Abstract
Anti–programmed cell death protein‐1 (anti‐PD‐1) therapy has greatly improved outcomes of patients with melanoma; however, many fail to respond. Although preclinical studies suggest a potentially synergistic relationship with anti‐PD‐1 therapy and certain concurrent medications, their clinical role remains unclear. Here, we retrospectively evaluated the use of nonsteroidal anti‐inflammatory drugs (NSAIDs) and other drugs in 330 patients with melanoma treated with anti‐PD‐1 therapy from four academic centers. In the cohort, 37% of patients used NSAIDs including aspirin (acetylsalicylic acid; ASA; 47%), cyclooxygenase (COX)‐2 inhibitors (2%), and non‐ASA/nonselective COX inhibitor NSAIDs (59%). The objective response rates (ORRs) were similar in patients with NSAID (43.4%) and no NSAID (41.3%) use with no significant difference in overall suvival (OS). There was a trend toward improved progression‐free survival (PFS) in patients who took NSAIDs (median PFS: 8.5 vs. 5.2 months; p = .054). Most patients (71.3%) took NSAIDs once daily or as needed. Multivariate analysis did not reveal an association with NSAID use with ORR, PFS, or OS. Concurrent use of metformin or beta blockers did not affect ORR, PFS, or OS. Our study found no conclusive association of concurrent NSAID or other medication use with improved outcomes in patients with melanoma treated with anti‐PD‐1 therapy. Larger and more systematic analysis is required to confirm these findings.
Short abstract
This retrospective study evaluated the association of nonsteroidal anti‐inflammatory drugs, beta blockers, and metformin with clinical outcomes in patients with advanced melanoma treated with anti‐PD‐1 therapy.
Introduction
Inflammation has been long recognized as a hallmark of cancer 1, 2. Malignant tumors are often surrounded by immune cells with similarities to other inflammatory conditions 3. Although an inflamed tumor suggests immune recognition of cancer neoantigens, cancers frequently evade the antitumor response through various escape mechanisms such as immune checkpoint upregulation or tumor‐promoting inflammation (e.g., tumor‐infiltrating macrophages). Although immune checkpoint inhibitors overcome some mechanisms of resistance, many tumors achieve immune evasion and fail to respond.
The use of aspirin and nonsteroidal anti‐inflammatory drugs (NSAIDs) is increasing in the U.S. population with recent estimates of 43 million and 29 million adults regularly taking aspirin (acetylsalicylic acid; ASA) and NSAIDs, respectively 4, 5, 6. Preclinical studies have demonstrated that a cyclooxygenase (COX)‐dependent mechanism drives tumor growth and that inhibition of COX, when synergized with programmed cell death protein‐1 (PD‐1) blockade, leads to tumor suppression, suggesting that NSAIDs could improve the efficacy of anti‐PD‐1 therapies 7, 8, 9. In addition, studies have suggested that other medications such as metformin and beta blockers may influence the outcomes of patients with advanced melanoma during treatment with PD‐1 blockade or in other settings 10, 11, 12, 13, 14, 15. In this retrospective study, we evaluated the association of NSAIDs, beta blockers, and metformin with clinical outcomes in patients with advanced melanoma treated with anti‐PD‐1 therapy.
Materials and Methods
We evaluated all patients with advanced melanoma treated with anti‐PD‐1 therapy (nivolumab or pembrolizumab) at four academic centers (n = 330) including Melanoma Institute Australia (n = 46), MD Anderson Cancer Center (n = 109), Westmead Hospital (n = 53), and Vanderbilt University Medical Center (n = 121) from October 2009 to January 2016, with institutional review board approval prior to the study. Waiver from patient's consent was approved because of the retrospective nature of this study. Baseline demographic, clinical, concurrent drug use, and treatment data were obtained from electronic medical records. The type of NSAIDs were divided into ASA; COX‐2 inhibitors; and non‐ASA, nonselective COX inhibitor NSAIDs (hereafter referred to as “other NSAIDs”). Patients were included if NSAIDs were started before anti‐PD‐1 therapy or within 6 weeks after anti‐PD‐1 treatment initiation. The use of other concurrent medications at baseline and within 6 weeks of anti‐PD‐1 treatment initiation, including beta blockers and metformin, was collected. Response was defined by the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria by cross‐sectional imaging and provided by medical oncologist at each collaborating institution. Progression‐free survival (PFS) and overall survival (OS) were described using the Kaplan‐Meier method and compared between groups using the log‐rank test. Comparison of response between groups was performed using chi‐square testing. Univariate and multivariable logistic regression models were used to test differences in response and survival with various concurrent medications controlled for age, gender, lactate dehydrogenase (LDH), number of prior therapies, center, stage, and body mass index (BMI). All statistical analyses were performed in R version 3.5.0.
Results
Baseline Characteristics
A total of 330 patients were included; 209 (63%) were male with a median age of 60 years (range, 16–86), and 237 (72%) were American Joint Committee on Cancer stage IV M1c (Table 1). Among these patients, 122 (37%) reported any NSAID use, including ASA and COX‐2 inhibitors. Of those patients who used NSAIDs (n = 122), 52% were on other NSAIDs only, 39% were on ASA only, 7% were on both NSAID and ASA, and 2% were on COX‐2 inhibitors only. Of the 72 patients who took other NSAIDs, 51 (71%) took ibuprofen and 15 (21%) took naproxen. Data on dosing were available in 37 patients with 28 (76%) patients on only as needed dosing and 9 (24%) patients on daily (or less frequent) dosing. Of patients on ASA, most (86%) received <100 mg daily.
Table 1.
Patient demographics
| Characteristic | (n = 330), n (%) |
|---|---|
| Mean age (range), years | 60 (16–86) |
| Male gender | 208 (63) |
| Mean BMI (range) | 27.6 (16.4–50.5) |
| Academic center | |
| MDACC | 109 (33) |
| MIA | 46 (14) |
| Vanderbilt | 121 (37) |
| WMH | 53 (16) |
| Elevated LDH | 113 (34) |
| Stage | |
| IIIC/M1a | 42 (13) |
| M1b | 50 (15) |
| M1c/d | 234 (72) |
| NSAID use | 122 (37) |
| NSAID only | 63 (19) |
| ASA only | 47 (14) |
| Both NSAID and ASA | 10 (3) |
| COX inhibitors | 2 (1) |
| Other drugs | |
| Metformin | 34 (10) |
| Beta blocker | 65 (20) |
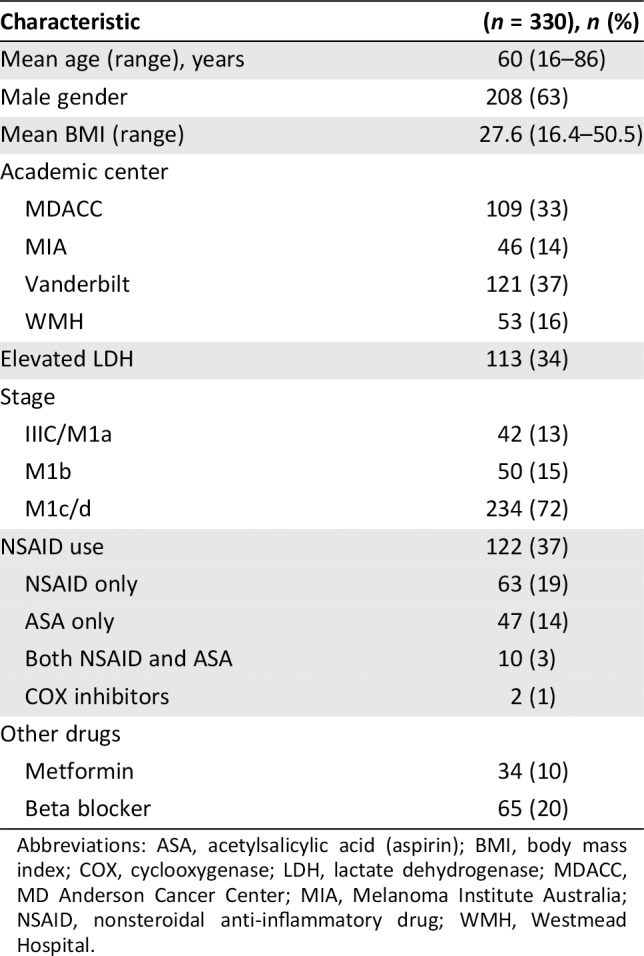
Abbreviations: ASA, acetylsalicylic acid (aspirin); BMI, body mass index; COX, cyclooxygenase; LDH, lactate dehydrogenase; MDACC, MD Anderson Cancer Center; MIA, Melanoma Institute Australia; NSAID, nonsteroidal anti‐inflammatory drug; WMH, Westmead Hospital.
In terms of timing of concurrent medication, 8 and 55 patients started ASA and NSAID, respectively, after initiation of anti‐PD‐1 therapy. Furthermore, metformin and beta blockers were started in six and five patients, respectively, after initiation of anti‐PD‐1 therapy. Notably, nine patients developed arthritis/arthralgia or rheumatoid arthritis flare while on therapy.
NSAID Use and Efficacy
In the entire group, the objective response rate (ORR) was 42.1% (partial response, 26.7%; complete response, 15.5%). Median PFS and OS were 5.8 months (range, 0.5–75.2 months) and 26.9 months (range, 0.9–75.2 months), respectively. There was no significant difference in ORR (43.4% vs. 41.3%; p = .71) in patients who used NSAIDs (including ASA and COX‐2 inhibitors) versus those who did not. Patients who used NSAIDs had a nonsignificant trend toward improved PFS (median PFS: 8.5 vs. 5.2 months; p = .054; hazard ratio [HR], 0.76; 95% confidence interval [CI], 0.57–1.00; Fig. 1A), but there was no difference in OS (median OS: 25.7 vs. 27.3 months; p = .58; HR, 0.91; 95% CI, 0.66–1.26; Fig. 1B).
Figure 1.
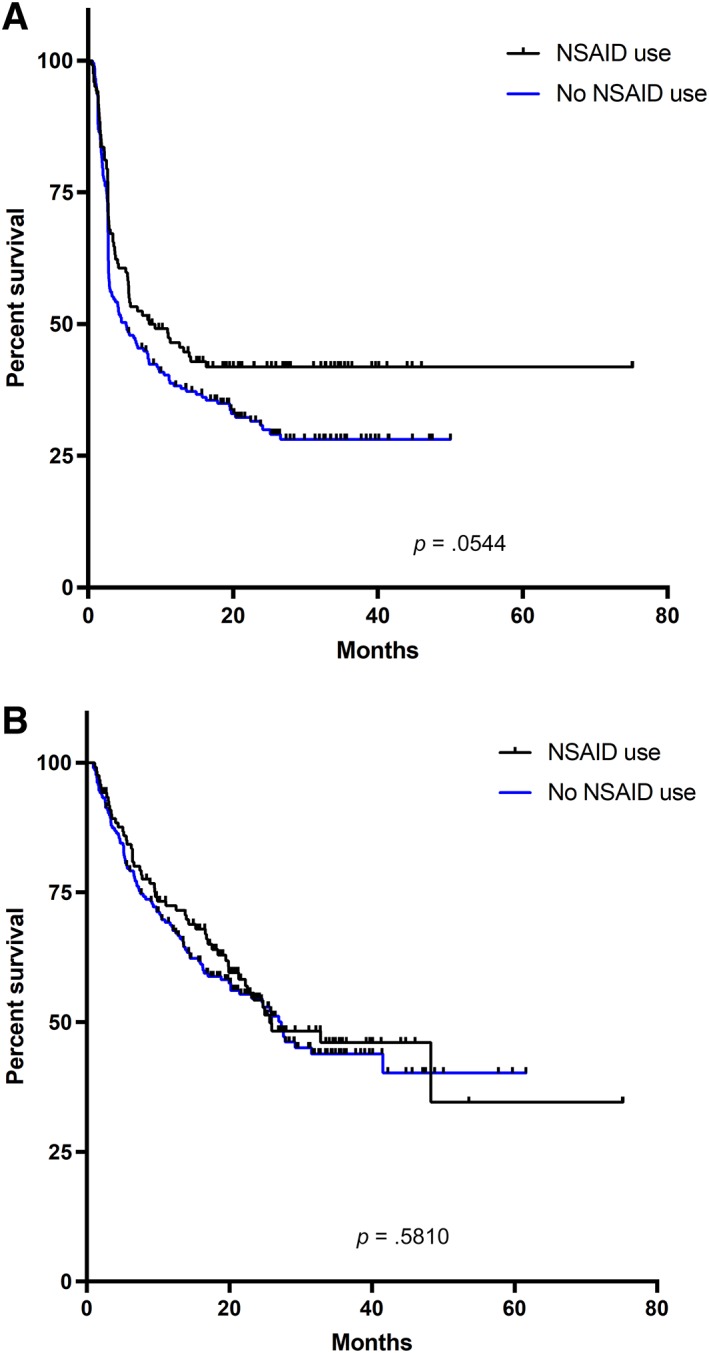
Progression‐free and overall survival based on NSAID usage. (A): Progression‐free survival based on NSAID usage. (B): Overall survival based on NSAID usage.Abbreviation: NSAID, nonsteroidal anti‐inflammatory drug.
Multivariable analysis adjusting for age, gender, stage LDH, prior therapies, aspirin dose, and BMI did not reveal an association between NSAID use with PFS (HR, 0.80; 95% CI, 0.59–1.09; p = .15) or OS (HR, 0.84; 95% CI, 0.59–1.20; p = .34). Furthermore, NSAID use did not lead to higher likelihood of response (HR, 1.08; 95% CI, 0.85–1.38; p = .76). Similarly, aspirin use at higher doses (≥100 mg) was not associated with improved PFS (HR, 2.19; 95% CI, 0.44–10.85; p = .34) or OS (HR, 1.45; 95% CI, 0.22–9.8).
Other Medications Use and Efficacy
Metformin and beta blocker use was also assessed. Among this cohort, only 34 (10.3%) patients used metformin. There was no improvement in PFS (median 11.1 vs. 5.6 months; p = .36; HR, 0.81; 95% CI, 0.54–1.23) or OS (median 27.6 vs. 26.0 month; p = .48; HR, 0.82; 95% CI, 0.50 – 1.35) with the use of metformin. Beta blockers were used in 65 (19.8%) patients. These included atenolol (12.3%), bisoprolol (6.2%), carvedilol (15.4%), labetalol (1.5%), metoprolol (60%), nebivolol (3.1%), and solatol (3.1%). Again, there was no significant difference in either PFS (median 11.2 vs. 5.5 months; p = .40; HR, 0.86; 95% CI, 0.62–1.20) or OS (median 27.8 vs. 25.8 months; p = .57; HR, 0.89; 95% CI, 0.60–1.32) with beta blocker use.
Discussion
Although the use of PD‐1 blockade has dramatically improved the clinical outcomes of patients with advanced melanoma, most patients experience intrinsic or acquired therapeutic resistance. Preclinical studies support a mechanism for COX‐2 inhibition to potentiate a response with PD‐1 blockade; however, our study did not show any significant improvement in response or survival with the concurrent use of NSAIDs in a real‐world population. Additionally, the use of other concurrent medications including metformin and beta blockers did not affect efficacy. Despite the largely negative statistical findings, we noted a trend toward improvement in PFS with NSAID use that may suggest a signal of efficacy and may need to be further explored in a larger prospective study.
Interestingly, preclinical models suggest COX‐2 inhibition leads to a significant reduction in distant metastasis along with inhibition of tumor growth, suggesting a benefit in the adjuvant setting after curative therapy 16, 17, 18. With the approval of anti‐PD‐1 therapy in the adjuvant setting for melanoma, further studies in this setting are needed to assess its role in preventing recurrence along with anti‐PD‐1 therapy. In addition, the the rising obesity epidemic in the U.S. has led to increase use of NSAIDs and other medications, which has important implications in the outcomes of patients with melanoma. A study from our groups demonstrated a significant survival benefit for obese men who were treated with immunotherapy and targeted therapy, which perhaps could be confounded by concurrent medication 19. However, we did not observe a clear contributor to such an association in this study.
The limitations of this study include small numbers of patients, potential underreporting of medication use and frequency, and retrospective data collection. In particular, the dose and type of NSAIDs were quite heterogeneous, with many patients taking intermittent or low doses of NSAIDs that may not potentiate antitumor immunity. Studies in melanoma xenografted mice showed tumor shrinkage with chronic daily aspirin administration, suggesting a dose‐dependent mechanism, which needs to be further explored 13. Along with pharmacodynamics studies of the drugs, further molecular studies of the tumors would help elucidate any prognostic biomarkers of response with these concurrent medications. Inherent issues with the variability in scan frequency and measurement techniques used at each site may also affect response and survival assessment. Additionally, 2.7% of patients developed inflammatory arthritis, which may have additionally counfounded the survival results, although this relationship needs to be confirmed prospectively. However, the percentage of cases seems in line with the reported 1% to 7% seen in clinical trials 20.
In conclusion, we observed that routine use of NSAIDs, beta blockers, and/or metformin did not significantly improve response or survival in a modest‐sized cohort of melanoma patients treated with anti‐PD‐1 therapy. These findings need to be confirmed with larger and more systematic analysis.
Disclosures
Jennifer L. McQuade: Merck, Bristol‐Myers Squibb (C/A); Georgina V. Long: Aduro, Amgen, Array, Bristol‐Myers Squibb, Merck Sharp Dohme, Novartis, Pierre‐Fabre, Oncosec, Roche (C/A); Matteo S. Carlino: Merck Sharp Dohme, Bristol‐Myers Squibb, Amgen, Novartis, Roche, IDEAYA (SAB); Alexander M. Menzies: BMD, Merck Sharp Dohme, Novartis, Roche, Pierre‐Fabre (C/A); Michael A. Davies: Bristol‐Myers Squibb, Novartis, Array, Roche/Genentech, GlaxoSmithKline (C/A), Bristol‐Myers Squibb, Roche/Genentech, GlaxoSmithKline (RF); Douglas B. Johnson: Array, Bristol‐Myers Squibb, Incyte, Merck, Novartis (C/A), Bristol‐Myers Squibb, Incyte (RF). The other authors indicated no financial relationships.
(C/A) Consulting/advisory relationship; (RF) Research funding; (E) Employment; (ET) Expert testimony; (H) Honoraria received; (OI) Ownership interests; (IP) Intellectual property rights/inventor/patent holder; (SAB) Scientific advisory board
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures
Acknowledgments
D.B.J received funding from the James C. Bradford Jr. Melanoma Fund and NIH K23CA204726. G.V.L. is supported by an Australian National Health and Medicine Research Council Practitioner Fellowship and the University of Sydney Medical Foundation.
Disclosures of potential conflicts of interest may be found at the end of this article.
Contributor Information
Daniel Y. Wang, Email: danielw@bcm.edu.
Douglas B. Johnson, Email: douglas.b.johnson@vumc.org.
References
- 1. Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor‐promoting chronic inflammation: A magic bullet? Science 2013;339:286–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144:646–674. [DOI] [PubMed] [Google Scholar]
- 3. Dvorak HF. Tumors: Wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med 1986;315:1650–1659. [DOI] [PubMed] [Google Scholar]
- 4. Zhou Y, Boudreau DM, Freedman AN. Trends in the use of aspirin and nonsteroidal anti‐inflammatory drugs in the general U.S. population. Pharmacoepidemiol Drug Saf 2014;23:43–50. [DOI] [PubMed] [Google Scholar]
- 5. Goulet A‐C, Einsphar JG, Alberts DS et al. Analysis of cyclooxygenase 2 (COX‐2) expression during malignant melanoma progression. Cancer Biol Ther 2003;2:713–718. [PubMed] [Google Scholar]
- 6. Panza E, De Cicco P, Ercolano G et al. Differential expression of cyclooxygenase‐2 in metastatic melanoma affects progression free survival. Oncotarget 2016;7:57077–57085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zelenay S, van der Veen AG, Bottcher JP et al. Cyclooxygenase‐dependent tumor growth through evasion of immunity. Cell 2015;162:1257–1270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Botti G, Fratangelo F, Cerrone M et al. COX‐2 expression positively correlates with PD‐L1 expression in human melanoma cells. J Transl Med 2017;15:46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Kumar D, Rahman H, Tyagi E et al. Aspirin suppresses PGE2 and activates AMP kinase to inhibit melanoma cell motility, pigmentation, and selective tumor growth in vivo. Cancer Prev Res (Phila) 2018;11:629–642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Afzal MZ, Mercado RR, Shirai K. Efficacy of metformin in combination with immune checkpoint inhibitors (anti‐PD‐1/anti‐CTLA‐4) in metastatic malignant melanoma. J Immunother cancer 2018;6:64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kokolus KM, Zhang Y, Sivik JM et al. Beta blocker use correlates with better overall survival in metastatic melanoma patients and improves the efficacy of immunotherapies in mice. Oncoimmunology 2018;7:e1405205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Giorgi V, Grazzini M, Benemei S et al. Propranolol for off‐label treatment of patients with melanoma: Results from a cohort study. JAMA Oncol 2018;4:e172908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Cha JH, Yang WH, Xia W et al. Metformin Promotes antitumor immunity via endoplasmic‐reticulum‐associated degradation of PD‐L1. Mol Cell 2018;71:606‐620.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Cerezo M, Tichet M, Abbe P et al. Metformin blocks melanoma invasion and metastasis development in AMPK/p53‐dependent manner. Mol Cancer Ther 2013;12:1605–1615. [DOI] [PubMed] [Google Scholar]
- 15. Tomic T, Botton T, Cerezo M et al. Metformin inhibits melanoma development through autophagy and apoptosis mechanisms. Cell Death Dis 2011;2:e199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Zhou P, Qin J, Li Y et al. Combination therapy of PKCzeta and COX‐2 inhibitors synergistically suppress melanoma metastasis. J Exp Clin Cancer Res 2017;36:115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim KM, Im AR, Kim SH et al. Timosaponin AIII inhibits melanoma cell migration by suppressing COX‐2 and in vivo tumor metastasis. Cancer Sci 2016;107:181–188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sadhu SS, Wang S, Averineni RK et al. In‐vitro and in‐vivo inhibition of melanoma growth and metastasis by the drug combination of celecoxib and dacarbazine. Melanoma Res 2016;26:572–579. [DOI] [PubMed] [Google Scholar]
- 19. McQuade JL, Daniel CR, Hess KR et al. Association of body‐mass index and outcomes in patients with metastatic melanoma treated with targeted therapy, immunotherapy, or chemotherapy: A retrospective, multicohort analysis. Lancet Oncol 2018;19:310–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Naidoo J, Cappelli LC, Forde PM et al. Inflammatory arthritis: A newly recognized adverse event of immune checkpoint blockade. The Oncologist 2017;22:627–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplemental Figures


