Abstract
Purpose:
Stereotactic Body Radiation Therapy (SBRT) is the standard of care for medically inoperable patients with early-stage non-small cell lung cancer (NSCLC). However, NSCLC is composed of several histological subtypes and the impact of this heterogeneity on SBRT treatments has yet to be established.
Patients and Methods:
We analyzed 740 early-stage NSCLC patients treated definitively with SBRT from 2003 through 2015. We calculated cumulative incidence curves using the competing risk method and identified predictors of local failure using Fine and Gray regression.
Results:
Overall, 72 patients had a local failure with a cumulative incidence of local failure at three years of 11.8%. On univariate analysis, squamous histology, younger age, fewer medical comorbidities, higher BMI, higher PET SUV, central tumors and lower radiation dose were associated with an increased risk of local failure. On multivariable analysis, squamous histology (HR 2.4 p = 0.008) was the strongest predictor of local failure. Patients with squamous cancers fail SBRT at a significantly higher rate than those with adenocarcinomas or NSCLC-not otherwise specified, with three-year cumulative incidences of local failure of 18.9% (95% CI= 12.7–25.1%), 8.7% (95% CI= 4.6–12.8%), 4.1% (95% CI= 0–9.6%), respectively.
Conclusion:
Our results demonstrate an increased rate of local failure in patients with squamous cell carcinoma. Standard approaches for radiotherapy that demonstrate efficacy for a population may not achieve optimal results for individual patients. Establishing the differential dose-effect of SBRT across histological groups is likely to improve efficacy and inform ongoing and future studies that aim to expand indications for SBRT.
INTRODUCTION
Clinical outcomes after surgery for patients with early-stage non-small cell lung cancer (NSCLC) are favorable, with five-year survival rates ranging from 60 to 80%.1 However, high rates of comorbid disease that could result in significant perioperative morbidity and impact long-term quality of life often disqualify many patients from surgery. In this milieu, non-invasive treatments like Stereotactic Body Radiotherapy (SBRT) to the lung were developed and expanded. To date, several prospective Phase II trials using SBRT have been conducted in medically inoperable patients with excellent results; namely, two to three year local tumor control between 93–98%.2–4 These successes have established SBRT as the standard of care in medically inoperable patients and studies have begun to explore a role for SBRT in healthier patients with early-stage NSCLC.5–10
The use SBRT for NSCLC has been optimized by the identification of tumor and treatment related factors that predict failure including larger tumor size and lower radiation doses.11–14 However, it remains unclear whether groups within NSCLC are more or less likely to respond to treatments. NSCLCs are characterized by substantial genetic diversity and the most optimal therapeutic approach is likely to vary based on the genetic features of individual cancers.15,16 Indeed, the stratification of NSCLC patients into more homogenous populations has resulted in a greater likelihood of response to specific agents.17–22 In the same manner, histological and molecular profiling of tumors may reveal subpopulations that are more or less likely to effect local control after SBRT. Elucidating the interaction between radiation dose and NSCLC subtypes is critical to SBRT’s optimization for indications beyond its current role in the treatment of early-stage, medically inoperable patients.
NSCLC is composed of two predominant histological subtypes, adenocarcinoma (~50%) and squamous cell carcinoma (SqCC) (~30%).23 Histology appears to separate NSCLC into more homogeneous subtypes24 and there are important differences in the genetic and microenvironments of tumors from glandular histologies (adenocarcinomas) compared to squamous differentiation.15,16,25 Therefore, we posited that the histological subtypes of lung cancer may impact the efficacy of SBRT. Herein, we used competing risk analysis to identify tumor and treatment factors that are predictive of local failure after SBRT.
MATERIAL AND METHODS
Patients
From an IRB approved database of 1084 patients treated with lung SBRT from 2003–2015, we included patients with clinical stages T1-T3N0M0 (AJCC 7th Ed.) lung cancer and excluded patients with tumors that invaded the chest wall, synchronous primary tumors and cases treated with the intent to salvage recurrent tumor after prior radiotherapy or surgery. Patients were treated based on either a pathologic or radiographic diagnosis. A diagnosis of lung cancer was confirmed by histological examination of biopsy specimens for 69.8% of the patients studied. A general surgical pathologist or a pulmonary pathologist diagnosed cases from 2003 to July 2014. A staff pulmonary pathologist classified all lung cancer cases after July 2014. The World Health Organization classification system was used from 2003–2011.26,27 After 2011, The International Association for the Study of Lung Cancer, the American Thoracic Society, and the European Respiratory Society (IASLC/ATS/ERS) classification system was used.28 In most cases, a diagnosis of adenocarcinoma and SqCC was made based solely on morphologic criteria. A histological or immunohistochemical (IHC) staining panel was used to better define the diagnosis if the morphology was not distinctly squamous or adenocarcinoma. In some cases, this resulted in diagnoses of NSCLC-favor adenocarcinoma or -favor SqCC. These cases were grouped with their respective “favored” diagnosis. NSCLC-not otherwise specified (NOS) was reserved for cases with strong, concurrent features of both adenocarcinoma and SqCC and cases without any definitive morphologic or IHC features of either adenocarcinoma or SqCC. Cases of large cell carcinoma before 2011 were re-classified as NSCLC-NOS for consistency with the current terminology.
In 30.1% (n = 223) of patients that did not have a confirmed pathologic diagnosis, an attempt at a biopsy was made in 39% (n = 87 or 11.8% of the overall population) without confirmation of malignant disease. A radiographic diagnosis was established in cases where a biopsy was contraindicated or was non-diagnostic. The criteria for a radiographic diagnosis included serial growth of a single lesion on computed tomography (CT) scans and positron emission tomography (PET) specific uptake values (SUV) that exceeded 3.0.
In all cases, an experienced thoracic surgeon and/or pulmonologist established medical inoperability. For each patient, the Charlson Commorbidity Index (CCI) was documented to assess comorbid illness and baseline pulmonary function testing was obtained. All patients were staged using CT of the chest, PET/CT and imaging of the brain (magnetic resonance imaging [MRI] or CT) was employed when clinically indicated. In cases where imaging revealed mediastinal or hilar lymph nodes enlarged by accepted radiographic criteria or where the standardized uptake value (SUV) exceeded a value of 3.0 on PET, pathologic mediastinal evaluation with endobronchial ultrasonography-guided sampling (EBUS) was requested.29 The use of chemotherapy after SBRT in patients considered at risk for distant failure was not routinely recommended given patient comorbidities. A small fraction of patients did receive chemotherapy (8.8%) at the discretion of the multidisciplinary treatment team.
Treatment
Our institutional approach for lung SBRT consists of immobilization in a Bodyfix (Elekta, Stockholm, Sweden) vacuum system with abdominal compression to restrict breathing motion, which is evaluated by fluoroscopy.30 In cases where motion could not be adequately restricted to less than 1 cm, Active Breathing Coordinator (Elekta, Stockholm, Sweden) was used. Tumors within a 2-cm expansion of the tracheobronchial tree were categorized as central.31 Our institutional SBRT approach used a risk-adapted approach.32 Initially, all patients received 50 Gy in five fractions. When the RTOG 0236 trial commenced, we elected to treat eligible patients with peripheral tumor to 60 Gy in three fractions as per protocol and continued to treat central tumors to 50 Gy in five fractions. In total, 70.8% of all patients analyzed received 50 Gy in five fractions or 60 Gy in three fractions (45.3% and 25.5%, respectively). Alternative fractionations [30 Gy in a single fraction (10%), 34 Gy in a single fraction (6.5%), 48 Gy in 4 fractions (7.3%), and other fractionation schemes (5.9%)] were employed for patients enrolled on clinical trial or if constraints for our standard fractionation schedules could not be met. Dose prescription and normal tissue constraint guidelines from national protocols including RTOG 0813, 0236, and 0915, and a protocol from the Roswell Park Cancer Institute at Buffalo, NY were used. All treatment plans were normalized to either the dose at the isocenter or maximum point dose. The dose was prescribed to an isodose line between 60–90% so that the PTV receives at least 95% coverage and the minimal dose to the PTV (D99) is 90% of the prescription. Heterogeneity correction was applied in dose calculations except for dose schemes of 60 Gy in three fractions and 30 Gy in a single fraction; these plans were calculated assuming homogeneous density within the patient. Patients were treated on a Novalis Platform (BrainLab, Munich, Germany) or NovalisTX (Varian Medical Systems, Palo Alto, CA) using either step and shoot IMRT, dynamic arc or, very rarely, volumetric arc therapy technique. Daily image guidance was performed with an orthogonal KV imaging (ExacTrac, BrainLab, Munich, Germany) with the addition of cone beam CT image guidance after 2011.
Endpoints
Local failure was defined as either radiographic progression with or without positive biopsy within 1 cm of the PTV to maintain a consistent definition of local/marginal failure in clinical trials of SBRT.3,4,33 CT scan(s), followed by at least one PET/CT, determined radiographic progression. In cases in which PET/CT revealed a value below the initial pre-treatment PET/CT scan, another PET/CT scan was obtained. A maximum SUV exceeding the initial pre-treatment PET scan or serial increases in SUV on post-treatment PET scan was considered a local failure. If PET/CT findings were consistent with failure, a biopsy was requested. A diagnosis of recurrent disease was confirmed histologically in 40.3% of the patients with local failure. All other patients were either medically unfit or unwilling to undergo a biopsy or the lesion was deemed to be inaccessible by a staff radiologist and/or pulmonologist. In these cases, a diagnosis of recurrence was made on the basis of radiographic progression alone. PET/CTPET/CTFailures within the same lobe of the lung but greater than 1 cm from the PTV of the initial treatment site were defined as lobar failure and was not considered in this analysis.
Statistical Analysis
Tumors were grouped into four categories: adenocarcinomas, SqCCs, unknown and NSCLC-NOS. The unknown group included patients that did not undergo a tissue biopsy and those with a non-diagnostic biopsy. The NSCLC-NOS group included patients with large cell carcinoma (before 2011) and NSCLC-NOS. Prescription radiation dose was adjusted for the number of fractions of radiation by calculating the biological equivalent dose (BED) with a standard α/β ratio of 10. BED RT dose was modeled as a categorical variable given its skewed distribution of prescription doses. Length of follow up was determined from end date of SBRT and patients who had not died were censored at the time of last chest imaging. Confidence intervals of the proportions were calculated by the Clopper and Pearson exact test. Confidence intervals of the median times to local failure were calculated by quantile estimates of the binomial distribution. Death without evidence of local failure was treated as a competing event, and Fine and Gray regression modeling was used to examine potential predictors of local failure. Age at the time of radiotherapy, gender, body mass index, current smoker status, CCI, PET/CT (SUV), tumor size, biologically effective dose and histological categories were subjected to univariate analyses. Variables with a p-value < 0.1 were included in a multivariable model. Cumulative incidence curves for local failure were estimated using the competing risk method, and Gray’s test was used to determine significance between cumulative incidence curves.34 Actuarial analysis was used to estimate rates of overall survival and the Kaplan-Meier method was used to generate overall survival curves. Statistical analysis was performed using SAS v9.4 (SAS Inc., Cary, NC) and R 3.2.4 (The R Foundation, Vienna, Austria).35
RESULTS
Patient Characteristics
740 patients met our eligibility criteria. The median survival time for the group as a whole was 30.5 months, with 1-, 2-, and 3- year actuarial rates of OS of 80.3% (95% confidence interval (CI)= 77.3– 83.3%), 59.6% (95% CI= 55.7– 63.5%), and 43.0% (95% CI= 38.7– 47.3%), respectively (Supplemental Figure 1). Adenocarcinoma, SqCC, unknown and NSCLC-NOS represented 32%, 29%, 30% and 8% of the overall population, respectively (Table 1). Patients were well balanced for patient age, gender, body mass index, performance status, length of follow up and radiotherapy dose. Only 9 patients (1.2%) did not undergo PET staging; one of these patients underwent mediastinal staging by EBUS. Patients with SqCC and in the unknown group consumed more tobacco in pack years (p=0.019) and patients in the unknown group had a higher CCI (p=0.041). Patients with SqCC were more likely to have tumors of larger size (p<0.001) and with higher PET SUV (p<0.001). The median follow-up overall was 15.5 months (IQR: 6.5–29.6). The median follow-up for SqCC, Adeno, unknown and NSCLC-NOS in months were 17.5 (IQR: 7.5–30.4), 13.6 (IQR: 5.0–27.9), 15.6 (IQR: 7.9–30.5) and 17.5 (IQR: 4.9–30.3), respectively, and were not significantly different (p = 0.23; calculated by the non-parametric test of medians). Among the 8.8% of total patients who received chemotherapy, there were no substantial differences in the percent of patients receiving chemotherapy by histology: SqCC 10.2%, adenocarcinoma 9.1%, unknown 8.1% and NOS 5.1%.
Table 1.
Baseline characteristics of the study population.
| Overall | Adeno | SqCC | Unknown | NSCLC-NOS | p | |
|---|---|---|---|---|---|---|
| n | 740 | 243 | 215 | 223 | 59 | |
| Characteristic | No. of Patients (%) or median [IQR] |
No. of Patients (%) or median [IQR] |
No. of Patients (%) or median [IQR] |
No. of Patients (%) or median [IQR] |
No. of Patients (%) or median [IQR] |
|
| Gender | 0.339 | |||||
| Female | 379 (51.2) | 125 (51.4) | 101 (47.0) | 118 (52.9) | 35 (59.3) | |
| Male | 361 (48.8) | 118 (48.6) | 114 (53.0) | 105 (47.1) | 24 (40.7) | |
| Ethnicity | 0.407 | |||||
| White | 615 (83.3) | 197 (81.4) | 179 (83.3) | 186 (83.8) | 53 (89.8) | |
| Black | 105 (14.2) | 36 (14.9) | 32 (14.9) | 32 (14.4) | 5 (8.5) | |
| Asian /Pacific Islander | 7 (0.9) | 4 (1.7) | 0 (0.0) | 3 (1.4) | 0 (0.0) | |
| Hispanic/Latino | 6 (0.8) | 4 (1.7) | 2 (0.9) | 0 (0.0) | 0 (0.0) | |
| Other | 3 (0.4) | 1 (0.4) | 0 (0.0) | 1 (0.5) | 1 (1.7) | |
|
American
Indian/Alaskan Native |
1 (0.1) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | |
| Multi-racial, -cultural | 1 (0.1) | 0 (0.0) | 1 (0.5) | 0 (0.0) | 0 (0.0) | |
| Age (years) | 74.7 [68.4, 81.3] | 75.0 [68.6, 81.2] | 74.5 [68.9, 81.8] | 74.7 [68.1, 80.8] | 73.0 [66.9, 80.8] | 0.772 |
| Tobacco (pack years) | 50.0 [30.0, 67.0] | 44.0 [25.0, 60.0] | 50.0 [30.0, 65.0] | 50.0 [31.5, 75.0] | 45.0 [20.0, 60.0] | 0.019* |
| KPS | 80.0 [70.0, 90.0] | 80.0 [70.0, 90.0] | 80.0 [70.0, 85.0] | 80.0 [70.0, 90.0] | 80.0 [70.0, 80.0] | 0.232 |
| BMI (kg/m2) | 26.0 [22.6, 30.1] | 26.0 [22.6, 30.5] | 25.8 [22.6, 29.6] | 26.5 [22.5, 30.5] | 26.6 [22.8, 29.7] | 0.727 |
| CCI | 3 [1, 4] | 2 [1, 4] | 2 [1, 3] | 3 [2, 4] | 2 [1, 3] | 0.041* |
| Reason for Inoperability | 0.431 | |||||
| Cardiac | 103 (13.9) | 35 (14.4) | 29 (13.5) | 32 (14.3) | 7 (11.9) | |
| Multifactorial | 21 (2.8) | 6 (2.5) | 13 (6.0) | 1 (0.4) | 1 (1.7) | |
| Other | 9 (1.2) | 2 (0.8) | 1 (0.5) | 5 (2.2) | 1 (1.7) | |
| Other Malignancy | 72 (9.7) | 24 (9.9) | 19 (8.8) | 25 (11.2) | 4 (6.8) | |
| Poor KPS | 22 (3.0) | 9 (3.7) | 7 (3.3) | 6 (2.7) | 0 (0.0) | |
| Pulmonary | 438 (59.2) | 138 (56.8) | 127 (59.1) | 133 (59.6) | 40 (67.8) | |
| Refused | 41 (5.5) | 17 (7.0) | 11 (5.1) | 10 (4.5) | 3 (5.1) | |
| Vascular | 30 (4.1) | 11 (4.5) | 6 (2.8) | 10 (4.5) | 3 (5.1) | |
| Unknown | 4 (0.5) | 1 (0.4) | 2 (0.9) | 1 (0.4) | 0 (0.0) | |
| Lung Cancer Histology | ||||||
| Adeno | 243 (32.8) | |||||
| SqCC | 215 (29.1) | |||||
| No biopsy | 136 (18.4) | |||||
| Non-diagnostic biopsy | 87 (11.8) | |||||
| NSCLC-NOS | 59 (8.0) | |||||
| Tumor Location | 0.059 | |||||
| Peripheral | 562 (75.9) | 184 (75.7) | 159 (74.0) | 176 (78.9) | 43 (72.9) | |
| Central | 172 (23.2) | 55 (22.6) | 56 (26.0) | 47 (21.1) | 14 (23.7) | |
| Unknown | 6 (0.8) | 4 (1.6) | 0 (0.0) | 0 (0.0) | 2 (3.4) | |
| Tumor Size (cm) | 2.2 [1.7, 3.1] | 2.3 [1.8, 3.2] | 2.7 [1.9, 4.0] | 1.9 [1.4, 2.5] | 2.4 [1.8, 3.1] | <0.001* |
| PET/CT (SUV) | 7.6 [4.3, 11.8] | 5.8 [3.6, 9.6] | 11.1 [7.8, 16.2] | 6.2 [4.0, 10.0] | 7.3 [4.8, 11.7] | <0.001* |
| T Classification | <0.001* | |||||
| T1a | 305 (41.3) | 84 (34.6) | 69 (32.1) | 128 (57.4) | 21 (35.6) | |
| T1b | 248 (33.5) | 93 (38.2) | 64 (29.8) | 71 (31.8) | 23 (39.0) | |
| T2a | 148 (20.0) | 58 (23.9) | 53 (24.7) | 22 (9.9) | 15 (25.4) | |
| T2b | 37 (5.0) | 9 (3.7) | 26 (12.1) | 2 (0.9) | 0 (0.0) | |
| T3 | 3 (0.4) | 0 (0.0) | 3 (1.4) | 0 (0.0) | 0 (0.0) | |
| Overall Stage | <0.001* | |||||
| IA | 546 (73.8) | 174 (71.6) | 131 (60.9) | 198 (88.8) | 43 (72.9) | |
| IB | 152 (20.5) | 59 (24.3) | 54 (25.1) | 22 (9.9) | 16 (27.1) | |
| IIA | 40 (5.4) | 9 (3.7) | 29 (13.5) | 2 (0.9) | 0 (0.0) | |
| IIB | 2 (0.3) | 1 (0.4) | 1 (0.5) | 1 (0.4) | 0 (0.0) | |
| Total Dose | 50 [50, 60] | 50 [48, 57.5] | 50 [50, 60] | 50 [48, 60] | 50 [50, 60] | 0.31 |
| No. of Fractions | 5.0 [3.0, 5.0] | 5.0 [3.0, 5.0] | 5.0 [3.0, 5.0] | 4.0 [3.0, 5.0] | 4.0 [3.0, 5.0] | 0.121 |
| BED | 105 [100, 180] | 106 [100, 150] | 105 [100, 150] | 105.6 [100, 180] | 105.6 [100, 180] | 0.797 |
Continuous variables are represented as median [IQR].
Abbreviations: BED, biologically effective dose; BMI, body mass index; CCI, Charlson comorbidity index; IQR, inter-quartile range; KPS, Karnofsky performance status; NSCLC-NOS, non-small cell carcinoma-not otherwise specified; OS, overall survival; SUV, standardized uptake values.
p values were calculated using Fisher’s exact for categorical variables and by the non-parametric test of medians for continuous variables.
p value of < 0.05 was considered statistically significant.
Tumor Control and Patterns of Recurrence
A total of 72 local failures were identified with a cumulative incidence of local failure at 3 years of 11.8% (95% CI= 9.1–14.5%) in the overall population. 90.3% of local failures were observed to occur within 25.1 months of initial treatment (Figure 1a). The median times to local failure for SqCCs and adenocarcinomas in months were 14.9 (95% CI=11.4–17.2) and 18.9 (95% CI: 11.9–20.1), respectively (Figure 1b). The proportion of local failure for patients with SqCC, adenocarcinoma, unknown and NOS were 15.3% (95% CI=10.8–20.9%), 7.4% (95% CI=4.4–11.4%), 8.5% (95% CI=5.2–13.0%) and 3.4% (95% CI=0.004–11.7%), respectively. The 1-, 2-, and 3-year actuarial rates of local failure for the group as a whole were 4.2%, 14.6%, and 16%, respectively. 54.2% (95% CI=42.0–65.9%) of patients with local failure experienced a nodal or distant metastasis, whereas 21.1% (95% CI=18.1–24.4%) of patients with local control experienced nodal or distant failure. The increased rate of nodal or distant metastasis associated with local failure did not vary based on histological categories (Supplemental Table 1). Taken together, SqCCs have a higher proportion of local failure than other lung cancer subtypes and the rate of nodal or distant metastases is significantly higher in patients who have a local failure than those with no evidence of local failure, suggesting that local failure is associated with nodal and/or distant recurrence.
Figure 1.
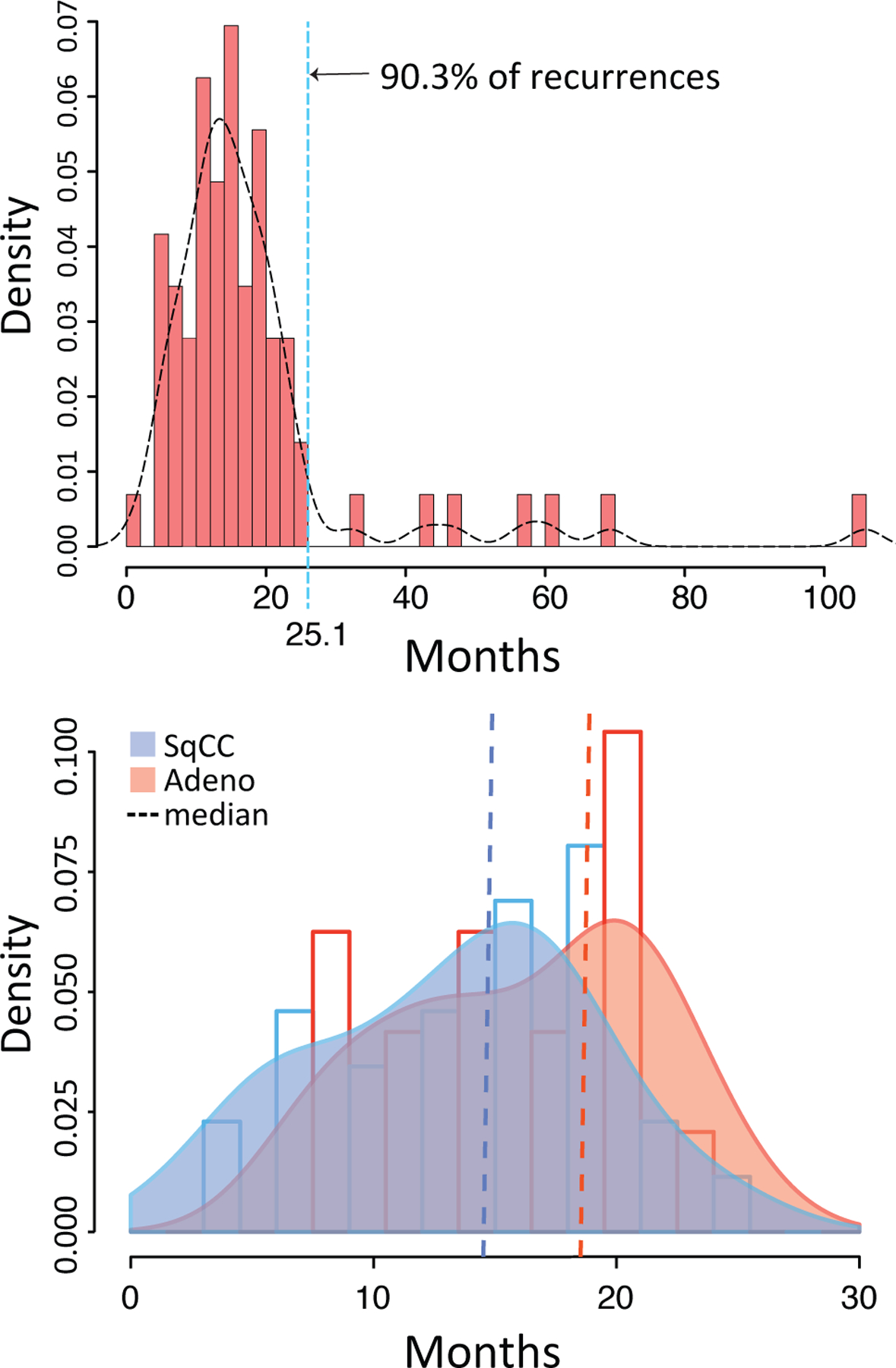
Probability density histogram(s) and curve(s) of time to local failure. (a) Overall (a) and by (b) Adeno= adenocarcinoma and SqCC= squamous cell carcinoma.
Predictive Factors
On univariate competing risk analysis, we demonstrated that squamous histology, younger age, fewer medical comorbidities, higher BMI, higher PET SUV, central tumors, and lower radiation dose were associated with an increased risk of local failure (Table 2). On multivariable analysis, squamous histology (HR 2.4, 95% CI= 1.3–4.5, p = 0.008) was the strongest predictor of local failure (Table 3). Lower radiation dose (HR 0.99, p = 0.008) and higher BMI (HR 1.07, p = <0.001) remained significantly associated with local failure. Estimated cumulative incidence curves (CICs) for death and local failure for each lung cancer histology group are shown in Figure 2. Gray’s test for equality across histological groups was highly significant for local failure (p=0.002). Patients with squamous cancers fail SBRT at a significantly higher rate than those with adenocarcinomas or NSCLC-NOS, with three-year cumulative incidences of local failure of 18.9% (95% CI= 12.7–25.1%), 8.7% (95% CI= 4.6–12.8%) and 4.1% (95% CI= 0–9.6%), respectively. Gray’s test for equality across the groups was not statistically significant for death (p=0.07), although the probability of death appeared to trend higher in the NSCLC-NOS group.
Table 2.
Predictors of local failure in univariate analysis.
| HR | 95% L | 95% U | p | |
|---|---|---|---|---|
| Age | 0.972 | 0.949 | 0.995 | 0.0191* |
| Gender (Female v. Male) | 1.061 | 0.669 | 1.683 | 0.8006 |
| BMI | 1.057 | 1.024 | 1.091 | 0.0007* |
| Current Smoker (No v. Yes) | 1.385 | 0.746 | 2.572 | 0.3019 |
| CCI | ||||
| 0–1 v. >3 | 1.722 | 0.795 | 3.732 | 0.1685 |
| 2–3 v. >3 | 2.605 | 1.311 | 5.179 | 0.0063* |
| 0–1 v. 2–3 | 0.661 | 0.384 | 1.139 | 0.1359 |
| PET SUV | 1.035 | 1.005 | 1.065 | 0.0203* |
| Central Tumor (Yes v. No) | 1.742 | 1.073 | 2.825 | 0.0246* |
| CT Size | 1.164 | 0.988 | 1.371 | 0.0691 |
| T stage (T1 v. T2)# | 0.829 | 0.499 | 1.376 | 0.4677 |
| Histology | ||||
| SqCC v. Adeno | 2.321 | 1.317 | 4.089 | 0.0036* |
| SqCC v. NSCLC-NOS | 5.660 | 1.380 | 23.223 | 0.0161* |
| SqCC v. Unknown | 1.996 | 1.133 | 3.517 | 0.0168* |
| Adeno v. NSCLC-NOS | 2.439 | 0.574 | 10.356 | 0.2268 |
| Adeno v. Unknown | 0.860 | 0.452 | 1.637 | 0.6463 |
| NSCLC-NOS v. Unknown | 0.353 | 0.083 | 1.495 | 0.1572 |
| BED (continuous) | 0.985 | 0.977 | 0.994 | 0.0009* |
| BED (categorical) | ||||
| 100 v. 180 | 3.913 | 1.703 | 8.994 | 0.0013* |
| 105–106 v. 180 | 3.262 | 1.148 | 9.267 | 0.0265* |
| 120–125 v. 180 | 1.940 | 0.600 | 6.273 | 0.2682 |
| 149.6 v. 180 | 3.748 | 1.171 | 11.998 | 0.0260* |
| BED (two groups only) | ||||
| 100 v. 180 | 3.917 | 1.703 | 9.007 | 0.0013* |
Abbreviations: Adeno, adenocarcinoma; BED, biologically effective dose; BMI, body mass index; CCI, Charlson comorbidity index; CT, computed tomography; NSCLC-NOS, non-small cell carcinoma-not otherwise specified; SqCC, squamous cell carcinoma; SUV, standardized uptake values.
Three patients with stage T3 disease were excluded from this analysis.
p value of < 0.05 was considered statistically significant.
Table 3.
Predictors of local failure in multivariable analysis.
| HR | 95% L | 95% U | p | |
|---|---|---|---|---|
| Age | 0.985 | 0.964 | 1.008 | 0.1943 |
| BMI (kg/m2) | 1.066 | 1.029 | 1.105 | 0.0004* |
| CCI | ||||
| 0–1 vs >3 | 1.419 | 0.63 | 3.197 | 0.3986 |
| 2–3 vs >3 | 2.127 | 1.029 | 4.396 | 0.0416* |
| 0–1 vs 2–3 | 0.667 | 0.378 | 1.178 | 0.1632 |
| CT Size | 1.049 | 0.858 | 1.283 | 0.6397 |
| PET/CT SUV | 1.006 | 0.968 | 1.046 | 0.7523 |
| Central Tumor (Yes v. No) | 1.067 | 0.589 | 1.93 | 0.8312 |
| Histology | ||||
| SqCC v. Adeno | 2.377 | 1.251 | 4.515 | 0.0082* |
| SqCC v. NSCLC-NOS | 4.705 | 1.221 | 18.129 | 0.0244* |
| SqCC v. Unknown | 1.808 | 0.951 | 3.435 | 0.0707 |
| Adeno v. NSCLC-NOS | 1.98 | 0.486 | 8.056 | 0.3403 |
| Adeno v. Unknown | 0.761 | 0.37 | 1.562 | 0.4562 |
| NSCLC-NOS v. Unknown | 0.384 | 0.096 | 1.541 | 0.177 |
| BED | 0.986 | 0.976 | 0.996 | 0.0079* |
Abbreviations: Adeno, adenocarcinoma; BED, biologically effective dose; BMI, body mass index; CCI, Charlson comorbidity index; CT, computed tomography; NSCLC-NOS, non-small cell carcinoma-not otherwise specified; SqCC, squamous cell carcinoma; SUV, standardized uptake values.
p value of < 0.05 was considered statistically significant.
Figure 2.
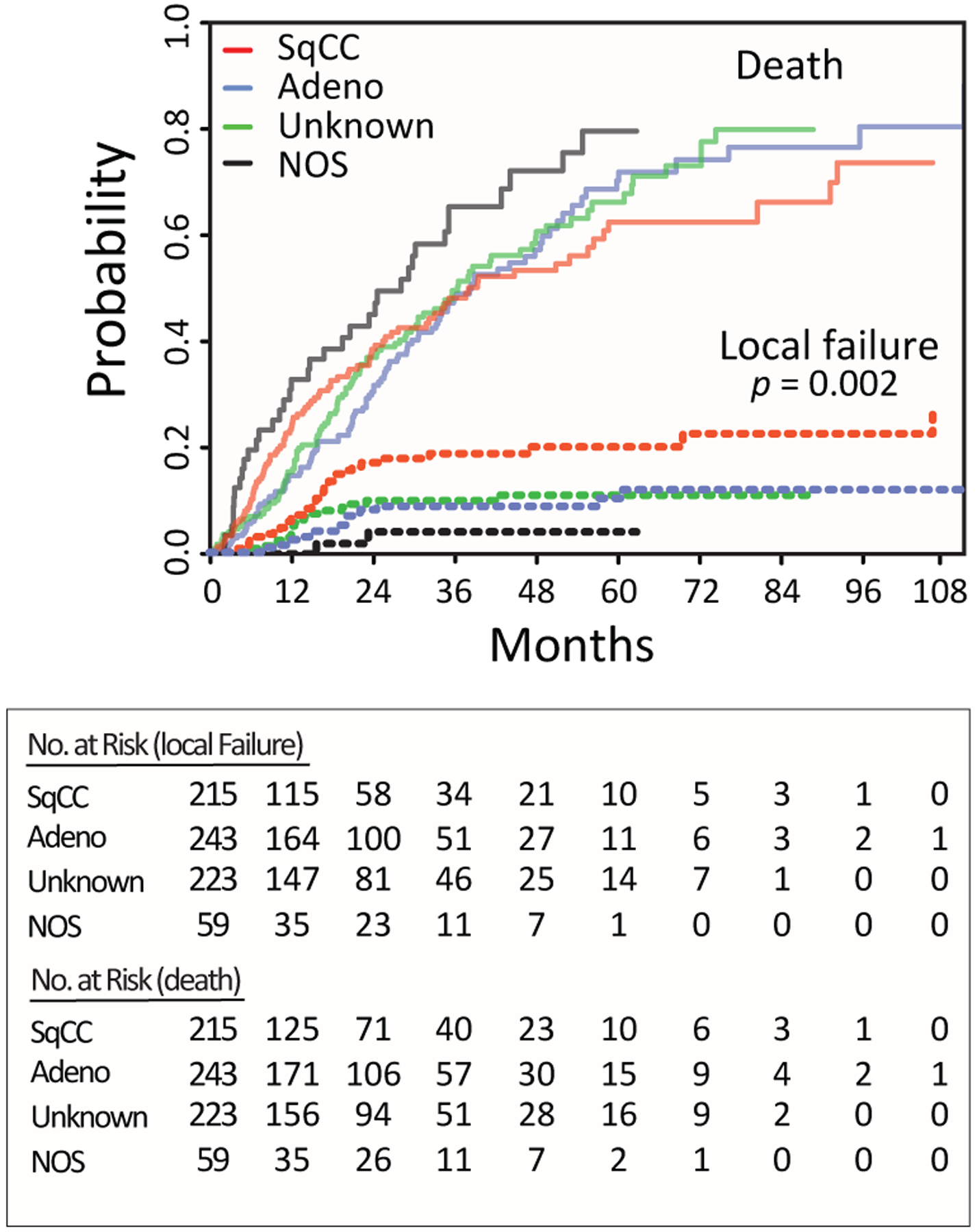
Estimated cumulative incidence curves with overall mortality and local failure for three categories of lung cancer. SqCC= squamous cell carcinoma; Adeno= adenocarcinoma; Unknown=no or non-diagnostic biopsy; NOS=NSCLC-not otherwise specified.
We plotted CICs for categories of radiation dose to assess the role of dose in local failure and generally observed a directly proportional increase in local control as a function of BED dose category (Supplemental Figure 2). Although patients treated to a BED of 149.6 Gy, which corresponded to a single fraction of SBRT at a dose of 34 Gy, fell below the trendline, they represented a small number of the patients treated (n=46). The two most common schedules used institutionally were 50 Gy in five fractions and 60 Gy in three fractions, or a BED of 100 and 180 Gy, respectively. To assess the impact of dose on local control between histological categories, we compared the CICs of adenocarcinomas and SqCCs within the two dose subgroups (100 Gy and 180 Gy BED) (Figure 3). Gray’s test for equality between the two histological groups was not statistically significant for local failure in the 180 Gy BED group (p=0.33), but was significant in the 100 Gy BED group (p=0.021). The three-year cumulative incidence of local failure for patients treated to a lower dose of 100 Gy BED was significantly higher in SqCCs (24.9%, 95% CI= 14.7– 35.1%) compared with adenocarcinomas (12.1%, 95% CI= 5.4– 18.9%). We examined the effect of histology on local control within the 100 Gy BED group by univariate competing risk subgroup analysis of T2 and T1 tumors (Supplemental Table 2). Within the T2, BED 100 Gy subgroup, SqCCs were more likely to fail compared with adenocarcinomas (HR 10.3, p = 0.02). Taken together, these results indicate that SqCCs are significantly more likely to fail compared with adenocarcinomas at the frequently used dose of 100 Gy BED and that the inferior local control rate is not explained solely by the larger tumor size of SqCCs.
Figure 3.
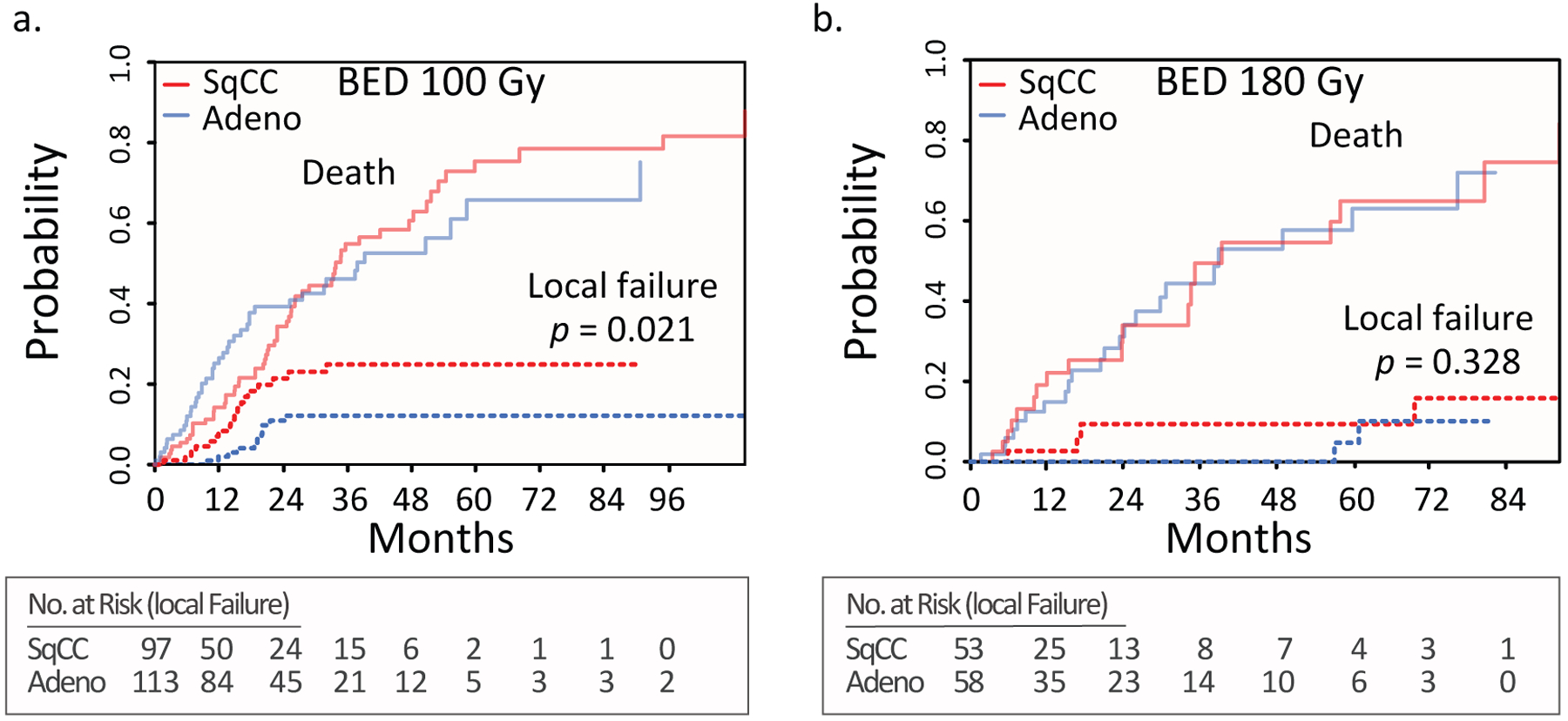
Estimated cumulative incidence curves with overall mortality and local failure as competing events for adenocarcinomas and squamous cell carcinoma for patients receiving a BED of 100 Gy (a) and 180 Gy (b). Adeno= adenocarcinoma; SqCC= squamous cell carcinoma.
DISCUSSION
Our results demonstrate that SqCCs have a significantly higher rate of local failure after SBRT than other NSCLCs. The local failure rate for SqCCs was 2-fold higher than adenocarcinomas. The three-year cumulative incidence of local failure for SqCCs was ~19%. For patients receiving the most frequent regimen of 50 Gy in five fractions, the cumulative rate of failure was ~25%. These results suggest that SqCCs are more resistant to SBRT than other NSCLCs. The clinically significant implication of our study is that SBRT treatments should be optimized on the basis of histology.
Our study’s three-year local tumor control rate of 88.2% is comparable to published values.2–4,29 The strengths of this study include the large number of patients evaluated, the homogeneity of treatment delivery, the extent of pathologic confirmation of NSCLCs, the length and extent of radiographic follow-up, and the completeness of the dataset. Although we addressed possible confounding factors in our analyses, we cannot fully account for all potential causes of biases. For example, a selection bias could have favored adenocarcinomas since they can be more likely to be peripheral and able to receive the higher radiation dose of 180 Gy BED. However, we did not find any evidence to that effect. There was no statistical difference in the proportion of central to peripheral tumors or the dose of radiation between adenocarcinomas and SqCCs (Table 1) and central tumors were not significantly more likely to experience local failure than peripherally located tumors (Table 3).
This study is the first demonstration of a histological basis for local failure after SBRT. Previous prospective and retrospective data have not identified differential local failure rates for SqCCs compared to other NSCLCs.32,36–40 Putative explanations include the smaller number of patients evaluated, insufficient pathologic confirmation of disease, a low proportion of SqCCs, and the apparent lack of evaluation of histology as a predictor of failure in those studies. In our study, 215 patients (29%) had a confirmed diagnosis of SqCCs, which to our knowledge is among the largest studies of SqCCs treated with SBRT to date.
Several questions remain to be answered. First, it is unclear why SqCCs are more likely to fail SBRT compared to adenocarcinomas. Genetic and/or microenviromental features may account for some of these differences. For example, SqCCs have distinct patterns of somatic alterations, a propensity for a higher mutational burden and a higher metabolic rate resulting in hypoxia; some or all of these features may contribute to radioresistance.41–43 Second, it is unclear why a subset of SqCCs fails SBRT. Comparative genomic evaluation of SqCCs that fail and those that do not may help elucidate the precise regulators of resistance and guide the selective reversal of that resistance by targeted therapies. Thirdly, it appears that higher doses of radiation may reduce the probability of failure after SBRT. However, dose-escalation needs to be balanced against toxicity, especially for central tumor located in close proximity to organs at risk.31 Alternative fractionation and dose schemes that increase BED while minimizing acute and delayed toxicity may optimize local control in cases where toxicity is a concern.
In conclusion, although highly effective in a diverse population of NSCLCs, SBRT can result in an unacceptably high local failure rate in patients with SqCCs. These findings should help motivate an evolution of SBRT from a generic population-based approach toward more nuanced patient selection and treatments.
Supplementary Material
ACKNOWLEDEMENTS
M.E.A. was supported by NIH KL2 TR000440 and the Lung Cancer Research Foundation. This publication was made possible in part by the Clinical and Translational Science Collaborative of Cleveland from the National Center for Advancing Translational Sciences (NCATS) component of the National Institutes of Health and NIH roadmap for Medical Research. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
Footnotes
CONFLICT OF INTEREST NOTIFICATION:
The authors of this study have no conflicts of interest pertaining to the conduct of this research.
REFERENCES
- 1.Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung Cancer Study Group. The Annals of thoracic surgery. September 1995;60(3):615–622; discussion 622–613. [DOI] [PubMed] [Google Scholar]
- 2.Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. Jama. March 17 2010;303(11):1070–1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Timmerman RD, Hu C, Michalski J, et al. Long-term Results of RTOG 0236: A Phase II Trial of Stereotactic Body Radiation Therapy (SBRT) in the Treatment of Patients with Medically Inoperable Stage I Non-Small Cell Lung Cancer. In: Int J Radiat Oncol Biol Phys 2014:S30. [Google Scholar]
- 4.Videtic GM, Hu C, Singh AK, et al. A Randomized Phase 2 Study Comparing 2 Stereotactic Body Radiation Therapy Schedules for Medically Inoperable Patients With Stage I Peripheral Non-Small Cell Lung Cancer: NRG Oncology RTOG 0915 (NCCTG N0927). Int J Radiat Oncol Biol Phys. November 15 2015;93(4):757–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Crabtree TD, Denlinger CE, Meyers BF, et al. Stereotactic body radiation therapy versus surgical resection for stage I non-small cell lung cancer. The Journal of thoracic and cardiovascular surgery. August 2010;140(2):377–386. [DOI] [PubMed] [Google Scholar]
- 6.Lagerwaard FJ, Verstegen NE, Haasbeek CJ, et al. Outcomes of stereotactic ablative radiotherapy in patients with potentially operable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. May 1 2012;83(1):348–353. [DOI] [PubMed] [Google Scholar]
- 7.Grills IS, Mangona VS, Welsh R, et al. Outcomes after stereotactic lung radiotherapy or wedge resection for stage I non-small-cell lung cancer. J Clin Oncol. February 20 2010;28(6):928–935. [DOI] [PubMed] [Google Scholar]
- 8.Chang JY, Senan S, Paul MA, et al. Stereotactic ablative radiotherapy versus lobectomy for operable stage I non-small-cell lung cancer: a pooled analysis of two randomised trials. Lancet Oncol. June 2015;16(6):630–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang JY, Senan S, Smit EF, Roth JA. Stereotactic radiotherapy or surgery for early-stage non-small-cell lung cancer - Authors’ reply. Lancet Oncol. February 2016;17(2):e42–43. [DOI] [PubMed] [Google Scholar]
- 10.Nagata Y, Hiraoka M, Shibata T, et al. Prospective Trial of Stereotactic Body Radiation Therapy for Both Operable and Inoperable T1N0M0 Non-Small Cell Lung Cancer: Japan Clinical Oncology Group Study JCOG0403. Int J Radiat Oncol Biol Phys. December 1 2015;93(5):989–996. [DOI] [PubMed] [Google Scholar]
- 11.Kestin L, Grills I, Guckenberger M, et al. Dose-response relationship with clinical outcome for lung stereotactic body radiotherapy (SBRT) delivered via online image guidance. Radiother Oncol. March 2014;110(3):499–504. [DOI] [PubMed] [Google Scholar]
- 12.Dunlap NE, Larner JM, Read PW, et al. Size matters: a comparison of T1 and T2 peripheral non-small-cell lung cancers treated with stereotactic body radiation therapy (SBRT). The Journal of thoracic and cardiovascular surgery. September 2010;140(3):583–589. [DOI] [PubMed] [Google Scholar]
- 13.Onishi H, Shirato H, Nagata Y, et al. Hypofractionated stereotactic radiotherapy (HypoFXSRT) for stage I non-small cell lung cancer: updated results of 257 patients in a Japanese multi-institutional study. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. July 2007;2(7 Suppl 3):S94–100. [DOI] [PubMed] [Google Scholar]
- 14.Koshy M, Malik R, Weichselbaum RR, Sher DJ. Increasing radiation therapy dose is associated with improved survival in patients undergoing stereotactic body radiation therapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. February 1 2015;91(2):344–350. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research N. Comprehensive genomic characterization of squamous cell lung cancers. Nature. September 27 2012;489(7417):519–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cancer Genome Atlas Research N. Comprehensive molecular profiling of lung adenocarcinoma. Nature. July 31 2014;511(7511):543–550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. J Clin Oncol. September 1 2005;23(25):5900–5909. [DOI] [PubMed] [Google Scholar]
- 18.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. May 20 2004;350(21):2129–2139. [DOI] [PubMed] [Google Scholar]
- 19.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. March 2012;13(3):239–246. [DOI] [PubMed] [Google Scholar]
- 20.Scagliotti GV, Parikh P, von Pawel J, et al. Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol. July 20 2008;26(21):3543–3551. [DOI] [PubMed] [Google Scholar]
- 21.Shaw AT, Kim DW, Nakagawa K, et al. Crizotinib versus chemotherapy in advanced ALK-positive lung cancer. N Engl J Med. June 20 2013;368(25):2385–2394. [DOI] [PubMed] [Google Scholar]
- 22.Solomon BJ, Mok T, Kim DW, et al. First-line crizotinib versus chemotherapy in ALK-positive lung cancer. N Engl J Med. December 4 2014;371(23):2167–2177. [DOI] [PubMed] [Google Scholar]
- 23.Cancer Facts & Figures. Atlanta, GA: American Cancer Society; 2016. [Google Scholar]
- 24.Hirsch FR, Spreafico A, Novello S, Wood MD, Simms L, Papotti M. The prognostic and predictive role of histology in advanced non-small cell lung cancer: a literature review. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. December 2008;3(12):1468–1481. [DOI] [PubMed] [Google Scholar]
- 25.Schuurbiers OC, Meijer TW, Kaanders JH, et al. Glucose metabolism in NSCLC is histology-specific and diverges the prognostic potential of 18FDG-PET for adenocarcinoma and squamous cell carcinoma. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. October 2014;9(10):1485–1493. [DOI] [PubMed] [Google Scholar]
- 26.Histological Typing of Lung and Pleural Tumors. 3rd ed ed. Berlin, Germany: Springer; 1999. [Google Scholar]
- 27.Pathology and Genetics of Tumours of the Lung, Pleura, Thymus and Heart. Lyon, France: IARCPress; 2004. [Google Scholar]
- 28.Travis WD, Brambilla E, Noguchi M, et al. International Association for the Study of Lung Cancer/American Thoracic Society/European Respiratory Society: international multidisciplinary classification of lung adenocarcinoma: executive summary. Proceedings of the American Thoracic Society. September 2011;8(5):381–385. [DOI] [PubMed] [Google Scholar]
- 29.Haasbeek CJ, Lagerwaard FJ, Slotman BJ, Senan S. Outcomes of stereotactic ablative radiotherapy for centrally located early-stage lung cancer. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. December 2011;6(12):2036–2043. [DOI] [PubMed] [Google Scholar]
- 30.Videtic GM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. International journal of radiation oncology, biology, physics. June 1 2010;77(2):344–349. [DOI] [PubMed] [Google Scholar]
- 31.Timmerman R, McGarry R, Yiannoutsos C, et al. Excessive toxicity when treating central tumors in a phase II study of stereotactic body radiation therapy for medically inoperable early-stage lung cancer. J Clin Oncol. October 20 2006;24(30):4833–4839. [DOI] [PubMed] [Google Scholar]
- 32.Lagerwaard FJ, Haasbeek CJ, Smit EF, Slotman BJ, Senan S. Outcomes of risk-adapted fractionated stereotactic radiotherapy for stage I non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. March 1 2008;70(3):685–692. [DOI] [PubMed] [Google Scholar]
- 33.Timmerman RD, Bizekis CS, Pass HI, et al. Local surgical, ablative, and radiation treatment of metastases. CA: a cancer journal for clinicians. May-Jun 2009;59(3):145–170. [DOI] [PubMed] [Google Scholar]
- 34.Scrucca L, Santucci A, Aversa F. Regression modeling of competing risk using R: an in depth guide for clinicians. Bone marrow transplantation. September 2010;45(9):1388–1395. [DOI] [PubMed] [Google Scholar]
- 35.Team R. R: A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; 2013. [Google Scholar]
- 36.Cuaron JJ, Yorke ED, Foster A, et al. Stereotactic body radiation therapy for primary lung cancers >3 centimeters. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. November 2013;8(11):1396–1401. [DOI] [PubMed] [Google Scholar]
- 37.Mak RH, Hermann G, Lewis JH, et al. Outcomes by tumor histology and KRAS mutation status after lung stereotactic body radiation therapy for early-stage non-small-cell lung cancer. Clin Lung Cancer. January 2015;16(1):24–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuo Y, Shibuya K, Nagata Y, et al. Prognostic factors in stereotactic body radiotherapy for non-small-cell lung cancer. Int J Radiat Oncol Biol Phys. March 15 2011;79(4):1104–1111. [DOI] [PubMed] [Google Scholar]
- 39.Wulf J, Baier K, Mueller G, Flentje MP. Dose-response in stereotactic irradiation of lung tumors. Radiother Oncol. October 2005;77(1):83–87. [DOI] [PubMed] [Google Scholar]
- 40.Grills IS, Hope AJ, Guckenberger M, et al. A collaborative analysis of stereotactic lung radiotherapy outcomes for early-stage non-small-cell lung cancer using daily online cone-beam computed tomography image-guided radiotherapy. Journal of thoracic oncology : official publication of the International Association for the Study of Lung Cancer. September 2012;7(9):1382–1393. [DOI] [PubMed] [Google Scholar]
- 41.Campbell JD, Alexandrov A, Kim J, et al. Distinct patterns of somatic genome alterations in lung adenocarcinomas and squamous cell carcinomas. Nat Genet. May 9 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lawrence MS, Stojanov P, Mermel CH, et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature. January 23 2014;505(7484):495–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ren W, Mi D, Yang K, et al. The expression of hypoxia-inducible factor-1alpha and its clinical significance in lung cancer: a systematic review and meta-analysis. Swiss medical weekly. 2013;143:w13855. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


