Abstract
Background
Treatment of delirium often includes haloperidol. Second‐generation antipsychotics like olanzapine have emerged as an alternative with possibly fewer side effects. The aim of this multicenter, phase III, randomized clinical trial was to compare the efficacy and tolerability of olanzapine with haloperidol for the treatment of delirium in hospitalized patients with advanced cancer.
Materials and Methods
Eligible adult patients (≥18 years) with advanced cancer and delirium (Delirium Rating Scale‐Revised‐98 [DRS‐R‐98] total score ≥17.75) were randomized 1:1 to receive either haloperidol or olanzapine (age‐adjusted, titratable doses). Primary endpoint was delirium response rate (DRR), defined as number of patients with DRS‐R‐98 severity score <15.25 and ≥4.5 points reduction. Secondary endpoints included time to response (TTR), tolerability, and delirium‐related distress.
Results
Between January 2011 and June 2016, 98 patients were included in the intention‐to‐treat analysis. DRR was 45% (95% confidence interval [CI], 31–59) for olanzapine and 57% (95% CI, 43–71) for haloperidol (Δ DRR −12%; odds ratio [OR], 0.61; 95% CI, 0.2–1.4; p = .23). Mean TTR was 4.5 days (95% CI, 3.2–5.9 days) for olanzapine and 2.8 days (95% CI, 1.9–3.7 days; p = .18) for haloperidol. Grade ≥3 treatment‐related adverse events occurred in 5 patients (10.2%) and 10 patients (20.4%) in the olanzapine and haloperidol arm, respectively. Distress rates were similar in both groups. The study was terminated early because of futility.
Conclusion
Delirium treatment with olanzapine in hospitalized patients with advanced cancer did not result in improvement of DRR or TTR compared with haloperidol. Clinical trial identification number. NCT01539733. Dutch Trial Register. NTR2559.
Implications for Practice
Guidelines recommend that pharmacological interventions for delirium treatment in adults with cancer should be limited to patients who have distressing delirium symptoms. It was suggested that atypical antipsychotics, such as olanzapine, outperform haloperidol in efficacy and safety. However, collective data comparing the efficacy and safety of typical versus atypical antipsychotics in patients with cancer are limited. If targeted and judicious use of antipsychotics is considered for the treatment of delirium in patients with advanced cancer, this study demonstrated that there was no statistically significant difference in response to haloperidol or olanzapine. Olanzapine showed an overall better safety profile compared with haloperidol, although this difference was not statistically significant.
Keywords: Delirium, Advanced cancer, Haloperidol, Olanzapine, Efficacy, Safety, Phase III
Short abstract
Pharmacological interventions for the treatment of delirium in patients with cancer is controversial. This article compares olanzapine and haloperidol for the treatment of delirium in hospitalized patients with advanced cancer.
Introduction
Delirium is a common, complex neuropsychiatric disorder with a high prevalence among hospitalized patients with advanced cancer 1, 2. This medical condition is characterized by changes in attention, awareness, and cognition, which develop over a short period of time and tend to fluctuate in severity during the course of a day 3. Delirium is associated with high morbidity and increased mortality 4, 5; it causes significant distress in patients and their caregivers and interferes with symptom assessment and decision making 6, 7. Therefore, early recognition and adequate management of delirium is of utmost importance in the care of hospitalized patients with advanced cancer.
New guidelines recommend that the use of pharmacological interventions in the management of delirium should be limited to patients with distressing symptoms (such as agitation, anxiety, or perceptual disturbances) or if there are safety concerns in which the patient is a potential risk to themselves or others 8, 9. In general, haloperidol, a typical or first‐generation antipsychotic, is recommended as the first line pharmacological option 10, 11. A recently performed systematic review and meta‐analysis of 15 randomized clinical trials suggested that atypical or second‐generation antipsychotics, including olanzapine, have a benefit with regard to efficacy and safety compared with haloperidol 12. Pooled atypical antipsychotics were associated with a shorter time to response (TTR; standard mean difference [SMD], −0.27) and a lower incidence (risk ratio [RR], 0.3) of extrapyramidal symptoms (EPS). However, most of the included studies were single center with small sample sizes, heterogeneous study populations, and at risk of bias.
Recently, a Cochrane systematic review included nine trials with 727 participants, assessing antipsychotics for delirium treatment in non‐ICU patients 9. Seven trials included a comparison of a typical to an atypical antipsychotic drug or placebo, including three studies evaluating patients with advanced cancer 13, 14, 15. Pooled analysis showed no significant difference in delirium severity (SMD −0.17; 95% confidence interval [CI], −0.4 to 0.02; seven studies; 542 participants), overall delirium resolution (RR, 1.1; 95% CI, 0.8–1.5; five studies; 349 participants), overall mortality (RR, 1.7; 95% CI, 0.8–3.5; four studies; 342 participants), or increased risk of EPS (RR, 12.2; 95% CI, 0.55–270; two studies; 198 participants) with atypical antipsychotics compared with typical antipsychotics. There was no evidence to support or refute the suggestion that antipsychotics shorten the course of delirium in hospitalized patients. However, the results were assessed as (very) low evidence (downgraded because of risk of bias, inconsistency, and/or imprecision). Moreover, important clinical endpoints, like duration of delirium, health‐related quality of life, and cardiac arrhythmia, were not reported for any trial comparing typical versus atypical antipsychotics.
Taken together, the efficacy and safety of pharmacological interventions for the treatment of delirium in patients with cancer is controversial. Effective and safe strategies for the management of delirium remain an unmet clinical need. Atypical antipsychotics may be an effective and safe alternative to haloperidol. Therefore, we conducted a multicenter, phase III randomized clinical trial (RCT) to compare the efficacy and tolerability of age‐adjusted and titratable doses of olanzapine versus haloperidol for the treatment of delirium in hospitalized patients with advanced cancer.
Subjects, Materials, and Methods
Study Design
This multicenter, randomized controlled, phase III trial (NCT01539733) was conducted at five sites (1 university cancer center, 2 teaching hospitals, 2 high‐care hospices) in The Netherlands between January 2011 and July 2016. At the time of their admission, patients and/or their legal representatives were asked for written informed consent to participate in this study in case the patient was diagnosed with delirium during hospitalization. The method for concealment of allocation was by enclosing assignments in sequentially numbered, opaque, sealed envelopes provided by an independent third party (university medical center pharmacist). The envelopes were opened sequentially, and only after the envelope had been irreversibly assigned to the participant. The study staff assessing the effect of antipsychotic treatment was blinded to the participant's treatment group for the entire duration of the study. The clinical staff administering the study medication and the patient being treated were not blinded. The study was conducted according to good clinical practice guidelines, the Declaration of Helsinki, and local laws and was approved by the institutional review boards of each participating study site.
Patients
Eligible patients were ≥18 years of age with advanced cancer, were admitted to a medical oncology ward or high‐care hospice facility, spoke the Dutch language fluently, and were diagnosed with delirium. Exclusion criteria included diagnosis of glaucoma, Parkinson's disease, dementia, psychiatric disorders interfering with delirium assessment, history of neuroleptic malignant syndrome or convulsions, delirium due to substance withdrawal, or cardiac conduction abnormalities (prolonged QTc interval of >500 msec on the electrocardiogram [ECG]). Patients being treated with other neuroleptic medication or lithium were also excluded from entering the study, because of the high probability of interactions (QTc prolongation, EPS, tardive dyskinesia, neuroleptic malignant syndrome etc.).
Procedures
Newly admitted patients were screened for delirium by the attending nurse using the delirium observation scale (DOS) on set days (Mondays and Thursdays) during each nursing shift (day, evening, night) or whenever delirium was suspected by the nursing or medical staff. The DOS is a 13‐item scale based on the Diagnostic and Statistical Manual of Mental Disorders fourth edition (DSM‐IV) criteria for delirium, designed to be completed by a nurse 16. The DOS is an accurate screening scale for delirium in patients with advanced cancer 17. The range of the total DOS score is 0–13; a total score of ≥3 indicates delirium. Patients with DOS score ≥3 were assessed on Delirium Rating Scale‐R‐98 (DRS‐R‐98) by a blinded assessor to confirm the diagnosis of delirium. The DRS‐R‐98 has 16 items, 13 of which assess the severity of symptoms, and 3 items are of diagnostic significance 18. The rating is applicable to the preceding 24 hours. Each severity item's rating levels are anchored with descriptions appropriate to that particular symptom. The severity ratings range from 0 (no impairment) to 3 (severe impairment), and a severity score of ≥15.25 or a total score of ≥17.75 is indicative of delirium; higher scores indicate higher severity of delirium. Delirium is considered cleared if the severity score is <15.25 with a decline of at least 4.5 points (d = 0.8) in the total score 18. Site initiation involved training of the clinical and study staff for standardized assessment of DOS and DRS‐R‐98 scores (performed by M.V. and E.N.).
Once delirium was diagnosed, potential precipitating factors for delirium, including changes in dose or type of opioids (<48 hours before diagnosis of delirium), dehydration, infection, intracranial malignancy, infection, and metabolic imbalances, were identified and scored (yes or no) by a comprehensive assessment. Delirium was categorized according to motor subtype (hypoactive, hyperactive, or mixed), based on DRS‐R‐98 item 7 (motor agitation) and item 8 (motor retardation). We also determined the predominance of certain “psychomotor features” by clinical observation. The hypoactive subtype is characterized by reduced alertness, sedation, and reduction of motor activity. The hyperactive form is associated with hypervigilance, overt psychotic features (e.g., hallucinations, delusions), and agitation. The mixed subtype has overlapping features of the hypoactive and hyperactive subtypes. All patients received tailored interventions targeted at the underlying causes of delirium, and appropriate nonpharmacological measures were taken according to clinical practice guidelines 8, 10, 11. Subsequently, all patients were randomized in a 1:1 ratio to receive haloperidol orally (PO) or subcutaneously (SC), or olanzapine PO or intramuscularly (IM). Dosing of antipsychotics was age adjusted and based on clinical practice guidelines 10, 11.
Patients <75 years of age assigned to haloperidol started with a loading dose of 1 mg. DOS scores were determined every 40 minutes thereafter. If the DOS score was ≥3, subsequent doses were increased by 1 mg up to a maximum dose of 20 mg PO or 10 mg SC on day 1 (supplemental online Table 1). Patients <75 years of age assigned to olanzapine started with a loading dose of 5 mg. DOS scores were determined every 2 hours thereafter. If the DOS score was ≥3, subsequent doses were increased to a maximum dose of 20 mg PO or IM (supplemental online Table 2). The loading, titration, and maximum doses of both haloperidol and olanzapine were halved for patients ≥75 years of age.
If the DOS score was <3, resolution of delirium was confirmed by DRS‐R‐98 assessment. Maintenance dose of haloperidol or olanzapine was one‐half of the total dose of the study drug administered during the first 24 h after initiation and divided in 1 or 2 doses. On days 2, 3, 4, and 7, DRS‐R‐98 assessment was repeated. If the DRS‐R‐98 severity score was ≥15.25, maintenance doses of haloperidol and olanzapine were adjusted (supplemental online Table 1 and 2).
Treatment with antipsychotics was discontinued if the maximum daily dose of the study drug was reached without resolution of delirium or if serious (grade ≥3) treatment‐related adverse events (TRAEs) occurred. TRAEs, including somnolence, dizziness, and EPS (including tremors and muscle stiffness), were monitored and graded daily according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03 19 by the study staff. ECG was performed before initiation of the study drug and on day 2. If QTc prolongation (>500 msec) occurred, treatment with the study drug was also discontinued. Administration of the benzodiazepine receptor agonist lorazepam PO or intravenously (IV) was allowed if the patient was deemed to require immediate intervention for safety or distress by the clinical staff. There was no predetermined dose schedule for the administration of PO or IV lorazepam. For optimal results, dose, frequency of administration, and duration of therapy with lorazepam were individualized according to patient response.
Patients who had recovered from delirium were asked to complete the Delirium Experience Questionnaire (DEQ). The DEQ is a face‐valid brief questionnaire asking six questions (three questions, yes or no; two questions, 0–4 numerical rating scale with 0 = not at all and 4 = extremely; one question, qualitative assessment) to assess recall of the delirium experience and the degree of distress related to the delirium episode 6. Spouses and/or caregivers and attending nurses were also asked to complete the DEQ to assess the level of caregiver distress.
Endpoint and Statistical Analysis
The primary endpoint was delirium response rate (DRR) on days 1–7 after randomization as defined by DRS‐R‐98 assessment (severity score <15.25; decline ≥4.5 points total score). Secondary endpoints included TTR, defined as the time from randomization to resolution of delirium (number of days); TRAEs according to CTCAE version 4.03; and delirium‐related distress for patients and their caregivers assessed by DEQ. An exploratory analysis of DRR was conducted for each motor subtype of delirium.
Assuming a 25% improvement in DRR (from 50% to 75%) with olanzapine compared with haloperidol 20, with α set to 5% and power to 90% and an expected dropout rate of 15%, the total sample size was 100 evaluable patients per treatment arm. DRR was compared between the two randomized groups by chi‐square tests and by calculating the 95% CI of the difference of the proportions in both the intention‐to‐treat (ITT) and the per protocol (PP) cohort (i.e., all patients who completed antipsychotic treatment). TTR outcomes in the ITT cohort were compared between the two treatment groups in the ITT by using stratified log‐rank tests and were plotted in a Kaplan‐Meier curve. TRAEs and DEQ scores were analyzed in an explorative or descriptive manner. All reported p values are two sided.
Futility analysis was conducted after 50% (n = 100) of the patients required for DRR analysis was included. The threshold for futility was set at a conditional power of 10%, which is usually applied as a stopping rule 21. All analyses were conducted by an independent statistician using IBM SPSS statistics version 22 (IBM, Chicago, IL).
Results
Between January 1, 2011, and June 15, 2016, a total 100 patients were randomly assigned: 50 patients per treatment arm (Fig. 1). Data were missing from one patient in each treatment arm. Ninety‐eight patients (49 patients per treatment arm) were included in the ITT cohort; 81 patients (40 in the olanzapine arm, and 41 in the haloperidol arm) were in the PP cohort.
Figure 1.

CONSORT diagram. *Data were missing from one patient in each treatment arm.
Table 1 describes the baseline characteristics of the ITT population, which were generally well balanced between the two arms. The majority of the patients were male (69.4%), with a mean age of approximately 69 years in both treatment groups. Mean DRS‐R‐98 total score at randomization was approximately 23 in both arms. Median number of precipitating factors for delirium was 2 (interquartile range [IQR], 1–3; range, 0–5). Most patients (n = 89; 91%) were admitted to a hospital ward. Reason for hospitalization was an emergency admission in 84.7% of the patients.
Table 1.
Baseline characteristics (intention‐to‐treat population)
| Characteristics | Olanzapine (n = 49) | Haloperidol (n = 49) |
|---|---|---|
| Mean age, years (SD) | 69.9 (9.3) | 68.4 (11.9) |
| Sex, n (%) | ||
| Male | 33 (67) | 35 (71) |
| Female | 16 (33) | 14 (29) |
| DRS‐R‐98a | ||
| Severity score, mean (SD) | 18.1 (3.8) | 17.6 (3.4) |
| Total score, mean (SD) | 23.5 (3.9) | 23.1 (3.4) |
| Delirium subtype, n (%) | ||
| Hyperactive | 16 (33) | 20 (41) |
| Hypoactive | 10 (20) | 17 (35) |
| Mixed | 21 (43) | 12 (24) |
| Unspecified | 2 (4) | 0 (0) |
| Use of opioids, n (%) | 33 (67) | 35 (71) |
| Use of benzodiazepines, n (%) | 18 (37) | 21 (43) |
| Use of neuropathic pain medication, n (%) | 7 (14) | 11 (22) |
| Use of psychotropic medication, n (%) | 3 (6) | 4 (8) |
| Precipitating factors,b median (IQR; range) | 2 (1‐3; 0‐5) | 2 (1‐3; 0‐5) |
| Admission type, n (%) | ||
| Hospital | 45 (92) | 43 (88) |
| Hospice | 4 (8) | 6 (12) |
| Reason for admission, n (%) | ||
| Emergency | 41 (84) | 42 (86) |
| Scheduled | 8 (16) | 7 (14) |
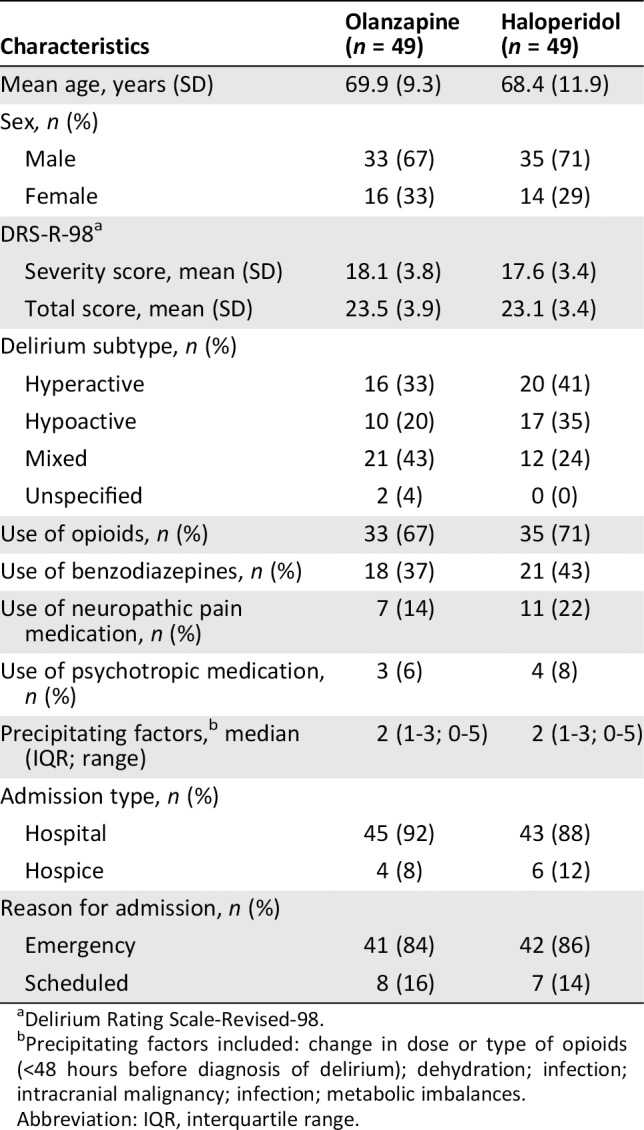
Delirium Rating Scale‐Revised‐98.
Precipitating factors included: change in dose or type of opioids (<48 hours before diagnosis of delirium); dehydration; infection; intracranial malignancy; infection; metabolic imbalances.
Abbreviation: IQR, interquartile range.
Efficacy
Median olanzapine dose was 8.8 mg (IQR, 5.0–15.0 mg) on day 1, 5.0 mg (IQR, 0.0–10.0 mg) on day 2, and 5.0 mg (IQR, 1.9–10.0 mg) at the end of study. For haloperidol, median dose was 2.5 mg (IQR, 1.0–4.8 mg) on day 1, 1.3 mg (IQR, 0.5–2.9 mg) on day 2, and 1.8 mg (IQR, 0.5–2.8 mg) at the end of study.
In the ITT cohort, DRR was 45% (95% CI, 31–59) for olanzapine and 57% (95% CI, 43–71) for haloperidol (ΔDRR −12%; odds ratio [OR], 0.61; 95% CI, 0.2–1.4; p = .23). DRR was 56% for olanzapine (95% CI, 41–72) and 68% (95% CI, 54–83) for haloperidol (ΔDRR −12%; OR, 0.61; 95% CI, 0.2–1.5; p = .27) in the PP cohort. Mean TTR in the olanzapine arm was 4.5 days (95% CI, 3.2–5.9), and it was 2.8 days (95% CI, 1.9–3.7; p = .18) in the haloperidol arm. (Fig. 2). Exploratory analysis did not demonstrate any significant benefit of olanzapine in DRR for hyperactive, hypoactive, or mixed subtypes (Table 2).
Figure 2.

Kaplan‐Meier estimates of time to response (TTR) according to treatment arm.
Table 2.
Delirium Resolution Rate (DRR) for delirium motor subtypes (intention‐to‐treat population)
| DRR | |||
|---|---|---|---|
| Motor subtypea | Olanzapine (n = 49) | Haloperidol (n = 49) | OR, 95% CI (p valuea) |
| Hyperactive, n | 16 | 20 | 0.5, 0.1–2.1 (.50) |
| Responders, n (%) | 8 (50) | 13 (65) | |
| Hypoactive, n | 10 | 17 | 0.2, 0.04–1.5 (.12) |
| Responders, n (%) | 3 (30) | 11 (65) | |
| Mixed, n | 21 | 12 | 1.8, 0.4–7.9 (.49) |
| Responders, n (%) | 10 (47) | 4 (33) | |
| Unknown, n | 2 | 0 |

p two‐sided chi‐square test (olanzapine vs. haloperidol).
Abbreviations: CI, confidence interval; OR, odds ratio.
Futility analysis was conducted, and at this time, the conditional power was 8.6%. As the conditional power was lower than the threshold for futility, this analysis indicated that this study was unlikely to reach the predefined efficacy criteria. Therefore, recruitment was terminated prematurely (June 15, 2016).
Safety
TRAEs were in line with previous data 9, with TRAEs of any grade occurring in 13 patients (26.5%) in the olanzapine arm and 16 patients (32.7%) in the haloperidol arm (Table 3). Grade ≥3 TRAEs (all leading to drug discontinuation) were reported in five patients (10.2%) in the olanzapine arm and 10 patients (20.4%) in the haloperidol arm (OR, 0.4; 95% CI, 0.1–1.4; p = .16). Sedation was the most reported grade ≥3 TRAE, in five (10.2%) and seven (14.3%) patients in the olanzapine and haloperidol arms, respectively. Grade ≥3 EPS (including tremors and muscle stiffness) occurred in two patients in the haloperidol arm; there was no reported grade ≥3 EPS in the olanzapine arm. One patient in the haloperidol arm experienced QTc prolongation (>500 msec), and none in the olanzapine arm. All grade ≥3 TRAEs occurred on day 1 or 2. There were no treatment‐related deaths in both treatment arms.
Table 3.
Incidence of therapy‐related adverse events (intention‐to‐treat population)
| Adverse event | Haloperidol (n = 49) | Olanzapine (n = 49) | ||||
|---|---|---|---|---|---|---|
| Any grade | Grade 3 | Grade 4/5 | Any grade | Grade 3 | Grade 4/5a | |
| All TRAEs, n (%) | 16 (32.7) | 10 (20.4) | 0 | 13 (26.5) | 4 (8.2) | 1 (2.0) |
| Sedation, n (%) | 10 (20.4) | 7 (14.3) | 0 | 9 (18.4) | 4 (8.2) | 1 (2.0) |
| EPS | ||||||
| Tremors, n (%) | 3 (6.1) | 1 (2.0) | 0 | 2 (4.1) | 0 | 0 |
| Muscle stiffness, n (%) | 2 (4.1) | 1 (2.0) | 0 | 1 (2.0) | 0 | 0 |
| Dizziness, n (%) | 0 | 0 | 0 | 1 (2.0) | 0 | 0 |
| QTc prolongation, n (%) | 1 (2.0) | 1 (2.0) | 0 | 0 | 0 | 0 |

Grade 5 TRAEs did not occur in both treatment arms.
Abbreviations: EPS, extrapyramidal symptom; TRAE, therapy‐related adverse event.
Delirium‐Related Distress
Sixteen patients in each treatment arm completed the DEQ. Mean delirium‐related distress level (on a 0–4 numerical rating scale) was 2.1 (SD 1.4) in the olanzapine arm and 2.3 (SD 1.4) in the haloperidol arm. The mean delirium‐related distress level rated by spouses and/or caregivers was 2.7 (SD 1.1) in the haloperidol arm and 3.0 (SD 1.2) in the olanzapine arm. Mean delirium‐related distress level rated by attending nurses was 0.9 (SD 0.9) in the haloperidol arm and 1.1 (SD 1.1) in the olanzapine arm.
Discussion
This multicenter, phase III RCT demonstrated no statistically significant difference in efficacy between olanzapine and haloperidol for the treatment of delirium in hospitalized adult patients with advanced cancer. Treatment with olanzapine did not result in a better DRR or shorter TTR compared with haloperidol. This trial met criteria for early stopping due to futility.
The management of delirium is complex because of the considerable heterogeneity in terms of etiology and clinical subtype 22, 23. A number of brain neural networks and pathways have been implicated, but underlying pathophysiological mechanisms remain poorly understood 24, 25. The complexity of delirium suggests that a variety of interventions is most likely needed, which combines both nonpharmacological and pharmacological strategies, as appropriate to the cancer trajectory and goals of care. The question remains whether antipsychotic drugs are clinically useful and safe for the treatment of delirium in patients with advanced cancer. If so, are atypical antipsychotics preferred because of their possibly better adverse effect profile and efficacy advantages in some patients?
Collective data remain limited on the activity and safety of antipsychotic drugs for the treatment of delirium in patients with advanced cancer. A recently performed Cochrane review on antipsychotics for treatment of delirium in hospitalized non‐ICU patients 9 included three studies comparing typical to atypical antipsychotics for the treatment of delirium in patients with advanced cancer 13, 14, 15. Lin et al. (n = 30; 100% with a cancer diagnosis) performed a single‐center, open RCT, and compared the efficacy between haloperidol and olanzapine to treat delirium (DSM‐IV criteria) in palliative and hospice center patients with cancer 13. Comparison of the scores of Delirium Rating Scale‐Chinese and Clinical Global Impression‐Severity between two groups showed no statistical difference. The study by Maneeton et al. (n = 52; 38.5% with cancer diagnosis) was a single‐center, prospective, double‐blind RCT which compared quetiapine versus haloperidol for the treatment of delirium (DSM‐IV‐TR and confusion assessment method criteria) [14]. The primary outcome measure was the DRS‐R‐98. They concluded that low‐dose quetiapine and haloperidol were equally effective and safe.
The 2018 European Society for Medical Oncology clinical practice guideline on delirium in adult cancer patients 8 identified 15 studies, including three RCTs 15, 26, 27. Kim et al. (n = 32; 72% with a cancer diagnosis) compared risperidone with olanzapine over a 7‐day period 26. The primary outcome measure was the DRS‐R‐98. There was no significant difference in either efficacy or adverse effects in this underpowered study. The second study by Hui et al. (n = 90; 100% with a cancer diagnosis) was a single‐center, double‐blind, parallel group RCT conducted in adult patients with advanced cancer, comparing the effect of lorazepam versus placebo as an adjuvant to haloperidol for persistent agitation (Richmond Agitation‐Sedation Scale [RASS] score of ≥ + 2) in patients with delirium (DSM‐IV‐TR criteria) 27. This study was designed to assess a different primary research objective than our RCT.
Recently, Agar et al. 15 conducted a three‐armed, multicenter, placebo‐controlled study of antipsychotic treatment of delirium in patients receiving palliative care (n = 247, 88% with a cancer diagnosis). Treatment with either risperidone or haloperidol was associated with significantly greater delirium symptom severity scores and mean extrapyramidal effects than placebo. There was no comparison of haloperidol versus risperidone in the Agar study. As a secondary outcome, haloperidol treatment was associated with poorer overall survival in long‐term follow‐up. However, there are some considerations that need to be addressed. First, although the therapeutic dose of haloperidol and the optimal dose titration schedule for delirium remain to be defined, starting and maintenance doses of haloperidol for patients >65 years of age (0.25 mg PO b.i.d., increased to a maximum of 2 mg per day) used in the Agar study were low compared with doses reported in previous studies in the oncology and palliative care setting 28. This may have underestimated the clinical effect of antipsychotic drugs to treat delirium in patients with advanced cancer. Furthermore, differences at baseline between the haloperidol and placebo arms in number of patients aged >65 years (90% vs. 80%, respectively) and the median dose of opioids (33 vs. 15 mg, respectively) are factors that may have affected study results. Other possible factors impacting on study results in the Agar study are older age and dementia or cognitive impairment, which are predictors of poor response to antipsychotics in the treatment of delirium 20, 29, 30, 31, 32. In the study by Agar et al., mean age of the patients in the haloperidol arm was 76.5 years (21% diagnosed with cognitive impairment), and in the risperidone arm mean age was 74.5 years (22% with cognitive impairment). Median IQCODE (a structured questionnaire to detect individuals who may go on to develop dementia) scores for cognitive impairment were ≥ 4, which shows that long‐term decline of cognitive status was highly prevalent. In our study, mean age was <70 years in both treatment arms, and patients with dementia were excluded. To conclude, prescribing of antipsychotic drugs for patients with delirium remains a matter of debate 33. Future studies need to identify baseline factors indicating which patients will benefit most from upfront treatment with antipsychotic drugs.
Overall, the safety profiles of haloperidol and olanzapine in the dose range tested were in line with previous studies in non‐ICU patients with delirium 9. TRAEs of any grade occurred in 32.7% and 26.5% of the patients in the haloperidol and olanzapine arm, respectively. TRAEs grade ≥3 (all leading to drug discontinuation) were reported more frequently in the haloperidol arm: 20.4% versus 10.2% in the olanzapine arm; however, this difference was not statistically significant. The most common grade ≥3 TRAE was sedation in both treatment arms. EPS grade ≥3 was uncommon, occurring in 4% of the patients in the haloperidol arm. Only three patients in the olanzapine arm experienced low grade EPS; EPS grade ≥3 did not occur in the olanzapine arm. All TRAEs resolved without sequelae when the study drug was discontinued. The use of antipsychotics is associated with QTc prolongation, which can lead to life‐threatening arrhythmia 34, 35, 36. In this study with routine ECG assessment, only one atrioventricular block episode was reported in the haloperidol arm.
Previous reports indicate high levels of delirium‐related distress in patients and their caregivers 5, 6, 7. In our study, exploratory analysis showed that mean stress scores reported by patients were just above 2 (on a 4‐point rating scale) in both treatment arms. As expected, stress scores reported by spouses and caregivers were high: 2.7 versus 3.0 in the haloperidol and olanzapine arm, respectively. Scores reported by attending nurses were low in both treatment arms. This could be the effect of improved professional education, providing nurses with educational resources and opportunities to apply knowledge with regard to delirium, which increases confidence in identification and management of delirium 37, 38.
This study has some limitations. First, our study did not include a placebo control group. The absence of a comparative placebo control group with active treatment groups limits the interpretation of our findings. Second, the DOS was completed not on a daily basis but at fixed times twice‐weekly, or whenever delirium was suspected. Daily assessments were not feasible given the high workload of the nursing staff. Consequently, some delirious cases may have remained undetected. Third, although the rater of the DRS‐R‐98 scores was blinded to the study drug, because the rater knew that all subjects were receiving active treatment, DRS‐R‐98 ratings could have been affected. Fourth, nonpharmacological interventions for the prevention and treatment of delirium were not standardized across the participating five sites in this study. However, it should be noted that for most nonpharmacological interventions, there is limited research evidence on which to base clinical recommendations. Interventions based on the Hospital Elder Life Program 39 have been successfully implemented in all Dutch health care institutions. Fifth, the decision of stopping this trial early for futility was adopted as a consequence of the results of the interim analysis, which demonstrated that it was highly unlikely that the trial would meet its primary objective of demonstrating superiority of olanzapine over haloperidol. With a probability rate of 8.6% to achieve its primary objective, this was well below the threshold of 10% for futility. Consequently, the number of included patients is relatively small, and the power of the analyses performed to assess secondary endpoints is low. Finally, the use of rescue interventions to manage agitation (e.g., benzodiazepines and physical restraints) was not prospectively recorded. Because these interventions are known to be associated with delirium, this could have introduced bias. However, it should be noted that retrospective analysis of the medical records showed that only very few patients (<3%) received benzodiazepines, and none received physical restraints.
Conclusion
The atypical antipsychotic olanzapine and haloperidol were equally effective and safe for the management of delirium in a broad population of hospitalized patients with advanced cancer. The focus of future placebo‐controlled RCTs should change to individualized, multimodal intervention strategies for managing delirium.
Author Contributions
Conception/design: Maurice J.D.L. van der Vorst, Elisabeth C.W. Neefjes, Manon S.A. Boddaert, Aartjan T.F. Beekman, Janneke A. Wilschut, Johannes Berkhof, Henk M.W. Verheul
Provision of study material or patients: Maurice J.D.L. van der Vorst, Elisabeth C.W. Neefjes, Bea A.T.T. Verdegaal, Aart Beeker, Saskia C.C. Teunissen, Wouter W.A. Zuurmond, Henk M.W. Verheul
Collection and/or assembly of data: Maurice J.D.L. van der Vorst, Elisabeth C.W. Neefjes, Bea A.T.T. Verdegaal, Aart Beeker, Saskia C.C. Teunissen, Janneke A. Wilschut, Johannes Berkhof, Henk M.W. Verheul
Data analysis and interpretation: Maurice J.D.L. van der Vorst, Elisabeth C.W. Neefje, Janneke A. Wilschut, Johannes Berkhof, Henk M.W. Verheul
Manuscript writing: Maurice J.D.L. van der Vorst, Elisabeth C.W. Neefjes, Manon S.A. Boddaert, Bea A.T.T. Verdegaal, Aart Beeker, Saskia C.C. Teunissen, Aartjan T.F. Beekman, Janneke A. Wilschut, Johannes Berkhof, Wouter W.A. Zuurmond, Henk M.W. Verheul
Final approval of manuscript: Maurice J.D.L. van der Vorst, Elisabeth C.W. Neefjes, Manon S.A. Boddaert, Bea A.T.T. Verdegaal, Aart Beeker, Saskia C.C. Teunissen, Aartjan T.F. Beekman, Janneke A. Wilschut, Johannes Berkhof, Wouter W.A. Zuurmond, Henk M.W. Verheul
Disclosures
The authors indicated no financial relationships.
Supporting information
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Tables
Acknowledgments
This study was supported by the Netherlands Organization for Health Research and Development (ZonMw) Palliative Care Program (No. 11510011).
Disclosures of potential conflicts of interest may be found at the end of this article.
References
- 1. Caraceni A, Grassi L. Delirium: Acute Confusional States in Palliative Medicine. 2nd ed. Oxford, U.K.: Oxford University Press, 2011. [Google Scholar]
- 2. Hosie A, Davidson PM, Agar M et al. Delirium prevalence, incidence, and implications for screening in specialist palliative care inpatient settings: A systematic review. Palliat Med 2013;27:486–498. [DOI] [PubMed] [Google Scholar]
- 3. American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition (DSM‐5). Washington, DC: American Psychiatric Association, 2013. Available at https://www.psychiatry.org/psychiatrists/practice/dsm. Accessed March 22, 2019. [Google Scholar]
- 4. Caraceni A, Nanni O, Maltoni M et al. Impact of delirium on the short‐term prognosis of advanced cancer patients. Italian Multicenter Study Group on Palliative Care . Cancer 2000;89:1145–1149. [DOI] [PubMed] [Google Scholar]
- 5. Lawlor PG, Bush SH. Delirium in patients with cancer: Assessment, impact, mechanisms and management. Nat Rev Clin Oncol 2015;12:77–79. [DOI] [PubMed] [Google Scholar]
- 6. Breitbart W, Gibson C, Tremblay A. The delirium experience: Delirium recall and delirium‐related distress in hospitalized patients with cancer, their spouses/caregivers, and their nurses. Psychosomatics 2002;43:183–194. [DOI] [PubMed] [Google Scholar]
- 7. Bruera E, Bush SH, Willey J et al. Impact of delirium and recall on the level of distress in patients with advanced cancer and their family caregivers. Cancer 2009;115:2004–2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Bush SH, Lawlor PG, Ryan K et al. Delirium in adult cancer patients: ESMO Clinical Practice Guidelines. Ann Oncol 2018;29(suppl 4):iv143–iv165. [DOI] [PubMed] [Google Scholar]
- 9. Burry L, Mehta S, Perreault MM et al. Antipsychotics for treatment of delirium in hospitalized non‐ICU patients. Cochrane Database Syst Rev 2018;CD005594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Clinical Guideline Centre . Delirium: Diagnosis, prevention and management. Clinical Guideline 103. NCGC, 2010. Available at https://www.nice.org.uk/CG103. Accessed March 22, 2019.
- 11. National Comprehensive Cancer Network . NCCN Clinical Practice guidelines in Oncology: Palliative care, Version 2.2013. Delirium PAL‐21. National Comprehensive Cancer Network (NCCN), 2013. Available at https://www.nccn.org/professionals/physician_gls/default.aspx. Accessed March 22, 2019. [DOI] [PubMed]
- 12. Kishi T, Hirota T, Matsunaga S, Iwata N. Antipsychotic medications for the treatment of delirium: A systematic review and meta‐analysis of randomised controlled trials. J Neurol Neurosurg Psychiatry 2016;87:767–774. [DOI] [PubMed] [Google Scholar]
- 13. Lin CJ, Sun FJ, Fang CK et al. An open trial comparing haloperidol with olanzapine for the treatment of delirium in palliative and hospice center cancer patients. J Int Med Taiwan 2008;19:345–354. [Google Scholar]
- 14. Maneeton B, Maneeton N, Srisurapanont M et al. Quetiapine versus haloperidol in the treatment of delirium: A double‐blind, randomized, controlled trial. Drug Des Devel Ther. 2013;24:657–667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Agar MR, Lawlor PG, Quinn S et al. Efficacy of oral risperidone, haloperidol, or placebo for symptoms of delirium among patients in palliative care: A randomized clinical trial. JAMA Intern Med 2017;177:34‐42. [DOI] [PubMed] [Google Scholar]
- 16. Schuurmans MJ, Shortridge‐Baggett LM, Duursma SA. The Delirium Observation Screening Scale: A screening instrument for delirium. Res Theory Nurs Pract 2003;17:31–50. [DOI] [PubMed] [Google Scholar]
- 17. Neefjes EC, van der Vorst MJ, Boddaert MS et al. Accuracy of the Delirium Observational Screening Scale (DOS) as a screening tool for delirium in patients with advanced cancer. BMC Cancer 2019;19:160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Trzepacz PT, Mittal D, Torres R et al. Validation of the Delirium Rating Scale‐revised‐98: Comparison with the delirium rating scale and the cognitive test for delirium. J Neuropsychiatry Clin Neurosci 2001;13:229–242. [DOI] [PubMed] [Google Scholar]
- 19. CTCAE version 4.03. Available at https://evs.nci.nih.gov/ftp1/CTCAE/About.html. Accessed March 22, 2019.
- 20. Breitbart W, Tremblay A, Gibson C. An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics 2002;43:175–182. [DOI] [PubMed] [Google Scholar]
- 21. Fuglsang A. Futility rules in bioequivalence trials with sequential designs. AAPS J 2014;16:79–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Neefjes EC, van der Vorst MJ, Verdegaal BA et al. Identification of patients with cancer with a high risk to develop delirium. Cancer Med 2017;6:1861–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ljubisavljevic V, Kelly B. Risk factors for development of delirium among oncology patients. Gen Hosp Psychiatry 2003;25:345–352. [DOI] [PubMed] [Google Scholar]
- 24. Maclullich AM, Anand A, Davis DH et al. New horizons in the pathogenesis, assessment and management of delirium. Age Ageing 2013;42:667–674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Maldonado JR. Delirium pathophysiology: An updated hypothesis of the etiology of acute brain failure. Int J Geriatr Psychiatry 2018;33:1428–1457. [DOI] [PubMed] [Google Scholar]
- 26. Kim SW, Yoo JA, Lee SY et al. Risperidone versus olanzapine for the treatment of delirium. Hum Psychopharmacol 2010;25:298–302. [DOI] [PubMed] [Google Scholar]
- 27. Hui D, Frisbee‐Hume S, Wilson A et al. Effect of lorazepam with haloperidol vs haloperidol alone on agitated delirium in patients with advanced cancer receiving palliative care: A randomized clinical trial. JAMA 2017;318:1047–1056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hui D, Dev R, Bruera A. Neuroleptics in the management of delirium in patients with advanced cancer. Curr Opin Support Palliat Care 2016:316–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gill SS, Bronskill SE, Normand SL et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med 2007;146:775–786. [DOI] [PubMed] [Google Scholar]
- 30. Wang PS, Schneeweiss S, Avorn J et al. Risk of death in elderly users of conventional vs. atypical antipsychotic medications. N Engl J Med 2005;353:2335–2341. [DOI] [PubMed] [Google Scholar]
- 31. Maust DT, Kim HM, Seyfried LS et al. Antipsychotics, other psychotropics, and the risk of death in patients with dementia: Number needed to harm. JAMA Psychiatry 2015;72:438–445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Yoon HJ, Park KM, Choi WJ et al. Efficacy and safety of haloperidol versus atypical antipsychotic medications in the treatment of delirium. BMC Psychiatry 2013;13:240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meagher D, Agar MR, Teodorczuk A. Debate article: Antipsychotic medications are clinically useful for the treatment of delirium. Int J Geriatr Psychiatry 2018;33:1420–1427. [DOI] [PubMed] [Google Scholar]
- 34. Stroup TS, Gray N. Management of common adverse effects of antipsychotic medications. World Psychiatry 2018;17:341–356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu CS, Tsai YT, Tsai HJ. Antipsychotic drugs and the risk of ventricular arrhythmia and/or sudden cardiac death: A nation‐wide case‐crossover study. J Am Heart Assoc 2015;4:e001568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ray WA, Chung CP, Murray KT et al. Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009;360:225–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Partridge JS, Martin FC, Harari D et al. The delirium experience: What is the effect on patients, relatives and staff and what can be done to modify this? Int J Geriatr Psychiatry 2018;28:804–812. [DOI] [PubMed] [Google Scholar]
- 38. Flagg B, Cox L, McDowell S et al. Nursing identification of delirium. Clin Nurse Spec 2010;24:260–266. [DOI] [PubMed] [Google Scholar]
- 39. Inouye SK, Bogardus ST Jr, Baker DI et al. The Hospital Elder Life Program: A model of care to prevent cognitive and functional decline in older hospitalized patients. Hospital Elder Life Program. J Am Geriatr Soc 2000;48:1697–1706. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
See http://www.TheOncologist.com for supplemental material available online.
Supplementary Tables


