Abstract
Background:
Adolescence is characterized by affective and cognitive changes that increase vulnerability to depression, especially in females. Neurodevelopmental models attribute adolescent depression to abnormal responses in amygdala, striatum, and prefrontal cortex (PFC). We examined whether the strength of functional brain networks involving these regions predict depression symptoms in adolescent females.
Methods:
In this longitudinal study, we recorded resting-state functional connectivity (RSFC) in 174 adolescent females. Using a cross-validation strategy, we related RSFC profiles that included 1) a network consisting of amygdala, striatum and PFC (within-circuit model), 2) connectivity of this network to the whole brain (extended-circuit model), and 3) a network consisting of the entire brain (whole-brain model) to depression symptoms assessed concurrently and 18 months later.
Results:
In testing subsets, the within-circuit RSFC profiles were associated with depression symptoms concurrently and 18 months later, while the extended-circuit and whole-brain model did not explain any additional variance in depression symptoms. Connectivity related to anterior cingulate and ventromedial prefrontal cortex contributed most to the association.
Conclusions:
Our results demonstrate that RSFC based brain networks that include amygdala, striatum and PFC are stable neural signatures of concurrent and future depression symptoms, representing a significant step towards identifying the neural mechanism of depression in adolescence.
Keywords: Depression, fMRI, neural network, adolescence
Introduction
Adolescence is a period marked by dynamic reorganization in functional neural networks implementing cognitive and affective functions (Galvan, 2017). Adolescence is also a period marked by a sharp increase in incidence of depression (Birmaher et al., 2007), especially in females (Salk et al., 2017). However, little is known regarding the neural circuitry associated with depression in adolescence. Here, we test whether the strength of functional connectivity in neural networks that include interconnections between 1) the amygdala, striatum and prefrontal cortex (PFC), 2) the amygdala, striatum, PFC and rest of the brain, and 3) the whole-brain can account for depression symptom in adolescents concurrently and in the future.
Impairments in affective and cognitive functioning are among the core symptoms of depression. In line with this, depression in adolescence is associated with alterations in the functioning of the 1) amygdala, implicated in emotional evaluation; 2) striatum, implicated in reward-processing and 3) PFC, implicated in the regulation of the above processes (Casey, 2015). However, affective and reward processing, and their control, do not map one-on-one onto specific regions, but are computed via rich interconnections within and across the affective, reward and control systems, as well as via their interactions with the whole brain (Pessoa, 2008). For example, decreased ability to use distal and complex rewards to guide behavior may be mediated via reduced recruitment of frontostriatal circuitry (Heller et al., 2013) and less regulation of emotional reactivity may be mediated via impairment in frontoamygdalar circuitry (Banks et al., 2007), potentially resulting in greater depression symptoms in adolescence. Not only are amygdala and PFC highly interconnected with each other, but they are also highly connected with the rest of the brain and considered “hub” regions (Pessoa, 2008).
Furthermore, adolescence is characterized not only by alterations in the functioning of amygdala, striatum and PFC, but also by critical changes in the structural and functional connectivity between these regions (Paus, 2005). Animal and human studies show that structural (Achterberg et al., 2016, Cunningham et al., 2002) and functional (Fareri et al., 2015, Gee et al., 2013, Jalbrzikowski et al., 2017) connectivity of amygdala and striatum with PFC are changing during adolescence. The alteration of amygdalar, striatal and prefrontal connections coinciding with the sharp increase in depression in adolescence strongly suggests that examining whether these networks contribute to depression symptoms in community dwelling adolescents may significantly advance our understanding of the neuropathology of adolescent depression.
Based on theoretical neurobiological models of emotional, reward, and cognitive processing in adolescence (Casey, 2015), we selected functionally defined nodes corresponding to amygdala, striatum and PFC (within-circuit model) from a larger 268-node functional brain atlas (Finn et al., 2015). Since the connectivity of hub regions such as amygdala and PFC may be established earlier in life while the development of long-range connections between hubs and non-hubs continues through adolescence (Di Martino et al., 2014), in another model we examined network connectivity not only within the network consisting of amygdala, striatum and the PFC, but also of this network to the rest of the brain (extended-circuit model), as well as a further expanded whole-brain model. Specifically, we were interested in examining whether including additional information from the extended and whole brain network accounted for additional variance in adolescent depression symptoms over and above the within-circuit model. We tested these models in a large (N=174) community dwelling sample of adolescent females using a connectome-based predictive modeling (CPM) approach (Shen et al., 2017, Finn et al., 2015) that has been utilized to predict personality traits and a wide range of psychological functions such as intelligence, sustained attention, and creative ability in normal function and disorders such as attention deficit hyperactivity disorder and anxiety (Hsu et al., 2018, Feng et al., 2018, Beaty et al., 2018, Rosenberg et al., 2016). In the present study, we used cross-validation approaches, deriving the neural network model in training subsets of the sample and then directly examining the performance of the model in predicting depression symptoms in the remaining (testing) subsets. By keeping the training and testing subsets independent, the cross-validation approach directly tested how well the models performed in new data, thereby facilitating future generalizability of neural network findings and their eventual development as neuroimaging-based markers of adolescent depression symptoms with real-world applicability.
Materials and Methods
Participants
The current study recruited 261 adolescent females (mean age = 15 years, 3 months, SD = 7 months) from the larger Adolescent Development of Emotions and Personality Traits (ADEPT) project. Participants and their parents gave informed assent and consent respectively, and all families were financially compensated for participation. The parent ADEPT project is a longitudinal project designed to examine personality traits and first onset of depression, approved by the Institutional Review Board at Stony Brook University. The parent ADEPT cohort consisted of 550 adolescent females (13.0 to 15.5 years of age at baseline assessment, mean age = 14 years, 5 months; SD = 7 months) and one of their biological parents as informants (93.1% mothers) that were recruited from the community. The cohort consisted of 80.5% Caucasian, 5.1% African-American, 8.4% Latino, 2.5% Asian, 0.4% Native American, and 3.1% “Others”. Inclusion criteria included fluency in English, capable of reading and comprehending questionnaires, and consent for participation from a biological parent. For additional description of recruitment procedures please refer to previous publications (Nelson et al., 2015, Speed et al., 2015).
At the baseline assessment (wave1), adolescents with lifetime history of major depressive disorder or dysthymia were excluded from further follow-up assessment waves. After completing the baseline assessment (wave1), participants were invited for follow-up clinical assessments for 3 years, and resting state fMRI (RSFC) data were acquired at the first follow-up (9 months after the baseline assessment). With the final assessment still under collection, the current study was based on assessment concurrent to and 18 months post-fMRI acquisition. The fMRI sample (N = 261) consisted of all participants who were willing to participate and met basic eligibility criteria for MRI (e.g., without metal implants, braces, claustrophobia, etc.). Difference in severity of depression symptoms between the fMRI subsample and participants who were not scanned were minimal suggesting little if any bias (Jin et al., 2017).
A total of 87 participants were excluded due to technical problems during data acquisition and incomplete fMRI data (N = 21), low fMRI image quality during initial quality control (N = 7), data processing errors due to crashed data (N = 2), subjects falling asleep (N=2). We also applied a stringent control of motion (see below) resulting in further exclusion of 55 participants. A final set of 174 participants were included for further analyses. Among these participants, depression symptom measures were completed for 173 at time of fMRI and 165 participants 18 months later.
Clinical measures
Mood and anxiety symptoms in adolescents were measured using the Inventory of Depressive and Anxiety Symptoms (IDAS-II), a new-generation factor-analytically derived measure of these symptoms (Watson et al., 2012). The IDAS-II measures individual symptoms of depression and anxiety, and also includes a general depression scale that converges strongly with commonly used measures of depression, such as Beck Depression Inventory (Beck et al., 1996). IDAS-II scales have demonstrated high internal consistency across multiple samples, with most showing coefficient alphas above .80 in adolescents (Watson et al., 2007) and other populations (Watson et al., 2012). Scales were computed as mean item response on a scale of 1–5 if at least 85% of items were endorsed.
Experimental design and statistical analyses
Image acquisition.
Structural and functional data were acquired using a Siemens Trio 3 T scanner with a 12-channel head coil at Stony Brook University SCAN Center. Resting-state data were acquired using a 6.02-min, T2-weighted echoplanar imaging (EPI) sequence (37 oblique-axial slices, in-plane resolution = 2.3 mm × 2.3 mm, slice thickness = 3.5 mm, field of view (FOV) = 224 mm x 224 mm, matrix = 96 × 96, TR = 2100 ms, TE = 23 ms, flip angle = 83°) while participants were asked to lay still with their eyes open to a fixation on the screen. A 5 to 6 min long acquisition sequence has been used to detect functional correlations between regions at rest in children and adults (Van Dijk et al., 2010, Braun et al., 2012, Thomason et al., 2011) and in psychiatric populations (Baker et al., 2019). Anatomical images were acquired using a T1-weighted MPRAGE sequence (in-plane resolution: 1 mm × 1 mm, FOV = 256 mm x 256 mm, with 176 slices). To ensure consistency among participants, brain images were aligned to the anterior-posterior commissure plane.
Image preprocessing.
General preprocessing.
Resting-state EPI data were preprocessed using SPM8 (http://www.fil.ion.ucl.ac.uk/spm/software/spm8/) and custom scripts in Matlab (https://www.mathworks.com/products/matlab.html) following standard procedures. After discarding the first 6 volumes for spin saturation, EPI data were slice-time corrected, realigned to the first volume, and coregistered to the MPRAGE. The structural MPRAGE data was used to derive normalization parameters by mapping onto T1 template in the Montreal Neurological Institute (MNI) space. The EPI data then underwent mode 1000 normalization (Power et al., 2012). The MPRAGE data were segmented into grey matter (GM), white matter (WM) and cerebrospinal fluid (CSF), and were normalized to MNI space by applying the transformation matrices derived above. Erosion using the erode3d function from the NeuroElf (http://neuroelf.net) was then applied to WM and CSF images to decrease the potential overlapping signal with GM (Power et al., 2017). Finally, timeseries data averaged across all voxels within each node was extracted from the GM mask. Following a previously established preprocessing pipeline, no further spatial smoothing was applied given that the timeseries was already averaged across voxels within each node (Finn et al., 2015).
Functional connectivity specific preprocessing.
Further processing included rigorous motion correction as well as bandpass filtering optimized for RSFC analyses. For motion control (Power et al., 2012), framewise displacement (FD) was calculated using the 6 rigid body motion parameter estimates, and volumes exceeding an FD values of 0.5 mm were censored from run timeseries. To balance the data quality and quantity in our study, the FD cutoff was chosen based on previous work using relatively motion-free participants showing that this value would flag high motion volumes above the norm (Power et al., 2012). Additionally, DVARS (D = temporal derivative of time courses, VARS = RMS variance over voxels) was computed and the cutoff for censoring was set as two standard deviations above the median across all participants. A volume was flagged if either the FD or the DVARS criteria was met. Variances that could be attributed to censored volumes were regressed out via regressors of no interest that contained binary coding [0s and 1] with a 1 assigned to the to-be-removed volume. If there were k flagged volumes, k orthogonal regressors were then included in the nuisance regressor matrix. The participant was excluded from further analyses, if less than 71 volumes were artifact-free. Results using a higher cutoff threshold of 99 volumes (~3.5 min) were reported in Appendix S1 in the Supporting Information. About 24% of participants were excluded from further analyses due to lack of sufficient artifact-free volumes. In addition to censored volume regressors, the nuisance regressors also consisted of 1) 24 motion regressors including the 6 rigid body motion parameter estimates and their first derivatives, and the square of these 12 linear parameter estimates, and 2) 3 nuisance signal regressors including mean signal averaged over CSF, mean signal averaged over WM, and mean signal averaged over the whole brain (Baker et al., 2014, Baker et al., 2019). Finally, for each subject, a temporal band filter of 0.009 HZ < f < 0.08 HZ was applied using butterworth filter (butter function in Matlab) to demeaned and detrended EPI data, as well as the 27 nuisance regressors.
In addition to using well-established preprocessing methods to minimize motion-related confounds, we examined whether frame-by-frame motion is correlated with depression symptoms.
Adolescent depression network models.
The details regarding selection of nodes (i.e., anatomical regions), network models and cross validation analyses are outlined in Appendix S2. In short, network nodes in the present study were a subset of nodes derived from a larger atlas that was based on RSFC of 45 healthy participants (Finn et al., 2015) (Figure 1–A). RSFC was measured as the Pearson’s correlation coefficient between time series extracted from all possible pairs of the 217 nodes from this atlas. The adolescent depression-circuit consisted of a subset of 40 nodes corresponding to dorsolateral prefrontal cortex (dlPFC), ventromedial prefrontal cortex (vmPFC), anterior cingulate cortex (ACC), amygdala (AMY), and striatum (STR) (Figure 1–B). See Table S1 for the complete list of the nodes. Three anatomical models, within-circuit, extended-circuit, and whole-brain (Figure 2) were constructed to investigate if the RSFC edges (the connectivity between two nodes) within the adolescent depression-circuit were associated with depressive severity concurrently and 18 months later. In order to control for the effect of developmental stage as well as the effect of movement, we obtained the residuals of each edge after regressing out age and number of artifact-free volumes across all participants. The residuals of the depression symptom severity measures were computed in the same way. We then tested the association between each of the network models with adolescent depression symptoms, using the well-established CPM method (Shen et al., 2017) that has been used to predict various individual difference measures (Hsu et al., 2018, Feng et al., 2018, Beaty et al., 2018, Rosenberg et al., 2016). Statistical significance was evaluated using a 10-fold-cross-validation (10fCV) approach (see Appendix S2 for details).
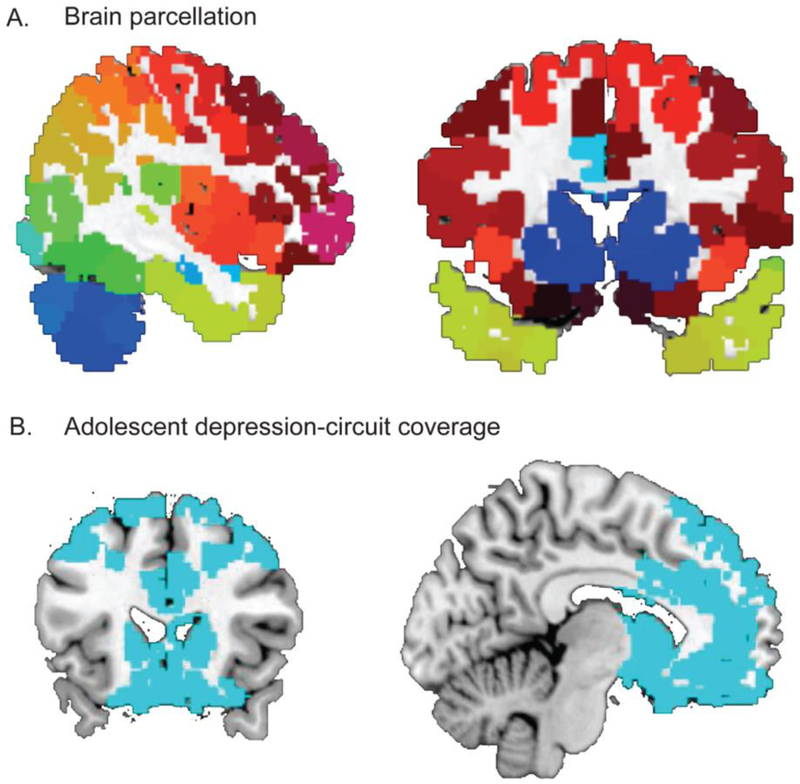
Figure 1. Nodal definition and circuit coverage.
A. Nodal definition. Whole-brain nodes based on a resting-state functional connectivity derived (Shen et al., 2013) atlas, which parcellated the brain into 268 regions. After removing nodes in the brain stem and cerebellum, 217 nodes were retained for the current study. B. Anatomical coverage of the depression-related circuit. From the nodes depicted in Figure 1 A, we selected all nodes whose centers fall within the anatomical boundaries of prefrontal cortex (PFC) including bilateral dorsolateral and ventromedial PFC and anterior cingulate cortex, as well as bilateral amygdala (AMY), and striatum (STR) including bilateral caudate, putamen, and pallidum.
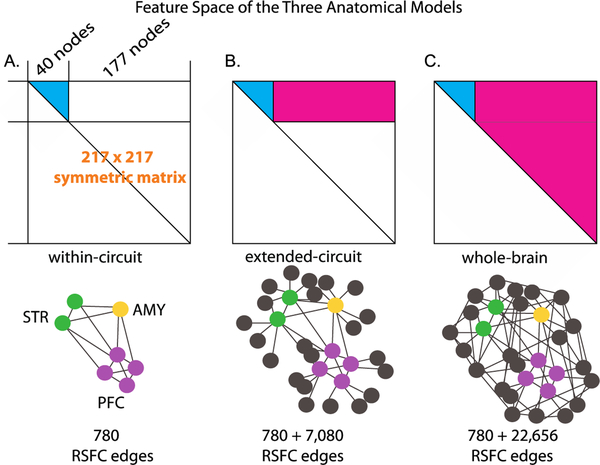
Figure 2. Illustration of the three anatomical models.
For each participant, the pairwise connectivity between the 217 nodes across the whole brain consisted of the 217 × 217 RSFC matrix symmetric about the diagonal. This matrix formed the initial connectivity feature space, with each cell of the matrix representing the connectivity strength between two nodes (i.e., an edge).The first two anatomical models involved a subsection of this connectivity space, and the third model covered the entire connectivity space. A. The within-circuit model. All possible pairs of connections between the PFC, and AMY nodes formed the RSFC connectivity space, resulting in 780 edges. B. The extended-circuit model. Connectivity between each of the nodes included in the within-circuit model (cyan) and of these within-circuit nodes to the rest of the brain (magenta), resulting in 7,080 additional edges. C. The whole-brain model. Connectivity between each of the nodes involved in the within-circuit model (cyan) and of these nodes to the rest of the RSFC connectivity space (magenta) were included, resulting in 22,656 additional edges compared to the within-circuit model. The bottom panel depicts these models using nodes and edges. For visual display clarity, not all possible nodes and edges were drawn.
Anatomical localization of the RSFC edges that predict depression symptoms.
After establishing the association between RSFC and depression symptoms, we identified edges that contributed to the successful prediction of concurrent and future depression symptoms for the successful anatomical model and then visualized these RSFC edges, i.e., the composition of the connectivity predictive of depression symptoms. The most reliable RSFC edges were identified as the ones selected in more than 95% of the 1,000 × 10-fold iterations. We also examined the edges using a lower threshold of 90% to qualitatively test if the RSFC profile varies due to different thresholds (Figure S2). To determine the anatomical locations of these edges we examined the MNI coordinates of the nodes which contributed to the identified edges.
Results
Depression symptom scores
IDAS-II General Depression scores concurrent with fMRI (M = 1.59, SD = .58) and 18 months later (M = 1.66, SD = .68) did not differ, t(164) = 1.50, p = .136.
Circuit-based RSFC profile explains concurrent and future depression symptoms
Given that head motion is a known confound for RSFC, we first confirmed that average frame-to-frame motion was not correlated with both concurrent (r = .05, p = .519) and future (r = .04, p = .587) depression symptoms in the participants included in our analyses. Similarly, the number of artifact-free volumes were not correlated with concurrent (r = −.05, p = .528) and future (r = .01, p = .851) depression symptoms in our sample.
By using a cross-validation approach, we examined whether the within-circuit RSFC profiles were associated with concurrent and future depression symptom severity scores. The within-circuit RSFC profiles were derived from the training sets and were tested in the testing sets by examining the correlation between observed and model-predicted depression severity scores (Figure 3 A–B). The results revealed a significant correlation, r = .22 (p = .032) between predicted and observed depression symptom scores for concurrent depression symptoms. The significance of the model remained when examined using another measure of association, i.e., mean squared error (MSE = .36, p = .033). Importantly, the same neural model that was derived based on relationship with concurrent depression symptoms in the training set also demonstrated a significant correlation with depression symptoms 18 months later, r = .20 (p = .042) and MSE = .47 (p = .022), in the testing set. Given that there is a substantial correlation between the concurrent and future depression symptoms across the entire sample (r = .61, p < .001), this finding suggests that the RSFC model trained using concurrent depression symptoms captures the individual differences in depression symptom severity that is stable over time, rather than specific to concurrent depression symptoms.
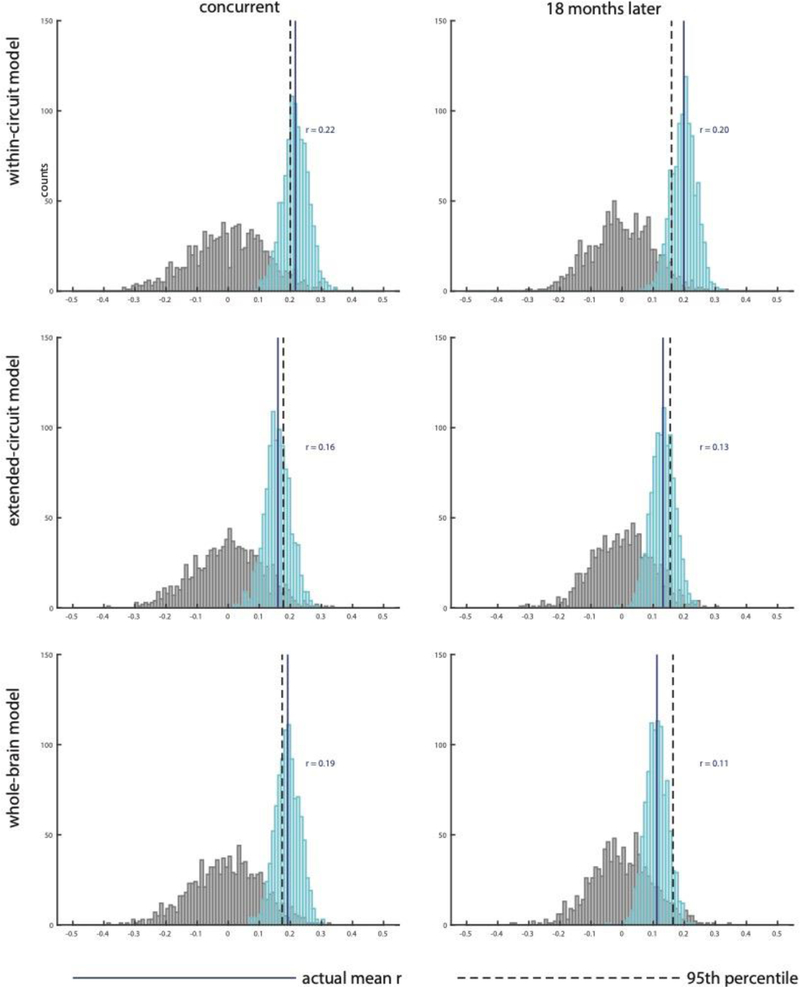
Figure 3. Circuit-based RSFC profiles explain concurrent and future depression.
Results of 10-fold-cross-validation (10fCV) shows correlation between observed depression scores and depression scores predicted by within-circuit model for A. concurrent and B. future depression. The score distributions of Pearson’s correlation coefficients are shown in cyan, with the solid blue line indicating the mean. The distribution of the correlation coefficients under the null hypothesis is shown in gray, with the dashed black line showing the 95th percentile of this distribution. The mean of the actual correlation coefficients exceeds the 95th percentile of null distribution establishing statistical significance in A and B. Results for extended-circuit model (C-D) and whole-brain model (E-F) show that these models do not account for additional variance after the variance accounted for by within-circuit model (see results for significance testing).
Next, we tested the model performance of the extended-circuit model and the whole-brain model to investigate whether extending the RSFC space outside of the depression-circuit led to higher association with depression symptom severity using the same cross-validation analyses. Our results showed that the extended-circuit model, which naturally included edges from the within-circuit model (Figure 3 C–D), did not correlate with concurrent depression symptoms, r = .16 (p = .069) and MSE = .36 (p = .080) or depression symptoms 18 months later, r = .13 (p = .091) and MSE = .49 (p = .129). The whole-brain model, which included both extended and within-circuit models (Figure 3 E–F), showed a significant correlation for concurrent depression symptoms, r = .19 (p = .038) and MSE = .34 (p = .047), but not for depression symptoms 18 months later, r = .11 (p = .131) and MSE = .49 (p = .240). Importantly, after accounting for the variance in depression symptoms due to within-circuit model, no additional variance in depression symptoms was accounted for by the additional edges in extended-circuit model for both concurrent depression symptoms (ΔR2 = −.01, p = .588) and depression symptoms 18 months later (ΔR2 = .03, p = .198) as well as by the additional edges in the whole-brain model for both concurrent depression symptoms (ΔR2 = −.00, p = .508) and depression symptoms 18 months later (ΔR2 = −.01, p = .64). The slight decrease in variance explained by the extended-circuit model and whole-brain model, compared to the simpler within-circuit model, indicates that additional edges increased amount of noise in predictors and diluted the signal, which was revealed in testing. In sum, 10FCV analyses revealed significant association for the within-circuit model with concurrent depression symptoms and depression symptoms 18 months later. Expanding the RSFC space to include regions outside of the depression-circuit did not increase the correlation with depression symptoms.
Functional anatomy of RSFC profiles associated with adolescent depression symptoms
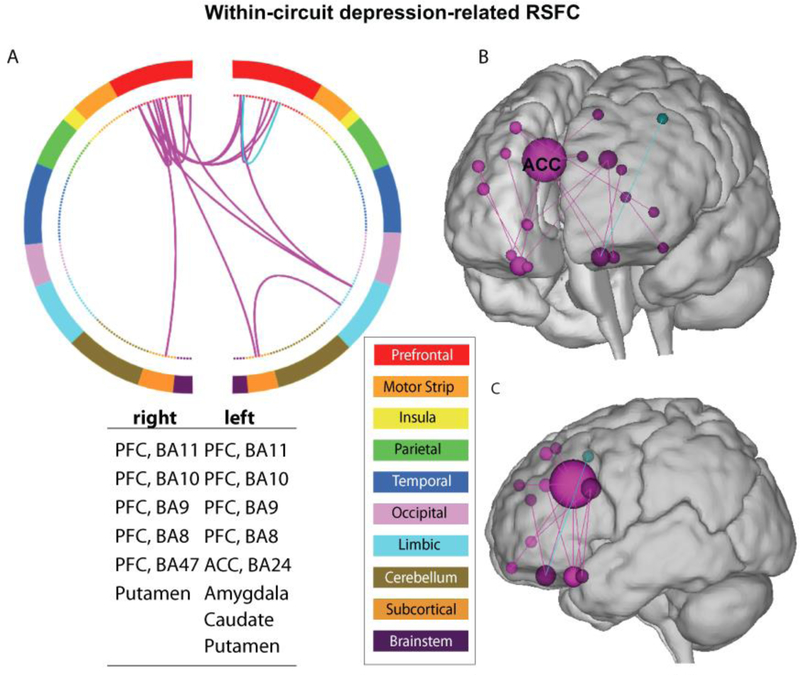
Next, we characterized the predictive model, i.e., the within-circuit model, anatomically by visualizing the specific edges that contributed most to the successful correlation with depression symptoms. It is important to note that due to the nature of cross-validation, a slightly different set of edges may be selected in each iteration of the cross-validation. Hence, we took a conservative approach of visualizing edges that were selected in at least 95% iterations of the analysis (i.e., the overlapping edges of 95% of the models derived in all iterations). As visualized in Figure 4, the node with the maximum contribution to correlating with concurrent depression symptoms in the within-circuit model was in right ACC (MNI coordinate: x = 7, y = 21, z = 32), with 7 connectivities originating from it. This node was followed by nodes contributing 3 connectivities, which were right vmPFC (MNI coordinate: x = 27, y = 18, z = −21), left vmPFC (MNI coordinate: x = −8, y = 40, z = −21), and left ACC (MNI coordinate: x = −5, y = 13, z = 29). The remaining nodes passed the threshold are shown in the corresponding figure with the region labels listed. Using a relaxed threshold of 90% overlapping edges revealed a similar anatomical profile, emphasizing the right ACC and vmPFC as being most contributive (Figure S2).
Figure 4. Key connectivity edges associated with adolescent depression.
A. circular plot organizes the 268 nodes (inner circle) from the original atlas into 10 anatomical regions (outer circle). These regions were organized roughly from anterior to posterior (top to bottom), with the right and left hemisphere in split halves. The color of the nodes and edges indicates positive (magenta) and negative (cyan) correlation with depression scores surviving the 95% thresholds. Regions between which the depicted edges connect were listed in the table under the circular plot. B. The same edges shown in A are shown on the glass brain. The size of the nodes indicates the number of edges connected with the corresponding node. C. The edge distribution showing the high-connectivity (>=3 connectivity) nodes with their edges in the glass brain. The Yale Connectivity Viewer, an online visualization tool was used to generate the corresponding figures (http://bisweb.yale.edu/build/connviewer.html).
Discussion
In the present study, we used a well-established data-driven approach derived from connectome analyses (Shen et al., 2017, Finn et al., 2015, Hsu et al., 2018, Feng et al., 2018, Beaty et al., 2018, Rosenberg et al., 2016) to test whether a highly influential yet, untested neural network consisting of amygdala, striatum and PFC (Ernst et al., 2006, Casey, 2015) predicts adolescent depression symptoms. Specifically, we examined whether RSFC 1) within this network and 2) extending into the rest of cortical-subcortical areas is associated with depression symptoms in a community sample of adolescent females both concurrently and 18 months later. Our results demonstrated that intrinsic functional connectivity models involving this neural network were stable correlates of concurrent and future adolescent depression symptoms. These models were tested using a cross-validation approach and represent a significant step towards understanding the neural mechanisms of depression in adolescence.
Recent studies show that alterations in RSFC of amygdala (Callaghan et al., 2017), striatum (Pan et al., 2017), as well as ACC and medial frontal gyrus (Ho et al., 2017) individually are associated with adolescent depression; however, no studies to our knowledge have examined whether the entire neural circuit comprising these regions is associated with increased depression symptoms during adolescence. The present study is the first to demonstrate the use of RSFC models involving the network consisting of all three regions for successful out-of-sample prediction of depression symptoms in adolescence. Importantly, neural network models that were associated with concurrent depression symptoms were also associated with future depression symptoms, indicating stability of the depression-related RSFC profile. Hence, our results demonstrate that rather than focusing solely on activity in striatum, amygdala or PFC, it is important to examine functional networks that connect these regions to each other to fully understand the mechanisms involved in adolescent depression symptoms. However, extending the network to include connections with the rest of the brain, or including RSFC of the whole-brain performed no better than the network consisting of striatum, amygdala and PFC only. These findings provide strong support for neurodevelopmental models proposing that striatum-based approach, amygdala-based avoidance and PFC-based regulatory components play a critical role in depression during adolescence (Casey, 2015, Ernst et al., 2006).
When examining the anatomical localization of the depression-related RSFC, we visualized connectivity edges that were present across most of the cross-validation iterations, and thereby were the edges correlated with depression symptoms consistently across various sub-samples. The results revealed that nodes in ACC had the highest number of connectivity that contributed to the out-of-sample prediction of adolescent depression symptoms, followed by vmPFC. Studies show that the dorsal-caudal portions of ACC and medial PFC play an important role in monitoring difficulty arising from choice between competing alternatives (Shenhav and Buckner, 2014) including monitoring of emotion-related competition (Etkin et al., 2006) and making choices based on reward value (Bush et al., 2002). The ventral-rostral portions of ACC and medial PFC, including vmPFC have been shown to be involved in regulating emotional conflict (Etkin et al., 2006), directing attention away from emotional information that is irrelevant to the task (Mohanty et al., 2007), and modulating emotional response via strategies such as reappraisal (Banks et al., 2007). The crucial role of ACC and vmPFC in regulation of affective and reward processing is also supported by evidence showing rich structural (Carmichael and Price, 1995) and functional (Etkin et al., 2006, Szekely et al., 2017) connectivity of ACC and vmPFC with limbic, striatal, and prefrontal regions. Finally, the ACC and vmPFC are core regions in the cortical-limbic network, which have long been linked to depression (Drevets, 2000, Drevets et al., 2008), and abnormal metabolic rate in ACC and vmPFC has been associated with baseline depression and treatment outcome (Drevets et al., 2002). Overall, our results highlight the importance of aberrant PFC-related regulatory mechanisms in explaining the increased rates of depression in adolescence
It is worthwhile to highlight that our findings of the association between RSFC profiles and depression symptoms were obtained using a well-established approach (Hsu et al., 2018, Feng et al., 2018, Beaty et al., 2018, Rosenberg et al., 2016) with cross-validation, following latest advancement in connectome methodology. We constructed RSFC models based on the correlation between the connectivity features and depression symptom scores in a subset of data, and estimated the predicted effect size in an independent testing subset (10fCV). In doing so, we ensured independence between feature selection and out-of-sample prediction, thus minimizing the chance that a significant association was driven by outliers, and increasing the chance of our results being generalizable in future data. These findings remain significant in 5-fold-cross-validation, and are robust to control of age, artifact-free volumes, and parental education, i.e., SES as well as using a subset of participants with more stringent motion control (see Appendix S1). Future work in which models are trained and tested on data from not only resting state, but also relevant tasks reflecting emotion processing, cognitive reappraisal, and reward learning, as well as various symptom dimensions, may help clarify the specificity and generalizability of the current model. For example, researchers can collaborate to identify edges that most consistently predict depression across tasks, settings, and comorbidities to reduce the risk of overfitting and improve generalizability. Furthermore, future work should examine the generalizability of these neural network models to depression in adolescent males. In addition, recent evidence has suggested that task-based connectivity models outperform resting-state connectivity models in predicting fluid intelligence (Greene et al., 2018), and resting-state connectivity models were associated with higher-order latent-factors stronger than lower-order cognitive task scores (Dubois et al., 2018). This indicates that engaging networks in targeted domains can improve the predictability of individual differences. Relatedly, while the current investigation tests the association of a neurodevelopmental theory-driven network model with depression it may be useful to compare this model with normatively derived resting-state functional connectivity networks (e.g., default mode or saliency networks) in future studies.
The advantage of using a community sample in the present study was to provide information that is broadly generalizable and largely uncontaminated by medications; however, a limitation is that only some participants had clinically significant depression. While examining low severity depression symptoms is informative because there is clear evidence of etiologic continuity between clinical and subthreshold depression (eg., (Ruscio, 2019)), our models need to be replicated in treatment-seeking samples. Hence, it is important to conduct future studies to examine whether adolescents with concurrent MDD diagnoses show a similar connectivity pattern as revealed in this study. It is possible that the association between RSFC and symptom severity may not be linear, such that there exists a steeper, flatter, or even reverse relationship in individuals above the clinical threshold of MDD. Furthermore, because male depression has been shown to have a distinct etiology (Kendler et al., 2006, Kendler et al., 2002) we sought to reduce heterogeneity in this study by testing our neural models in females only. Thus, future research is needed to determine whether these models perform equally well in male samples. Although to our knowledge, the current study has the largest adolescent female sample size to date examining depression symptom-relevant neural circuit concurrently and prospectively using cross-validation approach, it is still relatively a small sample. This challenge was partially addressed by using the cross-validation approach, however it is critical to test the current findings in larger samples in the future. Also, the role of these models in predicting pre-puberty and childhood-onset depression remains to be investigated. Analytically, while studies have conducted analyses using different thresholds for feature detection, for e.g., (Finn et al., 2015, Greene et al., 2018), these studies typically involve much larger sample sizes than ours and examine traits that are known to have approximately normal distribution, allowing them to overcome low power-related constraints that arise when the feature selection thresholds are high. Furthermore, the current findings were based on relatively short length of scanning time. The optimal scan time depends on multiple factors, including the metrics of RSFC, the scope of the connectivity (ranging from voxel-wise to whole-brain connectivity), the tradeoff between the quantity of data and participant-related factors such as fatigue, motion, sleepiness, etc. (Tomasi et al., 2017). Studies have recommended a single scan session between 7 (Tomasi et al., 2017) to 13 min (Bessette et al., 2018, Birn et al., 2013) for obtaining reliable resting state data.
In sum, by combining neurobiological models and a cross-validation analytical approach, the current study demonstrated the importance of intrinsic functional connectivity based on PFC, amygdala, and striatum in adolescent depression. The association was evident both concurrently and 18 months later. These intrinsic functional connectivity profiles not only contain information about connectivity between large-scale anatomical regions, but also connectivity between finer grained sub-regions. Furthermore, it is important that interconnections between the amygdala, striatum and PFC were critical in predicting adolescent depression symptoms and that extended connections of these regions or the whole brain circuit did not add additional variance. Finally, within this circuitry, connections within prefrontal regions and from prefrontal to subcortical regions were found to contribute the most to current and future depression symptoms, highlighting the importance of this circuitry in the etiology of depression in adolescence. These results advance our understanding of the neural mechanisms of depression in adolescence and demonstrate that neural measures derived from the basic developmental neuroscience literature can predict depression symptoms. Present findings also emphasize the importance of the neural circuitry connecting amygdala, striatum and PFC as potential target for early identification and treatment of depression.
Supplementary Material
Table 1.
Demographic information.
| Age at fMRI (mean and SD) |
IDAS-II General Depression at fMRI (mean and SD) and (percentile ranks) |
IDAS-II General Depression 18 months later (mean and SD) and (percentile ranks) |
Parental Education (frequency) |
Parental Marital Status |
|
|---|---|---|---|---|---|
| Resting-state fMRI sample | 15.29 (.65) | 1.59 (.58) 1.16 (25th) 1.42 (50th) 1.80 (75th) 3.59 (99th) |
1.66 (.68) 1.16 (25th) 1.42 (50th) 2.00 (75th) 4.23 (99th) |
7.5% neither parent attended college | 86.2% living together |
| 27.6% one parent at least attended college | 13.6% not living together | ||||
| 64.4% both parents at least attended college | |||||
Key points.
Several influential neurobiological models of normative development attribute affective and cognitive changes in adolescence to changes in amygdala, striatum and PFC functioning.
Using machine learning strategies, we demonstrate that the strength of resting-state functional connectivity in the network connecting these regions predicts future depression symptoms in a community sample of adolescent females.
Large-scale networks that include the whole brain do not account for additional variance in predicting depression.
These results advance our understanding of the neural mechanisms of depression in adolescence and demonstrate that neural measures derived from the basic developmental neuroscience literature can predict depression symptoms.
Present findings emphasize the importance of the neural circuitry connecting amygdala, striatum and PFC as potential target for early identification and treatment of depression.
Acknowledgements
This study was supported by a National Institute of Mental Health Program Project R01 MH093479. J.X.V.S. was supported by K01MH107763. The authors thank Molly Gromatsky (ADEPT coordinator) and Melissa Carr (fMRI coordinator), and the families who participated. The authors would also like to thank Dr. Edwin W. Cook III for technical advice.
Footnotes
Supporting information
Additional Supporting Information may be found online in the Supporting Information section at the end of this article:
Appendix S1. Supplementary Results.
Appendix S2. Supplementary Methods.
Table S1. Adolescent depression-circuit nodes.
Figure S1. 3.5 min artifact-free data.
Figure S2. Relaxed threshold edge distribution.
The authors have declared that they have no competing or potential conflicts of interest.
Conflict of interest statement: No conflicts declared.
References
- ACHTERBERG M, PEPER JS, VAN DUIJVENVOORDE AC, MANDL RC & CRONE EA (2016). Frontostriatal White Matter Integrity Predicts Development of Delay of Gratification: A Longitudinal Study. J Neurosci, 36, 1954–1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER JT, DILLON DG, PATRICK LM, ROFFMAN JL, BRADY RO JR., PIZZAGALLI DA, ONGUR D & HOLMES AJ (2019). Functional connectomics of affective and psychotic pathology. Proc Natl Acad Sci U S A, 116, 9050–9059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BAKER JT, HOLMES AJ, MASTERS GA, YEO BT, KRIENEN F, BUCKNER RL & ONGUR D (2014). Disruption of cortical association networks in schizophrenia and psychotic bipolar disorder. JAMA Psychiatry, 71, 109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BANKS SJ, EDDY KT, ANGSTADT M, NATHAN PJ & PHAN KL (2007). Amygdala-frontal connectivity during emotion regulation. Soc Cogn Affect Neurosci, 2, 303–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BEATY RE, KENETT YN, CHRISTENSEN AP, ROSENBERG MD, BENEDEK M, CHEN Q, FINK A, QIU J, KWAPIL TR, KANE MJ & SILVIA PJ (2018). Robust prediction of individual creative ability from brain functional connectivity. Proc Natl Acad Sci U S A, 115, 1087–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BECK AT, STEER RA & BROWN GK (1996). Beck depression inventory-II. San Antonio, 78, 490–498. [Google Scholar]
- BESSETTE KL, JENKINS LM, SKERRETT KA, GOWINS JR, DELDONNO SR, ZUBIETA JK, MCINNIS MG, JACOBS RH, AJILORE O & LANGENECKER SA (2018). Reliability, Convergent Validity and Time Invariance of Default Mode Network Deviations in Early Adult Major Depressive Disorder. Front Psychiatry, 9, 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BIRMAHER B, BRENT D, ISSUES AWGOQ, BERNET W, BUKSTEIN O, WALTER H, BENSON RS, CHRISMAN A, FARCHIONE T, GREENHILL L, HAMILTON J, KEABLE H, KINLAN J, SCHOETTLE U, STOCK S, PTAKOWSKI KK & MEDICUS J (2007). Practice parameter for the assessment and treatment of children and adolescents with depressive disorders. J Am Acad Child Adolesc Psychiatry, 46, 1503–1526. [DOI] [PubMed] [Google Scholar]
- BIRN RM, MOLLOY EK, PATRIAT R, PARKER T, MEIER TB, KIRK GR, NAIR VA, MEYERAND ME & PRABHAKARAN V (2013). The effect of scan length on the reliability of resting-state fMRI connectivity estimates. Neuroimage, 83, 550–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BRAUN U, PLICHTA MM, ESSLINGER C, SAUER C, HADDAD L, GRIMM O, MIER D, MOHNKE S, HEINZ A, ERK S, WALTER H, SEIFERTH N, KIRSCH P & MEYER-LINDENBERG A (2012). Test-retest reliability of resting-state connectivity network characteristics using fMRI and graph theoretical measures. Neuroimage, 59, 1404–1412. [DOI] [PubMed] [Google Scholar]
- BUSH G, VOGT BA, HOLMES J, DALE AM, GREVE D, JENIKE MA & ROSEN BR (2002). Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc Natl Acad Sci U S A, 99, 523–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CALLAGHAN BL, DANDASH O, SIMMONS JG, SCHWARTZ O, BYRNE ML, SHEEBER L, ALLEN NB & WHITTLE S (2017). Amygdala Resting Connectivity Mediates Association Between Maternal Aggression and Adolescent Major Depression: A 7-Year Longitudinal Study. J Am Acad Child Adolesc Psychiatry, 56, 983–991 e983. [DOI] [PubMed] [Google Scholar]
- CARMICHAEL ST & PRICE JL (1995). Limbic connections of the orbital and medial prefrontal cortex in macaque monkeys. J Comp Neurol, 363, 615–641. [DOI] [PubMed] [Google Scholar]
- CASEY BJ (2015). Beyond simple models of self-control to circuit-based accounts of adolescent behavior. Annu Rev Psychol, 66, 295–319. [DOI] [PubMed] [Google Scholar]
- CUNNINGHAM MG, BHATTACHARYYA S & BENES FM (2002). Amygdalo-cortical sprouting continues into early adulthood: implications for the development of normal and abnormal function during adolescence. J Comp Neurol, 453, 116–130. [DOI] [PubMed] [Google Scholar]
- DI MARTINO A, FAIR DA, KELLY C, SATTERTHWAITE TD, CASTELLANOS FX, THOMASON ME, CRADDOCK RC, LUNA B, LEVENTHAL BL, ZUO XN & MILHAM MP (2014). Unraveling the miswired connectome: a developmental perspective. Neuron, 83, 1335–1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DREVETS WC (2000). Neuroimaging studies of mood disorders. Biol Psychiatry, 48, 813–829. [DOI] [PubMed] [Google Scholar]
- DREVETS WC, BOGERS W & RAICHLE ME (2002). Functional anatomical correlates of antidepressant drug treatment assessed using PET measures of regional glucose metabolism. Eur Neuropsychopharmacol, 12, 527–544. [DOI] [PubMed] [Google Scholar]
- DREVETS WC, PRICE JL & FUREY ML (2008). Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct, 213, 93–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DUBOIS J, GALDI P, PAUL LK & ADOLPHS R (2018). A distributed brain network predicts general intelligence from resting-state human neuroimaging data. Philos Trans R Soc Lond B Biol Sci, 373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ERNST M, PINE DS & HARDIN M (2006). Triadic model of the neurobiology of motivated behavior in adolescence. Psychol Med, 36, 299–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ETKIN A, EGNER T, PERAZA DM, KANDEL ER & HIRSCH J (2006). Resolving emotional conflict: a role for the rostral anterior cingulate cortex in modulating activity in the amygdala. Neuron, 51, 871–882. [DOI] [PubMed] [Google Scholar]
- FARERI DS, GABARD-DURNAM L, GOFF B, FLANNERY J, GEE DG, LUMIAN DS, CALDERA C & TOTTENHAM N (2015). Normative development of ventral striatal resting state connectivity in humans. Neuroimage, 118, 422–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENG C, YUAN J, GENG H, GU R, ZHOU H, WU X & LUO Y (2018). Individualized prediction of trait narcissism from whole-brain resting-state functional connectivity. Hum Brain Mapp. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FINN ES, SHEN X, SCHEINOST D, ROSENBERG MD, HUANG J, CHUN MM, PAPADEMETRIS X & CONSTABLE RT (2015). Functional connectome fingerprinting: identifying individuals using patterns of brain connectivity. Nat Neurosci, 18, 1664–1671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GALVAN A (2017). Adolescence, brain maturation and mental health. Nat Neurosci, 20, 503–504. [DOI] [PubMed] [Google Scholar]
- GEE DG, HUMPHREYS KL, FLANNERY J, GOFF B, TELZER EH, SHAPIRO M, HARE TA, BOOKHEIMER SY & TOTTENHAM N (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci, 33, 4584–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREENE AS, GAO S, SCHEINOST D & CONSTABLE RT (2018). Task-induced brain state manipulation improves prediction of individual traits. Nat Commun, 9, 2807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HELLER AS, JOHNSTONE T, LIGHT SN, PETERSON MJ, KOLDEN GG, KALIN NH & DAVIDSON RJ (2013). Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. American Journal of Psychiatry, 170, 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HO TC, SACCHET MD, CONNOLLY CG, MARGULIES DS, TYMOFIYEVA O, PAULUS MP, SIMMONS AN, GOTLIB IH & YANG TT (2017). Inflexible Functional Connectivity of the Dorsal Anterior Cingulate Cortex in Adolescent Major Depressive Disorder. Neuropsychopharmacology, 42, 2434–2445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HSU WT, ROSENBERG MD, SCHEINOST D, CONSTABLE RT & CHUN MM (2018). Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc Cogn Affect Neurosci, 13, 224–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JALBRZIKOWSKI M, LARSEN B, HALLQUIST MN, FORAN W, CALABRO F & LUNA B (2017). Development of White Matter Microstructure and Intrinsic Functional Connectivity Between the Amygdala and Ventromedial Prefrontal Cortex: Associations With Anxiety and Depression. Biol Psychiatry, 82, 511–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JIN J, NARAYANAN A, PERLMAN G, LUKING K, DELORENZO C, HAJCAK G, KLEIN DN, KOTOV R & MOHANTY A (2017). Orbitofrontal Cortex Activity and Connectivity Predict Future Depression Symptoms in Adolescence. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KENDLER KS, GARDNER CO & PRESCOTT CA (2002). Toward a comprehensive developmental model for major depression in women. Am J Psychiatry, 159, 1133–1145. [DOI] [PubMed] [Google Scholar]
- KENDLER KS, GARDNER CO & PRESCOTT CA (2006). Toward a comprehensive developmental model for major depression in men. Am J Psychiatry, 163, 115–124. [DOI] [PubMed] [Google Scholar]
- MOHANTY A, ENGELS AS, HERRINGTON JD, HELLER W, HO MH, BANICH MT, WEBB AG, WARREN SL & MILLER GA (2007). Differential engagement of anterior cingulate cortex subdivisions for cognitive and emotional function. Psychophysiology, 44, 343–351. [DOI] [PubMed] [Google Scholar]
- NELSON BD, PERLMAN G, HAJCAK G, KLEIN DN & KOTOV R (2015). Familial risk for distress and fear disorders and emotional reactivity in adolescence: an event-related potential investigation. Psychol Med, 45, 2545–2556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAN PM, SATO JR, SALUM GA, ROHDE LA, GADELHA A, ZUGMAN A, MARI J, JACKOWSKI A, PICON F, MIGUEL EC, PINE DS, LEIBENLUFT E, BRESSAN RA & STRINGARIS A (2017). Ventral Striatum Functional Connectivity as a Predictor of Adolescent Depressive Disorder in a Longitudinal Community-Based Sample. Am J Psychiatry, 174, 1112–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PAUS T (2005). Mapping brain maturation and cognitive development during adolescence. Trends Cogn Sci, 9, 60–68. [DOI] [PubMed] [Google Scholar]
- PESSOA L (2008). On the relationship between emotion and cognition. Nat Rev Neurosci, 9, 148–158. [DOI] [PubMed] [Google Scholar]
- POWER JD, BARNES KA, SNYDER AZ, SCHLAGGAR BL & PETERSEN SE (2012). Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage, 59, 2142–2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- POWER JD, PLITT M, LAUMANN TO & MARTIN A (2017). Sources and implications of whole-brain fMRI signals in humans. Neuroimage, 146, 609–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSENBERG MD, FINN ES, SCHEINOST D, PAPADEMETRIS X, SHEN X, CONSTABLE RT & CHUN MM (2016). A neuromarker of sustained attention from whole-brain functional connectivity. Nat Neurosci, 19, 165–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RUSCIO AM (2019). Normal Versus Pathological Mood: Implications for Diagnosis. Annu Rev Clin Psychol, 15, 179–205. [DOI] [PubMed] [Google Scholar]
- SALK RH, HYDE JS & ABRAMSON LY (2017). Gender differences in depression in representative national samples: Meta-analyses of diagnoses and symptoms. Psychol Bull, 143, 783–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN X, FINN ES, SCHEINOST D, ROSENBERG MD, CHUN MM, PAPADEMETRIS X & CONSTABLE RT (2017). Using connectome-based predictive modeling to predict individual behavior from brain connectivity. Nat Protoc, 12, 506–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHEN X, TOKOGLU F, PAPADEMETRIS X & CONSTABLE RT (2013). Groupwise whole-brain parcellation from resting-state fMRI data for network node identification. Neuroimage, 82, 403–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHENHAV A & BUCKNER RL (2014). Neural correlates of dueling affective reactions to win-win choices. Proc Natl Acad Sci U S A, 111, 10978–10983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPEED BC, NELSON BD, PERLMAN G, KLEIN DN, KOTOV R & HAJCAK G (2015). Personality and emotional processing: A relationship between extraversion and the late positive potential in adolescence. Psychophysiology, 52, 1039–1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SZEKELY A, SILTON RL, HELLER W, MILLER GA & MOHANTY A (2017). Differential functional connectivity of rostral anterior cingulate cortex during emotional interference. Soc Cogn Affect Neurosci, 12, 476–486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- THOMASON ME, DENNIS EL, JOSHI AA, JOSHI SH, DINOV ID, CHANG C, HENRY ML, JOHNSON RF, THOMPSON PM, TOGA AW, GLOVER GH, VAN HORN JD & GOTLIB IH (2011). Resting-state fMRI can reliably map neural networks in children. Neuroimage, 55, 165–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TOMASI DG, SHOKRI-KOJORI E & VOLKOW ND (2017). Temporal Evolution of Brain Functional Connectivity Metrics: Could 7 Min of Rest be Enough? Cereb Cortex, 27, 4153–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VAN DIJK KR, HEDDEN T, VENKATARAMAN A, EVANS KC, LAZAR SW & BUCKNER RL (2010). Intrinsic functional connectivity as a tool for human connectomics: theory, properties, and optimization. J Neurophysiol, 103, 297–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WATSON D, O’HARA MW, SIMMS LJ, KOTOV R, CHMIELEWSKI M, MCDADE-MONTEZ EA, GAMEZ W & STUART S (2007). Development and validation of the Inventory of Depression and Anxiety Symptoms (IDAS). Psychol Assess, 19, 253–268. [DOI] [PubMed] [Google Scholar]
- WATSON D, O’HARA MW, NARAGON-GAINEY K, KOFFEL E, CHMIELEWSKI M, KOTOV R, STASIK SM & RUGGERO CJ (2012). Development and validation of new anxiety and bipolar symptom scales for an expanded version of the IDAS (the IDAS-II). Assessment, 19, 399–420. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.