Abstract
Objective
As a post‐approval commitment, this dose‐ranging study was undertaken to evaluate efficacy and safety of onabotulinumtoxinA in adolescents.
Background
In adolescents, migraine is often undiagnosed or misdiagnosed and can present unique management challenges. OnabotulinumtoxinA was approved for prevention of chronic migraine (CM) in adults in 2010.
Methods
This multicenter, double‐blind, parallel‐group, randomized trial assessed a single treatment of onabotulinumtoxinA (155 U or 74 U) vs placebo (intramuscular saline) administered via the recommended fixed‐dose fixed site paradigm in adolescents with CM aged 12 to <18 years. The primary efficacy measure was change in frequency of headache days from baseline at week 12; other measures included change in frequency of headache days at weeks 4 and 8 and change in frequency of severe headache days. Safety and tolerability were assessed.
Results
Of 125 randomized patients (onabotulinumtoxinA 155 U, n = 45; onabotulinumtoxinA 74 U, n = 43; placebo, n = 37), all were included in the primary efficacy analysis, and 115 (92.0%) completed the study. Lack of efficacy was the primary reason for discontinuing (n = 4; 3.2%); no patients discontinued because of adverse events. All treatments reduced frequency of headache days at week 12, with no significant differences between treatments. The mean (95% confidence interval) changes from baseline in the frequency of headache days during the 28‐day period ending at week 12 (primary endpoint) were −6.3 (−8.5, −4.2), −6.4 (−8.8, −4.0), and −6.8 (−9.6, −4.1) days in the onabotulinumtoxinA 155 U, onabotulinumtoxinA 74 U, and placebo groups, respectively (P ≥ .474). All treatments reduced frequency of severe headache days and were well‐tolerated; serious adverse events (n = 3) were considered unrelated to treatment and resolved without sequelae. The most commonly reported treatment‐emergent adverse events were neck pain (n = 8), upper respiratory tract infection (n = 7), migraine, and nasopharyngitis (n = 5 each).
Conclusion
Although this study did not meet its efficacy endpoints, onabotulinumtoxinA was well tolerated in this adolescent population. Given previous data demonstrating the benefits of onabotulinumtoxinA in adults with CM, additional studies with design modifications, including adequate statistical power, to assess the efficacy of multiple treatment cycles of onabotulinumtoxinA for CM prevention in adolescents may be informative.
Keywords: onabotulinumtoxinA, chronic migraine, adolescents, prevention, safety
Abbreviations
- ANCOVA
analysis of covariance
- BMI
body mass index
- CHAMP
Childhood and Adolescent Migraine Prevention trial
- CI
confidence interval
- CM
chronic migraine
- CSSRS
Columbia‐Suicide Severity Rating Scale
- FDA
Food and Drug Administration
- ICHD
International Classification of Headache Disorders
- IM
intramuscular
- PedMIDAS
Pediatric Migraine Disability Assessment
- PREEMPT
Phase 3 REsearch Evaluating Migraine Prophylaxis Therapy
- SD
standard deviation
- TEAE
treatment‐emergent adverse event
- TRAE
treatment‐related adverse event
Introduction
Migraine occurs in approximately 8% of individuals aged <20 years old1 and is the second highest cause of years lost due to disability globally across all age groups.2 Despite the prevalence of migraine and level of disability associated with it, migraine is often undiagnosed or misdiagnosed.3 Diagnosis of migraine in children and adolescents is based on criteria in the International Classification of Headache Disorders (ICHD), which has undergone slight revisions with each edition predominantly in relation to duration of headache attack. The second edition of the ICHD indicated that headache attacks may last 1 to 72 hours in childhood migraine,4 while the latest edition indicates that untreated headache attacks of <2 hours are not proven in children with migraine and has defined migraine based on headache attacks of 2 to 72 hours.5 In addition, ICHD guidelines have consistently noted that migraines in children are typically bilateral, whereas in adults, a unilateral location is more common.4, 5
Chronic migraine (CM) is defined as ≥15 headache days per month for >3 months, with migraine‐like headaches on ≥8 days per month.5 While CM is less common in children younger than 12 years,6 there is an estimated prevalence of 0.8% among 12‐ to 17‐year‐olds, with rates as high as 1.8% when CM associated with medication overuse was included.7 CM is severely disabling,7 negatively affecting school performance and attendance.8 To improve function and quality of life and to reduce disability, it is recommended that preventive treatment be started early.9 Like in adults, some adolescents with CM have intractable disease and fail to respond to ≥2 preventive treatments.9 Thus, effective and well‐tolerated treatments for prevention of CM in adolescents would be welcomed; currently, topiramate is the only preventive treatment approved in the United States for migraine in adolescents.10
In the Phase 3 Research Evaluating Migraine Prophylaxis Therapy (PREEMPT) trials, onabotulinumtoxinA 155 to 195 U was found to significantly reduce the frequency of headache days vs placebo in adults with CM,11 and effects continued and were sustained over a 32‐week open‐label extension of the trials.12 OnabotulinumtoxinA was approved by the U.S. Food and Drug Administration (FDA) in October 2010 to be used as preventive treatment for CM in adults. This study was undertaken to address a post‐approval commitment required under the Pediatric Research Equity Act to assess efficacy and safety of onabotulinumtoxinA in adolescents with CM. Preliminary findings of retrospective reviews of off‐label use of onabotulinumtoxinA 75 to 200 U in the treatment of children and adolescents with migraine with or without chronic daily headache,13 CM,14 or chronic daily headache or CM15 provided initial safety and tolerability data to support our study and to justify our dose selection. The FDA specifically requested that at least 2 different doses of onabotulinumtoxinA be assessed in the adolescent population; therefore, the approved dose of onabotulinumtoxinA 155 U was assessed, as was a dose approximately 50% of the approved dose (74 U). The objective of this post‐approval study was to evaluate efficacy and safety of a single treatment cycle of onabotulinumtoxinA 155 and 74 U vs placebo as preventive treatment for CM in adolescents (aged 12 to <18 years).
Methods
Study Design and Treatments
A single‐treatment, multicenter, double‐blind, randomized, placebo‐controlled, and parallel‐group study was conducted at 28 sites (Table S1) in the United States between October 2012 and August 2016 to assess the efficacy and safety of onabotulinumtoxinA in adolescents aged 12 to <18 years.
Investigators at each study site were responsible for evaluating and enrolling patients, and obtaining informed consent and local internal review board approval (if required by their specific institution). At each study site, patients were randomized in a 1:1:1 ratio using an interactive voice response system/interactive web response system administered centrally by Allergan plc (Dublin, Ireland) to receive a single dose of onabotulinumtoxinA 155 U, onabotulinumtoxinA 74 U, or placebo (intramuscular [IM] saline). Each treatment was administered by IM injection at fixed doses into 31 fixed sites across 7 specific head/neck muscles following the PREEMPT approach16 and per the current approved U.S. treatment paradigm (Table S2).17 To maintain the total injected volume at 3.1 mL (0.1 mL per injection site), the diluent concentration differed for the 3 treatment groups. To maintain blinding, neither the investigator, the person performing the injection, nor any other site personnel who interacted with patients were involved in the preparation of study medication; all doses were prepared by independent personnel who did not perform any other procedures or participate in any other aspects of the study. In addition to the study treatments, patients were permitted to take acute headache treatments as prescribed or directed by their physicians; no preventive treatments were permitted.
The total study duration was 16 weeks, including a baseline screening period of 4 weeks to establish that individuals met CM entry criteria (Fig. 1). Eligible patients were randomized and received treatment on day 1 and returned for follow‐up visits at weeks 1, 6, and 12 (exit visit). Electronic daily diaries were kept by patients for the duration of the study.
Figure 1.
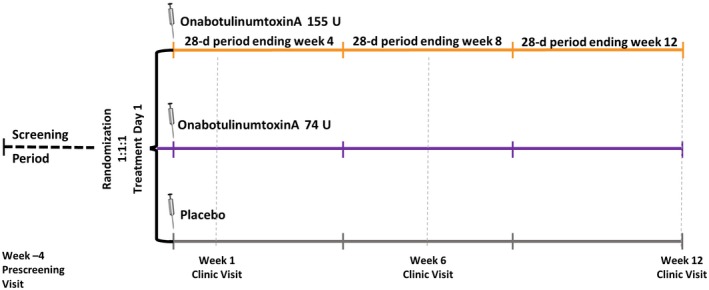
Overview of study design.
The study design was reviewed and approved by the FDA (Division of Neurology Products), was approved by local and central institutional review boards, and was conducted in compliance with Good Clinical Practice guidelines. Parental/representative written informed consent and written minor assent were obtained prior to any treatment being administered.
Patients
To be enrolled into the study, patients had to be aged 12 to <18 years on the day of randomization, have had CM for ≥6 months prior to screening, and, per ICHD‐2nd edition (first revision) guidelines in place at the time of the study,4 have had ≥15 headache days of ≥1 hour of total headache duration during the 4‐week baseline screening period. Patients with an unstable concomitant condition were excluded, as were patients with a headache diagnosis other than CM in the 52 weeks prior to screening, those with a body weight <30 kg or a body mass index (BMI) ≤5% or ≥95% of recommended, those who had previously received botulinum toxin for any reason, and those who were not in the baseline screening phase for ≥28 days or did not record ≥20 diary days during the screening period. Adolescent women who were pregnant, nursing, or planning a pregnancy during the study period were excluded; eligible patients who enrolled in the study were required to use a reliable form of contraception, including abstinence, as appropriate. Finally, patients with a significant risk of suicide, as assessed by the Columbia‐Suicide Severity Rating Scale (CSSRS), were excluded.
Efficacy Measures
Patients reported headache frequency, headache duration, headache severity, and use of acute headache treatment via electronic diaries completed at the end of each day. A 1‐day window for diary completion was allowed, to enable patients to report any further headache‐related activity that may have occurred on the previous day after completing that day’s diary entry. The primary efficacy measure was change from baseline in frequency of headache days during the 28‐day period ending with week 12; a headache day was defined as any day (00:00 to 23:59) with ≥1 total headache hour. Secondary efficacy measures included the change from baseline over a 28‐day period in the frequency of headache days at weeks 4 and 8, frequency of severe headache days, total cumulative hours of headache on headache days, the need for oral rescue treatment for an acute migraine attack, and the proportion of patients with a ≥50% decrease from baseline in the frequency of headache. Other efficacy measures included the change from baseline at week 12 in the Pediatric Migraine Disability Assessment (PedMIDAS), a tool that measures migraine disability via responses to 6 questions about the impact of migraine over the last 3 months.18
Safety and Tolerability
Adverse events were assessed throughout the study during the screening period and at each follow‐up visit initially by general, non‐directed questioning (eg, how have you been feeling since the last visit?) and directed questioning, including the 9‐item Patient Health Questionnaire and the CSSRS. Physical and neurologic examinations were undertaken as appropriate. Urine pregnancy tests were obtained for all female patients, and clinical laboratory tests (blood chemistry and hematology) were obtained for all patients. A treatment‐emergent adverse event (TEAE) was defined as a post‐baseline adverse event with onset after the initiation of study treatment or an adverse event with onset before study treatment that worsened in severity or became serious after the initiation of study treatment. A treatment‐related adverse event (TRAE) included any adverse event that in the investigator's opinion may have been caused by the study medication with reasonable possibility. Any adverse event determined to be drug related and not already listed as a TRAE in the investigator’s brochure was reported to the governing institutional review board. In addition, investigators rated the severity of any adverse event (mild, moderate, or severe). Adverse events were considered serious if they resulted in death, a life‐threatening adverse event, inpatient hospitalization, a persistent or significant disability/incapacity, or a congenital anomaly, or were associated with cancer or any abortion (spontaneous or nonspontaneous).
Statistical Analysis
Efficacy assessments were undertaken in the intent‐to‐treat population, including all randomized patients, regardless of the actual treatment received. The safety population, defined as all patients who received ≥1 injection of the study treatment, was used for all safety assessments. Based on a 2‐sided error level of α = 0.05, a sample size of 42 patients per treatment group had 82% power to detect a between‐group difference in mean change from baseline of 4 headache days assuming a standard deviation (SD) of 6.3 based on the results of phase 3 studies in adults.
For any enrolled patient with <28 days of diary data from the baseline screening period, a pro rata approach based on the diary data available (≥20 days) was used to account for missing data. In the follow‐up period, any patient with <20 days of diary data was set to missing, and these missing values were estimated using a modified last observation carried forward within treatment methodology, rounded to the nearest whole number.
Mean (SD) changes from baseline in the frequency of headache days during the 28‐day period ending with week 12 (primary endpoint) were compared between treatment groups using an analysis of covariance (ANCOVA) with baseline headache frequency as a covariate, investigator center as a stratifying cofactor, and treatment group as a main effect. A 2‐sided test was undertaken, and P values were generated for all pairwise comparisons; P < .05 was considered statistically significant. The secondary endpoints of mean (SD) change from baseline in the frequency of headache days during the 28‐day periods ending with weeks 4 and 8 were analyzed using the same methodology used for the primary efficacy analysis. To control for multiple pairwise comparisons, a gatekeeping approach was used: onabotulinumtoxinA 155 U was compared to placebo; if P < .05, then onabotulinumtoxinA 74 U was compared to placebo. If each onabotulinumtoxinA dose was significantly better than placebo, the higher dose was compared with the lower dose, also at the .05 level. Comparisons of the proportion of 50% headache day responders were performed using logistic regression, with baseline headache frequency as a covariate, investigator center as a stratifying cofactor, and treatment group as a main effect.
Sensitivity analyses were performed to explore how sensitive the primary efficacy results were to the method of imputation. As a primary sensitivity analysis, the primary ANCOVA analysis was performed on scores imputed by the reversion toward baseline method (in which missing scores were imputed as the average nonmissing score across all previous 28‐day periods including baseline). As a secondary sensitivity analysis, the primary ANCOVA analysis was performed on observed data (ie, data without imputation for missing counts when there were less than 20 days of reported data). This ANCOVA analysis of observed data was also performed with any postdropout diary data excluded. Additional sensitivity analyses using the mixed model for repeat measures and the last nonmissing observed score methods were also performed. Statistical analyses were conducted using SAS version 9.3 software (SAS Institute, Cary, NC).
Results
Patient Disposition and Demographics
A total of 221 patients were screened for eligibility; 125 met enrollment criteria, were randomized to receive treatment, and were included in the primary efficacy analysis (onabotulinumtoxinA 155 U, n = 45; onabotulinumtoxinA 74 U, n = 43; placebo, n = 37). Of these patients, 2 randomized to receive onabotulinumtoxinA 155 U were found to be ineligible for the study before receiving treatment, and therefore did not receive treatment. The safety population included 123 patients who were treated with at least 1 dose of study medication (onabotulinumtoxinA 155 U, n = 43; onabotulinumtoxinA 74 U, n = 43; placebo, n = 37). The study was completed by 115 of 125 patients (92.0%), with lack of efficacy as the main reason for not completing the study (4/125, 3.2%; Fig. 2). No patients discontinued due to adverse events.
Figure 2.
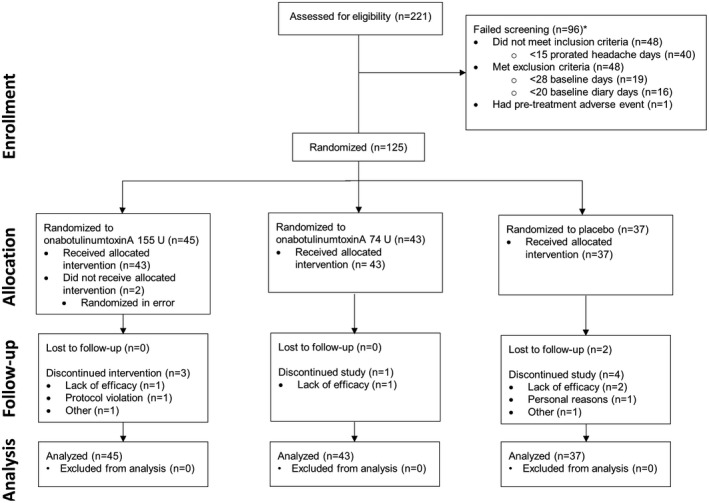
Study flow diagram indicating patient disposition. *Patients could fail screening for multiple reasons; patients with <20 baseline diary days only include those who completed the 28‐day baseline period; patients with <15 prorated headache days only included those who completed the 28‐day baseline period and had ≥20 baseline diary days.
Treatment groups were well‐balanced in terms of baseline demographics; the overall mean (SD) age was 15.1 (1.5) years, and the majority of patients were female (98/125; 78.4%) and Caucasian (101/125; 80.8%), with a mean (SD) BMI of 22.5 (3.9) kg/m2 (Table 1). The mean (SD) age of onset of CM was 10.5 (3.2) years and mean (SD) time since onset was 4.2 (3.0) years. Approximately one‐third of patients had allodynia (Allodynia Symptom Checklist score ≥3) at baseline.
Table 1.
Patient Demographics and Headache Characteristics at Baseline
| OnabotulinumtoxinA 155 U (n = 45) | OnabotulinumtoxinA 74 U (n = 43) | Placebo (n = 37) | Total (n = 125) | |
|---|---|---|---|---|
| Mean (SD) age, years | 15.1 (1.4) | 15.0 (1.5) | 15.2 (1.5) | 15.1 (1.5) |
| Female, n (%) | 37 (82) | 32 (74) | 29 (78) | 98 (78) |
| Caucasian, n (%) | 38 (84) | 37 (86) | 26 (70) | 101 (81) |
| Mean (SD) BMI, kg/m2 | 22.5 (3.8) | 22.0 (4.0) | 23.2 (3.9) | 22.5 (3.9) |
| Age of onset | ||||
| Mean (SD) age of onset, years | 10.7 (3.0) | 10.4 (2.9) | 10.3 (3.8) | 10.5 (3.2) |
| <12 years, n (%) | 26 (58) | 27 (63) | 20 (54) | 73 (58) |
| 12 to <15 years, n (%) | 16 (36) | 14 (33) | 14 (38) | 44 (35) |
| 15 to <18 years, n (%) | 3 (7) | 2 (5) | 3 (8) | 8 (6) |
| Time since onset | ||||
| Mean (SD) time, years | 4.1 (2.9) | 4.2 (2.7) | 4.5 (3.4) | 4.2 (3.0) |
| <3 years, n (%) | 13 (29) | 14 (33) | 13 (35) | 40 (32) |
| 3 to 5 years, n (%) | 18 (40) | 12 (28) | 9 (24) | 39 (31) |
| >5 years, n (%) | 14 (31) | 17 (40) | 15 (41) | 46 (37) |
| Allodynia, n (%) | 14 (31) | 13 (30) | 15 (41) | 42 (34) |
BMI = body mass index.
Efficacy Outcomes
All treatment groups reduced the frequency of headache days from baseline at week 12 (primary assessment point) after the single treatment cycle; there were no significant differences between treatment groups at week 12 or at weeks 4 or 8 (Fig. 3A). Similarly, all treatments reduced severe headache day frequency compared with baseline, and there were no significant differences between treatment groups at all time points except 1. OnabotulinumtoxinA 74 U treatment resulted in a significantly greater reduction in mean (SD; 95% confidence interval [CI]) frequency of severe headache days vs placebo at week 8 (−3.4 [5.0; −4.91 to −1.83] vs −1.4 [6.8; −3.68 to 0.87]; P = .037; Fig. 3B). However, as this was a single event with no supporting trends, this single difference was not deemed clinically meaningful. Results of the sensitivity analyses consistently demonstrated similarity across treatment groups and no significant between‐group differences emerged for any 28‐day period.
Figure 3.
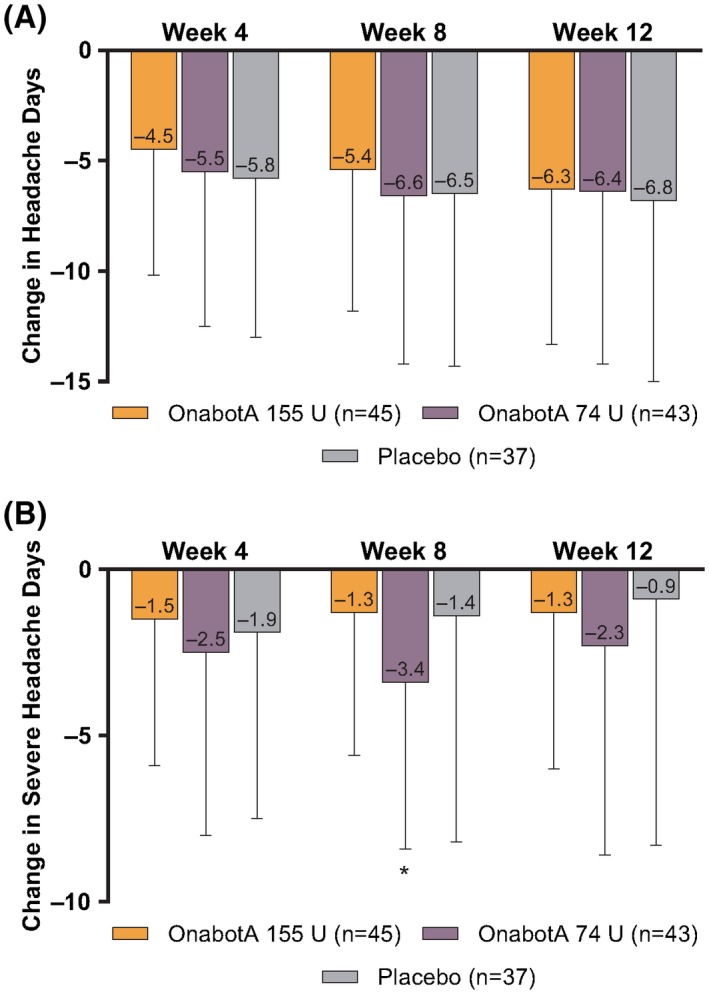
Change in mean (95% CI) (A) headache days and (B) severe headache days per 28‐day period vs baseline. OnabotA = onabotulinumtoxinA. *P = .037 for onabotulinumtoxinA 74 U vs placebo.
There was no significant difference between treatment arms in change from baseline in total cumulative headache hours; similarly, there was no significant difference between treatment arms in the proportion of responders (having at least a 50% decrease from baseline in frequency of headache days).
The mean (SD) changes from baseline in the PedMIDAS score at week 12 were –19.5 (53.0) and −36.1 (66.0) in the onabotulinumtoxinA 155 U and 74 U groups, respectively, compared with −32.6 (56.1) in the placebo group. Between‐group comparisons in PedMIDAS score were not statistically significant (P ≥ .114).
Safety and Tolerability
Treatments were well‐tolerated. At least 1 TEAE was reported by 21/43 patients (48.8%) in the onabotulinumtoxinA 155 U group, 23/43 patients (53.5%) in the onabotulinumtoxinA 74 U group, and 14/37 patients (37.8%) in the placebo group (Table 2). There was no apparent dose‐relationship for individual adverse events, although there is a suggestion of a dose response for any TRAE. The majority of TEAEs were deemed to be mild or moderate in severity (onabotulinumtoxinA 155 U: 18/21 [86%]; onabotulinumtoxinA 74 U: 17/23 [74%]; placebo: 13/14 [93%]) and were considered unrelated to treatment (Table 2). The most commonly reported TEAEs (≥3% overall incidence) among onabotulinumtoxinA recipients were neck pain (8/86 [9%] vs 0/37 [0%] with placebo), nasopharyngitis (4/86 [5%] vs 1/37 [3%] with placebo), migraine (4/86 [5%] vs 1/37 [3%] with placebo), musculoskeletal pain (4/86 [5%] vs 0/37 [0%] with placebo), dizziness (3/86 [3%] vs 1/37 [3%] with placebo), and upper respiratory tract infection (3/86 [3%] vs 4/37 [11%] with placebo; Table 2). All serious adverse events were considered unrelated to treatment, and all resolved without sequelae. One patient receiving onabotulinumtoxinA 155 U reported a serious adverse event (cellulitis), as did 2 patients receiving onabotulinumtoxinA 74 U (migraine and appendicitis). No patients in any treatment group discontinued the study due to adverse events; there were no deaths.
Table 2.
Summary of Adverse Events in Each Treatment Group, Safety Population
| Adverse Event, n (%)† | OnabotulinumtoxinA 155 U (n = 43) | OnabotulinumtoxinA 74 U (n = 43) | Placebo (n = 37) | Any OnabotulinumtoxinA (n = 86) |
|---|---|---|---|---|
| Any adverse event | 21 (49) | 23 (53) | 14 (38) | 44 (51) |
| Treatment‐unrelated | 14 (33) | 19 (44) | 14 (38) | 33 (38) |
| Treatment‐related | 10 (23) | 7 (16) | 4 (11) | 17 (20) |
| Serious adverse event | 1 (2) | 2 (5) | 0 | 3 (3) |
| Treatment‐unrelated | 1 (2)§ | 2 (5)‡ | 0 | 3 (3) |
| Treatment‐related | 0 | 0 | 0 | 0 |
| Discontinued study due to adverse event | 0 | 0 | 0 | 0 |
| Deaths | 0 | 0 | 0 | 0 |
| Adverse events occurring in ≥5% of any treatment group† | ||||
| Neck pain | 3 (7) | 5 (12) | 0 | 8 (9) |
| Nasopharyngitis | 2 (5) | 2 (5) | 1 (3) | 4 (5) |
| Migraine | 1 (2) | 3 (7) | 1 (3) | 4 (5) |
| Musculoskeletal pain | 1 (2) | 3 (7) | 0 | 4 (5) |
| Dizziness | 3 (7) | 0 | 1 (3) | 3 (3) |
| Upper respiratory tract infection | 0 | 3 (7) | 4 (11) | 3 (3) |
| Bronchitis | 1 (2) | 0 | 2 (5) | 1 (1) |
| Oropharyngeal pain | 0 | 0 | 2 (5) | 0 |
Patients may have had ≥1 adverse event.
Migraine and appendicitis.
Cellulitis.
Treatment‐related TEAEs were reported by 10/43 (23%) patients in the onabotulinumtoxinA 155 U group, 7/43 (16%) patients in the onabotulinumtoxinA 74 U group, and 4/37 (11%) patients in the placebo group. The majority of treatment‐related events were mild in severity in the onabotulinumtoxinA 155 U (8/10 [80%]) and onabotulinumtoxinA 74 U (5/7 [71%]) groups, and mild for 50% (2/4) in the placebo group. Treatment‐related TEAEs reported by >3% of onabotulinumtoxinA recipients were neck pain (5/86 [6%] vs 0/37 [0%] with placebo) and musculoskeletal pain (4/86 [5%] with onabotulinumtoxinA vs 0/37 [0%] with placebo), with no evidence of a dose‐response relationship for these events. Although 1 incident of facial paresis was determined to be a local pharmacologic effect of onabotulinumtoxinA, none of the reported TEAEs or treatment‐related TEAEs were associated with possible distant spread of toxin.
Discussion
This dose‐ranging study evaluated the safety and tolerability of onabotulinumtoxinA 155 U and 74 U in adolescents aged 12 to <18 years old. Treatment with onabotulinumtoxinA 155 U, onabotulinumtoxinA 74 U, and placebo reduced headache day frequency from baseline by 6.3, 6.4, and 6.8 days, respectively, for the 28‐day period ending at week 12. No statistically significant differences between onabotulinumtoxinA and placebo were found for primary or secondary efficacy endpoints. OnabotulinumtoxinA 74 U, but not 155 U, caused a significant reduction in frequency of severe headache days at week 8 but not at week 12. However, this was not part of an overall pattern in the efficacy results, and this difference between onabotulinumtoxinA and placebo in the present study does not appear to be clinically meaningful.
Case series and retrospective analyses of the off‐label use of onabotulinumtoxinA at doses of up to 215 U in adolescents with medically refractory CM suggest that onabotulinumtoxinA can provide subjective and clinically meaningful relief of symptoms, including reductions in headache frequency and severity (Table 3).13, 14, 15, 19, 20, 21, 22, 23, 24, 25, 26 One retrospective case series demonstrated a reduction in headache frequency after 1 treatment cycle to a level similar to that observed in the present study.14
Table 3.
OnabotulinumtoxinA in Pediatric Chronic Migraine: Prior Research
| N | Mean Age, Years (Range) | Dose | Outcomes | |
|---|---|---|---|---|
| Chan et al (2009)13 (case series) | 6 | (14‐18) | 100 U |
|
| Ahmed et al (2010)15 (case series) | 5 | NR | 100 U |
|
| Kabbouche et al (2012)14 (retrospective review) | 45 | 16.8 (11‐21) | Average: 188.5 U (±32 U) |
|
| Minimum: 75 U | ||||
| Maximum: 200 U | ||||
| Schroeder et al (2012)19 (case series: chronic daily headache) | 5 | 13 (10‐16) | Minimum: 40 U |
|
| Maximum: 150 U | ||||
| Bernhard et al (2014)20 (case series) | 10 | (13‐17) | 150 U |
|
| Choi and Bae (2016)21 (case series) | 5 | (14‐16) | 155 IU |
|
| Pezzuto et al (2016)23 (retrospective review) | 42 | (11‐18) | <50 U‐<110 U |
|
| Yonker et al (2016) (retrospective review)24 | 38 | 15.9 | NR |
|
| Calderon et al (2017)22 (retrospective review) | 10 | 15 (8‐18) | Mean: 167.5 U |
|
| Minimum: 75 U | ||||
| Maximum: 215 U | ||||
| Shah (2018)26 (randomized, placebo‐controlled, crossover study) | 6† | (8‐17) | NR |
|
| Shah (2018)25 (retrospective review) | 11‡ | 15 (8‐17) | Median: 165 U |
|
| Minimum: 155 U | ||||
| Maximum: 215 U |
NR = not reported; PedMIDAS = pediatric Migraine Disability Assessment questionnaire.
Preliminary data.
1 patient lost to follow‐up; analysis based on 10 patients.
Unlike previous retrospective evaluations, our study was a randomized double‐blind, placebo‐controlled trial. In controlled trials of acute and preventive medications in children and adolescents with migraine, the placebo response has consistently been higher than that typically observed in adults,27, 28, 29, 30, 31, 32 with some evidence of an inverse relationship between age and likelihood of a placebo response.32, 33 In the Childhood and Adolescent Migraine Prevention (CHAMP) trial of amitriptyline, topiramate, and placebo, for example, 61% of patients receiving placebo experienced a >50% reduction in headache, ultimately leading to the early cessation of the trial.34
A number of factors have been suggested to account for higher placebo response rates observed in children and adolescents with migraine compared with adults. These include possible differences in clinical presentation and underlying mechanisms of migraine in children and adults, as well as the impact of the study design.28, 35 Modifications in study design may increase the probability of detecting benefits of active treatment. For example, we suggest that to assess the efficacy of active treatment vs placebo in children and adolescents with migraine with the same level of assurance as for adults, sample sizes need to be larger than those in adult clinical trials. In this context, 1 aspect of our study was that it had a considerably smaller number of participants (n = 125) than were enrolled in the pivotal PREEMPT trials (n = 1384 across the pooled studies), and thus may have not been powered sufficiently to detect differences in efficacy between onabotulinumtoxinA and placebo treatments in this adolescent population that were comparable to those seen in the PREEMPT studies.12 The current study was only powered to detect a between‐group difference in the reduction of headache days of 4 days or greater. In the PREEMPT pooled primary analysis at 24 weeks, the change from baseline in number of headache days was −8.4 with onabotulinumtoxinA treatment and −6.6 for placebo, a mean between‐group difference of −1.8 headache days.11
Trial designs that allow the identification and exclusion of placebo responders before randomization may be more effective at detecting therapeutic gains in pediatric populations.35, 36 For example, one of the few triptan trials to demonstrate efficacy in pediatric patients utilized a blinded run‐in phase to identify placebo responders.37 Meta‐analyses of placebo‐controlled trials of triptans in children and adolescents with migraine38, 39, 40, 41 suggest that our parallel‐group study design may have contributed to the high placebo response rate we observed. Of note, preliminary data from an ongoing pediatric double‐blind crossover study of onabotulinumtoxinA have shown statistically significant decreases in the frequency and duration of migraine with onabotulinumtoxinA vs placebo, accompanied by numerical improvements in intensity and function ratings.26
The probability of receiving active treatment rather than placebo can also influence responses. For example, response rates have been shown to be higher in active‐comparator than in placebo‐controlled trials.42 In placebo‐controlled trials, a greater than 50% chance of receiving the active treatment or a greater expectation of a response have been associated with higher placebo response rates.42, 43, 44, 45, 46 Thus, a potential limitation of our study was that patients had a 2:1 chance of receiving active treatment; therefore, the high placebo response rate observed might have been expected.
An additional limitation of the current study is that the design provided for only 1 treatment cycle of onabotulinumtoxinA. In adults, it has been established in the pooled PREEMPT studies that a meaningful proportion of patients who failed to respond to the first treatment became responders after additional treatment cycles.47 In addition, the results of the long‐term, open‐label COMPEL study showed that the mean reduction in the number of headache days per 28 days was greater after 9 treatment cycles (108 weeks; −10.7 headaches) than after 2 treatment cycles (24 weeks; −7.4 headaches).48 Therefore, a longer study to explore the safety and efficacy of repeated exposure to onabotulinumtoxinA in the developing adolescent, with at least 2 treatment cycles, may be necessary in this population to detect significant differences between onabotulinumtoxinA and placebo treatment.
Additional potential limitations include that the instrument used to measure allodynia (the Allodynia Symptom Checklist)49 has not been validated in an adolescent population and that the last observation carried forward was used for missing data (although this was well controlled for by multiple sensitivity analyses described in the study).
For clinical studies to deliver on the overall objective of the Pediatric Research Equity Act, there must be a careful evaluation of both the benefits and the risks of treatment in the pediatric population.50, 51 Evaluations of off‐label use of onabotulinumtoxinA for migraine at doses of up to 215 U in pediatric populations have reported infrequent adverse events, which typically resolved spontaneously or with nonsteroidal anti‐inflammatory treatment.13, 14, 15, 20, 25 In the current trial, treatment‐related TEAEs occurred in 20% of patients exposed to onabotulinumtoxinA compared with 11% of patients in the placebo group. The majority of TEAEs were mild or moderate in severity, were considered unrelated to treatment, and did not lead to treatment discontinuation.
Conclusions
Although this post‐approval study did not meet its efficacy endpoints, the results provide evidence of the safety and tolerability of onabotulinumtoxinA in the adolescent population. The findings also offer insights on study design that can be used to guide future research into the efficacy of onabotulinumtoxinA for the prevention of migraine in adolescents with CM. In adults, the beneficial effects of onabotulinumtoxinA have been shown to increase with multiple treatments.48 Therefore, to fully assess the benefits of treatment in adolescents, we suggest that future pediatric trials evaluate at least 2 treatment cycles of onabotulinumtoxinA, enroll larger numbers of patients, and explore the use of a placebo run‐in or crossover design.
Statement of Authorship
Category 1
(a) Conception and Design
Mitchell F. Brin
(b) Acquisition of Data
Paul K. Winner, Marielle Kabbouche, Veronica Wangsadipura, Arlene Lum
(c) Analysis and Interpretation of Data
Paul K. Winner, Marielle Kabbouche, Marcy Yonker, Veronica Wangsadipura, Arlene Lum, Mitchell F. Brin
Category 2
(a) Drafting the Manuscript
Paul K. Winner, Marielle Kabbouche, Marcy Yonker, Veronica Wangsadipura, Arlene Lum, Mitchell F. Brin
(b) Revising It for Intellectual Content
Paul K. Winner, Marielle Kabbouche, Marcy Yonker, Veronica Wangsadipura, Arlene Lum, Mitchell F. Brin
Category 3
(a) Final Approval of the Completed Manuscript
Paul K. Winner, Marielle Kabbouche, Marcy Yonker, Veronica Wangsadipura, Arlene Lum, Mitchell F. Brin
Supporting information
Acknowledgments
Editorial support for development of this manuscript was provided by Lisa Feder, PhD of Peloton Advantage, LLC, an OPEN Health company, Parsippany, NJ, USA, and Lee B. Hohaia, PharmD, of Complete Healthcare Communications, LLC (North Wales, PA), a CHC Group company, and was funded by Allergan plc (Dublin, Ireland).
Conflict of Interest: Paul K. Winner has received consulting fees/honoraria from Adler, Allergan, Amgen, Supernus, and TEVA; has served on speaker bureaus for Allergan, Amgen, Avanir, Supernus, and TEVA; and has received research grants from Adler, Allergan, Amgen, NuPathe, AstraZeneca, Avanir, Eli Lilly, Novartis, and TEVA. Marielle Kabbouche has received consulting fees from Amgen, Impax, and Supernus. Marcy Yonker has received consulting fees from Impax, Upsher‐Smith, and Amgen. Veronica Wangsadipura, Arlene Lum, and Mitchell F. Brin are full‐time employees of Allergan and own stock in the company.
Funding: All phases of this study were fully supported by Allergan plc (Dublin, Ireland), and there were no other sources of funding.
ClinicalTrials.gov Identifier: NCT01662492.
References
- 1. Abu‐Arafeh I, Razak S, Sivaraman B, Graham C. Prevalence of headache and migraine in children and adolescents: A systematic review of population‐based studies. Dev Med Child Neurol. 2010;52:1088‐1097. [DOI] [PubMed] [Google Scholar]
- 2. GBD 2016 Disease and Injury Incidence and Prevalence Collaborators . Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990‐2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1211‐1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hershey AD, Winner PK. Pediatric migraine: Recognition and treatment. J Am Osteopath Assoc. 2005;105:2S‐8S. [PubMed] [Google Scholar]
- 4. Olesen J. The international classification of headache disorders, 2nd edition (ICHD‐II). Rev Neurol (Paris). 2005;161:689‐691. [DOI] [PubMed] [Google Scholar]
- 5. Headache Classification Committee of the International Headache Society . The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1‐211. [DOI] [PubMed] [Google Scholar]
- 6. Ozge A, Yalin OO. Chronic migraine in children and adolescents. Curr Pain Headache Rep. 2016;20:14. [DOI] [PubMed] [Google Scholar]
- 7. Lipton RB, Manack A, Ricci JA, Chee E, Turkel CC, Winner P. Prevalence and burden of chronic migraine in adolescents: Results of the chronic daily headache in adolescents study (C‐dAS). Headache. 2011;51:693‐706. [DOI] [PubMed] [Google Scholar]
- 8. Buse DC, Manack AN, Fanning KM, et al. Chronic migraine prevalence, disability, and sociodemographic factors: Results from the American Migraine Prevalence and Prevention Study. Headache. 2012;52:1456‐1470. [DOI] [PubMed] [Google Scholar]
- 9. O'Brien HL, Kabbouche MA, Kacperski J, Hershey AD. Treatment of pediatric migraine. Curr Treat Options Neurol. 2015;17:326. [DOI] [PubMed] [Google Scholar]
- 10. Trokendi XR [Package Insert]. Rockville, MD: Supernus Pharmaceuticals, Inc; 2018. [Google Scholar]
- 11. Dodick DW, Turkel CC, DeGryse RE, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled results from the double‐blind, randomized, placebo‐controlled phases of the PREEMPT clinical program. Headache. 2010;50:921‐936. [DOI] [PubMed] [Google Scholar]
- 12. Aurora SK, Winner P, Freeman MC, et al. OnabotulinumtoxinA for treatment of chronic migraine: Pooled analyses of the 56‐week PREEMPT clinical program. Headache. 2011;51:1358‐1373. [DOI] [PubMed] [Google Scholar]
- 13. Chan VW, McCabe EJ, MacGregor DL. Botox treatment for migraine and chronic daily headache in adolescents. J Neurosci Nurs. 2009;41:235‐243. [DOI] [PubMed] [Google Scholar]
- 14. Kabbouche M, O'Brien H, Hershey AD. OnabotulinumtoxinA in pediatric chronic daily headache. Curr Neurol Neurosci Rep. 2012;12:114‐117. [DOI] [PubMed] [Google Scholar]
- 15. Ahmed K, Oas KH, Mack KJ, Garza I. Experience with botulinum toxin type A in medically intractable pediatric chronic daily headache. Pediatr Neurol. 2010;43:316‐319. [DOI] [PubMed] [Google Scholar]
- 16. Blumenfeld A, Silberstein SD, Dodick DW, Aurora SK, Turkel CC, Binder WJ. Method of injection of onabotulinumtoxinA for chronic migraine: A safe, well‐tolerated, and effective treatment paradigm based on the PREEMPT clinical program. Headache. 2010;50:1406‐1418. [DOI] [PubMed] [Google Scholar]
- 17. Botox [Package Insert]. Dublin, Ireland: Allergan; 2019. [Google Scholar]
- 18. Hershey AD, Powers SW, Vockell AL, LeCates S, Kabbouche MA, Maynard MK. PedMIDAS: Development of a questionnaire to assess disability of migraines in children. Neurology. 2001;57:2034‐2039. [DOI] [PubMed] [Google Scholar]
- 19. Schroeder AS, Huss K, Blaschek A, et al. Ten‐year follow‐up in a case series of integrative botulinum toxin intervention in adolescents with chronic daily headache and associated muscle pain. Neuropediatrics. 2012;43:339‐345. [DOI] [PubMed] [Google Scholar]
- 20. Bernhard MK, Bertsche A, Syrbe S, Weise S, Merkenschlager A. Botulinum toxin injections for chronic migraine in adolescents – An early therapeutic option in the transition from neuropaediatrics to neurology [in German]. Fortschr Neurol Psychiatr. 2014;82:39‐42. [DOI] [PubMed] [Google Scholar]
- 21. Choi Y, Bae C. Onabotulinumtoxin a as one of the preventive treatments for chronic migraine in Korean adolescents In: 5th European Headache and Migraine Trust International Congress. Glasgow, UK; 2016:17‐18. [Google Scholar]
- 22. Calderon M‐D, Wu W, Ma M, et al. A longitudinal evaluation of the effectiveness of Botox® in pediatric patients experiencing migraines: A five‐year retrospective study Poster presented at: The Anesthesiology Annual Meeting; October 23, 2017; Boston, MA. [Google Scholar]
- 23. Pezzuto T, Beyderman L, Chugani D, Xie L. Is less more? Pediatric intractable migraine and botox treatment. Ann Neurol. 2016;80:S299‐S300. [Google Scholar]
- 24. Yonker M, Marian M. Effectiveness of prophylactic onabotulinumtoxin a in adolescents with chronic migraine. Headache. 2016;56:31‐32. [Google Scholar]
- 25. Shah S, Calderon M‐D, Wu WD, Grant J, Rinehart J. Onabotulinumtoxin A (BOTOX®) for prophylactic treatment of pediatric migraine: A retrospective longitudinal analysis. J Child Neurol. 2018;33:580‐586. [DOI] [PubMed] [Google Scholar]
- 26. Shah S. Effectiveness of onabotulinumtoxinA (Botox®) in pediatric patients experiencing migraines: A randomized double blinded placebo crossover study in the pediatric pain population In: ASRA Chronic Pain Grant Update. Pittsburgh, PA: American Society of Regional Anesthesia and Pain Medicine; 2018:2016. [DOI] [PubMed] [Google Scholar]
- 27. Lewis DW, Winner P, Wasiewski W. The placebo responder rate in children and adolescents. Headache. 2005;45:232‐239. [DOI] [PubMed] [Google Scholar]
- 28. El‐Chammas K, Keyes J, Thompson N, Vijayakumar J, Becher D, Jackson JL. Pharmacologic treatment of pediatric headaches: A meta‐analysis. JAMA Pediatr. 2013;167:250‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Kacperski J, Bazarsky A. New developments in the prophylactic drug treatment of pediatric migraine: What is new in 2017 and where does it leave us? Curr Pain Headache Rep. 2017;21:38. [DOI] [PubMed] [Google Scholar]
- 30. Loder E, Goldstein R, Biondi D. Placebo effects in oral triptan trials: The scientific and ethical rationale for continued use of placebo controls. Cephalalgia. 2005;25:124‐131. [DOI] [PubMed] [Google Scholar]
- 31. Faria V, Linnman C, Lebel A, Borsook D. Harnessing the placebo effect in pediatric migraine clinic. J Pediatr. 2014;165:659‐665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kacperski J, Hershey AD. Preventive drugs in childhood and adolescent migraine. Curr Pain Headache Rep. 2014;18:422. [DOI] [PubMed] [Google Scholar]
- 33. Maas HJ, Danhof M, Della Pasqua OE. Analysis of the relationship between age and treatment response in migraine. Cephalalgia. 2009;29:772‐780. [DOI] [PubMed] [Google Scholar]
- 34. Powers SW, Coffey CS, Chamberlin LA, et al. Trial of amitriptyline, topiramate, and placebo for pediatric migraine. N Engl J Med. 2017;376:115‐124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sun H, Bastings E, Temeck J, et al. Migraine therapeutics in adolescents: A systematic analysis and historic perspectives of triptan trials in adolescents. JAMA Pediatr. 2013;167:243‐249. [DOI] [PubMed] [Google Scholar]
- 36. Wasiewski WW. Preventive therapy in pediatric migraine. J Child Neurol. 2001;16:71‐78. [DOI] [PubMed] [Google Scholar]
- 37. Ho T, Pearlman E, Lewis D, et al. Efficacy and tolerability of rizatriptan for treatment of a single migraine attack in pediatric migraineurs: Results from a randomized, double‐blind, placebo‐controlled trial using a novel enrichment design. Cephalalgia. 2012;32:750‐765. [DOI] [PubMed] [Google Scholar]
- 38. Evers S. The efficacy of triptans in childhood and adolescence migraine. Curr Pain Headache Rep. 2013;17:342. [DOI] [PubMed] [Google Scholar]
- 39. Evers S. Controlled trials in pediatric migraine: Crossover versus parallel group. Curr Pain Headache Rep. 2007;11:241‐244. [DOI] [PubMed] [Google Scholar]
- 40. Evers S, Marziniak M, Frese A, Gralow I. Placebo efficacy in childhood and adolescence migraine: An analysis of double‐blind and placebo‐controlled studies. Cephalalgia. 2009;29:436‐444. [DOI] [PubMed] [Google Scholar]
- 41. Sakai F. Oral triptans in children and adolescents: An update. Curr Pain Headache Rep. 2015;19:8. [DOI] [PubMed] [Google Scholar]
- 42. Rutherford BR, Sneed JR, Roose SP. Does study design influence outcome? The effects of placebo control and treatment duration in antidepressant trials. Psychother Psychosom. 2009;78:172‐181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Papakostas GI, Fava M. Does the probability of receiving placebo influence clinical trial outcome? A meta‐regression of double‐blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol. 2009;19:34‐40. [DOI] [PubMed] [Google Scholar]
- 44. Nakamura Y, Donaldson GW, Kuhn R, Bradshaw DH, Jacobson RC, Chapman CR. Investigating dose‐dependent effects of placebo analgesia: A psychophysiological approach. Pain. 2012;153:227‐237. [DOI] [PubMed] [Google Scholar]
- 45. Sinyor M, Levitt AJ, Cheung AH, et al. Does inclusion of a placebo arm influence response to active antidepressant treatment in randomized controlled trials? Results from pooled and meta‐analyses. J Clin Psychiatry. 2010;71:270‐279. [DOI] [PubMed] [Google Scholar]
- 46. Rutherford BR, Wall MM, Brown PJ, et al. Patient expectancy as a mediator of placebo effects in antidepressant clinical trials. Am J Psychiatry. 2017;174:135‐142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Silberstein SD, Dodick DW, Aurora SK, et al. Per cent of patients with chronic migraine who responded per onabotulinumtoxinA treatment cycle: PREEMPT. J Neurol Neurosurg Psychiatry. 2015;86:996‐1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Blumenfeld AM, Stark RJ, Freeman MC, Orejudos A, Manack Adams A. Long‐term study of the efficacy and safety of onabotulinumtoxinA for the prevention of chronic migraine: COMPEL study. J Headache Pain. 2018;19:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Lipton RB, Bigal ME, Ashina S, et al. Cutaneous allodynia in the migraine population. Ann Neurol. 2008;63:148‐158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Frattarelli DA, Galinkin JL, Green TP, et al. Off‐label use of drugs in children. Pediatrics. 2014;133:563‐567. [DOI] [PubMed] [Google Scholar]
- 51. Weimer K, Gulewitsch MD, Schlarb AA, Schwille‐Kiuntke J, Klosterhalfen S, Enck P. Placebo effects in children: A review. Pediatr Res. 2013;74:96‐102. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


