Abstract
The fossil record indicates that early mammals had small brains with proportionately little neocortex. Here we consider what is known about the organization of the neocortex in species with the least expanded neocortex from 6 major clades of the mammalian radiation. Common features of the neocortex across these clades include primary and secondary sensory areas, retrosplenial and cingulate cortex, and frontal cortex. Overall, early mammals likely had a core of 15–20 cortical areas that have been retained in most present-day mammals.
Key Words: Brains, Olfaction, Evolution, Sensory cortex
Introduction
Humans everywhere have been curious about their origins. Thus, we have the various creation myths that characterize most cultures. Fortunately, evolutionary science provides a way of obtaining evidence-based answers to this question. Now we know that modern humans gradually evolved over millions of years from a sequence of ancestors who were less and less like ourselves as we go back in time. As it is arguably our brains that most make us human, it is obviously important to understand how the human brain, with all its marvelous functions, came to be.
Among mammal brains, human brains are one of the largest, and they contain more neurons than do the brains of elephants and killer whales, which are at least 3 times larger [Hart and Hart, 2007; Herculano-Houzel, 2009]. Most of our brain (80%) consists of the neocortex of the telencephalon [Avzevedo et al., 2009], which is divided into as many as 150–200 functionally distinct cortical areas [Changizi and Shimojo, 2005; Finlay and Brodsky, 2007]; Brodmann [1909] called these subdivisions of the cortex the organs of the brain. The brains of early mammals were very different as they had little neocortex in proportion to the rest of the brain and few cortical areas [Kaas, 2007a]. Thus, the evolution of the human brain was marked by a great expansion of especially one part of the brain, i.e. the neocortex [Baron, 2007], and a great increase in the complexity of this part [Kaas, 2006; Kaas and Preuss, 2008]. As the neocortex is responsible for our thoughts and dreams, it is fair to say that it made us human [Kaas, 2005]. Here, we consider an early phase in the evolution of the neocortex in humans and the neocortex of the early mammals that gave rise to all modern mammals. While it is reasonable to focus on the neocortex, modifications of other parts of the nervous system were clearly critical in the evolution of the human brain.
In all mammals, the neocortex is a thick structure with 6 traditionally defined layers. However, these layers vary greatly in distinctiveness and other characteristics across areas of the neocortex and across mammalian taxa. These histological features have long been used to suggest the locations and boundaries of functionally distinct subdivisions, the cortical areas, and general functions of areas by laminar specializations identifying these as having sensory or motor roles. The neocortex also varies greatly in size in proportion to the rest of the brain and in number of cortical areas. Given this variability in present-day mammals, what can be said about the organization of the neocortex in early mammals?
The Origin of the Neocortex
The neocortex is not a new part of the brain as the dorsal cortex of reptiles is recognized as homologous to the neocortex of mammals. However, mammals are not closely related to any of the reptiles that are living today. The immediate ancestors of mammals have been commonly called mammal-like reptiles [Colbert and Morales, 1991], but early reptiles are now referred to as stem amniotes, i.e. those descendants of amphibians that adapted to living on land by having eggs with amniotic membranes to keep them from drying out. Stem amniotes divided into 2 major clades about 320 million years ago (mya): the Sauropsids leading to modern reptiles and birds and the Synapsids leading to modern mammals [Butler and Hodos, 2005]. The dorsal cortex of present-day reptiles is a thin cap on the forebrain that consists of a single layer of neurons receiving inputs and providing outputs [Ulinski, 2007]. Much of this dorsal cortex receives visual inputs, while a smaller portion receives somatosensory inputs [Northcutt and Kaas, 1995; Striedter, 1997; Kaas, 2007b]. Apparently, none of the dorsal cortex receives auditory inputs [Bruce, 2007; Medina, 2007]. While early amniotes may have had something like the dorsal cortex of present-day reptiles, it is not clear how or when such a simple sheet of neurons like the dorsal cortex evolved into the larger, thicker, and highly laminated neocortex of mammals [Northcutt and Kaas, 1995; Kaas, 2007b; Molnár et al., 2007]. As present-day mammals are the only survivors of the synapsid amniotes that produced a range of ‘mammal-like reptiles’ and, some 230 mya, early mammals, we have no comparative information about brain organization in the other nonsurviving branches of the synapsid radiation. Early mammals persisted and radiated into 6 major clades, 20 orders, and over 4,500 species of present-day mammals [Bininda-Emonds et al., 2007].
What the Fossil Record Tells Us about the Brains of Early Mammals
As the soft tissue of brains is not preserved in the fossil record, we are greatly limited in what we can learn from this record. However, the interior of the skull, i.e. the brain case, often closely conforms to the shape of the brain. Thus, the endocasts of fossilized skulls of extinct mammaliaforms can reveal important details about their brains, especially the proportional sizes of major parts such as the neocortex, as often the trace of the rhinal fissure that separates the neocortex from the piriform cortex is present. Based on knowledge acquired from modern studies of present-day mammals, we can make functional inferences based on the brain shapes that are in- dicated by endocasts. For example, evidence of expanded temporal and occipital regions of the neocortex almost certainly reflect an expanded cortical visual system. Endocasts can indicate the locations of cortical fissures, and we know especially from the studies of Welker [1990] that cortical fissures sometimes divide the cortex into functional divisions of the somatosensory cortex corresponding to representations of the face, hand, and other body parts. Based on such evidence, Radinsky [1977] was able to make inferences about the somatotopic organization of the somatosensory cortex from the endocasts of the skulls of extinct carnivores and primates. More recently, Van Essen [2007] proposed that fissures often indicate the locations of borders between cortical areas. According to Van Essen's ‘tension-based’ hypothesis, connections between cortical areas resist the expansion of the cortical surface during development, causing buckling that results in gyri [Herculano-Houzel et al., 2010]. Thus, the fossil record may provide additional information about the functional organization of the neocortex based on information about cortical fissures [Kaas, 2009].
What the fossil record tells us about the brains of early mammaliaforms is quite limited but still quite useful. For example, the endocasts of the brain of premammal cynodonts were shaped in a way that indicated that the cerebral hemispheres were small and lacked the lateral expansion of mammalian hemispheres, suggesting that the 6-layered neocortex of mammals emerged with mammals, but not before [Kemp, 2009]. The fossil record also indicates that early mammals were generally small (mouse-to-rat sized) [Smith et al., 2010], and they had small brains with proportionately little neocortex [Jerison, 2007]. While the neocortex of the small brains of early mammals was not characterized by fissures, a shallow rhinal sulcus separating the neocortex from the piriform cortex was sometimes revealed in endocasts, thereby demarcating the lateral extent of the neocortex [Kielan-Jaworowska et al., 2004]. Overall, the neocortex was restricted to a small cap on the rest of the forebrain, whereas the olfactory bulb and olfactory (piriform) cortex were relatively large, indicating that olfaction was important in these early mammals, as Herrick [1948] deduced from early comparative studies. The small cap of neocortex that characterized the brains of early mammals contrasts greatly with the expansive neocortex found in the brains of many extant mammals, yet species of mammals continue to exist today with small brains and little neocortex. Thus, the lesson from the fossil rec- ord is that comparative studies of the cortical organization of extant mammals would likely be more informative about the organization of the neocortex of early mammals if they focused on present-day mammals with small brains and little neocortex.
Reconstructing the Organization of the Neocortex of Early Mammals
The organization of the neocortex of early mammals can be inferred from traits of the neocortex that are widely shared by members of the major branches of mammalian evolution. The basic assumptions are that shared traits are often present in 2 related species because they were inherited (retained) from a common ancestor and that traits distributed across more branches of the mammalian radiation are those inherited from earlier, more distant ancestors. As similar traits can evolve independently, the field of cladistics [Hennig, 1966] provides logical guidelines for distinguishing shared traits from independently evolved traits [Cunningham et al., 1998]. In addition, present-day mammals with little neocortex likely have brains that have changed the least from those of early ancestors, and these brains would be most useful in efforts to identify shared traits. This does not mean that the brain of any extant mammal fully resembles the ancestral brain of early mammals, and it is easy to demonstrate that some aspects of cortical organization are highly derived and specialized in some mammals with small brains. As an example, the neocortex of echolocating bats is dominated by a large auditory region that is subdivided and specialized for processing information from echo signals [Suga, 1990]. The specialized somatosensory cortex, partly involved in electroreception, in the duck-billed platypus is another example [Krubitzer et al., 1995]. Fortunately, these extreme specializations are easily revealed as such in comparative studies of cortical organization. Here we consider cortical organization in the 6 major branches of mammalian evolution in an effort to identify shared traits that were likely retained from a common ancestor.
Cortical Organization in Monotremes
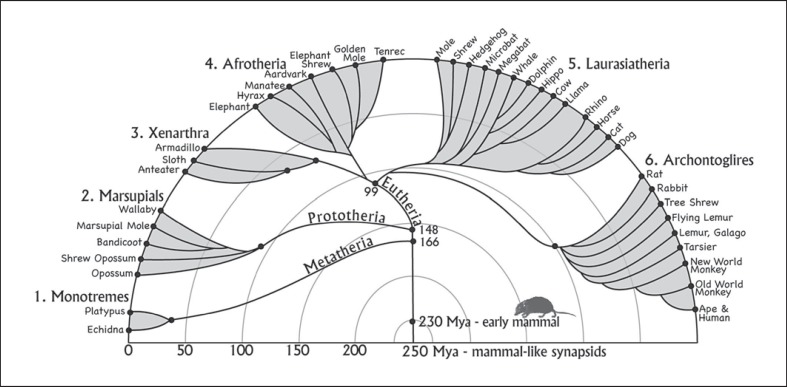
Primitive mammals evolved from mammal-like therapsids of the synapsid branch of early amniotes some 230 mya. Monotremes (mammalian subclass Prototheria) represent the earliest surviving branch of the mammalian radiation (fig. 1) as they diverged from therian mammals (marsupials and eutherians) about 170 million or more years ago [Woodburne et al., 2003]. Only 5 species of monotremes survive, i.e. the platypus and 4 species of echidnas. Monotremes hold great interest as they have a strange mix of retained and specialized features [Gould, 1985]. As in reptiles and birds, monotremes have only 1 ventral opening, i.e. the cloaca, and lay eggs. Females do secrete milk for hatched offspring, but they sweat milk from their ventral surface and have no nipples. The platypus is amphibious, and the echidna may have had semiaquatic ancestors [Phillips et al., 2009]. Both have a bill-like nose and as adults lack teeth. The most derived features of the brain relate to specializations for electroreception, which is especially important for the platypuses as they forage only in the water with eyes, ears, and nostrils closed and use their electro-receptive system to locate aquatic invertebrates and other prey.
Fig. 1.
The 6 major branches of the mammalian radiation. The evolution of present-day extant mammals is shown. The branches and times of divergence are from Murphy et al. [2001], and somewhat different estimates are from Bininda-Emonds et al. [2007]. See Woodburne et al. [2003] for evidence of a greater antiquity of mammalian clades.
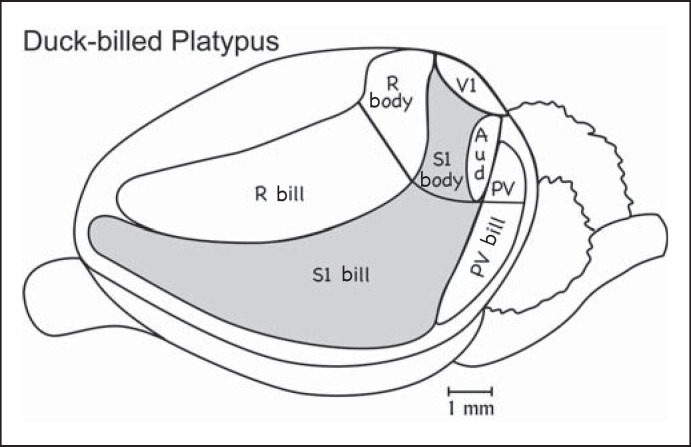
The neocortex of the platypus is proportionately larger than that of early mammals but not as expansive as in many extant mammals, and it lacks cortical fissures (fig. 2). As in other mammals, it has been possible to identify a region of auditory cortex, a primary visual area, and a primary somatosensory area (S1). These are features of all mammalian brains and therefore were retained from a common, early mammal ancestor. What is most unusual about the neocortex of the platypus is the representation of both the tactile and electro-receptors of the bill in S1 and the large representation of the bill in S1 and two other somatosensory areas [Krubitzer et al., 1995]. In addition to S1, a somatosensory area on the posterior border of S1 has been identified as the parietal ventral somatosensory area (PV) on the basis of somatotopic organization and position relative to S1. PV has been identified in the neocortex of a number of mammals, suggesting an ancient origin. However, the second somatosensory area (S2) has been more commonly identified in various mammals, and many mammals have both PV and S2. The possibility remains that the area identified as PV is S2, or that both S2 and PV exist in monotremes. A third somatosensory field, the rostral area (R), is located along the border of rostral S1, and again the representation of the bill occupies most of the area. R is activated by taps and other stimuli that activate receptors in muscles and deep in the skin, suggesting that R is involved in proprioception. In many mammals, a narrow strip of cortex just rostral to S1 is activated by muscle spindle receptors and is important in proprioception. However, this rostral somatosensory area, referred to as area 3a in primates and cats, is not as expansive as R in monotremes. Collectively, PV, S1, and R occupy about two thirds of the cortical sheet [Krubitzer and Campi, 2009], mostly with representations of the bill, a remarkable specialization of the neocortex.
Fig. 2.
Cortical organization in the platypus. Much of the cortex is occupied by the somatosensory areas S1, SR (rostral somatosensory area), and PV. Note the large representations of the bill compared to the body. The small amount of visual cortex is the primary visual area, V1. The auditory cortex (Aud) is also very small. The areal organization of other parts of the neocortex has not been established [based on Krubitzer et al., 1995].
Another unusual feature of the neocortex in monotremes is that S1, V1, and the primary auditory cortex are adjacent to one another without regions of other cortex separating them. Although the relative positions of S1, V1, and the auditory cortex are similar to those in other mammals (fig. 3, 4, 5, 6), secondary sensory areas separate these primary areas in most mammals. While a separate motor area (M) just rostral to R has been proposed [e.g. Rowe, 1990], there are reasons to question this conclusion. In the platypus, the cortical region from which movements can be evoked by electrical stimulation [Lende, 1969] appears to be largely coextensive with S1, R, and PV. As motor responses can be evoked from the somatosensory cortex in other mammals, there is no need to postulate a separate motor area in the platypus cortex. In the other monotreme, the echidna, there is stronger but still questionable evidence that the region where movements can be evoked by electrical stimulation extends past R into cortex with a reduced layer 4, a histological feature of the motor cortex [Lende, 1963; Ulinski, 1984]. As the possibility of a motor cortex in monotremes needs further consideration, we return to this issue in considering the organization of the neocortex of marsupials.
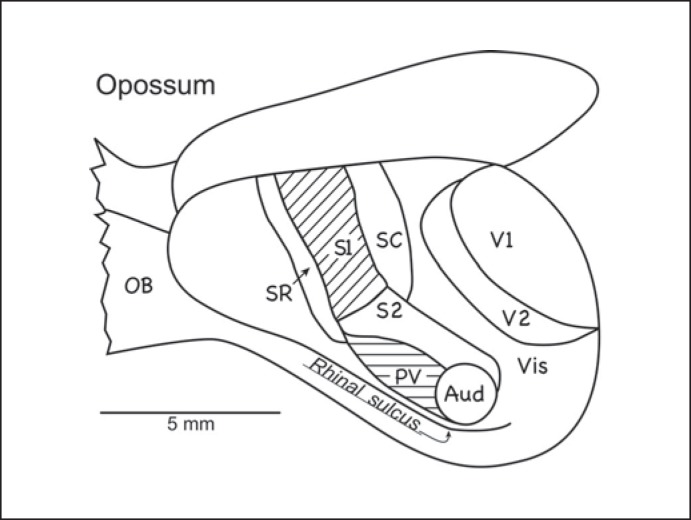
Fig. 3.
Cortical organization in the North American opossum. The somatosensory cortex includes S1, S2 (second somatosensory area), PV, SR, and SC (caudal somatosensory area). The visual cortex includes V1, V2 (the second visual area), visual area prostriata on the medial wall of the cerebral hemisphere, and VIS (visual cortex of the caudal temporal region). An auditory region (Aud) includes 1 or more primary areas. There is no compelling evidence of a separate motor area, M1. OB = Olfactory bulb [based on Beck et al., 1996].
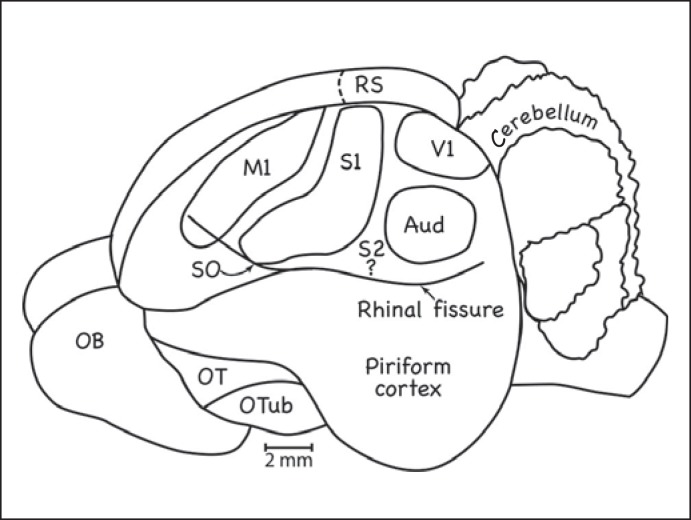
Fig. 4.
Cortical organization in the armadillo (Dasypus novemcinctus mexicanus). As in other placental mammals, a primary motor cortex (M1) is rostral to S1. Auditory (Aud) and primary visual (V1) areas were defined. Retrosplenial (RS) areas have been defined in the cortex of the medial wall. OT = Olfactory track; OTub = olfactory tubercle; SO = superorbital sulcus [based on Royce et al., 1975].
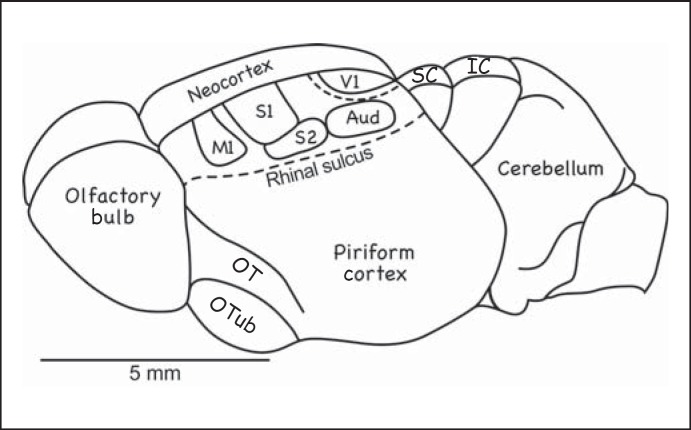
Fig. 5.
Cortical organization in the tenrec. The neocortex is proportionately very small in this mammal, as in early mammals. Most of the cortex is occupied by M1, S1, S2, V1, and the auditory core (Aud). A dashed line marks the expected location of V2. The cortex fails to cover the superior colliculus (SC) and the inferior colliculus (IC). OT = Olfactory track; OTub = olfactory tubercule [based on Krubitzer et al., 1997].
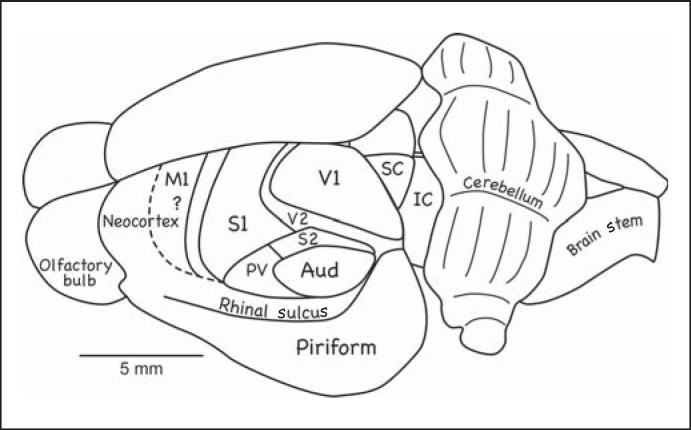
Fig. 6.
Cortical organization in the hedgehog. The dashed line marks the expected location of M1. Conventions are as in previous figures [based on Kaas et al., 1970; Catania et al., 2000].
While the platypus has a smooth, rather small brain with only a moderate amount of prefrontal cortex, echidnas have a larger brain with a number of fissures and much more prefrontal cortex. The large amount of prefrontal cortex in a slow-moving mammal with apparently limited cognitive abilities has always been a puzzle. However, echidnas have an enlarged piriform cortex and olfactory bulbs and they appear to depend on olfaction to find food. Krubitzer and Campi [2009] suggest that the expanded frontal cortex in echidnas is devoted to processing olfactory information and as such it is a uniquely specialized feature of the echidna brain.
In summary, monotreme brains appear to have primary somatosensory, auditory, and visual areas, secondary somatosensory areas adjoining S1 rostrally and caudally, and possibly secondary auditory and visual areas, but little cortex that could be devoted to higher-order sensory processing. The prefrontal (orbital frontal) cortex is not unusually large in the platypus. The cortex of the medial wall likely includes divisions of retrosplenial and cingulate cortex [Hassiotis et al., 2004]. Although somatosensory and other sensory areas are architectonically distinct, other areas of cortex do not appear to be highly differentiated. Allowing for an entorhinal region, a taste cortex, and the possibility of a motor cortex, the platypus appears to have roughly 15 or so subdivisions of the neocortex of functional significance.
Cortical Organization in Marsupials
Marsupials or metatherian mammals constitute the second oldest branch of mammalian evolution, having separated from the eutherian branch (placental mammals) some 150 million or more years ago (fig. 1). Marsupial reproduction represents an intermediate state between egg-laying monotremes and the longer gestation time of placental mammals. Marsupial eggs are large with a yolk and have a shell membrane that lasts over most of a very short gestation. While constituting only about 6% of mammalian species, marsupials have radiated to form 3 American orders and 4 Australasian orders, which emerged from a single marsupial migration from South America to Australia via Antarctica when they were joined forming Gondwana [Nilsson et al., 2010]. The 265 species of present-day marsupials are quite diverse in terms of body form, size, and behavior, ranging from the Tasmanian devil to the red kangaroo. American opossums and Australian possums are considered the most primitive as their body forms approximate those of early marsupials [Kemp, 2005]. In addition, their brains are small, with relatively little neocortex, and the cortex does not have fissures.
North American opossums have been often studied because they are considered to be generally primitive, they are available, and they are members of 1 of the 3 major branches of the mammalian radiation [for reviews see Johnson, 1990; Rowe, 1990]. The cortex of North American opossums (fig. 3) includes a primary somatosensory area, S1 [Beck et al., 1996], with a representation of the contralateral body surface that proceeds from hind limbs to the face in a mediolateral sequence, as in other mammals, and has a large representation of the face, facial vibrissae, and nose, as in many mammals. Two other somatosensory representations, i.e. the second somatosensory area, S2, and the parietal ventral area, PV, are located just ventral to S1 where they extend caudally to the auditory cortex. These 2 somatosensory areas have been described in a number of other mammals, but they are unusually large relative to S1 in opossums. S1 is bordered by rostral (SR) and caudal (SC) somatosensory fields that are densely interconnected with S1. The rostral field is in the position of a proprioceptive field, sometimes called area 3a [Krubitzer and Kahn, 2003], in other mammals. The auditory cortex has histological characteristics of the primary cortex, and it may contain more than 1 primary area, as in most placental mammals [Kaas, 2011]. However, evidence of only one primary area was obtained in a study of the brush-tailed possum [Gates and Aitkin, 1982]. Part of PV responds to auditory stimulation, and there may be a fringe of secondary auditory cortex outside of the primary region. The visual cortex includes the primary area, V1, the second visual area, V2, and possibly temporal cortex ventral to V2 [Rosa et al., 1999; Martinich et al., 2000]; a visual area, prostriata, borders V1 medially [Rosa and Krubitzer, 1999]. All of these visual areas appear to be common in placental mammals.
There is no evidence of a separate motor area rostral to SR in opossums, although movements can be evoked by electrically stimulating S1 and SR [Beck et al., 1996]; corticospinal projections appear to originate only from S1 and the S2-to-PV region [Lende, 1963; Nudo and Masterton, 1990]. In a similar manner, Frost et al. [2000] found ‘no evidence of a motor representation rostral to S1’ in the South American opossum Monodelphis domestica. In several other marsupials, such as the bush-tailed possum, investigators have argued for a partially separate motor area rostral to S1 [Rowe, 1990]. This more rostral ‘motor’ cortex likely corresponds to SR of opossums and R of monotremes rather than M1.
The cortical organization of the South American opossum (M. domestica) is very similar to that of the North American opossum, except that evidence of the somatosensory area PV was not found [Huffman et al., 1999; Catania et al., 2000b; Frost et al., 2000]. In addition, other subdivisions of the cortex have been identified architectonically [Wong and Kaas, 2009], including dorsal and ventral retrosplenial areas, the prostriata visual area, dorsal and ventral areas of cingulate cortex, and a densely myelinated division of the frontal cortex. There is also a strip of perirhinal cortex along the rhinal sulcus. The Australian marsupials, the brush-tailed possum [Elston and Manger, 1999], the northern quoll, and the striped possum [Huffman et al., 1999] also have visual, auditory, and somatosensory areas arranged much like those in North American opossums, and both S2 and PV were identified in these marsupials. Similarities between American and Australian marsupial brains are especially revealing as they represent the 2 major branches of the marsupial radiation that have been separate for much of the 150 million years since marsupial and eutherian mammals diverged [Nilsson et al., 2010]. Thus, shared similarities in neocortex organization likely reflect cortical features that were present near or before the separation of the marsupial and eutherian lines.
Cortical Organization in Xenarthra
Eutherian (placental) mammals distinguish themselves from metatherian marsupials and prototherian monotremes by nourishing a fetus in the womb rather than laying an egg or giving birth to underdeveloped offspring nourished outside the body. While all mammals have an anterior commissure connecting the neocortex of the 2 cerebral hemispheres, only eutherian mammals have a corpus callosum [Ebner, 1969]. Eutherian mammals diverged into 4 major groups; the Xenarthra and Afrotheria emerged in the southern hemisphere super continent of Gondwana, while the Laurasiatheria and Euarchontoglires arose in the northern hemisphere super continent Laurasia [Wildman et al., 2007]. The separation of Xenarthra from Afrotheria occurred approximately 100 mya with the separation of South America from Africa. Present-day Xenarthrans consist of sloths, anteaters, and armadillos of the New World. Their derived traits include adaptations for digging and burrowing, eating (the loss of incisors and canines), and, in armadillos, armor-like leathery plates to protect the body. Reproduction in armadillos is unique among mammals in that 1 sexually produced embryo divides into 4 genetically identical offspring [Loughry et al., 1998]. According to Sherwood et al. [2009], Xenarthrans and Afrotherians demonstrate more conserved architectural traits in the neocortex than do other eutherians.
The cortical organization in Xenarthrans is not well documented. Most of what is known comes from studies of the 9-banded armadillo [Royce et al., 1975]. However, armadillos have little neocortex in proportion to their large olfactory bulbs and piriform cortex [Reep et al., 2007]. The neocortex in armadillos has at least a primary somatosensory area, S1, and a more rostral motor area, M1 (fig. 4). A partial overlap in these areas, not shown here, probably reflects the limited resolution of brain surface electrodes as well as the motor role of the sensory cortex. A small primary visual area, i.e. V1, and an auditory area were also detected. The primary auditory and visual areas were densely myelinated, and a retrosplenial region was clear in the myelin preparation. Thus, armadillos appear to have primary sensory areas with little space between them for additional areas, although at least 1 secondary somatosensory area, S2, and a secondary visual area, V2, are likely. The retrosplenial cortex on the medial wall extends toward the border of V1 (striate cortex), although a prostriata visual area may separate them.
Sloths have larger brains with 2 main fissures. Cortical organization has been studied only with surface electrodes, but evidence for S1, S2, one auditory region, and one visual region was found [Meulders et al., 1966; Saraiva and Magalhaes-Castro, 1975]. The auditory and visual regions may contain more than 1 area.
Cortical Organization in Afrotheria
The Afrotherian clade diverged from other placentals about 100 mya (fig. 1). Afrotherian mammals soon diversified into 6 major branches that now differ greatly in their adaptations. They include elephants, manatees and dugongs, hyraxes, aardvarks, elephant shrews, golden moles, and tenrecs. Tenrecs and golden moles had long been considered members of the order Insectivora (shrews, moles, and hedgehogs) of the Laurasiatherian clade because these mammals resemble insectivores by retaining primitive features. However, recent molecular evidence places golden moles and tenrecs firmly in the Afrotherian clade and shows that they are thus more closely related to aardvarks and elephants than shrews and moles [Stanhope et al., 1998].
The focus here is on the hedgehog tenrec (Echinops) because tenrecs resemble early mammals in body size and skeletal features. Most importantly, they have small brains relative to body size, with proportionately little neocortex (fig. 5), much like early mammals. Tenrecs have primary and secondary somatosensory areas, i.e. S1 and S2, a primary auditory region, and a primary visual cortex [Krubitzer et al., 1997]. As in other eutherian mammals, they have a primary motor area, M1, rostral to S1 [Künzle, 2009]. Tenrecs have a separate motor thalamus with inputs from the cerebellar nuclei [Künzle, 1998], but it is uncertain how precisely the motor thalamus projects to the motor cortex. A narrow strip of cortex between S1 and M1 may correspond to the rostral belt of somatosensory cortex with proprioceptive inputs in other mammals. A narrow belt of secondary somatosensory cortex is also expected along the caudal border of S1. There is also architectonic and connectional evidence of visual area prostriata and retrosplenial cortex [see Künzle, 2009]. Overall, the small cap of neocortex in tenrecs is occupied by a few primary and secondary sensory areas, a motor area, cingulate and retrosplenial cortex, and a frontal region.
Members of other branches of Afrotheria have not been studied in any detail, and this is especially the case for those species with large, highly fissured brains. However, cortical organization has been studied in elephant shrews, named after their long, flexible snout rather than their shrew-like size. Elephant shrews are closely related to tenrecs and golden moles. They have a somewhat larger brain than tenrecs, with proportionately more neocortex. Their cortex is dominated by a primary somatosensory area, i.e. S1, a more lateral somatosensory region, i.e. S2 or S2 and PV, an auditory region, and a large visual region that extends to the borders of the somatosensory and auditory cortex [Dengler-Crish et al., 2006]. Elephant shrews have large eyes and a well-developed visual system. As the primary visual cortex, V1, has not been defined, the visual cortex may contain visual areas in addition to the commonly found V2. The frontal cortex is limited in size, and much of it may be occupied by the motor cortex. Overall, their cortical organization is similar to that of tenrecs, with more of their cortex devoted to vision.
Cortical Organization in Insectivores and Other Members of the Laurasiatherian Clade
The large and highly variable Laurasiatherian clade includes bats, pangolins, carnivores, odd-toed ungulates, even-toed ungulates, whales, hippos, and insectivores. The cortex is least expanded in insectivores and echolocating bats. Insectivores have long been considered an early offshoot of the placental radiation based on the retention of many primitive traits. However, other mammals, such as tenrecs, have also retained primitive traits, including a small brain with little neocortex. Tenrecs, golden moles, and elephant shrews are no longer grouped with insectivores. Presently, only hedgehogs, moles, shrews, and solenodons are considered members of the insectivore clade, with shrews being more closely related to hedgehogs than the more specialized moles [Gould, 1986; Douady et al., 2002]. Hedgehogs and shrews would appear to have brains more similar to those of early mammals than those of most members of the Laurasiatherian clade, including even the brains of echolocating bats as these bats have a specialized auditory system.
Hedgehogs have long generated research interest because of their small brains with little neocortex [Clark, 1932]. Their small cap of neocortex (fig. 6), which fails to cover the midbrain, is largely occupied by somatosensory areas (S1, S2, and PV), the primary auditory cortex, primary and secondary visual cortex (V1 and V2), and primary motor cortex [Kaas et al., 1970; Batzri-Izraeli et al., 1990; Nudo and Masterton, 1990; Dinopoulos, 1994; Catania et al., 2000a].
The cortical organization in shrews is not that much different, except that the visual cortex is reduced compared to that of hedgehogs, there is little or no visual cortex lateral to V1, and the secondary somatosensory cortex, i.e. S2 or S2 plus PV, is enlarged [Catania et al., 1999]. The auditory cortex is located near the caudolateral margin of the neocortex, while S2 separates the auditory cortex from the visual cortex. These sensory areas are all in the caudal half of the neocortex, with M1 taking up only part of the rostral half. Thus, the frontal cortex rostral to M1 is proportionately larger in shrews than in hedgehogs. The medial cortex likely includes retrosplenial and cingulate areas. Overall, there are few subdivisions of the cortex.
Moles differ from hedgehogs and shrews by having small eyes covered with skin, a very thin optic nerve, no external ear, and a narrow auditory ear canal. Thus, they appear to largely depend on somatosensory information. The neocortex in moles is proportionately small relative to the olfactory bulb and piriform cortex, as in other insectivores, but it is organized somewhat differently [Catania and Kaas, 1997; Catania, 2000, 2005]. The somatosensory cortex has a more caudal placement than in most mammals. There is evidence of at least 2 representations, i.e. S1 and S2. Motor cortex is medial and rostral to S1. Cortex caudal to S1 at the caudal pole of the neocortex responds to vibrations, suggesting that this is auditory cortex that has specialized for a tactile function. Frontal cortex of uncertain functions occupies about a third of the neocortex. Cortex responsive to visual stimuli has not been found. The large caudomedial region normally occupied by the visual cortex has unknown functions. In comparison, the unique star-nosed mole also has a large somatosensory region that extends rostrocaudally nearly to the caudal pole of the neocortex where the auditory cortex is located. This somatosensory region includes S1, S2, and a third representation of only the sensory rays of the nose. Architectonically, a small oval of cortex suggests the location of V1, but this cortex does not seem to be visually responsive.
In summary, hedgehogs, shrews, and moles all have small brains with proportionately little neocortex. The most generalized pattern of cortical organization is found in hedgehogs, where sensory and motor areas occupy most of the neocortex, and they are arranged in the pattern reflected in small-brained mammals in other taxa. Shrews have less a developed visual cortex, with V1 as the only remaining visual area in at least some species, while moles have small eyes covered with skin and no functional visual cortex. The auditory cortex is reduced or modified for vibration in shrews and moles.
Old World echolocating microbats have small brains that are highly specialized for processing auditory signals [Riquimaroux et al., 1991], while fruit-eating megabats have slightly larger brains with an expanded visual region and perhaps 6 visual areas [Rosa, 1999]. It was largely the features of the well-developed visual system that led some investigators to propose that megabats, but not microbats, are closely related to primates [for discussion see Kaas and Preuss, 1993; Rosa, 1999], but molecular evidence places both mega- and microbats firmly in Laurasiatheria (fig. 1).
Most of the other mammals of the Laurasiatherian radiation (horses, cows, dolphins, and cats) have large brains with many fissures, suggesting that the ancestral brain organization has been greatly modified. However, not much is known about their brains, except for those of cats and ferrets [Homman-Ludiye et al., 2010], which have been studied extensively. These carnivores have an expanded number of visual and auditory areas, while the somatosensory cortex includes at least S1 and S2 and bands of somatosensory cortex rostral and caudal to S1. The band rostral to S1 has been called area 3a, as in primates, and this area is involved in proprioception. An area along the caudal border of S1, i.e. 5B [Foxworthy and Meredith, 2011], likely corresponds to the caudal somatosensory area SC of other mammals. In cats, as many as 17 visual areas [see Lyon, 2007] and as many as 12 auditory areas [Kaas and Hackett, 2008; Kaas, 2011] have been described.
Rodents and Rabbits (Glires) and Other Archontoglires
The Archontoglire radiation of placental mammals emerged about 90 mya (fig. 1) and includes present-day rodents and lagomorphs (Glires), as well as flying lemurs, tree shrews, and primates (Archontans). Most of the clade is too specialized to be of prime value in providing information about the organization of cortex in early mammals. All primates have large brains relative to body size, with expanded neocortex, although this is most pronounced in anthropoid primates [Jerison, 1973]. Primates have a greatly increased number of cortical areas [Kaas, 2007b], with as many as 35 visual areas proposed for macaque monkeys [Felleman and Van Essen, 1991; Lyon, 2007]. The neocortex in tree shrews is moderately expanded and has as many as 10 visual areas [Lyon et al., 1998]. Flying lemurs, also called colugos as they are not lemurs and glide rather than fly, have large eyes, suggesting a well developed visual system, but their cortical organization has not been studied. Rats and mice, as well as rabbits (lagomorphs), have been well studied. They have a somewhat expanded neocortex but without the fissures of some of the larger rodents.
The proposed organization of the neocortex in rats (fig. 7) includes 2 motor areas, i.e. M1 and premotor area M2 [Wise and Donoghue, 1986], and somatosensory areas S1, PV, and S2 [Remple et al., 2003]. The rostral border of S1 is formed by a dysgranular region, extends into parts of S1, and is likely the target of proprioceptive inputs. Connections with S1 identify a band of cortex along the caudal border of S1 as a somatosensory area, SC. The cortex between the SC and visual cortex, the only cortex that can be considered posterior parietal cortex (PPC), has neurons that respond to both visual and somatosensory stimuli [Wallace et al., 2004]. The primary visual cortex (V1) is easily identified architectonically, and most of the lateral border of V1 is formed by V2 [Rosa and Krubitzer, 1999], although there are other interpretations [Wang et al., 2011]. A visual area medial to V1 has been called prostriata in other mammals. Other visual areas exist lateral to V2, including a temporal posterior area [Campi and Krubitzer, 2010]. Subdivisions of the retrosplenial cortex adjoin prostriata on the medial wall of the cerebral hemisphere where they abut subdivisions of the cingulate cortex rostrally [Zilles and Wree, 1995]. The auditory cortex has been extensively studied, and it includes two primary areas and several associated secondary areas [Kaas, 2011]. The frontal cortex includes anterior cingulate cortex medially and orbital frontal cortex laterally [Preuss, 1995], and additional divisions have been proposed [Reep et al., 1996]. The cortical organization in mice appears to be very similar, although some areas may be missing in this very small mammal.
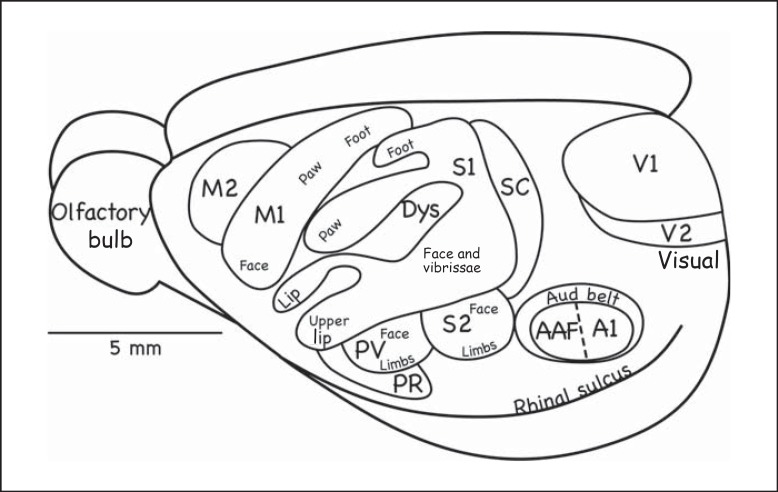
Fig. 7.
Cortical organization in the rat. The primary auditory cortex includes A1 and the anterior auditory field (AAF). The auditory belt (Aud belt) of the secondary auditory cortex includes several proposed areas. M2 = Second motor area; PR = parietal rostral area; Dys = dysgranular cortex (corresponding to SR in fig. 2, 3) [based on Remple et al., 2003].
Cortical organization is somewhat variable in rodents [Krubitzer et al., 2011]. Squirrels differ from rats and mice in that they have an expanded visual region of cortex that appears to contain a number of visual areas [Kaas, 2002; Wong and Kaas, 2008]. Mole rats, rodents that are adapted to an underground life in a network of tunnels, have expanded somatosensory areas to occupy the posterior pole of the neocortex where the visual cortex is normally located [Catania and Remple, 2002]. They seem to have little or no cortex devoted to vision, and little audition, so that perhaps half or more of the cortical surface is occupied by somatosensory and motor areas, with retrosplenial, cingulate, and frontal areas making up most of the rest. Larger rodents have not been extensively studied, but the overall arrangement of auditory, visual, and somatosensory areas in the South American agouti and capybara are similar to those in other rodents, although the temporal cortex, likely visual in function, is expanded [Santiago et al., 2007]. There are many more rodents that could be studied as there are at least 1,814 species distributed across 29 families and 3 suborders.
Lagomorphs (rabbits, hares, and pikas) form the other branch of the Glire radiation that separated from the ancestors of rodents about 67 mya [Asher et al., 2005]. Rabbits have a smooth brain with little frontal cortex and an expanded occipital-temporal region reflecting an emphasis on the visual cortex. Early studies established that visual areas V1 and V2 occupy a large region of the occipital cortex [Thompson et al., 1950]. Connections with the visual thalamus suggest the location of prostriata just medial to V1, followed by the retrosplenial cortex [Towns et al., 1982]. Visual connections also implicate the temporal cortex just lateral to V2 in visual processing. S1 is located just rostral to V1 and V2, with a second somatosensory area, S2, ventral to S1. Both somatosensory areas are highly devoted to representing facial hairs and lips [Gould, 1986]. Studies of the auditory cortex have been limited, but there is evidence of 1 primary area, A1, just caudal to S2 [Velenovsky et al., 2003].
Conclusions
The fossil record indicates that most early mammals were small, mouse-to-rat sized, and had small brains with little neocortex in proportion to body size. However, nothing is known directly from this fossil record about the functional organization of the neocortex in such early mammals. Thus, this early organization needs to be inferred from what is known about cortical organization in extant mammals. Present-day mammals have been grouped into 6 major clades that diverged from each other early in mammalian evolution from 90 to 180 mya. Logically, a reconstruction of the cortical organization of the earliest mammals would be based on features common to members of all 6 major clades. However, each of the 6 branches of mammalian evolution branched further, sometimes into many diverse families and species and sometimes into a more limited array of present-day species. Many of the present-day species and families are characterized by brains much larger than those of their early mammal ancestors, suggesting that their brains have not only enlarged but have also increased in complexity. This presumption is supported by experimental evidence of some of these mammals, such as well-studied cats and monkeys, where there is clear evidence of an increase in numbers of cortical areas. Thus, the focus here has been on the brains of mammals across the 6 major clades that appear to have changed the least from the small brains of their common ancestors. Toward that goal, the brains of Afrotherian tenrecs appear, superficially at least, to most closely resemble the brains of early mammals, as deduced from skull endocasts, in that tenrecs have proportionately the least neocortex, and the olfactory bulb and olfactory (piriform) cortex occupy a large part of the forebrain. These proportions approximate those of the brains of early mammals, supporting the conclusion that olfaction was very important for early mammals. In the other clades, present-day members generally had brains with at least somewhat more neocortex, but present-day insectivores and opossums have only slightly more neocortex. Armadillos (Xenarthra) have relatively little neocortex, but their external armor is clearly a specialization, and their brains have received only limited study. The platypus (Monotreme) has only a somewhat expanded the neocortex, but their somatosensory system is highly specialized and it incorporates electroreception. Rats and mice (Laurasiatheria) have the advantage of being well studied, and they have only a modest expansion of the neocortex. Other mammals (shrews, moles, rabbits, and bats) were only briefly considered here, and results from these species reveal both specializations and characters in common with other mammals.
Of the sensory and motor systems, somatosensory and visual cortex have been studied the most, and the results suggest that early mammals had 4 or 5 somatosensory fields and 3–5 visual fields counting the area prostriata. All mammals appear to have an S1, and there is reasonable evidence that S1 typically connects directly with narrow bands of cortex immediately rostral and caudal to S1, as well as with S2 and perhaps PV lateral and caudal to S1. It is possible and even likely that, near the rhinal sulcus in the insular cortex, most mammals have a region where neurons are responsive to both taste substances and touch, as described in rats [Katz et al., 2002], but results from other taxa are too limited to support such a conclusion. The auditory cortex, as outlined here, likely corresponds to the primary auditory cortex. The region is densely myelinated, and it has other architectonic features of primary sensory cortex. In many mammals, the auditory cortex includes 2 or more primary fields, as well as a number of secondary areas [Kaas, 2011]. Among the species illustrated here, the auditory cortex has been subdivided on the basis of experimental evidence only in rats, where 2 primary areas, AAF and A1, exist within the myelinated core, and several secondary auditory fields have been proposed. The other illustrated mammals clearly have an auditory core of the primary cortex, as well as perhaps secondary areas, but we do not know if 2 or more core areas are common to most mammals and thereby likely existed in early mammals. Except in mammals with small, skin-covered eyes that no longer have object vision, or with very small brains as in some shrews, all mammals appear to have V1, V2, and possibly prostriata, as well as at least a small segment of caudal temporal cortex which commonly receives inputs from V1 and V2 and is visual. Thus, early mammals likely had 4 visual areas and possibly more.
All placental mammals appear to have a primary motor area, M1. At least 1 premotor area also exists in a number of placental mammals, but the evidence is too limited to conclude that the premotor cortex is characteristic of all placental mammals. The presence or absence of a motor cortex in marsupials and monotremes is questionable, with claims made both ways. Perhaps the best evidence comes from opossums, where there is general agreement that somatosensory areas S1 and SR are involved in motor behavior as electrical stimulation of sites in these areas can evoke movements. However, no movements are evoked from the cortex rostral to SR in the expected location of M1, and movements can be evoked from S1 and SR in placental mammals. Thus, opossums appear to lack a separate motor area, M1. By implication, other marsupials would also lack a separate motor area, but there is not widespread agreement with regard to this as some evidence suggests that a separate or partially separate M1 exists in some of the marsupials with larger brains. As this evidence is not compelling, it seems most likely that the motor cortex evolved after the split between the marsupial and placental lines. Overall, it seems reasonable to propose that the motor cortex does not exist in opossums, it did not evolve independently in some other marsupials, and it was not present in early marsupials and lost in opossums [see Beck et al., 1996]. The evidence of a motor cortex in monotremes is limited and somewhat inconsistent, but it seems unlikely that M1 existed in early mammals, was retained in monotremes, but was lost in marsupials after they branched from placentals. M1 seems to be a valuable innovation of early placental mammals.
Other areas of cortex are more difficult to identify with certainty, and thus a cladistic analysis is more challenging. However, the retrosplenial cortex is architectonically distinct, and generally granular and agranular retrosplenial areas are identified. Although additional retrosplenial areas have been proposed, early mammals likely had 2 retrosplenial areas. Typically, 3 or more subdivisions of the cingulate cortex are described, and early mammals probably had 2–3 cingulate areas. The frontal cortex may have several divisions, but most often a lateral orbital-frontal division and 1 or more medial divisions are described. Other areas include the cortex along the rhinal sulcus, which may have several functional divisions [Burwell et al., 1995].
In summary, the common ancestor of all mammals had a small cap of neocortex on a forebrain dominated by olfaction. This small cap of neocortex was divided into as few as 15 cortical areas of functional significance, although the number could be larger (20–25). Most of this core of basic areas was passed on to subsequent mammals where the areas became variously adapted and specialized for the needs of the many branches of the mammalian radiation. Cortical sensory areas often became more differentiated and specialized for sensory processing, and various parts of the receptor sheet occupied proportionately more of the cortical representations according to the emerging sensory specialization of various mammals. A separate motor area, i.e. M1, and possibly 1 or more premotor areas, as well as the corpus callosum, emerged with eutherian mammals. Quite commonly, mammals with larger brains evolved, and in at least some mammals this was associated with an increase in the number of cortical areas. Human brains may have the greatest number of areas. The full number is hard to determine, but a number between 100 and 200 seems likely. This would roughly constitute a 10-fold increase in the number of areas over the course of evolution from the early mammal ancestors.
References
- Asher RJ, Meng J, Wible JR, McKenna MC, Rougier GW, Dashzeveg D, Novacek MJ. Stem Lagomorpha and the antiquity of Glires. Science. 2005;307:1091–1094. doi: 10.1126/science.1107808. [DOI] [PubMed] [Google Scholar]
- Avzevedo FA, Carvalho LR, Grinberg LT, Farfel JM, Ferretti RE, Leite RE, Filho WJ, Lent R, Herculano-Houzel S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J Comp Neurol. 2009;513:532–541. doi: 10.1002/cne.21974. [DOI] [PubMed] [Google Scholar]
- Baron G. Encephalization: comparative studies of brain size and structure volume in mammals. In: Kaas JH, Krubitzer LA, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 3. 2007. pp. pp 125–135. Mammals. [Google Scholar]
- Batzri-Izraeli R, Kelly JB, Glendenning KK, Masterton RB, Wollberg Z. Auditory cortex of the long-eared hedgehog (Hemiechinus auritus) Brain Behav Evol. 1990;36:237–248. doi: 10.1159/000115310. [DOI] [PubMed] [Google Scholar]
- Beck PD, Pospichal MW, Kaas JH. Topography, architecture, and connections of somatosensory cortex in opossums: evidence for five somatosensory areas. J Comp Neurol. 1996;366:109–133. doi: 10.1002/(SICI)1096-9861(19960226)366:1<109::AID-CNE8>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- Bininda-Emonds OR, Cardillo M, Jones KE, MacPhee RD, Beck RM, Grenyer R, Price SA, Vos RA, Gittleman JL, Purvis A. The delayed rise of present-day mammals. Nature. 2007;446:507–512. doi: 10.1038/nature05634. [DOI] [PubMed] [Google Scholar]
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde. Leipzig, Barth. 1909 [Google Scholar]
- Bruce LL. Evolution of the nervous system in reptiles. In: Kaas JH, Bullock TH, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 2. 2007. pp. pp 125–156. Non-Mammalian Vertebrates. [Google Scholar]
- Burwell RD, Witter MP, Amaral DG. The perirhinal and postrhinal cortices of the rat: a review of the neuroanatomical literature and comparisons with findings from the monkey brain. Hippocampus. 1995;5:390–408. doi: 10.1002/hipo.450050503. [DOI] [PubMed] [Google Scholar]
- Butler AB, Hodos W. Hoboken, Wiley. ed 2 2005. Comparative Vertebrate Neuroanatomy: Evolution and Adaptation. [Google Scholar]
- Campi KL, Krubitzer L. Comparative studies of diurnal and nocturnal rodents: differences in lifestyle result in alterations in cortical field size and number. J Comp Neurol. 2010;518:4491–4512. doi: 10.1002/cne.22466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catania KC. Cortical organization in moles: evidence of new areas and a specialized S2. Somatosens Mot Res. 2000;17:335–347. doi: 10.1080/08990220020002042. [DOI] [PubMed] [Google Scholar]
- Catania KC. Evolution of sensory specializations in insectivores. Anat Rec. 2005;287A:1038–1050. doi: 10.1002/ar.a.20265. [DOI] [PubMed] [Google Scholar]
- Catania KC, Collins CE, Kaas JH. Organization of sensory cortex in the East African hedge hog (Atelerix albiventris). J Comp Neurol. 2000a;421:256–274. doi: 10.1002/(sici)1096-9861(20000529)421:2<256::aid-cne10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- Catania KC, Jain N, Franca JG, Volchan E, Kaas JH. The organization of somatosensory cortex in the short-tailed opossum (Monodelphis domestica) Somatosens Mot Res. 2000b;17:39–51. doi: 10.1080/08990220070283. [DOI] [PubMed] [Google Scholar]
- Catania KC, Kaas JH. The organization of somatosensory cortex and distribution of corticospinal neurons in the eastern mole (Scalopus aquaticus) J Comp Neurol. 1997;378:337–353. doi: 10.1002/(sici)1096-9861(19970217)378:3<337::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Catania KC, Lyon DC, Mock OB, Kaas JH. Cortical organization in shrews: evidence from five species. J Comp Neurol. 1999;410:55–72. [PubMed] [Google Scholar]
- Catania KC, Remple MS. Somatosensory cortex dominated by the representation of teeth in the naked mole-rat brain. Proc Natl Acad Sci USA. 2002;99:5692–5697. doi: 10.1073/pnas.072097999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changizi MA, Shimojo S. Parcellation and area-area connectivity as a function of neocortex size. Brain Behav Evol. 2005;66:88–98. doi: 10.1159/000085942. [DOI] [PubMed] [Google Scholar]
- Clark WE. The brain of the insectivora. Prog Zool Soc Lond. 1932;102:975–1013. [Google Scholar]
- Colbert EH, Morales M. Evolution of the Vertebrates. New York, Wiley-Liss. 1991 [Google Scholar]
- Cunningham CW, Omland KE, Oakley TH. Reconstructing ancestral character states: a critical reappraisal. Trends Ecol Evol. 1998;13:361–366. doi: 10.1016/s0169-5347(98)01382-2. [DOI] [PubMed] [Google Scholar]
- Dengler-Crish CM, Crish SD, O'Brian MJ, Catania KC. Organization of the somatosensory cortex in elephant shrews (E. Edwardii). Anat Rec. 2006;288A:859–866. doi: 10.1002/ar.a.20357. [DOI] [PubMed] [Google Scholar]
- Dinopoulos A. Reciprocal connections of the motor neocortical area with the contralateral thalamus in the hedgehog (Erinaceus europaeus) brain. Euro J Neurosci. 1994;6:374–380. doi: 10.1111/j.1460-9568.1994.tb00280.x. [DOI] [PubMed] [Google Scholar]
- Douady CJ, Chatelier PI, Madsen O, de Jong WW, Catzellis F, Springer MS, Stanhope M. Molecular phylogenetic evidence confirming the eulipotyphia concept and in support of hedgehogs as the sister group to shrews. Mol Phylogenet Evol. 2002;25:200–209. doi: 10.1016/s1055-7903(02)00232-4. [DOI] [PubMed] [Google Scholar]
- Ebner FF. A comparison of primitive forebrain organization in metatherian and eutherian mammals. Ann NY Acad Sci. 1969;167:241. [Google Scholar]
- Elston GN, Manger PR. The organization and connections of somatosensory cortex in the brush-tailed possum (Trichosurus vulpecula): evidence for multiple, topographically organized and interconnected representations in an Australian marsupial. Somatosens Mot Res. 1999;16:312–337. doi: 10.1080/08990229970384. [DOI] [PubMed] [Google Scholar]
- Felleman DJ, Van Essen DC. Distributed hierarchical processing in the primate cerebral cortex. Cereb Cortex. 1991;1:1–47. doi: 10.1093/cercor/1.1.1-a. [DOI] [PubMed] [Google Scholar]
- Finlay B, Brodsky P. Cortical evolution as the expression of a program for disproportionate growth and the proliferation of areas. In: Kaas JH, Krubitzer LA, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 3. 2007. pp. pp 73–96. Mammals. [Google Scholar]
- Foxworthy WA, Meredith AM. An examination of somatosensory area SIII in ferret cortex. Somatosens Mot Res. 2011 doi: 10.3109/08990220.2010.548465. E-pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost SB, Milliken GW, Plautz EJ, Masterton RB, Nudo RJ. Somatosensory and motor representations in cerebral cortex of a primitive mammal (Monodelphis domestica): a window into the early evolution of sensorimotor cortex. J Comp Neurol. 2000;421:29–51. doi: 10.1002/(sici)1096-9861(20000522)421:1<29::aid-cne3>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Gates GR, Aitkin LM. Auditory cortex in the marsupial possum Trichosurus vulpecula. Hear Res. 1982;7:1–11. doi: 10.1016/0378-5955(82)90078-8. [DOI] [PubMed] [Google Scholar]
- Gould HJ. Body surface maps in the somatosensory cortex of rabbit. J Comp Neurol. 1986;243:207–233. doi: 10.1002/cne.902430206. [DOI] [PubMed] [Google Scholar]
- Gould SJ. To be a platypus. Natural History. 1985;94:10–15. [Google Scholar]
- Hart BL, Hart LA. Kaas JH, Krubitzer LA, Evolution of Nervous Systems, editors. Evolution of the elephant brains: a paradox between brain size and cognitive behavior. London, Elsevier. 2007;3:pp 491–497. Mammals. [Google Scholar]
- Hassiotis M, Paxinos G, Ashwell KWS. Cyto- and chemo-architecture of the cerebral cortex of the Australian echidna (Tachyglossus aculeatus). 1. Areal organization. J Comp Neurol. 2004;475:493–517. doi: 10.1002/cne.20193. [DOI] [PubMed] [Google Scholar]
- Hennig W. Phylogenetic Systematics. Urbana, University of Illinois Press. 1966 [Google Scholar]
- Herculano-Houzel S. The human brain in numbers: a linearly scaled-up primate brain. Front Hum Neurosci. 2009;3:31. doi: 10.3389/neuro.09.031.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herculano-Houzel S, Mota B, Wong P, Kaas JH. Connectivity-driven white matter scaling and folding in primate cerebral cortex. Proc Natl Acad Sci USA. 2010;107:19008–19013. doi: 10.1073/pnas.1012590107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrick CJ. The Brain of the Tiger Salamander. Chicago, University of Chicago Press. 1948 [Google Scholar]
- Homman-Ludiye J, Manger PR, Bourne JA. Immunohistochemical parcellation of the ferret (Mustela putorius) visual cortex reveals substantial homology with the cat (Felis catus) J Comp Neurol. 2010;518:4439–4462. doi: 10.1002/cne.22465. [DOI] [PubMed] [Google Scholar]
- Huffman KJ, Nelson J, Clarey J, Krubitzer L. The organization of somatosensory cortex in three species of marsupials: neural correlates of morphological specializations. J Comp Neurol. 1999;403:5–32. doi: 10.1002/(sici)1096-9861(19990105)403:1<5::aid-cne2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Jerison HJ. Evolution of the Brain and Intelligence. New York, Academic Press. 1973 [Google Scholar]
- Jerison HJ. What fossils tell us about the evolution of the neocortex. In: Kaas JH, Krubitzer LA, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 3. 2007. pp. pp 1–12. Mammals. [Google Scholar]
- Johnson JI. Comparative development of somatic sensory cortex. In: Jones EG, Peters A, Cerebral Cortex, editors. New York, Plenum Press. 8B. 1990. pp. pp 335–449. [Google Scholar]
- Kaas JH. Convergences in the modular and areal organization of the forebrain of mammals: implications for the reconstruction of forebrain evolution. Brain Behav Evol. 2002;59:262–272. doi: 10.1159/000063563. [DOI] [PubMed] [Google Scholar]
- Kaas JH. From mice to men: the evolution of the large, complex human brain. J Biosci. 2005;30:155–165. doi: 10.1007/BF02703695. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Evolution of the neocortex. Curr Biol. 2006;16:910–914. doi: 10.1016/j.cub.2006.09.057. [DOI] [PubMed] [Google Scholar]
- Kaas JH. Reconstructing the organization of the forebrain of the first mammals. In: Kaas JH, Krubitzer LA, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 3. 2007a. pp. pp 27–48. Mammals. [Google Scholar]
- Kaas JH. The evolution of sensory and motor systems in primates. In: Kaas JH, Preuss TM, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 4. 2007b. pp. pp 35–57. Primates. [Google Scholar]
- Kaas JH, Cerebral fissure patterns . Encyclopedia of Neuroscience. In: Squire LR, editor. San Diego, Elsevier. 2009. pp. pp 793–800. [Google Scholar]
- Kaas JH, The evolution of auditory cortex: the core areas . The Auditory Cortex. In: Winer JA, Schreiner CE, editors. New York, Springer. 2011. pp. pp 407–427. [Google Scholar]
- Kaas JH, Hackett TA. The functional neuroanatomy of the auditory cortex. In: Dallos P, Oertel D, The Senses: A Comprehensive Reference, editors. London, Elsevier. Vol. 3. 2008. pp. pp 765–780. Audition. [Google Scholar]
- Kaas JH, Hall WC, Diamond IT. Cortical visual area I and II in the hedgehog: relation between evoked potential maps and architectonic subdivisions. J Neurophysiol. 1970;33:595–615. doi: 10.1152/jn.1970.33.5.595. [DOI] [PubMed] [Google Scholar]
- Kaas JH, Preuss TM. Archontan affinities as reflected in the visual system. In: Szalay F, Novacek M, McKenna M, Mammalian Phylogeny, editors. New York, Springer. 1993. pp. pp 115–128. [Google Scholar]
- Kaas JH, Preuss TM, Human brain evolution . Fundamental Neuroscience. In: Squire LR, editor. San Diego, Elsevier. 2008. pp. pp 1027–1035. [Google Scholar]
- Katz DB, Nicolelis MA, Simon SA. Gustatory processing is dynamic and distributed. Curr Opin Neurobiol. 2002;12:448–454. doi: 10.1016/s0959-4388(02)00341-0. [DOI] [PubMed] [Google Scholar]
- Kemp TS. The Origin and Evolution of Mammals. Oxford, Oxford University Press. 2005 [Google Scholar]
- Kemp TS. The endocranial cavity of a nonmammalian eucynodont (Chiniquodon theotenicus) and its implications for the origin of the mammalian brain. J Vertebr Paleontol. 2009;29:1188–1198. [Google Scholar]
- Kielan-Jaworowska Z, Cifelli RL, Luo Z-X. Mammals from the Age of Dinosaurs. New York, Columbia University Press. 2004 [Google Scholar]
- Krubitzer LA, Campi K, Neocortical organization in monotremes . Encyclopedia of Neuroscience. In: Squire LR, editor. Oxford, Elsevier. 2009. pp. pp 51–59. [Google Scholar]
- Krubitzer LA, Campi KL. All rodents are not the same: a modern synthesis of cortical organization. Brain Behav Evol. 2011;78:51–93. doi: 10.1159/000327320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krubitzer LA, Kahn DM. Nature versus nurture revisited: an old idea with a new twist. Prog Neurobiol. 2003;70:33–52. doi: 10.1016/s0301-0082(03)00088-1. [DOI] [PubMed] [Google Scholar]
- Krubitzer L, Künzle H, Kaas JH. Organization of sensory cortex in a Madagascan insectivore, the tenrec (Echinops telfairi) J Comp Neurol. 1997;379:399–414. [PubMed] [Google Scholar]
- Krubitzer L, Manger P, Pettigrew J, Calford M. Organization of somatosensory cortex in monotremes: in search of the prototypical plan. J Comp Neurol. 1995;351:261–306. doi: 10.1002/cne.903510206. [DOI] [PubMed] [Google Scholar]
- Künzle H. Thalamic territories innervated by cerebellar nuclear afferents in the hedgehog tenrec, Echinops telfairi. J Comp Neurol. 1998;402:313–326. doi: 10.1002/(sici)1096-9861(19981221)402:3<313::aid-cne3>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- Künzle H. Tracing thalamo-cortical connections in tenrec a further attempt to characterize poorly differentiated neocortical regions, particularly the motor cortex. Brain Res. 2009;1253:35–47. doi: 10.1016/j.brainres.2008.11.052. [DOI] [PubMed] [Google Scholar]
- Lende RA. Cerbreal cortex: a sensorimotor amalgam in the Marsupialia. Science. 1963;141:730–732. doi: 10.1126/science.141.3582.730. [DOI] [PubMed] [Google Scholar]
- Lende RA. A comparative approach to neocortex: localization in monotremes, marsupials and insectivores. Ann NY Acad Sci. 1969;167:262–275. [Google Scholar]
- Loughry WJ, Prodöhl PA, McDonough CM, Avise JC. Polyembryony in armadillos. Am Sci. 1998;86:274–279. [Google Scholar]
- Lyon DC. The evolution of visual cortex and visual systems. In: Kaas JH, Krubitzer LA, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 3. 2007. pp. pp 267–306. Mammals. [Google Scholar]
- Lyon DC, Jain N, Kaas JH. Cortical connections of striate and extrastriate visual areas in tree shrews. J Comp Neurol. 1998;401:109–128. doi: 10.1002/(sici)1096-9861(19981109)401:1<109::aid-cne7>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- Martinich S, Pontes MN, Rocha-Miranda CE. Patterns of corticocortical, corticotectal, and commissural connections in the opossum visual cortex. J Comp Neurol. 2000;416:224–244. [PubMed] [Google Scholar]
- Medina L. Do birds and reptiles possess homologues of mammalian visual, somatosensory and motor cortices? In: Kaas JH, Bullock TH, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 2. 2007. pp. pp 163–194. Non-Mammalian Vertebrates. [Google Scholar]
- Meulders M, Gybels J, Bergmans J, Gerebtzoff MA, Goffart M. Sensory projections of somatic, auditory and visual origin to the cerebral cortex of the sloth (Choloepus hoffmanni peters). J Comp Neurol. 1966;126:535–546. [PubMed] [Google Scholar]
- Molnár Z, Tavare A, Cheurq AF. The origin of neocortex: lessons from comparative embryology; in Kaas JH, Krubitzer LA (eds): Evolution of Nervous Systems. Oxford, Elsevier. 2007;3:pp 13–26. Mammals. [Google Scholar]
- Murphy WJ, Eizirik E, O'Brien SJ, Madesen O, Scally M, Douady CJ, Teeling E, Ryder OA, Stanhope MJ, de Jong WW, Springer MS. Resolution of the early placental mammal radiation using Bayesian phylogenetics. Science. 2001;294:2348–2351. doi: 10.1126/science.1067179. [DOI] [PubMed] [Google Scholar]
- Nilsson MA, Churakov G, Sommer M, Van Tran N, Zemann A, Brosius J, Schmitz J. Tracking marsupial evolution using archaic genomic retroposon insertions. PLoS Biol. 2010;8:1–9. doi: 10.1371/journal.pbio.1000436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcutt RG, Kaas JH. The emergence and evolution of mammalian neocortex. Trends Neurosci. 1995;18:373–379. doi: 10.1016/0166-2236(95)93932-n. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Masterton RB, Descending pathways to the spinal cord 3. Sites of origin of the corticospinal tract. J Comp Neurol. 1990;296:559–583. doi: 10.1002/cne.902960405. [DOI] [PubMed] [Google Scholar]
- Phillips MJ, Bennett TH, Lee MS. Molecules, morphology, and ecology indicate a recent, amphibious ancestry for echidnas. Proc Natl Acad Sci USA. 2009;106:17089–17094. doi: 10.1073/pnas.0904649106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss TM. Do rats have prefrontal cortex? The Rose-Woolsey-Akert program reconsidered. J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Radinsky L. Brains of early carnivores. Paleobiology. 1977;3:333–349. [Google Scholar]
- Reep RL, Corwin JV, King V. Neuronal connections of orbital cortex in rats: topography of cortical and thalamic afferents. Exp Brain Res. 1996;111:215–232. doi: 10.1007/BF00227299. [DOI] [PubMed] [Google Scholar]
- Reep RL, Finlay BL, Darlington RB. The limbic system in mammalian brain evolution. Brain Behav Evol. 2007;70:57–70. doi: 10.1159/000101491. [DOI] [PubMed] [Google Scholar]
- Remple MS, Henry EC, Catania KC. Organization of somatosensory cortex in the laboratory rat (Rattus norvegicus): evidence for two lateral areas joined at the representation of the teeth. J Comp Neurol. 2003;467:105–118. doi: 10.1002/cne.10909. [DOI] [PubMed] [Google Scholar]
- Riquimaroux H, Gaioni SJ, Suga N. Cortical computational maps control auditory perception. Science. 1991;251:565–568. doi: 10.1126/science.1990432. [DOI] [PubMed] [Google Scholar]
- Rosa MG. Topographic organization of extrastriate areas in the flying fox: implications for the evolution of mammalian visual cortex. J Comp Neurol. 1999;411:503–523. doi: 10.1002/(sici)1096-9861(19990830)411:3<503::aid-cne12>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Krubitzer LA. The evolution of visual cortex: where is V2? Trends Neurosci. 1999;22:242–248. doi: 10.1016/s0166-2236(99)01398-3. [DOI] [PubMed] [Google Scholar]
- Rosa MG, Krubitzer LA, Molnar Z, Nelson JE. Organization of visual cortex in the northern quoll, Dasyurus hallucatus: evidence for a homologue of the second visual area in marsupials. Eur J Neurosci. 1999;11:907–915. doi: 10.1046/j.1460-9568.1999.00497.x. [DOI] [PubMed] [Google Scholar]
- Rowe M, Organization of the cerebral cortex in monotremes and marsupials Jones EG, Peters A. New York, Plenum Press, part II. 8B. 1990. Cerebral Cortex: Comparative Structures and Evolution of Cerebral Cortex; pp. pp 263–334. [Google Scholar]
- Royce JG, Martin GF, Dom RM. Functional localization and cortical architecture in the nine-banded armadillo (Dasypus novemcinctus mexicanus). J Comp Neurol. 1975;164:495–522. doi: 10.1002/cne.901640408. [DOI] [PubMed] [Google Scholar]
- Santiago LF, Rocha EG, Freire MA, Dias IA, Lent R, Houzel JC, Picanco-Diniz CW, Pereira A, Jr, Franca JG. The organizational variability of the rodent somatosensory cortex. Rev Neurosci. 2007;18:283–294. doi: 10.1515/revneuro.2007.18.3-4.283. [DOI] [PubMed] [Google Scholar]
- Saraiva PE, Magalhaes-Castro B. Sensory and motor representation in the cerebral cortex of the three-toed sloth (Bradypus tridactylus). Brain Res. 1975;90:181–193. doi: 10.1016/0006-8993(75)90300-5. [DOI] [PubMed] [Google Scholar]
- Sherwood CC, Stimpson CD, Butti C, Bonar CJ, Newton AL, Allman JM, Hof PR. Neocortical neuron types in Xenarthra and Afrotheria: implications for brain evolution in mammals. Brain Struc Funct. 2009;213:301–328. doi: 10.1007/s00429-008-0198-9. [DOI] [PubMed] [Google Scholar]
- Smith FA, Boyer AG, Brown JH, Costa DP, Dayan T, Ernest SK, Evans AR, Fortelius M, Gittleman JL, Hamilton MJ, Harding LE, Lintulaakso K, Lyons SK, McCain C, Okie JG, Saarinen JJ, Sibly RM, Stephens PR, Theodor J, Uhen MD. The evolution of maximum body size of terrestrial mammals. Science. 2010;330:12216–12219. doi: 10.1126/science.1194830. [DOI] [PubMed] [Google Scholar]
- Stanhope MJ, Waddell VG, Madsen O, de Jong W, Hedges SB, Cleven GC, Kao D, Springer MS. Molecular evidence for multiple origins of Insectivora and for a new order of endemic African insectivore mammals. Proc Natl Acad Sci USA. 1998;95:9967–9972. doi: 10.1073/pnas.95.17.9967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Striedter GF. The telencephalon of tetrapods in evolution. Brain Behav Evol. 1997;49:179–213. doi: 10.1159/000112991. [DOI] [PubMed] [Google Scholar]
- Suga N. Cortical computational maps for auditory imaging. Neurol Netw. 1990;3:3–21. [Google Scholar]
- Thompson JM, Woolsey CN, Talbot SA. Visual areas I and II of cerebral cortex of rabbit. J Neurophysiol. 1950;13:277–288. doi: 10.1152/jn.1950.13.4.277. [DOI] [PubMed] [Google Scholar]
- Towns LC, Burton CJ, Kimberly CJ, Fetterman MR. Projections of the dorsal lateral geniculate and lateral posterior nuclei to visual cortex in the rabbit. J Comp Neurol. 1982;210:87–98. doi: 10.1002/cne.902100110. [DOI] [PubMed] [Google Scholar]
- Ulinski PS. Thalamic projections to the somatosensory cortex of the echidna, (Tachyglossus aculeatus) J Comp Neurol. 1984;229:153–170. doi: 10.1002/cne.902290203. [DOI] [PubMed] [Google Scholar]
- Ulinski PS, Visual cortex of turtles Kaas JH, Bullock TH. London, Elsevier. Vol. 2. 2007. Evolution of Nervous Systems; pp. pp 195–203. Non-Mammalian Vertebrates. [Google Scholar]
- Van Essen DC. Cerebral cortical folding patterns in primates: why they vary and what they signify. In: Kaas JH, Preuss TM, Evolution of Nervous Systems, editors. London, Elsevier. Vol. 4. 2007. pp. pp 267–276. Primates. [Google Scholar]
- Velenovsky DS, Cetas JS, Price RO, Sinex DG, McMullen NT. Functional subregions in primary auditory cortex defined by thalamocortical terminal arbors: an electrophysiological and anterograde labeling study. J Neurosci. 2003;23:308–316. doi: 10.1523/JNEUROSCI.23-01-00308.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace MT, Ramachandran R, Stein BE. A revised view of sensory cortical parcellation. Proc Natl Acad Sci USA. 2004;101:2167–2772. doi: 10.1073/pnas.0305697101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Gao E, Burkhalter A. Gateways of ventral and dorsal streams in mouse visual cortex. J Neurosci. 2011;31:1905–1918. doi: 10.1523/JNEUROSCI.3488-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker WI. Why does cerebral cortex fissure and fold? In: Jones EG, Peters A, Cerebral Cortex, editors. New York, Plenum. 1990. [Google Scholar]
- Wildman DE, Uddin M, Opazo JC, Liu G, Lefort V, Guindon S, Gascuel O, Grossman LI, Romero R, Godman M. Genomics, biogeography, and the diversification of placental mammals. Proc Natl Acad Sci USA. 2007;104:14395–14400. doi: 10.1073/pnas.0704342104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Donoghue JP, Motor cortex of rodents . Cerebral Cortex. In: Jones EG, Peters A, editors. New York, Plenum. Vol. 5. 1986. pp. pp 243–270. [Google Scholar]
- Wong P, Kaas JH. Architectonic subdivisions of neocortex in the gray squirrel (Sciurus carolinensis). Anat Rec. 2008;10:1301–1333. doi: 10.1002/ar.20758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong P, Kaas JH. An architectonic study of the neocortex of the short-tailed opossum (Monodelphis domestica). Brain Behav Evol. 2009;73:206–228. doi: 10.1159/000225381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodburne MO, Rich TH, Springer MS. The evolution of tribospheny and the antiquity of mammalian clades. Mol Phylogenet Evol. 2003;28:360–385. doi: 10.1016/s1055-7903(03)00113-1. [DOI] [PubMed] [Google Scholar]
- Zilles K, Wree A. Cortex: areal and laminar structrure. In: Paxinos G, The Rat Nervous System, editor. Sydney, Academic Press. 1995. pp. pp 649–685. [Google Scholar]