Abstract
Background
The fluoropyrimidines, 5‐fluorouracil (5‐FU) and capecitabine, are commonly used chemotherapeutic agents that have been associated with coronary vasospasm.
Methods
In this retrospective case‐control study, we identified patients at our institution who received 5‐FU or capecitabine in 2018. We compared characteristics of patients who experienced cardiotoxicity with controls. We described phenotypes and outcomes of cardiotoxic cases.
Results
We identified 177 patients who received fluoropyrimidines. After adjudication, 4.5% of the cohort met the criteria for cardiovascular toxicity. Coronary artery disease was more common among cases than controls (38% vs. 7%, p < .05). There was also a trend toward increased prevalence of cardiovascular risk factors in cases compared with controls. Most cardiotoxic cases had chest pain, although a minority of cases presented with nonischemic cardiomyopathy.
Conclusion
Cardiotoxicity phenotypes associated with fluoropyrimidine use are not limited to coronary vasospasm. Cardiac risk factors and ischemic heart disease were highly prevalent among patients with cardiotoxicity.
Keywords: Fluorouracil, Capecitabine, Cardiotoxicity
Short abstract
Cardiotoxicity is a complication of treatment with fluoropyrimidines. This article identifies cardiotoxicity phenotypes associated with fluoropyrimidine use, compares clinical characteristics and concurrent medication use among patients with and without cardiotoxicity, and describes clinical outcomes in patients who experienced fluoropyrimidine‐associated complications.
Introduction
Cardiotoxicity is a feared complication of fluoropyrimidines, occurring in 0%–5% of patients treated with 5‐fluorouracil (5‐FU) or capecitabine 1. Although the most common manifestation of fluoropyrimidine cardiotoxicity is angina caused by coronary vasospasm 2, other severe adverse cardiac events, including cardiomyopathy, myocarditis, and sudden cardiac death, have been reported 3. Cardiotoxicity from fluoropyrimidines typically occurs shortly after initiation of therapy, with an onset time of several hours after exposure 4. The identification of patients at risk for adverse cardiac events remains a major challenge, partly because of limited data on baseline cardiac risk factors in published cancer treatment trials 1. Cardioprotective strategies during fluoropyrimidine treatment include concomitant use of aspirin, calcium channel blockers, and nitrates 5. Bolus dosing of 5‐FU may also provide a cardioprotective benefit compared with continuous infusions 6.
In this study, we sought to identify cardiotoxicity phenotypes associated with fluoropyrimidine use, to compare clinical characteristics and concurrent medication use among patients with and without cardiotoxicity, and to describe clinical outcomes in patients who experienced fluoropyrimidine‐associated cardiotoxicity.
Methods
We retrospectively studied all patients in our institution who received either 5‐FU or capecitabine in 2018 as identified by an online clinical query tool. Cases of cardiotoxicity were determined based on at least one of the following signs or symptoms of cardiotoxicity within 2 weeks of fluoropyrimidine treatment: (A) symptoms consistent with coronary vasospasm, where an alternative etiology was felt to be unlikely, (B) new evidence of ischemia on electrocardiography, (C) new left ventricular dysfunction on echocardiography, (D) acute change in cardiac biomarkers, or (E) angiographic evidence of coronary vasospasm. Cases were adjudicated by two cardiologists. Baseline characteristics, cardiovascular risk factors, history of coronary artery disease, pharmacologic information, and outcomes were obtained from the medical record. Coronary artery disease history was determined based on documentation of history of acute coronary syndrome, positive stress test, or revascularization history by percutaneous coronary intervention or coronary artery bypass graft. Fisher's exact test was used to compare baseline characteristics in cases and controls. This study was approved by the Institutional Review Board at Beth Israel Deaconess Medical Center.
Results
Incidence of Cardiotoxicity
A total of 177 patients received either 5‐FU or capecitabine at our institution during 2018. In this cohort, 47% were women and 53% were men. After adjudication, eight patients were identified as having an adverse cardiac event associated with fluoropyrimidine treatment, representing an overall incidence of 4.5%. Six patients received 5‐FU, and two patients received capecitabine. The incidence of cardiotoxicity was 4.2% among patients who received 5‐FU and 5.3% among patients who received capecitabine.
Baseline Characteristics
Of the eight patients who experienced fluoropyrimidine‐associated cardiotoxicity, seven were male and one was female (Table 1). The median age was similar in cases and controls and was 63 years across the cohort. There was a trend toward increased prevalence of cardiac risk factors in cases versus controls: 75% versus 35% had high cholesterol, 75% versus 43% had hypertension, 75% versus 39% were current or prior tobacco users, and 37.5% versus 16% had diabetes. In addition, 37.5% versus 7% had a history of coronary artery disease (p < .05).
Table 1.
Baseline characteristics, pharmacologic data, cardiotoxicity phenotypes, and clinical outcomes for patients treated with fluoropyrimidines
| Data | Cases (n = 8), n (%) | Controls (n = 169), n (%) |
|---|---|---|
| Baseline characteristics | ||
| Male | 7 (87.5) | 90 (53) |
| Hyperlipidemia | 6 (75) | 59 (35) |
| Hypertension | 6 (75) | 72 (43) |
| Tobacco use | 6 (75) | 66 (39) |
| Diabetes | 3 (37.5) | 27 (16) |
| Coronary artery disease | 3 (37.5)a | 12 (7)a |
| Age, median (IQR), years | 62 (67–77) | 63 (54–71) |
| Pharmacologic data | ||
| Type of fluoropyrimidine | ||
| 5‐FU | 6 (75) | 133 (79) |
| Capecitabine | 2 (25) | 32 (19) |
| 5‐FU and capecitabine | 0 (0) | 4 (2) |
| Type of administration | ||
| Bolus dosing + continuous infusion | 5 (83) | 93 (68) |
| Continuous infusion | 1 (17) | 38 (28) |
| Baseline cardiac medications | ||
| Aspirin | 5 (62.5) | 35 (21) |
| Statin | 4 (50) | 45 (27) |
| Calcium channel blockers | 5 (62.5) | 26 (16) |
| Nitrates | 2 (25) | 1 (1) |
| Cardiotoxicity phenotypes | ||
| Chest pain | 8 (100) | |
| Elevated troponin | 3 (37.5) | |
| Ischemic ECG changes | 3 (37.5) | |
| New left ventricular systolic dysfunction | 2 (25) | |
| Clinical outcomes | ||
| Fluoropyrimidine therapy discontinued | 4 (50) | |
| New cardiac medication initiated | 6 (75) |
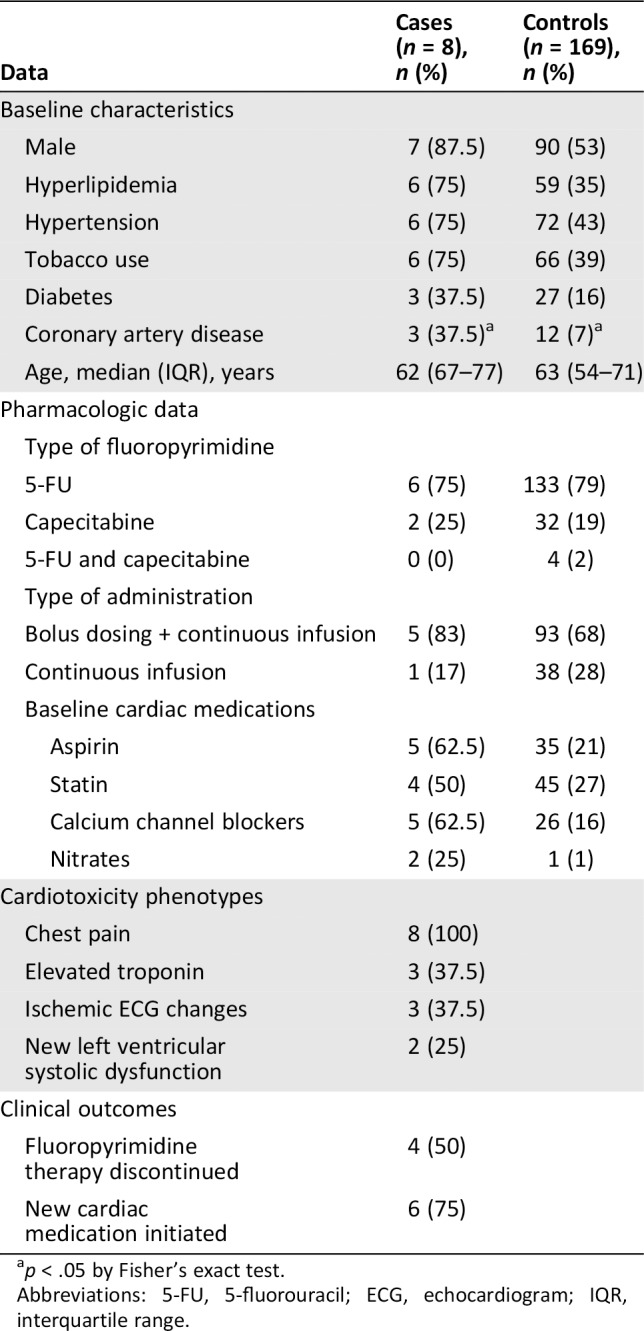
p < .05 by Fisher's exact test.
Abbreviations: 5‐FU, 5‐fluorouracil; ECG, echocardiogram; IQR, interquartile range.
Pharmacologic Data
Baseline use of cardiac medications was high across the cohort. More patients who experienced cardiotoxicity were prescribed cardiac medications, either for preexisting cardiovascular disease or for initiation of fluoropyrimidines, prior to the development of cardiotoxicity than their counterparts in the control group, including aspirin, statins, calcium channel blockers, and nitrates (Table 1). A majority of patients treated with 5‐FU received a combination of bolus dosing and continuous infusion (69%). The remainder received continuous infusions (27%) or bolus dosing alone (4%). Three cases of 5‐FU cardiotoxicity occurred within the first cycle of treatment, two cases occurred during the second cycle of treatment, and one case occurred during the third cycle of treatment. Capecitabine cardiotoxicity cases occurred after 3 days and 6 days of treatment. None of the patients who experienced a cardiotoxic event had received other cardiotoxic cancer medications, including anthracyclines and trastuzumab.
Clinical Outcomes
Anginal symptoms were the most common manifestation of cardiotoxicity and were present in all cases. Two patients (25% of cases) had new left ventricular dysfunction as determined by comparison with prior echocardiograms, although baseline echocardiography was not available for the entire cohort. After experiencing a cardiotoxic event, half of patients ultimately discontinued 5‐FU or capecitabine (Table 2). Of the four patients who stopped fluoropyrimidine treatment and were transitioned to alternate chemotherapies, three achieved remission, and one is still undergoing active treatment (Table 2). Of the patients who continued fluoropyrimidines, one patient completed the regimen, and three were transitioned to comfort care because of cancer progression and other side effects. A majority (75%) of all patients who developed cardiotoxicity were started on new cardiac medications, most commonly nitrates, calcium channel blockers, and aspirin.
Table 2.
Cardiac medications and clinical outcomes of patients with cardiotoxic events
| Patient | Cancer | Treatment | Cardiac medications | Cardiotoxic event | Clinical outcomes |
|---|---|---|---|---|---|
| Patient 1 | Esophageal adenocarcinoma | 5‐FU | Statin | Chest pain, troponin elevation, ischemic ECG changes, reduced ejection fraction | Discontinued 5‐FU; started on carboplatin, paclitaxel, trastuzumab |
| Patient 2 | Rectal adenocarcinoma | 5‐FU | Aspirin, Calcium channel blocker, Nitrates | Chest pain | Discontinued 5‐FU; no further treatment |
| Patient 3 | Rectal adenocarcinoma | Capecitabine | None | Chest pain, troponin elevation, ischemic ECG changes, reduced ejection fraction | Discontinued capecitabine; no further treatment |
| Patient 4 | Pancreatic ductal adenocarcinoma | Capecitabine | Aspirin, calcium channel blocker | Chest pain | Continued capecitabine |
| Patient 5 | Pancreatic ductal adenocarcinoma | 5‐FU | Aspirin, statin, calcium channel blocker, nitrates | Chest pain | Continued 5‐FU |
| Patient 6 | Pancreatic ductal adenocarcinoma | 5‐FU | Aspirin, statin | Chest pain | Continued 5‐FU |
| Patient 7 | Colon adenocarcinoma | 5‐FU | Aspirin, statin, calcium channel blocker | Chest pain, ischemic ECG changes | Discontinued 5‐FU; started on carboplatin, paclitaxel |
| Patient 8 | Pancreatic ductal adenocarcinoma | 5‐FU | Statin, calcium channel blocker | Chest pain, troponin elevation | Continued 5‐FU |
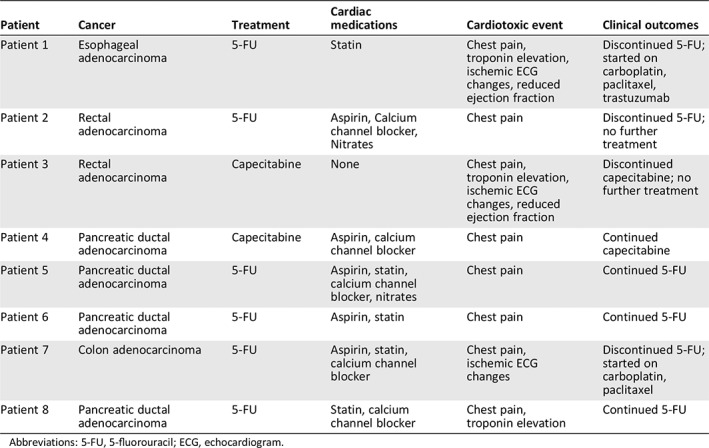
Abbreviations: 5‐FU, 5‐fluorouracil; ECG, echocardiogram.
Discussion
This retrospective case‐control study describes a number of findings that are hypothesis‐generating and worthy of future investigation. First, our event rate of 4.5% is consistent with contemporary incidence rates of fluoropyrimidine‐associated cardiotoxicity. Second, men were highly represented in cases of cardiotoxicity in our study. This is in contrast to prior observations implicating vasomotor dysfunction in the pathogenesis of fluoropyrimidine‐associated cardiotoxicity, an entity which is thought to disproportionately affect women compared with men 7. Third, patients in this study had relatively high rates of cardiovascular risk factors such as hyperlipidemia, hypertension, and smoking. We also found that patients with cardiotoxic events had higher rates of coronary artery disease at baseline. This is not readily explained by increased testing, as patients with and without cardiovascular risk factors had similar rates of electrocardiograms obtained prior to chemotherapy. Fourth, a majority of patients in our study who experienced cardiotoxicity were taking at least one cardiac medication prior to fluoropyrimidine initiation, including calcium channel blockers, nitrates, and aspirin. Fifth, we found that some patients with fluoropyrimidine cardiotoxicity had left ventricular dysfunction in the absence of overt ischemia, which may reflect a separate mechanism of cardiotoxicity 2, 8.
Cardiotoxicity from fluoropyrimidines can range in severity. Severe cases may require emergent administration of uridine triacetate, a pyrimidine analog that was approved as an antidote for fluoropyrimidine toxicity in 2015 9. When the diagnosis of fluoropyrimidine cardiotoxicity is unclear, testing for genetic variants in thymidylate synthase and dihydropyrimidine dehydrogenase may help support the diagnosis 10, 11. Patients who will be rechallenged with fluoropyrimidines should be initiated on aspirin, calcium channel blockers, and nitrates prior to administration of chemotherapy, although there are few data from randomized controlled trials to support the efficacy of this approach. Cardiology or cardio‐oncology consultation is encouraged to aid in the diagnosis, treatment, and prevention of fluoropyrimidine cardiotoxicity.
Study Limitations
This study was limited to a single institution and may not reflect broader patient populations and practice patterns. The small sample size may conceal clinically significant differences between cases and controls. The retrospective design of the study is subject to confounders.
Conclusion
Cardiotoxicity can be a life‐threatening complication of fluoropyrimidine therapy that limits appropriate treatment of highly morbid and metastatic cancers. Cardiac risk factors and history of ischemic heart disease were prevalent among patients who experienced cardiotoxic events after fluoropyrimidine use at our institution. Cardiotoxic events often resulted in discontinuation of fluoropyrimidine chemotherapy.
Disclosures
Mary Linton Peters: Agios (C/A), Bayer, AstraZeneca, Beigene, Berg, Taiho (RF), Exelixis (H), AstraZeneca, Exelixis, Halozyme (other—travel). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Acknowledgments
This study was supported by the Division of Cardiovascular Medicine and the Department of Medicine, Beth Israel Deaconess Medical Center.
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Upshaw JN, O'Neill A, Carver JR et al. Fluoropyrimidine cardiotoxicity: Time for a contemporaneous appraisal. Clin Colorectal Cancer 2019;18:44–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Mosseri M, Fingert HJ, Varticovski L et al. In vitro evidence that myocardial ischemia resulting from 5‐fluorouracil chemotherapy is due to protein kinase C‐mediated vasoconstriction of vascular smooth muscle. Cancer Res 1993;53:3028–3033. [PubMed] [Google Scholar]
- 3. Polk A, Vaage‐Nilsen M, Vistisen K et al. Cardiotoxicity in cancer patients treated with 5‐fluorouracil or Capecitabine: A systematic review of incidence, manifestations and predisposing factors. Cancer Treat Rev 2013;39:974–984. [DOI] [PubMed] [Google Scholar]
- 4. Becker K, Erckenbrecht JF, Haussinger D et al. Cardiotoxicity of the antiproliferative compound fluorouracil. Drugs 1999;57:475–484. [DOI] [PubMed] [Google Scholar]
- 5. Clasen SC, Ky B, O'Quinn R et al. Fluoropyrimidine‐induced cardiac toxicity: Challenging the current paradigm. J Gastrointest Oncol 2017;8:970–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Kosmas C, Kallistratos MS, Kopterides P et al. Cardiotoxicity of fluoropyrimidines in different schedules of administration: A prospective study. J Cancer Res Clin Oncol 2008;134:75–82. [DOI] [PubMed] [Google Scholar]
- 7. Aziz A, Hansen HS, Sechtem U et al. Sex‐related differences in vasomotor function in patients with angina and unobstructed coronary arteries. J Am Coll Cardiol 2017;70:2349–2358. [DOI] [PubMed] [Google Scholar]
- 8. Grunwald MR, Howie L, Diaz LA Jr. Takotsubo cardiomyopathy and fluorouracil: Case report and review of the literature. J Clin Oncol 2012;30:e11–e14. [DOI] [PubMed] [Google Scholar]
- 9. Ma WW, Saif MW, El‐Rayes BF et al. Emergency use of uridine triacetate for the prevention and treatment of life‐threatening 5‐fluorouracil and capecitabine toxicity. Cancer 2017;123:345–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lecomte T, Ferraz JM, Zinzindohoue F et al. Thymidylate synthase gene polymorphism predicts toxicity in colorectal cancer patients receiving 5‐fluorouracil‐based chemotherapy. Clin Cancer Res 2004;10:5880–5888. [DOI] [PubMed] [Google Scholar]
- 11. Caudle KE, Thorn CF, Klein TE et al. Clinical Pharmacogenetics Implementation Consortium guidelines for dihydropyrimidine dehydrogenase genotype and fluoropyrimidine dosing. Clin Pharmacol Ther 2013;94:640–645. [DOI] [PMC free article] [PubMed] [Google Scholar]


