Abstract
Background: Drug resistance severely reduces treatment efficiency of chemotherapy and leads to poor prognosis. However, regulatory factors of chemoresistant cancer cells are largely unknown.
Methods: The expression of estrogen receptor related receptors (ERRs) in chemoresistant cancer cells are checked. The roles of ERRγ in chemoresistance are confirmed by in vitro and in vivo studies. The mechanisms responsible for ERRγ-regulated expression of ABCB1 and CPT1B are investigated.
Results: The expression of ERRγ is upregulated in chemoresistant cancer cells. Targeted inhibition of ERRγ restores the chemosensitivity. ERRγ can directly bind to the promoter of ABCB1 to increase its transcription. An elevated interaction between ERRγ and p65 in chemoresistant cells further strengthens transcription of ABCB1. Further, ERRγ can increase the fatty acid oxidation (FAO) in chemoresistant cells via regulation of CPT1B, the rate-limiting enzyme of FAO. The upregulated ERRγ in chemoresistant cancer cells might be due to increased levels of N6-methyladenosine (m6A) can trigger the splicing of precursor ESRRG mRNA.
Conclusions: m6A induced ERRγ confers chemoresistance of cancer cells through upregulation of ABCB1 and CPT1B.
Keywords: Chemoresistance, ERRγ, ABCB1, CPT1B, FAO
Introduction
Chemotherapy using one or more anticancer drugs is the major strategy for cancer treatment, particularly for patients with advanced and/or metastatic tumors that cannot undergo surgery 1. It is generally preferred in developing countries due to high costs of targeted therapies 2. However, many patients gradually develop resistance to progressive chemotherapy, resulting in treatment failure that has become a serious clinical problem in cancer therapy. One important feature of chemoresistance is that cancer cells often become resistant to not just one drug, but also to different drugs. This is known as multidrug resistance (MDR) and will seriously affect the treatment efficiency.
Several mechanisms involved in chemoresistance have been identified in the last few decades 3-5. ATP-binding cassette (ABC) transporters with a family of 48 human members can regulate the absorption, disposition and elimination of drugs to mediate chemoresistance 6. ATP binding cassette subfamily B member 1 (ABCB1), which encodes multidrug resistance protein-1 (MDR-1)/P-glycoprotein (P-gp), is one of the best studied molecules in drug resistance 3. It can confer cancer cell resistance to numerous anticancer agents such as doxorubicin (Dox), taxol (Tax), colchicine, vincristine and even tyrosine kinase inhibitors 7.
Recently, emerging evidence indicates that metabolic properties of chemoresistant cancer cells diverge significantly from those of their parental cells 8-10. Dysregulation of glucose metabolism, fatty acid synthesis and glutaminolysis have been linked to therapeutic resistance in cancer treatment 11. Fatty acids (FAs) are important cellular energy resources utilized through FA oxidation (FAO), which has been shown to be involved in cancer stem cell self-renewal and chemoresistance of breast 10 and gastric 12 cancers. Inhibition of FAO can repress stemness and in vivo growth of cancer cells 13, 14. However, the roles and regulatory factors of FAO in chemoresistant cancer cells are largely unknown.
Estrogen receptor related receptors (ERRs), which include ERRα/β/γ, are orphan nuclear receptors and share sequence homology with estrogen receptor α (ERα) 15. It has been reported that ERRα is involved in chemotherapy resistance of osteosarcoma cells 16. ERRγ is a crucial mediator of multiple endocrine and metabolic signals 17 and mediates tamoxifen (TAM) resistance of invasive lobular breast cancer, in which knockdown of ERRγ restores TAM sensitivity 18. Androgen-dependent repression of ERRγ reprograms metabolic properties of prostate cancer 19, whereas miR-378 can inhibit the expression of ERRγ to suppress tricarboxylic acid cycle (TCA) gene expression and oxygen consumption as well as an increase in lactate production 20. All these data suggest that ERRs are likely involved in chemoresistance of cancer cells.
We found that ERRγ was significantly upregulated in chemoresistant cancer cells, with knockdown of ERRγ restoring the chemosensitivity. Mechanistically, ERRγ can mediate the chemoresistance of cancer cells via upregulation of ABCB1 and facilitation of FAO. Our results identify a new macromolecule that may serve as a predictive marker of chemotherapy and as an effective target for overcoming chemoresistance.
Results
ERRγ is upregulated in chemoresistant cancer cells
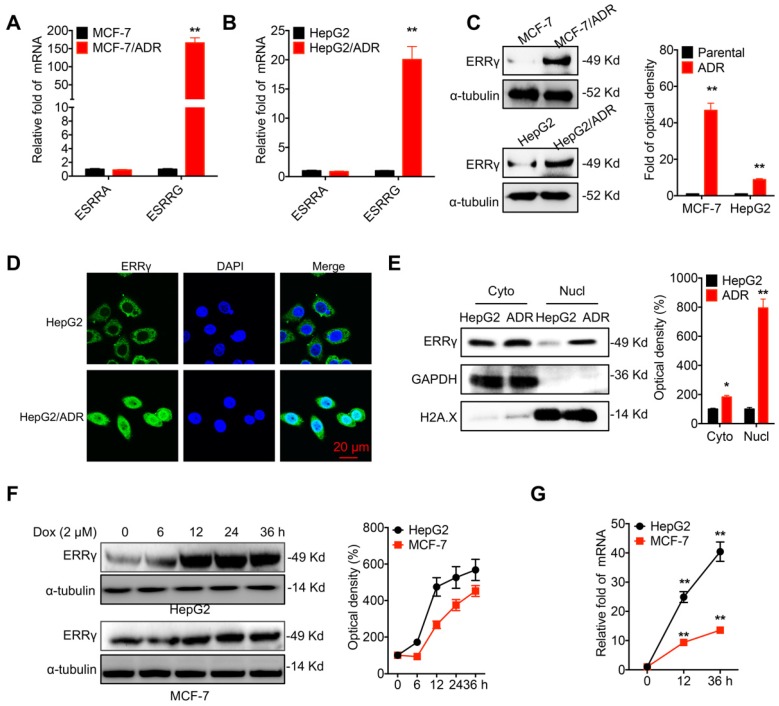
The chemoresistance of breast (MCF-7/ADR) and liver (HepG2/ADR) cancer cells was confirmed by evaluation of Dox sensitivity and compared with that of their corresponding parental cells (Figure S1A and S1B). A potential role of ERR signals in chemoresistance was then assessed by quantifying the mRNA levels of ERRα (ESRRA) and ERRγ (ESRRG). Quantitative (q) RT-PCR showed enhanced mRNA levels of ERRγ in MCF-7/ADR cells as compared to that in MCF-7 cells (Figure 1A). Increased expression of ERRγ was also observed in HepG2/ADR cells as compared to that in HepG2 cells (Figure 1B). An elevated protein level of ERRγ was also observed in MCF-7/ADR and HepG2/ADR cells by Western blot analysis (Figure 1C). In contrast, the protein levels of ERRα had no clear difference between chemoresistant and parental cells (Figure S1C). An increased mRNA level of ERRγ was also observed in other chemoresistant lines, including MCF-7/Tax (resistance to Tax), MDA-MB-231/Tax, and A549/Tax cells as compared to their corresponding parental cells (Figure S1D). Western blot analysis further confirmed that the protein level of ERRγ was increased in MCF-7/Tax and MDA-MB-231/Tax as compared to their controls (Figure S1E). Confocal microscopy showed that the expression of ERRγ was mainly located within the cytoplasm of HepG2 cells, while the expression and nuclear accumulation of ERRγ were increased in HepG2/ADR cells (Figure 1D). An increased nuclear localization of ERRγ in HepG2/ADR cells was confirmed by subcellular fractionation and Western blot analysis (Figure 1E) and further validated in MCF-7/ADR cells (Figure S1F). The expression levels of ERRα/γ in cancer cells were also examined following Dox treatment. Western blot analysis showed Dox treatment rapidly increased the expression of ERRγ (Figure 1F), but not ERRα (Figure S1G), in both HepG2 and MCF-7 cells. This might be due to that Dox treatment can increase the mRNA of ERRγ expression in cancer cells (Figure 1G). Collectively, these data showed that ERRγ is upregulated in chemoresistant cancer cells.
Figure 1.
ERRγ is upregulated in chemoresistant cancer cells. (A&B) Expression of ERRα (ESRRA) and ERRγ (ESRRG) measured in MCF-7/ADR (A), HepG2/ADR (B), and their corresponding parental cells by qRT-PCR; (C) Protein levels of ERRγ in MCF-7/ADR, HepG2/ADR, and their corresponding parental cells measured by Western blot analysis (left) and quantitatively analyzed (right); (D) Subcellular expression of ERRγ in HepG2/ADR and HepG2 cells visualized by confocal imaging; (E) The subcellular expression of ERRγ in HepG2/ADR and HepG2 cells was checked by Western blot analysis (left) and quantitatively analyzed (right); (F) Cells were treated with Dox (2 μM) for the indicated times, then the protein expression of ERRγ was checked by Western blot analysis (left) and quantitatively analyzed (right); (G) Cells were treated with Dox (2 μM) for the indicated times, then the mRNA expression of ERRγ was checked by qRT-PCR. Data were presented as means ± SD from three independent experiments. *p<0.05, **p< 0.01 compared with control.
ERRγ regulates chemoresistance of cancer cells
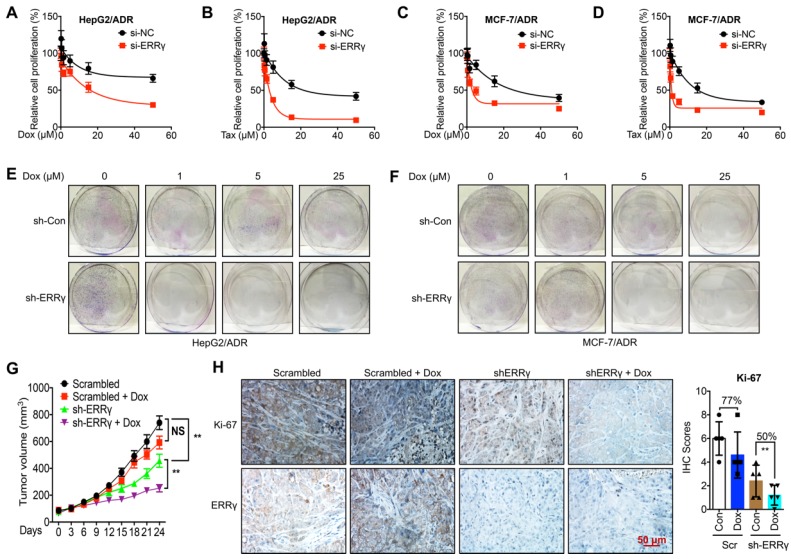
To determine whether ERRγ is involved in chemoresistance of cancer cells, we knocked down the expression of ERRγ by siRNA transfection (Figure S2A). Knockdown of ERRγ significantly restored Dox and Tax sensitivity in HepG2/ADR cells, with IC50 values of Dox for si-NC and si-ERRγ being 173 and 15.9 μM (Figure 2A) and of Tax for si-NC and si-ERRγ being 29.2 and 2.36 μM, respectively (Figure 2B). As to MCF-7/ADR cells, the IC50 values of Dox for si-NC and si-ERRγ were 30.2 and 4.96 μM (Figure 2C) and of Tax for si-NC and si-ERRγ were 23.4 and 2.76 μM, respectively (Figure 2D). Colony formation assay showed that knockdown of ERRγ significantly inhibited colonization of both HepG2/ADR (Figure 2E) and MCF-7/ADR (Figure 2F) cells. In contrast, overexpression of ERRγ in HepG2 and MCF-7 cells by transfection with pcDNA/ERRγ (Figure S2B) decreased the sensitivity of both cells to the treatment of Dox and Tax (Figure S2 C-F).
Figure 2.
ERRγ regulates chemoresistance of cancer cells. (A&B) Cell proliferation rate in si-NC- or si-ERRγ-1-transfected HepG2/ADR cells for 24 h and followed by treatment with increasing concentrations of Dox (A) or Tax (B) for 48 h; (C&D) Cell proliferation rate in si-NC- or si-ERRγ-1-transfected MCF-7/ADR cells for 24 h and followed by treatment with increasing concentrations of Dox (C) or Tax (D) for 48 h; (E&F) HepG2/ADR (E) or MCF-7/ADR (F) cells transfected with scrambled shRNA or sh-ERRγ were split and cultured in fresh medium for the next 15 days. The colonies were fixed with methanol/glacial acetic acid (7:1) and stained with 0.1% of crystal violet; (G) Tumor volume measurement in mouse xenografts. HepG2/ADR cells stably transfected with scrambled shRNA or sh-ERRγ were subcutaneously inoculated in nude mice. We randomly divided the mice into Scramble, sh-ERRγ, Dox + Scramble and Dox + sh-ERRγ groups and treated them as described in the Methods. Tumor growth curves were constructed based on the tumor volumes measured in the mice; (H) IHC analysis of mouse xenograft tissues. Expression of ERRγ and proliferation marker Ki-67 was determined in tumor tissue sections from the xenografts using IHC (scale bar, 50 μm) and quantitatively analyzed; Data were presented as means ± SD from three independent experiments. **p< 0.01. NS, no significant.
To evaluate whether ERRγ is essential for in vivo chemoresistance of cancer cells, we established mouse xenograft tumors by using sh-ERRγ-transfected HepG2/ADR cells (Figure S2G). The tumor growth rate and tumor size at the end of the experiments were significantly decreased in the sh-ERRγ group relative to the scrambled group (Figure 2G). When treated with Dox, the scrambled group showed no obvious decrease in tumor size as compared with that of the control group. As expected, sh-ERRγ increased in vivo Dox sensitivity of HepG2/Dox cells (Figure 2G). Subsequent IHC analysis confirmed the in vivo knockdown efficiency of sh-ERRγ (Figure 2H). Further, Dox treatment obviously reduced the expression of the proliferation marker Ki-67 in the sh-ERRγ group (50%) than in the scrambled group (77%) (Figure 2H). These data suggested that ERRγ regulates both in vitro and in vivo chemoresistance of cancer cells.
P-gp is involved in ERRγ-regulated chemoresistance of cancer cells
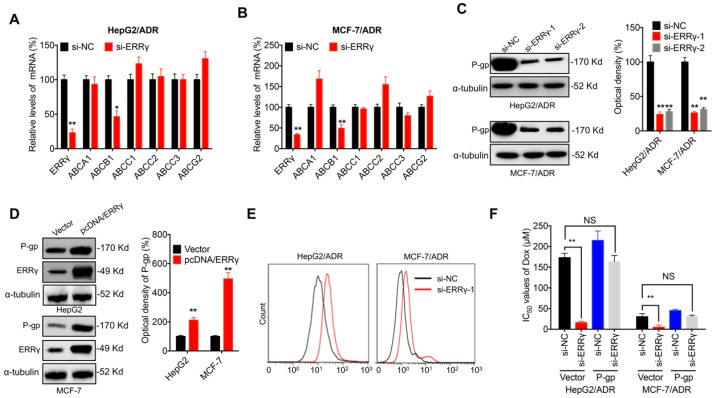
ABC transporters are critical for chemoresistance of cancer cells 6. Expression of the major ABC transporters, including ABCA1, ABCB1, ABCC1, ABCC2, ABCC3 and ABCG2, was assessed in chemoresistant cancer cells transfected with si-ERRγ. qRT-PCR showed that si-ERRγ significantly decreased the expression of ABCB1, but not others, in both HepG2/ADR (Figure 3A) and MCF-7/ADR (Figure 3B) cells. Both si-ERRγ-1 and si-ERRγ-2 decreased ABCB1 mRNA levels in MCF-7/Tax and MDA-MB-231/Tax cells (Figure S3A). Decreased protein expression of P-gp (encoded by ABCB1) was observed in both HepG2/ADR and MCF-7/ADR cells transfected with si-ERRγ-1 and si- ERRγ-2 (Figure 3C), while overexpression of ERRγ increased the expression of P-gp in both HpeG2 and MCF-7 cells (Figure 3D).
Figure 3.
P-gp is involved in ERRγ-regulated chemoresistance of cancer cells. (A&B) mRNA expression of ABC transporters measured in HepG2/ADR (A) or MCF-7/ADR (B) cells 24 h post transfection with si-NC or si-ERRγ-1; (C) Expression of P-gp protein measured by Western blot analysis (left) and quantitively analyzed (right) in HepG2/ADR or MCF-7/ADR cells 24 h post transfection with si-NC or si-ERRγ-1/2; (D) Expression of P-gp protein measured by Western blot analysis (left) and quantitively analyzed (right) in HepG2 or MCF-7 cells 24 h post transfection with vector control or pcDNA/ERRγ; (E) P-gp function analyzed by flow cytometric measurement of the intracellular accumulation of Rh123 in HepG2/ADR or MCF-7/ADR cells 24 h post transfection with scrambled siRNA or si-ERRγ-1; (F) IC50 values of Dox in HepG2/ADR or MCF-7/ADR cells co-transfected with si-ERRγ and P-gp construct. Data were presented as means ± SD from three independent experiments. *p< 0.05, **p< 0.01. NS, no significant.
Although P-gp is known to mediate tumor cell chemoresistance 6, its role in ERRγ-regulated chemosensitivity was further investigated. Our data confirmed that si-ERRγ increased cellular accumulation of Rh123, a well-known fluorescent P-gp substrate, in both HepG2/ADR and MCF-7/ADR cells (Figure 3E). Overexpression of P-gp (Figure S3B) restored si-ERRγ-induced upregulation of Dox sensitivity in both HepG2/ADR and MCF-7/ADR cells (Figure 3F). These data indicated that P-gp is indeed involved in ERRγ-regulated chemoresistance of cancer cells.
ERRγ interacts with p65 to regulate the transcription of ABCB1
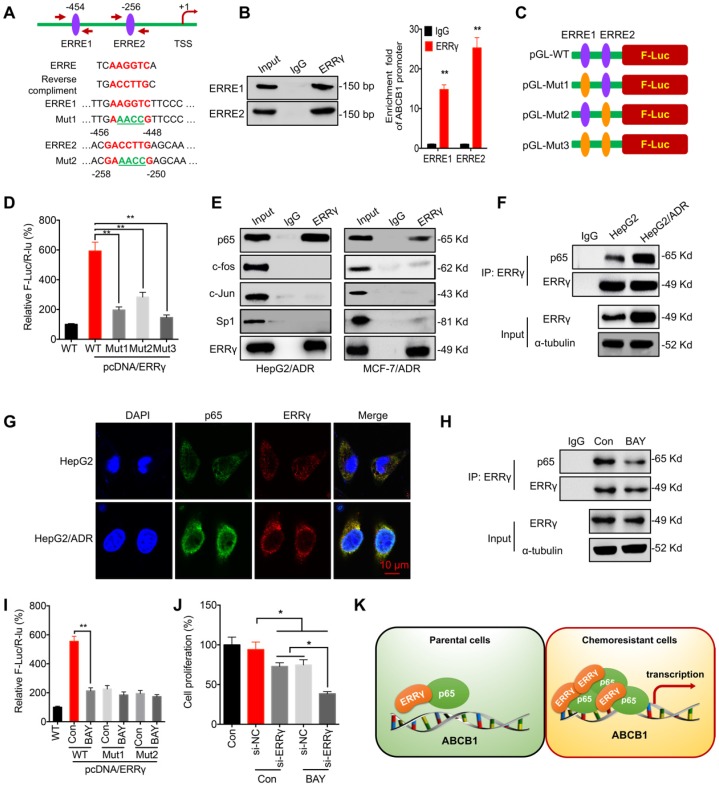
The mechanism responsible for ERRγ-regulated transcription of ABCB1 was further investigated. Computer-assisted searches of potential ERRγ-binding sites (ERR response element, ERRE, TNAAGGTCA) within the ABCB1 promoter region (-1 kb) was conducted by using the TESS database, which predicts transcription factor-binding sites. Two putative ERREs located at -454 and -256 bp upstream of the transcription start site of the ABCB1 promoter were identified (Figure 4A). ChIP-PCR confirmed that ERRγ binds to these two ERREs (Figure 4B). Next, an ABCB1 promoter (-1024 to -1)-driven luciferase reporter assay was conducted with reporters containing wild-type (WT) or mutated (Mut) ERRγ-binding sites (Figure 4A and 4C). As shown in Figure 4D, ERRγ induced robust luciferase expression in pGL3-ABCB1-WT, whereas mutation at ERRE1 and/or ERRE2 significantly decreased ERRγ-induced luciferase activity, suggesting that ERRγ binds directly to the ABCB1 promoter to regulate its transcription.
Figure 4.
ERRγ interacts with p65 to regulate ABCB1 transcription. (A) Schematic representation of ERREs in the promoter region of ABCB1 with changes of nucleotides in ERRE1 and ERRE2 shown as indicated; (B) ChIP-PCR assay showing ERRγ binding to ERRE1 and ERRE2 in ABCB1 promoter. The input (5%), binding between ERRγ and the promoter of ABCB1 at the potential binding site ERRE1/2, was amplified by qPCR (right) and confirmed by 2% agarose gel electrophoresis (left); (C) Schematic representation of mutated ERRE positions in pGL-ABCB1 vector; (D) Reporter gene assay performed in HepG2 cells 24 h post transfection with pGL-ABCB1-WT or pGL-ABCB1-Mut1/2/3 by dual-luciferase analysis; (E) Examination of ERRγ interaction with different transcription factors in HepG2/ADR and MCF-7/ADR cells following immunoprecipitation with ERRγ or control antibody and analyzed by Western blot analysis; (F) Interaction between ERRγ and p65 in HepG2 and HepG2/ADR cells monitored by immunoprecipitation using anti-ERRγ antibody; After ERRγ was immunoprecipitated, the binding between ERRγ and p65 was examined by Western blot analysis. An equal amount of ERRγ was loaded for normalization according to a pre-Western blot; (G) Expression and localization of p65 (green) and ERRγ (red) in HepG2 and HepG2/ADR cells visualized by confocal imaging; (H) Interaction between ERRγ and p65 in HepG2/ADR cells treated with or without BAY 11-7082 for 12 h and then analyzed by immunoprecipitation using an antibody against ERRγ; (I) Dual-luciferase reporter gene assay performed in HepG2 cells transfected with pGL-ABCB1-WT or pGL-ABCB1-Mut1/2, with or without pcDNA/ERRγ, for 12 h and then further treated with or without BAY 11-7082 for 12 h; (J) HepG2/ADR cells were treated with si-RNA or si-ERRγ combined with or without BAY 11-7082 for 12 h and then further treated with 5 μM Dox for 48 h; (K) Model for ERRγ/p65-promoted transcription of ABCB1 in chemoresistant cancer cells. Data were presented as means ± SD from three independent experiments. **p< 0.01. NS, no significant.
ERRγ can form homodimers and heterodimers via its ligand-binding domain (LBD) 21, 22. We hypothesized that transcription factors regulating ABCB1 expression, including c-Jun, c-Fos, NF-κB/p65, and Sp1 23, 24, might interact with ERRγ to increase its activity. Co-IP showed that ERRγ associated with endogenous p65, but not the other transcription factors, in HepG2/ADR and MCF-7/ADR cells (Figure 4E). Consistently, reciprocal co-IP showed that ERRγ was pulled down in HepG2/ADR and MCF-7/ADR cell lysates by anti-p65 antibody (Figure S4A). To compare the binding between ERRγ and p65 in chemoresistant and parental cells, an equal amount of ERRγ after immunoprecipitation by use of its antibody was loaded for normalization according to a pre-Western blot since the endogenous ERRγ was increased in HepG2/ADR cells. The data showed that the binding between ERRγ and p65 was increased in HepG2/ADR cells as compared with that in HepG2 cells (Figure 4F). Confocal imaging showed enhanced expression of ERRγ in HepG2/ADR cells and colocalized with p65 in both HepG2 and HepG2/ADR cells (Figure 4G). These results suggest that the interaction between ERRγ and p65 was upregulated in chemoresistant cells.
We further investigated whether p65 was involved in ERRγ-regulated transcription of ABCB1. An inhibitor of NF-κB, BAY 11-7082, suppressed the mRNA (Figure S4B) and protein (Figure S4C) expression of P-gp in HepG2/ADR and MCF-7/ADR cells. BAY 11-7082 also decreased the interaction between p65 and ERRγ in HepG2/ADR cells (Figure 4H). Moreover, BAY 11-7082 could decrease the promoter activity of pGL3-ABCB1-WT, while had no significant effect on the relative F-Luc/R-Luc for pGL-ABCB1-Mut-1/2 (Figure 4I), suggesting that ERRγ was involved in p65-regulated transcription of ABCB1. Further, our data showed that BAY 11-708 can significantly elevate the si-ERRγ-increased Dox sensitivity of HepG2/ADR cells (Figure 4 J). Our data suggest that ERRγ can interact with p65 to promote ABCB1 transcription in chemoresistant cells (Figure 4K).
ERRγ dictates metabolic reprogramming in chemoresistant cancer cells
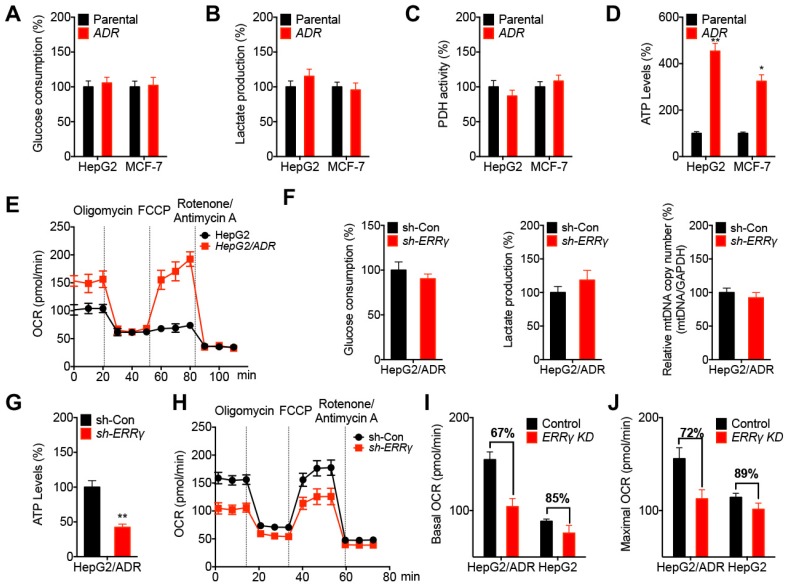
Chemoresistant cancer cells diverge metabolic properties such as aerobic glycolysis and mitochondrial respiration 25, 26. Our data showed that HepG2/ADR and MCF-7/ADR cells exhibited no significant difference in glucose consumption (Figure 5A) and lactate production (Figure 5B) rates compared to that of their corresponding parental cells. Further, the activity of pyruvate dehydrogenase (PDH), which converts pyruvate to acetyl-CoA (Ac-CoA) and then enters the TCA cycle 27, did not vary between chemoresistant and parental cells (Figure 5C). However, the extracellular ATP levels in chemoresistant cells were significantly greater than that in the parental cells (Figure 5D). Seahorse analysis showed that HepG2/ADR cells displayed an increased basal and maximal oxygen consumption rate (OCR), an indicator of mitochondrial oxidative respiration (Figure 5E), but had comparable levels of the extracellular acidification rate (ECAR), which reflects the overall glycolytic flux (Figure S5A), than that of HepG2 cells. However, the mitochondrial mass between HepG2/ADR and HepG2 cells had no significant difference (Figure S5B). These data suggested that chemoresistant cells showed increased ATP production and OCR than that of parental cells.
Figure 5.
ERRγ dictates the metabolic reprogramming in chemoresistant cancer cells. (A~D) Relative glucose consumption (A), lactate production (B), PDH activity (C), and ATP levels (D) measured in HepG2/ADR or MCF-7/ADR cells following comparison with those measured in their corresponding parental cells; (E) OCR in HepG2/ADR and HepG2 cells measured by Seahorse XF24 analyzer; (F) Relative glucose consumption, lactate production, and mitochondrial mass in HepG2/ADR cells transfected with sh-Con or sh-ERRγ; (G) Relative ATP levels in HepG2/ADR cells transfected with sh-Con or sh-ERRγ; (H) OCR in HepG2/ADR cells transfected with sh-Con or sh-ERRγ measured by Seahorse XF24 analyzer; (I~J) Basal (I) and maximal (J) OCR measured by Seahorse XF24 analyzer in HepG2/ADR or HepG2 cells transfected with sh-Con or sh-ERRγ. Data were presented as means ± SD from three independent experiments. *p< 0.05, **p< 0.01.
We further investigated the potential roles of ERRγ in metabolic programming of chemoresistant cancer cells. Our data showed that knockdown of ERRγ had no significant effect on glucose consumption, lactate production, or mitochondrial mass in HepG2/ADR cells (Figure 5F). However, knockdown of ERRγ decreased the ATP levels of HepG2/ADR cells (Figure 5G) as well as the basal and maximum OCRs (Figure 5H), but had no significant effect on ECAR (Figure S5C) in HepG2/ADR cells. This was further confirmed by overexpression of ERRγ resulting in increased basal and maximum OCRs without significantly altering ECAR in HepG2 cells (Figure S5D and S5E). Moreover, knockdown of ERRγ more effectively decreased the basal (Figure 5I) and maximum (Figure 5J) OCRs of HepG2/ADR cells than that in HepG2 cells. Both overexpression of ERRγ in HepG2 cells (Figure S5F) and knockdown of ERRγ in HepG2/ADR cells (Figure S5G) had no significant effect on key gene expression involved in oxidative phosphorylation (OxPhos) such as CS, NDUFA4, SDHB, COX5B, or ATP5B, suggesting that ERRγ-upregulated OCR and ATP production were not related to the OxPhos pathway. All the data indicated that ERRγ dictates metabolic reprogramming in chemoresistant cancer cells without affecting glycolysis, mitochondrial mass, and PDH activities, but it does increase ATP generation and OCR.
ERRγ regulates FAO via Cpt1b in chemoresistant cancer cells
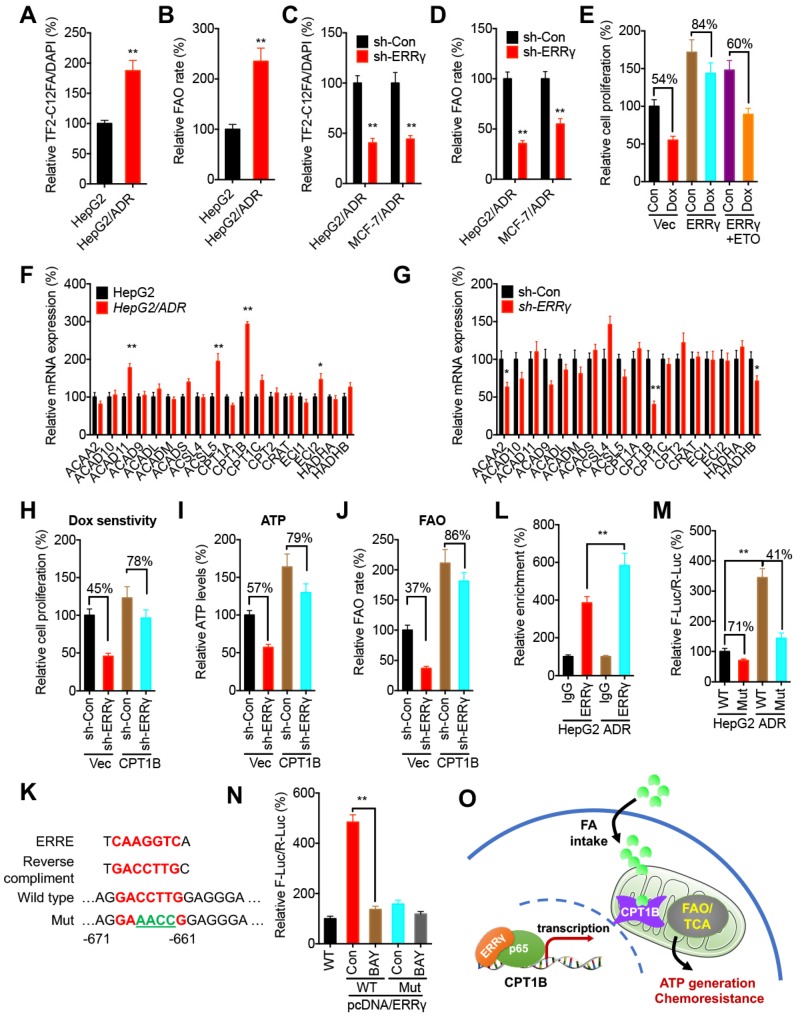
A recent study indicates that ERRγ in kidney cells can regulate mitochondrial FAO functions via direct binding to FAO genes such as CPT1B, CPT2, ACADM, and HADHA 28. We then investigated the potential effects of ERRγ on mitochondrial FAO functions in cancer cells. We found the FA uptake (Figure 6A) and FAO rate (Figure 6B) in HepG2/ADR cells were significantly greater than that in HepG2 cells. Knockdown of ERRγ significantly inhibited the FA uptake (Figure 6C) and FAO rate (Figure 6D) in both HepG2/ADR and MCF-7/ADR cells. Further, overexpression of ERRγ decreased Dox sensitivity of HepG2 cells, while this effect could be blocked by the FAO inhibitor etomoxir (ETO) (Figure 6E), suggesting that upregulation of FAO was involved in ERRγ-induced chemoresistance of cancer cells.
Figure 6.
ERRγ regulates the FAO via Cpt1b in chemoresistant cancer cells. (A~B) Relative FA uptake (A) and FA β oxidation rate (B) between HepG2 and HepG2/ADR cells; (C~D) Relative FA uptake (C) and FA β oxidation rate (D) in cells transfected with sh-Con or sh-ERRγ; (E) Cell proliferation measured by CCK-8 kit in HepG2 cells pre-transfected with vector control or pcDNA/ERRγ for 6 h and then treated with or without Dox (1 μM) combined with or without ETO for 24 h; (F) mRNA levels of FAO-related genes measured by qRT-PCR in HepG2 and HepG2/ADR cells; (G) mRNA levels of FAO-related genes measured by qRT-PCR in HepG2/ADR cells transfected with sh-Con or sh-ERRγ; (H) Cell proliferation measured by CCK-8 kit in HepG2/ADR cells pre-transfected with sh-Con or sh-ERRγ and then transfected with vector or a Cpt1b expression construct, followed by further treatment with Dox (10 μM) for 24 h; (I~J) ATP (I) and FA β oxidation rate (J) measured in HepG2/ADR cells transfected with sh-Con or sh-ERRγ with further transfection with vector or a Cpt1b expression construct for 24 h; (K) Nucleotide sequences of ERREs in CPT1B and the mutated (GACCTTG to AGAACCG) nucleotides in pGL3-CPT1B-Mut-Luc vector; (L) ChIP assay measuring ERRγ binding to CPT1B promoter in both HepG2 and HepG2/ADR cells; (M) Dual-luciferase reporter gene assay performed in HepG2 and HepG2/ADR cells transfected with pGL3-CPT1B-WT-Luc or pGL3-CPT1B-Mut-Luc; (N) Dual-luciferase reporter gene assay performed in HepG2 cells transfected with pGL-ABCB1-WT or pGL3-CPT1B-Mut reporter with or without pcDNA/ERRγ for 12 h and then further treated with or without BAY 11-7082 for 12 h; (O) Model for ERRγ-regulated FAO via Cpt1b in chemoresistant cancer cells. Data were presented as means ± SD from three independent experiments. *p< 0.05.
To investigate the mechanisms of ERRγ-regulated FAO in cancer cells, qRT-PCR was performed to evaluate the expression of genes involved in cellular FAO (Table S1) 29. Among all of the FAO-related genes examined, expression of CPT1B was significantly increased in HepG2/ADR cells as compared with that in HepG2 cells (Figure 6F). Knockdown performed with sh-ERRγ significantly decreased CPT1B expression in HepG2/ADR cells (Figure 6G). Consistently, overexpression of ERRγ increased the expression of CPT1B in HepG2 cells (Figure S6A), while both si-ERRγ-1 and si-ERRγ-2 significantly decreased the protein levels of Cpt1b in HepG2/ADR cells (Figure S6B). To investigate whether Cpt1b was involved in ERRγ-regulated FAO and chemoresistance, we overexpressed Cpt1b in HepG2/ADR cells transfected with sh-Con or sh-ERRγ (Figure S6C). Our data showed that overexpression of Cpt1b significantly attenuated sh-ERRγ-increased sensitivity of Dox in HepG2/ADR cells (Figure 6H), and also sh-ERRγ-downregulated ATP levels (Figure 6I) and FAO rate (Figure 6J), suggesting that Cpt1b is involved in ERRγ-regulated FAO and chemoresistance of cancer cells.
Cpt1b has been indicated as a direct transcriptional target of ERRγ 28, but the binding site(s) in the CPT1B promoter has not been well studied. Analysis of the region 1.0 kb upstream from the transcription start site in the CPT1B promoter revealed one putative ERRE, showing 78% homology (7/9) to the consensus ERRE (Figure 6K). ChIP assay confirmed that binding of ERRγ to CPT1B in HepG2/ADR cells was greater than that in HepG2 cells (Figure 6L). We then cloned the promoter of CPT1B to generate pGL3-CPT1B-WT-Luc and mutated the bind site (to GAAACCG) to generate pGL3-CPT1B-Mut-Luc (Figure 6K). The promoter activity of pGL3-CPT1B-WT-Luc in HepG2/ADR cells was significantly greater than that in HepG2 cells; however, pGL3-CPT1B-Mut-Luc attenuated this difference between HepG2/ADR and HepG2 cells (Figure 6M). In general, the promoter activity of pGL3-CPT1B-WT-Luc was greater than that of pGL3-CPT1B-Mut-Luc in both HepG2/ADR and HepG2 cells (Figure 6M).
We further investigated whether p65/ERRγ complex was involved in the upregulation of Cpt1b in chemoresistant cells. BAY 11-7082, the inhibitor of p65/NF-κB, suppressed the mRNA (Figure S6D) and protein (Figure S6E) levels of Cpt1b in both HepG2/ADR and MCF-7/ADR cells and also significantly decreased ERRγ-induced promoter activity of pGL3-CPT1B-WT-Luc, while it had less effect on the activity of pGL3-CPT1B-Mut-Luc in HepG2 cells (Figure 6N). Our data suggest that ERRγ forms a complex with p65, binds CPT1B promoter to increase its expression, elevates FAO, and thus mediate chemoresistance of cancer cells (Figure 6O).
The m6A-facilitated splicing increases the expression of ERRγ
The potential epigenetic mechanisms responsible for the upregulation of ERRγ in chemoresistant cells were investigated. Firstly, treatment with 5-aza-dC (a DNA methyltransferase inhibitor) had no significant effect on ERRγ expression in either HepG2 or HepG2/ADR cells (Figure S7 A), suggesting that DNA methylation might not be involved in ERRγ expression. Further, broad-spectrum HDAC inhibitors including SAHA and NaB also had no obvious effect on the expression of ERRγ in HepG2 or HepG2/ADR cells (Figure S7B), indicating histone acetylation might not be responsible for the upregulation of ERRγ in chemoresistant cells.
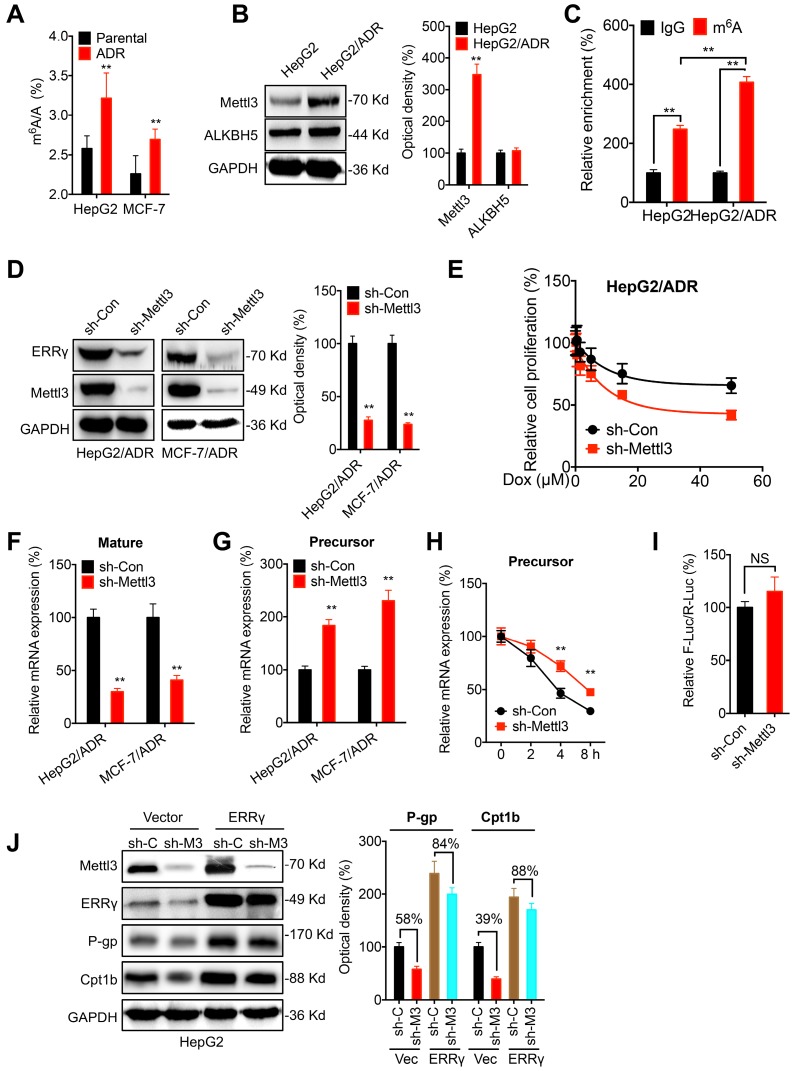
Recent investigations indicated that N6-methyladenosine (m6A) modification can regulate gene expression and be involved in chemoresistance of cancer cells 30, 31. Intriguingly, we found that the m6A of mRNA was increased in HepG2/ADR and MCF/ADR cells as compared to their parental cells (Figure 7 A). Western blot analysis showed that the expression of m6A methyltransferase Mettl3 was upregulated in HepG2/ADR cells, while the expression of demethylase ALKBH5 had no variation (Figure 7 B). m6A-RIP-qPCR confirmed that a 2-fold m6A antibody enriched ESRRG mRNA in HepG2 cells, while this enrichment was significantly increased in HepG2/ADR cells (Figure 7 C). We found that knockdown of Mettl3 can decrease the expression of ERRγ in both HepG2/ADR and MCF-7/ADR cells (Figure 7 D). Consistently, over expression of Mettl3 increased the expression of ERRγ in HepG2 cells (Figure S7 C). Further, knockdown of Metttl3 can significantly increase the Dox sensitivity of HepG2/ADR cells (Figure 7 E). It indicated that m6A can increase the expression of ERRγ in cancer cells.
Figure 7.
The m6A-facilitated splicing is responsible for the upregulation of ERRγ. (A) The m6A/A ratio of total mRNA in HepG2/ADR and MCF-7/ADR cells were determined by LC-MS/MS and compared with that in their parental cells; (B) The expression of Mettl3 and ALKBH5 in HepG2/ADR and HepG2 cells was checked by western blot analysis and quantitatively analyzed; (C) m6A RIP-qPCR analysis of ERRγ mRNA in HepG2/ADR and HepG2 cells; (D) The expression of ERRγ in HepG2/ADR and MCF-7/ADR cells transfected with sh-Con or sh-Mettl3 was checked by western blot analysis and quantitatively analyzed; (E) HepG2/ADR cells transfected with sh-Con or sh-Mettl3 were further treated with increasing concentrations of Dox, the cell proliferation was tested by CCK-8 kit; (F~G) The mature (E) and precursor (F) mRNA of ERRγ in HepG2/ADR cells transfected with sh-Con or sh-Mettl3 were checked by qRT-PCR; (H) HepG2/ADR cells transfected with sh-Con or sh-Mettl3 were pre-treated with Act-D for 90 min, then the precursor mRNA of ERRγ was checked by qRT-PCR; (I) The promoter activity of pGL-ESRRG-Basic in HepG2/ADR cells transfected with sh-Con or sh-Mettl3 was checked by dual luciferase assay; (J) HepG2 cells were transfected with sh-Con, sh-Mettl3, vector control or ERRγ construct alone or together for 24 h, the expression of targets was measured and quantitatively analyzed. Data were presented as means ± SD from three independent experiments. **p< 0.01. NS, no significant.
We then investigated the potential mechanisms responsible for m6A regulated expression of ERRγ. Firstly, knockdown of Mettl3 had no significant effect on the protein stability of ERRγ in HepG2/ADR cells (Figure S7 D). However, knockdown of Mettl3 can significantly decrease mature mRNA of ERRγ (Figure 7 F). Consistently, over expression of Mettl3 can increase the mRNA expression of ERRγ in both HepG2 and MCF-7 cells (Figure S7 E). The Mettl3 induced upregulation might not be due to the nuclear turnover or mRNA degradation since knockdown of Mettl3 had no significant effect on either the subcellular localization of mature mRNA (Figure S7 F) or the mRNA stability (Figure S7 G). Intriguingly, we found that knockdown of Mettl3 can increase the precursor mRNA of ERRγ (Figure 7 G), which might be due to that knockdown of Mettl3 can increase the half-life of precursor mRNA (Figure 7 H). It suggested that the decrease of m6A can delay the splicing of precursor mRNA of ERRγ to suppress its expression. This was confirmed by the promoter activity assay which showed that knockdown of Mettl3 had no significant effect on the pGL-ESRRG-Basic (Figure 7 I), suggesting that m6A had no effect on the transcription of ERRγ. Further, we found that in HepG2 cells, knockdown of Mettl3 resulted in the down regulation of P-gp and Cpt1b, however, over expression of ERRγ can significantly attenuate knockdown of Mettl3 induced down regulation of P-gp and Cpt1b (Figure 7 J). Collectively, these data suggested that m6A can trigger the splicing of ERRγ precursor mRNA and then regulate the phenotype of chemoresistance.
The m6A/ERRγ axis and in vivo cancer progression
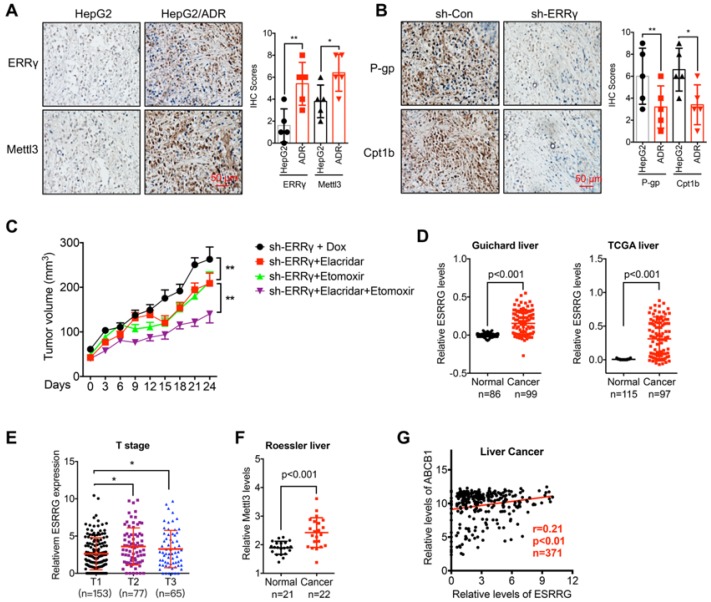
To define the molecular basis of the ERRγ in cancer progression, we checked the expression of Mettl3 and ERRγ in xenografts based on HepG2 and HepG2/ADR cells. Our data showed that when the tumor volume was comparable, the expression of ERRγ and Mettl3 was increased in HepG2/ADR groups as compared to that in parental cells (Figure 8 A). We further checked the in vivo effects of sh-ERRγ on the expression of P-gp and Cpt1b in chemoresistance cells. Our data showed that knockdown of ERRγ can further decrease the expression of P-gp and Cpt1b in HepG2/ADR xenograft model (Figure 8 B). These data indicated that m6A/ ERRγ/P-gp-Cpt1b axis was involved in the in vivo progression and chemoresistance of cancer cells. Further, we established mouse xenograft tumors by using sh-ERRγ-transfected HepG2/ADR cells. Then the xenograft tumors were then treated with Dox combined with elacridar (P-gp inhibitor) 32 or etomoxir (Cpt1 inhibitor) 8, our data showed that the combination of elacridar or etomoxir can increase the in vivo Dox sensitivity of HepG2/Dox cells. Further, the combination of elacridar and etomoxir had synergistic effects to increase the sh-ERRγ-restored in vivo Dox sensitivity of HepG2/Dox cells (Figure 8C). It confirmed that ERRγ can regulate the in vivo chemoresistance via modulation of P-gp and Cpt1b.
Figure 8.
The m6A/ ERRγ axis and in vivo cancer progression. (A) IHC (ERRγ and Mettl3)-stained paraffin-embedded sections obtained from HepG2 and HepG2/ADR xenografts when the tumor volumes were about 100 mm3 for each group; (B) The sh-control and sh-Mettl3 HepG2/ADR cells were subcutaneously inoculated in nude mice. IHC (P-gp and Cpt1b)-stained paraffin-embedded sections obtained at the end of experiment; (C) Tumor volume measurement in mouse xenografts. HepG2/ADR cells stably transfected with sh-ERRγ were subcutaneously inoculated in nude mice. We randomly divided the mice into sh-ERRγ, sh-ERRγ + Elacridar, sh-ERRγ + Etomoxir, and sh-ERRγ + Elacridar + Etomoxir and then treated with Dox as described in the Methods. Tumor growth curves were constructed based on the tumor volumes measured in the mice; (D) Expression of ESRRG in HCC tumor tissues and normal liver tissues from Oncomine database (Guichard and TCGA liver cancers); (E) ESRRG expression in liver cancers of T1 (n=153), T2 (n=77), and T3 (n=65) stages from TCGA database; (F) Expression of Mettl3 in HCC tumor tissues and normal liver tissues from Oncomine database (Roessler liver); (G) Correlation between ESRRG and ABCB1 in liver cancer patients (n=371) from TCGA database; Data were presented as means ± SD from three independent experiments. *p<0.05, **p< 0.01.
We then analyzed the expression of m6A/ERRγ axis and their correlation with clinical characteristics of breast and liver cancers. Increased expression of ERRγ in liver cancer versus normal tissue has been observed in Guichard and TCGA data from Oncomine database (Figure 8D). Consistently, increased expression of ERRγ was also observed in breast cancer versus normal tissues in Finak data from Oncomine database (Figure S8 A). Further, significant rising expression levels of ESRRG from T1 to T3 stage of liver cancer tissues were observed (Figure 8 E), implying an increasing tendency of ESRRG expression during malignant transformation.
In addition, increased expression of Mettl3 has also be found in Roessler liver cancer (Figure 8 F). Further, we assessed the correlation between ERRγ and P-gp in cancer patients with data downloaded from LinkedOmics (http://www.linkedomics.org). Our data showed that the expression of ERRγ was positively correlated with the ABCB1 in liver (Figure 8 G) and breast (Figure S8 B) cancer patients. All these data suggested that ERRγ regulated chemoresistance and metabolic reprogramming might be involved in the in vivo cancer progression.
Discussion
Previous studies indicated that ERR signaling can regulate the progression of various cancers 19, 33, 34. Our present study found that ERRγ was upregulated in chemoresistant cancer cells and targeted inhibition of ERRγ restored the chemosensitivity. Mechanistically, ERRγ can interact with p65 and bind to the promoter of ABCB1, which encodes a key transporter that pumps many foreign substances out of cells, to increase its transcription and expression. At the same time, ERRγ can facilitate FAO of chemoresistant cancer cells via upregulation of Cpt1b. Intriguingly, chemoresistant cells showed increased levels of m6A and expression of Mettl3 than that of parental cells, which can trigger the splicing of precursor of ESRRG mRNA to increase it expression. Collectively, we found that m6A-induced ERRγ is essential for chemoresistance of cancer cells through upregulation of ABCB1 and metabolic reprogramming.
Since ERRγ is upregulated in resistant cells, targeted inhibition of ERRγ can increase in vitro and in vivo sensitivity to chemotherapy. In hormone therapy of breast cancer cells, ERRγ is upregulated during the acquisition of TAM resistance in estrogen receptor-positive (ER+) breast cancer cells, thus overexpression of ERRγ is sufficient to induce TAM resistance 18 via cooperation with proline, glutamic acid and leucine rich protein 1 (PELP1) to inhibit TAM-mediated cell death 35. ERRγ target genes are poor prognostic factors in TAM-treated breast cancer 36. As to cancer progression, the role of ERRγ seems to be paradoxical. ERRγ acts as a tumor suppressor in gastric cancer by directly targeting the Wnt signaling pathway 37. Conversely, ERRγ is upregulated in liver cancer and exerts oncogenic potential by suppressing p21 and p27 38. The diverse roles of ERRγ is likely dependent on cell context and its functional interactions with cell-specific transcription factors and co-regulators. Our present study revealed that essential roles of ERRγ in chemoresistance of cancer cells. Nowadays, GSK5182, the inverse agonist of ERRγ, has been reported to inhibit the biological functions of ERRγ in cardiac hypertrophy 39, iron homeostasis 40, and cancer cell proliferation 41. The therapeutic potential of GSK5182 on cancer chemoresistance needs further investigations.
Continuing our finding that ERRγ interacts with p65 to trigger the transcription and expression of ABCB1, we identified two ERREs in the promoter region of ABCB1 and proved that both ERREs are involved in ERRγ-regulated expression of P-gp. NF-κB appears to play a dual role in the regulation of ABCB1 42. It can bind to -167 and -158 of the ABCB1 promoter to activate its transcription in liver cancer cells 43. In our study, the inhibitor (BAY) of NF-κB can attenuate ERRγ-induced transcription of ABCB1, potentially attributed to BAY-abrogated NF-κB binding to DNA 44 and thus reducing ERRγ/p65 association with the ABCB1 promoter. Our results also revealed an essential role of NF-κB in ERRγ-induced expression of ABCB1 and chemoresistance, which is further supported by clinical data confirming that expression of ABCB1 and ERRγ was positively correlated in liver cancer tissues.
Metabolic reprogramming is one of the hallmarks for cancer cell growth and progression, as well as resistance to chemotherapy 45. We found that chemoresistant cells reprogram metabolic pathways without affecting glycolysis, mitochondrial mass, and PDH activities but increasing ATP generation and OCR through acceleration of FAO. Recent evidence underscores the idea that FAO, also called β-oxidation, is an important energy resource required for cancer cell growth, survival, and metastasis 13, 46. Inhibition of FAO is identified as a new therapeutic approach for MYC-overexpressing triple-negative breast cancer (TNBC) 8. Some studies indicate that FAO is able to support breast cancer stem cell self-renewal and is a characteristic of chemoresistant cancer cells 10. The upregulation of FAO may thus confer the chemoresistance through maximizing ATP production, decrease intracellular ROS, and eventually protect cancer cells from death 13, 47. Our data confirmed that targeted FAO might be helpful in overcoming chemoresistance of cancer cells.
According to our data, ERRγ-regulated Cpt1b was responsible for facilitated FAO in chemoresistant cancer cells. ERRγ plays an important role in metabolism to promote energy-generating mitochondrial functions in several energy-demanding cell types 17. Genomic studies revealed that ERRγ binds directly to and activates the transcription of hundreds of genes involved in mitochondrial OxPhos/FAO functions, including CPT1B, CPT2, ACADM, and HADHA 28, 48. Consistently, we identified the ERRE located at -671 to -661 of the CPT1B promoter is involved in ERRγ-regulated transcription and expression. Cpt1b, which is responsible for fatty acid transport into mitochondria for β-oxidation, localizes at the outer mitochondrial membrane and acts as the rate-limiting FAO enzyme 49. We found that overexpression of Cpt1b can reverse sh-ERRγ-sensitized Dox treatment and downregulation of ATP and FAO, while elevated Cpt1b expression correlates with poorer response to chemotherapy 10. Considering ERRγ cistromes may exhibit cell-type-specific features to match the metabolic profiles of individual cell types 17, the roles of ERRγ/Cpt1b axis-regulated metabolic reprogramming in chemoresistance of other cancers will need more studies.
Intriguingly, we found that m6A was upregulated in chemoresistant cells and facilitated the splicing of precursor of ERSSG mRNA to elevate its expression. The roles of mRNA modification in controlling the cancer progression have just begun to be studied. Our recent study indicated that m6A can trigger the epithelial to mesenchymal transition of cancer cells via triggering the translation of Snail 50. In the present study, we showed that knockdown of Mettl3 can restore the chemosensitivity of HepG2/ADR cells, which is consistent with recent study that Mettl3 can promote the chemo- and radioresistance of pancreatic cancer cells 51. m6A can regulate the all stages in the life cycle of RNA including RNA processing, nuclear export and translation modulation 31, 52, 53. It has been reported that splicing regulators and m6A “reader” HNRNPC can regulate the splicing of target mRNAs in a m6A switch regulated manner 54. Whether HNRNPC is involved in Mettl3 and chemoresistance triggered splicing of ESRRG precursor mRNA need further studies.
In conclusion, we identified a key role of ERRγ in chemoresistance of cancer cells via upregulation of ABCB1 and facilitation of FAO. We further uncovered novel mechanisms for ERRγ regulated transcription of ABCB1, revealed that Cpt1b mediated FAO is essential for chemoresistance, and found that m6A can trigger the cleave of precursor mRNA of ESRRG to decrease chemosensitivity. Our results provided a potent target that may serve as a predictive marker of chemotherapy and as an effective target for overcome chemoresistance.
Supplementary Material
Supplementary figures, tables, materials and methods.
Acknowledgments
We thank Prof Feng Liu and Prof Junjiu Huang at Sun Yat-sen University for experimental skills and instrumental help.
Consent for publication
Written informed consent for publication was obtained from all participants.
Funding
This research was supported by the National Natural Science Foundation of China (Grant Nos. 81973343, 81673454, 81672608, 81871994, and 81701834), the Fundamental Research Funds for the Central Universities (Sun Yat-sen University) (Nos.19ykpy130 and 19ykzd24), the Guangdong Provincial Key Laboratory of Chiral Molecule and Drug Discovery (2019B030301005), the Guangdong Provincial Key Laboratory of Construction Foundation (No. 2017B030314030), the Guangdong Natural Science Foundation (2019B151502063), and the Guangzhou Science and Technology Planning Program (201902020018). CM Chiang's research was supported by US National Institutes of Health (CA103867), Cancer Prevention Research Institute of Texas (RP180349 and RP190077) and the Welch Foundation (I-1805).
Author Contributions
Conception and design: Hongsheng Wang, Weiling He, Long Wu, Zhuojia Chen.
Acquisition of data: Zhuojia Chen, Long Wu, Xinyao Lin, Jiawang Zhou, Lichen Ge, Yanxi Peng, Hui Huang.
Analysis and interpretation of data: Hongsheng Wang, Zhuojia Chen, Cheng-Ming Chiang, Feng Liu.
Writing, review, and/or revision of the manuscript: Hongsheng Wang, Wei Ling He, Zhuojia Chen, Cheng-Ming Chiang.
Abbreviations
- ABC
ATP-binding cassette
- ABCB1
ATP binding cassette subfamily B member 1
- Dox
doxorubicin
- ECAR
extracellular acidification rate
- ER
estrogen receptor
- ERRγ
estrogen related receptor γ
- ERRE
ERR response elements
- ETX
etomoxir
- FA
fatty acid
- FAO
fatty acid oxidation
- LBD
ligand-binding domain
- m6A
N6-methyladenosine
- MDR
multidrug resistance
- TAM
tamoxifen
- TCA
tricarboxylic acid cycle
- TNBC
triple-negative breast cancer
- OCR
oxygen consumption rate
- OxPhos
oxidative phosphorylation
- P-gp
P-glycoprotein
- PDH
pyruvate dehydrogenase
- PELP1
proline, glutamic acid and leucine rich protein 1
- Tax
taxol
- WT
wide-type
References
- 1.Holohan C, Van Schaeybroeck S, Longley DB, Johnston PG. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–26. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 2.Prasad V, De Jesus K, Mailankody S. The high price of anticancer drugs: origins, implications, barriers, solutions. Nat Rev Clin Oncol. 2017;14:381–90. doi: 10.1038/nrclinonc.2017.31. [DOI] [PubMed] [Google Scholar]
- 3.Robey RW, Pluchino KM, Hall MD, Fojo AT, Bates SE, Gottesman MM. Revisiting the role of ABC transporters in multidrug-resistant cancer. Nat Rev Cancer. 2018;18:452–64. doi: 10.1038/s41568-018-0005-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhao J. Cancer stem cells and chemoresistance: The smartest survives the raid. Pharmacol Ther. 2016;160:145–58. doi: 10.1016/j.pharmthera.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jo Y, Choi N, Kim K, Koo HJ, Choi J, Kim HN. Chemoresistance of Cancer Cells: Requirements of Tumor Microenvironment-mimicking In Vitro Models in Anti-Cancer Drug Development. Theranostics. 2018;8:5259–75. doi: 10.7150/thno.29098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nigam SK. What do drug transporters really do? Nat Rev Drug Discov. 2015;14:29–44. doi: 10.1038/nrd4461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gottesman MM, Lavi O, Hall MD, Gillet JP. Toward a Better Understanding of the Complexity of Cancer Drug Resistance. Annu Rev Pharmacol Toxicol. 2016;56:85–102. doi: 10.1146/annurev-pharmtox-010715-103111. [DOI] [PubMed] [Google Scholar]
- 8.Camarda R, Zhou AY, Kohnz RA, Balakrishnan S, Mahieu C, Anderton B. et al. Inhibition of fatty acid oxidation as a therapy for MYC-overexpressing triple-negative breast cancer. Nat Med. 2016;22:427–432. doi: 10.1038/nm.4055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sounni NE, Cimino J, Blacher S, Primac I, Truong A, Mazzucchelli G. et al. Blocking Lipid Synthesis Overcomes Tumor Regrowth and Metastasis after Antiangiogenic Therapy Withdrawal. Cell Metab. 2014;20:280–94. doi: 10.1016/j.cmet.2014.05.022. [DOI] [PubMed] [Google Scholar]
- 10.Wang T, Fahrmann JF, Lee H, Li YJ, Tripathi SC, Yue C. et al. JAK/STAT3-Regulated Fatty Acid beta-Oxidation Is Critical for Breast Cancer Stem Cell Self-Renewal and Chemoresistance. Cell Metab. 2018;27:136–50. doi: 10.1016/j.cmet.2017.11.001. e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhao Y, Butler EB, Tan M. Targeting cellular metabolism to improve cancer therapeutics. Cell Death Dis. 2013;4:e532. doi: 10.1038/cddis.2013.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.He W, Liang B, Wang C, Li S, Zhao Y, Huang Q. et al. MSC-regulated lncRNA MACC1-AS1 promotes stemness and chemoresistance through fatty acid oxidation in gastric cancer. Oncogene. 2019;38:4637–54. doi: 10.1038/s41388-019-0747-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carracedo A, Cantley LC, Pandolfi PP. Cancer metabolism: fatty acid oxidation in the limelight. Nat Rev Cancer. 2013;13:227–32. doi: 10.1038/nrc3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE. et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18:1350–8. doi: 10.1038/nm.2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deblois G, Giguere V. Oestrogen-related receptors in breast cancer: control of cellular metabolism and beyond. Nat Rev Cancer. 2013;13:27–36. doi: 10.1038/nrc3396. [DOI] [PubMed] [Google Scholar]
- 16.Chen Y, Zhang K, Li Y, Guo R, Zhang K, Zhong G. et al. Oestrogen-related receptor alpha mediates chemotherapy resistance of osteosarcoma cells via regulation of ABCB1. J Cell Mol Med. 2019;23:2115–24. doi: 10.1111/jcmm.14123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Misra J, Kim DK, Choi HS. ERRgamma: a Junior Orphan with a Senior Role in Metabolism. Trends Endocrinol Metab. 2017;28:261–72. doi: 10.1016/j.tem.2016.12.005. [DOI] [PubMed] [Google Scholar]
- 18.Riggins RB, Lan JP, Zhu Y, Klimach U, Zwart A, Cavalli LR. et al. ERRgamma mediates tamoxifen resistance in novel models of invasive lobular breast cancer. Cancer Res. 2008;68:8908–17. doi: 10.1158/0008-5472.CAN-08-2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Audet-Walsh E, Yee T, McGuirk S, Vernier M, Ouellet C, St-Pierre J. et al. Androgen-Dependent Repression of ERRgamma Reprograms Metabolism in Prostate Cancer. Cancer Res. 2017;77:378–89. doi: 10.1158/0008-5472.CAN-16-1204. [DOI] [PubMed] [Google Scholar]
- 20.Eichner LJ, Perry MC, Dufour CR, Bertos N, Park M, St-Pierre J. et al. miR-378( *) mediates metabolic shift in breast cancer cells via the PGC-1beta/ERRgamma transcriptional pathway. Cell Metab. 2010;12:352–61. doi: 10.1016/j.cmet.2010.09.002. [DOI] [PubMed] [Google Scholar]
- 21.Huppunen J, Aarnisalo P. Dimerization modulates the activity of the orphan nuclear receptor ERRgamma. Biochem Biophys Res Commun. 2004;314:964–70. doi: 10.1016/j.bbrc.2003.12.194. [DOI] [PubMed] [Google Scholar]
- 22.Hentschke M, Susens U, Borgmeyer U. Domains of ERR gamma that mediate homodimerization and interaction with factors stimulating DNA binding. Eur J Biochem. 2002;269:4086–97. doi: 10.1046/j.1432-1033.2002.03102.x. [DOI] [PubMed] [Google Scholar]
- 23.Labialle S, Gayet L, Marthinet E, Rigal D, Baggetto LG. Transcriptional regulators of the human multidrug resistance 1 gene: recent views. Biochem Pharmacol. 2002;64:943–8. doi: 10.1016/s0006-2952(02)01156-5. [DOI] [PubMed] [Google Scholar]
- 24.Requenez-Contreras JL, Lopez-Castillejos ES, Hernandez-Flores R, Moreno-Eutimio MA, Granados-Riveron JT, Martinez-Ruiz GU. et al. MiR-138 indirectly regulates the MDR1 promoter by NF-kappaB/p65 silencing. Biochem Biophys Res Commun. 2017;484:648–55. doi: 10.1016/j.bbrc.2017.01.168. [DOI] [PubMed] [Google Scholar]
- 25.Lukey MJ, Katt WP, Cerione RA. Targeting Therapy Resistance: When Glutamine Catabolism Becomes Essential. Cancer Cell. 2018;33:795–7. doi: 10.1016/j.ccell.2018.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wicki A, Mandala M, Massi D, Taverna D, Tang HF, Hemmings BA. et al. Acquired Resistance to Clinical Cancer Therapy: A Twist in Physiological Signaling. Physiol Rev. 2016;96:805–29. doi: 10.1152/physrev.00024.2015. [DOI] [PubMed] [Google Scholar]
- 27.DeBerardinis RJ, Lum JJ, Hatzivassiliou G, Thompson CB. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 2008;7:11–20. doi: 10.1016/j.cmet.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Zhao J, Lupino K, Wilkins BJ, Qiu C, Liu J, Omura Y. et al. Genomic integration of ERRgamma-HNF1beta regulates renal bioenergetics and prevents chronic kidney disease. Proc Natl Acad Sci U S A. 2018;115:E4910–E9. doi: 10.1073/pnas.1804965115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Tan Z, Xiao L, Tang M, Bai F, Li J, Li L. et al. Targeting CPT1A-mediated fatty acid oxidation sensitizes nasopharyngeal carcinoma to radiation therapy. Theranostics. 2018;8:2329–47. doi: 10.7150/thno.21451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou S, Bai ZL, Xia D, Zhao ZJ, Zhao R, Wang YY. et al. FTO regulates the chemo-radiotherapy resistance of cervical squamous cell carcinoma (CSCC) by targeting beta-catenin through mRNA demethylation. Mol Carcinog. 2018;57:590–7. doi: 10.1002/mc.22782. [DOI] [PubMed] [Google Scholar]
- 31.Roundtree IA, Evans ME, Pan T, He C. Dynamic RNA Modifications in Gene Expression Regulation. Cell. 2017;169:1187–200. doi: 10.1016/j.cell.2017.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.den Ouden D, van den Heuvel M, Schoester M, van Rens G, Sonneveld P. In vitro effect of GF120918, a novel reversal agent of multidrug resistance, on acute leukemia and multiple myeloma cells. Leukemia. 1996;10:1930–6. [PubMed] [Google Scholar]
- 33.Deblois G, St-Pierre J, Giguere V. The PGC-1/ERR signaling axis in cancer. Oncogene. 2013;32:3483–90. doi: 10.1038/onc.2012.529. [DOI] [PubMed] [Google Scholar]
- 34.Vargas G, Bouchet M, Bouazza L, Reboul P, Boyault C, Gervais M. et al. ERRalpha promotes breast cancer cell dissemination to bone by increasing RANK expression in primary breast tumors. Oncogene. 2019;38:950–64. doi: 10.1038/s41388-018-0579-3. [DOI] [PubMed] [Google Scholar]
- 35.Girard BJ, Regan Anderson TM, Welch SL, Nicely J, Seewaldt VL, Ostrander JH. Cytoplasmic PELP1 and ERRgamma protect human mammary epithelial cells from Tam-induced cell death. PLoS One. 2015;10:e0121206. doi: 10.1371/journal.pone.0121206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Madhavan S, Gusev Y, Singh S, Riggins RB. ERRgamma target genes are poor prognostic factors in Tamoxifen-treated breast cancer. J Exp Clin Cancer Res. 2015;34:45. doi: 10.1186/s13046-015-0150-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang MH, Choi H, Oshima M, Cheong JH, Kim S, Lee JH. et al. Estrogen-related receptor gamma functions as a tumor suppressor in gastric cancer (vol 9, 1920, 2018) Nat Commun. 2018;9:1920. doi: 10.1038/s41467-018-04244-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kim JH, Choi YK, Byun JK, Kim MK, Kang YN, Kim SH. et al. Estrogen-related receptor gamma is upregulated in liver cancer and its inhibition suppresses liver cancer cell proliferation via induction of p21 and p27. Exp Mol Med. 2016;48:e213. doi: 10.1038/emm.2015.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kwon DH, Eom GH, Kee HJ, Nam YS, Cho YK, Kim DK. et al. Estrogen-related receptor gamma induces cardiac hypertrophy by activating GATA4. J Mol Cell Cardiol. 2013;65:88–97. doi: 10.1016/j.yjmcc.2013.09.011. [DOI] [PubMed] [Google Scholar]
- 40.Kim DK, Jeong JH, Lee JM, Kim KS, Park SH, Kim YD. et al. Inverse agonist of estrogen-related receptor gamma controls Salmonella typhimurium infection by modulating host iron homeostasis. Nat Med. 2014;20:419–24. doi: 10.1038/nm.3483. [DOI] [PubMed] [Google Scholar]
- 41.Samimi H, Haghpanah V, Irani S, Arefian E, Sohi AN, Fallah P. et al. Transcript-level regulation of MALAT1-mediated cell cycle and apoptosis genes using dual MEK/Aurora kinase inhibitor "BI-847325" on anaplastic thyroid carcinoma. Daru. 2019;27:1–7. doi: 10.1007/s40199-018-0231-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen KG, Sikic BI. Molecular Pathways: Regulation and Therapeutic Implications of Multidrug Resistance. Clin Cancer Res. 2012;18:1863–9. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhou G, Kuo MT. NF-kappaB-mediated induction of mdr1b expression by insulin in rat hepatoma cells. J Biol Chem. 1997;272:15174–83. doi: 10.1074/jbc.272.24.15174. [DOI] [PubMed] [Google Scholar]
- 44.Xue W, Meylan E, Oliver TG, Feldser DM, Winslow MM, Bronson R. et al. Response and resistance to NF-kappaB inhibitors in mouse models of lung adenocarcinoma. Cancer Discov. 2011;1:236–47. doi: 10.1158/2159-8290.CD-11-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guerra F, Arbini AA, Moro L. Mitochondria and cancer chemoresistance. Biochim Biophys Acta Bioenerg. 2017;1858:686–99. doi: 10.1016/j.bbabio.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 46.Carracedo A, Weiss D, Leliaert AK, Bhasin M, de Boer VC, Laurent G. et al. A metabolic prosurvival role for PML in breast cancer. J Clin Invest. 2012;122:3088–100. doi: 10.1172/JCI62129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pike LS, Smift AL, Croteau NJ, Ferrick DA, Wu M. Inhibition of fatty acid oxidation by etomoxir impairs NADPH production and increases reactive oxygen species resulting in ATP depletion and cell death in human glioblastoma cells. Biochim Biophys Acta. 2011;1807:726–34. doi: 10.1016/j.bbabio.2010.10.022. [DOI] [PubMed] [Google Scholar]
- 48.Pei L, Mu Y, Leblanc M, Alaynick W, Barish GD, Pankratz M. et al. Dependence of hippocampal function on ERRgamma-regulated mitochondrial metabolism. Cell Metab. 2015;21:628–36. doi: 10.1016/j.cmet.2015.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Qu Q, Zeng F, Liu X, Wang QJ, Deng F. Fatty acid oxidation and carnitine palmitoyltransferase I: emerging therapeutic targets in cancer. Cell Death Dis. 2016;7:e2226. doi: 10.1038/cddis.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lin X, Chai G, WU Y, Li J, Chen F, Liu J. et al. RNA m6A methylation regulates the epithelial mesenchymal transition of cancer cells and translation of Snail. Nat Commun. 2019;10:e2065. doi: 10.1038/s41467-019-09865-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Taketo K, Konno M, Asai A, Koseki J, Toratani M, Satoh T. et al. The epitranscriptome m6A writer METTL3 promotes chemo- and radioresistance in pancreatic cancer cells. Int J Oncol. 2018;52:621–9. doi: 10.3892/ijo.2017.4219. [DOI] [PubMed] [Google Scholar]
- 52.Zhao BS, Roundtree IA, He C. Post-transcriptional gene regulation by mRNA modifications. Nat Rev Mol Cell Biol. 2017;18:31–42. doi: 10.1038/nrm.2016.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Li XC, Jin F, Wang BY, Yin XJ, Hong W, Tian FJ. The m6A demethylase ALKBH5 controls trophoblast invasion at the maternal-fetal interface by regulating the stability of CYR61 mRNA. Theranostics. 2019;9:3853–65. doi: 10.7150/thno.31868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Alarcon CR, Goodarzi H, Lee H, Liu XH, Tavazoie S, Tavazoie SF. HNRNPA2B1 Is a Mediator of m(6)A-Dependent Nuclear RNA Processing Events. Cell. 2015;162:1299–308. doi: 10.1016/j.cell.2015.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary figures, tables, materials and methods.