Abstract
Primary cilia are small, antenna-like structures that detect mechanical and chemical cues and transduce extracellular signals. While mammalian primary cilia were first reported in the late 1800s, scientific interest in these sensory organelles has burgeoned since the beginning of the twenty-first century with recognition that primary cilia are essential to human health. Among the most common clinical manifestations of ciliary dysfunction are renal cysts. The molecular mechanisms underlying renal cystogenesis are complex, involving multiple aberrant cellular processes and signaling pathways, while initiating molecular events remain undefined. Autosomal Dominant Polycystic Kidney Disease is the most common renal cystic disease, caused by disruption of polycystin-1 and polycystin-2 transmembrane proteins, which evidence suggests must localize to primary cilia for proper function. To understand how the absence of these proteins in primary cilia may be remediated, we review intracellular trafficking of polycystins to the primary cilium. We also examine the controversial mechanisms by which primary cilia transduce flow-mediated mechanical stress into intracellular calcium. Further, to better understand ciliary function in the kidney, we highlight the LKB1/AMPK, Wnt, and Hedgehog developmental signaling pathways mediated by primary cilia and misregulated in renal cystic disease.
11.1. Functions and Features of Primary Cilia
Almost all mammalian cells have an apical protrusion that is used to sense the extracellular environment. This protrusion, the cilium (also known as the flagellum), is an ancient organelle thought to be present on the last eukaryotic common ancestor (Mitchell 2007). Mammalian primary cilia were first reported in 1898 on various iron hematoxylin-stained epithelia, and at that time, primary cilia extending into renal tubules were speculated to sense fluid flow (Zimmermann 1898). Following detailed electron microscopic analyses of embryonic and adult cell types, cilia were further proposed to serve as conserved structures that receive and transduce extracellular cues (Poole et al. 1985). Indeed, the primary cilium functions as an antenna to receive light and mechanical and chemical signals. The ciliary membrane, while topologically continuous with the plasma membrane, is distinct in its composition of membrane and protein components. The increase in cell surface area provided by the ciliary membrane protrusion allows for a concentration of receptors that can transduce extracellular signals. Cilia can also shed or lose membrane from their distal tips in order to release materials to the extracellular environment (Hogan et al. 2009; Wood et al. 2013), signal to adjacent organisms (Wang et al. 2014), or turn over its spent protein, including molecular and membrane components (Young 1971; Young and Bok 1969), demonstrating their capacity for two-way communication.
11.1.1. Cilium Assembly
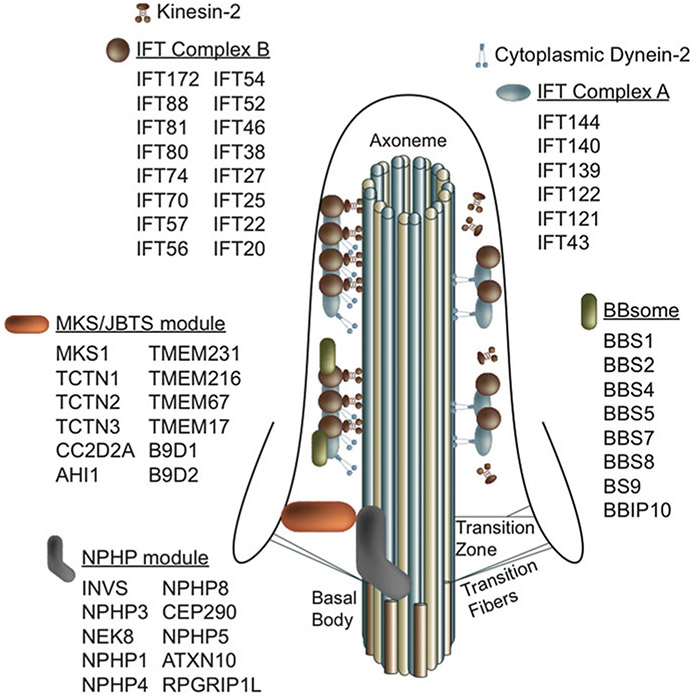
The cilium is composed of a cylindrical arrangement of nine microtubule doublets (A and B tubules) termed the axoneme, which is ensheathed by the cellular plasma membrane (Fig. 11.1). While motile cilia also contain a central pair of microtubules along with accessory structures such as dynein arms and radial spokes for force generation, nonmotile primary cilia lack these structures. Cilia extend and are maintained via intraflagellar transport (IFT), the bidirectional movement of motor and cargo proteins along the microtubular axoneme. IFT was first discovered in the green alga, Chlamydomonas, by differential interference contrast (DIC) visualization of particle movement along flagella (Kozminski et al. 1993). Trafficking of proteins from the base to the tip of the cilium in anterograde IFT is powered by motor proteins in the kinesin-2 family (Cole et al. 1998). This anterograde motor sedimented with a large group of proteins could be biochemically purified as two complexes, complex B and complex A (Cole et al. 1998). Complex B proteins are associated with the anterograde kinesin motor and complex A with retrograde transport from the tip to the ciliary base mediated by the cytoplasmic dynein motor (Pazour et al. 1998, 1999; Porter et al. 1999; Signor et al. 1999). Mutations in the complex B IFT proteins or IFT motors result in stunted cilia and flagella, suggesting their requirement for ciliary assembly (Brazelton et al. 2001; Deane et al. 2001; Follit et al. 2006; Fujiwara et al. 1999; Haycraft et al. 2003; Huangfu et al. 2003; Ishikawa et al. 2014; Pazour et al. 2000; Qin et al. 2007; Sun et al. 2004). Accumulation of complex B proteins in swollen ciliary distal tips and impaired retrograde IFT in complex A mutants indicate that complex A proteins are required for retrograde IFT (Blacque et al. 2006; Iomini et al. 2001, 2009; Perkins et al. 1986; Qin et al. 2011; Tran et al. 2008). Additionally, complex A proteins have also been shown to be required for ciliary entry of G-protein coupled receptors in mammalian cells (Mukhopadhyay et al. 2010).
Fig. 11.1.
Structure of primary cilium. Multi-protein complexes at the transition zone, basal body, and along the axoneme are required for building and maintaining the primary cilium
While each type of cilium is specialized to receive appropriate signals within a certain tissue, proteins common to most cilia include tubulins, comprising the structural units of the axoneme, microtubule motors and IFT complexes, the Bardet–Biedl Syndrome-related protein complex (BBsome), which traffics cargo in and out of the cilium, and vesicular trafficking-related small GTPases (ARFs, ARFs, Rabs). As we will discuss in more detail below, mutant proteins common to several types of cilia result in pleiotropic phenotypes due to ciliary dysfunction in multiple tissues.
11.1.2. Basal Bodies, Early Cilium Formation, and Cilium Disassembly
Cilia are anchored at their base by centriole-derived basal bodies (Fig. 11.1). The basal body is composed of nine microtubule triplets (A, B, and C tubules). The A and B tubules extend from the basal body during cilium formation and become the ciliary axoneme. The basal body is the mother centriole of the pair that forms the centrosome at spindle poles during cell division. During G1 or G0, the basal body acquires accessory structures (distal and subdistal appendages), migrates, and docks to the apical plasma membrane. Docking occurs by attachment of the distal appendages to Golgi-derived vesicles (Sorokin 1962) that fuse to one another and, subsequently, to the apical plasma membrane for cilium extension. This process is mediated by membrane-deforming proteins, EH-domain containing 1 (EHD1) and EHD3 for fusion of distal appendage vesicles into a ciliary vesicle, and by recruitment of GTPase and GTP exchange factors Rab11 and Rabin8 for activation of Rab8 in ciliary vesicle biogenesis (Knodler et al. 2010; Westlake et al. 2011). The basal body then matures through loss of centrosomal protein 110 (CP110) and recruitment of IFT proteins and those of the transition zone (Deane et al. 2001; Lu et al. 2015; Rosenbaum and Witman 2002), a compartment distal to the basal body that is proposed to act as a ciliary gate, regulating ciliary entry of proteins.
Just as there is a mechanism for maturation of centrioles to nucleate cilia, there is also a mechanism to disassemble cilia and release sequestered basal bodies for mitotic progression. Several proteins have been implicated in this process, most notably the Aurora A kinase (Pugacheva et al. 2007), which activates mitotic spindle assembling CDK1-Cyclin-B. Growth factors activate a pathway that stimulates Aurora A kinase and, subsequently, the histone deacetylase, HDAC6, responsible for tubulin deacetylation and cilium destabilization. Another group of kinases, the NIMA-related kinases (NEK), are also involved in ciliary resorption (Hilton et al. 2013; Wloga et al. 2006). Nek2, involved in centrosome separation, is basal body localized and activates a microtubule depolymerizing kinesin, kif24, for ciliary resorption in the G2/M transition (Kim et al. 2015; Spalluto et al. 2012). In Chlamydomonas, another microtubule depolymerizing kinesin, kinesin-13, is also required for flagellar disassembly (Piao et al. 2009).
11.1.3. Cilium Length Control
Another fundamental discovery made in Chlamydomonas that expanded our understanding of how cilia are maintained was that tubulin continuously turns over at flagellar tips (Marshall and Rosenbaum 2001; Song and Dentler 2001; Stephens 1997). This, along with evidence that flagella exhibit a rapid initial growth that slows as they approach steady state (Rosenbaum and Child 1967) and that flagella disassembly occurs at a constant rate independent of length (Kozminski et al. 1995; Marshall et al. 2005; Marshall and Rosenbaum 2001; Parker and Quarmby 2003), forms the basis of the balance point model of flagellar maintenance (Marshall et al. 2005; Marshall and Rosenbaum 2001). In this model, flagella reach their steady-state length when the assembly and disassembly rates are equal. The length dependence of flagellar assembly is based upon the variable quantity of IFT material entering the flagellum at different lengths (Craft et al. 2015; Engel et al. 2009; Ludington et al. 2013) as well as the variable quantity of IFT material accumulating at basal bodies prior to trafficking into flagella (Ludington et al. 2013). Cilia in different cell types have a characteristic length, which presumably are well suited for their function. A few examples of characteristic ciliary lengths are shown in Table 11.1.
Table 11.1.
Mammalian ciliary lengths
| Cell/tissue type | Cilium length (μm) | Citation |
|---|---|---|
| Embryonic neural tube | 0.97 ± 0.17 | He et al. (2014) |
| Rat tail tendon fascicles | 1.35 ± 0.11 | Lavagnino et al. (2013) |
| Mouse embryonic node | 2–2.5 | Alten et al. (2012) |
| Mouse olfactory bulb | 22 | Ying et al. (2014) |
| Rat cholangiocytes (large bile ducts) | 7.35±1.32 | Masyuk et al. (2008) |
| Rat cholangiocytes (small bile ducts) | 3.58±1.12 | Masyuk et al. (2008) |
| Pancreatic duct | 2 | Cano et al. (2004) |
| Pancreatic islet | 2.5 | Ait-Lounis et al. (2007) |
| Kidney | 3–3.5 | Pazour et al. (2000) |
| N1 hypothalamic neurons | 2–2.5 | Han et al. (2014) |
| Hypothalamic arcuate nucleus | 5.5 ± 0.44 | Han et al. (2014) |
| Ventromedial hypothalamus | 5.3 ± 0.39 | Han et al. (2014) |
Chemical and genetic screens (Avasthi et al. 2012; Kim et al. 2010, 2016a) along with single gene perturbation have identified many regulators of ciliary length. In addition to IFT motors, proteins, and cargo that are required for proper cilium assembly, proteins involved in tubulin modification (Pugacheva et al. 2007; Sanchez de Diego et al. 2014) and actin dynamics (Abdul-Majeed et al. 2012; Avasthi et al. 2014; Bershteyn et al. 2010; Kim et al. 2010; Oishi et al. 2006; Sharma et al. 2011) also regulate cilium length. A variety of cell surface receptors have also been identified as modulators of cilium length such as dopamine receptors D1, D5, and other G-protein coupled receptors (Abdul-Majeed et al. 2012; Abdul-Majeed and Nauli 2011; Avasthi et al. 2012). Regulators of protein phosphorylation such as GSK3β, MAP kinases (LF4, Dlk-1, DYF-5), cell cycle-related kinases (Nek1, Nek4, Nek8, Nrk2, Nrk17, Nrk30, Cdc42, Cnk2, LF2, LF5, AurA), and phosphatases (cdc14b) also modulate cilia length (Berman et al. 2003; Burghoorn et al. 2007; Coene et al. 2011; Pugacheva et al. 2007; Tam et al. 2013; Tam et al. 2007; Thiel et al. 2011; van der Vaart et al. 2015; White and Quarmby 2008; Wloga et al. 2006; Zuo et al. 2011). In addition to all of these, cilia respond to second messengers such as calcium, cAMP, inositol 1,4,5-trisphosphate, and associated enzymes protein kinase C, adenylate cyclase III, and inositol polyphosphate 5-phosphatase E, respectively (Abdul-Majeed et al. 2012; Besschetnova et al. 2010; Hatayama et al. 2011; Luo et al. 2012; Ou et al. 2009). Clearly, many different genes are involved in the response of cilia to external signals as well as in coordination with the cell cycle. Additional genes regulating cilium structure and length have been previously reviewed (Avasthi and Marshall 2012; Broekhuis et al. 2013; Keeling et al. 2016), and much is still unknown about how these pathways work together or, indeed, which of them are utilized concurrently. Along with axoneme structure, ciliary membrane must also be regulated for proper assembly, maintenance, and protein composition (Bloodgood 2012). Ciliary formation requires trafficking of Golgi-derived vesicles regulated by small GTPases including Rab8, Rab11, Arf4, and Arl3 (Kim et al. 2014; Knodler et al. 2010; Mazelova et al. 2009; Schwarz et al. 2012; Westlake et al. 2011) as well as membrane regulating proteins EHD1/3 (Lu et al. 2015). In mammalian photoreceptors and Chlamydomonas flagella, ciliary membrane is continuously renewed (Dentler 2013; Young 1971; Young and Bok 1969) and membrane/proteins can be shed via ectosome release (Avasthi and Marshall 2013; Hogan et al. 2009; Wang et al. 2014; Wood et al. 2013; Wood and Rosenbaum 2015). While these studies highlight mechanisms of membrane regulation, how ciliary membrane and axoneme dynamics are coordinated remains largely a mystery.
Within the kidney, cilium length increases during development, being shortest in renal vesicles and longest in mature fetal nephron segments (Saraga-Babic et al. 2012). Renal ciliary length is also modulated in response to various endogenous and extracellular factors, including hormones and changes in urinary shear stress, which are thought to alter the sensitivity of the ciliated cell to external cues (Besschetnova et al. 2010; Upadhyay et al. 2014). Alterations in length of primary cilia are also observed in response to renal injury and during regeneration/repair processes (Verghese et al. 2008, 2009; Han et al. 2016).
11.2. Ciliopathies: Linking Ciliary Dysfunction to Renal Cystogenesis
The discovery that the Oak Ridge polycystic kidney disease (orpk) mouse model resulted from a defect in Tg737/IFT88 marked a major breakthrough linking dysfunction of primary cilia to human disease (Pazour et al. 2000). Soon afterward, an explosion of discoveries followed demonstrating that mysterious disorders affecting multiple organ systems including the retina, kidney, skeletal system, reproductive systems, and others could be traced to defects in a single ciliary gene. These syndromic diseases, termed ciliopathies, include Meckel–Gruber Syndrome (MKS), Bardet–Biedl Syndrome (BBS), Joubert Syndrome, Jeune Syndrome, Nephronophthisis (NPHP), and others (Table 11.2). These pediatric diseases range in severity, causing death in infancy of individuals with MKS or during late teenage years in some individuals with NPHP, and have overlapping but varying degrees of clinical manifestations, including kidney cysts, intellectual disabilities, craniofacial and skeletal defects, polydactyly, retinal degeneration, hypogonadism, and obesity. Many of these diseases, such as MKS and NPHP, result from disruption of proteins at the transition zone, a proposed hotspot for disease gene networks (Chih et al. 2012; Garcia-Gonzalo et al. 2011; Williams et al. 2011). Disruption of these proteins alters cilia structure, resulting in cilia that are abnormally short, absent, or, less commonly, elongated (Williams et al. 2011). BBS is caused by mutations in one of the 21 genes (Bujakowska et al. 2015; Heon et al. 2016; Lindstrand et al. 2014; Schaefer et al. 2016; reviewed in Khan et al. 2016), most of which encode proteins comprising or facilitating formation or trafficking of the BBSome complex, which has been demonstrated to be a cargo of IFT in Chlamydomonas flagella (Lechtreck et al. 2009) and to transport signaling molecules to membrane compartments and within the ciliary membrane (Guo et al. 2016; Guo and Rahmouni 2011). The BBS core is an 8-subunit complex composed of BBS1, BBS2, BBS4, BBS5, BBS7, BBS8, BBS9, and BBS18/BBIP1 (Nachury et al. 2007; Scheidecker et al. 2014). Additionally, BBS3/ARL6 and BBS17/LZTFL1 regulate ciliary trafficking of the BBSome (Liew et al. 2014; Marion et al. 2012; Seo et al. 2011), while BBS6, BBS10, and BBS12 form a BBS-chaperonin complex together with BBS7 to promote BBSome assembly (Seo et al. 2010; Zhang et al. 2012). Causative mutations in IFT genes have also been identified in BBS patients. IFT27/BBS19 (Aldahmesh et al. 2014) facilitates ciliary exit of the BBSome (Eguether et al. 2014; Liew et al. 2014), and while IFT172/BBS20 (Bujakowska et al. 2015; Schaefer et al. 2016) has been shown to regulate IFT particle turnaround at the ciliary distal tip in Chlamydomonas (Pedersen et al. 2005), its connection to the BBSome remains to be explored. While disruption of some BBS proteins impairs ciliogenesis (Marion et al. 2009), mutations of other BBS proteins do not overtly affect cilia structure, but impede ciliary entry or exit of signaling molecules (Berbari et al. 2008; Zhang et al. 2011).
Table 11.2.
Ciliopathies
| Ciliopathy | Organ systems affected | References |
|---|---|---|
| Alström Syndrome | Visual, auditory, cardiovascular, endocrine, hepatic, renal, skeletal, neural | Hearn et al. (2005) |
| Bardet–Biedl Syndrome | Skeletal, neural, visual, renal, reproductive | Ansley et al. (2003) |
| Birt–Hogg–Dubé Syndrome | Renal (carcinoma and cysts), lung, integumentary | Luijten et al. (2013) |
| Ellis van Creveld Syndrome | Skeletal, cardiac | Ruiz-Perez and Goodship (2009) |
| Jeune Syndrome | Skeletal, renal | Beales et al. (2007) |
| Joubert Syndrome | Renal, visual, neural | Louie and Gleeson (2005) |
| Juvenile cystic kidney disease | Renal | Smith et al. (2006) |
| Kartagener Syndrome/Primary ciliary dyskinesia | Respiratory, neural, laterality, reproductive | Afzelius (1976), Camner et al. (1975) |
| Leber congenital amaurosis | Visual | den Hollander et al. (2007) |
| Meckel–Gruber Syndrome | Renal, neural, hepatic | Dawe et al. (2007) |
| Nephronophthisis | Renal | Otto et al. (2003) |
| Orofaciodigital Syndrome 1 | Skeletal | Romio et al. (2004) |
| Polycystic kidney disease | Renal, hepatic, cardiovascular | Pazour et al. (2000) |
| Retinitis pigmentosa | Visual | Hong et al. (2001) |
| Senior–Loken Syndrome | Renal, visual | Otto et al. (2005) |
| Sensenbrenner Syndrome | Skeletal | Walczak-Sztulpa et al. (2010) |
| Tuberous sclerosis | Neural, integumentary, cardiovascular, renal, respiratory | Hartman et al. (2009) |
| Usher Syndrome | Visual, auditory | Bonneau et al. (1993) |
| Von Hippel–Lindau | Tumors in blood vessels and adrenal glands, renal (carcinoma and cysts) | Esteban et al. (2006) |
Of the many clinical features of ciliopathies, a major hallmark is renal cystic disease (Quinlan et al. 2008). Renal cystic diseases are mostly inherited and can be classified as Polycystic Kidney Disease (PKD) or as non-PKD, depending on the mutated gene. While the non-PKD diseases comprise the ciliopathies mentioned above, PKD results from mutations in Polycystic Kidney Disease 1 (PKD1), PKD2, or Polycystic Kidney and Hepatic Disease 1 (PKHD1) genes. Mutations of PKD1 or PKD2 cause approximately 90% of cases of Autosomal Dominant Polycystic Kidney Disease (ADPKD), the most common fatal genetic disease, affecting 1:400–1:1000 adults worldwide. Renal cysts can initiate in the fetus and progressively grow, compressing and compromising surrounding parenchyma and causing end-stage renal disease in the 6th decade of life. Mutations in PKHD1 cause Autosomal Recessive PKD, which affects 1:20,000 children. The PKD1, PKD2, and PKHD1 gene products, polycystin 1 (PC1), PC2, and fibrocystin/polyductin (FPC), localize to various subcellular compartments, including the primary cilium (Barr and Sternberg 1999; Yoder et al. 2002). Ciliary localization of polycystin-GFP fusion proteins in C. elegans provided the first demonstration of a link between renal cystic disease and primary cilia (Barr and Sternberg 1999). The ciliary localization of mammalian PKD gene products together with evidence that ciliary defects cause renal cysts led to the proposal that ciliary dysfunction may present a unifying etiological mechanism for renal cystic diseases. Recently, mutations in Glucosidase Alpha; Neutral AB (GANAB), which catalyzes a step in the N-glycosylation pathway in the endoplasmic reticulum (ER), have been reported in ADPKD patients, reflecting a critical role for the ER protein maturation process for functional PC1 and its localization to the primary cilium (Porath et al. 2016), discussed in more detail in Sect. 3.
Multiple cellular processes are disrupted in renal cystogenesis that result in cell dedifferentiation, increased cell proliferation, increased fluid secretion of renal epithelial cells, and increased tubular cell apoptosis (reviewed in Calvet and Grantham 2001; Torres and Harris 2006; Zhou 2009). Studies using ADPKD primary renal cells revealed that the combination of high intracellular cAMP levels and low intracellular calcium (Ca2+) drives cell proliferation and fluid secretion of cyst-lining epithelial cells (Calvet 2008; Wallace 2011). In human ADPKD cells, low homeostatic intracellular Ca2+ enables aberrant cAMP-mediated activation of Protein Kinase A (PKA) and ERK, which, in turn, activates both cystic fibrosis transmembrane conductance regulator (CFTR) and ERK signaling pathways, causing fluid secretion by epithelial cells into the cyst lumen and increased proliferation of cyst-lining epithelial cells, respectively (Yamaguchi et al. 2004). Addition of a Ca2+ ionophore rescued these aberrant responses, and conversely, addition of a Ca2+ channel blocker to normal human kidney (NHK) cells caused ADPKD cellular responses, demonstrating the pivotal role of intracellular Ca2+ in influencing cellular phenotype (Yamaguchi et al. 2006). Elevated intracellular cAMP is common to cystic kidneys of both orthologous Pkd and ciliary mouse models: the former including Pkd1 (Hopp et al. 2012) and Pkd2 (Torres et al. 2004) mutant mice and the Pkhd1-mutant Polycystic Kidney (PCK) rat (Wang et al. 2008), while the latter group including pcy (Yamaguchi et al. 1997), cpk (Tao et al. 2015), jck (Smith et al. 2006), Kif3a (Choi et al. 2011), and Thm1/Ttc21b (Tran et al. 2014b). Such observations suggest that increased cAMP may be a universal and fundamental mechanism originating from ciliary dysfunction. Another unifying phenomenon is that loss of PKD or ciliary proteins during kidney development leads to severe, rapidly progressing renal cystic disease, while loss of these proteins after kidney maturation (P12–P14) causes a mild, slowly progressing disease, demonstrating that the developmental state of the kidney strongly influences renal cystogenesis (Piontek et al. 2007). A role for the primary cilium and PC1 in sensing tubular injury and in regulating the repair–regeneration–redifferentiation response has been proposed, and dysfunction of this adaptive mechanism in mutant kidneys may provide a trigger for cyst formation in the adult kidney (Weimbs 2007).
Yet, differences are evident between PKD and non-PKD renal cystic diseases. In PKD, growth of renal cysts greatly enlarges kidney size, while in non-PKD diseases, such as NPHP, kidney size tends to decrease due to abundant fibrosis. Another contrast between PKD and non-PKD diseases is that while most non-PKD proteins have a fundamental role in ciliogenesis, PC1, PC2, and FPC do not. This is also reflected by the majority of studies that do not report altered ciliary length in Pkd1/Pkd2 mutants, with the exception of two. In renal epithelia of the PC1RC/RC mouse model, which carries an ADPKD mutation (Hopp et al. 2012) and in cultured Pkd1−/− and Pkd2−/− derived mouse renal epithelial cells (Jin et al. 2014b), cilia were lengthened. This may relate to the role of PC1/PC2 in modulating intracellular cAMP and Ca2+, since pharmacological manipulation of intracellular cAMP and Ca2+ in mouse inner medullary collecting duct (IMCD) cells showed that low Ca2+ lengthens cilia, while high Ca2+ shortens cilia (Besschetnova et al. 2010).
Although loss of proteins required for ciliary structure usually causes NPHP in the human population, IFT mouse models and the Nek8 mutant, jck, manifest PKD-like disease with enlarged kidneys. Moreover, heterozygosity for both Pkd1 and the Nek8jck/+ mutation, which in the homozygous form causes longer cilia and an ADPKD phenotype (Smith et al. 2006), caused renal cysts in mice, while single heterozygosity did not, suggesting that molecular mechanisms stemming from deficiency of PC1 and jck mutant NEK8 protein may converge (Natoli et al. 2008). Intriguingly, despite that ablation of Kif3a, and in turn primary cilia, results in renal cysts, combined loss of primary cilia and PC1/2 ameliorated PC-mediated renal cystogenesis and decreased renal epithelial cell proliferation in mice, suggesting the presence of an as yet unidentified ciliary-mediated signaling pathway that is crucial for PC1/2 cyst development (Ma et al. 2013). Corroborating and extending these data, reduced ciliary length through inhibition of Cyclin Dependent Kinase 5 (CKD5) also ameliorated the renal cystic disease in jck mice (Husson et al. 2016). These data underscore the need to define the role of ciliary proteins individually and in PKD to understand mechanisms that exacerbate or attenuate PKD. Disease variability observed among ADPKD patients may be due to mutations/polymorphisms in genes affecting ciliary function.
11.3. Ciliary Localization of Polycystins Is Interdependent and Important for Their Function
Analysis of several ADPKD missense mutations in either PKD1 or PKD2 showed that they resulted in the absence of polycystins in cilia (Cai et al. 2014). One of these mutations, PC2W414G, present in the first extracellular loop, retained channel activity, suggesting that functional PC2 in the endoplasmic reticulum (ER) is insufficient to prevent cystic disease and that ciliary localization of polycystins is essential for this function. Thus, understanding ciliary-targeting mechanisms of polycystins is important for understanding PKD biology and finding therapeutic targets.
Several ciliary targeting sites have been uncovered in the PC1 C-terminal cystosolic tail, including a KVHPSST sequence (Ward et al. 2011) and sites within and upstream of the coiled-coiled domain required for interaction with PC2 (Su et al. 2015). An RVxP ciliary targeting sequence in the PC2 N-terminus has also been identified (Geng et al. 2006). Accumulating evidence suggests that ciliary localization of PC1 and PC2 is interdependent (Freedman et al. 2013; Gainullin et al. 2015; Kim et al. 2014; Nauli et al. 2003; Su et al. 2015). In ADPKD-derived, PKD1-mutant induced pluripotent stem cells and in Pkd1−/− MEFs, PC2 ciliary localization was reduced or undetectable, respectively, while in Pkd2−/− MEFs, ciliary PC1 was lacking (Freedman et al. 2013; Gainullin et al. 2015; Kim et al. 2013). Conversely, overexpression of PC2 enhanced both WT and mutant PC1 ciliary targeting, while increasing levels of WT PC1, but not of a PC1 construct lacking its C-terminus, promoted PC2 ciliary localization in mouse IMCD3 cells (Freedman et al. 2013; Su et al. 2015), consistent with a requirement for the PC1 C-terminus in ciliary localization.
To reach the primary cilium, PC1 protein must undergo maturation, which has also been shown to depend on PC2 (Gainullin et al. 2015; Kim et al. 2014). PC1 maturation involves N-linked core glycan addition and cleavage at its G-protein coupled proteolysis site (GPS), which generates extracellular N-terminal (NTF) and membrane-embedded C-terminal (CTF) fragments that remain non-covalently associated, and occurs within the ER prior to subsequent trafficking and N-glycan modification in the Golgi (Wei et al. 2007). PKD1 missense mutations that interfere with GPS cleavage prevent PC1 maturation and ciliary localization (Cai et al. 2014; Chapin et al. 2010; Kurbegovic et al. 2014). PC1–PC2 association occurs before GPS cleavage of PC1 (Gainullin et al. 2015) and is required for the PC complex to reach the trans-Golgi (Kim et al. 2014). In Pkd2−/− mouse embryonic fibroblasts (MEF), cleaved PC1 remained endoH sensitive, suggesting that PC1 must complex with PC2 to traffic through the Golgi. Further, cleavage of PC1 is essential for transport through the Golgi, since a non-cleavable PC1 mutant co-precipitated with PC2 remained endoH sensitive (Kim et al. 2014). Pkd1RC/RC hypomorphic mutant mice carrying various Pkd2-mutant alleles of differing deficiency revealed that renal cystic disease severity was determined by levels of mature PC1, which in turn correlated with PC2 levels, demonstrating the dependence of PC1 maturation on PC2 in vivo (Gainullin et al. 2015).
11.3.1. Polycystin Transport from the Golgi Apparatus to the Cilium
Golgi-derived vesicles convey proteins to the base of the primary cilium, a process termed polarized vesicle trafficking (reviewed by (Hsiao et al. 2012). This transport is essential for ciliogenesis and is mediated by the Rab and Arf families of small GTPases, which recruit vesicle coating complexes during vesicle budding, docking, and fusion. At the centrosome, Rab11 is essential in vesicular targeting of Rabin8 to recruit and activate Rab8 for ciliary targeting of cargo proteins (Knodler et al. 2010). Two 8-subunit protein complexes, the exocyst and the BBSome, are required for post-Golgi vesicular membrane trafficking and for ciliary entry and exit of signaling proteins, respectively. Within cilia, trafficking of the BBSome and its cargo is mediated by IFT.
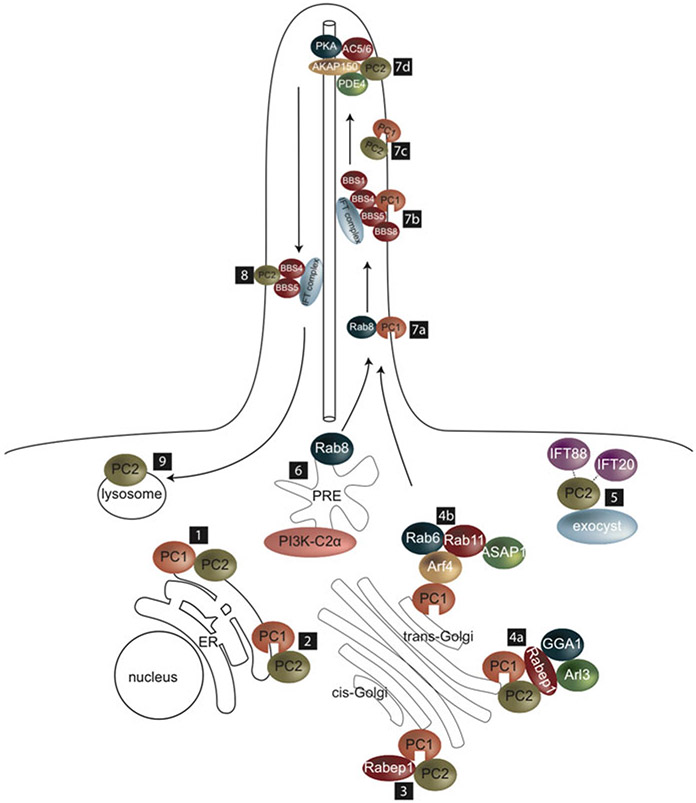
Evidence suggests that multi-protein complexes involving the Rab and Arf GTPases, the exocyst, and the BBSome mediate post-Golgi intracellular and ciliary trafficking of polycystins (Fig. 11.2). In photoreceptors, a protein complex consisting of Arf4 and the ArfGAP with SH3 domain, ankyrin repeat, and PH domain 1 GTPase-activating protein (ASAP1) traffics membrane proteins from the Golgi to the base of the modified cilium (Mazelova et al. 2009). Hypothesizing a role for such a complex in trafficking polycystins, Ward et al. (2011) found that via its KVHPSST ciliary targeting sequence, PC1 interacts with Arf4 and ASAP1 in a complex with Rab6 and Rab11 in the Golgi membranes of primary renal cortical epithelial (RCTE) cells (Ward et al. 2011). The RVxP ciliary targeting sequence of PC2 also allowed binding of PC2 to Arf4, which is localized in the trans-Golgi, suggesting that PC2 also passes through the Golgi network. In another study, a yeast two-hybrid screen identified RAB GTPase Binding Effector Protein 1 (Rabep1) to interact with PC1 in a region containing the coiled-coiled domain within the C-terminal tail (Kim et al. 2014). This interaction was confirmed in primary collecting duct (CD) cells of mouse postnatal kidneys and also occurred in Pkd2−/−- MEF. Since this study showed that PC1 exits the ER in a complex with PC2, the presence of PC1-Rabep1 complex in Pkd2−/− MEF indicates this interaction occurs in a pre-Golgi compartment. Subsequently, Rabep1 was found to bind to Golgi-localized, gamma adaptin ear-containing, ARF-binding protein 1 (GGA1) in CD cells, an interaction that had been shown previously in trans-Golgi vesicles (Mattera et al. 2003). GGA1 was subsequently found to bind to Arf-like 3 (Arl3), which facilitates ciliary localization of cargo proteins (Wright et al. 2011). Taken together, binding of Rabep1 to PC1 in a pre-Golgi compartment links the PC1/PC2 complex to GGA1 at the trans-Golgi, where GGA1 then interacts with Arl3. In contrast to findings of Ward et al. (2011), PC1 did not co-immunoprecipitate with Arf4 in CD cells (Kim et al. 2014), which may point to differences between cell types and requires further investigation.
Fig. 11.2.
Intracellular trafficking of polycystins to primary cilium. Intracellular and ciliary trafficking of polycystins involves the following steps: (1) PC1 and PC2 interact in the ER (Kim et al. 2014; Gainullin et al. 2015); (2) PC1 is cleaved (Kim et al. 2014; Gainullin et al. 2015); (3) PC1/PC2 complex interacts with Rabep1 in pre-Golgi complex (Kim et al. 2014); (4a) Rabep1 bridges PC1/PC2 complex to GGA1, which binds to Arl3 at the trans-Golgi (Kim et al. 2014); (4b) PC1 in a complex with Arf4, ASAP1, Rab6, and Rab11 in Golgi (Ward et al. 2011); (5) PC2 interacts with exocyst, in a complex with IFT88 and IFT20, which facilitates ciliary entry of PC2 (Fogelgren et al. 2011); (6) PI3K-C2α in the pericentriolar recycling endosome (PRE) activates Rab8 (Franco et al. 2014), which interacts with PC1 and also PC2 and (7a) facilitates ciliary entry of PC1 and also of PC2 (Ward et al. 2011; Hoffmeister et al. 2011; Franco et al. 2016); (7b) Binding of PC1 to BBS1 and the BBSome facilitates ciliary entry of PC1 (Su et al. 2014); (7c) PC1 and PC2 complex in the cilium (Nauli et al. 2003); (7d) PC2 is in a ciliary complex with AKAP150, AC5/6, PDE4, and PKA (Choi et al. 2011); (8) BBS4 and BBS5 mediate ciliary removal of PC2 (Xu et al. 2015); (9) PC2 is degraded in the lysosome (Hu et al. 2007; Xu et al. 2015)
In contrast to these two studies, glycosylation analysis of PC2 in whole cell lysates of porcine renal proximal tubular epithelial LLC-PK1 cells, together with live-imaging and electron microscopy, suggested that PC2 destined for primary cilia is trafficked to the cis-Golgi and, subsequently to the cilium, bypassing the mid- and trans-Golgi membranes (Hoffmeister et al. 2011). Supporting this, ciliary protein fractions of RCTE cells contained endoH-resistant PC1 that co-precipitated with endoH-sensitive PC2 (Gainullin et al. 2015). These data imply that PC2 vesicular trafficking is independent of that of PC1. Thus, two possible routes for trafficking of PC2 to the primary cilium are suggested by these studies.
PC1 also interacted with Rab8 (Ward et al. 2011), which is directed to the ciliary base by Abelson Helper Integration Site 1 (AHI1) and targets cargo to the primary cilium. Expression of a dominant-negative Rab8 prevented ciliary localization of PC1 (Ward et al. 2011) and also of PC2 (Hoffmeister et al. 2011). Rab8 is activated by Rab11, which is stimulated by Class II phosphoinositide 3-kinase-C2α (PI3K-C2α), which localizes to pericentriolar recycling endosomes (PRE) near the ciliary base (Franco et al. 2014). shRNA knockdown of PI3K-C2α in IMCD3 cells shortened cilia and resulted in a marked reduction of cells with PC2 ciliary localization, which was rescued upon expression of a constitutively active Rab8 (Franco et al. 2016). Pi3k-C2α+/− mice were sensitized to cyst formation on a Pkd1+/− or Pkd2+/− genetic background or when subjected to ischemia/reperfusion-induced renal injury, substantiating the importance of PC ciliary localization for PC function in vivo.
Both the exocyst and BBSome appear to mediate ciliary entry of polycystins. Deficiency of Sec10, an exocyst subunit, which localizes to the cilium and controls ciliogenesis (Zuo et al. 2009), resulted in undetectable ciliary localization of PC2 (Fogelgren et al. 2011). Morpholino knockdown of Sec10 in zebrafish phenocopied the PC2 morpholino mutant, resulting in a tail curled upwards, left–right laterality defects, and glomerular abnormalities. Sec10 was also shown to co-precipitate with PC2, IFT88, and IFT52, suggesting that the exocyst together in a complex with IFT proteins mediates ciliary entry of PC2. Using a yeast two-hybrid screen and GST pull-down experiments, BBS1, BBS4, BBS5, and BBS8 were identified as interacting with the PC1 C-terminus, although only loss of BBS1 and expression of a dominant-negative form of BBS3/Arl6 in IMCD cells shortened cilia and prevented PC1 ciliary localization (Su et al. 2014). Since Rab11 interacts with the exocyst and also promotes association of BBS1 with Rabin8 (Knodler et al. 2010), PC-BBSome and PC-exocyst complexes may form at the base of the cilium in connection with Rab11. The BBSome is also implicated in ciliary removal of PC2. In RPE cells deficient for both BBS4 and BBS5, PC2 accumulated in the primary cilium, although overall PC2 protein levels were unaffected, suggesting that BBS4 and BBS5 together mediate removal of PC2 from the primary cilium (Xu et al. 2015). PC2 further undergoes ubiquitination and lysosomal degradation (Hu et al. 2007). Treatment of RPE cells with a lysosomal inhibitor, chloroquine, increased ciliary localization of PC2, similar to the combined deficiency of BBS4 and BBS5 (Xu et al. 2015).
Most of these trafficking studies examined PC1 or PC2 alone. Further examination of whether PC-Rab/Arf, PC-BBSome, or PC-exocyst complexes consist of PC1/PC2 together would help ascertain whether PC1 and PC2 traffic independently or as a complex from the Golgi to the primary cilium. If the polycystins traffic independently, such experiments would further determine at what point of trafficking, e.g., prior to or following ciliary entry, the PC1/PC2 complex is formed.
11.4. Primary Cilium is a Mechanosensory Organelle
After more than 100 years, Zimmermann’s hypothesis that primary cilia lining the renal tubules act as flow sensors (Zimmermann 1898) was demonstrated. Fluid flow causes primary cilia of cultured renal epithelial cells to deflect and intracellular Ca2+ levels to increase via a mechanism that requires extracellular Ca2+ influx and ensuing release of intracellular Ca2+ stores (Praetorius and Spring 2001). In cells deciliated by chloral hydrate, Ca2+ response was abrogated, demonstrating that primary cilia are essential in flow-mediated elevation of cystosolic Ca2+ (Praetorius and Spring 2003). A role for cilia was further made evident by the absence of a luminal flow-induced Ca2+ response in microperfused collecting ducts of 2-week-old orpk mutant mice, which harbor an IFT88 hypomorphic mutation causing shortened cilia (Liu et al. 2005b). This abrogated response occurred in tubular segments that were not yet dilated, suggesting defective mechanotransduction might mark tubules sensitized to cystogenesis. In addition, magnitude of the Ca2+ response was observed to be twofold higher at P14 than at P7 in wild-type mice, an increase that was not observed in orpk mice. This suggests that the cilia-mediated, flow-induced intracellular Ca2+ response matures at P14. Interestingly, this time point coincides with the developmental window that influences renal cystic disease severity (Piontek et al. 2007).
Absence of PC1 or PC2 function in renal cells also obliterated flow-mediated intracellular Ca2+ increase (Nauli et al. 2003). This led to the proposal that ciliary PC1 senses flow and activates the Ca2+ channel activity of ciliary PC2, causing Ca2+ influx into the cilium, which, in turn, results in increased intracellular Ca2+. FPC, TRPV4, and NEK8 also complex with PC2 at the primary cilium, and cells deficient in these proteins have shown impaired mechanotransduction (Kottgen et al. 2008; Manning et al. 2013; Wang et al. 2007). Yet, the variable development of renal tubular cysts among various Pkhd1 mutant mouse models, with some displaying absence of cysts (Gallagher et al. 2008; Moser et al. 2005) and others presence of cysts (Garcia-Gonzalez et al. 2007; Woollard et al. 2007), the presence of glomerular but not tubular cysts in Nek8-null mice, which die at birth (Manning et al. 2012), contrasting with the Nekjck/jck mutant adults, which show a phenotype resembling human ADPKD (Smith et al. 2006), along with the lack of renal cyst formation in Trpv4-deficient mice and zebrafish, has questioned the contribution of impaired mechanotransduction to renal cystogenesis (Kottgen et al. 2008). In Pkhd1-mutant PCK rats, which model ARPKD and develop renal cysts, TRPV4 mechanosensory function was reduced in cyst-lining epithelial cells and TRPV4 activation attenuated the renal cystic disease (Zaika et al. 2013), demonstrating that impaired fluid flow-induced intracellular Ca2+ generation can modulate renal cystic disease, even if it may not be sufficient to initiate renal cystogenesis. Since mutations in PKHD1 cause ARPKD in humans and rats, but show variable effects in mice, these varying influences of FPC on renal cystogenesis across different species may reflect differential regulation of fluid flow-induced Ca2+ responses in mouse, rat, and human kidneys. Likewise, the requirement of TRPV4 in fluid flow-induced Ca2+ generation may be cell line or tubule segment specific as illustrated by the lack of involvement of TRPV4 in fluid flow-mediated, PC2- and calcium-dependent induction of endothelin-1 expression in IMCD cells (Pandit et al. 2015).
Dysfunctional flow sensing or mechanotransduction may contribute to renal cystogenesis via aberrant calcium-mediated or calcium-independent mechanisms. Collecting duct cells derived from the orpk mouse mutant exhibit blunted primary cilia, increased apical calcium entry in response to fluid flow (Siroky et al. 2006) and to epidermal growth factor (EGF) treatment, and an augmented PC2- and TRPV4-dependent proliferative response to EGF (Zhang et al. 2013). PC1 undergoes mechano-regulated cleavages within its C-terminal tail (CTT), generating fragments (CTT, P30, P15) that translocate to the nucleus, interact with various transcription transactivators (CHOP, TCF, STAT3, STAT6), and modulate proliferative, apoptotic, or cytokine-stimulated pathways. Cleavage of the CTT is stimulated by loss of mechanostimulation, as in MDCK cultures under static (no-flow) conditions (Low et al. 2006) or in wild-type mouse kidneys subjected to unilateral obstruction of the ureter, and by loss of mechanosensory function, as in Kif3a-null kidneys which lack primary cilia (Chauvet et al. 2004). PC2 influences cleavage and subsequent stabilization of PC1 CTT via a calcium-independent mechanism. Membrane-localized (non-cleaved) CTT binds and sequesters STAT6 and its coactivator P100 to prevent their nuclear activity (Low et al. 2006), or maintains canonical (JAK-activated, SOCS inhibitable) signaling via STAT1 and STAT3, thought to modulate cytokine-mediated STAT signaling (Talbot et al. 2011, 2014). Cleavage and nuclear translocation of CTT can be anti-cystogenic, by binding to CHOP, TCF, or β-catenin to prevent interaction with p300, thus inhibiting apoptosis or proliferative Wnt-signaling (Lal et al. 2008; Merrick et al. 2012), or can exacerbate cystic disease via Src-mediated or augmented cytokine-mediated STAT3 activation (Talbot et al. 2011, 2014). Observations of CTT fragments and nuclear staining with antibodies directed to the C-terminus of PC1 in human ADPKD kidneys (Talbot et al. 2011), along with the abilities of leflunomide and pyrimethamine, inhibitors of STAT6 and STAT3, respectively, to ameliorate cystic disease in PKD mouse models (Olsan et al. 2011; Takakura et al. 2011), support the relevance of these CTT-mediated functions in PKD.
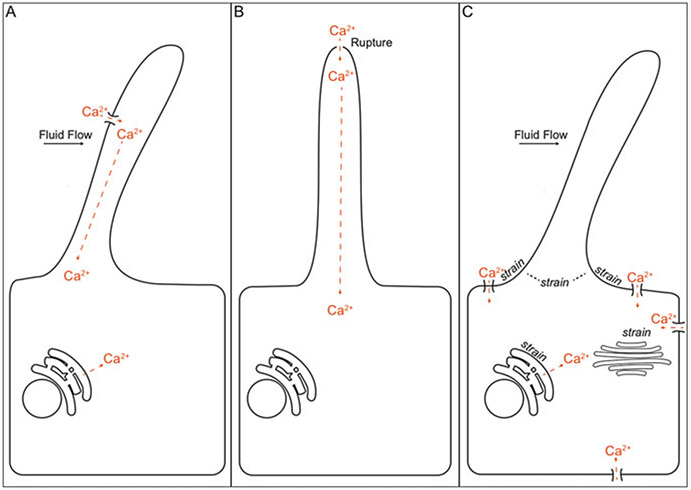
For more than a decade, Ca2+ influx into the cilium could only be speculated until strategies to visualize Ca2+ in the primary cilium were pioneered. Genetically encoded Ca2+ indicators were fused to a ciliary targeting sequence of ciliary localized proteins (Delling et al. 2013; Jin et al. 2014a; Su et al. 2013). Jin et al. (2014a) showed that upon mechanical bending of a primary cilium, Ca2+ increased in the cilium, which was followed by increased Ca2+ in the cytosol, at last providing experimental evidence that primary cilia convert fluid flow shear stress into ciliary Ca2+ signals (Fig. 11.3a). Additionally, Pkd2 siRNA knockdown impeded the increased ciliary Ca2+ response, supporting the role of PC2 in mediating flow-induced Ca2+ response. This sequence of events has been supported in vivo in the nonmotile cilia of zebrafish Kupffer’s vesicle, which is orthologous to the mammalian node. In the node, leftward fluid flow generated by nodal cilia elevates cytosolic calcium in cells on the left side of the node, triggering asymmetric gene expression which is required for left–right asymmetry. In response to leftward fluid flow, Ca2+ was shown to increase in nonmotile cilia and, subsequently, in the cytoplasm, supporting that primary cilia transduce mechanical forces into ciliary Ca2+ (Yuan et al. 2015). pkd2 and its putative partner pkd1-like1 were identified as the molecules required for mechanotransduction in Kupffer’s vesicle.
Fig. 11.3.
Models of fluid flow shear stress-induced intracellular Ca2+ generation. (a) Fluid flow induces ciliary Ca2+ influx, which releases intracellular Ca2+ stores (Jin et al. 2014a; Yuan et al. 2015). (b) Ciliary Ca2+ influx induced by ciliary membrane rupture increases Ca2+ at ciliary base but does not release intracellular Ca2+ stores (Delling et al. 2013). (c) Bending of primary cilium induced by fluid flow causes strain at base of cilium, which induces Ca2+ influx via apical and basolateral membranes and, subsequently, release of intracellular Ca+2 stores (Liu et al. 2003; Rydholm et al. 2010; Khayyeri et al. 2015)
However, by patch clamping the cilium, DeCaen et al. (2013) showed that ciliary ion currents were generated by the gene products of Pkd1L1 and Pkd2L1, while PC1 and PC2 activities were undetectable (DeCaen et al. 2013). In addition, laser disruption of the ciliary tip increased ciliary Ca2+, but caused only small increases in Ca2+ at the ciliary base, which did not significantly alter cytosolic Ca2+ levels (Delling et al. 2013) (Fig. 11.3b). By developing a transgenic mouse expressing a ratiometric genetically encoded Ca2+ indicator in primary cilia and using swept-field confocal microscopy capturing up to 500–1000 frames/s to image cells and tissues derived from this mouse, fluid flow was not observed to induce Ca2+ changes in primary cilia of cultured kidney epithelial cells nor in the renal thick ascending limb of loop of Henle, in crown cells of the embryonic node, or in kinocilia of inner ear hair cells (Delling et al. 2016). In incidences when ciliary Ca2+ increased, Ca2+ was observed first to increase in the cytoplasm and subsequently to diffuse into the cilium. At supraphysiological flow velocities, ciliary tips were observed to rupture, which was followed by extracellular Ca2+ entry. Collectively, these results challenge the notions that primary cilia and the polycystins transduce mechanical strain into ciliary Ca2+ and, moreover, that ciliary Ca2+ regulates intracellular Ca2+.
Mathematical modeling of ciliary deflection and cellular response has provided additional perspectives (Fig. 11.3c). By integrating data from measurements of shear stress-induced intracellular Ca2+ generation in microperfused and split-open rabbit cortical collecting ducts with theoretical modeling, Liu et al. (2003) proposed that activation of increased intracellular Ca2+ initiates at the base of the cilium where the cilium transfers mechanical forces to the cytoskeleton (Liu et al. 2003). Consistent with the ciliary base having an important role in mechanotransduction, another mathematical model suggested that ciliary deflection causes strain to build up at the base of the primary cilium and in the surrounding cell membrane (Rydholm et al. 2010). This strain buildup occurred approximately 30 s following induction of fluid flow coinciding with the 30 s time delay observed to generate a flow-induced intracellular Ca2+ response in MDCK cells (Praetorius and Spring 2001). In addition to strain accumulating at the ciliary base, results of another computational study showed that deflection of primary cilia causes strain to accumulate also at the nucleus and Golgi apparatus (Khayyeri et al. 2015). Thus, analysis of transcriptional or posttranslational changes upon fluid flow may merit investigation.
Taken together, the data suggest that the intermediary steps between ciliary deflection and increased intracellular Ca2+ require further study. Differences in cell lines, imaging techniques, use of cell monolayers versus perfused or split-open tubules, and species may contribute to the discrepancies. The debate on the role of the primary cilium as a mechanotransducer of ciliary Ca2+ continues (Tran and Lechtreck 2016). Super-resolution imaging and additional in vivo studies may be important in defining the mechanosensory role of primary cilia.
11.5. Primary Cilium is a Signaling Organelle
Multiple signaling pathways are disrupted in ADPKD (reviewed in Song et al. 2009; Torres and Harris 2006), and accumulating evidence suggests that a number of these are mediated by primary cilia. We discuss Ca2+/cAMP, LKB1/AMPK, and Wnt signaling, which play important roles in PKD. In addition, we review Hedgehog (Hh) signaling, whose regulation at the primary cilium is the most characterized, and thus, ciliary mechanisms learned from this pathway may enable ideas that can be applied to other signaling molecules relevant to PKD.
11.5.1. Ca2+/cAMP
Ca2+ levels are fivefold higher in the cilium than in the cytosol demonstrating that the cilium is a separate Ca2+ compartment (Delling et al. 2013). Additionally, studies by Choi et al. (2011) indicate that PC2 is integral to the formation of a complex that regulates cAMP and Ca2+ at the primary cilium. The PC2 C-terminus interacts with A-kinase anchoring protein 150 (AKAP150), which creates a scaffold for binding of protein kinase A (PKA); adenylyl cyclases 5 and 6 (AC5/6), which synthesize cAMP; and phosphodiesterase 4C (PDE4C), which catabolizes cAMP (Choi et al. 2011). Loss of PC2 in Pkd2−/− derived renal epithelial cells resulted in the absence of ciliary AC5/6 and PDE4C and elevated intracellular cAMP levels. Expression of wild-type PC2, but not of a PC2 mutant lacking Ca2+ channel activity, decreased cAMP levels, suggesting that the PC2 Ca2+ channel activity specifically regulates cAMP levels. Using proximity labeling, Mick et al. (2015) also detected the ciliary presence of PKA and AC5/6 in IMCD3 cells (Mick et al. 2015). Collectively, these data suggest that cilia are distinct Ca2+/cAMP signaling organelles. The influence of ciliary Ca2+ in modulating ADPKD has been demonstrated genetically in mice (Jin et al. 2014b). The voltage-dependent L-type calcium channel, CaV1.2, localizes to primary cilia, but is absent from cilia in Pkd1−/− and Pkd2−/− derived renal epithelial cells. Deletion of CaV1.2 in Pkd1 heterozygous mice caused the growth of few but extremely large renal cysts, while cysts did not occur in single Pkd1 heterozygotes.
Although mechanisms linking ciliary Ca2+/cAMP to cytosolic pools require further investigation, targeting cAMP formation in preclinical models of ADPKD has proven extremely effective (Gattone et al. 2003; Torres et al. 2004; Wang et al. 2005, 2008). Intracellular cAMP formation can be attenuated by inhibiting arginine vasopressin receptor 2 (AVPR2), which stimulates AC6, or activating the somatostatin receptor, which inhibits AC6. Administration of Tolvaptan, an inhibitor of AVPR2, together with pasireotide, an activator of somatostatin receptors in the Pkd1RC/RC mouse, resulted in lower renal cystic and fibrotic volumes than treatment with either Tolvaptan or pasireotide alone, demonstrating that targeting both receptors simultaneously is more effective than targeting one alone (Hopp et al. 2015). Knockout of Pde1a, Pde1c, or Pde3a, which catabolize cAMP, in a Pkd2−/WS25 mouse model exacerbated renal cystogenesis, suggesting that activating these enzymes may also be beneficial (Ye et al. 2015).
The Tolvaptan Efficacy and Safety in Management of Autosomal Dominant Polycystic Kidney Disease and its Outcomes (TEMPO) 3:4 study was a 3-year phase III clinical trial, in which early-stage ADPKD patients anticipated to have rapid renal cyst growth were randomly assigned to either a Tolvaptan or a placebo treatment group (Torres et al. 2011). ADPKD patients administered Tolvaptan showed reduced kidney volume and reduced symptoms of kidney malfunction, including flank pain, hypertension, and albuminuria, compared to patients administered a placebo (Torres et al. 2012). Tolvaptan has been approved for ADPKD patients in Japan, Canada, and Europe. In the USA, the Food and Drug Administration considered the side effects, such as polyuria, excessive thirst, and risk of liver injury, to outweigh the benefits. However, an open-label extension study (TEMPO 4:4) is ongoing (https://clinicaltrials.gov/ct2/show/record/NCT01214421).
11.5.2. LKB1/AMPK
The mammalian target of rapamycin (mTOR) pathway is aberrantly activated in cyst-lining epithelial cells of human ADPKD tissue (Shillingford et al. 2006), and administration of mTOR inhibitor, rapamycin, has attenuated renal cystogenesis in most PKD rodent models tested (Shillingford et al. 2006; Tao et al. 2005; Wahl et al. 2006; reviewed in Ibraghimov-Beskrovnaya and Natoli 2011). mTOR signaling is essential for cell metabolism and growth and is mediated by two multi-protein complexes, mTOR complex 1 (mTORC1) and mTORC2. mTORC1 phosphorylates and activates p706S kinase (S6K) and inhibits 4E-BP1 to stimulate protein synthesis, enabling cell growth. While nutrient and amino acid availability stimulates mTORC1, energy stress and the “energy sensor” molecule, AMP-activated protein kinase (AMPK) inhibits mTORC1 in a ciliary-dependent manner (Boehlke et al. 2010).
Upon fluid flow, the ciliary-localized liver kinase B1 (LKB1) tumor suppressor kinase phosphorylates AMPK, which accumulates at the ciliary base and suppresses mTORC1 and, in turn, cell growth (Boehlke et al. 2010). Kif3a cko mice were shown to have larger collecting duct cells, demonstrating the ciliary role in regulating mTOR signaling and cell growth. Further, MDCK cells deficient for Kif3a or Ift88 showed larger cell size only when subjected to fluid flow, demonstrating the importance of fluid shear stress as a stimulus. In LKB1-deficient cells, fluid flow also resulted in larger cells, and while P-AMPK levels of whole cell lysates were not altered, P-AMPK specifically at the basal body was reduced. Pkd2 kd MDCK cells subjected to fluid flow did not show larger cell size nor elevated levels of P-S6K, suggesting that fluid flow stimulation of LKB1/AMPK signaling is independent of flow-mediated intracellular Ca2+ generation.
In addition to flow-mediated suppression of mTOR signaling, other regulators of mTOR signaling have been identified. Lack of ubiquitination of the hepatocyte growth factor (HGF) receptor, c-Met, also activates mTOR signaling in a PKD mouse model, and administration of a c-Met inhibitor attenuated renal cystogenesis in Pkd1-null mutant embryos (Qin et al. 2010). Additionally, treatment of MDCK cells with metformin, which activates AMPK, suppressed mTOR signaling and CFTR activity, and administration of metformin to Pkd1 mouse mutants either prior to or during renal cyst formation attenuated renal cystic disease (Takiar et al. 2011).
Despite the success of mTor inhibitors in attenuating cystic disease in most preclinical models of PKD, clinical trials of sirolimus (rapamycin) and its derivative, everolimus, in ADPKD patients have not shown the same efficacy (Ruggenenti et al. 2016; Serra et al. 2010; Walz et al. 2010). In an 18-month study, no difference in total kidney volume was observed in patients treated with sirolimus compared to patients receiving standard care (Serra et al. 2010). In a 24-month study, ADPKD patients receiving everolimus showed a lower total kidney volume increase, but also a lower parenchymal volume increase, and kidney function was not better than those of patients receiving a placebo (Walz et al. 2010). In another study, ADPKD patients at stages 3b and 4 of chronic kidney disease received sirolimus for 1 year but showed greater decline in glomerular filtration rate (GFR), increased proteinuria, and higher total kidney volume than patients receiving conventional care (Ruggenenti et al. 2016). The study concluded that sirolimus was unsafe and ineffective in ADPKD patients. Another study is ongoing to determine if pulsed administration of sirolimus will be more effective (https://clinicaltrials.gov/ct2/show/NCT02055079). Rapamycin acts mostly on mTORC1, and feedback mechanisms may result in elevated mTOR signaling via mTORC2. A new mTOR inhibitor that inhibits both complexes has shown efficacy in a rat model of PKD (Ravichandran et al. 2015). Alternatively, targeting the pathway upstream of mTORC1/2 at the level of AMPK or cMet might also be more effective. Additionally, administration of lower doses of mTOR inhibitors as part of a combination therapy may also yield more beneficial results. Finally, targeting drug delivery to the kidney may be most effective at reducing toxicity and increasing therapeutic efficacy (Shillingford et al. 2012). Folate receptors are expressed at high levels in the kidney, and thus, folate receptor-mediated endocytosis may be exploited to target compounds to the kidney. Indeed, administration of folate-conjugated rapamycin (FC-rapamycin) to adult mice reduced mTOR signaling in the kidney and not in the spleen. FC-rapamycin also attenuated PKD in bpk (BALB/c polycystic kidneys) mice, mutant for Bicaudal C Family RNA-binding protein 1 (Bicc1) (Cogswell et al. 2003), which regulates Pkd2 via microRNA miR-17 (Tran et al. 2010).
11.5.3. Wnt
Wnt signaling is activated upon binding of Wnt ligand to a Frizzled (Fz) receptor. Downstream of this, Wnt signaling partitions into canonical and noncanonical pathways, determined by the presence of co-receptors. While canonical Wnt signaling regulates cell proliferation, differentiation, and cell fate, noncanonical signaling directs tissue organization and morphogenesis and causes transient increases in intracellular Ca2+. For the purpose of this review, we will focus predominantly on the canonical pathway. For a review on noncanonical signaling and ADPKD, see Tran et al. (2014a).
In the canonical pathway, in the absence of ligand, a destruction complex comprised of scaffolding protein, Axin2, adenomatous polyposis coli (APC), casein kinase 1 (CK1), and glycogen synthase kinase 3β (GSK3β) phosphorylates and targets β-catenin for degradation. In the presence of ligand, binding of Wnt to Fz recruits the low-density lipoprotein (LDL)-related protein 6 (LRP6) co-receptor, cytoplasmic Dishevelled (Dvl), Axin2, and GSK3β concomitantly disassembling the destruction complex. Stabilized β-catenin translocates to the nucleus and dimerizes with the T-cell factor/lymphoid enhancer factor (TCF/LEF) transcription factors to activate target genes, such as Lef1, Axin2, and c-myc, promoting cell proliferation (reviewed in Oh and Katsanis 2013).
Two studies show that fluid flow can regulate Wnt signaling. Fluid flow across IMCD cells increased the expression of inversin, which is mutated in NPHP2, and downregulated β-catenin (Simons et al. 2005). While experiments in Xenopus laevis embryos and mammalian renal epithelial cells suggested that inversin antagonizes canonical Wnt signaling while promoting the noncanonical Wnt/Planar Cell Polarity pathway (Simons et al. 2005), kidneys of inversin-null mouse mutants showed normal regulation of canonical Wnt signaling (Sugiyama et al. 2011), suggesting regulation of the balance between mammalian canonical and noncanonical Wnt branches may be more intricate in vivo. Fluid flow in the mouse embryonic node has also been shown to regulate Wnt signaling via feedback loops with Cerl2 (Nakamura et al. 2012). An early event in establishing left–right asymmetry is the decay of Cerl2 mRNA on the left side of crown cells in the mouse node induced by fluid flow. Wnt3 promotes Wnt3 expression and further Cerl2 mRNA degradation, and conversely, Cerl2 promotes Wnt3 degradation. These data suggest that flow-mediated decay of Cerl2 enables asymmetric Wnt signaling around the node. This contrasts with the effect of fluid flow over IMCD cells, which leads to downregulated β-catenin.
Pathway components, such as β-catenin, APC, and Dishevelled 3, localize to the ciliary axoneme, while P-β-catenin, which is targeted for degradation, is present at the basal body (Corbit et al. 2008; Mick et al. 2015). In Kif3a−/− embryos and in Wnt3a ligand-stimulated cells derived from Kif3a−/−, IFT88orpk/orpk, and Ofd1−/− mice harboring the Batgal Wnt reporter, reporter activity increased, suggesting that cilia act as a “brake” in canonical Wnt signal transduction (Corbit et al. 2008). In vitro studies suggest that Jouberin (Jbn), encoded by AHI1, which is mutated in Joubert Syndrome, facilitates nuclear translocation of β-catenin and that the primary cilium acts to sequester Jouberin and β-catenin away from the nucleus (Lancaster et al. 2011).
Yet, the functionality of primary cilia in mediating Wnt signaling has been unclear. IFT mutant embryos show Hedgehog (Hh) mutant phenotypes and not phenotypes characteristic of misregulated Wnt signaling (reviewed in Eggenschwiler and Anderson 2007). Additionally, although Kif3a−/−; Batgal and Ift88−/−;Batgal embryos showed increased Wnt reporter activity (Corbit et al. 2008), Thm1aln/aln; Batgal and Ahi−/−;Batgal embryos, which have shortened and absent cilia, respectively, showed normal reporter activity (Lancaster et al. 2011; Stottmann et al. 2009). Interestingly, Dnchc2−/−;Batgal embryos showed decreased reporter activity in the kidney but increased activity in the midbrain (Lancaster et al. 2011). This varying effect of Dnchc2 deficiency was demonstrated in vitro. Moderate inhibition of Dnchc2, which did not affect cilia structure, dampened Wnt response, while greater inhibition of Dnchc2, which shortened cilia, increased Wnt response. Thus, ciliary regulation of Wnt signaling is context dependent; some organs, such as the brain, are more sensitive to dosage of a particular ciliary gene. These data also suggest that deficiency of different ciliary genes differentially influences Wnt signaling output.
The correct balance of Wnt signaling is critical for maintaining renal tubular integrity, and both inappropriate activation and inhibition of the pathway can lead to renal cysts. Renal-specific overexpression of active β-catenin or loss of APC, which negatively regulates β-catenin, caused renal cysts in mice (Qian et al. 2005; Saadi-Kheddouci et al. 2001). Additionally, enhancing canonical Wnt signaling by ablating Aquaporin 1 (Aqp1) in a mouse model of PKD exacerbated renal cystogenesis (Wang et al. 2015). AQP1 interacts with β-catenin, GSK3β, LRP6, and Axin1, and deficiency of AQP1 increased the levels of stabilized β-catenin. Finally, in an Mks1 knockout mouse model of Meckel Syndrome with shortened cilia, canonical Wnt signaling was upregulated prior to cysts forming in embryonic mutant kidneys (Wheway et al. 2013). Conversely, in Ahi−/− mice, which model NPHP, canonical Wnt signaling was reduced at 5 months of age, and renal microcysts and tubular dilations ensued at 12 months of age (Lancaster et al. 2009).
However, misregulated Wnt signaling does not precede cystogenesis in all mouse models of cystic kidney disease. In mice with renal-specific deletion of Kif3a, Ift20, Ift140, or Tmem67, upregulation of canonical Wnt signaling was detected after renal cysts were formed, suggesting that other mechanisms underlie initiation of renal cystogenesis (Jonassen et al. 2008, 2012; Leightner et al. 2013; Lin et al. 2003). A direct role for loss of polycystins on Wnt signaling in vivo is also controversial. Pkd1−/−; TCF-lacZ embryonic kidneys and Pkd2/WS25; TCF-lacZ adult kidneys showed normal reporter activity in cyst-lining epithelia and normal levels of β-catenin in whole kidney lysates, even after cysts had formed (Miller et al. 2011). In direct contrast, canonical Wnt signaling was upregulated in cystlining epithelial cells of E17.5 Pkd1−/−; TCF-lacZ kidneys, and levels of total and active β-catenin of whole kidney lysates were also elevated (Qin et al. 2012). Varying expression levels and patterns by different Wnt reporter lines and the absence of Wnt reporter activity in sites of known canonical Wnt signaling have been reported. Different background strains may also possibly account for the discrepancies observed in reporter activity as well as β-catenin levels.
Recently, PC1 has been shown to bind various Wnt ligands. Binding of WNT9B resulted in increased intracellular Ca2+ via Ca2+ influx, and WNT9B and WNT3A induced whole-cell currents in WT MEFs but not in Pkd2−/− MEFs (Kim et al. 2016b). Combined morpholino knockdowns of dvl2 and pkd1 or of wnt9a and pkd1 in Xenopus showed synergistic effects on inducing a pronephric cystic phenotype, indicating these molecules function in the same pathway. These data are the first to identify ligands of PC1 and indicate that misregulated Wnt/Ca2+ signaling is causative in ADPKD.
11.5.4. Hedgehog
The first signaling pathway discovered to occur at the primary cilium was the mammalian Hh pathway. The first major discovery was made when ethylnitrosourea (ENU)-mutagenized mouse embryos with Hh signaling phenotypes, such as neural tube patterning defects and polydactyly, were found to have mutations in Ift genes (Huangfu et al. 2003). Hh signaling is essential for patterning and development of most vertebrate organs and is also critical for tissue homeostasis (reviewed in Ingham et al. 2011). Hh ligands initiate signaling by binding the transmembrane receptor, Patched (PTCH1), at the cilium. Once bound, PTCH1 diminishes from the cilium, and concomitantly, the transmembrane signal transducer, Smoothened (SMO), is enriched in the cilium and activated (Corbit et al. 2005; Rohatgi et al. 2007). A series of dephosphorylation and phosphorylation events activate full-length Glioblastoma transcription factors, GLI2 and GLI3 (Niewiadomski et al. 2014), which accumulate at the ciliary distal tip (Haycraft et al. 2005). In the absence of ligand, full-length GLI3 protein is processed into GLI3 repressor, which also requires the primary cilium. GLI transcription factors translocate to the nucleus, and the balance of GLI activators (GLIA) to GLI3 repressor determines the level of Hh signaling output (Christensen and Ott 2007; Eggenschwiler and Anderson 2007).
IFT, the BBSome, ciliary Ca2+, and the phospholipid content of the ciliary membrane tune this developmental pathway by ultimately regulating ciliary localization of Hh signaling components. In mice, loss of all IFT proteins misregulates Hh signaling (Nozawa et al. 2013). Loss of most complex B proteins, which mediate anterograde IFT, causes the absence of cilia and inability to transduce the Hh signal (Huangfu and Anderson 2005; Huangfu et al. 2003; Liu et al. 2005a). In contrast, loss of IFT complex A proteins, THM1 and IFT122, which largely mediate retrograde IFT, sequesters proteins in bulb-like structures at the distal tip of shortened cilia and causes inappropriate activation of the Hh pathway due to enhanced GLI2A and GLI3A activities (Qin et al. 2011; Tran et al. 2008). In addition to cilia compartmentalizing the Hh signaling cascade, IFT is integral to Hh transduction by trafficking signaling components within cilia. Unlike most complex B proteins, IFT25 and IFT27 are not essential for ciliogenesis but form a subcomplex that is critical for ciliary import of GLI2 and ciliary export of the BBSome and its cargoes, PTCH1, SMO, and Gpr161, a negative regulator of the pathway (Eguether et al. 2014; Keady et al. 2012; Liew et al. 2014). As a result, loss of IFT25 or IFT27 does not alter cilia structure but disrupts activation of the pathway. Ca2+ modulates IFT (Collingridge et al. 2013), and treatment of MEF with Smoothened Agonist (SAG) resulted in elevated ciliary Ca2+ after 24 h (Delling et al. 2013). Delling et al. (2013) propose that rather than SAG directly activating Ca2+ channels, SAG recruits PC2-L1 to primary cilia to “tune” Ca2+ levels for proper function of IFT proteins, such as IFT25, which has a Ca2+ binding site. Delling et al. (2013) further propose that other Hh signaling molecules be examined for Ca2+ dependence. More recently, the phospholipid content of the ciliary membrane has also been shown to be a critical regulator of the pathway. Phophoinositide phosphatase, INPP5E, in which mutations cause ciliopathies (Jacoby et al. 2009), creates a distinct phosphoinositide distribution in the ciliary membrane, whereby phosphoinositide 4-phosphate (PI(4)P) is present in the membrane that ensheathes the axoneme and phosphoinositide 4,5-bisphosphate (PI(4,5) P2) comprises the membrane at the ciliary base (Chavez et al. 2015; Garcia-Gonzalo et al. 2015). Loss of INPP5E caused mislocalization of PI(4,5)P2 to the ciliary membrane enclosing the axoneme, which increased ciliary localization of Hh negative modulators, PI(4,5)P2-binding Tulp3, Gpr161, and IFT140, resulting in inactivation of the pathway.
Like the Wnt pathway, regulation of Hh signaling has been studied mostly in cells that do not undergo shear stress. However, Hh signaling also has a role in chondrocytes, which do undergo mechanical strain. In embryonic chondrocytes, Hh signaling is essential for proliferation and differentiation in the growth plate, while in adult chondrocytes, aberrant activation of Hh signaling leads to degeneration of chondrocytes in osteoarthritis. Chondrocytes subjected to cyclic tensile strain (CTS) showed increased Ihh transcription and increased Hh activity as assessed by levels of Gli1 transcripts (Thompson et al. 2014). In Ift88orpk/orpk cells subjected to CTS, Ihh transcription increased but Gli1 transcription did not, demonstrating that the primary cilium is not required for Ihh transcription but for transduction of the pathway. Thus, in chondrocytes, Hh signaling is regulated in a mechanosensitive manner (Shao et al. 2012; Thompson et al. 2014).
Evidence that Hh signaling is increased in renal cystogenesis is emerging. In a genome-wide transcriptome analysis of human ADPKD renal cystic tissue, expression of Hh signaling components, including Gli2, was upregulated (Song et al. 2009). Additionally, several mouse models of renal cystic disease show increased Hh signaling. Mutation of the Glis2 transcription factor, a member of the Kruppel-like C2H2 zinc finger protein subfamily, including the GLI proteins, causes NPHP in humans and mice, and transcriptome analysis of Glis2−/− kidneys revealed an upregulation of Gli1, a direct target of the Hh pathway (Attanasio et al. 2007). In vitro, Glis2 repressed the Hh pathway and Glis2 knockdown resulted in transformation of renal epithelial cells to a fibroblast appearance, suggesting that reduced levels of Hh signaling may maintain renal tubular epithelial cells in a differentiated state (Li et al. 2011). Conditional deletion of IFT140, a complex A protein, resulted in cystic kidney disease and increased expression of Gli transcripts in cystic kidneys, suggesting increased Hh signaling (Jonassen et al. 2012). Similarly, perinatal deletion of Thm1, which also encodes a complex A protein (Tran et al. 2008) and is mutated in patients with ciliopathies (Davis et al. 2011), also caused cystic kidney disease in mice and increased expression of Gli transcripts in cystic kidneys (Tran et al. 2014b). Further, Gli transcripts were also increased in jck and Pkd1 cko cystic kidneys.
In a study examining the effects of corticosteroid overexposure on kidney development, addition of Hh inhibitor, cyclopamine, reduced hydrocortisone-induced cysts without affecting organ growth, implicating increased Hh signaling in this mechanism of cystogenesis (Chan et al. 2010). Hh inhibitors also prevented cyst formation in cultured embryonic kidneys of Thm1, jck, and Pkd1 mutant mice (Tran et al. 2014b). Downregulating Hh signaling in orthologous PKD mouse models in vivo will help determine a functional role for increased Hh signaling in PKD-mediated renal cystogenesis.
11.6. Perspectives
While aberrant regulation of multiple signaling pathways has been implicated in ADPKD, this complexity is not unique to ADPKD but occurs also in cancer and correlates with more advanced disease. This emphasizes the need to understand the molecular mechanisms of early disease and, ultimately, those that initiate disease processes. In ADPKD-derived, PKD1-mutant iPS cells, only the absence of ciliary PC1 and PC2, and not abnormalities in cell proliferation, apoptosis, or ciliogenesis, was observed, suggesting that ciliary mislocalization of proteins may be early events (Freedman et al. 2013). Thus, determining the proteins resident in cilia in healthy and disease states may help reveal initiating mechanisms. Advances in proteomics and imaging technologies may facilitate such investigations. Recently, proximity labeling of primary cilia using APEX technology was demonstrated to successfully uncover novel proteins in wild-type and Ift27−/− primary cilia (Mick et al. 2015). APEX technology provides remarkable sensitivity to allow temporal “snapshots” of both transient and stable residents of the dynamic ciliary proteome. Performing similar experiments in PC1/PC2-deficient cells, which also have primary cilia, seems feasible. Proximity labeling may also be applied to examining proteins at the ciliary base, the ER, and Golgi apparatus in response to fluid flow and in PKD1/2 mutant cells to further analyze the cellular events important in PKD biology.
Super-resolution imaging has refined the localization of proteins at the ciliary base. Combining stimulated emission depletion (STED) super-resolution imaging with transmission electron microscopy has provided a nanometer-scale view of the spatial distribution of seven proteins at the transition zone and distal appendices in mammalian cells (Yang et al. 2015). This level of resolution can be applied to proteins uncovered using proteomics technology in the axoneme, ciliary base, ER, and Golgi apparatus. Live imaging of renal proximal tubules of CiliaGFP mice that express ciliary-localized somatostatin receptor 3 (Sstr3)::GFP shows that fluid flow causes cilia to bend and lie almost parallel to the apical cell surface (O’Connor et al. 2013). These experiments can be extended to analyzing cilia behavior at various stages of renal cystogenesis and correlating with fluid flow-induced intracellular Ca2+ generation. The achievement of imaging ciliary Ca2+ in vivo can also be applied to possibly other organelles and possibly other pathways.
Thus far, targeting cAMP levels by administration of Tolvaptan has proven most effective in treating patients with ADPKD. Still this therapy can be ameliorated to improve efficacy and reduce side effects. Targeting other pathways in combination with Tolvaptan and directing drug delivery to the kidney may potentiate therapeutic benefits. Alternatively, targeting the PC protein defect and ciliary mislocalization has been proposed as a more proximal therapy (Cai et al. 2014; Trudel et al. 2016). Approximately, 30% of mutations are missense mutations for which the protein defect may be potentially targeted. Since ciliary localization of Hh signaling components is regulated by phospholipid content of ciliary membrane, investigations into the role of the ciliary membrane lipid content on PC ciliary localization may also be warranted. Glycosphingolipid metabolism modulates ADPKD in mouse models (Natoli et al. 2010) and is involved in the formation of apical membrane and primary cilia of differentiated MDCK cells (Pescio et al. 2012). Thus, the future of ADPKD research promises exciting discoveries toward finding effective therapies and ultimately a cure.
Contributor Information
Prachee Avasthi, Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS, USA.
Robin L. Maser, Department of Clinical Laboratory Sciences, University of Kansas Medical Center, Kansas City, KS, USA; Kidney Institute, University of Kansas Medical Center, 3901 Rainbow Blvd., MS #3038, Kansas City 66160, KS, USA
Pamela V. Tran, Department of Anatomy and Cell Biology, University of Kansas Medical Center, Kansas City, KS, USA; Kidney Institute, University of Kansas Medical Center, 3901 Rainbow Blvd., MS #3038, Kansas City 66160, KS, USA
References
- Abdul-Majeed S, Nauli SM (2011) Dopamine receptor type 5 in the primary cilia has dual chemo- and mechano-sensory roles. Hypertension 58:325–331 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdul-Majeed S, Moloney BC, Nauli SM (2012) Mechanisms regulating cilia growth and cilia function in endothelial cells. Cell Mol Life Sci 69:165–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afzelius BA (1976) A human syndrome caused by immotile cilia. Science 193:317–319 [DOI] [PubMed] [Google Scholar]
- Ait-Lounis A, Baas D, Barras E, Benadiba C, Charollais A, Nlend Nlend R, Liegeois D, Meda P, Durand B, Reith W (2007) Novel function of the ciliogenic transcription factor RFX3 in development of the endocrine pancreas. Diabetes 56:950–959 [DOI] [PubMed] [Google Scholar]
- Aldahmesh MA, Li Y, Alhashem A, Anazi S, Alkuraya H, Hashem M, Awaji AA, Sogaty S, Alkharashi A, Alzahrani S et al. (2014) IFT27, encoding a small GTPase component of IFT particles, is mutated in a consanguineous family with Bardet–Biedl syndrome. Hum Mol Genet 23:3307–3315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alten L, Schuster-Gossler K, Beckers A, Groos S, Ulmer B, Hegermann J, Ochs M, Gossler A (2012) Differential regulation of node formation, nodal ciliogenesis and cilia positioning by Noto and Foxj1. Development 139:1276–1284 [DOI] [PubMed] [Google Scholar]
- Ansley SJ, Badano JL, Blacque OE, Hill J, Hoskins BE, Leitch CC, Kim JC, Ross AJ, Eichers ER, Teslovich TM et al. (2003) Basal body dysfunction is a likely cause of pleiotropic Bardet–Biedl syndrome. Nature 425:628–633 [DOI] [PubMed] [Google Scholar]
- Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA et al. (2007) Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet 39:1018–1024 [DOI] [PubMed] [Google Scholar]
- Avasthi P, Marshall WF (2012) Stages of ciliogenesis and regulation of ciliary length. Differ Res Biol Divers 83:S30–S42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasthi P, Marshall W (2013) Ciliary secretion: switching the cellular antenna to ‘transmit’. Curr Biol 23:R471–R473 [DOI] [PubMed] [Google Scholar]
- Avasthi P, Marley A, Lin H, Gregori-Puigjane E, Shoichet BK, von Zastrow M, Marshall WF (2012) A chemical screen identifies class a g-protein coupled receptors as regulators of cilia. ACS Chem Biol 7:911–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avasthi P, Onishi M, Karpiak J, Yamamoto R, Mackinder L, Jonikas MC, Sale WS, Shoichet B, Pringle JR, Marshall WF (2014) Actin is required for IFT regulation in Chlamydomonas reinhardtii. Curr Biol 24:2025–2032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr MM, Sternberg PW (1999) A polycystic kidney-disease gene homologue required for male mating behaviour in C. elegans. Nature 401:386–389 [DOI] [PubMed] [Google Scholar]
- Beales PL, Bland E, Tobin JL, Bacchelli C, Tuysuz B, Hill J, Rix S, Pearson CG, Kai M, Hartley J et al. (2007) IFT80, which encodes a conserved intraflagellar transport protein, is mutated in Jeune asphyxiating thoracic dystrophy. Nat Genet 39:727–729 [DOI] [PubMed] [Google Scholar]
- Berbari NF, Lewis JS, Bishop GA, Askwith CC, Mykytyn K (2008) Bardet–Biedl syndrome proteins are required for the localization of G protein-coupled receptors to primary cilia. Proc Natl Acad Sci USA 105:4242–4246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman SA, Wilson NF, Haas NA, Lefebvre PA (2003) A novel MAP kinase regulates flagellar length in Chlamydomonas. Curr Biol 13:1145–1149 [DOI] [PubMed] [Google Scholar]
- Bershteyn M, Atwood SX, Woo WM, Li M, Oro AE (2010) MIM and cortactin antagonism regulates ciliogenesis and hedgehog signaling. Dev Cell 19:270–283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besschetnova TY, Kolpakova-Hart E, Guan Y, Zhou J, Olsen BR, Shah JV (2010) Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr Biol 20:182–187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blacque OE, Li C, Inglis PN, Esmail MA, Ou G, Mah AK, Baillie DL, Scholey JM, Leroux MR (2006) The WD repeat-containing protein IFTA-1 is required for retrograde intraflagellar transport. Mol Biol Cell 17:5053–5062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloodgood RA (2012) The future of ciliary and flagellar membrane research. Mol Biol Cell 23:2407–2411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boehlke C, Kotsis F, Patel V, Braeg S, Voelker H, Bredt S, Beyer T, Janusch H, Hamann C, Godel M et al. (2010) Primary cilia regulate mTORC1 activity and cell size through Lkb1. Nat Cell Biol 12:1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonneau D, Raymond F, Kremer C, Klossek JM, Kaplan J, Patte F (1993) Usher syndrome type I associated with bronchiectasis and immotile nasal cilia in two brothers. J Med Genet 30:253–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brazelton WJ, Amundsen CD, Silflow CD, Lefebvre PA (2001) The bld1 mutation identifies the Chlamydomonas osm-6 homolog as a gene required for flagellar assembly. Curr Biol 11:1591–1594 [DOI] [PubMed] [Google Scholar]
- Broekhuis JR, Leong WY, Jansen G (2013) Regulation of cilium length and intraflagellar transport. Int Rev Cell Mol Biol 303:101–138 [DOI] [PubMed] [Google Scholar]
- Bujakowska KM, Zhang Q, Siemiatkowska AM, Liu Q, Place E, Falk MJ, Consugar M, Lancelot ME, Antonio A, Lonjou C et al. (2015) Mutations in IFT172 cause isolated retinal degeneration and Bardet–Biedl syndrome. Hum Mol Genet 24:230–242 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghoorn J, Dekkers MP, Rademakers S, de Jong T, Willemsen R, Jansen G (2007) Mutation of the MAP kinase DYF-5 affects docking and undocking of kinesin-2 motors and reduces their speed in the cilia of Caenorhabditis elegans. Proc Natl Acad Sci USA 104:7157–7162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Y, Fedeles SV, Dong K, Anyatonwu G, Onoe T, Mitobe M, Gao JD, Okuhara D, Tian X, Gallagher AR et al. (2014) Altered trafficking and stability of polycystins underlie polycystic kidney disease. J Clin Invest 124:5129–5144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calvet JP (2008) Strategies to inhibit cyst formation in ADPKD. Clin J Am Soc Nephrol CJASN 3:1205–1211 [DOI] [PubMed] [Google Scholar]
- Calvet JP, Grantham JJ (2001) The genetics and physiology of polycystic kidney disease. Semin Nephrol 21:107–123 [DOI] [PubMed] [Google Scholar]
- Camner P, Mossberg B, Afzelius BA (1975) Evidence of congenitally nonfunctioning cilia in the tracheobronchial tract in two subjects. Am Rev Respir Dis 112:807–809 [DOI] [PubMed] [Google Scholar]
- Cano DA, Murcia NS, Pazour GJ, Hebrok M (2004) Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131:3457–3467 [DOI] [PubMed] [Google Scholar]
- Chan SK, Riley PR, Price KL, McElduff F, Winyard PJ, Welham SJ, Woolf AS, Long DA (2010) Corticosteroid-induced kidney dysmorphogenesis is associated with deregulated expression of known cystogenic molecules, as well as Indian hedgehog. Am J Physiol Renal Physiol 298: F346–F356 [DOI] [PubMed] [Google Scholar]
- Chapin HC, Rajendran V, Caplan MJ (2010) Polycystin-1 surface localization is stimulated by polycystin-2 and cleavage at the G protein-coupled receptor proteolytic site. Mol Biol Cell 21:4338–4348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauvet V, Tian X, Husson H, Grimm DH, Wang T, Hiesberger T, Igarashi P, Bennett AM, Ibraghimov-Beskrovnaya O, Somlo S et al. (2004) Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J Clin Invest 114:1433–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chavez M, Ena S, Van Sande J, de Kerchove d’Exaerde A, Schurmans S, Schiffmann SN (2015) Modulation of ciliary phosphoinositide content regulates trafficking and sonic hedgehog signaling output. Dev Cell 34:338–350 [DOI] [PubMed] [Google Scholar]
- Chih B, Liu P, Chinn Y, Chalouni C, Komuves LG, Hass PE, Sandoval W, Peterson AS (2012) A ciliopathy complex at the transition zone protects the cilia as a privileged membrane domain. Nat Cell Biol 14:61–72 [DOI] [PubMed] [Google Scholar]
- Choi YH, Suzuki A, Hajarnis S, Ma Z, Chapin HC, Caplan MJ, Pontoglio M, Somlo S, Igarashi P (2011) Polycystin-2 and phosphodiesterase 4C are components of a ciliary A-kinase anchoring protein complex that is disrupted in cystic kidney diseases. Proc Natl Acad Sci USA 108:10679–10684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen ST, Ott CM (2007) Cell signaling. A ciliary signaling switch. Science 317:330–331 [DOI] [PubMed] [Google Scholar]
- Coene KL, Mans DA, Boldt K, Gloeckner CJ, van Reeuwijk J, Bolat E, Roosing S, Letteboer SJ, Peters TA, Cremers FP et al. (2011) The ciliopathy-associated protein homologs RPGRIP1 and RPGRIP1L are linked to cilium integrity through interaction with Nek4 serine/threonine kinase. Hum Mol Genet 20:3592–3605 [DOI] [PubMed] [Google Scholar]
- Cogswell C, Price SJ, Hou X, Guay-Woodford LM, Flaherty L, Bryda EC (2003) Positional cloning of jcpk/bpk locus of the mouse. Mamm Genome 14:242–249 [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL (1998) Chlamydomonas kinesin-II-dependent intraflagellar transport (IFT): IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141:993–1008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collingridge P, Brownlee C, Wheeler GL (2013) Compartmentalized calcium signaling in cilia regulates intraflagellar transport. Curr Biol 23:2311–2318 [DOI] [PubMed] [Google Scholar]
- Corbit KC, Aanstad P, Singla V, Norman AR, Stainier DY, Reiter JF (2005) Vertebrate Smoothened functions at the primary cilium. Nature 437:1018–1021 [DOI] [PubMed] [Google Scholar]
- Corbit KC, Shyer AE, Dowdle WE, Gaulden J, Singla V, Chen MH, Chuang PT, Reiter JF (2008) Kif3a constrains beta-catenin-dependent Wnt signalling through dual ciliary and non-ciliary mechanisms. Nat Cell Biol 10:70–76 [DOI] [PubMed] [Google Scholar]
- Craft JM, Harris JA, Hyman S, Kner P, Lechtreck KF (2015) Tubulin transport by IFT is upregulated during ciliary growth by a cilium-autonomous mechanism. J Cell Biol 208:223–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis EE, Zhang Q, Liu Q, Diplas BH, Davey LM, Hartley J, Stoetzel C, Szymanska K, Ramaswami G, Logan CV et al. (2011) TTC21B contributes both causal and modifying alleles across the ciliopathy spectrum. Nat Genet 43:189–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawe HR, Smith UM, Cullinane AR, Gerrelli D, Cox P, Badano JL, Blair-Reid S, Sriram N, Katsanis N, Attie-Bitach T et al. (2007) The Meckel–Gruber syndrome proteins MKS1 and meckelin interact and are required for primary cilium formation. Hum Mol Genet 16:173–186 [DOI] [PubMed] [Google Scholar]
- Deane JA, Cole DG, Seeley ES, Diener DR, Rosenbaum JL (2001) Localization of intraflagellar transport protein IFT52 identifies basal body transitional fibers as the docking site for IFT particles. Curr Biol 11:1586–1590 [DOI] [PubMed] [Google Scholar]
- DeCaen PG, Delling M, Vien TN, Clapham DE (2013) Direct recording and molecular identification of the calcium channel of primary cilia. Nature 504:315–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M, DeCaen PG, Doerner JF, Febvay S, Clapham DE (2013) Primary cilia are specialized calcium signalling organelles. Nature 504:311–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delling M, Indzhykulian AA, Liu X, Li Y, Xie T, Corey DP, Clapham DE (2016) Primary cilia are not calcium-responsive mechanosensors. Nature 531:656–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- den Hollander AI, Koenekoop RK, Mohamed MD, Arts HH, Boldt K, Towns KV, Sedmak T, Beer M, Nagel-Wolfrum K, McKibbin M et al. (2007) Mutations in LCA5, encoding the ciliary protein lebercilin, cause Leber congenital amaurosis. Nat Genet 39:889–895 [DOI] [PubMed] [Google Scholar]
- Dentler W (2013) A role for the membrane in regulating Chlamydomonas flagellar length. PLoS One 8:e53366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggenschwiler JT, Anderson KV (2007) Cilia and developmental signaling. Annu Rev Cell Dev Biol 23:345–373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eguether T, San Agustin JT, Keady BT, Jonassen JA, Liang Y, Francis R, Tobita K, Johnson CA, Abdelhamed ZA, Lo CW et al. (2014) IFT27 links the BBSome to IFT for maintenance of the ciliary signaling compartment. Dev Cell 31:279–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engel BD, Ludington WB, Marshall WF (2009) Intraflagellar transport particle size scales inversely with flagellar length: revisiting the balance-point length control model. J Cell Biol 187:81–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esteban MA, Harten SK, Tran MG, Maxwell PH (2006) Formation of primary cilia in the renal epithelium is regulated by the von Hippel–Lindau tumor suppressor protein. J Am Soc Nephrol 17:1801–1806 [DOI] [PubMed] [Google Scholar]
- Fogelgren B, Lin SY, Zuo X, Jaffe KM, Park KM, Reichert RJ, Bell PD, Burdine RD, Lipschutz JH (2011) The exocyst protein Sec10 interacts with Polycystin-2 and knockdown causes PKD-phenotypes. PLoS Genet 7:e1001361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Follit JA, Tuft RA, Fogarty KE, Pazour GJ (2006) The intraflagellar transport protein IFT20 is associated with the Golgi complex and is required for cilia assembly. Mol Biol Cell 17:3781–3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco I, Gulluni F, Campa CC, Costa C, Margaria JP, Ciraolo E, Martini M, Monteyne D, De Luca E, Germena G et al. (2014) PI3K class II alpha controls spatially restricted endosomal PtdIns3P and Rab11 activation to promote primary cilium function. Dev Cell 28:647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franco I, Margaria JP, De Santis MC, Ranghino A, Monteyne D, Chiaravalli M, Pema M, Campa CC, Ratto E, Gulluni F, Perez-Morga D, Somlo S, Merlo GR, Boletta A, Hirsch E (2016) Phosphoinositide 3-Kinase-C2α regulates polycystin-2 ciliary entry and protects against kidney cyst formation. J Am Soc Nephrol 27(4):1135–1144. doi: 10.1681/ASN.2014100967. Epub 2015 Aug 13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman BS, Lam AQ, Sundsbak JL, Iatrino R, Su X, Koon SJ, Wu M, Daheron L, Harris PC, Zhou J et al. (2013) Reduced ciliary polycystin-2 in induced pluripotent stem cells from polycystic kidney disease patients with PKD1 mutations. J Am Soc Nephrol 24:1571–1586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujiwara M, Ishihara T, Katsura I (1999) A novel WD40 protein, CHE-2, acts cell-autonomously in the formation of C. elegans sensory cilia. Development 126:4839–4848 [DOI] [PubMed] [Google Scholar]
- Gainullin VG, Hopp K, Ward CJ, Hommerding CJ, Harris PC (2015) Polycystin-1 maturation requires polycystin-2 in a dose-dependent manner. J Clin Invest 125:607–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallagher AR, Esquivel EL, Briere TS, Tian X, Mitobe M, Menezes LF, Markowitz GS, Jain D, Onuchic LF, Somlo S (2008) Biliary and pancreatic dysgenesis in mice harboring a mutation in Pkhd1. Am J Pathol 172:417–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalez MA, Menezes LF, Piontek KB, Kaimori J, Huso DL, Watnick T, Onuchic LF, Guay-Woodford LM, Germino GG (2007) Genetic interaction studies link autosomal dominant and recessive polycystic kidney disease in a common pathway. Hum Mol Genet 16:1940–1950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Corbit KC, Sirerol-Piquer MS, Ramaswami G, Otto EA, Noriega TR, Seol AD, Robinson JF, Bennett CL, Josifova DJ et al. (2011) A transition zone complex regulates mammalian ciliogenesis and ciliary membrane composition. Nat Genet 43:776–784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Gonzalo FR, Phua SC, Roberson EC, Garcia G 3rd, Abedin M, Schurmans S, Inoue T, Reiter JF (2015) Phosphoinositides Regulate ciliary protein trafficking to modulate hedgehog signaling. Dev Cell 34:400–409 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gattone VH 2nd, Wang X, Harris PC, Torres VE (2003) Inhibition of renal cystic disease development and progression by a vasopressin V2 receptor antagonist. Nat Med 9:1323–1326 [DOI] [PubMed] [Google Scholar]
- Geng L, Okuhara D, Yu Z, Tian X, Cai Y, Shibazaki S, Somlo S (2006) Polycystin-2 traffics to cilia independently of polycystin-1 by using an N-terminal RVxP motif. J Cell Sci 119:1383–1395 [DOI] [PubMed] [Google Scholar]
- Guo DF, Rahmouni K (2011) Molecular basis of the obesity associated with Bardet–Biedl syndrome. Trends Endocrinol Metab TEM 22:286–293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo DF, Cui H, Zhang Q, Morgan DA, Thedens DR, Nishimura D, Grobe JL, Sheffield VC, Rahmouni K (2016) The BBSome controls energy homeostasis by mediating the transport of the leptin receptor to the plasma membrane. PLoS Genet 12:e1005890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han SJ, Jang HS, Kim JI, Lipschutz JH, Park KM (2016) Unilateral nephrectomy elongates primary cilia in the remaining kidney via reactive oxygen species. Sci Rep 6:22281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han YM, Kang GM, Byun K, Ko HW, Kim J, Shin MS, Kim HK, Gil SY, Yu JH, Lee B et al. (2014) Leptin-promoted cilia assembly is critical for normal energy balance. J Clin Investig 124:2193–2197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartman TR, Liu D, Zilfou JT, Robb V, Morrison T, Watnick T, Henske EP (2009) The tuberous sclerosis proteins regulate formation of the primary cilium via a rapamycin-insensitive and polycystin 1-independent pathway. Hum Mol Genet 18:151–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatayama M, Mikoshiba K, Aruga J (2011) IP3 signaling is required for cilia formation and left–right body axis determination in Xenopus embryos. Biochem Biophys Res Commun 410:520–524 [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK (2005) Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Schafer JC, Zhang Q, Taulman PD, Yoder BK (2003) Identification of CHE-13, a novel intraflagellar transport protein required for cilia formation. Exp Cell Res 284:251–263 [DOI] [PubMed] [Google Scholar]
- He M, Subramanian R, Bangs F, Omelchenko T, Liem KF Jr, Kapoor TM, Anderson KV (2014) The kinesin-4 protein Kif7 regulates mammalian Hedgehog signalling by organizing the cilium tip compartment. Nat Cell Biol 16:663–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hearn T, Spalluto C, Phillips VJ, Renforth GL, Copin N, Hanley NA, Wilson DI (2005) Subcellular localization of ALMS1 supports involvement of centrosome and basal body dysfunction in the pathogenesis of obesity, insulin resistance, and type 2 diabetes. Diabetes 54:1581–1587 [DOI] [PubMed] [Google Scholar]
- Heon E, Kim G, Qin S, Garrison JE, Tavares E, Vincent A, Nuangchamnong N, Scott CA, Slusarski DC, Sheffield VC (2016) Mutations in C8ORF37 cause Bardet Biedl syndrome (BBS21). Hum Mol Genet 25(11):2283–2294. Epub 2016 Mar 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilton LK, Gunawardane K, Kim JW, Schwarz MC, Quarmby LM (2013) The kinases LF4 and CNK2 control ciliary length by feedback regulation of assembly and disassembly rates. Curr Biol 23:2208–2214 [DOI] [PubMed] [Google Scholar]
- Hoffmeister H, Babinger K, Gurster S, Cedzich A, Meese C, Schadendorf K, Osten L, de Vries U, Rascle A, Witzgall R (2011) Polycystin-2 takes different routes to the somatic and ciliary plasma membrane. J Cell Biol 192:631–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogan MC, Manganelli L, Woollard JR, Masyuk AI, Masyuk TV, Tammachote R, Huang BQ, Leontovich AA, Beito TG, Madden BJ et al. (2009) Characterization of PKD protein-positive exosome-like vesicles. J Am Soc Nephrol 20:278–288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong DH, Yue G, Adamian M, Li T (2001) Retinitis pigmentosa GTPase regulator (RPGRr)-interacting protein is stably associated with the photoreceptor ciliary axoneme and anchors RPGR to the connecting cilium. J Biol Chem 276:12091–12099 [DOI] [PubMed] [Google Scholar]
- Hopp K, Hommerding CJ, Wang X, Ye H, Harris PC, Torres VE (2015) Tolvaptan plus pasireotide shows enhanced efficacy in a PKD1 model. J Am Soc Nephrol 26:39–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopp K, Ward CJ, Hommerding CJ, Nasr SH, Tuan HF, Gainullin VG, Rossetti S, Torres VE, Harris PC (2012) Functional polycystin-1 dosage governs autosomal dominant polycystic kidney disease severity. J Clin Invest 122:4257–4273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao YC, Tuz K, Ferland RJ (2012) Trafficking in and to the primary cilium. Cilia 1:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J, Wittekind SG, Barr MM (2007) STAM and Hrs down-regulate ciliary TRP receptors. Mol Biol Cell 18:3277–3289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV (2005) Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA 102:11325–11330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV (2003) Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87 [DOI] [PubMed] [Google Scholar]
- Husson H, Moreno S, Smith LA, Smith MM, Russo RJ, Pitstick R, Sergeev M, Ledbetter SR, Bukanov NO, Lane M et al. (2016) Reduction of ciliary length through pharmacologic or genetic inhibition of CDK5 attenuates polycystic kidney disease in a model of nephronophthisis. Hum Mol Genet 25(11):2245–2255. Epub 2016 Apr 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibraghimov-Beskrovnaya O, Natoli TA (2011) mTOR signaling in polycystic kidney disease. Trends Mol Med 17:625–633 [DOI] [PubMed] [Google Scholar]
- Ingham PW, Nakano Y, Seger C (2011) Mechanisms and functions of Hedgehog signalling across the metazoa. Nat Rev Genet 12:393–406 [DOI] [PubMed] [Google Scholar]
- Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G (2001) Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol 153:13–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iomini C, Li L, Esparza JM, Dutcher SK (2009) Retrograde intraflagellar transport mutants identify complex A proteins with multiple genetic interactions in Chlamydomonas reinhardtii. Genetics 183:885–896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa H, Ide T, Yagi T, Jiang X, Hirono M, Sasaki H, Yanagisawa H, Wemmer KA, Stainier DY, Qin H et al. (2014) TTC26/DYF13 is an intraflagellar transport protein required for transport of motility-related proteins into flagella. Elife 3:e01566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby M, Cox JJ, Gayral S, Hampshire DJ, Ayub M, Blockmans M, Pernot E, Kisseleva MV, Compere P, Schiffmann SN et al. (2009) INPP5E mutations cause primary cilium signaling defects, ciliary instability and ciliopathies in human and mouse. Nat Genet 41:1027–1031 [DOI] [PubMed] [Google Scholar]
- Jin X, Mohieldin AM, Muntean BS, Green JA, Shah JV, Mykytyn K, Nauli SM (2014a) Cilioplasm is a cellular compartment for calcium signaling in response to mechanical and chemical stimuli. Cell Mol Life Sci 71:2165–2178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin X, Muntean BS, Aal-Aaboda MS, Duan Q, Zhou J, Nauli SM (2014b) L-type calcium channel modulates cystic kidney phenotype. Biochim Biophys Acta 1842(9):1518–1526. doi: 10.1016/j.bbadis.2014.06.001. Epub 2014 Jun 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen JA, San Agustin J, Follit JA, Pazour GJ (2008) Deletion of IFT20 in the mouse kidney causes misorientation of the mitotic spindle and cystic kidney disease. J Cell Biol 183:377–384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonassen JA, SanAgustin J, Baker SP, Pazour GJ (2012) Disruption of IFT complex A causes cystic kidneys without mitotic spindle misorientation. J Am Soc Nephrol 23:641–651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keady BT, Samtani R, Tobita K, Tsuchya M, San Agustin JT, Follit JA, Jonassen JA, Subramanian R, Lo CW, Pazour GJ (2012) IFT25 links the signal-dependent movement of Hedgehog components to intraflagellar transport. Dev Cell 22:940–951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keeling J, Tsiokas L, Maskey D (2016) Cellular mechanisms of ciliary length control. Cells 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan SA, Muhammad N, Khan MA, Kamal A, Rehman ZU, Khan S (2016) Genetics of human Bardet–Biedl syndrome, an updates. Clin Genet 90(1):3–15. doi: 10.1111/cge.12737. Epub 2016 Feb 9 [DOI] [PubMed] [Google Scholar]
- Khayyeri H, Barreto S, Lacroix D (2015) Primary cilia mechanics affects cell mechanosensation: a computational study. J Theor Biol 379:38–46 [DOI] [PubMed] [Google Scholar]
- Kim JH, Ki SM, Joung JG, Scott E, Heynen-Genel S, Aza-Blanc P, Kwon CH, Kim J, Gleeson JG, Lee JE (2016a) Genome-wide screen identifies novel machineries required for both ciliogenesis and cell cycle arrest upon serum starvation. Biochim Biophys Acta 1863:1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee K, Choi JH, Ringstad N, Dynlacht BD (2015) Nek2 activation of Kif24 ensures cilium disassembly during the cell cycle. Nat Commun 6:8087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Lee JE, Heynen-Genel S, Suyama E, Ono K, Lee K, Ideker T, Aza-Blanc P, Gleeson JG (2010) Functional genomic screen for modulators of ciliogenesis and cilium length. Nature 464:1048–1051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Nie H, Nesin V, Tran U, Outeda P, Bai CX, Keeling J, Maskey D, Watnick T, Wessely O et al. (2016b) The polycystin complex mediates Wnt/Ca signalling. Nat Cell Biol 18 (7):752–764. doi: 10.1038/ncb3363. Epub 2016 May 23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H, Xu H, Yao Q, Li W, Huang Q, Outeda P, Cebotaru V, Chiaravalli M, Boletta A, Piontek K et al. (2014) Ciliary membrane proteins traffic through the Golgi via a Rabep1/GGA1/Arl3-dependent mechanism. Nat Commun 5:5482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knodler A, Feng S, Zhang J, Zhang X, Das A, Peranen J, Guo W (2010) Coordination of Rab8 and Rab11 in primary ciliogenesis. Proc Natl Acad Sci USA 107:6346–6351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kottgen M, Buchholz B, Garcia-Gonzalez MA, Kotsis F, Fu X, Doerken M, Boehlke C, Steffl D, Tauber R, Wegierski T et al. (2008) TRPP2 and TRPV4 form a polymodal sensory channel complex. J Cell Biol 182:437–447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Beech PL, Rosenbaum JL (1995) The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol 131:1517–1527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozminski KG, Johnson KA, Forscher P, Rosenbaum JL (1993) A motility in the eukaryotic flagellum unrelated to flagellar beating. Proc Natl Acad Sci USA 90:5519–5523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurbegovic A, Kim H, Xu H, Yu S, Cruanes J, Maser RL, Boletta A, Trudel M, Qian F (2014) Novel functional complexity of polycystin-1 by GPS cleavage in vivo: role in polycystic kidney disease. Mol Cell Biol 34:3341–3353 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lal M, Song X, Pluznick JL, Di Giovanni V, Merrick DM, Rosenblum ND, Chauvet V, Gottardi CJ, Pei Y, Caplan MJ (2008) Polycystin-1 C-terminal tail associates with beta-catenin and inhibits canonical Wnt signaling. Hum Mol Genet 17:3105–3117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Louie CM, Silhavy JL, Sintasath L, Decambre M, Nigam SK, Willert K, Gleeson JG (2009) Impaired Wnt-beta-catenin signaling disrupts adult renal homeostasis and leads to cystic kidney ciliopathy. Nat Med 15:1046–1054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lancaster MA, Schroth J, Gleeson JG (2011) Subcellular spatial regulation of canonical Wnt signalling at the primary cilium. Nat Cell Biol 13:700–707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavagnino M, Gardner K, Sedlak AM, Arnoczky SP (2013) Tendon cell ciliary length as a biomarker of in situ cytoskeletal tensional homeostasis. Muscles Ligaments Tendons J 3:118–121 [PMC free article] [PubMed] [Google Scholar]
- Lechtreck KF, Johnson EC, Sakai T, Cochran D, Ballif BA, Rush J, Pazour GJ, Ikebe M, Witman GB (2009) The Chlamydomonas reinhardtii BBSome is an IFT cargo required for export of specific signaling proteins from flagella. J Cell Biol 187:1117–1132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leightner AC, Hommerding CJ, Peng Y, Salisbury JL, Gainullin VG, Czarnecki PG, Sussman CR, Harris PC (2013) The Meckel syndrome protein meckelin (TMEM67) is a key regulator of cilia function but is not required for tissue planar polarity. Hum Mol Genet 22:2024–2040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li B, Rauhauser AA, Dai J, Sakthivel R, Igarashi P, Jetten AM, Attanasio M (2011) Increased hedgehog signaling in postnatal kidney results in aberrant activation of nephron developmental programs. Hum Mol Genet 20(21):4155–4166. doi: 10.1093/hmg/ddr339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liew GM, Ye F, Nager AR, Murphy JP, Lee JS, Aguiar M, Breslow DK, Gygi SP, Nachury MV (2014) The intraflagellar transport protein IFT27 promotes BBSome exit from cilia through the GTPase ARL6/BBS3. Dev Cell 31:265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, Igarashi P (2003) Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100:5286–5291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrand A, Davis EE, Carvalho CM, Pehlivan D, Willer JR, Tsai IC, Ramanathan S, Zuppan C, Sabo A, Muzny D et al. (2014) Recurrent CNVs and SNVs at the NPHP1 locus contribute pathogenic alleles to Bardet–Biedl syndrome. Am J Hum Genet 94:745–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Murcia NS, Duan Y, Weinbaum S, Yoder BK, Schwiebert E, Satlin LM (2005b) Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am J Physiol Renal Physiol 289:F978–F988 [DOI] [PubMed] [Google Scholar]
- Liu A, Wang B, Niswander LA (2005a) Mouse intraflagellar transport proteins regulate both the activator and repressor functions of Gli transcription factors. Development 132:3103–3111 [DOI] [PubMed] [Google Scholar]
- Liu W, Xu S, Woda C, Kim P, Weinbaum S, Satlin LM (2003) Effect of flow and stretch on the [Ca2+]i response of principal and intercalated cells in cortical collecting duct. Am J Physiol Renal Physiol 285:F998–F1012 [DOI] [PubMed] [Google Scholar]
- Louie CM, Gleeson JG (2005) Genetic basis of Joubert syndrome and related disorders of cerebellar development. Hum Mol Genet 14 Spec No. 2:R235–R242 [DOI] [PubMed] [Google Scholar]
- Low SH, Vasanth S, Larson CH, Mukherjee S, Sharma N, Kinter MT, Kane ME, Obara T, Weimbs T (2006) Polycystin-1, STAT6, and P100 function in a pathway that transduces ciliary mechanosensation and is activated in polycystic kidney disease. Dev Cell 10:57–69 [DOI] [PubMed] [Google Scholar]
- Lu Q, Insinna C, Ott C, Stauffer J, Pintado PA, Rahajeng J, Baxa U, Walia V, Cuenca A, Hwang YS et al. (2015) Early steps in primary cilium assembly require EHD1/EHD3-dependent ciliary vesicle formation. Nat Cell Biol 17:531. [DOI] [PubMed] [Google Scholar]
- Ludington WB, Wemmer KA, Lechtreck KF, Witman GB, Marshall WF (2013) Avalanche-like behavior in ciliary import. Proc Natl Acad Sci USA 110:3925–3930 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luijten MN, Basten SG, Claessens T, Vernooij M, Scott CL, Janssen R, Easton JA, Kamps MA, Vreeburg M, Broers JL et al. (2013) Birt–Hogg–Dube syndrome is a novel ciliopathy. Hum Mol Genet 22:4383–4397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo N, Lu J, Sun Y (2012) Evidence of a role of inositol polyphosphate 5-phosphatase INPP5E in cilia formation in zebrafish. Vis Res 75:98–107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Tian X, Igarashi P, Pazour GJ, Somlo S (2013) Loss of cilia suppresses cyst growth in genetic models of autosomal dominant polycystic kidney disease. Nat Genet 45:1004–1012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning DK, Sergeev M, van Heesbeen RG, Wong MD, Oh JH, Liu Y, Henkelman RM, Drummond I, Shah JV, Beier DR (2013) Loss of the ciliary kinase Nek8 causes left–right asymmetry defects. J Am Soc Nephrol 24:100–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V, Stoetzel C, Schlicht D, Messaddeq N, Koch M, Flori E, Danse JM, Mandel JL, Dollfus H (2009) Transient ciliogenesis involving Bardet–Biedl syndrome proteins is a fundamental characteristic of adipogenic differentiation. Proc Natl Acad Sci USA 106:1820–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion V, Stutzmann F, Gerard M, De Melo C, Schaefer E, Claussmann A, Helle S, Delague V, Souied E, Barrey C et al. (2012) Exome sequencing identifies mutations in LZTFL1, a BBSome and smoothened trafficking regulator, in a family with Bardet–Biedl syndrome with situs inversus and insertional polydactyly. J Med Genet 49:317–321 [DOI] [PubMed] [Google Scholar]
- Marshall WF, Rosenbaum JL (2001) Intraflagellar transport balances continuous turnover of outer doublet microtubules: implications for flagellar length control. J Cell Biol 155:405–414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall WF, Qin H, Rodrigo Brenni M, Rosenbaum JL (2005) Flagellar length control system: testing a simple model based on intraflagellar transport and turnover. Mol Biol Cell 16:270–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masyuk AI, Masyuk TV, LaRusso NF (2008) Cholangiocyte primary cilia in liver health and disease. Dev Dyn 237:2007–2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattera R, Arighi CN, Lodge R, Zerial M, Bonifacino JS (2003) Divalent interaction of the GGAs with the Rabaptin-5-Rabex-5 complex. EMBO J 22:78–88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazelova J, Astuto-Gribble L, Inoue H, Tam BM, Schonteich E, Prekeris R, Moritz OL, Randazzo PA, Deretic D (2009) Ciliary targeting motif VxPx directs assembly of a trafficking module through Arf4. EMBO J 28:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merrick D, Chapin H, Baggs JE, Yu Z, Somlo S, Sun Z, Hogenesch JB, Caplan MJ (2012) The gamma-secretase cleavage product of polycystin-1 regulates TCF and CHOP-mediated transcriptional activation through a p300-dependent mechanism. Dev Cell 22:197–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mick DU, Rodrigues RB, Leib RD, Adams CM, Chien AS, Gygi SP, Nachury MV (2015) Proteomics of primary cilia by proximity labeling. Dev Cell 35:497–512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller MM, Iglesias DM, Zhang Z, Corsini R, Chu L, Murawski I, Gupta I, Somlo S, Germino GG, Goodyer PR (2011) T-cell factor/beta-catenin activity is suppressed in two different models of autosomal dominant polycystic kidney disease. Kidney Int 80:146–153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell DR (2007) The evolution of eukaryotic cilia and flagella as motile and sensory organelles. Adv Exp Med Biol 607:130–140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser M, Matthiesen S, Kirfel J, Schorle H, Bergmann C, Senderek J, Rudnik-Schoneborn S, Zerres K, Buettner R (2005) A mouse model for cystic biliary dysgenesis in autosomal recessive polycystic kidney disease (ARPKD). Hepatology 41:1113–1121 [DOI] [PubMed] [Google Scholar]
- Mukhopadhyay S, Wen X, Chih B, Nelson CD, Lane WS, Scales SJ, Jackson PK (2010) TULP3 bridges the IFT-A complex and membrane phosphoinositides to promote trafficking of G protein-coupled receptors into primary cilia. Genes Dev 24:2180–2193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peranen J, Merdes A, Slusarski DC, Scheller RH, Bazan JF, Sheffield VC et al. (2007) A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell 129:1201–1213 [DOI] [PubMed] [Google Scholar]
- Nakamura T, Saito D, Kawasumi A, Shinohara K, Asai Y, Takaoka K, Dong F, Takamatsu A, Belo JA, Mochizuki A et al. (2012) Fluid flow and interlinked feedback loops establish left–right asymmetric decay of Cerl2 mRNA. Nat Commun 3:1322. [DOI] [PubMed] [Google Scholar]
- Natoli TA, Gareski TC, Dackowski WR, Smith L, Bukanov NO, Russo RJ, Husson H, Matthews D, Piepenhagen P, Ibraghimov-Beskrovnaya O (2008) Pkd1 and Nek8 mutations affect cell-cell adhesion and cilia in cysts formed in kidney organ cultures. Am J Physiol Renal Physiol 294:F73–F83 [DOI] [PubMed] [Google Scholar]
- Natoli TA, Smith LA, Rogers KA, Wang B, Komarnitsky S, Budman Y, Belenky A, Bukanov NO, Dackowski WR, Husson H et al. (2010) Inhibition of glucosylceramide accumulation results in effective blockade of polycystic kidney disease in mouse models. Nat Med 16:788–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nauli SM, Alenghat FJ, Luo Y, Williams E, Vassilev P, Li X, Elia AE, Lu W, Brown EM, Quinn SJ et al. (2003) Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat Genet 33:129–137 [DOI] [PubMed] [Google Scholar]
- Niewiadomski P, Kong JH, Ahrends R, Ma Y, Humke EW, Khan S, Teruel MN, Novitch BG, Rohatgi R (2014) Gli protein activity is controlled by multisite phosphorylation in vertebrate hedgehog signaling. Cell Rep 6:168–181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nozawa YI, Lin C, Chuang PT (2013) Hedgehog signaling from the primary cilium to the nucleus: an emerging picture of ciliary localization, trafficking and transduction. Curr Opin Genet Dev 23:429–437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor AK, Malarkey EB, Berbari NF, Croyle MJ, Haycraft CJ, Bell PD, Hohenstein P, Kesterson RA, Yoder BK (2013) An inducible CiliaGFP mouse model for in vivo visualization and analysis of cilia in live tissue. Cilia 2:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh EC, Katsanis N (2013) Context-dependent regulation of Wnt signaling through the primary cilium. J Am Soc Nephrol 24:10–18 [DOI] [PubMed] [Google Scholar]
- Oishi I, Kawakami Y, Raya A, Callol-Massot C, Izpisua Belmonte JC (2006) Regulation of primary cilia formation and left–right patterning in zebrafish by a noncanonical Wnt signaling mediator, duboraya. Nat Genet 38:1316–1322 [DOI] [PubMed] [Google Scholar]
- Olsan EE, Mukherjee S, Wulkersdorfer B, Shillingford JM, Giovannone AJ, Todorov G, Song X, Pei Y, Weimbs T (2011) Signal transducer and activator of transcription-6 (STAT6) inhibition suppresses renal cyst growth in polycystic kidney disease. Proc Natl Acad Sci USA 108:18067–18072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto EA, Loeys B, Khanna H, Hellemans J, Sudbrak R, Fan S, Muerb U, O’Toole JF, Helou J, Attanasio M et al. (2005) Nephrocystin-5, a ciliary IQ domain protein, is mutated in Senior–Loken syndrome and interacts with RPGR and calmodulin. Nat Genet 37:282–288 [DOI] [PubMed] [Google Scholar]
- Otto EA, Schermer B, Obara T, O’Toole JF, Hiller KS, Mueller AM, Ruf RG, Hoefele J, Beekmann F, Landau D et al. (2003) Mutations in INVS encoding inversin cause nephronophthisis type 2, linking renal cystic disease to the function of primary cilia and left–right axis determination. Nat Genet 34:413–420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ou Y, Ruan Y, Cheng M, Moser JJ, Rattner JB, van der Hoorn FA (2009) Adenylate cyclase regulates elongation of mammalian primary cilia. Exp Cell Res 315:2802–2817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandit MM, Gao Y, van Hoek A, Kohan DE (2015) Osmolar regulation of endothelin-1 production by the inner medullary collecting duct. Life Sci 159:135–139. doi: 10.1016/j.lfs.2015.10.037. Epub 2015 Nov 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker JD, Quarmby LM (2003) Chlamydomonas fla mutants reveal a link between deflagellation and intraflagellar transport. BMC Cell Biol 4:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, Cole DG (2000) Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol 151:709–718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Dickert BL, Witman GB (1999) The DHC1b (DHC2) isoform of cytoplasmic dynein is required for flagellar assembly. J Cell Biol 144:473–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pazour GJ, Wilkerson CG, Witman GB (1998) A dynein light chain is essential for the retrograde particle movement of intraflagellar transport (IFT). J Cell Biol 141:979–992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen LB, Miller MS, Geimer S, Leitch JM, Rosenbaum JL, Cole DG (2005) Chlamydomonas IFT172 is encoded by FLA11, interacts with CrEB1, and regulates IFT at the flagellar tip. Curr Biol 15:262–266 [DOI] [PubMed] [Google Scholar]
- Perkins LA, Hedgecock EM, Thomson JN, Culotti JG (1986) Mutant sensory cilia in the nematode Caenorhabditis elegans. Dev Biol 117:456–487 [DOI] [PubMed] [Google Scholar]
- Pescio LG, Favale NO, Marquez MG, Sterin-Speziale NB (2012) Glycosphingolipid synthesis is essential for MDCK cell differentiation. Biochim Biophys Acta 1821:884–894 [DOI] [PubMed] [Google Scholar]
- Piao T, Luo M, Wang L, Guo Y, Li D, Li P, Snell WJ, Pan J (2009) A microtubule depolymerizing kinesin functions during both flagellar disassembly and flagellar assembly in Chlamydomonas. Proc Natl Acad Sci USA 106:4713–4718 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piontek K, Menezes LF, Garcia-Gonzalez MA, Huso DL, Germino GG (2007) A critical developmental switch defines the kinetics of kidney cyst formation after loss of Pkd1. Nat Med 13:1490–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poole CA, Flint MH, Beaumont BW (1985) Analysis of the morphology and function of primary cilia in connective tissues: a cellular cybernetic probe? Cell Motil 5:175–193 [DOI] [PubMed] [Google Scholar]
- Porath B, Gainullin VG, Cornec-Le Gall E, Dillinger EK, Heyer CM, Hopp K, Edwards ME, Madsen CD, Mauritz SR, Banks CJ et al. (2016) Mutations in GANAB, encoding the glucosidase IIalpha subunit, cause autosomal-dominant polycystic kidney and liver disease. Am J Hum Genet 98:1193–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter ME, Bower R, Knott JA, Byrd P, Dentler W (1999) Cytoplasmic dynein heavy chain 1b is required for flagellar assembly in Chlamydomonas. Mol Biol Cell 10:693–712 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR (2001) Bending the MDCK cell primary cilium increases intracellular calcium. J Membr Biol 184:71–79 [DOI] [PubMed] [Google Scholar]
- Praetorius HA, Spring KR (2003) Removal of the MDCK cell primary cilium abolishes flow sensing. J Membr Biol 191:69–76 [DOI] [PubMed] [Google Scholar]
- Pugacheva EN, Jablonski SA, Hartman TR, Henske EP, Golemis EA (2007) HEF1-dependent Aurora A activation induces disassembly of the primary cilium. Cell 129:1351–1363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian CN, Knol J, Igarashi P, Lin F, Zylstra U, Teh BT, Williams BO (2005) Cystic renal neoplasia following conditional inactivation of apc in mouse renal tubular epithelium. J Biol Chem 280:3938–3945 [DOI] [PubMed] [Google Scholar]
- Qin J, Lin Y, Norman RX, Ko HW, Eggenschwiler JT (2011) Intraflagellar transport protein 122 antagonizes Sonic Hedgehog signaling and controls ciliary localization of pathway components. Proc Natl Acad Sci USA 108:1456–1461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Taglienti M, Cai L, Zhou J, Kreidberg JA (2012) c-Met and NF-kappaB-dependent overexpression of Wnt7a and −7b and Pax2 promotes cystogenesis in polycystic kidney disease. J Am Soc Nephrol 23:1309–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin S, Taglienti M, Nauli SM, Contrino L, Takakura A, Zhou J, Kreidberg JA (2010) Failure to ubiquitinate c-Met leads to hyperactivation of mTOR signaling in a mouse model of autosomal dominant polycystic kidney disease. J Clin Invest 120:3617–3628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H, Wang Z, Diener D, Rosenbaum J (2007) Intraflagellar transport protein 27 is a small G protein involved in cell-cycle control. Curr Biol 17:193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quinlan RJ, Tobin JL, Beales PL (2008) Modeling ciliopathies: primary cilia in development and disease. Curr Top Dev Biol 84:249–310 [DOI] [PubMed] [Google Scholar]
- Ravichandran K, Zafar I, Ozkok A, Edelstein CL (2015) An mTOR kinase inhibitor slows disease progression in a rat model of polycystic kidney disease. Nephrol Dial Transplant 30:45–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohatgi R, Milenkovic L, Scott MP (2007) Patched1 regulates hedgehog signaling at the primary cilium. Science 317:372–376 [DOI] [PubMed] [Google Scholar]
- Romio L, Fry AM, Winyard PJ, Malcolm S, Woolf AS, Feather SA (2004) OFD1 is a centrosomal/basal body protein expressed during mesenchymal-epithelial transition in human nephrogenesis. J Am Soc Nephrol 15:2556–2568 [DOI] [PubMed] [Google Scholar]
- Rosenbaum JL, Child FM (1967) Flagellar regeneration in protozoan flagellates. J Cell Biol 34:345–364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenbaum JL, Witman GB (2002) Intraflagellar transport. Nat Rev Mol Cell Biol 3:813–825 [DOI] [PubMed] [Google Scholar]
- Ruggenenti P, Gentile G, Perico N, Perna A, Barcella L, Trillini M, Cortinovis M, Ferrer Siles CP, Reyes Loaeza JA, Aparicio MC et al. (2016) Effect of sirolimus on disease progression in patients with autosomal dominant polycystic kidney disease and CKD stages 3b-4. Clin J Am Soc Nephrol 11(5):785–794. doi: 10.2215/CJN.09900915. Epub 2016 Feb 22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz-Perez VL, Goodship JA (2009) Ellis-van Creveld syndrome and Weyers acrodental dysostosis are caused by cilia-mediated diminished response to hedgehog ligands. Am J Med Genet C Semin Med Genet 151C:341–351 [DOI] [PubMed] [Google Scholar]
- Rydholm S, Zwartz G, Kowalewski JM, Kamali-Zare P, Frisk T, Brismar H (2010) Mechanical properties of primary cilia regulate the response to fluid flow. Am J Physiol Renal Physiol 298: F1096–F1102 [DOI] [PubMed] [Google Scholar]
- Saadi-Kheddouci S, Berrebi D, Romagnolo B, Cluzeaud F, Peuchmaur M, Kahn A, Vandewalle A, Perret C (2001) Early development of polycystic kidney disease in transgenic mice expressing an activated mutant of the beta-catenin gene. Oncogene 20:5972–5981 [DOI] [PubMed] [Google Scholar]
- Sanchez de Diego A, Alonso Guerrero A, Martinez AC, van Wely KH (2014) Dido3-dependent HDAC6 targeting controls cilium size. Nat Commun 5:3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saraga-Babic M, Vukojevic K, Bocina I, Drnasin K, Saraga M (2012) Ciliogenesis in normal human kidney development and post-natal life. Pediatr Nephrol 27:55–63 [DOI] [PubMed] [Google Scholar]
- Schaefer E, Stoetzel C, Scheidecker S, Geoffroy V, Prasad MK, Redin C, Missotte I, Lacombe D, Mandel JL, Muller J et al. (2016) Identification of a novel mutation confirms the implication of IFT172 (BBS20) in Bardet–Biedl syndrome. J Hum Genet 61(5):447–450. doi: 10.1038/jhg.2015.162. Epub 2016 Jan 14 [DOI] [PubMed] [Google Scholar]
- Scheidecker S, Etard C, Pierce NW, Geoffroy V, Schaefer E, Muller J, Chennen K, Flori E, Pelletier V, Poch O et al. (2014) Exome sequencing of Bardet–Biedl syndrome patient identifies a null mutation in the BBSome subunit BBIP1 (BBS18). J Med Genet 51:132–136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwarz N, Hardcastle AJ, Cheetham ME (2012) Arl3 and RP2 mediated assembly and traffic of membrane associated cilia proteins. Vis Res 75:2–4 [DOI] [PubMed] [Google Scholar]
- Seo S, Baye LM, Schulz NP, Beck JS, Zhang Q, Slusarski DC, Sheffield VC (2010) BBS6, BBS10, and BBS12 form a complex with CCT/TRiC family chaperonins and mediate BBSome assembly. Proc Natl Acad Sci USA 107:1488–1493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo S, Zhang Q, Bugge K, Breslow DK, Searby CC, Nachury MV, Sheffield VC (2011) A novel protein LZTFL1 regulates ciliary trafficking of the BBSome and Smoothened. PLoS Genet 7: e1002358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serra AL, Poster D, Kistler AD, Krauer F, Raina S, Young J, Rentsch KM, Spanaus KS, Senn O, Kristanto P et al. (2010) Sirolimus and kidney growth in autosomal dominant polycystic kidney disease. N Engl J Med 363:820–829 [DOI] [PubMed] [Google Scholar]
- Shao YY, Wang L, Welter JF, Ballock RT (2012) Primary cilia modulate Ihh signal transduction in response to hydrostatic loading of growth plate chondrocytes. Bone 50:79–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N, Kosan ZA, Stallworth JE, Berbari NF, Yoder BK (2011) Soluble levels of cytosolic tubulin regulate ciliary length control. Mol Biol Cell 22:806–816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillingford JM, Leamon CP, Vlahov IR, Weimbs T (2012) Folate-conjugated rapamycin slows progression of polycystic kidney disease. J Am Soc Nephrol 23:1674–1681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shillingford JM, Murcia NS, Larson CH, Low SH, Hedgepeth R, Brown N, Flask CA, Novick AC, Goldfarb DA, Kramer-Zucker A et al. (2006) The mTOR pathway is regulated by polycystin-1, and its inhibition reverses renal cystogenesis in polycystic kidney disease. Proc Natl Acad Sci USA 103:5466–5471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signor D, Wedaman KP, Orozco JT, Dwyer ND, Bargmann CI, Rose LS, Scholey JM (1999) Role of a class DHC1b dynein in retrograde transport of IFT motors and IFT raft particles along cilia, but not dendrites, in chemosensory neurons of living Caenorhabditis elegans. J Cell Biol 147:519–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons M, Gloy J, Ganner A, Bullerkotte A, Bashkurov M, Kronig C, Schermer B, Benzing T, Cabello OA, Jenny A et al. (2005) Inversin, the gene product mutated in nephronophthisis type II, functions as a molecular switch between Wnt signaling pathways. Nat Genet 37:537–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siroky BJ, Ferguson WB, Fuson AL, Xie Y, Fintha A, Komlosi P, Yoder BK, Schwiebert EM, Guay-Woodford LM, Bell PD (2006) Loss of primary cilia results in deregulated and unabated apical calcium entry in ARPKD collecting duct cells. Am J Physiol Renal Physiol 290:F1320–F1328 [DOI] [PubMed] [Google Scholar]
- Smith LA, Bukanov NO, Husson H, Russo RJ, Barry TC, Taylor AL, Beier DR, Ibraghimov-Beskrovnaya O (2006) Development of polycystic kidney disease in juvenile cystic kidney mice: insights into pathogenesis, ciliary abnormalities, and common features with human disease. J Am Soc Nephrol 17:2821–2831 [DOI] [PubMed] [Google Scholar]
- Song L, Dentler WL (2001) Flagellar protein dynamics in Chlamydomonas. J Biol Chem 276:29754–29763 [DOI] [PubMed] [Google Scholar]
- Song X, Di Giovanni V, He N, Wang K, Ingram A, Rosenblum ND, Pei Y (2009) Systems biology of autosomal dominant polycystic kidney disease (ADPKD): computational identification of gene expression pathways and integrated regulatory networks. Hum Mol Genet 18:2328–2343 [DOI] [PubMed] [Google Scholar]
- Sorokin S (1962) Centrioles and the formation of rudimentary cilia by fibroblasts and smooth muscle cells. J Cell Biol 15:363–377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spalluto C, Wilson DI, Hearn T (2012) Nek2 localises to the distal portion of the mother centriole/basal body and is required for timely cilium disassembly at the G2/M transition. Eur J Cell Biol 91:675–686 [DOI] [PubMed] [Google Scholar]
- Stephens RE (1997) Synthesis and turnover of embryonic sea urchin ciliary proteins during selective inhibition of tubulin synthesis and assembly. Mol Biol Cell 8:2187–2198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stottmann RW, Tran PV, Turbe-Doan A, Beier DR (2009) Ttc21b is required to restrict sonic hedgehog activity in the developing mouse forebrain. Dev Biol 335:166–178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Driscoll K, Yao G, Raed A, Wu M, Beales PL, Zhou J (2014) Bardet–Biedl syndrome proteins 1 and 3 regulate the ciliary trafficking of polycystic kidney disease 1 protein. Hum Mol Genet 23:5441–5451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su S, Phua SC, DeRose R, Chiba S, Narita K, Kalugin PN, Katada T, Kontani K, Takeda S, Inoue T (2013) Genetically encoded calcium indicator illuminates calcium dynamics in primary cilia. Nat Methods 10:1105–1107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su X, Wu M, Yao G, El-Jouni W, Luo C, Tabari A, Zhou J (2015) Regulation of polycystin-1 ciliary trafficking by motifs at its C-terminus and polycystin-2 but not by cleavage at the GPS site. J Cell Sci 128:4063–4073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugiyama N, Tsukiyama T, Yamaguchi TP, Yokoyama T (2011) The canonical Wnt signaling pathway is not involved in renal cyst development in the kidneys of inv mutant mice. Kidney Int 79:957–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N (2004) A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development 131:4085–4093 [DOI] [PubMed] [Google Scholar]
- Takakura A, Nelson EA, Haque N, Humphreys BD, Zandi-Nejad K, Frank DA, Zhou J (2011) Pyrimethamine inhibits adult polycystic kidney disease by modulating STAT signaling pathways. Hum Mol Genet 20:4143–4154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takiar V, Nishio S, Seo-Mayer P, King JD Jr, Li H, Zhang L, Karihaloo A, Hallows KR, Somlo S, Caplan MJ (2011) Activating AMP-activated protein kinase (AMPK) slows renal cystogenesis. Proc Natl Acad Sci USA 108:2462–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JJ, Shillingford JM, Vasanth S, Doerr N, Mukherjee S, Kinter MT, Watnick T, Weimbs T (2011) Polycystin-1 regulates STAT activity by a dual mechanism. Proc Natl Acad Sci USA 108:7985–7990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talbot JJ, Song X, Wang X, Rinschen MM, Doerr N, LaRiviere WB, Schermer B, Pei YP, Torres VE, Weimbs T (2014) The cleaved cytoplasmic tail of polycystin-1 regulates Src-dependent STAT3 activation. J Am Soc Nephrol 25:1737–1748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LW, Ranum PT, Lefebvre PA (2013) CDKL5 regulates flagellar length and localizes to the base of the flagella in Chlamydomonas. Mol Biol Cell 24:588–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tam LW, Wilson NF, Lefebvre PA (2007) A CDK-related kinase regulates the length and assembly of flagella in Chlamydomonas. J Cell Biol 176:819–829 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao S, Kakade VR, Woodgett JR, Pandey P, Suderman ED, Rajagopal M, Rao R (2015) Glycogen synthase kinase-3beta promotes cyst expansion in polycystic kidney disease. Kidney Int 87:1164–1175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao Y, Kim J, Schrier RW, Edelstein CL (2005) Rapamycin markedly slows disease progression in a rat model of polycystic kidney disease. J Am Soc Nephrol 16:46–51 [DOI] [PubMed] [Google Scholar]
- Thiel C, Kessler K, Giessl A, Dimmler A, Shalev SA, von der Haar S, Zenker M, Zahnleiter D, Stoss H, Beinder E et al. (2011) NEK1 mutations cause short-rib polydactyly syndrome type majewski. Am J Hum Genet 88:106–114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson CL, Chapple JP, Knight MM (2014) Primary cilia disassembly down-regulates mechanosensitive hedgehog signalling: a feedback mechanism controlling ADAMTS-5 expression in chondrocytes. Osteoarthr Cartil 22:490–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Harris PC (2006) Mechanisms of disease: autosomal dominant and recessive polycystic kidney diseases. Nat Clin Pract Nephrol 2:40–55 quiz 55 [DOI] [PubMed] [Google Scholar]
- Torres VE, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB, Ouyang J, Czerwiec FS (2012) Tolvaptan in patients with autosomal dominant polycystic kidney disease. N Engl J Med 367:2407–2418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres VE, Meijer E, Bae KT, Chapman AB, Devuyst O, Gansevoort RT, Grantham JJ, Higashihara E, Perrone RD, Krasa HB et al. (2011) Rationale and design of the TEMPO (tolvaptan efficacy and safety in management of autosomal dominant polycystic kidney disease and its outcomes) 3–4 study. Am J Kidney Dis 57:692–699 [DOI] [PubMed] [Google Scholar]
- Torres VE, Wang X, Qian Q, Somlo S, Harris PC, Gattone VH 2nd (2004) Effective treatment of an orthologous model of autosomal dominant polycystic kidney disease. Nat Med 10:363–364 [DOI] [PubMed] [Google Scholar]
- Tran PV, Lechtreck KF (2016) An age of enlightenment for cilia: the FASEB summer research conference on the “Biology of cilia and flagella”. Dev Biol 409:319–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Sharma M, Li X, Calvet JP (2014a) Developmental signaling: does it bridge the gap between cilia dysfunction and renal cystogenesis? Birth Defects Res C Embryo Today 102:159–173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Talbott GC, Turbe-Doan A, Jacobs DT, Schonfeld MP, Silva LM, Chatterjee A, Prysak M, Allard BA, Beier DR (2014b) Downregulating Hedgehog signaling reduces renal cystogenic potential of mouse models. J Am Soc Nephrol 25(10):2201–2212. doi: 10.1681/ASN.2013070735. Epub 2014 Apr 3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran PV, Haycraft CJ, Besschetnova TY, Turbe-Doan A, Stottmann RW, Herron BJ, Chesebro AL, Qiu H, Scherz PJ, Shah JV et al. (2008) THM1 negatively modulates mouse sonic hedgehog signal transduction and affects retrograde intraflagellar transport in cilia. Nat Genet 40:403–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran U, Zakin L, Schweickert A, Agrawal R, Doger R, Blum M, De Robertis EM, Wessely O (2010) The RNA-binding protein bicaudal C regulates polycystin 2 in the kidney by antagonizing miR-17 activity. Development 137:1107–1116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudel M, Yao Q, Qian F (2016) The role of G-protein-coupled receptor proteolysis site cleavage of polycystin-1 in renal physiology and polycystic kidney disease. Cells 5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Upadhyay VS, Muntean BS, Kathem SH, Hwang JJ, Aboualaiwi WA, Nauli SM (2014) Roles of dopamine receptor on chemosensory and mechanosensory primary cilia in renal epithelial cells. Front Physiol 5:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Vaart A, Rademakers S, Jansen G (2015) DLK-1/p38 MAP kinase signaling controls cilium length by regulating RAB-5 mediated endocytosis in Caenorhabditis elegans. PLoS Genet 11:e1005733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese E, Ricardo SD, Weidenfeld R, Zhuang J, Hill PA, Langham RG, Deane JA (2009) Renal primary cilia lengthen after acute tubular necrosis. J Am Soc Nephrol 20:2147–2153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verghese E, Weidenfeld R, Bertram JF, Ricardo SD, Deane JA (2008) Renal cilia display length alterations following tubular injury and are present early in epithelial repair. Nephrol Dial Transplant 23:834–841 [DOI] [PubMed] [Google Scholar]
- Wahl PR, Serra AL, Le Hir M, Molle KD, Hall MN, Wuthrich RP (2006) Inhibition of mTOR with sirolimus slows disease progression in Han:SPRD rats with autosomal dominant polycystic kidney disease (ADPKD). Nephrol Dial Transplant 21:598–604 [DOI] [PubMed] [Google Scholar]
- Walczak-Sztulpa J, Eggenschwiler J, Osborn D, Brown DA, Emma F, Klingenberg C, Hennekam RC, Torre G, Garshasbi M, Tzschach A et al. (2010) Cranioectodermal Dysplasia, Sensenbrenner syndrome, is a ciliopathy caused by mutations in the IFT122 gene. Am J Hum Genet 86:949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace DP (2011) Cyclic AMP-mediated cyst expansion. Biochim Biophys Acta 1812:1291–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz G, Budde K, Mannaa M, Nurnberger J, Wanner C, Sommerer C, Kunzendorf U, Banas B, Horl WH, Obermuller N et al. (2010) Everolimus in patients with autosomal dominant polycystic kidney disease. N Engl J Med 363:830–840 [DOI] [PubMed] [Google Scholar]
- Wang X, Gattone V 2nd, Harris PC, Torres VE (2005) Effectiveness of vasopressin V2 receptor antagonists OPC-31260 and OPC-41061 on polycystic kidney disease development in the PCK rat. J Am Soc Nephrol 16:846–851 [DOI] [PubMed] [Google Scholar]
- Wang W, Li F, Sun Y, Lei L, Zhou H, Lei T, Xia Y, Verkman AS, Yang B (2015) Aquaporin-1 retards renal cyst development in polycystic kidney disease by inhibition of Wnt signaling. FASEB J 29:1551–1563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Silva M, Haas LA, Morsci NS, Nguyen KC, Hall DH, Barr MM (2014) C. elegans ciliated sensory neurons release extracellular vesicles that function in animal communication. Curr Biol 24:519–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wu Y, Ward CJ, Harris PC, Torres VE (2008) Vasopressin directly regulates cyst growth in polycystic kidney disease. J Am Soc Nephrol 19:102–108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S, Zhang J, Nauli SM, Li X, Starremans PG, Luo Y, Roberts KA, Zhou J (2007) Fibrocystin/polyductin, found in the same protein complex with polycystin-2, regulates calcium responses in kidney epithelia. Mol Cell Biol 27:3241–3252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward HH, Brown-Glaberman U, Wang J, Morita Y, Alper SL, Bedrick EJ, Gattone VH 2nd, Deretic D, Wandinger-Ness A (2011) A conserved signal and GTPase complex are required for the ciliary transport of polycystin-1. Mol Biol Cell 22:3289–3305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei W, Hackmann K, Xu H, Germino G, Qian F (2007) Characterization of cis-autoproteolysis of polycystin-1, the product of human polycystic kidney disease 1 gene. J Biol Chem 282:21729–21737 [DOI] [PubMed] [Google Scholar]
- Weimbs T (2007) Polycystic kidney disease and renal injury repair: common pathways, fluid flow, and the function of polycystin-1. Am J Physiol Renal Physiol 293:F1423–F1432 [DOI] [PubMed] [Google Scholar]
- Westlake CJ, Baye LM, Nachury MV, Wright KJ, Ervin KE, Phu L, Chalouni C, Beck JS, Kirkpatrick DS, Slusarski DC et al. (2011) Primary cilia membrane assembly is initiated by Rab11 and transport protein particle II (TRAPPII) complex-dependent trafficking of Rabin8 to the centrosome. Proc Natl Acad Sci USA 108:2759–2764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wheway G, Abdelhamed Z, Natarajan S, Toomes C, Inglehearn C, Johnson CA (2013) Aberrant Wnt signalling and cellular over-proliferation in a novel mouse model of Meckel–Gruber syndrome. Dev Biol 377:55–66 [DOI] [PubMed] [Google Scholar]
- White MC, Quarmby LM (2008) The NIMA-family kinase, Nek1 affects the stability of centrosomes and ciliogenesis. BMC Cell Biol 9:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams CL, Li C, Kida K, Inglis PN, Mohan S, Semenec L, Bialas NJ, Stupay RM, Chen N, Blacque OE et al. (2011) MKS and NPHP modules cooperate to establish basal body/transition zone membrane associations and ciliary gate function during ciliogenesis. J Cell Biol 192:1023–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wloga D, Camba A, Rogowski K, Manning G, Jerka-Dziadosz M, Gaertig J (2006) Members of the NIMA-related kinase family promote disassembly of cilia by multiple mechanisms. Mol Biol Cell 17:2799–2810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CR, Rosenbaum JL (2015) Ciliary ectosomes: transmissions from the cell’s antenna. Trends Cell Biol 25:276–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood CR, Huang K, Diener DR, Rosenbaum JL (2013) The cilium secretes bioactive ectosomes. Curr Biol 23:906–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woollard JR, Punyashtiti R, Richardson S, Masyuk TV, Whelan S, Huang BQ, Lager DJ, vanDeursen J, Torres VE, Gattone VH et al. (2007) A mouse model of autosomal recessive polycystic kidney disease with biliary duct and proximal tubule dilatation. Kidney Int 72:328–336 [DOI] [PubMed] [Google Scholar]
- Wright KJ, Baye LM, Olivier-Mason A, Mukhopadhyay S, Sang L, Kwong M, Wang W, Pretorius PR, Sheffield VC, Sengupta P et al. (2011) An ARL3-UNC119-RP2 GTPase cycle targets myristoylated NPHP3 to the primary cilium. Genes Dev 25:2347–2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Q, Zhang Y, Wei Q, Huang Y, Li Y, Ling K, Hu J (2015) BBS4 and BBS5 show functional redundancy in the BBSome to regulate the degradative sorting of ciliary sensory receptors. Sci Rep 5:11855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi T, Hempson SJ, Reif GA, Hedge AM, Wallace DP (2006) Calcium restores a normal proliferation phenotype in human polycystic kidney disease epithelial cells. J Am Soc Nephrol 17:178–187 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Nagao S, Kasahara M, Takahashi H, Grantham JJ (1997) Renal accumulation and excretion of cyclic adenosine monophosphate in a murine model of slowly progressive polycystic kidney disease. Am J Kidney Dis 30:703–709 [DOI] [PubMed] [Google Scholar]
- Yamaguchi T, Wallace DP, Magenheimer BS, Hempson SJ, Grantham JJ, Calvet JP (2004) Calcium restriction allows cAMP activation of the B-Raf/ERK pathway, switching cells to a cAMP-dependent growth-stimulated phenotype. J Biol Chem 279:40419–40430 [DOI] [PubMed] [Google Scholar]
- Yang TT, Su J, Wang WJ, Craige B, Witman GB, Tsou MF, Liao JC (2015) Superresolution pattern recognition reveals the architectural map of the ciliary transition zone. Sci Rep 5:14096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye H, Wang X, Sussman CR, Hopp K, Irazabal MV, Bakeberg JL, LaRiviere WB, Manganiello VC, Vorhees CV, Zhao H et al. (2015) Modulation of polycystic kidney disease severity by phosphodiesterase 1 and 3 subfamilies. J Am Soc Nephrol 27(5):1312–1320. doi: 10.1681/ASN.2015010057. Epub 2015 Sep 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ying G, Avasthi P, Irwin M, Gerstner CD, Frederick JM, Lucero MT, Baehr W (2014) Centrin 2 is required for mouse olfactory ciliary trafficking and development of ependymal cilia planar polarity. J Neurosci 34:6377–6388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder BK, Hou X, Guay-Woodford LM (2002) The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J Am Soc Nephrol 13:2508–2516 [DOI] [PubMed] [Google Scholar]
- Young RW (1971) The renewal of rod and cone outer segments in the rhesus monkey. J Cell Biol 49:303–318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young RW, Bok D (1969) Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol 42:392–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan S, Zhao L, Brueckner M, Sun Z (2015) Intraciliary calcium oscillations initiate vertebrate left–right asymmetry. Curr Biol 25:556–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaika O, Mamenko M, Berrout J, Boukelmoune N, O’Neil RG, Pochynyuk O (2013) TRPV4 dysfunction promotes renal cystogenesis in autosomal recessive polycystic kidney disease. J Am Soc Nephrol 24:604–616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang ZR, Chu WF, Song B, Gooz M, Zhang JN, Yu CJ, Jiang S, Baldys A, Gooz P, Steele S et al. (2013) TRPP2 and TRPV4 form an EGF-activated calcium permeable channel at the apical membrane of renal collecting duct cells. PLoS One 8:e73424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Nishimura D, Seo S, Vogel T, Morgan DA, Searby C, Bugge K, Stone EM, Rahmouni K, Sheffield VC (2011) Bardet–Biedl syndrome 3 (Bbs3) knockout mouse model reveals common BBS-associated phenotypes and Bbs3 unique phenotypes. Proc Natl Acad Sci USA 108:20678–20683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Q, Yu D, Seo S, Stone EM, Sheffield VC (2012) Intrinsic protein–protein interaction-mediated and chaperonin-assisted sequential assembly of stable Bardet–Biedl syndrome protein complex, the BBSome. J Biol Chem 287:20625–20635 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J (2009) Polycystins and primary cilia: primers for cell cycle progression. Annu Rev Physiol 71:83–113 [DOI] [PubMed] [Google Scholar]
- Zimmermann KW (1898) Beitrage zur Kenntniss einiger Drusen und Epithelien. Arch Mikrosk Anat 52:552–706 [Google Scholar]
- Zuo X, Fogelgren B, Lipschutz JH (2011) The small GTPase Cdc42 is necessary for primary ciliogenesis in renal tubular epithelial cells. J Biol Chem 286:22469–22477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo X, Guo W, Lipschutz JH (2009) The exocyst protein Sec10 is necessary for primary ciliogenesis and cystogenesis in vitro. Mol Biol Cell 20:2522–2529 [DOI] [PMC free article] [PubMed] [Google Scholar]