Abstract
Background
IgA nephropathy is the most common glomerulonephritis world‐wide. IgA nephropathy causes end‐stage kidney disease (ESKD) in 15% to 20% of affected patients within 10 years and in 30% to 40% of patients within 20 years from the onset of disease. This is an update of a Cochrane review first published in 2003 and updated in 2015.
Objectives
To determine the benefits and harms of immunosuppression strategies for the treatment of IgA nephropathy.
Search methods
We searched the Cochrane Kidney and Transplant Register of Studies up to 9 September 2019 through contact with the Information Specialist using search terms relevant to this review. Studies in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE, conference proceedings, the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Selection criteria
We included randomised controlled trials (RCTs) and quasi‐RCTs of treatment for IgA nephropathy in adults and children and that compared immunosuppressive agents with placebo, no treatment, or other immunosuppressive or non‐immunosuppressive agents.
Data collection and analysis
Two authors independently assessed study risk of bias and extracted data. Estimates of treatment effect were summarised using random effects meta‐analysis. Treatment effects were expressed as relative risk (RR) and 95% confidence intervals (95% CI) for dichotomous outcomes and mean difference (MD) and 95% CI for continuous outcomes. Risks of bias were assessed using the Cochrane tool. Evidence certainty was evaluated using GRADE methodology.
Main results
Fifty‐eight studies involving 3933 randomised participants were included. Six studies involving children were eligible. Disease characteristics (kidney function and level of proteinuria) were heterogeneous across studies. Studies evaluating steroid therapy generally included patients with protein excretion of 1 g/day or more. Risk of bias within the included studies was generally high or unclear for many of the assessed methodological domains.
In patients with IgA nephropathy and proteinuria > 1 g/day, steroid therapy given for generally two to four months with a tapering course probably prevents the progression to ESKD compared to placebo or standard care (8 studies; 741 participants: RR 0.39, 95% CI 0.23 to 0.65; moderate certainty evidence). Steroid therapy may induce complete remission (4 studies, 305 participants: RR 1.76, 95% CI 1.03 to 3.01; low certainty evidence), prevent doubling of serum creatinine (SCr) (7 studies, 404 participants: RR 0.43, 95% CI 0.29 to 0.65; low certainty evidence), and may lower urinary protein excretion (10 studies, 705 participants: MD ‐0.58 g/24 h, 95% CI ‐0.84 to ‐0.33;low certainty evidence). Steroid therapy had uncertain effects on glomerular filtration rate (GFR), death, infection and malignancy. The risk of adverse events with steroid therapy was uncertain due to heterogeneity in the type of steroid treatment used and the rarity of events.
Cytotoxic agents (azathioprine (AZA) or cyclophosphamide (CPA) alone or with concomitant steroid therapy had uncertain effects on ESKD (7 studies, 463 participants: RR 0.63, 95% CI 0.33 to 1.20; low certainty evidence), complete remission (5 studies; 381 participants: RR 1.47, 95% CI 0.94 to 2.30; very low certainty evidence), GFR (any measure), and protein excretion. Doubling of serum creatinine was not reported.
Mycophenolate mofetil (MMF) had uncertain effects on the progression to ESKD, complete remission, doubling of SCr, GFR, protein excretion, infection, and malignancy. Death was not reported.
Calcineurin inhibitors compared with placebo or standard care had uncertain effects on complete remission, SCr, GFR, protein excretion, infection, and malignancy. ESKD and death were not reported.
Mizoribine administered with renin‐angiotensin system inhibitor treatment had uncertain effects on progression to ESKD, complete remission, GFR, protein excretion, infection, and malignancy. Death and SCr were not reported.
Leflunomide followed by a tapering course with oral prednisone compared to prednisone had uncertain effects on the progression to ESKD, complete remission, doubling of SCr, GFR, protein excretion, and infection. Death and malignancy were not reported.
Effects of other immunosuppressive regimens (including steroid plus non‐immunosuppressive agents or mTOR inhibitors) were inconclusive primarily due to insufficient data from the individual studies in low or very low certainty evidence. The effects of treatments on death, malignancy, reduction in GFR at least of 25% and adverse events were very uncertain. Subgroup analyses to determine the impact of specific patient characteristics such as ethnicity or disease severity on treatment effectiveness were not possible.
Authors' conclusions
In moderate certainty evidence, corticosteroid therapy probably prevents decline in GFR or doubling of SCr in adults and children with IgA nephropathy and proteinuria. Evidence for treatment effects of immunosuppressive agents on death, infection, and malignancy is generally sparse or low‐quality. Steroid therapy has uncertain adverse effects due to a paucity of studies. Available studies are few, small, have high risk of bias and generally do not systematically identify treatment‐related harms. Subgroup analyses to identify specific patient characteristics that might predict better response to therapy were not possible due to a lack of studies. There is no evidence that other immunosuppressive agents including CPA, AZA, or MMF improve clinical outcomes in IgA nephropathy.
Plain language summary
Immunosuppressive agents for treating IgA nephropathy
What is the issue? IgA nephropathy is a common kidney disease that often leads to decreased kidney function and may result ultimately in kidney failure for one‐third of affected people. The cause of IgA nephropathy is not known, although most people with the disease have abnormalities in their immune system.
What did we do? We searched for all the research trials that assessed the effect of immunosuppressive therapy in people with IgA nephropathy in September 2019. We measured the certainty we could have about the treatments using a system called "GRADE".
What did we find? We found 58 studies involving 3933 adults and children who were treated with immunosuppressive therapy. Patients in the studies were given either steroids or other forms of therapy to reduce the actions of their immune system. The treatment they got was decided by random chance. Steroid therapy taken for 2 to 4 months appeared to slow damage to the kidney and probably prevents patients from developing kidney failure. It is really uncertain whether steroids cause side effects such as serious infection. One study was stopped early because patients who received steroid therapy had more infections than those patients who were given placebo. Other medications like cyclophosphamide, azathioprine, and mycophenolate mofetil did not clearly protect kidney function in people with IgA nephropathy.
Conclusions
Steroid therapy may prevent kidney failure in IgA nephropathy but the risks of serious infections are uncertain with treatment.
Summary of findings
Background
Description of the condition
IgA nephropathy was first described in 1968 by Dr J. Berger. Characterised by prominent mesangial IgA deposits seen diffusely on immunofluorescence microscopy, the condition was initially thought to be a rare and benign cause of recurrent haematuria (Berger 1968). It has since become apparent, however, that IgA nephropathy is neither rare nor benign. Although biopsy practices differ from region to region, thus affecting the frequency of diagnosis of IgA nephropathy, it has been demonstrated that IgA nephropathy is the most common glomerular disease world‐wide (D'Amico 1987; Han 2010) with a variable prevalence ranging from 5% to more than 40% (Schena 2009).
The natural history of IgA nephropathy is now known to be highly heterogeneous and far from benign in many patients. While up to 50% of patients experience lasting remission (Kim 2016; Nolin 1999), 40% can develop end‐stage kidney disease (ESKD) within 20 years (Manno 2007), while another 30% to 40% experience decreased kidney function (Inagaki 2017; Rekola 1991). Overall, as many as 15% to 50% of those affected develop chronic kidney disease (CKD) and eventually ESKD (Rostoker 1995; Schena 2001). Studies have demonstrated that risk factors associated with disease progression include evidence of proteinuria, especially in people with proteinuria < 1 g/day (Reich 2007), hypertension (Liu 2019) or elevated serum creatinine (SCr) at the time of kidney biopsy, microhematuria at diagnosis (Gallo 1988; Manno 2007; Neelakantappa 1988), and specific histological lesions (as reported in the Oxford classification) (Cattran 2009; Haas 2017; Trimarchi 2017). These prognostic data may help stratify those patients at highest need for effective therapy.
Evidence suggests that IgA nephropathy is a consequence of abnormal glycosylation of O‐linked glycans in the hinge region of IgA1, resulting in increased circulation of galactose‐deficient IgA1 (Gd‐IgA1) (Gale 2017; Mestecky 1993). Most patients have some abnormalities of the immune system some time in their disease course, including increased circulating IgA or some other humoral or cellular abnormality. It has been shown that the IgA molecules deposited in the glomerular mesangium have the same abnormalities of glycosylation (Hiki 2001). Altered IgA glycosylation may enhance mesangial deposition due to the formation of pathogenic immune complexes or by promoting IgA molecular interactions with kidney matrix proteins and/or mesangial cell immune receptors.
Complement system activation occurs in IgA nephropathy through the alternative and lectin pathways, with complement components identified in pathogenic mesangial deposits (Maillard 2015), Evidence for complement activity in the progression of IgA nephropathy glomerular injury has led to the development of short interfering RNA molecules (siRNA) against complement component 5 (C5) which is undergoing evaluation in a phase 2 randomised controlled trial (RCT) (NCT03841448).
Description of the intervention
Despite better understanding of the pathogenic mechanisms causing IgA nephropathy, there is no established disease‐targeted treatment for IgA nephropathy and various treatments have been applied, including corticosteroid, azathioprine (AZA), calcineurin inhibitors (CNIs), cyclophosphamide (CPA), mycophenolate mofetil (MMF), rituximab and leflunomide (Hou 2017; Lafayette 2017; Locatelli 1999; Pozzi 2010; Song 2017).
IgA nephropathy has been identified as having an inflammatory basis leading to the biological rationale of corticosteroid therapy (Coppo 2018). Over the last decades, some studies have reported that intravenous steroid pulse therapy in combination with oral prednisolone are effective for reducing proteinuria and preventing ESKD, as well as increasing 10‐year survival (Pozzi 1999). Evidence from observational studies (Tesar 2015) and RCTs (TESTING 2017) showed potential benefits of corticosteroid treatment in patients with proteinuric IgA nephropathy, although severe infectious complications and a higher mortality risk has suggested the need to evaluate intervention strategies that have lower toxicity.
Tonsillectomy combined with steroid pulse therapy has been shown to induce had a significant impact on clinical remission of IgA proteinuria and may be beneficial for long‐term kidney survival (Hotta 2001). In Asian countries, tonsillectomy is performed in at least 50% of adults with IgA nephropathy, however genetic variation may impact on IgA susceptibility and therapeutic response to this intervention strategy (Hirano 2019). By contrast, some studies have shown no therapeutic effect of corticosteroid (Lai 1986) and tonsillectomy (Piccoli 2010) in patients with IgA nephropathy leading to therapeutic uncertainty.
The recent focus on the role of gut–kidney axis in IgA nephropathy has led to development of selective corticosteroid formulations targeting the intestinal mucosal immune system, aiming to reduce proteinuria and stabilise kidney function with fewer systemic adverse events from steroid therapy (NEFIGAN 2017).
Patients may not always respond to corticosteroid therapy leading to consideration of additive immunosuppressive therapies to obtain a synergistic effect. Although IgA nephropathy is likely an autoimmune kidney disease, there is uncertainty about whether some immunosuppressive agents such as AZA or CPA suppress disease activity, reduce proteinuria or protect kidney function particularly in the absence of rapidly progressive glomerulonephritis (Locatelli 1999; Walker 1990a). The supportive versus immunosuppressive therapy for the treatment of progressive IgA nephropathy (STOP‐IgAN 2008) RCT showed that combined corticosteroid and immunosuppressive therapy may be superior to supportive care alone.
CNIs possess potent immunosuppressive properties, suppressing the activation and proliferation of T cells to inhibit synthesis of interleukin (IL)‐2. This suppresses secondary synthesis of various cytokines, including IL‐4 and tumour necrosis factor‐alpha. Despite these immunomodulating effects, there are limited data for protection of kidney function and evidence of increased side effects with CNIs (Song 2017).
MMF selectively inhibits the proliferation of T and B lymphocytes, antibody production, generation of cytotoxic T cells and the recruitment of leukocytes to sites of inflammation. However, experimental evidence has not clearly shown that the anti‐inflammatory properties of MMF, by attenuating glomerular and interstitial injury, are beneficial in the treatment of progressive IgA nephropathies with an acceptable safety profile (Maes 2004).
Few RCTs have evaluated the efficacy of leflunomide in the treatment of IgA nephropathy to demonstrate reduction in proteinuria and protection of kidney function (Cheng 2015). Leflunomide, generally evaluated in China, has very limited efficacy data (Lou 2006).
There has been limited stratification by risk of ESKD or disease severity in studies evaluating IgA nephropathy management. Substantial disease heterogeneity suggests a validated tool for IgA nephropathy could support accurate prediction of disease progression and enrich trial populations with patients at highest risk of ESKD (Barbour 2019). Although clinical evidence suggests that treatment of IgA nephropathy with either single and combined treatments regimen can lead to partial or complete remission and prevent loss of kidney function, some patients still experience progressive kidney injury (Moriyama 2019). The protective role of immunosuppressive therapy has been uncertain in part due to the small sample sizes and short duration therapy and follow‐up in available studies. In addition, global heterogeneity in disease activity and susceptibility based on ethnicity may impact on interpretation of treatment efficacy in different ethnicity groups and international regions (Kiryluk 2012). As a consequence of fewer data and heterogeneous disease activity in existing studies, the longer term effects of immunosuppression have been uncertain.
How the intervention might work
IgA nephropathy often progresses very slowly, taking decades to reach the clinical outcomes usually studied in clinical studies (death and need for dialysis or kidney transplantation). It has thus been difficult to establish the most effective treatment regimen for IgA nephropathy. Reviews have examined the evidence for treatment of both adults (Nolin 1999) and children (Wyatt 2001) with IgA nephropathy to find optimal regimens. These analyses included studies of varying methodological quality, and are mostly case series and other forms of non‐randomised evaluation. These data have resulted in conflicting information regarding the optimal therapy. The most commonly used regimens include immunosuppressive agents such as glucocorticoids (steroids), cyclosporin A (CSA), or CPA. Additionally, non‐immunosuppressive medications including fish oils, anticoagulants, antihypertensive agents and surgical tonsillectomy with and without immunosuppression have been tested in a variety of studies including RCTs.
Why it is important to do this review
Given the burden of disease and the known risks of progression, as well as the lack of an accepted effective therapy, a systematic review of these treatments was necessary to aid healthcare providers in managing this condition. The present review focuses on the benefits and harms of immunosuppressive treatment for IgA nephropathy. The initial review was published in 2003 (Samuels 2003b; Samuels 2004) and was updated in 2015 (Vecchio 2015).
A separate review summarises the benefits and harms of non‐immunosuppressive treatments for IgA nephropathy (Reid 2011).
Objectives
To determine the benefits and harms of immunosuppression for the treatment of IgA nephropathy.
Methods
Criteria for considering studies for this review
Types of studies
RCTs and quasi‐RCTs (RCTs in which allocation to treatment was obtained by alternation, use of alternate medical records, date of birth or other predictable methods) that compared immunosuppressive therapy (corticosteroids, cytotoxic agents, MMF, leflunomide, or other) with other immunosuppressive agents, non‐immunosuppressive treatment (including antihypertensive agents and anticoagulants), or placebo or no treatment/standard care for the treatment of IgA nephropathy were included.
Types of participants
Adult and children with biopsy‐proven IgA nephropathy.
Types of interventions
Immunosuppressive agent versus placebo, no treatment/standard care, or other non‐immunosuppressive agent (including renin‐angiotensin system (RAS) inhibitors)
Head to head comparisons between immunosuppressive agents.
Types of outcome measures
Primary outcomes
ESKD requiring kidney replacement therapy (KRT) (dialysis or kidney transplantation)
Complete remission: defined by a reduction in urinary protein excretion to less than 1 g/24 hours in three consecutive daily samples or as defined by the investigators
Doubling of SCr
SCr (µmol/L)
Estimated or measured glomerular filtration rate (GFR) (either creatinine clearance (CrCl) (mL/min) or Cockcroft clearance (mL/min/1.73 m2)
Urinary protein excretion (g/24 hours)
Secondary outcomes
Death
Infection
Malignancy
Where possible, time to reach the above end‐points in each treatment arm was included in the analysis.
Adverse effects
Dropout rate due to treatment‐related adverse events
Bone density, fracture or shorter stature
Search methods for identification of studies
Electronic searches
We searched the Cochrane Kidney and Transplant Register of Studies up to 9 September 2019 through contact with the Information Specialist using search terms relevant to this review. The Register contains studies identified from the following sources.
Monthly searches of the Cochrane Central Register of Controlled Trials (CENTRAL)
Weekly searches of MEDLINE OVID SP
Searches of kidney and transplant journals, and the proceedings and abstracts from major kidney and transplant conferences
Searching of the current year of EMBASE OVID SP
Weekly current awareness alerts for selected kidney and transplant journals
Searches of the International Clinical Trials Register (ICTRP) Search Portal and ClinicalTrials.gov.
Studies contained in the Register are identified through searches of CENTRAL, MEDLINE, and EMBASE based on the scope of Cochrane Kidney and Transplant. Details of search strategies, as well as a list of handsearched journals, conference proceedings and current awareness alerts, are available on the Cochrane Kidney and Transplant website.
See Appendix 1 for search terms used in strategies for this review.
Searching other resources
Reference lists of review articles, relevant studies and clinical practice guidelines.
Contacting relevant individuals/organisations seeking information about unpublished or incomplete studies.
Grey literature sources (e.g. abstracts, dissertations and theses), in addition to those already included in the Cochrane Kidney and Transplant Register of Studies, were searched.
Data collection and analysis
The initial review was undertaken by five authors (JAS, GFMS, JCC, FPS, DAM) and was updated by 10 authors (PN, SCP, MR, VS, JCC, MV, JAS, DAM, FPS, GFMS).
Selection of studies
The search strategy described was used to obtain titles and abstracts of studies that may be relevant to the review. The titles and abstracts were screened independently by at least two authors, who discarded studies that were not applicable; however, studies and reviews that may have included relevant data or information on studies were retained initially. Two authors independently assessed retrieved abstracts and, where necessary the full text, of these studies to determine which studies satisfied the inclusion criteria.
Data extraction and management
Data extraction was carried out independently by at least two authors using standard data extraction forms. Studies reported in non‐English language journals were translated before assessment. Where more than one publication of one study existed, reports be grouped together and the publication with the most complete data was used in the analyses. When relevant outcomes were only published in earlier versions these data were used. Any discrepancies between published versions were to highlighted.
Assessment of risk of bias in included studies
The following items were assessed independently by two authors using the risk of bias assessment tool (Higgins 2011) (seeAppendix 2).
Was there adequate sequence generation (selection bias)?
Was allocation adequately concealed (selection bias)?
-
Was knowledge of the allocated interventions adequately prevented during the study?
Participants and personnel (performance bias)
Outcome assessors (detection bias)
Were incomplete outcome data adequately addressed (attrition bias)?
Are reports of the study free of suggestion of selective outcome reporting (reporting bias)?
Was the study apparently free of other problems that could put it at a risk of bias?
Measures of treatment effect
For dichotomous outcomes (mortality, infection, ESKD, doubling of SCr, malignancy, reduction in GFR at least 25 or 50%, complete remission, adverse events) results were expressed as relative risk (RR) with 95% confidence intervals (CI) for individual studies. When continuous scales of measurement were used, we assessed the effects of treatment (SCr, CrCl, annual GFR loss and urinary protein excretion), using the mean difference (MD), or the standardised mean difference (SMD) if different scales had been used. Adverse events were summarised descriptively. As measures of proteinuria and albuminuria were reported using various measures, including relative to urinary creatinine, we have harmonised all endpoints to a single measure of milligrams per day or excretion. We followed the methods reported by Lambers Heerspink 2015 to convert the albumin excretion rate per day to protein excretion rate by dividing the albumin excretion by 0.6, recognising that a total daily protein excretion of 500 mg/day is approximately equal to 300 mg/day of albumin.
Dealing with missing data
Any further information required from the original author was requested by written correspondence (e.g. emailing or writing to corresponding author) and any relevant information obtained in this manner was included in the review.
Assessment of heterogeneity
We first assessed the heterogeneity by visual inspection of the forest plot. We then quantified statistical heterogeneity using the I2 statistic, which describes the percentage of total variation across studies that is due to heterogeneity rather than sampling error (Higgins 2003). A guide to the interpretation of I2 values was as follows:
0% to 40%: might not be important
30% to 60%: may represent moderate heterogeneity
50% to 90%: may represent substantial heterogeneity
75% to 100%: considerable heterogeneity.
The importance of the observed value of I2 depends on the magnitude and direction of treatment effects and the strength of evidence for heterogeneity (e.g. P‐value from the Chi2 test, or a confidence interval for I2) (Higgins 2011).
Assessment of reporting biases
It was planned that if sufficient RCTs were identified, an attempt would be made to assess for publication bias using a funnel plot (Egger 1997). However, insufficient data precluded subgroup analyses in this review update.
Data synthesis
Treatment effects were summarised using a random effects model. For each analysis, the fixed effects model was also evaluated to ensure robustness of the model chosen and susceptibility to outliers.
Subgroup analysis and investigation of heterogeneity
Subgroup analysis was planned to explore how possible sources of heterogeneity (paediatric versus adult population, stage of renal biopsy, race of participants) might have influenced the treatment effects observed. However, due to the small number of studies, subgroup analyses to determine the impact of patient characteristics on treatment effectiveness were not possible.
Post hoc subgroup analysis
We performed a post hoc subgroup analysis to assess the effect of the background of treatments with and without RAS blockade and blood pressure (BP) control (ACE inhibitor and/or ARB) on risks of ESKD.
'Summary of findings' tables
We presented the main results of the review in 'Summary of findings' tables. These tables present key information concerning the quality of the evidence, the magnitude of the effects of the interventions examined, and the sum of the available data for the main outcomes (Schunemann 2011a). The 'Summary of findings' tables also include an overall grading of the evidence related to each of the main outcomes using the GRADE (Grades of Recommendation, Assessment, Development and Evaluation) approach (GRADE 2008; GRADE 2011). The GRADE approach defines the quality of a body of evidence as the extent to which one can be confident that an estimate of effect or association is close to the true quantity of specific interest. The quality of a body of evidence involves consideration of within‐trial risk of bias (methodological quality), directness of evidence, heterogeneity, precision of effect estimates and risk of publication bias (Schunemann 2011b). We presented the following outcomes in the 'Summary of findings' tables.
We presented the following outcomes in the 'Summary of findings' table:
ESKD
Complete remission
≥ 50% GFR loss
Annual GFR loss (mL/min/1.73 m2)
Death (any cause)
Infection
Malignancy
Results
Description of studies
Results of the search
Search results are shown in Figure 1. For this 2020 review update, we identified 69 new reports. There were 36 new studies (56 reports) and 13 new reports of 10 existing studies. Seventeen new studies (38 reports) were eligible (BRIGHT‐SC 2016; CAST‐IgA 2015; Cheung 2018; Hirai 2017; Hou 2017; Koitabashi 1996; Lee 2003; Masutani 2016; Min 2017; NEFIGAN 2017; Shen 2013; Shi 2012a; Shima 2018; STOP‐IgAN 2008; TESTING 2017; Wu 2016; Yamauchi 2001) and three studies (three reports) were excluded (GloMY 2010; Imai 2006; Yonemura 2000b).
1.
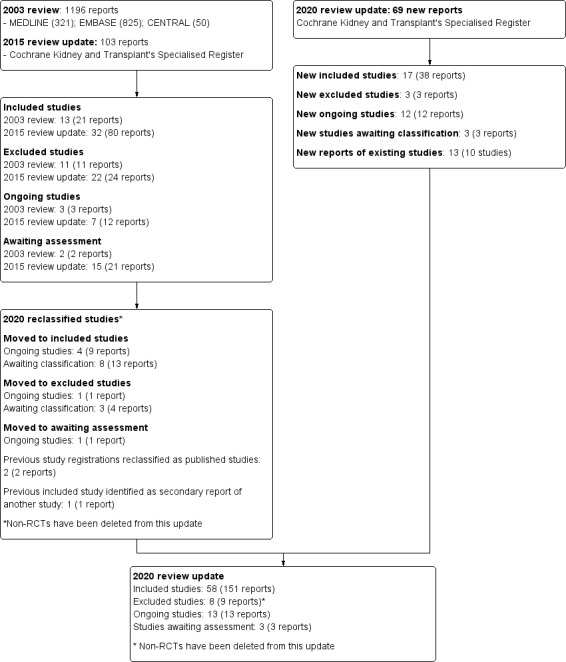
Study flow diagram.
There are 13 ongoing studies (AIGA 2016; ARTEMIS‐IgAN 2018; ChiCTR1800014442; MAIN 2013; NCT00657059; NCT02808429; NCT03468972; NEFIGARD 2018; PIRAT 2015; SIGN 2014; TIGER 2017; TOPplus‐IgAN 2013; UMIN000032031) that have not yet been completed according to details held within the www.ClinicalTrials.gov registry, www.chictr.org.cn and https://upload.umin.ac.jp/; and three studies are awaiting classification while we try to determine if they meet our inclusion criteria (NCT00301600; NCT02160132; NCT02571842). These 16 studies will be assessed in a future update of this review.
In addition, four previous ongoing studies (2nd NA IgAN 2004; Hou 2017; Lafayette 2017; STOP‐IgAN 2008) and eight studies awaiting assessment (Chen 2002; Cruzado 2011; Kawamura 2014; Kim 2013b; Liu 2010a; Liu 2014; Stangou 2011; Xie 2011) have been reclassified as included. One ongoing study (Dal Canton 2005) and three studies awaiting classification have been reclassified as excluded (Chen 2009b; Czock 2007; Shen 2009).
For this 2020 update there are 58 included studies, 13 ongoing studies, 3 studies awaiting assessment and 8 excluded studies. Non‐RCTs have been removed from this update.
Included studies
The characteristics of the participants and the interventions in included studies are detailed in the Characteristics of included studies. Overall, 58 studies (151 publications) enrolling a total of 3933 patients, were included in this review update (2nd NA IgAN 2004; Ballardie 2002; BRIGHT‐SC 2016; Cao 2008; CAST‐IgA 2015; Chen 2002; Cheung 2018; Cruzado 2011; Frisch 2005; Harmankaya 2002; Hirai 2017; Horita 2007; Hou 2017; Julian 1993; Kanno 2003; Katafuchi 2003; Kawamura 2014; Kim 2013b; Kobayashi 1996; Koike 2008; Koitabashi 1996; Lafayette 2017; Lai 1986; Lai 1987; Lee 2003; Liu 2010a; Liu 2014; Locatelli 1999; Lou 2006; Lv 2009; Maes 2004; Manno 2001; Masutani 2016; Min 2017; NA IgAN 1995; NEFIGAN 2017; Ni 2005; Nuzzi 2009; Pozzi 1999; Segarra 2006; Shen 2013; Shi 2012a; Shima 2018; Shoji 2000; Stangou 2011; STOP‐IgAN 2008; Takeda 1999; Tang 2005; TESTING 2017; Walker 1990a; Welch 1992; Woo 1987; Wu 2016; Xie 2011; Yamauchi 2001; Yoshikawa 1999; Yoshikawa 2006; Zhang 2004). Ten authors were contacted for clarifications relating to their publications and to request additional unpublished information. Four authors replied to our request.
Six studies included paediatric participants (Kobayashi 1996; Nuzzi 2009; Shima 2018; Welch 1992; Yoshikawa 1999; Yoshikawa 2006). Twenty‐six studies included people with daily protein excretion > 1 g/24 hours (Cao 2008; Chen 2002; Cruzado 2011; Frisch 2005; Horita 2007; Hou 2017; Kawamura 2014; Lee 2003; Kobayashi 1996; Lai 1987; Liu 2014; Locatelli 1999; Lou 2006; Lv 2009; Maes 2004; Manno 2001; Min 2017; Ni 2005; Pozzi 1999; Segarra 2006; Shen 2013; Shi 2012a; Stangou 2011; Tang 2005; TESTING 2017; Walker 1990a). Thirteen studies (BRIGHT‐SC 2016; Chen 2002; Cheung 2018; Kawamura 2014; Koitabashi 1996; Lafayette 2017; Nuzzi 2009; Segarra 2006; Shi 2012a; Shima 2018; Takeda 1999; Welch 1992; Yamauchi 2001) did not report data in an extractable format that could be included in our meta‐analysis.
We identified five study of head‐to‐head comparisons between different immunosuppressive agents (Chen 2002; Hou 2017; Liu 2010a; Shen 2013; Wu 2016) and there were no studies that compared different doses of the same immunosuppressive agents.
Excluded studies
We excluded eight studies (nine reports) as they did not include all participants with IgA nephropathy (Imai 2006; Sulimani 2001; Yonemura 2000b), did not evaluate a immunosuppressive agent intervention (Chen 2009b; Czock 2007; Shen 2009), or did not complete the participant recruitment (Dal Canton 2005; GloMY 2010). See Characteristics of excluded studies.
Risk of bias in included studies
The risks of bias in the included studies are summarised in Figure 2. Risks of bias in individual studies are shown in Figure 3 and described in the Characteristics of included studies.
2.
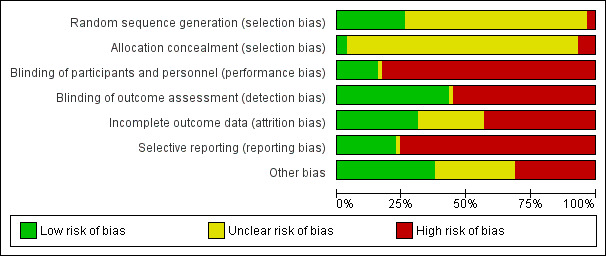
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
3.
Risk of bias summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Random sequence generation was considered at low risk of bias in 15 studies (2nd NA IgAN 2004; Hirai 2017; Julian 1993; Kawamura 2014; Kim 2013b; Locatelli 1999; Lv 2009; Manno 2001; NEFIGAN 2017; Pozzi 1999; Shoji 2000; Stangou 2011; TESTING 2017; Welch 1992; Wu 2016), at high risk in two studies (Kobayashi 1996; Lai 1987), and unclear in the remaining 41 studies.
Allocation concealment was adjudicated as low risk of bias in two studies (Manno 2001; TESTING 2017), at high risk in four studies (Kawamura 2014; Kobayashi 1996; Lai 1986; Lai 1987); and unclear in the remaining 52 studies.
Blinding
Nine studies (2nd NA IgAN 2004; BRIGHT‐SC 2016; Cheung 2018; Frisch 2005; Kim 2013b; NEFIGAN 2017; TESTING 2017; Welch 1992; Wu 2016) were blinded and considered to be at low risk of bias and one study (Lee 2003) was assessed as unclear risk of performance bias. The remaining 48 studies were not blinded and were considered at high risk of performance bias.
Outcome assessment was considered to be at low risk of detection bias in 25 studies (Ballardie 2002; BRIGHT‐SC 2016; Cao 2008; CAST‐IgA 2015; Horita 2007; Hou 2017; Kanno 2003; Katafuchi 2003; Kobayashi 1996; Koike 2008; Koitabashi 1996; Lai 1986; NEFIGAN 2017; Nuzzi 2009; Shoji 2000; Takeda 1999; TESTING 2017; Walker 1990a; Welch 1992; Woo 1987; Wu 2016; Xie 2011; Yamauchi 2001; Yoshikawa 1999; Yoshikawa 2006), unclear in one study (Lee 2003), and high risk the remaining 32 studies.
Incomplete outcome data
Eighteen studies were judged to be a low risk of attrition bias (Ballardie 2002; Cruzado 2011; Frisch 2005; Horita 2007; Hou 2017; Kim 2013b; Koike 2008; Lai 1986; Liu 2010a; Lv 2009; Manno 2001; Masutani 2016; Shima 2018; STOP‐IgAN 2008; Tang 2005; Walker 1990a; Welch 1992; Yoshikawa 2006), 25 studies were at high risk of attrition bias (2nd NA IgAN 2004; BRIGHT‐SC 2016; Harmankaya 2002; Hirai 2017; Julian 1993; Kanno 2003; Katafuchi 2003; Kobayashi 1996; Lafayette 2017; Lai 1987; Liu 2014; Locatelli 1999; Lou 2006; Maes 2004; Min 2017; NA IgAN 1995; NEFIGAN 2017; Ni 2005; Pozzi 1999; Segarra 2006; Shoji 2000; TESTING 2017; Wu 2016; Xie 2011; Yoshikawa 1999), and the remaining 15 studies were unclear.
Selective reporting
Thirteen studies were judged to be at low risk of reporting bias (Cruzado 2011; Frisch 2005; Locatelli 1999; Lv 2009; Maes 2004; Manno 2001; NEFIGAN 2017; Pozzi 1999; STOP‐IgAN 2008; Tang 2005; TESTING 2017; Walker 1990a; Wu 2016), one study was unclear (Lee 2003), and 44 were at high risk of reporting bias.
Other potential sources of bias
We adjudicated 22 studies as low risk of bias from other potential sources (Harmankaya 2002; Hirai 2017; Horita 2007; Hou 2017; Kanno 2003; Katafuchi 2003; Kawamura 2014; Kim 2013b; Liu 2010a; Liu 2014; Locatelli 1999; Lv 2009; Maes 2004; Min 2017; Shima 2018; Shoji 2000; STOP‐IgAN 2008; Tang 2005; Walker 1990a; Wu 2016; Xie 2011; Yoshikawa 2006) considering balance of participant characteristics and co‐interventions, governmental or academic sources of funding and balanced timing of outcome assessment for all treatment groups. Eighteen studies (2nd NA IgAN 2004; Cruzado 2011; Frisch 2005; Kobayashi 1996; Koike 2008; Lafayette 2017; Lai 1986; Lai 1987; Lou 2006; Masutani 2016; NA IgAN 1995; NEFIGAN 2017; Pozzi 1999; Segarra 2006; Stangou 2011; TESTING 2017; Woo 1987; Yoshikawa 1999) was assessed as high risk of bias. Risk of bias was unclear in the remaining 18 studies.
Effects of interventions
See: Table 1; Table 2; Table 3; Table 4; Table 5; Table 6
Summary of findings for the main comparison. Systemic corticosteroid versus no corticosteroid regimen for IgA nephropathy.
| Systemic corticosteroid versus no corticosteroid regimen for IgA nephropathy | |||||
|
Patient or population: adults and children who have IgA nephropathy proven on renal biopsy Setting: Australia, China, Europe, Japan, USA Intervention: corticosteroid regimen (includes steroids alone or with RAS inhibitors) Comparison: no corticosteroid regimen | |||||
| Outcomes | Anticipated absolute benefits* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with no steroids | Risk with steroids | ||||
|
End‐stage kidney disease Follow‐up: 2 to 10 years |
141 per 1000 | 55 per 1000 (32 to 92) |
RR 0.39 (0.23 to 0.65) |
741 (8) | ⊕⊕⊕⊝ moderate 1 |
|
Complete remission Follow‐up: 2 to 5 years |
364 per 1000 | 641 per 1000 (375 to 1000) | RR 1.76 (1.03 to 3.01) |
305 (4) | ⊕⊕⊝⊝ low 1,3 |
|
GFR loss ≥ 50% Follow‐up: 2 to 2.1 years |
96 per 1000 | 54 per 1000 (24 to 119) |
RR 0.56 (0.25 to 1.24) |
326 (2) | ⊕⊕⊝⊝ low 1,2 |
|
Annual GFR loss (mL/min/1.73 m2) Follow‐up: 2.1 to 5 years |
The mean annual GFR loss ranged across control groups from 6.17 to 6.95 mL/min/1.73 m2 | The mean annual GFR loss in the intervention group was ‐5.40 mL/min/1.73 m2 less than the control group (95% CI ‐8.55 less to ‐2.25 less) | ‐‐ | 359 (2) | ⊕⊕⊕⊝ moderate 1 |
|
Death (any cause) Median follow‐up: 2.1 years |
8 per 1000 | 15 per 1000 (1 to 162) |
RR 1.85 (0.17 to 20.19) |
262 (1) | ⊕⊝⊝⊝ very low 1,4 |
|
Infection Median follow‐up: 2.1 years |
No events | 11/136** | RR 21.32 (1.27, 358.10) | 262 (1) | ⊕⊝⊝⊝ very low 1,2,3 |
|
Malignancy Follow‐up: 6 years |
23 per 1000 | 23 per 1000 (1 to 356) |
RR 1.00 (0.06 to 15.48) |
86 (1) | ⊕⊝⊝⊝ very low 1,2,4 |
|
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded due to study limitations including lack of allocation concealment and lack of blinding
2 Downgraded due to imprecision in treatment estimate (consistent with appreciable benefit or harm)
3 Downgraded due to evidence of important statistical heterogeneity
4 Downgraded two levels due to severe imprecision in treatment estimate (consistent with appreciable benefit or harm)
Summary of findings 2. Cytotoxic regimen versus no cytotoxic regimen for IgA nephropathy.
| Cytotoxic regimen versus no cytotoxic regimen for IgA nephropathy | |||||
|
Patient or population: adults and children who have IgA nephropathy proven on renal biopsy Settings: Australia, China, Europe, Japan Intervention: cytotoxic therapy (including combinations of cyclophosphamide or azathioprine with steroid therapy) Comparison: no cytotoxic therapy | |||||
| Outcomes | Anticipated absolute benefits* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk with no cytotoxic therapy | Risk with cytotoxic therapy | ||||
|
End‐stage kidney disease Follow‐up: 1 to 7 years |
166 per 1000 | 105 per 1000 (55 to 199) |
RR 0.63 (0.33 to 1.20) |
463 (7) | ⊕⊕⊝⊝ low 1,3 |
|
Complete remission Follow‐up: 0.5 to 5 years |
337 per 1000 | 495 per 1000 (317 to 775) |
RR 1.47 (0.94 to 2.30) |
381 (5) | ⊕⊝⊝⊝ very low 1,3,4 |
| GFR loss ≥ 50% | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Annual GFR loss (mL/min/1.73 m2) Follow‐up: 3 years |
The mean GFR loss was 0.01 mL/min/1.73 m2 in the control group | The mean GFR loss in the intervention group was 0.01 mL/min/1.73 m2 lower than the control group (95% CI ‐0.03 to 0.01) | ‐‐ | 162 (1) | ⊕⊕⊝⊝ low 1,3 |
|
Death (any cause) Follow‐up: 3 years |
13 per 1000 | 13 per 1000 (1 to 199) |
RR 0.98 (0.06 to 15.33) |
162 (1) | ⊕⊝⊝⊝ very low 1,2 |
|
Infection Follow‐up: 1 to 7 years |
22 per 1000 | 37 per 1000 (10 to 149) |
RR 1.70 (0.43 to. 6.76) |
268 (4) | ⊕⊝⊝⊝ very low 1,2 |
|
Malignancy Follow‐up: 3 years |
No events | 2/82** | RR 4.88 (0.24 to 100.08) |
162 (1) | ⊕⊝⊝⊝ very low 1,2 |
|
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded due to study limitations including lack of allocation concealment and lack of blinding
2 Downgraded two levels due to severe imprecision in treatment estimate (consistent with appreciable benefit or harm)
3 Downgraded due to imprecision in treatment estimate (consistent with appreciable benefit or harm)
4 Downgraded due to evidence of important statistical heterogeneity
Summary of findings 3. MMF regimen versus no MMF regimen for IgA nephropathy.
| MMF regimen versus no MMF regimen for IgA nephropathy | |||||
|
Patient or population: adults and children who have IgA nephropathy proven on renal biopsy Settings: Australia, China, Europe Intervention: MMF regimen (includes MMF alone, or in combination with RAS inhibitors or steroids) Comparison: mo MMF regimen | |||||
| Outcomes | Anticipated absolute benefits* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk without MMF | Risk with MMF | ||||
|
End‐stage kidney disease Follow‐up: 1 to 3 years |
96 per 1000 | 70 per 1000 (15 to 310) |
RR 0.73 (0.16 to 3.23) |
280 (4) | ⊕⊝⊝⊝ very low 1,2,3 |
|
Complete remission Follow‐up: 1 to 2 years |
267 per 1000 | 280 per 1000 (195 to 406) |
RR 1.05 (0.73 to 1.52) |
271 (4) | ⊕⊝⊝⊝ very low 1,2 |
|
GFR loss ≥ 50% Follow‐up: 2 years |
133 per 1000 | 294 per 1000 (67 to 1000) |
RR 2.21 (0.50 to 9.74) |
32 (1) | ⊕⊝⊝⊝ very low 1,2 |
|
Annual GFR loss (mL/min/1.73 m2) Follow‐up: 1 year |
The mean GFR loss was 10.6 mL/min/1.73 m2 in the control group | The mean GFR loss in the intervention group was 2.00 mL/min/1.73 m2 lower than the control group (95% CI ‐25.15 to 29.15) | ‐‐ | 28 (1) | ⊕⊝⊝⊝ very low 1,2 |
| Death (any cause) | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Infection Follow‐up: 1 to 3 years |
169 per 1000 | 230 per 1000 (147 to 358) |
RR 1.36 (0.87 to 2.12) |
301 (4) | ⊕⊝⊝⊝ very low 1,2 |
|
Malignancy Follow‐up: 1 to 3 years |
50 per 1000 | 14 per 1000 (2 to 127) |
RR 0.28 (0.03 to 2.54) |
86 (2) | ⊕⊝⊝⊝ very low 1,2 |
| The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded due to study limitations including lack of allocation concealment and lack of blinding
2 Downgraded two levels due to severe imprecision in treatment estimate (consistent with appreciable benefit or harm)
3 Downgraded due to evidence of important statistical heterogeneity
Summary of findings 4. Calcineurin inhibitor regimen versus no calcineurin inhibitor regimen for IgA nephropathy.
| Calcineurin inhibitor regimen versus no calcineurin inhibitor regimen for IgA nephropathy | |||||
|
Patient or population: adults and children who have IgA nephropathy proven on renal biopsy Settings: China Intervention: calcineurin inhibitor regimen (includes calcineurin inhibitor alone or in combination with steroids) Comparison: no calcineurin inhibitor regimen | |||||
| Outcomes | Anticipated absolute benefits* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk without calcineurin inhibitor | Risk with calcineurin inhibitor | ||||
| End‐stage kidney disease | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Complete remission Follow‐up: 0.5 to 1 year |
541 per 1000 | 492 per 1000 (325 to 752) |
RR 0.91 (0.60 to 1.39) |
72 (2) | ⊕⊝⊝⊝ very low 1,2 |
| GFR loss ≥ 50% | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Annual GFR loss (mL/min/ 1.73 m2) |
No data observations | Not estimable | No studies | No studies | Not estimable |
| Death (any cause) | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Infection Follow‐up: 1 year |
130 per 1000 | 40 per 1000 (4 to 356) |
RR 0.31 (0.03 to 2.74) |
48 (1) | ⊕⊝⊝⊝ very low 1,2 |
|
Malignancy Follow‐up: 1 year |
40 per 1000 | 14 per 1000 (1 to 338) |
RR 0.36 (0.02 to 8.45) |
48 (1) | ⊕⊝⊝⊝ very low 1,2 |
| The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded due to study limitations including lack of allocation concealment and lack of blinding
2 Downgraded two levels due to severe imprecision in treatment estimate (consistent with appreciable benefit or harm)
Summary of findings 5. Mizoribine regimen versus no mizoribine regimen for IgA nephropathy.
| Mizoribine regimen compared with no mizoribine regimen for IgA nephropathy | |||||
|
Patient or population: adults and children who have IgA nephropathy proven on renal biopsy Settings: Japan Intervention: mizoribine regimen (includes mizoribine alone or with RAS inhibitors) Comparison: no mizoribine regimen | |||||
| Outcomes | Anticipated absolute benefits* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk without mizoribine | Risk with mizoribine | ||||
|
End‐stage kidney disease Follow‐up: 3 years |
48 per 1000 | 48 per 1000 (3 to 718) |
RR 1.00 (0.07 to 14.95) |
42 (1) | ⊕⊝⊝⊝ very low 1,2 |
|
Complete remission Follow‐up: 3 years |
467 per 1000 | 887 per 1000 (495 to 1000) |
RR 1.90 (1.06 to 3.43) |
24 (1) | ⊕⊝⊝⊝ very low 1,2 |
| GFR loss ≥ 50% | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Annual GFR loss (mL/min/1.73 m2) |
No data observations | Not estimable | No studies | No studies | Not estimable |
| Death (any cause) | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Infection Follow‐up: 1 to 2.1 years |
60 per 1000 | 91 per 1000 (8 to 969) |
RR 1.52 (0.14 to 16.15) |
104 (2) | ⊕⊝⊝⊝ very low 1,2,3 |
|
Malignancy Follow‐up: 3 years |
No events | 1/21** | RR 3.00 (0.13 to 69.70) |
42 (1) | ⊕⊝⊝⊝ very low 1,2 |
|
The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. ** Event rate derived from the raw data. A 'per thousand' rate is non‐informative in view of the scarcity of evidence and zero events in the control group | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded due to study limitations including lack of allocation concealment and lack of blinding
2 Downgraded two levels due to severe imprecision in treatment estimate (consistent with appreciable benefit or harm)
3 Downgraded due to evidence of important statistical heterogeneity
Summary of findings 6. Leflunomide regimen versus no leflunomide regimen for IgA nephropathy.
| Leflunomide regimen compared with no leflunomide regimen for IgA nephropathy | |||||
|
Patient or population: adults and children who have IgA nephropathy proven on renal biopsy Settings: China Intervention: leflunomide regimen (includes leflunomide alone or with steroids or RAS inhibitor) Comparison: no leflunomide regimen | |||||
| Outcomes | Anticipated absolute benefits* (95% CI) | Relative effect (95% CI) | No. of participants (studies) | Quality of the evidence (GRADE) | |
| Risk without leflunomide | Risk with leflunomide | ||||
|
End‐stage kidney disease Follow‐up: 7.3 years |
111 per 1000 | 76 per 1000 (19 to 294) |
RR 0.68 (0.17 to 2.65) |
85 (1) | ⊕⊝⊝⊝ very low 1,2 |
|
Complete remission Follow‐up: 0.25 to 7.3 years |
357 per 1000 | 386 per 1000 (286 to 521) |
RR 1.08 (0.80 to 1.46) |
282 (4) | ⊕⊝⊝⊝ very low 1,2 |
| GFR loss ≥ 50% | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Annual GFR loss (mL/min/1.73 m2) |
No data observations | Not estimable | No studies | No studies | Not estimable |
| Death (any cause) | No data observations | Not estimable | No studies | No studies | Not estimable |
|
Infection Follow‐up: 0.5 to 7.3 years |
56 per 1000 | 54 per 1000 (25 to 117) |
RR 0.97 (0.45 to 2.09) |
387 (3) | ⊕⊝⊝⊝ very low 1,2 |
| Malignancy | No data observations | Not estimable | No studies | No studies | Not estimable |
| The risk in the intervention group (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; RR: Risk Ratio. | |||||
| GRADE Working Group grades of evidence High certainty: We are very confident that the true effect lies close to that of the estimate of the effect. Moderate certainty: We are moderately confident in the effect estimate: The true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different. Low certainty: Our confidence in the effect estimate is limited: The true effect may be substantially different from the estimate of the effect. Very low certainty: We have very little confidence in the effect estimate: The true effect is likely to be substantially different from the estimate of effect. | |||||
1 Downgraded due to study limitations including lack of allocation concealment and lack of blinding
2 Downgraded two levels due to severe imprecision in treatment estimate (consistent with appreciable benefit or harm)
See: Table 1: Steroid regimen versus no steroid regimen for treating IgA nephropathy; Table 2: Cytotoxic regimen versus no cytotoxic regiment for treating IgA nephropathy; Table 3: MMF regimen versus no MMF regimen for IgA nephropathy; Table 4: CNI regimen versus no CNI regimen for IgA nephropathy; Table 5: Mizoribine regimen versus no mizoribine regimen for IgA nephropathy; Table 6: Leflunomide regimen versus no leflunomide regimen for IgA nephropathy.
We grouped the included studies into nine treatment comparisons.
Systemic corticosteroid versus no corticosteroid regimen (Julian 1993; Kanno 2003; Katafuchi 2003; Lee 2003; Kobayashi 1996; Koike 2008; Lai 1986; Lv 2009; Manno 2001; NA IgAN 1995; Nuzzi 2009; Pozzi 1999; Shoji 2000; Takeda 1999; TESTING 2017; Welch 1992; Yamauchi 2001)
Locally‐acting steroid versus no locally‐acting steroid (NEFIGAN 2017)
Cytotoxic (CPA, AZA or belimumab) versus no cytotoxic regimen (Ballardie 2002; BRIGHT‐SC 2016; Cheung 2018: Harmankaya 2002; Koitabashi 1996; Lafayette 2017; Locatelli 1999; Stangou 2011; STOP‐IgAN 2008; Yoshikawa 1999; Yoshikawa 2006; Walker 1990a; Woo 1987)
MMF versus no MMF regimen (2nd NA IgAN 2004; Chen 2002; Frisch 2005; Hou 2017; Maes 2004; Tang 2005)
CNI versus no CNI regimen (Kim 2013b; Lai 1987; Liu 2014; Shen 2013)
Mizoribine versus no mizoribine regimen (Hirai 2017; Masutani 2016; Shima 2018; Xie 2011)
Leflunomide versus no leflunomide regimen (Cao 2008; Liu 2010a; Lou 2006; Min 2017; Ni 2005; Shi 2012a; Wu 2016; Zhang 2004)
Steroid plus non‐immunosuppressive agents versus steroid alone (CAST‐IgA 2015; Horita 2007; Kawamura 2014; Segarra 2006)
mTOR inhibitor versus no mTOR inhibitor regimen (Cruzado 2011).
End‐stage kidney disease requiring kidney replacement therapy
In patients mostly with mild to moderate kidney disease and protein excretion of over 1 g/24 hours, steroid treatment was administered generally as oral prednisolone 0.6 to 1 mg/kg during 2 to 4 months of therapy followed by a tapering course for a median follow‐up of 54 months (between 24 and 120 months). Participant follow‐up for occurrence of ESKD was generally between 2 and 10 years. In eight studies, steroid therapy probably reduces the absolute risk of reaching ESKD compared with standard care without steroid therapy or placebo (Analysis 1.1 (8 studies, 741 participants): RR 0.39, 95% CI 0.23 to 0.65; I2 = 0%; moderate certainty evidence). There was moderate statistical heterogeneity in the treatment effects between the studies.
1.1. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 1 ESKD.
CPA or AZA alone or with concomitant steroid treatment for 3 to 6 months had uncertain effects on ESKD over 2 to 7 years of follow‐up (Analysis 3.1 (7 studies; 463 participants): RR 0.63, 95% CI 0.33 to 1.20; I2 = 34%; low certainty evidence) compared to standard care or placebo without steroid therapy.
3.1. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 1 ESKD.
MMF (1.5 to 2 g/day) with or without steroid therapy administered for between 24 weeks and 3 years had uncertain effects on progression to ESKD when compared with placebo, standard care or steroid alone (Analysis 4.1 (4 studies; 280 participants): RR 0.73, 95% CI 0.16 to 3.23; I2 = 54%; very low certainty evidence). There was moderate statistical heterogeneity in the treatment effects between the studies.
4.1. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 1 ESKD.
Mizoribine administered at 150 mg/day for 12 months had uncertain effects within a single study in which two ESKD events (one in each group) occurred over 36 months (Analysis 6.1 (42 participants): RR 1.00, 95% CI 0.07 to 14.95; very low certainty evidence).
6.1. Analysis.
Comparison 6 Mizoribine versus no mizoribine regimen, Outcome 1 ESKD.
Leflunomide (20 mg/day) for 12 months in conjunction with oral prednisone (0.8 mg/day) for 4 to 6 weeks versus prednisone (1.0 mg/day) for 8 to 12 weeks had uncertain effects on ESKD in a single study (Analysis 7.1 (85 participants): RR 0.68, 95% CI 0.17 to 2.65; very low certainty evidence).
7.1. Analysis.
Comparison 7 Leflunomide versus no leflunomide regimen, Outcome 1 ESKD.
There was no evidence for the effects of CNIs, steroids combined with non‐immunosuppressive agents, or mTOR inhibitors on ESKD.
Complete remission
Prednisone (0.8 to 1 mg/kg/d or 40 to 60 mg/day), methylprednisolone (0.6 to 0.8 mg/kg/day), or prednisolone (40 to 60 mg/day) were administered during 10 weeks to 8 months of therapy followed by a tapering course. Steroid therapy may incur complete remission compared with placebo, standard care or RAS inhibitor therapy during 2 to 5 years follow‐up (Analysis 1.2 (4 studies, 305 participants): RR 1.76, 95% CI 1.03 to 3.01; I2 = 69%; low certainty evidence). There was substantial heterogeneity in treatment effects observed between the studies.
1.2. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 2 Complete remission.
CPA or AZA with concomitant steroid treatment given for 4 months to 2 years had uncertain effects on complete remission compared to steroid alone, standard care or anticoagulant/antiplatelet during 6 months to 5 years follow‐up (Analysis 3.2 (5 studies, 381 participants): RR 1.47, 95% CI 0.94 to 2.30; I2 = 72%; very low certainty evidence). There was substantial heterogeneity in treatment effects observed between the studies.
3.2. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 2 Complete remission.
MMF (1.5 to 2 g/day) with or without steroid therapy administered given for 6 months to 1 year had uncertain effects on complete remission when compared with placebo, standard care or steroid alone (Analysis 4.2 (4 studies, 271 participants): RR 1.05, 95% CI 0.73 to 1.52; I2 = 0%; very low certainty evidence).
4.2. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 2 Complete remission.
CNIs (CSA 3 mg/day or tacrolimus 0.05 to 0.1 mg/kg/day) were administered during 6 months to 1 year with concomitant steroid treatment had uncertain effects on complete remission (Analysis 5.1 (2 studies, 72 participants): RR 0.91, 95% CI 0.60 to 1.39; I2 = 0%; very low certainty evidence).
5.1. Analysis.
Comparison 5 Calcineurin inhibitor (CNI) versus no CNI regimen, Outcome 1 Complete remission.
Mizoribine administered at 150 mg/day for 12 months had uncertain effects within a single study in which 15 complete remissions occurred during 36 months follow‐up (Analysis 6.2 (24 participants): RR 1.90, 95% CI 1.06 to 3.43; very low certainty evidence).
6.2. Analysis.
Comparison 6 Mizoribine versus no mizoribine regimen, Outcome 2 Complete remission.
Leflunomide (10 to 60 mg/day) for 3 to 12 months with or without oral prednisone had uncertain effects on complete remission over 3 to 88 months follow‐up (Analysis 7.2 (4 studies, 282 participants): RR 1.08, 95% CI 0.80 to 1.46; I2 = 0%; very low certainty evidence) compared to prednisone alone or RAS inhibitor.
7.2. Analysis.
Comparison 7 Leflunomide versus no leflunomide regimen, Outcome 2 Complete remission.
Steroid (steroid pulse followed by prednisolone or prednisolone alone 30 mg followed by a tapering course) for 6 to 24 months with RAS inhibitor or ARB had uncertain effects on complete remission for 24 months follow‐up (Analysis 8.1 (2 studies, 115 participants): RR 1.05, 95% CI 0.83 to 1.31; I2 = 0%; low certainty evidence) compared to prednisolone with or without steroid pulse and tonsillectomy.
8.1. Analysis.
Comparison 8 Steroid plus non‐immunosuppressive agents versus steroid alone, Outcome 1 Complete remission.
There was no evidence for the effects of mTOR inhibitors on complete remission.
Doubling of serum creatinine
Prednisone (0.5 to 1 mg/kg/day or 40 to 60 mg/day) and prednisolone (0.8 mg/kg/day or 20 to 60 mg/day) with or without methylprednisolone (1 g IV) were administered during 10 weeks to 2 years of therapy followed by a tapering course. Steroid therapy may prevent the doubling of SCr compared with standard care or RAS inhibitor therapy during 1 to 10 years follow‐up (Analysis 1.3 (7 studies, 404 participants): RR 0.43, 95% CI 0.29 to 0.65; I2 = 0%; low certainty evidence).
1.3. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 3 Doubling of serum creatinine.
MMF (2 g/day) for up to 3 years had uncertain effects on occurrence of doubling of SCr when compared with placebo or standard care (Analysis 4.3 (2 studies, 74 participants): RR 2.01, 95% CI 0.28 to 14.44; I2 = 0%; low certainty evidence).
4.3. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 3 Doubling of serum creatinine.
Leflunomide (40 mg/day) for 12 months with oral prednisone had uncertain effects on occurrence of doubling of SCr over 88 months follow‐up in a single study (Analysis 7.3 (85 participants): RR 0.50, 95% CI 0.17 to 1.50; low certainty evidence) compared to prednisone alone.
7.3. Analysis.
Comparison 7 Leflunomide versus no leflunomide regimen, Outcome 3 Doubling of serum creatinine.
There was no evidence for the effects of cytotoxic agents, CNIs, mizoribine, steroids combined with non‐immunosuppressive agents or of mTOR inhibitors on doubling of SCr.
Serum creatinine
Prednisone (0.5 to 1 mg/kg/day or 40 to 60 mg/day) and prednisolone (0.5 to 0.8 mg/kg/day or 20 to 60 mg/day) with or without methylprednisolone (1 g IV) were administered during 4 to 36 months of therapy followed by a tapering course. Steroid therapy had uncertain effects on SCr compared with standard care or other non‐immunosuppressive treatment during 1 to 6 years follow‐up (Analysis 1.4 (7 studies, 211 participants) MD ‐21.07 µmol/L, 95% CI ‐44.12 to 1.99; I2 = 78%; very low certainty evidence). There was substantial heterogeneity in treatment effects observed between the studies.
1.4. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 4 Serum creatinine.
MMF (1.5 g/day) with steroid therapy administered for 6 months of therapy followed by a tapering course had uncertain effects on SCr when compared with steroid combined with leflunomide in a single study (Analysis 4.4 (40 participants): MD ‐1.58 µmol/L, 95% CI ‐19.29 to 16.13; low certainty evidence).
4.4. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 4 Serum creatinine.
CSA (5 mg/kg/day) or tacrolimus (0.1 mg/kg/day) administered for 3 to 4 months followed by a tapering course had uncertain effects on SCr when compared with placebo during 4 to 6 months follow‐up (Analysis 5.2 (2 studies, 62 participants): MD 7.75 µmol/L, 95% CI ‐6.76 to 22.27; I2 = 0%; low certainty evidence).
5.2. Analysis.
Comparison 5 Calcineurin inhibitor (CNI) versus no CNI regimen, Outcome 2 Serum creatinine.
Leflunomide (40 to 50 mg/day) for 6 to 12 months followed by a tapering course with oral prednisone had uncertain effects on SCr over 6 to 88 months follow‐up (Analysis 7.4 (2 studies, 125 participants): MD ‐4.29 µmol/L, 95% CI ‐15.81 to 7.24; I2 = 0%; low certainty evidence) compared to prednisone with or without MMF.
7.4. Analysis.
Comparison 7 Leflunomide versus no leflunomide regimen, Outcome 4 Serum creatinine.
There was no evidence for the effects of cytotoxic agents, mizoribine, steroids combined with non‐immunosuppressive agents or of mTOR inhibitors on SCr.
Glomerular filtration rate
Reduction in glomerular filtration rate (at least 50%)
In the two studies evaluating steroid treatment and reporting this outcome, steroids were administered as prednisone (initially 60 mg/m2) on alternate days or methylprednisolone (0.6 to 0.8 mg/kg/day) were administered during 6 to 24 months of therapy. Participant follow‐up for reduction in GFR of at least 50% was generally over two years. Steroid therapy had uncertain effects on risks of a ≥ 50% reduction compared to fish oil or placebo (Analysis 1.5 (2 studies; 326 participants): RR 0.56, 95% CI 0.25 to 1.24; I2 = 0%; low certainty evidence).
1.5. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 5 GFR loss: ≥ 50%.
MMF administered at 2000 mg for 52 weeks had uncertain effects on the risk of GFR reduction ≥ 50% at 2 years of follow‐up in a single study (Analysis 4.5 (32 participants) RR 2.21, 95% CI 0.50 to 9.74; very low certainty evidence).
4.5. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 5 GFR loss: ≥ 50%.
Risks of reduction in GFR of at least 50% was not reported for cytotoxic agents, CNIs, mizoribine, leflunomide, steroids combined with non‐immunosuppressive agents, or mTOR inhibitors.
Reduction glomerular filtration rate (at least 25%)
MMF (2 g/day) had uncertain effects on the risk of GFR reduction ≥ 35% over 3 years in a single study (Analysis 4.6 (34 participants): RR 2.17, 95% CI 0.53 to 8.88; low certainty evidence).
4.6. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 6 GFR loss: ≥ 25%.
Risks of reduction in GFR of at least 25% was not reported for steroids, cytotoxic agents, CNIs, mizoribine, leflunomide, steroids combined with non‐immunosuppressive agents, or mTOR inhibitors.
Annual glomerular filtration loss
Prednisone (1 mg/kg/day) or methylprednisolone (0.6 to 0.8 mg/kg/d were administered during 6 to 8 months of therapy followed by a tapering course. Steroid therapy probably prevents annual GFR loss compared with placebo or RAS inhibitors during 2.1 to 5 years follow‐up (Analysis 1.6 (2 studies, 359 participants): MD ‐5.40 mL/min/1.73 m2, 95% CI ‐8.55 to ‐2.25; I2 = 0%; moderate certainty evidence).
1.6. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 6 Annual GFR loss [mL/min/1.73 m2].
CPA followed by AZA with concomitant steroid treatment given for 6 months had uncertain effects on annual GFR loss compared to standard care during 3 years follow‐up in a single study (Analysis 3.3 (162 participants): MD ‐0.01 mL/min/1.73 m2, 95% CI ‐0.03 to 0.01; low certainty evidence).
3.3. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 3 Annual GFR loss [mL/min/1.73 m2].
MMF (2 g/day) had uncertain effects on annual GFR loss compared to placebo during 12 months follow‐up in a single study (Analysis 4.7 (28 participants): MD 2.0 mL/min/1.73 m2, 95% CI ‐25.15 to 29.15; very low certainty evidence).
4.7. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 7 Annual GFR loss [mL/min/1.73 m2].
There was no evidence for the effects of CNIs, mizoribine, leflunomide, steroids combined with non‐immunosuppressive agents, or mTOR inhibitors on annual GFR loss.
Glomerular filtration rate (any measure)
Prednisolone (0.8 mg/kg/day or 40 to 60 mg/day) and prednisone (0.5 mg/kg/day or 40 to 60 mg/day) with or without methylprednisolone (1 g IV) were administered during 4 to 18 months of therapy followed by a tapering course. Steroid therapy had uncertain effects on GFR compared with standard care or other non‐immunosuppressive treatment during 1 to 10 years follow‐up (Analysis 1.7 (4 studies, 138 participants): MD 17.87 mL/min/1.73 m2, 95% CI 4.93 to 30.82; I2 = 53%; very low certainty evidence). There was moderate heterogeneity in treatment effects observed between the studies.
1.7. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 7 GFR (any measure).
AZA (1 to 2 mg/kg/day) with concomitant steroid treatment given for 1 to 2 years had uncertain effects on GFR compared to steroid alone or anticoagulant/antiplatelet therapy (Analysis 3.4 (3 studies, 174 participants): MD 3.07 mL/min/1.73 m2, 95% CI ‐6.57 to 12.72; I2 = 0%; low certainty evidence).
3.4. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 4 GFR (any measure) [mL/min/1.73 m2].
MMF (2 g/day) administered for 12 months had uncertain effects on GFR when compared with placebo in a single study (Analysis 4.8 (28 participants): MD ‐2.50 mL/min/1.73 m2, 95% CI ‐30.79 to 25.79; low certainty evidence).
4.8. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 8 GFR (any measure) [mL/min/1.73 m2].
CSA (3 to 5 mg/day) with or without concomitant steroid treatment and tacrolimus (0.1 mg/kg/day) for 3 to 12 months had uncertain effects on GFR during 4 to 60 months follow‐up (Analysis 5.3 (3 studies, 110 participants): MD ‐0.18 mL/min/1.73 m2, 95% CI ‐7.42 to 7.07; I2 = 0%; low certainty evidence).
5.3. Analysis.
Comparison 5 Calcineurin inhibitor (CNI) versus no CNI regimen, Outcome 3 GFR (any measure).
Mizoribine (150 to 250 mg/day) with RAS inhibitor treatment had uncertain effects on GFR when compared with RAS inhibitor alone in a single study (Analysis 6.3 (65 participants): MD 2.05 mL/min/1.73 m2, 95% CI ‐10.16 to 14.26; low certainty evidence).
6.3. Analysis.
Comparison 6 Mizoribine versus no mizoribine regimen, Outcome 3 GFR (any measure).
Leflunomide (40 to 60 mg/day) for 6 to 12 months with or without oral prednisone had uncertain effects on GFR over 6 to 88 months follow‐up (Analysis 7.5 (2 studies, 131 participants): MD 11.11 mL/min/1.73 m2, 95% CI ‐3.32 to 25.55; I2 = 62%; very low certainty evidence) compared to prednisone alone or RAS inhibitor. There was substantial heterogeneity in treatment effects observed between the studies.
7.5. Analysis.
Comparison 7 Leflunomide versus no leflunomide regimen, Outcome 5 GFR (any measure).
Prednisolone (30 mg) followed by a tapering course for 24 months combined with ARB had uncertain effects on GFR in a single study (Analysis 8.2 (38 participants): MD 16.00 mL/min/1.73 m2, 95% CI ‐6.89 to 38.89; low certainty evidence) compared to prednisolone alone.
8.2. Analysis.
Comparison 8 Steroid plus non‐immunosuppressive agents versus steroid alone, Outcome 2 GFR (any measure) [mL/min/1.73 m2].
There was no evidence for the effects of mTOR inhibitors on GFR.
Urinary protein excretion
Methylprednisolone (0.6 to 0.8 mg/kg/day), prednisolone (0.4 to 0.8 mg/kg/day or 20 to 60 mg/day) and prednisone (0.5 mg/kg/day or 40 to 60 mg/day) with or without methylprednisolone (1 g IV) were administered during 4 to 24 months of therapy followed by a tapering course. Steroid therapy may lower urinary protein excretion compared with placebo, standard care or other non‐immunosuppressive treatment during 1 to 10 years follow‐up (Analysis 1.8 (10 studies, 705 participants): MD ‐0.58 g/24 h, 95% CI ‐0.84 to ‐0.33; I2 = 60%;low certainty evidence). There was substantial heterogeneity in treatment effects observed between the studies.
1.8. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 8 Urinary protein excretion.
CPA and/or AZA with concomitant steroid treatment given for 3 to 24 months had uncertain effects on urinary protein excretion compared to standard care, steroid alone or other non‐immunosuppressive treatment (Analysis 3.5 (5 studies, 255 participants): MD ‐0.77 g/24 h, 95% CI ‐1.80 to 0.26; I2 = 98%; very low certainty evidence). There was substantial heterogeneity in treatment effects observed between the studies.
3.5. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 5 Urinary protein excretion.
MMF (1.5 to 2 g/day) with or without steroid therapy administered for up to 3 years had uncertain effects on urinary protein excretion when compared with placebo, standard care or steroid with leflunomide over 6 months to 3 years follow‐up (Analysis 4.9 (5 studies, 172 participants): MD ‐0.06 g/24 h, 95% CI ‐0.92 to 0.81; I2 = 96%; very low certainty evidence). There was substantial heterogeneity in treatment effects observed between the studies.
4.9. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 9 Urinary protein excretion.
CSA (3 to 5 mg/day) with or without concomitant steroid treatment or tacrolimus (0.1 mg/kg/day) for 3 to 12 months had uncertain effects on urinary protein excretion during 4 to 60 months follow‐up (Analysis 5.4 (3 studies, 110 participants): MD ‐0.50 g/24 h, 95% CI ‐1.12 to 0.12; I2 = 82%; very low certainty evidence). There was substantial heterogeneity in treatment effects observed between the studies.
5.4. Analysis.
Comparison 5 Calcineurin inhibitor (CNI) versus no CNI regimen, Outcome 4 Urinary protein excretion.
Mizoribine (150 to 250 mg/day) with RAS inhibitor or steroid treatment had uncertain effect on reduction of urinary protein excretion when compared with RAS inhibitor or steroid alone (Analysis 6.4 (2 studies, 105 participants): MD ‐0.04 g/24 h, 95% CI ‐0.30 to 0.22; low certainty evidence).
6.4. Analysis.
Comparison 6 Mizoribine versus no mizoribine regimen, Outcome 4 Urinary protein excretion.
Leflunomide (20 to 50 mg/day) with or without oral prednisone had uncertain effects on urinary protein excretion over 3 to 6 months follow‐up (Analysis 7.6 (3 studies, 125 participants): MD 0.20 g/24 h, 95% CI ‐0.60 to 1.00; I2 = 69%; very low certainty evidence) compared to steroid with or without MMF. There was substantial heterogeneity in treatment effects observed between the studies.
7.6. Analysis.
Comparison 7 Leflunomide versus no leflunomide regimen, Outcome 6 Urinary protein excretion.
Prednisolone (30 mg) followed by a tapering course for 24 months combined with ARB had uncertain effect on reduction of urinary protein excretion in a single study (Analysis 8.3 (38 participants): MD ‐0.20 g/24 h, 95% CI ‐0.26 to ‐0.14; low certainty evidence) compared to prednisolone alone.
8.3. Analysis.
Comparison 8 Steroid plus non‐immunosuppressive agents versus steroid alone, Outcome 3 Urinary protein excretion.
Sirolimus (1 mg/day) had uncertain effect on reduction of urinary protein excretion during 12 months follow‐up compared with no mTOR inhibitors in a single study (Analysis 9.1 (23 participants): MD ‐0.80 g/24 h, 95% CI ‐1.83 to 0.23; low certainty evidence).
9.1. Analysis.
Comparison 9 mTORi versus no mTORi regimen, Outcome 1 Urinary protein excretion.
Death (any cause)
Due to the rarity of death during follow‐up with this condition, the effects of all treatment strategies on the outcome of total death were either imprecisely known or not reported.
One parallel‐group study measured the effects of steroid versus placebo on the risks of death (any cause). During a median of 25 months, 3 deaths among 262 participants were reported. The comparative effects of treatment on death (any cause) was uncertain with an imprecise treatment effect (Analysis 1.9: RR 1.85, 95% CI 0.17 to 20.19; very low certainty evidence). Similarly, in a parallel‐group study evaluating CPA followed by AZA plus steroid versus steroid alone for 36 months, two deaths (one in each group) were recorded and treatment effects were uncertain (Analysis 3.6 (162 participants): RR 0.98, 95% CI 0.06 to 15.33; very low certainty evidence).
1.9. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 9 Death (any cause).
3.6. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 6 Death (any cause).
Death (any cause) was not reported for other treatment regimens including MMF, CNIs, mizoribine, leflunomide, steroid therapy combined with non‐immunosuppressive agents and mTOR inhibitors.
Infection
Methylprednisolone (0.6 to 0.8 mg/kg/day) was administered during 6 months of therapy followed by a tapering course. Corticosteroid therapy had uncertain effects on infection compared with placebo during 2 years follow‐up with very low imprecision in the estimated effect in a single study (Analysis 1.10 (262 participants): RR 21.32, 95% CI 1.27 to 358.10; very low certainty evidence). TESTING 2017 study was terminated early on the recommendation of the Data Safety Monitoring Committee due to an excess of serious adverse events in the corticosteroid group (mainly infections).
1.10. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 10 Infection.
NEFIGAN 2017, a novel targeted release formulation of budesonide, a glucocorticoid which is released in the distal ileum, with low systemic availability, had very uncertain effects on increasing infection adverse events during 12 months (Analysis 2.1 (150 participants): RR 0.83, 95% CI 0.21 to 3.35; very low certainty evidence). Budesonide (8 to 16 mg/day) was administered during 9 months of therapy.
2.1. Analysis.
Comparison 2 Locally‐acting steroid versus no locally‐acting steroid, Outcome 1 Infection.
CPA or AZA with concomitant steroid treatment given for 3 to 12 months had uncertain effects on infection compared to placebo or standard care (Analysis 3.7 (4 studies, 268 participants): RR 1.70, 95% CI 0.43 to 6.76; I2 = 0%; very low certainty evidence).
3.7. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 7 Infection.
MMF (1.5 to 2 g/day) with or without steroid therapy administered for up to 3 years had uncertain effects on occurrence of infection when compared with placebo, standard care or steroid alone (Analysis 4.10 (4 studies, 301 participants): RR 1.36, 95% CI 0.87 to 2.12; I2 = 0%; very low certainty evidence).
4.10. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 10 Infection.
CSA (3 mg/day) with concomitant steroid treatment for 12 months had uncertain effects in a single study in which four infections occurred during 60 months follow‐up (Analysis 5.5 (48 participants): RR 0.31, 95% CI 0.03 to 2.74; very low certainty evidence).
5.5. Analysis.
Comparison 5 Calcineurin inhibitor (CNI) versus no CNI regimen, Outcome 5 Infection.
Mizoribine (150 to 250 mg/day) with concomitant steroid or RAS inhibitor treatment had uncertain effects on occurrence of infection when compared with steroid or RAS inhibitor alone (Analysis 6.5 (2 studies, 104 participants): RR 1.52, 95% CI 0.14 to 16.15; I2 = 52%; very low certainty evidence). There was moderate statistical heterogeneity in the treatment effects between the studies
6.5. Analysis.
Comparison 6 Mizoribine versus no mizoribine regimen, Outcome 5 Infection.
Leflunomide (20 to 40 mg/day) for 6 to 12 months with or without oral prednisone had uncertain effects on occurrence of infection over 6 to 24 months follow‐up (Analysis 7.7 (3 studies, 387 participants): RR 0.97, 95% CI 0.45 to 2.09; I2 = 0%; very low certainty evidence) compared to prednisone alone or placebo.
7.7. Analysis.
Comparison 7 Leflunomide versus no leflunomide regimen, Outcome 7 Infection.
There was no evidence for the effects of steroids combined with non‐immunosuppressive agents or of mTOR inhibitors on infection.
Malignancy
Prednisone (0.5 mg/kg/day) for 6 months and methylprednisolone (1g IV) had uncertain effects in a single study in which 2 malignancies (1 in each group) occurred during 6 years follow‐up (Analysis 1.11 (86 participants): RR 1.00, 95% CI 0.06 to 15.48; very low certainty evidence).
1.11. Analysis.
Comparison 1 Systemic corticosteroid versus no corticosteroid regimen, Outcome 11 Malignancy.
CPA followed by AZA with concomitant steroid treatment given for 6 months had uncertain effects on malignancy compared to standard care during 3 years follow‐up in a single study (Analysis 3.8 (162 participants): RR 4.88, 95% CI 0.24 to 100.08; very low certainty evidence).
3.8. Analysis.
Comparison 3 Cytotoxic versus no cytotoxic regimen, Outcome 8 Malignancy.
MMF (2 g/day) administered for up to 3 years had uncertain effects on occurrence of malignancies when compared with placebo (Analysis 4.11 (2 studies, 86 participants): RR 0.28, 95% CI 0.03 to 2.54; I2 = 0%; very low certainty evidence).
4.11. Analysis.
Comparison 4 MMF versus no MMF regimen, Outcome 11 Malignancy.
CSA (3 mg/day) with concomitant steroid treatment for 12 months had uncertain effects in a single study in which one malignancy occurred (Analysis 5.6 (48 participants): RR 0.36, 95% CI 0.02 to 8.45; very low certainty evidence).
5.6. Analysis.
Comparison 5 Calcineurin inhibitor (CNI) versus no CNI regimen, Outcome 6 Malignancy.
Mizoribine (150 mg/day) for 12 months had uncertain effects on occurrence of malignancy when compared with standard care in a single study (Analysis 6.6 (42 participants): RR 3.00, 95% CI 0.13 to 69.70; very low certainty evidence). There was no evidence for the effects of leflunomide, steroids combined with non‐immunosuppressive agents or of mTOR inhibitors on malignancy.
6.6. Analysis.
Comparison 6 Mizoribine versus no mizoribine regimen, Outcome 6 Malignancy.
Adverse events
Table 7 details the adverse events in studies when they were described.
1. Reports of adverse events in individual studies.
| Study ID | Intervention | Reported side effect | Number of events in treatment group (N) | Number of events in control group (N) |
| 2nd NA IgAN 2004 | MMF versus placebo | Malignant melanoma, multiple intra‐abdominal injuries | 0+1 (13) | 1+0 (15) |
| Ballardie 2002 | Steroids + CPA versus no treatment | Pulmonary TB, overt diabetes, bone marrow toxicity, gastrointestinal toxicity | 1+1+1+1 (19) | 0+0+0+0 (19) |
| BRIGHT‐SC 2016 | Carboxylic acids (Blisibimod) versus placebo | Not reported | Not reported | Not reported |
| Cao 2008 | Steroids + leflunomide versus steroids | None reported | 0 (18) | 0 (18) |
| CAST‐IgA 2015 | Steroid + prednisolone + tonsillectomy + ARB versus steroid + prednisolone | Not reported | Not reported | Not reported |
| Chen 2002 | MMF versus prednisone | Diarrhoea, herpes zoster, nausea | % participants reported diarrhoea, herpes zoster, nausea (31) | Not reported |
| Cheung 2018 | Belimumab versus placebo | Not reported | Not reported | Not reported |
| Cruzado 2011 | Macrolide lactams (sirolimus) versus usual care | Fever, reversible hyperkalaemia, prostatic syndrome, unspecific acute gastritis, cholesterol increase, anaemia, oedema, mild facial rash | 0+1+1+1+2+1+2+2 (14) | 1+1+0+0+0+0+0+0 (9) |
| Frisch 2005 | MMF versus placebo | Gastrointestinal effects, deep vein thrombosis | 2+0 (17) | 2+1 (15) |
| Harmankaya 2002 | Steroids + AZA versus no treatment | Increased transaminase levels, minor cushingoid features, gastric pain | 1+2+1 (21) | 0 (22) |
| Hirai 2017 | Mizoribine versus usual care | Hepatotoxicity, low‐grade fever, malignant lymphoma | 1+1+1 (21) | 0+0+0 (21) |
| Horita 2007 | Steroids + RAS inhibitors versus steroids | Hypotension | 2 (20) | 0 (20) |
| Hou 2017 | MMF versus prednisone | Pneumonia, acute kidney injuries, ophthalmos‐neuritis, osteonecrosis of the femoral head, ESKD, gastric perforation, newly diagnosed diabetes, impaired glucose tolerance, infections, hepatic dysfunction, gastrointestinal symptoms, cushing syndrome, acne, cramps, insomnia, alopecia, tremors | 3+1+1+0+0+0+1+12+27+9+7+16+2+5+12+5+5 (87) | 4+0+0+1+1+1+12+15+20+14+10+42+5+11+17+11+7 (88) |
| Julian 1993 | Steroids versus no treatment | Overt diabetes, insomnia, acne | 2+2+3 (18) | 1 (17) |
| Kanno 2003 | Steroids versus warfarin | None reported | 0 (6) | 0 (4) |
| Katafuchi 2003 | Steroids + dipyridamole versus dipyridamole (in an abstract (Katafuchi 1997) that reported steroids versus antiplatelet agent in 80 participants, no adverse events were reported) |
Palpitations/insomnia | 3 (43) | 1 (47) |
| Kawamura 2014 | Methylprednisolone + tonsillectomy versus steroid | None reported | 0 (33) | 0 (39) |
| Lee 2003 | Steroids + ARB versus ARB | Not reported | Not reported | Not reported |
| Kim 2013b | Tacrolimus versus placebo | Cardiovascular, gastrointestinal, genitourinary, hematologic, musculoskeletal, neurologic, respiratory, dermatologic | 2+21+4+1+3+12+4+2 (20) | 1+4+0+0+3+1+5+1 (20) |
| Kobayashi 1996 | Steroids versus no treatment | None reported | 0 (20) | 0 (26) |
| Koike 2008 | Prednisolone + dipyridamole versus dipyridamole | None reported | 0 (24) | 0 (24) |
| Koitabashi 1996 | Chinese medicine (Saireito) versus no treatment versus prednisolone + AZA + anticoagulants + dipyridamole versus anticoagulants + dipyridamole | Not reported | Not reported | Not reported |
| Lafayette 2017 | Rituximab versus usual care | Cough, fever, flu, gastrointestinal symptoms, embolism, infections, rush, nasal congestion, eye problems, wart left 5th digit, right flank tenderness, headache, haemorrhage, pruritus, confusion, fatigue, muscle problems, back pain, dyspnoea, heartburn and cardiac problems, anorexia, sore throat, flushing, hypertension, photosensitivity, vaginal bleeding, left hand numbness | Not reported (17) | Not reported (17) |
| Lai 1986 | Steroids versus no treatment | Gastritis, hypertension | 1+3 (17) | 0+0 (17) |
| Lai 1987 | CSA versus placebo | Dyspepsia, headache, hypertension, hirsutism | 6+7+1+3+7 (12) | 0+0+0+0+1 (12) |
| Liu 2010a | Prednisone + leflunomide versus prednisone + MMF | Not reported | Not reported | Not reported |
| Liu 2014 | Methylprednisolone + CSA versus methylprednisolone | Severe pneumonia, recurrent urinary tract infection, elevated blood sugar, eyesight degradation | 3+2+2+5 (23) | 1+3+2+9 (25) |
| Locatelli 1999 | Steroids + AZA versus steroids | Hepatotoxicity, leukopenia, GI symptoms, bacterial Infections, viral Infections, Pneumocystis carinii infection, type 2 diabetes, hypertension | 5+3+3+3+1+1+1+0 (101) | 0+0+0+2+1+0+2+1 (106) |
| Lou 2006 | Leflunomide versus RAS inhibitors | Serum transaminases, mild alopecia, severe diarrhoea, cough | 2+1+1+0 (24) | 0+0+0+2 (22) |
| Lv 2009 | Steroids + RAS inhibitors versus RAS inhibitors | Cough, hyperkalaemia, palpitation, arthralgia | 2+0+1+1 (33) | 1+0+0+0 (30) |
| Maes 2004 | MMF versus placebo | Reactivation of pulmonary TB, gastrointestinal complaints, leukopenia, rectal carcinoma | 1+2+1+0 (21) | 0+0+0+1 (19) |
| Manno 2001 | Steroids + RAS inhibitors versus RAS inhibitors | Striae, glucidic intolerance, cough | 3+1+0 (48) | 0+0+2 (49) |
| Masutani 2016 | Prednisolone followed by mizoribine versus prednisolone | Pneumonia, herpes zoster, severe drug allergy | 2+0+0 (20) | 0+1+1 (20) |
| Min 2017 | Leflunomide + prednisone versus full dose prednisone | Hepatotoxicity, upper respiratory infection, pulmonary infection, diarrhoea, herpes‐zoster virus infection, pruritus, insomnia, alopecia, abnormal glucose metabolism | 3+4+2+1+0+1+0+1+0 (40) | 2+4+1+0+2+0+2+0+2 (45) |
| NA IgAN 1995 | Steroids versus placebo | Heartburn, increased appetite, weight gain | 15+24+22 (33) | 5+10+13 (31) |
| NEFIGAN 2017 | Low dose TRF‐budesonide versus high dose TRF‐budesonide versus placebo | Nasopharyngitis, acne, joint swelling, cushingoid, insomnia, diarrhoea, dyspepsia, headache, alopecia, back pain, mood swings, oedema peripheral, blood creatine phosphokinase increased, hirsutism, hypertension, muscle spasms, abdominal pain, nausea, upper respiratory tract infection | Treatment group 1 8+8+8+5+6+1+2+3+4+6+3+2+3+3+3+5+4+4+2 (51) Treatment group 2 10+9+9+8+8+5+7+6+4+3+5+6+3+5+5+2+3+3+3 (49) |
10+3+2+3+2+7+4+3+2+1+2+2+3+1+1+2+1+1+3 (50) |
| Ni 2005 | Steroids + leflunomide versus steroids | Elevated liver enzyme, infection, diarrhoea, nausea, rash, insomnia, blood glucose increase | 4+8+2+1+1+0+0 (51) | 4+10+0+0+1+1+1 (51) |
| Nuzzi 2009 | Steroids versus no treatment | None reported | 0 (15) | 0 (12) |
| Pozzi 1999 | Steroids versus no treatment | None reported | 0 (43) | 0 (43) |
| Segarra 2006 | Immunoglobulin + steroids versus steroids | Cutaneous rush, DM | 1+0 (19) | 0+1 (17) |
| Shen 2013 | Corticosteroid + CPA versus corticosteroid + tacrolimus versus corticosteroid | Not reported | Not reported | Not reported |
| Shi 2012a | Steroids + leflunomide versus steroids | Adverse events were assessed but not clearly reported | Not reported | Not reported |
| Shima 2018 | Prednisolone + mizoribine + warfarin + dipyridamole versus prednisolone + mizoribine | Obesity, hyperuricaemia, hypertension, headache, steroid‐induced gastric ulcer, glaucoma, steroid acne, stretch marks, bleeding, decreased bone mineral density, cataract, elevation of serum bilirubin, psychosis | 6+2+1+6+2+2+2+1+2+0+0+0+0 | 7+5+6+0+3+2+2+1+0+1+1+1+1 |
| Shoji 2000 | Steroids versus dipyridamole | Headache | 0 (11) | 1 (8) |
| Stangou 2011 | AZA + methylprednisolone versus methylprednisolone | Not reported | Not reported | Not reported |
| STOP‐IgAN 2008 | Methylprednisolone versus no treatment | Diverticulitis or appendicitis, pneumonia or respiratory tract infection, viral exanthema, knee empyema, death, malignant neoplasm, impaired glucose tolerance or DM, gastrointestinal bleeding, fracture, osteonecrosis, weight gain | 3+3+1+1+1+2+9+0+1+0+14 (82) | 1+1+1+0+1+0+1+0+0+0+5 (80) |
| Takeda 1999 | Steroids + antiplatelet agent versus antiplatelet agent | None reported | 0 (13) | 0 (12) |
| Tang 2005 | MMF + RAS inhibitors versus RAS inhibitors | Fall in haemoglobin level, diarrhoea, upper gastrointestinal upset, infective episodes | 3+1+1+3 (20) | None reported (20) |
| TESTING 2017 | Methylprednisolone versus placebo | Respiratory infection, pneumocystis pneumonia, cryptococcal meningitis, nocardia infection of skin and knee joint, perianal abscess, urinary tract infection, fever, duodenal ulcer, gastrointestinal bleeding, gastric perforation, vascular necrosis, osteochondroma, pulmonary embolism, deep vein thrombosis, hepatotoxicity, haemoptysis, acute right upper quadrant pain, arthralgia, symptomatic incarcerated paraumbilical hernia, uremia, soft tissue injury, new‐onset DM, vascular necrosis, fracture | 4+3+1+1+1+1+1+1+2+1+2+1+1+2+1+1+1+1+0+0+ 1+2+1+1 (136) |
0+0+0+0+0+0+0+0+0+1+0+0+0+0+0+0+0+0+1+2+ 0+3+0+0 (126) |
| Walker 1990a | CPA + dipyridamole + warfarin versus no treatment | Gonadal toxicity, headache | 2+1 (25) | 0+0 (27) |
| Welch 1992 | Steroids versus placebo | None reported | 0 (20) | 0 (20) |
| Woo 1987 | CPA + dipyridamole + warfarin versus no treatment | Gum bleeding | 2 (27) | 0 (21) |
| Wu 2016 | Telmisartan + clopidogrel placebo + leflunomide placebo versus telmisartan + clopidogrel + leflunomide placebo versus telmisartan + clopidogrel placebo + leflunomide versus telmisartan + clopidogrel + leflunomide | Death, abnormal liver function, hypotension, hyperkalaemia, neutropenia, rash, skin purpura, upper gastrointestinal bleeding, herpes zoster, urinary tract infection, upper respiratory tract infection | Treatment group 1 0+0+0+1+1+0+0+0+1+0+1 (100) Treatment group 2 0+3+1+2+0+0+0+0+0+1+0 (100) Treatment group 3 0+1+2+1+0+0+0+0+0+0+0 (100) |
0+3+0+0+2+2+1+1+0+0+0 (99) |
| Xie 2011 | Mizoribine versus losartan versus combination group | Serious adverse events, hyperuricaemia, upper respiratory tract infection, herpes zoster, leukopenia, elevation of transaminases, vertigo, alopecia | Treatment group 1 0+3+1+0+1+1+0+0 (35) Treatment group 2 0+1+3+0+1+1+1+0 (30) |
0+3+2+1+0+0+1+1 (34) |
| Yoshikawa 1999 | Steroids + AZA + dipyridamole versus dipyridamole | Alopecia, anaemia, leukopenia, cataract, ulcer, depression | 1+0+3+1+1+1 (40) | 0+1+0+0+0+0 (38) |
| Yoshikawa 2006 | Steroids + dipyridamole + AZA + warfarin versus steroids | Hypertension, glucosuria, aseptic necrosis of femur, glaucoma, cataract, headache, leukopenia, bleeding, anaemia, elevated transaminase concentration | 0+0+1+2+0+3+3+1+1+2 (40) | 5+3+1+2+2+0+0+0+0+1 (40) |
| Zhang 2004 | Leflunomide versus steroids | Elevate liver enzyme, nausea, lose hair, leukopenia | 3+1+1+1 (27) | None reported (22) |
ARB ‐ angiotensin receptor blocker; AZA ‐ azathioprine; CPA ‐ cyclophosphamide; CSA ‐ cyclosporin A; DM ‐ diabetes mellitus; MMF ‐ mycophenolate mofetil; RAS ‐ renin‐angiotensin system; TB ‐ tuberculosis; TRF‐budesonide ‐ targeted‐release formulation of budesonide
Publication bias
Due to the insufficient number of studies in each meta‐analysis, we were not able to assess for evidence of missing data due to small study effects or publication bias.
Subgroup analysis
We planned subgroup analysis assessing the treatment effects in studies involving various ethnicities, but due to limitations in the number of available studies, a subgroup analysis based on ethnicity was not possible
Post hoc subgroup analysis
There was no evidence that treatment effects of steroid therapy on risk of ESKD was different among participants receiving concomitant RAS blockade and BP control (ACEi and/or ARB) and those participants in whom additional background therapy was not specifically prescribed.
Discussion
The aim of this Cochrane review was to evaluate the effectiveness and safety of immunosuppression for treatment of IgA nephropathy to prevent progression to ESKD needing dialysis or kidney transplantation. In addition to the clinical endpoint of ESKD, we also examined the effects of various immunosuppression strategies on intermediate kidney endpoints including at least 50% reduction in eGFR, annual GFR loss, SCr, urinary protein excretion, and IgA nephropathy disease remission. Potential harms of treatment were evaluated including infection and malignancy. This is an update of a Cochrane review first published in 2003 and updated in early 2015 (which included 32 studies involving 1781 participants).
Summary of main results
In this substantive review update, 58 studies involving 3933 randomised participants were included. The major Immunosuppressive strategies were systemic corticosteroids and local corticosteroids (including budesonide), were frequently heterogeneous, and were allocated to nine intervention‐containing regimens:
Systemic corticosteroids
Locally‐acting steroid
Cytotoxic therapy (CPA or AZA)
MMF
CNI (CSA or tacrolimus)
Mizoribine
Leflunomide
Steroid plus non‐immunosuppressive agents
mTOR inhibitor (sirolimus).
Systemic corticosteroid therapy given for two to four months followed by a tapering dose has beneficial effects on a range of clinical and intermediate renal outcomes in people with IgA nephropathy and proteinuria. In people with generally moderate to severe proteinuria > 1 g/24 hours and mild to moderate CKD, corticosteroid treatment probably prevents ESKD requiring dialysis or transplantation (moderate certainty evidence); reduces annual loss of GFR for two to five years; incurs complete disease remission; and may reduce protein excretion by 0.5 g/24 hours. The effects of steroid therapy on preventing 50% loss in eGFR, infection, death (any cause), and malignancy were uncertain as there were few studies that reported these outcomes.
The effects of all other immunosuppressive regimens on clinical outcomes of IgA nephropathy (locally‐acting steroid, cytotoxic agents, MMF, CNIs, mizoribine, leflunomide, and mTOR inhibitors alone or with steroids) was uncertain. In general data were sparse due to few studies or intervention effects did not reach clinical or statistical significance. The various immunosuppression strategies had uncertain treatment effects on risks of ESKD, infection, complete remission, malignancy, GFR, SCr or doubling of SCr and urinary protein excretion. Steroids given together with non‐immunosuppressive agents similarly had uncertain effects on complete remission, GFR and urinary protein excretion compared to steroids administered alone.
There was no evidence that treatment effects of steroid therapy on risk of ESKD was different among participants receiving concomitant RAS blockade and BP control (ACE inhibitor and/or ARB) and those participants in whom additional background therapy was not specifically prescribed.
Overall completeness and applicability of evidence
In this substantive update, we were able to include an additional 26 studies with almost 2000 additional participants to the previous Cochrane review. Despite this now larger number of studies, limitations in the existing available studies include the rarity of many clinical events such as death and malignancy, precluding certainty of the impact of treatment on these clinical outcomes. Although ESKD tends to be a relatively rare outcome, studies evaluating steroid therapy tended to include participants with moderate or severe levels of proteinuria who are at higher risk for kidney failure. Intermediate kidney outcomes including change in eGFR, complete clinical disease remission, and reduction in protein excretion rate were improved with steroid therapy. Concordance between effects on clinical (ESKD) and surrogate outcomes (doubling SCr, reduction in proteinuria) strengthens the findings of the contributing studies. Studies measured effects of treatment on ESKD for between two and 10 years. providing sufficient statistical power to evaluate a hard renal endpoint. The findings of the review may not apply to those patients with milder clinical presentations with lower levels of proteinuria (< 1 gram) at the time of diagnosis. The benefits and risks of treatment in patients with marked impairment of kidney disease (GFR category 4) may be less certain. The treatment estimates are provided by evidence of moderate certainty. Due to disease heterogeneity in contributing studies and a lack of capacity to conduct subgroup analyses by subgroups of disease severity and ethnicity, knowledge of treatment efficacy based on specific patient characteristics is limited. It is unclear whether the findings of steroid effectiveness are applicable to patients with crescentic or rapidly progressive IgA nephropathy who are underrepresented in the available studies, and who may warrant a more aggressive treatment strategy. It was not possible to estimate whether treatment strategies had different effects based on age, ethnicity, or other clinical factors. We planned subgroup analysis assessing the treatment effects in studies involving various ethnicities, but due to limitations in the number of available studies, a subgroup analysis based on ethnicity was not possible.
We have included in the systemic corticosteroid meta‐analysis, only participants in STOP‐IgAN 2008 that received systemic corticosteroids alone. We analysed participants receiving combined systemic corticosteroids with additional cytotoxic agents in a separate relevant meta‐analysis.
Quality of the evidence
Due to the heterogeneity of participants, interventions and comparators, we have attempted to reduce complexity by categorising therapeutic strategies into specific groups. This may have over‐simplified the treatment interventions used and drawn differing treatment approaches into a single analysis, when important therapeutic effects might have existed. Despite combining treatment groups into overarching categories, we did not observe substantial statistical heterogeneity in the analyses, with the key finding of steroid effects on risk of ESKD having moderate certainty. There has been limited stratification by risk of ESKD in people with IgA nephropathy. Substantial disease heterogeneity suggests a validated tool for IgA nephropathy could predict the disease progression and enrich trial populations with patients at highest risk of ESKD.
The majority of studies in this review were at unclear risk of bias for many of the risk of bias domains, lowering certainty in the results due to study limitations. It was likely that most studies were not blinded, which may have impacted on treatment adherence and outcome assessment. For assessment of steroid therapy, GRADE assessment of outcomes led to trials being downgraded due to study limitations, while imprecision was present in some estimates due to a small number of studies or the rarity of clinical endpoints.
Potential biases in the review process
The evidence for this review is derived from a systematic search of the Cochrane Kidney and Transplant's specialised register, which provides literature from grey sources including conference proceedings and handsearched journals. This approach may help to minimise omission of potentially relevant trials. We additionally requested data from authors. The literature search was screened independently by two review authors who were involved in the process of the whole review, to limit errors in data management and analysis, and determining the risks of bias in contributing studies.
There was a high degree of heterogeneity between the available trials in the study interventions, clinical presentation and severity of IgA nephropathy, follow‐up duration, and measurement of outcome data. Despite this, we were able to summarise treatment effects across many trials with limited evidence of statistical heterogeneity. We have made generalisations about the dose and duration of corticosteroid therapy in the interests of assisting in clinical application of the findings of this review, that contained an element of subjectivity.
Agreements and disagreements with other studies or reviews
The findings in this review are consistent with a recently published systematic review with network meta‐analysis that identified treatment with steroid therapy plus RAS inhibition was the most effective treatment to prevent ESKD among patients with proteinuria more than 1 gram per day (Yang 2018). Similarly, the finding in this study that MMF has uncertain effects on renal outcomes in IgA nephropathy is consistent with a recently updated meta‐analysis of RCTs (Zheng 2018) and a second meta‐analysis which found that MMF did not reduce proteinuria significantly in patients with IgA nephropathy and persistent proteinuria after RAS blockade (Hogg 2015).
The findings of this systematic review are consistent with global guideline recommendations for the management of IgA nephropathy (KDIGO 2012). These guidelines suggest:
Patients with IgA nephropathy who have persistent proteinuria above 1 g/day despite three to six months of conservative management and who have an estimated GFR above 50 mL/min might receive benefit from steroid therapy (six months) based on low‐quality evidence
Patients with IgA nephropathy do not receive combined corticosteroid and CPA or AZA treatment unless there is crescentic IgA nephropathy with deteriorating kidney function
Not using MMF in the treatment of IgA nephropathy.
Authors' conclusions
Implications for practice.
The findings of this review are consistent with global guidelines and existing systematic reviews.
In adults and children with biopsy‐proven IgA nephropathy, proteinuria of 1 g/day or higher and mild to moderate kidney disease, steroid therapy given for 2 to 4 months with a tapering course probably prevents ESKD (moderate certainty) and slows annual progression of kidney failure (moderate certainty), while corticosteroids may decrease proteinuria (low certainty). Caution with corticosteroid therapy is needed due to the potential for serious infections, and at present evidence certainty about risks of adverse infection events in available trials is very low.
Other immunosuppression strategies do not appear to have detectable benefits on kidney function among adults and children with IgA nephropathy. Specifically, treatment with CPA, AZA, or MMF do not appear to be indicated to treat IgA nephropathy.
Treatment strategies for aggressive forms of IgA nephropathy have limited evidence, and the findings in this review may not be generalisable to patients with mild disease.
Implications for research.
While available data suggest steroid therapy might be effective to reduce ESKD and improve complete remission, additional specific data would be informative.
Due to the wide heterogeneity of disease in IgA nephropathy, stratification of study populations using risk stratification scoring may assist to improve precision in treatment estimates and identify populations with specific disease risks or severity that are most responsive to evaluated treatments.
Based on available data, and the promising utility of steroid therapy in IgA nephropathy, a larger study comparing targeted release formulation of budesonide against placebo and prednisone and sufficiently powered to evaluate infection‐related adverse events would help to inform clinical practice. The absence of studies among patients with lower GFR suggests additional studies that include patients with rapidly progressive disease and with lower GFRs may be informative.
In addition, studies of steroid treatment with evaluation of patient‐relevant endpoints that focus on the following questions would be helpful.
Effect of baseline proteinuria level on treatment effectiveness (appropriate threshold for initiating therapy)
Duration of treatment
Effects of ethnicity on treatment effectiveness.
A trials network that provides a multinational multicentre approach (as is utilised in research of rare glomerulonephritides) may increase the feasibility of studies in this clinical setting that are powered to evaluate treatment effects on patient‐relevant outcomes.
Based on this evidence synthesis showing potential harm from steroid therapy, further evaluation of newer targeted therapies such as budesonide and eculizumab and other complement‐targeted therapies is warranted.
What's new
| Date | Event | Description |
|---|---|---|
| 18 March 2020 | Amended | Risk of bias judgements added for Yamauchi 2001 |
History
Protocol first published: Issue 1, 2003 Review first published: Issue 4, 2003
| Date | Event | Description |
|---|---|---|
| 9 September 2019 | New search has been performed | Search update and update of included studies and outcome data |
| 9 September 2019 | New citation required and conclusions have changed | New studies and interventions added |
| 15 July 2015 | New search has been performed | Review updated |
| 15 July 2015 | New citation required and conclusions have changed | New interventions identified |
| 22 July 2008 | Amended | Converted to new review format. |
Notes
Risk of bias judgements added for Yamauchi 2001.
Acknowledgements
The authors are grateful to the following peer reviewers for their time and comments: Ainslie M. Hildebrand MD MSc, Professor Jonathan Barratt PhD FRCP (The Mayer Professor of Renal Medicine, Department of Cardiovascular Sciences, University of Leicester; Honorary Consultant Nephrologist, John Walls Renal Unit, Leicester General Hospital, UK), Richard J. Glassock MD (Emeritus Professor, Geffen School of Medicine at UCLA, Los Angeles, CA, USA).
The authors are particularly indebted to Drs C Pozzi, RG Walker, R Katafuchi, and O Harmankaya for providing additional data relating to their studies upon request.
We also thank Professor Vlado Perkovic for providing additional data for the TESTING 2017 study in the 2020 update.
Bibiana Bonerba was an author on the 2015 update.
Appendices
Appendix 1. Electronic search strategies
| Database | Search terms |
| CENTRAL |
|
| MEDLINE |
|
| EMBASE |
|
Appendix 2. Assessment of source of bias
| Potential source of bias | Assessment criteria |
|
Random sequence generation Selection bias (biased allocation to interventions) due to inadequate generation of a randomised sequence |
Low risk of bias: Random number table; computer random number generator; coin tossing; shuffling cards or envelopes; throwing dice; drawing of lots; minimization (minimization may be implemented without a random element, and this is considered to be equivalent to being random). |
| High risk of bias: Sequence generated by odd or even date of birth; date (or day) of admission; sequence generated by hospital or clinic record number; allocation by judgement of the clinician; by preference of the participant; based on the results of a laboratory test or a series of tests; by availability of the intervention. | |
| Unclear: Insufficient information about the sequence generation process to permit judgement. | |
|
Allocation concealment Selection bias (biased allocation to interventions) due to inadequate concealment of allocations prior to assignment |
Low risk of bias: Randomisation method described that would not allow investigator/participant to know or influence intervention group before eligible participant entered in the study (e.g. central allocation, including telephone, web‐based, and pharmacy‐controlled, randomisation; sequentially numbered drug containers of identical appearance; sequentially numbered, opaque, sealed envelopes). |
| High risk of bias: Using an open random allocation schedule (e.g. a list of random numbers); assignment envelopes were used without appropriate safeguards (e.g. if envelopes were unsealed or non‐opaque or not sequentially numbered); alternation or rotation; date of birth; case record number; any other explicitly unconcealed procedure. | |
| Unclear: Randomisation stated but no information on method used is available. | |
|
Blinding of participants and personnel Performance bias due to knowledge of the allocated interventions by participants and personnel during the study |
Low risk of bias: No blinding or incomplete blinding, but the review authors judge that the outcome is not likely to be influenced by lack of blinding; blinding of participants and key study personnel ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding or incomplete blinding, and the outcome is likely to be influenced by lack of blinding; blinding of key study participants and personnel attempted, but likely that the blinding could have been broken, and the outcome is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Blinding of outcome assessment Detection bias due to knowledge of the allocated interventions by outcome assessors. |
Low risk of bias: No blinding of outcome assessment, but the review authors judge that the outcome measurement is not likely to be influenced by lack of blinding; blinding of outcome assessment ensured, and unlikely that the blinding could have been broken. |
| High risk of bias: No blinding of outcome assessment, and the outcome measurement is likely to be influenced by lack of blinding; blinding of outcome assessment, but likely that the blinding could have been broken, and the outcome measurement is likely to be influenced by lack of blinding. | |
| Unclear: Insufficient information to permit judgement | |
|
Incomplete outcome data Attrition bias due to amount, nature or handling of incomplete outcome data. |
Low risk of bias: No missing outcome data; reasons for missing outcome data unlikely to be related to true outcome (for survival data, censoring unlikely to be introducing bias); missing outcome data balanced in numbers across intervention groups, with similar reasons for missing data across groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk not enough to have a clinically relevant impact on the intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes not enough to have a clinically relevant impact on observed effect size; missing data have been imputed using appropriate methods. |
| High risk of bias: Reason for missing outcome data likely to be related to true outcome, with either imbalance in numbers or reasons for missing data across intervention groups; for dichotomous outcome data, the proportion of missing outcomes compared with observed event risk enough to induce clinically relevant bias in intervention effect estimate; for continuous outcome data, plausible effect size (difference in means or standardized difference in means) among missing outcomes enough to induce clinically relevant bias in observed effect size; ‘as‐treated’ analysis done with substantial departure of the intervention received from that assigned at randomisation; potentially inappropriate application of simple imputation. | |
| Unclear: Insufficient information to permit judgement | |
|
Selective reporting Reporting bias due to selective outcome reporting |
Low risk of bias: The study protocol is available and all of the study’s pre‐specified (primary and secondary) outcomes that are of interest in the review have been reported in the pre‐specified way; the study protocol is not available but it is clear that the published reports include all expected outcomes, including those that were pre‐specified (convincing text of this nature may be uncommon). |
| High risk of bias: not all of the study’s pre‐specified primary outcomes have been reported; one or more primary outcomes is reported using measurements, analysis methods or subsets of the data (e.g. subscales) that were not pre‐specified; one or more reported primary outcomes were not pre‐specified (unless clear justification for their reporting is provided, such as an unexpected adverse effect); one or more outcomes of interest in the review are reported incompletely so that they cannot be entered in a meta‐analysis; the study report fails to include results for a key outcome that would be expected to have been reported for such a study. | |
| Unclear: Insufficient information to permit judgement | |
|
Other bias Bias due to problems not covered elsewhere in the table |
Low risk of bias: The study appears to be free of other sources of bias. |
| High risk of bias: Had a potential source of bias related to the specific study design used; stopped early due to some data‐dependent process (including a formal‐stopping rule); had extreme baseline imbalance; has been claimed to have been fraudulent; had some other problem. | |
| Unclear: Insufficient information to assess whether an important risk of bias exists; insufficient rationale or evidence that an identified problem will introduce bias. |
Data and analyses
Comparison 1. Systemic corticosteroid versus no corticosteroid regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ESKD | 8 | 741 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.23, 0.65] |
| 1.1 Steroid (oral) versus placebo or usual care | 5 | 495 | Risk Ratio (IV, Random, 95% CI) | 0.48 [0.27, 0.85] |
| 1.2 Steroid (IV + oral) versus placebo or usual care | 1 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.14 [0.01, 2.68] |
| 1.3 Steroid (oral) plus RASi versus RASi alone | 2 | 160 | Risk Ratio (IV, Random, 95% CI) | 0.16 [0.04, 0.59] |
| 2 Complete remission | 4 | 305 | Risk Ratio (IV, Random, 95% CI) | 1.76 [1.03, 3.01] |
| 2.1 Steroid (oral) versus placebo or usual care | 2 | 145 | Risk Ratio (IV, Random, 95% CI) | 3.47 [0.71, 17.08] |
| 2.2 Steroid plus RASi versus RASi alone | 2 | 160 | Risk Ratio (IV, Random, 95% CI) | 1.41 [0.80, 2.48] |
| 3 Doubling of serum creatinine | 7 | 404 | Risk Ratio (IV, Random, 95% CI) | 0.43 [0.29, 0.65] |
| 3.1 Steroid (oral) versus placebo or usual care | 6 | 341 | Risk Ratio (IV, Random, 95% CI) | 0.45 [0.29, 0.69] |
| 3.2 Steroid (oral) plus RASi versus RASi alone | 1 | 63 | Risk Ratio (IV, Random, 95% CI) | 0.26 [0.06, 1.15] |
| 4 Serum creatinine | 7 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 4.1 Steroid versus no treatment or placebo or other non‐immunosuppressive treatment | 7 | 211 | Mean Difference (IV, Random, 95% CI) | ‐21.07 [‐44.12, 1.99] |
| 5 GFR loss: ≥ 50% | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 5.1 Steroid (oral) versus placebo or usual care | 2 | 326 | Risk Ratio (IV, Random, 95% CI) | 0.56 [0.25, 1.24] |
| 6 Annual GFR loss [mL/min/1.73 m2] | 2 | 359 | Mean Difference (IV, Random, 95% CI) | ‐5.40 [‐8.55, ‐2.25] |
| 6.1 Steroid (oral) versus placebo or usual care | 1 | 262 | Mean Difference (IV, Random, 95% CI) | ‐5.16 [‐9.79, ‐0.53] |
| 6.2 Steroid (oral) plus RASi versus RASi alone | 1 | 97 | Mean Difference (IV, Random, 95% CI) | ‐5.61 [‐9.91, ‐1.31] |
| 7 GFR (any measure) | 4 | Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 7.1 Steroid versus no treatment or placebo or other non‐immunosuppressive treatment | 4 | 138 | Mean Difference (IV, Random, 95% CI) | 17.87 [4.93, 30.82] |
| 8 Urinary protein excretion | 10 | 705 | Mean Difference (IV, Random, 95% CI) | ‐0.58 [‐0.84, ‐0.33] |
| 8.1 Steroid plus dipyridamole versus dipyridamole alone | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.37 [‐0.78, 0.04] |
| 8.2 Steroid versus no treatment or placebo or other non‐immunosuppressive treatment | 9 | 657 | Mean Difference (IV, Random, 95% CI) | ‐0.63 [‐0.92, ‐0.33] |
| 9 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 9.1 Steroid (oral) versus placebo or usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Infection | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 10.1 Steroid (oral) versus placebo or usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Malignancy | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 11.1 Steroid (IV + oral) versus placebo or usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 2. Locally‐acting steroid versus no locally‐acting steroid.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Infection | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected |
Comparison 3. Cytotoxic versus no cytotoxic regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ESKD | 7 | 463 | Risk Ratio (IV, Random, 95% CI) | 0.63 [0.33, 1.20] |
| 1.1 Cyclophosphamide then azathioprine plus steroid versus usual care | 2 | 200 | Risk Ratio (IV, Random, 95% CI) | 0.49 [0.14, 1.75] |
| 1.2 Cyclophosphamide plus antiplatelet/anticoagulant versus usual care | 2 | 100 | Risk Ratio (IV, Random, 95% CI) | 0.31 [0.03, 2.85] |
| 1.3 Azathioprine plus steroid versus placebo/usual care | 1 | 43 | Risk Ratio (IV, Random, 95% CI) | 3.14 [0.13, 72.96] |
| 1.4 Azathioprine plus steroid versus steroid alone | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.59, 2.32] |
| 1.5 Azathioprine plus steroid plus anticoagulant/antiplatelet versus anticoagulant/antiplatelet | 1 | 74 | Risk Ratio (IV, Random, 95% CI) | 0.34 [0.07, 1.64] |
| 2 Complete remission | 5 | 381 | Risk Ratio (IV, Random, 95% CI) | 1.47 [0.94, 2.30] |
| 2.1 Cyclophosphamide then azathioprine plus steroid versus usual care | 1 | 162 | Risk Ratio (IV, Random, 95% CI) | 3.41 [1.17, 9.93] |
| 2.2 Cyclophosphamide plus steroid versus steroid | 1 | 24 | Risk Ratio (IV, Random, 95% CI) | 0.78 [0.44, 1.39] |
| 2.3 Azathioprine plus steroids versus placebo/usual care | 1 | 43 | Risk Ratio (IV, Random, 95% CI) | 5.94 [2.03, 17.34] |
| 2.4 Azathioprine plus steroids plus anticoagulants versus steroids alone | 1 | 78 | Risk Ratio (IV, Random, 95% CI) | 1.24 [1.01, 1.52] |
| 2.5 Azathioprine plus steroid plus anticoagulant/antiplatelet versus anticoagulant/antiplatelet | 1 | 74 | Risk Ratio (IV, Random, 95% CI) | 1.13 [0.76, 1.70] |
| 3 Annual GFR loss [mL/min/1.73 m2] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Cyclophosphamide then azathioprine plus steroid versus usual care | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 GFR (any measure) [mL/min/1.73 m2] | 3 | 174 | Mean Difference (IV, Random, 95% CI) | 3.07 [‐6.57, 12.72] |
| 4.1 Azathioprine plus steroid plus anticoagulant/antiplatelet versus anticoagulant/antiplatelet | 1 | 74 | Mean Difference (IV, Random, 95% CI) | 2.0 [‐15.98, 19.98] |
| 4.2 Azathioprine plus steroids plus anticoagulants versus steroids alone | 2 | 100 | Mean Difference (IV, Random, 95% CI) | 3.51 [‐7.92, 14.94] |
| 5 Urinary protein excretion | 5 | 255 | Mean Difference (IV, Random, 95% CI) | ‐0.77 [‐1.80, 0.26] |
| 5.1 Cytotoxic agents plus steroids versus placebo, no treatment or anticoagulant/antiplatelet | 3 | 155 | Mean Difference (IV, Random, 95% CI) | ‐1.25 [‐2.71, 0.21] |
| 5.2 Cytotoxic agents plus steroids plus anticoagulants versus steroids alone | 2 | 100 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.09, 0.05] |
| 6 Death (any cause) | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Cyclophosphamide then azathioprine plus steroid versus steroid | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Infection | 4 | 268 | Risk Ratio (IV, Random, 95% CI) | 1.70 [0.43, 6.76] |
| 7.1 Cyclophosphamide then azathioprine plus steroid versus usual care | 2 | 200 | Risk Ratio (IV, Random, 95% CI) | 4.65 [0.54, 39.85] |
| 7.2 Azathioprine plus steroid versus steroid alone | 2 | 68 | Risk Ratio (IV, Random, 95% CI) | 0.85 [0.14, 5.10] |
| 8 Malignancy | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 8.1 Cyclophosphamide then azathioprine plus steroid versus usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 4. MMF versus no MMF regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ESKD | 4 | 280 | Risk Ratio (IV, Random, 95% CI) | 0.73 [0.16, 3.23] |
| 1.1 MMF versus placebo/usual care | 2 | 66 | Risk Ratio (IV, Random, 95% CI) | 2.37 [0.63, 8.96] |
| 1.2 MMF plus steroid versus steroid alone | 1 | 174 | Risk Ratio (IV, Random, 95% CI) | 0.20 [0.01, 4.20] |
| 1.3 MMF plus RASi versus RASi alone | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 0.22 [0.05, 0.90] |
| 2 Complete remission | 4 | 271 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.73, 1.52] |
| 2.1 MMF versus placebo/usual care | 3 | 97 | Risk Ratio (IV, Random, 95% CI) | 2.02 [0.55, 7.38] |
| 2.2 MMF plus steroid versus steroid alone | 1 | 174 | Risk Ratio (IV, Random, 95% CI) | 0.99 [0.68, 1.46] |
| 3 Doubling of serum creatinine | 2 | 74 | Risk Ratio (IV, Random, 95% CI) | 2.01 [0.28, 14.44] |
| 3.1 MMF versus placebo/usual care | 1 | 34 | Risk Ratio (IV, Random, 95% CI) | 4.45 [0.25, 79.87] |
| 3.2 MMF plus RASi versus RASi alone | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 1.0 [0.07, 14.90] |
| 4 Serum creatinine | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 5 GFR loss: ≥ 50% | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5.1 MMF versus placebo/usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 GFR loss: ≥ 25% | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.1 MMF versus placebo/usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 7 Annual GFR loss [mL/min/1.73 m2] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 7.1 MMF versus placebo/usual care | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 GFR (any measure) [mL/min/1.73 m2] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 9 Urinary protein excretion | 5 | 172 | Mean Difference (IV, Random, 95% CI) | ‐0.06 [‐0.92, 0.81] |
| 9.1 MMF versus placebo | 3 | 92 | Mean Difference (IV, Random, 95% CI) | 0.59 [0.20, 0.98] |
| 9.2 MMF plus RASi versus RASi alone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐1.26 [‐1.46, ‐1.06] |
| 9.3 MMF versus leflunomide | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.02 [‐0.20, 0.16] |
| 10 Infection | 4 | 301 | Risk Ratio (IV, Random, 95% CI) | 1.36 [0.87, 2.12] |
| 10.1 MMF versus placebo/usual care | 3 | 126 | Risk Ratio (IV, Random, 95% CI) | 1.35 [0.50, 3.64] |
| 10.2 MMF plus steroid versus steroid alone | 1 | 175 | Risk Ratio (IV, Random, 95% CI) | 1.37 [0.83, 2.24] |
| 11 Malignancy | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 11.1 MMF versus placebo/usual care | 2 | 86 | Risk Ratio (IV, Random, 95% CI) | 0.28 [0.03, 2.54] |
Comparison 5. Calcineurin inhibitor (CNI) versus no CNI regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 2 | Risk Ratio (IV, Random, 95% CI) | Subtotals only | |
| 1.1 CNI plus steroid versus steroid | 2 | 72 | Risk Ratio (IV, Random, 95% CI) | 0.91 [0.60, 1.39] |
| 2 Serum creatinine | 2 | 62 | Mean Difference (IV, Random, 95% CI) | 7.75 [‐6.76, 22.27] |
| 2.1 Cyclosporin versus placebo or usual care | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 0.0 [‐32.39, 32.39] |
| 2.2 Tacrolimus versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 9.70 [‐6.53, 25.93] |
| 3 GFR (any measure) | 3 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.18 [‐7.42, 7.07] |
| 3.1 Cyclosporin versus placebo or no treatment [mL/min/1.73 m2] | 1 | 22 | Mean Difference (IV, Random, 95% CI) | 4.5 [‐7.36, 16.36] |
| 3.2 Tacrolimus versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐5.70 [‐20.27, 8.87] |
| 3.3 CNI plus steroid versus steroid | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐1.17 [‐12.92, 10.58] |
| 4 Urinary protein excretion | 3 | 110 | Mean Difference (IV, Random, 95% CI) | ‐0.50 [‐1.12, 0.12] |
| 4.1 Cyclosporin versus placebo or no treatment | 1 | 22 | Mean Difference (IV, Random, 95% CI) | ‐1.60 [‐2.43, ‐0.77] |
| 4.2 Tacrolimus versus placebo | 1 | 40 | Mean Difference (IV, Random, 95% CI) | ‐0.11 [‐0.52, 0.30] |
| 4.3 CNI plus steroid versus steroid | 1 | 48 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.46, 0.12] |
| 5 Infection | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 5.1 CNI plus steroid versus steroid | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 6 Malignancy | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.1 CNI plus steroid versus steroid | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 6. Mizoribine versus no mizoribine regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ESKD | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Mizoribine versus placebo/usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Complete remission | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Mizoribine versus placebo/usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 GFR (any measure) | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 4 Urinary protein excretion | 2 | 105 | Mean Difference (IV, Random, 95% CI) | ‐0.04 [‐0.30, 0.22] |
| 4.1 Mizoribine versus ACEi | 1 | 65 | Mean Difference (IV, Random, 95% CI) | ‐0.17 [‐0.39, 0.05] |
| 4.2 Mizoribine plus steroid versus steroid alone | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.10 [‐0.15, 0.35] |
| 5 Infection | 2 | 104 | Risk Ratio (IV, Random, 95% CI) | 1.52 [0.14, 16.15] |
| 5.1 Mizoribine plus steroid (IV + oral) versus steroid alone | 1 | 40 | Risk Ratio (IV, Random, 95% CI) | 7.0 [0.38, 127.32] |
| 5.2 Mizoribine plus RASi versus RASi | 1 | 64 | Risk Ratio (IV, Random, 95% CI) | 0.59 [0.11, 3.29] |
| 6 Malignancy | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 6.1 Mizoribine versus placebo/usual care | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 7. Leflunomide versus no leflunomide regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ESKD | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 1.1 Leflunomide plus low dose steroid versus high dose steroid | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 2 Complete remission | 4 | 282 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.80, 1.46] |
| 2.1 Leflunomide versus RASi | 1 | 46 | Risk Ratio (IV, Random, 95% CI) | 1.17 [0.68, 2.00] |
| 2.2 Leflunomide plus low dose steroid versus high dose steroid | 2 | 187 | Risk Ratio (IV, Random, 95% CI) | 1.00 [0.64, 1.57] |
| 2.3 Leflunomide versus steroid | 1 | 49 | Risk Ratio (IV, Random, 95% CI) | 1.63 [0.56, 4.70] |
| 3 Doubling of serum creatinine | 1 | Risk Ratio (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Leflunomide plus low dose steroid versus high dose steroid | 1 | Risk Ratio (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 4 Serum creatinine | 2 | 125 | Mean Difference (IV, Random, 95% CI) | ‐4.29 [‐15.81, 7.24] |
| 5 GFR (any measure) | 2 | 131 | Mean Difference (IV, Random, 95% CI) | 11.11 [‐3.32, 25.55] |
| 5.1 Leflunomide plus low dose steroid versus high dose steroid | 1 | 85 | Mean Difference (IV, Random, 95% CI) | 3.77 [‐8.82, 16.36] |
| 5.2 Leflunomide versus RASi | 1 | 46 | Mean Difference (IV, Random, 95% CI) | 18.5 [5.81, 31.19] |
| 6 Urinary protein excretion | 3 | 125 | Mean Difference (IV, Random, 95% CI) | ‐0.03 [‐0.32, 0.25] |
| 6.1 Leflunomide plus steroid versus steroid alone | 2 | 85 | Mean Difference (IV, Random, 95% CI) | 0.03 [‐0.66, 0.72] |
| 6.2 Leflunomide vs MMF | 1 | 40 | Mean Difference (IV, Random, 95% CI) | 0.02 [‐0.16, 0.20] |
| 7 Infection | 3 | 387 | Risk Ratio (IV, Random, 95% CI) | 0.97 [0.45, 2.09] |
| 7.1 Leflunomide plus low dose steroid versus high dose steroid | 2 | 187 | Risk Ratio (IV, Random, 95% CI) | 0.90 [0.41, 1.99] |
| 7.2 Leflunomide versus placebo | 1 | 200 | Risk Ratio (IV, Random, 95% CI) | 3.0 [0.12, 72.77] |
Comparison 8. Steroid plus non‐immunosuppressive agents versus steroid alone.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Complete remission | 2 | 115 | Risk Ratio (IV, Random, 95% CI) | 1.05 [0.83, 1.31] |
| 1.1 Steroid plus RASi versus steroid alone | 1 | 38 | Risk Ratio (IV, Random, 95% CI) | 1.08 [0.84, 1.39] |
| 1.2 Steroid plus tonsillectomy plus ARB versus steroid plus tonsillectomy | 1 | 77 | Risk Ratio (IV, Random, 95% CI) | 0.93 [0.56, 1.53] |
| 2 GFR (any measure) [mL/min/1.73 m2] | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 2.1 Steroid plus RASi versus steroid alone | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] | |
| 3 Urinary protein excretion | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected | |
| 3.1 Steroid plus RASi versus steroid alone | 1 | Mean Difference (IV, Random, 95% CI) | 0.0 [0.0, 0.0] |
Comparison 9. mTORi versus no mTORi regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Urinary protein excretion | 1 | Mean Difference (IV, Random, 95% CI) | Totals not selected |
Comparison 10. Subgroup analysis for ESKD: steroid versus no steroid regimen.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 ESKD | 8 | 741 | Risk Ratio (IV, Random, 95% CI) | 0.39 [0.23, 0.65] |
| 1.1 Baseline ACEi/ARB | 4 | 484 | Risk Ratio (IV, Random, 95% CI) | 0.35 [0.17, 0.75] |
| 1.2 No baseline ACEi/ARB | 4 | 257 | Risk Ratio (IV, Random, 95% CI) | 0.42 [0.20, 0.87] |
10.1. Analysis.
Comparison 10 Subgroup analysis for ESKD: steroid versus no steroid regimen, Outcome 1 ESKD.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
2nd NA IgAN 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised scheme that was constructed with a computer‐based pseudo‐random number generator |
| Allocation concealment (selection bias) | Unclear risk | The biostatistician determined the treatment group assignment for all eligible patients |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind placebo‐controlled study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/25 participants assigned to MMF (fall in GFR (2); patient choice (2); prolonged hospitalisation (1); non‐adherence (1); trial termination (6)) and 12/27 participants assigned to placebo (loss‐to‐follow up (1); patient choice (4); malignant melanoma (1); pregnancy (1); trial terminated (5)) did not complete study at 12 months |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause) and ESKD) were not reported |
| Other bias | High risk | An independent Data and Safety Monitoring Committee met in person or by teleconference at least annually. In year 5 of the trial (2007), following careful consideration of the unblinded trial data, the committee concluded that it was extremely unlikely that any efficacy could be demonstrated from the limited additional enrolment and follow‐up that was going to be possible. The committee therefore recommended termination of the trial. There were no safety issues leading to this decision. Baseline characteristics were balanced across treatment groups |
Ballardie 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants remained in study and were included in analysis for ESKD at 24 months |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), infection) |
| Other bias | Unclear risk | Not reported in sufficient detail to perform adjudication. Methods of randomisation, baseline characteristics were not reported to assess quality of randomisation |
BRIGHT‐SC 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was not reported. However, the study outcomes of interest were objectively measured, therefore this was adjudicated as low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 10/57 participants did not complete 6 months of study follow‐up and were not included in analysis. It was not clear whether there was differential loss in the treatment groups and the reasons for dropout were not provided |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | Unclear risk | Insufficient information to permit judgement. Methods of randomisation, baseline characteristics were not reported to assess quality of randomisation |
Cao 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no report of blinding. As the treatments were physically different, it was likely that participants and investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was not reported. However, the study outcomes of interest were objectively measured, therefore this was adjudicated as low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | Unclear risk | Insufficient information to permit judgement. Methods of randomisation, baseline characteristics were not reported to assess quality of randomisation |
CAST‐IgA 2015.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessment was not reported. However, the study outcomes of interest were objectively measured, therefore this was adjudicated as low risk of bias |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | Unclear risk | Insufficient information to permit judgement. Methods of randomisation, baseline characteristics were not reported to assess quality of randomisation |
Chen 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | Unclear risk | Insufficient information to permit judgement. Methods of randomisation, baseline characteristics were not reported to assess quality of randomisation |
Cheung 2018.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Interpretation of subjective outcomes may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), infection, malignancy, ESKD, doubling of SCr, complete remission, SCr) |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Cruzado 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Permuted‐block randomisation with a block size of six and an allocation ratio of 1:1 |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data were available |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes, except death, were reported |
| Other bias | High risk | Recruitment terminated early due to lack of recruitment (achieved recruitment of 23 out of 30 planned participants). Participants in control group were older (imbalance of baseline characteristics) |
Frisch 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised using permuted blocks of four. Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Known only to the research pharmacy. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Patients and physicians were blinded to the therapy by use of identical capsules. Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. Key outcomes were objective laboratory measures or clinical events and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data were available |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | High risk | The study was terminated early after the second scheduled interim analysis done by the independent study monitor revealed a trend towards a worse outcome in the MMF group that would have made it highly unlikely to show a benefit for MMF given our rate of recruitment and our target sample size. Follow‐up stopped in July 2003 |
Harmankaya 2002.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Not all participant data were reported |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hirai 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done at the registration centre of Jichi Medical University using a computer‐based allocation program |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/21 participants lost to follow up in mizoribine group (including for adverse events); 6/21 participants lost to follow up in control group. Imbalance in discontinuation between groups |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), ESKD, GFR loss, infection) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Horita 2007.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/22 patients dropped out of the prednisolone group due to postural hypotension |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), ESKD, GFR loss, infection) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Hou 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Hangzhou Tigermed Consulting Co Ltd created the randomisation list |
| Allocation concealment (selection bias) | Unclear risk | Sequentially numbered concealed envelopes containing group assignments were provided to investigators. Not stated if envelopes were opaque |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | The outcomes were adjudicated by an independent Clinical End Points Committee, blinded to the treatment regimen |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/87 in treatment group not included in primary analysis. 1/89 in control group not included in primary analysis |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), ESKD, GFR loss, infection) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Julian 1993.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | This is a preliminary report ‐ only 3 patients had completed the full 2 year study; 24 remain in the study and 21 of these have completed at least 6 months and 16 have completed 12 months |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection) |
| Other bias | Unclear risk | Insufficient information to permit judgement. Methods of randomisation, baseline characteristics were not reported to assess quality of randomisation |
Kanno 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 5/15 patients did not complete study (2 in the treatment group and 3 in the control group) |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Katafuchi 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/49 participants lost to follow up in intervention group; 7/54 participants lost to follow up in control group |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), malignancy, infection) |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Kawamura 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was done by a technical assistant in the registration centre using a computer‐based allocation program with a minimisation method, which was developed by an outside company |
| Allocation concealment (selection bias) | High risk | Allocation was based on the presence or absence of tonsillectomy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Since the allocation was based on the presence or absence of tonsillectomy, neither the patients nor the physicians were blinded to the group assignment |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Although those assessing the outcomes were not blinded, they assessed the data regarding the pre‐defined outcomes using pre‐specified statistical analyses. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), infection) were not reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Kim 2013b.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation |
| Allocation concealment (selection bias) | Unclear risk | Conducted by the independent statistical committee (independent from the researcher (doctors, nurses, and pharmacists related to this study) and patients)) |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blinded study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/20 participants assigned to tacrolimus did not complete study. 1/20 participants assigned to control did not complete study |
| Selective reporting (reporting bias) | High risk | The reported study outcomes matched the outcomes reported in the published study protocol (published with the study). Key outcomes expected for this type of study (death (any cause), ESKD, change in GFR, infection) were not reported |
| Other bias | Low risk | The study appeared to be free from other sources of bias |
Kobayashi 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Prospectively divided into two groups according to the order of renal biopsy |
| Allocation concealment (selection bias) | High risk | Prospectively divided into two groups according to the order of renal biopsy. This is a quasi‐randomised study design |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 11/31 patients in the treatment group and 33/59 patients from the control group were excluded from the analyses |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), malignancy, infection) were not reported |
| Other bias | High risk | The participants received differential prescribing of anti‐thrombotic drugs during follow‐up. There was imbalance in sex and history of hypertension at baseline |
Koike 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Two doctors who did not know the histological scores randomly assigned the patients to either the steroid or control group. The doctors used two envelopes consisting of A (steroid group) or B (control group) and containing study instructions" |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Unable to blind due to the tapering of the prednisolone |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in study follow‐up |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), malignancy, infection) were not reported |
| Other bias | High risk | Co‐intervention with antihypertensive therapy was imbalanced between groups (administered to intervention group participants only). There was imbalance in kidney function between groups (the control group participants had a higher mean SCr) |
Koitabashi 1996.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Control group
Co‐intervention: not reported |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Lafayette 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Central randomisation |
| Allocation concealment (selection bias) | Unclear risk | Random assignment by prefilled envelopes |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. As the treatment assignment was unblinded and the outcomes included adverse events and reactions to the infusions, it is possible that outcome assessment was influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/17 drop‐out in control group; 2/17 drop‐out in rituximab group + 1 patient randomised but treatment not given |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | High risk | Different use of co‐interventions (acetaminophen and diphenhydramine plus dexamethasone with rituximab infusion). There was imbalance in age, race, SCr, eGFR, and proteinuria between treatment groups |
Lai 1986.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | High risk | The patients were divided into two groups according to the treatment regime |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data were available |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | High risk | Imbalance between groups at baseline (SCr higher in control group, CrCl lower in control group, urinary protein excretion lower in control group) |
Lai 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Computer generated. However, "All patients were given a number in the trial based on their order of entry" |
| Allocation concealment (selection bias) | High risk | All patients were given a number in the trial based on their order of entry, which determined their allocation to the treatment or placebo group |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Initially blinded however "We decided to reduce the dose of cyclosporin by 20% if plasma creatinine concentration exceeded 25% of the baseline value or the plasma cyclosporin trough concentration (concentration measured 12 hours after administration) reached 150 µg/l (evaluated by radioimmunoassay with a Sandoz kit). Similarly we decided to increase the dose of cyclosporin by 20% if the plasma cyclosporin trough concentration fell below 45 µg/l." |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Not reported. The patients were interviewed by telephone weekly about any side effects. Interpretation of subjective outcomes may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Outcomes were reported for 9 participants in cyclosporin group and 10 participants in placebo group. However, in a secondary publication, there were 11 participants allocated to each treatment group |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | High risk | Imbalance in baseline characteristics (age, plasma creatinine, urinary protein) |
Lee 2003.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group:
Control group:
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | Unclear risk | Insufficient information to permit judgement |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Liu 2010a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | No drug withdrawal or termination occurred in any of the patients in the two groups |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), infection, ESKD) were not reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Liu 2014.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions (both groups)
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Three patients were lost to follow‐up (one from the steroid group, two from the combination group), and two patients in the combination group discontinued cyclosporin A after three months of treatment due to severe pulmonary infections. After six months of treatment, one patient in the steroid group without a response to treatment was converted to cyclosporin A |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), infection, ESKD) were not reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Locatelli 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Two centralised, computer‐generated randomisation lists (1 for each stratum) |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 7/20 participants allocated to steroids and AZA did not complete follow up (6 due to side effects); 4/26 participants allocated to steroids only did not complete follow up (4 due to side effects) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes, except mortality, were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
Lou 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventionS
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | "Two patients were lost to follow up (one was from experimental group, one from control group), one withdrew from study because of side‐effects." |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), infection, ESKD) were not reported |
| Other bias | High risk | Imbalance in baseline characteristics suggesting problems with randomisation. Control treatment and balance of co‐interventions not reported adequately |
Lv 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated randomisation code |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 2/33 participants withdrawn from combination group and 1/30 withdrawn from control group |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes that would be expected in a study of this type, except mortality, were reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Maes 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Several dropouts and exclusions: treatment group (ESKD (2), adverse events (1), emigration (2)); control group (death (1), adverse events (1)) |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes expected for this type of study were reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Manno 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐intervention
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "An allocation assignment sequence was generated at the coordinating centre by random number tables; a list divided into blocks of 10 was adequately concealed to prevent attempts to subvert randomisation" |
| Allocation concealment (selection bias) | Low risk | Quote: "Central telephone randomisation for every eligible patient was performed by the Scientific Secretariat." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patients were analysed for the primary outcome |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes, except death (any cause), was reported |
| Other bias | Unclear risk | The study appeared to be free of other sources of bias. Non‐random block allocation may have led to prediction of treatment within centres |
Masutani 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Patients were allocated using a minimisation method in which stratifying factors were UPCR ≥ 2.0 g/g Cr), serum Cr levels (≥ 1.2 mg/dL for males and ≥ 1.0 mg/dL for females), and adding tonsillectomy |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 3/20 participants allocated to mizoribine and prednisolone did not complete follow‐up; 2/20 participants allocated to prednisolone did not complete follow up. However, "Although 5 patients did not complete study medication because of the side effects, retracted consent or deviation from the tapering schedule, no patient was lost to follow‐up, and we could perform ITT analyses." |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), infection, ESKD) were not reported |
| Other bias | High risk | Imbalance at baseline in age, BP, and eGFR |
Min 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 37/44 participants assigned to leflunomide completed the study follow‐up. 43/46 patients assigned to steroid therapy completed study follow‐up |
| Selective reporting (reporting bias) | High risk | All relevant outcomes, except death (any cause), were reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
NA IgAN 1995.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group 1
Control group 2
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote: "72 completed 2 years of trial drugs and 18 patients exited prematurely. Six patients dropped out of the trial after randomisation but before the start of study drugs." |
| Selective reporting (reporting bias) | High risk | The primary outcome was changed between the protocol (CrCl < 70% of baseline value) and the final study publication (CrCl <of 60% of baseline value) |
| Other bias | High risk | Interim analyses were planned but not clearly reported. The methods for interim analyses were different in the protocol and the final study publication. Imbalance at baseline for level of proteinuria between study groups |
NEFIGAN 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Patients were randomly allocated to treatment groups using a computer algorithm method of permuted blocks |
| Allocation concealment (selection bias) | Unclear risk | Treatment code envelopes were provided for each randomised patient. In case of emergency, the code envelope could be opened. Any unmasked patient had to be withdrawn from the trial |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation; however participants were unaware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 46/51 patients completed treatment and follow‐up in placebo group; 40/51 and 34/51 completed treatment and follow‐up in TRF‐budesonide 8 mg and 16 mg group, respectively. There was differential loss to follow up due to severe adverse events which were higher in the higher dose budesonide group |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | High risk | Imbalance between groups in eGFR, weight, and time from diagnosis at baseline |
Ni 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Preliminary reports, unsure of final number enrolled. 73/102 participants completed 12 months, 28/102 completed 24 months |
| Selective reporting (reporting bias) | High risk | Data for outcomes such as ESKD, death (any cause), and malignancy were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Nuzzi 2009.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Data for outcomes such as ESKD, death (any cause), and malignancy were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Pozzi 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centralised table of random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | All patients in the steroid group completed the 6 months of therapy; high dropout in both groups after this period |
| Selective reporting (reporting bias) | Low risk | Data for outcomes such as death (any cause), and infection were not reported. All other outcomes that would be expected for this type of study were reported |
| Other bias | High risk | Four patients in the control group received steroids as rescue therapy |
Segarra 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐intervention: not reported |
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study were not reported |
| Other bias | High risk | Insufficient information to permit judgement |
Shen 2013.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Shi 2012a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | There was no report of blinding. As the treatments were physically different, it was likely that participants and/or investigators were aware of treatment allocation |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Data for outcomes such as ESKD, death (any cause), malignancy, and infections were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Shima 2018.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Sealed envelopes. Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Non‐double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. However, reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1 patient in the treatment group withdrew consent after allocation. all other patients completed the study |
| Selective reporting (reporting bias) | High risk | Data for outcomes such as ESKD, death (any cause), malignancy, and infections were not reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Shoji 2000.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated random numbers |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 2/10 patients from the control group withdrew ‐ refused repeat biopsy |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study were not reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Stangou 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated sequence |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), ESKD) were not reported |
| Other bias | High risk | Imbalance at baseline in eGFR and time since diagnosis |
STOP‐IgAN 2008.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes | The study was funded through German Federal Ministry of Education and Research grant GFVT01044604 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Randomisation codes that were used to assign patients in a 1:1 ratio were generated by means of covariate adaptive randomisation with respect to factors that had the potential to modify the treatment effect (i.e., eGFR and proteinuria). Telephone randomisation by the study secretary. After the investigator establishes the eligibility of the patient to participant in the study, the study centre sends a fax to the Trial Office. The Trial Office assigned a treatment to the patient after being sent the following information: initials, gender, age, CrCl, degree of proteinuria |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 4/82 assigned to immunosuppression were lost to follow up. 4/80 assigned to supportive care were lost to follow up |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Takeda 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | No numeric data were available |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Tang 2005.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data were reported |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | The study appears to be free of other sources of bias |
TESTING 2017.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was performed using a minimization algorithm based on the stratification variables; the algorithm was centrally generated and used by all centres to minimize any imbalances in key variables |
| Allocation concealment (selection bias) | Low risk | Randomly assigned 1:1 via a password‐protected encrypted web site interface |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation; however participants were unaware of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | There were only 2/136 lost to follow up in methylprednisolone group. Imbalance in discontinuation between groups |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | High risk | Trial terminated early because of excess serious adverse events |
Walker 1990a.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All patient data were reported |
| Selective reporting (reporting bias) | Low risk | Adverse events and death (any cause) were not reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Welch 1992.
| Methods |
|
|
| Participants |
|
|
| Interventions | Two, 3‐month courses of therapy separated by a 3‐month rest period Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The first course for each patient was assigned by a random‐numbers table." |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Quote: "The drugs were dispensed by the Children's Hospital Medical Center pharmacy with a coded label, so that neither patients nor investigators were aware of the identity of the medication." |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | All participants were included in analyses |
| Selective reporting (reporting bias) | High risk | Relevant numeric data were not available. Patient‐centred outcomes of relevance were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Woo 1987.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Outcomes relevant to study design were not reported such as death (any cause) and infection/malignancy |
| Other bias | High risk | Imbalance in duration of follow up and proteinuria between treatment groups |
Wu 2016.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Treatment group 4
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer‐generated randomisation list was produced by a staff member at the Peking University Clinical Research Institute (Beijing, China) who was not otherwise involved in the study |
| Allocation concealment (selection bias) | Unclear risk | Detailed blind coding was recorded and covertly preserved in the coordinating centre. Each study centre was randomly stratified according to the enrolment order |
| Blinding of participants and personnel (performance bias) All outcomes | Low risk | Double‐blind study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | In total, 25/400 patients were lost to follow‐up. However the proportion was low, the proportion of loss to follow‐up was different for each treatment group |
| Selective reporting (reporting bias) | Low risk | All relevant outcomes were reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Xie 2011.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 6/35 participants in mizoribine group did not complete study. 4/34 participants in combination group did not complete study. 5/30 participants in the losartan group did not complete study |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause), ESKD) were not reported |
| Other bias | Low risk | The study appeared to be free of other source of bias |
Yamauchi 2001.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | There was no pre‐specified protocol identified for this study. The study did not report extractable data for the key outcomes that would be expected for a study of this type (e.g., death (any cause), GFR loss, infection, malignancy) |
| Other bias | Unclear risk | Insufficient information to permit judgement |
Yoshikawa 1999.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement. |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Sealed envelope technique in blocks of four." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Blinding of outcome assessment not specifically reported. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 4/38 participants allocated to control group did not complete trial. 0/40 participants allocated to immunosuppression completed trial. Imbalance in discontinuation between groups |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause) and ESKD) were not reported |
| Other bias | High risk | Imbalance in urine protein excretion at baseline |
Yoshikawa 2006.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | "Sealed envelope technique in blocks of four." |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Two independent investigators who were blinded to the treatment status reviewed the second biopsies. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 1/40 participants did not complete study from each treatment group |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause) and ESKD) were not reported |
| Other bias | Low risk | The study appeared to be free of other sources of bias |
Zhang 2004.
| Methods |
|
|
| Participants |
|
|
| Interventions | Treatment group
Control group
Co‐interventions
|
|
| Outcomes |
|
|
| Notes |
|
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Allocation concealment (selection bias) | Unclear risk | Insufficient information to permit judgement |
| Blinding of participants and personnel (performance bias) All outcomes | High risk | Open‐label study |
| Blinding of outcome assessment (detection bias) All outcomes | High risk | Two independent investigators who were blinded to the treatment status reviewed the second biopsies. Key outcomes were objective laboratory measures and were unlikely to be affected by any knowledge of treatment allocation. Reporting of adverse events may have been influenced by knowledge of treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Insufficient information to permit judgement |
| Selective reporting (reporting bias) | High risk | Key outcomes expected for this type of study (death (any cause) and ESKD) were not reported |
| Other bias | Unclear risk | Insufficient information to permit judgement |
ACEi ‐ angiotensin‐converting enzyme inhibitor/s; ALT ‐ alanine aminotransferase; ARB ‐ angiotensin receptor blockers; ASP ‐ aspartate aminotransferase; AZA ‐ azathioprine; BP ‐ blood pressure; BUN ‐ blood urea nitrogen; CKD ‐ chronic kidney disease; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin A; DBP ‐ diastolic blood pressure; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; (e)GFR ‐ (estimated) glomerular filtration rate; GN ‐ glomerulonephritis; HCT ‐ haematocrit; HIV ‐ human immunodeficiency virus; HSP ‐ Henoch‐Schönlein Purpura; IgAN ‐ IgA nephropathy; IQR ‐ interquartile range; IV ‐ intravenous; KRT ‐ kidney replacement therapy; LDL ‐ low density lipoprotein; M/F ‐ male/female; MAP ‐ mean arterial pressure; MMF ‐ mycophenolate mofetil; QoL ‐ quality of life; RAS ‐ renin‐angiotensin system; RCT ‐ randomised controlled trial; SBP ‐ systolic blood pressure; SCr ‐ serum creatinine; SD ‐ standard deviation; SEM ‐ standard error of the mean; SLE ‐ systemic lupus erythematosus; UACR ‐ urinary albumin:creatinine ratio; UPCR ‐ urinary protein:creatinine ratio
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Chen 2009b | Wrong intervention: not immunosuppressive agent intervention; the study evaluated Tripterygium wilfordii Hook F. This treatment was adjudicated as not immunosuppression |
| Czock 2007 | Wrong intervention: not immunosuppressive agent intervention; the study evaluated the pharmacokinetics of two different mycophenolic acid formulations. As the study did not compare two different immunosuppression agents, this study was adjudicated as not fulfilling the eligibility criteria on the basis of intervention |
| Dal Canton 2005 | This study was abandoned without completing participant recruitment. |
| GloMY 2010 | This study was adjudicated as not completing recruitment target. The study authors let us know that the trial was closed to recruitment on 21 August 2012 with 3 patients with IgAN randomised. The trial was intended as a pilot for feasibility for a larger trial. |
| Imai 2006 | Wrong population: not all participants with biopsy‐proven IgAN; the study included participants with a range of crescentics glomerulopathies. Data for those participants with IgAN were not available separately. |
| Shen 2009 | Wrong intervention: not immunosuppressive agent intervention; the study compared combined regime of Tripterygium glycosides and benazepril. These treatments were adjudicated as not immunosuppression. |
| Sulimani 2001 | Wrong population: not all patients had IgAN; data for those participants with IgAN were not available separately. |
| Yonemura 2000b | Wrong population: not all participants had IgAN; the study included participants with minimal change disease. |
IgAN ‐ IgA nephropathy
Characteristics of studies awaiting assessment [ordered by study ID]
NCT00301600.
| Methods | Single centre parallel RCT |
| Participants | 40 patients with crescentics IgAN |
| Interventions | Pulse IV CPA or oral MMF |
| Outcomes | Efficacy, safety, tolerability and relapse of MMF |
| Notes | Study completed in 2006. Written to investigators to request update/data. As study is completed >10 years previously, trial data are unlikely to be available or obtained. |
NCT02160132.
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Notes | Study completed on December 2016 Emailed investigators on 21.5.2018 to request update on trial status, but not answer was provided Clinicaltrials.gov identifier: NCT02160132 No study results available |
NCT02571842.
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Notes | Study completed on December 2016 Email investigators to request update on trial status, but not answer was provided Clinicaltrials.gov identifier: NCT02571842 No study results available |
ACEi ‐ angiotensin‐converting enzyme inhibitor; ARB ‐ angiotensin receptor blocker; BP ‐ blood pressure; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin A; (e)GFR ‐ (estimated) glomerular filtration rate; GN ‐ glomerulonephritis; HIV ‐ human immunodeficiency virus; HSP ‐ Henoch‐Schönlein Purpura; IgAN ‐ IgA nephropathy; IV ‐ intravenous; MMF ‐ mycophenolate mofetil; RCT ‐ randomised controlled study; SLE ‐ systemic lupus erythematosus; UACR ‐ urine albumin creatinine ratio
Characteristics of ongoing studies [ordered by study ID]
AIGA 2016.
| Trial name or title | Efficacy and safety of a combination of mycophenolate mofetil and corticosteroid in advanced IgA nephropathy (AIGA) |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | June 2016 |
| Contact information | Eunju Jung oakly74@nate.com; Jonghyuk Lee leejongh@ckdpharm.com |
| Notes | Study completion date: October 2018 No study results available |
ARTEMIS‐IgAN 2018.
| Trial name or title | Study of the safety and efficacy of OMS721 in patients with immunoglobulin A (IgA) nephropathy |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | February 2018 |
| Contact information | Laura Haas (206) 676‐0886 lhaas@omeros.com Fay Wang (206) 676‐0863 fwang@omeros.com |
| Notes | Estimated study completion date: April 2023 No study results available |
ChiCTR1800014442.
| Trial name or title | Prospective study of the efficacy and safety of improved Italy scheme therapy for IgA nephropathy |
| Methods | Not reported |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | Not reported |
| Contact information | Li Y: Telephon number and email were not reported |
| Notes | Estimated study completion date: not reported No study results available |
MAIN 2013.
| Trial name or title | The Effects of mycophenolate mofetil (MMF) on renal outcomes in advanced immunoglobulin A (IgA) nephropathy patients (MAIN) |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | June 2013 |
| Contact information | Fan Fan Hou 0086‐20‐61641591 ffhouguangzhou@163.com |
| Notes | Estimated study completion date: June 2018 No study results available |
NCT00657059.
| Trial name or title | Mycophenolate mofetil (MMF) in patients With IgA nephropathy |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 3
Co‐interventions
|
| Outcomes |
|
| Starting date | September 2007 |
| Contact information | Xueqing Yu 8620‐87766335 yuxq@mail.sysu.edu.cn Qiongqiong Yan 8620‐87755766 ext 8843 qqyzzm@yahoo.com.cn |
| Notes | Estimated study completion date: April 2019 No study results available |
NCT02808429.
| Trial name or title | Efficacy and safety of atacicept in IgA nephropathy |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Treatment group 2
Control group
|
| Outcomes |
|
| Starting date | January 2017 |
| Contact information | Study Director: EMD Serono Research & Development Institute, Inc., a subsidiary of Merck KGaA, Darmstadt, Germany US Medical Information 888‐275‐7376 service@emdgroup.com Merck KGaA Communication Center 49 6151 72 5200 service@merckgroup.com |
| Notes | Estimated completion date: July 2020 No study results available |
NCT03468972.
| Trial name or title | Effect of immunosuppression in IgA nephropathy |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | Estimated March 2019 |
| Contact information | Seung Hyeok Han 82‐2‐2228‐1984 hansh@yuks.ac |
| Notes | Estimated study completion date: May 2023 No study results available |
NEFIGARD 2018.
| Trial name or title | Efficacy and safety of nefecon in patients with primary IgA (immunoglobulin A) nephropathy (NEFIGARD) |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | August 2018 |
| Contact information | Medpace Research, Inc +1 800 730 5779 info@medpace.com |
| Notes | Estimated study completion date: December 2024 No study results available |
PIRAT 2015.
| Trial name or title | Prevention in recipients with Primary IgA Nephropathy of Recurrence After Kidney Transplantation: ATG‐F versus basiliximab as induction immunosuppressive treatment (PIRAT) |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | January 2011 |
| Contact information | Principal Investigator: Francois Berthoux |
| Notes | Estimated study completion date: December 2019 No study results available Sponsor: Centre Hospitalier Universitaire de Saint Etienne |
SIGN 2014.
| Trial name or title | Safety and efficacy study of fostamatinib to treat immunoglobulin A (IgA) nephropathy |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Control group
Co‐interventions
|
| Outcomes |
|
| Starting date | October 2014 |
| Contact information | Study director: Rigel Pharmaceuticals Inc (no other specific information available) |
| Notes | Estimated study completion date: November 2018 No study results available Responsible party: Rigel Pharmaceuticals |
TIGER 2017.
| Trial name or title | Treatment of IgA Nephropathy According to Renal Lesions (TIGER) |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group
Control group
|
| Outcomes |
|
| Starting date | February 2018 |
| Contact information | Dominique Joly +33 1 44 49 54 12 dominique.joly@nck.aphp.fr Sandra Colas 01 71 19 64 32 sandra.colas@aphp.fr |
| Notes | Estimated study completion: June 2019 No study results available |
TOPplus‐IgAN 2013.
| Trial name or title | Treatment of Prednisone Plus Cyclophosphamide in Patients With Advanced‐stage IgA Nephropathy (TOPplus‐IgAN) |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
Co‐interventions
|
| Outcomes |
|
| Starting date | December 2012 |
| Contact information | Principal Investigator: Wei Shi. Guangdong General Hospital |
| Notes | Study completion: December 2019 No study results available |
UMIN000032031.
| Trial name or title | The steroid internal use method for patients with IgA nephropathy |
| Methods | Parallel RCT |
| Participants |
|
| Interventions | Treatment group 1
Treatment group 2
|
| Outcomes |
|
| Starting date | April 2018 |
| Contact information | Hitoshi Suzuki 03‐5802‐1065 shitoshi@juntendo.ac.jp |
| Notes | Estimated study completion date: not yet available No study results available |
ACEi ‐ angiotensin‐converting enzyme inhibitor; AKI ‐ acute kidney injury; ARB ‐ angiotensin receptor blocker; AZA ‐ azathioprine; BMI ‐ body mass index; BP ‐ blood pressure; CKD‐EPI ‐ Chronic Kidney Disease Epidemiology Collaboration; CPA ‐ cyclophosphamide; CrCl ‐ creatinine clearance; CSA ‐ cyclosporin; DBP ‐ diastolic blood pressure; DM ‐ diabetes mellitus; ESKD ‐ end‐stage kidney disease; (e)GFR ‐ (estimated) glomerular filtration rate; GN ‐ glomerulonephritis; HCT ‐ hematocrit; HIV ‐ human immunodeficiency virus; HSP ‐ Henoch‐Schönlein purpura; IgAN ‐ IgA nephropathy; KRT ‐ kidney replacement therapy; M/F ‐ male/female; MDRD ‐ Modified Diet in Renal Disease; MMF ‐ mycophenolate mofetil; PRA ‐ panel reactive antibody; RAS ‐ renin‐angiotensin system; RCT ‐ randomised controlled trial; SBP ‐ systemic blood pressure; SC ‐ subcutaneous; SCr ‐ serum creatinine; SD ‐ standard deviation; SLE ‐ systemic lupus erythematosus; TB ‐ tuberculosis; WBC ‐ white blood cell count; UPCR ‐ urinary protein:creatinine ratio
Differences between protocol and review
2015 review update: Risk of bias assessment has replaced the quality checklist.
2020 review update: We have added additional outcomes including annual change in eGFR and malignancy.
Contributions of authors
2003 review
This review is the product of an equal contribution from Joshua Samuels and Giovanni FM Strippoli, who conceived it, developed the protocol, designed and conducted the review, performed the data extraction, data analysis and wrote the final review
Jonathan C Craig was involved in the conduct, data‐analysis and writing of the review
Donald Molony reviewed the final draft
Francesco P Schena reviewed the final draft
2015 review update
This review is the product of an equal contribution from Joshua Samuels and Giovanni FM Strippoli, who conceived it, developed the protocol, designed and conducted the review, performed the data extraction, data analysis and wrote the final review
Mariacristina Vecchio data extraction, data analysis and wrote the final review
Bibiana Bonerba data extraction, data analysis and wrote the final review
Marinella Ruospo data extraction, data analysis and wrote the final review
Jonathan C Craig reviewed and commented on the final draft
Donald Molony reviewed and commented on the final draft.
Francesco P Schena reviewed and commented on the final draft
2020 review update
The update of the review was conducted by Patrizia Natale and Marinella Ruospo, who performed the data extraction, data analysis and wrote the final review
Suetonia C Palmer provided intellectual input throughout the review update process
Giovanni FM Strippoli reviewed and commented on the final draft
Valeria Saglimbene reviewed and commented on the final draft
Mariacristina Vecchio reviewed and commented on the final draft
Joshua A Samuels reviewed and commented on the final draft
Jonathan C Craig reviewed and commented on the final draft
Donald Molony reviewed and commented on the final draft.
Francesco P Schena reviewed and commented on the final draft
Sources of support
Internal sources
Cochrane Renal Group, Australia.
External sources
No sources of support supplied
Declarations of interest
No author has a vested interest in any of the products or procedures included in the analysis.
Edited (no change to conclusions)
References
References to studies included in this review
2nd NA IgAN 2004 {published data only}
- Hogg R, Bay C. Reduction of proteinuria observed in response to high dose ACE inhibition and omega‐3 fatty acids in pts with IgA nephropathy. Report from the second North American IgA nephropathy trial [abstract no: SA‐FC059]. Journal of the American Society of Nephrology 2007;18(Abstracts):48A. [CENTRAL: CN‐00740507] [Google Scholar]
- Hogg RJ. Preliminary report from the second North American IgA nephropathy (IgAN) trial [abstract no: F‐PO1098]. Journal of the American Society of Nephrology 2006;17(Abstracts):567A. [CENTRAL: CN‐00615827] [Google Scholar]
- Hogg RJ, Bay C. Dose‐dependency of the effect of omega‐3 fatty acids (O3FA) on proteinuria in patients with IgA nephropathy: report from the 2nd North American IgA nephropathy trial [abstract no: F‐PO860]. Journal of the American Society of Nephrology 2005;16:523A‐4A. [CENTRAL: CN‐00615826] [Google Scholar]
- Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, et al. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. American Journal of Kidney Diseases 2015;66(5):783‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogg RJ, Fitzgibbons L, Atkins C, Nardelli N, Bay RC, North American IgA Nephropathy Study Group. Efficacy of omega‐3 fatty acids in children and adults with IgA nephropathy is dosage‐ and size‐dependent. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(6):1167‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogg RJ, SouthWest Pediatric Nephrology Study Group. A randomized controlled trial of mycophenolate mofetil in patients with IgA nephropathy: study protocol. Personal communication 2003. [DOI] [PMC free article] [PubMed]
- Hogg RJ, Wyatt RJ, Scientific Planning Committee of the North American IgA Nephropathy Study. A randomized controlled trial of mycophenolate mofetil in patients with IgA nephropathy [ISRCTN62574616]. BMC Nephrology 2004;5:3. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Ballardie 2002 {published data only}
- Ballardie FW, Roberts IS. A controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy [abstract]. Nephrology Dialysis Transplantation 1996;11(8):1684. [CENTRAL: CN‐00261200] [DOI] [PubMed] [Google Scholar]
- Ballardie FW, Roberts IS. Controlled prospective trial of prednisolone and cytotoxics in progressive IgA nephropathy. Journal of the American Society of Nephrology 2002;13(1):142‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
BRIGHT‐SC 2016 {published data only}
- Barratt J, Hislop C, Pennington J, Gangal M, Martin R, Liew A. Effects of blisibimod, a selective inhibitor of b‐cell activating factor, in patients with IgA nephropathy [abstract no: FR‐PO1128]. Journal of the American Society of Nephrology 2016;27(Abstracts):4B. [CENTRAL: CN‐01658029] [Google Scholar]
Cao 2008 {published data only}
- Cao L, Ni Z, Qian J, Fang W, Lin A, Zhang W, et al. Leflunomide plus low dose prednisone reduced urinary VCAM‐1 level in progressive IgA nephropathy [abstract no: F‐PO1851]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):527A. [CENTRAL: CN‐00740501] [Google Scholar]
- Zhou M, Ni Z, Cao L, Qian J, Fang W, Shan M, et al. Leflunomide plus low dose prednisone reduced serum interleukin‐18 level in progressive IgA nephropathy [abstract no: TH‐PO515]. Journal of the American Society of Nephrology 2009;20(Abstract Suppl):232A. [Google Scholar]
CAST‐IgA 2015 {published data only}
- Kohagura K, Arima H, Miyasato H, Chang TH, Kobori H, Iseki K, et al. Effects of candesartan on clinical remission in IgA nephropathy treated with steroid pulse therapy and tonsilectomy (CAST IgA Study) ‐ a randomized control study [abstract no: TH‐PO661]. Journal of the American Society of Nephrology 2015;26(Abstracts):240A. [CENTRAL: CN‐01658023] [Google Scholar]
Chen 2002 {published data only}
- Chen X, Cai G, Zhang Y, Qiu Q, Cheng Q. Control study of effects of mycophenolate mofetil on IgA nephropathy [abstract no: A0311]. Journal of the American Society of Nephrology 2000;11(Sept):57A. [CENTRAL: CN‐00550413] [Google Scholar]
- Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, et al. A randomized control study of mycophenolate mofetil treatment in severe IgA nephropathy [abstract no: F‐FC065]. Journal of the American Society of Nephrology 2002;13(September, Program & Abstracts):14A. [CENTRAL: CN‐00444783] [Google Scholar]
- Chen X, Chen P, Cai G, Wu J, Cui Y, Zhang Y, et al. A randomized control trial of mycophenolate mofeil treatment in severe IgA nephropathy. Chung‐Hua i Hsueh Tsa Chih [Chinese Medical Journal] 2002;82(12):796‐801. [MEDLINE: ] [PubMed] [Google Scholar]
- Chen X, Wu J, Zhang Y, Liu S, Tang L. 72 weeks follow‐up study of effects of mycophenolate mofetil on IgA nephropathy [abstract no: A0353]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):66‐7A. [CENTRAL: CN‐00444784] [Google Scholar]
Cheung 2018 {published data only}
- Cheung CK, Szklarzewicz J, Rawal R, Barratt J. A phase II study evaluating the safety and efficacy of belimumab in patients with IgA nephropathy [abstract]. Kidney Diseases 2018;4(3):142‐3. [EMBASE: 624069857] [Google Scholar]
Cruzado 2011 {published data only}
- Cruzado JM, Poveda R, Ibernon M, Diaz M, Fulladosa X, Carrera M, et al. Low‐dose sirolimus combined with angiotensin‐converting enzyme inhibitor and statin stabilizes renal function and reduces glomerular proliferation in poor prognosis IgA nephropathy. Nephrology Dialysis Transplantation 2011;26(11):3596‐602. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Curzado JM, Poveda R, Fulladosa X, Torras J, Ibernon M, Diaz M, et al. Low dose of sirolimus for the treatment of poor‐prognosis IgA nephropathy: a prospective controlled trial [abstract no: F‐PO1964]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):555A. [CENTRAL: CN‐00757192] [Google Scholar]
Frisch 2005 {published data only}
- Frisch G, Lin J, Rosenstock J, Markowitz G, D'Agati V, Radhakrishnan J, et al. Mycophenolate mofetil (MMF) vs placebo in patients with moderately advanced IgA nephropathy: a double‐blind randomized controlled trial. Nephrology Dialysis Transplantation 2005;20(10):2139‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Frisch G, Lin J, Rosenstock J, Markowitz G, D'Agati V, Radhakrishnan J, et al. Mycophenolate mofetil vs placebo in patients at high risk for progressive IgA nephropathy: a double blind RCT [abstract no: SU‐PO987]. Journal of the American Society of Nephrology 2003;14(Nov):753A. [CENTRAL: CN‐00644287] [Google Scholar]
Harmankaya 2002 {published data only}
- Harmankaya O, Ozturk Y, Basturk T, Obek A, Kilicarslan I. Efficacy of immunosuppressive therapy in IgA nephropathy presenting with isolated hematuria. International Urology & Nephrology 2002;33(1):167‐71. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hirai 2017 {published data only}
- Hirai K, Ookawara S, Kitano T, Miyazawa H, Ito K, Ueda Y, et al. Efficacy and safety of adding mizoribine to standard treatment in patients with immunoglobulin A nephropathy: a randomized controlled trial. Kidney Research & Clinical Practice 2017;36(2):159‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Horita 2007 {published data only}
- Horita Y, Tadokoro M, Taura K, Ashida R, Hiu M, Taguchi T, et al. Prednisolone co‐administered with losartan confers renoprotection in patients with IgA nephropathy. Renal Failure 2007;29(4):441‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horita Y, Tadokoro M, Taura K, Taguchi T, Kohno S. Effects of co‐administration of prednisolone plus losartan in moderate proteinuric IgA nephropathy [abstract no: MP099]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv332. [CENTRAL: CN‐00615873] [Google Scholar]
Hou 2017 {published data only}
- Hou JH, Le WB, Chen N, Wang WM, Liu ZS, Liu D, et al. Mycophenolate mofetil combined with prednisone versus full‐dose prednisone in IgA nephropathy with active proliferative lesions: a randomized controlled trial. American Journal of Kidney Diseases 2017;69(6):788‐95. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Julian 1993 {published data only}
- Julian BA, Barker C. Alternate‐day prednisone therapy in IgA nephropathy. Preliminary analysis of a prospective, randomized, controlled trial. Contributions to Nephrology 1993;104:198‐206. [MEDLINE: ] [PubMed] [Google Scholar]
- Julian BA, Barker CV, Woodford SY. Alternate‐day prednisone treatment of patients with IgA nephropathy [abstract no: 99P]. Journal of the American Society of Nephrology 1993;4(Program & Abstracts):681. [CENTRAL: CN‐00484554] [Google Scholar]
Kanno 2003 {published data only}
- Kanno Y, Witt M, Okada H, Nemoto H, Sugahara S, Nakamoto H, et al. A comparison of corticosteroid and warfarin therapy in IgA nephropathy with crescent formation: preliminary trial. Clinical & Experimental Nephrology 2003;7(1):48‐51. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Katafuchi 2003 {published data only}
- Katafuchi R, Ikeda K, Yanase T, Yanagida T, Yoshida T, Hirakata H, et al. A randomized prospective control study of low dose prednisolone therapy for IgA nephropathy: its usefulness and limitations [abstract]. Nephrology 1999;5(Suppl):A18. [CENTRAL: CN‐01657782] [Google Scholar]
- Katafuchi R, Kiyoshi I, Mizumasa T, Tanaka H, Ando T, Yanase T, et al. Controlled, prospective trial of steroid treatment in IgA nephropathy: a limitation of low‐dose prednisolone therapy. American Journal of Kidney Diseases 2003;41(5):972‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Katafuchi R, Yoshida T, Yanase T, Ikeda K, Fujimi S. Low dose steroid therapy in IgA nephropathy: a randomized prospective control study [abstract no: P1126]. Nephrology 1997;3(Suppl 1):S355. [CENTRAL: CN‐00461044] [Google Scholar]
Kawamura 2014 {published data only}
- Katafuchi R, Kawamura T, Hashiguchi A, Hisano S, Shimizu A, Miyazaki Y, et al. Pathological sub‐analysis of randomized controlled trial of tonsillectomy combined with steroid pulse therapy vs steroid pulse monotherapy in IGA nephropathy [abstract no: PS1‐096]. Nephrology 2014;19(Suppl 2):108. [EMBASE: 71617003] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katafuchi R, Kawamura T, Joh K, Hashiguchi A, Hisano S, Shimizu A, et al. Pathological sub‐analysis of a multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy versus steroid pulse monotherapy in patients with immunoglobulin A nephropathy. Clinical & Experimental Nephrology 2016;20(2):244‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with IgA nephropathy [abstract no: SY8‐05]. Nephrology 2014;19(Suppl 2):18‐9. [EMBASE: 71616742] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Yoshimura M, Miyazaki Y, Okamoto H, Kimura K, Hirano K, et al. A multicenter randomized controlled trial of tonsillectomy combined with steroid pulse therapy in patients with immunoglobulin A nephropathy. Nephrology Dialysis Transplantation 2014;29(8):1546‐53. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimura M, Imasawa T, Nakayama M, Wada A, Katahuti R, Kawamura T. Tonsillectomy and steroid pulse therapy in IgA nephropathy: a randomized, controlled trial versus a multicenter prospective controlled study [abstract no: SU329]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐01912327]
Kim 2013b {published data only}
- Kim YC, Chin HJ, Koo HS, Kim S. Tacrolimus decreases albuminuria in patients with IgA nephropathy and normal blood pressure: a double‐blind randomized controlled trial of efficacy of tacrolimus on IgA nephropathy. PLoS ONE [Electronic Resource] 2013;8(8):e71545. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim YC, Koo HS, Lee H, Chin HJ, Kim S. Double‐blind, randomized placebo‐controlled clinical trial for the effiicacy of tacrolimus in the patients with albuminuric, normotensive IgA nephropathy [abstract no: TH‐PO410]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):192A. [Google Scholar]
- Yu MY, Kim YC, Koo HS, Chin HJ. Short‐term anti‐proteinuric effect of tacrolimus is not related to preservation of the glomerular filtration rate in IgA nephropathy: a 5‐year follow‐up study.[Erratum in: PLoS One. 2018 Jan 29;13(1):e0192266; PMID: 29377948]. PLoS ONE [Electronic Resource] 2017;12(11):e0188375. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Kobayashi 1996 {published data only}
- Kobayashi Y, Hiki Y, Kokubo T, Horii A, Tateno S. Steroid therapy during the early stage of progressive IgA nephropathy. A 10‐year follow‐up study. Nephron 1996;72(2):237‐42. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koike 2008 {published data only}
- Koike M, Takei T, Uchida K, Honda K, Moriyama T, Horita S, et al. Clinical assessment of low‐dose steroid therapy for patients with IgA nephropathy: a prospective study in a single center. Clinical & Experimental Nephrology 2008;12(4):250‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Koitabashi 1996 {published data only}
- Koitabashi Y, Yoshikawa N, Ito H. Randomized, prospective, multcenter trial for treatment of IgA nephropathy in children [abstract no: S‐5]. Pediatric Nephrology 1996;10(1):C4. [CENTRAL: CN‐01657792] [Google Scholar]
Lafayette 2017 {published data only}
- Lafayette RA, Canetta PA, Rovin BH, Appel GB, Hogan MC, Erickson SB, et al. A randomized trial of rituximab in advanced IgA nephropathy [abstract no: SA‐PO1098]. Journal of the American Society of Nephrology 2015;26(Abstracts):B5. [CENTRAL: CN‐01658018] [Google Scholar]
- Lafayette RA, Canetta PA, Rovin BH, Appel GB, Novak J, Nath KA, et al. A randomized, controlled trial of rituximab in IgA nephropathy with proteinuria and renal dysfunction. Journal of the American Society of Nephrology 2017;28(4):1306‐13. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lai 1986 {published data only}
- Lai KN, Lai FM, Ho CP, Chan KW. Corticosteroid therapy in IgA nephropathy with nephrotic syndrome: a long‐term controlled trial. Clinical Nephrology 1986;26(4):174‐80. [MEDLINE: ] [PubMed] [Google Scholar]
Lai 1987 {published data only}
- Lai KN, Lai FM. Short‐term controlled trial of cyclosporin A therapy in IgA nephropathy [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:73. [CENTRAL: CN‐00626035]
- Lai KN, Lai FM, Chui SH. Effect of cyclosporin A on cellular immunity in IgA nephropathy [abstract]. 10th International Congress of Nephrology; 1987 Jul 26‐31; London, UK. 1987:388. [CENTRAL: CN‐00626034]
- Lai KN, Lai FM, Chui SH, Leung KN, Lam CW. Effect of ciclosporin on lymphocyte subpopulations and immunoglobulin production in IgA nephropathy. Nephron 1989;52(4):307‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lai KN, Lai FM, Li PK, Vallance‐Owen J. Cyclosporin treatment of IgA nephropathy: a short term controlled trial. British Medical Journal Clinical Research Ed 1987;295(6607):1165‐8. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai KN, Lai FM, Vallance‐Owen J. Short‐term controlled trial of cyclosporin A in therapy in IgA nephropathy [abstract no: 12]. 23rd Annual Meeting Australasian Society of Nephrology; 1987 Apr 1‐3; Adelaide, SA. 1987:13.
- Lai KN, Lam CW, Cheng IK, Tam JS, Lai FM. Effect of cyclosporine A on circulating immune complexes in IgA nephropathy. International Urology & Nephrology 1991;23(3):265‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lai KN, Mac‐Moune LF, Vallance‐Owen J. A short‐term controlled trial of cyclosporine A in IgA nephropathy. Transplantation Proceedings 1988;20(3 Suppl 4):297‐303. [MEDLINE: ] [PubMed] [Google Scholar]
Lee 2003 {published data only}
- Kim YK, Lee SH, Lee TW, Kim MJ, Yang MH, Ihm CG. Renoprotective effect of the combined use of steroid and angiotensin II receptor blocker in IgA Nephropathy. Korean Journal of Nephrology 2005;24(1):71‐9. [CENTRAL: CN‐01045483] [Google Scholar]
- Lee SH, Shim JJ, Lee SH, Lee TW, Kim MJ, Yang MH. Effect of combined treatment of steroid and angiotensin II receptor blocker (ARB) in proteinuric IgA nephropathy. Korean Journal of Nephrology 2003;22(5):539‐45. [CENTRAL: CN‐01045911] [Google Scholar]
Liu 2010a {published data only}
- Liu XW, Li DM, Xu GS, Sun SR. Comparison of the therapeutic effects of leflunomide and mycophenolate mofetil in the treatment of immunoglobulin A nephropathy manifesting with nephrotic syndrome. International Journal of Clinical Pharmacology & Therapeutics 2010;48(8):509‐13. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Liu 2014 {published data only}
- Liu H, Ding X, Fang Y, Xu X, Zhang X, Zhong Y. Medium dose of cyclosporine combined with methylprednisolone in IgA nephropathy: prospective randomized controlled trial [abstract no: SA‐PO2300]. Journal of the American Society of Nephrology 2010;21(Abstract Suppl):636A. [Google Scholar]
- Liu H, Xu X, Fang Y, Ji J, Zhang X, Yuan M, et al. Comparison of glucocorticoids alone and combined with cyclosporine A in patients with IgA nephropathy: a prospective randomized controlled trial. Internal Medicine 2014;53(7):675‐81. [EMBASE: 2014229755] [DOI] [PubMed] [Google Scholar]
Locatelli 1999 {published data only}
- Vecchio L, Pozzi C, Andrulli S, Pani A, Scaini P, Fogazzi G, et al. Corticosteroids and azathioprine vs corticosteroids alone in IgA nephropathy: a randomised, controlled trial [abstract no: SA770]. World Congress of Nephrology; 2009 May 22‐26; Milan, Italy. 2009. [CENTRAL: CN‐01912332]
- Locatelli F, Pozzi C, Vecchio L, Andrulli S, Pani A, Fogazzi G, et al. Combined treatment with steroids and azathioprine in IgA nephropathy: design of a prospective randomised multicentre trial. Journal of Nephrology 1999;12(5):308‐11. [MEDLINE: ] [PubMed] [Google Scholar]
- Pozzi C, Andrulli S, Pani A, Scaini P, Vecchio L, Fogazzi G, et al. Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. Journal of the American Society of Nephrology 2010;21(10):1783‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pozzi C, Andrulli S, Pani A, Scaini P, Roccatello D, Fogazzi G, et al. IgA nephropathy with severe chronic renal failure: a randomized controlled trial of corticosteroids and azathioprine. Journal of Nephrology 2013;26(1):86‐93. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lou 2006 {published data only}
- Lou T, Wang C, Chen Z, Shi C, Tang H, Liu X, et al. Randomised controlled trial of leflunomide in the treatment of immunoglobulin A nephropathy. Nephrology 2006;11(2):113‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Lv 2009 {published data only}
- Lv J, Zhang H, Chen Y, Li G, Jiang L, Singh AK, et al. Combination therapy of prednisone and ACE inhibitor versus ACE‐inhibitor therapy alone in patients with IgA nephropathy: a randomized controlled trial. American Journal of Kidney Diseases 2009;53(1):26‐32. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Lv J, Zhang H, Chen Y, Li G, Wang H. Addition of steroids to ACE inhibitors is more preferred to patients with IgA nephropathy: a prospective randomized controlled trial [abstract no: SA‐FC105]. Journal of the American Society of Nephrology 2007;18(Abstracts):57A. [CENTRAL: CN‐00740506] [Google Scholar]
Maes 2004 {published data only}
- Maes B, Claes K, Evenepoel P, Kuypers D, Oyen R, Vanwalleghem J, et al. A prospective placebo‐controlled randomized study of mycophenolate mofetil treatment for IGA nephropathy: lack of clinical efficacy after three years [abstract no: T194]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):343‐4. [CENTRAL: CN‐00446533] [Google Scholar]
- Maes BD, Evenepoel P, Kuypers D, Messiaen T, Vanrenterghem Y. A prospective placebo‐controlled randomized single centre study of mycophenolate mofetil treatment for IGA nephropathy: lack of clinical efficacy after two years [abstract no: A0599]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):114A. [CENTRAL: CN‐00446534] [Google Scholar]
- Maes BD, Oyen R, Claes K, Evenepoel P, Kuypers D, Vanwalleghem J, et al. Mycophenolate mofetil in IgA nephropathy: results of a 3‐year prospective placebo‐controlled randomized study. Kidney International 2004;65(5):1842‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Manno 2001 {published data only}
- Manno C, Gesualdo L, D'Altri C, Rossini M, Grandaliano G, Schena FP. Prospective randomized controlled multicenter trial on steroids plus ramipril in proteinuric IgA nephropathy. Journal of Nephrology 2001;14(4):248‐52. [MEDLINE: ] [PubMed] [Google Scholar]
- Manno C, Torres DD, Pesce F, Rossini M, Schena FP. Long‐term prospective randomized controlled multicentre trial on steroids plus ramipril in proteinuric IgA nephropathy [abstract no: LB‐001]. American Society of Nephrology (ASN) Renal Week; 2008 Nov 4‐9; Philadelphia,PA. 2008. [CENTRAL: CN‐00740468]
- Manno C, Torres DD, Rossini M, Pesce F, Schena FP. Randomized controlled clinical trial of corticosteroids plus ACE‐inhibitors with long‐term follow‐up in proteinuric IgA nephropathy.[Erratum in: Nephrol Dial Transplant. 2010 Apr;25(4):1363‐4]. Nephrology Dialysis Transplantation 2009;24(12):3694‐701. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Torres D, Rossini M, Manno C, Gesualdo L, Grandaliano G, Schena FP, et al. Steroids plus ramipril versus ramipril alone in the treatment of IgA nephropathy: interim analysis of a prospective, controlled, randomized, multicenter trial [abstract no: MO27]. 41st Congress. European Renal Association. European Dialysis and Transplantation Association; 2004 May 15‐18; Lisbon, Portugal. 2004:222‐3. [CENTRAL: CN‐00636151]
Masutani 2016 {published data only}
- Masutani K, Tsuchimoto A, Yamada T, Hirakawa M, Mitsuiki K, Katafuchi R, et al. Comparison of steroid‐pulse therapy and combined with mizoribine in IgA nephropathy: a randomized controlled trial. Clinical & Experimental Nephrology 2016;20(6):896‐903. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Masutani K, Tsuchimoto A, Yamada T, Mitsuiki K, Katafuchi R, Hirakata HN, et al. No additional effect of oral immunosuppressive agents, mizoribine, with steroid pulse therapy in patients with IgA nephropathy: a prospective randomized controlled trial [abstract no: TH‐PO441]. Journal of the American Society of Nephrology 2014;25(Abstracts):207A. [CENTRAL: CN‐01658024] [Google Scholar]
Min 2017 {published data only}
- Min L, Wang Q, Cao L, Zhou W, Yuan J, Zhang M, et al. Comparison of combined leflunomide and low‐dose corticosteroid therapy with full‐dose corticosteroid monotherapy for progressive IgA nephropathy. Oncotarget 2017;8(29):48375‐84. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
NA IgAN 1995 {published data only}
- Hogg R, Fitzgibbons L, Lee JD, Julian BA, Holub BJ. Omega‐3 fatty acids (O3FA) for patients with IgA nephropathy (IgAN): efficacy is dose‐dependent. Report from the North American (NA) IgAN trial [abstract no: SA‐PO171]. Journal of the American Society of Nephrology 2004;15(Oct):337A. [CENTRAL: CN‐00676029] [Google Scholar]
- Hogg RJ. A randomized, placebo‐controlled, multicenter trial evaluating alternate‐day prednisone and fish oil supplements in young patients with immunoglobulin A nephropathy. Scientific Planning Committee of the IgA Nephropathy Study. American Journal of Kidney Diseases 1995;26(5):792‐6. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogg RJ, Fitzgibbons L, Atkins C, Nardelli N, Bay RC, North American IgA Nephropathy Study Group. Efficacy of omega‐3 fatty acids in children and adults with IgA nephropathy is dosage‐ and size‐dependent. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(6):1167‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogg RJ, Lee J, Nardelli N, Julian BA, Cattran D, Waldo B, et al. Clinical trial to evaluate omega‐3 fatty acids and alternate day prednisone in patients with IgA nephropathy: report from the Southwest Pediatric Nephrology Study Group. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(3):467‐74. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Hogg RJ, Lee J, Nardelli NA, Cattran D, Hirschman G, Julian BA. Clinical trial of alternate‐day prednisone or daily omega‐3 fatty acids in patients with IgA nephropathy [abstract no: OFC10]. Pediatric Nephrology 2004;19(9):C64. [CENTRAL: CN‐00583302] [Google Scholar]
- Hogg RJ, Lee J, Nardelli NA, Cattran D, Hirschman G, Julian BA. Multicenter, placebo‐controlled trial of alternate‐day prednisone (QOD‐PRED) or daily omega‐3 fatty acids (OM‐3 FA) in children and young adults with IgA nephropathy (IgAN). Report from the Southwest Pediatric Nephrology Study Group [abstract no: SU‐PO979]. Journal of the American Society of Nephrology 2003;14(Nov):751A. [CENTRAL: CN‐00583125] [Google Scholar]
NEFIGAN 2017 {published data only}
- Bhachu JS, Scionti K, Muto M, Molyneux K, Barratt J. Targeted release‐budesonide (NEFECON) modifies circulating IGA‐IGG immune complex levels and levels of poorly O‐galactosylated IgA in IgAN [abstract]. Kidney Diseases 2018;4(3):121‐2. [EMBASE: 624087998] [Google Scholar]
- Fellstrom B, Barratt J, Cook H, Coppo R, Feehally J, Fijter J, et al. Proteinuria reduction in IgA nephropathy by nefecon, a targeted release formulation of budesonide ‐ results from the NEFIGAN trial [abstract no: TO013]. Nephrology Dialysis Transplantation 2017;32(Suppl 3):iii82‐3. [EMBASE: 617291389] [Google Scholar]
- Fellstrom B, Barratt J, Floege J. Treatment of IgA nephropathy with nefecon, a targeted‐release formulation of budesonide‐extended posthoc results from the NEFIGAN trial [abstract]. Kidney Diseases 2018;4(3):140. [EMBASE: 624069810] [Google Scholar]
- Fellstrom B, Coppo R, Feehally J, Floege J, Jardine A, Locatelli F, et al. The NEFIGAN trial: a randomized, placebo‐controlled study to evaluate the efficacy and safety of NEFECON in IgA nephropathy patients at risk of developing ESRD: preliminary data from the run‐in phase [abstract no: TH‐PO442]. Journal of the American Society of Nephrology 2014;25(Abstracts):207A. [CENTRAL: CN‐01658027] [Google Scholar]
- Fellstrom B, Coppo R, Feehally J, Floege J, Fijter JW, Jardine AG, et al. The NEFIGAN trial: NEFECON, a novel targeted release formulation of budesonide, reduces proteinuria and stabilizes eGFR in IgA nephropathy patients at risk of ESRD [abstract no: HI‐OR04]. Journal of the American Society of Nephrology 2015;26(Abstracts):B1. [CENTRAL: CN‐01658026] [Google Scholar]
- Fellstrom BC, Barratt J, Cook H, Coppo R, Feehally J, Fijter JW, et al. Targeted‐release budesonide versus placebo in patients with IgA nephropathy (NEFIGAN): a double‐blind, randomised, placebo‐controlled phase 2b trial. Lancet 2017;389(10084):2117‐27. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Fellstrom BC, Coppo R, Feehally J, Floege J, Jardine AG, Koskinen PK, et al. The NEFIGAN trial: a phase 2b multicenter, randomized, placebo‐controlled study to evaluate the efficacy and safety of two doses of NEFECON in primary IgA nephropathy patients at risk of developing ESRD: rationale and study design [abstract no: PUB167]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):932A. [Google Scholar]
- Floege J. Mucosal corticosteroid therapy of IgA nephropathy. Kidney International 2017;92(2):278‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Muto M, Bhachu J, Brown J, Molyneux K, Coppo R, Barratt J. Targeted release‐budesonide (Nefecon) modifies mucosal IgA responses and possibly gut permeability in IgA nephropathy [abstract]. Kidney Diseases 2018;4(3):138‐9. [EMBASE: 624069793] [Google Scholar]
Ni 2005 {published data only}
- Ni Z, Qian J, Lu F, Jiang G, He L, Yao J, et al. Leflunomide plus low dose prednisone could reduce proteinuria and stabilize kidney function in progressive IgA nephropathy at 2 year follow‐up study [abstract no: F‐PO1967]. Journal of the American Society of Nephrology 2008;19(Abstracts Issue):555A. [CENTRAL: CN‐00740503] [Google Scholar]
- Ni Z, Qian JQ, Lu F, Yao J, Yuan WJ, Zhu H, et al. Leflunomide plus low dose prednisone therapy in progressive IgA nephropathy at 2 year follow‐up: a multi‐center, perspective, randomized control study [abstract no: SU‐PO1054]. Journal of the American Society of Nephrology 2007;18(Abstracts Issue):819A. [CENTRAL: CN‐00740500] [Google Scholar]
- Ni Z, Qian JQ, Lu F, Yao J, Yuan WJ, Zhu W, et al. Leflunomide treatment in progressive IgA nephropathy: interim analysis from a multi‐center, perspective, randomized control study [abstract no: SA‐PO1080]. Journal of the American Society of Nephrology 2006;17(Abstracts):800A. [CENTRAL: CN‐00740499] [Google Scholar]
- Ni Z, Qian JQ, Lu FM, Yao J, He L, Zhu H, et al. Leflunomide treatment in progressive IgA nephropathy: preliminary results from a multi‐center, perspective, randomized control study [abstract no: F‐PO859]. Journal of the American Society of Nephrology 2005;16:523A. [CENTRAL: CN‐00740496] [Google Scholar]
Nuzzi 2009 {published data only}
- Nuzzi F, D'Armiento M, Balletta MM, Malgieri G, Pecoraro C. Early corticosteroid treatment (CT) in children with IgA nephropathy (IGAn): a randomized and controlled trial [abstract]. Pediatric Nephrology 2010;25(9):1880. [EMBASE: 70438531] [Google Scholar]
- Nuzzi F, D'Armiento M, Malgieri G, Ferretti A, Marzano L, Pecoraro C. Early corticosteroid treatment in children with IGA nephropathy: a randomized and controlled trial [abstract no: OC005]. 27th Annual Scientific Meeting; Transplantation Society of Australia & New Zealand; 2009 June 17‐19; Canberra, Australia. 2009:28. [CENTRAL: CN‐00756254]
Pozzi 1999 {published data only}
- Vecchio L, Pozzi C, Fogazzi GB, Andrulli S, Pani A, Rustichelli R, et al. Renal histological picture and steroid treatment in IGA nephropathy [abstract no: SU‐PO984]. Journal of the American Society of Nephrology 2003;14(Nov):752A. [CENTRAL: CN‐00447275] [Google Scholar]
- Locatelli F, Pozzi C, Vecchio L, Bolasco PG, Fogazzi GB, Andrulli S, et al. Role of proteinuria reduction in the progression of IgA nephropathy. Renal Failure 2001;23(3‐4):495‐505. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pozzi C. Randomized trial of steroids in IgA nephropathy with moderate proteinuria at 5 years of follow up [abstract]. Nephrology Dialysis Transplantation 1997;12(9):A77. [CENTRAL: CN‐00250589] [Google Scholar]
- Pozzi C, Andrulli S, Vecchio L, Melis P, Fogazzi GB, Altieri P, et al. Corticosteroid effectiveness in IgA nephropathy: long‐term results of a randomized, controlled trial. Journal of the American Society of Nephrology 2004;15(1):157‐63. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pozzi C, Bolasco PG, Fogazzi GB, Andrulli S, Altieri P, Ponticelli C, et al. Corticosteroids in IgA nephropathy: a randomised controlled trial. Lancet 1999;353(9156):883‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Pozzi C, Vecchio L, Andrulli S, Melis P, Fogazzi G, Altieri P, et al. Steroid therapy in IgA nephropathy [abstract no: A0439]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):86A. [CENTRAL: CN‐00583670] [Google Scholar]
- Pozzi C, Vecchio L, Andrulli S, Pani A, Fogazzi GB, Rustichelli R, et al. Steroid effectiveness on proteinuria reduction in IgA nephropathy [abstract no: O94]. Nephrology Dialysis Transplantation 2002;17(Suppl 12):29. [CENTRAL: CN‐00509427] [Google Scholar]
- Pozzi C, Vecchio L, Fogazzi GB, Andrulli S, Pani A, Rustichelli R, et al. Renal histological picture and steroid treatment in IgA nephropathy [abstract no: T193]. Nephrology Dialysis Transplantation 2003;18(Suppl 4):343. [CENTRAL: CN‐01912329] [Google Scholar]
Segarra 2006 {published data only}
- Segarra A, Vila J, Montoro B, Sunye P, Calero F, Orfila MA, et al. A multicenter randomized study to analyze the efficacy and safety of high‐dose immunoglobulin therapy associated with steroids vs steroid monotherapy in patients with IgA nephropathy [abstract no: MP085]. Nephrology Dialysis Transplantation 2006;21(Suppl 4):iv327. [CENTRAL: CN‐00755295] [Google Scholar]
Shen 2013 {published data only}
- Shen P, Li Y, Wang Z, Wang W, Ren H, Zhang W, et al. A prospective randomized study on the efficacy of corticosteroid combined with cyclophosphamide or FK506 in primary IGA nephropathy with mild or moderate renal injury [abstract no: SP308]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i175. [EMBASE: 71075508] [Google Scholar]
Shi 2012a {published data only}
- Shi B, Ni Z, Cao L, Mou S, Zhang M, Wang Q, et al. Mannose‐binding lectin gene polymorphism may predict response to leflunomide in patients with progressive IgA nephropathy [abstract no: FR‐PO819]. Journal of the American Society of Nephrology 2012;23(Abstract Suppl):556A. [Google Scholar]
Shima 2018 {published data only}
- Shima Y, Nakanishi K, Kaku Y, Ishikura K, Hataya H, Matsuyama T, et al. Combination therapy with or without warfarin and dipyridamole for severe childhood IgA nephropathy: an RCT. Pediatric Nephrology 2018;33(11):2103‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shoji 2000 {published data only}
- Shoji T, Nakanishi I, Saito N, Hayashi T, Togawa M, Okada N, et al. The corticosteroid treatment of diffuse mesangial proliferative IgA nephropathy: a one‐year prospective trial [abstract no: A0466]. Journal of the American Society of Nephrology 1997;8(Program & Abstracts):98A. [Google Scholar]
- Shoji T, Nakanishi I, Suzuki A, Hayashi T, Togawa M, Okada N, et al. Early treatment with corticosteroids ameliorates proteinuria, proliferative lesions, and mesangial phenotypic modulation in adult diffuse proliferative IgA nephropathy. American Journal of Kidney Diseases 2000;35(2):194‐201. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Stangou 2011 {published data only}
- Stangou M, Ekonomidou D, Giamalis P, Liakou H, Tsiantoulas A, Pantzaki A, et al. Steroids and azathioprine in the treatment of IgA nephropathy. Clinical & Experimental Nephrology 2011;15(3):373‐80. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
STOP‐IgAN 2008 {published data only}
- Eitner F, Ackermann D, Hilgers RD, Floege J. Supportive versus immunosuppressive therapy of progressive IgA nephropathy (STOP) IgAN trial: rationale and study protocol. Journal of Nephrology 2008;21(3):284‐9. [MEDLINE: ] [PubMed] [Google Scholar]
- Floege J, Rauen T, Eitner F, Fitzner C, Hilgers RD. Corticosteroid monotherapy versus combined immunosuppression in IgA nephropathy: insights from the STOP‐IgAN trial [abstract no: SA‐PO1097]. Journal of the American Society of Nephrology 2015;26(Abstracts):B4‐5. [CENTRAL: CN‐01658032] [Google Scholar]
- Floege J, Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, et al. Supportive versus immunosuppressive therapy for progressive IgA nephropathy (STOP‐IGAN): a randomized, controlled, open‐label multicenter trial [abstract]. 52nd Congress ERA‐EDTA; 2015 May 28‐31; London, UK. 2015. [CENTRAL: CN‐01658031]
- Floege J, Rauen T, Schindler J, Fitzner C, Eitner F, Groene HJ, et al. The MEST kidney biopsy score predicts renal outcome in STOP‐IgAN trial patients ‐ a post‐hoc study [abstract no: TH‐OR057]. Journal of the American Society of Nephrology 2016;27(Abstract Suppl):14A. [CENTRAL: CN‐01658030] [Google Scholar]
- Lennartz D, Rauen T, Fitzner C, Eitner F, Hilgers RD, Floege J. Dual blockade of the renin‐angiotensin system in patients with IgA nephropathy ‐ insights from the STOP‐IGAN Trial [abstract no: SUN‐021]. Kidney International Reports 2019;4(7 Suppl):S161. [EMBASE: 2002179482] [Google Scholar]
- Rauen T, Eitner F, Fitzner C, Sommerer C, Zeier M, Otte B, et al. Intensive supportive care plus immunosuppression in IgA nephropathy. New England Journal of Medicine 2015;373(23):2225‐36. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Rauen T, Fitzner C, Eitner F, Sommerer C, Zeier M, Otte B, et al. Effects of two immunosuppressive treatment protocols for IgA nephropathy. Journal of the American Society of Nephrology 2018;29(1):317‐25. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauen T, Lennartz DP, Fitzner C, Eitner F, Hilgers RD, Floege J. Dual blockade of the renin‐angiotensin system in patients with IgA nephropathy‐insights from the Stop‐IgAN Trial [abstract]. Kidney Diseases 2018;4(3):141. [EMBASE: 624069825] [Google Scholar]
- Schimpf JI, Klein T, Fitzner C, Eitner F, Porubsky S, Hilgers RD, et al. Renal outcomes of STOP‐IgAN trial patients in relation to baseline histology (MEST‐C scores). BMC Nephrology 2018;19(1):328. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Takeda 1999 {published data only}
- Takeda T, Muso E, Maeda M, Ono T, Higashi Y, Takeshita K, et al. Two‐year randomized controlled trial of steroid therapy for adult patients with moderately active IgA nephropathy (IgAN) [abstract no: A0463]. Journal of the American Society of Nephrology 1999;10(Program & Abstracts):90A‐1A. [CENTRAL: CN‐00583221] [Google Scholar]
Tang 2005 {published data only}
- Tang S, Leung JC, Chan LY, Lui YH, Tang CS, Kan CH, et al. Mycophenolate mofetil alleviates persistent proteinuria in IgA nephropathy. Kidney International 2005;68(2):802‐12. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Tang S, Leung JC, Tang AW, Ho YW, Chan LY, Chan TM, et al. A prospective, randomized, case‐controlled study on the efficacy of mycophenolate mofetil (MMF) for IgA nephropathy (IgAN) patients with persistent proteinuria despite angiotensin blockade [abstract no: SU‐PO986]. Journal of the American Society of Nephrology 2003;14(Nov):752A. [CENTRAL: CN‐00583218] [Google Scholar]
- Tang SC, Tang AW, Wong SS, Leung JC, Ho YW, Lai KN. Long‐term study of mycophenolate mofetil treatment in IgA nephropathy. Kidney International 2010;77(6):543‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
TESTING 2017 {published data only}
- Lv J, Zhang H, Perkovic V, TESTING Study Group. The therapeutic evaluation of steroids in IgA nephropathy global (TESTING) study [abstract no: LB01]. 53rd Congress ERA‐EDTA; 2016 May 21‐24; Vienna, Austria. 2016. [CENTRAL: CN‐01658019]
- Lv J, Zhang H, Wong MG, Jardine MJ, Hladunewich M, Jha V, et al. Effect of oral methylprednisolone on clinical outcomes in patients with IgA nephropathy: the TESTING randomized clinical trial. JAMA 2017;318(5):432‐42. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Walker 1990a {published data only}
- Walker RG, Yu SH, Owen JE, Kincaid‐Smith P. The treatment of mesangial IgA nephropathy with cyclophosphamide, dipyridamole and warfarin: a two‐year prospective trial. Clinical Nephrology 1990;34(3):103‐7. [MEDLINE: ] [PubMed] [Google Scholar]
Welch 1992 {published data only}
- Welch TR, Fryer C, Shely E, Witte DP, Quinlan M. Double‐blind, controlled trial of short‐term prednisone therapy in immunoglobulin A glomerulonephritis. Journal of Pediatrics 1992;121(3):474‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Woo 1987 {published data only}
- Woo KT, Chiang GS, Lim CH. Follow‐up renal biopsies in IgA nephritic patients on triple therapy. Clinical Nephrology 1987;28(6):304‐5. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Chiang GS, Yap HK, Lim CH. Controlled therapeutic trial of IgA nephritis with follow‐up renal biopsies. Annals of the Academy of Medicine, Singapore 1988;17(2):226‐31. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Edmondson RP, Yap HK, Wu AY, Chiang GS, Lee EJ, et al. Effects of triple therapy on the progression of mesangial proliferative glomerulonephritis. Clinical Nephrology 1987;27(2):56‐64. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Lee GS, Lau YK, Chiang GS, Lim CH. Effects of triple therapy in IgA nephritis: a follow‐up study 5 years later. Clinical Nephrology 1991;36(2):60‐6. [MEDLINE: ] [PubMed] [Google Scholar]
- Woo KT, Lee GS, Lau YK, Chiang GSC Lim CH. Anti platelet therapy in IgA nephritis [abstract]. 11th International Congress of Nephrology; 1990 Jul 15‐20; Tokyo, Japan. 1990:13. [CENTRAL: CN‐00448412]
Wu 2016 {published data only}
- Chen XM, Wu J, Duan S, Zheng Y. Efficacy and safety of telmisartan, clopidogrelin and leflunomide in patients with IgA nephropathy ‐ a multicentre, prospective, randomized, double‐blind, double‐dummy controlled clinical trial [abstract no: SA‐PO848]. Journal of the American Society of Nephrology 2013;24(Abstracts):821A. [CENTRAL: CN‐01658021] [Google Scholar]
- Wu J, Duan S, Li W, Wang Y, Liu W, Zhang J, et al. Efficacy and safety of telmisartan, clopidogrelin, and leflunomide in patients with IgA nephropathy ‐ a multicentre, prospective, randomized, double‐blind and‐dummy controlled clinical trial [abstract no: SP307]. Nephrology Dialysis Transplantation 2013;28(Suppl 1):i175. [EMBASE: 71075507] [Google Scholar]
- Wu J, Duan SW, Sun XF, Li WG, Wang YP, Liu WH, et al. Efficacy of leflunomide, telmisartan, and clopidogrel for immunoglobulin A nephropathy: a randomized controlled trial. Chinese Medical Journal 2016;129(16):1894‐903. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Xie 2011 {published data only}
- Xie Y, Huang S, Wang L, Miao L, Zhang A, Li Y, et al. Efficacy and safety of mizoribine combined with losartan in the treatment of IgA nephropathy: a multicenter, randomized, controlled study. American Journal of the Medical Sciences 2011;341(5):367‐72. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yamauchi 2001 {published data only}
- Yamauchi A, Uzu T, Yamato M, Ko M, Takahara K. Effect of steroid therapy on the progression of IgA nephropathy [abstract no: A1329]. Journal of the American Society of Nephrology 2001;12(Program & Abstracts):259A. [CENTRAL: CN‐00448450] [Google Scholar]
Yoshikawa 1999 {published data only}
- Ito H, Yoshikawa N. Prospective multicenter controlled therapeutic trial in IgA nephropathy in Japanese children: a preliminary report [abstract no: S‐I‐2]. Pediatric Nephrology 1992;6(6):C208. [CENTRAL: CN‐01658022] [Google Scholar]
- Kamei K, Nakanishi K, Ito S, Saito M, Sako M, Ishikura K, et al. Long‐term results of a randomized controlled trial in childhood IgA nephropathy. Clinical Journal of the American Society of Nephrology: CJASN 2011;6(6):1301‐7. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa N, Ito H. Combined therapy with prednisolone, azathioprine, heparin‐warfarin, and dipyridamole for paediatric patients with severe IgA nephropathy‐‐is it relevant for adult patients?. Nephrology Dialysis Transplantation 1999;14(5):1097‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yoshikawa N, Ito H. Corticosteroids and immunosuppressive drugs [abstract]. Pediatric Nephrology 2001;16(8):C31. [CENTRAL: CN‐00448482] [Google Scholar]
- Yoshikawa N, Ito H, Sakai T, Takekoshi Y, Honda M, Awazu, M, et al. A controlled trial of combined therapy for newly diagnosed severe childhood IgA nephropathy. The Japanese Pediatric IgA Nephropathy Treatment Study Group. Journal of the American Society of Nephrology 1999;10(1):101‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yoshikawa N, Itoh H, Japanese Pediatric IgA Nephropathy Treatment Study Group. A controlled trial of prednisolone (P), azathioprine (A), heparin‐warfarin (H‐W) and dipyridamole (D) in newly diagnosed severe childhood IgA nephropathy (IGAN) [abstract no: A0779]. Journal of the American Society of Nephrology 1996;7(9):1401. [CENTRAL: CN‐00583182] [DOI] [PubMed] [Google Scholar]
Yoshikawa 2006 {published data only}
- Yoshikawa N. Treatment of IGA nephropathy in children [abstract no: FCP04]. Pediatric Nephrology 2004;19(9):C57. [CENTRAL: CN‐00583685] [Google Scholar]
- Yoshikawa N, Honda M, Iijima K, Awazu M, Hattori S, Nakanishi K, et al. Steroid treatment for severe childhood IgA nephropathy: a randomized, controlled trial. Clinical Journal of the American Society of Nephrology: CJASN 2006;1(3):511‐7. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Yoshikawa N, Ito H. Corticosteroids and immunosuppressive drugs [abstract]. Pediatric Nephrology 2001;16(8):C31. [CENTRAL: CN‐00448482] [Google Scholar]
- Yoshikawa N, Ito H. Prednisolone therapy versus combined therapy with prednisolone, azathioprine, warfarin and dipyridamole for newly diagnosed severe childhood IgA nephropathy: a controlled trial by the Japanese Pediatric IgA Nephropathy Treatment study group [abstract no: A0430]. Journal of the American Society of Nephrology 2000;11(Sept):79A. [CENTRAL: CN‐00550736] [DOI] [PubMed] [Google Scholar]
Zhang 2004 {published data only}
- Zhang XZ, He Q, Luo TC, Lin ST. Efficacy and safety of leflunomide in the treatment of IgA nephropathy: preliminary results from a randomized, corticosteroid controlled, multi‐center clinical trial [abstract no: SA‐PO168]. Journal of the American Society of Nephrology 2004;15(Oct):337A. [CENTRAL: CN‐00583916] [Google Scholar]
- Zhang XZ, He YC, Luo Q, Yang TC, Li XG, Lin SY. Efficacy and safety of leflunomide in the treatment of IgA nephropathy: a perspective, corticosteroid controlled, multi‐center clinical trial [abstract no: F‐PO1097]. Journal of the American Society of Nephrology 2006;17(Abstracts):567A. [CENTRAL: CN‐00644205] [Google Scholar]
References to studies excluded from this review
Chen 2009b {published data only}
- Chen Y, Qin Y. Clinical effects of triple therapy in treatment of IgA nephropathy patients with moderate proteinuria. Xian Dai Yi Yao Wei Sheng 2009;25:1645‐6. [CENTRAL: CN‐01912326] [Google Scholar]
Czock 2007 {published data only}
- Czock D, Rasche FM, Carius A, Glander P, Budde K, Bauer S, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid after enteric‐coated mycophenolate versus mycophenolate mofetil in patients with progressive IgA nephritis. Journal of Clinical Pharmacology 2007;47(7):850‐9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
- Keller F, Mueller L, Rasche M, Carius A, Glander P, Bauer S, et al. Pharmacokinetics and pharmacodynamics of mycophenolic acid after enteric‐coated mycophenolate sodium and mycophenolate mofetil in patients with IgA nephritis and renal impairment [abstract no: F‐PO1099]. Journal of the American Society of Nephrology 2006;17(Abstracts):568A. [CENTRAL: CN‐00615866] [Google Scholar]
Dal Canton 2005 {published data only}
- Dal Canton A, Amore A, Barbano G, Coppo R, Emma F, Grandaliano G, et al. One‐year angiotensin‐converting enzyme inhibition plus mycophenolate mofetil immunosuppression in the course of early IgA nephropathy: a multicenter, randomised, controlled study. Journal of Nephrology 2005;18(2):136‐40. [MEDLINE: ] [PubMed] [Google Scholar]
GloMY 2010 {published data only}
- Harper L. Randomised pilot trial of myfortic for the treatment of primary proteinuric glomerulonephritis (Short title: proteinuria in glomerulonephritis: Myfortic (GloMY)) Trial protocol – version 3.2. www.birmingham.ac.uk/Documents/college‐mds/trials/bctu/glomy/GloMY‐protocol‐Version‐3‐2‐030112.pdf (accessed prior to 13 January 2020).
Imai 2006 {published data only}
- Imai H, Hotta O, Yoshimura M, Konta T, Tsubakihara Y, Miyazaki M, et al. Deoxyspergualin, an immunosuppressant, in patients suffering from nephropathies with crescent formation: an open‐label trial to evaluate safety and efficacy. Clinical & Experimental Nephrology 2006;10(1):40‐54. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Shen 2009 {published data only}
- Shen SJ, Hu ZX, Wang SM, Li QH. Effects of a combined regime of Tripterygium wilfordii glycosides and benazepril in treatment of IgA nephropathy. Zhong Guo Zhong Xi Yi jie He Shen Bing Za Zhi 2009;10:154‐5. [CENTRAL: CN‐01912324] [Google Scholar]
Sulimani 2001 {published data only}
- Sulimani FM, Alhomssi M, Mitwalli A, al Wakeel J, Alam A, Tarif N, et al. Difficult nephropathies: a multicenter randomized trial on the treatment [abstract]. Saudi Journal of Kidney Diseases & Transplantation 2001;12(2):229. [CENTRAL: CN‐00402775] [Google Scholar]
Yonemura 2000b {published data only}
- Yonemura K, Kimura M, Miyaji T, Hishida A. Short‐term effect of vitamin K administration on prednisolone‐induced loss of bone mineral density in patients with chronic glomerulonephritis. Calcified Tissue International 2000;66(2):123‐8. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
NCT00301600 {published data only}
- Li LS. Mycophenolate mofetil versus intravenous cyclophosphamide pulses in the treatment of crescentic IgA nephropathy. www.clinicaltrials.gov/ct2/show/NCT00301600 (first received 13 March 2006).
NCT02160132 {published data only}
- Deng Y. A controlled study of steroids therapy for patients of IgA nephropathy with active pathological changes. www.ClinicalTrials.gov/show/NCT02160132 2014; Vol. (first received 10 June 2014).
NCT02571842 {published data only}
- Chancharoenthana W. Rituximab in recurrent IgA nephropathy. www.ClinicalTrials.gov/show/NCT02571842 (first received 8 October 2015).
References to ongoing studies
AIGA 2016 {published data only}
- Kim C, Lee D, Lee S, Choi B, Han S, Lee S. Efficacy and safety of a combination of mycophenolate mofetil and corticosteroid in advanced IgA nephropathy (AIGA). www.clinicalTrials.gov/show/NCT02981212 (first received 5 December 2016).
ARTEMIS‐IgAN 2018 {published data only}
- NCT03608033. Study of the safety and efficacy of OMS721 in patients with immunoglobulin A (IgA) nephropathy. www.clinicaltrials.gov/show/nct03608033 (first received 31 July 2018).
ChiCTR1800014442 {published data only}
- Li Y, Fu RG. Prospective study of the efficacy and safety of improved Italy scheme therapy for IgA nephropathy. www.chictr.org.cn/showprojen.aspx?proj=24628 (first received 13 January 2018).
MAIN 2013 {published data only}
- Hou F. The effects of mycophenolate mofetil (MMF) on renal outcomes in advanced immunoglobulin A (IgA) nephropathy patients. www.ClinicalTrials.gov/show/NCT01854814 (first received 16 May 2013).
NCT00657059 {published data only}
- Yu X, Yang Q. Mycophenolate mofetil (MMF) in patients with IgA nephropathy (IgAN). www.clinicaltrials.gov/ct2/show/NCT00657059 (first received 14 April 2008).
NCT02808429 {published data only}
- NCT02808429. Efficacy and safety of atacicept in IgA nephropathy. www.ClinicalTrials.gov/show/NCT02808429 (first received 21 June 2016).
NCT03468972 {published data only}
- Han SH. Effect of immunosuppression in IgA nephropathy. www.clinicaltrials.gov/show/nct03468972 (first received 19 March 2018).
NEFIGARD 2018 {published data only}
- NCT03643965. Efficacy and safety of Nefecon in patients with primary IgA (immunoglobulin A) nephropathy (Nefigard). www.clinicaltrials.gov/ct2/show/NCT03643965 (first received 23 August 2018).
PIRAT 2015 {published data only}
- Berthoux F. Prevention in recipients with primary IgA Nephropathy of recurrence after kidney transplantation: ATG‐F versus basiliximab as induction immunosuppressive treatment (PIRAT). www.ClinicalTrials.gov/show/NCT02523768 (first received 14 August 2015).
SIGN 2014 {published data only}
- Tam WK, Tumlin J, Barratt J, Rovin HB, Roberts SD, Roufosse C, et al. Spleen tyrosine kinase (SYK) inhibition in IgA nephropathy: a global, phase ii, randomised placebo‐controlled trial of fostamatinib [abstract no: SUN‐036]. Kidney International Reports 2019;4(7 Suppl):S168. [EMBASE: 2002180224] [Google Scholar]
TIGER 2017 {published data only}
- Joly D, Alarmartine E. Treatment of IgA nephropathy according to renal lesions (TIGER). www.ClinicalTrials.gov/show/NCT03188887 (first received 16 June 2017).
TOPplus‐IgAN 2013 {published data only}
- Shi W. Extended follow‐up of treatment of prednisone plus cyclophosphamide in patients with advanced‐stage IgA nephropathy (e‐TOPplus). www.ClinicalTrials.gov/show/NCT03218852 (first received 17 July 2017}.
- Shi W. Treatment of prednisone plus cyclophosphamide in patients with advanced‐stage IgA nephropathy (TOPplus‐IgAN). www.ClinicalTrials.gov/show/NCT01758120 (first received 1 January 2013).
UMIN000032031 {published data only}
- Suzuki H. The steroid internal use method for patients with IgA nephropathy. upload.umin.ac.jp/cgi‐open‐bin/ctr_e/ctr_view.cgi?recptno=R000036551 (first received 1 April 2018).
Additional references
Barbour 2019
- Barbour SJ, Coppo R, Zhang H, Liu ZH, Suzuki Y, Matsuzaki Y, et al. Evaluating a new international risk‐prediction tool in IgA nephropathy. JAMA Internal Medicine 2019;179(7):942‐52. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Berger 1968
- Berger J, Hinglais N. Intercapillary deposits of IgA‐IgG [Les ddpots intercapillaires d'IgA‐IgG]. Journal d Urologie et de Nephrologie 1968;74(9):694‐5. [MEDLINE: ] [PubMed] [Google Scholar]
Cattran 2009
- Working Group of the International IgA Nephropathy Network and the Renal Pathology Society, Cattran DC, Coppo R, Cook HT, Feehally J, Roberts IS, at al. The Oxford classification of IgA nephropathy: rationale, clinicopathological correlations, and classification. Kidney International 2009;76(5):535‐45. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Cheng 2015
- Cheng G, Liu D, Margetts P, Liu L, Zhao Z, Liu Z, et al. Valsartan combined with clopidogrel and/or leflunomide for the treatment of progressive immunoglobulin A nephropathy. Nephrology 2015;20(2):77‐84. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Coppo 2018
- Coppo R. Treatment of IgA nephropathy: recent advances and prospects. Nephrologie et Therapeutique 2018;14 Suppl 1:S13–21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
D'Amico 1987
- D'Amico G. The commonest glomerulonephritis in the world: IgA nephropathy. Quarterly Journal of Medicine 1987;64(245):709‐27. [MEDLINE: ] [PubMed] [Google Scholar]
Egger 1997
- Egger M, Davey‐Smith G, Schneider M, Minder C. Bias in meta‐analysis detected by a simple graphical test. BMJ 1997;315(7109):629‐34. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gale 2017
- Gale DP, Molyneux K, Wimbury D, Higgins P, Levine AP, Caplin B, et al. Galactosylation of IgA1 Is Associated with Common Variation in C1GALT1. Journal of the American Society of Nephrology 2017;28(7):2158‐66. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gallo 1988
- Gallo GR, Katafuchi R, Neelakantappa K, Baldwin DS. Prognostic pathologic markers in IgA nephropathy. American Journal of Kidney Diseases 1988;12(5):362‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
GRADE 2008
- Guyatt GH, Oxman AD, Vist GE, Kunz R, Falck‐Ytter Y, Alonso‐Coello P, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ 2008;336(7650):924‐6. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
GRADE 2011
- Guyatt G, Oxman AD, Akl EA, Kunz R, Vist G, Brozek J, et al. GRADE guidelines: 1. Introduction‐GRADE evidence profiles and summary of findings tables. Journal of Clinical Epidemiology 2011;64(4):383‐94. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Haas 2017
- Haas M, Verhave JC, Liu ZH, Alpers CE, Barratt J, Becker JU, et al. A multicenter study of the predictive value of crescents in IgA nephropathy.[Erratum in: J Am Soc Nephrol. 2017 May;28(5):1665; PMID: 28455358]. Journal of the American Society of Nephrology 2017;28(2):691‐701. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Han 2010
- Han SH, Kang EW, Park JK, Kie JH, Han DS, Kang SW. Spontaneous remission of nephrotic syndrome in patients with IgA nephropathy. Nephrology Dialysis Transplantation 2010;26(5):1570–75. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Higgins 2003
- Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327(7414):557‐60. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Hiki 2001
- Hiki Y, Odani H, Takahashi M, Yasuda Y, Nishimoto A, Iwase H, et al. Mass spectrometry proves under‐O‐glycosylation of glomerular IgA1 in IgA nephropathy. Kidney International 2001;59(3):1077‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hirano 2019
- Hirano K, Matsuzaki K, Yasuda T, Nishikawa M, Yasuda Y, Koike K, et al. Association between tonsillectomy and outcomes in patients with immunoglobulin A nephropathy. JAMA Network Open 2019;2(5):e194772. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Hogg 2015
- Hogg RJ, Bay RC, Jennette JC, Sibley R, Kumar S, Fervenza FC, et al. Randomized controlled trial of mycophenolate mofetil in children, adolescents, and adults with IgA nephropathy. American Journal of Kidney Diseases 2015;66(5):783‐91. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Hotta 2001
- Hotta O, Miyazaki M, Furuta T, Tomioka S, Chiba S, Horigome I, et al. Tonsillectomy and steroid pulse therapy significantly impact on clinical remission in patients with IgA nephropathy. American Journal of Kidney Diseases 2001;38(4):736–43. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Inagaki 2017
- Inagaki K, Yasuda Y, Ando M, Kaihan AB, Hachiya A, Ozeki T, et al. Seasonal proteinuria changes in IgA nephropathy patients after proteinuria remission. PLoS ONE [Electronic Resource] 2017;12(11):e0187607. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
KDIGO 2012
- Kidney Disease: Improving Global Outcomes (KDIGO) Glomerulonephritis Work Group. KDIGO clinical practice guideline for glomerulonephritis. Kidney International ‐ Supplement 2012;2(2):139‐274. [https://kdigo.org/wp‐content/uploads/2017/02/KDIGO‐2012‐GN‐Guideline‐English.pdf] [Google Scholar]
Kim 2016
- Kim J, Park JH, Kim DH, Kim HY, Kim SH, Park WD. A patient with IgA nephropathy: 5 years after complete remission of minimal change nephrotic syndrome. EWHA Medical Journal 2016;39(4):118‐21. [EMBASE: 613536188] [Google Scholar]
Kiryluk 2012
- Kiryluk K, Li Y, Sanna‐Cherchi S, Rohanizadegan M, Suzuki H, Eitner F, et al. Geographic differences in genetic susceptibility to IgA nephropathy: GWAS replication study and geospatial risk analysis. PLoS Genetics 2012;8(6):e1002765. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Lambers Heerspink 2015
- Lambers Heerspink HJ, Kropelin TF, Hoekman J, Zeeuw D, Reducing Albuminuria as Surrogate Endpoint (REASSURE) Consortium. Drug‐induced reduction in albuminuria is associated with subsequent renoprotection: a meta‐analysis. American Journal of Kidney Diseases 2015;26(8):2055‐64. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Liu 2019
- Liu L, Yin Z, Ma J, Duan S, Chen X. Potential association of body constitution with the prognosis of IgA nephropathy: a long‐time follow‐up of 203 cases in China. Evidence‐Based Complementary & Alternative Medicine: eCAM 2019:6289478. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Maillard 2015
- Maillard N, Wyatt RJ, Julian BA, Kiryluk K, Gharavi A, Fremeaux‐Bacchi V, et al. Current understanding of the role of complement in IgA nephropathy. Journal of the American Society of Nephrology 2015;26(7):1503‐12. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Manno 2007
- Manno C, Strippoli GF, D'Altri C, Torres D, Rossini M, Schena FP. A novel simpler histological classification for renal survival in IgA nephropathy: a retrospective study. American Journal of Kidney Diseases 2007;49(6):763‐5. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Mestecky 1993
- Mestecky J, Tomana M, Crowley‐Nowick PA, Moldoveanu Z, Julian BA, Jackson S. Defective galactosylation and clearance of IgA1 molecules as a possible etiopathogenic factor in IgA nephropathy. Contributions to Nephrology 1993;104:172‐82. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Moriyama 2019
- Moriyama T. Clinical and histological features and therapeutic strategies for IgA nephropathy. Clinical & Experimental Nephrology 2019;23(9):1089‐99. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
NCT03841448
- A study of cemdisiran in adults with immunoglobulin A nephropathy (IgAN). www.clinicaltrials.gov/ct2/show/NCT03841448 (first posted 15 February 2019).
Neelakantappa 1988
- Neelakantappa K, Gallo GR, Baldwin DS. Proteinuria in IgA nephropathy. Kidney International 1988;33(3):716‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Nolin 1999
- Nolin L, Courteau M. Management of IgA nephropathy: evidence‐based recommendations. Kidney International ‐ Supplement 1999;70:S56‐62. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Piccoli 2010
- Piccoli A, Codognotto M, Tabbi MG, Favaro E, Rossi B. Influence of tonsillectomy on the progression of mesangioproliferative glomerulonephritis. Nephrology Dialysis Transplantation 2010;25(8):2583–9. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Pozzi 2010
- Pozzi C, Andrulli S, Pani A, Scaini P, Vecchio L, Fogazzi G, et al. Addition of azathioprine to corticosteroids does not benefit patients with IgA nephropathy. Journal of the American Society of Nephrology 2010;21(10):1783–90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Reich 2007
- Reich HN, Troyanov S, Scholey JW, Cattran DC, Toronto Glomerulonephritis Registry. Remission of proteinuria improves prognosis in IgA nephropathy. Journal of the American Society of Nephrology 2007;18(12):3177‐83. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Reid 2011
- Reid S, Cawthon PM, Craig JC, Samuels JA, Molony DA, Strippoli GF. Non‐immunosuppressive treatment for IgA nephropathy. Cochrane Database of Systematic Reviews 2011, Issue 3. [DOI: 10.1002/14651858.CD003962.pub2] [DOI] [PubMed] [Google Scholar]
Rekola 1991
- Rekola S, Bergstrand A, Bucht H. Deterioration of GFR in IgA nephropathy as measured by 51Cr‐EDTA clearance. Kidney International 1991;40(6):1050‐4. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Rostoker 1995
- Rostoker G, Desvaux‐Belghiti D, Pilatte Y, Petit‐Phar M, Philippon C, Deforges L, et al. Immunomodulation with low‐dose immunoglobulins for moderate IgA nephropathy and Henoch‐Schonlein purpura. Preliminary results of a prospective uncontrolled trial. Nephron 1995;69(3):327‐34. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schena 2001
- Schena FP. Immunoglobulin A nephropathy with mild renal lesions: a call in the forest for physicians and nephrologists. American Journal of Medicine 2001;110(6):499‐500. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Schena 2009
- Schena FP, Pesce F. Chapter 2: Epidemiology and ancestral difference. In: Kar Neng Lai editor(s). Recent advances in IgA nephropathy. Singapore: World Scientific, 2009:9‐19. [Google Scholar]
Schunemann 2011a
- Schünemann HJ, Oxman AD, Higgins JP, Vist GE, Glasziou P, Guyatt GH. Chapter 11: Presenting results and 'Summary of findings' tables. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Schunemann 2011b
- Schünemann HJ, Oxman AD, Higgins JP, Deeks JJ, Glasziou P, Guyatt GH. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JP, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Song 2017
- Song YH, Cai GY, Xiao YF, Wang YP, Yuan BS, Xia YY, et al. Efficacy and safety of calcineurin inhibitor treatment for IgA nephropathy: a meta‐analysis. BMC Nephrology 2017;18(1):61. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Tesar 2015
- Tesar V, Troyanov S, Bellur S, Verhave JC, Cook HT, Feehally J, et al. Corticosteroids in IgA nephropathy: a retrospective analysis from the VALIGA study. Journal of the American Society of Nephrology 2015;26(9):2248–58. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Trimarchi 2017
- Trimarchi H, Barratt J, Cattran DC, Cook HT, Coppo R, Haas M, et al. Oxford Classification of IgA nephropathy 2016: an update from the IgA Nephropathy Classification Working Group. Kidney International 2017;91(5):1014‐21. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Wyatt 2001
- Wyatt RJ, Hogg RJ. Evidence‐based assessment of treatment options for children with IgA nephropathies. Pediatric Nephrology 2001;16(2):156‐67. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Yang 2018
- Yang P, Honghong Z, Xiao B, Xu G. Comparative efficacy and safety of therapies in IgA nephropathy: a network meta‐analysis of randomized controlled trials. KI Reports 2018;3(4):794‐803. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
Zheng 2018
- Zheng JN, Bi TD, Zhu LB, Liu LL. Efficacy and safety of mycophenolate mofetil for IgA nephropathy: An updated meta‐analysis of randomized controlled trials. Experimental & Therapeutic Medicine 2018;16(3):1882‐90. [MEDLINE: ] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Samuels 2003a
- Samuels JA, Strippoli GF, Craig JC, Schena FP, Malony DA. Immunosuppressive and cytotoxic agents for treating IgA nephropathy. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD003965] [DOI] [PubMed] [Google Scholar]
Samuels 2003b
- Samuels JA, Strippoli GF, Craig JC, Schena FP, Molony DA. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database of Systematic Reviews 2003, Issue 4. [DOI: 10.1002/14651858.CD003965] [DOI] [PubMed] [Google Scholar]
Samuels 2004
- Samuels JA, Strippoli GF, Craig JC, Schena FP, Molony DA. Immunosuppressive treatments for immunoglobulin A nephropathy: a meta‐analysis of randomized controlled trials. Nephrology 2004;9(4):177‐85. [MEDLINE: ] [DOI] [PubMed] [Google Scholar]
Vecchio 2015
- Vecchio M, Bonerba B, Palmer SC, Craig JC, Ruospo M, Samuels JA, et al. Immunosuppressive agents for treating IgA nephropathy. Cochrane Database of Systematic Reviews 2015, Issue 8. [DOI: 10.1002/14651858.CD003965.pub2] [DOI] [PubMed] [Google Scholar]


