Abstract
Resistance to meticillin and vancomycin in Staphylococcus aureus significantly complicates the management of severe infections like bacteraemia, endocarditis or osteomyelitis. Here, we review the molecular mechanisms and genomic epidemiology of resistance to these agents, with a focus on how genomics has provided insights into the emergence and evolution of major meticillin-resistant S. aureus clones. We also provide insights on the use of bacterial whole-genome sequencing to inform management of S. aureus infections and for control of transmission at the hospital and in the community.
Keywords: Staphylococcus aureus, genomics, antibiotic resistance, MRSA, vancomycin
Data Summary
Fig. 1 was generated using genomic data and geographical metadata extracted from the Staphopia platform (https://staphopia.emory.edu/) using the Staphopia R package (https://github.com/staphopia/staphopia-r).
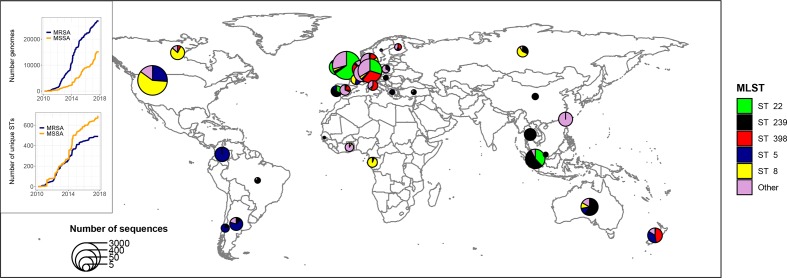
Fig. 1.
Snapshot of the genomic epidemiology of MRSA based on 42 948 publicly available S. aureus genomes (27 120 mec-positive) processed through the Staphopia platform (https://staphopia.emory.edu/). The inset shows the explosion of sequenced genomes and the constant increase in genetic diversity with 1099 different STs found in Staphopia. Despite this diversity, 70 % of MRSA sequences belonged to five STs (ST22, ST8, ST5, ST239 and ST398). It should be noted that publicly available S. aureus genomics do not accurately represent S. aureus epidemiology at this stage, due to sequencing and availability bias and lack of metadata in a large proportion of the dataset.
Impact Statement.
Meticillin- and vancomycin-resistant Staphylococcus aureus have been included by the World Health Organization in the global priority list of antibiotic-resistant bacteria, given the high mortality and morbidity associated with invasive S. aureus infections such as endocarditis and osteomyelitis, and the suboptimal outcome of treatment when anti-staphylococcal β-lactams are not available. Whole-genome sequencing (WGS) studies have not only highlighted how meticillin-resistant S. aureus spreads in the community and at the hospital, but also shown how use of antibiotics and biocides in the community initiates and amplifies the establishment of drug-resistant S. aureus . Moreover, emerging resistance to last-line antibiotics like vancomycin, daptomycin and linezolid can now be dissected at the molecular level by genomic studies. Through increased understanding of the genomic basis of resistance and emerging work on S. aureus virulence and persistence, there is likely to be a growing role of WGS in the direct clinical management of S. aureus infections.
Introduction
The facultative pathogen Staphylococcus aureus is associated with asymptomatic carriage in 25 % of adults [1] and with a wide spectrum of clinical conditions ranging from skin and soft tissue infections, through to invasive infections such as pneumonia, bacteraemia, infective endocarditis, septic arthritis and osteomyelitis [2]. Invasive S. aureus infections still carry a high mortality (for example around 20 and 10 % for endocarditis [3, 4] and pneumonia [5], respectively) and their management can be very complex, particularly when complicated by antimicrobial resistance [6].
The clinical introduction of penicillin in the 1940s dramatically improved the outcome of S. aureus infections (the mortality of S. aureus bacteraemia in the pre-antibiotic era was as high as 80 %); however, after the introduction of penicillin, resistance spread rapidly, and by 1948 more than the half of tested isolates in one centre were resistant to penicillin [7]. Interestingly, the rise of penicillin-resistant S. aureus was subsequently found to be linked to the spread of a single clone, phage type 80/81 [8], the first example of the ‘epidemic waves’ that now characterize the molecular epidemiology of resistant S. aureus [9]. A similar phenomenon was observed after the introduction of penicillinase-resistant penicillins (e.g. meticillin, oxacillin) in 1959. Two years later, a report described three clinical S. aureus isolates that were resistant to this newly introduced anti-staphylococcal antibiotic [10]. Recent work has established that meticillin-resistant S. aureus (MRSA) was already circulating prior to the introduction of meticillin and was likely selected for by penicillin [11]. MRSA subsequently disseminated in the hospital environment, and then separate epidemic waves occurred in the community. By contrast, resistance to the last-line antibiotic vancomycin has developed slowly following its introduction in 1958, with the first report of vancomycin-intermediate S. aureus (VISA) by Hiramatsu et al. in 1997 [12]. The relatively late emergence of vancomycin resistance was probably related to limited use of vancomycin until the 1980s, when the surge in MRSA infections boosted its use [13]. Resistance to the most recently introduced anti-staphylococcal antibiotics (daptomycin and linezolid) has also been readily acquired: for example, secondary resistance under treatment was described in the randomized controlled trial that led to FDA (Food and Drug Administration, USA) approval of daptomycin [14]; and linezolid resistance, albeit rare, has been reported in series of isolates [15].
In this mini-review, we provide an overview of the major genomic-based insights into the two major clinically relevant mechanisms of staphylococcal resistance (resistance to meticillin and vancomycin), and highlight the contribution of genomic epidemiology to the understanding of the establishment and spread of resistant clones (especially MRSA). Finally, we provide an outline for the future use of genomics beyond resistance research and epidemiology, towards improved individual patient management of invasive S. aureus infections, by prediction of antibiotic response, persistence and virulence.
Genomic insights into MRSA
Genetic basis of meticillin resistance
S. aureus acquires resistance to anti-staphylococcal penicillins through expression of an additional penicillin-binding protein (PBP) (PBP2a) [16]. Unlike other PBPs, PBP2a is resistant to the inhibitory effects of all β-lactams (with the exception of ceftaroline and ceftobiprole) and is almost always encoded by the accessory gene mecA [17]. The expression of mecA is inducible and controlled by a signal-inducer protein and a repressor located within the mecA operon [17]. Accordingly, most MRSA strains express PBP2a at low level, but harbour highly resistant subpopulations (heteroresistance) [18]. High-level resistance can be expressed in special circumstances. An example is the stringent response, i.e. the intracellular accumulation of the second messenger (p)ppGpp secondary to nutritional stress [19, 20]. In vitro studies identified genes involved in the stringent response (such as relA ) as ‘auxiliary genes’ that alter the expression of oxacillin resistance, along with several other determinants including the fem (factors essential for meticillin resistance) genes [21, 22].
Recently, alternative mec alleles have been described. For example, mecC shares 70 % nucleotide identity with mecA and is typically found in livestock-associated MRSA (LA-MRSA) [23]. Based on two reviews of epidemiological studies [24, 25], this mec variant appears to be infrequent and restricted to Europe (with one single case report from Australia [26]). Interestingly, mecC-MRSA appears to have a low oxacillin minimum inhibitory concentration (MIC) due to the distinctive characteristics of its PBP2a-homologue (PBP2c), including higher binding affinity for oxacillin than cefoxitin [27], and susceptibility to penicillin-β-lactam inhibitor combinations [28]. Accordingly, mecC-MRSA was successfully treated with β-lactams in an experimental endocarditis model [29]. Rarer mec homologues are also reported in other staphylococci or related species, such as mecA1 ( Staphylococcus sciuri ), mecA2 ( Staphylococcus vitulinus ) or mecB and mecD ( Macrococcus spp.) [30–32]. Based on genomic studies, it is hypothesized that mecA was acquired several years prior to the first clinical detection of MRSA in 1961. Harkins et al. applied a Bayesian phylogenetic inference to a collection of early MRSA isolates (1960–1980) and concluded that meticillin resistance emerged in the mid-1940s, suggesting that the introduction of penicillin may have contributed to the selective pressure that lead to the advent of MRSA [19].
Horizontal transfer of mecA is made possible by carriage on a specific mobile genetic element (MGE) ranging between 23 and 68 kb in size, the staphylococcal cassette chromosome (SCC) [33]. The association of mecA with the SCC (termed the SCCmec) not only is important for mecA acquisition or transfer, but also is a key factor mediating antimicrobial co-resistance, since genes conferring resistance to non-β-lactam antibiotics can be co-located in the same locus [34–36]. To date, 13 variants of SCCmec have been described [37], but with the increasing number of sequenced strains, new variants are likely to be discovered in the future. Beyond SCCmec, other MGEs are critical for acquisition and dissemination of resistance to antibiotics and biocides (particularly plasmids and transposons, see the review by Firth and colleagues [38]) and virulence determinants (particularly bacteriophages, see the review by Xia and Wolz [39]).
The conserved structure of SCCmec and mecA has facilitated the molecular detection of meticillin resistance; molecular point-of-care tests have streamlined the rapid detection of MRSA from clinical samples. However, correlation between the presence of mecA or mecC and phenotypic resistance to oxacillin is not absolute – approximately 3 % of S. aureus strains harbouring mecA are phenotypically susceptible to oxacillin [40, 41]. As mentioned above, this phenomenon has been previously explained by heterogeneous synthesis of PBP2a [42], but a genomic study provided an interesting alternative mechanism. The authors investigated two clinical isolates of mecA-positive meticillin-susceptible S. aureus (MSSA) and demonstrated that mecA expression was suppressed by disruption of the gene through insertion of IS1181 in one case and a mecA frameshift mutation in the other [41]. β-Lactam sensitivity in MRSA has been investigated in a recently published study by Harrison et al., where they identified a subset of MRSA strains that were susceptible to penicillin/clavulanic acid combinations. The genomic basis of this phenomenon was found to be the association of mutations both in the promoter and coding sequence of mecA [43].
Conversely, oxacillin resistance can be mediated by other mechanisms than PBP2a production. Such mecA- (and mecC)-negative, oxacillin-resistant strains [borderline oxacillin-resistant S. aureus (BORSA)] are increasingly recognized and may be associated with failure of oxacillin clinical therapy, typically in complex, deep-seated infections [44, 45]. While previous work has investigated β-lactamase hyperproduction or PBP mutations [46], recent genomic studies of BORSA isolates have linked this phenotype to mutations in the regulatory gene gdpP [47, 48], which degrades the second messenger c-di-AMP. gdpP mutations have been linked to changes in cell-wall metabolism and increased resistance to antibiotics targeting the cell wall like β-lactams and vancomycin [49].
Genomic epidemiology of MRSA
The genomic epidemiology of MRSA is multifaceted. MRSA is typically clonal, with epidemic waves of infections characterized by the temporal rise and decline of clones [50, 51]. Parallel to these chronological changes, geographical segregation can be observed, with some adaptation to specific environments, including health-care facilities, community settings and animal populations. These broad groupings form the basis for the often used classifications of health-care-associated MRSA (HA-MRSA), community-associated MRSA (CA-MRSA) and livestock-associated MRSA (LA-MRSA). Previously, molecular epidemiology using lower-resolution approaches, such as pulsed-field gel electrophoresis (PFGE), multilocus sequence typing (MLST) or spa typing, helped delineate dominant MRSA clones and track their emergence, expansion and spread [52]; however, over the past 10 years, whole-genome sequencing (WGS)-based studies have defined the complex epidemiology of MRSA, from tracking global dissemination of successful clones, to dissecting chains of transmission in hospitals and the community, and between livestock and humans.
Harris et al. performed the first study that applied large-scale bacterial WGS to explore global dissemination of the HA-MRSA clone sequence type (ST)239 [53]. While the core-genome phylogeny was consistent with PFGE and spa typing, genomic data provided detailed insights into the phylogeographical structure of the ST239 lineage, the emergence of antibiotic-resistance mutations and transmission within a health-care facility. The impact of genomics on the investigation and control of hospital-associated MRSA outbreaks has also been demonstrated in subsequent papers [54–56], while others have analysed MRSA transmission networks in the community setting [57] or described the emergence of LA-MRSA and the complex interplay associated with transmission from humans to farm animals and vice versa [58, 59]. Table 1 provides a selection of the major genomic epidemiological studies associated with MRSA and their key findings. Given the large number of studies that have applied WGS to address MRSA epidemiology, we have selected studies based on (i) their novelty at the time they were published, and (ii) the range of MRSA genomic epidemiology (e.g. global or local transmission, adaptive evolution, source attribution in LA-MRSA).
Table 1.
Selection of studies that used WGS to investigate the genomic epidemiology of MRSA
|
Setting |
Publication |
MRSA clone |
No. of isolates (timeframe) |
Main findings |
Rationale for inclusion |
|---|---|---|---|---|---|
|
International studies |
|||||
|
Health-care associated |
Harris et al., Science 2010 [53] |
ST239 |
63 (1982–2007) |
Phylogeographical analysis highlighting global dissemination of ST239 HA-MRSA and transmission within one hospital in Thailand. Comparison to spa typing and PFGE shows that WGS has higher resolution. |
|
|
Livestock associated |
Price et al., mBio 2012 [58] |
ST398 |
89 (1993–2010) |
Worldwide genomic study of ST398 revealing that the ancestral clades were human-associated and meticillin susceptible, and that after human to livestock transition the clone underwent host adaptation. Adaptation to livestock was marked by acquisition of meticillin and tetracycline resistance, and loss of the phage carrying human-associated immune evasion cluster (IEC). |
First genomic description of the LA-MRSA clone ST398. |
|
Health-care associated |
Holden et al., Genome Res 2013 [69] |
ST22 (EMRSA-15) |
193 (1990–2009) |
Detailed reconstruction of the evolution of the ST22 clone using Bayesian methods showed: (i) dissemination first throughout the UK and then globally, and (ii) progressive acquisition of antibiotic-resistance determinants (SCCmec IV and grlA and gyrA mutations associated with fluoroquinolone resistance). Acquisition of resistance to β-lactams and fluoroquinolones has likely contributed to the success of the clones, leading to an expansion of the subclade ST22-A2. |
An in-depth genomic study of the emerging HA-MRSA clone ST22 using a Bayesian framework to decipher genetic determinants of clonal dissemination. |
|
Multiple settings |
Aanensen et al., mBio 2016 [60] |
Multiple STs |
308 (2006–2007) |
Genomic snapshot of invasive S. aureus isolates collected across 186 hospitals in Europe. This study provided a framework to study genomic epidemiology of S. aureus at a continental level. It showed high genetic diversity, especially among MSSA. MRSA clones (but not MSSA) were geographically clustered with evidence of international transmission. |
This study showed the potential use of genomics for surveillance of S. aureus epidemiology at continental scale. |
|
Community associated |
Ward et al, Genome Biol 2016 [76] |
ST59 |
120 (1998–2011) |
Global study of CA-MRSA clone ST59 that is dominant in East Asia. Two distinct clades were identified that emerged in the USA and in Taiwan. Transitions between countries were mapped using a ‘Markov jumps’ method based on a Bayesian phylogenetic approach. This analysis identified Taiwan and the USA as ‘source countries’ as opposed to the ‘sink countries’ the UK, Australia and the Netherlands. |
Innovative use of Bayesian methods to track transmission between countries. |
|
Community associated |
Van Hal et al., Front Microbiol 2018 [73] |
ST93 |
459 (1991–2012) |
Global study of CA-MRSA clone ST93 that determined ST93-MRSA emerged from indigenous communities in northern Australia and subsequently spread to the east coast of Australia, New Zealand and Europe. A locally adapted clone to NZ was evident. |
Example of global dissemination of a CA-MRSA clone. |
|
Community associated |
Steinig et al., mBio 2019 [159] |
ST772 |
340 (2004–2013) |
Global study of CA-MRSA clone ST772 that determined ST772 emerged from the Indian subcontinent in the 1970s with sporadic transmission overseas. Acquisition of a multidrug-resistance plasmid was instrumental in the emergence and dissemination of a globally disseminated clade in the 1990s. |
Genomic epidemiological study applied to an emergent community-associated clone from South Asia |
|
Country-wide studies |
|||||
|
Community associated |
Stinear et al., Genome Biol Evol 2014 [71] |
ST93 |
56 (2000–2009) |
Comprehensive study of the adaptive evolution of the Australian CA-MRSA clone ST93, showing progressive reduction in secretion of exotoxins and decrease in oxacillin MIC. |
Example of an in-depth study combining genomics and phenotypic analysis to study adaptive evolution. |
|
Health-care associated |
Baines et al., mBio 2015 [70] |
ST239 |
123 (1980–2012) |
Genomic and phenotypic study of the adaptive evolution of the hospital-adapted MRSA clone ST239 in Australia. The analysis showed that adaptive changes (virulence attenuation and increased antibiotic resistance) and the introduction of a previously undetected ST239 clade from Asia promoted ST239 persistence in the hospital environment. |
Another example of adaptive evolution study applied to a hospital-associated clone. |
|
Community associated |
Baines et al., Antimicrob Agent Chemother 2016 [34] |
ST1 |
46 (2005–2013) |
Genomic investigation of the expansion of the fusidic acid-resistant CA-MRSA ST5 clone in New Zealand, which was associated with increased use of topical fusidic acid. The key finding was the consistent co-location of the fusidic acid-resistance determinant fusC with the SCC. This study indicates that topic antimicrobials can drive the emergence of MRSA through co-resistance and co-location of resistance genes. |
Genomic demonstration of the role of co-resistance in promoting the emergence of CA-MRSA. |
|
Health-care associated |
Reuter et al., Genome Res 2016 [61] |
Multiple STs |
1013 (2001–2010) |
Large genomic surveillance study of MRSA bloodstream infections in the UK and Ireland used to infer transmission and resistance acquisition. The study showed a dominance of ST22 (EMRSA-15), and ST36 (EMRSA-16) and phylogeographical clustering around referral networks. |
First demonstration of systematic WGS of invasive MRSA infections. |
|
Livestock associated |
Gonçalves da Silva et al., Microb Genom 2017 [59] |
ST398 |
147 (2004–2015) |
This study investigated the emergence of the LA-MRSA clone ST398 in New Zealand. A Bayesian phylogenetic approach was used to infer that the clone originated in swine from western Europe. |
Example of use of Bayesian phylogenomics for source attribution in LA-MRSA. |
|
Mixed setting |
Coll et al., Sci Transl Med 2017 [64] |
ST22 and others |
1465 (2012–2013) |
Genomic investigation of MRSA transmission within a single hospital referral network in the UK. The study revealed several clusters of transmission both at the hospital and community (general practice) level. |
Powerful demonstration of the use of integrated genomic and epidemiological data (extracted from hospital administration, patient postcodes, general practice attendance) for MRSA transmission surveillance. |
|
Single institution studies / communities |
|||||
|
Hospital associated |
Köser et al., N Engl J Med 2012 [54] |
ST22 |
14 (2009) |
Demonstration of the use of rapid WGS to investigate and manage a MRSA outbreak in a neonatal intensive care unit in the UK. |
First use of WGS to track hospital transmission of MRSA. |
|
Community associated |
Uhlemann et al., Proc Natl Acad Sci USA 2014 [57] |
ST8 (USA300) |
378 (2009–2011) |
Genomic investigation of transmission networks of ST8 CA-MRSA (USA300) within a New York community, showing that community households played a major role in maintaining and disseminating carriage and infection. |
This study used WGS to track transmission of USA300 in the community. |
|
Health-care associated |
Tong et al., Gen Research 2015 [75] |
ST239 |
79 (2008) |
Investigation of endemic MRSA in a developing world hospital setting that demonstrated considerable diversity within two intensive care units in a Thailand hospital. Also demonstrated that individuals can be colonized by a cloud of strains. |
First genomic description of MRSA in a developing world hospital setting. |
|
Health-care associated |
Senn et al., mBio 2016 [55] |
ST228 |
228 (2008–2012) |
WGS study investigation of a sustained MRSA outbreak in a Swiss teaching hospital. Genomic and epidemiological data revealed that the outbreak was due to a single ST228 clone. Low genetic diversity within the isolates suggested direct transmission between patients, possibly due to increased transmissibility and to more frequent rectal carriage, as compared to other clones. |
This in-depth genomic investigation of a single-institution MRSA outbreak showed that genomics can be used to identify hidden MRSA reservoirs and investigate transmission dynamics. |
|
Hospital associated |
Price et al., Lancet Infect Dis 2017 [63] |
Multiple STs |
1819 (2011–2012) |
Comprehensive longitudinal sampling of S. aureus strains from patients, a health-care worker and the environment in a single-centre intensive-care unit. Genomics showed constant introduction of novel subtypes, with infrequent transmission to patients. |
First use of WGS to track S. aureus transmission between patients, a health-care worker and the environment. |
More recent S. aureus genomic epidemiological studies have evolved in two complementary directions: (i) expanding the breadth of the analysis by providing local, national or international surveillance frameworks for MRSA (and S. aureus in general); or (ii) performing in-depth investigations of single clones or lineages, and exploring the interplay between adaptive evolution and antibiotic pressure. For example, Aanansen et al. performed a population genomic study of 308 S . aureus isolates across 21 European countries. Their data provided a ‘snapshot’ view of the genetic diversity of S. aureus across a continent, and allowed the investigation of evolution within single clones and intercontinental transmission, as well as identification of both MSSA and MRSA ‘high-risk clones’ (e.g. CC22, CC30) based on speed of expansion as assessed from phylogenomics, phylogeographical structure and the presence of key resistance or virulence genes. Their study also showed that the population structures of MRSA and MSSA are fundamentally different, with the former being more clonal and geographically clustered [60]. Building on a similar approach, Reuter et al. described the population structure of over 1000 invasive MRSA isolates from the UK and Ireland [61]. An important finding of their study was strong phylogeographical clustering around hospital referral networks, highlighting the potential for the use of WGS in epidemiological surveillance and early identification of new hospital outbreaks [61, 62]. Two recent studies expanded the framework of surveillance of MRSA transmission: Price et al. showed that genomics can be applied to interrogate the complex transmission interplay between patients, health-care workers and the environment in the health-care setting [63], while a study by Coll et al. integrated genomic data with epidemiological data retrieved from various sources (hospital admissions, postcodes, general practice attendance) to reconstruct MRSA transmission networks both in the hospital and the community [64].
With over 40 000 S . aureus genomes now publicly available, large-scale genomic surveillance is now possible [65, 66]. Fig. 1 demonstrates the global distribution of MRSA clones based on publicly available S. aureus genomes processed through the Staphopia platform [65]. Despite the great interest of this large and ever-growing public dataset of S. aureus sequences, it should be noted that these data are not necessarily representative of the actual S. aureus epidemiology (sources of bias include the larger availability of sequencing in a small group of developed countries, increased sequencing of MRSA for public-health reasons and lack of metadata including country of collection for a large proportion of the isolates). Other available genomic platforms for S. aureus (and other bacteria) offer access to publicly available genomes and allow comparison of sequences uploaded by the user through analysis pipelines, e.g. patric (https://www.patricbrc.org), National Center for Biotechnology Information (NCBI) Pathogen detection (https://www.ncbi.nlm.nih.gov/pathogens/; however, S. aureus is not included yet) and Pathogenwatch (https://pathogen.watch). These repositories and new computational approaches allow high-throughput analysis of stored sequence data for both rapid and efficient genomic surveillance [67] and discovery of genetic determinants of resistance or pathogenesis [68].
In-depth genomic studies of specific MRSA lineages
An early example of an in-depth, clone-specific approach is reported in a study by Holden et al., who applied Bayesian phylogenetics methods to dissect the evolutionary history of the hospital-associated ST22 clone, the dominant MRSA clone in the UK. The analysis reconstructed the acquisition of important antibiotic-resistance determinants (mecA and resistance-associated mutations in the gyrA and grlA genes) and showed that rapid expansion and dissemination of sublineage ST22-A2 was likely promoted by acquisition of fluoroquinolone resistance [69]. Other authors have combined population genomics and phenotypic studies to dissect adaptive micro-evolution of MRSA both in the hospital [70] and community environment [71–73]. For example, two genomics studies of ST93, a community-acquired MRSA clone in Australia, have revealed how this lineage likely arose in a remote area in North-West Australia and subsequently disseminated across the continent and overseas [71, 73]. One study also showed that this high-virulence clone changed its phenotype towards reduced virulence (e.g. expression of alpha-toxin) and increased susceptibility to oxacillin [71], possibly indicating adaptive evolution to the health-care environment, as previously shown in other clones such as CC30 [74]. Another Australian study explored adaptive evolution of the hospital-associated ST239 clone. Phylogenetic analysis showed that the epidemics had been enhanced by the introduction of a previously unrecognized sublineage from Asia. Both the Australian and the Asian sublineage of ST239 exhibited patterns of convergent evolution, namely decreased virulence and increased resistance to antibiotics (including vancomycin) – both characteristics of hospital adaptation. The transmission potential of ST239 was also highlighted in a study performed in an hospital in Thailand [75]. Further, in a worldwide study of the CA-MRSA clone ST59 (dominant in East Asia), the authors applied a Bayesian phylogenetic method (‘Markov jumps’) to identify ‘source’ countries (USA, Taiwan) and ‘sink’ countries (Australia, the UK, the Netherlands) of the ST59 epidemic [76]. Collectively, these studies have provided clear examples of the utility of population genomics (complemented with relevant phenotypic testing) in understanding the evolutionary mechanisms that underpin the success of MRSA clones.
Co-resistance in MRSA
An area of ongoing interest is the role of co-resistance to non-β-lactam antibiotics in the spread and expansion of MRSA clones [77]. Co-resistance is of both epidemiological and clinical relevance, since it has been shown that use of selected antibiotics (e.g. fluoroquinolones) may drive the MRSA epidemic [78]; thus, offering potential targets of preventive interventions through antibiotic stewardship in human health or in agriculture [79]. This is not only true for systemic antibiotics, but also for topical antibiotics and biocides [80].
From a genomic perspective, co-resistance can arise via four different mechanisms: (i) the same genetic determinant (gene or mutation) can confer resistance to multiple antibiotics (e.g. the pleiomorphic effects of rpoB mutations, which include decreased susceptibility to vancomycin and daptomycin [66]); (ii) compensatory mutations that counterbalance the fitness cost associated with resistance to one antibiotic can alter susceptibility to another drug (e.g. increased β-lactam susceptibility in VISA, see below [81]); (iii) the genetic resistance determinants are co-located on the same mobile element (e.g. within the SCCmec) [36]; or (iv) the resistance determinants are co-located within the same strain (e.g. fluoroquinolone resistance in MRSA).
Co-location on the same MGE is important, because it is associated with a risk of horizontal transmission of both genetic determinants. In MRSA, it is enabled by the plasticity of the SCCmec element that can host several genes associated with resistance to antibiotics or heavy metals [82]. For example, the erythromycin-resistance gene erm(A) is found on transposon Tn554, which is in turn nested within type II, III and VIII SCCmec elements [33], while the tetracycline-resistance plasmid pT181 is integrated in type III SCCmec. Similarly, the aminoglycoside-resistance gene aacA-aphD is carried on transposon Tn4001, which can in turn be found not only on several multi-resistance plasmids, but also on some SCCmec elements [83]. An illustrative example of the effect of resistance co-location on MRSA epidemiology is provided by the rapid emergence of fusidic acid-resistant MRSA and MSSA in New Zealand that was likely fuelled by the unrestricted use of topical fusidic acid. Genomic studies showed that fusidic acid resistance was restricted to two dominant MRSA clones (ST5 and ST1) and one MSSA clone (ST1) that had acquired the fusidic acid-resistance determinant, fusC. Crucially, the fusC operon was exclusively located in SCC elements, in both MSSA and MRSA [34, 84]. Important MRSA co-resistance determinants located outside SCCmec are the quinolone-resistance genes gyrAB and grlAB, encoding the DNA gyrase and DNA topoisomerase, respectively [85]. Population genomics studies show that a single acquisition of quinolone resistance in the 1990s drove clonal expansion in both ST8 (USA300) [57] and the ST22 lineage (EMRSA-15) [69].
Tolerance to biocides and resistance to topical antibiotics can also be mediated by genes located on plasmids. The qacA gene encodes an efflux pump that is associated with tolerance to monovalent and divalent cations such as chlorhexidine, a widely used disinfectant in the hospital setting. It is generally carried on pSK1 family plasmids, often in combination with other resistance genes such as the β-lactamase blaZ. A recent adaptive evolution study has shown a progressive decrease of chlorhexidine sensitivity among ST239 isolates. This phenotypic change was associated with a complex rearrangement of the pSK1 plasmids [86]. Mupirocin resistance is linked to mutations in the chromosomal gene ileRS (low-level resistance) or the plasmid-located gene mupA [87]. A recent genomic study showed that mutations in the essential gene ileS appear to have pleiotropic effects [88], highlighting the complexity of antibiotic resistance in S. aureus .
Using genomics to explore virulence in MRSA
The complexity of virulence has been recently highlighted [89]. It has been very difficult to identify single genomic determinants of clinical outcome in S. aureus infections [90], with the exception of some toxin-mediated syndromes [91, 92]. Nevertheless, it is possible to classify genetic determinants of virulence based on broad phenotypic characterization in experimental models (e.g. adhesion, toxin production, immune evasion and gene regulation) and their genetic context (i.e. core genome or accessory genome). Although a detailed description of virulence determinants is beyond the scope of this review, we will highlight some recent insights into S. aureus virulence that have been specifically provided by genomic studies.
A striking feature of CA-MRSA clones (such as ST8 and ST93) has been increased virulence in animal models and clinical examples of severe diseases (such as necrotizing skin or lung infections) [93, 94]. This has also been demonstrated in vitro as increased cytotoxicity against human lymphocytes or macrophages [95]. While the genetic basis of increased virulence remains elusive, these clones were characterized by the presence of the Panton-Valentine leukocidin (PVL) toxin encoded by two genes lukS and lukF, located on a bacteriophage [96]. There remains controversy around the true clinical relevance of PVL [97]; however, a recent genome-wide association study (GWAS) showed that it was strongly associated with S. aureus pyomyositis among children in Cambodia [98]. Further, it has been shown that cell toxicity resulting from exotoxin production in MRSA might be related to regulatory mechanisms rather that a single gene or locus. For example, there is an inverse relationship between PBP2a expression and toxicity; generally, classic CA-MRSA clones such as ST8 and ST93 have a lower oxacillin MIC and higher toxicity [99].
Other virulence determinants identified in genomic studies are the arginine catabolic mobile element (ACME), a large genetic segment possibly enhancing colonization in ST8 MRSA [100] and sasX, carried on a prophage in ST239 MRSA [101]. It is expected that genomic studies will continue to identify previously unrecognized virulence determinants. For example, a recent analysis of 92 USA300 isolates from an outbreak in a New York community identified mutations in the pyrimidine nucleotide biosynthetic operon regulator pyrR that were associated with enhanced fitness in vitro and enhanced colonization and transmission in a mouse model [72]. Furthermore, genomic analysis revealed that the acquisition of a bacteriophage was associated with larger skin abscesses in an animal model, emphasizing the impact of structural variants and MGEs on clone success and staphylococcal pathogenesis [72].
Genomic insights into vancomycin-resistant S. aureus
The first report of vancomycin resistance was published in 1997 [12], 39 years after vancomycin was first introduced [102]. The authors isolated an MRSA strain with a vancomycin MIC of 8 mg l−1 from a patient with a persistent sternal wound infection, who had been exposed to vancomycin for several weeks [12]. From a molecular perspective, vancomycin resistance in S. aureus can arise through acquisition of the vancomycin-resistance determinant vanA, or more commonly via an array of vanA-independent mechanisms, mostly mutations in genes involved in cell-wall biosynthesis [103]. vanA-mediated resistance is associated with high-level resistance (VRSA, with a vancomycin MIC of 16 mg l−1 and higher) and is due to acquisition of the vanA operon, located on transposon Tn1546 [104], more commonly associated with vancomycin resistance in enterococci [105]. It was first described in 2002 in a patient with end-stage renal failure and diabetic foot infection [106]. Subsequent molecular studies demonstrated that the VRSA isolate carried a conjugative plasmid that had acquired Tn1546 from a co-infecting vancomycin-resistant Enterococcus faecalis [104]. While this report and previous experimental work [107] raised concerns of dissemination of high-level vancomycin resistance, VRSA remains very rare, with a total of only 14 cases reported in the USA [108], and a few reports from Iran [109] and India [110]. Although most VRSA strains to date belong to clonal complex 5, genomic analyses of 12 VRSA strains from the USA showed that they were genetically distant, with the most recent common ancestor around 1960 and likely independent acquisition of the plasmid-born vanA operon in each isolate [111].
Since its first description in 1997, several studies have investigated the complex background of vanA-independent vancomycin resistance. Phenotypically, these strains have low-level vancomycin resistance (VISA, vancomycin MIC 4–8 mg l−1) or may not be resistant when tested with conventional methods, yet harbour vancomycin heteroresistance (hVISA) [103]. They are also characterized by a thickened cell wall [112], slower growth and increased autolysis [113]. The molecular basis of these changes is complex and polygenic (extensive reviews have been published by Howden et al. [114] and McGuinness et al. [108]). Most mutations involve regulators of cell-wall biosynthesis, such as the two-component regulators vraRS [115], graRS [116] and walKR [117]. However, mutations in the rpoB gene (with or without co-resistance to rifampicin) [118] and in the PP2C phosphatase prpC [119] can also be associated with the VISA phenotype. Interestingly, in one case, decreased vancomycin susceptibility was linked to insertion of the transposon IS256 upstream of walKR [120], possibly altering its expression [121]. To date, two GWASs have assessed putative mutations associated with the VISA phenotype, both on ST239 isolates. The first study of 123 isolates found an association with a SNP in walkR [70], while the second (75 isolates) pointed to the H481Y/L/N rpoB mutation [122]. Further, a study using a machine-learning approach found that the VISA phenotype could be predicted with 84 % accuracy [123]. Although reduced vancomycin susceptibility can be found in any genetic background [124], ST239 strains tend to have a higher vancomycin MIC [125]. ST5 seems also to be more often associated with VISA [126].
Despite different genetic resistance mechanisms and phenotypes, VRSA and VISA share common features that distinguish them from MRSA. Unlike MRSA, VRSA and VISA are generally polyclonal, and no significant dissemination has been documented. To date, vancomycin resistance has occurred secondarily, during treatment for complicated S. aureus infections. As such, prevention of this resistance is likely best achieved through optimising the management of complex MRSA infections (including appropriate source control) and implementing antibiotic stewardship, rather than through infection control. However, there remains an omnipresent risk that vancomycin resistance could disseminate more effectively, especially with widespread transfer and expansion of a vanA-harbouring clone [127].
Unfortunately, resistance to newer anti-staphylococcal antibiotics is also emerging. Daptomycin has been proposed as a possible alternative to vancomycin for the treatment of invasive MRSA infections (with the exception of pneumonia) [128]; however, VISA/hVISA are often co-resistant to daptomycin [129] and secondary resistance can develop in vivo, especially in the case of deep-seated infections and poor source control [130]. Genetically, the main mechanism of daptomycin resistance is considered to be gain-of-function mutations in mprF, which encodes for a lysyltransferase producing lysylphosphatidylglycerol, a positively charged cell-membrane lipid that mediates resistance to host antimicrobial peptides [131]. It is hypothesized that mutations associated with daptomycin resistance increase cell-membrane positivity and, hence, impair binding of daptomycin, which is positively charged [132]. Similar to the VISA phenotype, comparative genomics studies of closely related isolates (either from cases of daptomycin treatment failure or from in vitro exposure experiments) have been instrumental in identifying further genetic determinants of daptomycin resistance. Strikingly, some of these genes are the same as those implicated in the VISA phenotype, such as walKR [133], rpoB [118] or prpC [119]. Furthermore, both daptomycin and vancomycin resistance are associated with the ‘see-saw’ effect, where increased resistance to glycopeptides and lipopeptides leads to reduced resistance to β-lactams [81]. The molecular basis of this phenomenon is complex and only partially investigated; for example, some studies have shown compensatory changes in the mecA gene [134] and reduced mecA expression [135].
Linezolid is a potential alternative anti-MRSA antibiotic that is not known to be affected by co-resistance to vancomycin. Resistance to linezolid can arise through to point mutations in 23S ribosomal RNA [136] and the ribosomal proteins L3/L4 [137]; however, it can also be acquired through transfer of the accessory gene cfr, which produces a 23S rRNA methylase [138]. This gene is often carried by a plasmid [139] and a small multi-clonal outbreak of cfr-positive MRSA has been described in a Spanish hospital [140]. Ceftaroline, a next-generation cephalosporin with specific activity against PBP2a, might be used either as salvage therapy or as part of combination treatment for invasive MRSA infections [141]. However, ceftaroline resistance has been described in several MRSA lineages, both at baseline and on treatment [142], mainly through point mutations in mecA or in pbp4 [143, 144]. Interestingly, mecA polymorphisms associated with ceftaroline resistance were found in a Korean study, despite the fact that ceftaroline had not yet been used in the country [145]. In a study of 421 strains, 17 % were non-susceptible to ceftaroline (>1 mg l−1), with a higher proportion in ST239 MRSA [146].
Applying genomics to the management of invasive S. aureus infections
In addition to population-level studies, genomics has been increasingly used in the clinical microbiology laboratory at the patient level, mainly in the prediction of phenotypic resistance from genotypic data. Several translational studies have shown that genomic prediction of antibiotic resistance is highly accurate in the case of S. aureus [147, 148]. The main issue with this approach is related to yet unknown resistance mechanisms [149]; however, it is likely that prediction accuracy will further improve as databases of genetic determinants of resistance are constantly updated, provided that careful genotype–phenotype association studies are also performed.
From a clinical perspective, one area where genomics offers considerable potential is through the use of WGS data to predict clinical outcomes and inform patient management, beyond considerations of antimicrobial resistance [90]. Previous molecular studies using multiple PCR or DNA arrays have suggested an association between certain clonal types and clinical manifestations or outcomes of S. aureus bacteraemia; however, with a few exceptions [150], no consistent link was demonstrated between the presence/absence of specific genes or mutations and clinical outcomes. More recently, Recker et al. used WGS data and applied a machine-learning algorithm to a S. aureus bacteraemia cohort in the UK to map associations between bacterial genetics, phenotypes potentially associated with virulence (cytotoxicity and biofilm formation) and clinical outcome [151]. The main finding of the study was that bacterial phenotype and genotype contributed to infection outcome; however, the effect appeared to be clone-specific, highlighting the complexity of outcome predictions in this setting. Another study from Denmark was not able to determine bacterial genomic predictors of infective endocarditis in S. aureus bacteraemia, despite using multiple genomic approaches [152]. Prediction might be more straightforward for rarer, specific clinical S. aureus syndromes. For example, Young et al. successfully applied GWAS to further highlight the role of PVL in the pathogenesis of pyomyositis [98]. However, to provide findings that can be implemented in clinical management, larger studies of genetic determinants of S. aureus infection outcomes are needed. Crucially, these studies will require additional validation either in independent cohorts or through functional tests [153], as well as integration of clinical covariables and, ideally, host genomics [154].
An alternative approach to uncover bacterial genetic determinants of disease is to investigate bacterial host adaptation through within-host evolution studies [155]. The study of genetically closely related isolates from the same patient offers a unique opportunity to identify new bacterial molecular markers of resistance, virulence or persistence without the need for large patient cohorts and without the analytical problems associated with GWAS approaches. These studies have played an essential part in establishing the genetic factors underlying VISA [114], but may also offer insights into the pathogenesis of invasive S. aureus infections [120, 156]. Furthermore, comparative genomics of multiple patient isolates could help manage treatment failure by a reliable differentiation between true relapse and reinfection, or by the identification of de novo resistance mutations, especially if novel techniques are used that allow accurate detection of low-frequency populations [157].
Conclusion and future directions
S. aureus remains a considerable clinical burden, in both hospital and community settings. This is aggravated by resistance to key anti-staphylococcal antibiotics like flucloxacillin and vancomycin. Molecular and genomic studies have provided invaluable insights into how resistance arises. For MRSA, they have demonstrated how MGEs have facilitated the selection and dissemination of distinct clones in hospital wards, community networks and at a global scale. Further, genomic datasets are now available, allowing the prediction of resistance to common antimicrobials, with ongoing work trying to accurately predict genotypic resistance to last-line antibiotics such as vancomycin, daptomycin, linezolid and ceftaroline. Future studies should also investigate whether bacterial genomics can be used to predict antibiotic synergism and response to combination therapy (e.g. vancomycin/daptomycin combination with β-lactams [158]). However, this large amount of genomic information can only be exploited if high-quality metadata are collected and (where possible) made publicly available. For example, phenotypic antibiotic susceptibility should be submitted along with other metadata (an approach encouraged by the NCBI, as described at: https://www.ncbi.nlm.nih.gov/biosample/docs/antibiogram/). Even more importantly, clinical phenotypes (including relevant outcomes and relevant treatment and confounder factors) should be mapped from carefully designed, prospective cohorts [90]. This integrative approach combining publicly available databases, curated microbiological and clinical phenotypes and powerful computational tools will pave the way for bacterial genomics to move from population studies to patient management.
Data bibliography
1. Petit RA III, Read TD. Staphylococcus aureus viewed from the perspective of 40,000+ genomes. PeerJ 2018;6:e5261. doi.org/10.7717/peerj.5261 (2018).
Funding information
Funding: S.Y.C.T. (grant no. 1145033) was supported by a Career Development Fellowship from the National Health and Medical Research Council (NHMRC) of Australia. D.A.W. (grant no. GNT1123854) was supported by an Early Career Fellowship from the NHMRC of Australia.
Conflicts of interest
The authors declare that there are no conflicts of interest.
Footnotes
Abbreviations: BORSA, borderline oxacillin-resistant Staphylococcus aureus; CA-MRSA, community-associated meticillin-resistant Staphylococcus aureus; GWAS, genome-wide association study; HA-MRSA, health-care-associated meticillin-resistant Staphylococcus aureus; hVISA, vancomycin heteroresistance; LA-MRSA, livestock-associated meticillin-resistant Staphylococcus aureus; MGE, mobile genetic element; MIC, minimum inhibitory concentration; MRSA, meticillin-resistant Staphylococcus aureus; MSSA, meticillin-susceptible Staphylococcus aureus; NCBI, National Center for Biotechnology Information; PBP, penicillin-binding protein; PVL, Panton-Valentine leukocidin; SCC, staphylococcal cassette chromosome; ST, sequence type; VISA, vancomycin-intermediate Staphylococcus aureus; VRSA, vancomycin-resistant Staphylococcus aureus; WGS, whole-genome sequencing.
All supporting data, code and protocols have been provided within the article or through supplementary data files.
References
- 1.Wertheim HFL, Melles DC, Vos MC, van Leeuwen W, van Belkum A, et al. The role of nasal carriage in Staphylococcus aureus infections. Lancet Infect Dis. 2005;5:751–762. doi: 10.1016/S1473-3099(05)70295-4. [DOI] [PubMed] [Google Scholar]
- 2.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev. 2015;28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miro JM, Anguera I, Cabell CH, Chen AY, Stafford JA, et al. Staphylococcus aureus native valve infective endocarditis: report of 566 episodes from the International Collaboration on Endocarditis Merged Database. Clin Infect Dis. 2005;41:507–514. doi: 10.1086/431979. [DOI] [PubMed] [Google Scholar]
- 4.Fowler VG, Miro JM, Hoen B, Cabell CH, Abrutyn E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA. 2005;293:3012–3021. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 5.Self WH, Wunderink RG, Williams DJ, Zhu Y, Anderson EJ, et al. Staphylococcus aureus community-acquired pneumonia: prevalence, clinical characteristics, and outcomes. Clin Infect Dis. 2016;63:300–309. doi: 10.1093/cid/ciw300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holmes NE, Robinson JO, van Hal SJ, Munckhof WJ, Athan E, et al. Morbidity from in-hospital complications is greater than treatment failure in patients with Staphylococcus aureus bacteraemia. BMC Infect Dis. 2018;18:107. doi: 10.1186/s12879-018-3011-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barber M, Rozwadowska-Dowzenko M. Infection by penicillin-resistant staphylococci. The Lancet. 1948;252:641–644. doi: 10.1016/S0140-6736(48)92166-7. [DOI] [PubMed] [Google Scholar]
- 8.Jessen O, Rosendal K, Bülow P, Faber V, Eriksen KR. Changing staphylococci and staphylococcal infections. A ten-year study of bacteria and cases of bacteremia. N Engl J Med. 1969;281:627–635. doi: 10.1056/NEJM196909182811201. [DOI] [PubMed] [Google Scholar]
- 9.Chambers HF, Deleo FR. Waves of resistance: Staphylococcus aureus in the antibiotic era. Nat Rev Microbiol. 2009;7:629–641. doi: 10.1038/nrmicro2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barber M. Methicillin-resistant staphylococci. J Clin Pathol. 1961;14:385–393. doi: 10.1136/jcp.14.4.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harkins CP, Pichon B, Doumith M, Parkhill J, Westh H, et al. Methicillin-resistant Staphylococcus aureus emerged long before the introduction of methicillin into clinical practice. Genome Biol. 2017;18:130. doi: 10.1186/s13059-017-1252-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hiramatsu K, Hanaki H, Ino T, Yabuta K, Oguri T, et al. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J Antimicrob Chemother. 1997;40:135–136. doi: 10.1093/jac/40.1.135. [DOI] [PubMed] [Google Scholar]
- 13.Moellering RC. Vancomycin: a 50-year reassessment. Clin Infect Dis. 2006;42 (Suppl. 1):S3–S4. doi: 10.1086/491708. [DOI] [PubMed] [Google Scholar]
- 14.Fowler VG, Boucher HW, Corey GR, Abrutyn E, Karchmer AW, et al. Daptomycin versus standard therapy for bacteremia and endocarditis caused by Staphylococcus aureus . N Engl J Med. 2006;355:653–665. doi: 10.1056/NEJMoa053783. [DOI] [PubMed] [Google Scholar]
- 15.Mendes RE, Hogan PA, Jones RN, Sader HS, Flamm RK. Surveillance for linezolid resistance via the Zyvox® annual appraisal of potency and spectrum (ZAAPS) programme (2014): evolving resistance mechanisms with stable susceptibility rates. J Antimicrob Chemother. 2016;71:1860–1865. doi: 10.1093/jac/dkw052. [DOI] [PubMed] [Google Scholar]
- 16.Hartman BJ, Tomasz A. Low-affinity penicillin-binding protein associated with beta-lactam resistance in Staphylococcus aureus . J Bacteriol. 1984;158:513–516. doi: 10.1128/jb.158.2.513-516.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang HZ, Hackbarth CJ, Chansky KM, Chambers HF. A proteolytic transmembrane signaling pathway and resistance to beta-lactams in staphylococci. Science. 2001;291:1962–1965. doi: 10.1126/science.1055144. [DOI] [PubMed] [Google Scholar]
- 18.Matthews PR, Stewart PR. Resistance heterogeneity in methicillin-resistant Staphylococcus aureus . FEMS Microbiol Lett. 1984;22:161–166. doi: 10.1111/j.1574-6968.1984.tb00718.x. [DOI] [Google Scholar]
- 19.Aedo S, Tomasz A. Role of the stringent stress response in the antibiotic resistance phenotype of methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2016;60:2311–2317. doi: 10.1128/AAC.02697-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gaca AO, Colomer-Winter C, Lemos JA. Many means to a common end: the intricacies of (p)ppGpp metabolism and its control of bacterial homeostasis. J Bacteriol. 2015;197:1146–1156. doi: 10.1128/JB.02577-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pardos de la Gandara M, Borges V, Chung M, Milheiriço C, Gomes JP, et al. Genetic determinants of high-level oxacillin resistance in methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2018;62:e00206-18. doi: 10.1128/AAC.00206-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Berger-Bächi B, Barberis-Maino L, Strässle A, Kayser FH, FemA KFH. Fema, a host-mediated factor essential for methicillin resistance in Staphylococcus aureus: molecular cloning and characterization. Mol Gen Genet. 1989;219:263–269. doi: 10.1007/BF00261186. [DOI] [PubMed] [Google Scholar]
- 23.García-Álvarez L, Holden MTG, Lindsay H, Webb CR, Brown DFJ, et al. Meticillin-resistant Staphylococcus aureus with a novel mecA homologue in human and bovine populations in the UK and Denmark: a descriptive study. Lancet Infect Dis. 2011;11:595–603. doi: 10.1016/S1473-3099(11)70126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paterson GK, Harrison EM, Holmes MA. The emergence of mecC methicillin-resistant Staphylococcus aureus . Trends Microbiol. 2014;22:42–47. doi: 10.1016/j.tim.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diaz R, Ramalheira E, Afreixo V, Gago B. Methicillin-resistant Staphylococcus aureus carrying the new mecC gene – a meta-analysis. Diagn Microbiol Infect Dis. 2016;84:135–140. doi: 10.1016/j.diagmicrobio.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 26.Worthing KA, Coombs GW, Pang S, Abraham S, Saputra S, et al. Isolation of mecC MRSA in Australia. J Antimicrob Chemother. 2016;71:2348–2349. doi: 10.1093/jac/dkw138. [DOI] [PubMed] [Google Scholar]
- 27.Kim CK, Milheiriço C, de Lencastre H, Tomasz A. Antibiotic resistance as a stress response: recovery of high-level oxacillin resistance in methicillin-resistant Staphylococcus aureus “auxiliary” (fem) mutants by induction of the stringent stress response. Antimicrob Agents Chemother. 2017;61:e00313-17. doi: 10.1128/AAC.00313-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ba X, Harrison EM, Lovering AL, Gleadall N, Zadoks R, et al. Old drugs to treat resistant bugs: methicillin-resistant Staphylococcus aureus isolates with mecC are susceptible to a combination of penicillin and clavulanic acid. Antimicrob Agents Chemother. 2015;59:7396–7404. doi: 10.1128/AAC.01469-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mancini S, Laurent F, Veloso TR, Giddey M, Vouillamoz J, et al. In vivo effect of flucloxacillin in experimental endocarditis caused by mecC-positive Staphylococcus aureus showing temperature-dependent susceptibility in vitro. Antimicrob Agents Chemother. 2015;59:2435–2438. doi: 10.1128/AAC.04733-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ito T, Hiramatsu K, Tomasz A, de Lencastre H, Perreten V, et al. Guidelines for reporting novel mecA gene homologues. Antimicrob Agents Chemother. 2012;56:4997–4999. doi: 10.1128/AAC.01199-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwendener S, Cotting K, Perreten V. Novel methicillin resistance gene mecD in clinical Macrococcus caseolyticus strains from bovine and canine sources. Sci Rep. 2017;7:43797. doi: 10.1038/srep43797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rolo J, Worning P, Nielsen JB, Bowden R, Bouchami O, et al. Evolutionary origin of the staphylococcal cassette chromosome mec (SCC mec) Antimicrob Agents Chemother. 2017;61:e02302-16. doi: 10.1128/AAC.02302-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.International Working Group on the Classification of Staphylococcal Cassette Chromosome Elements (IWG-SCC) Classification of staphylococcal cassette chromosome mec (SCCmec): guidelines for reporting novel SCCmec elements. Antimicrob Agents Chemother. 2009;53:4961–4967. doi: 10.1128/AAC.00579-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Baines SL, Howden BP, Heffernan H, Stinear TP, Carter GP, et al. Rapid emergence and evolution of Staphylococcus aureus clones harboring fusC-containing staphylococcal cassette chromosome elements. Antimicrob Agents Chemother. 2016;60:2359–2365. doi: 10.1128/AAC.03020-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ellington MJ, Reuter S, Harris SR, Holden MTG, Cartwright EJ, et al. Emergent and evolving antimicrobial resistance cassettes in community-associated fusidic acid and meticillin-resistant Staphylococcus aureus . Int J Antimicrob Agents. 2015;45:477–484. doi: 10.1016/j.ijantimicag.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Harris TM, Bowen AC, Holt DC, Sarovich DS, Stevens K, et al. Investigation of trimethoprim/sulfamethoxazole resistance in an emerging sequence type 5 methicillin-resistant Staphylococcus aureus clone reveals discrepant resistance reporting. Clin Microbiol Infect. 2018;24:1027–1029. doi: 10.1016/j.cmi.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 37.Kaya H, Hasman H, Larsen J, Stegger M, Johannesen TB, et al. SCCmecFinder, a web-based tool for typing of staphylococcal cassette chromosome mec in Staphylococcus aureus using whole-genome sequence data. mSphere. 2018;3:e00612-17. doi: 10.1128/mSphere.00612-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Firth N, Jensen SO, Kwong SM, Skurray RA, Ramsay JP. Staphylococcal plasmids, transposable and integrative elements. Microbiol Spectr. 2018;6:GPP3-0030-2018. doi: 10.1128/microbiolspec.GPP3-0030-2018. [DOI] [PubMed] [Google Scholar]
- 39.Xia G, Wolz C. Phages of Staphylococcus aureus and their impact on host evolution. Infect Genet Evol. 2014;21:593–601. doi: 10.1016/j.meegid.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 40.Jones D, Elshaboury RH, Munson E, Dilworth TJ. A retrospective analysis of treatment and clinical outcomes among patients with methicillin-susceptible Staphylococcus aureus bloodstream isolates possessing detectable mecA by a commercial PCR assay compared to patients with methicillin-resistant Staphylococcus aureus bloodstream isolates. Antimicrob Agents Chemother. 2018;62:e01396-17. doi: 10.1128/AAC.01396-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Proulx MK, Palace SG, Gandra S, Torres B, Weir S, et al. Reversion from methicillin susceptibility to methicillin resistance in Staphylococcus aureus during treatment of bacteremia. J Infect Dis. 2016;213:1041–1048. doi: 10.1093/infdis/jiv512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chung M, Kim CK, Conceição T, Aires-De-Sousa M, de Lencastre H, et al. Heterogeneous oxacillin-resistant phenotypes and production of PBP2a by oxacillin-susceptible/mecA-positive MRSA strains from Africa. J Antimicrob Chemother. 2016;71:2804–2809. doi: 10.1093/jac/dkw209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrison EM, Ba X, Coll F, Blane B, Restif O, et al. Genomic identification of cryptic susceptibility to penicillins and β-lactamase inhibitors in methicillin-resistant Staphylococcus aureus . Nat Microbiol. 2019;4:1680–1691. doi: 10.1038/s41564-019-0471-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skinner S, Murray M, Walus T, Karlowsky JA. Failure of cloxacillin in treatment of a patient with borderline oxacillin-resistant Staphylococcus aureus endocarditis. J Clin Microbiol. 2009;47:859–861. doi: 10.1128/JCM.00571-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Burd EM, Alam MT, Passalacqua KD, Kalokhe AS, Eaton ME, et al. Development of oxacillin resistance in a patient with recurrent Staphylococcus aureus bacteremia. J Clin Microbiol. 2014;52:3114–3117. doi: 10.1128/JCM.00615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ba X, Harrison EM, Edwards GF, Holden MTG, Larsen AR, et al. Novel mutations in penicillin-binding protein genes in clinical Staphylococcus aureus isolates that are methicillin resistant on susceptibility testing, but lack the mec gene. J Antimicrob Chemother. 2014;69:594–597. doi: 10.1093/jac/dkt418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ba X, Kalmar L, Hadjirin NF, Kerschner H, Apfalter P, et al. Truncation of GdpP mediates β-lactam resistance in clinical isolates of Staphylococcus aureus . J Antimicrob Chemother. 2019;74:1182–1191. doi: 10.1093/jac/dkz013. [DOI] [PubMed] [Google Scholar]
- 48.Argudín MA, Roisin S, Nienhaus L, Dodémont M, de Mendonça R, et al. Genetic diversity among Staphylococcus aureus isolates showing oxacillin and/or cefoxitin resistance not linked to the presence of mec genes. Antimicrob Agents Chemother. 2018;62:e00091-18. doi: 10.1128/AAC.00091-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Corrigan RM, Gründling A. Cyclic di-AMP: another second messenger enters the fray. Nat Rev Microbiol. 2013;11:513–524. doi: 10.1038/nrmicro3069. [DOI] [PubMed] [Google Scholar]
- 50.Planet PJ. Life after USA300: the rise and fall of a superbug. J Infect Dis. 2017;215:S71–S77. doi: 10.1093/infdis/jiw444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Turner NA, Sharma-Kuinkel BK, Maskarinec SA, Eichenberger EM, Shah PP, et al. Methicillin-resistant Staphylococcus aureus: an overview of basic and clinical research. Nat Rev Microbiol. 2019;17:203–218. doi: 10.1038/s41579-018-0147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Grundmann H, Aanensen DM, van den Wijngaard CC, Spratt BG, Harmsen D, et al. Geographic distribution of Staphylococcus aureus causing invasive infections in Europe: a molecular-epidemiological analysis. PLoS Med. 2010;7:e1000215. doi: 10.1371/journal.pmed.1000215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Harris SR, Feil EJ, Holden MTG, Quail MA, Nickerson EK, et al. Evolution of MRSA during hospital transmission and intercontinental spread. Science. 2010;327:469–474. doi: 10.1126/science.1182395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Köser CU, Holden MTG, Ellington MJ, Cartwright EJP, Brown NM, et al. Rapid whole-genome sequencing for investigation of a neonatal MRSA outbreak. N Engl J Med. 2012;366:2267–2275. doi: 10.1056/NEJMoa1109910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Senn L, Clerc O, Zanetti G, Basset P, Prod'hom G, et al. The stealthy superbug: the role of asymptomatic enteric carriage in maintaining a long-term hospital outbreak of ST228 methicillin-resistant Staphylococcus aureus . mBio. 2016;7:e02039-15. doi: 10.1128/mBio.02039-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harris SR, Cartwright EJP, Török ME, Holden MTG, Brown NM, et al. Whole-genome sequencing for analysis of an outbreak of meticillin-resistant Staphylococcus aureus: a descriptive study. Lancet Infect Dis. 2013;13:130–136. doi: 10.1016/S1473-3099(12)70268-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uhlemann A-C, Dordel J, Knox JR, Raven KE, Parkhill J, et al. Molecular tracing of the emergence, diversification, and transmission of S. aureus sequence type 8 in a New York community. Proc Natl Acad Sci USA. 2014;111:6738–6743. doi: 10.1073/pnas.1401006111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Price LB, Stegger M, Hasman H, Aziz M, Larsen J, et al. Staphylococcus aureus CC398: host adaptation and emergence of methicillin resistance in livestock. mBio. 2012;3:e00305-11. doi: 10.1128/mBio.00305-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gonçalves da Silva A, Baines SL, Carter GP, Heffernan H, French NP, et al. A phylogenomic framework for assessing the global emergence and evolution of clonal complex 398 methicillin-resistant Staphylococcus aureus . Microb Genom. 2017;3:e000105. doi: 10.1099/mgen.0.000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Aanensen DM, Feil EJ, Holden MTG, Dordel J, Yeats CA, et al. Whole-genome sequencing for routine pathogen surveillance in public health: a population snapshot of invasive Staphylococcus aureus in Europe. mBio. 2016;7:e00444-16. doi: 10.1128/mBio.00444-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Reuter S, Török ME, Holden MTG, Reynolds R, Raven KE, et al. Building a genomic framework for prospective MRSA surveillance in the United Kingdom and the Republic of Ireland. Genome Res. 2016;26:263–270. doi: 10.1101/gr.196709.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Toleman MS, Reuter S, Jamrozy D, Wilson HJ, Blane B, et al. Prospective genomic surveillance of methicillin-resistant Staphylococcus aureus (MRSA) associated with bloodstream infection, England, 1 October 2012 to 30 September 2013. Euro Surveill. 2019;24:1800215. doi: 10.2807/1560-7917.ES.2019.24.4.1800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Price JR, Cole K, Bexley A, Kostiou V, Eyre DW, et al. Transmission of Staphylococcus aureus between health-care workers, the environment, and patients in an intensive care unit: a longitudinal cohort study based on whole-genome sequencing. Lancet Infect Dis. 2017;17:207–214. doi: 10.1016/S1473-3099(16)30413-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Coll F, Harrison EM, Toleman MS, Reuter S, Raven KE, et al. Longitudinal genomic surveillance of MRSA in the UK reveals transmission patterns in hospitals and the community. Sci Transl Med. 2017;9:eaak9745. doi: 10.1126/scitranslmed.aak9745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Petit RA, Read TD. Staphylococcus aureus viewed from the perspective of 40,000+ genomes. PeerJ. 2018;6:e5261. doi: 10.7717/peerj.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Guérillot R, Gonçalves da Silva A, Monk I, Giulieri S, Tomita T, et al. Convergent evolution driven by rifampin exacerbates the global burden of drug-resistant Staphylococcus aureus . mSphere. 2018;3:e00550-17. doi: 10.1128/mSphere.00550-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bradley P, den Bakker HC, Rocha EPC, McVean G, Iqbal Z. Ultrafast search of all deposited bacterial and viral genomic data. Nat Biotechnol. 2019;37:152–159.:152. doi: 10.1038/s41587-018-0010-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Copin R, Shopsin B, Torres VJ. After the deluge: mining Staphylococcus aureus genomic data for clinical associations and host-pathogen interactions. Curr Opin Microbiol. 2018;41:43–50. doi: 10.1016/j.mib.2017.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Holden MTG, Hsu L-Y, Kurt K, Weinert LA, Mather AE, et al. A genomic portrait of the emergence, evolution, and global spread of a methicillin-resistant Staphylococcus aureus pandemic. Genome Res. 2013;23:653–664. doi: 10.1101/gr.147710.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Baines SL, Holt KE, Schultz MB, Seemann T, Howden BO, et al. Convergent adaptation in the dominant global hospital clone ST239 of methicillin-resistant Staphylococcus aureus . mBio. 2015;6:e00080. doi: 10.1128/mBio.00080-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stinear TP, Holt KE, Chua K, Stepnell J, Tuck KL, et al. Adaptive change inferred from genomic population analysis of the ST93 epidemic clone of community-associated methicillin-resistant Staphylococcus aureus . Genome Biol Evol. 2014;6:366–378. doi: 10.1093/gbe/evu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Copin R, Sause WE, Fulmer Y, Balasubramanian D, Dyzenhaus S, et al. Sequential evolution of virulence and resistance during clonal spread of community-acquired methicillin-resistant Staphylococcus aureus . Proc Natl Acad Sci USA. 2019;116:1745–1754. doi: 10.1073/pnas.1814265116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.van Hal SJ, Steinig EJ, Andersson P, Holden MTG, Harris SR, et al. Global scale dissemination of ST93: a divergent Staphylococcus aureus epidemic lineage that has recently emerged from remote Northern Australia. Front Microbiol. 2018;9:1453. doi: 10.3389/fmicb.2018.01453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.DeLeo FR, Kennedy AD, Chen L, Bubeck Wardenburg J, Kobayashi SD, et al. Molecular differentiation of historic phage-type 80/81 and contemporary epidemic Staphylococcus aureus . Proc Natl Acad Sci USA. 2011;108:18091–18096. doi: 10.1073/pnas.1111084108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Tong SYC, Holden MTG, Nickerson EK, Cooper BS, Köser CU, et al. Genome sequencing defines phylogeny and spread of methicillin-resistant Staphylococcus aureus in a high transmission setting. Genome Res. 2015;25:111–118. doi: 10.1101/gr.174730.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ward MJ, Goncheva M, Richardson E, McAdam PR, Raftis E, et al. Identification of source and sink populations for the emergence and global spread of the East-Asia clone of community-associated MRSA. Genome Biol. 2016;17:160. doi: 10.1186/s13059-016-1022-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lindsay JA. Hospital-associated MRSA and antibiotic resistance – what have we learned from genomics? Int J Med Microbiol. 2013;303:318–323. doi: 10.1016/j.ijmm.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 78.Couderc C, Jolivet S, Thiébaut ACM, Ligier C, Remy L, et al. Fluoroquinolone use is a risk factor for methicillin-resistant Staphylococcus aureus acquisition in long-term care facilities: a nested case-case-control study. Clin Infect Dis. 2014;59:206–215. doi: 10.1093/cid/ciu236. [DOI] [PubMed] [Google Scholar]
- 79.Dweba CC, Zishiri OT, El Zowalaty ME. Methicillin-resistant Staphylococcus aureus: livestock-associated, antimicrobial, and heavy metal resistance. Infect Drug Resist. 2018;11:2497–2509. doi: 10.2147/IDR.S175967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Williamson DA, Carter GP, Howden BP. Current and emerging topical antibacterials and antiseptics: agents, action, and resistance patterns. Clin Microbiol Rev. 2017;30:827–860. doi: 10.1128/CMR.00112-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ortwine JK, Werth BJ, Sakoulas G, Rybak MJ. Reduced glycopeptide and lipopeptide susceptibility in Staphylococcus aureus and the "seesaw effect": Taking advantage of the back door left open? Drug Resist Updat. 2013;16:73–79. doi: 10.1016/j.drup.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 82.Liu J, Chen D, Peters BM, Li L, Li B, et al. Staphylococcal chromosomal cassettes mec (SCCmec): a mobile genetic element in methicillin-resistant Staphylococcus aureus . Microb Pathog. 2016;101:56–67. doi: 10.1016/j.micpath.2016.10.028. [DOI] [PubMed] [Google Scholar]
- 83.Byrne ME, Gillespie MT, Skurray RA. Molecular analysis of a gentamicin resistance transposonlike element on plasmids isolated from North American Staphylococcus aureus strains. Antimicrob Agents Chemother. 1990;34:2106–2113. doi: 10.1128/AAC.34.11.2106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Carter GP, Schultz MB, Baines SL, Gonçalves da Silva A, Heffernan H, et al. Topical antibiotic use coselects for the carriage of mobile genetic elements conferring resistance to unrelated antimicrobials in Staphylococcus aureus . Antimicrob Agents Chemother. 2018;62:e02000–02017. doi: 10.1128/AAC.02000-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schmitz FJ, Jones ME, Hofmann B, Hansen B, Scheuring S, et al. Characterization of grlA, grlB, gyrA, and gyrB mutations in 116 unrelated isolates of Staphylococcus aureus and effects of mutations on ciprofloxacin MIC. Antimicrob Agents Chemother. 1998;42:1249–1252. doi: 10.1128/AAC.42.5.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Baines SL, Jensen SO, Firth N, Gonçalves da Silva A, Seemann T, et al. Remodeling of pSK1 family plasmids and enhanced chlorhexidine tolerance in a dominant hospital lineage of methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2019;63:e02356-18. doi: 10.1128/AAC.02356-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Patel JB, Gorwitz RJ, Jernigan JA. Mupirocin resistance. Clin Infect Dis. 2009;49:935–941. doi: 10.1086/605495. [DOI] [PubMed] [Google Scholar]
- 88.Yokoyama M, Stevens E, Laabei M, Bacon L, Heesom K, et al. Epistasis analysis uncovers hidden antibiotic resistance-associated fitness costs hampering the evolution of MRSA. Genome Biol. 2018;19:94. doi: 10.1186/s13059-018-1469-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Laabei M, Massey R. Using functional genomics to decipher the complexity of microbial pathogenicity. Curr Genet. 2016;62:523. doi: 10.1007/s00294-016-0576-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Giulieri SG, Holmes NE, Stinear TP, Howden BP. Use of bacterial whole-genome sequencing to understand and improve the management of invasive Staphylococcus aureus infections. Expert Rev Anti Infect Ther. 2016;14:1023–1036. doi: 10.1080/14787210.2016.1233815. [DOI] [PubMed] [Google Scholar]
- 91.Bergdoll M, Crass BA, Reiser RF, Robbins RN, Davis JP. A new staphylococcal enterotoxin, enterotoxin F, associated with toxic-shock-syndrome Staphylococcus aureus isolates. The Lancet. 1981;317:1017–1021. doi: 10.1016/S0140-6736(81)92186-3. [DOI] [PubMed] [Google Scholar]
- 92.Gillet Y, Henry T, Vandenesch F. Fulminant staphylococcal infections. Microb Spectr. 2018;6 doi: 10.1128/microbiolspec.GPP3-0036-2018. [DOI] [PubMed] [Google Scholar]
- 93.Chua KYL, Monk IR, Lin Y-H, Seemann T, Tuck KL, et al. Hyperexpression of α-hemolysin explains enhanced virulence of sequence type 93 community-associated methicillin-resistant Staphylococcus aureus . BMC Microbiol. 2014;14:31. doi: 10.1186/1471-2180-14-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Gillet Y, Issartel B, Vanhems P, Fournet J-C, Lina G, et al. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. The Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 95.Laabei M, Uhlemann A-C, Lowy FD, Austin ED, Yokoyama M, et al. Evolutionary trade-offs underlie the multi-faceted virulence of Staphylococcus aureus . PLoS Biol. 2015;13:e1002229. doi: 10.1371/journal.pbio.1002229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vandenesch F, Lina G, Henry T. Staphylococcus aureus hemolysins, Bi-component leukocidins, and cytolytic peptides: a redundant arsenal of membrane-damaging virulence factors? Front Cell Infect Microbiol. 2012;2:12. doi: 10.3389/fcimb.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Otto M. Community-Associated MRSA: what makes them special? Int J Med Microbiol. 2013;303:324–330. doi: 10.1016/j.ijmm.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Young BC, Earle SG, Soeng S, Sar P, Kumar V, et al. Panton–Valentine leucocidin is the key determinant of Staphylococcus aureus pyomyositis in a bacterial GWAS. eLife. 2019;8:e42486. doi: 10.7554/eLife.42486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Rudkin JK, Laabei M, Edwards AM, Joo H-S, Otto M, et al. Oxacillin alters the toxin expression profile of community-associated methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2014;58:1100–1107. doi: 10.1128/AAC.01618-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin Microbiol Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Li M, Du X, Villaruz AE, Diep BA, Wang D, et al. MRSA epidemic linked to a quickly spreading colonization and virulence determinant. Nat Med. 2012;18:816–819. doi: 10.1038/nm.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Levine DP. Vancomycin: a history. Clin Infect Dis. 2006;42 (Suppl. 1):S5–S12. doi: 10.1086/491709. [DOI] [PubMed] [Google Scholar]
- 103.Lee JYH, Howden BP. Vancomycin in the treatment of methicillin-resistant Staphylococcus aureus – a clinician’s guide to the science informing current practice. Expert Rev Anti Infect Ther. 2015;13:855–869. doi: 10.1586/14787210.2015.1041924. [DOI] [PubMed] [Google Scholar]
- 104.Weigel LM, Clewell DB, Gill SR, Clark NC, McDougal LK, et al. Genetic analysis of a high-level vancomycin-resistant isolate of Staphylococcus aureus . Science. 2003;302:1569–1571. doi: 10.1126/science.1090956. [DOI] [PubMed] [Google Scholar]
- 105.Périchon B, Courvalin P. VanA-type vancomycin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2009;53:4580. doi: 10.1128/AAC.00346-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Chang S, Sievert DM, Hageman JC, Boulton ML, Tenover FC, et al. Infection with vancomycin-resistant Staphylococcus aureus containing the vanA resistance gene. N Engl J Med. 2003;348:1342–1347. doi: 10.1056/NEJMoa025025. [DOI] [PubMed] [Google Scholar]
- 107.Noble WC, Virani Z, Cree RG. Co-transfer of vancomycin and other resistance genes from Enterococcus faecalis NCTC 12201 to Staphylococcus aureus . FEMS Microbiol Lett. 1992;72:195–198. doi: 10.1016/0378-1097(92)90528-v. [DOI] [PubMed] [Google Scholar]
- 108.McGuinness WA, Malachowa N, DeLeo FR. Vancomycin resistance in Staphylococcus aureus . Yale J Biol Med. 2017;90:269–281. [PMC free article] [PubMed] [Google Scholar]
- 109.Ghahremani M, Jazani NH, Sharifi Y. Emergence of vancomycin-intermediate and -resistant Staphylococcus aureus among methicillin-resistant S. aureus isolated from clinical specimens in the northwest of Iran. J Glob Antimicrob Resist. 2018;14:4–9. doi: 10.1016/j.jgar.2018.01.017. [DOI] [PubMed] [Google Scholar]
- 110.Kumar M. Multidrug-Resistant Staphylococcus aureus, India, 2013-2015. Emerg Infect Dis. 2016;22:1666–1667. doi: 10.3201/eid2209.160044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kos VN, Desjardins CA, Griggs A, Cerqueira G, Van Tonder A, et al. Comparative genomics of vancomycin-resistant Staphylococcus aureus strains and their positions within the clade most commonly associated with methicillin-resistant S. aureus hospital-acquired infection in the United States. mBio. 2012;3:e00112-12. doi: 10.1128/mBio.00112-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Howden BP, Johnson PDR, Ward PB, Stinear TP, Davies JK. Isolates with low-level vancomycin resistance associated with persistent methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2006;50:3039–3047. doi: 10.1128/AAC.00422-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pfeltz RF, Singh VK, Schmidt JL, Batten MA, Baranyk CS, et al. Characterization of passage-selected vancomycin-resistant Staphylococcus aureus strains of diverse parental backgrounds. Antimicrob Agents Chemother. 2000;44:294–303. doi: 10.1128/AAC.44.2.294-303.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Howden BP, Davies JK, Johnson PDR, Stinear TP, Grayson ML. Reduced vancomycin susceptibility in Staphylococcus aureus, including vancomycin-intermediate and heterogeneous vancomycin-intermediate strains: resistance mechanisms, laboratory detection, and clinical implications. Clin Microbiol Rev. 2010;23:99–139. doi: 10.1128/CMR.00042-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proc Natl Acad Sci USA. 2007;104:9451–9456. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Cui L, Neoh H-M, Shoji M, Hiramatsu K. Contribution of vraSR and graSR point mutations to vancomycin resistance in vancomycin-intermediate Staphylococcus aureus . Antimicrob Agents Chemother. 2009;53:1231–1234. doi: 10.1128/AAC.01173-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Howden BP, McEvoy CRE, Allen DL, Chua K, Gao W, et al. Evolution of multidrug resistance during Staphylococcus aureus infection involves mutation of the essential two component regulator WalKR. PLoS Pathog. 2011;7:e1002359. doi: 10.1371/journal.ppat.1002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cui L, Isii T, Fukuda M, Ochiai T, Neoh H-M, et al. An rpoB mutation confers dual heteroresistance to daptomycin and vancomycin in Staphylococcus aureus . Antimicrob Agents Chemother. 2010;54:5222–5233. doi: 10.1128/AAC.00437-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Passalacqua KD, Satola SW, Crispell EK, Read TD. A mutation in the PP2C phosphatase gene in a Staphylococcus aureus USA300 clinical isolate with reduced susceptibility to vancomycin and daptomycin. Antimicrob Agents Chemother. 2012;56:5212–5223. doi: 10.1128/AAC.05770-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Giulieri SG, Baines SL, Guerillot R, Seemann T, Gonçalves da Silva A, et al. Genomic exploration of sequential clinical isolates reveals a distinctive molecular signature of persistent Staphylococcus aureus bacteraemia. Genome Med. 2018;10:65. doi: 10.1186/s13073-018-0574-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McEvoy CRE, Tsuji B, Gao W, Seemann T, Porter JL, et al. Decreased vancomycin susceptibility in Staphylococcus aureus caused by IS256 tempering of WalKR expression. Antimicrob Agents Chemother. 2013;57:3240–3249. doi: 10.1128/AAC.00279-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Alam MT, Petit RA, Crispell EK, Thornton TA, Conneely KN, et al. Dissecting vancomycin-intermediate resistance in Staphylococcus aureus using genome-wide association. Genome Biol Evol. 2014;6:1174–1185. doi: 10.1093/gbe/evu092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Rishishwar L, Petit RA, Kraft CS, Jordan IK. Genome sequence-based discriminator for vancomycin-intermediate Staphylococcus aureus . J Bacteriol. 2014;196:940–948. doi: 10.1128/JB.01410-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhang S, Sun X, Chang W, Dai Y, Ma X, et al. Systematic review and meta-analysis of the epidemiology of vancomycin-intermediate and heterogeneous vancomycin-intermediate Staphylococcus aureus isolates. PLoS One. 2015;10:e0136082. doi: 10.1371/journal.pone.0136082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Holmes NE, Turnidge JD, Munckhof WJ, Robinson JO, Korman TM, et al. Genetic and molecular predictors of high vancomycin MIC in Staphylococcus aureus bacteremia isolates. J Clin Microbiol. 2014;52:3384–3393. doi: 10.1128/JCM.01320-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Howden BP, Peleg AY, Stinear TP. The evolution of vancomycin intermediate Staphylococcus aureus (VISA) and heterogenous-VISA. Infect Genet Evol. 2014;21:575–582. doi: 10.1016/j.meegid.2013.03.047. [DOI] [PubMed] [Google Scholar]
- 127.Gardete S, Tomasz A. Mechanisms of vancomycin resistance in Staphylococcus aureus . J Clin Invest. 2014;124:2836–2840. doi: 10.1172/JCI68834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Humphries RM, Pollett S, Sakoulas G. A current perspective on daptomycin for the clinical microbiologist. Clin Microbiol Rev. 2013;26:759–780. doi: 10.1128/CMR.00030-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Kelley PG, Gao W, Ward PB, Howden BP. Daptomycin non-susceptibility in vancomycin-intermediate Staphylococcus aureus (VISA) and heterogeneous-VISA (hVISA): implications for therapy after vancomycin treatment failure. J Antimicrob Chemother. 2011;66:1057–1060. doi: 10.1093/jac/dkr066. [DOI] [PubMed] [Google Scholar]
- 130.Sharma M, Riederer K, Chase P, Khatib R. High rate of decreasing daptomycin susceptibility during the treatment of persistent Staphylococcus aureus bacteremia. Eur J Clin Microbiol Infect Dis. 2008;27:433–437. doi: 10.1007/s10096-007-0455-5. [DOI] [PubMed] [Google Scholar]
- 131.Bayer AS, Schneider T, Sahl H-G. Mechanisms of daptomycin resistance in Staphylococcus aureus: role of the cell membrane and cell wall. Ann NY Acad Sci. 2013;1277:139–158. doi: 10.1111/j.1749-6632.2012.06819.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yang S-J, Mishra NN, Rubio A, Bayer AS. Causal role of single nucleotide polymorphisms within the mprF gene of Staphylococcus aureus in daptomycin resistance. Antimicrob Agents Chemother. 2013;57:5658–5664. doi: 10.1128/AAC.01184-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Friedman L, Alder JD, Silverman JA. Genetic changes that correlate with reduced susceptibility to daptomycin in Staphylococcus aureus . Antimicrob Agents Chemother. 2006;50:2137–2145. doi: 10.1128/AAC.00039-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Adhikari RP, et al. Vancomycin-induced deletion of the methicillin resistance gene mecA in Staphylococcus aureus . J Antimicrob Chemother. 2004;54:360–363. doi: 10.1093/jac/dkh350. [DOI] [PubMed] [Google Scholar]
- 135.Renzoni A, Kelley WL, Rosato RR, Martinez MP, Roch M, et al. Molecular bases determining daptomycin resistance-mediated resensitization to β-lactams (seesaw effect) in methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2017;61:e01634-16. doi: 10.1128/AAC.01634-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Wang G, Hindler JF, Ward KW, Bruckner DA. Increased vancomycin MICs for Staphylococcus aureus clinical isolates from a university hospital during a 5-year period. J Clin Microbiol. 2006;44:3883–3886. doi: 10.1128/JCM.01388-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Locke JB, Hilgers M, Shaw KJ. Novel ribosomal mutations in Staphylococcus aureus strains identified through selection with the oxazolidinones linezolid and torezolid (TR-700) Antimicrob Agents Chemother. 2009;53:5265–5274. doi: 10.1128/AAC.00871-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Locke JB, Rahawi S, LaMarre J, Mankin AS, Shaw KJ. Genetic environment and stability of cfr in methicillin-resistant Staphylococcus aureus CM05. Antimicrob Agents Chemother. 2012;56:332–340. doi: 10.1128/AAC.05420-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Locke JB, Zuill DE, Scharn CR, Deane J, Sahm DF, et al. Identification and characterization of linezolid-resistant cfr-positive Staphylococcus aureus USA300 isolates from a New York City medical center. Antimicrob Agents Chemother. 2014;58:6949–6952. doi: 10.1128/AAC.03380-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Sánchez García M, De la Torre MA, Morales G, Peláez B, José Tolón M, et al. Clinical outbreak of linezolid-resistant Staphylococcus aureus in an intensive care unit. JAMA. 2010;303:2260–2264. doi: 10.1001/jama.2010.757. [DOI] [PubMed] [Google Scholar]
- 141.Geriak M, Haddad F, Rizvi K, Rose W, Kullar R, et al. Clinical data on daptomycin plus ceftaroline versus standard of care monotherapy in the treatment of methicillin-resistant Staphylococcus aureus bacteremia. Antimicrob Agents Chemother. 2019;63:e02483-18. doi: 10.1128/AAC.02483-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Long SW, Olsen RJ, Mehta SC, Palzkill T, Cernoch PL, et al. Pbp2A mutations causing high-level ceftaroline resistance in clinical methicillin-resistant Staphylococcus aureus isolates. Antimicrob Agents Chemother. 2014;58:6668–6674. doi: 10.1128/AAC.03622-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Wüthrich D, Cuénod A, Hinic V, Morgenstern M, Khanna N, et al. Genomic characterization of inpatient evolution of MRSA resistant to daptomycin, vancomycin and ceftaroline. J Antimicrob Chemother. 2019;74:1452–1454. doi: 10.1093/jac/dkz003. [DOI] [PubMed] [Google Scholar]
- 144.Alm RA, McLaughlin RE, Kos VN, Sader HS, Iaconis JP, et al. Analysis of Staphylococcus aureus clinical isolates with reduced susceptibility to ceftaroline: an epidemiological and structural perspective. J Antimicrob Chemother. 2014;69:2065–2075. doi: 10.1093/jac/dku114. [DOI] [PubMed] [Google Scholar]
- 145.Lee H, Yoon E-J, Kim D, Kim JW, Lee K-J, et al. Ceftaroline resistance by clone-specific polymorphism in penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus . Antimicrob Agents Chemother. 2018;62:e00485-18. doi: 10.1128/AAC.00485-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Abbott IJ, Jenney AWJ, Jeremiah CJ, Mirčeta M, Kandiah JP, et al. Reduced in vitro activity of ceftaroline by Etest among clonal complex 239 methicillin-resistant Staphylococcus aureus clinical strains from Australia. Antimicrob Agents Chemother. 2015;59:7837–7841. doi: 10.1128/AAC.02015-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Bradley P, Gordon NC, Walker TM, Dunn L, Heys S, et al. Rapid antibiotic-resistance predictions from genome sequence data for Staphylococcus aureus and Mycobacterium tuberculosis . Nat Commun. 2015;6:10063. doi: 10.1038/ncomms10063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Gordon NC, Price JR, Cole K, Everitt R, Morgan M, et al. Prediction of Staphylococcus aureus antimicrobial resistance by whole-genome sequencing. J Clin Microbiol. 2014;52:1182–1191. doi: 10.1128/JCM.03117-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Williamson DA, Heffernan H, Nimmo G. Contemporary genomic approaches in the diagnosis and typing of Staphylococcus aureus . Pathology. 2015;47:270–275. doi: 10.1097/PAT.0000000000000236. [DOI] [PubMed] [Google Scholar]
- 150.Lower SK, Lamlertthon S, Casillas-Ituarte NN, Lins RD, Yongsunthon R, et al. Polymorphisms in fibronectin binding protein A of Staphylococcus aureus are associated with infection of cardiovascular devices. Proc Natl Acad Sci USA. 2011;108:18372–18377. doi: 10.1073/pnas.1109071108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Recker M, Laabei M, Toleman MS, Reuter S, Saunderson RB, et al. Clonal differences in Staphylococcus aureus bacteraemia-associated mortality. Nat Microbiol. 2017;2:1381–1388. doi: 10.1038/s41564-017-0001-x. [DOI] [PubMed] [Google Scholar]
- 152.Lilje B, Rasmussen RV, Dahl A, Stegger M, Skov RL, et al. Whole-genome sequencing of bloodstream Staphylococcus aureus isolates does not distinguish bacteraemia from endocarditis. Microb Genom. 2017;3:mgen.0.000138. doi: 10.1099/mgen.0.000138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Read TD, Massey RC. Characterizing the genetic basis of bacterial phenotypes using genome-wide association studies: a new direction for bacteriology. Genome Med. 2014;6:109. doi: 10.1186/s13073-014-0109-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Scott WK, Medie FM, Ruffin F, Sharma-Kuinkel BK, Cyr DD, et al. Human genetic variation in GLS2 is associated with development of complicated Staphylococcus aureus bacteremia. PLoS Genet. 2018;14:e1007667. doi: 10.1371/journal.pgen.1007667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Didelot X, Walker AS, Peto TE, Crook DW, Wilson DJ. Within-host evolution of bacterial pathogens. Nat Rev Microbiol. 2016;14:150–162. doi: 10.1038/nrmicro.2015.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Young BC, Wu C-H, Gordon NC, Cole K, Price JR, et al. Severe infections emerge from commensal bacteria by adaptive evolution. eLife. 2017;6:e30637. doi: 10.7554/eLife.30637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Guérillot R, Li L, Baines S, Howden B, Schultz MB, et al. Comprehensive antibiotic-linked mutation assessment by resistance mutation sequencing (RM-seq) Genome Med. 2018;10:63. doi: 10.1186/s13073-018-0572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Davis JS, Sud A, O'Sullivan MVN, Robinson JO, Ferguson PE, et al. Combination of vancomycin and β-lactam therapy for methicillin-resistant Staphylococcus aureus bacteremia: a pilot multicenter randomized controlled trial. Clin Infect Dis. 2016;62:173–180. doi: 10.1093/cid/civ808. [DOI] [PubMed] [Google Scholar]
- 159.Steinig EJ, Duchene S, Robinson DA, Monecke S, Yokoyama M, et al. Evolution and global transmission of a multidrug-resistant, community-associated methicillin-resistant Staphylococcus aureus lineage from the Indian subcontinent. mBio. 2019;10:e01105-19. doi: 10.1128/mBio.01105-19. [DOI] [PMC free article] [PubMed] [Google Scholar]