Abstract
The Bombyx mori nucleopolyhedrovirus (BmNPV) baculovirus expression system (BES) is a eukaryotic expression system. It possesses great capability for post‐translation modification in expression of foreign proteins. With the counterselection cassette rpsL‐neo and phage λ‐Red recombinase, the defective‐rescue BmNPV BES reBmBac can be employed for efficient heterologous multigene coexpression at different gene sites in one baculovirus genome. In the present study, a recombinant baculovirus, reBm‐Cαγ, carrying two types of chicken interferon (IFN) genes (chIFN‐α and chIFN‐γ) was constructed using the reBmBac system. The chIFN‐α and chIFN‐γ genes were inserted into the same baculovirus genome at the polyhedron and p10 gene sites, respectively. The recombinant baculovirus was capable of coexpressing both chIFN‐α and chIFN‐γ. The expression levels of the two types of IFN in the coexpression product were exponentially high, at approximately 1.7 and 2.5 times higher, respectively, than those in the corresponding single‐expression products. The increase in expression level corresponds to replacement of the nonessential p10 gene in the reBm‐Cαγ recombinant baculovirus. This coexpression of recombinant chicken IFNs showed superior antiviral activity.
Keywords: baculovirus expression system, coexpression, reBmBac system, recombinant chicken interferon
In this study, we developed a construction strategy of recombinant Bombyx mori nucleopolyhedrovirus for multigene coexpression. This strategy provides a method for gene deletion or insertion at any site of the baculovirus genome in Escherichia coli. Using this strategy, we constructed the recombinant baculovirus containing two types of chicken interferon genes (interferon‐α and interferon‐γ) at different gene sites. The production of silkworm infected with this recombinant baculovirus shown higher expression level and antiviral activity.
1. INTRODUCTION
Since its inception more than 30 years ago, the baculovirus expression system (BES) has been widely employed for recombinant protein expression at massive levels (van Oers, Pijlman, & Vlak, 2015; Smith, Summers, & Fraser, 1983). Depending on posttranslational modifications in insect cells and larvae, the BES is markedly suitable for eukaryotic protein expression (Kidd & Emery, 1993). In recent years, multigene expression employing the BES has been reported (Berger, Fitzgerald, & Richmond, 2004; Kanai, Athmaram, Stewart, & Roy, 2013; Yao et al., 2012). Multigene expression in a single recombinant baculovirus has advantages in expression applications. The expression of double‐chain antibodies and packaging of recombinant adeno‐associated virus (rAAV) are a few examples of this expression system (Furuta, Ogawa, Katsuda, Fujii, & Yamaji, 2010; Negrete, Yang, Mendez, Levy, & Kotin, 2007).
Bombyx mori nucleopolyhedrovirus (BmNPV) is one of the most widely employed baculoviruses for gene expression. In the last 3 decades, BmNPV has undergone modifications in many ways to generate recombinant BmNPV that is more convenient and enhances the expression efficiency of foreign genes (Kato, Kajikawa, Maenaka, & Park, 2010; Maeda et al., 1985). Compared with the AcMNPV‐cell expression system, the BmNPV‐silkworm system possesses better posttranslational processing and greater expression efficiency (Dojima et al., 2009; Usami et al., 2011). Previously, we successfully constructed a reBmBac system for recombinant BmNPV with increased efficiency (Liu et al., 2016). By utilizing this system, researchers can proficiently and rapidly obtain recombinant baculoviruses and target proteins in silkworms.
Interferons (IFNs) were first discovered in the 1950s, and since then, they have been researched deeply in many fields. Interferons are categorized into three major classes. Type I IFNs are generated by almost any type of cell in response to invading pathogens (Alsharifi, Mullbacher, & Regner, 2008). They can induce the expression of specific antiviral proteins and related physiological responses by binding with specific receptors on the cell membrane (Levy, Marie, & Durbin, 2011). Type II IFNs possess strong immune regulation ability and can regulate the activity of lymphocytes (Muller et al., 1994; Platanias, 2005). Type III IFNs have been recently discovered. They have functions similar to those of type I IFNs (Kotenko, 2011; Sheppard et al., 2003). Some studies indicate that type I and II IFNs demonstrate synergy in the establishment of an antiviral state (Muller et al., 1994; Platanias, 2005; Sekellick, Lowenthal, O'Neil, & Marcus, 1998).
Chicken IFN‐α (chIFN‐α) is a type I IFN. Research has revealed its antiviral potential against Rous sarcoma virus, Newcastle disease virus, infectious bursal disease virus, and avian influenza virus in vitro and in vivo (Jiang, Yang, & Kapczynski, 2011; Marcus, van der Heide, & Sekellick, 1999; Meng et al., 2011; O'Neill, Livant, & Ewald, 2010). Chicken IFN‐γ (chIFN‐γ) is a type II IFN. It demonstrates avian virus inhibition both in vitro and in vivo and has the capability to prevent poultry coccidiosis (Cardenas‐Garcia et al., 2016; Khatri & Sharma, 2008; Shah et al., 2010). Studies also illustrate that chIFN‐γ enhances the growth performance of reared broilers (Lowenthal, 2001). These two IFNs have an immune synergism effect. The combination of chIFN‐α and chIFN‐γ can significantly enhance viral inhibition and elicit an antiviral state (Plachy et al., 1999; Sekellick et al., 1998).
In the current study, recombinant BmNPV simultaneously carrying the chIFN‐α and chIFN‐γ genes at distinct gene sites was constructed using the reBmBac system. This recombinant baculovirus was employed for the coexpression of two types of IFN and can provide a foundation for the combination of IFNs and their possible future therapeutic application.
2. MATERIALS AND METHODS
A Bombyx mori‐derived cell line, Bm5, was cultured in TC100 insect cell culture medium (Applichem) with 10% fetal bovine serum (FBS, Gibco, USA) at 27°C according to published procedures (Summers & Smith, 1987). DMEM (Dulbecco's modified Eagle's medium) and Trypsin‐EDTA were obtained from Thermo Fisher Scientific. Specific pathogen‐free (SPF) fertilized eggs (8–10 days) were obtained from Beijing Vital River Laboratory Animal Technology Co., Ltd. The recombinant vesicular stomatitis virus VSV‐GFP was acquired from Lanzhou Veterinary Research Institute, CAAS.
Escherichia coli BW25113/pKD46 was obtained from the Molecular, Cellular and Developmental Biology Department, Kline Biology Tower 830, Yale University. The reBmBac vector was constructed in our laboratory. The pVL1393 transfer vector and Lipofectin were acquired from Invitrogen. Rabbit anti‐chicken IFN‐α and IFN‐γ antibodies were obtained from RayBiotech. HRP‐conjugated goat anti‐rabbit IgG was obtained from Abcam.
2.1. Construction of the gene‐targeting vector
The pP10 vector (GenBank ID: MN702625) was used to transfer the target gene into the baculovirus at the p10 gene site. Homologous targeting arms 232 bp upstream and 118 bp downstream of the p10 gene were amplified and inserted into the pMD18‐simple vector.
The pP10‐rpsL‐neo vector (GenBank ID: MN702626) was utilized to knock out the p10 gene. The counterselection cassette rpsL‐neo (GenBank ID: GU084141.1) was synthesized and inserted into the pP10 vector.
The pVL1393‐Cα and pVL1393‐Cγ vectors were employed to individually transfer the chicken interferon‐α gene and interferon‐γ gene into the recombinant baculovirus genome at the polyhedron site. The chIFN‐α and chIFN‐γ genes were codon optimized according to amino acid sequences (GenBank IDs: ADU60333.1 and ABI83735.1) and synthesized. These two genes were individually inserted into the pVL1393 transfer vector, in which there are baculovirus recombination sequences on both sides of the MCS. The pP10‐Cγ vector was used to transfer the chIFN‐γ gene into the recombinant baculovirus genome at the p10 gene site. The chIFN‐γ gene was inserted into the pP10 vector via BamH I/Not I digestion.
2.2. Construction of the recombinant baculovirus
The reBmBac vector was modified from BmNPV. An E. coli CopyControl origin of replication was inserted into the genome at chi‐cat gene site. And tetracycline resistance gene was inserted at polyhedrin gene site. These two make sure the baculovirus DNA can be edited and amplified in E. coli. The essential ORF1629 gene was also partial deleted to make sure the highly recombination efficiency.
According to a published protocol (Datsenko & Wanner, 2000; Liu et al., 2016), the p10 gene of reBmBac baculovirus genomic DNA was replaced by the rpsL‐neo cassette by phage λ‐Red recombinase in E. coli. Then, the rpsL‐neo cassette was replaced by the chIFN‐γ gene. The recombinant baculovirus shuttle vector containing the chIFN‐γ gene at the p10 gene site was named reBmBac‐p10Cγ.
According to published procedures (Liu et al., 2016), the pVL1393‐Cα or pVL1393‐Cγ vector was mixed with the reBmBac vector. The mixture was used to cotransfect Bm5 cells. chIFN‐α and chIFN‐γ genes were inserted into the baculovirus genome at the polyhedron gene site. The recombinant baculoviruses were named reBm‐Cα and reBm‐Cγ. A mixture of the pVL1393‐Cα vector and reBmBac‐p10Cγ was then utilized to cotransfect Bm5 cells. chIFN‐α was transferred into the reBmBac‐p10Cγ genome at the polyhedron gene site. The recombinant baculovirus, which contained the chIFN‐α gene at the polyhedron site and the chIFN‐γ gene at the p10 site, was named reBm‐Cαγ. The recombinant baculoviruses reBm‐Cαγ, reBm‐Cα, and reBm‐Cγ were purified by plaque screening (Pen, Welling, & Welling‐Wester, 1989).
2.3. Expression of interferon in silkworm larvae and pupae
Fifth instar silkworm larvae or pupae were injected with 5 μl of cell culture media containing recombinant BmNPV (107–108 plaque‐forming units (PFU) mL−1) between abdominal knobs on the backside. Silkworm larvae and pupae were reared at 25 ~ 27°C and 65% humidity for 108–120 hr. Larval hemolymph was collected by cutting prolegs, and 1‐phenyl‐2‐thiourea was added at 0.1 mM to prevent melanization. Larval hemolymph and pupae were stored at −20°C for subsequent assays.
2.4. Antiviral activity assay of recombinant interferon in chicken embryo fibroblasts
According to published procedures (Rein & Rubin, 1968), chicken embryo fibroblasts (CEFs) were prepared. A CEF cell suspension was isolated from 9‐ to 11‐day‐old chicken embryos. The cell concentration was approximately 1 × 106 cells per milliliter. CEF cells were seeded in 96‐well plates at a constant cell density of 1 × 105 cells per well for 8–12 hr at 37°C in 5% CO2 in air.
A GFP‐reduction assay with VSV‐GFP was used to assay the antiviral activity of recombinant chicken IFNs in CEFs. Larval hemolymph containing IFN expression product was treated by ultrasonication. After centrifugation, the supernatant of the IFN product was filtered with a 0.22 μm syringe filter. Interferon supernatant was gradient diluted in DMEM with 7% FBS. After incubation with diluted IFN for 18–24 hr at 37°C in 5% CO2 in air, CEF cells were infected with VSV‐GFP at 10 TCID50 per well. After 24 hr, cell cytopathic effect (CPE) and green fluorescence were detected. The antiviral activity of IFNs was calculated according to the CPE reduction method (Ge et al., 2006).
2.5. Inactivation of chicken interferon γ
IFN‐α is both acid and heat resistant. However, IFN‐γ is easily deactivated by both acid (pH 2.0) and temperature (56°C) (Ho, Armstrong, & Breinig, 1975). Thus, the antiviral activity of IFN‐α in the coexpression product was studied. The pH value of larval hemolymph or pupa product was adjusted to 2.0 ± 0.2 using a hydrochloric acid solution (0.1 M). After 24 hr of static incubation at 4°C and filtration, the pH value was adjusted to 7.0 ± 0.1 using a sodium hydroxide solution (1 M). After 1 hr of static incubation at 4°C and filtration, the sample was heat treated (56°C) for 30 min. After filtration with a 0.22 μm syringe filter, IFN‐γ of the product was deactivated.
The expression of IFN was detected by western blotting according to the Protein Blotting Guide (Bio‐Rad). Relative expression levels of IFN‐α and IFN‐γ in these products were detected by indirect ELISA.
3. RESULTS
3.1. Generation of the recombinant baculoviruses reBm‐Cαγ, reBm‐Cα and reBm‐Cγ
Two transfer plasmids, pVL1393‐Cα and pVL1393‐Cγ, were used to transfer the chIFN‐α and chIFN‐γ genes into reBmBac for single expression of IFN. The recombinant baculoviruses reBm‐Cα and reBm‐Cγ, constructed though cotransfection of transfer plasmids and reBmBac genome DNA (Figure 1a), were capable of expressing chIFN‐α and chIFN‐γ, respectively. In the IFN coexpression recombinant baculovirus reBm‐Cαγ, the chIFN‐γ gene was inserted into the reBmBac genome downstream of the p10 promoter sequence through homologous recombination in E. coli. The chIFN‐α gene was inserted into the same reBmBac genome downstream of the polyhedron promoter sequence through cotransfection in cells (Figure 1b).
Figure 1.
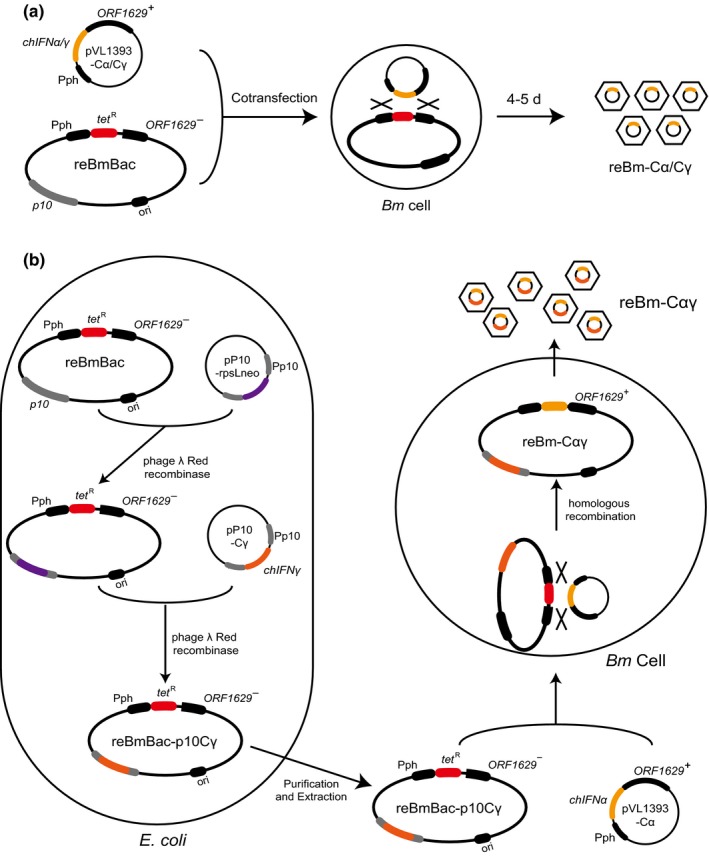
Schematic representation for the generation of recombinant viruses containing chicken IFNs. (a) Construction of recombinant baculoviruses containing a single interferon gene. During cotransfection of reBmBac DNA and foreign gene transfer vector, the interferon gene was inserted into baculovirus genome at the polyhedrin site. The tetracycline resistance (tetR) gene was replaced. (b) construction of a coexpression recombinant baculovirus containing chIFN‐α and chIFN‐γ at distinct gene sites. In Escherichia coli, chIFN‐γ was firstly inserted into reBmBac at p10 gene site by using phage λ‐Red recombinase. Then, the reBmBac‐p10Cγ vector was purified and cotransfected with chIFN‐α transfer vector to prepare the coexpression recombinant baculovirus in Bm cells
3.2. Expression and antiviral activity analyses of coexpression IFNs and single‐expression IFNs
The expression products of chIFN‐α and chIFN‐γ were analyzed by Western blotting (Figure 2). Figure 2 demonstrates that an approximately 22 kDa protein band that reacted with an anti‐chIFN‐α antibody was detected in reBm‐Cαγ and reBm‐Cα expression samples. Likewise, an approximately 19 kDa protein band that reacted with an anti‐chIFN‐γ antibody was observed in reBm‐Cαγ and reBm‐Cγ expression samples. No corresponding immunoreactive protein was detected in the negative control sample from larval hemolymph infected with control BmNPV.
Figure 2.
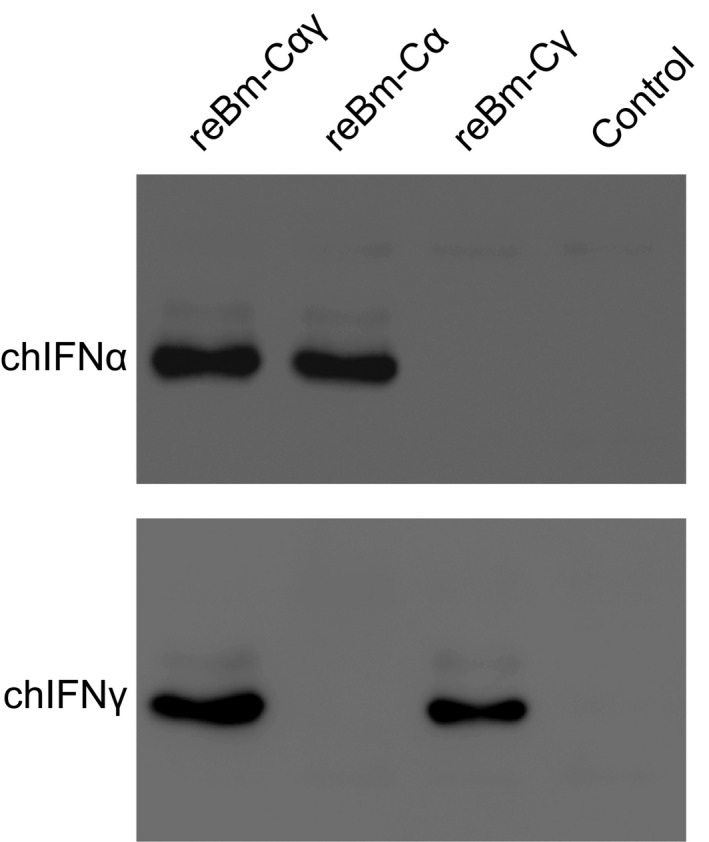
Western blot assay of chIFN‐α and chIFN‐γ expression in single‐ and coexpression products. The reBm‐Cαγ sample is larval hemolymph infected with the coexpression recombinant baculovirus reBm‐Cαγ. The reBm‐Cα and reBm‐Cγ samples are larval haemolymphs infected with the single‐expression recombinant baculovirus reBm‐Cα or reBm‐Cγ. chIFN‐α proteins were detected in reBm‐Cαγ and reBm‐Cα samples at approximately 22 kDa. ChIFN‐γ proteins were detected in reBm‐Cαγ and reBm‐Cγ samples at approximately 19 kDa. No corresponding immunoreactive protein was detected in control samples
Antiviral activity of recombinant IFNs was assayed utilizing a CPE inhibition assay with CEF cells. Recombinant IFN products could inhibit VSV‐GFP infection in CEFs (Figure 3). The antiviral activity assay results indicated that the antiviral potential of reBm‐Cα, reBm‐Cγ, and reBm‐Cαγ products were 3.26 ± 0.61 × 106 IU/mL, 5.08 ± 0.43 × 106 IU/mL, and 3.27 ± 0.50 × 107 IU/mL in hemolymph, respectively. These recombinant baculovirus expression products were then acid (pH 2.0) and heat treated (56°C). IFN‐α is acid and heat resistant, but IFN‐γ is acid and heat labile (Ho et al., 1975). Therefore, the antiviral activity of the reBm‐Cα product remained almost the same before and after treatment. The antiviral activity of the reBm‐Cγ product declined to an undetectable level. The antiviral activity of the reBm‐Cαγ product was still 5.78 ± 0.88 × 106 IU/mL, which was due to IFN‐α activity. This activity was 2 times greater than that of the reBm‐Cα product. This enhanced expression level of IFN‐α must be due to p10 gene deletion in coexpression recombinant baculovirus (Hitchman et al., 2010).
Figure 3.
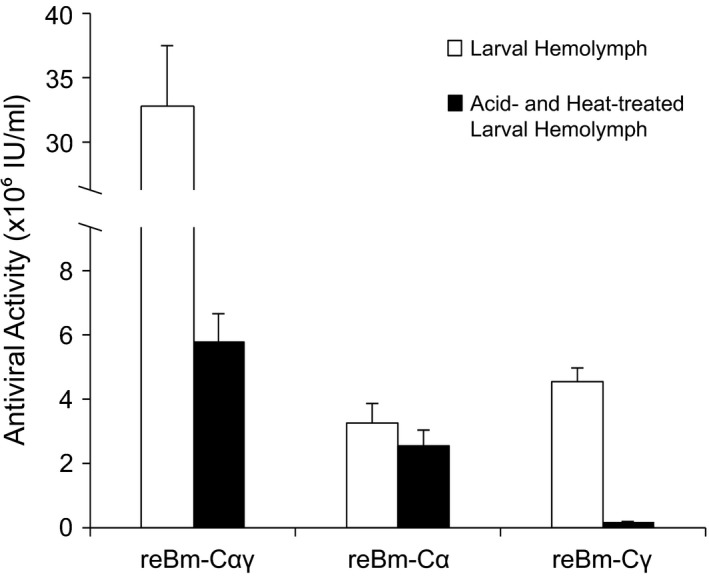
Antiviral activity assay of single‐expression IFNs versus coexpression IFNs. The antiviral activity of the reBm‐Cαγ product was approximately 5–10 times greater than that of the reBm‐Cα or reBm‐Cγ product. After acid and heat treatment (pH 2.0, 56°C), the antiviral activity of the reBm‐Cα product was almost the same as that before the treatments. The antiviral activity of the treated reBm‐Cγ product was negligible. The antiviral activity of the treated reBm‐Cαγ product was greater than that of chIFN‐α. It was approximately 1.7 times greater than that of the reBm‐Cα product
The ELISA results for chIFN‐γ illustrate that the expression level of chIFN‐γ in reBm‐Cαγ‐infected larval hemolymph was approximately 2.5 times greater than that in reBm‐Cγ‐infected larval hemolymph (Figure A1).
3.3. The synergistic antiviral effect of interferon type I and II
The reBm‐Cα and reBm‐Cγ products were diluted to 10 × 104 IU/mL and mixed in different proportions. The antiviral activity of the mixtures was detected. The results revealed that chIFN‐α and chIFN‐γ (at a ratio of 1:2) enhanced the maximum antiviral activity, that is 17.92 ± 1.07 × 104 IU/mL (Table 1). This result indicated that an increased ratio of IFN type II caused an obvious synergistic antiviral effect of IFN type I and II.
Table 1.
Antiviral activity of a mixture of two types of interferon
| Ratio of the two types interferon | Theoretical value (×104 IU/ml) | Measured value (×104 IU/ml) | |
|---|---|---|---|
| chIFN‐α | chIFN‐γ | ||
| 4 | 1 | 10 | 10.04 ± 0.75 |
| 2 | 1 | 10 | 10.22 ± 0.59 |
| 1 | 1 | 10 | 11.41 ± 0.78 |
| 1 | 2 | 10 | 17.92 ± 1.07 |
| 1 | 4 | 10 | 14.05 ± 0.99 |
3.4. Inhibitory effect of coexpression interferons on in vitro replication of Marek's disease virus
The highly oncogenic strain RB1B of Marek's disease virus (MDV) has the ability to replicate and develop plaques in CEFs. Chicken embryo fibroblasts treated with co‐ or single‐expression chIFNs were employed to determine the inhibitory effect on the in vitro replication of MDV. Mean PFU is listed in Table 2. The results revealed that 800 IU of coexpression product completely inhibited MDV replication in CEFs. The same titer of single‐expression IFN products containing one of the two types of IFN could only partially inhibit MDV replication. This result indicated that chicken IFNs expressed in our baculovirus‐silkworm system have excellent anti‐MDV activity. This study further validated the synergistic antiviral potential of type I and type II IFNs.
Table 2.
Inhibitory effect of interferons on MDV
| Larval hemolymph sample | CK | reBm‐Cα | reBm‐Cγ | reBm‐Cαγ |
|---|---|---|---|---|
| Interferon dose (IU/well) | 0 | 800 | 800 | 800 |
| Plaque (PFU/well) | 18.5 ± 1.8 | 11.8 ± 0.9 | 10.3 ± 1.4 | 0 |
| 16.0 ± 2.5 | 9.8 ± 1.8 | 9.5 ± 1.5 | 0 | |
| 20.0 ± 4.0 | 12.5 ± 1.5 | 12.8 ± 1.7 | 0 |
4. DISCUSSION
In our previous studies, the reBmBac recombinant baculovirus construction strategy was successfully established, and recombinant porcine IFN‐α was efficiently expressed (Liu et al., 2016). Then, IFNs from several other species were successively expressed utilizing this system. All of the IFN products exhibited prominent antiviral activity.
In the present study, the coexpression of type I and II IFNs at different gene sites was successfully achieved using a single recombinant baculovirus. The type I IFN chIFN‐α gene was introduced at the polyhedron gene site. The chIFN‐γ gene, a type II IFN‐encoding gene, was inserted at the p10 gene site. The antiviral potential of the coexpression product was five to ten times higher than that of any single‐expression product. After heat and acid treatment, the remaining IFN‐α antiviral activity of the coexpression product was approximately two times greater than that of the chIFN‐α single‐expression product. This finding indicates that the expression level of IFN‐α in the reBm‐Cαγ product was significantly higher than that in the reBm‐Cα product. ELISA results further augmented that multi‐IFN expression in the coexpression product was higher than that in the single‐expression product. This result was consistent with the antiviral assay. The increase in expression level is due to deletion of the p10 gene. Just like polyhedrin gene, p10 is a nonessential gene for baculovirus replication and budded virus production. And also, it is nonessential for recombinant protein expression, but with high expression level (Hitchman et al., 2010). In recombinant baculovirus, polyhedrin promoter is the preference for foreign gene expression. The polyhedrin gene is replaced by a foreign gene. The saved cost of polyhedrin expression provides for the recombinant protein expression. In reBm‐Cαγ, the deletion of p10 gene can also save a certain degree of cost of gene expression, even though the p10 promoter is weaker than the polyhedrin promoter. And these saving costs could be used for foreign gene expression. Thus, in reBm‐Cαγ, the deletion of p10 gene enhances the chIFN‐α expression which driven by polyhedrin promoter, indirectly. This finding also provides an optimal direction for enhanced expression levels. However, the antiviral assay against VSV and MDV showed that the antiviral potential of the coexpression product was more than two times greater than that of any single‐expression product. This difference must be due to the synergistic effect of the two types of IFN. Type I and II IFNs display diverse antiviral mechanisms. Here, coadministration of these two IFNs exhibited synergistic effects and elevated antiviral potential. Hence, the coexpression of the two IFNs by employing the BES is significant and beneficial. The coexpression products displayed greater antiviral activity and synergistic effects than the single‐expression products. This strategy of combined administration of chicken IFNs can suppress viral diseases in the poultry industry.
The insertion of the chIFN‐γ gene at the p10 gene site was mediated by the counterselection cassette rpsL‐neo and phage λ‐Red recombinase. There was no fundamental sequence or antibiotic resistance gene residue in the target gene site of the baculoviral genome. By employing this method, multiple gene insertion into various gene sites in one recombinant baculovirus can be achieved easily. This multigene baculovirus expression system can be a potential tool in many research fields. The BES is advantageous for antibody expression (Verma, Boleti, & George, 1998). Previously, heavy‐ and light‐chain genes were inserted in the same gene site (Furuta et al., 2010). Using the multigene expression strategy in the present study, heavy‐ and light‐chain genes can be introduced into different sites in one recombinant baculovirus, and enhanced expression levels will be achieved. rAAV packaging is another application area of the BES. In preceding studies, rAAV packaging required two or three recombinant baculoviruses, which individually contained cap, rep, and target genes (Aslanidi, Lamb, & Zolotukhin, 2009; Negrete et al., 2007). By employing our multigene expression strategy, rep, cap, and target genes can be inserted separately into the same recombinant baculovirus at the egt, p10, and polyhedron gene sites, respectively. The packaging efficiency of rAAV would thus be greater than that when using two or three recombinant baculoviruses (Galibert & Merten, 2011).
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTIONS
Xingjian Liu: Conceptualization‐Lead, Data curation‐Lead, Formal analysis‐Lead, Investigation‐Lead, Methodology‐Lead, Project administration‐Lead, Resources‐Equal, Validation‐Lead, Writing‐original draft‐Lead, Writingreview & editing‐Lead.
Xin Yang: Data curation‐Equal, Investigation‐Equal, Validation‐Equal, Writing‐review & editing‐Supporting.
Arslan Mehboob: Investigation‐Equal, Methodology‐Supporting, Validation‐Equal, Writing‐review & editing‐Supporting.
Xiaoyuan Hu: Investigation‐Supporting, Resources‐Equal, Writing‐review & editing‐Supporting.
Yongzhu Yi: Investigation‐Supporting, Writing‐original draft‐Supporting.
Yinü Li: Conceptualization‐Equal, Funding acquisition‐Equal, Methodology‐Supporting, Project administration‐Equal, Resources‐Supporting.
Zhifang Zhang: Conceptualization‐Equal, Funding acquisition‐Equal, Methodology‐Supporting, Project administration‐Equal, Writing‐original draft‐Supporting, Writing‐review & editing‐Supporting.
ETHICS STATEMENT
None required.
ACKNOWLEDGMENTS
This work was supported by The National Key Research and Development Program of China (No. 2016YFD0500108 and 2017YFD0500706), National Natural Sciences Foundation of China (No. 31670156 and 31872430).
APPENDIX 1.
Figure A1.
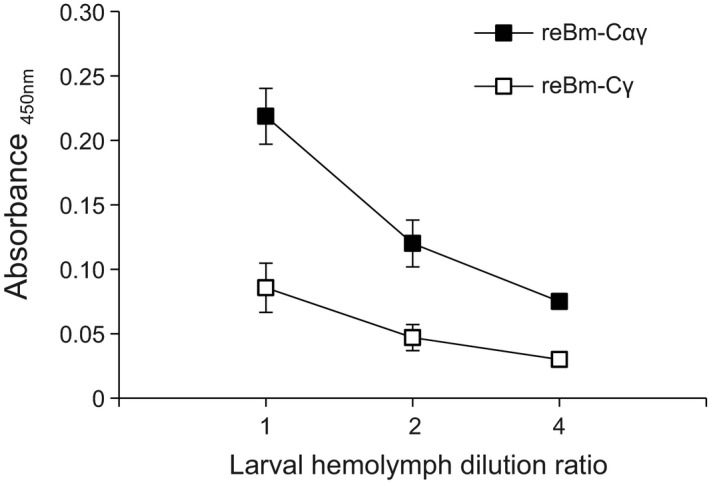
ELISA assay of chIFN‐γ expression in reBm‐Cαγ and reBm‐Cγ product. The reBm‐Cαγ sample is larval haemolymph infected with coexpression recombinant baculovirus reBm‐Cαγ. reBm‐Cγ sample is larval haemolymph infected with the single‐expression recombinant baculovirus reBm‐Cγ. The mean absorbance (450 nm) of reBm‐Cαγ sample was around 2.5 times as much as that of reBm‐Cγ sample
Liu X, Yang X, Mehboob A, et al. A construction strategy for a baculovirus‐silkworm multigene expression system and its application for coexpression of type I and type II interferons. MicrobiologyOpen. 2020;9:e979 10.1002/mbo3.979
Contributor Information
Yinü Li, Email: liyinv@caas.cn.
Zhifang Zhang, Email: bri-zhangzhifang@caas.cn.
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this published article.
REFERENCES
- Alsharifi, M. , Mullbacher, A. , & Regner, M. (2008). Interferon type I responses in primary and secondary infections. Immunology and Cell Biology, 86(3), 239–245. 10.1038/sj.icb.7100159 [DOI] [PubMed] [Google Scholar]
- Aslanidi, G. , Lamb, K. , & Zolotukhin, S. (2009). An inducible system for highly efficient production of recombinant adeno‐associated virus (rAAV) vectors in insect Sf9 cells. Proceedings of the National Academy of Sciences of the United States of America, 106(13), 5059–5064. 10.1073/pnas.0810614106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, I. , Fitzgerald, D. J. , & Richmond, T. J. (2004). Baculovirus expression system for heterologous multiprotein complexes. Nature Biotechnology, 22(12), 1583–1587. 10.1038/nbt1036 [DOI] [PubMed] [Google Scholar]
- Cardenas‐Garcia, S. , Dunwoody, R. P. , Marcano, V. , Diel, D. G. , Williams, R. J. , Gogal, R. M. , … Afonso, C. L. (2016). Effects of chicken interferon gamma on newcastle disease virus vaccine immunogenicity. PLoS ONE, 11(7), e0159153 10.1371/journal.pone.0159153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datsenko, K. A. , & Wanner, B. L. (2000). One‐step inactivation of chromosomal genes in Escherichia coli K‐12 using PCR products. Proceedings of the National Academy of Sciences of the United States of America, 97(12), 6640–6645. 10.1073/pnas.120163297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dojima, T. , Nishina, T. , Kato, T. , Uno, T. , Yagi, H. , Kato, K. , & Park, E. Y. (2009). Comparison of the N‐linked glycosylation of human beta1,3‐N‐acetylglucosaminyltransferase 2 expressed in insect cells and silkworm larvae. Journal of Biotechnology, 143(1), 27–33. 10.1016/j.jbiotec.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Furuta, T. , Ogawa, T. , Katsuda, T. , Fujii, I. , & Yamaji, H. (2010). Efficient production of an antibody Fab fragment using the baculovirus‐insect cell system. Journal of Bioscience and Bioengineering, 110(5), 577–581. 10.1016/j.jbiosc.2010.06.001 [DOI] [PubMed] [Google Scholar]
- Galibert, L. , & Merten, O. W. (2011). Latest developments in the large‐scale production of adeno‐associated virus vectors in insect cells toward the treatment of neuromuscular diseases. Journal of Invertebrate Pathology, 107(Suppl), S80–93. 10.1016/j.jip.2011.05.008 [DOI] [PubMed] [Google Scholar]
- Ge, J. , Wen, Z. , Wang, X. , Hu, S. , Liu, Y. , Kong, X. , … Bu, Z. (2006). Generating vesicular stomatitis virus pseudotype bearing the severe acute respiratory syndrome coronavirus spike envelope glycoprotein for rapid and safe neutralization test or cell‐entry assay. Annals of the New York Academy of Sciences, 1081, 246–248. 10.1196/annals.1373.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hitchman, R. B. , Possee, R. D. , Crombie, A. T. , Chambers, A. , Ho, K. , Siaterli, E. , … King, L. A. (2010). Genetic modification of a baculovirus vector for increased expression in insect cells. Cell Biology and Toxicology, 26(1), 57–68. 10.1007/s10565-009-9133-y [DOI] [PubMed] [Google Scholar]
- Ho, M. , Armstrong, J. A. , & Breinig, M. (1975). Interferon. Annual Review of Microbiology, 29(1), 131–161. 10.1146/annurev.mi.29.100175.001023 [DOI] [PubMed] [Google Scholar]
- Jiang, H. , Yang, H. , & Kapczynski, D. R. (2011). Chicken interferon alpha pretreatment reduces virus replication of pandemic H1N1 and H5N9 avian influenza viruses in lung cell cultures from different avian species. Virology Journal, 8, 447 10.1186/1743-422X-8-447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai, Y. , Athmaram, T. N. , Stewart, M. , & Roy, P. (2013). Multiple large foreign protein expression by a single recombinant baculovirus: A system for production of multivalent vaccines. Protein Expression and Purification, 91(1), 77–84. 10.1016/j.pep.2013.07.005 [DOI] [PubMed] [Google Scholar]
- Kato, T. , Kajikawa, M. , Maenaka, K. , & Park, E. Y. (2010). Silkworm expression system as a platform technology in life science. Applied Microbiology and Biotechnology, 85(3), 459–470. 10.1007/s00253-009-2267-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khatri, M. , & Sharma, J. M. (2008). IFN‐gamma upregulation and protection by macrophage‐adapted infectious bursal disease virus. Vaccine, 26(36), 4740–4746. 10.1016/j.vaccine.2008.06.053 [DOI] [PubMed] [Google Scholar]
- Kidd, I. M. , & Emery, V. C. (1993). The use of baculoviruses as expression vectors. Applied Biochemistry and Biotechnology, 42(2–3), 137–159. 10.1007/BF02788049 [DOI] [PubMed] [Google Scholar]
- Kotenko, S. V. (2011). IFN‐lambdas. Current Opinion in Immunology, 23(5), 583–590. 10.1016/j.coi.2011.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy, D. E. , Marie, I. J. , & Durbin, J. E. (2011). Induction and function of type I and III interferon in response to viral infection. Current Opinion in Virology, 1(6), 476–486. 10.1016/j.coviro.2011.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu, X. , Wei, Y. , Li, Y. , Li, H. , Yang, X. , Yi, Y. , & Zhang, Z. (2016). A highly efficient and simple construction strategy for producing recombinant Baculovirus Bombyx mori Nucleopolyhedrovirus. PLoS ONE, 11(3), e0152140 10.1371/journal.pone.0152140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenthal, J. W. (2001). New therapeutics for poultry: therapeutic applications of chicken interferon gamma (ChIFN‐y) in poultry: A report for the Rural Industries Research and Development Corporation. [Google Scholar]
- Maeda, S. , Kawai, T. , Obinata, M. , Fujiwara, H. , Horiuchi, T. , Saeki, Y. , … Furusawa, M. (1985). Production of human alpha‐interferon in silkworm using a baculovirus vector. Nature, 315(6020), 592–594. [DOI] [PubMed] [Google Scholar]
- Marcus, P. I. , van der Heide, L. , & Sekellick, M. J. (1999). Interferon action on avian viruses. I. Oral administration of chicken interferon‐alpha ameliorates Newcastle disease. Journal of Interferon and Cytokine Research, 19(8), 881–885. 10.1089/107999099313406 [DOI] [PubMed] [Google Scholar]
- Meng, S. , Yang, L. , Xu, C. , Qin, Z. , Xu, H. , Wang, Y. , … Liu, W. (2011). Recombinant chicken interferon‐alpha inhibits H9N2 avian influenza virus replication in vivo by oral administration. Journal of Interferon and Cytokine Research, 31(7), 533–538. 10.1089/jir.2010.0123 [DOI] [PubMed] [Google Scholar]
- Muller, U. , Steinhoff, U. , Reis, L. F. , Hemmi, S. , Pavlovic, J. , Zinkernagel, R. M. , & Aguet, M. (1994). Functional role of type I and type II interferons in antiviral defense. Science, 264(5167), 1918–1921. [DOI] [PubMed] [Google Scholar]
- Negrete, A. , Yang, L. C. , Mendez, A. F. , Levy, J. R. , & Kotin, R. M. (2007). Economized large‐scale production of high yield of rAAV for gene therapy applications exploiting baculovirus expression system. Journal of Gene Medicine, 9(11), 938–948. 10.1002/jgm.1092 [DOI] [PubMed] [Google Scholar]
- O'Neill, A. M. , Livant, E. J. , & Ewald, S. J. (2010). Interferon alpha‐induced inhibition of infectious bursal disease virus in chicken embryo fibroblast cultures differing in Mx genotype. Avian Diseases, 54(2), 802–806. 10.1637/9001-072309-Reg.1 [DOI] [PubMed] [Google Scholar]
- Pen, J. , Welling, G. W. , & Welling‐Wester, S. (1989). An efficient procedure for the isolation of recombinant baculovirus. Nucleic Acids Research, 17(1), 451 10.1093/nar/17.1.451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plachy, J. , Weining, K. C. , Kremmer, E. , Puehler, F. , Hala, K. , Kaspers, B. , & Staeheli, P. (1999). Protective effects of type I and type II interferons toward Rous sarcoma virus‐induced tumors in chickens. Virology, 256(1), 85–91. 10.1006/viro.1999.9602 [DOI] [PubMed] [Google Scholar]
- Platanias, L. C. (2005). Mechanisms of type‐I‐and type‐II‐interferon‐mediated signalling. Nature Reviews Immunology, 5(5), 375–386. 10.1038/nri1604 [DOI] [PubMed] [Google Scholar]
- Rein, A. , & Rubin, H. (1968). Effects of local cell concentrations upon the growth of chick embryo cells in tissue culture. Experimental Cell Research, 49(3), 666–678. 10.1016/0014-4827(68)90213-9 [DOI] [PubMed] [Google Scholar]
- Sekellick, M. J. , Lowenthal, J. W. , O'Neil, T. E. , & Marcus, P. I. (1998). Chicken interferon types I and II enhance synergistically the antiviral state and nitric oxide secretion. Journal of Interferon and Cytokine Research, 18(6), 407–414. 10.1089/jir.1998.18.407 [DOI] [PubMed] [Google Scholar]
- Shah, M. A. A. , Song, X. , Xu, L. , Yan, R. , Song, H. , Ruirui, Z. , … Li, X. (2010). The DNA‐induced protective immunity with chicken interferon gamma against poultry coccidiosis. Parasitology Research, 107(3), 747–750. 10.1007/s00436-010-1940-9 [DOI] [PubMed] [Google Scholar]
- Sheppard, P. , Kindsvogel, W. , Xu, W. , Henderson, K. , Schlutsmeyer, S. , Whitmore, T. E. , … Klucher, K. M. (2003). IL‐28, IL‐29 and their class II cytokine receptor IL‐28R. Nature Immunology, 4(1), 63–68. 10.1038/ni873 [DOI] [PubMed] [Google Scholar]
- Smith, G. E. , Summers, M. D. , & Fraser, M. J. (1983). Production of human beta interferon in insect cells infected with a baculovirus expression vector. Molecular and Cellular Biology, 3(12), 2156–2165. 10.1128/MCB.3.12.2156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers, M. D. , & Smith, G. E. (1987). A manual of methods for baculovirus vectors and insect cell culture procedures. Bulletin - Texas Agricultural Experiment Station, no. 1555.
- Usami, A. , Ishiyama, S. , Enomoto, C. , Okazaki, H. , Higuchi, K. , Ikeda, M. , … Nagaya, H. (2011). Comparison of recombinant protein expression in a baculovirus system in insect cells (Sf9) and silkworm. Journal of Biochemistry, 149(2), 219–227. 10.1093/jb/mvq138 [DOI] [PubMed] [Google Scholar]
- van Oers, M. M. , Pijlman, G. P. , & Vlak, J. M. (2015). Thirty years of baculovirus‐insect cell protein expression: From dark horse to mainstream technology. Journal of General Virology, 96(Pt 1), 6–23. 10.1099/vir.0.067108-0 [DOI] [PubMed] [Google Scholar]
- Verma, R. , Boleti, E. , & George, A. J. (1998). Antibody engineering: Comparison of bacterial, yeast, insect and mammalian expression systems. Journal of Immunological Methods, 216(1–2), 165–181. 10.1016/S0022-1759(98)00077-5 [DOI] [PubMed] [Google Scholar]
- Yao, L. , Wang, S. , Su, S. , Yao, N. , He, J. , Peng, L. , & Sun, J. (2012). Construction of a baculovirus‐silkworm multigene expression system and its application on producing virus‐like particles. PLoS ONE, 7(3), e32510 10.1371/journal.pone.0032510 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


