Abstract
Background
Calcium channel blockers are the most commonly prescribed drugs for people with primary Raynaud's phenomenon. Primary Raynaud's phenomenon is a common condition characterised by an exaggerated vasospastic response to cold or emotion: classically the digits (fingers and toes) turn white, then blue, then red. This is an update of the review first published in 2014.
Objectives
To assess the effects of different calcium channel blockers for primary Raynaud's phenomenon as determined by attack rates, severity scores, participant‐preference scores and physiological measurements.
Search methods
For this update the Cochrane Vascular Trial Search Co‐ordinator searched the Specialised Register (last searched January 2016) and the Cochrane Register of Studies (CENTRAL) (2015, Issue 12). In addition the TSC searched clinical trials databases.
Selection criteria
Randomised controlled trials evaluating the effects of oral calcium channel blockers for the treatment of primary Raynaud's phenomenon.
Data collection and analysis
Three review authors independently assessed the trials for inclusion and their quality, and extracted the data. Data extraction included adverse events. We contacted trial authors for missing data.
Main results
We included seven randomised trials with 296 participants. Four trials examined nifedipine and the remainder nicardipine. Comparisons were with placebo in six trials and with both dazoxiben and placebo in one trial (only the nifedipine versus placebo data were used within this review). Treatment with oral calcium channel blockers was minimally effective in primary Raynaud's phenomenon at decreasing the frequency of attacks (standardised mean difference of 0.23; 95% confidence interval (CI) 0.08 to 0.38, P = 0.003). This translates to 1.72 (95% CI 0.60 to 2.84) fewer attacks per week on calcium channel blockers compared to placebo. One trial provided details on duration of attacks reporting no statistically significant difference between the nicardipine and placebo groups (no P value reported). Only two trials provided any detail of statistical comparisons of (unvalidated) severity scores between treatment groups: one of these trials (60 participants) reported a mean severity score of 1.55 on placebo and 1.36 on nicardipine, difference 0.2 (95% CI of difference 0 to 0.4, no P value reported) and the other trial (three participants only with primary Raynaud's phenomenon) reported a median severity score of 2 on both nicardipine and placebo treatment (P > 0.999) suggesting little effect on severity. Participant‐preference scores were included in four trials, but in only two were results specific to participants with primary Raynaud's phenomenon, and scoring systems differed between trials: scores differed between treatments in only one trial, in which 33% of participants on placebo and 73% on nifedipine reported improvement in symptoms (P < 0.001). Physiological measurements were included as outcome measures in five trials (different methodologies were used in each): none of these trials found any statistically significant between‐treatment group differences. Treatment with calcium channel blockers appeared to be associated with a number of adverse reactions, including headaches, flushing and oedema (swelling). Overall, the trials were classed as being at low or unclear risk of bias; and the quality of the evidence presented was moderate for number of attacks, very low for duration of attacks, high for severity scores and low for patient preference scores.
Authors' conclusions
The randomised controlled trials included in this review provide moderate quality evidence that oral calcium channel blockers are minimally effective in the treatment of primary Raynaud's phenomenon as measured by the frequency of attacks and high‐quality evidence that they have little effect on severity. We are unable to comment on duration of attacks or on patient preference due to the very low and low quality of evidence as a result of small sample sizes in the included studies and the variable data quality of outcome measures.
Keywords: Humans, Calcium Channel Blockers, Calcium Channel Blockers/therapeutic use, Nicardipine, Nicardipine/therapeutic use, Nifedipine, Nifedipine/therapeutic use, Randomized Controlled Trials as Topic, Raynaud Disease, Raynaud Disease/drug therapy
Plain language summary
Calcium channel blockers for primary Raynaud's phenomenon
Background
Raynaud's phenomenon is a disorder whereby blood vessels in the fingers and toes constrict and reduce blood flow, causing pain and discolouration. This is usually in response to cold exposure or emotional stress. In a small number of cases, Raynaud's phenomenon is associated with an underlying disease but, for most people, it is idiopathic (of uncertain cause, or 'primary'). Primary Raynaud's phenomenon is extremely common (especially in women), with one UK study suggesting that over 15% of the population are affected. For people with primary Raynaud's phenomenon who do not respond to conservative measures (e.g. keeping warm), calcium channel blockers represent the first line in drug treatment. Calcium channel blockers (sometimes called calcium antagonists) are drugs that affect the way calcium passes into certain muscle cells and they are the most commonly prescribed medication for primary Raynaud's phenomenon.
Study characteristics and key results
This review examined seven randomised trials which included 296 participants. Although overall all the trials were classed as being at low or unclear risk of bias, the sample size of the included trials was small and there was unclear reporting of outcomes. Two different calcium channel blockers were included: nifedipine and nicardipine. Comparisons in six trials were with placebo and in one trial with both placebo and another type of drug (although only data relating to the calcium channel blocker and placebo were used in this case). Treatment with oral calcium channel blockers was found to be minimally effective in primary Raynaud's phenomenon, reducing the frequency of attacks by around 1.7 attacks per person per week. One trial provided information on duration of attacks reporting no difference between the calcium channel blocker and placebo groups . Oral calcium channel blockers had no effect on severity scores in the two trials in which these were assessed. Only two trials reported preference scores (whereby participants are asked which treatment they prefer) specifically in those with primary Raynaud's phenomenon, and in only one of these was there a between‐treatment group difference (participants preferred nifedipine to placebo). Physiological measurements (for example measurement of finger blood flow) were performed in five trials, data could not be combined as the methods were too different, no differences found between calcium channel blocker and placebo treatment were seen in any trial. Treatment with calcium channel blockers was associated with a number of adverse events including headaches, flushing and ankle swelling.
Quality of the evidence
The results of this review were limited by the low number of participants recruited to the studies and by the limitations of currently used outcome measures. This review shows moderate quality evidence that oral calcium channel blockers are minimally effective in the treatment of primary Raynaud's phenomenon, as measured by the frequency of attacks, and high quality evidence that they have little effect on severity. We are unable to comment on duration of attacks and patient preference due to the very low and low quality of evidence provided by the trials in relation to these outcomes.
Summary of findings
Summary of findings for the main comparison. Calcium channel blockers for primary Raynauds's phenomenon.
| Calcium channel blockers for primary Raynauds's phenomenon | ||||||
| Patient or population: primary Raynaud's phenomenon Setting: hospital and hospital outpatient clinics Intervention: calcium channel blockers Comparison: placebo | ||||||
| Outcomes | Anticipated absolute effects* (95% CI) | Relative effect (95% CI) | № of participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Risk with placebo | Risk with Calcium channel blockers | |||||
| Number of attacks | The mean number of attacks in the intervention group was 0.23 standard deviations more (0.08 more to 0.38 more) than the placebo group. This translates to 1.72 (0.60 to 2.84) fewer attacks per week. | ‐ | 296 (7 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | ||
| Duration of attacks | One study reported mean attack duration of 11 minutes in placebo group compared to 13 minutes in Nicardipine group. | ‐ | 16 (1 RCT) |
⊕⊝⊝⊝ VERY LOW 2 3 | ||
| Severity scores | One study reported difference in mean (95% CI) severity score of 0.2 (0 to 0.4) in favour of nicardipine for 60 patients in a crossover study. One study had a median score of 2 for patients taking both placebo and nicardipine. One reported 'no significant difference' with no other information. Unable to make between‐group comparisons using information reported in other studies. | ‐ | 291 (6 RCTs) |
⊕⊕⊕⊕ HIGH | Please see Quality of the evidence | |
| Patient preference scores | One study reported mean improvement score of 0.6 vs 2.2 in favour of nifedipine, P = 0.25. One reported that 73% of patients considered symptoms to have improved on nifedipine compared to 33% on placebo. | ‐ | 185 (4 RCTs) |
⊕⊕⊝⊝ LOW 4 5 | ||
| *The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk ratio; OR: Odds ratio; | ||||||
| GRADE Working Group grades of evidence High quality: We are very confident that the true effect lies close to that of the estimate of the effect Moderate quality: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different Low quality: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect Very low quality: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect | ||||||
1 Small crossover studies given high weighting due to high correlation between treatment periods. 95% CI for pooled treatment estimate consistent with negligible to moderate treatment effectiveness. 2 Information from one study of 13 participants. 3 Only group means provided. No information on variability of response, so unable to establish precision. 4 Information on variability missing. Substantively different methods of recording prevent pooling. 5 Two studies did not report separately for primary Raynaud's phenomenon participants.
Background
Description of the condition
Raynaud's phenomenon (episodic colour changes of the fingers and toes, usually in response to cold exposure or to emotional stress) may be primary (idiopathic); or secondary to a variety of conditions including connective tissue diseases (especially systemic sclerosis‐spectrum disorders), extrinsic vascular compression, use of vibratory equipment (hand‐arm‐vibration‐syndrome), hyperviscosity states, and certain drugs or chemicals (Block 2001). The classic colour changes are white (ischaemia), blue (deoxygenation) and red (reperfusion).
This review of calcium channel blockers is concerned only with primary Raynaud's phenomenon. A key differentiating feature between primary and secondary Raynaud's phenomenon is that in primary Raynaud's phenomenon vasospasm is entirely reversible: irreversible tissue injury does not occur (LeRoy 1992). This is in contrast to the situation in systemic sclerosis, for example, when digital ischaemia can be severe and result in ulceration, scarring, and sometimes even gangrene. Although primary Raynaud's phenomenon is therefore often considered 'benign', it can cause considerable discomfort and distress in severely affected people, with a negative impact on perceived quality of life comparable to in patients with secondary Raynaud's (Hughes 2015).
The pathogenesis of primary Raynaud's phenomenon is not known but it is likely that abnormalities in the neural control elements of vascular tone play a key role (Herrick 2005). Primary Raynaud's phenomenon is common; its reported prevalence varies, in part related to geography, being less prevalent in warmer climates (Maricq 1997). A UK general practice‐based study suggested that 15% to 19% of the population experience Raynaud's phenomenon (Silman 1990), and of these most will have primary Raynaud's phenomenon. Women are more affected than men.
A careful history and examination will usually indicate whether or not a person with Raynaud's phenomenon is likely to have the primary form or whether there is an underlying cause. The key investigations to perform are an erythrocyte sedimentation rate (normal in primary Raynaud's phenomenon), antinuclear antibody (ANA) (negative or only weakly positive in people with primary Raynaud's phenomenon) and nailfold capillaroscopy (normal in people with primary Raynaud's phenomenon) (LeRoy 1992; Wigley 2002). In the person with Raynaud's phenomenon, systemic sclerosis‐specific autoantibodies and nailfold capillaroscopic abnormalities are independent risk factors for systemic sclerosis (Koenig 2008).
Many people with primary Raynaud's phenomenon are reassured to know that they do not have a serious underlying disease and respond well to conservative measures, including keeping warm and avoiding drugs with vasoconstrictive effects (Suter 2005). Smoking cessation should be strongly encouraged. Although smoking has not been proven to adversely affect primary Raynaud's phenomenon, it is likely that Raynaud's could be exacerbated via a variety of possible mechanisms including endothelial injury and increased plasma viscosity. Although some sufferers have reported benefit from temperature biofeedback, this was shown to be ineffective in a large randomised controlled trial of 313 participants with primary Raynaud's phenomenon (RTS 2000).
For those people with primary Raynaud's phenomenon who do not respond to conservative (i.e. 'non‐drug') measures, drug treatment will often be required. The aim of drug treatment is to prevent vasospasm or to increase vasodilation, or both. Despite the high prevalence of primary Raynaud's phenomenon, there have been relatively few controlled clinical trials, probably reflecting how difficult clinical trials of Raynaud's phenomenon are to set up. These have to be run over the winter months, there is a large placebo effect, and there is a lack of reliable outcome measures which are sensitive to change. The Raynaud's Condition Score, a self‐assessment score (0 to 10) which incorporates frequency, duration and severity of attacks, is increasingly used in clinical trials (Merkel 2002).
Description of the intervention
Calcium channel blockers are generally considered first‐line in the drug treatment of primary Raynaud's phenomenon, and are the group of drugs which have been most researched. A meta‐analysis published in 2005 concluded that calcium channel blockers had some effect in primary Raynaud's phenomenon in reducing the frequency and severity of Raynaud's attacks, but that this was small (2.8 to 5.0 fewer attacks per week with a 33% reduction in severity) (Thompson 2005). Other drugs currently used include angiotensin converting enzyme (ACE) inhibitors, angiotensin II receptor antagonists (Dziadzio 1999) and alpha‐adrenergic blockers (Harding 1998). Intravenous prostanoids, used to treat severe exacerbations of digital ischaemia and ulceration in people with systemic sclerosis‐spectrum disorders, are not generally indicated for primary Raynaud's phenomenon. Similarly, surgical intervention, for example digital (palmar) sympathectomy, may be indicated for severe secondary Raynaud's phenomenon but not in people with primary Raynaud's phenomenon.
How the intervention might work
Calcium channel blockers bind to L‐type voltage‐gated calcium channels on cells, preventing influx of extracellular calcium. There are two main categories of calcium channel blocker: the dihydropyridines (these include nifedipine, nicardipine and amlodipine) and the non‐dihydropyridines (these include diltiazem and verapamil) (Sturgill 1998). The dihydropyridines are more selective for vascular smooth muscle than the non‐dihydropyridines: they are potent vasodilators with minimal effect on cardiac contractility or conduction, and can cause reflex tachycardia. In contrast, verapamil and diltiazem are negatively inotropic and can slow cardiac conduction, and are less potent vasodilators than the dihydropyridines. The calcium channel blockers which have been most studied in primary Raynaud's phenomenon, and forming the basis of this review, are dihydropyridines.
Why it is important to do this review
Calcium channel blockers are currently the most commonly prescribed drug treatment for primary Raynaud's phenomenon.
Objectives
To assess the effects of different calcium channel blockers for primary Raynaud's phenomenon as determined by attack rates, severity scores, participant‐preference scores and physiological measurements.
Methods
Criteria for considering studies for this review
Types of studies
We considered all randomised and blinded trials of the effects of orally‐administered calcium channel blockers compared with placebo, or other drug therapy, in people with primary Raynaud's phenomenon, using any method of randomisation. We included trials not analysed on an intention‐to‐treat basis, provided all randomised participants were accounted for. We included cross‐over trials only if they incorporated (a) a placebo or no‐treatment 'run‐in' of at least one week (or provided baseline data for quantification of the severity of the condition), and (b) a 'wash‐out' period of at least one week between treatment arms for all participants. We identified but did not include in the meta‐analyses studies in which primary and secondary cases could not be resolved. We also excluded open studies and single‐dose studies. There was no restriction on language, with publications in languages other than English translated and assessed for eligibility.
Types of participants
Adults, over 18 years of age, with clinical features of primary Raynaud's phenomenon. Trials including a mixture of primary and secondary participants were included if primary participants could be identified and analysed separately.
Types of interventions
Experimental intervention
Oral administration of a calcium channel blocker.
Comparator intervention
Placebo
Another pharmacological therapy
Other treatments
Types of outcome measures
Primary outcomes
Attack rates of Raynaud's phenomenon
Duration of attacks
Severity scores
Participant‐preference scores
Secondary outcomes
Physiological measurements (including digital temperature and blood flow response to hand cooling)
Adverse reactions (e.g. flushing, headache, tachycardia and ankle swelling), and withdrawal of medication.
Search methods for identification of studies
There were no language or publication status restrictions.
Electronic searches
For this update the Cochrane Vascular Trial Search Co‐ordinator (TSC) searched the Specialised Register (January 2016). In addition the TSC searched the Cochrane Central Register of Controlled Trials (CENTRAL) 2015, Issue 12, part of theCochrane Library, www.cochranelibrary.com.
See Appendix 1 for details of the search strategy used to search CENTRAL. The Specialised Register is maintained by the TSC and is constructed from weekly electronic searches of MEDLINE, EMBASE, CINAHL, AMED, and through handsearching relevant journals. The full list of the databases, journals and conference proceedings which have been searched, as well as the search strategies used are described in the Specialised Register section of the Cochrane Vascular module in theCochrane Library (www.cochranelibrary.com).
The TSC searched the following trial databases (January 2016) for details of ongoing and unpublished studies using the term 'Raynaud';
World Health Organization International Clinical Trials Registry (apps.who.int/trialsearch/) ClinicalTrials.gov (clinicaltrials.gov/) ISRCTN registry (www.controlled‐trials.com/) Nederlands Trials Register (www.trialregister.nl/trialreg/admin/rctsearch.asp)
Searching other resources
For this update all trials listed under 'channel blockers' on www.trialscentral.org were checked by the authors for reference to Raynaud's phenomenon (January 2016) with no relevant results found.
Reference lists of relevant studies and reviews were also checked for potentially relevant studies.
Data collection and analysis
Selection of studies
We obtained full‐text articles of all the references identified and where necessary translated them. For this update, three review authors (MH, MA, AH) independently assessed the articles identified by the search. For the previous version, three review authors (HE, MA and AH) independently reviewed the articles and resolved disagreements by discussion and consensus. One review author (HE) contacted six trial authors or co‐authors for additional information on articles where data from subgroups could not be identified. We obtained email addresses by searching the relevant article, the author's or co‐author's most recent reference in PubMed, and 'Google'. We received replies from four.
Data extraction and management
No new studies were identified for this update. In the previous version, three review authors (HE, MA and AH) independently reviewed each study identified as being eligible for inclusion, and extracted data from included studies using the Cochrane Peripheral Vascular Diseases Group 'Data Extraction Table'. This includes method of allocation, degree of blinding, power calculations, exclusions post‐randomisation, losses to follow‐up, source of funding, number of participants, age and sex of participants, inclusion and exclusion criteria, treatment, control group, duration of study, and outcome measures. We resolved disagreements by discussion and consensus. Where a trial was described in multiple publications, we extracted data from the most complete report.
Assessment of risk of bias in included studies
No new studies were identified for this update. In the previous version, three review authors (HE, MA, AH) independently assessed trial quality as being at 'low', 'high' or 'unclear' risk of bias across the following areas, with reference to the criteria listed in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011):
1. Adequate sequence generation. 2. Allocation concealment. 3. Blinding of participants and personnel, and of outcome assessment. 4. Incomplete outcome data addressed. 5. Free of selective reporting. 6. Free of any other bias: i.e. if no other potential sources of bias were identified.
We resolved disagreement about whether or not a trial fulfilled certain quality criteria by consensus, in discussion with the other review authors (HE, JW). All quality criteria ratings and supporting information are listed in the 'Risk of bias' tables (See Characteristics of included studies).
Measures of treatment effect
The rate of attacks was the only outcome for which we deemed it appropriate to conduct meta‐analysis. The results of the remaining outcomes were described separately for each study. For attack rate, the measure of treatment effect used was the difference in mean number of Raynaud's attacks in the treatment and placebo groups. Mean numbers of attacks were reported over different time periods across the studies and, in the case of RTS 2000, geometric means were reported, requiring log transformation in order to make the comparison. Accordingly, we used a standardised mean difference as the parameterisation of treatment effect, calculated by the difference in means divided by the pooled standard deviation (SD) for each study. We calculated the individual study estimates of treatment effect in R (R Development Core Team 2012).
Numbers of attacks were reported on different scales across trials. We calculated standardised mean differences for each trial, in order to provide commensurable estimates of treatment effect. Following data synthesis, we transformed the pooled estimate of treatment effect and its 95% confidence interval to an interpretable scale by multiplying by a typical SD, obtained by pooling the standard deviations of the trials in the analysis (Higgins 2011, section 12.6.4).
Unit of analysis issues
The individual participant was the unit of analysis. For cross‐over trials, the calculation of standard errors accounted for the fact that observations were paired (Elbourne 2002).
Dealing with missing data
Five trials had missing data due to participant withdrawal. In three of these (Sarkozi 1986; Vayssairat 1991; Wollersheim 1991), participant‐level data were not reported and no intention‐to‐treat analysis had been attempted. For these studies, we analysed the data as reported based on all participants retained in the study. As such, we assumed missing data to be missing completely at random. In one of the five studies (Ettinger 1984), participant‐level data were reported, but the number of participants with primary Raynaud's phenomenon was too low to permit meaningful imputation of missing values. One study (RTS 2000), presented results having conducted multiple imputation to account for missing data. We used these results in the meta‐analysis. We contacted two authors for missing data or if the reporting of data was unclear and, while both responded, no additional data were available.
Assessment of heterogeneity
We examined statistical heterogeneity using a Cochrane's Q test and the I² statistic (Higgins 2003).
Wherever there appeared to be at least moderate statistical heterogeneity (I² > 30%), we performed a random‐effects analysis to test the robustness of the results derived from the fixed‐effect approach. Assessment of clinical heterogeneity was based on consideration of the protocols of the studies included in the analysis and their individual estimates of effect size. Wherever we suspected clinical heterogeneity, we performed a sensitivity analysis by repeating the meta‐analysis with the clinically heterogeneous trials excluded.
Assessment of reporting biases
We entered primary outcome data from all included studies into a funnel plot (trial effect against trial size) to investigate the possibility of publication bias, but the small number of studies (seven) meant that we could reach no definitive conclusion.
Data synthesis
We performed statistical analysis according to the statistical guidelines provided for authors by the Cochrane Peripheral Vascular Diseases Group and from the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
We combined the results of five cross‐over trials and two parallel‐group trials in a meta‐analysis. These compared the calcium channel blockers nifedipine and nicardipine with placebo for the outcome 'frequency of Raynaud's attacks'. We calculated estimates of treatment effect and standard errors for each of the studies.
Two studies did not report SDs for treatment or placebo groups, and did not contain sufficient information to calculate them. This did not, however, prevent the calculation of a standardised mean difference for either study. For one study (Wollersheim 1991), the mean difference in the number of attacks, and therefore the standardised difference, was zero. We made a conservative assumption that there was no correlation between participants' measurements, resulting in a larger standard error and a relatively low weighting for the study within the meta‐analysis. The other study (RTS 2000) reported geometric means for attack rates and adjusted P values. By using the upper limit of the P value and log‐transforming the geometric means, it was possible to calculate a standard error for the difference in means. From this, it was possible to calculate a standardised mean difference and a corresponding standard error.
We then pooled the estimates using the generic inverse variance method, whereby each study is weighted according to the precision of its estimate of treatment effect. Following synthesis, we back‐transformed the pooled estimate to an interpretable scale, as described above.
We used a forest plot to show individual study estimates and 95% confidence intervals (CI), together with the pooled estimate. We conducted the meta‐analysis using the Review Manager 5 software (RevMan 2012) provided by The Cochrane Collaboration.
Subgroup analysis and investigation of heterogeneity
We performed separate subgroup analyses for the nifedipine and nicardipine trials. To investigate the influence of clinically heterogeneous trials, we performed sensitivity analyses with the trials in question excluded.
Sensitivity analysis
We conducted sensitivity analyses to examine the robustness of the meta‐analysis as follows:
If a study had a discordant estimate of treatment effect, we repeated the analysis with that study removed.
If a study had a weighting that seemed disproportionate to its sample size, we repeated the analysis with that study removed.
If an analysis displayed at least moderate statistical heterogeneity (e.g. I² > 30%) we repeated the analysis using the random‐effects method.
Summary of findings
We produced a summary of findings table for the comparison calcium channel blockers versus placebo for patients with primary Raynaud’s phenomenon, using the GRADEpro GDT software. We used the GRADE approach to assess the quality of the evidence for the primary review outcomes of attack rates, duration of attacks, severity and patient preference. We downgraded the evidence from 'high quality' for serious or very serious study limitations (risk of bias, indirectness and inconsistency of evidence, imprecision of effect estimates or potential publication bias) according to the Cochrane Handbook for Systematic Reviews (Higgins 2011) and the GRADE Working Group (GRADE Working Group 2004).
To highlight, the number of participants in the summary of findings table does differ from the outcome number of attacks because the participants in the crossover studies are counted twice by RevMan 2012, due to the fact that each acts as their own control. Our analysis takes this into account, by allowing for the correlation between the paired measurements in the crossover studies.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
See Figure 1.
1.
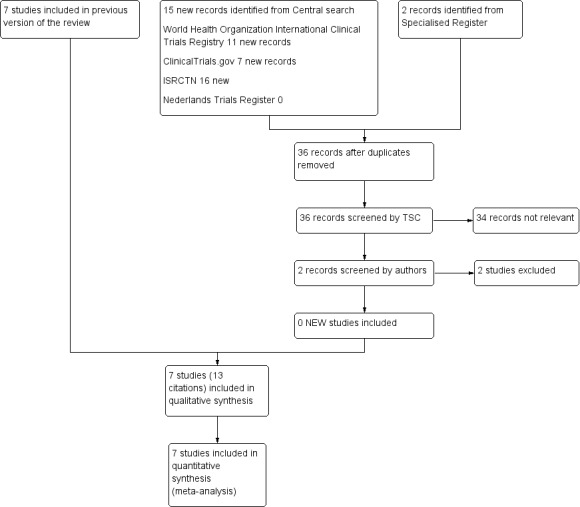
Study flow diagram.
For this update two additional studies were excluded.
Included studies
Seven trials were eligible for inclusion in the review. Three trials had multiple publications (Ettinger 1984; Kahan 1987; RTS 2000). In two cases (Ettinger 1984; RTS 2000), we extracted data from the main trial report and in one case (Kahan 1987) from the English language publication. See the Characteristics of included studies table. The seven included trials had a total of 296 participants with primary Raynaud's phenomenon. The largest study (RTS 2000) involved 158 participants, three (Sarkozi 1986; Vayssairat 1991; Wollersheim 1991) had between 69 and 16 participants and the remaining three studies (Rodeheffer 1983; Ettinger 1984; Kahan 1987) had six or fewer participants. The studies were all published between 1983 and 2000.
In the 87 full‐text articles assessed for eligibility, we identified a total of 11 calcium channel blockers (48 studies involved nifedipine, eight nicardipine, five nisoldipine, four diltiazem, three felodipine, three flunarizine, two isradipine, one amlodipine, one verapamil, one phendilin and one drug in development, with some examining more than one calcium channel blocker). The seven included trials represented two different drugs (four nifedipine (Rodeheffer 1983; Ettinger 1984; Sarkozi 1986; RTS 2000) and three nicardipine (Kahan 1987; Vayssairat 1991; Wollersheim 1991)).
Types of participants
Four trials had a mixture of participants with primary and secondary Raynaud's phenomenon (Rodeheffer 1983; Ettinger 1984; Kahan 1987; Wollersheim 1991). However, results were presented separately for the two groups. The remaining trials included only participants with primary Raynaud's phenomenon. The definitions of primary Raynaud's phenomenon used within the seven trials varied substantially: two referenced specific criteria (Wollersheim 1991 used Allen 1932; Vayssairat 1991 used Priollet 1987), four provided descriptions only (Rodeheffer 1983: symptomatic Raynaud's phenomenon relating to cold or stress without demonstrable systemic disease; Ettinger 1984: episodic digital pallor and cyanosis on cold exposure without associated demonstrable disease; Sarkozi 1986: episodic, well‐demarcated, digital pallor or cyanosis in response to cold or emotional stimuli, episodes associated with numbness and pain and no clinical evidence of primary disease; and Kahan 1987: symptomatic, bilateral Raynaud's without secondary disease) while RTS 2000 screened participants for primary Raynaud's using colour charts (Maricq 1988) and, along with Vayssairat 1991, used nailfold capillaroscopy, antinuclear antibody (ANA) testing and physical examinations to rule out secondary Raynaud's. In two trials (Rodeheffer 1983; Sarkozi 1986), references to positive ANA titres or the development of digital gangrene amongst trial participants indicate that some participants diagnosed as primary Raynaud's phenomenon may not have fulfilled what are now the generally accepted criteria.
Major exclusion criteria were secondary Raynaud's phenomenon in three trials (Sarkozi 1986; Vayssairat 1991; RTS 2000) although, as above, there were concerns that some participants with secondary Raynaud's phenomenon may have been included in one of these studies (Sarkozi 1986). Participants were required to be free of vasoactive medications one week prior in one case (Sarkozi 1986), four weeks prior in one case (Wollersheim 1991), and at the start of the trial in four cases (Rodeheffer 1983; Ettinger 1984; Kahan 1987; Vayssairat 1991). Ettinger 1984 also required participants not to take non‐steroidal anti‐inflammatory drugs (NSAIDS), aspirin and drugs affecting the nervous system. One trial did not list medications as exclusion criteria (RTS 2000).
Baseline frequency of Raynaud's attacks was provided in only one trial (RTS 2000; as an average daily attack rate during the previous cold season) and five trials had a minimum attack rate as an inclusion criterion. Participants were required to have at least two attacks per day during the previous cold season in one trial (RTS 2000), one attack per day in two trials (Ettinger 1984; Wollersheim 1991), two per week in one trial (Sarkozi 1986) and three per week in one trial (Vayssairat 1991). Two trials (Rodeheffer 1983; Kahan 1987) did not stipulate a minimum attack rate.
One study (Rodeheffer 1983) had only female participants and four trials had more female than male participants (Ettinger 1984; Sarkozi 1986; Vayssairat 1991; RTS 2000), which would reflect the prevalence of primary Raynaud's phenomenon in the population. In two trials (Kahan 1987; Wollersheim 1991), no breakdown by gender was available for participants with primary Raynaud's phenomenon. The age range across the studies also reflected the age distribution of Raynaud's phenomenon in the population by including both young and older participants: three trials included participants aged 18 to 65 years, one trial 18 to 68 years, one trial 20 to 49 years and in two cases the mean age was 39 and 45 years. Ethnicity data were provided in only one trial (RTS 2000). All seven trials were based within a hospital setting: three in the USA, two in France, one in Canada and one in the Netherlands. Five trials were conducted during the cold season, one trial ran from January to June (Kahan 1987) and in one trial (Wollersheim 1991) the timing was not specified. Data on smoking habits were provided in only one trial (Sarkozi 1986).
Interventions
The included trials represented two different drugs: four trials with nifedipine and three with nicardipine. Four trials compared a calcium channel blocker with placebo using a cross‐over design, two trials compared a calcium channel blocker with placebo using a parallel design and one study compared a calcium channel blocker with another active drug (dazoxiben) and with placebo using a cross‐over design (Ettinger 1984).
Length of studies
The duration of the treatment periods varied between studies: two weeks (Rodeheffer 1983; Ettinger 1984; Kahan 1987; Vayssairat 1991), three weeks (Wollersheim 1991), 10 weeks (Sarkozi 1986) and 12 to 13 months (RTS 2000). Three trials had a run‐in period on no medication lasting either one week (Sarkozi 1986; Kahan 1987) or three weeks (Wollersheim 1991). One trial had a one‐week run‐in period with single‐blind placebo treatment (Vayssairat 1991) and two trials had a two‐week run‐in period with single‐blind placebo treatment (Rodeheffer 1983; Ettinger 1984). In one trial, there was a one‐month baseline observation period but it was not clear if participants were required to be off medication (RTS 2000).
Outcomes
All the trials were published prior to 2001 and so predated the use of the Raynaud's Condition Score (Merkel 2002). Frequency of Raynaud's attacks was recorded in all seven trials using participant diaries. In one trial (RTS 2000) participants were provided with a colour chart and each verified attack had to be at least 30 minutes apart.
Duration of attacks was recorded in only one trial (Wollersheim 1991).
Severity of Raynaud's attacks was recorded in six of the seven trials (Ettinger 1984; Sarkozi 1986; Kahan 1987; Vayssairat 1991; Wollersheim 1991; RTS 2000). Severity was recorded in five trials using participant diaries whereby each attack was rated according to a scale, although the scales used differed: mild, moderate or severe (Ettinger 1984), 0 to 3 with 0 = mild and 3 = severe (Sarkozi 1986), 0 to 10 scale (Wollersheim 1991), 1 to 4 scale with 1 = mild and 4 = highly severe (Vayssairat 1991) and a four‐point scale (RTS 2000). In one trial, severity of attacks was graded at the end of each treatment period using a single scale of 1 to 4 with 1 = slight to 4 = very severe (Kahan 1987). One trial (Rodeheffer 1983) did not record severity of Raynaud's attacks.
Four trials (Rodeheffer 1983; Ettinger 1984; Wollersheim 1991; RTS 2000) used some form of participant‐preference scores although again scales used differed substantially across trials. Rodeheffer 1983 asked participants to rate the overall effectiveness of treatment at the end of each treatment period on a five‐point scale (marked improvement, moderate improvement, minimal improvement, no change, worse). Ettinger 1984 asked participants to rate overall response to treatment and side effects at the end of each treatment period on a four‐point scale (worse or no change, slight change, moderate change, marked change). Wollersheim 1991 asked participants to rate drug effectiveness relative to baseline at the end of each treatment period on a five‐point scale (much worse, worse, no difference, better, much better). Finally, RTS 2000 asked participants to rate improvement compared to baseline at quarterly visits.
Two trials (Vayssairat 1991; RTS 2000) included quality of life measures, although data were reported only in one (Vayssairat 1991).
Physiological measurements were used in five trials (Rodeheffer 1983; Ettinger 1984; Sarkozi 1986; Vayssairat 1991; Wollersheim 1991). Ettinger 1984 examined finger systolic pressure after local cooling at the end of each treatment period but no data were available specifically for participants with primary Raynaud's phenomenon. Rodeheffer 1983 assessed digital‐artery systolic pressure at 30 and 15 degrees C. Sarkozi 1986 assessed pulse amplitude of digital blood flow (assessed by photoplethysmography) and time to return to baseline pulse amplitude following cold pressor challenge at baseline and on the last day of treatment. Vayssairat 1991 assessed cold‐reactive hyperaemia as measured by skin temperature using cold challenge tests at each visit with the exception of the 'wash‐out' period. Wollersheim 1991 examined response to a finger‐cooling test as measured by finger skin temperature, laser Doppler flux and transcutaneous oxygen tension.
Excluded studies
See: Characteristics of excluded studies and Figure 1.
For this update two additional studies were excluded (Fontenelle 2008; Lee 2014), making a total of sixty‐four excluded studies. Of these, twenty‐three were excluded because they did not meet the review inclusion criteria of a minimum one week wash‐out within cross‐over designs and a minimum one week run‐in at baseline or baseline data on the severity of Raynaud's phenomenon (Smith 1982; Baadsgaard 1983; Kahan 1983a; Kahan 1983b; Winston 1983; Agnelli 1984; Kahan 1985a; Kahan 1985b; Corbin 1986; Finch 1986; Gjorup 1986a; Gjorup 1986b; Hawkins 1986; Kahan 1986; Redondo 1986; White 1986; Challenor 1987; Kallenberg 1987; Rupp 1987; Wigley 1987a; Wise 1987; Van Heereveld 1988; Challenor 1989). Ten trials included participants with both primary and secondary Raynaud's phenomenon and did not present data which allowed data extraction for the primary group (and no additional data could be obtained) (Müller‐Bühl 1983; Rhedda 1985; Aldoori 1986; Waller 1986; Costantini 1987; Finch 1988; Ferri 1992; La Civita 1993; La Civita 1996; Martinez 1999). In eight trials the drug was administered only once (single‐dose trials) (Kahan 1981; Gush 1987; Lewis 1987; Morgan 1987; Wollersheim 1987; Weber 1990; Dompeling 1992; Fontenelle 2008), six trials were open studies (Shcherbakov 1987; Codella 1989; Denton 1999; Dziadzio 1999a; Coleiro 2001; Choi 2009) and participants within seven trials did not have primary Raynaud's phenomenon (Sauza 1984; Da Costa 1987; Schmidt 1989; Mascagni 1994; Varela‐Aguilar 1997; Scorza 2001; Lee 2014). In four trials the active drug was not a calcium channel blocker, or a calcium channel blocker was used in combination with another therapy (Brotzu 1989; Moriau 1993; Csiki 2011; Caglayan 2012). Four trials were not randomised (Creager 1984; Pisenti 1984; Stefenelli 1986; Leppert 1989). In two trials, the definition of the outcome and description of the data collection were lacking (Nilsson 1987; Wasir 1983). Many trials had more than one reason for exclusion. The principle reasons for exclusion only are listed in Characteristics of excluded studies.
Risk of bias in included studies
See: Figure 2. All included studies were described as randomised and double‐blinded. No studies were classed as being at high risk of bias.
2.
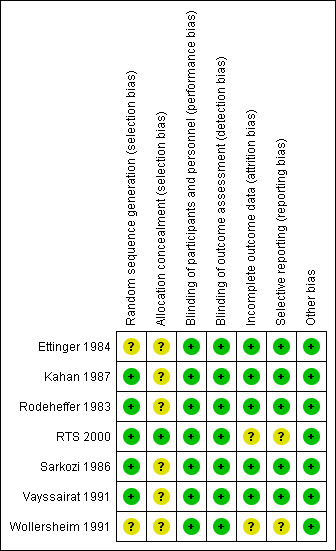
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Six of the seven included trials (Rodeheffer 1983; Ettinger 1984; Sarkozi 1986; Kahan 1987; Vayssairat 1991; Wollersheim 1991), did not provide full details of the method of allocation and, as a result, these were classed as having an unclear risk of bias.
Blinding
All included studies were described as being double‐blind with both participants and personnel unaware of the treatment allocation. Data regarding Raynaud's attack frequency, severity and duration were all collected from participant diaries. Overall, from the descriptions provided by authors, the blinding appeared to be adequate.
Incomplete outcome data
In two trials (Wollersheim 1991; RTS 2000), the reporting of outcome data and side effects associated with treatment was difficult to interpret.
Selective reporting
In RTS 2000, participant progress through the study was not clear. In both Wollersheim 1991 and RTS 2000 not all outcomes described in the methods were reported in full.
Other potential sources of bias
We identified no other potential sources of bias.
Follow‐up and exclusions
Only one trial (RTS 2000) discussed intention‐to‐treat analysis. This study had forty‐three drop‐outs after randomisation (27%). Two trials (Rodeheffer 1983; Kahan 1987) had no drop‐outs or exclusions. Four trials reported loss to follow‐up. Ettinger 1984 had three withdrawals (50%) which, in all cases, were due to symptomatic orthostatic hypotension while on nifedipine. Sarkozi 1986 had seven withdrawals (18%), of which five were on nifedipine (two for digital gangrene, two for non‐compliance and one for severe flushing) and two were on placebo (one for pregnancy and one for non‐compliance). Vayssairat 1991 had nine withdrawals (13%), of which two were for personal reasons and seven due to side effects (five on placebo and two on nicardipine for headache/nausea and malaise/vertigo). Wollersheim 1991 had three (18%) withdrawals due to side effects (one on placebo and two on nicardipine) although the reasons were not recorded.
Power calculations
Power calculations for a comparison of a calcium channel blocker with a placebo for participants with primary Raynaud's phenomenon were given by only one trial (Vayssairat 1991). Overall, the sample sizes in the included trials were small.
Cross‐over, carry‐over and period effect
All five cross‐over studies included in the review (Rodeheffer 1983; Ettinger 1984; Kahan 1987; Vayssairat 1991; Wollersheim 1991) incorporated a wash‐out period of at least one week. In three cases (Rodeheffer 1983; Ettinger 1984; Vayssairat 1991), this involved single‐blind treatment with placebo and in the remaining two cases (Kahan 1987; Wollersheim 1991) it involved no vasoactive treatment. One study reported that there was no observed order effect (Kahan 1987). In the remaining studies, no testing for period and carry‐over effect was reported.
Funding
Five trials (Rodeheffer 1983; Ettinger 1984; Vayssairat 1991; Wollersheim 1991; RTS 2000) had support from a pharmaceutical company (two (Vayssairat 1991; Wollersheim 1991) received financial support and three (Rodeheffer 1983; Ettinger 1984; RTS 2000) received donations of the active drug), three trials also had support from charitable bodies (Rodeheffer 1983; Wollersheim 1991; RTS 2000) and the remaining two (Sarkozi 1986; Kahan 1987) did not state their source of funding.
Effects of interventions
See: Table 1
Attack rates of Raynaud's phenomenon
The meta‐analysis suggested a statistically significant advantage of treatment with calcium channel blockers compared to placebo as measured by the number of Raynaud's attacks (standardised mean difference (SMD) of 0.23; 95% confidence interval (CI) 0.08 to 0.38, P = 0.003) although the effect size was small and the 95% CI indicated that the actual treatment effect may be very small to moderate (Figure 3; Analysis 1.1). The transformed results, using a pooled SD of 7.48, indicated a decrease in the number of Raynaud's attacks per week on calcium channel blockers as compared to placebo (1.72; 95% CI 0.60 to 2.84). Calcium channel blockers, therefore, could reduce the weekly number of attacks by as few as 0.6, or as many as 2.8.
3.
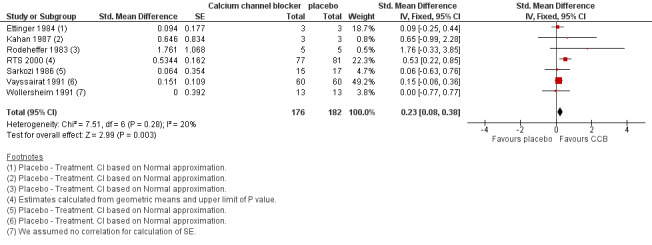
Comparison 1 Calcium channel blockers versus placebo, Outcome: 1 Number of attacks.
1.1. Analysis.
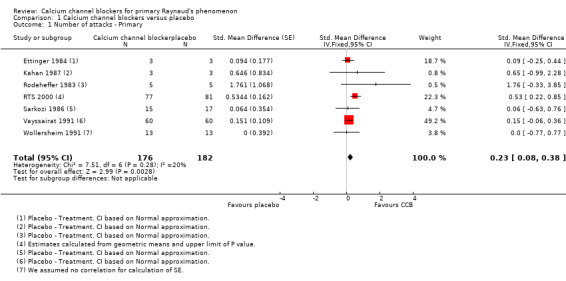
Comparison 1 Calcium channel blockers versus placebo, Outcome 1 Number of attacks ‐ Primary.
Within the meta‐analysis, Ettinger 1984 received a high weighting inconsistent with the number of participants but when we conducted a sensitivity analysis the effect of excluding this study made little difference to the overall results (Analysis 1.2). RTS 2000 was the only trial with a 95% CI for the SMD which unequivocally suggested a beneficial effect of treatment (the interval did not span zero). Accordingly, the meta‐analysis was repeated with RTS 2000 removed. The effect was a reduction of the point estimate and confidence limits of the overall estimate of treatment effect. We no longer observed a statistically significant treatment effect (Figure 4; Analysis 1.3).
1.2. Analysis.
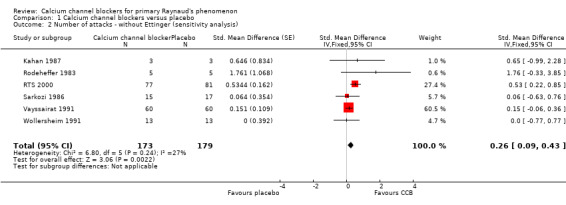
Comparison 1 Calcium channel blockers versus placebo, Outcome 2 Number of attacks ‐ without Ettinger (sensitivity analysis).
4.
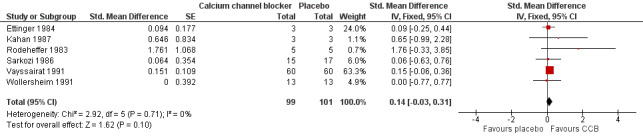
Comparison: 1 Calcium channel blockers versus placebo, Outcome: 1.5 Number of attacks ‐ without RTS 2000 (sensitivity analysis).
1.3. Analysis.
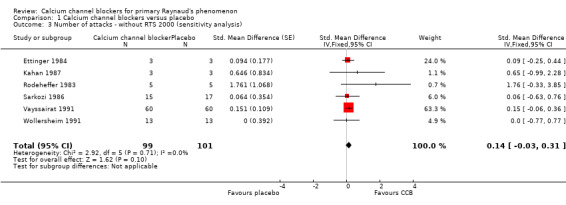
Comparison 1 Calcium channel blockers versus placebo, Outcome 3 Number of attacks ‐ without RTS 2000 (sensitivity analysis).
The results are also presented for each drug separately.
Nifedipine
(ATC classification code C08CA05: Selective calcium channel blocker with mainly vascular effects; dihydropyridine derivatives)
Four trials were included (Rodeheffer 1983; Ettinger 1984; Sarkozi 1986; RTS 2000): two were parallel (Sarkozi 1986; RTS 2000) and two were cross‐over (Rodeheffer 1983; Ettinger 1984). One parallel trial compared 10 mg nifedipine three times a day rising to 20 mg three times a day if no improvement shown at five weeks (Sarkozi 1986) with placebo for a total of 10 weeks. The other parallel trial compared 30 mg sustained‐release nifedipine once a day rising to 60 mg if well tolerated for a period of one year (RTS 2000). One cross‐over trial compared 20 mg nifedipine three times a day with placebo for a period of two weeks (Ettinger 1984), while the other cross‐over trial compared 10 mg nifedipine three times a day rising to 20 mg if well tolerated with placebo for a period of two weeks (Rodeheffer 1983).
The effect of excluding the three nicardipine trials was to increase the point estimate and confidence intervals for the size of the treatment effect (Figure 5; Analysis 1.4). A significant treatment effect was found at the 5% level (P = 0.004). A pooled SD of 7.51 was calculated for the nifedipine trials to give a difference in means of 2.41; 95% CI 0.75 to 4.06.
5.
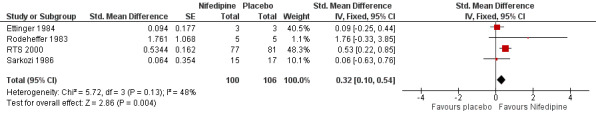
Comparison 1: Calcium channel blockers versus placebo, Outcome: 1.2 Number of attacks ‐ Nifedipine trials only.
1.4. Analysis.
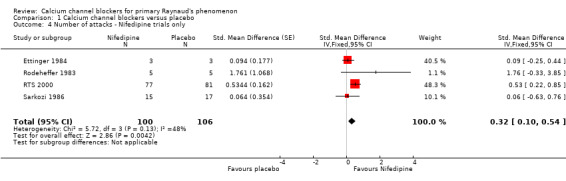
Comparison 1 Calcium channel blockers versus placebo, Outcome 4 Number of attacks ‐ Nifedipine trials only.
Nifedipine therefore could reduce the weekly number of attacks by as few as 0.8, or by as many as 4.1. There was a considerable amount of statistical heterogeneity in the meta‐analysis of nifedipine trials alone (I² = 48%). We performed a random‐effects analysis to investigate whether the results were robust to heterogeneity and, while this had a negligible effect on the point estimate of treatment effect, the confidence interval was wider and the estimate of treatment effect was no longer statistically significant at the 5% level (Analysis 1.5). The difference in means was 2.33 (95% CI ‐0.38 to 5.11).
1.5. Analysis.
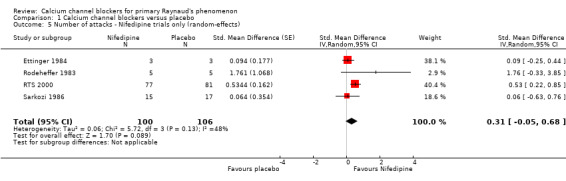
Comparison 1 Calcium channel blockers versus placebo, Outcome 5 Number of attacks ‐ Nifedipine trials only (random‐effects).
Nicardipine
(ATC classification code C08CA04: Selective calcium channel blocker with mainly vascular effects; dihydropyridine derivatives)
Three cross‐over trials were included (Kahan 1987; Vayssairat 1991; Wollersheim 1991): one compared 30 mg nicardipine three times a day with placebo for three weeks (Wollersheim 1991), one compared 50 mg long‐acting nicardipine twice a day with placebo for two weeks (Vayssairat 1991) and one compared 20 mg nicardipine three times a day with placebo for two weeks (Kahan 1987). Two trials included participants with both primary and secondary Raynaud's phenomenon, and only one was restricted to primary Raynaud's only.
We found no overall effect of nicardipine (Figure 6; Analysis 1.6). Multiplying the estimate and CI by the baseline SD reported in Vayssairat 1991 gave a result of 1.11 (95% CI ‐0.44 to 2.59). Nicardipine therefore could increase the weekly number of attacks by as much as 0.4, could decrease the weekly number of attacks by as much as 2.6 or have no effect whatsoever.
6.
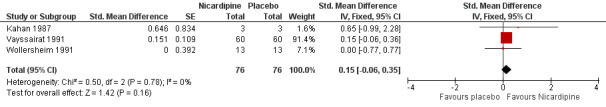
Comparison 1: Calcium channel blockers versus placebo, Outcome: 1.3 Number of attacks ‐ Nicardipine trials only.
1.6. Analysis.
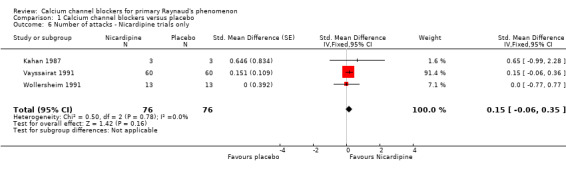
Comparison 1 Calcium channel blockers versus placebo, Outcome 6 Number of attacks ‐ Nicardipine trials only.
Duration of attacks
Duration of attacks was recorded in only one trial (Wollersheim 1991): the average duration of attacks was 11 minutes in participants in the placebo group and 13 minutes in the nicardipine group but this difference was not statistically significant. Insufficient information was available to reproduce this analysis, and no P values or confidence intervals were provided.
Severity scores
Severity of Raynaud's attacks was recorded in six of the seven trials (Ettinger 1984; Sarkozi 1986; Kahan 1987; Vayssairat 1991; Wollersheim 1991; RTS 2000). In one trial (Ettinger 1984), severity scores were not reported specifically for participants with primary Raynaud's and no results were provided in another trial (RTS 2000). Wollersheim 1991 reported no significant difference between placebo and treatment although insufficient information was available to reproduce the analysis, and no P values or confidence intervals were reported. Vayssairat 1991 reported no significant difference between 60 participants receiving placebo and nicardipine in a cross‐over design (mean score ± SD: 1.55 ± 0.7 in placebo versus 1.36 ± 0.8 in nicardipine; difference 0.2 (95% CI of difference 0 to 0.4)). No P value was reported and it was not possible to reproduce the Wilcoxon signed rank test due to a lack of participant‐level data. One trial (Sarkozi 1986) reported average percentage change in number of attacks, broken down by severity. A reduction in the average number of mild, moderate and severe attacks was reported for participants on nifedipine, although no statistical comparison was made with the placebo group. In one trial (Kahan 1987) a comparison of severity scores in primary Raynaud's participants was not made, but the authors of this review were able to make a comparison using reported participant‐level data with a median score of 2 for three participants while on placebo versus 2 while on nicardipine (Wilcoxon signed rank test, P > 0.9999). We did not pool severity data for meta‐analysis for the following reasons: scales were generally described and unvalidated, the timing of assessment differed in one trial (Kahan 1987), and no data on severity were reported in one trial (RTS 2000).
Participant‐preference scores
Four trials (Rodeheffer 1983; Ettinger 1984; Wollersheim 1991; RTS 2000) used some form of participant‐preference scores although scales used differed substantially across trials. Rodeheffer 1983 asked participants to rate the overall effectiveness of treatment at the end of each treatment period on a five‐point scale (marked improvement, moderate improvement, minimal improvement, no change, worse) with no significant difference between placebo and nifedipine (mean score of 0.6 in placebo group versus 2.2 in nifedipine group, P = 0.25 by Wilcoxon signed rank test). Ettinger 1984 asked participants to rate overall response to treatment and side effects at the end of each treatment period on a four‐point scale (worse or no change, slight change, moderate change, marked change) and found no significant difference between nicardipine and placebo although no breakdown was available for participants with primary Raynaud's phenomenon. Wollersheim 1991 asked participants to rate drug effectiveness relative to baseline at the end of each treatment period on a five‐point scale (much worse, worse, no difference, better, much better) and found no preference for nicardipine over placebo, although again no breakdown was available for participants with primary Raynaud's phenomenon. Finally, RTS 2000 asked participants to rate improvement compared to baseline at quarterly visits and found participants rated symptoms as better on nifedipine in comparison to placebo (33% in placebo group versus 73% in nifedipine group, P < 0.001). We did not pool participant‐preference data for meta‐analysis for the following reasons: scales were generally described and unvalidated, definitions of participant‐preference differed slightly across all four trials, and one trial (RTS 2000) collected data at a different time point.
Two trials (Vayssairat 1991; RTS 2000) included quality of life measures, although data were reported only in one (Vayssairat 1991).
Physiological measurements (including digital temperature and blood flow response to hand cooling)
Physiological measurements were used in five trials (Rodeheffer 1983; Ettinger 1984; Sarkozi 1986; Vayssairat 1991; Wollersheim 1991). Ettinger 1984 examined finger systolic pressure after local cooling at the end of each treatment period but no data were available specifically for participants with primary Raynaud's phenomenon. Rodeheffer 1983 assessed digital‐artery systolic pressure at 30 and 15 degrees C and found no significant change in the mean cold‐induced fall in digital artery systolic blood pressure between nifedipine and placebo (P = 0.625 and P > 0.999 respectively, from Wilcoxon signed rank test conducted by the review authors). Sarkozi 1986 assessed pulse amplitude of digital blood flow (assessed by photoplethysmography) and time to return to baseline pulse amplitude following cold pressor challenge at baseline and on the last day of treatment. No significant differences were found between baseline and at the end of treatment in either the nifedipine or placebo groups. Between‐group comparisons of physiological measurements after 10 weeks of treatment conducted by the authors of this review found no significant differences for pulse amplitudes (P = 0.896 and P = 0.815 by unpaired t‐tests, right and left hands respectively) or for responses to the cold pressor test (P = 0.542 and P = 0.066) for right index and middle fingers respectively). Vayssairat 1991 assessed cold‐reactive hyperaemia as measured by skin temperature using cold challenge tests at each visit with the exception of the 'wash‐out' period but found no significant increase in reactive hyperaemia between the nicardipine and placebo groups (mean of 0.49 ± ‐2.4 in placebo versus 0.77 ± ‐1.6 in treatment group, difference of ‐0.3 ± ‐3, 95% CI ‐1.0 to 0.5, but no P value given and insufficient information to reproduce the analysis). Wollersheim 1991 examined response to a finger cooling test as measured by finger skin temperature, laser Doppler flux and transcutaneous oxygen tension. No effects of nicardipine were found for any of these three parameters, nor were there any differences between participants with primary and secondary Raynaud's phenomenon. P values were not reported but were calculated by the review authors using unpaired t‐tests, as insufficient data were available to calculate the standard error of the mean difference required for paired t‐tests (finger skin temperature P = 0.830, laser Doppler flux P = 0.570, transcutaneous oxygen tension P = 0.812). Accordingly, we acknowledge that these tests will have reduced power. We did not pool physiological data for meta‐analysis because the methodology used varied substantially and the results could not be synthesised.
Adverse reactions
Adverse reactions for participants with primary Raynaud's phenomenon were reported differently across the seven trials. Rodeheffer 1983 reported a significantly higher incidence of mild transient headaches in the nifedipine group versus the placebo group but no breakdown was available for participants with primary Raynaud's phenomenon. Ettinger 1984 provided no adverse event breakdown but (as above) referred to three withdrawals in the nifedipine group due to hypotension. Sarkozi 1986 indicated that all participants on nifedipine reported side effects and, in addition, there was a significantly higher incidence of palpitations in the nifedipine group versus the placebo group (1/18 in placebo and 7/18 in treatment group, P = 0.020) as well as flushing (0/18 in placebo and 6/18 in treatment group, P = 0.010). Kahan 1987 did not provide a breakdown by treatment group. Wollersheim 1991 did not provide a breakdown but flushing, palpitations and headaches were referred to as common adverse events – 39 adverse events/16 participants in placebo group and 50 adverse events/16 participants in treatment group plus 1 discontinued therapy/16 participants in placebo group and 2 discontinued therapy/16 participants in treatment group. Vayssairat 1991 reported that the number of participants with at least one side effect was significantly higher in the nifedipine group versus placebo (19 versus 7, P < 0.05) with headaches (12 versus 1, P < 0.05) and oedema (9 versus 2, P < 0.05) listed as occurring significantly more often when taking nifedipine compared to placebo. They reported no significant differences in occurrence of flushing and/or erythema, vertigo, nausea, cutaneous rash, 'various' and withdrawal from the trial (no P values reported from McNemar's test, with insufficient detail to reproduce the analysis). RTS 2000 reported a higher incidence of oedema, flushing and headaches (8/77 headaches in placebo group versus 13/77 in treatment group, P < 0.001) in the nifedipine group compared to the placebo group, and two participants in the nifedipine group reported tachycardia.
Discussion
Summary of main results
This review summarises the evidence for the use of calcium channel blockers in the treatment of primary Raynaud's phenomenon. Of the 69 studies identified by the search (which examined 11 different calcium channel blockers), seven trials with a total of 296 participants were included in the review. The remaining 62 studies did not match the inclusion criteria of this review. Four of the seven included trials investigated nifedipine, and three investigated nicardipine.
Only two trials (Kahan 1987; Vayssairat 1991), provided any detail of statistical comparisons of (unvalidated) severity scores between treatment groups: one of these trials (60 participants) reported a mean severity score of 1.55 on placebo and 1.36 on nicardipine, difference 0.2 (95% CI of difference 0 to 0.4, no P value reported) and the other trial (three participants only with primary Raynaud's phenomenon) reported a median severity score of 2 on both nicardipine and placebo treatment (P > 0.999). The contrasting results may be partially explained by differences in how severity was quantified across the trials, using unvalidated scales. Participant‐preference scores were included in four trials, but in only two were results specific to participants with primary Raynaud's phenomenon, and scoring systems differed between trials: scores differed between treatments in only one trial, in which 33% of participants on placebo and 73% on nifedipine reported improvement in symptoms (P < 0.001). Physiological measurements were included as outcome measures in five trials (different methodologies were used in each): in none of these trials were any between‐treatment group differences found. Treatment with calcium channel blockers was associated with a number of adverse reactions including headaches, flushing and oedema.
The meta‐analysis of the seven trials indicated a statistically significant advantage of treatment with calcium channel blockers compared to placebo as measured by the number of Raynaud's attacks per week. However, this significant treatment effect was no longer observed when the analysis was repeated without RTS 2000. This trial included the largest number of participants (158), was conducted over a longer period and was the one which suggested most benefit from a calcium channel blocker. It is possible that the larger effect size was attributable to the longer follow‐up period. The use of multiple imputation and the number of participants within the treatment arm who received 0 mg of nifedipine may contribute to the different findings in RTS 2000, although these factors would be expected to decrease rather than increase the estimate of effect.
When the meta‐analysis was repeated for each of the two calcium channel blockers individually, the estimate of treatment effect was statistically significant for the four nifedipine trials but this result was not robust to a secondary analysis using a random‐effects method. No overall effect of nicardipine was found.
All the studies reported adverse events although it was not always possible within studies including participants with both primary and secondary Raynaud's phenomenon to determine the adverse reactions for the primary Raynaud's group alone.
Overall completeness and applicability of evidence
This meta‐analysis highlighted several difficulties inherent in the existing clinical trials of calcium channel blockers in primary Raynaud's phenomenon:
a) The overall quality of the data was very variable with the result that the only outcome measure that could be compared using a meta‐analysis across all seven included trials was the frequency of Raynaud's attacks. This meta‐analysis therefore emphasises the need for validated outcome measures for Raynaud's phenomenon.
b) A large number of trials were excluded because it was not possible to separate out participants with primary and secondary Raynaud's phenomenon, or because of inadequate wash‐out periods within cross‐over trial designs.
c) Sample size was small, resulting from the small number of trials which could be included, and the small numbers of participants included in most of these seven trials.
d) The dose of calcium channel blockers used may not reflect maximum doses used in current clinical practice: for example, many clinicians increase the nifedipine dose to 80 mg daily. Also, in only one study was treatment duration longer than 10 weeks, with most studies having much shorter treatment periods.
e) The categorisation and assessment of some of the participants classed as having primary Raynaud's phenomenon was sometimes unsatisfactory with reports of digital gangrene and positive ANA titres suggesting that some participants had secondary rather than primary Raynaud's phenomenon. However, this has to be set against the 'real‐world' background in which the distinction between primary and secondary Raynaud's phenomenon can be difficult; around 1% to 2% per year of those who present with what initially seems to be primary Raynaud's phenomenon go on to develop a systemic sclerosis spectrum disorder or some other underlying disease (Hirschl 2006).
Quality of the evidence
We conducted an assessment of overall quality of the evidence according to the GRADE approach (GRADE Working Group 2004; Schunemann 2006; Guyatt 2008a; Guyatt 2008b; Higgins 2011). All seven trials included in the review were described as randomised, double‐blind, placebo‐controlled trials and were judged to be sound in terms of design. Allocation concealment was not fully described in six out of the seven trials, and although described as 'double‐blinded', details of how blinding was ensured were not provided. Although detail was lacking in this regard, the review authors did not feel that there was a high likelihood of bias as a result.
Our population of interest was people with primary Raynaud's phenomenon and as such we excluded trials where results for primary Raynaud's participants were not identifiable. Although the demographic information reported across the seven included trials was relatively limited, the sample was broadly representative in terms of gender balance and age range. It should be noted, however, that this sample was predominantly hospital‐based and all seven trials recruited from hospital clinics, with the exception of RTS 2000 where participants were also recruited via advertisement. A significant proportion of people with primary Raynaud's phenomenon are not referred to hospital for evaluation and treatment: it is likely that those who are may be more severely affected or may not have responded to conservative measures, or both, and these are the participants mainly presented in this meta‐analysis. Another potential concern (highlighted above) is that the doses of calcium channel blockers used within the seven trials are lower than the maximum doses administered in clinical practice. The estimates of treatment effect observed in these seven trials may therefore underestimate the effect of calcium channel blockers.
Further comments regarding quality of the evidence relating to specific outcomes considered in the review follow:
Number of attacks
With the exception of RTS 2000, which had a higher 95% lower confidence limit for the standardised mean difference in the number of Raynaud's attacks, the 95% CIs were generally consistent across the trials. A sensitivity analysis with RTS 2000 excluded failed to reproduce the significant treatment difference observed in the meta‐analysis of all seven trials. In this review, the longer follow‐up time of RTS 2000 has been proposed as a possible explanation for this potentially discordant interval (the difference is not so severe that the intervals do not overlap). Small numbers of participants with primary Raynaud's phenomenon were included in several trials (Rodeheffer 1983; Ettinger 1984; Kahan 1987). The 95% confidence intervals for the standardised mean difference in the number of Raynaud's attacks in both Rodeheffer 1983 and Kahan 1987 were wide, corresponding to a lack of precision in the estimates of treatment effect. The standard error for the estimate of treatment effect in Ettinger 1984, however, was small due to the high within‐participant correlation (ρ = 0.953) and the 95% confidence interval calculated from this study was not too wide. Although Rodeheffer 1983 and Kahan 1987 received a very low weighting in the meta‐analysis on account of their large standard errors, the presence of studies with small samples constitutes a limiting feature of these trials as a body of evidence. On account of this, the review authors revised their assessment of the quality of evidence provided by these trials to 'moderate' for number of attacks.
Duration of attacks
The quality of evidence for the outcome duration of attacks was considered to be very low due to the fact that only information on 13 participants in one trial was provided (Wollersheim 1991), and no information on variation in response (precision) was available.
Severity scores
While six trials reported on severity scores, only one trial reported on this outcome in a manner that allowed for robust conclusions to be drawn (Vayssairat 1991). This was a well‐designed cross‐over study of 60 participants and the evidence arising from this study was consistent with the limited information provided by the remainder. As such, we believe the quality of evidence in relation to the outcome severity scores to be high.
Patient preference scores
We considered the quality of evidence in relation to patient preference scores to be low. No information on variation in response was available, incommensurate scales were used between studies and two studies did not report results separately for primary Raynaud's phenomenon participants. Accordingly, it is difficult to comment on the effect of calcium channel blockers on patient preference scores.
Due to the small number of trials meeting the inclusion criteria of this review, a definitive assessment of publication bias using a funnel plot was not possible. However, the search strategy employed in this review was robust, including conference abstracts in addition to published papers. Furthermore, several of the studies included here contained results best described as null findings. We did not deem the risk of publication bias to be substantial enough to downgrade our assessment of the quality of this body of evidence.
Potential biases in the review process
None of the review authors was involved in any of the included or excluded studies, and none of us has any relevant commercial or other conflicts of interest. The Trial Search Co‐ordinator conducted extensive searches for relevant articles and for details of ongoing studies, to reduce the risk of publication bias. Three review authors independently assessed all studies. As described in the review, we also attempted to deal with missing information and data by contacting authors. No further information was available, however, and missing data could introduce bias into the results of four of the seven studies where multiple imputation had not been attempted and could not be performed by the review authors. This, in turn, could introduce bias into the results of the meta‐analyses presented here. Two trials (Ettinger 1984; Vayssairat 1991), are of concern in this respect, as both received high weighting in the meta‐analysis of all trials.
Agreements and disagreements with other studies or reviews
A previous meta‐analysis (Thompson 2005), indicated a statistically significant advantage of treatment with calcium channel blockers over placebo in primary Raynaud's phenomenon, with a decrease of between 2.8 to 5 attacks per week. The estimate of treatment effect was statistically significant for nifedipine but not significant for nicardipine or nisoldipine. Inclusion criteria, however, differed from those for this Cochrane review. Trials were included if the number of participants with primary Raynaud's phenomenon exceeded 75%, and trials with a cross‐over design but no wash‐out period were also included. As a result, the following trials were included by Thompson 2005 but excluded from this review: Kahan 1983a; Kahan 1985a; Kahan 1985b; Aldoori 1986; Corbin 1986; Gjorup 1986a; Gjorup 1986b; Redondo 1986; Waller 1986; Challenor 1987; Nilsson 1987; Challenor 1989. In one case, a trial was excluded by Thompson 2005 on the grounds of insufficient data for primary Raynaud's participants but was included within this review (Wollersheim 1991). Despite these different inclusion criteria, the results presented here can be seen as consistent with the findings of Thompson 2005 .
Thompson 2005 also included data on the severity of Raynaud's attacks within the meta‐analysis. However, we decided not to pool these data because of variation across the seven included trials in terms of scale, description and usage. Instead, we have reported the severity results separately for each trial in which they were reported.
Authors' conclusions
Implications for practice.
There is moderate‐quality evidence that oral calcium channel blockers are minimally effective in the treatment of primary Raynaud's phenomenon as measured by the frequency of attacks. Participants experienced 1.72 (95% CI 0.60 to 2.84) fewer attacks per week on calcium channel blockers compared to placebo. This effect size was small, although may be greater with longer duration of treatment. There is high‐quality evidence that calcium channel blockers have little effect on severity. We are unable to comment on duration of attacks or on patient preference due to the very low and low quality of evidence available for these outcomes. The results of this review were limited by small sample size and variable overall data quality.
Implications for research.
Given that calcium channel blockers are considered by most clinicians as a first‐line treatment for primary Raynaud's phenomenon (and are likely to be the comparator drug in clinical trials of new therapies), there is a need to better define their efficacy in adequately powered, well‐designed clinical trials. One concern is the current lack of validated, objective outcome measures for the assessment of Raynaud's attacks. The Raynaud's Condition Score (Merkel 2002) has been validated as an outcome measure for Raynaud's phenomenon and future clinical trials should at least include this.
Feedback
Query number of participants, 19 April 2016
Summary
Minor query ‐ for the outcome number of attacks, there are 7 RCTs with 358 participants in the data and analysis section but the Summary of findings reports 7 RCTs with 296 people ‐ I can't quite figure out why the numbers are different.
Reply
Many thanks for the feedback and minor query.
The discrepancy (between the summary of findings table and the table for the outcome number of attacks) is because the participants in the crossover studies are counted twice by RevMan 2012, due to the fact that each acts as their own control. Our analysis takes this into account, by allowing for the correlation between the paired measurements in the crossover studies.
We have added clarification to this effect in the body of the review for completeness.
Contributors
Feedback: Ms Karen Pettersen, Editor EBM, Wiley Reply: Dr Michael Hughes and Mr Jack Wilkinson on behalf of review authors
What's new
| Date | Event | Description |
|---|---|---|
| 12 July 2016 | Feedback has been incorporated | Feedback and authors' response added |
History
Protocol first published: Issue 2, 2000 Review first published: Issue 1, 2014
| Date | Event | Description |
|---|---|---|
| 14 January 2016 | New search has been performed | New search run. No new studies included. Two new studies excluded. |
| 14 January 2016 | New citation required but conclusions have not changed | New search run. No new studies included. Two new studies excluded. Text amended to reflect current Cochrane policies and Summary of Findings table added. Conclusions not changed. |
| 13 May 2008 | New citation required and minor changes | New team of authors. |
| 13 May 2008 | Amended | Converted to new review format. |
| 15 November 2004 | Amended | Re‐published with a new team of authors led by Mr Ian Quirk (issue 1, 2005). |
| 19 January 2000 | Amended | First published version, contact author Dr Ed Housley (Issue 2, 2000). |
Acknowledgements
We would like to thank the editorial team for the Cochrane Vascular Group for their advice and comments on this review.
The first published version of the protocol for this review was conceived and written by Dr Ed Housley. A revised version of the protocol was published in Issue 1, 2005 with a new team of authors led by Mr Ian Quirk.
Appendices
Appendix 1. CENTRAL search strategy
| #1 | MeSH descriptor: [Raynaud Disease] explode all trees | 279 |
| #2 | raynaud* | 640 |
| #3 | #1 or #2 | 641 |
| #4 | MeSH descriptor: [Calcium Channel Blockers] explode all trees | 2699 |
| #5 | calcium near/3 antagonist* | 2703 |
| #6 | calcium near/3 blocker* | 4487 |
| #7 | calcium near/3 inhibit* | 657 |
| #8 | amlodipine or amrinone or azelnidipine | 2588 |
| #9 | bencyclan* or bepridil or AT877 or “AT 877” | 660 |
| #10 | cilnidipine or cinnarizine or conotoxin* | 312 |
| #11 | daropidine or diltiazem | 1669 |
| #12 | efonidipine or felodipine or fendiline or flunarizine | 1094 |
| #13 | gallopamil or isradipine or lacidopine or lidoflazine | 654 |
| #14 | mibefradil or nicardipine or nifedipine or nimodipine or nisoldipine or nitrendipine | 5609 |
| #15 | perhexiline or prenylamine or verapamil | 2223 |
| #16 | magnesium next sulph* | 629 |
| #17 | #4 or #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12 or #13 or #14 or #15 or #16 | 15223 |
| #18 | #3 and #17 in Trials (Word variations have been searched) | 125 |
Data and analyses
Comparison 1. Calcium channel blockers versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Number of attacks ‐ Primary | 7 | 358 | Std. Mean Difference (Fixed, 95% CI) | 0.23 [0.08, 0.38] |
| 2 Number of attacks ‐ without Ettinger (sensitivity analysis) | 6 | 352 | Std. Mean Difference (Fixed, 95% CI) | 0.26 [0.09, 0.43] |
| 3 Number of attacks ‐ without RTS 2000 (sensitivity analysis) | 6 | 200 | Std. Mean Difference (Fixed, 95% CI) | 0.14 [‐0.03, 0.31] |
| 4 Number of attacks ‐ Nifedipine trials only | 4 | 206 | Std. Mean Difference (Fixed, 95% CI) | 0.32 [0.10, 0.54] |
| 5 Number of attacks ‐ Nifedipine trials only (random‐effects) | 4 | 206 | Std. Mean Difference (Random, 95% CI) | 0.31 [‐0.05, 0.68] |
| 6 Number of attacks ‐ Nicardipine trials only | 3 | 152 | Std. Mean Difference (Fixed, 95% CI) | 0.15 [‐0.06, 0.35] |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ettinger 1984.
| Methods | Study design: Randomised, double‐blind, placebo‐controlled, cross‐over trial. Trial duration: 10 weeks (2 weeks run‐in, 2 weeks treatment/placebo, 1 week wash‐out, 2 weeks treatment/placebo, 1 week wash‐out, 2 weeks treatment/placebo). Method of randomisation: Participants described as randomly assigned but no description. Concealment of randomisation: Treatment phases double‐blind but no further description. Exclusions post‐randomisation: 3 Losses to follow‐up: 0 |
|
| Participants | Country: USA. Setting: Hospital, cold season months. No: 6 primary Raynaud's phenomenon (total no in study: 25 ‐ 19 with secondary Raynaud's). Age: Restricted to 18 ‐ 65 years of age. Aged 22 ‐ 55 years. Sex: 5 women; 1 man. Other: No smoking data. Inclusion criteria: One episode of Raynaud's phenomenon per day. Exclusion criteria: Serious renal, cardiac, hepatic, pulmonary, hematologic or metabolic disease or active digital ulceration, concomitant therapy with aspirin, NSAIDs, dipyridamole, sulphinpyrazone, vasodilators or drugs interfering with sympathetic nervous system function. Primary Raynaud's definition: Episodic digital pallor and cyanosis on cold exposure relieved by rewarming without associated demonstrable disease. |
|
| Interventions | Treatment 1: 20 mg nifedipine 3 times a day. Treatment 2: 100 mg dazoxiben 4 times a day Control: Matching placebo. Only data relating to nifedipine versus placebo were used in this review. Duration: 3 x 2 weeks. Wash‐out period: 1‐week single‐blind placebo. Run‐in period: 2‐week single‐blind placebo. |
|
| Outcomes | 1. Number of attacks per 2‐week period. 2. Severity on a 3‐point scale (mild, moderate, severe). 3. Duration of attacks in minutes. 4. Pain intensity on a visual analogue scale from 0 ‐ 12. 5. Participant preference and side effects. 6. Finger systolic pressure after local cooling at end of each treatment period. |
|
| Notes | 3 participants (all with primary Raynaud's phenomenon) did not complete the trial due to symptomatic orthostatic hypotension while on nifedipine. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Participants described as "randomly assigned". No further information. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind. No further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. No further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Majority of outcomes participant‐reported. Participants blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None noted. |
Kahan 1987.
| Methods | Study design: Randomised, double‐blind, placebo‐controlled, cross‐over trial. Trial duration: 6 weeks (1 week observation no treatment, 2 weeks treatment/placebo, 1 week observation no treatment, 2 weeks treatment/placebo). Method of randomisation: Table of random numbers. Concealment of randomisation: Treatment phases double‐blind but no further description. Exclusions post‐randomisation: 0 Losses to follow‐up: 0 |
|
| Participants | Country: France. Setting: Hospital, January to June. No: 3 primary Raynaud's phenomenon (total no. in study: 20; 17 with secondary Raynaud's). Age: Restricted to 18 ‐ 68 years of age. No breakdown for primary Raynaud's participants. Sex: No breakdown for primary Raynaud's participants. Other: No smoking data. Inclusion criteria: Symptomatic, bilateral Raynaud's phenomenon. Exclusion criteria: Vasoactive medication. Primary Raynaud's definition: Symptomatic, bilateral Raynaud's phenomenon, idiopathic. |
|
| Interventions | Treatment: 20 mg nicardipine 3 times a day. Control: Matching placebo. Duration: 2 x 2 weeks. Wash‐out period: 1 week no treatment. Run‐in period: 1 week no treatment. |
|
| Outcomes | 1. Frequency of Raynaud's attacks over 2‐week period. 2. Severity of attacks on a 4‐point scale (slight, moderate, severe, very severe). |
|
| Notes | No side effects information specifically for the primary Raynaud's group. One participant with primary Raynaud's responded very well to treatment and the other two participants did not respond. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Determined with a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind. No further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. No further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes participant‐reported. Participants blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Authors describe compliance as 100% with no missing data. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None noted. |
Rodeheffer 1983.
| Methods | Study design: Randomised, double‐blind, placebo‐controlled, cross‐over trial. Trial duration: 7 weeks (2 weeks placebo run‐in, 2 weeks treatment/placebo, 1 week placebo wash‐out, 2 weeks treatment/placebo). Method of randomisation: Table of random numbers. Concealment of randomisation: Treatment phases double‐blind, run‐in and wash‐out single‐blind but no further description. Exclusions post‐randomisation: 0 Losses to follow‐up: 0 |
|
| Participants | Country: USA. Setting: Hospital outpatient clinics, cold season. No: 5 primary Raynaud's phenomenon (total no: 15; 10 with secondary Raynaud's). Age: 20 ‐ 49 years of age. Sex: All women. Other: No smoking data. Inclusion criteria: Bilateral episodic digital pallor and cyanosis relieved by warming. Exclusion criteria: Drugs influencing vascular tone, causes of Raynaud's other than connective tissue disease. Primary Raynaud's definition: Symptomatic Raynaud's phenomenon relating to cold or stress without demonstrable systemic disease. |
|
| Interventions | Treatment: 10 mg nifedipine 3 times a day for 3 days, followed by 20 mg 3 times a day if free from severe side effects. Control: Matching placebo with matching increase to 20 mg if free from severe side effects. Duration: 2 x 2 weeks. Wash‐out period: 1 week single‐blind placebo. Run‐in period: 1 week single‐blind placebo. |
|
| Outcomes | 1. Total number of attacks in 2‐week period from a participant diary. 2. Participant assessment of treatment on a 5‐point scale (marked improvement, moderate improvement, minimal improvement, no change, worse). 3. Digital‐artery systolic pressure at 30 and 15 degrees C. |
|
| Notes | Limited data on side effects and no data specifically for participants with primary Raynaud's. 2/5 had significant ANA titres suggestive of possible secondary Raynaud's. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation sequence determined with a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind. No further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. No further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Majority of outcomes participant‐reported. Participants blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None noted. |
RTS 2000.
| Methods | Study design: Randomised, double‐blind (for drug and placebo arm), placebo‐controlled, parallel‐group trial. One arm compared nifedipine with placebo and a second arm (not double‐blind) compared temperature biofeedback with electromyographic biofeedback. Only the drug/placebo arm is considered here. Trial duration: 12 ‐ 13 months. Method of randomisation: Automated telephone randomisation system. Concealment of randomisation: Double‐blind. Central allocation. Exclusions post‐randomisation: 0 Losses to follow‐up: 43 |
|
| Participants | Country: USA. Setting: Hospital clinics, cold season months. No: 158, all with primary Raynaud's phenomenon. Age: No breakdown for the nifedipine versus placebo arm. Sex: No breakdown for the nifedipine versus placebo arm. 70% women. Other: No smoking data. Inclusion criteria: Primary Raynaud's confirmed by clinical examination, colour charts, nailfold capillary microscopy and ANA testing, at least 2 attacks per day in the previous cold season. Exclusion criteria: Secondary Raynaud's phenomenon, contraindications to nifedipine, completion of less than 75% of 1‐month baseline attack record. Primary Raynaud's definition: Idiopathic primary Raynaud's phenomenon. |
|
| Interventions | Treatment: 30 mg sustained‐release nifedipine daily, increased to 60 mg per day after first week if no adverse reactions. Based on tolerance, dosage adjusted to 2, 1 or 0 x 30 mg capsules per day over 4 subsequent weeks and continued for 12 ‐ 13 months. Placebo: Matching placebo. Duration: 12 ‐ 13 months. Wash‐out period: Not applicable. Run‐in period: Four week baseline period. |
|
| Outcomes | 1. Daily Raynaud's attack rate during 4 weeks immediately after the 1‐year assessment. Attacks recorded in participant diaries, with a subgroup of verified attacks (code matching example photographs and occurring at least 30 minutes after previously recorded attack). 2. Severity of Raynaud's on a 4‐point scale (recorded by participant and investigator). 3. Impact of Raynaud's on daily life (recorded by participant and investigator). 4. Improvement compared to baseline (recorded by participant and investigator). 5. General health (recorded by participant and investigator). 6. Quality of life and physical symptoms assessed by a modified Short Form Health Survey and a modified Cohen‐Hoberman Inventory of Physical Symptoms (recorded by participant). |
|
| Notes | Limited side effect information. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Automated telephone randomisation service. |
| Allocation concealment (selection bias) | Low risk | Central allocation system. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. Adequately described. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcomes participant‐reported or clinician‐reported. Participants and clinicians blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Missing data handled using intention‐to‐treat analysis. Loss to follow‐up figures provided but not clear if this included participants with adverse events, participants taking 0 mg nifedipine or placebo and those failing to complete diaries. |
| Selective reporting (reporting bias) | Unclear risk | Not all secondary outcomes are reported. |
| Other bias | Low risk | None noted. |
Sarkozi 1986.
| Methods | Study design: Randomised, double‐blind, placebo‐controlled parallel‐group trial. Trial duration: 11 weeks (1 week baseline assessment, 10 week treatment or placebo). Method of randomisation: Table of random numbers. Concealment of randomisation: Double‐blind but no further description. Exclusions post‐randomisation: 0 Losses to follow‐up: 7 |
|
| Participants | Country: Canada. Setting: Hospital, Raynaud's research clinics. Winter months. No: 39, all with primary Raynaud's phenomenon. Age: Mean age 42.1 years in nifedipine group and 37.6 years in placebo group. Sex: 29 women; 10 men. Other: 12/39 current smokers. Inclusion criteria: At least 2 vasospastic attacks per week with a stable frequency and/or severity for 3 months prior to entry. Exclusion criteria: No vasoactive drugs 1 week prior to entry. No reserpine 3 months prior to entry. Seconday Raynaud's, pregnancy or nursing mothers, child‐bearing potential, hepatic or renal insufficiency. Primary Raynaud's definition: Episodic, well‐demarcated, digital pallor or cyanosis in response to cold exposure or emotional stimuli, episodes associated with numbness and/or pain and no clinical evidence of primary disease. |
|
| Interventions | Treatment:10 mg 3 times a day, increasing to 20 mg 3 times a day at 5 weeks if no improvement. Control: Matching placebo with increase at 5 weeks if no improvement. Duration: 10 weeks. Wash‐out period: Not applicable. Run‐in period: 1 week on no vasoactive treatment. |
|
| Outcomes | 1. Weekly Raynaud's attack rates. 2. Severity of attacks assessed on a 3‐point scale (mild, moderate, severe). 3. Change in pulse amplitude of digital blood flow assessed by photoplethysmography and time to return to baseline pulse amplitude following cold pressor challenge using photography. |
|
| Notes | Reference to development of digital gangrene in 2 participants suggestive of secondary Raynaud's. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomization sequence determined from a table of random numbers. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind. No further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. No further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Majority of outcomes participant‐reported. Participants blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None noted. |
Vayssairat 1991.
| Methods | Study design: Randomised, double‐blind, placebo‐controlled cross‐over trial. Trial duration: 6 weeks (1 week placebo run‐in, 2 weeks treatment/placebo, 1 week placebo wash‐out, 2 weeks treatment/placebo). Method of randomisation: Randomisation code sent to participating centres by trial co‐ordinator. Concealment of randomisation: Double‐blind. Research teams knew randomisation code as Treatment A or Treatment B but no further description. Exclusions post‐randomisation: 0 Losses to follow‐up: 9 |
|
| Participants | Country: France. Setting: Hospital, cold season months. No: 69, all with primary Raynaud's phenomenon. Age: Restricted to 18 ‐ 65 years of age. Mean age 38 ± 15 years. Sex: 51 women; 18 men Other: No smoking data. Inclusion criteria: At least 3 attacks per week, primary Raynaud's confirmed by clinical examination, nailfold capillary microscopy, ANA testing, chest and hand x‐rays. Exclusion criteria: Secondary Raynaud's, pregnancy, coexisting illness or drug therapy. Primary Raynaud's definition: Defined according to Priollet's 1987 classification (Priollet 1987). |
|
| Interventions | Treatment: 50 mg long‐acting nicardipine twice a day. Control: Matching placebo. Duration: 2 x 2 weeks. Wash‐out period: 1 week single‐blind placebo. Run‐in period: 1 week single‐blind placebo. |
|
| Outcomes | 1.Weekly Raynaud's attack rate. 2. Severity of Raynaud's attacks on a 4‐point scale (mild, moderate, severe, highly severe). 3. Overall disability on a 10 cm visual analogue scale. 4. Cold‐reactive hyperaemia (measured by skin temperature). |
|
| Notes | 9 did not complete trial ‐ 2 for personal reasons and 7 due to side effects (5 during placebo and 2 during nifedipine treatment periods). | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation code supplied by trial coordinator. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind. Allocation method partially described: research teams knew randomisation code as Treatment A or Treatment B but no further description. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. No further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Majority of outcomes participant‐reported. Participants blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants accounted for. |
| Selective reporting (reporting bias) | Low risk | All outcomes reported. |
| Other bias | Low risk | None noted. |
Wollersheim 1991.
| Methods | Study design: Double‐blind, placebo‐controlled, randomised, cross‐over trial. Trial duration: 11 weeks (3 weeks run‐in, 3 weeks treatment/placebo, 2 weeks wash‐out, 3 weeks treatment/placebo). Method of randomisation: Delivered "at random" but method not described. Concealment of randomisation: Allocation per participant provided in sealed envelope. No further information. Exclusions post‐randomisation: 0 Losses to follow‐up: 7 (due to incomplete diary data, but unclear if primary or secondary). 3 with primary Raynaud's phenomenon withdrew due to side effects. |
|
| Participants | Country: Netherlands. Setting: Hospital outpatient clinic, season not stated. No: 16 with primary Raynaud's phenomenon (total no: 25; 9 with secondary Raynaud's). Age: Restricted to 18 ‐ 65 years of age. No breakdown for primary Raynaud's participants. Sex: No breakdown for primary Raynaud's participants. Other: No smoking data for primary Raynaud's participants. Inclusion: Raynaud's phenomenon according to Allen and Brown criteria (Allen 1932), at least 1 attack per day, normal body habitus, normal electrocardiogram and normotensive. Exclusion: Concomitant medications, vasoactive medications during 4 weeks prior to baseline, causes of Raynaud's other than connective tissue disease. Primary Raynaud's definition: Allen and Brown criteria (Allen 1932). |
|
| Interventions | Treatment: 30 mg nicardipine 3 times a day. Control: Matching placebo. Duration: 2 x 3 weeks. Wash‐out period: 3 weeks with no drug treatment. Run‐in period: 3 weeks baseline observation with no vasoactive drug treatment. |
|
| Outcomes | 1. Daily Raynaud's attack rate. 2. Duration of Raynaud's attack in minutes. 3. Severity of Raynaud's attacks on an 11‐point (0 ‐ 10) scale. 4. Participant assessment of drug effectiveness on a 5‐point scale (much worse, worse, no difference, better, much better). 5. Participant and investigator opinion of ischaemic changes on a 3‐point scale (worse, no difference, better). 6. Participant assessment of side effect severity and frequency on a visual analogue scale, to produce an adverse effect score. 7. Participant preference. 8. Response to finger cooling test as measured by systolic and diastolic blood pressure, heart rate, ECG and ECG‐triggered venous occlusion plethysmography, finger skin temperature, laser Doppler flux and transcutaneous oxygen tension. |
|
| Notes | 3 (all with primary Raynaud's) withdrew with side effects. Mean values for Raynaud's attack rate, duration, severity and adverse effects number given for primary Raynaud's participants. However, no participant preference data or finger cooling test data beyond (for finger cooling) a statement that there was no difference found between primary and secondary Raynaud's phenomenon participants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Delivered "at random" but method not described. |
| Allocation concealment (selection bias) | Unclear risk | Double‐blind. Allocation method partially described; allocation per participant provided in sealed envelope. No further information. |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind. No further information. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Majority of outcomes participant‐reported. Participants blinded to treatment allocation. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All data for a given participant excluded from analysis if data missing in any phase. 7 excluded due to missing data but unclear how many had primary Raynaud's phenomenon. |
| Selective reporting (reporting bias) | Unclear risk | Most but not all outcomes reported. |
| Other bias | Low risk | None noted. |
ANA: antinuclear antibody ECG: electrocardiogram/graphic
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agnelli 1984 | No/insufficient wash‐out period. |
| Aldoori 1986 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| Baadsgaard 1983 | No/insufficient wash‐out period. |
| Brotzu 1989 | Calcium channel blocker used in combination with another agent. |
| Caglayan 2012 | Not a calcium channel blocker. |
| Challenor 1987 | No/insufficient wash‐out period. |
| Challenor 1989 | No/insufficient wash‐out period. |
| Choi 2009 | Open study. |
| Codella 1989 | Open study. |
| Coleiro 2001 | Open study. |
| Corbin 1986 | No/insufficient wash‐out period. |
| Costantini 1987 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| Creager 1984 | Not a randomised controlled trial. |
| Csiki 2011 | Calcium channel blocker used in combination with another agent. |
| Da Costa 1987 | Not a study of primary Raynaud's phenomenon. |
| Denton 1999 | Open study. |
| Dompeling 1992 | Single‐dose study. |
| Dziadzio 1999a | Open study. |
| Ferri 1992 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| Finch 1986 | No/insufficient wash‐out period. |
| Finch 1988 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| Fontenelle 2008 | Single dose study. |
| Gjorup 1986a | No/insufficient wash‐out period. |
| Gjorup 1986b | No/insufficient wash‐out period. |
| Gush 1987 | Single‐dose study. |
| Hawkins 1986 | No/insufficient wash‐out period. |
| Kahan 1981 | Single‐dose study. |
| Kahan 1983a | No/insufficient wash‐out period. |
| Kahan 1983b | No/insufficient wash‐out period. |
| Kahan 1985a | No/insufficient wash‐out period. |
| Kahan 1985b | No/insufficient wash‐out period. |
| Kahan 1986 | No/insufficient wash‐out period. |
| Kallenberg 1987 | No/insufficient wash‐out period. |
| La Civita 1993 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| La Civita 1996 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| Lee 2014 | Not a study of primary Raynaud's phenomenon. |
| Leppert 1989 | Not a randomised controlled trial. |
| Lewis 1987 | Single‐dose study. |
| Martinez 1999 | No separate analysis of primary and secondary Raynaud's. Unclear if there was a wash‐in period. |
| Mascagni 1994 | Not a study of primary Raynaud's phenomenon. |
| Morgan 1987 | Single‐dose study. |
| Moriau 1993 | Calcium channel blocker used in combination with another agent. |
| Müller‐Bühl 1983 | No separate analysis of pimary and secondary Raynaud's. |
| Nilsson 1987 | No data on any of the four primary outcome measures (attack rate, duration of attacks, severity scores, participant‐preference scores). |
| Pisenti 1984 | Not a randomised controlled trial. |
| Redondo 1986 | No/insufficient wash‐out period. |
| Rhedda 1985 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| Rupp 1987 | No/insufficient wash‐out period. |
| Sauza 1984 | Not a study of primary Raynaud's phenomenon. |
| Schmidt 1989 | Not a study of primary Raynaud's phenomenon. |
| Scorza 2001 | Not a study of primary Raynaud's phenomenon. |
| Shcherbakov 1987 | Open study. |
| Smith 1982 | No/insufficient wash‐out period. |
| Stefenelli 1986 | Not a randomised controlled trial. |
| Van Heereveld 1988 | No/insufficient wash‐out period and IV calcium channel blockers. |
| Varela‐Aguilar 1997 | Not a study of primary Raynaud's phenomenon. |
| Waller 1986 | No separate analysis of primary and secondary Raynaud's. Authors contacted and confirmed no further data are available. |
| Wasir 1983 | Abstract only, no data presented. |
| Weber 1990 | Single‐dose study. |
| White 1986 | No/insufficient wash‐out period. |
| Wigley 1987a | No/insufficient wash‐out period. |
| Winston 1983 | No/insufficient wash‐out period. |
| Wise 1987 | No/insufficient wash‐out period. |
| Wollersheim 1987 | Single‐dose study. |
Differences between protocol and review
Subgroup analyses for smoking status, pre‐interventional severity, gender and age were proposed in the protocol but this was not deemed possible due to the data quality of the included trials.
The outcomes 'physiological measurements for example, finger systolic pressure response to digital cooling' and 'digital temperature and blood flow response to hand cooling' have been combined into the outcome 'Physiological measurements (including digital temperature and blood flow response to hand cooling)'.
Contributions of authors
HE: identified trials, confirmed eligibility, assessed the quality of trials, extracted data and co‐authored the review. MH: identified trials, confirmed eligibility, assessed the quality of trials, and co‐authored the review. MA: identified trials, confirmed eligibility, assessed the quality of trials, extracted data and co‐authored the review. AH identified trials, confirmed eligibility, assessed the quality of trials, extracted data and co‐authored the review. JW assessed the quality of trials, performed the statistical analysis and co‐authored the review.
Sources of support
Internal sources
No sources of support supplied
External sources
-
Chief Scientist Office, Scottish Government Health Directorates, The Scottish Government, UK.
The Cochrane Vascular editorial base is supported by the Chief Scientist office.
Declarations of interest
HE: declares that she is currently employed by the University of Edinburgh with no known conflicts. MH: none known. MA: has received honoraria and financial support to attend educational national and international meetings from Actelion Pharmaceuticals and Bristol‐Meyers Squibb. These included Coronary artery hypertension meeting and Consensus best practice for digital ulcer management meeting presentations (Actelion Pharmaceuticals) and national presentations (Bristol‐Meyers Squibb). MA received support to attend Advisory board and consensus best practice for digital ulcer management meeting organised and supported by Actelion Pharmaceutical. MA's institution receives National Digital Ulcer Registry participation payments (supported by Actelion Pharmaceuticals). AH: declares that she has undertaken consultancy work for Actelion and Apricus (Data Safety Monitoring Board). AH's institution has received research grant funding from Actelion and she has spoken at meetings sponsored by Actelion. AH has been a PI on a study sponsored by Orion (this study was on systemic sclerosis‐related Raynaud's phenomenon), and is a PI on studies sponsored by Actelion (these studies are on systemic sclerosis‐related Raynaud's phenomenon). JW: none known.
Edited (no change to conclusions), comment added to review
References
References to studies included in this review
Ettinger 1984 {published data only}
- Ettinger WH, Wise RA, Schaffhauser D, Wigley FM. Controlled double‐blind trial of dazoxiben and nifedipine in the treatment of Raynaud's phenomenon. American Journal of Medicine 1984;77(3):451‐6. [DOI] [PubMed] [Google Scholar]
- Malamet R, Wise RA, Ettinger EH, Wigley FM. Nifedipine in the treatment of Raynaud's phenomenon. Evidence for inhibition of platelet activation. American Journal of Medicine 1985;78(4):602‐8. [DOI] [PubMed] [Google Scholar]
- Wigley FM, Malamet R, Wise RA. Reproducibility of cold provocation in patients with Raynaud's phenomenon. Journal of Rheumatology 1987;14(4):751‐5. [PubMed] [Google Scholar]
Kahan 1987 {published data only}
- Kahan A, Amor B, Menkes CJ. Nicardipine in the treatment of Raynaud's phenomenon. Revue du Rhumatisme et des Maladies Osteo‐Articulaires 1987;54(6):487‐90. [PubMed] [Google Scholar]
- Kahan A, Amor B, Menkes CJ, Weber S, Guerin F, Degeorges M. Nicardipine in the treatment of Raynaud's phenomenon: A randomized double‐blind trial. Angiology 1987;38(4):333‐7. [DOI] [PubMed] [Google Scholar]
Rodeheffer 1983 {published data only}
- Rodeheffer RJ, Rommer JA, Wigley F, Smith CR. Controlled double‐blind trial of nifedipine in the treatment of Raynaud's phenomenon. New England Journal of Medicine 1983;308(15):880‐3. [DOI] [PubMed] [Google Scholar]
RTS 2000 {published data only}
- Maricq HR, Jennings JR, Valter I, Frederick M, Thompson B, Smith EA, et al. Evaluation of treatment efficacy of Raynaud phenomenon by digital blood pressure response to cooling. Raynaud's Treatment Study Investigators. Vascular Medicine 2000;5(3):135‐40. [DOI] [PubMed] [Google Scholar]
- Middaugh SJ, Haythornthwaite JA, Thompson B, Hill R, Brown KM, Freedman RR, et al. The Raynaud's Treatment Study: biofeedback protocols and acquisition of temperature biofeedback skills. Applied Psychophysiology and Biofeedback 2001;26(4):251‐78. [DOI] [PubMed] [Google Scholar]
- Raynaud's Treatment Study Investigators. Comparison of sustained‐release nifedipine and temperature biofeedback for treatment of primary Raynaud phenomenon. Results from a randomized clinical trial with 1‐year follow‐up. Archives of Internal Medicine 2000;160(8):1101‐8. [DOI] [PubMed] [Google Scholar]
- Thompson B, Geller NL, Hunsberger S, Frederick M, Hill R, Jacob RG, et al. Behavioral and pharmacologic interventions: the Raynaud's Treatment Study. Controlled Clinical Trials 1999;20(1):52‐63. [DOI] [PubMed] [Google Scholar]
Sarkozi 1986 {published data only}
- Sarkozi J, Bookman AA, Mahon W, Ramsay C, Detsky AS, Keystone EC. Nifedipine in the treatment of idiopathic Raynaud's syndrome. Journal of Rheumatology 1986;13(2):331‐6. [PubMed] [Google Scholar]
Vayssairat 1991 {published data only}
- Vayssairat M, Boccalon H, Ginestet MC, Priollet P, Franco A, Carpentier P, et al. Controlled multicenter double‐blind trial of nicardipine in the treatment of primary Raynaud phenomenon. American Heart Journal 1991;122(1 Suppl 2):352‐5. [DOI] [PubMed] [Google Scholar]
Wollersheim 1991 {published data only}
- Wollersheim H, Thien T. Double‐blind placebo‐controlled crossover study of oral nicardipine in the treatment of Raynaud's phenomenon. Journal of Cardiovascular Pharmacology 1991;18(6):813‐8. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Agnelli 1984 {published data only}
- Agnelli G, Parise P, Colangeli C. Pilot evaluation of flunarizine in Raynaud's disease: A placebo‐ controlled, double blind cross‐over study. Acta Therapeutica 1984;10(2):153‐62. [Google Scholar]
Aldoori 1986 {published data only}
- Aldoori M, Campbell WB, Dieppe PA. Nifedipine in the treatment of Raynaud's syndrome. Cardiovascular Research 1986;20(6):466‐70. [DOI] [PubMed] [Google Scholar]
Baadsgaard 1983 {published data only}
- Baadsgaard O, Schmidt JF, Ironmann L. The effect of nifedipin (adalat) on Raynaud's phenomenon. Ugeskrift for Laeger 1983;145(49):3814‐6. [PubMed] [Google Scholar]
Brotzu 1989 {published data only}
- Brotzu G, Falchi S, Mannu B, Montisci R, Petruzzo P, Staico R. The importance of presynaptic beta receptors in Raynaud's disease. Journal of Vascular Surgery 1989;9(6):767‐71. [PubMed] [Google Scholar]
Caglayan 2012 {published data only}
- Caglayan E, Axmann S, Hellmich M, Moinzadeh P, Rosenkranz S. Vardenafil for the treatment of Raynaud phenomenon: A randomized, double‐blind, placebo‐controlled crossover study. Archives of Internal Medicine 2012;172(15):1182‐4. [DOI] [PubMed] [Google Scholar]
Challenor 1987 {published data only}
- Challenor VF, Waller DG, Francis DA, Francis JL, Mani R, Roath S. Nisoldipine in primary Raynaud's phenomenon. European Journal of Clinical Pharmacology 1987;33(1):27‐30. [DOI] [PubMed] [Google Scholar]
- Francis JL, Roath OS, Challenor VF, Waller DG. The effect of nisoldipine on whole blood platelet aggregation in patients with Raynaud's phenomenon. British Journal of Clinical Pharmacology 1988;25(6):751‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Challenor 1989 {published data only}
- Challenor VF, Waller DG, Hayward RA, Griffin MJ, Roath OS. Vibrotactile sensation and response to nifedipine dose titration in primary Raynaud's phenomenon. Angiology 1989;40(2):122‐8. [DOI] [PubMed] [Google Scholar]
Choi 2009 {published data only}
- Choi WS, Choi CJ, Kim KS, Lee JH, Song CH, Chung JH, et al. To compare the efficacy and safety of nifedipine sustained release with Ginkgo biloba extract to treat patients with primary Raynaud's phenomenon in South Korea; Korean Raynaud study (KOARA study). Clinical Rheumatology 2009;28(5):553‐9. [DOI] [PubMed] [Google Scholar]
Codella 1989 {published data only}
- Codella O, Caramaschi P, Olivieri O, Perbellini L, Perbellini A, Bambara LM, et al. Controlled comparison of ketanserin and nifedipine in Raynaud's phenomenon. Angiology 1989;40(2):114‐21. [DOI] [PubMed] [Google Scholar]
Coleiro 2001 {published data only}
- Coleiro B, Marshall SE, Denton CP, Howell K, Blann A, Welsh KI, et al. Treatment of Raynaud's phenomenon with the selective serotonin reuptake inhibitor fluoxetine. Rheumatology 2001;40(9):1038‐43. [DOI] [PubMed] [Google Scholar]
Corbin 1986 {published data only}
- Corbin DO, Wood DA, Macintyre CC, Housley E. A randomized double blind cross‐over trial of nifedipine in the treatment of primary Raynaud's phenomenon. European Heart Journal 1986;7(2):165‐70. [DOI] [PubMed] [Google Scholar]
- Corbin DOC, Wood DA, Macintyre CCA, Housley El. A randomised double blind cross‐over trial of nifedipine in the treatment of primary Raynaud's phenomenon [abstract]. Clinical Science 1985; Vol. 69, issue Suppl 12:21P. [DOI] [PubMed]
Costantini 1987 {published data only}
- Costantini A, Martelli E, Bavera P, Agus GB. Slow release nifedipine in the treatment of Raynaud's phenomenon. International Angiology 1987;6(4):359‐63. [PubMed] [Google Scholar]
Creager 1984 {published data only}
- Creager MA, Pariser KM, Winston EM, Rasmussen HM, Miller KB, Coffman JD. Nifedipine‐induced fingertip vasodilation in patients with Raynaud's phenomenon. American Heart Journal 1984;108(2):370‐3. [DOI] [PubMed] [Google Scholar]
Csiki 2011 {published data only}
- Csiki Z, Garai I, Shemirani AH, Papp G, Zsori KS, Andras C, et al. The effect of metoprolol alone and combined metoprolol‐felodipin on the digital microcirculation of patients with primary Raynaud's syndrome. Microvascular Research 2011;82(1):84‐7. [DOI] [PubMed] [Google Scholar]
Da Costa 1987 {published data only}
- Costa J, Gomes JA, Espirito Santo J, Queiros M. Inefficacy of diltiazem in the treatment of Raynaud's Phenomenon with associated connective tissue disease: a double blind placebo controlled study. Journal of Rheumatology 1987;14(4):858‐9. [PubMed] [Google Scholar]
Denton 1999 {published data only}
- Denton CP, Bunce TD, Dorado MB, Roberts Z, Wilson H, Howell K, et al. Probucol improves symptoms and reduces lipoprotein oxidation susceptibility in patients with Raynaud's phenomenon. Rheumatology 1999;38(4):309‐15. [DOI] [PubMed] [Google Scholar]
Dompeling 1992 {published data only}
- Dompeling EC, Smit AJ. Assessment of pinacidil in patients with primary Raynaud's phenomenon. Vasa Supplementum 1992;34:34‐7. [PubMed] [Google Scholar]
Dziadzio 1999a {published data only}
- Dziadzio M, Denton CP, Smith R, Howell K, Blann A, Bowers E, et al. Losartan therapy for Raynaud's phenomenon and scleroderma: clinical and biochemical findings in a fifteen‐week, randomized, parallel‐group, controlled trial. Arthritis and Rheumatism 1999;42(12):2646‐55. [DOI] [PubMed] [Google Scholar]
Ferri 1992 {published data only}
- Ferri C, Cecchetti R, Cini G, Gambini I, Civita L, Bernini L, et al. Slow‐releasing nicardipine in the treatment of Raynaud's phenomena without underlying diseases. Clinical Rheumatology 1992;11(1):76‐80. [DOI] [PubMed] [Google Scholar]
Finch 1986 {published data only}
- Finch MB, Johnston GD, Dawson J. The peripheral vascular effects of nifedipine in Raynaud's disease. British Journal of Clinical Pharmacology 1985; Vol. 21:100P‐1P.
- Finch MB, Johnston GD, Dawson J. The peripheral vascular effects of nifedipine in Raynaud's disease associated with scleroderma: a double blind crossover study. Clinical Rheumatology 1986; Vol. 5, issue 4:493‐8. [PubMed]
Finch 1988 {published data only}
- Finch MB, Copeland S, Passmore AP, Johnston GD. A double‐blind cross‐over study of nifedipine retard in patients with Raynaud's phenomenon. Clinical Rheumatology 1988;7(3):359‐65. [DOI] [PubMed] [Google Scholar]
Fontenelle 2008 {published data only}
- Fontenelle SM, Kayser C, Pucinelli ML, Andrade LE. Cold stimulus fingertip lacticemy test ‐ an effective method to monitor acute therapeutic intervention on primary Raynaud's phenomenon and systemic sclerosis. Rheumatology (Oxford, England) 2008;47(1):80‐3. [DOI] [PubMed] [Google Scholar]
Gjorup 1986a {published data only}
- Gjorup T, Kelbaek H, Hartling OJ, Nielsen SL. Controlled double‐blind trial of the clinical effect of nifedipine in the treatment of idiopathic Raynaud's phenomenon. American Heart Journal 1986;111(4):742‐5. [DOI] [PubMed] [Google Scholar]
Gjorup 1986b {published data only}
- Gjorup T, Hartling OJ, Kelbaek H, Nielsen SL. Controlled double blind trial of nisoldipine in the treatment of idiopathic Raynaud's phenomenon. European Journal of Clinical Pharmacology 1986;31(4):387‐9. [DOI] [PubMed] [Google Scholar]
Gush 1987 {published data only}
- Gush RJ, Taylor LJ, Jayson MI. Acute effects of sublingual nifedipine in patients with Raynaud's phenomenon. Journal of Cardiovascular Pharmacology 1987;9(5):628‐31. [DOI] [PubMed] [Google Scholar]
Hawkins 1986 {published data only}
- Hawkins SJ, Black CM, Hall ND, McGregor A, Ring EF, Maddison PJ. Clinical and laboratory effects of nifedipine in Raynaud's phenomenon. Rheumatology International 1986;6(2):85‐8. [DOI] [PubMed] [Google Scholar]
Kahan 1981 {published data only}
- Kahan A, Weber S, Amor B, Saporta L, Hodara M, Degoerges M. Nifedipine and Raynaud's phenomenon. Annals of Internal Medicine 1981;94(41):546. [DOI] [PubMed] [Google Scholar]
Kahan 1983a {published data only}
- Kahan A, Weber S, Amor B, Menkes CJ, Hodara M, Degeorges M. Nifedipine and Raynaud's phenomenon associated with connective tissue diseases. International Angiology 1985;4(2):221‐3. [PubMed] [Google Scholar]
- Kahan A, Weber S, Amor B, Menkes CJ, Saporta L, Hodara M, et al. Calcium entry blocking agents in digital vasospasm (Raynaud's phenomenon). European Heart Journal 1983;4(Suppl C):123‐9. [DOI] [PubMed] [Google Scholar]
- Kahan A, Weber S, Amor B, Saporta L, Hodara M, Degeorges M. Controlled study of nifedipine in the treatment of Raynaud's phenomenon. Revue du Rhumatisme et des Maladies Osteo‐Articulaires 1982;49(5):337‐43. [PubMed] [Google Scholar]
Kahan 1983b {published data only}
- Kahan A, Weber S, Amor B, Guerin F, Degeorges M. Nifedipine in the treatment of migraine in patients with Raynaud's phenomenon. New England Journal of Medicine 1983;308(18):1102‐3. [PubMed] [Google Scholar]
Kahan 1985a {published data only}
- Kahan A, Amor B, Menkes CJ. A randomised double‐blind trial of diltiazem in the treatment of Raynaud's phenomenon. Annals of the Rheumatic Diseases 1985;44(1):30‐3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Kahan 1985b {published data only}
- Kahan A, Foult JM, Weber S, Amor B, Menkes CJ, Degeorges M. Nifedipine and alpha1‐adrenergic blockade in Raynaud's phenomenon. European Heart Journal 1985;6(8):702‐5. [DOI] [PubMed] [Google Scholar]
Kahan 1986 {published data only}
- Kahan A, Amor B, Menkes CJ, Weber S, Guerin F, Degeorges M. Controlled double‐blind trial of nicardipine in the treatment of Raynaud's phenomenon. International Journal of Clinical Pharmacology and Therapeutics 1986; Vol. 39, issue 2:202.
Kallenberg 1987 {published data only}
- Kallenberg CG, Wouda AA, Kuitert JJ, Tijssen J, Wesseling H. Nifedipine in Raynaud's phenomenon: Relationship between immediate, short term and longterm effects. Journal of Rheumatology 1987;14(2):284‐90. [PubMed] [Google Scholar]
- Kallenberg CG, Wouda AA, Kuitert JJ, Tijssen J, Wesseling H. Treatment of Raynaud's phenomenon with nifedipine short‐term and long‐term effects. Vasa Supplementum 1987;18:68‐70. [PubMed] [Google Scholar]
La Civita 1993 {published data only}
- Civita L, Pitaro N, Rossi M, Gambini I, Giuggioli D, Cini G, et al. Amlodipine in the treatment of Raynaud's phenomenon. British Journal of Rheumatology 1993;32(6):524‐5. [DOI] [PubMed] [Google Scholar]
- Civita L, Pitaro N, Rossi M, Giuggioli D, Gambini I, Cini G, et al. Amlodipine in the treatment of Raynaud's phenomenon. A double‐blind placebo‐controlled crossover study. Clinical Drug Investigation 1997;13(Suppl 1):126‐31. [Google Scholar]
La Civita 1996 {published data only}
- Civita L, Giuggioli D, Chicca MG, Longombardo G, Pasero G, Ferri C. Effect of isradipine on endothelin‐1 plasma concentrations in patients with Raynaud's phenomenon [letter]. Annals of the Rheumatic Diseases 1996;55(5):331‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Lee 2014 {published data only}
- Lee EY, Park JK, Lee W, Kim YK, Park CS‐Y, Giles JT, et al. Head‐to‐head comparison of udenafil vs amlodipine in the treatment of secondary raynaud's phenomenon: A double‐blind, randomized, cross‐over study. Rheumatology (Oxford, England) 2014;53(4):658‐64. [DOI] [PubMed] [Google Scholar]
Leppert 1989 {published data only}
- Leppert J, Jonasson T, Nilsson H, Ringqvist I. The effect of isradipine, a new calcium‐channel antagonist, in patients with primary Raynaud's phenomenon: A single‐blind dose‐response study. Cardiovascular Drugs Therapy 1989;3(3):397‐401. [DOI] [PubMed] [Google Scholar]
Lewis 1987 {published data only}
- Lewis P, Psaila JV, Morgan RH, Davies WT, Woodcock JP. Nifedipine in patients with Raynaud's syndrome ‐ effects on radial artery flow. European Heart Journal 1987;8(Suppl K):83‐6. [DOI] [PubMed] [Google Scholar]
Martinez 1999 {published data only}
- Martinez S, Lozano P, Pallares L, Julia J, Artigues I, Plaza A, et al. Usefulness of photoplethysmography and indexes of digital pressure in Raynaud phenomenon. Medicina Clinica 1999;113(9):327‐30. [PubMed] [Google Scholar]
Mascagni 1994 {published data only}
- Mascagni B, Sardina M, Alvino S, Berruti V, Bazzi S, Scorza R. Effects of iloprost in comparison to nifedipine in patients with Raynaud's phenomenon secondary to progressive systemic sclerosis. International Angiology 1994; Vol. 13, issue Suppl 1:81.
Morgan 1987 {published data only}
- Morgan RH, Psaila JV, Davies WT, Carolan G, Woodcock JP. Digital and radial artery blood flow in patients with Raynaud's phenomenon in response to nifedipine. European Journal of Vascular Surgery 1987;1(6):403‐8. [DOI] [PubMed] [Google Scholar]
Moriau 1993 {published data only}
- Moriau M, Lavenne‐Pardonge E, Crasborn L, Frenckell R, Col‐Debeys C. Treatment of Raynaud's phenomenon with piracetam. Arzneimittel‐Forschung 1993;43(5):526‐35. [PubMed] [Google Scholar]
Müller‐Bühl 1983 {published data only}
- Diehm C, Müller‐Bühl U, Mörl H, Schettler G. Calcium antagonists in Raynaud's phenomenon [Calciumantagonistum beim Raynaud‐phanomen]. Zeitschrift fur Kardiologie 1982; Vol. 71, issue 9:Abstract 86.
- Müller‐Bühl U, Diehm C, Scheuermann W, Mörl H. Calcium antagonists for the treatment of Raynaud's phenomenon. Deutsche Medizinische Wochenschrift 1983;108(47):1795‐7. [DOI] [PubMed] [Google Scholar]
Nilsson 1987 {published data only}
- Nilsson H, Jonason T, Leppert J, Ringqvist I. The effect of the calcium‐entry blocker nifedipine on cold‐induced digital vasospasm. A double‐blind crossover study versus placebo. Acta Medica Scandinavica 1987;221(1):53‐60. [DOI] [PubMed] [Google Scholar]
Pisenti 1984 {published data only}
- Pisenti L, Hart LL. DIAS Rounds. Nifedipine and prazosin in Raynaud's phenomenon. Drug Intelligence and Clinical Pharmacy 1984;18(3):213‐4. [PubMed] [Google Scholar]
Redondo 1986 {published data only}
- Rivera Redondo J, Noguerado Asensio A, López Bote JP, Alvaro‐Gracia Alvaro JM, Ossorio Castellanos C. Nifedipine treatment of Raynaud's phenomenon: a double blind controlled clinical study. Revista Espanola de Reumatologia 1986;13:121‐3. [Google Scholar]
Rhedda 1985 {published data only}
- Rhedda A, McCans J, Willan AR, Ford PM. A double blind placebo controlled crossover randomized trial of diltiazem in Raynaud's phenomenon. Journal of Rheumatology 1985;12(4):724‐7. [PubMed] [Google Scholar]
Rupp 1987 {published data only}
- Rupp PA, Mellinger S, Kohler J, Dorsey JK, Furst DE. Nicardipine for the treatment of Raynaud's phenomena: A double blind crossover trial of a new calcium entry blocker. Journal of Rheumatology 1987;14(4):745‐50. [PubMed] [Google Scholar]
Sauza 1984 {published data only}
- Sauza J, Kraus A, González‐Amaro R, Alarcón‐Segovia D. Effect of the calcium channel blocker nifedipine on Raynaud's Phenomenon. A controlled double blind trial. Journal of Rheumatology 1984;11(3):362‐4. [PubMed] [Google Scholar]
Schmidt 1989 {published data only}
- Schmidt JF, Valentin N, Nielsen SL. The clinical effect of felodipine and nifedipine in Raynaud's phenomenon. European Journal of Clinical Pharmacology 1989;37(2):191‐2. [DOI] [PubMed] [Google Scholar]
Scorza 2001 {published data only}
- Scorza R, Caronni M, Mascagni B, Berruti V, Bazzi S, Micallef E, et al. Effects of long‐term cyclic iloprost therapy in systemic sclerosis with Raynaud's phenomenon. A randomized, controlled study. Clinical and Experimental Rheumatology 2001;19(5):503‐8. [PubMed] [Google Scholar]
Shcherbakov 1987 {published data only}
- Shcherbakov AB, Guseva NG, Mach ES. Treatment of Raynaud's syndrome with calcium entry blockers. Terapevticheskii Arkhiv 1987;59(4):89‐92. [PubMed] [Google Scholar]
Smith 1982 {published data only}
- Smith CD, McKendry RJ. Controlled trial of nifedipine in the treatment of Raynaud's phenomenon. Lancet 1982;2(8311):1299‐301. [DOI] [PubMed] [Google Scholar]
- Smith D, McKendry R. Treatment of Raynaud's phenomenon with nifedipine. Annals of the Royal College of Physicians and Surgeons of Canada 1982;15(4):Abstract 282. [Google Scholar]
Stefenelli 1986 {published data only}
- Stefenelli T, Silberbauer K, Glogar D. Pentoxifylline/placebo/nifedipine in patients with Raynaud's phenomenon: influence on frequency of attacks and rewarming time. Klinische Wochenschrift 1986;64:1155‐6. [Google Scholar]
Van Heereveld 1988 {published data only}
- Heereveld H, Wollersheim H, Gough K, Thien T. Intravenous nicardipine in Raynaud's phenomenon: A controlled trial. Journal of Cardiovascular Pharmacology 1988;11(1):68‐74. [DOI] [PubMed] [Google Scholar]
Varela‐Aguilar 1997 {published data only}
- Varela‐Aguilar JM, Sanchez‐Roman J, Talegon Melendez A, Castillo Palma MJ. [Comparative study of misoprostol and nifedipine in the treatment of Raynaud's phenomenon secondary to systemic diseases. Hemodynamic assessment with Doppler duplex]. Revista Clinica Espanola 1997;197(2):77‐83. [PubMed] [Google Scholar]
Waller 1986 {published data only}
- Francis DA, Waller DG, Challenor VF, Roath OS. Red cell deformability in patients with Raynaud's phenomenon treated with nifedipine. Clinical Hemorheology 1985; Vol. 5, issue 5:742.
- Waller DG, Challenor VF, Francis DA, Roath OS. Clinical and rheological effects of nifedipine in Raynaud's phenomenon. British Journal of Clinical Pharmacology 1986;22(4):449‐54. [DOI] [PMC free article] [PubMed] [Google Scholar]
Wasir 1983 {published data only}
- Wasir HS, Singh G, Kaul R, Sachdeva U, Khan WA, Chhina GS, et al. A comparative study of trifluoperazine a calmodulin inhibitor, nifedipine, dipyridamole and intraarterial reserpine in the treatment of Raynaud's phenomenon: A double blind randomised controlled trial. Indian Heart Journal 1983; Vol. 35, issue 5:306.
Weber 1990 {published data only}
- Weber A, Bounameaux H. Effects of low‐dose nifedipine on a cold provocation test in patients with Raynaud's disease. Journal of Cardiovascular Pharmacology 1990;15(5):853‐5. [DOI] [PubMed] [Google Scholar]
White 1986 {published data only}
- White CJ, Phillips WA, Abrahams LA, Watson TD, Singleton PT Jr. Objective benefit of nifedipine in the treatment of Raynaud's phenomenon. American Journal of Medicine 1986;80(4):623‐5. [DOI] [PubMed] [Google Scholar]
Wigley 1987a {published data only}
- Wigley FM, Wise RA, Malamet R, Scott TE. Nicardipine in the treatment of Raynaud's phenomenon. Dissociation of platelet activation from vasospasm. Arthritis and Rheumatism 1987;30(3):281‐6. [DOI] [PubMed] [Google Scholar]
Winston 1983 {published data only}
- Winston EL, Pariser KM, Miller KB, Salem DN, Creager MA. Nifedipine as a therapeutic modality for Raynaud's phenomenon. Arthritis and Rheumatism 1983;26(10):1177‐80. [DOI] [PubMed] [Google Scholar]
Wise 1987 {published data only}
- Wise RA, Malamet R, Wigley FM. Acute effects of nifedipine on digital blood flow in human subjects with Raynaud's phenomenon: a double blind placebo controlled trial. Journal of Rheumatology 1987;14(2):278‐83. [PubMed] [Google Scholar]
Wollersheim 1987 {published data only}
- Wollersheim H, Thien T, Van't Laar A. Acute and chronic effects of nifedipine in Raynaud's phenomenon. European Journal of Clinical Investigation 1986;16(Suppl):A16. [Google Scholar]
- Wollersheim H, Thien T, Van't Laar A. Nifedipine in primary Raynaud's phenomenon and in scleroderma: oral versus sublingual hemodynamic effects. Journal of Clinical Pharmacology 1987;27(11):907‐13. [DOI] [PubMed] [Google Scholar]
Additional references
Allen 1932
- Allen EV, Brown GE. Raynaud's disease: a critical review of minimal requisites for diagnosis. American Journal of Medical Science 1932;183(2):187‐200. [Google Scholar]
ATC classification
- ATC 2013. Anatomical Therapeutical Chemical Classification. WHO. http://www.whocc.no/atcddd (accessed 21 February 2013).
Block 2001
- Block JA, Sequeira W. Raynaud's phenomenon. Lancet 2001;357(9273):2042‐8. [DOI] [PubMed] [Google Scholar]
Dziadzio 1999
- Dziadzio M, Denton CP, Smith R, Howell K, Blann A, Bowers E, et al. Losartan therapy for Raynaud's phenomenon and scleroderma: clinical and biochemical findings in a fifteen‐week, randomized, parallel‐group, controlled trial. Arthritis and Rheumatism 1999;42(12):2646‐55. [DOI] [PubMed] [Google Scholar]
Elbourne 2002
- Elbourne DR, Altman DG, Higgins JPT, Curtin F, Worthington HV, Vail A. Meta‐analyses involving cross‐over trials: methodological issues. International Journal of Epidemiology 2002;31(1):140–9. [DOI] [PubMed] [Google Scholar]
GRADE Working Group 2004
- GRADE Working Group. Grading quality of evidence and strength of recommendations. British Medical Journal 2004;328:1490‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008a
- Guyatt GH, Oxman AD, Kunz R, Vist GE, Falck‐Ytter Y, Schunemann HJ, GRADE Working Group. What is 'quality of evidence' and why is it so important to clinicians?. British Medical Journal 2008;336(7651):995‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Guyatt 2008b
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE Working Group. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. British Medical Journal 2008;336(7650):924‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Harding 1998
- Harding SE, Tingey PC, Pope J, Fenlon D, Furst D, Shea B, et al. Prazosin for Raynaud's phenomenon in progressive systemic sclerosis. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000956] [DOI] [PMC free article] [PubMed] [Google Scholar]
Herrick 2005
- Herrick AL. Pathogenesis of Raynaud's phenomenon. Rheumatology 2005;44(5):587‐96. [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. British Medical Journal 2003;327(7414):557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hirschl 2006
- Hirschl M, Hirschl K, Lenz M, Katzenschlager R, Hutter HP, Kundi M. Transition from primary Raynaud's phenomenon to secondary Raynaud's phenomenon identified by diagnosis of an associated disease; results of ten years of prospective surveillance. Arthritis and Rheumatism 2006;54(6):1974‐81. [DOI] [PubMed] [Google Scholar]
Hughes 2015
- Hughes M, Snapir A, Wilkinson J, Snapir D, Wigley FM, Herrick AL. Prediction and impact of attacks of Raynaud's phenomenon, as judged by patient perception. Rheumatology (Oxford, England) 2015;54(8):1443‐7. [10.1093/rheumatology/kev002. Epub 2015 Mar 9] [DOI] [PubMed] [Google Scholar]
Koenig 2008
- Koenig M, Joyal F, Fritzler MJ, Roussin A, Abrahamowicz M, Boire G, et al. Autoantibodies and microvascular damage are independent predictive factors for the progression of Raynaud's phenomenon to systemic sclerosis: a twenty year prospective study of 586 patients, with validation of proposed criteria for early systemic sclerosis. Arthritis and Rheumatism 2008;58(12):3902‐12. [DOI] [PubMed] [Google Scholar]
LeRoy 1992
- LeRoy EC, Medsger TA Jr. Raynaud's phenomenon: a proposal for classification. Clinical and Experimental Rheumatology 1992;10(5):458‐8. [PubMed] [Google Scholar]
Maricq 1988
- Maricq HR, Weinrich MC. Diagnosis of Raynaud's phenomenon assisted by colour charts. Journal of Rheumatology 1988;15(3):454‐9. [PubMed] [Google Scholar]
Maricq 1997
- Maricq HR, Carpentier PH, Weinrich MC, Keil JE, Palesch Y, Biro C, et al. Geographic variation in the prevalence of Raynaud's phenomenon: a 5 region comparison. Journal of Rheumatology 1997;24(5):879‐89. [PubMed] [Google Scholar]
Merkel 2002
- Merkel PA, Herlyn K, Martin RW, Anderson JJ, Mayes MD, Bell P, et al. Scleroderma Clinical Trials Consortium. Measuring disease activity and functional status in patients with scleroderma and Raynaud's phenomenon. Arthritis and Rheumatism 2002;46(9):2410‐20. [DOI] [PubMed] [Google Scholar]
Priollet 1987
- Priollet P, Vayssairat M, Housset E. How to classify Raynaud's phenomenon. Long‐term follow up study of 73 cases. American Journal of Medicine 1987;83(3):494‐8. [DOI] [PubMed] [Google Scholar]
R Development Core Team 2012
- R Development Core Team. R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing, 2012. [Google Scholar]
RevMan 2012 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.3. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
Schunemann 2006
- Schunemann HJ, Jaeschke R, Cook DJ, Bria WF, El‐Solh AA, Ernst A, et al. ATS Documents Development and Implementation Committee. An official ATS statement: grading the quality of evidence and strength of recommendations in ATS guidelines and recommendations. American Journal of Respiratory and Critical Care Medicine 2006;174(5):605‐14. [DOI] [PubMed] [Google Scholar]
Silman 1990
- Silman A, Holligan S, Brennan P, Maddison P. Prevalence of symptoms of Raynaud's phenomenon in general practice. British Medical Journal 1990;301(6752):590‐2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Sturgill 1998
- Sturgill MG, Seibold JR. Rational use of calcium‐channel antagonists in Raynaud's phenomenon. Current Opinion in Rheumatology 1998;10(6):584‐8. [DOI] [PubMed] [Google Scholar]
Suter 2005
- Suter LG, Murabito JM, Felson DT, Fraenkel L. The incidence and natural history of Raynaud's phenomenon in the community. Arthritis and Rheumatism 2005;52(4):1259‐63. [DOI] [PubMed] [Google Scholar]
Thompson 2005
- Thompson AE, Pope JE. Calcium channel blockers for primary Raynaud's phenomenon: a meta‐analysis. Rheumatology 2005;44(2):145‐50. [DOI] [PubMed] [Google Scholar]
Wigley 2002
- Wigley FM. Raynaud's phenomenon. Clinical practice Raynaud's phenomenon. New England Journal of Medicine 2002;347(13):1001‐8. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Ennis 2014
- Ennis H, Anderson ME, Wilkinson J, Herrick AL. Calcium channel blockers for primary Raynaud's phenomenon. Cochrane Database of Systematic Reviews 2014, Issue 1. [DOI: 10.1002/14651858.CD002069.pub4] [DOI] [PubMed] [Google Scholar]


