Abstract
Overnight pulse oximetry (OPO) has proven to be an effective and beneficial technique to determine the cardiorespiratory status of patients in both the inpatient and outpatient settings. It is a cheap, safe, reliable, simple, and accurate method of patient monitoring as compared to the expensive and labor-intensive method of multichannel polysomnography for detecting sleep-disordered breathing. It provides accurate information about patient's oxygenation status and also helps in monitoring the response to continuous positive airway pressure and in the surgical treatment of obstructive sleep apnea (OSA). Nocturnal hypoxemia portends a poor prognosis in patients of chronic obstructive pulmonary disease (COPD), interstitial lung disease (ILD), and neuromuscular diseases. OPO can help its early detection and management.
KEY WORDS: Chronic obstructive pulmonary disease, desaturation, interstitial lung disease, obstructive sleep apnea, overnight pulse oximetry
INTRODUCTION
Hypoxemia results in a poor clinical outcome, making pulse oximetry an important tool to monitor patient oxygenation routinely both in primary care and in hospital.[1] Overnight pulse oximetry (OPO) also has emerged as one of the most widely used techniques to determine a patient's cardiopulmonary status, as it provides adequate information about patient's oxygenation and respiratory patterns.
PRINCIPLE
Pulse oximetry is a spectrophotometry technology which determines the arterial oxygen saturation by the detection of pulsatile blood flow and is based on differing absorption spectra of oxyhemoglobin and deoxyhemoglobin which includes the red and near-infrared wavelengths of light.[2] The oxyhemoglobin absorbs near-infrared light and dissipates the red component of light which makes it appear bright red. On the contrary, deoxyhemoglobin absorbs the red component of light more and scatters the near infrared, making it look less red.[3]
Other wavelengths of the light such as the yellow, green, blue, and far infrared are absorbed by nonvascular tissues. Based on this principle of differential absorption of light, pulse oximeters emit two wavelengths of light, red at 660 nm and near infrared at 940 nm from two light-emitting diodes (LEDs) located in one arm of the probe. A photoreceptor is present opposite to the LEDs with patient tissues with a higher vascular density such as the finger, toes, nose, forehead, or earlobe held in between. This photodiode detects the amount of red and near-infrared light absorbed by the vascular tissues from the light transmitted through the finger held in between the arms of the probe. A large number of measurements of relative light absorption are made multiple times every second, and these are fed into a microprocessor that compares the ratio of absorption of the two spectra against a set of stored reference values in the form of a calibration curve obtained empirically by measuring the relative red: infra-red modulation ratios in healthy volunteers whose saturations were altered from 100% to approximately 70%. These values are accepted or rejected using specific formulas. The microprocessor gives a new reading every 0.5–1 s that averages out the readings over the last 3–6 s.[3]
Over the past few years, the advancement in technology has resulted in the possibility of continuous pulse oximetry recording which gives a better idea of a patient's oxygenation and respiratory status. Generally, continuous oximetry is done during the night time; hence, it is called OPO.
During a routine OPO, one can get an idea about the mean overnight saturation (mean SaO2) and lowest SaO2 during the entire night recording. In general healthy controls, the normal overnight mean oxygen saturation is 96%.[4] A decreased value points to an underlying cardiopulmonary problem.
Another parameter which is clinically helpful and is widely reported in most studies is time spent by the patient, under 90% oxygen saturation (T-90) during the duration of the study. An elevated T-90 during overnight sleep study represents the hypoxic burden during the study. It provides the clinician a clue of the presence of coexistence of pulmonary hypertension, heart failure, and hypoventilation symptoms.
OPO has been used extensively in the field of sleep medicine and classified as a type 4 monitoring device.[5,6] Its low cost, easy availability, and being able to perform it on an outpatient basis make this an attractive alternative to polysomnographic studies for the screening of obstructive sleep apnea (OSA) in patients with a high pretest suspicion.
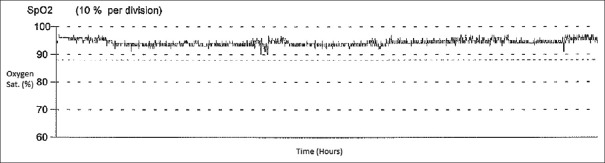
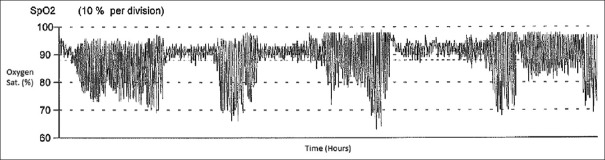
Most clinicians also look at the oxygen saturation waveform patterns, which provide a good view of the oxygenation status of the patient for the entire night's recording. In healthy controls, on OPO, the oxygen saturation is maintained above 90% throughout the night [Figure 1]. One of the most typical patterns that one can appreciate on OPO is the recognition of the relationship between rapid eye movement (REM) sleep stage and occurrence of multiple clustered episodes of deep oxygen desaturation, also called sawtooth pattern or “icicles from the rooftop” pattern [Figure 2], which is generally seen in the presence of OSA.
Figure 1.
Normal pulse oximetry
Figure 2.
Abnormal pulse oximetry
One can also report on the number of oxygen desaturations (decrease of oxygen saturation by 4% from the baseline per hour of recording) as an index, generally called oxygen desaturation index (ODI).[7] Various authors have used different cutoff points for reporting ODI. The threshold for an abnormal ODI can be either ≥5 desaturations per hour, ≥10 desaturations per hour, or ≥15 desaturations per hour.[1] It has been demonstrated that in patients with a high pretest probability of OSA, ODI does correlate with the apnea-hypopnea index.[7,8,9]
Role of overnight pulse oximetry in screening for sleep-disordered breathing
OSA syndrome or sleep-disordered breathing (SDB) is a major public health problem and has a rather higher prevalence rate. Although polysomnography (PSG) is the gold standard for studying the sleep quality and diagnosing SDB, it is expensive, time consuming, labor intensive, less readily available, and requires regular monitoring and expertise for the interpretation. On the other hand, OPO can be used as a quick and valuable screening tool.[10,11,12] It can be used as a case-selection technique to detect patients who need PSG.[1,13]
Nocturnal pulse oximetry is a low-cost, safe, simple, convenient, noninvasive, and effective screening method for SDB in adults.[11,14] It is readily and easily accessible with no risks or side effects to the patients and has decreased the waiting time of OSA diagnosis.[11]
Apnea and hypopnea cause an arousal from sleep causing sympathetic activation, sleep disruption, snoring, fatigue, nocturia, morning headaches, and excessive daytime sleepiness.[15] SDB leads to increased risk of motor-vehicle crashes.[16] Several studies have shown that sleep apnea is associated with hypertension, stroke, heart failure, obesity, metabolic syndrome,[17] diabetes,[18] neurocognitive deficits,[19] gastroesophageal reflux disease,[20] and erectile dysfunction.[21] Such health problems can reduce the quality of life. Chiang et al. have found OSA prevalence to be 60.9% among adult primary care population who are at risk of OSA.[10] Hence, due to these effects of OSA and its increasing prevalence, quick and accurate screening, diagnosis and treatment becomes very important.
Several studies have been done to evaluate the validity and usefulness of OPO in the screening of OSA [Table 1]. Hang et al. studied a total of 616 patients with nocturnal oximetry and PSG study and showed that OPO is a satisfactory diagnostic tool for patients with severe OSA.[11]
Table 1.
A selective review of overnight pulse oximetry in the patients of obstructive sleep apnea
| Author/years/study type | Dataset (n) | Condition | Aim | Gold standard (cutoff) | Technique | Results |
|---|---|---|---|---|---|---|
| Hang et al./2015[11] | 616 patients with suspected OSAS | OSAS | Test the validity of overnight oximetry for moderate-to-severe OSA | Moderate OSA - AHI=30, Severe OSA - AHI=15 | Overnight oximetry and Alice 4 PSG recorder | ODI derived from oximetry showed Severe OSA accuracy 90.42%-90.55%, sensitivity 89.36-89.87%, and specificity 91.08%-93.05%; moderate-to-severe OSA accuracy 87.33-87.77%, sensitivity 87.71-88.53%, and specificity 86.38-86.56% |
| Vázquez et al./2000[9] | 246 patients with suspected OSAS | OSAS | Comparison of results of pulse oximetry and PSG | AHI=15 | In-laboratory PSG and digital oximetry recording | AHI obtained from PSG and RDI obtained from oximetry are highly correlated (R=0.97), oximetry sensitivity=98%, specificity=88% |
| Chiang et al./2017[10]/Prospective study | 305 males229 females with risk factors for OSAS | OSAS | Testing overnight pulse oximetry as a screening tool for OSAS and comparing results with PSG | PSG AHI 5-14 events/h=mild OSA, 15-30=moderate OSA, >30=severe OSAOxygen desaturation defined as a decrease of 4% or more from the baseline SaO2. Patients with 5 or more such events per hour were called screening positive patients | PSG and digital oximetry recording | 60.9% (325) patients screened positive for OSASensitivity of OSA diagnosis with overnight pulse oximetry=94.4% and specificity=78.9% |
| Kunisaki et al./2016[22]/Prospective observation-al study | 234 sleep study patients with suspected OSAS | OSAS | Determine if overnight oximetry has high PPV for OSA diagnosis | RDI ≥15/h | Assessed area under ROC and PPV of STOP-BANG score and ODI | 65% with ODI ≥15, 49% had an ODI >7/h, which had PPV of 92% |
| Chiang et al./2011[23]/Prospective cross-sectional study | 60 patients with suspected OSAS | OSAS | Usefulness of overnight oximetry as a screening tool for OSA | PSG AHI 5-14 events/h=mild OSA, 15-30=moderate OSA, >30=severe OSAOxygen desaturation defined as a decrease of 4% or more from the baseline SaO2. Patients with 5 or more such events per hour were called screening positive patients | PSG and overnight oximetry recording | 85% had OSA by PSG study, 14.9 events/h detected by overnight pulse oximetry-derived ODI, and 24.6% events/h detected by PSG derived AHI, With ODI, sensitivity, and specificity of OSA diagnosis was 92% and 88%, respectively |
| Linz et al./2018[24]/Prospective study | 439 patients with documented AF, 69% of males | OSAS | The utility of overnight oximetry in the diagnosis of SDB in AF | SDB severity based on PSG-derived AHI, AHI 15-29/h moderate SDB, ≥15/h is moderate to severe SDB, AHI >30 is severe SDB | ODI was compared with PSG derived AHI | ODI was able to detect moderate-to-severe SDB and severe SDB in AF patients. ODI cutoff of 4.1/h had a sensitivity of 91% and specificity of 83% in diagnosis of moderate SDB, ODI of 7.6/h had a sensitivity and specificity of 89% and 83%, respectively, in patients with severe SDB |
| Romem et al./2014[6] | 73 patients with suspected OSAS | OSAS | To detect the accuracy of PPG-based finger pulse oximetry for diagnosis of OSA | Mild OSA with AHI 5-14.9, moderate OSA - AHI 15-29.9 and severe OSA - AHI ≥30 | PSG results and digital oximetry recording compared | Valid results in 65 patients, positive correlation obtained between pulse oximetry- and PSG-derived data, for AHI >5/h, sensitivity 80% and specificity 86%, for AHI >15/h sensitivity 70% and specificity 91% |
| Dumitrache-Rujinski et al./2013[14]/Prospective study | 199 patients with suspected OSAS, 123 morbidly obese (Group A) and 76 (Group B) nonobese | OSAS | Role of OPO in screening of OSA in obese patients with BMI >40 kg/m2 | NA | PSG was performed and correlation between desaturation index and AHI*** was done in the 2 Groups | In group A, 94.3% diagnosed with OSAS, mean ODI 47.2/h, mean AHI 46.5/h, mean SaO2 88.5%. In group B, 85.52% diagnosed with OSA, mean ODI 23.12/h, mean AHI 28.8/h, mean SaO2 93.7%. There was a significant positive correlation between ODI and AHI in both groups |
| Scott et al./2014[12] | 59 COPD patients, 27 males | COPD | Use of pulse oximetry to detect OSA in COPD patients | OSA was diagnosed if AHI was >15 events/h, sustained desaturation ≥4% in 1 h time scale was called a desaturation “event”, ≥3 desaturation/saturation cycles over 15 min time scale was called a “pattern” | Pulse oximetry tracings were used to diagnose OSA in COPD patients | 53% COPD patients had OSA, 35 patients were correctly diagnosed as having OSA with an accuracy of 59%, sensitivity was 59% and specificity 60% showing only a modest role of pulse oximetry in diagnosis of OSA in COPD |
OSA: Obstructive sleep apnea, OSAS: OSA syndrome, AHI: Apnea-Hypopnea index, PSG: Polysomnography, ODI: Oxygen desaturation index, RDI: Respiratory disturbance index, ROC: Receiver-operating characteristic curve, PPV: Positive predictive value; SDB: Sleep-disordered breathing, OPO: Overnight pulse oximetry, PPG: Photoplethysmography, AF: Atrial fibrillation, COPD: Chronic Obstructive Pulmonary Disease, NA: Not available, BMI: Body mass index
Others have also reported that in a population at high risk for OSA, OPO can be an effective screening tool.[9,10,22,23,24] Romem et al. evaluated the accuracy of photoplethysmography (PPG)-based finger pulse oximeter in the diagnosis of OSA. The study was performed on 73 patients with a likely diagnosis of OSA using the PPG-based device and simultaneous in-laboratory PSG. It was concluded that the pulse oximetry data were compared well with the PSG data.[6]
OPO has a role in the screening of OSA in obese patients too. Morbid obesity (BMI >40 kg/m2) leads to a decreased basal nocturnal saturation due to the supine hypoventilation, hypoxemia, and hypercapnia during sleep secondary to a large abdomen, reduced diaphragmatic excursions, and low chest wall compliance.[25] These factors result in increased susceptibility to SDB. The OPO shows a combination of low baseline oximetry (i.e., low T-90) and evidence of intermittent hypoxemia.
A study by Dumitrache-Rujinski et al. assessed the role of OPO in the screening of OSA in morbidly obese patients. They prospectively evaluated 123 morbidly obese patients and 76 nonobese patients and assessed the correlation between the overnight desaturation index obtained by the pulse oximetry and apnea-hypopnea index measured by the PSG. ODI proved to be a useful tool as a screening method in obese and nonobese patients.[14]
Netzer et al. in a review of OPO reported a wide range of sensitivity and specificity for predicting SDB ranging from 31% to 98% and 41% to 100%, respectively.[1] Several possible reasons can be put forward to explain these variations.[26] As reported by Böhning et al., there is an interdevice variability between different pulse oximeters which may affect the quality of data and hence the study results.[27] In addition, there is variability in how physicians interpret oximetry tracings and appears to be another limiting step towards its wider use.[28]
In general, from the literature, it is clear that OPO allows for the screening for OSA in patients with moderate-to-severe cases of OSA but is rather inadequate for the exclusion of milder cases. It is also important to point however that oximetry testing cannot distinguish between OSA and central sleep apnea, so it is insufficient for the definitive diagnosis of OSA or qualify patients for therapy.[29] It should be considered as a screening tool only for patients with high pretest suspicion. Patients who are found to have suspicion of OSA will still need an overnight PSG or a home sleep study to confirm their diagnosis.
Others have also suggested that OPO can also be used to assess the response to continuous positive airway pressure (CPAP) therapy and surgical treatment of OSA.[1,10] This is particularly helpful to establish the improvement in ODI and T-90 once the patient has been treated for OSA.
Role of overnight pulse oximetry in the management of chronic obstructive pulmonary disease
OSA and hypoxemia are commonly encountered in chronic obstructive pulmonary disease (COPD) patients.[12] The sleep-related respiratory changes can prove to be dangerous in a patient of COPD due to the reduced diaphragmatic excursion, ventilation-perfusion mismatch, and decreased chest wall compliance.[30] Nocturnal hypoxemia can lead to cardiovascular complications and secondary pulmonary hypertension.[31] The presence of nocturnal hypoxemia can easily be assessed with home OPO which allows one to measure the T-90 value, which is the time at night spent below 90% oxygen saturation. In general, one single OPO recording may not be enough to assess the nocturnal desaturation in COPD, as it shows night-to-night variability.[32]
The overlap of COPD with OSA makes the diagnosis and prognosis very challenging.[33] It leads to poor sleep and lower quality of life with increased morbidity and mortality. About 5–10% of COPD patients have OSA, which is about 0.5–1% of the general population ≥40 years.[34,35] Patients with OSA and hypoxemic COPD receiving long term oxygen therapy (LTOT) have higher survival rates when treated with CPAP, as compared with those treated with LTOT only.[36] Nocturnal oximetry is an inexpensive method to screen for OSA in patients with advanced COPD.[12] In addition, patients with COPD and OSA that are being treated with CPAP can also be monitored with OPO for the adequacy of nocturnal oxygenation.
Role of overnight pulse oximetry in the management of neuromuscular disorders
Neuromuscular respiratory failure in disorders such as amyotrophic lateral sclerosis (ALS) and muscular dystrophies is characterized by daytime hypercapnia, dyspnea, and nocturnal hypoventilation (NH).[37] This NH is an indication for noninvasive ventilation (NIV).[38,39] Hence, early detection of hypercapnia and NH is important so that NIV can be timely initiated which would improve the quality of life in patients of ALS.[40] NH is detected by lung function testing, PSG, or by exhaled PCO2 monitoring.[41,42] However, PSG is not always feasible because it requires overnight stay in the sleep laboratories, and patient at times may not be willing due to their special needs. Therefore, recently, a monitor that measures transcutaneous PCO2 and SpO2 by pulse oximetry in a home setting has gained popularity. In a study by Bauman et al. on 35 patients of neuromuscular disorders, home-based unsupervised monitoring of PCO2 and SpO2 proved to be a useful method for diagnosing NH.[37]
In a study by Elman et al., 87 nocturnal oximetry evaluations were done on 78 ALS patients symptomatic for SDB. These measurements were compared for those with forced vital capacity (FVC) >50% versus those with FVC of <50% of normal. A considerable number of these symptomatic patients showed evidence of nocturnal hypoxemia by oximetry proving that FVC <50% is not the only criteria for starting nocturnal nasal ventilation. Many patients can be hypoxemic and symptomatic at a higher FVC. Hence, nocturnal oximetry helps to identify these patients for earlier respiratory intervention.[43] Nocturnal pulse oximetry indirectly provides information about the arterial saturation in the night time and indirectly provides information about the respiratory status. In patients with neuromuscular disorders, there is a significant relationship between the decreased nocturnal saturation and daytime hypercapnia.[44]
Although OPO is not a substitute for PSG, the low cost and convenience of this technique has stimulated its application in ALS.[43,45,46] A mean nocturnal O2 saturation below 93% as detected by OPO is of prognostic value in ALS.[46] Furthermore, NIV adapted to patients with early changes as registered by OPO permitted a longer survival compared with a group of patients who started NIV when FVC declined to 50% or less.[45]
The presence of significant nocturnal desaturation has been proposed as a parameter in decision-making with regard to initiating home mechanical ventilation (HMV) or monitoring HMV effectiveness in patients with neuromuscular disease, obesity, or chest wall deformity. The data obtained from nocturnal home pulse oximetry and used in decision-making are the duration of the time with oxygen saturation SaO2 <90% expressed as percentage (T-90) or in minutes (Tm90), mean SaO2, and lowest SaO2.[47]
Role of overnight pulse oximetry in the management of interstitial lung diseases
OPO becomes increasingly important in the evaluation of patients with interstitial lung diseases (ILDs) because they exhibit abnormal sleep architecture with sleep fragmentation, SDB, and nocturnal oxygen desaturation. Hypoxemia at night is common in patients of ILD even if there is no daytime resting hypoxemia[48] and is linked to pulmonary hypertension and early mortality.[49,50] Therefore, correcting nocturnal hypoxemia improves the quality of life and prevents pulmonary hypertension, which is one of the strongest markers of a poor prognosis in ILD.[51] There exist no standard universally accepted criteria of nocturnal hypoxemia in patients of ILD, but according to the Australia and New Zealand guidelines of the treatment of idiopathic pulmonary fibrosis, nocturnal oxygen therapy is required in patients who desaturate below 88% for at least one-third of the total sleep time in the absence of OSA.[52] Studies are required to clarify the benefits of oxygen and to assess whether different thresholds for initiating nocturnal oxygen are more appropriate, for example, SpO2 <90% for >10% of sleep time, according to the studies in pulmonary hypertension.[50]
The patients of ILD have a rapid, shallow breathing pattern due to the impaired gas exchange across the thickened, collagen-dense interstitium. The incidence of OSA is also high due to the increased upper airway collapsibility and ventilatory control instability.[53,54]
Pihtili et al. studied the incidence of OSA in 17 patients with idiopathic pulmonary fibrosis (IPF), 15 patients with stage II–III sarcoidosis, and 18 patients with pulmonary fibrosis due to scleroderma. The study showed 68% of the patients with OSA in this population. The prevalence of OSA was 82.3% in the IPF patients, 66.6% in the sarcoidosis patients, and 55.5% in the scleroderma patients.[55] The OSA-related morbidity and mortality is high in ILD patients.[56]
Elevated nocturnal desaturation index strongly predicts the increased mortality in ILD patients, as the nocturnal desaturation is proportional to the underlying interstitial fibrosis.[49] Hence, early diagnosis and management is essential. PSG being an expensive procedure cannot be performed in all patients of OSA. Therefore, nocturnal oximetry can be used to screen patients of ILD for the presence of SDB, and those detected can be evaluated with an overnight PSG so that early CPAP treatment can be initiated.[57]
OTHER USES OF OVERNIGHT PULSE OXIMETRY
Pulse oximetry is safe to use in monitoring all patients, and generally, there are no contraindications. Continuous pulse oximetry has a wide application in preoperative assessment, operating room surgeries, postoperative recovery room, intensive care units, and stroke units.[1]
LIMITATIONS OF OVERNIGHT PULSE OXIMETRY
Interpretation of the pulse oximetry data can be a challenging task. The user should have the knowledge of normal oxygen saturation values during sleep.[1] Ayache and Strohl used a structured template for pulse oximetry data interpretation by pulmonary and critical care fellows and concluded that the use of a structured template produced a high interrater agreement and accuracy.[58]
As pulse oximetry measures pulsatile blood flow, poor perfusion and poor peripheral arterial blood flow due to hypovolemic shock, hypothermia, cardiac failure, dysarrythmia, and peripheral vascular disease affects its measurements.[1,3] Body movements such as during tremors and convulsions, vasoconstriction and hypotension can cause artifacts as it interrupts the pulse signal. It does not always detect movement artifacts so can overestimate desaturations.[1,3] Venous pulsations can also cause a falsely low SpO2 recording as the venous oxyhemoglobin saturation measured by the pulse oximeter lowers the arterial saturation.[3] Pulse oximetry readings also depend on hemoglobin structure, so methemoglobinemia and carboxyhemoglobinemia can cause high readings and severe anemia can lead to low readings. Dyes such as methylene blue, indocyanine green, and indigo carmine used in diagnostic testing mimic the red light absorption like deoxyhemoglobin and hence could cause falsely low readings.[3] Other causes of false low readings are application of fingernail polish and the presence of abnormal hemoglobin variants in blood.[3]
In addition, pulse oximetry does not detect other types of SDB such as upper airway resistance syndrome and pure central sleep apnea.[1] It is unable to score sleep quality.[11] It is important to recognize that the accuracy of pulse oximeters deteriorates when the SpO2 falls below 80%.
CONCLUSION
OPO however still is a simple and low-cost “home monitoring device.”[59] It provides a rapid turnover of nocturnal oxygen data in managing various cardiopulmonary disorders. With advancement in home monitoring devices, this technology not only is cost-effective but also allows timely screening of patients at risk of hypoxemia.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Netzer N, Eliasson AH, Netzer C, Kristo DA. Overnight pulse oximetry for sleep-disordered breathing in adults: A review. Chest. 2001;120:625–33. doi: 10.1378/chest.120.2.625. [DOI] [PubMed] [Google Scholar]
- 2.Talwar A, Feinsilver SH. Respiratory monitoring. Respir Care Clin N Am. 2000;6:523–43. doi: 10.1016/s1078-5337(05)70088-5. [DOI] [PubMed] [Google Scholar]
- 3.Chan ED, Chan MM, Chan MM. Pulse oximetry: Understanding its basic principles facilitates appreciation of its limitations. Respir Med. 2013;107:789–99. doi: 10.1016/j.rmed.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 4.Gries RE, Brooks LJ. Normal oxyhemoglobin saturation during sleep. How low does it go? Chest. 1996;110:1489–92. doi: 10.1378/chest.110.6.1489. [DOI] [PubMed] [Google Scholar]
- 5.Collop NA, Anderson WM, Boehlecke B, Claman D, Goldberg R, Gottlieb DJ, et al. Clinical guidelines for the use of unattended portable monitors in the diagnosis of obstructive sleep apnea in adult patients. Portable Monitoring Task Force of the American Academy of Sleep Medicine. J Clin Sleep Med. 2007;3:737–47. [PMC free article] [PubMed] [Google Scholar]
- 6.Romem A, Romem A, Koldobskiy D, Scharf SM. Diagnosis of obstructive sleep apnea using pulse oximeter derived photoplethysmographic signals. J Clin Sleep Med. 2014;10:285–90. doi: 10.5664/jcsm.3530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chung F, Liao P, Elsaid H, Islam S, Shapiro CM, Sun Y. Oxygen desaturation index from nocturnal oximetry: A sensitive and specific tool to detect sleep-disordered breathing in surgical patients. Anesth Analg. 2012;114:993–1000. doi: 10.1213/ANE.0b013e318248f4f5. [DOI] [PubMed] [Google Scholar]
- 8.Malbois M, Giusti V, Suter M, Pellaton C, Vodoz JF, Heinzer R. Oximetry alone versus portable polygraphy for sleep apnea screening before bariatric surgery. Obes Surg. 2010;20:326–31. doi: 10.1007/s11695-009-0055-9. [DOI] [PubMed] [Google Scholar]
- 9.Vázquez JC, Tsai WH, Flemons WW, Masuda A, Brant R, Hajduk E, et al. Automated analysis of digital oximetry in the diagnosis of obstructive sleep apnoea. Thorax. 2000;55:302–7. doi: 10.1136/thorax.55.4.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chiang LK, Kam CW, Ng LV. Overnight pulse oximetry for screening of obstructive sleep apnea in at-risk adult patients in the primary care setting: Prospective case series. Fam Med Community Health. 2017;5:215–22. [Google Scholar]
- 11.Hang LW, Wang HL, Chen JH, Hsu JC, Lin HH, Chung WS, et al. Validation of overnight oximetry to diagnose patients with moderate to severe obstructive sleep apnea. BMC Pulm Med. 2015;15:24. doi: 10.1186/s12890-015-0017-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scott AS, Baltzan MA, Wolkove N. Examination of pulse oximetry tracings to detect obstructive sleep apnea in patients with advanced chronic obstructive pulmonary disease. Can Respir J. 2014;21:171–5. doi: 10.1155/2014/948717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hussain SF, Fleetham JA. Overnight home oximetry: Can it identify patients with obstructive sleep apnea-hypopnea who have minimal daytime sleepiness? Respir Med. 2003;97:537–40. doi: 10.1053/rmed.2003.1480. [DOI] [PubMed] [Google Scholar]
- 14.Dumitrache-Rujinski S, Calcaianu G, Zaharia D, Toma CL, Bogdan M. The role of overnight pulse-oximetry in recognition of obstructive sleep apnea syndrome in morbidly obese and non obese patients. Maedica (Buchar) 2013;8:237–42. [PMC free article] [PubMed] [Google Scholar]
- 15.Jordan AS, McSharry DG, Malhotra A. Adult obstructive sleep apnoea. Lancet. 2014;383:736–47. doi: 10.1016/S0140-6736(13)60734-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tregear S, Reston J, Schoelles K, Phillips B. Obstructive sleep apnea and risk of motor vehicle crash: Systematic review and meta-analysis. J Clin Sleep Med. 2009;5:573–81. [PMC free article] [PubMed] [Google Scholar]
- 17.Garvey JF, Pengo MF, Drakatos P, Kent BD. Epidemiological aspects of obstructive sleep apnea. J Thorac Dis. 2015;7:920–9. doi: 10.3978/j.issn.2072-1439.2015.04.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kendzerska T, Gershon AS, Hawker G, Tomlinson G, Leung RS. Obstructive sleep apnea and incident diabetes. A historical cohort study. Am J Respir Crit Care Med. 2014;190:218–25. doi: 10.1164/rccm.201312-2209OC. [DOI] [PubMed] [Google Scholar]
- 19.Osorio RS, Gumb T, Pirraglia E, Varga AW, Lu SE, Lim J, et al. Sleep-disordered breathing advances cognitive decline in the elderly. Neurology. 2015;84:1964–71. doi: 10.1212/WNL.0000000000001566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jaimchariyatam N, Tantipornsinchai W, Desudchit T, Gonlachanvit S. Association between respiratory events and nocturnal gastroesophageal reflux events in patients with coexisting obstructive sleep apnea and gastroesophageal reflux disease. Sleep Med. 2016;22:33–8. doi: 10.1016/j.sleep.2016.04.013. [DOI] [PubMed] [Google Scholar]
- 21.Chen CM, Tsai MJ, Wei PJ, Su YC, Yang CJ, Wu MN, et al. Erectile dysfunction in patients with sleep apnea-a nationwide population-based study. PLoS One. 2015;10:e0132510. doi: 10.1371/journal.pone.0132510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kunisaki KM, Bohn OA, Wetherbee EE, Rector TS. High-resolution wrist-worn overnight oximetry has high positive predictive value for obstructive sleep apnea in a sleep study referral population. Sleep Breath. 2016;20:583–7. doi: 10.1007/s11325-015-1251-6. [DOI] [PubMed] [Google Scholar]
- 23.Chiang LK, Ng PT, Kam CW, Ng LV, Wong CY, Yee KS, et al. Usefulness in using portable overnight pulse oximeter for screening obstructive sleep apnea in adult patients in primary health care setting. Hong Kong Pract. 2011;33:146–52. [Google Scholar]
- 24.Linz D, Kadhim K, Brooks AG, Elliott AD, Hendriks JM, Lau DH, et al. Diagnostic accuracy of overnight oximetry for the diagnosis of sleep-disordered breathing in atrial fibrillation patients. Int J Cardiol. 2018;272:155–61. doi: 10.1016/j.ijcard.2018.07.124. [DOI] [PubMed] [Google Scholar]
- 25.Piper AJ, Grunstein RR. Obesity hypoventilation syndrome: mechanisms and management. Am J Respir Crit Care Med. 2011;183:292–8. doi: 10.1164/rccm.201008-1280CI. [DOI] [PubMed] [Google Scholar]
- 26.Bahgat YS, Khalil YM, El Maghraby RA, El Sayed Mohamed E, Elsayed MM. The use of overnight pulse oximetry and phoniatrics parameters in the screening protocol of obstructive sleep apnea. Egypt J Chest Dis Tuberc. 2012;61:459–68. [Google Scholar]
- 27.Böhning N, Schultheiss B, Eilers S, Penzel T, Böhning W, Schmittendorf E. Comparability of pulse oximeters used in sleep medicine for the screening of OSA. Physiol Meas. 2010;31:875–88. doi: 10.1088/0967-3334/31/7/001. [DOI] [PubMed] [Google Scholar]
- 28.Ramsey R, Mehra R, Strohl KP. Variations in physician interpretation of overnight pulse oximetry monitoring. Chest. 2007;132:852–9. doi: 10.1378/chest.07-0312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Epstein LJ, Kristo D, Strollo PJ, Jr, Friedman N, Malhotra A, Patil SP, et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J Clin Sleep Med. 2009;5:263–76. [PMC free article] [PubMed] [Google Scholar]
- 30.Krachman S, Minai OA, Scharf SM. Sleep abnormalities and treatment in emphysema. Proc Am Thorac Soc. 2008;5:536–42. doi: 10.1513/pats.200708-134ET. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chaouat A, Naeije R, Weitzenblum E. Pulmonary hypertension in COPD. Eur Respir J. 2008;32:1371–85. doi: 10.1183/09031936.00015608. [DOI] [PubMed] [Google Scholar]
- 32.Lewis CA, Eaton TE, Fergusson W, Whyte KF, Garrett JE, Kolbe J. Home overnight pulse oximetry in patients with COPD: More than one recording may be needed. Chest. 2003;123:1127–33. doi: 10.1378/chest.123.4.1127. [DOI] [PubMed] [Google Scholar]
- 33.Jelic S. Diagnostic and therapeutic approach to coexistent chronic obstructive pulmonary disease and obstructive sleep apnea. Int J Chron Obstruct Pulmon Dis. 2008;3:269–75. doi: 10.2147/copd.s2168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Owens RL, Malhotra A. Sleep-disordered breathing and COPD: the overlap syndrome. Respir Care. 2010;55:1333–44. [PMC free article] [PubMed] [Google Scholar]
- 35.Weitzenblum E, Chaouat A, Kessler R, Canuet M. Overlap syndrome: Obstructive sleep apnea in patients with chronic obstructive pulmonary disease. Proc Am Thorac Soc. 2008;5:237–41. doi: 10.1513/pats.200706-077MG. [DOI] [PubMed] [Google Scholar]
- 36.Machado MC, Vollmer WM, Togeiro SM, Bilderback AL, Oliveira MV, Leitão FS, et al. CPAP and survival in moderate-to-severe obstructive sleep apnoea syndrome and hypoxaemic COPD. Eur Respir J. 2010;35:132–7. doi: 10.1183/09031936.00192008. [DOI] [PubMed] [Google Scholar]
- 37.Bauman KA, Kurili A, Schmidt SL, Rodriguez GM, Chiodo AE, Sitrin RG. Home-based overnight transcutaneous capnography/pulse oximetry for diagnosing nocturnal hypoventilation associated with neuromuscular disorders. Arch Phys Med Rehabil. 2013;94:46–52. doi: 10.1016/j.apmr.2012.08.215. [DOI] [PubMed] [Google Scholar]
- 38.McKim DA, Road J, Avendano M, Abdool S, Cote F, Duguid N, et al. Home mechanical ventilation: A Canadian Thoracic Society clinical practice guideline. Can Respir J. 2011;18:197–215. doi: 10.1155/2011/139769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonds AK. Recent advances in respiratory care for neuromuscular disease. Chest. 2006;130:1879–86. doi: 10.1378/chest.130.6.1879. [DOI] [PubMed] [Google Scholar]
- 40.Bourke SC, Tomlinson M, Williams TL, Bullock RE, Shaw PJ, Gibson GJ. Effects of non-invasive ventilation on survival and quality of life in patients with amyotrophic lateral sclerosis: A randomised controlled trial. Lancet Neurol. 2006;5:140–7. doi: 10.1016/S1474-4422(05)70326-4. [DOI] [PubMed] [Google Scholar]
- 41.Finder JD, Birnkrant D, Carl J, Farber HJ, Gozal D, Iannaccone ST, et al. Respiratory care of the patient with Duchenne muscular dystrophy: ATS consensus statement. Am J Respir Crit Care Med. 2004;170:456–65. doi: 10.1164/rccm.200307-885ST. [DOI] [PubMed] [Google Scholar]
- 42.Hamada S, Ishikawa Y, Aoyagi T, Ishikawa Y, Minami R, Bach JR. Indicators for ventilator use in Duchenne muscular dystrophy. Respir Med. 2011;105:625–9. doi: 10.1016/j.rmed.2010.12.005. [DOI] [PubMed] [Google Scholar]
- 43.Elman LB, Siderowf AD, McCluskey LF. Nocturnal oximetry: utility in the respiratory management of amyotrophic lateral sclerosis. Am J Phys Med Rehabil. 2003;82:866–70. doi: 10.1097/01.PHM.0000091985.22659.30. [DOI] [PubMed] [Google Scholar]
- 44.de Carvalho M, Costa J, Pinto S, Pinto A. Percutaneous nocturnal oximetry in amyotrophic lateral sclerosis: Periodic desaturation. Amyotroph Lateral Scler. 2009;10:154–61. doi: 10.1080/17482960802382305. [DOI] [PubMed] [Google Scholar]
- 45.Pinto A, de Carvalho M, Evangelista T, Lopes A, Sales-Luís L. Nocturnal pulse oximetry: A new approach to establish the appropriate time for non-invasive ventilation in ALS patients. Amyotroph Lateral Scler Other Motor Neuron Disord. 2003;4:31–5. doi: 10.1080/14660820310006706. [DOI] [PubMed] [Google Scholar]
- 46.Velasco R, Salachas F, Munerati E, Le Forestier N, Pradat PF, Lacomblez L, et al. Nocturnal oxymetry in patients with amyotrophic lateral sclerosis: Role in predicting survival. Rev Neurol (Paris) 2002;158:575–8. [PubMed] [Google Scholar]
- 47.Fernández R, Rubinos G, Cabrera C, Galindo R, Fumero S, Sosa A, et al. Nocturnal home pulse oximetry: Variability and clinical implications in home mechanical ventilation. Respiration. 2011;82:142–7. doi: 10.1159/000322671. [DOI] [PubMed] [Google Scholar]
- 48.Schiza S, Mermigkis C, Margaritopoulos GA, Daniil Z, Harari S, Poletti V, et al. Idiopathic pulmonary fibrosis and sleep disorders: No longer strangers in the night. Eur Respir Rev. 2015;24:327–39. doi: 10.1183/16000617.00009114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corte TJ, Wort SJ, Talbot S, Macdonald PM, Hansel DM, Polkey M, et al. Elevated nocturnal desaturation index predicts mortality in interstitial lung disease. Sarcoidosis Vasc Diffuse Lung Dis. 2012;29:41–50. [PubMed] [Google Scholar]
- 50.Minai OA, Pandya CM, Golish JA, Avecillas JF, McCarthy K, Marlow S, et al. Predictors of nocturnal oxygen desaturation in pulmonary arterial hypertension. Chest. 2007;131:109–17. doi: 10.1378/chest.06-1378. [DOI] [PubMed] [Google Scholar]
- 51.Wijsenbeek MS, Holland AE, Swigris JJ, Renzoni EA. Comprehensive supportive care for patients with fibrosing interstitial lung disease. Am J Respir Crit Care Med. 2019;200:152–9. doi: 10.1164/rccm.201903-0614PP. [DOI] [PubMed] [Google Scholar]
- 52.Jo HE, Troy LK, Keir G, Chambers DC, Holland A, Goh N, et al. Treatment of idiopathic pulmonary fibrosis in australia and new zealand: A position statement from the Thoracic Society of Australia and New Zealand and the Lung Foundation Australia. Respirology. 2017;22:1436–58. doi: 10.1111/resp.13146. [DOI] [PubMed] [Google Scholar]
- 53.Mermigkis C, Bouloukaki I, Schiza SE. Sleep as a new target for improving outcomes in idiopathic pulmonary fibrosis. Chest. 2017;152:1327–38. doi: 10.1016/j.chest.2017.07.019. [DOI] [PubMed] [Google Scholar]
- 54.Troy LK, Corte TJ. Sleep disordered breathing in interstitial lung disease: A review. World J Clin Cases. 2014;2:828–34. doi: 10.12998/wjcc.v2.i12.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Pihtili A, Bingol Z, Kiyan E, Cuhadaroglu C, Issever H, Gulbaran Z. Obstructive sleep apnea is common in patients with interstitial lung disease. Sleep Breath. 2013;17:1281–8. doi: 10.1007/s11325-013-0834-3. [DOI] [PubMed] [Google Scholar]
- 56.Mermigkis C, Bouloukaki I, Schiza SE. Obstructive sleep apnea in patients with interstitial lung diseases: Past and future. Sleep Breath. 2013;17:1127–8. doi: 10.1007/s11325-013-0836-1. [DOI] [PubMed] [Google Scholar]
- 57.Pitsiou G, Bagalas V, Boutou A, Stanopoulos I, Argyropoulou-Pataka P. Should we routinely screen patients with idiopathic pulmonary fibrosis for nocturnal hypoxemia? Sleep Breath. 2013;17:447–8. doi: 10.1007/s11325-012-0716-0. [DOI] [PubMed] [Google Scholar]
- 58.Ayache M, Strohl KP. High interrater reliability of overnight pulse oximetry interpretation among inexperienced physicians using a structured template. J Clin Sleep Med. 2018;14:541–8. doi: 10.5664/jcsm.7040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gay PC. Overnight pulse oximetry unwoven. J Clin Sleep Med. 2018;14:497–8. doi: 10.5664/jcsm.7026. [DOI] [PMC free article] [PubMed] [Google Scholar]