Abstract
The National Heart, Lung, and Blood Institute (NHLBI) convened a multidisciplinary working group of hypertension researchers on December 6-7, 2018, in Bethesda, Maryland to share current scientific knowledge in hypertension and to identify barriers to translation of basic into clinical science/trials and implementation of clinical science into clinical care of patients with hypertension. The goals of the working group were: 1) to provide an overview of recent discoveries that may be ready for testing in pre-clinical and clinical studies; 2) to identify gaps in knowledge that impede translation; 3) to highlight the most promising scientific areas in which to pursue translation; 4) to identify key challenges and barriers for moving basic science discoveries into translation, clinical studies and trials; and 5) to identify roadblocks for effective dissemination and implementation of basic and clinical science in real world settings. The working group addressed issues that were responsive to many of the objectives of the NHLBI Strategic Vision. The working group identified major barriers and opportunities for translating research to improved control of hypertension. This review summarizes the discussion and recommendations of the working group.
Keywords: hypertension, clinical trial, translational research, immunology, microbiome, sex differences, clinical practice
Introduction
Hypertension remains a leading cause of global death and disability from cardiovascular disease (CVD) and stroke.1 The 2017 American College of Cardiology (ACC)/American Heart Association (AHA) clinical practice guidelines on hypertension reported a markedly increased number of people with diagnosed and undetected hypertension.2 The Systolic Blood Pressure Intervention Trial (SPRINT) data suggest that intensive lowering of blood pressure (BP) can significantly lower CVD events and mortality.3 Despite extensive research and the existence of multiple effective therapeutic interventions, hypertension remains an important public health challenge in the United States, and hypertension-related mortality continues to increase. This suggests that in spite of the commitment and significant investments made by NHLBI and other funding agencies over the past three decades, the translation of basic science discoveries and knowledge of pathophysiology into better treatments that can reach diverse patient population groups remains inadequate.
The goals of the working group were: 1) to provide an overview of recent discoveries that may be ready for testing in pre-clinical and clinical studies; 2) to identify gaps in knowledge that impede translation; 3) to highlight the most promising scientific areas in which to pursue translation; 4) to identify key challenges and barriers for moving basic science discoveries into translation, clinical studies and trials; 5) to identify roadblocks for effective dissemination and implementation in real world settings.
The working group brought together 16 experts in hypertension research from a broad range of disciplines in basic, translational, clinical, population, and implementation sciences. The members were selected from diverse areas in hypertension research in order to facilitate cross-cutting discussion, spark innovative ideas for future research pathways, and identify major barriers in hypertension research and clinical application. NHLBI staff planned, convened, and participated in these discussion.
The working group focused on key challenges and barriers that hinder progress of basic science translation through clinical trials and implementation. The five topic-specific sessions examined potential barriers in the way of: basic science discovery leading to new therapeutic interventions; moving forward from experimental animal model studies to human studies; translation into clinical trials; advancing from clinical trials to clinical practice; and moving from clinical findings and guidelines to real world settings and implementation.
The workshop was responsive to many of the objectives in the NHLBI Strategic Vision including:4 1) Understand normal biological function and resilience, 2) Investigate newly discovered pathobiological mechanisms important to the onset and progression of HLBS (heart, lung, blood, and sleep) diseases, 3) Investigate factors that account for differences in health among populations, 4) Identify factors that account for individual differences in pathobiology and in responses to treatments, 5) Develop and optimize novel diagnostic and therapeutic strategies to prevent, treat, and cure HLBS diseases, 6) Optimize clinical and implementation research to improve health and reduce disease, and 7) Leverage emerging opportunities in data science to open new frontiers in HLBS research. The workshop did not directly address the 8th objective - Further develop, diversify, and sustain a scientific workforce capable of accomplishing the NHLBI’s mission.
TRANSLATION OF DISCOVERY SCIENCE
To identify problems, find solutions and uncover potential opportunities for effective translation of basic science into clinical science, we asked the working group several important questions: “Could identification of additional contributors to the pathophysiology of hypertension lead to effective new therapeutic interventions?” “Are new treatments on the horizon, and how do findings from basic science laboratories evolve to new clinical treatments?” “What are the challenges associated with assessing next steps after identifying a new regulator of arterial BP?” Several (of the many) recent discoveries which have advanced our understanding of the fundamental mechanisms regulating arterial pressure and causing hypertension and its CVD consequences and their potential to enable development of new therapeutic agents were discussed. The discussion was not intended to be a comprehensive accounting of all gains from recent hypertension research, but to provide specific examples.
The T0 to T4 classification system is a method describing research within the translational science spectrum as follows: T0: basic biomedical research, including preclinical and animal studies; T1: translation to humans, including proof of concept studies, Phase 1 clinical trials, and focus on new methods of diagnosis, treatment, and prevention in highly-controlled settings; T2: translation to patients, including Phase 2 and 3 clinical trials and controlled studies leading to clinical application and evidence-based guidelines; T3: translation to practice, including comparative effectiveness research, post-marketing studies, clinical outcomes research, health services research, and dissemination and implementation research; and T4: translation to communities, including population level outcomes research, monitoring of morbidity, mortality, benefits and risks, and impacts of policy change. Many basic science researchers work solely in the T0 space and find it challenging to move into and through the T1-T4 continuum. Forming and maintaining teams of investigators that cover the spectrum from basic discovery, development of therapeutics and clinical research remains a barrier.
The Immunological Basis for Hypertension
An important development in the field of hypertension has been the realization that there is an immunological contribution to the disease. For decades, cells of both the innate and adaptive immune system have been observed in blood vessels and kidneys of hypertensive humans and in experimental animal models.5 Historically, it was observed that immunosuppression can lower BP in rats with unilateral renal infarction, that transfer of lymphocytes from these animals conferred hypertension to recipient rats, that thymectomy lowered BP, and that thymus transplant from non-hypertensive rats lowered BP in hypertensive rats.6,7
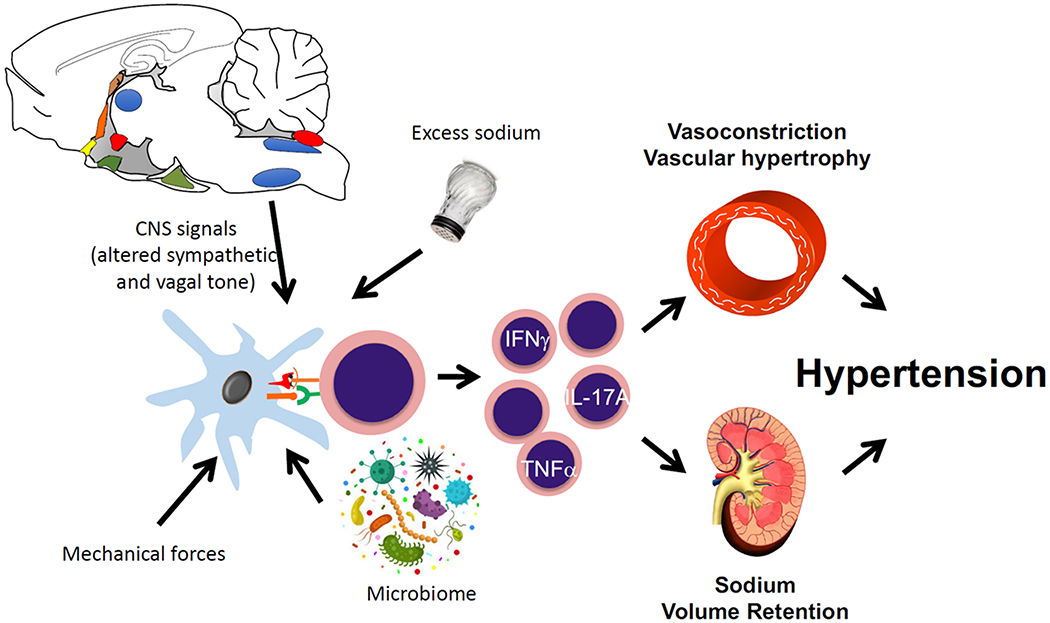
With the marked advances in immunology during the past decade, our understanding of the immunological basis of hypertension has expanded dramatically. Virtually every cell in the immune system, including macrophages, B and T lymphocytes, monocytes, T cells and dendritic cells have been implicated in this disease. The emerging paradigm is that various types of immune cells infiltrate the kidney, vasculature and brain, releasing potent mediators including cytokines, matrix metalloproteinases and reactive oxygen species that have profound effects on these target organs (Figure 1). These effects take two major forms. First, they alter function. For example, cytokines like TNFα, IL-1β and IL-17A impair endothelium-dependent vasodilation, enhance vasoconstriction, and alter the expression and function of renal tubular sodium transporters.8–10 Second, these mediators promote irreversible tissue damage, fibrosis and cell death. In several experimental models in which cytokines or immune cells are eliminated, target organ damage is virtually eliminated, even though BP is only modestly reduced.11 For a review of this topic see Norlander et al.12
Figure 1: Immune mechanisms causing hypertension.
Several stimuli common to hypertension stimulate antigen presenting cells to activate T cells, which in turn produce cytokines including TNFα, IL-17A and IFNγ. These activated T cells infiltrate the kidney and vasculature, where they promote sodium retention, vasoconstriction and local tissue damage, leading to worsening hypertension.
These studies in animals point to new therapeutic avenues that need to be studied in translational experiments and humans. Our phenotypic and genetic analytical capacity has greatly expanded our understanding of immune cells. New methodologies, including mass cytometry (CyTOF), single cell RNA sequencing, and Cytoseq have identified new types and subsets of immune cells that need to be defined in experimental animals and humans.13 A major hurdle for hypertension investigators is that these methods are costly and often cannot be replicated. Another related issue is that data from such experiments require expert analysis beyond the capability of investigators traditionally trained in hypertension and related CVD research. Initiatives to fund these sophisticated kinds of analyses in hypertension are highly desirable. A central repository to streamline analysis of these dense data sets, would increase research productivity. As such data sets become available, a central site to facilitate access to and analysis of such data would be ideal.
The potential of small molecules that modify the inflammatory response to lower blood pressure in humans without causing undue immune suppression suggests a new class of therapeutic agents for hypertension. Such exploration could involve repurposing of existing agents used for other diseases. In some cases of severe hypertension or hypertension associated with aggressive end organ damage, biologic agents that block inflammatory mediators (anti-IL-1β or IL-17A for examples) should be studied. 2-Hydroxybenzylamine (2-HOBA) and agents that prevent inflammasome activation should also be studied.
A hurdle to current research in this field is that the surface markers that identify human immune cells are not uniformly shared by other species. As an example, human monocyte subsets are characterized by the surface markers CD14 and CD16, while mouse monocytes express CD115 and Ly6C. Likewise, memory T cells in humans are characterized by the presence of CD45RO, while mouse memory T cells express CD44. There are different markers for effector vs. central memory T cells in mice and humans. The proportion of circulating lymphocytes and granulocytes varies substantially between these species. This does not mean that there are fundamental differences between these cells, and indeed Ly6C has been proposed to serve the same subset of monocytes as human cells that have both CD14 and CD16, and the role of memory T cells seems similar in both species.14 These differences make it difficult to directly extend knowledge gained from studies in mice to humans. Even more troublesome is the fact that the reagents for studying the rat immune system are limited and there has not been an extensive characterization of the transcriptional profile of various rat immune cells. Rats have otherwise proven extraordinarily valuable in understanding the pathophysiology of hypertension. Despite these limitations, studies of hypertensive rat models have confirmed an important role of immune cells and inflammation in hypertension.11,15–17 Efforts to develop additional reagents like surface marker antibodies and studies of the rat immune system using transcriptional profiling would be extremely helpful in this regard.
Another concern is that there are differences in the susceptibility of different strains of mice to various hypertensive interventions. For example, C57BL/6 mice are relatively resistant to elevations of blood pressure caused by salt feeding, while salt induces hypertension in other strains. This could be considered an advantage, because such variability is also observed in human subpopulations and comparison of mouse strains might be informative.
A powerful approach to overcome these species differences involves the use of humanized mice.18 These animals have a full gamut of human immune cells and can be made hypertensive by either infusion of Ang II, sodium feeding or by using the L-NAME/ high sodium feeding paradigm.19 The activation, homing and phenotypic changes of human immune cells can be monitored in these animals. The effect of therapeutic interventions can also be monitored. Such studies are costly but will provide a powerful approach to translational studies of immune activation in hypertension.
New Mediators of Systemic Vasoconstriction and Arterial Stiffness
Arterial stiffness is a risk factor for cardiovascular morbidity and mortality.20 Between 2010 and 2015 NHLBI supported 11 R01 awards in response to the RFA entitled “Cellular and Molecular Mechanisms of Arterial Stiffening and Its Relationship to Development of Hypertension” to clarify temporal and causal relationships between arterial stiffening and hypertension. A summary of the results have been published.21,22 One of the accomplishments of the initiative was the demonstration that increased arterial stiffness can precede systemic hypertension in several different animal models. This is consistent with data from the Framingham Heart Study.23 An extensive list of the 275 publications derived from the initiative is provided in the online supplement. Recent reviews also detail new advances in our understanding of the relationship between arterial stiffness and hypertension.24,25
Findings from human genetic studies and results of clinical trials can seed the next generation of informative animal models that will uncover underlying pathophysiological mechanisms and molecular pathways. As an example, PPARγ was identified as a new hypertension gene by virtue of its genetic linkage with hypertension and from clinical studies showing that drugs (thiazolidinediones) that activate PPARγ can lower BP.26,27 Studies of a unique animal model in which a hypertension-causing mutation in human PPARγ was specifically targeted to vascular smooth muscle led to the identification of the RhoBTB1-Cullin-3 pathway as an important regulator of BP.28 Mutations in Cullin-3 cause hypertension in humans, and additional studies revealed that loss of vascular smooth muscle Cullin-3 in mice causes severe and progressive hypertension and arterial stiffness.29,30 Importantly, re-expression of the PPARγ target gene RhoBTB1 in mice reversed the hypertension and arterial stiffness caused by interference with PPARγ.31 Biochemical studies revealed that the molecular target of RhoBTB1 is phosphodiesterase 5 (PDE5), an important component of the cGMP pathway that controls vasodilation. PDE5 inhibitors, already approved for erectile dysfunction, are in clinical trials for male urogenital dysfunction, optic neuropathy, contrast media-induced nephropathy, cancer, heart failure, metabolic syndrome, type 2 diabetes, cardiomyopathies, and chronic kidney disease, among others (see ClinicialTrials.gov). Moreover, there are numerous completed and ongoing trials to examine effectiveness of PDE5 inhibitors as treatments for pulmonary arterial hypertension. Other clinical trials are focusing on PDE5 related to exercise capacity in hypertension, the association between erectile dysfunction and endothelial dysfunction, and improving diastolic function in patients with resistant hypertension.
Identification of a gene that rapidly regresses arterial stiffness is potentially groundbreaking, but taking this to the next step remains a challenge as many unknowns remain. First, is RhoBTB1 relevant in humans? Second, what is the most physiologically relevant site of RhoBTB1 expression, given that it is ubiquitously expressed? Third and perhaps most importantly, is RhoBTB1 a druggable target? Current data suggest that RhoBTB1-deficiency leads to disease, thus classical inhibitors are unlikely to be useful therapeutically.
Role of Microbiota in the Pathophysiology of Hypertension
Another area of recent discovery in hypertension research was discussed - whether understanding the microbiome could lead to new and improved treatments for hypertension? The primary focus of this inquiry has been microbiota composition in the gastrointestinal tract. The gastrointestinal tract presents a vast interface between the external environment and symbiotic and pathogenic factors such as food and microbes that interact with the host. It is an initial point of entry for many deleterious risk factors for hypertension. In addition, the gastrointestinal tract is enriched with endocrine, immune and enteric nervous systems, highly innervated and harbors trillions of microorganisms. The microbiome’s potential interactions with the host fine tune the gastrointestinal tract to regulate diverse physiological functions. Despite these implications and the fact that environmental influences play critical roles in hypertension, the impact of the gut on BP control was not fully appreciated until a decade ago as a result of advances in genomic and metabolomic technologies and by two publications demonstrating dysbiosis in hypertension.32,33
Persuasive evidence now exists for gut dysbiosis in animal models and in human hypertension.34 Emerging data suggest that altered gut microbiota in hypertension could be responsible for high BP and associated pathophysiology. These data include: 1) Dysbiosis is found in prehypertensive patients; 2) Fecal matter transplant from hypertensive patients or animals results in increases in BP in normotensive animals; and 3) Fecal matter transplant from spontaneously hypertensive rats (SHR) into normotensive Wistar Kyoto (WKY) rats increases BP, neuroinflammation, sympathetic nervous system activity, circulating T cells, aortic infiltration by circulating T cells and impaired endothelial function.34–36 Conversely, fecal matter transplant from WKY rats into SHR reduces BP, restores inflammatory cell imbalance and improves endothelial function. Proof of this concept in human subjects would be an important contribution in clinical hypertension research. Another significant development has been the observation of profound alterations in gut pathology and increased intestinal permeability in hypertension. These findings have important implications in the regulation of mucin production and subsequently gut wall interactions with microbiota, thus impacting overall host-microbiota communication in hypertension. Rescue of gut pathology by captopril, an ACE inhibitor, further strengthens the gut-BP link.33
Diet-originated and microbiota-produced metabolic products such as short chain fatty acids (SCFA) have attracted attention in delineating the role of microbiota in BP control and hypertension.37 Decreased abundance of short-chain fatty acids (SCFA) producing bacterial communities have been demonstrated in hypertension.34,38 Supplementation with butyrate or propionate decreases BP.39,40 This suggests that beneficial effects of a high fiber diet could be due to microbiota-mediated fermentation of the fiber to produce SCFA. This concept warrants further investigation to strengthen the link between diet and gut microbiota and explore whether there are diet-dependent microbiota signatures with implications for BP regulation.
Sodium intake is an independent risk factor for hypertension, and it is likely that gut microbiota play a role in sodium sensitivity. The find that high sodium intake alters gut microbiota and increases gut inflammation in Dahl-SS rats supports this view.41 High sodium intake decreases Lactobacillus murinus and treatment of mice with this bacterium prevents sodium-sensitive hypertension.42 Furthermore, Lactobacillus reuteri is suggested to be protective against high sodium diets in some salt sensitive people.42 However, much more research is needed to determine if there are gut microbial signatures unique to sodium sensitive and sodium resistant hypertensive patients to establish their therapeutic importance.
Finally, microbiota in other organs particularly in the oral cavity, has been gaining interest in recent years since early observations showed an association between poor oral health with greater prevalence of cardiovascular disease.43 A relationship between high blood pressure and subgingival periodontal bacteria has been observed.44,45 Both relative abundance of certain oral bacterial species in older women with high blood pressure and an association of periodontal disease with high blood pressure strengthen the concept that oral dysbiosis could be linked to hypertension.44,46,47 Additionally, oral nitrate producing bacteria have been found to play a beneficial role blood pressure regulation and cardiometabolic diseases.48 This is supported by evidence that a nitrate-rich diet lowers blood pressure, while tongue cleaning that eliminates nitrate-producing bacteria, increases it.48 These observations strongly implicate involvement of oral microbiota in blood pressure control. Further research is needed to fully understand this relationship.
Despite above advances supporting the involvement of gut microbiota many important questions need to be answered. Some of these questions are:
We have inadequate information on microbiota signatures in hypertensive and normotensive phenotypes, and little understanding of sex and ethnic diversity signatures. Furthermore, are microbiota signatures associated with prehypertensive states? Large cohort studies with metagenomic analyses are required.
Mechanisms involved in epithelial-microbiota communication need to be investigated from a bidirectional signaling perspective.
How do sympathetic and vagal pathways interact with the gut to coordinate their influence on BP control?
Do hypertension signals such as high sodium intake and high fat diet directly influence gut epithelium or microbiota or both to alter host-microbiota crosstalk?
What role, if any, do gut microbiota play in drug-resistant hypertension? Is the metabolism of antihypertensive drugs higher in resistant hypertensive patients?
What is the role of microbiota in other organs such as oral cavity, skin, and heart in blood pressure regulation?
Sodium (Na+)/potassium (K+) Ratio in Hypertension
The working group discussed whether increased understanding of sodium and potassium handling can lead to new approaches to reduce BP. The increase in hypertension-related mortality since 1980 is often attributed to increased consumption of sodium-enriched processed and restaurant food. Early land dwelling animals, including humans, faced a sodium-scarce, potassium-rich environment, and the renin angiotensin aldosterone system (RAAS) evolved to conserve sodium and drive appetite for salty food, and excretion of excess dietary K+ in order to maintain homeostasis of circulating volume and K+ levels.49 Industrialized humans now satisfy their craving for salty food with readily available sodium-rich foods, and have no apparent sensory drive to consume K+. NaCl intake varies widely (0.05 g/day in Yanomami to 6-10 g/day in US) and predicts BP, yet, hypertension correlates better with dietary Na+/ K+ ratio than with Na+ alone.50–52 The recently published report, Dietary Reference Intakes for Sodium and Potassium, defines the Chronic Disease Risk Reduction (CDRR) intake values of sodium for adults 19 years and older as 2.3 g per day, recommending intakes above this level be reduced to reduce risk of hypertension and cardiovascular disease.53 The report indicates that median intakes of US and Canadian populations ranged between 3-4 g per day, well above the 2.3g per day CDRR. The DRI committee reviewed research on association of sodium intake and cardiovascular disease outcomes and mortality and noted that validation studies of sodium intake estimation methods using spot urine had a “systematic bias across the range of sodium intakes, such that spot urine sodium estimates are particularly biased estimates of 24-hour urine sodium at the lower and upper extremes of intake” (NASEM, 2019, pp.226-227).53 The Committee did not define CDRR intake values for potassium, citing the “heterogeneity of studies, lack of a intake-response relationship, and low or insufficient evidence for the chronic disease endpoints”. Prospective studies suggest that higher K+ intake is associated with decreased incidence of hypertension (Prevention of Renal and Vascular Endstage Disease Intervention Trial, PREVEND), and slower progression of chronic kidney disease.54,55 A recent Dietary Approaches to Stop Hypertension (DASH) diet study of ~400 participants with hypertension showed that a low sodium DASH diet (rich in fruits, vegetables, low-fat dairy products and naturally high in K+), lowered systolic BP (SBP) more effectively than sodium reduction alone, with striking reductions in the highest-BP group.56
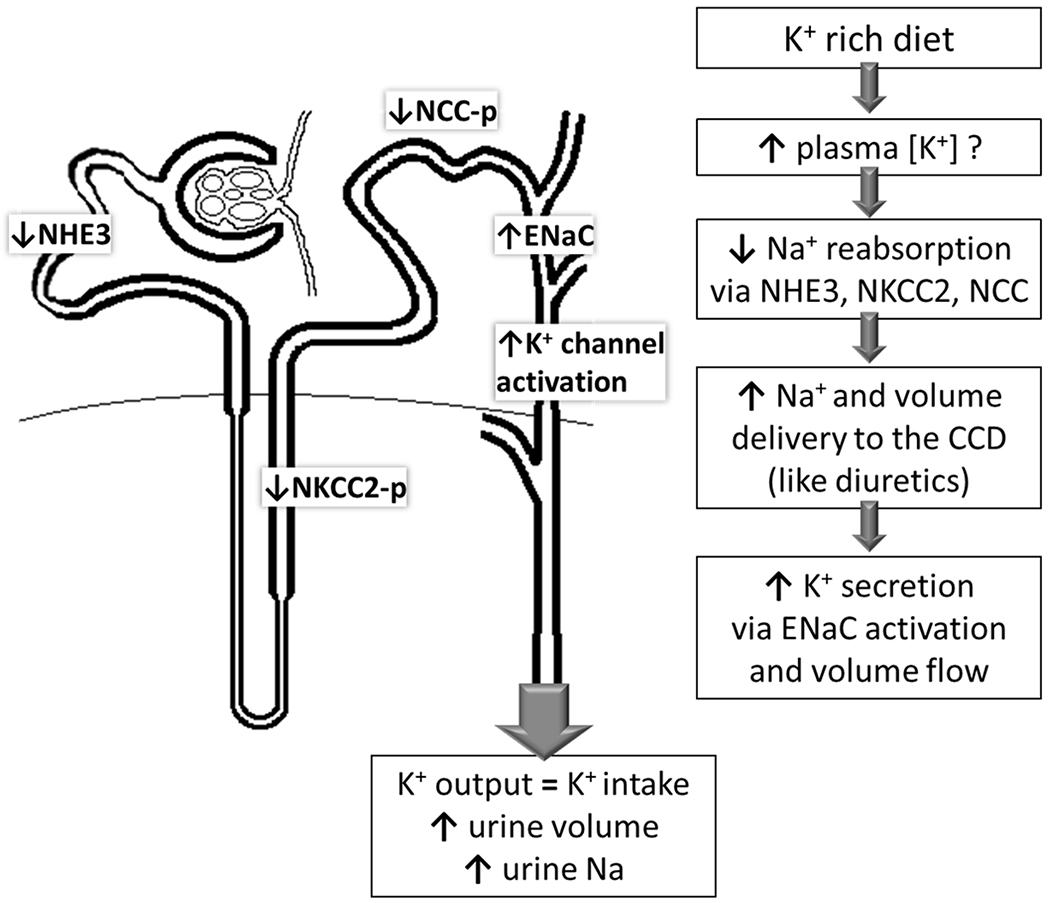
Recent studies in rats and mice provide mechanistic insight into the BP lowering potential of raising dietary K+ (Figure 2).51 Mice consuming K+ enriched diets exhibit lower proximal tubular fluid reabsorption and 40% lower Na+/H+ exchanger isoform 3 (NHE-3) abundance. Along the distal nephron, a rise in plasma [K+] inactivates the Na+-Cl- cotransporter (NCC) by dephosphorylation, thus, increasing Na+ delivery downstream to the epithelial Na+ channels (ENaC) where Na+ reabsorption drives K+ secretion via collecting duct K+ channels.57,58 These mechanisms complete the homeostatic response to increased K+ intake by raising urinary K+ and lowering plasma K+. Conversely, low dietary K+ intake activates NCC to minimize downstream K+ secretion even when Na+ intake is high; thus, a K+ -poor diet blunts the impact of lowering dietary Na+ intake on BP due to NCC activation to conserve K+.58 Importantly, eating a K+-rich diet, similar to that consumed by our pre-industrial ancestors, is predicted to reduce Na+ reabsorption along the proximal and distal nephron and significantly augment the impact of lowering dietary Na+ on BP.40,58
Figure 2: Mechanisms of Potassium induced Natriuresis.
Raising dietary potassium reduces sodium transporter activation along the nephron by: reducing proximal tubule (PT) Na/H exchanger isoform 3 (NHE3), reducing phosphorylation (-p, activation marker) of the thick ascending limb (TAL) Na-K-2Cl co transporter (NKCC2) and distal convoluted tubule (DCT) Na-Cl-cotransporter (NCC). Reducing these transporters increases volume flow and sodium delivery to the cortical collecting duct (CCD), as occurs with loop and thiazide diuretics. In CCD, sodium reabsorption via epithelial Na+ channels (ENaC) increases which drives potassium secretion via K+ channels, and higher volume flow activates K+ channels. This potassium provoked natriuresis and diuresis has the potential to reduce effective circulating volume and blood pressure, like thiazide and loop diuretics.
The recently published dietary reference index for potassium asserts “lack of evidence for intake-response relationship, and lack of supporting evidence for benefit of potassium on CVD”.59 While there is evidence that increasing dietary potassium is associated with reducing BP, there are no available CVD outcome studies that support long term benefit. Thus, a need exists for CVD outcome studies in humans to test the hypothesis that raising dietary potassium promotes natriuresis, lowers BP and improves CVD outcomes.53,60 Two relevant studies are underway. First, the Salt Substitute and Stroke Study (SSaSS) of >20,000 people in rural China is a 5-year trial investigating the impact of a potassium enriched salt substitute on stroke. Second, a randomized placebo-controlled clinical trial in the Netherlands (n= 400) is addressing reno-protective effects of potassium including BP as an end point.61 Filling this knowledge gap can drive feasible strategies to raise the dietary potassium / Na+ ratio (e.g., potassium enriched table salt, subsidizing potassium -rich foods) in order to reduce the prevalence of hypertension and associated medical costs.62,63
TRANSLATION TO HUMANS
This session focused on how researchers can translate fundamental discoveries to understand human hypertension.
Importance of Sex Differences in Hypertension
Genetic, epigenetic and hormonal mechanisms contribute to sex differences in BP and the effects of increased BP on target organs.64 There is heterogeneity in the timing of BP transition over the life course in women versus men.65 Men tend to develop BP elevations earlier in life, while in women hypertension is often diagnosed at later, postmenopausal time points. Further, in animal models, there is a sexual dimorphism such that BP is much higher in male than female hypertensive mice and rats.66,67 This has led to most preclinical hypertension research being carried out in male rodents; omission of female animals from these studies denies us access to fundamental knowledge about the pathogenesis of hypertension in the female.
Sex hormones and their receptors, as well as differential expression of the sex chromosome genes, contribute to the development of hypertension and related CVD. Estrogen has beneficial effects (vasodilation, decreased inflammatory activation and growth factor expression and reduced lesion progression) on healthy arteries of young women but harmful effects (vasoconstriction, inflammatory activation and growth factor expression and decreased plaque stability) on arteries of older postmenopausal women with established atherosclerosis.68 Further mechanistic work is needed to elucidate the cellular and molecular pathways through which estrogen mediates its anti-inflammatory and vasoprotective actions in the young and how these pathways are affected by aging. These studies may reveal strategies for estrogen rescue in both sexes. Additional studies on how sex hormones and sex chromosomes differentially affect gene networks and how this influences CVD susceptibility are also needed.
Pre-eclampsia and gestational hypertension have been associated with increased risk of chronic hypertension and various forms of cardiovascular disease, including ischemic heart disease, stroke and venous thromboembolism, as well as all-cause mortality in later life.69–72 These risks are further elevated in the presence of a small-for-gestational-age infant and/or preterm delivery. There is also an unmet need for further studies of the risks of hypertension in pregnancy and the possible benefit or harm of antihypertensive treatment in pregnant women. Assessment of these benefits and risks for both mother and fetus is needed both short-term (during gestation and the immediate post-postpartum period) and long-term in order to assess CVD outcomes in the mother and developmental outcomes in the child. For example, a recently published randomized clinical trial from India demonstrated that nifedipine, methyldopa and labetalol are effective options in the treatment of severe hypertension of pregnancy in low resource settings.73 Thresholds and goals for medical treatment of hypertension in pregnancy also need to be established in randomized controlled trials. The ongoing NHLBI-sponsored pragmatic multicenter randomized clinical trial of antihypertensive therapy for mild chronic hypertension during pregnancy, the Chronic Hypertension and Pregnancy (CHAP) Project, is a good example of a much-needed study (ClinicalTrials.gov NCT02299414). Long-term follow-up of CHAP participants and other cohorts of women with gestational hypertension is needed to assess the long-term effects, both benefits and possible harms, of antihypertensive treatment in pregnancy.
Key challenges and barriers to progress in sex-related research in hypertension include the belief that sex differences in BP and responses to treatment are unimportant and not deserving of further study. Further, the belief of the public and health care community that estrogen is harmful for older persons and should not be studied further has created a major impediment to further research in the area. Another particularly vexing challenge is the controversy regarding the relative benefits and risks of BP lowering in pregnant women. The controversy concerns the extent of BP lowering and what impact it may have on the fetus in terms of miscarriage and risk for growth restriction. Both have biologic plausibility. If maternal mean arterial pressure is reduced, exchange of O2 and nutrients at the maternal-fetal interface may be impaired, leading to stillbirth or fetal growth restriction. The developmental origins of health and disease suggest that fetal growth restriction may be associated with future CVD risk for the child.74 The impact of lowering BP at all, and what the appropriate thresholds and goals for BP lowering treatment should be on the pregnancy and the future life of the child are poorly understood. Therefore, many obstetricians are reluctant to treat hypertensive pregnant women with BP lowering medications. Further study in this area, as in the CHAP Project, are urgently needed.
In order to address these gaps in knowledge about sex differences in the pathogenesis of hypertension and its vascular complications, more support is needed to examine these issues in both animal models and humans. There is a need to improve the experimental models of hypertension in female animals since most existing preclinical research has been conducted in males. Using retired female breeder animals would provide a far better model to study postmenopausal hypertension than is currently commonly used.
Role of Sympathetic Nervous System Activity in Hypertension
Increased activity of the sympathetic nervous system is a major contributor to the pathogenesis and maintenance of hypertension.75 However, at the present time there are no diagnostic methods available in the clinic to assess sympathetic nervous system activity in patients. Moreover, preclinical studies suggest that the mechanisms whereby the sympathetic nervous system chronically increases arterial pressure are not generalized to the entire body but likely involve only key organs and tissues such as the renal and splanchnic vascular beds.76 These studies also suggest that the contribution of increased sympathetic nerve activity to one target (e.g., kidney) versus another may vary from patient to patient. Therefore, in addition to development of methods to measure “whole body” activity of the sympathetic nervous system, it is also necessary to assess organ/tissue-based sympathetic nerve activity in the clinic.
Improved diagnostics for organ/tissue based sympathetic activity will help guide novel treatments for organ targeted inhibition of the sympathetic nervous system. This approach is desirable since it avoids unwanted side-effects associated with global blockade using traditional drug-based therapies. This was the goal of the first device-based therapy for organ specific targeting of the sympathetic nervous system; catheter-based renal nerve ablation (CBRNA).77 The first proof-of-principle for CBRNA was the Symplicity HTN-1 trial conducted in 45 patients with drug resistant hypertension.78,79 Mean office blood pressure was decreased at 1 month (−21/−10 mmHg) and up to 36 months (−32/14 mmHg) after a single procedure. Symplicity HTN-2 was a larger randomized controlled trial in 106 patients with drug resistant hypertension conducted in 24 centers in Europe, Australia and New Zealand. Patients were randomized 1:1 for treatment or control, but control subjects did not receive a sham procedure.80 The results of this trial were similar to those of Symplicity HTN-1, where a sustained decreased in office blood pressure was observed in patients treated with CBRNA compared to controls.
The outcome of a larger U.S. randomized, blinded, sham-controlled trial, Symplicity HTN-3, was highly anticipated. Symplicity HTN-3 was conducted in 535 patients randomly assigned in a 2:1 ratio to undergo CBRNA or a sham procedure.81 CBRNA decreased office systolic blood pressure by an average of 14mmHg at six months post-CBRNA. However, the primary efficacy endpoint was not met as the decrease in office systolic blood pressure was not different between the CBRNA and the sham group, suggesting either a placebo or Hawthorne effect. An extensive post-hoc analysis of Symplicity HTN-3 suggests failure to properly perform the procedure may have been major factor in the failure of the trial. In addition, 39% of patients changed medication during the trial, which may have influenced responses. Moreover, African Americans in the sham control group receiving a vasodilator had a marked decrease in systolic pressure (−21.9 mmHg) that was not observed in the other subgroups, perhaps reflecting a change in pharmacological adherence.
Although the failure of Symplicity HTN-3 was viewed by some to indicate CBRNA is not an effective therapy for hypertension, recent improvements in catheter technology and clinical trial design has validated this approach.82 More than a decade after the publication of the original proof-of-concept study, three recent trials have sparked renewed interest in CBRNA: the SPYRAL HTN-OFF MED, the RADIANCE-HTN SOLO (endovascular ultra- sound) as well as the SPYRAL HTN-ON MED trial in hypertensive patients on concurrent antihypertensive therapy.82 All demonstrated a convincing and clinically significant reduction of ambulatory blood pressure in comparison with respective sham control groups. Evidence is therefore now available from three adequately designed, randomized, sham-controlled trials confirming the blood pressure lowering efficacy of CBRNA.
Finally, a recent case report demonstrated a substantial a reduction in blood pressure by combining CBRNA and celiac plexus block (thereby reducing sympathetic activity to the splanchnic vascular bed) in a patient with treatment resistant hypertension.83 This demonstrates the potential for targeting nerves to organs other than the kidney for the treatment of hypertension. Therefore, organ-based modulation of sympathetic activity represents an emerging area for development of novel hypertension therapies.
Relevance of Animal Models in Understanding and Hypertension
The value of basic animal research for advancing our understanding of hypertension and CVD and for testing potential therapies is well established. Virtually all major advances in hypertension therapy have been preceded by studies in experimental animals. Animal models have been especially informative for unraveling the pathophysiology of secondary forms of hypertension such as those caused by renal artery stenosis and various endocrine disorders.84 Animal models of genetic hypertension have also revealed potential pathways for heritable hypertension in humans.84 Development of animal models that recapitulate the pathophysiology of human primary (essential) hypertension, has been challenging, however. Choosing appropriate animal models, using rigorous methods for phenotyping, and studying diverse experimental models under appropriate conditions are all important factors in determining the relevance of preclinical studies for understanding the etiology, treatment and prevention of human hypertension.
Are commonly used animal models the most appropriate for understanding human hypertension? Current evidence suggests that 65-75% of the risk for human hypertension is related to excessive weight gain and obesity, which are often associated with a complex cascade of metabolic disorders and only modest activation of known hypertensinogenic systems (e.g. renin-angiotensin-aldosterone and sympathetic nervous systems).85 Moreover, obesity initially produces only mild hypertension, with increasing severity and onset of CVD, stroke and chronic kidney disease generally requiring several years to develop.86 Large and small animal models of obesity-induced hypertension have been developed and, in some cases, display many of the cardiovascular, renal and metabolic changes observed on humans with primary hypertension, including slow onset of mild to moderate hypertension.86 However, the most commonly used experimental models of genetic and secondary hypertension develop full blown hypertension very rapidly, with onset of major increases in BP and target organ injury in 1-2 weeks.84
An analysis by Galis et al. indicated that approximately 72% of animal studies of hypertension funded by NHLBI included angiotensin II infusion (48%) or genetic models (24%).87 Angiotensin II infusion rates in many studies were high, producing plasma concentrations far in excess of those observed in human primary hypertension, and were associated with rapid onset of large increases in BP. Commonly used genetic models of hypertension, such as spontaneously hypertensive rats or Dahl salt-sensitive rats, develop severe hypertension with complex genetic etiologies that are not yet fully understood and may not have direct counterparts in humans. Although the choice of appropriate animal models depends on the specific objectives of the studies, excessive reliance on well-established genetic, endocrine and surgical models of hypertension may be an important barrier for translation.
Excessive reliance on specific strains of inbred rodents with limited genetic diversity may also be a barrier to translation. The large menagerie of animal models that once formed the basis for physiological studies of hypertension has been whittled down to mainly rats and mice. The ability to manipulate the genome of mice, which have a short gestation period and large litter size, has been especially advantageous. Mice also require minimal quantities of compounds for treatment and have relatively simple housing requirements, and fewer federal regulations than large animals. These considerations have led to an exponential increase in the number of papers published using mice, whereas the number of hypertension studies using large animals have declined. Although hundreds of inbred strains of rats and mice are available commercially, the C57BL/6 mouse is, by far, the most widely used mouse strain for biomedical research. Inclusion of additional strains with diverse genetic backgrounds may improve the ability to generalize results and discern mechanisms of disease etiology and progression.
Additional emphasis should also be directed toward use of large animal models that closely mimic the pathophysiology of human primary hypertension. The gradual decline in use of dogs, primates, and other large animals may have a negative impact on translation of preclinical studies to understanding human pathophysiology. Although cost and convenience are often overriding considerations, the advantages and disadvantages of large and small animal models in meeting specific research aims should be carefully weighed.
Age and sex of experimental animals and humans have important influences on BP regulation and development of CVD. The NIH has appropriately emphasized the importance of studying both males and females. However, most studies of experimental hypertension are conducted in young animals, whereas human primary hypertension is usually associated with aging, obesity and metabolic disorders. Studies in aged animals may offer translational advantages compared to the common practice of using mainly young animals.
Are the most commonly used animal models studied under appropriate conditions? A limitation of many experimental studies, especially in rodents, is that the animals are permitted to eat ad libitum and therefore are over-fed, are usually relatively inactive, and are therefore “metabolically morbid”.88 Thus, “control” animals used for most studies are actually “couch potatoes”, which may limit their usefulness. Also, most rodent studies are conducted at temperatures that induce cold stress, with important effects on metabolism, BP and heart rate. In mice, the thermoneutral zone lies at 30°C, whereas most laboratories and animal facilities are maintained at 18-22°C.89 These abnormal environmental conditions induce metabolic morbidity and cold stress that can be important confounders in studies of hypertension, obesity, and associated metabolic disorders.
Are the phenotyping methods used in experimental (and clinical) hypertension studies rigorous enough? A key phenotype for hypertension studies is BP. Yet, many studies of experimental animals and humans use sub-optimal methods for BP measurement that may not be rigorous enough to adequately address the proposed aims. Studies in experimental animals may also be compromised by measuring BP during anesthesia or stress or by using techniques that do not permit assessment of BP during normal daily activities, or BP variability, which can influence risk for CVD.90 The same concerns exist for many studies in humans that rely on casual office BP measurements made under non-standardized conditions.90
Extensive phenotyping of BP is now feasible in small and large animals using radiotelemetry. Further development of improved technologies for telemetric assessment of cardiovascular, nervous system and metabolic function as well as in vivo imaging of molecular events will greatly increase the rigor and reproducibility of studies in experimental animals.
Genomics (and other “omics”), “big data”, electronic health records, clinical trials, and population studies are providing opportunities for translating results from experimental studies to understanding human hypertension, but there will always be a need for animal models of hypertension. Continuous reevaluation of animal models, comparison with human hypertension and CVD, and more rigorous phenotyping (of humans as well as experimental models) will inform future research and enhance translation for improved treatment and prevention strategies.
TRANSLATION TO PATIENTS, CLINICAL PRACTICE AND REAL-WORLD SETTINGS
These three sessions focused on how the understanding of hypertension mechanisms improves the health of patients, the implementation of effective hypertension treatments in clinical practice and the effect of controlling blood pressure on populations, particularly the underserved.
Barriers to the Elimination of Resistant Hypertension
This session focused on the potential to eliminate resistant hypertension (RH). RH is defined as BP of a hypertensive patient that remains above the therapeutic goal despite the concurrent use of three or more antihypertensive drugs of different classes administered at maximal or maximal tolerated doses or BP that is controlled at or below the therapeutic target but requires at least four antihypertensive agents of different classes to achieve control.91,92 The therapeutic goal defining the separation of RH from non-RH was BP <140/90 mmHg, or <130/80 mmHg in patients with diabetes or CKD, according to the recommendations of the 2003 Report of the Joint National Committee on the Prevention, Detection, Evaluation and Treatment of High BP in Adults (JNC-7).93 In 2017, the ACC/AHA clinical practice guideline recommended a lower BP target, < 130/80 mmHg, for adults below 65 years of age and SBP < 130 mmHg for adults at or over 65 years of age.2 The 2018 AHA Scientific Statement on RH adopted the lower BP target (<130/80 mmHg) from the 2017 ACC/AHA guideline to define RH.92
Pseudo-RH is a term applied when BP is elevated above the therapeutic goal but is not truly resistant to pharmacologic therapy.92 Most common causes of pseudo-RH are: 1) inaccurate BP measurement commonly resulting in falsely elevated BP values; 2) the “white coat effect” (office BP above goal but out-of-office BP at or below goal in a patient taking at least one antihypertensive medication), which is associated with minimal or no increased risk of CVD and stroke; 3) under-treatment of hypertension, including “clinical inertia”, in which there is lack of appropriate treatment escalation in a patient with uncontrolled hypertension; and 4) medication nonadherence. These causes of pseudo-resistance must be excluded before the diagnosis of true RH can be confirmed.92 When pseudo-resistance cannot be excluded, the term “apparent treatment RH” (aTRH) is usually employed.92 Virtually all clinic- and cohort-based studies defining the prevalence of RH have de facto reported the prevalence of aTRH, and the prevalence of true RH represents a major gap in knowledge. A recent study demonstrated that, using the 2008 definition of RH (BP ≥ 140/90 mmHg or ≥ 130/80 mmHg for adults with diabetes or CKD) the prevalence of aTRH was 17.7% of hypertensives, whereas using the 2018 definition (BP ≥ 130/80 mmHg for all adults or systolic BP ≥ 130 mmHg for adults ≥ age 65) the prevalence was only marginally increased at 19.7%. 94
Medication nonadherence is a substantial problem in the diagnosis and management of RH.95 In a recent meta-analysis of 24 studies, 31.2% of patients with RH were shown to be non-adherent.94 The highest pooled nonadherence estimates were for therapeutic drug monitoring and directly observed therapy (47.9%) and the lowest estimates were for indirect methods such as questionnaires and self-reporting (3.3%). Thus, objective measures of drugs and/or drug metabolites are the most reliable method of assessment but due to lack of resources and/or unavailability, these methods are rarely employed in clinical practice.
The diagnosis, evaluation and treatment of true RH includes identification and reversal of contributing lifestyle factors (i.e., obesity, poor dietary pattern, high dietary sodium intake, alcohol intake, and low physical activity), discontinuation or minimization of interfering substances (e.g., non-steroidal anti-inflammatory agents, oral contraceptives, hormone replacement therapy, sympathomimetic amines), and screening for secondary causes of hypertension (especially primary aldosteronism, renal parenchymal disease and renal artery stenosis).92 Evidence-based management of RH includes the substitution of a long-acting thiazide-like diuretic (i.e., chlorthalidone or indapamide) for hydrochlorothiazide and the addition of a mineralocorticoid receptor antagonist (MRA; spironolactone or eplerenone) or amiloride to the antihypertensive drug regimen.92 Beyond that point, recommendations for addition of antihypertensive drugs are based on expert opinion, as no clinical trials are available upon which to base evidence.92 Despite the evidence for the efficacy of a thiazide-like diuretic and MRA, a recent analysis demonstrated a low rate of usage (3.2-9%) of these agents in the management of resistant RH.94
Major gaps in knowledge that are critical in the management of RH include: 1) the prevalence of true RH, 2) optimal methods for detection and reversal of non-adherence in the context of RH, 3) prognosis of controlled vs uncontrolled true RH, 4) causes of clinical inertia, 5) optimal BP targets for RH and their relationship to target organ damage, 6) identification of intermediate phenotypes of RH in whom to explore pathophysiology and biomarkers to identify early target organ damage, 7) pharmacogenetic/genomic prediction of BP and target organ damage responses to antihypertensive agents, 8) evidence-based approaches to the therapeutic decision algorithm, and 9) the role, if any, of device-based therapy.
The most promising scientific areas in RH include: 1) elucidation of the pathophysiology of true RH using clinical research protocols to identify intermediate phenotypes (e.g., salt-sensitivity of BP, obesity-induced RH, autonomous aldosterone production, “refractory” hypertension) and mechanisms of severe BP elevation and target organ damage, 2) identification of circulating or urinary biomarkers predictive of early target organ damage (e.g., circulating dp-ucMGP, urinary mucin 1, and CKD273), 3) Randomized controlled trials (RCTs) to identify the optimal BP target for patients with true RH, 4) RCTs to evaluate the BP lowering efficacy of pharmacologic management beyond thiazide-like diuretics, MRAs and amiloride, 5) RCTs to determine the effects of intensive versus standard BP lowering on CVD and stroke morbidity and mortality, and 6) rigorous evaluation of device-based therapeutic options.
Key challenges and barriers hindering progress in RH include: 1) the shifting definitions of RH, 2) the poor infrastructure for patient-oriented research (lack of General Clinical Research Centers and their resources for clinical investigation), 3) the expense of large-scale RCTs and the limited funding resources for these trials, 4) the lack of knowledge of who is at risk for developing RH, the absence of validated prediction biomarkers; and lack of a strategy for prevention based on early intervention, 5) the lack of a basis for personalized antihypertensive drug selection based on genetic/genomic information, and 6) the absence of new classes of antihypertensive drugs during the past two decades.
Major Unanswered Questions in Hypertension Treatment and Control
The working group first discussed the major unanswered questions in hypertension treatment and control. A number of the unanswered questions related to hypertension treatment and control were described in detail in the 2017 ACC/AHA Hypertension Guideline “Evidence gaps and future directions.”2 It is important to emphasize the first item in their list: Does hypertension prevention or earlier treatment of hypertension improve outcomes? This could be tested with non-pharmacologic interventions or drug therapy to prevent the development of sustained hypertension, major CVD outcomes and mortality in persons at high risk for developing hypertension.
Several important RCTs including the Veterans Administration Cooperative Study of the treatment of hypertension in the 1960s and the NHLBI-sponsored Hypertension Detection and Follow-up Program (HDFP) of the 1970s, have shown that treating to a diastolic BP (DBP) goal <90 mm Hg reduces CVD outcomes.96,97 However, we do not have definitive clinical trial evidence that treatment to a lower DBP goal, e.g., <80 mmHg, in a general hypertensive population would reduce events, even though recent guidelines have recommended such a goal based on “expert opinion”.2 Several trials, including the NHLBI-sponsored Systolic Hypertension in the Elderly Program (SHEP), have demonstrated CVD benefits of targeting a systolic BP (SBP) goal <150 mmHg, and the NIH-sponsored SPRINT study has shown that targeting a SBP goal <120 mmHg versus <140 mmHg reduces major CVD events and total mortality by 25-30%.3,98 These results need to be confirmed in other populations, such as Asians, and in hypertensive patients with comorbidities such as diabetes, CKD, post-stroke, or heart failure. There are data suggesting benefit for some of these groups, including the standard glycemia subgroup of the NHLBI-sponsored Action to Control Cardiovascular Risk in Diabetes (ACCORD) BP trial and the CKD subgroup of SPRINT.99,100 In contrast, the NINDS-sponsored Secondary Prevention of Small Subcortical Strokes (SPS3) Study did not show benefit of intensive treatment (SBP goal <130 mmHg) for the primary stroke outcome and ACCORD BP did not show significant benefit for overall CVD outcomes.101 However, both of these trials were underpowered because of lower than expected event rates.
An important question raised by observational data from 24-hour ambulatory BP monitoring (ABPM) is whether screening for, or treatment of, hypertension using nighttime BP or some other ABPM parameter or daytime home BP measurements reduces major CVD events more than using office BP measurements alone. It is also not clear how nighttime or home BP is best treated. Another issue is whether and how higher BP variability, which is associated with higher CVD risk, can best be reduced and if that reduction would improve CVD outcomes.
A number of RCTs, such as the NHLBI-sponsored Antihypertensive and Lipid Lowering for the Prevention of Heart Attack Trial (ALLHAT), have compared major antihypertensive drug classes, but questions remain about whether one class or combination of classes is superior to other classes or combinations, and whether the addition of particular drugs or classes, such as a MRA or neutral endopeptidase inhibitor would be superior.102 Other major unanswered questions pertain to comparative efficacy of drugs in the same or similar classes. Some of these questions may be more appropriately tested in pragmatic studies, such as the VA-sponsored Diuretic Comparison Project (DCP), which is comparing the effect of chlorthalidone versus hydrochlorothiazide on major CVD outcomes.103
Other important clinical research topics include how to improve adherence to lifestyle/non-pharmacologic interventions and antihypertensive drug therapy, especially in community settings and the related question - how to improve BP control in various settings, systems, and populations. Finally, a major outstanding question is whether device therapy makes a clinically important addition to other non-pharmacologic (lifestyle modification) therapies and long-term antihypertensive drug therapy in controlling BP and preventing CVD.104
Gaps in Implementing Lifestyle Change to Control BP
The two core strategies to prevent and control high BP are - lifestyle modification and BP lowering medications. Lifestyle modifications that lower BP include weight loss, sodium reduction, potassium supplementation, healthy dietary patterns, increased physical activity, and moderation of alcohol intake. While much BP treatment focuses on medical management of adults with elevated BP, the vast scope of the BP epidemic argues for public health strategies that broadly and efficiently deliver lifestyle modification to the general population. These strategies include technology-facilitated interventions, and team-based care that engages a variety of health professionals (e.g., dentists, pharmacists, medical assistants, community health workers) and others (e.g., peers, barbers). To date, most studies of lifestyle modification have focused on adults. However, since BP tracks from younger ages into adulthood and elevated BP at younger ages is already associated with subclinical disease, there is compelling rationale to focus research on developing and testing BP-lowering strategies at younger ages. Further, the burden of high BP disproportionately affects certain groups, e.g. blacks and individuals with lower socioeconomic status. Targeted BP lowering strategies are needed for these groups, which may not have access to traditional sources of medical care or may prefer alternative venues.
Roadblocks to Use of Precision Medicine in Hypertension
While there is great hope in “-omic” strategies for prevention and treatment of hypertension, progress in translation of -omics into practice has been challenging. It is important to identify factors that are perceived to stand between our current -omic understanding of hypertension and real-world hypertension precision medicine strategies. Generally, published reviews of hypertension -omics outline recent developments and characterize the state of the field. Many authors conclude their reviews by describing the limitations of current knowledge and suggesting important avenues for future research. In short, reviewers often identify what they perceive to be roadblocks to precision medicine in hypertension.
The PubMed database (www.ncbi.nlm.nih.gov/pubmed/) was queried for reviews published from late 2015-2018 using the following search criteria: hypertension OR “blood pressure” OR “arterial pressure” AND genetics OR genomics OR pharmacogenetics OR pharmacogenomics OR “precision medicine” OR “personalized medicine.” Of the search returns, 52 reviews were freely available as full text, and of these, 41 were deemed to assess the limits of current knowledge and avenues for future research (references provided in Online Supplement). These assessments were compiled, categorized, and tallied. Our review of the literature revealed a range of challenges in both the research and clinical domains. There was, however, some agreement among experts: the most-noted roadblock was mentioned in 10 reviews. The 10 most frequently noted roadblocks included the following (in descending order of times noted):
Gene x gene and gene x environment interactions are probably critically important but are difficult to study and have been understudied.
A paucity of good pharmacogenetic evidence and no validated gene panels exist to guide treatment.
Documented genotype-phenotype associations have not provided viable drug targets.
The BP phenotype may be problematic, and hard outcome studies are rare.
Other -omics, compared to genomics, have been understudied. Other -omics studies are not as standardized as genomic studies, thereby making comparisons among studies and meta-analyses difficult.
Hypertension is a complex condition and difficult to study.
Existing -omic data have not been fully exploited.
Replication studies for potentially informative genotype-phenotype associations have not been conducted.
Genotype-phenotype association findings often do not replicate across racial groups.
Replicated genotype-phenotype associations have low predictive value.
Several workshop recommendations were made to overcome these roadblocks in the next 5-10 years: incorporating DNA collection and genome-wide association analyses into every BP clinical trial; creating ancillary genomics committees for trials funded by NHLBI; using the TOPMed, EMERGE, and All of Us studies to build a genetic screening tool for antihypertensive medication efficacy and safety; potentially partnering with commercial, direct-to-consumer genomics enterprises (e.g., 23andMe, MyHeritage DNA, etc.); funding further analyses of extant trials with BP data (e.g., SPRINT, Look Ahead, GenHAT).
Implementation of Multilevel/Population Interventions to Control Hypertension
This topic focused on the important question: “Why can we not successfully implement multilevel/population interventions to improve BP control?” A strong association exists between social determinants of health and CVD.105,106 Neighborhood characteristics may affect hypertension prevalence. Individuals residing in the most economically deprived neighborhoods have greater odds of having high BP.107 An association also exists between residence in certain geographic areas, such as the southeastern United States, and the prevalence of hypertension.108 Taken together, these and other social determinants of BP are critically important to the prevention and control of hypertension in the population.
Low adherence to medication is common and is a major contributing factor to uncontrolled BP and RH. For example, in one study, 21.3% of 6,627 older U.S. adults initiating antihypertensive medication in 2012 discontinued treatment within one year.109 Also, 31.7% of patients who had not discontinued their antihypertensive medication had low treatment adherence, defined by having medication available to take for <80% of days in the year following treatment initiation. Barriers to achieving high medication adherence are multifactorial and include complex medication regimens (e.g., multipill regimens), convenience factors (e.g., dosing frequency), behavioral factors, and issues with adverse effects of medications administered to asymptomatic patients. Additional factors commonly associated with low antihypertensive medication adherence are limited access to care and cost.110
Consistently effective intervention strategies include: 1) facilitating patient-provider communication, 2) using mHealth technologies with emphasis on two-way communication, 3) providing patient education in tandem with lifestyle and behavioral counseling, and 4) providing psychosocial support. Regarding medication adherence phases, all studies examined implementation (i.e., taking medications as prescribed over time) and one also addressed treatment initiation (i.e., beginning a new medication).111
Clinician therapeutic inertia is another barrier preventing patients from achieving guideline-recommended goals. Data from the U.S. National Ambulatory Medical Care Survey indicate that there were 41.7 million primary care visits in the United States annually between 2005 and 2012 wherein a patient had SBP ≥140 mm Hg or DBP ≥90 mm Hg.110 However, a new antihypertensive medication was initiated in only 7 million (16.8%) of these visits.112 There are a number of reasons why clinicians may not initiate or intensify antihypertensive medication, including workflow constraints and insufficient time to conduct a patient evaluation, concern about side effects, lack of knowledge to make dosing decisions, and uncertainty regarding a patient’s out-of-office BP.113
The United States health care organizations and agencies in the public and private sectors spend between $70 billion and $120 billion on research each year. Yet, in many routine clinical situations, stakeholders do not have enough information to make decisions about the most effective treatments under particular circumstances or for particular patients. When evidence is available, it often takes a long time to use it to make decisions.114 Moreover, evidence is not always useful or might not address questions that decision makers need answered. Finding ways to enhance awareness and knowledge of useful and relevant information (dissemination) to help people and organizations make decisions and put it into practice (implementation) is an increasingly important area of focus. Engagement of diverse stakeholders, such as payers and insurers, should enhance the uptake and feasibility of clinical trials and trial findings. To enhance communication, we propose a meeting to address implementation science in hypertension, including leaders from NHLBI, healthcare systems, payers, industry, insurers and other agencies.
NHLBI has recently funded 10 sites to train the next cohort of investigators in the field of implementation science via RFA-HL-17-016. Dissemination and implementation generally begins at the evidence-based stage and includes engaging stakeholders to provide input on relevant research and become partners in the execution of research and dissemination of findings. Engagement is most effective when it is bidirectional, with stakeholders providing input and feedback and receiving information in turn. Lessons learned by partners and stakeholders from planning and conducting dissemination and implementation activities should inform subsequent efforts. One of the working group’s recommendations was to prioritize the funding of initiatives that focus on multi-level system approaches. In support of the NHLBI’s strategic goal to speed up the adoption of findings into real-world settings, NHLBI published two funding opportunities to support implementation research studies related to cardiovascular and pulmonary health disparities, titled “Disparities Elimination through Coordinated Interventions to Prevent and Control Heart and Lung Disease Risk (DECIPHeR)” in March 2019. It is anticipated that research aimed at identifying strategies to achieve sustainable uptake of proven-effective interventions in routine clinical and public health practice and community-based settings will be funded through the DECIPHeR initiative. Dissemination and implementation efforts occur concurrent with and build upon other research and foundational activities. The identification of target audiences and the formation of partnerships begin at the evidence-based stage. The framework emphasizes that, when evidence is assessed, additional work to refine target audiences and engage partners should occur. The notion that the processes through which evidence is adopted will vary by context or setting and type of evidence is especially important. Thus, the DECIPHeR initiative appears to be timely given the favorable outcomes of an NHLBI-funded study that paired African-American-owned barbershops with pharmacists in an effort to address the high incidence of hypertension in African-American men.115 This important study was the first to provide clear evidence that a pharmacist intervention in collaboration with a barber can make significant differences and lower cardiovascular health risks in the barber’s regular patrons.
In general, multilevel, multicomponent strategies, followed by patient-level strategies, are most effective for BP control in patients with hypertension and should be used to improve hypertension control.116 Issues addressing the multi-level barriers of medication adherence and clinical inertia need further attention, and the field of implementation science will be essential to ensure that successful programs reach those who need them in a timely manner.
Summary
The working group reviewed presentations with an eye on identifying and clarifying key barriers to overcome and to identify research opportunities for consideration. Table 1 illustrates several of the key barriers where creative solutions are necessary. Table 2 provides research opportunities suggested by the workgroup to remove barriers impeding translation. The reader should recognize that neither is an exhaustive list but reflects the opinions of the working group based on the issues presented and discussed. The authors apologize in advance if specific areas important to hypertension about which the reader may be passionate are not included.
Table 1:
Major Barriers Impeding Translation of Hypertension Research
| Communication gaps among basic science researchers, clinical researchers, and clinicians in the field of hypertension are preventing translation of fundamental discoveries to better BP control. |
| Limited understanding of the antecedents of hypertension such as fetal programming and epigenetics. |
| Limited understanding of gene-environment and gene-gene interactions to improve therapeutic decision making in hypertension. |
| While animal models are crucial for identifying therapeutic targets and testing new drugs, not all experimental and genetic animal models are applicable to humans. |
| Poor understanding and validation of methods for detection and reversal of antihypertensive medication non-adherence. |
| Limited understanding of age-related changes in BP in children, adolescents and young adults, determinants of BP for early prevention and effectiveness of antihypertensive therapy to improve clinical outcomes. |
| Insufficient engagement of diverse stakeholders such as payers and insurers through all study phases to enhance uptake and feasibility of clinical trials and clinical trial findings. |
| Insufficient knowledge of how efficacious community-based interventions can be brought to scale |
Table 2:
Research Opportunities to Enhance Translation of Hypertension Research
| Encourage innovative translational research that requires collaboration among basic and clinical scientists and includes patient-oriented research. |
| Facilitate training that encourages collaboration and cross-training in basic science and clinical application. |
| Develop new drugs and treatments (such as potassium-rich diets) to target diverse hypertensive patient populations, such as patients with resistant hypertension. |
| Capitalize on resources currently or previously supported by NHLBI such as databases, clinical populations, and clinical trial data that will facilitate discovery. |
| Develop new technologies for better phenotyping of humans and animals through: in vivo imaging, single cell analysis (central repository and analysis), analysis of large datasets, validation of surrogate endpoints and biomarkers, robust long term follow-up, and assessment of tissue and organ-based sympathetic activity. |
| Support studies on hypertension and aging, including arterial aging, cognition, medication adherence, and complications of antihypertensive therapy. |
| Support studies related to the role of sex differences in the complications of hypertension and hypertension in pregnancy and preeclampsia. |
| Develop and use animal models that are best suited to the scientific question posed irrespective of cost. |
| Develop approaches to optimally detect and reverse antihypertensive medication non-adherence. |
| Strengthen the evidence base for genetic screening tools for both risk of hypertension and optimal treatment options, with collection of genetic data in clinical trials and population-based studies across the lifespan. |
| Support clinical trials for early intervention in high BP, particularly in stage 1 hypertension and in younger populations, with long term tracking of outcomes. |
| Develop strategies to engage health care practitioners in strong patient relationship bonds and trust to promote lifestyle modification in high risk populations. |
| Support studies that focus on multi-level, collaborative system-based approaches including patients, providers, and/or health systems (at a minimum of two levels). |
| Encourage researchers to incorporate implementation science methodologies that can look broadly to bridge healthcare and community settings. |
| Support clinical trials designed to use quasi-experimental or mixed methodologies and those that specifically address the questions, such as “who does it work for?” and “when does it work?” |
| Convene representatives and leaders from NHLBI, healthcare systems, payers, industry, insurers, and other government agencies to address implementation science in hypertension. |
| Support training for the next generation of health disparities and implementation science researchers, including lay persons and community health workers. |
Supplementary Material
Acknowledgments:
The authors wish to thank NIH staff (Marc Charette, Paula Einhorn, Larry Fine, Lucy Hsu, Michael Wolz, and David Goff from NHLBI and Christine Maric-Bilkan from NIDDK) and Drs. Cheryl Dennison-Himmelfarb, Jay Horton, and Ricardo Rocha, who participated in the Working Group.
Sources of Funding: The proceedings of the “Hypertension: Barriers to Translation” Working Group were supported through funds provided by the NHLBI.
Disclosures: No disclosures other than research grants from the AHA and/or NIH for the following authors: Sigmund, Carey, Arnett, Galis, Green Parker, Hall, Harrison, McDonough, Nicastro, Raizada, Wright, Oh.
Appel: No financial relationships related to this paper.
Wrote and received royalties for 3 chapters in ‘UpToDate’ for Wolers Kluwer on the effects of sodium, exercise and smoking on blood pressure.
Bosworth: No financial relationships related to this paper.
Research funds from the Veterans Affairs Health Services Research & Development, VA Office of Rural Health, NIH (K12 HL138030, U01 HL142099), Sanofi, Novo Nordisk, Improved Patient Outcomes, Preventic Diagnostic, Otsuka and consulting funds from Sanofi, Novartis
Cushman:
Steering Committee for a diabetes hypoglycemic drug CV outcome trial (Eli Lilly)
Consultation: Sanofi (2018); Novartis (2017) both focused on hypertension.
Oparil: No financial relationships related to this paper.
Personal fees from 98point6, Inc, CinCor Pharma, Pfizer, Inc, and Medtronic.
Research grants from ROX Medical, Inc, Bayer, Idorsia Pharmaceuticals, Ltd.
Non-financial support and other support from Preventric Diagnostics, LLC.
Serves as Editor-in-Chief of Current Hypertension Reports with an annual stipend of $5,000 (Springer), term until 12/2020.
Osborn: Consultant fees from Medtronic and ReCor
Footnotes
Conflict of Interest: The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
Contributor Information
Curt D. Sigmund, Medical College of Wisconsin, Milwaukee, WI.
Robert M. Carey, University of Virginia, Charlottesville, VA.
Lawrence Appel, Johns Hopkins University, Baltimore, MD.
Donna Arnett, University of Kentucky, Lexington, KY.
Hayden B. Bosworth, Duke University, Durham, NC.
William C. Cushman, Veterans Affairs Medical Center, Memphis, TN.
Zorina S. Galis, Vascular Biology & Hypertension Branch, DCVS, NHLBI
Melissa Green Parker, Health Inequities and Global Health Branch, CTRIS, NHLBI.
John E. Hall, University of Mississippi Medical Center, Jackson, MS.
David G. Harrison, Vanderbilt University, Nashville, TN.
Alicia A. McDonough, University of Southern California, Los Angeles, CA.
Holly L. Nicastro, Clinical Applications and Prevention Branch, DCVS, NHLBI.
Suzanne Oparil, University of Alabama at Birmingham, Birmingham, AL.
John W. Osborn, Jr, University of Minnesota, Minneapolis, MN.
Mohan K. Raizada, University of Florida, Gainesville, FL.
Jacqueline D. Wright, Epidemiology Branch, DCVS, NHLBI.
Young S. Oh, Vascular Biology & Hypertension Branch, DCVS, NHLBI.
References
- 1.Forouzanfar MH, Liu P, Roth GA, Ng M, Biryukov S, Marczak L, Alexander L, Estep K, Hassen Abate K, Akinyemiju TF, Ali R, Alvis-Guzman N, Azzopardi P, Banerjee A, Barnighausen T, Basu A, Bekele T, Bennett DA, Biadgilign S, Catala-Lopez F, Feigin VL, Fernandes JC, Fischer F, Gebru AA, Gona P, Gupta R, Hankey GJ, Jonas JB, Judd SE, Khang YH, Khosravi A, Kim YJ, Kimokoti RW, Kokubo Y, Kolte D, Lopez A, Lotufo PA, Malekzadeh R, Melaku YA, Mensah GA, Misganaw A, Mokdad AH, Moran AE, Nawaz H, Neal B, Ngalesoni FN, Ohkubo T, Pourmalek F, Rafay A, Rai RK, Rojas-Rueda D, Sampson UK, Santos IS, Sawhney M, Schutte AE, Sepanlou SG, Shifa GT, Shiue I, Tedla BA, Thrift AG, Tonelli M, Truelsen T, Tsilimparis N, Ukwaja KN, Uthman OA, Vasankari T, Venketasubramanian N, Vlassov VV, Vos T, Westerman R, Yan LL, Yano Y, Yonemoto N, Zaki ME and Murray CJ. Global Burden of Hypertension and Systolic Blood Pressure of at Least 110 to 115 mm Hg, 1990-2015. JAMA. 2017;317:165–182. [DOI] [PubMed] [Google Scholar]
- 2.Whelton PK, Carey RM, Aronow WS, Casey DE Jr, Collins KJ, Dennison Himmelfarb C, DePalma SM, Gidding S, Jamerson KA, Jones DW, MacLaughlin EJ, Muntner P, Ovbiagele B, Smith SC Jr., Spencer CC, Stafford RS, Taler SJ, Thomas RJ, Williams KA Sr., Williamson JD and Wright JT Jr. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of High Blood Pressure in Adults: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Hypertension. 2018;71:1269–1324. [DOI] [PubMed] [Google Scholar]
- 3.Group SR, Wright JT Jr., Williamson JD, Whelton PK, Snyder JK, Sink KM, Rocco MV, Reboussin DM, Rahman M, Oparil S, Lewis CE, Kimmel PL, Johnson KC, Goff DC Jr., Fine LJ, Cutler JA, Cushman WC, Cheung AK and Ambrosius WT. A Randomized Trial of Intensive versus Standard Blood-Pressure Control. N Engl J Med. 2015;373:2103–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Writing Group for the Division of Cardiovascular Sciences’ Strategic Vision Implementation P, Goff DC Jr., Buxton DB, Pearson GD, Wei GS, Gosselin TE, Addou EA, Stoney CM, Desvigne-Nickens P, Srinivas PR, Galis ZS, Pratt C, Kit KBK, Maric-Bilkan C, Nicastro HL, Wong RP, Sachdev V, Chen J and Fine L Implementing the National Heart, Lung, and Blood Institute’s Strategic Vision in the Division of Cardiovascular Sciences. Circ Res. 2019;124:491–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olsen F Inflammatory cellular reaction in hypertensive vascular disease in man. Acta Pathol Microbiol Scand A. 1972;80:253–256. [PubMed] [Google Scholar]
- 6.Okuda T and Grollman A. Passive transfer of autoimmune induced hypertension in the rat by lymph node cells. Tex Rep Biol Med. 1967;25:257–264. [PubMed] [Google Scholar]
- 7.White FN and Grollman A. Autoimmune Factors Associated with Infarction of the Kidney. Nephron. 1964;1:93–102. [DOI] [PubMed] [Google Scholar]
- 8.Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C and Harrison DG. Role of the T cell in the genesis of angiotensin II induced hypertension and vascular dysfunction. J Exp Med. 2007;204:2449–2460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Madhur MS, Lob HE, McCann LA, Iwakura Y, Blinder Y, Guzik TJ and Harrison DG. Interleukin 17 promotes angiotensin II-induced hypertension and vascular dysfunction. Hypertension. 2010;55:500–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang J, Rudemiller NP, Patel MB, Karlovich NS, Wu M, McDonough AA, Griffiths R, Sparks MA, Jeffs AD and Crowley SD. Interleukin-1 Receptor Activation Potentiates Salt Reabsorption in Angiotensin II-Induced Hypertension via the NKCC2 Co-transporter in the Nephron. Cell Metab. 2016;23:360–368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mattson DL, Lund H, Guo C, Rudemiller N, Geurts AM and Jacob H. Genetic mutation of recombination activating gene 1 in Dahl salt-sensitive rats attenuates hypertension and renal damage. Am J Physiol Regul Integr Comp Physiol. 2013;304:R407–414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Norlander AE, Madhur MS and Harrison DG. The immunology of hypertension. J Exp Med. 2018;215:21–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Villani AC, Satija R, Reynolds G, Sarkizova S, Shekhar K, Fletcher J, Griesbeck M, Butler A, Zheng S, Lazo S, Jardine L, Dixon D, Stephenson E, Nilsson E, Grundberg I, McDonald D, Filby A, Li W, De Jager PL, Rozenblatt-Rosen O, Lane AA, Haniffa M, Regev A and Hacohen N. Single-cell RNA-seq reveals new types of human blood dendritic cells, monocytes, and progenitors. Science. 2017;356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gerhardt T and Ley K. Monocyte trafficking across the vessel wall. Cardiovasc Res. 2015;107:321–330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rudemiller N, Lund H, Jacob HJ, Geurts AM, Mattson DL and PhysGen Knockout P. CD247 modulates blood pressure by altering T-lymphocyte infiltration in the kidney. Hypertension. 2014;63:559–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harwani SC, Chapleau MW, Legge KL, Ballas ZK and Abboud FM. Neurohormonal modulation of the innate immune system is proinflammatory in the prehypertensive spontaneously hypertensive rat, a genetic model of essential hypertension. Circ Res. 2012;111:1190–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Raikwar N, Braverman C, Snyder PM, Fenton RA, Meyerholz DK, Abboud FM and Harwani SC. Renal denervation and CD161a immune ablation prevent cholinergic hypertension and renal sodium retention. Am J Physiol Heart Circ Physiol. 2019;317:H517–H530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ito R, Takahashi T and Ito M. Humanized mouse models: Application to human diseases. J Cell Physiol. 2018;233:3723–3728. [DOI] [PubMed] [Google Scholar]
- 19.Itani HA, McMaster WG Jr., Saleh MA, Nazarewicz RR, Mikolajczyk TP, Kaszuba AM, Konior A, Prejbisz A, Januszewicz A, Norlander AE, Chen W, Bonami RH, Marshall AF, Poffenberger G, Weyand CM, Madhur MS, Moore DJ, Harrison DG and Guzik TJ. Activation of Human T Cells in Hypertension: Studies of Humanized Mice and Hypertensive Humans. Hypertension. 2016;68:123–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Townsend RR, Wilkinson IB, Schiffrin EL, Avolio AP, Chirinos JA, Cockcroft JR, Heffernan KS, Lakatta EG, McEniery CM, Mitchell GF, Najjar SS, Nichols WW, Urbina EM, Weber T and American Heart Association Council on H. Recommendations for Improving and Standardizing Vascular Research on Arterial Stiffness: A Scientific Statement From the American Heart Association. Hypertension. 2015;66:698–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oh YS, Berkowitz DE, Cohen RA, Figueroa CA, Harrison DG, Humphrey JD, Larson DF, Leopold JA, Mecham RP, Ruiz-Opazo N, Santhanam L, Seta F, Shyy JYJ, Sun Z, Tsao PS, Wagenseil JE and Galis ZS. A Special Report on the NHLBI Initiative to Study Cellular and Molecular Mechanisms of Arterial Stiffness and Its Association With Hypertension. Circ Res. 2017;121:1216–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oh YS. Arterial stiffness and hypertension. Clin Hypertens. 2018;24:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaess BM, Rong J, Larson MG, Hamburg NM, Vita JA, Levy D, Benjamin EJ, Vasan RS and Mitchell GF. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308:875–881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dumor K, Shoemaker-Moyle M, Nistala R and Whaley-Connell A. Arterial Stiffness in Hypertension: an Update. Curr Hypertens Rep. 2018;20:72. [DOI] [PubMed] [Google Scholar]
- 25.Safar ME, Asmar R, Benetos A, Blacher J, Boutouyrie P, Lacolley P, Laurent S, London G, Pannier B, Protogerou A, Regnault V and French Study Group on Arterial S. Interaction Between Hypertension and Arterial Stiffness. Hypertension. 2018;72:796–805. [DOI] [PubMed] [Google Scholar]
- 26.Barroso I, Gurnell M, Crowley VE, Agostini M, Schwabe JW, Soos MA, Maslen GL, Williams TD, Lewis H, Schafer AJ, Chatterjee VK and O’Rahilly S. Dominant negative mutations in human PPARgamma associated with severe insulin resistance, diabetes mellitus and hypertension. Nature. 1999;402:880–883. [DOI] [PubMed] [Google Scholar]
- 27.Dormandy JA, Charbonnel B, Eckland DJ, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefebvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Koranyi L, Laakso M, Mokan M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Skrha J, Smith U, Taton J and Investigators PR. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;366:1279–1289. [DOI] [PubMed] [Google Scholar]
- 28.Pelham CJ, Ketsawatsomkron P, Groh S, Grobe JL, de Lange WJ, Ibeawuchi SR, Keen HL, Weatherford ET, Faraci FM and Sigmund CD. Cullin-3 regulates vascular smooth muscle function and arterial blood pressure via PPARgamma and RhoA/Rho-kinase. Cell Metab. 2012;16:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Agbor LN, Nair AR, Wu J, Lu KT, Davis DR, Keen HL, Quelle FW, McCormick JA, Singer JD and Sigmund CD. Conditional deletion of smooth muscle Cullin-3 causes severe progressive hypertension. JCI Insight. 2019;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boyden LM, Choi M, Choate KA, Nelson-Williams CJ, Farhi A, Toka HR, Tikhonova IR, Bjornson R, Mane SM, Colussi G, Lebel M, Gordon RD, Semmekrot BA, Poujol A, Valimaki MJ, De Ferrari ME, Sanjad SA, Gutkin M, Karet FE, Tucci JR, Stockigt JR, Keppler-Noreuil KM, Porter CC, Anand SK, Whiteford ML, Davis ID, Dewar SB, Bettinelli A, Fadrowski JJ, Belsha CW, Hunley TE, Nelson RD, Trachtman H, Cole TR, Pinsk M, Bockenhauer D, Shenoy M, Vaidyanathan P, Foreman JW, Rasoulpour M, Thameem F, Al-Shahrouri HZ, Radhakrishnan J, Gharavi AG, Goilav B and Lifton RP. Mutations in kelch-like 3 and cullin 3 cause hypertension and electrolyte abnormalities. Nature. 2012;482:98–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mukohda M, Fang S, Wu J, Agbor LN, Nair AR, Ibeawuchi SC, Hu C, Liu X, Lu KT, Guo DF, Davis DR, Keen HL, Quelle FW and Sigmund CD. RhoBTB1 protects against hypertension and arterial stiffness by restraining phosphodiesterase 5 activity. J Clin Invest. 2019;129:2318–2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mell B, Jala VR, Mathew AV, Byun J, Waghulde H, Zhang Y, Haribabu B, Vijay-Kumar M, Pennathur S and Joe B. Evidence for a link between gut microbiota and hypertension in the Dahl rat. Physiol Genomics. 2015;47:187–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Santisteban MM, Qi Y, Zubcevic J, Kim S, Yang T, Shenoy V, Cole-Jeffrey CT, Lobaton GO, Stewart DC, Rubiano A, Simmons CS, Garcia-Pereira F, Johnson RD, Pepine CJ and Raizada MK. Hypertension-Linked Pathophysiological Alterations in the Gut. Circ Res. 2017;120:312–323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zubcevic J, Richards EM, Yang T, Kim S, Sumners C, Pepine CJ and Raizada MK. Impaired Autonomic Nervous System-Microbiome Circuit in Hypertension. Circ Res. 2019;125:104–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B and Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Toral M, Robles-Vera I, de la Visitacion N, Romero M, Sanchez M, Gomez-Guzman M, Rodriguez-Nogales A, Yang T, Jimenez R, Algieri F, Galvez J, Raizada MK and Duarte J. Role of the immune system in vascular function and blood pressure control induced by faecal microbiota transplantation in rats. Acta Physiol (Oxf). 2019;227:e13285. [DOI] [PubMed] [Google Scholar]
- 37.Pluznick JL. Microbial Short-Chain Fatty Acids and Blood Pressure Regulation. Curr Hypertens Rep. 2017;19:25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Marques FZ, Nelson E, Chu PY, Horlock D, Fiedler A, Ziemann M, Tan JK, Kuruppu S, Rajapakse NW, El-Osta A, Mackay CR and Kaye DM. High-Fiber Diet and Acetate Supplementation Change the Gut Microbiota and Prevent the Development of Hypertension and Heart Failure in Hypertensive Mice. Circulation. 2017;135:964–977. [DOI] [PubMed] [Google Scholar]
- 39.Bartolomaeus H, Balogh A, Yakoub M, Homann S, Marko L, Hoges S, Tsvetkov D, Krannich A, Wundersitz S, Avery EG, Haase N, Kraker K, Hering L, Maase M, Kusche-Vihrog K, Grandoch M, Fielitz J, Kempa S, Gollasch M, Zhumadilov Z, Kozhakhmetov S, Kushugulova A, Eckardt KU, Dechend R, Rump LC, Forslund SK, Muller DN, Stegbauer J and Wilck N. Short-Chain Fatty Acid Propionate Protects From Hypertensive Cardiovascular Damage. Circulation. 2019;139:1407–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L, Xu S, Guo X, Uchida S, Weinstein AM, Wang T and Palmer LG. Regulation of renal Na transporters in response to dietary K. Am J Physiol Renal Physiol. 2018;315:F1032–F1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bier A, Braun T, Khasbab R, Di Segni A, Grossman E, Haberman Y and Leibowitz A. A High Salt Diet Modulates the Gut Microbiota and Short Chain Fatty Acids Production in a Salt-Sensitive Hypertension Rat Model. Nutrients. 2018;10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wilck N, Matus MG, Kearney SM, Olesen SW, Forslund K, Bartolomaeus H, Haase S, Mahler A, Balogh A, Marko L, Vvedenskaya O, Kleiner FH, Tsvetkov D, Klug L, Costea PI, Sunagawa S, Maier L, Rakova N, Schatz V, Neubert P, Fratzer C, Krannich A, Gollasch M, Grohme DA, Corte-Real BF, Gerlach RG, Basic M, Typas A, Wu C, Titze JM, Jantsch J, Boschmann M, Dechend R, Kleinewietfeld M, Kempa S, Bork P, Linker RA, Alm EJ and Muller DN. Salt-responsive gut commensal modulates TH17 axis and disease. Nature. 2017;551:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Carrizales-Sepulveda EF, Ordaz-Farias A, Vera-Pineda R and Flores-Ramirez R. Periodontal Disease, Systemic Inflammation and the Risk of Cardiovascular Disease. Heart Lung Circ. 2018;27:1327–1334. [DOI] [PubMed] [Google Scholar]
- 44.Macedo Paizan ML and Vilela-Martin JF. Is there an association between periodontitis and hypertension? Curr Cardiol Rev. 2014;10:355–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Martin-Cabezas R, Seelam N, Petit C, Agossa K, Gaertner S, Tenenbaum H, Davideau JL and Huck O. Association between periodontitis and arterial hypertension: A systematic review and meta-analysis. Am Heart J. 2016;180:98–112. [DOI] [PubMed] [Google Scholar]
- 46.Gordon JH, LaMonte MJ, Genco RJ, Zhao J, Li L, Hovey KM, Tsompana M, Buck MJ, Andrews CA, McSkimming DI, Zheng W, Sun Y and Wactawski-Wende J. Is the Oral Microbiome Associated with Blood Pressure in Older Women? High Blood Press Cardiovasc Prev. 2019;26:217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Desvarieux M, Demmer RT, Jacobs DR Jr., Rundek T, Boden-Albala B, Sacco RL and Papapanou PN. Periodontal bacteria and hypertension: the oral infections and vascular disease epidemiology study (INVEST). J Hypertens. 2010;28:1413–1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Grant MM and Jonsson D. Next Generation Sequencing Discoveries of the Nitrate-Responsive Oral Microbiome and Its Effect on Vascular Responses. J Clin Med. 2019;8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rossier BC, Baker ME and Studer RA. Epithelial sodium transport and its control by aldosterone: the story of our internal environment revisited. Physiol Rev. 2015;95:297–340. [DOI] [PubMed] [Google Scholar]
- 50.Elliott P, Dyer A and Stamler R. The INTERSALT study: results for 24 hour sodium and potassium, by age and sex. INTERSALT Co-operative Research Group. J Hum Hypertens. 1989;3:323–330. [PubMed] [Google Scholar]
- 51.Gritter M, Rotmans JI and Hoorn EJ. Role of Dietary K(+) in Natriuresis, Blood Pressure Reduction, Cardiovascular Protection, and Renoprotection. Hypertension. 2019;73:15–23. [DOI] [PubMed] [Google Scholar]
- 52.Yang Q, Liu T, Kuklina EV, Flanders WD, Hong Y, Gillespie C, Chang MH, Gwinn M, Dowling N, Khoury MJ and Hu FB. Sodium and potassium intake and mortality among US adults: prospective data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2011;171:1183–1191. [DOI] [PubMed] [Google Scholar]
- 53.National Academies of Sciences E, and Medicine. Dietary Reference Intakes for Sodium and Potassium. Washington, DC: 2019; The National Academies Press: 10.17226/25353. [DOI] [PubMed] [Google Scholar]
- 54.Kieneker LM, Gansevoort RT, Mukamal KJ, de Boer RA, Navis G, Bakker SJ and Joosten MM. Urinary potassium excretion and risk of developing hypertension: the prevention of renal and vascular end-stage disease study. Hypertension. 2014;64:769–776. [DOI] [PubMed] [Google Scholar]
- 55.Kim HW, Park JT, Yoo TH, Lee J, Chung W, Lee KB, Chae DW, Ahn C, Kang SW, Choi KH, Han SH and Investigators K-CS. Urinary Potassium Excretion and Progression of CKD. Clin J Am Soc Nephrol. 2019;14:330–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Juraschek SP, Miller ER 3rd, Weaver CM and Appel LJ. Effects of Sodium Reduction and the DASH Diet in Relation to Baseline Blood Pressure. J Am Coll Cardiol. 2017;70:2841–2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McDonough AA and Youn JH. Potassium Homeostasis: The Knowns, the Unknowns, and the Health Benefits. Physiology (Bethesda). 2017;32:100–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Terker AS, Zhang C, McCormick JA, Lazelle RA, Zhang C, Meermeier NP, Siler DA, Park HJ, Fu Y, Cohen DM, Weinstein AM, Wang WH, Yang CL and Ellison DH. Potassium modulates electrolyte balance and blood pressure through effects on distal cell voltage and chloride. Cell Metab. 2015;21:39–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Newberry SJ, Chung M, Anderson CAM, Chen C, Fu Z, Tang A, Zhao N, Booth M, Marks J, Hollands S, Motala A, Larkin J, Shanman R and Hempel S. Sodium and Potassium Intake: Effects on Chronic Disease Outcomes and Risks Rockville (MD); 2018. [PubMed] [Google Scholar]
- 60.Clase CM, Carrero JJ, Ellison DH, Grams ME, Hemmelgarn BR, Jardine MJ, Kovesdy CP, Kline GA, Lindner G, Obrador GT, Palmer BF, Cheung M, Wheeler DC, Winkelmayer WC, Pecoits-Filho R and Conference P. Potassium homeostasis and management of dyskalemia in kidney diseases: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2019; 97:42–61. [DOI] [PubMed] [Google Scholar]
- 61.Gritter M, Vogt L, Yeung SMH, Wouda RD, Ramakers CRB, de Borst MH, Rotmans JI and Hoorn EJ. Rationale and Design of a Randomized Placebo-Controlled Clinical Trial Assessing the Renoprotective Effects of Potassium Supplementation in Chronic Kidney Disease. Nephron. 2018;140:48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drewnowski A, Rehm CD, Maillot M and Monsivais P. The relation of potassium and sodium intakes to diet cost among U.S. adults. J Hum Hypertens. 2015;29:14–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Pearson-Stuttard J, Bandosz P, Rehm CD, Penalvo J, Whitsel L, Gaziano T, Conrad Z, Wilde P, Micha R, Lloyd-Williams F, Capewell S, Mozaffarian D and O’Flaherty M. Reducing US cardiovascular disease burden and disparities through national and targeted dietary policies: A modelling study. PLoS Med. 2017;14:e1002311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Colafella KMM and Denton KM. Sex-specific differences in hypertension and associated cardiovascular disease. Nat Rev Nephrol. 2018;14:185–201. [DOI] [PubMed] [Google Scholar]
- 65.Hardy ST, Holliday KM, Chakladar S, Engeda JC, Allen NB, Heiss G, Lloyd-Jones DM, Schreiner PJ, Shay CM, Lin D, Zeng D and Avery CL. Heterogeneity in Blood Pressure Transitions Over the Life Course: Age-Specific Emergence of Racial/Ethnic and Sex Disparities in the United States. JAMA Cardiol. 2017;2:653–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Barsha G, Denton KM and Mirabito Colafella KM. Sex- and age-related differences in arterial pressure and albuminuria in mice. Biol Sex Differ. 2016;7:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Reckelhoff JF. Sex Differences in Regulation of Blood Pressure. Adv Exp Med Biol. 2018;1065:139–151. [DOI] [PubMed] [Google Scholar]
- 68.Mendelsohn ME and Karas RH. Molecular and cellular basis of cardiovascular gender differences. Science. 2005;308:1583–1587. [DOI] [PubMed] [Google Scholar]
- 69.Riise HKR, Sulo G, Tell GS, Igland J, Nygard O, Iversen AC and Daltveit AK. Association Between Gestational Hypertension and Risk of Cardiovascular Disease Among 617 589 Norwegian Women. J Am Heart Assoc. 2018;7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Ray JG, Vermeulen MJ, Schull MJ and Redelmeier DA. Cardiovascular health after maternal placental syndromes (CHAMPS): population-based retrospective cohort study. Lancet. 2005;366:1797–1803. [DOI] [PubMed] [Google Scholar]
- 71.Mosca L, Appel LJ, Benjamin EJ, Berra K, Chandra-Strobos N, Fabunmi RP, Grady D, Haan CK, Hayes SN, Judelson DR, Keenan NL, McBride P, Oparil S, Ouyang P, Oz MC, Mendelsohn ME, Pasternak RC, Pinn VW, Robertson RM, Schenck-Gustafsson K, Sila CA, Smith SC Jr., Sopko G, Taylor AL, Walsh BW, Wenger NK, Williams CL and Aha. Evidence-based guidelines for cardiovascular disease prevention in women. American Heart Association scientific statement. Arterioscler Thromb Vasc Biol. 2004;24:e29–50. [DOI] [PubMed] [Google Scholar]
- 72.Mosca L, Benjamin EJ, Berra K, Bezanson JL, Dolor RJ, Lloyd-Jones DM, Newby LK, Pina IL, Roger VL, Shaw LJ, Zhao D, Beckie TM, Bushnell C, D’Armiento J, Kris-Etherton PM, Fang J, Ganiats TG, Gomes AS, Gracia CR, Haan CK, Jackson EA, Judelson DR, Kelepouris E, Lavie CJ, Moore A, Nussmeier NA, Ofili E, Oparil S, Ouyang P, Pinn VW, Sherif K, Smith SC Jr, ., Sopko G, Chandra-Strobos N, Urbina EM, Vaccarino V and Wenger NK. Effectiveness-based guidelines for the prevention of cardiovascular disease in women--2011 update: a guideline from the american heart association. Circulation. 2011;123:1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Easterling T, Mundle S, Bracken H, Parvekar S, Mool S, Magee LA, von Dadelszen P, Shochet T and Winikoff B. Oral antihypertensive regimens (nifedipine retard, labetalol, and methyldopa) for management of severe hypertension in pregnancy: an open-label, randomised controlled trial. Lancet. 2019;394:1011–1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Robinson JS, Moore VM, Owens JA and McMillen IC. Origins of fetal growth restriction. Eur J Obstet Gynecol Reprod Biol. 2000;92:13–19. [DOI] [PubMed] [Google Scholar]
- 75.Malpas SC. Sympathetic nervous system overactivity and its role in the development of cardiovascular disease. Physiol Rev. 2010;90:513–557. [DOI] [PubMed] [Google Scholar]
- 76.Osborn JW, Fink GD and Kuroki MT. Neural mechanisms of angiotensin II-salt hypertension: implications for therapies targeting neural control of the splanchnic circulation. Curr Hypertens Rep. 2011;13:221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Osborn JW and Banek CT. Catheter-Based Renal Nerve Ablation as a Novel Hypertension Therapy: Lost, and Then Found, in Translation. Hypertension. 2018;71:383–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Krum H, Schlaich M, Whitbourn R, Sobotka PA, Sadowski J, Bartus K, Kapelak B, Walton A, Sievert H, Thambar S, Abraham WT and Esler M. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373:1275–1281. [DOI] [PubMed] [Google Scholar]
- 79.Krum H, Schlaich MP, Sobotka PA, Bohm M, Mahfoud F, Rocha-Singh K, Katholi R and Esler MD. Percutaneous renal denervation in patients with treatment-resistant hypertension: final 3-year report of the Symplicity HTN-1 study. Lancet. 2014;383:622–629. [DOI] [PubMed] [Google Scholar]
- 80.Symplicity HTNI, Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE and Bohm M Renal sympathetic denervation in patients with treatment-resistant hypertension (The Symplicity HTN-2 Trial): a randomised controlled trial. Lancet. 2010;376:1903–1909. [DOI] [PubMed] [Google Scholar]
- 81.Kandzari DE, Bhatt DL, Sobotka PA, O’Neill WW, Esler M, Flack JM, Katzen BT, Leon MB, Massaro JM, Negoita M, Oparil S, Rocha-Singh K, Straley C, Townsend RR and Bakris G. Catheter-based renal denervation for resistant hypertension: rationale and design of the SYMPLICITY HTN-3 Trial. Clin Cardiol. 2012;35:528–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kiuchi MG, Esler MD, Fink GD, Osborn JW, Banek CT, Bohm M, Denton KM, DiBona GF, Everett THt, Grassi G, Katholi RE, Knuepfer MM, Kopp UC, Lefer DJ, Lohmeier TE, May CN, Mahfoud F, Paton JFR, Schmieder RE, Pellegrino PR, Sharabi Y and Schlaich MP. Renal Denervation Update From the International Sympathetic Nervous System Summit: JACC State-of-the-Art Review. J Am Coll Cardiol. 2019;73:3006–3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lee CJ, Kim BK, Yoon KB, Lee HY, Dominiczak AF, Touyz RM, Jennings GLR, Cho EJ, Hering D and Park S. Case of Refractory Hypertension Controlled by Repeated Renal Denervation and Celiac Plexus Block: A Case of Refractory Sympathetic Overload. Hypertension. 2017;69:978–984. [DOI] [PubMed] [Google Scholar]
- 84.Lerman LO, Kurtz TW, Touyz RM, Ellison DH, Chade AR, Crowley SD, Mattson DL, Mullins JJ, Osborn J, Eirin A, Reckelhoff JF, Iadecola C and Coffman TM. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension. 2019;73:e87–e120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hall JE, do Carmo JM, da Silva AA, Wang Z and Hall ME. Obesity, kidney dysfunction and hypertension: mechanistic links. Nat Rev Nephrol. 2019;15:367–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Hall JE, do Carmo JM, da Silva AA, Wang Z and Hall ME. Obesity-induced hypertension: interaction of neurohumoral and renal mechanisms. Circ Res. 2015;116:991–1006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Galis ZS, Thrasher T, Reid DM, Stanley DV and Oh YS. Investing in high blood pressure research: a national institutes of health perspective. Hypertension. 2013;61:757–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martin B, Ji S, Maudsley S and Mattson MP. “Control” laboratory rodents are metabolically morbid: why it matters. Proc Natl Acad Sci U S A. 2010;107:6127–6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fischer AW, Cannon B and Nedergaard J. Optimal housing temperatures for mice to mimic the thermal environment of humans: An experimental study. Mol Metab. 2018;7:161–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves J, Hill MN, Jones DW, Kurtz T, Sheps SG, Roccella EJ, Subcommittee of P and Public Education of the American Heart Association Council on High Blood Pressure R. Recommendations for blood pressure measurement in humans and experimental animals: Part 1: blood pressure measurement in humans: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Hypertension. 2005;45:142–161. [DOI] [PubMed] [Google Scholar]
- 91.Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B and Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 92.Carey RM, Calhoun DA, Bakris GL, Brook RD, Daugherty SL, Dennison-Himmelfarb CR, Egan BM, Flack JM, Gidding SS, Judd E, Lackland DT, Laffer CL, Newton-Cheh C, Smith SM, Taler SJ, Textor SC, Turan TN, White WB, American Heart Association Professional/Public E, Publications Committee of the Council on H, Council on C, Stroke N, Council on Clinical C, Council on G, Precision M, Council on Peripheral Vascular D, Council on Quality of C, Outcomes R and Stroke C. Resistant Hypertension: Detection, Evaluation, and Management: A Scientific Statement From the American Heart Association. Hypertension. 2018;72:e53–e90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL Jr., Jones DW, Materson BJ, Oparil S, Wright JT Jr, Roccella EJ, Joint National Committee on Prevention DE, Treatment of High Blood Pressure. National Heart L, Blood I and National High Blood Pressure Education Program Coordinating C. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. [DOI] [PubMed] [Google Scholar]
- 94.Carey RM, Sakhuja S, Calhoun DA, Whelton PK and Muntner P. Prevalence of Apparent Treatment-Resistant Hypertension in the United States. Hypertension. 2019;73:424–431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Durand H, Hayes P, Morrissey EC, Newell J, Casey M, Murphy AW and Molloy GJ. Medication adherence among patients with apparent treatment-resistant hypertension: systematic review and meta-analysis. J Hypertens. 2017;35:2346–2357. [DOI] [PubMed] [Google Scholar]
- 96.Effects of treatment on morbidity in hypertension. II. Results in patients with diastolic blood pressure averaging 90 through 114 mm Hg. JAMA. 1970;213:1143–1152. [PubMed] [Google Scholar]
- 97.Five-year findings of the hypertension detection and follow-up program. I. Reduction in mortality of persons with high blood pressure, including mild hypertension. Hypertension Detection and Follow-up Program Cooperative Group. JAMA. 1979;242:2562–2571. [PubMed] [Google Scholar]
- 98.Prevention of stroke by antihypertensive drug treatment in older persons with isolated systolic hypertension. Final results of the Systolic Hypertension in the Elderly Program (SHEP). SHEP Cooperative Research Group. JAMA. 1991;265:3255–3264. [PubMed] [Google Scholar]
- 99.Beddhu S, Chertow GM, Greene T, Whelton PK, Ambrosius WT, Cheung AK, Cutler J, Fine L, Boucher R, Wei G, Zhang C, Kramer H, Bress AP, Kimmel PL, Oparil S, Lewis CE, Rahman M and Cushman WC. Effects of Intensive Systolic Blood Pressure Lowering on Cardiovascular Events and Mortality in Patients With Type 2 Diabetes Mellitus on Standard Glycemic Control and in Those Without Diabetes Mellitus: Reconciling Results From ACCORD BP and SPRINT. J Am Heart Assoc. 2018;7:e009326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cheung AK, Rahman M, Reboussin DM, Craven TE, Greene T, Kimmel PL, Cushman WC, Hawfield AT, Johnson KC, Lewis CE, Oparil S, Rocco MV, Sink KM, Whelton PK, Wright JT Jr., Basile J, Beddhu S, Bhatt U, Chang TI, Chertow GM, Chonchol M, Freedman BI, Haley W, Ix JH, Katz LA, Killeen AA, Papademetriou V, Ricardo AC, Servilla K, Wall B, Wolfgram D, Yee J and Group SR. Effects of Intensive BP Control in CKD. J Am Soc Nephrol. 2017;28:2812–2823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Group SPSS, Benavente OR, Coffey CS, Conwit R, Hart RG, McClure LA, Pearce LA, Pergola PE and Szychowski JM. Blood-pressure targets in patients with recent lacunar stroke: the SPS3 randomised trial. Lancet. 2013;382:507–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Officers A, Coordinators for the ACRGTA and Lipid-Lowering Treatment to Prevent Heart Attack T. Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–2997. [DOI] [PubMed] [Google Scholar]
- 103.Lederle FA, Cushman WC, Ferguson RE, Brophy MT and Fiore Md LD. Chlorthalidone Versus Hydrochlorothiazide: A New Kind of Veterans Affairs Cooperative Study. Ann Intern Med. 2016;165:663–664. [DOI] [PubMed] [Google Scholar]
- 104.Weber MA, Mahfoud F, Schmieder RE, Kandzari DE, Tsioufis KP, Townsend RR, Kario K, Bohm M, Sharp ASP, Davies JE, Osborn JW, Fink GD, Euler DE, Cohen DL, Schlaich MP and Esler MD. Renal Denervation for Treating Hypertension: Current Scientific and Clinical Evidence. JACC Cardiovasc Interv. 2019;12:1095–1105. [DOI] [PubMed] [Google Scholar]
- 105.Carey RM, Muntner P, Bosworth HB and Whelton PK. Prevention and Control of Hypertension: JACC Health Promotion Series. J Am Coll Cardiol. 2018;72:1278–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Bird CE, Seeman T, Escarce JJ, Basurto-Davila R, Finch BK, Dubowitz T, Heron M, Hale L, Merkin SS, Weden M and Lurie N. Neighbourhood socioeconomic status and biological ‘wear and tear’ in a nationally representative sample of US adults. J Epidemiol Community Health. 2010;64:860–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Keita AD, Judd SE, Howard VJ, Carson AP, Ard JD and Fernandez JR. Associations of neighborhood area level deprivation with the metabolic syndrome and inflammation among middle- and older- age adults. BMC Public Health. 2014;14:1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kershaw KN, Diez Roux AV, Carnethon M, Darwin C, Goff DC, Jr., Post W, Schreiner PJ and Watson K. Geographic variation in hypertension prevalence among blacks and whites: the multi-ethnic study of atherosclerosis. Am J Hypertens. 2010;23:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tajeu GS, Kent ST, Kronish IM, Huang L, Krousel-Wood M, Bress AP, Shimbo D and Muntner P. Trends in Antihypertensive Medication Discontinuation and Low Adherence Among Medicare Beneficiaries Initiating Treatment From 2007 to 2012. Hypertension. 2016;68:565–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Krousel-Wood M, Joyce C, Holt E, Muntner P, Webber LS, Morisky DE, Frohlich ED and Re RN. Predictors of decline in medication adherence: results from the cohort study of medication adherence among older adults. Hypertension. 2011;58:804–810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zullig LL, Ramos K and Bosworth HB. Improving Medication Adherence in Coronary Heart Disease. Curr Cardiol Rep. 2017;19:113. [DOI] [PubMed] [Google Scholar]
- 112.Mu L and Mukamal KJ. Treatment Intensification for Hypertension in US Ambulatory Medical Care. J Am Heart Assoc. 2016;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Milman T, Joundi RA, Alotaibi NM and Saposnik G. Clinical inertia in the pharmacological management of hypertension: A systematic review and meta-analysis. Medicine (Baltimore). 2018;97:e11121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Morris ZS, Wooding S and Grant J. The answer is 17 years, what is the question: understanding time lags in translational research. J R Soc Med. 2011;104:510–520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Victor RG, Lynch K, Li N, Blyler C, Muhammad E, Handler J, Brettler J, Rashid M, Hsu B, Foxx-Drew D, Moy N, Reid AE and Elashoff RM. A Cluster-Randomized Trial of Blood-Pressure Reduction in Black Barbershops. N Engl J Med. 2018;378:1291–1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mills KT, Obst KM, Shen W, Molina S, Zhang HJ, He H, Cooper LA and He J. Comparative Effectiveness of Implementation Strategies for Blood Pressure Control in Hypertensive Patients: A Systematic Review and Meta-analysis. Ann Intern Med. 2018;168:110–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.