank the entire medical, nursing, and technical staff actively involved in the health surveillance program. Special thanks go to C. Bilato (PFAS Team, Local Health Unit 8 Berica), K. Grego (Local Health Unit 9 Scaligera), V. Mecenero (PFAS Team, Local Health Unit 8 Berica), E. Narne (Screening and Health Impact Assessment Unit, Azienda Zero), G. Scanelli (PFAS Team, Local Health Unit 8 Berica), L. Tagliapietra (Veneto Region), and A. Vantini (Regional Agency for Environmental Prevention and Protection).
Footnotes
Supplemental Material is available online (https://doi.org/10.1289/EHP5337).
The authors declare they have no actual or potential competing financial interests.
Note to readers with disabilities: EHP strives to ensure that all journal content is accessible to all readers. However, some figures and Supplemental Material published in EHP articles may not conform to 508 standards due to the complexity of the information being presented. If you need assistance accessing journal content, please contact ehponline@niehs.nih.gov. Our staff will work with you to assess and meet your accessibility needs within 3 working days.
References
- Andersen ME, Clewell HJ, Tan YM, Butenhoff JL, Olsen GW. 2006. Pharmacokinetic modeling of saturable, renal resorption of perfluoroalkylacids in monkeys—probing the determinants of long plasma half-lives. Toxicol 227(1–2):156–164, PMID: 16978759, 10.1016/j.tox.2006.08.004. [DOI] [PubMed] [Google Scholar]
- Ballesteros V, Costa O, Iñiguez C, Fletcher T, Ballester F, Lopez-Espinosa MJ. 2017. Exposure to perfluoroalkyl substances and thyroid function in pregnant women and children: a systematic review of epidemiologic studies. Environ Int 99:15–28, PMID: 27884404, 10.1016/j.envint.2016.10.015. [DOI] [PubMed] [Google Scholar]
- Bartolomé M, Gallego-Picó A, Cutanda F, Huetos O, Esteban M, Pérez-Gómez B, et al. . 2017. Perfluorinated alkyl substances in Spanish adults: geographical distribution and determinants of exposure. Sci Total Environ 603–604:352–360, PMID: 28633112, 10.1016/j.scitotenv.2017.06.031. [DOI] [PubMed] [Google Scholar]
- Brantsæter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, et al. . 2013. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int 54:74–84, PMID: 23419425, 10.1016/j.envint.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butenhoff JL, Kennedy GL Jr, Hinderliter PM, Lieder PH, Jung R, Hansen KJ, et al. . 2004. Pharmacokinetics of perfluorooctanoate in cynomolgus monkeys. Toxicol Sci 82(2):394–406, PMID: 15470233, 10.1093/toxsci/kfh302. [DOI] [PubMed] [Google Scholar]
- Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham L. 2007. Polyfluoroalkyl chemicals in the U.S. population: data from the National Health and Nutrition Examination Survey (NHANES) 2003–2004 and comparisons to NHANES 1999–2000. Environ Health Perspect 115(11):1596–1602, PMID: 18007991, 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas A, Gold DR, Hauser R, Kleinman KP, Hivert MF, Calafat AM, et al. . 2017. Plasma concentrations of per and polyfluoroalkyl substances at baseline and associations with glycemic indicators and diabetes incidence among high-risk adults in the Diabetes Prevention Program Trial. Environ Health Perspect 125(10):107001, PMID: 28974480, 10.1289/EHP1612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, et al. . 2015. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int 84:71–81, PMID: 26232143, 10.1016/j.envint.2015.07.014. [DOI] [PubMed] [Google Scholar]
- CDC (Centers for Disease Control and Prevention). 2017. Fourth National Report on Human Exposure to Environmental Chemicals. Updated Tables, January 2017, vol. 1. https://www.cdc.gov/exposurereport/pdf/FourthReport_UpdatedTables_Volume1_Jan2017.pdf [accessed 1 March 2019].
- Chang SC, Das K, Ehresman DJ, Ellefson ME, Gorman GS, Hart JA, et al. . 2008. Comparative pharmacokinetics of perfluorobutyrate in rats, mice, monkeys, and humans and relevance to human exposure via drinking water. Toxicol Sci 104(1):40–53, PMID: 18353799, 10.1093/toxsci/kfn057. [DOI] [PubMed] [Google Scholar]
- Darrow LA, Stein CR, Steenland K. 2013. Serum perfluorooctanoic acid and perfluorooctane sulfonate concentrations in relation to birth outcomes in the Mid-Ohio Valley, 2005–2010. Environ Health Perspect 121(10):1207–1213, PMID: 23838280, 10.1289/ehp.1206372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA CONTAM Panel (European Food Safety Authority Panel on Contaminants in the Food Chain), Knutsen HK, Alexander J, Barregård L, Bignami M, Brüschweiler B, Ceccatelli S, et al. . 2018. Scientific Opinion on the risk to human health related to the presence of perfluorooctane sulfonic acid and perfluorooctanoic acid in food. EFSA J 16(12):5194, 10.2903/j.efsa.2018.5194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmett EA, Shofer FS, Zhang H, Freeman D, Desai C, Shaw LM. 2006. Community exposure to perfluorooctanoate: relationships between serum concentrations and exposure sources. J Occup Environ Med 48(8):759–770, PMID: 16902368, 10.1097/01.jom.0000232486.07658.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser AJ, Webster TF, Watkins DJ, Strynar MJ, Kato K, Calafat AM, et al. . 2013. Polyfluorinated compounds in dust from homes, offices, and vehicles as predictors of concentrations in office workers’ serum. Environ Int 60:128–136, PMID: 24041736, 10.1016/j.envint.2013.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ, Brooks AP Jr, Maher A, Flensborg P, Arnold S, Fletcher T, et al. . 2009. The C8 Health Project: design, methods, and participants. Environ Health Perspect 117(12):1873–1882, PMID: 20049206, 10.1289/ehp.0800379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frisbee SJ, Shankar A, Knox SS, Steenland K, Savitz DA, Fletcher T, et al. . 2010. Perfluorooctanoic acid, perfluorooctane sulfonate, and serum lipids in children and adolescents: results from the C8 Health Project. Arch Pediatr Adolesc Med 164(9):860–869, PMID: 20819969, 10.1001/archpediatrics.2010.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girardi P, Merler E. 2019. A mortality study on male subjects exposed to polyfluoroalkyl acids with high internal dose of perfluorooctanoic acid. Environ Res 179(Part A):108743, PMID: 31542491, 10.1016/j.envres.2019.108743. [DOI] [PubMed] [Google Scholar]
- Harada K, Inoue K, Morikawa A, Yoshinaga T, Saito N, Koizumi A. 2005. Renal clearance of perfluorooctane sulfonate and perfluorooctanoate in humans and their species-specific excretion. Environ Res 99(2):253–261, PMID: 16194675, 10.1016/j.envres.2004.12.003. [DOI] [PubMed] [Google Scholar]
- Herrick RL, Buckholz JM, Biro FM, Calafat AM, Ye X, Xie C, et al. . 2017. Polyfluoroalkyl substance exposure in the Mid-Ohio River Valley, 1991–2012. Environ Pollut 228:50–60, PMID: 28505513, 10.1016/j.envpol.2017.04.092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hölzer J, Midasch O, Rauchfuss K, Kraft M, Reupert R, Angerer J, et al. . 2008. Biomonitoring of perfluorinated compounds in children and adults exposed to perfluorooctanoate-contaminated drinking water. Environ Health Perspect 116(5):651–657, PMID: 18470314, 10.1289/ehp.11064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingelido AM, Abballe A, Gemma S, Dellatte E, Iacovella N, De Angelis G, et al. . 2018. Biomonitoring of perfluorinated compounds in adults exposed to contaminated drinking water in the Veneto Region, Italy. Environ Int 110:149–159, PMID: 29108835, 10.1016/j.envint.2017.10.026. [DOI] [PubMed] [Google Scholar]
- Ingelido AM, Marra V, Abballe A, Valentini S, Iacovella N, Barbieri P, et al. . 2010. Perfluorooctanesulfonate and perfluorooctanoic acid exposures of the Italian general population. Chemosphere 80(10):1125–1130, PMID: 20633921, 10.1016/j.chemosphere.2010.06.025. [DOI] [PubMed] [Google Scholar]
- Kärrman A, Ericson I, van Bavel B, Darnerud PO, Aune M, Glynn A, et al. . 2007. Exposure of perfluorinated chemicals through lactation: levels of matched human milk and serum and a temporal trend, 1996–2004, in Sweden. Environ Health Perspect 115(2):226–230, PMID: 17384769, 10.1289/ehp.9491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudo N, Katakura M, Sato Y, Kawashima Y. 2002. Sex hormone-regulated renal transport of perfluorooctanoic acid. Chem Biol Interact 139(3):301–316, PMID: 11879818, 10.1016/s0009-2797(02)00006-6. [DOI] [PubMed] [Google Scholar]
- Lauritzen HB, Larose TL, Øien T, Odland JØ, van de Bor M, Jacobsen GW, et al. . 2016. Factors associated with maternal serum levels of perfluoroalkyl substances and organochlorines: a descriptive study of parous women in Norway and Sweden. PLoS One 11(11):e0166127, PMID: 27824939, 10.1371/journal.pone.0166127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee CK, Suh CH, Kang HS, Hong CP, Choi SN. 2017. Serum concentrations of per- and poly-fluoroalkyl substances and factors associated with exposure in the general adult population in South Korea. Int J Hyg Environ Health 220(7):1195–1198, PMID: 28821426, 10.1016/j.ijheh.2017.07.009. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, et al. . CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration). 2009. A new equation to estimate glomerular filtration rate. Ann Intern Med 150(9):604–612, PMID: 19414839, 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Fletcher T, Mucs D, Scott K, Lindh CH, Tallving P, et al. . 2018. Half-lives of PFOS, PFHxS and PFOA after end of exposure to contaminated drinking water. Occup Environ Med 75(1):46–51, PMID: 29133598, 10.1136/oemed-2017-104651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong B, Bloom MS, Fletcher T. 2012. Thyroid function and perfloroalkyl acids in children living near a chemical plant. Environ Health Perspect 120(7):1036–1041, PMID: 22453676, 10.1289/ehp.1104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorber M, Egeghy PP. 2011. Simple intake and pharmacokinetic modeling to characterize exposure of Americans to perfluoroctanoic acid, PFOA. Environ Sci Technol 45(19):8006–8014, PMID: 21517063, 10.1021/es103718h. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Basterrechea M, Grimalt JO, et al. . 2015. Transfer of perfluoroalkyl substances from mother to fetus in a Spanish birth cohort. Environ Res 142:471–478, PMID: 26257032, 10.1016/j.envres.2015.07.020. [DOI] [PubMed] [Google Scholar]
- Manzano-Salgado CB, Casas M, Lopez-Espinosa MJ, Ballester F, Martinez D, Ibarluzea J, et al. . 2016. Variability of perfluoroalkyl substance concentrations in pregnant women by socio-demographic and dietary factors in a Spanish birth cohort. Environ Int 92–93:357–365, PMID: 27132161, 10.1016/j.envint.2016.04.004. [DOI] [PubMed] [Google Scholar]
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, et al. . 2014. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect 122(2):187–192, PMID: 24280536, 10.1289/ehp.1306613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson JW, Hatch EE, Webster TF. 2010. Exposure to polyfluoroalkyl chemicals and cholesterol, body weight, and insulin resistance in the general U.S. population. Environ Health Perspect 118(2):197–202, PMID: 20123614, 10.1289/ehp.0901165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Burris JM, Ehresman DJ, Froehlich JW, Seacat AM, Butenhoff JL, et al. . 2007. Half-life of serum elimination of perfluorooctanesulfonate, perfluorohexanesulfonate, and perfluorooctanoate in retired fluorochemical production workers. Environ Health Perspect 115(9):1298–1305, PMID: 17805419, 10.1289/ehp.10009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen GW, Chang SC, Noker PE, Gorman GS, Ehresman DJ, Lieder PH, et al. . 2009. A comparison of the pharmacokinetics of perfluorobutanesulfonate (PFBS) in rats, monkeys, and humans. Toxicol 256(1–2):65–74, PMID: 19059455, 10.1016/j.tox.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Polesello S, Valsecchi S. 2013. Rischio associato alla presenza di sostanza perfluoro-alchiliche (PFAS) nelle acque potabili e nei corpi idrici recettori di aree industriali nella Provincia di Vicenza e aree limitrofe. http://www.regione.veneto.it/web/sanita/informazione-e-comunicazione [accessed 1 March 2019].
- Russell MH, Nilsson H, Buck RC. 2013. Elimination kinetics of perfluorohexanoic acid in humans and comparison with mouse, rat and monkey. Chemosphere 93(10):2419–2425, PMID: 24050716, 10.1016/j.chemosphere.2013.08.060. [DOI] [PubMed] [Google Scholar]
- Steenland K, Jin C, MacNeil J, Lally C, Ducatman A, Vieira V, et al. . 2009. Predictors of PFOA Levels in a community surrounding a chemical plant. Environ Health Perspect 117(7):1083–1088, PMID: 19654917, 10.1289/ehp.0800294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderland EM, Hu XC, Dassuncao C, Tokranov AK, Wagner CC, Allen JG. 2019. A review of the pathways of human exposure to poly- and perfluoroalkyl substances (PFASs) and present understanding of health effects. J Expo Sci Environ Epidemiol 29(2):131–147, PMID: 30470793, 10.1038/s41370-018-0094-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valsecchi S, Rusconi M, Mazzoni M, Viviano G, Pagnotta R, Zaghi C, et al. . 2015. Occurrence and sources of perfluoroalkyl acids in Italian river basins. Chemosphere 129:126–134, PMID: 25108894, 10.1016/j.chemosphere.2014.07.044. [DOI] [PubMed] [Google Scholar]
- Williams B, Mancia G, Spiering W, Agabiti Rosei E, Azizi M, Burnier M, et al. . 2018. 2018 ESC/ESH Guidelines for the management of arterial hypertension: The Task Force for the Management of Arterial Hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens 36(10):1953–2041, PMID: 30234752, 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- Winquist A, Steenland K. 2014a. Modeled PFOA Exposure and coronary artery disease, hypertension, and high cholesterol in community and worker cohorts. Environ Health Perspect 122(12):1299–1305, PMID: 25260175, 10.1289/ehp.1307943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winquist A, Steenland K. 2014b. Perfluorooctanoic acid (PFOA) exposure and thyroid disease in community and worker cohorts. Epidemiology 25(2):255–264, PMID: 24407430, 10.1097/EDE.0000000000000040. [DOI] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2017. Keeping our water clean: the case of water contamination in the Veneto Region, Italy. Müller A, ed. Copenhagen, Denmark: World Health Organization. [Google Scholar]
- Zhang C, Sundaram R, Maisog J, Calafat A, Barr DB, Buck Louis GM. 2015. A prospective study of pre-pregnancy serum concentrations of perfluorochemicals and the risk of gestational diabetes. Fertil Steril 103(1):184–189, PMID: 25450302, 10.1016/j.fertnstert.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang T, Sun H, Lin Y, Qin X, Zhang Y, Geng X, et al. . 2013. Distribution of poly- and perfluoroalkyl substances in matched samples from pregnant women and carbon chain length related maternal transfer. Environ Sci Technol 47(14):7974–7981, PMID: 23777259, 10.1021/es400937y. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.