Abstract
Natural killer (NK) cells play an important role in antiviral resistance. The integrin α2 (α2), which dimerizes with integrin β1 (β1), distinguishes NK cells from Innate Lymphoid Cells 1 (ILC1) and other leukocytes. Despite its use as an NK cell marker, little is known about the role of α2β1 in NK cell biology. Here we show that in mice, α2β1 deficiency does not alter the balance of NK cell/ILC1 generation, and slightly decrease the number of NK cells in the bone marrow and spleen without affecting NK cell maturation. NK cells deficient in α2β1 had no impairment at entering or distributing within the draining lymph node (dLN) of ectromelia virus (ECTV) infected mice or at becoming effectors, but proliferated poorly in response to ECTV and did not increase in numbers following infection with mouse cytomegalovirus (MCMV). Still, α2β1 deficient NK cells efficiently protected from lethal mousepox and controlled MCMV titers in the spleen. Thus, α2β1 is required for optimal NK cell proliferation but is dispensable for protection against ECTV and MCMV, two well established models of viral infection where NK cells are known to be important.
Introduction
Natural killer (NK) cells are leukocytes of the innate immune system that belong to the Group 1 of innate lymphoid cells (ILCs). After developing in the bone marrow, NK cells circulate between blood and tissues patrolling the body for infections and tumors. Following viral infections, NK cells are rapidly recruited from the blood to secondary draining lymph nodes and other infected tissues where they produce interferon gamma (IFN-γ), kill infected cells and proliferate, playing a critical role as a first line of anti-viral defense (1, 2). For example, C57BL/6 (B6) mice, which are normally resistant to mouse cytomegalovirus (MCMV) and ectromelia virus (ECTV), become susceptible when NK cells are eliminated or dysfunctional (3-7). Also in humans, NK cell-deficient individuals become sick or succumb to normally non-life-threatening infections with human cytomegalovirus (HCMV) or with varicella zoster (8-10).
The phenotypes and functions of NK cells overlap extensively with those of ILC1s, the only other member of the Group1 ILCs. For example, both ILC1s and NK cells express the typical NK cell activating receptors NKp46 and, in B6 mice, NK1.1. Both ILC1s and NK cells need the transcription factor T-bet for their development (11). Moreover, NK cells and ILC1s produce IFN-γ upon stimulation. Because of this, ILC1s can participate in virus control in tissues (12). Yet, ILC1s and NK cells also exhibit differences that make them unique. ILC1s are tissue resident and do not circulate in the blood, whereas NK cells circulate in the blood and are transient within tissues. NK cells but not ILC1s are cytolytic and express the transcription factor eomesodermin (Eomes) (13-16). At the cell surface, the major differences between ILC1s and NK cells is in the expression of integrins. ILC1s but not NK cells express the integrin β3 and some, but not all ILC1s, express integrin α1 (12). On the other hand, NK cells, but not ILC1s, express the integrin αM (CD11b, also expressed by other unrelated leukocytes) and the integrin α2 (herein α2, aka CD49b).
Integrins are cell surface heterodimeric receptors conformed by an ɑ and a β chain (17). Integrins function in the adhesion of leukocytes to the endothelium and their extravasation into lymph nodes (LNs) and inflamed tissues. Integrins are also important for the displacement of leukocytes within tissues and can participate in their development and activation (18). α2 dimerizes with the integrin β1 (herein β1, aka CD29) to form α2β1 (aka VLA-2), a receptor for collagens Type I and III. Of note, β1 is thought to be the only partner of α2 while β1 can heterodimerize with 12 different α chains (herein xβ1) (19, 20) including α1 (α1β1) in ILC1s. Notably, α2β1 is also expressed by human NK cells (21).
Despite α2 being a reliable NK cell marker, the role of α2β1 in the biology of NK cells is not well understood. When the Lanier group discovered α2 on murine NK cells, they also showed that two different anti-α2 mAbs, DX5 and HMα2, do not block the binding of NK cells to collagen-coated plates, despite that HMα2 blocks the α2β1-dependent binding of platelets to collagen. In addition, Lanier’s group demonstrated that immobilized anti-α2 mAbs did not stimulate IFN-γ production in NK cells, nor did they block NK cell killing of YAC-1 cells (22, 23). Together, these data suggested that α2β1 may not be essential for NK cell function. Later on, others showed that positive purification of NK cells using DX5 mAb negatively affected their ability to produce IFN-γ in vitro, kill target cells in vitro and in vivo, and impaired their motility in LNs (24). However, these data did not directly demonstrate a physiological role for α2β1. More recently, it was shown that NK cells interacted with collagen fibers in the draining LNs (dLNs) of mice infected in the ear flaps with Toxoplasma gondii (T gondii), and that administration of HMα2 in the ear flap decreased the presence of NK cells in T gondii infected foci in dLNs (25). This suggested that α2β1 interactions with collagen might be involved in NK cells migration to infected foci. Similarly, a study with human NK cells in vitro also suggested that α2β1 might participate in the interaction of NK cells with collagen but the data was correlative (21).
To further understand the role of α2β1 in NK cell biology, we produced mice with specific genetic ablations of α2 in NKp46+ cells. Using these mice, we show that α2β1 affects but is not critical for NK development and maturation. Moreover, we also show that during ECTV infection, α2β1 is not necessary for NK recruitment to or distributing within the dLN of ECTV infected mice or at becoming effectors after ECTV or MCMV infection. Notably, α2β1 is required for optimal NK proliferation in response to ECTV and increase in numbers in response to MCMV, but is dispensable for survival to ECTV and the control of MCMV titers in the spleen. These results indicate that α2β1 is not essential for NK cell differentiation or effector function. Moreover, we demonstrate that NK cells with defective proliferation can still protect from ECTV lethality and can control MCMV replication.
Materials and Methods
Mice
All the procedures involving mice were carried out in strict accordance with the recommendations in the Eight Edition of the Guide for the Care and Use of Laboratory Animals of the National Research Council of the National Academies. All protocols were approved by Thomas Jefferson University’s Institutional Animal Care and Use Committee. All mice used in experiments were 6-12 weeks old Ncr1Cre+-Itgb1fl/fl or Ncr1Cre+-Itga2fl/fl with appropriate littermate controls. No sex differences were observed. C57BL/6 (B6) and B6.CD45.1 mice were purchased from Charles river directly for experiments or as breeders. Aged B6 mice (18-20 months) were obtained from the National Institute of Aging (NIA) aged colony at Charles river. B6.Cg-Itga2tm1.1Tkun/J (α2fl/fl), and B6;129-Itgb1tm1Efu/J (β1fl/fl) mice were purchased from Jackson Laboratories. Of note, the β1fl/fl mice originally obtained from Jackson were in mixed 129/B6 background and carried the NK complex in chromosome 6 from the 129 strain. Therefore, they lacked NK1.1. To solve this problem, the original β1fl/fl mice were backcrossed to C57BL/6 (B6) mice for two generations to generate β1fl/fl mice expressing NK1.1 homozygously. C57BL/6-Ncr1tm1.1(iCre)Viv/Orl mice (Ncr1-Cre) were a gift of Dr. Eric Vivier (Marseille, France). Colonies were bred at Thomas Jefferson University under specific pathogen free conditions.
Viruses and Infection
ECTV-Moscow strain (ATCC VR-1374) and ECTV-mCherry were propagated in tissue culture as previously described (26). Mice were infected in the footpad with 3,000 or 100,000 plaque forming units (PFUs) ECTV as indicated. For the determination of survival, mice were monitored daily and, to avoid unnecessary suffering, mice were euthanized and counted as dead when imminent death was certain as determined by lack of activity and unresponsiveness to touch. Euthanasia was according to the 2013 edition of the AVMA Guideline for the Euthanasia of Animals. For virus titers, the entire spleen or portions of the liver were homogenized in 2.5% FBS RPMI (Corning) using a Tissue Lyser (QIAGEN). Virus titers were determined on BSC-1 cells as previously described (26).
For MCMV infections, indicated strains were infected with 2.5 × 105 pfu I.P. of MCMV K181 (K181) or 1.0 × 104 pfu I.P. of MCMV V70 (V70) for 120 hours. K181 stocks were produced by infecting 4 × 106 M210-B4 (ATCC CRL-1972TM) with a multiplicity of infection of 0.01 for 4-5 dpi, or until cytopathic effect in culture was observed. Cells and supernatant were collected, centrifuged at 2600 x g for 10 minutes, and resuspended in 10 mL complete RPMI 1640 (Corning) supplemented with 10% FBS, 10mM Hepes (Corning), 1mM Sodium Pyruvate (Corning), 100 units Penicillin (Corning), and 100 μg/mL Streptomycin (Corning). Debris were then lysed with a Dounce homogenizer while on ice and spun. Supernatant was collected, transferred to ultracentrifuge tubes (Beckman), and spun at 50,000 x g for 1 hour at 4° C. The supernatant was discarded and the pellet resuspend pellet in 500 μL of complete RPMI. V70 stocks were produced by infecting BALB/c mice ( > 3 weeks) with 10 pfu of tissue-cultured V70 for 21 days. Whole salivary glands were harvested and placed in 1 mL sterile PBS (Corning), processed with a tissue grinder, and diluted to a 10% mixture of homogenate to media with a final concentration of 5% DMSO. Virus titers were determined by infecting M210-B4 monolayers for 4-5 days and counting the resulting plaques. This protocol was adapted from a published protocol (27).
Cell Depletions
NK cells were depleted by i.p. inoculation of 100 μg NK1.1 mAb (PK136, BioXcell) one day before injection and one dpi with ECTV. For MCMV infection, NK cells were depleted similarly one day before, one and three dpi with MCMV.
Immunofluorescent Microscopy
Preparation of ndLNs and dLNs was as previously described (28). 10-micron sections were stained with APC-NK1.1 (PK136; Biolegend) and mounted in Prolong Diamond plus DAPI (Thermo-Fisher). Images were collected with a Nikon A1R laser scanning microscope (LSCM). For better visualization, all the photographs were assembled in a single file and the contrast and brightness was increased in unison using Adobe Photoshop. In addition, APC-NK1.1 is shown in the green channel to distinguish NK cells more clearly from virally infected cells.
Cell isolation
Mice were euthanized by cervical dislocation. Single-cell suspensions were prepared from spleen and bone marrow and lysed for red blood cells using Ammonium-Chloride-Potassium (ACK) lysis buffer, and cells were washed with RPMI (Corning) supplemented with 5% FCS and later used for flow cytometric analysis. To obtain single-cell suspensions, LNs were first incubated in Liberase TM (1.67 Wünsch units/mL) (Sigma) in PBS with 25 mM HEPES for 30 minutes at 37°C before adding PBS with 25 mM HEPES + 10% FBS to halt the digestion process, followed by mechanical disruption of the tissue through a 70-μm filter.
Flow Cytometry
Flow cytometry to characterize NK cells in the bone marrow and spleen was performed as previously described (29). To determine NK cell responses in the LNs, intact LNs were incubated at 37°C for 1 h in media containing10 μg/ml brefeldin A and then made into single cell suspensions. The cells were then stained for cell surface molecules, fixed, permeabilized, and stained for intracellular molecules using the Cytofix/Cytoperm kit (BD) or eBiosciences™ Foxp3/Transcription factor staining kit (Invitrogen) according to the manufacturer’s instructions. The following Abs were used: FITC-CD3ε (Clone 145-2C11; Biolegend), APC/Fire™750-CD11b/ BV605-CD11b (Clone M1/70; Biolegend), PerCP/Cy5.5-CD27 (Clone LG.3A10; Biolegend), PE/Cy7-CD29 ( Clone HMβ1-1; Biolegend), PE-CD45.1 (Clone A20; biologend), Percp-cy5.5-CD45.2 (104; Biolegend), BV421-CD49b (Clone RB6-8C5; Biolegend), AF488-Eomes (Clone Dan11mag; eBiosciences), PE/Cy7-IFNγ (XMG1.2; Biolegend), APC-Ki67 (16A8; Biolegend), PE/ FITC-Ly49H (3D10; Biolegend), APC-NK1.1/BV605-NK1.1 (PK136; Biolegend), APC-NKp46 (Clone 29A1.4; Biolegend), BV786-TCRβ (Clone H57-597; BD), and either Pacific Blue-GzmB (Clone GB11; Biolegend) or PE-labeled anti–human GzmB (Thermo Fisher) that cross-reacts with mouse GzmB. For analysis, samples were acquired using a BD Fortessa flow cytometer (BD Biosciences), and data were analyzed with FlowJo software (TreeStar).
Adoptive Transfers of Lymphocytes
Splenocytes were obtained from the indicated strains of mice, mixed in a 1:1 ratio, then labeled with 4 μM CFSE (Thermo Fisher), and a total of 2 × 107 cells (1 × 107 cells each) in 0.2 ml PBS was inoculated i.v. into the recipient mice.
RNA and DNA Preparation and RT-qPCR
Total RNA from LNs was obtained with the RNeasy Mini Kit (QIAGEN) as previously described (30, 31). First-strand cDNA was synthesized with High Capacity cDNA Reverse Transcription Kit (Life Technologies). For EVM003 and GAPDH RT-qPCR was performed using iTaq Universal SYBR Green with the following primers: Gapdh: tgtccgtcgtggatctgac and cctgcttcaccaccttcttg, EVM003: tctgtcctttaacagcatagatgtaga and tgttaactcggaagttgatatggta. For viral loads, RNA (ECTV) from naïve mice were used as controls and no amplification was observed. Thus, for quantification purposes, their CT values were adjusted to 40.
Total DNA was extracted from the spleen using the Gentra Puregene Tissue Kit (QIAGEN). and following the manufacturer’s instructions for extraction of DNA from tissues. The spleen was homogenized in 10 mL of 2.5% FBS RPMI (Corning) and 1 mL was taken for DNA extraction. RNA and protein were removed by adding RNase A solution and Proteinase K. Extracted DNA was quantified by nanodrop and two microliters DNA were used as a template in each qPCR reaction using the TaqMan Fast Advanced Master Mix (Thermo-Fisher) with the following primer for MCMV-E1: tcgcccatcgtttcgaga and tctcgtaggtccactgacgga. Genome copy numbers were calculated based on a standard curve of a plasmid containing the MCMV-E1 gene.
Statistical analysis
Statistical analysis was performed with Prism software (Graphpad Software Inc., La Jolla, CA, USA) software. For survival studies, P-values were obtained with the log-rank (Mantel-Cox) test. P-values were determined using Mann–Whitney test, and when multiple groups had to be compared, we used one-way ANOVA and Fisher ‘s LSD Test for multiple comparisons.
Results
Generation and characterization of mice with NK cells deficient in α2β1
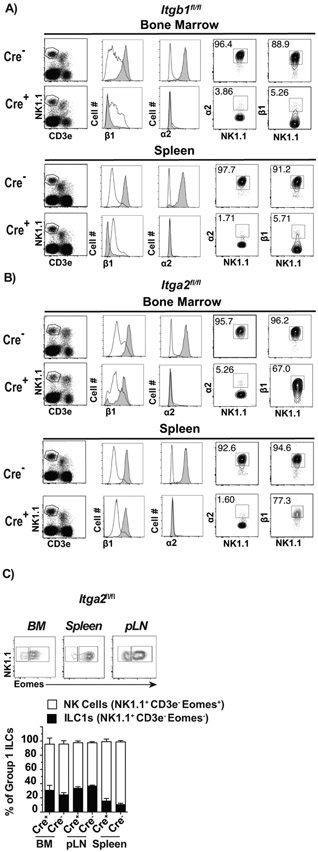
To investigate the role of α2β1 in NK cell maturation and function, we crossed Ncr1Cre+ mice (Ncr1 encodes NKp46) (32) with either Itgb1 fl/fl or Itga2 fl/fl mice to respectively generate mice with NK cells deficient in β1 (Ncr1Cre+-Itgb1fl/fl, herein Cre+-Itgb1fl/fl) or α2 (Ncr1Cre+-Itga2fl/fl, herein Cre+-Itga2 fl/fl). Flow cytometry analysis of bone marrow and spleen cells showed that most but not all CD3− NK1.1+ cells in Cre−-Itgb1fl/fl littermate control mice but only a few Cre+-Itgb1fl/fl mice expressed α2 and β1. This indicates that in NK1.1+ cells, α2 is only expressed at the cell surface if β1 is also expressed. T-cells (CD3e+ NK1.1−) did not express α2 and had low levels of β1 in either Cre− or Cre+ -Itgb1fl/fl mice (Figure 1A). Meanwhile, α2 in CD3− NK1.1+ was expressed at high levels in Cre−-Itga2fl/fl mice but was absent in Cre+-Itga2fl/fl mice, which mostly maintained β1. On the other hand, α2 was not expressed by T-cells in either Cre− or Cre+-itga2fl/fl mice (Figure 1B). This demonstrates that α2 is efficiently deleted in NK1.1+ cells of Cre+-Itga2fl/fl mice. Thus, Cre+-Itga2fl/fl mice have α2β1 deficiency at the surface of NK1.1+ cells. However, because β1was still expressed, β1 likely pairs with other integrins at the surface of NK1.1+ cells. Thus, subsequent experiments are described with Cre+ and Cre −-Itga2fl/fl mice as in addition to α2β1 loss, NK1.1+ cells in Cre+-Itgb1fl/fl have deficiencies in additional β1 pairs. Also, Itgb1fl/fl mice are in a B6;129 mixed background, which could complicate the analysis. Of note, the deficiency of α2 in NK1.1+ cells did not redirect NK cells to the ILC1 linage because the frequencies of NK cells (Eomes+) and ILC1s (Eomes−) within Group 1 ILCs (NK1.1+ CD3−) was similar in the bone marrow, spleen and peripheral LN (pLN) of Cre− and Cre+ -Itga2fl/fl (Figure 1C) where NK cells were the predominant population.
Figure 1: Generation and characterization of mice with NK cells deficient in α2β1.
Ncr1Cre+ mice were crossed with Itgb1fl/fl or Itga2fl/fl mice to generate Cre+-Itgb1fl/fl, Cre+-Itga2fl/f and Cre− littermate controls. A-B) Representative flow cytometry analysis in the indicated tissues and mouse strains: NK1.1 and T-cells were distinguished by CD3e and NK1.1 staining (left panels) on cells previously gated on FSC-A vs. FSC-H to eliminate doublets and on lymphocytes by FSC-H and SSC-H. From these plots, expression of α2 and β1 was determined on gated NK cells (CD3e− NK1.1+, gray histogram) or T-cells (CD3e+ NK1.1−, white histograms). These were used to establish the definitive gates for NK cells shown as contour plots on the right. C) Representative flow plots showing the gating strategy to identify ILC1s and NK cells in the indicated tissues based on NK1.1 and Eomes, and stacked columns depicting the frequencies of ILC1s and NK cells as mean ± SEM in different tissues. Data correspond to 2 independent experiments combined with a total of 6-8 mice/group.
α2β1 affects, but not critically, the development of NK cells.
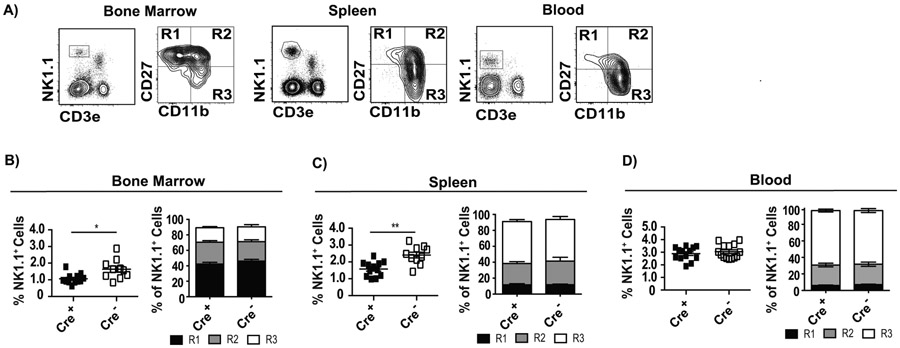
We next investigated whether intrinsic α2β1 deficiency affects NK cell development. Expression of CD27 and CD11b defines different maturational stages of NK cells where CD27+ CD11b− (herein R1) are immature, CD27+ CD11b+ (herein R2) are transitional but with effector capacity, and CD27− CD11b+ (herein R3) are the most mature and have the strongest cytolytic function (33) (Figure 2A) . We found that in Cre+-Itga2fl/fl mice, the frequency of NK1.1+ cells were decreased in the bone marrow (Figure 2B) and in the spleen (Figure 2C), but not in the blood (Figure 2D). However, the frequencies of the different maturation stages (R1, R2 and R3) were not altered in any of the organs (Figure 2 B, C, and D). Thus, the absence of α2β1 seems to slightly decrease the frequency of NK cells in the bone marrow and spleen, but has no effect on NK cell maturation.
Figure 2: α2β1 affects, but not critically, the development of NK cells.
The indicated tissues from Cre+ and Cre− Itga2fl/fl mice were analyzed for the frequency of NK1.1+ cells and their maturation stages (R1, R2 and R3) according to CD27 and CD11b expression. A) Representative flow cytometry plots indicating the gating strategy. B-D) Summary graphs indicating the frequency of NK1.1+ cells in individual mice with mean ± SEM (left panels) and the mean ± SEM frequencies of R1 (CD27+ CD11b−), R2 (CD27+ CD11b+), and R3 (CD27− CD11b+) cells within the NK1.1+ CD3e− gate in the indicated tissues and mice. Data correspond to three or four independent experiments combined with a total of 11-27 mice/group. P-values were calculated using the Mann-Whitney U statistical test or ANOVA as necessary. For all, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Response to viral infection
Deficiency in α2β1 does not impair the constitutive or ECTV-induced accumulation of NK cells in LNs, their distribution within the LN or the acquisition of an effector phenotype
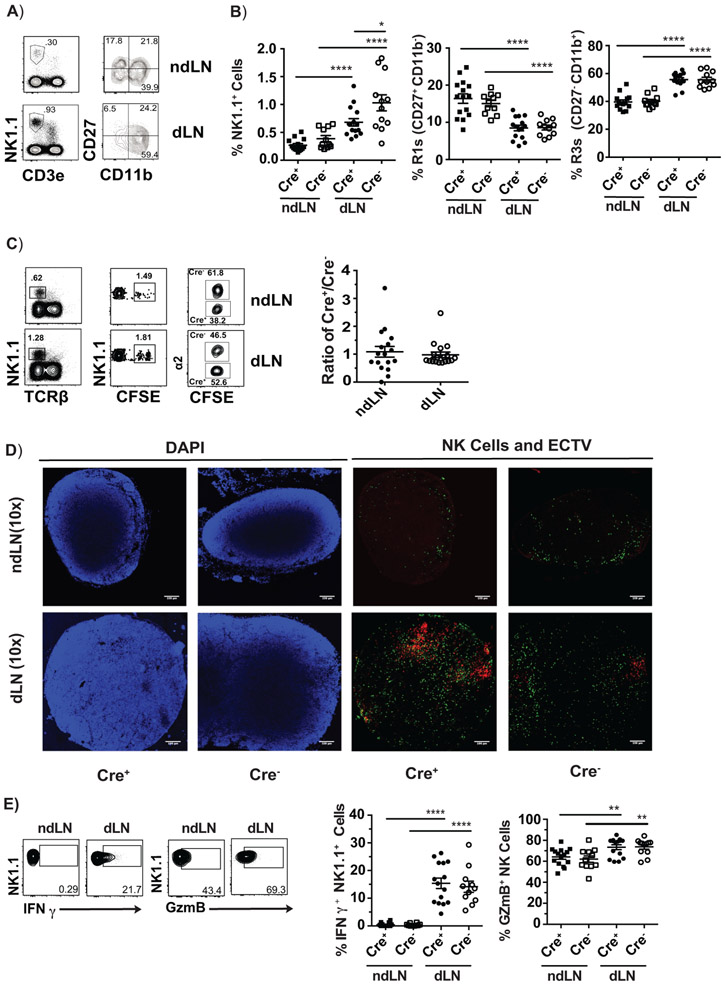
NK cells are present in LNs at low numbers at steady state and increase their numbers by migrating from the circulation during inflammation. We have previously shown that mature R3 NK cells rapidly enter the dLN after ECTV infection (7). Given the importance of integrins in lymphocyte adhesion and migration it was possible that α2β1 was required in this process. Cre+ and Cre− -Itga2fl/fl mice were challenged with ECTV and the recruitment of NK cell into the dLNs was measured by comparing frequencies of NK cells in the non-draining LN (ndLN) (i.e. at steady state) and dLN at 48 hours post infection (hpi). We observed that Cre+-Itga2fl/fl mice preferentially recruited NK cells to the dLN but slightly albeit significantly less than Cre−-Itga2fl/fl mice, which could be an effect of the decreased number of NK cells in the bone marrow and spleen of Cre+-Itga2fl/fl. Nevertheless, similar to Cre− controls, the accumulation of NK cells in the dLN of Cre+ mice resulted in a relative increase in the frequency of mature R3 NK cells and concomitant decrease in immature R1 NK cells (Figure 3A-B).
Figure 3: Deficiency in α2β1 does not impair the constitutive or ECTV-induced accumulation of NK cells in LNs, their distribution within the LN or the acquisition of an effector phenotype.
A-E) Cre+ and Cre− Itga2fl/fl mice were infected with 3,000 pfu WT ECTV or ECTV mCherry and their ndLN and dLNs were analyzed at 48-72 hpi. A) Representative flow cytometry plots showing the gating strategy. B) Summary graphs indicating the frequency of NK1.1+ cells in individual mice with mean ± and frequencies of R1 and R3 cells in individual mice with mean ± SEM in Cre− and Cre+ Itga2fl/fl mice. In A and B, data correspond to three individual experiments combined with a total n of 11-15 mice/group. C) Splenocytes from Cre+ Itga2fl/fl and Cre−- Itga2fl/fl were mixed at a 1:1 ratio labeled with 4 uM CFSE and transferred into recipient B6 mice. The mice were infected with 3,000 pfu WT ECTV one day post transfer and their ndLN and dLNs analyzed at 48 hpi. Representative flow plots show the gating strategy for identification of adoptively transferred cells and WT and α2β1-deficient NK cells. Dot plots show the ratio of α2/WT NK cells in the ndLNs and dLNs of individual mice with mean ± SEM. All experiments were repeated 2-3 times. Data are displayed as a combination of all the repeats with a total n of 17 or 18 mice/group. D) Cre+ and Cre− Itga2fl/fl mice were infected with ECTV-mCherry. At 72 hpi, their ndLN and dLN were visualized by confocal microscopy with 10X magnification. Blue: DAPI, Green: NK1.1 staining. Red: mCherry. Pictures are from the ndLN or dLN of ECTV infected mice. Experiments were repeated 2 times with a total n of 6 mice/group. Representative lymph nodes from one mouse in each group are shown. E) Cre+ and Cre− Itga2fl/fl infected with 3,000 pfu WT ECTV and their ndLN and dLNs were analyzed at 48 hpi. Representative flow cytometry plots showing the gating strategy for expression of intracellular IFN-γ or GzmB. Dot plots depict the frequencies with mean ± SEM of NK1.1+ cells that were IFN-γ+ (left) or GzmB+ (right) in the ndLN and dLN of individual Cre− or Cre+ Itga2fl/fl mice as indicated. Data corresponds to three individual experiments combined with a total n of 11-15 mice/group. P-values were calculated using the Mann-Whitney U statistical test or ANOVA when necessary. For all, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
It was possible that in Cre+-Itga2fl/fl mice NK cells deficient in α2β1 migrated normally to the dLN because of a lack of competition. Thus, splenocytes from Cre+ and Cre− -Itga2fl/fl mice were mixed in a 1:1 ratio, labeled with CFSE, transferred into B6 mice, which were subsequently infected with ECTV in the footpad. At 48 hpi, the ratios of Cre+/Cre− Itga2fl/fl NK cells in the ndLN and the dLN were similar, confirming that deficiency in α2β1 does not affect the constitutive or virus induce entry of NK cells into the dLN (Figure 3C).
Next, we looked at NK cell localization within LNs. As mentioned previously, antibody blockade suggested that α2β1 directs NK cells to sites of bacterial infection within the dLN via interactions with collagen (24). Thus, we analyzed whether absence of α2β1 results in NK cell mislocalization within the dLN during a viral infection. Cre+ and Cre− -Itga2fl/fl mice were infected with ECTV expressing mCherry and ndLNs and dLNs were visualized by confocal microscopy after staining with anti-NK1.1 mAb. The analysis confirmed recruitment because there was an increase in NK1.1 + cells (green) in the dLN vs the ndLN of both, Cre+ and Cre− mice. Furthermore, similar to Cre− controls, NK1.1+ cells in Cre+ mice distributed throughout the dLN including infected and uninfected areas (Figure 3D).
We also analyzed effector functions. At 60 hpi, the NK cells in the dLN of B6 mice are highly activated as determined by granzyme B (GzmB) and interferon gamma (IFN-γ ) expression and these effector molecules are critical for the control of ECTV dissemination from the dLN to the liver and the spleen (7). Thus, we tested whether α2β1 deficiency affects expression of GzmB and IFN-γ in NK cells. We found that Cre− and Cre+-Itga2fl/fl had similarly increased frequencies of both, IFN-γ+ and GzmB+ NK cells in the dLN at 60 hpi with ECTV when compared to the ndLN (Figure 3E).
α2β1 is necessary for optimal NK cell proliferation in the spleen during ECTV and MCMV infections but is not required for the acquisition of effector functions
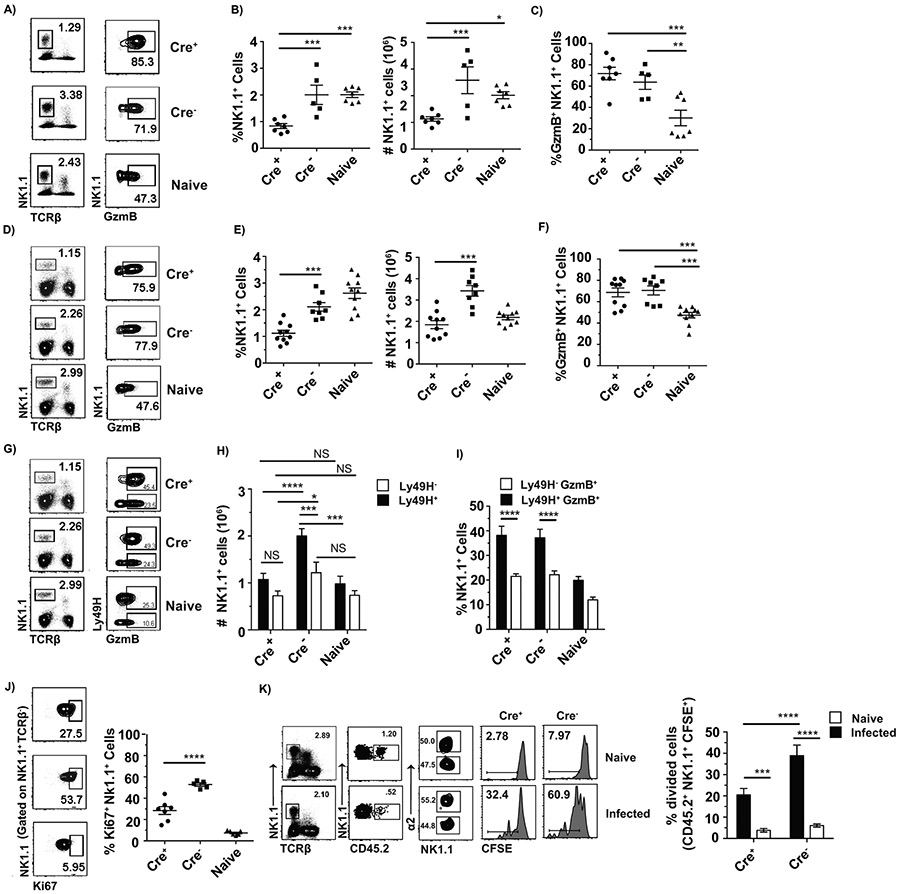
In addition to curbing virus spread from LNs, NK cells play an important role in the control of ECTV by becoming activated in the spleen and liver until the T-cell response develops (3, 7). Thus, we also looked at NK cell responses in the spleen. At 120 hpi with ECTV, which is the peak of the NK response, the frequency and absolute numbers of NK cells in the spleens of Cre+ mice were significantly lower than in Cre− -Itga2fl/fl and naïve mice (Figure 4A and B). A similar effect was observed in the liver and blood (Figure S1). However, the frequency of GzmB+ NK cells was not significantly different between Cre+ and Cre− mice and was significantly higher than in naïve mice (Figure 4A and C), indicating that NK cells do not need α2β1to acquire effector functions in the spleen or liver during ECTV infection.
Figure 4: α2β1 is necessary for optimal NK cell proliferation in the spleen during ECTV and MCMV infections but is not required for the acquisition of NK cell effector functions.
Cre+ and Cre− Itga2fl/fl mice were infected with either 3,000 pfu of WT ECTV or 250,000 pfu of MCMV K181 (MCMV), and their spleens were analyzed at 120 hpi. Naïve B6 mice were used as an additional control. A) Representative flow cytometry plots showing the gating strategy for identification of total NK cells in indicated mice infected with ECTV, as well as intracellular expression of GzmB in total NK1.1+ cells. B) Dot plots depict the frequencies and total numbers with mean ± SEM of NK1.1+ cells that were in the spleen of ECTV infected individual Cre− or Cre+ Itga2fl/fl mice as indicated. Data are displayed as a combination of two individual experiments with a total n of 5-7 mice/group C) Dot plots depict the frequency with mean ± SEM of GzmB+ NK1.1+ cells that were in the spleen of ECTV infected individual Cre− or Cre+ Itga2fl/fl mice as indicated. Data correspond to two individual experiments combined with a total n of 5-7 mice/group. D) Representative flow cytometry plots showing the gating strategy for identification of total NK cells in indicated mice infected with MCMV, as well as intracellular expression of GzmB in total NK1.1+ cells. E) Dot plots depict the frequencies and total numbers with mean ± SEM of NK1.1+ cells in the spleen of MCMV infected individual Cre− or Cre+ Itga2fl/fl mice as indicated. Data are displayed as a combination of three individual experiments with a total n of 8-10 mice/group. F) Dot plots depict the frequency with mean ± SEM of GzmB+ NK1.1+ cells in the spleen of MCMV infected individual Cre− or Cre+ Itga2fl/fl mice as indicated. Data are displayed as a combination of three individual experiments with a total n of 8-10 mice/group. G) Representative flow cytometry plots showing the gating strategy for identification of total NK cells, as well as intracellular expression of GzmB in Ly49H+ or Ly49H− cells within the NK1.1+ population in mice infected with MCMV. H) Bar graphs depict the number of Ly49H+ NK1.1+ or Ly49H− NK1.1+ cells with mean ± SEM of NK1.1+ in the spleen of MCMV infected individual Cre− or Cre+ Itga2fl/fl mice as indicated. I) Bar graphs depict the frequency of GzmB+ cells with mean ± SEM in NK1.1+ Ly49H+ or y49H− cells in the spleen of MCMV infected individual Cre− or Cre+ Itga2fl/fl mice as indicated. Data are displayed as a combination of three individual experiments with a total n of 8-10 mice/group J) Dot plots depict the frequency with mean ± SEM of Ki67+ NK1.1+ cells in the spleen of ECTV infected individual Cre− or Cre+ Itga2fl/fl mice as indicated. Data are displayed as a combination of two individual experiments with a total n of 5-7 mice/group. K) Splenocytes from Cre+ Itga2fl/fl and Cre−- Itga2fl/fl were mixed at a 1:1 ratio labeled with 4 uM CFSE and transferred into recipient CD45.1 mice. Recipient mice were either naïve or infected with 3,000 pfu WT ECTV one day post transfer and their spleens analyzed at 120 hpi. Representative flow plots show the gating strategy for identification of adoptively transferred cells either WT and α2β1-deficient NK cells and the dilution of CFSE by either WT or α2β1-deficient NK cells. Bar graphs show the frequency of WT or α2β1-deficient NK cells that diluted their CFSE in the spleen of individual mice with mean ± SEM. Data corresponds to three individual experiments combined with a total n of 8-11 (naïve) and 10-12 (infected) mice/group. P-values were calculated using the Mann-Whitney U statistical test or ANOVA when necessary. For all, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
To determine whether reduced numbers of NK cells with efficient effector differentiation was a characteristic of the ECTV NK cell response or could be extended to other viral infections, we infected Cre+ and Cre− -Itga2fl/fl mice with MCMV. As with ECTV, Cre+ -Itga2fl/fl mice had reduced frequencies and numbers of NK cells than Cre− -Itga2fl/fl mice at 120 hpi. Compared to naïve mice, MCMV-infected Cre− -Itga2fl/fl had increased total number (but not frequency) of NK cells (Figure 4D and E). As with ECTV, the upregulation of GzmB in Cre+ -Itga2fl/fl mice remained intact (Figure 4D and F).
During MCMV infection of B6 mice, NK cells expand in two phases, the early phase occurs within the first two days of infection, is dependent on cytokines and most NK cells expand (34, 35). The late phase occurs ~120 hpi and happens mostly in Ly49H+ NK cells that recognized the viral protein m157 at the surface of infected cells (36-40). Analysis of Ly49H+ and Ly49H− NK cells revealed that Ly49H+ NK cells from Cre+-Itga2fl/fl mice did not increase in number whereas the Ly49H+ NK cells from Cre− mice did (Figure 4G and H). Yet, similar frequencies of NK1.1+ Ly49H+ cells produced GzmB in Cre− and Cre+ Itga2fl/fl mice (Figure 4G and I). Therefore, during MCMV infection, Ly49H+ NK cells deficient in α2β1 failed to increase in numbers but MCMV-specific NK cells (i.e., Ly49H+) still acquired an effector phenotypes.
Given the reduced NK cell numbers, we next used the ECTV model to test whether α2β1 is necessary for NK cell proliferation. At 120 hpi with ECTV, Cre+ -Itga2fl/fl mice had significantly lower frequency of Ki67+ NK1.1+ cells in the spleen than Cre−-Itga2fl/fl mice indicating that in the absence of α2β1, fewer NK cells entered into G1 (Figure 4J). To determine whether reduced Ki67 expression translated to impaired cell division, we adoptively transferred CFSE-labeled splenocytes from Cre+ and Cre− Itga2fl/fl mice in a 1:1 ratio into a CD45.1 host, which were then infected with ECTV in the footpad. Proliferation of Cre+ and Cre− NK cells (identified by α2 staining) was assessed by CFSE dilution at 144 hpi. The results indicated significantly decreased proliferation in Cre+ compared to Cre− -Itga2fl/fl mice (Figure 4K). This indicates that α2β1 is required for optimal proliferation of NK cells during ECTV infection.
α2β1-deficient NK cells protect from lethal mousepox and control MCMV titers in the spleen
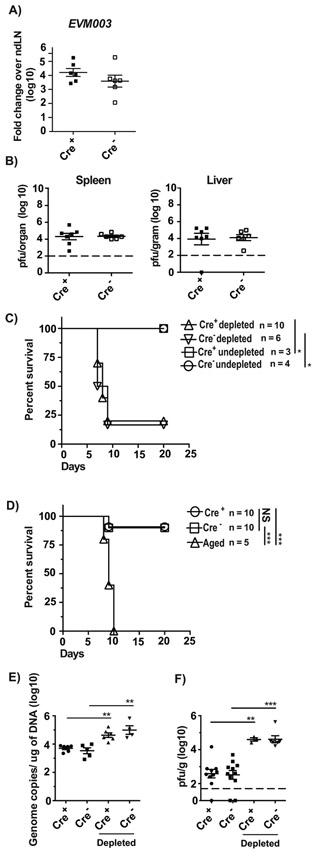
B6 mice are naturally resistant to lethal mousepox but become highly susceptible when depleted of NK cells with anti-NK1.1 or Asialo-GM antibody or when NK cells are dysfunctional as demonstrated by increased virus titers in spleen and liver at 3 and 5 dpi, and by high lethality (29, 41). When challenged with ECTV, Cre− and Cre+ -Itga2fl/fl had similar levels of viral gene expression in the dLNs at 72 hpi (Figure 5A) and similar titers of replicating virus in the spleen and liver at 120 hpi as determined by plaque assay (Figure 5B). Treatment with anti-NK1.1 mAb resulted in a significant increase in lethality in both Cre− and Cre+ -Itga2fl/fl mice, while undepleted Cre− and Cre+ -Itga2fl/fl mice were resistant indicating that α2β1 is not required for NK cell-mediated resistance to lethal mousepox (Figure 5C). Moreover, Cre+ -Itga2fl/fl mice were resistant when challenged with a significantly higher dose of ECTV, but control aged B6 mice, which have immature and dysfunctional NK cells, succumbed to ECTV infection (Figure 5D). Also, at 5 dpi with tissue cultured (K181) and salivary gland passaged (V70) MCMV, Cre− and Cre+ mice had similar virus loads in the spleen, which increased similarly after NK cell depletion (Figure 5E and F). These results indicate that the α2β1 in NK cells and optimal NK cell proliferation are dispensable for effective NK-cell mediated control of two mouse-specific viruses in the acute phase of infection.
Figure 5. α2β1-deficient NK cells protect from lethal mousepox and from MCMV:
A) The indicated mice were infected with ECTV and at 72 hpi, the expression of the viral gene EVM003 in individual mice with mean ± SEM was determined in the dLN by RT-qPCR. Data correspond to two individual experiments combined with a total of 6-8 mice/group. B) The indicated mice were infected with ECTV and at 120 hpi, the virus loads in spleens or livers of individual mice with mean ± SEM were determined by plaque assay. Data correspond to two individual experiments combined with a total of 6-9 mice/group. The limit of detection is indicated by the dotted line (2 log). C) The indicated mice were depleted or not of NK cells with anti-NK1.1 mAb PK136 one day before and one day after infection with 3,000 pfu ECTV in the footpad. Survival was monitored. Data are displayed as a combination of two independent experiments with a total of 3-10 mice/group Statistical analysis was performed by Log-Rank test. For all, *p < 0.05; **p < 0.01. D) The indicated mice infected with 100,000 pfu ECTV in the footpad. Survival was monitored. Data are displayed as a combination of two independent experiments with a total of 5-10 mice/group Statistical analysis was performed by Log-Rank test. For all, *p < 0.05; **p < 0.01; ***p < 0.001. E-F) The indicated mice were depleted or not of NK cells with anti-NK1.1 mAb PK136 one day before and one, three, and five days after infection with 250,000 pfu of tissue-cultured MCMV K181 (E) or 10,000 pfu of salivary gland passaged MCMV V70 (F) i.p. Spleens were collected at 120 hpi and genome copies of MCMV E1 gene (E) or pfu (F) were calculated. Data are displayed as a combination of two or three independent experiments with a total of 3-11 mice/group. The limit of detection for pfu is indicated by the dotted line (1.7 log). P-values were calculated using the Mann-Whitney U statistical test or ANOVA when necessary. For all, *p < 0.05; **p < 0.01; ***p < 0.001; ****p < 0.0001.
Discussion
In this study, we show that α2β1 expression by NK cells is not essential for their maturation or commitment to the NK cell lineage, entry to and distribution within lymph nodes, or the induction of an anti-ECTV effector response. However, α2β1 is required for optimal NK cell proliferation during ECTV infection and for increase in NK cell during MCMV infection. Despite this, α2β1-deficient NK cells were still able to protect from ECTV and control MCMV titers in the spleen, demonstrating that mature and functional NK cells can mediate protection without extensive proliferation in two viral infection models where the anti-viral role of NK cells is very well established.
Previous research has shown that the VLA family of integrin heterodimers has roles in metastasis of cancer cells, immunomodulation, and leukocyte extravasation and retention in tissue (42-47). While α2β1 is used as a marker to identify NK cells across mouse strains as well as to distinguish NK cells from ILC1s, its role in NK cell biology remains unclear. Bone marrow stromal cells synthesize types 1 and 3 collagen fibers (48) and it has been shown their reduction in bone marrow stromal cells leads to decreased collagen synthesis (49, 50). Previously, we have shown that the NK cells in the bone marrow and other organs of aged mice are immature with increased R1 and decreased R3 populations (29, 51). We also showed that NK cells in the bone marrow of aged mice have lower expression of α2β1 (51) and that NK-cell extrinsic factors in the bone marrow stroma are responsible for the defective maturation of NK cells in aged mice, leading us to hypothesize that α2β1 and its interaction with collagen are necessary for NK cell maturation. Thus, we also hypothesized that expression of α2β1 on developing NK cells and their interaction with collagen could be required for their proper linage commitment and maturation in the bone marrow. However, we found that α2β1 does not influence lineage commitment of the common innate lymphoid progenitor to NK cells vs. ILC1s. Also, while α2β1 deficiency slightly decreases the frequency of NK cells in bone marrow and spleen, it does not affect NK cell maturation because the proportions of R1s and R3s were similar within the bone marrow, peripheral blood, and spleen of Cre+- and Cre− -Itga2 fl/fl mice. Thus, the data strongly suggests that the deficient NK cell maturation in aged mice (51) is not due to deficient α2β1 expression.
It is known that CXCR3 directs homing of NK cells to the dLN during viral infection (52, 53), but the identity of the integrin(s) involved in entry into the dLN remains unknown. We show that Cre+-Itga2 fl/fl NK cells enter ndLN and dLNs normally, whether or not they are in competition with Cre− Itga2 fl/fl NK cells. This indicates that α2β1 is not required for NK cell constitutive entry into the ndLN and ECTV-induced entry into the dLN. Also, using confocal microscopy, we found that α2β1-deficient NK cells distribute similarly within the ndLN and dLN including areas of virus infection, indicating that α2β1 is not required for intra-nodal displacement. Thus, adhesion molecules other than α2β1 may be responsible for the NK cell extravasation into and distribution within ndLNs and dLNs.
It has been reported that α2 in mouse NK cells and β1 in human NK cells are needed to control Toxoplasma gondii and cryptococcal neoformans infections respectively, via direct or indirect activation of NK cells (25, 54). Here we show that during viral infections, α2β1 is not required for NK cell activation because Cre+ -Itga2fl/fl NK cells upregulated IFN-γ or GzmB normally during ECTV and MCMV infections. Furthermore, Cre− and Cre+ -Itga2fl/fl mice controlled ECTV and MCMV similarly while depletion of α2β1 NK cells resulted in high susceptibility to lethal mousepox and higher MCMV titers in spleen, indicating α2β1 is dispensable at conferring NK cell-mediated control against ECTV and MCMV.
Interactions with β1 and collagen have previously been shown to affect the expansion of pancreas β-cells as well as the morphogenesis of submandibular epithelial cells (55, 56). Thus, we believe α2β1 induces proliferation of NK cells but does not affect their trafficking or localization during viral infections because of the global reduction in NK cells within the blood, liver, and spleen of infected Cre+ -Itga2fl/fl mice. It remains possible, however, that NK cells are increased at other sites that we did not test. Importantly, we showed that α2β1 is required for optimal NK cell proliferation. Future experiments should identify the ligands and signaling mechanisms of this α2β1-dependent proliferation and whether β1 plays a role in the proliferation of other leukocytes.
It is well established that survival to ECTV and efficient control of MCMV requires NK cells, and that the NK cell responses to both viruses includes acquisition of effector functions and proliferation. Our results show that the NK cell-mediated resistance to ECTV and control of MCMV virus loads can still occur with significantly decreased proliferation. It is also known that innate immune mechanisms including NK cell effector functions in the dLN play a critical role in protection against lethal ECTV infection (29, 59). Notably, at 48-72 hpi the frequency of NK cells in the dLN of Cre+ -Itga2fl/fl is only slightly decreased. Thus, the successful migration of mature and functional α2β1-deficient NK cells to the dLN at 48-72 hpi and their activation in the spleen allows for ECTV control even when NK numbers are altered. These findings further highlight the importance of controlling viral replication in the dLN during an acute virus infection.
During primary infection with MCMV, Ly49H+ NK cells interacting with the viral protein m157 expand and protect mice from MCMV (5, 39, 60, 61). These MCMV-specific NK cells then contract and give rise to long-lived memory NK cells that contribute to protection upon reinfection (57, 62). Our data suggests that in addition to Ly49H interacting with m157, α2β1 acts as an additional signal for optimal Ly49H+ NK cell proliferation. Future experiments could look whether this affects the induction of memory NK cells.
In summary, α2β1 is viewed as a phenotypic hallmark of NK cells, but its role in NK cell biology is not well understood. Our work shows that α2β1 is not required for NK cell maturation, migration to LNs or acquisition of effector functions. However, α2β1 is required for optimal virus-induced proliferation and accumulation of NK cells. Unexpectedly, despite impaired proliferation, α2β1-defficient NK cells still protected from two viral infections in their natural hosts.
Supplementary Material
Key Points.
α2β1 deficiency does not alter the balance of NK cell/ILC1 generation
α2β1 deficiency does not alter NK cell maturation
α2β1 deficient NK cells efficiently protect from lethal mousepox and control MCMV
Acknowledgements
We thank Lingjuan Tang for technical assistance and the Flow and Animal Laboratory Facilities at Thomas Jefferson University for their services.
This work was supported by grants from the National Institute of Allergy and Infectious Diseases (NIAID) (R01AI110457 and R01AI065544) and National Institute on Aging (NIA) (AG048602) to (L.J.S.). E.W. was partially supported by F32AI129352 from the NIAID. BM and CK were supported by T32 AI134646 from NIAID. PAP was partially supported by a PhD fellowship (PD/BD/128078/2016) from the MD/PhD Program of the University of Minho-School of Medicine funded by the Fundação para a Ciência e Tecnologia (FCT). Research reported in this publication utilized the Flow Cytometry and Laboratory Animal facilities at Sidney Kimmel Cancer Center at Jefferson Health and was supported by the National Cancer Institute of the National Institutes of Health under Award Number P30CA056036.
References
- 1.French AR, and Yokoyama WM. 2003. Natural killer cells and viral infections. Curr. Opin. Immunol 15: 45–51. [DOI] [PubMed] [Google Scholar]
- 2.Biron CA, Nguyen KB, Pien GC, Cousens LP, and Salazar-mather TP. 1999. Natural Killer Cells in Antiviral Defense : Function and Regulation by Innate Cytokines. Annu. Rev. Immunol 17: 189–220. [DOI] [PubMed] [Google Scholar]
- 3.Parker AK, Parker S, Yokoyama WM, Corbett JA, and Buller RML. 2007. Induction of Natural Killer Cell Responses by Ectromelia Virus Controls Infection ᰔ. 81: 4070–4079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delano ML, and Brownstein DG. 1995. Innate resistance to lethal mousepox is genetically linked to the NK gene complex on chromosome 6 and correlates with early restriction of virus replication by cells with an NK phenotype. J. Virol 69: 5875–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukowski JF, Woda BA, and Welsh RM. 1984. Pathogenesis of murine cytomegalovirus infection in natural killer cell-depleted mice. J. Virol 52: 119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bukowski JF, Woda BA, Habu S, Okumura K, and Welsh R. 1983. Natural killer cell depletion enhances virus synthesis and virus-induced hepatitis. J. Immunol 131: 1531–1538. [PubMed] [Google Scholar]
- 7.Fang M, Lanier LL, and Sigal LJ. 2008. A Role for NKG2D in NK Cell – Mediated Resistance to Poxvirus Disease. PLoS Pathog. 4: e30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Biron CA, Byron KS, and Sullivan JL. 1989. Severe Herpesvirus Infections in an Adolescent without Natural Killer Cells. N. Engl. J. Med 320: 1731–1735. [DOI] [PubMed] [Google Scholar]
- 9.Etzioni A, Eidenschenk C, Katz R, Beck R, Casanova JL, and Pollack S. 2005. Fatal varicella associated with selective natural killer cell deficiency. J. Pediatr 146: 423–425. [DOI] [PubMed] [Google Scholar]
- 10.Eidenschenk C, Jouanguy E, Alcaïs A, Mention J-J, Pasquier B, Fleckenstein IM, Puel A, Gineau L, Carel J-C, Vivier E, Le Deist F, and Casanova J-L. 2006. Familial NK cell deficiency associated with impaired IL-2- and IL-15-dependent survival of lymphocytes. J. Immunol 177: 8835–43. [DOI] [PubMed] [Google Scholar]
- 11.Diefenbach A, Colonna M, and Koyasu S. 2014. Development, differentiation, and diversity of innate lymphoid cells. Immunity 41: 354–365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weizman O. El, Adams NM, Schuster IS, Krishna C, Pritykin Y, Lau C, Degli-Esposti MA, Leslie CS, Sun JC, and O’Sullivan TE. 2017. ILC1 Confer Early Host Protection at Initial Sites of Viral Infection. Cell 171: 795–808.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daussy C, Faure F, Mayol K, Viel S, Gasteiger G, Charrier E, Bienvenu J, Henry T, Debien E, Hasan UA, Marvel J, Yoh K, Takahashi S, Prinz I, de Bernard S, Buffat L, and Walzer T. 2014. T-bet and Eomes instruct the development of two distinct natural killer cell lineages in the liver and in the bone marrow. J. Exp. Med 211: 563–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gordon SM, Chaix J, Rupp LJ, Wu J, Madera S, Sun JC, Lindsten T, and Reiner SL. 2012. The Transcription Factors T-bet and Eomes Control Key Checkpoints of Natural Killer Cell Maturation. Immunity 36: 55–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pikovskaya O, Chaix J, Rothman NJ, Collins A, Chen Y-H, Scipioni AM, Vivier E, and Reiner SL. 2016. Cutting Edge: Eomesodermin Is Sufficient To Direct Type 1 Innate Lymphocyte Development into the Conventional NK Lineage. J. Immunol 196: 1449–1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fuchs A 2016. ILC1s in tissue inflammation and infection. Front. Immunol 7: 3–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leitinger B, and Hohenester E. 2007. Mammalian collagen receptors. Matrix Biol. 26: 146–155. [DOI] [PubMed] [Google Scholar]
- 18.Ley K, Laudanna C, Cybulsky MI, and Nourshargh S. 2007. Getting to the site of inflammation: The leukocyte adhesion cascade updated. Nat. Rev. Immunol 7: 678–689. [DOI] [PubMed] [Google Scholar]
- 19.Zutter MM, and Santoro SA. 1990. Widespread histologic distribution of the alpha 2 beta 1 integrin cell-surface collagen receptor. Am. J. Pathol 137: 113–20. [PMC free article] [PubMed] [Google Scholar]
- 20.Elices MJ, and Hemler ME. 1989. The human integrin VLA-2 is a collagen receptor on some cells and a collagen/laminin receptor on others. Proc. Natl. Acad. Sci. U. S. A 86: 9906–9910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Goda S, Inoue H, Umehara H, Miyaji M, Nagano Y, Harakawa N, Imai H, Lee P, Macarthy JB, Ikeo T, Domae N, Shimizu Y, and Iida J. 2006. Matrix metalloproteinase-1 produced by human CXCL12-stimulated natural killer cells. Am. J. Pathol 169: 445–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arase H, Saito T, Phillips JH, and Lanier LL. 2001. Cutting Edge: The Mouse NK Cell-Associated Antigen Recognized by DX5 Moncoclonal Antibody is CD49b ( α 2 Integrin, Very Late Antigen-2). J. Immunol 167: 1141–1144. [DOI] [PubMed] [Google Scholar]
- 23.Miyake S, Sakurai T, Okumura K, and Yagita H. 1994. Identification of collagen and laminin receptor integrins on murine T lymphocytes. Eur. J. Immunol 24: 2000–2005. [DOI] [PubMed] [Google Scholar]
- 24.Garrod KR, Wei SH, Parker I, and Cahalan MD. 2007. Natural killer cells actively patrol peripheral lymph nodes forming stable conjugates to eliminate MHC-mismatched targets. Proc. Natl. Acad. Sci. U. S. A 104: 12081–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Coombes JL, Han S, Van Rooijen N, Raulet DH, and Robey EA. 2012. Infection-induced regulation of NK cells by macrophages and collagen at the lymph node subcapsular sinus. Cell Rep. 2: 124–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Xu R-H, Cohen M, Tang Y, Lazear E, Whitbeck JC, Eisenberg RJ, Cohen GH, and Sigal LJ. 2008. The orthopoxvirus type I IFN binding protein is essential for virulence and an effective target for vaccination. J. Exp. Med 205: 981–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zurbach KA, Moghbeli T, and Snyder CM. 2014. Resolving the titer of murine cytomegalovirus by plaque assay using the M2-10B4 cell line and a low viscosity overlay. Virol. J 11: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wong E, Xu RH, Rubio D, Lev A, Stotesbury C, Fang M, and Sigal LJ. 2018. Migratory Dendritic Cells, Group 1 Innate Lymphoid Cells, and Inflammatory Monocytes Collaborate to Recruit NK Cells to the Virus-Infected Lymph Node. Cell Rep. 24: 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang M, Roscoe F, and Sigal LJ. 2010. Age-dependent susceptibility to a viral disease due to decreased natural killer cell numbers and trafficking. J. Exp. Med 207: 2369–2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rubio D, Xu R, Remakus S, Krouse TE, Truckenmiller ME, Thapa RJ, Norbury CC, Sigal LJ, Balachandran S, and Alcamı A. 2013. Article Crosstalk between the Type 1 Interferon and Nuclear Factor Kappa B Pathways Confers Resistance to a Lethal Virus Infection. Cell Host Microbe 13: 701–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu R-H, Wong EB, Rubio D, Roscoe F, Ma X, Nair S, Remakus S, Schwendener R, Shinu J, Shlomchik M, and Sigal LJ. 2015. Sequential Activation of Two Pathogen-Sensing Pathways Required for Type I Interferon Expression and Resistance to an Acute DNA Virus Infection Article Sequential Activation of Two Pathogen-Sensing Pathways Required for Type I Interferon Expression and Re. Immunity 43: 1148–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Narni-Mancinelli E, Chaix J, Fenis A, Kerdiles YM, Yessaad N, Reynders A, Gregoire C, Luche H, Ugolini S, Tomasello E, Walzer T, and Vivier E. 2011. Fate mapping analysis of lymphoid cells expressing the NKp46 cell surface receptor. Proc. Natl. Acad. Sci 108: 18324–18329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hayakawa Y, and Smyth MJ. 2006. CD27 Dissects Mature NK Cells into Two Subsets with Distinct Responsiveness and Migratory Capacity. J. Immunol 1517–1524. [DOI] [PubMed] [Google Scholar]
- 34.Nguyen KB, Salazar-Mather TP, Dalod MY, Van Deusen JB, Wei X, Liew FY, Caligiuri MA, Durbin JE, and Biron CA. 2002. Coordinated and Distinct Roles for IFN-αβ, IL-12, and IL-15 Regulation of NK Cell Responses to Viral Infection. J. Immunol 169: 4279–4287. [DOI] [PubMed] [Google Scholar]
- 35.Dokun AO, Kim S, Smith HRC, Kang HSP, Chu DT, and Yokoyama WM. 2001. Specific and nonspecific NK cell activation during virus infection. Nat. Immunol 2: 951–956. [DOI] [PubMed] [Google Scholar]
- 36.Daniels KA, Devora G, Lai WC, O’Donnell CL, Bennett M, and Welsh RM. 2001. Murine Cytomegalovirus Is Regulated by a Discrete Subset of Natural Killer Cells Reactive with Monoclonal Antibody to Ly49h. J. Exp. Med 194: 29–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tripathy SK, Smith HRC, Holroyd EA, Pingel JT, and Yokoyama WM. 2006. Expression of m157, a Murine Cytomegalovirus-Encoded Putative Major Histocompatibility Class I (MHC-I)-Like Protein, Is Independent of Viral Regulation of Host MHC-I. J. Virol 80: 545–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Voigt V, Forbes CA, Tonkin JN, Degli-Esposti MA, Smith HRC, Yokoyama WM, and Scalzo AA. 2003. Murine cytomegalovirus m157 mutation and variation leads to immune evasion of natural killer cells. Proc. Natl. Acad. Sci 100: 13483–13488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Arase H, Mocarski ES, Campbell AE, Hill AB, and Lanier LL. 2002. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science (80-. ). 296: 1323–1326. [DOI] [PubMed] [Google Scholar]
- 40.Adams EJ, Juo ZS, Venook RT, Boulanger MJ, Arase H, Lanier LL, and Garcia KC. 2007. Structural elucidation of the m157 mouse cytomegalovirus ligand for Ly49 natural killer cell receptors. Proc. Natl. Acad. Sci 104: 10128–10133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fang M, Orr MT, Spee P, Egebjerg T, Lanier LL, and Luis J. 2012. CD94 is essential for NK cell-mediated resistance to a lethal viral disease. Immunity 34: 579–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gagliani N, Magnani CF, Huber S, Gianolini ME, Pala M, Licona-Limon P, Guo B, Herbert DR, Bulfone A, Trentini F, Di Serio C, Bacchetta R, Andreani M, Brockmann L, Gregori S, Flavell RA, and Roncarolo MG. 2013. Coexpression of CD49b and LAG-3 identifies human and mouse T regulatory type 1 cells. Nat. Med. 19: 739–746. [DOI] [PubMed] [Google Scholar]
- 43.Yan X, Johnson BD, and Orentas RJ. 2008. Induction of a VLA-2 (CD49b)-Expressing Effector T Cell Population by a Cell-Based Neuroblastoma Vaccine Expressing CD137L. J. Immunol 181: 4621–4631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshimura K, Meckel KF, Laird LS, Chia CY, Park JJ, Olino KL, Tsunedomi R, Harada T, Iizuka N, Hazama S, Kato Y, Keller JW, Thompson JM, Chang F, Romer LH, Jain A, Iacobuzio-Donahue C, Oka M, Pardoll DM, and Schulick RD. 2009. Integrin α2 mediates selective metastasis to the liver. Cancer Res. 69: 7320–7328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Geherin SA, Gómez D, Glabman RA, Ruthel G, Hamann A, and Debes GF. 2016. IL-10 + Innate-like B Cells Are Part of the Skin Immune System and Require α 4 β 1 Integrin To Migrate between the Peritoneum and Inflamed Skin. J. Immunol 196: 2514–2525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Becker HM, Rullo J, Chen M, Bak S, Xiao H, Hay JB, and Cybulsky MI. 2013. α 1 β 1 Integrin-Mediated Adhesion Inhibits Macrophage Exit from a Peripheral Inflammatory Lesion. J. Immunol 190: 4305–4314. [DOI] [PubMed] [Google Scholar]
- 47.Yokoyama WM, Maxfield SR, and Shevach EM. 1989. Very Early (VEA) and Very Late (VLA) Activation Antigens have Distinct Functions in T Lymphocyte Activation. Immunol. Rev 109: 153–176. [DOI] [PubMed] [Google Scholar]
- 48.Bentley SA 1982. Collagen synthesis by bone marrow stromal cells: a quantitative study. Br. J. Haematol 50: 491–497. [DOI] [PubMed] [Google Scholar]
- 49.Chen XD, Shi S, Xu T, Robey PG, and Young MF. 2002. Age-related osteoporosis in biglycan-deficient mice is related to defects in bone marrow stromal cells. J. Bone Miner. Res 17: 331–340. [DOI] [PubMed] [Google Scholar]
- 50.Zhang W, Ou G, Hamrick M, Hill W, Borke J, Wenger K, Chutkan N, Yu J, Mi QS, Isales CM, and Shi XM. 2008. Age-related changes in the osteogenic differentiation potential of mouse bone marrow stromal cells. J. Bone Miner. Res 23: 1118–1128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nair S, Fang M, and Sigal LJ. 2015. The natural killer cell dysfunction of aged mice is due to the bone marrow stroma and is not restored by IL-15/IL-15Ra treatment. Aging Cell 14: 180–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wong E, Xu RH, Rubio D, Lev A, Stotesbury C, Fang M, and Sigal LJ. 2018. Migratory Dendritic Cells, Group 1 Innate Lymphoid Cells, and Inflammatory Monocytes Collaborate to Recruit NK Cells to the Virus-Infected Lymph Node. Cell Rep. 24: 142–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pak-wittel MA, Yang L, Sojka DK, Rivenbark JG, and Yokoyama WM. 2012. Interferon-γ mediates chemokine-dependent recruitment of natural killer cells during viral infection. Proc. Natl. Acad. Sci 110: 50–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Xiang RF, Li S, Ogbomo H, Stack D, and Mody CH. 2018. β1 Integrins Are Required To Mediate NK Cell Killing of Cryptococcus neoformans. J. Immunol 201: 2369–2376. [DOI] [PubMed] [Google Scholar]
- 55.Rebustini IT, Myers C, Lassiter KS, Surmak A, Szabova L, Holmbeck K, Pedchenko V, Hudson BG, and Hoffman MP. 2009. MT2-MMP-Dependent Release of Collagen IV NC1 Domains Regulates Submandibular Gland Branching Morphogenesis. Dev. Cell [DOI] [PMC free article] [PubMed]
- 56.Diaferia GR, Jimenez-Caliani AJ, Ranjitkar P, Yang W, Hardiman G, Rhodes CJ, Crisa L, and Cirulli V. 2013. β1 Integrin Is a Crucial Regulator of Pancreatic β-Cell Expansion. Development 140: 3360–3372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Min-Oo G, and Lanier LL. 2014. Cytomegalovirus generates long-lived antigen-specific NK cells with diminished bystander activation to heterologous infection. J. Exp. Med 211: 2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sun JC, Madera S, Bezman NA, Beilke JN, Kaplan MH, and Lanier LL. 2012. Proinflammatory cytokine signaling required for the generation of natural killer cell memory. J. Exp. Med 209: 947–954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kastenmüller W, Torabi-Parizi P, Subramanian N, Lämmermann T, and Germain RN. 2012. A spatially-organized multicellular innate immune response in lymph nodes limits systemic pathogen spread. Cell . [DOI] [PMC free article] [PubMed]
- 60.Lee S-H, Zafer A, de Repentigny Y, Kothary R, Tremblay ML, Gros P, Duplay P, Webb JR, and Vidal SM. 2003. Transgenic Expression of the Activating Natural Killer Receptor Ly49H Confers Resistance to Cytomegalovirus in Genetically Susceptible Mice. J. Exp. Med 197: 515–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cheng TP, French AR, Plougastel BFM, Pingel JT, Orihuela MM, Buller ML, and Yokoyama WM. 2008. Ly49h is necessary for genetic resistance to murine cytomegalovirus. Immunogenetics 60: 565–573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sun JC, Beilke JN, and Lanier LL. 2009. Adaptive immune features of natural killer cells. Nature 457: 557–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.