Abstract
Introduction:
Spatial navigation deficits are observed in Alzheimer disease (AD) cross-sectionally, but prediction of longitudinal clinical decline has been less examined.
Methods:
Cognitive mapping (CM) was assessed in 95 participants and route Learning (RL) was assessed in 65 participants at baseline. Clinical progression over an average of 4-5 years was assessed using the Clinical Dementia Rating scale. Relative predictive ability was compared to episodic memory, hippocampus and cerebrospinal fluid biomarkers (ptau181/Aβ42 ratio).
Results:
CM and RL were predictors of clinical progression (ps<.032). All measures, except RL-Learning, remained predictors with episodic memory in models (ps<.048). Only RL-Retrieval remained a predictor when ptau181/Aβ42 was included (p<.001). CM interacted with hippocampus and ptau181/Aβ42 in prediction (ps<.013). CM, RL and episodic memory evidenced strong diagnostic accuracy (AUCs=.894, .794 and .735, respectively); CM tended to perform better than episodic memory (p=.056).
Discussion:
Baseline spatial navigation performance may be appropriate for assessing risk of clinical progression.
Keywords: allocentric, egocentric, response learning, place learning, amyloid, preclinical Alzheimer’s disease
1. Introduction
There is a current research emphasis on developing cognitive measures that are sensitive to preclinical Alzheimer disease (AD), which is associated with an increased risk of developing symptomatic AD [1,2]. During preclinical AD, individuals are clinically normal but evidence AD-related pathological changes, determined using biomarkers for beta-amyloid deposition and neurofibrillary tangles [1,3–5]. Thus, preclinical AD is associated with decreased cerebrospinal fluid (CSF) Aβ42, increased CSF phosphorylated tau (ptau181), and elevations in PET measures of amyloid and tau [5]. Additionally, smaller brain volumes are observed in preclinical AD, including smaller hippocampal volumes [6–8, but see 9,10]. Sensitive cognitive measures for preclinical AD are important for determining trajectories of cognitive decline and response to intervention, as future disease modifying treatments may be most effective during the earliest stages [11]. Considering the expense and/or invasiveness of current methods in identifying preclinical AD (i.e., lumbar puncture, PET), cognitive tasks may represent an opportunity for a more accessible and lower risk initial screening procedure [1].
Existing methods for delineating cognitive deficits in mild cognitive impairment (MCI) and symptomatic AD tend to focus on traditional psychometric measures, with an emphasis on episodic memory. However, these may not be sufficiently sensitive to subtler cognitive difficulties present in preclinical AD [12–14]. There is emerging interest in targeting spatial navigation abilities that may prove more sensitive to preclinical AD than current psychometric measures used in identifying MCI and symptomatic AD [15,16]. This interest is consistent with amyloid and tau deposition, and volumetric declines, occurring early in brain regions that subserve navigation [7, 17,18]. For example, hippocampal place and entorhinal grid cells support spatial navigation [19], with evidence of AD-related dysfunction in these neuronal substrates [18].
Deficits in multiple aspects of spatial navigation are consistently observed in MCI and symptomatic AD [18, 20], including impairments in route learning and cognitive mapping. Route learning is based on an egocentric representation in which an individual learns a specific path within the environment based on a sequence of body-turns relative to landmarks (e.g., repeatedly taking same path between the grocery store and post office based on turning right at the gas station, left at the church, etc.). Cognitive mapping involves a world-centered representation wherein an individual acquires survey-like knowledge incorporating inter-relationships amongst landmarks (e.g., developing a mental map of the grocery store being south of the church, which is west of the post office). Route learning involves striatal circuits whereas cognitive mapping involves hippocampal circuits [21,22]. Importantly, recent work suggests that individuals in the preclinical AD continuum (i.e., low CSF Aβ42) have differential deficits in cognitive map formation relative to route learning [23]. This cognitive mapping task demonstrated high sensitivity (92%; 57% specificity) in detecting the preclinical AD continuum [23], was more sensitive than a standard episodic memory task or route learning [23] and had strong psychometric properties [24].
This past work was cross-sectional and longitudinal investigations are necessary to confirm the utility of spatial navigation tasks for predicting clinical progression. We examined whether spatial navigation tasks were significant predictors of clinical progression with the hypothesis that the cognitive mapping task would be a more robust predictor than route learning. We also examined the relative predictive ability of these tasks in comparison to previously reported predictors of clinical progression, including AD biomarkers (CSF ptau181/Aβ42 ratio), hippocampal volume and standard measures of episodic memory. Lastly, we examined the diagnostic accuracy of the cognitive measures.
2. Methods
2.1. Participants.
Participants were recruited from the Knight Alzheimer Disease Research Center (ADRC) at Washington University and initially participated in previous spatial navigation studies [23,24]. Ninety-eight participants completed the cognitive mapping (CM) task and sixty-seven completed the route learning (RL) task. Three individuals who completed the CM task and two who completed the RL task did not have longitudinal data. The final sample was 95 for the CM cohort and 65 for the RL cohort with 63 individuals completing both tasks (see Tables 1 and 2 for sample descriptions). Three individuals were in both initial studies; data from initial administration are included here.
Table 1.
Sample characteristics: cognitive mapping task
| Total Sample | No Progression | Yes Progression | |
|---|---|---|---|
| N | 95 | 78 | 17 |
| Gender (M/F) | 49/46 | 39/39 | 10/7 |
| Age (years) (M, SD)* | 72.10 (8.40) | 70.60 (7.86) | 78.94 (7.52) |
| Age range | 50-90 | 50-85 | 66-90 |
| Education (years) (M, SD) | 16.57 (2.38) | 16.40 (2.44) | 17.19 (2.07) |
| Education (range) | 12-21 | 12-21 | 13-20 |
| Health Composite (M, SD)* | .77 (.82) | .67 (.77) | 1.24 (.90) |
| Visuomotor Expertise Time (M, SD) | 53.94 (3.68) | 53.71 (3.25) | 54.99 (5.23) |
| Visuomotor Expertise Time (range) | 48-64 | 48-61 | 48-64 |
| Computer Experience (M, SD) | 6.53 (3.39) | 6.56 (3.50) | 6.38 (2.88) |
| Computer Experience (range) | 0-15 | 0-15 | 1-11 |
| Baseline CDR-SB (M, SD)* | .54 (1.47) | .17 (.78) | 2.21 (2.47) |
| Baseline CDR-SB (range | 0-8 | 0-4.5 | 0-8 |
| Time in study (years) (M, SD) | 4.16 (1.84) | 4.11 (1.86) | 4.04 (1.83) |
| Time in study (range) | 1.02-7.07 | 1.19-7.07 | 1.02-7.02 |
| Number CDR follow ups (M, SD) | 4.40 (1.84) | 4.35 (1.97) | 5.00 (1.84) |
| Number CDR follow ups (range) | 2-8 | 2-8 | 2-8 |
| Episodic Memory (M, SD)* | .04 (.86) | .13 (.69) | −.65 (1.09) |
| Hippocampus (cm3) (N, M, SD)* | 74; 739 (111) | 64; 759 (97) | 10; 613 (114) |
| ptau181/Aβ42 (N, M, SD) | 64; .026 (.022) | 57; .023 (.019) | 7; .049 (.031) |
Notes. CDR=Clinical Dementia Rating scale; CDR-SB=Clinical Dementia Rating Scale-Sum of Boxes; M=mean; SD=standard deviation. Time in study=years between baseline assessment and most recent CDR. At baseline, 81 participants were CDR=0, 10 were CDR=0.5, and 4 were CDR=1. Among the 17 progressors, 7 went from CDR=0 to CDR>0, 6 went from CDR=0.5 to CDR=1, 1 went from CDR=0.5 to CDR=2, 3 went from CDR=1 to CDR=2;
p<.05 difference between no progression and yes progression groups using a t-test or Chi-squared test.
N=67 from Allison et al., 2016; N=28 from Allison et al., in press). Participants had CSF (M=.87, range=−1.97-1.68) and MRI (M=.90, range=−2.00-1.99) collection within 2 years of the cognitive mapping condition and memory assessment within 1.02 years (M=.47, range=.10-1.02).
Table 2.
Sample characteristics: route learning task
| Total Sample | No Progression |
Yes Progression |
|
|---|---|---|---|
| N | 65 | 48 | 17 |
| Gender (M/F) | 32/33 | 21/27 | 11/6 |
| Age (years) (M, SD)* | 71.98 (9.35) | 69.60 (8.82) | 78.71 (7.48) |
| Age (range) | 50-90 | 50-85 | 66-90 |
| Education (years) (M, SD) | 16.35 (2.38) | 16.04 (2.41) | 17.31 (2.09) |
| Education range | 12-20 | 12-20 | 12-20 |
| Health Composite (M, SD)* | .85 (.85) | .69 (.80) | 1.29 (.85) |
| Visuomotor Expertise Time (M, SD) | 54.29 (4.40) | 54.17 (4.22) | 54.65 (4.99) |
| Visuomotor Expertise Time (range) | 47-63 | 48-63 | 47-62 |
| Computer Experience (M, SD) | 7.11 (3.37) | 7.23 (3.63) | 6.77 (2.59) |
| Computer Experience (range) | 0-15 | 0-15 | 2-11 |
| Baseline CDR-SB (M, SD)* | .89 (1.88) | .28 (.98) | 2.62 (2.66) |
| Baseline CDR-SB (range) | 0-8 | 0-4.5 | 0-8 |
| Time in study (years) (M, SD)* | 4.95 (1.60) | 5.26 (1.37) | 4.06 (1.91) |
| Time in study (range) | 1.02-7.07 | 1.19-7.07 | 1.02-7.07 |
| Number CDR follow ups (M, SD) | 5.00 (1.83) | 5.19 (1.72) | 4.47 (2.07) |
| Number CDR follow ups (range) | 2-8 | 2-8 | 2-8 |
| Episodic Memory (M, SD)* | −.03 (.88) | .19 (.72) | −.65 (.99) |
| Hippocampus (cm3) (N, M, SD)* | 49; 725 (131) | 38; 763 (106) | 11; 593 (127) |
| ptau181/Aβ42 ratio (N, M, SD) | 41; .023 (.018) | 34; .018 (.011) | 7; .044 (.028) |
Notes. CDR=Clinical Dementia Rating Scale; CDR-SB=Clinical Dementia Rating Scale-Sum of Boxes; M=mean; SD=standard deviation. Time in study=years between baseline assessment and most recent CDR. At baseline, 50 participants were CDR=0, 10 were CDR=0.5, and 5 were CDR=1. Among the 17 progressors, 6 went from CDR=0 to CDR>0, 6 went from CDR=0.5 to CDR=1, 1 went from CDR=.5 to CDR=2, 4 went from CDR=1 to CDR=2;
p<.05 difference between no progression and yes progression groups using a t-test or Chi-squared test.
N=65 from Allison et al., 2016. Participants had CSF (M=.72, range=−1.97-1.66) and MRI (M=.90, range=−2.15-2.03) collection within 2.15 years of the route learning condition and memory assessment within 1 year (M=.42, range=.11-.92).
Participants were screened for major medical conditions, including Parkinson’s disease, Huntington’s disease, stroke, seizures and major head injury. Participants had normal vision or wore corrective lenses. Participants consented to participation in accordance with Washington University Human Research Protection Office guidelines.
2.2. Clinical Dementia Rating Scale (CDR).
The CDR global score was used to determine absence or presence, as well as severity, of dementia (CDR of 0=clinical normality; 0.5=very mild dementia; 1=mild dementia; 2=moderate dementia; 3=severe dementia; [25]. Clinical diagnosis of symptomatic AD is made in accordance with criteria reported by NINCDS-ADRDA [26]. Individuals clinically diagnosed with AD at the Knight ADRC, including those meeting MCI criteria, have AD pathology in 93% of cases [27,28].
The CDR-Sum of Boxes score (CDR-SB; range: 0-18) provides a quantitative estimate of clinical impairment and is based on scores in six cognitive and functional domains (memory, orientation, judgment and problem solving, community affairs, home and hobbies, personal care) used to generate the global CDR score. The larger range of CDR-SB scores allows for increased precision and additional information regarding impairment, especially for mild impairment [29–31].
Clinical progression was defined as: a) an increase in global CDR score from the time of the spatial navigation tasks to the most recent follow-up; and b) increase in CDR-SB across measurement occasions. CDR global scores collected between baseline and most recent follow-up were not used in determining clinical progression.
2.3. Virtual Navigation Tasks.
Administration of navigation tasks are described fully in prior work [23,24]. The experimental maze environments consisted of a series of interconnected hallways with landmarks and wallpapers that differed in color. A joystick was used to maneuver through the environment. Participants completed practice and a visuomotor expertise test in a separate environment with the latter requiring traversal of a simple environment within a specified time.
2.3.1. Cognitive Mapping Task.
A CM task was administered in prior studies [23,24] using similar procedures with differences in administration noted below. Across both studies, there were learning and retrieval phases. There were 3 study-test trials during the learning phase. During study, participants freely explored the environment for 7 [23] or 4 minutes [24]. Participants were then given a blank 2D map of the environment and placed Xs at landmark locations. The learning score (CM-Learning) was the average number of correct locations across trials ([23]: range=0-20; [24]: range=0-18). After a 10-minute [23] or 15-minute delay [24], participants completed 12 [23] or 6 trials [24] in which they were presented with a picture of a landmark and navigated to the landmark using the shortest path as quickly as possible. The retrieval score (CM-Retrieval) was the average amount of time taken to find landmarks. To account for differences in administration, scores within each sample were standardized (z-transformed) to obtain an estimate of each individual’s relative ranking and have the same scale across studies.
2.3.2. Route Learning Task.
The RL task included learning and delayed retrieval phases [23]. During learning, participants followed the same route marked by arrows repeatedly for 5 minutes. Next, participants drew the path on a blank 2D map of the environment. These study-test trials were completed 4 times. The learning score (RL-Learning) was the average proportion of correctly drawn turns at intersections relative to the total number of intersections along the route across 4 trials (range=0-1). After a 10-minute delay, participants traversed the designated route without arrows 3 times. The average amount of time taken to traverse the route across trials was the retrieval phase variable (RL-Retrieval).
2.4. Episodic Memory Composite.
` An episodic memory composite was created from preexisting ADRC psychometric data. A measure of list learning was free recall from the Selective Reminding Task[32]. A measure of paragraph memory was immediate and delayed recall on the Logical Memory test from Wechsler Memory scales [33,34]. A measure of paired associates learning was the Associate Learning task from Wechsler Memory Scales. Standardized scores for each participant were averaged to create the composite score. Different versions of the Wechsler Scales [33,34] have been administered over time and across cohorts. To account for multiple versions of a test, raw scores from each subsample were standardized separately and then combined across data sets.
2.5. CSF Collection and Processing.
CSF collection has been previously described [35]. All CSF samples were analyzed using next generation Elecsys electrochemiluminescent immunoassays for Aβ42 and ptau181 developed by Roche Diagnostics (Basel, Switzerland) and run on the automated Roche Cobas e 601 analyzer [36]. Values for Aβ42 above 1,700 pg/ml have not been validated and are not to be used in clinical decision making. The ratio between ptau181 and Aβ42 was used as the AD biomarker measure because it has been found to best map onto PET imaging results [37].
2.6. Structural MRI Acquisition and Processing.
MRI scans were acquired using one of two Siemens 3T scanners (TIM Trio: TE=3ms, TR=2400ms, TI=1000ms, FA=8°, 256x256 mm acquisition matrix, 1x1x1mm voxels; BioGraph scanner: TE=2.95ms, TR=2300ms, TI=900ms, FA=9°, 240x256 mm acquisition matrix, 1x1x1.2mm voxels). The FreeSurfer image analysis suite v5.3 was used for image processing and delineation of the hippocampus [38, 39]. FreeSurfer implements an automated probabilistic labeling procedure where individual voxels are assigned a neuroanatomical label based on data from a manually labeled training set. Volumes were summed across hemispheres and estimated intracranial volume was used to adjust for body size differences using an analysis of covariance approach [40].
2.7. Computer Experience.
Participants self-reported their experience with computers, computer games and virtual reality games (Likert scale 0-7). A measure of computer experience was created by summing the experience scores (0-21).
2.8. Health Composite.
A health composite (0-5) was created using the sum of past or present mild head trauma, heart problems, hypertension, diabetes and depression.
2.9. Statistical Analyses.
2.9.1. General.
CM-Learning, CM-Retrieval, RL-Learning and RL-Retrieval variables were examined in separate models. When a spatial navigation variable was a significant predictor, previously established predictors were added to the model to assess whether there was unique variance explained by the spatial navigation variable. Established predictors (CSF ptau181/Aβ42, hippocampal volume or episodic memory composite) were examined in separate models. Age, sex, education and the health composite were covariates. For logistic and linear mixed effects models, the learning variables and the episodic memory composite were reverse scored so that higher scores reflected worse performance. Standardized variables were used in all analyses.
2.9.2. CDR Progression.
Logistic regression analyses were conducted using IBM SPSS Statistics 25. These analyses were used to determine whether baseline spatial navigation performance predicted global CDR progression (1=progression; 0=no-progression). Only episodic memory was additionally examined in these models considering the sample size issues with a categorical outcome [41].
2.9.3. Longitudinal Change in CDR-SB.
Linear mixed effects models were used to determine whether spatial navigation performance predicted change in CDR-SB over time. These models were conducted using the nlme package [42] in R version 3.5.1 [43]. Time (years in study) and intercept were random effects. The interaction between the spatial navigation variables and time were the independent variables of primary interest. In models examining relative predictive utility, the other predictor variable (i.e., CSF ptau181/Aβ42, hippocampal volume, episodic memory) and their interaction with time were added to the model. Finally, three-way interactions between spatial navigation variables, CSF ptau181/Aβ42 or hippocampal volume, and time were included in a model to examine whether there was moderation of any relationship observed between CM performance and CDR-SB progression.
In order to assess whether spatial navigation performance may be a sensitive marker of the preclinical AD stage, secondary analyses were conducted including only participants that were clinically normal at baseline (Table 3).
Table 3.
Sample characteristics: CDR=0 at baseline
| Cognitive Mapping Condition | Route Learning Condition | |
|---|---|---|
| N | 81 | 50 |
| Yes Progression/No Progression | 7/74 | 6/44 |
| Gender (M/F) | 40/41 | 22/28 |
| Age (years) (M, SD) | 71.11 (7.73) | 71.99 (9.35) |
| Age range | 50-84 | 50-84 |
| Education (years) (M, SD) | 16.59 (2.40) | 16.35 (2.38) |
| Education (range) | 12-21 | 12-20 |
| Health Composite (M, SD) | .72 (.81) | .85 (.85) |
| Visuomotor Expertise Time (M, SD) | 53.65 (3.23) | 54.56 (4.27) |
| Visuomotor Expertise Time (range) | 48-61 | 48-63 |
| Computer Experience (M, SD) | 6.55 (3.30) | 7.32 (3.23) |
| Computer Experience (range) | 1-15 | 3-15 |
| Baseline CDR-SB (M, SD) | .01 (.06) | .01 (.07) |
| Baseline CDR-SB (range) | 0-.5 | 0-.5 |
| Time in study (years) (M, SD)* | 4.35 (1.84) | 5.53 (1.13) |
| Time in study (range) | 1.33-7.07 | 2.80-7.07 |
| Number CDR follow ups (M, SD)* | 4.54 (1.86) | 5.38 (1.70) |
| Number CDR follow ups (range) | 2-8 | 2-8 |
| Episodic Memory (M, SD) | −.18 (.63) | −.03 (.88) |
| Hippocampus (cm3) (N, M, SD) | 68; 756 (92) | 42; 725 (131) |
| ptau181/Aβ42 (N, M, SD) | 62; .025 (.020) | 39; .021 (.015) |
Notes. CDR=Clinical Dementia Rating scale; CDR-SB=Clinical Dementia Rating Scale-Sum of Boxes; M=mean; SD=standard deviation. Time in study=years between baseline assessment and most recent CDR.
2.9.4. Diagnostic Accuracy.
Receiver operating characteristic analyses were conducted to assess diagnostic accuracy in predicting clinical progression according to global CDR. Standardized composites were created combining learning and retrieval phases within each task. Area under the curve (AUC) values were compared amongst CM, RL and episodic memory composites using the DeLong, DeLong and Clarke-Pearson method [44] with the paired data option. Analyses were conducted for individuals who completed CM, RL and episodic memory tasks (n=63 from the total sample with 15 demonstrating a worse CDR at most recent assessment; n=49 for CDR=0 subsample with 5 converting to CDR>0 at most recent assessment).
2.9.5. Outliers.
Outliers were defined as values >3 STD from the group mean. Analyses were conducted with and without outliers. Unless otherwise specified, results were unchanged when outliers were removed.
3. Results
3.1. Cognitive Mapping.
CM-Learning (OR=3.131, CI=1.376-7.123, p=.007) and CM-Retrieval (OR=2.950, CI=1.444-6.024, p=.003) predicted global CDR progression and these relationships remained with episodic memory added to models (CM-Learning: OR=2.591, CI=1.007-6.667, p=.048; CM-Retrieval: OR=2.409, CI=1.104-5.257, p=.027; Supplementary Table 1). Both also predicted CDR-SB progression (CM-Learning: β=.266, p<.001; CM-Retrieval: β=.250, p=.002; Supplementary Tables 2–3; Supplementary Figure 1), including with episodic memory (CM-Learning: β=.216, p=.003; CM-Retrieval: β=.184, p=.021) or hippocampal volume (CM-Learning: β=.236, p=.004; CM-Retrieval: β=.233, p=.033) included. Neither CM-Learning (β=.122, p=.197) nor CM-Retrieval (β=.123, p=.369) remained significant with ptau181/Aβ42 included.
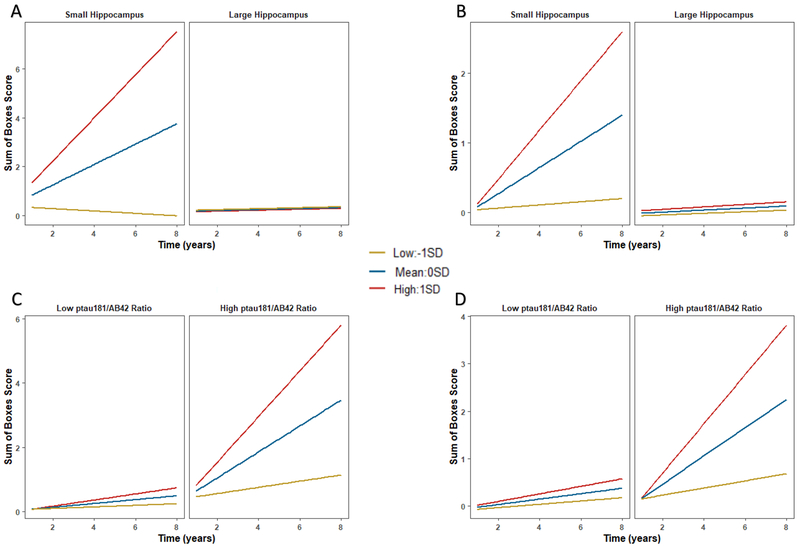
Hippocampal volume significantly moderated the association of CM-Learning (β=−.276, p<.001) and CM-Retrieval (β=−.270, p=.002) with longitudinal CDR-SB progression (Figure 1). With one outlier removed, the ptau181/Aβ42 ratio significantly moderated the association of CM-Learning (without outlier: β=.309, p=.001; with outlier: β=.110, p=.194) and CM-Retrieval (without outlier: β=.355, p=.013; with outlier: β=.176, p=.190) with progression. Individuals with both lower performance and smaller hippocampus (or elevated ptau181/Aβ42) evidenced greater clinical progression (Figure 1; Supplementary Table 4 for simple slopes).
Figure 1.
Interactive effects with the learning phase of the cognitive mapping task. Plots depict a split at 0STD for hippocampal volume and CSF ptau181/Aβ42, and −1STD, 0STD, 1STD for the cognitive mapping variable for illustration purposes. The 0STD value for CSF ptau181/Aβ42 corresponds to cut-off values reported in Hansson et al., 2018 [45]. Analyses were conducted with continuous variables. Higher CM-Learning scores reflect worse performance. A) Hippocampal volume and CM-Learning in total sample. B) Hippocampal volume and CM-Learning in CDR=0 subsample. C) CSF ptau181/Aβ42 and CM-Learning in total sample without an outlier. D) CSF ptau181/Aβ42 and CM-Learning in total sample in CDR=0 subsample without an outlier. A similar pattern was observed for the retrieval phase, but not depicted. See Supplementary Table 4 for results of the simple slopes analyses.
In the clinically normal subsample, CM-Learning (β=.155, p=.024) remained a significant predictor of CDR-SB progression (Supplementary Table 5), including with episodic memory (β=.152, p=.031) or hippocampal volume (β=.170, p=.032) added to models, but not with ptau181/Aβ42 (β=.090, p=.290) included. Again, hippocampal volume moderated the association of CM-Learning with progression (without one outlier β=−.211, p=.057; with outlier β=−.219, p=.009). With one outlier removed, the ptau181/Aβ42 ratio moderated the association of CM-Learning with progression (without outlier: β=.305, p=.002; with outlier: β=.090, p=.268). CM-Retrieval (β=.121, p=.195) was not significant (Figure 1; Supplementary Tables 4 and 6).
3.2. Route Learning.
RL-Learning (OR=3.293, CI=1.307-8.298, p=.011) and RL-Retrieval (OR=6.301, CI=1.941-20.461, p=.002) predicted global CDR progression (Supplementary Table 7). RL-Retrieval remained significant with episodic memory in the model (OR=4.781, CI=1.341-17.045, p=.016), but RL-Learning did not (OR=1.911, CI=.631-5.792, p=.252).
A similar pattern was observed for CDR-SB with both RL-Learning (β=.202, p=.032) and RL-Retrieval (β=.467, p<.001) predicting progression (Supplementary Tables 8–9; Supplemental Figure 1), and only RL-Retrieval predicting with episodic memory included (RL-Learning: β=.015, p=.886; RL-Retrieval: β=.377, p<.001). Neither RL-Learning (β=.035, p=.734) or RL-Retrieval (β=.176, p=.309) were significant with hippocampal volume included. RL-Retrieval remained significant when ptau181/Aβ42 was added to the model (β=.468, p<.001), and RL-Learning was significant with two outliers removed (without outliers: β=.201, p=.010; with outliers: β=.137, p=.103).
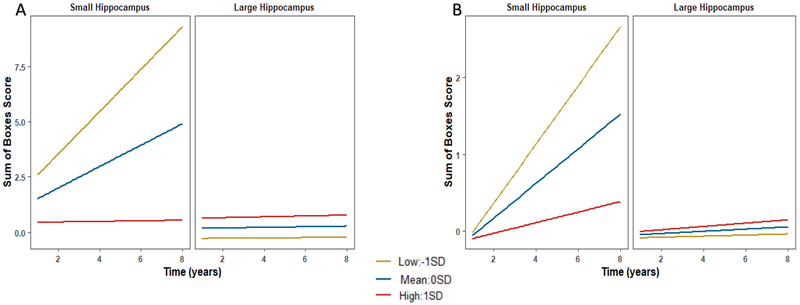
Hippocampal volume did not moderate the association of either RL-Learning (without outlier: β=−.129, p=.276; with outlier: β=−.194, p=.044) or RL-Retrieval (without outlier: β=−.217, p=.059; with outlier: β=−.254, p=.024; Figure 2) with CDR-SB progression when one outlier was removed (Supplementary Table 4). CSF ptau181/Aβ42 did not significantly moderate the association of either RL-Learning or RL-Retrieval with progression (β=.028, p=.691; β=−.037, p=.689, respectively).
Figure 2.
Interactive effects with the route learning task. Plots depict a split at 0STD for hippocampal volume and -1STD, 0STD, 1STD for the route learning variable for illustration purposes. Analyses were conducted with continuous variables. Higher RL-Retrieval scores reflect worse performance. A) Hippocampal volume and RL-Retrieval in total sample without an outlier. B) Hippocampal volume and RL-Retrieval in CDR=0 subsample without an outlier. See Supplementary Table 4 for results of the simple slopes analyses.
In the clinically normal subsample (Supplementary Tables 10–11), RL-Learning was not a significant predictor of CDR-SB progression (β=.009, p=.911). RL-Retrieval was a significant predictor (β=.340, p=.015), and remained significant with episodic memory (β=.350, p=.014) included. With two outliers removed, RL-Retrieval remained significant when ptau181/Aβ42 was added to the model (without outliers: β=.405, p=.002; with outliers: β=.202, p=.225), but not with hippocampal volume (β=−.082, p=.635) included. However, hippocampal volume moderated the relationship of RL-Retrieval with progression (β=−.439, p=.005; Supplementary Table 4; Figure 2). CSF ptau181/Aβ42 was not a significant moderator with one outlier removed (without outlier: β=−.734, p=.525; with outlier: β=−.176, p=.035).
3.3. Diagnostic Accuracy: Global CDR Progression in Full Sample.
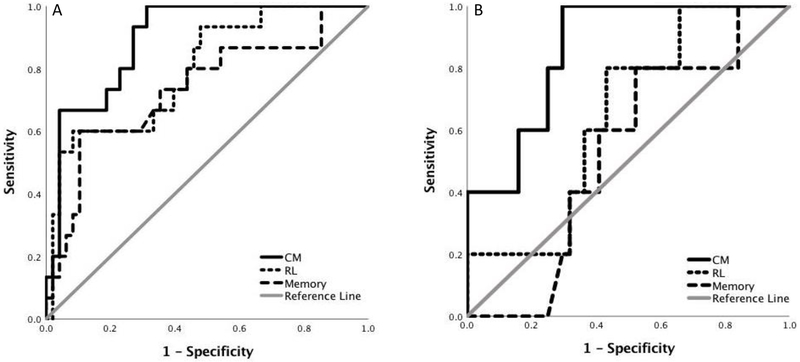
The AUCs for CM (.894 (SE=.041), p<.001; Youden index=.687; sensitivity=1, specificity=.687), RL (.794 (SE=.067), p=.001; Youden index=.517; sensitivity=.600, specificity=.817) and episodic memory (.735 (SE=.080), p=.006; Youden index=.496; sensitivity=.600, specificity=.896) were significant (Figure 3). CM and RL AUCs were not significantly different (χ2=1.59, p=.207). RL and episodic memory AUCs were not significantly different (χ2=.91, p=.339). There was a non-significant trend for the CM AUC to be significantly higher than the memory AUC (χ2=3.67, p=.056).
Figure 3.
ROC curves for cognitive mapping, route learning and episodic memory tasks. A) Results for the clinically heterogenous sample. B) Results for the clinically normal sample. See text for details.
3.4. Diagnostic Accuracy: Conversion from Global CDR=0 to CDR>0.
The AUC for CM (.859 (SE=.067), p=.009; Youden index=.705; sensitivity=1, specificity=.705) was significant, but the AUCs for RL (.645 (SE=.109), p=.291; Youden index=.368; sensitivity=.800, specificity=.568) and episodic memory (.527 (SE=.12), p=.843; Youden index=.277; sensitivity=.800, specificity=.477) were not. The CM AUC was significantly higher than the memory AUC (χ2=8.12, p=.004). The AUCs for CM and RL, and for RL and episodic memory, were not significantly different (CM vs RL: χ2=1.66, p=.198; RL vs episodic memory: χ2=.86, p=.389).
4. Discussion
This longitudinal study examined whether tasks assessing the ability to form, retain and use a cognitive map or the ability to learn and retrieve a novel route predicted clinical progression. In a clinically heterogenous sample, with individuals ranging from clinically normal to mild symptomatic AD, both CM and RL predicted clinical progression and discriminated between progressors and non-progressors. This is consistent with progressive involvement of hippocampal and striatal circuits with disease progression [46], as well as with deficits in both CM and RL in symptomatic AD [18,20,23]. Thus, when seeking to predict clinical progression in individuals with varying levels of clinical impairment, deficits in multiple aspects of spatial navigation may serve to predict later disease progression. Although not examined here, there is some research that spatial navigation may also prove useful in differential diagnosis of dementia subtypes (e.g., symptomatic AD vs frontotemporal dementia; [47]).
Notably, results highlight that in terms of cognitive measures, CM may be more useful than psychometric measures of episodic memory given the trend for greater diagnostic accuracy in the clinically heterogenous sample. Furthermore, individuals with lower CM performance in addition to elevated AD pathology or reduced hippocampal volume evidenced the greatest degree of clinical progression. Thus, not only may CM be useful in prediction in isolation, but it may have added value when used in conjunction with hippocampal volume and/or AD biomarkers. In contrast, while RL evidenced incremental utility relative to AD biomarkers and did discriminate, it was not better than episodic memory and did not as robustly interact with hippocampal volume or AD pathology. These findings place limits on the utility of the RL task.
A particularly critical issue is developing cognitive tasks sensitive to preclinical AD. Importantly, results also provide evidence that CM may be particularly useful relative to RL and episodic memory for identifying individuals at risk in this phase. That is, results suggest that identifying clinically normal individuals with both AD pathology (or reduced hippocampal volume) as well as CM-Learning deficits may be particularly useful for assessing who is at most risk for clinical progression. Again, RL-Retrieval did evidence some positive features (e.g., incremental utility relative to ptau181/Aβ42, interaction with hippocampus). However, CM was better than episodic memory in discriminating progression to symptomatic AD in individuals who were clinically normal at baseline, and RL did not significantly discriminate. This is consistent with cross-sectional findings of a significant deficit in CM, but not RL, in individuals in the preclinical AD continuum [23]. Differential utility of CM measures is also consistent with neuropathology occurring early in CM-relevant brain regions, such as entorhinal cortex and hippocampus [7, 17–19].
Overall, current findings are consistent with two prior investigations examining the ability of spatial navigation tasks to predict conversion to MCI, and conversion from MCI to symptomatic AD [48, 49; but see 50]. Of note, these studies mainly utilized CM tasks for assessing spatial navigation and did not incorporate comparisons to AD biomarkers or episodic memory. Thus, current findings add substantively to this literature by supporting the incremental utility of both CM and RL as well as by providing more direct evidence that CM may be particularly useful.
Limitations include the relatively small number of clinically normal individuals converting to AD. While we did observe significant findings for CM consistent with cross-sectional work, replication in a larger sample is warranted. Administration of CM tasks varied across baseline studies, potentially leading to differences in absolute performance level. However, both studies observed task associations with CSF Aβ42 and evidence of utility relative to episodic memory [23,24], providing support that the versions were capturing the same construct. While we robustly estimated episodic memory with three tasks, there was some variation in Wechsler Memory Scale version. Lastly, the sample consisted of highly educated individuals limiting generalizability.
Collectively, current findings indicate that baseline CM and RL performance were associated with future clinical progression. These findings highlight the potential utility of spatial navigation tasks as assessment tools for identifying risk of progression to more advanced stages of AD. Measures of CM may be particularly useful considering evidence that these may be more powerful than standard episodic memory measures. Furthermore, recent work has established strong psychometric properties of CM [24]. Longitudinal investigations of spatial navigation would be important to determine its ability to track subtle cognitive changes in the preclinical AD phase [15,16]. In addition, future work should incorporate longitudinal imaging and biomarker data to assess whether baseline spatial navigation predicts changes in AD biomarkers and brain structure.
Supplementary Material
Supplementary Figure 1. Spaghetti plots of individual trajectories. Plots depict tertiles for illustration purposes. Analyses were conducted with continuous variables.
5. Acknowledgements
This work was supported by NIH grants P50 AG05861, P01 AG03991, P01 AG026276, Spring 2015 Association for Psychological Science Student Grant, 2015 Society for Clinical Neuropsychology Dissertation Award and 2015 Psychological and Brain Sciences Dissertation Grant. T. F. Levine was supported by the National Science Foundation DGE-1745038. S. Allison was supported by National Institute on Aging 5T32AG00030. We thank the Knight ADRC Biomarker Core for CSF analysis, the Clinical Core for participant assessments and the Imaging Core for MRI data. We thank Chauncey Scott, Tyler Blazey and Chiharu Johnston for assistance with the development of the virtual maze environments and programming the virtual maze tasks.
6. Declaration of Interest
Taylor F Levine: none
Samantha L Allison: none
Marta Stojanovic: none
Anne M Fagan is supported by NIH grants including P50AG005681, P01AG003991, P01AG026276 and UF01AG03243807. She is on the Scientific Advisory Boards for Roche Diagnostics, AbbVie and Genentech and consults for Araclon Biotech/Griffols, Biogen, and DiamiR.
John C Morris is funded by NIH grants # P50AG005681; P01AG003991; P01AG026276 and UF1AG032438. Neither Dr. Morris nor his family owns stock or has equity interest (outside of mutual funds or other externally directed accounts) in any pharmaceutical or biotechnology company.
Denise Head: none
7. References
- [1].Jack CR, Bennett DA, Blennow K, Carrillo MC, Dunn B, Haeberlein SB, et al. NIA-AA Research Framework: Toward a biological defition of Alzheimer’s disease. Alzheimer’s & Dementia 2018;14:535–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Vos SJ, Xiong C, Visser PJ, Jasielec MS, Hassenstab J, Grant EA, et al. Preclinical Alzheimer’s disease and its outcome: A longitudinal cohort study. Lancet Neurol 2013;12:957–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Bloom GS Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol 2014;71:505–508. [DOI] [PubMed] [Google Scholar]
- [4].Price JL, McKeel DW, Buckles VD, Roe CM, Xiong C, Grundman M, et al. Neuropathology of nondemented aging: Presumptive evidence for preclinical Alzheimer disease. Neurobiology of Aging 2009;30:1026–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Brier MR, Gordon B, Friedrichsen K, McCarthy J, Stern A, Christensen J, et al. Tau and abeta imaging, CSF measures, and cognition in Alzheimer’s disease. SciTransl Med 2016;8:338–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Bernard CC, Helmer B, Dilharreguy H, Amieva S, Auriacombe JF, Dartigues M, et al. Time course of brain volume changes in the preclinical phase of Alzheimer’s disease. Alzheimers ement 2014;10:143–151. [DOI] [PubMed] [Google Scholar]
- [7].Storandt M, Mintun MA, Head D, Morris JC Cognitive decline and brain volume loss as sigatures of cerebral amyloid-beta peptide deposition identified with Pittsburgh compound B: Cognitive decline associated with abeta deposition. Arch Neurol 2009;66:1476–1481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Gordon BA, Blazey T, Su Y, Fagan AM, Holtzman DM, Morris JC, Benzinger TL Longitudinal β-amyloid deposition and hippocampal volume in preclinical Alzheimer disease and suspected non-Alzheimer disease pathophysiology. JAMA Neurol 2016;73:1192–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Clark LR, Berman SE, Norton D, Koscik RL, Jonaitis E, Blennow K, et al. Age-accelerated cognitive decline in asymptomatic adults with CSF β-amyloid. Neurology 2018;90:1306–1315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Schoonenboom NS, van der Flier WM, Blankenstein MA, Bouwman FH, Van Kamp GJ, Barkhof F, Schelten P CSF and MRI markers independetly contribute to the diagnosis of Alzheimer’s disease. Neurobiol Aging 2008;29:669–675. [DOI] [PubMed] [Google Scholar]
- [11].Garcia-Alloza MM, Subramanian D, Thyssen LA, Borrelli A, Fauq P, Das TE, et al. Existing plaques and neuritic abnormalities in APP:PS1 mice are not affected by administration of the gamma-secretase inhibitor LY-411575. Mol Neurodegener 2009;6:4: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hedden T, Oh H, Younger AP, Patel TA Meta-analysis of amyloid-cognition relations in cognively normal older adults. Neurology 2013;80:1341–1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Loewenstein DA, A. D, R.E. Curiel, R. Duara, H. Buschke Novel cognitive paradigms for the detection of memory impairment in preclinical Alzheimer’s disease. Assessment 2018;25:348–359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Rentz DM, Para Rodriguez MA, Amariglio R, Stern Y, Sperling R, Ferris S Promising developments in neuropsychological approaches for the detection of preclinical Alzheimer’s disease: A selective review. Alzheimers Res Ther 2013;5:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ritchie KM, Ropacki B, Albala J, Harrison J, Kaye J, Kramer C, et al. Recommended cognitive outcomes in preclinical Alzheimer’s disease: Consensus statement from the European Prevention of Alzheimer’s Dementia project. Alzheimers Dement 2017;13:186–195. [DOI] [PubMed] [Google Scholar]
- [16].Weintraub S, Carrillo MC, Farias ST, Goldberg TE, Hendrix JA, Jaeger J, et al. Measuring cognition and function in the preclinical stage of Alzheimer’s disease. Alzheimers Dement 2018;4:64–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Braak H, & Del Tredici K The preclinical phase of the pathological process underlying sporadic Alzheimer’s disease. Brain 2015;138:2814–2833. [DOI] [PubMed] [Google Scholar]
- [18].Coughlan G, Laczó J, Hort J, Minihane AM, Hornberger M Spatial navigation deficits overlooked cognitive marker for preclinical Alzheimer disease? Nat Rev Neurol 2018;14:496–506. [DOI] [PubMed] [Google Scholar]
- [19].Moser EI, Kropff E, Moser M Place cells, grid cells and the brain’s spatial representation system. Annu Rev Neurosci 2008;31:69–89. [DOI] [PubMed] [Google Scholar]
- [20].Lithfous S, Dufour A, Despres O Spatial navigation in normal aging and the prodromal stage of Alzheimer’s disease: Insights from imaging and behavioral studies. Ageing Res Rev 2013;12:201–213. [DOI] [PubMed] [Google Scholar]
- [21].Packard MG, McGaugh JL Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol Learn Mem 1996;65:65–72. [DOI] [PubMed] [Google Scholar]
- [22].Iaria G, Petrides M, Dagher A, Pike B, Bohbot VD Cognitive strategies dependent on the hippocampus and caudate nucleus in human navigation: Variability and change with practice. J Neurosci 2003;23:5945–5952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Allison SL, Fagan AM, Morris JC, Head D Spatial navigation in preclinical Alzheimer’s disease. J Alzheimers Dis 2016;52:77–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Allison SL, Rodebaugh TL, Johnston C, Fagan AM, Morris JC, Head D Developing a spatial navigation screeing tool sensitive to the preclinical Alzheimer disease continuum. Arch Clin Neuropsych In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morris JC The Clinical Dementia Rating (CDR): Current version and scoring rules. Neurology 1993;43:2412–2414. [DOI] [PubMed] [Google Scholar]
- [26].McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM Clinical diagnosis of Alzheimer’s disease: Report of the NINCDS-ADRDA Work Group under the auspices of Department of Health and Human Services Task Force on Alzheimer’s Disease. Neurology 1984;34:939–944. [DOI] [PubMed] [Google Scholar]
- [27].Berg L, McKeel DW, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathological studies in cognitively healthy aging and Alzheimer disease. Arch Neurol 1998;55:326–335. [DOI] [PubMed] [Google Scholar]
- [28].Storandt M, Grant EA, Miller JP, Morris JC Longitudinal course and neuropathological outcomes in original vs revised MCI and in pre-MCI. Neurology 2006;67:467–473. [DOI] [PubMed] [Google Scholar]
- [29].Lynch CA, Walsh C, Blanco A, Moran M, Coen RF, Walsh JB, Lawlor BA The Clinical Dementia Rating sum of box score in mild dementia. Dement Geriatr Cogn Disord 2006;21:40–43. [DOI] [PubMed] [Google Scholar]
- [30].O’Bryant SE, Lacritz LH, Hall J, Waring SC, Chan W, Khodr ZG, et al. Validation of the new interpretive guidelines for the Clinical Dementia Rating scale sum of boxes score in the National Alzheimer’s Coordinating Center database. Arch Neurol 2010;67:746–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Williams M, Storandt M, Roe CM, Morris JC Progression of Alzheimer’s disease as measured by Clinical Dementia Rating sum of boxes scores. Alzheimers Dement 2013;9(1 Suppl):S39–S44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Grober E, Buschke H, Crystal H, Bang S, Dresner R Screening for dementia by memory testing. Neurology 1988;38:900–903. [DOI] [PubMed] [Google Scholar]
- [33].Wechsler D, & Stone CP 1973. Wechsler Memory Scale. New York: Psychological Corporation. [Google Scholar]
- [34].Wechsler D 1997. Wechsler Memory Scale (3rd edition). San Antonio: Psychological Corporation. [Google Scholar]
- [35].Fagan AM, Mintun MA, Mach RH, Lee SY, Dence CS, Shah AR, et al. Inverse relation between in vivo amyloid imaging load and cerebrospinal fluid Abeta42 in humans. Ann Neurol 2006;59:512–519. [DOI] [PubMed] [Google Scholar]
- [36].Bittner T, Zetterberg H, Teunissen CE, Ostlund RE, Militello M, Andreasson U, et al. Technical performance of a novel, fully automated electrochemiluminescence immunoassay for the quantitation of beta amyloid (1-42) in human cerebrospinal fluid. Alzheimers Dement 2016;12:517–526. [DOI] [PubMed] [Google Scholar]
- [37].Schindler SE, Gray JD, Gordon BA, Xiong C, Batrla-Utermann R, Quan M, et al. Cerebrospinal fluid biomarkers measured by Elecsys assays compared to amyloid imaging. Alzheimers Dement 2018;14:1460–1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, et al. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–55. [DOI] [PubMed] [Google Scholar]
- [39].Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, et al. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage 2006;31:968–980. [DOI] [PubMed] [Google Scholar]
- [40].Buckner RL, Head D, Parker J, Fotenos AF, Marcus D, Morris JC, Snyder AZ A unified approach for morphometric and functional data analysis in young, old, and demented adults using automated atlas-based head size normalization: Reliability and validation against manual measurement of total intracranial volume. NeuroImage 2004;23:724–738. [DOI] [PubMed] [Google Scholar]
- [41].Altman DG & Rosyston P The cost ofdichotomising continuous variables. BMJ 2006; 332(7549):1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. nlme: Linear and nonlinear mixed effects models. R package version 3.1. 2013;3(1):111. [Google Scholar]
- [43].Allaire J RStudio: Integrated development environment for R. Boston, MA: 2012. [Google Scholar]
- [44].DeLong ER, DeLong DM, & Clarke-Pearson DL Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1998;44:837–845. [PubMed] [Google Scholar]
- [45].Hansson O, Seibyl K, Stomrud E, Zetterberg H, Trojanowski JQ, Bittner T, et al. CSF biomarkers of Alzheimer’s idsease concord with amyloid-β PET and predict clinical progression: A study of fully automated immunoassays in BioFINDER and ADNI cohorts. Alzheimers Dement 2018;14(11):1470–1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Madsen SK, Ho AJ, Hua X, Saharan PS, Toga AW, Jack CR Jr., et al. 3D maps localize caudate nucleus atrophy in 400 Alzhiemer’s disease, mild cognitive impairment, and healthy elderly subjects. Neurobiol Aging 2010;31(8):1312–1325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Tu S, Spiers HJ, Hodger JR, Piguet O, Hornberger M Egocentric versus allocentric spatial memory in behavioral variant frontotemporal dementia and Alzheimer’s disease. J Alzheimers Dis 2017;59(3)883–892 [DOI] [PubMed] [Google Scholar]
- [48].Verghese J, Lipton R, Ayers E Spatial navigation and risk of cognitive impairment: A prospective cohort study. Alzheimers Dement 2017;13:985–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Wood RA, Moodley KK, Lever C, Minati L, Chan D Allocentric spatial memory testing predicts conversion from mild cognitive impairment to dementia: An initial proof-of concept study. Front Neurol 2016;7:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Weniger G, Ruhleder M, Lange C, Wolf S, Irle E Egocentric and allocentric memory as assessed by virtual reality in individuals with amnestic mild cognitive impairment. Neuropsychologia 2011;49:518–527. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Spaghetti plots of individual trajectories. Plots depict tertiles for illustration purposes. Analyses were conducted with continuous variables.