Abstract
Serotonin (5‐HT) contributes to the pathogenesis of experimental neonatal pulmonary hypertension (PH) associated with bronchopulmonary dysplasia (BPD). Platelets are the primary source of circulating 5‐HT and is released upon platelet activation. Platelet transfusions are associated with neonatal mortality and increased rates of BPD. As BPD is often complicated by PH, we tested the hypothesis that circulating platelets are activated and also increased in the lungs of neonatal mice with bleomycin‐induced PH associated with BPD. Newborn wild‐type mice received intraperitoneal bleomycin (3 units/kg) three times weekly for 3 weeks. Platelets from mice with experimental PH exhibited increased adhesion to collagen under flow (at 300 s−1 and 1,500 s−1) and increased expression of the αIIbβ3 integrin and phosphatidylserine, markers of platelet activation. Platelet‐derived factors 5‐HT and platelet factor 4 were increased in plasma from mice with experimental PH. Pharmacologic blockade of the 5‐HT 2A receptor (5‐HT 2A R) prevents bleomycin‐induced PH and pulmonary vascular remodeling. Here, platelets from mice with bleomycin‐induced PH demonstrate increased 5‐HT 2A R expression providing further evidence of both platelet activation and increased 5‐HT signaling in this model. In addition, bleomycin treatment increased lung platelet accumulation. In summary, platelets are activated, granule factors are released, and are increased in numbers in the lungs of mice with experimental neonatal PH. These results suggest platelet activation and release of platelet‐derived factors may increase vascular tone, promote aberrant angiogenesis, and contribute to the development of neonatal PH.
Keywords: neonate, platelets, pulmonary hypertension, serotonin
Our study utilized a murine bleomycin model of pulmonary hypertension (PH) to study the hypothesis that platelets from mice with PH circulate in an activated state and that circulating and lung platelet‐derived factors, as well as the number of platelets in the lungs of neonatal mice with PH, is significantly increased. Through an extensive characterization of the functional status of platelets from bleomycin‐treated mice, we demonstrate that mice with bleomycin‐induced PH exhibit qualitative but not quantitative platelet changes. Circulating platelets from mice with PH exhibit hyperactivity as measured directly by adhesion and aggregation under flow and the presence of activation markers and indirectly by quantification of platelet‐specific proteins in plasma. Interestingly, we have also found that the absolute number of platelets within the lungs of mice with PH is significantly higher all together suggesting that platelets could directly be promoting PH.

1. INTRODUCTION
Pulmonary hypertension (PH) is a life‐threatening condition that develops in 14%–25% of preterm infants with the lung disease of prematurity known as bronchopulmonary dysplasia (BPD) (Bhat, Salas, Foster, Carlo, & Ambalavanan, 2012; Mourani et al., 2015). The pathophysiology of neonatal PH associated with BPD involves alterations in numerous signaling pathways, including the serotonin (5‐HT) pathway (Alvira, 2016; Bhatt et al., 2001; Delaney et al., 2018; Le Cras et al., 1999; Le Cras, Markham, Tuder, Voelkel, & Abman, 2002). We have previously shown that pharmacologic inhibition of 5‐HT signaling via the 5‐HT 2A receptor (5‐HT 2A R) increases pulmonary blood flow in fetal sheep with PH and protects against the development of murine bleomycin‐induced neonatal PH (Delaney et al., 2013, 2018). The vast majority of peripheral 5‐HT (98%) is synthesized by enterochromaffin cells of the small intestine and taken up by platelets via the serotonin transporter (SERT) where it is stored within dense granules (Barter & Pearse, 1953). This mechanism results in plasma levels of 5‐HT in the low nanomolar range while in platelet dense granules, the concentration of 5‐HT reaches the millimolar range (Holmsen & Weiss, 1979). Selective serotonin reuptake inhibitors, via blockade of SERT, increase the plasma 5‐HT levels and have been associated with PH in newborns exposed to these agents during the third trimester of fetal development (Chambers et al., 2006).
Increased plasma 5‐HT and activation of circulating platelets are reported in adults with PH (Damas et al., 2004; Diehl et al., 2011; Herve et al., 1990, 1995; Kazimierczyk & Kaminski, 2018; Kereveur et al., 2000; Nakonechnicov, Gabbasov, Chazova, Popov, & Belenkov, 1996). While no studies have evaluated whether platelets within the lungs of patients who died or received a lung transplant for PH were activated, pulmonary artery thromboses are increased in patients with PH and anti‐platelet therapies targeting the platelet hemostatic response demonstrate clear benefit in patients with chronic thromboembolic PH (Chaouat, Weitzenblum, & Higenbottam, 1996; Moser & Bloor, 1993; Wagenvoort, 1980). Antibody‐induced thrombocytopenia and treatment with pharmacologic platelet inhibitors (aspirin and dipyridamole) protect rats from monocrotaline and hypoxia‐induced PH (Gao et al., 2017; Keith, Will, Huxtable, & Weir, 1987; Mlczoch, Tucker, Weir, Reeves, & Grover, 1978; Shen, Shen, Pu, & He, 2011). Additionally, mice with a platelet‐specific deletion of toll‐like receptor 4 are protected from hypoxia‐induced PH (Bauer et al., 2014). Whether activation of platelets and increased circulating platelet‐derived factors such as 5‐HT are associated with experimental neonatal PH is unknown.
Platelets are small anucleated cells derived from megakaryocytes and are essential for hemostasis. Platelets are also integral mediators of other physiologic processes including immune regulation, vascular inflammation, and wound healing (Golebiewska & Poole, 2015; Kubes, 2016; Opneja, Kapoor, & Stavrou, 2019; Projahn & Koenen, 2012; Rondina & Garraud, 2014; Smyth et al., 2009). Aberrant platelet activation mediates several pathologic conditions in adults including atherosclerosis, sepsis, asthma, and acute lung injury (Lievens & Hundelshausen, 2011; Looney et al., 2009; Middleton, Weyrich, & Zimmerman, 2016; Pitchford, Cleary, Arkless, & Amison, 2019). In neonates, elevated platelet counts after birth are an independent predictor of moderate and severe BPD, which is often associated with PH (Chen, Li, Qiu, Yang, & Walther, 2019). Furthermore, increased plasma platelet‐derived protein, platelet factor 4 (PF4), after birth is associated with higher rates of later pulmonary vascular disease in former preterm infants (Wagner et al., 2018). Interestingly, recent published randomized clinical trials have raised concern about the effect of platelet transfusions on major neonatal outcomes. Preterm neonates transfused with platelets to maintain a higher platelet threshold have higher rates of mortality, BPD, and intraventricular hemorrhage (Curley et al., 2019; Kumar, 2019; Sola‐Visner & Bercovitz, 2016).
The mechanism by which platelets may adversely affect these neonatal outcomes is unknown and raises suspicion for the role of platelet activation in the pathogenesis of other neonatal conditions including PH. Each platelet contains numerous growth factors, vasoactive mediators, chemokines, cytokines, and angiogenic agents that have been implicated in the pathogenesis of PH (Balabanian et al., 2002; Christman et al., 1992; Clave, Maeda, Thomaz, Bydlowski, & Lopes, 2019; Duncan et al., 2012; Flaumenhaft & Sharda, 2019; Hundelshausen, Petersen, & Brandt, 2007; Italiano et al., 2008; Jurasz, Ng, Granton, Courtman, & Stewart, 2010; Kawut et al., 2005; Lopes et al., 2011; Tantawy, Adly, Ismail, Habeeb, & Farouk, 2013). These factors are stored within three types of granules: alpha (PF4 (CXCL4), CXCL7, CXCL5, PDGF, TGF‐ß), dense (5‐HT, Ca, ADP, ATP), and lysosomal (Flaumenhaft & Sharda, 2019). Resting platelets circulate at the margins of blood vessels and are maintained in their resting state primarily by the release of endothelial‐derived mediators such as nitric oxide (NO) and prostacyclin (PGI2) (Andrews & Berndt, 2004; Aytekin et al., 2012; Willems & Aken, 1979). With endothelial dysfunction and injury, NO and PGI2 release by the endothelium is decreased, platelets adhere to the subendothelial matrix, aggregate and release their granule contents including 5‐HT via exocytosis (Aytekin et al., 2012; Koupenova & Freedman, 2019; Ranchoux et al., 2018). Platelet 5‐HT activates pulmonary vascular receptors increasing pulmonary vascular tone and smooth muscle cell proliferation. In addition, platelet 5‐HT can act in autocrine way by enhancing local platelet activation and aggregation through the platelet 5‐HT 2A R (Mammadova‐Bach, Mauler, Braun, & Duerschmied, 2018).
Whether platelets contribute to the pathogenesis of neonatal PH and are a source of increased circulating mediators known to cause PH is unknown. Our study utilized a murine bleomycin model of PH to study the hypothesis that platelets from mice with PH circulate in an activated state and that circulating and lung platelet‐derived factors, as well as the number of platelets in the lungs of neonatal mice with PH, is significantly increased.
2. METHODS
2.1. Mouse model
The University of Colorado Denver Institutional Animal Care and Use Committee (IACUC) approved all animal studies. Beginning on days 1–2 of life, C57BL/6 wild‐type mice (Jackson Laboratory) were injected with intraperitoneal phosphate‐buffered saline (PBS) or bleomycin (3 units/kg, dissolved in PBS) (Hospira) three times per week for 3 weeks (total nine injections, 10 μl). This murine injury model of PH and BPD produces similar major pathologic findings to infants with PH and BPD including; impaired alveolar development (decreased radial alveolar counts, increased mean linear intercept and increased air space area), vascular remodeling (decreased vessel density, muscularization of small vessels, medial wall thickening), and PH (right ventricular hypertrophy and elevated right ventricular systolic pressure Delaney et al., 2018; Delaney et al., 2015; Sherlock et al., 2018). Bleomycin doses were adjusted for body weight at each injection. Mice were euthanized for tissue harvesting at 3 weeks of age.
2.2. Preparation of mouse blood, platelets, and plasma
Mice were anesthetized with isoflurane and blood was obtained via cardiac puncture of the right ventricle after performing a bilateral thoracotomy using a 21‐gauge needle containing the appropriate anticoagulant (3.8% ACD or heparin). Complete blood counts were obtained within 60 min after blood collection using the veterinarian hematologic analyzer Heska HT5. Platelet‐rich plasma (PRP) was obtained by centrifugation of whole blood at 100 × g for 10 min. PRP was supplemented with PGI2 (1 µg/ml) and incubated at room temperature for 3 min prior to centrifugation at 2,000 g × 2 min to obtain platelet poor plasma (PPP) or platelet pellets for further washing using PGI2‐containing Tyrodes buffer at 2,000 g × 2 min.
2.3. Whole blood microfluidic flow assays
Clean glass slides were functionalized with (tridecafluoro‐1,1,2,2tetrahydrooctyl) trichlorosilane (FOTS) via vapor deposition (Mayer, Boer, Shinn, Clews, & Michalske, 2000). Collagen‐related peptides (CRP), integrin α2β1 ligand (GFOGER), and von Willebrand factor binding peptide (VWF‐BP) were patterned on glass to simulate the major interactions between platelets and type I collagen. These peptides mimic the binding domains on type I collagen for platelets’ GPVI and integrin α2β1 receptors as well as the binding domain for the A3 domain of VWF. The peptides were mixed to a final concentration of 250 µg/ml each in 10 mm acetic acid, incubated for 2 hr at room temperature in a microfluidic channel (l = 49 mm, w = 100 µm, h = 50 µm), and rinsed with 0.1% (w/v) Texas Red in 10 mM acetic acid to locate the strip by fluorescence. A microfluidic device consisting of 32 parallel channels (w = 300 µm, h = 50 µm) was placed perpendicular to the strip of peptides. Channels were blocked with 2% bovine serum albumin in PBS pH 7.4 for 45 min. Mouse blood samples were incubated with DiOC6 in DMSO (final concentration 1 µM) at 37°C for 10 min. Blood was then added to reservoirs on the device and perfused for 5 min at 300 s−1 and 1,500 s−1 using a syringe pump (Harvard PhD Ultra) in withdraw mode. Four technical replicates were performed on each sample and each condition was repeated twice on separate days. Platelet accumulation was measured by fluorescent images captured in each channel using motorized stages on an inverted microscope (Olympus IX83—40X objective—NA 0.6).
2.4. Assessment of platelet activation by flow cytometry
Assessment of platelet activation by flow cytometry was performed by diluting washed platelets (1 × 106 platelets/ml) in Tyrodes buffer containing 1 mM CaCl2. Murine platelets were activated with thrombin (0.1 IU/ml) in the presence of anti‐mouse CD41‐BV421 antibody (Biolegend, Clone # MWReg30; 1:50), anti αIIbβ3 in active conformation (Emfret; clone JON/A‐PE; 1:25), P‐selectin‐APC (Biolegend, clone APM‐1; 1:25), or bovine Lactadherin‐FITC (Haematologic Technologies; 10 µg/ml). The activation was quenched at 5 min using ice‐cold 1% PFA Tyrodes buffer. Samples were run in the Gallios analyzer (Beckman Coulter). Studies were performed with n = 3–5 mice/day per group and repeated at least twice. Flow cytometry data were analyzed using Kaluza flow analysis software (Beckman Coulter) and Flowjo (Flowjo, LLC). Gating strategy as previously described (Davizon‐Castillo et al., 2019 ).
2.5. Measurement of 5‐HT and PF4 by ELISA
Platelet‐rich plasma and PPP samples were obtained as described above. Studies were performed with n = 8–19 mice and ELISAs were run on two separate days. Total lung homogenates were prepared in lysis buffer containing protease and phosphatase inhibitors. PF4 and 5‐HT levels were measured using mouse ELISA kits (Abcam, Cambridge, MA and GenWay Biotech, respectively) following the manufacturer's instructions.
2.6. Platelet 2A and SERT protein expression
Platelets were pooled from several mice from different litters to obtain 20 million platelets per sample and Western blot was performed on a single day with an n of 4–5 as previously described (Nozik‐Grayck et al., 2014). One sample from the bleomycin group was excluded from analysis as the result was 2 standard deviations outside of the mean. The following antibodies were used: 5HT 2A R (1:500, Santa Cruz), SERT (1:500, Abcam), and β‐actin (1:10,000, Sigma‐Aldrich). The same membrane was cut in two and probed separately for the 5‐HT 2A R and SERT. The full representative blots are shown in Figure 5. The 5‐HT 2A R antibody has been previously validated (Lofdahl et al., 2016) and the SERT antibody has been validated using a transfected cell line by the manufacturer. The species‐appropriate secondary IgG antibody was used (1:2,000, Millipore).
Figure 5.
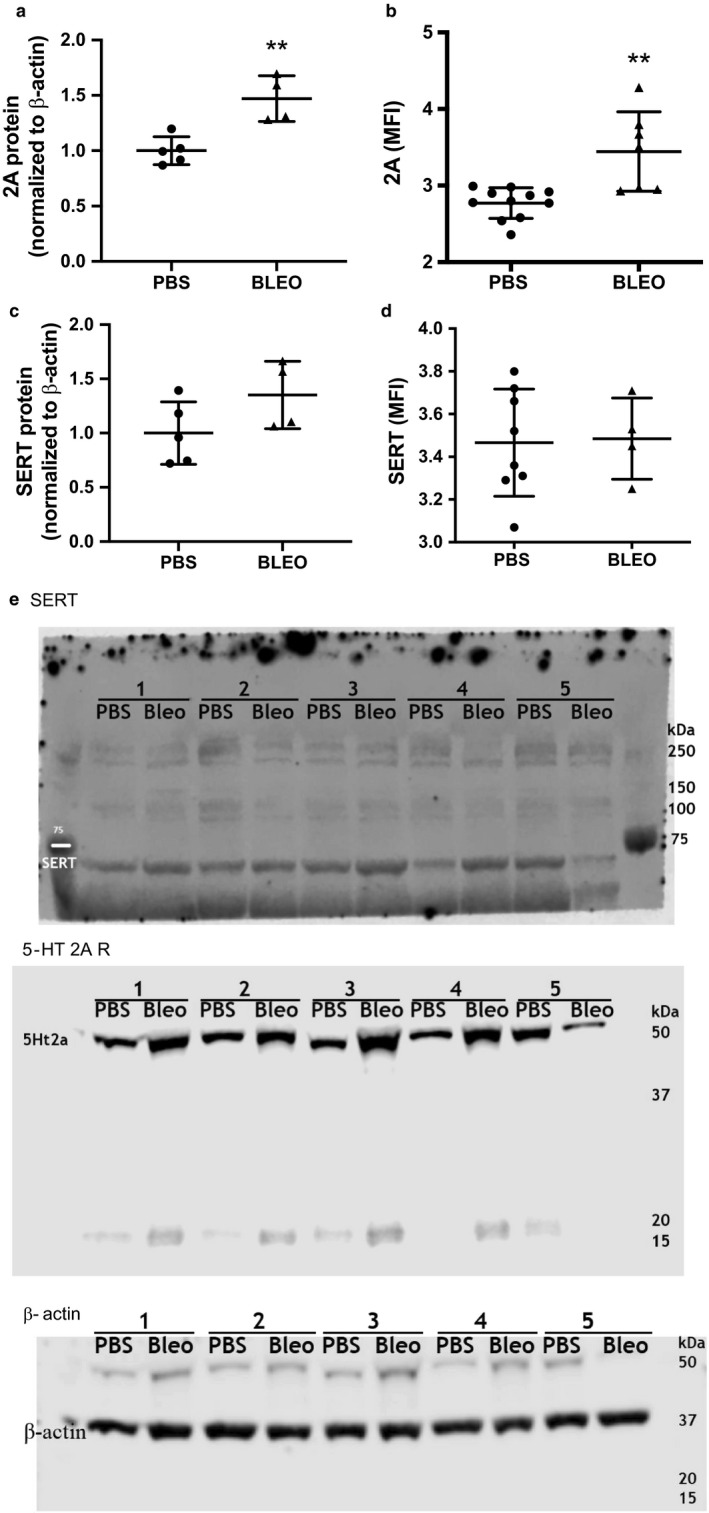
Western blot and FACS analysis for platelet 5‐HT 2A R and platelet SERT in mice treated with IP PBS or bleomycin. Bleomycin treatment increases platelet protein expression of the 5‐HT 2A R. (a) 5‐HT 2A R expression relative to β‐actin, **p < .005, by unpaired t test, n = 4–5 (pooled platelets from several mice, varied sex). One sample from the bleomycin group was excluded from analysis as the result was 2 standard deviations outside of the mean. (b) 5‐HT 2A receptor positive platelets in whole blood, **p < .005, by unpaired t test, n = 7 PBS (7M, 4F), n = 7 Bleo (4M, 7F). (c) SERT protein expression by relative to β‐actin, ns, n = 4–5 (pooled platelets from several mice, varied sex). One sample from the bleomycin group was excluded from analysis as the result was 2 standard deviations outside of the mean. (d) SERT‐positive platelets in whole blood, ns, n = 7 PBS (5M, 2F), n = 4 Bleo (2M, 2F). (e) Representative full‐length Western blots. PBS, phosphate‐buffered saline; SERT, serotonin transporter
FACS: 15 µl of whole blood was incubated with 2 µl of CD41‐BV421 (MWReg30, 1:50, Biolegend), 5‐HT 2A R‐FITC (1:50, Abcam), and SERT‐PE (1:50, LS Bio) for 15 min at room temperature in the dark. Whole blood was then fixed and lysed using 500 µl 1‐step Fix/Lyse solution for 20 min. Cells were then analyzed on a Beckman Coulter Gallios flow cytometer. Platelets were defined by their overall low forward and side scatter along with positive staining for CD41‐BV421. After which, 2A and SERT expression was determined using shifts in the Mean Fluorescence Intensity. Flow studies were performed on 2 separate days for 5‐HT 2A R and a single day for SERT with an n of 4–7.
2.7. Lung platelet quantification
Histology and Immunohistochemistry: Lungs were flushed with PBS then inflation‐fixed at 25 cm H2O for 30 min with 4% paraformaldehyde for paraffin embedding. Immunohistochemistry was performed for CD41 using the rabbit polyclonal antibody (1:200, GTX113758, GeneTex) diluted in Dako Antibody Diluent (S0809 Agilent Dako). Sections were developed with Dako EnVision+ Dual Link System‐HRP (DAB+) (K4065 Agilent Dako) and counterstained with Light Green (STLGC100 American MasterTech Scientific).
FACS: 100 µl of a 1:10 dilution of CD41‐BV421 (Biolegend, Clone # MWReg30) in PBS was retro‐orbitally injected into the mice 5 min before collecting lungs to label intravascular platelets. Studies were performed on a single day with an n of 5–7. Lungs were homogenized as previously described (PMID 30024304). Whole lung digests were then stained with CD41‐APC (Clone # MWReg30, BD Biosciences) and CD42b‐FITC (Clone # Xia.G5, Emfret) to label whole lung platelets. Interstitial platelets were defined for positive staining for CD41‐APC and CD42b‐FITC but negative staining for CD41‐BV421 (CD41‐APCHi, CD42b‐FITCHi, CD41‐BV421Lo). Quantification was performed using 123eCount beads (Thermofisher) as previously described (Good et al., 2018).
2.8. Antibody validation
All antibodies used in this study have undergone validation in the course of this study or have been previously validated. Representative full‐length blots and details of antibody validation are presented in the methods.
2.9. Statistical analysis
Data were analyzed using Prism (GraphPad Software) by unpaired t test, or two‐tailed t test. Data were expressed as mean ± SE and significance defined as p < .05.
3. RESULTS
3.1. Bleomycin induces greater platelet accumulation in whole blood microfluidic flow assays
We performed whole blood microfluidic flow assays comparing platelets from mice treated with bleomycin to those treated with PBS. Blood was perfused over a patterned substrate of the collagen‐related peptides CRP, GFOGER, and VWF‐BP that mimic the functionality of type I collagen in terms of platelet adhesion, activation, and aggregation (Pugh et al., 2010). Assays were run for 5 min at 300 s−1 and 1,500 s−1 to mimic venous and arterial shear rates. At both shear rates, we observed an increase in platelet buildup in platelets from mice treated with bleomycin (Figure 1a). By quantifying the maximum fluorescence intensity of DiOC6‐labeled platelets, we saw an approximate twofold increase in platelet fluorescence in blood from mice with experimental PH and BPD (p < 1E‐3 for 1,500 s−1 and p < 1E‐4 for 300 s−1) (Figure 1b,c).
Figure 1.
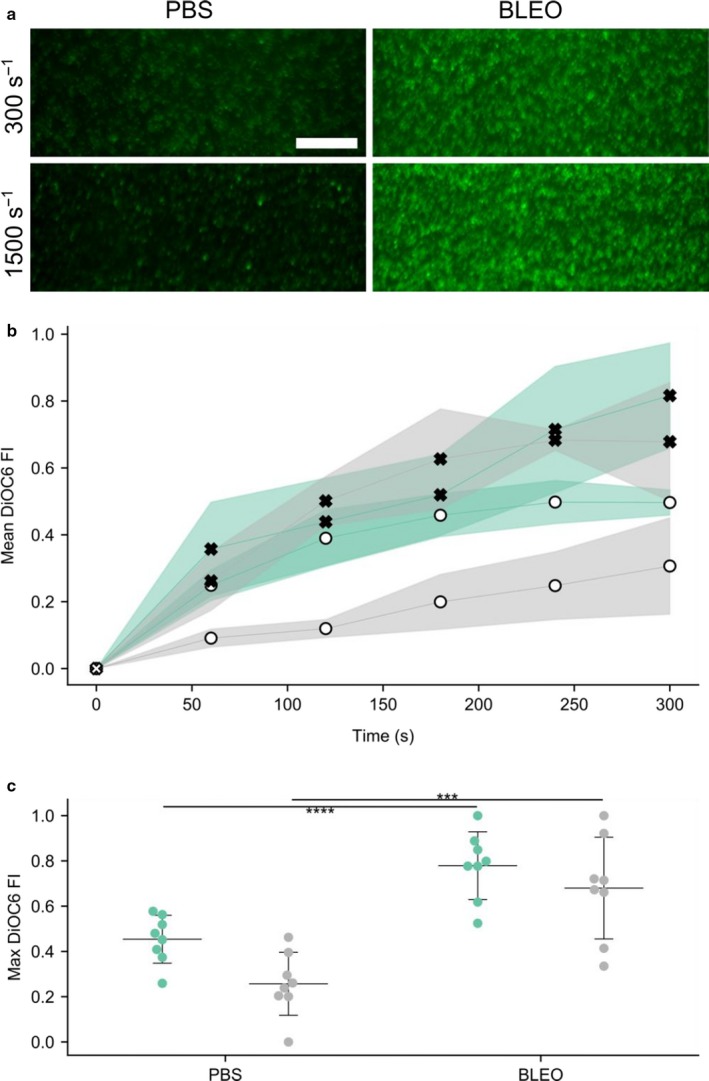
Platelets from mice with bleomycin‐induced PH demonstrate increased buildup during in vitro flow assays. Assays were run for 5 min at 300 s−1 and 1,500 s−1 to mimic venous and arterial shear rates. (a) Representative images of DiOC6‐labeled mouse platelets (scale bar = 50 µm) (b) Representative time series curves of platelet DiOC6 fluorescence. Green and grey regions depict assay shear rates of 300 s−1 and 1,500 s−1, respectively. Filled x's represent bleomycin‐treated mice, while open circles represent PBS treated mice. (c) Summary statistics of maximum DiOC6 FI for each assay. A single dot represents one assay, middle line shows the mean, and error bars display SD. At a given shear rate, blood from mice with experimental PH show an approximate twofold increase in platelet fluorescence. *** and **** denotes p < 10−3 and p < 10−4, respectively. Four technical replicates were performed on each sample and each condition was repeated twice on separate days, n = 4, PBS (1M, 1F), Bleo (1M, 1F), analysis by two‐tailed t test. PBS, phosphate‐buffered saline; PH, pulmonary hypertension
3.2. Platelet surface markers of activation are increased in neonatal murine PH
The activation profile of washed platelets from mice with experimental PH showed a subtle but significant increase in baseline (circulating) activation of the αIIbβ3 integrin, the main fibrinogen receptor (Figure 2a). Platelets from PH mice and control mice exhibit similar active αIIbβ3 integrin on their surfaces after activation with thrombin (0.1 IU/ml) for 5 min (Figure 2b). In addition to exhibiting higher levels of active αIIbβ3 at baseline, platelets from PH mice have significantly higher levels of phosphatidylserine (PS), whose primary role is to provide a phospholipid platform for the assembly, activation, and amplification of the coagulation cascade in vivo. This difference is evident at baseline and upon activation with thrombin (Figure 2c,d). Despite these significant differences in phosphatidylserine and active αIIbβ3 integrin, we did not observe differences in P‐selectin at baseline or upon activation with thrombin (0.1 IU/ml) (Figure 2e,f). To determine whether bleomycin itself activates platelets, we incubated washed platelets with comparable plasma concentrations of bleomycin and found that bleomycin does not lead to platelet activation of the αIIbβ3 integrin or increased exposure of PS or P‐selectin (Figure 2g).
Figure 2.
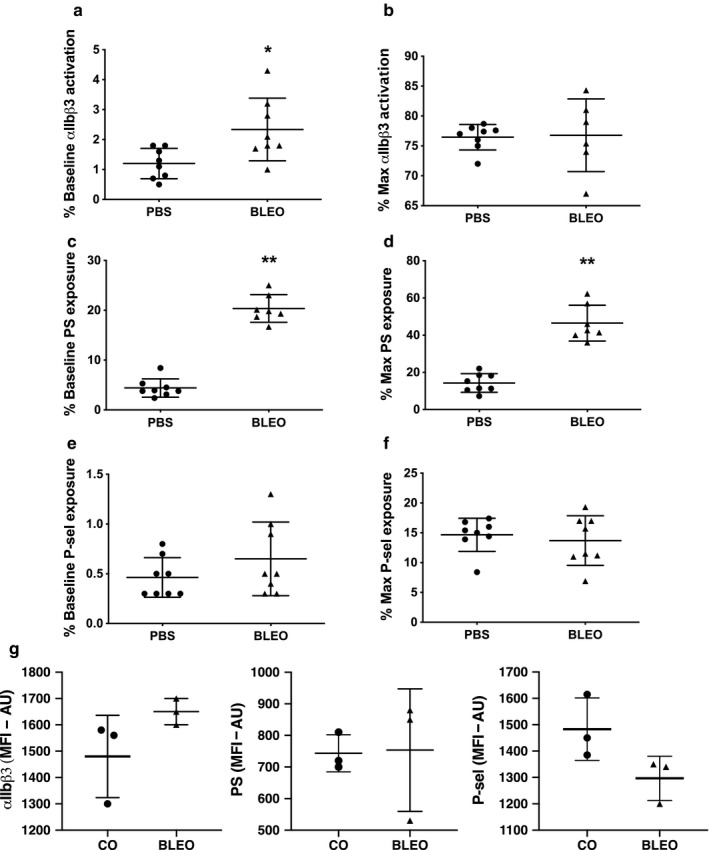
Platelets from mice are activated at baseline. (a) Platelets from mice with bleomycin‐induced PH have higher levels of active αIIbβ3 (main fibrinogen receptor) at baseline (unstimulated), *p < .05 by unpaired t test, n = 8 PBS (sex, not recorded), n = 8 Bleo (sex, not recorded). (b) Platelet activation after thrombin stimulation (0.1 IU/ml) for 5 min is similar between PBS and bleomycin. (c) Platelets from bleomycin‐induced PH exhibit significantly higher procoagulant potential by exposing higher amounts of phosphatidylserine on their surface at (c) baseline and after activation with (d) thrombin, **p < .0001 by unpaired t test, n = 7–8. Platelet degranulation as determined by measuring surface P‐selectin at (e) baseline and (f) after activation with thrombin are similar between groups, groups n = 8 PBS, n = 8 Bleo. (g) Incubation of pooled washed platelets from neonatal mice with comparable plasma concentrations of bleomycin does not lead to platelet activation of the αIIbβ3 integrin, PS or P‐selectin, n = 6 mice/group, pooled whole blood from 2 mice for each data point (4M, 2F). PBS, phosphate‐buffered saline; PH, pulmonary hypertension
3.3. Plasma levels of platelet‐specific alpha and dense granule factors are increased in experimental neonatal PH
To further assess whether experimental PH induces platelet activation, we measured PPP levels of the platelet‐specific alpha granule protein PF4 and the dense granule factor 5‐HT. In our murine model of bleomycin‐induced PH, platelet‐poor plasma levels of PF4 and 5‐HT are significantly elevated suggesting that baseline platelet activation of the αIIbβ3 integrin and PS exposure are accompanied with alpha and dense granule release (Figure 3a,b).
Figure 3.
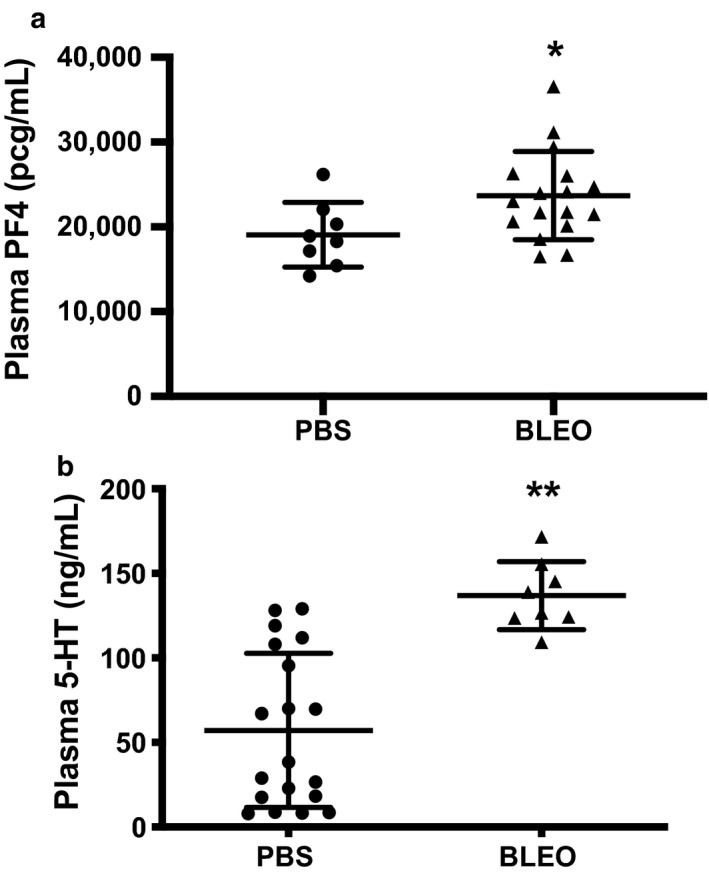
Plasma levels of platelet‐specific alpha (PF4) and dense granule (5‐HT) proteins are increased in experimental neonatal PH. (a) Platelet‐poor plasma PF4 levels from neonatal mice following IP PBS or bleomycin treatment, *p < .05 by unpaired t test, n = 8 PBS (6M,2F), n = 16 Bleo (9M, 7F). (b) Platelet‐poor plasma 5‐HT levels from neonatal mice following IP PBS or bleomycin treatment, **p < .0001 by unpaired t test, n = 8–19 (sex, not recorded). PBS, phosphate‐buffered saline; PF4, platelet factor 4; PH, pulmonary hypertension
3.4. Platelet hematologic indices from mice with experimental PH are similar to controls
To determine whether bleomycin‐induced PH is associated with significant changes in bone marrow output, we obtained complete blood counts from control and PH mice. Total numbers of leukocytes, hemoglobin, platelets, and the mean platelet volume were not different between groups and suggests that bleomycin‐induced PH has no significant effect on bone marrow output of neonatal mice and that the increased platelet adhesion observed in the microfluidics assay is not due to the presence of higher platelet numbers in mice with PH (Figure 4a–d).
Figure 4.
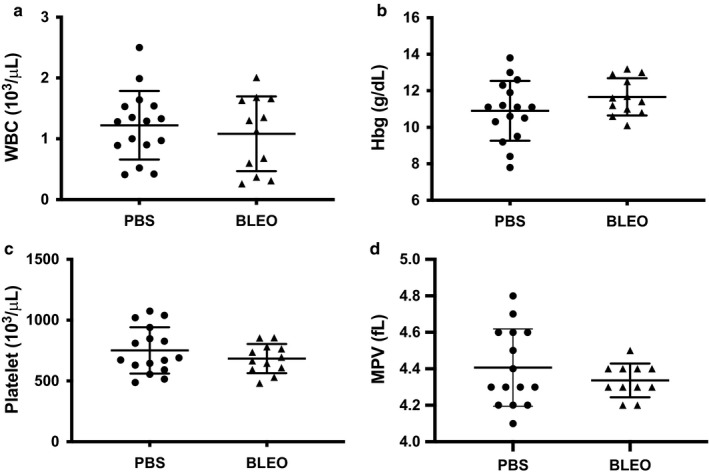
Platelet hematologic indices from mice with experimental PH are similar to controls. (a) Leukocyte counts in neonatal mice following IP PBS or bleomycin treatment, ns, n = 16 PBS (8M,8F), n = 11 Bleo (6M, 5F). (b) Hemoglobin levels in neonatal mice following IP PBS or bleomycin treatment, ns, n = 16 PBS (8M,8F), n = 11 Bleo (6M, 5F). (c) Platelet counts in neonatal mice following IP PBS or bleomycin treatment, ns, n = 16 PBS (8M,8F), n = 11 Bleo (6M, 5F). (d) Mean platelet volume (mpv) in neonatal mice following IP PBS or bleomycin treatment, ns, n = 15 PBS (7M, 8F), n = 10 Bleo (5M, 5F). PBS, phosphate‐buffered saline; PH, pulmonary hypertension
3.5. Platelet 5‐HT 2A receptor expression is increased in murine PH and BPD
The SERT is responsible for platelet 5‐HT uptake and the 5‐HT 2A R enhances local platelet aggregation and activation. After 3 weeks of treatment with either PBS or bleomycin, we analyzed protein expression of washed platelets by Western blot and platelets within whole blood by FACS to determine whether changes in 5‐HT 2A R and/or SERT were associated with the development of bleomycin‐induced neonatal PH. We found increased expression of the platelet 5‐HT 2A R protein by both Western blot of isolated platelets and FACs of circulating platelets (Figure 5a,b). Bleomycin‐induced PH did not change platelet SERT expression (Figure 5c,d).
3.6. Platelets are increased in the lungs of mice with experimental neonatal PH
Lung sections of bleomycin‐treated mice showed increased amounts of platelets by immunohistochemistry (Figure 6a–d). Therefore, we quantitatively analyzed the absolute numbers of intravascular and interstitial platelets present in homogenized lung tissues using reference counting beads. Intravascular platelets were labeled with anti‐CD41‐BV421. We considered CD41‐APC/CD42b‐FITC double‐positive and CD41‐BV421‐negative platelets as interstitial platelets. We report that the number of interstitial platelets was significantly higher in lungs from bleomycin‐induced PH (Figure 6e). These results are in accordance with the elevated lung levels of the platelet‐specific protein, PF4 (Figure 6f).
Figure 6.
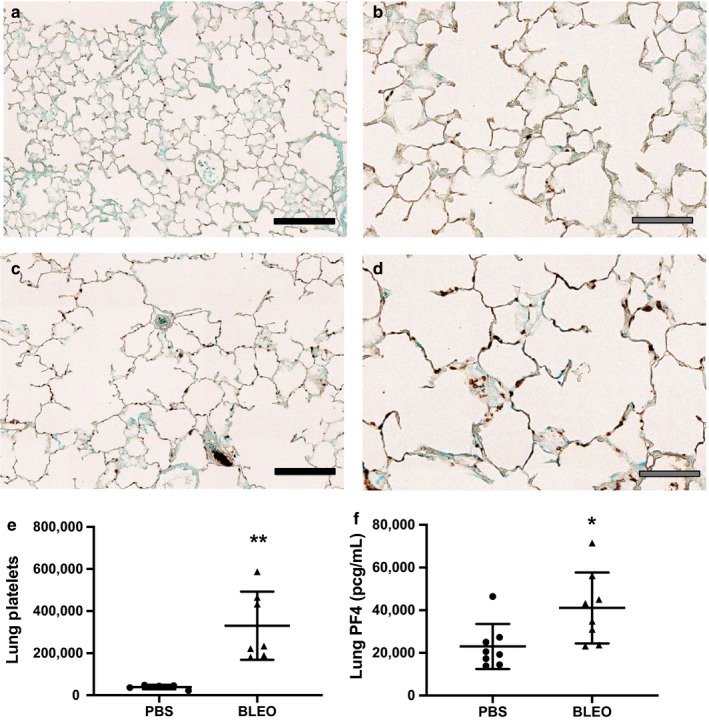
Platelets are increased in the lungs of mice with bleomycin‐induced PH. (a–d) Representative CD41 staining in 3‐week‐old mice treated with IP (a) PBS‐20 × magnification, (b) PBS‐40× magnification, (c) bleomycin‐20× magnification, or (d) bleomycin‐40× magnification, black filled scale bars = 100μm, grey filled scale bars = 50μm. (e) Interstitial platelets in neonatal mice following IP PBS or bleomycin treatment, **p < .005 by unpaired t test, n = 5 PBS (4M,1F), n = 7 Bleo (5M, 2F). (f) PF4 levels in whole lung homogenates from neonatal mice following IP PBS or bleomycin treatment, *p < .05 by unpaired t test, n = 8 PBS (6M, 2F), n = 8 Bleo (3M, 5F). PBS, phosphate‐buffered saline; PF4, platelet factor 4; PH, pulmonary hypertension
4. DISCUSSION
We previously reported that pharmacologic blockade of the 5‐HT 2A R prevents bleomycin‐induced PH and pulmonary vascular remodeling (Delaney et al., 2018). As platelets contain 99% of circulating 5‐HT, which is released upon activation, we tested the hypothesis that circulating platelets are activated and increased in the lungs of neonatal mice with bleomycin‐induced PH. Through an extensive characterization of the functional status of platelets from bleomycin‐treated mice, we demonstrate that mice with bleomycin‐induced PH exhibit qualitative but not quantitative changes in circulating platelets. We show that circulating platelets from mice with PH exhibit a subtle but significant increase in platelet activation at baseline as evidenced by the higher percentage of circulating platelets with active αIIbβ3, the main platelet integrin involved in platelet aggregation. Moreover, we also show that these differences are functionally relevant as platelets from PH mice demonstrate greater accumulation than control littermates using our microfluidic assays. Similarly, significantly elevated plasma levels of the platelet‐specific proteins PF4 and 5‐HT further demonstrate higher baseline platelet activation in mice with PH. We also found that the absolute number of platelets within the lungs of mice with PH is significantly higher, altogether suggesting that platelets could directly be promoting PH. Ongoing work in our group focuses on the elucidation of the specific aspects of platelet function that directly favor the development of PH. Whether platelet adhesion to endothelial vasculature, platelet degranulation, transmigration, or platelet aggregation are required to promote PH remain to be studied.
Our data show that platelets from mice with PH appear to circulate in a “primed” state as evidenced by significantly higher levels of active αIIbβ3 and PS at baseline. Active αIIbβ3 has a high affinity for fibrinogen and fibrin and elevated baseline levels of active αIIbβ3 may favor platelet aggregation and adhesion to endothelial surfaces (Huang et al., 2019). Although the difference in baseline activation of the αIIbβ3 integrin is significant but subtle, higher baseline levels of active αIIbβ3 integrin detected by flow cytometry directly and functionally correlate with increased platelet accumulation on collagen. Upon platelet activation, platelets also expose PS. Exposed PS provides the phospholipid surface required for the assembly and amplification of the coagulation system. Therefore, the elevated amounts of PS on the surface of platelets from mice with PH in addition to the elevated levels of active αIIbβ3 are not only suggestive of higher platelet activation but also of increased procoagulant potential of platelets from mice with PH. Interestingly, recent work has shown that platelet PS can mediate the formation of neutrophil macroaggregates that promote pulmonary thrombosis in a model of murine intestinal ischemia/reperfusion (Yuan et al., 2017). Altogether, our results demonstrate that circulating platelets from mice with experimental PH have higher levels of active αIIbβ3 integrins that favor aggregation with other platelets and potentially to endothelial surfaces through immobilized fibrinogen or von Willebrand Factor (Kauskot & Hoylaerts, 2012). Ongoing work in our laboratory is aimed at investigating the mechanisms for increased platelet αIIbβ3 and PS observed in mice with PH and the functional significance as it relates to the pathogenesis of PH.
In addition to the functional platelet changes, we observed significantly higher levels of the platelet‐specific molecules PF4 and 5‐HT in the plasma of bleomycin‐treated mice suggesting ongoing alpha and dense granule release. As platelet activation is usually associated with release of all alpha granule contents, we were surprised by our results that despite observing higher plasma levels of the alpha granule, PF4, there is no difference in platelet expression of alpha granule, P‐selectin, between control and bleomycin‐induced PH mice. It is conceivable that this is due to previously described age‐related low expression and storage of P‐selectin in alpha granules of platelets from human neonates, murine fetal and neonatal platelets or due to neonatal agonist‐specific degranulation hypo‐responsiveness (Baker‐Groberg, Lattimore, Recht, McCarty, & Haley, 2016; Stolla et al., 2019).
Platelet‐derived PF4 inhibits endothelial cell proliferation in vitro and increased levels after birth are associated with higher rates of later pulmonary vascular disease in former preterm infants (Gengrinovitch, 1995; Maione., 1990; Wagner et al., 2018). 5‐HT release by activated platelets promotes pulmonary vasoconstriction, mitogenesis, and further platelet activation (Delaney, Gien, Grover, Roe, & Abman, 2011; Dunn, Lorch, & Sinha, 1989; Fanburg & Lee, 1997; Mammadova‐Bach et al., 2018; Walther et al., 2003; Yabanoglu et al., 2009). While higher levels of plasma PF4 and 5‐HT could reflect differences in clearance between control and bleomycin‐induced mice, it is more likely that these are the result of in vivo platelet degranulation as we observed a significantly higher pool of circulating activated platelets in bleomycin‐treated mice and no difference in SERT expression in platelets isolated from bleomycin‐treated mice. 5‐HT further enhances local platelet aggregation and activation via the platelet 5‐HT 2A R (McBride, 1990; Meuleman et al., 1983). We have previously reported that pharmacologic blockade of the 5‐HT 2A R with ketanserin prevents bleomycin‐induced PH and pulmonary vascular remodeling. Interestingly, we now show that platelets from mice treated with bleomycin demonstrate increased 5‐HT 2A R expression providing further evidence for platelet activation and serotonin signaling in this model and leads to the possibility that one mechanism for protection observed in ketanserin‐treated mice is blockade of platelet serotonin signaling.
A key finding of our study is the increased accumulation of platelets in the lungs of mice with experimental PH. This observation strongly suggests that platelets from bleomycin‐induced PH either adhere to the pulmonary vasculature or transmigrate into the perivascular space where they could directly deliver their granule contents. Increased lung intravascular and extravascular platelets have been reported in experimental models of acute lung injury, allergic lung inflammation, and sepsis (Cleary et al., 2019; Ortiz‐Munoz et al., 2014; Pitchford et al., 2008; Yuan et al., 2017). To our knowledge, this is the first report of increased extravascular lung platelets in a model of PH.
Our knowledge about platelet and megakaryocyte biology in neonates is relatively scarce. Findings from recent clinical studies suggest that elevated platelet counts at baseline in preterm infants and platelet transfusions may have deleterious effects on neonatal outcomes. High platelet counts after birth are an independent predictor of moderate and severe BPD and increased PF4 after birth is associated with increased rates of later pulmonary vascular disease in former preterm infants (Chen et al., 2019; Wagner et al., 2018). Preterm neonates randomized to receive platelet transfusions to maintain a higher baseline platelet count (50K) showed increased mortality when compared to preterm neonates with a lower transfusion threshold (25K) (Curley et al., 2019). Moreover, preterm neonates in the high transfusion baseline group had an increased incidence of BPD. Whether these complications are directly caused by platelets is difficult to establish; however, they illustrate the need to expand our knowledge on neonatal platelet biology and the interplay between platelets and neonatal lung disease.
There are a few potential limitations that warrant further investigation. While we measured plasma PF4 and 5‐HT as indicators of platelet activation, we recognize that platelets store hundreds of factors, including chemokines, growth factors, and vasoactive substances. Many of these factors have been implicated in the pathogenesis of neonatal PH promoting aberrant angiogenesis, increasing vascular tone, and inducing inflammation. However, it is conceivable to think that platelets could have a protective role in PH and BPD and future studies will focus on determining whether platelet activation is protective or promotes the development of neonatal PH. Another limitation of this study was the inability to definitely determine the source of increased lung platelets and the mechanisms leading to increased platelets within the lung including whether exposed collagen, fibrinogen, or VWF mediate increased platelet adherence. Our data demonstrate significantly higher numbers of platelets in the lungs of mice with PH; however, the number of circulating platelets remained comparable between control and PH mice. Given that the lung is a known site for extramedullary platelet biogenesis, it is possible that the increased number of lung platelets in mice with PH was due to higher lung megakaryocyte platelet production and not necessarily due to the recruitment of circulating platelets (Kaufman, Airo, Pollack, & Crosby, 1965; Lefrancais et al., 2017; Zucker‐Franklin, 2000). Future experiments utilizing transfusion of radiolabeled platelets will help address this question. Lastly, while our data demonstrate a clear association between platelet activation and neonatal PH, it remains unanswered whether platelets themselves or a secreted factor influence the phenotype of pulmonary vascular cells directly contributing to the pathogenesis of PH. Future studies by our laboratory will address these remaining questions.
In summary, we report that platelets are activated in neonatal murine PH in the setting of BPD, demonstrated by increased accumulation to collagen under physiologic flow conditions, increased platelet markers of activation, and increased plasma levels of alpha and dense granule stored factors. Our observations are in concordance with previously reported work in adults with PH where platelets exhibit increased in vivo activation and platelet hyperreactivity ex vivo, supporting our hypothesis that platelets are key players in the pathogenesis of PH (Maeda, Bydlowski, & Lopes, 2005; Nakonechnicov et al., 1996; Yaoita et al., 2014). In addition, we show that platelets from mice with experimental PH have increased expression of the 5‐HT 2A R which could further enhance local platelet aggregation and activation. Finally, we demonstrate that platelets are increased in the lung interstitium of mice with PH. We speculate that pharmacologic strategies targeting platelet activation in PH in the setting of BPD may provide a novel therapeutic strategy.
Davizon‐Castillo P, Allawzi A, Sorrells M, et al. Platelet activation in experimental murine neonatal pulmonary hypertension. Physiol Rep. 2020;8:e14386 10.14814/phy2.14386
REFERENCES
- Alvira, C. M. (2016). Aberrant pulmonary vascular growth and remodeling in bronchopulmonary dysplasia. Frontiers in Medicine, 3, 21 10.3389/fmed.2016.00021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews, R. K. , & Berndt, M. C. (2004). Platelet physiology and thrombosis. Thrombosis Research, 114, 447–453. 10.1016/j.thromres.2004.07.020 [DOI] [PubMed] [Google Scholar]
- Aytekin, M. , Aulak, K. S. , Haserodt, S. , Chakravarti, R. , Cody, J. , Minai, O. A. , & Dweik, R. A. (2012). Abnormal platelet aggregation in idiopathic pulmonary arterial hypertension: Role of nitric oxide. American Journal of Physiology. Lung Cellular and Molecular Physiology, 302, L512–L520. 10.1152/ajplung.00289.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker‐Groberg, S. M. , Lattimore, S. , Recht, M. , McCarty, O. J. , & Haley, K. M. (2016). Assessment of neonatal platelet adhesion, activation, and aggregation. Journal of Thrombosis and Haemostasis, 14, 815–827. 10.1111/jth.13270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanian, K. , Foussat, A. , Dorfmuller, P. , Durand‐Gasselin, I. , Capel, F. , Bouchet‐Delbos, L. , … Humbert, M. (2002). CX(3)C chemokine fractalkine in pulmonary arterial hypertension. American Journal of Respiratory and Critical Care Medicine, 165, 1419–1425. [DOI] [PubMed] [Google Scholar]
- Barter, R. , & Pearse, A. G. (1953). Detection of 5‐hydroxytryptamine in mammalian enterochromaffin cells. Nature, 172, 810 10.1038/172810a0 [DOI] [PubMed] [Google Scholar]
- Bauer, E. M. , Chanthaphavong, R. S. , Sodhi, C. P. , Hackam, D. J. , Billiar, T. R. , & Bauer, P. M. (2014). Genetic deletion of toll‐like receptor 4 on platelets attenuates experimental pulmonary hypertension. Circulation Research, 114, 1596–1600. 10.1161/CIRCRESAHA.114.303662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat, R. , Salas, A. A. , Foster, C. , Carlo, W. A. , & Ambalavanan, N. (2012). Prospective analysis of pulmonary hypertension in extremely low birth weight infants. Pediatrics, 129, e682–e689. 10.1542/peds.2011-1827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatt, A. J. , Pryhuber, G. S. , Huyck, H. , Watkins, R. H. , Metlay, L. A. , & Maniscalco, W. M. (2001). Disrupted pulmonary vasculature and decreased vascular endothelial growth factor, Flt‐1, and TIE‐2 in human infants dying with bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine, 164, 1971–1980. 10.1164/ajrccm.164.10.2101140 [DOI] [PubMed] [Google Scholar]
- Chambers, C. D. , Hernandez‐Diaz, S. , Van Marter, L. J. , Werler, M. M. , Louik, C. , Jones, K. L. , & Mitchell, A. A. (2006). Selective serotonin‐reuptake inhibitors and risk of persistent pulmonary hypertension of the newborn. New England Journal of Medicine, 354, 579–587. 10.1056/NEJMoa052744 [DOI] [PubMed] [Google Scholar]
- Chaouat, A. , Weitzenblum, E. , & Higenbottam, T. (1996). The role of thrombosis in severe pulmonary hypertension. European Respiratory Journal, 9, 356–363. 10.1183/09031936.96.09020356 [DOI] [PubMed] [Google Scholar]
- Chen, X. , Li, H. , Qiu, X. , Yang, C. , & Walther, F. J. (2019). Neonatal hematological parameters and the risk of moderate‐severe bronchopulmonary dysplasia in extremely premature infants. BMC Pediatrics, 19, 138 10.1186/s12887-019-1515-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christman, B. W. , McPherson, C. D. , Newman, J. H. , King, G. A. , Bernard, G. R. , Groves, B. M. , & Loyd, J. E. (1992). An imbalance between the excretion of thromboxane and prostacyclin metabolites in pumonary hypertension. New England Journal of Medicine, 327, 70–75. [DOI] [PubMed] [Google Scholar]
- Clave, M. M. , Maeda, N. Y. , Thomaz, A. M. , Bydlowski, S. P. , & Lopes, A. A. (2019). Phosphodiesterase type 5 inhibitors improve microvascular dysfunction markers in pulmonary arterial hypertension associated with congenital heart disease. Congenital Heart Disease, 14, 246–255. 10.1111/chd.12688 [DOI] [PubMed] [Google Scholar]
- Cleary, S. J. , Hobbs, C. , Amison, R. T. , Arnold, S. , O'Shaughnessy, B. G. , Lefrancais, E. , … Pitchford, S. C. (2019). LPS‐induced lung platelet recruitment occurs independently from neutrophils, PSGL‐1, and P‐selectin. American Journal of Respiratory Cell and Molecular Biology, 61, 232–243. 10.1165/rcmb.2018-0182OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curley, A. , Stanworth, S. J. , Willoughby, K. , Fustolo‐Gunnink, S. F. , Venkatesh, V. , Hudson, C. , … PlaNe, T. M. C. (2019). Randomized trial of platelet‐transfusion thresholds in neonates. New England Journal of Medicine, 380, 242–251. 10.1056/NEJMoa1807320 [DOI] [PubMed] [Google Scholar]
- Damas, J. K. , Otterdal, K. , Yndestad, A. , Aass, H. , Solum, N. O. , Froland, S. S. , … Andreassen, A. K. (2004). Soluble CD40 ligand in pulmonary arterial hypertension: Possible pathogenic role of the interaction between platelets and endothelial cells. Circulation, 110, 999–1005. [DOI] [PubMed] [Google Scholar]
- Davizon‐Castillo, P. , McMahon, B. , Aguila, S. , Bark, D. , Ashworth, K. , Allawzi, A. , … Di Paola, J. (2019). TNF‐alpha driven inflammation and mitochondrial dysfunction define the platelet hyperreactivity of aging. Blood, 134, 727–740. 10.1182/blood.2019000200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, C. , Gien, J. , Grover, T. R. , Roe, G. , & Abman, S. H. (2011). Pulmonary vascular effects of serotonin and selective serotonin reuptake inhibitors in the late‐gestation ovine fetus. American Journal of Physiology. Lung Cellular and Molecular Physiology, 301, L937–L944. 10.1152/ajplung.00198.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, C. , Gien, J. , Roe, G. , Isenberg, N. , Kailey, J. , & Abman, S. H. (2013). Serotonin contributes to high pulmonary vascular tone in a sheep model of persistent pulmonary hypertension of the newborn. American Journal of Physiology. Lung Cellular and Molecular Physiology, 304, L894–L901. 10.1152/ajplung.00043.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, C. , Sherlock, L. , Fisher, S. , Maltzahn, J. , Wright, C. , & Nozik‐Grayck, E. (2018). Serotonin 2A receptor inhibition protects against the development of pulmonary hypertension and pulmonary vascular remodeling in neonatal mice. American Journal of Physiology. Lung Cellular and Molecular Physiology, 314, L871–L881. 10.1152/ajplung.00215.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delaney, C. , Wright, R. H. , Tang, J. R. , Woods, C. , Villegas, L. , Sherlock, L. , … Nozik‐Grayck, E. (2015). Lack of EC‐SOD worsens alveolar and vascular development in a neonatal mouse model of bleomycin‐induced bronchopulmonary dysplasia and pulmonary hypertension. Pediatric Research, 78, 634–640. 10.1038/pr.2015.166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diehl, P. , Aleker, M. , Helbing, T. , Sossong, V. , Germann, M. , Sorichter, S. , … Moser, M. (2011). Increased platelet, leukocyte and endothelial microparticles predict enhanced coagulation and vascular inflammation in pulmonary hypertension. Journal of Thrombosis and Thrombolysis, 31, 173–179. 10.1007/s11239-010-0507-z [DOI] [PubMed] [Google Scholar]
- Duncan, M. , Wagner, B. D. , Murray, K. , Allen, J. , Colvin, K. , Accurso, F. J. , & Ivy, D. D. (2012). Circulating cytokines and growth factors in pediatric pulmonary hypertension. Mediators of Inflammation, 2012, 143428 10.1155/2012/143428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn. (1989). Responses of small intrapulmonary arteries to vasoactive compounds in the fetal and neonatal lamb: NE, Epi, 5‐HT, K channel. Pediatric Research, 25, 360–363. [DOI] [PubMed] [Google Scholar]
- Fanburg, B. L. , & Lee, S. L. (1997). A new role for an old molecule: Serotonin as a mitogen. American Journal of Physiology, 272, L795–L806. [DOI] [PubMed] [Google Scholar]
- Flaumenhaft, R. , & Sharda, A. (2019).Platelet Secretion. In Platelets (pp. 349–370).
- Gao, H. , Cheng, Y. , Zong, L. , Huang, L. , Qiao, C. , Li, W. , … Zhao, C. (2017). Aspirin attenuates monocrotaline‐induced pulmonary arterial hypertension in rats by suppressing the ERK/MAPK pathway. Clinical and Experimental Hypertension, 39, 34–41. 10.1080/10641963.2016.1210620 [DOI] [PubMed] [Google Scholar]
- Gengrinovitch, S. , Greenberg, S. M. , Cohen, T. , Gitay‐Goren, H. , Rockwell, P. , … Neufeld, G. (1995). Platelet factor‐4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. Journal of Biological Chemistry, 270, 15059–15065. [DOI] [PubMed] [Google Scholar]
- Gengrinovitch, S. , Greenberg, S. M. , Cohen, T. , Gitay‐Goren, H. , Rockwell, P. , … Neufeld, G. (1995). Platelet factor‐4 inhibits the mitogenic activity of VEGF121 and VEGF165 using several concurrent mechanisms. Journal of Biological Chemistry, 270, 15059–15065. [DOI] [PubMed] [Google Scholar]
- Golebiewska, E. M. , & Poole, A. W. (2015). Platelet secretion: From haemostasis to wound healing and beyond. Blood Reviews, 29, 153–162. 10.1016/j.blre.2014.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good, R. J. , Hernandez‐Lagunas, L. , Allawzi, A. , Maltzahn, J. K. , Vohwinkel, C. U. , Upadhyay, A. K. , … Nozik‐Grayck, E. (2018). MicroRNA dysregulation in lung injury: The role of the miR‐26a/EphA2 axis in regulation of endothelial permeability. American Journal of Physiology. Lung Cellular and Molecular Physiology, 315, L584–L594. 10.1152/ajplung.00073.2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herve, P. , Drouet, L. , Dosquet, C. , Launay, J. M. , Rain, B. , Simonneau, G. , … Duroux, P. (1990). Primary pulmonary hypertension in a patient with a familial platelet storage pool disease: Role of serotonin. American Journal of Medicine, 89, 117–120. 10.1016/0002-9343(90)90111-p [DOI] [PubMed] [Google Scholar]
- Herve, P. , Launay, J. M. , Scrobohaci, M. L. , Brenot, F. , Simonneau, G. , Petitpretz, P. , … Drouet, L. (1995). Increased plasma serotonin in primary pulmonary hypertension. American Journal of Medicine, 99, 249–254. 10.1016/S0002-9343(99)80156-9 [DOI] [PubMed] [Google Scholar]
- Holmsen, H. , & Weiss, H. J. (1979). Secretable storage pools in platelets. Annual Review of Medicine, 30, 119–134. 10.1146/annurev.me.30.020179.001003 [DOI] [PubMed] [Google Scholar]
- Huang, J. , Li, X. , Shi, X. , Zhu, M. , Wang, J. , Huang, S. , … Jin, J. (2019). Platelet integrin alphaIIbbeta3: Signal transduction, regulation, and its therapeutic targeting. Journal of Hematology & Oncology, 12, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Italiano, J. E. Jr , Richardson, J. L. , Patel‐Hett, S. , Battinelli, E. , Zaslavsky, A. , Short, S. , … Klement, G. L. (2008). Angiogenesis is regulated by a novel mechanism: Pro‐ and antiangiogenic proteins are organized into separate platelet alpha granules and differentially released. Blood, 111, 1227–1233. 10.1182/blood-2007-09-113837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurasz, P. , Ng, D. , Granton, J. T. , Courtman, D. W. , & Stewart, D. J. (2010). Elevated platelet angiostatin and circulating endothelial microfragments in idiopathic pulmonary arterial hypertension: A preliminary study. Thrombosis Research, 125, 53–60. 10.1016/j.thromres.2009.04.005 [DOI] [PubMed] [Google Scholar]
- Kaufman, R. M. , Airo, R. , Pollack, S. , & Crosby, W. H. (1965). Circulating megakaryocytes and platelet release in the lung. Blood, 26(6), 720–731. 10.1182/blood.V26.6.720.720 [DOI] [PubMed] [Google Scholar]
- Kauskot, A. , & Hoylaerts, M. F. (2012). Platelet receptors. Handbook of Experimental Pharmacology, 210, 23–57. [DOI] [PubMed] [Google Scholar]
- Kawut, S. M. , Horn, E. M. , Berekashvili, K. K. , Widlitz, A. C. , Rosenzweig, E. B. , & Barst, R. J. (2005). von Willebrand factor independently predicts long‐term survival in patients with pulmonary arterial hypertension. Chest, 128, 2355–2362. [DOI] [PubMed] [Google Scholar]
- Kazimierczyk, R. , & Kaminski, K. (2018). The role of platelets in the development and progression of pulmonary arterial hypertension. Advances in Medical Sciences, 63, 312–316. 10.1016/j.advms.2018.04.013 [DOI] [PubMed] [Google Scholar]
- Keith, I. M. , Will, J. A. , Huxtable, R. J. , & Weir, K. (1987). Anti‐platelet agents reduce morphological changes of chronic hypoxic pulmonary hypertension. Histology and Histopathology, 2, 203–206. [PubMed] [Google Scholar]
- Kereveur, A. , Callebert, J. , Humbert, M. , Herve, P. , Simonneau, G. , Launay, J.‐M. , & Drouet, L. (2000). High plasma serotonin levels in primary pulmonary hypertension. Effect of long‐term epoprostenol (prostacyclin) therapy. Arteriosclerosis, Thrombosis, and Vascular Biology, 20, 2233–2239. 10.1161/01.ATV.20.10.2233 [DOI] [PubMed] [Google Scholar]
- Koupenova, M. , & Freedman, J. E. (2019). Inhibition of platelet function by the endothelium. Platelets, 311–327. 10.1016/b978-0-12-813456-6.00017-5 [DOI] [Google Scholar]
- Kubes, P. (2016). The versatile platelet contributes to inflammation, infection, hemostasis, coagulation and cancer. Seminars in Immunology, 28, 535 10.1016/j.smim.2016.11.002 [DOI] [PubMed] [Google Scholar]
- Kumar, R. (2019). Platelet transfusion for PDA closure ‐ Pediatrics.pdf.Pediatrics. [DOI] [PubMed]
- Le Cras, T. D. , Kim, D. H. , Gebb, S. , Markham, N. E. , Shannon, J. M. , Tuder, R. M. , & Abman, S. H. (1999). Abnormal lung growth and the development of pulmonary hypertension in the Fawn‐Hooded rat. American Journal of Physiology, 277, L709–L718. 10.1152/ajplung.1999.277.4.L709 [DOI] [PubMed] [Google Scholar]
- Le Cras, T. D. , Markham, N. E. , Tuder, R. M. , Voelkel, N. F. , & Abman, S. H. (2002). Treatment of newborn rats with a VEGF receptor inhibitor causes pulmonary hypertension and abnormal lung structure. American Journal of Physiology. Lung Cellular and Molecular Physiology, 283, L555–L562. 10.1152/ajplung.00408.2001 [DOI] [PubMed] [Google Scholar]
- Lefrancais, E. , Ortiz‐Munoz, G. , Caudrillier, A. , Mallavia, B. , Liu, F. , Sayah, D. M. , … Looney, M. R. (2017). The lung is a site of platelet biogenesis and a reservoir for haematopoietic progenitors. Nature, 544, 105–109. 10.1038/nature21706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lievens, D. , & von Hundelshausen, P. (2011). Platelets in atherosclerosis. Thrombosis and Haemostasis, 106, 827–838. 10.1160/TH11-08-0592 [DOI] [PubMed] [Google Scholar]
- Lofdahl, A. , Rydell‐Tormanen, K. , & Muller, C. Martina Holst, C. , Thiman, L. , Ekstrom, G. , … Westergren‐Thorsson, G. (2016). 5‐HT2B receptor antagonists attenuate myofibroblast differentiation and subsequent fibrotic responses in vitro and in vivo. Physiological Reports, 4, e12873 10.14814/phy2.12873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Looney, M. R. , Nguyen, J. X. , Hu, Y. , Van Ziffle, J. A. , Lowell, C. A. , & Matthay, M. A. (2009). Platelet depletion and aspirin treatment protect mice in a two‐event model of transfusion‐related acute lung injury. Journal of Clinical Investigation, 119, 3450–3461. 10.1172/JCI38432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopes, A. A. , Barreto, A. C. , Maeda, N. Y. , Cicero, C. , Soares, R. P. , Bydlowski, S. P. , & Rich, S. (2011). Plasma von Willebrand factor as a predictor of survival in pulmonary arterial hypertension associated with congenital heart disease. Brazilian Journal of Medical and Biological Research, 44, 1269–1275. 10.1590/S0100-879X2011007500149 [DOI] [PubMed] [Google Scholar]
- Maeda, N. Y. , Bydlowski, S. P. , & Lopes, A. A. (2005). Increased tyrosine phosphorylation of platelet proteins including pp125(FAK) suggests endogenous activation and aggregation in pulmonary hypertension. Clinical and applied thrombosis/hemostasis, 11, 411–415. [DOI] [PubMed] [Google Scholar]
- Maione, T. E. , Gray, G. S. , Petro, J. , Hunt, A. J. , Donner, A. L. , Bauer, S. I. , … Sharpe, R. J. . (1990). Inhibition of angiogenesis by recombinant human platelet factor‐4 and related peptides. Science, 247(4938), 77–79. [DOI] [PubMed] [Google Scholar]
- Mammadova‐Bach, E. , Mauler, M. , Braun, A. , & Duerschmied, D. (2018). Autocrine and paracrine regulatory functions of platelet serotonin. Platelets, 29, 541–548. 10.1080/09537104.2018.1478072 [DOI] [PubMed] [Google Scholar]
- Mayer, T. M. , de Boer, M. P. , Shinn, N. D. , Clews, P. J. , & Michalske, T. A. (2000). Chemical vapor deposition of fluoroalkylsilane monolayer films for adhesion control in microelectromechanical systems. Journal of Vacuum Science & Technology B: Microelectronics and Nanometer Structures Processing, Measurement, and Phenomena, 18, 2433–2440. 10.1116/1.1288200 [DOI] [Google Scholar]
- McBride. (1990). Inhibition of serotonin amplified human platelet aggregation by. ketanserin.pdf>. [DOI] [PubMed]
- Meuleman, T. R. , Hill, D. C. , Port, J. D. , Stanley, T. H. , Pace, N. L. , & Mohammad, S. F. (1983). Ketanserin prevents platelet aggregation and endotoxin‐induced pulmonary vasoconstriction. Critical Care Medicine, 11, 606–611. 10.1097/00003246-198308000-00005 [DOI] [PubMed] [Google Scholar]
- Middleton, E. A. , Weyrich, A. S. , & Zimmerman, G. A. (2016). Platelets in pulmonary immune responses and inflammatory lung diseases. Physiological Reviews, 96, 1211–1259. 10.1152/physrev.00038.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mlczoch, J. , Tucker, A. , Weir, E. K. , Reeves, J. T. , & Grover, R. F. (1978). Platelet‐mediated pulmonary hypertension and hypoxia during pulmonary microembolism: Reduction by platelet inhibition. Chest, 74, 648–653. [DOI] [PubMed] [Google Scholar]
- Moser, K. M. , & Bloor, C. M. (1993). Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest, 103, 685–692. 10.1378/chest.103.3.685 [DOI] [PubMed] [Google Scholar]
- Mourani, P. M. , Sontag, M. K. , Younoszai, A. , Miller, J. I. , Kinsella, J. P. , Baker, C. D. , … Abman, S. H. (2015). Early pulmonary vascular disease in preterm infants at risk for bronchopulmonary dysplasia. American Journal of Respiratory and Critical Care Medicine, 191, 87–95. 10.1164/rccm.201409-1594OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakonechnicov, S. , Gabbasov, Z. , Chazova, I. , Popov, E. , & Belenkov, Y. (1996). Platelet aggregation in patients with primary pulmonary hypertension. Blood Coagulation & Fibrinolysis, 7, 225–227. 10.1097/00001721-199603000-00029 [DOI] [PubMed] [Google Scholar]
- Nozik‐Grayck, E. , Woods, C. , Taylor, J. M. , Benninger, R. K. , Johnson, R. D. , Villegas, L. R. , … Farrow, K. N. (2014). Selective depletion of vascular EC‐SOD augments chronic hypoxic pulmonary hypertension. American Journal of Physiology. Lung Cellular and Molecular Physiology, 307, L868–L876. 10.1152/ajplung.00096.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opneja, A. , Kapoor, S. , & Stavrou, E. X. (2019). Contribution of platelets, the coagulation and fibrinolytic systems to cutaneous wound healing. Thrombosis Research, 179, 56–63. 10.1016/j.thromres.2019.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortiz‐Munoz, G. , Mallavia, B. , Bins, A. , Headley, M. , Krummel, M. F. , & Looney, M. R. (2014). Aspirin‐triggered 15‐epi‐lipoxin A4 regulates neutrophil‐platelet aggregation and attenuates acute lung injury in mice. Blood, 124, 2625–2634. 10.1182/blood-2014-03-562876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pitchford, S. , Cleary, S. , Arkless, K. , & Amison, R. (2019). Pharmacological strategies for targeting platelet activation in asthma. Current Opinion in Pharmacology, 46, 55–64. 10.1016/j.coph.2019.03.012 [DOI] [PubMed] [Google Scholar]
- Pitchford, S. C. , Momi, S. , Baglioni, S. , Casali, L. , Giannini, S. , Rossi, R. , … Gresele, P. (2008). Allergen induces the migration of platelets to lung tissue in allergic asthma. American Journal of Respiratory and Critical Care Medicine, 177, 604–612. 10.1164/rccm.200702-214OC [DOI] [PubMed] [Google Scholar]
- Projahn, D. , & Koenen, R. R. (2012). Platelets: Key players in vascular inflammation. Journal of Leukocyte Biology, 92, 1167–1175. 10.1189/jlb.0312151 [DOI] [PubMed] [Google Scholar]
- Pugh, N. , Simpson, A. M. , Smethurst, P. A. , de Groot, P. G. , Raynal, N. , & Farndale, R. W. (2010). Synergism between platelet collagen receptors defined using receptor‐specific collagen‐mimetic peptide substrata in flowing blood. Blood, 115, 5069–5079. 10.1182/blood-2010-01-260778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ranchoux, B. , Harvey, L. D. , Ayon, R. J. , Babicheva, A. , Bonnet, S. , Chan, S. Y. , … Perez, V. J. (2018). Endothelial dysfunction in pulmonary arterial hypertension: An evolving landscape (2017 Grover Conference Series). Pulmonary Circulation, 8, 2045893217752912 10.1177/2045893217752912 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rondina, M. T. , & Garraud, O. (2014). Emerging evidence for platelets as immune and inflammatory effector cells. Frontiers in Immunology, 5, 653 10.3389/fimmu.2014.00653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen, L. , Shen, J. , Pu, J. , & He, B. (2011). Aspirin attenuates pulmonary arterial hypertension in rats by reducing plasma 5‐hydroxytryptamine levels. Cell Biochemistry and Biophysics, 61, 23–31. 10.1007/s12013-011-9156-x [DOI] [PubMed] [Google Scholar]
- Sherlock, L. G. , Trumpie, A. , Hernandez‐Lagunas, L. , McKenna, S. , Fisher, S. , Bowler, R. , … Nozik‐Grayck, E. (2018). Redistribution of extracellular superoxide dismutase causes neonatal pulmonary vascular remodeling and PH but protects against experimental bronchopulmonary dysplasia. Antioxidants, 7(3), 42 10.3390/antiox7030042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smyth, S. S. , McEver, R. P. , Weyrich, A. S. , Morrell, C. N. , Hoffman, M. R. , Arepally, G. M. , … Platelet, C. P. (2009). Platelet functions beyond hemostasis. Journal of Thrombosis and Haemostasis, 7, 1759–1766. 10.1111/j.1538-7836.2009.03586.x [DOI] [PubMed] [Google Scholar]
- Sola‐Visner, M. , & Bercovitz, R. S. (2016). Neonatal platelet transfusions and future areas of research. Transfusion Medicine Reviews, 30, 183–188. 10.1016/j.tmrv.2016.05.009 [DOI] [PubMed] [Google Scholar]
- Stolla, M. C. , Catherman, S. C. , Kingsley, P. D. , Rowe, R. G. , Koniski, A. D. , Fegan, K. , … Palis, J. (2019). Lin28b regulates age‐dependent differences in murine platelet function. Blood Advances, 3, 72–82. 10.1182/bloodadvances.2018020859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tantawy, A. A. , Adly, A. A. , Ismail, E. A. , Habeeb, N. M. , & Farouk, A. (2013). Circulating platelet and erythrocyte microparticles in young children and adolescents with sickle cell disease: Relation to cardiovascular complications. Platelets, 24, 605–614. 10.3109/09537104.2012.749397 [DOI] [PubMed] [Google Scholar]
- von Hundelshausen, P. , Petersen, F. , & Brandt, E. (2007). Platelet‐derived chemokines in vascular biology. Thrombosis and Haemostasis, 97, 704–713. 10.1160/TH07-01-0066 [DOI] [PubMed] [Google Scholar]
- Wagenvoort, C. A. (1980). Lung biopsy specimens in the evaluation of pulmonary vascular disease. Chest, 77, 614–625. 10.1378/chest.77.5.614 [DOI] [PubMed] [Google Scholar]
- Wagner, B. D. , Babinec, A. E. , Carpenter, C. , Gonzalez, S. , O'Brien, G. , Rollock, K. , … Abman, S. H. (2018). Proteomic profiles associated with early echocardiogram evidence of pulmonary vascular disease in preterm infants. American Journal of Respiratory and Critical Care Medicine, 197, 394–397. 10.1164/rccm.201703-0654LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walther, D. J. , Peter, J. U. , Winter, S. , Holtje, M. , Paulmann, N. , Grohmann, M. , … Bader, M. (2003). Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha‐granule release. Cell, 115, 851–862. [DOI] [PubMed] [Google Scholar]
- Willems, C. , & van Aken, W. G. (1979). Production of prostacyclin by vascular endothelial cells. Haemostasis, 8, 266–273. [DOI] [PubMed] [Google Scholar]
- Yabanoglu, S. , Akkiki, M. , Seguelas, M. H. , Mialet‐Perez, J. , Parini, A. , & Pizzinat, N. (2009). Platelet derived serotonin drives the activation of rat cardiac fibroblasts by 5‐HT2A receptors. Journal of Molecular and Cellular Cardiology, 46, 518–525. 10.1016/j.yjmcc.2008.12.019 [DOI] [PubMed] [Google Scholar]
- Yaoita, N. , Shirakawa, R. , Fukumoto, Y. , Sugimura, K. , Miyata, S. , Miura, Y. , … Horiuchi, H. (2014). Platelets are highly activated in patients of chronic thromboembolic pulmonary hypertension. Arteriosclerosis, Thrombosis, and Vascular Biology, 34, 2486–2494. 10.1161/ATVBAHA.114.304404 [DOI] [PubMed] [Google Scholar]
- Yuan, Y. , Alwis, I. , Wu, M. C. L. , Kaplan, Z. , Ashworth, K. , Bark, D. , … Jackson, S. P. (2017). Neutrophil macroaggregates promote widespread pulmonary thrombosis after gut ischemia. Science Translational Medicine, 9(409), eaam5861 10.1126/scitranslmed.aam5861 [DOI] [PubMed] [Google Scholar]
- Zucker‐Franklin. (2000). Platelet production pulmonary capillary bed.pdf. American Journal Pathology, 157, 69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]


