Abstract
Background
Milk feedings can be given via nasogastric tube either intermittently, typically over 10 to 20 minutes every two or three hours, or continuously, using an infusion pump. Although theoretical benefits and risks of each method have been proposed, effects on clinically important outcomes remain uncertain.
Objectives
To examine the evidence regarding the effectiveness of continuous versus intermittent bolus nasogastric milk feeding in premature infants less than 1500 grams.
Search methods
Searches were performed of the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2011), MEDLINE, CINAHL and HealthSTAR up to July 2011.
Selection criteria
Randomised and quasi‐randomised clinical trials comparing continuous versus intermittent bolus nasogastric milk feeding in premature infants less than 1500 grams.
Data collection and analysis
Two review authors independently assessed all trials for relevance and methodologic quality. The standard methods of the Cochrane Neonatal Review Group were used to extract data.
Main results
Overall, the seven included trials, involving 511 infants, found no differences in time to achieve full enteral feeds between feeding methods (weighted mean difference (WMD) 2 days; 95% CI ‐0.3 to 3.9) . In the subgroup analysis of those studies comparing continuous nasogastric versus intermittent bolus nasogastric milk feedings the findings remained unchanged (WMD 2 days, 95% CI ‐0.4 to 4.1). There was no significant difference in somatic growth and incidence of NEC between feeding methods irrespective of tube placement. One study noted a trend toward more apneas during the study period in infants fed by the continuous tube feeding method compared to those fed by intermittent feedings delivered predominantly by orogastric tube placements [mean difference (MD) 14.0 apneas during study period; 95% CI ‐0.2 to 28.2]. In subgroup analysis based on weight groups, one study suggested that infants less than 1000 grams and 1000 to 1250 grams birth weight gained weight faster when fed by the continuous nasogastric tube feeding method compared to intermittent nasogastric tube feeding method (MD 2.0 g/day; 95% CI 0.5 to 3.5; MD 2.0 g/day; 95% CI 0.2 to 3.8, respectively). A trend toward earlier discharge for infants less than 1000 grams birth weight fed by the continuous tube feeding method compared to intermittent nasogastric tube feeding method (MD ‐11 days; 95% CI ‐21.8 to ‐0.2).
Authors' conclusions
Small sample sizes, methodologic limitations, inconsistencies in controlling variables that may affect outcomes, and conflicting results of the studies to date make it difficult to make universal recommendations regarding the best tube feeding method for premature infants less than 1500 grams. The clinical benefits and risks of continuous versus intermittent nasogastric tube milk feeding cannot be reliably discerned from the limited information available from randomised trials to date.
Plain language summary
Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams
There is no difference in time to achieve full feedings in low birth weight premature infants fed milk through a tube into the stomach either on a continuous basis or over 10 to 20 minutes every two to three hours. Premature infants born weighing less than 1500 grams are not able to coordinate sucking, swallowing, and breathing. Feeding into the gastrointestinal tract (enteral feeding) helps with gastrointestinal tract development and growth. Therefore, in addition to feeding through a tube into a vein (parenterally), premature infants may be fed milk through a tube placed either up their nose and into the stomach (nasogastric feeding) or through their mouth and into the stomach (orogastric feeding). Usually a set amount of milk is given over 10 to 20 minutes every two to three hours (intermittent bolus gavage feeding). Some clinicians prefer to feed premature infants continuously. Each feeding method has beneficial effects (e.g., achieve full feedings sooner) but can also have harmful effects (destructive inflammation of the gastrointestinal tract or necrotizing enterocolitis. There was no difference in time to achieve full feedings between feeding methods regardless of tube placement. Reports of the incidence of destructive inflammation of the gastrointestinal tract (necrotizing enterocolitis) were similar. However, there is not enough evidence to determine the best feeding method for low birth weight premature infants. More research is required in this area.
Background
Description of the condition
Tube feeding is necessary for most premature infants less than 1500 grams because of their inability to coordinate sucking, swallowing, and breathing (Schanler 1999) and the danger of aspiration (Valman 1972).
Description of the intervention
The conventional tube feeding method is intermittent bolus gavage feeding, where a prescribed volume of milk is given over a short period of time (Aynsley‐Green 1982), usually over 10 to 20 minutes by gravity. The first reported use of the continuous nasogastric tube feeding method for preterm infants was in 1972 (Valman 1972). Some clinicians prefer the continuous nasogastric feeding method for feeding premature infants less than 1300 grams birth weight, although intermittent bolus gavage feeding is the method more commonly used in practice (Toce 1987).
How the intervention might work
Theoretical risks and benefits of both continuous nasogastric milk feeding and intermittent bolus milk feeding have been proposed. Continuous nasogastric feedings may improve energy efficiency (by increasing energy absorbed and decreasing energy expenditure) (Grant 1991), reduce feeding intolerance, improve nutrient absorption, and improve growth (Toce 1987). However, continuous infusion of milk into the gastrointestinal tract could alter the cyclical pattern of release of gastrointestinal tract hormones, which might affect metabolic homeostasis, and growth (Aynsley‐Green 1982). Furthermore, a properly functioning lower oesophageal sphincter is an important barrier against the reflux of stomach contents into the oesophagus and aspiration. Reflux and aspiration may be compounded in the premature infant receiving continuous nasogastric feedings. Not only do these infants have reduced lower oesophageal sphincter pressure (Newell 1988), but the nasogastric tube remains in situ preventing complete closure of the sphincter.
Milk feedings given by the intermittent bolus gavage method are thought to be more physiologic because they promote the cyclical surges of gastrointestinal tract hormones normally seen in healthy term infants (Aynsley‐Green 1982; Aynsley‐Green 1990). Gastrointestinal hormones such as gastrin, gastric inhibitory peptide, and enteroglucagon are trophic and require the presence of intraluminal nutrients to stimulate secretion. Surges in plasma concentrations of gastrointestinal tract hormones postnatally may be important for gastrointestinal tract development (Lucas 1986; Aynsley‐Green 1989). On the other hand, functional limitations of the premature infant's gastrointestinal system such as delayed gastric emptying or intestinal transit could hinder the premature infant's ability to handle bolus milk feeds, resulting in feeding intolerance. Additionally, this feeding regimen alternates between periods of feeding and fasting which may challenge the premature infant's ability to maintain metabolic homeostasis and, therefore, decrease growth (Aynsley‐Green 1982).
The effects of the feeding method on feeding tolerance, weight gain or days to regain birth weight were examined in two non‐randomised controlled trials (Krishnan 1981; Urrutia 1983). In a retrospective study, Krishnan 1981 found that infants fed milk by continuous nasogastric tube feeding reached enteral intakes of 90 kcal/kg/day almost twice as quickly as those infants fed milk by intermittent bolus gavage feeding (16 +/‐ 6 versus 26 +/‐ 17 days, respectively). In addition, infants in the continuous group achieved steady weight gain sooner than infants in the intermittent group (24 +/‐ 10 versus 32 +/‐ 14 days). Unfortunately, these findings are difficult to interpret due to study design and methodologic limitations. First, the non‐random assignment of patients allows for selection bias. Second, energy intake was not controlled and may have influenced feeding tolerance and weight gain. Third, a convenience sample rather than a predetermined sample size was used, making it difficult to achieve both clinical and statistical significance in a study. Hence, it is difficult to make generalizations regarding these findings to similar populations of infants (Raudonis 1995).
Urrutia 1983 conducted a non‐randomised prospective study of continuous versus intermittent nasogastric tube milk feedings. They found no difference between groups in days to regain birth weight. These findings are also difficult to interpret because patients were allocated to the continuous or intermittent group based on neonatologists' preference rather than random assignment, and a convenience sample was used.
Why it is important to do this review
It is important to determine the clinical risks and benefits of each method of feeding to enable clinicians to make informed decisions regarding the most appropriate feeding method for an individual infant. Therefore, a systematic review of trials which compare the two methods of milk feedings was performed.
Objectives
To examine the evidence from randomised trials regarding the effectiveness of continuous versus intermittent bolus nasogastric tube milk feeding as primary feeding strategies in preterm infants less than 1500 grams by:
i) identifying all experimental and quasi‐experimental trials of continuous versus intermittent nasogastric tube milk feeding in this population;
ii) assessing the methodologic quality of each study;
iii) examining the risks and benefits of continuous versus intermittent nasogastric tube milk feeding in preterm infants < 1500 grams on clinically relevant outcomes including:
Primary Outcomes: a) feeding intolerance as measured by number of days of feeding interruptions and days on total parenteral nutrition (TPN); b) days to regain birth weight; c) age at full enteral feedings (days); d) age at discharge to referral hospital or home (days); e) somatic growth including rates of gain in weight, length, and head circumference; f) necrotizing enterocolitis (NEC) including suspected and confirmed (Bell's Stage II or greater).
Secondary Outcomes: a) Apnea
iv) conducting a subgroup analyses based on weight groups including < 750 grams, 750 to 999 grams, and 1000 to < 1500 grams.
Methods
Criteria for considering studies for this review
Types of studies
All randomised and quasi‐randomised trials which compared continuous versus intermittent nasogastric tube milk feeding as primary feeding strategies in preterm infants less than 1500 grams.
Types of participants
Infants born with birth weight less than 1500 grams who have no prior history of feeding or feeding intolerance, and no congenital anomalies that might interfere with establishing enteral feeds.
Types of interventions
Continuous nasogastric feeding versus intermittent bolus nasogastric feeding with human milk or infant formula for the initiation of feeds and advancement to full enteral feeds.
Types of outcome measures
Primary outcomes
a) Feeding intolerance as measured by number of days of feeding interruptions and days on TPN. b) Days to regain birth weight. c) Age at full enteral feedings (days). d) Age at discharge to referral hospital or home (days). e) Anthropometric measurements including rate of gain in weight, length and head circumference. f) NEC, including suspected and confirmed (Bell's Stage II or greater).
Secondary outcomes
a) Apnea.
Search methods for identification of studies
Computerized searches were conducted by both review authors up to July 2011. The databases that were searched included the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 3, 2011), MEDLINE back to 1966, CINAHL back to 1982 and HealthSTAR back to 1975. The following MeSH headings were used to conduct the searches: continuous, intermittent, enteral nutrition, enteral feeding, feeding, enteral nursing, enteroinsular axis, infant‐premature‐metabolism, feeding methods, gastric residuals, feeding intolerance. The searches were limited with terms such as infant‐newborn and infant, very low birth weight.
We also searched: www.clinicaltrials.gov and www.controlled‐trials.com; terms: (infant OR newborn) AND (continuous OR intermittent) AND (nutrition OR feeding OR nursing OR enteroinsular OR metabolism OR gastric).
All potentially relevant titles and abstracts identified in the searches by either review author were retrieved. The reference list of each article was reviewed independently for additional relevant titles and abstracts and these were also retrieved.
Data collection and analysis
The systematic review followed the method described in the Cochrane Collaboration Handbook.
Selection of studies
All the articles that were retrieved from the complete search were assessed for relevance independently by the two review authors. Criteria for relevance included trials that utilized experimental or quasi‐experimental designs, compared continuous nasogastric tube milk feeding versus intermittent bolus nasogastric tube milk feeding, and reviewed clinically relevant outcomes as stated in the objectives. Two studies (Schanler 1999; Dsilna 2005) compared continuous versus intermittent feeding methods; however, both nasogastric and orogastric tube feedings were used with the intermittent feeding method. In the Dsilna 2005 study, data from the intermittent nasogastric feeding group and the intermittent orogastric feeding group were taken together as these two groups did not differ in characteristics (e.g., demographic, birth related factors, and duration of feeding) and primary outcome of time to achieve full enteral feedings. In the Schanler 1999 study, infants in the intermittent feeding method group received primarily (approximately 90% of time) orogastric tube feedings (personal communication). This systematic review includes studies comparing continuous versus intermittent feeding methods and aims to undertake a subgroup analysis of studies comparing only nasogastric tube feedings.
Differences were resolved through discussion and consensus of the review authors.
Data extraction and management
Data were extracted independently by the two review authors. Posteriori subgroup analysis based on tube feeding method (i.e., nasogastric versus orogastric) and birth weight groups (< 1000 grams, 1000 to 1249 grams and 1250 to 1499 grams) was conducted where available. The birth weight groups differed from the subgroups proposed a priori (< 750 grams, 750 to 999 grams, 1000 to 1500 grams).
Investigators were contacted for additional information and/or clarification regarding six studies (Macdonald 1992; Silvestre 1996; Toce 1987; Schanler 1999; Dollberg 2000; Dsilna 2005). See characteristics of included studies for details. Individual group standard deviation for data on days to full feeds from Macdonald 1992 (reported pooled standard deviations), subgroup data from Toce 1987; Schanler 1999; Dollberg 2000 and Dsilna 2005 were not available to include in this update. The Wang 2005 study published in a Chinese journal awaits assessment as the English abstract does not provide enough information to appraise its relevance and methodology. Additionally, limited data is shared in the abstract. Assessment is pending receipt of full translated paper.
Assessment of risk of bias in included studies
Methodologic quality was assessed using the following key criteria: blindness of randomisation, blindness of intervention, completeness of follow‐up and blinding of outcome measurement. Additional criteria of study quality included evidence of confounders, objective criteria of measuring outcomes, and defined exclusion/inclusion criteria. The interventions being compared could not be blinded to those providing care, but should have been blinded to the assessors of the outcomes.
In addition, for the update in 2011, the following issues were evaluated and entered into the Risk of Bias table (Higgins 2011):
(1) Sequence generation (checking for possible selection bias). Was the allocation sequence adequately generated? For each included study, we categorized the method used to generate the allocation sequence as: low risk (any truly random process e.g. random number table; computer random number generator); high risk (any non random process e.g. odd or even date of birth; hospital or clinic record number) or unclear risk.
(2) Allocation concealment (checking for possible selection bias). Was allocation adequately concealed? For each included study, we categorized the method used to conceal the allocation sequence as: low risk (e.g. telephone or central randomisation; consecutively numbered sealed opaque envelopes); high risk (open random allocation; unsealed or non‐opaque envelopes, alternation; date of birth);or unclear risk.
(3) Blinding (checking for possible performance bias). Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment? For each included study, we categorized the methods used to blind study participants and personnel from knowledge of which intervention a participant received. Blinding was assessed separately for different outcomes or classes of outcomes. We categorized the methods as: low risk, high risk or unclear for participants; adequate, inadequate or unclear for personnel; adequate, inadequate or unclear risk for outcome assessors.
(4) Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations). Were incomplete outcome data adequately addressed? For each included study and for each outcome, we described the completeness of data including attrition and exclusions from the analysis. We noted whether attrition and exclusions were reported, the numbers included in the analysis at each stage (compared with the total randomised participants), reasons for attrition or exclusion where reported, and whether missing data were balanced across groups or were related to outcomes. Where sufficient information was reported or supplied by the trial authors, we re‐included missing data in the analyses. We categorized the methods as: low risk (< 20% missing data); high risk (≥ 20% missing data) or unclear risk.
(5) Selective reporting bias. Are reports of the study free of suggestion of selective outcome reporting? For each included study, we described how we investigated the possibility of selective outcome reporting bias and what we found. We assessed the methods as: low risk (where it is clear that all of the study’s pre‐specified outcomes and all expected outcomes of interest to the review have been reported); high risk (where not all the study’s prespecified outcomes have been reported; one or more reported primary outcomes were not pre‐specified; outcomes of interest are reported incompletely and so cannot be used; study fails to include results of a key outcome that would have been expected to have been reported); or unclear risk.
(6) Other sources of bias. Was the study apparently free of other problems that could put it at a high risk of bias? For each included study, we described any important concerns we had about other possible sources of bias (for example, whether there was a potential source of bias related to the specific study design or whether the trial was stopped early due to some data‐dependent process). We assessed whether each study was free of other problems that could put it at risk of bias as: low risk; high risk; or unclear risk.
If needed, we planned to explore the impact of the level of bias through undertaking sensitivity analyses.
Measures of treatment effect
Those articles judged to have the appropriate quality by both review authors were included in the analysis.
The standard methods of the Neonatal Review Group were used. Statistical analyses were performed using Review Manager software. Categorical outcomes such as the incidence of NEC were analysed using relative risk (RR) and risk difference (RD). For statistically significant results, we reported the number needed to treat (NNT). Weighted mean differences are reported for continuous outcomes such as days of feeding intolerance. The comparisons have been displayed with 95% confidence intervals in Cochrane plots.
Dealing with missing data
See "Data extraction and management" above.
Assessment of heterogeneity
We estimated the treatment effects of individual trials and examined heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If we detected statistical heterogeneity, we explored the possible causes (for example, differences in study quality, participants, intervention regimens, or outcome assessments) using post hoc subgroup analyses. We used a fixed effects model for meta‐analysis.
Data synthesis
The meta‐analysis was been performed using Review Manager software (RevMan 5), supplied by the Cochrane Collaboration. For estimates of typical relative risk and risk difference, we used the Mantel‐Haenszel method. For measured quantities, we used the inverse variance method. All meta‐analyses were done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
A posteriori subgroup analyses based on tube feeding method (i.e., nasogastric versus orogastric) and birth weight groups (< 1000 grams, 1000 to 1249 grams and 1250 to 1499 grams) was conducted where available. The birth weight groups differed from the subgroups proposed a priori (< 750 grams, 750 to 999 grams, 1000 to 1500 grams).
Results
Description of studies
There were 12 reports that met the eligibility criteria. However, one study had three reports and another study had two reports but the outcomes showed in the second report were not clinically relevant to this review. Hence, there were nine studies that met the criteria for relevance. Two of the potentially eligible studies (Berseth 1992; Baker 1997) were excluded because the outcomes reported were not clinically relevant to this review. As a result, seven studies (complete data from four and partial data from three) were included in the review (Akintorin 1997; Dollberg 2000; Dsilna 2005 (partial); Macdonald 1992 (partial), Schanler 1999; Silvestre 1996 (partial), and Toce 1987). The table 'Characteristics of Included Studies', outlines details of the seven studies described below.
Akintorin 1997 included clinically stable infants 700 to 1250 grams who were able to start feeding before day 10 of life. Continuous feeds (N = 39) were delivered by an infusion pump while bolus feeds (N = 41) were given every three hours over 15 to 30 minutes by gravity via an indwelling nasogastric feeding tube. Feeding protocols were developed for each 50 to100 gram weight category and strategies were identified for managing feeding intolerance. Infants were followed until they were tolerating full feeds, defined as 100 kcal/kg/d of enteral feeds for at least 48 hours. In addition to the primary outcome of days to full feeds, the authors reported feeding intolerance (feeds withheld for > 12 hours), days to regain birth weight, and days to discharge weight of 2040 grams.
Dollberg 2000 included infants 500 to 1250 grams who were < 48 hours postnatal age. Feeds were initiated between day two and five as per feeding protocol that specified increase in feed volume and included guidelines to manage feeding intolerance (gastric residual volume (GRV) > 20% volume of feeds over previous four hours). Continuous feeds (N = 10) were delivered by infusion pump. Infants 501 to 750 grams received intermittent bolus feeding (nasogastric tube placement) every two hours while infants > 750 grams received feedings every three hours by gravity (N = 13). The major outcome variable was "delay in reaching full feeds", defined as the difference between the expected time to reach full feeds as per feeding protocol and the actual time taken by infants to attain full feeds, defined as 160 cc/kg/d. Days to full feeds, and days to regain birth weight (mean + standard error) were also reported.
Dsilna 2005 (partial) included infants with gestational age 24 to 29 weeks and birth weight < 1200 grams. Human milk feedings were initiated within 30 hours of birth as per pre‐established guidelines. Infants were initially started on intravenous glucose infusion and then within 72 hours were supplemented with total parenteral nutrition. Fortification of human milk was initiated for all infants when total parenteral nutrition was discontinued. Continuous feeds (N = 22) were delivered by infusion pump. The control groups, intermittent orogastric (N = 24) and intermittent nasogastric feeding (N = 22), received feedings every three hours over a period of 15 to 40 minutes which was tailored based on the infant's feed volume and tolerance. The assigned feeding method was used until postmenstrual age of 32 weeks when intermittent orogastric feedings were changed to nasogastric feedings and infants in the continuous feeding group were weaned over a period of 10 to 14 days to intermittent feeding every three hours. Infants in the continuous feeding method group were compared with infants from the two control groups as a collective (i.e. the intermittent orogastric group and intermittent nasogastric group were combined). The primary outcome was time to achieve full enteral feedings defined as the number of days from birth to the time when infants tolerated the prescribed total fluid intake enterally. Secondary outcomes included time to regain birth weight, anthropometric measurements, enteral intolerance, necrotizing enterocolitis, and septicaemia.
Macdonald 1992 (partial) included infants < 1400 grams whose route of feed was determined on day two of life when milk feedings were started. Infants were fed by bolus nasogastric (N = 12), continuous nasogastric (N = 12), or transpyloric (N = 10) feeding method. Infants were supplemented with total parenteral nutrition. Although a feeding protocol was described, it appears to pertain to continuous feeding method as initial volume of feed and increments are described in mls/hour. The delivery equipment for continuous feeds, the bolus feeding method, transpyloric feeding method, and management of feeding intolerance was not described. The selected method of feeding was used until infants reached a weight of 1600 grams after which all infants were switched to bolus nasogastric feeding method. Energy supplements were not provided during the trial period. The primary outcome of this study was growth including length, weight, and occipitofrontal circumference. Standard deviations for days to full enteral feedings was not reported and we are awaiting response from the co‐investigator of this study. Triceps and quadriceps skinfold thickness, and chosen biochemical indices (e.g., alkaline phosphatase, urea, albumin, prealbumin, and transferrin) were also measured but were not included in this review.
Schanler 1999 included infants 26 to 30 weeks gestation who were < 96 hours of age and whose fraction of inspired oxygen was < 0.6 by 72 hours. Feeding protocols were developed for feeding schedules and management of feeding intolerance. The continuous feeding method (N = 83) was not described. Intermittent feedings (N = 88) were given every three hours over 20 minutes by orogastric/nasogastric tube feeding method (note: predominantly by orogastric tube feedings). The primary outcome was days to attain full oral feeds, defined as eight bottle or breast feeds per day. Other outcomes reported in this study included days to full feeding (150 cc/kg/d), weight gain, head circumference gain, length gain, skinfold thickness (five sites), feeding intolerance, nutritional balance studies, bone mineral content and serum indices of protein and mineral status.
Silvestre 1996 (partial) included infants 750 to < 1500 grams who were able to start nasogastric feeds on day two or three of life. Infants in the continuous feeding group (N = 42) received a feeding over a three hour period. Infants in the intermittent bolus feeding group (N = 40) were fed every three hours over 15 to 30 minutes up to a maximum of one hour if feeding difficulties were encountered. Feeding protocols were developed for advancement of feeds and for the management of feeding intolerance (GRV >= 2 hours feed volume for continuous; >= 2 mL feed for intermittent bolus). The primary outcome was rate of weight gain. Days to full feeds (75 kcal/kg/day) and days to discharge (criteria not defined) were also reported. Anthropometric measurements (change in head circumference, length, midtricipetal skin‐fold thickness, skin‐fold thickness, and subscapular skin‐fold thickness) and retention rates of nitrogen, fat, total carbohydrate, and lactose were also compared between groups. There appears to be a significant difference between the continuous and intermittent feeding method groups in head circumference data; however, the difference is reported as being insignificant. Clarification has been requested.
Toce 1987 included infants < 1500 grams who were ready for enteral nutrition. The timing of feeds was not specified. Continuous feeds (N = 30) were delivered by an infusion pump. Intermittent nasogastric feeds (N = 23) were given every three hours by gravity. Feeding protocols and management of feeding intolerance were standardized. The following outcomes were reported: somatic growth including weight gain, change in length, occipitofrontal circumference, and skin fold thickness, total protein, bilirubin, feeding related complications such as NEC, suspected NEC, feeding intolerance, hours NPO per day, number of apnoea episodes per day, number of stools per day, and fraction heme‐positive stools.
Risk of bias in included studies
Of the seven studies included in the review, six were randomised. One study (Toce 1987) used alternate assignment. Blinding of allocation was achieved for six of the seven studies (Macdonald 1992; Silvestre 1996; Akintorin 1997; Schanler 1999; Dollberg 2000; Dsilna 2005).
Caregivers were not blinded to the intervention as this would not be feasible. In one study (Silvestre 1996) outcome assessors were blinded and in one study they were not (Dollberg 2000). In the Dsilna 2005 study, only radiographic assessors for the outcome of necrotizing enterocolitis were blinded to patient group assignment. For the remaining four studies blinding of the outcome assessors could not be determined.
In each of the trials, several infants were removed from the assigned feeding protocols for clinical reasons including, but not limited to, feeding intolerance. An intention‐to‐treat analysis should include outcome results for such infants.
Only one study (Schanler 1999) had complete follow‐up. In this study, infants were removed from the treatment protocol if they were not able to adhere to the feeding protocol for more than one week. Ten infants in the continuous group and one in the intermittent group were removed from their assigned feeding protocols because of feeding intolerance. However, data from these infants were included in the analysis.
Silvestre 1996 reported complete follow‐up only for stratified groups. In this study, 11 infants were removed from treatment protocols and excluded from overall analyses. Of three infants excluded from the continuous group, none were excluded for feeding intolerance, although one was excluded for protocol violation. Of eight infants excluded from the intermittent group, three were excluded for feeding intolerance and another two for protocol violation. Follow‐up was incomplete in the remaining three studies.
Akintorin 1997 excluded nine infants from analysis. Of four patients excluded from the continuous group, one had been switched to breastfeeding, but none were excluded for feeding intolerance. Of five infants excluded from the intermittent group, three infants were excluded for protocol violation (feeding intolerance not specified) and one had been switched to breastfeeding.
Dollberg 2000 excluded five infants from analysis, one for protocol violation (the infant was switched to bolus feeding method because of failure to establish full feedings) and the other four because of death.
Dsilna 2005 excluded two infants post randomisation because of diagnosed malformations. One infant in the control group was switched to the continuous feeding method group because of severe apnoea and bradycardia secondary to gastroesophageal reflux. Data for this infant was included as randomised. In the early intervention phase, three infants died (one infant in each feeding group) due to respiratory and circulatory collapse. Two infants in the continuous feeding method group died at postmenstrual ages of 33 and 47 weeks secondary to septicaemia and severe respiratory and circulatory distress and chronic lung disease.
Macdonald 1992 enrolled 13 babies in the continuous feeding method group but only 12 completed the study as one infant was transferred to another hospital at age two weeks of life. Three of the 15 infants enrolled in the bolus feeding method group died within the first week of life, before milk feedings were established and were therefore excluded from the analysis.
In the Toce 1987 study, 30 infants were excluded because they did not complete seven days in the study. In these studies, the post‐randomisation exclusion of infants from the analysis has resulted in loss to follow‐up.
The overall methodologic quality of the studies was fair. Refer to the table, Characteristics of Included Studies, for quality assessment.
Effects of interventions
CONTINUOUS versus INTERMITTENT BOLUS (NASOGASTRIC AND OROGASTRIC TUBE) MILK FEEDING ‐ ALL INFANTS (COMPARISON 1)
Feeding performance (Outcome 1.1):
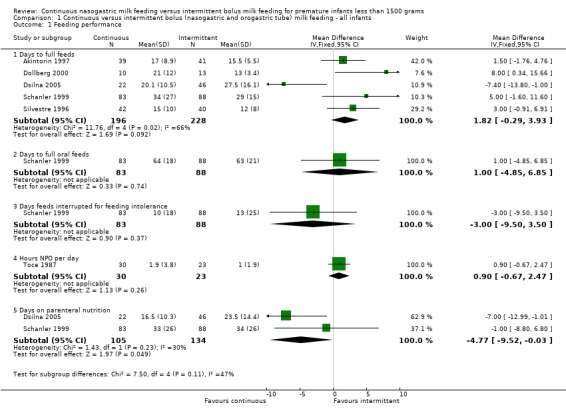
Days to full feeds (five trials) (Outcome 1.1.1):Dollberg 2000 and Schanler 1999 found that it took significantly longer for infants fed by the continuous feeding method to reach full feeds while Dsilna 2005 found that infants fed by continuous feeding method took a shorter time to attain full enteral feedings. Akintorin 1997 and Silvestre 1996 found no significant difference in the time taken to achieve full feeds. The meta‐analysis revealed no significant difference in days to reach full feeds between feeding methods (WMD 2 days; 95% CI ‐0.3 to 3.9). However, it is difficult to draw a conclusion from these data because there was significant statistical heterogeneity suggesting the studies may have assessed this outcome differently.
Days to full oral feeds (one trial) (Outcome 1.1.2):Schanler 1999 found no difference between groups.
Feeding intolerance (four trials) (Outcomes 1.1.3 and 1.1.4):Schanler 1999 found no difference between groups in the number of days on which feedings were interrupted for feeding intolerance. Toce 1987 found no difference between groups in the average number of hours spent NPO per day for feeding intolerance. Similarly, Akintorin 1997 found no difference in the number of infants who experienced feeding intolerance, defined as feeds held longer than 12 hours. Dsilna 2005 found no difference in feeding intolerance defined as number of occasions the infant was diagnosed with suspected necrotizing enterocolitis (Bell Stage I) followed by interruption of enteral feeds for at least 8 hours. A meta‐analysis could not be performed because the measures of feeding intolerance were not comparable.
Days on TPN (two trials) (Outcome 1.1.5):Dsilna 2005 found that infants in the intermittent feeding group required parenteral nutrition (total and partial) for a significantly longer duration. Schanler 1999 found no difference in the number of days on parenteral nutrition for infants fed by either method. The meta‐analysis revealed no significant difference in the number of days on parenteral nutrition between feeding methods (WMD ‐5 days; 95% CI ‐9.5 to ‐0.03).
Growth Outcomes (Outcome 1.2):
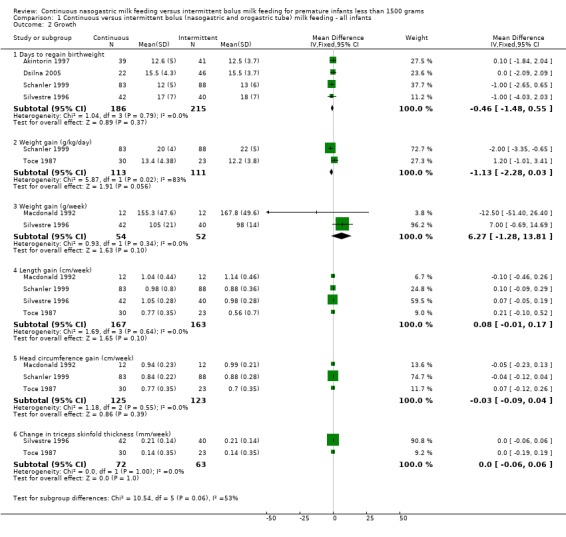
Days to regain birth weight (four trials) (Outcome 1.2.1):Akintorin 1997; Dsilna 2005; Schanler 1999 and Silvestre 1996 found no difference in time to regain birth weight between the two feeding groups. The meta‐analysis found no evidence of a difference between feeding methods (WMD ‐0.5 days; 95% CI ‐1.5 to 0.6).
Weight gain (four trials) (Outcomes 1.2.2 and 1.2.3): Two studies (Schanler 1999, and Toce 1987) reported weight gain in grams per kg per day while the other two studies (Macdonald 1992, and Silvestre 1996) reported weight gain in grams per week. Schanler 1999 found that infants fed by the continuous feeding method gained weight slower than infants fed by the intermittent bolus feeding method. However, Toce 1987 did not find a difference in weight gain between the two groups. The meta‐analysis did not support a difference in growth rates between the two groups (WMD ‐1.1 g per kg per day; 95% CI ‐2.3 to 0.03); however, significant statistical heterogeneity was noted. Similarly, Macdonald 1992 and Silvestre 1996 did not find a difference in weight gain (grams per week) between the two groups. The meta‐analysis once again did not support a difference in growth rates between the two groups (WMD 6.3 g/week 95% CI ‐1.3 to 13.8).
Length gain (four trials) (Outcome 1.2.4): None of the trials (Macdonald 1992; Schanler 1999; Silvestre 1996; and Toce 1987) showed a difference in length gain between the two groups. The meta‐analysis of the four trials (Macdonald 1992; Schanler 1999; Silvestre 1996; and Toce 1987) that reported length gain found there was no evidence of a difference in length gain between the two groups (WMD 0.08 cm/week; 95% CI ‐0.01 to 0.17). Dsilna 2005 did not measure length gain but rather growth rate (mm/day) of the lower leg from birth to 32 weeks postmenstrual age and birth to 36 weeks postmenstrual age and reported significantly faster growth rate in infants in the continuous nasogastric feeding method group at these two time periods; P value .002 and .012, respectively.
Head circumference gain (four trials) (Outcome 1.2.5):Macdonald 1992; Schanler 1999 and Toce 1987 did not find any difference between groups, and the meta‐analysis did not support a difference (WMD ‐0.03 cm/week; 95% CI ‐0.1 to 0.04). Data from Silvestre 1996 have been excluded from the meta‐analysis as it is not clear if there is a typographical error in the data (see Characteristics of Included Studies).
Change in triceps skinfold thickness (three trials) (Outcome 1.2.6): No difference in skinfold thickness was seen between groups in trials by Silvestre 1996 and Toce 1987 (WMD 0 mm/week; 95% CI ‐0.1 to 0.1). Macdonald 1992 found marked interobserver variability in measurements of skinfold thickness and concluded that this was not a reliable method of assessing growth in low birth weight infants. The findings were not reported.
Utilization of Resources (Outcome 1.3):
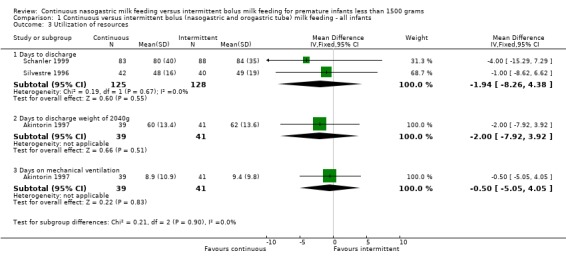
Days to discharge (two trials) (Outcome 1.3.1):Schanler 1999 and Silvestre 1996 were unable to find any difference between groups in time to discharge. The meta‐analysis supports this conclusion (WMD ‐2 days; 95% CI ‐8.3 to 4.4).
Days to discharge weight of 2040 grams (one trial) (Outcome 1.3.2):Akintorin 1997 did not find any difference between groups.
Days on mechanical ventilation (two trials) (Outcome 1.3.3):Akintorin 1997 found that infants were ventilated for similar lengths of time regardless of the method of feeding. Dsilna 2005 reported no difference in the numbers of infants who required mechanical ventilatory support between the two groups. Since the measures of mechanical ventilation were not comparable, a meta‐analysis could not be performed.
Complications (categorical) (Outcome 1.4):
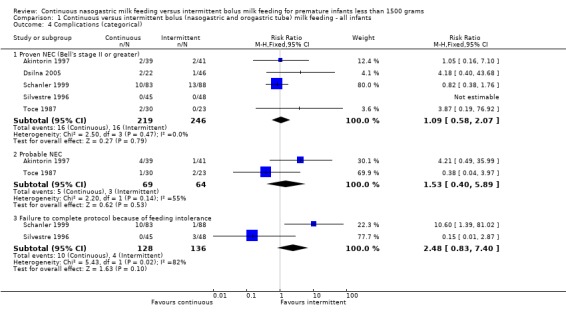
Proven necrotizing enterocolitis (NEC), Bell's stage II or greater (6 trials) (Outcome 1.4.1): In the trials by Akintorin 1997; Dsilna 2005; Schanler 1999 and Toce 1987 there were no differences between groups in the incidence of proven NEC. Silvestre 1996 had no cases of NEC in study infants. In the meta‐analysis of these five studies, there was no evidence of effect (typical RR 1.1; 95% CI 0.6 to 2.1). Macdonald 1992 reported cases of proven and suspected NEC determined solely on radiographic findings which were not described. Hence, the data were not included in the meta‐analysis. There was one case of proven NEC and one case of probable NEC in the continuous group. Infants in the bolus group had no cases of NEC, proven or probable.
Probable NEC (two trials) (Outcome 1.4.2): Neither Akintorin 1997 nor Toce 1987 found any difference between groups in the incidence of probable NEC (Bell Stage I). Dsilna 2005 defined feeding intolerance as probable NEC (Bell Stage I) plus interruption of enteral feeds for at least 8 hours. It is not clear in the Dsilna 2005 study how many infants met the criteria of Bell Stage I only. Hence, their data could not be included in the meta‐analysis. The meta‐analysis of the two trials (Akintorin 1997 and Toce 1987) showed no evidence of effect (typical RR 1.5; 95% CI 0.4 to 5.9). Significant heterogeneity of treatment effect was noted; hence data need to be interpreted prudently.
Failure to complete protocol because of feeding intolerance (two trials) (Outcome 1.4.3):Schanler 1999 and Silvestre 1996 reported the number of infants who were unable to complete the feeding protocol due to feeding intolerance. Schanler 1999 found that infants allocated to continuous feeds were significantly more likely to be withdrawn from the assigned feeding protocol. The meta‐analysis found no evidence of a difference between groups in infants removed from the assigned feeding protocol due to feeding intolerance (typical RR 2.5; 95% CI 0.8 to 7.4). However, it is difficult to draw a conclusion from these data because there was significant statistical heterogeneity suggesting the two studies may have assessed this outcome differently.
Complications (continuous) (Outcome 1.5):
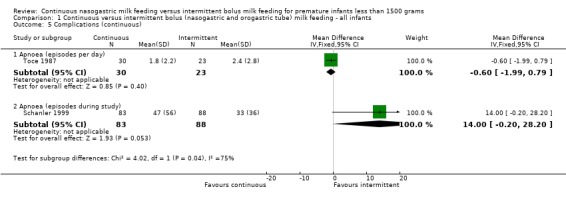
Apnoea (four trials) (Outcomes 1.5.1 and 1.5.2):Toce 1987 did not find a significant difference in the number of apneas per infant per day between groups. Schanler 1999 did show a trend toward increased number of apneic episodes during the study period in infants fed by continuous feeding method (MD 14.0 apneas; 95% CI ‐0.2 to 28.2). A meta‐analysis could not be performed as the two studies reported episodes differently. The results of Schanler 1999 are in contrast to observations of Akintorin 1997 in which apnoea was more commonly observed in the intermittent feeding group (data not provided). Similarly, Silvestre 1996 reported that only infants in the intermittent feeding group (750 to 999 gram weight category) had feedings held for recurrent apnoea.
CONTINUOUS versus INTERMITTENT BOLUS (NASOGASTRIC TUBE) MILK FEEDING ‐ ALL INFANTS (COMPARISON 2)
Feeding performance (Outcome 2.1):
Days to full feeds (four trials) (Outcome 2.1.1):Dollberg 2000 found that it took significantly longer for infants fed by the continuous feeding method to reach full feeds. Akintorin 1997 and Silvestre 1996 found no significant difference in the time taken to achieve full feeds. Dsilna 2005 provided mean and standard deviations which indicate that infants in the intermittent group took longer to attain full feeds. However, statistical analysis was reported comparing the continuous and intermittent groups (intermittent orogastric feeding and intermittent nasogastric feeding) combined. The meta‐analysis of the four studies (Akintorin 1997; Dollberg 2000; Dsilna 2005; and Silvestre 1996) revealed no significant difference in days to achieve full feeds between the continuous tube feeding method as compared to those infants fed by the intermittent bolus nasogastric feeding method (WMD 2 days; 95% CI ‐0.4 to 4.1).
Days to full oral feeds (Outcome 2.1.2):No study reported this outcome.
Feeding intolerance (two trials) (Outcomes 2.1.3 and 2.1.4):Toce 1987 found no difference between groups in the average number of hours spent NPO per day for feeding intolerance. Similarly, Akintorin 1997 found no difference in the number of infants who experienced feeding intolerance, defined as feeds held longer than 12 hours. A meta‐analysis could not be performed because the measures of feeding intolerance were not comparable.
Days on TPN (Outcome 2.1.5): No study addressed this outcome.
Growth Outcomes (Outcome 2.2):
Days to regain birth weight (three trials) (Outcome 2.2.1):Akintorin 1997; and Silvestre 1996 found no difference in time to regain birth weight between the two feeding groups. Dsilna 2005 provided mean and standard deviations for the 2 control groups (intermittent nasogastric and intermittent orogastric), however reported statistical analysis comparing the continuous and intermittent groups combined. The meta‐analysis of the three studies (Akintorin 1997; Dsilna 2005; and Silvestre 1996) found no evidence of a difference between feeding methods (WMD ‐0.3 days; 95% CI ‐1.7 to 1.0).
Weight gain (three trials) (Outcomes 2.2.2 and 2.2.3):Toce 1987 reported weight gain in grams per kg per day while the other two studies (Macdonald 1992, and Silvestre 1996) reported weight gain in grams per week. Toce 1987 did not find a difference in weight gain between the two groups. Similarly, Macdonald 1992 and Silvestre 1996 did not find a difference in weight gain (grams per week) between the two groups. The meta‐analysis of the two studies (Macdonald 1992, and Silvestre 1996) once again did not support a difference in growth rates between the two groups (WMD 6.3 g/week 95% CI ‐1.3 to 13.8).
Length gain (three trials) (Outcome 2.2.4): None of the trials (Macdonald 1992; Silvestre 1996; and Toce 1987) showed a difference in length gain between the two groups. Dsilna 2005 did not measure length gain but rather growth rate (mm/day) of the lower leg from birth to 32 weeks postmenstrual age and birth to 36 weeks postmenstrual age. A significant difference in growth rate of the lower leg, in favor of continuous nasogastric feeding method, was reported only for the birth to 36 weeks postmenstrual age time period. The meta‐analysis of the three trials (Macdonald 1992; Silvestre 1996; and Toce 1987) that reported length gain found there was no evidence of a difference in length gain between the two groups (WMD 0.07 cm/week; 95% CI ‐0.04 to 0.2).
Head circumference gain (three trials) (Outcome 2.2.5):Macdonald 1992; and Toce 1987 did not find any difference between groups, and the meta‐analysis did not support a difference (WMD 0.01 cm/week; 95% CI ‐0.1, 0.1). Data from Silvestre 1996 have been excluded from the meta‐analysis until clarified by the author (see Characteristics of Included Studies).
Change in triceps skinfold thickness (three trials) (Outcome 2.2.6): No difference in skinfold thickness was seen between groups in trials by Silvestre 1996 and Toce 1987 (WMD 0 mm/week; 95% CI ‐0.1 to 0.1). Macdonald 1992 found marked interobserver variability in measurements of skinfold thickness and concluded that this was not a reliable method of assessing growth in low birth weight infants. The findings were not reported.
Utilization of Resources (Outcome 2.3):
Days to discharge (one trial) (Outcome 2.3.1):Silvestre 1996 was unable to find any difference between groups in time to discharge.
Days to discharge weight of 2040 grams (one trial) (Outcome 2.3.2):Akintorin 1997 did not find any difference between groups.
Days on mechanical ventilation (two trial) (Outcome 2.3.3):Akintorin 1997 found that infants were ventilated for similar lengths of time regardless of the method of feeding. Dsilna 2005 found that the need for mechanical ventilation (number of infants needing intermittent positive pressure ventilation or high frequency oscillatory ventilation), continuous positive airway pressure and supplemental oxygen was comparable among the groups. Since the measures of mechanical ventilation were not comparable, a meta‐analysis could not be performed.
Complications (categorical) (Outcome 2.4):
Proven necrotizing enterocolitis (NEC), Bell's stage II or greater (five trials) (Outcome 2.4.1): In the trials by Akintorin 1997 and Toce 1987 there were no differences between groups in the incidence of proven NEC. Silvestre 1996 had no cases of NEC in study infants. Dsilna 2005 reported 2 cases of NEC in the continuous nasogastric feeding group and no cases of NEC in the intermittent nasogastric feeding method group. In the meta‐analysis of these four studies, there was no evidence of effect (typical RR 2.2; 95% CI 0.6 to 8.6). Macdonald 1992 reported cases of proven and suspected NEC determined solely on radiographic findings which were not described. Hence, the data were not included in the meta‐analysis. There was one case of proven NEC and one case of probable NEC in the continuous group. Infants in the bolus group had no cases of NEC, proven or probable.
Probable NEC (two trials) (Outcome 2.4.2): Neither Akintorin 1997 nor Toce 1987 found any difference between groups in the incidence of probable NEC. The meta‐analysis showed no evidence of effect (typical RR 1.5; 95% CI 0.4 to 6). Significant heterogeneity of treatment effect was noted; hence data need to be interpreted prudently. Failure to complete protocol because of feeding intolerance (one trial) (Outcome 2.4.3):Silvestre 1996 reported that 3 infants in the intermittent nasogastric tube feeding method were excluded post‐randomisation because of feeding intolerance.
Complications (continuous) (Outcome 2.5):
Apnoea (three trials) (Outcomes 2.5.1 and 2.5.2):Toce 1987 did not find a significant difference in the number of apneas per infant per day between groups. Akintorin 1997 more commonly observed apnoea in the intermittent feeding group (data not provided). Similarly, Silvestre 1996 reported that only infants in the intermittent feeding group (750 to 999 gram weight category) had feedings held for recurrent apnoea (data not provided). A meta‐analysis could not be performed because of missing data.
CONTINUOUS versus INTERMITTENT BOLUS (NASOGASTRIC AND OROGASTRIC TUBE) MILK FEEDING ‐ INFANTS < 1000 GRAMS (COMPARISON 3)
Feeding Performance (Outcome 3.1):
Days to full feeds (three trials) (Outcome 3.1.1): Trials by Akintorin 1997 and Silvestre 1996 did not find a difference in days to full feeds between groups while Dsilna 2005 found that infants fed by continuous feeding method took a shorter time to attain full enteral feedings. The meta‐analysis found no evidence of difference in days to full feeds between feeding methods (WMD ‐1 day; 95% CI 4.6 to 1.8). There was significant heterogeneity consequently it is difficult to draw a conclusion from these data.
Feeding intolerance (one trial). Dsilna 2005 did not report number of days of feeding intolerance but rather number of occasions the infant was diagnosed with suspected necrotizing enterocolitis (Bell Stage I) followed by interruption of enteral feeds for at least 8 hours and found no difference between groups.
Days on TPN (one trials) (Outcome 3.1.2):Dsilna 2005 found that infants in the intermittent feeding group required parenteral nutrition (total and partial) for a significantly longer duration.
Growth Outcomes (Outcome 3.2):
Days to regain birthweight (three trials) (Outcome 3.2.1):Akintorin 1997; Dsilna 2005 and Silvestre 1996 did not show a difference between groups. The meta‐analysis did not show a difference between groups (WMD ‐0.1 day; 95% CI ‐2.1 to 1.8).
Weight gain (one trial) (Outcome 3.2.2): Data from Silvestre 1996 suggested that infants < 1000 grams birth weight gained weight faster when fed by continuous tube feeding method (mean difference 2.0 g/day; 95% CI 0.5 to 3.5).
Length gain (one trial) (Outcome 3.2.3):Silvestre 1996 found no difference between groups. Dsilna 2005 did not measure length gain but rather growth rate (mm/day) of the lower leg from birth to 32 weeks postmenstrual age and birth to 36 weeks postmenstrual age and reported significant difference in growth rate of the lower leg at these two time periods in favor of the continuous nasogastric feeding method.
Head circumference gain (one trial) (Outcome 3.2.4):Silvestre 1996 found no difference between groups.
Utilization of Resources (Outcome 3.3):
Days to discharge (one trial) (Outcome 3.3.1): Data from Silvestre 1996 suggested a trend toward earlier discharge in infants with birth weight less than 1000 grams fed by continuous tube feeding method (mean difference ‐11 days; 95% CI ‐21.8 to ‐0.2).
Days to discharge weight of 2040 grams (one trial) (Outcome 3.3.2):Akintorin 1997 did not find any difference between groups (0.0; ‐5.85 to 5.85).
Days on mechanical ventilation (one trial) (Outcome 3.3.3):Dsilna 2005 did not report the number of days of mechanical ventilation but rather number of infants who required mechanical ventilation. Dsilna 2005 reported no difference in the numbers of infants who required mechanical ventilatory support between the two groups.
Complications (categorical) (Outcome 3.4):
Proven necrotizing enterocolitis (NEC), Bell's stage II or greater (one trial) (Outcome 3.4.1):Dsilna 2005 reported no differences between groups in the incidence of proven NEC.
Probable NEC (one trial) (Outcome 3.4.2):Dsilna 2005 defined feeding intolerance as probable NEC (Bell Stage I) plus interruption of enteral feeds for at least 8 hours. It is not clear in the Dsilna 2005 study how many infants met the criteria of Bell Stage I only.
CONTINUOUS versus INTERMITTENT BOLUS (NASOGASTRIC TUBE) MILK FEEDING ‐ INFANTS < 1000 GRAMS (COMPARISON 4) Feeding Performance (Outcome 4.1):
Days to full feeds (two trials) (Outcome 4.1.1): Trials by Akintorin 1997 and Silvestre 1996 did not find a difference in days to full feeds between groups. The meta‐analysis found no evidence of difference in days to full feeds between feeding methods (WMD 0.8 days; 95% CI ‐2.8 to 4.3).
Growth Outcomes (Outcome 4.2):
Days to regain birthweight (two trials) (Outcome 4.2.1):Akintorin 1997 and Silvestre 1996 did not show a difference between groups. The meta‐analysis did not show a difference between groups (WMD ‐0.5 days; 95% CI ‐3.5 to 2.6).
Weight gain (one trial) (Outcome 4.2.2): Data from Silvestre 1996 suggested that infants < 1000 grams birth weight gained weight faster when fed by continuous tube feeding method (mean difference 2.0 g/day; 95% CI 0.5 to 3.5).
Head circumference gain (one trial) (Outcome 4.2.4):Silvestre 1996 found no difference between groups.
Utilization of Resources (Outcome 4.3):
Days to discharge (one trial) (Outcome 4.3.1): Data from Silvestre 1996 suggested a trend toward earlier discharge in infants with birth weight less than 1000 grams fed by continuous tube feeding method (mean difference ‐11 days; 95% CI ‐21.8 to ‐0.2).
Days to discharge weight of 2040 grams (one trial) (Outcome 4.3.2):Akintorin 1997 did not find any difference between groups (0.00; ‐5.85 to 5.85).
Days on mechanical ventilation (one trial) (Outcome 3.3.3):Dsilna 2005 did not report the number of days of mechanical ventilation but rather the number of infants who required mechanical ventilation. Dsilna 2005 reported no difference in the number of infants who required mechanical ventilatory support between the two groups.
CONTINUOUS versus INTERMITTENT BOLUS (NASOGASTRIC TUBE) MILK FEEDING ‐ INFANTS 1000 ‐ 1249 GRAMS (COMPARISON 5)
Feeding Performance (Outcome 5.1):
Days to full feeds (two trials). (Outcome 5.1.1): Neither Akintorin 1997 nor Silvestre 1996 found a difference between groups. The meta‐analysis also found no difference (WMD ‐0.2 days; 95% CI 2.5 to 2.2).
Growth Outcomes (Outcome 5.2):
Days to regain birthweight (two trials). (Outcome 5.2.1): Neither Akintorin 1997 nor Silvestre 1996 found a difference in days to regain birthweight between infants fed by continuous or intermittent tube feeding method. The meta‐analysis also showed no evidence of a difference (WMD ‐0.4 days; 95% CI ‐2.5 to 1.7).
Weight gain (one trial). (Outcome 5.2.2): Data from Silvestre 1996 suggested that infants with birth weight 1000 to 1249 grams gained weight faster when fed by continuous tube feeding method (mean difference 2.0 grams/day; 95% CI 0.2 to 3.8).
Length gain (one trial). (Outcome 5.2.3):Silvestre 1996 found no difference in length gain between groups.
Head circumference gain (one trial). (Outcome 5.2.4):Silvestre 1996 found no difference in head circumference gain between groups.
Utilization of Resources (Outcome 5.3):
Days to discharge (one trial) (Outcome 5.3.1):Silvestre 1996 found no difference in days to discharge for infants fed by continuous versus intermittent tube feeding methods.
Days to discharge weight of 2040 grams (one trial) (Outcome 5.3.2):Akintorin 1997 found no difference between groups in days to reach discharge weight.
CONTINUOUS versus INTERMITTENT BOLUS (NASOGASTRIC TUBE) MILK FEEDING ‐ INFANTS 1250 ‐ 1499 GRAMS (COMPARISON 6)
Feeding Performance (Outcome 6.1):
Days to full feeds (one trial) (Outcome 6.1.1):Silvestre 1996 found no difference between groups in days to full feeds.
Growth Outcomes (Outcome 6.2):
Days to regain birthweight (one trial) (Outcome 6.2.1):Silvestre 1996 found no difference between groups in the days to regain birthweight.
Weight gain (one trial) (Outcome 6.2.2):Silvestre 1996 found no difference between groups.
Length gain (one trial) (Outcome 6.2.3):Silvestre 1996 found no difference between groups.
Head circumference gain (one trial) (Outcome 6.2.4):Silvestre 1996 found no difference between groups.
Utilization of Resources (Outcome 6.3):
Days to discharge (one trial) (Outcome 6.3.1):Silvestre 1996 found no difference between groups.
Discussion
Current practice varies with regards to use of continuous versus intermittent bolus gavage feeding methods. There are theoretical advantages to each method, and practice appears to be based more on individual assessment rather than scientific evidence. There are seven clinical trials comparing the effectiveness of continuous versus intermittent milk feedings methods in premature infants less than 1500 grams. Six studies (Toce 1987; Silvestre 1996; Akintorin 1997; Schanler 1999; Dollberg 2000; Dsilna 2005) compared continuous infusion with intermittent feeds given every two or three hours. One study (Macdonald 1992) compared continuous infusion, intermittent feeds (frequency unknown), and transpyloric method of feeding. All but one study (Macdonald 1992) stratified participants, either by birth weight or gestational age, in order to obtain more homogeneous samples for comparison.
Gavage feedings may be given via nasogastric or orogastric feeding tube placement. Some clinicians prefer the orogastric tube placement given the potential of apnoea secondary to airflow obstruction with nasogastric tubes. Five trials (Toce 1987; Macdonald 1992; Silvestre 1996; Akintorin 1997; Dollberg 2000) focused strictly on the nasogastric tube feeding method. One trial (Schanler 1999) compared continuous gavage feeding with intermittent feedings that were delivered predominantly (> 90%) by the orogastric tube feeding placement (personal communication). Although Dsilna 2005 had two control groups, intermittent nasogastric tube feeding method and intermittent orogastric tube feeding method, data were combined when the analysis was undertaken for the comparison. All studies were included in this systematic review with a subgroup analysis presented for only those trials which compared continuous nasogastric feeding method with intermittent bolus nasogastric feeding method. Complete data from four (Toce 1987; Akintorin 1997; Schanler 1999; Dollberg 2000) and partial data from three studies (Dsilna 2005; Macdonald 1992; and Silvestre 1996) were included in the review.
Overall, the evidence suggests that there is no significant difference in the time taken to achieve full feeds between continuous versus intermittent gavage feeding methods irrespective of tube placement (i.e., nasogastric or orogastric). Dsilna 2005 reported findings in contrast to Dollberg 2000 and Schanler 1999 which suggested that infants fed by the continuous tube feeding method took longer to reach full feeds. Dsilna 2005 defined full feeds as enteral intake of the total prescribed volume based on postnatal age and weight (< 140 to 160 cc/kg/day) for 48 hours. Dollberg 2000 defined full feeds as reaching an enteral intake of 160 cc/kg/day within a specific time frame based on weight group (e.g., eight days in the largest weight group). In the study by Schanler 1999, an enteral intake of 150 cc/kg/day was considered full feeds. Differences in the way the primary outcome of full feeds was measured may account for the statistically heterogeneity noted in the overall meta‐analysis (i.e., including nasogastric and orogastric tube placement and examining data for all infants). Other potential sources of heterogeneity might include differences in definition and management of feeding intolerance, participants, intervention, and study quality which are addressed later. Consequently, it is difficult to draw conclusions from these data.
Days on parenteral nutrition was examined only in studies which compared continuous nasogastric feeding method with intermittent bolus (nasogastric and orogastric tube) milk feeding. Although not significant difference in the number of days on parenteral nutrition was reported (WMD ‐5 days; 95% CI ‐9.5, ‐0.03) between feeding methods, the difference of five days is clinically significant as it has workload implications (e.g. nursing care and medical management), as well could potentially facilitate transfer of the infant to a center which does not manage total parenteral nutrition. Days on parenteral nutrition was not examined in studies comparing continuous nasogastric feeding method with intermittent nasogastric bolus milk feeding method.
There was no difference in growth (weight, length, head circumference) of infants fed by either method regardless of tube placement. Although infants with birth weight < 1000 grams and 1000 to 1249 grams had better weight gain on continuous feeds, these results were only reported in one study with a small sample size. To assess this further, subgroup data has been requested from Toce 1987; Schanler 1999; Dollberg 2000 and Dsilna 2005 . The lack of an effect on growth may, in part, be attributed to the consistent provision of nutrients within studies with the use of parenteral nutrition (Toce 1987; Macdonald 1992; Akintorin 1997; Schanler 1999; Dsilna 2005). There was a trend toward increased number of apneas per patient in infants fed by the continuous tube feeding method (p = 0.05), but this trend is in contrast to results observed in other studies. Dsilna 2005 noted that one infant in the intermittent nasogastric feeding group was switched to intermittent orogastric feedings because of carbon dioxide retention secondary to nasal obstruction most likely caused by the feeding tube. Finally, there was a trend toward earlier discharge in infants < 1000 grams birth weight fed by the continuous tube feeding method (p = 0.05), but this trend was seen only in one study, again with a small sample size. Overall, there was no difference in days to discharge between groups. These results, particularly time to achieve full feeds, should be interpreted with caution as findings may be confounded by the definition and management of feeding intolerance. For example, six studies (Toce 1987; Silvestre 1996; Akintorin 1997; Schanler 1999; Dollberg 2000; Dsilna 2005) had predetermined criteria to advance feeds and to identify and manage feeding intolerance. Gastric residual volume was a major criterion in determining feeding tolerance. Three of the studies (Akintorin 1997; Schanler 1999; Dollberg 2000) reported a greater incidence of residuals in infants fed by continuous tube feeding, and this may have resulted in a greater number of interruptions of feeds and / or slower increases in feeds, thereby increasing the number of days taken to reach full feeds. Dollberg 2000 reported that regression analysis suggested that only the method of feeding affected feeding intolerance defined as presence of residual > 20 % of the feed volume. In this study, continuous feeding was associated with increased feeding intolerance. In contrast, Dsilna 2005 reported no difference in rates of feeding intolerance, gastric residuals, or vomiting between infants in the continuous nasogastric feeding and intermittent nasogastric feeding method. However, infants in the intermittent orogastric feeding method had higher rates of feeding intolerance, gastric residuals, and vomiting. Schanler 1999 reported an association between the greater number of residuals in the continuous group and increased time to full feeds. Akintorin 1997 similarly reported that there was an association between feeding intolerance and time to full feeds and time to reach discharge weight. On the other hand, Silvestre 1996 found that only infants in the intermittent group were removed from the study protocol for feeding‐related reasons, including significant residuals. Depending on the individual tolerance for and response to gastric residuals, the management of feeding intolerance may significantly affect the reported feeding tolerance and the time taken to reach full feeds. The need for an evidence based approach to management of gastric residual volumes is apparent. Furthermore, only one study (Schanler 1999) included all infants in the results whether or not they were able to adhere to the feeding protocol. Silvestre 1996 included all infants in the analysis for subgroup analysis but not for overall analysis. Unfortunately, there were several infants in each study who were removed from the treatment protocol for various reasons including feeding intolerance and protocol violation. Exclusion of these infants might influence study outcomes such as days to full feeds.
There was statistical heterogeneity in the outcomes of weight gain and the number of infants who failed to complete the protocol because of feeding intolerance. Potential sources of heterogeneity might include differences in participants, intervention, and study quality.
Participants In five studies (Toce 1987; Silvestre 1996; Akintorin 1997; Schanler 1999; Dollberg 2000) infants were stratified into groups, either by birth weight or gestational age prior to randomisation because the feeding method may influence outcomes differently across different weight groups. However, stratification varied between these studies (see Characteristics of Included Studies). Dsilna 2005 had two control groups in order to identify the influence of feeding tube placement (i.e., nasal versus oral).
Intervention Although feeding protocols had been predetermined for all studies, both the timing of initiation of feeds and the type of feeding varied between studies. In two studies (Silvestre 1996; Dollberg 2000), it is not clear when feeds were initiated, or if they were initiated at the same postnatal age for all infants. Although one study (Macdonald 1992) states on day two of life milk route was determined and milk feedings initiated, it is unclear if all study infants were able to start feeds on day two. In the study by Schanler 1999, infants within each feeding group were randomised to early or late feedings to be initiated on postnatal day four or 14 respectively (actual initiation day six and 16 respectively). The number of infants who started feeds early versus late was similar between the two groups. Toce 1987; Akintorin 1997 and Dsilna 2005 reported similar timing of feeds for infants in the intermittent and continuous feeding method groups. Infants in the Dsilna 2005 study were maintained nil per os for a mean of 2.5 days (SD = 4.0) in the intermittent group and a mean of 3.3 days (SD = 6.7). There was no significant difference between groups. Akintorin 1997 reported similar timing of feeds for infants in the two groups (intermittent 5.6 +/‐ 2.2 days; continuous 5.7 +/‐ 2.1 days). Toce 1987 initiated feeds later (intermittent 7.3 +/‐ 4.8 days; continuous 9.7 +/‐ 7.1 days), but the difference between groups was not significant.
The type of feeding might also influence feeding tolerance and advancement to full enteral feeds. One study (Dsilna 2005) excluded infants fed infant formula. Three studies (Macdonald 1992; Silvestre 1996; Akintorin 1997) excluded infants fed human milk. The other three studies (Toce 1987; Schanler 1999; Dollberg 2000) included infants fed either human milk or infant formula. In the studies by Dollberg 2000 and Toce 1987, there were more infants fed human milk in the intermittent bolus group as compared with the continuous group (Dollberg 2000 4 versus 2 respectively, and Toce 1987 5 versus 0 respectively). Both the timing and the type of feedings might influence outcomes such as days to reach full feeds, feeding intolerance and somatic growth.
Study quality Only four studies (Silvestre 1996; Akintorin 1997; Schanler 1999; Dsilna 2005) reported use of a power calculation to determine sample size. The primary outcome used to determine the sample size differed in each of the three studies and included days to full feeds, days to full oral feeds, and rate of weight gain. This systematic review includes 511 infants. The mean and standard deviation for days to full feeds from the largest study, Schanler 1999, was used to determine the sample size required to show a significant difference between Continuous and Intermittent bolus milk feeding. Based on a 2‐tailed unpaired t‐test, alpha level of 0.05 and power of 0.8, the sample size was estimated at 400 per group.
Only three studies included infants dropped from the feeding protocols in the analyses. Dsilna 2005 and Schanler 1999 included all infants in the analysis. Silvestre 1996 included all infants in the analyses of stratified groups, but excluded the infants dropped from the feeding protocols from the overall analysis. In each of the six studies included in this review, several infants were dropped from the treatment groups for various reasons, including feeding intolerance, protocol violations, and transfer to another hospital at two weeks of life. Exclusion of these infants who were unable to feed according to the assigned protocol might have impacted on outcomes including feeding tolerance, days to full feeds and days to discharge, as well as incidence of NEC and apnoea.
Caregivers were not blinded to the intervention as this would not be feasible. This may have introduced bias in decisions related to feeding management of infants.
Finally, although all studies attempted to compare demographic data and various indices of acuity between groups, the health state of the infant might affect some of the outcomes of interest. For example, smaller, sicker infants may take longer to reach full feeds, and be discharged later. Akintorin 1997 reported that infants with feeding intolerance were more likely to weigh less than 1000 grams and to require ventilatory support for a longer period of time. In that study, 20 of 28 infants with feeding intolerance were < 1000 grams birth weight.
Authors' conclusions
Implications for practice.
There is no difference in time to achieve full enteral feeds between continuous and intermittent bolus tube feeding method regardless of tube feed placement. However, the clinical risks and benefits of continuous and intermittent nasogastric tube milk feeding cannot be reliably discerned from current available randomised trials.
Implications for research.
Further research is needed to determine if either feeding method is more appropriate for the initiation of feeds, and if either method may be better tolerated by infants who experience feeding intolerance, a question not addressed in the current review. A rigorous methodology should be adopted, defining feeding protocols and feeding intolerance consistently for all infants. Infants should be stratified according to birthweight and gestation, and possibly according to illness.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2011 | New citation required but conclusions have not changed | Search was updated in July 2011. No new trials identified. Risk of Bias tables completed. No changes to conclusions. |
| 4 August 2011 | New search has been performed | This review updates the existing review "Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams", published in the Cochrane Database of Systematic Reviews (Premji 2004). |
History
Protocol first published: Issue 4, 1999 Review first published: Issue 1, 2001
| Date | Event | Description |
|---|---|---|
| 5 February 2008 | New search has been performed | This review updates the existing review "Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams", published in the Cochrane Database of Systematic Reviews, Issue 4, 2004 (Premji 2004). Two new trials were identified as a result of the most recent search completed October 26, 2007. The previous conclusion of no significant difference in somatic growth of infants fed by continuous versus intermittent bolus tube feeds remains unchanged. |
| 15 January 2008 | Amended | Converted to new review format. |
| 5 March 2004 | New search has been performed | Substantive amendment |
Acknowledgements
Hamilton Health Sciences Corporation Foundation.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Feeding performance | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days to full feeds | 5 | 424 | Mean Difference (IV, Fixed, 95% CI) | 1.82 [‐0.29, 3.93] |
| 1.2 Days to full oral feeds | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐4.85, 6.85] |
| 1.3 Days feeds interrupted for feeding intolerance | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | ‐3.0 [‐9.50, 3.50] |
| 1.4 Hours NPO per day | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.67, 2.47] |
| 1.5 Days on parenteral nutrition | 2 | 239 | Mean Difference (IV, Fixed, 95% CI) | ‐4.77 [‐9.52, ‐0.03] |
| 2 Growth | 6 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Days to regain birthweight | 4 | 401 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐1.48, 0.55] |
| 2.2 Weight gain (g/kg/day) | 2 | 224 | Mean Difference (IV, Fixed, 95% CI) | ‐1.13 [‐2.28, 0.03] |
| 2.3 Weight gain (g/week) | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | 6.27 [‐1.28, 13.81] |
| 2.4 Length gain (cm/week) | 4 | 330 | Mean Difference (IV, Fixed, 95% CI) | 0.08 [‐0.01, 0.17] |
| 2.5 Head circumference gain (cm/week) | 3 | 248 | Mean Difference (IV, Fixed, 95% CI) | ‐0.03 [‐0.09, 0.04] |
| 2.6 Change in triceps skinfold thickness (mm/week) | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 3 Utilization of resources | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Days to discharge | 2 | 253 | Mean Difference (IV, Fixed, 95% CI) | ‐1.94 [‐8.26, 4.38] |
| 3.2 Days to discharge weight of 2040g | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐7.92, 3.92] |
| 3.3 Days on mechanical ventilation | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐5.05, 4.05] |
| 4 Complications (categorical) | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Proven NEC (Bell's stage II or greater) | 5 | 465 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.09 [0.58, 2.07] |
| 4.2 Probable NEC | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.40, 5.89] |
| 4.3 Failure to complete protocol because of feeding intolerance | 2 | 264 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.48 [0.83, 7.40] |
| 5 Complications (continuous) | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Apnoea (episodes per day) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.99, 0.79] |
| 5.2 Apnoea (episodes during study) | 1 | 171 | Mean Difference (IV, Fixed, 95% CI) | 14.0 [‐0.20, 28.20] |
1.1. Analysis.
Comparison 1 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants, Outcome 1 Feeding performance.
1.2. Analysis.
Comparison 1 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants, Outcome 2 Growth.
1.3. Analysis.
Comparison 1 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants, Outcome 3 Utilization of resources.
1.4. Analysis.
Comparison 1 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants, Outcome 4 Complications (categorical).
1.5. Analysis.
Comparison 1 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding ‐ all infants, Outcome 5 Complications (continuous).
Comparison 2. Continuous versus intermittent bolus (nasogastric tube) milk feeding ‐ all infants.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Feeding performance | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days to full feeds | 4 | 229 | Mean Difference (IV, Fixed, 95% CI) | 1.82 [‐0.44, 4.08] |
| 1.2 Days to full oral feeds | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.3 Days feeds interrupted for feeding intolerance | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Hours NPO per day | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [‐0.67, 2.47] |
| 1.5 Days on parenteral nutrition | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Growth | 5 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Days to regain birthweight | 3 | 206 | Mean Difference (IV, Fixed, 95% CI) | ‐0.31 [‐1.65, 1.03] |
| 2.2 Weight gain (g/kg/day) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | 1.20 [‐1.01, 3.41] |
| 2.3 Weight gain (g/week) | 2 | 106 | Mean Difference (IV, Fixed, 95% CI) | 6.27 [‐1.28, 13.81] |
| 2.4 Length gain (cm/week) | 3 | 159 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.04, 0.18] |
| 2.5 Head circumference gain (cm/week) | 2 | 77 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [‐0.12, 0.13] |
| 2.6 Change in triceps skinfold thickness (mm/week) | 2 | 135 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.06, 0.06] |
| 3 Utilization of resources | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Days to discharge | 1 | 82 | Mean Difference (IV, Fixed, 95% CI) | ‐1.0 [‐8.62, 6.62] |
| 3.2 Days to discharge weight of 2040g | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐7.92, 3.92] |
| 3.3 Days on mechanical ventilation | 1 | 80 | Mean Difference (IV, Fixed, 95% CI) | ‐0.5 [‐5.05, 4.05] |
| 4 Complications (categorical) | 4 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Proven NEC (Bell's stage II or greater) | 4 | 270 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.23 [0.58, 8.57] |
| 4.2 Probable NEC | 2 | 133 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.53 [0.40, 5.89] |
| 4.3 Failure to complete protocol because of feeding intolerance | 1 | 93 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.15 [0.01, 2.87] |
| 5 Complications (continuous) | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 5.1 Apnoea (episodes per day) | 1 | 53 | Mean Difference (IV, Fixed, 95% CI) | ‐0.60 [‐1.99, 0.79] |
| 5.2 Apnoea (episodes during study) | 0 | 0 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
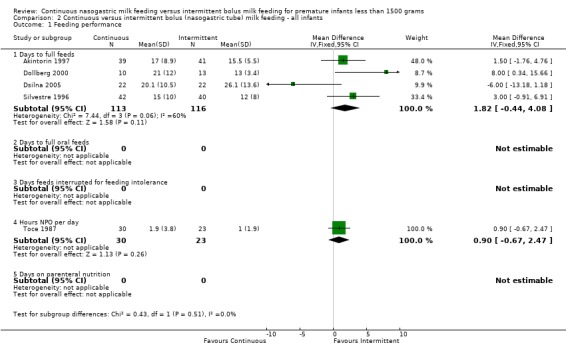
Comparison 2 Continuous versus intermittent bolus (nasogastric tube) milk feeding ‐ all infants, Outcome 1 Feeding performance.
2.2. Analysis.
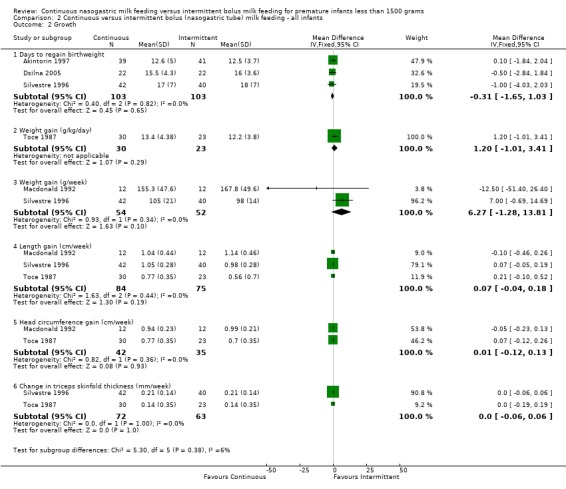
Comparison 2 Continuous versus intermittent bolus (nasogastric tube) milk feeding ‐ all infants, Outcome 2 Growth.
2.3. Analysis.
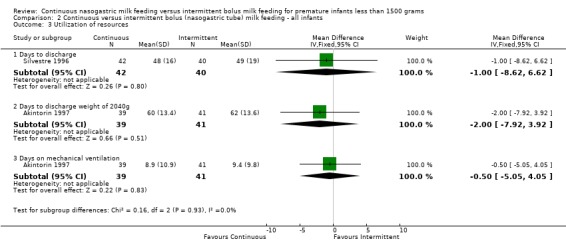
Comparison 2 Continuous versus intermittent bolus (nasogastric tube) milk feeding ‐ all infants, Outcome 3 Utilization of resources.
2.4. Analysis.
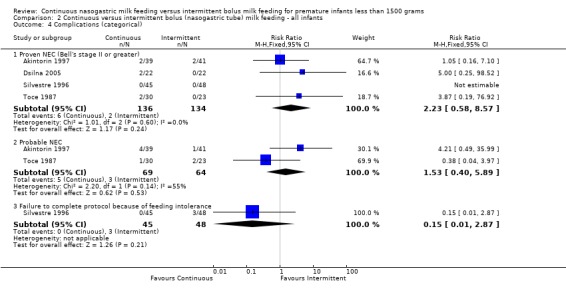
Comparison 2 Continuous versus intermittent bolus (nasogastric tube) milk feeding ‐ all infants, Outcome 4 Complications (categorical).
2.5. Analysis.
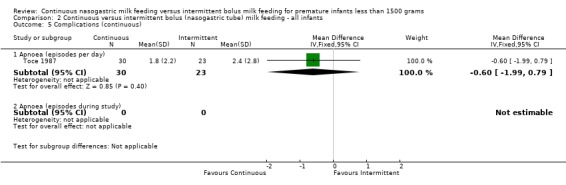
Comparison 2 Continuous versus intermittent bolus (nasogastric tube) milk feeding ‐ all infants, Outcome 5 Complications (continuous).
Comparison 3. Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding in infants < 1000g.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Feeding performance | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days to full feeds | 3 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐1.37 [‐4.59, 1.84] |
| 1.2 Days on parenteral nutrition | 1 | 55 | Mean Difference (IV, Fixed, 95% CI) | ‐10.5 [‐17.16, ‐3.84] |
| 2 Growth | 3 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Days to regain birthweight | 3 | 120 | Mean Difference (IV, Fixed, 95% CI) | ‐0.13 [‐2.11, 1.84] |
| 2.2 Weight gain (g/day) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.54, 3.46] |
| 2.3 Length gain (cm/week) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.08, 0.22] |
| 2.4 Head circumference gain (cm/week) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| 3 Utilization of resources | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐7.63, 2.66] |
| 3.1 Days to discharge | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐21.83, ‐0.17] |
| 3.2 Days to discharge weight of 2040 grams | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐5.85, 5.85] |
| 4 Complications (categorical) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Proven NEC (Bell's stage II or greater) | 1 | 44 | Risk Ratio (M‐H, Fixed, 95% CI) | 5.0 [0.25, 98.52] |
| 4.2 Probable NEC | 0 | 0 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
3.1. Analysis.
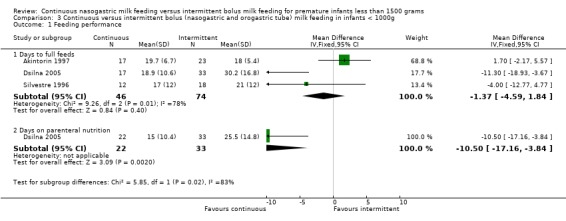
Comparison 3 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding in infants < 1000g, Outcome 1 Feeding performance.
3.2. Analysis.
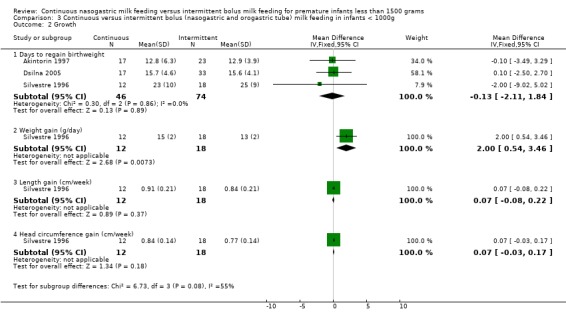
Comparison 3 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding in infants < 1000g, Outcome 2 Growth.
3.3. Analysis.
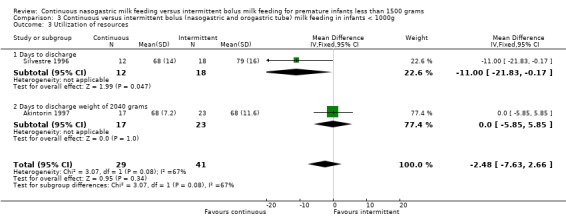
Comparison 3 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding in infants < 1000g, Outcome 3 Utilization of resources.
3.4. Analysis.
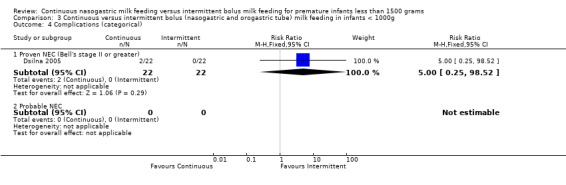
Comparison 3 Continuous versus intermittent bolus (nasogastric and orogastric tube) milk feeding in infants < 1000g, Outcome 4 Complications (categorical).
Comparison 4. Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants < 1000g.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Feeding performance | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days to full feeds | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | 0.77 [‐2.78, 4.31] |
| 2 Growth | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Days to regain birthweight | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐0.46 [‐3.51, 2.60] |
| 2.2 Weight gain (g/day) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.54, 3.46] |
| 2.3 Length gain (cm/week) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.08, 0.22] |
| 2.4 Head circumference gain (cm/week) | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | 0.07 [‐0.03, 0.17] |
| 3 Utilization of resources | 2 | 70 | Mean Difference (IV, Fixed, 95% CI) | ‐2.48 [‐7.63, 2.66] |
| 3.1 Days to discharge | 1 | 30 | Mean Difference (IV, Fixed, 95% CI) | ‐11.0 [‐21.83, ‐0.17] |
| 3.2 Days to discharge weight of 2040 grams | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐5.85, 5.85] |
4.1. Analysis.
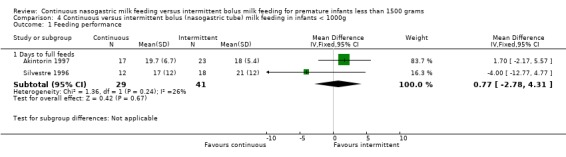
Comparison 4 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants < 1000g, Outcome 1 Feeding performance.
4.2. Analysis.
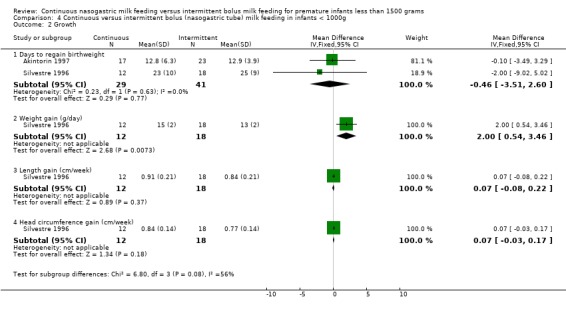
Comparison 4 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants < 1000g, Outcome 2 Growth.
4.3. Analysis.
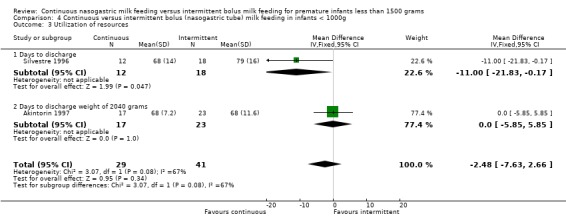
Comparison 4 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants < 1000g, Outcome 3 Utilization of resources.
Comparison 5. Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1000g and < 1249g.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Feeding performance | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days to full feeds | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.17 [‐2.54, 2.21] |
| 2 Growth | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Days to regain birthweight | 2 | 71 | Mean Difference (IV, Fixed, 95% CI) | ‐0.40 [‐2.45, 1.66] |
| 2.2 Weight gain (g/day) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 2.0 [0.16, 3.84] |
| 2.3 Length gain (cm/week) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.15, 0.15] |
| 2.4 Head circumference gain (cm/week) | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.52, 0.52] |
| 3 Utilization of resources | 2 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Days to discharge | 1 | 31 | Mean Difference (IV, Fixed, 95% CI) | ‐2.0 [‐9.18, 5.18] |
| 3.2 Days to discharge weight of 2040 grams | 1 | 40 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐3.01, 5.01] |
5.1. Analysis.
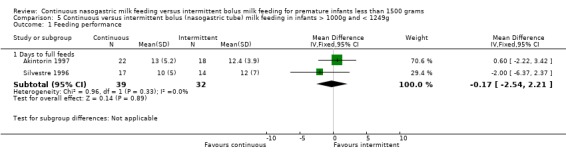
Comparison 5 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1000g and < 1249g, Outcome 1 Feeding performance.
5.2. Analysis.
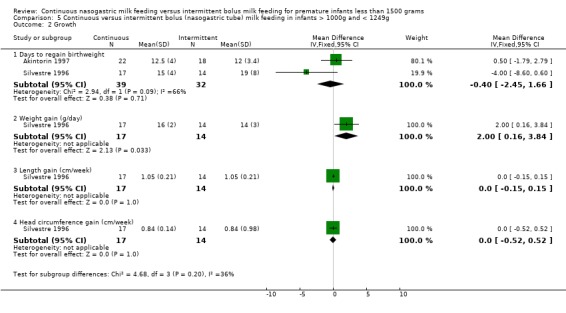
Comparison 5 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1000g and < 1249g, Outcome 2 Growth.
5.3. Analysis.
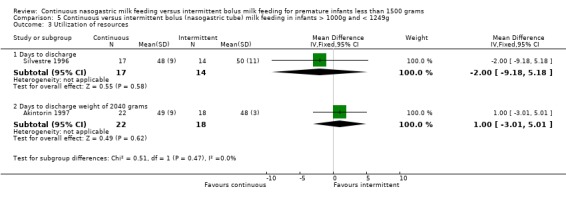
Comparison 5 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1000g and < 1249g, Outcome 3 Utilization of resources.
Comparison 6. Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1250g and < 1499g.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Feeding performance | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 1.1 Days to full feeds | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 5.0 [‐0.48, 10.48] |
| 2 Growth | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2.1 Days to regain birthweight | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐3.53, 3.53] |
| 2.2 Weight gain (g/day) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐1.77, 1.77] |
| 2.3 Length gain (cm/week) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.14 [‐0.08, 0.36] |
| 2.4 Head circumference gain (cm/week) | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 0.0 [‐0.10, 0.10] |
| 3 Utilization of resources | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 3.1 Days to discharge | 1 | 32 | Mean Difference (IV, Fixed, 95% CI) | 1.0 [‐3.85, 5.85] |
6.1. Analysis.
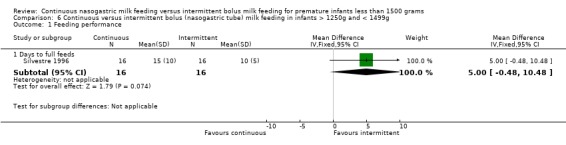
Comparison 6 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1250g and < 1499g, Outcome 1 Feeding performance.
6.2. Analysis.
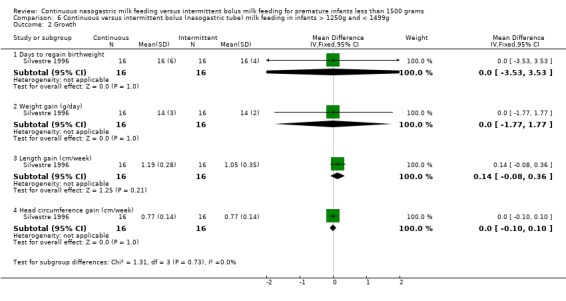
Comparison 6 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1250g and < 1499g, Outcome 2 Growth.
6.3. Analysis.
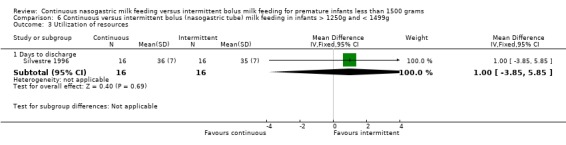
Comparison 6 Continuous versus intermittent bolus (nasogastric tube) milk feeding in infants > 1250g and < 1499g, Outcome 3 Utilization of resources.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Akintorin 1997.
| Methods | Randomised ‐ Yes. Blindness of randomisation ‐ Yes. Blindness of intervention ‐ No. Complete follow‐up ‐ No. Blinding of outcome measurement ‐ Can't tell. |
|
| Participants | 89 infants randomised. 9 post‐randomisation exclusions.
80 infants analysed. Inclusion: Infants 700‐1250g, haemodynamically stable and ready to start enteral feeds. Exclusion: Apgar score < 3 at 5 minutes, to receive breast milk, documented sepsis, NEC or unable to start feeding before day 10 of life. |
|
| Interventions | Feeding did not begin until umbilical arterial catheter removed. Continuous feeding by infusion pump. Intermittent feeding given every 3 hours for 15‐30 minutes by gravity via indwelling feeding tube. Feeding protocol for each 50‐100 g weight category. Protocol to manage feeding intolerance (feeds held > 12 hours). Energy and protein intake kept identical between groups. Feeds: undiluted preterm formula (20 Kcal/oz). Timing of Feeds: Protocol was < 10 postnatal days. Actual for Continuous group was 5.7 +/‐ 2.1 days and for Intermittent group was 5.6 +/‐ 2.2 days. |
|
| Outcomes | Primary: Days to full feeds (100 Kcal/kg/d). Secondary: Feeding intolerance, days to regain birth weight, days to discharge weight of 2040 g, NEC, and apnoea (>15 seconds). |
|
| Notes | Sample size calculation based on 35% decrease in number of days to full feeds in continuous group. Did not exclude SGA infants. Uncertain when feeds changed from continuous to bolus feeding. Numbers unbalanced. Exclusions: 4 Continuous (none due to protocol violation) and 5 Bolus (3 due to protocol violation). Larger proportion of infants whose feeds were held in the continuous group had residuals, whereas in the bolus group infants had apnea/bradycardia. Guidelines for residuals may allow larger volumes than some other studies. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Stratified according to birth weight into one of two groups (700‐1000g and 1001‐1250g). Randomly assigned within each weight group by using sequentially numbered opaque sealed envelopes using a table of random numbers. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Caregivers not blinded as would not be feasible. |
Dollberg 2000.
| Methods | Randomised ‐ Yes Blindness of randomisation ‐ Yes. Blindness of intervention ‐ No. Complete follow‐up ‐ No. Blinding of outcome measurement ‐ No. |
|
| Participants | 28 infants randomised.
5 post‐randomisation exclusions.
23 infants analysed. Inclusion: AGA, < 48 hours postnatal age, no major congenital malformations, and informed consent. |
|
| Interventions | Continuous feeding by syringe pump.
Intermittent feeding by gravity every 2 hours in 501 ‐ 750g group; every 3 hours in other infants. Feeding protocol for each weight group. Protocol to manage feeding intolerance (gastric residual > 20% of the volume fed over the previous 4 hr). Feeds: undiluted human milk, preterm formula, (initially diluted), or both. Timing of Feeds: Protocol was day 2‐5. Actual was not stated. |
|
| Outcomes | Days to full feeds (160 mL/kg/day). Days to regain birth weight. Delay between expected time to full feeds vs actual time to full feeds. | |
| Notes | Pilot study. Additional data re: intervention, sample size and methodologic criteria provided by investigator as study initially available as abstract (now published). No sample size calculation. 5 post‐randomisation exclusions. Regression analysis suggested mode of feeding as the only variable affecting feeding tolerance. Awaiting subgroup data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Stratified by birth weight (501 ‐ 750g; 751 ‐ 1000g; 1001 ‐ 1250g). Assigned randomly (using random numbers). The randomisation assignment was performed using sealed opaque envelopes that were grouped in an even blocked design, by the stratification variable (birthweight). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Investigators were not blinded to the study group assignment, but caregivers responsible for the infants' care and for feeding protocol performance were not part of the investigation. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Outcome assessors were not blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excluded nine infants from analysis. Of four patients excluded from the continuous group, one had been switched to breastfeeding, but none were excluded for feeding intolerance. Of five infants excluded from the intermittent group, three infants were excluded for protocol violation (feeding intolerance not specified) and one had been switched to breastfeeding. |
Dsilna 2005.
| Methods | Blindness of randomisation ‐ Yes. Blindness of intervention ‐ No. Complete follow‐up ‐ No. Blinding of outcome measurements ‐ for outcome of necrotizing enterocolitis only. | |
| Participants | 70 infants randomised.
2 post‐randomisation exclusion. 68 infants analysed. Inclusion: gestational age 24 to 29 weeks and birth weight < 1200 grams, stable respiratory status (i.e., arterial‐alveolar oxygen tension ratio >= 0.18), no major congenital malformations, maternal ability to read in Swedish, and residing within geographical catchment area of 3 independent neonatal units at Karolinska University Hospital. |
|
| Interventions | Feeding initiated before 30 hours postnatal age. Actual not stated. Feeds: mother's own milk or frozen pasturized human milk from the local milk bank. Continuous feedings by electric infusion pump and intermittent orogastric or nasogastric feedings given every third hour over a period of 15 to 40 minutes. Duration of feeding based on volume given and feeding difficulties experienced by infant. Protocol, based on infant's birth weight, followed for increasing feedings. At postmenstrual age of 32 weeks continuous nasogastric feedings weaned to intermittent feeding. Feeding intolerance managed by "clinical routine" which included reducing volume of feeding or temporarily withholding feeding. Fortification of human milk was initiated for all infants when total parenteral nutrition was discontinued. |
|
| Outcomes | Primary: Days to achieve full enteral feedings. Secondary: Time to regain birth weight, anthropometric measurements, enteral intolerance, necrotizing enterocolitis, and septicaemia. |
|
| Notes | Infants in the continuous feeding method group compared with infants from the 2 control groups (intermittent orogastric group and intermittent nasogastric group) combined as the two control groups did not "differ in primary outcome, demographic and birth‐related factors, and duration of feedings" (p. 45).
No significant difference in protein and energy intakes. Sample size calculation based on 40% difference in time to achieving full enteral feedings. Did not exclude SGA infants. Exclusions ‐ 2 post‐randomisation because of diagnosed malformations. Switched intermittent orogastric feeding to continuous nasogastric feeding for 14 days (N=1). Switched intermittent orogastric feeding to intermittent nasogastric feeding for 13 days (N=1). Switched intermittent nasogastric feeding to intermittent orogastric feeding for 6 days (N=1). (Not clear if intention‐to‐treat). Mortality ‐ one infant in each feeding group (total 3) died in the early intervention phase due to respiratory and circulatory collapse (not accounted for in sample of the study). Two infants died after postmenstrual ages of 33 and 47 weeks due to septicaemia combined with sever respiratory and circulatory distress and chronic lung disease. Both infants were in the continuous nasogastric feeding group (accounted for in the sample of the study). |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Randomised ‐ do not indicate what method was used (e.g., random number table; however, used sealed opaque envelopes. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Caregivers not blinded as would not be feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | Only radiographic assessors for the outcome of NEC were blinded to patient group assignment. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Excluded two infants post randomisation because of diagnosed malformations. One infant in the control group was switched to the continuous feeding method group because of severe apnoea and bradycardia secondary to gastroesophageal reflux. Data for this infant was included as randomised. In the early intervention phase, three infants died (one infant in each feeding group) due to respiratory and circulatory collapse. Two infants in the continuous feeding method group died at postmenstrual ages of 33 and 47 weeks secondary to septicaemia and severe respiratory and circulatory distress and chronic lung disease. |
Macdonald 1992.
| Methods | Randomised. No Stratification. Blindness of randomisation ‐ Yes. Blindness of intervention ‐ No. Complete follow‐up ‐ No. Blinding of outcome measurement ‐ Can't tell. |
|
| Participants | 43 infants randomised. 9 post‐randomisation exclusions. 34 infants analysed. Inclusion: Infants < 1400 g. Exclusion: Infants who received expressed breast milk, major congenital malformations, developed hydrocephalus, and if there was intrauterine viral infections. |
|
| Interventions | Milk feeding started on day 2 of life with 1 mL/hr of SMA low birthweight formula (Wyeth). Increased 0.5 to 1.0 mL/hr until tolerating 150 mL/kg/day (supplemented with total parenteral nutrition). Unclear frequency of bolus feeding, frequency of increase in feeds, equipment for delivery of continuous feeds. Selected method used until infant attained weight of 1600 g. No energy supplements during study period. |
|
| Outcomes | Growth rate including weight gain (grams/week), length gain (mm/week), and occipitofrontal circumference (mm/week), and triceps and quadriceps skinfold thickness, oral energy input, days to full feedings, and chosen biochemical indices (e.g., alkaline phosphatase, urea, albumin, prealbumin, and transferrin). Complications: extra abdominal radiographs, proved aspiration, NEC (proved and probable ‐ ?used Bell's staging), septicaemia, gastric bleeding. |
|
| Notes | No sample size calculation. Did not exclude SGA infants. Feeding intervention not described in detail. 5 exclusions in transpyloric group, 1 in continuous group and 3 in bolus group. Report pooled standard deviations for days to full feedings (awaiting mean and standard deviation). Extra abdominal radiographs (n=31) were performed for transpyloric tube placements. Aspiration (1 in continuous group) occurred when babies were over 1600 grams, hence were being fed by bolus nasogastric route. Gastric bleeding ( 1 in bolus group) occurred before milk feedings were started. Staphylococcus epidermidis ‐ 1 case in each group ‐ unclear if this was during study period or during length of stay in NICU. Sample size for each group obtained from author. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | On day 2 the feeding route was determined for each baby by opening a sealed envelope. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Caregivers not blinded as would not be feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Enrolled 13 babies in the continuous feeding method group but only 12 completed the study as one infant was transferred to another hospital at age two weeks of life. Three of the 15 infants enrolled in the bolus feeding method group died within the first week of life, before milk feedings were established and were therefore excluded from the analysis. |
Schanler 1999.
| Methods | Randomised ‐ Yes Blindness of randomisation ‐ yes. Blindess of intervention ‐ no. Complete follow‐up ‐ yes. Blinding of outcome measurements ‐ can't tell. |
|
| Participants | 171 infants randomised. Inclusion: 26 to 30 weeks gestation, AGA, postnatal age < or = 96 hours, no congenital anomalies, fraction of inspired oxygen < 0.6 by 72 hours, and written informed consent. Removed infants from treatment protocol if unable to adhere to feeding protocol for > 1 week. |
|
| Interventions | GI priming vs no enteral intake day 4 to 14 and continuous vs bolus nasogastric tube feedings. 4 Groups: NPO continuous, NPO bolus, GI priming continuous, and GI prime bolus. Bolus feeding given every 3 hours over 20 minutes. Continuous feeding method not described. Feeding protocol for infants. Protocol to manage feeding intolerance based on excess gastric residual volume. Nutrient intakes similar between groups. Feeds: undiluted human milk or initially diluted preterm infant formula. Timing of Feeds: Protocol was 4 ‐ 14 days. Actual was 6 ‐ 16 days. | |
| Outcomes | Primary: time to full oral feeding (8 breast/bottle feeding per day). Secondary: days to full enteral feeding (150cc/kg/d), weight gain, head circumference gain, length gain, skinfold thickness (5 sites), feeding intolerance, NEC, apnoea (>20 seconds), nutritional balance studies, bone mineral content and serum indices of protein and mineral status. |
|
| Notes | Data comparing continuous vs intermittent groups obtained from investigator.
Intent‐to‐treat analysis. Sample size calculation based on a 2 week difference in the time to full oral feeds. Infants randomised to early vs late enteral feeds (day 4 vs 14). 11 infants switched protocol (10 continuous and 1 bolus). Observed greater incidence of residuals with continuous feeds, and more infants unable to adhere to feeding protocols. Infants in the intermittent bolus feeding group ‐ tube placement predominantly (> 90%) orogastric (personal communication). Small sample size given the number of effects being examined (NPO, early feeding, and stratification of gestation age and feed type). Have established criteria for transition to oral feeds, however, not well described, some criteria subjective (e.g. favourable oral motor assessment, increased apnoea, or oxygen needs). Methods section states "The assigned orogastric/nasogastric tube‐feeding method (continuous vs bolus) was maintained throughout the three phases". There is no further mention of tube placement, and no way to know how the babies were in the intermittent group were fed. Awaiting subgroup data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Stratified by gestational age (26‐27 vs 28‐30 weeks) and by diet (human milk vs preterm formula) and assigned randomly among four treatment combinations in a balanced two‐way design where the two treatments were the use of GI priming versus "non per os", or no enteral intake (NPO) from day 4 through 14 after birth and the method of tube‐feeding, continuous infusion versus bolus. Randomisation was performed using sealed opaque envelopes that were grouped, in an uneven blocked design, by stratification variables (gestational age, intent to feed human milk). |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Caregivers not blinded as would not be feasible. |
| Blinding of outcome assessment (detection bias) All outcomes | Unclear risk | To ensure the objective assessment of the major outcome variable, the time required by the infant to attain full oral feeding (8 breast and/or bottle feedings per day), oral‐motor function was assessed serially, using a method designed specially for this study. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Complete follow‐up. Infants were removed from the treatment protocol if they were not able to adhere to the feeding protocol for more than one week. Ten infants in the continuous group and one in the intermittent group were removed from their assigned feeding protocols because of feeding intolerance. However, data from these infants were included in the analysis. |
Silvestre 1996.
| Methods | Randomised. Stratified: 750‐999g, 1000‐1249g, and 1250‐1499g. Blindness of randomisation ‐ Yes. Blindness of intervention ‐ No. Complete follow‐up ‐ Partial (only for stratified group). Blinding of outcome measurement ‐ Yes. |
|
| Participants | 93 infants randomised. 11 post randomisation exclusions. 82 infants analysed (all 93 infants included in analysis of stratified groups). Inclusion: Infants AGA with birth weight 750‐1500 grams, born between 27‐34 weeks gestation, had no major congenital malformations and stable to start feeds on day 2 or 3 of life. |
|
| Interventions | Continuous feeds administered over 3 hours, every 3 hours. Intermittent bolus feeds every 3 hours over 15‐30 minutes. Feeding protocol for infants. Criteria to define feeding intolerance predetermined (gastric residual volume >= 2 hr feed for continuous or >= 2mL bolus feeds). Feeds: water, initially diluted preterm infant formula. Timing of Feeds: Protocol was day 2‐3. Actual was not stated. | |
| Outcomes | Primary: rate of weight gain. (*growth data was converted to grams/week). Secondary: days to full feeds, days to regain birth weight, days to discharge, length gain, and head circumference gain. |
|
| Notes | Clarification of data for head circumference requested (data printed in the article appears to be significant but was reported as insignificant ‐ ? typographical error in data). Data on complete study sample not "intent‐to‐treat". Intent‐to‐treat analysis by weight groups. Sample size calculation based on >/= 10% increase rate of weight gain in continuous group. Full feeds not defined. 11 exclusions: 3 in Continuous group (1 protocol violation), 8 in Bolus group (3 feeding intolerance, 2 protocol violations). Unbalanced numbers between groups. ?criteria for discharge. Initial feed of water given for different duration (2 hours in continuous group vs 6 hours in bolus group). Nipple feeding 34 wks or 1500 grams. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | Infants were randomly assigned to either continuous or intermittent feedings. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | In this study, investigators were not unaware of the feeding group assignment. |
| Blinding of outcome assessment (detection bias) All outcomes | Low risk | Outcome assessors were blinded. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reported complete follow‐up only for stratified groups. In this study, 11 infants were removed from treatment protocols and excluded from overall analyses. Of three infants excluded from the continuous group, none were excluded for feeding intolerance, although one was excluded for protocol violation. Of eight infants excluded from the intermittent group, three were excluded for feeding intolerance and another two for protocol violation. Follow‐up was incomplete in the remaining three studies. |
Toce 1987.
| Methods | Quasi‐experimental. Alternate assignment within 16 groups.
Stratified:
<1250g,
1250‐1500g,
sex,
IUGR, and prior need for ventilation. Blindness of randomisation ‐ Can't tell. Blindness of intervention ‐ No. Complete follow‐up ‐ No. Blinding of outcome measurement ‐ Can't tell. |
|
| Participants | 83 infants
(obtained consent). 30 excluded (completed less than 7 days). 53 analysed. Inclusion: Preterm infants <= 1500grams, no major congenital anomalies, no longer ventilated, and ready for enteral nutrition. |
|
| Interventions | Continuous feeds delivered by infusion pump. Intermittent feeds every 3 hours by gravity. Feeding protocol for infants. Predetermined criteria to manage feeding intolerance (feeds held > 16 hours). Energy intake constant between groups. Feeds: sterile water, initially diluted formula. Timing of Feeds: Protocol was not stated. Actual for Continuous group was 9.7 +/‐ 7.1 days and for Intermittent group was 7.3 +/‐ 4.8 days. | |
| Outcomes | Somatic growth (weight, length, head circumference, and skinfold thickness gains), feeding related complications, changes in total protein, bilirubin, and albumin. | |
| Notes | Subjective eligibility criteria, no sample size calculation, and not intent‐to‐treat. Definition of feeding intolerance not described. Significant differences in demographic factors between groups: low one‐minute Apgar scores in the Continous group, and increase frequency of human milk feeding in the Intermittent bolus gavage feeding method. Awaiting subgroup data. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | High risk | Quasi‐experimental. Alternate assignment within 16 groups. Stratified: <1250g, 1250‐1500g, sex, IUGR, and prior need for ventilation. |
| Blinding of participants and personnel (performance bias) All outcomes | Unclear risk | Caregivers not blinded as would not be feasible. |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Post‐randomisation exclusion of infants from the analysis resulted in loss to follow‐up. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Baker 1997 | Outcomes not clinically relevant to this review ‐ duodenal motor responses. |
| Berseth 1992 | Population unknown (i.e. weight). Outcomes not clinically relevant to this review ‐ intestinal motor activity. |
| Dsilna 2008 | Outcomes reported are not clinically relevant to this review. |
Contributions of authors
S. Premji (SSP) and L. Chessell (CL) ‐ wrote protocol, searched for trials, selected studies, extracted data, wrote review.
SSP ‐ input data into RevMan, performed data‐analyses.
CL ‐ corresponded with authors, double checked data entry.
Sources of support
Internal sources
Hamilton Health Sciences Corporation Foundation, Canada.
External sources
No sources of support supplied
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Akintorin 1997 {published data only}
- Akintorin SM, Kamat M, Pildes RS, Kling P, Andes S, Hill J, Pyati S. A prospective randomized trial of feeding methods in very low birth weight infants. Pediatrics 1997;100:E4. [DOI] [PubMed] [Google Scholar]
Dollberg 2000 {published and unpublished data}
- Dollberg S, Kuint J, Mazkereth R, et al. Feeding tolerance in preterm infants: Randomized trial of bolus and continuous feeding. Journal of the American College of Nutrition 2000;19(6):797‐800. [DOI] [PubMed] [Google Scholar]
Dsilna 2005 {published and unpublished data}
- Dsilna A, Christensson K, Alfredsson L, et al. Continuous feeding promotes gastrointestinal tolerance and growth in very low birth weight infants. Journal of Pediatrics 2005;147:43‐9. [DOI] [PubMed] [Google Scholar]
Macdonald 1992 {published and unpublished data}
- Macdonald PD, Skeoch CH, Carse H, Dryburgh F, Alroomi LG, Galea P, Gettinby G. Randomised trial of continuous nasogastric, bolus nasogastric, and transpyloric feeding in infants of birth weight under 1400 g. Archives of Disease in Childhood 1992;67:429‐31. [DOI] [PMC free article] [PubMed] [Google Scholar]
Schanler 1999 {published and unpublished data}
- Schanler RJ, Shulman RJ, Lau C. Feeding strategies for premature infants: Beneficial outcomes of feeding fortified milk versus preterm formula. Pediatrics 1999;103:1150‐7. [DOI] [PubMed] [Google Scholar]
- Schanler RJ, Shulman RJ, Lau C, Smith EO, Heitkemper MM. Feeding strategies for premature infants: Randomized trial of gastrointestinal priming and tube‐feeding method. Pediatrics 1999;103(2):434‐9. [DOI] [PubMed] [Google Scholar]
- Shulman RJ, Schanler RJ, Lau C, Heitkemper M, Ou CN, Smith EO. Early feeding, antenatal glucocorticoids, and human milk decrease intestinal permeability in preterm infants. Pediatric Research 1998;44:519‐23. [DOI] [PubMed] [Google Scholar]
Silvestre 1996 {published data only}
- Silvestre MA, Morbach CA, Brans YW, Shankaran S. A prospective randomized trial comparing continuous versus intermittent feeding method in very low birth weight neonates. Journal of Pediatrics 1996;128:748‐52. [DOI] [PubMed] [Google Scholar]
Toce 1987 {published data only}
- Toce SS, Keenan WJ, Homan SM. Enteral feeding in very‐low‐birth‐weight infants. A comparison of two nasogastric methods. American Journal of Diseases of Children 1987;141:439‐44. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Baker 1997 {published data only}
- Baker JH, Berseth CL. Duodenal motor responses in preterm infants fed formula with varying concentrations and rates of infusion. Pediatric Research 1997;42:618‐22. [DOI] [PubMed] [Google Scholar]
Berseth 1992 {published data only}
- Berseth CL, Baker J, DeVille K, et al. Rate of feeding and nutrient concentration affect preterm small intestinal motor responses to orogastric feedings. Gastroenterology 1992;103:1385(Abstract). [Google Scholar]
Dsilna 2008 {published data only}
- Dsilna A, Christensson K, Gustafsson AS, Lagercrantz H, Alfredsson L. Behavioural stress is affected by the mode of tube feeding in very low birth weight infants. Clinical Journal of Pain 2008;24:447‐55. [DOI] [PubMed] [Google Scholar]
Additional references
Aynsley‐Green 1982
- Aynsley‐Green A, Adrian TE, Bloom SR. Feeding and the development of enteroinsular hormone secretion in the preterm infant: Effects of continuous gastric infusions of human milk compared with intermittent boluses. Acta Paediatrica Scandinavica 1982;71:379‐83. [DOI] [PubMed] [Google Scholar]
Aynsley‐Green 1989
- Aynsley‐Green A. New insights into the nutritional management of newborn infants derived from studies of metabolic and endocrine inter‐relations during the adaptation of postnatal life. The Proceedings of the Nutrition Society 1989;48:283‐92. [DOI] [PubMed] [Google Scholar]
Aynsley‐Green 1990
- Aynsley‐Green A, Lucas A, Lawson GR, Bloom SR. Gut hormones and regulatory peptides in relation to enteral feeding, gastroenteritis, and necrotizing enterocolitis in infancy. Journal of Pediatrics 1990;117:S24‐32. [DOI] [PubMed] [Google Scholar]
Grant 1991
- Grant J, Denne SC. Effect of intermittent versus continuous enteral feeding on energy expenditure in premature infants. Journal of Pediatrics 1991;118:928‐32. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org, [updated March 2011]. [Google Scholar]
Krishnan 1981
- Krishnan V, Satish M. Continuous (C) vs. intermittent (I) nasogastric (N/G) feeding in very low birth weight (VLBW) infants. Pediatric Research 1981;15:537 (Abstract). [Google Scholar]
Lucas 1986
- Lucas A, Bloom SR, Aynsley‐Green A. Gut hormones and "minimal enteral feeding". Acta Paediatrica Scandinavica 1986;75:719‐23. [DOI] [PubMed] [Google Scholar]
Newell 1988
- Newell SJ, Sarkar PK, Durbin GM, Booth IW, McNeish AS. Maturation of the lower esophageal sphincter in the preterm baby. Gut 1988;29:167‐72. [DOI] [PMC free article] [PubMed] [Google Scholar]
Raudonis 1995
- Raudonis BM, Talbot LA. Use of critical thinking in research: The research critique. In: Talbot LA editor(s). Principles and practice of nursing research. St. Louis: Mosby, 1995:513‐534. [Google Scholar]
Urrutia 1983
- Urrutia J, Poole E. Continuous nasogastric versus intermittent gavage feedings in very low birth weight infants. Pediatric Research 1983;17:203A (Abstract). [Google Scholar]
Valman 1972
- Valman HB, Heath CD, Brown RJK. Continuous intragastric milk feeds in infants of low birth weight. BMJ 1972;3:547‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Premji 2001
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2001, Issue 1. [DOI: 10.1002/14651858.CD001819] [DOI] [PubMed] [Google Scholar]
Premji 2003
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2003, Issue 1. [DOI: 10.1002/14651858.CD001819] [DOI] [PubMed] [Google Scholar]
Premji 2004
- Premji S, Chessell L. Continuous nasogastric milk feeding versus intermittent bolus milk feeding for premature infants less than 1500 grams. Cochrane Database of Systematic Reviews 2004, Issue 4. [DOI: 10.1002/14651858.CD001819] [DOI] [PMC free article] [PubMed] [Google Scholar]


