Abstract
Introduction
The long-term effect of potato consumption on mortality and cardiovascular (CV) and cardiometabolic risk factors is still largely unknown. Using the National Health and Nutrition Examination Surveys (NHANES) 1999–2010, we evaluted the long-term impact of potato intake on total and cause-specific (cardiovascular disease (CVD), cerebrovascular disease and cancer) mortality, and the results were next validated in a systematic review and meta-analysis of cohort studies investigating pooled associations of potato consumption with all-cause and cause-specific death.
Material and methods
Vital status up to December 31, 2011 was ascertained in NHANES. Cox proportional hazards were applied to determine the hazard ratios (HRs) and 95% confidence intervals (95% CI) of mortality for each quartile of the potato intake, with the lowest quartile (Q1 – with the lowest intake) used as a reference. In the meta-analysis we used adjusted Cox regression to determine the risk ratio (RR) and 95% CI, as well as random effects models and generic inverse variance methods to synthesize quantitative and pooled data, followed by a leave-one-out method for sensitivity analysis.
Results
Among 24,856 participants included, 3433 deaths occurred during the mean follow-up of 6.4 years. In multivariate adjusted models, total (42%), CVD (65%), cerebrovascular (26%) and cancer (52%) mortality risk was greater in individuals with higher potato consumption than those with the lowest intake (p < 0.001 for all comparisons). However, this link disappeared after adjustment for confounding factors. Results from pooling current prospective studies revealed a non-significant association between total (RR = 1.25, 0.98–1.60, p = 0.066), CVD (RR = 0.99, 0.90–1.08, p = 0.845) and stroke mortality (RR = 0.94, 0.85–1.03, p = 0.214) with potato consumption. Individuals with a higher potato intake had a less favorable profile of cardiometabolic factors, including greater waist circumference (97.2 vs. 99.5 cm, p < 0.001) and a less favorable profile of systolic and diastolic blood pressure, levels of triglycerides (TG), high-density lipoprotein-cholesterol (HDL-C) and TG/HDL-C ratio (p < 0.001 for all comparisons).
Conclusions
Our results revealed no significant effects of potato intake on long-term mortality rates, whereas higher potato consumption was adversely related to cardiometabolic risk factors. These findings should be taken into consideration for public health strategies, establishing the position for potatoes in the food pyramid.
Keywords: mortality, potato, stroke, cardiovascular disease, cardiometabolic, meta-analysis
Introduction
Non-communicable diseases, including the most important ones, such as cardiovascular disease (CVD) and stroke, are the leading causes of death globally [1, 2]. Based on the recent data, each year more than 17 million people die due to CVD worldwide, accounting for 30% of global mortality [3]. It is also very well known that a well-balanced and healthy diet might essentially improve CV health, which has been demonstrated in many available large cohort studies [4–6].
White potatoes have often been a part of many traditional diets of Western countries [7]. They are rich in starch and have a high glycemic index and load, which, based on available data, have been associated with an increased risk of CVD and mortality [7]. However, compared with other common carbohydrate sources, potatoes have a low energy density because of their high water content [8]. They also provide important micronutrients, which are all associated with a decreased risk of morbidity and mortality [9]. Thus, overall it is hard to assess their potential harmful or beneficial effects [8].
There are very limited data on potato consumption and its impact on public health and clinical outcomes, such as mortality. A study by Muraki et al. that included three North American cohorts reported that greater consumption of potatoes was associated with a 33% higher risk of type 2 diabetes (T2DM), independently of several potential confounders [10]. Likewise, results from prospective US cohorts showed that high consumption of potatoes was associated with a higher risk of hypertension (HTN) [11]. In contrast, a study conducted in Sweden in 69,313 men and women failed to find any significant link between higher potato intake and the risk of CVD morbidity and mortality during a 13-year follow-up [12]. A systematic review, which included 5 observational studies with a total of 170,413 healthy subjects, also did not provide any conclusive evidence that could suggest an association between potato intake and the risk of developing obesity and type 2 DM [13]. Similarly, a longitudinal analysis that included 4,440 participants (with 8-year follow-up) reported that participants with the highest potato consumption did not have an increased risk of all-cause mortality [10]. However, there were several limitations of this study, including the lack of assessment of cause-specific mortality and the influence of biochemical parameters, which might have been important confounding factors [14]. To the best of our knowledge, no previous prospective study has investigated the association between potato consumption and cancer mortality [15].
Taking into account still limited data and inconsistent results of available studies, a controversy was raised in the US and UK regarding the recognition of potatoes as vegetables in dietary recommendations [8, 15]. Currently, in the US national food guide potatoes are considered a vegetable [8], whereas they are grouped with cereals in the UK national food guide [8, 15]. The US Institute of Medicine recently recommended that white potatoes should be allowed as an eligible vegetable of the Special Supplemental Nutrition Program for Women, Infants, and Children [8, 10, 15]. Such inconsistencies may originate from different considerations, including the historical nature of potatoes and policies of trade, agriculture, and food, as well as the limited and mixed evidence of the association of potato consumption with health outcomes such as mortality and cardiometabolic factors [16–21].
Given that potatoes are widely consumed in both North America and Europe as well as due to still inconsistent knowledge on their health effects, as well as conflicting findings reported, we prospectively examined the association of potato consumption with total and cause-specific mortality, including cancer, stroke and CVD, using population-based cohorts in the US. As a secondary objective, to validate the cohort results, we conducted a systematic review and meta-analysis by pooling existing prospective cohort studies.
Material and methods
NHANES Study
Main study characteristics
This was a prospective cohort study using data from the US National Health and Nutrition Examination Survey (NHANES). The National Center for Health Statistics (NCHS) Research Ethics Review Board approved the underlying protocol and written informed consent was obtained from all participants. The current study is based on the analysis of data from 2-year NHANES survey cycles between 1999 and 2010, restricted to participants aged ≥ 20 years. Details on the NHANES Laboratory/Medical Technologists Procedures and Anthropometry Procedures were described elsewhere [22].
Dietary intake was assessed via 24 h recalls obtained by a trained interviewer, using a computer-assisted dietary interview system with standardized probes, i.e. the United States Department of Agriculture Automated Multiple-Pass Method (AMPM) [23]. Briefly, the type and quantity of all foods and beverages consumed in a single 24 h period before the dietary interview (from midnight to midnight) were collected using the AMPM. It is designed to enhance complete and accurate data collection while reducing respondent burden. The United States Department of Agriculture (USDA) Food and Nutrient Database for Dietary Studies was used to determine the nutrient content of foods.
Cardiometabolic risk factors
A blood specimen was drawn from an antecubital vein. Glycated hemoglobin (HbA1c) was measured using a Tosoh A1C 2.2 Plus Glycohemoglobin Analyzer (Tosoh Bioscience, San Francisco, USA). Fasting blood glucose (FBG) was measured by a hexokinase method using a Roche/Hitachi 911 Analyzer and Roche Modular P Chemistry Analyzer (NJ, USA). Insulin was measured using an ELISA immunoassay (Mercodia, Uppsala, Sweden). Other laboratory test details are available in the NHANES Laboratory/Medical Technologists Procedures Manual [24]. Homeostasis model assessment of insulin resistance (HOMA-IR) was calculated as: (FBG (mg/dl) × insulin (mU/ml)/22.5) using fasting values. Details on C-reactive protein (CRP) measurement are available elsewhere [25]. The anthropometrically predicted visceral adipose tissue (apVAT) was measured with gender-specific validated equations that included age, body mass index (BMI), and circumferences of the waist (WC) and thigh [26]. The equation for men was: 6 × WC – 4.41 × proximal thigh circumference + 1.19 × age – 213.65; and the equation for women was: 2.15 × WC – 3.63 × proximal thigh + 1.46 × age + 6:22 × BMI – 92.713 [26]. Visceral adiposity index (VAI) was calculated using gender-specific formulas: men (WC/39.68 + (1.88 × BMI)) × (triglycerides (TG)/1.03) × (1.31/high-density lipoprotein cholesterol (HDL-C)); women: (WC/36.58 + (1.89 × BMI)) × (TG/0.81) × (1.52/HDL-C), where both TG and HDL-C levels are expressed in mmol/l [26]. TG to HDL-C ratio was calculated as the ratio of TG (mg/dl) to HDL-C (mg/dl). The lipid accumulation product (LAP) index was calculated as (WC – 65) × (TG (mmol/l)) in men, and (WC – 58) × (TG (mmol/l)) in women [26].
A digital scale was used to measure weight to the nearest 100 g and a fixed stadiometer to measure height to the nearest mm. BMI was calculated as weight in kg divided by the square of height in m. WC was measured at the iliac crest to the nearest mm, using a steel tape.
Mortality
The de-identified and anonymized data of NHANES 1999–2010 participants were linked to longitudinal Medicare and mortality data using the NHANES assigned sequence number. Mortality follow-up data are available from the date of survey participation until December 31, 2011. We examined all-cause mortality, as well as mortality due to CVD (I00-I09, I11, I13, I20-I51, I60-I69), cancer (C00-C97), and cerebrovascular disease (I60-I69). Cause of death was determined using the 10th revision of the International Classification of Diseases (ICD-10).
Statistical analysis
Analyses were conducted according to the guidelines set by the Centers for Disease Control and Prevention for analysis of the NHANES dataset, accounting for the masked variance and using their suggested weighting methodology [27, 28]. Continuous and categorical demographic variables were compared across tertiles of potato consumption using analysis of variance (ANOVA) and χ2 tests, respectively. Multivariable Cox proportional hazards were applied to determine the hazard ratios (HRs) and 95 % confidence intervals (CIs) of mortality for each tertile of potato consumption, with the lowest tertile (T1 = lowest potato intake) always used as a reference. To derive the HR and 95% CI, we used 3 different models, Model 1: adjusted for age, gender, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking and alcohol consumption; Model 2: adjusted additionally for BMI, dietary fat, carbohydrates, saturated fat, protein and dietary fiber; and Model 3: Model 2 adjusted for hypertension and DM. A two-sided p < 0.05 was used to characterize significant results. Data were analyzed using SPSS complex sample module version 22.0 (IBM Corp, Armonk, NY).
Systematic review and meta-analysis
Literature search and study selection
This meta-analysis was designed, conducted and reported according to Meta-analysis Of Observational Studies in Epidemiology (MOOSE) guidelines [29]. The primary exposure of interest was potato consumption while the primary outcome of interest was changes in total and cause-specific mortality subsequent to potato intake. Prospective cohort studies published up to June 2018 (without language restriction) were searched using PubMed/Medline, Embase, and Scopus database. This was complemented by hand searches of the reference list of eligible articles, and email correspondence with authors for additional data, where relevant.
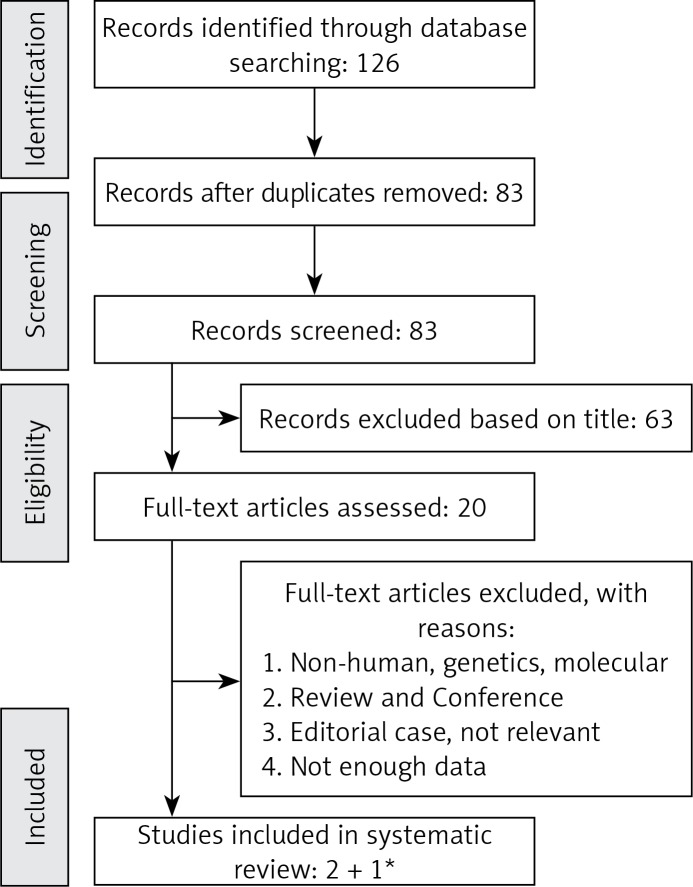
After excluding duplicates and based on titles and abstracts, we excluded studies on animals, baseline age < 18 years, or populations with prior coronary heart disease (CHD), DM or any other chronic diseases. Eligible studies were selected by using predefined inclusion criteria of prospective cohort studies, healthy populations and original articles on the association of potato intake and all-cause and cause-specific mortality (CVD/CHD, cerebrovascular and cancer). In addition, supplementary hand searching of reference lists of previous reviews or meta-analyses was conducted. Overall, of 20 eligible full articles, 2 studies met the inclusion criteria (Figure 1). To increase the power and credibility of the obtained results, the recent data based on the NHANES study were included in the final meta-analysis.
Figure 1.
Flow chart of literature search for metaanalysis on potato consumption with total and cause-specific mortality for the selection of studies
*Recent data based on NHANES analysis by Mazidi et al.
Study selection
Study selection started with the removal of duplicates, followed by screening of titles and abstracts by two reviewers (MM and NK). To avoid bias, the reviewers were blinded to the names, qualifications and the institutional affiliations of the study authors. Agreement between the reviewers was excellent (𝛋 index: 0.89; p < 0.001). Disagreements were resolved at a meeting between reviewers prior to selected articles being retrieved (a flow chart is available in Figure 1). We included studies if they met all the following criteria: (1) the studies of interest concerned potato intake; (2) the studies were population-based cohort studies and reported all-cause and cause-specific mortality data; (3) RR, HR or odds ratio (OR) estimates with 95% CI adjusted for multivariable factors were available or could be calculated; (4) original articles with full texts in English.
Studies were excluded according to the following criteria: (1) reviews, letters, unpublished data or comments; (2) those published in languages other than English; (3) not population-based cohort studies; (4) RR, HR or OR estimates with 95% CI were not available or could not be calculated. Narrative reviews, comments, opinion pieces, methodological, editorials, letters or any other publications lacking primary data and/or explicit method descriptions were also excluded.
Data extraction and management
The full texts of studies meeting the inclusion criteria were retrieved and screened to determine eligibility by 2 reviewers (MM, NK). The study quality assessment was performed according to the Newcastle-Ottawa Scale [30]. By evaluation of selection, comparability and outcome, the rating system scores for studies range from 0 (highest degree of bias) to 9 (lowest degree of bias). Additionally, we investigated the funding sources of all the eligible studies. Following assessment of methodological quality, the reviewers (MM & NK) extracted data using a purpose-designed data extraction form and independently summarized what they consider to be the most important results from each study. These summaries were compared and any differences of opinion were resolved by discussion and consultation with a third reviewer (MB). Any further calculations on study data considered necessary were conducted by the first reviewer and checked by the second reviewer. Information extracted from each eligible study included the following items: author, year and references, country, study name, men (%), mean age, follow-up time (years), number of cases, number of participants, parameter, outcome and main confounders.
Data synthesis and statistical analyses
For studies that reported results from different multivariable-adjusted models, the model including the most confounding factors was extracted for the meta-analysis. The random-effect model was applied to calculate pooled RRs, 95% CI and p-value for heterogeneity. RRs comparing the highest with the lowest intake category were combined across studies to generate the summary associations. The extent of heterogeneity across studies was examined using the I2 test and I2 > 50 % together with a two-sided p < 0.05 indicated significant heterogeneity [31].
Publication bias
Potential publication bias was explored using visual inspection of Begg’s funnel plot asymmetry, Begg’s rank correlation and Egger’s weighted regression tests. The Duval and Tweedie trim method was used to adjust the analysis for the effects of publication bias [32]. The meta-analysis was conducted using the Comprehensive Meta-Analysis (CMA) V3 software (Biostat, NJ) [33].
Results
NHANES study
Overall, 24,856 participants were included, with a mean age of 47.7 years, comprising 48.7% men and 51.3% women. Demographic characteristics of the participants according to potato consumption are shown in Table I. Individuals with a higher potato intake (1–2 serving(s)/day) were significantly younger compared with those with no potato consumption (0 servings/day) (46.3 vs. 47.7 years, respectively, p < 0.001, Table I). Males represented the majority in the highest amount of potato consumption group (59.1 vs. 40.9%, respectively, p < 0.001), whereas females represented the highest percentage in the lowest category (52.0 vs. 48.0%, respectively, p < 0.001, Table I). With regard to race/ethnicity, in the category with the highest potato intake the distribution was as follows: Non-Hispanic White (52.2%), Non-Hispanic Black (22.0%) and Mexican-American (13.8%, p < 0.001, Table I). Regarding education level, the majority of individuals with “more than high school” (47.9%) were in the category with no potato consumption, while most of those with “less than high school” (29.9%) education were in the “1–2 serving(s)/day” category.
Table I.
Characteristics of study participants according to potato consumption
| Parameter | Potato consumption1 | P-value | ||
|---|---|---|---|---|
| 0 servings2/day (n = 11369) | < 1 serving/day (n = 8856) | 1–2 serving(s)/day (n = 4631) | ||
| Age [years] | 47.7 ±0.2 | 47.1 ±0.2 | 46.3 ±0.3 | < 0.001 |
| Gender (%): | ||||
| Men | 48.0 | 45.9 | 59.1 | < 0.001 |
| Women | 52.0 | 54.1 | 40.9 | |
| Race/ethnicity (%): | ||||
| Mexican-American | 20.6 | 18.5 | 13.8 | < 0.001 |
| Other Hispanic | 9.0 | 8.4 | 5.3 | |
| Non-Hispanic white | 45.4 | 47.1 | 55.2 | |
| Non-Hispanic black | 20.4 | 21.4 | 22.0 | |
| Other | 4.6 | 4.6 | 3.7 | |
| Marital status (%): | ||||
| Married | 50.5 | 52.7 | 51.6 | < 0.001 |
| Widowed | 8.4 | 8.8 | 6.9 | |
| Divorced | 10.9 | 9.3 | 10.5 | |
| Never married | 19.0 | 18.8 | 19.1 | |
| Education status (%): | ||||
| Less than high school | 26.4 | 28.1 | 29.9 | < 0.001 |
| Completed high school | 22.2 | 25.6 | 26.8 | |
| More than high school | 47.9 | 46.1 | 46.7 | |
| Mortality status: | ||||
| Total mortality | 1028 (29.9) | 1164 (33.9) | 1241 (36.1) | < 0.001 |
| Cancer mortality | 286 (34.5) | 246 (29.7) | 295 (35.6) | < 0.001 |
| Coronary heart disease mortality | 298 (31.8) | 329 (35.1) | 310 (33.0) | < 0.001 |
| Cerebrovascular disease mortality | 69 (30.2) | 78 (34.2) | 81 (35.5) | < 0.001 |
Groups across the quartiles were compared by either χ2 test or analysis of variance. Values expressed as mean and standard deviation (SD) or %.
Potato in the diet included baked, boiled, fried, hash-browned, home-fried, mashed, roasted, scalloped, stuffed, with sauce, potato salad, and potato chips.
One serving consisted of 30–149 g and two servings consisted of at least 150 g.
We also calculated adjusted (for age, gender, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, dietary fat, carbohydrates, saturated fat, protein and dietary fiber) mean of cardiovascular and cardiometabolic risk factors across increasing intake of potatoes (Table II). With increasing potato consumption, participants had a greater WC (97.2 vs. 99.5 cm, p < 0.001) and a less favorable profile of systolic (SBP) and diastolic blood pressure (DBP), TG, HDL-C and TG/HDL-C ratio (p < 0.001 for all comparisons). For both SBP (“0 serving/day = “122.1 vs. “1–2 serving/day “= 123.5 mm Hg) and DBP (“0 servings/day “= 68.2 vs. “1–2 serving(s)/day “= 69.3 mm Hg) the increase of potato intake was associated with significant BP increase (Table II). However, no significant differences were observed for BMI, apVAT or apolipoprotein B (apoB) across potato intake tertiles (Table II). With regard to glucose/insulin homeostasis parameters, insulin and HOMA-IR increased as the potato consumption became higher (both p < 0.001), while there was no significant difference in FBG, HOMA-β, HbA1c or TG to FBG ratio (all p > 0.086). We also found a non-significant slight increase in CRP levels across the tertiles of potato consumption (“0 servings/day “= 0.35 vs. “1–2 serving(s)/day “= 0.38, p = 0.125, Table II).
Table II.
Characteristics of study participants by potato consumption and its influence on cardiometabolic and cardiovascular risk factors
| Parameter | Potato consumption1 | P-value | ||
|---|---|---|---|---|
| 0 servings2/day (n = 11369) | < 1 serving/day (n = 8856) | 1–2 serving(s)/day (n = 4631) | ||
| Body mass index [kg/m2] | 28.8 ±0.1 | 28.7 ±0.1 | 28.9 ±0.1 | 0.143 |
| Waist circumference [cm] | 97.2 ±0.4 | 98.7 ±0.3 | 99.5 ±0.4 | < 0.001 |
| apVAT* | 180.2 ±3.4 | 180.7 ±2.5 | 179.8 ±2.6 | 0.283 |
| Systolic blood pressure [mm Hg] | 122.1 ±0.3 | 122.9 ±0.4 | 123.5 ±0.3 | < 0.001 |
| Diastolic blood pressure [mm Hg] | 68.2 ±0.4 | 68.9 ±0.39 | 69.3 ±0.3 | < 0.001 |
| TG [mg/dl] | 153.3 ±3.5 | 154.6 ±2.7 | 157.0 ±4.1 | < 0.001 |
| HDL-C [mg/dl] | 53.6 ±0.4 | 53.1 ±0.4 | 52.6 ±0.3 | < 0.001 |
| TG to HDL-C ratio | 3.52 ±0.1 | 3.59 ±0.09 | 3.85 ±0.1 | < 0.001 |
| FBG [mg/dl] | 99.1 ±0.6 | 99.9 ±0.7 | 100.1 ±0.8 | 0.142 |
| Insulin | 13.4 ±0.2 | 13.5 ±0.2 | 14.2 ±0.3 | < 0.001 |
| HOMA-IR | 0.75 ±0.03 | 0.91 ±0.03 | 1.09 ±0.02 | < 0.001 |
| HOMA-β | 151.2 ±4.2 | 158.2 ±5.9 | 154.3 ±8.8 | 0.086 |
| HbA1c (%) | 5.64 ±0.02 | 5.65 ±0.02 | 5.64 ±0.02 | 0.436 |
| TG to FBG ratio | 8.43 ±0.01 | 8.72 ±0.01 | 8.52 ±0.02 | 0.246 |
| CRP [mg/dl] | 0.35 ±0.01 | 0.38 ±0.01 | 0.38 ±0.01 | 0.125 |
| Apolipoprotein B [mg/dl] | 0.94 ±0.01 | 0.93 ±0.01 | 0.94 ±0.01 | 0.539 |
| LAP | 68.2 ±1.2 | 69.3 ±2.0 | 71.4 ±1.9 | < 0.001 |
| VAI | 2.50 ±0.02 | 2.56 ±0.01 | 2.53 ±0.02 | 0.436 |
Adjusted (for age, gender, race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, dietary fat, carbohydrates, saturated fat, protein and dietary fiber) means were compared across potato consumption by using analysis of co-variance (ANCOVA).
Potato in the diet included baked, boiled, fried, hash-browned, home-fried, mashed, roasted, scalloped, stuffed, with sauce, potato salad, and potato chips.
One serving consisted of 30–149 g and two servings consisted of at least 150 g. HOMA-IR – homeostatic model assessment of insulin resistance, HOMA-β – homeostatic model assessment of B-cell function, LAP – lipid accumulation product, VAI – visceral adiposity index, apVAT – anthropometrically predicted visceral adipose tissue, TG – triglyceride: FBG – fasting blood glucose, HDL-C – high density lipoprotein cholesterol, CRP – C-reactive protein, HbA1c – glycated hemoglobin.
During the follow-up period of 76.4 months (6.4 years), 3,433 total deaths were recorded, including 827 cancer deaths, 937 CVD deaths, and 228 cerebrovascular disease deaths. The distributions of total and cause-specific mortality across quartiles of potato consumption are depicted in Table I. Detailed results from 3 multivariable Cox regression models for risk of death (total, cancer, CVD, cerebrovascular disease) across potato intake are shown in Table III. With regard to total mortality, there was a positive link (42% higher) between potato consumption and total mortality in Model 1, whereas after more adjustment (Model 2 and Model 3), the association between total mortality and potato intake disappeared (p > 0.421). In the first (52% higher risk) and second (35% higher risk) models we found a positive association between cancer mortality and potato consumption (both p < 0.001), while this link disappeared after more adjustment (in Model 3) (p = 0.235). A similar pattern was observed for CVD mortality – in the first and second model, CVD mortality was positively correlated with potato intake (RR = 1.65, 95% CI: 1.53–1.95, and RR = 1.51, 95% CI: 1.19–1.91, respectively, p < 0.001, Table III), whereas no link was present after correction in Model 3 (RR = 1.14, 95% CI: 0.99–1.32) (p = 0.418). There was also a positive association between cerebrovascular disease mortality and potato consumption in Model 1 of adjustment (highest intake = 1.26, 1.14–1.39, p < 0.001, Table III), while the link was significantly attenuated once we adjusted for more confounders in Model 2 (plus dietary factors) and Model 3 (plus BMI, HTN and T2DM) (both p > 0.476, Table III). Finally, with regard to cancer mortality, we found a positive and significant association between potato intake and cancer mortality in Model 1 (52%) and 2 (35%), while this link disappeared after more correction (RR = 1.09, 95% CI: 0.99–1.028, p = 0.235 for trend).
Table III.
Multivariable-adjusted hazard ratios (95% CIs) for mortality across potato consumption
| Parameter | Potato consumption | P-value | ||
|---|---|---|---|---|
| 0 servings/day (n = 11369) | < 1 serving/day (n = 8856) | 1–2 serving(s)/day (n = 4631) | ||
| Total mortality: | ||||
| Model 1 | 1 (Reference) | 1.23 (1.11–1.43) | 1.42 (1.27–1.96) | < 0.001 |
| Model 2 | 1 (Reference) | 1.22 (0.95–1.63) | 1.20 (1.01–1.64) | 0.421 |
| Model 3 | 1 (Reference) | 1.10 (0.98–1.42) | 1.30 (0.98–1.70) | 0.523 |
| Cancer mortality: | ||||
| Model 1 | 1 (Reference) | 1.19 (1.04–1.42) | 1.52 (1.23–1.51) | < 0.001 |
| Model 2 | 1 (Reference) | 1.13 (1.02–1.28) | 1.35 (1.06–1.69) | < 0.001 |
| Model 3 | 1 (Reference) | 0.99 (0.75–1.26) | 1.09 (0.99–1.28) | 0.235 |
| Cardiovascular disease mortality: | ||||
| Model 1 | 1 (Reference) | 1.39 (1.25–1.62) | 1.65 (1.53–1.95) | < 0.001 |
| Model 2 | 1 (Reference) | 1.30 (1.02–1.92) | 1.51 (1.19–1.91) | < 0.001 |
| Model 3 | 1 (Reference) | 1.02 (0.76–1.28) | 1.14 (0.99–1.32) | 0.418 |
| Cerebrovascular disease mortality: | ||||
| Model 1 | 1 (Reference) | 1.36 (1.19–1.73) | 1.26 (1.14–1.39) | < 0.001 |
| Model 2 | 1 (Reference) | 1.01 (0.98–1.16) | 1.09 (0.99–1.23) | 0.476 |
| Model 3 | 1 (Reference) | 1.01 (0.69–1.43) | 0.97 (0.78–1.20) | 0.863 |
1Potato in the diet included baked, boiled, fried, hash-browned, home-fried, mashed, roasted, scalloped, stuffed, with sauce, potato salad, and potato chips.
2One serving consisted of 30–149 g and two servings consisted of at least 150 g. Model 1 – Adjusted for age, gender, and race, education, marital status, poverty to income ratio, total energy intake, physical activity, smoking and alcohol consumption; Model 2 – Adjusted for age, gender, race, education, and marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, dietary fat, carbohydrates, saturated fat, protein and dietary fiber; Model 3 – Adjusted for age, gender, race, education, and marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, dietary fat, carbohydrates, saturated fat, protein, dietary fiber, body mass index, hypertension and diabetes.
Meta-analysis and systematic review
Overviews of key characteristics of the 3 prospective cohort studies (4 cohorts) are shown in Table IV. A total of 98,569 participants, with 7672 mortality cases were included in the analysis. The duration of follow-up ranged from 6.4 to 13 years. Results of quality assessment showed that for all included studies scoring was ≥ 7.
Table IV.
Characteristics of prospective cohort studies included in the analysis
| Author, year and reference | Country, region/cohort | Men (%) | Age | Follow-up time [years] | No. of cases | No. of subjects | Parameter | Outcome | Main confounders |
|---|---|---|---|---|---|---|---|---|---|
| Larsson, 2016 [12] | Sweden, Cohort of Swedish Men and the Swedish Mammography Cohort | 52.0 | 45–83 | 13 | 4003 | 69,313 | Potato consumption | CVD* and stroke death | Age, education, family history of MI before 60 years, smoking status and pack-years of smoking, aspirin use, walking or bicycling, exercise, BMI, history of hypertension, history of hypercholesterolemia, alcohol consumption, total energy intake and DASH diet score |
| Veronese, 2017 [14] | USA, Osteoarthritis Initiative cohort study, | 42.1 | 61.3 | 8 | 236 | 4,400 | Potato consumption | Total death | Age, gender, race/ethnicity, BMI, education, smoking habits, yearly income, Physical Activity Scale for Elderly score, Charlson comorbidity index, daily energy intake, alcohol consumption, adherence to a Mediterranean diet, and Center for Epidemiologic Studies Depression scale |
| Mazidi, 2019 | US National Health and Nutrition Examination Survey | 48.7 | 47.7 | 6.4 | 3433 | 24,856 | Potato consumption | Total death, CVD, stroke and cancer death | Age, gender, race, education, and marital status, poverty to income ratio, total energy intake, physical activity, smoking, alcohol consumption, dietary fat, carbohydrates, saturated fat, protein, dietary fiber, BMI, hypertension and diabetes |
CVD – cardiovascular disease, MI – myocardial infarction, BMI – body mass index, DASH – Dietary Approaches to Stop Hypertension.
Associations of potato consumption with all-cause, CVD and stroke mortality
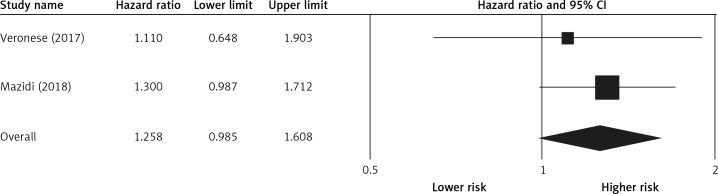
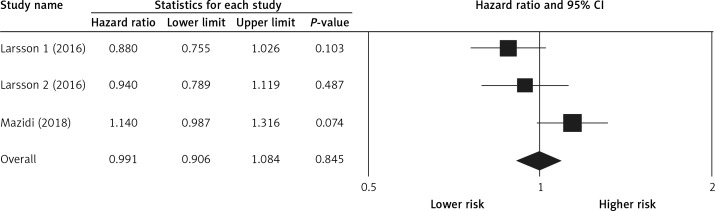
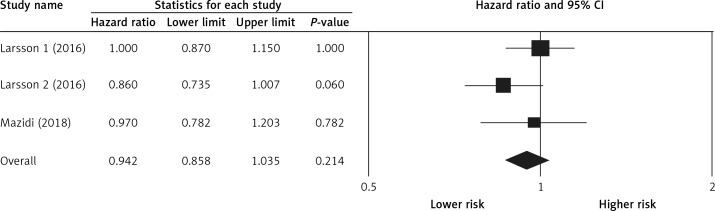
Pooling the data on the association between potato consumption and total mortality revealed a positive and non-significant association (RR = 1.25, 0.98–1.60, p = 0.066, n = 2 studies, Figure 2), with no risk of heterogeneity, I2 = 1.2, p = 0.968. There was no association between potato consumption and CVD mortality (RR = 0.99, 0.90–1.08, p = 0.845, n = 3 studies, no heterogeneity, I2 = 3.2, p = 0.825, Figure 3) or between potato intake and stroke mortality (RR = 0.94, 0.85–1.03, p = 0.214, n = 3 studies, Figure 4), with no sign of heterogeneity, I2 = 1.9, p = 0.876.
Figure 2.
Forest plot of potato consumption and risk of total mortality
Figure 3.
Forest plot of potato consumption and risk of cardiovascular disease mortality
Figure 4.
Forest plot of potato consumption and risk of stroke mortality
Publication bias
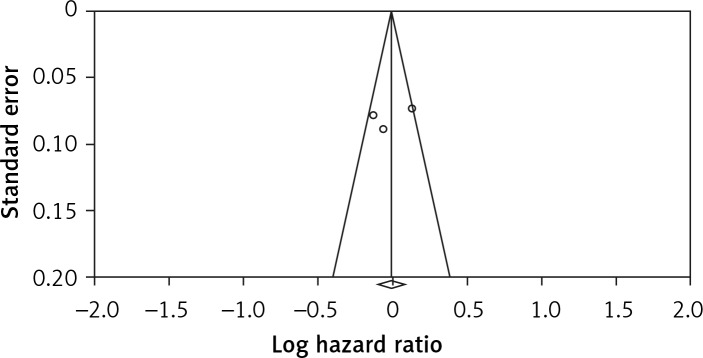
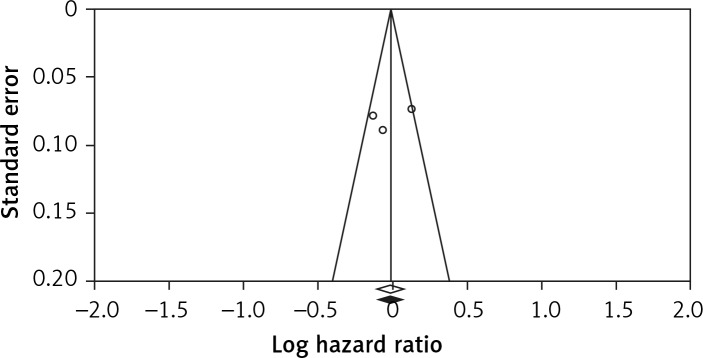
Visual inspection of the funnel plot symmetry suggested no potential publication bias for the comparison of potato consumption and CVD mortality (Figure 5). Moreover, Egger’s linear regression indicated absence of publication bias (intercept = –2.3, 95% CI: –6.91 to 6.00, two-tailed p = 0.542), as well as the Begg’s rank correlation test (Kendall’s τ with continuity correction = 1.00, z = 0.342, two-tailed p = 0.436). After adjustment of the effect size for potential publication bias using the ‘trim and fill’ correction, no potentially missing studies were imputed in the funnel plot (RR = 0.99, 0.90–1.08) (Figure 6). The ‘fail-safe N’ test showed that 102 studies would be needed to bring the obtained results down to a non-significant (p > 0.05) value.
Figure 5.
Funnel plots for studies on the association between potato consumption and risk of cardiovascular disease mortality. Open circles represent observed published studies; open diamond represents observed effect size
Figure 6.
Trim and fill method was used to impute for potentially missing studies (potato consumption and risk of cardiovascular disease mortality); no potentially missing study was imputed in funnel plot. Open circles represent observed published studies; open diamond represents observed effect size; closed diamond represents imputed effect size
Discussion
Based on both individual data and a meta-analysis of available prospective studies we evaluated the impact of potato consumption on total and cause-specific mortality. Furthermore, we investigated the changes in cardiometabolic risk factors across the tertiles of potato intake. We found that in minimally adjusted models there was a positive association of total, CVD, stroke and cancer mortality with potato consumption (i.e. higher intake was linked to higher risk) but this association disappeared after more adjustments (dietary factors, BMI, HTN and T2DM). The results of pooling studies showed a non-significant association between potato intake and risk of total, CVD and stroke mortality. However, participants with higher potato consumption had a much less favorable profile of cardiovascular and cardiometabolic risk factors; these associations were independent of demographic, lifestyle and dietary factors.
Our findings showing a non-significant link between total and cause-specific mortality and potato intake are in line with the few other available studies [12, 14, 34]. However, to the best of our knowledge, only one study, with 4,440 participants (aged between 45 and 79 years) followed up for 8 years, examined whether frequent potato consumption was associated with total mortality risk [14]. They evaluated potato intake using a Block Brief 2000 food-frequency questionnaire (FFQ) and categorized it as ≤ 1 time/month, 2–3 times/month, 1 time/week, 2 times/week, or ≥ 3 times/week. They found that potato intake was not associated with an increased risk of all-cause mortality (HR = 1.11; 95% CI: 0.65–1.91) [14]. This might have happened because of using different co-variants, since in our fully adjusted model we failed to find any association between total and cause-specific mortally after adjusting for more variables. Similarly, only one study investigated the association of potato consumption with CVD and stroke mortality, by pooling the results of 2 prospective cohorts of Swedish adults (the Cohort of Swedish Men and the Swedish Mammography Cohort), involving 69,313 men and women, free of CVD and DM, followed up for 13 years [12]. They evaluated the potato consumption with FFQ as 2.4 times/week, 2.4–7.9 times/week and > 7.9 times/week, and found no link between potato intake, CVD (HR = 0.99; 95% CI: 0.95–1.03) or stroke mortality (HR = 1.01; 95% CI: 0.97–1.05) [12]. Similarly, a study consisting of 2 large American cohort populations (i.e. 75,596 women aged 34 to 59 years in the Nurses’ Health Study with 14 years of follow-up, and 38,683 men aged 40 to 75 years in the Health Professionals’ Follow-up Study with 8 years of follow-up) reported no associations between potato intake and risk of stroke morbidity [34].
We hypothesize that the neutral impact of potato consumption on mortality risk might be due to the unique composition of potatoes. In this context, it is possible that the high content of fiber, vitamins, and micronutrients in white potatoes might have counterbalanced the detrimental effects of their high glycemic index [8]. In this context, we have recently reported on the beneficial effects of fiber, minerals and vitamins on glucose and insulin homeostasis [35]. However, larger prospective studies are required to further investigate this relationship.
Our findings indicated no link between potato consumption and cancer mortality. One case-control study found potato consumption to be protective against rectal cancer risk among women, while no associations were found for men [36]. In a cohort study, potato fiber was found to have a protective effect against colon cancer [37]. In contrast, other studies reported that individuals with high potato consumption tended to have higher risk of colon cancer [38], increased risk of gastric cancer among women but not among men [39] or risk of rectal cancer among Whites but not among African-Americans [40]. Regarding the insignificant impact of potato intake on the risk of cancer, some short-term studies have even suggested that potatoes contain antitumor agents [15]. The potential health benefits of potato consumption have been related to fiber, niacin, folate, vitamin C, and minerals (e.g. potassium, magnesium and iron) contained in potatoes [8]. Fiber is known to have a protective effect against cancer, but the association between the other nutrients in potatoes and cancer are either inconsistent or incomplete [41]. In recent years, there has been a more critical focus on potato consumption, as potatoes have a high glycemic index and glycemic load [42, 43]. On the other hand, several studies have found high dietary glycemic index and glycemic load to be associated with an increased risk of several cancers [44]. However, it is possible that the beneficial compounds of the potato mentioned above may compensate for the detrimental effect of their high glycemic index and glycemic load [45].
We need to bear in mind that the preparation method is also relevant for the association between potato consumption and risk of mortality. Boiling is assumed to be the healthiest preparation method. Roasted and fried potatoes are carriers of salt and fats, and acrylamide formation may be an additional problem in potatoes cooked at temperatures above 120°C [46]. However, we did not have clear information on the way of preparation in this analysis, and which potatoes dominated for given subjects. This is a limitation of our study. Prospective studies focusing on this matter are needed.
We also examined the link between cardiometabolic and CV risk factors and potato consumption. Participants with higher potato consumption had worse lipid profile, BP, glucose/insulin homeostasis markers and anthropometric variables, while we found no link between CRP, an independent predictor of CVD, and potato intake. The association of potato intake with hypertension is a critical public health problem in the US, mainly because potatoes have recently been included in the government sponsored food programs [11]. Their inclusion was justified by the potential CVD benefits attributed to their high potassium content, leading to improvements in blood pressure [11]. Briefly, the Institute of Medicine and the US Department of Agriculture recommended white potatoes to be allowed as part of the fruit and vegetable cash voucher Women, Infants & Children (WIC) program, with most states having already implemented this regulation. The Institute of Medicine’s report emphasized the high potassium content of potatoes, which is a desired feature of foods for the WIC population [11]; a higher potassium intake has been associated with a lower BP. In a meta-analysis of 22 randomized controlled trials increased potassium intake (as a supplement) produced a 3.5 mm Hg reduction in SBP and 2.0 mm Hg reduction in DBP in hypertensive patients [47]. However, in line with another study, which was carried out in US adults [11], the authors found that a higher potato intake was associated with increased, rather than decreased, SBP and DBP. In a prospective longitudinal cohort study which involved altogether almost 200,000 participants from the Nurses’ Health Study I and II, and from the Health Professionals Follow-up Study, who were non-hypertensive at baseline, higher potato consumption was independently and prospectively associated with increased risk of developing hypertension [11].
Evidence of potato intake in relation to glucose/insulin control and management is sparse [48]. In 3 cross-sectional and case-control studies, potato intake was positively associated with worse glucose/insulin control, higher insulin resistance and prevalent type 2 DM [16–21]. In a Finnish cohort comprising 4,303 men and women, participants who consumed 283 g/day of total potatoes had 42% higher use of antidiabetic drugs than those consuming 132 g/day of potatoes [19]. A study that included 3 North American cohorts also reported that greater potato consumption was associated with a 33% higher risk of T2DM, independently of several potential confounders [10]. In contrast, in the Women’s Health Study, which comprised 39,876 female health professionals, total potato consumption was not associated with DM risk after multivariable adjustment [20]. The results are still inconsistent; however, in most of the available studies, especially in those where the final results were adjusted for multiple variables that might have also affected T2DM risk, the authors found no association between potato intake and T2DM [49–54].
We found that participants with higher potato consumption had a greater WC, which is in accordance with some other studies. For example, Mozaffarian et al., who conducted a large, prospective pooled cohort study, found that increased consumption of 1 serving/day of all types of potatoes combined was associated with a mean body weight gain of 0.58 kg over a period of 4 years [53]. In a similar cohort study, an increase of 0.1 cm in WC over 5 years was observed in women for each increase in potato intake of 60 kcal/day (not including intake of French fries) [54]. In contrast, Halkjaer et al. looked at WC and found no association between potato intake and WC [55].
Our analysis has some strength and obvious limitations, mainly due to the observational character of the study. Because of the prospective design, the misclassification of exposure, which is unavoidable in dietary assessments, is most likely non-differential. It would be better to analyze the risk of mortality and potato intake with regard to different ways of cooking the potatoes. Although we included major possible confounders of lifestyle and dietary factors in the multivariable analysis, residual or unmeasured confounding may still exist. Furthermore, since this is an observational study, we cannot establish causality between potato consumption and the outcomes. In clinical trials, maintaining high adherence to a dietary intervention for a long time is typically difficult, in part because of dietary changes contradicting participants’ long-term dietary preferences [56–58]. Hence, poor adherence may dilute the true effect of an intervention.
As the data collection was performed on all days of the week throughout the year in NHANES, the potential for day-specific information bias is very low [59]. We observed a very low level of heterogeneity, indicating the validity of our results. Furthermore, to the best of our knowledge, this is the first meta-analysis (although it was based only on 3 cohort studies) on the long-term impact of potato consumption on total and cause-specific mortality.
In conclusion, our results suggest no effect of potato intake on total or cause-specific mortality, although potato consumption was associated with a detrimental impact on cardiometabolic factors. These results are important for policy makers, contributing to increased public awareness about the role of the diet on health and the controversy regarding the correct place of potatoes in the food pyramid.
Acknowledgments
The material presented in this manuscript is original and has not been submitted for publication elsewhere.
Conflict of interest
NK has given talks, attended conferences and participated in trials sponsored by Amgen, Angelini, Astra Zeneca, Boehringer Ingelheim, MSD, Novartis, NovoNordisk, Sanofi and WinMedica. DPM has given talks and attended conferences sponsored by MSD, AstraZeneca and Libytec. The other authors declare no conflict of interest.
References
- 1.GBD 2017 Causes of Death Collaborators Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1736–88. doi: 10.1016/S0140-6736(18)32203-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.GBD 2016 Lifetime Risk of Stroke Collaborators Feigin VL, Nguyen G, Cercy K, et al. Global, Regional, and Country-Specific Lifetime Risks of Stroke, 1990 and 2016. N Engl J Med. 2018;379:2429–37. doi: 10.1056/NEJMoa1804492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Benjamin EJ, Blaha MJ, Chiuve SE, et al. Heart disease and stroke statistics-2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–603. doi: 10.1161/CIR.0000000000000485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Global, regional, and national comparative risk assessment of 84 behavioural, environmental and occupational, and metabolic risks or clusters of risks for 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392:1923–94. doi: 10.1016/S0140-6736(18)32225-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mazidi M, Katsiki N, Mikhailidis DP, Sattar N, Banach M. Lower carbohydrate diets and all-cause and cause-specific mortality: a population-based cohort study and pooling of prospective studies. Eur Heart J. 2019;40:2870–9. doi: 10.1093/eurheartj/ehz174. [DOI] [PubMed] [Google Scholar]
- 6.Banach M, Mikhailidis DP, Mazidi M. Low-carbohydrate diet: forget restriction, replace with balance! Eur Heart J. 2020 doi: 10.1093/eurheartj/ehz927. [DOI] [PubMed] [Google Scholar]
- 7.McGill CR, Kurilich AC, Davignon J. The role of potatoes and potato components in cardiometabolic health: a review. Ann Med. 2013;45:467–73. doi: 10.3109/07853890.2013.813633. [DOI] [PubMed] [Google Scholar]
- 8.Anderson GH, Soeandy CD, Smith CE. White vegetables: glycemia and satiety. Adv Nutrition. 2013;4:356s–67s. doi: 10.3945/an.112.003509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mazidi M, Katsiki N, Mikhailidis DP, Banach M. International Lipid Expert Panel (ILEP) Effect of dietary insulinemia on all-cause and cause-specific mortality: results from a cohort study. J Am Coll Nutr. 2019 Nov;25:1–7. doi: 10.1080/07315724.2019.1646167. [DOI] [PubMed] [Google Scholar]
- 10.Muraki I, Rimm EB, Willett WC, Manson JE, Hu FB, Sun Q. Potato consumption and risk of type 2 diabetes: results from three prospective cohort studies. Diabetes Care. 2016;39:376–84. doi: 10.2337/dc15-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borgi L, Rimm EB, Willett WC, Forman JP. Potato intake and incidence of hypertension: results from three prospective US cohort studies. BMJ. 2016;353:i2351. doi: 10.1136/bmj.i2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larsson SC, Wolk A. Potato consumption and risk of cardiovascular disease: 2 prospective cohort studies. Am J Clin Nutr. 2016;104:1245–52. doi: 10.3945/ajcn.116.142422. [DOI] [PubMed] [Google Scholar]
- 13.Borch D, Juul-Hindsgaul N, Veller M, Astrup A, Jaskolowski J, Raben A. Potatoes and risk of obesity, type 2 diabetes, and cardiovascular disease in apparently healthy adults: a systematic review of clinical intervention and observational studies. Am J Clin Nutr. 2016;104:489–98. doi: 10.3945/ajcn.116.132332. [DOI] [PubMed] [Google Scholar]
- 14.Veronese N, Stubbs B, Noale M, et al. Fried potato consumption is associated with elevated mortality: an 8-y longitudinal cohort study. Am J Clin Nutr. 2017;106:162–7. doi: 10.3945/ajcn.117.154872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Camire ME, Kubow S, Donnelly DJ. Potatoes and human health. Crit Rev Food Sci Nutr. 2009;49:823–40. doi: 10.1080/10408390903041996. [DOI] [PubMed] [Google Scholar]
- 16.Ylonen SK, Virtanen SM, Groop L. The intake of potatoes and glucose metabolism in subjects at high risk for type 2 diabetes. Diabetic Med. 2007;24:1049–50. doi: 10.1111/j.1464-5491.2007.02206.x. [DOI] [PubMed] [Google Scholar]
- 17.Khosravi-Boroujeni H, Saadatnia M, Shakeri F, Keshteli AH, Esmaillzadeh A. A case-control study on potato consumption and risk of stroke in central Iran. Arch Iran Med. 2013;16:172–6. [PubMed] [Google Scholar]
- 18.Khosravi-Boroujeni H, Mohammadifard N, Sarrafzadegan N, et al. Potato consumption and cardiovascular disease risk factors among Iranian population. Int J Food Sci Nutr. 2012;63:913–20. doi: 10.3109/09637486.2012.690024. [DOI] [PubMed] [Google Scholar]
- 19.Montonen J, Jarvinen R, Heliovaara M, Reunanen A, Aromaa A, Knekt P. Food consumption and the incidence of type II diabetes mellitus. Eur J Clin Nutr. 2005;59:441–8. doi: 10.1038/sj.ejcn.1602094. [DOI] [PubMed] [Google Scholar]
- 20.Liu S, Serdula M, Janket SJ, et al. A prospective study of fruit and vegetable intake and the risk of type 2 diabetes in women. Diabetes Care. 2004;27:2993–6. doi: 10.2337/diacare.27.12.2993. [DOI] [PubMed] [Google Scholar]
- 21.Halton TL, Willett WC, Liu S, Manson JE, Stampfer MJ, Hu FB. Potato and french fry consumption and risk of type 2 diabetes in women. Am J Clin Nutr. 2006;83:284–90. doi: 10.1093/ajcn/83.2.284. [DOI] [PubMed] [Google Scholar]
- 22.Mazidi M, Shivappa N, Wirth MD, Hebert JR, Vatanparast H, Kengne AP. The association between dietary inflammatory properties and bone mineral density and risk of fracture in US adults. Eur J Clin Nutr. 2017;71:1273–7. doi: 10.1038/ejcn.2017.133. [DOI] [PubMed] [Google Scholar]
- 23.Mazidi M, Gao HK, Kengne AP. Food patterns are associated with likelihood of CKD in US adults. Sci Rep. 2018;8:10696. doi: 10.1038/s41598-018-27365-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. https://www.cdc.gov/nchs/data/nhanes/nhanes_11_12/2011-12_laboratory_procedures_manual.pdf (last assessed 27th February 2019)
- 25.Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. Inverse association between serum antioxidant levels and inflammatory markers is moderated by adiposity: a report based on a large representative population sample of American adults. Br J Nutr. 2018;120:1272–8. doi: 10.1017/S0007114518002581. [DOI] [PubMed] [Google Scholar]
- 26.Mazidi M, Kengne AP, Katsiki N, Mikhailidis DP, Banach M. Lipid accumulation product and triglycerides/glucose index are useful predictors of insulin resistance. J Diabetes Compl. 2018;32:266–70. doi: 10.1016/j.jdiacomp.2017.10.007. [DOI] [PubMed] [Google Scholar]
- 27.NHANES Statistics . Analytic And Reporting Guidelines. http://www.cdc.gov/nchs/data/nhanes/ nhanes 03 04/nhanes analytic guidelines dec 2005.pdf. [Google Scholar]
- 28.Mazidi M, Wong ND, Katsiki N, Mikhailidis DP, Banach M. Dietary patterns, plasma vitamins and Trans fatty acids are associated with peripheral artery disease. Lipids Health Disease. 2017;16:254. doi: 10.1186/s12944-017-0635-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stroup DF, Berlin JA, Morton SC, et al. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–12. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 30.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25:603–5. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 31.Mazidi M, Kengne AP, Banach M. Effects of coenzyme Q10 supplementation on plasma C-reactive protein concentrations: a systematic review and meta-analysis of randomized controlled trials. Pharmacol Res. 2018;128:130–6. doi: 10.1016/j.phrs.2017.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Duval S, Tweedie R. Trim and fill: a simple funnel-plot-based method of testing and adjusting for publication bias in meta-analysis. Biometrics. 2000;56:455–63. doi: 10.1111/j.0006-341x.2000.00455.x. [DOI] [PubMed] [Google Scholar]
- 33.Borenstein M, Hedges L, Higgins J, Rothstein H. Englewood Cliffs. NJ: Biostat. Inc; 2005. Comprehensive Metaanalysis (Vers. 2) [Google Scholar]
- 34.Joshipura KJ, Ascherio A, Manson JE, et al. Fruit and vegetable intake in relation to risk of ischemic stroke. JAMA. 1999;282:1233–9. doi: 10.1001/jama.282.13.1233. [DOI] [PubMed] [Google Scholar]
- 35.Mazidi M, Kengne AP, Mikhailidis DP, Toth PP, Ray KK, Banach M. Dietary food patterns and glucose/insulin homeostasis: a cross-sectional study involving 24,182 adult Americans. Lipids Health Dis. 2017;16:192. doi: 10.1186/s12944-017-0571-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Deneo-Pellegrini H, Boffetta P, De Stefani E, Ronco A, Brennan P, Mendilaharsu M. Plant foods and differences between colon and rectal cancers. Eur J Cancer Prev. 2002;11:369–75. doi: 10.1097/00008469-200208000-00009. [DOI] [PubMed] [Google Scholar]
- 37.Hansen L, Skeie G, Landberg R, et al. Intake of dietary fiber, especially from cereal foods, is associated with lower incidence of colon cancer in the HELGA cohort. Int J Cancer. 2012;131:469–78. doi: 10.1002/ijc.26381. [DOI] [PubMed] [Google Scholar]
- 38.Steinmetz KA, Potter JD. Food-group consumption and colon cancer in the Adelaide Case-Control Study. I. Vegetables and fruit. Int J Cancer. 1993;53:711–9. doi: 10.1002/ijc.2910530502. [DOI] [PubMed] [Google Scholar]
- 39.De Stefani E, Correa P, Boffetta P, Deneo-Pellegrini H, Ronco AL, Mendilaharsu M. Dietary patterns and risk of gastric cancer: a case-control study in Uruguay. Gastric Cancer. 2004;7:211–20. doi: 10.1007/s10120-004-0295-2. [DOI] [PubMed] [Google Scholar]
- 40.Williams CD, Satia JA, Adair LS, et al. Dietary patterns, food groups, and rectal cancer risk in Whites and African-Americans. Cancer Epidemiol Biomarkers Prev. 2009;18:1552–61. doi: 10.1158/1055-9965.EPI-08-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Asli LA, Olsen A, Braaten T, Lund E, Skeie G. Potato consumption and risk of colorectal cancer in the Norwegian women and cancer cohort. Nutr Cancer. 2017;69:564–72. doi: 10.1080/01635581.2017.1295086. [DOI] [PubMed] [Google Scholar]
- 42.Ludwig DS. The glycemic index: physiological mechanisms relating to obesity, diabetes, and cardiovascular disease. JAMA. 2002;287:2414–23. doi: 10.1001/jama.287.18.2414. [DOI] [PubMed] [Google Scholar]
- 43.van Bakel MM, Kaaks R, Feskens EJ, et al. Dietary glycaemic index and glycaemic load in the European Prospective Investigation into Cancer and Nutrition. Eur J Clin Nutr. 2009;(63 Suppl 4):S188–205. doi: 10.1038/ejcn.2009.81. [DOI] [PubMed] [Google Scholar]
- 44.Sieri S, Krogh V, Agnoli C, et al. Dietary glycemic index and glycemic load and risk of colorectal cancer: results from the EPIC-Italy study. Int J Cancer. 2015;136:2923–31. doi: 10.1002/ijc.29341. [DOI] [PubMed] [Google Scholar]
- 45.Mazidi M, Kengne AP, Banach M. Mineral and vitamin consumption and telomere length among adults in the United States. Pol Arch Intern Med. 2017;127:87–90. doi: 10.20452/pamw.3927. [DOI] [PubMed] [Google Scholar]
- 46.Mottram DS, Wedzicha BL, Dodson AT. Acrylamide is formed in the Maillard reaction. Nature. 2002;419:448–9. doi: 10.1038/419448a. [DOI] [PubMed] [Google Scholar]
- 47.Aburto NJ, Hanson S, Gutierrez H, Hooper L, Elliott P, Cappuccio FP. Effect of increased potassium intake on cardiovascular risk factors and disease: systematic review and meta-analyses. BMJ. 2013;346:f1378. doi: 10.1136/bmj.f1378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mazidi M, Shivappa N, Wirth MD, et al. Dietary inflammatory index and cardiometabolic risk in US adults. Atherosclerosis. 2018;276:23–7. doi: 10.1016/j.atherosclerosis.2018.02.020. [DOI] [PubMed] [Google Scholar]
- 49.Hu FB, Stampfer MJ, Rimm E, et al. Dietary fat and coronary heart disease: a comparison of approaches for adjusting for total energy intake and modeling repeated dietary measurements. Am J Epidemiol. 1999;149:531–40. doi: 10.1093/oxfordjournals.aje.a009849. [DOI] [PubMed] [Google Scholar]
- 50.Chiuve SE, Fung TT, Rimm EB, et al. Alternative dietary indices both strongly predict risk of chronic disease. J Nutr. 2012;142:1009–18. doi: 10.3945/jn.111.157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bernstein AM, Sun Q, Hu FB, Stampfer MJ, Manson JE, Willett WC. Major dietary protein sources and risk of coronary heart disease in women. Circulation. 2010;122:876–83. doi: 10.1161/CIRCULATIONAHA.109.915165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu FB, Leitzmann MF, Stampfer MJ, Colditz GA, Willett WC, Rimm EB. Physical activity and television watching in relation to risk for type 2 diabetes mellitus in men. Arch Intern Med. 2001;161:1542–8. doi: 10.1001/archinte.161.12.1542. [DOI] [PubMed] [Google Scholar]
- 53.Mozaffarian D, Hao T, Rimm EB, Willett WC, Hu FB. Changes in diet and lifestyle and long-term weight gain in women and men. N Engl J Med. 2011;364:2392–404. doi: 10.1056/NEJMoa1014296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Halkjaer J, Tjonneland A, Overvad K, Sorensen TI. Dietary predictors of 5-year changes in waist circumference. J Am Dietetic Assoc. 2009;109:1356–66. doi: 10.1016/j.jada.2009.05.015. [DOI] [PubMed] [Google Scholar]
- 55.Halkjaer J, Sorensen TI, Tjonneland A, Togo P, Holst C, Heitmann BL. Food and drinking patterns as predictors of 6-year BMI-adjusted changes in waist circumference. Br J Nutr. 2004;92:735–48. doi: 10.1079/bjn20041246. [DOI] [PubMed] [Google Scholar]
- 56.Mazidi M, Katsiki N, Mikhailidis DP, Banach M. Ideal cardiovascular health associated with fatty liver: results from a multi-ethnic survey. Atherosclerosis. 2019;284:129–35. doi: 10.1016/j.atherosclerosis.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 57.Mazidi M, Katsiki N, Mikhailidis DP, Banach M. Adiposity may moderate the link between choline intake and non-alcoholic fatty liver disease. J Am Coll Nutr. 2019;38:633–9. doi: 10.1080/07315724.2018.1507011. [DOI] [PubMed] [Google Scholar]
- 58.Mazidi M, Katsiki N, Mikhailidis DP, Bartłomiejczyk MA, Banach M. Association of empirical dietary atherogenic indices with all-cause and cause-specific mortality in a multi-ethnic adult population of the United States. Nutrients. 2019;11(10) doi: 10.3390/nu11102323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guenther PM, Ding EL, Rimm EB. Alcoholic beverage consumption by adults compared to dietary guidelines: results of the National Health and Nutrition Examination Survey, 2009-2010. J Acad Nutr Diet. 2013;113:546–50. doi: 10.1016/j.jand.2012.12.015. [DOI] [PubMed] [Google Scholar]