Abstract
Systemic sclerosis (SSc) is a complex, multiorgan, autoimmune disease. Lung fibrosis occurs in ∼80% of patients with SSc; 25% to 30% develop progressive interstitial lung disease (ILD). The pathogenesis of fibrosis in SSc-associated ILD (SSc-ILD) involves cellular injury, activation/differentiation of mesenchymal cells, and morphological/biological changes in epithelial/endothelial cells. Risk factors for progressive SSc-ILD include older age, male sex, degree of lung involvement on baseline high-resolution computed tomography imaging, reduced DlCO, and reduced FVC. SSc-ILD does not share the genetic risk architecture observed in idiopathic pulmonary fibrosis (IPF), with key risk factors yet to be identified. Presence of anti–Scl-70 antibodies and absence of anti-centromere antibodies indicate increased likelihood of progressive ILD. Elevated levels of serum Krebs von den Lungen-6 and C-reactive protein are both associated with SSc-ILD severity and predict SSc-ILD progression. A promising prognostic indicator is serum chemokine (C-C motif) ligand 18. SSc-ILD shares similarities with IPF, although clear differences exist. Histologically, a nonspecific interstitial pneumonia pattern is commonly observed in SSc-ILD, whereas IPF is defined by usual interstitial pneumonia. The course of SSc-ILD is variable, ranging from minor, stable disease to a progressive course, whereas all patients with IPF experience progression of disease. Although appropriately treated patients with SSc-ILD have better chances of stabilization and survival, a relentlessly progressive course, akin to IPF, is seen in a minority. Better understanding of cellular and molecular pathogenesis, genetic risk, and distinctive features of SSc-ILD and identification of robust prognostic biomarkers are needed for optimal disease management.
Keywords: systemic sclerosis, interstitial lung diseases, autoimmune diseases, risk factors, biomarkers
Systemic sclerosis (SSc) is a complex autoimmune disease with a range of manifestations, including vasculopathy, Raynaud’s phenomenon, immune dysfunction, and fibrosis of the skin and internal organs (1–3). It is a rare disease, with an estimated global prevalence of 3 to 24 per 100,000 (4). Diagnostic criteria for SSc were published jointly by the European League against Rheumatism and the American College of Rheumatology in 2013, with a scoring system based on a range of possible signs, symptoms, and autoantibodies (5).
Lung fibrosis occurs in up to approximately 80% of patients with SSc, with varying prevalence depending on ascertainment methods, and 25% to 30% of patients develop progressive interstitial lung disease (ILD) (2). In a large international cohort study, 35% of SSc-related deaths were attributed to pulmonary fibrosis, making it the leading cause of mortality in this patient population (6). The course of SSc-associated ILD (SSc-ILD) is highly variable; some patients have limited or stable lung involvement, whereas in others, lung disease progresses inexorably. Because of the largely irreversible and potentially progressive nature of ILD, it is important that diagnostic tests are performed early, so that treatment can be initiated with minimal delay.
In this article, we review SSc-ILD with a focus on pathogenesis, risk factors, and patient characteristics associated with the condition, with a view to identifying patients most at risk for the disease and its progression. We also highlight similarities and differences between SSc-ILD and idiopathic pulmonary fibrosis (IPF), the most frequent and deadly of the idiopathic ILDs.
Pathogenesis
The architectural disruption and collagen-rich extracellular matrix (ECM) in SSc-ILD results from the interaction of cells in the epithelial, endothelial, and interstitial compartments with components of the innate and adaptive immune system and the ECM, after chronic microinjuries in the lung. The first step in the pathological process is believed to comprise repetitive endothelial and epithelial cell injury. This leads to activation of the innate and adaptive immune system, recruitment and activation of fibroblasts, and differentiation of fibroblasts to a myofibroblast phenotype (7), with accumulation of ECM and development of fibrosis (8). Apoptosis is triggered in some epithelial cells, and others undergo epithelial–mesenchymal transition (7). Many of the phenotypic changes occurring in respiratory epithelial cells in the context of fibrosis remain unknown and require further study. Cells undergoing epithelial–mesenchymal transition exhibit profound morphological and biological changes, such as loss of polarity, increased capacity for migration, increased production of ECM components, and increased resistance to apoptosis (7). Resistance to apoptosis is also characteristic of certain myofibroblasts, which may contribute to the rate and extent of fibrosis (7) in SSc-ILD.
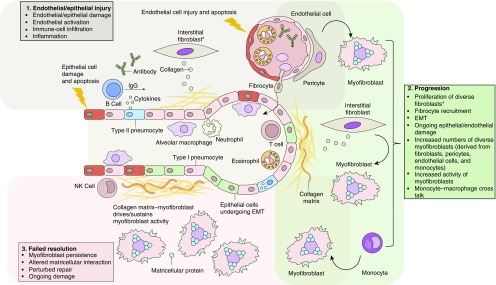
A plausible model of pathogenesis for parenchymal lung involvement in connective tissue disease, which consolidates current evidence on SSc-ILD pathology and describes initial alveolar epithelial and endothelial injuries that are triggered by environmental factors, pathogens, or inflammation, is shown in Figure 1 (9). The latter event results in damage to the lung tissue and initiation of repair pathways including the recruitment of fibroblasts and myofibroblasts; close anatomical and functional interactions between alveolar epithelial and endothelial compartments result in recruitment of circulating cellular components and mediators, such as platelets and progenitor cells. In this model, myofibroblasts are key profibrotic cells that persist in affected lung tissue; the extent of their persistence determines the pattern and type of fibrotic reaction. Interplay of myofibroblasts with the ECM via matricellular proteins, such as integrins and microfibrils, together with soluble factors, such as connective tissue growth factor, drive the fibrotic process. The degree of irreversible architectural disruption likely determines the progression or reversibility of the lung condition (9).
Figure 1.
Cellular pathogenesis of fibrotic lung injury in systemic sclerosis. *Including SPINT2hi, MFAP5hi, and few WIF1hi fibroblasts. EMT = epithelial–mesenchymal transition; NK cell = natural killer T cell.
TGF-β (transforming growth factor-β) is believed to be one of the key factors in the process of fibrosis. It has been implicated in ECM accumulation and the regulation of immune response (7, 8). Injured cells secrete TGF-β, which leads to the recruitment of immune cells, including macrophages, which in turn release more TGF-β (7). Increased expression of genes regulated by TGF-β has been confirmed in patients with progressive lung fibrosis (10). Type 2 helper T cells that secrete IL (e.g., IL-4 and IL-13) are also believed to play a role in the development of fibrosis (8). Moreover, levels of thrombin are increased in the lungs of patients with SSc-ILD (7), probably as a consequence of cellular injury. In addition to its role in the coagulation cascade, thrombin may contribute to fibrosis by increasing proliferation of fibroblasts in response to fibrinogen and facilitating differentiation of fibroblasts into myofibroblasts (7). The Wnt/β-catenin pathway has been implicated in the activation of fibroblasts and in pulmonary tissue remodeling (7).
Elements involved in the pathogenesis of SSc, such as IL-6 and M2-like macrophages, may also contribute to the development of SSc-ILD, especially early in the disease (11–13). Increases in macrophage polarization, elevated C-reactive protein, and serum IL-6 levels have been associated with the progression of early SSc-ILD (10, 12, 14).
Genetics and Epigenetics
SSc-ILD has been associated with a number of HLA-dependent genes and non-HLA genes (see Tables E1 and E2 in the online supplement) (15). After the analyses of at least 200 patients with SSc-ILD, only two variants conferred an odds ratio of at least 2.0 with statistical significance: HLA-DRB1*3 (Han Chinese population) and CTGF rs6918698 (GG genotype; UK population) (15).
Despite the number of reported associations, genetic biomarkers relevant to the risk of ILD in patients with SSc are yet to be established with certainty (15). Many of the individual studies reporting associations of genetic variants with SSc-ILD have been small, and follow-up studies of specific associations are either lacking or have reported conflicting data. Therefore, a concerted effort is needed, involving large numbers of patients of different ethnicities, to establish more definite genetic risk factors for SSc-ILD and its progression.
A few studies have investigated the epigenetics of SSc-ILD (7). Epigenetic factors that may play a role in the pathogenesis of SSc-ILD include CpG methylation, which is related to increased DNA methyltransferase expression in fibroblasts. Increased DNA methyltransferase expression may affect the activities of nitric oxide synthase or the collagen transcription suppression factor Fli1 (Friend leukemia virus integration 1). Fli1 appears to play a role in protecting against ILD, by upregulating the expression of genes, including autoimmune regulator and CXCL13 (7, 16). A genome-wide study of genes in peripheral blood mononuclear cells identified four methylation-regulated genes (F2R, FYN, PAG1, and PRKCH) as being underexpressed in patients with SSc-ILD versus patients with SSc and no ILD (17). Significantly increased expression of the XRCC4 DNA repair gene was reported in patients with SSc with versus without ILD (18). Micro-RNA (miRNA) expression has also been assessed in animal models and in lung tissue and peripheral blood mononuclear cells derived from patients with SSc-ILD. Studies have shown that increased expression of miR-155 is associated with worsened lung function and increased lung fibrosis (19).
Risk Factors for the Development and Progression of SSc-ILD
Risk factors associated with progressive ILD among patients with SSc include diffuse cutaneous SSc, male sex, African American race, and the presence of anti–Scl-70 antibodies, also known as antitopoisomerase I antibodies or ATA, discussed previously in the section on genetics and epigenetics (20–22). Other indices of SSc-ILD severity have also been associated with progressive disease, including the extent of disease on high-resolution computed tomography (HRCT) imaging, reduced DlCO (% predicted), and decreased FVC (% predicted) (23, 24).
Similarly, risk factors for mortality in SSc-ILD include older age, male sex, extent of disease on HRCT imaging, lower FVC, and lower DlCO (23). Several models, including the Composite Physiologic Index; Interstitial Lung Disease–Gender, Age, Physiology Index; du Bois index; and modified du Bois index, have been reported to help predict mortality in patients with SSc-ILD (25). These models are based on readily available clinical details, such as age, sex, and FVC. HRCT imaging is routinely performed at most centers, and the findings can be integrated with pulmonary function test results as per the Limited/Extensive Staging System developed by Goh and colleagues for SSc-ILD (26). This staging system, which is based on the visual estimation of extent of disease on HRCT and, as necessary, integrated with FVC (% predicted), appears to predict the patient’s risk of mortality more accurately than either of the component variables when used in isolation (26). This validated staging system proposes the rapid identification of limited or extensive lung disease using HRCT on the basis of a disease extent threshold of 20%. In cases in which disease extent remains indeterminate on HRCT, FVC is used to classify lung disease as either limited or extensive on the basis of an FVC threshold of 70%. This system represents a practical means of integrating HRCT extent and functional severity in routine prognostic evaluation (26). HRCT images from patients with SSc-ILD are provided in Figures 2, 3, and 4 to demonstrate examples of ILD with limited, indeterminate, and extensive disease on CT imaging, according to the Goh and colleagues 20% threshold (26). Stratification of patients using this system has been shown to be predictive of both progression-free survival and mortality.
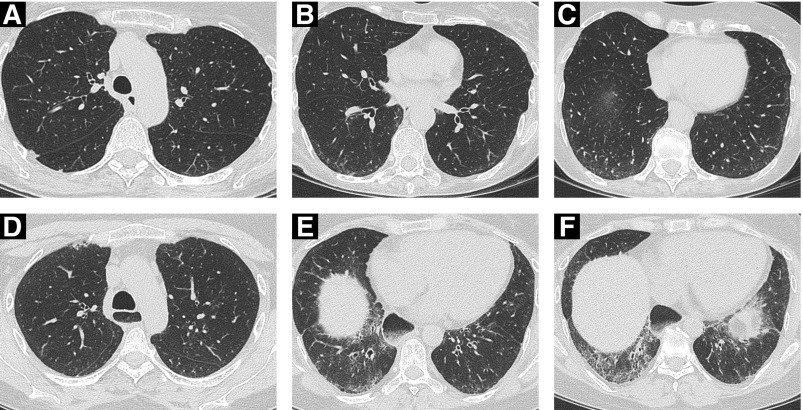
Figure 2.
(A–C) Limited disease (<20% extent) on high-resolution computed tomography (HRCT) imaging in a 72-year-old female nonsmoker. HRCT images at the level of (A) the aortic arch show no convincing interstitial lung disease (ILD), and (B and C) very limited subpleural ground-glass opacification. (D–F) ILD of “indeterminate” extent on HRCT imaging in a 46-year-old female nonsmoker with systemic sclerosis. (A–D) The upper zones show minor reticulation, (E) just below the level of the right hemidiaphragm, and (F) the costophrenic recesses demonstrating reticulation, ground-glass opacification, and traction bronchiectasis/bronchiolectasis. The morphologic features are in keeping with a fibrotic nonspecific interstitial pneumonia pattern. Disease extent on HRCT imaging with regard to the 20% threshold is difficult to gauge (i.e., “indeterminate” according to the Goh staging); FVC in this patient was 60% predicted, thereby indicating “extensive” ILD. Note the marked esophageal dilatation containing food residue.
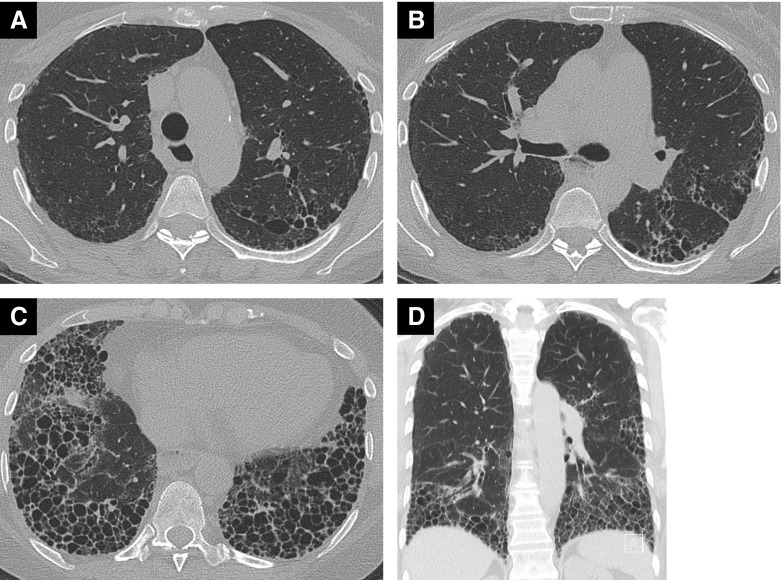
Figure 3.
High-resolution computed tomography images in a 58-year-old woman with systemic sclerosis who never smoked; DlCO 32% predicted and FVC 76% predicted. Axial images at (A) the level of the aortic arch, (B) the carina, and (C) the lower lobes demonstrate extensive disease (>20% extent by visual estimation) and (D) coronal reconstruction. There is marked honeycombing, particularly in the lower lobes, indicating a usual interstitial pneumonia pattern. The coronal image shows striking lower zone preponderance of disease.
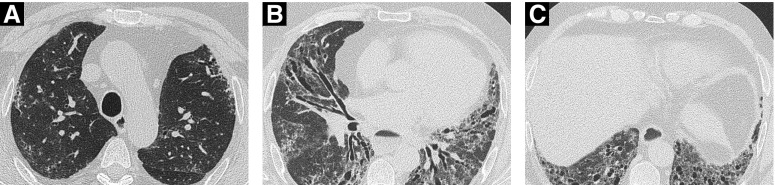
Figure 4.
Computed tomography imaging in a 52-year-old man, ex-smoker, with a DlCO of 22% and FVC 56% predicted. Axial images at (A) the level of the arch, (B) the pulmonary venous confluence, and (C) the costophrenic recesses show extensive (>20%) disease. There is predominant ground-glass opacification with fine reticulation, no honeycombing, but severe traction bronchiectasis. The computed tomography features are consistent with a fibrotic nonspecific interstitial pneumonia pattern. Note also the marked esophageal dilatation.
The 6-minute-walk test has also been demonstrated to be an independent predictor of mortality in SSc-ILD. Certain blood biomarkers may also be used to predict the risk of disease progression (27, 28), although they are not routinely used in clinical practice.
In the SLS (Scleroderma Lung Study) I (NCT00004563) and II (NCT00883129), higher baseline skin score, older age, and a decline in FVC and DlCO over 2 years were independently associated with an increased risk of mortality (29). A decline in the FVC and the DlCO over 2 years was a better predictor of mortality than the baseline FVC and DlCO (29). In a long-term study of the prognostic significance of pulmonary function test changes, the strongest 1-year predictor of future mortality in patients with SSc-ILD was a composite endpoint defined either by a decline from baseline in FVC of ≥10% or a decline of 5% to 9% in FVC with a decrease in DlCO of ≥15% (30). Thus, short-term changes in measurements of SSc-ILD progression appear to have important implications regarding long-term outcomes. The overlap between risk factors for ILD progression and for increased mortality is unsurprising.
Treatment of SSc-ILD is beyond the scope of this review; however, several landmark studies have indicated that some treatments may be able to stabilize or slow down disease progression and, therefore, improve patient outcomes. All these trials focused on patients with clinically meaningful ILD, defined as a combination of moderate to severe ILD on HRCT and abnormal pulmonary physiology with symptoms. SLS I showed that 12 months of treatment of SSc-ILD with cyclophosphamide (CYC) improved FVC % predicted by 2.53% versus placebo (P < 0.03). A modest benefit was also reported in TLC, dyspnea, skin thickening, and health-related quality of life (31, 32). SLS II was a 24-month study comparing 2-year treatment with mycophenolate mofetil (MMF) with 1 year of treatment with CYC followed by 1 year of placebo in patients with SSc-ILD. The two treatment approaches showed similar efficacy in terms of FVC % predicted (mean improvement of 2.19% and 2.88%, respectively) at 24 months. However, MMF treatment was reported to be better tolerated (e.g., lower rates of leucopenia and thrombocytopenia) (33). The Fibrosing Alveolitis in Scleroderma Trial was a randomized, placebo-controlled study of low-dose prednisolone and six-monthly doses of intravenous CYC and oral azathioprine. Compared with placebo, study intervention showed a nonsignificant trend toward improving FVC (treatment difference, 4.19%; P = 0.08) (34). Recently, nintedanib became the first U.S. Food and Drug Administration–approved treatment for SSc-ILD; it is indicated for slowing the rate of decline in pulmonary function in patients with SSc-associated ILD on the basis of the results of the phase III, randomized, double-blind placebo-controlled SENSCIS (Safety and Efficacy of Nintedanib in Systemic Sclerosis) trial (35). Primary endpoint analysis in the SENSCIS trial showed that the adjusted annual rate of decline in FVC was 52.4 ml/yr in nintedanib-treated patients versus 93.3 ml/yr in placebo-treated patients (difference, 41.0 ml/yr; 95% confidence interval, 2.9–79.0 ml/yr; P = 0.04) over a 1-year period in the total study population. Subgroups analyses reported that nintedanib reduced the progression of ILD irrespective of mycophenolate use at baseline. Statistical testing did not indicate heterogeneity in the treatment effect of nintedanib between those who were or were not receiving mycophenolate at baseline (P = 0.45 for treatment-by-time-by-subgroup interaction). The absolute effect of nintedanib versus placebo in reducing the rate of decline in FVC was numerically lower in patients who were receiving mycophenolate at baseline compared with those who were not receiving mycophenolate at baseline (26.3 ml/yr vs. 55.4 ml/yr). The relative treatment effect of nintedanib was similar between these subgroups (40% and 46%, respectively) and consistent with that observed in the overall population (44%). No other significant clinical benefits were observed (36).
Blood Serum and BAL Fluid Biomarkers
Blood serum or BAL fluid (BALF) biomarkers may be of value in diagnosing SSc-ILD and in prognostication. A number of potential biomarkers have been identified, which could be indicative of lung involvement in patients with SSc (Tables 1 and E3) (27). Autoantibodies are the only blood markers currently available in routine clinical practice (Tables 1 and E3). The presence of anti–Scl-70 antibodies and the absence of anticentromere antibodies in SSc indicate an increased likelihood of progressive ILD (20, 22, 37). Associations of these antibodies with major histocompatibility complex II antigens support the genetic basis of SSc-ILD (37).
Table 1.
Clinically Used Biomarkers and Biomarkers under Investigation in SSc-ILD
| Mechanistic Pathway and Biomarker | References |
|---|---|
| Clinically used biomarkers | |
| Immune dysregulation or inflammation | |
| Anti-centromere | 20, 22, 37 |
| Anti–Scl-70 | 22, 37 |
| Nucleolar pattern on ANA (representing anti-Th/To, U3 RNP) | 83 |
| Biomarkers supported by significant clinical data | |
| Epithelial cell injury or barrier dysfunction | |
| CCL-18 | 40, 61 |
| KL-6* | 23, 38, 39 |
| SP-D | 84 |
| Immune dysfunction or inflammation | |
| IL-6/CRP | 12, 41 |
| Biomarkers under investigation | |
| Epithelial cell injury or barrier dysfunction | |
| APOAI | 55 |
| CC16 | 85 |
| ET-1 | 86 |
| Isoprostane | 86 |
| SP-A | 87 |
| sE-selectin | 86 |
| sVCAM-1 | 86 |
| SPFA2 | 55 |
| S100A6 | 55 |
| TGF-β | 86 |
| VEGF | 86 |
| 14-3-3ε | 55 |
| Immune dysfunction or inflammation | |
| Anti-CXCR4 | 48 |
| Anti-CXCR3 | 48 |
| CCL2 | 43 |
| CRP | 88 |
| CXCL4 | 45 |
| CXCL10 | 89 |
| CX3CL1 | 90 |
| C3a | 55 |
| IL-10 | 86 |
| IL-15 | 86 |
| IL-17† | 65 |
| IL-22† | 65 |
| IL-23 | 86 |
| miR-155 | 19 |
| Remodeling and fibrosis | |
| Chitinase-1 | 50 |
| CTGF | 86 |
| Circulating fibrocytes | 88 |
| GDF-15 | 88 |
| MMP7 | 27 |
| MMP12 | 42 |
| MMP13 | 88 |
| miR-21 | 19 |
| miR-92A | 91 |
| miR-200c | 88 |
| PMN elastase | 86 |
| TIMP-1 | 86 |
| TIMP-2 | 88 |
| YKL-40 | 49 |
Definition of abbreviations: 14-3-3ε = tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein epsilon; ANA = antinuclear antibody; anti-Th/To = Th/To ribonucleoprotein antibody; APOAI = apolipoprotein A-I; C3a = complement 3 anaphylatoxin; CC16 = club cell secretory protein 16; CCL = chemokine (C-C motif) ligand; CRP = C-reactive protein; CTGF = connective tissue growth factor; CX3CL1 = chemokine (C-X3-C motif) ligand 1; CXCL = chemokine (C-X-C motif) ligand; CXCR3 = chemokine (C-X-C motif) receptor 3; ET-1 = endothelin-1; GDF-15 = growth differentiation factor–15; KL-6 = Krebs von den Lungen-6; miR = microRNA; MMP = matrix metalloproteinase; PMN = polymorphonuclear; S100A6 = S100 calcium-binding protein A6; Scl-70 = topoisomerase 1; sE-selectin = soluble E selectin; SP-A = surfactant protein A; SP-D = surfactant protein D; SPFA2 = pulmonary surfactant–associated protein A2; SSc-ILD = systemic sclerosis–associated interstitial lung disease; sVCAM-1 = soluble vascular cell adhesion molecule 1; TGF-β = transforming growth factor-β; TIMP-1 = tissue inhibitors of metalloproteinases-1; U3 RNP = fibrillarin; VEGF = vascular endothelial growth factor; YKL-40 = chitinase-3-like protein 1.
Approved by Japan’s Health Insurance Program as a diagnostic marker for interstitial lung diseases in 1999.
Circulating IL-producing T cells.
A number of biomarkers are being investigated in clinical research (Tables 1 and E3), although they are not currently available for use in routine clinical practice, with the exception of KL-6 (Krebs von den Lungen-6), which is available but only in Japan. Among biomarkers under clinical investigation, high plasma levels of KL-6 appear to be predictive of lung involvement and ILD progression in patients with SSc (23, 38, 39), including in SLS-II. Serum CCL18 (chemokine [C-C motif] ligand 18), a macrophage 2–derived protein that is chemotactic for a number of immune cells, has also been shown to be a good prognostic marker, even after adjustment for baseline ILD severity (40, 41). Analysis of serum CCL18 was able to differentiate the impact of tocilizumab versus placebo in SSc with early ILD on FVC% (14).
Serum levels of MMP7 (matrix metalloproteinase-7) are higher in patients with SSc-ILD versus SSc without ILD, and combined measurements of KL-6 and MMP7 have been suggested for identifying patients at risk of developing clinically significant ILD (27). Higher levels of MMP12 (matrix metalloproteinase-12) have been found in patients with SSc-ILD versus those without lung involvement; in the population with SSc-ILD, increased MMP12 levels appear to be associated with lower FVC (42). Data from two cohorts of patients with SSc showed that high plasma concentrations of CCL2 are predictive of ILD progression and shorter survival (43). Elevated acute-phase reactants, such as high plasma C-reactive protein levels, have been associated with an increased likelihood of progressive early SSc-ILD (44). Also, elevated serum IL-6 levels have been reported to be predictive of early disease progression (specifically, declines in DlCO and FVC or death within 12 mo) in patients with SSc-ILD (12). However, IL-6 would provide only low specificity for diagnosing SSc-ILD, because its levels are elevated in a range of inflammatory diseases.
A proteome-wide analysis in SSc identified CXCL4 (chemokine [C-X-C motif] ligand 4) as the principal protein secreted by plasmacytoid dendritic cells (45). Plasmacytoid dendritic cells in the BALF are associated with the severity of disease on HRCT in SSc-ILD (46). Plasma levels of CXCL4 correlate with the occurrence of ILD in patients with SSc, and higher levels of this biomarker are associated with more rapid decline in DlCO (45). Volkmann and colleagues found that plasma CXCL4 levels were higher in patients with SSc-ILD compared with healthy control subjects in SLS II; however, the levels did not correlate with severity of ILD at baseline (47). Plasma CXCL4 levels reduced with immunosuppressive therapy; larger declines observed over the first 12 months of treatment were associated with greater improvements in lung function over the subsequent 12 months (47). Moreover, levels of antibodies against CXCL3 (chemokine [C-X-C motif] ligand 4) and CXCL4 have been reported to be increased in patients with SSc-ILD versus healthy control subjects but lower in patients with deteriorating versus stable lung function (48). Serum levels of chitinase-3–like protein 1, also known as YKL-40, have been shown to be higher in patients with SSc with versus those without pulmonary involvement (49). Levels of chitinase 1 have been reported to be significantly higher in patients with SSc-ILD than in patients with SSc and no lung involvement; as well as being a candidate biomarker, this enzyme could be considered as a therapeutic target (50).
Currently, BAL is not routinely performed in patients with SSc-ILD; the previously observed link between BALF neutrophilia and mortality was subsequently found to be mainly related to disease severity (51, 52). However, BAL has been shown to be useful in identifying clinically unsuspected infections in a small minority of patients with SSc-ILD. If not appropriately treated, such infections have the potential to be aggravated by immunosuppressive therapy (53). In routine clinical practice, BAL is not considered to provide additional meaningful prognostic information; however, this could change if biomarkers independent of disease severity and without an equivalent correlate in the peripheral blood are identified. BALF inflammatory cytokines have been described as potential predictive biomarkers of SSc-ILD deterioration; this, however, has so far only been reported in small patient cohorts (54). Furthermore, proteomic and gene expression analysis of BALF is likely to provide insights that are specific to SSc-ILD pathogenesis that may not be possible in the peripheral blood. Proteomic analysis of BALF has also identified the differential expression of a number of potential biomarkers including C3a (complement 3 anaphylatoxin), APOAI (apolipoprotein A-I), 14-3-3ε, SPFA2 (pulmonary surfactant–associated protein A2), and S100A6 (S100 calcium-binding protein A6), involved in fibrosis, innate immune responses, and vascular damage (55).
Comparison with IPF
Respiratory clinicians are often more familiar with IPF than SSc-ILD, IPF being the prototypic ILD; IPF affects a greater number of patients and has been researched more extensively than SSc-ILD. Not surprisingly, there is a larger literature and clinical experience in IPF compared with SSc-ILD; therefore, it is logical to explore the similarities and differences between SSc-ILD and IPF. A comparative summary is provided in Tables E3 and E4.
Although ILD occurs in a large proportion of patients with SSc, only some will experience disease that worsens over time (2). Spontaneous regression can occur, albeit rarely, in SSc-ILD, and the disease course is likely to be stabilized by treatment with immunosuppressants or as part of natural history of the disease—changing from a declining trend to stability or, in a small percentage of cases, improving over time (13, 56). In contrast, all patients with IPF have progressive fibrosis, albeit at different rates (57), which never undergoes spontaneous regression.
Immunological involvement appears to differ between SSc-ILD and IPF (Tables E3 and E4), although adaptive and innate immune mechanisms are implicated in both diseases. Most patients with SSc-ILD are positive for autoantibodies (e.g., antinuclear antibodies), whereas clinically relevant levels of autoantibodies are believed to be absent from patients with IPF (13). A single study has reported a link between anti-HSP70 antibodies and poor survival in IPF, although currently this is not considered in routine clinical practice (58). The existence of specific activation mechanisms for different macrophage subpopulations has been described in IPF, whereby M1 macrophages (inducers include LPS, IFN-γ, and granulocyte–stimulating colony–stimulating factor) and M2 macrophages (inducers include IL-4, IL-10, IL-13, and TGF-β) are both involved in the pathogenesis of the disease (59). IL-4+ T cells in the BALF are associated with the severity of disease on HRCT imaging in SSc-ILD (60). Levels of CCL18 are increased in BALF and serum of patients with either IPF or SSc-ILD. In both diseases, serum CCL18 has been linked to worse prognosis independent of disease severity (40, 61), and levels of serum CCL18 appear to decrease in response to anti-IL6 therapy (14), with stabilization in lung function.
A study of lung tissue showed increased mast cell density in patients with IPF compared with healthy control subjects, whereas mast cell density was similar in patients with SSc-ILD and healthy control subjects (62). Regarding adaptive immunity, numbers of CD4+ CD25+ regulatory T cells in the lungs appear to be increased in SSc-ILD but not in IPF (63, 64). Also, increased numbers of IL-22–producing T-helper cells have been observed in SSc-ILD but not in IPF (65, 66). Consistent with these findings, individuals with SSc-ILD, but not those with IPF, benefit from CYC treatment (13). There is, therefore, good evidence to suggest that adaptive immune mechanisms play a reduced role in IPF compared with SSc-ILD. In fact, few patients with IPF are likely to respond to any immunosuppressant therapy, whereas most patients with SSc-ILD respond to such treatment. Further understanding of the phenotypes, activation mechanisms, and roles of macrophages in lung fibrosis, both in IPF and SSc-ILD, may help in the development of therapeutic targets.
Some of the pathological pathways involved in fibrogenesis in IPF are similar to those in SSc-ILD. The initial trigger of fibrosis in both diseases appears to be epithelial and/or endothelial cell injury (13). The associated cell death has several effects, including the activation of TGF-β, which then triggers immune responses and causes fibroblast activation, proliferation, and differentiation into myofibroblasts. These processes culminate in the excess deposition of ECM (11).
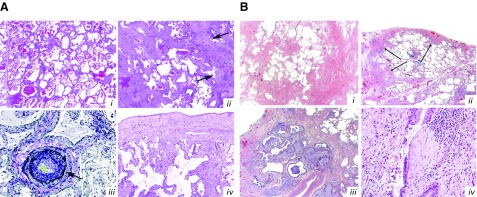
On histopathologic analysis, patients with SSc-ILD usually exhibit fibrotic (rarely cellular) nonspecific interstitial pneumonia (NSIP; Figure 5) (67), whereas usual interstitial pneumonia (UIP) may be observed only in a minority of patients with SSc-ILD. In contrast, UIP is the defining morphological pattern in patients with IPF (68). Patients with SSc-ILD and a UIP pattern have a better prognosis than patients with IPF; moreover, patients with SSc and a UIP pattern do not appear to have a significantly worse survival than patients with SSc and NSIP (69, 70). Although the reasons for this are unclear, UIP in patients with a connective tissue disease is characterized by higher numbers of lymphoid follicles, smaller honeycomb cysts, and fewer fibroblastic foci compared with UIP in IPF (71).
Figure 5.
Histopathology of systemic sclerosis–associated interstitial lung disease (SSc-ILD) and idiopathic pulmonary fibrosis (13, 68). (A) SSc-ILD. (Ai) Nonspecific interstitial pneumonia; note the diffuse alveolar septal thickening throughout the lobule with lack of peripheral accentuation in the area of an interlobular septum on the left. (Aii) Usual interstitial pneumonia; note the peripheral involvement of a pulmonary lobule sparing the centrilobular area containing the bronchovascular bundle. Arrows indicate fibroblastic foci. (Aiii) Pulmonary arterial hypertension; note the hypertensive arterial changes with prominent intimal fibrosis. Arrow indicates separation of the media and intima by the internal elastic lamina. (Aiv) Pleural fibrosis; its presence supports the diagnosis of SSc-ILD in the appropriate clinical setting. Hematoxylin and eosin (H&E)-stained sections are shown in Ai, Aii, and Aiv; Verhoeff–van Gieson–stained sections in Aiii. Original magnification ×40 in i and ii; ×200 in iii; ×100 in iv. (B) Usual interstitial pneumonia. (Bi) At low magnification, the diagnostic key is the abrupt alternating of scarred and normal lung (patchwork pattern: scar-normal-scar-normal). In the scarred areas, the alveolar architecture is obliterated. (Bii) The fibrosis frequently prevails at the periphery of the lobule in the subpleural paraseptal regions (arrows), with relative sparing of the centrolobule. This is a useful diagnostic clue, particularly in early cases like this (H&E 20). (Biii) Honeycomb consists of enlarged airspaces lined by bronchiolar epithelium, frequently filled by mucus and surrounded by dense scars. Note the architectural distortion and the abrupt transition with residual normal lung seen in the right upper corner. (Biv) A fibroblastic focus consisting of a dome-shaped proliferation of myofibroblasts immersed in a myxoid matrix. Fibroblastic foci can be covered by bronchiolar epithelium, as here, or by hyperplasic pneumocytes. H&E-stained sections are shown in Bi–Biv. (A) Reprinted from Arthritis & Rheumatology, Vol. 66, Herzog EL, et al., Review: Interstitial Lung Disease Associated With Systemic Sclerosis and Idiopathic Pulmonary Fibrosis: How Similar and Distinct? 1967–1978, Copyright (2014), by permission from John Wiley & Sons. (B) Reprinted from Respiratory Medicine, Vol. 104, Cavazza A, et al., The role of histology in idiopathic pulmonary fibrosis: An update, S11–S22, Copyright (2010), by permission from Elsevier.
Genetic variants associated with SSc-ILD and IPF do not appear to overlap. The association with the MUC5B (mucin 5B) promoter variant rs35705950, observed in sporadic IPF and familial idiopathic interstitial pneumonias (IIPs), is one notable example that is absent in SSc-ILD (72, 73). MUC5B expression is increased in the small airways and honeycomb cysts in UIP/IPF but similar to control subjects in the small airways of patients with SSc with an NSIP pattern (74). More generally, the genetic susceptibility loci identified in IIPs were not observed in a large North American cohort of patients with SSc-ILD (75). It is possible that the underlying genetics of ILDs are related to the different histopathological patterns. For example, rheumatoid arthritis–associated ILD with a UIP pattern is associated with the MUC5B promoter variant rs35705950 (76); however, the same variant has also been associated with idiopathic NSIP (77). Further studies are needed to characterize the link between genetic characteristics and ILD patterns. A number of HLA alleles have been associated with SSc-ILD, as discussed previously. Although associations between HLA alleles and IIP have been reported (78, 79), specific HLA allele associations do not overlap between SSc-ILD and IPF. For instance, HLA DRB1*1501, observed to be associated with IPF (78), has been reported as protective against SSc (80).
Epigenetic changes may underpin bronchiolar remodeling and the associated formation of enlarged bronchiolized airspaces (i.e., honeycombing, which occurs to differing extents in IPF and SSc-ILD). Chilosi and colleagues were the first to highlight the importance of the bronchioloalveolar junction and to report overexpression of markers of the Wnt pathway (e.g., β-catenin and MMP7) in IPF but not in NSIP (81). Differences between SSc-ILD and IPF are likely in specific miRNA profiles as well as in other epigenetic parameters; further studies are needed to characterize these differences and their relevance.
Despite treatment not being the focus of this review, we briefly mention some important differences and similarities in terms of treatment of SSc-ILD and IPF as highlighted by key clinical trials. The antifibrotic agents nintedanib and pirfenidone have shown benefit and are approved as treatments in IPF. In SSc-ILD, nintedanib has been granted U.S. Food and Drug Administration approval to slow the rate of decline in pulmonary function in patients with SSc-ILD on the basis of the results of the phase III SENSCIS trial, similar to its effect in patients with IPF. Furthermore, and in line with the known safety profile of nintedanib in patients with IPF, diarrhea was the most common adverse event (AE); all reported AEs were at worst mild or moderate in severity, as reported in 49.5% and 45.0% of patients, respectively (36). The phase II LOTUSS (Safety and Tolerability of Pirfenidone in Participants with SSc-ILD) trial showed that pirfenidone administered either as monotherapy or in combination with MMF had an acceptable tolerability profile in patients with SSc-ILD. The most common AEs were nausea, headache, and fatigue, which is consistent with its tolerability profile in patients with IPF (82). SLS III (NCT03221257), for which recruitment was ongoing at the time of writing, was designed to compare pirfenidone plus MMF with MMF alone in SSc-ILD. The results of this study, due in May 2021, may provide further data regarding the similarities and differences between treatment response in SSc-ILD and IPF.
Conclusions
ILD is a common complication of SSc and a significant cause of morbidity and mortality. Differentiation from IPF is particularly important, because IPF is the most common fibrosing ILD. This is usually straightforward in the context of the classic extrapulmonary SSc manifestations but can be more difficult in patients with SSc sine scleroderma. Knowledge of SSc-ILD is important in our community to ensure that affected patients are managed optimally. Greater extent of lung fibrosis on HRCT, lower FVC, and early lung function decline are predictors of early mortality. Familiarity with key clinical features (including established risk factors of progressive lung disease) may prove useful in raising our alertness to the possibility of SSc-ILD in relevant patients. Perhaps most importantly, high awareness of the disease and its characteristics will be needed to realize the potential of new treatment options.
Supplementary Material
Acknowledgments
Acknowledgment
Writing support and formatting assistance was provided by Leon Newman, Ph.D., and Ken Sutor, B.Sc., of GeoMed, an Ashfield company, part of UDG Healthcare plc, which was contracted and funded by Boehringer Ingelheim Pharmaceuticals, Inc. (BIPI). BIPI was given the opportunity to review the manuscript for medical and scientific accuracy as well as intellectual property considerations.
Footnotes
Supported by Boehringer Ingelheim Pharmaceuticals, Inc. None of the authors received financial compensation from an external source in return for writing or publishing this paper.
Author Contributions: Only the named authors of this manuscript contributed to the content of this manuscript. All authors have contributed in the preparation of this manuscript. The authors meet criteria for authorship as defined by the International Committee of Medical Journal Editors (ICMJE).
CME will be available for this article at www.atsjournals.org.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201903-0563CI on December 16, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Denton CP, Hughes M, Gak N, Vila J, Buch MH, Chakravarty K, et al. BSR and BHPR Standards, Guidelines and Audit Working Group. BSR and BHPR guideline for the treatment of systemic sclerosis. Rheumatology (Oxford) 2016;55:1906–1910. doi: 10.1093/rheumatology/kew224. [DOI] [PubMed] [Google Scholar]
- 2.Denton CP, Khanna D. Systemic sclerosis. Lancet. 2017;390:1685–1699. doi: 10.1016/S0140-6736(17)30933-9. [DOI] [PubMed] [Google Scholar]
- 3.Khanna D, Tashkin DP, Denton CP, Lubell MW, Vazquez-Mateo C, Wax S. Ongoing clinical trials and treatment options for patients with systemic sclerosis-associated interstitial lung disease. Rheumatology (Oxford) 2019;58:567–579. doi: 10.1093/rheumatology/key151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ranque B, Mouthon L. Geoepidemiology of systemic sclerosis. Autoimmun Rev. 2010;9:A311–A318. doi: 10.1016/j.autrev.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 5.van den Hoogen F, Khanna D, Fransen J, Johnson SR, Baron M, Tyndall A, et al. 2013 classification criteria for systemic sclerosis: an American College of Rheumatology/European League against Rheumatism collaborative initiative. Arthritis Rheum. 2013;65:2737–2747. doi: 10.1002/art.38098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tyndall AJ, Bannert B, Vonk M, Airò P, Cozzi F, Carreira PE, et al. Causes and risk factors for death in systemic sclerosis: a study from the EULAR Scleroderma Trials and Research (EUSTAR) database. Ann Rheum Dis. 2010;69:1809–1815. doi: 10.1136/ard.2009.114264. [DOI] [PubMed] [Google Scholar]
- 7.Akter T, Silver RM, Bogatkevich GS. Recent advances in understanding the pathogenesis of scleroderma-interstitial lung disease. Curr Rheumatol Rep. 2014;16:411. doi: 10.1007/s11926-014-0411-1. [DOI] [PubMed] [Google Scholar]
- 8.Giacomelli R, Liakouli V, Berardicurti O, Ruscitti P, Di Benedetto P, Carubbi F, et al. Interstitial lung disease in systemic sclerosis: current and future treatment. Rheumatol Int. 2017;37:853–863. doi: 10.1007/s00296-016-3636-7. [DOI] [PubMed] [Google Scholar]
- 9.Wells AU, Denton CP. Interstitial lung disease in connective tissue disease--mechanisms and management. Nat Rev Rheumatol. 2014;10:728–739. doi: 10.1038/nrrheum.2014.149. [DOI] [PubMed] [Google Scholar]
- 10.Christmann RB, Sampaio-Barros P, Stifano G, Borges CL, de Carvalho CR, Kairalla R, et al. Association of interferon- and transforming growth factor β-regulated genes and macrophage activation with systemic sclerosis-related progressive lung fibrosis. Arthritis Rheumatol. 2014;66:714–725. doi: 10.1002/art.38288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bagnato G, Harari S. Cellular interactions in the pathogenesis of interstitial lung diseases. Eur Respir Rev. 2015;24:102–114. doi: 10.1183/09059180.00003214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Lauretis A, Sestini P, Pantelidis P, Hoyles R, Hansell DM, Goh NS, et al. Serum interleukin 6 is predictive of early functional decline and mortality in interstitial lung disease associated with systemic sclerosis. J Rheumatol. 2013;40:435–446. doi: 10.3899/jrheum.120725. [DOI] [PubMed] [Google Scholar]
- 13.Herzog EL, Mathur A, Tager AM, Feghali-Bostwick C, Schneider F, Varga J. Review: interstitial lung disease associated with systemic sclerosis and idiopathic pulmonary fibrosis: how similar and distinct? Arthritis Rheumatol. 2014;66:1967–1978. doi: 10.1002/art.38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016;387:2630–2640. doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 15.Stock CJW, Renzoni EA. Genetic predictors of systemic sclerosis-associated interstitial lung disease: a review of recent literature. Eur J Hum Genet. 2018;26:765–777. doi: 10.1038/s41431-018-0104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Taniguchi T, Miyagawa T, Toyama S, Yamashita T, Nakamura K, Saigusa R, et al. CXCL13 produced by macrophages due to Fli1 deficiency may contribute to the development of tissue fibrosis, vasculopathy and immune activation in systemic sclerosis. Exp Dermatol. 2018;27:1030–1037. doi: 10.1111/exd.13724. [DOI] [PubMed] [Google Scholar]
- 17.Zhu H, Zhu C, Mi W, Chen T, Zhao H, Zuo X, et al. Integration of genome-wide DNA methylation and transcription uncovered aberrant methylation-regulated genes and pathways in the peripheral blood mononuclear cells of systemic sclerosis. Int J Rheumatol. 2018;2018:7342472. doi: 10.1155/2018/7342472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mak AC, Tang PL, Cleveland C, Smith MH, Kari Connolly M, Katsumoto TR, et al. Brief report: whole-exome sequencing for identification of potential causal variants for diffuse cutaneous systemic sclerosis. Arthritis Rheumatol. 2016;68:2257–2262. doi: 10.1002/art.39721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Christmann RB, Wooten A, Sampaio-Barros P, Borges CL, Carvalho CR, Kairalla RA, et al. miR-155 in the progression of lung fibrosis in systemic sclerosis. Arthritis Res Ther. 2016;18:155. doi: 10.1186/s13075-016-1054-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ashmore P, Tikly M, Wong M, Ickinger C. Interstitial lung disease in South Africans with systemic sclerosis. Rheumatol Int. 2018;38:657–662. doi: 10.1007/s00296-017-3893-0. [DOI] [PubMed] [Google Scholar]
- 21.Steen V. Predictors of end stage lung disease in systemic sclerosis. Ann Rheum Dis. 2003;62:97–99. doi: 10.1136/ard.62.2.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wangkaew S, Euathrongchit J, Wattanawittawas P, Kasitanon N, Louthrenoo W. Incidence and predictors of interstitial lung disease (ILD) in Thai patients with early systemic sclerosis: inception cohort study. Mod Rheumatol. 2016;26:588–593. doi: 10.3109/14397595.2015.1115455. [DOI] [PubMed] [Google Scholar]
- 23.Winstone TA, Assayag D, Wilcox PG, Dunne JV, Hague CJ, Leipsic J, et al. Predictors of mortality and progression in scleroderma-associated interstitial lung disease: a systematic review. Chest. 2014;146:422–436. doi: 10.1378/chest.13-2626. [DOI] [PubMed] [Google Scholar]
- 24.Wu W, Jordan S, Becker MO, Dobrota R, Maurer B, Fretheim H, et al. Prediction of progression of interstitial lung disease in patients with systemic sclerosis: the SPAR model. Ann Rheum Dis. 2018;77:1326–1332. doi: 10.1136/annrheumdis-2018-213201. [DOI] [PubMed] [Google Scholar]
- 25.Ryerson CJ, O’Connor D, Dunne JV, Schooley F, Hague CJ, Murphy D, et al. Predicting mortality in systemic sclerosis-associated interstitial lung disease using risk prediction models derived from idiopathic pulmonary fibrosis. Chest. 2015;148:1268–1275. doi: 10.1378/chest.15-0003. [DOI] [PubMed] [Google Scholar]
- 26.Goh NS, Desai SR, Veeraraghavan S, Hansell DM, Copley SJ, Maher TM, et al. Interstitial lung disease in systemic sclerosis: a simple staging system. Am J Respir Crit Care Med. 2008;177:1248–1254. doi: 10.1164/rccm.200706-877OC. [DOI] [PubMed] [Google Scholar]
- 27.Kennedy B, Branagan P, Moloney F, Haroon M, O’Connell OJ, O’Connor TM, et al. Biomarkers to identify ILD and predict lung function decline in scleroderma lung disease or idiopathic pulmonary fibrosis. Sarcoidosis Vasc Diffuse Lung Dis. 2015;32:228–236. [PubMed] [Google Scholar]
- 28.Khanna D, Nagaraja V, Tseng CH, Abtin F, Suh R, Kim G, et al. Predictors of lung function decline in scleroderma-related interstitial lung disease based on high-resolution computed tomography: implications for cohort enrichment in systemic sclerosis-associated interstitial lung disease trials. Arthritis Res Ther. 2015;17:372. doi: 10.1186/s13075-015-0872-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Volkmann ER, Tashkin DP, Sim M, Li N, Goldmuntz E, Keyes-Elstein L, et al. SLS I and SLS II study groups. Short-term progression of interstitial lung disease in systemic sclerosis predicts long-term survival in two independent clinical trial cohorts. Ann Rheum Dis. 2019;78:122–130. doi: 10.1136/annrheumdis-2018-213708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goh NS, Hoyles RK, Denton CP, Hansell DM, Renzoni EA, Maher TM, et al. Short-term pulmonary function trends are predictive of mortality in interstitial lung disease associated with systemic sclerosis. Arthritis Rheumatol. 2017;69:1670–1678. doi: 10.1002/art.40130. [DOI] [PubMed] [Google Scholar]
- 31.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Scleroderma Lung Study Research Group. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354:2655–2666. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 32.Khanna D, Yan X, Tashkin DP, Furst DE, Elashoff R, Roth MD, et al. Scleroderma Lung Study Group. Impact of oral cyclophosphamide on health-related quality of life in patients with active scleroderma lung disease: results from the scleroderma lung study. Arthritis Rheum. 2007;56:1676–1684. doi: 10.1002/art.22580. [DOI] [PubMed] [Google Scholar]
- 33.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Sclerodema Lung Study II Investigators. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016;4:708–719. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis Rheum. 2006;54:3962–3970. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 35.US Food & Drug Administration. FDA approves first treatment for patients with rare type of lung disease. 2019 [accessed 10 Sep 2019]. Available from: https://www.fda.gov/news-events/press-announcements/fda-approves-first-treatment-patients-rare-type-lung-disease.
- 36.Distler O, Highland KB, Gahlemann M, Azuma A, Fischer A, Mayes MD, et al. SENSCIS Trial Investigators. Nintedanib for systemic sclerosis-associated interstitial lung disease. N Engl J Med. 2019;380:2518–2528. doi: 10.1056/NEJMoa1903076. [DOI] [PubMed] [Google Scholar]
- 37.Ho KT, Reveille JD. The clinical relevance of autoantibodies in scleroderma. Arthritis Res Ther. 2003;5:80–93. doi: 10.1186/ar628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuwana M, Shirai Y, Takeuchi T. Elevated serum Krebs von den Lungen-6 in early disease predicts subsequent deterioration of pulmonary function in patients with systemic sclerosis and interstitial lung disease. J Rheumatol. 2016;43:1825–1831. doi: 10.3899/jrheum.160339. [DOI] [PubMed] [Google Scholar]
- 39.Volkmann ER, Tashkin DP, Kuwana M, Li N, Roth MD, Charles J, et al. Pneumoproteins KL-6 and CCL-18 predict progression of interstitial lung disease in systemic sclerosis. Arthritis Rheumatol. 2019;71:2059–2067. doi: 10.1002/art.41020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Prasse A, Pechkovsky DV, Toews GB, Schäfer M, Eggeling S, Ludwig C, et al. CCL18 as an indicator of pulmonary fibrotic activity in idiopathic interstitial pneumonias and systemic sclerosis. Arthritis Rheum. 2007;56:1685–1693. doi: 10.1002/art.22559. [DOI] [PubMed] [Google Scholar]
- 41.Elhai M, Hoffmann-Vold AM, Avouac J, Pezet S, Cauvet A, Leblond A, et al. Performance of candidate serum biomarkers for systemic sclerosis-associated interstitial lung disease. Arthritis Rheumatol. 2019;71:972–982. doi: 10.1002/art.40815. [DOI] [PubMed] [Google Scholar]
- 42.Manetti M, Guiducci S, Romano E, Bellando-Randone S, Conforti ML, Ibba-Manneschi L, et al. Increased serum levels and tissue expression of matrix metalloproteinase-12 in patients with systemic sclerosis: correlation with severity of skin and pulmonary fibrosis and vascular damage. Ann Rheum Dis. 2012;71:1064–1072. doi: 10.1136/annrheumdis-2011-200837. [DOI] [PubMed] [Google Scholar]
- 43.Wu M, Baron M, Pedroza C, Salazar GA, Ying J, Charles J, et al. CCL2 in the circulation predicts long-term progression of interstitial lung disease in patients with early systemic sclerosis: data from two independent cohorts. Arthritis Rheumatol. 2017;69:1871–1878. doi: 10.1002/art.40171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chowaniec M, Skoczyńska M, Sokolik R, Wiland P. Interstitial lung disease in systemic sclerosis: challenges in early diagnosis and management. Reumatologia. 2018;56:249–254. doi: 10.5114/reum.2018.77977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Bon L, Affandi AJ, Broen J, Christmann RB, Marijnissen RJ, Stawski L, et al. Proteome-wide analysis and CXCL4 as a biomarker in systemic sclerosis. N Engl J Med. 2014;370:433–443. doi: 10.1056/NEJMoa1114576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kafaja S, Valera I, Divekar AA, Saggar R, Abtin F, Furst DE, et al. pDCs in lung and skin fibrosis in a bleomycin-induced model and patients with systemic sclerosis. JCI Insight. 2018;3:98380. doi: 10.1172/jci.insight.98380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Volkmann ER, Tashkin DP, Roth MD, Clements PJ, Khanna D, Furst DE, et al. Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis-related interstitial lung disease. Arthritis Res Ther. 2016;18:305. doi: 10.1186/s13075-016-1203-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Weigold F, Günther J, Pfeiffenberger M, Cabral-Marques O, Siegert E, Dragun D, et al. Antibodies against chemokine receptors CXCR3 and CXCR4 predict progressive deterioration of lung function in patients with systemic sclerosis. Arthritis Res Ther. 2018;20:52. doi: 10.1186/s13075-018-1545-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nordenbaek C, Johansen JS, Halberg P, Wiik A, Garbarsch C, Ullman S, et al. High serum levels of YKL-40 in patients with systemic sclerosis are associated with pulmonary involvement. Scand J Rheumatol. 2005;34:293–297. doi: 10.1080/03009740510018598. [DOI] [PubMed] [Google Scholar]
- 50.Lee CG, Herzog EL, Ahangari F, Zhou Y, Gulati M, Lee CM, et al. Chitinase 1 is a biomarker for and therapeutic target in scleroderma-associated interstitial lung disease that augments TGF-β1 signaling. J Immunol. 2012;189:2635–2644. doi: 10.4049/jimmunol.1201115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goh NS, Veeraraghavan S, Desai SR, Cramer D, Hansell DM, Denton CP, et al. Bronchoalveolar lavage cellular profiles in patients with systemic sclerosis-associated interstitial lung disease are not predictive of disease progression. Arthritis Rheum. 2007;56:2005–2012. doi: 10.1002/art.22696. [DOI] [PubMed] [Google Scholar]
- 52.Strange C, Bolster MB, Roth MD, Silver RM, Theodore A, Goldin J, et al. Scleroderma Lung Study Research Group. Bronchoalveolar lavage and response to cyclophosphamide in scleroderma interstitial lung disease. Am J Respir Crit Care Med. 2008;177:91–98. doi: 10.1164/rccm.200705-655OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Clements PJ, Goldin JG, Kleerup EC, Furst DE, Elashoff RM, Tashkin DP, et al. Regional differences in bronchoalveolar lavage and thoracic high-resolution computed tomography results in dyspneic patients with systemic sclerosis. Arthritis Rheum. 2004;50:1909–1917. doi: 10.1002/art.20265. [DOI] [PubMed] [Google Scholar]
- 54.Schmidt K, Martinez-Gamboa L, Meier S, Witt C, Meisel C, Hanitsch LG, et al. Bronchoalveoloar lavage fluid cytokines and chemokines as markers and predictors for the outcome of interstitial lung disease in systemic sclerosis patients. Arthritis Res Ther. 2009;11:R111. doi: 10.1186/ar2766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Landi C, Bargagli E, Carleo A, Refini RM, Bennett D, Bianchi L, et al. Bronchoalveolar lavage proteomic analysis in pulmonary fibrosis associated with systemic sclerosis: S100A6 and 14-3-3ε as potential biomarkers. Rheumatology (Oxford) 2019;58:165–178. doi: 10.1093/rheumatology/key223. [DOI] [PubMed] [Google Scholar]
- 56.Kim HJ, Tashkin DP, Gjertson DW, Brown MS, Kleerup E, Chong S, et al. Transitions to different patterns of interstitial lung disease in scleroderma with and without treatment. Ann Rheum Dis. 2016;75:1367–1371. doi: 10.1136/annrheumdis-2015-208929. [DOI] [PubMed] [Google Scholar]
- 57.Sharif R. Overview of idiopathic pulmonary fibrosis (IPF) and evidence-based guidelines. Am J Manag Care. 2017;23:S176–S182. [PubMed] [Google Scholar]
- 58.Kahloon RA, Xue J, Bhargava A, Csizmadia E, Otterbein L, Kass DJ, et al. Patients with idiopathic pulmonary fibrosis with antibodies to heat shock protein 70 have poor prognoses. Am J Respir Crit Care Med. 2013;187:768–775. doi: 10.1164/rccm.201203-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zhang L, Wang Y, Wu G, Xiong W, Gu W, Wang CY. Macrophages: friend or foe in idiopathic pulmonary fibrosis? Respir Res. 2018;19:170. doi: 10.1186/s12931-018-0864-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Divekar AA, Khanna D, Abtin F, Maranian P, Saggar R, Saggar R, et al. Treatment with imatinib results in reduced IL-4-producing T cells, but increased CD4(+) T cells in the broncho-alveolar lavage of patients with systemic sclerosis. Clin Immunol. 2011;141:293–303. doi: 10.1016/j.clim.2011.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Schupp JC, Binder H, Jäger B, Cillis G, Zissel G, Müller-Quernheim J, et al. Macrophage activation in acute exacerbation of idiopathic pulmonary fibrosis. PLoS One. 2015;10:e0116775. doi: 10.1371/journal.pone.0116775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Cha SI, Chang CS, Kim EK, Lee JW, Matthay MA, Golden JA, et al. Lung mast cell density defines a subpopulation of patients with idiopathic pulmonary fibrosis. Histopathology. 2012;61:98–106. doi: 10.1111/j.1365-2559.2012.04197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Frantz C, Auffray C, Avouac J, Allanore Y. Regulatory T cells in systemic sclerosis. Front Immunol. 2018;9:2356. doi: 10.3389/fimmu.2018.02356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hou Z, Ye Q, Qiu M, Hao Y, Han J, Zeng H. Increased activated regulatory T cells proportion correlate with the severity of idiopathic pulmonary fibrosis. Respir Res. 2017;18:170. doi: 10.1186/s12931-017-0653-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Truchetet ME, Brembilla NC, Montanari E, Allanore Y, Chizzolini C. Increased frequency of circulating Th22 in addition to Th17 and Th2 lymphocytes in systemic sclerosis: association with interstitial lung disease. Arthritis Res Ther. 2011;13:R166. doi: 10.1186/ar3486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilson MS, Madala SK, Ramalingam TR, Gochuico BR, Rosas IO, Cheever AW, et al. Bleomycin and IL-1beta-mediated pulmonary fibrosis is IL-17A dependent. J Exp Med. 2010;207:535–552. doi: 10.1084/jem.20092121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.du Bois RM. Mechanisms of scleroderma-induced lung disease. Proc Am Thorac Soc. 2007;4:434–438. doi: 10.1513/pats.200608-152MS. [DOI] [PubMed] [Google Scholar]
- 68.Cavazza A, Rossi G, Carbonelli C, Spaggiari L, Paci M, Roggeri A. The role of histology in idiopathic pulmonary fibrosis: an update. Respir Med. 2010;104:S11–S22. doi: 10.1016/j.rmed.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 69.Bouros D, Wells AU, Nicholson AG, Colby TV, Polychronopoulos V, Pantelidis P, et al. Histopathologic subsets of fibrosing alveolitis in patients with systemic sclerosis and their relationship to outcome. Am J Respir Crit Care Med. 2002;165:1581–1586. doi: 10.1164/rccm.2106012. [DOI] [PubMed] [Google Scholar]
- 70.Park JH, Kim DS, Park IN, Jang SJ, Kitaichi M, Nicholson AG, et al. Prognosis of fibrotic interstitial pneumonia: idiopathic versus collagen vascular disease-related subtypes. Am J Respir Crit Care Med. 2007;175:705–711. doi: 10.1164/rccm.200607-912OC. [DOI] [PubMed] [Google Scholar]
- 71.Song JW, Do KH, Kim MY, Jang SJ, Colby TV, Kim DS. Pathologic and radiologic differences between idiopathic and collagen vascular disease-related usual interstitial pneumonia. Chest. 2009;136:23–30. doi: 10.1378/chest.08-2572. [DOI] [PubMed] [Google Scholar]
- 72.Peljto AL, Steele MP, Fingerlin TE, Hinchcliff ME, Murphy E, Podlusky S, et al. The pulmonary fibrosis-associated MUC5B promoter polymorphism does not influence the development of interstitial pneumonia in systemic sclerosis. Chest. 2012;142:1584–1588. doi: 10.1378/chest.12-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Stock CJ, Sato H, Fonseca C, Banya WA, Molyneaux PL, Adamali H, et al. Mucin 5B promoter polymorphism is associated with idiopathic pulmonary fibrosis but not with development of lung fibrosis in systemic sclerosis or sarcoidosis. Thorax. 2013;68:436–441. doi: 10.1136/thoraxjnl-2012-201786. [DOI] [PubMed] [Google Scholar]
- 74.Conti C, Montero-Fernandez A, Borg E, Osadolor T, Viola P, De Lauretis A, et al. Mucins MUC5B and MUC5AC in distal airways and honeycomb spaces: comparison among idiopathic pulmonary fibrosis/usual interstitial pneumonia, fibrotic nonspecific interstitial pneumonitis, and control lungs. Am J Respir Crit Care Med. 2016;193:462–464. doi: 10.1164/rccm.201507-1322LE. [DOI] [PubMed] [Google Scholar]
- 75.Wu M, Assassi S, Salazar GA, Pedroza C, Gorlova OY, Chen WV, et al. Genetic susceptibility loci of idiopathic interstitial pneumonia do not represent risk for systemic sclerosis: a case control study in Caucasian patients. Arthritis Res Ther. 2016;18:20. doi: 10.1186/s13075-016-0923-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Juge PA, Lee JS, Ebstein E, Furukawa H, Dobrinskikh E, Gazal S, et al. MUC5B promoter variant and rheumatoid arthritis with interstitial lung disease. N Engl J Med. 2018;379:2209–2219. doi: 10.1056/NEJMoa1801562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Horimasu Y, Ohshimo S, Bonella F, Tanaka S, Ishikawa N, Hattori N, et al. MUC5B promoter polymorphism in Japanese patients with idiopathic pulmonary fibrosis. Respirology. 2015;20:439–444. doi: 10.1111/resp.12466. [DOI] [PubMed] [Google Scholar]
- 78.Fingerlin TE, Murphy E, Zhang W, Peljto AL, Brown KK, Steele MP, et al. Genome-wide association study identifies multiple susceptibility loci for pulmonary fibrosis. Nat Genet. 2013;45:613–620. doi: 10.1038/ng.2609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Xue J, Gochuico BR, Alawad AS, Feghali-Bostwick CA, Noth I, Nathan SD, et al. The HLA class II Allele DRB1*1501 is over-represented in patients with idiopathic pulmonary fibrosis. PLoS One. 2011;6:e14715. doi: 10.1371/journal.pone.0014715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Arnett FC, Gourh P, Shete S, Ahn CW, Honey RE, Agarwal SK, et al. Major histocompatibility complex (MHC) class II alleles, haplotypes and epitopes which confer susceptibility or protection in systemic sclerosis: analyses in 1300 Caucasian, African-American and Hispanic cases and 1000 controls. Ann Rheum Dis. 2010;69:822–827. doi: 10.1136/ard.2009.111906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Chilosi M, Poletti V, Murer B, Lestani M, Cancellieri A, Montagna L, et al. Abnormal re-epithelialization and lung remodeling in idiopathic pulmonary fibrosis: the role of deltaN-p63. Lab Invest. 2002;82:1335–1345. doi: 10.1097/01.lab.0000032380.82232.67. [DOI] [PubMed] [Google Scholar]
- 82.Khanna D, Albera C, Fischer A, Khalidi N, Raghu G, Chung L, et al. An open-label, phase II study of the safety and tolerability of pirfenidone in patients with scleroderma-associated interstitial lung disease: the LOTUSS trial. J Rheumatol. 2016;43:1672–1679. doi: 10.3899/jrheum.151322. [DOI] [PubMed] [Google Scholar]
- 83.Steen VD. Autoantibodies in systemic sclerosis. Bull Rheum Dis. 1996;45:6–8. [PubMed] [Google Scholar]
- 84.Yamakawa H, Hagiwara E, Kitamura H, Yamanaka Y, Ikeda S, Sekine A, et al. Serum KL-6 and surfactant protein-D as monitoring and predictive markers of interstitial lung disease in patients with systemic sclerosis and mixed connective tissue disease. J Thorac Dis. 2017;9:362–371. doi: 10.21037/jtd.2017.02.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rivière S, Hua-Huy T, Tiev KP, Cabane J, Dinh-Xuan AT. High baseline serum Clara cell 16 kDa predicts subsequent lung disease worsening in systemic sclerosis. J Rheumatol. 2018;45:242–247. doi: 10.3899/jrheum.170440. [DOI] [PubMed] [Google Scholar]
- 86.Lota HK, Renzoni EA. Circulating biomarkers of interstitial lung disease in systemic sclerosis. Int J Rheumatol. 2012;2012:121439. doi: 10.1155/2012/121439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ogawa F, Shimizu K, Muroi E, Hara T, Hasegawa M, Takehara K, et al. Serum levels of 8-isoprostane, a marker of oxidative stress, are elevated in patients with systemic sclerosis. Rheumatology (Oxford) 2006;45:815–818. doi: 10.1093/rheumatology/kel012. [DOI] [PubMed] [Google Scholar]
- 88.Bonhomme O, André B, Gester F, de Seny D, Moermans C, Struman I, et al. Biomarkers in systemic sclerosis-associated interstitial lung disease: review of the literature. Rheumatology (Oxford) 2019;58:1534–1546. doi: 10.1093/rheumatology/kez230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Antonelli A, Ferri C, Fallahi P, Ferrari SM, Giuggioli D, Colaci M, et al. CXCL10 (alpha) and CCL2 (beta) chemokines in systemic sclerosis--a longitudinal study. Rheumatology (Oxford) 2008;47:45–49. doi: 10.1093/rheumatology/kem313. [DOI] [PubMed] [Google Scholar]
- 90.Hoffmann-Vold AM, Weigt SS, Palchevskiy V, Volkmann E, Saggar R, Li N, et al. Augmented concentrations of CX3CL1 are associated with interstitial lung disease in systemic sclerosis. PLoS One. 2018;13:e0206545. doi: 10.1371/journal.pone.0206545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sing T, Jinnin M, Yamane K, Honda N, Makino K, Kajihara I, et al. microRNA-92a expression in the sera and dermal fibroblasts increases in patients with scleroderma. Rheumatology (Oxford) 2012;51:1550–1556. doi: 10.1093/rheumatology/kes120. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.