Abstract
This study describes results of PCR and viral RNA testing for SARS-CoV-2 in bronchoalveolar fluid, sputum, feces, blood, and urine specimens from patients with COVID-19 infection in China to identify possible means of non-respiratory transmission.
An epidemic of respiratory disease caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) began in China and has spread to other countries.1 Real-time reverse transcriptase–polymerase chain reaction (rRT-PCR) of nasopharyngeal swabs typically has been used to confirm the clinical diagnosis.2 However, whether the virus can be detected in specimens from other sites, and therefore potentially transmitted in other ways than by respiratory droplets, is unknown.
Methods
We investigated the biodistribution of SARS-CoV-2 among different tissues of inpatients with coronavirus disease 2019 (COVID-19) diagnosed based on symptoms and radiology and confirmed by SARS-CoV-2 detection. This study was approved by the ethics commissions of the participating hospitals, with a waiver of informed consent.
Patients with specimens collected based on clinical indications from 3 hospitals in the Hubei and Shandong provinces and Beijing, China, from January 1 through February 17, 2020, were included. Pharyngeal swabs were collected from most patients 1 to 3 days after hospital admission. Blood, sputum, feces, urine, and nasal samples were collected throughout the illness. Bronchoalveolar lavage fluid and fibrobronchoscope brush biopsy were sampled from patients with severe illness or undergoing mechanical ventilation. RNA was extracted from clinical specimens and determined by rRT-PCR targeting the open reading frame 1ab gene of SARS-CoV-2 as previously described.2 The cycle threshold values of rRT-PCR were used as indicators of the copy number of SARS-CoV-2 RNA in specimens with lower cycle threshold values corresponding to higher viral copy numbers. A cycle threshold value less than 40 is interpreted as positive for SARS-CoV-2 RNA. Four SARS-CoV-2 positive fecal specimens with high copy numbers were cultured, and then electron microscopy was performed to detect live virus. Patterns in a subgroup of patients with multiple specimens collected during hospitalization were explored.
Results
There were 1070 specimens collected from 205 patients with COVID-19 who were a mean age of 44 years (range, 5-67 years) and 68% male. Most of the patients presented with fever, dry cough, and fatigue; 19% of patients had severe illness. Bronchoalveolar lavage fluid specimens showed the highest positive rates (14 of 15; 93%), followed by sputum (72 of 104; 72%), nasal swabs (5 of 8; 63%), fibrobronchoscope brush biopsy (6 of 13; 46%), pharyngeal swabs (126 of 398; 32%), feces (44 of 153; 29%), and blood (3 of 307; 1%). None of the 72 urine specimens tested positive (Table).
Table. Detection Results of Clinical Specimens by Real-Time Reverse Transcriptase–Polymerase Chain Reaction.
| Specimens and values | Bronchoalveolar lavage fluid (n = 15) |
Fibrobronchoscope brush biopsy (n = 13) |
Sputum (n = 104) |
Nasal swabs (n = 8) |
Pharyngeal swabs (n = 398) |
Feces (n = 153) |
Blood (n = 307) |
Urine (n = 72) |
|---|---|---|---|---|---|---|---|---|
| Positive test result, No. (%) | 14 (93) | 6 (46) | 75 (72) | 5 (63) | 126 (32) | 44 (29) | 3 (1) | 0 |
| Cycle threshold, mean (SD) | 31.1 (3.0) | 33.8 (3.9) | 31.1 (5.2) | 24.3 (8.6) | 32.1 (4.2) | 31.4 (5.1) | 34.6 (0.7) | ND |
| Range | 26.4-36.2 | 26.9-36.8 | 18.4-38.8 | 16.9-38.4 | 20.8-38.6 | 22.3-38.4 | 34.1-35.4 | |
| 95% CI | 28.9-33.2 | 29.8-37.9 | 29.3-33.0 | 13.7-35.0 | 31.2-33.1 | 29.4-33.5 | 0.0-36.4 |
Abbreviation: ND, no data.
The mean cycle threshold values of all specimen types were more than 30 (<2.6 × 104 copies/mL) except for nasal swabs with a mean cycle threshold value of 24.3 (1.4 × 106 copies/mL), indicating higher viral loads (Table).
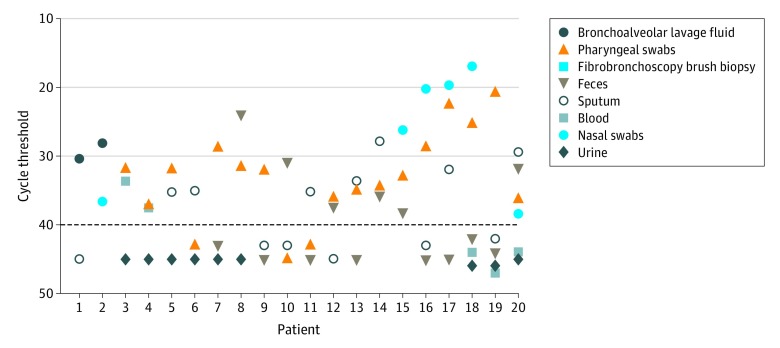
Twenty patients had 2 to 6 specimens collected simultaneously (Figure). Viral RNA was detected in single specimens from 6 patients (respiratory specimens, feces, or blood), while 7 patients excreted virus in respiratory tract specimens and in feces (n = 5) or blood (n = 2). Live SARS-CoV-2 was observed in the stool sample from 2 patients who did not have diarrhea.
Figure. Severe Acute Respiratory Syndrome Coronavirus 2 Distribution and Shedding Patterns Among 20 Hospitalized Patients.
The specimen with a cycle threshold value above the dashed line is interpreted as positive for SARS-CoV-2 RNA; those under, negative.
Discussion
In this study, SARS-CoV-2 was detected in specimens from multiple sites of 205 patients with COVID-19, with lower respiratory tract samples most often testing positive for the virus. Importantly, the live virus was detected in feces, implying that SARS-CoV-2 may be transmitted by the fecal route. A small percentage of blood samples had positive PCR test results, suggesting that infection sometimes may be systemic. Transmission of the virus by respiratory and extrarespiratory routes may help explain the rapid spread of disease. In addition, testing of specimens from multiple sites may improve the sensitivity and reduce false-negative test results. Two smaller studies reported the presence of SARS-CoV-2 in anal or oral swabs and blood from 16 patients in Hubei Province,3 and viral load in throat swabs and sputum from 17 confirmed cases.4
The limitations of this study include that some patients did not have detailed clinical information available, so the data could not be correlated with symptoms or disease course and that the number of some types of samples was small. Further investigation of patients with detailed temporal and symptom data and consecutively collected specimens from different sites is warranted.
Section Editor: Jody W. Zylke, MD, Deputy Editor.
References
- 1.Tan W, Zhao X, Ma X, et al. . Notes from the field: a novel coronavirus genome identified in a cluster of pneumonia cases—Wuhan, China 2019-2020. China CDC Weekly. 2020;2(4):61-62. [PMC free article] [PubMed] [Google Scholar]
- 2.Wang D, Hu B, Hu C, et al. . Clinical characteristics of 138 hospitalized patients with 2019 novel coronavirus–infected pneumonia in Wuhan, China. JAMA. Published February 7, 2020. doi: 10.1001/jama.2020.1585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang W, Du RH, Li B, et al. . Molecular and serological investigation of 2019-nCoV infected patients: implication of multiple shedding routes. Emerg Microbes Infect. 2020;9(1):386-389. doi: 10.1080/22221751.2020.1729071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Y, Zhang D, Yang P, Poon LLM, Wang Q. Viral load of SARS-CoV-2 in clinical samples. Lancet Infect Dis. Published online February 24, 2020. doi: 10.1016/S1473-3099(20)30113-4 [DOI] [PMC free article] [PubMed] [Google Scholar]