We highlight different approaches used to induce boron deficiency in plants and how this has led to differences in interpretation of boron’s role in the cell wall versus the cytosol.
Keywords: Arabidopsis, BOR1, boron deficiency, boron transport, boronic acids, maize, NIP5, 1, phenylboronic acid, rice, rottenear, tassel-less1
Abstract
Deficiency of the essential nutrient boron (B) in the soil is one of the most widespread micronutrient deficiencies worldwide, leading to developmental defects in root and shoot tissues of plants, and severe yield reductions in many crops. Despite this agricultural importance, the underlying mechanisms of how B shapes plant developmental and morphological processes are still not unequivocally understood in detail. This review evaluates experimental approaches that address our current understanding of how B influences plant morphological processes by focusing on developmental defects observed under B deficiency. We assess what is known about mechanisms that control B homeostasis and specifically highlight: (i) limitations in the methodology that is used to induce B deficiency; (ii) differences between mutant phenotypes and normal plants grown under B deficiency; and (iii) recent research on analyzing interactions between B and phytohormones. Our analysis highlights the need for standardized methodology to evaluate the roles of B in the cell wall versus other parts of the cell.
Introduction
The micronutrient boron (B) is one of the essential plant micronutrients (Warington, 1923). B is taken up from the soil in the form of the uncharged boric acid (BA) (Fig. 1A). BA can be taken up either passively (via diffusion) or actively under B-deficient conditions in the soil (Fig. 1A). The range between B deficiency and toxicity is very narrow and therefore proper B homeostasis is required in the plant. B deficiency in plants is often caused by low soil B content, which occurs worldwide, and is reportedly one of the most widespread micronutrient deficiencies (Shorrocks, 1997). Currently, there is an effort in various research laboratories worldwide to understand the effects of B deficiency and toxicity in different plant species including, but not limited to, Arabidopsis thaliana (Arabidopsis), Zea mays (maize), Brassica napus (Brassica), Oryza sativa (rice), Malus domestica (apple), citrus, Hordeum vulgare (barley), and wheat (Noguchi et al., 1997; Takano et al., 2006; Nakagawa et al., 2007; Sutton et al., 2007; Wimmer et al., 2009; Schnurbusch et al., 2010; Tanaka et al., 2011; Miwa et al., 2013; Yang et al., 2013; Abreu et al., 2014; Chatterjee et al., 2014, 2017; Durbak et al., 2014; Hanaoka et al., 2014; Leonard et al., 2014; Pallotta et al., 2014; Liu et al., 2015; Fang et al., 2016; Matthes and Torres-Ruiz, 2016; Eggert and von Wirén, 2017; Sotta et al., 2017; Zhang et al., 2017; Duran et al., 2018; Routray et al., 2018; Shao et al., 2018; Diehn et al., 2019; Gómez-soto et al., 2019).
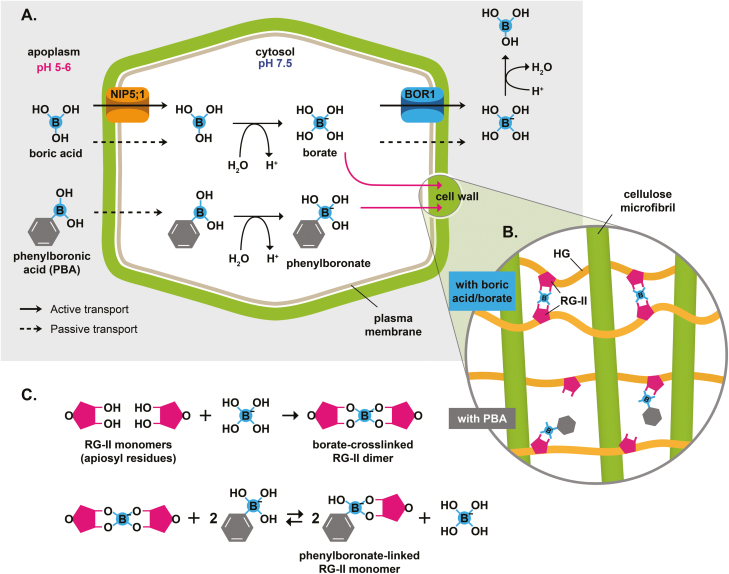
Fig. 1.
Boric acid (BA)/phenylboronic acid (PBA) uptake into plant cells. (A) Passive and active uptake mechanisms of BA and PBA through either diffusion or the NIP5;1 channel protein. Due to the cytosolic pH, BA and PBA are diverted into borate and phenylboronate anions, which react with the apiosyl residues of rhamnogalacturonan-II (RG-II) in the cell wall. Export of borate and phenylboronate out of a plant cell occurs via diffusion or the BOR1 export transporter. (B) Close-up of a cell wall pectin matrix with borate cross-linking two RG-II monomers (only the apiosyl residues are shown for simplicity) and phenylboronate interfering with the cross-linking by competitively binding to only one RG-II monomer. (C) Reaction of the borate anion and the phenylboronate anion with apiosyl residues of RG-II monomers.
B deficiency has negative consequences on plant growth, development, and performance. One of the earliest defects detected upon B deficiency is a cessation of growth at the growing tips, or meristems (groups of stem cells, that give rise to all above- and below-ground organs). This growth arrest due to a reduction in cell elongation and cell division (Dell and Huang, 1997; Poza-viejo et al., 2018), in both the root and the shoot, leads to a balanced reduction of the shoot to root biomass. This response is in contrast to other macronutrient deficiencies, for example nitrogen or phosphorus which lead to enhanced root growth, and therefore an increase in the root to shoot biomass (Hermans et al., 2006). While a general growth arrest has also been reported in plants subjected to many other nutrient deficiencies, a negative effect on meristematic tissues is known to occur for only a few others, including calcium, copper, iron, and zinc (Marschner, 2012; Mendoza-Cózatl et al., 2019). Therefore, understanding the molecular mechanisms underlying B deficiency is of interest not only to plant physiologists, but also to developmental biologists.
B has been shown to be functionally involved in the stability of the primary cell wall by cross-linking two of the rhamnogalacturonan-II (RG-II) pectic subunits via their apiosyl residues (Ishii and Matsunaga, 1996; Kobayashi et al., 1996; O’Neill et al., 1996) (Fig. 1B). As cell wall biosynthesis and stability have to be maintained for proper plant development, B has to be continuously delivered to growing tissues from the soil through the root and vascular tissues by directional cell to cell transport. The cross-linking of pectin subunits in the cell wall is so far the best understood, characterized, and, although recently challenged (Lewis, 2019), also the accepted primary role of B (Gonzales-Fontes, 2019; Wimmer et al., 2019). Additional roles for B have been proposed and demonstrated related to metabolism, membrane processes, and phytohormone signaling (discussed below), though it is difficult to separate primary and secondary effects of B deficiency (Blevins and Lukaszewski, 1998; Bolaños et al., 2004; Goldbach and Wimmer, 2007; Wimmer et al., 2009; Voxeur and Fry, 2014). Hypotheses regarding additional roles of B beyond the cell wall are, at least in part, founded on the observation that the amount of B that is localized in cell walls stays relatively constant with varying B concentration of the soil or media, while the amount of cytosolic B follows the B concentration of the soil, suggesting that the cytosolic B pool could be involved in early B deficiency reactions (Goldbach et al., 2000).
In the membrane, roles of B in influencing the function and the localization of plasma membrane (PM) proteins, transport processes across the membrane, and membrane integrity have been demonstrated (Goldbach and Wimmer, 2007; Wimmer et al., 2009; Voxeur and Fry, 2014; Matthes and Torres-Ruiz, 2016). The identification of membrane-associated, B-interacting proteins and the in vitro formation of a glycosylinositol phosphorylceramides (GIPC)–B–RG-II complex provide the first molecular explanations for a function of B in the stabilization of membranes/membrane domains and possible wall–membrane attachment sites (Wimmer et al., 2009; Voxeur and Fry, 2014). Although the experimental evidence of the physiological significance of the identified B membrane proteins and the in vivo existence of a GIPC–B–RG-II complex is missing, these studies provide pivotal insights into the biochemical basis for the B dependency of plant growth and development.
In recent years, progress in B research has been made in identifying and characterizing mutants with impaired B transport (Noguchi et al., 1997; Takano et al., 2006; Nakagawa et al., 2007; Tanaka et al., 2008; Miwa et al., 2013, 2014; Chatterjee et al., 2014, 2017; Durbak et al., 2014; Hanaoka et al., 2014; Leonard et al., 2014; Liu et al., 2015; Zhang et al., 2017; Shao et al., 2018), understanding the underlying mechanisms of B homeostasis and regulation (Sotta et al., 2017; Yoshinari and Takano, 2017; Yoshinari et al., 2019), development of B imaging techniques (Fukuda et al., 2018; Wang et al., 2018; Zhou et al., 2018; Housh et al., 2020), and studying possible interactions of B with phytohormones (Abreu et al., 2014; Camacho-Cristóbal et al., 2015; Li et al., 2015; Matthes and Torres-Ruiz, 2016; Zhou et al., 2016; Eggert and von Wirén, 2017; Poza-viejo et al., 2018; Gómez-soto et al., 2019). Additionally, B-mediated changes in plant water relations and implications in interactions of B with other abiotic factors, such as drought, is gaining interest in the B research community (Wimmer and Eichert, 2013; Macho-Rivero et al., 2017).
With this review, we evaluate differences and limitations of the published methodologies used to induce B deficiency, in the context of our current understanding of the effects of B deficiency on plant development.
B homeostasis through concerted action of boric acid facilitators and borate exporter proteins
The B requirement of plants is species specific, yet the range between deficient and toxic soil B concentrations is small (Goldberg, 1997). Therefore, plants regulate B homeostasis in order to maintain optimal B concentrations within their cells. Research in recent years has shed light on the molecular mechanisms that underly the maintenance of B homeostasis (as reviewed in Yoshinari and Takano, 2017). In short, plants control B uptake and transport through rapid regulation of two different classes of B transporters, namely BA channels of the major intrinsic protein (MIP) family and borate transporters of the BOR family (Fig. 1A).
For B to be used by the plant, it has to be (i) in a plant-accessible form, which is either the uncharged BA or the borate anion; and (ii) transported from the soil and through the roots to the shoot. B is taken up by the plant in the form of BA (Fig. 1A), which is the main form of B in solution at physiological pH (5–6). Within a plant cell, the cytosolic pH (7.5) leads to the formation of the borate anion, which readily interacts with apiosyl residues of RG-II in the cell wall (Fig. 1B). Since biological membranes are quite permeable for BA, passive uptake of BA via diffusion prevails when B concentrations are adequate or high (Raven, 1980; Dordas and Brown, 2000). The identification of a necessity for an active B transport mechanism (Dordas et al., 2000) was refined by the characterization of Arabidopsis mutants that require higher B concentrations in the growing medium (Noguchi et al., 1997; Takano et al., 2006). These mutant studies helped establish that under low soil B concentrations, BA is taken up by the plant with the help of BA channels of the MIP family and transported within the plant by borate exporters of the BOR family. The BA channels belong to the nodulin 26-like intrinsic protein (NIP) subfamily of MIPs. NIPs are further divided into the subclasses I, II, and III, of which the subclass II is important for BA uptake (Roberts and Routray, 2017). While Arabidopsis NIP5;1 (AtNIP5;1) transports B into the root from the soil (Fig. 1A), AtHIGH BORON REQUIRING1 (AtBOR1) is involved in the export of B out of the cell (Fig. 1A). Both of these transporter families show polar subcellular localization, which so far has only been shown in Arabidopsis. While AtBOR1 displays polar localization towards the inner/stele side in various root cell types, the hypocotyl, and cotyledons (Takano et al., 2010; Yoshinari et al., 2019), AtNIP5;1 shows polar localization towards the soil side in Arabidopsis root epidermal and root cap cells (Takano et al., 2010). Due to their polar localization, the concerted action of AtBOR1 and AtNIP5;1 leads to a directional, radial transport of B from the soil into the stele. From there, B is loaded into the xylem and transported through the transpiration stream to the shoot. To regulate B levels within a plant, the abundance of both transporter classes is highly controlled and depends on the cytosolic B concentration. While the abundance of AtNIP5;1 is primarily regulated via ribosome stalling and subsequent mRNA degradation (Tanaka et al., 2011, 2016), the abundance of AtBOR1 is regulated via degradation through ubiquitination and translational suppression (Takano et al., 2010; Aibara et al., 2018).
The regulation of B transport occurs on a fast time scale. Endocytotic AtBOR1 degradation is apparent 30 min after the transfer from low to high B medium and it is suggested that AtBOR1’s half-life is under an hour (Takano et al., 2005). On the other hand, AtNIP5;1 mRNA’s half-life is 10–15 min (Tanaka et al., 2011). Using a mathematical modeling approach, it was recently shown that such rapid (also termed ‘swift’ by Sotta et al., 2017) regulation of transporter abundance secures a constant B concentration within root cells. Delaying the transporter regulation swiftness in silico leads to (i) oscillations of B concentrations in the simulated cells; (ii) traffic jam-like increases of cellular B concentrations propagating back from the xylem to the soil against the B flow from the soil to the xylem; and (iii) a reduction in total B throughput through the tissue and ultimately reduced xylem loading of B. As the threshold for B toxicity is narrow, increases in B concentrations caused by such oscillations would lead to DNA damage and cell death. This rapid regulation of B transporter abundance therefore is suggested to be one mechanism by which plants avoid high intracellular B levels to limit B-induced damage and to maintain optimal constant B xylem loading rates over time (Sotta et al., 2017). Experimental data substantiating the occurrence of oscillations in B concentrations due to a lack of rapid regulation of B transporters would be a valuable contribution to support this modeling approach.
Other NIP and BOR family members have also been shown to be involved in either B uptake, distribution, or relocation, particularly in Arabidopsis. While AtNIP6;1 is involved in B transfer from the xylem to the phloem (Tanaka et al., 2008), AtNIP7;1 was shown to be a B uptake facilitator in developing anthers (Li et al., 2011). AtBOR1’s closest homolog, namely AtBOR2, as well as AtBOR4 were also shown to be involved in B export (Miwa et al., 2013, 2014).
B can be found in various locations in the plant cell including the cell wall, the cytoplasm, and the vacuole (Thellier et al., 1979; Dannel et al., 1998). Additional cellular locations of B, such as the PM, have also been demonstrated (Pollard et al., 1977; Cakmak and Römheld, 1997; Wimmer et al., 2009; Voxeur and Fry, 2014). While the majority of a plant’s B content is reported to be found in the cell wall bound to RG-II (Matoh et al., 1996), it is the cytosolic B content that varies, when B contents in the growth media are altered (Goldbach et al., 2000). Interestingly, the 5'-untranslated region (UTR) of AtNIP5;1 ‘responds’ to alterations in the cytosolic B content, and was therefore recently used to develop a cytosolic B sensor (Fukuda et al., 2018). To date, the cytosolic B content is suggested to be the sole site of B sensing, but detailed studies of varying B concentrations in other cellular locations are lacking. Water-soluble B (cytosolic B) is usually measured when reporting B contents/concentrations in plants (see Supplementary Table S1 at JXB online), and reports that quantify B–RG-II dimers together with B content/concentrations are scarce (Supplementary Table S1), but are necessary to elucidate a more complete picture of B’s cellular function(s) in plants.
How to study B deficiency
B is ubiquitously present, making it difficult to experimentally induce B deficiency in a controlled manner on a plant level. Typically there are three approaches that are used by the research community, namely (i) making use of particular mutants in either the BA import facilitators or the borate export proteins (as outlined in the first section); (ii) using chemicals, such as boronic acids [e.g. phenylboronic acid (PBA), Fig. 1] to mimic B deficiency; or (iii) transfer assays from B-containing medium onto medium with reduced B (either solid medium or hydroponic cultures). The mutant approach has successfully been used in various plant species, including Arabidopsis (Noguchi et al., 1997; Takano et al., 2006), maize (Chatterjee et al., 2014, 2017; Durbak et al., 2014; Leonard et al., 2014), Brassica (Zhang et al., 2017), and rice (Nakagawa et al., 2007; Hanaoka et al., 2014; Liu et al., 2015). Due to reduced uptake or distribution of B, these mutants typically have a reduced cytosolic B content and, for some of them, reduced B–RG-II cross-linking has been reported as well (Supplementary Table S1). Therefore, the mutant approach can be used to study the effects of the reduction of cytosolic B content as well as the effects of the reduction in B–RG-II cross-linking. An exception to this is the Atbor2 mutant, which was shown to not influence cytosolic B content, but rather solely interferes with B–RG-II cross-linking in the cell wall (Miwa et al., 2013) (Supplementary Table S1). Unfortunately, analysis of B–RG-II complexes in addition to cytosolic B content/concentration measurements has been reported in only a few studies (Noguchi et al., 2000, 2003; Miwa et al., 2013; Durbak et al., 2014) (Supplementary Table S1), yet would provide important data for elucidating potential cell wall-independent roles of B.
Limitations of using a mutant approach are that (i) deciphering cause versus consequence of B deficiency is difficult, since typically the end-product is examined, for example, the formation of a particular structure or organ; and (ii) phenotypes can be highly variable, especially when studying crops, due to non-standardized or non-controllable conditions in the field (Fig. 3, note asterisks in B, C, and E). In order to gain deeper insights into the primary roles of B on a cellular level, different approaches are needed. Recently, it has been suggested that chemical approaches, which interfere with RG-II dimerization, might provide an avenue for that. Suitable chemicals are, for example, boronic acids (Bassil et al., 2004; Matthes and Torres-Ruiz, 2017) or inhibitors of fucosylation of cell wall components (Dumont et al., 2015), since they can interfere with specific biochemical processes in a transient way and therefore allow a precise induction of B deficiency in a timely manner. Boronic acids, such as PBA, interfere with BA’s capability to bind and cross-link cis-diol groups, specifically RG-II in the cell wall (Bassil et al., 2004) (Fig. 1B, C). They interfere in this process by only binding two cis-diols and, therefore, inhibiting the cross-linking of two RG-II molecules in the cell wall (Fig. 1B, C). Boronic acids have so far been used to induce B deficiency in tobacco (Bassil et al., 2004), petunia (Bassil et al., 2004), Arabidopsis (Matthes and Torres-Ruiz, 2016; Duran et al., 2018), and apple (Fang et al., 2016). The chemical approach will lead to an increase in cytosolic B content, rather than a decrease, as boronic acids will still deliver B into the cell (Duran et al., 2018) and B deficiency-like symptoms are assumed to be solely due to an interference with cell wall cross-linking (Bassil et al., 2004; Matthes and Torres-Ruiz, 2016). However, direct effects of boronic acids on transcription or other processes/molecules that offer cis-diols have not been excluded. Additionally, boronic acids have been introduced as small-molecule auxin inhibitors that target YUCCA auxin biosynthesis enzymes (Kakei et al., 2015), which might provide evidence of a direct interaction between B and auxin function which is disrupted by the boronic acid. Alternatively, boronic acids might not be specific to inducing B deficiency which should be a target of future research. By combining a mutant and a chemical approach, a dissection of the effects of cytosolic B deficiency versus disruption of cell wall stability/integrity might be possible.
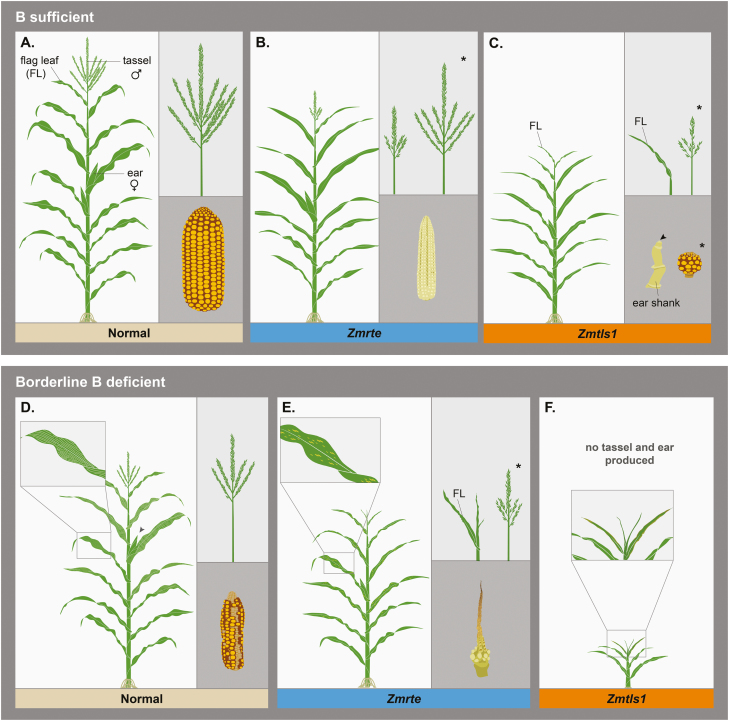
Fig. 3.
Phenotypes of normal maize plants, and Zmrte (co-ortholog of Atbor1) and Zmtls1 (co-ortholog of Atnip5;1) transporter mutants, when grown under B-sufficient and B-deficient conditions. Vegetative and reproductive phenotypes, when grown under B-sufficient conditions of (A) normal maize plants, (B) Zmrte, and (C) Zmtls1 (note that the arrowhead points to a missing ear; depicted is only the ear shank, a structure typically subtending an ear). Vegetative and reproductive phenotypes, when grown under borderline B-deficient conditions of (D) normal maize plants, (E) Zmrte, and (F) Zmtls1. The asterisk indicates phenotypic variability. FL=flag leaf; male and female signs indicate a male and female inflorescence, respectively.
In the third approach, transfer assays make use of solution cultures, where plants are initially grown with adequate levels of B and afterwards transferred to conditions with an insufficient amount of B. The plant’s response to B withdrawal is measured. Solution cultures are performed in either solid or liquid medium. In contrast to conventional solution cultures, B-free medium is used to which specific amounts of B are subsequently added. In order to obtain B-free medium, the use of glassware is avoided in the preparation of buffers, and B-chelating resins such as Amberlite® IRA743 are used (Bell et al., 2002). The use of Amberlite® IRA743 additionally allows buffering of B in the nutrient solution (Asad et al., 1997) and is reported to maintain B concentrations over multiple days (Huang et al., 1999), which is not the case in conventional solution cultures, where nutrient concentrations may vary due to infrequent renewal of the culture medium. The effectiveness of Amberlite® IRA743 in maintaining B concentrations depends on various factors and therefore should be determined for each experimental set-up. Like the chemical approach, the analysis of short periods of B withdrawal in B-buffered solution cultures using Amberlite® IRA743 allows the study of primary or early secondary effects upon B deficiency. In combination with a mutant approach, B-buffered solution cultures are therefore a powerful technique for controlled investigations on B nutrition.
In addition to the aforementioned approaches to induce B deficiency, there are several breeding approaches and strategies that are being applied or tested to enhance B availability and uptake in plants, which add to the techniques to study B nutrition and B deficiency in plants. Among those techniques are methods to modify root traits, grafting of suitable rootstocks, application of biostimulators (materials other than fertilizers that influence plant metabolic processes) or nano-fertilizers, and inoculation with mycorrhizal fungi or plant growth-promoting rhizobacteria, as reviewed in Shireen et al. (2018). In addition, transgenic approaches using B transport proteins appear promising for enhancing crop productivity under both B deficiency and toxicity conditions (Miwa et al., 2006, 2007; Kato et al., 2009; Takada et al., 2014; Uraguchi et al., 2014; Wakuta et al., 2016).
Effects of B deficiency on plant development
The study of B deficiency shows that B is necessary for proper plant development, as B deficiency leads to root, leaf, flower, and meristem defects in various species, including Arabidopsis, maize, and rice (Figs 2, 3). Reports on the absolute internal and external B requirements for a given plant species (Hu et al., 1996; Bell et al., 2002; Lordkaew et al., 2011; Eggert and von Wirén, 2017) point out several common findings: (i) B requirements for growing organs exceed the requirement of mature organs; (ii) B requirements in reproductive plant parts are higher than in vegetative plant parts; and (iii) internal B requirements appear to be highly species specific, which seems to be correlated with the composition of the primary cell wall (Hu et al., 1996). The higher B requirement of reproductive tissues might explain why reproductive growth is particularly sensitive to B limitation and the fact that seed yield reductions have been reported without any other symptoms during vegetative growth (Dell and Huang, 1997; Lordkaew et al., 2011).
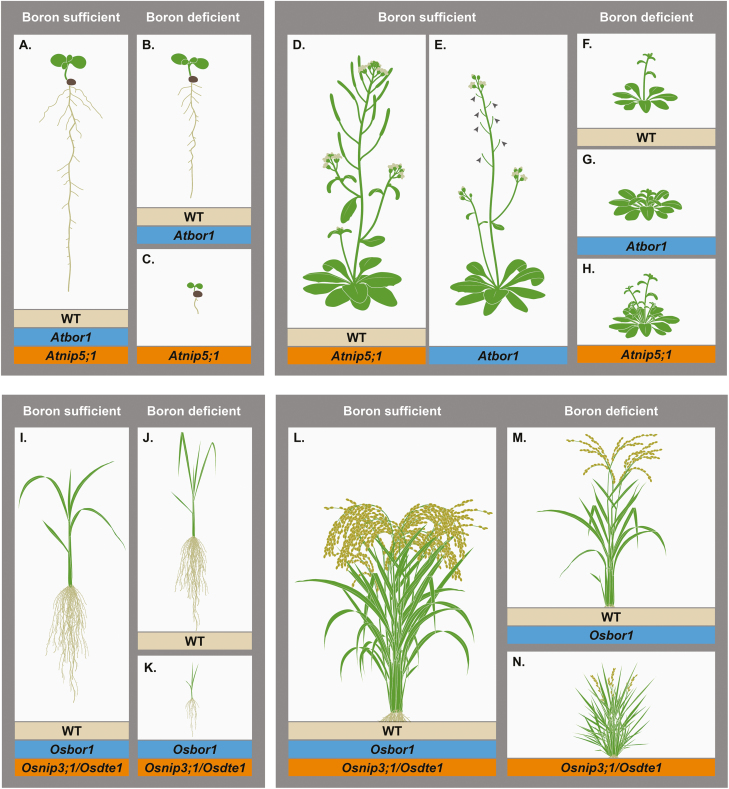
Fig. 2.
Phenotypes of the Arabidopsis and rice bor1 and nip5;1 transporter mutants when grown under B-sufficient and B-deficient conditions. Root phenotypes, when grown under B-sufficient conditions of (A) wild-type Arabidopsis (WT), Atnip5;1, and Atbor1. Root phenotypes, when grown under B-deficient conditions of (B) WT, Atbor1 and (C) Atnip5;1. Reproductive phenotypes, when grown under B-sufficient conditions of (D) WT, Atnip5;1 and (E) Atbor1 (arrowheads depict reduced seed set due to female sterility). Reproductive phenotypes, when grown under B-deficient conditions of (F) WT, (G) Atbor1, and (H) Atnip5;1. (I) Vegetative phenotypes, when grown under B-sufficient conditions of WT rice, Osbor1, and Osdte1/Osnip3;1 mutants. Vegetative phenotypes, when grown under B-deficient conditions of (J) WT rice, and (K) Osbor1 and Osdte/Osnip3;1. (L) Reproductive phenotypes, when grown under B-sufficient conditions of WT rice, Osbor1, and Osdte1/Osnip3;1. Reproductive phenotypes, when grown under B-deficient conditions of (M) WT rice, Osbor1, and (N) Osdte1/Osnip3;1.
Interestingly, mutant phenotypes of characterized B transporters only partially match up with the phenotypes observed when normal plants are grown under B deficiency (Figs 2, 3). For example, wild-type (WT) Arabidopsis plants grown without any B (hydroponic culture: no added B) retain apical dominance, and exhibit severe defects in rosette leaf expansion (Noguchi et al., 1997) (Fig. 2F). When grown under B-deficient conditions (solid medium: 0.1 µM B), WT Arabidopsis plants show only a moderate reduction in primary root length and still develop lateral roots (Takano et al., 2006) (Fig. 2B). In contrast, Atbor1 mutants display severe repression of apical dominance and a moderate reduction in the expansion of the rosette leaves, when grown under B-deficient conditions (hydroponic culture: 3 µM B) (Fig. 2G). Root growth is only altered once leaf growth becomes severely affected (Noguchi et al., 1997). In addition, Atbor1 fails to set seed due to female sterility (Noguchi et al., 1997) (Fig. 2E; note arrowheads that point to sterile flowers) when grown under B-sufficient conditions (hydroponic culture: 30 µM B). On the other hand, Atnip5;1 mutants display severe root defects when grown under B-deficient conditions (hydroponic culture: 3 µM B) (Fig. 2C) including a cessation of primary root growth, absence of lateral roots, an increase in root hair density, no further expansion of the rosette leaves, and a striking inhibition of the development of flowers and siliques (Takano et al., 2006) (Fig. 2C, H). The ‘nude’ root phenotype (no lateral roots) and a reduction in expansion of the rosette leaves persist with the addition of moderate B levels. With adequate B levels (hydroponic culture: 30 µM B), the Atnip5;1 mutant does not display any developmental phenotypes and grows similarly to WT Arabidopsis accessions (Takano et al., 2006) (Fig. 2D). In conclusion, Atbor1 and Atnip5 mutants have different phenotypes from each other that are more severe than those of WT Arabidopsis plants grown in B-deficient conditions (Fig. 2).
Young rice seedlings do not show any plant height or root lengths defects when grown under B deficiency (hydroponics: 0.03 µM B) for <4 weeks (Uraguchi and Fujiwara, 2011). Prolonged growth under B deficiency (hydroponics: 0.18 µM B or 0.03 µM B) leads to a reduction in root length, plant height, and grain yield, due to low fertility and reduction in the number of panicles (inflorescence of rice) and spikelets (flower-harboring structure in grasses) (Uraguchi and Fujiwara, 2011) (Fig. 2J–M). T-DNA insertion lines in the rice ortholog of AtBOR1, namely Osbor1, show severe reductions in both shoot and root growth in comparison with WT rice plants when grown for 4 weeks under B-deficient conditions (hydroponics: 0 µM B) (Nakagawa et al., 2007) (Fig. 2K). OsNIP3;1/DWARF AND TILLER ENHANCING1 (DTE1) is the rice homolog of AtNIP5;1 (Hanaoka et al., 2014; Liu et al., 2015). When grown under B-sufficient conditions (hydroponics: 18 µM B), Osnip3;1/Osdte1 RNAi knockdown lines do not differ morphologically from WT rice, whereas when grown for 3 weeks under B-deficient conditions (hydroponics: 0 µM B), Osnip3;1/Osdte1 RNAi lines showed severe shoot growth reduction in contrast to WT rice (Fig. 2K) (Hanaoka et al., 2014). Under low B field conditions, Osnip3;1/Osdte1 mutants show enhanced tillering, a reduction in plant height, reduced seed set, and reduced pollen viability (Fig. 2N), while B-sufficient field conditions do not lead to any obvious growth defects of Osnip3;1/Osdte1 in comparison with WT rice (Liu et al., 2015).
In maize, B deficiency negatively influences yield, yet, in comparison with other cereals, maize has been reported to have a relatively low B requirement for maximum yield (Martens and Westermann, 1991). When grown under B deficiency (e.g. in sand without additional B), no vegetative phenotypes are seen in normal maize plants, although B concentrations can already be significantly reduced within the plant (Lordkaew et al., 2011). When entering the reproductive stage, the upper leaves exhibit translucent streaks (Fig. 3D) and plants produce multiple ears (female inflorescence in maize), that might harbor a tassel-like (male inflorescence in maize) structure, with few short silks (stigmas of maize ears). In addition, tassels are visibly smaller (Fig. 3D), with small, shriveled anthers, that can in extreme cases be devoid of pollen (Lordkaew et al., 2011). Similar phenotypes are seen in maize mutants co-orthologous to Atbor1 and Atnip5;1, namely Zmrotten ear (Zmrte) (Chatterjee et al., 2014, 2017) and Zmtassel-less1 (Zmtls1) (Durbak et al., 2014; Leonard et al., 2014; Matthes et al., 2018). Zmrte shows defects in both male and female reproductive development (Fig. 3B, E). In severe cases, tassels are shorter and less branched with no spikelets (Fig. 3E). Severe ear defects include being shorter, shrunken, withered, and not producing mature spikelets (Chatterjee et al., 2014). Zmrte phenotypes are variable depending on the genetic background and the B content in the soil, so that both tassel and ear can appear rather normal (note the asterisk in Fig. 3B), but are always sterile irrespective of B concentration in the soil (Fig. 3B, E). Zmrte also displays loss of apical dominance and shows occasional necrotic lesions on leaves, and shorter, discolored, or wrinkly leaves (Chatterjee et al., 2014) (Fig. 3E). The vegetative leaf phenotypes are different in morphology from the reported transparent streaks on leaves, when normal maize is grown in sand without added B (Lordkaew et al., 2011) (compare Fig. 3D and E). The defects seen in Zmrte mutants, grown under B-deficient conditions, are enhanced when the action of its duplicated gene Zmrte2 is also impaired (Chatterjee et al., 2017). Zmrte;rte2 double mutants produce only few leaves and die after a few weeks when grown in B-deficient soil (Chatterjee et al., 2017). The severe vegetative defect is reminiscent of Zmtls1 mutants, when grown under borderline B-deficient conditions (Durbak et al., 2014) (Fig. 3F), where Zmtls1 mutants develop only 4–8 narrow, stiff leaves, and die at the seedling stage (Durbak et al., 2014; Matthes et al., 2018). A similarly severe vegetative phenotype can be obtained by growing normal maize in a nutrient solution without added B (Eltinge, 1936). In contrast to reported Zmrte or Zmtls1 phenotypes, the first visible defect in normal maize grown in nutrient solution without added B is a yellow discoloration between the veins of older leaves and an unrolling of the youngest visible leaf (Eltinge, 1936). In B-rich soils, Zmtls1 displays variable reproductive defects, including not developing a tassel or producing a highly reduced tassel (Fig. 3C; note asterisk). In addition, the ear is either completely absent or short and ball-shaped (Fig. 3C). While both Zmtls1 and Zmrte can develop multiple ears on the same node like normal maize grown in sand without added B, neither mutant has been reported to develop a tassel instead of an ear.
The study of developmental and growth defects in different species under B deficiency shows interesting differences in phenotypes and in regulation of B transporters. Such differences might indicate that regulatory responses upon B deficiency are species specific, therefore necessitating future research efforts for B regulation in various species, from both a physiological and a developmental point of view. Examples for species-specific differences are: (i) Zmrte shows defects in both male and female reproductive development, which is different from the Atbor1 mutant; (ii) the root phenotype of the Osbor1 T-DNA insertion lines is in contrast to both Atbor1 and Zmrte mutants, which do not show strong root phenotypes; (iii) OsBOR1 is involved in xylem loading, but, unlike AtBOR1, is also functionally important for B uptake into roots (Nakagawa et al., 2007); and (iv) OsBOR1’s role in xylem loading is independent of the B conditions, whereas AtBOR1 is reported to be less important under B-sufficient conditions (Nakagawa et al., 2007). It has to be noted, though, that AtBOR1’s role under B-sufficient conditions might have been overlooked, as B concentrations in the Atbor1 mutant are severely reduced specifically in the upper portion of the inflorescence even under B-sufficient conditions (hydroponics: 30 µM B) (Noguchi et al., 1997). (v) While WT rice plants typically accumulate B in the leaf sheath in comparison with the leaf blade, the Osnip3;1 RNAi knockdown mutant accumulates more B in the leaf blades compared with the leaf sheath (Hanaoka et al., 2014). Indeed, it was recently shown that OsNIP3;1 is not involved in B uptake or translocation, but rather in preferentially delivering B to developing tissues (Shao et al., 2018).
It was recently shown by using a chemical approach with PBA that B deficiency leads to very specific developmental defects when applied during Arabidopsis embryogenesis. The application of PBA was shown to specifically lead to the disruption of the asymmetric cell division of the hypophysis (the precursor cell for the root apical meristem; RAM) (Matthes and Torres-Ruiz, 2016). Consequently, RAM formation was blocked, leading to phenocopies of the AtAUXIN RESPONSE FACTOR 5/MONOPTEROS (mp) mutants. Atmp mutants fail to perform the correct cell division of the hypophysis and give rise to seedlings with no primary root (Berleth and Jürgens, 1993). The primary defect upon PBA treatment in Arabidopsis embryos was hypothesized to be a transient delocalization of the polarly localized auxin efflux carrier AtPINFORMED1 (PIN1), leading to a disruption of the directional flow of the phytohormone auxin. The very specific induction of Atmp phenocopies upon inducing B deficiency in Arabidopsis embryos suggests an interaction of B with auxin and adds data to support the long-standing hypothesis of additional roles of B beyond its cell wall function (Brown et al., 2002).
Interactions of B and hormones
Potential interactions between B and in particular the hormones auxin, cytokinin (CK), ethylene, and abscisic acid (ABA) have been hypothesized (Blevins and Lukaszewski, 1998; Wang et al., 2006; Abreu et al., 2014; Li et al., 2015; Matthes and Torres-Ruiz, 2016; Macho-Rivero et al., 2017; Poza-viejo et al., 2018; Gómez-soto et al., 2019). While direct evidence for such interactions is missing, they would lead to the elucidation of additional roles of B beyond the cell wall and help determine if hormonal defects are a secondary effect of an altered cell wall and consequently an altered morphology, or if B interacts with hormones directly. Specifically, there is a long-standing interest in studying interactions of B with auxin, as B deprivation was shown to cause alterations of levels of the main active auxin indole 3-acetic acid (IAA) as reviewed in Blevins and Lukaszewski (1998). These alterations are hypothesized to be due to (i) enhancement of the activity of IAA oxidase, an enzyme involved in the catabolism of IAA (Jarvis et al., 1983); or (ii) the involvement of B in auxin transport (Li et al., 2001; Wang et al., 2006; Li et al., 2015; Matthes and Torres-Ruiz, 2016). Interestingly, numerous studies in various plant species have either directly or indirectly shown alterations of auxin levels upon B deficiency, but with different results (Blevins and Lukaszewski, 1998; Martín-Rejano et al., 2011; Li et al., 2015; Matthes and Torres-Ruiz, 2016; Zhou et al., 2016; Eggert and von Wirén, 2017), hindering a general reasoning of the relationship between B deficiency and hormones, such as auxin. Reasons for such potential discrepancies could be attributed to different levels of B deficiencies, different organs being analyzed, and different methods of inducing B deficiency. This indicates not only the necessity for standardized methodologies, but also the necessity of reporting B deficiency symptoms/effects in defined cellular locations, tissues, and developmental stages in order to make studies comparable and reproducible.
A recent study in Brassica seedlings (Eggert and von Wirén, 2017), which are highly sensitive to B deficiency (Zhang et al., 2014), highlights this importance. Eggert and von Wirén (2017) took a physiological approach, where they simultaneously measured active phytohormone species, including their precursors and catabolites, in association with the shoot B nutritional status to detect B-dependent growth responses. This study revealed that until the presence of B deficiency symptoms are manifest (Fig. 4; note the asterisk), IAA levels slightly decrease, but afterwards the concentration of all tested auxin species increased with progressing B deficiency (Eggert and von Wirén, 2017) (Fig. 4). When crossing a certain threshold of B deficiency, however, IAA levels significantly dropped, although they were still higher than under B-sufficient conditions (Fig. 4). The authors used these opposing behaviors of IAA levels depending on the B nutritional status of a plant to explain contradictions between their study and the study by Zhou et al. (2016), which reported reduction in shoot IAA concentrations in Brassica under low B supply.
Fig. 4.
Trends of IAA, ABA, and CK levels with increasing B deficiency in Brassica napus plants. Modified from Eggert and von Wirén (2017) with permission.
Contradictions about the level of IAA in B-deficient conditions have also been reported in Arabidopsis (Li et al., 2015; Matthes and Torres-Ruiz, 2016). For example, by using the synthetic auxin signaling marker DR5:GFP (green fluorescent protein) in Arabidopsis embryos, a reduction of GFP in the RAM was observed upon PBA application to siliques (Matthes and Torres-Ruiz, 2016), indicating reduced auxin levels in the embryonic RAM upon B deficiency. A different study suggested an overaccumulation of auxin in the Arabidopsis root stele, as detected by the absence of the DII-Venus reporter line, which is degraded when auxin is present (Li et al., 2015). In this study, seedlings were grown on agar plates with different B concentrations (normal, 30 µM B; and low, 0.1 µM B) and analyzed 10 d after sowing. While likely reasons for the opposing changes in IAA levels between the two studies are (i) differences in the methods of inducing B deficiency and (ii) differences in the tissue analyzed, it is possible that the observed differences between the two studies lies in the difference of B nutritional status within the plants, as suggested by Eggert and von Wirén (2017). Both Arabidopsis studies nevertheless point to effects of B deficiency on polar auxin transport and the PIN proteins, particularly AtPIN1. Although the effect of B deficiency on AtPIN1 leads to different outcomes in the different tissues/cells (overaccumulation of auxin versus a decrease of auxin levels), these findings open up the possibility that AtPIN1 protein abundance/localization might be one of the first targets upon B deficiency. Although direct evidence for this speculation is missing, it is supported by the observation that other nutrient deficiencies, such as of manganese or calcium, do not affect the abundance of AtPIN1 (Li et al., 2015). Another hypothesis is that the effect of AtPIN1 abundance and localization upon B deficiency might be secondary due to the modulation of other hormones such as CK, for example (Li et al., 2015). Elucidating cause versus consequence between B deficiency and IAA metabolism/transport remains a subject for future research.
Although interactions between CK and B status are not as widely explored as those with B and auxin, there are a number of reports demonstrating an involvement of CK in the regulation of the B stress response in plants. In Pisum sativum (pea) shoots, a reduction of CK was reported, when grown under B deficiency (hydroponics: 0 µM added B) (Li et al., 2001). In Arabidopsis, it was observed that a CK receptor gene, CYTOKININ RESPONSE 1 (CRE1)/WOODEN LEG (WOL)/ARABIDOPSIS HISTIDINE KINASE4 (AHK4), is down-regulated upon B deficiency and this down-regulation is correlated with a phenotype of abnormal protoxylem differentiation, proposed to be due to failure in the transition from cell division to cell differentiation (Abreu et al., 2014). A dependence of cytokinin receptor expression on the B nutritional status was also shown in navel oranges (Citrus sinensis Osb.) (Yang et al., 2013). Interestingly, the cytokinin receptor gene WOL was up-regulated at all time points of increasing B deficiency when compared with the control conditions. The analytical approach in Brassica, that was mentioned earlier (Eggert and von Wirén, 2017), revealed that the active CK forms isopentenyladenine and cis-zeatin, as well as the precursors and transport forms, isopentenyladenine riboside and cis-zeatin riboside, significantly decreased with increasing B deficiency in Brassica shoots (Eggert and von Wirén, 2017) (Fig. 4). It will be interesting to determine in the future whether the expression of the receptor genes depends on CK levels, which would allow the hypothesis that the transcriptional response of the CK receptors might be a secondary response of the down-regulation of CK levels upon B deficiency. In support of this hypothesis, recent work by Poza-viejo et al. (2018) showed increases in CK signaling components as early events after Arabidopsis seedlings had been transferred to B deficiency conditions (solid medium; 0.1 µM B).
An interaction of B with various steps in phytohormone pathways is also highlighted by results from (i) transcriptome studies in B deficiency mutants (–/+ B) versus normal plants or normal plants growing under B-sufficient conditions and then transferred onto B-deficient conditions; and (ii) from the similarity of B-deficient and hormone-deficient phenotypes. For example, transcriptome studies in maize seedlings of the Zmtls1 mutant revealed that MADS box transcription factors as well as genes involved in the auxin pathway are enriched in untreated Zmtls1 plants in comparison with untreated normal plants or B-treated Zmtls1 mutants (Leonard et al., 2014). In addition, some weaker alleles of Zmtls1 show enhanced tillering, resembling auxin biosynthesis mutants in maize (Durbak et al., 2014).
A novel approach to understanding the interactions between B and various phytohormones comes from a study that analyzed the hormone-responsive motifs in the AtNIP5;1 promoter (pAtNIP5;1) (Gómez-soto et al., 2019). pAtNIP5;1 was shown to harbor multiple cis-related regulatory elements associated with ABA, auxins, CK, salicylic acid, ethylene, gibberellins, and brassinosteroids, suggesting hormonal control of AtNIP5;1 expression. Indeed, the authors showed induction of AtNIP5;1 expression with ABA and the ethylene precursor 1-aminocyclopropane-1-carboxylic acid in different parts of the Arabidopsis root especially under B deficiency (Gómez-soto et al., 2019). On the contrary, auxin as well as blocking auxin export most prominently induced AtNIP5;1 when transferred from B-deficient (solid medium: no added B) to control conditions. While the addition of CK did not alter AtNIP5;1 expression, inhibiting CRE1 led to an induction of AtNIP5;1 expression when seedlings were transferred to B-deficient conditions (solid medium: no added B) (Gómez-soto et al., 2019). The findings in this study are interesting, because the induction of AtNIP5;1 expression by ABA correlated with an increased B uptake (while the induction of AtNIP5;1 expression by ethylene did not). The correlation between ABA-induced AtNIP5;1 expression and increased B uptake was speculated to function as an early B stress response. Indications of B interactions with ABA initially came from B toxicity studies, where it was shown that upon B toxicity, ABA content in the shoot increased, which was correlated with an increased expression of the ABA biosynthesis gene AtNINE-CIS-EPOXYCAROTENOID DIOXYGENASE 3, in the root (Macho-Rivero et al., 2017). It was also recently shown that ABA and its glycosylester both increased upon severe B deficiency in Brassica (Eggert and von Wirén, 2017) (Fig. 4).
In addition, recent studies indicate the participation of a signaling pathway involving auxin and ethylene in the response of root growth to B deficiency (Camacho-Cristóbal et al., 2015) though it remains challenging to unequivocally distinguish between primary and secondary effects. Approaches to separating out the complexity of primary and secondary effects of B deficiency include the analysis of hormone mutants under short-term B deprivation in solution culture or using a chemical approach by adding hormones to the solution culture and analyzing their effects on plant development under B deficiency (Camacho-Cristóbal et al., 2015; Li et al., 2015; Poza-viejo et al., 2018; Gómez-soto et al., 2019).
Conclusion and future directions
Significant progress has been made over the last few years particularly in understanding how B homeostasis is achieved/maintained and how/why B deficiency and toxicity affect plant development. Nevertheless, the understanding of the regulatory mechanisms underlying B deficiency is still incomplete. This is in part due to non-standardized methodologies for inducing B deficiency or toxicity, yet standardized methodologies are urgently needed in order to make experiments between groups comparable and repeatable.
To date, B transporters and channels have been associated with regulating B homeostasis in plants, yet their mutant phenotypes do not completely match up with phenotypes observed when normal plants are grown under B deficiency, as outlined above. These observations have been attributed to cell-specific transporter functions and might further indicate that there are additional genes responsible for regulating the B homeostasis in plants. The characterization of mutants that can tolerate low B concentrations in the soil, yet are not B transporters, such as the low boron tolerance (lbt) mutant in Arabidopsis (Huai et al., 2018) or mutants that interfere with the RG-II cross-linking, such as murus1 (O’Neill et al., 2001) or the GDP-sugar transporter mutant gonst3/gglt1 (Sechet et al., 2018), point to a more complex B regulation in plants than currently understood. These findings necessitate future research to elucidate additional molecular players, which will close gaps in our current understanding of how B functions within a plant.
One of the least understood factors in B research is how B is sensed in a cell. While studies with AtNIP5;1 and specifically its B-dependent regulation suggest that B sensing happens through detection of the intercellular B concentrations, there are several observations that challenge a sensing mechanism that is solely based upon the detection of the intercellular B concentrations. Studies on BOR1–GFP Arabidopsis plants using PBA and BA in the same medium show a strong localization of BOR1–GFP at the PM (Matthes and Torres-Ruiz, 2016), which allows the speculation that B is at least in part sensed at the cell wall, since cytosolic BA levels are still high yet BOR1–GFP is retained at the PM, instead of being degraded. It cannot be excluded that cells employ multiple B-sensing mechanisms, the detection and molecular basis of which should be the focus of future research in the field.
B sensing and deciphering the additional roles of B is still challenging, because subcellular visualization of B or B–RG-II within plants remains demanding, mainly due to the low abundance of B that falls below typical detection levels. Instrumental in tackling these challenges will be the recent development of a B sensor in Arabidopsis, based on the AtNIP5;1 5'-UTR that responds to cytosolic B concentrations, which allows visualization of cytosolic B content at a cellular resolution (Fukuda et al., 2018). Additional recent advances in B imaging include the development of an RG-II antibody (Zhou et al., 2018), the employment of quantitative neutron capture radiography on developing maize meristems (Wang et al., 2018), and the development of a B radiotracer based on a PBA derivative (Housh et al., 2020)
Soon the discovery of the essentiality of B will celebrate its 100th anniversary (Warington, 1923), and we still lack a detailed understanding of how B shapes morphological processes. In particular, the dissection of the complexity between primary and secondary responses of B withdrawal on plant development needs in-depth characterization. Treatments that exclusively disrupt meristem growth or B–RG-II formation should be tested and the effects on subsequent hormonal regulation should be contrasted with B deficiency effects. Although B is still the least understood micronutrient, its importance in agriculture continues to advance research into this perplexing element.
Supplementary data
Supplementary data are available at JXB online.
Table S1. B concentration and B-RGII cross-linking in various B transporter mutants under B-deficient and B-sufficient conditions
Acknowledgements
Boron research in the McSteen lab is supported by the Agriculture and Food Research Initiative Grant 2015-06592 from the USDA National Institute of Food and Agriculture and a University of Missouri, Bond Life Sciences Center, Early Concept Grant to PM and MSM. We are indebted to Norman Best and Ruediger Matthes for critical assessment of the manuscript, and to Dale Blevins for valuable discussions. In addition, we thank the anonymous reviewers for their insightful comments. We apologize to colleagues whose work we could not cite due to page restrictions, and declare no conflict of interest.
Glossary
Abbreviations:
- ABA
abscisic acid
- AHK4
ARABIDOPSIS HISTIDINE KINASE4
- B
boron
- BA
boric acid
- BOR1
high boron requiring1
- CK
cytokinin
- CRE1
CYTOKININ RESPONSE1
- DTE
dwarf and tiller enhancing1
- GFP
green fluorescent protein
- GIPC
glycosylinositol phophorylceramide
- IAA
indole 3-acetic acid
- MIP
major intrinsic protein
- MP
MONOPTEROS
- NIP
nodulin 26-like intrinsic protein
- PBA
phenylboronic acid
- PIN1
PINFORMED1
- PM
plasma membrane
- RAM
root apical meristem
- RG-II
rhamnogalacturonan-II
- rte
rottenear
- SAM
shoot apical meristem
- tls1
tassel-less1
- UTR
untranslated region
- WOL
WOODEN LEG
- WT
wild type
References
- Abreu I, Poza L, Bonilla I, Bolaños L. 2014. Boron deficiency results in early repression of a cytokinin receptor gene and abnormal cell differentiation in the apical root meristem of Arabidopsis thaliana. Plant Physiology and Biochemistry 77, 117–121. [DOI] [PubMed] [Google Scholar]
- Aibara I, Hirai T, Kasai K, Takano J, Onouchi H, Naito S, Fujiwara T, Miwa K. 2018. Boron-dependent translational suppression of the borate exporter BOR1 contributes to the avoidance of boron toxicity. Plant Physiology 177, 759–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asad A, Bell RW, Dell B, Huang L. 1997. Development of a boron buffered solution culture system for controlled studies of plant boron nutrition. Plant and Soil 188, 21–32. [Google Scholar]
- Bassil E, Hu H, Brown PH. 2004. Use of phenylboronic acids to investigate boron function in plants. Possible role of boron in transvacuolar cytoplasmic strands and cell-to-wall adhesion. Plant Physiology 136, 3383–3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell R, Dell B, Huang L. 2002. Boron requirements of plants. In: Goldbach H, ed. Boron in plant and animal nutrition. New York: Kluwer Academic/Plenum Publishers, 63–84. [Google Scholar]
- Berleth T, Jürgens G. 1993. The role of the monopteros gene in organising the basal body region of the Arabidopsis embryo. Development 118, 575–587. [Google Scholar]
- Blevins DG, Lukaszewski KM. 1998. Boron in plant structure and function. Annual Review of Plant Physiology and Plant Molecular Biology 49, 481–500. [DOI] [PubMed] [Google Scholar]
- Bolaños L, Lukaszewski K, Bonilla I, Blevins D. 2004. Why boron? Plant Physiology and Biochemistry 42, 907–912. [DOI] [PubMed] [Google Scholar]
- Brown PH, Bellaloui N, Wimmer MA, Bassil ES, Ruiz J, Hu H, Pfeffer H, Dannel F, Römheld V. 2002. Boron in plant biology. Plant Biology 4, 205–223. [Google Scholar]
- Cakmak I, Römheld V. 1997. Boron deficiency-induced impairments of cellular functions in plants. Plant and Soil 193, 71–83. [Google Scholar]
- Camacho-Cristóbal JJ, Martín-Rejano EM, Herrera-Rodríguez MB, Navarro-Gochicoa MT, Rexach J, González-Fontes A. 2015. Boron deficiency inhibits root cell elongation via an ethylene/auxin/ROS-dependent pathway in Arabidopsis seedlings. Journal of Experimental Botany 66, 3831–3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Liu Q, Menello C, Galli M, Gallavotti A. 2017. The combined action of duplicated boron transporters is required for maize growth in boron-deficient conditions. Genetics 206, 2041–2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee M, Tabi Z, Galli M, Malcomber S, Buck A, Muszynski M, Gallavotti A. 2014. The boron efflux transporter ROTTEN EAR is required for maize inflorescence development and fertility. The Plant Cell 26, 2962–2977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dannel F, Pfeffer H, Römheld V. 1998. Compartmentation of boron in roots and leaves of sunflower as affected by boron supply. Journal of Plant Physiology 153, 615–622. [Google Scholar]
- Dell B, Huang L. 1997. Physiological response of plants to low boron. Plant and Soil 193, 103–120. [Google Scholar]
- Diehn TA, Bienert MD, Pommerrenig B, et al. 2019. Boron demanding tissues of Brassica napus express specific sets of functional Nodulin26-like Intrinsic Proteins and BOR1 transporters. The Plant Journal 100, 68–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dordas C, Brown P. 2000. Membrane biology permeability of boric acid across lipid bilayers and factors affecting it. Journal of Membrane Biology 105, 95–105. [DOI] [PubMed] [Google Scholar]
- Dordas C, Chrispeels MJ, Brown PH. 2000. Permeability and channel-mediated transport of boric acid across membrane vesicles isolated from squash roots. Plant Physiology 124, 1349–1362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumont M, Lehner A, Bardor M, Burel C, Vauzeilles B, Lerouxel O, Anderson CT, Mollet J, Lerouge P. 2015. Inhibition of fucosylation of cell wall components by 2-fluoro 2-deoxy-l-fucose induces defects in root cell elongation. The Plant Journal 84, 1137–1151. [DOI] [PubMed] [Google Scholar]
- Duran C, Arce P, Felipe J. 2018. Methylboronic acid fertilization alleviates boron deficiency symptoms in Arabidopsis thaliana. Planta 248, 221–229. [DOI] [PubMed] [Google Scholar]
- Durbak AR, Phillips KA, Pike S, O’Neill MA, Mares J, Gallavotti A, Malcomber ST, Gassmann W, McSteen P. 2014. Transport of boron by the tassel-less1 aquaporin is critical for vegetative and reproductive development in maize. The Plant Cell 26, 2978–2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eggert K, von Wirén N. 2017. Response of the plant hormone network to boron deficiency. New Phytologist 216, 868–881. [DOI] [PubMed] [Google Scholar]
- Eltinge ET. 1936. Effect of boron deficiency upon the structure of Zea mays. Plant Physiology 11, 765–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang K, Gao S, Zhang W, Xing Y, Cao Q, Qin L. 2016. Addition of phenylboronic acid to Malus domestica pollen tubes alters calcium dynamics, disrupts actin filaments and affects cell wall architecture. PLos One 11, e0149232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuda M, Wakuta S, Kamijo J, Fujiwara T, Takano J. 2018. Establishment of genetically encoded biosensors for cytosolic boric acid in plant cells. The Plant Journal 95, 763–774. [DOI] [PubMed] [Google Scholar]
- Goldbach HE, Wimmer MA. 2007. Boron in plants and animals: is there a role beyond cell-wall structure? Journal of Plant Nutrition and Soil Science 170, 39–48. [Google Scholar]
- Goldbach HE, Wimmer MA, Findeklee P. 2000. Discussion paper: Boron—how can the critical level be defined? Journal of Plant Nutrition and Soil Science 163, 115–121. [Google Scholar]
- Goldberg S. 1997. Reactions of boron with soils. Plant and Soil 193, 35–48. [Google Scholar]
- Gómez-Soto D, Galván S, Rosales E, Bienert P, Abreu I, Bonilla I, Bolaños L, Reguera M. 2019. Insights into the role of phytohormones regulating pAtNIP5;1 activity and boron transport in Arabidopsis thaliana. Plant Science 287, 110198. [DOI] [PubMed] [Google Scholar]
- Gonzales-Fontes A. 2019. Why boron is an essential element for vascular plants. New Phytologist doi: 10.1111/nph.16033. [DOI] [PubMed] [Google Scholar]
- Hanaoka H, Uraguchi S, Takano J, Tanaka M, Fujiwara T. 2014. OsNIP3;1, a rice boric acid channel, regulates boron distribution and is essential for growth under boron-deficient conditions. The Plant Journal 78, 890–902. [DOI] [PubMed] [Google Scholar]
- Hermans C, Hammond JP, White PJ, Verbruggen N. 2006. How do plants respond to nutrient shortage by biomass allocation? Trends in Plant Science 11, 610–617. [DOI] [PubMed] [Google Scholar]
- Housh AB, Matthes MS, Gerheart A, Wilder SL, Kil K, Schueller M, Guthrie JM, McSteen P, Ferrieri R. 2020. Assessment of a 18F-phenylboronic acid radiotracer for imaging boron in maize. International Journal of Molecular Sciences (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Brown PH, Labavitch JM. 1996. Species variability in boron requirement is correlated with cell wall pectin. Journal of Experimental Botany 47, 227–232. [Google Scholar]
- Huai Z, Peng L, Wang S, Zhao H, Shi L, Xu F. 2018. Identification and characterization of an Arabidopsis thaliana mutant lbt with high tolerance to boron deficiency. Frontiers in Plant Science 9, 736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang L, Bell RW, Dell B. 1999. Factors controlling equilibrium boron (B) concentration in nutrient solution buffered with B-specific resin (Amberlite IRA-743). Plant and Soil 208, 233–241. [Google Scholar]
- Ishii T, Matsunaga T. 1996. Isolation and characterization of a rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydrate Research 284, 1–9. [Google Scholar]
- Jarvis B, Ali A, Shaheed A. 1983. Auxin and boron in relation to the rooting response and ageing of mung bean cuttings. New Phytologist 95, 509–518. [Google Scholar]
- Kakei Y, Yamazaki C, Suzuki M, Nakamura A, Sato A, Ishida Y, Kikuchi R. 2015. Small-molecule auxin inhibitors that target YUCCA are powerful tools for studying auxin function. The Plant Journal 84, 827–837. [DOI] [PubMed] [Google Scholar]
- Kato Y, Miwa K, Takano J, Wada M, Fujiwara T. 2009. Highly boron deficiency-tolerant plants generated by enhanced expression of NIP5;1, a boric acid channel. Plant & Cell Physiology 50, 58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Matoh T, Azuma J. 1996. Two chains of Rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiology 110, 1017–1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leonard A, Holloway B, Guo M, et al. 2014. tassel-less1 encodes a boron channel protein required for inflorescence development in maize. Plant & Cell Physiology 55, 1044–1054. [DOI] [PubMed] [Google Scholar]
- Lewis DH. 2019. Boron: the essential element for vascular plants that never was. New Phytologist 221, 1685–1690. [DOI] [PubMed] [Google Scholar]
- Li C, Pfeffer H, Dannel F, Römheld V, Bangerth F. 2001. Effects of boron starvation on boron compartmentation, and possibly hormone-mediated elongation growth and apical dominance of pea (Pisum sativum) plants. Physiologia Plantarum 111, 212–219. [Google Scholar]
- Li K, Kamiya T, Fujiwara T. 2015. Differential roles of PIN1 and PIN2 in root meristem maintenance under low-B conditions in Arabidopsis thaliana. Plant & Cell Physiology 56, 1205–1214. [DOI] [PubMed] [Google Scholar]
- Li T, Choi WG, Wallace IS, Baudry J, Roberts DM. 2011. Arabidopsis thaliana NIP7;1: an anther-specific boric acid transporter of the aquaporin superfamily regulated by an unusual tyrosine in helix 2 of the transport pore. Biochemistry 50, 6633–6641. [DOI] [PubMed] [Google Scholar]
- Liu K, Liu L, Ren Y, et al. 2015. Dwarf and tiller-enhancing 1 regulates growth and development by influencing boron uptake in boron limited conditions in rice. Plant Science 236, 18–28. [DOI] [PubMed] [Google Scholar]
- Lordkaew S, Dell B, Jamjod S, Rerkasem B. 2011. Boron deficiency in maize. Plant and Soil 342, 207–220. [Google Scholar]
- Macho-Rivero MÁ, Camacho-Cristóbal JJ, Herrera-Rodríguez MB, Müller M, Munné-Bosch S, González-Fontes A. 2017. Abscisic acid and transpiration rate are involved in the response to boron toxicity in Arabidopsis plants. Physiologia Plantarum 160, 21–32. [DOI] [PubMed] [Google Scholar]
- Marschner P. 2012. Marschner’s mineral nutrition of higher plants. Amsterdam: Academic Press. [Google Scholar]
- Martens DC, Westermann DT. 1991. Fertilizer applications for correcting micronutrient deficiencies. Micronutrients in agriculture. Madison, WI: Soil Science Society of America, 549–592. [Google Scholar]
- Martín-Rejano EM, Camacho-Cristóbal JJ, Herrera-Rodríguez MB, Rexach J, Navarro-Gochicoa MT, González-Fontes A. 2011. Auxin and ethylene are involved in the responses of root system architecture to low boron supply in Arabidopsis seedlings. Physiologia Plantarum 142, 170–178. [DOI] [PubMed] [Google Scholar]
- Matoh T, Kawaguchi S, Kobayashi M. 1996. Ubiquity of a borate–rhamnogalacturonan II complex in the cell walls of higher plants. Plant & Cell Physiology 37, 636–640. [Google Scholar]
- Matthes M, Torres-Ruiz RA. 2016. Boronic acid treatment phenocopies monopteros by affecting PIN1 membrane stability and polar auxin transport in Arabidopsis thaliana embryos. Development 143, 4053–4062. [DOI] [PubMed] [Google Scholar]
- Matthes M, Torres-Ruiz RA. 2017. Boronic acids as tools to study (plant) developmental processes? Plant Signaling & Behavior 12, e1321190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthes MS, Robil JM, Tran T, Kimble A, Mcsteen P. 2018. Increased transpiration is correlated with reduced boron deficiency symptoms in the maize tassel-less1 mutant. Physiologia Plantarum 163, 344–355. [DOI] [PubMed] [Google Scholar]
- Mendoza-Cózatl DG, Gokul A, Carelse MF, Jobe TO, Long TA, Keyster M. 2019. Keep talking: crosstalk between iron and sulfur networks fine-tunes growth and development to promote survival under iron limitation. Journal of Experimental Botany 70, 4197–4210. [DOI] [PubMed] [Google Scholar]
- Miwa K, Aibara I, Fujiwara T, Miwa K, Aibara I, Fujiwara T. 2014. Arabidopsis thaliana BOR4 is upregulated under high boron conditions and confers tolerance to high boron. Soil Science and Plant Nutrition 60, 349–355. [Google Scholar]
- Miwa K, Takano J, Fujiwara T. 2006. Improvement of seed yields under boron-limiting conditions through overexpression of BOR1, a boron transporter for xylem loading, in Arabidopsis thaliana. The Plant Journal 46, 1084–1091. [DOI] [PubMed] [Google Scholar]
- Miwa K, Takano J, Omori H, Seki M, Shinozaki K, Fujiwara T. 2007. Plants tolerant of high boron levels. Science 318, 1417. [DOI] [PubMed] [Google Scholar]
- Miwa K, Wakuta S, Takada S, Ide K, Takano J, Naito S, Omori H, Matsunaga T, Fujiwara T. 2013. Roles of BOR2, a boron exporter, in cross linking of Rhamnogalacturonan II and root elongation under boron limitation in Arabidopsis. Plant Physiology 163, 1699–1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa Y, Hanaoka H, Kobayashi M, Miyoshi K, Miwa K, Fujiwara T. 2007. Cell-type specificity of the expression of Os BOR1, a rice efflux boron transporter gene, is regulated in response to boron availability for efficient boron uptake and xylem loading. The Plant Cell 19, 2624–2635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noguchi K, Dannel F, Pfeffer H, Römheld V, Hayashi H, Fujiwara T. 2000. Defect in root–shoot translocation of boron in Arabidopsis thaliana mutant bor 1-1. Journal of Plant Physiology 156, 751–755. [Google Scholar]
- Noguchi K, Ishii T, Matsunaga T, Kakegawa K, Hayashi H, Fujiwara T. 2003. Biochemical properties of the cell wall in the Arabidopsis mutant bor1-1 in relation to boron nutrition. Journal of Plant Nutrition and Soil Science 166, 175–178. [Google Scholar]
- Noguchi K, Yasumori M, Imai T, Naito S, Matsunaga T, Oda H, Hayashi H, Chino M, Fujiwara T. 1997. bor1-1, an Arabidopsis thaliana mutant that requires a high level of boron. Plant Physiology 115, 901–906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Neill MA, Eberhard S, Albersheim P, Darvill AG. 2001. Requirement of borate cross-linking of cell wall Rhamnogalacturonan II for Arabidopsis growth. Science 294, 846–849. [DOI] [PubMed] [Google Scholar]
- O’Neill MA, Warrenfeltz D, Kates K, Pellerin P, Doco T, Darvill AG, Albersheim P, De P, Viala P. 1996. Rhamnogalacturonan-II, a pectic polysaccharide in the walls of growing plant cell, forms a dimer that is covalently cross-linked by a borate ester. Journal of Biological Chemistry 271, 22923–22930. [DOI] [PubMed] [Google Scholar]
- Pallotta M, Schnurbusch T, Hayes J, Hay A, Baumann U, Paull J, Langridge P, Sutton T. 2014. Molecular basis of adaptation to high soil boron in wheat landraces and elite cultivars. Nature 514, 88–91. [DOI] [PubMed] [Google Scholar]
- Pollard AS, Parr AJ, Loughman BC. 1977. Boron in relation to membrane function in higher plants. Journal of Experimental Botany 28, 831–841. [Google Scholar]
- Poza-viejo L, Abreu I, González-garcía MP, Allauca P, Bonilla I, Bolanos L, Reguera M. 2018. Boron deficiency inhibits root growth by controlling meristem activity under cytokinin regulation. Plant Science 270, 176–189. [DOI] [PubMed] [Google Scholar]
- Raven JA. 1980. Short- and long-distance transport of boric acid in plants. New Phytologist 84, 231–249. [Google Scholar]
- Roberts DM, Routray P. 2017. The nodulin 26 intrinsic protein subfamily. In: Chaumont F, Stephen T, eds. Plant aquaporins. Signaling and communication in plants. Cham: Springer, 267–296. [Google Scholar]
- Routray P, Li T, Yamasaki A, Yoshinari A, Takano J, Choi G. 2018. Nodulin intrinsic protein 7;1 is a tapetal boric acid channel involved in pollen cell wall formation. Plant Physiology 178, 1269–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnurbusch T, Hayes J, Hrmova M, Baumann U, Ramesh SA, Tyerman SD, Langridge P, Sutton T. 2010. Boron toxicity tolerance in barley through reduced expression of the multifunctional aquaporin HvNIP2;1. Plant Physiology 153, 1706–1715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sechet J, Htwe S, Urbanowicz B, et al. 2018. Suppression of Arabidopsis GGLT1 affects growth by reducing the l-galactose content and borate cross-linking of rhamnogalacturonan-II. The Plant Journal 96, 1036–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JF, Yamaji N, Liu XW, Yokosho K, Shen RF, Ma JF. 2018. Preferential distribution of boron to developing tissues is mediated by the intrinsic protein OsNIP3. Plant Physiology 176, 1739–1750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shireen F, Nawaz MA, Chen C, Zhang Q, Zheng Z, Sohail H, Sun J, Cao H, Huang Y, Bie Z. 2018. Boron: functions and approaches to enhance its availability in plants for sustainable agriculture. International Journal of Molecular Sciences 19, 1856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shorrocks VM. 1997. The occurrence and correction of boron deficiency. Plant and Soil 193, 121–148. [Google Scholar]
- Sotta N, Duncan S, Tanaka M, Sato T, Maree AF, Fujiwara T, Grieneisen VA. 2017. Rapid transporter regulation prevents substrate flow traffic jams in boron transport. eLife 6, e27038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton T, Baumann U, Hayes J, et al. 2007. Boron-toxicity tolerance in barley arising from efflux transporter amplification. Science 318, 1446–1449. [DOI] [PubMed] [Google Scholar]
- Takada S, Miwa K, Omori H, Fujiwara T, Naito S, Takano J. 2014. Improved tolerance to boron deficiency by enhanced expression of the boron transporter BOR2. Soil Science and Plant Nutrition 60, 341–348. [Google Scholar]
- Takano J, Miwa K, Yuan L, von Wirén N, Fujiwara T. 2005. Endocytosis and degradation of BOR1, a boron transporter of Arabidopsis thaliana, regulated by boron availability. Proceedings of the National Academy of Sciences, USA 102, 12276–12281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Tanaka M, Toyoda A, Miwa K, Kasai K, Fuji K, Onouchi H, Naito S, Fujiwara T. 2010. Polar localization and degradation of Arabidopsis boron transporters through distinct trafficking pathways. Proceedings of the National Academy of Sciences, USA 107, 5220–5225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano J, Wada M, Ludewig U, Schaaf G, von Wirén N, Fujiwara T. 2006. The Arabidopsis major intrinsic protein NIP5;1 is essential for efficient boron uptake and plant development under boron limitation. The Plant Cell 18, 1498–1509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Sotta N, Yamazumi Y, et al. 2016. The minimum open reading frame, AUG-stop, induces boron-dependent ribosome stalling and mRNA degradation. The Plant Cell 28, 2830–2849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Takano J, Chiba Y, Lombardo F, Ogasawara Y, Onouchi H, Naito S, Fujiwara T. 2011. Boron-dependent degradation of NIP5;1 mRNA for acclimation to excess boron conditions in Arabidopsis. The Plant Cell 23, 3547–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Wallace IS, Takano J, Roberts DM, Fujiwara T. 2008. NIP6;1 is a boric acid channel for preferential transport of boron to growing shoot tissues in Arabidopsis. The Plant Cell 20, 2860–2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thellier M, Duval Y, Demarty M. 1979. Borate exchanges of Lemna minor L. as studied with the help of the enriched stable isotopes and of a (n,α) nuclear reaction. Plant Physiology 63, 283–288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uraguchi S, Fujiwara T. 2011. Significant contribution of boron stored in seeds to initial growth of rice seedlings. Plant and Soil 340, 435–442. [Google Scholar]
- Uraguchi S, Kato Y, Hanaoka H, Miwa K, Fujiwara T. 2014. Generation of boron-deficiency-tolerant tomato by overexpressing an Arabidopsis thaliana borate transporter AtBOR1. Frontiers in Plant Science 5, 125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voxeur A, Fry SC. 2014. Glycosylinositol phosphorylceramides from Rosa cell cultures are boron-bridged in the plasma membrane and form complexes with rhamnogalacturonan II. The Plant Journal 79, 139–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakuta S, Fujikawa T, Naito S, Takano J. 2016. Tolerance to excess-boron conditions acquired by stabilization of a BOR1 variant with weak polarity in Arabidopsis. Frontiers in Cell and Developmental Biology 4, 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Römheld V, Li C, Bangerth F. 2006. Involvement of auxin and CKs in boron deficiency induced changes in apical dominance of pea plants (Pisum sativum L.). Journal of Plant Physiology 163, 591–600. [DOI] [PubMed] [Google Scholar]
- Wang X, Brockman JD, Guthrie JM, Lever SZ. 2018. Analysis and imaging of boron distribution in maize by quantitative neutron capture radiography. Applied Radiation and Isotopes 140, 252–261. [DOI] [PubMed] [Google Scholar]
- Warington K. 1923. The effect of boric acid and borax on the broad bean and certain other plants. Annals of Botany 37, 629–672. [Google Scholar]
- Wimmer M, Abreu I, Bell R, et al. 2019. Boron : an essential element for vascular plants. New Phytologist doi: 10.1111/nph.16127. [DOI] [PubMed] [Google Scholar]
- Wimmer MA, Eichert T. 2013. Review: mechanisms for boron deficiency-mediated changes in plant water relations. Plant Science 203–204, 25–32. [DOI] [PubMed] [Google Scholar]
- Wimmer MA, Lochnit G, Bassil E, Mühling KH, Goldbach HE. 2009. Membrane-associated, boron-interacting proteins isolated by boronate affinity chromatography. Plant & Cell Physiology 50, 1292–1304. [DOI] [PubMed] [Google Scholar]
- Yang CQ, Liu YZ, An JC, et al. 2013. Digital gene expression analysis of corky split vein caused by boron deficiency in ‘Newhall’ Navel Orange (Citrus sinensis Osbeck) for selecting differentially expressed genes related to vascular hypertrophy. PLoS One 8, e65737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari A, Hosokawa T, Amano T, Beier MP, Kunieda T, Shimada T, Hara-Nishimura I, Naito S, Takano J. 2019. Polar localization of the borate exporter BOR1 requires AP2-dependent endocytosis. Plant Physiology 179, 1569–1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshinari A, Takano J. 2017. Insights into the mechanisms underlying boron homeostasis in plants. Frontiers in Plant Science 8, 1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D, Zhao H, Shi L, Xu F. 2014. Physiological and genetic responses to boron deficiency in Brassica napus: a review. Soil Science and Plant Nutrition 60, 304–313. [Google Scholar]
- Zhang Q, Chen H, He M, Zhao Z, Cai H, Ding G, Shi L, Xu F. 2017. The boron transporter BnaC4.BOR1;1c is critical for inflorescence development and fertility under boron limitation in Brassica napus. Plant, Cell & Environment 40, 1819–1833. [DOI] [PubMed] [Google Scholar]
- Zhou T, Hua Y, Huang Y, Ding G, Shi L, Xu F. 2016. Physiological and transcriptional analyses reveal differential phytohormone responses to boron deficiency in Brassica napus genotypes. Frontiers in Plant Science 7, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Kobayashi M, Awano T, Matoh T, Takabe K. 2018. A new monoclonal antibody against rhamnogalacturonan II and its application to immunocytochemical detection of rhamnogalacturonan II in Arabidopsis roots. Bioscience, Biotechnology, and Biochemistry 82, 1780–1789. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.