Abstract
Background
Toxoplasma gondii (T. gondii) is an intracellular pathogen that can lead to abortion in pregnant women infected with this parasite. Therefore, the present study aimed to estimate the global seroprevalence of anti-T. gondii antibodies in women who had spontaneous abortion based on the results of published articles and evaluate the relationship between seroprevalence of anti-T. gondii antibodies and abortion via a systematical review and meta-analysis.
Methods
Different databases were searched in order to gain access to all studies on the seroprevalence of anti- T. gondii antibodies in women who had spontaneous abortion and association between seroprevalence of anti-T. gondii antibodies and abortion published up to April 25th, 2019. Odds ratio (OR) and the pooled rate seroprevalence of T. gondii with a 95% confidence interval (CI) were calculated using the random effects model.
Results
In total, 8 cross-sectional studies conducted on 1275 women who had abortion in present pregnancy, 40 cross-sectional studies performed on 9122 women who had a history of abortion, and 60 articles (involving 35 cross-sectional studies including 4436 women who had spontaneous abortion as case and 10398 as control and 25 case-control studies entailing 4656 cases and 3178 controls) were included for the final analyses. The random-effects estimates of the prevalence of anti-T. gondii IgG antibody in women who had abortion in present pregnancy and women who had a history of abortion were 33% (95% CI: 17%-49%) and 43% (95% CI: 27%-60%), respectively. In addition, the pooled OR for anti-T. gondii IgG antibody in cross-sectional and case-control studies among women who had spontaneous abortion were 1.65 (95% CI: 1.31–2.09) and 2.26 (95% CI: 1.56–3.28), respectively. Also, statistical analysis showed that the pooled OR of the risk of anti-T. gondii IgM antibody 1.39 (95% CI: 0.61–3.15) in cross-sectional and 4.33 (95% CI: 2.42–7.76) in case-control studies.
Conclusion
Based on the results of the current study, T. gondii infection could be considered a potential risk factor for abortion. It is recommended to carry out further and more comprehensive investigations to determine the effect of T. gondii infection on abortion to prevent and control toxoplasmosis among pregnant women around the world.
Author summary
Toxoplasma gondii (T. gondii) is of utmost importance during pregnancy since it can pass through the placental barrier and infect the embryo’s tissues. Consequences of passing the placental barrier and infecting the fetus is abortion, fetal death or severe congenital defects, such as hydrocephaly and chorioretinitis. Although extensive studies have been conducted on the seroprevalence of anti-T. gondii antibodies in women and its role in abortion, the diversity of studies design, sample size, and the various quality of studies pose daunting challenges to the available information. Therefore, it is essential that this information be updated and synthesized to help physicians and healthcare providers. The results of the current study revealed the high seroprevalence of anti-T. gondii antibodies in women who had spontaneous abortion and the positive relationship between seroprevalence of anti-T. gondii antibodies and abortion.
Introduction
Toxoplasmosis is a serious endemic disease caused by an intracellular parasite called Toxoplasma gondii (T. gondii). According to the seroepidemiological studies, this parasite infects about 15–85% of the total population of the world [1–3]. The only known definitive hosts for T. gondii are members of family Felidae, including domestic and wild cats. On the other hand, various warm-blooded mammals, including humans and rodents can be the intermediate host of this parasite [4]. Human infections are acquired through several major ways: 1) consumption of undercooked meat especially pork and mutton and unpasteurized milk from infected animals, 2) direct or indirect contact with oocysts from the environment, 3) vertical transmission during pregnancy, 4) blood transfusions, and 5) organ transplants [5–8]. T. gondii infection is generally asymptomatic in immunologically healthy adults. However, it can cause a variety of life-threatening clinical complications in immunocompromised patients [9]. This parasite is of utmost importance during pregnancy since it can cross the placental barrier to infect embryonic tissues [10, 11]. If this infection occurs during the first and second trimester of pregnancy, it may manifest in severe symptoms, such as low birth weight, hydrocephaly, intracranial calcifications, and retinochoroiditis that are recognizable at birth [12]. On the other hand, infections in the third trimester of pregnancy do generally not show symptoms at birth; however, they may develop intracranial calcifications, hearing impairment, visual disorders, and developmental delay later in life [13]. The global annual incidence rate of congenital toxoplasmosis (CT) is estimated to be 190,100 cases with an approximate incidence rate of 1.5 cases per 1000 live births [14]. Effective factor in transplacental transmission and severity of CT depends on the time of maternal infection [15]. There are two types of miscarriage: sporadic and recurrent [16]. Spontaneous pregnancy loss is a clinical problem of pregnancy occurring in 15% of all clinically recognized pregnancies [17]. The diagnosis of CT for the prevention of abortion is based on laboratory techniques, monitoring the immune response, direct detection of the parasite by animal or tissue inoculation, and molecular techniques [18].
Some of the risk factors of abortion cited in different studies include ethnicity, stress, use of non-steroidal anti-inflammatory or some antidepressant drugs, smoking, use of cocaine, caffeine and alcohol abuse, and obesity [19–29]. Specifically, 15% of early abortions and 66% of late abortions are attributed to infections [30, 31]. T. gondii, Toxocara cati, Toxocara canis, Plasmodium falciparum, Escherichia coli, Listeria monocytogenes, Brucella species, Klebsiella pneumonia, Rubella, Cytomegalovirus, Varicella—Zoster Virus, human immunodeficiency virus, and human papillomavirus are the most common causes of intrauterine infections [32, 33]. Congenital infections, such as CT, are the main causes of miscarriage [33]. Habitual abortion or recurrent miscarriage is the three consecutive pregnancy loss prior to 20 weeks from the last menstrual period [17]. The common causes of habitual abortion include untreated hypothyroidism [34], parental chromosomal abnormalities [35], certain uterine anatomic abnormalities [36], uncontrolled diabetes mellitus [37], immunologic abnormalities [38], infections [39], and environmental factors [40]. The prevalence of T. gondii infection in pregnant women varies significantly across different continents and countries around the world. Seroprevalence of T. gondii is reported to be high in Europe, up to 54% in Southern European countries, whereas this value was found to be within 18.5%-92.5% in sub-Saharan Africa [41, 42]. Given the important role of T. gondii infection in abortion and indefinite rate of T. gondii-associated abortion around the world, the present study aimed to determine the rate of seroprevalence of anti-T. gondii antibodies among women who had abortion or a history of abortion, and evaluate the relationship between seroprevalence of anti-T. gondii antibodies and abortion in women.
Methods
Design and protocol registration
A protocol was registered with PROSPERO (No. CRD42019124531) and published and the methods are briefly reported here [43]. We used the preferred reporting items for systematic reviews and meta-analysis guidelines for the performance of this study (S1 Table) [44].
Search strategy
A literature search was performed using the following electronic databases: PubMed, Scopus, EMBASE, ProQuest, ScienceDirect, Web of Science, and Google Scholar search engine from inception until 25th of April 2019. Search terms are applied alone or in combination as follows: (Toxoplasma gondii OR T. gondii OR toxoplasmosis) AND (abortion OR miscarriage OR fetal loss) AND pregnant women. In addition, the search was restricted to English language articles; therefore, published articles in non-English languages and unpublished studies were not investigated. Moreover, citation lists of relevant papers were checked.
Inclusion and exclusion criteria
Inclusion criteria entailed English language articles evaluating the effects of T. gondii on abortion only among human subjects in cross-sectional and case-control studies. On the other hand, we excluded non-original papers (reviews, systematic reviews, editorials or letters), and conference papers. Also, the studies that T. gondii infection was diagnosed by molecular methods in women who had spontaneous abortion, paraffin-embedded blocks and placenta tissues were excluded from our study.
Study selection and data extraction
All retrieved articles were stored in EndNote X9 to organize the summaries. Search results were merged, duplicates were removed automatically and all the titles and abstracts and identified relevant articles were independently scanned by two team members (Fig 1). Two reviewers independently extracted the data using a standardized form. The extracted variables included the name of the first author, year of publication, location of the study, diagnostic method, serological results, number of seropositive cases, as well as the age of the participants.
Fig 1. Flow diagram of the study design process.
Quality assessment
The quality of the articles was assessed independently in terms of selection, comparability, and exposure. Quality was scored using the Newcastle-Ottawa Scales (NOS) [45]. The quality scale was within the range of 0–9 points with a score of ≤ 3 representing a low-quality study. On the other hand, studies with a NOS score > 6 in case-control and > 5 in cross-sectional studies are considered high quality.
Statistical analysis
The data were analyzed in Stata software (version 14; Stata Corp, College Station, TX, USA). The odds ratio (ORs) was used for this meta-analysis with 95% confidence intervals (CIs) to assess the relationship between seroprevalence of anti-T. gondii antibodies and abortion in women who had spontaneous abortion. Moreover, the pooled seroprevalence of anti-T. gondii antibodies was calculated in women with either a recent abortion or a history of abortion. In the forest plots, OR > 1 denotes the positive effect of T. gondii on abortion, whereas an OR < 1 is indicative of the protective effect of T. gondii on abortion. In addition, I2 statistics were applied to represent the heterogeneity index [46]. Only when I2 > 50%, the heterogeneity was considered significant. Publication bias was evaluated through Egger’s test and it was considered significant when P-value was less than 0.05 [47]. Additionally, the sensitivity analysis test was performed by removing the effect of each study on the overall results. In addition, the method of “trim and fill” has been used for a comprehensive evaluation of the possible effects of publication bias. The subgroup analysis and meta-regression test were conducted according to the diagnostic methods and type of study.
Results
Study identification and selection
A total of 35105 publications were identified and archived in EndNote (version X9) to organize the resources used in the research process. Duplicates were removed by automation followed by a manual duplicate search. The duplication search included a comparison of the authors’ name, journals name, year of publication, volume number, issue number, page number, and the titles. In the next step, the titles and abstracts of full texts were independently reviewed by two researchers (TN and ZH). Finally, 244 papers were selected for the accurate evaluation of full texts out of which 72 papers were included in the systematic review. One study was not analyzed because it did not accurately quantify the number of IgM and IgG positive cases separately [48], and its data were only systematically presented; therefore, 71 papers were included in the meta-analysis. Eight articles were related to the seroprevalence of anti-T. gondii antibodies in women who had spontaneous abortion in present pregnancy [49–56]. Moreover, serologic methods were used in six of these studies [49–52, 55, 56]. Two out of 8 articles used both serological and molecular methods in the diagnosis of T. gondii [53, 54]. The seroprevalence of anti-T. gondii antibodies in women who had a history of abortion (one or more times) was investigated in 40 articles. In addition, 60 articles were reviewed to estimate the relationship between seroprevalence of anti-T. gondii antibodies and abortion, out of which 35 articles were cross-sectional studies that examined the seroprevalence of anti-T. gondii antibodies in women who had a history of abortion; however, it was possible to calculate the ORs for them due to the nature of the studies. Therefore, they were also used to evaluate the relationship between seroprevalence of anti-T. gondii antibodies and abortion. Fig 1 demonstrates the process of articles screening and selection.
General characteristics of included studies
General characteristics of included studies are depicted in Tables 1–3. These studies which were published between 1971 and 2019 included 25 case-control and 47 cross-sectional studies. As shown in the Tables 1–3, various studies have used the different diagnostic methods, such as enzyme-linked immunosorbent assay (ELISA), direct agglutination test (DAT), complement fixation test (CFT), latex agglutination test (LAT), indirect haemagglutination (IHA), haemagglutination (HA), indirect immunofluorescence assay (IFA), enzyme immunoassay (EIA), lateral flow immunoassay (LFIA), avidity test, Remington test, mini VIDAS technique, and one step advanced quality. Participants in the majority of articles were ascertained by ELISA (50 studies). Some studies used two or more diagnostic methods for T. gondii infection. It is noted that most researchers performed the tests using commercial kits and some of them used in house tests.
Table 1. Characteristics of the included studies for seroprevalence of anti-T. gondii antibodies in women who had a history of abortion.
| First author | Publication year | Place of study | Type of study | Method (s) | Test | Sample size (n) | IgG+ n (%) | IgM+ n (%) | Age (years) |
|---|---|---|---|---|---|---|---|---|---|
| Kimball, 1971 [55] | 1971 | USA | Cs | DAT CFT |
IgG IgM |
941 | 355 (37.72) | -- | -- |
| Stray-Pedersen, 1979 [57] | 1979 | Norway | Cs | DAT IFA |
IgG | 2048 | 279 (13.62) | -- | ≥ 35 |
| Decavalas, 1990 [58] | 1990 | Greece | Cs | IFA for IgG Remington for IgM |
IgG IgM |
126 | 66 (52.38) | 0 (0) | -- |
| Singh, 1998 [59] | 1998 | United Arab Emirates | Cs | IFA | IgG IgM |
1823 | 547 (30) | 3 (0.16) | -- |
| Qublan, 2002 [60] | 2002 | Amman | Cs | IFA | IgG IgM |
104 | 64 (61.53) | -- | 15–46 |
| Elnahas, 2003 [5] | 2003 | Sudan | Cs | ELISA | IgG IgM |
129 | 46 (35.65) | -- | -- |
| Nissapatorn, 2003 [61] | 2003 | Malaysia | Cs | ELISA | IgG IgM |
14 | 10 (71.42) | -- | 15–44 |
| Chopra, 2004 [62] | 2004 | India | Cs | ELISA | IgM | 118 | 61 (51.69) | 15–45 | |
| Ertug, 2005 [63] | 2005 | Turkey | Cs | ELISA IFA DAT Avidity test |
IgG | 90 | 33 (36.7) | -- | 15–40 |
| Barbosa, 2009 [64] | 2009 | Brazil | Cs | EIA | IgG IgM |
71 | 46 (64.78) | -- | 13–40 |
| Nijem, 2009 [65] | 2009 | Palestine | Cs | ELISA | IgG IgM |
76 | 25 (32.89) | 14 (18.42) | 16–43 |
| Mousa, 2011 [66] | 2011 | Libya | Cs | ELISA | IgG IgM |
117 | 55 (47.1) | -- | 18–44 |
| Drueish, 2011 [67] | 2011 | Iraq | Cs | ELISA | IgG IgM |
122 | 25 (20.49) | 17 (13.93) | 15–45 |
| Pavlinová, 2011 [32] | 2011 | Slovak Republic | Cs | EIA | IgG IgM |
221 | 93 (42.1) | 4 (1.8) | 31.3 ± 5.6 |
| Jasim, 2011 [68] | 2011 | Iraq | Cs | ELISA | IgG IgM |
162 | 144 (88.88) | 148 (91.35) | 15–65 |
| Nissapatorn, 2011 [69] | 2011 | Thailand | Cs | ELISA | IgG IgM |
147 | 43 (29.25) | -- | 15–45 |
| Hajsoleimani, 2012 [6] | 2012 | Iran | Cs | ELISA | IgG IgM |
423 | 159 (37.6) | -- | > 30 |
| Malarvizhi, 2012 [70] | 2012 | India | Cs | ELISA | IgG IgM |
67 | 12 (17.91) | (11.94) | > 40 |
| Padmavathy, 2013 [71] | 2013 | India | Cs | ELISA | IgG IgM |
47 | 27 (57.44) | 4 (8.51) | 19.36 |
| Ebrahimzadeh, 2013 [72] | 2013 | Iran | Cs | ELISA | IgG IgM |
71 | 17 (23.94) | -- | 14–44 |
| Moura, 2013 [73] | 2013 | Brasil | Cs | IFA ELISA |
IgG IgM |
92 | 59 (64.13) | -- | 14–45 |
| Babaie, 2013 [74] | 2013 | Iran | Cs | ELISA | IgG IgM |
82 | 31 (37.80) | -- | 16–47 |
| Chintapalli, 2013 [75] | 2013 | India | Cs | ELISA | IgG IgM |
20 | 15 (75) | 5 (25) | 15–34 |
| Alvarado-Esquivel, 2014 [76] | 2014 | Mexico | Cs | EIA | IgG IgM |
326 | 22 (6.7) | 2 (0.6) | 35.57 ± 12.43 |
| Almushait, 2014 [77] | 2014 | Saudi Arabia | Cs | ELISA | IgG IgM |
162 | 71 (43.82) | 12 (7.40) | 16–41 |
| Abedi, 2015 [78] | 2015 | Iran | Cs | ELISA | IgG | 300 | 111 (37) | -- | 16–39 |
| Awoke, 2015 [42] | 2015 | Ethiopia | Cs | LAT | IgG IgM |
95 | 29 (30.5) | -- | 15–44 |
| Gelaye, 2015 [79] | 2015 | Ethiopia | Cs | LAT | IgG IgM |
71 | 62 (87.3) | -- | 15–35 |
| Alvarado-Esquivel, 2015 [80] | 2015 | Mexico | Cs | EIA | IgG IgM |
43 | 2 (4.7) | -- | 16–50 |
| Anubhuti, 2015 [81] | 2015 | India | Cs | LFIA | IgG IgM |
60 | 12 (20) | 0 (0) | 21–35 |
| Mohamed, 2016 [82] | 2016 | Saudi Arabia | Cs | EIA | IgG IgM |
126 | 23 (18.25) | 0 (0) | 16–40 |
| Mohaghegh, 2016 [83] | 2016 | Iran | Cs | ELISA | IgG IgM |
35 | 35 (100) | -- | 18–45 |
| Imam, 2016 [84] | 2016 | Egypt | Cs | ELISA | IgG IgM |
112 | 22 (19.6) | -- | 15–49 |
| Nazir, 2017 [85] | 2017 | Pakistan | Cs | ELISA | IgG | 93 | 31 (33.33) | -- | ≥ 36 |
| Yasmeen, 2017 [86] | 2017 | India | Cs | ELISA | IgG IgM |
39 | 10 (25.6) | -- | 18–35 |
| Negero, 2017 [41] | 2017 | Ethiopia | Cs | LAT | IgG IgM |
207 | 176 (83.8) | -- | 15–34 |
| Matin, 2017 [87] | 2017 | Iran | Cs | ELISA Nested-PCR |
IgG IgM |
200 | 86 (43) | 8 (4) | 16–41 |
| Costa, 2018 [88] | 2018 | Brazil | Cs | ELISA | IgG IgM |
89 | 64 (71.9) | -- | > 19 |
| Hafez Hassanain [89] | 2018 | Egypt | Cs | ELISA | IgG IgM IgG avidity |
47 | 20 (42.5) | -- | 15–44 |
| Rashno, 2019 [90] | 2019 | Iran | Cs | ELISA PCR |
IgG IgM |
6 | 3 (50) | 0 (0) | -- |
Cs: cross-sectional, DAT: direct agglutination test, CFT: complement fixation test, LAT: latex agglutination test, ELISA: enzyme-linked immunosorbent assay, IFA: indirect immunofluorescence assay, EIA: enzyme immunoassay, LFIA: lateral flow immunoassay, PCR: polymerase chain reaction, IgG: immunoglobulin G, IgM: immunoglobulin M
Table 3. Description of the studies included looking for an association between seroprevalence of anti-T. gondii antibodies and abortion.
| First author | Publication year | Place of study | Type of study | Method (s) | Test | N | Case (n) | Case & IgG+ (n, %) | Case & IgM+ (n, %) | Control (n) | Control & IgG+ (n, %) | Control & IgM+ (n, %) | Age (years) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kimball, 1971 [55] | 1971 | USA | Cs | DAT CFT |
IgG IgM |
4832 | 941 | 355 (37.72) | -- | 3891 | 1206 (31) | -- | P: -- C: 25 |
| Stray-Pedersen, 1977 [91] | 1977 | Norway | Cc | DAT IFA CFT |
IgG | 216 | 157 | 39 (25) | -- | 59 | 11 (19) | -- | P: 29.4 C: 29.4 |
| Lolis, 1978 [92] | 1978 | Greece | Cc | HA IFA |
IgG IgM IgA |
232 | 152 | 62 (40.8) | -- | 80 | 10 (12.5) | -- | -- |
| Decavalas, 1990 [58] | 1990 | Greece | Cs | IFA for IgG Remington for IgM |
IgG IgM | 303 | 126 | 66 (52.38) | 0 (0) | 177 | 88 (49.71) | 0 (0) | P: 27.8 C: 25.9 |
| Galvan Ramirez, 1995 [93] | 1995 | Mexico | Cc | ELISA | IgG IgM | 155 | 105 | 48 (44.9) | 35 (33.3) | 50 | 13(26.01) | 1 (1.9) | P: 32.3 ± 7 C: 31.7 ± 7.1 |
| Sahwi, 1995 [94] | 1995 | Egypt | Cc | IHA | IgG IgM | 140 | 100 | 37 (37) | 19 (19) | 40 | 4 (10) | 3 (7.5) | P: 30 ± 5.975 C: 28 ± 5.236 |
| Djurkovic-Djakovic, 1995 [95] | 1995 | Yogoslavi | Cc | DAT | IgG IgM | 2315 | 1747 | 570 (32.62) | 94 (5.38) | 568 | 193 (33.97) | 27 (4.75) | 14–45 |
| Al Hamdani, 1997 [96] | 1997 | Iraq | Cc | IHA IFA |
IgG IgM | 200 | 81 | 15 (18.5) | -- | 119 | 7 (5.9) | -- | P: 29 ± 7.9 C: 27 ±6.93 |
| Zargar, 1998 [97] | 1998 | India | Cc | ELISA | IgM | 454 | 285 | -- | 141 (49.47) | 169 | -- | 15 (8.87) | P: 27.73 ± 4.57 C: 28.59 ±5.13 |
| Qublan, 2002 [60] | 2002 | Amman | Cs | IFA | IgG IgM | 280 | 104 | 64 (61.53) | -- | 176 | 68 (38.63) | -- | 15–46 |
| Elnahas, 2003 [5] | 2003 | Sudan | Cs | ELISA | IgG IgM | 487 | 129 | 46 (35.65) | -- | 358 | 120 (33.51) | -- | -- |
| Nissapatorn, 2003 [61] | 2003 | Malaysia | Cs | ELISA | IgG IgM | 200 | 14 | 10 (71.42) | 186 | 88 (47.3) | 15–44 | ||
| Chopra, 2004 [62] | 2004 | India | Cs | ELISA | IgM | 218 | 118 | 61 (51.69) | 100 | 0 (0) | 15–45 | ||
| Nimri, 2004 [98] | 2004 | France | Cc | ELISA Nested PCR |
IgG IgM | 248 | 148 | 80 (54.0) | 4 (2.7) | 100 | 12 (12.0) | 0 (0) | P: 28 C: 28 |
| Ertug, 2005 [63] | 2005 | Turkey | Cs | ELISA IFA DAT Avidity Test |
IgG | 357 | 90 | 33 (36.7) | -- | 267 | 78 (29.2) | -- | 15–40 |
| Surpam, 2006 [99] | 2006 | India | Cc | ELISA | IgM | 119 | 44 | -- | 12 (27.27) | 75 | -- | 1 (1.33) | 20–38 |
| Sebastian, 2008 [100] | 2008 | India | Cc | ELISA | IgG IgM | 101 | 71 | 36 (50.7) | 30 | -- | 6 (20) | 15–34 | |
| Al-Saeed, 2008 [101] | 2008 | Iraq | Cc | LAT | IgG IgM | 140 | 120 | 50 (41.7) | -- | 20 | 0 (0) | -- | -- |
| Barbosa, 2009 [64] | 2009 | Brazil | Cs | EIA | IgG IgM | 190 | 71 | 46 (64.78) | -- | 119 | 81 (68.06) | -- | 13–40 |
| Nijem, 2009 [65] | 2009 | Palestine | Cs | ELISA | IgG IgM | 204 | 76 | 25 (32.89) | 14 (18.42) | 128 | 32 (25) | 22 (17.18) | 16–43 |
| AL–Taie, 2010 [102] | 2009 | Iraq | Cc | ELISA | IgM | 88 | 38 | -- | 15 (39.4) | 50 | -- | 6 (12) | > 41 |
| Hassan, 2009 [103] | 2009 | Iraq | Cc | ELISA | IgG IgM | 119 | 96 | 7 (7.29) | 20 (20.83) | 23 | 2 (8.7) | 0 (0) | 23.9–28.5 |
| Drueish, 2011 [67] | 2011 | Iraq | Cs | ELISA | IgG IgM | 177 | 122 | 25 (20.49) | 17 (13.93) | 50 | 12(24) | 0 (0) | 15–45 |
| Pavlinová, 2011 [32] | 2011 | Slovak Republic | Cs | EIA | IgG IgM | 537 | 221 | 93 (42.1) | 4 (1.8) | 179 | 45 (25.1) | 5 (2.8) | P: 31.3 ± 5.6 C: 29.4 ± 5.6 |
| Jasim, 2011 [68] | 2011 | Iraq | Cs | ELISA | IgG IgM | 300 | 162 | 144 (88.88) | 148 (91.35) | 138 | 123 (89.13) | 122 (88.40) | 15–65 |
| Mousa, 2011 [66] | 2011 | Libya | Cs | ELISA | IgG IgM | 143 | 117 | 55 (47.1) | -- | 26 | 9 (34.6) | -- | 18–44 |
| Nissapatorn, 2011 [69] | 2011 | Thailand | Cs | ELISA | IgG IgM | 640 | 147 | 43 (29.25) | -- | 493 | 138 (28) | -- | 15–45 |
| Hajsoleimani, 2012 [6] | 2012 | Iran | Cs | ELISA | IgG IgM | 500 | 423 | 159 (37.6) | -- | 77 | 30 (39) | -- | > 30 |
| Malarvizhi, 2012 [70] | 2012 | India | Cs | ELISA | IgG IgM | 232 | 67 | 12 (17.91) | 8 (11.94) | 165 | 11 (4.74) | 1 (0.43) | > 40 |
| Elamin, 2012 [104] | 2012 | Saudi Arabia | Cc | ELISA PCR |
IgG IgM | 188 | 94 | 33 (35.1) | 5 (15.2) | 94 | 37 (39.4) | 6 (16.2) | -- |
| Ebrahimzadeh, 2013 [72] | 2013 | Iran | Cs | ELISA | IgG IgM | 221 | 71 | 17 (23.94) | -- | 150 | 51 (34) | -- | 14–44 |
| Babaie, 2013 [74] | 2013 | Iran | Cs | ELISA | IgG IgM | 419 | 82 | 31 (37.80) | -- | 337 | 113 (33.5) | -- | 16–47 |
| Moura, 2013 [73] | 2013 | Brazil | Cs | IFA ELISA |
IgG IgM | 400 | 92 | 59 (64.13) | -- | 308 | 175 (56.81) | -- | 14–45 |
| Chintapalli, 2013 [75] | 2013 | India | Cs | ELISA | IgG IgM | 32 | 20 | 15 (75) | 5 (25) | 12 | 1 (8.33) | 1 (8.33) | 15–34 |
| Hussan, 2013 [105] | 2013 | Iraq | Cc | ELISA | IgG IgM | 255 | 210 | 46 (22) | 32 (15.23) | 45 | 3 (6.66) | 0 (0) | -- |
| Abou-Gabal, 2013 [48] | 2013 | Egypt | Cc | One step advanced quality | IgG IgM | 80 | 40 | -- | -- | 40 | -- | -- | P: 33.13 ± 10.341 C: 33.05 ± 8.941 |
| Siddiqui, 2014 [106] | 2014 | India | Cc | ELISA | IgG IgM | 63 | 48 | -- | 17 (35.4) | 15 | -- | 0 (0) | > 30 |
| Almushait, 2014 [77] | 2014 | Saudi Arabia | Cs | ELISA | IgG IgM | 487 | 162 | 71 (43.82) | 12 (7.40) | 325 | 118 (36.3) | 18 (5.5) | 16–41 |
| Sultana, 2014 [107] | 2014 | Bangladesh | Cc | ELISA | IgG IgM | 91 | 46 | 8 (17.4) | 7 (15.2) | 45 | 4 (8.9) | 0 (0) | P: 24.43 ± 4.17 C: 24.56 ±4.36 |
| Abbas, 2014 [108] | 2014 | Iraq | Cc | ELISA | IgG IgM | 130 | 100 | 42 (42) | 12 (12) | 30 | 0 (0) | 0 (0) | -- |
| Awoke, 2015 [42] | 2015 | Ethiopia | Cs | LAT | IgG IgM | 384 | 95 | 29 (30.5) | -- | 289 | 42 (14.5) | -- | 15–44 |
| Gelaye, 2015 [79] | 2015 | Ethiopia | Cs | LAT | IgG IgM | 288 | 71 | 62 (87.3) | -- | 217 | 184 (84.8) | -- | 15–35 |
| Saki, 2015 [109] | 2015 | Iran | Cc | ELISA | IgG IgM | 260 | 130 | 32 (24.6) | 1 (0.76) | 130 | 28 (21.5) | 0 (0) | -- |
| Kamal, 2015 [110] | 2015 | Egypt | Cc | ELISA | IgG IgM | 149 | 29 | 18 (62.0) | -- | 120 | 8 (6.66) | -- | 18–40 |
| Anubhuti, 2015 [81] | 2015 | India | Cs | LFIA | IgG IgM | 120 | 60 | 12 (20) | 0 (0) | 60 | 3 (5) | 0 (0) | 21–35 |
| Alvarado-Esquivel, 2015 [80] | 2015 | Mexico | Cs | EIA | IgG IgM | 150 | 43 | 2 (4.7) | -- | 107 | 12 (11.2) | -- | 16–50 |
| Ghasemi, 2016 [111] | 2016 | Iran | Cc | ELISA PCR |
IgG IgM | 192 | 82 | 22 (26.8) | 3 (3.6) | 110 | 29 (26.4) | 1 (0.9) | P: 28.29 C: 28.58 |
| Mohaghegh, 2016 [83] | 2016 | Iran | Cs | ELISA | IgG IgM | 350 | 35 | 35 (100) | -- | 315 | 75 (23.8) | -- | 18–45 |
| Mohamed, 2016 [82] | 2016 | Saudi Arabia | Cs | ELISA | IgG IgM | 326 | 126 | 23 (18.25) | 0 (0) | 200 | 46 (23) | 4 (2) | 16–40 |
| Nazir, 2017 [85] | 2016 | Pakistan | Cs | ELISA | IgG | 403 | 93 | 31 (33.33) | -- | 310 | 40 (12.90) | -- | ≥ 36 |
| Imam, 2016 [84] | 2016 | Egypt | Cs | ELISA | IgG IgM | 138 | 112 | 22 (19.6) | -- | 26 | 4 (15.4) | -- | 15–49 |
| Rasti, 2016 [112] | 2016 | Iran | Cc | ELISA | IgG IgM | 179 | 81 | 22 (27.2) | 1 (1.2) | 98 | 28 (28.6) | 2 (2) | P: 28.2 C: 28.6 |
| Yasmeen, 2017 [86] | 2017 | India | Cs | ELISA | IgG IgM | 251 | 39 | 10 (25.6) | -- | 212 | 43 (20.3) | -- | 18–35 |
| Negero, 2017 [41] | 2017 | Ethiopia | Cs | LAT | IgG IgM | 369 | 207 | 176 (83.8) | -- | 162 | 34 (16.2) | -- | 15–34 |
| Matin, 2017 [87] | 2017 | Iran | Cs | ELISA Nested-PCR | IgG IgM | 200 | 58 | 31 (53.44) | 0 (0) | 142 | 55 (38.73) | 8 (5.63) | 16–41 |
| Costa, 2018 [88] | 2018 | Brazil | Cs | ELISA | IgG IgM | 352 | 89 | 64 (71.9) | -- | 263 | 189 (71.8) | -- | > 19 |
| Çakmak, 2018 [113] | 2018 | Turkey | Cc | ELISA | IgG IgM | 1240 | 412 | 125 (30.6) | 27 (6.6) | 828 | 157 (19.2) | 35 (4.2) | P: 27.6 ± 11.4 C: 29.1 ± 9.87 |
| Hafez Hassanain, 2018 [89] | 2018 | Egypt | Cs | ELISA | IgG IgM IgG avidity |
388 | 47 | 20 (42.5) | -- | 341 | 59 (17.3) | -- | 15–44 |
| Rashno, 2019 [90] | 2019 | Iran | Cs | ELISA PCR |
IgG IgM | 98 | 6 | 3 (50) | 0 (0) | 92 | 31 (33.69) | 0 (0) | -- |
| Kheirandish, 2019 [114] | 2019 | Iran | Cc | ELISA | IgG IgM | 480 | 240 | 114 (47.5) | 8 (3.3) | 240 | 111 (46.3) | 1 (0.4) | P: 27 ± 6.499 C: 27.01 ± 6.459 |
Cs: cross-sectional, CC: case-control, DAT: direct agglutination test, CFT: complement fixation test, LAT: latex agglutination test, ELISA: enzyme-linked immunosorbent assay, IHA: indirect haemagglutination, HA: haemagglutination, IFA: indirect immunofluorescence assay, EIA: enzyme immunoassay, LFIA: lateral flow immunoassay, PCR: polymerase chain reaction, IgG: immunoglobulin G, IgM: immunoglobulin M, Case: women who had abortion, Control: women who had not abortion
Table 2. Characteristics of the included studies for seroprevalence of anti-T. gondii antibodies in women who had abortion in present pregnancy.
| First author | Publication year | Place of study | Type of study | Method (s) | Test | Sample size (n) | IgG+ n (%) | IgM+ n (%) | Age (years) |
|---|---|---|---|---|---|---|---|---|---|
| Kimball, 1971 [55] | 1971 | USA | Cs | DAT CFT |
IgG IgM |
260 | 109 (41.9) | 0 (0) | -- |
| Sanghi, 1997 [52] | 1997 | United Kingdom | Cs | LAT ELISA |
IgG IgM |
85 | 0 (0) | 0 (0) | -- |
| Hadi, 2011 [56] | 2011 | Iraq | Cs | Mini VIDAS technique | IgG IgM |
190 | 11 (5.78) | 24 (12.63) | 15–45 |
| Amin, 2012 [49] | 2012 | Iran | Cs | ELISA | IgG IgM IgA |
264 | 99 (37.5) | 21 (8.0) | 14–57 |
| Tammam, 2013 [50] | 2013 | Egypt | Cs | ELISA | IgG IgM |
76 | 35 (46.1) | 14 (18.4) | 19–36 |
| Vado-Solis, 2013 [53] | 2013 | Mexico | Cs | ELISA PCR |
IgG IgM |
100 | 32 (32) | 2 (2) | 25.3 ± 7.3 |
| Hernández-Cortazar, 2016 [54] | 2016 | Mexico | Cs | ELISA Q-PCR Nested PCR |
IgG IgM |
161 | 95 (59) | 6 (3.72) | -- |
| El Aal, 2018 [51] | 2018 | Egypt | Cs | ELISA Q-PCR LAMP |
IgG IgM |
139 | 62 (44.6) | -- | 21–34 |
Cs: cross-sectional, DAT: direct agglutination test, CFT: complement fixation test, LAT: latex agglutination test, ELISA: enzyme-linked immunosorbent assay, PCR: polymerase chain reaction, Q-PCR: quantitative PCR, LAMP: loop-mediated isothermal amplification, IgG: immunoglobulin G, IgM: immunoglobulin M
In addition, the included articles in the present meta-analysis showed an acceptable quality (i.e., ≥ 3 for cross-sectional studies and ≥ 4 for case-control studies). S2 Table represents the quality score of different eligible studies.
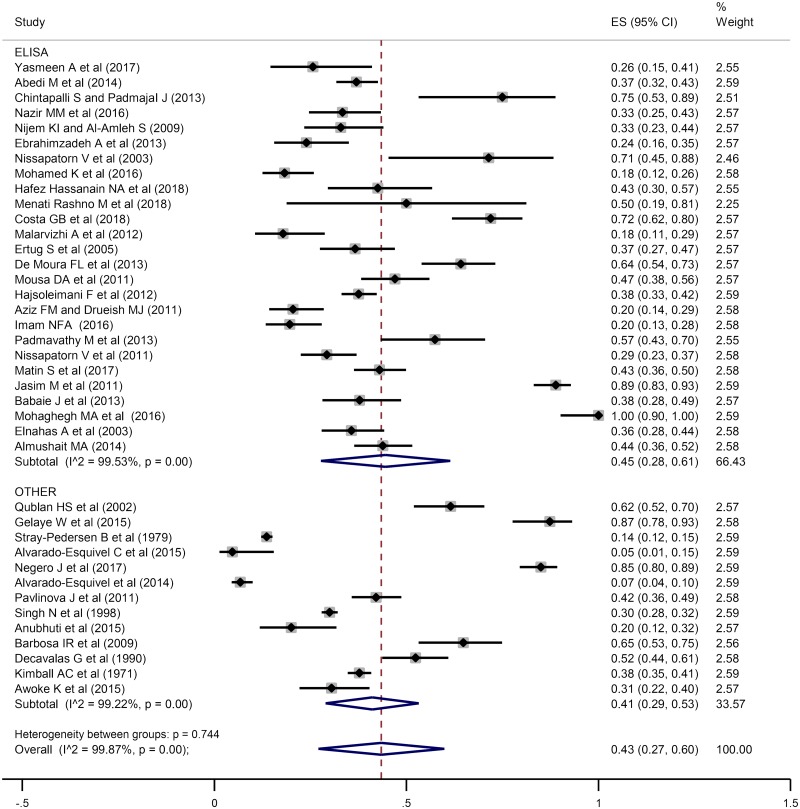
Seroprevalence of anti-T. gondii antibodies in women who had a history of abortion
A total of 9004 women who had a history of abortion were investigated for the seroprevalence of anti-T. gondii IgG antibody in 39 articles, out of which 2930 were positive using several serological methods. In addition, 16 studies entailing 3662 women who had spontaneous abortion were reviewed for the seroprevalence of anti-T. gondii IgM antibody out of which 286 women were seropositive. According to forest plot diagram in Fig 2 and S1 Fig, the pooled seroprevalence of anti- T. gondii IgG and IgM antibodies in women who had a history of abortion based on a random effects model was estimated at 43% (95% CI: 27%-60%) and 3% (95% CI: 3%-4%), respectively. Furthermore, I2 statistic revealed a significant heterogeneity among the studies (I2 = 99.87%, P = 0.00). Moreover, subgroup analysis results based on diagnostic methods showed that heterogeneity for ELISA and other methods (DAT, CFT, LAT, IFA, EIA, LFIA, and Remington test) were I2 = 99.53%, P = 0.00 and I2 = 99.22%, P = 0.00, respectively. In addition, heterogeneity between groups was P = 0.744 (Fig 2).
Fig 2. The reported seroprevalence of anti- T. gondii IgG antibody in women who had a history of abortion.
The horizontal lines define the reported 95% confidence interval for the seroprevalence in each study, and the diamond below the graph shows the pooled seroprevalence.
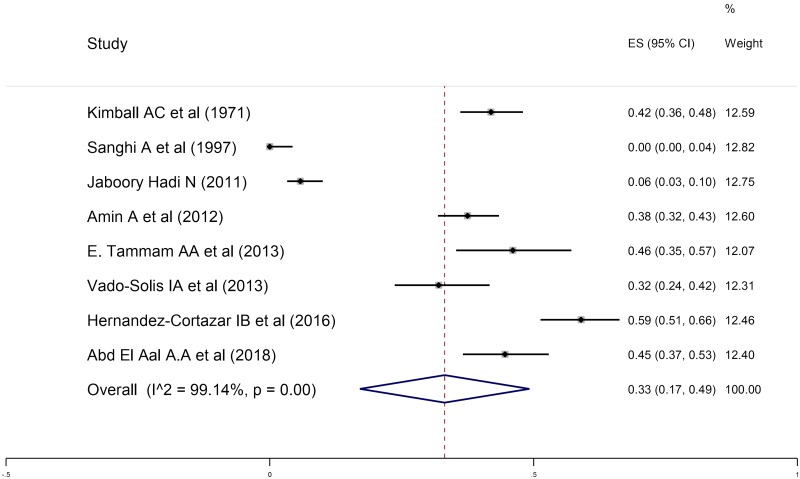
Seroprevalence of anti-T. gondii antibodies in women who had abortion in present pregnancy
A total number of 1275 women who had spontaneous abortion in present pregnancy were examined for the seroprevalence of anti- T. gondii antibodies using different serologic tests. 1275 serum samples in eight studies and 1136 serum samples of women who had abortion in present pregnancy in seven studies were evaluated for anti- T. gondii IgG and IgM antibodies using serologic tests out of which 443 and 67 cases were positive for anti- T. gondii IgG and IgM antibodies, respectively. The pooled seroprevalence rates of anti- T. gondii IgG and IgM antibodies in women who had abortion in present pregnancy using a random-effects model were determined to be 33% (95% CI: 17%-49%) and 1% (95% CI: 1%-2%), respectively (Fig 3 and S2 Fig). The results of heterogeneity test in different studies for IgG and IgM antibodies were I2 = 99.14%, P = 0.00; I2 = 92.06%, P = 0.00, respectively.
Fig 3. The reported seroprevalence of anti- T. gondii IgG antibody in women who had abortion in present pregnancy.
Relationship between anti-T. gondii IgG antibody and abortion
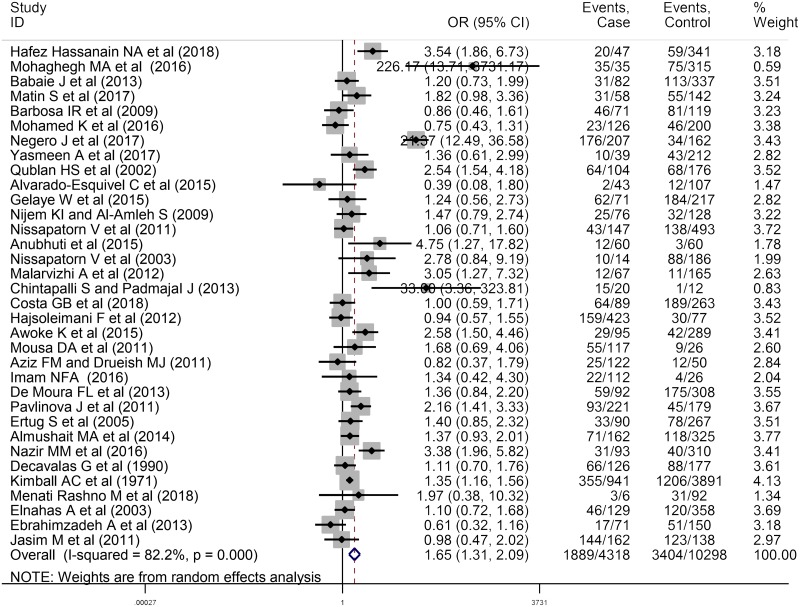
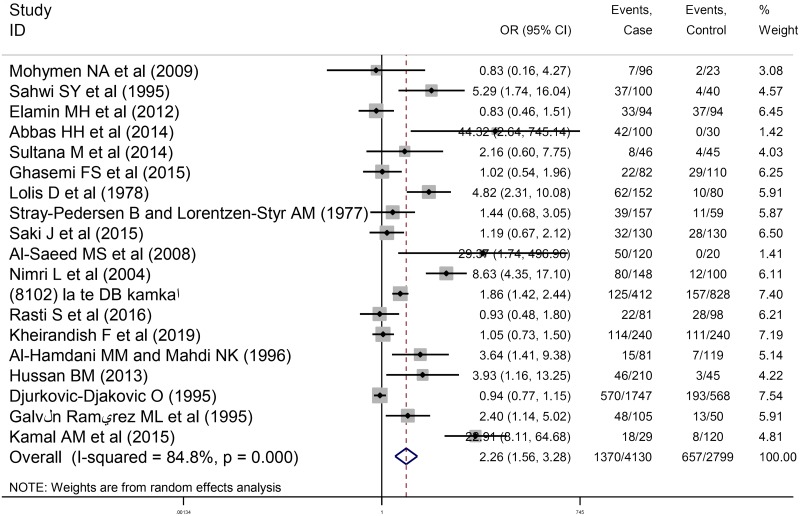
A total of 53 studies entailing 8448 women who had spontaneous abortion and 13097 control individuals were evaluated in the current study. This meta-analysis included 34 cross-sectional studies evaluating anti-T. gondii IgG antibody and abortion with 4318 women who had spontaneous abortion (1889 positive for anti-T. gondii antibodies) and 10298 women as the control group (3404 positive for anti-T. gondii antibodies). Moreover, 19 case-control articles evaluating anti-T. gondii IgG antibody and abortion were entered into the meta-analysis including 4130 women who had spontaneous abortion (1370 positive for anti- T. gondii antibodies) and 2799 controls (657 positive for anti- T. gondii antibodies). As depicted in the forest plot diagram, the pooled ORs of the risk of anti-T. gondii IgG antibody investigated in women who had spontaneous abortion in cross-sectional and case-control studies were 1.65 (95% CI: 1.31–2.09) and 2.26 (95% CI: 1.56–3.28), respectively (Figs 4 and 5). Additionally, the test of heterogeneity revealed significant heterogeneity among cross-sectional and case-control studies as I2 = 82.2%, P = 0.00 and I2 = 84.8%, P = 0.00, respectively. Publication bias was assessed using Egger’s test in cross-sectional studies (P = 0.194) and no publication bias was observed (S3 Fig). On the other hand, the results of Eggert’s test were statistically significant in case-control studies (P = 0.007) (S4 Fig). In addition, there was no change in the results of pooled random-effects analysis corrected by using “trim and fill” method (S5 Fig). The robustness and reliability of the results of this meta-analysis were indicated by sensitivity analysis (S6 and S7 Figs). In order to improve the interpretation of the meta-analysis results, a meta-regression test was performed based on the type of study revealing the significant effect of type of study on the heterogeneity of the studies (P = 0.032).
Fig 4. Forest plot diagram of cross-sectional studies showing IgG seropositivity rates of T. gondii.
Fig 5. Forest plot diagram of case-control studies showing IgG seropositivity rates of T. gondii.
Relationship between anti-T. gondii IgM antibody and abortion
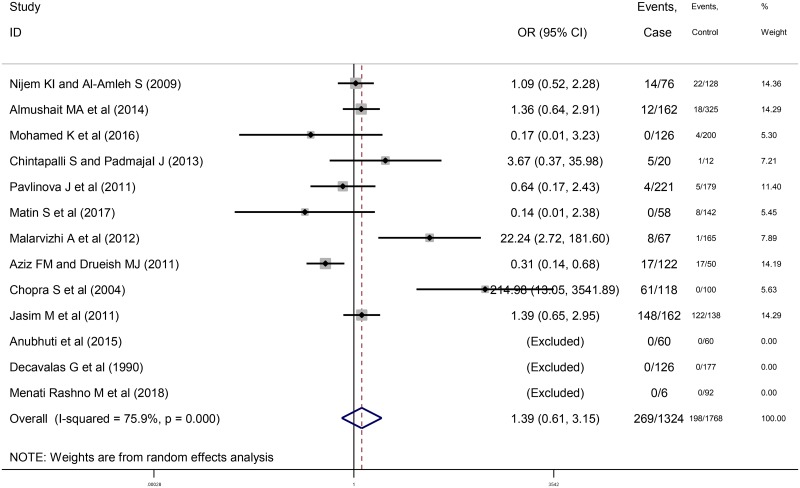
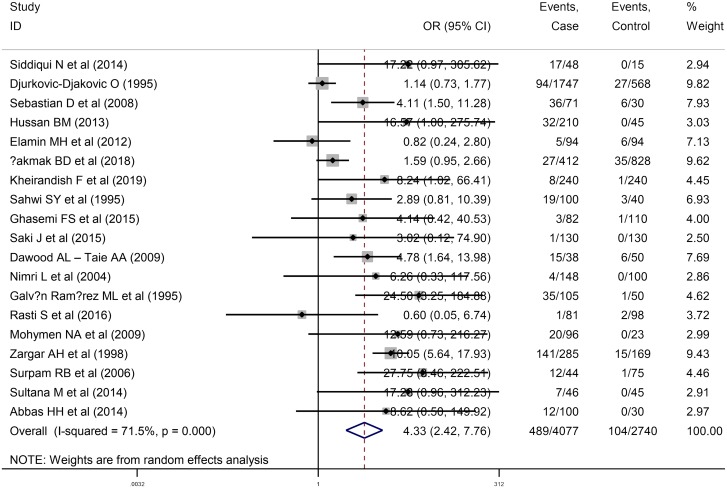
A total of 29 papers were entered into the meta-analysis including 1132 women who had spontaneous abortion (269 positive for anti- T. gondii antibodies) and 1439 controls (198 positive for anti- T. gondii antibodies) in 10 cross-sectional studies, as well as 4077 women who had spontaneous abortion (489 positive for anti- T. gondii antibodies) and 2740 controls (104 positive for anti- T. gondii antibodies) in 19 case-control studies evaluating anti-T. gondii IgM antibody. The results of the meta-analysis indicated a common OR of the risk of anti-T. gondii IgM antibody 1.39 (95% CI: 0.61-3.15) in cross-sectional studies (Fig 6) and 4.33 (95% CI: 2.42–7.76) in case-control ones (Fig 7). Moreover, the results illustrated a significant heterogeneity in cross-sectional and case-control studies (I2 = 75.9%, P = 0.00 and I2 = 71.5%, P = 0.00), respectively.
Fig 6. Forest plot diagram of cross-sectional studies showing IgM seropositivity rates of T. gondii.
Fig 7. Forest plot diagram of case-control studies showing IgM seropositivity rates of T. gondii.
The results of Egger’s test were not statistically significant in cross-sectional studies (P = 0.36), which indicates no publication bias. However, the funnel plot shows asymmetric pattern (S8 Fig). Based on the results of “trim and fill” method, publication bias does not appear to have a significant impact on the results (S9 Fig). On the other hand, the results of Egger’s test were statistically significant in case-control studies (P = 0.02) (S10 Fig). According to the results of “trim and fill” method, publication bias does not have a significant effect on the results of the pooled random-effects analysis (S11 Fig). The results confirmed the reliability and stability of the present meta-analysis after the performance of sensitivity analyses (S12 and S13 Figs). In addition, we performed the meta-regression analysis based on the type of study suggesting type of study had no significant effect on the heterogeneity among the studies included in the present meta-analysis (P = 0.77).
Discussion
Fetuses are at high risk of this parasite during acute infection due to Toxoplasma's ability to cross the placental barrier and infect the fetus before it can acquire immunity [115]. This systematic review and meta-analysis investigated the seroprevalence of anti- T. gondii antibodies in women who had abortion in present pregnancy or a history of abortion and the relationship between the seroprevalence of anti- T. gondii antibodies and abortion. The obtained results indicated that the overall estimation for the prevalence of anti- T. gondii IgG antibodies in women who had a history of abortion (43%; 95% CI: 27%-60%) was higher than those with present abortion (33%; 95% CI: 17%-49%). According to Figs 2 and 3, the highest and lowest seroprevalence rates of anti- T. gondii antibodies in women who had a history of abortion were related to the studies performed by Mohaghegh et al. as 100% (95% CI: 0–100%) [83] and Alvarado-Esquivel et al. as 5% (95% CI: 1–15%) [80]. Additionally, the highest and lowest seroprevalence rates of anti- T. gondii antibodies in women who had abortion in present pregnancy were observed in studies conducted by Hernández-Cortazar et al. as 59% (95% CI: 51–66%) [54] and Sanghi et al. as 0% (95% CI: 0–4%) [52]. IgG seropevalence is dependent on age and the risk of fetal loss increases after the age of 35 years. The risk of fetal loss is 9% in women aged 20–24 years and increases to 75% among women aged 45 and over. Because in the included articles the relationship of age and the seropositivity of T.gondii antibodies was not evaluated, we avoided the analysis of this main risk factor and this can be considered as a basic gap.
The prevalence of this infection varies according to geographical area and differences in climate, dietary habits, hygiene, and susceptibility of the host [116]. The global prevalence of T. gondii infection in pregnant women is within the range of 7%-51.3% and seroprevalence in women with abnormal pregnancies is within the range of 17.5–52.3% [93, 97]. The overall prevalence of T. gondii varies from country to country. Moreover, in some countries it has declined dramatically over the years. For example, in France, the prevalence of T. gondii infection in pregnant women in the 1960s was 84%, in 1995, it was 54% and in 2003, it was 44%. Since France operates a congenital toxoplasmosis surveillance system; so, this system appears to be an essential tool for evaluating the efficacy of new screening strategies [117].
Moreover, this study assessed the relationship between seroprevalence of anti- T. gondii antibodies and abortion. The results revealed that the ORs of prevalence of anti-T. gondii IgG antibodies in women who had spontaneous abortion were 1.65 (95% CI: 1.31–2.09) in cross-sectional and 2.26 (95% CI: 1.56–3.28) in case-control studies, compared to control group. In all of these analyses, heterogeneity was significant (I2 > 50%). Variation in these studies in terms of inclusion and exclusion criteria, the diverse populations, the difference in the methods of selection of the target populations, the age of the participants in the studies, the type of study (cross-sectional or case-control studies) may contribute to heterogeneity among studies. Therefore, a meta-regression test was performed to investigate the impact of the type of study on heterogeneity. The results suggested that the type of study had a significant effect on the assessment of the relationship between anti-T. gondii IgG antibody and abortion. However, this effect was not significant for anti-T. gondii IgM antibody. Based on results of the Egger’s test, there is no significant publication bias in cross-sectional studies. However, the results of Eggert’s test were statistically significant in case-control studies. These findings are likely to be related to the variability of the results of preliminary studies entered the meta-analysis. It is worthy to note that usually language-bias can be a possible reason for publication bias since only English-language articles were used in this study. As the results of the "trim and fill" method showed that the publication bias did not have a significant effect on the study results; so, the results presented in this paper seem to be valid and it is not necessary to use other languages. Furthermore, English abstracts of articles with non- English languages, due to the lack of sufficient information for analysis, were not included in our study.
Out of 72 studies, 43 were conducted in Asia, 12 in Africa, 9 in America and 8 in Europe and none in Australia and many European and American countries have no published articles in this field. There are several risk factors for the prevalence of T. gondii in women who had spontaneous abortion, such as ethnicity, socioeconomic status, history of contact with cats, raw meat consumption, strain’s virulence of T. gondii, immunological competence of the mother, smoking, and alcohol abuse. However, these risk factors were not specifically investigated in most studies and it was not possible to perform meta-analysis these risk factors.
It is important to determine the timing of abortion in relation to the measurement of seroprevalence of anti- T. gondii antibodies; because, occurrence of infection in the first trimester, when hormone levels are low and there is little helper T cell type 2 (Th2) bias, reduces the chance of transmission to the fetus but increases the likelihood of miscarriage. In contrast, the occurrence of infection in the third trimester, when there is a strong Th2 bias, makes abortion unlikely, but increases congenital transmission. The helper T cell type 1 responses caused by T. gondii infection in early pregnancy may induce miscarriage. In contrast, the strong Th2 bias and decreased natural killer cells, macrophages and CD8+ T cells function occur in the late stages of pregnancy that may contribute to parasite survival and increase the chance of congenital transmission [118]. However, due to the lack of evaluation of this risk factor in most studies assessing the relationship between abortion and the seroprevalence of anti- T. gondii antibodies, or incomplete data in some studies, the timing of abortion was not analyzed and this is considered as a major gap. On the other hand, in cross-sectional studies, it is not known when the abortion or the seroconversion happened.
Results of IgG calculated in this study (43% and 33%) do not provide sufficient data for the evaluation of CT. This can be due to the fact that IgG appears approximately 2 weeks after IgM, reaches peak levels within 6–8 weeks, and starts to slowly decrease to lower levels after 1 year until the end of infected subject’s life due to the persistence of latent cysts in immune-privileged organs [18, 119, 120].
In the current study, the overall prevalence of anti- T. gondii IgM antibodies in women who had abortion in present pregnancy or a history of abortion was estimated at 1% (95% CI: 1%-2%) and 3% (95% CI: 3%-4%), respectively. IgM antibody is an indicator of recent infection and detection of specific IgM antibody can assist in the determination of acute infection. It is usually detectable 1 week after infection and declines more rapidly, compared to IgG antibody. Level of IgM antibody increases to reach peak levels after 1–3 months. There is a slow decline in antibody levels over the next 9 months until negativation [121, 122]. It was revealed that IgM antibodies can be detected for 12 years following the acute phase of infection [121]. On the other hand, autoimmune antibodies, such as rheumatoid factor and antinuclear antibodies, non-specific in vitro binding and acute viral infection, can cause false-positive IgM results up to 60% and results of commercial kits used in reference laboratories. This can be viewed as the limitation of this method since it cannot be determined whether the patient has contracted this infection a few months ago or is in the acute phase of the disease which can put the fetus at risk [123, 124].
Positive IgM test results should be confirmed in reference laboratories using specific tests, such as IgG avidity, or by evaluation of IgA and IgE antibodies [125]. When the IgM serologic reaction is positive in an asymptomatic patient, the IgG avidity test is an auxiliary test to distinguish between acute and chronic infection. Avidity antibodies are low in pregnant women who were infected at least 3–5 months earlier. Low-avidity may persist for 1 year. In these cases, the interpretation of the results needs more precision [125, 126].
It is better to study IgA antibody in pregnant women to continue the evaluation of the acute form of T.gondii infection. IgA production is similar to that of IgM and it appears during the first week, whereas IgA antibody peak occurs later than IgM and persist more than 3 or 4 months following primary infection; therefore, they disappear earlier than IgM [127]. IgA may persist for more than a year [18, 120, 127]. Therefore, it is not also sufficient in the detection of acute infection in adults [128].
On the other hand, specific IgE antibodies are produced rapidly and remain detectable less than 4 months after infection by ELISA in sera of adults with acute infection, neonates infected with congenital infection, and children with chorioretinitis [129]. IgE antibody may be helpful in the diagnosis of lymphadenopathies, and the persistence of IgE can be an indicator of active toxoplasmosis [130]. Although IgE seropositivity occurs for a briefer period than IgM or IgA antibodies and it is useful for the diagnosis of the acute form [131, 132], it does not have enough sensitivity [130].
In pregnant women, confirmation of active toxoplasmosis in reference laboratories can be achieved by the inoculation of body fluids or tissues in mouse or cell culture [18]. Mouse inoculation is absolutely sensitive; however, it requires the use of live animals, housing, euthanasia, autopsy and antibody testing. In addition, it takes a maximum of 6 weeks to obtain a diagnosis and is not widely available method for modem clinical laboratories [133, 134]. Cell culture is a practical method for the detection of T. gondii than mouse inoculation; however, it is relatively slow and may not be sensitive [135].
Unfortunately, in spite of the importance of cell culture and mouse inoculation for the detection of T. gondii, most researchers have not used these techniques in their researches. Moreover, polymerase chain reaction can be performed on amniotic fluid to detect T. gondii-specific DNA after 18 weeks of pregnancy [136].
Limitations
The limitations of the present study include: 1) lack of large cohort of women with T. gondii infection, as compared to controls for the investigation of the association between abortion and T. gondii infection (because cross-sectional and case-control studies due to their nature, do not provide the possibility to explore the causal relationship), 2) the diversity of the diagnostic methods with different sensitivity and specificity, 3) The use of English articles due to lack of fluency in other languages.
Conclusion
Since the pooled ORs for anti-T. gondii IgG and IgM antibodies in different studies among women who had spontaneous abortion were higher than controls, it shows a possible relationship between T. gondii and spontaneous abortion. Hence, emphasize on health education especially on the Toxoplasma transmission routes in the childhood, and performance of screening program using regular serologic tests during pregnancy could help physicians in the diagnosis, prevention, and treatment of toxoplasmosis and reduction of the economic burden of the disease on society.
Supporting information
(DOC)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Acknowledgments
This article is an approved plan from Student Research Committee of Mazandaran University of Medical Sciences, Sari, Iran (number: 6101). The code of ethics of this plan is (IR.MAZUMS.REC.1398.6101).
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Fuentes I, Rodriguez M, Domingo CJ, del Castillo F, Juncosa T, Alvar J. Urine sample used for congenital toxoplasmosis diagnosis by PCR. J Clin Microbiol. 1996;34(10):2368–71. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pappas G, Roussos N, Falagas ME. Toxoplasmosis snapshots: global status of Toxoplasma gondii seroprevalence and implications for pregnancy and congenital toxoplasmosis. Int J Parasitol. 2009;39(12):1385–94. https://doi.10.1016/j.ijpara.2009.04.003 . [DOI] [PubMed] [Google Scholar]
- 3.Smith JL. Documented outbreaks of toxoplasmosis: transmission of Toxoplasma gondii to humans. J Food Prot. 1993;56(7):630–9. https://doi.10.4315/0362-028x-56.7.630 . [DOI] [PubMed] [Google Scholar]
- 4.Dubey JP. Advances in the life cycle of Toxoplasma gondii. Int J Parasitol. 1998;28(7):1019–24. 10.1016/s0020-7519(98)00023-x . [DOI] [PubMed] [Google Scholar]
- 5.Elnahas A, Gerais AS, Elbashir MI, Eldien ES, Adam I. Toxoplasmosis in pregnant Sudanese women. Saudi Med J. 2003;24(8):868–70. . [PubMed] [Google Scholar]
- 6.Hajsoleimani F, Ataeian A, Nourian A, Mazloomzadeh S. Seroprevalence of Toxoplasma gondii in pregnant women and bioassay of IgM positive cases in Zanjan, northwest of Iran. Iran J Parasitol. 2012;7(2):82–6. . [PMC free article] [PubMed] [Google Scholar]
- 7.Hill D, Dubey JP. Toxoplasma gondii: transmission, diagnosis and prevention. Clin Microbiol Infect. 2002;8(10):634–40. https://doi.10.1046/j.1469-0691.2002.00485.x . [DOI] [PubMed] [Google Scholar]
- 8.Alvarado-Esquivel C, Rascon-Careaga A, Hernandez-Tinoco J, Corella-Madueno MA, Sanchez-Anguiano LF, Aldana-Madrid ML, et al. Seroprevalence and associated risk factors for Toxoplasma gondii infection in healthy blood donors: a cross-sectional study in Sonora, Mexico. Biomed Res Int. 2016;2016:9597276 https://doi.10.1155/2016/9597276 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmadpour E, Daryani A, Sharif M, Sarvi S, Aarabi M, Mizani A, et al. Toxoplasmosis in immunocompromised patients in Iran: a systematic review and meta-analysis. J Infect Dev Ctries 2014;8(12):1503–10. https://doi.10.3855/jidc.4796 . [DOI] [PubMed] [Google Scholar]
- 10.Tenter AM, Heckeroth AR, Weiss LM. Toxoplasma gondii: from animals to humans. Int J Parasitol Parasites Wildl. 2000;30(12–13):1217–58. 10.1016/s0020-7519(00)00124-7 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sukthana Y. Toxoplasmosis: beyond animals to humans. Trends Parasitol. 2006;22(3):137–42. https://doi.10.1016/j.pt.2006.01.007 . [DOI] [PubMed] [Google Scholar]
- 12.Song KJ, Shin JC, Shin HJ, Nam HW. Seroprevalence of toxoplasmosis in Korean pregnant women. Korean J Parasitol. 2005;43(2):69–71. https://doi.10.3347/kjp.2005.43.2.69 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Campello Porto L, Duarte EC. Association between the risk of congenital toxoplasmosis and the classification of toxoplasmosis in pregnant women and prenatal treatment in Brazil, 1994–2009. Int J Infect Dis 2012;16(7):e480–6. https://doi.10.1016/j.ijid.2012.01.016 . [DOI] [PubMed] [Google Scholar]
- 14.Torgerson PR, Mastroiacovo P. The global burden of congenital toxoplasmosis: a systematic review. Bull World Health Organ. 2013;91(7):501–8. https://doi.10.2471/blt.12.111732 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Eslamirad Z, Mosayebi M, Hajihossein R. Molecular testing for Toxoplasma diagnosis in aborted fetuses-taleghani maternity hospital-Arak-Iran. Res Mol Med. 2014;2(3):41–4. [Google Scholar]
- 16.Stirrat GM. Recurrent miscarriage. Lancet. 1990;336(8716):673–5. https://doi.10.1016/0140-6736(90)92159-f . [DOI] [PubMed] [Google Scholar]
- 17.Ford HB, Schust DJ. Recurrent pregnancy loss: etiology, diagnosis, and therapy. Rev Obstet Gynecol. 2009;2(2):76–83. . [PMC free article] [PubMed] [Google Scholar]
- 18.Rorman E, Zamir CS, Rilkis I, Ben-David H. Congenital toxoplasmosis—prenatal aspects of Toxoplasma gondii infection. Reprod Toxicol. 2006;21(4):458–72. https://doi.10.1016/j.reprotox.2005.10.006 . [DOI] [PubMed] [Google Scholar]
- 19.Coste J, Job-Spira N, Fernandez H. Risk factors for spontaneous abortion: a case-control study in France. Hum Reprod. 1991;6(9):1332–7. https://doi.10.1093/oxfordjournals.humrep.a137535 . [DOI] [PubMed] [Google Scholar]
- 20.Nielsen GL, Sorensen HT, Larsen H, Pedersen L. Risk of adverse birth outcome and miscarriage in pregnant users of non-steroidal anti-inflammatory drugs: population based observational study and case-control study. BMJ. 2001;322(7281):266–70. https://doi.10.1136/bmj.322.7281.266 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sopori M. Effects of cigarette smoke on the immune system. Nat Rev Immunol. 2002;2(5):372–7. https://doi.10.1038/nri803 . [DOI] [PubMed] [Google Scholar]
- 22.Lashen H, Fear K, Sturdee DW. Obesity is associated with increased risk of first trimester and recurrent miscarriage: matched case-control study. Hum Reprod. 2004;19(7):1644–6. https://doi.10.1093/humrep/deh277 . [DOI] [PubMed] [Google Scholar]
- 23.Maconochie N, Doyle P, Prior S, Simmons R. Risk factors for first trimester miscarriage—results from a UK-population-based case-control study. BJOG. 2007;114(2):170–86. https://doi.10.1111/j.1471-0528.2006.01195.x . [DOI] [PubMed] [Google Scholar]
- 24.Lindbohm ML, Sallmen M, Taskinen H. Effects of exposure to environmental tobacco smoke on reproductive health. Scand J Work Environ Health. 2002;28 (2):84–96. . [PubMed] [Google Scholar]
- 25.Ness RB, Grisso JA, Hirschinger N, Markovic N, Shaw LM, Day NL, et al. Cocaine and tobacco use and the risk of spontaneous abortion. N Engl J Med. 1999;340(5):333–9. https://doi.10.1056/nejm199902043400501 . [DOI] [PubMed] [Google Scholar]
- 26.Kesmodel U, Wisborg K, Olsen SF, Henriksen TB, Secher NJ. Moderate alcohol intake in pregnancy and the risk of spontaneous abortion. Alcohol and Alcoholism. 2002;37(1):87–92. https://doi.10.1093/alcalc/37.1.87 . [DOI] [PubMed] [Google Scholar]
- 27.Rasch V. Cigarette, alcohol, and caffeine consumption: risk factors for spontaneous abortion. Acta Obstet Gynecol Scand. 2003;82(2):182–8. https://doi.10.1034/j.1600-0412.2003.00078.x . [DOI] [PubMed] [Google Scholar]
- 28.Hemels ME, Einarson A, Koren G, Lanctot KL, Einarson TR. Antidepressant use during pregnancy and the rates of spontaneous abortions: a meta-analysis. Ann Pharmacother. 2005;39(5):803–9. https://doi.10.1345/aph.1E547 . [DOI] [PubMed] [Google Scholar]
- 29.Clifford K, Rai R, Watson H, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod. 1994;9(7):1328–32. https://doi.10.1093/oxfordjournals.humrep.a138703 . [DOI] [PubMed] [Google Scholar]
- 30.Srinivas SK, Ma Y, Sammel MD, Chou D, McGrath C, Parry S, et al. Placental inflammation and viral infection are implicated in second trimester pregnancy loss. Am J Obstet Gynecol MFM. 2006;195(3):797–802. https://doi.10.1016/j.ajog.2006.05.049 . [DOI] [PubMed] [Google Scholar]
- 31.Baud D, Regan L, Greub G. Emerging role of Chlamydia and Chlamydia-like organisms in adverse pregnancy outcomes. Curr Opin Infect Dis. 2008;21(1):70–6. https://doi.10.1097/QCO.0b013e3282f3e6a5 . [DOI] [PubMed] [Google Scholar]
- 32.Pavlinová J, Kinčeková J, Ostró A, Saksun L, Vasilková Z, Königová AJH. Parasitic infections and pregnancy complications. Helminthologia. 2011;48(1):8–12. https://doi.10.2478/s11687-011-0002-x [Google Scholar]
- 33.Nigro G, Mazzocco M, Mattia E, Di Renzo GC, Carta G, Anceschi MM. Role of the infections in recurrent spontaneous abortion. J Matern Fetal Med. 2011;24(8):983–9. https://doi.10.3109/14767058.2010.547963 . [DOI] [PubMed] [Google Scholar]
- 34.Vaquero E, Lazzarin N, De Carolis C, Valensise H, Moretti C, Ramanini C. Mild thyroid abnormalities and recurrent spontaneous abortion: diagnostic and therapeutical approach. Am J Reprod Immunol. 2000; 43(4):204–8. https://doi.10.1111/j.8755-8920.2000.430404.x . [DOI] [PubMed] [Google Scholar]
- 35.Rubio C, Simón C, Vidal F, Rodrigo L, Pehlivan T, Remohí J, Pellicer A. Chromosomal abnormalities and embryo development in recurrent miscarriage couples. Hum Reprod. 2003; 18(1):182–8. https://doi.10.1093/humrep/deg015 . [DOI] [PubMed] [Google Scholar]
- 36.Weltz JI. Low molecular weight heparins. N Engl J Med. 1997; 337(10):688–98. https://doi.10.1056/NEJM199709043371007 . [DOI] [PubMed] [Google Scholar]
- 37.Glueck CJ, Wang P, Goldenberg N, Sieve-Smith L. Pregnancy outcomes among women with polycystic ovary syndrome treated with metformin. Hum Reprod. 2002; 17(11):2858–64. https://doi.10.1093/humrep/17.11.2858 . [DOI] [PubMed] [Google Scholar]
- 38.Derksen RH, de Groot PG. The obstetric antiphospholipid syndrome. J Reprod Immunol. 2008; 77(1):41–50. https://doi.10.1016/j.jri.2006.12.003 . [DOI] [PubMed] [Google Scholar]
- 39.Matovina M, Husnjak K, Milutin N, Ciglar S, Grce M (2004) Possible role of bacterial and viral infections in miscarriages. Fertil Steril. 2004; 81(3):662–9. https://doi.10.1016/j.fertnstert.2003.08.020 . [DOI] [PubMed] [Google Scholar]
- 40.Polifka JE, Friedman JM (1991) Environmental toxins and recurrent pregnancy loss. Infertil Reprod Med Clin North Am. 1991; 2:175–213. [Google Scholar]
- 41.Negero J, Yohannes M, Woldemichael K, Tegegne D. Seroprevalence and potential risk factors of T. gondii infection in pregnant women attending antenatal care at Bonga Hospital, Southwestern Ethiopia. Int J Infect Dis. 2017;57:44–9. https://doi.10.1016/j.ijid.2017.01.013 . [DOI] [PubMed] [Google Scholar]
- 42.Awoke K, Nibret E, Munshea A. Sero-prevalence and associated risk factors of Toxoplasma gondii infection among pregnant women attending antenatal care at Felege Hiwot Referral Hospital, northwest Ethiopia. Asian Pac J Trop Med. 2015;8(7):549–54. https://doi.10.1016/j.apjtm.2015.06.014 . [DOI] [PubMed] [Google Scholar]
- 43.Daryani A, Nayeri Chegeni T, Sarvi S, Hosseininejad Z, Amouei A, Moosazadeh M. Toxoplasma gondii and human abortion: a systematic review and meta-analysis. PROSPERO. 2019. [DOI] [PMC free article] [PubMed]
- 44.Moher D, Liberati A, Tetzlaff J, Altman DG. Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–80. . [PubMed] [Google Scholar]
- 45.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–5. https://doi.10.1007/s10654-010-9491-z . [DOI] [PubMed] [Google Scholar]
- 46.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–58. https://doi.10.1002/sim.1186 . [DOI] [PubMed] [Google Scholar]
- 47.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–34. https://doi.10.1136/bmj.315.7109.629 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abou-Gabal KM, El-Fayoumi H, El-Shabrawy E, Hammed A, Roshdi A. Prevalence and causes of recurrent abortion among women in beni-suef governorate. Life Sci J. 2013;10(3). [Google Scholar]
- 49.Amin A, Mazloomzadeh S, Haniloo A, Mohammadian F, Fazaeli A. Evaluation of anti-Toxoplasma IgG, IgM, and IgA in mothers with spontaneous abortion in Zanjan, Northwest Iran. Korean J Parasitol. 2012;50(4):371–4. https://doi.10.3347/kjp.2012.50.4.371 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tammam AE, Haridy MA, Abdellah AH, Ahmed SR, Fayed HM, Alsammani MA. Seroepidemiology of Toxoplasma gondii infection in women with first trimester spontaneous miscarriage in qena governorate, egypt. J Clin Diagn Res. 2013;7(12):2870–3. https://doi.10.7860/jcdr/2013/6480.3780 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.El Aal AAA, Nahnoush RK, Elmallawany MA, El-Sherbiny WS, Badr MS, Nasr GM. Isothermal PCR for feasible molecular diagnosis of primary toxoplasmosis in women recently experienced spontaneous abortion. Open Access Maced J Med Sci. 2018;6(6):982–7. https://doi.10.3889/oamjms.2018.227 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sanghi A, Morgan-Capner P, Hesketh L, Elstein M. Zoonotic and viral infection in fetal loss after 12 weeks. Br J Obstet Gynaecol. 1997;104(8):942–5. 10.1111/j.1471-0528.1997.tb14355.x . [DOI] [PubMed] [Google Scholar]
- 53.Vado-Solis IA, Suarez-Solis V, Jimenez-Delgadillo B, Zavala-Velazquez JE, Segura-Correa JC. Toxoplasma gondii presence in women with spontaneous abortion in Yucatan, Mexico. J Protozool. 2013;99(2):383–5. https://doi.10.1645/ge-3189.1 . [DOI] [PubMed] [Google Scholar]
- 54.Hernández-Cortazar IB, Acosta-Viana KY, Guzman-Marin E, Segura-Correa JC, Ortega-Pacheco A, Carrillo-Martínez JR, et al. Toxoplasma gondii in women with recent abortion from Southern Mexico. Asian Pac J Trop Dis. 2016;6(3):193–8. https://doi.10.1016/S2222-1808(15)61012-X. [Google Scholar]
- 55.Kimball AC, Kean BH, Fuchs F. The role of toxoplasmosis in abortion. Am J Obstet Gynecol. 1971;111(2):219–26. https://doi.10.1016/0002-9378(71)90893-3 . [DOI] [PubMed] [Google Scholar]
- 56.Hadi NJ. Prevalence of Antibodies to Cytomegalovirus, Rubella Virus and Toxoplasma gondii among aborted women in Thiqar province. JEPS. 2011;1(5):3–9. [Google Scholar]
- 57.Stray-Pedersen B, Lorentzen-Styr AM. The prevalence of Toxoplasma antibodies among 11,736 pregnant women in Norway. Scand J Infect Dis. 1979;11(2):159–65. https://doi.10.3109/inf.1979.11.issue-2.12 . [DOI] [PubMed] [Google Scholar]
- 58.Decavalas G, Papapetropoulou M, Giannoulaki E, Tzigounis V, Kondakis XG. Prevalence of Toxoplasma gondii antibodies in gravidas and recently aborted women and study of risk factors. Eur J Epidemiol. 1990;6(2):223–6. https://doi.10.1007/bf00145798 . [DOI] [PubMed] [Google Scholar]
- 59.Singh N. Status of Toxoplasma antibodies in recurrent fetal loss in U.A.E. women. Indian J Pediatr. 1998;65(6):891–7. https://doi.10.1007/bf02831357 . [DOI] [PubMed] [Google Scholar]
- 60.Qublan HS, Jumaian N, Abu-Salem A, Hamadelil FY, Mashagbeh M, Abdel-Ghani F. Toxoplasmosis and habitual abortion. J Obstet Gynaecol. 2002;22(3):296–8. https://doi.10.1080/01443610220130616 . [DOI] [PubMed] [Google Scholar]
- 61.Nissapatorn V, Noor Azmi M, Cho S, Fong M, Init I, Rohela M, et al. Toxoplasmosis: prevalence and risk factors. J Obstet Gynaecol. 2003;23(6):618–24. https://doi.10.1080/01443610310001604376 . [DOI] [PubMed] [Google Scholar]
- 62.Chopra S, Arora U, Aggarwal A. Prevalence of IgM antibodies to Toxoplasma, Rubella and Cytomegalovirus infections during pregnancy. JK science. 2004;6(4):190–2. [Google Scholar]
- 63.Ertug S, Okyay P, Turkmen M, Yuksel H. Seroprevalence and risk factors for Toxoplasma infection among pregnant women in Aydin province, Turkey. BMC Public Health. 2005;5:66 https://doi.10.1186/1471-2458-5-66 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barbosa IR, de Carvalho Xavier Holanda CM, de Andrade-Neto VF. Toxoplasmosis screening and risk factors amongst pregnant females in Natal, northeastern Brazil. Trans R Soc Trop Med Hyg. 2009;103(4):377–82. https://doi.10.1016/j.trstmh.2008.11.025 . [DOI] [PubMed] [Google Scholar]
- 65.Nijem KI, Al-Amleh S. Seroprevalence and associated risk factors of toxoplasmosis in pregnant women in Hebron district, Palestine. East Mediterr Health J. 2009;15(5):1278–84. . [PubMed] [Google Scholar]
- 66.Mousa D, Mohammad M, Toboli A. Toxoplasma gondii infection in pregnant women with previous adverse pregnancy outcome. Med J Islamic World Acad Sci. 2011;109(412):1–8. [Google Scholar]
- 67.Drueish MJ, Aziz FM. Toxoplasmosis: serious disease during pregnancy. Baghdad Sci J. 2011;8(1):91–5. [Google Scholar]
- 68.Jasim M, Majeed HA, Ali AI. Performance of serological diagnosis of TORCH agents in aborted versus non aborted women of waset province in Iraq. MJOTU. 2011;2(172):141–7. [Google Scholar]
- 69.Nissapatorn V, Suwanrath C, Sawangjaroen N, Ling LY, Chandeying V. Toxoplasmosis-serological evidence and associated risk factors among pregnant women in southern Thailand. The Am J Trop Med Hyg. 2011;85(2):243–7. https://doi.10.4269/ajtmh.2011.10-0633 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Malarvizhi A, Viswanathan T, Lavanya V, Moorthy K. Seroprevalence of Toxoplasma gondii in pregnant women. J Public Health Epidemiol. 2012;4(6):170–7. [Google Scholar]
- 71.Padmavathy M, Gowri M, Malini J, Umapathy B, Navaneeth B, Bhatia M, et al. Seroprevalence of TORCH infections and adverse reproductive outcome in current pregnancy with bad obstetric history. J Clin Biomed Sci. 2013;3(2):62–71. [Google Scholar]
- 72.Ebrahimzadeh A, Mohammadi S, Salimi-Khorashad A, Jamshidi A. Seroprevalence of toxoplasmosis among pregnant women referring to the reference laboratory of Zahedan, Iran. ZJRMS. 2013;15:32–5. [Google Scholar]
- 73.Moura FL, Amendoeira MR, Bastos OM, Mattos DP, Fonseca AB, Nicolau JL, et al. Prevalence and risk factors for Toxoplasma gondii infection among pregnant and postpartum women attended at public healthcare facilities in the city of niteroi, state of Rio de Janeiro, Brazil. Rev Soc Bras Med Trop. 2013;46(2):200–7. https://doi.10.1590/0037-8682-1613-2013 . [DOI] [PubMed] [Google Scholar]
- 74.Babaie J, Amiri S, Mostafavi E, Hassan N, Lotfi P, Esmaeili Rastaghi AR, et al. Seroprevalence and risk factors for Toxoplasma gondii infection among pregnant women in Northeast Iran. Clin Vaccine Immunol. 2013;20(11):1771–3. https://doi.10.1128/CVI.00125-13 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chintapalli S, Padmaja IJ. Seroprevalence of toxoplasmosis in antenatal women with bad obstetric history. Trop Parasitol. 2013;3(1):62–6. https://doi.10.4103/2229-5070.113915 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Alvarado-Esquivel C, Pacheco-Vega SJ, Hernandez-Tinoco J, Centeno-Tinoco MM, Beristain-Garcia I, Sanchez-Anguiano LF, et al. Miscarriage history and Toxoplasma gondii infection: a cross-sectional study in women in Durango City, Mexico. Eur J Microbiol Immunol. 2014;4(2):117–22. https://doi.10.1556/EuJMI.4.2014.2.4 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Almushait MA, Dajem SM, Elsherbiny NM, Eskandar MA, Al Azraqi TA, Makhlouf LM. Seroprevalence and risk factors of Toxoplasma gondii infection among pregnant women in south 0western, Saudi Arabia. J Parasit Dis. 2014;38(1):4–10. https://doi.10.1007/s12639-012-0195-z . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Abedi M, Heidari H, Dehkordi ZS, Youssefi MR. Serological study of toxoplasmosis in women with previous history of abortion at Hamedan’s medical centers during 2012–2013. Comp Clin Path. 2015;24(3):589–92. [Google Scholar]
- 79.Gelaye W, Kebede T, Hailu A. High prevalence of anti-Toxoplasma antibodies and absence of Toxoplasma gondii infection risk factors among pregnant women attending routine antenatal care in two Hospitals of Addis Ababa, Ethiopia. Int J Infect Dis. 2015;34:41–5. https://doi.10.1016/j.ijid.2015.03.005 . [DOI] [PubMed] [Google Scholar]
- 80.Alvarado-Esquivel C, Pacheco-Vega SJ, Salcedo-Jaquez M, Sanchez-Anguiano LF, Hernandez-Tinoco J, Rabago-Sanchez E, et al. Stillbirth history and Toxoplasma gondii infection in women attending public health centers in a northern Mexican City. Eur J Microbiol Immunol. 2015;5(2):164–71. https://doi.10.1556/1886.2015.00009 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anubhuti, Roy RR, Mittra J, Begum SJ. Seroprevalence of Toxoplasma gondii in spontaneous abortions in pregnant women. J Evol Med Dent Sci. 2015;4(39):6763–68. [Google Scholar]
- 82.Mohamed K, Bahathiq A, Degnah N, Basuni S, Al Malki A, Babalghith A. Detection of Toxoplasma gondii infection and associated risk factors among pregnant women in Makkah Al Mukarramah, Saudi Arabia. Asian Pac J Trop Dis. 2016;6(2):113–9. 10.1016/S2222-1808(15)60995-1. [DOI] [Google Scholar]
- 83.Mohaghegh MA, Kalani H, Hashemi M, Hashemi S, Yazdnezhad SK, Hejazi SH, et al. Toxoplasmosis-related risk factors in pregnant women in the north Khorasan province, Iran. IJMRHS. 2016;5(8):370–4. [Google Scholar]
- 84.Imam N F.A, Azzam Esra’a A.A, Attia AA. Seroprevalence of Toxoplasma gondii among pregnant women in Almadinah Almunawwarah KSA. J Taibah Univ Med Sci. 2016;11(3):255–9. [Google Scholar]
- 85.Nazir M, Akhtar M, Maqbool A, Waheed A, Sajid M, Ali M, et al. Antibody prevalence and risk factors for Toxoplasma gondii infection in women from Multan, Pakistan. Zoonoses Public Health. 2017;64(7):537–42. https://doi.10.1111/zph.12336 [DOI] [PubMed] [Google Scholar]
- 86.Yasmeen A, Prasa SR, Sheela SR, Krishnappa J. Screening of pregnant women for anti-Toxoplasma antibodies and their newborn for vertical transmission. J Clin Diagn Res. 2017;11(10). https://doi.10.7860/JCDR/2017/28231.10742. [Google Scholar]
- 87.Matin S, Shahbazi G, Namin ST, Moradpour R, Feizi F, Piri-Dogahe H. Comparison of placenta PCR and maternal serology of aborted women for detection of Toxoplasma gondii in Ardabil, Iran. Korean J Parasitol. 2017;55(6):607–11. https://doi.10.3347/kjp.2017.55.6.607 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Costa GB, de Oliveira MC, Gadelha SR, Albuquerque GR, Teixeira M, Da Silva Raiol MR, et al. Infectious diseases during pregnancy in Brazil: seroprevalence and risk factors. J Infect Dev Ctries. 2018;12(08):657–65. 10.3855/jidc.9492 [DOI] [PubMed] [Google Scholar]
- 89.Hafez Hassanain NA, Shaapan RM, Hafez Hassanain MA. Associated antenatal health risk factors with incidence of toxoplasmosis in Egyptian pregnant women. Pak J Biol Sci. 2018;21(9):463–8. https://doi.10.3923/pjbs.2018.463.468 . [DOI] [PubMed] [Google Scholar]
- 90.Rashno MM, Fallahi S, Arab-Mazar Z, Dana H. Seromolecular assess of Toxoplasma gondii infection in pregnant women and neonatal umbilical cord blood. EXCLI J. 2019;18:1–7. . [PMC free article] [PubMed] [Google Scholar]
- 91.Stray-Pedersen B, Lorentzen-Styr AM. Uterine Toxoplasma infections and repeated abortions. Am J Obstet Gynecol. 1977;128(7):716–21. https://doi.10.1016/0002-9378(77)90709-8 . [DOI] [PubMed] [Google Scholar]
- 92.Lolis D, Tzigounis V, Michalas S, Koumentakou E, Kaskarelis D. Toxoplasma antibodies and spontaneous abortion. Int J Gynaecol Obstet. 1978;15(4):299–301. 10.1002/j.1879-3479.1977.tb00697.x [DOI] [PubMed] [Google Scholar]
- 93.Galvan Ramirez ML, Soto Mancilla JL, Velasco Castrejon O, Perez Medina R. Incidence of anti-Toxoplasma antibodies in women with high-risk pregnancy and habitual abortions. Rev Soc Bras Med Trop.1995;28(4):333–7. 10.1590/s0037-86821995000400005 . [DOI] [PubMed] [Google Scholar]
- 94.Sahwi SY, Zaki MS, Haiba NY, Elsaid OK, Anwar MY, AbdRabbo SA. Toxoplasmosis as a cause of repeated abortion. J Obstet Gynaecol. 1995;21(2):145–8. 10.1111/j.1447-0756.1995.tb01087.x . [DOI] [PubMed] [Google Scholar]
- 95.Djurkovic-Djakovic O JG, investigation o. Toxoplasma infection and pathological outcome of pregnancy. Gynecol Obstet Invest. 1995;40(1):36–41. https://doi.10.1159/000292299 . [DOI] [PubMed] [Google Scholar]
- 96.Al Hamdani MM, Mahdi NK. Toxoplasmosis among women with habitual abortion. East Mediterr Health J. 1996;3(2):310–315. [Google Scholar]
- 97.Zargar AH, Masoodi SR, Laway BA, Sofi BA, Wani AI. Seroprevalence of toxoplasmosis in women with repeated abortions in Kashmir. J Epidemiol Community Health. 1998;52(2):135–6. https://doi.10.1136/jech.52.2.135 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nimri L, Pelloux H, Elkhatib L. Detection of Toxoplasma gondii DNA and specific antibodies in high-risk pregnant women. Am J Trop Med Hyg. 2004;71(6):831–5. . [PubMed] [Google Scholar]
- 99.Surpam RB, Kamlakar UP, Khadse R, Qazi M, Jalgaonkar SV. Serological study for TORCH infections in women with bad obstetric history. J Obstet Gynecol Indi. 2006;56(1):41–3. [Google Scholar]
- 100.Sebastian D, Zuhara K, Sekaran K. Influence of TORCH infections in first trimester miscarriage in the Malabar region of Kerala. Afr J Microbiol Res. 2008;2(3):56–9. [Google Scholar]
- 101.Al-Saeed MS, Muhsin MA, AL-Juburi GJ. Study the role of Toxoplasma gondii, Cytomegalovirus and anti-phospholipids antibodies in cases of abortion among women in Hilla city. QMJ. 2008;4(6):27–34. [Google Scholar]
- 102.AL-Taie AAD. Serological study for TORCH infections in women with high delivery risk factors in Mosul. Tikrit Journal of Pure Science. 2010;15(1):193–8. [Google Scholar]
- 103.Mohymen NA, Hussien A, Hassan FK. Association between TORCH agents and recurrent spontaneous abortion. Iraqi J Med Sci. 2009;7(4):40–6. [Google Scholar]
- 104.Elamin MH, Al-Olayan EM, Omer SA, Alagaili AN, Mohammed OB. Molecular detection and prevalence of Toxoplasma gondii in pregnant women in Sudan. Afr J Microbiol Res. 2012;6(2):308–11. https://doi.10.5897/AJMR11.1314. [Google Scholar]
- 105.Hussan BM. Study the prevalence of ACL, APL, CMV, HSV, Rubella and Toxoplasma gondii in aborted women in Baghdad. MJB. 2013;10(2):455–64. [Google Scholar]
- 106.Siddiqui N, Shujatullah F, Khan HM, Rabbani T, Khan PA. IgG avidity antibodies against Toxoplasma gondii in high risk females of reproductive age group in India. Korean J Parasitol. 2014;52(5):487–91. https://doi.10.3347/kjp.2014.52.5.487 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sultana M, Hossain MS, Dewan F, Sultana J, Rashid M. Association of Toxoplasma gondii infection with spontaneous abortion. Bangladesh J Obstet Gynaeco. 2014;29(2):87–93. [Google Scholar]
- 108.Abbas HH, Alasadiy Y, Al-Tememi MJ. Detection Toxoplasma gondii by real-time PCR in abortive and pregnant women in Almuthanna province. JIARM. 2014;2:310–7. [Google Scholar]
- 109.Saki J, Mohammadpour N, Moramezi F, Khademvatan S. Seroprevalence of Toxoplasma gondii in women who have aborted in comparison with the women with normal delivery in Ahvaz, southwest of Iran. ScientificWorldJournal. 2015;2015:764369 https://doi.10.1155/2015/764369 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Kamal AM, Ahmed AK, Abdellatif MZ, Tawfik M, Hassan EE. Seropositivity of toxoplasmosis in pregnant women by ELISA at Minia university hospital, Egypt. Korean J Parasitol. 2015;53(5):605–10. https://doi.10.3347/kjp.2015.53.5.605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ghasemi FS, Rasti S, Piroozmand A, Bandehpour M, Kazemi B, Mousavi SG, et al. Toxoplasmosis-associated abortion and stillbirth in Tehran, Iran. J Matern Fetal Neonatal Med. 2016;29(2):248–51. https://doi.10.3109/14767058.2014.996127 . [DOI] [PubMed] [Google Scholar]
- 112.Rasti S, Ghasemi FS, Abdoli A, Piroozmand A, Mousavi SG, Fakhrie-Kashan Z. ToRCH "co-infections" are associated with increased risk of abortion in pregnant women. Congenit Anom. 2016;56(2):73–8. https://doi.10.1111/cga.12138 . [DOI] [PubMed] [Google Scholar]
- 113.Çakmak BD, Dündar B, Bayram F, Özgen G. Toxoplasma gondii seropositivity in pregnancies with normal delivery and complicated with abortion. EuRJ. 2018;4(4):275–9. https://doi.10.18621/eurj.339882. [Google Scholar]
- 114.Kheirandish F, Ezatpour B, Fallahi SH, Tarahi MJ, Hosseini P, Karimi Rouzbahani A, et al. Toxoplasma serology status and risk of miscarriage, a case-control study among women with a history of spontaneous abortion. Int J Fertil Steril. 2019;13(3):184–9. https://doi.10.22074/ijfs.2019.5740 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Weiss LM, Dubey JP. Toxoplasmosis: A history of clinical observations. Int J Parasitol. 2009;39(8):895–901. https://doi.10.1016/j.ijpara.2009.02.004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dard C, Fricker-Hidalgo H, Brenier-Pinchart MP, Pelloux H. Relevance of and new developments in serology for toxoplasmosis. Trends Parasitol. 2016;32(6):492–506. https://doi.10.1016/j.pt.2016.04.001 . [DOI] [PubMed] [Google Scholar]
- 117.Villena I, Ancelle T, Delmas C, Garcia P, Brezin AP, Thulliez P, et al. Congenital toxoplasmosis in France in 2007: first results from a national surveillance system. Euro Surveill. 2010;15(25). https://doi.10.2807/ese.15.25.19600-en . [DOI] [PubMed] [Google Scholar]
- 118.Roberts CW, Walker W, Alexander J. Sex-associated hormones and immunity to protozoan parasites. Clin Microbiol Rev. 2001;14(3):476–88. 10.1128/CMR.14.3.476-488.2001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Robert-Gangneux F, Darde ML. Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev. 2012;25(2):264–96. https://doi.10.1128/cmr.05013-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bessieres MH, Roques C, Berrebi A, Barre V, Cazaux M, Seguela JP. IgA antibody response during acquired and congenital toxoplasmosis. J Clin Pathol. 1992;45(7):605–8. https://doi.10.1136/jcp.45.7.605 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bobic B, Sibalic D, Djurkovic-Djakovic O. High levels of IgM antibodies specific for Toxoplasma gondii in pregnancy 12 years after primary Toxoplasma infection. Case report. Gynecol Obstet Invest. 1991;31(3):182–4. https://doi.10.1159/000293151 . [DOI] [PubMed] [Google Scholar]
- 122.Villard O, Cimon B, L’Ollivier C, Fricker-Hidalgo H, Godineau N, Houze S, et al. Serological diagnosis of Toxoplasma gondii infection: recommendations from the French national reference center for toxoplasmosis. Diagn Microbiol Infect Dis. 2016;84(1):22–33. https://doi.10.1016/j.diagmicrobio.2015.09.009 . [DOI] [PubMed] [Google Scholar]
- 123.Montoya JG, Liesenfeld O. Toxoplasmosis. Lancet. 2004;363(9425):1965–76. https://doi.10.1016/s0140-6736(04)16412-x . [DOI] [PubMed] [Google Scholar]
- 124.De Paschale M, Agrappi C, Belvisi L, Cagnin D, Cerulli T, Clerici P, et al. Revision of the positive predictive value of IgM anti-Toxoplasma antibodies as an index of recent infection. New Microbiol. 2008;31(1):105–11. . [PubMed] [Google Scholar]
- 125.Lopes FM, Goncalves DD, Mitsuka-Bregano R, Freire RL, Navarro IT. Toxoplasma gondii infection in pregnancy. Braz J Infect Dis. 2007;11(5):496–506. 10.1590/s1413-86702007000500011 . [DOI] [PubMed] [Google Scholar]
- 126.Remington JS, Thulliez P, Montoya JG. Recent developments for diagnosis of toxoplasmosis. J Clin Microbiol. 2004;42(3):941–5. 10.1128/JCM.42.3.941-945.2004 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Murat JB, Souvignet A, Fricker-Hidalgo H, Brenier-Pinchart MP, Bost-Bru C, Pelloux H. Assessment of the IgA immunosorbent agglutination assay for the diagnosis of congenital toxoplasmosis on a series of 145 toxoplasmic seroconversions. Clin Vaccine Immunol. 2015;22(4):456–8. https://doi.10.1128/cvi.00666-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Stepick-Biek P, Thulliez P, Araujo FG, Remington JS. IgA antibodies for diagnosis of acute congenital and acquired toxoplasmosis. J Infect Dis. 1990;162(1):270–3. https://doi.10.1093/infdis/162.1.270 . [DOI] [PubMed] [Google Scholar]
- 129.Pinon JM, Toubas D, Marx C, Mougeot G, Bonnin A, Bonhomme A, et al. Detection of specific immunoglobulin E in patients with toxoplasmosis. J Clin Microbiol. 1990;28(8):1739–43 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Foudrinier F, Villena I, Jaussaud R, Aubert D, Chemla C, Martinot F, et al. Clinical value of specific immunoglobulin E detection by enzyme-linked immunosorbent assay in cases of acquired and congental toxoplasmosis. J Clin Microbiol. 2003;41(4):1681–6. 10.1128/JCM.41.4.1681-1686.2003 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Montoya JG, Remington JS. Studies on the serodiagnosis of toxoplasmic lymphadenitis. Clin Infect Dis. 1995;20(4):781–9. https://doi.10.1093/clinids/20.4.781 . [DOI] [PubMed] [Google Scholar]
- 132.Wong SY, Hajdu MP, Ramirez R, Thulliez P, McLeod R, Remington JS. Role of specific immunoglobulin E in diagnosis of acute Toxoplasma infection and toxoplasmosis. J Clin Microbiol. 1993;31(11):2952–9. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Derouin F, Mazeron MC, Garin YJ. Comparative study of tissue culture and mouse inoculation methods for demonstration of Toxoplasma gondii. J Clin Microbiol.1987;25(9):1597–600. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hitt JA, Filice GA. Detection of Toxoplasma gondii parasitemia by gene amplification, cell culture, and mouse inoculation. J Clin Microbiol. 1992;30(12):3181–4. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.James GS, Sintchenko VG, Dickeson DJ, Gilbert GL. Comparison of cell culture, mouse inoculation, and PCR for detection of Toxoplasma gondii: effects of storage conditions on sensitivity. J Clin Microbiol. 1996;34(6):1572–5. . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Hohlfeld P, Daffos F, Costa JM, Thulliez P, Forestier F, Vidaud M. Prenatal diagnosis of congenital toxoplasmosis with a polymerase-chain-reaction test on amniotic fluid. N Engl J Med. 1994;331(11):695–9. https://doi.10.1056/nejm199409153311102 . [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(DOCX)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
(TIF)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.