Abstract
Objective.
To evaluate the association between body mass index (BMI) and mortality in women with endometrial cancer.
Methods.
A systematic review was performed utilizing a Medline search with Mesh keywords ‘endometrial neoplasms’ and (‘body mass index’ or ‘obesity’) and (‘survival analysis’ or ‘mortality’ or ‘survivor’ or ‘survival’) for studies published prior to June 2013. Inclusion criteria included studies that assessed associations between BMI and survival in endometrial cancer patients. Two investigators independently reviewed the title and abstract and full-text of articles for inclusion or exclusion decision; discordant decisions were adjudicated by a third reviewer. A random-effects model was constructed that was comparable to the standard random-effects models used in the meta-analysis of odds ratios. The model was fitted using SAS PROC NLMIXED.
Results.
1451 studies were identified and reviewed in duplicate, 18 met inclusion criteria. A random-effects meta-analysis demonstrated significantly higher odds of mortality with increasing BMI in endometrial cancer patients. Specifically the odds ratios were 1.01, 1.17, 1.26, and 1.66 for BMI categories of 25–29.9, 30–34.9, 35–39.9, and 40+, respectively. The odds ratio for all-cause mortality in endometrial cancer patients with a BMI ≥ 40 compared to those with a BMI < 25 was 1.66 (CI: 1.10–2.51, p = 0.02). A single dose–response model indicated that a 10% increase in BMI resulted in a 9.2% increase in the odds of all-cause mortality (p = 0.007).
Conclusion.
Increased BMI is significantly associated with increased all-cause mortality in women with endometrial cancer, with the highest risk for those with a BMI ≥ 40.
Keywords: Body mass index, Obesity, Mortality, Survival, Endometrial cancer
1. Introduction
Obesity is a known risk factor for endometrial cancer with obese women having a 2–5 fold higher incidence of endometrial cancer [1]. In addition, 62% of American women are overweight or obese [2]. While the relationship between obesity, measured by body mass index (BMI), and increased risk of endometrial is well established, there is conflicting data regarding BMI and survival in women diagnosed with endometrial cancer. Understanding the relationship between BMI and survival outcomes in women with endometrial cancer is extremely important as endometrial carcinoma is the most common gynecologic malignancy in the United States. Approximately 54,870 new endometrial cancer diagnoses are estimated in 2015 with 10,170 deaths expected from this disease [3]. There has been an alarming increase in endometrial cancer cases; incidence rates increased by 2.4% from 2007 to 2011 according to the latest report form the American Cancer Society Cancer Statistics [3]. The increase in the prevalence of obesity and endometrial cancer highlights the need to understand the effects of obesity on endometrial cancer outcomes and mortality.
There have been conflicting results in the literature regarding the association between BMI and survival in women with endometrial cancer. Some studies have demonstrated either improved survival (the obesity paradox) or no difference in survival between non-obese and obese endometrial cancer survivors. However, other studies have demonstrated a significant association between BMI and decreased survival. Calle and colleagues conducted a prospective study to evaluate the relationship between BMI and the risk of death from all cancers and reported that the endometrial cancer survivors with a BMI > 40 had a 6.25 fold increased relative risk (RR) of death compared to those who were of normal weight [4].
While a systematic review regarding survival outcomes and obesity in endometrial cancer has been published, a meta-analysis has not been performed [5]. Therefore, we performed a systematic review and meta-analysis, to evaluate the association between BMI and survival in women with endometrial cancer. Information gleaned from this analysis will help determine if BMI is associated with survival in endometrial cancer patients. In addition, our results, if positive, will inform clinical trials to evaluate BMI-reducing strategies aimed at improving survival in women with endometrial cancer.
2. Methods
2.1. Sources
This systematic review and meta-analysis was conducted in accord with guidelines for Meta-Analysis of Observational Studies in Epidemiology guidelines (http://edmgr.ovid.com/ong/accounts/moose.pdf). A systematic review was performed utilizing a Medline search using exploded Mesh keywords ‘endometrial neoplasms’ and (‘body mass index’ or ‘obesity’) and (‘survival analysis’ or ‘mortality’ or ‘survivor’ or ‘survival’). Furthermore, we obtained additional sources by manually reviewing references in papers and from American Society of Clinical Oncology (ASCO) Communications: Cancer in the News.
Inclusion and exclusion criteria were developed based on patient population, comparators, outcomes, and language criteria. Study inclusion criteria were as follows: study included women with endometrial cancer; study evaluated survival outcomes based on BMI, a surrogate for obesity; the study included a comparison group; the study reported a quantitative association between BMI and survival outcomes; the study was peer-reviewed and written in the English. There were no time limitations or exclusion based on study design. There also were no limitations regarding sample size, treatment type, or selection of controls. Study exclusion criteria were as follows: study results could not be interpreted in the context of hazard ratios (HR); or publication type is editorial, review, or letter to the editor.
Titles and abstracts of identified articles were reviewed by one reviewer (AAS) and independently confirmed by a second reviewer (LH, SM, VV, VBJ, PAG) for potential inclusion in the study. Articles included by either reviewer were subjected to full-text screening. All articles were independently reviewed by two investigators who determined if each article was included or excluded for data abstraction. One researcher (AAS) abstracted the data from all the studies, and the second reviewer (LH, SM, VV, VBJ, PAG) completed a second independent abstraction file. The abstraction files were merged and compared along-side the original article to assess for accuracy and completeness. Quality of individual studies was assessed using the approach described in Agency for Healthcare and Research Quality’s Methods Guide for Effectiveness and Comparative Effectiveness Reviews and Guyatt et al. [6,7] The quality of the individual studies were graded by 2 authors (AAS, LH) and summary quality ratings of high, moderate, low, and very low were assigned to each study [7].
The quantitative synthesis for survival outcomes was challenging based on heterogeneity of the studies, statistical design, reporting of results, and observational study designs. There was substantial heterogeneity in BMI (continuous or categorical variables); the type of BMI categories; and adjustment variables. Performing a meta-analysis on the effect of BMI on total mortality was challenging as individual studies reported the hazard ratios/odds ratios for different BMI intervals. Therefore, we assumed that the logarithm of each odds ratio could be described by a linear model. The model included a random effects term, σ2, as well as terms for BMI categories: less than 25, 25 to 29.9, 30 to 34.9, 35 to 39.9, and 40+. Independent variables were used to create the BMI category desired.
If every study used the same five intervals for BMI, then the analysis would be relatively straightforward. However, most studies used a subset of those intervals. For example, the Arem et al. study used the first three categories (<25 kg/m2, 25 to <30, 30 to <35), but grouped the remaining values into a single category, 35 + (35 to 39.9, and 40+) [8]. In order to use this study as reported it was necessary to estimate the fraction of the sample in each of the last two categories. The primary assumption was that BMI values are lognormally distributed. The maximum likelihood methods were used to estimate the parameters of the distribution based on the observed frequencies and intervals reported [9]. From this we estimated the fractions to be 0.154 and 0.077 for the 35 to 39.9, and the 40+ categories, respectively. Instead of using independent variables with all zeros except for a one for either the 35 to 39.9 category or for the 40+ categories, the normalized values of 0.667 and 0.333 for those variables were used.
Survival outcome measures varied between the studies and included progression-free survival (PFS), disease-free survival (DFS), overall survival (OS), all-cause mortality, relative risk of mortality, death, death-rate, recurrence frequency, recurrence risk, recurrence-free survival (RFS), and cancer-specific survival (CSS). Studies were required to report hazard ratios (HRs) or odds ratios (ORs) with 95% confidence intervals (CIs) or to provide adequate data to allow the 95% CI to be calculated. The primary analysis was based on all-cause mortality, because not all studies uniformly reported PFS, DFS, RFS, and/or CSS.
The general strategy for analysis was to construct a random-effects model that was comparable to the standard random-effects models used in the meta-analysis of odds ratios as described by Hasselblad [10]. We assumed that each odds ratio, ORij, could be described by the following model:
where i denoted the study, j denoted the specific time interval, and k was the number of BMI intervals used in the model. The αi were assumed to be random and normal with mean 0 and variance . SEij was the standard error of the jth odds ratio from the ith study. σ2 was the extra variation from the random effects model. The xij were the fixed terms that describe the BMI interval covered by that particular odds ratio. The βj (j = 1, …, k) were the odds ratios to be estimated for each BMI interval. A sensitivity analyses was conducted in which the meta-analyses was repeated excluding very low quality studies. The models were fitted using SAS PROC NLMIXED (SAS Institute Inc.; Cary, NC; 2009) with “subject” set to the particular study [11].
3. Results
In the literature search (Fig. 1), 1451 distinct citations were identified and reviewed in duplicate. After exclusions, 18 articles (8 moderate, 6 low quality, and 4 very low quality studies) remained that reported the association of BMI and endometrial cancer outcomes relevant to this study. The study set was comprised of 665,694 women with endometrial cancer. BMI was stratified into a variety of BMI categories (continuous or categorical variables) and the type of BMI categories varied substantially (Table 1).
Fig. 1.
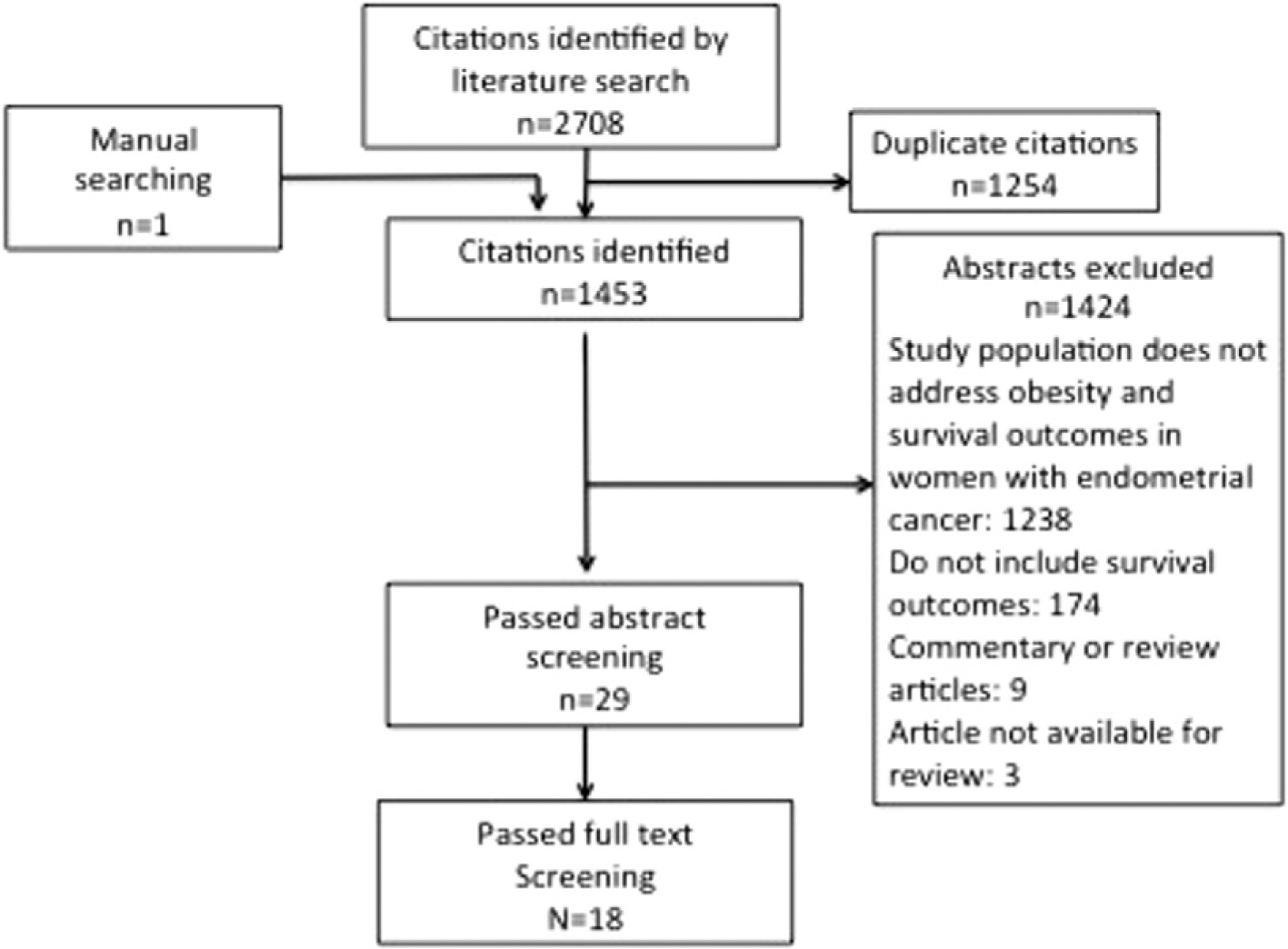
A systematic review was performed utilizing a Medline search using exploded Mesh keywords ‘endometrial neoplasms’ and (‘body mass index’ (n = 250) or ‘obesity’ (n = 272)) and (‘survival analysis’ (n = 1145) or ‘mortality’ (n = 986) or ‘survivor’ (n = 45) or ‘survival’ (n = 10)).
Table 1.
Study characteristics and association between body mass index and survival.
| Study | N | Referent BMI | Comparison BMI | Hazard ratio | Confidence interval | p-Value | Multivariate analysis | Median follow-up | Geographic setting |
| Akbayir 2012 | 3461 | <25 | 25–30 | 0.82 | 0.15–4.29 | 0.81 | Yes | 59.1 months | Turkey |
| >30 | 0.55 | 0.13–2.34 | 0.42 | ||||||
| 25–30 | 1.212 | 0.78–1.85 | |||||||
| Arem 2013 | 983 | <25 | 30–35 | 1.572 | 1.00–2.45 | 0.003 | Yes | 5.2 years | United States3 |
| >35 | 1.852 | 1.19–2.88 | |||||||
| 25–30 | 1.914 | 1.25–2.95 | 5.1 years | United States5 | |||||
| Arem 2013 | 1400 | 18.5 < 25 | 30–35 | 2.124 | 1.37–3.29 | <.001 | Yes | ||
| >35 | 2.784 | 1.78–4.34 | |||||||
| 22 | 1.73 | 0.71–4.26 | |||||||
| Bjorge 2010 | 287,320 | 20 | 24 | 1.45 | 0.59–3.57 | <.001 | Yes | 10 years | Norway, Austria, Sweden |
| 26 | 1.99 | 0.85–4.67 | |||||||
| 32 | 5.35 | 2.43–11.8 | |||||||
| 25–29.9 | 1.5 | 1.26–1.78 | |||||||
| Calle 2003 | 364,760 | 18.5 < 25 | 30–34.9 | 2.53 | 2.02–3.18 | <.001 | Yes | 16 years | United States6 |
| 35–39.9 | 2.77 | 1.83–4.18 | |||||||
| ≥40 | 6.25 | 3.75–10.4 | |||||||
| Chia 2007 | 745 | <25 | 25–29.9 | 1.2 | 0.8–2.0 | 0.05 | Yes | 9.3 years | United States7 |
| ≥30 | 1.6 | 1.0–2.5 | |||||||
| <18.5 | 1.018 | 0.13–7.76 | |||||||
| 25.0–29.9 | 1.008 | 0.56–1.79 | United Kingdom, South Africa, Poland, New Zealand | ||||||
| Crosbie 2012 | 1070 | 18.5–24.9 | 30.0–34.9 | 0.848 | 0.44–1.61 | 0.38 | Yes | 34.3 months | |
| 35.0–39.9 | 0.628 | 0.27–1.42 | |||||||
| ≥40 | 1.178 | 0.49–2.76 | |||||||
| Gates 2006 | 181 | <25 | >25 | 5.4 | 0.49–59.0 | 0.17 | Yes | Not given | United States9 |
| <18.5 | 3.42 | 0.75–15.6 | 0.17 | Yes | Not given | Korea | |||
| Jeong 2010 | 937 | <23 | 18.5 < 25 | 1.04 | 0.53–2.05 | ||||
| 23≤25 | 0.93 | 0.37–2.29 | 0.70 | ||||||
| ≥25 | 0.87 | 0.41–1.83 | |||||||
| Kodama 2005 | 242 | <25 | ≥25 | 0.425 | 0.14–1.32 | 0.07 | No | 54 months | Japan |
| Martra 2008 | 766 | ≥30 | <30 | 1.04 | 1.01–1.07 | <0.001 | Yes | 38 months | Italy, Ohio |
| Mauland 2011 | 1129 | ≥25 | <25 | 0.9310 | 0.68–1.2710 | 0.6510 | Yes | 4.9 years | Norway |
| 25.0–29.9 | 0.96 | 0.69–1.33 | 0.81 | ||||||
| Modesitt 2007 | 949 | <25 | 30.0–39.9 | 1.33 | 0.96–1.84 | 0.08 | Yes | 13.6 months | United States |
| ≥40 | 1.86 | 1.16–2.99 | 0.01 | ||||||
| Munstedt 2008 | 1180 | NA11 | NA11 | 0.98 | 0.95–1.00 | 0.09 | No | 6.2 years | Germany |
| Rabischong 2011 | 207 | <30 | ≥30 | 0.835 | .30–2.32 | 0.73 | No | 69–71 months | France |
| <22.5 | 0.81 | 0.57–1.17 | |||||||
| Reeves 2007 | 2657 | 22.5–24.9 | 25–27.4 | 1.09 | 0.82–1.45 | Not Given | Yes | 7 years | England, Scotland |
| 27.5–29.5 | 1.21 | 0.85–1.71 | |||||||
| ≥30 | 2.28 | 1.81–2.87 | |||||||
| Temkin 2007 | 442 | <25 | 25.0 ≤ 30 | 0.16 | 0.14–0.19 | 0.003 | Yes | Not given | United States12 |
| ≥30 | 0.16 | 0.14–0.18 | |||||||
| von Gruenigen 2006 | 380 | <30 | 30–39 | 1.47 | 0.81–2.67 | 0.21 | Yes | 65 months | United States5 |
| ≥40 | 2.76 | 1.20–6.33 | 0.02 |
The total number of patients in the study was 370, but only 346 had information regarding body mass index.
The authors also included an age-adjusted model. The hazard ratios (HR) and confidence intervals (CI) were 1.19 (0.78–1.83), 1.43(0.92–2.22), and 1.86 (1.20–2.88) for women with BMI 25–30, 30–35, and >35 compared to BMI < 25, respectively (p = 0.004).
Geographic settings included California, Florida, Pennsylvania, New York, North Carolina, Arizona, Massachusetts, Washington D.C., Ohio, and Iowa.
Data presented was determined using a multivariate model adjusting for grade, stage, chemotherapy; and surgery. The authors also included a second model adjusting for grade, stage, race, chemotherapy, surgery, smoking, diabetes, and family history of breast cancer. Using this model the hazard ratios (HR) and confidence intervals (CI) were 1.74 (1.13–2.66), 1.84(1.17–2.88), and 2.35 (1.48–3.73) for women with BMI 25–30, 30–35, and >35 compared to BMI < 25, respectively (p < 0.001).
Geographic settings included California, Florida, Pennsylvania, New Jersey, North Carolina, Louisiana, Georgia, Michigan, Texas, Arizona and Nevada.
Geographic settings within the United States, not further specified.
Geographic setting: Wisconsin.
The data represents survival outcomes for women with Type I endometrial cancer. The authors also included a separate analysis for women with Type II endometrial cancers. The hazard ratios (HR) and confidence intervals (CI) were 0.96 (0.46–2.02), 0.76 (0.31–1.87), and 0.88 (0.33–2.35) for women with BMI 25–29.9, 30–34.9, and 35.0–39.9 compared to BMI 8.5–24.9, respectively (p = 0.664).
Geographic setting: Georgia.
Only data from the adjusted analysis is listed.
BMI was only evaluated as a continuous variable.
Geographic setting: New York.
The full list of included articles is provided in Table 1. The quality of data and length of follow-up varied among the studies. Fifteen studies included a multivariate analysis. Eight of the included studies reported a statistically significant association between higher BMI and higher mortality among endometrial cancer survivors [4,8, 12–17], while 8 other studies showed no association between BMI and survival [18–25]. Two other studies demonstrated improved survival in women with higher BMIs [26,27]. The studies collectively cover a time period from 1974 to 2009 with follow-up times ranging from 1.6 to 16 years. The geographic setting of the studies included Austria, Germany; France, Italy, Japan, Korea, New Zealand, Norway, Poland, South Africa, Sweden, Turkey, the United Kingdom; and the United States with representation from Arizona, California, Florida, Georgia, Iowa, Louisiana, Massachusetts, Michigan, New Jersey, New York, Nevada, North Carolina, Ohio, Pennsylvania, Texas, Washington, Washington D.C., and Wisconsin, as well as states participating in Gynecologic Oncology Group (GOG) studies.
The odds ratios for each of the four BMI intervals relative to the baseline interval (less than 25) were estimated (Table 2). The odds ratio for all-cause mortality increased for each BMI category exhibiting a clear dose–response relationship even though none was assumed. Specifically the odds ratios were 1.01, 1.17, 1.26, and 1.66 for BMI categories of 25–29.9, 30–34.9, 35–39.9, and 40+, respectively. The odds ratio for all-cause mortality in endometrial cancer patients with a BMI ≥ 40 compared to those with a BMI < 25 was 1.66 (CI: 1.10–2.51, p = 0.02).
Table 2.
Estimated odds ratios of all-cause mortality by BMI category.
| BMI interval | Odds ratio | Lower CI limit | Upper CI limit | p-Value |
|---|---|---|---|---|
| 25–29.9 | 1.01 | 0.77 | 1.32 | 0.9 |
| 30–34.9 | 1.17 | 0.85 | 1.61 | 0.3 |
| 35–39.9 | 1.26 | 0.78 | 2.03 | 0.3 |
| 40+ | 1.66 | 1.10 | 2.51 | 0.02 |
| Extra VARIATION (σ) | 0.27 | NA | NA | <0.0001 |
A model that used the logarithm of the median of each BMI interval as the dose was calculated and fitted a single dose–response model (including a similar term for extra variation) (Table 3). Increasing BMI by 10% increased the odds of all-cause mortality by 9.2% (CI: 2.7–16.1, p = 0.007). The interval estimates model in Table 3 provided a comparable fit compared to the original model denoted in Table 2, but with two parameters instead of five. The interval estimates confirm that a linear logistic model provides a good description for the effect of BMI on all-cause mortality. The odds of all-cause mortality were significantly increased with increasing BMI (Fig. 2).
Table 3.
Estimated odds ratio of all-cause mortality per 10% Increase in BMI.
| Parameter | Estimate | Lower CI limit | Upper CI limit | p-Value |
|---|---|---|---|---|
| Per 10% increase in BMI | 1.092 | 1.027 | 1.161 | 0.007 |
| Extra variation (σ) | 0.265 | NA | NA | <0.0001 |
Fig. 2.
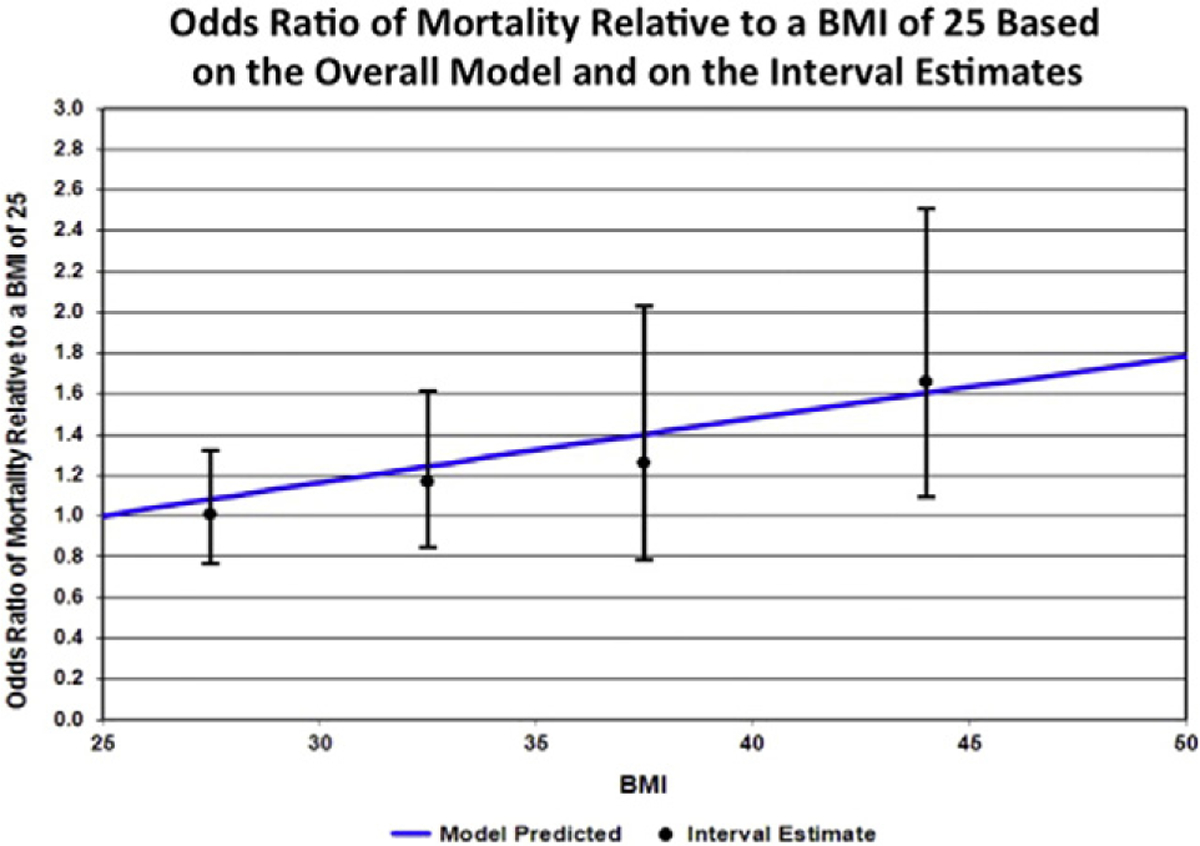
The figure depicts the odds ratio of all-cause mortality relative to a BMI of 25 based on the overall model and on the interval estimates. The odds of all-cause mortality were significantly increased with increasing BMI.
A sensitivity analysis was performed removing the 4 “very low” quality studies. Almost identical estimates and p-values were obtained. Furthermore, the estimate of the random variation term was not reduced in the sensitivity analysis.
4. Discussion
Our findings demonstrated a clear increased risk of all-cause mortality in obese endometrial cancer patients. The odds of all-cause mortality were significantly increased with increasing BMI and women at highest risk for death were those that suffered from class III obesity, or the morbidly or super morbidly obese defined as those with BMI ≥ 40 or ≥50, respectively. Others have also reported an association between obesity and increased risk of death in women with endometrial cancer [4,8, 12–17]. These studies either investigated clinical outcomes from incident endometrial cancers identified in large prospective cohort studies [4,8,12,13,15] and a registry study [14], or ancillary studies from GOG therapeutic clinical trials for women with endometrial cancer [16,17].
Arem and colleagues evaluated the association between obesity and clinical outcome in incident endometrial cancer cases identified in two large prospective cohort studies conducted in the United States; the Women’s Health Initiative (WHI) Observational Study and Clinical Trials, and the National Institutes of Health (NIH) — American Association of Retired Persons (AARP) Diet and Health Study [8,12]. In both studies, comprised of 2383 incident cases, they noted a significant increased risk of all-cause mortality (1.43–2.35 fold increase) and endometrial cancer-specific mortality (1.91–3.0 fold increase) in obese women [8,12]. In the prospective Cancer Prevention Study II, Calle et al. reported a 6.25 fold increase in the relative risk of death in obese women with a BMI ≥40.0 [4]. Similar findings have also been reported in European women [13,15]. Reeves evaluated cancer incidence and mortality in the Million Women Study conducted in the United Kingdom that enrolled 1.2 million women, aged 50–64, during 1996–2001. Endometrial cancer survivors with a BMI ≥ 30 had a 2.28 (CI 1.81 to 2.87) relative risk of mortality compared to women with a BMI < 22.5 [15].
While these large prospective American and European cohort studies provided important insights into the association between BMI and survival they were limited with regard to information about clinical demographics, as well as variable therapeutic management. The GOG studies provide valuable clinical information in cohorts of women with early and advanced stage disease as well as recurrent endometrial cancer who were treated in a uniform manner on GOG clinical trials [16, 17]. In women with early stage disease enrolled on GOG99, a randomized controlled trial of surgery with or without adjuvant radiation therapy, a 2.77 fold increased risk of death was found in women with a BMI ≥ 40 compared to those with a BMI < 30 (CI, 1.21–6.36; p = .016) [16]. Increased BMI was also significantly associated with an increased risk of death in women with advanced endometrial cancer (stage III and IV) with a BMI ≥ 40 compared to those with a BMI < 25 (HR = 1.86; CI 1.16–2.99; p = 0.01) [17]. One hypothesis for the worse outcomes noted in morbidly obese women was the common practice of capping chemotherapy doses at a body surface area of 2 m2, which was specified in at least two of the protocols included in the ancillary analysis. Capping chemotherapy doses may result in inferior survival outcomes and currently this practice has been discouraged [28]. An alternative theory is that radiation therapy may be compromised in obese patients. Pichon and colleagues reported that obesity affects the delivery of radiotherapy for cancer patients due to physical, technical, and dosimetric constraints [29]. Lin et al. reported that obese endometrial cancer patients were more likely to have higher frequencies of daily shifts and systematic errors, and greater planning target margin requirements [30]. Fortunately, for obese patients the use of image guided radiotherapy reduced setup error and resultant margin requirements. In GOG 99, BMI was associated with greater cutaneous toxicity, but less gastrointestinal toxicity in endometrial cancer patients who received adjuvant whole pelvic radiation therapy [16]. There was no interaction between BMI and treatment arm in their multivariate analysis on recurrence-free interval or overall survival indicating that the prognostic significance of BMI was consistent in the two treatment arms [16]. Technical difficulties are probably not relevant for the delivery of vaginal brachytherapy and the affect of BMI on vaginal brachytherapy is most likely minimal. Overall, there is a paucity of information regarding radiation oncology specific outcomes in obese women with endometrial cancer.
Others have reported that obesity was associated with improved outcomes [26,27]. However, after adjusting for confounding factors in multivariate analyses the associations between BMI and survival were no longer significant [26,27]. For example, Temkin et al. conducted a multi-institutional retrospective study and reported increased BMI was associated with improved overall survival (117 vs 85 months for women with BMI ≥ 30 compared to BMI < 25; p = 0.003). However, after adjusting for BMI, age, race, grade, stage, and chemotherapy in the multivariate analyses, BMI was no longer associated with survival(p = 0.147). The authors also conducted a propensity score analysis that suggested that the association between BMI and survival is most likely attributed to the fact that obesity is often associated with younger age, lower grade, decreased stage, and less postoperative chemotherapy, all of which are associated with a favorable prognosis [27]. These findings may account for the obesity paradox phenomenon that has been noted in a variety of cancers as well as medical conditions, wherein obesity is a major risk factor for the disease, but obese patients have a survival benefit [31].
Conversely, several studies showed no association between obesity and survival but may have been limited by sample size [18–25]. All of the studies that revealed an association between increased BMI and increased risk of mortality included a multivariate analysis [4,8,12–17]. Many of the studies that demonstrated no association between obesity and survival also included a multivariate analysis [18–21,23,24,26,27], but two studies did not include BMI as a factor in their multivariate models [22,25]. We hypothesize that the conflicting results between the various studies are multifactorial and may be due to the different types of study design, lack of power, BMI classification and/or statistical analyses.
Comparable increased all-cause mortality has also been reported in the general population with higher BMI who do not have cancer [32–34]. Barrington de Gonzalez et al. pooled data from 19 prospective studies encompassing 1.46 million adults, and reported an estimated HR for all-cause mortality of 1.99 (CI, 1.90–2.09) in patients with a BMI 40–49.4 compared to 22.5–24.9 [32]. The HR for mortality was highest in women who were healthy never smokers. Similarly, Adams and colleagues reported that the relative risk of death in women with a BMI ≥ 40 was 1.94 (CI, 1.79–2.09) compared to women with a BMI 23.5–24.9. The relative risk of death did decrease after accounting for pre-existing chronic disease (HR 1.29: CI, 1.15–1.45), current smoking (HR 1.61: CI, 1.33–1.93), and age greater than 60 (HR 1.89: CI, 1.65–2.16) [33]. The lowest risk was reported by Flegal et al. with hazard ratios for all-cause mortality in women with BMI ≥ 35 of 1.26 (CI, 0.96–1.64) in all age groups, and 1.63 (CI, 0.77–3.46) for those ≥65 years old [34]. The increased all-cause mortality noted in our study and others in most likely multifactorial and may be related to obesity-related comorbidities; treatment related issues such as chemotherapy dose capping and optimal delivery of radiation; as well as underlying obesity-related carcinogenesis and molecular aberrations that may contribute to worse outcomes.
There is a need to explore causes of death in obese endometrial cancer patients in order to implement strategies to improve survival outcomes. Robbins and colleagues reported that over 80% of the deaths in women with stage I-II endometrial cancer were secondary to causes other than endometrial cancer [35]. Furthermore, women with higher Age-Adjusted Charlson Comorbidity index scores were at higher risk for death, but not disease-specific mortality, or recurrence [35]. Cardiovascular disease has been reported to be the leading cause of death among endometrial cancer survivors [36]. Illuminating data from the NIH-AARP study revealed that women with BMI ≥ 35 had a statistically significant 4.77 fold increased risk of cardiovascular related mortality 10 years after their endometrial cancer diagnosis (CI = 1.88 to 12.10; p < 0.001) [12]. A new field of medicine known as Cardio-oncology has been developed to explore and address cancer-related cardiovascular side effects and improve cardiovascular outcomes of cancer survivors. Clinical trials evaluating weight loss and physical activity interventions in colon and breast cancer survivors have demonstrated favorable changes in intermediary markers such as insulin, adipokines, sex hormones, and inflammatory biomarkers which may be surrogates for survival [37,38]. It is yet to be determined if cardiovascular risk in endometrial cancer patients can be reduced through weight loss strategies including dietary modifications and physical activity [39]. In addition, obesity increases the risk of a variety of other cancers (particularly breast and colon), as well as type 2 diabetes, hypertension, and respiratory disease that can contribute to increased mortality [40].
Furthermore, obesity may lead to chronic inflammation; aberrant adipokine signaling and insulin signaling; lipid deregulation; and increased tumor angiogenesis that may contribute to endometrial carcinogenesis and disease progression [41]. However, the molecular mechanisms underlying the increased obesity-related risk of endometrial cancer as well as disease progression have not been completely elucidated. Berg et al. reported an association between increased KRAS and immunological activation in complex atypical hyperplastic tissue specimens from obese women compared to non-obese women [42]. Several authors have shown that aberrations in the PTEN pathway may impact cancer prognosis in the context of obesity [43,44]. Dellas and colleagues found that obese women with endometrioid endometrial carcinoma were more likely to have tumors that demonstrated combined loss of PTEN/p27kip1 protein expression (51%, BMI ≥ 30; 30%, BMI 25–29.9; and 23%, BMI < 25; p < 0.005). Moreover PTEN/p27kip1 protein expression was associated with significantly better prognosis in obese patients, but not in non-obese patients (p = 0.01) [43]. These data imply that obesity may promote carcinogenesis and tumor progression via differential modulation of gene pathways.
The strengths of our study are the comprehensive evaluation of the literature, meticulous data collection, and detailed statistical analysis. However, there are limitations to our study design. Specifically, there was a great deal of heterogeneity in the various studies as measured by the random variation which limited our ability to assess for publication bias and to develop Forest plots. The heterogeneity is to be expected because of the use of different designs, the variation in the factors used for adjustment, and the populations being studied. The variation associated with our BMI estimates is discounted, because the BMI distributions are closely approximated by a lognormal distribution, that produces estimates of the median value that are much closer than any of the standard estimates. In any event, the random variation added to the independent variable reduces the estimated effect, and so our estimates of the true effect are probably conservative.
Our stringent systematic review of the literature and meta-analysis indicate that obesity is associated with an increased risk for all-cause mortality. Endometrial cancer survivors with higher BMI have worse overall survival compared to non-obese women. Strikingly, those who suffer from class III obesity are at the highest risk for mortality. Further evaluation is needed to identify causes of death in endometrial cancer patients in order to understand if obesity is the causative factor and institute preventive methods.
HIGHLIGHTS.
Increased BMI is associated with increased all-cause mortality in women with endometrial cancer.
Women with a BMI ≥ 40 have the highest risk of death.
Footnotes
Conflict of interest statement
The authors declare that there are no conflicts of interest.
References
- [1].Kaaks R, Lukanova A, Kurzer MS, Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review, Cancer Epidemiol. Biomark. Prev 11 (12) (2002) 1531–1543. [PubMed] [Google Scholar]
- [2].Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M, Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies, Lancet 371 (9612) (2008) 569–578. [DOI] [PubMed] [Google Scholar]
- [3].American Cancer Society, Cancer Facts & Figures 2015, American Cancer Society, Atlanta, 2015. [Google Scholar]
- [4].Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ, Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults, N. Engl. J. Med 348 (17) (2003) 1625–1638. [DOI] [PubMed] [Google Scholar]
- [5].Arem H, Irwin ML, Obesity and endometrial cancer survival: a systematic review, Int. J. Obes 37 (5) (2013) 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Methods guide for effectiveness and comparative effectiveness reviews, AHRQ Publication No. 10 (14)-EHC063-EF, Agency for Healthcare Research and Quality, Rockville, MD, January 2014 (Chapters available at: www.effectivehealthcare.ahrq.gov. [cited 2015 June 16, 2015]). [PubMed] [Google Scholar]
- [7].Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck-Ytter Y, Alonso-Coello P, et al. , GRADE: an emerging consensus on rating quality of evidence and strength of recommendations, BMJ 336 (7650) (2008) 924–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Arem H, Chlebowski R, Stefanick ML, Anderson G, Wactawski-Wende J, Sims S, et al. , Body mass index, physical activity, and survival after endometrial cancer diagnosis: results from the Women’s Health Initiative, Gynecol. Oncol 128 (2) (2013) 181–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Hasselblad V, Stead AG, Galke W, Analysis of coarsely grouped data from the lognormal distribution, J. Am. Stat. Assoc 75 (372) (1980) 771–778. [Google Scholar]
- [10].Hasselblad V, Meta-analysis of multitreatment studies, Med. Decis. Mak 18 (1) (1998) 37–43. [DOI] [PubMed] [Google Scholar]
- [11].Inc. SI, SAS/STAT ® 9.2 User’s Guide, 2009. (Cary, NC: ). [Google Scholar]
- [12].Arem H, Park Y, Pelser C, Ballard-Barbash R, Irwin ML, Hollenbeck A, et al. , Prediagnosis body mass index, physical activity, and mortality in endometrial cancer patients, J. Natl. Cancer Inst 105 (5) (2013) 342–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Bjorge T, Stocks T, Lukanova A, Tretli S, Selmer R, Manjer J, et al. , Metabolic syndrome and endometrial carcinoma, Am. J. Epidemiol 171 (8) (2010) 892–902. [DOI] [PubMed] [Google Scholar]
- [14].Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM, Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis, Int. J. Gynecol. Cancer 17 (2) (2007) 441–446. [DOI] [PubMed] [Google Scholar]
- [15].Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D, et al. , Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study, BMJ 335 (7630) (2007) 1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Von Gruenigen VE, Tian C, Frasure H, Waggoner S, Keys H, Barakat RR, Treatment effects, disease recurrence, and survival in obese women with early endometrial carcinoma: a Gynecologic Oncology Group study, Cancer 107 (12) (2006) 2786–2791. [DOI] [PubMed] [Google Scholar]
- [17].Modesitt SC, Tian C, Kryscio R, Thigpen JT, Randall ME, Gallion HH, et al. , Impact of body mass index on treatment outcomes in endometrial cancer patients receiving doxorubicin and cisplatin: a Gynecologic Oncology Group study, Gynecol. Oncol 105 (1) (2007) 59–65. [DOI] [PubMed] [Google Scholar]
- [18].Akbayir O, Corbacioglu Esmer A, Numanoglu C, Cilesiz Goksedef BP, Akca A, Bakir LV, et al. , Influence of body mass index on clinicopathologic features, surgical morbidity and outcome in patients with endometrial cancer, Arch. Gynecol. Obstet 286 (5) (2012) 1269–1276. [DOI] [PubMed] [Google Scholar]
- [19].Crosbie EJ, Roberts C, Qian W, Swart AM, Kitchener HC, Renehan AG, Body mass index does not influence post-treatment survival in early stage endometrial cancer: results from the MRC ASTEC trial, Eur. J. Cancer 48 (6) (2012) 853–864. [DOI] [PubMed] [Google Scholar]
- [20].Gates EJ, Hirschfield L, Matthews RP, Yap OW, Body mass index as a prognostic factor in endometrioid adenocarcinoma of the endometrium, J. Natl. Med. Assoc 98 (11) (2006) 1814–1822. [PMC free article] [PubMed] [Google Scholar]
- [21].Jeong NH, Lee JM, Lee JK, Kim JW, Cho CH, Kim SM, et al. , Role of body mass index as a risk and prognostic factor of endometrioid uterine cancer in Korean women, Gynecol. Oncol 118 (1) (2010) 24–28. [DOI] [PubMed] [Google Scholar]
- [22].Kodama J, Seki N, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y, Correlation of presenting symptoms and patient characteristics with endometrial cancer prognosis in Japanese women, Int. J. Gynaecol. Obstet 91 (2) (2005) 151–156. [DOI] [PubMed] [Google Scholar]
- [23].Martra F, Kunos C, Gibbons H, Zola P, Galletto L, DeBernardo R, et al. , Adjuvant treatment and survival in obese women with endometrial cancer: an international collaborative study, Am. J. Obstet. Gynecol 198 (1) (2008) 89 (e1–8). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Mauland KK, Trovik J, Wik E, Raeder MB, Njolstad TS, Stefansson IM, et al. , High BMI is significantly associated with positive progesterone receptor status and clinico-pathological markers for non-aggressive disease in endometrial cancer, Br. J. Cancer 104 (6) (2011) 921–926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Rabischong B, Larrain D, Canis M, Le Bouedec G, Pomel C, Jardon K, et al. , Long-term follow-up after laparoscopic management of endometrial cancer in the obese: a fifteen-year cohort study, J. Minim. Invasive Gynecol 18 (5) (2011) 589–596. [DOI] [PubMed] [Google Scholar]
- [26].Munstedt K, Wagner M, Kullmer U, Hackethal A, Franke FE, Influence of body mass index on prognosis in gynecological malignancies, Cancer Causes Control 19 (9) (2008) 909–916. [DOI] [PubMed] [Google Scholar]
- [27].Temkin SM, Pezzullo JC, Hellmann M, Lee YC, Abulafia O, Is body mass index an independent risk factor of survival among patients with endometrial cancer? Am. J. Clin. Oncol 30 (1) (2007) 8–14. [DOI] [PubMed] [Google Scholar]
- [28].Griggs JJ, Mangu PB, Anderson H, Balaban EP, Dignam JJ, Hryniuk WM, et al. , Appropriate chemotherapy dosing for obese adult patients with cancer: American Society of Clinical Oncology clinical practice guideline, J. Clin. Oncol 30 (13) (2012) 1553–1561. [DOI] [PubMed] [Google Scholar]
- [29].Pichon B, Thureau S, Delpon G, Barillot I, Mahé MA, Obesity and radiation: technical difficulties, toxicity and efficacy, Cancer Radiother. 17 (5–6) (2013) 543–548. [DOI] [PubMed] [Google Scholar]
- [30].Lin LL, Hertan L, Rengan R, B-KK T, Effect of body mass index on magnitude of setup errors in patients treated with adjuvant radiotherapy for endometrial cancer with daily image guidance, Int. J. Radiat. Oncol. Biol. Phys 83 (2012) 670–675. [DOI] [PubMed] [Google Scholar]
- [31].Amundson DE, Djurkovic S, Matwiyoff GN, The obesity paradox, Crit. Care Clin 26 (4) (2010) 583–596. [DOI] [PubMed] [Google Scholar]
- [32].Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, RJ MI, et al. , Body-mass index and mortality among 1.46 million white adults, N. Engl. J. Med 363 (23) (2010) 2211–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Adams KF, Schatzkin A, Harris TB, Kipnis V, Mouw T, Ballard-Barbash R, Hollenbeck A, Leitzmann MF, Overweight, obesity, and mortality in a large prospective cohort of persons 50 to 71 years old, N. Engl. J. Med 355 (8) (2006) 763–778. [DOI] [PubMed] [Google Scholar]
- [34].Flegal KM, Kit BK, Orpana H, Graubard BI, Association of mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis, JAMA 309 (1) (2013) 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Robbins JR, Gayar OH, Zaki M, Mahan M, Buekers T, Elshaikh MA, Impact of age-adjusted Charlson comorbidity score on outcomes for patients with early-stage endometrial cancer, Gynecol. Oncol 131 (3) (2013) 593–597. [DOI] [PubMed] [Google Scholar]
- [36].Ward KK, Shah NR, Saenz CC, McHale MT, Alvarez EA, Plaxe SC, Cardiovascular disease is the leading cause of death among endometrial cancer patients, Gynecol. Oncol 126 (2) (2012) 176–179. [DOI] [PubMed] [Google Scholar]
- [37].Fader AN, Arriba LN, Frasure HE, von Gruenigen VE, Endometrial cancer and obesity: epidemiology, biomarkers, prevention and survivorship, Gynecol. Oncol 114 (1) (2009) 121–127. [DOI] [PubMed] [Google Scholar]
- [38].Irwin ML, Mayne ST, Impact of nutrition and exercise on cancer survival, Cancer J. 14 (6) (2008) 435–441. [DOI] [PubMed] [Google Scholar]
- [39].McCarroll ML, Armbruster S, Frasure HE, Gothard MD, Gil KM, Kavanagh MB, et al. , Self-efficacy, quality of life, and weight loss in overweight/obese endometrial cancer survivors (SUCCEED): a randomized controlled trial, Gynecol. Oncol 132 (2) (2014) 397–402. [DOI] [PubMed] [Google Scholar]
- [40].Folsom AR, Kaye SA, Potter JD, Prineas RJ, Association of incident carcinoma of the endometrium with body weight and fat distribution in older women: early findings of the Iowa Women’s Health Study, Cancer Res. 49 (23) (1989) 6828–6831. [PubMed] [Google Scholar]
- [41].Khandekar MJ, Cohen P, Spiegelman BM, Molecular mechanisms of cancer development in obesity, Nat. Rev. Cancer 11 (12) (2011) 886–895. [DOI] [PubMed] [Google Scholar]
- [42].Berg A, Hoivik EA, Mjos S, Holst F, Werner HM, Tangen IL, et al. , Molecular profiling of endometrial carcinoma precursor, primary and metastatic lesions suggests different targets for treatment in obese compared to non-obese patients, Oncotarget 6 (2) (Ja20 2015) 1327–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Dellas A, Jundt G, Sartorius G, Schneider M, Moch H, Combined PTEN and p27kip1 protein expression patterns are associated with obesity and prognosis in endometrial carcinomas, Clin. Cancer Res 15 (7) (2009) 2456–2462. [DOI] [PubMed] [Google Scholar]
- [44].Westin SN, Ju Z, Broaddus RR, Krakstad C, Li J, Pal N, et al. , PTEN loss is a context-dependent outcome determinant in obese and non-obese endometrioid endometrialcancer patients, Mol. Oncol 9 (8) (2015) 1694–1703. [DOI] [PMC free article] [PubMed] [Google Scholar]


