Abstract
Background
Elderly patients often suffer from postoperative cognitive impairment which increases mortality, morbidity, and the economic burden. However, how continuous femoral nerve block (cFNB) influence the incidence of postoperative cognitive dysfunction (POCD) has never been reported. This study tried to explore how cFNB affects the incidence of POCD among low-risk and high-risk patients with femoral neck fractures.
Material/Methods
We conducted a retrospective propensity score-matched study and allocated matched patients (n=172) with femoral neck fractures into the cFNB group (n=86) and the control group (n=86). Demographical and clinical data were collected and compared, including the visual analog scale (VAS) score, the morphine consumption, and the POCD incidence. Subgroup analysis of high-risk patients (Mini-Cog score ≤2) and low-risk patients (Mini-Cog score ≥3) was also carried out.
Results
After matching, baseline characteristics of 2 groups were comparable between the 2 groups (all P>0.05). Compared with the control group, the cFNB group had significantly lower visual analog scale (VAS) score and morphine consumption in the postoperative 3 days (P<0.05). For high-risk patients, the Kaplan-Meier survival curve suggested that the incidence of POCD the cFNB group was significantly lower than the control group (P=0.005), without statistical difference for total or low-risk patients (P>0.05). Multivariate Cox hazard regression analysis showed that the adoption of cFNB conferred a protective effect on POCD (HR=0.556, 95% CI 0.316–0.981, P=0.043).
Conclusions
For patients undergoing femoral neck fracture surgery, perioperative cFNB administration is useful in decreasing the incidence of POCD, especially for high-risk patients with a Mini-Cog score equal to or less than 2 points.
MeSH Keywords: Femoral Neck Fractures, Femoral Nerve, Mild Cognitive Impairment, Propensity Score
Background
Postoperative cognitive dysfunction (POCD) is a condition when the memory and intellectual function of the patient are impaired by the process of anesthesia and operation, which manifests as a reduction in neuropsychological test scores after surgery [1,2]. POCD is a common surgical complication in orthopedics, including total knee arthroplasty (TKA) [3] and total hip arthroplasty (THR) [4]. Femoral neck fracture is a common injury in elderly patients, and typically requires orthopedic surgery, such as internal fixation, arthroplasty, and hip replacement [5], which is a risk factor for POCD [6]. The occurrence of POCD greatly increases the mortality, morbidity, and medical burden of patients with femoral neck fractures. Previous studies have confirmed the risk factors for POCD, including the modifiable and non-modifiable ones [7], which has laid the foundation for multiple preemptive measures for preventing POCD.
Different tools have been developed to assess the cognitive function of patients and to stratify the risk for POCD, including the Montreal Cognitive Assessment (MoCA), Mini-Mental State Examination (MMSE), and Mini-Cog© score [8]. The Mini-Cog score is a brief and validated tool used in the evaluation of cognitive function of elderly patients undergoing elective surgery [9] which demonstrates good performance in predicting postoperative complications [10]; while the MMSE has been widely used as the diagnostic tool for POCD [2]. A study showed that preemptive application of continuous femoral nerve block (cFNB) could enhance analgesia and decrease the incidence of POCD, which assumes that relief of pain could reduce the sensitization of pain receptors [11]. However, there is rarely evidence investigating the influence of cFNB on the onset of POCD among patients undergoing femoral neck fracture surgery. In this study, we retrospectively compared the analgesia effect and POCD incidence between patients receiving cFNB and control patients by using propensity score match. In addition, a subgroup analysis of patients of different risk levels and a Cox regression analysis was also conducted.
Material and Methods
Study population
We retrospectively collected the clinical data of patients meeting the following criteria: 1) patient diagnosed with femoral neck fracture and received elective surgeries; 2) patient older than 65 years old; 30 American Society of Anesthesiologists (ASA) classification of the patient resulted of ASA grade I or II; 4) patient complete data including MMSE score and Mini-Cog score. Exclusion criteria: 1) patients not adherent to or dropped from MMSE evaluation; and 2) patients with severe postoperative delirium. From June 2012 to June 2018, a total of 468 patients diagnosed with femoral neck fractures and received surgeries in our hospital, among whom 326 patients fit the criteria and were recruited in this study. The propensity score match excluded 154 patients, while the other 172 patients remained in the study, who were classified into the cFNB group and the control group (patient-controlled intravenous analgesia, PCIA) according to the treatment type. Figure 1 showed the study design.
Figure 1.

Flow chart of study design.
Demographic characteristics from the hospital’s electronic database were collected for analysis. The signed informed consent of the patients was also acquired at admission. There was no patient demonstrating symptoms of cognitive dysfunction and all patients were aware when signed the consent. All authors had no access to information that could identify individual participants during or after data collection. The Medical Ethics Committee of The Third Affiliated Hospital of Wenzhou Medical University approved this study.
Anesthesia
All patients were given intravenous anesthesia; 1 mg penehyclidine hydrochloride was injected intramuscularly before the anesthetic. A 20-gauge intravascular catheter was placed in the basilica vein, and routine monitors including noninvasive blood pressure, peripheral capillary oxygen saturation, bispectral index (BIS), and electrocardiogram were also applied. Induction of anesthesia was completed with midazolam (0.05–0.1 mg/kg), sufentanil (0.3–0.4 μg/kg), etomidate (0.1–0.2 mg/kg), and cis-atracurium (0.15–0.2 mg/kg), followed by application of a laryngeal mask airway. The parameters were usually set as follows: tidal volume 6–8 mL/kg, oxygen flow 1.5 L/minute, PETCO2 35–45 mm Hg and respiratory rate (RR) 12–14 times/minute. Anesthesia was maintained with a continuous infusion of propofol (Sichuan Guorui Pharmaceutical Co., Ltd., No. H20040079, 4–8 mg/kg/hour) and remifentanil (Yichang Renfu Pharmaceutical Co., Ltd., No. H20030197, 0.1–0.2 μg/kg/minute), and muscle relaxation was maintained with intermittent injection of cis-atracurium (Jiangsu Hengrui Pharmaceutical Co., Ltd., No. H20060869, 0.05 mg/kg). During the surgery, the BIS was kept at 40–60, and incidents including hypotension and bradycardia were dealt with using the appropriate drugs. Before the completion of the surgery, propofol, remifentanil, and cis-atracurium were stopped, and intravenous injection of neostigmine (0.02 mg/kg) and atropine (0.01 mg/kg) were conducted to relieve the residual effects of the muscle relaxants.
Perioperative analgesia
As previously described [11], supine position was placed for patients of cFNB group the with the target leg a little abducted. Mark entry point 1 cm lateral to the femoral artery. A Braun nerve stimulator (pulse duration 0.1 ms, stimulation frequency 2 Hz, initial current 1 mA, threshold current 0.3 mA) was used for positioning the entry. Injection of 15 mL mixture of 2% lidocaine and 0.75% ropivacaine into the previously marked point when a current <0.3 mA could still induce quadricep contraction and patella fluctuation was conducted. The needle was slowly inserted to a depth of 2 mm at the entry point at a cephalic angle of 30° and then the core was replaced with a 20-gauge cannula. Next, the mixture of 5 mL lidocaine/ropivacaine was injected for another time. We used a disposable patient-controlled analgesia pump (containing 0.75% ropivacaine 25 mL+sufentanil 50 μg) connected to the cannula, which lasted for 50 hours following surgery.
For patients given traditional PCIA, the disposable patient-controlled analgesia pump (containing bolus dose, oxycodone 1 mg and nefopam 1 mg) was connected to the deep venous catheter. Intravenous morphine injection and oral COX-2 inhibitors, including parecoxib sodium or celecoxib for pain management were given for all patients.
Data collection
Demographic data of age, gender, body mass index (BMI), education, as well as clinical characteristics including ASA grade, comorbidities, Garden classification, and surgery type were all collected from the hospital’s electronic database.
We recorded the visual analog scale (VAS) score and postoperative usage dose of morphine. We also made the VAS score and dose of morphine evaluation at 9 time points (post-anesthesia care unit [PACU] on first day, first evening, second night, second day, second evening, third night, third day, third evening).
Cognitive function was evaluated by MMSE and Mini-Cog score. MMSE was measured prior to surgery and every day after the surgery, and a decrease of 2 points compared to the baseline level was considered as POCD. Mini-Cog score was evaluated before the surgery to divide the patients into a high-risk subgroup (Mini-Cog score ≤2) and low-risk subgroup (Mini-Cog score ≥3).
Statistical analysis
We used IBM SPSS Statistics, version 19.0 (SPSS, Inc., Armonk, NY, USA) for statistical analysis. Normality distribution test of the variables was conducted first to check the variable distribution condition. When meeting the normal distribution, the continuous variables were described as mean±standard deviation, while not they were described as median (lower quarter, upper quarter). The categorical variables were presented as proportions.
Comparison of continuous variables of different groups was conducted with independent sample t-test. We used the chi-square test to compare differences between categorical variables. Multivariable logistic regression was used in propensity score calculation after considering demographic and clinical variables. The “greedy match” method were used for the match of patients with the closest propensity. Following the propensity score match, a Student’s t-test for paired samples and a McNemar test were used for comparison of demographic and clinical date. Repeated measurement analysis of variance was used for comparison of VAS and morphine consumption of different time point.
As for the survival analysis, Cox hazard ratios (HR) regression analysis was conducted for the covariates and the incidence of POCD. The POCD incidence in the cFNB group and the control group was compared via Kaplan-Meier survival curves and log rank tests. A P-value less than 0.05 was considered statistically significant.
Results
Demographic and clinical characteristics
There were obvious differences between the cFNB group and the control group in age (P<0.001), BMI (P<0.001), cardiovascular diseases (P=0.010), and Mini-Cog score (P=0.001) before being matched, as shown in Table 1. However, the propensity score match eliminated these differences. After the match, demographic and clinical data was similar without statistical significance between the 2 groups (all P>0.05).
Table 1.
Demographical and clinical characteristics of patients undergoing femoral neck fracture surgery.
| Variables | Before the match | After the match | ||||
|---|---|---|---|---|---|---|
| PCIA (n=184) | cFNB (n=142) | P value | PCIA (n=86) | cFNB (n=86) | P value | |
| Demographics | ||||||
| Age (y) | 68.8±5.7 | 71.8±6.7 | <0.001 | 70.0±6.4 | 70.5±6.2 | 0.672 |
| Gender (% Male) | 51 (27.2%) | 41 (28.9%) | 0.804 | 28 (32.6%) | 27 (31.4%) | 1.000 |
| BMI (kg/m−2) | 26.8±4.7 | 25.6±4.3 | <0.001 | 25.7±3.6 | 25.3±4.2 | 0.488 |
| Education | 0.943 | 0.573 | ||||
| Illiteracy | 47 (25.5%) | 37 (26.1%) | 22 (25.6%) | 22 (25.6%) | ||
| Elementary school | 59 (32.1%) | 47 (33.1%) | 32 (37.2%) | 25 (29.1%) | ||
| High school | 52 (28.3%) | 36 (25.4%) | 18 (20.9%) | 25 (29.1%) | ||
| College or higher | 26 (14.1%) | 22 (15.5%) | 14 (16.3%) | 14 (16.3%) | ||
| ASA grade | 0.595 | 0.358 | ||||
| I | 44 (23.9%) | 30 (21.1%) | 22 (25.6%) | 16 (18.6%) | ||
| II | 140 (76.1%) | 112 (78.9%) | 64 (74.4%) | 70 (81.4%) | ||
| Comorbidities | ||||||
| Hypertension | 79 (42.9%) | 60 (42.3%) | 0.911 | 31 (36.0%) | 40 (46.5%) | 0.215 |
| Diabetes | 52 (28.3%) | 41 (28.9%) | 0.902 | 26 (30.2%) | 24 (27.9%) | 0.867 |
| Cerebrovascular Disease | 13 (7.1%) | 13 (9.2%) | 0.540 | 7 (8.1%) | 10 (11.6%) | 0.611 |
| Cardiovascular disease | 34 (18.5%) | 12 (8.5%) | 0.010 | 15 (17.4%) | 7 (8.1%) | 0.108 |
| Renal dysfunction | 8 (4.3%) | 7 (4.9%) | 0.797 | 4 (4.7%) | 5 (5.8%) | 1.000 |
| Garden classification | ||||||
| I | 62 (33.7%) | 37 (26.1%) | 0.082 | 19 (22.1%) | 27 (31.4%) | 0.145 |
| II | 78 (42.4%) | 53 (37.3%) | 42 (48.8%) | 42 (48.8%) | ||
| III | 20 (10.9%) | 21 (14.8%) | 12 (14.0%) | 4 (4.7%) | ||
| IV | 24 (13.0%) | 31 (21.8%) | 13 (15.1%) | 13 (15.1%) | ||
| Surgery type | 0.086 | 0.133 | ||||
| Dynamic hip screw | 53 (32.5%) | 35 (24.6%) | 27 (31.4%) | 24 (27.9%) | ||
| Dynamic locking plate | 56 (18.8%) | 36 (25.4%) | 22 (25.6%) | 30 (34.9%) | ||
| Hemi-arthroplasty | 35 (48.7%) | 22 (15.5%) | 15 (17.4%) | 6 (7.0%) | ||
| Total hip replacement | 40 (48.7%) | 49 (34.5%) | 22 (25.6%) | 26 (30.2%) | ||
| Mini-Cog score | 0.001 | 0.176 | ||||
| ≤2 points | 36 (19.6%) | 52 (36.6%) | 20 (23.3%) | 29 (33.7%) | ||
| ≥3 points | 148 (80.4%) | 90 (63.4%) | 66 (76.7%) | 57 (66.3%) | ||
PCIA – patient-controlled intravenous analgesia; cFNB – continuous femoral nerve block; BMI – Body mass index; ASA – American Society of Anesthesiologists.
VAS score and opioid consumption
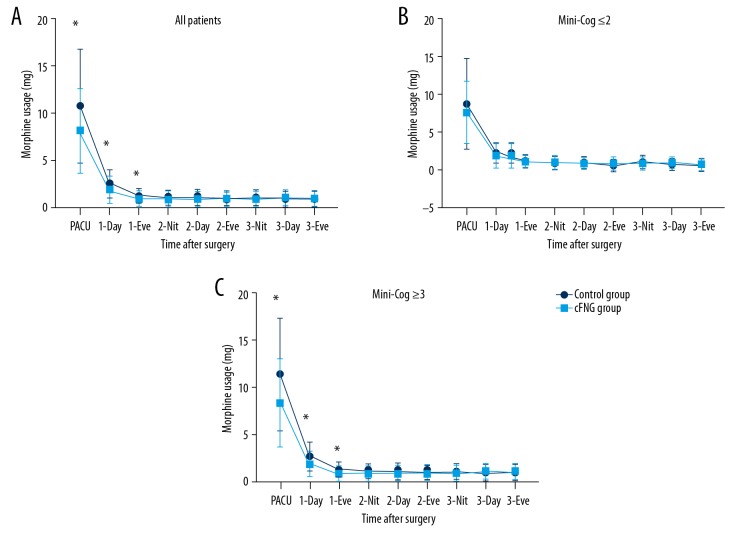
As demonstrated in Figure 2, the mean VAS score of the cFNB group was much lower than the control group during all 3 days after the surgery (P<0.05). Figure 3 shows that the morphine consumption of the cFNB group was also lower than the control group while in the PACU and during the first day after the surgery (P<0.05). However, morphine consumption was similar between the 2 groups on day 2 and day 3 (P>0.05).
Figure 2.
(A–C) Postoperative VAS score of cFNB group and Control group. PACU, post-anesthesia care unit; Eve, evening; Nit, night; * P<0.05. VAS – visual analog scale; cFNB – continuous femoral nerve block; PACU – post-anesthesia care unit.
Figure 3.
Postoperative morphine consumption of cFNB group and Control group. * P<0.05. cFNB – continuous femoral nerve block; PACU – post-anesthesia care unit; Eve – evening; Nit – night.
Incidence of POCD
The incidence of POCD was assessed within 7 days after the surgery by evaluating the MMSE score. When comparing the cFNB group and the control group, the Kaplan-Meier curve (Figure 4) and the log-rank test showed that no significant difference was found in the incidence of POCD (P=0.102). In terms of subgroup analysis for patients with different Mini-Cog score, the results were different. For patients with Mini-Cog score ≥3, the incidence of POCD was comparable between the cFNB group and the control group (Figure 5). However, Figure 6 demonstrated that the incidence of POCD for the patients with Mini-Cog score ≤2 in the cFNB group was significantly lower (P=0.005).
Figure 4.

Kaplan-Meier curve of POCD in total. POCD – postoperative cognitive dysfunction.
Figure 5.

Kaplan-Meier curve of POCD in low risk patients (Mini-Cog ≥3). POCD – postoperative cognitive dysfunction.
Figure 6.

Kaplan-Meier curve of POCD in high risk patients (Mini-Cog ≤2). POCD – postoperative cognitive dysfunction.
Cox regression analysis
The results of Cox regression analysis are shown in Tables 2 and 3. We conducted the univariate regression analysis and the results showed that age, Garden classification, and surgery type were statistically significant risk factors. These factors were included in the multivariate regression model together with Mini-Cog score and cFNB use. The final model showed that age, hemi-arthroplasty, and total hip replacement (compared to dynamic hip screw) were independent risk factors, while the use of cFNB was the protecting factor for the incidence of POCD (HR 0.556 95% CI 0.316–0.981, P=0.043).
Table 2.
Univariate Cox regression analysis between covariates and the incidence of POCD.
| Covariates | HR | 95%CI | P value |
|---|---|---|---|
| Age | 1.069 | 1.021–1.118 | 0.004 |
| Gender (Male to Female) | 0.896 | 0.500–1.607 | 0.713 |
| BMI | 1.039 | 0.971–1.113 | 0.269 |
| Education | |||
| Illiteracy | – | – | – |
| Elementary school | 1.058 | 0.530–2.110 | 0.873 |
| High school | 0.642 | 0.278–1.483 | 0.299 |
| College or higher | 1.394 | 0.645–3.015 | 0.398 |
| ASA (II to I) | 1.096 | 0.587–2.045 | 0.774 |
| Hypertension | 0.971 | 0.564–1.671 | 0.916 |
| Diabetes mellitus | 1.046 | 0.583–1.875 | 0.881 |
| Cerebrovascular disease | 0.717 | 0.259–1.985 | 0.522 |
| Cardiovascular disease | 0.995 | 0.450–2.202 | 0.991 |
| Renal dysfunction | 1.063 | 0.332–3.407 | 0.917 |
| Garden classification | |||
| I | – | – | – |
| II | 1.094 | 0.491–2.434 | 0.827 |
| III | 2.383 | 0.887–6.402 | 0.085 |
| IV | 4.801 | 2.175–10.598 | <0.001 |
| Surgery type | |||
| Dynamic hip screw | – | – | – |
| Proximal femoral nail | 1.822 | 0.610–5.436 | 0.282 |
| Hemi-arthroplasty | 4.301 | 1.406–13.158 | 0.011 |
| Total hip replacement | 8.391 | 3.255–21.629 | <0.001 |
| Mini-Cog score (≥3 to ≤2) | 0.643 | 0.370–1.118 | 0.118 |
| Use of cFNB | 0.664 | 0.386–1.143 | 0.139 |
POCD – postoperative cognitive dysfunction; HR – hazard ratio; CI – confidence interval; BMI – body mass index; ASA – American Society of Anesthesiologists; cFNB – continuous femoral nerve block.
Table 3.
Multivariate Cox regression analysis between covariates and the incidence of POCD.
| Covariates | HR | 95%CI | P value |
|---|---|---|---|
| Age | 1.058 | 1.012–1.107 | 0.014 |
| Garden classification | |||
| I | – | – | – |
| II | 0.913 | 0.380–2.194 | 0.839 |
| III | 0.838 | 0.242–2.907 | 0.781 |
| IV | 1.185 | 0.423–3.323 | 0.747 |
| Surgery type | |||
| Dynamic hip screw | – | – | – |
| Proximal femoral nail | 2.032 | 0.644–6.411 | 0.226 |
| Hemi-arthroplasty | 4.974 | 1.402–17.655 | 0.013 |
| Total hip replacement | 7.656 | 2.620–22.371 | <0.001 |
| Mini-Cog score (≥3 to ≤2) | 0.601 | 0.339–1.064 | 0.081 |
| Use of cFNB | 0.556 | 0.316–0.981 | 0.043 |
POCD – postoperative cognitive dysfunction; HR – hazard ratio; CI – confidence interval; cFNB – continuous femoral nerve block.
Discussion
Ever since it was first reported in 1955 as cognitive impairment cases, POCD has gained worldwide attention for its related morbidity, increased healthcare burden and even mortality, especially in elderly patients [12]. However, the underlying mechanism of POCD remains elusive, which leads to continuous controversy over the diagnosis, prevention and treatment [13]. One proposed explanation is that receptor sensitization in the brain is induced by surgery pain [14], which lays the basis for prevention with local blockade. Several studies suggested that the femoral nerve block could interrupt the transmission of pain to the spinal cord, and hence reduce the sensitization of spinal cord and brain pain receptors, which share common pathways with cognitive function [15,16]. Intended for pain relief, cFNB has been applied in TKA surgery and has obvious effect [17]. We applied cFNB in surgery for femoral neck fractures, which are common and hazardous for the elderly [18]. Perioperative analgesia plays a critical role in femoral neck fracture treatment [19]. Our results showed that cFNB achieved a much more satisfying analgesic effect after the surgery compared to traditional PCIA. As a result, the additional use of intravenous morphine sharply decreased on the first day when cFNB was used. It has been reported that patients with hip fractures, including those patients with dementia, who received femoral nerve block had lower pain scores and required lower doses of opioids before surgery compared with those receiving conventional pain management [20], which correlated well with our study results. Another potential mechanism includes postoperative inflammation and NF-kappaB/P65 signaling pathway, which has been proven to be important in the development of POCD [21]. Ren et al. demonstrated that Ulinastatin had the effect of reducing oxidative stress as well as inflammatory response, improving neurological functions, and promoting postoperative recovery for elderly patients with femoral neck fracture after hip arthroplasty [22].
The diagnosis criteria of POCD has varied between different studies; however, all criteria required the evaluation of the cognitive function before and after the study [23]. MMSE is one of the most widely used scales in evaluating cognitive function, and a previous study defined POCD as a postoperative MMSE score >1 standard deviation below the preoperative score [24]. Deng et al. used the MMSE score >1 standard (equal to 2 points) and reported that preemptive use of cFNB could enhance the recovery of cognitive function in patients undergoing TKA surgery [11]. We also adopted the latter criteria and achieved similar results. However, we found differences between high-risk patients and low-risk patients defined by the preoperative Mini-Cog score. Compared to MMSE, Mini-Cog is more convenient and timesaving (3 to 4 minutes), and less affected by age and education level [25]. Patients with a preoperative Mini-Cog score equal to or lower than 2 point might already have underlying mild cognitive impairment (MCI) and might be easily affected by the surgery. Our study showed that in all 49 patients with a Mini-Cog score ≤2 enrolled in our study, 20 cases (40.8%) developed POCD, which was much higher than the prevalence among patients with Mini-Cog scores ≥3 (27.6%). The final Cox regression model also demonstrated that a Mini-Cog score ≤2 was a strong risk factor for POCD (HR 0.601 95% CI 0.339–1.064, P=0.081), however, without statistical significance. The preoperative evaluation made it possible to follow subgroup analysis. Although the incidence of POCD between the cFNB group and the control group didn’t differ in the total group of patients or in the subgroup of patients with Mini-Cog ≥3, it was significantly different for the subgroup of patients with Mini-Cog ≤2, which meant that the cFNB could be helpful in preventing POCD for high-risk patients. We only evaluated the short-term incidence of POCD within 7 days using survival analysis, which could avoid the influence of rehabilitation and hospital stay.
The subsequent multivariate regression analysis also validated the protective role of cFNB with respect to POCD development (HR 0.556 95% CI 0.316–0.981, P=0.043). We should recognize that both Mini-Cog ≤2 and use of cFNB was not significant in univariate analysis. Univariate analysis was used for first screen rather than final decision, while multivariate analysis considered all possible variables in total, which was more accurate and related to realities. Variables with whether statistical significance or clinical implication could be included in final model.
Several risk factors for onset of POCD were identified in this study. Aside from the Mini-Cog score and use of cFNB discussed previously, 2 factors remained statistically significant: age and surgery type. Undoubtedly, age is one of the most essential risk factors in POCD incidence, or even the only important risk factor, as suggested by some reports [26], which might be attributed to the neurovascular disease-related risk factors, more cerebral white matter damage and less cognitive reserve present in older patients [27]. Another significant risk factor is the surgery type; we found that the risk of POCD for patients undergoing hemi-arthroplasty is nearly 4 times higher than patients undergoing dynamic hip screw, while the risk in patients undergoing total hip replacement is 7 times higher. The obvious differences between surgery types might be explained by the longer duration of surgery and anesthesia, and greater quantity of anesthetic drugs used, which have been reported to be associated with higher incidence of POCD [27]. Notably, our results found that surgery type was a significantly risk factor, while Garden classification was not, which might be attributed to its association with the surgery type, and Cox regression analysis can distinguish dependent variables.
Several limitations of this study must be specified. First, despite propensity score match, disadvantages of non-randomization, lack of complete clinical data, and retrospective views still existed, which inevitable reduced the evidence power. Secondly, the sample size was still not optimal to determine other possible risk factors and the effect of cFNB on all patients. Also, since MMSE was evaluated several times after the surgery, the results might have been affected by the repeated measurements. Finally, long-term results of POCD were absent from this study.
Conclusions
In summary, by retrospectively comparing the incidence of POCD in patients receiving cFNB to controls, we can reach the conclusion that perioperative cFNB can help prevent POCD, especially for high-risk patients with Mini-Cog scores equal to or less than 2 points. We also demonstrated that age and surgery type were highly associated with development of POCD.
Footnotes
Source of support: Departmental sources
Conflict of interests
None.
References
- 1.Laalou FZ, Jochum D, Pain L. [Postoperative cognitive dysfunction (POCD): Strategy of prevention, assessment and management]. Ann Fr Anesth Reanim. 2011;30(10):e49–53. doi: 10.1016/j.annfar.2011.08.009. [in French] [DOI] [PubMed] [Google Scholar]
- 2.Chi YL, Li ZS, Lin CS, et al. Evaluation of the postoperative cognitive dysfunction in elderly patients with general anesthesia. Eur Rev Med Pharmacol Sci. 2017;21:1346–54. [PubMed] [Google Scholar]
- 3.Scott JE, Mathias JL, Kneebone AC. Postoperative cognitive dysfunction after total joint arthroplasty in the elderly: A meta-analysis. J Arthroplasty. 2014;29:261–67.e261. doi: 10.1016/j.arth.2013.06.007. [DOI] [PubMed] [Google Scholar]
- 4.Mei B, Zha H, Lu X, et al. Peripheral nerve block as a supplement to light or deep general anesthesia in elderly patients receiving total hip arthroplasty: A prospective randomized study. Clin J Pain. 2017;33:1053–59. doi: 10.1097/AJP.0000000000000502. [DOI] [PubMed] [Google Scholar]
- 5.Gallo J, Cechova I, Zapletalova J. [Early complications associated with total hip arthroplasty due to femoral neck fracture]. Acta Chir Orthop Traumatol Cech. 2010;77:389–94. [PubMed] [Google Scholar]
- 6.Zhao Y, Fu D, Chen K, et al. Outcome of hemiarthroplasty and total hip replacement for active elderly patients with displaced femoral neck fractures: A meta-analysis of 8 randomized clinical trials. PLoS One. 2014;9:e98071. doi: 10.1371/journal.pone.0098071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Berger M, Nadler JW, Browndyke J, et al. Postoperative cognitive dysfunction: Minding the gaps in our knowledge of a common postoperative complication in the elderly. Anesthesiol Clin. 2015;33:517–50. doi: 10.1016/j.anclin.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robinson TN, Wu DS, Pointer LF, et al. Preoperative cognitive dysfunction is related to adverse postoperative outcomes in the elderly. J Am Col Surg. 2012;215:12–17. doi: 10.1016/j.jamcollsurg.2012.02.007. discussion 17–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Culley DJ, Flaherty D, Reddy S, et al. Preoperative cognitive stratification of older elective surgical patients: A cross-sectional study. Anesth Analg. 2016;123:186–92. doi: 10.1213/ANE.0000000000001277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Culley DJ, Flaherty D, Fahey MC, et al. Poor performance on a preoperative cognitive screening test predicts postoperative complications in older orthopedic surgical patients. Anesthesiology. 2017;127:765–74. doi: 10.1097/ALN.0000000000001859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Deng LQ, Hou LN, Song FX, et al. Effect of pre-emptive analgesia by continuous femoral nerve block on early postoperative cognitive function following total knee arthroplasty in elderly patients. Exp Ther Med. 2017;13:1592–97. doi: 10.3892/etm.2017.4099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bedford PD. Adverse cerebral effects of anaesthesia on old people. Lancet. 1955;269:259–63. doi: 10.1016/s0140-6736(55)92689-1. [DOI] [PubMed] [Google Scholar]
- 13.Fontes MT, Swift RC, Phillips-Bute B, et al. Predictors of cognitive recovery after cardiac surgery. Anesth Analg. 2013;116:435–42. doi: 10.1213/ANE.0b013e318273f37e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie G, Zhang W, Chang Y, Chu Q. Relationship between perioperative inflammatory response and postoperative cognitive dysfunction in the elderly. Med Hypotheses. 2009;73:402–3. doi: 10.1016/j.mehy.2009.01.056. [DOI] [PubMed] [Google Scholar]
- 15.Shackman AJ, Salomons TV, Slagter HA, et al. The integration of negative affect, pain and cognitive control in the cingulate cortex. Nat Rev Neurosci. 2011;12:154–67. doi: 10.1038/nrn2994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Devinney MJ, Mathew JP, Berger M. Postoperative delirium and postoperative cognitive dysfunction: Two sides of the same coin? Anesthesiology. 2018;9(129):389–91. doi: 10.1097/ALN.0000000000002338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Paul JE, Arya A, Hurlburt L, et al. Femoral nerve block improves analgesia outcomes after total knee arthroplasty: A meta-analysis of randomized controlled trials. Anesthesiology. 2010;113:1144–62. doi: 10.1097/ALN.0b013e3181f4b18. [DOI] [PubMed] [Google Scholar]
- 18.Ye Y, Chen K, Tian K, et al. Medial buttress plate augmentation of cannulated screw fixation in vertically unstable femoral neck fractures: Surgical Technique and Preliminary Results. Injury. 2017;48:2189–93. doi: 10.1016/j.injury.2017.08.017. [DOI] [PubMed] [Google Scholar]
- 19.Candal-Couto JJ, McVie JL, Haslam N, et al. Pre-operative analgesia for patients with femoral neck fractures using a modified fascia iliaca block technique. Injury. 2005;36:505–10. doi: 10.1016/j.injury.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 20.Unneby A, Svensson O, Gustafson Y, Olofsson B. Femoral nerve block in a representative sample of elderly people with hip fracture: A randomised controlled trial. Injury. 2017;48:1542–49. doi: 10.1016/j.injury.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 21.Zheng JW, Meng B, Li XY, et al. NF-kappaB/P65 signaling pathway: A potential therapeutic target in postoperative cognitive dysfunction after sevoflurane anesthesia. Eur Rev Med Pharmacol Sci. 2017;21:394–407. [PubMed] [Google Scholar]
- 22.Ren KW, Shen N, Tang JL, et al. Effects of ulinastatin on inflammatory response and cognitive function after hip arthroplasty for the elderly patients with femoral neck fracture. Eur Rev Med Pharmacol Sci. 2018;22:1126–32. doi: 10.26355/eurrev_201802_14401. [DOI] [PubMed] [Google Scholar]
- 23.Duff K. Evidence-based indicators of neuropsychological change in the individual patient: relevant concepts and methods. Arch Clin Neuropsychol. 2012;27:248–61. doi: 10.1093/arclin/acr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li J, Zhou J, Wan Y, et al. Association between ABO blood type and postoperative cognitive dysfunction in elderly patients undergoing unilateral total hip arthroplasty surgery in China. Med Sci Monit. 2017;23:2584–89. doi: 10.12659/MSM.901736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li X, Dai J, Zhao S, et al. Comparison of the value of Mini-Cog and MMSE screening in the rapid identification of Chinese outpatients with mild cognitive impairment. Medicine. 2018;97:e10966. doi: 10.1097/MD.0000000000010966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Newman MF, Kirchner JL, Phillips-Bute B, et al. Longitudinal assessment of neurocognitive function after coronary-artery bypass surgery. N Engl J Med. 2001;344:395–402. doi: 10.1056/NEJM200102083440601. [DOI] [PubMed] [Google Scholar]
- 27.Griebe M, Amann M, Hirsch JG, et al. Reduced functional reserve in patients with age-related white matter changes: A preliminary FMRI study of working memory. PLoS One. 2014;9:e103359. doi: 10.1371/journal.pone.0103359. [DOI] [PMC free article] [PubMed] [Google Scholar]