Abstract
The oral mucosa is a primary barrier site and a portal for entry of microbes, food and airborne particles into the gastrointestinal tract. Nonetheless, mucosal immunity at this barrier remains understudied compared to other anatomical barrier sites. Here, we review basic aspects of oral mucosal histology, the oral microbiome and common and clinically significant diseases that that present at oral mucosal barriers. We particularly focus on the role of IL-17/Th17 responses in protective immunity and inflammation in the oral mucosa. IL-17/Th17 responses are highly relevant to maintaining barrier integrity and preventing pathogenic infections by the oral commensal fungus Candida albicans. On the other hand, aberrant IL-17/Th17 responses are implicated in driving the pathogenesis of periodontitis and consequent bone and tooth loss. We discuss distinct IL-17-secreting T cell subsets, emphasizing their regulation and function in oropharyngeal candidiasis and periodontitis.
Keywords: mucosal immunity, oral immunology, periodontal disease, candidiasis, Th17, IL-17
One Sentence Summary
Preserving a homeostatic balance with resident microbial communities of the oropharynx depends on a measured dose of type 17 immunity.
Introduction
The concept that immune activities vary depending on tissue context has become much more appreciated in recent years. Far more is now understood about unique immunological networks that serve the specific requirements and demands of a given tissue environment. Such local immunological systems typically arise in response to tissue-specific environmental cues and stressors and perform local specialized immune surveillance to infection as well as playing critical roles in the preservation of tissue integrity and functionality.
Particularly important are tissue-specific immune responses at barrier sites, which are continuously exposed to diverse environmental stimuli. It is fair to say that principles of barrier immunity have been derived dominantly from studies of the gastrointestinal tract. However, more recent studies of skin, lung and other mucosal sites have demonstrated that lessons from the gut do not necessarily translate to other tissues. In this regard, the oral mucosal barrier has been largely understudied, especially in the immunology community, and is consequently far less understood (1). In this review we discuss unique aspects of the oral mucosal immune system, with a particular focus on IL-17/Th17-dependent pathways that control two major microbe-triggered diseases that occur at this barrier, periodontitis and candidiasis. Principles learned in these disease settings have often been unexpected, and are likely to apply to other diseases and potentially other tissue sites.
Unique aspects of oral mucosal immunity
Together with the nasopharynx, the oral mucosa is the site of first encounter to environmental elements prior to entry into the gastrointestinal tract and, at least partially, into the airway (Fig. 1). Yet while the oral mucosa is constantly exposed to a plethora of environmental stimuli, the immune system at this site mediates protection to pathogens typically without overt inflammation. Furthermore, the oral mucosa is a site of optimal wound healing (2). However, how environmental and microbial stimuli influence development and activity of local specialized tissue immune responses is not well understood at the oral barrier. Furthermore, how these interactions shape systemic immunity is also not well characterized. Another unique but poorly-understood facet of the mouth is the constant mechanical stimulation experienced by this tissue during mastication. We have only a rudimentary understanding of how mechanical events influence immunity in this tissue.
Figure 1. Histology of the oral mucosa, a barrier for environmental stimuli and commensal microbiota.
The oral mucosa is a frequent site of first encounter. Airborne particles (allergens), commensal microbes and food are exposed to the mouth before transiting to the gastrointestinal tract or airway. The oral mucosa is composed of a stratified squamous epithelium with varying characteristics at different oral anatomical locations. Lining epithelium (buccal, inner lip, floor of mouth) is multilayered, non-keratinized and with rich vascularity in the submucosal areas. Immune cells are present scattered within and underlying the epithelium. In the dorsum and lateral borders of the tongue, the oral mucosa becomes specialized, with discrete formations called papillae. Within the papillae are taste buds, the chemoreceptors of taste. Within the tonsils the epithelium becomes invaginated, forming crypts. Crypt epithelium is often just a single layer with interspersed M cells, which can sample antigens and transfer them to underlying immune cells, which form germinal centers. Another area of vulnerable epithelium lies in the gingival crevice. Crevicular epithelium becomes very thin at the base of the pocket where it connects to the tooth surface. This epithelial barrier is constantly exposed to the tooth-adherent microbial biofilm. Immune cells are abundant in this region and neutrophils continuously transmigrate through from the tissue into the crevice.
Oral Epithelial Organization and Histology
The cellular biology of the mucosal epithelium informs its functions in terms of immunological responses (Fig. 1). Mucosal tissues of the oral cavity are multilayered squamous epithelia, which are divided into 3 main categories; masticatory mucosa, lining mucosa and specialized mucosa(3). Masticatory mucosa is found in areas that are subjected to constant mechanical stimulation from chewing, and are thus keratinized to withstand ongoing mechanical stimulation and injury. These tissues include the external gingiva (areas surrounding the teeth), hard palate and dorsum of tongue. Specialized mucosa is found specifically in the regions of the taste buds on the dorsal surface of the tongue and contains nerve endings for general sensory reception and taste perception. Lining mucosa is found in all remaining areas. Epithelia in these sites are partially keratinized or non-keratinized and include the mucosa of the inner surface of lips, the buccal mucosa, the soft palate and the floor of mouth. Because of their minimal keratinization, these areas are more exposed to the environment. A particularly permeable epithelium is the sublingual space, which has a thin, non-keratinized epithelium and contains high submucosal vascularity. Due to these properties, the sublingual space is used for sublingual mucosal vaccination and for delivery of emergency medications for rapid systemic dissemination (4).
Areas of the oral cavity that are particularly exposed to the outside environment that are especially vulnerable to infections or environmental stimuli are:
1. Crevicular epithelium and Junctional epithelium
Crevicular epithelium is the inner lining of the gingiva. This mucosal tissue is opposite of the tooth and in close approximation to the tooth-adherent microbial biofilm. The crevicular epithelium becomes progressively thinner and transitions into the junctional epithelium (JE). The JE is a 3–4 cell layer thick epithelium connecting the tooth to the gingival submucosa tissue. This area is particularly vulnerable to microbial translocation from the tooth adherent biofilm. It is thin, consisting of just a few layers of epithelium. The JE is connected to the tooth surface solely through hemi-desmosomes, which become transiently interrupted due to local irritations. In fact, the JE is constantly perturbed by the local microbiome and continuously subjected to micro-damage from chewing. This site is considered a primary portal for microbial translocation during chewing/flossing or in the presence of local inflammation (gingivitis/periodontitis)
2. Tonsil crypt epithelium
Tonsils are mucosal-associated lymphoid tissues (MALT) which form a ring at the opening of the respiratory and digestive tract, the Waldeyer’s ring. They include the pharyngeal, lingual, nasal and tubular tonsils, with the palatine and lingual tonsils being those associated with the oral cavity. Tonsils are secondary lymphoid organs positioned as a first line of defense against invading pathogens, particularly during early life. The lining epithelium of the tonsils consists of squamous cell epithelium which forms multiple invaginations (crypts), and is often a one-cell-layered epithelium within the crypt area. Within the surface epithelium of the tonsil, specialized antigen-capture cells called M cells drive the efficient uptake of antigens from the environment and facilitate their presentation to the adaptive immune system (1, 3).
The Oral Microbiome
While most of the recent attention of the microbiome field has focused on the gut, the oral cavity harbors some of the most rich and diverse microbial communities inhabiting the human body. Commensal microbiota are thought to be main drivers of barrier immune function, typically shaping protective/homeostatic immune responses at barrier tissues (5). However, while the role of the oral commensal microbiome in the induction of protective oral immunity is not completely defined, oral microbiota are known to play key roles in induction of local disease.
The healthy microbiota in the oral environment is primarily composed of rich bacterial communities but also includes fungi, viruses, archaea and protozoa (6). Thus far, the oral bacterial communities have been best studied in most detail, and approximately 600 species/phylotypes have been identified in the oral cavity (7). Predominant oral phyla are Actinobacteria, Bacteroidetes, Chlamydiae, Chloroflexi, Euryarchaeota, Firmicutes, Fusobacteria, Proteobacteria, Spirochaetes, Absconditabacteria (SR1), Synergistetes, Tenericutes, and Saccharibacteria (TM7).
Within the oral cavity multiple ecological niches with distinct microbial communities exist. There is a major distinction between mucosal-associated microbial communities and those that are tooth-associated (8)(9). Tooth adherent communities are particularly complex and form elaborate biofilm formations, which differ significantly in health and disease (10). Consistent with the presence of unique microbial communities, different tissue locations in the oral cavity are susceptible to disparate microbe-triggered oral pathologies. Dental caries (leading to cavities) occur on the tooth surface, gingivitis and periodontal disease target tooth-associated mucosa (gingiva) and candidiasis primarily affects the lining mucosa, primarily the tongue dorsum (Table I). In all these diseases, shifts in the local microbial communities are thought to be the trigger and/or perpetuator of disease pathology. Dental caries are caused by acid-producing bacteria that form resistant biofilms (‘oral plaque’) that erode tooth enamel and associated sequelae(11). Oral candidiasis is the result of overgrowth of the commensal fungus Candida albicans (12) and periodontal disease (PD) is associated with microbial dysbiosis in tooth-associated microbial communities (13). Beyond, traditionally thought of as microbe- triggered oral diseases, emerging evidence also supports a role for the oral microbiome in oral mucosal carcinogenesis (14, 15).
TABLE I.
Clinically significant diseases of the mouth
| Dental caries: One of the most prevalent human diseases, caused by acid-producing bacteria which destroy the tooth surface and can lead to dental infection once they reach the tooth nerve-vascular interior bundle. |
| Periodontitis: Microbe-triggered inflammation leading to destruction of tooth-supporting structures (including soft tissue, bone, and ligaments). See text for more details. |
| Oral candidiasis (thrush): Oropharyngeal candidiasis (OPC, thrush) is typically caused by the commensal fungus Candida albicans. See text for more details. |
| Recurrent oral ulcers:Common painful ulcerations of the oral mucosa, without clear etiopathogenesis. Have been associated with various triggers including infection (primarily viral), immunodeficiency (neutropenia), autoimmunity (Bechet’s disease) and tobacco use. |
| Oral viral infections: Most common is herpetic stomatitis, a painful ulcerative condition caused by herpes simplex virus, typically in young children. Oral human papilloma virus (HPV) infection is a sexually transmitted oral viral condition associated with oral cancer risk |
| Sjögren’s syndrome: Autoimmune disorder affecting exocrine glands (including salivary glands) and leading to xerostomia (dry mouth) and related sequelae. |
| Oral sauamous cell carcinoma: Epithelial tumors of the oral mucosa and throat. Major risk factors include tobacco and alcohol use and HPV infection (most commonly HPV16). Most prevalent in men above age 50. >50% 5-year survival. |
|
Systemic inflammatory diseases manifesting in the mouth: Dermatological bullous diseases (pemphigus, pemphigoid, lichen planus), Crohn’s disease, systemic lupus erythematosus, graft-versus-host disease. |
Oral microbiota are implicated in tissue pathology at distal sites (Fig. 2). Oral microbiota continuously enter the gastrointestinal tract during feeding and swallowing, but their relative proportions are very different in these sites. Systemic translocation or oral microbiota also occurs constitutively through the gingival crevice, and can be exacerbated by dental infection or tissue disruption during dental procedures (16). Indeed, constituents of the oral microbiome are found in the gut and lung and systemic bacteremia with oral microbiota has been documented transiently after dental procedures or in the presence of oral disease (16).
Figure 2. Oral mucosal histology.
Oral microbiota are linked to the initiation and/or pathogenesis of systemic diseases such as colon cancer, infectious endocarditis, ventilator-associated pneumonia and Alzheimer’s disease. Periodontitis has been associated with systemic conditions such as cardiovascular disease, diabetes, pregnancy complications and rheumatoid arthritis.
There are several recognized and potentially fatal systemic infection associated with oral microbiota. For example, infectious endocarditis occurs when oral bacteria enter the bloodstream and settle in the heart lining, a heart valve or a blood vessel. This typically has occurred in the presence of dental infection and associated with a dental procedure in patients with prosthetic heart valves or a history of previous endocarditis. Prophylactic antibiotic for at-risk individuals has mitigated the incidence of this disease, but still remains a risk for patients with undiagnosed cardiovascular susceptibility (16). Oral microbiota implicated in ventilation-associated pneumonia (VAP) (17). Consequently, oral antiseptics are now commonly used for prevention of VAP. Oral microbiota has been detected in distal tissues in the setting of diseases including atheromatous plaques, colorectal cancer lesions and Alzheimer’s disease brains (18–20).
One well-understood oral microbe implicated in triggering distal disease is Fusobacterium nucleatum, an anaerobe that is among the most prevalent bacterial species in colorectal cancer tissues (20). This “commensal turned pathogen”, has been suggested to potentiate intestinal tumorigenesis and modulate the tumor-immune microenvironment (21, 22). Surprisingly, F. nucleatum and its associated microbiome are maintained in distal metastases from colorectal lesions (23). Oral microbes have also been linked to the pathogenesis of Rheumatoid Arthritis. Both P. gingivalis and Aggregatibacter actinomycetencomitans (Aa) have been linked to rheumatoid arthritis (RA) pathogenesis (24, 25). Recent work has uncovered the role of Aa in inducing changes in neutrophil function, including hypercitrullination of host proteins, an abnormality also observed in joints of patients with rheumatoid arthritis and typically thought of as an early event in RA initiation of disease (19). Clearly, oral microbes alone are probably insufficient to initiate distal disease, since bacterial translocation of oral microbes occurs in most individuals without disease sequelae. It is likely that additional factors such as an underlying genetic susceptibility (13) are necessary to establish a setting where oral bacteria potentiate disease at distal sites. The above examples convey the scientific imperative to better understand the oral microbial environment and its connection to human health.
Oral Diseases
Clinically significant diseases of the oral cavity can be divided into those that in which most of the pathology is localized to the oropharynx and those that are systemic in nature and include manifestations in the mouse (Table I). We will discuss the epidemiology and pathophysiology of two common oral diseases, periodontal disease and candidiasis, in detail.
Periodontal Disease
Periodontal disease (PD) is a common inflammatory disease of the tooth-supporting structures. PD typically starts as mucosal tissue inflammation (gingivitis) and progresses in susceptible individuals to a chronic destructive inflammation that damages tooth-supporting structures (gingiva, supporting alveolar bone and ligament). In severe cases this leads to loss of bone surrounding the teeth and ultimately tooth loss (26). Indeed, PD is the major cause for tooth loss in adults. Periodontitis is a very prevalent human disease. In adults, approximately 8–10% of the general population, globally, present with severe forms of disease, which are associated to need for surgical interventions, high medical costs and impaired quality of life (particularly when associated with tooth loss) (27, 28). Although periodontitis usually is a chronic disease which develops over decades, aggressive forms of periodontitis also exist and are primarily encountered in teenagers and younger adults (26).
Periodontitis results from an imbalance between the microbiome and host at the gingival mucosal barrier interface. Dysbiosis in periodontitis is associated with transition from health-associated tooth adherent microbiome to a disease-associated microbiome (29, 30). In health, facultative aerobes dominate the gingival microbiome, with a high representation of Streptococci and Actinomyces species. In disease, the microbiome shifts to anaerobic genera with overrepresentation of periodontitis-associated species, including Porphyromonas gingivalis, Tanerella forsythia, Treponema denticola (a triad of bacteria known as the “red complex”) and Filifactor alocis (13, 31, 32). Increases in total microbial biomass and community diversity are considered cardinal features of a periodontitis- associated microbiomes (13). Microbiome shifts in periodontitis result both from interspecies interactions and from the adaptation of the microbial community to the changing environment in the presence of inflammation(13). This dysbiotic microbiome is thought to have increased virulence and an improved capacity to thrive in an inflammatory environment. In turn, local inflammation is thought to provide nutrients to the microbial community and further perpetuate microbial dysbiosis, creating a vicious self-reinforcing cycle (33).
Microbial dysbiosis alone does not necessarily precipitate periodontitis, but it could initiate disease in the context of other risk factors such as baseline genetic predisposition (34). Hence, accumulation of bacterial plaque due to lack of oral care and hygiene does not necessarily lead to periodontitis. However, microbial triggering is a key event for the initiation and perpetuation of PD pathogenesis, and all current standard of care treatments rely on the removal of microbial biofilm by mechanical removal, or treatment with antibiotics in aggressive disease (26). Even so, removal of oral biofilm typically will not be curative, but aim at arresting disease for periods of time.
Genetic susceptibility is a key factor both for onset and progression of periodontitis. A genetic basis for periodontitis is supported by twin studies and familial aggregation of severe forms of the disease, with heritability estimates as high as 50% (35). However, the nine genome-wide association studies performed so far have failed to consistently identify specific single-nucleotide polymorphisms across populations (26, 36). In contrast, Mendelian diseases have clearly demonstrated that host genetics dictates susceptibility to periodontal disease(37). Genetic defects affecting neutrophils are strongly linked to PD. Specially, patients with congenital neutropenia (Kostman Syndrome caused by HAX1 mutations and severe congenital neutropenia caused by ELA2/ELANE mutations), or defects in neutrophil egress from the bone marrow (WHIM syndrome caused by CXCR4 gain-of-function mutations), defects in neutrophil recruitment (Chediak Higashi, LYST mutations), defects in neutrophil extravasation into tissues (Leukocyte Adhesion Deficiency I, caused by ITGB2 mutations) and neutrophil function (Papillon-Lefèvre syndrome, CTSC mutations) all present with extremely severe periodontitis at an early age. In fact, patients with severe neutrophil genetic defects will often lose their entire dentition during adolescence, despite standard of care and antibiotic treatment (37).
Beyond genetics, cigarette smoking is the number one environmental risk factor for development of periodontitis, with risk estimates ranging between 2.5–7.0 (26). Smokers have higher prevalence, increased severity and higher rate of progression of disease. Treatment studies also reveal inferior treatment outcomes for both surgical and non-surgical treatment in smokers. Given that smoking is a modifiable risk factor, smoking cessation studies also reveal improved outcomes after smoking cessation. Pre-existing and particularly uncontrolled diabetes mellitus is also a significant and a well-documented underlying disease risk factor for the development of periodontal disease. The prevalence and severity of periodontitis are increased in patients with long-standing and poorly controlled diabetes (26).
One of the often-unappreciated consequences of PD is the impact on other tissues, and increasing emerging data illustrates how far-reaching these consequences can be. The disease periodontitis has specifically been clinically associated with various systemic conditions (Fig. 2) and theorized to be a potential risk factor for the triggering or progression of these conditions (including preterm labor, cardiovascular disease/atherosclerosis, RA, and diabetes mellitus) (25, 29). Whether periodontal microbiota and inflammation play a role in triggering distal disease, or common host disease susceptibility and shared mechanisms of pathology underlie these associations is still under investigation.
Oral Candidiasis
The most common fungal infection of humans is oropharyngeal candidiasis (OPC, thrush), typically caused by the dimorphic commensal fungus Candida albicans, or less frequently other Candida species. OPC is rare in healthy individuals, but common in extremes of age and settings of immune compromise. The latter may result from oral-specific immune-altering regimens, such as radiation for head-neck cancers, inhaled steroids for asthma or even denture use. Alternatively, induced or acquired immunosuppression affecting T cells such as HIV/AIDS, certain chemotherapies, many transplant drugs or certain anti-cytokine biologics are also associated with OPC. Some congenital immunodeficiencies also cause OPC, accompanied by dermal candidiasis and onchomycosis (fungal infection of nails), collectively termed chronic mucocutaneous candidiasis (CMC) (38). Although not life-threatening, oral thrush can significantly impact nutritional intake due to pain during eating and swallowing; in extreme cases in infants, OPC can cause failure to thrive (39). OPC has also been associated with esophageal cancer (40, 41). As discussed in detail in sections to follow, most or even all of these conditions are linked to deficiencies in the Th17 pathway in some capacity (42).
In addition to OPC, C. albicans is the causative agent of vulvovaginal candidiasis (VVC), affecting most of women of childbearing years, often recurrently. Unlike OPC, VVC is considered to be a disease caused by an overactive immune response rather than impaired host defense, consistent with its incidence in otherwise healthy women (12). Candida also cause infrequent but life-threatening bloodstream infections. Disseminated candidiasis has an average mortality rate exceeding 50% and is the third most common hospital acquired infection (43). To date, there are no licensed vaccines to any fungal pathogens, though an experimental vaccine against C. albicans (targeting a fungal adhesin, Als1/3) has been evaluated in VVC with good safety and hints of efficacy (44). The effect of this vaccine in preventing OPC has not been determined, but may be challenging to implement given the immunocompromised state of susceptible individuals (45).
C. albicans is a commensal microbe of the human oral cavity. Colonization estimates based on culture studies range from 50–80%, but fungal microbiome studies detect C. albicans DNA in most or all healthy individuals (15). This fungus is also found in the GI tract, where it can disseminate from an abdominal breach, as during surgery or abdominal injury. Although beyond the scope of this review, C. albicans has a complex relationship with the intestinal microbiome, with potentially far-reaching consequences on host immunity (33). Recent data in humans and mice suggest there may be evolutionary pressure to maintain C. albicans as a commensal microbe, as a high proportion of C. albicans-reactive T cells show cross-reactivity to other pathogenic fungi. Following antifungal treatment, these cross-reactive T cells diminish, and hence their continued presence in the gut may provide heterologous immunity to non-Candida fungal pathogens (46, 47).
The IL-17/Th17 pathway – a primer
IL-17 (IL-17A) was discovered in 1993 but was largely overlooked until 2005 with the revamping of the Th1/Th2 paradigm to include the Th17 subset of CD4+ T cells (48). IL-17-expressing populations include the conventional adaptive Th17 subset of T cells as well as innate-acting lymphocyte populations, including γδ T cells, NKT cells, “innate lymphoid cells” (ILC), TCRαβ+ “natural” Th17 cells (nTh17) and Foxp3+ Treg-like cells, collectively termed “type 17” (49). Some evidence for IL-17 expression in B cells and myeloid cells is also reported, though this is controversial. While requirements for inducing IL-17 in type 17 cell types vary, IL-23 and/or IL-1 are usually implicated, in conjunction with signals from pathogen-derived PAMPs or the inflammatory environment. Type 17 cells also produce other cytokines, such as IL-17F, IL-22 and GM-CSF. The IL17F gene is closely linked to IL17A; these cytokines form homodimers but also heterodimers, and signal through the same receptor, with varying affinities (IL-17A>IL-17A/F>IL-17F). The transcription factor RORγt is viewed as a ‘master regulator’ of Th17 cells, and, in combination with STAT3 and other transcription factors, is required for IL-17A and IL-17F expression. Type 17 cells express the IL-23 receptor, but IL-23 per se is not essential for IL-17 expression (50). Type 17 cells are also characterized by expression of CCR6, which binds the mucosal chemokine CCL20 (51). Consequently, type 17 cells are highly enriched at mucosal surfaces, and play key roles in barrier immunity. This is especially evident for oral immune responses, as outlined below.
Although produced almost exclusively by lymphocytes, IL-17 signals in the non-hematopoietic compartment, thus bridging the immune system and inflamed tissues, especially mucosae. The IL-17 signaling pathway has recently been described in extensive detail (52). Briefly, IL-17 engages a heterodimeric receptor (IL-17RA and IL-17RC) found mainly in mesenchymal and epithelial cell types. All known downstream IL-17 signaling events are initiated by the multifunctional adaptor Act1, which is recruited to the IL-17R after ligand engagement. Act1 recruits diverse TRAF proteins, which regulate inflammatory mRNA expression through transcriptional and post-transcriptional pathways. Best described is TRAF6-dependent activation of the NF-κB/IκBξ, C/EBP and AP-1 transcription factors, which orchestrate new gene transcription. Often less appreciated, IL-17 is also a potent regulator of mRNA fate (stabilization, degradation, translation), a pathway initiated by TRAF2/5 and orchestrated by a suite of RNA-binding proteins including Act1 itself. (47). The ability of IL-17 to stabilize mRNA post-transcriptionally explains how this cytokine is able to synergize promiscuously with diverse inflammatory signals. That is, regardless of how target mRNAs are upregulated by specific transcription factors, IL-17-mediated signals that stabilize or destabilize the resulting mRNA determine the magnitude of downstream target gene expression. The circuitry involved can be quite complex, and interestingly several of the relevant RNA-binding proteins were first identified in studies of IL-17-dependent oral candidiasis (52).
IL-17 function has been inferred quite accurately from its target genes. Transcriptomic studies have revealed a consistent core IL-17 “gene signature,” which includes neutrophil-activating genes (CXC chemokines, G-CSF), antimicrobial proteins (β-defensins, S100A8/9, lipocalin-2), cytokines (IL-6, GM-CSF) and transcription factors (IκBξ, C/EBPβ, C/EBPδ) (52). IL-17 induces many tissue- or cell type-specific genes as well. Consistently, IL-17 is a potent inducer of barrier host defense against microbes that are sensitive to neutrophils and antimicrobial protein (AMP) activity, including periodontal pathogens and C. albicans, among others. While a potent inducer of potentially damaging inflammation, IL-17 also drives tissue repair, a property evident in some of the genes that it regulates (53). In the gut, barrier tissue repair appears to be a dominant response, perhaps explaining why anti-IL-17 therapy in humans failed in IBD trials (54). In the oral mucosa, far less is known about the extent to which IL-17 drives tissue repair. Although the majority of documented IL-17 signaling events occur at barrier surfaces, recent data indicate roles in controlling metabolism in stromal lymph node tissue as well. Roles in cancer have also been described (52).
IL-17/Th17 host responses in oral disease: periodontal disease and candidiasis
IL-17 in periodontal (gingival) immunity
Homeostatic Th17 in periodontal health
IL-17-secreting cells are a small cell population in healthy oral tissues, both in mice and humans. In healthy human gingiva the majority of IL-17-secreting cells are Th17 cells and constitute about 1% of the total CD4 T cell pool (55). In mice however, at steady state, the dominant IL-17-secreting cells are γδ T cells, followed by TCRβ+ Th17 cells and a small but detectable number of group 3 innate lymphoid cells (ILC3s) (56, 57). The difference between mice and humans could be attributed to the clean environment in most mouse facilities, where a lower level of antigenic exposure may occur and responses are mainly innate (58). Alternatively or in addition, available human data are derived from studies of adult populations who have experienced years of local exposure allowing an adaptive response to predominate. In keeping with this idea, murine gingival Th17 cells increase with age(5).
Th development of Th17 cells in barrier compartments is largely dependent on commensal microbiota (59). In this regard, specific bacterial taxa are responsible for amplifying Th17 cells in the gastrointestinal tract, skin and the eye(53). Surprisingly, in the gingiva, Th17 cells are present at comparable levels in germ-free (GF) versus SPF mice (5), suggesting a unique regulation of gingival Th17 cells. This is also in contrast to γδ T cells in the gingiva, which depend on commensal microbiota (60)(61). Consistent with this, unlike other barrier compartments where development of IL-17-secreting T cells depends largely on IL-1(62), in the gingiva development and accumulation of Th17 in health depends solely on the cytokine IL-6 and not IL-1 or IL-23 (5).
We discovered that a key driver of Th17 accumulation in the gingiva is physiological damage caused by constant low-level mechanical stimulation during mastication (5)(Fig. 3). Supporting this idea is the observation that a soft (liquid) diet in mice leads to decreased Th17 cells in gingiva, whereas a hard food diet or local abrasion increases Th17 cell accumulation. Mechanistically, mechanical damage induces IL-6 from gingival epithelial cells, which is required for this accumulation of Th17 cells. Triggering of Th17 also requires antigen, although the source of antigen is for the induction of Th17 cells is unclear, particularly in GF settings. Th17 cells in gingiva are associated with recruitment of neutrophils, which are required to clear acute bacterial infections (5, 63). Furthermore, accumulation of Th17 at steady state is linked to a barrier-protective IL-17 transcriptional program in the gingiva, including the induction of antimicrobial peptides and neutrophil chemo-attractants, suggesting a homeostatic function for these damage-induced Th17 cells.
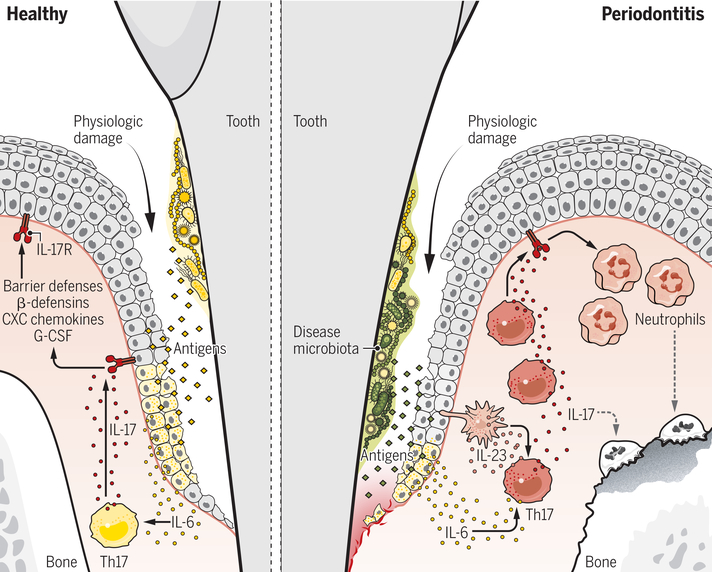
Figure 3. Regulation/function of Th17 in periodontal immunity and inflammation.
In health, the trigger for Th17 cell accumulation in the gingiva is ongoing damage that occurs during mastication. Resulting local tissue damage triggers IL-6 production from epithelial cells, which induces Th17 cell accumulation in an antigen-dependent manner. These health-associated Th17 cells secrete IL-17 and mediate IL-17-dependent protective barrier responses, such as antimicrobial defense. In periodontitis, Th17 cells are expanded in the gingiva in response to a dysbiotic microbiome. Disease-associated microbiota trigger IL-6- and IL-23-dependent accumulation of Th17 cells. Disease-causing Th17 cells drive periodontal bone loss through excessive neutrophil recruitment and related immunopathology.
Th17 cells in periodontitis
While inhibition of IL-17 signaling is linked to periodontal infection in animal models (63), the physiologic role of Th17 cells in humans is less evident. In contrast, impaired development of Th17 cells as in STAT3-deficiency does not cause an overt periodontal phenotype (33, 57). Even so, increased IL-17 has been documented in the lesions of patients with periodontitis by multiple groups (64–66). In periodontitis, Th17 cells become abundant within the lesion, and a subset co-express cytokines typically associated with Th17 pathogenicity such as GM-CSF and IFN-γ (Fig. 3). Th17 expansion is also a dominant feature of the murine ligature-induced model of periodontitis, which is considered a relevant model of human PD (57, 67). Studies in experimental periodontitis reveal that triggering of gingival Th17 cells during disease is distinct compared to health. Gingival Th17 cells in health accumulate independently of commensal microbiota and require IL-6.
In contrast, in disease, Th17 cells are microbiota-dependent and need both IL-6 and IL-23 for their induction (57). While no specific bacterial taxa have been conclusively identified as inducing Th17 cells in periodontitis, disease-associated microbial dysbiosis rather than a non-specific accumulation of microbiota appears to be critical. Specifically, microbial shifts towards anaerobic bacteria appear to be critical for triggering Th17-dependent disease, as treatment with metronidazole (which targets anaerobic bacteria) inhibits periodontal Th17 induction and disease progression. Importantly, metronidazole is also the antibiotic of choice (in combination with amoxicillin) for treating periodontitis in humans.
Several studies in experimental periodontitis suggest a pathogenic role for the Th17 subset. Genetic or pharmacologic inhibition of Th17 cells or IL-17 protects from periodontal bone loss, hence suggesting that these cells and their related pathways could potentially be exploited therapeutically (5, 67–69) (70). In this regard, patients with genetic defects in Th17 differentiation (due to loss-of-function STAT3 mutations) were shown to have reduced periodontal inflammation and bone loss compared to the general population (57).
To date, treatment targeting the Th17 pathway for periodontitis has only been attempted in the setting of a rare monogenic disease that causes highly aggressive periodontal disease, Leukocyte Adhesion Deficiency I (LAD-I). LAD-I is caused by single gene mutations in ITGB2, which encodes CD18, the common chain of β2 integrins. When CD18 is dysfunctional, neutrophils are unable to transmigrate from the vasculature into tissues. This prototypic Mendelian defect revealed that loss of tissue neutrophils predisposes to microbial dysbiosis and amplification of tissue Th17 responses (71, 72). Mechanistically, this was explained through the “neutrostat theory” which posits that: (i) IL-17/Th17 responses are key for neutrophil recruitment; (ii) once recruited, tissue-localized neutrophils play a critical role in downregulating the IL-23/IL-17 pathway. Therefore, in the absence of tissue neutrophils, other IL-17 inflammatory responses become exaggerated in a frustrated effort to control inflammation. In LAD-I, exaggerated tissue Th17 responses promote barrier immunopathology (73).
Based on this concept, ustekinumab (an antibody that blocks the shared IL-12/IL-23 p40 subunit) was used for the treatment of mucosal and cutaneous immunopathology. In this single case, a 19-year-old male presented with LAD-1 and severe periodontitis as well as a deep intractable cutaneous wound which was not healing for over 2 years. He was treated with ustekinumab for over a year, which led to reduction of gingival inflammation and a complete and uneventful healing of his cutaneous wound. Based on this, proof of concept case, a clinical trial has been initiated for the treatment of LAD-1 associated immunopathology (including periodontitis) with ustekinumab. The more recent approval of IL-23-based biologics may also be fruitful in these types of severe, albeit rare situations (74). Additional patient data is of course necessary to evaluate the safety and efficacy of IL-23/Th17 blockage in the treatment of LAD-1 associated PD. Furthermore, genetic factors that predispose to Th17 amplification in more common forms of periodontitis are not understood and whether inhibitors of IL-23/Th17 will prove to be efficacious in the treatment of common forms of periodontitis remains to be seen.
IL-17 in oral candidiasis
The observation that nearly all HIV/AIDS patients exhibit oral thrush, especially as CD4+ cell numbers decline, implicated CD4+ Th cells in OPC pathogenesis. In contrast, HIV+ women do not experience VVC at unusual levels, nor is systemic candidiasis commonly seen in HIV/AIDS (75), collectively suggesting that CD4+ cells play a far more important role in OPC than other forms of candidiasis. (75)The CD4+Th cell response in OPC was originally attributed to Th1 cells, in part based on susceptibility of knockout mice lacking the IL-12p40 subunit. However, IFNγ-deficient mice (lacking Th1 activity) were resistant (76), a conundrum often seen in studies of the Th1/Th2 paradigm (48). Findings from analysis of C. albicans colonization in the gastric mucosa supported a protective role for Th1 cells and damaging role for IL-17 (77). Mice lacking IL-23p19, IL-17RA or IL-17RC, but not the IL-12-specific subunit IL-12p35, were shown to be highly susceptible to oral infections with C. albicans, thus implicating the type 17 pathway rather than Th1 cells in preventing OPC (78, 79). Type 17 cytokines were also found to be host-protective in transgenic mice expressing HIV (80). IL-17 is required for immunity to numerous strains of C. albicans (81) and for C. glabrata/C. albicans co-infections (82).
During this time frame, emerging studies of human primary immune deficiencies (PIDs) corroborated the potent protective role for IL-17/Th17 in oral mucosal C. albicans (42). Job’s syndrome (hyper-IgE syndrome; HIES) is a characterized by a spectrum of symptoms including CMC/OPC and caused by dominant-negative mutations in STAT3 (or other JAK-STAT genes), which correlates with reduced Th17 frequencies in these patients (83, 84). Patients with mutations in AIRE have autoimmune endocrinopathy usually accompanied by CMC (known as APECED or APS-1). Several reports described neutralizing antibodies against type 17 cytokines in these patients, including IL-17A, IL-17F and IL-22, which was postulated to explain OPC susceptibility (85–87). Another PID associated with CMC is due to gain-of-function mutations in STAT1, which is also characterized by reduced (though not absent) Th17 cell numbers (88). A direct demonstration of the importance of IL-17 signaling in mucosal candidiasis came from whole exome sequencing studies of families with unexplained CMC. Casanova and Puel et al. discovered loss-of-function (LOF) mutations in IL-17RA, IL-17F, IL-17RC and Act1 (89–93). Many patients with IL-17R-related mutations also experience Staphylococcal infections, but surprisingly few other disease manifestations (89, 94); this could be related to the propensity of these organisms to form polymicrobial biofilms or the intriguing cross-reactivity of Candida and Staphylococcus surface antigens (95). Human mutations in genes encoding RORγt (RORC) and IL-23 also cause CMC, and are additionally linked to Th1-dependent Mycobacterial infections (92, 96). Consistent with these findings, Candida-specific T cells in healthy humans are nearly all Th17 (51). Studies of HIV and SIV showed profound early loss of Th17+ cells upon infection, potentially explaining why oral thrush is an AIDS-defining opportunistic infection (97). Collectively, these studies indicated that mice and humans show strong concordance in terms of the importance of the Th17 pathway in immunity to OPC.
In orchestrating antifungal immunity in the oral mucosa, IL-17 regulates multiple immune events that have potential to protect against C. albicans (Fig. 4), as illustrated by the induction of a classic IL-17 gene signature in the oral mucosa in murine OPC (98, 99). Prominent among these genes are neutrophil-recruiting CXC chemokines (CXCL1, CXCL2, and CXCL5) and G-CSF, which is consistent with the “neutrostat theory” described above. Mice lacking the common receptor for these chemokines (CXCR2) exhibit severe susceptibility to OPC, and neutrophil depletion has a similar outcome (100). Neutrophils have potent anti-Candida activity both in vitro and in vivo (33). However, the importance of IL-17 in controlling the oral antifungal neutrophil response has been controversial, with differing relative contributions observed among groups (78, 101, 102). IL-1 is also implicated in regulating the oral neutrophil response (103).
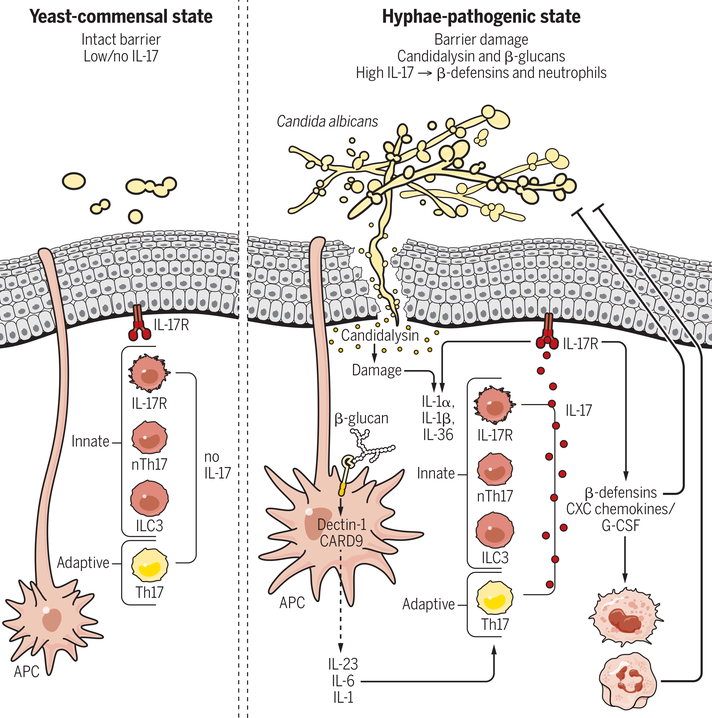
Figure 4. Regulation/function of IL-17 secreting cells during candidiasis.
C. albicans is a dimorphic commensal fungus that colonizes the human oral cavity. In health, this organism is found mainly in an avirulent yeast form, and triggers little or no immune activity in the oral mucosa. Upon conversion to a hyphal state, C. albicans exposes β-glucans in the cell wall that activate the Dectin-1/CARD9 pathway in APCs. APCs consequently secrete Th17-skewing cytokines such as IL-1, IL-6, and IL-23, driving IL-17 production from antigen-specific Th17 cells. Hyphae also secrete the peptide candidalysin, which damages oral epithelial cells and triggers secretion of DAMPs such as IL-1 and IL-36. These signals activate innate IL-17-producing cells. IL-17 binds to its receptor on oral epithelial cells and induces antimicrobial effectors, including CXC chemokines and G-CSF that promote a neutrophil response and β-defensins that have direct fungicidal activity.
AMPs have potent candidacidal activity and are key products of the mucosal epithelia or saliva that control oral Candida infections. IL-17 is a particularly strong inducer of β-defensins (BDs) (78) (Fig. 4). Loss of murine BD3 (the orthologue of human BD2) causes susceptibility to OPC comparable to loss of IL-17RA, and deletion of BD1 similarly rendered mice susceptible to infection (102, 104). Intriguingly, some chemokines induced by IL-17 have antimicrobial properties such as CCL20 (105), although surprisingly the latter is not essential for immunity to acute OPC in mice (62). Dry mouth (xerostomia) caused by drugs or Sjögren’s syndrome, is linked to OPC, due to diminution of the rich antimicrobial composition of saliva. Histatins are primate-specific salivary AMPs that also have strong antifungal activity (106). BDs and histatins are reduced in saliva from HIES patients and are inversely correlated with oral C. albicans colonization (107).
IL-17 responsiveness is dictated by expression of its receptor. IL-17RA is ubiquitously expressed, while the IL-17RC subunit is more restricted (52). Most analyses of IL-17RA or Act1 in vivo indicated roles only in the non-hematopoietic system (52). In OPC, deletion of IL-17RA in superficial keratin 13+ oral epithelial cells caused OPC almost as severe as a complete IL-17RA-knockout, and bone marrow chimera experiments ruled out essential functions in hematopoietic cells (98). In this regard, studies of the inflammasome by Hise et al. revealed distinct compartmentalization of oral immunity to C. albicans; whereas NLRP3 is active in multiple compartments, NLRC4 acts only in mucosal stroma (108, 109). A new report implicates IL-17RD in IL-17 signaling (110), but the role of this receptor in OPC is unknown. Hence it is likely that there is much more to be learned with regards to the full spectrum of IL-17 signaling activities.
Despite the conservation of IL-17-driven immunity between mice and humans, a major distinction is that mice do not harbor C. albicans as a commensal organism, either in the mouth or intestine. Consequently, most studies of OPC reflect the first encounter with this organism, i.e., an innate rather than an adaptive response (62). Accordingly, the strong IL-17- and IL-23-dependence seen in most of these early studies were attributable to innate cells rather than conventional, antigen-specific Th17 responses. In these settings, IL-23 is produced by Langerin+ DCs (111). IL-17 in an acute setting is detected in several innate immune cell types, including γδ-T cells, ILC3s and, dominantly, an unusual innate-acting population of CD4+TCRαβ+ ‘natural’ Th17 cells (nTh17) (112–114) (Fig. 4). Their importance is supported by susceptibility of Rag1−/− mice to OPC (115). Surprisingly, the only lymphocyte population to expand after oral C. albicans infection was nTh17 cells (62, 116), which contrasts with skin where γδ-T cells dominate the murine anti-Candida response (117). The bona fide innate properties of nTh17 cells were demonstrated in recall infection studies where secondary infections showed antigen-specificity and improved fungal clearance compared to primary infections (118). Further evidence came from use of a Nur77-GFP-reporter mouse that reports active TCR signaling; in primary oral C. albicans infections there was no detectable activation of the Nur77 reporter, consistent with a lack of antigen-specific TCR recognition, whereas the reporter was expressed when mice were subjected to a secondary re-infection (114). There are no accepted markers to distinguish nTh17 cells from conventional Th17 cells, and therefore the nature of innate IL-17 sources in humans in candidiasis is not known. However, since mouse nTh17 cells express CD4, one might speculate that such cells, if they exist in humans, would be depleted by HIV infection and thus help explain the high incidence of OPC in AIDS (75).
Studies to define the activation requirements of nTh17 cells revealed unexpectedly that prototypical fungal pattern receptors such as dectins or TLR2, implicated in driving adaptive Th17 responses to fungi (119), were dispensable for the innate IL-17 response (114, 120). Rather, IL-17 induction in acute OPC requires a recently described fungal toxin, candidalysin (114, 121) (Fig. 4). Candidalysin is a pore-forming peptide produced only by the invasive, hyphal form of C. albicans. This peptide acts on oral epithelial cells to induce cell damage and a c-Fos-driven innate inflammatory response characterized by production of IL-1, IL-36, β-defensins and other cytokines (121). This candidalysin-mediated IL-1/36 response is essential for induction of IL-17 and proliferation of nTh17 cells. However, whereas IL-1 drives expression of IL-17, IL-36 promotes IL-23, unexpectedly revealing distinct and separable functions of IL-17 and IL-23 pathways in OPC (103, 122). This could have implications for infection risk associated with different anti-cytokine biologic therapies.
Surprisingly, the innate TCRαβ+ ‘nTh17’ cells activated in murine OPC are not the same as those seen in the gingiva during periodontal disease (5, 114), though there are parallels. In OPC, activation of nTh17 cells is IL-1-dependent but IL-6- and STAT3-independent; the opposite is true in periodontal disease. Both populations increase with age (5). Likely there is much more to learn about tissue-specific subpopulations of innate type 17 cells, which could impact or inform therapeutic interventions for oral diseases.
In humans, in contrast to mice, exposure to C. albicans occurs early in life and hence the adaptive response predominates. Immunocompetent mice exposed orally to C. albicans clear the fungus rapidly, but show enhanced clearance upon re-exposure, indicative of an effective recall response (118). Detailed studies of this adaptive response revealed an immune-dominant Th cell epitope corresponding to the C. albicans adhesin Als1/3; intriguingly, this is the same epitope used in the experimental Candida vaccine (112, 123). Moreover, Th17 cells recognizing this epitope a cross-reactive against multiple Candida species, paralleling recent findings in humans (47).
Th17-mediated inflammation is counteracted by Treg cells, and there is a complex interrelationship between these cell types. In OPC, Tregs enhance activity of Th17 cells by virtue of IL-2 consumption, reversing the inhibitory effects of IL-2 on Th17 cell differentiation. Tregs thus act to enhance Th17-mediated clearance of C. albicans from the mouth (115). The activity of Tregs is regulated by the oral microbiota, as antibiotic treatment decreases both Tregs and Th17 cells. This was attributable to short chain fatty acids (SCFA) produced by local oral flora (124).
A major consequence of the overhaul of the Th1/Th2 paradigm was the recognition that some autoimmune diseases are driven by the Th17 pathway. Consequently, antibodies against IL-17 or its receptor were approved recently and became transformative for plaque psoriasis. Anti-IL-23 biologics were also recently approved. Predictably, OPC is a side-effect of anti-IL-17 drugs, but at surprisingly low frequencies, and is usually clinically manageable with antifungal treatment (125). Analogous studies in mice similarly indicated that blockade of IL-17A alone does not render mice strongly susceptible to OPC, although combined blockade with anti-IL-17F antibodies (but not with anti-TNF-α antibodies) somewhat increases fungal burdens (126–128). Still, these do not reach the high levels seen in individuals or mice with a complete loss of IL-17 signaling, suggesting that residual IL-17 is apparently sufficient to control oral candidiasis. Alternatively, there may be additional IL-17RA-dependent ligands that are not affected by these regimens. However, studies assessing IL-17C (which uses IL-17RA) or its receptor IL-17RE found no role, at least in mice (129).
Concluding remarks
Host-microbe interactions are not well characterized at the oral mucosal barrier. However, distinct mucosal environments with unique microbial communities exist in the oral cavity. Importantly, imbalance between microbiome and host underlies common diseases of oral mucosa. IL-17/Th17 dependent pathways are also central for control of infection and inflammation at the oral mucosa, but in complex and sometimes surprising ways. Novel therapeutic approaches have begun to be implemented based on findings in this field, but it is likely that much more remains to be learned that could ultimately be translated to patients with oral disorders. Hence studies of this important yet often neglected mucosal compartment revealed unexpected properties of this immune axis, and emphasize a need for more studies in this area.
Acknowledgments
We thank Julian Naglik and David Moyes (King’s College London, UK) for helpful discussions.
Funding. SLG was supported by NIH grant R37-DE022550. NMM was supported by the Intramural program of NIDCR. Opinions expressed herein are those of the authors and do not necessarily reflect views of the NIH.
Footnotes
Competing interests. SLG is recipient of US Patent #10,160,974, “Engineered cytokine- and chemokine-expressing Candida albicans strains and methods of use.” SLG has consulted for Merck, Abbvie and Eli Lilly. The authors declare no other competing interests.
References and Notes
- 1.Moutsopoulos NM, Konkel JE, Tissue-Specific Immunity at the Oral Mucosal Barrier. Trends in immunology 39, 276–287 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Iglesias-Bartolome R et al. , Transcriptional signature primes human oral mucosa for rapid wound healing. Sci Transl Med 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanci A, Ten Cate’s Oral Histology: Development, Structure, and Function. (Elsevier, St Louis, Missouri, ed. 8th, 2013). [Google Scholar]
- 4.Presland RB, Jurevic RJ, Making sense of the epithelial barrier: what molecular biology and genetics tell us about the functions of oral mucosal and epidermal tissues. J Dent Educ 66, 564–574 (2002). [PubMed] [Google Scholar]
- 5.Dutzan N et al. , On-going Mechanical Damage from Mastication Drives Homeostatic Th17 Cell Responses at the Oral Barrier. Immunity 46, 133–147 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wade WG, The oral microbiome in health and disease. Pharmacol Res 69, 137–143 (2013). [DOI] [PubMed] [Google Scholar]
- 7.Dewhirst FE et al. , The human oral microbiome. J Bacteriol 192, 5002–5017 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.C. Human Microbiome Project, A framework for human microbiome research. Nature 486, 215–221 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Proctor DM, Relman DA, The Landscape Ecology and Microbiota of the Human Nose, Mouth, and Throat. Cell host & microbe 21, 421–432 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mark Welch JL, Rossetti BJ, Rieken CW, Dewhirst FE, Borisy GG, Biogeography of a human oral microbiome at the micron scale. Proceedings of the National Academy of Sciences of the United States of America 113, E791–800 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tanner AC, Kressirer CA, Faller LL, Understanding Caries From the Oral Microbiome Perspective. J Calif Dent Assoc 44, 437–446 (2016). [PubMed] [Google Scholar]
- 12.Jabra-Rizk MA et al. , Candida albicans Pathogenesis: Fitting Within the “Host-Microbe Damage Response Framework”. Infect Immun 84, 2724–2739 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abusleme L et al. , The subgingival microbiome in health and periodontitis and its relationship with community biomass and inflammation. The ISME journal 7, 1016–1025 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tezal M et al. , Chronic periodontitis and the incidence of head and neck squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev 18, 2406–2412 (2009). [DOI] [PubMed] [Google Scholar]
- 15.Peters BA et al. , Oral Microbiome Composition Reflects Prospective Risk for Esophageal Cancers. Cancer Res 77, 6777–6787 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Squire JD, Gardner PJ, Moutsopoulos NM, Leiding JW, Antibiotic Prophylaxis for Dental Treatment in Patients with Immunodeficiency. J Allergy Clin Immunol Pract 7, 819–823 (2019). [DOI] [PubMed] [Google Scholar]
- 17.Bahrani-Mougeot FK et al. , Molecular analysis of oral and respiratory bacterial species associated with ventilator-associated pneumonia. J Clin Microbiol 45, 1588–1593 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dominy SS et al. , Porphyromonas gingivalis in Alzheimer’s disease brains: Evidence for disease causation and treatment with small-molecule inhibitors. Sci Adv 5, eaau3333 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koren O et al. , Human oral, gut, and plaque microbiota in patients with atherosclerosis. Proceedings of the National Academy of Sciences of the United States of America 108 Suppl 1, 4592–4598 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kostic AD et al. , Genomic analysis identifies association of Fusobacterium with colorectal carcinoma. Genome Res 22, 292–298 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Han YW, Fusobacterium nucleatum: a commensal-turned pathogen. Curr Opin Microbiol 23, 141–147 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kostic AD et al. , Fusobacterium nucleatum potentiates intestinal tumorigenesis and modulates the tumor-immune microenvironment. Cell host & microbe 14, 207–215 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bullman S et al. , Analysis of Fusobacterium persistence and antibiotic response in colorectal cancer. Science 358, 1443–1448 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Koziel J, Mydel P, Potempa J, The link between periodontal disease and rheumatoid arthritis: an updated review. Curr Rheumatol Rep 16, 408 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Konig MF et al. , Aggregatibacter actinomycetemcomitans-induced hypercitrullination links periodontal infection to autoimmunity in rheumatoid arthritis. Sci Transl Med 8, 369ra176 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kinane DF, Stathopoulou PG, Papapanou PN, Periodontal diseases. 3, 17038 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Eke PI et al. , Update on Prevalence of Periodontitis in Adults in the United States: NHANES 2009 to 2012. J Periodontol 86, 611–622 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Papapanou PN, The prevalence of periodontitis in the US: forget what you were told. Journal of dental research 91, 907–908 (2012). [DOI] [PubMed] [Google Scholar]
- 29.Hajishengallis G, Periodontitis: from microbial immune subversion to systemic inflammation. Nature reviews. Immunology 15, 30–44 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hajishengallis G, Lamont RJ, Dancing with the Stars: How Choreographed Bacterial Interactions Dictate Nososymbiocity and Give Rise to Keystone Pathogens, Accessory Pathogens, and Pathobionts. Trends in microbiology 24, 477–489 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Socransky SS, Haffajee AD, Cugini MA, Smith C, Kent RL Jr., Microbial complexes in subgingival plaque. J Clin Periodontol 25, 134–144 (1998). [DOI] [PubMed] [Google Scholar]
- 32.Griffen AL et al. , Distinct and complex bacterial profiles in human periodontitis and health revealed by 16S pyrosequencing. The ISME journal 6, 1176–1185 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abusleme L et al. , Human defects in STAT3 promote oral mucosal fungal and bacterial dysbiosis. JCI Insight 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Darveau RP, Periodontitis: a polymicrobial disruption of host homeostasis. Nat Rev Microbiol 8, 481–490 (2010). [DOI] [PubMed] [Google Scholar]
- 35.Michalowicz BS et al. , Evidence of a substantial genetic basis for risk of adult periodontitis. J Periodontol 71, 1699–1707 (2000). [DOI] [PubMed] [Google Scholar]
- 36.Divaris K et al. , Genome-wide association study of periodontal pathogen colonization. Journal of dental research 91, 21S–28S (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Silva LM, Brenchley L, Moutsopoulos NM, Primary immunodeficiencies reveal the essential role of tissue neutrophils in periodontitis. Immunological reviews 287, 226–235 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Li J, Vinh DC, Casanova JL, Puel A, Inborn errors of immunity underlying fungal diseases in otherwise healthy individuals. Curr Opin Microbiol 40, 46–57 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lanternier F et al. , Primary immunodeficiencies underlying fungal infections. Current opinion in pediatrics 25, 736–747 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Delsing CE et al. , Association of esophageal candidiasis and squamous cell carcinoma. Medical mycology case reports 1, 5–8 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhu F et al. , Autoreactive T Cells and Chronic Fungal Infection Drive Esophageal Carcinogenesis. Cell Host Microbe 21, 478–493 e477 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Milner J, Holland S, The cup runneth over: lessons from the ever-expanding pool of primary immunodeficiency diseases. Nat Rev Immunol 13, 635–648 (2013). [DOI] [PubMed] [Google Scholar]
- 43.Brown GD et al. , Hidden killers: human fungal infections. Sci Transl Med 4, 165rv113 (2012). [DOI] [PubMed] [Google Scholar]
- 44.Ibrahim AS et al. , NDV-3 protects mice from vulvovaginal candidiasis through T- and B-cell immune response. Vaccine 31, 5549–5556 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Cassone A, Development of vaccines for Candida albicans: Fighting a skilled transformer. Nat Rev Microbiol 11, 884–891 (2013). [DOI] [PubMed] [Google Scholar]
- 46.Shao TY et al. , Commensal Candida albicans Positively Calibrates Systemic Th17 Immunological Responses. Cell Host Microbe 25, 404–417 e406 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bacher P et al. , Human Anti-fungal Th17 Immunity and Pathology Rely on Cross-Reactivity against Candida albicans. Cell 176, 1340–1355 e1315 (2019). [DOI] [PubMed] [Google Scholar]
- 48.Steinman L, A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nature Med 13, 139–145 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Veldoen M, Interleukin 17 is a chief orchestrator of immunity. Nature immunology 18, 612–621 (2017). [DOI] [PubMed] [Google Scholar]
- 50.McGeachy MJ et al. , TGF-beta and IL-6 drive the production of IL-17 and IL-10 by T cells and restrain T(H)-17 cell-mediated pathology. Nature immunology 8, 1390–1397 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Acosta-Rodriguez EV et al. , Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nature immunology 8, 639–646 (2007). [DOI] [PubMed] [Google Scholar]
- 52.Li X, Bechara R, Zhao J, McGeachy MJ, Gaffen SL, Interleukin 17 receptor-based signaling and implications for disease. Nat Immunol 20, 1594–1602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stockinger B, Omenetti S, The dichotomous nature of T helper 17 cells. Nature reviews. Immunology, (2017). [DOI] [PubMed] [Google Scholar]
- 54.Hueber W et al. , Secukinumab, a human anti-IL-17A monoclonal antibody, for moderate to severe Crohn’s disease: unexpected results of a randomised, double-blind placebo-controlled trial. Gut 61, 1693–1700 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dutzan N, Konkel JE, Greenwell-Wild T, Moutsopoulos NM, Characterization of the human immune cell network at the gingival barrier. Mucosal immunology 9, 1163–1172 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dutzan N, Abusleme L, Konkel JE, Moutsopoulos NM, Isolation, Characterization and Functional Examination of the Gingival Immune Cell Network. J Vis Exp, 53736 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dutzan N et al. , A dysbiotic microbiome triggers TH17 cells to mediate oral mucosal immunopathology in mice and humans. Sci Transl Med 10, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Beura LK et al. , Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hooper LV, Littman DR, Macpherson AJ, Interactions between the microbiota and the immune system. Science 336, 1268–1273 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wilharm A et al. , Mutual interplay between IL-17-producing gammadeltaT cells and microbiota orchestrates oral mucosal homeostasis. Proc Natl Acad Sci U S A 116, 2652–2661 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Krishnan S et al. , Amphiregulin-producing gammadelta T cells are vital for safeguarding oral barrier immune homeostasis. Proceedings of the National Academy of Sciences of the United States of America 115, 10738–10743 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Verma AH et al. , Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor candidalysin. Sci Immunol 2, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yu JJ et al. , An essential role for IL-17 in preventing pathogen-initiated bone destruction: recruitment of neutrophils to inflamed bone requires IL-17 receptor-dependent signals. Blood 109, 3794–3802 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zenobia C, Hajishengallis G, Basic biology and role of interleukin-17 in immunity and inflammation. Periodontol 2000 69, 142–159 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cardoso CR et al. , Evidence of the presence of T helper type 17 cells in chronic lesions of human periodontal disease. Oral Microbiol Immunol 24, 1–6 (2009). [DOI] [PubMed] [Google Scholar]
- 66.Moutsopoulos NM et al. , Porphyromonas gingivalis promotes Th17 inducing pathways in chronic periodontitis. Journal of autoimmunity 39, 294–303 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tsukasaki M et al. , Host defense against oral microbiota by bone-damaging T cells. Nat Commun 9, 701 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Eskan MA et al. , The leukocyte integrin antagonist Del-1 inhibits IL-17-mediated inflammatory bone loss. Nature immunology 13, 465–473 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Xiao E et al. , Diabetes Enhances IL-17 Expression and Alters the Oral Microbiome to Increase Its Pathogenicity. Cell host & microbe 22, 120–128 e124 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bostanci N, Abe T, Belibasakis GN, Hajishengallis G, TREM-1 Is Upregulated in Experimental Periodontitis, and Its Blockade Inhibits IL-17A and RANKL Expression and Suppresses Bone loss. J Clin Med 8, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moutsopoulos NM et al. , Defective neutrophil recruitment in leukocyte adhesion deficiency type I disease causes local IL-17-driven inflammatory bone loss. Sci Transl Med 6, 229ra240 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Moutsopoulos NM et al. , Subgingival microbial communities in Leukocyte Adhesion Deficiency and their relationship with local immunopathology. PLoS pathogens 11, e1004698 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ley K, Breaking a Vicious Cycle. The New England journal of medicine 376, 1172–1174 (2017). [DOI] [PubMed] [Google Scholar]
- 74.Markham A, Guselkumab: First Global Approval. Drugs 77, 1487–1492 (2017). [DOI] [PubMed] [Google Scholar]
- 75.Fidel PL Jr., Candida-Host Interactions in HIV Disease: Implications for Oropharyngeal Candidiasis. Adv Dent Res 23, 45–49 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Farah C, Hu Y, Riminton S, Ashman R, Distinct roles for interleukin-12p40 and tumour necrosis factor in resistance to oral candidiasis defined by gene targeting. Oral Microbiol Immunol 21, 252–255 (2006). [DOI] [PubMed] [Google Scholar]
- 77.Zelante T et al. , IL-23 and the Th17 pathway promote inflammation and impair antifungal immune resistance. Eur J Immunol 37, 2695–2706 (2007). [DOI] [PubMed] [Google Scholar]
- 78.Conti H et al. , Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine 206, 299–311 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ho A et al. , IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J. Immunol. 185, 1063–1070 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Goupil M et al. , Defective IL-17- and IL-22-dependent mucosal host response to Candida albicans determines susceptibility to oral candidiasis in mice expressing the HIV-1 transgene. BMC immunology 15, 49 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schonherr FA et al. , The intraspecies diversity of C. albicans triggers qualitatively and temporally distinct host responses that determine the balance between commensalism and pathogenicity. Mucosal Immunol in press, (2017). [DOI] [PubMed] [Google Scholar]
- 82.Tati S et al. , Candida glabrata Binding to Candida albicans Hyphae Enables Its Development in Oropharyngeal Candidiasis. PLoS Pathog 12, e1005522 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ma CS et al. , Deficiency of Th17 cells in hyper IgE syndrome due to mutations in STAT3. The Journal of experimental medicine 205, 1551–1557 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Milner JD et al. , Impaired T(H)17 cell differentiation in subjects with autosomal dominant hyper-IgE syndrome. Nature 452, 773–776 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kisand K et al. , Chronic mucocutaneous candidiasis in APECED or thymoma patients correlates with autoimmunity to Th17-associated cytokines. The Journal of experimental medicine 207, 299–308 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Puel A et al. , Autoantibodies against IL-17A, IL-17F, and IL-22 in patients with chronic mucocutaneous candidiasis and autoimmune polyendocrine syndrome type I. The Journal of experimental medicine 207, 291–297 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Browne SK, Holland SM, Anti-cytokine autoantibodies explain some chronic mucocutaneous candidiasis. Immunol Cell Biol 88, 614–615 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.van de Veerdonk FL, Netea MG, Treatment options for chronic mucocutaneous candidiasis. J Infect 72 Suppl, S56–60 (2016). [DOI] [PubMed] [Google Scholar]
- 89.Puel A et al. , Chronic mucocutaneous candidiasis in humans with inborn errors of interleukin-17 immunity. Science 332, 65–68 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Boisson B et al. , A biallelic ACT1 mutation selectively abolishes interleukin-17 responses in humans with chronic mucocutaneous candidiasis. Immunity 39, 676–686 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Ling Y et al. , Inherited IL-17RC deficiency in patients with chronic mucocutaneous candidiasis. The Journal of experimental medicine 212, 619–631 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Martinez-Barricarte R et al. , Human IFN-gamma immunity to mycobacteria is governed by both IL-12 and IL-23. Sci Immunol 3, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Bhattad S, Dinakar C, Pinnamaraju H, Ganapathy A, Mannan A, Chronic Mucocutaneous Candidiasis in an Adolescent Boy Due to a Novel Mutation in TRAF3IP2. J Clin Immunol 39, 596–599 (2019). [DOI] [PubMed] [Google Scholar]
- 94.Levy R et al. , Genetic, immunological, and clinical features of patients with bacterial and fungal infections due to inherited IL-17RA deficiency. Proc Natl Acad Sci U S A 113, E8277–E8285 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Harriott MM, Noverr MC, Importance of Candida-bacterial polymicrobial biofilms in disease. Trends in microbiology 19, 557–563 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Okada S et al. , Impairment of immunity to Candida and Mycobacterium in humans with bi-allelic RORC mutations. Science 349, 606–613 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brenchley JM et al. , Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood 112, 2826–2835 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Conti HR et al. , IL-17 Receptor Signaling in Oral Epithelial Cells Is Critical for Protection against Oropharyngeal Candidiasis. Cell host & microbe 20, 606–617 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Conti HR et al. , Th17 cells and IL-17 receptor signaling are essential for mucosal host defense against oral candidiasis. The Journal of experimental medicine 206, 299–311 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Huppler AR et al. , Role of neutrophils in IL-17-dependent immunity to mucosal candidiasis. J Immunol 192, 1745–1752 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Trautwein-Weidner K, Gladiator A, Nur S, Diethelm P, LeibundGut-Landmann S, IL-17-mediated antifungal defense in the oral mucosa is independent of neutrophils. Mucosal Immunol 8, 221–231 (2015). [DOI] [PubMed] [Google Scholar]
- 102.Conti H et al. , IL-17RA signaling in oral epithelium is critical for protection against oropharyngeal candidiasis. Cell Host Microbe 20, 606–617 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Altmeier S et al. , IL-1 Coordinates the Neutrophil Response to C. albicans in the Oral Mucosa. PLoS Pathog 12, e1005882 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Tomalka J et al. , beta-Defensin 1 plays a role in acute mucosal defense against Candida albicans. J Immunol 194, 1788–1795 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Oppenheim JJ, Biragyn A, Kwak LW, Yang D, Roles of antimicrobial peptides such as defensins in innate and adaptive immunity. Annals of the rheumatic diseases 62 Suppl 2, ii17–21 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Edgerton M et al. , Candidacidal activity of salivary histatins. J. Biol. Chem. 273, 20438–20447 (1998). [DOI] [PubMed] [Google Scholar]
- 107.Conti HR et al. , New mechanism of oral immunity to mucosal candidiasis in hyper-IgE syndrome. Mucosal immunology 4, 448–455 (2011) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hise AG et al. , An essential role for the NLRP3 inflammasome in host defense against the human fungal pathogen Candida albicans. Cell Host Microbe 5, 487–497 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tomalka J et al. , A novel role for the NLRC4 inflammasome in mucosal defenses against the fungal pathogen Candida albicans. PLoS Pathog 7, e1002379 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Su Y et al. , Interleukin-17 receptor D constitutes an alternative receptor for interleukin-17A important in psoriasis-like skin inflammation. Sci Immunol 4, (2019). [DOI] [PubMed] [Google Scholar]
- 111.Sparber F et al. , Langerin+ DCs regulate innate IL-17 production in the oral mucosa during Candida albicans-mediated infection. PLoS Pathog 14, e1007069 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bär E et al. , A novel Th cell epitope of Candida albicans mediates protection from fungal infection. J Immunol 188, 5636–5643 (2012). [DOI] [PubMed] [Google Scholar]
- 113.Conti H et al. , Oral-resident ‘natural’ Th17 cells and γδ-T cells control opportunistic Candida albicans infections. The Journal of experimental medicine 211, 2075–2084 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Verma A et al. , Oral epithelial cells orchestrate innate Type 17 responses to Candida albicans through the virulence factor Candidalysin. Sci Immunol 2, eeam8834 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Pandiyan P et al. , CD4+CD25+Foxp3+ regulatory T cells promote Th17 cells in vitro and enhance host resistance in mouse Candida albicans Th17 infection model. Immunity 34, 422–434 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Conti HR et al. , Oral-resident natural Th17 cells and gammadelta T cells control opportunistic Candida albicans infections. The Journal of experimental medicine 211, 2075–2084 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kashem SW et al. , Candida albicans Morphology and Dendritic Cell Subsets Determine T Helper Cell Differentiation. Immunity 42, 356–366 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Hernández-Santos N et al. , Th17 cells confer long term adaptive immunity to oral mucosal Candida albicans infections. Mucosal Immunol 6, 900–910 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Netea MG et al. , Immune sensing of Candida albicans requires cooperative recognition of mannans and glucans by lectin and Toll-like receptors. The Journal of clinical investigation 116, 1642–1650 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bishu S et al. , CARD9 is required for adaptive but not innate immunity to oral mucosal Candida albicans infections. Infect Immun 82, 1173–1180 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Moyes D et al. , Candidalysin: A fungal peptide toxin critical for mucosal infection. Nature 532, 64–68 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Verma AH et al. , IL-36 and IL-1/IL-17 Drive Immunity to Oral Candidiasis via Parallel Mechanisms. J Immunol 201, 627–634 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Spellberg B et al. , The antifungal vaccine derived from the recombinant N terminus of Als3p protects mice against the bacterium Staphylococcus aureus. Infect Immun 76, 4574–4580 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bhaskaran N et al. , Role of Short Chain Fatty Acids in Controlling Tregs and Immunopathology During Mucosal Infection. Frontiers in microbiology 9, 1995 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Saunte DM, Mrowietz U, Puig L, Zachariae C, Candida infections in psoriasis and psoriatic arthritis patients treated with IL-17 inhibitors and their practical management. Br J Dermatol, (2016). [DOI] [PubMed] [Google Scholar]
- 126.Whibley N et al. , Antibody blockade of IL-17-family cytokines in immunity to acute murine oral mucosal candidiasis. J Leukoc Biol 99, 1153–1164 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Shen F et al. , Combined Blockade of TNF-alpha and IL-17A Alleviates Progression of Collagen-Induced Arthritis without Causing Serious Infections in Mice. J Immunol 202, 2017–2026 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Gladiator A, Wangler N, Trautwein-Weidner K, Leibundgut-Landmann S, Cutting Edge: IL-17-Secreting Innate Lymphoid Cells Are Essential for Host Defense against Fungal Infection. J Immunol 190, 521–525 (2013). [DOI] [PubMed] [Google Scholar]
- 129.Conti HR et al. , Signaling through IL-17C/IL-17RE is dispensable for immunity to systemic, oral and cutaneous candidiasis. PLoS One 10, e0122807 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]