Abstract
Study Objectives
To document the rates of persistent, remitted, and intermittent excessive daytime sleepiness (EDS) in a longitudinal 5-year community study of adults and to assess how changes in risk factors over time can predict improvement of daytime sleepiness (DS).
Methods
Participants were recruited in 2007–2008 as part of a population-based epidemiological study implemented in Canada. They completed postal assessments at baseline and at each yearly follow-up. An Epworth Sleepiness Scale total score >10 indicated clinically significant EDS; a 4-point reduction between two consecutive evaluations defined DS improvement. Socio-demographic, lifestyle, health characteristics, and sleep-related measures (e.g. insomnia symptoms, sleep duration, sleep medication) were self-reported at each time point. Cox proportional-hazard models were used to predict EDS and DS remissions over 5 years.
Results
Among the 2167 participants, 33% (n = 714) met criteria for EDS at baseline, of whom 33% had persistent EDS, 44% intermittent EDS, and 23% remitted EDS over the follow-up. Furthermore, 61.4% of 2167 initial participants had stable DS, 27.1% sustained DS improvement and 8.5% transient improvement over the follow-up. The main predictors of EDS remission or DS improvement were normal weight, taking less hypnotics, having hypertension, increased nighttime sleep duration, and decreased insomnia, and depressive symptoms.
Conclusions
EDS waxes and wanes over time with frequent periods of remission and is influenced by behavioral characteristics and changes in psychological, metabolic, and nighttime sleep patterns. Targeting these predictors in future interventions is crucial to reduce DS in the general adult population.
Keywords: cohort studies, epidemiology, sleepiness, remission, natural history, insomnia, chronic, disease, psychological factors
Statement of Significance.
Excessive daytime sleepiness (EDS) is a highly prevalent symptom in the general population often associated with low quality of life. It is costly and represents a substantial burden to the health care system, as it is linked to an increased risk of accidents and injuries. In a large general population sample with a wide age range, followed up for 5 years, we reported that 33% of the participants reported EDS and that its natural course fluctuated significantly over time. Behavioral characteristics and changes in psychological, metabolic, and nighttime sleep patterns were associated with EDS remission and/or daytime sleepiness (DS) improvement and could be targets for interventions to reduce DS in the general adult population.
Introduction
Excessive daytime sleepiness (EDS) is a common symptom in the general population, with severity and duration greatly affecting its prevalence which ranges from 5% to 30% in several epidemiological studies [1–5]. EDS is not only the cardinal symptom of central disorders of hypersomnolence but also the primary concern for many subjects presenting with sleep disorders or other conditions. Several studies report that EDS is often associated with voluntary behaviors leading to poor sleep and sleep debt but also with metabolic, cardiovascular, neurological and psychiatric disorders, or side effects of medications [5–10]. The pathophysiology of EDS remains unclear, with a lack of reliable biological markers. EDS is often multifactorial, and is frequently associated with low quality of life, and an increased risk of accidents and injuries [11, 12]. EDS represents therefore a substantial cost burden to the health care system.
Despite its high prevalence and negative consequences, the natural history of EDS remains unclear. The few longitudinal studies indicate that the trajectories of EDS fluctuate over time with persistence rates around 35% for periods ranging from 5 to 7.5 years [1, 13, 14] and incidence rates ranging from 1.9% to 28% for 1- and 5-year follow-ups, respectively [1, 13–16]. This heterogeneity is partly due to the lack of a standard definition of EDS, with some studies asking subjects to estimate the severity of their daytime sleepiness (DS), others the number of days per week they reported EDS and others using the Epworth Sleepiness scale (ESS) to assess daytime sleep propensity. The ESS is a self-report questionnaire widely used to evaluate overall EDS in general and clinical populations; the subject is instructed to make a probability judgment about the expectation of “dozing” in eight different circumstances (e.g. sitting and reading). A score above 10 is considered to reflect clinically significant EDS [17].
Prospective studies aiming to identify individual predictors of these fluctuations are scarce. Overall, these studies found that young and older age, insomnia and depressive symptoms, chronic pain, lifestyle factors (e.g. lower coffee consumption, smoking), obesity and weight gain, and some medical conditions (diabetes mellitus and anemia) were risk factors for EDS [1, 13, 14, 18]. In contrast, only one prospective study exploring multiple potential factors associated with remitted EDS showed weight loss as the single significant predictor [13]. The understanding of these fluctuations is key to prevention, treatment, and policy decisions regarding current and future health care services. But to date, there is a lack of studies allowing the identification of factors associated with EDS improvement.
The aim of the present study was to document the rates of persistent, remitted, and intermittent EDS assessed by the ESS in a 5-year follow-up population-based study. We also sought to determine whether baseline socio-demographic and lifestyle characteristics, and time-dependent variables related to behavioral, psychological, metabolic, and nighttime sleep changes could predict DS improvement over time.
Methods
Study population
Subjects included were recruited as part of a larger epidemiological study aiming to assess prevalence and incidence, risk factors, natural history, and burden of insomnia in Canada. Detailed design and sampling procedures for this study have been reported elsewhere [19]. Briefly, subjects aged ≥18 years were recruited from a random selection of more than 12,000 subjects who completed a telephone interview about their sleep between 2007 and 2008. Participants were then asked if they wanted to take part in the longitudinal phase of the study, which involved completion of seven postal evaluations over a 5-year period: the baseline evaluation was sent 1 month after the telephone interview, the second at 6 months and the remaining evaluations scheduled every year. Overall, 3006 completed the baseline postal assessment, of which 82% participated at the 1-year and 71% at the 5-year follow-up. All participants provided written informed consent to participate in the study, prior to the study protocol and approved by the ethical committee of the Université Laval-Quebec, Canada. The methods in the current study were implemented in accordance with the approved guidelines.
Definition of EDS and its changes during the 5-year follow-up
EDS was assessed at baseline and at each follow-up wave (after 6 months, 1, 2, 3, 4, and 5 years) by the ESS. A total score >10 indicates a clinically significant EDS [17].
Subjects with EDS at baseline were further considered as persistent EDS cases when EDS persisted until the end of the follow-up, as remitted EDS cases in the absence of further EDS (i.e. ESS total score ≤10) over the follow-up, and as intermittent EDS cases for others (i.e. remission followed by reoccurrence of EDS).
A 4-point ESS total score reduction between two consecutive assessments was considered as the minimally significant difference, defining DS improvement. This 4-point change was already considered as clinically significant in previous randomized controlled trials on narcolepsy and central hypersomnias [20, 21]. However, a reduction of 4 points from a previous ESS total score below 8 was not taken into account as it was considered to be non-clinically significant (n = 74). Stable DS improvement was defined by an absence of further ESS total score increase in the subsequent follow-up (i.e. change of ≤ 3 points) and a transient DS improvement by ESS total score fluctuations (i.e. of > 3 points) during the follow-up.
Other sleep-related measures
At each evaluation, sleep measurements were self-reported by the participant. Sleep duration was divided into four categories: <6.0 h/night, between 6.0 and 6.9, 7.0 and 7.9, ≥8 [4]. The number of naps was recorded as none; <3 per week; ≥3 [4]. The severity of insomnia symptoms was evaluated using the Insomnia Severity Index (ISI) with higher scores suggesting more severe symptoms (score between 0 and 7: absence of insomnia; between 8 and 14: subthreshold insomnia; between 15 and 28 moderate-severe insomnia 15–28) [22].
Sleep medication included prescriptions and over-the-counter (OTC) drugs used in the previous month. They were classified as benzodiazepine (BZD), BZD-like compounds (zolpidem, zopiclone, zaleplon), antidepressants and miscellaneous medications (including barbiturates, OTC, and neuroleptics).
Socio-demographic, lifestyle, and health measures
Demographic characteristics, education level (categorized as “secondary level,” “degree below bachelor,” and “bachelor or above”), and marital status (single, divorced/separated or widowed; married, common-law couple) were assessed at baseline.
At baseline and at each follow-up, a standardized evaluation included self-reported measures related to the past month on daily life behavior such as alcohol consumption (none; 1 drink per day; ≥2 drinks), caffeine intake (none; 1–2 cups per day; ≥3), smoking status (never; present or past users), and physical activity of more than 30 min (never or less than one per week; between 1 and 3 per week, more than four-times per week); anthropometric data with height and weight to calculate body mass index (BMI) (classified as <25 kg/m2: normal; 25–30: overweight; ≥30: obese). The presence of diabetes, endocrine and metabolic disorders (e.g. cholesterol, dysthyroidism), hypertension, cardiovascular diseases, chronic pain, neurological diseases, and other nonspecific diseases (allergies, cancer, digestive, bone, lung, otorhinolaryngology, skin, urinary, or genital problems) was assessed by a list of 14 diseases, with additional self-reported conditions at baseline and at each follow-up, using the question “Currently, do you suffer from one or more of the following health problems?”
Depressive symptoms were evaluated using the Beck Depression Inventory II (BDI-II), with higher scores suggesting more severe depressive symptoms (≤13: minimal; 14–19: mild, 20–63: moderate-severe depression) [23].
Statistical analysis
Cox proportional hazard models with delayed entry and age of the participants as the time scale were used to estimate the hazard ratios (HR) and their 95% confidence intervals (CI) for the associations between subject characteristics and the probability of the first EDS remission. Proportional hazards assumptions were tested for baseline covariables. Participants with criteria for persistent EDS were taken as the reference category. For participants with criteria for EDS remission, age of event was taken to be the age at questionnaire completion. Participants without criteria for EDS remission were censored at the last follow-up visit. Variables associated at p < 0.15 in the univariate analysis and the ESS total score at baseline were included in the multivariate analysis to evaluate independent predictors associated with EDS remission. The 0.15 p-value cutoff was chosen based on the recommendations of several authors who argue that the 0.05 threshold can fail to identify variables known to be important [24]. In this study, several covariates (i.e. gender, level of education) were treated as time-constant, others as time-dependent covariates with multiple changes (i.e. behavioral, psychological, metabolic and other disorders, and nighttime sleep duration). For the latter covariates, the survival analysis was based on the last observation available before occurrence of the event. For continuous variables (Var) (e.g. sleep duration, ISI, Beck depression inventory score), a change was defined as the relative difference and was obtained as follows: (ΔR) = (Vart–Vart−1)/Vart−1. Instead of considering ΔR as a continuous variable, we chose to categorize ΔR into three classes to distinguish those with a significant gain from those with a significant loss. A significant gain was defined as the highest quartile of ΔR distribution (i.e. based on the relative differences between all two-by-two consecutive follow-up waves for all subjects regardless of any occurrence of EDS/DS improvement) and a significant loss as the lowest quartile of ΔR distribution. Values in the interquartile range were grouped together as the reference category. For categorical variables (e.g. changes in chronic pain status), an increase was defined as a change from a given category to the category just above, and a decrease to the category just below. A similar approach was used to study the predictors of (1) intermittent EDS vs. persistent EDS, (2) sustained DS improvement vs. no ESS total score change, and (3) transient DS improvement vs. no ESS total score change over the 5-year follow-up. Significance level was set at a two-sided p < 0.05. Analyses were performed using SAS-version 9.4 (SAS Inc., Cary, NC, USA).
Results
Subject characteristics
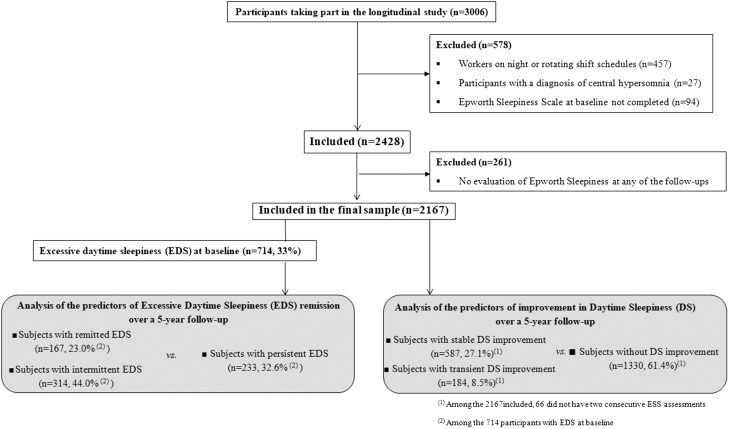
The study sample included 2,167 participants with a median baseline age of 51 years (range 18–89) of whom 64.1% were women, 97% were Caucasian, 40% lived alone, around one third had a secondary level of education (28.5%) and one third a bachelor degree or above (34.75%). As detailed in the flow-chart diagram (Figure 1), these subjects were free of central hypersomnia disorders (i.e. mainly narcolepsy and idiopathic hypersomnia), were day workers, and had completed the ESS at baseline and at least one of the five annual follow-up evaluations. Participants excluded from the study (N = 261) had a significantly lower educational level, were younger, and more likely to be current smokers and depressed. No significant differences were found for sleep duration, number of naps, insomnia, EDS, or associated chronic diseases.
Figure 1.
Flow chart diagram.
Regarding baseline sleep characteristics in the entire population, 714 (33%) had criteria for EDS at baseline (ESS total score >10), 13.1% slept less than 6 h per night, 13.7% took three naps or more during the week, and 16.1% had a moderate to severe level of insomnia (ISI > 14). Only 14.1% took sleep medications: 50.2% took BZD, 19.6% BZD-like compounds, 12.6% antidepressants, and 29.4 % OTC medication (23.8% antihistaminics and 5.6% melatonin). Furthermore, 9.4% had moderate to severe depressive symptoms (BDI ≥ 20), and 20.9% were obese.
Predictors of remission of EDS over a 5-year follow-up
Among the population of 714 subjects with criteria for EDS at baseline, 17.8% slept less than 6 h per night, 19.8% took three naps or more during the week, and 21.6% had a moderate to severe level of insomnia (ISI > 14). Only 10.8% took sleep medication as following: 47.4% took BZD, 14.7% BZD-like compounds, 21.3% took antidepressants, and 29.3% OTC medication (22.4% antihistaminics and 6.6% melatonin). Moreover, 13.3% had moderate to severe depressive symptoms (BDI ≥ 20), and 24.6% were obese.
Among the 714 baseline EDS subjects, 233 (33%) met criteria for persistent EDS, 167 (23%) for remitted EDS, and 314 (44%) for intermittent EDS improvement. The median onset of the first occurrence of remission was at 1.21 years of follow-up (range: 0.60–4.71).
In the univariate analysis, compared to subjects with persistent EDS, those with remitted EDS were more likely to have normal BMI, hypertension, change in insomnia severity, and depressive symptoms at the time of EDS remission, and were less likely to have chronic pain increase (Table 1). In the multivariate analysis including baseline ESS total score, and covariates associated with a p < 0.15, only having a normal weight, hypertension and changes in insomnia and depressive symptoms were independent predictors of remitted EDS (Table 2).
Table 1.
Estimated hazard ratios for potential predictors of the first episode of remitted excessive daytime sleepiness (EDS) compared to persistent EDS, and of the first episode of sustained daytime sleepiness (DS) improvement compared to those with a stable condition
| Variable | Persistent EDS N = 233 | Remitted EDS N = 167 | HR [95% CI]* | P-Value | No ESS change N = 1330 | Sustained DS improvement N = 587 | HR [95% CI]* | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||||
| Baseline characteristics | ||||||||||||
| Gender, women (vs. men) | 138 | 59.23 | 107 | 64.07 | 1.03 [0.74–1.43] | 0.85 | 849 | 63.83 | 383 | 65.25 | 1.04 [0.87–1.23] | 0.68 |
| Educational level | ||||||||||||
| Secondary level | 76 | 32.62 | 47 | 28.14 | 1 | 0.43 | 387 | 29.10 | 154 | 26.24 | 1 | 0.79 |
| Degree below bachelor | 82 | 35.19 | 65 | 38.92 | 1.26 [0.85–1.88] | 478 | 35.94 | 223 | 37.99 | 1.07 [0.87–1.32] | ||
| Bachelor or above | 75 | 32.19 | 55 | 32.93 | 1.04 [0.69–1.58] | 465 | 34.96 | 210 | 35.78 | 1.02 [0.82–1.26] | ||
| Living alone, Yes (vs. No) | 79 | 33.91 | 69 | 41.57 | 1.21 [0.88–1.67] | 0.24 | 514 | 38.65 | 248 | 42.32 | 1.19 [1.01–1.41] | 0.04 |
| Smoking status | ||||||||||||
| Not a smoker | 181 | 78.02 | 130 | 78.31 | 1 | 0.80 | 1055 | 79.38 | 468 | 79.73 | 1 | 0.96 |
| Current smoker | 37 | 15.95 | 28 | 16.87 | 1.08 [0.71–1.65] | 199 | 14.97 | 82 | 13.97 | 1.03 [0.81–1.30] | ||
| Occasional smoker | 14 | 6.03 | 8 | 4.82 | 0.83 [0.40–1.72] | 75 | 5.64 | 37 | 6.30 | 1.04 [0.74–1.45] | ||
| Coffee consumption | ||||||||||||
| No | 41 | 17.67 | 25 | 14.97 | 1 | 0.33 | 221 | 16.63 | 107 | 18.23 | 1 | 0.54 |
| One–two cups per day | 103 | 44.40 | 79 | 47.31 | 1.37 [0.85–2.21] | 637 | 47.93 | 266 | 45.32 | 0.93 [0.74–1.17] | ||
| Three or more cups per day | 88 | 37.93 | 63 | 37.72 | 1.45 [0.88–2.41] | 471 | 35.44 | 214 | 36.46 | 1.03 [0.81–1.31] | ||
| Alcohol consumption | ||||||||||||
| No | 176 | 77.19 | 123 | 75.00 | 0.84 [0.51–1.39] | 0.70 | 999 | 75.74 | 456 | 78.08 | 1.26 [0.93–1.71] | 0.32 |
| One drink per day | 28 | 12.28 | 23 | 14.02 | 0.96 [0.51–1.81] | 179 | 13.57 | 81 | 13.87 | 1.25 [0.87–1.80] | ||
| Two or more drinks per day | 24 | 10.53 | 18 | 10.98 | 1 | 141 | 10.69 | 47 | 8.05 | 1 | ||
| Physical activity | ||||||||||||
| ≥4 times per week | 63 | 27.16 | 41 | 24.55 | 0.88 [0.57–1.36] | 0.85 | 393 | 29.68 | 173 | 29.47 | 1.02 [0.81–1.28] | 0.90 |
| [1–3] times per week | 107 | 46.12 | 80 | 47.90 | 0.94 [0.64–1.36] | 621 | 46.90 | 287 | 48.89 | 1.05 [0.85–1.29] | ||
| < 1 per week/never | 62 | 26.72 | 46 | 27.54 | 1 | 310 | 23.41 | 127 | 21.64 | 1 | ||
| Hypnotic intake, Yes (vs. No) | 29 | 12.61 | 27 | 16.46 | 1.23 [0.81–1.88] | 0.33 | 206 | 15.69 | 64 | 11.00 | 0.71 [0.54–0.92] | 0.009 |
| Time-dependent covariates 1-year before the first EDS/DS improvement | ||||||||||||
| Sleep duration (h) | ||||||||||||
| ≥8 | 49 | 21.03 | 47 | 28.48 | 1.33 [0.81–2.17] | 0.07 | 412 | 31.07 | 169 | 28.89 | 0.93 [0.70–1.22] | 0.45 |
| [7–8] | 80 | 34.33 | 55 | 33.33 | 1.00 [0.62–1.61] | 482 | 36.35 | 197 | 33.68 | 0.87 [0.66–1.15] | ||
| [6–7] | 62 | 26.61 | 35 | 21.21 | 0.72 [0.43–1.21] | 281 | 21.19 | 145 | 24.79 | 1.03 [0.77–1.37] | ||
| <6 | 42 | 18.03 | 28 | 16.97 | 1 | 151 | 11.39 | 74 | 12.65 | 1 | ||
| Insomnia severity index | ||||||||||||
| <8 | 86 | 36.91 | 71 | 42.77 | 1.27 [0.83–1.95] | 0.10 | 781 | 58.77 | 268 | 45.73 | 0.52 [0.42–0.64] | <0.0001 |
| [8–14] | 100 | 42.92 | 60 | 36.14 | 0.87 [0.56–1.35] | 391 | 29.42 | 198 | 33.79 | 0.70 [0.55–0.88] | ||
| ≥15 | 47 | 20.17 | 35 | 21.08 | 1 | 157 | 11.81 | 120 | 20.48 | 1 | ||
| Beck depression Inventory score | ||||||||||||
| ≤13 | 165 | 70.82 | 125 | 75.30 | 1.41 [0.87–2.28] | 0.34 | 1141 | 85.79 | 452 | 77.00 | 0.57 [0.44–0.74] | <0.0001 |
| [14–19] | 21 | 9.01 | 20 | 12.05 | 1.20 [0.63–2.29] | 100 | 7.52 | 68 | 11.58 | 0.86 [0.61–1.21] | ||
| [20–63] | 47 | 20.17 | 21 | 12.65 | 1 | 89 | 6.69 | 67 | 11.41 | 1 | ||
| Body mass index (kg/m2) | ||||||||||||
| <25 | 83 | 35.62 | 84 | 50.30 | 1.65 [1.07–2.56] | 0.07 | 567 | 42.63 | 245 | 41.74 | 0.81 [0.66–1.00] | 0.13 |
| [25–30] | 78 | 33.48 | 55 | 32.93 | 1.55 [0.98–2.46] | 503 | 37.82 | 204 | 34.75 | 0.84 [0.68–1.05] | ||
| ≥30 | 72 | 30.90 | 28 | 16.77 | 1 | 260 | 19.55 | 138 | 23.51 | 1 | ||
| Chronic pain, Yes (vs. No) | 81 | 34.76 | 45 | 26.95 | 1.00 [0.71–1.40] | 0.98 | 311 | 23.42 | 163 | 27.82 | 1.23 [1.03–1.48] | 0.02 |
| Cardiovascular disease, Yes (vs. No) | 26 | 11.21 | 8 | 4.88 | 0.76 [0.36–1.59] | 0.47 | 126 | 9.61 | 41 | 7.06 | 0.99 [0.71–1.37] | 0.94 |
| Hypertension, Yes (vs. No) | 58 | 25.66 | 49 | 29.88 | 1.62 [1.10–2.40] | 0.02 | 347 | 26.65 | 127 | 22.32 | 0.99 [0.80–1.23] | 0.94 |
| Endocrine and metabolic disease, Yes (vs. No) | 58 | 25.00 | 36 | 21.69 | 1.10 [0.75–1.63] | 0.62 | 317 | 24.07 | 111 | 19.07 | 0.99 [0.80–1.22] | 0.92 |
| Diabetes, Yes (vs. No) | 23 | 9.96 | 11 | 6.71 | 0.93 [0.50–1.74] | 0.82 | 128 | 9.76 | 33 | 5.69 | 0.75 [0.52–1.07] | 0.11 |
| Neurological disease, Yes (vs. No) | 48 | 20.87 | 23 | 14.02 | 0.86 [0.55–1.36] | 0.52 | 183 | 13.96 | 81 | 13.94 | 1.20 [0.95–1.52] | 0.12 |
| Other diseases, Yes (vs. No) | 135 | 57.94 | 86 | 51.81 | 0.93 [0.67–1.28] | 0.65 | 619 | 46.61 | 278 | 47.52 | 1.10 [0.93–1.31] | 0.25 |
| Changes observed at the time of the first EDS/DS improvement | ||||||||||||
| Change in sleep duration | ||||||||||||
| % of decrease (−7.21%)† | 53 | 24.20 | 41 | 27.33 | 1.12 [0.73–1.73] | 0.49 | 307 | 23.36 | 143 | 24.96 | 1.16 [0.94–1.43] | 0.03 |
| No change | 104 | 47.49 | 65 | 43.33 | 1 | 723 | 55.02 | 281 | 49.04 | 1 | ||
| % of increase (+9.09%)† | 62 | 28.31 | 44 | 29.33 | 1.29 [0.85–1.94] | 284 | 21.61 | 149 | 26.00 | 1.31 [1.07–1.61] | ||
| Change in insomnia severity index | ||||||||||||
| % of decrease (−27.27%)† | 5 | 2.25 | 36 | 23.23 | 3.90 [2.50–6.08] | <0.0001 | 45 | 3.39 | 104 | 18.02 | 1.68 [1.33–2.11] | <0.0001 |
| No change | 190 | 85.59 | 94 | 60.65 | 1 | 1089 | 82.13 | 403 | 69.84 | 1 | ||
| % of increase (+33.33%)† | 27 | 12.16 | 25 | 16.13 | 1.69 [1.03–2.77] | 192 | 14.48 | 70 | 12.13 | 0.82 [0.63–1.06] | ||
| Change in Beck Depression Inventory score | ||||||||||||
| % of decrease (−33.33%)† | 44 | 19.73 | 56 | 36.36 | 1.64 [1.10–2.43] | 0.04 | 309 | 23.27 | 191 | 33.04 | 1.30 [1.07–1.57] | 0.0004 |
| No change | 129 | 57.85 | 66 | 42.86 | 1 | 661 | 49.77 | 278 | 48.10 | 1 | ||
| % of increase (+42.86%)† | 50 | 22.42 | 32 | 20.78 | 1.10 [0.70–1.72] | 358 | 26.96 | 109 | 18.86 | 0.81 [0.65–1.02] | ||
| Change in body mass index | ||||||||||||
| % of decrease (−2.44%)† | 57 | 25.68 | 38 | 25.00 | 0.88 [0.58–1.33] | 0.41 | 299 | 22.62 | 148 | 25.56 | 1.18 [0.96–1.45] | 0.29 |
| % of no change | 117 | 52.70 | 83 | 54.61 | 1 | 721 | 54.54 | 297 | 51.30 | 1 | ||
| % of increase (+3.23%)† | 48 | 21.62 | 31 | 20.39 | 0.74 [0.47–1.16] | 302 | 22.84 | 134 | 23.14 | 1.07 [0.87–1.32] | ||
| Change in chronic pain | ||||||||||||
| Decrease | 25 | 11.21 | 14 | 9.21 | 0.74 [0.40–1.38] | 0.05 | 99 | 7.47 | 66 | 11.46 | 1.40 [1.07–1.83] | 0.03 |
| No change | 173 | 77.58 | 131 | 86.18 | 1 | 1114 | 84.08 | 454 | 78.82 | 1 | ||
| Increase | 25 | 11.21 | 7 | 4.61 | 0.38 [0.17–0.87] | 112 | 8.45 | 56 | 9.72 | 1.20 [0.90–1.61] | ||
DS, daytime sleepiness; EDS, Excessive Daytime Sleepiness; ESS: Epworth Severity Scale.
*Adjusted for age.
†For a given variable, the numbers in brackets correspond to the lowest (for decrease) or the highest quartile (for increase) of all relative differences between two consecutives evaluations.
Table 2.
Multivariate proportional hazards model of potential predictors of remitted EDS and sustained DS improvement
| Variable | Remitted EDS vs. persistent EDS | Sustained of DS improvement vs. No ESS change | ||
|---|---|---|---|---|
| HR [95% CI]* | P-Value | HR [95% CI]* | P-Value | |
| Living alone, Yes | 1.22 [1.04–1.45] | 0.02 | ||
| Hypnotic intake during the last month, Yes | 0.79 [0.60–1.03] | 0.08 | ||
| Sleep duration (h) | ||||
| ≥8 | 0.98 [0.52–1.83] | 0.19 | ||
| [6–8] | 0.80 [0.44–1.47] | |||
| [6–8] | 0.57 [0.30–1.07] | |||
| <6 | 1 | |||
| Body mass index (kg/m2) | ||||
| <25 | 1.81 [1.08–3.03] | 0.04 | 0.97 [0.78–1.21] | 0.34 |
| [25–30] | 1.13 [0.66–1.94] | 0.86 [0.69–1.08] | ||
| ≥30 | 1 | 1 | ||
| Hypertension, Yes (vs. No) | 1.98 [1.20–3.27] | 0.007 | ||
| Diabetes, Yes (vs. No) | 0.94 [0.70–1.26] | 0.66 | ||
| Neurological disease, Yes (vs. No) | 1.10 [0.89–1.37] | 0.39 | ||
| Change in sleep duration | ||||
| % of decrease (−7.21%)† | 1.07 [0.87–1.31] | 0.41 | ||
| No change | 1 | |||
| % of increase (+9.09%)† | 1.15 [0.93–1.41] | |||
| Change in insomnia severity index | ||||
| % of decrease (−27.27%)† | 3.49 [2.10–5.81] | <0.0001 | 1.23 [0.65–2.32] | 0.49 |
| No change | 1 | 1 | ||
| % of increase (+33.33%)† | 1.79 [1.03–3.11] | 1.13 [0.90–1.43] | ||
| Change in Beck Depression Inventory score | ||||
| % of decrease (−33.33%)† | 1.69 [1.09–2.61] | 0.04 | 1.08 [0.88–1.33] | 0.77 |
| No change | 1 | 1 | ||
| % of increase (+42.86%)† | 1.07 [0.65–1.75] | 1.02 [0.84–1.25] | ||
| Change in chronic pain | ||||
| Decrease | 0.97 [0.50–1.89] | 0.23 | 1.25 [0.94–1.66] | 0.24 |
| No change | 1 | 1 | ||
| Increase | 0.47 [0.20–1.11] | 1.14 [0.87–1.50] | ||
DS, Daytime sleepiness; EDS, Excessive Daytime Sleepiness; ESS, Epworth Severity Scale.
*Variables associated at p < 0.15 in univariate analysis (Table 1) at baseline were included in the model and were adjusted for baseline ESS score and age.
†For a given variable, the numbers in brackets correspond to the lowest (for decrease) or the highest quartile (for increase) of all relative differences between two consecutives evaluations.
In the univariate analysis, compared to subjects with persistent EDS, those with intermittent EDS, took less hypnotics, had less nonspecific comorbid diseases and changes in insomnia symptoms over the follow-up (Table 3). Multivariate analysis showed that taking less hypnotics and reporting decrease in insomnia symptoms were independent predictors of intermittent EDS (Table 4).
Table 3.
Estimated hazard ratio for potential predictors of the first episode of intermittent excessive daytime sleepiness (EDS) compared to persistent EDS, and of the first episode of transient daytime sleepiness (DS) decrease compared to those with a stable condition
| Variable | Persistent EDS N = 233EDS | Intermittent N = 314 | HR [95% CI]* | P-Value | No ESS change N = 1330 | Transient DS improvement N = 184 | HR [95% CI]* | P-Value | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | n | % | |||||
| Baseline characteristics | ||||||||||||
| Gender, women | 138 | 59.23 | 202 | 64.33 | 1.07 [0.84–1.36] | 0.59 | 849 | 63.83 | 118 | 64.13 | 1.00 [0.74–1.35] | 0.99 |
| Educational level | ||||||||||||
| Secondary level | 76 | 32.62 | 80 | 25.48 | 1 | 0.59 | 387 | 29.10 | 61 | 33.15 | 1 | 0.12 |
| Degree below bachelor | 82 | 35.19 | 120 | 38.22 | 1.17 [0.87–1.57] | 478 | 35.94 | 67 | 36.41 | 0.81 [0.57–1.16] | ||
| Bachelor or above | 75 | 32.19 | 114 | 36.31 | 1.12 [0.83–1.51] | 465 | 34.96 | 56 | 30.43 | 0.68 [0.47–0.98] | ||
| Living alone, Yes (vs. No) | 79 | 33.91 | 124 | 39.49 | 1.19 [0.94–1.50] | 0.14 | 514 | 38.65 | 76 | 41.30 | 1.19 [0.88–1.60] | 0.27 |
| Smoking status | ||||||||||||
| Not a smoker | 181 | 78.02 | 253 | 80.57 | 1 | 0.91 | 1055 | 79.38 | 137 | 74.46 | 1 | 0.05 |
| Current smoker | 37 | 15.95 | 46 | 14.65 | 0.95 [0.69–1.31] | 199 | 14.97 | 38 | 20.65 | 1.54 [1.07–2.22] | ||
| Occasional smoker | 14 | 6.03 | 15 | 4.78 | 0.92 [0.54–1.56] | 75 | 5.64 | 9 | 4.89 | 0.86 [0.43–1.69] | ||
| Coffee consumption | ||||||||||||
| No | 41 | 17.67 | 52 | 16.56 | 1 | 0.54 | 221 | 16.63 | 37 | 20.22 | 1 | 0.39 |
| One–two cups per day | 103 | 44.40 | 146 | 46.50 | 1.20 [0.86–1.65] | 637 | 47.93 | 76 | 41.53 | 0.82 [0.55–1.23] | ||
| Three or more cups per day | 88 | 37.93 | 116 | 36.94 | 1.18 [0.84–1.67] | 471 | 35.44 | 70 | 38.25 | 1.01 [0.66–1.53] | ||
| Alcohol consumption | ||||||||||||
| No | 176 | 77.19 | 234 | 74.76 | 0.86 [0.59–1.23] | 0.36 | 999 | 75.74 | 140 | 76.92 | 1.00 [0.61–1.65] | 0.99 |
| One drink per day | 28 | 12.28 | 45 | 14.38 | 1.05 [0.67–1.66] | 179 | 13.57 | 24 | 13.19 | 0.97 [0.52–1.79] | ||
| Two or more drinks per day | 24 | 10.53 | 34 | 10.86 | 1 | 141 | 10.69 | 18 | 9.89 | 1 | ||
| Physical activity | ||||||||||||
| ≥4 times per week | 63 | 27.16 | 76 | 24.20 | 0.92 [0.66–1.27] | 0.78 | 393 | 29.68 | 45 | 24.46 | 0.70 [0.47–1.05] | 0.22 |
| [1–3] times per week | 107 | 46.12 | 163 | 51.91 | 1.01 [0.77–1.34] | 621 | 46.90 | 90 | 48.91 | 0.85 [0.60–1.20] | ||
| < 1 per week/never | 62 | 26.72 | 75 | 23.89 | 1 | 310 | 23.41 | 49 | 26.63 | 1 | ||
| Hypnotic intake, Yes (vs. No) | 29 | 12.61 | 20 | 6.49 | 0.58 [0.36–0.92] | 0.02 | 206 | 15.69 | 24 | 13.04 | 0.84 [0.55–1.30] | 0.44 |
| Time-dependent covariates 1-year before the first intermittent EDS/Transient DS improvement | ||||||||||||
| Sleep duration (h) | ||||||||||||
| ≥8 | 49 | 21.03 | 72 | 23.30 | 1.23 [0.83–1.81] | 0.37 | 412 | 31.07 | 40 | 21.98 | 0.48 [0.30–0.76] | 0.007 |
| [7–8] | 80 | 34.33 | 123 | 39.81 | 1.23 [0.86–1.75] | 482 | 36.35 | 59 | 32.42 | 0.57 [0.37–0.87] | ||
| [6–7] | 62 | 26.61 | 71 | 22.98 | 0.98 [0.67–1.44] | 281 | 21.19 | 47 | 25.82 | 0.77 [0.50–1.20] | ||
| <6 | 42 | 18.03 | 43 | 13.92 | 1 | 151 | 11.39 | 36 | 19.78 | 1 | ||
| Insomnia severity index | ||||||||||||
| <8 | 86 | 36.91 | 141 | 44.90 | 1.14 [0.85–1.54] | 0.03 | 781 | 58.77 | 69 | 37.50 | 0.33 [0.23–0.47] | <0.0001 |
| [8–14] | 100 | 42.92 | 110 | 35.03 | 0.81 [0.59–1.12] | 391 | 29.42 | 64 | 34.78 | 0.55 [0.38–0.80] | ||
| ≥15 | 47 | 20.17 | 63 | 20.06 | 1 | 157 | 11.81 | 51 | 27.72 | 1 | ||
| Beck Depression Inventory score | ||||||||||||
| ≤13 | 165 | 70.82 | 234 | 74.52 | 1.35 [0.94–1.94] | 0.26 | 1141 | 85.79 | 122 | 66.30 | 0.34 [0.23–0.51] | <0.0001 |
| [14–19] | 21 | 9.01 | 45 | 14.33 | 1.35 [0.86–2.13] | 100 | 7.52 | 28 | 15.22 | 0.74 [0.44–1.24] | ||
| [20–63] | 47 | 20.17 | 35 | 11.15 | 1 | 89 | 6.69 | 34 | 18.48 | 1 | ||
| Body mass index (kg/m2) | ||||||||||||
| <25 | 83 | 35.62 | 122 | 38.98 | 1.01 [0.76–1.33] | 0.85 | 567 | 42.63 | 80 | 43.48 | 0.78 [0.54–1.12] | 0.23 |
| [25–30] | 78 | 33.48 | 107 | 34.19 | 1.08 [0.80–1.44] | 503 | 37.82 | 58 | 31.52 | 0.72 [0.49–1.06] | ||
| ≥30 | 72 | 30.90 | 84 | 26.84 | 1 | 260 | 19.55 | 46 | 25.00 | 1 | ||
| Chronic pain, Yes (vs. No) | 87 | 37.34 | 88 | 28.03 | 0.85 [0.66–1.10] | 0.21 | 311 | 23.42 | 52 | 28.26 | 1.30 [0.94–1.80] | 0.11 |
| Cardiovascular disease, Yes (vs. No) | 26 | 11.21 | 23 | 7.37 | 1.09 [0.70–1.71] | 0.71 | 126 | 9.61 | 11 | 6.01 | 0.93 [0.50–1.73] | 0.81 |
| Hypertension, Yes (vs. No) | 58 | 25.66 | 59 | 19.22 | 0.87 [0.64–1.18] | 0.38 | 347 | 26.65 | 52 | 28.89 | 1.52 [1.07–2.16] | 0.02 |
| Endocrine and metabolic disease, Yes (vs. No) | 58 | 25.00 | 55 | 17.63 | 0.87 [0.65–1.18] | 0.38 | 317 | 24.07 | 43 | 23.50 | 1.37 [0.96–1.97] | 0.08 |
| Diabetes, Yes (vs. No) | 23 | 9.96 | 23 | 7.37 | 0.93 [0.60–1.44] | 0.75 | 128 | 9.76 | 19 | 10.38 | 1.47 [0.90–2.39] | 0.13 |
| Neurological disease, Yes (vs. No) | 48 | 20.87 | 44 | 14.10 | 0.83 [0.60–1.16] | 0.28 | 183 | 13.96 | 33 | 18.03 | 1.54 [1.06–2.26] | 0.02 |
| Other diseases, Yes (vs. No) | 140 | 60.09 | 143 | 45.69 | 0.78 [0.62–0.98] | 0.04 | 619 | 46.61 | 92 | 50.00 | 1.28 [0.95–1.73] | 0.11 |
| Changes observed at the time of the first intermittent EDS/transient DS improvement | ||||||||||||
| Change in sleep duration | ||||||||||||
| % of decrease (−7.21%)† | 53 | 24.20 | 59 | 20.27 | 0.81 [0.59–1.11] | 0.38 | 307 | 23.36 | 41 | 23.16 | 1.19 [0.80–1.77] | <0.0001 |
| No change | 104 | 47.49 | 153 | 52.58 | 1 | 723 | 55.02 | 66 | 37.29 | 1 | ||
| % of increase (+9.09%)† | 62 | 28.31 | 79 | 27.15 | 0.99 [0.74–1.33] | 284 | 21.61 | 70 | 39.55 | 2.28 [1.62–3.20] | ||
| Change in insomnia severity index | ||||||||||||
| % of decrease (−27.27%)† | 5 | 2.25 | 67 | 22.64 | 3.11 [2.26–4.27] | <0.0001 | 45 | 3.39 | 24 | 13.41 | 1.20 [0.76–1.90] | 0.02 |
| No change | 190 | 85.59 | 176 | 59.46 | 1 | 1089 | 82.13 | 141 | 78.77 | 1 | ||
| % of increase (+33.33%)† | 27 | 12.16 | 53 | 17.91 | 1.61 [1.16–2.24] | 192 | 14.48 | 14 | 7.82 | 0.46 [0.26–0.82] | ||
| Change in Beck Depression Inventory score | ||||||||||||
| % of decrease (−33.33%)† | 44 | 19.73 | 91 | 30.64 | 1.29 [0.97–1.73] | 0.21 | 309 | 23.27 | 75 | 41.44 | 1.73 [1.24–2.43] | 0.002 |
| No change | 129 | 57.85 | 139 | 46.80 | 1 | 661 | 49.77 | 71 | 39.23 | 1 | ||
| % of increase (+42.86%)† | 50 | 22.42 | 67 | 22.56 | 1.06 [0.77–1.44] | 358 | 26.96 | 35 | 19.34 | 1.02 [0.68–1.52] | ||
| Change in body mass index | ||||||||||||
| % of decrease (−2.44%)† | 57 | 25.68 | 71 | 24.07 | 1.04 [0.77–1.41] | 0.97 | 299 | 22.62 | 50 | 27.93 | 1.28 [0.90–1.81] | 0.09 |
| % of no change | 117 | 52.70 | 143 | 48.47 | 1 | 721 | 54.54 | 96 | 53.63 | 1 | ||
| % of increase (+3.23%)† | 48 | 21.62 | 81 | 27.46 | 1.01 [0.75–1.36] | 302 | 22.84 | 33 | 18.44 | 0.78 [0.52–1.16] | ||
| Change in chronic pain status | ||||||||||||
| Decrease | 25 | 11.21 | 26 | 8.78 | 0.76 [0.50–1.16] | 0.09 | 99 | 7.47 | 14 | 7.82 | 0.76 [0.43–1.35] | 0.59 |
| No change | 173 | 77.58 | 247 | 83.45 | 1 | 1114 | 84.08 | 148 | 82.68 | 1 | ||
| Increase | 25 | 11.21 | 23 | 7.77 | 0.64 [0.40–1.01] | 112 | 8.45 | 17 | 9.50 | 1.09 [0.65–1.83] | ||
DS, daytime sleepiness; EDS, Excessive Daytime Sleepiness; ESS: Epworth Severity Scale.
*Adjusted for age.
†For a given variable, the numbers in brackets correspond to the lowest (for decrease) or the highest quartile (for increase) of all relative differences between two consecutives evaluations.
Table 4.
Multivariate proportional hazards model of potential predictors to intermittent excessive daytime sleepiness (EDS) and transient daytime sleepiness (DS) improvement
| Variable | Intermittent EDS vs. persistent EDS | Transient DS improvement vs. No ESS change | ||
|---|---|---|---|---|
| HR [95% CI]* | P-Value | HR [95% CI]* | P-Value | |
| Educational level | ||||
| Secondary level | 1 | 0.93 | ||
| Degree below bachelor | 0.98 [0.66–1.44] | |||
| Bachelor or above | 0.84 [0.55–1.26] | |||
| Living alone, Yes | 1.09 [0.83–1.42] | 0.55 | ||
| Hypnotic intake during the last month, Yes (vs. No) | 0.47 [0.27–0.80] | 0.006 | ||
| Smoking status | ||||
| Not a smoker | 1 | 0.16 | ||
| Current smoker | 1.50 [0.99–2.27] | |||
| Occasional smoker | 1.02 [0.49–2.13] | |||
| Chronic pain, Yes (vs. No) | 0.86 [0.60–1.25] | 0.43 | ||
| Hypertension, Yes (vs. No) | 1.11 [0.75–1.64] | 0.61 | ||
| Endocrine and metabolic disease, Yes (vs. No) | 1.17 [0.78–1.74] | 0.45 | ||
| Diabetes, Yes (vs. No) | 1.29 [0.75–2.22] | 0.37 | ||
| Neurological disease, Yes (vs. No) | 1.13 [0.75–1.72] | 0.56 | ||
| Other diseases, Yes (vs. No) | 0.90 [0.68–1.18] | 0.43 | 1.18 [0.85–1.65] | 0.33 |
| Change in sleep duration | ||||
| % of decrease (−7.21%)† | 1.14 [0.75–1.74] | 0.0007 | ||
| No change | 1 | |||
| % of increase (+9.09%)† | 1.96 [1.37–2.81] | |||
| Change in insomnia severity index | ||||
| % of decrease (−27.27%)† | 2.73 [1.95–3.82] | <0.0001 | 2.10 [0.98–4.49] | 0.11 |
| No change | 1 | 1 | ||
| % of increase (+33.33%)† | 1.30 [0.92–1.83] | 1.91 [1.01–3.59] | ||
| Change in Beck Depression Inventory score | ||||
| % of decrease (−33.33%)† | 1.86 [1.30–2.65] | 0.002 | ||
| No change | 1 | |||
| % of increase (+42.86%)† | 1.11 [0.72–1.70] | |||
| Change in body mass index | ||||
| % of decrease (−2.44%)† | 1.12 [0.77–1.62] | 0.13 | ||
| % of no change | 1 | |||
| % of increase (+3.23%)† | 0.70 [0.46–1.06] | |||
| Change in chronic pain | ||||
| Decrease | 0.82 [0.53–1.28] | 0.46 | ||
| No change | 1 | |||
| Increase | 0.78 [0.48–1.28] | |||
DS, daytime sleepiness; EDS, Excessive Daytime Sleepiness; ESS, Epworth Severity Scale.
*Variables associated at p < 0.15 in univariate analysis (Table 3) at baseline were included in the model and were adjusted for baseline ESS score and age.
†For a given variable, the numbers in brackets correspond to the lowest (for decrease) or the highest quartile (for increase) of all relative differences between two consecutives evaluations.
Predictors of improvement of DS over a 5-year follow-up
Among the 2,167 participants at baseline, 1,330 (61.4%) had criteria for a stable DS (<4-point change on ESS total score over time), 587 (27.1%) for a sustained improvement in DS, and 184 (8.5%) for a transient improvement in DS (Figure 1). The median delay of the first occurrence of decrease was 2.06 years (range: 0.34–4.70).
In the univariate analysis, compared to subjects with stable ESS total score, those with sustained DS improvement were more likely to live alone, take less hypnotics, and have more insomnia depressive and pain symptoms 1-year before the DS improvement but increased changes in severity symptoms at the time of DS improvement. They also had increase in sleep duration at the time of DS improvement (Table 1). Multivariate analysis showed that living alone with a trend for less hypnotic intake (p = 0.08) favored sustained DS improvement (Table 2).
Compared to subjects with a stable ESS total score, those with a transient DS improvement were more likely to be current smokers, had more hypertension and neurological diseases, slept less at night, had higher insomnia and depressive symptoms with higher changes at the time of DS improvement (Table 3). In multivariate analysis, an increase in nighttime sleep duration and a decrease in depressive symptoms were independently associated with a transient DS improvement (Table 4).
Discussion
This study examined the rates and risk factors of persistent, remitted and intermittent EDS, and stable and transient improvement in DS over a 5-year follow-up in a large population-based cohort study. Among the 33% of the participants with criteria for EDS, 33% had persistent EDS, 44% intermittent EDS, and 23% remitted EDS. Among the whole sample, 61.4% had stable DS (ESS total score change of < 4-point), 27.1% sustained DS and 8.5% transient DS improvement over the 5-year follow-up. The main predictors for remission of EDS or DS improvement were normal weight, taking less hypnotic, having hypertension, increased nighttime sleep duration, and decreased insomnia and depressive symptoms.
Our results confirmed that EDS follows a waxing and waning pattern in its natural course, and is often an unstable condition in the general adult population [1, 13]. Using the same criteria for EDS (ESS total score >1017) and DS (change of ≥ 4-points on the ESS; a clinically relevant change often used as the primary endpoint in pharmacological studies on central hypersomnias [20, 21]), we previously reported within the same cohort that 28% of subjects developed incident EDS, and 31% increased DS over time [1]. This unstable state of sleepiness has also been highlighted by a study over a 7.5-year follow-up, classifying participants as excessive daytime sleepers using two single questions referring to feelings of sleepiness/tiredness and/or irresistible sleep attacks, instead of using ESS [13]. They found a prevalence of EDS of 15.9%, an incidence around 8%, a persistence rate of 38%, and a remission rate of 62%.
The waxing and waning of self-reported EDS appears to be consistent across different studies with variable populations and designs. Such fluctuation in self-reported EDS sounds like a striking result, however it has only rarely been formally studied. In this study, we examined this phenomenon by comparing subjects exhibiting a remittent/intermittent EDS pattern to those with stable conditions over the 5-year follow-up. As main predictors, we found that having normal weight, hypertension and decreasing depressive symptoms were associated with remission of EDS whereas taking less hypnotics and increasing nighttime sleep duration were associated with intermittent EDS, and decreasing insomnia symptoms were related to both. To the best of our knowledge, this is the first study to report such findings. A previous study already reported that obesity and weight gain predicted incident EDS while weight loss was associated with its remission in the general population [13]. The low proportion of participants with obesity (around 25% of participants with EDS at baseline) in our sample and the relative low BMI changes during the follow-up could explain the absence of significant effect of weight loss. However, we reported that a normal BMI was an independent predictor of remitted EDS as only non-obese subjects were at risk of normalizing EDS. Several other clinical factors such as depression, diabetes, and allergy/asthma were reported as clinical predictors of incident EDS, but were not associated with remission of EDS [13]. We also found other risk factors associated with a sustained improvement of DS: living alone, taking less hypnotic. In contrast, an increase in nighttime sleep duration and having less depressive symptoms were independently associated with a transient improvement in DS. Most of these factors share some similarities between the different conditions associated with EDS or DS improvement that further underline the complex relationships between nighttime sleep disturbances, hypertension, depressive symptoms, and DS [25]. These findings should be further confirmed using objective daytime and nighttime sleep measures. One study previously reported that objective sleep disturbances were associated with incident EDS in depressed subjects [13]; however to our knowledge such association was not reported for remitted EDS.
A healthy lifestyle with no sleep deprivation, no metabolic, cardiovascular, or neurological diseases without hypnotic intake strengthened the potential to decrease the levels of DS. Of interest, some of these factors (i.e. depressive and insomnia symptoms) have already been identified as risk factors for incident EDS or DS increase; this is in keeping with a mirror image of EDS risk and protective factors. Moreover, most of them were potentially modifiable factors by means of pharmacologic and behavioral treatments. Indeed, improving insomnia and depressive symptoms, taking less hypnotics, and increasing nighttime sleep duration may be our priority in terms of public health policies to decrease EDS and the levels of DS in the general adult population. Policy decisions proposing interventions specifically targeting non-obese populations may be more successful at reducing the risk of low academic or professional performance, road, domestic or work accidents [26, 27], and morbi-mortality related to cardiovascular and neurodegenerative pathologies [28, 29].
The strengths of this study were the prospective design, the sample size, the large number of potential predictors including socio-demographic, lifestyle, and health characteristics, some of which were evaluated according to standardized scales using in clinical practice. The study also had in-depth yearly follow-ups over 5 years which enabled us to take into account covariates that changed over time. The evaluation of EDS and DS changes was assessed using the ESS which is the most commonly used tool in sleep research and clinical setting.
This study had some limitations. Selection bias inherent in all epidemiological studies may exist even if precautions were taken to have a representative sample of the Canadian population. The data were exclusively self-reported causing potentially recall bias and a lack of accuracy in responses. Even if the ESS and its threshold have been largely validated in the general population, its measurement remains subjective with some potential for overlap with fatigue symptom, despite being of different pathophysiology and etiology. Objective measurements of sleep quality and EDS evaluated by polysomnography and Multiple Sleep Latency Test were not available; however, performing such tests is very difficult within the context of a large epidemiological survey. Moreover, there are little or no correlations between ESS total scores and objective mean sleep latencies obtained either in patients suffering from sleep pathologies or in the general population [30].
In conclusion, EDS is a frequent waxing and waning condition with a high occurrence of remission in the general adult population. The main factors associated with these outcomes were identifiable and potentially modifiable characteristics such as nighttime sleep duration, insomnia and depressive symptoms, hypnotic intake, and overweight/obesity. Further studies should examine whether targeted interventions may decrease the levels of DS and potentially related outcomes in the general population.
Acknowledgments
The authors thank Joanna Norton for her careful review of the English.
Funding
This study was supported by a Canadian Institutes of Health Research grant (# 42504). Charles M Morin received research support from Idorsia.
Conflict of interest statement. C.M.M. has served on advisory board for Abbott, Merck, and Phillips. Y.D. has received funds for seminars, board engagements and travel to conferences by UCB Pharma, Jazz, Theranexus, Flamel, and Bioprojet.
Authors’ contributions
I.J., C.M., and Y.D. participated in the conception and design of the study. I.J. conducted the analyses and wrote the first draft of the manuscript. I.J., C.M., and Y.D. participated in the interpretation of the data. I.J., C.M., and Y.D. contributed to the writing of the manuscript. C.M., and H.I. participated in the acquisition of the data.
References
- 1. Jaussent I, et al.. Incidence, worsening and risk factors of daytime sleepiness in a population-based 5-year longitudinal study. Sci Rep. 2017;7(1):1372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Joo S, et al.. Prevalence of excessive daytime sleepiness and associated factors in the adult population of Korea. Sleep Med. 2009;10(2):182–188. [DOI] [PubMed] [Google Scholar]
- 3. Klink M, et al.. Prevalence of reported sleep disturbances in a general adult population and their relationship to obstructive airways diseases. Chest. 1987;91(4): 540–546. [DOI] [PubMed] [Google Scholar]
- 4. Ohayon MM, et al.. Operational definitions and algorithms for excessive sleepiness in the general population: implications for DSM-5 nosology. Arch Gen Psychiatry. 2012;69(1):71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Tsuno N, et al.. Determinants of excessive daytime sleepiness in a French community-dwelling elderly population. J Sleep Res. 2007;16(4):364–371. [DOI] [PubMed] [Google Scholar]
- 6. Akbaraly TN, et al.. Sleep complaints and metabolic syndrome in an elderly population: the Three-City Study. Am J Geriatr Psychiatry. 2015;23(8):818–828. [DOI] [PubMed] [Google Scholar]
- 7. Bixler EO, et al.. Excessive daytime sleepiness in a general population sample: the role of sleep apnea, age, obesity, diabetes, and depression. J Clin Endocrinol Metab. 2005;90(8):4510–4515. [DOI] [PubMed] [Google Scholar]
- 8. Empana JP, et al.. Excessive daytime sleepiness is an independent risk indicator for cardiovascular mortality in community-dwelling elderly: the three city study. Stroke. 2009;40(4):1219–1224. [DOI] [PubMed] [Google Scholar]
- 9. Ohayon MM, et al.. Daytime sleepiness and cognitive impairment in the elderly population. Arch Intern Med. 2002;162(2):201–208. [DOI] [PubMed] [Google Scholar]
- 10. Vermeeren A. Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs. 2004;18(5): 297–328. [DOI] [PubMed] [Google Scholar]
- 11. Connor J, et al.. Driver sleepiness and risk of serious injury to car occupants: population based case control study. BMJ. 2002;324(7346):1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohayon MM. From wakefulness to excessive sleepiness: what we know and still need to know. Sleep Med Rev. 2008;12(2):129–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Fernandez-Mendoza J, et al.. Natural history of excessive daytime sleepiness: role of obesity, weight loss, depression, and sleep propensity. Sleep. 2015;38(3):351–360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Theorell-Haglöw J, et al.. Predictors for development of excessive daytime sleepiness in women: a population-based 10-year follow-up. Sleep. 2015;38(12):1995–2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Breslau N, et al.. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39(6):411–418. [DOI] [PubMed] [Google Scholar]
- 16. Ford DE, et al.. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? JAMA. 1989;262(11):1479–1484. [DOI] [PubMed] [Google Scholar]
- 17. Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14(6): 540–545. [DOI] [PubMed] [Google Scholar]
- 18. Hasler G, et al.. Excessive daytime sleepiness in young adults: a 20-year prospective community study. J Clin Psychiatry. 2005;66(4):521–529. [DOI] [PubMed] [Google Scholar]
- 19. Morin CM, et al.. The natural history of insomnia: a population-based 3-year longitudinal study. Arch Intern Med. 2009;169(5):447–453. [DOI] [PubMed] [Google Scholar]
- 20. Dauvilliers Y, et al. Pitolisant versus placebo or modafinil in patients with narcolepsy: a double-blind, randomised trial. Lancet Neurol. 2013;12(11):1068–1075. [DOI] [PubMed] [Google Scholar]
- 21. US Modafinil in Narcolepsy Multicenter Study Group. Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy: US Modafinil in Narcolepsy Multicenter Study Group. Neurology 2000;54:1166–1175. [DOI] [PubMed] [Google Scholar]
- 22. Bastien CH, et al.. Validation of the Insomnia Severity Index as an outcome measure for insomnia research. Sleep Med. 2001;2(4):297–307. [DOI] [PubMed] [Google Scholar]
- 23. Beck AT, et al. Inventaire de dépression de Beck-deuxième édition. Toronto, Ontario: Pyschological Corporation ed;1996. [Google Scholar]
- 24. Hosmer D, et al. Applied Logistic Regression, 2nd ed. Hoboken, NJ: Wiley Inter-science, 2000. [Google Scholar]
- 25. Roehrs T, et al.. Sleep and pain: interaction of two vital functions. Semin Neurol. 2005;25(1):106–116. [DOI] [PubMed] [Google Scholar]
- 26. Philip P, et al.. Sleep disorders and accidental risk in a large group of regular registered highway drivers. Sleep Med. 2010;11(10):973–979. [DOI] [PubMed] [Google Scholar]
- 27. Ohayon MM, et al.. Excessive sleep duration and quality of life. Ann Neurol. 2013;73(6):785–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Jaussent I, et al.. Excessive sleepiness is predictive of cognitive decline in the elderly. Sleep. 2012;35(9): 1201–1207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Jaussent I, et al.. Insomnia, daytime sleepiness and cardio-cerebrovascular diseases in the elderly: a 6-year prospective study. PLoS One. 2013;8(2):e56048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kendzerska TB, et al.. Evaluation of the measurement properties of the Epworth sleepiness scale: a systematic review. Sleep Med Rev. 2014;18(4):321–331. [DOI] [PubMed] [Google Scholar]