Abstract
Background/Aims:
Accumulating evidence from a relatively small number of prospective studies indicates that exposure to prenatal stress profoundly influences the developing human fetus with consequences that persist into childhood and very likely forever.
Methods:
Maternal/fetal dyads are assessed at ∼20, ∼25, ∼31 and ∼36 weeks of gestation. Infant assessments begin 24 h after delivery with the collection of cortisol and behavioral responses to the painful stress of the heel-stick procedure and measures of neonatal neuromuscular maturity. Infant cognitive, neuromotor development, stress and emotional regulation are evaluated at 3, 6 12 and 24 months of age. Maternal psychosocial stress and demographic information is collected in parallel with infant assessments. Child neurodevelopment is assessed with cognitive tests, measures of adjustment and brain imaging between 5 and 8 years of age.
Results:
Psychobiological markers of stress during pregnancy, especially early in gestation, result in delayed fetal maturation, disrupted emotional regulation and impaired cognitive performance during infancy and decreased brain volume in areas associated with learning and memory in 6- to 8-year-old children. We review findings from our projects that maternal endocrine alterations that accompany pregnancy and influence fetal/infant/child development are associated with decreased affective responses to stress, altered memory function and increased risk for postpartum depression.
Conclusions:
Our findings indicate that the mother and her fetus both are influenced by exposure to psychosocial and biological stress. The findings that fetal and maternal programming occur in parallel may have important implications for long-term child development and mother/child interactions.
Key Words: Fetal programming, Developmental origins of disease, Cortisol, Stress, CRH, Sex differences, Prenatal stress, Pregnancy, Infant development, Anxiety, Fetal development, Maternal programming, Postpartum depression
Introduction
Overview
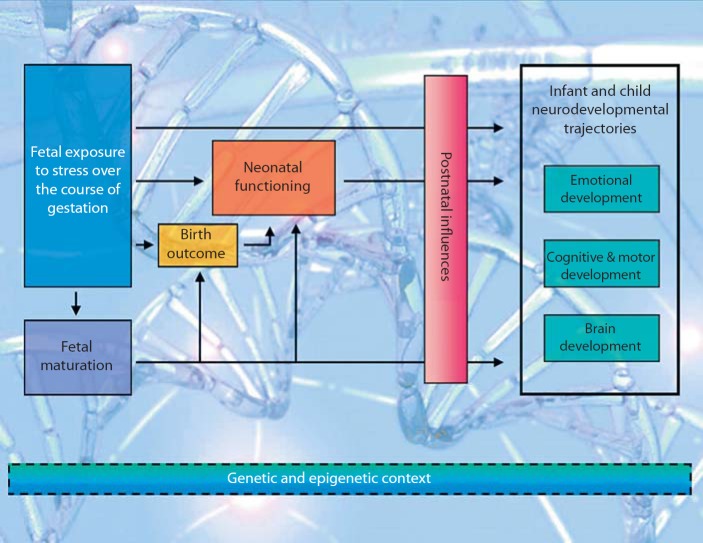
One central assumption of our program of research is that fetal exposure to maternal signals of stress has a significant (programming) influence on the trajectory of fetal development. Findings from our projects indicate that the human fetus is exquisitely sensitive to the physiological and psychological effects of maternal stress and that these influences can be measured. Moreover, our findings indicate that the timing of fetal exposure to maternal psychobiological stress is associated with unique profiles of birth outcomes, fetal reactivity, and infant and child outcomes. We will review here recent findings that both psychosocial and biological markers of stress during pregnancy influence (i) the behavior of the fetus, (ii) the temperament of the neonate and young infant, (iii) the neurobehavioral characteristics of the developing infant/toddler, and (iv) the structure of the nervous system in the child (fig. 1).
Fig. 1.
Schematic representation of the psychobiological stress, fetal programming model that guides our research program. Fetal exposure to stress can influence infant/child development directly or indirectly (though fetal behavior, birth outcomes and neonatal functioning). The consequences of prenatal and very early development on later outcomes can be mediated or moderated by postnatal influences. Genetic and epigenetic influences interact at all stages of the model.
A second assumption of our program of research is that the maternal endocrine alterations that accompany pregnancy and influence fetal/infant/child development also have implications for the maternal brain and behavior. Less is known about ‘maternal programming’ however, we will review here evidence that (i) maternal behavior is altered during pregnancy, (ii) maternal prenatal exposure to stress hormones influences postpartum adaptation, and (iii) reproductive experience alters the nervous system.
Fetal Programming
The human placenta is both a sensory and effector organ that incorporates information from its maternal host environment into the fetal developmental program. Stress signals detected by the placental/fetal unit may prime or advance the placental clock [1] and activate the promoter region of the corticotropin-releasing hormone (CRH) gene resulting in an increase in placental synthesis of this ‘master’ stress hormone [2]. The accelerated increase in circulating placental CRH (pCRH) associated with stress initiates a cascade of events initiating parturition and resulting in early departure (i.e. preterm birth) from the inhospitable host.
Preterm birth is one potential outcome of fetal exposure to stress during gestation; however, there are other lifelong consequences of exposure to intrauterine or gestational sources of stress. Because prenatal life is a time of unprecedented growth, the human fetal nervous system is particularly vulnerable both to organizing and disorganizing influences. Fetal exposure to adverse intrauterine events including those associated with maternal anxiety, psychosocial stress and depression result in subsequent risk for cardiovascular disease, hypertension, hyperlipidemia, insulin resistance, non-insulin-dependent diabetes mellitus, obesity, higher serum cholesterol concentrations, shortened lifespan, asthma and other poor health outcomes [3,4,5,6,7,8]. These influences on the fetus have been described as ‘programming’.
Human Pregnancy and the Stress System
Physiological stress systems change dramatically during human pregnancy [9]. The differences in reproductive and stress physiology, even in very closely related species such as humans and non-human primates, limit the validity of generalizing from animal models to the conditions experienced in humans [10]. The ‘fight or flight’ stress system is altered during human pregnancy with the growth and development of the placenta. The placenta expresses the genes for CRH (hCRHmRNA) and proopiomelanocortin, the precursor for ACTH and β-endorphin. All of these stress hormones increase as pregnancy advances, but the exponential increase in pCRH in maternal plasma is especially dramatic, reaching levels observed only in the hypothalamic portal system during physiological stress [11]. Moreover, in contrast to the well-known negative feedback regulation of hypothalamic CRH, cortisol stimulates the expression of hCRHmRNA in the placenta, establishing a positive feedback loop that allows for the simultaneous increase of pCRH, ACTH, β-endorphin and cortisol over the course of gestation [12,13]. As pregnancy advances toward term and these stress hormones increase, the positive feedback loop becomes dampened because the hypophyseal corticotrophs are downregulated [14,15].
The effects of these hormones however are modulated by the activities of binding proteins and enzymes. For example, concurrent with increases in pCRH, maternal CRH-binding protein rises and then falls abruptly around the 36th week of gestation [1]. Maternal plasma cortisol-binding globulin (CBG) levels also change across pregnancy. CBG is stimulated by estrogen and levels increase progressively with advancing gestation until 36 gestational weeks when there is a significant decline in CBG [16]. Variations in CBG may contribute to individual differences in developmental outcomes because levels have been shown to be lower in women with growth-restricted fetuses [16]. Activity of the placental enzyme 11β-hydroxysteroid dehydrogenase (11β-HSD2) also contributes to the influence of cortisol on mother and fetus. This enzyme oxidizes maternal cortisol into its inactive form, cortisone [17,18]. The levels of placental 11β-HSD2 rise as gestation progresses before falling precipitously near term ensuring maturation of the fetal lungs, central nervous system (CNS) and other organ systems in full-term births [19,20]. Elevated maternal stress downregulates 11β-HSD2 activity in the placenta allowing a greater proportion of maternal cortisol to cross the placenta [21].
These changes have significant implications for the human fetus and for the mother. First, because of the massive changes in stress hormones (two- to fortyfold) over the course of gestation, pregnancy can be considered a major physiological stressor. Second, because of the positive feedback between cortisol and pCRH that develops during human pregnancy, the fetus will be exposed simultaneously to the increases of both stress hormones. Third, because the receptors in the maternal stress system are downregulated as pregnancy advances, during late gestation environmental stress is less effective in triggering the endocrine axis and women become less responsive to the effects of stress [15,22,23,24,25,26]. Thus, stressful events early in pregnancy are experienced by the mother as more unpleasant and may exert greater influences on the fetus than events closer to term. The increased exposure to maternal and placental stress hormones and the change in maternal perceptions of stress during gestation play a fundamental role in the organization of the fetal nervous system and in maternal adaptation during pregnancy.
Assessment Protocol for Our Research Program
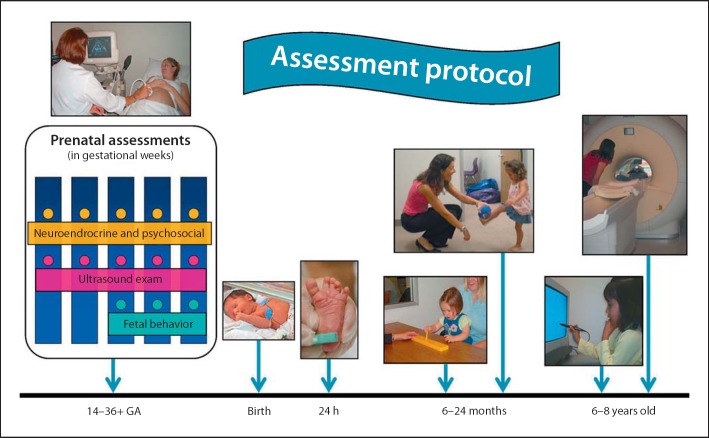
We have developed a prospective protocol for the assessment of prenatal exposure to maternal stress and stress hormones on fetal, infant and child development (fig. 2). Maternal psychosocial and biological stress measures are collected at five gestational intervals beginning between 14 and 16 weeks. Maternal/fetal dyads are assessed at ∼20, ∼25, ∼31 and ∼36 weeks of gestation. At ∼25, ∼31 and ∼36 gestational weeks, fetal neurodevelopment is evaluated with a measure of startle and habituation. At delivery, information on length of gestation and birth weight is abstracted from medical records. Infant assessments begin 24 h after delivery with the collection of cortisol and behavioral responses to the painful stress of the heel-stick procedure and measures of neonatal neuromuscular maturity. Infant cognitive, neuromotor development, stress and emotional regulation are evaluated at 3, 6, 12 and 24 months of age. Maternal psychosocial stress and demographic information is collected in parallel with infant assessments. Child neurodevelopment is assessed with cognitive tests, measures of adjustment and brain imaging between 5 and 8 years of age.
Fig. 2.
As described in the text, our prospective, longitudinal assessment protocol is designed to follow fetuses at regular intervals from ∼15 weeks' gestation to birth and then to follow the child at regular intervals through 8 years of age. It is important to acknowledge that measures of maternal behavior and self-report also are collected at each child visit.
The findings described in all of the studies from our project that are reviewed here were observed in healthy low-risk cohorts of children born at term and remained significant after considering an extensive list of prenatal and postnatal controls (including birth outcome and postnatal maternal stress and depression).
Gestational Stress Influences Human Fetal Behavior
In humans, the most well-documented effects of exposure to maternal stress are on birth outcomes including preterm delivery [24,27,28] and the resulting adverse developmental consequences [29]. However, intrauterine exposures to biological and psychosocial stress contribute to developmental impairments independently of preterm birth or growth restriction. The study of human fetal behavior is important because it provides the opportunity to assess the effects of gestational stress on development before the effects of external influences are exerted, such as birth outcome, parenting and socialization.
Several studies reported that increased maternal anxiety or psychosocial stress is associated with hyperactive fetuses and fetal tachycardia [30,31], a sudden fall in fetal heart rate (FHR) followed by over-swing recovery [32,33], significant FHR increases [34], increased fetal motor activity [35], more time in quiet sleep [36], and higher pulsatility index in the fetal middle cerebral artery [37]. Conversely, reduced anxiety or positive emotional states result in decreased fetal breathing and increased body movements [38,39].
Measures of fetal responses to external stimulation have been used in our projects to directly assess the developmental consequences of exposure to biological and psychosocial indices of stress [40]. With measures of FHR we discovered that fetuses of women with elevated pCRH during the third trimester were less responsive to the presence of a novel stimulus [41]. In a subsequent study, we reported that FHR habituation was delayed when fetuses were exposed to over-expression of maternal endogenous opiates [42]. To evaluate programming influences on the fetus, we assessed the consequences of gestational stress during the early second trimester on fetal behavior in the early third trimester. We found that low pCRH at 15 gestational weeks, but not later, predicted a more mature FHR pattern at 25 gestational weeks [43,44]. This is evidence of gestational stress exerting programming influences on the developing nervous system that is independent of postnatal experiences.
Gestational Stress Influences Infant Development
Results from animal models have established that fetal exposure to stress is associated with life-long compromised neurodevelopment, enhanced stress reactivity and increased fearful or anxious behavior [45,46,47,48,49,50,51,52]. A small number of human studies have focused primarily on the consequences of prenatal stress for behavioral and emotional regulation. Our studies of the effects of exposure to prenatal stress on infants and children have included measures of both temperament and cognition.
Prenatal Stress and Temperament
Prenatal exposure to elevated levels of maternal psychosocial stress and stress hormones has been reported to be associated with behavioral and emotional disturbances during infancy and childhood that are independent of birth outcome and postpartum maternal stress or depression [53,54,55,56,57,58,59,60,61]. Our studies have shown that elevated levels of prenatal maternal anxiety and depression were associated with increased infant fearful temperament after controlling for the influence of postpartum maternal state in both maternal report and laboratory observational measures of temperament [56,61]. In these same studies, maternal and placental stress-related hormones also were associated with more fearful infant temperament [53,61]; however, the biological stress associations with infant temperament were independent of the associations between maternal psychosocial stress and infant temperament.
In a second cohort of 179 mothers and their full-term infants, we evaluated the association between prenatal maternal anxiety and infant temperament. Maternal state anxiety and pregnancy-specific anxiety (PSA) were evaluated at five gestational intervals and at 3 months postpartum (fig. 2). Mothers reported their infants' temperament using the Infant Behavior Questionnaire at 3 months. Consistent with our previously published work, elevated maternal state anxiety was associated with increased negative affectivity among healthy 3-month-old infants born at term (partial r = 0.15, p < 0.05), after controlling for postnatal maternal psychological distress (anxiety and depression). As shown in figure 3, elevated PSA throughout gestation was associated with more negative infant temperament after controlling for postnatal maternal psychological distress (partial r ranged from 0.15 to 0.24, p < 0.05). A stepwise and hierarchical regression indicated that after accounting for relevant covariates (i) PSA more strongly predicted infant temperament than state anxiety and (ii) the association between state anxiety and infant temperament was not significant after accounting for the effects of PSA. These findings underscore the growing recognition that PSA may be a particularly potent influence on adverse birth and infant outcomes.
Fig. 3.
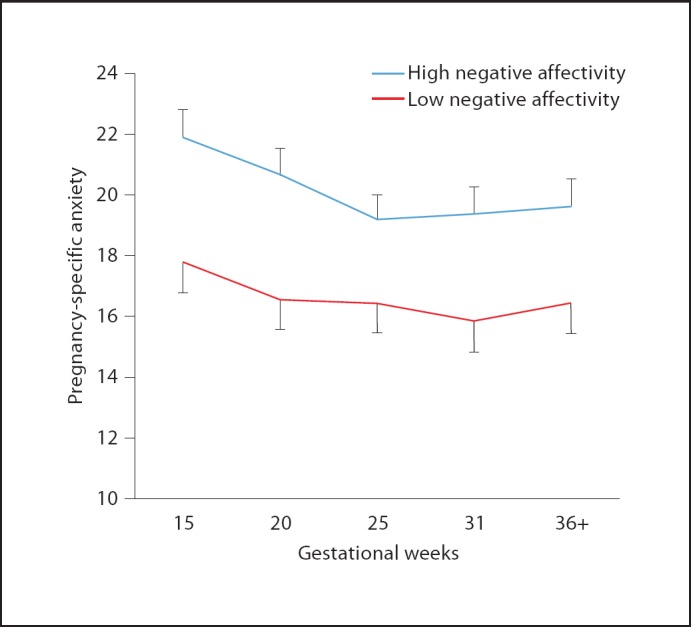
Maternal reports of high levels of PSA throughout pregnancy are associated with maternal reports of elevated negative affectivity in the infant at 3 months of age independent of the effects of postpartum maternal psychological states.
In addition to identifying an association between prenatal exposure to maternal psychosocial and biological stress signals and infant temperament, we recently reported [62] in a sample of 116 mothers and their healthy full-term infants assessed at five gestational intervals that prenatal maternal cortisol and psychosocial stress each exerted influences on neonatal stress regulation, independent of baseline functioning, and these influences were dependent upon the gestational period during which the fetus was exposed. Specifically we found that elevated maternal cortisol early in gestation was associated with slower behavioral recovery from the painful stress of a heel-stick procedure within 24 h of birth. Elevated maternal cortisol during the second half of gestation was associated with a larger and more prolonged neonatal cortisol response to stress. Moreover, higher levels of maternal perceived stress throughout gestation predicted a slower rate of recovery of behavioral stress responses in the newborn. These findings, as with the results of fetal behavior, suggest that the effects of fetal exposures (programming) are observed before the influences of postnatal experiences and exposures are exerted. These data are consistent with evidence that prenatal exposure to synthetic glucocorticoids during the late second and early third trimester is associated with an amplified cortisol response to stress among healthy full-term neonates [63]. Together, these data provide compelling evidence that gestational exposure to excess glucocorticoids alters the developmental trajectory of the fetal hypothalamic-pituitary-adrenal (HPA) axis with consequences for postnatal stress regulation.
Prenatal Stress and Cognition
Few studies have examined the effects of prenatal stress on cognitive development. Although there is evidence that maternal self-report of elevated stress, depression and anxiety during the prenatal period is associated with delayed infant cognitive and neuromotor development [61,64] and that these deficits may persist into adolescence [65], the findings across studies are not consistent [66,67].
In the largest study conducted (125 subjects) with repeated evaluations at five prenatal intervals and three intervals during infancy, we reported that the consequences of fetal exposure to maternal cortisol and PSA were dependent upon when during gestation these two indicators of stress were elevated [9]. Fetal exposure to cortisol early in pregnancy resulted in significantly lower scores on measures of mental development. Conversely, elevated maternal cortisol late in gestation was associated with significantly higher scores on measures of mental development. Similar results were observed for levels of maternal PSA. Despite the similar effects of maternal cortisol and anxiety on infant cognition at 1 year of age, these two measures of prenatal stress were not related and exerted independent effects on developmental outcomes. The consequences for the infant were confined to cognitive outcomes. Motor performance was unaffected by either exposure to cortisol or maternal anxiety.
These findings linking cortisol to infant cognitive development are consistent with its function in the maturation of the human fetus. As described above, early in pregnancy the fetus is partially protected from maternal cortisol because it is oxidized and inactivated by 11β-HSD2. However, because 11β-HSD2 is only a partial barrier, excessive synthesis and release of maternal cortisol exposes the fetus to concentrations that may have detrimental neurological consequences. As pregnancy advances toward term, fetal exposure to elevated cortisol is necessary for maturation of the fetal nervous system and lungs [68]. Fetal exposure to cortisol during the third trimester is facilitated by the sharp drop in 11β-HSD2 which allows a greater proportion of maternal cortisol to cross the placental barrier [69,70].
Gestational Stress Influences the Developing Brain
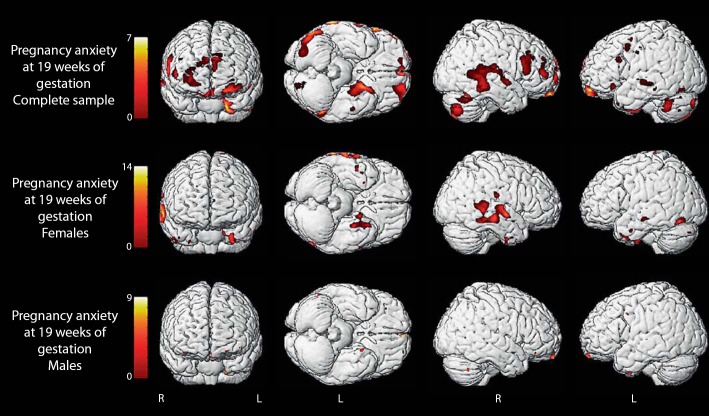
Low birth weight and preterm birth have been related to reductions in regional brain volumes [71,72,73,74,75]. However, because adverse birth outcomes may be markers of in utero stress exposure, it has been difficult to separate the effects of fetal stress exposures on brain morphology from perinatal complications. Recently, our group published the first study to show that fetal exposure to PSA was related to specific changes in brain morphology at 6–9 years of age independent of birth phenotype [76]. Specifically, PSA early in gestation was associated with gray matter volume reductions in the prefrontal cortex, the premotor cortex, the medial temporal lobe, the lateral temporal cortex, the postcentral gyrus as well as the cerebellum extending to the middle occipital gyrus and the fusiform gyrus (fig. 4). These brain regions are associated with a variety of cognitive functions. Specifically, the prefrontal cortex is involved in executive cognitive functions such as reasoning, planning, attention, working memory, and some aspects of language [77]. Structures in the medial temporal lobe, including areas connected to the hippocampus (entorhinal, perirhinal, parahippocampal cortex), constitute a medial temporal lobe memory system [78]. The temporal polar cortex is involved in social and emotional processing including recognition and semantic memory [79,80]. A network in the temporal-parietal cortex consisting of the middle temporal gyrus, the superior temporal gyrus and the angular gyrus has been shown to be important in processes related to auditory language processing in children [81]. Brain systems involved in language learning including the inferior frontal gyrus, the middle temporal gyrus and the parahippocampal gyrus also are reduced in children ‘exposed’ to high levels of PSA [82]. This is the first prospective study in healthy children to show that prenatal maternal anxiety (PSA in this case) is related to distinctive patterns of structural brain development.
Fig. 4.
Areas of reduced gray matter volume in 6- to 8-year-old children in association with elevated PSA at ∼19–20 weeks' gestation. The primary effect is observed among girls. Voxels with p < 0.001 (uncorrected) are displayed.
Mechanisms of Stress Effects on the Developing Nervous System
Our understanding of the potential mechanisms by which prenatal stress and biological effectors of stress may produce long-lasting changes in brain structure and function come primarily from animal studies. These mechanisms include changes in neurotransmitter levels, adult neurogenesis, as well as cell growth and survival. Pre- and postnatal stress exposure has been associated with changes in N-methyl-D-aspartate (NMDA) receptor expression in the hippocampus and frontal cortex [83,84,85], reduced adult neurogenesis [52,86,87,88,89] and reduced BDNF mRNA in the hippocampus and prefrontal cortex [84,90,91,92].
We described briefly the normal endocrine changes that occur during pregnancy and indicated that they are exacerbated under conditions of stress. We presented evidence that fetal exposure to elevated levels of stress and to stress-related hormones, particularly CRH and cortisol, are associated with abbreviated gestation, disturbed emotional regulation, poorer cognitive performance and reduced brain volume in critical areas. It is possible that fetal exposure to these markers of stress may directly influence the developing nervous system. For instance, elevated concentrations of pCRH may directly affect the developing brain by excessive (upregulation) expression of CRH receptors throughout the brain, including, but not limited to, hippocampus, amygdala and prefrontal cortex [93], and indirectly by stimulating production of fetal cortisol. Exogenously administered CRH has been shown to increase limbic neuronal excitation leading to seizures [93,94,95] and may participate in mechanisms of neuronal injury [96,97]. CRH has neurotoxic effects on hippocampal neurons [96,98,99,100,101], and these effects seem to be more pronounced in the immature hippocampus [96,100,102]. The decline in density of dendritic spines in the hippocampus has been prevented by selective blockade of the CRH1 receptor [103].
The sources of cortisol in the fetal compartment are from the fetal and maternal adrenals. pCRH is secreted into the fetal circulation and may affect fetal adrenal cortisol production; the CRH1 receptor is present in human fetal adrenal tissue from mid-gestation onwards [104]. As mentioned above, fetal exposure to maternal cortisol is regulated by 11β-HSD2, but this placental enzyme is only a partial barrier so that a proportion of maternal cortisol passes through the placenta [105,106]. Glucocorticoid receptors are present throughout the CNS [107,108,109,110] and glucocorticoids easily pass through the blood-brain barrier [111] and influence multiple brain regions, including, but not limited to, the hippocampus, amygdala and prefrontal cortex. At high concentrations, cortisol may inhibit growth and differentiation of the developing nervous system; considerable evidence indicates that glucocorticoids are neurotoxic to hippocampal CA3 pyramidal cells [112,113,114]. Furthermore, in embryonic hippocampal neurons cortiscosterone induces neuronal death, which is mediated by a decrease in BDNF and prevented by BDNF administration [115]. Cortisol also affects myelination in the developing brain because glucocorticoid receptors are expressed in oligodendrocytes, the glia cells that manufacture myelin sheets in the CNS. Specifically, delayed myelination has been reported in the corpus callosum in association with prenatal exogenous glucocorticoid exposure [116], which is consistent with findings that prenatal stress exposure affects the size of the corpus callosum [117].
One remaining question is whether or not the effects of maternal responses to stress on the fetus are related to shared genetic factors. In studies of naturally occurring variations in maternal stress it is difficult to separate the association between the predisposition to respond to stress and the neurodevelopmental patterns observed in the fetus and child, from the consequences of other factors that might contribute to this association, such as shared genes. The programming findings reported here, however, are consistent with animal models where random assignment is possible [118] and with human studies that evaluated the consequences of randomly occurring traumatic events, such as natural disasters [25,119,120] and with studies of exogenous administration of GCs. Further, recent human studies have documented developmental consequences of prenatal stress among children conceived by in vitro fertilization who were not genetically related to their mother [121]. Thus, genetic mechanisms cannot be completely ruled out on experimental grounds as a possible explanation for the effects of maternal experience on fetal/child development. There is reasonable evidence, however, to warrant the conclusion that maternal stress is translated into direct effects on the fetal nervous system.
Sex-Specific Programming Effects
There is a rapidly expanding literature in animal models indicating that there are sexually dimorphic responses to stress and adversity [122,123,124] including and perhaps especially associated with stress during the prenatal period [125,126,127]. In the study of human FHR responses to external stimulation reported above [44], we discovered that female fetuses displayed more mature responses than males at 31 and 36 gestational weeks. We reported delayed neuromotor development associated with fetal exposure to cortisol early in gestation and CRH late in gestation was confined to male neonates [128]. In this review, we report that the reduction in brain volumes in children exposed to elevated PSA early in gestation [76] primarily were observed in girls (fig. 4). These findings are consistent with findings of sex-specific trajectories of fetal development [129,130] and the sexually dimorphic risk of neurological impairment associated with neonatal complications [131].
There is evidence that sexually specific patterns are formed very early in development and are reflected in the function and response to stress of the placenta. The female placenta appears to be more responsive to changes in glucocorticoid concentration than the male placenta. Clifton [132] has argued that this sexually dimorphic placental sensitivity to signals of adversity (elevated glucocorticoids) results in different patterns of response and in particular in different patterns of growth. Male fetuses, Clifton suggests, do not alter their patterns of development in response to adversity and continue to grow despite reduced resources. Because the male fetus has not adjusted to the initial adversity and has not conserved its resources, it is more susceptible to later stress with increases in morbidity and mortality. In contrast, the female placenta responds or adjusts to an adverse maternal environment in multiple ways (gene and protein changes) resulting in reduced growth. If exposed to stress that reduces nutrients and resources later in gestation, the female fetus has conserved its energy needs which increases the probability of survival. By this mechanism, sexually specific patterns of response to stress may be programmed very early in fetal development.
Maternal Programming
The dramatic maternal endocrine alterations that accompany pregnancy have implications not only for the maintenance of gestation, successful parturition and optimal fetal/infant/child development, but also have ramifications for the maternal brain and behavior. In 1971, Diamond et al. [133] demonstrated that pregnancy in rats resulted in larger cortical size – providing the first empirical evidence that pregnancy is a critical period of development in the female lifespan during which neural architecture is remodeled. More recent studies with rodent models have confirmed that pregnancy and reproduction produce changes in brain structure and function that persist throughout the lifespan [134,135,136,137,138,139]. These changes are evident in brain regions involved both directly (e.g. recognition of young and attachment) and indirectly in maternal caretaking (e.g. spatial memory and stress responsivity). Our studies have examined the influence of a range of reproductive experience on the structure and function of women's brains – a process we term ‘maternal programming’ [26].
Affective Programming
Our studies were the first in humans to demonstrate diminished psychological response to major life events during pregnancy [25,140]. Specifically, we found that events occurring early in pregnancy were experienced as more stressful than those same events occurring later in pregnancy. Our findings were consistent with reports that HPA axis, blood pressure, heart rate and catecholamine responses to stress are dampened as pregnancy progresses [141]. There are several reports in humans that variations in prenatal levels of estrogen, cortisol and oxytocin influence the quality of early postpartum maternal care [142,143,144]. Further, a recent fMRI study reported that women who had given birth vaginally exhibited greater activation in brain regions involved in the regulation of empathy, arousal, motivation and reward circuits in response to their baby's cries compared to those who had not [145].
The changes in physiological stress responding as gestation advances are adaptive and promote survival [24.] Specifically, downregulated psychological and physiological maternal stress responding provides protection for mother and fetus from the effects of adversity as pregnancy progresses. For instance, stress experienced early in gestation, but not later, is associated with preterm birth [140,146]. Moreover, women who fail to show the expected decrease in generalized stress and anxiety or dampening in the cortisol awakening response during pregnancy are at increased risk for preterm delivery [24,147].
Cognitive Programming
Estimates of the percent of women who report impaired cognitive function during pregnancy range from 48 to 81% [148,149]. A recent meta-analysis of 17 relatively small studies published over the last decade indicated deficits in two components of memory during pregnancy: recall memory (both immediate and delayed) and the executive component of working memory [150]. In the largest longitudinal study of cognitive function during pregnancy (254 pregnant women, 60 non-pregnant women), we assessed memory function and hormones five times during pregnancy and once postpartum. The pregnant women exhibited poorer performance than the non-pregnant women during gestation and these effects were still apparent 3 months after delivery. Further, the diminished memory function during pregnancy and postpartum was associated with prenatal trajectories of both estradiol and cortisol [26]. These findings represent the first demonstration of potential biological mediators of diminished memory associated with pregnancy in humans.
Psychopathology
In the non-pregnant state, CRH is believed to play a role in the etiology of depression. Depressed individuals have an increased number and hypersensitivity of CRH neurons in the paraventricular nucleus of the hypothalamus [16,17]. Because of the dramatic increase in pCRH during pregnancy and the link between CRH and depression, our group has examined the possible risk pCRH may present for postpartum depression (PPD). In a cohort of 100 women followed prospectively five times beginning early in pregnancy, elevations in pCRH at 25 weeks' gestation were associated with an increased risk of developing symptoms of PPD [18]. These findings add new support to the small but emerging literature indicating that the maternal brain is susceptible to changes associated with normal human pregnancy.
In another large study from our laboratory, plasma levels of β-endorphin in 307 pregnant women with a singleton pregnancy were determined at regular intervals, and again at 9 weeks postpartum. Symptoms of depression were assessed at the last four pregnancy visits and postpartum with standardized questionnaires. We found that increased β-endorphin levels at any time point during pregnancy were associated with a more than threefold increase in the risk of developing PPD symptoms among women who were euthymic at 25 weeks' gestation. This relation was not observed among women reporting symptoms of depression during pregnancy. These findings with different biostress markers suggested that 25 weeks' gestation may be a sensitive time period during which endocrine factors may program postnatal maternal mood [151].
Possible Mechanisms
Currently, virtually nothing is known about how reproduction alters human brain structure. It is likely that the dramatic hormonal changes during pregnancy have direct influences on the nervous system. In humans, estrogen alterations due to menopause and the menstrual cycle are associated with transient alterations in brain structure [152,153]. Moreover, primates who are sensitive to stress-induced amenorrhea exhibit greater evidence of CRH activity in the paraventricular nucleus and thalamus and high CRH fiber density in the central nucleus of the amygdala than resilient animals [154]. These exposures which are of a relatively small magnitude strongly suggest that in humans, as in rodents, the massive hormonal changes of pregnancy alter brain function and morphology. Despite this, only one study has examined the influence of pregnancy on human brain structure. Oatridge et al. [155], using MRI in a small group of women, documented decreased total brain volumes over the course of pregnancy and into the postpartum period. Future work examining maternal programming in humans is essential for a complete understanding of women's mental health and also for a comprehensive understanding of prenatal influences on the health of their offspring.
Conclusions
The results from our research studies indicate that the mother and her fetus both are susceptible to exposure to elevated levels of psychosocial and biological stress. It is important, however, to acknowledge the independent and joint influences of prenatal exposure to psychosocial and biological stress on development. The human placenta integrates numerous sources of maternal stress signals and responds with a dose-dependent release of stress hormones [156]. Because the HPA and placental system is responsive to both psychosocial and physiological stress and because these two sources often are independent, the correlation with stress biomarkers often is low. Thus, maternal psychosocial stress does not exclusively determine fetal exposure to biological stress signals and elevated levels of stress hormones do not necessarily reflect the experience of increased maternal stress. The evidence indicates that both biological and psychosocial sources of stress, especially pregnancy-specific stress, have significant influences on the fetus with long-term consequences in the infant, child and perhaps beyond. Moreover, several studies reported in this review found that PSA was a stronger predictor of various outcomes than generalized anxiety. The experience of pregnancy presents unique fears and concerns and these dimensions are captured by items that are included in our measures (e.g., ‘I am fearful regarding the health of my baby’ ‘I am concerned or worried about losing my baby’ ‘I am concerned or worried about developing medical problems during my pregnancy’).
The finding that fetal and maternal programming may occur in parallel raises interesting possibilities related to long-term consequences. One possibility is that infants/children who are products of pregnancies characterized by elevated stress levels may be subjected to double jeopardy. Fetuses with compromised developmental trajectories by exposures to high stress also are at increased risk for receiving parenting from a depressed mother. Thus, the infant who is already at risk for adverse developmental outcomes, and who has the greatest need of competent mothering, is most likely to receive compromised quality of maternal care. A second possibility involves the adaptive significance of fetal programming. Just as the tadpole adjusts its development to maximize its chances of survival in a hostile environment [157], the human fetus may adjust its development in response to prenatal maternal stress signals in anticipation of a hostile or non-nurturing postnatal environment. The fetus that is stressed in utero and adjusts its development accordingly to prepare for a hostile environment may cope better in the presence of lower quality of maternal care than the fetus that was not exposed to prenatal stress signals and did not make this anticipatory adjustment to its trajectory.
Acknowledgements
Supported by awards NS-41298, HD-51852 and HD-28413 to C.A.S., HD-50662 to E.P.D. and HD-40967 to L.M.G. The assistance of Cheryl Crippen, Megan Blair, Christina Canino, Natalie Hernandez, Kendra Leak and Christine Cordova is gratefully acknowledged.
References
- 1.McLean M, et al. A placental clock controlling the length of human pregnancy. Nat Med. 1995;1:460–463. doi: 10.1038/nm0595-460. [DOI] [PubMed] [Google Scholar]
- 2.Sandman CA, et al. Elevated maternal cortisol early in pregnancy predicts third trimester levels of placental corticotropin-releasing hormone: priming the placental clock. Peptides. 2006;27:1457–1463. doi: 10.1016/j.peptides.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 3.Barker DJP. Mothers, Babies and Health in Later Life. Edinburgh, Churchill Livingstone. 1998 [Google Scholar]
- 4.Barker DJ, et al. The relation of small head circumference and thinness at birth to death from cardiovascular disease in adult life. BMJ. 1993;306:422–426. doi: 10.1136/bmj.306.6875.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McCormack VA, et al. Fetal growth and subsequent risk of breast cancer: results from long-term follow-up of Swedish cohort. BMJ. 2003;326:248. doi: 10.1136/bmj.326.7383.248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Roseboom TJ, et al. Coronary heart disease after prenatal exposure to the Dutch famine 1944–45. Heart. 2000;84:595–598. doi: 10.1136/heart.84.6.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richards M, et al. Birth weight and cognitive function in the British 1946 birth cohort: longitudinal population-based study. BMJ. 2001;322:199–203. doi: 10.1136/bmj.322.7280.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cookson H, et al. Mothers' anxiety during pregnancy is associated with asthma in their children. J Allergy Clin Immunol. 2009;123:847–853. doi: 10.1016/j.jaci.2009.01.042. e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis EP, Sandman CA. The timing of prenatal exposure to maternal cortisol and psychosocial stress is associated with human infant cognitive development. Child Dev. 2010;81:131–148. doi: 10.1111/j.1467-8624.2009.01385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith R, et al. Corticotropin-releasing hormone in chimpanzee and gorilla pregnancies. J Clin Endocrinol Metab. 1999;84:2820–2825. doi: 10.1210/jcem.84.8.5906. [DOI] [PubMed] [Google Scholar]
- 11.Lowry PJ. Corticotropin-releasing factor and its binding protein in human plasma. Ciba Found Symp. 1993;172:108–115. [PubMed] [Google Scholar]
- 12.Robinson BG, et al. Glucocorticoid stimulates expression of corticotropin-releasing hormone gene in human placenta. Proc Natl Acad Sci USA. 1988;85:5244–5248. doi: 10.1073/pnas.85.14.5244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Petraglia F, et al. Peptide signaling in human placenta and membranes: autocrine, paracrine, and endocrine mechanisms. Endocr Rev. 1996;17:156–186. doi: 10.1210/edrv-17-2-156. [DOI] [PubMed] [Google Scholar]
- 14.Suda T, et al. Responses to corticotropin-releasing hormone and its bound and free forms in pregnant and nonpregnant women. J Clin Endocrinol Metab. 1989;69:38–42. doi: 10.1210/jcem-69-1-38. [DOI] [PubMed] [Google Scholar]
- 15.Schulte HM, Weisner D, Allolio B. The corticotrophin-releasing hormone test in late pregnancy: lack of adrenocorticotrophin and cortisol response. Clin Endocrinol (Oxf) 1990;33:99–106. doi: 10.1111/j.1365-2265.1990.tb00470.x. [DOI] [PubMed] [Google Scholar]
- 16.Ho JT, Lewis JG, O'Loughlin P. Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clin Endocrinol (Oxf) 2007;66:869–877. doi: 10.1111/j.1365-2265.2007.02826.x. [DOI] [PubMed] [Google Scholar]
- 17.King BR, Nicholson RC, Smith R. Placental corticotrophin-releasing hormone, local effects and fetomaternal endocrinology. Stress. 2001;4:219–233. doi: 10.3109/10253890109014747. [DOI] [PubMed] [Google Scholar]
- 18.Kajantie E, et al. Placental 11β-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J Clin Endocrinol Metab. 2003;88:493–500. doi: 10.1210/jc.2002-021378. [DOI] [PubMed] [Google Scholar]
- 19.Murphy VE, Clifton VL. Alterations in human placental 11β-hydroxysteroid dehydrogenase type 1 and 2 with gestational age and labour. Placenta. 2003;24:739–744. doi: 10.1016/s0143-4004(03)00103-6. [DOI] [PubMed] [Google Scholar]
- 20.Ma XH, Wu WX, Nathanielsz PW. Gestation-related and betamethasone-induced changes in 11β-hydroxysteroid dehydrogenase types 1 and 2 in the baboon placenta. Am J Obstet Gynecol. 2003;188:13–21. doi: 10.1067/mob.2003.62. [DOI] [PubMed] [Google Scholar]
- 21.Mairesse J, et al. Maternal stress alters endocrine function of the feto-placental unit in rats. Am J Physiol Endocrinol Metab. 2007;292:E1526–E1533. doi: 10.1152/ajpendo.00574.2006. [DOI] [PubMed] [Google Scholar]
- 22.De Weerth C, Buitelaar JK. Cortisol awakening response in pregnant women. Psychoneuroendocrinology. 2005;30:902–907. doi: 10.1016/j.psyneuen.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Entringer S, et al. Attenuation of maternal psychophysiological stress responses and the maternal cortisol awakening response over the course of human pregnancy. Stress. 2010;13:258–268. doi: 10.3109/10253890903349501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Glynn LM, et al. Pattern of perceived stress and anxiety in pregnancy predicts preterm birth. Health Psychol. 2008;27:43–51. doi: 10.1037/0278-6133.27.1.43. [DOI] [PubMed] [Google Scholar]
- 25.Glynn L, et al. When stress happens matters: the effects of earthquake timing on stress responsivity in pregnancy. Am J Obstet Gynecol. 2001;184:637–642. doi: 10.1067/mob.2001.111066. [DOI] [PubMed] [Google Scholar]
- 26.Glynn L, Implications of maternal programming for fetal neurodevelopment . Maternal Influences on Fetal Neurodevelopment: Clinical and Research Aspects. In: Zimmerman A, Conners S, editors. New York, Springer. 2010. pp. pp 33–53. [Google Scholar]
- 27.Dunkel Schetter C, Glynn LM. Stress in pregnancy: empirical evidence and theoretical issues to guide interdisciplinary research. In: Contrada R, Baum A, Handbook of Stress, editors. New York, Springer. 2010. [Google Scholar]
- 28.Dunkel Schetter C. Stress processes in pregnancy and preterm birth. Curr Dir Psychol Sci. 2009;18:204–209. [Google Scholar]
- 29.Glynn LM, Sandman CA. The influence of prenatal stress and adverse birth outcomes on human cognitive and neurological development; in International Review of Research in Mental Retardation. New York, Academic Press. 2006:pp 109–129. [Google Scholar]
- 30.Ferreira A. Prenatal Environment. Springfield, Thomas. 1969 [Google Scholar]
- 31.McDonald RL. The role of emotional factors in obstetric complications: a review. Psychosom Med. 1968;30:222–237. doi: 10.1097/00006842-196803000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Benson P, et al. Foetal heart rate and maternal emotional state. Br J Med Psychol. 1987;60:151–154. doi: 10.1111/j.2044-8341.1987.tb02725.x. [DOI] [PubMed] [Google Scholar]
- 33.Talbert D, Benson P, et al. Fetal response to maternal anxiety: a factor in antepartum heart rate monitoring. J Obstet Gynecol. 1982;3:34–38. [Google Scholar]
- 34.Monk C, et al. Maternal stress responses and anxiety during pregnancy: effects on fetal heart rate. Dev Psychobiol. 2000;36:67–77. [PubMed] [Google Scholar]
- 35.Van den Bergh BR, et al. The effect of (induced) maternal emotions on fetal behavior: a controlled study. Early Hum Dev. 1989;19:9–19. doi: 10.1016/0378-3782(89)90100-x. [DOI] [PubMed] [Google Scholar]
- 36.Groome L, et al. Maternal anxiety during pregnancy: effect on fetal behavior at 38–40 weeks of gestation. J Dev Behav Pediatr. 1995;16:391–396. [PubMed] [Google Scholar]
- 37.Sjöström K, et al. Maternal anxiety in late pregnancy and fetal hemodynamics. Eur J Obstet Gynecol Reprod Biol. 1997;74:149–155. doi: 10.1016/s0301-2115(97)00100-0. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer EZ. Fetal vibroacoustic stimulation. Obstet Gynecol. 1993;81:451–457. [PubMed] [Google Scholar]
- 39.Field T, et al. Effects of ultrasound feedback on pregnancy anxiety, fetal activity, and neonatal outcome. Obstet Gynecol. 1985;66:525–528. [PubMed] [Google Scholar]
- 40.Sandman CA, et al. Maternal stress, HPA activity, and fetal/infant outcome. Ann N Y Acad Sci. 1997;814:266–275. doi: 10.1111/j.1749-6632.1997.tb46162.x. [DOI] [PubMed] [Google Scholar]
- 41.Sandman CA, et al. Maternal corticotropin-releasing hormone and habituation in the human fetus. Dev Psychobiol. 1999;34:163–173. doi: 10.1002/(sici)1098-2302(199904)34:3<163::aid-dev1>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 42.Sandman CA, et al. Maternal hypothalamic-pituitary-adrenal dysregulation during the third trimester influences human fetal responses. Dev Neurosci. 2003;25:41–49. doi: 10.1159/000071467. [DOI] [PubMed] [Google Scholar]
- 43.Class QA, et al. Low levels of corticotropin-releasing hormone during early pregnancy are associated with precocious maturation of the human fetus. Dev Neurosci. 2008;30:419–426. doi: 10.1159/000191213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Buss C, et al. Maturation of the human fetal startle response: evidence for sex-specific maturation of the human fetus. Early Hum Dev. 2009;85:633–638. doi: 10.1016/j.earlhumdev.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dickerson PA, et al. Early emergence of increased fearful behavior in prenatally stressed rats. Physiol Behav. 2005;86:586–593. doi: 10.1016/j.physbeh.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 46.Maccari S, Morley-Fletcher S. Effects of prenatal restraint stress on the hypothalamus-pituitary-adrenal axis and related behavioural and neurobiological alterations. Psychoneuroendocrinology. 2007;32:10–15. doi: 10.1016/j.psyneuen.2007.06.005. [DOI] [PubMed] [Google Scholar]
- 47.Van Den Hove DLA, Blanco CE, Aendekerk B. Prenatal restraint stress and long-term affective consequences. Dev Neurosci. 2005;27:313–320. doi: 10.1159/000086711. [DOI] [PubMed] [Google Scholar]
- 48.Weinstock M. Alterations induced by gestational stress in brain morphology and behaviour of the offspring. Prog Neurobiol. 2001;65:427–451. doi: 10.1016/s0301-0082(01)00018-1. [DOI] [PubMed] [Google Scholar]
- 49.Schneider ML, Coe CL. Repeated social stress during pregnancy impairs neuromotor development of the primate infant. J Dev Behavior Pediatr. 1993;14:81–87. [PubMed] [Google Scholar]
- 50.Schneider ML, et al. Growth and development following prenatal stress exposure in primates: an examination of ontogenetic vulnerability. Child Dev. 1999;70:263–274. doi: 10.1111/1467-8624.00020. [DOI] [PubMed] [Google Scholar]
- 51.Clarke AS, et al. Long-term effects of prenatal stress on HPA activity in juvenile rhesus monkeys. Dev Psychobiol. 1994;27:257–269. doi: 10.1002/dev.420270502. [DOI] [PubMed] [Google Scholar]
- 52.Coe CL, et al. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol Psychiatry. 2003;54:1025–1034. doi: 10.1016/s0006-3223(03)00698-x. [DOI] [PubMed] [Google Scholar]
- 53.Davis EP, et al. Maternal plasma corticotropin-releasing hormone levels during pregnancy are associated with infant temperament. Dev Neurosci. 2005;27:299–305. doi: 10.1159/000086709. [DOI] [PubMed] [Google Scholar]
- 54.Huizink AC, et al. Psychological measures of prenatal stress as predictors of infant temperament. J Am Acad Child Adolesc Psychiatry. 2002;41:1078–1085. doi: 10.1097/00004583-200209000-00008. [DOI] [PubMed] [Google Scholar]
- 55.O'Connor TG, Heron J, Glover V. Antenatal anxiety predicts child behavioral/emotional problems independently of postnatal depression. J Am Acad Child Adolesc Psychiatry. 2002;41:1470–1477. doi: 10.1097/00004583-200212000-00019. [DOI] [PubMed] [Google Scholar]
- 56.Davis EP, et al. Prenatal maternal anxiety and depression predict negative behavioral reactivity in infancy. Infancy. 2004;6:319–331. [Google Scholar]
- 57.Bergman K, et al. Maternal stress during pregnancy predicts cognitive ability and fearfulness in infancy. J Am Acad Child Adolesc Psychiatry. 2007;46:1454–1463. doi: 10.1097/chi.0b013e31814a62f6. [DOI] [PubMed] [Google Scholar]
- 58.De Weerth C, Van Hees Y, Buitelaar J. Prenatal maternal cortisol levels and infant behavior during the first 5 months. Early Hum Dev. 2003;74:139–151. doi: 10.1016/s0378-3782(03)00088-4. [DOI] [PubMed] [Google Scholar]
- 59.Grant KA, et al. Maternal prenatal anxiety, postnatal caregiving and infants' cortisol responses to the still-face procedure. Dev Psychobiol. 2009;51:625–637. doi: 10.1002/dev.20397. [DOI] [PubMed] [Google Scholar]
- 60.Kinsella MT, Monk C. Impact of maternal stress, depression and anxiety on fetal neurobehavioral development. Clin Obstet Gynecol. 2009;52:425–440. doi: 10.1097/GRF.0b013e3181b52df1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Davis EP, et al. Prenatal exposure to maternal depression and cortisol influences infant temperament. J Am Acad Child Adolesc Psychiatry. 2007;46:737–746. doi: 10.1097/chi.0b013e318047b775. [DOI] [PubMed] [Google Scholar]
- 62.Davis EP, et al. Prenatal maternal stress programs infant stress regulation. J Child Psychol Psychiatry. 2011;52:119–129. doi: 10.1111/j.1469-7610.2010.02314.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Davis EP, Waffarn F, Sandman CA. Prenatal exposure to synthetic glucocorticoids impairs stress regulation among healthy full-term infants. Dev Psychobiol. 2011;53:175–183. doi: 10.1002/dev.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huizink AC, et al. Stress during pregnancy is associated with developmental outcome in infancy. J Child Psychol Psychiatry. 2003;44:810–818. doi: 10.1111/1469-7610.00166. [DOI] [PubMed] [Google Scholar]
- 65.Mennes M, et al. Long-term cognitive sequelae of antenatal maternal anxiety: involvement of the orbitofrontal cortex. Neurosci Biobehav Rev. 2006;30:1078–1086. doi: 10.1016/j.neubiorev.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 66.DiPietro JA, et al. Maternal psychological distress during pregnancy in relation to child development at age two. Child Dev. 2006;77:573–587. doi: 10.1111/j.1467-8624.2006.00891.x. [DOI] [PubMed] [Google Scholar]
- 67.Van den Bergh B. The influence of maternal emotion during pregnancy on fetal and neonatal behavior. Pre- Peri-Nat Psychol J. 1990;5:119–130. [Google Scholar]
- 68.Hacking D, et al. Respiratory distress syndrome and antenatal corticosteroid treatment in premature twins. Arch Dis Child Fetal Neonatal Ed. 2001;85:F77–F78. doi: 10.1136/fn.85.1.F75g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Giannopoulos G, Jackson K, Tulchinsky D. Glucocorticoid metabolism in human placenta, decidua, myometrium, and fetal membranes. J Steroid Biochem. 1982;17:371–374. doi: 10.1016/0022-4731(82)90628-8. [DOI] [PubMed] [Google Scholar]
- 70.Murphy VE, et al. Endocrine regulation of human fetal growth: the role of the mother, placenta, and fetus. Endocr Rev. 2006;27:141–169. doi: 10.1210/er.2005-0011. [DOI] [PubMed] [Google Scholar]
- 71.Peterson BS, et al. Regional brain volume abnormalities and long-term cognitive outcome in preterm infants. JAMA. 2000;284:1939–1947. doi: 10.1001/jama.284.15.1939. [DOI] [PubMed] [Google Scholar]
- 72.Buss C, et al. Maternal care modulates the relationship between prenatal risk and hippocampal volume in women but not in men. J Neurosci. 2007;27:2592–2595. doi: 10.1523/JNEUROSCI.3252-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Abernethy LJ, Palaniappan M, Cooke RW. Quantitative magnetic resonance imaging of the brain in survivors of very low birth weight. Arch Dis Child. 2002;87:279–283. doi: 10.1136/adc.87.4.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nosarti C, et al. Adolescents who were born very preterm have decreased brain volumes. Brain. 2002;125:1616–1623. doi: 10.1093/brain/awf157. [DOI] [PubMed] [Google Scholar]
- 75.Beauchamp MH, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 76.Buss C, et al. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6- to 9-year-old children. Psychoneuroendocrinology. 2010;35:141–153. doi: 10.1016/j.psyneuen.2009.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Connolly JD, et al. Human fMRI evidence for the neural correlates of preparatory set. Nat Neurosci. 2002;5:1345–1352. doi: 10.1038/nn969. [DOI] [PubMed] [Google Scholar]
- 78.Squire LR, Stark CE, Clark RE. The medial temporal lobe. Annu Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- 79.Hoistad M, Barbas H. Sequence of information processing for emotions through pathways linking temporal and insular cortices with the amygdala. Neuroimage. 2008;40:1016–1033. doi: 10.1016/j.neuroimage.2007.12.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nakamura K, Kubota K. The primate temporal pole: its putative role in object recognition and memory. Behav Brain Res. 1996;77:53–77. doi: 10.1016/0166-4328(95)00227-8. [DOI] [PubMed] [Google Scholar]
- 81.Ahmad Z, et al. Auditory comprehension of language in young children: neural networks identified with fMRI. Neurology. 2003;60:1598–1605. doi: 10.1212/01.wnl.0000059865.32155.86. [DOI] [PubMed] [Google Scholar]
- 82.Mestres-Misse A, et al. Functional neuroanatomy of meaning acquisition from context. J Cogn Neurosci. 2008;20:2153–2166. doi: 10.1162/jocn.2008.20150. [DOI] [PubMed] [Google Scholar]
- 83.Kinnunen AK, Koenig JI, Bilbe G. Repeated variable prenatal stress alters pre- and postsynaptic gene expression in the rat frontal pole. J Neurochem. 2003;86:736–748. doi: 10.1046/j.1471-4159.2003.01873.x. [DOI] [PubMed] [Google Scholar]
- 84.Roceri M, et al. Early maternal deprivation reduces the expression of BDNF and NMDA receptor subunits in rat hippocampus. Mol Psychiatry. 2002;7:609–616. doi: 10.1038/sj.mp.4001036. [DOI] [PubMed] [Google Scholar]
- 85.Pickering C, et al. Repeated maternal separation of male Wistar rats alters glutamate receptor expression in the hippocampus but not the prefrontal cortex. Brain Res. 2006;1099:101–108. doi: 10.1016/j.brainres.2006.04.136. [DOI] [PubMed] [Google Scholar]
- 86.Lemaire V, et al. Prenatal stress produces learning deficits associated with an inhibition of neurogenesis in the hippocampus. Proc Natl Acad Sci USA. 2000;97:11032–11037. doi: 10.1073/pnas.97.20.11032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fujioka A, et al. Differential effects of prenatal stress on the morphological maturation of hippocampal neurons. Neuroscience. 2006;141:907–915. doi: 10.1016/j.neuroscience.2006.04.046. [DOI] [PubMed] [Google Scholar]
- 88.Odagiri K, et al. Psychological prenatal stress reduced the number of BrdU immunopositive cells in the dorsal hippocampus without affecting the open field behavior of male and female rats at one month of age. Neurosci Lett. 2008;446:25–29. doi: 10.1016/j.neulet.2008.09.028. [DOI] [PubMed] [Google Scholar]
- 89.Lemaire V, et al. Postnatal stimulation of the pups counteracts prenatal stress-induced deficits in hippocampal neurogenesis. Biol Psychiatry. 2006;59:786–792. doi: 10.1016/j.biopsych.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 90.Fumagalli F, et al. Corticostriatal brain-derived neurotrophic factor dysregulation in adult rats following prenatal stress. Eur J Neurosci. 2004;20:1348–1354. doi: 10.1111/j.1460-9568.2004.03592.x. [DOI] [PubMed] [Google Scholar]
- 91.Van den Hove DL, et al. Prenatal stress and neonatal rat brain development. Neuroscience. 2006;137:145–155. doi: 10.1016/j.neuroscience.2005.08.060. [DOI] [PubMed] [Google Scholar]
- 92.Aisa B, et al. Effects of neonatal stress on markers of synaptic plasticity in the hippocampus: implications for spatial memory. Hippocampus. 2009;19:1222–1231. doi: 10.1002/hipo.20586. [DOI] [PubMed] [Google Scholar]
- 93.Ehlers CL, et al. Corticotropin-releasing factor produces increases in brain excitability and convulsive seizures in rats. Brain Res. 1983;278:332–336. doi: 10.1016/0006-8993(83)90266-4. [DOI] [PubMed] [Google Scholar]
- 94.Baram TZ, et al. The CRF1 receptor mediates the excitatory actions of corticotropin-releasing factor (CRF) in the developing rat brain: in vivo evidence using a novel, selective, non-peptide CRF receptor antagonist. Brain Res. 1997;770:89–95. doi: 10.1016/s0006-8993(97)00759-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Baram TZ, et al. Corticotropin-releasing hormone-induced seizures in infant rats originate in the amygdala. Ann Neurol. 1992;31:488–494. doi: 10.1002/ana.410310505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baram TZ, Hatalski CG. Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain. Trends Neurosci. 1998;21:471–476. doi: 10.1016/s0166-2236(98)01275-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Joels M, Baram TZ. The neuro-symphony of stress. Nat Rev Neurosci. 2009;10:459–466. doi: 10.1038/nrn2632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Maecker H, et al. Astressin, a novel and potent CRF antagonist, is neuroprotective in the hippocampus when administered after a seizure. Brain Res. 1997;744:166–170. doi: 10.1016/s0006-8993(96)01207-3. [DOI] [PubMed] [Google Scholar]
- 99.Strijbos PJ, Relton JK, Rothwell NJ. Corticotrophin-releasing factor antagonist inhibits neuronal damage induced by focal cerebral ischaemia or activation of NMDA receptors in the rat brain. Brain Res. 1994;656:405–408. doi: 10.1016/0006-8993(94)91485-0. [DOI] [PubMed] [Google Scholar]
- 100.Baram TZ, Ribak CE. Peptide-induced infant status epilepticus causes neuronal death and synaptic reorganization. Neuroreport. 1995;6:277–280. doi: 10.1097/00001756-199501000-00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Brunson KL, et al. Long-term, progressive hippocampal cell loss and dysfunction induced by early-life administration of corticotropin-releasing hormone reproduce the effects of early-life stress. Proc Natl Acad Sci USA. 2001;98:8856–8861. doi: 10.1073/pnas.151224898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hollrigel GS, et al. The pro-convulsant actions of corticotropin-releasing hormone in the hippocampus of infant rats. Neuroscience. 1998;84:71–79. doi: 10.1016/s0306-4522(97)00499-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen Y, et al. Rapid loss of dendritic spines after stress involves derangement of spine dynamics by corticotropin-releasing hormone. J Neurosci. 2008;28:2903–2911. doi: 10.1523/JNEUROSCI.0225-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith R, et al. Corticotropin-releasing hormone directly and preferentially stimulates dehydroepiandrosterone sulfate secretion by human fetal adrenal cortical cells. J Clin Endocrinol Metab. 1998;83:2916–2920. doi: 10.1210/jcem.83.8.5020. [DOI] [PubMed] [Google Scholar]
- 105.Gitau R, et al. Fetal exposure to maternal cortisol. Lancet. 1998;352:707–708. doi: 10.1016/S0140-6736(05)60824-0. [DOI] [PubMed] [Google Scholar]
- 106.Gitau R, et al. Fetal hypothalamic-pituitary-adrenal stress responses to invasive procedures are independent of maternal responses. J Clin Endocrinol Metab. 2001;86:104–109. doi: 10.1210/jcem.86.1.7090. [DOI] [PubMed] [Google Scholar]
- 107.Jacobson L, Sapolsky R. The role of the hippocampus in feedback regulation of the hypothalamic-pituitary-adrenocortical axis. Endocr Rev. 1991;12:118–134. doi: 10.1210/edrv-12-2-118. [DOI] [PubMed] [Google Scholar]
- 108.Diorio D, Viau V, Meaney MJ. The role of the medial prefrontal cortex (cingulate gyrus) in the regulation of hypothalamic-pituitary-adrenal responses to stress. J Neurosci. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Sanchez MM, et al. Distribution of corticosteroid receptors in the rhesus brain: relative absence of glucocorticoid receptors in the hippocampal formation. J Neurosci. 2000;20:4657–4668. doi: 10.1523/JNEUROSCI.20-12-04657.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Noorlander CW, et al. Ontogeny of hippocampal corticosteroid receptors: effects of antenatal glucocorticoids in human and mouse. J Comp Neurol. 2006;499:924–932. doi: 10.1002/cne.21162. [DOI] [PubMed] [Google Scholar]
- 111.Zarrow MX, Philpott JE, Denenberg VH. Passage of 14C-4-corticosterone from the rat mother to the foetus and neonate. Nature. 1970;226:1058–1059. doi: 10.1038/2261058a0. [DOI] [PubMed] [Google Scholar]
- 112.Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sapolsky RM, et al. Hippocampal damage associated with prolonged glucocorticoid exposure in primates. J Neurosci. 1990;10:2897–2902. doi: 10.1523/JNEUROSCI.10-09-02897.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Uno H, et al. Neurotoxicity of glucocorticoids in the primate brain. Horm Behav. 1994;28:336–348. doi: 10.1006/hbeh.1994.1030. [DOI] [PubMed] [Google Scholar]
- 115.Nitta A, et al. Brain-derived neurotrophic factor prevents neuronal cell death induced by corticosterone. J Neurosci Res. 1999;57:227–235. doi: 10.1002/(SICI)1097-4547(19990715)57:2<227::AID-JNR8>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 116.Huang WL, et al. Repeated prenatal corticosteroid administration delays myelination of the corpus callosum in fetal sheep. Int J Dev Neurosci. 2001;19:415–425. doi: 10.1016/s0736-5748(01)00026-0. [DOI] [PubMed] [Google Scholar]
- 117.Coe CL, Lulbach GR, Schneider ML. Prenatal disturbance alters the size of the corpus callosum in young monkeys. Dev Psychobiol. 2002;41:178–185. doi: 10.1002/dev.10063. [DOI] [PubMed] [Google Scholar]
- 118.Kapoor A, Petropoulos S, Matthews SG. Fetal programming of hypothalamic-pituitary-adrenal axis function and behavior by synthetic glucocorticoids. Brain Res Rev. 2008;57:586–595. doi: 10.1016/j.brainresrev.2007.06.013. [DOI] [PubMed] [Google Scholar]
- 119.LaPlante DP, et al. Stress during pregnancy affects general intellectual and language functioning in human toddlers. Pediatr Res. 2004;56:400–410. doi: 10.1203/01.PDR.0000136281.34035.44. [DOI] [PubMed] [Google Scholar]
- 120.Yehuda R, et al. Transgenerational effects of posttraumatic stress disorder in babies of mothers exposed to the World Trade Center attacks during pregnancy. J Clin Endocrinol Metab. 2005;90:4115–4118. doi: 10.1210/jc.2005-0550. [DOI] [PubMed] [Google Scholar]
- 121.Rice F, et al. The links between prenatal stress and offspring development and psychopathology: disentangling environmental and inherited influences. Psychol Med. 2009;40:1–11. doi: 10.1017/S0033291709005911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Bale TL. Neuroendocrine and immune influences on the CNS: it's a matter of sex. Neuron. 2009;64:13–16. doi: 10.1016/j.neuron.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 123.Lin Y, et al. Sex differences in the effects of acute and chronic stress and recovery after long-term stress on stress-related brain regions of rats. Cereb Cortex. 2009;19:1978–1989. doi: 10.1093/cercor/bhn225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Boukouvalas G, et al. High-fat feeding influences the endocrine responses of pubertal rats to an acute stress. Neuroendocrinology. 2010;92:235–245. doi: 10.1159/000321393. [DOI] [PubMed] [Google Scholar]
- 125.Goel N, Bale TL. Identifying early behavioral and molecular markers of future stress sensitivity. Endocrinology. 2007;148:4585–4591. doi: 10.1210/en.2007-0479. [DOI] [PubMed] [Google Scholar]
- 126.Mueller BR, Bale TL. Sex-specific programming of offspring emotionality after stress early in pregnancy. J Neurosci. 2008;28:9055–9065. doi: 10.1523/JNEUROSCI.1424-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gabory A, Attig L, Junien C. Sexual dimorphism in environmental epigenetic programming. Mol Cell Endocrinol. 2009;304:8–18. doi: 10.1016/j.mce.2009.02.015. [DOI] [PubMed] [Google Scholar]
- 128.Ellman LM, et al. Timing of fetal exposure to stress hormones: effects on newborn physical and neuromuscular maturation. Dev Psychobiol. 2008;50:232–241. doi: 10.1002/dev.20293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Bernardes J, et al. Linear and complex heart rate dynamics vary with sex in relation to fetal behavioural states. Early Hum Dev. 2008;84:433–439. doi: 10.1016/j.earlhumdev.2007.12.001. [DOI] [PubMed] [Google Scholar]
- 130.DiPietro JA, et al. Fetal neurobehavioral development: associations with socioeconomic class and fetal sex. Dev Psychobiol. 1998;33:79–91. [PubMed] [Google Scholar]
- 131.Kesler SR, et al. Brain volume reductions within multiple cognitive systems in male preterm children at age twelve. J Pediatr. 2008;152:513–520. doi: 10.1016/j.jpeds.2007.08.009. e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Clifton VL. Sex and the human placenta: mediating differential strategies of fetal growth and survival. Placenta. 2010;31((suppl 1)):S33–S39. doi: 10.1016/j.placenta.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 133.Diamond MC, Johnson RE, Ingram C. Brain plasticity induced by environment and pregnancy. Int J Neurosci. 1971;2:171–178. doi: 10.3109/00207457109146999. [DOI] [PubMed] [Google Scholar]
- 134.Keyser-Marcus L, et al. Alterations of medial preoptic area neurons following pregnancy and pregnancy-like steroidal treatment in the rat. Brain Res Bull. 2001;55:737–745. doi: 10.1016/s0361-9230(01)00554-8. [DOI] [PubMed] [Google Scholar]
- 135.Kinsely CH, Lambert KG. The maternal brain. Sci Am. 2006;294:72–79. doi: 10.1038/scientificamerican0106-72. [DOI] [PubMed] [Google Scholar]
- 136.Kinsely CH, et al. Motherhood improves learning and memory. Nature. 1999;402:137–138. doi: 10.1038/45957. [DOI] [PubMed] [Google Scholar]
- 137.Kinsely CH, et al. Motherhood and the hormones of pregnancy modify concentrations of hippocampal neuronal dendritic spines. Horm Behav. 2006;49:131–142. doi: 10.1016/j.yhbeh.2005.05.017. [DOI] [PubMed] [Google Scholar]
- 138.Pawluski JL, Galea LAM. Hippocampal morphology is differentially affected by reproductive experience in the mother. J Neurobiol. 2006;66:71–81. doi: 10.1002/neu.20194. [DOI] [PubMed] [Google Scholar]
- 139.Gatewood JD, et al. Motherhood mitigates aging-related decrements in learning and memory and positively affects brain aging in the rat. Brain Res Bull. 2005;66:91–98. doi: 10.1016/j.brainresbull.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 140.Glynn LM, et al. Pregnancy affects appraisal of negative life events. J Psychosom Res. 2004;56:47–52. doi: 10.1016/S0022-3999(03)00133-8. [DOI] [PubMed] [Google Scholar]
- 141.De Weerth C, Buitelaar JK. Physiological stress reactivity in human pregnancy – a review. Neurosci Biobehav Rev. 2005;29:295–312. doi: 10.1016/j.neubiorev.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 142.Feldman R, et al. Evidence for a neuroendocrinological foundation of human affiliation: plasma oxytocin levels across pregnancy and the postpartum period predict mother-infant bonding. Psychol Sci. 2007;18:965–970. doi: 10.1111/j.1467-9280.2007.02010.x. [DOI] [PubMed] [Google Scholar]
- 143.Fleming AS, et al. Hormonal and experiential correlates of maternal responsiveness during pregnancy and the puerperium in human mothers. Horm Behav. 1997;31:145–158. doi: 10.1006/hbeh.1997.1376. [DOI] [PubMed] [Google Scholar]
- 144.Fleming AS, Steiner M, Corter C. Cortisol, hedonics and maternal responsiveness. Horm Behav. 1997;32:85–98. doi: 10.1006/hbeh.1997.1407. [DOI] [PubMed] [Google Scholar]
- 145.Swain JE, et al. Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry. 2008;49:1042–1052. doi: 10.1111/j.1469-7610.2008.01963.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lederman SA, et al. The effects of the World Trade Center event on birth outcomes among term deliveries at three lower Manhattan hospitals. Environ Health Perspect. 2004;112:1772–1778. doi: 10.1289/ehp.7348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Buss C, et al. The maternal cortisol awakening response in human pregnancy is associated with the length of gestation. Am J Obstet Gynecol. 2010;201:e1–e8. doi: 10.1016/j.ajog.2009.06.063. [DOI] [PubMed] [Google Scholar]
- 148.Brindle PM, et al. Objective and subjective memory impairment in pregnancy. Psychol Med. 1991;21:647–653. doi: 10.1017/s0033291700022285. [DOI] [PubMed] [Google Scholar]
- 149.Poser CM, Kassirer MR, Peyser JM. Benign encephalopathy of pregnancy. Acta Neurol Scand. 1986;73:39–43. doi: 10.1111/j.1600-0404.1986.tb03239.x. [DOI] [PubMed] [Google Scholar]
- 150.Henry JD, Rendell PG. A review of the impact of pregnancy on memory function. J Clin Exp Neuropsychol. 2007;29:793–803. doi: 10.1080/13803390701612209. [DOI] [PubMed] [Google Scholar]
- 151.Yim IS, et al. Prenatal β-endorphin as an early predictor of postpartum depressive symptoms in euthymic women. J Affect Disord. 2010;125:128–133. doi: 10.1016/j.jad.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Lord C, et al. Hippocampal volumes are larger in postmenopausal women using estrogen therapy compared to past users, never users and men: a possible window of opportunity effect. Neurobiol Aging. 2008;29:95–101. doi: 10.1016/j.neurobiolaging.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 153.Protopopescu X, et al. Hippocampal structural changes across the menstrual cycle. Hippocampus. 2008;18:985–988. doi: 10.1002/hipo.20468. [DOI] [PubMed] [Google Scholar]
- 154.Weissheimer KB, et al. Interactions of corticotropin-releasing factor, urocortin and citalopram in a primate model of stress-induced amenorrhea. Neuroendocrinology. 2010;92:224–234. doi: 10.1159/000319257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oatridge A, Holdcroft A, et al. Change in brain size during and after pregnancy: study in healthy women and women with preeclampsia. Am J Neuroradiol. 2002;13:19–26. [PMC free article] [PubMed] [Google Scholar]
- 156.Petraglia F, et al. Neuroendocrinology of the human placenta. Front Neuroendocrinol. 1990;11:6–37. [Google Scholar]
- 157.Denver RJ. Environmental stress as a developmental cue: corticotropin-releasing hormone is a proximate mediator of adaptive phenotypic plasticity in amphibian metamorphosis. Horm Behav. 1997;31:169–179. doi: 10.1006/hbeh.1997.1383. [DOI] [PubMed] [Google Scholar]