Abstract
Background
A test that helps predict the time to the final menstrual period (FMP) has been sought for many years.
Objective
To assess the ability of antimullerian hormone (AMH) measurements to predictions the time to FMP.
Design
Prospective longitudinal cohort study.
Setting
The Study of Women’s Health Across the Nation.
Participants and Measurements
AMH and FSH were measured in 1537 pre- or early perimenopausal women, mean age 47.5 ± 2.6 years at baseline, then serially until 12 months of amenorrhea occurred. AMH was measured using a 2-site ELISA with a detection limit of 1.85 pg/mL.
Main Outcome Measure
Areas under the receiver operating curves (AUC) for AMH-based and FSH-based predictions of time to FMP, stratified by age. Probabilities that women would undergo their FMP in the next 12, 24, or 36 months across a range of AMH values were assessed.
Results
AUCs for predicting that the FMP will occur within the next 24 months were significantly greater for AMH-based than FSH-based models. The probability that a woman with an AMH <10 pg/mL would undergo her FMP within the next 12 months ranged from 51% at h<48 years of age to 79% at ≥51 years. The probability that a woman with an AMH >100 pg/mL would not undergo her FMP within the next 12 months ranged from 97% in women <48 years old to 90% in women ≥51 years old.
Conclusions
AMH measurement helps estimate when a woman will undergo her FMP, and, in general, does so better than FSH.
Keywords: female reproductive endocrinology, aging, gonadotropins, inhibin/activin/follistatin/AMH, menopause, ovaries
Although the average life expectancy for women in the United States has increased from approximately 47 to 79 years since 1900 (1) the mean age at which women have their final menstrual period (FMP) has only increased from 45 to 51 years (2, 3). As a result, women currently live for approximately 30 years after their FMP compared with just 2 years in the early 1900s (1). The dramatic increase in the lifespan of postmenopausal women has increased the importance of determining the impact of the menopause on a wide variety of physiologic and psychosocial measures. Additionally, the increase in postmenopausal lifespan has increased the importance of identifying biomarkers that facilitate accurate and precise predictions of the time until the FMP (4).
Currently, FSH is a widely used biomarker of ovarian age but has several significant limitations. First, because FSH is a pituitary, and not an ovarian hormone, FSH measures ovarian reserve indirectly (4). Second, FSH varies reciprocally with changes in estradiol and the inhibins, its key regulators across the menstrual cycle (5). Additionally, the 10-fold variation in FSH across the menstrual cycle and its further variability when cycles become irregular during the menopausal transition has a major impact on the interpretation of a single level.
Antimullerian hormone (AMH, also known as mullerian inhibiting substance) has advantages over FSH as an index of ovarian aging (6–9). Because it is produced by secondary, preantral, and early antral follicles up to about 8 mm in diameter (10), AMH provides a direct index of ovarian activity (4). AMH levels decline progressively with age (9, 11–14), and reflect ovarian reserve (15–18). Importantly, AMH levels are stable across the menstrual cycle and can be measured at any time without affecting interpretation of its level (19, 20). However, prior evaluations of AMH as a predictor of time to FMP (11, 13, 21–25) were not able to predict timing of the FMP with precision. This paper evaluates the ability of AMH to predict the FMP, using a new, ultrasensitive AMH assay (picoAMH ELISA, Ansh Labs, Webster, TX), which has a lower limit of detection than prior AMH assays.
To assess the ability of AMH to predict the time of the FMP, AMH was measured in women participating in the Study of Women’s Health Across the Nation (SWAN), beginning when they were pre- or early perimenopausal and, whenever possible, serially until they experienced their FMP. We first assessed the ability of AMH to predict whether the FMP would occur within the next 12, 24, or 36 months and compared those predictions with FSH-based predictions. Second, we determined the sensitivity, specificity, and positive and negative predictive values of AMH concentrations of <10 and >100 pg/mL for predicting the time to FMP. Finally, we calculated probabilities of reaching the FMP within various time periods across a range of AMH values, stratified by age.
Methods
Study cohort
SWAN is a multicenter, multiethnic, community-based, longitudinal study of the menopause transition (26). From 1996 to 1998, 3302 women ages 42 to 52, who had at least 1 menstrual period in the past 3 months, and who self-identified as being white or of a predesignated race/ethnic group, were enrolled. White women (n = 1550) were recruited at all 7 sites; black women (n = 935) were recruited in the Boston, Pittsburgh, Chicago, and the Detroit areas; and Japanese (n = 281), Chinese (n = 250), and Hispanic (n = 286) women were recruited in Los Angeles, Oakland, and northern New Jersey, respectively (26).
Study visits and collection of blood samples
Baseline and follow-up visits included in-person interviews, questionnaires, anthropometrics, and blood draws. Body mass index (BMI), menopause transition stage (determined using previously described bleeding criteria) (27), and cigarette smoking were assessed at each visit. The bleeding episode preceding 12 months of amenorrhea was designated as the FMP even if subsequent bleeding occurred; in other words, the FMP was not reset if another bleed occurred once the criterion of 12 months’ amenorrhea had been met (28). At each visit, a fasting blood draw was scheduled between 8:00 and 10:00 am on menstrual cycle days 2 through 5 to measure AMH, FSH, estradiol, and inhibin B. If 60 days passed without being able to collect blood during this window because of menstrual cycle irregularity accompanying progress through the menopausal transition, blood was collected at any time during the next 30 days. Serum was processed promptly, frozen, and stored at -80°C until thawed for measurements.
Analysis sample
We analyzed 7407 blood samples from 1537 women ages 42 to 63 who had their FMP without having a hysterectomy, bilateral ovariectomy, or taking hormone therapy; had a documented FMP date; and had at least 1 blood sample available while pre- or early perimenopausal. Women who had menstrual bleeding in the previous 3 months with no change in cycle predictability in the past year were classified premenopausal, whereas women who had menstrual bleeding in the previous 3 months with a decrease in cycle regularity in the past year were classified as early perimenopausal. The initial blood sample was obtained at the baseline visit in 121 women, the first follow-up visit in 1311 women, the second follow-up visit in 69 women, and the third through the tenth follow-up visits in the remaining 36 women. Whenever possible, subsequent samples were obtained annually until women became postmenopausal. In 1108 women, a blood sample was obtained after the FMP but before 365 days without menstrual bleeding had elapsed, so that, at the time these blood samples were obtained, the date of the FMP had not yet been established; these samples were excluded from the analysis. The protocol was approved by the institutional review board at each SWAN site and the SWAN Repository. All participants provided written informed consent.
Hormone assays
Estradiol (E2) and FSH were measured using immunoassays (23, 29) Intra- and interassay coefficients of variation (CVs) averaged 10.6% and 6.4% at an E2 level of 50 pg/mL (29) and 9.4% and 7.2% at FSH levels of 8.3 and 13.7 IU /L (23). The limit of detection (LOD), the least amount of an analyte detectable with 95% probability, ranged from 1 to 7 pg/mL for E2 (29) and was ~1 IU/L for FSH (23). AMH was measured using a 2-site ELISA (MenoCheck picoAMH ELISA, Ansh Labs) with intra- and interassay CVs ranging from 2.5% to 5.1% and 3.4% to 4.9%, respectively, at levels of 91 and 290 pg/mL. The LOD was 1.85 pg/mL (30). AMH was measured at Ansh Labs under the joint supervision of the Ansh Laboratory Director and the Director of Special Chemistry, Clinical Pathology Core Laboratory at Massachusetts General Hospital. Inhibin B was measured using an ELISA (Ansh Labs) with intra- and interassay CVs of <4% and an LOD of 1.6 pg/mL (31).
Data analysis
Participant characteristics and crude values of study analytes at baseline were summarized using means (SD) for normally distributed variables, medians (interquartile range) for nonnormally distributed variables and percentages for categorical variables. Because there were only 91 Hispanic participants, all of whom classified their race as white, we combined Hispanic women with the white women for the analyses.
Selection of covariates
Initial models included age, race/ethnicity, body mass index (BMI < vs. > 25 kg/m2), cigarette smoking, estradiol, and inhibin B levels. In multivariable models, other than AMH (or FSH), only age and BMI contributed independently to the prediction of the FMP. Moreover, the independent contribution of BMI to FMP prediction, although statistically significant, was clinically small. Hence, primary results on AMH-based predictions are reported separately by age group. Cigarette smoking, race/ethnicity, and estradiol and inhibin levels (data not shown) were not included in the final multivariable models.
AMH and FSH as predictors of the FMP
To examine patterns of change in AMH and FSH as women approached the FMP, we plotted mean values of the hormones in deciles of time to FMP. To compare the ability of the 2 hormones to predict the time to the FMP, we calculated the area under the receiver operating characteristic curves (AUC) for predicting that the FMP will occur within 12, 24, and 36 months using logistic regression (32). In 6 separate models, AMH and FSH were the primary predictors, and age and BMI were the only covariates, and all 3 terms were treated as continuous variables. Generalized estimating equations were used to account for multiple observations per woman.
Age-stratified AMH-based prediction of FMP
The sensitivity and specificity of AMH less than 10 pg/mL or 100 pg/mL for the FMP occurring within the next 12, 24, or 36 months were determined separately in 3 age groups: <48 years (n = 2152), 48 to <51 years (n = 2616), and >51 years (n = 2639). Cut points for age were selected to generate 3 groups of approximately equal size with cut points at integer years. Bootstrapping (with 3000 repetitions) was used to calculate 95% confidence intervals (CI) for the test statistics. Values of 10 and 100 pg/mL have been suggested as testing thresholds for determining if the FMP is imminent (33). We selected a range of AMH thresholds (2, 5, 10, 25, 50, 100, 200, and 400 pg/mL) by examining plots of the probability of reaching the FMP within 12, 24, and 36 months as a function of AMH within each age stratum. For each AMH interval (eg, <2, 2 to <5, 5 to <10) we calculated the age-stratified probability of the FMP occurring in the next 12, 24, and 36 months as the proportion of samples in the AMH-by-age group for which the FMP occurred within the specified period, and used bootstrapping (with 3000 repetitions) to calculate 95% CIs. Because of small cell sizes, groups at the extremes of the AMH distribution (low end in the younger age group and at the high end in the older age group) were combined. All analyses were conducted using SAS, version 9.4.
Results
Clinical characteristics
At the time of their first AMH measurement, the cohort comprised 761 white women (including 91 Hispanics), 460 black women, 146 Chinese women, and 170 Japanese women who were 47.5 ± 2.6 (mean ± SD) years old; 30.6% were premenopausal; and 69.4% were early perimenopausal (Table 1). The mean time to the FMP was 57 ± 38 months, and varied by race/ethnicity from 54 + 37 months in black women to 62 ± 37 months in Japanese women.
Table 1.
Clinical Characteristics for All Participants and by Race/Ethnicity at the Time of the Baseline Blood Draw
| Characteristic | All Women (n = 1537) | Whitea (n = 761) | Black (n = 460) | Chinese (n = 146) | Japanese (n = 170) |
|---|---|---|---|---|---|
| Age (y) | 47.5 ± 2.6 | 47.3 ± 2.6 | 47.6 ± 2.6 | 47.6 ± 2.5 | 47.6 ± 2.5 |
| BMI (kg/m2) | 28.3 ± 7.4 | 27.2 ± 7.4 | 32.3 ± 10.1 | 23.2 ± 4.3 | 23.1 ± 3.6 |
| Premenopausal (%) | 30.6 | 33.5 | 27.3 | 33.6 | 26.2 |
| Early perimenopausal (%) | 69.4 | 66.5 | 72.7 | 66.4 | 73.8 |
| Time to FMP (mo) | 57 ± 38 | 57 ± 37 | 59 ± 36 | 55 ± 38 | 62 ± 39 |
| AMH (pg/mL) | 142 (16, 472) | 158 (25, 502) | 97 (6, 357) | 62 (6, 382) | 255 (26, 992) |
| FSH (IU/L) | 19 (12, 35) | 19 (13, 35) | 20 (13, 36) | 23 (13, 42) | 17 (13, 28) |
| Inhibin B (pg/mL) | 31 (3, 86) | 31 (5, 86) | 14 (2, 64) | 45 (8, 96) | 67 (14, 111) |
| Estradiol (pg/mL) | 49 (30, 88) | 52 (31, 92) | 47 (29, 87) | 39 (24, 72) | 49 (30, 81) |
Age, BMI, and time to FMP are expressed as the mean ± SD.
AMH, FSH, inhibin B, and estradiol are expressed as the median (25th, 75th percentiles).
aIncludes 91 Hispanic women.
AMH, antimullerian hormone; BMI, body mass index; FMP, final menstrual period.
AMH and FSH change in relation to the FMP
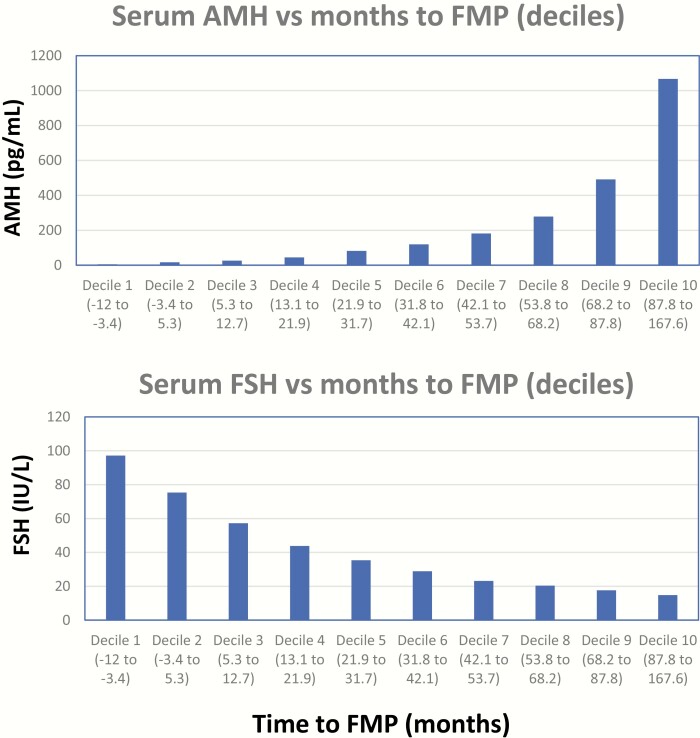
AMH decreased and FSH increased progressively as the participants approached their FMP (Fig. 1a and b). Decreases in mean AMH levels were progressive, with AMH levels below the assay detection limit in 14% of samples collected 12 to 24 months before the FMP, 25% of samples collected 0 to 12 months before the FMP, and 42% of samples collected 0 to 12 months after the FMP.
Figure 1.
Mean serum AMH (upper panel) and serum FSH (lower panel) levels vs deciles of months to the FMP. AMH, antimullerian hormone; FMP, final menstrual period.
AUCs using AMH and FSH as predictors of the time to the FMP
The AUCs for AMH and FSH, each combined with age and BMI in separate logistic models for predicting the occurrence of the FMP within 12 months, were 0.881 (95% CI, 0.873-0.889) for AMH and 0.885 (95% CI, 0.876-0.893) for FSH (P = NS). The AUCs were 0.891 (95% CI, 0.884-0.900) for AMH and 0.877 (95% CI, 0.869-0.885) for FSH for predicting FMP occurrence within 24 months, and 0.896 (95% CI, 0.889-0.903) for AMH and 0.871 (95% CI, 0.864-0.880) for FSH for predicting FMP occurrence within 36 months. For both the 24- and 36-month predictions, AUCs with AMH as primary predictor were significantly greater than the corresponding AUCs with FSH as primary predictor (P < .05).
AMH-based tests of the FMP occurring within specified periods, stratified by age
AMH was <10 pg/mL in 2675 samples (36%). The sensitivities of AMH <10 pg/mL for experiencing the FMP in the next 12 months were 71%, 73%, and 82% in women <48, 48 to <51, and >51 years old. The positive predictive values for reaching the FMP in the next 12 months were 51%, 63%, and 79% in the 3 age strata and increased to 78%, 89%, and 97% when the prediction was extended to 36 months. (Tables 2 and 3).
Table 2.
Properties of AMH-Based Tests for FMP Occurring Within 12, 24, and 36 Months, Stratified by Age
| Age Groups | 12 Months | 24 Months | 36 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <48 y | AMH | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |||
| Sensitivity | <10 | 0.71 | 0.64 | 0.78 | 0.60 | 0.53 | 0.66 | 0.47 | 0.42 | 0.53 |
| PPV | <10 | 0.51 | 0.44 | 0.58 | 0.65 | 0.58 | 0.72 | 0.78 | 0.72 | 0.85 |
| Specificity | <100 | 0.65 | 0.61 | 0.68 | 0.70 | 0.66 | 0.73 | 0.76 | 0.72 | 0.80 |
| NPV | <100 | 0.97 | 0.96 | 0.98 | 0.94 | 0.93 | 0.96 | 0.86 | 0.83 | 0.89 |
| 48 to <51 y | ||||||||||
| Sensitivity | <10 | 0.73 | 0.68 | 0.77 | 0.62 | 0.58 | 0.66 | 0.54 | 0.50 | 0.57 |
| PPV | <10 | 0.63 | 0.58 | 0.68 | 0.81 | 0.77 | 0.85 | 0.89 | 0.86 | 0.92 |
| Specificity | <100 | 0.46 | 0.42 | 0.50 | 0.57 | 0.52 | 0.62 | 0.68 | 0.62 | 0.74 |
| NPV | <100 | 0.96 | 0.93 | 0.98 | 0.87 | 0.83 | 0.91 | 0.73 | 0.68 | 0.79 |
| ≥51 y | ||||||||||
| Sensitivity | <10 | 0.82 | 0.78 | 0.85 | 0.75 | 0.72 | 0.79 | 0.70 | 0.66 | 0.73 |
| PPV | <10 | 0.79 | 0.75 | 0.83 | 0.92 | 0.89 | 0.95 | 0.97 | 0.95 | 0.99 |
| Specificity | <100 | 0.27 | 0.22 | 0.32 | 0.39 | 0.31 | 0.47 | 0.53 | 0.42 | 0.64 |
| NPV | <100 | 0.90 | 0.83 | 0.95 | 0.76 | 0.67 | 0.85 | 0.56 | 0.45 | 0.67 |
Test statistics (and 95% CI) were generated by bootstrapping (3000 repetitions).
The specificity of AMH <100 for FMP within 12 months can be interpreted as the sensitivity of AMH ≥100 for FMP later than 12 months. Similarly, the NPV of AMH <100 for FMP within 12 months is also the PPV of AMH ≥100 for FMP later than 12 months.
AMH, antimullerian hormone (pg/mL); CI, confidence interval; FMP, final menstrual period; NPV, negative predictive value; PPV, positive predictive value.
Table 3.
Properties of AMH-Based Tests for FMP Occurring Within 12, 24, and 36 Months When Age <45 Years (N = 421)
| Age Groups | 12 Months | 24 Months | 36 Months | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| <45 y | AMH | Estimate | 95% CI | Estimate | 95% CI | Estimate | 95% CI | |||
| Sensitivity | <10 | 0.59 | 0.48 | 0.70 | 0.49 | 0.35 | 0.63 | 0.35 | 0.26 | 0.46 |
| PPV | <10 | 0.47 | 0.33 | 0.66 | 0.55 | 0.40 | 0.74 | 0.64 | 0.47 | 0.84 |
| Specificity | <100 | 0.77 | 0.70 | 0.84 | 0.80 | 0.73 | 0.87 | 0.83 | 0.76 | 0.90 |
| NPV | <100 | 0.95 | 0.93 | 0.96 | 0.93 | 0.90 | 0.96 | 0.86 | 0.82 | 0.90 |
Test statistics (and 95% confidence interval) were generated by bootstrapping (3000 repetitions).
The specificity of AMH <100 for FMP within 12 months can be interpreted as the sensitivity of AMH ≥100 for FMP later than 12 months. Similarly, the NPV of AMH <100 for FMP within 12 months is also the PPV of AMH ≥100 for FMP later than 12 months.
AMH, antimullerian hormone (pg/mL); CI, confidence interval; FMP, final menstrual period; NPV, negative predictive value; PPV, positive predictive value.
AMH was >100 pg/mL in 2839 (38%) of samples. The specificities of AMH <100 pg/mL for having the FMP in the next 36 months (or the sensitivities of AMH >100 for FMP not occurring in 36 months) were 76%, 68%, and 53% in the 3 age groups. The negative predictive values of AMH <100 pg/mL for the FMP occurring in the next 12 months (or the positive predictive value of AMH >100 for FMP not occurring in 12 months) were 97%, 96%, and 90%, in women <48, 48 to <51, and >51 years old.
The probabilities of reaching the FMP within various periods across a range of AMH values, stratified by age, are shown in Table 4. The positive predictive value of a given AMH level greater than a prespecified threshold for the FMP being distant is the same as the negative predictive value of an AMH level below a prespecified threshold for the FMP being imminent. The probabilities increase as the prediction time horizon increases (from 12 to 36 months) and as age increases. For example, in women >51 years whose AMH is <2 pg/mL, the probability of reaching the FMP increases from 82% for the 12-month prediction to 98% for the 36-month prediction. Similarly, in women whose AMH is between 10 and 24.9 pg/mL, the probability of reaching the FMP in 36 months increases from 71% in women <48 years to 90% in women >51 years. A high AMH value is particularly good at excluding an imminent FMP. The probability of the FMP not occurring in the next 12 months is 77% in women >51 years, 81% in women 48 to <51 years, and 91% in women <48 years if AMH is between 50 and 99.9 pg/mL.
Table 4.
Probabilities of Reaching the FMP Within Specified Time Periods by AMH Level, Stratified by Age
| 12 Months | 24 Months | 36 Months | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Age Groups | AMH (pg/mL) | Prob | 95% CI | Prob | 95% CI | Prob | 95% CI | |||
| <48 y | <10 | 0.51 | 0.44 | 0.58 | 0.65 | 0.58 | 0.72 | 0.78 | 0.72 | 0.85 |
| 10 to <25 | 0.22 | 0.11 | 0.35 | 0.48 | 0.34 | 0.64 | 0.71 | 0.57 | 0.85 | |
| 25 to <50 | 0.12 | 0.04 | 0.22 | 0.25 | 0.13 | 0.37 | 0.52 | 0.38 | 0.66 | |
| 50 to <100 | 0.09 | 0.03 | 0.15 | 0.20 | 0.11 | 0.30 | 0.39 | 0.28 | 0.51 | |
| 100 to <200 | 0.05 | 0.02 | 0.09 | 0.12 | 0.05 | 0.19 | 0.28 | 0.18 | 0.38 | |
| 200 to <400 | 0.02 | 0.00 | 0.04 | 0.05 | 0.02 | 0.08 | 0.16 | 0.10 | 0.22 | |
| 400+ | 0.02 | 0.01 | 0.04 | 0.03 | 0.02 | 0.05 | 0.06 | 0.04 | 0.09 | |
| 48 to <51 y | <5 | 0.67 | 0.62 | 072 | 0.82 | 0.78 | 0.87 | 0.90 | 0.86 | 0.93 |
| 5 to <10 | 0.43 | 0.30 | 0.58 | 0.77 | 0.64 | 0.88 | 0.87 | 0.76 | 0.96 | |
| 10 to <25 | 0.40 | 0.30 | 0.51 | 0.66 | 0.56 | 0.77 | 0.87 | 0.79 | 0.94 | |
| 25 to <50 | 0.23 | 0.15 | 0.33 | 0.51 | 0.39 | 0.64 | 0.74 | 0.63 | 0.86 | |
| 50 to <100 | 0.19 | 0.11 | 0.28 | 0.45 | 0.34 | 0.57 | 0.69 | 0.59 | 0.79 | |
| 100 to <200 | 0.10 | 0.05 | 0.16 | 0.25 | 0.16 | 0.34 | 0.45 | 0.35 | 0.55 | |
| 200+ | 0.01 | 0.00 | 0.03 | 0.06 | 0.02 | 0.09 | 0.17 | 0.11 | 0.23 | |
| ≥51 y | <2 | 0.82 | 0.78 | 0.86 | 0.94 | 0.91 | 0.96 | 0.98 | 0.96 | 0.99 |
| 2 to <5 | 0.67 | 0.54 | 0.80 | 0.88 | 0.79 | 0.96 | 0.95 | 0.89 | 1.00 | |
| 5 to <10 | 0.72 | 0.60 | 0.85 | 0.87 | 0.78 | 0.96 | 0.95 | 0.88 | 1.00 | |
| 10 to <25 | 0.53 | 0.42 | 0.64 | 0.75 | 0.65 | 0.85 | 0.90 | 0.82 | 0.97 | |
| 25 to <50 | 0.35 | 0.22 | 0.48 | 0.65 | 0.52 | 0.78 | 0.88 | 0.78 | 0.96 | |
| 50 to <100 | 0.23 | 0.12 | 0.36 | 0.48 | 0.34 | 0.62 | 0.71 | 0.58 | 0.84 | |
| 100+ | 0.10 | 0.05 | 0.17 | 0.24 | 0.15 | 0.33 | 0.44 | 0.33 | 0.55 | |
Probabilities (and 95% CI) were generated by bootstrapping (3000 repetitions). Probability of not reaching the FMP in the specified period can be calculated as 1 minus the probability listed above.
AMH, antimullerian hormone (pg/mL); CI, confidence interval; FMP, final menstrual period.
Discussion
The ability to predict the FMP accurately and precisely has long been a “Holy Grail” of menopause research. However, using menstrual bleeding patterns (34), serum FSH levels (13, 22, 35), or previous AMH assays (11, 13, 21–25), the FMP can only be predicted within a window of approximately 4 years (36, 37), a time window that is too great to be clinically useful. Using a 2-site ELISA whose LOD (1.85 pg/mL) is substantially lower than that of prior AMH ELISAs (15, 30, 38), it is now possible to predict the FMP within a window of 12 to 24 months in late-reproductive aged women, a marked improvement compared with less sensitive AMH assays, serum FSH levels, or menstrual bleeding patterns. Using this assay, more than one-half of samples with previously undetectable AMH levels are now measurable (15). Until recently, the LOD of AMH ELISAs was so high, typically 50 to 100 pg/mL (11, 20–25, 39), that AMH could not be measured in blood samples from many regularly cycling women, making accurate and precise predictions of when the FMP would occur extremely difficult.
Although ultrasensitive AMH measurements improve the ability to predict when the FMP will occur in women in their early 40s and older, a substantial proportion of samples still have values below the LOD long before the FMP. In some of these women, menstrual status based on bleeding patterns is likely misclassified (40). In addition, biological intercycle variability in AMH of up to 15% has been reported in women aged 18 to 44 (41). Although not well understood, such variation may have affected our ability to predict the FMP. Because AMH is a marker of activated follicles, and not a direct marker of the primordial follicle pool, natural variability in the patterns of AMH decline in individual women is to be expected. Because ovarian function waxes and wanes during the menopausal transition, AMH may be relatively lower during periods of amenorrhea, and may increase when the small remaining ovarian follicle pool initiates a menstrual cycle (42).
Age had a major impact on the relationship between AMH and the time to the FMP, as previously observed (13, 38, 43). The observed sensitivity and positive predictive value of an AMH value <10 pg/mL for experiencing the FMP within 12 months were 11% and 28% greater in women age 51 years or older compared with those younger than 48 years. In women younger than 48, an AMH value >100 pg/mL essentially guaranteed that the FMP would not occur within the next 12 months, with a positive predictive value of 97%. Data on women younger than age 42 are not included in SWAN, and therefore attempts to extrapolate these data to younger groups of women are not warranted.
Although AMH is believed to largely measure the quantity of ovarian follicles, the number and health of the AMH-producing granulosa cells within the remaining ovarian follicles of perimenopausal women may also be declining. Women in their mid to late 40s have more granulosa cell apoptosis (44), depletion of mitochondria (45), and smaller preovulatory follicles (46), than younger women, all of which imply less functional ovarian follicles over time. Thus, despite a low AMH level, younger women may have qualitatively better follicle function, and therefore a longer duration of time to their FMP, a notion supported by evidence that AMH does not predict pregnancy potential in women aged 30 to 44, who are at least 3 to 5 years before the onset of the menopausal transition (47).
When forecasting whether the FMP will occur in the next 24 or 36 months, AUCs were greater when AMH was used as the primary predictor than when FSH was used. This finding indicates that AMH-based predictions of the time to the FMP are more likely to be correct than FSH-based predictions, though differences were modest. The differences between AMH-based and FSH-based predictions of time to FMP would be expected to be larger if blood samples were collected at random across the menstrual cycle, rather than focusing sample collection on cycle days 2 to 5, as was done in this study.
Several features of this study enabled better predictions of time to the FMP than in other cohorts. First, the larger number of women and the longer duration of follow-up in the SWAN cohort favor the generation of more accurate and precise predictions. Furthermore, all women in the cohort were followed until they had experienced at least 12 consecutive months of amenorrhea, eliminating a potential source of bias if the relationship between AMH levels and the time to the FMP differs between women who transition early and those who transition late. Finally, an ultrasensitive AMH assay was essential for improving the prediction of time to the FMP (15, 30, 38).
Despite these advances, there are several potential reasons why AMH did not predict the time of the FMP with even higher accuracy and better precision. First, determination of the FMP based on bleeding patterns may not always be correct (40). Women who develop secondary amenorrhea before their true FMP because of low body weight, stress, excess physical activity, hyperprolactinemia, or other disorders could have been classified as postmenopausal despite adequate ovarian reserve. An AMH value >10 pg/mL provides strong evidence that such women are not really postmenopausal. In contrast, in some women postmenopausal bleeding from undiagnosed urogenital pathology occurring well after their true FMP may be confused with perimenopausal bleeding. In those women, an undetectable AMH level while still experiencing vaginal bleeding may provide a valuable clue to their diagnosis. Although most women older than age 45 with 12 consecutive months of amenorrhea are postmenopausal, about 10% will experience postmenopausal bleeding (28). Measuring AMH should help reduce FMP misclassification due to non-menstrual vaginal bleeding or isolated spontaneous menstrual cycle recovery. Additionally, using 2 or more serial AMH determinations to calculate rates of change may provide more accurate predictions of the FMP (48).
There are many potential additional clinical applications for AMH measurements (42). AMH measurements may help women predict when vasomotor symptoms are likely to begin (49), or when heavy menstrual bleeding is likely to end. In women with heavy bleeding resulting from uterine pathology, such as leiomyomata or adenomyosis, the ability to predict the FMP accurately may help women to decide whether to undergo a hysterectomy or temporize with medical management.
Information that indicates when the menopause is likely to occur also has important implications for major nonreproductive health issues. Later age at menopause is associated with a lower risk of cardiovascular disease and a greater life expectancy (50, 51). Women who undergo menopause early are more likely to develop osteoporosis (52) and/or cardiovascular disease (53–55), whereas women who undergo menopause late have higher risks of endometrial (56) and breast cancer (57). In a meta-analysis including more than 415 000 women, 119 000 of whom had invasive breast cancer, the risk of developing breast cancer increased 3% for each year older a woman was at menopause (58). Understanding the risk factor profile of each woman should allow health care providers to focus screening efforts and apply preventive measures in an individualized manner that facilitates healthy aging.
In summary, using an ultrasensitive ELISA with a limit of detection of 1.85 pg/mL, together with a woman’s age, clinically useful predictions of the time to FMP are now feasible for many women. Ultrasensitive measurements of AMH provide a reliable index of ovarian aging that should prove useful in both clinical and research settings.
Acknowledgments
The authors thank the study staff at each site and all the women who participated in Study of Women’s Health Across the Nation (SWAN).
Glossary
Abbreviations
- AMH
antimullerian hormone
- AUC
area under the curve
- BMI
body mass index
- CI
confidence interval
- CV
coefficient of variation
- E2
estradiol
- FMP
final menstrual period
- LOD
limit of detection
- SWAN
Study of Women’s Health Across the Nation
SWAN has grant support from the National Institutes of Health (NIH), DHHS, through the National Institute on Aging (NIA), the National Institute of Nursing Research (NINR) and the NIH Office of Research on Women’s Health (ORWH) (Grants U01NR004061; U01AG012505, U01AG012535, U01AG012531, U01AG012539, U01AG012546, U01AG012553, U01AG012554, U01AG012495, U01AG017719, and Administrative Supplement to U01AG012531 from NIA). The content of this manuscript is solely the responsibility of the authors and does not necessarily represent the official views of the NIA, NINR, ORWH or the NIH.
Clinical Centers: University of Michigan, Ann Arbor—Siobán Harlow, PI 2011–present, MaryFran Sowers, principal investigator (PI) 1994–2011; Massachusetts General Hospital, Boston, MA—Joel Finkelstein, PI 1999–present; Robert Neer, PI 1994–1999; Rush University, Rush University Medical Center, Chicago, IL—Howard Kravitz, PI 2009–present; Lynda Powell, PI 1994–2009; University of California, Davis/Kaiser—Ellen Gold, PI; University of California, Los Angeles—Gail Greendale, PI; Albert Einstein College of Medicine, Bronx, NY—Carol Derby, PI 2011–present, Rachel Wildman, PI 2010–2011; Nanette Santoro, PI 2004–2010; University of Medicine and Dentistry—New Jersey Medical School, Newark—Gerson Weiss, PI 1994–2004; and the University of Pittsburgh, Pittsburgh, PA—Karen Matthews, PI.
NIH Program Office: National Institute on Aging, Bethesda, MD—Chanda Dutta 2016–present; Winifred Rossi 2012–present; Sherry Sherman 1994–2012; Marcia Ory 1994–2001; National Institute of Nursing Research, Bethesda, MD—Program Officers.
Central Laboratory: University of Michigan, Ann Arbor—Daniel McConnell (Central Ligand Assay Satellite Services).
SWAN Repository: University of Michigan, Ann Arbor—Siobán Harlow 2013–present; Dan McConnell 2011–2013; MaryFran Sowers 2000–2011.
Coordinating Center: University of Pittsburgh, Pittsburgh, PA—Maria Mori Brooks, PI 2012–present; Kim Sutton-Tyrrell, PI 2001–2012; New England Research Institutes, Watertown, MA—Sonja McKinlay, PI 1995–2001.
Steering Committee: Susan Johnson, Current Chair & Chris Gallagher, Former Chair
Ansh Labs, LLC: Webster, TX—Bhanu Kalra (Research Development and Quality Control)
Additional Information
Disclosures: Ajay Kumar is an employee of Ansh Labs. Anthony Morrison was an employee of Ansh Labs at the time this study was started; he is currently with Motive Biosciences.
Data Availability
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.
References and Notes
- 1. Oeppen J, Vaupel JW. Demography. Broken limits to life expectancy. Science. 2002;296(5570):1029–1031. [DOI] [PubMed] [Google Scholar]
- 2. Gold EB, Bromberger J, Crawford S, et al. Factors associated with age at natural menopause in a multiethnic sample of midlife women. Am J Epidemiol. 2001;153(9):865–874. [DOI] [PubMed] [Google Scholar]
- 3. Gold EB, Crawford SL, Avis NE, et al. Factors related to age at natural menopause: longitudinal analyses from SWAN. Am J Epidemiol. 2013;178(1):70–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Roudebush WE, Kivens WJ, Mattke JM. Biomarkers of ovarian reserve. Biomark Insights. 2008;3:259–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hurwitz JM, Santoro N. Inhibins, activins, and follistatin in the aging female and male. Semin Reprod Med. 2004;22(3):209–217. [DOI] [PubMed] [Google Scholar]
- 6. Baker ML, Metcalfe SA, Hutson JM. Serum levels of müllerian inhibiting substance in boys from birth to 18 years, as determined by enzyme immunoassay. J Clin Endocrinol Metab. 1990;70(1):11–15. [DOI] [PubMed] [Google Scholar]
- 7. Hudson PL, Dougas I, Donahoe PK, et al. An immunoassay to detect human müllerian inhibiting substance in males and females during normal development. J Clin Endocrinol Metab. 1990;70(1):16–22. [DOI] [PubMed] [Google Scholar]
- 8. Josso N, Legeai L, Forest MG, Chaussain JL, Brauner R. An enzyme linked immunoassay for anti-müllerian hormone: a new tool for the evaluation of testicular function in infants and children. J Clin Endocrinol Metab. 1990;70(1):23–27. [DOI] [PubMed] [Google Scholar]
- 9. Cate RL, Mattaliano RJ, Hession C, et al. Isolation of the bovine and human genes for Müllerian inhibiting substance and expression of the human gene in animal cells. Cell. 1986;45(5):685–698. [DOI] [PubMed] [Google Scholar]
- 10. Weenen C, Laven JS, Von Bergh AR, et al. Anti-Müllerian hormone expression pattern in the human ovary: potential implications for initial and cyclic follicle recruitment. Mol Hum Reprod. 2004;10(2):77–83. [DOI] [PubMed] [Google Scholar]
- 11. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC. Antimüllerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77(2):357–362. [DOI] [PubMed] [Google Scholar]
- 12. Kelsey TW, Wright P, Nelson SM, Anderson RA, Wallace WH. A validated model of serum anti-müllerian hormone from conception to menopause. PLoS One. 2011;6(7):e22024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kim C, Slaughter JC, Wang ET, et al. Anti-Müllerian hormone, follicle stimulating hormone, antral follicle count, and risk of menopause within 5 years. Maturitas. 2017;102:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Lee MM, Donahoe PK, Hasegawa T, et al. Mullerian inhibiting substance in humans: normal levels from infancy to adulthood. J Clin Endocrinol Metab. 1996;81(2):571–576. [DOI] [PubMed] [Google Scholar]
- 15. Iwase A, Osuka S, Nakamura T, et al. Usefulness of the ultrasensitive anti-Müllerian hormone assay for predicting true ovarian reserve. Reprod Sci. 2016;23(6):756–760. [DOI] [PubMed] [Google Scholar]
- 16. Kevenaar ME, Meerasahib MF, Kramer P, et al. Serum anti-mullerian hormone levels reflect the size of the primordial follicle pool in mice. Endocrinology. 2006;147(7): 3228–3234. [DOI] [PubMed] [Google Scholar]
- 17. Seifer DB, MacLaughlin DT, Christian BP, Feng B, Shelden RM. Early follicular serum müllerian-inhibiting substance levels are associated with ovarian response during assisted reproductive technology cycles. Fertil Steril. 2002;77(3):468–471. [DOI] [PubMed] [Google Scholar]
- 18. van Rooij IA, Broekmans FJ, te Velde ER, et al. Serum anti-Müllerian hormone levels: a novel measure of ovarian reserve. Hum Reprod. 2002;17(12):3065–3071. [DOI] [PubMed] [Google Scholar]
- 19. Sowers M, McConnell D, Gast K, et al. Anti-Müllerian hormone and inhibin B variability during normal menstrual cycles. Fertil Steril. 2010;94(4):1482–1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hehenkamp WJ, Looman CW, Themmen AP, de Jong FH, Te Velde ER, Broekmans FJ. Anti-Müllerian hormone levels in the spontaneous menstrual cycle do not show substantial fluctuation. J Clin Endocrinol Metab. 2006;91(10):4057–4063. [DOI] [PubMed] [Google Scholar]
- 21. Broer SL, Eijkemans MJ, Scheffer GJ, et al. Anti-mullerian hormone predicts menopause: a long-term follow-up study in normoovulatory women. J Clin Endocrinol Metab. 2011;96(8):2532–2539. [DOI] [PubMed] [Google Scholar]
- 22. Freeman EW, Sammel MD, Lin H, Gracia CR. Anti-mullerian hormone as a predictor of time to menopause in late reproductive age women. J Clin Endocrinol Metab. 2012;97(5): 1673–1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Sowers MR, Eyvazzadeh AD, McConnell D, et al. Anti-mullerian hormone and inhibin B in the definition of ovarian aging and the menopause transition. J Clin Endocrinol Metab. 2008;93(9):3478–3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tehrani FR, Shakeri N, Solaymani-Dodaran M, Azizi F. Predicting age at menopause from serum antimüllerian hormone concentration. Menopause. 2011;18(7):766–770. [DOI] [PubMed] [Google Scholar]
- 25. van Rooij IA, Tonkelaar Id, Broekmans FJ, et al. Anti-müllerian hormone is a promising predictor for the occurrence of the menopausal transition. Menopause. 2004;11(6 Pt 1): 601–606. [DOI] [PubMed] [Google Scholar]
- 26. Sowers M, Crawford S, Sternfeld B, et al. SWAN: a multicenter, multiethnic, community-based cohort study of women and the menopausal transition. In: Lobo R, Kelsey J, Marcus R, eds. Menopause: Biology and Pathobiology. San Diego: Academic Press; 2000:175–188. [Google Scholar]
- 27. Finkelstein JS, Brockwell SE, Mehta V, et al. Bone mineral density changes during the menopause transition in a multiethnic cohort of women. J Clin Endocrinol Metab. 2008;93(3):861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wallace RB, Sherman BM, Bean JA, Treloar AE, Schlabaugh L. Probability of menopause with increasing duration of amenorrhea in middle-aged women. Am J Obstet Gynecol. 1979;135(8):1021–1024. [DOI] [PubMed] [Google Scholar]
- 29. England BG, Parsons GH, Possley RM, McConnell DS, Midgley AR. Ultrasensitive semiautomated chemiluminescent immunoassay for estradiol. Clin Chem. 2002;48(9): 1584–1586. [PubMed] [Google Scholar]
- 30. Robertson DM, Kumar A, Kalra B, et al. Detection of serum antimüllerian hormone in women approaching menopause using sensitive antimüllerian hormone enzyme-linked immunosorbent assays. Menopause. 2014;21(12):1277–1286. [DOI] [PubMed] [Google Scholar]
- 31. Liu DM, Torchen LC, Sung Y, et al. Evidence for gonadotrophin secretory and steroidogenic abnormalities in brothers of women with polycystic ovary syndrome. Hum Reprod. 2014;29(12):2764–2772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44(3):837–845. [PubMed] [Google Scholar]
- 33. Laboratories A. MenoCheck picoAMH ELISA. 2018; https://www.anshlabs.com/product/picoamh-elisa/. Accessed April 6, 2019.
- 34. Harlow SD, Cain K, Crawford S, et al. Evaluation of four proposed bleeding criteria for the onset of late menopausal transition. J Clin Endocrinol Metab. 2006;91(9):3432–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Randolph JF Jr, Crawford S, Dennerstein L, et al. The value of follicle-stimulating hormone concentration and clinical findings as markers of the late menopausal transition. J Clin Endocrinol Metab. 2006;91(8):3034–3040. [DOI] [PubMed] [Google Scholar]
- 36. Greendale GA, Ishii S, Huang MH, Karlamangla AS. Predicting the timeline to the final menstrual period: the study of women’s health across the nation. J Clin Endocrinol Metab. 2013;98(4):1483–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Santoro N, Brockwell S, Johnston J, et al. Helping midlife women predict the onset of the final menses: SWAN, the Study of Women’s Health Across the Nation. Menopause. 2007;14(3 Pt 1):415–424. [DOI] [PubMed] [Google Scholar]
- 38. Nair S, Slaughter JC, Terry JG, et al. Anti-mullerian hormone (AMH) is associated with natural menopause in a population-based sample: the CARDIA Women’s Study. Maturitas. 2015;81(4):493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. La Marca A, Stabile G, Artenisio AC, Volpe A. Serum anti-Mullerian hormone throughout the human menstrual cycle. Hum Reprod. 2006;21(12):3103–3107. [DOI] [PubMed] [Google Scholar]
- 40. Guthrie J, Dennerstein L, Burger H. How reliably does 12-month amenorrhea define final menstrual period? Data from a longitudinal study. Climacteric. 2002;5(1):92. [PubMed] [Google Scholar]
- 41. Kissell KA, Danaher MR, Schisterman EF, et al. Biological variability in serum anti-Müllerian hormone throughout the menstrual cycle in ovulatory and sporadic anovulatory cycles in eumenorrheic women. Hum Reprod. 2014;29(8): 1764–1772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Seifer DB, Maclaughlin DT. Mullerian inhibiting substance is an ovarian growth factor of emerging clinical significance. Fertil Steril. 2007;88(3):539–546. [DOI] [PubMed] [Google Scholar]
- 43. Bastian LA, Smith CM, Nanda K. Is this woman perimenopausal? JAMA. 2003;289(7):895–902. [DOI] [PubMed] [Google Scholar]
- 44. Sadraie SH, Saito H, Kaneko T, Saito T, Hiroi M. Effects of aging on ovarian fecundity in terms of the incidence of apoptotic granulosa cells. J Assist Reprod Genet. 2000;17(3): 168–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Seifer DB, DeJesus V, Hubbard K. Mitochondrial deletions in luteinized granulosa cells as a function of age in women undergoing in vitro fertilization. Fertil Steril. 2002;78(5):1046–1048. [DOI] [PubMed] [Google Scholar]
- 46. Santoro N, Isaac B, Neal-Perry G, et al. Impaired folliculogenesis and ovulation in older reproductive aged women. J Clin Endocrinol Metab. 2003;88(11):5502–5509. [DOI] [PubMed] [Google Scholar]
- 47. Steiner AZ, Pritchard D, Stanczyk FZ, et al. Association between biomarkers of ovarian reserve and infertility among older women of reproductive age. JAMA. 2017;318(14):1367–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Freeman EW, Sammel MD, Lin H, Boorman DW, Gracia CR. Contribution of the rate of change of antimüllerian hormone in estimating time to menopause for late reproductive-age women. Fertil Steril. 2012;98(5):1254–1259.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Avis NE, Crawford SL, Greendale G, et al. ; Study of Women’s Health Across the Nation . Duration of menopausal vasomotor symptoms over the menopause transition. JAMA Intern Med. 2015;175(4):531–539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Jacobsen BK, Heuch I, Kvåle G. Age at natural menopause and all-cause mortality: a 37-year follow-up of 19 731 Norwegian women. Am J Epidemiol. 2003;157(10):923–929. [DOI] [PubMed] [Google Scholar]
- 51. Ossewaarde ME, Bots ML, Verbeek AL, et al. Age at menopause, cause-specific mortality and total life expectancy. Epidemiology. 2005;16(4):556–562. [DOI] [PubMed] [Google Scholar]
- 52. Kritz-Silverstein D, Barrett-Connor E. Early menopause, number of reproductive years, and bone mineral density in postmenopausal women. Am J Public Health. 1993;83(7):983–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Cui R, Iso H, Toyoshima H, et al. ; JACC Study Group . Relationships of age at menarche and menopause, and reproductive year with mortality from cardiovascular disease in Japanese postmenopausal women: the JACC study. J Epidemiol. 2006;16(5):177–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Hu FB, Grodstein F, Hennekens CH, et al. Age at natural menopause and risk of cardiovascular disease. Arch Intern Med. 1999;159(10):1061–1066. [DOI] [PubMed] [Google Scholar]
- 55. Muka T, Oliver-Williams C, Kunutsor S, et al. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol. 2016;1(7):767–776. [DOI] [PubMed] [Google Scholar]
- 56. Gao Y, Zhao M, Dai X, Tong M, Wei J, Chen Q. The prevalence of endometrial cancer in pre- and postmenopausal Chinese women. Menopause. 2016;23(8):884–887. [DOI] [PubMed] [Google Scholar]
- 57. Kelsey JL, Gammon MD, John EM. Reproductive factors and breast cancer. Epidemiol Rev. 1993;15(1):36–47. [DOI] [PubMed] [Google Scholar]
- 58. Collaborative Group on Hormonal Factors in Breast Cancer. Menarche, menopause, and breast cancer risk: individual participant meta-analysis, including 118 964 women with breast cancer from 117 epidemiological studies. Lancet Oncol. 2012;13(11):1141–1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Restrictions apply to the availability of data generated or analyzed during this study to preserve patient confidentiality or because they were used under license. The corresponding author will on request detail the restrictions and any conditions under which access to some data may be provided.